User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Continuous glucose monitoring benefits patients with type 1 diabetes
, according to two separate randomized trials reported online Jan. 24 in JAMA.
Compared with usual care, continuous glucose monitoring decreased mean HbA1c levels by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden. Both research groups noted that lengthier trials are needed to assess longer-term effectiveness of continuous glucose monitoring in this patient population, the possible adverse effects of long-term use, and whether the reduction in HbA1c levels translates into improved clinical outcomes.
The first trial, which was conducted at 24 U.S. endocrinology practices, involved patients aged 25 and older (mean age, 48 years) who had had type 1 diabetes for a median of 19 years and whose baseline HbA1c levels ranged from 7.5% to 10%. A total of 105 of these participants were randomly assigned to use continuous glucose monitoring (CGM group) and 53 to receive usual care (control group) for 24 weeks, said Roy W. Beck, MD, PhD, of the Jaeb Center for Health Research, Tampa, and his associates.
The CGM group was instructed to wear the device on at least 85% of days and to calibrate it at least twice per day, and they were to verify their glucose level by doing blood glucose meter testing at least three times daily before injecting insulin. The control group was instructed to do blood glucose meter testing at least four times per day.
The primary outcome, reduction in HbA1c level, was 1.1% at 12 weeks and 1% at 24 weeks with CGM, compared with 0.5% at 12 weeks and 0.4% at 24 weeks with usual care. At the end of the treatment period, the mean difference between the two study groups in HbA1c reduction was 0.6%.
Secondary outcomes also favored CGM, including the time spent with glucose levels within the target range of 70-180 mg/dL, duration of hypoglycemia, duration of hyperglycemia, and glycemic variability. In addition, patients reported a high level of satisfaction with CGM, Dr. Beck and his associates said (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19975).
The second trial was conducted at 15 medical centers in Sweden and involved 161 adults aged 18 and older (mean age, 44 years) whose baseline HbA1c levels were 7.5% or higher. The participants served as their own controls, randomly assigned to use either CGM or usual care for 26 weeks and then to crossover to the other group for 26 weeks, said Marcus Lind, MD, PhD, of the diabetes outpatient clinic, Uddevalla (Sweden) Hospital, and his associates.
The primary outcome, reduction in HbA1c level, was lower by 0.4% with CGM than with usual care. In addition, secondary outcomes also favored CGM, including treatment satisfaction, patient concern about having a hypoglycemic episode, overall well-being, and mean glucose levels. However, in this study, patients measured their blood glucose levels less often with CGM (2.7 measurements per day) than with usual care (3.7 measurements per day).
One patient developed an allergic reaction to the device’s internal sensor and had it removed, according to Dr. Lind and his associates (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19976).
Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
Both of these studies show a clear benefit with continuous glucose monitoring in patients with type 1 diabetes, but there are a few concerns.
First, CGM is expensive, and insurers may be reluctant to pay for this device in light of the relatively modest benefits reported by Beck et al. and Lind et al. Second, CGM is invasive and still requires patients to monitor their blood glucose with needle sticks several times per day – two factors that may limit its acceptability to many patients.
Third, the clinicians in these trials were experienced with using CGM and instructing patients in its use. Most clinicians who are not endocrinologists, and even some endocrinologists, would not have the time to manage the volume of data generated by the device and to guide patients’ lifestyle and insulin dosage changes accordingly, given the current time constraints in managing diabetes care.
Mayer B. Davidson, MD, is at Charles R. Drew University of Medicine and Science, Los Angeles. He reported having no relevant financial disclosures. Dr. Davidson made these remarks in an editorial accompanying the two reports (JAMA. 2017;317:363-4).
Both of these studies show a clear benefit with continuous glucose monitoring in patients with type 1 diabetes, but there are a few concerns.
First, CGM is expensive, and insurers may be reluctant to pay for this device in light of the relatively modest benefits reported by Beck et al. and Lind et al. Second, CGM is invasive and still requires patients to monitor their blood glucose with needle sticks several times per day – two factors that may limit its acceptability to many patients.
Third, the clinicians in these trials were experienced with using CGM and instructing patients in its use. Most clinicians who are not endocrinologists, and even some endocrinologists, would not have the time to manage the volume of data generated by the device and to guide patients’ lifestyle and insulin dosage changes accordingly, given the current time constraints in managing diabetes care.
Mayer B. Davidson, MD, is at Charles R. Drew University of Medicine and Science, Los Angeles. He reported having no relevant financial disclosures. Dr. Davidson made these remarks in an editorial accompanying the two reports (JAMA. 2017;317:363-4).
Both of these studies show a clear benefit with continuous glucose monitoring in patients with type 1 diabetes, but there are a few concerns.
First, CGM is expensive, and insurers may be reluctant to pay for this device in light of the relatively modest benefits reported by Beck et al. and Lind et al. Second, CGM is invasive and still requires patients to monitor their blood glucose with needle sticks several times per day – two factors that may limit its acceptability to many patients.
Third, the clinicians in these trials were experienced with using CGM and instructing patients in its use. Most clinicians who are not endocrinologists, and even some endocrinologists, would not have the time to manage the volume of data generated by the device and to guide patients’ lifestyle and insulin dosage changes accordingly, given the current time constraints in managing diabetes care.
Mayer B. Davidson, MD, is at Charles R. Drew University of Medicine and Science, Los Angeles. He reported having no relevant financial disclosures. Dr. Davidson made these remarks in an editorial accompanying the two reports (JAMA. 2017;317:363-4).
, according to two separate randomized trials reported online Jan. 24 in JAMA.
Compared with usual care, continuous glucose monitoring decreased mean HbA1c levels by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden. Both research groups noted that lengthier trials are needed to assess longer-term effectiveness of continuous glucose monitoring in this patient population, the possible adverse effects of long-term use, and whether the reduction in HbA1c levels translates into improved clinical outcomes.
The first trial, which was conducted at 24 U.S. endocrinology practices, involved patients aged 25 and older (mean age, 48 years) who had had type 1 diabetes for a median of 19 years and whose baseline HbA1c levels ranged from 7.5% to 10%. A total of 105 of these participants were randomly assigned to use continuous glucose monitoring (CGM group) and 53 to receive usual care (control group) for 24 weeks, said Roy W. Beck, MD, PhD, of the Jaeb Center for Health Research, Tampa, and his associates.
The CGM group was instructed to wear the device on at least 85% of days and to calibrate it at least twice per day, and they were to verify their glucose level by doing blood glucose meter testing at least three times daily before injecting insulin. The control group was instructed to do blood glucose meter testing at least four times per day.
The primary outcome, reduction in HbA1c level, was 1.1% at 12 weeks and 1% at 24 weeks with CGM, compared with 0.5% at 12 weeks and 0.4% at 24 weeks with usual care. At the end of the treatment period, the mean difference between the two study groups in HbA1c reduction was 0.6%.
Secondary outcomes also favored CGM, including the time spent with glucose levels within the target range of 70-180 mg/dL, duration of hypoglycemia, duration of hyperglycemia, and glycemic variability. In addition, patients reported a high level of satisfaction with CGM, Dr. Beck and his associates said (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19975).
The second trial was conducted at 15 medical centers in Sweden and involved 161 adults aged 18 and older (mean age, 44 years) whose baseline HbA1c levels were 7.5% or higher. The participants served as their own controls, randomly assigned to use either CGM or usual care for 26 weeks and then to crossover to the other group for 26 weeks, said Marcus Lind, MD, PhD, of the diabetes outpatient clinic, Uddevalla (Sweden) Hospital, and his associates.
The primary outcome, reduction in HbA1c level, was lower by 0.4% with CGM than with usual care. In addition, secondary outcomes also favored CGM, including treatment satisfaction, patient concern about having a hypoglycemic episode, overall well-being, and mean glucose levels. However, in this study, patients measured their blood glucose levels less often with CGM (2.7 measurements per day) than with usual care (3.7 measurements per day).
One patient developed an allergic reaction to the device’s internal sensor and had it removed, according to Dr. Lind and his associates (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19976).
Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
, according to two separate randomized trials reported online Jan. 24 in JAMA.
Compared with usual care, continuous glucose monitoring decreased mean HbA1c levels by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden. Both research groups noted that lengthier trials are needed to assess longer-term effectiveness of continuous glucose monitoring in this patient population, the possible adverse effects of long-term use, and whether the reduction in HbA1c levels translates into improved clinical outcomes.
The first trial, which was conducted at 24 U.S. endocrinology practices, involved patients aged 25 and older (mean age, 48 years) who had had type 1 diabetes for a median of 19 years and whose baseline HbA1c levels ranged from 7.5% to 10%. A total of 105 of these participants were randomly assigned to use continuous glucose monitoring (CGM group) and 53 to receive usual care (control group) for 24 weeks, said Roy W. Beck, MD, PhD, of the Jaeb Center for Health Research, Tampa, and his associates.
The CGM group was instructed to wear the device on at least 85% of days and to calibrate it at least twice per day, and they were to verify their glucose level by doing blood glucose meter testing at least three times daily before injecting insulin. The control group was instructed to do blood glucose meter testing at least four times per day.
The primary outcome, reduction in HbA1c level, was 1.1% at 12 weeks and 1% at 24 weeks with CGM, compared with 0.5% at 12 weeks and 0.4% at 24 weeks with usual care. At the end of the treatment period, the mean difference between the two study groups in HbA1c reduction was 0.6%.
Secondary outcomes also favored CGM, including the time spent with glucose levels within the target range of 70-180 mg/dL, duration of hypoglycemia, duration of hyperglycemia, and glycemic variability. In addition, patients reported a high level of satisfaction with CGM, Dr. Beck and his associates said (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19975).
The second trial was conducted at 15 medical centers in Sweden and involved 161 adults aged 18 and older (mean age, 44 years) whose baseline HbA1c levels were 7.5% or higher. The participants served as their own controls, randomly assigned to use either CGM or usual care for 26 weeks and then to crossover to the other group for 26 weeks, said Marcus Lind, MD, PhD, of the diabetes outpatient clinic, Uddevalla (Sweden) Hospital, and his associates.
