User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
The Importance of Service Learning in Dermatology Residency: An Actionable Approach to Improve Resident Education and Skin Health Equity
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
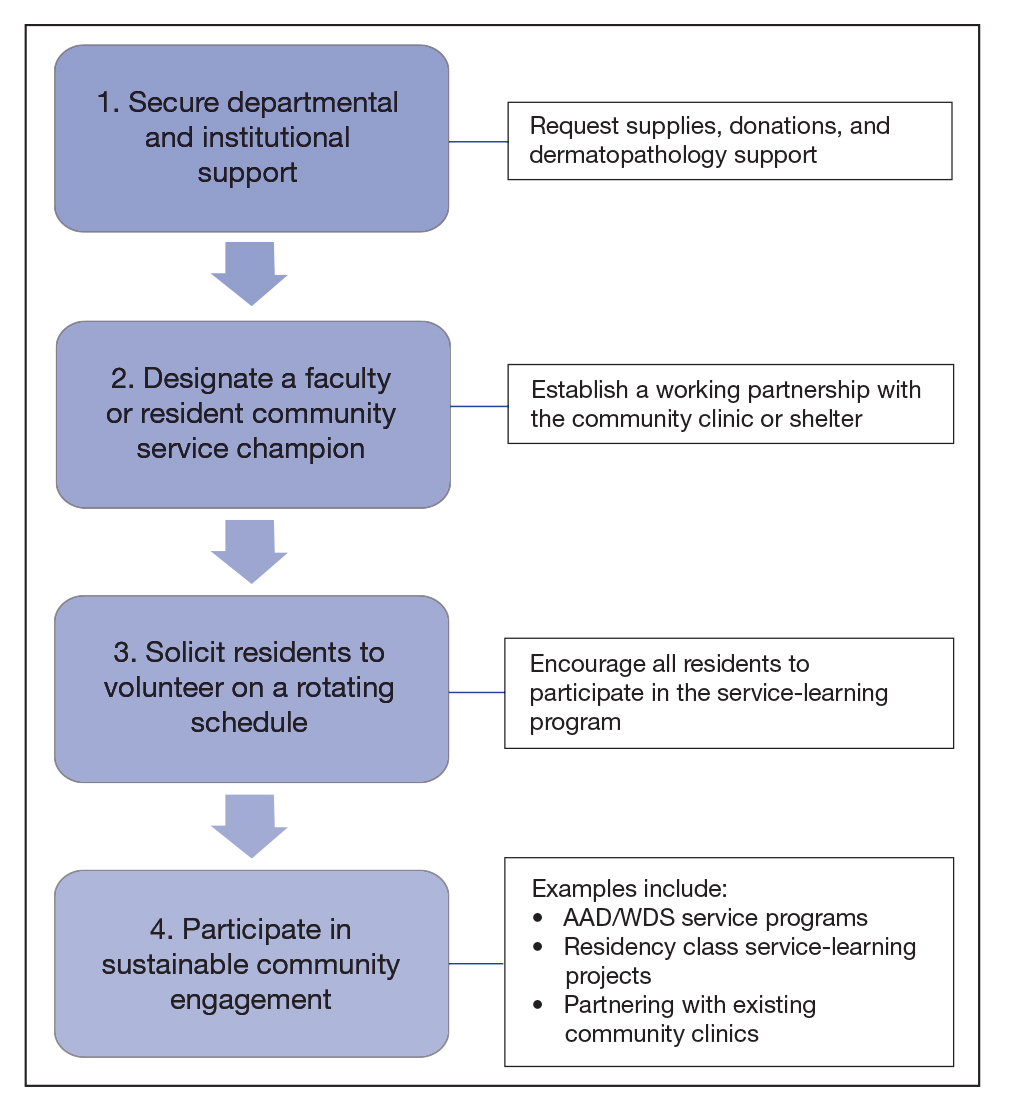
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
Access to specialty care such as dermatology is a challenge for patients living in underserved communities.1 In 2019, there were 29.6 million individuals without health insurance in the United States—9.2% of the population—up from 28.6 million the prior year.2 Furthermore, Black and Hispanic patients, American Indian and Alaskan Natives, and Native Hawaiian and other Pacific Islanders are more likely to be uninsured than their White counterparts.3 Community service activities such as free skin cancer screenings, partnerships with community practices, and teledermatology consultations through free clinics are instrumental in mitigating health care disparities and improving access to dermatologic care. In this article, we build on existing models from dermatology residency programs across the country to propose actionable methods to expand service-learning opportunities in dermatology residency training and increase health care equity in dermatology.
Why Service Learning?
Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive scholastic experience and meet societal needs.4 In pilot studies of family medicine residents, service-learning initiatives enhanced the standard residency curriculum by promoting clinical practice resourcefulness.5 Dermatology Accreditation Council for Graduate Medical Education requirements mandate that residents demonstrate an awareness of the larger context of health care, including social determinants of health.6 Likewise, dermatology residents must recognize the impact of socioeconomic status on health care utilization, treatment options, and patient adherence. With this understanding, residents can advocate for quality patient care and improve community-based health care systems.6
Service-learning projects can effectively meet the specific health needs of a community. In a service-learning environment, residents will understand a community-based health care approach and work with attending physician role models who exhibit a community service ethic.7 Residents also can gain interprofessional experience through collaborating with a team of social workers, community health workers, care coordinators, pharmacists, nurses, medical students, and attending physicians. Furthermore, residents can practice communicating effectively with patients and families across a range of socioeconomic and cultural backgrounds. Interprofessional, team-based care and interpersonal skill acquisition are both Accreditation Council for Graduate Medical Education requirements for dermatology training.6 Through increased service-learning opportunities, dermatology trainees will learn to recognize and mitigate social determinants of health with a holistic, patient-centered treatment plan.
Free or low-cost medical clinics provide health care to more than 15 million Americans, many of whom identify with marginalized racial and ethnic groups.8 In a dermatology access study, a sample of clinics listed in the National Association of Free and Charitable Clinics database were contacted regarding the availability of dermatologic care; however, more than half of the sites were unresponsive or closed, and the remaining clinics offered limited access to dermatology services.9 The scarcity of free and low-cost dermatologic services likely contributes to adverse skin health outcomes for patients in underserved communities.10 By increasing service learning within dermatology residency training programs, access to dermatologic care will improve for underserved and uninsured populations.
Actionable Methods to Increase Service Learning in Dermatology Residency Training Programs
Utilize Programming Offered Through National Dermatology Associations and Societies
The American Academy of Dermatology (AAD) has developed programming through which faculty, residents, and private practice dermatologists perform community service targeting underserved populations. SPOT me , a skin cancer screening program, is the AAD’s longest-standing public health program through which it provides complimentary screening forms, handouts, and advertisements to facilitate skin cancer screening. AccessDerm is the AAD’s philanthropic teledermatology program that delivers dermatologic care to underserved communities. Camp Discovery and the Shade Structure Grant Program are additional initiatives promoted by the AAD to support volunteer services for communities while learning about dermatology. Residents may apply for AAD grants to subsidize participation in the Native American Health Service Resident Rotation Program, the Skin Care for Developing Countries program, or an international grant.
The Women’s Dermatologic Society hosts 3 primary umbrella community outreach initiatives: Play Safe in the Sun, Coast-2-Coast, and the Transforming Interconnecting Project Program Women’s Shelter Initiative. From uplifting and educating individuals in women’s shelters about skin care, oral hygiene, self-care, nutrition, and social skills to providing complimentary skin cancer screenings, the Women’s Dermatologic Society provides easily accessible tool kits and syllabi to facilitate project composition and completion by its members.
Implement Residency Class Service-Learning Projects
Incoming dermatology residents are regularly encouraged to draft research proposals at the beginning of each academic year. Encouraging residency classes to work collectively on a dermatology service-learning project likely will increase resident camaraderie and project success while minimizing internal competition. In developing a service-learning proposal, residents should engage with community leaders and groups to best understand how to meet the skin health needs of underserved communities. The project should have clear objectives, benchmarks, and full support of the dermatology department. Short-term service-learning projects are completed when set goals are achieved, while sustainable projects continue with each new resident class.
Partner With Existing Community or Federally Funded Clinics
Establishing partnerships with free or federally funded health centers is a reliable way to increase service-learning opportunities in dermatology residency training. Personal malpractice carriers often include free clinic coverage, and most states offer limited liability or immunity for physicians who volunteer their professional services or subsidize malpractice insurance purchases.11 In light of the global coronavirus disease 2019 pandemic, teledermatology options should be explored alongside in-person services. Although logistics may vary based on institutional preference, the following are our recommendations for building community partnerships for dermatology service learning (Figure):
• Secure departmental and institutional support. This includes requesting supplies, donations, and dermatopathology support
• Designate a resident or faculty community service champion to lead clinic correspondence and oversee operative logistics. This individual will establish a working partnership with the community clinic, assess the needs of the patient population, and manage the clinic schedule. The champion also will initiate and maintain open lines of communication with community providers for continuity of care. This partnership with community providers allows for shared resources and mutual learning
• Solicit residents to volunteer on a rotating schedule. Although some residents are fully committed to community service and health care justice, all residents need to participate in the service-learning program
• Participate in sustainable community engagement on a schedule that suits the needs of the community and takes into consideration resident and attending availability
Final Thoughts
Service learning in dermatology residency training is essential to improve access to equitable dermatologic care and train clinically competent dermatologists who have experience practicing in resource-limited settings. Service learning places cultural awareness and an understanding of socioeconomic determinants of health at the forefront.12 Some dermatology residency programs treat a high percentage of medically underserved patients; others have integrated service learning into dermatology rotations, and a few programs offer community engagement–focused residency tracks.13-16 Each dermatology program should evaluate its workforce, resources, and nearby underserved communities to strategically develop a program-specific service-learning program. Service-learning clinics often are the sole means by which patients from underserved communities receive dermatologic care.17 A commitment to service learning in dermatology residency programs will improve skin health equity and improve dermatology residency education.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Cook NL, Hicks LS, O’Malley J, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459-1468.
- Broaddus M, Aron-Dine A. Uninsured rate rose again in 2019, further eroding earlier progress. Center on Budget and Policy Priorities website. Published September 15, 2020. Accessed February 9, 2021. https://www.cbpp.org/research/health/uninsured-rate-rose-again-in-2019-further-eroding-earlier-progress
- Artiga S, Orgera K, Damico A. Changes in health coverage by race and ethnicity since the ACA, 2010-2018. Kaiser Family Foundation website. Published March 5, 2020. Accessed February 9, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/changes-in-health-coverage-by-race-and-ethnicity-since-the-aca-2010-2018/
- Martinez MG. H.R.2010 - 103rd Congress (1993-1994): National and Community Service Trust Act of 1993. AmeriCorps website. Accessed November 24, 2020. https://www.congress.gov/bill/103rd-congress/house-bill/2010
- Gefter L, Merrell SB, Rosas LG, et al. Service-based learning for residents: a success for communities and medical education. Fam Med. 2015;47:803-806.
- ACGME Program Requirements for Graduate Medical Education in Dermatology. Accreditation Council for Graduate Medical Education website. Updated July 1, 2020. Accessed February 9, 2021. https://acgme.org/Portals/0/PFAssets/ProgramRequirements/080_Dermatology_2020.pdf?ver=2020-06-29-161626-133
- 7. Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946.
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246.
- Shi L, Stevens GD. Vulnerability and unmet health care needs: the influence of multiple risk factors. J Gen Intern Med. 2005;20:148-154.
- Benrud L, Darrah J, Johnson A. Liability considerations for physician volunteers in the US. Virtual Mentor. 2010;12:207-212.
- Service-learning plays vital role in understanding social determinants of health. AAMC website. Published September 27, 2016. Accessed February 22, 2021. https://www.aamc.org/news-insights/service-learning-plays-vital-role-understanding-social-determinants-health
- Sheu J, Gonzalez E, Gaeta JM, et al. Boston Health Care for the Homeless Program–Harvard Dermatology collaboration: a service-learning model providing care for an underserved population. J Grad Med Educ. 2014;6:789-790.
- Ojeda VD, Romero L, Ortiz A. A model for sustainable laser tattoo removal services for adult probationers. Int J Prison Health. 2019;15:308-315.
