User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
How to Avoid Freaking Out About Kidney Function
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams.
We had a great discussion with Kidney Boy, Dr Joel Topf, everyone’s favorite nephrologist, and he taught us how to manage blood pressure in chronic kidney disease (CKD).
Paul N. Williams, MD: Dr Topf focuses more on albuminuria than we are used to doing. It’s probably one of the most important prognostic indicators of how a patient is going to do from a renal standpoint.
Historically, I’ve tended to focus on the estimated glomerular filtration rate (eGFR), and the lower that number gets, the more I sweat, but albuminuria is probably equally, if not more, important as a way of prognosticating whether a patient is going to progress to dialysis or transplant. He directed us towards this nifty little calculator, kidneyfailurerisk.com, where you plug in the patient’s age, eGFR, and degree of albuminuria, and it spits out their risk of progressing to hemodialysis or renal transplantation over the next 5 years. It’s a nice way to concretely explain to patients their risk for progression.
Instead of telling the patient, “You are high risk,” Dr Topf will say, “Your risk is 6% of needing dialysis in the next 5 years.” You can even use these thresholds to gauge when to refer a patient. If someone has a 5-year risk between 3% and 5% or higher, that patient should probably be seeing a nephrologist.
If their 2-year risk is greater than 20%, that patient probably should be evaluated for transplantation. This gives us have more concrete numbers to work with rather than just saying, “Your kidneys aren’t working as well as we would like and you should see a kidney doctor.” Patients have a better sense of how serious things might be.
Watto: It’s just easier for them to understand. Dr Topf made the point that we used to have a heat map based on the stage of CKD that would tell you how high a patient’s risk was compared with other people. But patients don’t really understand relative risk, so Dr Topf tells them their absolute risk for ending up on dialysis over the next 2-5 years.
Patients come in and they are worried because they looked at their lab results and see that their creatinine level is red, or their eGFR is low. They think, It says I have stage 3a CKD.
We should probably have the stages of CKD start at stage 3, which should be called stage 1 so it doesn’t sound as bad. Patients think they are halfway to dialysis; they are already at stage 3 and didn’t even know their kidneys were a problem.
Dr Topf said that cystatin C (something I only recently started ordering) can be obtained, and sometimes you can recategorize the patient, especially those with an eGFR between 45 and 60. The cystatin C can predict their renal function better than the creatinine-based equations. If you are using the creatinine equation, he recommends using the 2021 equations.
Another nice thing about cystatin C is that it isn’t tripped up in younger patients with a lot of muscle mass. You just have to watch out for inflammation, which can throw the test off. For example, when a patient is in the intensive care unit, it’s probably not that helpful, but for your outpatients, cystatin C works well.
Williams: I’ve been using it a fair amount in my patients with more muscle mass. And some patients have been taking creatine as a supplement, and that can alter the numbers as well. This is a nice way to get them out of CKD stage 2 or 3 and back where they belong, with normal healthy functioning kidneys.
Watto: Now, Paul, if we find a patient with more advanced CKD — let’s say stage 4, whether by cystatin C or serum creatinine, and their eGFR is less than 30 — should we start peeling off the angiotensin-converting enzyme ACE inhibitor or the angiotensin receptor blocker (ARB)? Those drugs can raise potassium. What should we do here?
Williams: That’s the temptation, Matt, and I feel like that was the old orthodoxy, back in residency. It didn’t take much for us to start taking off ACE inhibitors or ARBs once the kidney function started to drop, but it turns out you may be doing more harm than good.
Some data have shown that if you peel off those medications, you actually increase mortality and cardiovascular risk. So, in general, if you can keep them going, the patient will be better off. Hang onto the ACE inhibitors or ARBs as long as you are able to, because they confer a fair amount of benefit.
Watto: As long as the potassium isn’t in red on your lab’s range. It might go up to 5.2 or 5.4, but as long as it’s stable, that should be OK. You probably wouldn’t initiate an ACE inhibitor or ARB or spironolactone with a potassium level above 5, but if it’s below 5 when you start and it goes up slightly after you start the drug, that could be acceptable.
Another thing we talked about was when a patient progresses to CKD and ends up on dialysis, how helpful are those intradialysis blood pressures in predicting cardiovascular outcomes?
Williams: For someone who’s performing the dialysis, probably really helpful. In the outpatient setting to predict cardiovascular risk, probably less so. Dr Topf makes the point that the readings are done either shortly after or right when the patient is about to have a large-bore catheter inserted into their arm. And then they have liters of fluid drained out of them. So those numbers are going to have huge amounts of variability. You would not base the patient’s blood pressure treatment solely on those numbers. But regardless of what the numbers are, or even regardless of your office numbers, hopefully you’re working with a nephrologist to make sure that you’re actually in concert and not fighting each other with the blood pressure medications.
Watto: Dr Topf said that a lot of the hypertension in dialysis is because of too much volume. If you can get the volume down, you might be able to peel off blood pressure medications instead of adding more. But some patients have issues with cramping; it’s uncomfortable and not everyone tolerates it.
I was really surprised to learn that beta blockers, specifically atenolol, have some evidence of improving cardiovascular outcomes in patients on dialysis. Dr Topf speculated that this was because they are largely dying of cardiovascular disease, so maybe that’s why, but that’s one of the places, the only places I can think of aside from thyroid disease, where atenolol really shines.
If you want to hear this fantastic episode and all the great pearls, then click on this link.
Matthew F. Watto, MD, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, disclosed no relevant financial relationships. Paul N. Williams, MD, has disclosed ties with The Curbsiders.
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams.
We had a great discussion with Kidney Boy, Dr Joel Topf, everyone’s favorite nephrologist, and he taught us how to manage blood pressure in chronic kidney disease (CKD).
Paul N. Williams, MD: Dr Topf focuses more on albuminuria than we are used to doing. It’s probably one of the most important prognostic indicators of how a patient is going to do from a renal standpoint.
Historically, I’ve tended to focus on the estimated glomerular filtration rate (eGFR), and the lower that number gets, the more I sweat, but albuminuria is probably equally, if not more, important as a way of prognosticating whether a patient is going to progress to dialysis or transplant. He directed us towards this nifty little calculator, kidneyfailurerisk.com, where you plug in the patient’s age, eGFR, and degree of albuminuria, and it spits out their risk of progressing to hemodialysis or renal transplantation over the next 5 years. It’s a nice way to concretely explain to patients their risk for progression.
Instead of telling the patient, “You are high risk,” Dr Topf will say, “Your risk is 6% of needing dialysis in the next 5 years.” You can even use these thresholds to gauge when to refer a patient. If someone has a 5-year risk between 3% and 5% or higher, that patient should probably be seeing a nephrologist.
If their 2-year risk is greater than 20%, that patient probably should be evaluated for transplantation. This gives us have more concrete numbers to work with rather than just saying, “Your kidneys aren’t working as well as we would like and you should see a kidney doctor.” Patients have a better sense of how serious things might be.
Watto: It’s just easier for them to understand. Dr Topf made the point that we used to have a heat map based on the stage of CKD that would tell you how high a patient’s risk was compared with other people. But patients don’t really understand relative risk, so Dr Topf tells them their absolute risk for ending up on dialysis over the next 2-5 years.
Patients come in and they are worried because they looked at their lab results and see that their creatinine level is red, or their eGFR is low. They think, It says I have stage 3a CKD.
We should probably have the stages of CKD start at stage 3, which should be called stage 1 so it doesn’t sound as bad. Patients think they are halfway to dialysis; they are already at stage 3 and didn’t even know their kidneys were a problem.
Dr Topf said that cystatin C (something I only recently started ordering) can be obtained, and sometimes you can recategorize the patient, especially those with an eGFR between 45 and 60. The cystatin C can predict their renal function better than the creatinine-based equations. If you are using the creatinine equation, he recommends using the 2021 equations.
Another nice thing about cystatin C is that it isn’t tripped up in younger patients with a lot of muscle mass. You just have to watch out for inflammation, which can throw the test off. For example, when a patient is in the intensive care unit, it’s probably not that helpful, but for your outpatients, cystatin C works well.
Williams: I’ve been using it a fair amount in my patients with more muscle mass. And some patients have been taking creatine as a supplement, and that can alter the numbers as well. This is a nice way to get them out of CKD stage 2 or 3 and back where they belong, with normal healthy functioning kidneys.
Watto: Now, Paul, if we find a patient with more advanced CKD — let’s say stage 4, whether by cystatin C or serum creatinine, and their eGFR is less than 30 — should we start peeling off the angiotensin-converting enzyme ACE inhibitor or the angiotensin receptor blocker (ARB)? Those drugs can raise potassium. What should we do here?
Williams: That’s the temptation, Matt, and I feel like that was the old orthodoxy, back in residency. It didn’t take much for us to start taking off ACE inhibitors or ARBs once the kidney function started to drop, but it turns out you may be doing more harm than good.
Some data have shown that if you peel off those medications, you actually increase mortality and cardiovascular risk. So, in general, if you can keep them going, the patient will be better off. Hang onto the ACE inhibitors or ARBs as long as you are able to, because they confer a fair amount of benefit.
Watto: As long as the potassium isn’t in red on your lab’s range. It might go up to 5.2 or 5.4, but as long as it’s stable, that should be OK. You probably wouldn’t initiate an ACE inhibitor or ARB or spironolactone with a potassium level above 5, but if it’s below 5 when you start and it goes up slightly after you start the drug, that could be acceptable.
Another thing we talked about was when a patient progresses to CKD and ends up on dialysis, how helpful are those intradialysis blood pressures in predicting cardiovascular outcomes?
Williams: For someone who’s performing the dialysis, probably really helpful. In the outpatient setting to predict cardiovascular risk, probably less so. Dr Topf makes the point that the readings are done either shortly after or right when the patient is about to have a large-bore catheter inserted into their arm. And then they have liters of fluid drained out of them. So those numbers are going to have huge amounts of variability. You would not base the patient’s blood pressure treatment solely on those numbers. But regardless of what the numbers are, or even regardless of your office numbers, hopefully you’re working with a nephrologist to make sure that you’re actually in concert and not fighting each other with the blood pressure medications.
Watto: Dr Topf said that a lot of the hypertension in dialysis is because of too much volume. If you can get the volume down, you might be able to peel off blood pressure medications instead of adding more. But some patients have issues with cramping; it’s uncomfortable and not everyone tolerates it.
I was really surprised to learn that beta blockers, specifically atenolol, have some evidence of improving cardiovascular outcomes in patients on dialysis. Dr Topf speculated that this was because they are largely dying of cardiovascular disease, so maybe that’s why, but that’s one of the places, the only places I can think of aside from thyroid disease, where atenolol really shines.
If you want to hear this fantastic episode and all the great pearls, then click on this link.
Matthew F. Watto, MD, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, disclosed no relevant financial relationships. Paul N. Williams, MD, has disclosed ties with The Curbsiders.
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams.
We had a great discussion with Kidney Boy, Dr Joel Topf, everyone’s favorite nephrologist, and he taught us how to manage blood pressure in chronic kidney disease (CKD).
Paul N. Williams, MD: Dr Topf focuses more on albuminuria than we are used to doing. It’s probably one of the most important prognostic indicators of how a patient is going to do from a renal standpoint.
Historically, I’ve tended to focus on the estimated glomerular filtration rate (eGFR), and the lower that number gets, the more I sweat, but albuminuria is probably equally, if not more, important as a way of prognosticating whether a patient is going to progress to dialysis or transplant. He directed us towards this nifty little calculator, kidneyfailurerisk.com, where you plug in the patient’s age, eGFR, and degree of albuminuria, and it spits out their risk of progressing to hemodialysis or renal transplantation over the next 5 years. It’s a nice way to concretely explain to patients their risk for progression.
Instead of telling the patient, “You are high risk,” Dr Topf will say, “Your risk is 6% of needing dialysis in the next 5 years.” You can even use these thresholds to gauge when to refer a patient. If someone has a 5-year risk between 3% and 5% or higher, that patient should probably be seeing a nephrologist.
If their 2-year risk is greater than 20%, that patient probably should be evaluated for transplantation. This gives us have more concrete numbers to work with rather than just saying, “Your kidneys aren’t working as well as we would like and you should see a kidney doctor.” Patients have a better sense of how serious things might be.
Watto: It’s just easier for them to understand. Dr Topf made the point that we used to have a heat map based on the stage of CKD that would tell you how high a patient’s risk was compared with other people. But patients don’t really understand relative risk, so Dr Topf tells them their absolute risk for ending up on dialysis over the next 2-5 years.
Patients come in and they are worried because they looked at their lab results and see that their creatinine level is red, or their eGFR is low. They think, It says I have stage 3a CKD.
We should probably have the stages of CKD start at stage 3, which should be called stage 1 so it doesn’t sound as bad. Patients think they are halfway to dialysis; they are already at stage 3 and didn’t even know their kidneys were a problem.
Dr Topf said that cystatin C (something I only recently started ordering) can be obtained, and sometimes you can recategorize the patient, especially those with an eGFR between 45 and 60. The cystatin C can predict their renal function better than the creatinine-based equations. If you are using the creatinine equation, he recommends using the 2021 equations.
Another nice thing about cystatin C is that it isn’t tripped up in younger patients with a lot of muscle mass. You just have to watch out for inflammation, which can throw the test off. For example, when a patient is in the intensive care unit, it’s probably not that helpful, but for your outpatients, cystatin C works well.
Williams: I’ve been using it a fair amount in my patients with more muscle mass. And some patients have been taking creatine as a supplement, and that can alter the numbers as well. This is a nice way to get them out of CKD stage 2 or 3 and back where they belong, with normal healthy functioning kidneys.
Watto: Now, Paul, if we find a patient with more advanced CKD — let’s say stage 4, whether by cystatin C or serum creatinine, and their eGFR is less than 30 — should we start peeling off the angiotensin-converting enzyme ACE inhibitor or the angiotensin receptor blocker (ARB)? Those drugs can raise potassium. What should we do here?
Williams: That’s the temptation, Matt, and I feel like that was the old orthodoxy, back in residency. It didn’t take much for us to start taking off ACE inhibitors or ARBs once the kidney function started to drop, but it turns out you may be doing more harm than good.
Some data have shown that if you peel off those medications, you actually increase mortality and cardiovascular risk. So, in general, if you can keep them going, the patient will be better off. Hang onto the ACE inhibitors or ARBs as long as you are able to, because they confer a fair amount of benefit.
Watto: As long as the potassium isn’t in red on your lab’s range. It might go up to 5.2 or 5.4, but as long as it’s stable, that should be OK. You probably wouldn’t initiate an ACE inhibitor or ARB or spironolactone with a potassium level above 5, but if it’s below 5 when you start and it goes up slightly after you start the drug, that could be acceptable.
Another thing we talked about was when a patient progresses to CKD and ends up on dialysis, how helpful are those intradialysis blood pressures in predicting cardiovascular outcomes?
Williams: For someone who’s performing the dialysis, probably really helpful. In the outpatient setting to predict cardiovascular risk, probably less so. Dr Topf makes the point that the readings are done either shortly after or right when the patient is about to have a large-bore catheter inserted into their arm. And then they have liters of fluid drained out of them. So those numbers are going to have huge amounts of variability. You would not base the patient’s blood pressure treatment solely on those numbers. But regardless of what the numbers are, or even regardless of your office numbers, hopefully you’re working with a nephrologist to make sure that you’re actually in concert and not fighting each other with the blood pressure medications.