The primary outcome, reduction in HbA1c level, was lower by 0.4% with CGM than with usual care. In addition, secondary outcomes also favored CGM, including treatment satisfaction, patient concern about having a hypoglycemic episode, overall well-being, and mean glucose levels. However, in this study, patients measured their blood glucose levels less often with CGM (2.7 measurements per day) than with usual care (3.7 measurements per day).
One patient developed an allergic reaction to the device’s internal sensor and had it removed, according to Dr. Lind and his associates (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19976).
Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: A 6-month course of continuous glucose monitoring modestly reduced HbA1c levels in patients with type 1 diabetes who used multiple daily insulin injections.
Major finding: Compared with usual care, continuous glucose monitoring decreased mean HbA1c by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden.
Data source: Two separate short-term randomized trials comparing the effect of continuous glucose monitoring against usual care in 319 adults with type 1 diabetes.
Disclosures: Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
Unique microbiota mix found in guts of T1D patients
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: Patients with type 1 diabetes (T1D) show signs of unique inflammation and microbiota in the duodenal mucosa, compared with controls and celiac patients.
Major finding: T1D patients had a “peculiar” inflammation signature and a unique microbiota composition, and there’s a sign of a link between inflammation and bacteria levels.
Data source: An analysis of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls.
Disclosures: The study was supported by institutional funds, and the authors report no relevant disclosures.
CKD death rate highest in Mexico
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.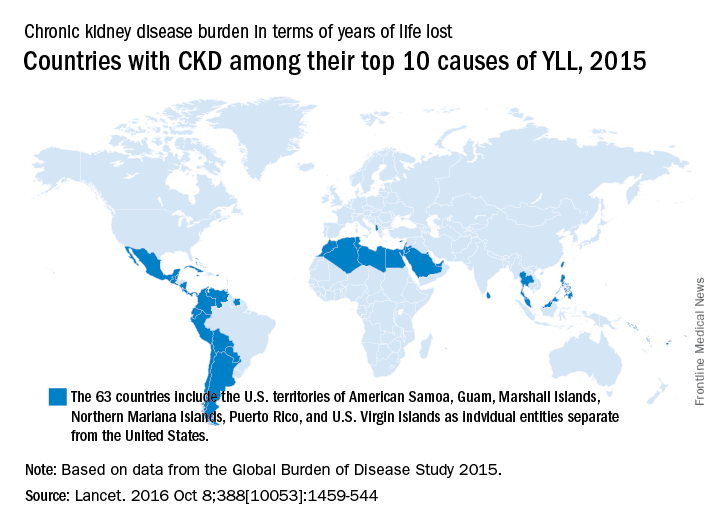
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
FROM THE LANCET
VIDEO: Protein-rich diet can help manage type 2 diabetes, NAFLD
Patients with type 2 diabetes should be put on diets rich in either animal or plant protein to reduce not only liver fat, but insulin resistance and hepatic necroinflammation markers as well, according to a study published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.007).
“High-protein diets have shown variable and sometimes even favorable effects on glucose metabolism and insulin sensitivity in people with type 2 diabetes and it is unclear which metabolic pathways are involved,” wrote the authors of the study, led by Mariya Markova, MD, of the German Institute of Human Nutrition Potsdam-Rehbrücke in Nuthetal, Germany.
SOURCE: American Gastroenterological Association
Obesity and insulin resistance have long been linked to liver fat, with excessive amounts of the latter causing nonalcoholic fatty liver disease (NAFLD), with a significant risk of nonalcoholic steatohepatitis (NASH) developing as well. Compounding this issue, at least in the United States, are widespread dietary and nutritional habits that promote consumption of animal protein, carbohydrates, and saturated fats. This “hypercaloric Western style diet,” as the authors call it, exacerbates the accumulation of fat deposits in the liver and complicates the health of patients across the country, regardless of weight.
“Remarkably, diets restricted in methionine were shown to prevent the development of insulin resistance and of the metabolic syndrome in animal models [so] the type of protein may elicit different metabolic responses depending on the amino acid composition,” Dr. Markova and her coinvestigators noted. “It is therefore hypothesized that high-plant-protein diets exert favorable effects on hepatic fat content and metabolic responses as compared to high intake of animal protein rich in BCAA [branched-chain amino acids] and methionine,” both of which can be found in suitably low levels via plant protein.
Dr. Markova and her team devised a prospective, randomized, open-label clinical trial involving 44 patients with type 2 diabetes and NAFLD, all of whom were recruited at the department of clinical nutrition of the German Institute of Human Nutrition Potsdam-Rehbrücke between June 2013 and March 2015. Subjects were randomized into one of two cohorts, each of which were assigned a diet rich in either animal protein (AP) or plant protein (PP) for a period of 6 weeks. Median body mass index in the AP cohort was 31.0 ± 0.8, and was 29.4 ± 1.0 in the PP cohort.
The AP cohort diet consisted mainly of meat and dairy products, while legumes constituted the bulk of the PP cohort diet. Both diets were isocaloric and had the same macronutrient makeup: 30% protein, 40% carbohydrates, and 30% fat. Seven subjects dropped out prior to completion of the study; of the 37 that remained all the way through – 19 in the AP cohort, 18 in the PP cohort – the age range was 49-78 years. Subjects maintained the same physical exercise regimens throughout the study that they had beforehand, and were asked not to alter them. Hemoglobin A1c levels ranged from 5.8% to 8.8% at baseline, and evaluations were carried out at fasting levels for each subject.
Patients in both cohorts saw significant decreases in intrahepatic fat content by the end of the trial period. Those in the AP cohort saw decreases of 48.0% (P = .0002), while those in the PP cohort saw a decrease of 35.7% (P = .001). Perhaps most importantly, the reductions in both cohorts were not correlated to body weight. In addition, levels of fibroblast growth factor 21 (FGF21), which has been shown to be a predictive marker of NAFLD, decreased by nearly 50% for both AP and PP cohorts (P less than .0002 for both).
“Despite the elevated intake and postprandial uptake of methionine and BCAA in the AP group, there was no indication of negative effects of these components,” the authors stated in the study. “The origin of protein – animal or plant – did not play a major role. Both high-protein diets unexpectedly induced strong reductions of FGF21, which was associated with metabolic improvements and the decrease of IHL.”
Despite these findings, however, the 6-week time span used here is not sufficient to determine just how viable this diet may be in the long term, according to the authors. Further studies will be needed, and will need to take place over longer periods of time, to “show the durability of the responses and eventual adverse effects of the diets.” Furthermore, different age groups must be examined to find out if the benefits observed by Dr. Markova and her coinvestigators were somehow related to the age of these subjects.
The study was funded by grants from German Federal Ministry of Food and Agriculture and German Center for Diabetes Research. Dr. Markova and her coauthors did not report any financial disclosures.
Human studies to assess the effects of isocaloric macronutrient substitution are fraught with difficulty. If one macronutrient is increased, what happens to the others? If you observe an effect, is it the phenomenon you were seeking due to the macronutrient you altered, or an epiphenomenon due to changes in the others?
Markova et al. attempted to study a 6-week “isocaloric” increase of animal vs. plant protein (from 17% to 30% of calories as protein). However, a decrease of percent fat from 41% to 30%, and a reduction in carbohydrate from 42% to 40% occurred commensurately. This brings up three concerns. First, despite the diet’s being “isocaloric,” weight and body mass index decreased by 2 kg and 0.8 kg/m2, respectively. Reductions in intrahepatic, visceral, and subcutaneous fat, and an increase in lean body mass were noted. So was the diet isocaloric? Protein reduces plasma ghrelin levels and is more satiating. Furthermore, metabolism of protein to ATP is inefficient compared to that of carbohydrate or fat. The authors say only that calories were “unrestricted.” These issues do not engender “isocaloric” confidence.
Lastly, the type of carbohydrate was not controlled for. Fructose is significantly more lipogenic than glucose. Yet they were lumped together as “carbohydrate,” and were uncontrolled. So what macronutrient really caused the reduction in liver fat? These methodological issues detract from the author’s message, and this study must be considered preliminary.
Robert H. Lustig, MD, MSL, is in the division of pediatric endocrinology, UCSF Benioff Children’s Hospital, San Francisco; member, UCSF Institute for Health Policy Studies. Dr. Lustig declared no conflicts of interest.
Human studies to assess the effects of isocaloric macronutrient substitution are fraught with difficulty. If one macronutrient is increased, what happens to the others? If you observe an effect, is it the phenomenon you were seeking due to the macronutrient you altered, or an epiphenomenon due to changes in the others?
Markova et al. attempted to study a 6-week “isocaloric” increase of animal vs. plant protein (from 17% to 30% of calories as protein). However, a decrease of percent fat from 41% to 30%, and a reduction in carbohydrate from 42% to 40% occurred commensurately. This brings up three concerns. First, despite the diet’s being “isocaloric,” weight and body mass index decreased by 2 kg and 0.8 kg/m2, respectively. Reductions in intrahepatic, visceral, and subcutaneous fat, and an increase in lean body mass were noted. So was the diet isocaloric? Protein reduces plasma ghrelin levels and is more satiating. Furthermore, metabolism of protein to ATP is inefficient compared to that of carbohydrate or fat. The authors say only that calories were “unrestricted.” These issues do not engender “isocaloric” confidence.
Lastly, the type of carbohydrate was not controlled for. Fructose is significantly more lipogenic than glucose. Yet they were lumped together as “carbohydrate,” and were uncontrolled. So what macronutrient really caused the reduction in liver fat? These methodological issues detract from the author’s message, and this study must be considered preliminary.
Robert H. Lustig, MD, MSL, is in the division of pediatric endocrinology, UCSF Benioff Children’s Hospital, San Francisco; member, UCSF Institute for Health Policy Studies. Dr. Lustig declared no conflicts of interest.