- Diversity & Community Track (Dermatology Diversity and Community Engagement residency position). Penn Medicine Dermatology website. Accessed February 9, 2021. https://dermatology.upenn.edu/residents/diversity-community-track/
- Duke Dermatology Diversity and Community Engagement residency position (1529080A2). Duke Dermatology website. Accessed February 9, 2021. https://dermatology.duke.edu/node/4742
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
Practice Points
- In 2019, nearly 30 million Americans did not have health insurance. Dermatologists in the United States should be cognizant of the challenges faced by underserved patients when accessing dermatologic care.
- Service learning is an educational approach that combines learning objectives with community service to provide a comprehensive learning experience, meet societal needs, and fulfill Accreditation Council for Graduate Medical Education requirements.
- Actionable methods to increase service learning in dermatology residency training include volunteering in community service programs offered by national dermatology organizations, implementing service-learning projects, and partnering with free and federally funded community practices.
- Dermatology residents who participate in service learning will help increase access to equitable dermatologic care and experience practicing in settings with limited resources.
Onychomycosis: New Developments in Diagnosis, Treatment, and Antifungal Medication Safety
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
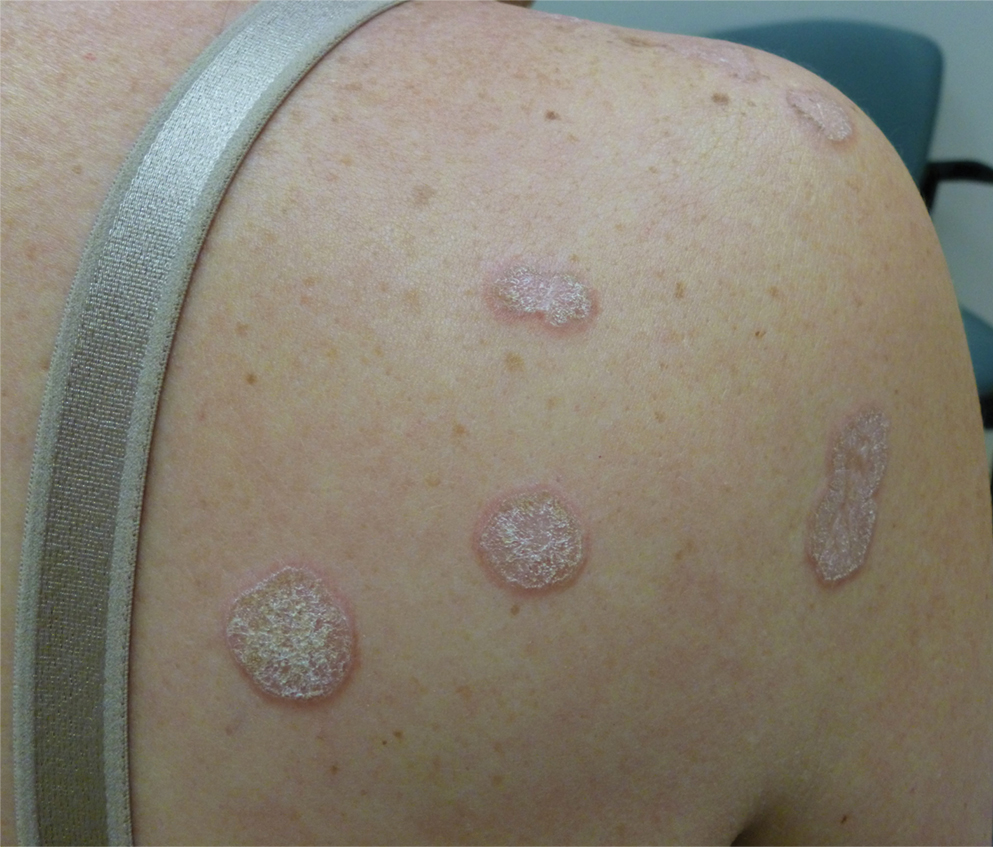
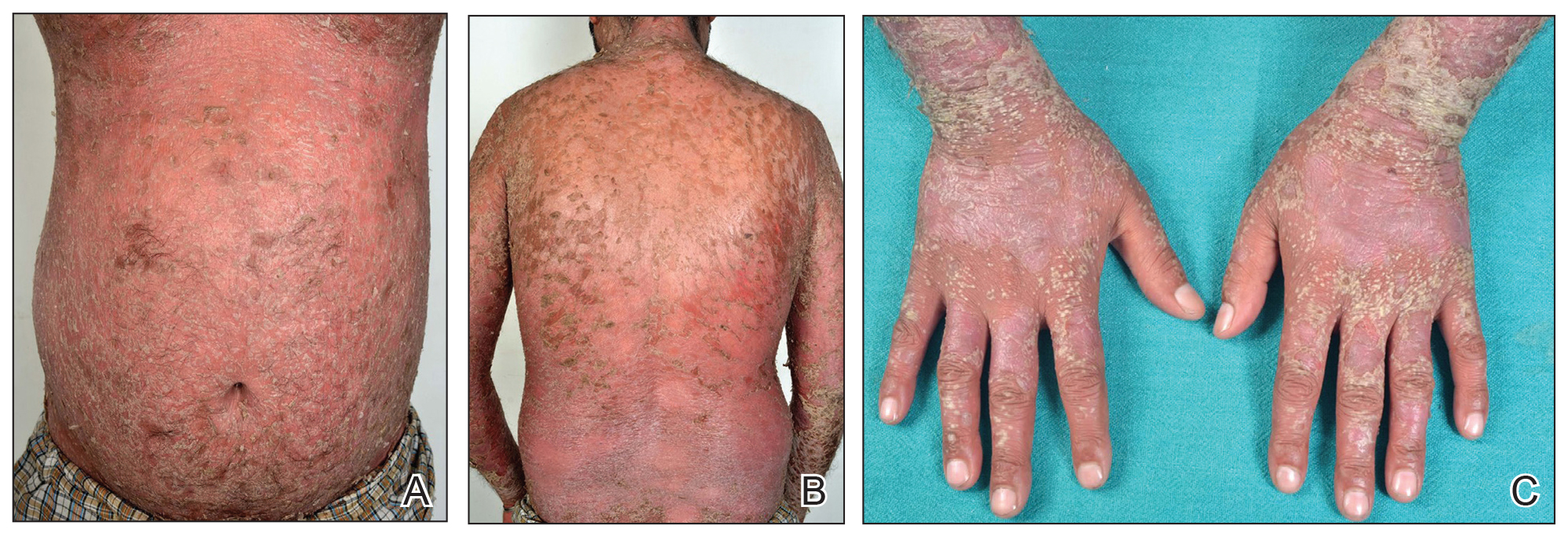
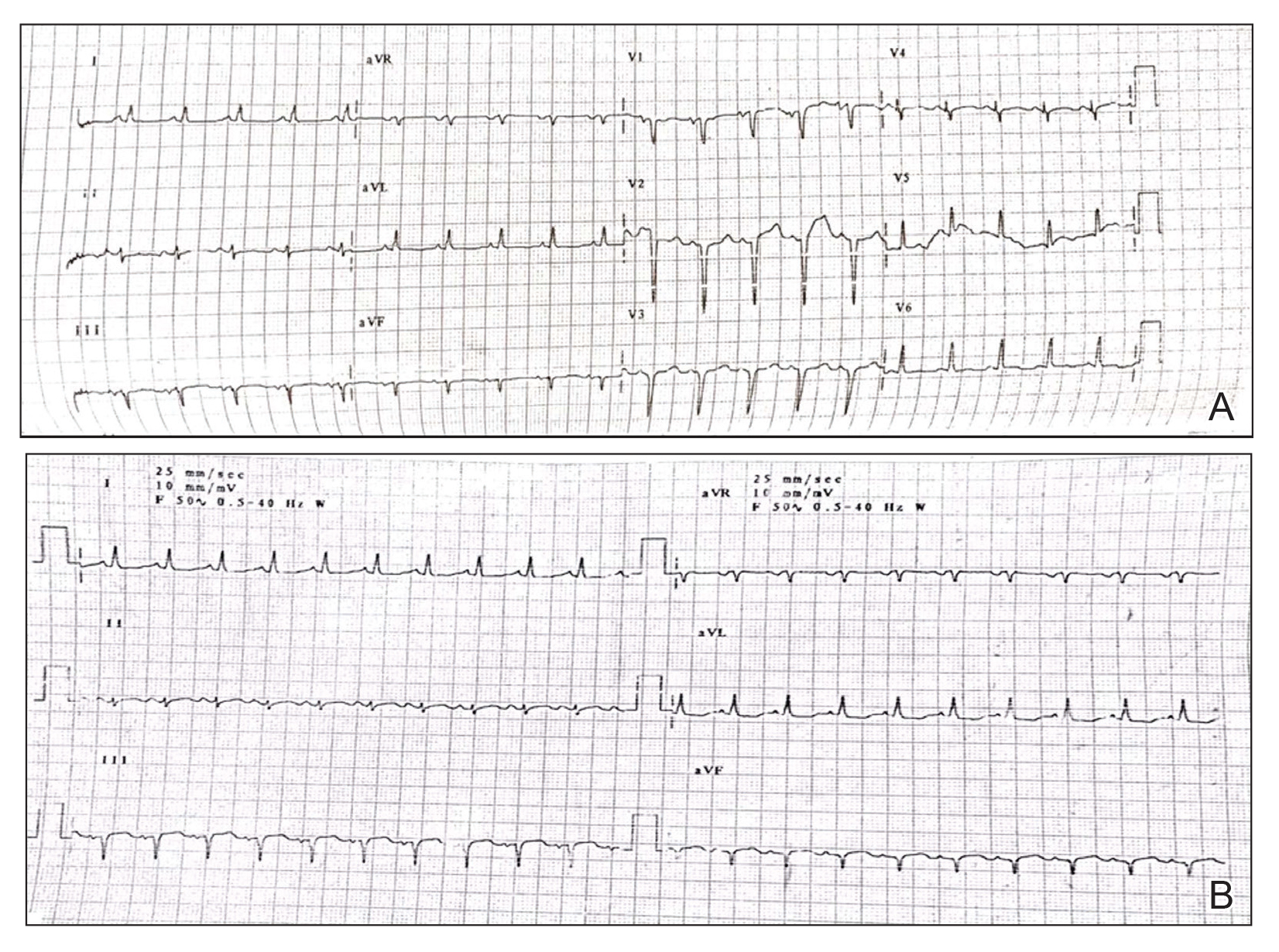
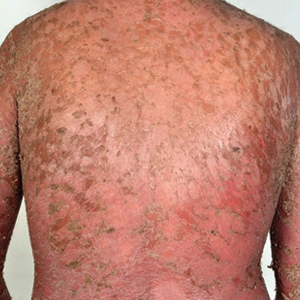
Hyperkeratotic Nummular Plaques on the Upper Trunk
The Diagnosis: Extragenital Lichen Sclerosus Et Atrophicus
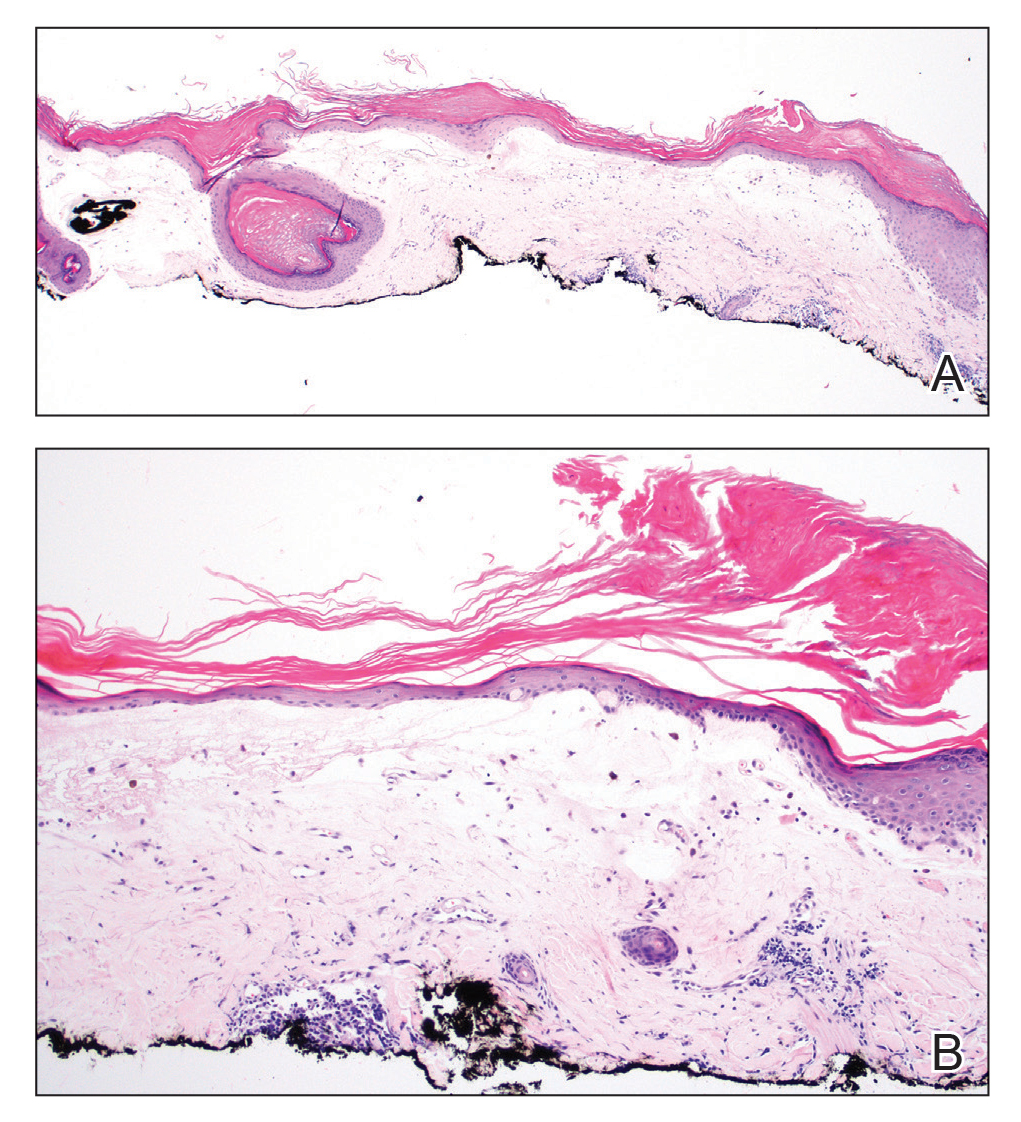
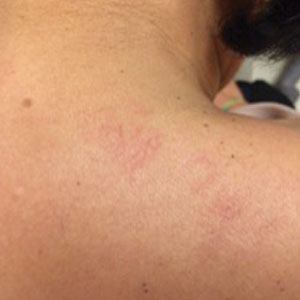
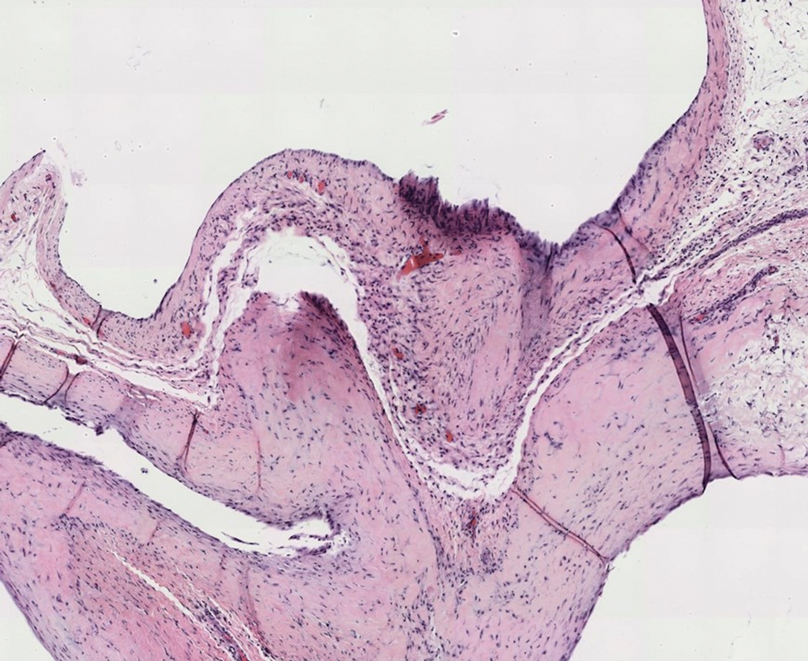
Histopathologic evaluation revealed hyperkeratosis, follicular plugging, epidermal atrophy, and homogenization of papillary dermal collagen with an underlying lymphocytic infiltrate (Figure 1). Direct immunofluorescence of a plaque with a superimposed bulla was negative for deposition of C3, IgG, IgA, IgM, or fibrinogen. Accordingly, clinicopathologic correlation supported a diagnosis of extragenital lichen sclerosus et atrophicus (LSA). Of note, the patient's history of genital irritation was due to genital LSA that preceded the extragenital manifestations.
