User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
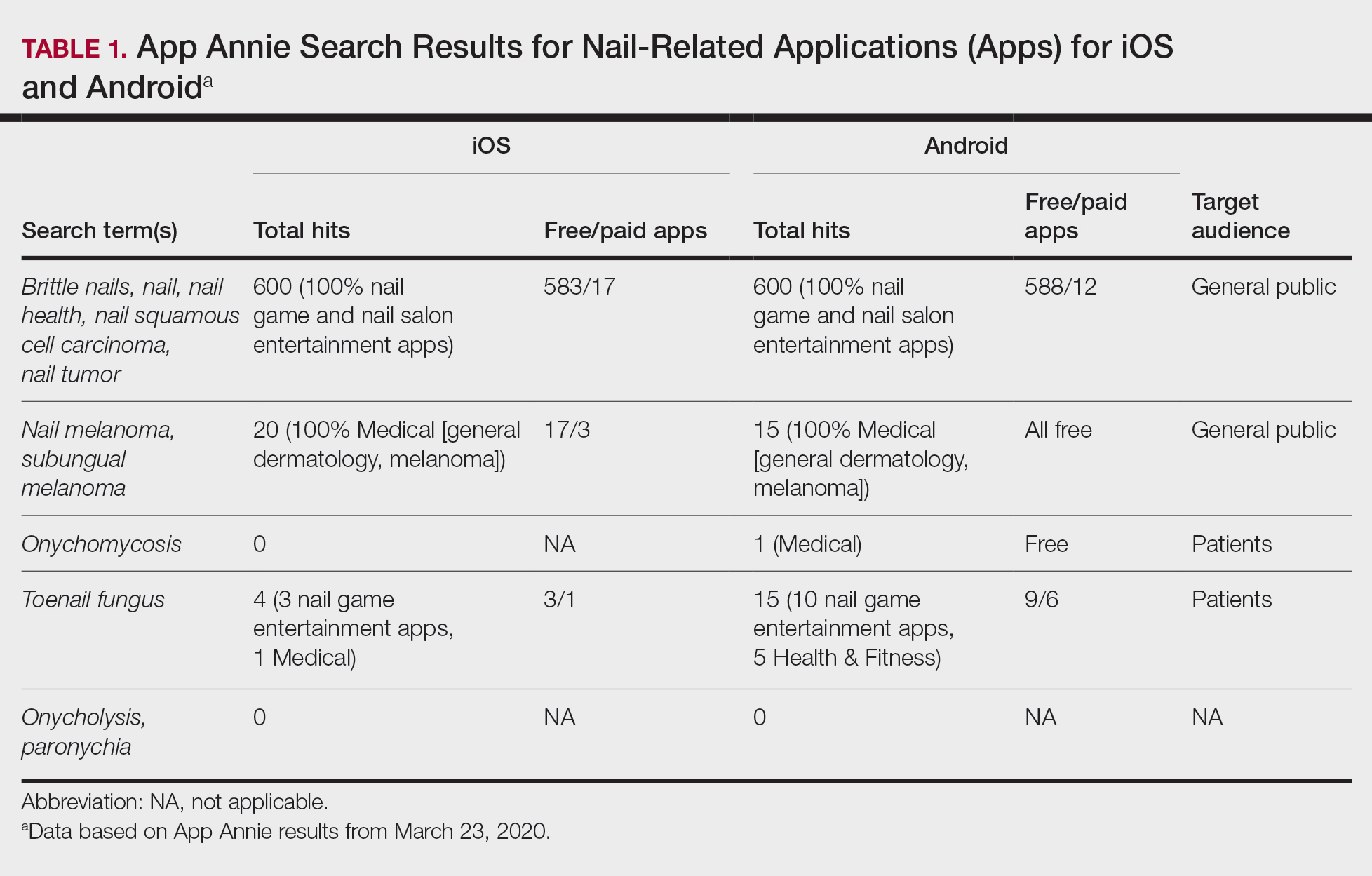
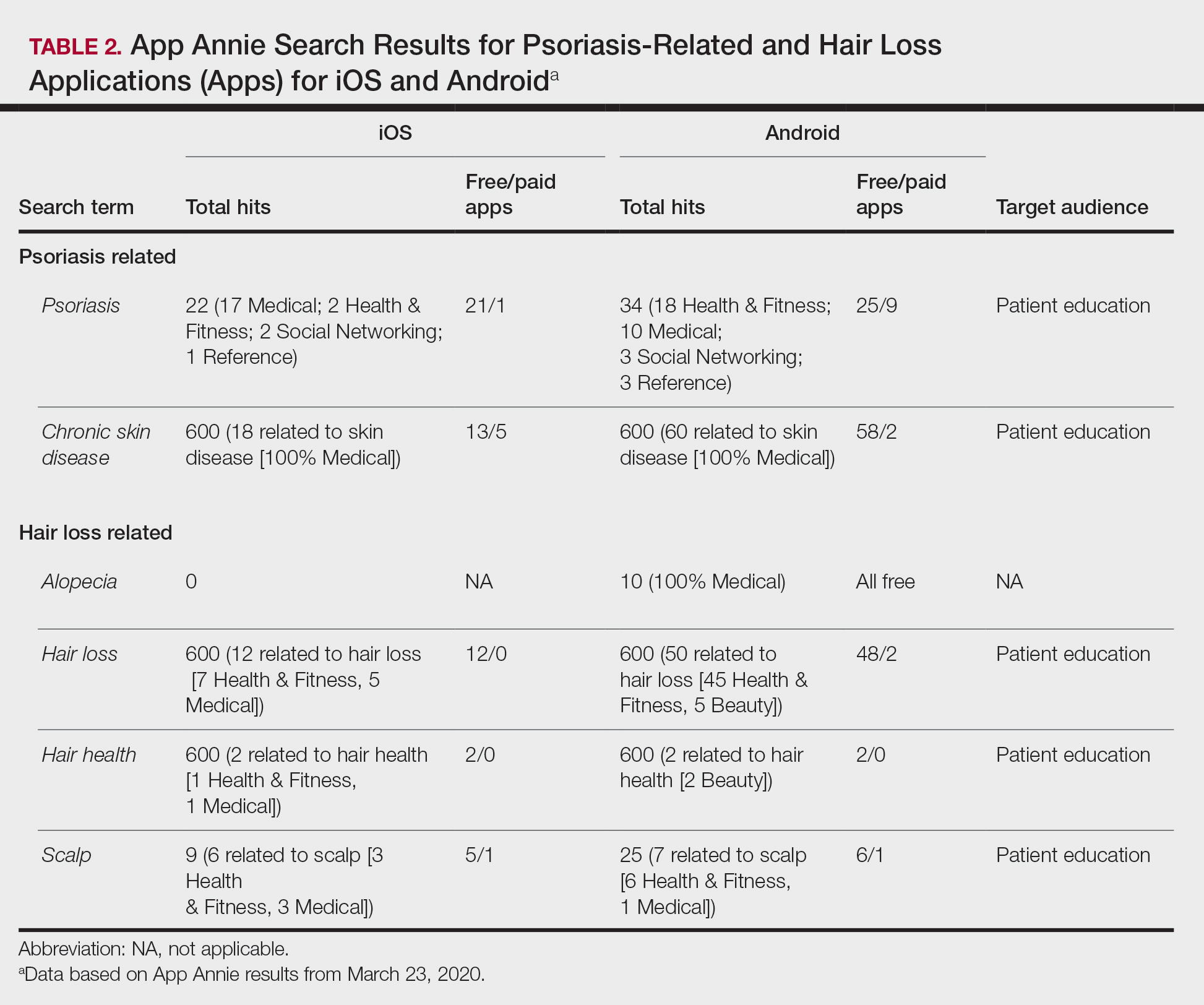
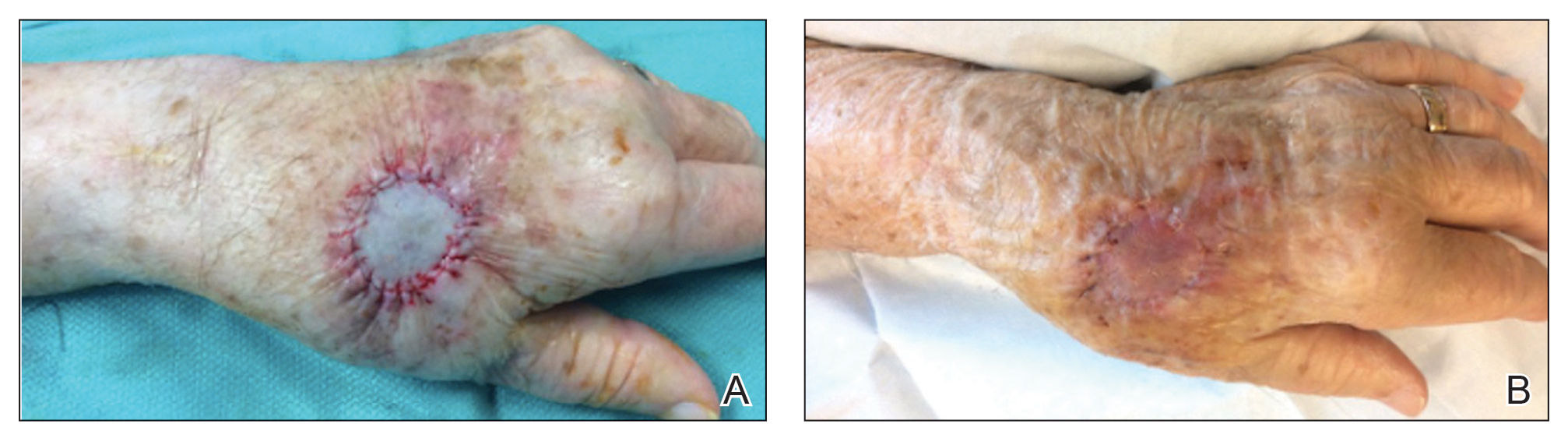
Thick Hyperkeratotic Plaques on the Palms and Soles
The Diagnosis: Keratoderma Climactericum
Keratoderma climactericum was first reported in 1934 by Haxthausen1 as nonpruritic circumscribed hyperkeratosis located mainly on the palms and soles. The initial eruption was described as discrete lesions with an oval or round shape that progressed to less-defined, confluent, hyperkeratotic patches with fissures.1 Keratoderma climactericum also may be referred to as Haxthausen disease and is considered an acquired palmoplantar keratoderma.2
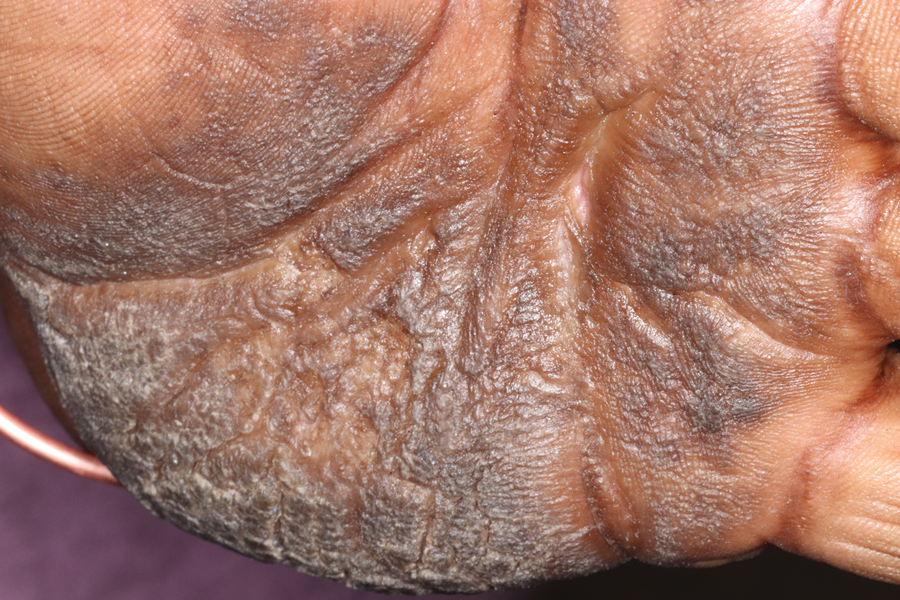
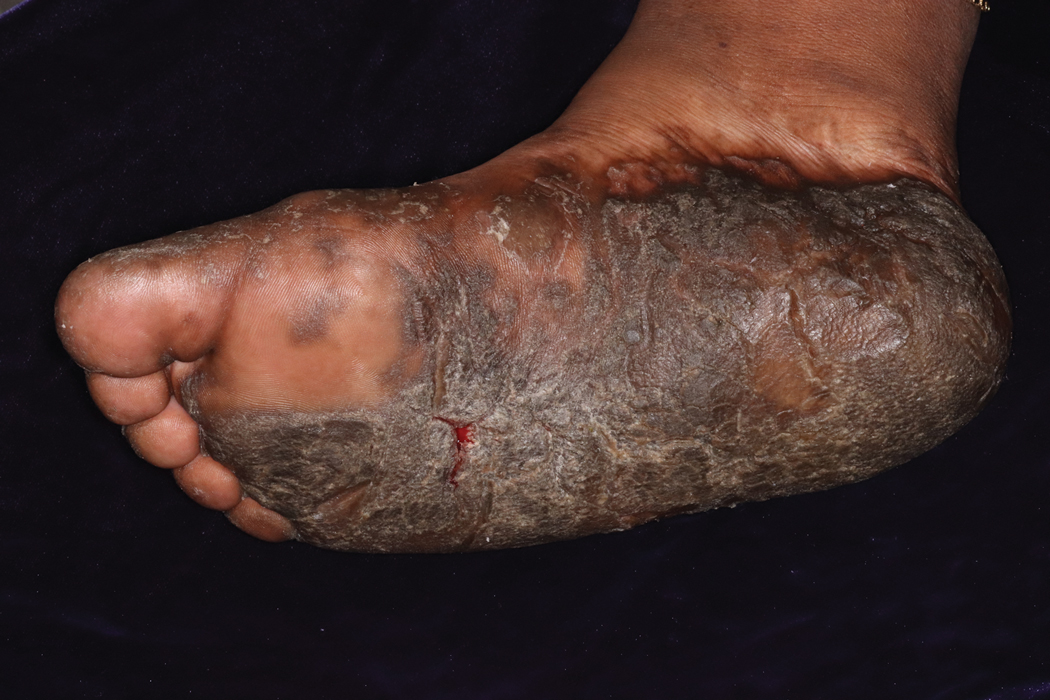
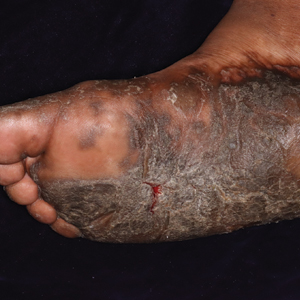
Keratoderma climactericum is a rare dermatologic disorder that presents in women of menopausal age who have no family or personal history of skin disease. Keratoderma climactericum is associated with hypertension and obesity.2 Keratotic lesions usually first occur on the plantar surfaces with eventual development of fissuring and hyperkeratosis that causes painful walking. The keratotic lesions on the plantar surfaces often are nonpruritic and gradually become confluent over time. As the disease progresses, keratotic lesions appear on the central palms, which can lead to confluent hyperkeratosis on the palmar surfaces (Figure 1).2 The exact mechanism of keratoderma climactericum has not been described but is believed to be due to hormonal dysregulation.2
