User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
For AFib cardioversion in obesity, dual energy might be the answer
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
AT AHA 2023
Blood pressure lowering reduces dementia risk
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
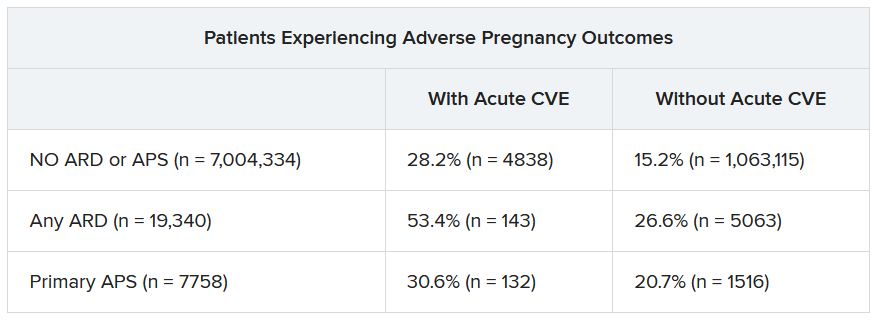
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Salt intake associated with increased type 2 diabetes risk
TOPLINE:
, even after adjustment for confounding factors.
METHODOLOGY:
- Researchers identified 402,982 participants in the UK Biobank from March 2006 to October 2010 who had completed a questionnaire about the frequency with which they added salt to food and who did not have diabetes, chronic kidney disease, cancer, or cardiovascular disease at baseline.
- Urine samples were collected at baseline, sodium and potassium levels were measured, and 24-hour sodium excretion was estimated.
- Investigators followed participants from baseline to diagnosis of diabetes, death, or the censoring date (May 23, 2021), whichever occurred first. Information on T2D events were collected through medical history linkage to data on hospital admissions, questionnaire, and the death register.
TAKEAWAY:
- During a mean follow-up of 11.9 years, 13,120 incident cases of T2D were documented.
- Compared with people who reported “never/rarely” adding salt to food, the sex- and age-adjusted hazard ratios (HRs) for developing T2D were 1.20, 1.32, and 1.86 for those who reported “sometimes,” “usually,” and “always” adding salt, respectively (P-trend < .001).
- After further adjustment for the Townsend deprivation index, education level, income, smoking, drinking, physical activity, and high cholesterol, the association was attenuated but remained significant, with HRs of 1.11, 1.18, and 1.28 for “sometimes,” “usually,” and “always” responses, respectively (P-trend < .001).
- After full adjustment, there was also a dose-dependent relationship across quintiles of urinary sodium and higher T2D risk, with HRs of 1 (reference), 1.12, 1.17, 1.28, and 1.34 for quintiles 2-5, respectively (P-trend < .001).
- Body fat percentage and body fat mass significantly mediated the association of adding salt with T2D, by estimated effects of 37.9% and 39.9%, respectively (both P < .001).
IN PRACTICE:
“These findings provide support that reduction of adding salt to foods may act as a potential behavioral intervention approach for preventing T2D. Future clinical trials are needed to further validate our findings,” the authors wrote.
SOURCE:
The study by Xuan Wang, MD, PhD, department of epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, and colleagues was published in the November 2023 issue of Mayo Clinic Proceedings.
LIMITATIONS:
The researchers could not completely exclude the possibility that high frequency of adding salt to foods is a marker for an unhealthy lifestyle. Self-reported frequency of adding salt to food might be subject to information bias and did not provide quantitative information on total sodium intake. In addition, participants were mainly of European descent, making application of the findings to other ethnic groups unclear; the observational design meant researchers could not rule out residual confounding; and information on addition of salt to food was available only at baseline, so potential changes in salt consumption during follow-up could not be considered.
DISCLOSURES:
The study was supported by grants from the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the Fogarty International Center; and Tulane Research Centers of Excellence Awards. The authors reported no potential competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
, even after adjustment for confounding factors.
METHODOLOGY:
- Researchers identified 402,982 participants in the UK Biobank from March 2006 to October 2010 who had completed a questionnaire about the frequency with which they added salt to food and who did not have diabetes, chronic kidney disease, cancer, or cardiovascular disease at baseline.
- Urine samples were collected at baseline, sodium and potassium levels were measured, and 24-hour sodium excretion was estimated.
- Investigators followed participants from baseline to diagnosis of diabetes, death, or the censoring date (May 23, 2021), whichever occurred first. Information on T2D events were collected through medical history linkage to data on hospital admissions, questionnaire, and the death register.