Lichen sclerosus et atrophicus is an inflammatory dermatosis that typically presents as atrophic white papules of the anogenital area that coalesce into pruritic plaques; the exact etiology remains to be elucidated, yet various circulating autoantibodies have been identified, suggesting a role for autoimmunity.1,2 Lichen sclerosus et atrophicus is more common in women than in men, with a bimodal peak in the age of onset affecting postmenopausal and prepubertal populations.1 In women, affected areas include the labia minora and majora, clitoris, perineum, and perianal skin; LSA spares the mucosal surfaces of the vagina and cervix.2 In men, uncircumscribed genital skin more commonly is affected. Involvement is localized to the foreskin and glans with occasional urethral involvement.2
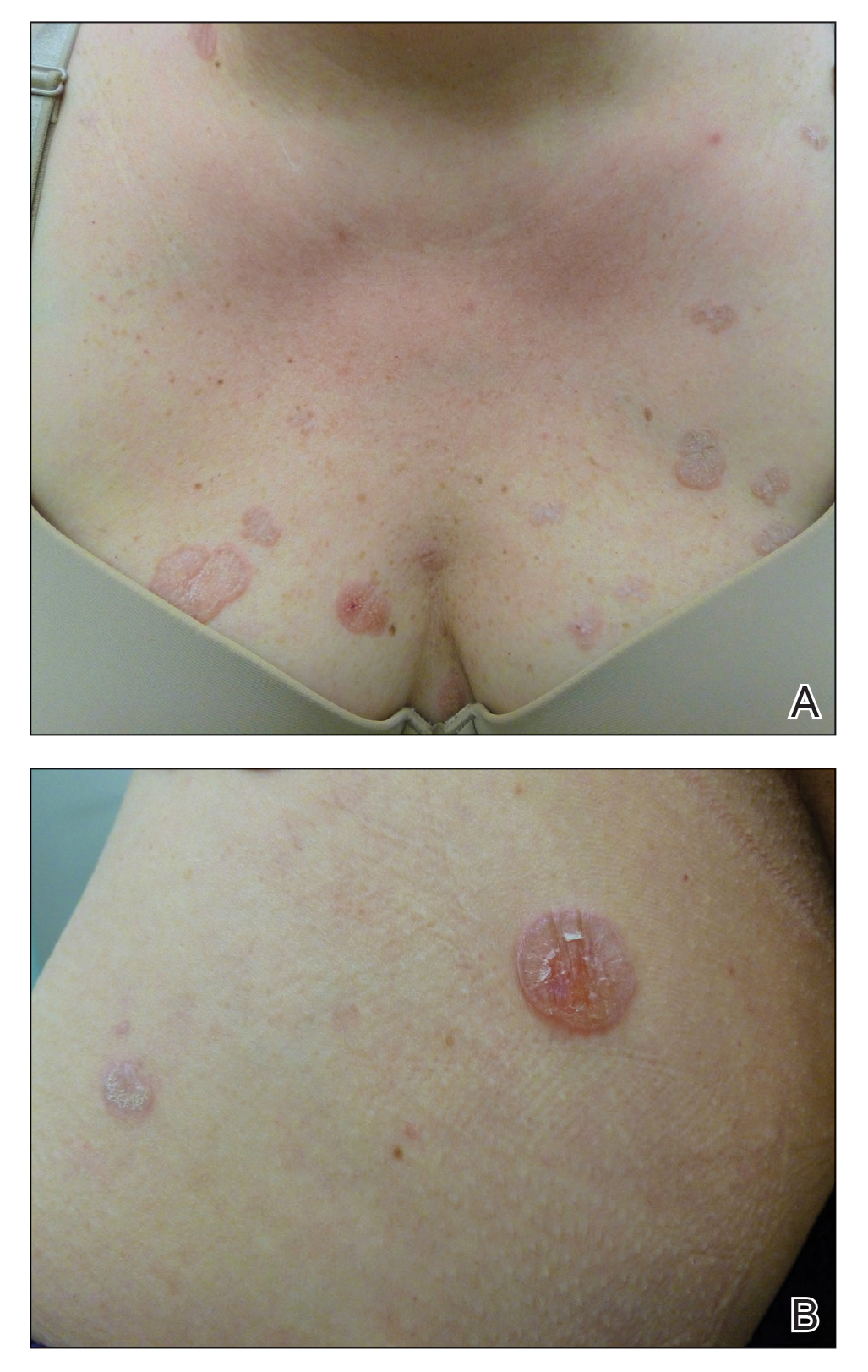
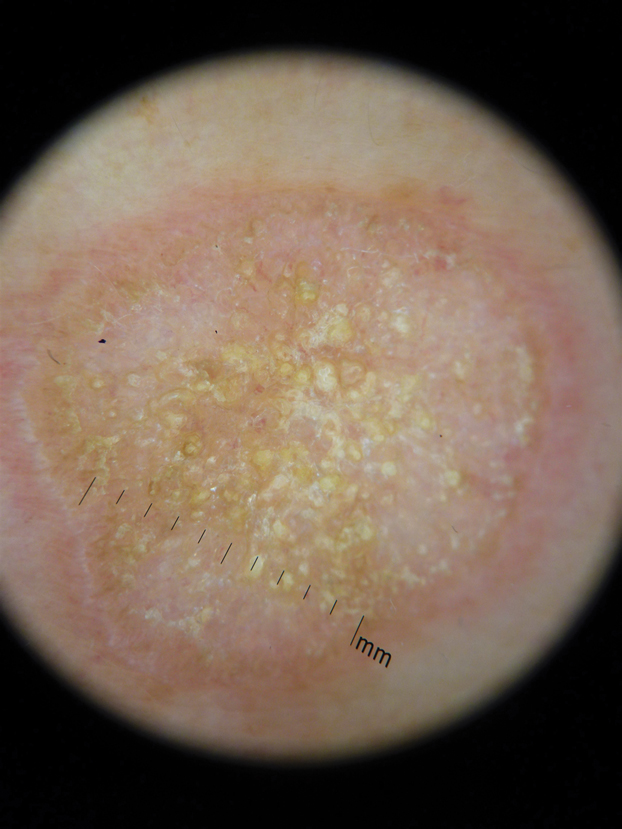
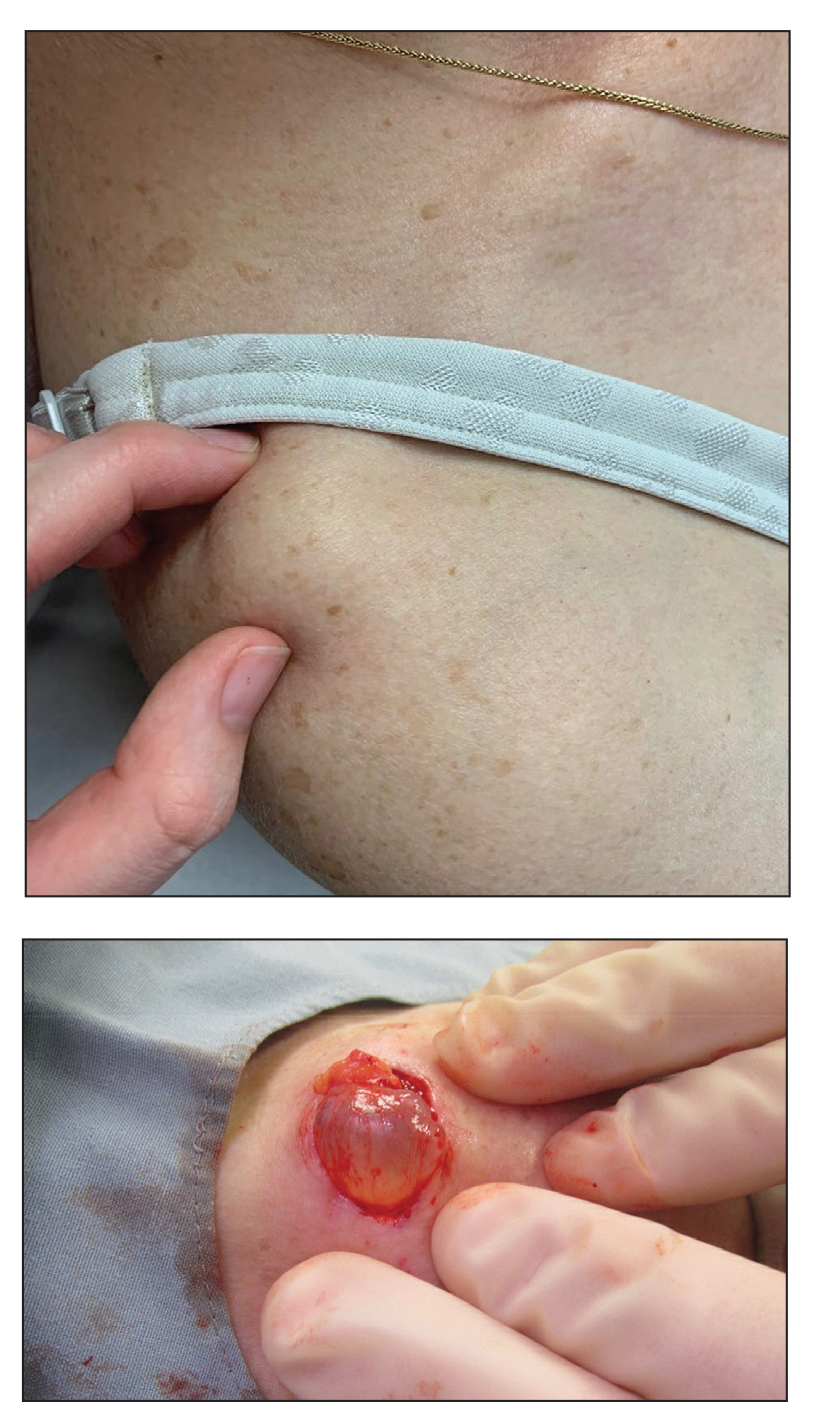
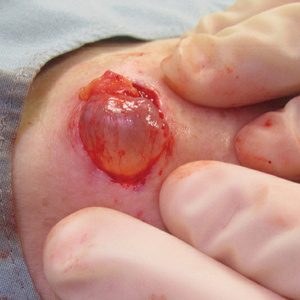
In contrast, extragenital LSA tends to present as asymptomatic papules and plaques that develop atrophy with time, involving the back, shoulders, neck, chest, thighs, axillae, and flexural wrists2,3; an erythematous rim often is present,4 and hyperkeratosis with follicular plugging may be prominent.5 Our patient's case emphasizes the predilection of plaques for the chest and intermammary skin (Figure 2A). Approximately 15% of LSA cases have extragenital involvement, and extragenital-limited disease accounts for roughly 5% of cases.6,7 Unlike genital LSA, extragenital disease has not been associated with an increased risk for squamous cell carcinoma.1 Bullae formation within plaques of genital or extragenital LSA has been reported3,8 and is exemplified in our patient (Figure 2B). Intralesional bullae formation likely is due to a combination of internal and external factors, mainly the inability to withstand shear forces due to an atrophic epidermis with basal vacuolar injury overlying an edematous papillary dermis with altered collagen.8 Dermatoscopic findings may aid in recognizing extragenital LSA9,10; our patient's plaques demonstrated the characteristic findings of comedolike openings, structureless white areas, and pink borders (Figure 3).