Watto: Dr Topf said that a lot of the hypertension in dialysis is because of too much volume. If you can get the volume down, you might be able to peel off blood pressure medications instead of adding more. But some patients have issues with cramping; it’s uncomfortable and not everyone tolerates it.
I was really surprised to learn that beta blockers, specifically atenolol, have some evidence of improving cardiovascular outcomes in patients on dialysis. Dr Topf speculated that this was because they are largely dying of cardiovascular disease, so maybe that’s why, but that’s one of the places, the only places I can think of aside from thyroid disease, where atenolol really shines.
If you want to hear this fantastic episode and all the great pearls, then click on this link.
Matthew F. Watto, MD, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, disclosed no relevant financial relationships. Paul N. Williams, MD, has disclosed ties with The Curbsiders.
Novel Digital Intervention Shows Promise for Depression
TOPLINE:
InterRhythmic care (IRC), a novel digital intervention, was linked to greater improvements in depressive symptoms, anxiety, interpersonal relationships, and social functioning in patients with major depressive disorder (MDD), compared with internet general psychoeducation in new research.
METHODOLOGY:
- The randomized, single-blind trial included 120 outpatients from the Shanghai Mental Health Center between March and November 2021 with MDD (mean age, 28.2 years; 99% Han Chinese; 83% women) who were randomly assigned to receive either IRC or internet general psychoeducation (control group).
- IRC included computer-based psychoeducation on stabilizing social rhythm regularity and resolution of interpersonal problems plus brief online interactions with clinicians. Patients received 10 minutes of IRC daily, Monday through Friday, for 8 weeks.
- The researchers assessed participants’ depressive symptoms, anxiety symptoms, interpersonal relationships, social function, and biological rhythms using the 17-item Hamilton Depression Rating Scale, Hamilton Anxiety Scale, Interpersonal Comprehensive Diagnostic Scale, Sheehan Disability Scale, and Morning and Evening Questionnaire at baseline and at 8 weeks.
TAKEAWAY:
- The participants who received IRC had significantly lower Hamilton Depression Rating total scores than those who received internet general psychoeducation (P < .001).
- The IRC group demonstrated improved anxiety symptoms, as evidenced by lower Hamilton Anxiety Scale total scores, than those observed for the control group (P < .001).
- The IRC group also showed improved outcomes in interpersonal relationships, as indicated by lower Interpersonal Comprehensive Diagnostic Scale total scores (P < .001).
- Social functioning improved significantly in the IRC group, as measured by the Sheehan Disability Scale subscores for work/school (P = .03), social life (P < .001), and family life (P = .001).
IN PRACTICE:
“This study demonstrated that IRC can improve clinical symptoms such as depressive symptoms, anxiety symptoms, interpersonal problems, and social function in patients with MDD. Our study suggested that the IRC can be used in clinical practice,” the investigators wrote.
SOURCE:
The study was led by Chuchen Xu, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine in China. It was published online on November 20, 2024, in The Journal of Psychiatric Research.
LIMITATIONS:
The 8-week follow-up period was considered too short to comprehensively evaluate the intervention’s long-term impact. Additionally, the researchers had to check and supervise assignment completion, which increased research costs and may, therefore, potentially limit broader implementation.
DISCLOSURES:
The investigators reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
InterRhythmic care (IRC), a novel digital intervention, was linked to greater improvements in depressive symptoms, anxiety, interpersonal relationships, and social functioning in patients with major depressive disorder (MDD), compared with internet general psychoeducation in new research.
METHODOLOGY:
- The randomized, single-blind trial included 120 outpatients from the Shanghai Mental Health Center between March and November 2021 with MDD (mean age, 28.2 years; 99% Han Chinese; 83% women) who were randomly assigned to receive either IRC or internet general psychoeducation (control group).
- IRC included computer-based psychoeducation on stabilizing social rhythm regularity and resolution of interpersonal problems plus brief online interactions with clinicians. Patients received 10 minutes of IRC daily, Monday through Friday, for 8 weeks.
- The researchers assessed participants’ depressive symptoms, anxiety symptoms, interpersonal relationships, social function, and biological rhythms using the 17-item Hamilton Depression Rating Scale, Hamilton Anxiety Scale, Interpersonal Comprehensive Diagnostic Scale, Sheehan Disability Scale, and Morning and Evening Questionnaire at baseline and at 8 weeks.
TAKEAWAY:
- The participants who received IRC had significantly lower Hamilton Depression Rating total scores than those who received internet general psychoeducation (P < .001).
- The IRC group demonstrated improved anxiety symptoms, as evidenced by lower Hamilton Anxiety Scale total scores, than those observed for the control group (P < .001).
- The IRC group also showed improved outcomes in interpersonal relationships, as indicated by lower Interpersonal Comprehensive Diagnostic Scale total scores (P < .001).
- Social functioning improved significantly in the IRC group, as measured by the Sheehan Disability Scale subscores for work/school (P = .03), social life (P < .001), and family life (P = .001).
IN PRACTICE:
“This study demonstrated that IRC can improve clinical symptoms such as depressive symptoms, anxiety symptoms, interpersonal problems, and social function in patients with MDD. Our study suggested that the IRC can be used in clinical practice,” the investigators wrote.
SOURCE:
The study was led by Chuchen Xu, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine in China. It was published online on November 20, 2024, in The Journal of Psychiatric Research.
LIMITATIONS:
The 8-week follow-up period was considered too short to comprehensively evaluate the intervention’s long-term impact. Additionally, the researchers had to check and supervise assignment completion, which increased research costs and may, therefore, potentially limit broader implementation.
DISCLOSURES:
The investigators reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
InterRhythmic care (IRC), a novel digital intervention, was linked to greater improvements in depressive symptoms, anxiety, interpersonal relationships, and social functioning in patients with major depressive disorder (MDD), compared with internet general psychoeducation in new research.
METHODOLOGY:
- The randomized, single-blind trial included 120 outpatients from the Shanghai Mental Health Center between March and November 2021 with MDD (mean age, 28.2 years; 99% Han Chinese; 83% women) who were randomly assigned to receive either IRC or internet general psychoeducation (control group).
- IRC included computer-based psychoeducation on stabilizing social rhythm regularity and resolution of interpersonal problems plus brief online interactions with clinicians. Patients received 10 minutes of IRC daily, Monday through Friday, for 8 weeks.
- The researchers assessed participants’ depressive symptoms, anxiety symptoms, interpersonal relationships, social function, and biological rhythms using the 17-item Hamilton Depression Rating Scale, Hamilton Anxiety Scale, Interpersonal Comprehensive Diagnostic Scale, Sheehan Disability Scale, and Morning and Evening Questionnaire at baseline and at 8 weeks.
TAKEAWAY:
- The participants who received IRC had significantly lower Hamilton Depression Rating total scores than those who received internet general psychoeducation (P < .001).
- The IRC group demonstrated improved anxiety symptoms, as evidenced by lower Hamilton Anxiety Scale total scores, than those observed for the control group (P < .001).
- The IRC group also showed improved outcomes in interpersonal relationships, as indicated by lower Interpersonal Comprehensive Diagnostic Scale total scores (P < .001).
- Social functioning improved significantly in the IRC group, as measured by the Sheehan Disability Scale subscores for work/school (P = .03), social life (P < .001), and family life (P = .001).
IN PRACTICE:
“This study demonstrated that IRC can improve clinical symptoms such as depressive symptoms, anxiety symptoms, interpersonal problems, and social function in patients with MDD. Our study suggested that the IRC can be used in clinical practice,” the investigators wrote.
SOURCE:
The study was led by Chuchen Xu, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine in China. It was published online on November 20, 2024, in The Journal of Psychiatric Research.
LIMITATIONS:
The 8-week follow-up period was considered too short to comprehensively evaluate the intervention’s long-term impact. Additionally, the researchers had to check and supervise assignment completion, which increased research costs and may, therefore, potentially limit broader implementation.
DISCLOSURES:
The investigators reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Some Antihypertensives Linked to HCC Risk in Patients With MASLD and Cirrhosis
SAN DIEGO — according to new research.
In particular, the use of calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) was associated with a higher risk of developing HCC, compared with not using these medications.
About half of patients with MASLD have hypertension, and the use of antihypertensives in these patients is beneficial to reduce the risk for cardiovascular disease and complications related to MASLD, said lead author Ahmed Elhariri, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston, who conducted the study as a research assistant in gastroenterology and hepatology at the Baylor College of Medicine, also in Houston.
However, previous studies have suggested a possible link between these medications and cancer development, “especially CCBs and breast and lung cancer,” said Elhariri, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Analyzing Potential Risks
In a case-control study, Elhariri and colleagues analyzed antihypertensive medication use among patients with MASLD-induced HCC, as defined by histology or radiology based on the Liver Imaging Reporting & Data System, and control patients with MASLD but without HCC.
Between 2020 and 2024, the research team recruited 153 newly diagnosed HCC cases with different etiologies and 170 patients with MASLD but without HCC from Baylor College of Medicine’s outpatient clinics. For this study, they selected 47 age- and sex-matched pairs, all of whom had cirrhosis. Only those with a history of hypertension were included, however. Data on risk factors of metabolic syndrome (including diabetes) and HCC were collected, along with details about medication use such as metformin and statins.
A total of 42 patients with MASLD and HCC and 39 MASLD control individuals had a history of hypertension and were treated with antihypertensive medications. The mean age was 66.5 years for the HCC group and 63.5 years for the control group, and the mean body mass index (BMI) was 31.1 for the HCC group and 31.7 for the control group.
After adjusting for age, sex, BMI, Hispanic ethnicity, and use of other medications, patients taking CCBs had an increased HCC risk (odds ratio [OR], 2.76), compared with those not taking CCBs. Patients taking ACE inhibitors or ARBs also had an increased HCC risk (OR, 2.54), compared with those not taking ACE inhibitors or ARBs.
However, there wasn’t a statistically significant difference in HCC risk among patients taking beta-blockers (OR, 0.87).
“Patients with fatty liver in the presence of metabolic syndrome, especially in the presence of cirrhosis and antihypertensives, need to have stricter surveillance for liver cancer,” Elhariri said.
“We need to carefully review blood pressure medications in patients with MASLD and cirrhosis,” he said. CCBs, ACE inhibitors, and ARBs can be replaced with beta-blockers, “which have been shown to reduce progression of cirrhosis-related complications.”
Considering Clinical Implications
“Although our study showed some association between the use of some commonly used antihypertensives and the risk for HCC in this high-risk population, it is based on data collected retrospectively on a small number of selected patients with advanced liver disease,” Elhariri noted.
The associations and underlying mechanisms should be studied in larger populations and prospective trials, he said. “Until we have more data with a significantly larger sample size, it’s premature to raise the concern in the general population.”
“The cardiovascular benefits of controlling blood pressure far outweigh the risk of liver cancer in patients with metabolic syndrome,” Elhariri added.
In ongoing studies, researchers are investigating ways to improve patient outcomes and reduce the negative effects of cirrhosis-associated complications among patients with MASLD and metabolic dysfunction–associated steatohepatitis (MASH), Muhammad Ali Butt, MD, a hepatology fellow at Beth Israel Lahey Hospital & Medical Center in Burlington, Massachusetts, said in an interview.
Butt, who wasn’t involved with this study, presented separate research on statins in MASH patients with cirrhosis, which indicated statistically significant decreases in portal hypertension, thrombosis, hepatorenal syndrome, hepatic encephalopathy, and mortality.
“We know patients with MASLD- and MASH-associated cirrhosis commonly have other comorbidities, including high cardiovascular risks, diabetes, and hyperlipidemia,” he said. “All of these conditions indicate patients to be on other medications such as antihypertensives or statins. It’s important to know the role these medications play, especially given the high-risk profile of these patients.”
Elhariri and Butt reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN DIEGO — according to new research.
In particular, the use of calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) was associated with a higher risk of developing HCC, compared with not using these medications.
About half of patients with MASLD have hypertension, and the use of antihypertensives in these patients is beneficial to reduce the risk for cardiovascular disease and complications related to MASLD, said lead author Ahmed Elhariri, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston, who conducted the study as a research assistant in gastroenterology and hepatology at the Baylor College of Medicine, also in Houston.
However, previous studies have suggested a possible link between these medications and cancer development, “especially CCBs and breast and lung cancer,” said Elhariri, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Analyzing Potential Risks
In a case-control study, Elhariri and colleagues analyzed antihypertensive medication use among patients with MASLD-induced HCC, as defined by histology or radiology based on the Liver Imaging Reporting & Data System, and control patients with MASLD but without HCC.
Between 2020 and 2024, the research team recruited 153 newly diagnosed HCC cases with different etiologies and 170 patients with MASLD but without HCC from Baylor College of Medicine’s outpatient clinics. For this study, they selected 47 age- and sex-matched pairs, all of whom had cirrhosis. Only those with a history of hypertension were included, however. Data on risk factors of metabolic syndrome (including diabetes) and HCC were collected, along with details about medication use such as metformin and statins.
A total of 42 patients with MASLD and HCC and 39 MASLD control individuals had a history of hypertension and were treated with antihypertensive medications. The mean age was 66.5 years for the HCC group and 63.5 years for the control group, and the mean body mass index (BMI) was 31.1 for the HCC group and 31.7 for the control group.
After adjusting for age, sex, BMI, Hispanic ethnicity, and use of other medications, patients taking CCBs had an increased HCC risk (odds ratio [OR], 2.76), compared with those not taking CCBs. Patients taking ACE inhibitors or ARBs also had an increased HCC risk (OR, 2.54), compared with those not taking ACE inhibitors or ARBs.
However, there wasn’t a statistically significant difference in HCC risk among patients taking beta-blockers (OR, 0.87).
“Patients with fatty liver in the presence of metabolic syndrome, especially in the presence of cirrhosis and antihypertensives, need to have stricter surveillance for liver cancer,” Elhariri said.
“We need to carefully review blood pressure medications in patients with MASLD and cirrhosis,” he said. CCBs, ACE inhibitors, and ARBs can be replaced with beta-blockers, “which have been shown to reduce progression of cirrhosis-related complications.”
Considering Clinical Implications
“Although our study showed some association between the use of some commonly used antihypertensives and the risk for HCC in this high-risk population, it is based on data collected retrospectively on a small number of selected patients with advanced liver disease,” Elhariri noted.
The associations and underlying mechanisms should be studied in larger populations and prospective trials, he said. “Until we have more data with a significantly larger sample size, it’s premature to raise the concern in the general population.”
“The cardiovascular benefits of controlling blood pressure far outweigh the risk of liver cancer in patients with metabolic syndrome,” Elhariri added.
In ongoing studies, researchers are investigating ways to improve patient outcomes and reduce the negative effects of cirrhosis-associated complications among patients with MASLD and metabolic dysfunction–associated steatohepatitis (MASH), Muhammad Ali Butt, MD, a hepatology fellow at Beth Israel Lahey Hospital & Medical Center in Burlington, Massachusetts, said in an interview.