Human studies to assess the effects of isocaloric macronutrient substitution are fraught with difficulty. If one macronutrient is increased, what happens to the others? If you observe an effect, is it the phenomenon you were seeking due to the macronutrient you altered, or an epiphenomenon due to changes in the others?
Markova et al. attempted to study a 6-week “isocaloric” increase of animal vs. plant protein (from 17% to 30% of calories as protein). However, a decrease of percent fat from 41% to 30%, and a reduction in carbohydrate from 42% to 40% occurred commensurately. This brings up three concerns. First, despite the diet’s being “isocaloric,” weight and body mass index decreased by 2 kg and 0.8 kg/m2, respectively. Reductions in intrahepatic, visceral, and subcutaneous fat, and an increase in lean body mass were noted. So was the diet isocaloric? Protein reduces plasma ghrelin levels and is more satiating. Furthermore, metabolism of protein to ATP is inefficient compared to that of carbohydrate or fat. The authors say only that calories were “unrestricted.” These issues do not engender “isocaloric” confidence.
Lastly, the type of carbohydrate was not controlled for. Fructose is significantly more lipogenic than glucose. Yet they were lumped together as “carbohydrate,” and were uncontrolled. So what macronutrient really caused the reduction in liver fat? These methodological issues detract from the author’s message, and this study must be considered preliminary.
Robert H. Lustig, MD, MSL, is in the division of pediatric endocrinology, UCSF Benioff Children’s Hospital, San Francisco; member, UCSF Institute for Health Policy Studies. Dr. Lustig declared no conflicts of interest.
Patients with type 2 diabetes should be put on diets rich in either animal or plant protein to reduce not only liver fat, but insulin resistance and hepatic necroinflammation markers as well, according to a study published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.007).
“High-protein diets have shown variable and sometimes even favorable effects on glucose metabolism and insulin sensitivity in people with type 2 diabetes and it is unclear which metabolic pathways are involved,” wrote the authors of the study, led by Mariya Markova, MD, of the German Institute of Human Nutrition Potsdam-Rehbrücke in Nuthetal, Germany.
SOURCE: American Gastroenterological Association
Obesity and insulin resistance have long been linked to liver fat, with excessive amounts of the latter causing nonalcoholic fatty liver disease (NAFLD), with a significant risk of nonalcoholic steatohepatitis (NASH) developing as well. Compounding this issue, at least in the United States, are widespread dietary and nutritional habits that promote consumption of animal protein, carbohydrates, and saturated fats. This “hypercaloric Western style diet,” as the authors call it, exacerbates the accumulation of fat deposits in the liver and complicates the health of patients across the country, regardless of weight.
“Remarkably, diets restricted in methionine were shown to prevent the development of insulin resistance and of the metabolic syndrome in animal models [so] the type of protein may elicit different metabolic responses depending on the amino acid composition,” Dr. Markova and her coinvestigators noted. “It is therefore hypothesized that high-plant-protein diets exert favorable effects on hepatic fat content and metabolic responses as compared to high intake of animal protein rich in BCAA [branched-chain amino acids] and methionine,” both of which can be found in suitably low levels via plant protein.
Dr. Markova and her team devised a prospective, randomized, open-label clinical trial involving 44 patients with type 2 diabetes and NAFLD, all of whom were recruited at the department of clinical nutrition of the German Institute of Human Nutrition Potsdam-Rehbrücke between June 2013 and March 2015. Subjects were randomized into one of two cohorts, each of which were assigned a diet rich in either animal protein (AP) or plant protein (PP) for a period of 6 weeks. Median body mass index in the AP cohort was 31.0 ± 0.8, and was 29.4 ± 1.0 in the PP cohort.
The AP cohort diet consisted mainly of meat and dairy products, while legumes constituted the bulk of the PP cohort diet. Both diets were isocaloric and had the same macronutrient makeup: 30% protein, 40% carbohydrates, and 30% fat. Seven subjects dropped out prior to completion of the study; of the 37 that remained all the way through – 19 in the AP cohort, 18 in the PP cohort – the age range was 49-78 years. Subjects maintained the same physical exercise regimens throughout the study that they had beforehand, and were asked not to alter them. Hemoglobin A1c levels ranged from 5.8% to 8.8% at baseline, and evaluations were carried out at fasting levels for each subject.
Patients in both cohorts saw significant decreases in intrahepatic fat content by the end of the trial period. Those in the AP cohort saw decreases of 48.0% (P = .0002), while those in the PP cohort saw a decrease of 35.7% (P = .001). Perhaps most importantly, the reductions in both cohorts were not correlated to body weight. In addition, levels of fibroblast growth factor 21 (FGF21), which has been shown to be a predictive marker of NAFLD, decreased by nearly 50% for both AP and PP cohorts (P less than .0002 for both).
“Despite the elevated intake and postprandial uptake of methionine and BCAA in the AP group, there was no indication of negative effects of these components,” the authors stated in the study. “The origin of protein – animal or plant – did not play a major role. Both high-protein diets unexpectedly induced strong reductions of FGF21, which was associated with metabolic improvements and the decrease of IHL.”
Despite these findings, however, the 6-week time span used here is not sufficient to determine just how viable this diet may be in the long term, according to the authors. Further studies will be needed, and will need to take place over longer periods of time, to “show the durability of the responses and eventual adverse effects of the diets.” Furthermore, different age groups must be examined to find out if the benefits observed by Dr. Markova and her coinvestigators were somehow related to the age of these subjects.
The study was funded by grants from German Federal Ministry of Food and Agriculture and German Center for Diabetes Research. Dr. Markova and her coauthors did not report any financial disclosures.
Patients with type 2 diabetes should be put on diets rich in either animal or plant protein to reduce not only liver fat, but insulin resistance and hepatic necroinflammation markers as well, according to a study published in the February issue of Gastroenterology (doi: 10.1053/j.gastro.2016.10.007).
“High-protein diets have shown variable and sometimes even favorable effects on glucose metabolism and insulin sensitivity in people with type 2 diabetes and it is unclear which metabolic pathways are involved,” wrote the authors of the study, led by Mariya Markova, MD, of the German Institute of Human Nutrition Potsdam-Rehbrücke in Nuthetal, Germany.
SOURCE: American Gastroenterological Association
Obesity and insulin resistance have long been linked to liver fat, with excessive amounts of the latter causing nonalcoholic fatty liver disease (NAFLD), with a significant risk of nonalcoholic steatohepatitis (NASH) developing as well. Compounding this issue, at least in the United States, are widespread dietary and nutritional habits that promote consumption of animal protein, carbohydrates, and saturated fats. This “hypercaloric Western style diet,” as the authors call it, exacerbates the accumulation of fat deposits in the liver and complicates the health of patients across the country, regardless of weight.
“Remarkably, diets restricted in methionine were shown to prevent the development of insulin resistance and of the metabolic syndrome in animal models [so] the type of protein may elicit different metabolic responses depending on the amino acid composition,” Dr. Markova and her coinvestigators noted. “It is therefore hypothesized that high-plant-protein diets exert favorable effects on hepatic fat content and metabolic responses as compared to high intake of animal protein rich in BCAA [branched-chain amino acids] and methionine,” both of which can be found in suitably low levels via plant protein.
Dr. Markova and her team devised a prospective, randomized, open-label clinical trial involving 44 patients with type 2 diabetes and NAFLD, all of whom were recruited at the department of clinical nutrition of the German Institute of Human Nutrition Potsdam-Rehbrücke between June 2013 and March 2015. Subjects were randomized into one of two cohorts, each of which were assigned a diet rich in either animal protein (AP) or plant protein (PP) for a period of 6 weeks. Median body mass index in the AP cohort was 31.0 ± 0.8, and was 29.4 ± 1.0 in the PP cohort.
The AP cohort diet consisted mainly of meat and dairy products, while legumes constituted the bulk of the PP cohort diet. Both diets were isocaloric and had the same macronutrient makeup: 30% protein, 40% carbohydrates, and 30% fat. Seven subjects dropped out prior to completion of the study; of the 37 that remained all the way through – 19 in the AP cohort, 18 in the PP cohort – the age range was 49-78 years. Subjects maintained the same physical exercise regimens throughout the study that they had beforehand, and were asked not to alter them. Hemoglobin A1c levels ranged from 5.8% to 8.8% at baseline, and evaluations were carried out at fasting levels for each subject.
Patients in both cohorts saw significant decreases in intrahepatic fat content by the end of the trial period. Those in the AP cohort saw decreases of 48.0% (P = .0002), while those in the PP cohort saw a decrease of 35.7% (P = .001). Perhaps most importantly, the reductions in both cohorts were not correlated to body weight. In addition, levels of fibroblast growth factor 21 (FGF21), which has been shown to be a predictive marker of NAFLD, decreased by nearly 50% for both AP and PP cohorts (P less than .0002 for both).
“Despite the elevated intake and postprandial uptake of methionine and BCAA in the AP group, there was no indication of negative effects of these components,” the authors stated in the study. “The origin of protein – animal or plant – did not play a major role. Both high-protein diets unexpectedly induced strong reductions of FGF21, which was associated with metabolic improvements and the decrease of IHL.”
Despite these findings, however, the 6-week time span used here is not sufficient to determine just how viable this diet may be in the long term, according to the authors. Further studies will be needed, and will need to take place over longer periods of time, to “show the durability of the responses and eventual adverse effects of the diets.” Furthermore, different age groups must be examined to find out if the benefits observed by Dr. Markova and her coinvestigators were somehow related to the age of these subjects.
The study was funded by grants from German Federal Ministry of Food and Agriculture and German Center for Diabetes Research. Dr. Markova and her coauthors did not report any financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: Animal- and plant-protein diets reduced liver fat for type 2 diabetes patients by 36%-48% over the course of 6 months (P = .0002 and P = .001, respectively).
Data source: Prospective study of 37 type 2 diabetes patients from June 2013 to March 2015.
Disclosures: The German Federal Ministry of Food and Agriculture and German Center for Diabetes Research supported the study. The authors did not report any financial disclosures.
