User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Antithrombotic therapy not warranted in COVID-19 outpatients
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The compass that points toward food
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
No benefit from lower temps for out-of-hospital cardiac arrest
The results “do not support the use of moderate therapeutic hypothermia to improve neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest,” write the investigators led by Michel Le May, MD, from the University of Ottawa Heart Institute, Ontario, Canada.
The CAPITAL CHILL results were first presented at the American College of Cardiology (ACC) 2021 Scientific Sessions in May.
They have now been published online, October 19, in JAMA.
High rates of brain injury and death
Comatose survivors of OHCA have high rates of severe brain injury and death. Current guidelines recommend targeted temperature management at 32°C to 36°C for 24 hours. However, small studies have suggested a potential benefit of targeting lower body temperatures.
In the CAPITAL CHILL study of 367 OHCA patients who were comatose on admission, there were no statistically significant differences in the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days with mild-versus-moderate hypothermia.
The primary composite outcome occurred in 89 of 184 (48.4%) patients in the moderate hypothermia group and 83 of 183 (45.4%) patients in the mild hypothermia group — a risk difference of 3.0% (95% confidence interval [CI], 7.2% - 13.2%) and relative risk of 1.07 (95% CI, 0.86 - 1.33; P = .56).
There was also no significant difference when looking at the individual components of mortality (43.5% vs 41.0%) and poor neurologic outcome (Disability Rating Scale score >5: 4.9% vs 4.4%).
The baseline characteristics of patients were similar in the moderate and mild hypothermia groups. The lack of a significant difference in the primary outcome was consistent after adjusting for baseline covariates as well as across all subgroups.
The rates of secondary outcomes were also similar between the two groups, except for a longer length of stay in the intensive care unit in the moderate hypothermia group compared with the mild hypothermia group, which would likely add to overall costs.
The researchers note that the Targeted Hypothermia vs Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial recently reported that targeted hypothermia at 33°C did not improve survival at 180 days compared with targeted normothermia at 37.5°C or less.
The CAPITAL CHILL study “adds to the spectrum of target temperature management, as it did not find any benefit of even further lowering temperatures to 31°C,” the study team says.
They caution that most patients in the trial had cardiac arrest secondary to a primary cardiac etiology and therefore the findings may not be applicable to cardiac arrest of all etiologies.
It’s also possible that the trial was underpowered to detect clinically important differences between moderate and mild hypothermia. Also, the number of patients presenting with a nonshockable rhythm was relatively small, and further study may be worthwhile in this subgroup, they say.
For now, however, the CAPITAL CHILL results provide no support for a lower target temperature of 31°C to improve outcomes in OHCA patients, Dr. Le May and colleagues conclude.
CAPITAL CHILL was an investigator-initiated study and funding was provided by the University of Ottawa Heart Institute Cardiac Arrest Program. Dr. Le May has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results “do not support the use of moderate therapeutic hypothermia to improve neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest,” write the investigators led by Michel Le May, MD, from the University of Ottawa Heart Institute, Ontario, Canada.
The CAPITAL CHILL results were first presented at the American College of Cardiology (ACC) 2021 Scientific Sessions in May.
They have now been published online, October 19, in JAMA.
High rates of brain injury and death
Comatose survivors of OHCA have high rates of severe brain injury and death. Current guidelines recommend targeted temperature management at 32°C to 36°C for 24 hours. However, small studies have suggested a potential benefit of targeting lower body temperatures.
In the CAPITAL CHILL study of 367 OHCA patients who were comatose on admission, there were no statistically significant differences in the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days with mild-versus-moderate hypothermia.
The primary composite outcome occurred in 89 of 184 (48.4%) patients in the moderate hypothermia group and 83 of 183 (45.4%) patients in the mild hypothermia group — a risk difference of 3.0% (95% confidence interval [CI], 7.2% - 13.2%) and relative risk of 1.07 (95% CI, 0.86 - 1.33; P = .56).
There was also no significant difference when looking at the individual components of mortality (43.5% vs 41.0%) and poor neurologic outcome (Disability Rating Scale score >5: 4.9% vs 4.4%).
The baseline characteristics of patients were similar in the moderate and mild hypothermia groups. The lack of a significant difference in the primary outcome was consistent after adjusting for baseline covariates as well as across all subgroups.
The rates of secondary outcomes were also similar between the two groups, except for a longer length of stay in the intensive care unit in the moderate hypothermia group compared with the mild hypothermia group, which would likely add to overall costs.
The researchers note that the Targeted Hypothermia vs Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial recently reported that targeted hypothermia at 33°C did not improve survival at 180 days compared with targeted normothermia at 37.5°C or less.
The CAPITAL CHILL study “adds to the spectrum of target temperature management, as it did not find any benefit of even further lowering temperatures to 31°C,” the study team says.
They caution that most patients in the trial had cardiac arrest secondary to a primary cardiac etiology and therefore the findings may not be applicable to cardiac arrest of all etiologies.
It’s also possible that the trial was underpowered to detect clinically important differences between moderate and mild hypothermia. Also, the number of patients presenting with a nonshockable rhythm was relatively small, and further study may be worthwhile in this subgroup, they say.
For now, however, the CAPITAL CHILL results provide no support for a lower target temperature of 31°C to improve outcomes in OHCA patients, Dr. Le May and colleagues conclude.
CAPITAL CHILL was an investigator-initiated study and funding was provided by the University of Ottawa Heart Institute Cardiac Arrest Program. Dr. Le May has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results “do not support the use of moderate therapeutic hypothermia to improve neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest,” write the investigators led by Michel Le May, MD, from the University of Ottawa Heart Institute, Ontario, Canada.
The CAPITAL CHILL results were first presented at the American College of Cardiology (ACC) 2021 Scientific Sessions in May.
They have now been published online, October 19, in JAMA.
High rates of brain injury and death
Comatose survivors of OHCA have high rates of severe brain injury and death. Current guidelines recommend targeted temperature management at 32°C to 36°C for 24 hours. However, small studies have suggested a potential benefit of targeting lower body temperatures.
In the CAPITAL CHILL study of 367 OHCA patients who were comatose on admission, there were no statistically significant differences in the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days with mild-versus-moderate hypothermia.