The clinical differential diagnosis for well-demarcated, pink, scaly plaques is broad. Nummular eczema usually presents as coin-shaped eczematous plaques on the dorsal aspects of the hands or lower extremities, and histology shows epidermal spongiosis.11 Nummular eczema may be considered due to the striking round morphology of various plaques, yet our patient's presentation was better served by a consideration of several papulosquamous disorders.
Lichen planus (LP) presents as intensely pruritic, violaceous, polygonal, flat-topped papules with overlying reticular white lines, or Wickham striae, that favor the flexural wrists, lower back, and lower extremities. Lichen planus also may have oral and genital mucosal involvement. Similar to LSA, LP is more common in women and preferentially affects the postmenopausal population.12 Additionally, hypertrophic LP may obscure Wickham striae and mimic extragenital LSA; distinguishing features of hypertrophic LP are intense pruritus and a predilection for the shins. Histology is defined by orthohyperkeratosis, hypergranulosis, sawtooth acanthosis, and vacuolar degeneration of the basal layer with Civatte bodies or dyskeratotic basal keratinocytes overlying a characteristic bandlike infiltrate of lymphocytes.12
Lichen simplex chronicus (LSC) is characterized by intense pruritus and presents as hyperkeratotic plaques with a predilection for accessible regions such as the posterior neck and extremities.13 The striking annular demarcation of this case makes LSC unlikely. Comparable to LSA and LP, LSC also may present with both genital and extragenital findings. Histology of LSC is characterized by irregular acanthosis or thickening of the epidermis with vertical streaking of collagen and vascular bundles of the papillary dermis.13
Subacute cutaneous lupus erythematosus (SCLE) is important to consider for a new papulosquamous eruption with a predilection for the sun-exposed skin of a middle-aged woman. The presence of papules on the volar wrist and history of genital irritation, however, make this entity less likely. Similar to LSA, histologic examination of SCLE reveals epidermal atrophy, basal layer degeneration, and papillary dermal edema with lymphocytic inflammation. However, SCLE lacks the band of inflammation underlying pale homogenized papillary dermal collagen, the most distinguishing feature of LSA; instead, SCLE shows superficial and deep perivascular and periadnexal lymphocytes and mucin in the dermis.14
Lichen sclerosus et atrophicus may be chronic and progressive in nature or cycle through remissions and relapses.2 Treatment is not curative, and management is directed to alleviating symptoms and preventing the progression of disease. First-line management of extragenital LSA is potent topical steroids.1 Adjuvant topical calcineurin inhibitors may be used as steroid-sparing agents.2 Phototherapy is a second-line therapy and even narrowband UVB phototherapy has demonstrated efficacy in managing extragenital LSA.15,16 Our patient was started on mometasone ointment and calcipotriene cream with slight improvement after a 6-month trial. Ongoing management is focused on optimizing application of topical therapies.
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus. Am J Clin Dermatol. 2013;14:27-47.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Surkan M, Hull P. A case of lichen sclerosus et atrophicus with distinct erythematous borders. J Cutan Med Surg. 2015;19:600-603.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41-46.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Hallel-Halevy D, Grunwald MH, Yerushalmi J, et al. Bullous lichen sclerosus et atrophicus. J Am Acad Dermatol. 1998;39:500-501.
- Garrido-Ríos AA, Álvarez-Garrido H, Sanz-Muñoz C, et al. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468.
- Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433-1439.
- Rudikoff D. Differential diagnosis of round or discoid lesions. Clin Dermatol. 2011;29:489-497.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Shaffer B, Beerman H. Lichen simplex chronicus and its variants: a discussion of certain psychodynamic mechanisms and clinical and histopathologic correlations. AMA Arch Derm Syphilol. 1951;64:340-351.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. Am J Clin Dermatol. 2009;10:365-381.
- Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71:981-984.
- Colbert RL, Chiang MP, Carlin CS, et al. Progressive extragenital lichen sclerosus successfully treated with narrowband UV-B phototherapy. Arch Dermatol. 2007;143:19-20.
The Diagnosis: Extragenital Lichen Sclerosus Et Atrophicus
Histopathologic evaluation revealed hyperkeratosis, follicular plugging, epidermal atrophy, and homogenization of papillary dermal collagen with an underlying lymphocytic infiltrate (Figure 1). Direct immunofluorescence of a plaque with a superimposed bulla was negative for deposition of C3, IgG, IgA, IgM, or fibrinogen. Accordingly, clinicopathologic correlation supported a diagnosis of extragenital lichen sclerosus et atrophicus (LSA). Of note, the patient's history of genital irritation was due to genital LSA that preceded the extragenital manifestations.
Lichen sclerosus et atrophicus is an inflammatory dermatosis that typically presents as atrophic white papules of the anogenital area that coalesce into pruritic plaques; the exact etiology remains to be elucidated, yet various circulating autoantibodies have been identified, suggesting a role for autoimmunity.1,2 Lichen sclerosus et atrophicus is more common in women than in men, with a bimodal peak in the age of onset affecting postmenopausal and prepubertal populations.1 In women, affected areas include the labia minora and majora, clitoris, perineum, and perianal skin; LSA spares the mucosal surfaces of the vagina and cervix.2 In men, uncircumscribed genital skin more commonly is affected. Involvement is localized to the foreskin and glans with occasional urethral involvement.2
In contrast, extragenital LSA tends to present as asymptomatic papules and plaques that develop atrophy with time, involving the back, shoulders, neck, chest, thighs, axillae, and flexural wrists2,3; an erythematous rim often is present,4 and hyperkeratosis with follicular plugging may be prominent.5 Our patient's case emphasizes the predilection of plaques for the chest and intermammary skin (Figure 2A). Approximately 15% of LSA cases have extragenital involvement, and extragenital-limited disease accounts for roughly 5% of cases.6,7 Unlike genital LSA, extragenital disease has not been associated with an increased risk for squamous cell carcinoma.1 Bullae formation within plaques of genital or extragenital LSA has been reported3,8 and is exemplified in our patient (Figure 2B). Intralesional bullae formation likely is due to a combination of internal and external factors, mainly the inability to withstand shear forces due to an atrophic epidermis with basal vacuolar injury overlying an edematous papillary dermis with altered collagen.8 Dermatoscopic findings may aid in recognizing extragenital LSA9,10; our patient's plaques demonstrated the characteristic findings of comedolike openings, structureless white areas, and pink borders (Figure 3).
The clinical differential diagnosis for well-demarcated, pink, scaly plaques is broad. Nummular eczema usually presents as coin-shaped eczematous plaques on the dorsal aspects of the hands or lower extremities, and histology shows epidermal spongiosis.11 Nummular eczema may be considered due to the striking round morphology of various plaques, yet our patient's presentation was better served by a consideration of several papulosquamous disorders.
Lichen planus (LP) presents as intensely pruritic, violaceous, polygonal, flat-topped papules with overlying reticular white lines, or Wickham striae, that favor the flexural wrists, lower back, and lower extremities. Lichen planus also may have oral and genital mucosal involvement. Similar to LSA, LP is more common in women and preferentially affects the postmenopausal population.12 Additionally, hypertrophic LP may obscure Wickham striae and mimic extragenital LSA; distinguishing features of hypertrophic LP are intense pruritus and a predilection for the shins. Histology is defined by orthohyperkeratosis, hypergranulosis, sawtooth acanthosis, and vacuolar degeneration of the basal layer with Civatte bodies or dyskeratotic basal keratinocytes overlying a characteristic bandlike infiltrate of lymphocytes.12
Lichen simplex chronicus (LSC) is characterized by intense pruritus and presents as hyperkeratotic plaques with a predilection for accessible regions such as the posterior neck and extremities.13 The striking annular demarcation of this case makes LSC unlikely. Comparable to LSA and LP, LSC also may present with both genital and extragenital findings. Histology of LSC is characterized by irregular acanthosis or thickening of the epidermis with vertical streaking of collagen and vascular bundles of the papillary dermis.13
Subacute cutaneous lupus erythematosus (SCLE) is important to consider for a new papulosquamous eruption with a predilection for the sun-exposed skin of a middle-aged woman. The presence of papules on the volar wrist and history of genital irritation, however, make this entity less likely. Similar to LSA, histologic examination of SCLE reveals epidermal atrophy, basal layer degeneration, and papillary dermal edema with lymphocytic inflammation. However, SCLE lacks the band of inflammation underlying pale homogenized papillary dermal collagen, the most distinguishing feature of LSA; instead, SCLE shows superficial and deep perivascular and periadnexal lymphocytes and mucin in the dermis.14
Lichen sclerosus et atrophicus may be chronic and progressive in nature or cycle through remissions and relapses.2 Treatment is not curative, and management is directed to alleviating symptoms and preventing the progression of disease. First-line management of extragenital LSA is potent topical steroids.1 Adjuvant topical calcineurin inhibitors may be used as steroid-sparing agents.2 Phototherapy is a second-line therapy and even narrowband UVB phototherapy has demonstrated efficacy in managing extragenital LSA.15,16 Our patient was started on mometasone ointment and calcipotriene cream with slight improvement after a 6-month trial. Ongoing management is focused on optimizing application of topical therapies.
