User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Dupilumab under FDA review for atopic dermatitis in children aged 6 months to 5 years
The and Sanofi.
If approved, dupilumab would be the first biologic approved for children in this age group in the United States, according to the statement. The proposed indication is as add-on therapy for children with moderate to severe AD not adequately controlled with topical prescription therapies or for whom topical therapies are not advised. The FDA granted breakthrough therapy designation for dupilumab for the treatment of severe AD in children aged 6 months to 11 years in 2016.
Approximately 85%-95% of atopic dermatitis patients develop symptoms before 5 years of age, and these symptoms often continue into adulthood, with an increased risk of skin infections and a significant impact on quality of life, according to the statement.
The sBLA is based on data from a phase 3 pivotal study of 162 children aged 6 months to 5 years in which dupilumab was added to standard-of-care topical corticosteroids, presented in December 2021. In the study, dupilumab plus standard of care significantly improved skin clearance and reduced overall disease severity and itch at 16 weeks compared with standard of care alone. Overall, 28% of the children randomized to dupilumab achieved the primary endpoint of clear or almost-clear skin, compared with 4% with those on standard of care alone (P < .0001), according to the manufacturers. Patients in the dupilumab group received either 200 mg (for children weighing ≥ 5 to < 15 kg) or 300 mg (for children weighing ≥ 15 to < 30 kg) every 4 weeks. Safety results were similar to those seen with dupilumab for children aged 6 years and older.
Conjunctivitis and herpes infections were among the most common adverse events associated with dupilumab in the study, according to the statement.
The target action date for the FDA decision on this application is June 9, 2022.
The and Sanofi.
If approved, dupilumab would be the first biologic approved for children in this age group in the United States, according to the statement. The proposed indication is as add-on therapy for children with moderate to severe AD not adequately controlled with topical prescription therapies or for whom topical therapies are not advised. The FDA granted breakthrough therapy designation for dupilumab for the treatment of severe AD in children aged 6 months to 11 years in 2016.
Approximately 85%-95% of atopic dermatitis patients develop symptoms before 5 years of age, and these symptoms often continue into adulthood, with an increased risk of skin infections and a significant impact on quality of life, according to the statement.
The sBLA is based on data from a phase 3 pivotal study of 162 children aged 6 months to 5 years in which dupilumab was added to standard-of-care topical corticosteroids, presented in December 2021. In the study, dupilumab plus standard of care significantly improved skin clearance and reduced overall disease severity and itch at 16 weeks compared with standard of care alone. Overall, 28% of the children randomized to dupilumab achieved the primary endpoint of clear or almost-clear skin, compared with 4% with those on standard of care alone (P < .0001), according to the manufacturers. Patients in the dupilumab group received either 200 mg (for children weighing ≥ 5 to < 15 kg) or 300 mg (for children weighing ≥ 15 to < 30 kg) every 4 weeks. Safety results were similar to those seen with dupilumab for children aged 6 years and older.
Conjunctivitis and herpes infections were among the most common adverse events associated with dupilumab in the study, according to the statement.
The target action date for the FDA decision on this application is June 9, 2022.
The and Sanofi.
If approved, dupilumab would be the first biologic approved for children in this age group in the United States, according to the statement. The proposed indication is as add-on therapy for children with moderate to severe AD not adequately controlled with topical prescription therapies or for whom topical therapies are not advised. The FDA granted breakthrough therapy designation for dupilumab for the treatment of severe AD in children aged 6 months to 11 years in 2016.
Approximately 85%-95% of atopic dermatitis patients develop symptoms before 5 years of age, and these symptoms often continue into adulthood, with an increased risk of skin infections and a significant impact on quality of life, according to the statement.
The sBLA is based on data from a phase 3 pivotal study of 162 children aged 6 months to 5 years in which dupilumab was added to standard-of-care topical corticosteroids, presented in December 2021. In the study, dupilumab plus standard of care significantly improved skin clearance and reduced overall disease severity and itch at 16 weeks compared with standard of care alone. Overall, 28% of the children randomized to dupilumab achieved the primary endpoint of clear or almost-clear skin, compared with 4% with those on standard of care alone (P < .0001), according to the manufacturers. Patients in the dupilumab group received either 200 mg (for children weighing ≥ 5 to < 15 kg) or 300 mg (for children weighing ≥ 15 to < 30 kg) every 4 weeks. Safety results were similar to those seen with dupilumab for children aged 6 years and older.
Conjunctivitis and herpes infections were among the most common adverse events associated with dupilumab in the study, according to the statement.
The target action date for the FDA decision on this application is June 9, 2022.
FROM THE FDA
Scientists see hope in new therapy for COVID-19 brain fog patients
People with long-COVID “brain fog” may be able to recover mental abilities that were dulled or stolen from them by the virus through an approach that has improved the effects of stroke, traumatic brain injury, and other post-viral disorders, doctors and scientists say.
For a lucky portion of the population, COVID-19 lasts a handful of days with minor symptoms. But for an estimated 37% who contract the virus, symptoms can linger for weeks, months, or even years. One of the most common symptoms of long COVID is brain fog: a life-altering condition characterized by slow thinking, confusion, difficulty remembering things, and poor concentration.
The approaches are based on the concept of neuroplasticity: The ability of neural networks in the brain to change, adapt, and strengthen, much like a muscle in the body that has been trained and exercised.
“The brain’s ability to bounce back from injury is what neuroplasticity is, and I’ve worked with people in our rehab clinic who have had brain tumors or suffer the effects of surgery or radiation on the brain, and people who have had West Nile virus, HIV, and meningitis,” said Tom Bergquist, PhD, clinical neuropsychologist at Mayo Clinic in Rochester, Minn. “There’s not a week that goes by that I don’t see someone recovering from COVID-19.”
One of the approaches used in the clinic is errorless learning, or having a patient with memory problems repeat information a certain number of times without error. The repetition helps rebuild those memory skills that were weakened during infection, Dr. Bergquist says.
People who have experienced brain fog after other viral infections have seen improvements with these approaches. Ben Ahrens, co-founder and CEO of re-origin – a company that offers neuroplasticity therapy – says he had long-term cognitive issues after a Lyme disease infection. Posttreatment Lyme disease syndrome, or chronic Lyme disease, occurs in about 1 in 10 people who are infected.
Mr. Ahrens says he was struck with Lyme 10 years ago and had brain fog, joint pain, and brain lesions detectable on scans for several years after infection.
According to Mr. Ahrens, neuroplasticity-based therapies help combat what researchers have found may be a lingering memory of past infections that lead to a heightened immune response, causing lingering symptoms.
“Essentially, what we believe is happening here, is the brain has learned that these symptoms are life-threatening – because, in fact, they can be,” Mr. Ahrens said. “The brain’s one job is to protect the body, and once it’s learned to associate these symptoms with that potentially very dangerous pathogen, even after it’s gone, things like a normal headache can trigger an immune cascade.”
Studies are underway at the University of Alabama at Birmingham to examine whether constraint-induced therapy – an approach rooted in neuroplasticity and historically used for loss of limb and speech function – is also effective for cognitive impairments like brain fog.
One technique they use is called shaping, which requires a person to repeatedly carry out their personal best function of impaired use – for example, remembering household tasks they have previously forgotten. That is done multiple times over several weeks in the clinic, and patients are given ways to transfer those skills to real-life use.
So far, the results are promising, said Edward Taub, PhD, researcher and professor of psychology at the University of Alabama at Birmingham.
When used in the past for physical impairments, researchers have noted not just clinical improvements, but structural changes. It led to an increase in the brain’s gray matter – which allows individuals to control movement, memory, and emotions – and improved white matter, which helps communication between gray matter areas.
Though results of the cognitive studies have not been published, Dr. Taub said patients with brain fog have shown improvement after just 35 hours of therapy and are nearly 100% improved after 6 months.
“The idea behind this is that the brain is responsive to use,” Dr. Taub said. “The amount of brain territory that’s dedicated to supporting or mediating a given behavioral function depends on the demands placed on the brain.”
A version of this article first appeared on WebMD.com.
People with long-COVID “brain fog” may be able to recover mental abilities that were dulled or stolen from them by the virus through an approach that has improved the effects of stroke, traumatic brain injury, and other post-viral disorders, doctors and scientists say.
For a lucky portion of the population, COVID-19 lasts a handful of days with minor symptoms. But for an estimated 37% who contract the virus, symptoms can linger for weeks, months, or even years. One of the most common symptoms of long COVID is brain fog: a life-altering condition characterized by slow thinking, confusion, difficulty remembering things, and poor concentration.
The approaches are based on the concept of neuroplasticity: The ability of neural networks in the brain to change, adapt, and strengthen, much like a muscle in the body that has been trained and exercised.
“The brain’s ability to bounce back from injury is what neuroplasticity is, and I’ve worked with people in our rehab clinic who have had brain tumors or suffer the effects of surgery or radiation on the brain, and people who have had West Nile virus, HIV, and meningitis,” said Tom Bergquist, PhD, clinical neuropsychologist at Mayo Clinic in Rochester, Minn. “There’s not a week that goes by that I don’t see someone recovering from COVID-19.”
One of the approaches used in the clinic is errorless learning, or having a patient with memory problems repeat information a certain number of times without error. The repetition helps rebuild those memory skills that were weakened during infection, Dr. Bergquist says.
People who have experienced brain fog after other viral infections have seen improvements with these approaches. Ben Ahrens, co-founder and CEO of re-origin – a company that offers neuroplasticity therapy – says he had long-term cognitive issues after a Lyme disease infection. Posttreatment Lyme disease syndrome, or chronic Lyme disease, occurs in about 1 in 10 people who are infected.
Mr. Ahrens says he was struck with Lyme 10 years ago and had brain fog, joint pain, and brain lesions detectable on scans for several years after infection.
According to Mr. Ahrens, neuroplasticity-based therapies help combat what researchers have found may be a lingering memory of past infections that lead to a heightened immune response, causing lingering symptoms.
“Essentially, what we believe is happening here, is the brain has learned that these symptoms are life-threatening – because, in fact, they can be,” Mr. Ahrens said. “The brain’s one job is to protect the body, and once it’s learned to associate these symptoms with that potentially very dangerous pathogen, even after it’s gone, things like a normal headache can trigger an immune cascade.”
Studies are underway at the University of Alabama at Birmingham to examine whether constraint-induced therapy – an approach rooted in neuroplasticity and historically used for loss of limb and speech function – is also effective for cognitive impairments like brain fog.
One technique they use is called shaping, which requires a person to repeatedly carry out their personal best function of impaired use – for example, remembering household tasks they have previously forgotten. That is done multiple times over several weeks in the clinic, and patients are given ways to transfer those skills to real-life use.
So far, the results are promising, said Edward Taub, PhD, researcher and professor of psychology at the University of Alabama at Birmingham.
When used in the past for physical impairments, researchers have noted not just clinical improvements, but structural changes. It led to an increase in the brain’s gray matter – which allows individuals to control movement, memory, and emotions – and improved white matter, which helps communication between gray matter areas.
Though results of the cognitive studies have not been published, Dr. Taub said patients with brain fog have shown improvement after just 35 hours of therapy and are nearly 100% improved after 6 months.
“The idea behind this is that the brain is responsive to use,” Dr. Taub said. “The amount of brain territory that’s dedicated to supporting or mediating a given behavioral function depends on the demands placed on the brain.”
A version of this article first appeared on WebMD.com.
People with long-COVID “brain fog” may be able to recover mental abilities that were dulled or stolen from them by the virus through an approach that has improved the effects of stroke, traumatic brain injury, and other post-viral disorders, doctors and scientists say.
For a lucky portion of the population, COVID-19 lasts a handful of days with minor symptoms. But for an estimated 37% who contract the virus, symptoms can linger for weeks, months, or even years. One of the most common symptoms of long COVID is brain fog: a life-altering condition characterized by slow thinking, confusion, difficulty remembering things, and poor concentration.
The approaches are based on the concept of neuroplasticity: The ability of neural networks in the brain to change, adapt, and strengthen, much like a muscle in the body that has been trained and exercised.
“The brain’s ability to bounce back from injury is what neuroplasticity is, and I’ve worked with people in our rehab clinic who have had brain tumors or suffer the effects of surgery or radiation on the brain, and people who have had West Nile virus, HIV, and meningitis,” said Tom Bergquist, PhD, clinical neuropsychologist at Mayo Clinic in Rochester, Minn. “There’s not a week that goes by that I don’t see someone recovering from COVID-19.”
One of the approaches used in the clinic is errorless learning, or having a patient with memory problems repeat information a certain number of times without error. The repetition helps rebuild those memory skills that were weakened during infection, Dr. Bergquist says.
People who have experienced brain fog after other viral infections have seen improvements with these approaches. Ben Ahrens, co-founder and CEO of re-origin – a company that offers neuroplasticity therapy – says he had long-term cognitive issues after a Lyme disease infection. Posttreatment Lyme disease syndrome, or chronic Lyme disease, occurs in about 1 in 10 people who are infected.
Mr. Ahrens says he was struck with Lyme 10 years ago and had brain fog, joint pain, and brain lesions detectable on scans for several years after infection.
According to Mr. Ahrens, neuroplasticity-based therapies help combat what researchers have found may be a lingering memory of past infections that lead to a heightened immune response, causing lingering symptoms.
“Essentially, what we believe is happening here, is the brain has learned that these symptoms are life-threatening – because, in fact, they can be,” Mr. Ahrens said. “The brain’s one job is to protect the body, and once it’s learned to associate these symptoms with that potentially very dangerous pathogen, even after it’s gone, things like a normal headache can trigger an immune cascade.”
Studies are underway at the University of Alabama at Birmingham to examine whether constraint-induced therapy – an approach rooted in neuroplasticity and historically used for loss of limb and speech function – is also effective for cognitive impairments like brain fog.
One technique they use is called shaping, which requires a person to repeatedly carry out their personal best function of impaired use – for example, remembering household tasks they have previously forgotten. That is done multiple times over several weeks in the clinic, and patients are given ways to transfer those skills to real-life use.
So far, the results are promising, said Edward Taub, PhD, researcher and professor of psychology at the University of Alabama at Birmingham.
When used in the past for physical impairments, researchers have noted not just clinical improvements, but structural changes. It led to an increase in the brain’s gray matter – which allows individuals to control movement, memory, and emotions – and improved white matter, which helps communication between gray matter areas.
Though results of the cognitive studies have not been published, Dr. Taub said patients with brain fog have shown improvement after just 35 hours of therapy and are nearly 100% improved after 6 months.
“The idea behind this is that the brain is responsive to use,” Dr. Taub said. “The amount of brain territory that’s dedicated to supporting or mediating a given behavioral function depends on the demands placed on the brain.”
A version of this article first appeared on WebMD.com.