In 1986, Deschamps et al3 presented 10 cases of keratoderma climactericum occurring in menopausal women with an average age of 57 years. The lesions began on the soles at areas of greatest pressure. Histopathology for each patient showed orthokeratotic hyperkeratosis, irregular hyperplasia, interpapillary ridges, and exocytosis of lymphocytes in the epidermis. Seven patients were treated with etretinate, which first led to the removal of palmar lesions, followed by improvement in plantar lesions and pain when walking. There was no association of keratoderma climactericum and sex hormones, as hormone levels were negative or normal for each patient.3
Three cases of keratoderma climactericum following bilateral oophorectomy in young women were reported by Wachtel4 in 1981. Unlike in women of menopausal age, there was no association of keratoderma climactericum with hypertension or obesity. Additionally, the lesions on the palms and soles were more diffusely distributed than in women of menopausal age. Estrogen administration completely reversed each patient's hyperkeratotic palms and soles.4 A definitive pathogenic role of estrogens in the development of keratoderma climactericum has yet to be determined.2
Histopathology is not specific for keratoderma climactericum, making the disease a clinical diagnosis. However, a biopsy may be useful to rule out palmoplantar psoriasis.2 Clinical information such as the age and sex of the patient, distribution of disease, presence of fissuring, and progression of disease from soles to palms should be considered when making a diagnosis of keratoderma climactericum. The differential diagnosis of keratoderma climactericum should include fungal infections, contact dermatitis, irritant dermatitis, psoriasis, atopic dermatitis, underlying malignancy, and pityriasis rubra pilaris.