The Diagnosis: Extragenital Lichen Sclerosus Et Atrophicus
Histopathologic evaluation revealed hyperkeratosis, follicular plugging, epidermal atrophy, and homogenization of papillary dermal collagen with an underlying lymphocytic infiltrate (Figure 1). Direct immunofluorescence of a plaque with a superimposed bulla was negative for deposition of C3, IgG, IgA, IgM, or fibrinogen. Accordingly, clinicopathologic correlation supported a diagnosis of extragenital lichen sclerosus et atrophicus (LSA). Of note, the patient's history of genital irritation was due to genital LSA that preceded the extragenital manifestations.
Lichen sclerosus et atrophicus is an inflammatory dermatosis that typically presents as atrophic white papules of the anogenital area that coalesce into pruritic plaques; the exact etiology remains to be elucidated, yet various circulating autoantibodies have been identified, suggesting a role for autoimmunity.1,2 Lichen sclerosus et atrophicus is more common in women than in men, with a bimodal peak in the age of onset affecting postmenopausal and prepubertal populations.1 In women, affected areas include the labia minora and majora, clitoris, perineum, and perianal skin; LSA spares the mucosal surfaces of the vagina and cervix.2 In men, uncircumscribed genital skin more commonly is affected. Involvement is localized to the foreskin and glans with occasional urethral involvement.2
In contrast, extragenital LSA tends to present as asymptomatic papules and plaques that develop atrophy with time, involving the back, shoulders, neck, chest, thighs, axillae, and flexural wrists2,3; an erythematous rim often is present,4 and hyperkeratosis with follicular plugging may be prominent.5 Our patient's case emphasizes the predilection of plaques for the chest and intermammary skin (Figure 2A). Approximately 15% of LSA cases have extragenital involvement, and extragenital-limited disease accounts for roughly 5% of cases.6,7 Unlike genital LSA, extragenital disease has not been associated with an increased risk for squamous cell carcinoma.1 Bullae formation within plaques of genital or extragenital LSA has been reported3,8 and is exemplified in our patient (Figure 2B). Intralesional bullae formation likely is due to a combination of internal and external factors, mainly the inability to withstand shear forces due to an atrophic epidermis with basal vacuolar injury overlying an edematous papillary dermis with altered collagen.8 Dermatoscopic findings may aid in recognizing extragenital LSA9,10; our patient's plaques demonstrated the characteristic findings of comedolike openings, structureless white areas, and pink borders (Figure 3).
The clinical differential diagnosis for well-demarcated, pink, scaly plaques is broad. Nummular eczema usually presents as coin-shaped eczematous plaques on the dorsal aspects of the hands or lower extremities, and histology shows epidermal spongiosis.11 Nummular eczema may be considered due to the striking round morphology of various plaques, yet our patient's presentation was better served by a consideration of several papulosquamous disorders.
Lichen planus (LP) presents as intensely pruritic, violaceous, polygonal, flat-topped papules with overlying reticular white lines, or Wickham striae, that favor the flexural wrists, lower back, and lower extremities. Lichen planus also may have oral and genital mucosal involvement. Similar to LSA, LP is more common in women and preferentially affects the postmenopausal population.12 Additionally, hypertrophic LP may obscure Wickham striae and mimic extragenital LSA; distinguishing features of hypertrophic LP are intense pruritus and a predilection for the shins. Histology is defined by orthohyperkeratosis, hypergranulosis, sawtooth acanthosis, and vacuolar degeneration of the basal layer with Civatte bodies or dyskeratotic basal keratinocytes overlying a characteristic bandlike infiltrate of lymphocytes.12
Lichen simplex chronicus (LSC) is characterized by intense pruritus and presents as hyperkeratotic plaques with a predilection for accessible regions such as the posterior neck and extremities.13 The striking annular demarcation of this case makes LSC unlikely. Comparable to LSA and LP, LSC also may present with both genital and extragenital findings. Histology of LSC is characterized by irregular acanthosis or thickening of the epidermis with vertical streaking of collagen and vascular bundles of the papillary dermis.13
Subacute cutaneous lupus erythematosus (SCLE) is important to consider for a new papulosquamous eruption with a predilection for the sun-exposed skin of a middle-aged woman. The presence of papules on the volar wrist and history of genital irritation, however, make this entity less likely. Similar to LSA, histologic examination of SCLE reveals epidermal atrophy, basal layer degeneration, and papillary dermal edema with lymphocytic inflammation. However, SCLE lacks the band of inflammation underlying pale homogenized papillary dermal collagen, the most distinguishing feature of LSA; instead, SCLE shows superficial and deep perivascular and periadnexal lymphocytes and mucin in the dermis.14
Lichen sclerosus et atrophicus may be chronic and progressive in nature or cycle through remissions and relapses.2 Treatment is not curative, and management is directed to alleviating symptoms and preventing the progression of disease. First-line management of extragenital LSA is potent topical steroids.1 Adjuvant topical calcineurin inhibitors may be used as steroid-sparing agents.2 Phototherapy is a second-line therapy and even narrowband UVB phototherapy has demonstrated efficacy in managing extragenital LSA.15,16 Our patient was started on mometasone ointment and calcipotriene cream with slight improvement after a 6-month trial. Ongoing management is focused on optimizing application of topical therapies.
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus. Am J Clin Dermatol. 2013;14:27-47.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Surkan M, Hull P. A case of lichen sclerosus et atrophicus with distinct erythematous borders. J Cutan Med Surg. 2015;19:600-603.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41-46.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Hallel-Halevy D, Grunwald MH, Yerushalmi J, et al. Bullous lichen sclerosus et atrophicus. J Am Acad Dermatol. 1998;39:500-501.
- Garrido-Ríos AA, Álvarez-Garrido H, Sanz-Muñoz C, et al. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468.
- Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433-1439.
- Rudikoff D. Differential diagnosis of round or discoid lesions. Clin Dermatol. 2011;29:489-497.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Shaffer B, Beerman H. Lichen simplex chronicus and its variants: a discussion of certain psychodynamic mechanisms and clinical and histopathologic correlations. AMA Arch Derm Syphilol. 1951;64:340-351.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. Am J Clin Dermatol. 2009;10:365-381.
- Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71:981-984.
- Colbert RL, Chiang MP, Carlin CS, et al. Progressive extragenital lichen sclerosus successfully treated with narrowband UV-B phototherapy. Arch Dermatol. 2007;143:19-20.
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus. Am J Clin Dermatol. 2013;14:27-47.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Surkan M, Hull P. A case of lichen sclerosus et atrophicus with distinct erythematous borders. J Cutan Med Surg. 2015;19:600-603.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41-46.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Hallel-Halevy D, Grunwald MH, Yerushalmi J, et al. Bullous lichen sclerosus et atrophicus. J Am Acad Dermatol. 1998;39:500-501.
- Garrido-Ríos AA, Álvarez-Garrido H, Sanz-Muñoz C, et al. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468.
- Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2013;27:1433-1439.
- Rudikoff D. Differential diagnosis of round or discoid lesions. Clin Dermatol. 2011;29:489-497.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Shaffer B, Beerman H. Lichen simplex chronicus and its variants: a discussion of certain psychodynamic mechanisms and clinical and histopathologic correlations. AMA Arch Derm Syphilol. 1951;64:340-351.
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus. Am J Clin Dermatol. 2009;10:365-381.
- Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71:981-984.
- Colbert RL, Chiang MP, Carlin CS, et al. Progressive extragenital lichen sclerosus successfully treated with narrowband UV-B phototherapy. Arch Dermatol. 2007;143:19-20.
A 48-year-old woman with a history of type 2 diabetes mellitus and nonalcoholic steatohepatitis presented with papules and plaques on the upper trunk, proximal extremities, and volar wrists. Clear fluid–filled bullae occasionally developed within the plaques and subsequently ruptured and healed. Aside from intermittent lesion tenderness and irritation with the formation and rupture of the bullae, the patient’s plaques were asymptomatic, and she specifically denied pruritus. A review of systems revealed a history of genital irritation evaluated by a gynecologist; nystatin–triamcinolone cream 0.1% applied as needed provided relief. The patient denied any recent flares or any new or changing oral mucosa findings or symptoms, preceding medications, or family history of similar lesions. Physical examination revealed well-demarcated, round, pink plaques with keratotic scale scattered across the upper trunk and central chest. The bilateral volar wrists were surfaced by well-circumscribed, thin, pink to violaceous, hyperkeratotic papules.
The Genital Examination in Dermatologic Practice
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
Resident Pearls
- Dermatologists should offer a genital examination to all patients who present for a routine total-body skin examination.
- It is critical to educate patients about the importance of examining the genital skin by discussing that skin diseases can arise in all areas of the body including the genital area. Encouraging genital self-examination also is helpful.
- If a patient declines, the dermatologist should strive to ensure that another provider is examining the genital skin.
Acquired Unilateral Nevoid Telangiectasia With Pruritus and Unknown Etiology
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.
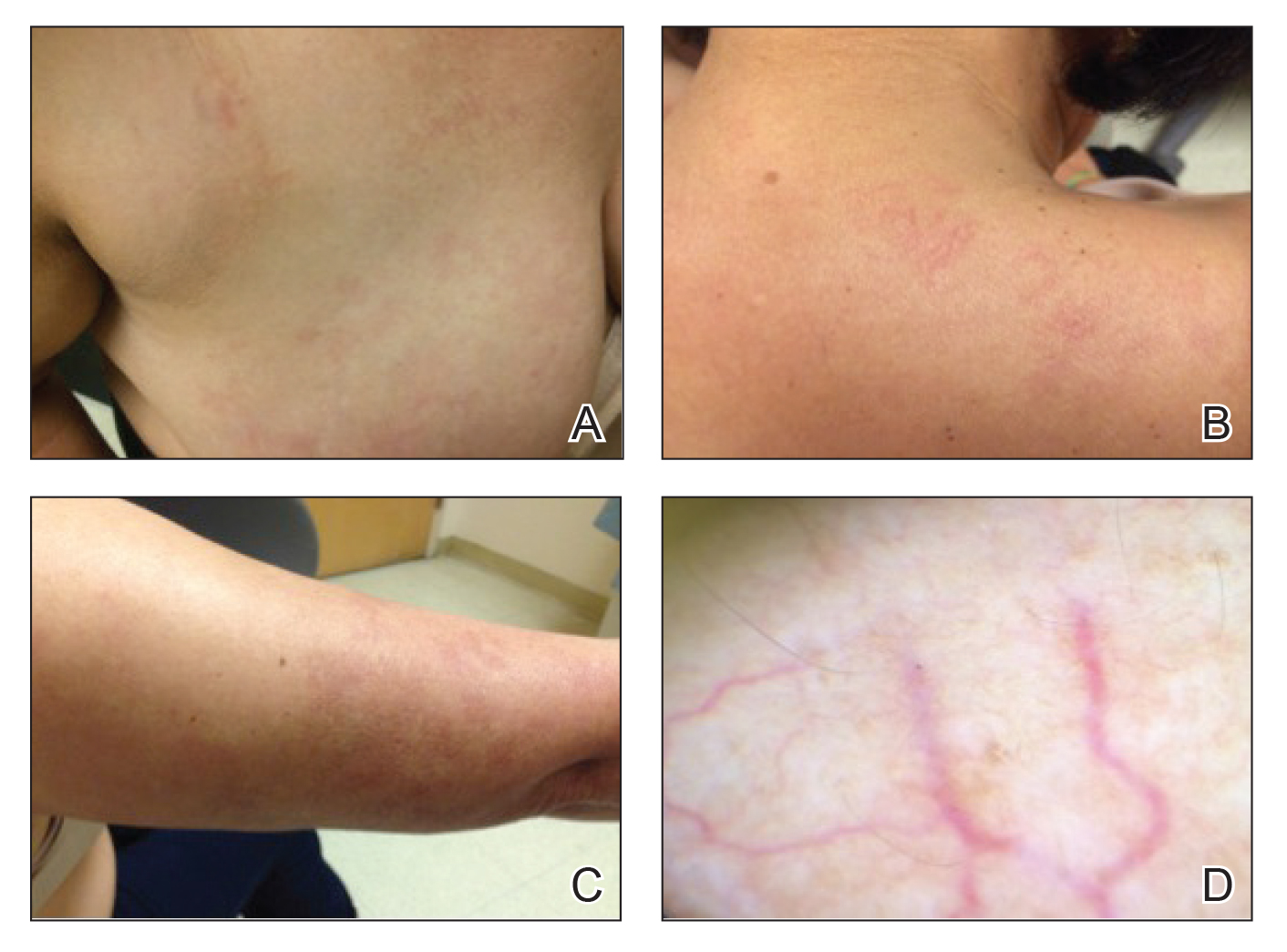
Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.

Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.

Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
Practice Points
- Unilateral nevoid telangiectasia may present in patients without an underlying hyperestrogenic state.
- Unilateral nevoid telangiectasia may present with symptoms including pruritus.
Painless Mobile Nodule on the Shoulder
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
A 70-year-old woman presented to the outpatient dermatology clinic with an acute-onset lesion on the right shoulder. She first noticed a “cyst” developing in the area approximately 3 weeks prior but noted that it may have been present longer. The lesion was bothersome when her undergarments rubbed against it, but she otherwise denied pain, increase in size, or drainage from the site. Her medical history was remarkable for a proliferating trichilemmal tumor on the right parietal scalp treated with Mohs surgery approximately 13 years prior to presentation. She had no personal or family history of skin cancer. Physical examination revealed a 2.5-cm, mobile, nontender, flesh-colored subcutaneous nodule on the right shoulder (top); no ulceration, bleeding, or drainage was present. The surrounding skin demonstrated no clinical changes. The patient was scheduled for outpatient surgical excision of the nodule, which initially was suspected to be a lipoma. During the excision, a translucent cystlike nodule (bottom) was gently dissected and sent for histopathologic examination.
Sudden Cardiac Death in a Young Patient With Psoriasis
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).
The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).
The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).
The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
Practice Points
- Low-grade chronic inflammation in patients with psoriasis can lead to vascular inflammation, which can further lead to the development of major adverse cardiovascular events (CVEs) and arrhythmia.
- The need for a multidisciplinary approach and close monitoring of cardiovascular risk factors in patients with psoriasis to prevent a CVE is vital.
- Baseline electrocardiogram and biomarkers for cardiovascular disease also should be performed in young patients with severe or unstable psoriasis.
Crusted Scabies Presenting as White Superficial Onychomycosislike Lesions
To the Editor:
We report the case of an 83-year-old male nursing home resident with a history of end-stage renal disease who presented with multiple small white islands on the surface of the nail plate, similar to those seen in white superficial onychomycosis (Figure 1). Minimal subungual hyperkeratosis of the fingernails also was observed. Three digits were affected with no toenail involvement. Wet mount examination with potassium hydroxide 20% showed a mite (Figure 2A) and multiple eggs (Figure 2B). Treatment consisted of oral ivermectin 3 mg immediately and permethrin solution 5% applied under occlusion to each of the affected nails for 5 consecutive nights, which resulted in complete clearance of the lesion on the nail plate after 2 weeks.


Crusted scabies was first described as Norwegian scabies in 1848 by Danielsen and Boeck,1 and the name was later changed to crusted scabies in 1976 by Parish and Lumholt2 because there was no inherent connection between Norway and Norwegian scabies. It is a skin infestation of Sarcoptes scabiei var hominis and more commonly is seen in immunocompromised individuals such as the elderly and malnourished patients as well as those with diabetes mellitus and alcoholism.3,4 Patients typically present with widespread hyperkeratosis, mostly involving the palms and soles. Subungual hyperkeratosis and nail dystrophy also can be seen when nail involvement is present, and the scalp rarely is involved.5 Unlike common scabies, skin burrows and pruritus may be minimal or absent, thus making the diagnosis of crusted scabies more difficult than normal scabies.6 Diagnosis of crusted scabies is confirmed by direct microscopy, which demonstrates mites, eggs, or feces. Strict isolation of the patient is necessary, as the disease is very contagious. Treatment with oral ivermectin (1–3 doses of 3 mg at 14-day intervals) in combination with topical permethrin is effective.7
We present a case of crusted scabies with nail involvement that presented with white superficial onychomycosislike lesions. The patient’s nails were successfully treated with a combination of oral ivermectin and topical permethrin occlusion of the nails. In cases with subungual hyperkeratosis, nonsurgical nail avulsion with 40% urea cream or ointment has been used to improve the penetration of permethrin. Partial nail avulsion may be necessary if subungual hyperkeratosis or nail dystrophy becomes extreme.8
- Danielsen DG, Boeck W. Treatment of Leprosy or Greek Elephantiasis. JB Balliere; 1848.
- Parish L, Lumholt G. Crusted scabies: alias Norwegian scabies. Int J Dermatol. 1976;15:747-748.
- Centers for Disease Control and Prevention. Parasites: scabies. Updated November 2, 2010. Accessed January 17, 2021. https://www.cdc.gov/parasites/scabies/
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patient and a review of the literature. J Infect. 2005;50:375-381.
- Dourmisher AL, Serafimova DK, Dourmisher LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234.
- Barnes L, McCallister RE, Lucky AW. Crusted (Norwegian) scabies: occurrence in a child undergoing a bone marrow transplant. Arch Dermatol. 1987;123:95-97.
- Huffam SE, Currie BJ. Ivermectin for Sarcoptes scabiei hyperinfestation. Int J Infect Dis. 1998;2:152-154.
- De Paoli R, Mark SV. Crusted (Norwegian) scabies: treatment of nail involvement. J Am Acad Dermatol. 1987;17:136-138.
To the Editor:
We report the case of an 83-year-old male nursing home resident with a history of end-stage renal disease who presented with multiple small white islands on the surface of the nail plate, similar to those seen in white superficial onychomycosis (Figure 1). Minimal subungual hyperkeratosis of the fingernails also was observed. Three digits were affected with no toenail involvement. Wet mount examination with potassium hydroxide 20% showed a mite (Figure 2A) and multiple eggs (Figure 2B). Treatment consisted of oral ivermectin 3 mg immediately and permethrin solution 5% applied under occlusion to each of the affected nails for 5 consecutive nights, which resulted in complete clearance of the lesion on the nail plate after 2 weeks.


Crusted scabies was first described as Norwegian scabies in 1848 by Danielsen and Boeck,1 and the name was later changed to crusted scabies in 1976 by Parish and Lumholt2 because there was no inherent connection between Norway and Norwegian scabies. It is a skin infestation of Sarcoptes scabiei var hominis and more commonly is seen in immunocompromised individuals such as the elderly and malnourished patients as well as those with diabetes mellitus and alcoholism.3,4 Patients typically present with widespread hyperkeratosis, mostly involving the palms and soles. Subungual hyperkeratosis and nail dystrophy also can be seen when nail involvement is present, and the scalp rarely is involved.5 Unlike common scabies, skin burrows and pruritus may be minimal or absent, thus making the diagnosis of crusted scabies more difficult than normal scabies.6 Diagnosis of crusted scabies is confirmed by direct microscopy, which demonstrates mites, eggs, or feces. Strict isolation of the patient is necessary, as the disease is very contagious. Treatment with oral ivermectin (1–3 doses of 3 mg at 14-day intervals) in combination with topical permethrin is effective.7
We present a case of crusted scabies with nail involvement that presented with white superficial onychomycosislike lesions. The patient’s nails were successfully treated with a combination of oral ivermectin and topical permethrin occlusion of the nails. In cases with subungual hyperkeratosis, nonsurgical nail avulsion with 40% urea cream or ointment has been used to improve the penetration of permethrin. Partial nail avulsion may be necessary if subungual hyperkeratosis or nail dystrophy becomes extreme.8
To the Editor:
We report the case of an 83-year-old male nursing home resident with a history of end-stage renal disease who presented with multiple small white islands on the surface of the nail plate, similar to those seen in white superficial onychomycosis (Figure 1). Minimal subungual hyperkeratosis of the fingernails also was observed. Three digits were affected with no toenail involvement. Wet mount examination with potassium hydroxide 20% showed a mite (Figure 2A) and multiple eggs (Figure 2B). Treatment consisted of oral ivermectin 3 mg immediately and permethrin solution 5% applied under occlusion to each of the affected nails for 5 consecutive nights, which resulted in complete clearance of the lesion on the nail plate after 2 weeks.


Crusted scabies was first described as Norwegian scabies in 1848 by Danielsen and Boeck,1 and the name was later changed to crusted scabies in 1976 by Parish and Lumholt2 because there was no inherent connection between Norway and Norwegian scabies. It is a skin infestation of Sarcoptes scabiei var hominis and more commonly is seen in immunocompromised individuals such as the elderly and malnourished patients as well as those with diabetes mellitus and alcoholism.3,4 Patients typically present with widespread hyperkeratosis, mostly involving the palms and soles. Subungual hyperkeratosis and nail dystrophy also can be seen when nail involvement is present, and the scalp rarely is involved.5 Unlike common scabies, skin burrows and pruritus may be minimal or absent, thus making the diagnosis of crusted scabies more difficult than normal scabies.6 Diagnosis of crusted scabies is confirmed by direct microscopy, which demonstrates mites, eggs, or feces. Strict isolation of the patient is necessary, as the disease is very contagious. Treatment with oral ivermectin (1–3 doses of 3 mg at 14-day intervals) in combination with topical permethrin is effective.7
We present a case of crusted scabies with nail involvement that presented with white superficial onychomycosislike lesions. The patient’s nails were successfully treated with a combination of oral ivermectin and topical permethrin occlusion of the nails. In cases with subungual hyperkeratosis, nonsurgical nail avulsion with 40% urea cream or ointment has been used to improve the penetration of permethrin. Partial nail avulsion may be necessary if subungual hyperkeratosis or nail dystrophy becomes extreme.8
- Danielsen DG, Boeck W. Treatment of Leprosy or Greek Elephantiasis. JB Balliere; 1848.
- Parish L, Lumholt G. Crusted scabies: alias Norwegian scabies. Int J Dermatol. 1976;15:747-748.
- Centers for Disease Control and Prevention. Parasites: scabies. Updated November 2, 2010. Accessed January 17, 2021. https://www.cdc.gov/parasites/scabies/
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patient and a review of the literature. J Infect. 2005;50:375-381.
- Dourmisher AL, Serafimova DK, Dourmisher LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234.
- Barnes L, McCallister RE, Lucky AW. Crusted (Norwegian) scabies: occurrence in a child undergoing a bone marrow transplant. Arch Dermatol. 1987;123:95-97.
- Huffam SE, Currie BJ. Ivermectin for Sarcoptes scabiei hyperinfestation. Int J Infect Dis. 1998;2:152-154.
- De Paoli R, Mark SV. Crusted (Norwegian) scabies: treatment of nail involvement. J Am Acad Dermatol. 1987;17:136-138.
- Danielsen DG, Boeck W. Treatment of Leprosy or Greek Elephantiasis. JB Balliere; 1848.
- Parish L, Lumholt G. Crusted scabies: alias Norwegian scabies. Int J Dermatol. 1976;15:747-748.
- Centers for Disease Control and Prevention. Parasites: scabies. Updated November 2, 2010. Accessed January 17, 2021. https://www.cdc.gov/parasites/scabies/
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patient and a review of the literature. J Infect. 2005;50:375-381.
- Dourmisher AL, Serafimova DK, Dourmisher LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234.
- Barnes L, McCallister RE, Lucky AW. Crusted (Norwegian) scabies: occurrence in a child undergoing a bone marrow transplant. Arch Dermatol. 1987;123:95-97.
- Huffam SE, Currie BJ. Ivermectin for Sarcoptes scabiei hyperinfestation. Int J Infect Dis. 1998;2:152-154.
- De Paoli R, Mark SV. Crusted (Norwegian) scabies: treatment of nail involvement. J Am Acad Dermatol. 1987;17:136-138.
Practice Points
- Crusted scabies is asymptomatic; therefore, any white lesion at the surface of the nail should be scraped and examined with potassium hydroxide.
- Immunosuppressed patients are at risk for infection.