Butt, who wasn’t involved with this study, presented separate research on statins in MASH patients with cirrhosis, which indicated statistically significant decreases in portal hypertension, thrombosis, hepatorenal syndrome, hepatic encephalopathy, and mortality.
“We know patients with MASLD- and MASH-associated cirrhosis commonly have other comorbidities, including high cardiovascular risks, diabetes, and hyperlipidemia,” he said. “All of these conditions indicate patients to be on other medications such as antihypertensives or statins. It’s important to know the role these medications play, especially given the high-risk profile of these patients.”
Elhariri and Butt reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN DIEGO — according to new research.
In particular, the use of calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) was associated with a higher risk of developing HCC, compared with not using these medications.
About half of patients with MASLD have hypertension, and the use of antihypertensives in these patients is beneficial to reduce the risk for cardiovascular disease and complications related to MASLD, said lead author Ahmed Elhariri, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston, who conducted the study as a research assistant in gastroenterology and hepatology at the Baylor College of Medicine, also in Houston.
However, previous studies have suggested a possible link between these medications and cancer development, “especially CCBs and breast and lung cancer,” said Elhariri, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Analyzing Potential Risks
In a case-control study, Elhariri and colleagues analyzed antihypertensive medication use among patients with MASLD-induced HCC, as defined by histology or radiology based on the Liver Imaging Reporting & Data System, and control patients with MASLD but without HCC.
Between 2020 and 2024, the research team recruited 153 newly diagnosed HCC cases with different etiologies and 170 patients with MASLD but without HCC from Baylor College of Medicine’s outpatient clinics. For this study, they selected 47 age- and sex-matched pairs, all of whom had cirrhosis. Only those with a history of hypertension were included, however. Data on risk factors of metabolic syndrome (including diabetes) and HCC were collected, along with details about medication use such as metformin and statins.
A total of 42 patients with MASLD and HCC and 39 MASLD control individuals had a history of hypertension and were treated with antihypertensive medications. The mean age was 66.5 years for the HCC group and 63.5 years for the control group, and the mean body mass index (BMI) was 31.1 for the HCC group and 31.7 for the control group.
After adjusting for age, sex, BMI, Hispanic ethnicity, and use of other medications, patients taking CCBs had an increased HCC risk (odds ratio [OR], 2.76), compared with those not taking CCBs. Patients taking ACE inhibitors or ARBs also had an increased HCC risk (OR, 2.54), compared with those not taking ACE inhibitors or ARBs.
However, there wasn’t a statistically significant difference in HCC risk among patients taking beta-blockers (OR, 0.87).
“Patients with fatty liver in the presence of metabolic syndrome, especially in the presence of cirrhosis and antihypertensives, need to have stricter surveillance for liver cancer,” Elhariri said.
“We need to carefully review blood pressure medications in patients with MASLD and cirrhosis,” he said. CCBs, ACE inhibitors, and ARBs can be replaced with beta-blockers, “which have been shown to reduce progression of cirrhosis-related complications.”
Considering Clinical Implications
“Although our study showed some association between the use of some commonly used antihypertensives and the risk for HCC in this high-risk population, it is based on data collected retrospectively on a small number of selected patients with advanced liver disease,” Elhariri noted.
The associations and underlying mechanisms should be studied in larger populations and prospective trials, he said. “Until we have more data with a significantly larger sample size, it’s premature to raise the concern in the general population.”
“The cardiovascular benefits of controlling blood pressure far outweigh the risk of liver cancer in patients with metabolic syndrome,” Elhariri added.
In ongoing studies, researchers are investigating ways to improve patient outcomes and reduce the negative effects of cirrhosis-associated complications among patients with MASLD and metabolic dysfunction–associated steatohepatitis (MASH), Muhammad Ali Butt, MD, a hepatology fellow at Beth Israel Lahey Hospital & Medical Center in Burlington, Massachusetts, said in an interview.
Butt, who wasn’t involved with this study, presented separate research on statins in MASH patients with cirrhosis, which indicated statistically significant decreases in portal hypertension, thrombosis, hepatorenal syndrome, hepatic encephalopathy, and mortality.
“We know patients with MASLD- and MASH-associated cirrhosis commonly have other comorbidities, including high cardiovascular risks, diabetes, and hyperlipidemia,” he said. “All of these conditions indicate patients to be on other medications such as antihypertensives or statins. It’s important to know the role these medications play, especially given the high-risk profile of these patients.”
Elhariri and Butt reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM AASLD 2024
Diabetes Drugs and Eye Disease: These Protect, These Don’t
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of electronic medical records from the TriNetX health research network to evaluate how systemic medications, such as GLP-1 RAs, fenofibrates, thiazolidinediones, and calcium channel blockers, influence the risk of developing DME in patients with type 2 diabetes.
- They included patients with a 5-year history of type 2 diabetes and an absence of DME at baseline.
- The treatment group included patients who initiated treatment with calcium channel blockers (n = 107,193), GLP-1 RAs (n = 76,583), thiazolidinediones (n = 25,657), or fenofibrates (n = 18,606) after a diagnosis of diabetes. The control group received none of these medications within 1 year of being diagnosed with the condition.
- The researchers used propensity score matching to balance baseline characteristics and comorbidities between both groups.
- The primary outcome was the incidence of diagnoses of DME within a 2-year follow-up period after the initiation of systemic medications.
TAKEAWAY:
- Patients treated with calcium channel blockers showed an increased risk for incident DME (hazard ratio [HR], 1.66; 95% CI, 1.54-1.78) compared with control individuals.
- Treatment with GLP-1 RAs was associated with a reduced risk for DME (HR, 0.77; 95% CI, 0.70-0.85), as was treatment with fenofibrates (HR, 0.83; 95% CI, 0.68-0.98).
- No significant difference in risk for DME was observed between patients taking thiazolidinediones and control individuals.
IN PRACTICE:
“We found a possible protective effect for GLP-1 RA medications and fenofibrate for DME and an adverse effect for calcium channel blockers with regard to the development of DME in patients” with type 2 diabetes, the authors wrote.
“Our preliminary data suggests a protective effect with regard to GLP-1 RA drugs and the development of DME. Clinical studies examining a potential therapeutic effect of GLP-1 RA drugs on DME do seem warranted. A single orally administered drug could conceivably lower blood sugar, reduce weight, offer cardiovascular protection, and treat DME” in patients with type 2 diabetes, they added.
SOURCE:
The study was led by Jawad Muayad, BS, of the School of Medicine at Texas A&M University, in Houston. It was published online on December 5, 2024, in Ophthalmology Retina.
LIMITATIONS:
The study was retrospective in nature. It relied on electronic medical records for the diagnosis of DME instead of directly assessing retinal images or measuring retinal thickness. Moreover, patients on certain medications may have been monitored more closely, potentially influencing the likelihood of a diagnosis of DME being recorded.
DISCLOSURES:
The study did not receive any funding support. One author disclosed receiving consulting fees from various institutions and pharmaceutical companies. The other authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of electronic medical records from the TriNetX health research network to evaluate how systemic medications, such as GLP-1 RAs, fenofibrates, thiazolidinediones, and calcium channel blockers, influence the risk of developing DME in patients with type 2 diabetes.
- They included patients with a 5-year history of type 2 diabetes and an absence of DME at baseline.
- The treatment group included patients who initiated treatment with calcium channel blockers (n = 107,193), GLP-1 RAs (n = 76,583), thiazolidinediones (n = 25,657), or fenofibrates (n = 18,606) after a diagnosis of diabetes. The control group received none of these medications within 1 year of being diagnosed with the condition.
- The researchers used propensity score matching to balance baseline characteristics and comorbidities between both groups.
- The primary outcome was the incidence of diagnoses of DME within a 2-year follow-up period after the initiation of systemic medications.
TAKEAWAY:
- Patients treated with calcium channel blockers showed an increased risk for incident DME (hazard ratio [HR], 1.66; 95% CI, 1.54-1.78) compared with control individuals.
- Treatment with GLP-1 RAs was associated with a reduced risk for DME (HR, 0.77; 95% CI, 0.70-0.85), as was treatment with fenofibrates (HR, 0.83; 95% CI, 0.68-0.98).
- No significant difference in risk for DME was observed between patients taking thiazolidinediones and control individuals.
IN PRACTICE:
“We found a possible protective effect for GLP-1 RA medications and fenofibrate for DME and an adverse effect for calcium channel blockers with regard to the development of DME in patients” with type 2 diabetes, the authors wrote.
“Our preliminary data suggests a protective effect with regard to GLP-1 RA drugs and the development of DME. Clinical studies examining a potential therapeutic effect of GLP-1 RA drugs on DME do seem warranted. A single orally administered drug could conceivably lower blood sugar, reduce weight, offer cardiovascular protection, and treat DME” in patients with type 2 diabetes, they added.
SOURCE:
The study was led by Jawad Muayad, BS, of the School of Medicine at Texas A&M University, in Houston. It was published online on December 5, 2024, in Ophthalmology Retina.
LIMITATIONS:
The study was retrospective in nature. It relied on electronic medical records for the diagnosis of DME instead of directly assessing retinal images or measuring retinal thickness. Moreover, patients on certain medications may have been monitored more closely, potentially influencing the likelihood of a diagnosis of DME being recorded.
DISCLOSURES:
The study did not receive any funding support. One author disclosed receiving consulting fees from various institutions and pharmaceutical companies. The other authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of electronic medical records from the TriNetX health research network to evaluate how systemic medications, such as GLP-1 RAs, fenofibrates, thiazolidinediones, and calcium channel blockers, influence the risk of developing DME in patients with type 2 diabetes.
- They included patients with a 5-year history of type 2 diabetes and an absence of DME at baseline.
- The treatment group included patients who initiated treatment with calcium channel blockers (n = 107,193), GLP-1 RAs (n = 76,583), thiazolidinediones (n = 25,657), or fenofibrates (n = 18,606) after a diagnosis of diabetes. The control group received none of these medications within 1 year of being diagnosed with the condition.
- The researchers used propensity score matching to balance baseline characteristics and comorbidities between both groups.
- The primary outcome was the incidence of diagnoses of DME within a 2-year follow-up period after the initiation of systemic medications.
TAKEAWAY:
- Patients treated with calcium channel blockers showed an increased risk for incident DME (hazard ratio [HR], 1.66; 95% CI, 1.54-1.78) compared with control individuals.
- Treatment with GLP-1 RAs was associated with a reduced risk for DME (HR, 0.77; 95% CI, 0.70-0.85), as was treatment with fenofibrates (HR, 0.83; 95% CI, 0.68-0.98).
- No significant difference in risk for DME was observed between patients taking thiazolidinediones and control individuals.
IN PRACTICE:
“We found a possible protective effect for GLP-1 RA medications and fenofibrate for DME and an adverse effect for calcium channel blockers with regard to the development of DME in patients” with type 2 diabetes, the authors wrote.
“Our preliminary data suggests a protective effect with regard to GLP-1 RA drugs and the development of DME. Clinical studies examining a potential therapeutic effect of GLP-1 RA drugs on DME do seem warranted. A single orally administered drug could conceivably lower blood sugar, reduce weight, offer cardiovascular protection, and treat DME” in patients with type 2 diabetes, they added.
SOURCE:
The study was led by Jawad Muayad, BS, of the School of Medicine at Texas A&M University, in Houston. It was published online on December 5, 2024, in Ophthalmology Retina.
LIMITATIONS:
The study was retrospective in nature. It relied on electronic medical records for the diagnosis of DME instead of directly assessing retinal images or measuring retinal thickness. Moreover, patients on certain medications may have been monitored more closely, potentially influencing the likelihood of a diagnosis of DME being recorded.
DISCLOSURES:
The study did not receive any funding support. One author disclosed receiving consulting fees from various institutions and pharmaceutical companies. The other authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Your Guide to COVID Vaccines for 2024-2025
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
Clopidogrel Tops Aspirin Post-PCI, Even in High-Risk Cases
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
CGM Use, GLP-1s, Drinking Water Key of 2025 ADA Standards
plus a strong endorsement for drinking water and much more.
The Standards of Care — 2025 were published December 9 as a supplement to Diabetes Care. The standards “incorporate the latest information from clinical trial data and knowledge of diabetes management into a comprehensive guidelines document that will assist physicians in managing patients with diabetes in their practices,” said Mandeep Bajaj, MBBS, ADA’s President, Medicine & Science.
In an interview, Bajaj highlighted some of the most important of the clinical updates in 2024, including the following:
- Consideration of the use of continuous glucose monitoring devices in adults with type 2 diabetes (T2D) who don’t use insulin. Medicare and many other payers currently only cover CGM for people who use insulin or are otherwise at risk for hypoglycemia. However, some CGMs are now available over the counter, Bajaj pointed out.
- Actions to be taken in the event of medication shortages. The ADA published guidance for this in the case of GLP-1 RAs on December 2. Essentially ADA advised substituting a different GLP-1 RA if possible. Nonapproved products aren’t recommended, but guidance is provided for people who choose to use them.
- Use of GLP-1 RAs for heart and kidney health. Recommendations were revised to explicitly advise on choice of pharmacotherapy for individuals with T2D, based on new data on those with established or high risk for atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and chronic kidney disease.
- Treatment of MAFLD with moderate or advanced liver fibrosis. A new recommendation for use of a thyroid hormone receptor–beta agonist is based on trial data for resmetirom. Moreover, Bajaj noted, “we’ve adopted the new nomenclature, which was previously NAFLD and NASH, and now is MAFLD and MASH [metabolic-associated steatohepatitis].”
- Advice to continue weight management therapy beyond achieving weight loss goals. This is based on a large amount of evidence that “stopping these therapies are associated with weight regain and increased cardiovascular risk,” Bajaj said, adding that this recommendation was made in collaboration with the Obesity Society.
- Antibody-based screening for presymptomatic T1D in family members of people with T2D and others who may be at risk. “Individuals who test autoantibody positive should be provided with or referred for counseling about the risk of developing diabetes, diabetes symptoms, and [diabetic ketoacidosis] prevention and should be given consideration for referral to a specialized center for further evaluation and/or consideration of a clinical trial or approved therapy to potentially delay development of clinical diabetes,” the document says.
- Screen for psychosocial issues. People with diabetes should be screened for concerns including diabetes distress, depression, anxiety, fear of hypoglycemia, and disordered eating behaviors. “People on insulin or sulfonylureas may have fear of hypoglycemia, but diabetes distress can happen to anyone with diabetes,” Bajaj pointed out. Caregivers and family members should be screened as well, the document advises.
- Drink water, not soda. In the nutrition section, a new recommendation strongly advises drinking water instead of nutritive or nonnutritive sweetened beverages. “This is an important recommendation. So, when patients ask what’s the best thing to drink, our answer is drink water rather than Coca Cola or Diet Coke,” Bajaj said. But, what about people with diabetes who can’t quit their diet soda habit? “We’ve said that the nonnutritive sweetener is preferred over sugar sweetener, provided it’s in moderation and short term ... but the best is water.”
Bajaj has received grant support from ADA. He had no further disclosures.
A version of this article first appeared on Medscape.com.
plus a strong endorsement for drinking water and much more.