TAKEAWAY:
- During a mean follow-up of 11.9 years, 13,120 incident cases of T2D were documented.
- Compared with people who reported “never/rarely” adding salt to food, the sex- and age-adjusted hazard ratios (HRs) for developing T2D were 1.20, 1.32, and 1.86 for those who reported “sometimes,” “usually,” and “always” adding salt, respectively (P-trend < .001).
- After further adjustment for the Townsend deprivation index, education level, income, smoking, drinking, physical activity, and high cholesterol, the association was attenuated but remained significant, with HRs of 1.11, 1.18, and 1.28 for “sometimes,” “usually,” and “always” responses, respectively (P-trend < .001).
- After full adjustment, there was also a dose-dependent relationship across quintiles of urinary sodium and higher T2D risk, with HRs of 1 (reference), 1.12, 1.17, 1.28, and 1.34 for quintiles 2-5, respectively (P-trend < .001).
- Body fat percentage and body fat mass significantly mediated the association of adding salt with T2D, by estimated effects of 37.9% and 39.9%, respectively (both P < .001).
IN PRACTICE:
“These findings provide support that reduction of adding salt to foods may act as a potential behavioral intervention approach for preventing T2D. Future clinical trials are needed to further validate our findings,” the authors wrote.
SOURCE:
The study by Xuan Wang, MD, PhD, department of epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, and colleagues was published in the November 2023 issue of Mayo Clinic Proceedings.
LIMITATIONS:
The researchers could not completely exclude the possibility that high frequency of adding salt to foods is a marker for an unhealthy lifestyle. Self-reported frequency of adding salt to food might be subject to information bias and did not provide quantitative information on total sodium intake. In addition, participants were mainly of European descent, making application of the findings to other ethnic groups unclear; the observational design meant researchers could not rule out residual confounding; and information on addition of salt to food was available only at baseline, so potential changes in salt consumption during follow-up could not be considered.
DISCLOSURES:
The study was supported by grants from the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the Fogarty International Center; and Tulane Research Centers of Excellence Awards. The authors reported no potential competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
, even after adjustment for confounding factors.
METHODOLOGY:
- Researchers identified 402,982 participants in the UK Biobank from March 2006 to October 2010 who had completed a questionnaire about the frequency with which they added salt to food and who did not have diabetes, chronic kidney disease, cancer, or cardiovascular disease at baseline.
- Urine samples were collected at baseline, sodium and potassium levels were measured, and 24-hour sodium excretion was estimated.
- Investigators followed participants from baseline to diagnosis of diabetes, death, or the censoring date (May 23, 2021), whichever occurred first. Information on T2D events were collected through medical history linkage to data on hospital admissions, questionnaire, and the death register.
TAKEAWAY:
- During a mean follow-up of 11.9 years, 13,120 incident cases of T2D were documented.
- Compared with people who reported “never/rarely” adding salt to food, the sex- and age-adjusted hazard ratios (HRs) for developing T2D were 1.20, 1.32, and 1.86 for those who reported “sometimes,” “usually,” and “always” adding salt, respectively (P-trend < .001).
- After further adjustment for the Townsend deprivation index, education level, income, smoking, drinking, physical activity, and high cholesterol, the association was attenuated but remained significant, with HRs of 1.11, 1.18, and 1.28 for “sometimes,” “usually,” and “always” responses, respectively (P-trend < .001).
- After full adjustment, there was also a dose-dependent relationship across quintiles of urinary sodium and higher T2D risk, with HRs of 1 (reference), 1.12, 1.17, 1.28, and 1.34 for quintiles 2-5, respectively (P-trend < .001).
- Body fat percentage and body fat mass significantly mediated the association of adding salt with T2D, by estimated effects of 37.9% and 39.9%, respectively (both P < .001).
IN PRACTICE:
“These findings provide support that reduction of adding salt to foods may act as a potential behavioral intervention approach for preventing T2D. Future clinical trials are needed to further validate our findings,” the authors wrote.
SOURCE:
The study by Xuan Wang, MD, PhD, department of epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, and colleagues was published in the November 2023 issue of Mayo Clinic Proceedings.
LIMITATIONS:
The researchers could not completely exclude the possibility that high frequency of adding salt to foods is a marker for an unhealthy lifestyle. Self-reported frequency of adding salt to food might be subject to information bias and did not provide quantitative information on total sodium intake. In addition, participants were mainly of European descent, making application of the findings to other ethnic groups unclear; the observational design meant researchers could not rule out residual confounding; and information on addition of salt to food was available only at baseline, so potential changes in salt consumption during follow-up could not be considered.