The primary composite outcome occurred in 89 of 184 (48.4%) patients in the moderate hypothermia group and 83 of 183 (45.4%) patients in the mild hypothermia group — a risk difference of 3.0% (95% confidence interval [CI], 7.2% - 13.2%) and relative risk of 1.07 (95% CI, 0.86 - 1.33; P = .56).
There was also no significant difference when looking at the individual components of mortality (43.5% vs 41.0%) and poor neurologic outcome (Disability Rating Scale score >5: 4.9% vs 4.4%).
The baseline characteristics of patients were similar in the moderate and mild hypothermia groups. The lack of a significant difference in the primary outcome was consistent after adjusting for baseline covariates as well as across all subgroups.
The rates of secondary outcomes were also similar between the two groups, except for a longer length of stay in the intensive care unit in the moderate hypothermia group compared with the mild hypothermia group, which would likely add to overall costs.
The researchers note that the Targeted Hypothermia vs Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial recently reported that targeted hypothermia at 33°C did not improve survival at 180 days compared with targeted normothermia at 37.5°C or less.
The CAPITAL CHILL study “adds to the spectrum of target temperature management, as it did not find any benefit of even further lowering temperatures to 31°C,” the study team says.
They caution that most patients in the trial had cardiac arrest secondary to a primary cardiac etiology and therefore the findings may not be applicable to cardiac arrest of all etiologies.
It’s also possible that the trial was underpowered to detect clinically important differences between moderate and mild hypothermia. Also, the number of patients presenting with a nonshockable rhythm was relatively small, and further study may be worthwhile in this subgroup, they say.
For now, however, the CAPITAL CHILL results provide no support for a lower target temperature of 31°C to improve outcomes in OHCA patients, Dr. Le May and colleagues conclude.
CAPITAL CHILL was an investigator-initiated study and funding was provided by the University of Ottawa Heart Institute Cardiac Arrest Program. Dr. Le May has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves combo pill for severe, acute pain
enough to require an opioid analgesic and for which alternative treatments fail to provide adequate pain relief.
Celecoxib is a nonsteroidal anti-inflammatory drug and tramadol is an opioid agonist. Seglentis contains 56 mg of celecoxib and 44 mg of tramadol.
“The unique co-crystal formulation of Seglentis provides effective pain relief via a multimodal approach,” Craig A. Sponseller, MD, chief medical officer of Kowa Pharmaceuticals America, said in a news release.
Esteve Pharmaceuticals has entered into an agreement with Kowa Pharmaceuticals America to commercialize the pain medicine in the United States, with a launch planned for early 2022.
“Seglentis uses four different and complementary mechanisms of analgesia and offers healthcare providers an important option to treat acute pain in adults that is severe enough to require opioid treatment and for which alternative treatments are inadequate,” Dr. Sponseller said.
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, the FDA will require a Risk Evaluation and Mitigation Strategy (REMS) for Seglentis.
The label states that the drug should be initiated as two tablets every 12 hours as needed and should be prescribed for the shortest duration consistent with individual patient treatment goals.
Patients should be monitored for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with Seglentis.
Prescribers should discuss naloxone (Narcan) with patients and consider prescribing the opioid antagonist naloxone based on the patient’s risk factors for overdose.
Full prescribing information is available online.
A version of this article was first published on Medscape.com.
enough to require an opioid analgesic and for which alternative treatments fail to provide adequate pain relief.
Celecoxib is a nonsteroidal anti-inflammatory drug and tramadol is an opioid agonist. Seglentis contains 56 mg of celecoxib and 44 mg of tramadol.
“The unique co-crystal formulation of Seglentis provides effective pain relief via a multimodal approach,” Craig A. Sponseller, MD, chief medical officer of Kowa Pharmaceuticals America, said in a news release.
Esteve Pharmaceuticals has entered into an agreement with Kowa Pharmaceuticals America to commercialize the pain medicine in the United States, with a launch planned for early 2022.
“Seglentis uses four different and complementary mechanisms of analgesia and offers healthcare providers an important option to treat acute pain in adults that is severe enough to require opioid treatment and for which alternative treatments are inadequate,” Dr. Sponseller said.
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, the FDA will require a Risk Evaluation and Mitigation Strategy (REMS) for Seglentis.
The label states that the drug should be initiated as two tablets every 12 hours as needed and should be prescribed for the shortest duration consistent with individual patient treatment goals.
Patients should be monitored for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with Seglentis.
Prescribers should discuss naloxone (Narcan) with patients and consider prescribing the opioid antagonist naloxone based on the patient’s risk factors for overdose.
Full prescribing information is available online.
A version of this article was first published on Medscape.com.
enough to require an opioid analgesic and for which alternative treatments fail to provide adequate pain relief.
Celecoxib is a nonsteroidal anti-inflammatory drug and tramadol is an opioid agonist. Seglentis contains 56 mg of celecoxib and 44 mg of tramadol.
“The unique co-crystal formulation of Seglentis provides effective pain relief via a multimodal approach,” Craig A. Sponseller, MD, chief medical officer of Kowa Pharmaceuticals America, said in a news release.
Esteve Pharmaceuticals has entered into an agreement with Kowa Pharmaceuticals America to commercialize the pain medicine in the United States, with a launch planned for early 2022.
“Seglentis uses four different and complementary mechanisms of analgesia and offers healthcare providers an important option to treat acute pain in adults that is severe enough to require opioid treatment and for which alternative treatments are inadequate,” Dr. Sponseller said.
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, the FDA will require a Risk Evaluation and Mitigation Strategy (REMS) for Seglentis.
The label states that the drug should be initiated as two tablets every 12 hours as needed and should be prescribed for the shortest duration consistent with individual patient treatment goals.
Patients should be monitored for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with Seglentis.
Prescribers should discuss naloxone (Narcan) with patients and consider prescribing the opioid antagonist naloxone based on the patient’s risk factors for overdose.
Full prescribing information is available online.
A version of this article was first published on Medscape.com.
Expensive insulins, pen devices dominate U.S. diabetes care
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccinations lower than ever as cases continue to drop
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.