The Standards of Care — 2025 were published December 9 as a supplement to Diabetes Care. The standards “incorporate the latest information from clinical trial data and knowledge of diabetes management into a comprehensive guidelines document that will assist physicians in managing patients with diabetes in their practices,” said Mandeep Bajaj, MBBS, ADA’s President, Medicine & Science.
In an interview, Bajaj highlighted some of the most important of the clinical updates in 2024, including the following:
- Consideration of the use of continuous glucose monitoring devices in adults with type 2 diabetes (T2D) who don’t use insulin. Medicare and many other payers currently only cover CGM for people who use insulin or are otherwise at risk for hypoglycemia. However, some CGMs are now available over the counter, Bajaj pointed out.
- Actions to be taken in the event of medication shortages. The ADA published guidance for this in the case of GLP-1 RAs on December 2. Essentially ADA advised substituting a different GLP-1 RA if possible. Nonapproved products aren’t recommended, but guidance is provided for people who choose to use them.
- Use of GLP-1 RAs for heart and kidney health. Recommendations were revised to explicitly advise on choice of pharmacotherapy for individuals with T2D, based on new data on those with established or high risk for atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and chronic kidney disease.
- Treatment of MAFLD with moderate or advanced liver fibrosis. A new recommendation for use of a thyroid hormone receptor–beta agonist is based on trial data for resmetirom. Moreover, Bajaj noted, “we’ve adopted the new nomenclature, which was previously NAFLD and NASH, and now is MAFLD and MASH [metabolic-associated steatohepatitis].”
- Advice to continue weight management therapy beyond achieving weight loss goals. This is based on a large amount of evidence that “stopping these therapies are associated with weight regain and increased cardiovascular risk,” Bajaj said, adding that this recommendation was made in collaboration with the Obesity Society.
- Antibody-based screening for presymptomatic T1D in family members of people with T2D and others who may be at risk. “Individuals who test autoantibody positive should be provided with or referred for counseling about the risk of developing diabetes, diabetes symptoms, and [diabetic ketoacidosis] prevention and should be given consideration for referral to a specialized center for further evaluation and/or consideration of a clinical trial or approved therapy to potentially delay development of clinical diabetes,” the document says.
- Screen for psychosocial issues. People with diabetes should be screened for concerns including diabetes distress, depression, anxiety, fear of hypoglycemia, and disordered eating behaviors. “People on insulin or sulfonylureas may have fear of hypoglycemia, but diabetes distress can happen to anyone with diabetes,” Bajaj pointed out. Caregivers and family members should be screened as well, the document advises.
- Drink water, not soda. In the nutrition section, a new recommendation strongly advises drinking water instead of nutritive or nonnutritive sweetened beverages. “This is an important recommendation. So, when patients ask what’s the best thing to drink, our answer is drink water rather than Coca Cola or Diet Coke,” Bajaj said. But, what about people with diabetes who can’t quit their diet soda habit? “We’ve said that the nonnutritive sweetener is preferred over sugar sweetener, provided it’s in moderation and short term ... but the best is water.”
Bajaj has received grant support from ADA. He had no further disclosures.
A version of this article first appeared on Medscape.com.
plus a strong endorsement for drinking water and much more.
The Standards of Care — 2025 were published December 9 as a supplement to Diabetes Care. The standards “incorporate the latest information from clinical trial data and knowledge of diabetes management into a comprehensive guidelines document that will assist physicians in managing patients with diabetes in their practices,” said Mandeep Bajaj, MBBS, ADA’s President, Medicine & Science.
In an interview, Bajaj highlighted some of the most important of the clinical updates in 2024, including the following:
- Consideration of the use of continuous glucose monitoring devices in adults with type 2 diabetes (T2D) who don’t use insulin. Medicare and many other payers currently only cover CGM for people who use insulin or are otherwise at risk for hypoglycemia. However, some CGMs are now available over the counter, Bajaj pointed out.
- Actions to be taken in the event of medication shortages. The ADA published guidance for this in the case of GLP-1 RAs on December 2. Essentially ADA advised substituting a different GLP-1 RA if possible. Nonapproved products aren’t recommended, but guidance is provided for people who choose to use them.
- Use of GLP-1 RAs for heart and kidney health. Recommendations were revised to explicitly advise on choice of pharmacotherapy for individuals with T2D, based on new data on those with established or high risk for atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and chronic kidney disease.
- Treatment of MAFLD with moderate or advanced liver fibrosis. A new recommendation for use of a thyroid hormone receptor–beta agonist is based on trial data for resmetirom. Moreover, Bajaj noted, “we’ve adopted the new nomenclature, which was previously NAFLD and NASH, and now is MAFLD and MASH [metabolic-associated steatohepatitis].”
- Advice to continue weight management therapy beyond achieving weight loss goals. This is based on a large amount of evidence that “stopping these therapies are associated with weight regain and increased cardiovascular risk,” Bajaj said, adding that this recommendation was made in collaboration with the Obesity Society.
- Antibody-based screening for presymptomatic T1D in family members of people with T2D and others who may be at risk. “Individuals who test autoantibody positive should be provided with or referred for counseling about the risk of developing diabetes, diabetes symptoms, and [diabetic ketoacidosis] prevention and should be given consideration for referral to a specialized center for further evaluation and/or consideration of a clinical trial or approved therapy to potentially delay development of clinical diabetes,” the document says.
- Screen for psychosocial issues. People with diabetes should be screened for concerns including diabetes distress, depression, anxiety, fear of hypoglycemia, and disordered eating behaviors. “People on insulin or sulfonylureas may have fear of hypoglycemia, but diabetes distress can happen to anyone with diabetes,” Bajaj pointed out. Caregivers and family members should be screened as well, the document advises.
- Drink water, not soda. In the nutrition section, a new recommendation strongly advises drinking water instead of nutritive or nonnutritive sweetened beverages. “This is an important recommendation. So, when patients ask what’s the best thing to drink, our answer is drink water rather than Coca Cola or Diet Coke,” Bajaj said. But, what about people with diabetes who can’t quit their diet soda habit? “We’ve said that the nonnutritive sweetener is preferred over sugar sweetener, provided it’s in moderation and short term ... but the best is water.”
Bajaj has received grant support from ADA. He had no further disclosures.
A version of this article first appeared on Medscape.com.
Freezing the Pain: A New Way to Treat Rib Fractures
This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today to discuss a novel way to treat pain related to conditions such as rib fractures and burns is Dr. Sergey Motov, an emergency physician with expertise in pain management and research director in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York.
Also joining me is Dr. Gary Schwartz, vice chair of pain and anesthesiology at Maimonides Medical Center. Dr. Schwartz is board certified in anesthesiology and interventional pain management.
Welcome, Sergey and Gary.
Sergey M. Motov, MD: Thank you, Robert.
Gary S. Schwartz, MD: Thank you, Robert.
Traditional Approaches to Pain Relief
Glatter: It’s a pleasure to have you both. Sergey, we were chatting earlier this week and you had mentioned a novel approach to treating a common condition we encounter in the emergency department — rib fractures.
As we all know, they’re very painful and can lead to pulmonary complications, including atelectasis, pneumonia due to splinting and lack of proper pain management, along with the use of incentive spirometry.
Sergey and Gary, can you describe traditional approaches to alleviating the pain associated with rib fractures? What do we typically use? Then we’ll get to some novel treatments that we’re here to discuss.
Motov: I’m going to use the emergency medicine approach to rib fractures. As you pointed out, pain relief is of utmost importance.
With the advent and acquiring of the amazing technique of interventional pain management, physicians, for the most part, are very astute about providing nerve blocks to alleviate pain, at least in immediate need. I’m talking about the relatively short term, 1-5 hours, in the emergency department.
Primarily, we focus on fascial plane blocks such as serratus anterior plane block. Traditionally, ED physicians don’t use much of the intercostal blocks. At times, we can direct the spinal block to cover the lateral aspect of the chest wall.
As part of the multimodal approach, we can use NSAIDs. If there’s a contraindication, we can use opioids. There are some data to support consideration of using topical formularies such as a lidocaine patch, but they are somewhat conflicting.
The question becomes what you’re going to send a patient home with. Again, traditional teaching is either opioids, immediate release with a short course, plus or minus NSAIDs, plus or minus acetaminophen.
The issue with rib fractures is that, while we can manage immediate and super-acute pain presentation in the ED and then discharge up to 24-72 hours, what happens afterwards is very challenging. Acute intercostal neuralgia related to traumatic rib fractures is semi-manageable, but if it’s inappropriately treated, it has a great tendency to transform into chronic intercostal neuralgia. It contributes a great deal of disability and morbidity.
Several years ago, I came across an entity called cryoneurolysis (cryo ─ cold temperature; neurolysis ─ freezing the nerve). I’m excited to be here today because Gary is the one who’s pioneering and championing this technique in our institution.
Cryoneurolysis: Mechanisms of Action and Benefits
Glatter: Gary, what do you see as the main role for this procedure at this time?
Schwartz: As Sergey alluded to, the traditional approach of opiates has side effects (ie, constipation, addiction, and tolerance). Unfortunately, many of these rib fractures occur in older patients. They come in anticoagulated, so they can’t have NSAIDs.
Sergey and his team in the ER have been pioneers in giving short-acting local anesthetic blocks that could last anywhere from 12 to 24 hours. There are long-acting local anesthetics that we can get out to 72 hours.
Unfortunately, these rib fractures and the pain associated with them, in addition to the intercostal neuralgia, could take weeks to heal. That’s where cryoneurolysis comes in. We’re all used to ice or cold temperature. For example, if your child gets an ear piercing, they put some ice on their earlobe beforehand, it numbs it up, and they don’t feel pain. It allows them to get their ears pierced without pain, but it’s short-acting.
What we have now are handheld devices with tips about as long as a pen, 3.5 inches, that allow you to go down precisely to these intercostal nerves that innervate the ribs and give a cold lesion that freezes these nerves.
The benefit of it is it’s not permanent like cryoablation, like we’ve seen for tumor necrosis, which destroys outside tissues. It’s really a small lesion, about 16 mm x 8 mm, which is enough to engulf the nerve and pretty much stun it.
It causes axonotmesis, but the epineurium, the endoneurium, and the perineurium — the inner workings of the nerve — stay intact, so it regrows. It just destroys the myelin sheath and the axon.
Glatter: You’re creating a scarring effect; is that what you’re saying? In other words, you’re doing a cold-temperature freeze and stunning the nerve. My question is, does it regrow? Is this a permanent type of injury?
Schwartz: With Wallerian degeneration, nerves do regrow after injuries.
Unfortunately, as you two probably see in the ER for big traumas, where the nerve is transected, those unfortunately do not grow back. This is considered a grade 2 lesion, so the Wallerian degeneration recurs. The nerves grow, depending on the literature you look at, about 0.5-2 mm per day.
This intervention gives us at least 3 months of relief for the patient, which is in the time frame where the rib fracture will heal, hopefully with no damage to the nerve from the fracture, and they go on living their life without having to take opiates or having to stop their anticoagulation.
Because prior to this, when I was a pain fellow, we used to put epidurals in many of these patients. The problem with that is patients can’t go home, and if they’re anticoagulated, you can’t place it because of the risk of a spinal hematoma.
Potential Use in Ventilation Weaning
Glatter: This is something we encounter daily, and certainly for those patients who have more numerous rib fractures or flail chest, this could be even more devastating, as well as for those who get intubated.
Do you see any role, in terms of ventilator weaning, in using this technique specifically in the ICU setting?
Schwartz: That’s an interesting concept. I’m not so sure about ventilator weaning, but we’ve used this in the hospital for rib fractures from traumas where patients had such severe fractures and had to go to the operating room for rib plating, and did necessitate an epidural. We’ve used this to discontinue their epidural and transition them to get the patient home.
I think that is part of the care, not only in the ER but in the hospital as well. We need to treat the patients, but we also have to have a transition plan to get them out of the hospital. Not that we don’t want to treat our patients, but we have to have a plan to get them home. I’m guessing that might be an interesting stage of research in the future if it does help with weaning from a ventilator.
Glatter: There are some studies out there suggesting that there can be some utility in terms of ventilator weaning using this technique. The ability of this to change how we manage pain is just incredible.
Sergey, do you feel that this is something that you could implement in your ED with your patients in the near future?
Motov: Definitely. I have personally been a very big proponent of it. I’m the theoreticist because I’ve covered a great deal of literature, and now having Gary and his team doing this in our institution, it’s a shame not to capitalize on it. I’m slowly moving toward figuring out the way of collaborative effort to have Gary and his team help my team and our colleagues, bring him on board, and maybe broaden the integration for pain management.
I believe, as Gary emphasized, that geriatric traumatic pain injuries are critically important due to the presence of comorbidities, potential drug interactions, and the challenges of managing these factors effectively.
There is one thing I want to bring up, and Gary, please support me on it. The procedure itself is fascinating because it provides long-term pain relief and reduces morbidity. I wouldn’t say mortality, just reduced morbidity. However, we need to be very conscious of the fact that this blockade, this ice-ball freezing of the nerve, can be detrimental to motor nerves. If your whole goal or idea of faster recovery after postoperative knee or hip replacements, or any traumatic lower- or upper-extremity surgery, includes blockade of motor nerves, it’s not going to be beneficial.
I believe the primary therapeutic application of this technology lies in targeting sensory nerves. For instance, intercostal nerves could be a focus in cases of rib fractures. Additionally, this approach shows promise for treating burns, particularly in the lower and upper extremities. Specifically, targeting nerves such as the lateral femoral cutaneous nerve or the anterior femoral cutaneous nerve could effectively neutralize pain and provide significant relief for weeks, if not months.
Based on additional predilection to what particular indications would be, maybe occipital headache with cervicalgia, occipital nerve block — it’s a sensory block — can benefit from it. Slowly but surely, there’s a slew of painful syndromes for which cryoneurolysis might have a great deal of use in the emergency department.
Cryoneurolysis for Other Pain Syndromes
Glatter: Gary, I’ll let you expand upon additional uses that you see. You did mention one on our chat earlier this week, which was postmastectomy pain syndrome with the intercostal brachial nerve. That’s a very compelling area of interest, certainly for the number of women that go through mastectomies or lumpectomies and that have axillary dissection or nerve injury.
Schwartz: Post-mastectomy is one way you could use this device and technology to attack painful syndromes, such as postmastectomy syndrome. Mastectomies are one of the most common surgeries performed in the United States, but I believe it’s a top three for post-op chronic pain, which we don’t normally think of.
There was a great study by a team in San Diego where they did intercostal brachial and intercostal nerve blocks on multiple nerves, and they decreased pain up to 3 months after the surgery and decreased opiates.
As Sergey alluded to, it’s approved for any peripheral nerve in the body. We’ve used it in our pain office for occipital neuralgia, postherpetic neuralgia, chronic rib pain after fractures, and surgery. Some of the most common uses are for superficial, sensory, genicular nerves, the lateral femoral cutaneous nerve, the anterior femoral cutaneous nerve, and the infrapatellar branch of the saphenous.
You could numb the skin preoperatively before a painful surgery, such as a total knee replacement — or as we like to call it, a total knee arthroplasty — to reduce opiates, improve function, and decrease length of stay. You could attack any sensory nerve.
