User login
Old drug is new treatment for chronic prostatitis
SAN DIEGO – Oral fosfomycin, a drug used for more than 4 decades to treat urinary tract infections in women, has gained a new life as a promising treatment for chronic prostatitis.
In the largest patient series reported to date, a 6-week course of fosfomycin resulted in an 85% clinical cure rate in 20 men with chronic prostatitis due to multidrug-resistant pathogens, Dr. Ilias Karaiskos reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This is a most welcome development because chronic prostatitis is a common condition and Escherichia coli – the number-one pathogen – is becoming increasingly resistant to fluoroquinolones, long considered the first-line therapy. The quinolone resistance issue is of particular concern because most other antibiotics lack the pharmacokinetics required to penetrate the prostate gland, explained Dr. Karaiskos of Hygeia General Hospital in Athens.
A recent study by other investigators showing that fosfomycin penetrates the prostate and achieves potentially therapeutic levels (Clin Infect Dis. 2014 Feb;58[4]:e101-5) inspired Dr. Karaiskos and coworkers to conduct their open 20-patient trial. Participants averaged 2.25 prior episodes of prostatitis.
Urine cultures showed that the most common pathogen was indeed E. coli, and that 15 of the 20 strains were resistant to fluoroquinolones. Most strains were also resistant to minocycline and trimethoprim-sulfamethoxazole. However, all strains were sensitive to fosfomycin (Monurol).
Dosing of fosfomycin in the study was 3 g once daily for the first week, then 3 g every 48 hours for the next 5 weeks.
Seventeen of 20 patients experienced clinical cure, defined as resolution of all symptoms plus absence of any evidence of inflammation upon follow-up imaging of the prostate by transrectal ultrasound or MRI upon treatment completion after 6 weeks of fosfomycin. Two patients failed to respond, and one discontinued treatment due to diarrhea.
Diarrhea was the most common treatment-emergent adverse event, affecting 5 of 20 patients. In most instances, diarrhea subsided when the dosing intervals were extended.
Further studies are needed to clarify the best fosfomycin dosing regimen for chronic prostatitis, Dr. Karaiskos said. For uncomplicated urinary tract infections the drug is typically given in a single megadose.
Dr. Karaiskos reported having no financial conflicts regarding this study, conducted free of commercial support.
SAN DIEGO – Oral fosfomycin, a drug used for more than 4 decades to treat urinary tract infections in women, has gained a new life as a promising treatment for chronic prostatitis.
In the largest patient series reported to date, a 6-week course of fosfomycin resulted in an 85% clinical cure rate in 20 men with chronic prostatitis due to multidrug-resistant pathogens, Dr. Ilias Karaiskos reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This is a most welcome development because chronic prostatitis is a common condition and Escherichia coli – the number-one pathogen – is becoming increasingly resistant to fluoroquinolones, long considered the first-line therapy. The quinolone resistance issue is of particular concern because most other antibiotics lack the pharmacokinetics required to penetrate the prostate gland, explained Dr. Karaiskos of Hygeia General Hospital in Athens.
A recent study by other investigators showing that fosfomycin penetrates the prostate and achieves potentially therapeutic levels (Clin Infect Dis. 2014 Feb;58[4]:e101-5) inspired Dr. Karaiskos and coworkers to conduct their open 20-patient trial. Participants averaged 2.25 prior episodes of prostatitis.
Urine cultures showed that the most common pathogen was indeed E. coli, and that 15 of the 20 strains were resistant to fluoroquinolones. Most strains were also resistant to minocycline and trimethoprim-sulfamethoxazole. However, all strains were sensitive to fosfomycin (Monurol).
Dosing of fosfomycin in the study was 3 g once daily for the first week, then 3 g every 48 hours for the next 5 weeks.
Seventeen of 20 patients experienced clinical cure, defined as resolution of all symptoms plus absence of any evidence of inflammation upon follow-up imaging of the prostate by transrectal ultrasound or MRI upon treatment completion after 6 weeks of fosfomycin. Two patients failed to respond, and one discontinued treatment due to diarrhea.
Diarrhea was the most common treatment-emergent adverse event, affecting 5 of 20 patients. In most instances, diarrhea subsided when the dosing intervals were extended.
Further studies are needed to clarify the best fosfomycin dosing regimen for chronic prostatitis, Dr. Karaiskos said. For uncomplicated urinary tract infections the drug is typically given in a single megadose.
Dr. Karaiskos reported having no financial conflicts regarding this study, conducted free of commercial support.
SAN DIEGO – Oral fosfomycin, a drug used for more than 4 decades to treat urinary tract infections in women, has gained a new life as a promising treatment for chronic prostatitis.