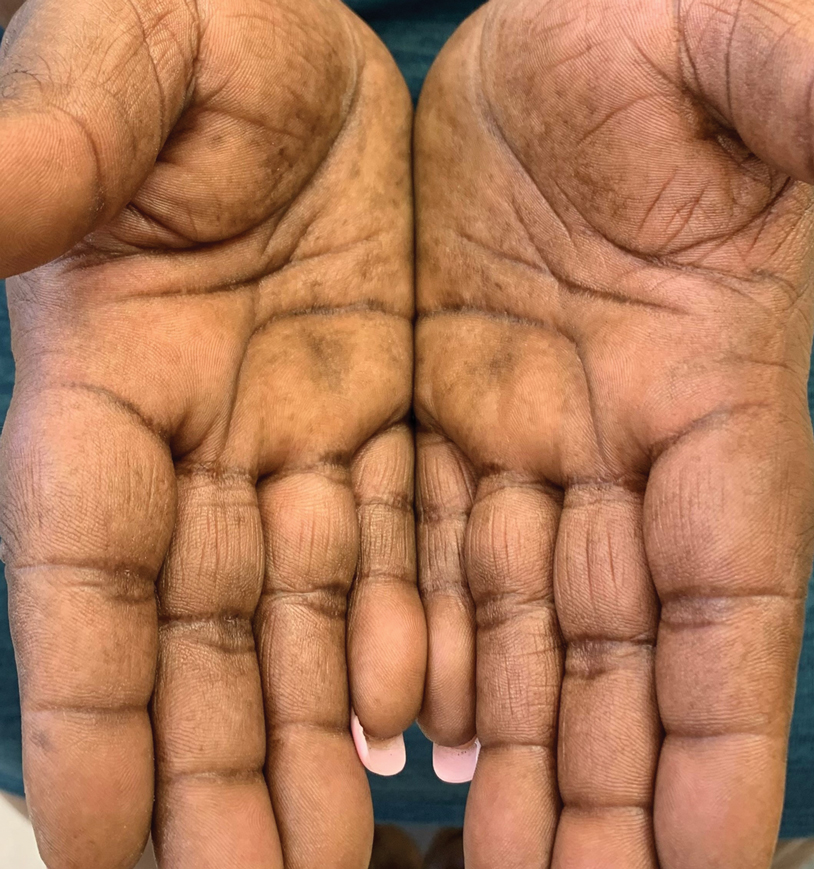
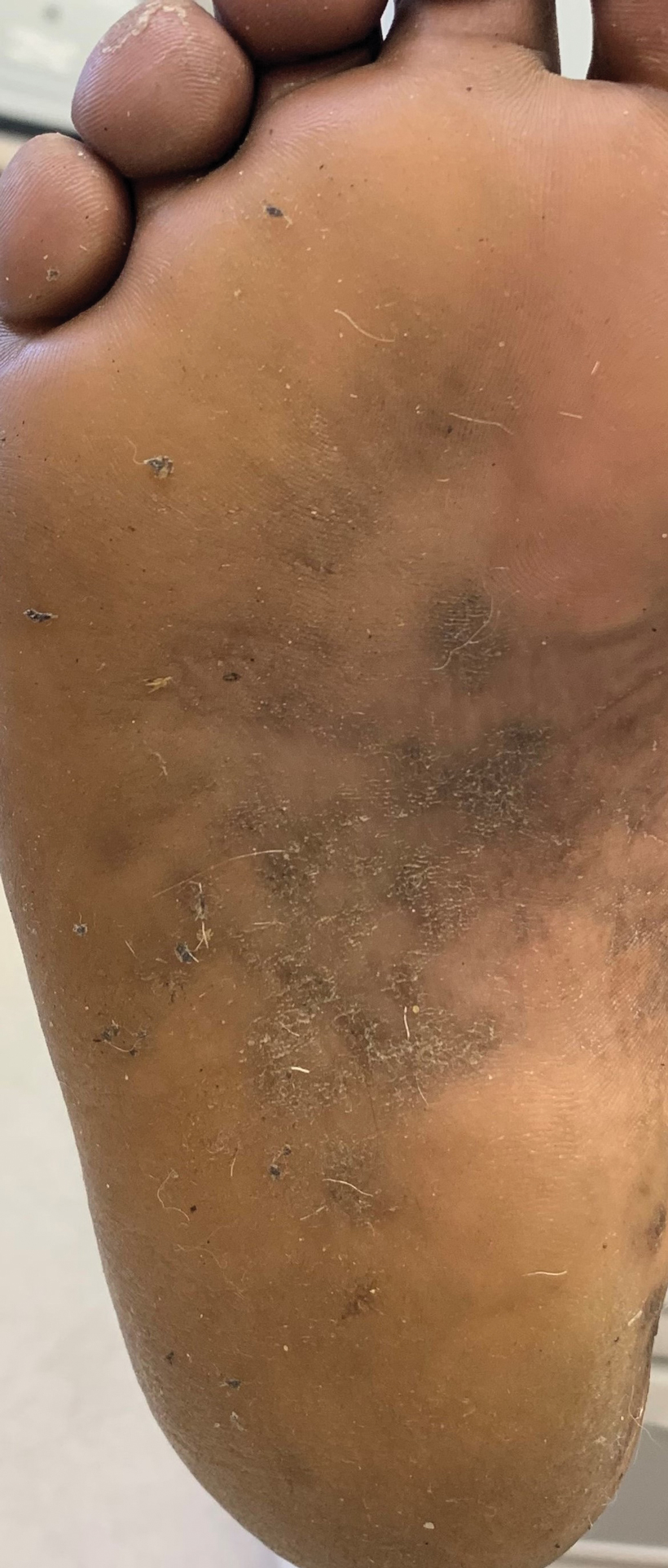
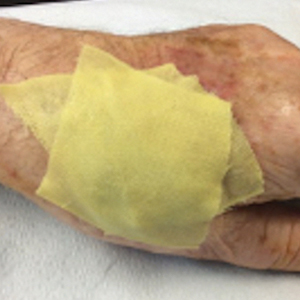
Treatment options for keratoderma climactericum include salicylic acid, emollients, oral retinoids, urea ointments, estriol cream, and topical steroids.5,6 Our patient was prescribed acitretin 25 mg daily and ammonium lactate to apply topically as needed for dry skin. Five months after the initial presentation, fissures and dry skin on the bilateral soles were still present. Ammonium lactate was discontinued, and the patient was prescribed urea cream 40%. Fifteen months after the initial presentation, the patient reported substantial improvement on the hands and feet and noted that she no longer needed the urea cream. Physical examination revealed no presence of hyperkeratosis or fissuring on the palms (Figure 2), and mild hyperkeratosis was present on the plantar surfaces of the feet (Figure 3). The patient continued to use acitretin to prevent disease relapse.
Keratoderma climactericum is an unusual and debilitating condition that occurs in women of menopausal age. It is diagnosed by its specific clinical presentation. More common diagnoses such as tinea and dermatitis should be ruled out before considering keratoderma climactericum.
- Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161-167.
- Patel S, Zirwas M, English JC. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Deschamps P, Leroy D, Pedailles S, et al. Keratoderma climactericum (Haxthausen's disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258-262.
- Wachtel TJ. Plantar and palmar hyperkeratosis in young castrated women. Int J Dermatol. 1981;20:270-271.
- Bristow I. The management of heel fissures using a steroid impregnated tape (Haelan) in a patient with Keratoderma climactericum. Podiatry Now. 2008;11:22-23.
- Mendes-Bastos P. Plantar keratoderma climactericum: successful improvement with a topical estriol cream. J Cosmet Dermatol. 2018;17:811-813.
The Diagnosis: Keratoderma Climactericum
Keratoderma climactericum was first reported in 1934 by Haxthausen1 as nonpruritic circumscribed hyperkeratosis located mainly on the palms and soles. The initial eruption was described as discrete lesions with an oval or round shape that progressed to less-defined, confluent, hyperkeratotic patches with fissures.1 Keratoderma climactericum also may be referred to as Haxthausen disease and is considered an acquired palmoplantar keratoderma.2
Keratoderma climactericum is a rare dermatologic disorder that presents in women of menopausal age who have no family or personal history of skin disease. Keratoderma climactericum is associated with hypertension and obesity.2 Keratotic lesions usually first occur on the plantar surfaces with eventual development of fissuring and hyperkeratosis that causes painful walking. The keratotic lesions on the plantar surfaces often are nonpruritic and gradually become confluent over time. As the disease progresses, keratotic lesions appear on the central palms, which can lead to confluent hyperkeratosis on the palmar surfaces (Figure 1).2 The exact mechanism of keratoderma climactericum has not been described but is believed to be due to hormonal dysregulation.2
In 1986, Deschamps et al3 presented 10 cases of keratoderma climactericum occurring in menopausal women with an average age of 57 years. The lesions began on the soles at areas of greatest pressure. Histopathology for each patient showed orthokeratotic hyperkeratosis, irregular hyperplasia, interpapillary ridges, and exocytosis of lymphocytes in the epidermis. Seven patients were treated with etretinate, which first led to the removal of palmar lesions, followed by improvement in plantar lesions and pain when walking. There was no association of keratoderma climactericum and sex hormones, as hormone levels were negative or normal for each patient.3
Three cases of keratoderma climactericum following bilateral oophorectomy in young women were reported by Wachtel4 in 1981. Unlike in women of menopausal age, there was no association of keratoderma climactericum with hypertension or obesity. Additionally, the lesions on the palms and soles were more diffusely distributed than in women of menopausal age. Estrogen administration completely reversed each patient's hyperkeratotic palms and soles.4 A definitive pathogenic role of estrogens in the development of keratoderma climactericum has yet to be determined.2
Histopathology is not specific for keratoderma climactericum, making the disease a clinical diagnosis. However, a biopsy may be useful to rule out palmoplantar psoriasis.2 Clinical information such as the age and sex of the patient, distribution of disease, presence of fissuring, and progression of disease from soles to palms should be considered when making a diagnosis of keratoderma climactericum. The differential diagnosis of keratoderma climactericum should include fungal infections, contact dermatitis, irritant dermatitis, psoriasis, atopic dermatitis, underlying malignancy, and pityriasis rubra pilaris.