We’ve utilized that already in our private practice. We’re trying to transition into the hospital to have everyone who gets a knee arthroplasty have this technology to decrease opiates, improve function, and recover faster.
This is quite interesting and motivating for me because when I first started, we had a femoral catheter to block the motor femoral nerve or an epidural. Patients were in the hospital for 3-5 days with the CPM [continuous passive motion] machine, which is like a medieval torture device that you might see in Mad Max — where you’re kind of moving the patient’s knee back and forth after surgery, and they were miserable, taking patient-controlled analgesia and high-dose opiates. Now, we’re freezing these nerves beforehand, doing our nerve blocks in the operating room with long-acting local anesthetic, and patients are going home the same day with minimal or even no opiates sometimes.
Implications for Patient Mobility and DVT Risk
Glatter: You’re getting up to 3 months of relief in that setting, doing it as you described?
Schwartz: Yes, up to 3 months of relief, which is huge, because most patients recovering from a knee arthroplasty, at about the 6- to 8-week mark, have improved range of motion, they have their 110° flexion, they have their extension, and they’re getting back to their normal life.
You cover the whole postoperative rehab, where patients don’t have to get recurring refills, they can participate in physical therapy. As you both know, part of the recovery process is to be able to interact with family and friends without being sleepy, angry, and in pain all day, so they can get back to their normal function.
Glatter: In terms of this procedure, would there be any increase in deep vein thrombosis (DVT) in relation to this, by chance?
Schwartz: Actually, there’s less of a risk of DVT because patients have less pain, so they can get up and move faster. Some of my surgical colleagues who have implemented this in their practice have gotten away from using the stronger anticoagulation like Xarelto (rivaroxaban) or Coumadin (warfarin), and they just give them baby aspirin postoperatively because their patients are going home the same day and walking. It’s probably safer for patients. There’s no research out there yet to show that, but we all know that the more you move and the more you’re not lying around, the lower the risk of having a DVT or a blood clot.
There are studies showing that there’s no damage to blood vessels, other than if you stick it with the needle, because the nitrogen gas in this that allows the ice ball to form does not get injected into the body. It’s all resorbed in the machine. The only thing the body sees is this ice ball, which would melt if you hit a blood vessel because we should be 98 °F and the ice ball is -88 °F. There’s no gas injected into the body either, so there’s no risk of a gas embolism.
Training and Implementation
Glatter: I was going to ask you about air emboli, and you perfectly led right into that.
In terms of training requirements, currently, what do you envision as a way we can train residents and fellows to do this? Is this currently something being considered in curriculum?
Schwartz: We are going to train our residents first. I’m training the attendings. Before you use this technology, you should have a basic understanding of ultrasound, how to use the device, the different settings, and what the risks are for each procedure you’re doing.
Let’s say, as Sergey alluded to, with an intercostal nerve block, you could have a pneumothorax. You have to be able to identify the rib, where the nerve should lie, the innermost intercostal muscle you could see on the newer ultrasounds, and where the pleura lies. People should start with just basic ultrasound training and then advance to a typical intercostal nerve block.
Once you master that, the procedure with the device is not much different than an intercostal nerve block, except you have a handheld device and the needle is just as long as a pen, 3.5 inches.
If you could do a nerve block with a spinal needle, you could do the procedure. Once people have the technical ultrasound skills, then they can advance to needle-based procedures, and once you have that training, you could use this procedure safely and efficaciously.
Glatter: Sergey, do you see this as requiring quite a bit of time and training in your program?
Motov: I mentioned earlier, before we started, that with the advent of ultrasound-guided nerve blocks, the vast majority of physicians are becoming very comfortable and fairly effective with maneuvering a needle and the ultrasound probe. The learning curve is essentially the same. The only difference is, as Gary pointed out, some of the nerves could be new to ED folks, but the technique, the understanding, the visualization, and the knowledge of anatomy are essentially the same.
As he pointed out, if you can use it with a spinal needle and local anesthetic, the procedure becomes exactly the same. It’s a slightly different drug and a different needle, and instead of local anesthetic, you’re using a gas at cold temperatures, and that’s pretty much it.
Glatter: Are there any other barriers to adoption in terms of cost, the device itself, or the companies that manufacture these handheld devices?
Schwartz: There’s always cost associated with the new device, needles, and the gas. Thankfully, they’re covered by Medicare, Medicaid, and most commercial insurances in the current framework, which I think is important. I think Congress is seeing the benefits of opiate sparing that Sergey helped lead in the ED.
At AABP Integrative Pain Care and Wellness and Maimonides, we’re doing this intraoperatively as well. I think the government is seeing that. There was a NOPAIN Act passed in 2023 that, starting January 1, 2025, will allow certain approved companies, devices, and medications to have to be repaid by CMS, Centers for Medicare & Medicaid Services, in the hospital setting and in the outpatient departments. In the inpatient surgical stays, we could have less opiates. I think that’s important. It is reimbursed now. Obviously, there is a cost associated.
The other benefit of this procedure and these techniques is, as Sergey alluded to, it’s done under ultrasound. The way we all learn procedures, whether it be central lines or chest tubes, is the blind technique. There is no good way to practice. In my interventional pain practice, many of our original techniques were done under fluoroscopy, and we don’t want to get extra radiation during practice.
The benefit of ultrasound and the advent of handheld ultrasound devices is that we can practice scanning and techniques on ourselves and on colleagues, without the fear of radiation. Other than the fact that we need to shower after the surgical lube is on from the scanning gel, you could practice your techniques in a safe way without harming a patient or yourself.
Future Directions in Pain Management Techniques
Glatter: Absolutely. Do you see any role for possibly stellate ganglion blocks, which are a bit riskier and have greater depth?
Schwartz: People are looking at different studies because, again, it’s a needle-based technology. We do many stellate ganglion blocks. I have not done it for this procedure yet, but that’s the next step of what I try. Under ultrasound, we could see the longus colli muscle and we could see the carotid artery. Obviously, we don’t see the ganglion per se, but anatomically, we know where it lies. You could drop a couple of lesions on there and give a theoretic prolonged sympathetic block, which might help with symptoms of complex regional pain syndrome.
I know there are some studies that have looked at stellate ganglion blocks for long-COVID symptoms. Unfortunately, it looks like we’re back in another wave right now. I think that’s the next step of the technology.
Glatter: Getting back to the emergency department, burns are something we see commonly — such painful conditions. This is something that could really provide significant relief, especially with burns that involve the chest wall, not just extremity burns.
Motov: I agree with you. Burns would be a very good indication to utilize this technique. Just listening to you and Gary, another thing that pops into my head, which may have actually some science behind it, would be any traumatic amputations done in a civilian environment or even in the military in a combat situation.
A person who has either an upper or lower extremity that is partially or completely severed or amputated, and the pain — God knows how bad it is — if not properly treated, it is going to be a very long recovery. That’s, I believe, another percutaneous condition where cryoneurolysis will be very beneficial to freeze those nerves, allowing patients to recover through rehab acute care, acute phases, rehabilitation, and move on with their lives.
Glatter: In the setting of a painful distal radius fracture, a femur fracture, and things of that nature, Gary, do you see this as a modality in conjunction with emergency medicine colleagues as being something that’s going to really become an important part of our armamentarium?
Schwartz: I do think it’s going to become more important in the future, as there are more studies to show what nerves you could block with cryoneurolysis in the longer term. I think you might see people start using these for fractures, especially for fractures that are not operable at the time or if a patient needs to be optimized prior to surgery.
As Sergey alluded to, it’s optimal in burns. People have been looking for relief of stump pain or postamputation pain. There’s a big researcher in Canada who’s been looking at pain with spasticity for people with cerebral palsy and poststroke issues, where they can’t move and they have pain moving an extremity after these conditions. We’re at just the tip of the iceberg as to where people are going to use this hand-held technology in the future.
Glatter: We use long-acting nerve blocks for hip fractures already in the emergency department. Why not employ this technique, which would have longer effects and limit opiate use?
Schwartz: It might even help a certain subset of the population, at least in Brooklyn, where we have a large elderly population. I believe it’s one of the oldest boroughs in the country, and definitely in New York.
There are some people that go on to surgery just because they might be bedbound, but it’s the pain that is dictating their surgical procedure, not that they’re ever going to walk again.
It’s maybe the next step to look for. If you could block this nerve for 3 months or longer, they’re still going to be bedbound, but maybe you could avoid a surgical procedure that carries its own morbidity and mortality, which I see a big interest in in the future.
Glatter: Absolutely. The idea behind treating spasticity is very important from an occupational therapy standpoint — eating, activities of daily living — just the basics.
Getting someone’s fingers released, being able to move their legs again, and getting them out of contracture states, I think, has a huge role.
Schwartz: Not only for the patient but also for the caregivers. For many of these patients, if they’re contracted fully and the pain from the spasticity is preventing their caregivers from moving them, it’s difficult to put on a shirt, pants, and so on.
One other point I’d like to make is that it’s reproducible. It’s not one-and-done. If the pain comes back from any of these conditions, you could treat again with another cryoneurolysis treatment. The current literature to date shows that it’s just as effective time and time again. I’ve seen clinically that you can repeat this procedure, whereas some of our other procedures that we do in medicine are not as reproducible, which is important for some of these chronic conditions.
Glatter: You had mentioned reimbursement earlier. Currently, this procedure is reimbursed under Medicare, Medicaid, and third-party payers, I assume?
Schwartz: Not all, but many commercial insurers. Yes for Medicare.
Final Takeaways
Glatter: Reimbursement has to be really universal because if this is shown to be more effective and limits opiate use, then there’s no question in my mind that this is such a groundbreaking procedure.
I’ll let you both give a few pearls for our audience to summarize our discussion.
Motov: I’d say it’s somewhat long overdue that this technique and pain-relieving modality should enter the emergency department, with the auspices and the beautiful collaborative effort between emergency department folks and interventional anesthesiologists, pain management specialists, collaborative training, and a collaborative goal of improving patients’ pain throughout the entire journey during the healthcare system.
That would be my only pearl. Just reach out to your colleagues within your respective institutions who you believe have aptitude, knowledge, and expertise. Reach out, get trained, and start passing down the knowledge to your faculty, and by virtue of extension, to your fellow residents and colleagues.
Schwartz: He took the words right out of my mouth. Communication and collaboration are the two most important things. There’s a shortage of physicians in this country. We can only each do so much, so we should each utilize and implement this technology to affect and help as many patients as possible.
We can decrease the amount of opiates, help our patients, help our family members in our community live with decreased pain, improve their function, and just get back to their lives and keep pushing the envelope of what’s the next step in treatment.
Again, like we went from giving opiates for this and that’s it — maybe an epidural, maybe a 5- to 6-hour intercostal nerve block — to fascial plane blocks like Sergey said, to more advanced procedures, to now we can give months of relief.
I think the communication, the collaboration, and the camaraderie among our different specialties are important to push the envelope to help our patients.
Glatter: That’s so well put. I completely agree.
I want to thank both of you for a very lively discussion. It was very informative. Your expertise is greatly appreciated and will certainly benefit our audience. Thank you both again.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. Dr. Schwartz is co-owner and primary clinic director at AABP Integrative Pain Care in Brooklyn, New York. Schwartz currently serves as the co-director of AABP Integrative Pain Care and Wellness and the vice chair of pain and anesthesiology for Maimonides Medical Center. Dr. Schwartz reported conflicts of interest with Pacira Biosciences and Dorsal Health; neither Dr. Glatter nor Dr. Motov reported relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today to discuss a novel way to treat pain related to conditions such as rib fractures and burns is Dr. Sergey Motov, an emergency physician with expertise in pain management and research director in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York.
Also joining me is Dr. Gary Schwartz, vice chair of pain and anesthesiology at Maimonides Medical Center. Dr. Schwartz is board certified in anesthesiology and interventional pain management.
Welcome, Sergey and Gary.
Sergey M. Motov, MD: Thank you, Robert.
Gary S. Schwartz, MD: Thank you, Robert.
Traditional Approaches to Pain Relief
Glatter: It’s a pleasure to have you both. Sergey, we were chatting earlier this week and you had mentioned a novel approach to treating a common condition we encounter in the emergency department — rib fractures.
As we all know, they’re very painful and can lead to pulmonary complications, including atelectasis, pneumonia due to splinting and lack of proper pain management, along with the use of incentive spirometry.
Sergey and Gary, can you describe traditional approaches to alleviating the pain associated with rib fractures? What do we typically use? Then we’ll get to some novel treatments that we’re here to discuss.
Motov: I’m going to use the emergency medicine approach to rib fractures. As you pointed out, pain relief is of utmost importance.
With the advent and acquiring of the amazing technique of interventional pain management, physicians, for the most part, are very astute about providing nerve blocks to alleviate pain, at least in immediate need. I’m talking about the relatively short term, 1-5 hours, in the emergency department.
Primarily, we focus on fascial plane blocks such as serratus anterior plane block. Traditionally, ED physicians don’t use much of the intercostal blocks. At times, we can direct the spinal block to cover the lateral aspect of the chest wall.
As part of the multimodal approach, we can use NSAIDs. If there’s a contraindication, we can use opioids. There are some data to support consideration of using topical formularies such as a lidocaine patch, but they are somewhat conflicting.
The question becomes what you’re going to send a patient home with. Again, traditional teaching is either opioids, immediate release with a short course, plus or minus NSAIDs, plus or minus acetaminophen.
The issue with rib fractures is that, while we can manage immediate and super-acute pain presentation in the ED and then discharge up to 24-72 hours, what happens afterwards is very challenging. Acute intercostal neuralgia related to traumatic rib fractures is semi-manageable, but if it’s inappropriately treated, it has a great tendency to transform into chronic intercostal neuralgia. It contributes a great deal of disability and morbidity.
Several years ago, I came across an entity called cryoneurolysis (cryo ─ cold temperature; neurolysis ─ freezing the nerve). I’m excited to be here today because Gary is the one who’s pioneering and championing this technique in our institution.
Cryoneurolysis: Mechanisms of Action and Benefits
Glatter: Gary, what do you see as the main role for this procedure at this time?
Schwartz: As Sergey alluded to, the traditional approach of opiates has side effects (ie, constipation, addiction, and tolerance). Unfortunately, many of these rib fractures occur in older patients. They come in anticoagulated, so they can’t have NSAIDs.
Sergey and his team in the ER have been pioneers in giving short-acting local anesthetic blocks that could last anywhere from 12 to 24 hours. There are long-acting local anesthetics that we can get out to 72 hours.
Unfortunately, these rib fractures and the pain associated with them, in addition to the intercostal neuralgia, could take weeks to heal. That’s where cryoneurolysis comes in. We’re all used to ice or cold temperature. For example, if your child gets an ear piercing, they put some ice on their earlobe beforehand, it numbs it up, and they don’t feel pain. It allows them to get their ears pierced without pain, but it’s short-acting.
What we have now are handheld devices with tips about as long as a pen, 3.5 inches, that allow you to go down precisely to these intercostal nerves that innervate the ribs and give a cold lesion that freezes these nerves.
The benefit of it is it’s not permanent like cryoablation, like we’ve seen for tumor necrosis, which destroys outside tissues. It’s really a small lesion, about 16 mm x 8 mm, which is enough to engulf the nerve and pretty much stun it.