Diabetes, ischemic heart disease, pain lead health spending
Diabetes, ischemic heart disease, and back and neck pain are the top three conditions accounting for the highest spending on personal health care in the United States, according to a report published online Dec. 27 in JAMA.
In addition, spending on pharmaceuticals – particularly diabetes therapies, antihypertensive drugs, and medications for hyperlipidemia – drove much of the massive increase in health care spending during the past 2 decades, said Joseph L. Dieleman, PhD, of the Institute for Health Metrics and Evaluation, University of Washington, Seattle, and his associates.
Recent increases in health care spending are well documented, but less is known about what is spent for individual conditions, in different health care settings, and in various patient age groups. To assess health care spending across these categories, the investigators collected and analyzed data for 1996 through 2013 from nationally representative surveys of households, nationally representative surveys of medical facilities, insurance claims, government budgets, and other official records.
They grouped the data into six type-of-care categories: inpatient care, ambulatory care, emergency department care, nursing facility care, dental care, and prescribed pharmaceuticals. “Spending on the six types of personal health care was then disaggregated across 155 mutually exclusive and collectively exhaustive conditions and 38 age and sex groups,” with each sex being divided into 5-year age groups, the researchers noted.
Based on these data, the investigators came to the following conclusions:
• Twenty conditions accounted for approximately 58% of personal health care spending, which totaled an estimated $1.2 trillion in 2013.
• More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion. Prescribed medications accounted for nearly 60% of diabetes costs.
• The second-highest amount of health care spending was for ischemic heart disease, which accounted for $88.1 billion in 2013. Most such spending occurred in inpatient settings.
• Low-back and neck pain, comprising the third-highest level of spending, cost an estimated $87.6 billion. Approximately 60% of this spending occurred in ambulatory settings.
• Among all 155 conditions, spending for diabetes and low-back and neck pain increased the most during the 18-year study period.
• Among all six types of care, spending on pharmaceuticals and emergency care increased the most during the study period.
It is important to note that for the purposes of this study, cancer was disaggregated into 29 separate conditions, and none of them placed in the top 20 for health care spending, Dr. Dieleman and his associates noted (JAMA. 2016;316[24]:2627-46. doi: 10.1001/jama.2016.16885).
When spending was categorized by patient age groups, working-age adults accounted for the greatest amount spent in 2013, estimated at $1,070.1 billion. But that was followed closely by patients aged 65 and older, who accounted for an estimated $796.5 billion, much of which was spent on care in nursing facilities. The smallest amount of health care spending was in children over age 1 and adolescents, who accounted for an estimated $233.5 billion.
Among the other study findings:
• Spending on pharmaceutical treatment of two conditions, hypertension and hyperlipidemia, increased at more than double the rate of total health care spending. It totaled an estimated $135.7 billion in 2013.
• Other top-20 conditions included falls, depression, skin disorders such as acne and eczema, sense disorders such as vision correction and hearing loss, dental care, urinary disorders, and lower respiratory tract infection.
This work was supported by the National Institute on Aging and the Vitality Institute. Dr. Dieleman and his associates reported having no relevant financial disclosures.
Dieleman et al. have “followed” the health care money, and the trail could ultimately lead to the United States changing how it spends a staggering, almost unimaginable amount – roughly $3.2 trillion in 2015 – on health care.
At the very least, their data indicate that the United States should pay more attention to managing physical pain, controlling the costs of pharmaceuticals, and promoting lifestyle interventions that prevent or ameliorate obesity and other factors contributing to diabetes and heart disease.
Ezekiel J. Emanuel, MD, is provost of the department of medical ethics and health policy at the Perelman School of Medicine and in the department of health care management at The Wharton School, University of Pennsylvania, Philadelphia. He reported receiving speaking fees from numerous industry sources. Dr. Emanuel made these remarks in an editorial comment accompanying Dr. Dieleman’s report (JAMA 2016;316:2604-6. doi: 10.1001/jama.2016.16739).
Dieleman et al. have “followed” the health care money, and the trail could ultimately lead to the United States changing how it spends a staggering, almost unimaginable amount – roughly $3.2 trillion in 2015 – on health care.
At the very least, their data indicate that the United States should pay more attention to managing physical pain, controlling the costs of pharmaceuticals, and promoting lifestyle interventions that prevent or ameliorate obesity and other factors contributing to diabetes and heart disease.
Ezekiel J. Emanuel, MD, is provost of the department of medical ethics and health policy at the Perelman School of Medicine and in the department of health care management at The Wharton School, University of Pennsylvania, Philadelphia. He reported receiving speaking fees from numerous industry sources. Dr. Emanuel made these remarks in an editorial comment accompanying Dr. Dieleman’s report (JAMA 2016;316:2604-6. doi: 10.1001/jama.2016.16739).
Dieleman et al. have “followed” the health care money, and the trail could ultimately lead to the United States changing how it spends a staggering, almost unimaginable amount – roughly $3.2 trillion in 2015 – on health care.
At the very least, their data indicate that the United States should pay more attention to managing physical pain, controlling the costs of pharmaceuticals, and promoting lifestyle interventions that prevent or ameliorate obesity and other factors contributing to diabetes and heart disease.
Ezekiel J. Emanuel, MD, is provost of the department of medical ethics and health policy at the Perelman School of Medicine and in the department of health care management at The Wharton School, University of Pennsylvania, Philadelphia. He reported receiving speaking fees from numerous industry sources. Dr. Emanuel made these remarks in an editorial comment accompanying Dr. Dieleman’s report (JAMA 2016;316:2604-6. doi: 10.1001/jama.2016.16739).
Diabetes, ischemic heart disease, and back and neck pain are the top three conditions accounting for the highest spending on personal health care in the United States, according to a report published online Dec. 27 in JAMA.
In addition, spending on pharmaceuticals – particularly diabetes therapies, antihypertensive drugs, and medications for hyperlipidemia – drove much of the massive increase in health care spending during the past 2 decades, said Joseph L. Dieleman, PhD, of the Institute for Health Metrics and Evaluation, University of Washington, Seattle, and his associates.
Recent increases in health care spending are well documented, but less is known about what is spent for individual conditions, in different health care settings, and in various patient age groups. To assess health care spending across these categories, the investigators collected and analyzed data for 1996 through 2013 from nationally representative surveys of households, nationally representative surveys of medical facilities, insurance claims, government budgets, and other official records.
They grouped the data into six type-of-care categories: inpatient care, ambulatory care, emergency department care, nursing facility care, dental care, and prescribed pharmaceuticals. “Spending on the six types of personal health care was then disaggregated across 155 mutually exclusive and collectively exhaustive conditions and 38 age and sex groups,” with each sex being divided into 5-year age groups, the researchers noted.
Based on these data, the investigators came to the following conclusions:
• Twenty conditions accounted for approximately 58% of personal health care spending, which totaled an estimated $1.2 trillion in 2013.
• More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion. Prescribed medications accounted for nearly 60% of diabetes costs.
• The second-highest amount of health care spending was for ischemic heart disease, which accounted for $88.1 billion in 2013. Most such spending occurred in inpatient settings.
• Low-back and neck pain, comprising the third-highest level of spending, cost an estimated $87.6 billion. Approximately 60% of this spending occurred in ambulatory settings.
• Among all 155 conditions, spending for diabetes and low-back and neck pain increased the most during the 18-year study period.
• Among all six types of care, spending on pharmaceuticals and emergency care increased the most during the study period.
It is important to note that for the purposes of this study, cancer was disaggregated into 29 separate conditions, and none of them placed in the top 20 for health care spending, Dr. Dieleman and his associates noted (JAMA. 2016;316[24]:2627-46. doi: 10.1001/jama.2016.16885).
When spending was categorized by patient age groups, working-age adults accounted for the greatest amount spent in 2013, estimated at $1,070.1 billion. But that was followed closely by patients aged 65 and older, who accounted for an estimated $796.5 billion, much of which was spent on care in nursing facilities. The smallest amount of health care spending was in children over age 1 and adolescents, who accounted for an estimated $233.5 billion.
Among the other study findings:
• Spending on pharmaceutical treatment of two conditions, hypertension and hyperlipidemia, increased at more than double the rate of total health care spending. It totaled an estimated $135.7 billion in 2013.
• Other top-20 conditions included falls, depression, skin disorders such as acne and eczema, sense disorders such as vision correction and hearing loss, dental care, urinary disorders, and lower respiratory tract infection.
This work was supported by the National Institute on Aging and the Vitality Institute. Dr. Dieleman and his associates reported having no relevant financial disclosures.
Diabetes, ischemic heart disease, and back and neck pain are the top three conditions accounting for the highest spending on personal health care in the United States, according to a report published online Dec. 27 in JAMA.
In addition, spending on pharmaceuticals – particularly diabetes therapies, antihypertensive drugs, and medications for hyperlipidemia – drove much of the massive increase in health care spending during the past 2 decades, said Joseph L. Dieleman, PhD, of the Institute for Health Metrics and Evaluation, University of Washington, Seattle, and his associates.
Recent increases in health care spending are well documented, but less is known about what is spent for individual conditions, in different health care settings, and in various patient age groups. To assess health care spending across these categories, the investigators collected and analyzed data for 1996 through 2013 from nationally representative surveys of households, nationally representative surveys of medical facilities, insurance claims, government budgets, and other official records.
They grouped the data into six type-of-care categories: inpatient care, ambulatory care, emergency department care, nursing facility care, dental care, and prescribed pharmaceuticals. “Spending on the six types of personal health care was then disaggregated across 155 mutually exclusive and collectively exhaustive conditions and 38 age and sex groups,” with each sex being divided into 5-year age groups, the researchers noted.
Based on these data, the investigators came to the following conclusions:
• Twenty conditions accounted for approximately 58% of personal health care spending, which totaled an estimated $1.2 trillion in 2013.
• More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion. Prescribed medications accounted for nearly 60% of diabetes costs.