Treatment options for keratoderma climactericum include salicylic acid, emollients, oral retinoids, urea ointments, estriol cream, and topical steroids.5,6 Our patient was prescribed acitretin 25 mg daily and ammonium lactate to apply topically as needed for dry skin. Five months after the initial presentation, fissures and dry skin on the bilateral soles were still present. Ammonium lactate was discontinued, and the patient was prescribed urea cream 40%. Fifteen months after the initial presentation, the patient reported substantial improvement on the hands and feet and noted that she no longer needed the urea cream. Physical examination revealed no presence of hyperkeratosis or fissuring on the palms (Figure 2), and mild hyperkeratosis was present on the plantar surfaces of the feet (Figure 3). The patient continued to use acitretin to prevent disease relapse.
Keratoderma climactericum is an unusual and debilitating condition that occurs in women of menopausal age. It is diagnosed by its specific clinical presentation. More common diagnoses such as tinea and dermatitis should be ruled out before considering keratoderma climactericum.
The Diagnosis: Keratoderma Climactericum
Keratoderma climactericum was first reported in 1934 by Haxthausen1 as nonpruritic circumscribed hyperkeratosis located mainly on the palms and soles. The initial eruption was described as discrete lesions with an oval or round shape that progressed to less-defined, confluent, hyperkeratotic patches with fissures.1 Keratoderma climactericum also may be referred to as Haxthausen disease and is considered an acquired palmoplantar keratoderma.2
Keratoderma climactericum is a rare dermatologic disorder that presents in women of menopausal age who have no family or personal history of skin disease. Keratoderma climactericum is associated with hypertension and obesity.2 Keratotic lesions usually first occur on the plantar surfaces with eventual development of fissuring and hyperkeratosis that causes painful walking. The keratotic lesions on the plantar surfaces often are nonpruritic and gradually become confluent over time. As the disease progresses, keratotic lesions appear on the central palms, which can lead to confluent hyperkeratosis on the palmar surfaces (Figure 1).2 The exact mechanism of keratoderma climactericum has not been described but is believed to be due to hormonal dysregulation.2
In 1986, Deschamps et al3 presented 10 cases of keratoderma climactericum occurring in menopausal women with an average age of 57 years. The lesions began on the soles at areas of greatest pressure. Histopathology for each patient showed orthokeratotic hyperkeratosis, irregular hyperplasia, interpapillary ridges, and exocytosis of lymphocytes in the epidermis. Seven patients were treated with etretinate, which first led to the removal of palmar lesions, followed by improvement in plantar lesions and pain when walking. There was no association of keratoderma climactericum and sex hormones, as hormone levels were negative or normal for each patient.3
Three cases of keratoderma climactericum following bilateral oophorectomy in young women were reported by Wachtel4 in 1981. Unlike in women of menopausal age, there was no association of keratoderma climactericum with hypertension or obesity. Additionally, the lesions on the palms and soles were more diffusely distributed than in women of menopausal age. Estrogen administration completely reversed each patient's hyperkeratotic palms and soles.4 A definitive pathogenic role of estrogens in the development of keratoderma climactericum has yet to be determined.2
Histopathology is not specific for keratoderma climactericum, making the disease a clinical diagnosis. However, a biopsy may be useful to rule out palmoplantar psoriasis.2 Clinical information such as the age and sex of the patient, distribution of disease, presence of fissuring, and progression of disease from soles to palms should be considered when making a diagnosis of keratoderma climactericum. The differential diagnosis of keratoderma climactericum should include fungal infections, contact dermatitis, irritant dermatitis, psoriasis, atopic dermatitis, underlying malignancy, and pityriasis rubra pilaris.
Treatment options for keratoderma climactericum include salicylic acid, emollients, oral retinoids, urea ointments, estriol cream, and topical steroids.5,6 Our patient was prescribed acitretin 25 mg daily and ammonium lactate to apply topically as needed for dry skin. Five months after the initial presentation, fissures and dry skin on the bilateral soles were still present. Ammonium lactate was discontinued, and the patient was prescribed urea cream 40%. Fifteen months after the initial presentation, the patient reported substantial improvement on the hands and feet and noted that she no longer needed the urea cream. Physical examination revealed no presence of hyperkeratosis or fissuring on the palms (Figure 2), and mild hyperkeratosis was present on the plantar surfaces of the feet (Figure 3). The patient continued to use acitretin to prevent disease relapse.
Keratoderma climactericum is an unusual and debilitating condition that occurs in women of menopausal age. It is diagnosed by its specific clinical presentation. More common diagnoses such as tinea and dermatitis should be ruled out before considering keratoderma climactericum.
- Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161-167.
- Patel S, Zirwas M, English JC. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Deschamps P, Leroy D, Pedailles S, et al. Keratoderma climactericum (Haxthausen's disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258-262.
- Wachtel TJ. Plantar and palmar hyperkeratosis in young castrated women. Int J Dermatol. 1981;20:270-271.
- Bristow I. The management of heel fissures using a steroid impregnated tape (Haelan) in a patient with Keratoderma climactericum. Podiatry Now. 2008;11:22-23.
- Mendes-Bastos P. Plantar keratoderma climactericum: successful improvement with a topical estriol cream. J Cosmet Dermatol. 2018;17:811-813.
- Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161-167.
- Patel S, Zirwas M, English JC. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Deschamps P, Leroy D, Pedailles S, et al. Keratoderma climactericum (Haxthausen's disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258-262.
- Wachtel TJ. Plantar and palmar hyperkeratosis in young castrated women. Int J Dermatol. 1981;20:270-271.
- Bristow I. The management of heel fissures using a steroid impregnated tape (Haelan) in a patient with Keratoderma climactericum. Podiatry Now. 2008;11:22-23.
- Mendes-Bastos P. Plantar keratoderma climactericum: successful improvement with a topical estriol cream. J Cosmet Dermatol. 2018;17:811-813.
A 52-year-old woman with a history of rheumatoid arthritis presented with a rash on the palms and soles of 7 years' duration that started around the onset of menopause. Physical examination revealed thick hyperkeratotic plaques with multiple deep fissures on the palms and soles. The patient's current medications included methotrexate for rheumatoid arthritis. She previously had been prescribed adalimumab by an outside physician for the rash, which provided no relief, and currently was using urea ointment, which caused a burning sensation on the palms and soles. The patient denied a personal or family history of psoriasis.
Management of a Child vs an Adult Presenting With Acral Lesions During the COVID-19 Pandemic: A Practical Review
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
Practice Points
- Children with chilblainslike lesions generally have a favorable prognosis. As lesions self-resolve, treatment should focus on symptom management and education.
- In children with chilblainslike lesions and no systemic symptoms, further workup for coronavirus disease 2019 (COVID-19) is not necessary for the care of the individual patient.
- In adults with acral lesions, it is important to distinguish between chilblainslike lesions, true acral ischemia, and retiform purpura. Chilblainslike lesions have been associated with mild COVID-19 disease, whereas acral ischemia and retiform purpura have been associated with severe and fatal disease.
- Biopsy and COVID-19 testing should be obtained in adults if there is diagnostic uncertainty or if there are worsening symptoms.