Guselkumab controls axial involvement in PsA through 2 years
Guselkumab (Tremfya) received Food and Drug Administration approval for the treatment of psoriatic arthritis (PsA) almost 2 years ago on the basis of a phase 3 trial, but a new substudy from that trial has now demonstrated long-term benefit in the subgroup of patients who entered the trial with axial involvement, according to data presented at the annual meeting of the Canadian Rheumatology Association.
“The symptom relief was clinically meaningful and durable through week 100,” reported Dafna D. Gladman, MD, professor of medicine and director of the psoriatic arthritis program at the University of Toronto.
In the previously published double-blind, placebo-controlled DISCOVER-2 trial, two dosing regimens of the interleukin-23 (IL-23) inhibitor guselkumab were compared with placebo in biologic-naive patients. The active regimens were similarly effective relative to placebo for the primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 24.
In this new long-term subgroup analysis, outcomes at 2 years were evaluated in the 246 patients with axial involvement (33.3% of the total number of 739 evaluable patients). Baseline characteristics across treatment groups in this subset of the DISCOVER-2 trial were similar.
Guselkumab exhibits nearly twofold advantage
At 24 weeks relative to baseline, the improvement on multiple axial involvement outcome measures was approximately twofold greater with active therapy than with placebo. For example, the mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score fell 2.6 points in both active treatment arms versus 1.4 on placebo.
The same relative advantage was observed when the BASDAI spinal pain subscore was isolated. There were also comparable responses on a modified BASDAI that excluded the peripheral pain response, and the Ankylosing Spondylitis Disease Activity Score (ASDAS).
When evaluated at week 52 and again at week 100, all outcomes employed to evaluate change in axial involvement were sustained. Many were further improved. In patients who initiated therapy on placebo, all of whom were switched to guselkumab at the end of the 24-week double-blind period, at least the same degree of axial symptom control relative to baseline was achieved at both time periods.
Incremental improvement observed over time
“For most measures there was further improvement at 2 years, and there was generally consistent response across patient groups stratified by HLA [human leucocyte antigen] status,” Dr. Gladman reported.
Relative to the 2.6-point reduction in the BASDAI score in the two guselkumab arms at week 24, the reductions reached 3.0, 3.1, and 3.3 at 100 weeks in the every-4-week guselkumab group, every-8-week guselkumab group, and the crossed-over placebo group, respectively. For ASDAS, the reductions at week 24 were 1.4, 1.5, and 0.7 points and reached 1.6, 1.7, and 1.6 points at 100 weeks in the every-4-week, every-8-week, and placebo crossover groups, respectively.
The sustained improvement is consistent with a previous post hoc analysis in which data from the phase 3 DISCOVER-1 trial were pooled with those from DISCOVER-2. This analysis focused on the 312 patients in these studies with axial disease documented by imaging. Again, the study showed improvement at week 24 was sustained at week 52 independent of HLA-B27 status.
Need for MRI confirmation seen
The potential problem with this new analysis as well as the previous analysis is the absence of MRI to provide objective evidence of axial involvement, according to Walter P. Maksymowych, MD, professor in the division of rheumatology at the University of Alberta, Edmonton.
Noting that previous studies have indicated that axial involvement assessed by investigators is not reliable even when performed with x-rays, Dr. Maksymowych said these data would be much more reassuring with MRI controls.
“We have seen little correlation between clinical symptoms and MRI manifestations of disease,” he said.
Dr. Gladman conceded this point. Baseline MRI was performed in some of the patients in this subgroup analysis, but it was not mandated. As a result, the data support benefit from guselkumab for symptomatic axial involvement, but she indicated that better evidence of a disease-modifying effect is expected from a more rigorous placebo-controlled trial of guselkumab called STAR.
This trial is requiring MRI at baseline and at week 24 and is using the Spondyloarthritis Research Consortium of Canada (SPARCC) score to assess change. Dr. Gladman said the trial is enrolling “as we speak.”
Overall, Dr. Gladman agreed with Dr. Maksymowych that objective biomarkers are important for demonstrating that treatments are improving long-term outcomes, not just relieving symptoms.
Guselkumab manufacturer Janssen supported the post hoc analysis. Dr. Gladman reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Janssen, Novartis, Pfizer, and UCB. Dr. Maksymowych reported financial relationships with AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB.
Guselkumab (Tremfya) received Food and Drug Administration approval for the treatment of psoriatic arthritis (PsA) almost 2 years ago on the basis of a phase 3 trial, but a new substudy from that trial has now demonstrated long-term benefit in the subgroup of patients who entered the trial with axial involvement, according to data presented at the annual meeting of the Canadian Rheumatology Association.
“The symptom relief was clinically meaningful and durable through week 100,” reported Dafna D. Gladman, MD, professor of medicine and director of the psoriatic arthritis program at the University of Toronto.
In the previously published double-blind, placebo-controlled DISCOVER-2 trial, two dosing regimens of the interleukin-23 (IL-23) inhibitor guselkumab were compared with placebo in biologic-naive patients. The active regimens were similarly effective relative to placebo for the primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 24.
In this new long-term subgroup analysis, outcomes at 2 years were evaluated in the 246 patients with axial involvement (33.3% of the total number of 739 evaluable patients). Baseline characteristics across treatment groups in this subset of the DISCOVER-2 trial were similar.
Guselkumab exhibits nearly twofold advantage
At 24 weeks relative to baseline, the improvement on multiple axial involvement outcome measures was approximately twofold greater with active therapy than with placebo. For example, the mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score fell 2.6 points in both active treatment arms versus 1.4 on placebo.
The same relative advantage was observed when the BASDAI spinal pain subscore was isolated. There were also comparable responses on a modified BASDAI that excluded the peripheral pain response, and the Ankylosing Spondylitis Disease Activity Score (ASDAS).
When evaluated at week 52 and again at week 100, all outcomes employed to evaluate change in axial involvement were sustained. Many were further improved. In patients who initiated therapy on placebo, all of whom were switched to guselkumab at the end of the 24-week double-blind period, at least the same degree of axial symptom control relative to baseline was achieved at both time periods.
Incremental improvement observed over time
“For most measures there was further improvement at 2 years, and there was generally consistent response across patient groups stratified by HLA [human leucocyte antigen] status,” Dr. Gladman reported.
Relative to the 2.6-point reduction in the BASDAI score in the two guselkumab arms at week 24, the reductions reached 3.0, 3.1, and 3.3 at 100 weeks in the every-4-week guselkumab group, every-8-week guselkumab group, and the crossed-over placebo group, respectively. For ASDAS, the reductions at week 24 were 1.4, 1.5, and 0.7 points and reached 1.6, 1.7, and 1.6 points at 100 weeks in the every-4-week, every-8-week, and placebo crossover groups, respectively.
The sustained improvement is consistent with a previous post hoc analysis in which data from the phase 3 DISCOVER-1 trial were pooled with those from DISCOVER-2. This analysis focused on the 312 patients in these studies with axial disease documented by imaging. Again, the study showed improvement at week 24 was sustained at week 52 independent of HLA-B27 status.
Need for MRI confirmation seen
The potential problem with this new analysis as well as the previous analysis is the absence of MRI to provide objective evidence of axial involvement, according to Walter P. Maksymowych, MD, professor in the division of rheumatology at the University of Alberta, Edmonton.
Noting that previous studies have indicated that axial involvement assessed by investigators is not reliable even when performed with x-rays, Dr. Maksymowych said these data would be much more reassuring with MRI controls.
“We have seen little correlation between clinical symptoms and MRI manifestations of disease,” he said.
Dr. Gladman conceded this point. Baseline MRI was performed in some of the patients in this subgroup analysis, but it was not mandated. As a result, the data support benefit from guselkumab for symptomatic axial involvement, but she indicated that better evidence of a disease-modifying effect is expected from a more rigorous placebo-controlled trial of guselkumab called STAR.
This trial is requiring MRI at baseline and at week 24 and is using the Spondyloarthritis Research Consortium of Canada (SPARCC) score to assess change. Dr. Gladman said the trial is enrolling “as we speak.”
Overall, Dr. Gladman agreed with Dr. Maksymowych that objective biomarkers are important for demonstrating that treatments are improving long-term outcomes, not just relieving symptoms.
Guselkumab manufacturer Janssen supported the post hoc analysis. Dr. Gladman reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Janssen, Novartis, Pfizer, and UCB. Dr. Maksymowych reported financial relationships with AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB.
Guselkumab (Tremfya) received Food and Drug Administration approval for the treatment of psoriatic arthritis (PsA) almost 2 years ago on the basis of a phase 3 trial, but a new substudy from that trial has now demonstrated long-term benefit in the subgroup of patients who entered the trial with axial involvement, according to data presented at the annual meeting of the Canadian Rheumatology Association.
“The symptom relief was clinically meaningful and durable through week 100,” reported Dafna D. Gladman, MD, professor of medicine and director of the psoriatic arthritis program at the University of Toronto.
In the previously published double-blind, placebo-controlled DISCOVER-2 trial, two dosing regimens of the interleukin-23 (IL-23) inhibitor guselkumab were compared with placebo in biologic-naive patients. The active regimens were similarly effective relative to placebo for the primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 24.
In this new long-term subgroup analysis, outcomes at 2 years were evaluated in the 246 patients with axial involvement (33.3% of the total number of 739 evaluable patients). Baseline characteristics across treatment groups in this subset of the DISCOVER-2 trial were similar.
Guselkumab exhibits nearly twofold advantage
At 24 weeks relative to baseline, the improvement on multiple axial involvement outcome measures was approximately twofold greater with active therapy than with placebo. For example, the mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score fell 2.6 points in both active treatment arms versus 1.4 on placebo.
The same relative advantage was observed when the BASDAI spinal pain subscore was isolated. There were also comparable responses on a modified BASDAI that excluded the peripheral pain response, and the Ankylosing Spondylitis Disease Activity Score (ASDAS).
When evaluated at week 52 and again at week 100, all outcomes employed to evaluate change in axial involvement were sustained. Many were further improved. In patients who initiated therapy on placebo, all of whom were switched to guselkumab at the end of the 24-week double-blind period, at least the same degree of axial symptom control relative to baseline was achieved at both time periods.
Incremental improvement observed over time
“For most measures there was further improvement at 2 years, and there was generally consistent response across patient groups stratified by HLA [human leucocyte antigen] status,” Dr. Gladman reported.
Relative to the 2.6-point reduction in the BASDAI score in the two guselkumab arms at week 24, the reductions reached 3.0, 3.1, and 3.3 at 100 weeks in the every-4-week guselkumab group, every-8-week guselkumab group, and the crossed-over placebo group, respectively. For ASDAS, the reductions at week 24 were 1.4, 1.5, and 0.7 points and reached 1.6, 1.7, and 1.6 points at 100 weeks in the every-4-week, every-8-week, and placebo crossover groups, respectively.
The sustained improvement is consistent with a previous post hoc analysis in which data from the phase 3 DISCOVER-1 trial were pooled with those from DISCOVER-2. This analysis focused on the 312 patients in these studies with axial disease documented by imaging. Again, the study showed improvement at week 24 was sustained at week 52 independent of HLA-B27 status.
Need for MRI confirmation seen
The potential problem with this new analysis as well as the previous analysis is the absence of MRI to provide objective evidence of axial involvement, according to Walter P. Maksymowych, MD, professor in the division of rheumatology at the University of Alberta, Edmonton.
Noting that previous studies have indicated that axial involvement assessed by investigators is not reliable even when performed with x-rays, Dr. Maksymowych said these data would be much more reassuring with MRI controls.
“We have seen little correlation between clinical symptoms and MRI manifestations of disease,” he said.
Dr. Gladman conceded this point. Baseline MRI was performed in some of the patients in this subgroup analysis, but it was not mandated. As a result, the data support benefit from guselkumab for symptomatic axial involvement, but she indicated that better evidence of a disease-modifying effect is expected from a more rigorous placebo-controlled trial of guselkumab called STAR.
This trial is requiring MRI at baseline and at week 24 and is using the Spondyloarthritis Research Consortium of Canada (SPARCC) score to assess change. Dr. Gladman said the trial is enrolling “as we speak.”
Overall, Dr. Gladman agreed with Dr. Maksymowych that objective biomarkers are important for demonstrating that treatments are improving long-term outcomes, not just relieving symptoms.
Guselkumab manufacturer Janssen supported the post hoc analysis. Dr. Gladman reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Janssen, Novartis, Pfizer, and UCB. Dr. Maksymowych reported financial relationships with AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB.
FROM THE ANNUAL MEETING OF THE CANADIAN RHEUMATOLOGY ASSOCIATION
If you’ve got 3 seconds, then you’ve got time to work out
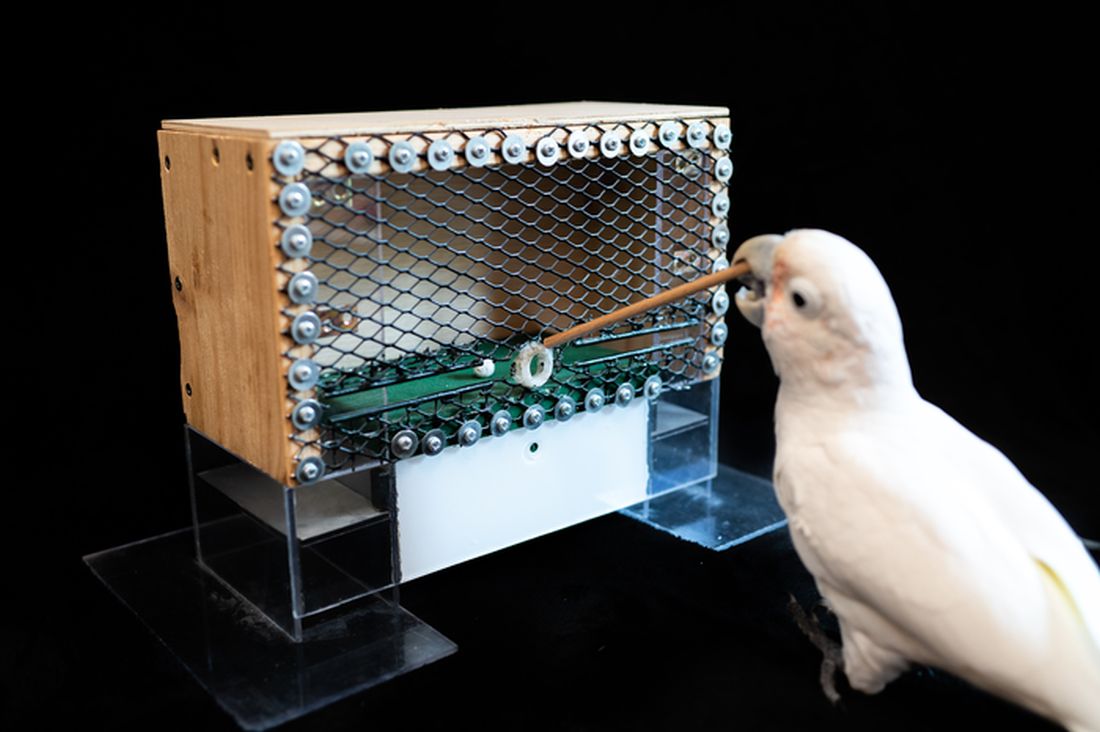
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Expert shares workup pearls for children with severe atopic dermatitis
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
FROM ODAC 2022
Boxed warning for JAK inhibitors belies their durability in real-world registry studies
Several relatively large real-world analyses of Janus kinase inhibitors (JAKi) in patients with rheumatoid arthritis appear to show that the oral small-molecule drugs are discontinued and retained at rates similar to or better than biologic disease-modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor inhibitors (TNFi), according to studies presented at the annual meeting of the Canadian Rheumatology Association.
