User login
ICAAC: Synergistic effects of two pediatric vaccines highlighted
SAN DIEGO – The potent synergistic benefits of coadministration of rotavirus vaccine and pneumococcal conjugate vaccine in young children are uniquely highlighted by a natural experiment conducted in southern Israel as described by Dr. Ron Dagan at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Dagan is professor of pediatrics and infectious diseases at Soroka University Medical Center in Beersheba, Israel. It’s the sole hospital in a large, well-defined area of southern Israel. All children in that part of Israel are born in the hospital and receive their health care there, making it possible to generate highly reliable disease incidence data.
At any given time, physicians at the hospital are responsible for the care of roughly 30,000 children less than 2 years of age. So there’s a huge study population, comprehensive follow-up, and – the final element in this prospective, population-based study – the rotavirus and pneumococcal conjugate vaccines were introduced in Israel just a few years ago and at roughly the same time. This enabled Dr. Dagan and his coinvestigators to compare hospitalization rates and pediatric emergency department outpatient visits for diarrheal and lower respiratory viral illnesses among children less than 2 years old during the prevaccine period of April 2006-March 2009 with rates during April 2013-March 2014, when uptake of the two vaccines in that age group exceeded 90%.
This was an unusual study in that it looked at the global impact of two major vaccines. In contrast, vaccine clinical trials and postmarketing studies typically evaluate only those outcomes directly related to that particular vaccine.
The results of the Israeli study were startling: in the 3 years prior to introduction of the vaccines, one in five children under age 2 years admitted to the hospital had as an admitting diagnosis either rotavirus gastroenteritis confirmed by a positive stool ELISA test or pneumococcal pneumonia as evidenced by alveolar pneumonia on chest x-ray. After the vaccines became available, the hospitalization rates for rotavirus gastroenteritis and alveolar pneumonia plummeted by 78% and 46%, respectively. Moreover, outpatient pediatric emergency department visits for rotavirus gastroenteritis dropped by 71% and visits for alveolar pneumonia fell by 67%.
But that’s not all. Smaller yet clinically meaningful reductions were also documented in nonrotavirus gastroenteritis and nonalveolar lower respiratory tract infections. Specifically, the hospitalization rate for nonrotavirus diarrheal illnesses and nonalveolar pneumonia lower respiratory infections dropped by 21% and 7%, respectively, while outpatient emergency visits for those disorders fell by 16% and 14%.
This translates to an estimated 1,890 fewer hospitalizations and 4,030 fewer outpatient emergency department visits for diarrheal disease or lower respiratory infection per 100,000 children under age 2 per year, Dr. Dagan reported.
Michael Schmidt, Ph.D., who chaired a press conference highlighting the Israeli study, declared, “These data are absolutely phenomenal. It really shows the global value of these vaccines for society.”
Dr. Schmidt, professor and vice chairman of microbiology and immunology at the Medical University of South Carolina, Charleston, posed a question: What’s the explanation for the reductions in diseases not directly addressed by those two vaccines?
“We believe that one success can favorably affect the other. If you are weakened by diarrhea, you may be more likely to get pneumonia, and vice versa,” according to Dr. Dagan.
He added that the results actually pack a significantly greater wallop than is apparent at first look because rotavirus gastroenteritis and pneumococcal pneumonia in young children are seasonal diseases. They occur chiefly during October-March. So those 5,920 fewer hospitalizations and outpatient visits/100,000 per young children per year are concentrated during pediatricians’ busiest half of the year.
“In most places in the world, winter is a time of so much illness that pediatricians can’t deliver appropriate care. We knew that in our hospital we couldn’t deliver appropriate care to children in the winter because there were so many sick children piled on top of each other. But now, because of these two vaccines, we are less crowded in the winter, we have more time for children, we make fewer mistakes,” he said.
The study was funded by vaccine manufacturers and the Israel Ministry of Health. Dr. Dagan reported serving as a consultant, adviser to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
SAN DIEGO – The potent synergistic benefits of coadministration of rotavirus vaccine and pneumococcal conjugate vaccine in young children are uniquely highlighted by a natural experiment conducted in southern Israel as described by Dr. Ron Dagan at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Dagan is professor of pediatrics and infectious diseases at Soroka University Medical Center in Beersheba, Israel. It’s the sole hospital in a large, well-defined area of southern Israel. All children in that part of Israel are born in the hospital and receive their health care there, making it possible to generate highly reliable disease incidence data.
At any given time, physicians at the hospital are responsible for the care of roughly 30,000 children less than 2 years of age. So there’s a huge study population, comprehensive follow-up, and – the final element in this prospective, population-based study – the rotavirus and pneumococcal conjugate vaccines were introduced in Israel just a few years ago and at roughly the same time. This enabled Dr. Dagan and his coinvestigators to compare hospitalization rates and pediatric emergency department outpatient visits for diarrheal and lower respiratory viral illnesses among children less than 2 years old during the prevaccine period of April 2006-March 2009 with rates during April 2013-March 2014, when uptake of the two vaccines in that age group exceeded 90%.
This was an unusual study in that it looked at the global impact of two major vaccines. In contrast, vaccine clinical trials and postmarketing studies typically evaluate only those outcomes directly related to that particular vaccine.
The results of the Israeli study were startling: in the 3 years prior to introduction of the vaccines, one in five children under age 2 years admitted to the hospital had as an admitting diagnosis either rotavirus gastroenteritis confirmed by a positive stool ELISA test or pneumococcal pneumonia as evidenced by alveolar pneumonia on chest x-ray. After the vaccines became available, the hospitalization rates for rotavirus gastroenteritis and alveolar pneumonia plummeted by 78% and 46%, respectively. Moreover, outpatient pediatric emergency department visits for rotavirus gastroenteritis dropped by 71% and visits for alveolar pneumonia fell by 67%.
But that’s not all. Smaller yet clinically meaningful reductions were also documented in nonrotavirus gastroenteritis and nonalveolar lower respiratory tract infections. Specifically, the hospitalization rate for nonrotavirus diarrheal illnesses and nonalveolar pneumonia lower respiratory infections dropped by 21% and 7%, respectively, while outpatient emergency visits for those disorders fell by 16% and 14%.
This translates to an estimated 1,890 fewer hospitalizations and 4,030 fewer outpatient emergency department visits for diarrheal disease or lower respiratory infection per 100,000 children under age 2 per year, Dr. Dagan reported.
Michael Schmidt, Ph.D., who chaired a press conference highlighting the Israeli study, declared, “These data are absolutely phenomenal. It really shows the global value of these vaccines for society.”
Dr. Schmidt, professor and vice chairman of microbiology and immunology at the Medical University of South Carolina, Charleston, posed a question: What’s the explanation for the reductions in diseases not directly addressed by those two vaccines?
“We believe that one success can favorably affect the other. If you are weakened by diarrhea, you may be more likely to get pneumonia, and vice versa,” according to Dr. Dagan.
He added that the results actually pack a significantly greater wallop than is apparent at first look because rotavirus gastroenteritis and pneumococcal pneumonia in young children are seasonal diseases. They occur chiefly during October-March. So those 5,920 fewer hospitalizations and outpatient visits/100,000 per young children per year are concentrated during pediatricians’ busiest half of the year.
“In most places in the world, winter is a time of so much illness that pediatricians can’t deliver appropriate care. We knew that in our hospital we couldn’t deliver appropriate care to children in the winter because there were so many sick children piled on top of each other. But now, because of these two vaccines, we are less crowded in the winter, we have more time for children, we make fewer mistakes,” he said.
The study was funded by vaccine manufacturers and the Israel Ministry of Health. Dr. Dagan reported serving as a consultant, adviser to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
SAN DIEGO – The potent synergistic benefits of coadministration of rotavirus vaccine and pneumococcal conjugate vaccine in young children are uniquely highlighted by a natural experiment conducted in southern Israel as described by Dr. Ron Dagan at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Dagan is professor of pediatrics and infectious diseases at Soroka University Medical Center in Beersheba, Israel. It’s the sole hospital in a large, well-defined area of southern Israel. All children in that part of Israel are born in the hospital and receive their health care there, making it possible to generate highly reliable disease incidence data.
At any given time, physicians at the hospital are responsible for the care of roughly 30,000 children less than 2 years of age. So there’s a huge study population, comprehensive follow-up, and – the final element in this prospective, population-based study – the rotavirus and pneumococcal conjugate vaccines were introduced in Israel just a few years ago and at roughly the same time. This enabled Dr. Dagan and his coinvestigators to compare hospitalization rates and pediatric emergency department outpatient visits for diarrheal and lower respiratory viral illnesses among children less than 2 years old during the prevaccine period of April 2006-March 2009 with rates during April 2013-March 2014, when uptake of the two vaccines in that age group exceeded 90%.
This was an unusual study in that it looked at the global impact of two major vaccines. In contrast, vaccine clinical trials and postmarketing studies typically evaluate only those outcomes directly related to that particular vaccine.
The results of the Israeli study were startling: in the 3 years prior to introduction of the vaccines, one in five children under age 2 years admitted to the hospital had as an admitting diagnosis either rotavirus gastroenteritis confirmed by a positive stool ELISA test or pneumococcal pneumonia as evidenced by alveolar pneumonia on chest x-ray. After the vaccines became available, the hospitalization rates for rotavirus gastroenteritis and alveolar pneumonia plummeted by 78% and 46%, respectively. Moreover, outpatient pediatric emergency department visits for rotavirus gastroenteritis dropped by 71% and visits for alveolar pneumonia fell by 67%.