It causes axonotmesis, but the epineurium, the endoneurium, and the perineurium — the inner workings of the nerve — stay intact, so it regrows. It just destroys the myelin sheath and the axon.
Glatter: You’re creating a scarring effect; is that what you’re saying? In other words, you’re doing a cold-temperature freeze and stunning the nerve. My question is, does it regrow? Is this a permanent type of injury?
Schwartz: With Wallerian degeneration, nerves do regrow after injuries.
Unfortunately, as you two probably see in the ER for big traumas, where the nerve is transected, those unfortunately do not grow back. This is considered a grade 2 lesion, so the Wallerian degeneration recurs. The nerves grow, depending on the literature you look at, about 0.5-2 mm per day.
This intervention gives us at least 3 months of relief for the patient, which is in the time frame where the rib fracture will heal, hopefully with no damage to the nerve from the fracture, and they go on living their life without having to take opiates or having to stop their anticoagulation.
Because prior to this, when I was a pain fellow, we used to put epidurals in many of these patients. The problem with that is patients can’t go home, and if they’re anticoagulated, you can’t place it because of the risk of a spinal hematoma.
Potential Use in Ventilation Weaning
Glatter: This is something we encounter daily, and certainly for those patients who have more numerous rib fractures or flail chest, this could be even more devastating, as well as for those who get intubated.
Do you see any role, in terms of ventilator weaning, in using this technique specifically in the ICU setting?
Schwartz: That’s an interesting concept. I’m not so sure about ventilator weaning, but we’ve used this in the hospital for rib fractures from traumas where patients had such severe fractures and had to go to the operating room for rib plating, and did necessitate an epidural. We’ve used this to discontinue their epidural and transition them to get the patient home.
I think that is part of the care, not only in the ER but in the hospital as well. We need to treat the patients, but we also have to have a transition plan to get them out of the hospital. Not that we don’t want to treat our patients, but we have to have a plan to get them home. I’m guessing that might be an interesting stage of research in the future if it does help with weaning from a ventilator.
Glatter: There are some studies out there suggesting that there can be some utility in terms of ventilator weaning using this technique. The ability of this to change how we manage pain is just incredible.
Sergey, do you feel that this is something that you could implement in your ED with your patients in the near future?
Motov: Definitely. I have personally been a very big proponent of it. I’m the theoreticist because I’ve covered a great deal of literature, and now having Gary and his team doing this in our institution, it’s a shame not to capitalize on it. I’m slowly moving toward figuring out the way of collaborative effort to have Gary and his team help my team and our colleagues, bring him on board, and maybe broaden the integration for pain management.
I believe, as Gary emphasized, that geriatric traumatic pain injuries are critically important due to the presence of comorbidities, potential drug interactions, and the challenges of managing these factors effectively.
There is one thing I want to bring up, and Gary, please support me on it. The procedure itself is fascinating because it provides long-term pain relief and reduces morbidity. I wouldn’t say mortality, just reduced morbidity. However, we need to be very conscious of the fact that this blockade, this ice-ball freezing of the nerve, can be detrimental to motor nerves. If your whole goal or idea of faster recovery after postoperative knee or hip replacements, or any traumatic lower- or upper-extremity surgery, includes blockade of motor nerves, it’s not going to be beneficial.
I believe the primary therapeutic application of this technology lies in targeting sensory nerves. For instance, intercostal nerves could be a focus in cases of rib fractures. Additionally, this approach shows promise for treating burns, particularly in the lower and upper extremities. Specifically, targeting nerves such as the lateral femoral cutaneous nerve or the anterior femoral cutaneous nerve could effectively neutralize pain and provide significant relief for weeks, if not months.
Based on additional predilection to what particular indications would be, maybe occipital headache with cervicalgia, occipital nerve block — it’s a sensory block — can benefit from it. Slowly but surely, there’s a slew of painful syndromes for which cryoneurolysis might have a great deal of use in the emergency department.
Cryoneurolysis for Other Pain Syndromes
Glatter: Gary, I’ll let you expand upon additional uses that you see. You did mention one on our chat earlier this week, which was postmastectomy pain syndrome with the intercostal brachial nerve. That’s a very compelling area of interest, certainly for the number of women that go through mastectomies or lumpectomies and that have axillary dissection or nerve injury.
Schwartz: Post-mastectomy is one way you could use this device and technology to attack painful syndromes, such as postmastectomy syndrome. Mastectomies are one of the most common surgeries performed in the United States, but I believe it’s a top three for post-op chronic pain, which we don’t normally think of.
There was a great study by a team in San Diego where they did intercostal brachial and intercostal nerve blocks on multiple nerves, and they decreased pain up to 3 months after the surgery and decreased opiates.
As Sergey alluded to, it’s approved for any peripheral nerve in the body. We’ve used it in our pain office for occipital neuralgia, postherpetic neuralgia, chronic rib pain after fractures, and surgery. Some of the most common uses are for superficial, sensory, genicular nerves, the lateral femoral cutaneous nerve, the anterior femoral cutaneous nerve, and the infrapatellar branch of the saphenous.
You could numb the skin preoperatively before a painful surgery, such as a total knee replacement — or as we like to call it, a total knee arthroplasty — to reduce opiates, improve function, and decrease length of stay. You could attack any sensory nerve.
We’ve utilized that already in our private practice. We’re trying to transition into the hospital to have everyone who gets a knee arthroplasty have this technology to decrease opiates, improve function, and recover faster.
This is quite interesting and motivating for me because when I first started, we had a femoral catheter to block the motor femoral nerve or an epidural. Patients were in the hospital for 3-5 days with the CPM [continuous passive motion] machine, which is like a medieval torture device that you might see in Mad Max — where you’re kind of moving the patient’s knee back and forth after surgery, and they were miserable, taking patient-controlled analgesia and high-dose opiates. Now, we’re freezing these nerves beforehand, doing our nerve blocks in the operating room with long-acting local anesthetic, and patients are going home the same day with minimal or even no opiates sometimes.
Implications for Patient Mobility and DVT Risk
Glatter: You’re getting up to 3 months of relief in that setting, doing it as you described?
Schwartz: Yes, up to 3 months of relief, which is huge, because most patients recovering from a knee arthroplasty, at about the 6- to 8-week mark, have improved range of motion, they have their 110° flexion, they have their extension, and they’re getting back to their normal life.
You cover the whole postoperative rehab, where patients don’t have to get recurring refills, they can participate in physical therapy. As you both know, part of the recovery process is to be able to interact with family and friends without being sleepy, angry, and in pain all day, so they can get back to their normal function.
Glatter: In terms of this procedure, would there be any increase in deep vein thrombosis (DVT) in relation to this, by chance?
Schwartz: Actually, there’s less of a risk of DVT because patients have less pain, so they can get up and move faster. Some of my surgical colleagues who have implemented this in their practice have gotten away from using the stronger anticoagulation like Xarelto (rivaroxaban) or Coumadin (warfarin), and they just give them baby aspirin postoperatively because their patients are going home the same day and walking. It’s probably safer for patients. There’s no research out there yet to show that, but we all know that the more you move and the more you’re not lying around, the lower the risk of having a DVT or a blood clot.
There are studies showing that there’s no damage to blood vessels, other than if you stick it with the needle, because the nitrogen gas in this that allows the ice ball to form does not get injected into the body. It’s all resorbed in the machine. The only thing the body sees is this ice ball, which would melt if you hit a blood vessel because we should be 98 °F and the ice ball is -88 °F. There’s no gas injected into the body either, so there’s no risk of a gas embolism.
Training and Implementation
Glatter: I was going to ask you about air emboli, and you perfectly led right into that.
In terms of training requirements, currently, what do you envision as a way we can train residents and fellows to do this? Is this currently something being considered in curriculum?
Schwartz: We are going to train our residents first. I’m training the attendings. Before you use this technology, you should have a basic understanding of ultrasound, how to use the device, the different settings, and what the risks are for each procedure you’re doing.
Let’s say, as Sergey alluded to, with an intercostal nerve block, you could have a pneumothorax. You have to be able to identify the rib, where the nerve should lie, the innermost intercostal muscle you could see on the newer ultrasounds, and where the pleura lies. People should start with just basic ultrasound training and then advance to a typical intercostal nerve block.
Once you master that, the procedure with the device is not much different than an intercostal nerve block, except you have a handheld device and the needle is just as long as a pen, 3.5 inches.
If you could do a nerve block with a spinal needle, you could do the procedure. Once people have the technical ultrasound skills, then they can advance to needle-based procedures, and once you have that training, you could use this procedure safely and efficaciously.
Glatter: Sergey, do you see this as requiring quite a bit of time and training in your program?
Motov: I mentioned earlier, before we started, that with the advent of ultrasound-guided nerve blocks, the vast majority of physicians are becoming very comfortable and fairly effective with maneuvering a needle and the ultrasound probe. The learning curve is essentially the same. The only difference is, as Gary pointed out, some of the nerves could be new to ED folks, but the technique, the understanding, the visualization, and the knowledge of anatomy are essentially the same.
As he pointed out, if you can use it with a spinal needle and local anesthetic, the procedure becomes exactly the same. It’s a slightly different drug and a different needle, and instead of local anesthetic, you’re using a gas at cold temperatures, and that’s pretty much it.
Glatter: Are there any other barriers to adoption in terms of cost, the device itself, or the companies that manufacture these handheld devices?
Schwartz: There’s always cost associated with the new device, needles, and the gas. Thankfully, they’re covered by Medicare, Medicaid, and most commercial insurances in the current framework, which I think is important. I think Congress is seeing the benefits of opiate sparing that Sergey helped lead in the ED.
At AABP Integrative Pain Care and Wellness and Maimonides, we’re doing this intraoperatively as well. I think the government is seeing that. There was a NOPAIN Act passed in 2023 that, starting January 1, 2025, will allow certain approved companies, devices, and medications to have to be repaid by CMS, Centers for Medicare & Medicaid Services, in the hospital setting and in the outpatient departments. In the inpatient surgical stays, we could have less opiates. I think that’s important. It is reimbursed now. Obviously, there is a cost associated.
The other benefit of this procedure and these techniques is, as Sergey alluded to, it’s done under ultrasound. The way we all learn procedures, whether it be central lines or chest tubes, is the blind technique. There is no good way to practice. In my interventional pain practice, many of our original techniques were done under fluoroscopy, and we don’t want to get extra radiation during practice.
The benefit of ultrasound and the advent of handheld ultrasound devices is that we can practice scanning and techniques on ourselves and on colleagues, without the fear of radiation. Other than the fact that we need to shower after the surgical lube is on from the scanning gel, you could practice your techniques in a safe way without harming a patient or yourself.
Future Directions in Pain Management Techniques
Glatter: Absolutely. Do you see any role for possibly stellate ganglion blocks, which are a bit riskier and have greater depth?
Schwartz: People are looking at different studies because, again, it’s a needle-based technology. We do many stellate ganglion blocks. I have not done it for this procedure yet, but that’s the next step of what I try. Under ultrasound, we could see the longus colli muscle and we could see the carotid artery. Obviously, we don’t see the ganglion per se, but anatomically, we know where it lies. You could drop a couple of lesions on there and give a theoretic prolonged sympathetic block, which might help with symptoms of complex regional pain syndrome.
I know there are some studies that have looked at stellate ganglion blocks for long-COVID symptoms. Unfortunately, it looks like we’re back in another wave right now. I think that’s the next step of the technology.
Glatter: Getting back to the emergency department, burns are something we see commonly — such painful conditions. This is something that could really provide significant relief, especially with burns that involve the chest wall, not just extremity burns.
Motov: I agree with you. Burns would be a very good indication to utilize this technique. Just listening to you and Gary, another thing that pops into my head, which may have actually some science behind it, would be any traumatic amputations done in a civilian environment or even in the military in a combat situation.
A person who has either an upper or lower extremity that is partially or completely severed or amputated, and the pain — God knows how bad it is — if not properly treated, it is going to be a very long recovery. That’s, I believe, another percutaneous condition where cryoneurolysis will be very beneficial to freeze those nerves, allowing patients to recover through rehab acute care, acute phases, rehabilitation, and move on with their lives.
Glatter: In the setting of a painful distal radius fracture, a femur fracture, and things of that nature, Gary, do you see this as a modality in conjunction with emergency medicine colleagues as being something that’s going to really become an important part of our armamentarium?
Schwartz: I do think it’s going to become more important in the future, as there are more studies to show what nerves you could block with cryoneurolysis in the longer term. I think you might see people start using these for fractures, especially for fractures that are not operable at the time or if a patient needs to be optimized prior to surgery.
As Sergey alluded to, it’s optimal in burns. People have been looking for relief of stump pain or postamputation pain. There’s a big researcher in Canada who’s been looking at pain with spasticity for people with cerebral palsy and poststroke issues, where they can’t move and they have pain moving an extremity after these conditions. We’re at just the tip of the iceberg as to where people are going to use this hand-held technology in the future.
Glatter: We use long-acting nerve blocks for hip fractures already in the emergency department. Why not employ this technique, which would have longer effects and limit opiate use?
Schwartz: It might even help a certain subset of the population, at least in Brooklyn, where we have a large elderly population. I believe it’s one of the oldest boroughs in the country, and definitely in New York.
There are some people that go on to surgery just because they might be bedbound, but it’s the pain that is dictating their surgical procedure, not that they’re ever going to walk again.
It’s maybe the next step to look for. If you could block this nerve for 3 months or longer, they’re still going to be bedbound, but maybe you could avoid a surgical procedure that carries its own morbidity and mortality, which I see a big interest in in the future.
Glatter: Absolutely. The idea behind treating spasticity is very important from an occupational therapy standpoint — eating, activities of daily living — just the basics.
Getting someone’s fingers released, being able to move their legs again, and getting them out of contracture states, I think, has a huge role.
Schwartz: Not only for the patient but also for the caregivers. For many of these patients, if they’re contracted fully and the pain from the spasticity is preventing their caregivers from moving them, it’s difficult to put on a shirt, pants, and so on.
One other point I’d like to make is that it’s reproducible. It’s not one-and-done. If the pain comes back from any of these conditions, you could treat again with another cryoneurolysis treatment. The current literature to date shows that it’s just as effective time and time again. I’ve seen clinically that you can repeat this procedure, whereas some of our other procedures that we do in medicine are not as reproducible, which is important for some of these chronic conditions.
Glatter: You had mentioned reimbursement earlier. Currently, this procedure is reimbursed under Medicare, Medicaid, and third-party payers, I assume?
Schwartz: Not all, but many commercial insurers. Yes for Medicare.
Final Takeaways
Glatter: Reimbursement has to be really universal because if this is shown to be more effective and limits opiate use, then there’s no question in my mind that this is such a groundbreaking procedure.
I’ll let you both give a few pearls for our audience to summarize our discussion.
Motov: I’d say it’s somewhat long overdue that this technique and pain-relieving modality should enter the emergency department, with the auspices and the beautiful collaborative effort between emergency department folks and interventional anesthesiologists, pain management specialists, collaborative training, and a collaborative goal of improving patients’ pain throughout the entire journey during the healthcare system.
That would be my only pearl. Just reach out to your colleagues within your respective institutions who you believe have aptitude, knowledge, and expertise. Reach out, get trained, and start passing down the knowledge to your faculty, and by virtue of extension, to your fellow residents and colleagues.