The findings of these studies, although conducted prior to the Food and Drug Administration’s September 2021 announcement of a boxed warning for JAKi, do not lend support to the warning’s message of higher risks of major adverse cardiovascular events (MACE), blood clots, cancer, and death associated with JAKi.
In one study, discontinuation of JAKi-class drugs was less common than discontinuation of bDMARD-class drugs, including tumor necrosis factor inhibitors (TNFi), according to a multicenter team of investigators led by Janet Pope, MD, a professor in the division of rheumatology at the University of Western Ontario, London.
The greater durability of the JAKi relative to TNFi “seem to be driven by a greater loss of efficacy in bDMARDs over time,” reported Samir Magdy Iskander, a medical student at the university, who presented the data.
JAKi rival bDMARDs for long-term retention
In a separate but larger analysis, the retention rates with the JAKi tofacitinib (Xeljanz) and TNFi in two RA registries in Canada were about the same after a mean follow-up of 23.2 months (36.9% vs. 37.5%), but the tofacitinib group was at a relative disadvantage. Relative to the bDMARD group, patients taking JAKi were more likely to have had prior treatment with a bDMARD (66.9% vs. 33.9%), to have a higher median baseline Clinical Disease Activity Index (CDAI) score (22.1 vs. 20.0; P < .05), and to be older (59.5 vs. 57.6 years).
In this study, 1,318 patients with RA enrolled in the Ontario Best Practices Research Initiative (OBRI) or a Quebec cohort called RHUMADATA were evaluated, reported Mohammad Movahedi, MD, PhD, of the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
“We have not yet analyzed the reasons for discontinuation, but the data show that retention is about the same, meaning that selection of one agent over the other should be tailored according to patient characteristics,” Dr. Movahedi said.
Reasons for discontinuation were presented in the other observational study, which included 333 adult patients with RA from two centers in Ontario. The discontinuation rate for adverse events was approximately 20% in both groups (HR, 1.0005; P = .98). However, the discontinuation rate for lack of efficacy favored the JAKi, reaching statistical significance.
TNFi failure for lack of efficacy is higher
“For lack of efficacy, the discontinuation rate was about 35% lower on the JAKi [HR, 0.6543; P = .029],” Mr. Iskander reported. Relative to those taking a TNFi, those on a JAKi demonstrated “greater durability regardless of gender, age, disease duration, and prior lines of therapy.”
In a population of patients who have not achieved an adequate response to conventional synthetic DMARDs (csDMARDs), which describes the study population from the two Ontario centers, JAKi “may be considered as a preferable method of treatment,” Mr. Iskander said.
Pointing out that many clinicians have interpreted the boxed warning as a relative contraindication for use of JAKi as first-line therapy in patients with an inadequate response to csDMARDs, Marinka Twilt, MD, PhD, the moderator of the scientific session where these data were presented, questioned the conclusion. In the boxed warning, clinicians and patients are advised to consider an increased risk of serious infections, malignancy, and cardiovascular-related mortality in individuals older than 50 years.
In response, Mr. Iskander said that the data were collected and analyzed prior to the change in labeling. He acknowledged that this study was not designed to capture long-term risks, such as cardiovascular disease or malignancy. In this analysis, the safety and tolerability of JAKi and bDMARDs appeared comparable.
NEJM published study leading to boxed warning
Just a week prior to the CRA annual meeting, the New England Journal of Medicine published an FDA-mandated postmarketing trial of tofacitinib that was used by the agency to justify the boxed warning for JAKi with indications for artitis and other inflammatory diseases. In that open-label trial, more than 4,000 patients aged 50 years or older with at least one additional cardiovascular risk factor were randomized to 5 mg tofacitinib twice daily, 10 mg tofacitinib twice daily, or a TNFi (adalimumab or etanercept).
The efficacy of the therapies was similar, but tofacitinib failed to meet predefined noninferiority criteria for the co–primary endpoints of MACE or cancer (excluding nonmelanoma skin cancer). For tofacitinib relative to TNFi, the hazard ratio was 1.33 for MACE and 1.48 for cancers. The JAKi was also associated with higher incidences of opportunistic infections.
Mr. Iskander noted that Canadian clinical practice guidelines currently identify JAKi as a reasonable first-line alternative to bDMARDs after inadequate response to csDMARDs. While his data support that position, Dr. Twilt indicated that the benefit-to-risk ratio of JAKi might need recalculation based on the data that led the FDA to issue its boxed warning. She questioned whether the language regarding the relative role of JAKi and bDMARDs will change in coming RA guideline revisions.
Dr. Iskander reported no potential conflicts of interest. Dr. Movahedi did not list any personal conflicts of interest but acknowledged that OBRI received unrestricted grants from a variety of pharmaceutical companies, including those that manufacture bDMARDs and JAKi. Dr. Twilt reported no potential conflicts of interest.
Several relatively large real-world analyses of Janus kinase inhibitors (JAKi) in patients with rheumatoid arthritis appear to show that the oral small-molecule drugs are discontinued and retained at rates similar to or better than biologic disease-modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor inhibitors (TNFi), according to studies presented at the annual meeting of the Canadian Rheumatology Association.
The findings of these studies, although conducted prior to the Food and Drug Administration’s September 2021 announcement of a boxed warning for JAKi, do not lend support to the warning’s message of higher risks of major adverse cardiovascular events (MACE), blood clots, cancer, and death associated with JAKi.
In one study, discontinuation of JAKi-class drugs was less common than discontinuation of bDMARD-class drugs, including tumor necrosis factor inhibitors (TNFi), according to a multicenter team of investigators led by Janet Pope, MD, a professor in the division of rheumatology at the University of Western Ontario, London.
The greater durability of the JAKi relative to TNFi “seem to be driven by a greater loss of efficacy in bDMARDs over time,” reported Samir Magdy Iskander, a medical student at the university, who presented the data.
JAKi rival bDMARDs for long-term retention
In a separate but larger analysis, the retention rates with the JAKi tofacitinib (Xeljanz) and TNFi in two RA registries in Canada were about the same after a mean follow-up of 23.2 months (36.9% vs. 37.5%), but the tofacitinib group was at a relative disadvantage. Relative to the bDMARD group, patients taking JAKi were more likely to have had prior treatment with a bDMARD (66.9% vs. 33.9%), to have a higher median baseline Clinical Disease Activity Index (CDAI) score (22.1 vs. 20.0; P < .05), and to be older (59.5 vs. 57.6 years).
In this study, 1,318 patients with RA enrolled in the Ontario Best Practices Research Initiative (OBRI) or a Quebec cohort called RHUMADATA were evaluated, reported Mohammad Movahedi, MD, PhD, of the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
“We have not yet analyzed the reasons for discontinuation, but the data show that retention is about the same, meaning that selection of one agent over the other should be tailored according to patient characteristics,” Dr. Movahedi said.
Reasons for discontinuation were presented in the other observational study, which included 333 adult patients with RA from two centers in Ontario. The discontinuation rate for adverse events was approximately 20% in both groups (HR, 1.0005; P = .98). However, the discontinuation rate for lack of efficacy favored the JAKi, reaching statistical significance.
TNFi failure for lack of efficacy is higher
“For lack of efficacy, the discontinuation rate was about 35% lower on the JAKi [HR, 0.6543; P = .029],” Mr. Iskander reported. Relative to those taking a TNFi, those on a JAKi demonstrated “greater durability regardless of gender, age, disease duration, and prior lines of therapy.”
In a population of patients who have not achieved an adequate response to conventional synthetic DMARDs (csDMARDs), which describes the study population from the two Ontario centers, JAKi “may be considered as a preferable method of treatment,” Mr. Iskander said.
Pointing out that many clinicians have interpreted the boxed warning as a relative contraindication for use of JAKi as first-line therapy in patients with an inadequate response to csDMARDs, Marinka Twilt, MD, PhD, the moderator of the scientific session where these data were presented, questioned the conclusion. In the boxed warning, clinicians and patients are advised to consider an increased risk of serious infections, malignancy, and cardiovascular-related mortality in individuals older than 50 years.
In response, Mr. Iskander said that the data were collected and analyzed prior to the change in labeling. He acknowledged that this study was not designed to capture long-term risks, such as cardiovascular disease or malignancy. In this analysis, the safety and tolerability of JAKi and bDMARDs appeared comparable.
NEJM published study leading to boxed warning
Just a week prior to the CRA annual meeting, the New England Journal of Medicine published an FDA-mandated postmarketing trial of tofacitinib that was used by the agency to justify the boxed warning for JAKi with indications for artitis and other inflammatory diseases. In that open-label trial, more than 4,000 patients aged 50 years or older with at least one additional cardiovascular risk factor were randomized to 5 mg tofacitinib twice daily, 10 mg tofacitinib twice daily, or a TNFi (adalimumab or etanercept).
The efficacy of the therapies was similar, but tofacitinib failed to meet predefined noninferiority criteria for the co–primary endpoints of MACE or cancer (excluding nonmelanoma skin cancer). For tofacitinib relative to TNFi, the hazard ratio was 1.33 for MACE and 1.48 for cancers. The JAKi was also associated with higher incidences of opportunistic infections.
Mr. Iskander noted that Canadian clinical practice guidelines currently identify JAKi as a reasonable first-line alternative to bDMARDs after inadequate response to csDMARDs. While his data support that position, Dr. Twilt indicated that the benefit-to-risk ratio of JAKi might need recalculation based on the data that led the FDA to issue its boxed warning. She questioned whether the language regarding the relative role of JAKi and bDMARDs will change in coming RA guideline revisions.
Dr. Iskander reported no potential conflicts of interest. Dr. Movahedi did not list any personal conflicts of interest but acknowledged that OBRI received unrestricted grants from a variety of pharmaceutical companies, including those that manufacture bDMARDs and JAKi. Dr. Twilt reported no potential conflicts of interest.
Several relatively large real-world analyses of Janus kinase inhibitors (JAKi) in patients with rheumatoid arthritis appear to show that the oral small-molecule drugs are discontinued and retained at rates similar to or better than biologic disease-modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor inhibitors (TNFi), according to studies presented at the annual meeting of the Canadian Rheumatology Association.
The findings of these studies, although conducted prior to the Food and Drug Administration’s September 2021 announcement of a boxed warning for JAKi, do not lend support to the warning’s message of higher risks of major adverse cardiovascular events (MACE), blood clots, cancer, and death associated with JAKi.
In one study, discontinuation of JAKi-class drugs was less common than discontinuation of bDMARD-class drugs, including tumor necrosis factor inhibitors (TNFi), according to a multicenter team of investigators led by Janet Pope, MD, a professor in the division of rheumatology at the University of Western Ontario, London.
The greater durability of the JAKi relative to TNFi “seem to be driven by a greater loss of efficacy in bDMARDs over time,” reported Samir Magdy Iskander, a medical student at the university, who presented the data.
JAKi rival bDMARDs for long-term retention
In a separate but larger analysis, the retention rates with the JAKi tofacitinib (Xeljanz) and TNFi in two RA registries in Canada were about the same after a mean follow-up of 23.2 months (36.9% vs. 37.5%), but the tofacitinib group was at a relative disadvantage. Relative to the bDMARD group, patients taking JAKi were more likely to have had prior treatment with a bDMARD (66.9% vs. 33.9%), to have a higher median baseline Clinical Disease Activity Index (CDAI) score (22.1 vs. 20.0; P < .05), and to be older (59.5 vs. 57.6 years).
In this study, 1,318 patients with RA enrolled in the Ontario Best Practices Research Initiative (OBRI) or a Quebec cohort called RHUMADATA were evaluated, reported Mohammad Movahedi, MD, PhD, of the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
“We have not yet analyzed the reasons for discontinuation, but the data show that retention is about the same, meaning that selection of one agent over the other should be tailored according to patient characteristics,” Dr. Movahedi said.
Reasons for discontinuation were presented in the other observational study, which included 333 adult patients with RA from two centers in Ontario. The discontinuation rate for adverse events was approximately 20% in both groups (HR, 1.0005; P = .98). However, the discontinuation rate for lack of efficacy favored the JAKi, reaching statistical significance.
TNFi failure for lack of efficacy is higher
“For lack of efficacy, the discontinuation rate was about 35% lower on the JAKi [HR, 0.6543; P = .029],” Mr. Iskander reported. Relative to those taking a TNFi, those on a JAKi demonstrated “greater durability regardless of gender, age, disease duration, and prior lines of therapy.”
In a population of patients who have not achieved an adequate response to conventional synthetic DMARDs (csDMARDs), which describes the study population from the two Ontario centers, JAKi “may be considered as a preferable method of treatment,” Mr. Iskander said.
Pointing out that many clinicians have interpreted the boxed warning as a relative contraindication for use of JAKi as first-line therapy in patients with an inadequate response to csDMARDs, Marinka Twilt, MD, PhD, the moderator of the scientific session where these data were presented, questioned the conclusion. In the boxed warning, clinicians and patients are advised to consider an increased risk of serious infections, malignancy, and cardiovascular-related mortality in individuals older than 50 years.
In response, Mr. Iskander said that the data were collected and analyzed prior to the change in labeling. He acknowledged that this study was not designed to capture long-term risks, such as cardiovascular disease or malignancy. In this analysis, the safety and tolerability of JAKi and bDMARDs appeared comparable.
NEJM published study leading to boxed warning
Just a week prior to the CRA annual meeting, the New England Journal of Medicine published an FDA-mandated postmarketing trial of tofacitinib that was used by the agency to justify the boxed warning for JAKi with indications for artitis and other inflammatory diseases. In that open-label trial, more than 4,000 patients aged 50 years or older with at least one additional cardiovascular risk factor were randomized to 5 mg tofacitinib twice daily, 10 mg tofacitinib twice daily, or a TNFi (adalimumab or etanercept).
The efficacy of the therapies was similar, but tofacitinib failed to meet predefined noninferiority criteria for the co–primary endpoints of MACE or cancer (excluding nonmelanoma skin cancer). For tofacitinib relative to TNFi, the hazard ratio was 1.33 for MACE and 1.48 for cancers. The JAKi was also associated with higher incidences of opportunistic infections.
