User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
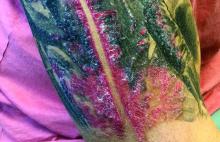
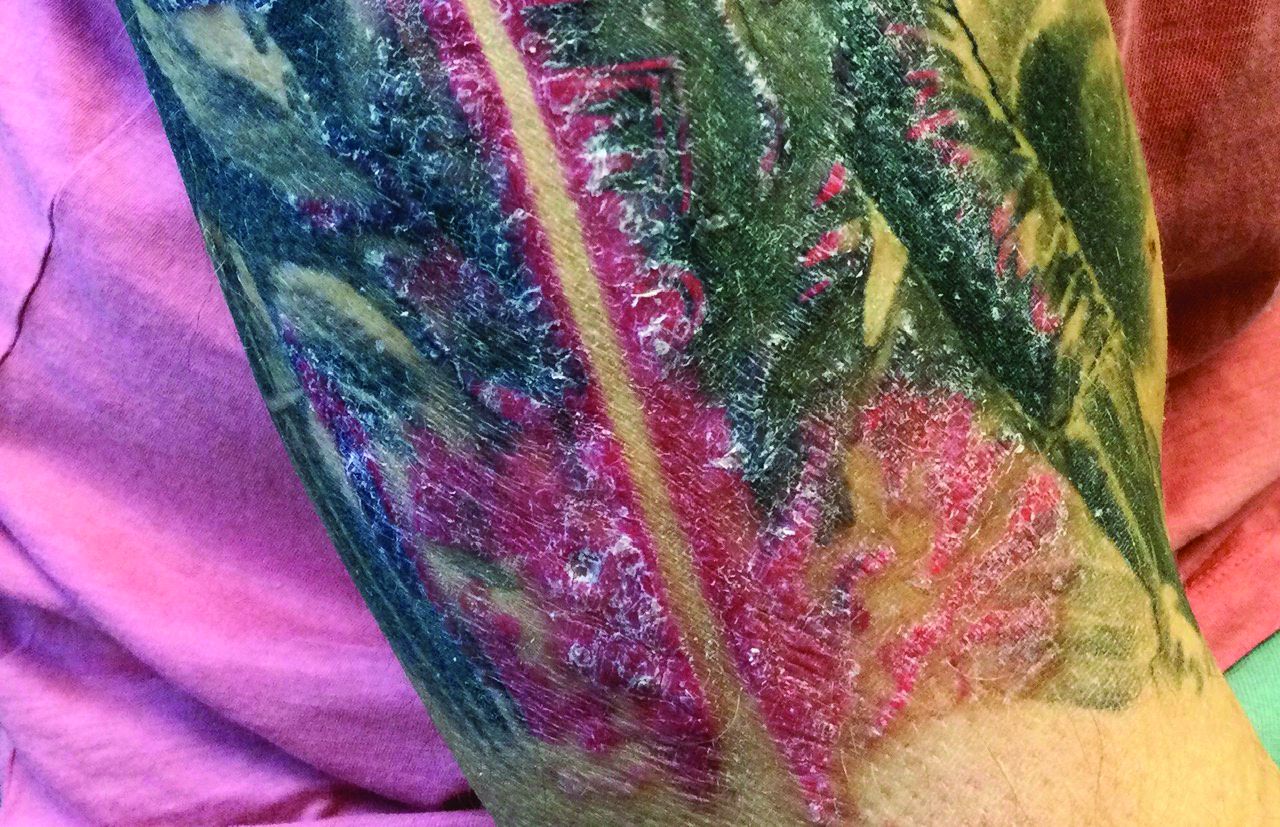
A male with pruritic scaling and bumps in the red area of a tattoo placed months earlier
, photoallergic reactions, infectious processes because of contaminated ink or a nonsterile environment, or as a Koebner response.
Dermatitis is commonly seen in patients with a sensitivity to certain pigments. Mercury sulfide or cinnabar in red, chromium in green, and cobalt in blue are common offenders. Cadmium, which is used for yellow, may cause a photoallergic reaction following exposure to ultraviolet light. Other inorganic salts of metals used for tattooing include ferric hydrate for ochre, ferric oxide for brown, manganese salts for purple. Reactions may be seen within a few weeks up to years after the tattoo is placed.
Reactions are often confined to the tattoo and may present as erythematous papules or plaques, although lesions may also present as scaly and eczematous patches. Psoriasis, vitiligo, and lichen planus may Koebnerize and appear in the tattoo. Sarcoidosis may occur in tattoos and can be seen upon histopathologic examination. Allergic contact dermatitis may also be seen in people who receive temporary henna tattoos in which the henna dye is mixed with paraphenylenediamine (PPD).
Histologically, granulomatous, sarcoidal, and lichenoid patterns may be seen. A punch biopsy was performed in this patient that revealed a lichenoid and interstitial lymphohistiocytic infiltrate with red tattoo pigment. Special stains for PAS, GMS, FITE, and AFB were negative. There was no polarizable foreign material identified.
Treatment includes topical steroids, which may be ineffective, intralesional kenalog, and surgical excision. Laser must be used with caution, as it may aggravate the allergic reaction and cause a systemic reaction.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
, photoallergic reactions, infectious processes because of contaminated ink or a nonsterile environment, or as a Koebner response.
Dermatitis is commonly seen in patients with a sensitivity to certain pigments. Mercury sulfide or cinnabar in red, chromium in green, and cobalt in blue are common offenders. Cadmium, which is used for yellow, may cause a photoallergic reaction following exposure to ultraviolet light. Other inorganic salts of metals used for tattooing include ferric hydrate for ochre, ferric oxide for brown, manganese salts for purple. Reactions may be seen within a few weeks up to years after the tattoo is placed.