Schwartz: He took the words right out of my mouth. Communication and collaboration are the two most important things. There’s a shortage of physicians in this country. We can only each do so much, so we should each utilize and implement this technology to affect and help as many patients as possible.
We can decrease the amount of opiates, help our patients, help our family members in our community live with decreased pain, improve their function, and just get back to their lives and keep pushing the envelope of what’s the next step in treatment.
Again, like we went from giving opiates for this and that’s it — maybe an epidural, maybe a 5- to 6-hour intercostal nerve block — to fascial plane blocks like Sergey said, to more advanced procedures, to now we can give months of relief.
I think the communication, the collaboration, and the camaraderie among our different specialties are important to push the envelope to help our patients.
Glatter: That’s so well put. I completely agree.
I want to thank both of you for a very lively discussion. It was very informative. Your expertise is greatly appreciated and will certainly benefit our audience. Thank you both again.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. Dr. Schwartz is co-owner and primary clinic director at AABP Integrative Pain Care in Brooklyn, New York. Schwartz currently serves as the co-director of AABP Integrative Pain Care and Wellness and the vice chair of pain and anesthesiology for Maimonides Medical Center. Dr. Schwartz reported conflicts of interest with Pacira Biosciences and Dorsal Health; neither Dr. Glatter nor Dr. Motov reported relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today to discuss a novel way to treat pain related to conditions such as rib fractures and burns is Dr. Sergey Motov, an emergency physician with expertise in pain management and research director in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York.
Also joining me is Dr. Gary Schwartz, vice chair of pain and anesthesiology at Maimonides Medical Center. Dr. Schwartz is board certified in anesthesiology and interventional pain management.
Welcome, Sergey and Gary.
Sergey M. Motov, MD: Thank you, Robert.
Gary S. Schwartz, MD: Thank you, Robert.
Traditional Approaches to Pain Relief
Glatter: It’s a pleasure to have you both. Sergey, we were chatting earlier this week and you had mentioned a novel approach to treating a common condition we encounter in the emergency department — rib fractures.
As we all know, they’re very painful and can lead to pulmonary complications, including atelectasis, pneumonia due to splinting and lack of proper pain management, along with the use of incentive spirometry.
Sergey and Gary, can you describe traditional approaches to alleviating the pain associated with rib fractures? What do we typically use? Then we’ll get to some novel treatments that we’re here to discuss.
Motov: I’m going to use the emergency medicine approach to rib fractures. As you pointed out, pain relief is of utmost importance.
With the advent and acquiring of the amazing technique of interventional pain management, physicians, for the most part, are very astute about providing nerve blocks to alleviate pain, at least in immediate need. I’m talking about the relatively short term, 1-5 hours, in the emergency department.
Primarily, we focus on fascial plane blocks such as serratus anterior plane block. Traditionally, ED physicians don’t use much of the intercostal blocks. At times, we can direct the spinal block to cover the lateral aspect of the chest wall.
As part of the multimodal approach, we can use NSAIDs. If there’s a contraindication, we can use opioids. There are some data to support consideration of using topical formularies such as a lidocaine patch, but they are somewhat conflicting.
The question becomes what you’re going to send a patient home with. Again, traditional teaching is either opioids, immediate release with a short course, plus or minus NSAIDs, plus or minus acetaminophen.
The issue with rib fractures is that, while we can manage immediate and super-acute pain presentation in the ED and then discharge up to 24-72 hours, what happens afterwards is very challenging. Acute intercostal neuralgia related to traumatic rib fractures is semi-manageable, but if it’s inappropriately treated, it has a great tendency to transform into chronic intercostal neuralgia. It contributes a great deal of disability and morbidity.
Several years ago, I came across an entity called cryoneurolysis (cryo ─ cold temperature; neurolysis ─ freezing the nerve). I’m excited to be here today because Gary is the one who’s pioneering and championing this technique in our institution.
Cryoneurolysis: Mechanisms of Action and Benefits
Glatter: Gary, what do you see as the main role for this procedure at this time?
Schwartz: As Sergey alluded to, the traditional approach of opiates has side effects (ie, constipation, addiction, and tolerance). Unfortunately, many of these rib fractures occur in older patients. They come in anticoagulated, so they can’t have NSAIDs.
Sergey and his team in the ER have been pioneers in giving short-acting local anesthetic blocks that could last anywhere from 12 to 24 hours. There are long-acting local anesthetics that we can get out to 72 hours.
Unfortunately, these rib fractures and the pain associated with them, in addition to the intercostal neuralgia, could take weeks to heal. That’s where cryoneurolysis comes in. We’re all used to ice or cold temperature. For example, if your child gets an ear piercing, they put some ice on their earlobe beforehand, it numbs it up, and they don’t feel pain. It allows them to get their ears pierced without pain, but it’s short-acting.
What we have now are handheld devices with tips about as long as a pen, 3.5 inches, that allow you to go down precisely to these intercostal nerves that innervate the ribs and give a cold lesion that freezes these nerves.
The benefit of it is it’s not permanent like cryoablation, like we’ve seen for tumor necrosis, which destroys outside tissues. It’s really a small lesion, about 16 mm x 8 mm, which is enough to engulf the nerve and pretty much stun it.
It causes axonotmesis, but the epineurium, the endoneurium, and the perineurium — the inner workings of the nerve — stay intact, so it regrows. It just destroys the myelin sheath and the axon.
Glatter: You’re creating a scarring effect; is that what you’re saying? In other words, you’re doing a cold-temperature freeze and stunning the nerve. My question is, does it regrow? Is this a permanent type of injury?
Schwartz: With Wallerian degeneration, nerves do regrow after injuries.
Unfortunately, as you two probably see in the ER for big traumas, where the nerve is transected, those unfortunately do not grow back. This is considered a grade 2 lesion, so the Wallerian degeneration recurs. The nerves grow, depending on the literature you look at, about 0.5-2 mm per day.
This intervention gives us at least 3 months of relief for the patient, which is in the time frame where the rib fracture will heal, hopefully with no damage to the nerve from the fracture, and they go on living their life without having to take opiates or having to stop their anticoagulation.
Because prior to this, when I was a pain fellow, we used to put epidurals in many of these patients. The problem with that is patients can’t go home, and if they’re anticoagulated, you can’t place it because of the risk of a spinal hematoma.
Potential Use in Ventilation Weaning
Glatter: This is something we encounter daily, and certainly for those patients who have more numerous rib fractures or flail chest, this could be even more devastating, as well as for those who get intubated.
Do you see any role, in terms of ventilator weaning, in using this technique specifically in the ICU setting?
Schwartz: That’s an interesting concept. I’m not so sure about ventilator weaning, but we’ve used this in the hospital for rib fractures from traumas where patients had such severe fractures and had to go to the operating room for rib plating, and did necessitate an epidural. We’ve used this to discontinue their epidural and transition them to get the patient home.
I think that is part of the care, not only in the ER but in the hospital as well. We need to treat the patients, but we also have to have a transition plan to get them out of the hospital. Not that we don’t want to treat our patients, but we have to have a plan to get them home. I’m guessing that might be an interesting stage of research in the future if it does help with weaning from a ventilator.
Glatter: There are some studies out there suggesting that there can be some utility in terms of ventilator weaning using this technique. The ability of this to change how we manage pain is just incredible.
Sergey, do you feel that this is something that you could implement in your ED with your patients in the near future?
Motov: Definitely. I have personally been a very big proponent of it. I’m the theoreticist because I’ve covered a great deal of literature, and now having Gary and his team doing this in our institution, it’s a shame not to capitalize on it. I’m slowly moving toward figuring out the way of collaborative effort to have Gary and his team help my team and our colleagues, bring him on board, and maybe broaden the integration for pain management.
I believe, as Gary emphasized, that geriatric traumatic pain injuries are critically important due to the presence of comorbidities, potential drug interactions, and the challenges of managing these factors effectively.
There is one thing I want to bring up, and Gary, please support me on it. The procedure itself is fascinating because it provides long-term pain relief and reduces morbidity. I wouldn’t say mortality, just reduced morbidity. However, we need to be very conscious of the fact that this blockade, this ice-ball freezing of the nerve, can be detrimental to motor nerves. If your whole goal or idea of faster recovery after postoperative knee or hip replacements, or any traumatic lower- or upper-extremity surgery, includes blockade of motor nerves, it’s not going to be beneficial.
I believe the primary therapeutic application of this technology lies in targeting sensory nerves. For instance, intercostal nerves could be a focus in cases of rib fractures. Additionally, this approach shows promise for treating burns, particularly in the lower and upper extremities. Specifically, targeting nerves such as the lateral femoral cutaneous nerve or the anterior femoral cutaneous nerve could effectively neutralize pain and provide significant relief for weeks, if not months.
Based on additional predilection to what particular indications would be, maybe occipital headache with cervicalgia, occipital nerve block — it’s a sensory block — can benefit from it. Slowly but surely, there’s a slew of painful syndromes for which cryoneurolysis might have a great deal of use in the emergency department.
Cryoneurolysis for Other Pain Syndromes
Glatter: Gary, I’ll let you expand upon additional uses that you see. You did mention one on our chat earlier this week, which was postmastectomy pain syndrome with the intercostal brachial nerve. That’s a very compelling area of interest, certainly for the number of women that go through mastectomies or lumpectomies and that have axillary dissection or nerve injury.
Schwartz: Post-mastectomy is one way you could use this device and technology to attack painful syndromes, such as postmastectomy syndrome. Mastectomies are one of the most common surgeries performed in the United States, but I believe it’s a top three for post-op chronic pain, which we don’t normally think of.
There was a great study by a team in San Diego where they did intercostal brachial and intercostal nerve blocks on multiple nerves, and they decreased pain up to 3 months after the surgery and decreased opiates.
As Sergey alluded to, it’s approved for any peripheral nerve in the body. We’ve used it in our pain office for occipital neuralgia, postherpetic neuralgia, chronic rib pain after fractures, and surgery. Some of the most common uses are for superficial, sensory, genicular nerves, the lateral femoral cutaneous nerve, the anterior femoral cutaneous nerve, and the infrapatellar branch of the saphenous.
You could numb the skin preoperatively before a painful surgery, such as a total knee replacement — or as we like to call it, a total knee arthroplasty — to reduce opiates, improve function, and decrease length of stay. You could attack any sensory nerve.
We’ve utilized that already in our private practice. We’re trying to transition into the hospital to have everyone who gets a knee arthroplasty have this technology to decrease opiates, improve function, and recover faster.
This is quite interesting and motivating for me because when I first started, we had a femoral catheter to block the motor femoral nerve or an epidural. Patients were in the hospital for 3-5 days with the CPM [continuous passive motion] machine, which is like a medieval torture device that you might see in Mad Max — where you’re kind of moving the patient’s knee back and forth after surgery, and they were miserable, taking patient-controlled analgesia and high-dose opiates. Now, we’re freezing these nerves beforehand, doing our nerve blocks in the operating room with long-acting local anesthetic, and patients are going home the same day with minimal or even no opiates sometimes.
Implications for Patient Mobility and DVT Risk
Glatter: You’re getting up to 3 months of relief in that setting, doing it as you described?
Schwartz: Yes, up to 3 months of relief, which is huge, because most patients recovering from a knee arthroplasty, at about the 6- to 8-week mark, have improved range of motion, they have their 110° flexion, they have their extension, and they’re getting back to their normal life.
You cover the whole postoperative rehab, where patients don’t have to get recurring refills, they can participate in physical therapy. As you both know, part of the recovery process is to be able to interact with family and friends without being sleepy, angry, and in pain all day, so they can get back to their normal function.
Glatter: In terms of this procedure, would there be any increase in deep vein thrombosis (DVT) in relation to this, by chance?
Schwartz: Actually, there’s less of a risk of DVT because patients have less pain, so they can get up and move faster. Some of my surgical colleagues who have implemented this in their practice have gotten away from using the stronger anticoagulation like Xarelto (rivaroxaban) or Coumadin (warfarin), and they just give them baby aspirin postoperatively because their patients are going home the same day and walking. It’s probably safer for patients. There’s no research out there yet to show that, but we all know that the more you move and the more you’re not lying around, the lower the risk of having a DVT or a blood clot.
There are studies showing that there’s no damage to blood vessels, other than if you stick it with the needle, because the nitrogen gas in this that allows the ice ball to form does not get injected into the body. It’s all resorbed in the machine. The only thing the body sees is this ice ball, which would melt if you hit a blood vessel because we should be 98 °F and the ice ball is -88 °F. There’s no gas injected into the body either, so there’s no risk of a gas embolism.
Training and Implementation
Glatter: I was going to ask you about air emboli, and you perfectly led right into that.
In terms of training requirements, currently, what do you envision as a way we can train residents and fellows to do this? Is this currently something being considered in curriculum?
Schwartz: We are going to train our residents first. I’m training the attendings. Before you use this technology, you should have a basic understanding of ultrasound, how to use the device, the different settings, and what the risks are for each procedure you’re doing.
Let’s say, as Sergey alluded to, with an intercostal nerve block, you could have a pneumothorax. You have to be able to identify the rib, where the nerve should lie, the innermost intercostal muscle you could see on the newer ultrasounds, and where the pleura lies. People should start with just basic ultrasound training and then advance to a typical intercostal nerve block.
Once you master that, the procedure with the device is not much different than an intercostal nerve block, except you have a handheld device and the needle is just as long as a pen, 3.5 inches.
If you could do a nerve block with a spinal needle, you could do the procedure. Once people have the technical ultrasound skills, then they can advance to needle-based procedures, and once you have that training, you could use this procedure safely and efficaciously.
Glatter: Sergey, do you see this as requiring quite a bit of time and training in your program?
Motov: I mentioned earlier, before we started, that with the advent of ultrasound-guided nerve blocks, the vast majority of physicians are becoming very comfortable and fairly effective with maneuvering a needle and the ultrasound probe. The learning curve is essentially the same. The only difference is, as Gary pointed out, some of the nerves could be new to ED folks, but the technique, the understanding, the visualization, and the knowledge of anatomy are essentially the same.
As he pointed out, if you can use it with a spinal needle and local anesthetic, the procedure becomes exactly the same. It’s a slightly different drug and a different needle, and instead of local anesthetic, you’re using a gas at cold temperatures, and that’s pretty much it.
Glatter: Are there any other barriers to adoption in terms of cost, the device itself, or the companies that manufacture these handheld devices?
Schwartz: There’s always cost associated with the new device, needles, and the gas. Thankfully, they’re covered by Medicare, Medicaid, and most commercial insurances in the current framework, which I think is important. I think Congress is seeing the benefits of opiate sparing that Sergey helped lead in the ED.
At AABP Integrative Pain Care and Wellness and Maimonides, we’re doing this intraoperatively as well. I think the government is seeing that. There was a NOPAIN Act passed in 2023 that, starting January 1, 2025, will allow certain approved companies, devices, and medications to have to be repaid by CMS, Centers for Medicare & Medicaid Services, in the hospital setting and in the outpatient departments. In the inpatient surgical stays, we could have less opiates. I think that’s important. It is reimbursed now. Obviously, there is a cost associated.