• The second-highest amount of health care spending was for ischemic heart disease, which accounted for $88.1 billion in 2013. Most such spending occurred in inpatient settings.
• Low-back and neck pain, comprising the third-highest level of spending, cost an estimated $87.6 billion. Approximately 60% of this spending occurred in ambulatory settings.
• Among all 155 conditions, spending for diabetes and low-back and neck pain increased the most during the 18-year study period.
• Among all six types of care, spending on pharmaceuticals and emergency care increased the most during the study period.
It is important to note that for the purposes of this study, cancer was disaggregated into 29 separate conditions, and none of them placed in the top 20 for health care spending, Dr. Dieleman and his associates noted (JAMA. 2016;316[24]:2627-46. doi: 10.1001/jama.2016.16885).
When spending was categorized by patient age groups, working-age adults accounted for the greatest amount spent in 2013, estimated at $1,070.1 billion. But that was followed closely by patients aged 65 and older, who accounted for an estimated $796.5 billion, much of which was spent on care in nursing facilities. The smallest amount of health care spending was in children over age 1 and adolescents, who accounted for an estimated $233.5 billion.
Among the other study findings:
• Spending on pharmaceutical treatment of two conditions, hypertension and hyperlipidemia, increased at more than double the rate of total health care spending. It totaled an estimated $135.7 billion in 2013.
• Other top-20 conditions included falls, depression, skin disorders such as acne and eczema, sense disorders such as vision correction and hearing loss, dental care, urinary disorders, and lower respiratory tract infection.
This work was supported by the National Institute on Aging and the Vitality Institute. Dr. Dieleman and his associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point:
Major finding: More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion.
Data source: A comprehensive estimate of U.S. spending on personal health care, based on information collected from nationally representative surveys of households and medical facilities, government budgets, insurance claims, and official records from 1996 through 2013.
Disclosures: The National Institute on Aging and the Vitality Institute supported the work. Dr. Dieleman and his associates reported having no relevant financial disclosures.
Prepregnancy overweight boosts risk of depressive symptoms in pregnancy
NEW ORLEANS – Prepregnancy overweight and obesity are associated with increased incidence and severity of depressive symptoms during pregnancy, independent of preeclampsia and other hypertensive pregnancy disorders or gestational diabetes, Satu Kumpulainen reported at Obesity Week 2016.
The implications of this novel finding are clear: “Prepregnancy interventions targeting overweight and obesity and mental health will not only benefit the pregnant mother’s health but will also provide optimal odds for healthy development of the fetus as well,” said Ms. Kumpulainen, a doctoral student at the University of Helsinki Institute of Behavioral Sciences.
It’s well established that prepregnancy obesity is a risk factor for gestational diabetes, preeclampsia, and depression during pregnancy. This study was carried out to learn if a high prepregnancy BMI boosts the risk of prenatal depression independent of the cardiometabolic complications of pregnancy, Ms. Kumpulainen explained at the meeting, which was presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
This proved to be the case in the Finnish women, 67.3% of whom were normal weight before pregnancy; 19.1% were overweight and 13.6% obese. Gestational diabetes occurred in 10.6% of the PREDO participants, and hypertension-spectrum disorders of pregnancy occurred in 8.2%.
The women who were obese or overweight prepregnancy reported higher rates of clinically meaningful depressive symptoms throughout pregnancy, compared with women who were normal weight. Using a CES-D score of 16 or more to define clinically significant depressive symptoms, such symptoms were reported as early as gestational week 12 and on multiple occasions thereafter by 19.9% of the women who were normal weight before pregnancy, 23.3% of those who were overweight, and 27.4% of those who were obese. The differences were statistically significant.
The risk of clinically significant depressive symptoms during pregnancy was no higher in prepregnancy normal-weight women who developed gestational diabetes or preeclampsia than in those who did not, Ms. Kumpulainen reported.
“Our findings suggest that cardiometabolic pregnancy disorders per se don’t trigger higher levels of depressive symptoms, but women with prepregnancy overweight and obesity feel more depressed right from the beginning of pregnancy,” Ms. Kumpulainen said.
She reported having no financial conflicts of interest related to the study, which was supported by Finnish scientific research grants.
NEW ORLEANS – Prepregnancy overweight and obesity are associated with increased incidence and severity of depressive symptoms during pregnancy, independent of preeclampsia and other hypertensive pregnancy disorders or gestational diabetes, Satu Kumpulainen reported at Obesity Week 2016.
The implications of this novel finding are clear: “Prepregnancy interventions targeting overweight and obesity and mental health will not only benefit the pregnant mother’s health but will also provide optimal odds for healthy development of the fetus as well,” said Ms. Kumpulainen, a doctoral student at the University of Helsinki Institute of Behavioral Sciences.
It’s well established that prepregnancy obesity is a risk factor for gestational diabetes, preeclampsia, and depression during pregnancy. This study was carried out to learn if a high prepregnancy BMI boosts the risk of prenatal depression independent of the cardiometabolic complications of pregnancy, Ms. Kumpulainen explained at the meeting, which was presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
This proved to be the case in the Finnish women, 67.3% of whom were normal weight before pregnancy; 19.1% were overweight and 13.6% obese. Gestational diabetes occurred in 10.6% of the PREDO participants, and hypertension-spectrum disorders of pregnancy occurred in 8.2%.
The women who were obese or overweight prepregnancy reported higher rates of clinically meaningful depressive symptoms throughout pregnancy, compared with women who were normal weight. Using a CES-D score of 16 or more to define clinically significant depressive symptoms, such symptoms were reported as early as gestational week 12 and on multiple occasions thereafter by 19.9% of the women who were normal weight before pregnancy, 23.3% of those who were overweight, and 27.4% of those who were obese. The differences were statistically significant.
The risk of clinically significant depressive symptoms during pregnancy was no higher in prepregnancy normal-weight women who developed gestational diabetes or preeclampsia than in those who did not, Ms. Kumpulainen reported.
“Our findings suggest that cardiometabolic pregnancy disorders per se don’t trigger higher levels of depressive symptoms, but women with prepregnancy overweight and obesity feel more depressed right from the beginning of pregnancy,” Ms. Kumpulainen said.
She reported having no financial conflicts of interest related to the study, which was supported by Finnish scientific research grants.
NEW ORLEANS – Prepregnancy overweight and obesity are associated with increased incidence and severity of depressive symptoms during pregnancy, independent of preeclampsia and other hypertensive pregnancy disorders or gestational diabetes, Satu Kumpulainen reported at Obesity Week 2016.
The implications of this novel finding are clear: “Prepregnancy interventions targeting overweight and obesity and mental health will not only benefit the pregnant mother’s health but will also provide optimal odds for healthy development of the fetus as well,” said Ms. Kumpulainen, a doctoral student at the University of Helsinki Institute of Behavioral Sciences.
It’s well established that prepregnancy obesity is a risk factor for gestational diabetes, preeclampsia, and depression during pregnancy. This study was carried out to learn if a high prepregnancy BMI boosts the risk of prenatal depression independent of the cardiometabolic complications of pregnancy, Ms. Kumpulainen explained at the meeting, which was presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
This proved to be the case in the Finnish women, 67.3% of whom were normal weight before pregnancy; 19.1% were overweight and 13.6% obese. Gestational diabetes occurred in 10.6% of the PREDO participants, and hypertension-spectrum disorders of pregnancy occurred in 8.2%.
The women who were obese or overweight prepregnancy reported higher rates of clinically meaningful depressive symptoms throughout pregnancy, compared with women who were normal weight. Using a CES-D score of 16 or more to define clinically significant depressive symptoms, such symptoms were reported as early as gestational week 12 and on multiple occasions thereafter by 19.9% of the women who were normal weight before pregnancy, 23.3% of those who were overweight, and 27.4% of those who were obese. The differences were statistically significant.
The risk of clinically significant depressive symptoms during pregnancy was no higher in prepregnancy normal-weight women who developed gestational diabetes or preeclampsia than in those who did not, Ms. Kumpulainen reported.
“Our findings suggest that cardiometabolic pregnancy disorders per se don’t trigger higher levels of depressive symptoms, but women with prepregnancy overweight and obesity feel more depressed right from the beginning of pregnancy,” Ms. Kumpulainen said.
She reported having no financial conflicts of interest related to the study, which was supported by Finnish scientific research grants.
AT OBESITY WEEK 2016
Key clinical point:
Major finding: Women who were obese prior to pregnancy were over 50% more likely to experience clinically significant depressive symptoms throughout pregnancy, compared with women who were normal weight before pregnancy, independent of whether the women developed gestational diabetes or preeclampsia.
Data source: This was a secondary analysis from a prospective study of more than 3,000 pregnant Finnish women.
Disclosures: The study was supported by Finnish scientific research grants. The presenter reported having no financial conflicts of interest related to the study.
Unless it is diagnosed, obesity won’t be treated
NEW ORLEANS – Obesity has been formally diagnosed in less than half of patients with a body-mass index of 30 kg/m2 or higher in the Cleveland Clinic’s large multispecialty database, and Bartolome Burguera, MD, believes it’s the same story elsewhere.
“I think pretty much all over the country obesity is really not well diagnosed,” Dr. Burguera, director of obesity programs at the Cleveland Clinic, said at Obesity Week 2016.
And that which hasn’t been diagnosed doesn’t get treated.
The diagnosis rate went up with higher BMIs; still, of the 25,137 patients with obesity class 3 as defined by a BMI of 40 kg/m2 or higher, only 75% had a formal diagnosis of obesity in their record, the endocrinologist said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
“For many years, physicians thought that obesity is not a disease. And even though it was considered a disease by some, they didn’t feel they had the tools, the knowledge, the support, the medications, or the time to take care of obesity, especially when they thought of it as a self-inflicted disease,” Dr. Burguera explained in an interview. He believes physician attitudes are slowly changing.
“In our clinic we’ve taken measures to change attitudes, for sure. Now, when we look in the electronic health record we get an automatic alert if the patient has a BMI of 30 or more,” he said.