But that’s not all. Smaller yet clinically meaningful reductions were also documented in nonrotavirus gastroenteritis and nonalveolar lower respiratory tract infections. Specifically, the hospitalization rate for nonrotavirus diarrheal illnesses and nonalveolar pneumonia lower respiratory infections dropped by 21% and 7%, respectively, while outpatient emergency visits for those disorders fell by 16% and 14%.
This translates to an estimated 1,890 fewer hospitalizations and 4,030 fewer outpatient emergency department visits for diarrheal disease or lower respiratory infection per 100,000 children under age 2 per year, Dr. Dagan reported.
Michael Schmidt, Ph.D., who chaired a press conference highlighting the Israeli study, declared, “These data are absolutely phenomenal. It really shows the global value of these vaccines for society.”
Dr. Schmidt, professor and vice chairman of microbiology and immunology at the Medical University of South Carolina, Charleston, posed a question: What’s the explanation for the reductions in diseases not directly addressed by those two vaccines?
“We believe that one success can favorably affect the other. If you are weakened by diarrhea, you may be more likely to get pneumonia, and vice versa,” according to Dr. Dagan.
He added that the results actually pack a significantly greater wallop than is apparent at first look because rotavirus gastroenteritis and pneumococcal pneumonia in young children are seasonal diseases. They occur chiefly during October-March. So those 5,920 fewer hospitalizations and outpatient visits/100,000 per young children per year are concentrated during pediatricians’ busiest half of the year.
“In most places in the world, winter is a time of so much illness that pediatricians can’t deliver appropriate care. We knew that in our hospital we couldn’t deliver appropriate care to children in the winter because there were so many sick children piled on top of each other. But now, because of these two vaccines, we are less crowded in the winter, we have more time for children, we make fewer mistakes,” he said.
The study was funded by vaccine manufacturers and the Israel Ministry of Health. Dr. Dagan reported serving as a consultant, adviser to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
AT ICAAC 2015
Key clinical point: Pediatric rotavirus and pneumococcal conjugate vaccines provide global benefits that extend well beyond the diseases they specifically target.
Major finding: When rotavirus and pneumococcal conjugate vaccines were introduced in southern Israel, hospitalization rates for rotavirus gastroenteritis and nonrotavirus gastroenteritis dropped by 78% and 21%, respectively, in children less than 2 years old, while hospitalizations for alveolar pneumonia, which is mainly pneumococcal, and other lower respiratory tract infections unlikely to be pneumococcal fell by 46% and 7%, respectively.
Data source: This study is part of an ongoing, prospective, population-based trial in southern Israel comparing rates of hospitalization and outpatient visits for diarrheal and lower respiratory tract illnesses in roughly 30,000 children, who were less than 2 years old during the 3 years just prior to introduction of the rotavirus and pneumococcal conjugate vaccines to rates after the vaccines became available.
Disclosures: The study was funded by vaccine manufacturers and the Israel Ministry of Health. The presenter reported serving as a consultant, advisor to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
Reported pertussis underestimates true incidence by up to 93-fold
SAN DIEGO – The true incidence of pertussis in recent years in Americans less than 50 years old is estimated to be 58- to 93-fold greater than the laboratory-confirmed reported case count, Philip O. Buck, Ph.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
It’s widely accepted that national surveillance systems vastly underestimate the incidence of pertussis because most cases don’t get reported. In order to obtain a more complete picture of the situation, Dr. Buck utilized a regression equation to estimate the proportion of cough illnesses attributable to laboratory-confirmed pertussis.
A closely similar regression model has previously been utilized by other investigators in published studies that provided estimates of the true burdens of influenza (Epidemiol Infect. 2002 Aug;129:99-106) and respiratory syncytial virus (Eur J Pediatr. 2010 Aug;169:997-1008), noted Dr. Buck, director of U.S. Health Outcomes at GlaxoSmithKline in Philadelphia.
He applied the regression model to medical claims for ICD-9-CM–diagnosed pertussis in individuals under age 50 in the IMS PharmMetric Plus claims database for the years 2008-2013. The database includes more than 150 million enrollees. The average reported incidence of pertussis in individuals less than 50 years old during the study years was 9 cases per 100,000 per year; however, the average regression-estimated incidence was 649 per 100,000, a 72-fold greater figure.
During 2011-2013, the 3 most recent years covered by the study, the regression-estimated incidence of pertussis was 93-fold, 62-fold, and 87-fold greater than the reported rates.
Dr. Buck is employed by GlaxoSmithKline, which funded the study and markets pertussis vaccine.
SAN DIEGO – The true incidence of pertussis in recent years in Americans less than 50 years old is estimated to be 58- to 93-fold greater than the laboratory-confirmed reported case count, Philip O. Buck, Ph.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
It’s widely accepted that national surveillance systems vastly underestimate the incidence of pertussis because most cases don’t get reported. In order to obtain a more complete picture of the situation, Dr. Buck utilized a regression equation to estimate the proportion of cough illnesses attributable to laboratory-confirmed pertussis.
A closely similar regression model has previously been utilized by other investigators in published studies that provided estimates of the true burdens of influenza (Epidemiol Infect. 2002 Aug;129:99-106) and respiratory syncytial virus (Eur J Pediatr. 2010 Aug;169:997-1008), noted Dr. Buck, director of U.S. Health Outcomes at GlaxoSmithKline in Philadelphia.
He applied the regression model to medical claims for ICD-9-CM–diagnosed pertussis in individuals under age 50 in the IMS PharmMetric Plus claims database for the years 2008-2013. The database includes more than 150 million enrollees. The average reported incidence of pertussis in individuals less than 50 years old during the study years was 9 cases per 100,000 per year; however, the average regression-estimated incidence was 649 per 100,000, a 72-fold greater figure.
During 2011-2013, the 3 most recent years covered by the study, the regression-estimated incidence of pertussis was 93-fold, 62-fold, and 87-fold greater than the reported rates.
Dr. Buck is employed by GlaxoSmithKline, which funded the study and markets pertussis vaccine.
SAN DIEGO – The true incidence of pertussis in recent years in Americans less than 50 years old is estimated to be 58- to 93-fold greater than the laboratory-confirmed reported case count, Philip O. Buck, Ph.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
It’s widely accepted that national surveillance systems vastly underestimate the incidence of pertussis because most cases don’t get reported. In order to obtain a more complete picture of the situation, Dr. Buck utilized a regression equation to estimate the proportion of cough illnesses attributable to laboratory-confirmed pertussis.
A closely similar regression model has previously been utilized by other investigators in published studies that provided estimates of the true burdens of influenza (Epidemiol Infect. 2002 Aug;129:99-106) and respiratory syncytial virus (Eur J Pediatr. 2010 Aug;169:997-1008), noted Dr. Buck, director of U.S. Health Outcomes at GlaxoSmithKline in Philadelphia.
He applied the regression model to medical claims for ICD-9-CM–diagnosed pertussis in individuals under age 50 in the IMS PharmMetric Plus claims database for the years 2008-2013. The database includes more than 150 million enrollees. The average reported incidence of pertussis in individuals less than 50 years old during the study years was 9 cases per 100,000 per year; however, the average regression-estimated incidence was 649 per 100,000, a 72-fold greater figure.
During 2011-2013, the 3 most recent years covered by the study, the regression-estimated incidence of pertussis was 93-fold, 62-fold, and 87-fold greater than the reported rates.
Dr. Buck is employed by GlaxoSmithKline, which funded the study and markets pertussis vaccine.
AT ICAAC 2015
Key clinical point: The annual rate of pertussis in Americans less than 50 years old is up to 93-fold greater than the reported incidence.
Major finding: The true incidence of pertussis in Americans less than 50 years old is estimated to be 58-93 times greater per year than the reported annual rates during recent years.
Data source: This was a retrospective cohort study which utilized a regression model to estimate the true fraction of cough illness attributable to laboratory-confirmed pertussis through analysis of 6 years worth of medical claims data from a large national database.
Disclosures: The study was funded by GlaxoSmithKline. The presenter is a company employee.
New assay may be a game changer in invasive candidiasis
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
EXPERT ANALYSIS FROM ICAAC 2015
Diabetic foot ulcer: Early closure post debridement best
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
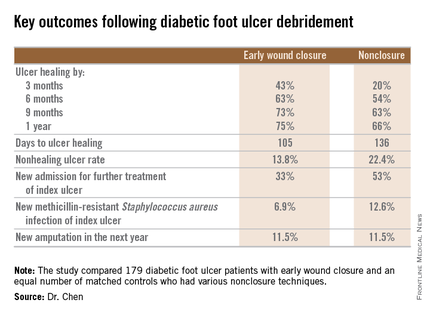
He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
AT ICAAC 2015
Key clinical point: Diabetic foot ulcers are more likely to heal with early wound closure following surgical debridement than with nonclosure techniques.
Major finding: Healing of diabetic foot ulcers after surgical debridement took an average of 105 days in patients who underwent early wound closure prior to hospital discharge, compared with 136 days in those whose wounds were managed with nonclosure techniques.
Data source: A retrospective, nonrandomized study featuring two propensity score–matched groups, with 179 patients in each, who were followed for 1 year post discharge for surgical debridement of a diabetic foot ulcer.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
Community hospitals harness rapid testing to slash bacteremia mortality
SAN DIEGO – Adoption of rapid diagnostic testing in conjunction with an antimicrobial stewardship program reduced time to initiation of optimal antibiotic therapy and markedly improved clinical outcomes in patients admitted with Gram-negative bacteremia at two Houston-area community hospitals.