DISCLOSURES:
The study was supported by grants from the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the Fogarty International Center; and Tulane Research Centers of Excellence Awards. The authors reported no potential competing interests.
A version of this article appeared on Medscape.com.
Sustained reductions in Lp(a) achieved with novel siRNA drug
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Dropping aspirin cuts bleeding in LVAD patients: ARIES-HM3
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
AT AHA 2023
Classification identifies four stages of heart attack
Relying on more than 50 years of data on acute MI with reperfusion therapy, the society has identified the following four stages of progressively worsening myocardial tissue injury:
- Aborted MI (no or minimal myocardial necrosis).
- MI with significant cardiomyocyte necrosis but without microvascular injury.
- Cardiomyocyte necrosis and microvascular dysfunction leading to microvascular obstruction (that is, “no reflow”).
- Cardiomyocyte and microvascular necrosis leading to reperfusion hemorrhage.
The classification is described in an expert consensus statement that was published in the Canadian Journal of Cardiology.
The new classification will allow for better risk stratification and more appropriate treatment and provide refined endpoints for clinical trials and translational research, according to the authors.
Currently, all patients with acute MI receive the same treatment, even though they may have different levels of tissue injury severity, statement author Andreas Kumar, MD, chair of the writing group and associate professor of medicine at Northern Ontario School of Medicine University, Sudbury, said in an interview.
“In some cases, treatment for a mild stage 1 acute MI may be deadly for someone with stage 4 hemorrhagic MI,” said Dr. Kumar.
Technological advances
The classification is based on decades of data. “The initial data were obtained with pathology studies in the 1970s. When cardiac MRI came around, around the year 2000, suddenly there was a noninvasive imaging method where we could investigate patients in vivo,” said Dr. Kumar. “We learned a lot about tissue changes in acute MI. And especially in the last 2 to 5 years, we have learned a lot about hemorrhagic MI. So, this then gave us enough knowledge to come up with this new classification.”
The idea of classifying acute MI came to Dr. Kumar and senior author Rohan Dharmakumar, PhD, executive director of the Krannert Cardiovascular Research Center at Indiana University, Indianapolis, when both were at the University of Toronto.
“This work has been years in the making,” Dr. Dharmakumar said in an interview. “We’ve been thinking about this for a long time, but we needed to get substantial layers of evidence to support the classification. We had a feeling about these stages for a long time, but that feeling needed to be substantiated.”
In 2022, Dr. Dharmakumar and Dr. Kumar observed that damage to the heart from MI was not only a result of ischemia caused by a blocked artery, but also a result of bleeding in the myocardium after the artery had been opened. Their findings were published in the Journal of the American College of Cardiology.
The author of an accompanying editorial lauded the investigators “for providing new, mechanistic insights into a difficult clinical problem that has an unmet therapeutic need.”
“Hemorrhagic MI is a very dangerous injury because hemorrhage itself causes a lot of problems,” said Dr. Kumar. “We reported that there is infarct expansion after reperfusion, so once you open up the vessel, the heart attack actually gets larger. We also showed that the remodeling of these hearts is worse. These patients take a second hit with hemorrhage occurring in the myocardium.”
Classification and staging
“The standard guideline therapy for somebody who comes into the hospital is to put in a stent, open the artery, have the patient stay in the hospital for 48-72 hours, and then be released home,” said Dr. Dharmukumar. “But here’s the problem. These two patients who are going back home have different levels of injury, yet they are taking the same medications. Even inside the hospital, we have heterogeneity in mortality risk. But we are not paying attention to one patient differently than the other, even though we should, because their injuries are very different.”
The CCS classification may provide endpoints and outcome measures beyond the commonly used clinical markers, which could lead to improved treatments to help patients recover from their cardiac events.
“We have this issue of rampant heart failure in acute MI survivors. We’ve gotten really good at saving patients from immediate death, but now we are just postponing some of the serious problems survivors are going to face, said Dr. Dharmukumar. “What are we doing for these patients who are really at risk? We’ve been treating every single patient the same way and we have not been paying attention to the very different stages of injury.”
In an accompanying editorial, Prakriti Gaba, MD, a clinical fellow in medicine at Brigham and Women’s Hospital, Boston, and Deepak L. Bhatt, MD, MPH, director of the Mount Sinai Fuster Heart Hospital, New York, wrote: “There is no doubt that the classification system proposed by the investigators is important and timely, as acute MI continues to account for substantial morbidity and mortality worldwide.”