“I think, in general, many more people now think of obesity as a disease. But it’s a chronic disease and you have to have chronic therapy. We have to make sure we make the diagnosis, and once you make the diagnosis you have to discuss treatment with the patient. If you don’t feel comfortable for whatever reason, I think you have to refer the patient to a colleague to take care of the obesity. Because when you take care of the obesity all the comorbidities get better: the diabetes, the blood pressure, the cholesterol. Obesity is the primary problem in so many other comorbidities. We have put little effort to this point in taking care of the obesity. We’ve put more effort into treating the diabetes and the other comorbidities,” Dr. Burguera said.
The Medicare obesity benefit provides reimbursement in primary care settings for intensive behavioral therapy with face-to-face counseling and motivational interviewing. The billing code is G0447. Coverage is provided for 22 visits over the course of a year, each lasting 15 minutes.
Dr. Batsis presented highlights of his published serial cross-sectional analysis of fee-for-service Medicare claims data for 2012 and 2013. Among Medicare beneficiaries eligible for the obesity benefit because they had a BMI of 30 kg/m2 or above, only 0.35% used the benefit in 2012. There was a tiny uptick to 0.6% in 2013, but even in the tiny fraction of eligible patients who availed themselves of the benefit, the average number of behavioral therapy sessions was just 2.1 visits out of the 22 for which physician reimbursement is available (Obesity. 2016 Sep;24[9]:1983-8).
“Let’s hope the 2014 data look a little better,” commented Dr. Batsis of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H.
There was marked regional variation in utilization of the Medicare obesity benefit across the U.S. in 2013. Rates were highest in Colorado – the state with the lowest obesity rate in the country – as well as Nebraska, Wisconsin, Vermont, and New Hampshire. Rates were lowest across the Southwest.
Dr. Burguera’s study was funded by Novo Nordisk. Dr. Batsis reported having no financial conflicts of interest.
NEW ORLEANS – Obesity has been formally diagnosed in less than half of patients with a body-mass index of 30 kg/m2 or higher in the Cleveland Clinic’s large multispecialty database, and Bartolome Burguera, MD, believes it’s the same story elsewhere.
“I think pretty much all over the country obesity is really not well diagnosed,” Dr. Burguera, director of obesity programs at the Cleveland Clinic, said at Obesity Week 2016.
And that which hasn’t been diagnosed doesn’t get treated.
The diagnosis rate went up with higher BMIs; still, of the 25,137 patients with obesity class 3 as defined by a BMI of 40 kg/m2 or higher, only 75% had a formal diagnosis of obesity in their record, the endocrinologist said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
“For many years, physicians thought that obesity is not a disease. And even though it was considered a disease by some, they didn’t feel they had the tools, the knowledge, the support, the medications, or the time to take care of obesity, especially when they thought of it as a self-inflicted disease,” Dr. Burguera explained in an interview. He believes physician attitudes are slowly changing.
“In our clinic we’ve taken measures to change attitudes, for sure. Now, when we look in the electronic health record we get an automatic alert if the patient has a BMI of 30 or more,” he said.
“I think, in general, many more people now think of obesity as a disease. But it’s a chronic disease and you have to have chronic therapy. We have to make sure we make the diagnosis, and once you make the diagnosis you have to discuss treatment with the patient. If you don’t feel comfortable for whatever reason, I think you have to refer the patient to a colleague to take care of the obesity. Because when you take care of the obesity all the comorbidities get better: the diabetes, the blood pressure, the cholesterol. Obesity is the primary problem in so many other comorbidities. We have put little effort to this point in taking care of the obesity. We’ve put more effort into treating the diabetes and the other comorbidities,” Dr. Burguera said.
The Medicare obesity benefit provides reimbursement in primary care settings for intensive behavioral therapy with face-to-face counseling and motivational interviewing. The billing code is G0447. Coverage is provided for 22 visits over the course of a year, each lasting 15 minutes.
Dr. Batsis presented highlights of his published serial cross-sectional analysis of fee-for-service Medicare claims data for 2012 and 2013. Among Medicare beneficiaries eligible for the obesity benefit because they had a BMI of 30 kg/m2 or above, only 0.35% used the benefit in 2012. There was a tiny uptick to 0.6% in 2013, but even in the tiny fraction of eligible patients who availed themselves of the benefit, the average number of behavioral therapy sessions was just 2.1 visits out of the 22 for which physician reimbursement is available (Obesity. 2016 Sep;24[9]:1983-8).
“Let’s hope the 2014 data look a little better,” commented Dr. Batsis of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H.
There was marked regional variation in utilization of the Medicare obesity benefit across the U.S. in 2013. Rates were highest in Colorado – the state with the lowest obesity rate in the country – as well as Nebraska, Wisconsin, Vermont, and New Hampshire. Rates were lowest across the Southwest.
Dr. Burguera’s study was funded by Novo Nordisk. Dr. Batsis reported having no financial conflicts of interest.
NEW ORLEANS – Obesity has been formally diagnosed in less than half of patients with a body-mass index of 30 kg/m2 or higher in the Cleveland Clinic’s large multispecialty database, and Bartolome Burguera, MD, believes it’s the same story elsewhere.
“I think pretty much all over the country obesity is really not well diagnosed,” Dr. Burguera, director of obesity programs at the Cleveland Clinic, said at Obesity Week 2016.
And that which hasn’t been diagnosed doesn’t get treated.
The diagnosis rate went up with higher BMIs; still, of the 25,137 patients with obesity class 3 as defined by a BMI of 40 kg/m2 or higher, only 75% had a formal diagnosis of obesity in their record, the endocrinologist said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
“For many years, physicians thought that obesity is not a disease. And even though it was considered a disease by some, they didn’t feel they had the tools, the knowledge, the support, the medications, or the time to take care of obesity, especially when they thought of it as a self-inflicted disease,” Dr. Burguera explained in an interview. He believes physician attitudes are slowly changing.
“In our clinic we’ve taken measures to change attitudes, for sure. Now, when we look in the electronic health record we get an automatic alert if the patient has a BMI of 30 or more,” he said.
“I think, in general, many more people now think of obesity as a disease. But it’s a chronic disease and you have to have chronic therapy. We have to make sure we make the diagnosis, and once you make the diagnosis you have to discuss treatment with the patient. If you don’t feel comfortable for whatever reason, I think you have to refer the patient to a colleague to take care of the obesity. Because when you take care of the obesity all the comorbidities get better: the diabetes, the blood pressure, the cholesterol. Obesity is the primary problem in so many other comorbidities. We have put little effort to this point in taking care of the obesity. We’ve put more effort into treating the diabetes and the other comorbidities,” Dr. Burguera said.
The Medicare obesity benefit provides reimbursement in primary care settings for intensive behavioral therapy with face-to-face counseling and motivational interviewing. The billing code is G0447. Coverage is provided for 22 visits over the course of a year, each lasting 15 minutes.
Dr. Batsis presented highlights of his published serial cross-sectional analysis of fee-for-service Medicare claims data for 2012 and 2013. Among Medicare beneficiaries eligible for the obesity benefit because they had a BMI of 30 kg/m2 or above, only 0.35% used the benefit in 2012. There was a tiny uptick to 0.6% in 2013, but even in the tiny fraction of eligible patients who availed themselves of the benefit, the average number of behavioral therapy sessions was just 2.1 visits out of the 22 for which physician reimbursement is available (Obesity. 2016 Sep;24[9]:1983-8).
“Let’s hope the 2014 data look a little better,” commented Dr. Batsis of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H.
There was marked regional variation in utilization of the Medicare obesity benefit across the U.S. in 2013. Rates were highest in Colorado – the state with the lowest obesity rate in the country – as well as Nebraska, Wisconsin, Vermont, and New Hampshire. Rates were lowest across the Southwest.
Dr. Burguera’s study was funded by Novo Nordisk. Dr. Batsis reported having no financial conflicts of interest.
AT OBESITY WEEK 2016
Key clinical point:
Major finding: Only 48% of a large group of patients with a BMI of 30 kg/m2 or higher had a formal diagnosis of obesity in their medical record.
Data source: This was a cross-sectional study of the electronic health records of nearly 325,000 active patients in the Cleveland Clinic database, 41.5% of whom had a BMI of 30 kg/m2 or higher.
Disclosures: The study was funded by Novo Nordisk.
Bariatric surgery or total joint replacement: which first?
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
AT OBESITY WEEK 2016
Key clinical point:
Major finding: When total joint replacement in obese patients was performed after bariatric surgery, mean hospital length of stay was a full day less than when the orthopedic surgery preceded the bariatric surgery.
Data source: This retrospective observational study included 102 obese patients who underwent bariatric surgery and total knee or hip replacement.
Disclosures: The study presenter reported having no financial conflicts of interest.
Landmark psychosocial guidelines for diabetes spark debate over the ideal vs. the practical
, but some question whether endocrinologists and others are up to the task of providing the extensive mental health services called for in the document.
Although the American Diabetes Association has often addressed the specific psychosocial concerns of persons with diabetes, the ADA’s first-ever position statement on the subject reflects a state-of-the-art approach to delivering integrated mental health and specialty services to this patient population. That alone makes the document a milestone in diabetes care, according to Yehuda Handelsman, MD, , medical director of the Metabolic Institute of America in Tarzana, Calif., and chair of the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines. “It raises the importance of the psychological being in diabetes. This has been mentioned before in guidelines, but it has never been the focus. In that respect, this [document] is very important,” Dr. Handelsman said in an interview.
The guidelines detail the most common psychological factors facing persons with diabetes throughout the life span, including diabetes distress, depression, anxiety, eating disorders, and diabetes-related cognitive dysfunction later in life. There is also a section addressing considerations such as mental and emotional preparation before and after bariatric surgery. Clinicians are urged to practice preventive care by assessing patients’ mental states regularly. A list of age-appropriate resources for screens and other measurement tools is included in the guidelines.