Indeed, in-hospital mortality in patients with Gram-negative bacteremia plummeted from 26% immediately prior to acquisition of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry, when lab testing of bloodstream organisms was done the traditional way, to just 2% post intervention, Ashley M. Lockwood, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Moreover, average overall hospital costs dropped from $18,644 to $15,234, for a savings of $3,410 per patient attributable to MALDI-TOF plus the pharmacist-led antimicrobial stewardship program, added Dr. Lockwood, who performed the before-and-after comparison study while at Houston Methodist Hospital System and is now the infectious disease pharmacist at Bayfront Health in St. Petersburg, Fla.
Prior studies have shown big benefits for MALDI-TOF and other rapid diagnostic tests in combination with antimicrobial stewardship, but all the research was done at tertiary medical centers. It was unclear whether the results were generalizable to the thousands of community hospitals across the nation. The new study removes any doubts on that score, Dr. Lockwood said in an interview.
“The use of MALDI-TOF coupled with near real-time antimicrobial stewardship should be incorporated in the community setting to improve time to therapeutic optimization in patients with Gram-negative bacteremia,” she declared.
The study compared outcomes in 149 patients with Gram-negative bacteremia admitted to the two community hospitals prior to acquisition of a shared MALDI-TOF system and 241 admitted post acquisition. Prior to MALDI-TOF, when the traditional culture and susceptibility results came back, the information would simply be entered into the electronic medical record. Post-MALDI-TOF, when the lab results get entered into the EMR, a third-party surveillance program known as Vigilanz Real-Time Surveillance notifies a clinical pharmacist, who then goes into the EMR to make sure the patient is on appropriate antibiotic therapy. If not, a call is made to the attending physician, who can either accept or reject the pharmacist’s recommended medication change.
Both pre- and post-MALDI-TOF it took about 20 hours from the time of the blood draw to a positive culture. But the time from that point to identification of the infecting pathogen dropped from 32 hours using the traditional method to 6.5 hours with MALDI-TOF. Time to results of susceptibility testing decreased from 48 to 22 hours. Time to antibiotic optimization for patients who weren’t already on effective therapy dropped from an average of 71 hours to 30 hours – and that’s what accounts for the dramatic reduction in mortality, according to Dr. Lockwood.
“With Gram-negative bacteremia, every hour counts. The mortality increases by 7.6% each hour, so I definitely think that reduction in mortality is real,” she said.
Although Dr. Lockwood and her coinvestigators expected to see a reduction in hospital length of stay post-MALDI-TOF, the average stay turned out to be 6.4 days in both groups. That’s probably because 6 days is already at the low end of the spectrum for patients admitted to a community hospital for bacteremia, she observed.
The initial investment for MALDI-TOF technology is roughly $250,000. That’s a drop in the bucket considering the reduced overall per-patient hospital cost and marked reduction in mortality, Dr. Lockwood observed.
She reported having no financial conflicts regarding her study, which was an unfunded research project.
SAN DIEGO – Adoption of rapid diagnostic testing in conjunction with an antimicrobial stewardship program reduced time to initiation of optimal antibiotic therapy and markedly improved clinical outcomes in patients admitted with Gram-negative bacteremia at two Houston-area community hospitals.
Indeed, in-hospital mortality in patients with Gram-negative bacteremia plummeted from 26% immediately prior to acquisition of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry, when lab testing of bloodstream organisms was done the traditional way, to just 2% post intervention, Ashley M. Lockwood, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Moreover, average overall hospital costs dropped from $18,644 to $15,234, for a savings of $3,410 per patient attributable to MALDI-TOF plus the pharmacist-led antimicrobial stewardship program, added Dr. Lockwood, who performed the before-and-after comparison study while at Houston Methodist Hospital System and is now the infectious disease pharmacist at Bayfront Health in St. Petersburg, Fla.
Prior studies have shown big benefits for MALDI-TOF and other rapid diagnostic tests in combination with antimicrobial stewardship, but all the research was done at tertiary medical centers. It was unclear whether the results were generalizable to the thousands of community hospitals across the nation. The new study removes any doubts on that score, Dr. Lockwood said in an interview.
“The use of MALDI-TOF coupled with near real-time antimicrobial stewardship should be incorporated in the community setting to improve time to therapeutic optimization in patients with Gram-negative bacteremia,” she declared.
The study compared outcomes in 149 patients with Gram-negative bacteremia admitted to the two community hospitals prior to acquisition of a shared MALDI-TOF system and 241 admitted post acquisition. Prior to MALDI-TOF, when the traditional culture and susceptibility results came back, the information would simply be entered into the electronic medical record. Post-MALDI-TOF, when the lab results get entered into the EMR, a third-party surveillance program known as Vigilanz Real-Time Surveillance notifies a clinical pharmacist, who then goes into the EMR to make sure the patient is on appropriate antibiotic therapy. If not, a call is made to the attending physician, who can either accept or reject the pharmacist’s recommended medication change.
Both pre- and post-MALDI-TOF it took about 20 hours from the time of the blood draw to a positive culture. But the time from that point to identification of the infecting pathogen dropped from 32 hours using the traditional method to 6.5 hours with MALDI-TOF. Time to results of susceptibility testing decreased from 48 to 22 hours. Time to antibiotic optimization for patients who weren’t already on effective therapy dropped from an average of 71 hours to 30 hours – and that’s what accounts for the dramatic reduction in mortality, according to Dr. Lockwood.
“With Gram-negative bacteremia, every hour counts. The mortality increases by 7.6% each hour, so I definitely think that reduction in mortality is real,” she said.
Although Dr. Lockwood and her coinvestigators expected to see a reduction in hospital length of stay post-MALDI-TOF, the average stay turned out to be 6.4 days in both groups. That’s probably because 6 days is already at the low end of the spectrum for patients admitted to a community hospital for bacteremia, she observed.
The initial investment for MALDI-TOF technology is roughly $250,000. That’s a drop in the bucket considering the reduced overall per-patient hospital cost and marked reduction in mortality, Dr. Lockwood observed.
She reported having no financial conflicts regarding her study, which was an unfunded research project.
SAN DIEGO – Adoption of rapid diagnostic testing in conjunction with an antimicrobial stewardship program reduced time to initiation of optimal antibiotic therapy and markedly improved clinical outcomes in patients admitted with Gram-negative bacteremia at two Houston-area community hospitals.
Indeed, in-hospital mortality in patients with Gram-negative bacteremia plummeted from 26% immediately prior to acquisition of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectrometry, when lab testing of bloodstream organisms was done the traditional way, to just 2% post intervention, Ashley M. Lockwood, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Moreover, average overall hospital costs dropped from $18,644 to $15,234, for a savings of $3,410 per patient attributable to MALDI-TOF plus the pharmacist-led antimicrobial stewardship program, added Dr. Lockwood, who performed the before-and-after comparison study while at Houston Methodist Hospital System and is now the infectious disease pharmacist at Bayfront Health in St. Petersburg, Fla.
Prior studies have shown big benefits for MALDI-TOF and other rapid diagnostic tests in combination with antimicrobial stewardship, but all the research was done at tertiary medical centers. It was unclear whether the results were generalizable to the thousands of community hospitals across the nation. The new study removes any doubts on that score, Dr. Lockwood said in an interview.
“The use of MALDI-TOF coupled with near real-time antimicrobial stewardship should be incorporated in the community setting to improve time to therapeutic optimization in patients with Gram-negative bacteremia,” she declared.
The study compared outcomes in 149 patients with Gram-negative bacteremia admitted to the two community hospitals prior to acquisition of a shared MALDI-TOF system and 241 admitted post acquisition. Prior to MALDI-TOF, when the traditional culture and susceptibility results came back, the information would simply be entered into the electronic medical record. Post-MALDI-TOF, when the lab results get entered into the EMR, a third-party surveillance program known as Vigilanz Real-Time Surveillance notifies a clinical pharmacist, who then goes into the EMR to make sure the patient is on appropriate antibiotic therapy. If not, a call is made to the attending physician, who can either accept or reject the pharmacist’s recommended medication change.
Both pre- and post-MALDI-TOF it took about 20 hours from the time of the blood draw to a positive culture. But the time from that point to identification of the infecting pathogen dropped from 32 hours using the traditional method to 6.5 hours with MALDI-TOF. Time to results of susceptibility testing decreased from 48 to 22 hours. Time to antibiotic optimization for patients who weren’t already on effective therapy dropped from an average of 71 hours to 30 hours – and that’s what accounts for the dramatic reduction in mortality, according to Dr. Lockwood.
“With Gram-negative bacteremia, every hour counts. The mortality increases by 7.6% each hour, so I definitely think that reduction in mortality is real,” she said.
Although Dr. Lockwood and her coinvestigators expected to see a reduction in hospital length of stay post-MALDI-TOF, the average stay turned out to be 6.4 days in both groups. That’s probably because 6 days is already at the low end of the spectrum for patients admitted to a community hospital for bacteremia, she observed.
The initial investment for MALDI-TOF technology is roughly $250,000. That’s a drop in the bucket considering the reduced overall per-patient hospital cost and marked reduction in mortality, Dr. Lockwood observed.
She reported having no financial conflicts regarding her study, which was an unfunded research project.
AT ICAAC 2015
Key clinical point: The use of rapid diagnostic testing in community hospitals sharply cuts time to antibiotic optimization and saves lives in patients with Gram-negative bacteremia.
Major finding: Hospital mortality in patients with Gram-negative bacteremia not on active empiric therapy prior to the results of susceptibility testing dropped from 26% to 2.1% following adoption of rapid diagnostic testing plus an antimicrobial stewardship program at two community hospitals.
Data source: This case-control study included 149 patients admitted with Gram-negative bacteremia prior to the intervention and 241 others admitted afterwards.