Assessing Psychological Interventions for Hidradenitis Suppurativa as a First Step Toward Patient-Centered Practice
Hidradenitis suppurativa (HS)(also known as acne inversa) is a chronic, recurrent, and debilitating inflammatory dermatologic disease of the hair follicle. It usually presents after puberty, with painful, deep-seated, inflamed lesions in apocrine gland–bearing areas of the body, most commonly the axillae and inguinal and anogenital regions.1
Hidradenitis suppurativa patients have a high rate of psychologic and psychiatric comorbidities that often are interrelated and multidirectional. Approximately 1 in 4 adults with HS also experience depression (prevalence among all HS patients, 16.9%), and 1 in 5 experience anxiety (prevalence, 4.9%).2,3 Hidradenitis suppurativa has been associated with bipolar disorder, schizophrenia, and suicidality.2,4
These comorbidity factors have a remarkable impact on HS patients’ quality of life (QOL). Compared to other diseases, including psoriasis, stroke, and conditions that create candidacy for heart transplantation, HS was identified as the most impairing condition.5,6 It is estimated that more than 50% of HS patients experience a very or extremely large effect on their QOL, as measured by the dermatology life quality index.6
Pain, a major component of low QOL in HS patients, has an adverse impact on emotional health. Hidradenitis suppurativa causes body image dissatisfaction, leading to shame, embarrassment, lack of self-confidence, stigmatization, and social isolation.7-9 Furthermore, patients with HS have an increased risk for antidepressant drug use, completed suicide, and suicidal behavior compared to the general population.10
Focusing therapy on physical manifestations of HS only while ignoring the psychologic aspect could lead to a vicious cycle in which stress triggers flares, leading to worsening HS, leading to more stress, and so on.11 Therefore, psychological support for HS patients is critical, and we believe it should be an integral part of managing the disease.
There is no evidence to support effective therapeutic intervention for psychological aspects of HS. We conducted a PubMed search of articles indexed for MEDLINE using the term hidradenitis in combination with psychology, psychological, mindfulness, and cognitive behavioral therapy. No relevant articles were found. Most articles on HS focused on the low QOL associated with the disease and patient coping mechanisms. However, there are a number of psychological therapies to consider and evaluate for the management of HS.
Psychological Therapies to Consider in HS
Cognitive Behavioral Treatment
Cognitive behavioral treatment has been successfully used to manage skin diseases other than HS.12 Patients’ shame and stigmatization due to body dissatisfaction often cause social isolation, which might appear as social anxiety.9,13 Cognitive behavioral treatment, or compassion-focused therapy, could increase patients’ self-acceptance and reduce shameful feelings.13
Group Therapy
Alternatively, group therapy might be beneficial for HS patients. Research has shown that most HS patients know others affected by the same disease or attend an HS support group, and patients value the support of peers with the disease.13 Therefore, group therapy meetings with HS patients that are directed by a health care professional might reduce feelings of shame and stigmatization and increase feelings of social acceptance.
Mindfulness
Another approach for managing psychological aspects of skin diseases that might be useful in HS is mindfulness-based stress reduction (MBSR), developed by Kabat-Zinn and colleagues,14 which helps patients develop mindfulness through training in meditation. It is an intensive, structured, patient-centered approach that has been successfully used in a variety of settings.14,15
Current evidence supports the use of MBSR in the adjunct treatment of chronic pain, anxiety, and depression—symptoms that have a great impact on HS patients’ QOL.16 Furthermore, MBSR is offered in a group setting, which is potentially an opportunity for peer support and understanding; social support has been reported to be highly beneficial for HS patients.17
Can the Placebo Effect Aid in Managing HS?
A recent review that assessed the placebo effect in randomized clinical trials (RCTs) of treatments for cutaneous disease demonstrated that the placebo effect in HS therapy trials is higher than in RCTs of therapies for psoriasis and eczema. This finding highlights the importance of the physician-patient relationship when managing HS, which can result in greater treatment adherence and more patient education, empowerment, and encouragement toward beneficial lifestyle changes.18
Complementary psychological interventions for managing HS might maximize the placebo effect in clinical practice.18 The placebo effect in RCTs is higher for HS treatments than for psoriasis treatments, and if patients with psoriasis improved with psychological interventions,12 it would be reasonable to expect an improvement in QOL with psychological interventions for HS.
Final Thoughts
Although a number of studies have been published in the medical literature regarding psychological intervention in psoriasis management,12 we found no clinical studies assessing the psychological management of HS. We conclude that more research is necessary to develop psychological interventions targeting HS patients because a multidisciplinary and patient-centered approach is essential for the management of HS.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:737-744.
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Sampogna F, Fania L, Mazzanti C, et al. The broad-spectrum impact of hidradenitis suppurativa on quality of life: a comparison with psoriasis. Dermatology. 2019;235:308-314.
- von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809-813.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332.
- Schneider-Burrus S, Jost A, Peters EMJ, et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 2018;154:447-451.
- Koumaki D, Efthymiou O, Bozi E, et al. Perspectives on perceived stigma and self-stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol. 2019;12:785-790.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112.
- Qureshi AA, Awosika O, Baruffi F, et al. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol. 2019;20:607-624.
- Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol. 2020;182:342-347.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936-943.
- Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716-721.
- Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Golbari NM, Porter ML, Kimball AM. Online communications among hidradenitis suppurativa patients reflect community needs. J Am Acad Dermatol. 2019;80:1760-1762.
- Ali AA, Seng EK, Alavi A, et al. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82:45-53.
Hidradenitis suppurativa (HS)(also known as acne inversa) is a chronic, recurrent, and debilitating inflammatory dermatologic disease of the hair follicle. It usually presents after puberty, with painful, deep-seated, inflamed lesions in apocrine gland–bearing areas of the body, most commonly the axillae and inguinal and anogenital regions.1
Hidradenitis suppurativa patients have a high rate of psychologic and psychiatric comorbidities that often are interrelated and multidirectional. Approximately 1 in 4 adults with HS also experience depression (prevalence among all HS patients, 16.9%), and 1 in 5 experience anxiety (prevalence, 4.9%).2,3 Hidradenitis suppurativa has been associated with bipolar disorder, schizophrenia, and suicidality.2,4
These comorbidity factors have a remarkable impact on HS patients’ quality of life (QOL). Compared to other diseases, including psoriasis, stroke, and conditions that create candidacy for heart transplantation, HS was identified as the most impairing condition.5,6 It is estimated that more than 50% of HS patients experience a very or extremely large effect on their QOL, as measured by the dermatology life quality index.6
Pain, a major component of low QOL in HS patients, has an adverse impact on emotional health. Hidradenitis suppurativa causes body image dissatisfaction, leading to shame, embarrassment, lack of self-confidence, stigmatization, and social isolation.7-9 Furthermore, patients with HS have an increased risk for antidepressant drug use, completed suicide, and suicidal behavior compared to the general population.10
Focusing therapy on physical manifestations of HS only while ignoring the psychologic aspect could lead to a vicious cycle in which stress triggers flares, leading to worsening HS, leading to more stress, and so on.11 Therefore, psychological support for HS patients is critical, and we believe it should be an integral part of managing the disease.
There is no evidence to support effective therapeutic intervention for psychological aspects of HS. We conducted a PubMed search of articles indexed for MEDLINE using the term hidradenitis in combination with psychology, psychological, mindfulness, and cognitive behavioral therapy. No relevant articles were found. Most articles on HS focused on the low QOL associated with the disease and patient coping mechanisms. However, there are a number of psychological therapies to consider and evaluate for the management of HS.
Psychological Therapies to Consider in HS
Cognitive Behavioral Treatment
Cognitive behavioral treatment has been successfully used to manage skin diseases other than HS.12 Patients’ shame and stigmatization due to body dissatisfaction often cause social isolation, which might appear as social anxiety.9,13 Cognitive behavioral treatment, or compassion-focused therapy, could increase patients’ self-acceptance and reduce shameful feelings.13
Group Therapy
Alternatively, group therapy might be beneficial for HS patients. Research has shown that most HS patients know others affected by the same disease or attend an HS support group, and patients value the support of peers with the disease.13 Therefore, group therapy meetings with HS patients that are directed by a health care professional might reduce feelings of shame and stigmatization and increase feelings of social acceptance.
Mindfulness
Another approach for managing psychological aspects of skin diseases that might be useful in HS is mindfulness-based stress reduction (MBSR), developed by Kabat-Zinn and colleagues,14 which helps patients develop mindfulness through training in meditation. It is an intensive, structured, patient-centered approach that has been successfully used in a variety of settings.14,15
Current evidence supports the use of MBSR in the adjunct treatment of chronic pain, anxiety, and depression—symptoms that have a great impact on HS patients’ QOL.16 Furthermore, MBSR is offered in a group setting, which is potentially an opportunity for peer support and understanding; social support has been reported to be highly beneficial for HS patients.17
Can the Placebo Effect Aid in Managing HS?
A recent review that assessed the placebo effect in randomized clinical trials (RCTs) of treatments for cutaneous disease demonstrated that the placebo effect in HS therapy trials is higher than in RCTs of therapies for psoriasis and eczema. This finding highlights the importance of the physician-patient relationship when managing HS, which can result in greater treatment adherence and more patient education, empowerment, and encouragement toward beneficial lifestyle changes.18
Complementary psychological interventions for managing HS might maximize the placebo effect in clinical practice.18 The placebo effect in RCTs is higher for HS treatments than for psoriasis treatments, and if patients with psoriasis improved with psychological interventions,12 it would be reasonable to expect an improvement in QOL with psychological interventions for HS.