Despite the guidelines’ thoroughness, Dr. Handelsman said he is not optimistic they will change much in the way of practice. “The people who wrote this are psychologists and other mental health professionals. This is what they do. When we endocrinologists see patients, we don’t have these [skills]. It’s not so easy to incorporate these suggestions into daily practice,” Dr. Handelsman, said.
“The paper addresses all service providers who help care for people with diabetes. That presumes a starting point of primary care physicians, but includes specialists and team members such as certified diabetes educators, registered nurses, nutritionists, behavioral practitioners, and so on.”
Plenty of integrated care models for diabetes care [are] already in existence, said Dr. Young-Hyman, who is also a certified diabetes educator.
One such clinic is operated by Richard Hellman, MD, , a past president of the American Association of Clinical Endocrinologists. His North Kansas City diabetes specialty clinic has offered psychosocial services to patients for much of its 30 years. The clinic’s focus is not on primary care, but many of his patients’ health needs are met by approaching their chronic illness care in a comprehensive way, according to Dr. Hellman. The clinic’s multidisciplinary team includes certified diabetes educators, nurse practitioners, physician assistants, dietitians, a clinical psychologist, and registered nurses.
Since passage of the Affordable Care Act, the zeitgeist has been a move away from fee for service care provided by a single clinician, to collaborative models. While talk of the incoming U.S. president and Congress dismantling the law has caused some uncertainty over how physician reimbursements will be structured in the future, pressure from health insurers to keep in place value-based care models – particularly for chronic illness management – may remain regardless of the ACA’s ultimate fate.
“The evidence is that when people with diabetes who also have stress or mood disorders get the care they need, they are more productive and healthier, and both insurers and employers save money,” Dr. Hellman said.
It is what might happen should primary care physicians find themselves facing either having to meet standards of care for a number of chronic illnesses or forfeit reimbursements. Such a scenario should concern policymakers dealing with the delivery of chronic illness care, according to Dr. Hellman, who has experience relevant to these issues as a member of the Physician Consortium for Performance Improvement since 2000 and the National Quality Forum Diabetes/Metabolism Technical Advisory Panel from 2009 to 2012.
“Payment strategies to force change are a blunt tool that often don’t work well. There is so much complexity. People often have kidney or heart disease. It’s hard to write policy with so much variation going on,” he said.
Adding mental health screening and referrals likely works well for all models of chronic illness care, according to Victor L. Roberts, MD, MBA, a clinical endocrinologist in Winter Park, Fla.
However, “I don’t see how a primary care doctor will have the time to [follow all the guidelines] and determine what is going on with the patient’s mental health,” observed Dr. Roberts, who works with many central Florida primary care clinics.
“But they can tell if someone is mildly or moderately depressed, and they can refer the patient for evaluation just like you would refer them for an EKG, a blood test, or a consultation to an endocrinologist,” he added. “Look at depression as a comorbidity.”
Not treating depression and anxiety as medical conditions means patient outcomes are almost guaranteed to be poor, he said.
“If someone is depressed, they are not listening. They’re worried, they’re not paying attention, their ability to incorporate new information is impaired,” Dr. Roberts added. That leads to less facility for self care and can contribute to a bidirectional conundrum of depression and worsening health, particularly in diabetes.
An embrace of value-based care as envisioned by the guidelines’ authors is irrelevant, however, if qualified mental health specialists – particularly those trained specifically in the psychosocial needs of people with diabetes – are nowhere to be found. “I [practice] in the middle of Los Angeles, and I can tell you that in a 30-mile radius, there is not a psychologist anywhere that I can refer a diabetes patient to,” Dr. Handelsman said.
To that end, the ADA has developed a partnership with the American Psychological Association to educate psychologists about the kinds of mental health challenges specific to patients with diabetes. The curriculum will be introduced later this year during the ADA’s scientific sessions meeting. At present, none of the classes are accredited, but Dr. Young-Hyman said her “pie-in-the-sky dream” would be to expand the program and continuing medical education units.
“We see the capacity issues and want to address them,” Dr. Young-Hyman said. That alone may not be enough to change practice in the specialty setting where integrated care, as provided by Dr. Hellman’s clinic, currently is the exception, according to Dr. Handelsman.
Although he said it was likely that guidelines issued by ACE will be expanded to incorporate the ADA’s recommendations, he challenged the ADA to advocate directly to endocrinology societies to educate them on the practical application of their recommendations.
“Take the guidelines and make us use them,” he said in the interview. Otherwise, because most clinical endocrinologists are not trained to address psychosocial concerns, unless a specialist already has an interest in mental health, Dr. Handelsman said that specialist largely will ignore this document. “Some in the field will read it ... but we will not take it to the streets.”
Despite his “guarded optimism,” neither does Dr. Roberts see how practice for primary care physicians will change much – at least, not in the near future, given what he called the already “bone crushing” constraints on their time.
Yet, he warned that not dealing with mental health issues means not delivering complete care.
“Depression is a complication of diabetes, in my expert opinion,” Dr. Roberts cautioned.
“Primary care physicians need to not sidestep this. They need to make it clear to their patients that dealing with depression is part and parcel of dealing with their chronic disease. The position statement can at least be a clarion call to consider mental health a medical condition that we can address in a matter-of-fact way.”
The American Diabetes Association’s recommendations for psychosocial care in diabetes are as follows:
• Integrate psychosocial care with collaborative, patient-centered medical care, and provide psychosocial care to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. (Evidence level A.)
• Consider assessing symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Include caregivers and family members in this assessment. (Evidence level B.)
• Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. (Evidence level E.)
• Consider assessing life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. (Evidence level E.)
• Address psychosocial problems upon identification. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. (Evidence level E.)
Dr. Handelsman chaired the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines committee and is the immediate past president of ACE. Dr. Hellman is an editorial board member of Diabetes Care. Dr. Young-Hyman had no relevant disclosures.
, but some question whether endocrinologists and others are up to the task of providing the extensive mental health services called for in the document.
Although the American Diabetes Association has often addressed the specific psychosocial concerns of persons with diabetes, the ADA’s first-ever position statement on the subject reflects a state-of-the-art approach to delivering integrated mental health and specialty services to this patient population. That alone makes the document a milestone in diabetes care, according to Yehuda Handelsman, MD, , medical director of the Metabolic Institute of America in Tarzana, Calif., and chair of the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines. “It raises the importance of the psychological being in diabetes. This has been mentioned before in guidelines, but it has never been the focus. In that respect, this [document] is very important,” Dr. Handelsman said in an interview.
The guidelines detail the most common psychological factors facing persons with diabetes throughout the life span, including diabetes distress, depression, anxiety, eating disorders, and diabetes-related cognitive dysfunction later in life. There is also a section addressing considerations such as mental and emotional preparation before and after bariatric surgery. Clinicians are urged to practice preventive care by assessing patients’ mental states regularly. A list of age-appropriate resources for screens and other measurement tools is included in the guidelines.
Despite the guidelines’ thoroughness, Dr. Handelsman said he is not optimistic they will change much in the way of practice. “The people who wrote this are psychologists and other mental health professionals. This is what they do. When we endocrinologists see patients, we don’t have these [skills]. It’s not so easy to incorporate these suggestions into daily practice,” Dr. Handelsman, said.
“The paper addresses all service providers who help care for people with diabetes. That presumes a starting point of primary care physicians, but includes specialists and team members such as certified diabetes educators, registered nurses, nutritionists, behavioral practitioners, and so on.”
Plenty of integrated care models for diabetes care [are] already in existence, said Dr. Young-Hyman, who is also a certified diabetes educator.
One such clinic is operated by Richard Hellman, MD, , a past president of the American Association of Clinical Endocrinologists. His North Kansas City diabetes specialty clinic has offered psychosocial services to patients for much of its 30 years. The clinic’s focus is not on primary care, but many of his patients’ health needs are met by approaching their chronic illness care in a comprehensive way, according to Dr. Hellman. The clinic’s multidisciplinary team includes certified diabetes educators, nurse practitioners, physician assistants, dietitians, a clinical psychologist, and registered nurses.
Since passage of the Affordable Care Act, the zeitgeist has been a move away from fee for service care provided by a single clinician, to collaborative models. While talk of the incoming U.S. president and Congress dismantling the law has caused some uncertainty over how physician reimbursements will be structured in the future, pressure from health insurers to keep in place value-based care models – particularly for chronic illness management – may remain regardless of the ACA’s ultimate fate.
“The evidence is that when people with diabetes who also have stress or mood disorders get the care they need, they are more productive and healthier, and both insurers and employers save money,” Dr. Hellman said.
It is what might happen should primary care physicians find themselves facing either having to meet standards of care for a number of chronic illnesses or forfeit reimbursements. Such a scenario should concern policymakers dealing with the delivery of chronic illness care, according to Dr. Hellman, who has experience relevant to these issues as a member of the Physician Consortium for Performance Improvement since 2000 and the National Quality Forum Diabetes/Metabolism Technical Advisory Panel from 2009 to 2012.
“Payment strategies to force change are a blunt tool that often don’t work well. There is so much complexity. People often have kidney or heart disease. It’s hard to write policy with so much variation going on,” he said.
Adding mental health screening and referrals likely works well for all models of chronic illness care, according to Victor L. Roberts, MD, MBA, a clinical endocrinologist in Winter Park, Fla.
However, “I don’t see how a primary care doctor will have the time to [follow all the guidelines] and determine what is going on with the patient’s mental health,” observed Dr. Roberts, who works with many central Florida primary care clinics.
“But they can tell if someone is mildly or moderately depressed, and they can refer the patient for evaluation just like you would refer them for an EKG, a blood test, or a consultation to an endocrinologist,” he added. “Look at depression as a comorbidity.”
Not treating depression and anxiety as medical conditions means patient outcomes are almost guaranteed to be poor, he said.
“If someone is depressed, they are not listening. They’re worried, they’re not paying attention, their ability to incorporate new information is impaired,” Dr. Roberts added. That leads to less facility for self care and can contribute to a bidirectional conundrum of depression and worsening health, particularly in diabetes.