Disclosures: The presenter reported having no financial conflicts regarding this unfunded study.
Cefazolin outperforms nafcillin for staphylococcal bacteremia
SAN DIEGO – Cefazolin is more effective, less toxic, easier to dose, and far less expensive than nafcillin; the current guideline-recommended first-line standard therapy for methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, Marguerite L. Monogue, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“These findings, coupled with the cost savings involved with using cefazolin over nafcillin, make it an appealing first-line agent for most MSSA bloodstream infections,” asserted Dr. Monogue of Hartford (Conn.) Hospital.
She presented a retrospective, nonrandomized cohort study involving 142 patients admitted to Parkland Hospital in Dallas for treatment of MSSA bacteremia due to endocarditis, osteomyelitis, pneumonia, or deep abscesses. Half started on nafcillin, the other half on cefazolin.
The treatment failure rate was 8.4% in the cefazolin group compared with 14% in the nafcillin-treated patients.
Moreover, the adverse event rate was 7% with cefazolin versus 19.7% with nafcillin. Nephrotoxicity was the main side effect; it occurred in 16.9% of the nafcillin group compared with 2.8% of those on cefazolin.
These study results are sure to draw the attention of hospital administrators because nafcillin costs a hefty 10-13 times more than cefazolin. Dr. Monogue estimated that Parkland Hospital would have saved nearly $100,000 if the 71 nafcillin-treated patients had instead received cefazolin.
“Some of these endocarditis and osteomyelitis patients are being treated for 6 weeks,” she noted.
Both drugs are beta-lactam antimicrobials. Their mechanisms of action are similar. However, nafcillin is classified as a penicillin, while cefazolin is considered a first-generation cephalosporin.
Nafcillin is dosed every 4 hours or by continuous infusion. Cefazolin is dosed once every 8 hours. Advantage cefazoline. Another dosing advantage favoring cefazolin is that at Parkland Hospital, patients can be discharged on cefazaolin and complete their treatment course at home, while if they’re on nafcillin they must remain in-hospital to finish their regimen.
Dr. Monogue reported having no financial conflicts with regard to this investigator-initiated, unfunded study.
bjancin@frontlinemedcom.com
SAN DIEGO – Cefazolin is more effective, less toxic, easier to dose, and far less expensive than nafcillin; the current guideline-recommended first-line standard therapy for methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, Marguerite L. Monogue, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“These findings, coupled with the cost savings involved with using cefazolin over nafcillin, make it an appealing first-line agent for most MSSA bloodstream infections,” asserted Dr. Monogue of Hartford (Conn.) Hospital.
She presented a retrospective, nonrandomized cohort study involving 142 patients admitted to Parkland Hospital in Dallas for treatment of MSSA bacteremia due to endocarditis, osteomyelitis, pneumonia, or deep abscesses. Half started on nafcillin, the other half on cefazolin.
The treatment failure rate was 8.4% in the cefazolin group compared with 14% in the nafcillin-treated patients.
Moreover, the adverse event rate was 7% with cefazolin versus 19.7% with nafcillin. Nephrotoxicity was the main side effect; it occurred in 16.9% of the nafcillin group compared with 2.8% of those on cefazolin.
These study results are sure to draw the attention of hospital administrators because nafcillin costs a hefty 10-13 times more than cefazolin. Dr. Monogue estimated that Parkland Hospital would have saved nearly $100,000 if the 71 nafcillin-treated patients had instead received cefazolin.
“Some of these endocarditis and osteomyelitis patients are being treated for 6 weeks,” she noted.
Both drugs are beta-lactam antimicrobials. Their mechanisms of action are similar. However, nafcillin is classified as a penicillin, while cefazolin is considered a first-generation cephalosporin.
Nafcillin is dosed every 4 hours or by continuous infusion. Cefazolin is dosed once every 8 hours. Advantage cefazoline. Another dosing advantage favoring cefazolin is that at Parkland Hospital, patients can be discharged on cefazaolin and complete their treatment course at home, while if they’re on nafcillin they must remain in-hospital to finish their regimen.
Dr. Monogue reported having no financial conflicts with regard to this investigator-initiated, unfunded study.
bjancin@frontlinemedcom.com
SAN DIEGO – Cefazolin is more effective, less toxic, easier to dose, and far less expensive than nafcillin; the current guideline-recommended first-line standard therapy for methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, Marguerite L. Monogue, Pharm.D., reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“These findings, coupled with the cost savings involved with using cefazolin over nafcillin, make it an appealing first-line agent for most MSSA bloodstream infections,” asserted Dr. Monogue of Hartford (Conn.) Hospital.
She presented a retrospective, nonrandomized cohort study involving 142 patients admitted to Parkland Hospital in Dallas for treatment of MSSA bacteremia due to endocarditis, osteomyelitis, pneumonia, or deep abscesses. Half started on nafcillin, the other half on cefazolin.
The treatment failure rate was 8.4% in the cefazolin group compared with 14% in the nafcillin-treated patients.
Moreover, the adverse event rate was 7% with cefazolin versus 19.7% with nafcillin. Nephrotoxicity was the main side effect; it occurred in 16.9% of the nafcillin group compared with 2.8% of those on cefazolin.
These study results are sure to draw the attention of hospital administrators because nafcillin costs a hefty 10-13 times more than cefazolin. Dr. Monogue estimated that Parkland Hospital would have saved nearly $100,000 if the 71 nafcillin-treated patients had instead received cefazolin.
“Some of these endocarditis and osteomyelitis patients are being treated for 6 weeks,” she noted.
Both drugs are beta-lactam antimicrobials. Their mechanisms of action are similar. However, nafcillin is classified as a penicillin, while cefazolin is considered a first-generation cephalosporin.
Nafcillin is dosed every 4 hours or by continuous infusion. Cefazolin is dosed once every 8 hours. Advantage cefazoline. Another dosing advantage favoring cefazolin is that at Parkland Hospital, patients can be discharged on cefazaolin and complete their treatment course at home, while if they’re on nafcillin they must remain in-hospital to finish their regimen.
Dr. Monogue reported having no financial conflicts with regard to this investigator-initiated, unfunded study.
bjancin@frontlinemedcom.com
AT ICAAC 2015
Key clinical point: The time is nigh for cefazolin to replace nafcillin as first-line therapy for methicillin-susceptible Staphylococcus aureus bacteremia.
Major finding: Treatment failure and adverse event rates were 8.4% and 7%, respectively, with cefazolin, compared with 14% and 19.7% with nafcillin.
Data source: This retrospective, nonrandomized cohort study included 142 patients hospitalized for methicillin-suscueptible Staphylococcus aureus bacteremia, with half started on current first-line nafcillin, the other half on cefazolin.
Disclosures: The presenter reported having no financial conflicts with regard to this investigator-initiated, unfunded study.
WCD: Look for TNF inhibitor–induced psoriasis in kids
VANCOUVER – Tumor necrosis factor inhibitor–induced psoriasiform dermatitis can occur in pediatric patients after any length of treatment, with documented cases emerging after the very first dose and as late as 63 months into anti-TNF therapy, Dr. Amy S. Paller said at the World Congress of Dermatology.
Histopathologically, this medication-induced condition is psoriasis. But it tends to follow a distinctive pattern, favoring the scalp, dorsal hands and feet, nails, and periorificial skin. Palmoplantar pustulosis is not uncommon. The lesions are often secondarily infected with Staphylococcus aureus, according to Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University in Chicago.
The phenomenon was first described in adults. But in the past several years, as the use of tumor necrosis factor (TNF) antagonists has gained increasing traction for treatment of pediatric inflammatory bowel disease and rheumatologic diseases, the dermatologic disorder has become better characterized in youths. In a retrospective study at McMaster Children’s Hospital in Hamilton, Ont., 17 of 172 (10%) infliximab-treated patients with Crohn’s disease developed new-onset psoriasis and another (0.6%) experienced worsening of preexisting psoriasis after anywhere from 1 to 25 infusions. Most patients responded well to topical steroids; however, three discontinued the biologic because of this complication (J Pediatr Gastroenterol Nutr. 2013 May;56[5]:512-8).
It’s now clear that the emergence of TNF inhibitor–induced psoriasis does not adversely affect the response of a patient’s inflammatory bowel disease or juvenile arthritis to the biologic. Also, the risk of recurrent psoriatic eruption is not reduced by concurrent methotrexate.
Researchers find TNF inhibitor–induced psoriasis to be an intriguing puzzle because of its paradoxical nature. After all, the TNF inhibitors are a highly effective treatment for moderate to severe plaque psoriasis. The leading theory as to the underlying basis for TNF inhibitor–induced psoriasis is that it may have a genetic basis, Dr. Paller noted.
The McMaster group found that their pediatric Crohn’s disease patients who developed psoriasis in conjunction with infliximab (Remicade) therapy were more likely than disease-matched controls to be homozygous for one of several specific polymorphisms in the interleukin-23R gene. And investigators at the University of Helsinki have reported that children with inflammatory bowel disease who developed psoriasiform dermatitis while on infliximab only rarely possessed the HLA-Cw*0602 genotype, which is commonly associated with psoriasis (Inflamm Bowel Dis. 2014 Aug;20[8]:1309-15).
The possibility of streptococcal or staphylococcal infection serving as a trigger for TNF inhibitor–induced psoriasis is also being explored, according to Dr. Paller.
She has received research funding from LEO Pharma and Amgen and serves as a consultant to AbbVie.