Reactions are often confined to the tattoo and may present as erythematous papules or plaques, although lesions may also present as scaly and eczematous patches. Psoriasis, vitiligo, and lichen planus may Koebnerize and appear in the tattoo. Sarcoidosis may occur in tattoos and can be seen upon histopathologic examination. Allergic contact dermatitis may also be seen in people who receive temporary henna tattoos in which the henna dye is mixed with paraphenylenediamine (PPD).
Histologically, granulomatous, sarcoidal, and lichenoid patterns may be seen. A punch biopsy was performed in this patient that revealed a lichenoid and interstitial lymphohistiocytic infiltrate with red tattoo pigment. Special stains for PAS, GMS, FITE, and AFB were negative. There was no polarizable foreign material identified.
Treatment includes topical steroids, which may be ineffective, intralesional kenalog, and surgical excision. Laser must be used with caution, as it may aggravate the allergic reaction and cause a systemic reaction.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
, photoallergic reactions, infectious processes because of contaminated ink or a nonsterile environment, or as a Koebner response.
Dermatitis is commonly seen in patients with a sensitivity to certain pigments. Mercury sulfide or cinnabar in red, chromium in green, and cobalt in blue are common offenders. Cadmium, which is used for yellow, may cause a photoallergic reaction following exposure to ultraviolet light. Other inorganic salts of metals used for tattooing include ferric hydrate for ochre, ferric oxide for brown, manganese salts for purple. Reactions may be seen within a few weeks up to years after the tattoo is placed.
Reactions are often confined to the tattoo and may present as erythematous papules or plaques, although lesions may also present as scaly and eczematous patches. Psoriasis, vitiligo, and lichen planus may Koebnerize and appear in the tattoo. Sarcoidosis may occur in tattoos and can be seen upon histopathologic examination. Allergic contact dermatitis may also be seen in people who receive temporary henna tattoos in which the henna dye is mixed with paraphenylenediamine (PPD).
Histologically, granulomatous, sarcoidal, and lichenoid patterns may be seen. A punch biopsy was performed in this patient that revealed a lichenoid and interstitial lymphohistiocytic infiltrate with red tattoo pigment. Special stains for PAS, GMS, FITE, and AFB were negative. There was no polarizable foreign material identified.
Treatment includes topical steroids, which may be ineffective, intralesional kenalog, and surgical excision. Laser must be used with caution, as it may aggravate the allergic reaction and cause a systemic reaction.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Expert shares her tips for an effective cosmetic consultation
The way Kelly Stankiewicz, MD, sees it,
“I can’t tell you how many times I’ve walked into a room and thought the patient would say they’re concerned about one thing, but they’re concerned about something totally different,” Dr. Stankiewicz, a dermatologist in private practice in Park City, Utah, said during the annual conference of the American Society for Laser Medicine and Surgery. “The first question I ask is, ‘What would you like to improve?’ ‘What’s bothering you?’ ‘What would you like to make look better?’ Frequently, it’s not what you think.”
Next, she tries to get a sense of their lifestyle by asking patients about their occupation, hobbies, and outdoor activities they may engage in. “Here in Park City, it’s very sunny most all the time, so treatments need to be tailored to when those outdoor activities are being done, or perhaps they can be avoided for a period of time,” she said. “This gives you an idea of what kind of downtime people will tolerate. I also like to hear about their history of cosmetic procedures. If someone has had a lot of cosmetic procedures done, you can talk with them on a more detailed level. If someone is completely unaware of treatment options, you have to keep it simple.”
Dr. Stankiewicz also reviews their personal history of cosmetic procedures when considering safety of treatment. “For instance, if somebody has had a neck lift, you want to be very cautious doing any ablative procedures along the jawline,” she said. “I also like to know if anyone has had any reactions to dermal fillers or neuromodulators that they did not like. It’s very helpful to hear from patients what’s worked for them and what hasn’t. I also like to keep my ear open for pricing concerns. Not everyone will bring up the pricing issues, but sometimes they will, and it’s an important piece of information. Lastly, it’s important to look for any warning signs like irrational behavior or unrealistic expectations. These are patients you want to try to avoid treating.”
She shared four other key components to an effective cosmetic consultation, including the examination itself, which she prefers to separate from the discussion portion of the visit. “I lean the patient back in the exam chair and shine the light on their skin, which is important for evaluating for conditions you may not have discussed that could be easily improved,” Dr. Stankiewicz said.
“If the patient is concerned about pigmented lesions, I’ll pull out my dermatoscope to make sure there isn’t any concern for skin cancer. After the examination, I’ll sit the patient up again so that there is a very distinct start and finish to the examination portion of my cosmetic consultation.”
Surgery vs. noninvasive treatments
Step three in her consultative process is to review treatment options with patients. “I never hold back if surgery is their best treatment option,” she said. “I don’t perform surgery, but I have a list of people I can refer them to.”
Once she addresses the potential for surgery, she reviews noninvasive treatment options, including topical products, injectables, lasers, and chemical peels. “Everyone who comes in for a cosmetic consultation leaves with some sort of topical recommendation, even if it’s as simple as a sunscreen I think they would like or a prescription for generic tretinoin,” she said. “I always present options in a framework starting with those that require lower downtime, higher number of treatment options, and lower cost. Then I move up the scale to tell them more about treatments that require higher downtime, a lower number of treatments, but have a higher cost.”
Step four in her consultative process involves discussing her final treatment recommendations. She’ll say something like, “I’ve been through all these options with you and my final recommendation is X,” and the patient walks away with a clear understanding of the recommendations, she said. “When I leave the room after giving my final recommendation, I’ll write everything down outside of the room, or I’ll have a member of my staff write down everything I’ve said outside the room.”