Final Thoughts
Although a number of studies have been published in the medical literature regarding psychological intervention in psoriasis management,12 we found no clinical studies assessing the psychological management of HS. We conclude that more research is necessary to develop psychological interventions targeting HS patients because a multidisciplinary and patient-centered approach is essential for the management of HS.
Hidradenitis suppurativa (HS)(also known as acne inversa) is a chronic, recurrent, and debilitating inflammatory dermatologic disease of the hair follicle. It usually presents after puberty, with painful, deep-seated, inflamed lesions in apocrine gland–bearing areas of the body, most commonly the axillae and inguinal and anogenital regions.1
Hidradenitis suppurativa patients have a high rate of psychologic and psychiatric comorbidities that often are interrelated and multidirectional. Approximately 1 in 4 adults with HS also experience depression (prevalence among all HS patients, 16.9%), and 1 in 5 experience anxiety (prevalence, 4.9%).2,3 Hidradenitis suppurativa has been associated with bipolar disorder, schizophrenia, and suicidality.2,4
These comorbidity factors have a remarkable impact on HS patients’ quality of life (QOL). Compared to other diseases, including psoriasis, stroke, and conditions that create candidacy for heart transplantation, HS was identified as the most impairing condition.5,6 It is estimated that more than 50% of HS patients experience a very or extremely large effect on their QOL, as measured by the dermatology life quality index.6
Pain, a major component of low QOL in HS patients, has an adverse impact on emotional health. Hidradenitis suppurativa causes body image dissatisfaction, leading to shame, embarrassment, lack of self-confidence, stigmatization, and social isolation.7-9 Furthermore, patients with HS have an increased risk for antidepressant drug use, completed suicide, and suicidal behavior compared to the general population.10
Focusing therapy on physical manifestations of HS only while ignoring the psychologic aspect could lead to a vicious cycle in which stress triggers flares, leading to worsening HS, leading to more stress, and so on.11 Therefore, psychological support for HS patients is critical, and we believe it should be an integral part of managing the disease.
There is no evidence to support effective therapeutic intervention for psychological aspects of HS. We conducted a PubMed search of articles indexed for MEDLINE using the term hidradenitis in combination with psychology, psychological, mindfulness, and cognitive behavioral therapy. No relevant articles were found. Most articles on HS focused on the low QOL associated with the disease and patient coping mechanisms. However, there are a number of psychological therapies to consider and evaluate for the management of HS.
Psychological Therapies to Consider in HS
Cognitive Behavioral Treatment
Cognitive behavioral treatment has been successfully used to manage skin diseases other than HS.12 Patients’ shame and stigmatization due to body dissatisfaction often cause social isolation, which might appear as social anxiety.9,13 Cognitive behavioral treatment, or compassion-focused therapy, could increase patients’ self-acceptance and reduce shameful feelings.13
Group Therapy
Alternatively, group therapy might be beneficial for HS patients. Research has shown that most HS patients know others affected by the same disease or attend an HS support group, and patients value the support of peers with the disease.13 Therefore, group therapy meetings with HS patients that are directed by a health care professional might reduce feelings of shame and stigmatization and increase feelings of social acceptance.
Mindfulness
Another approach for managing psychological aspects of skin diseases that might be useful in HS is mindfulness-based stress reduction (MBSR), developed by Kabat-Zinn and colleagues,14 which helps patients develop mindfulness through training in meditation. It is an intensive, structured, patient-centered approach that has been successfully used in a variety of settings.14,15
Current evidence supports the use of MBSR in the adjunct treatment of chronic pain, anxiety, and depression—symptoms that have a great impact on HS patients’ QOL.16 Furthermore, MBSR is offered in a group setting, which is potentially an opportunity for peer support and understanding; social support has been reported to be highly beneficial for HS patients.17
Can the Placebo Effect Aid in Managing HS?
A recent review that assessed the placebo effect in randomized clinical trials (RCTs) of treatments for cutaneous disease demonstrated that the placebo effect in HS therapy trials is higher than in RCTs of therapies for psoriasis and eczema. This finding highlights the importance of the physician-patient relationship when managing HS, which can result in greater treatment adherence and more patient education, empowerment, and encouragement toward beneficial lifestyle changes.18
Complementary psychological interventions for managing HS might maximize the placebo effect in clinical practice.18 The placebo effect in RCTs is higher for HS treatments than for psoriasis treatments, and if patients with psoriasis improved with psychological interventions,12 it would be reasonable to expect an improvement in QOL with psychological interventions for HS.
Final Thoughts
Although a number of studies have been published in the medical literature regarding psychological intervention in psoriasis management,12 we found no clinical studies assessing the psychological management of HS. We conclude that more research is necessary to develop psychological interventions targeting HS patients because a multidisciplinary and patient-centered approach is essential for the management of HS.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:737-744.
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Sampogna F, Fania L, Mazzanti C, et al. The broad-spectrum impact of hidradenitis suppurativa on quality of life: a comparison with psoriasis. Dermatology. 2019;235:308-314.
- von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809-813.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332.
- Schneider-Burrus S, Jost A, Peters EMJ, et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 2018;154:447-451.
- Koumaki D, Efthymiou O, Bozi E, et al. Perspectives on perceived stigma and self-stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol. 2019;12:785-790.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112.
- Qureshi AA, Awosika O, Baruffi F, et al. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol. 2019;20:607-624.
- Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol. 2020;182:342-347.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936-943.
- Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716-721.
- Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Golbari NM, Porter ML, Kimball AM. Online communications among hidradenitis suppurativa patients reflect community needs. J Am Acad Dermatol. 2019;80:1760-1762.
- Ali AA, Seng EK, Alavi A, et al. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82:45-53.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:737-744.
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Sampogna F, Fania L, Mazzanti C, et al. The broad-spectrum impact of hidradenitis suppurativa on quality of life: a comparison with psoriasis. Dermatology. 2019;235:308-314.
- von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809-813.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332.
- Schneider-Burrus S, Jost A, Peters EMJ, et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 2018;154:447-451.
- Koumaki D, Efthymiou O, Bozi E, et al. Perspectives on perceived stigma and self-stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol. 2019;12:785-790.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112.
- Qureshi AA, Awosika O, Baruffi F, et al. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol. 2019;20:607-624.
- Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol. 2020;182:342-347.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936-943.
- Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716-721.
- Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Golbari NM, Porter ML, Kimball AM. Online communications among hidradenitis suppurativa patients reflect community needs. J Am Acad Dermatol. 2019;80:1760-1762.
- Ali AA, Seng EK, Alavi A, et al. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82:45-53.
PRACTICE POINTS
- Although hidradenitis suppurativa (HS) has high rates of psychological comorbidities, management of the psychological aspects of the disease has not been studied extensively.
- Complementary psychological interventions should be evaluated for the management of HS.
Distribution of Skin-Type Diversity in Photographs in AAD Online Educational Modules
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
PRACTICE POINTS
- Recent studies have highlighted poor representation of darker skin types in textbooks.
- The Basic Dermatology Curriculum of the American Academy of Dermatology has a low (16%) representation of darker skin types in photographs; more than one-quarter of curriculum lectures had no such images.
- Darker skin types were underrepresented for skin cancers and overrepresented for sexually transmitted infections, raising questions about how photographs were chosen.
- Educators should consider using existing resources of photographs of diverse skin types when designing future curricula.
Are There Mobile Applications Related to Nail Disorders?
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
Practice Points
- Patient-targeted mobile applications (apps) might aid with clinical referencing and education.
- There are patient-directed psoriasis and hair loss apps on iOS and Android platforms, but informative apps related to nail disorders are limited.
- There is a need to develop apps related to nail health for patient education.
24-7 Dressing Technique to Optimize Wound Healing After Mohs Micrographic Surgery
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
Comparison of Shave and Punch Biopsy Utilization Among Dermatology Practices
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
Practice Points
- Dermatologists should be aware that skin biopsy billing codes and reimbursements were changed in 2019 to reflect their level of complexity, which may impact how often each type of biopsy is used.
- Even small shifts in biopsy utilization behavior among dermatologists in the context of reimbursement changes can have a large impact on net reimbursements.
Upper Lip Anatomy, Mechanics of Local Flaps, and Considerations for Reconstruction
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
The upper lip poses challenges during reconstruction. Distortion of well-defined anatomic structures, including the vermilion border, oral commissures, Cupid’s bow, and philtrum, leads to noticeable deformities. Furthermore, maintenance of upper and lower lip function is essential for verbal communication, facial expression, and controlled opening of the oral cavity.