Imaging and staging could be useful in guiding appropriate therapy, Bhatt said in an interview. “The authors’ hope, which I think is a very laudable one, is that more finely characterizing exactly what the extent of damage is and what the mechanism of damage is in a heart attack will make it possible to develop therapies that are particularly targeted to each of the stages,” he said.
“It is quite common to have the ability to do cardiac MRI at experienced cardiovascular centers, although this may not be true for smaller community hospitals,” Dr. Bhatt added. “But at least at larger hospitals, this will allow for much finer evaluation and assessment of exactly what is going on in that particular patient and how extensive the heart muscle damage is. Eventually, this will facilitate the development of therapies that are specifically targeted to treat each stage.”
Dr. Kumar is partly supported by a research grant from the Northern Ontario Academic Medicine Association. Dr. Dharmakumar was funded in part by grants from the U.S. National Institutes of Health. Dr. Dharmakumar has an ownership interest in Cardio-Theranostics. Dr. Bhatt has served on advisory boards for Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys. He is a member of the board of directors of or holds stock in Angiowave, Boston VA Research Institute, Bristol-Myers Squibb, DRS.LINQ, High Enroll, Society of Cardiovascular Patient Care, and TobeSoft. He has worked as a consultant for Broadview Ventures, and Hims. He has received honoraria from the American College of Cardiology, Arnold and Porter law firm, Baim Institute for Clinical Research, Belvoir Publications, Canadian Medical and Surgical Knowledge Translation Research Group, Cowen and Company, Duke Clinical Research Institute, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Oakstone CME, Piper Sandler, Population Health Research Institute, Slack Publications, Society of Cardiovascular Patient Care, WebMD, and Wiley.
A version of this article appeared on Medscape.com.
Relying on more than 50 years of data on acute MI with reperfusion therapy, the society has identified the following four stages of progressively worsening myocardial tissue injury:
- Aborted MI (no or minimal myocardial necrosis).
- MI with significant cardiomyocyte necrosis but without microvascular injury.
- Cardiomyocyte necrosis and microvascular dysfunction leading to microvascular obstruction (that is, “no reflow”).
- Cardiomyocyte and microvascular necrosis leading to reperfusion hemorrhage.
The classification is described in an expert consensus statement that was published in the Canadian Journal of Cardiology.
The new classification will allow for better risk stratification and more appropriate treatment and provide refined endpoints for clinical trials and translational research, according to the authors.
Currently, all patients with acute MI receive the same treatment, even though they may have different levels of tissue injury severity, statement author Andreas Kumar, MD, chair of the writing group and associate professor of medicine at Northern Ontario School of Medicine University, Sudbury, said in an interview.
“In some cases, treatment for a mild stage 1 acute MI may be deadly for someone with stage 4 hemorrhagic MI,” said Dr. Kumar.
Technological advances
The classification is based on decades of data. “The initial data were obtained with pathology studies in the 1970s. When cardiac MRI came around, around the year 2000, suddenly there was a noninvasive imaging method where we could investigate patients in vivo,” said Dr. Kumar. “We learned a lot about tissue changes in acute MI. And especially in the last 2 to 5 years, we have learned a lot about hemorrhagic MI. So, this then gave us enough knowledge to come up with this new classification.”
The idea of classifying acute MI came to Dr. Kumar and senior author Rohan Dharmakumar, PhD, executive director of the Krannert Cardiovascular Research Center at Indiana University, Indianapolis, when both were at the University of Toronto.
“This work has been years in the making,” Dr. Dharmakumar said in an interview. “We’ve been thinking about this for a long time, but we needed to get substantial layers of evidence to support the classification. We had a feeling about these stages for a long time, but that feeling needed to be substantiated.”
In 2022, Dr. Dharmakumar and Dr. Kumar observed that damage to the heart from MI was not only a result of ischemia caused by a blocked artery, but also a result of bleeding in the myocardium after the artery had been opened. Their findings were published in the Journal of the American College of Cardiology.
The author of an accompanying editorial lauded the investigators “for providing new, mechanistic insights into a difficult clinical problem that has an unmet therapeutic need.”
“Hemorrhagic MI is a very dangerous injury because hemorrhage itself causes a lot of problems,” said Dr. Kumar. “We reported that there is infarct expansion after reperfusion, so once you open up the vessel, the heart attack actually gets larger. We also showed that the remodeling of these hearts is worse. These patients take a second hit with hemorrhage occurring in the myocardium.”