Finally, she and her staff record all the relevant information for the patient as a customized handout, including the treatment options discussed, how many will be required, whether they have to come in early for numbing cream or not, and the per treatment price tag. “Once we’ve written down everything we’ve discussed, I’ll circle or I’ll star my recommended treatment,” Dr. Stankiewicz said. They also have a handout for topical products, and she checks off the topical products that she discussed with the patient. The third handout she provides to patients is a recommended skin care regimen.
Dr. Stankiewicz reported having no relevant financial disclosures.
The way Kelly Stankiewicz, MD, sees it,
“I can’t tell you how many times I’ve walked into a room and thought the patient would say they’re concerned about one thing, but they’re concerned about something totally different,” Dr. Stankiewicz, a dermatologist in private practice in Park City, Utah, said during the annual conference of the American Society for Laser Medicine and Surgery. “The first question I ask is, ‘What would you like to improve?’ ‘What’s bothering you?’ ‘What would you like to make look better?’ Frequently, it’s not what you think.”
Next, she tries to get a sense of their lifestyle by asking patients about their occupation, hobbies, and outdoor activities they may engage in. “Here in Park City, it’s very sunny most all the time, so treatments need to be tailored to when those outdoor activities are being done, or perhaps they can be avoided for a period of time,” she said. “This gives you an idea of what kind of downtime people will tolerate. I also like to hear about their history of cosmetic procedures. If someone has had a lot of cosmetic procedures done, you can talk with them on a more detailed level. If someone is completely unaware of treatment options, you have to keep it simple.”
Dr. Stankiewicz also reviews their personal history of cosmetic procedures when considering safety of treatment. “For instance, if somebody has had a neck lift, you want to be very cautious doing any ablative procedures along the jawline,” she said. “I also like to know if anyone has had any reactions to dermal fillers or neuromodulators that they did not like. It’s very helpful to hear from patients what’s worked for them and what hasn’t. I also like to keep my ear open for pricing concerns. Not everyone will bring up the pricing issues, but sometimes they will, and it’s an important piece of information. Lastly, it’s important to look for any warning signs like irrational behavior or unrealistic expectations. These are patients you want to try to avoid treating.”
She shared four other key components to an effective cosmetic consultation, including the examination itself, which she prefers to separate from the discussion portion of the visit. “I lean the patient back in the exam chair and shine the light on their skin, which is important for evaluating for conditions you may not have discussed that could be easily improved,” Dr. Stankiewicz said.
“If the patient is concerned about pigmented lesions, I’ll pull out my dermatoscope to make sure there isn’t any concern for skin cancer. After the examination, I’ll sit the patient up again so that there is a very distinct start and finish to the examination portion of my cosmetic consultation.”
Surgery vs. noninvasive treatments
Step three in her consultative process is to review treatment options with patients. “I never hold back if surgery is their best treatment option,” she said. “I don’t perform surgery, but I have a list of people I can refer them to.”
Once she addresses the potential for surgery, she reviews noninvasive treatment options, including topical products, injectables, lasers, and chemical peels. “Everyone who comes in for a cosmetic consultation leaves with some sort of topical recommendation, even if it’s as simple as a sunscreen I think they would like or a prescription for generic tretinoin,” she said. “I always present options in a framework starting with those that require lower downtime, higher number of treatment options, and lower cost. Then I move up the scale to tell them more about treatments that require higher downtime, a lower number of treatments, but have a higher cost.”
Step four in her consultative process involves discussing her final treatment recommendations. She’ll say something like, “I’ve been through all these options with you and my final recommendation is X,” and the patient walks away with a clear understanding of the recommendations, she said. “When I leave the room after giving my final recommendation, I’ll write everything down outside of the room, or I’ll have a member of my staff write down everything I’ve said outside the room.”
Finally, she and her staff record all the relevant information for the patient as a customized handout, including the treatment options discussed, how many will be required, whether they have to come in early for numbing cream or not, and the per treatment price tag. “Once we’ve written down everything we’ve discussed, I’ll circle or I’ll star my recommended treatment,” Dr. Stankiewicz said. They also have a handout for topical products, and she checks off the topical products that she discussed with the patient. The third handout she provides to patients is a recommended skin care regimen.
Dr. Stankiewicz reported having no relevant financial disclosures.
The way Kelly Stankiewicz, MD, sees it,
“I can’t tell you how many times I’ve walked into a room and thought the patient would say they’re concerned about one thing, but they’re concerned about something totally different,” Dr. Stankiewicz, a dermatologist in private practice in Park City, Utah, said during the annual conference of the American Society for Laser Medicine and Surgery. “The first question I ask is, ‘What would you like to improve?’ ‘What’s bothering you?’ ‘What would you like to make look better?’ Frequently, it’s not what you think.”
Next, she tries to get a sense of their lifestyle by asking patients about their occupation, hobbies, and outdoor activities they may engage in. “Here in Park City, it’s very sunny most all the time, so treatments need to be tailored to when those outdoor activities are being done, or perhaps they can be avoided for a period of time,” she said. “This gives you an idea of what kind of downtime people will tolerate. I also like to hear about their history of cosmetic procedures. If someone has had a lot of cosmetic procedures done, you can talk with them on a more detailed level. If someone is completely unaware of treatment options, you have to keep it simple.”
Dr. Stankiewicz also reviews their personal history of cosmetic procedures when considering safety of treatment. “For instance, if somebody has had a neck lift, you want to be very cautious doing any ablative procedures along the jawline,” she said. “I also like to know if anyone has had any reactions to dermal fillers or neuromodulators that they did not like. It’s very helpful to hear from patients what’s worked for them and what hasn’t. I also like to keep my ear open for pricing concerns. Not everyone will bring up the pricing issues, but sometimes they will, and it’s an important piece of information. Lastly, it’s important to look for any warning signs like irrational behavior or unrealistic expectations. These are patients you want to try to avoid treating.”