Similar to a prior review focused on the lower lip,1 we conducted a review of the literature using the PubMed database (1976-2017) and the following search terms: upper lip, lower lip, anatomy, comparison, cadaver, histology, local flap, and reconstruction. We reviewed studies that assessed anatomic and histologic characteristics of the upper and the lower lips, function of the upper lip, mechanics of local flaps, and upper lip reconstruction techniques including local flaps and regional flaps. Articles with an emphasis on free flaps were excluded.
The initial search resulted in 1326 articles. Of these, 1201 were excluded after abstracts were screened. Full-text review of the remaining 125 articles resulted in exclusion of 85 papers (9 foreign language, 4 duplicates, and 72 irrelevant). Among the 40 articles eligible for inclusion, 12 articles discussed anatomy and histology of the upper lip, 9 examined function of the upper lip, and 19 reviewed available techniques for reconstruction of the upper lip.
In this article, we review the anatomy and function of the upper lip as well as various repair techniques to provide the reconstructive surgeon with greater familiarity with the local flaps and an algorithmic approach for upper lip reconstruction.
Anatomic Characteristics of the Upper Lip
The muscular component of the upper lip primarily is comprised of the orbicularis oris (OO) muscle divided into 2 distinct concentric components: pars peripheralis and pars marginalis.2,3 It is discontinuous in some individuals.4 Although OO is the primary muscle of the lower lip, the upper lip is remarkably complex. Orbicularis oris and 3 additional muscles contribute to upper lip function: depressor septi nasi, the alar portion of the nasalis, and levator labii superioris alaeque nasi (LLSAN).5
The modiolus, a muscular structure located just lateral to the commissures, serves as a convergence point for facial muscle animation and lip function while distributing contraction forces between the lips and face.6 It is imperative to preserve its location in reconstruction to allow for good functional and aesthetic outcomes.
The upper lip is divided into 3 distinct aesthetic subunits: the philtrum and 1 lateral subunit on each side.7,8 Its unique surface features include the Cupid’s bow, vermilion tubercle, and philtral columns. The philtral columns are created by the dermal insertion on each side of the OO, which originates from the modiolus, decussates, and inserts into the skin of the contralateral philtral groove.2,9-11 The OO has additional insertions into the dermis lateral to the philtrum.5 During its course across the midline, it decreases its insertions, leading to the formation and thinness of the philtral dimple.9 The philtral shape primarily is due to the intermingling of LLSAN and the pars peripheralis in an axial plane. The LLSAN enters superolateral to the ipsilateral philtral ridge and courses along this ridge to contribute to the philtral shape.2 Formation of the philtrum’s contour arises from the opposing force of both muscles pulling the skin in opposite directions.2,5 The vermilion tubercle arises from the dermal insertion of the pars marginalis originating from the ipsilateral modiolus and follows the vermilion border.2 The Cupid’s bow is part of the white roll at the vermilion-cutaneous junction produced by the anterior projection of the pars peripheralis.10 The complex anatomy of this structure explains the intricacy of lip reconstructions in this area.
Function of the Upper Lip
Although the primary purpose of OO is sphincteric function, the upper lip’s key role is coverage of dentition and facial animation.12 The latter is achieved through the relationship of multiple muscles, including levator labii superioris, levator septi nasi, risorius, zygomaticus minor, zygomaticus major, levator anguli oris, and buccinator.7,13-17 Their smooth coordination results in various facial expressions. In comparison, the lower lip is critical for preservation of oral competence, prevention of drooling, eating, and speech due to the actions of OO and vertical support from the mentalis muscle.1,18-22
Reconstructive Methods for the Upper Lip
Multiple options are available for reconstruction of upper lip defects, with the aim to preserve facial animation and coverage of dentition. When animation muscles are involved, restoring function is the goal, which can be achieved by placing sutures to reapproximate the muscle edges in smaller defects or anchor the remaining muscle edge to preserve deep structures in larger defects, respecting the vector of contraction and attempting simulation of the muscle function. Additionally, restoration of the continuity of OO also is important for good aesthetic and functional outcomes.
Janis23 proposed the rule of thirds to approach upper and lower lip reconstruction. Using these rules, we briefly analyze the available flaps focusing on animation, OO restoration, preservation of the modiolus position, and sensation for each (eTable).
The perialar crescentic flap, an advancement flap, can be utilized for laterally located partial-thickness defects affecting up to one-third of the upper lip, especially those adjacent to the alar base, as well as full-thickness defects affecting up to two-thirds of the upper lip.7,24 The OO continuity and position of the modiolus often are preserved, sensation is maintained, and muscles of animation commonly are unaffected by this flap, especially in partial-thickness defects. In males, caution should be exercised where non–hair-bearing skin of the cheek is advanced to the upper lip region. Other potential complications include obliteration of the melolabial crease and pincushioning.7
Nasolabial (ie, melolabial) flaps are suggested for repair of defects up to one-third of the upper lip, especially when the vermilion is unaffected, or in lateral defects with or without commissure involvement.7,24-28 This flap is based on the facial artery and may be used as a direct transposition, V-Y advancement, or island flap with good aesthetic and functional outcomes (Figure 1).29,30 There is limited literature regarding the effects on animation. However, it may be beneficial in avoiding microstomia, as regional tissue is transferred from the cheek area, maintaining upper lip length. Additionally, the location of the modiolus often is unaffected, especially when the flap is harvested above the level of the muscle, providing superior facial animation function. Flap design is critical in areas lateral to the commissure and over the modiolus, as distortion of its position can occur.26 Similar to crescentic advancement, it is important to exercise caution in male patients, as non–hair-bearing tissue can be transferred to the upper lip. Reported adverse outcomes of the nasolabial flap include a thin flat upper lip, obliteration of the Cupid’s bow, and hypoesthesia that may improve over time.30
The Abbe flap is suitable for reconstruction of upper lip defects affecting up to two-thirds of the upper lip and lateral defects, provided the commissure or philtrum is unaffected.7,8 It is a 2-stage lip-switch flap based on the inferior labial artery, where tissue is harvested and transferred from the lower lip (Figure 2).23,31 It is particularly useful for philtral reconstruction, as incision lines at the flap edges can recreate the skin folds of the philtrum. Moreover, incision lines are better concealed under the nose, making it favorable for female patients. Surgeons should consider the difference in philtral width between sexes when designing this flap for optimal aesthetic outcome, as males have larger philtral width than females.21 The Abbe flap allows preservation of the Cupid’s bow, oral commissure, and modiolus position; however, it is an insensate flap and does not establish continuity of OO.23 For central defects, the function of animation muscles is not critically affected. In philtral reconstruction using an Abbe flap, a common adverse outcome is widening of the central segment because of tension and contraction forces applied by the adjacent OO. Restoration of the continuity of the muscle through dissection and advancement in small defects or anchoring of muscle edges on deeper surfaces may avoid direct pull on the flap. In larger central defects extending beyond the native philtrum, it is important to recreate the philtrum proportional to the remaining upper and lower lips. The recommended technique is a combination of a thin Abbe flap with bilateral perialar crescentic advancement flaps to maintain a proportional philtrum. Several variations have been described, including 3D planning with muscular suspension for natural raised philtral columns, avoiding a flat upper lip.5
The Yu flap, a sensate single-stage rotational advancement flap, can be used in a variety of ways for repair of upper lip defects, depending on the size and location.26 Lateral defects up to one-half of the upper lip should be repaired with a unilateral reverse Yu flap, central defects up to one-half of the upper lip can be reconstructed with bilateral reverse Yu flaps, and defects up to two-thirds of the upper lip can be repaired with bilateral Yu flaps. This flap restores OO continuity and thus preserves sphincter function, minimizes oral incompetence, and has a low risk of microstomia. The muscles of facial animation are preserved, yet the modiolus is not. Good aesthetic outcomes have been reported depending on the location of the Yu flap because scars can be placed in the nasolabial sulcus, commissures, or medially to recreate the philtrum.26
The Estlander flap is a single-stage flap utilizing donor tissue from the opposing lip for reconstruction of lateral defects up to two-thirds of the upper lip with commissure and philtrum involvement (Figure 3).8,23,32 It is an insensate flap that alters the position of the modiolus, distorting oral and facial animation.23 The superomedial position of the modiolus is better tolerated in the upper lip because it increases the relaxation tone of the lower lip and simulates the vector of contraction of major animation muscles, positively impacting the sphincteric function of the reconstructed lip. Sphincteric function action is not as impaired compared with the lower lip because the new position of the modiolus tightens the lower lip and prevents drooling.33 When designing the flap, one should consider that the inferior labial artery has been reported to remain with 10 mm of the superior border of the lower lip; therefore, pedicles of the Abbe and Estlander flaps should be at least 10 mm from the vermilion border to preserve vascular supply.34,35
The Karapandzic flap, a modified Gilles fan flap, can be employed for repair of central defects up to two-thirds of the upper lip.8,23,32,36-39 The bilateral advancement of full-thickness adjacent tissue edges preserves neurovascular structures allowing sensation and restores OO continuation.40 Prior studies have shown the average distance of the superior labial artery emergence from the facial artery and labial commissure is 12.1 mm; thus, at least 12.1 mm of tissue from the commissure should be preserved to prevent vascular compromise in Karapandzic flaps.34,35 The modiolus position is altered, and facial animation muscles are disrupted, consequently impairing facial animation, especially elevation of the lip.36 The philtrum is obliterated, producing unfavorable aesthetic outcomes. Finally, the upper lip is thinner and smaller in volume than the lower lip, increasing the risk for microstomia compared with the lower lip with a similar reconstructive technique.36
Defects larger than two-thirds of the upper lip require a Bernard Burrow flap, distant free flap, or combination of multiple regional and local flaps dependent on the characteristics of the defect.36,41 Distant free flaps are beyond the scope of this review. The Bernard Burrow flap consists of bilaterally opposing cheek advancement flaps. It is an insensate flap that does not restore OO continuity, producing minimal muscle function and poor animation. Microstomia is a common adverse outcome.36
Conclusion
Comprehensive understanding of labial anatomy and its intimate relationship to function and aesthetics of the upper lip are critical. Flap anatomy and mechanics are key factors for successful reconstruction. The purpose of this article is to utilize knowledge of histology, anatomy, and function of the upper lip to improve the outcomes of reconstruction. The Abbe flap often is utilized for reconstruction of the philtrum and central upper lip defects, though it is a less desirable option for lower lip reconstruction. The Karapandzic flap, while sensate and restorative of OO continuity, may have less optimal functional and cosmetic results compared with its use in the lower lip. Regarding lateral defects involving the commissure, the Estlander flap provides a reasonable option for the upper lip when compared with its use in lower lip defects, where outcomes are usually inferior.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
- Boukovalas S, Boson AL, Hays JP, et al. A systematic review of lower lip anatomy, mechanics of local flaps, and special considerations for lower lip reconstruction. J Drugs Dermatol. 2017;16:1254-1261.