An embrace of value-based care as envisioned by the guidelines’ authors is irrelevant, however, if qualified mental health specialists – particularly those trained specifically in the psychosocial needs of people with diabetes – are nowhere to be found. “I [practice] in the middle of Los Angeles, and I can tell you that in a 30-mile radius, there is not a psychologist anywhere that I can refer a diabetes patient to,” Dr. Handelsman said.
To that end, the ADA has developed a partnership with the American Psychological Association to educate psychologists about the kinds of mental health challenges specific to patients with diabetes. The curriculum will be introduced later this year during the ADA’s scientific sessions meeting. At present, none of the classes are accredited, but Dr. Young-Hyman said her “pie-in-the-sky dream” would be to expand the program and continuing medical education units.
“We see the capacity issues and want to address them,” Dr. Young-Hyman said. That alone may not be enough to change practice in the specialty setting where integrated care, as provided by Dr. Hellman’s clinic, currently is the exception, according to Dr. Handelsman.
Although he said it was likely that guidelines issued by ACE will be expanded to incorporate the ADA’s recommendations, he challenged the ADA to advocate directly to endocrinology societies to educate them on the practical application of their recommendations.
“Take the guidelines and make us use them,” he said in the interview. Otherwise, because most clinical endocrinologists are not trained to address psychosocial concerns, unless a specialist already has an interest in mental health, Dr. Handelsman said that specialist largely will ignore this document. “Some in the field will read it ... but we will not take it to the streets.”
Despite his “guarded optimism,” neither does Dr. Roberts see how practice for primary care physicians will change much – at least, not in the near future, given what he called the already “bone crushing” constraints on their time.
Yet, he warned that not dealing with mental health issues means not delivering complete care.
“Depression is a complication of diabetes, in my expert opinion,” Dr. Roberts cautioned.
“Primary care physicians need to not sidestep this. They need to make it clear to their patients that dealing with depression is part and parcel of dealing with their chronic disease. The position statement can at least be a clarion call to consider mental health a medical condition that we can address in a matter-of-fact way.”
The American Diabetes Association’s recommendations for psychosocial care in diabetes are as follows:
• Integrate psychosocial care with collaborative, patient-centered medical care, and provide psychosocial care to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. (Evidence level A.)
• Consider assessing symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Include caregivers and family members in this assessment. (Evidence level B.)
• Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. (Evidence level E.)
• Consider assessing life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. (Evidence level E.)
• Address psychosocial problems upon identification. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. (Evidence level E.)
Dr. Handelsman chaired the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines committee and is the immediate past president of ACE. Dr. Hellman is an editorial board member of Diabetes Care. Dr. Young-Hyman had no relevant disclosures.
, but some question whether endocrinologists and others are up to the task of providing the extensive mental health services called for in the document.
Although the American Diabetes Association has often addressed the specific psychosocial concerns of persons with diabetes, the ADA’s first-ever position statement on the subject reflects a state-of-the-art approach to delivering integrated mental health and specialty services to this patient population. That alone makes the document a milestone in diabetes care, according to Yehuda Handelsman, MD, , medical director of the Metabolic Institute of America in Tarzana, Calif., and chair of the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines. “It raises the importance of the psychological being in diabetes. This has been mentioned before in guidelines, but it has never been the focus. In that respect, this [document] is very important,” Dr. Handelsman said in an interview.
The guidelines detail the most common psychological factors facing persons with diabetes throughout the life span, including diabetes distress, depression, anxiety, eating disorders, and diabetes-related cognitive dysfunction later in life. There is also a section addressing considerations such as mental and emotional preparation before and after bariatric surgery. Clinicians are urged to practice preventive care by assessing patients’ mental states regularly. A list of age-appropriate resources for screens and other measurement tools is included in the guidelines.
Despite the guidelines’ thoroughness, Dr. Handelsman said he is not optimistic they will change much in the way of practice. “The people who wrote this are psychologists and other mental health professionals. This is what they do. When we endocrinologists see patients, we don’t have these [skills]. It’s not so easy to incorporate these suggestions into daily practice,” Dr. Handelsman, said.
“The paper addresses all service providers who help care for people with diabetes. That presumes a starting point of primary care physicians, but includes specialists and team members such as certified diabetes educators, registered nurses, nutritionists, behavioral practitioners, and so on.”
Plenty of integrated care models for diabetes care [are] already in existence, said Dr. Young-Hyman, who is also a certified diabetes educator.
One such clinic is operated by Richard Hellman, MD, , a past president of the American Association of Clinical Endocrinologists. His North Kansas City diabetes specialty clinic has offered psychosocial services to patients for much of its 30 years. The clinic’s focus is not on primary care, but many of his patients’ health needs are met by approaching their chronic illness care in a comprehensive way, according to Dr. Hellman. The clinic’s multidisciplinary team includes certified diabetes educators, nurse practitioners, physician assistants, dietitians, a clinical psychologist, and registered nurses.
Since passage of the Affordable Care Act, the zeitgeist has been a move away from fee for service care provided by a single clinician, to collaborative models. While talk of the incoming U.S. president and Congress dismantling the law has caused some uncertainty over how physician reimbursements will be structured in the future, pressure from health insurers to keep in place value-based care models – particularly for chronic illness management – may remain regardless of the ACA’s ultimate fate.
“The evidence is that when people with diabetes who also have stress or mood disorders get the care they need, they are more productive and healthier, and both insurers and employers save money,” Dr. Hellman said.
It is what might happen should primary care physicians find themselves facing either having to meet standards of care for a number of chronic illnesses or forfeit reimbursements. Such a scenario should concern policymakers dealing with the delivery of chronic illness care, according to Dr. Hellman, who has experience relevant to these issues as a member of the Physician Consortium for Performance Improvement since 2000 and the National Quality Forum Diabetes/Metabolism Technical Advisory Panel from 2009 to 2012.
“Payment strategies to force change are a blunt tool that often don’t work well. There is so much complexity. People often have kidney or heart disease. It’s hard to write policy with so much variation going on,” he said.
Adding mental health screening and referrals likely works well for all models of chronic illness care, according to Victor L. Roberts, MD, MBA, a clinical endocrinologist in Winter Park, Fla.
However, “I don’t see how a primary care doctor will have the time to [follow all the guidelines] and determine what is going on with the patient’s mental health,” observed Dr. Roberts, who works with many central Florida primary care clinics.
“But they can tell if someone is mildly or moderately depressed, and they can refer the patient for evaluation just like you would refer them for an EKG, a blood test, or a consultation to an endocrinologist,” he added. “Look at depression as a comorbidity.”
Not treating depression and anxiety as medical conditions means patient outcomes are almost guaranteed to be poor, he said.
“If someone is depressed, they are not listening. They’re worried, they’re not paying attention, their ability to incorporate new information is impaired,” Dr. Roberts added. That leads to less facility for self care and can contribute to a bidirectional conundrum of depression and worsening health, particularly in diabetes.
An embrace of value-based care as envisioned by the guidelines’ authors is irrelevant, however, if qualified mental health specialists – particularly those trained specifically in the psychosocial needs of people with diabetes – are nowhere to be found. “I [practice] in the middle of Los Angeles, and I can tell you that in a 30-mile radius, there is not a psychologist anywhere that I can refer a diabetes patient to,” Dr. Handelsman said.
To that end, the ADA has developed a partnership with the American Psychological Association to educate psychologists about the kinds of mental health challenges specific to patients with diabetes. The curriculum will be introduced later this year during the ADA’s scientific sessions meeting. At present, none of the classes are accredited, but Dr. Young-Hyman said her “pie-in-the-sky dream” would be to expand the program and continuing medical education units.
“We see the capacity issues and want to address them,” Dr. Young-Hyman said. That alone may not be enough to change practice in the specialty setting where integrated care, as provided by Dr. Hellman’s clinic, currently is the exception, according to Dr. Handelsman.
Although he said it was likely that guidelines issued by ACE will be expanded to incorporate the ADA’s recommendations, he challenged the ADA to advocate directly to endocrinology societies to educate them on the practical application of their recommendations.
“Take the guidelines and make us use them,” he said in the interview. Otherwise, because most clinical endocrinologists are not trained to address psychosocial concerns, unless a specialist already has an interest in mental health, Dr. Handelsman said that specialist largely will ignore this document. “Some in the field will read it ... but we will not take it to the streets.”
Despite his “guarded optimism,” neither does Dr. Roberts see how practice for primary care physicians will change much – at least, not in the near future, given what he called the already “bone crushing” constraints on their time.
Yet, he warned that not dealing with mental health issues means not delivering complete care.
“Depression is a complication of diabetes, in my expert opinion,” Dr. Roberts cautioned.
“Primary care physicians need to not sidestep this. They need to make it clear to their patients that dealing with depression is part and parcel of dealing with their chronic disease. The position statement can at least be a clarion call to consider mental health a medical condition that we can address in a matter-of-fact way.”
The American Diabetes Association’s recommendations for psychosocial care in diabetes are as follows:
• Integrate psychosocial care with collaborative, patient-centered medical care, and provide psychosocial care to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. (Evidence level A.)
• Consider assessing symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Include caregivers and family members in this assessment. (Evidence level B.)
• Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. (Evidence level E.)
• Consider assessing life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. (Evidence level E.)
• Address psychosocial problems upon identification. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. (Evidence level E.)
Dr. Handelsman chaired the American College of Endocrinology 2011 Comprehensive Diabetes Guidelines committee and is the immediate past president of ACE. Dr. Hellman is an editorial board member of Diabetes Care. Dr. Young-Hyman had no relevant disclosures.
FROM DIABETES CARE
Diabetes treatment costs doubled in Sweden since 2006
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT EASD 2016
Key clinical point:
Major finding: Treatment costs jumped from €608 million in 2006 to €1.27 billion in 2014.
Data source: The 8-year study used national health care data.
Disclosures: The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.

