Mr. Iskander noted that Canadian clinical practice guidelines currently identify JAKi as a reasonable first-line alternative to bDMARDs after inadequate response to csDMARDs. While his data support that position, Dr. Twilt indicated that the benefit-to-risk ratio of JAKi might need recalculation based on the data that led the FDA to issue its boxed warning. She questioned whether the language regarding the relative role of JAKi and bDMARDs will change in coming RA guideline revisions.
Dr. Iskander reported no potential conflicts of interest. Dr. Movahedi did not list any personal conflicts of interest but acknowledged that OBRI received unrestricted grants from a variety of pharmaceutical companies, including those that manufacture bDMARDs and JAKi. Dr. Twilt reported no potential conflicts of interest.
FROM THE ANNUAL MEETING OF THE CANADIAN RHEUMATOLOGY ASSOCIATION
How to make the most of your time with psoriasis patients
In the clinical experience of George Han, MD, PhD, .
“They come in with bags of topical products to show you what they’ve tried,” Dr. Han, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said during the ODAC Dermatology, Aesthetic & Surgical Conference. “And you’re supposed to see this patient, talk to them, and counsel them in about 10 minutes. How do you make time to conduct an efficient psoriasis visit?”
Patients have a long-term battle to get clear, and spending a little longer on the initial visit “pays a lot of dividends,” he said. “Some of these patients are the most thankful patients in our practices, and it truly is gratifying” to see how much they can improve.
Questions about diet
Dr. Han said that psoriasis patients often ask him if, what, or how much they’re eating affects their disease. “But how do you counsel patients about diet when we’re not dietitians? We can at least give some guidance based on available data.”
He referred to a nationwide study of psoriasis patient-reported outcomes and dietary behaviors, which found that the percentage of patients who reported skin improvement was greatest after reducing intake of alcohol (53.8%); gluten (53.4%); and nightshade vegetables, such as tomatoes, potatoes, and peppers (52.1%); and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). He noted that there is a threefold increased incidence of celiac disease in patients with psoriasis.
As for nightshade vegetables, intake leads to increased alkaloids, “which have been known to worsen bowel inflammation such as in IBD [inflammatory bowel disease], but there is a lack of controlled trials examining this in the overall psoriasis population,” Dr. Han said. The Mediterranean diet, he added, “is sensible, and adding olive oil to your diet seems to have a positive effect on ... PASI, while fish oil seems to reduce C-reactive protein.” The data on the effect of vitamin D supplements are mixed, he said.
A separate randomized study evaluated the impact of weight loss in overweight or obese patients with psoriasis, who had not achieved clearance after 4 weeks of systemic treatment. Significantly more of those in the dietary intervention arm reached the weight loss goal of 5% at 20 weeks, and patients in this arm had a median reduction in the Psoriasis Area and Severity Index (PASI) score of almost 50%, compared with almost 26% among those without an active dietary intervention.
Joint pain, PsA
For psoriasis patients who complain of joint pain, he recommends administering quick measures like the five-question Psoriasis Epidemiology Screening Test (PEST) to screen for psoriatic arthritis (PsA), which is available on the National Psoriasis Foundation web site. “I ask patients about swollen, tender joints – specifically hands, wrists, ankles, feet, and toes,” Dr. Han said. Joint stiffness in the morning is a “concerning finding,” which is “more indicative of psoriatic arthritis than vague knee or back pain that worsens with use. If you have a younger patient with back pain who has a reduced ability to flex their spine, think axial disease.”
Tumor necrosis factor (TNF)–alpha inhibitors are considered first- and second-line treatment for PsA, but interleukin (IL)–17 inhibitors are generally considered just as effective overall. “The IL-23 inhibitors have mixed signals,” said Dr. Han, who is also on the NPF’s medical board. “We know that guselkumab is effective against psoriatic arthritis, but there is no inhibition of joint progression at the approved dosage on the label – though it was pretty close.”
Risankizumab (Skyrizi), an IL-23 inhibitor, was approved in January 2022 for adults with PsA and while the American College of Rheumatology response data “look reasonably good, the results for inhibition of radiographic progression are quite far off and it’s not in the label,” he said. Tildrakizumab (Ilumya), an IL-23 inhibitor, “looks impressive in phase 2b trials. It will be interesting to see if there is differentiation between the IL-23 agents in treating joint disease going forward.”
Dr. Han considers biologic therapy a good option for patients with questionable joint involvement or very limited joint disease. “If the patient has some evidence of PsA, as long as it’s a medication that has approval for that, I’m OK with starting it,” he said. “However, for patients whose joint pain dominates over the skin, or [who] have severe joint disease at presentation, I would prioritize the TNF-alpha inhibitors and IL-17s and refer them to rheumatology for shared management.”
Topical, oral treatments
As for topical approaches to treating psoriasis, adding halobetasol propionate 0.01% to tazarotene 0.045% may have a synergistic effect, while tapinarof 1% cream holds promise, he said. Tapinarof, which is expected to be approved this year, is an investigative aryl hydrocarbon agonist that inhibits an array of proinflammatory cytokines, including interferon-gamma and TNF-alpha. “It has been shown to have inhibitory effects both on Th17 cytokines and Th2 cytokines,” Dr. Han said. “What’s nice about this is that patients still appear to have treatment effect 1-2 months after stopping the drug.”
Another topical agent now under FDA review for psoriasis, is roflumilast, a phosphodiesterase type 4 (PDE4) inhibitor, which has been shown to have a treatment efficacy of 30% or more. “We’ll see how this works into our treatment regimen for psoriasis,” he said, as strategies targeting PDE4 have already been reported to help treat psoriasis.
With regards to oral therapies, he said that there are concerns about the efficacy of the oral PDE4 inhibitor apremilast, approved for psoriasis, compared with other biologics. Deucravacitinib, an oral selective tyrosine kinase 2 (TYK2) inhibitor also under FDA review for psoriasis, “may fill this gap, because its efficacy seems much stronger and really capitalizes on blocking IL-23, which we know is a central pathway in the pathogenesis of psoriasis.”
Phototherapy is another treatment option. Home narrowband-UVB devices cost $3,000-$5,000, “which is a fraction of 1 year of biologic treatment,” Dr. Han said. Older data on phototherapy suggest that “lesions can clear within 2-3 months, depending on how often you do the phototherapy, while newer data suggest that 75% of patients can achieve clear or minimal disease” with phototherapy.
Biologic therapy
If patients meet criteria for treatment with a biologic, he begins the conversation by saying, “I don’t want to give you an immunosuppressant, but your psoriasis represents an overactivation of inflammation in your body, so in some way we have to bring that down. Ideally, we would target your immune system in a way that targets psoriasis very narrowly, while leaving it to do what it needs to: protecting against infections and neoplasia.”
XXXIL-17 inhibitors generally have the fastest onset of action, Dr. Han noted. Authors of a review paper found that achievement of Psoriasis Area and Severity Index (PASI) 50 was 1.8 weeks with brodalumab, 1.9 weeks for ixekizumab, 3 weeks for high-dose secukinumab, 3.5 weeks for adalimumab, 3.7 weeks for infliximab, 5.1 weeks for low-dose ustekinumab, 6.5 weeks for high-dose etanercept, and 10.9 weeks with low-dose etanercept, while achievement of PASI 50 was closer to 1 month for IL-23 inhibitors.
“The conversation I have with patients on IL-23 inhibitors is, ‘we’re in this for the long haul,’ otherwise they come in 2 months later,” he said. “They may have gotten clearer but we’re talking about getting well over half of our patients to PASI 100, or to clear or minimal disease, and they may not have gotten there yet. It helps to frame expectations.”
Dr. Han disclosed that he is a consultant to, a speaker for, or has received research support from Beiersdorf, CeraVe, Celgene, Janssen, Lilly, MC2, Pfizer, UCB, Boehringer Ingelheim, Bond Avillion, Athenex, Amgen, AbbVie, Regeneron/Sanofi, LEO Pharma, Ortho Dermatologics, BMS, Sun Pharma, Dermavant, Dermtech, MedX, Novartis, and Castle Biosciences.
In the clinical experience of George Han, MD, PhD, .
“They come in with bags of topical products to show you what they’ve tried,” Dr. Han, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said during the ODAC Dermatology, Aesthetic & Surgical Conference. “And you’re supposed to see this patient, talk to them, and counsel them in about 10 minutes. How do you make time to conduct an efficient psoriasis visit?”
Patients have a long-term battle to get clear, and spending a little longer on the initial visit “pays a lot of dividends,” he said. “Some of these patients are the most thankful patients in our practices, and it truly is gratifying” to see how much they can improve.
Questions about diet
Dr. Han said that psoriasis patients often ask him if, what, or how much they’re eating affects their disease. “But how do you counsel patients about diet when we’re not dietitians? We can at least give some guidance based on available data.”
He referred to a nationwide study of psoriasis patient-reported outcomes and dietary behaviors, which found that the percentage of patients who reported skin improvement was greatest after reducing intake of alcohol (53.8%); gluten (53.4%); and nightshade vegetables, such as tomatoes, potatoes, and peppers (52.1%); and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). He noted that there is a threefold increased incidence of celiac disease in patients with psoriasis.
As for nightshade vegetables, intake leads to increased alkaloids, “which have been known to worsen bowel inflammation such as in IBD [inflammatory bowel disease], but there is a lack of controlled trials examining this in the overall psoriasis population,” Dr. Han said. The Mediterranean diet, he added, “is sensible, and adding olive oil to your diet seems to have a positive effect on ... PASI, while fish oil seems to reduce C-reactive protein.” The data on the effect of vitamin D supplements are mixed, he said.
A separate randomized study evaluated the impact of weight loss in overweight or obese patients with psoriasis, who had not achieved clearance after 4 weeks of systemic treatment. Significantly more of those in the dietary intervention arm reached the weight loss goal of 5% at 20 weeks, and patients in this arm had a median reduction in the Psoriasis Area and Severity Index (PASI) score of almost 50%, compared with almost 26% among those without an active dietary intervention.
Joint pain, PsA
For psoriasis patients who complain of joint pain, he recommends administering quick measures like the five-question Psoriasis Epidemiology Screening Test (PEST) to screen for psoriatic arthritis (PsA), which is available on the National Psoriasis Foundation web site. “I ask patients about swollen, tender joints – specifically hands, wrists, ankles, feet, and toes,” Dr. Han said. Joint stiffness in the morning is a “concerning finding,” which is “more indicative of psoriatic arthritis than vague knee or back pain that worsens with use. If you have a younger patient with back pain who has a reduced ability to flex their spine, think axial disease.”
Tumor necrosis factor (TNF)–alpha inhibitors are considered first- and second-line treatment for PsA, but interleukin (IL)–17 inhibitors are generally considered just as effective overall. “The IL-23 inhibitors have mixed signals,” said Dr. Han, who is also on the NPF’s medical board. “We know that guselkumab is effective against psoriatic arthritis, but there is no inhibition of joint progression at the approved dosage on the label – though it was pretty close.”
Risankizumab (Skyrizi), an IL-23 inhibitor, was approved in January 2022 for adults with PsA and while the American College of Rheumatology response data “look reasonably good, the results for inhibition of radiographic progression are quite far off and it’s not in the label,” he said. Tildrakizumab (Ilumya), an IL-23 inhibitor, “looks impressive in phase 2b trials. It will be interesting to see if there is differentiation between the IL-23 agents in treating joint disease going forward.”
Dr. Han considers biologic therapy a good option for patients with questionable joint involvement or very limited joint disease. “If the patient has some evidence of PsA, as long as it’s a medication that has approval for that, I’m OK with starting it,” he said. “However, for patients whose joint pain dominates over the skin, or [who] have severe joint disease at presentation, I would prioritize the TNF-alpha inhibitors and IL-17s and refer them to rheumatology for shared management.”
Topical, oral treatments
As for topical approaches to treating psoriasis, adding halobetasol propionate 0.01% to tazarotene 0.045% may have a synergistic effect, while tapinarof 1% cream holds promise, he said. Tapinarof, which is expected to be approved this year, is an investigative aryl hydrocarbon agonist that inhibits an array of proinflammatory cytokines, including interferon-gamma and TNF-alpha. “It has been shown to have inhibitory effects both on Th17 cytokines and Th2 cytokines,” Dr. Han said. “What’s nice about this is that patients still appear to have treatment effect 1-2 months after stopping the drug.”
Another topical agent now under FDA review for psoriasis, is roflumilast, a phosphodiesterase type 4 (PDE4) inhibitor, which has been shown to have a treatment efficacy of 30% or more. “We’ll see how this works into our treatment regimen for psoriasis,” he said, as strategies targeting PDE4 have already been reported to help treat psoriasis.
With regards to oral therapies, he said that there are concerns about the efficacy of the oral PDE4 inhibitor apremilast, approved for psoriasis, compared with other biologics. Deucravacitinib, an oral selective tyrosine kinase 2 (TYK2) inhibitor also under FDA review for psoriasis, “may fill this gap, because its efficacy seems much stronger and really capitalizes on blocking IL-23, which we know is a central pathway in the pathogenesis of psoriasis.”
Phototherapy is another treatment option. Home narrowband-UVB devices cost $3,000-$5,000, “which is a fraction of 1 year of biologic treatment,” Dr. Han said. Older data on phototherapy suggest that “lesions can clear within 2-3 months, depending on how often you do the phototherapy, while newer data suggest that 75% of patients can achieve clear or minimal disease” with phototherapy.
Biologic therapy
If patients meet criteria for treatment with a biologic, he begins the conversation by saying, “I don’t want to give you an immunosuppressant, but your psoriasis represents an overactivation of inflammation in your body, so in some way we have to bring that down. Ideally, we would target your immune system in a way that targets psoriasis very narrowly, while leaving it to do what it needs to: protecting against infections and neoplasia.”
XXXIL-17 inhibitors generally have the fastest onset of action, Dr. Han noted. Authors of a review paper found that achievement of Psoriasis Area and Severity Index (PASI) 50 was 1.8 weeks with brodalumab, 1.9 weeks for ixekizumab, 3 weeks for high-dose secukinumab, 3.5 weeks for adalimumab, 3.7 weeks for infliximab, 5.1 weeks for low-dose ustekinumab, 6.5 weeks for high-dose etanercept, and 10.9 weeks with low-dose etanercept, while achievement of PASI 50 was closer to 1 month for IL-23 inhibitors.
“The conversation I have with patients on IL-23 inhibitors is, ‘we’re in this for the long haul,’ otherwise they come in 2 months later,” he said. “They may have gotten clearer but we’re talking about getting well over half of our patients to PASI 100, or to clear or minimal disease, and they may not have gotten there yet. It helps to frame expectations.”
Dr. Han disclosed that he is a consultant to, a speaker for, or has received research support from Beiersdorf, CeraVe, Celgene, Janssen, Lilly, MC2, Pfizer, UCB, Boehringer Ingelheim, Bond Avillion, Athenex, Amgen, AbbVie, Regeneron/Sanofi, LEO Pharma, Ortho Dermatologics, BMS, Sun Pharma, Dermavant, Dermtech, MedX, Novartis, and Castle Biosciences.