She shared four other key components to an effective cosmetic consultation, including the examination itself, which she prefers to separate from the discussion portion of the visit. “I lean the patient back in the exam chair and shine the light on their skin, which is important for evaluating for conditions you may not have discussed that could be easily improved,” Dr. Stankiewicz said.
“If the patient is concerned about pigmented lesions, I’ll pull out my dermatoscope to make sure there isn’t any concern for skin cancer. After the examination, I’ll sit the patient up again so that there is a very distinct start and finish to the examination portion of my cosmetic consultation.”
Surgery vs. noninvasive treatments
Step three in her consultative process is to review treatment options with patients. “I never hold back if surgery is their best treatment option,” she said. “I don’t perform surgery, but I have a list of people I can refer them to.”
Once she addresses the potential for surgery, she reviews noninvasive treatment options, including topical products, injectables, lasers, and chemical peels. “Everyone who comes in for a cosmetic consultation leaves with some sort of topical recommendation, even if it’s as simple as a sunscreen I think they would like or a prescription for generic tretinoin,” she said. “I always present options in a framework starting with those that require lower downtime, higher number of treatment options, and lower cost. Then I move up the scale to tell them more about treatments that require higher downtime, a lower number of treatments, but have a higher cost.”
Step four in her consultative process involves discussing her final treatment recommendations. She’ll say something like, “I’ve been through all these options with you and my final recommendation is X,” and the patient walks away with a clear understanding of the recommendations, she said. “When I leave the room after giving my final recommendation, I’ll write everything down outside of the room, or I’ll have a member of my staff write down everything I’ve said outside the room.”
Finally, she and her staff record all the relevant information for the patient as a customized handout, including the treatment options discussed, how many will be required, whether they have to come in early for numbing cream or not, and the per treatment price tag. “Once we’ve written down everything we’ve discussed, I’ll circle or I’ll star my recommended treatment,” Dr. Stankiewicz said. They also have a handout for topical products, and she checks off the topical products that she discussed with the patient. The third handout she provides to patients is a recommended skin care regimen.
Dr. Stankiewicz reported having no relevant financial disclosures.
FROM ASLMS 2021
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
Cellular senescence, skin aging, and cosmeceuticals
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.
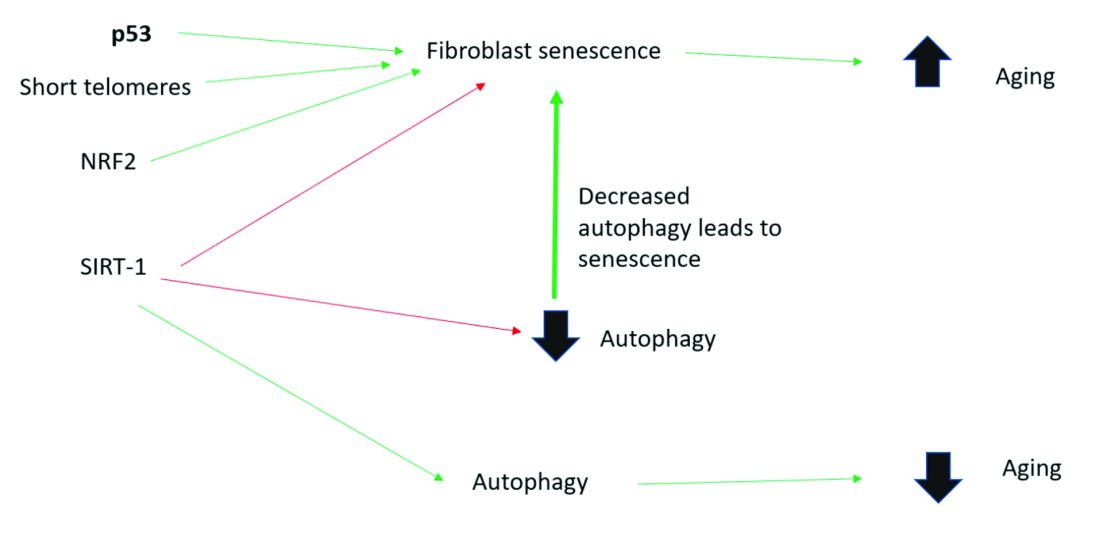
Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
Judge tosses hospital staff suit over vaccine mandate
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
OSHA issues new rules on COVID-19 safety for health care workers
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
Topical histone deacetylase inhibitor reduced BCC size in phase 2 study
according to research presented at the annual meeting of the Society for Investigative Dermatology.
“Our results demonstrate a clinically significant decrease in tumor size in response to 6 weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 55% reaching complete pathological resolution,” James M. Kilgour, MD, a postdoctoral research fellow at the Sarin Lab at Stanford (Calif.) University, said at the meeting.
Surgical excision is the preferred treatment for BCC, but there is still a need for noninvasive treatment options, Dr. Kilgour said. “Given the potential morbidity associated with excision, particularly for patients experiencing multiple or recurrent tumors, such as the immunosuppressed or patients with Gorlin syndrome, an effective and tolerable topical therapy would be a significant benefit,” he noted.
Previously, in an in silico screen experiment, Dr. Kilgour and colleagues identified HDAC inhibitors as a “top predicted therapeutic” for BCC treatment, and found that in mice studies, HDAC inhibitors were able to suppress the growth of BCC cell lines and BCC allografts.
Remetinostat, a pan-HDAC inhibitor, is being investigated as a treatment for cutaneous T-cell lymphoma.
HDAC inhibitors are thought to “alter expression of key oncogenes and tumor suppressors through epigenetic modification of histone and nonhistone proteins,” he noted.
To evaluate the efficacy of topical remetinostat for BCC, the investigators enrolled 30 patients with 49 BCC tumors in a phase 2, open-label, single-arm trial. Participants had tumors that were greater than 5 mm in diameter and had been referred to surgery at Stanford before enrollment. Patients were a mean of 59 years old, 63% were men, and 90% were White; 59.2% of participants had tumors with a diameter greater than 10 mm, the rest had tumors with a diameter of 10 mm or less.