Classification and staging
“The standard guideline therapy for somebody who comes into the hospital is to put in a stent, open the artery, have the patient stay in the hospital for 48-72 hours, and then be released home,” said Dr. Dharmukumar. “But here’s the problem. These two patients who are going back home have different levels of injury, yet they are taking the same medications. Even inside the hospital, we have heterogeneity in mortality risk. But we are not paying attention to one patient differently than the other, even though we should, because their injuries are very different.”
The CCS classification may provide endpoints and outcome measures beyond the commonly used clinical markers, which could lead to improved treatments to help patients recover from their cardiac events.
“We have this issue of rampant heart failure in acute MI survivors. We’ve gotten really good at saving patients from immediate death, but now we are just postponing some of the serious problems survivors are going to face, said Dr. Dharmukumar. “What are we doing for these patients who are really at risk? We’ve been treating every single patient the same way and we have not been paying attention to the very different stages of injury.”
In an accompanying editorial, Prakriti Gaba, MD, a clinical fellow in medicine at Brigham and Women’s Hospital, Boston, and Deepak L. Bhatt, MD, MPH, director of the Mount Sinai Fuster Heart Hospital, New York, wrote: “There is no doubt that the classification system proposed by the investigators is important and timely, as acute MI continues to account for substantial morbidity and mortality worldwide.”
Imaging and staging could be useful in guiding appropriate therapy, Bhatt said in an interview. “The authors’ hope, which I think is a very laudable one, is that more finely characterizing exactly what the extent of damage is and what the mechanism of damage is in a heart attack will make it possible to develop therapies that are particularly targeted to each of the stages,” he said.
“It is quite common to have the ability to do cardiac MRI at experienced cardiovascular centers, although this may not be true for smaller community hospitals,” Dr. Bhatt added. “But at least at larger hospitals, this will allow for much finer evaluation and assessment of exactly what is going on in that particular patient and how extensive the heart muscle damage is. Eventually, this will facilitate the development of therapies that are specifically targeted to treat each stage.”
Dr. Kumar is partly supported by a research grant from the Northern Ontario Academic Medicine Association. Dr. Dharmakumar was funded in part by grants from the U.S. National Institutes of Health. Dr. Dharmakumar has an ownership interest in Cardio-Theranostics. Dr. Bhatt has served on advisory boards for Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys. He is a member of the board of directors of or holds stock in Angiowave, Boston VA Research Institute, Bristol-Myers Squibb, DRS.LINQ, High Enroll, Society of Cardiovascular Patient Care, and TobeSoft. He has worked as a consultant for Broadview Ventures, and Hims. He has received honoraria from the American College of Cardiology, Arnold and Porter law firm, Baim Institute for Clinical Research, Belvoir Publications, Canadian Medical and Surgical Knowledge Translation Research Group, Cowen and Company, Duke Clinical Research Institute, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Oakstone CME, Piper Sandler, Population Health Research Institute, Slack Publications, Society of Cardiovascular Patient Care, WebMD, and Wiley.
A version of this article appeared on Medscape.com.
Relying on more than 50 years of data on acute MI with reperfusion therapy, the society has identified the following four stages of progressively worsening myocardial tissue injury:
- Aborted MI (no or minimal myocardial necrosis).
- MI with significant cardiomyocyte necrosis but without microvascular injury.
- Cardiomyocyte necrosis and microvascular dysfunction leading to microvascular obstruction (that is, “no reflow”).
- Cardiomyocyte and microvascular necrosis leading to reperfusion hemorrhage.
The classification is described in an expert consensus statement that was published in the Canadian Journal of Cardiology.
The new classification will allow for better risk stratification and more appropriate treatment and provide refined endpoints for clinical trials and translational research, according to the authors.
Currently, all patients with acute MI receive the same treatment, even though they may have different levels of tissue injury severity, statement author Andreas Kumar, MD, chair of the writing group and associate professor of medicine at Northern Ontario School of Medicine University, Sudbury, said in an interview.
“In some cases, treatment for a mild stage 1 acute MI may be deadly for someone with stage 4 hemorrhagic MI,” said Dr. Kumar.
Technological advances
The classification is based on decades of data. “The initial data were obtained with pathology studies in the 1970s. When cardiac MRI came around, around the year 2000, suddenly there was a noninvasive imaging method where we could investigate patients in vivo,” said Dr. Kumar. “We learned a lot about tissue changes in acute MI. And especially in the last 2 to 5 years, we have learned a lot about hemorrhagic MI. So, this then gave us enough knowledge to come up with this new classification.”