In the clinical experience of George Han, MD, PhD, .
“They come in with bags of topical products to show you what they’ve tried,” Dr. Han, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said during the ODAC Dermatology, Aesthetic & Surgical Conference. “And you’re supposed to see this patient, talk to them, and counsel them in about 10 minutes. How do you make time to conduct an efficient psoriasis visit?”
Patients have a long-term battle to get clear, and spending a little longer on the initial visit “pays a lot of dividends,” he said. “Some of these patients are the most thankful patients in our practices, and it truly is gratifying” to see how much they can improve.
Questions about diet
Dr. Han said that psoriasis patients often ask him if, what, or how much they’re eating affects their disease. “But how do you counsel patients about diet when we’re not dietitians? We can at least give some guidance based on available data.”
He referred to a nationwide study of psoriasis patient-reported outcomes and dietary behaviors, which found that the percentage of patients who reported skin improvement was greatest after reducing intake of alcohol (53.8%); gluten (53.4%); and nightshade vegetables, such as tomatoes, potatoes, and peppers (52.1%); and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). He noted that there is a threefold increased incidence of celiac disease in patients with psoriasis.
As for nightshade vegetables, intake leads to increased alkaloids, “which have been known to worsen bowel inflammation such as in IBD [inflammatory bowel disease], but there is a lack of controlled trials examining this in the overall psoriasis population,” Dr. Han said. The Mediterranean diet, he added, “is sensible, and adding olive oil to your diet seems to have a positive effect on ... PASI, while fish oil seems to reduce C-reactive protein.” The data on the effect of vitamin D supplements are mixed, he said.
A separate randomized study evaluated the impact of weight loss in overweight or obese patients with psoriasis, who had not achieved clearance after 4 weeks of systemic treatment. Significantly more of those in the dietary intervention arm reached the weight loss goal of 5% at 20 weeks, and patients in this arm had a median reduction in the Psoriasis Area and Severity Index (PASI) score of almost 50%, compared with almost 26% among those without an active dietary intervention.
Joint pain, PsA
For psoriasis patients who complain of joint pain, he recommends administering quick measures like the five-question Psoriasis Epidemiology Screening Test (PEST) to screen for psoriatic arthritis (PsA), which is available on the National Psoriasis Foundation web site. “I ask patients about swollen, tender joints – specifically hands, wrists, ankles, feet, and toes,” Dr. Han said. Joint stiffness in the morning is a “concerning finding,” which is “more indicative of psoriatic arthritis than vague knee or back pain that worsens with use. If you have a younger patient with back pain who has a reduced ability to flex their spine, think axial disease.”
Tumor necrosis factor (TNF)–alpha inhibitors are considered first- and second-line treatment for PsA, but interleukin (IL)–17 inhibitors are generally considered just as effective overall. “The IL-23 inhibitors have mixed signals,” said Dr. Han, who is also on the NPF’s medical board. “We know that guselkumab is effective against psoriatic arthritis, but there is no inhibition of joint progression at the approved dosage on the label – though it was pretty close.”
Risankizumab (Skyrizi), an IL-23 inhibitor, was approved in January 2022 for adults with PsA and while the American College of Rheumatology response data “look reasonably good, the results for inhibition of radiographic progression are quite far off and it’s not in the label,” he said. Tildrakizumab (Ilumya), an IL-23 inhibitor, “looks impressive in phase 2b trials. It will be interesting to see if there is differentiation between the IL-23 agents in treating joint disease going forward.”
Dr. Han considers biologic therapy a good option for patients with questionable joint involvement or very limited joint disease. “If the patient has some evidence of PsA, as long as it’s a medication that has approval for that, I’m OK with starting it,” he said. “However, for patients whose joint pain dominates over the skin, or [who] have severe joint disease at presentation, I would prioritize the TNF-alpha inhibitors and IL-17s and refer them to rheumatology for shared management.”
Topical, oral treatments
As for topical approaches to treating psoriasis, adding halobetasol propionate 0.01% to tazarotene 0.045% may have a synergistic effect, while tapinarof 1% cream holds promise, he said. Tapinarof, which is expected to be approved this year, is an investigative aryl hydrocarbon agonist that inhibits an array of proinflammatory cytokines, including interferon-gamma and TNF-alpha. “It has been shown to have inhibitory effects both on Th17 cytokines and Th2 cytokines,” Dr. Han said. “What’s nice about this is that patients still appear to have treatment effect 1-2 months after stopping the drug.”
Another topical agent now under FDA review for psoriasis, is roflumilast, a phosphodiesterase type 4 (PDE4) inhibitor, which has been shown to have a treatment efficacy of 30% or more. “We’ll see how this works into our treatment regimen for psoriasis,” he said, as strategies targeting PDE4 have already been reported to help treat psoriasis.
With regards to oral therapies, he said that there are concerns about the efficacy of the oral PDE4 inhibitor apremilast, approved for psoriasis, compared with other biologics. Deucravacitinib, an oral selective tyrosine kinase 2 (TYK2) inhibitor also under FDA review for psoriasis, “may fill this gap, because its efficacy seems much stronger and really capitalizes on blocking IL-23, which we know is a central pathway in the pathogenesis of psoriasis.”
Phototherapy is another treatment option. Home narrowband-UVB devices cost $3,000-$5,000, “which is a fraction of 1 year of biologic treatment,” Dr. Han said. Older data on phototherapy suggest that “lesions can clear within 2-3 months, depending on how often you do the phototherapy, while newer data suggest that 75% of patients can achieve clear or minimal disease” with phototherapy.
Biologic therapy
If patients meet criteria for treatment with a biologic, he begins the conversation by saying, “I don’t want to give you an immunosuppressant, but your psoriasis represents an overactivation of inflammation in your body, so in some way we have to bring that down. Ideally, we would target your immune system in a way that targets psoriasis very narrowly, while leaving it to do what it needs to: protecting against infections and neoplasia.”
XXXIL-17 inhibitors generally have the fastest onset of action, Dr. Han noted. Authors of a review paper found that achievement of Psoriasis Area and Severity Index (PASI) 50 was 1.8 weeks with brodalumab, 1.9 weeks for ixekizumab, 3 weeks for high-dose secukinumab, 3.5 weeks for adalimumab, 3.7 weeks for infliximab, 5.1 weeks for low-dose ustekinumab, 6.5 weeks for high-dose etanercept, and 10.9 weeks with low-dose etanercept, while achievement of PASI 50 was closer to 1 month for IL-23 inhibitors.
“The conversation I have with patients on IL-23 inhibitors is, ‘we’re in this for the long haul,’ otherwise they come in 2 months later,” he said. “They may have gotten clearer but we’re talking about getting well over half of our patients to PASI 100, or to clear or minimal disease, and they may not have gotten there yet. It helps to frame expectations.”
Dr. Han disclosed that he is a consultant to, a speaker for, or has received research support from Beiersdorf, CeraVe, Celgene, Janssen, Lilly, MC2, Pfizer, UCB, Boehringer Ingelheim, Bond Avillion, Athenex, Amgen, AbbVie, Regeneron/Sanofi, LEO Pharma, Ortho Dermatologics, BMS, Sun Pharma, Dermavant, Dermtech, MedX, Novartis, and Castle Biosciences.
FROM ODAC 2022
Real-world data reinforce stem cell transplant for progressive systemic sclerosis
Current selection criteria for autologous hematopoietic stem cell transplant (AHSCT) in patients with rapidly progressing systemic sclerosis were validated in a study presented at the annual meeting of the Canadian Rheumatology Association.
The study, which associated AHSCT with improvement in overall survival and an acceptable risk of adverse events, “provides valuable real-world, long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with rapidly progressing systemic sclerosis,” reported Nancy Maltez, MD, a rheumatologist and clinical investigator who is on the faculty of the University of Ottawa.
The prospective study enrolled 85 patients in Canada and 41 patients in France with rapidly progressing systemic sclerosis. The patients in both countries were enrolled with the same eligibility criteria for AHSCT, but patients in France underwent AHSCT while the patients in Canada were treated with conventional therapies, such as cyclophosphamide.
On the primary outcome of overall survival, the Kaplan-Meier curve split almost immediately in favor of AHSCT. At 4 years, more than 25% of patients in the conventional therapy group had died versus less than 5% of those who underwent AHSCT. Although the mortality curve did slope downwards in the AHSCT group over the subsequent 6 years of follow-up, it largely paralleled and remained superior to convention therapy.
About 50% survival advantage seen for AHSCT
In this nonrandomized study, the statistical survival advantage of AHSCT was not provided, but the survival graph showed about 75% survival at 8 years of follow-up in the AHSCT group, compared with about 50% survival in the conventional-therapy group.
Many of the secondary outcomes, including those evaluating skin involvement, preservation of lung function, and absence of renal complications also favored AHSCT, according to Dr. Maltez.
On the modified Rodnan skin score, a significant difference (P < .001) observed at 12 months was sustained at 36 months, when the score was 4.48 points lower among patients treated with AHSCT. The difference in forced vital capacity (FVC) was about 10% higher (P < .0001) in the AHSCT group.
Over long-term follow-up, the incidence of scleroderma renal crisis per 100 person-years was 6.02 cases in the conventional therapy group versus 0.58 cases (P < .001) in the AHSCT group. There was no significant difference in the proportion of patients in the two groups receiving a pacemaker over the course of follow-up, but the rate of new malignancies per 100 person-years was 3.71 in the conventional care group versus 0.58 (P < .001) in the AHSCT group.
Significant complications attributed to AHSCT were uncommon. This is important, because AHSCT was not uniformly well tolerated in the initial trials. The first of three randomized trials with AHSCT in progressive systemic sclerosis was published more than 10 years ago after a series of promising early phase trials. Each associated AHSCT with benefit, but patient selection appeared to be important.
In the ASSIST trial of 2011, AHSCT was associated with significant reductions in skin involvement and improvements in pulmonary function relative to cyclophosphamide, but enrollment was stopped after only 19 patients, and follow-up extended to only 2 years.
Substantial AHSCT-related mortality in ASTIS
In the second trial, called ASTIS, AHSCT was associated with a higher rate of mortality than cyclophosphamide after 1 year of follow-up, although there was a significantly greater long-term event-free survival for AHSCT when patients were followed out to 4 years. This study reinforced the need for cardiac screening because of because of concern that severe cardiac compromise contributed to the increased risk of AHSCT-related mortality.
The SCOT trial employed a high-intensity myeloablative conditioning regimen and total body irradiation prior to AHSCT. It is not clear that these contributed to improved survival, particularly because of the risk for irradiation to exacerbate complications in the lung and kidney, but AHSCT-related mortality was only 3% at 54 months. Patient enrollment criteria in this trial were also suspected of having played a role in the favorable results.
In the Canadian-French collaborative study, patients were considered eligible for AHSCT if they met the enrollment criteria used in the ASTIS trial, according to Dr. Maltez. She attributed the low rates of early mortality and relative absence of transplant-related death to the lessons learned in the published trials.
Overall, the data support the routine but selective use of AHSCT in rapidly progressing systemic sclerosis, Dr. Maltez concluded.
Maria Carolina Oliveira, MD, of the department of internal medicine at the University of São Paulo, generally agreed. A coauthor of a recent review of AHSCT for systemic sclerosis, Dr. Oliveira emphasized that patient selection is critical.
“AHSCT for systemic sclerosis has very specific inclusion criteria. Indeed, it is indicated for patients with severe and progressive disease but under two specific conditions: severe and progressive diffuse skin involvement and/or progressive interstitial lung disease,” she said in an interview.
Because of the thin line between benefit and risk according to disease subtypes and comorbidities, she said that it is important to be aware of relative contraindications and to recognize the risks of AHSCT.
At this time, and in the absence of better biomarkers to identify those most likely to benefit, “patients with other forms of severe scleroderma, such as those with pulmonary hypertension, scleroderma renal crisis, or severe cardiac involvement, for example, are not eligible,” she said.
Dr. Maltez and Dr. Oliveira reported having no potential conflicts of interest.
Current selection criteria for autologous hematopoietic stem cell transplant (AHSCT) in patients with rapidly progressing systemic sclerosis were validated in a study presented at the annual meeting of the Canadian Rheumatology Association.
The study, which associated AHSCT with improvement in overall survival and an acceptable risk of adverse events, “provides valuable real-world, long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with rapidly progressing systemic sclerosis,” reported Nancy Maltez, MD, a rheumatologist and clinical investigator who is on the faculty of the University of Ottawa.
The prospective study enrolled 85 patients in Canada and 41 patients in France with rapidly progressing systemic sclerosis. The patients in both countries were enrolled with the same eligibility criteria for AHSCT, but patients in France underwent AHSCT while the patients in Canada were treated with conventional therapies, such as cyclophosphamide.
On the primary outcome of overall survival, the Kaplan-Meier curve split almost immediately in favor of AHSCT. At 4 years, more than 25% of patients in the conventional therapy group had died versus less than 5% of those who underwent AHSCT. Although the mortality curve did slope downwards in the AHSCT group over the subsequent 6 years of follow-up, it largely paralleled and remained superior to convention therapy.
About 50% survival advantage seen for AHSCT
In this nonrandomized study, the statistical survival advantage of AHSCT was not provided, but the survival graph showed about 75% survival at 8 years of follow-up in the AHSCT group, compared with about 50% survival in the conventional-therapy group.
Many of the secondary outcomes, including those evaluating skin involvement, preservation of lung function, and absence of renal complications also favored AHSCT, according to Dr. Maltez.
On the modified Rodnan skin score, a significant difference (P < .001) observed at 12 months was sustained at 36 months, when the score was 4.48 points lower among patients treated with AHSCT. The difference in forced vital capacity (FVC) was about 10% higher (P < .0001) in the AHSCT group.
Over long-term follow-up, the incidence of scleroderma renal crisis per 100 person-years was 6.02 cases in the conventional therapy group versus 0.58 cases (P < .001) in the AHSCT group. There was no significant difference in the proportion of patients in the two groups receiving a pacemaker over the course of follow-up, but the rate of new malignancies per 100 person-years was 3.71 in the conventional care group versus 0.58 (P < .001) in the AHSCT group.
Significant complications attributed to AHSCT were uncommon. This is important, because AHSCT was not uniformly well tolerated in the initial trials. The first of three randomized trials with AHSCT in progressive systemic sclerosis was published more than 10 years ago after a series of promising early phase trials. Each associated AHSCT with benefit, but patient selection appeared to be important.
In the ASSIST trial of 2011, AHSCT was associated with significant reductions in skin involvement and improvements in pulmonary function relative to cyclophosphamide, but enrollment was stopped after only 19 patients, and follow-up extended to only 2 years.