After the tumors were photographed and measured, participants received 6 weeks of topical remetinostat therapy, followed by final measurement and photography of the tumors at 8 weeks and surgical excision. Topical remetinostat 1% gel was applied three times per day under bandage occlusion.
Overall, 25 participants with 33 tumors were included in the per-protocol analysis. At 8 weeks, there was at least a 30% decrease in diameter from baseline for 69.7% of tumors in these patients, with 17 of 33 tumors showing a complete response by week 8.
Regarding tumor subtypes, there was a 100% overall response rate for the 6 superficial BCC tumors (1 partial response, 5 complete responses), a 68.2% ORR for the 22 nodular tumors (5 partial responses, 10 complete responses), and a 66.7% ORR for the 3 infiltrative tumors (no partial responses, 2 complete responses). There were no partial or complete responses for the two micronodular tumors.
Most adverse events in the study were localized drug reactions, with no serious or systemic adverse events, Dr. Kilgour noted, with 10 tumors demonstrating either no reaction or a grade 1 reaction, and 23 tumors having a grade 2 or grade 3 response.
The investigators also used imaging (ImageJ) software to evaluate the average decrease in cross-sectional tumor area. The results of the analysis showed an average decrease at 8 weeks from baseline of 71.5%. In addition, histological assessment at 8 weeks demonstrated that 54.8% of tumors had complete pathological resolution.
“In the future, we advocate for a follow-up blinded, randomized, controlled trial of remetinostat with greater participant diversity,” Dr. Kilgour said. “Specifically, greater power is needed to understand which histological subtypes of BCC will respond best to the treatment and we need to understand the long-term durability of tumor resolution.”
Remetinostat promising as topical BCC therapy
In an interview, Beth G. Goldstein, MD, a dermatologist and Mohs surgeon in Chapel Hill, N.C., noted that the preliminary study was “an exciting report of a safe, well-tolerated nonsurgical option for patients with superficial BCCs on the trunk and extremities,” which has potential to be used in the future for nonsuperficial BCCs. The study shows that superficial BCCs can respond at a rate of 100% on nonfacial areas, said Dr. Goldstein, who was not involved in the research. “These lesions can be quite large with higher chances of recurrence and difficulty with wound care.
“This type of directed therapy hopefully continues to be perfected for treatment of BCC that avoids scarring and is well tolerated,” she added.
Dr. Goldstein commented that complete pathological resolution of 54.8% “was still unacceptably low for the remaining tumor types.” For those cases, she said remetinostat could possibly “provide a topical option as an adjunctive treatment for potentially reducing the size of the BCC prior to surgical removal,” as an alternative to a systemic therapy like vismodegib (Erivedge).
Dr. Goldstein said the strengths of the study were in the variety of tumors, close follow-up with histologic evaluation and safety signals. In terms of limitations, she said whether there were any cases of Gorlin syndrome was not clear. In addition, at least 30% of BCCs have a mixed tumor type on Mohs surgery that differs from the original biopsy, and “there was no mention if the residual tumor remained with the same histology,” she said.
In the future, a large, randomized trial is warranted to stratify for Gorlin syndrome, patients who are immunosuppressed, and additional tumor types that were underrepresented in this study, such as micronodular tumors, Dr. Goldstein said. Future studies also should examine how remetinostat impacts BCC in facial areas, the effect of multiple applications, and how the therapy performs as an adjunctive treatment before surgery.
This study was funded by Medivir, the Damon Runyon Foundation, National Cancer Institute, an American Skin Association Hambrick Medical Student grant, and Stanford Medical Scholars. Dr. Kilgour and Dr. Goldstein reported no relevant financial disclosures.
according to research presented at the annual meeting of the Society for Investigative Dermatology.
“Our results demonstrate a clinically significant decrease in tumor size in response to 6 weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 55% reaching complete pathological resolution,” James M. Kilgour, MD, a postdoctoral research fellow at the Sarin Lab at Stanford (Calif.) University, said at the meeting.
Surgical excision is the preferred treatment for BCC, but there is still a need for noninvasive treatment options, Dr. Kilgour said. “Given the potential morbidity associated with excision, particularly for patients experiencing multiple or recurrent tumors, such as the immunosuppressed or patients with Gorlin syndrome, an effective and tolerable topical therapy would be a significant benefit,” he noted.
Previously, in an in silico screen experiment, Dr. Kilgour and colleagues identified HDAC inhibitors as a “top predicted therapeutic” for BCC treatment, and found that in mice studies, HDAC inhibitors were able to suppress the growth of BCC cell lines and BCC allografts.
Remetinostat, a pan-HDAC inhibitor, is being investigated as a treatment for cutaneous T-cell lymphoma.
HDAC inhibitors are thought to “alter expression of key oncogenes and tumor suppressors through epigenetic modification of histone and nonhistone proteins,” he noted.
To evaluate the efficacy of topical remetinostat for BCC, the investigators enrolled 30 patients with 49 BCC tumors in a phase 2, open-label, single-arm trial. Participants had tumors that were greater than 5 mm in diameter and had been referred to surgery at Stanford before enrollment. Patients were a mean of 59 years old, 63% were men, and 90% were White; 59.2% of participants had tumors with a diameter greater than 10 mm, the rest had tumors with a diameter of 10 mm or less.
After the tumors were photographed and measured, participants received 6 weeks of topical remetinostat therapy, followed by final measurement and photography of the tumors at 8 weeks and surgical excision. Topical remetinostat 1% gel was applied three times per day under bandage occlusion.
Overall, 25 participants with 33 tumors were included in the per-protocol analysis. At 8 weeks, there was at least a 30% decrease in diameter from baseline for 69.7% of tumors in these patients, with 17 of 33 tumors showing a complete response by week 8.