VANCOUVER – Tumor necrosis factor inhibitor–induced psoriasiform dermatitis can occur in pediatric patients after any length of treatment, with documented cases emerging after the very first dose and as late as 63 months into anti-TNF therapy, Dr. Amy S. Paller said at the World Congress of Dermatology.
Histopathologically, this medication-induced condition is psoriasis. But it tends to follow a distinctive pattern, favoring the scalp, dorsal hands and feet, nails, and periorificial skin. Palmoplantar pustulosis is not uncommon. The lesions are often secondarily infected with Staphylococcus aureus, according to Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University in Chicago.
The phenomenon was first described in adults. But in the past several years, as the use of tumor necrosis factor (TNF) antagonists has gained increasing traction for treatment of pediatric inflammatory bowel disease and rheumatologic diseases, the dermatologic disorder has become better characterized in youths. In a retrospective study at McMaster Children’s Hospital in Hamilton, Ont., 17 of 172 (10%) infliximab-treated patients with Crohn’s disease developed new-onset psoriasis and another (0.6%) experienced worsening of preexisting psoriasis after anywhere from 1 to 25 infusions. Most patients responded well to topical steroids; however, three discontinued the biologic because of this complication (J Pediatr Gastroenterol Nutr. 2013 May;56[5]:512-8).
It’s now clear that the emergence of TNF inhibitor–induced psoriasis does not adversely affect the response of a patient’s inflammatory bowel disease or juvenile arthritis to the biologic. Also, the risk of recurrent psoriatic eruption is not reduced by concurrent methotrexate.
Researchers find TNF inhibitor–induced psoriasis to be an intriguing puzzle because of its paradoxical nature. After all, the TNF inhibitors are a highly effective treatment for moderate to severe plaque psoriasis. The leading theory as to the underlying basis for TNF inhibitor–induced psoriasis is that it may have a genetic basis, Dr. Paller noted.
The McMaster group found that their pediatric Crohn’s disease patients who developed psoriasis in conjunction with infliximab (Remicade) therapy were more likely than disease-matched controls to be homozygous for one of several specific polymorphisms in the interleukin-23R gene. And investigators at the University of Helsinki have reported that children with inflammatory bowel disease who developed psoriasiform dermatitis while on infliximab only rarely possessed the HLA-Cw*0602 genotype, which is commonly associated with psoriasis (Inflamm Bowel Dis. 2014 Aug;20[8]:1309-15).
The possibility of streptococcal or staphylococcal infection serving as a trigger for TNF inhibitor–induced psoriasis is also being explored, according to Dr. Paller.
She has received research funding from LEO Pharma and Amgen and serves as a consultant to AbbVie.
VANCOUVER – Tumor necrosis factor inhibitor–induced psoriasiform dermatitis can occur in pediatric patients after any length of treatment, with documented cases emerging after the very first dose and as late as 63 months into anti-TNF therapy, Dr. Amy S. Paller said at the World Congress of Dermatology.
Histopathologically, this medication-induced condition is psoriasis. But it tends to follow a distinctive pattern, favoring the scalp, dorsal hands and feet, nails, and periorificial skin. Palmoplantar pustulosis is not uncommon. The lesions are often secondarily infected with Staphylococcus aureus, according to Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University in Chicago.
The phenomenon was first described in adults. But in the past several years, as the use of tumor necrosis factor (TNF) antagonists has gained increasing traction for treatment of pediatric inflammatory bowel disease and rheumatologic diseases, the dermatologic disorder has become better characterized in youths. In a retrospective study at McMaster Children’s Hospital in Hamilton, Ont., 17 of 172 (10%) infliximab-treated patients with Crohn’s disease developed new-onset psoriasis and another (0.6%) experienced worsening of preexisting psoriasis after anywhere from 1 to 25 infusions. Most patients responded well to topical steroids; however, three discontinued the biologic because of this complication (J Pediatr Gastroenterol Nutr. 2013 May;56[5]:512-8).
It’s now clear that the emergence of TNF inhibitor–induced psoriasis does not adversely affect the response of a patient’s inflammatory bowel disease or juvenile arthritis to the biologic. Also, the risk of recurrent psoriatic eruption is not reduced by concurrent methotrexate.
Researchers find TNF inhibitor–induced psoriasis to be an intriguing puzzle because of its paradoxical nature. After all, the TNF inhibitors are a highly effective treatment for moderate to severe plaque psoriasis. The leading theory as to the underlying basis for TNF inhibitor–induced psoriasis is that it may have a genetic basis, Dr. Paller noted.
The McMaster group found that their pediatric Crohn’s disease patients who developed psoriasis in conjunction with infliximab (Remicade) therapy were more likely than disease-matched controls to be homozygous for one of several specific polymorphisms in the interleukin-23R gene. And investigators at the University of Helsinki have reported that children with inflammatory bowel disease who developed psoriasiform dermatitis while on infliximab only rarely possessed the HLA-Cw*0602 genotype, which is commonly associated with psoriasis (Inflamm Bowel Dis. 2014 Aug;20[8]:1309-15).
The possibility of streptococcal or staphylococcal infection serving as a trigger for TNF inhibitor–induced psoriasis is also being explored, according to Dr. Paller.
She has received research funding from LEO Pharma and Amgen and serves as a consultant to AbbVie.
EXPERT ANALYSIS FROM WCD 2015
Nail psoriasis therapies lack supporting evidence
VANCOUVER – Evidence-based therapy for nail psoriasis is in a sorry state because of a lack of consensus on a reliable nail psoriasis scoring system for use in clinical trials, according to a coauthor of the Cochrane systematic review of interventions for nail psoriasis.
“The last 12 randomized clinical trials used 21 ways of scoring the results of treatment, so comparing the studies means comparing apples to oranges. Which is the most effective treatment? What should we advise our patients? We don’t know. Comparison is impossible,” Dr. Marcel C. Pasch said at the World Congress of Dermatology.
The Cochrane report (Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633) deemed the evidence for topical therapies as “inconclusive and weak,” even though topicals are the treatment mainstay for this localized expression of psoriasis. Indeed, Dr. Pasch and his coauthors found that no topical therapy has been shown effective in improving nail psoriasis. The Cochrane group concluded that just five therapies rise to the standard of being evidence based in terms of efficacy: the tumor necrosis factor (TNF) inhibitors infliximab (Remicade) and golimumab (Simponi), superficial radiation therapy, Grenz rays, and electron beam therapy. All five are strikingly impractical for use in clinical practice.
“The findings are quite disappointing because nobody sends a patient with psoriasis to the radiotherapist, and while giving an anti-TNF biologic only for the nails will be effective, at least in my country it won’t be reimbursed,” wrote Dr. Pasch, a dermatologist at Radboud University Nijmegen (the Netherlands) Medical Centre.
The presence and severity of nail psoriasis is unrelated to the severity of cutaneous psoriasis. Moreover, nail psoriasis without cutaneous involvement occurs in 5%-10% of psoriasis patients.
Since publication of the Cochrane systematic review, 12 new randomized controlled trials of treatments for nail psoriasis have appeared. Six focused on biologics: the anti-TNF agents certolizumab (Cimzia), etanercept (Enbrel), and adalimumab (Humira); the anti–interleukin-12/23 agent ustekinumab (Stelara); and the interleukin-17A inhibitor secukiumab (Cosentyx). Dr. Pasch said in his opinion all five biologics were supported by convincing studies and now can be added to the short list of evidence-based nail psoriasis therapies.
Of the six recent studies of topical therapies, two provided persuasive evidence of efficacy, in his view: tacrolimus ointment and indigo naturalis extract in oil (Lindioil), a variant of a traditional Chinese medicine therapy, which at this time isn’t commercially available.
In contrast, studies of clobetasol nail lacquer, pulsed dye laser therapy, a nail lacquer based upon chitin from crab shells, and a study of calcitriol ointment versus betamethasone dipropionate ointment failed to be convincing either because of methodologic problems or lack of efficacy, he continued.
These 12 recent randomized clinical trials utilized 21 different nail psoriasis scoring systems.
“Which scoring system is best? The answer is, we don’t know,” Dr. Pasch said.
He and his coinvestigators compared eight different scoring systems in a prospective study and concluded that the Nijmegen–Nail Psoriasis Activity Index Tool (N-NAIL), which Dr. Pasch helped develop, best reflected the clinical severity of nail psoriasis (J Am Acad Dermatol. 2014 Jun;70[6]:1061-6).
However, he added that at present there is no validated scoring system for nail psoriasis. And creation of a single validated scoring system that researchers can agree on as the standard is a prerequisite for making major advances in the treatment of nail psoriasis, in Dr. Pasch’s view.
He is so convinced of this that he has created an organization whose goal is to achieve consensus on one reliable, validated nail psoriasis scoring system for use in clinical trials. At the World Congress of Dermatology, he invited stakeholders – including academic and community dermatologists, patient organizations, and the pharmaceutical industry – to join (www.nailinitiative.org).
Session chair Dr. Peter van de Kerkhof, chairman of dermatology at Radboud University, said he sees the NAPSI (Nail Psoriasis Severity Index) being used in lots of clinical trials in psoriasis. What’s wrong with building a consensus around NAPSI? he asked.
“The problem is not the NAPSI score,” Dr. Pasch replied. “The problem is that in each trial a modified NAPSI score is used, but they are all modified in different ways. We have the single-hand NAPSI, the eight-finger NAPSI, the 10-finger NAPSI, the target NAPSI. The NAPSI doesn’t exist anymore.”
He reported receiving research grants from Pfizer and Janssen-Cilag.
VANCOUVER – Evidence-based therapy for nail psoriasis is in a sorry state because of a lack of consensus on a reliable nail psoriasis scoring system for use in clinical trials, according to a coauthor of the Cochrane systematic review of interventions for nail psoriasis.