Substantial AHSCT-related mortality in ASTIS
In the second trial, called ASTIS, AHSCT was associated with a higher rate of mortality than cyclophosphamide after 1 year of follow-up, although there was a significantly greater long-term event-free survival for AHSCT when patients were followed out to 4 years. This study reinforced the need for cardiac screening because of because of concern that severe cardiac compromise contributed to the increased risk of AHSCT-related mortality.
The SCOT trial employed a high-intensity myeloablative conditioning regimen and total body irradiation prior to AHSCT. It is not clear that these contributed to improved survival, particularly because of the risk for irradiation to exacerbate complications in the lung and kidney, but AHSCT-related mortality was only 3% at 54 months. Patient enrollment criteria in this trial were also suspected of having played a role in the favorable results.
In the Canadian-French collaborative study, patients were considered eligible for AHSCT if they met the enrollment criteria used in the ASTIS trial, according to Dr. Maltez. She attributed the low rates of early mortality and relative absence of transplant-related death to the lessons learned in the published trials.
Overall, the data support the routine but selective use of AHSCT in rapidly progressing systemic sclerosis, Dr. Maltez concluded.
Maria Carolina Oliveira, MD, of the department of internal medicine at the University of São Paulo, generally agreed. A coauthor of a recent review of AHSCT for systemic sclerosis, Dr. Oliveira emphasized that patient selection is critical.
“AHSCT for systemic sclerosis has very specific inclusion criteria. Indeed, it is indicated for patients with severe and progressive disease but under two specific conditions: severe and progressive diffuse skin involvement and/or progressive interstitial lung disease,” she said in an interview.
Because of the thin line between benefit and risk according to disease subtypes and comorbidities, she said that it is important to be aware of relative contraindications and to recognize the risks of AHSCT.
At this time, and in the absence of better biomarkers to identify those most likely to benefit, “patients with other forms of severe scleroderma, such as those with pulmonary hypertension, scleroderma renal crisis, or severe cardiac involvement, for example, are not eligible,” she said.
Dr. Maltez and Dr. Oliveira reported having no potential conflicts of interest.
Current selection criteria for autologous hematopoietic stem cell transplant (AHSCT) in patients with rapidly progressing systemic sclerosis were validated in a study presented at the annual meeting of the Canadian Rheumatology Association.
The study, which associated AHSCT with improvement in overall survival and an acceptable risk of adverse events, “provides valuable real-world, long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with rapidly progressing systemic sclerosis,” reported Nancy Maltez, MD, a rheumatologist and clinical investigator who is on the faculty of the University of Ottawa.
The prospective study enrolled 85 patients in Canada and 41 patients in France with rapidly progressing systemic sclerosis. The patients in both countries were enrolled with the same eligibility criteria for AHSCT, but patients in France underwent AHSCT while the patients in Canada were treated with conventional therapies, such as cyclophosphamide.
On the primary outcome of overall survival, the Kaplan-Meier curve split almost immediately in favor of AHSCT. At 4 years, more than 25% of patients in the conventional therapy group had died versus less than 5% of those who underwent AHSCT. Although the mortality curve did slope downwards in the AHSCT group over the subsequent 6 years of follow-up, it largely paralleled and remained superior to convention therapy.
About 50% survival advantage seen for AHSCT
In this nonrandomized study, the statistical survival advantage of AHSCT was not provided, but the survival graph showed about 75% survival at 8 years of follow-up in the AHSCT group, compared with about 50% survival in the conventional-therapy group.
Many of the secondary outcomes, including those evaluating skin involvement, preservation of lung function, and absence of renal complications also favored AHSCT, according to Dr. Maltez.
On the modified Rodnan skin score, a significant difference (P < .001) observed at 12 months was sustained at 36 months, when the score was 4.48 points lower among patients treated with AHSCT. The difference in forced vital capacity (FVC) was about 10% higher (P < .0001) in the AHSCT group.
Over long-term follow-up, the incidence of scleroderma renal crisis per 100 person-years was 6.02 cases in the conventional therapy group versus 0.58 cases (P < .001) in the AHSCT group. There was no significant difference in the proportion of patients in the two groups receiving a pacemaker over the course of follow-up, but the rate of new malignancies per 100 person-years was 3.71 in the conventional care group versus 0.58 (P < .001) in the AHSCT group.
Significant complications attributed to AHSCT were uncommon. This is important, because AHSCT was not uniformly well tolerated in the initial trials. The first of three randomized trials with AHSCT in progressive systemic sclerosis was published more than 10 years ago after a series of promising early phase trials. Each associated AHSCT with benefit, but patient selection appeared to be important.
In the ASSIST trial of 2011, AHSCT was associated with significant reductions in skin involvement and improvements in pulmonary function relative to cyclophosphamide, but enrollment was stopped after only 19 patients, and follow-up extended to only 2 years.
Substantial AHSCT-related mortality in ASTIS
In the second trial, called ASTIS, AHSCT was associated with a higher rate of mortality than cyclophosphamide after 1 year of follow-up, although there was a significantly greater long-term event-free survival for AHSCT when patients were followed out to 4 years. This study reinforced the need for cardiac screening because of because of concern that severe cardiac compromise contributed to the increased risk of AHSCT-related mortality.
The SCOT trial employed a high-intensity myeloablative conditioning regimen and total body irradiation prior to AHSCT. It is not clear that these contributed to improved survival, particularly because of the risk for irradiation to exacerbate complications in the lung and kidney, but AHSCT-related mortality was only 3% at 54 months. Patient enrollment criteria in this trial were also suspected of having played a role in the favorable results.
In the Canadian-French collaborative study, patients were considered eligible for AHSCT if they met the enrollment criteria used in the ASTIS trial, according to Dr. Maltez. She attributed the low rates of early mortality and relative absence of transplant-related death to the lessons learned in the published trials.
Overall, the data support the routine but selective use of AHSCT in rapidly progressing systemic sclerosis, Dr. Maltez concluded.
Maria Carolina Oliveira, MD, of the department of internal medicine at the University of São Paulo, generally agreed. A coauthor of a recent review of AHSCT for systemic sclerosis, Dr. Oliveira emphasized that patient selection is critical.
“AHSCT for systemic sclerosis has very specific inclusion criteria. Indeed, it is indicated for patients with severe and progressive disease but under two specific conditions: severe and progressive diffuse skin involvement and/or progressive interstitial lung disease,” she said in an interview.
Because of the thin line between benefit and risk according to disease subtypes and comorbidities, she said that it is important to be aware of relative contraindications and to recognize the risks of AHSCT.
At this time, and in the absence of better biomarkers to identify those most likely to benefit, “patients with other forms of severe scleroderma, such as those with pulmonary hypertension, scleroderma renal crisis, or severe cardiac involvement, for example, are not eligible,” she said.
Dr. Maltez and Dr. Oliveira reported having no potential conflicts of interest.
FROM THE ANNUAL MEETING OF THE CANADIAN RHEUMATOLOGY ASSOCIATION
How to advance equity, diversity, and inclusion in dermatology: Recommendations from an expert panel
– so he launched community outreach efforts with local businesses to attract patients from diverse backgrounds.
“For instance, I worked with U.S. Bank to give lectures on minorities in medicine and talked about outreach options and possible ways to include more ethnicities in medicine overall,” Dr. Qutub said during a panel discussion on equity, diversity, and inclusion (EDI) that took place at the ODAC Dermatology, Aesthetic & Surgical Conference. “I also did outreach with medical clinics in the area. Once patients are referred to you, they start to talk to people in their communities about you, and before you know it, you get people from their church and family members in your clinic.”
Dr. Qutub, who is ODAC’s director of Equity, Diversity, and Inclusion, kept EDI in mind when hiring staff for his practice, “to include candidates with varying experiences and backgrounds,” he said. “The idea was to make sure that when patients came into the clinic, they saw a varied group of individuals that were working together to help improve their health care outcomes. I found that made patients more comfortable in the clinic. It’s also important to have that representation daily in a larger setting like residency programs or multispecialty groups.”
Educational resources
Another panelist, Adam Friedman, MD, emphasized inclusivity of educational resources to ensure a dermatology workforce that can take care of all patients. “How can we expect the dermatology community to be able to treat anyone who comes through the door of their clinic if we don’t provide the resources that highlight and showcase the nuances and the diversity that skin disease has to offer?” asked Dr. Friedman, professor and chair of dermatology at George Washington University, Washington. “It comes down to educational tools and being purposeful when you’re putting together a talk or writing a paper, to be inclusive and have that on the top of your mind. It’s about saying right here, right now, we have to purposefully make a decision to be inclusive, to be welcoming to all so that we can practice at the highest level of our calling to treat everyone effectively and equitably.”
A unique feature of the atlas “is that we have taken multiple skin conditions, even common features such as erythema, and placed different skin tones side by side at the same angle to appreciate the full spectrum, and highlight those nuances,” Dr. Friedman said. “When you’re in clinic, when you see even common things like acne or seborrheic dermatitis,” he recommended taking photos to create a repository, “because you never know when that will be helpful when you want to show a medical student or a patient what something can look like on someone with a similar skin tone, or even to share with them how diverse skin conditions can appear across populations.”
Clinical research
Another way to help close racial gaps in dermatology is to improve access to mentorships and clinical research, according to panelist Chesahna Kindred, MD, of Kindred Hair & Skin Center in Columbia, Md. “We should be thoroughly embarrassed by the lack of diversity in our clinical trials,” she said.
In her role as chair of the dermatology section of the National Medical Association (NMA Derm), Dr. Kindred helped launch the NMA Derm research committee, which trains members to run clinical trials in their practices – an undertaking that was largely prompted by claims from pharmaceutical industry representatives that they struggle to find Black participants for clinical trials. “The truth of the matter is, if a Black patient doesn’t choose to go to Dr. Smith as a patient, they’re certainly not going to choose to go to Dr. Smith as a research participant,” Dr. Kindred said. “We have to meet those diverse populations where they are. By and large for Black patients, those are Black dermatologists.
In addition to meeting with primary investigators, she has been meeting with industry representatives, who she said are very interested in improving clinical trial diversity. “When a trial does not include a diverse population, we can call it out and say it is subpar,” she said.
In 2020, the Food and Drug Administration announced the availability of a guidance document, “Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs,” which includes recommendations for sponsors on how to increase enrollment of underrepresented populations in their clinical trials of medical products.
Dr. Kindred has created a clinical research unit in her own practice, in partnership with Howard University’s department of dermatology and NMA Dermatology.
Studies Dr. Kindred is involved with include those looking at the relationship between hair care products targeted to Black women and the development of central centrifugal cicatricial alopecia (CCCA). CCCA is getting worse with each generation, “and we think the cause might be environmental,” she said. “Studies show that there are almost zero percent carcinogens in hair care products that target Whites. But close to 100% of hair care products that target Blacks contain carcinogens and endocrine disrupting chemicals, the most common being phthalates, which are found in relaxers, chemicals that patients use to straighten their hair.”
Urinary phthalate concentrations have been found to be much higher in Black women than in White women, and one of the pilot studies she is involved with is checking the urinary phthalate levels in Black women with and without CCCA, to see if there is a correlation.
Mentorships
DiAnne S. Davis, MD, of North Dallas Dermatology Associates, rounded out the panel discussion by underscoring the importance of mentorships for underrepresented minority medical students, which includes providing guidance through the application process. “Mentorship is key to closing some of these gaps, particularly in our field of dermatology,” Dr. Davis said.
Through NMA Derm, Dr. Davis was tasked by one of her mentors, Dr. Kindred, to spearhead a mentorship program that pairs medical students with a mentor in the dermatology field, “so we can help guide them not only on their medical school process but help in coordinating research projects, and make them successful in matching to dermatology,” she said. “When students reach out to you, it’s important to take them under your wing or connect them to somebody you know so that we can increase the number of minority dermatologists.”
None of the panelists reported having disclosures relevant to their presentations.
– so he launched community outreach efforts with local businesses to attract patients from diverse backgrounds.
“For instance, I worked with U.S. Bank to give lectures on minorities in medicine and talked about outreach options and possible ways to include more ethnicities in medicine overall,” Dr. Qutub said during a panel discussion on equity, diversity, and inclusion (EDI) that took place at the ODAC Dermatology, Aesthetic & Surgical Conference. “I also did outreach with medical clinics in the area. Once patients are referred to you, they start to talk to people in their communities about you, and before you know it, you get people from their church and family members in your clinic.”
Dr. Qutub, who is ODAC’s director of Equity, Diversity, and Inclusion, kept EDI in mind when hiring staff for his practice, “to include candidates with varying experiences and backgrounds,” he said. “The idea was to make sure that when patients came into the clinic, they saw a varied group of individuals that were working together to help improve their health care outcomes. I found that made patients more comfortable in the clinic. It’s also important to have that representation daily in a larger setting like residency programs or multispecialty groups.”
Educational resources
Another panelist, Adam Friedman, MD, emphasized inclusivity of educational resources to ensure a dermatology workforce that can take care of all patients. “How can we expect the dermatology community to be able to treat anyone who comes through the door of their clinic if we don’t provide the resources that highlight and showcase the nuances and the diversity that skin disease has to offer?” asked Dr. Friedman, professor and chair of dermatology at George Washington University, Washington. “It comes down to educational tools and being purposeful when you’re putting together a talk or writing a paper, to be inclusive and have that on the top of your mind. It’s about saying right here, right now, we have to purposefully make a decision to be inclusive, to be welcoming to all so that we can practice at the highest level of our calling to treat everyone effectively and equitably.”
A unique feature of the atlas “is that we have taken multiple skin conditions, even common features such as erythema, and placed different skin tones side by side at the same angle to appreciate the full spectrum, and highlight those nuances,” Dr. Friedman said. “When you’re in clinic, when you see even common things like acne or seborrheic dermatitis,” he recommended taking photos to create a repository, “because you never know when that will be helpful when you want to show a medical student or a patient what something can look like on someone with a similar skin tone, or even to share with them how diverse skin conditions can appear across populations.”
Clinical research
Another way to help close racial gaps in dermatology is to improve access to mentorships and clinical research, according to panelist Chesahna Kindred, MD, of Kindred Hair & Skin Center in Columbia, Md. “We should be thoroughly embarrassed by the lack of diversity in our clinical trials,” she said.
In her role as chair of the dermatology section of the National Medical Association (NMA Derm), Dr. Kindred helped launch the NMA Derm research committee, which trains members to run clinical trials in their practices – an undertaking that was largely prompted by claims from pharmaceutical industry representatives that they struggle to find Black participants for clinical trials. “The truth of the matter is, if a Black patient doesn’t choose to go to Dr. Smith as a patient, they’re certainly not going to choose to go to Dr. Smith as a research participant,” Dr. Kindred said. “We have to meet those diverse populations where they are. By and large for Black patients, those are Black dermatologists.
In addition to meeting with primary investigators, she has been meeting with industry representatives, who she said are very interested in improving clinical trial diversity. “When a trial does not include a diverse population, we can call it out and say it is subpar,” she said.