- Wu J, Yin N. Detailed anatomy of the nasolabial muscle in human fetuses as determined by micro-CT combined with iodine staining. Ann Plast Surg. 2016;76:111-116.
- Pepper JP, Baker SR. Local flaps: cheek and lip reconstruction. JAMA Facial Plast Surg. 2013;15:374-382.
- Rogers CR, Weinberg SM, Smith TD, et al. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518-524.
- Yin N, Wu D, Wang Y, et al. Complete philtrum reconstruction on the partial-thickness cross-lip flap by nasolabial muscle tension line group reconstruction in the same stage of flap transfer. JAMA Facial Plast Surg. 2017;19:496-501.
- Al-Hoqail RA, Abdel Meguid EM. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plast Surg. 2009;33:147-152.
- Galyon SW, Frodel JL. Lip and perioral defects. Otolaryngol Clin North Am. 2001;34:647-666.
- Massa AF, Otero-Rivas M, González-Sixto B, et al. Combined cutaneous rotation flap and myomucosal tongue flap for reconstruction of an upper lip defect. Actas Dermosifiliogr. 2014;105:869-871.
- Latham RA, Deaton TG. The structural basis of the philtrum and the contour of the vermilion border: a study of the musculature of the upper lip. J Anat. 1976;121:151-160.
- Garcia de Mitchell CA, Pessa JE, Schaverien MV, et al. The philtrum: anatomical observations from a new perspective. Plast Reconstr Surg. 2008;122:1756-1760.
- Bo C, Ningbei Y. Reconstruction of upper lip muscle system by anatomy, magnetic resonance imaging, and serial histological sections. J Craniofac Surg. 2014;25:48-54.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453.
- Hur MS, Youn KH, Hu KS, et al. New anatomic considerations on the levator labii superioris related with the nasal ala. J Craniofac Surg. 2010;21:258-260.
- Song R, Ma H, Pan F. The “levator septi nasi muscle” and its clinical significance. Plast Reconstr Surg. 2002;109:1707-1712; discussion 1713.
- Choi DY, Hur MS, Youn KH, et al. Clinical anatomic considerations of the zygomaticus minor muscle based on the morphology and insertion pattern. Dermatol Surg. 2014;40:858-863.
- Youn KH, Park JT, Park DS, et al. Morphology of the zygomaticus minor and its relationship with the orbicularis oculi muscle. J Craniofac Surg. 2012;23:546-548.
- Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Craniomaxillofac Surg. 2016;44:1917-1921.
- Vinkka-Puhakka H, Kean MR, Heap SW. Ultrasonic investigation of the circumoral musculature. J Anat. 1989;166:121-133.
- Ferrario VF, Rosati R, Peretta R, et al. Labial morphology: a 3-dimensional anthropometric study. J Oral Maxillofac Surg. 2009;67:1832-1839.
- Ferrario VF, Sforza C, Schmitz JH, et al. Normal growth and development of the lips: a 3-dimensional study from 6 years to adulthood using a geometric model. J Anat. 2000;196:415-423.
- Sforza C, Grandi G, Binelli M, et al. Age- and sex-related changes in three-dimensional lip morphology. Forensic Sci Int. 2010;200:182.e181-187.
- Wilson DB. Embryonic development of the head and neck: part 3, the face. Head Neck Surg. 1979;2:145-153.
- Janis JE, ed. Essentials of Plastic Surgery. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2014.
- Burusapat C, Pitiseree A. Advanced squamous cell carcinoma involving both upper and lower lips and oral commissure with simultaneous reconstruction by local flap: a case report. J Med Case Rep. 2012;6:23.
- El-Marakby HH. The versatile naso-labial flaps in facial reconstruction. J Egypt Natl Canc Inst. 2005;17:245-250.
- Li ZN, Li RW, Tan XX, et al. Yu’s flap for lower lip and reverse Yu’s flap for upper lip reconstruction: 20 years experience. Br J Oral Maxillofac Surg. 2013;51:767-772.
- Wollina U. Reconstructive surgery in advanced perioral non-melanoma skin cancer. Results in elderly patients. J Dermatol Case Rep. 2014;8:103-107.
- Younger RA. The versatile melolabial flap. Otolaryngol Head Neck Surg. 1992;107:721-726.
- Włodarkiewicz A, Wojszwiłło-Geppert E, Placek W, et al. Upper lip reconstruction with local island flap after neoplasm excision. Dermatol Surg. 1997;23:1075-1079.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Kriet JD, Cupp CL, Sherris DA, et al. The extended Abbé flap. Laryngoscope. 1995;105:988-992.
- Khan AA, Kulkarni JV. Karapandzic flap. Indian J Dent. 2014;5:107-109.
- Raschke GF, Rieger UM, Bader RD, et al. Lip reconstruction: an anthropometric and functional analysis of surgical outcomes. Int J Oral Maxillofac Surg. 2012;41:744-750.
- Maǧden O, Edizer M, Atabey A, et al. Cadaveric study of the arterial anatomy of the upper lip. Plast Reconstr Surg. 2004;114:355-359.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arterial supply of the lips: an anatomical and analytical approach. J Craniofac Surg. 2008;19:785-794.
- Kim JC, Hadlock T, Varvares MA, et al. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001;3:170-177.
- Teemul TA, Telfer A, Singh RP, et al. The versatility of the Karapandzic flap: a review of 65 cases with patient-reported outcomes. J Craniomaxillofac Surg. 2017;45:325-329.
- Matteini C, Mazzone N, Rendine G, et al. Lip reconstruction with local m-shaped composite flap. J Craniofac Surg. 2010;21:225-228.
- Williams EF, Setzen G, Mulvaney MJ. Modified Bernard-Burow cheek advancement and cross-lip flap for total lip reconstruction. Arch Otolaryngol Head Neck Surg. 1996;122:1253-1258.
- Jaquet Y, Pasche P, Brossard E, et al. Meyer’s surgical procedure for the treatment of lip carcinoma. Eur Arch Otorhinolaryngol. 2005;262:11-16.
- Dang M, Greenbaum SS. Modified Burow’s wedge flap for upper lateral lip defects. Dermatol Surg. 2000;26:497-498.
Contact Dermatitis of the Hands: Is It Irritant or Allergic?
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Practice Points
- For the hands, irritant contact dermatitis (ICD) is more common than allergic contact dermatitis in both occupational and nonoccupational settings. Because of overlapping clinical features, it can be difficult to differentiate between these conditions.
- Use of hand hygiene products, frequent handwashing, wet work, mechanical trauma, and occlusion can contribute to ICD of the hands.
- Common hand contact allergens include preservatives, metals, fragrances, and rubber accelerators.
- Patch testing often is necessary for diagnosis of hand dermatitis, and both screening and supplemental allergen series may be required.