Recurrent Painful Nodules Following Synthol Injection to Enhance Bicep Volume
To the Editor:
A 28-year-old man presented to the dermatology clinic with red, tender, swollen nodules on the left arm of 5 days’ duration, which had been a recurrent issue involving both arms. He also experienced intermittent fatigue and mild myalgia but denied associated fevers or chills. Oral clindamycin prescribed by a local emergency department provided some improvement. Upon further questioning, the patient admitted to injecting an unknown substance into the muscles 10 years prior for the purpose of enhancing their volume and appearance. Physical examination revealed large bilateral biceps with firm, mobile, nontender, subcutaneous nodules and mild erythema on the inner aspects of the arms. An incisional biopsy of a left arm nodule was performed with tissue culture (Figure 1). Microscopic evaluation revealed mild dermal sclerosis with edema and sclerosis of fat septae (Figure 2A). The fat lobules contained granulomas with surrounding lymphocytes and clear holes noted within the histiocytic giant cells, indicating a likely foreign substance (Figure 2B). Immunohistochemical staining of the histiocytes with CD68 highlighted the clear vacuoles (Figure 3). Polarization examination, Alcian blue, periodic acid–Schiff, and acid-fast bacilli staining were negative. Bacterial, fungal, and mycobacterial tissue cultures and staining also were negative. The histologic findings of septal and lobular panniculitis with sclerosis and granulomatous inflammation in the clinical setting were consistent with a foreign body reaction secondary to synthol injection.
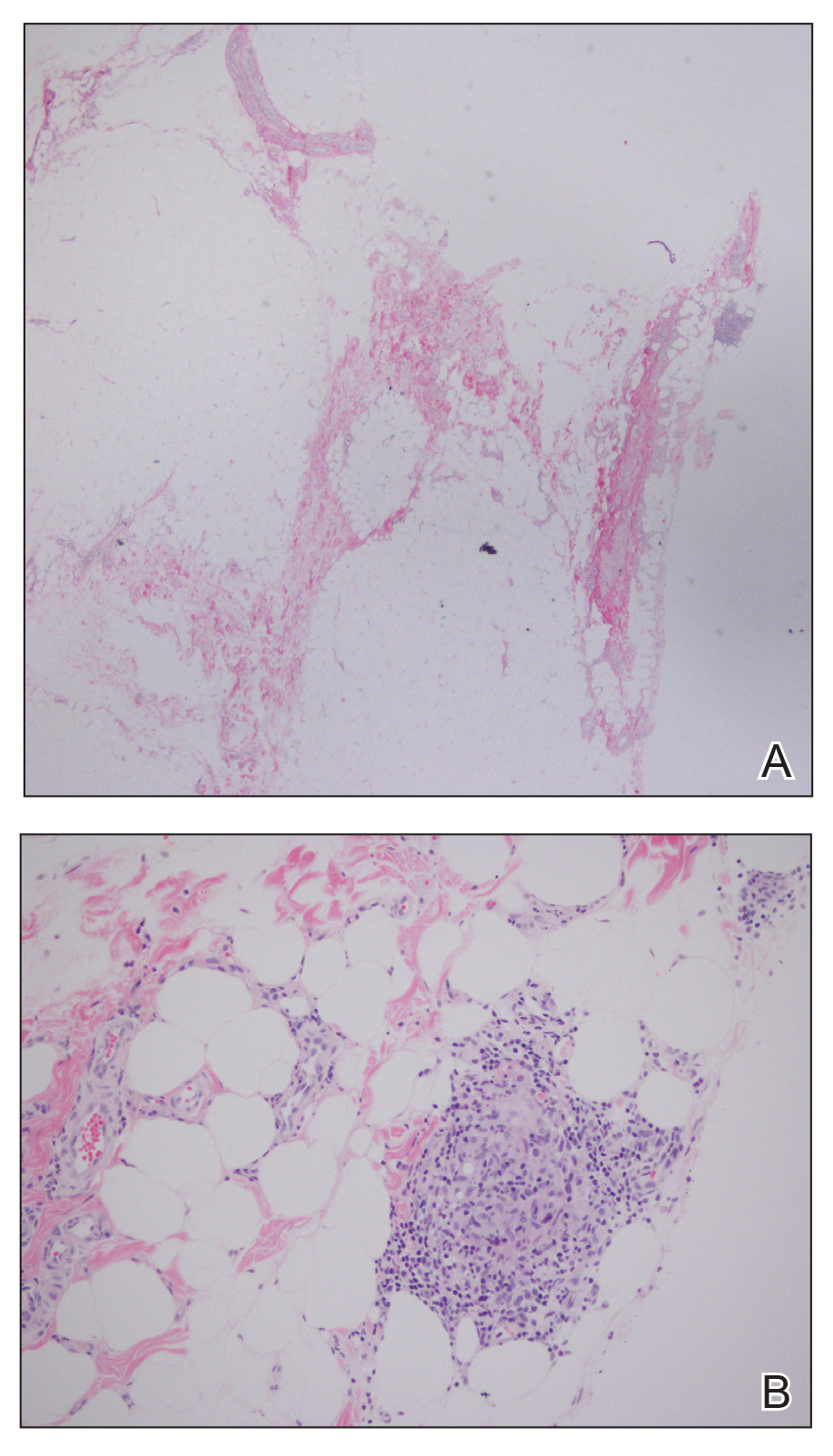
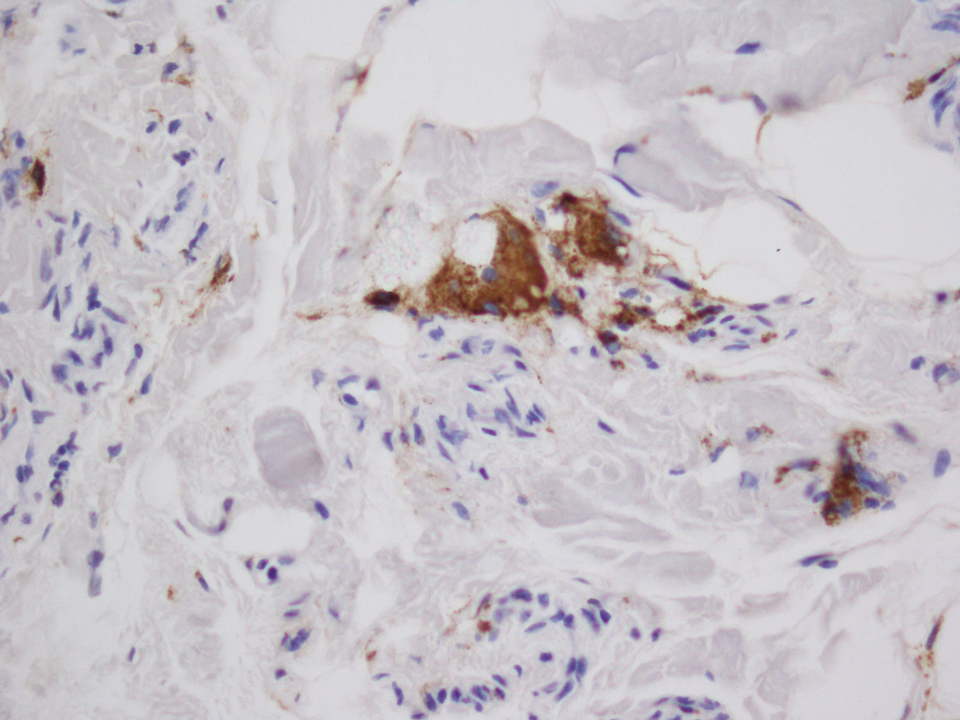
The willingness of athletes in competitive sports to undergo procedures or utilize substances for a competitive advantage despite both immediate and long-term consequences is well documented.1,2 In bodybuilding, use of anabolic steroids and intramuscular oil injections has been documented.3 The use of site enhancements in the form of “fillers” such as petroleum jelly and paraffin have been used for more than 100 years.4 The use of oil for volumetric site enhancement began in the 1960s in Italy with formebolone and evolved to the use of synthol in the 1990s.5 Synthol is a substance composed of 85% oil in the form of medium-chain triglycerides, 7.5% alcohol, and 7.5% lidocaine.6 The presumed mechanism of action of injected oils consists of an initial inflammatory response followed by fibrosis and chronic macrophagocytosis, ultimately leading to expanded volume in the subcutaneous tissue.7 These procedures are purely aesthetic with no increase in muscle strength or performance.
There are few cases in the literature of side effects from intramuscular synthol injections. In one report, a 29-year-old man presented with painful muscle fibrosis requiring open surgical excision of massively fibrotic bicep tissue.8 Another report documented a 45-year-old man who presented with spontaneous ulcerations on the biceps that initially were treated with antibiotics and compression therapy but eventually required surgical intervention and skin grafting.9 Complications have been more frequently reported from injections of other oils such as paraffin and sesame.10,11 Given the similar underlying mechanisms of action, injected oils share the local side effects of inflammation, infection, chronic wounds, and ulceration,9,10 as well as a systemic risk for embolization leading to pulmonary emboli, myocardial infarction, and stroke.6 Although no standard of care exists for the management of complications arising from intramuscular oil injections, treatments that have been employed include antibiotics, corticosteroids, wound care, and compression therapy; definitive treatment typically is surgical excision.6,8,9,11,12 Psychiatric evaluation also should be considered to evaluate for the possibility of body dysmorphic disorder and other associated psychiatric conditions.11
Pressure for a particular aesthetic appearance, both within and outside the world of competitive sports, has driven individuals to various methods of muscular enhancement. Volumetric site enhancements have become increasingly popular, in part due to the perceived lack of systemic side effects, such as those associated with anabolic steroids.8 However, most users are unaware of the notable short-term and long-term risks associated with intramuscular oil injections. Synthol is widely available on the Internet and easily can be purchased and injected by anyone.13 Medical providers should be aware of the possibility of aesthetic site enhancement use in their patients and be able to recognize and intervene in these cases to prevent chronic damage to muscle tissue and accompanying complications. Despite extensive commercialization of these products, few reports in the medical literature exist detailing the side effects of intramuscular oil injections, which may be contributing to the trivialization of these procedures by the general public.12
- Baron DA, Martin DM, Abol Magd S. Doping in sports and its spread to at-risk populations: an international review. World Psychiatry. 2007;6:118-123.
- Holt RIG, Erotokritou-Mulligan I, Sönksen PH. The history of doping and growth hormone abuse in sport. Growth Horm IGF Res. 2009;19:320-326.
- Figueiredo VC, Pedroso da Silva PR. Cosmetic doping—when anabolic-androgenic steroids are not enough. Subst Use Misuse. 2014;49:1163-1167.
- Glicenstein J. The first “fillers,” vaseline and paraffin. from miracle to disaster [in French]. Ann Chir Plast Esthet. 2007;52:157-161.
- Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54-58.
- Pupka A, Sikora J, Mauricz J, et al. The usage of synthol in the body building [in Polish]. Polim Med. 2009;39:63-65.
- Di Benedetto G, Pierangeli M, Scalise A, et al. Paraffin oil injection in the body: an obsolete and destructive procedure. Ann Plast Surg. 2002;49:391-396.
- Ghandourah S, Hofer MJ, Kiessling A, et al. Painful muscle fibrosis following synthol injections in a bodybuilder: a case report. J Med Case Rep. 2012;6:248.
- Ikander P, Nielsen AM, Sørensen JA. Injection of synthol in a bodybuilder can cause chronic wounds and ulceration [in Danish]. Ugeskr Laeger. 2015;177:V12140642.
- Henriksen TF, Løvenwald JB, Matzen SH. Paraffin oil injection in bodybuilders calls for preventive action [in Danish]. Ugeskr Laeger. 2010;172:219-220.
- Darsow U, Bruckbauer H, Worret WI, et al. Subcutaneous oleomas induced by self-injection of sesame seed oil for muscle augmentation. J Am Acad Dermatol. 2000;42(2, pt 1):292-294.
- Banke IJ, Prodinger PM, Waldt S, et al. Irreversible muscle damage in bodybuilding due to long-term intramuscular oil injection. Int J Sports Med. 2012;33:829-834.
- Hall M, Grogan S, Gough B. Bodybuilders’ accounts of synthol use: the construction of lay expertise online. J Health Psychol. 2016;21:1939-1948.