“The last 12 randomized clinical trials used 21 ways of scoring the results of treatment, so comparing the studies means comparing apples to oranges. Which is the most effective treatment? What should we advise our patients? We don’t know. Comparison is impossible,” Dr. Marcel C. Pasch said at the World Congress of Dermatology.
The Cochrane report (Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633) deemed the evidence for topical therapies as “inconclusive and weak,” even though topicals are the treatment mainstay for this localized expression of psoriasis. Indeed, Dr. Pasch and his coauthors found that no topical therapy has been shown effective in improving nail psoriasis. The Cochrane group concluded that just five therapies rise to the standard of being evidence based in terms of efficacy: the tumor necrosis factor (TNF) inhibitors infliximab (Remicade) and golimumab (Simponi), superficial radiation therapy, Grenz rays, and electron beam therapy. All five are strikingly impractical for use in clinical practice.
“The findings are quite disappointing because nobody sends a patient with psoriasis to the radiotherapist, and while giving an anti-TNF biologic only for the nails will be effective, at least in my country it won’t be reimbursed,” wrote Dr. Pasch, a dermatologist at Radboud University Nijmegen (the Netherlands) Medical Centre.
The presence and severity of nail psoriasis is unrelated to the severity of cutaneous psoriasis. Moreover, nail psoriasis without cutaneous involvement occurs in 5%-10% of psoriasis patients.
Since publication of the Cochrane systematic review, 12 new randomized controlled trials of treatments for nail psoriasis have appeared. Six focused on biologics: the anti-TNF agents certolizumab (Cimzia), etanercept (Enbrel), and adalimumab (Humira); the anti–interleukin-12/23 agent ustekinumab (Stelara); and the interleukin-17A inhibitor secukiumab (Cosentyx). Dr. Pasch said in his opinion all five biologics were supported by convincing studies and now can be added to the short list of evidence-based nail psoriasis therapies.
Of the six recent studies of topical therapies, two provided persuasive evidence of efficacy, in his view: tacrolimus ointment and indigo naturalis extract in oil (Lindioil), a variant of a traditional Chinese medicine therapy, which at this time isn’t commercially available.
In contrast, studies of clobetasol nail lacquer, pulsed dye laser therapy, a nail lacquer based upon chitin from crab shells, and a study of calcitriol ointment versus betamethasone dipropionate ointment failed to be convincing either because of methodologic problems or lack of efficacy, he continued.
These 12 recent randomized clinical trials utilized 21 different nail psoriasis scoring systems.
“Which scoring system is best? The answer is, we don’t know,” Dr. Pasch said.
He and his coinvestigators compared eight different scoring systems in a prospective study and concluded that the Nijmegen–Nail Psoriasis Activity Index Tool (N-NAIL), which Dr. Pasch helped develop, best reflected the clinical severity of nail psoriasis (J Am Acad Dermatol. 2014 Jun;70[6]:1061-6).
However, he added that at present there is no validated scoring system for nail psoriasis. And creation of a single validated scoring system that researchers can agree on as the standard is a prerequisite for making major advances in the treatment of nail psoriasis, in Dr. Pasch’s view.
He is so convinced of this that he has created an organization whose goal is to achieve consensus on one reliable, validated nail psoriasis scoring system for use in clinical trials. At the World Congress of Dermatology, he invited stakeholders – including academic and community dermatologists, patient organizations, and the pharmaceutical industry – to join (www.nailinitiative.org).
Session chair Dr. Peter van de Kerkhof, chairman of dermatology at Radboud University, said he sees the NAPSI (Nail Psoriasis Severity Index) being used in lots of clinical trials in psoriasis. What’s wrong with building a consensus around NAPSI? he asked.
“The problem is not the NAPSI score,” Dr. Pasch replied. “The problem is that in each trial a modified NAPSI score is used, but they are all modified in different ways. We have the single-hand NAPSI, the eight-finger NAPSI, the 10-finger NAPSI, the target NAPSI. The NAPSI doesn’t exist anymore.”
He reported receiving research grants from Pfizer and Janssen-Cilag.
VANCOUVER – Evidence-based therapy for nail psoriasis is in a sorry state because of a lack of consensus on a reliable nail psoriasis scoring system for use in clinical trials, according to a coauthor of the Cochrane systematic review of interventions for nail psoriasis.
“The last 12 randomized clinical trials used 21 ways of scoring the results of treatment, so comparing the studies means comparing apples to oranges. Which is the most effective treatment? What should we advise our patients? We don’t know. Comparison is impossible,” Dr. Marcel C. Pasch said at the World Congress of Dermatology.
The Cochrane report (Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633) deemed the evidence for topical therapies as “inconclusive and weak,” even though topicals are the treatment mainstay for this localized expression of psoriasis. Indeed, Dr. Pasch and his coauthors found that no topical therapy has been shown effective in improving nail psoriasis. The Cochrane group concluded that just five therapies rise to the standard of being evidence based in terms of efficacy: the tumor necrosis factor (TNF) inhibitors infliximab (Remicade) and golimumab (Simponi), superficial radiation therapy, Grenz rays, and electron beam therapy. All five are strikingly impractical for use in clinical practice.
“The findings are quite disappointing because nobody sends a patient with psoriasis to the radiotherapist, and while giving an anti-TNF biologic only for the nails will be effective, at least in my country it won’t be reimbursed,” wrote Dr. Pasch, a dermatologist at Radboud University Nijmegen (the Netherlands) Medical Centre.
The presence and severity of nail psoriasis is unrelated to the severity of cutaneous psoriasis. Moreover, nail psoriasis without cutaneous involvement occurs in 5%-10% of psoriasis patients.
Since publication of the Cochrane systematic review, 12 new randomized controlled trials of treatments for nail psoriasis have appeared. Six focused on biologics: the anti-TNF agents certolizumab (Cimzia), etanercept (Enbrel), and adalimumab (Humira); the anti–interleukin-12/23 agent ustekinumab (Stelara); and the interleukin-17A inhibitor secukiumab (Cosentyx). Dr. Pasch said in his opinion all five biologics were supported by convincing studies and now can be added to the short list of evidence-based nail psoriasis therapies.
Of the six recent studies of topical therapies, two provided persuasive evidence of efficacy, in his view: tacrolimus ointment and indigo naturalis extract in oil (Lindioil), a variant of a traditional Chinese medicine therapy, which at this time isn’t commercially available.
In contrast, studies of clobetasol nail lacquer, pulsed dye laser therapy, a nail lacquer based upon chitin from crab shells, and a study of calcitriol ointment versus betamethasone dipropionate ointment failed to be convincing either because of methodologic problems or lack of efficacy, he continued.
These 12 recent randomized clinical trials utilized 21 different nail psoriasis scoring systems.
“Which scoring system is best? The answer is, we don’t know,” Dr. Pasch said.
He and his coinvestigators compared eight different scoring systems in a prospective study and concluded that the Nijmegen–Nail Psoriasis Activity Index Tool (N-NAIL), which Dr. Pasch helped develop, best reflected the clinical severity of nail psoriasis (J Am Acad Dermatol. 2014 Jun;70[6]:1061-6).
However, he added that at present there is no validated scoring system for nail psoriasis. And creation of a single validated scoring system that researchers can agree on as the standard is a prerequisite for making major advances in the treatment of nail psoriasis, in Dr. Pasch’s view.
He is so convinced of this that he has created an organization whose goal is to achieve consensus on one reliable, validated nail psoriasis scoring system for use in clinical trials. At the World Congress of Dermatology, he invited stakeholders – including academic and community dermatologists, patient organizations, and the pharmaceutical industry – to join (www.nailinitiative.org).
Session chair Dr. Peter van de Kerkhof, chairman of dermatology at Radboud University, said he sees the NAPSI (Nail Psoriasis Severity Index) being used in lots of clinical trials in psoriasis. What’s wrong with building a consensus around NAPSI? he asked.
“The problem is not the NAPSI score,” Dr. Pasch replied. “The problem is that in each trial a modified NAPSI score is used, but they are all modified in different ways. We have the single-hand NAPSI, the eight-finger NAPSI, the 10-finger NAPSI, the target NAPSI. The NAPSI doesn’t exist anymore.”
He reported receiving research grants from Pfizer and Janssen-Cilag.
EXPERT ANALYSIS FROM WCD 2015
When to think ‘primary aldosteronism’
ESTES PARK, COLO. – Primary aldosteronism, traditionally seen as a rare, stump-the-experts-type disorder, is now accepted as the cause of 5%-10% of all cases of what has been considered essential hypertension.
Primary aldosteronism is a readily treatable disorder, either surgically or medically, depending upon the subtype. So it’s essential to know which hypertensive patients to screen and how to confirm the diagnosis and then to move forward to identify the subtype in play, Dr. Michael T. McDermott said at a conference on internal medicine sponsored by the University of Colorado.
Primary aldosteronism occurs when the adrenal gland produces excessive aldosterone without being stimulated by renin. Aldosterone causes sodium retention in the kidney in exchange for potassium and hydrogen ions. The result is the triple harms of hypertension, hypokalemia, and metabolic acidosis, explained Dr. McDermott, professor of medicine and clinical pharmacology and director of endocrinology and diabetes practice at University of Colorado Hospital in Aurora.
The two main subtypes of primary aldosteronism are idiopathic hyperaldosteronism (IHA), also known as bilateral adrenal hyperplasia, which accounts for two-thirds of all cases, and unilateral aldosterone-producing adenoma, which accounts for the remaining third.