The idea of classifying acute MI came to Dr. Kumar and senior author Rohan Dharmakumar, PhD, executive director of the Krannert Cardiovascular Research Center at Indiana University, Indianapolis, when both were at the University of Toronto.
“This work has been years in the making,” Dr. Dharmakumar said in an interview. “We’ve been thinking about this for a long time, but we needed to get substantial layers of evidence to support the classification. We had a feeling about these stages for a long time, but that feeling needed to be substantiated.”
In 2022, Dr. Dharmakumar and Dr. Kumar observed that damage to the heart from MI was not only a result of ischemia caused by a blocked artery, but also a result of bleeding in the myocardium after the artery had been opened. Their findings were published in the Journal of the American College of Cardiology.
The author of an accompanying editorial lauded the investigators “for providing new, mechanistic insights into a difficult clinical problem that has an unmet therapeutic need.”
“Hemorrhagic MI is a very dangerous injury because hemorrhage itself causes a lot of problems,” said Dr. Kumar. “We reported that there is infarct expansion after reperfusion, so once you open up the vessel, the heart attack actually gets larger. We also showed that the remodeling of these hearts is worse. These patients take a second hit with hemorrhage occurring in the myocardium.”
Classification and staging
“The standard guideline therapy for somebody who comes into the hospital is to put in a stent, open the artery, have the patient stay in the hospital for 48-72 hours, and then be released home,” said Dr. Dharmukumar. “But here’s the problem. These two patients who are going back home have different levels of injury, yet they are taking the same medications. Even inside the hospital, we have heterogeneity in mortality risk. But we are not paying attention to one patient differently than the other, even though we should, because their injuries are very different.”
The CCS classification may provide endpoints and outcome measures beyond the commonly used clinical markers, which could lead to improved treatments to help patients recover from their cardiac events.
“We have this issue of rampant heart failure in acute MI survivors. We’ve gotten really good at saving patients from immediate death, but now we are just postponing some of the serious problems survivors are going to face, said Dr. Dharmukumar. “What are we doing for these patients who are really at risk? We’ve been treating every single patient the same way and we have not been paying attention to the very different stages of injury.”
In an accompanying editorial, Prakriti Gaba, MD, a clinical fellow in medicine at Brigham and Women’s Hospital, Boston, and Deepak L. Bhatt, MD, MPH, director of the Mount Sinai Fuster Heart Hospital, New York, wrote: “There is no doubt that the classification system proposed by the investigators is important and timely, as acute MI continues to account for substantial morbidity and mortality worldwide.”
Imaging and staging could be useful in guiding appropriate therapy, Bhatt said in an interview. “The authors’ hope, which I think is a very laudable one, is that more finely characterizing exactly what the extent of damage is and what the mechanism of damage is in a heart attack will make it possible to develop therapies that are particularly targeted to each of the stages,” he said.
“It is quite common to have the ability to do cardiac MRI at experienced cardiovascular centers, although this may not be true for smaller community hospitals,” Dr. Bhatt added. “But at least at larger hospitals, this will allow for much finer evaluation and assessment of exactly what is going on in that particular patient and how extensive the heart muscle damage is. Eventually, this will facilitate the development of therapies that are specifically targeted to treat each stage.”
Dr. Kumar is partly supported by a research grant from the Northern Ontario Academic Medicine Association. Dr. Dharmakumar was funded in part by grants from the U.S. National Institutes of Health. Dr. Dharmakumar has an ownership interest in Cardio-Theranostics. Dr. Bhatt has served on advisory boards for Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys. He is a member of the board of directors of or holds stock in Angiowave, Boston VA Research Institute, Bristol-Myers Squibb, DRS.LINQ, High Enroll, Society of Cardiovascular Patient Care, and TobeSoft. He has worked as a consultant for Broadview Ventures, and Hims. He has received honoraria from the American College of Cardiology, Arnold and Porter law firm, Baim Institute for Clinical Research, Belvoir Publications, Canadian Medical and Surgical Knowledge Translation Research Group, Cowen and Company, Duke Clinical Research Institute, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Oakstone CME, Piper Sandler, Population Health Research Institute, Slack Publications, Society of Cardiovascular Patient Care, WebMD, and Wiley.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Potential dapagliflozin benefit post MI is not a ‘mandate’
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
AT AHA 2023
Long COVID and mental illness: New guidance
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
FROM PHYSICAL MEDICINE AND REHABILITATION