Regarding tumor subtypes, there was a 100% overall response rate for the 6 superficial BCC tumors (1 partial response, 5 complete responses), a 68.2% ORR for the 22 nodular tumors (5 partial responses, 10 complete responses), and a 66.7% ORR for the 3 infiltrative tumors (no partial responses, 2 complete responses). There were no partial or complete responses for the two micronodular tumors.
Most adverse events in the study were localized drug reactions, with no serious or systemic adverse events, Dr. Kilgour noted, with 10 tumors demonstrating either no reaction or a grade 1 reaction, and 23 tumors having a grade 2 or grade 3 response.
The investigators also used imaging (ImageJ) software to evaluate the average decrease in cross-sectional tumor area. The results of the analysis showed an average decrease at 8 weeks from baseline of 71.5%. In addition, histological assessment at 8 weeks demonstrated that 54.8% of tumors had complete pathological resolution.
“In the future, we advocate for a follow-up blinded, randomized, controlled trial of remetinostat with greater participant diversity,” Dr. Kilgour said. “Specifically, greater power is needed to understand which histological subtypes of BCC will respond best to the treatment and we need to understand the long-term durability of tumor resolution.”
Remetinostat promising as topical BCC therapy
In an interview, Beth G. Goldstein, MD, a dermatologist and Mohs surgeon in Chapel Hill, N.C., noted that the preliminary study was “an exciting report of a safe, well-tolerated nonsurgical option for patients with superficial BCCs on the trunk and extremities,” which has potential to be used in the future for nonsuperficial BCCs. The study shows that superficial BCCs can respond at a rate of 100% on nonfacial areas, said Dr. Goldstein, who was not involved in the research. “These lesions can be quite large with higher chances of recurrence and difficulty with wound care.
“This type of directed therapy hopefully continues to be perfected for treatment of BCC that avoids scarring and is well tolerated,” she added.
Dr. Goldstein commented that complete pathological resolution of 54.8% “was still unacceptably low for the remaining tumor types.” For those cases, she said remetinostat could possibly “provide a topical option as an adjunctive treatment for potentially reducing the size of the BCC prior to surgical removal,” as an alternative to a systemic therapy like vismodegib (Erivedge).
Dr. Goldstein said the strengths of the study were in the variety of tumors, close follow-up with histologic evaluation and safety signals. In terms of limitations, she said whether there were any cases of Gorlin syndrome was not clear. In addition, at least 30% of BCCs have a mixed tumor type on Mohs surgery that differs from the original biopsy, and “there was no mention if the residual tumor remained with the same histology,” she said.
In the future, a large, randomized trial is warranted to stratify for Gorlin syndrome, patients who are immunosuppressed, and additional tumor types that were underrepresented in this study, such as micronodular tumors, Dr. Goldstein said. Future studies also should examine how remetinostat impacts BCC in facial areas, the effect of multiple applications, and how the therapy performs as an adjunctive treatment before surgery.
This study was funded by Medivir, the Damon Runyon Foundation, National Cancer Institute, an American Skin Association Hambrick Medical Student grant, and Stanford Medical Scholars. Dr. Kilgour and Dr. Goldstein reported no relevant financial disclosures.
according to research presented at the annual meeting of the Society for Investigative Dermatology.
“Our results demonstrate a clinically significant decrease in tumor size in response to 6 weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 55% reaching complete pathological resolution,” James M. Kilgour, MD, a postdoctoral research fellow at the Sarin Lab at Stanford (Calif.) University, said at the meeting.
Surgical excision is the preferred treatment for BCC, but there is still a need for noninvasive treatment options, Dr. Kilgour said. “Given the potential morbidity associated with excision, particularly for patients experiencing multiple or recurrent tumors, such as the immunosuppressed or patients with Gorlin syndrome, an effective and tolerable topical therapy would be a significant benefit,” he noted.
Previously, in an in silico screen experiment, Dr. Kilgour and colleagues identified HDAC inhibitors as a “top predicted therapeutic” for BCC treatment, and found that in mice studies, HDAC inhibitors were able to suppress the growth of BCC cell lines and BCC allografts.
Remetinostat, a pan-HDAC inhibitor, is being investigated as a treatment for cutaneous T-cell lymphoma.
HDAC inhibitors are thought to “alter expression of key oncogenes and tumor suppressors through epigenetic modification of histone and nonhistone proteins,” he noted.
To evaluate the efficacy of topical remetinostat for BCC, the investigators enrolled 30 patients with 49 BCC tumors in a phase 2, open-label, single-arm trial. Participants had tumors that were greater than 5 mm in diameter and had been referred to surgery at Stanford before enrollment. Patients were a mean of 59 years old, 63% were men, and 90% were White; 59.2% of participants had tumors with a diameter greater than 10 mm, the rest had tumors with a diameter of 10 mm or less.
After the tumors were photographed and measured, participants received 6 weeks of topical remetinostat therapy, followed by final measurement and photography of the tumors at 8 weeks and surgical excision. Topical remetinostat 1% gel was applied three times per day under bandage occlusion.
Overall, 25 participants with 33 tumors were included in the per-protocol analysis. At 8 weeks, there was at least a 30% decrease in diameter from baseline for 69.7% of tumors in these patients, with 17 of 33 tumors showing a complete response by week 8.
Regarding tumor subtypes, there was a 100% overall response rate for the 6 superficial BCC tumors (1 partial response, 5 complete responses), a 68.2% ORR for the 22 nodular tumors (5 partial responses, 10 complete responses), and a 66.7% ORR for the 3 infiltrative tumors (no partial responses, 2 complete responses). There were no partial or complete responses for the two micronodular tumors.