In the largest patient series reported to date, a 6-week course of fosfomycin resulted in an 85% clinical cure rate in 20 men with chronic prostatitis due to multidrug-resistant pathogens, Dr. Ilias Karaiskos reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This is a most welcome development because chronic prostatitis is a common condition and Escherichia coli – the number-one pathogen – is becoming increasingly resistant to fluoroquinolones, long considered the first-line therapy. The quinolone resistance issue is of particular concern because most other antibiotics lack the pharmacokinetics required to penetrate the prostate gland, explained Dr. Karaiskos of Hygeia General Hospital in Athens.
A recent study by other investigators showing that fosfomycin penetrates the prostate and achieves potentially therapeutic levels (Clin Infect Dis. 2014 Feb;58[4]:e101-5) inspired Dr. Karaiskos and coworkers to conduct their open 20-patient trial. Participants averaged 2.25 prior episodes of prostatitis.
Urine cultures showed that the most common pathogen was indeed E. coli, and that 15 of the 20 strains were resistant to fluoroquinolones. Most strains were also resistant to minocycline and trimethoprim-sulfamethoxazole. However, all strains were sensitive to fosfomycin (Monurol).
Dosing of fosfomycin in the study was 3 g once daily for the first week, then 3 g every 48 hours for the next 5 weeks.
Seventeen of 20 patients experienced clinical cure, defined as resolution of all symptoms plus absence of any evidence of inflammation upon follow-up imaging of the prostate by transrectal ultrasound or MRI upon treatment completion after 6 weeks of fosfomycin. Two patients failed to respond, and one discontinued treatment due to diarrhea.
Diarrhea was the most common treatment-emergent adverse event, affecting 5 of 20 patients. In most instances, diarrhea subsided when the dosing intervals were extended.
Further studies are needed to clarify the best fosfomycin dosing regimen for chronic prostatitis, Dr. Karaiskos said. For uncomplicated urinary tract infections the drug is typically given in a single megadose.
Dr. Karaiskos reported having no financial conflicts regarding this study, conducted free of commercial support.
AT ICAAC 2015
Key clinical point: Oral fosfomycin is an effective alternative to fluoroquinolones in chronic prostatitis patients.
Major finding: Six weeks of oral fosfomycin resulted in an 85% clinical cure rate in 20 men with multidrug-resistant chronic prostatitis.
Data source: This was an open-label, uncontrolled study.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
ESC: Cancer itself may cause cardiotoxicity
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
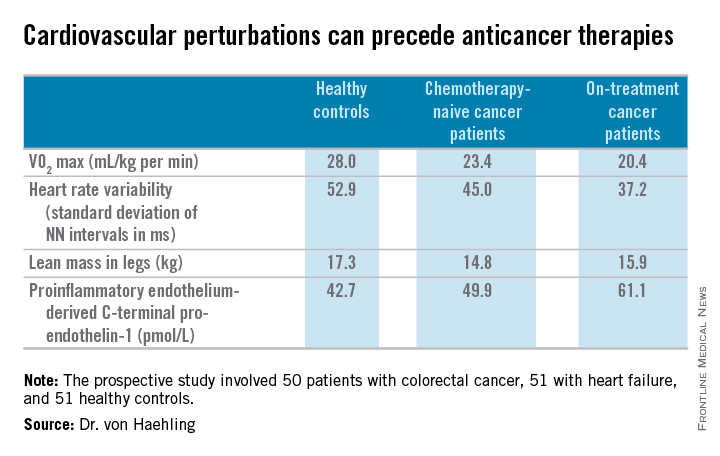
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.
For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
EXPERT ANALYSIS FROM THE ESC CONGRESS 2015
Progress in Treating Diabetic Foot Osteomyelitis
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
EXPERT ANALYSIS FROM ICAAC 2015
Progress in treating diabetic foot osteomyelitis
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
EXPERT ANALYSIS FROM ICAAC 2015
ESC: Mechanical dyssynchrony in narrow-QRS heart failure spells trouble
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
AT THE ESC CONGRESS 2015
Key clinical point: Persistent mechanical dyssynchrony in patients with heart failure and a narrow QRS interval appears to be a risk factor for poor outcomes rather than a modifiable therapeutic target.
Major finding: Patients with heart failure, narrow QRS width, and mechanical dyssynchrony whose dyssynchrony persisted or worsened during 6 months of follow-up were 54% more likely to experience death or heart failure hospitalization.
Data source: A secondary analysis of the large, multicenter, prospective, EchoCRT trial, which randomized patients to cardiac resynchronization therapy turned on or off.
Disclosures: The EchoCRT trial was sponsored by Biotronik. The study presenter reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
ESC: Mechanical dyssynchrony in narrow-QRS heart failure spells trouble
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
AT THE ESC CONGRESS 2015
Key clinical point: Persistent mechanical dyssynchrony in patients with heart failure and a narrow QRS interval appears to be a risk factor for poor outcomes rather than a modifiable therapeutic target.
Major finding: Patients with heart failure, narrow QRS width, and mechanical dyssynchrony whose dyssynchrony persisted or worsened during 6 months of follow-up were 54% more likely to experience death or heart failure hospitalization.