To the Editor:
A 28-year-old man presented to the dermatology clinic with red, tender, swollen nodules on the left arm of 5 days’ duration, which had been a recurrent issue involving both arms. He also experienced intermittent fatigue and mild myalgia but denied associated fevers or chills. Oral clindamycin prescribed by a local emergency department provided some improvement. Upon further questioning, the patient admitted to injecting an unknown substance into the muscles 10 years prior for the purpose of enhancing their volume and appearance. Physical examination revealed large bilateral biceps with firm, mobile, nontender, subcutaneous nodules and mild erythema on the inner aspects of the arms. An incisional biopsy of a left arm nodule was performed with tissue culture (Figure 1). Microscopic evaluation revealed mild dermal sclerosis with edema and sclerosis of fat septae (Figure 2A). The fat lobules contained granulomas with surrounding lymphocytes and clear holes noted within the histiocytic giant cells, indicating a likely foreign substance (Figure 2B). Immunohistochemical staining of the histiocytes with CD68 highlighted the clear vacuoles (Figure 3). Polarization examination, Alcian blue, periodic acid–Schiff, and acid-fast bacilli staining were negative. Bacterial, fungal, and mycobacterial tissue cultures and staining also were negative. The histologic findings of septal and lobular panniculitis with sclerosis and granulomatous inflammation in the clinical setting were consistent with a foreign body reaction secondary to synthol injection.



The willingness of athletes in competitive sports to undergo procedures or utilize substances for a competitive advantage despite both immediate and long-term consequences is well documented.1,2 In bodybuilding, use of anabolic steroids and intramuscular oil injections has been documented.3 The use of site enhancements in the form of “fillers” such as petroleum jelly and paraffin have been used for more than 100 years.4 The use of oil for volumetric site enhancement began in the 1960s in Italy with formebolone and evolved to the use of synthol in the 1990s.5 Synthol is a substance composed of 85% oil in the form of medium-chain triglycerides, 7.5% alcohol, and 7.5% lidocaine.6 The presumed mechanism of action of injected oils consists of an initial inflammatory response followed by fibrosis and chronic macrophagocytosis, ultimately leading to expanded volume in the subcutaneous tissue.7 These procedures are purely aesthetic with no increase in muscle strength or performance.
There are few cases in the literature of side effects from intramuscular synthol injections. In one report, a 29-year-old man presented with painful muscle fibrosis requiring open surgical excision of massively fibrotic bicep tissue.8 Another report documented a 45-year-old man who presented with spontaneous ulcerations on the biceps that initially were treated with antibiotics and compression therapy but eventually required surgical intervention and skin grafting.9 Complications have been more frequently reported from injections of other oils such as paraffin and sesame.10,11 Given the similar underlying mechanisms of action, injected oils share the local side effects of inflammation, infection, chronic wounds, and ulceration,9,10 as well as a systemic risk for embolization leading to pulmonary emboli, myocardial infarction, and stroke.6 Although no standard of care exists for the management of complications arising from intramuscular oil injections, treatments that have been employed include antibiotics, corticosteroids, wound care, and compression therapy; definitive treatment typically is surgical excision.6,8,9,11,12 Psychiatric evaluation also should be considered to evaluate for the possibility of body dysmorphic disorder and other associated psychiatric conditions.11
Pressure for a particular aesthetic appearance, both within and outside the world of competitive sports, has driven individuals to various methods of muscular enhancement. Volumetric site enhancements have become increasingly popular, in part due to the perceived lack of systemic side effects, such as those associated with anabolic steroids.8 However, most users are unaware of the notable short-term and long-term risks associated with intramuscular oil injections. Synthol is widely available on the Internet and easily can be purchased and injected by anyone.13 Medical providers should be aware of the possibility of aesthetic site enhancement use in their patients and be able to recognize and intervene in these cases to prevent chronic damage to muscle tissue and accompanying complications. Despite extensive commercialization of these products, few reports in the medical literature exist detailing the side effects of intramuscular oil injections, which may be contributing to the trivialization of these procedures by the general public.12
To the Editor:
A 28-year-old man presented to the dermatology clinic with red, tender, swollen nodules on the left arm of 5 days’ duration, which had been a recurrent issue involving both arms. He also experienced intermittent fatigue and mild myalgia but denied associated fevers or chills. Oral clindamycin prescribed by a local emergency department provided some improvement. Upon further questioning, the patient admitted to injecting an unknown substance into the muscles 10 years prior for the purpose of enhancing their volume and appearance. Physical examination revealed large bilateral biceps with firm, mobile, nontender, subcutaneous nodules and mild erythema on the inner aspects of the arms. An incisional biopsy of a left arm nodule was performed with tissue culture (Figure 1). Microscopic evaluation revealed mild dermal sclerosis with edema and sclerosis of fat septae (Figure 2A). The fat lobules contained granulomas with surrounding lymphocytes and clear holes noted within the histiocytic giant cells, indicating a likely foreign substance (Figure 2B). Immunohistochemical staining of the histiocytes with CD68 highlighted the clear vacuoles (Figure 3). Polarization examination, Alcian blue, periodic acid–Schiff, and acid-fast bacilli staining were negative. Bacterial, fungal, and mycobacterial tissue cultures and staining also were negative. The histologic findings of septal and lobular panniculitis with sclerosis and granulomatous inflammation in the clinical setting were consistent with a foreign body reaction secondary to synthol injection.



The willingness of athletes in competitive sports to undergo procedures or utilize substances for a competitive advantage despite both immediate and long-term consequences is well documented.1,2 In bodybuilding, use of anabolic steroids and intramuscular oil injections has been documented.3 The use of site enhancements in the form of “fillers” such as petroleum jelly and paraffin have been used for more than 100 years.4 The use of oil for volumetric site enhancement began in the 1960s in Italy with formebolone and evolved to the use of synthol in the 1990s.5 Synthol is a substance composed of 85% oil in the form of medium-chain triglycerides, 7.5% alcohol, and 7.5% lidocaine.6 The presumed mechanism of action of injected oils consists of an initial inflammatory response followed by fibrosis and chronic macrophagocytosis, ultimately leading to expanded volume in the subcutaneous tissue.7 These procedures are purely aesthetic with no increase in muscle strength or performance.
There are few cases in the literature of side effects from intramuscular synthol injections. In one report, a 29-year-old man presented with painful muscle fibrosis requiring open surgical excision of massively fibrotic bicep tissue.8 Another report documented a 45-year-old man who presented with spontaneous ulcerations on the biceps that initially were treated with antibiotics and compression therapy but eventually required surgical intervention and skin grafting.9 Complications have been more frequently reported from injections of other oils such as paraffin and sesame.10,11 Given the similar underlying mechanisms of action, injected oils share the local side effects of inflammation, infection, chronic wounds, and ulceration,9,10 as well as a systemic risk for embolization leading to pulmonary emboli, myocardial infarction, and stroke.6 Although no standard of care exists for the management of complications arising from intramuscular oil injections, treatments that have been employed include antibiotics, corticosteroids, wound care, and compression therapy; definitive treatment typically is surgical excision.6,8,9,11,12 Psychiatric evaluation also should be considered to evaluate for the possibility of body dysmorphic disorder and other associated psychiatric conditions.11
Pressure for a particular aesthetic appearance, both within and outside the world of competitive sports, has driven individuals to various methods of muscular enhancement. Volumetric site enhancements have become increasingly popular, in part due to the perceived lack of systemic side effects, such as those associated with anabolic steroids.8 However, most users are unaware of the notable short-term and long-term risks associated with intramuscular oil injections. Synthol is widely available on the Internet and easily can be purchased and injected by anyone.13 Medical providers should be aware of the possibility of aesthetic site enhancement use in their patients and be able to recognize and intervene in these cases to prevent chronic damage to muscle tissue and accompanying complications. Despite extensive commercialization of these products, few reports in the medical literature exist detailing the side effects of intramuscular oil injections, which may be contributing to the trivialization of these procedures by the general public.12
- Baron DA, Martin DM, Abol Magd S. Doping in sports and its spread to at-risk populations: an international review. World Psychiatry. 2007;6:118-123.
- Holt RIG, Erotokritou-Mulligan I, Sönksen PH. The history of doping and growth hormone abuse in sport. Growth Horm IGF Res. 2009;19:320-326.
- Figueiredo VC, Pedroso da Silva PR. Cosmetic doping—when anabolic-androgenic steroids are not enough. Subst Use Misuse. 2014;49:1163-1167.
- Glicenstein J. The first “fillers,” vaseline and paraffin. from miracle to disaster [in French]. Ann Chir Plast Esthet. 2007;52:157-161.
- Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54-58.
- Pupka A, Sikora J, Mauricz J, et al. The usage of synthol in the body building [in Polish]. Polim Med. 2009;39:63-65.
- Di Benedetto G, Pierangeli M, Scalise A, et al. Paraffin oil injection in the body: an obsolete and destructive procedure. Ann Plast Surg. 2002;49:391-396.
- Ghandourah S, Hofer MJ, Kiessling A, et al. Painful muscle fibrosis following synthol injections in a bodybuilder: a case report. J Med Case Rep. 2012;6:248.
- Ikander P, Nielsen AM, Sørensen JA. Injection of synthol in a bodybuilder can cause chronic wounds and ulceration [in Danish]. Ugeskr Laeger. 2015;177:V12140642.
- Henriksen TF, Løvenwald JB, Matzen SH. Paraffin oil injection in bodybuilders calls for preventive action [in Danish]. Ugeskr Laeger. 2010;172:219-220.
- Darsow U, Bruckbauer H, Worret WI, et al. Subcutaneous oleomas induced by self-injection of sesame seed oil for muscle augmentation. J Am Acad Dermatol. 2000;42(2, pt 1):292-294.
- Banke IJ, Prodinger PM, Waldt S, et al. Irreversible muscle damage in bodybuilding due to long-term intramuscular oil injection. Int J Sports Med. 2012;33:829-834.
- Hall M, Grogan S, Gough B. Bodybuilders’ accounts of synthol use: the construction of lay expertise online. J Health Psychol. 2016;21:1939-1948.
- Baron DA, Martin DM, Abol Magd S. Doping in sports and its spread to at-risk populations: an international review. World Psychiatry. 2007;6:118-123.
- Holt RIG, Erotokritou-Mulligan I, Sönksen PH. The history of doping and growth hormone abuse in sport. Growth Horm IGF Res. 2009;19:320-326.
- Figueiredo VC, Pedroso da Silva PR. Cosmetic doping—when anabolic-androgenic steroids are not enough. Subst Use Misuse. 2014;49:1163-1167.
- Glicenstein J. The first “fillers,” vaseline and paraffin. from miracle to disaster [in French]. Ann Chir Plast Esthet. 2007;52:157-161.
- Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54-58.
- Pupka A, Sikora J, Mauricz J, et al. The usage of synthol in the body building [in Polish]. Polim Med. 2009;39:63-65.
- Di Benedetto G, Pierangeli M, Scalise A, et al. Paraffin oil injection in the body: an obsolete and destructive procedure. Ann Plast Surg. 2002;49:391-396.
- Ghandourah S, Hofer MJ, Kiessling A, et al. Painful muscle fibrosis following synthol injections in a bodybuilder: a case report. J Med Case Rep. 2012;6:248.
- Ikander P, Nielsen AM, Sørensen JA. Injection of synthol in a bodybuilder can cause chronic wounds and ulceration [in Danish]. Ugeskr Laeger. 2015;177:V12140642.
- Henriksen TF, Løvenwald JB, Matzen SH. Paraffin oil injection in bodybuilders calls for preventive action [in Danish]. Ugeskr Laeger. 2010;172:219-220.
- Darsow U, Bruckbauer H, Worret WI, et al. Subcutaneous oleomas induced by self-injection of sesame seed oil for muscle augmentation. J Am Acad Dermatol. 2000;42(2, pt 1):292-294.
- Banke IJ, Prodinger PM, Waldt S, et al. Irreversible muscle damage in bodybuilding due to long-term intramuscular oil injection. Int J Sports Med. 2012;33:829-834.
- Hall M, Grogan S, Gough B. Bodybuilders’ accounts of synthol use: the construction of lay expertise online. J Health Psychol. 2016;21:1939-1948.
Practice Points
- The use of injectable volumetric site enhancers in the form of oils to improve the aesthetic appearance of muscles has been prevalent for decades despite potentially serious adverse reactions.
- Complications from these procedures are underrecognized in the medical setting, perhaps owing to the trivialization of these procedures by the general public.