Most adverse events in the study were localized drug reactions, with no serious or systemic adverse events, Dr. Kilgour noted, with 10 tumors demonstrating either no reaction or a grade 1 reaction, and 23 tumors having a grade 2 or grade 3 response.
The investigators also used imaging (ImageJ) software to evaluate the average decrease in cross-sectional tumor area. The results of the analysis showed an average decrease at 8 weeks from baseline of 71.5%. In addition, histological assessment at 8 weeks demonstrated that 54.8% of tumors had complete pathological resolution.
“In the future, we advocate for a follow-up blinded, randomized, controlled trial of remetinostat with greater participant diversity,” Dr. Kilgour said. “Specifically, greater power is needed to understand which histological subtypes of BCC will respond best to the treatment and we need to understand the long-term durability of tumor resolution.”
Remetinostat promising as topical BCC therapy
In an interview, Beth G. Goldstein, MD, a dermatologist and Mohs surgeon in Chapel Hill, N.C., noted that the preliminary study was “an exciting report of a safe, well-tolerated nonsurgical option for patients with superficial BCCs on the trunk and extremities,” which has potential to be used in the future for nonsuperficial BCCs. The study shows that superficial BCCs can respond at a rate of 100% on nonfacial areas, said Dr. Goldstein, who was not involved in the research. “These lesions can be quite large with higher chances of recurrence and difficulty with wound care.
“This type of directed therapy hopefully continues to be perfected for treatment of BCC that avoids scarring and is well tolerated,” she added.
Dr. Goldstein commented that complete pathological resolution of 54.8% “was still unacceptably low for the remaining tumor types.” For those cases, she said remetinostat could possibly “provide a topical option as an adjunctive treatment for potentially reducing the size of the BCC prior to surgical removal,” as an alternative to a systemic therapy like vismodegib (Erivedge).
Dr. Goldstein said the strengths of the study were in the variety of tumors, close follow-up with histologic evaluation and safety signals. In terms of limitations, she said whether there were any cases of Gorlin syndrome was not clear. In addition, at least 30% of BCCs have a mixed tumor type on Mohs surgery that differs from the original biopsy, and “there was no mention if the residual tumor remained with the same histology,” she said.
In the future, a large, randomized trial is warranted to stratify for Gorlin syndrome, patients who are immunosuppressed, and additional tumor types that were underrepresented in this study, such as micronodular tumors, Dr. Goldstein said. Future studies also should examine how remetinostat impacts BCC in facial areas, the effect of multiple applications, and how the therapy performs as an adjunctive treatment before surgery.
This study was funded by Medivir, the Damon Runyon Foundation, National Cancer Institute, an American Skin Association Hambrick Medical Student grant, and Stanford Medical Scholars. Dr. Kilgour and Dr. Goldstein reported no relevant financial disclosures.
FROM SID 2021
Cross-sectional study finds chronic skin conditions have highest opioid prescribing rates
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
FROM SID 2021
COVID-19 death toll higher for international medical graduates
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
Lenabasum missed mark for systemic sclerosis but may show promise for adjunctive therapy
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Although a phase 3 trial of lenabasum did not meet its primary endpoint for treatment of diffuse cutaneous systemic sclerosis (dcSSc), the drug led to more improvement in participants who were not receiving background immunosuppressant therapy during the trial than that seen in participants who received the placebo. Lenabasum also had a favorable safety profile, according to findings presented at the annual European Congress of Rheumatology.
The double-blind, randomized, placebo-controlled trial involved 363 adults who had had dcSSc for up to 6 years. One third of the participants received 5 mg of oral lenabasum, one third received 20 mg, and one third received a placebo. Patients already receiving immunosuppressant therapy could continue to receive it during the trial if the dose had been stable for at least 8 weeks before screening and corticosteroid therapy did not exceed 10 mg prednisone per day or the equivalent.
“The decision to allow background immunosuppressant therapies was made to reflect real-world clinical practice,” coprincipal investigator Robert Dr. Spiera, MD, director of the Vasculitis and Scleroderma Program at the Hospital for Special Surgery, New York, told attendees.
“It is surprising that we do not see any added efficacy of lenabasum in this trial, given the fact that the previous phase 2 trial in 42 patients did show a clear benefit of lenabasum over placebo in the same population,” Jeska K. de Vries-Bouwstra, MD, PhD, a rheumatologist at Leiden (the Netherlands) University Medical Center told this news organization. “Even more, the clinical response in the phase 2 study was supported by a greater change in gene expression in skin tissue of pathways involved in inflammation and fibrosis with lenabasum as compared to placebo.”
Background immunosuppressants contribute to unprecedented placebo responses
The researchers compared the ACR CRISS (Combined Response Index in Diffuse Cutaneous Systemic Sclerosis) score and several secondary endpoints at 52 weeks between the 123 participants who received the placebo and the 120 participants who received 20 mg of lenabasum. A total of 60% of the lenabasum group and 66% of the placebo group had a disease duration of 3 or fewer years, and the modified Rodnan skin score (mRSS) was 22 in the lenabasum group and 23.3 in the placebo group at baseline.
A large majority of participants in both groups – 89% in the lenabasum group and 84% in the placebo group – were receiving background immunosuppressant therapy during the trial. Specifically, 53% of each group was taking mycophenolate, and 23% of the lenabasum group and 32% of the placebo group were taking corticosteroids. In addition, 22% of the lenabasum group and 12% of the placebo group were on methotrexate, and 27% of the lenabasum group and 22% of the placebo group were on another immunosuppressant therapy.
Half of the placebo group and 58% of the lenabasum group were taking only one immunosuppressive therapy. About one-third of the lenabasum (32%) and placebo (34%) groups were taking two or more immunosuppressive therapies.