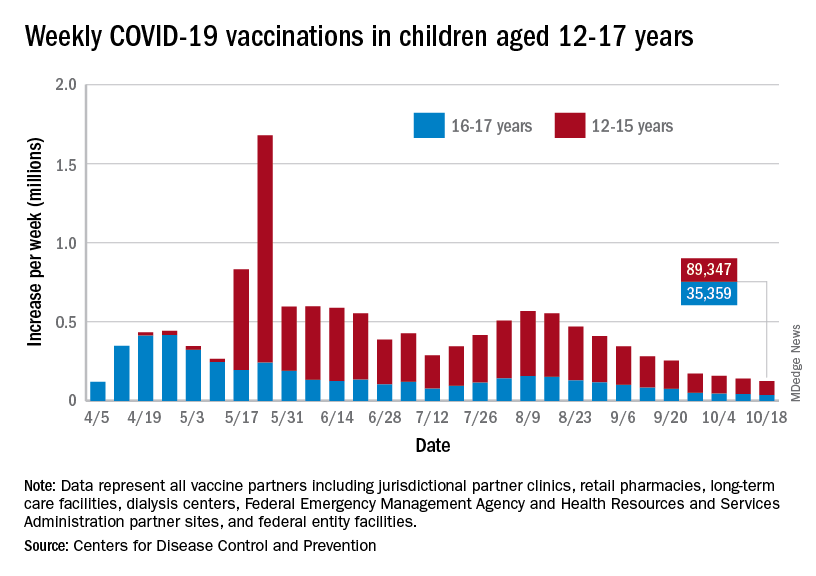
Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
Tramadol linked to higher risk of mortality, compared with codeine
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Patient loses prostate after biopsy slide switched; more
It’s difficult enough when a patient’s prostate is removed because of cancer. But it’s another thing altogether when the prostate is removed because of a medical error, as a report on 3 CBS Philly, among other news outlets, makes clear.
The patient, Eric Spangs, lives in southeastern Pennsylvania. Testing indicated an elevation in prostate-specific antigen (PSA) level. He subsequently underwent biopsy of the prostate, which appeared to indicate cancer. In time, though, Mr. Spangs learned there had been an error: the tissue section used in the microscopic diagnosis had come from the biopsy specimen of a different patient. Mr. Spangs himself didn’t actually have cancer.
Ordinarily, such news would be cause for celebration. But this was far from a normal situ ation: Following his initial cancer diagnosis, Mr. Spangs underwent a radical laparoscopic prostatectomy at a local hospital.
“It’s devastated me emotionally and physically,” Mr. Spangs said. It has also been emotionally devastating for his wife, Melissa. (The couple has five children.)
Their attorney, Aaron Freiwald, has filed a suit against the health system to which the local hospital belongs and the area’s largest urologic practice.
The Spangs wish to caution other patients not to make the same mistake they did: they failed to get a second opinion from an oncology specialist, as recommended by the American Cancer Society. (Eric Spangs did receive a second opinion from someone at the urologic practice, but that practice doesn’t specialize in oncology.)
The Spangs also worry about the patient who received the false-negative biopsy result. They have been assured, however, that that patient will be properly notified of his actual cancer status.
Fertility specialist uses own sperm to impregnate patients
A suit claims that a Rochester, N.Y., gynecologist and fertility specialist used his own sperm to inseminate multiple patients, according to a story reported by the Associated Press and other news outlets.
The suit was filed last month by the daughter — call her “Harriet Jones” — of one of the women who received fertility services from the doctor during the 1980s. Ms. Jones’s suit alleges that at the time, the doctor told her mother that the sperm donor would be a medical student at the University of Rochester. In fact, the donor was the doctor himself. He kept that fact a secret for years, even after Ms. Jones — his own daughter — sought him out for gynecologic services.
The secret gradually began to come to light in 2016, when Ms. Jones’s nonbiological father — the man who had helped to raise her — died. Curious about her biological father, Ms. Jones sought to learn his identity from the Rochester gynecologist who had treated her mother and was now her own gynecologist. The doctor said he couldn’t be of help; he claimed he hadn’t kept the relevant records.
Ms. Jones then submitted a blood sample to a direct-to-consumer genetic testing company. The results surprised her: Not only did she learn of her ethnicity, but she also discovered the existence of two half siblings, who were donor-conceived in 1984 and 1985, respectively, the very period when her own mother was undergoing insemination procedures. Ms. Jones subsequently discovered the existence of additional half siblings, all born in the first half of the 1980s.
Initially elated by the discoveries, Ms. Jones soon grew despondent and anxious. She suffered from migraine headaches, among other symptoms. Her biological father, it seemed, had been “a serial sperm donor.”
Still, she continued to go to her Rochester doctor for treatment, having no reason to suspect anything untoward about him. Her visits, including those for prolonged menstrual bleeding, involved routine breast and pelvic exams, transvaginal ultrasounds, and intrauterine contraceptive placements under sedation.
Over this period, her doctor was friendly, asking her a variety of questions about her personal life. During one especially strange visit, however, he began chuckling and said, “You’re a really good kid, such a good kid.” During this visit, he invited his wife into the exam room, presumably to meet Ms. Jones.
It was at this moment that Ms. Jones had a revelation: Could her gynecologist actually be her biological father?
In May 2021, Ms. Jones and a half brother with whom she had been in touch contacted the gynecologist’s daughter from his first marriage. All three underwent genetic testing. The results showed a 99.99% chance of a genetic link.
Ms. Jones has said in her suit that “no reasonable woman” would have submitted to pelvic examinations and other examinations by a doctor whom she knew to be her father.
Besides fraud, her suit alleges medical malpractice, battery, infliction of mental distress, and lack of informed consent. She is seeking compensation for all harm caused to her, including past and future economic damages, past unreimbursed medical expenses, and future expenses related to her mental health treatment and care.
The story included no further details about the civil litigation. As for criminal charges, it’s unlikely Ms. Jones’s biological father — her gynecologist — will face criminal charges for his alleged crimes because they fall outside of the state’s statute of limitations.
Parents say daughter’s stroke wasn’t identified
The Georgia parents of a young woman who died from a stroke following a series of alleged misdiagnoses are suing multiple practitioners, reports Legal Newswire and other news outlets
In June 2019, Michaela Smith was training for her job as a detention officer when she began experiencing a variety of symptoms, including headache, shortness of breath, throat swelling, and slurred speech. She was taken to the emergency department (ED) at Hamilton Medical Center, in Dalton, Ga.
There, she was examined by an attending ED doctor, who ordered a CT scan. The results were read by radiologist Michael J. Cooney, MD. In his reading, the Smith family’s lawsuit alleges, Dr. Cooney failed to identify the basilar artery sign, which is a key indicator of a vessel occlusion in stroke patients. Dr. Cooney concluded that Ms. Smith’s scan showed no acute intracranial abnormality. He sent her home without further discharge instructions.
At home, Ms. Smith fell asleep but awoke in an altered mental state, one of several classic stroke symptoms that she had been experiencing. She returned to the ED. This time, she was examined by David F. Hawkins, MD, an ED physician. Although his differential diagnosis identified Ms. Smith’s symptoms as most likely stroke related, Dr. Hawkins allegedly failed to immediately corroborate his findings with additional vascular imaging. Later in the day, Ms. Smith did undergo an MRI, which a second radiologist, Kevin F. Johnson, MD, misread as showing no signs of ischemia in her basilar artery, according to the lawsuit.
That same day, Dr. Hawkins conferred with a second neurologist, Jeffrey T. Glass, MD, who recommended that Ms. Smith be admitted to the hospital because of her deteriorating condition. The Smiths’ suit claims that Dr. Glass also failed to diagnosis their daughter’s underlying condition, although he did sign off on her transfer to Baroness Erlanger Hospital, in Chattanooga, Tenn.
There, Ms. Smith’s condition continued to worsen. She soon required mechanical ventilation and tube feeding. On July 3, 2019, she was pronounced dead.
“This is an egregious case of negligence,” said the attorney representing the Smiths, who are suing the physicians involved and their practices, as well as Hamilton Medical Center and several unnamed defendants.
“Although two radiology studies and her clinical presentation indicated that Michaela was having a catastrophic stroke, her doctors repeatedly misread the studies as normal, failed to diagnose the stroke, and failed to treat her deficits as a neurological emergency,” the family’s lawyer stated.
At press time, there had been no response from any of the defendants or their attorneys.
A version of this article first appeared on Medscape.com.
It’s difficult enough when a patient’s prostate is removed because of cancer. But it’s another thing altogether when the prostate is removed because of a medical error, as a report on 3 CBS Philly, among other news outlets, makes clear.
The patient, Eric Spangs, lives in southeastern Pennsylvania. Testing indicated an elevation in prostate-specific antigen (PSA) level. He subsequently underwent biopsy of the prostate, which appeared to indicate cancer. In time, though, Mr. Spangs learned there had been an error: the tissue section used in the microscopic diagnosis had come from the biopsy specimen of a different patient. Mr. Spangs himself didn’t actually have cancer.
Ordinarily, such news would be cause for celebration. But this was far from a normal situ ation: Following his initial cancer diagnosis, Mr. Spangs underwent a radical laparoscopic prostatectomy at a local hospital.
“It’s devastated me emotionally and physically,” Mr. Spangs said. It has also been emotionally devastating for his wife, Melissa. (The couple has five children.)
Their attorney, Aaron Freiwald, has filed a suit against the health system to which the local hospital belongs and the area’s largest urologic practice.
The Spangs wish to caution other patients not to make the same mistake they did: they failed to get a second opinion from an oncology specialist, as recommended by the American Cancer Society. (Eric Spangs did receive a second opinion from someone at the urologic practice, but that practice doesn’t specialize in oncology.)
The Spangs also worry about the patient who received the false-negative biopsy result. They have been assured, however, that that patient will be properly notified of his actual cancer status.
Fertility specialist uses own sperm to impregnate patients
A suit claims that a Rochester, N.Y., gynecologist and fertility specialist used his own sperm to inseminate multiple patients, according to a story reported by the Associated Press and other news outlets.
The suit was filed last month by the daughter — call her “Harriet Jones” — of one of the women who received fertility services from the doctor during the 1980s. Ms. Jones’s suit alleges that at the time, the doctor told her mother that the sperm donor would be a medical student at the University of Rochester. In fact, the donor was the doctor himself. He kept that fact a secret for years, even after Ms. Jones — his own daughter — sought him out for gynecologic services.
The secret gradually began to come to light in 2016, when Ms. Jones’s nonbiological father — the man who had helped to raise her — died. Curious about her biological father, Ms. Jones sought to learn his identity from the Rochester gynecologist who had treated her mother and was now her own gynecologist. The doctor said he couldn’t be of help; he claimed he hadn’t kept the relevant records.
Ms. Jones then submitted a blood sample to a direct-to-consumer genetic testing company. The results surprised her: Not only did she learn of her ethnicity, but she also discovered the existence of two half siblings, who were donor-conceived in 1984 and 1985, respectively, the very period when her own mother was undergoing insemination procedures. Ms. Jones subsequently discovered the existence of additional half siblings, all born in the first half of the 1980s.
Initially elated by the discoveries, Ms. Jones soon grew despondent and anxious. She suffered from migraine headaches, among other symptoms. Her biological father, it seemed, had been “a serial sperm donor.”
Still, she continued to go to her Rochester doctor for treatment, having no reason to suspect anything untoward about him. Her visits, including those for prolonged menstrual bleeding, involved routine breast and pelvic exams, transvaginal ultrasounds, and intrauterine contraceptive placements under sedation.
Over this period, her doctor was friendly, asking her a variety of questions about her personal life. During one especially strange visit, however, he began chuckling and said, “You’re a really good kid, such a good kid.” During this visit, he invited his wife into the exam room, presumably to meet Ms. Jones.
It was at this moment that Ms. Jones had a revelation: Could her gynecologist actually be her biological father?
In May 2021, Ms. Jones and a half brother with whom she had been in touch contacted the gynecologist’s daughter from his first marriage. All three underwent genetic testing. The results showed a 99.99% chance of a genetic link.
Ms. Jones has said in her suit that “no reasonable woman” would have submitted to pelvic examinations and other examinations by a doctor whom she knew to be her father.
Besides fraud, her suit alleges medical malpractice, battery, infliction of mental distress, and lack of informed consent. She is seeking compensation for all harm caused to her, including past and future economic damages, past unreimbursed medical expenses, and future expenses related to her mental health treatment and care.
The story included no further details about the civil litigation. As for criminal charges, it’s unlikely Ms. Jones’s biological father — her gynecologist — will face criminal charges for his alleged crimes because they fall outside of the state’s statute of limitations.
Parents say daughter’s stroke wasn’t identified
The Georgia parents of a young woman who died from a stroke following a series of alleged misdiagnoses are suing multiple practitioners, reports Legal Newswire and other news outlets
In June 2019, Michaela Smith was training for her job as a detention officer when she began experiencing a variety of symptoms, including headache, shortness of breath, throat swelling, and slurred speech. She was taken to the emergency department (ED) at Hamilton Medical Center, in Dalton, Ga.
There, she was examined by an attending ED doctor, who ordered a CT scan. The results were read by radiologist Michael J. Cooney, MD. In his reading, the Smith family’s lawsuit alleges, Dr. Cooney failed to identify the basilar artery sign, which is a key indicator of a vessel occlusion in stroke patients. Dr. Cooney concluded that Ms. Smith’s scan showed no acute intracranial abnormality. He sent her home without further discharge instructions.
At home, Ms. Smith fell asleep but awoke in an altered mental state, one of several classic stroke symptoms that she had been experiencing. She returned to the ED. This time, she was examined by David F. Hawkins, MD, an ED physician. Although his differential diagnosis identified Ms. Smith’s symptoms as most likely stroke related, Dr. Hawkins allegedly failed to immediately corroborate his findings with additional vascular imaging. Later in the day, Ms. Smith did undergo an MRI, which a second radiologist, Kevin F. Johnson, MD, misread as showing no signs of ischemia in her basilar artery, according to the lawsuit.
That same day, Dr. Hawkins conferred with a second neurologist, Jeffrey T. Glass, MD, who recommended that Ms. Smith be admitted to the hospital because of her deteriorating condition. The Smiths’ suit claims that Dr. Glass also failed to diagnosis their daughter’s underlying condition, although he did sign off on her transfer to Baroness Erlanger Hospital, in Chattanooga, Tenn.
There, Ms. Smith’s condition continued to worsen. She soon required mechanical ventilation and tube feeding. On July 3, 2019, she was pronounced dead.
“This is an egregious case of negligence,” said the attorney representing the Smiths, who are suing the physicians involved and their practices, as well as Hamilton Medical Center and several unnamed defendants.
“Although two radiology studies and her clinical presentation indicated that Michaela was having a catastrophic stroke, her doctors repeatedly misread the studies as normal, failed to diagnose the stroke, and failed to treat her deficits as a neurological emergency,” the family’s lawyer stated.
At press time, there had been no response from any of the defendants or their attorneys.
A version of this article first appeared on Medscape.com.
It’s difficult enough when a patient’s prostate is removed because of cancer. But it’s another thing altogether when the prostate is removed because of a medical error, as a report on 3 CBS Philly, among other news outlets, makes clear.
The patient, Eric Spangs, lives in southeastern Pennsylvania. Testing indicated an elevation in prostate-specific antigen (PSA) level. He subsequently underwent biopsy of the prostate, which appeared to indicate cancer. In time, though, Mr. Spangs learned there had been an error: the tissue section used in the microscopic diagnosis had come from the biopsy specimen of a different patient. Mr. Spangs himself didn’t actually have cancer.
Ordinarily, such news would be cause for celebration. But this was far from a normal situ ation: Following his initial cancer diagnosis, Mr. Spangs underwent a radical laparoscopic prostatectomy at a local hospital.
“It’s devastated me emotionally and physically,” Mr. Spangs said. It has also been emotionally devastating for his wife, Melissa. (The couple has five children.)
Their attorney, Aaron Freiwald, has filed a suit against the health system to which the local hospital belongs and the area’s largest urologic practice.
The Spangs wish to caution other patients not to make the same mistake they did: they failed to get a second opinion from an oncology specialist, as recommended by the American Cancer Society. (Eric Spangs did receive a second opinion from someone at the urologic practice, but that practice doesn’t specialize in oncology.)
The Spangs also worry about the patient who received the false-negative biopsy result. They have been assured, however, that that patient will be properly notified of his actual cancer status.
Fertility specialist uses own sperm to impregnate patients
A suit claims that a Rochester, N.Y., gynecologist and fertility specialist used his own sperm to inseminate multiple patients, according to a story reported by the Associated Press and other news outlets.
The suit was filed last month by the daughter — call her “Harriet Jones” — of one of the women who received fertility services from the doctor during the 1980s. Ms. Jones’s suit alleges that at the time, the doctor told her mother that the sperm donor would be a medical student at the University of Rochester. In fact, the donor was the doctor himself. He kept that fact a secret for years, even after Ms. Jones — his own daughter — sought him out for gynecologic services.
The secret gradually began to come to light in 2016, when Ms. Jones’s nonbiological father — the man who had helped to raise her — died. Curious about her biological father, Ms. Jones sought to learn his identity from the Rochester gynecologist who had treated her mother and was now her own gynecologist. The doctor said he couldn’t be of help; he claimed he hadn’t kept the relevant records.
Ms. Jones then submitted a blood sample to a direct-to-consumer genetic testing company. The results surprised her: Not only did she learn of her ethnicity, but she also discovered the existence of two half siblings, who were donor-conceived in 1984 and 1985, respectively, the very period when her own mother was undergoing insemination procedures. Ms. Jones subsequently discovered the existence of additional half siblings, all born in the first half of the 1980s.
Initially elated by the discoveries, Ms. Jones soon grew despondent and anxious. She suffered from migraine headaches, among other symptoms. Her biological father, it seemed, had been “a serial sperm donor.”
Still, she continued to go to her Rochester doctor for treatment, having no reason to suspect anything untoward about him. Her visits, including those for prolonged menstrual bleeding, involved routine breast and pelvic exams, transvaginal ultrasounds, and intrauterine contraceptive placements under sedation.
Over this period, her doctor was friendly, asking her a variety of questions about her personal life. During one especially strange visit, however, he began chuckling and said, “You’re a really good kid, such a good kid.” During this visit, he invited his wife into the exam room, presumably to meet Ms. Jones.
It was at this moment that Ms. Jones had a revelation: Could her gynecologist actually be her biological father?
In May 2021, Ms. Jones and a half brother with whom she had been in touch contacted the gynecologist’s daughter from his first marriage. All three underwent genetic testing. The results showed a 99.99% chance of a genetic link.
Ms. Jones has said in her suit that “no reasonable woman” would have submitted to pelvic examinations and other examinations by a doctor whom she knew to be her father.
Besides fraud, her suit alleges medical malpractice, battery, infliction of mental distress, and lack of informed consent. She is seeking compensation for all harm caused to her, including past and future economic damages, past unreimbursed medical expenses, and future expenses related to her mental health treatment and care.
The story included no further details about the civil litigation. As for criminal charges, it’s unlikely Ms. Jones’s biological father — her gynecologist — will face criminal charges for his alleged crimes because they fall outside of the state’s statute of limitations.
Parents say daughter’s stroke wasn’t identified
The Georgia parents of a young woman who died from a stroke following a series of alleged misdiagnoses are suing multiple practitioners, reports Legal Newswire and other news outlets
In June 2019, Michaela Smith was training for her job as a detention officer when she began experiencing a variety of symptoms, including headache, shortness of breath, throat swelling, and slurred speech. She was taken to the emergency department (ED) at Hamilton Medical Center, in Dalton, Ga.
There, she was examined by an attending ED doctor, who ordered a CT scan. The results were read by radiologist Michael J. Cooney, MD. In his reading, the Smith family’s lawsuit alleges, Dr. Cooney failed to identify the basilar artery sign, which is a key indicator of a vessel occlusion in stroke patients. Dr. Cooney concluded that Ms. Smith’s scan showed no acute intracranial abnormality. He sent her home without further discharge instructions.
At home, Ms. Smith fell asleep but awoke in an altered mental state, one of several classic stroke symptoms that she had been experiencing. She returned to the ED. This time, she was examined by David F. Hawkins, MD, an ED physician. Although his differential diagnosis identified Ms. Smith’s symptoms as most likely stroke related, Dr. Hawkins allegedly failed to immediately corroborate his findings with additional vascular imaging. Later in the day, Ms. Smith did undergo an MRI, which a second radiologist, Kevin F. Johnson, MD, misread as showing no signs of ischemia in her basilar artery, according to the lawsuit.
That same day, Dr. Hawkins conferred with a second neurologist, Jeffrey T. Glass, MD, who recommended that Ms. Smith be admitted to the hospital because of her deteriorating condition. The Smiths’ suit claims that Dr. Glass also failed to diagnosis their daughter’s underlying condition, although he did sign off on her transfer to Baroness Erlanger Hospital, in Chattanooga, Tenn.
There, Ms. Smith’s condition continued to worsen. She soon required mechanical ventilation and tube feeding. On July 3, 2019, she was pronounced dead.
“This is an egregious case of negligence,” said the attorney representing the Smiths, who are suing the physicians involved and their practices, as well as Hamilton Medical Center and several unnamed defendants.
“Although two radiology studies and her clinical presentation indicated that Michaela was having a catastrophic stroke, her doctors repeatedly misread the studies as normal, failed to diagnose the stroke, and failed to treat her deficits as a neurological emergency,” the family’s lawyer stated.
At press time, there had been no response from any of the defendants or their attorneys.
A version of this article first appeared on Medscape.com.
AHA: Quality of STEMI care has stalled, needs improvement
Following up on its 2007 initiative to improve care for people who have ST-segment elevation myocardial infarction (STEMI), the American Heart Association has issued a policy statement that includes a host of recommendations to further overcome barriers to optimal care for this most severe type of heart attack.
The statement recommends steps for designing what the writing committee calls “the ideal STEMI system of care” for patients who have these severe heart attacks.
The focus of the policy statement is the AHA’s Mission: Lifeline national initiative to coordinate and improve the quality of care to patients with STEMI, which was introduced in 2007. Since then, the number or participating hospitals has increased from 485 to 857, now covering more than 85% of the U.S. population, noted the new statement, published online in Circulation.
“Bringing STEMI referring hospitals, STEMI receiving centers and emergency medical services [EMS] together in the development of local and regional systems of care within the AHA’s Mission: Lifeline program has led to significant improvement in time to treatment and outcomes for patients with STEMI,” Alice K. Jacobs, MD, lead statement author and vice chair for clinical affairs in the department of medicine at Boston Medical Center and a professor at Boston University, said in an interview.
“Yet,” Dr. Jacobs added, “opportunities exist to further improve the coordination of care and address remaining barriers to providing ideal care. Moreover, Mission: Lifeline systems of care have been extended to other time-sensitive cardiovascular disorders including stroke and out-of-hospital cardiac arrest.”
The statement itself noted, “Although there have been significant improvements in patients with STEMI receiving guideline-recommended care, progress has slowed during the past few years.” From 2008 to 2012, a number of key quality care measures at participating hospitals had improved markedly. For example, door-in-door-out (DIDO) transfers improved from a median of 76 to 62 minutes (P < .001).
However, from 2012 to 2019, with more hospitals participating, while many key measures improved, a few either plateaued or worsened slightly. Median DIDO time, for example – again, with more hospitals participating, compared with the earlier dataset – went from 45 in 2012 to 48 in 2019, according to AHA data.
Key recommendations aim to impact and improve hospital care for patients with STEMI, Dr. Jacobs said. “In addition to avoiding patient delay at the onset of recognized symptoms of a heart attack, accessing 911 and following EMS destination protocols, the prehospital activation of the cardiac catheterization lab and providing a 911 response for interhospital transport as well as direct-to-cardiac-catheterization-lab transport bypassing the emergency department when appropriate would all impact and improve hospital care.”
Other key recommendations of the statement include:
- Increasing public awareness of heart attack signs and symptoms and the importance for calling 911.
- Addressing post-MI care, including use of evidence-based practices for cardiac rehabilitation and even getting insurance companies to encourage cardiac rehab through incentives.
- Engaging rural hospitals by leveraging telemedicine to expedite percutaneous coronary intervention (PCI) and by developing systems for treatment protocols and rapid transport among facilities.
- Tearing down financial barriers with a global reimbursement model that encompasses each stop in a patient’s journey through the care system: the referring hospital, receiving center, EMS transport and transfer, and ancillary services.
The statement also took into account improving disparities in the quality of care women with STEMI receive. “It has been reported that women with STEMI may have less typical symptoms than men and arrive later [delay longer] than men after symptom onset,” Dr. Jacobs said. “Educating the public and all members of the health-care team about issues specific to women will be helpful in improving care in women. Of note, STEMI systems of care have been shown to reduce sex and age disparities in care.”
The statement also addressed implications of the COVID-19 pandemic, stating that PCI should remain the dominant treatment for patients with classic STEMI. “Patients must be reassured that appropriate precautions have been implemented by EMS and hospital to protect them and health care workers from COVID-19 infection,” the statement noted.
Dr. Jacobs has no relevant relationships to disclose.
Following up on its 2007 initiative to improve care for people who have ST-segment elevation myocardial infarction (STEMI), the American Heart Association has issued a policy statement that includes a host of recommendations to further overcome barriers to optimal care for this most severe type of heart attack.
The statement recommends steps for designing what the writing committee calls “the ideal STEMI system of care” for patients who have these severe heart attacks.
The focus of the policy statement is the AHA’s Mission: Lifeline national initiative to coordinate and improve the quality of care to patients with STEMI, which was introduced in 2007. Since then, the number or participating hospitals has increased from 485 to 857, now covering more than 85% of the U.S. population, noted the new statement, published online in Circulation.
“Bringing STEMI referring hospitals, STEMI receiving centers and emergency medical services [EMS] together in the development of local and regional systems of care within the AHA’s Mission: Lifeline program has led to significant improvement in time to treatment and outcomes for patients with STEMI,” Alice K. Jacobs, MD, lead statement author and vice chair for clinical affairs in the department of medicine at Boston Medical Center and a professor at Boston University, said in an interview.
“Yet,” Dr. Jacobs added, “opportunities exist to further improve the coordination of care and address remaining barriers to providing ideal care. Moreover, Mission: Lifeline systems of care have been extended to other time-sensitive cardiovascular disorders including stroke and out-of-hospital cardiac arrest.”
The statement itself noted, “Although there have been significant improvements in patients with STEMI receiving guideline-recommended care, progress has slowed during the past few years.” From 2008 to 2012, a number of key quality care measures at participating hospitals had improved markedly. For example, door-in-door-out (DIDO) transfers improved from a median of 76 to 62 minutes (P < .001).
However, from 2012 to 2019, with more hospitals participating, while many key measures improved, a few either plateaued or worsened slightly. Median DIDO time, for example – again, with more hospitals participating, compared with the earlier dataset – went from 45 in 2012 to 48 in 2019, according to AHA data.
Key recommendations aim to impact and improve hospital care for patients with STEMI, Dr. Jacobs said. “In addition to avoiding patient delay at the onset of recognized symptoms of a heart attack, accessing 911 and following EMS destination protocols, the prehospital activation of the cardiac catheterization lab and providing a 911 response for interhospital transport as well as direct-to-cardiac-catheterization-lab transport bypassing the emergency department when appropriate would all impact and improve hospital care.”
Other key recommendations of the statement include:
- Increasing public awareness of heart attack signs and symptoms and the importance for calling 911.
- Addressing post-MI care, including use of evidence-based practices for cardiac rehabilitation and even getting insurance companies to encourage cardiac rehab through incentives.
- Engaging rural hospitals by leveraging telemedicine to expedite percutaneous coronary intervention (PCI) and by developing systems for treatment protocols and rapid transport among facilities.
- Tearing down financial barriers with a global reimbursement model that encompasses each stop in a patient’s journey through the care system: the referring hospital, receiving center, EMS transport and transfer, and ancillary services.
The statement also took into account improving disparities in the quality of care women with STEMI receive. “It has been reported that women with STEMI may have less typical symptoms than men and arrive later [delay longer] than men after symptom onset,” Dr. Jacobs said. “Educating the public and all members of the health-care team about issues specific to women will be helpful in improving care in women. Of note, STEMI systems of care have been shown to reduce sex and age disparities in care.”
The statement also addressed implications of the COVID-19 pandemic, stating that PCI should remain the dominant treatment for patients with classic STEMI. “Patients must be reassured that appropriate precautions have been implemented by EMS and hospital to protect them and health care workers from COVID-19 infection,” the statement noted.
Dr. Jacobs has no relevant relationships to disclose.
Following up on its 2007 initiative to improve care for people who have ST-segment elevation myocardial infarction (STEMI), the American Heart Association has issued a policy statement that includes a host of recommendations to further overcome barriers to optimal care for this most severe type of heart attack.
The statement recommends steps for designing what the writing committee calls “the ideal STEMI system of care” for patients who have these severe heart attacks.
The focus of the policy statement is the AHA’s Mission: Lifeline national initiative to coordinate and improve the quality of care to patients with STEMI, which was introduced in 2007. Since then, the number or participating hospitals has increased from 485 to 857, now covering more than 85% of the U.S. population, noted the new statement, published online in Circulation.
“Bringing STEMI referring hospitals, STEMI receiving centers and emergency medical services [EMS] together in the development of local and regional systems of care within the AHA’s Mission: Lifeline program has led to significant improvement in time to treatment and outcomes for patients with STEMI,” Alice K. Jacobs, MD, lead statement author and vice chair for clinical affairs in the department of medicine at Boston Medical Center and a professor at Boston University, said in an interview.
“Yet,” Dr. Jacobs added, “opportunities exist to further improve the coordination of care and address remaining barriers to providing ideal care. Moreover, Mission: Lifeline systems of care have been extended to other time-sensitive cardiovascular disorders including stroke and out-of-hospital cardiac arrest.”
The statement itself noted, “Although there have been significant improvements in patients with STEMI receiving guideline-recommended care, progress has slowed during the past few years.” From 2008 to 2012, a number of key quality care measures at participating hospitals had improved markedly. For example, door-in-door-out (DIDO) transfers improved from a median of 76 to 62 minutes (P < .001).
However, from 2012 to 2019, with more hospitals participating, while many key measures improved, a few either plateaued or worsened slightly. Median DIDO time, for example – again, with more hospitals participating, compared with the earlier dataset – went from 45 in 2012 to 48 in 2019, according to AHA data.
Key recommendations aim to impact and improve hospital care for patients with STEMI, Dr. Jacobs said. “In addition to avoiding patient delay at the onset of recognized symptoms of a heart attack, accessing 911 and following EMS destination protocols, the prehospital activation of the cardiac catheterization lab and providing a 911 response for interhospital transport as well as direct-to-cardiac-catheterization-lab transport bypassing the emergency department when appropriate would all impact and improve hospital care.”
Other key recommendations of the statement include:
- Increasing public awareness of heart attack signs and symptoms and the importance for calling 911.
- Addressing post-MI care, including use of evidence-based practices for cardiac rehabilitation and even getting insurance companies to encourage cardiac rehab through incentives.
- Engaging rural hospitals by leveraging telemedicine to expedite percutaneous coronary intervention (PCI) and by developing systems for treatment protocols and rapid transport among facilities.
- Tearing down financial barriers with a global reimbursement model that encompasses each stop in a patient’s journey through the care system: the referring hospital, receiving center, EMS transport and transfer, and ancillary services.
The statement also took into account improving disparities in the quality of care women with STEMI receive. “It has been reported that women with STEMI may have less typical symptoms than men and arrive later [delay longer] than men after symptom onset,” Dr. Jacobs said. “Educating the public and all members of the health-care team about issues specific to women will be helpful in improving care in women. Of note, STEMI systems of care have been shown to reduce sex and age disparities in care.”
The statement also addressed implications of the COVID-19 pandemic, stating that PCI should remain the dominant treatment for patients with classic STEMI. “Patients must be reassured that appropriate precautions have been implemented by EMS and hospital to protect them and health care workers from COVID-19 infection,” the statement noted.
Dr. Jacobs has no relevant relationships to disclose.
FROM CIRCULATION
FDA OKs new high-dose naloxone product for opioid overdose
ZIMHI from Adamis Pharmaceuticals is administered using a single-dose, prefilled syringe that delivers 5 mg of naloxone hydrochloride solution through intramuscular or subcutaneous injection.
Naloxone is an opioid antagonist that works by blocking or reversing the effects of the opioid, including extreme drowsiness, slowed breathing, or loss of consciousness.
Opioid-related overdose deaths — driven partly by prescription drug overdoses — remain a leading cause of death in the United States.
ZIMHI “provides an additional option in the treatment of opioid overdoses,” the FDA said in a statement announcing approval.
In a statement from Adamis Pharmaceuticals, Jeffrey Galinkin, MD, an anesthesiologist and former member of the FDA advisory committee for analgesics and addiction products, said he is “pleased to see this much-needed, high-dose naloxone product will become part of the treatment tool kit as a countermeasure to the continued surge in fentanyl related deaths.”
“The higher intramuscular doses of naloxone in ZIMHI should result in more rapid and higher levels of naloxone in the systemic circulation, which in turn, should result in more successful resuscitations,” Dr. Galinkin said.
Last spring the FDA approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of opioid overdose.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
The FDA approved ZIMHI (and Kloxxado) through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company plans to launch ZIMHI in the first quarter of 2022.
A version of this article first appeared on Medscape.com.
ZIMHI from Adamis Pharmaceuticals is administered using a single-dose, prefilled syringe that delivers 5 mg of naloxone hydrochloride solution through intramuscular or subcutaneous injection.
Naloxone is an opioid antagonist that works by blocking or reversing the effects of the opioid, including extreme drowsiness, slowed breathing, or loss of consciousness.
Opioid-related overdose deaths — driven partly by prescription drug overdoses — remain a leading cause of death in the United States.
ZIMHI “provides an additional option in the treatment of opioid overdoses,” the FDA said in a statement announcing approval.
In a statement from Adamis Pharmaceuticals, Jeffrey Galinkin, MD, an anesthesiologist and former member of the FDA advisory committee for analgesics and addiction products, said he is “pleased to see this much-needed, high-dose naloxone product will become part of the treatment tool kit as a countermeasure to the continued surge in fentanyl related deaths.”
“The higher intramuscular doses of naloxone in ZIMHI should result in more rapid and higher levels of naloxone in the systemic circulation, which in turn, should result in more successful resuscitations,” Dr. Galinkin said.
Last spring the FDA approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of opioid overdose.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
The FDA approved ZIMHI (and Kloxxado) through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company plans to launch ZIMHI in the first quarter of 2022.
A version of this article first appeared on Medscape.com.
ZIMHI from Adamis Pharmaceuticals is administered using a single-dose, prefilled syringe that delivers 5 mg of naloxone hydrochloride solution through intramuscular or subcutaneous injection.
Naloxone is an opioid antagonist that works by blocking or reversing the effects of the opioid, including extreme drowsiness, slowed breathing, or loss of consciousness.
Opioid-related overdose deaths — driven partly by prescription drug overdoses — remain a leading cause of death in the United States.
ZIMHI “provides an additional option in the treatment of opioid overdoses,” the FDA said in a statement announcing approval.
In a statement from Adamis Pharmaceuticals, Jeffrey Galinkin, MD, an anesthesiologist and former member of the FDA advisory committee for analgesics and addiction products, said he is “pleased to see this much-needed, high-dose naloxone product will become part of the treatment tool kit as a countermeasure to the continued surge in fentanyl related deaths.”
“The higher intramuscular doses of naloxone in ZIMHI should result in more rapid and higher levels of naloxone in the systemic circulation, which in turn, should result in more successful resuscitations,” Dr. Galinkin said.
Last spring the FDA approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of opioid overdose.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
The FDA approved ZIMHI (and Kloxxado) through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company plans to launch ZIMHI in the first quarter of 2022.
A version of this article first appeared on Medscape.com.