The other benefit of this procedure and these techniques is, as Sergey alluded to, it’s done under ultrasound. The way we all learn procedures, whether it be central lines or chest tubes, is the blind technique. There is no good way to practice. In my interventional pain practice, many of our original techniques were done under fluoroscopy, and we don’t want to get extra radiation during practice.
The benefit of ultrasound and the advent of handheld ultrasound devices is that we can practice scanning and techniques on ourselves and on colleagues, without the fear of radiation. Other than the fact that we need to shower after the surgical lube is on from the scanning gel, you could practice your techniques in a safe way without harming a patient or yourself.
Future Directions in Pain Management Techniques
Glatter: Absolutely. Do you see any role for possibly stellate ganglion blocks, which are a bit riskier and have greater depth?
Schwartz: People are looking at different studies because, again, it’s a needle-based technology. We do many stellate ganglion blocks. I have not done it for this procedure yet, but that’s the next step of what I try. Under ultrasound, we could see the longus colli muscle and we could see the carotid artery. Obviously, we don’t see the ganglion per se, but anatomically, we know where it lies. You could drop a couple of lesions on there and give a theoretic prolonged sympathetic block, which might help with symptoms of complex regional pain syndrome.
I know there are some studies that have looked at stellate ganglion blocks for long-COVID symptoms. Unfortunately, it looks like we’re back in another wave right now. I think that’s the next step of the technology.
Glatter: Getting back to the emergency department, burns are something we see commonly — such painful conditions. This is something that could really provide significant relief, especially with burns that involve the chest wall, not just extremity burns.
Motov: I agree with you. Burns would be a very good indication to utilize this technique. Just listening to you and Gary, another thing that pops into my head, which may have actually some science behind it, would be any traumatic amputations done in a civilian environment or even in the military in a combat situation.
A person who has either an upper or lower extremity that is partially or completely severed or amputated, and the pain — God knows how bad it is — if not properly treated, it is going to be a very long recovery. That’s, I believe, another percutaneous condition where cryoneurolysis will be very beneficial to freeze those nerves, allowing patients to recover through rehab acute care, acute phases, rehabilitation, and move on with their lives.
Glatter: In the setting of a painful distal radius fracture, a femur fracture, and things of that nature, Gary, do you see this as a modality in conjunction with emergency medicine colleagues as being something that’s going to really become an important part of our armamentarium?
Schwartz: I do think it’s going to become more important in the future, as there are more studies to show what nerves you could block with cryoneurolysis in the longer term. I think you might see people start using these for fractures, especially for fractures that are not operable at the time or if a patient needs to be optimized prior to surgery.
As Sergey alluded to, it’s optimal in burns. People have been looking for relief of stump pain or postamputation pain. There’s a big researcher in Canada who’s been looking at pain with spasticity for people with cerebral palsy and poststroke issues, where they can’t move and they have pain moving an extremity after these conditions. We’re at just the tip of the iceberg as to where people are going to use this hand-held technology in the future.
Glatter: We use long-acting nerve blocks for hip fractures already in the emergency department. Why not employ this technique, which would have longer effects and limit opiate use?
Schwartz: It might even help a certain subset of the population, at least in Brooklyn, where we have a large elderly population. I believe it’s one of the oldest boroughs in the country, and definitely in New York.
There are some people that go on to surgery just because they might be bedbound, but it’s the pain that is dictating their surgical procedure, not that they’re ever going to walk again.
It’s maybe the next step to look for. If you could block this nerve for 3 months or longer, they’re still going to be bedbound, but maybe you could avoid a surgical procedure that carries its own morbidity and mortality, which I see a big interest in in the future.
Glatter: Absolutely. The idea behind treating spasticity is very important from an occupational therapy standpoint — eating, activities of daily living — just the basics.
Getting someone’s fingers released, being able to move their legs again, and getting them out of contracture states, I think, has a huge role.
Schwartz: Not only for the patient but also for the caregivers. For many of these patients, if they’re contracted fully and the pain from the spasticity is preventing their caregivers from moving them, it’s difficult to put on a shirt, pants, and so on.
One other point I’d like to make is that it’s reproducible. It’s not one-and-done. If the pain comes back from any of these conditions, you could treat again with another cryoneurolysis treatment. The current literature to date shows that it’s just as effective time and time again. I’ve seen clinically that you can repeat this procedure, whereas some of our other procedures that we do in medicine are not as reproducible, which is important for some of these chronic conditions.
Glatter: You had mentioned reimbursement earlier. Currently, this procedure is reimbursed under Medicare, Medicaid, and third-party payers, I assume?
Schwartz: Not all, but many commercial insurers. Yes for Medicare.
Final Takeaways
Glatter: Reimbursement has to be really universal because if this is shown to be more effective and limits opiate use, then there’s no question in my mind that this is such a groundbreaking procedure.
I’ll let you both give a few pearls for our audience to summarize our discussion.
Motov: I’d say it’s somewhat long overdue that this technique and pain-relieving modality should enter the emergency department, with the auspices and the beautiful collaborative effort between emergency department folks and interventional anesthesiologists, pain management specialists, collaborative training, and a collaborative goal of improving patients’ pain throughout the entire journey during the healthcare system.
That would be my only pearl. Just reach out to your colleagues within your respective institutions who you believe have aptitude, knowledge, and expertise. Reach out, get trained, and start passing down the knowledge to your faculty, and by virtue of extension, to your fellow residents and colleagues.
Schwartz: He took the words right out of my mouth. Communication and collaboration are the two most important things. There’s a shortage of physicians in this country. We can only each do so much, so we should each utilize and implement this technology to affect and help as many patients as possible.
We can decrease the amount of opiates, help our patients, help our family members in our community live with decreased pain, improve their function, and just get back to their lives and keep pushing the envelope of what’s the next step in treatment.
Again, like we went from giving opiates for this and that’s it — maybe an epidural, maybe a 5- to 6-hour intercostal nerve block — to fascial plane blocks like Sergey said, to more advanced procedures, to now we can give months of relief.
I think the communication, the collaboration, and the camaraderie among our different specialties are important to push the envelope to help our patients.
Glatter: That’s so well put. I completely agree.
I want to thank both of you for a very lively discussion. It was very informative. Your expertise is greatly appreciated and will certainly benefit our audience. Thank you both again.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. Dr. Schwartz is co-owner and primary clinic director at AABP Integrative Pain Care in Brooklyn, New York. Schwartz currently serves as the co-director of AABP Integrative Pain Care and Wellness and the vice chair of pain and anesthesiology for Maimonides Medical Center. Dr. Schwartz reported conflicts of interest with Pacira Biosciences and Dorsal Health; neither Dr. Glatter nor Dr. Motov reported relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Skin Stress Biomarker May Predict Nerve Damage in Early T2D
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
The Cause of All That Stress: Tonsillectomy?
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.
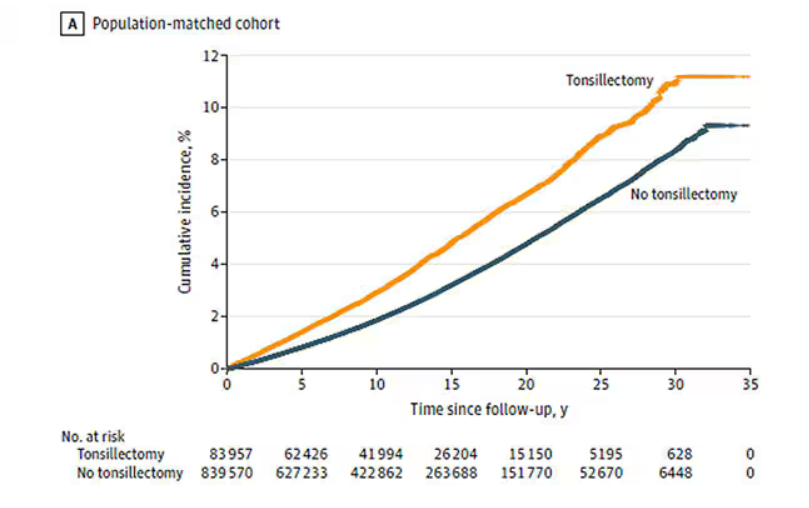
That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.
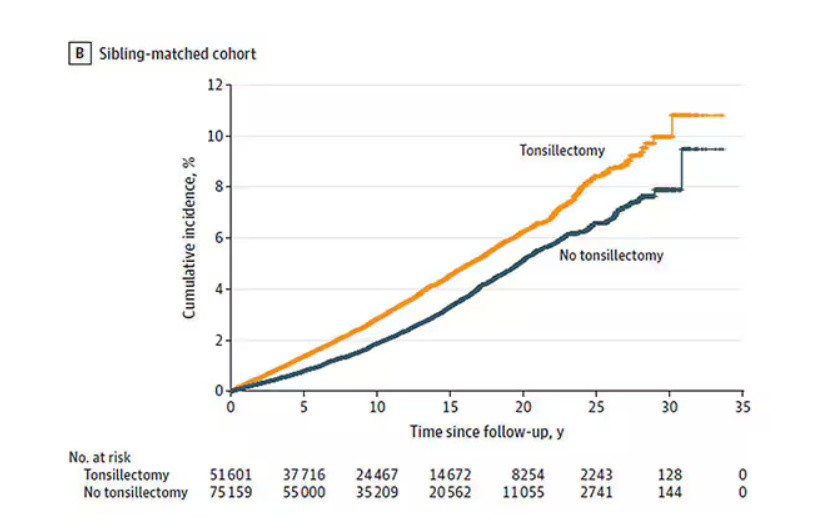
Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
You know those times in your life when you’re just feeling ... stressed? You’re on the edge; you have no chill; everything just sort of gets to you. If you can step away from the anxiety for a moment, you might ask yourself where it’s all coming from. Is it really the stuff in your inbox at work or is it money issues at home? Is it something with your relationship, or maybe it’s your sleep quality or your diet? One thing you probably won’t blame for those acute stress reactions is the tonsillectomy you had as a kid. But according to new research, maybe you should.
Tonsillectomy and adenoidectomy are among the most common surgical procedures young people in the United States undergo, with about 300,000 cases a year, according to recent numbers. That’s down a bit from numbers a decade or so ago, but suffice it to say, a good chunk of the population is walking around right now without their tonsils.
The data supporting tonsillectomy have never been great. The two big indications for the surgery are recurrent sore throat — data show that tonsillectomy reduces this by about 0.7 sore throats per year— and obstructive sleep apnea (OSA). The data for improvement of OSA are a bit better than the data for sore throats.
Also, tonsillectomy is a relatively quick, relatively well-reimbursed surgery with indications that are — let’s be honest — somewhat subjective, and so variation is high. One study found that in a single Vermont town, nearly 60% of the population had had their tonsils removed by the time they turned 18. A few towns over, the rate was 20%.
A few factors have led to the decline of tonsillectomy in recent years. Reimbursement rates have gone down a bit. Additionally, better data collection and statistical analysis have shown that the benefits of the procedure are relatively modest.
And then there is a body of medical literature that at first struck me as surprising and almost bizarre: data linking tonsillectomy to subsequent physical and psychiatric disorders.
I teach a course on interpretation of the medical literature, and one of the first things I teach my students is to check their gut when they see the conclusion of a study.
Basically, even before you read the data, have a sense in your own mind if the hypothesis seems reasonable. If a paper is going to conclude that smoking leads to increased risk for bone cancer, I’d say that seems like a reasonable thing to study. If a paper purports to show a link between eating poultry and bone cancer, I’m going to be reading it with quite a bit more skepticism.
The technical term for that process is assessing “biologic plausibility.” If we’re talking tonsils, we have to ask ourselves: Is it plausible that removing someone’s tonsils when they are young should lead to major problems in the future?
At first blush, it didn’t seem very plausible to me.
But the truth is, there are quite a few studies out there demonstrating links like this: links between tonsillectomy and irritable bowel syndrome; links between tonsillectomy and cancer; links between tonsillectomy and depression.
And this week, appearing in JAMA Network Open, is a study linking tonsillectomy with stress disorders.
Researchers leveraged Sweden’s health database, which contains longitudinal data on basically every person who has lived in Sweden since 1981. This database let them know who had a tonsillectomy or adenoidectomy, and when, and what happened to them later in life.
I think the best way to present these data is to show you what they found, and then challenge that finding, and then show you what they did in anticipation of the challenges we would have to their findings. It’s a pretty thorough study.
So, topline results here. The researchers first identified 83,957 individuals who had their tonsils removed. They matched each of them with 10 controls who did not have their tonsils removed but were the same age and sex.
Over around 30 years of follow-up, those people who had their tonsils removed were 43% more likely to develop a stress-related disorder. Among the specific disorders, the risk for PTSD was substantially higher: 55% higher in the tonsillectomy group.

That’s pretty surprising, but I bet you already want to push back against this. Sure, the control group was the same age and sex, but other factors might be different between the two groups. You’d be right to think so. People who got their tonsils out were more likely to have parents with a history of stress-related disorders and who had lower educational attainment. But the primary results were adjusted for those factors.
There’s more to a family than parental educational attainment, of course. To account for household factors that might be harder to measure, the researchers created a second control group, this one comprising the siblings of people who had their tonsils removed but who hadn’t themselves had their tonsils removed.
The relationship between tonsillectomy and stress disorders in this population was not quite as robust but still present: a 34% increase in any stress disorder and a 41% increase in the risk for PTSD.

Maybe kids who get their tonsils out are just followed more closely thereafter, so doctors might notice a stress disorder and document it in the medical record; whereas with other kids it might go unnoticed. This is known as ascertainment bias. The researchers addressed this in a sensitivity analysis where they excluded new diagnoses of stress disorders that occurred in the first 3 years after tonsillectomy. The results were largely unchanged.
So how do we explain these data? We observe a correlation between tonsillectomy in youth and stress disorders in later life. But correlation is not causation. One possibility, perhaps even the most likely possibility, is that tonsillectomy is a marker of some other problem. Maybe these kids are more prone to infections and are therefore more likely to need their tonsils removed. Then, after a lifetime of more infections than average, their stress responses are higher. Or maybe kids with a higher BMI are more likely to have their tonsils removed due to sleep apnea concerns, and it’s that elevated BMI that leads to higher stress in later life.
Or maybe this is causal. Maybe there actually is biological plausibility here. The authors suggest that removal of tonsils might lead to broader changes in the immune system; after all, tonsillar tissue is on the front line of our defense against pathogens that might enter our bodies through our mouths or noses. Immunologic changes lead to greater inflammation over time, and there is decent evidence to link chronic inflammation to a variety of physical and psychological disorders.
In support of this, the authors show that the kids with tonsillectomy were more likely to be hospitalized for an infectious disease in the future as well, in magnitudes similar to the increased risk for stress. But they don’t actually show that the relationship between tonsillectomy and stress is mediated by that increased risk for infectious disease.
In the end, I find these data really intriguing. Before I dug into the literature, it seemed highly unlikely that removal of these small lumps of tissue would have much of an effect on anything. Now I’m not so sure. A few things can be removed from the human body without any consequences, but it can be hard to know exactly what those consequences are.
That said, given the rather marginal benefits of tonsillectomy and the growing number of studies expanding on the risks, I expect that we’ll see the rates of the surgery decline even further in the future.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Connecticut. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.