In 2020, the Food and Drug Administration announced the availability of a guidance document, “Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs,” which includes recommendations for sponsors on how to increase enrollment of underrepresented populations in their clinical trials of medical products.
Dr. Kindred has created a clinical research unit in her own practice, in partnership with Howard University’s department of dermatology and NMA Dermatology.
Studies Dr. Kindred is involved with include those looking at the relationship between hair care products targeted to Black women and the development of central centrifugal cicatricial alopecia (CCCA). CCCA is getting worse with each generation, “and we think the cause might be environmental,” she said. “Studies show that there are almost zero percent carcinogens in hair care products that target Whites. But close to 100% of hair care products that target Blacks contain carcinogens and endocrine disrupting chemicals, the most common being phthalates, which are found in relaxers, chemicals that patients use to straighten their hair.”
Urinary phthalate concentrations have been found to be much higher in Black women than in White women, and one of the pilot studies she is involved with is checking the urinary phthalate levels in Black women with and without CCCA, to see if there is a correlation.
Mentorships
DiAnne S. Davis, MD, of North Dallas Dermatology Associates, rounded out the panel discussion by underscoring the importance of mentorships for underrepresented minority medical students, which includes providing guidance through the application process. “Mentorship is key to closing some of these gaps, particularly in our field of dermatology,” Dr. Davis said.
Through NMA Derm, Dr. Davis was tasked by one of her mentors, Dr. Kindred, to spearhead a mentorship program that pairs medical students with a mentor in the dermatology field, “so we can help guide them not only on their medical school process but help in coordinating research projects, and make them successful in matching to dermatology,” she said. “When students reach out to you, it’s important to take them under your wing or connect them to somebody you know so that we can increase the number of minority dermatologists.”
None of the panelists reported having disclosures relevant to their presentations.
– so he launched community outreach efforts with local businesses to attract patients from diverse backgrounds.
“For instance, I worked with U.S. Bank to give lectures on minorities in medicine and talked about outreach options and possible ways to include more ethnicities in medicine overall,” Dr. Qutub said during a panel discussion on equity, diversity, and inclusion (EDI) that took place at the ODAC Dermatology, Aesthetic & Surgical Conference. “I also did outreach with medical clinics in the area. Once patients are referred to you, they start to talk to people in their communities about you, and before you know it, you get people from their church and family members in your clinic.”
Dr. Qutub, who is ODAC’s director of Equity, Diversity, and Inclusion, kept EDI in mind when hiring staff for his practice, “to include candidates with varying experiences and backgrounds,” he said. “The idea was to make sure that when patients came into the clinic, they saw a varied group of individuals that were working together to help improve their health care outcomes. I found that made patients more comfortable in the clinic. It’s also important to have that representation daily in a larger setting like residency programs or multispecialty groups.”
Educational resources
Another panelist, Adam Friedman, MD, emphasized inclusivity of educational resources to ensure a dermatology workforce that can take care of all patients. “How can we expect the dermatology community to be able to treat anyone who comes through the door of their clinic if we don’t provide the resources that highlight and showcase the nuances and the diversity that skin disease has to offer?” asked Dr. Friedman, professor and chair of dermatology at George Washington University, Washington. “It comes down to educational tools and being purposeful when you’re putting together a talk or writing a paper, to be inclusive and have that on the top of your mind. It’s about saying right here, right now, we have to purposefully make a decision to be inclusive, to be welcoming to all so that we can practice at the highest level of our calling to treat everyone effectively and equitably.”
A unique feature of the atlas “is that we have taken multiple skin conditions, even common features such as erythema, and placed different skin tones side by side at the same angle to appreciate the full spectrum, and highlight those nuances,” Dr. Friedman said. “When you’re in clinic, when you see even common things like acne or seborrheic dermatitis,” he recommended taking photos to create a repository, “because you never know when that will be helpful when you want to show a medical student or a patient what something can look like on someone with a similar skin tone, or even to share with them how diverse skin conditions can appear across populations.”
Clinical research
Another way to help close racial gaps in dermatology is to improve access to mentorships and clinical research, according to panelist Chesahna Kindred, MD, of Kindred Hair & Skin Center in Columbia, Md. “We should be thoroughly embarrassed by the lack of diversity in our clinical trials,” she said.
In her role as chair of the dermatology section of the National Medical Association (NMA Derm), Dr. Kindred helped launch the NMA Derm research committee, which trains members to run clinical trials in their practices – an undertaking that was largely prompted by claims from pharmaceutical industry representatives that they struggle to find Black participants for clinical trials. “The truth of the matter is, if a Black patient doesn’t choose to go to Dr. Smith as a patient, they’re certainly not going to choose to go to Dr. Smith as a research participant,” Dr. Kindred said. “We have to meet those diverse populations where they are. By and large for Black patients, those are Black dermatologists.
In addition to meeting with primary investigators, she has been meeting with industry representatives, who she said are very interested in improving clinical trial diversity. “When a trial does not include a diverse population, we can call it out and say it is subpar,” she said.
In 2020, the Food and Drug Administration announced the availability of a guidance document, “Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs,” which includes recommendations for sponsors on how to increase enrollment of underrepresented populations in their clinical trials of medical products.
Dr. Kindred has created a clinical research unit in her own practice, in partnership with Howard University’s department of dermatology and NMA Dermatology.
Studies Dr. Kindred is involved with include those looking at the relationship between hair care products targeted to Black women and the development of central centrifugal cicatricial alopecia (CCCA). CCCA is getting worse with each generation, “and we think the cause might be environmental,” she said. “Studies show that there are almost zero percent carcinogens in hair care products that target Whites. But close to 100% of hair care products that target Blacks contain carcinogens and endocrine disrupting chemicals, the most common being phthalates, which are found in relaxers, chemicals that patients use to straighten their hair.”
Urinary phthalate concentrations have been found to be much higher in Black women than in White women, and one of the pilot studies she is involved with is checking the urinary phthalate levels in Black women with and without CCCA, to see if there is a correlation.
Mentorships
DiAnne S. Davis, MD, of North Dallas Dermatology Associates, rounded out the panel discussion by underscoring the importance of mentorships for underrepresented minority medical students, which includes providing guidance through the application process. “Mentorship is key to closing some of these gaps, particularly in our field of dermatology,” Dr. Davis said.
Through NMA Derm, Dr. Davis was tasked by one of her mentors, Dr. Kindred, to spearhead a mentorship program that pairs medical students with a mentor in the dermatology field, “so we can help guide them not only on their medical school process but help in coordinating research projects, and make them successful in matching to dermatology,” she said. “When students reach out to you, it’s important to take them under your wing or connect them to somebody you know so that we can increase the number of minority dermatologists.”
None of the panelists reported having disclosures relevant to their presentations.
FROM ODAC 2022
Q&A: Long COVID symptoms, management, and where we’re headed
Long COVID continues to be a moving target – continuously evolving and still surprising doctors and patients who have sometimes incapacitating long-term symptoms.
Little about the disorder seems predictable at this point. People can have long COVID after asymptomatic, mild, or severe COVID-19, for example. And when a person gets long COVID – also known as long-haul COVID – symptoms can vary widely.
To address all the uncertainty, the New York State Department of Health gathered experts in primary care, pediatrics, physical medicine, rehabilitation, and pulmonology to answer some pressing questions.
New York in 2020 was the first epicenter of the pandemic in the United States, making it also the center of the long COVID epidemic, says Emily Lutterloh, MD, director of the Division of Epidemiology at the New York State Department of Health.
What do you do when you’re seeing a patient with long COVID for the first time?
The first exam varies because there are so many different ways long COVID presents itself, says Benjamin Abramoff, MD, a physical medicine and rehabilitation specialist at Penn Medicine in Philadelphia.
I’ve now been seriously ill with #LongCovid for 11 months. I was never hospitalized. I didn’t even have a “mild” covid case. Instead, I developed Long Covid from an asymptomatic infection.
I’m far from unique. Up to 1/5 of asymptomatic patients go on to have long-term symptoms.
— Ravi Veriah Jacques (@RaviHVJ) February 3, 2022
Assessing their previous and current care also helps to direct their ongoing management, says Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai Health System in New York.
Can vaccination help people with long COVID?
Anything that we can do to help prevent people from being critically ill or being hospitalized with COVID-19 is helpful to prevent long COVID, says Dr. Abramoff, who is also director of the long COVID clinic at the University of Pennsylvania, Philadelphia.
“So that’s something I always discuss with patients. In some research, sometimes patients do feel better after the vaccine,” he says.
What kind of therapies do you find helpful for your patients?
Rehabilitation is a key part of recovery from long COVID, Dr. Abramoff says. “It is very important to make this very patient-specific.”
“We have patients that are working. They’re already going to the gym in some cases but don’t feel like they have the same endurance,” he says. “And then we have patients who are so crippled by their fatigue that they can’t get out of bed.”
1/ What is #LongCOVID?!
A disabling malady from ongoing inflammation, autoimmunity, & potential viral reservoirs (GI, brain?)
NEW DATA: The Lungs “light up” on special MRI Scans 3 to 9 months later in patients never hospitalized for COVID.https://t.co/I2kyZ4cK5F pic.twitter.com/dL1P67L2DK
— WesElyMD (@WesElyMD) February 2, 2022
An exercise program can help people who have long COVID.
“There’s a big role for therapy services in the recovery of these patients,” says John Baratta, MD, of the department of physical medicine and rehabilitation at the University of North Carolina at Chapel Hill.
But the limited number of long COVID clinics can mean some people are unable to get to therapists trained on the needs of patients with lingering COVID symptoms. Educating community physical and occupational therapists is one solution.
How long does it take for people with long COVID to recover and get back to 100% if they can?
Specific numbers aren’t really available, Dr. Baratta says.
“But I can tell you the general trend that I see is that a lot of patients have a gradual improvement of symptoms. The slow but steady improvement with time may be the body’s natural healing process, a result of medical interventions, or both.”
It can help to reassure people with long COVID that they will not be discharged from care until they feel they’ve maximized their health, says Sharagim Kemp, DO, medical director of the COVID Recovery Program for Nuvance Health, a health system in New York and Connecticut.
It’s essential to set realistic recovery expectations and tell patients that not everyone will return to 100% of their pre-COVID functioning, she says.
“Once we are able to help them reset their expectations, there’s almost an accelerated recovery because they are not putting that pressure on themselves anymore,” Dr. Kemp says.
What are the most common symptoms you’re seeing in long COVID?
It’s helpful to think of long COVID as a very broad umbrella term, Dr. Abramoff says.
Echoing what many others have observed, fatigue, cognitive dysfunction or “brain fog,“ and shortness of breath or troubled breathing appear to be the most common symptoms, he says.
Some reported vague symptoms, Dr. Kemp says.
People may go to the doctor “not even realizing that they had COVID. That’s one of the important points here – to have a high index of suspicion for patients who come in with multiple symptoms,” she says.
For this reason, patients can report symptoms that don’t necessarily fit into any specialty, says Sarah J. Ryan, MD, an internal medicine doctor at Columbia University Irving Medical Center in New York. People say they are “just not themselves” or they are tired after their COVID-19 recovery.
Is there a connection between severe COVID cases and severe long COVID?
“It’s not like that at all. I would say that more than 80% of the patients that we see had mild to moderate illness and they were not hospitalized,” Dr. Baratta says.
Long COVID is a bit different in children and teenagers, says Ixsy Ramirez, MD, a pediatric pulmonologist at University of Michigan Health, Ann Arbor. Most patients in the long COVID clinic at the University of Michigan were previously healthy, and not children with asthma or other lung conditions as one might expect. In fact, many are student athletes, or were before they had long COVID.
In this population, shortness of breath is most common, followed by chest pain and fatigue. Unfortunately, the symptoms are so serious for many kids that their performance is limited, even if they can return to competitive play.
Are there defined criteria you use to diagnose long COVID? How do you give someone a diagnosis?
That’s an ever-evolving question, Dr. Kemp says. The generally accepted definition centers on persistent or new symptoms 4 weeks or more after the original COVID-19 illness, but there are exceptions.
Researchers are working on lab tests to help confirm the diagnosis. But without a definitive blood biomarker, getting to the diagnosis requires “some thorough detective work,” Dr. Ryan says.
Do you bring in mental health providers to help with treatment?
“We focus on mental health quite a bit actually,” says, Dr. Chen, cofounder of his institution’s COVID recovery clinic. Mount Sinai offers one-on-one and group mental health services, for example.
“Personally, I’ve seen patients that I did not expect to have such severe mental health changes” with long COVID.
One of the most powerful accounts and testimonies I have seen on what most #LongCovid patients experience when interacting with their doctors.
“I did not fit in a box, so they chose not to see me, even worse they made me feel like it was my fault for not fitting in their box” pic.twitter.com/7GQLBucuO5
— charlos (@loscharlos) February 3, 2022
Examples include severe depression, cases of acute psychosis, hallucinations, and other problems “that are really unexpected after a viral illness.”
Stony Brook University Hospital in New York has a long COVID clinic staffed by multiple primary care doctors who do exams and refer patients to services. A bonus of offering psychological services to all post-COVID patients is doctors get a more complete picture of each person and a better understanding of what they are going through, says Abigail Chua, MD, a pulmonologist at Stony Brook.
Some empathy is essential, Dr. Baratta says. “It’s important to recognize that a lot of these patients present with a sense of grief or loss for their prior life.”
What does the future hold?
A simple test to diagnose long COVID, combined with an effective treatment that helps people feel better within a week, would be ideal, Dr. Abramoff says.
“That would be lovely. But you know, we’re just not at that point.”
And it would be helpful to start identifying subtypes of long COVID so diagnosis and treatment can be more targeted, Dr. Abramoff says. Otherwise, “It’s going to be a very challenging approach to try to treat all of our patients with long COVID symptoms the same way.”
Good clinical trials likewise are needed to address all the subtleties of long COVID.
A number of long COVID centers are collaborating on research to find out more, Dr. Chen says. Actions include setting up a bank of tissue samples from people with long COVID so researchers can continue to figure out the condition.
One goal, Dr. Chen says, would be the ability to treat long COVID rather than just its symptoms.
Long COVID emphasizes the need to prevent people from getting COVID in the first place, Dr. Ramirez says. This will continue to be important, particularly when some people dismiss the seriousness of COVID, comparing it to a cold if they get it. That attitude discounts the large number of people who unfortunately go on to develop long-term, often debilitating, symptoms.
A version of this article first appeared on WebMD.com.
Long COVID continues to be a moving target – continuously evolving and still surprising doctors and patients who have sometimes incapacitating long-term symptoms.
Little about the disorder seems predictable at this point. People can have long COVID after asymptomatic, mild, or severe COVID-19, for example. And when a person gets long COVID – also known as long-haul COVID – symptoms can vary widely.
To address all the uncertainty, the New York State Department of Health gathered experts in primary care, pediatrics, physical medicine, rehabilitation, and pulmonology to answer some pressing questions.