The primary endpoint at 52 weeks was not significantly different between the two groups: a CRISS score of 0.888 in the lenabasum group and 0.887 in the placebo group. A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Patients with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
“We had very high CRISS scores in all three groups, and they were comparable in all three groups,” Dr. Spiera reported. Because improvement in placebo group far exceeded expectations, the researchers were unable to discern the treatment effect of lenabasum on top of the placebo effect.
The placebo group had better outcomes than expected because of the background immunosuppressant therapy, particularly the use of mycophenolate. When the researchers looked only at placebo participants, the CRISS score was 0.936 in the 97 patients receiving background immunosuppressant therapy of any kind and 0.935 in the 29 patients taking only mycophenolate with no other immunosuppressant therapy, compared with 0.417 in the 16 patients not receiving any background therapy.
In a prespecified analysis, the researchers investigated background immunosuppressive therapy as a mediator. The CRISS score for the 10 lenabasum participants not receiving background therapy was 0.811, compared with 0.417 seen in the placebo group patients not on background therapy.
Among the 173 participants taking mycophenolate in particular, the mycophenolate “had a statistically significant improvement on CRISS score that increased with each visit,” Dr. Spiera reported. The duration of mycophenolate therapy also affected efficacy results.
Patients who had been taking mycophenolate longer saw less improvement in their CRISS score over time. Those taking it more than 2 years at baseline had a CRISS score of 0.86, compared with 0.96 for those taking it for 1-2 years at baseline and 0.98 for those taking it from 6 months to 1 year at baseline. Those who had only been taking mycophenolate for up to 6 months at baseline had a CRISS score of 0.99. Meanwhile, patients not taking any background immunosuppressant therapies only had a CRISS score of about 0.35.
Changes in secondary endpoints followed same pattern as CRISS
The secondary endpoints similarly showed no statistically significant difference when comparing the lenabasum and placebo groups overall. These endpoints included change in mRSS score, change in forced vital capacity (FVC) percentage and volume, and change in the Health Assessment Questionnaire Disability Index (HAQ-DI) score.
However, the researchers again found that duration of background therapy affected FVC.
“You were more likely to have declined [in FVC] if you were on placebo and more likely to have improved or stayed stable if you were on lenabasum if you were a patient on more than 2 years of immunomodulatory therapy at baseline,” Dr. Spiera reported. “There was evidence for an effect of lenabasum on FVC suggested by post-hoc analyses that considered the effect of background immunosuppressive therapies on outcomes, but those results would require confirmation in additional studies to determine the potential of lenabasum for treating patients with diffuse cutaneous systemic sclerosis,” Dr. Spiera noted in his conclusions.
Serious adverse events occurred in 9.2% of the lenabasum group and 5.8% of the placebo group. Rates of severe adverse events were similar between the lenabasum (14.6%) and placebo (13%) groups.
Is there a subgroup for whom lenabasum would be efficacious?
Although De Vries-Bouwstra of Leiden University Medical Center acknowledged the role of mycophenolate in the trial, she does not think background therapy can totally explain the observation and speculated on other possibilities.
“For example, there were fewer males in the placebo group as compared to the phase 2 study. From previous cohort studies we know that males have higher risk of worsening of skin disease,” she said. “In addition, it could be worthwhile to evaluate antibody profiles of the population under study; some subpopulations defined by autoantibody have higher risk for skin progression, while others can show spontaneous improvement.”
Dr. De Vries-Bouwstra said that, although it’s not currently appropriate to advocate for lenabasum to treat dcSSc, it may eventually become an additional treatment in those who still show active skin or lung disease after 2 years of mycophenolate treatment if future research identifies a benefit from that application. She would also like to see an evaluation of lenabasum’s possible benefits in patients with very early and active inflammatory disease. “Ideally, one could stratify patients based on biomarkers reflecting activation in relevant pathways, for example by using gene expression analysis from skin tissue to stratify,” she said.
Jacob M. van Laar, MD, PhD, professor of rheumatology at University Medical Center Utrecht (the Netherlands), also commented on the potential differences in using the drug in early versus later disease.
“Based on ex vivo analyses of skin samples from systemic sclerosis patients, one would expect such a mechanism of action to be particularly relevant in very early disease, so the observation that it might also be effective at a later disease stage is interesting,” Dr. van Laar told this news organization. “We still have a lot to learn about this complex disease.”
Given that safety does not appear to be a major concern and that there may be a benefit in a subgroup of patients, Dr. van Laar also said he hoped “the company is not deterred by the seemingly negative result of the primary endpoint.”
Dr. Spiera expressed optimism about what this trial’s findings have revealed about management of dcSSc.
“Independent of what lenabasum did or didn’t do in this trial, I think there’s going to be a lot that we’re going to learn from this trial and that we’re already learning and analyzing right now about treating scleroderma,” he said in an interview.
He reiterated the value of allowing background therapy in the trial to ensure it better replicated real-world clinical practice.
“You’re not withholding therapies that we think are probably active from patients with active disease that, once you incur organ damage, is probably not going to be reversible,” Dr. Spiera said. “The downside is that it makes it harder to see an effect of a drug on top of the background therapy if that background therapy is effective. So what we saw in terms of this absence of benefit from lenabasum really may have been a ceiling effect.”
Nevertheless, Dr. Spiera said the findings still strongly suggest that lenabasum is an active compound.
“It’s not an enormously powerful effect, but it probably has a role as an adjunctive therapy in people on stable background therapy who have either plateaued or are getting worse,” he said. “The thing we have to keep in mind also is this was an incredibly safe therapy. It’s not immunosuppressive.”
The trial was funded by Corbus. Dr. Spiera has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Inflarx, Kadmon, AstraZeneca, AbbVie, CSL Behring, Sanofi, and Janssen. Dr. De Vries-Bouwstra has received consulting fees from AbbVie and Boehringer Ingelheim and research grants from Galapagos and Janssen. Dr. Van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.