Which hypertensive patients should be screened for primary aldosteronism? Anyone with resistant hypertension as defined by inadequate blood pressure control while on three or more antihypertensive drugs; those with severe hypertension, meaning readings greater than 160/100 mm Hg; patients with onset of hypertension before age 20; and any hypertensive patient with hypokalemia, which can either be provoked by diuretic therapy or in some cases occurs spontaneously, the endocrinologist continued.
Screening for primary aldosteronism entails getting a morning blood sample to measure plasma aldosterone and plasma renin activity after having the patient sit for 10 minutes. A positive screen requires both a plasma aldosterone level greater than 15 ng/dL and a plasma aldosterone/plasma renin activity ratio greater than 20.
“You can do this screening test in a patient on any medication except spironolactone. They have to stop that drug for at least 2 weeks first,” according to Dr. McDermott.
Confirmation of a positive screening test requires demonstration that the elevated aldosterone can’t be suppressed via volume expansion. This volume expansion can be accomplished in two ways: putting the patient on a high-salt diet for 3 days, which can generally be accomplished simply by eating some potato chips daily on top of a typically high-salt American diet, or by intravenous infusion of 2 L of normal saline over the course of 4 hours.
If a 24-hour urine collection on day 3 of a high-salt diet shows an aldosterone level in excess of 12 mcg, the diagnosis of primary aldosteronism is confirmed.
“If anyone in this room who doesn’t have primary aldosteronism had a high-salt diet for 3 days and we did a 24-hour urine on the third day, your aldosterone would be zero. So a level over 12 mcg is clearly abnormal,” Dr. McDermott emphasized.
Likewise, a plasma aldosterone greater than 10 ng/dL following the IV saline infusion is also confirmatory.
A good clinical clue that primary aldosteronism is due to an aldosterone-producing tumor is severe hypertension and/or severe hypokalemia in a patient under 40 years of age. A plasma aldosterone greater than 25 ng/dL or a urine aldosterone in excess of 30 mcg/24 hours is another useful clue because a tumor produces a lot more aldosterone than does bilateral adrenal hyperplasia.
If, and only if, a patient is willing to undergo surgery in the event further work-up establishes the presence of an aldosterone-producing tumor, the next step is an abdominal CT scan. If it reveals a unilateral hypodense nodule greater than 1 cm in size and the patient is less than 35 years old, referral for unilateral laparoscopic or open adrenalectomy is warranted. Preoperatively the patient should be placed on either spironolactone or eplerenone; these aldosterone antagonists block the effects of aldosterone on the kidney, with resultant normalization of hypertension and hypokalemia.
Bilateral adrenal hyperplasia is much more common than an aldosterone-producing tumor in patients older than age 35, so even when a unilateral nodule is found in such a patient it’s worthwhile to consider adrenal vein sampling. If the sampling lateralizes to one side then adrenalectomy can be offered. If there is no lateralization, however, the patient has IHA and medical management is appropriate. An aldosterone antagonist will normalize the hypokalemia but may not be sufficient to control the elevated blood pressure, in which case a calcium channel blocker, ACE inhibitor, and/or angiotensin receptor blocker can be added.
Dr. McDermott reported having no financial conflicts with regard to his presentation.
ESTES PARK, COLO. – Primary aldosteronism, traditionally seen as a rare, stump-the-experts-type disorder, is now accepted as the cause of 5%-10% of all cases of what has been considered essential hypertension.
Primary aldosteronism is a readily treatable disorder, either surgically or medically, depending upon the subtype. So it’s essential to know which hypertensive patients to screen and how to confirm the diagnosis and then to move forward to identify the subtype in play, Dr. Michael T. McDermott said at a conference on internal medicine sponsored by the University of Colorado.
Primary aldosteronism occurs when the adrenal gland produces excessive aldosterone without being stimulated by renin. Aldosterone causes sodium retention in the kidney in exchange for potassium and hydrogen ions. The result is the triple harms of hypertension, hypokalemia, and metabolic acidosis, explained Dr. McDermott, professor of medicine and clinical pharmacology and director of endocrinology and diabetes practice at University of Colorado Hospital in Aurora.
The two main subtypes of primary aldosteronism are idiopathic hyperaldosteronism (IHA), also known as bilateral adrenal hyperplasia, which accounts for two-thirds of all cases, and unilateral aldosterone-producing adenoma, which accounts for the remaining third.
Which hypertensive patients should be screened for primary aldosteronism? Anyone with resistant hypertension as defined by inadequate blood pressure control while on three or more antihypertensive drugs; those with severe hypertension, meaning readings greater than 160/100 mm Hg; patients with onset of hypertension before age 20; and any hypertensive patient with hypokalemia, which can either be provoked by diuretic therapy or in some cases occurs spontaneously, the endocrinologist continued.
Screening for primary aldosteronism entails getting a morning blood sample to measure plasma aldosterone and plasma renin activity after having the patient sit for 10 minutes. A positive screen requires both a plasma aldosterone level greater than 15 ng/dL and a plasma aldosterone/plasma renin activity ratio greater than 20.
“You can do this screening test in a patient on any medication except spironolactone. They have to stop that drug for at least 2 weeks first,” according to Dr. McDermott.
Confirmation of a positive screening test requires demonstration that the elevated aldosterone can’t be suppressed via volume expansion. This volume expansion can be accomplished in two ways: putting the patient on a high-salt diet for 3 days, which can generally be accomplished simply by eating some potato chips daily on top of a typically high-salt American diet, or by intravenous infusion of 2 L of normal saline over the course of 4 hours.
If a 24-hour urine collection on day 3 of a high-salt diet shows an aldosterone level in excess of 12 mcg, the diagnosis of primary aldosteronism is confirmed.
“If anyone in this room who doesn’t have primary aldosteronism had a high-salt diet for 3 days and we did a 24-hour urine on the third day, your aldosterone would be zero. So a level over 12 mcg is clearly abnormal,” Dr. McDermott emphasized.
Likewise, a plasma aldosterone greater than 10 ng/dL following the IV saline infusion is also confirmatory.
A good clinical clue that primary aldosteronism is due to an aldosterone-producing tumor is severe hypertension and/or severe hypokalemia in a patient under 40 years of age. A plasma aldosterone greater than 25 ng/dL or a urine aldosterone in excess of 30 mcg/24 hours is another useful clue because a tumor produces a lot more aldosterone than does bilateral adrenal hyperplasia.
If, and only if, a patient is willing to undergo surgery in the event further work-up establishes the presence of an aldosterone-producing tumor, the next step is an abdominal CT scan. If it reveals a unilateral hypodense nodule greater than 1 cm in size and the patient is less than 35 years old, referral for unilateral laparoscopic or open adrenalectomy is warranted. Preoperatively the patient should be placed on either spironolactone or eplerenone; these aldosterone antagonists block the effects of aldosterone on the kidney, with resultant normalization of hypertension and hypokalemia.
Bilateral adrenal hyperplasia is much more common than an aldosterone-producing tumor in patients older than age 35, so even when a unilateral nodule is found in such a patient it’s worthwhile to consider adrenal vein sampling. If the sampling lateralizes to one side then adrenalectomy can be offered. If there is no lateralization, however, the patient has IHA and medical management is appropriate. An aldosterone antagonist will normalize the hypokalemia but may not be sufficient to control the elevated blood pressure, in which case a calcium channel blocker, ACE inhibitor, and/or angiotensin receptor blocker can be added.
Dr. McDermott reported having no financial conflicts with regard to his presentation.
ESTES PARK, COLO. – Primary aldosteronism, traditionally seen as a rare, stump-the-experts-type disorder, is now accepted as the cause of 5%-10% of all cases of what has been considered essential hypertension.
Primary aldosteronism is a readily treatable disorder, either surgically or medically, depending upon the subtype. So it’s essential to know which hypertensive patients to screen and how to confirm the diagnosis and then to move forward to identify the subtype in play, Dr. Michael T. McDermott said at a conference on internal medicine sponsored by the University of Colorado.
Primary aldosteronism occurs when the adrenal gland produces excessive aldosterone without being stimulated by renin. Aldosterone causes sodium retention in the kidney in exchange for potassium and hydrogen ions. The result is the triple harms of hypertension, hypokalemia, and metabolic acidosis, explained Dr. McDermott, professor of medicine and clinical pharmacology and director of endocrinology and diabetes practice at University of Colorado Hospital in Aurora.
The two main subtypes of primary aldosteronism are idiopathic hyperaldosteronism (IHA), also known as bilateral adrenal hyperplasia, which accounts for two-thirds of all cases, and unilateral aldosterone-producing adenoma, which accounts for the remaining third.
Which hypertensive patients should be screened for primary aldosteronism? Anyone with resistant hypertension as defined by inadequate blood pressure control while on three or more antihypertensive drugs; those with severe hypertension, meaning readings greater than 160/100 mm Hg; patients with onset of hypertension before age 20; and any hypertensive patient with hypokalemia, which can either be provoked by diuretic therapy or in some cases occurs spontaneously, the endocrinologist continued.
Screening for primary aldosteronism entails getting a morning blood sample to measure plasma aldosterone and plasma renin activity after having the patient sit for 10 minutes. A positive screen requires both a plasma aldosterone level greater than 15 ng/dL and a plasma aldosterone/plasma renin activity ratio greater than 20.
“You can do this screening test in a patient on any medication except spironolactone. They have to stop that drug for at least 2 weeks first,” according to Dr. McDermott.
Confirmation of a positive screening test requires demonstration that the elevated aldosterone can’t be suppressed via volume expansion. This volume expansion can be accomplished in two ways: putting the patient on a high-salt diet for 3 days, which can generally be accomplished simply by eating some potato chips daily on top of a typically high-salt American diet, or by intravenous infusion of 2 L of normal saline over the course of 4 hours.