New York in 2020 was the first epicenter of the pandemic in the United States, making it also the center of the long COVID epidemic, says Emily Lutterloh, MD, director of the Division of Epidemiology at the New York State Department of Health.
What do you do when you’re seeing a patient with long COVID for the first time?
The first exam varies because there are so many different ways long COVID presents itself, says Benjamin Abramoff, MD, a physical medicine and rehabilitation specialist at Penn Medicine in Philadelphia.
I’ve now been seriously ill with #LongCovid for 11 months. I was never hospitalized. I didn’t even have a “mild” covid case. Instead, I developed Long Covid from an asymptomatic infection.
I’m far from unique. Up to 1/5 of asymptomatic patients go on to have long-term symptoms.
— Ravi Veriah Jacques (@RaviHVJ) February 3, 2022
Assessing their previous and current care also helps to direct their ongoing management, says Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai Health System in New York.
Can vaccination help people with long COVID?
Anything that we can do to help prevent people from being critically ill or being hospitalized with COVID-19 is helpful to prevent long COVID, says Dr. Abramoff, who is also director of the long COVID clinic at the University of Pennsylvania, Philadelphia.
“So that’s something I always discuss with patients. In some research, sometimes patients do feel better after the vaccine,” he says.
What kind of therapies do you find helpful for your patients?
Rehabilitation is a key part of recovery from long COVID, Dr. Abramoff says. “It is very important to make this very patient-specific.”
“We have patients that are working. They’re already going to the gym in some cases but don’t feel like they have the same endurance,” he says. “And then we have patients who are so crippled by their fatigue that they can’t get out of bed.”
1/ What is #LongCOVID?!
A disabling malady from ongoing inflammation, autoimmunity, & potential viral reservoirs (GI, brain?)
NEW DATA: The Lungs “light up” on special MRI Scans 3 to 9 months later in patients never hospitalized for COVID.https://t.co/I2kyZ4cK5F pic.twitter.com/dL1P67L2DK
— WesElyMD (@WesElyMD) February 2, 2022
An exercise program can help people who have long COVID.
“There’s a big role for therapy services in the recovery of these patients,” says John Baratta, MD, of the department of physical medicine and rehabilitation at the University of North Carolina at Chapel Hill.
But the limited number of long COVID clinics can mean some people are unable to get to therapists trained on the needs of patients with lingering COVID symptoms. Educating community physical and occupational therapists is one solution.
How long does it take for people with long COVID to recover and get back to 100% if they can?
Specific numbers aren’t really available, Dr. Baratta says.
“But I can tell you the general trend that I see is that a lot of patients have a gradual improvement of symptoms. The slow but steady improvement with time may be the body’s natural healing process, a result of medical interventions, or both.”
It can help to reassure people with long COVID that they will not be discharged from care until they feel they’ve maximized their health, says Sharagim Kemp, DO, medical director of the COVID Recovery Program for Nuvance Health, a health system in New York and Connecticut.
It’s essential to set realistic recovery expectations and tell patients that not everyone will return to 100% of their pre-COVID functioning, she says.
“Once we are able to help them reset their expectations, there’s almost an accelerated recovery because they are not putting that pressure on themselves anymore,” Dr. Kemp says.
What are the most common symptoms you’re seeing in long COVID?
It’s helpful to think of long COVID as a very broad umbrella term, Dr. Abramoff says.
Echoing what many others have observed, fatigue, cognitive dysfunction or “brain fog,“ and shortness of breath or troubled breathing appear to be the most common symptoms, he says.
Some reported vague symptoms, Dr. Kemp says.
People may go to the doctor “not even realizing that they had COVID. That’s one of the important points here – to have a high index of suspicion for patients who come in with multiple symptoms,” she says.
For this reason, patients can report symptoms that don’t necessarily fit into any specialty, says Sarah J. Ryan, MD, an internal medicine doctor at Columbia University Irving Medical Center in New York. People say they are “just not themselves” or they are tired after their COVID-19 recovery.
Is there a connection between severe COVID cases and severe long COVID?
“It’s not like that at all. I would say that more than 80% of the patients that we see had mild to moderate illness and they were not hospitalized,” Dr. Baratta says.
Long COVID is a bit different in children and teenagers, says Ixsy Ramirez, MD, a pediatric pulmonologist at University of Michigan Health, Ann Arbor. Most patients in the long COVID clinic at the University of Michigan were previously healthy, and not children with asthma or other lung conditions as one might expect. In fact, many are student athletes, or were before they had long COVID.
In this population, shortness of breath is most common, followed by chest pain and fatigue. Unfortunately, the symptoms are so serious for many kids that their performance is limited, even if they can return to competitive play.
Are there defined criteria you use to diagnose long COVID? How do you give someone a diagnosis?
That’s an ever-evolving question, Dr. Kemp says. The generally accepted definition centers on persistent or new symptoms 4 weeks or more after the original COVID-19 illness, but there are exceptions.
Researchers are working on lab tests to help confirm the diagnosis. But without a definitive blood biomarker, getting to the diagnosis requires “some thorough detective work,” Dr. Ryan says.
Do you bring in mental health providers to help with treatment?
“We focus on mental health quite a bit actually,” says, Dr. Chen, cofounder of his institution’s COVID recovery clinic. Mount Sinai offers one-on-one and group mental health services, for example.
“Personally, I’ve seen patients that I did not expect to have such severe mental health changes” with long COVID.
One of the most powerful accounts and testimonies I have seen on what most #LongCovid patients experience when interacting with their doctors.
“I did not fit in a box, so they chose not to see me, even worse they made me feel like it was my fault for not fitting in their box” pic.twitter.com/7GQLBucuO5
— charlos (@loscharlos) February 3, 2022
Examples include severe depression, cases of acute psychosis, hallucinations, and other problems “that are really unexpected after a viral illness.”
Stony Brook University Hospital in New York has a long COVID clinic staffed by multiple primary care doctors who do exams and refer patients to services. A bonus of offering psychological services to all post-COVID patients is doctors get a more complete picture of each person and a better understanding of what they are going through, says Abigail Chua, MD, a pulmonologist at Stony Brook.
Some empathy is essential, Dr. Baratta says. “It’s important to recognize that a lot of these patients present with a sense of grief or loss for their prior life.”
What does the future hold?
A simple test to diagnose long COVID, combined with an effective treatment that helps people feel better within a week, would be ideal, Dr. Abramoff says.
“That would be lovely. But you know, we’re just not at that point.”
And it would be helpful to start identifying subtypes of long COVID so diagnosis and treatment can be more targeted, Dr. Abramoff says. Otherwise, “It’s going to be a very challenging approach to try to treat all of our patients with long COVID symptoms the same way.”
Good clinical trials likewise are needed to address all the subtleties of long COVID.
A number of long COVID centers are collaborating on research to find out more, Dr. Chen says. Actions include setting up a bank of tissue samples from people with long COVID so researchers can continue to figure out the condition.
One goal, Dr. Chen says, would be the ability to treat long COVID rather than just its symptoms.
Long COVID emphasizes the need to prevent people from getting COVID in the first place, Dr. Ramirez says. This will continue to be important, particularly when some people dismiss the seriousness of COVID, comparing it to a cold if they get it. That attitude discounts the large number of people who unfortunately go on to develop long-term, often debilitating, symptoms.
A version of this article first appeared on WebMD.com.
Long COVID continues to be a moving target – continuously evolving and still surprising doctors and patients who have sometimes incapacitating long-term symptoms.
Little about the disorder seems predictable at this point. People can have long COVID after asymptomatic, mild, or severe COVID-19, for example. And when a person gets long COVID – also known as long-haul COVID – symptoms can vary widely.
To address all the uncertainty, the New York State Department of Health gathered experts in primary care, pediatrics, physical medicine, rehabilitation, and pulmonology to answer some pressing questions.
New York in 2020 was the first epicenter of the pandemic in the United States, making it also the center of the long COVID epidemic, says Emily Lutterloh, MD, director of the Division of Epidemiology at the New York State Department of Health.
What do you do when you’re seeing a patient with long COVID for the first time?
The first exam varies because there are so many different ways long COVID presents itself, says Benjamin Abramoff, MD, a physical medicine and rehabilitation specialist at Penn Medicine in Philadelphia.
I’ve now been seriously ill with #LongCovid for 11 months. I was never hospitalized. I didn’t even have a “mild” covid case. Instead, I developed Long Covid from an asymptomatic infection.
I’m far from unique. Up to 1/5 of asymptomatic patients go on to have long-term symptoms.
— Ravi Veriah Jacques (@RaviHVJ) February 3, 2022
Assessing their previous and current care also helps to direct their ongoing management, says Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai Health System in New York.
Can vaccination help people with long COVID?
Anything that we can do to help prevent people from being critically ill or being hospitalized with COVID-19 is helpful to prevent long COVID, says Dr. Abramoff, who is also director of the long COVID clinic at the University of Pennsylvania, Philadelphia.
“So that’s something I always discuss with patients. In some research, sometimes patients do feel better after the vaccine,” he says.
What kind of therapies do you find helpful for your patients?
Rehabilitation is a key part of recovery from long COVID, Dr. Abramoff says. “It is very important to make this very patient-specific.”
“We have patients that are working. They’re already going to the gym in some cases but don’t feel like they have the same endurance,” he says. “And then we have patients who are so crippled by their fatigue that they can’t get out of bed.”
1/ What is #LongCOVID?!
A disabling malady from ongoing inflammation, autoimmunity, & potential viral reservoirs (GI, brain?)
NEW DATA: The Lungs “light up” on special MRI Scans 3 to 9 months later in patients never hospitalized for COVID.https://t.co/I2kyZ4cK5F pic.twitter.com/dL1P67L2DK
— WesElyMD (@WesElyMD) February 2, 2022
An exercise program can help people who have long COVID.
“There’s a big role for therapy services in the recovery of these patients,” says John Baratta, MD, of the department of physical medicine and rehabilitation at the University of North Carolina at Chapel Hill.
But the limited number of long COVID clinics can mean some people are unable to get to therapists trained on the needs of patients with lingering COVID symptoms. Educating community physical and occupational therapists is one solution.
How long does it take for people with long COVID to recover and get back to 100% if they can?
Specific numbers aren’t really available, Dr. Baratta says.
“But I can tell you the general trend that I see is that a lot of patients have a gradual improvement of symptoms. The slow but steady improvement with time may be the body’s natural healing process, a result of medical interventions, or both.”
It can help to reassure people with long COVID that they will not be discharged from care until they feel they’ve maximized their health, says Sharagim Kemp, DO, medical director of the COVID Recovery Program for Nuvance Health, a health system in New York and Connecticut.
It’s essential to set realistic recovery expectations and tell patients that not everyone will return to 100% of their pre-COVID functioning, she says.
“Once we are able to help them reset their expectations, there’s almost an accelerated recovery because they are not putting that pressure on themselves anymore,” Dr. Kemp says.
What are the most common symptoms you’re seeing in long COVID?
It’s helpful to think of long COVID as a very broad umbrella term, Dr. Abramoff says.
Echoing what many others have observed, fatigue, cognitive dysfunction or “brain fog,“ and shortness of breath or troubled breathing appear to be the most common symptoms, he says.
Some reported vague symptoms, Dr. Kemp says.
People may go to the doctor “not even realizing that they had COVID. That’s one of the important points here – to have a high index of suspicion for patients who come in with multiple symptoms,” she says.
For this reason, patients can report symptoms that don’t necessarily fit into any specialty, says Sarah J. Ryan, MD, an internal medicine doctor at Columbia University Irving Medical Center in New York. People say they are “just not themselves” or they are tired after their COVID-19 recovery.
Is there a connection between severe COVID cases and severe long COVID?
“It’s not like that at all. I would say that more than 80% of the patients that we see had mild to moderate illness and they were not hospitalized,” Dr. Baratta says.
Long COVID is a bit different in children and teenagers, says Ixsy Ramirez, MD, a pediatric pulmonologist at University of Michigan Health, Ann Arbor. Most patients in the long COVID clinic at the University of Michigan were previously healthy, and not children with asthma or other lung conditions as one might expect. In fact, many are student athletes, or were before they had long COVID.
In this population, shortness of breath is most common, followed by chest pain and fatigue. Unfortunately, the symptoms are so serious for many kids that their performance is limited, even if they can return to competitive play.
Are there defined criteria you use to diagnose long COVID? How do you give someone a diagnosis?
That’s an ever-evolving question, Dr. Kemp says. The generally accepted definition centers on persistent or new symptoms 4 weeks or more after the original COVID-19 illness, but there are exceptions.
Researchers are working on lab tests to help confirm the diagnosis. But without a definitive blood biomarker, getting to the diagnosis requires “some thorough detective work,” Dr. Ryan says.
Do you bring in mental health providers to help with treatment?
“We focus on mental health quite a bit actually,” says, Dr. Chen, cofounder of his institution’s COVID recovery clinic. Mount Sinai offers one-on-one and group mental health services, for example.
“Personally, I’ve seen patients that I did not expect to have such severe mental health changes” with long COVID.
One of the most powerful accounts and testimonies I have seen on what most #LongCovid patients experience when interacting with their doctors.
“I did not fit in a box, so they chose not to see me, even worse they made me feel like it was my fault for not fitting in their box” pic.twitter.com/7GQLBucuO5
— charlos (@loscharlos) February 3, 2022
Examples include severe depression, cases of acute psychosis, hallucinations, and other problems “that are really unexpected after a viral illness.”
Stony Brook University Hospital in New York has a long COVID clinic staffed by multiple primary care doctors who do exams and refer patients to services. A bonus of offering psychological services to all post-COVID patients is doctors get a more complete picture of each person and a better understanding of what they are going through, says Abigail Chua, MD, a pulmonologist at Stony Brook.
Some empathy is essential, Dr. Baratta says. “It’s important to recognize that a lot of these patients present with a sense of grief or loss for their prior life.”
What does the future hold?
A simple test to diagnose long COVID, combined with an effective treatment that helps people feel better within a week, would be ideal, Dr. Abramoff says.
“That would be lovely. But you know, we’re just not at that point.”
And it would be helpful to start identifying subtypes of long COVID so diagnosis and treatment can be more targeted, Dr. Abramoff says. Otherwise, “It’s going to be a very challenging approach to try to treat all of our patients with long COVID symptoms the same way.”
Good clinical trials likewise are needed to address all the subtleties of long COVID.
A number of long COVID centers are collaborating on research to find out more, Dr. Chen says. Actions include setting up a bank of tissue samples from people with long COVID so researchers can continue to figure out the condition.
One goal, Dr. Chen says, would be the ability to treat long COVID rather than just its symptoms.
Long COVID emphasizes the need to prevent people from getting COVID in the first place, Dr. Ramirez says. This will continue to be important, particularly when some people dismiss the seriousness of COVID, comparing it to a cold if they get it. That attitude discounts the large number of people who unfortunately go on to develop long-term, often debilitating, symptoms.
A version of this article first appeared on WebMD.com.