Data source: A secondary analysis of the large, multicenter, prospective, EchoCRT trial, which randomized patients to cardiac resynchronization therapy turned on or off.
Disclosures: The EchoCRT trial was sponsored by Biotronik. The study presenter reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
Ocular syphilis on the rise: What clinicians must know
SAN DIEGO – Cases of ocular syphilis are on the upswing in 2015, and many physicians are not up to speed regarding this serious complication, experts asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“I know that at my institution there’s been a lack of education about ocular syphilis. The housestaff are unaware that you can have ocular syphilis with a normal lumbar puncture,” said Dr. Kimberly A. Workowski, professor of medicine at Emory University in Atlanta and lead author of the Centers for Disease Control and Prevention 2015 STD guidelines (MMWR Recomm Rep. 2015;64[RR-03]:1-137).
When she asked for a show of hands by physicians in the audience who had seen a case of ocular syphilis in the past 6 months, a lot of arms shot up.
“We’ve had a large increase in ocular syphilis at our institution, too,” Dr. Workowski noted.
Dr. Juliet Stoltey of the University of California, San Francisco, said that since the initial January 2015 report of an outbreak of ocular syphilis in the Seattle area, cases have been reported from around the country, prompting the CDC to issue a clinical advisory in April.
It remains unclear whether the increase in ocular syphilis cases is the result of a true outbreak of a more oculo/neurotrophic strain of Treponema pallidum or simply the result of greater awareness of the complication in the setting of a steep rise in syphilis overall, according to Dr. Stoltey.
Circulation of a more neurotrophic strain is biologically plausible based on animal studies. Data from the University of Washington, Seattle, point to strain type 14d/f as a potential culprit.
California surveillance data show a more than 140% increase in syphilis cases overall during 2006-2014. And while it appears that the proportion of cases with ocular or other forms of neurologic syphilis has increased to an even greater extent than the overall rise in syphilis, the significance of this observation is uncertain given that the surveillance system lacks a standard mechanism for reporting ocular syphilis.
Regardless, Dr. Stoltey continued, here’s what physicians really need to know about ocular syphilis: Syphilis can affect virtually all parts of the eye, neurosyphilis can occur at any stage of the infection, and delayed identification of ocular syphilis has been associated with visual loss.
A syphilis serology test should be ordered in patients who have visual complaints along with risk factors for syphilis, such as men who have sex with men, as well as in those who have ophthalmologic findings compatible with syphilis. Both a treponemal and nontreponemal test should be ordered since the false-negative prozone effect has been documented in patients with ocular syphilis.
Clinical characteristics of ocular syphilis include eye redness, eye pain, visual field deficits or a decline in visual acuity, and headache accompanying eye symptoms. Ophthalmologic findings can include uveitis, iritis, vitrial detachment, optic neuritis, and marked vision loss.
Any patient with visual complaints plus a positive syphilis serology should undergo ophthalmologic evaluation immediately, Dr. Stoltey emphasized. A lumbar puncture and CSF analysis is recommended for syphilis patients with eye or neurologic symptoms such as cranial nerve dysfunction, auditory disease, meningitis, loss of vibration sensation, stroke, or altered mental status.
“I see signs of potential neurosyphilis being missed all the time,” Dr. Workowski said. “And here is a really important point: Treat for neurosyphilis if a patient with syphilis has the signs or symptoms of neurologic, audiologic, or ophthalmologic involvement regardless of what the CSF shows.”
The guideline-recommended treatment for ocular or neurosyphilis is aqueous crystalline penicillin G, 18-24 million units daily, administered as 3-4 million units IV every 4 hours for 10-14 days.
Dr. Stoltey urged physicians to store frozen pre–antibiotic therapy clinical samples from their patients with ocular syphilis and to contact the CDC, which, together with investigators at the University of Washington, Seattle, is conducting a study aimed at determining whether an oculotrophic strain of T. pallidum is circulating.
Dr. Stoltey and Dr. Workowski reported having no financial conflicts of interest.
SAN DIEGO – Cases of ocular syphilis are on the upswing in 2015, and many physicians are not up to speed regarding this serious complication, experts asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“I know that at my institution there’s been a lack of education about ocular syphilis. The housestaff are unaware that you can have ocular syphilis with a normal lumbar puncture,” said Dr. Kimberly A. Workowski, professor of medicine at Emory University in Atlanta and lead author of the Centers for Disease Control and Prevention 2015 STD guidelines (MMWR Recomm Rep. 2015;64[RR-03]:1-137).
When she asked for a show of hands by physicians in the audience who had seen a case of ocular syphilis in the past 6 months, a lot of arms shot up.
“We’ve had a large increase in ocular syphilis at our institution, too,” Dr. Workowski noted.
Dr. Juliet Stoltey of the University of California, San Francisco, said that since the initial January 2015 report of an outbreak of ocular syphilis in the Seattle area, cases have been reported from around the country, prompting the CDC to issue a clinical advisory in April.
It remains unclear whether the increase in ocular syphilis cases is the result of a true outbreak of a more oculo/neurotrophic strain of Treponema pallidum or simply the result of greater awareness of the complication in the setting of a steep rise in syphilis overall, according to Dr. Stoltey.
Circulation of a more neurotrophic strain is biologically plausible based on animal studies. Data from the University of Washington, Seattle, point to strain type 14d/f as a potential culprit.
California surveillance data show a more than 140% increase in syphilis cases overall during 2006-2014. And while it appears that the proportion of cases with ocular or other forms of neurologic syphilis has increased to an even greater extent than the overall rise in syphilis, the significance of this observation is uncertain given that the surveillance system lacks a standard mechanism for reporting ocular syphilis.
Regardless, Dr. Stoltey continued, here’s what physicians really need to know about ocular syphilis: Syphilis can affect virtually all parts of the eye, neurosyphilis can occur at any stage of the infection, and delayed identification of ocular syphilis has been associated with visual loss.
A syphilis serology test should be ordered in patients who have visual complaints along with risk factors for syphilis, such as men who have sex with men, as well as in those who have ophthalmologic findings compatible with syphilis. Both a treponemal and nontreponemal test should be ordered since the false-negative prozone effect has been documented in patients with ocular syphilis.
Clinical characteristics of ocular syphilis include eye redness, eye pain, visual field deficits or a decline in visual acuity, and headache accompanying eye symptoms. Ophthalmologic findings can include uveitis, iritis, vitrial detachment, optic neuritis, and marked vision loss.
Any patient with visual complaints plus a positive syphilis serology should undergo ophthalmologic evaluation immediately, Dr. Stoltey emphasized. A lumbar puncture and CSF analysis is recommended for syphilis patients with eye or neurologic symptoms such as cranial nerve dysfunction, auditory disease, meningitis, loss of vibration sensation, stroke, or altered mental status.
“I see signs of potential neurosyphilis being missed all the time,” Dr. Workowski said. “And here is a really important point: Treat for neurosyphilis if a patient with syphilis has the signs or symptoms of neurologic, audiologic, or ophthalmologic involvement regardless of what the CSF shows.”
The guideline-recommended treatment for ocular or neurosyphilis is aqueous crystalline penicillin G, 18-24 million units daily, administered as 3-4 million units IV every 4 hours for 10-14 days.
Dr. Stoltey urged physicians to store frozen pre–antibiotic therapy clinical samples from their patients with ocular syphilis and to contact the CDC, which, together with investigators at the University of Washington, Seattle, is conducting a study aimed at determining whether an oculotrophic strain of T. pallidum is circulating.
Dr. Stoltey and Dr. Workowski reported having no financial conflicts of interest.
SAN DIEGO – Cases of ocular syphilis are on the upswing in 2015, and many physicians are not up to speed regarding this serious complication, experts asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“I know that at my institution there’s been a lack of education about ocular syphilis. The housestaff are unaware that you can have ocular syphilis with a normal lumbar puncture,” said Dr. Kimberly A. Workowski, professor of medicine at Emory University in Atlanta and lead author of the Centers for Disease Control and Prevention 2015 STD guidelines (MMWR Recomm Rep. 2015;64[RR-03]:1-137).
When she asked for a show of hands by physicians in the audience who had seen a case of ocular syphilis in the past 6 months, a lot of arms shot up.
“We’ve had a large increase in ocular syphilis at our institution, too,” Dr. Workowski noted.
Dr. Juliet Stoltey of the University of California, San Francisco, said that since the initial January 2015 report of an outbreak of ocular syphilis in the Seattle area, cases have been reported from around the country, prompting the CDC to issue a clinical advisory in April.
It remains unclear whether the increase in ocular syphilis cases is the result of a true outbreak of a more oculo/neurotrophic strain of Treponema pallidum or simply the result of greater awareness of the complication in the setting of a steep rise in syphilis overall, according to Dr. Stoltey.
Circulation of a more neurotrophic strain is biologically plausible based on animal studies. Data from the University of Washington, Seattle, point to strain type 14d/f as a potential culprit.
California surveillance data show a more than 140% increase in syphilis cases overall during 2006-2014. And while it appears that the proportion of cases with ocular or other forms of neurologic syphilis has increased to an even greater extent than the overall rise in syphilis, the significance of this observation is uncertain given that the surveillance system lacks a standard mechanism for reporting ocular syphilis.
Regardless, Dr. Stoltey continued, here’s what physicians really need to know about ocular syphilis: Syphilis can affect virtually all parts of the eye, neurosyphilis can occur at any stage of the infection, and delayed identification of ocular syphilis has been associated with visual loss.
A syphilis serology test should be ordered in patients who have visual complaints along with risk factors for syphilis, such as men who have sex with men, as well as in those who have ophthalmologic findings compatible with syphilis. Both a treponemal and nontreponemal test should be ordered since the false-negative prozone effect has been documented in patients with ocular syphilis.
Clinical characteristics of ocular syphilis include eye redness, eye pain, visual field deficits or a decline in visual acuity, and headache accompanying eye symptoms. Ophthalmologic findings can include uveitis, iritis, vitrial detachment, optic neuritis, and marked vision loss.
Any patient with visual complaints plus a positive syphilis serology should undergo ophthalmologic evaluation immediately, Dr. Stoltey emphasized. A lumbar puncture and CSF analysis is recommended for syphilis patients with eye or neurologic symptoms such as cranial nerve dysfunction, auditory disease, meningitis, loss of vibration sensation, stroke, or altered mental status.
“I see signs of potential neurosyphilis being missed all the time,” Dr. Workowski said. “And here is a really important point: Treat for neurosyphilis if a patient with syphilis has the signs or symptoms of neurologic, audiologic, or ophthalmologic involvement regardless of what the CSF shows.”
The guideline-recommended treatment for ocular or neurosyphilis is aqueous crystalline penicillin G, 18-24 million units daily, administered as 3-4 million units IV every 4 hours for 10-14 days.
Dr. Stoltey urged physicians to store frozen pre–antibiotic therapy clinical samples from their patients with ocular syphilis and to contact the CDC, which, together with investigators at the University of Washington, Seattle, is conducting a study aimed at determining whether an oculotrophic strain of T. pallidum is circulating.
Dr. Stoltey and Dr. Workowski reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM ICAAC 2015
ESC: New support for aspirin’s anticancer effect
LONDON – The new U.S. Preventive Services Task Force draft recommendation for daily low-dose aspirin to reduce the risk of colorectal cancer has been shored up by three large new positive case-control studies presented at the annual congress of the European Society of Cardiology.
The studies weren’t considered by the USPSTF in its deliberations. They’re too new. But the three studies, each with a different methodologic design, collectively included more than 2 million subjects. And each study showed roughly a 30% reduction in the risk of colorectal cancer in daily aspirin users.
Dr. Luis A. Garcia Rodriguez presented the three studies, each one an analysis based on data extracted from the Health Improvement Network U.K. primary care database.
The first study included 170,336 new users of low-dose aspirin for primary or secondary prevention of cardiovascular disease and an equal number of individually matched non–aspirin-user controls. This study was designed to simulate enrollment into a placebo-controlled clinical trial of aspirin. During a median 5.1 years of follow-up and after adjustment for numerous potential confounders including smoking, body mass index, and use of nonsteroidal anti-inflammatory drugs, current users of aspirin were found to have a 34% relative risk reduction for colorectal cancer, reported Dr. Garcia Rodriguez, director of the Spanish Center for Pharmacoepidemiologic Research in Madrid.
The second study compared 171,527 new users of low-dose aspirin with 149,597 new users of acetaminophen. Acetaminophen users were selected as a control group to minimize any healthy-user bias arising from differences between aspirin users and nonusers in the first study. In study two, over a median 5.8 years of follow-up, the aspirin users had an adjusted 29% relative risk reduction for colorectal cancer.
The third study used a nested case-control methodology. Dr. Garcia Rodriguez and his associates began with a pool of 1,935,801 patients naive to daily low-dose aspirin. They then identified the individuals within that cohort who were diagnosed with colorectal cancer during 7.5 years of follow-up, matched them to 20,000 controls by age and gender, and determined who had initiated daily low-dose aspirin for cardiovascular prevention during the follow-up period. The daily aspirin users proved to be at an adjusted 31% lower risk of colorectal cancer than nonusers.
The fact that a consistent protective effect against colorectal cancer was seen in all three studies argues against the findings being explainable on the basis of selection bias, Dr. Garcia Rodriguez asserted.
In all three studies, the protective effect against colorectal cancer was greater in patients on low-dose aspirin for secondary rather than primary cardiovascular prevention. Patients taking aspirin for secondary prevention in studies one, two, and three had relative risk reductions in colorectal cancer of 40%, 38%, and 39%, respectively, compared with non–aspirin-using controls. Among individuals on aspirin for primary cardiovascular prevention, the relative risk reductions for colorectal cancer were 29%, 22%, and 25% across the three studies.
The studies were funded by Bayer Pharma. Dr. Garcia Rodriguez has served as an advisory board member for the company.
This article was updated 10/14/2015.
LONDON – The new U.S. Preventive Services Task Force draft recommendation for daily low-dose aspirin to reduce the risk of colorectal cancer has been shored up by three large new positive case-control studies presented at the annual congress of the European Society of Cardiology.
The studies weren’t considered by the USPSTF in its deliberations. They’re too new. But the three studies, each with a different methodologic design, collectively included more than 2 million subjects. And each study showed roughly a 30% reduction in the risk of colorectal cancer in daily aspirin users.
Dr. Luis A. Garcia Rodriguez presented the three studies, each one an analysis based on data extracted from the Health Improvement Network U.K. primary care database.
The first study included 170,336 new users of low-dose aspirin for primary or secondary prevention of cardiovascular disease and an equal number of individually matched non–aspirin-user controls. This study was designed to simulate enrollment into a placebo-controlled clinical trial of aspirin. During a median 5.1 years of follow-up and after adjustment for numerous potential confounders including smoking, body mass index, and use of nonsteroidal anti-inflammatory drugs, current users of aspirin were found to have a 34% relative risk reduction for colorectal cancer, reported Dr. Garcia Rodriguez, director of the Spanish Center for Pharmacoepidemiologic Research in Madrid.
The second study compared 171,527 new users of low-dose aspirin with 149,597 new users of acetaminophen. Acetaminophen users were selected as a control group to minimize any healthy-user bias arising from differences between aspirin users and nonusers in the first study. In study two, over a median 5.8 years of follow-up, the aspirin users had an adjusted 29% relative risk reduction for colorectal cancer.
The third study used a nested case-control methodology. Dr. Garcia Rodriguez and his associates began with a pool of 1,935,801 patients naive to daily low-dose aspirin. They then identified the individuals within that cohort who were diagnosed with colorectal cancer during 7.5 years of follow-up, matched them to 20,000 controls by age and gender, and determined who had initiated daily low-dose aspirin for cardiovascular prevention during the follow-up period. The daily aspirin users proved to be at an adjusted 31% lower risk of colorectal cancer than nonusers.
The fact that a consistent protective effect against colorectal cancer was seen in all three studies argues against the findings being explainable on the basis of selection bias, Dr. Garcia Rodriguez asserted.
In all three studies, the protective effect against colorectal cancer was greater in patients on low-dose aspirin for secondary rather than primary cardiovascular prevention. Patients taking aspirin for secondary prevention in studies one, two, and three had relative risk reductions in colorectal cancer of 40%, 38%, and 39%, respectively, compared with non–aspirin-using controls. Among individuals on aspirin for primary cardiovascular prevention, the relative risk reductions for colorectal cancer were 29%, 22%, and 25% across the three studies.
The studies were funded by Bayer Pharma. Dr. Garcia Rodriguez has served as an advisory board member for the company.
This article was updated 10/14/2015.
LONDON – The new U.S. Preventive Services Task Force draft recommendation for daily low-dose aspirin to reduce the risk of colorectal cancer has been shored up by three large new positive case-control studies presented at the annual congress of the European Society of Cardiology.
The studies weren’t considered by the USPSTF in its deliberations. They’re too new. But the three studies, each with a different methodologic design, collectively included more than 2 million subjects. And each study showed roughly a 30% reduction in the risk of colorectal cancer in daily aspirin users.
Dr. Luis A. Garcia Rodriguez presented the three studies, each one an analysis based on data extracted from the Health Improvement Network U.K. primary care database.
The first study included 170,336 new users of low-dose aspirin for primary or secondary prevention of cardiovascular disease and an equal number of individually matched non–aspirin-user controls. This study was designed to simulate enrollment into a placebo-controlled clinical trial of aspirin. During a median 5.1 years of follow-up and after adjustment for numerous potential confounders including smoking, body mass index, and use of nonsteroidal anti-inflammatory drugs, current users of aspirin were found to have a 34% relative risk reduction for colorectal cancer, reported Dr. Garcia Rodriguez, director of the Spanish Center for Pharmacoepidemiologic Research in Madrid.
The second study compared 171,527 new users of low-dose aspirin with 149,597 new users of acetaminophen. Acetaminophen users were selected as a control group to minimize any healthy-user bias arising from differences between aspirin users and nonusers in the first study. In study two, over a median 5.8 years of follow-up, the aspirin users had an adjusted 29% relative risk reduction for colorectal cancer.
The third study used a nested case-control methodology. Dr. Garcia Rodriguez and his associates began with a pool of 1,935,801 patients naive to daily low-dose aspirin. They then identified the individuals within that cohort who were diagnosed with colorectal cancer during 7.5 years of follow-up, matched them to 20,000 controls by age and gender, and determined who had initiated daily low-dose aspirin for cardiovascular prevention during the follow-up period. The daily aspirin users proved to be at an adjusted 31% lower risk of colorectal cancer than nonusers.
The fact that a consistent protective effect against colorectal cancer was seen in all three studies argues against the findings being explainable on the basis of selection bias, Dr. Garcia Rodriguez asserted.
In all three studies, the protective effect against colorectal cancer was greater in patients on low-dose aspirin for secondary rather than primary cardiovascular prevention. Patients taking aspirin for secondary prevention in studies one, two, and three had relative risk reductions in colorectal cancer of 40%, 38%, and 39%, respectively, compared with non–aspirin-using controls. Among individuals on aspirin for primary cardiovascular prevention, the relative risk reductions for colorectal cancer were 29%, 22%, and 25% across the three studies.
The studies were funded by Bayer Pharma. Dr. Garcia Rodriguez has served as an advisory board member for the company.
This article was updated 10/14/2015.
AT THE ESC CONGRESS 2015
Key clinical point: Each of three new case-control studies concluded that daily low-dose aspirin for cardiovascular prevention reduced the risk of developing colorectal cancer by about 30%.
Major finding: Relative risk reductions for colorectal cancer among aspirin users during median follow-up periods of 5.1-7.5 years in the three studies were 34%, 29%, and 31%.
Data source: The three studies, which included well over 2 million patients, were based on data extracted from a large U.K. primary care network database.
Disclosures: The studies were funded by Bayer Pharma. Dr. Rodriguez has served as an advisory board member for the company.
ICAAC: 2015 brings three major systematic reviews of diabetic foot infection therapy
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ICAAC 2015
ICAAC: Enhanced disinfection practices in day care reduce antibiotic use
SAN DIEGO – Use of antibiotics was cut by one-third among 3- to 5-year-olds attending child care centers that used enhanced hard surface disinfection practices in a multicenter randomized trial.
The intervention involved the use of commercially available wipes and sprays containing quaternary ammonium chloride compounds as well as a combined cleaner plus 1.9% sodium hypochlorite.
“Our intervention involved providing easier-to-use products that are more likely to provide an effective dose of the disinfectant, and the disinfection intervention appeared to reduce antibiotic use,” Charles P. Gerba, Ph.D., observed at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The 10-week study included 402 children at a dozen Arizona day care centers. Six centers were randomized to serve as controls, with the staff continuing to follow standard disinfection protocols recommended by the Arizona state health department: namely, a two-step process entailing the use of soapy water followed by bleach.
The other six centers were provided with the commercial hard surface disinfectants and instructed in using them effectively to kill germs. For example, the disinfecting wipes were to be used daily on refrigerator door handles and after every use of diaper-changing areas and high chairs. The one-step cleanser/dilute bleach product was to be applied daily to the sink, toilet, and countertops.
“These quat ammonium-containing wipes and sprays typically require 2-10 minutes of contact time in order to disinfect,” explained Dr. Gerba, professor of microbiology and environmental sciences at the University of Arizona, Tucson.
Hand-washing practices continued as usual throughout the study.
Every time a child missed school during the 10-week study, Dr. Gerba or a coinvestigator phoned the parents to learn if the child was sick, had sought medical attention, or was taking antibiotics.
The key finding: In a multivariate Poisson regression analysis, the use of antibiotics was 32% lower in the intervention arm than in controls.
Children attending the day care centers in the intervention arm also had fewer medical visits. Moreover, microbiologic sampling of hard surfaces at the participating centers documented that fewer antibiotic-resistant bacteria were present at the centers using enhanced disinfection practices.
Asked if he expects his study findings to result in a widespread change in cleaning practices at child care centers, Dr. Gerba noted that the commercially available enhanced disinfectant products are costlier than a bucket of soapy water and another bucket of dilute bleach.
“Child care centers are sensitive to cost,” he said. “Our current studies are examining the economic benefit of using products that are more convenient and contain the right doses for disinfection.”
He dismissed audience concerns that microbes might develop resistance to the disinfectants used in this study.
“In 120 years, no one has ever seen resistance to chlorine bleach by any microorganisms in continuous exposure. The same can be said for quat compounds. The literature shows that if they are used properly for disinfection, microorganisms don’t develop resistance because basically you’re using a stick of dynamite to kill a cockroach,” Dr. Gerba said.
The study was partially funded by a research grant provided by the Clorox Company to the University of Arizona, Tucson.
SAN DIEGO – Use of antibiotics was cut by one-third among 3- to 5-year-olds attending child care centers that used enhanced hard surface disinfection practices in a multicenter randomized trial.
The intervention involved the use of commercially available wipes and sprays containing quaternary ammonium chloride compounds as well as a combined cleaner plus 1.9% sodium hypochlorite.
“Our intervention involved providing easier-to-use products that are more likely to provide an effective dose of the disinfectant, and the disinfection intervention appeared to reduce antibiotic use,” Charles P. Gerba, Ph.D., observed at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The 10-week study included 402 children at a dozen Arizona day care centers. Six centers were randomized to serve as controls, with the staff continuing to follow standard disinfection protocols recommended by the Arizona state health department: namely, a two-step process entailing the use of soapy water followed by bleach.
The other six centers were provided with the commercial hard surface disinfectants and instructed in using them effectively to kill germs. For example, the disinfecting wipes were to be used daily on refrigerator door handles and after every use of diaper-changing areas and high chairs. The one-step cleanser/dilute bleach product was to be applied daily to the sink, toilet, and countertops.
“These quat ammonium-containing wipes and sprays typically require 2-10 minutes of contact time in order to disinfect,” explained Dr. Gerba, professor of microbiology and environmental sciences at the University of Arizona, Tucson.
Hand-washing practices continued as usual throughout the study.
Every time a child missed school during the 10-week study, Dr. Gerba or a coinvestigator phoned the parents to learn if the child was sick, had sought medical attention, or was taking antibiotics.
The key finding: In a multivariate Poisson regression analysis, the use of antibiotics was 32% lower in the intervention arm than in controls.
Children attending the day care centers in the intervention arm also had fewer medical visits. Moreover, microbiologic sampling of hard surfaces at the participating centers documented that fewer antibiotic-resistant bacteria were present at the centers using enhanced disinfection practices.
Asked if he expects his study findings to result in a widespread change in cleaning practices at child care centers, Dr. Gerba noted that the commercially available enhanced disinfectant products are costlier than a bucket of soapy water and another bucket of dilute bleach.
“Child care centers are sensitive to cost,” he said. “Our current studies are examining the economic benefit of using products that are more convenient and contain the right doses for disinfection.”
He dismissed audience concerns that microbes might develop resistance to the disinfectants used in this study.
“In 120 years, no one has ever seen resistance to chlorine bleach by any microorganisms in continuous exposure. The same can be said for quat compounds. The literature shows that if they are used properly for disinfection, microorganisms don’t develop resistance because basically you’re using a stick of dynamite to kill a cockroach,” Dr. Gerba said.
The study was partially funded by a research grant provided by the Clorox Company to the University of Arizona, Tucson.
SAN DIEGO – Use of antibiotics was cut by one-third among 3- to 5-year-olds attending child care centers that used enhanced hard surface disinfection practices in a multicenter randomized trial.
The intervention involved the use of commercially available wipes and sprays containing quaternary ammonium chloride compounds as well as a combined cleaner plus 1.9% sodium hypochlorite.
“Our intervention involved providing easier-to-use products that are more likely to provide an effective dose of the disinfectant, and the disinfection intervention appeared to reduce antibiotic use,” Charles P. Gerba, Ph.D., observed at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The 10-week study included 402 children at a dozen Arizona day care centers. Six centers were randomized to serve as controls, with the staff continuing to follow standard disinfection protocols recommended by the Arizona state health department: namely, a two-step process entailing the use of soapy water followed by bleach.
The other six centers were provided with the commercial hard surface disinfectants and instructed in using them effectively to kill germs. For example, the disinfecting wipes were to be used daily on refrigerator door handles and after every use of diaper-changing areas and high chairs. The one-step cleanser/dilute bleach product was to be applied daily to the sink, toilet, and countertops.
“These quat ammonium-containing wipes and sprays typically require 2-10 minutes of contact time in order to disinfect,” explained Dr. Gerba, professor of microbiology and environmental sciences at the University of Arizona, Tucson.
Hand-washing practices continued as usual throughout the study.
Every time a child missed school during the 10-week study, Dr. Gerba or a coinvestigator phoned the parents to learn if the child was sick, had sought medical attention, or was taking antibiotics.
The key finding: In a multivariate Poisson regression analysis, the use of antibiotics was 32% lower in the intervention arm than in controls.
Children attending the day care centers in the intervention arm also had fewer medical visits. Moreover, microbiologic sampling of hard surfaces at the participating centers documented that fewer antibiotic-resistant bacteria were present at the centers using enhanced disinfection practices.
Asked if he expects his study findings to result in a widespread change in cleaning practices at child care centers, Dr. Gerba noted that the commercially available enhanced disinfectant products are costlier than a bucket of soapy water and another bucket of dilute bleach.
“Child care centers are sensitive to cost,” he said. “Our current studies are examining the economic benefit of using products that are more convenient and contain the right doses for disinfection.”
He dismissed audience concerns that microbes might develop resistance to the disinfectants used in this study.
“In 120 years, no one has ever seen resistance to chlorine bleach by any microorganisms in continuous exposure. The same can be said for quat compounds. The literature shows that if they are used properly for disinfection, microorganisms don’t develop resistance because basically you’re using a stick of dynamite to kill a cockroach,” Dr. Gerba said.
The study was partially funded by a research grant provided by the Clorox Company to the University of Arizona, Tucson.
AT ICAAC 2015
Key clinical point: Children attending day care centers that adopted enhanced hard surface disinfecting practices using commercially available disinfectant wipes and sprays experienced a 32% reduction in antibiotic use.
Major finding: The use of antibiotics by children attending day care centers using commercially available disinfecting sprays and wipes with staff instruction in their effective use was 32% less than at centers using the traditional soapy water and bleach.
Data source: This 10-week prospective study included 402 children aged 3-5 years at 12 day care centers, half of which received the disinfection intervention while the other half used standard cleaning protocols.
Disclosures: The study was funded in part by a research grant from the Clorox Company to the University of Arizona, Tucson.