If a 24-hour urine collection on day 3 of a high-salt diet shows an aldosterone level in excess of 12 mcg, the diagnosis of primary aldosteronism is confirmed.
“If anyone in this room who doesn’t have primary aldosteronism had a high-salt diet for 3 days and we did a 24-hour urine on the third day, your aldosterone would be zero. So a level over 12 mcg is clearly abnormal,” Dr. McDermott emphasized.
Likewise, a plasma aldosterone greater than 10 ng/dL following the IV saline infusion is also confirmatory.
A good clinical clue that primary aldosteronism is due to an aldosterone-producing tumor is severe hypertension and/or severe hypokalemia in a patient under 40 years of age. A plasma aldosterone greater than 25 ng/dL or a urine aldosterone in excess of 30 mcg/24 hours is another useful clue because a tumor produces a lot more aldosterone than does bilateral adrenal hyperplasia.
If, and only if, a patient is willing to undergo surgery in the event further work-up establishes the presence of an aldosterone-producing tumor, the next step is an abdominal CT scan. If it reveals a unilateral hypodense nodule greater than 1 cm in size and the patient is less than 35 years old, referral for unilateral laparoscopic or open adrenalectomy is warranted. Preoperatively the patient should be placed on either spironolactone or eplerenone; these aldosterone antagonists block the effects of aldosterone on the kidney, with resultant normalization of hypertension and hypokalemia.
Bilateral adrenal hyperplasia is much more common than an aldosterone-producing tumor in patients older than age 35, so even when a unilateral nodule is found in such a patient it’s worthwhile to consider adrenal vein sampling. If the sampling lateralizes to one side then adrenalectomy can be offered. If there is no lateralization, however, the patient has IHA and medical management is appropriate. An aldosterone antagonist will normalize the hypokalemia but may not be sufficient to control the elevated blood pressure, in which case a calcium channel blocker, ACE inhibitor, and/or angiotensin receptor blocker can be added.
Dr. McDermott reported having no financial conflicts with regard to his presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
Attack PCOS in adolescents hard
ESTES PARK, COLO. – The sweet spot for intervention in polycystic ovary syndrome occurs early: in the adolescent who doesn’t yet desire pregnancy and who is just starting to experience the classic PCOS progressive weight gain.
“The obstetricians don’t want to see these patients when they’re already 220 lb and they’re trying to induce ovulation. It’s our job to pick them up in their adolescence. You want to clamp the hormones – suppress the androgens – so that things will be better down the road,” Dr. Margaret E. Wierman urged at a conference on internal medicine sponsored by the University of Colorado.
Aggressive treatment at this stage will reduce the risk of a host of potential health problems later. In addition to infertility issues, these include increased long-term risks of diabetes, hypertension, dyslipidemia, metabolic syndrome, endometrial cancer, obstructive sleep apnea, and nonalcoholic fatty liver disease, noted Dr. Wierman, professor of medicine, ob.gyn., and physiology at the university and chief of endocrinology at the Denver VA Medical Center.
PCOS is by far the most common cause of hyperandrogenic anovulation. Indeed, 6%-8% of all women in their childbearing years have PCOS. The condition is defined by clinical and/or biochemical hyperandrogenism, oligomenorrhea or amenorrhea, and the presence of ovarian cysts or increased ovarian volume on ultrasound.
In the adolescent with PCOS who doesn’t desire pregnancy, Dr. Wierman takes a three-pronged treatment approach: regularize menses with a less androgenic oral contraceptive, such as one containing norethindrone, or by using cyclic progesterone; treat hirsutism with spironolactone at 50-100 mg once daily coupled with electrolysis for local control; and consider using short-acting metformin as an insulin-sensitizing agent.
The use of metformin in treating PCOS is controversial. It is off-label therapy. There have been no prospective, randomized, placebo-controlled, double-blind studies supporting this application of the drug. Nevertheless, Dr. Wierman is a believer.
“If you take these adolescent girls, especially as they’re beginning to start the weight gain, and you give them metformin, it will stop the weight gain, improve their androgen level and their insulin resistance, and it will help them when they’re ready to have kids later on. I use metformin, and I call it Antabuse for fat: You cheat, you pay. You have no side effects if you eat healthy. If you eat fatty foods, you will have nausea and diarrhea,” she explained.
The endocrinologist added that she finds metformin works best in the adolescent with PCOS who has a family history of diabetes, is gaining weight, but is not yet diabetic herself.
“And that’s based on clinical experience, no prospective studies,” Dr. Wierman emphasized.
She reported having no financial conflicts regarding her presentation.
ESTES PARK, COLO. – The sweet spot for intervention in polycystic ovary syndrome occurs early: in the adolescent who doesn’t yet desire pregnancy and who is just starting to experience the classic PCOS progressive weight gain.
“The obstetricians don’t want to see these patients when they’re already 220 lb and they’re trying to induce ovulation. It’s our job to pick them up in their adolescence. You want to clamp the hormones – suppress the androgens – so that things will be better down the road,” Dr. Margaret E. Wierman urged at a conference on internal medicine sponsored by the University of Colorado.
Aggressive treatment at this stage will reduce the risk of a host of potential health problems later. In addition to infertility issues, these include increased long-term risks of diabetes, hypertension, dyslipidemia, metabolic syndrome, endometrial cancer, obstructive sleep apnea, and nonalcoholic fatty liver disease, noted Dr. Wierman, professor of medicine, ob.gyn., and physiology at the university and chief of endocrinology at the Denver VA Medical Center.
PCOS is by far the most common cause of hyperandrogenic anovulation. Indeed, 6%-8% of all women in their childbearing years have PCOS. The condition is defined by clinical and/or biochemical hyperandrogenism, oligomenorrhea or amenorrhea, and the presence of ovarian cysts or increased ovarian volume on ultrasound.
In the adolescent with PCOS who doesn’t desire pregnancy, Dr. Wierman takes a three-pronged treatment approach: regularize menses with a less androgenic oral contraceptive, such as one containing norethindrone, or by using cyclic progesterone; treat hirsutism with spironolactone at 50-100 mg once daily coupled with electrolysis for local control; and consider using short-acting metformin as an insulin-sensitizing agent.
The use of metformin in treating PCOS is controversial. It is off-label therapy. There have been no prospective, randomized, placebo-controlled, double-blind studies supporting this application of the drug. Nevertheless, Dr. Wierman is a believer.
“If you take these adolescent girls, especially as they’re beginning to start the weight gain, and you give them metformin, it will stop the weight gain, improve their androgen level and their insulin resistance, and it will help them when they’re ready to have kids later on. I use metformin, and I call it Antabuse for fat: You cheat, you pay. You have no side effects if you eat healthy. If you eat fatty foods, you will have nausea and diarrhea,” she explained.
The endocrinologist added that she finds metformin works best in the adolescent with PCOS who has a family history of diabetes, is gaining weight, but is not yet diabetic herself.
“And that’s based on clinical experience, no prospective studies,” Dr. Wierman emphasized.
She reported having no financial conflicts regarding her presentation.
ESTES PARK, COLO. – The sweet spot for intervention in polycystic ovary syndrome occurs early: in the adolescent who doesn’t yet desire pregnancy and who is just starting to experience the classic PCOS progressive weight gain.
“The obstetricians don’t want to see these patients when they’re already 220 lb and they’re trying to induce ovulation. It’s our job to pick them up in their adolescence. You want to clamp the hormones – suppress the androgens – so that things will be better down the road,” Dr. Margaret E. Wierman urged at a conference on internal medicine sponsored by the University of Colorado.
Aggressive treatment at this stage will reduce the risk of a host of potential health problems later. In addition to infertility issues, these include increased long-term risks of diabetes, hypertension, dyslipidemia, metabolic syndrome, endometrial cancer, obstructive sleep apnea, and nonalcoholic fatty liver disease, noted Dr. Wierman, professor of medicine, ob.gyn., and physiology at the university and chief of endocrinology at the Denver VA Medical Center.
PCOS is by far the most common cause of hyperandrogenic anovulation. Indeed, 6%-8% of all women in their childbearing years have PCOS. The condition is defined by clinical and/or biochemical hyperandrogenism, oligomenorrhea or amenorrhea, and the presence of ovarian cysts or increased ovarian volume on ultrasound.
In the adolescent with PCOS who doesn’t desire pregnancy, Dr. Wierman takes a three-pronged treatment approach: regularize menses with a less androgenic oral contraceptive, such as one containing norethindrone, or by using cyclic progesterone; treat hirsutism with spironolactone at 50-100 mg once daily coupled with electrolysis for local control; and consider using short-acting metformin as an insulin-sensitizing agent.
The use of metformin in treating PCOS is controversial. It is off-label therapy. There have been no prospective, randomized, placebo-controlled, double-blind studies supporting this application of the drug. Nevertheless, Dr. Wierman is a believer.
“If you take these adolescent girls, especially as they’re beginning to start the weight gain, and you give them metformin, it will stop the weight gain, improve their androgen level and their insulin resistance, and it will help them when they’re ready to have kids later on. I use metformin, and I call it Antabuse for fat: You cheat, you pay. You have no side effects if you eat healthy. If you eat fatty foods, you will have nausea and diarrhea,” she explained.
The endocrinologist added that she finds metformin works best in the adolescent with PCOS who has a family history of diabetes, is gaining weight, but is not yet diabetic herself.
“And that’s based on clinical experience, no prospective studies,” Dr. Wierman emphasized.
She reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM