User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Alcohol ups risk for atrial fibrillation episode hours later
Consuming alcohol increases the risk for an atrial fibrillation (AF) episode hours later, according to a study published online Aug. 30 in Annals of Internal Medicine.
Past research has associated long-term alcohol consumption with the development of AF, and abstinence from alcohol has been associated with a lower overall AF burden. However, lead study author Greg Marcus, MD, a cardioelectrophysiolgist at the University of California, San Francisco, noted that many patients say that alcohol is a trigger for discrete AF episodes.
To test whether that was possible, the researchers enrolled 100 patients who had a history of AF events and who consumed at least one drink per month. Participants wore a transdermal alcohol sensor and an ambulatory, single-lead electrocardiogram device for 4 weeks. They were instructed to press a button on the electrocardiogram device each time they consumed a standard alcoholic beverage. In addition, blood samples were tested for phosphatidylethanol (PEth) at the participants’ 2-week and 4-week visits. PEth is a phospholipid formed in the blood after alcohol intake. It remains in the blood for up to 4 weeks after alcohol consumption.
The study findings confirmed what the patients had reported. The odds of an AF episode were 38% greater with every 0.1% increase in peak blood alcohol concentration over the previous 12 hours (odds ratio [OR], 1.38; 95% confidence interval, 1.04-1.83; P = .024). Moreover, an episode of AF was associated with twofold greater odds (OR, 2.02; 95% CI, 1.38-3.17) of having consumed one alcoholic drink in the past 4 hours. It was associated with more than threefold greater odds of having consumed two or more drinks (OR, 3.58; 95% CI, 1.63-7.89).
“The major takeaway is, among atrial fibrillation patients, consuming alcohol substantially heightened their risk for any given atrial fibrillation event in the subsequent few hours,” Dr. Marcus said. “The more alcohol consumed, the higher that risk.”
The acute effect of alcohol on these arrhythmias also means that modifying alcohol consumption could immediately benefit some patients. “These data combined with other evidence suggest that recommending minimizing or completely eliminating alcohol will likely be helpful to them,” Dr. Marcus said.
The study’s reliance on wearables and sensors was impressive, said Mariann R. Piano, PhD, director of the Center for Research Development and Scholarship, Vanderbilt University, Nashville, Tenn. Often, these types of studies are “self-reported and confounded by recall bias,” she said. But this study passively documented arrhythmia events and blood alcohol level without any patient input. The additional measures of alcohol consumption were used to validate the blood alcohol sensor.
The study’s focus on patients with a history of AF highlighted a high-risk patient group, according to Dr. Piano, who coauthored an editorial about the study. However, the findings may not be applicable to the general population.
Dr. Marcus said alcohol’s role in causing these types of arrhythmias is probably a matter of degree. AF patients are more prone to events than is the general population and are therefore more sensitive to alcohol, he said. But excessive alcohol consumption could increase the chance of AF in the general population.
The study is not without its limitations, however. For instance, “it would have been really ideal if we knew what that blood alcohol was” before an episode, Dr. Piano said. The number of drinks is a good start, but two drinks can affect persons differently, depending on their weight and height. Also, baseline PEth values suggest that patients had been drinking before the study, she said. Ideally, patients could have been asked to abstain from alcohol for a period before the study to determine a negative baseline PEth value and minimize the effects of previous drinking on AF episodes.
Moving forward, this research should inform how clinicians care for their AF patients, both experts agree. “We need to talk to patients about how much they drink,” Dr. Piano said. In addition, patients should be advised to closely monitor what they’re drinking.
“This definitely sharpens the focus of the importance of a thorough alcohol history when we see an atrial fibrillation patient and to counsel them to reduce or eliminate alcohol, even among those that don’t have alcohol use disorders,” Dr. Marcus said.
Preliminary results of the study were presented as a late-breaking clinical trials presentation at the American College of Cardiology meeting in May.
Dr. Marcus has received grants from Baylis, Jawbone, and Eight Sleep and has received personal fees from InCarda and Johnson & Johnson. Coauthors have received personal fees from VivaLNK, Huba Pharmaceuticals, Johnson & Johnson, and Merck and grants from Samsung and Amgen Inc. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Consuming alcohol increases the risk for an atrial fibrillation (AF) episode hours later, according to a study published online Aug. 30 in Annals of Internal Medicine.
Past research has associated long-term alcohol consumption with the development of AF, and abstinence from alcohol has been associated with a lower overall AF burden. However, lead study author Greg Marcus, MD, a cardioelectrophysiolgist at the University of California, San Francisco, noted that many patients say that alcohol is a trigger for discrete AF episodes.
To test whether that was possible, the researchers enrolled 100 patients who had a history of AF events and who consumed at least one drink per month. Participants wore a transdermal alcohol sensor and an ambulatory, single-lead electrocardiogram device for 4 weeks. They were instructed to press a button on the electrocardiogram device each time they consumed a standard alcoholic beverage. In addition, blood samples were tested for phosphatidylethanol (PEth) at the participants’ 2-week and 4-week visits. PEth is a phospholipid formed in the blood after alcohol intake. It remains in the blood for up to 4 weeks after alcohol consumption.
The study findings confirmed what the patients had reported. The odds of an AF episode were 38% greater with every 0.1% increase in peak blood alcohol concentration over the previous 12 hours (odds ratio [OR], 1.38; 95% confidence interval, 1.04-1.83; P = .024). Moreover, an episode of AF was associated with twofold greater odds (OR, 2.02; 95% CI, 1.38-3.17) of having consumed one alcoholic drink in the past 4 hours. It was associated with more than threefold greater odds of having consumed two or more drinks (OR, 3.58; 95% CI, 1.63-7.89).
“The major takeaway is, among atrial fibrillation patients, consuming alcohol substantially heightened their risk for any given atrial fibrillation event in the subsequent few hours,” Dr. Marcus said. “The more alcohol consumed, the higher that risk.”
The acute effect of alcohol on these arrhythmias also means that modifying alcohol consumption could immediately benefit some patients. “These data combined with other evidence suggest that recommending minimizing or completely eliminating alcohol will likely be helpful to them,” Dr. Marcus said.
The study’s reliance on wearables and sensors was impressive, said Mariann R. Piano, PhD, director of the Center for Research Development and Scholarship, Vanderbilt University, Nashville, Tenn. Often, these types of studies are “self-reported and confounded by recall bias,” she said. But this study passively documented arrhythmia events and blood alcohol level without any patient input. The additional measures of alcohol consumption were used to validate the blood alcohol sensor.
The study’s focus on patients with a history of AF highlighted a high-risk patient group, according to Dr. Piano, who coauthored an editorial about the study. However, the findings may not be applicable to the general population.
Dr. Marcus said alcohol’s role in causing these types of arrhythmias is probably a matter of degree. AF patients are more prone to events than is the general population and are therefore more sensitive to alcohol, he said. But excessive alcohol consumption could increase the chance of AF in the general population.
The study is not without its limitations, however. For instance, “it would have been really ideal if we knew what that blood alcohol was” before an episode, Dr. Piano said. The number of drinks is a good start, but two drinks can affect persons differently, depending on their weight and height. Also, baseline PEth values suggest that patients had been drinking before the study, she said. Ideally, patients could have been asked to abstain from alcohol for a period before the study to determine a negative baseline PEth value and minimize the effects of previous drinking on AF episodes.
Moving forward, this research should inform how clinicians care for their AF patients, both experts agree. “We need to talk to patients about how much they drink,” Dr. Piano said. In addition, patients should be advised to closely monitor what they’re drinking.
“This definitely sharpens the focus of the importance of a thorough alcohol history when we see an atrial fibrillation patient and to counsel them to reduce or eliminate alcohol, even among those that don’t have alcohol use disorders,” Dr. Marcus said.
Preliminary results of the study were presented as a late-breaking clinical trials presentation at the American College of Cardiology meeting in May.
Dr. Marcus has received grants from Baylis, Jawbone, and Eight Sleep and has received personal fees from InCarda and Johnson & Johnson. Coauthors have received personal fees from VivaLNK, Huba Pharmaceuticals, Johnson & Johnson, and Merck and grants from Samsung and Amgen Inc. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Consuming alcohol increases the risk for an atrial fibrillation (AF) episode hours later, according to a study published online Aug. 30 in Annals of Internal Medicine.
Past research has associated long-term alcohol consumption with the development of AF, and abstinence from alcohol has been associated with a lower overall AF burden. However, lead study author Greg Marcus, MD, a cardioelectrophysiolgist at the University of California, San Francisco, noted that many patients say that alcohol is a trigger for discrete AF episodes.
To test whether that was possible, the researchers enrolled 100 patients who had a history of AF events and who consumed at least one drink per month. Participants wore a transdermal alcohol sensor and an ambulatory, single-lead electrocardiogram device for 4 weeks. They were instructed to press a button on the electrocardiogram device each time they consumed a standard alcoholic beverage. In addition, blood samples were tested for phosphatidylethanol (PEth) at the participants’ 2-week and 4-week visits. PEth is a phospholipid formed in the blood after alcohol intake. It remains in the blood for up to 4 weeks after alcohol consumption.
The study findings confirmed what the patients had reported. The odds of an AF episode were 38% greater with every 0.1% increase in peak blood alcohol concentration over the previous 12 hours (odds ratio [OR], 1.38; 95% confidence interval, 1.04-1.83; P = .024). Moreover, an episode of AF was associated with twofold greater odds (OR, 2.02; 95% CI, 1.38-3.17) of having consumed one alcoholic drink in the past 4 hours. It was associated with more than threefold greater odds of having consumed two or more drinks (OR, 3.58; 95% CI, 1.63-7.89).
“The major takeaway is, among atrial fibrillation patients, consuming alcohol substantially heightened their risk for any given atrial fibrillation event in the subsequent few hours,” Dr. Marcus said. “The more alcohol consumed, the higher that risk.”
The acute effect of alcohol on these arrhythmias also means that modifying alcohol consumption could immediately benefit some patients. “These data combined with other evidence suggest that recommending minimizing or completely eliminating alcohol will likely be helpful to them,” Dr. Marcus said.
The study’s reliance on wearables and sensors was impressive, said Mariann R. Piano, PhD, director of the Center for Research Development and Scholarship, Vanderbilt University, Nashville, Tenn. Often, these types of studies are “self-reported and confounded by recall bias,” she said. But this study passively documented arrhythmia events and blood alcohol level without any patient input. The additional measures of alcohol consumption were used to validate the blood alcohol sensor.
The study’s focus on patients with a history of AF highlighted a high-risk patient group, according to Dr. Piano, who coauthored an editorial about the study. However, the findings may not be applicable to the general population.
Dr. Marcus said alcohol’s role in causing these types of arrhythmias is probably a matter of degree. AF patients are more prone to events than is the general population and are therefore more sensitive to alcohol, he said. But excessive alcohol consumption could increase the chance of AF in the general population.
The study is not without its limitations, however. For instance, “it would have been really ideal if we knew what that blood alcohol was” before an episode, Dr. Piano said. The number of drinks is a good start, but two drinks can affect persons differently, depending on their weight and height. Also, baseline PEth values suggest that patients had been drinking before the study, she said. Ideally, patients could have been asked to abstain from alcohol for a period before the study to determine a negative baseline PEth value and minimize the effects of previous drinking on AF episodes.
Moving forward, this research should inform how clinicians care for their AF patients, both experts agree. “We need to talk to patients about how much they drink,” Dr. Piano said. In addition, patients should be advised to closely monitor what they’re drinking.
“This definitely sharpens the focus of the importance of a thorough alcohol history when we see an atrial fibrillation patient and to counsel them to reduce or eliminate alcohol, even among those that don’t have alcohol use disorders,” Dr. Marcus said.
Preliminary results of the study were presented as a late-breaking clinical trials presentation at the American College of Cardiology meeting in May.
Dr. Marcus has received grants from Baylis, Jawbone, and Eight Sleep and has received personal fees from InCarda and Johnson & Johnson. Coauthors have received personal fees from VivaLNK, Huba Pharmaceuticals, Johnson & Johnson, and Merck and grants from Samsung and Amgen Inc. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Self-described ‘assassin,’ now doctor, indicted for 1M illegal opioid doses
A according to the U.S. Department of Justice. The substances include oxycodone and morphine.
Adrian Dexter Talbot, MD, 55, of Slidell, La., is also charged with maintaining a medical clinic for the purpose of illegally distributing controlled substances, per the indictment.
Because the opioid prescriptions were filled using beneficiaries’ health insurance, Dr. Talbot is also charged with defrauding Medicare, Medicaid, and Blue Cross and Blue Shield of Louisiana of more than $5.1 million.
When contacted by this news organization for comment on the case via Twitter, Dr. Talbot or a representative responded with a link to his self-published book on Amazon. In his author bio, Dr. Talbot refers to himself as “a former assassin,” “retired military commander,” and “leader of the Medellin Cartel’s New York operations at the age of 16.” The Medellin Cartel is a notorious drug distribution empire.
Dr. Talbot is listed as the author of another book on Google Books detailing his time as a “former teenage assassin” and leader of the cartel, told as he struggles with early onset Alzheimer’s.
Dr. Talbot could spend decades in prison
According to the National Institute on Drug Abuse, 444 residents of the Bayou State lost their lives because of an opioid-related drug overdose in 2018. During that year, the state’s health care providers wrote more than 79.4 opioid prescriptions for every 100 persons, which puts the state in the top five in the United States in 2018, when the average U.S. rate was 51.4 prescriptions per 100 persons.
Charged with one count each of conspiracy to unlawfully distribute and dispense controlled substances and maintaining drug-involved premises and conspiracy to commit health care fraud, Dr. Talbot is also charged with four counts of unlawfully distributing and dispensing controlled substances. He is scheduled for a federal court appearance on September 10.
In addition to presigning prescriptions for individuals he didn’t meet or examine, federal officials allege Dr. Talbot hired another health care provider to similarly presign prescriptions for people who weren’t examined at a medical practice in Slidell, where Dr. Talbot was employed. The DOJ says Dr. Talbot took a full-time job in Pineville, La., and presigned prescriptions while no longer physically present at the Slidell clinic.
A speaker’s bio for Dr. Talbot indicates he worked as chief of medical services for the Alexandria Veterans Affairs Health Care System in Pineville.
According to the DOJ’s indictment, Dr. Talbot was aware that patients were filling the prescriptions that were provided outside the scope of professional practice and not for a legitimate medical purpose. This is what triggered the DOJ’s fraudulent billing claim.
Dr. Talbot faces a maximum penalty of 10 years for conspiracy to commit health care fraud and 20 years each for the other counts, if convicted.
Dr. Talbot was candidate for local coroner
In February 2015, Dr. Talbot announced his candidacy for coroner for St. Tammany Parish, about an hour’s drive south of New Orleans, reported the Times Picayune. The seat was open because the previous coroner had resigned and ultimately pleaded guilty to a federal corruption charge.
The Times Picayune reported at the time that Dr. Talbot was a U.S. Navy veteran, in addition to serving as medical director and a primary care physician at the Medical Care Center in Slidell. Among the services provided to his patients were evaluations and treatment for substance use and mental health disorders, according to a press release issued by Dr. Talbot’s campaign.
Dr. Talbot’s medical license was issued in 1999 and inactive as of 2017, per the Louisiana State Board of Medical Examiners.
Louisiana expects $325M in multistate settlement with opioid companies
Louisiana is a party to a multistate and multijurisdictional lawsuit where the state is expected to receive more than $325 million in a settlement reached with drug distributors Cardinal, McKesson, and AmerisourceBergen, and drug manufacturer Johnson & Johnson, reported the Louisiana Illuminator in July. The total settlement may reach $26 billion dollars.
The Associated Press reported in July that there have been at least $40 billion in completed or proposed settlements, penalties, and fines between governments as a result of the opioid epidemic since 2007.
That total doesn’t include a proposed settlement involving members of the Sackler family, who own Purdue Pharmaceuticals, which manufactured and marketed the opioid painkiller OxyContin. The Sackler family have agreed to pay approximately $4.3 billion and surrender ownership of their bankrupt company, reported NPR. The family’s proposed settlement is part of a deal involving Purdue Pharmaceuticals worth more than $10 billion, reported Reuters.
In 2020, there were more than 81,000 drug overdose deaths, the highest number recorded in a 12-month period, per the U.S. Centers for Disease Control and Prevention. Fentanyl, an illicitly manufactured synthetic opioid, was the lead driver of those deaths.
A version of this article first appeared on Medscape.com.
A according to the U.S. Department of Justice. The substances include oxycodone and morphine.
Adrian Dexter Talbot, MD, 55, of Slidell, La., is also charged with maintaining a medical clinic for the purpose of illegally distributing controlled substances, per the indictment.
Because the opioid prescriptions were filled using beneficiaries’ health insurance, Dr. Talbot is also charged with defrauding Medicare, Medicaid, and Blue Cross and Blue Shield of Louisiana of more than $5.1 million.
When contacted by this news organization for comment on the case via Twitter, Dr. Talbot or a representative responded with a link to his self-published book on Amazon. In his author bio, Dr. Talbot refers to himself as “a former assassin,” “retired military commander,” and “leader of the Medellin Cartel’s New York operations at the age of 16.” The Medellin Cartel is a notorious drug distribution empire.
Dr. Talbot is listed as the author of another book on Google Books detailing his time as a “former teenage assassin” and leader of the cartel, told as he struggles with early onset Alzheimer’s.
Dr. Talbot could spend decades in prison
According to the National Institute on Drug Abuse, 444 residents of the Bayou State lost their lives because of an opioid-related drug overdose in 2018. During that year, the state’s health care providers wrote more than 79.4 opioid prescriptions for every 100 persons, which puts the state in the top five in the United States in 2018, when the average U.S. rate was 51.4 prescriptions per 100 persons.
Charged with one count each of conspiracy to unlawfully distribute and dispense controlled substances and maintaining drug-involved premises and conspiracy to commit health care fraud, Dr. Talbot is also charged with four counts of unlawfully distributing and dispensing controlled substances. He is scheduled for a federal court appearance on September 10.
In addition to presigning prescriptions for individuals he didn’t meet or examine, federal officials allege Dr. Talbot hired another health care provider to similarly presign prescriptions for people who weren’t examined at a medical practice in Slidell, where Dr. Talbot was employed. The DOJ says Dr. Talbot took a full-time job in Pineville, La., and presigned prescriptions while no longer physically present at the Slidell clinic.
A speaker’s bio for Dr. Talbot indicates he worked as chief of medical services for the Alexandria Veterans Affairs Health Care System in Pineville.
According to the DOJ’s indictment, Dr. Talbot was aware that patients were filling the prescriptions that were provided outside the scope of professional practice and not for a legitimate medical purpose. This is what triggered the DOJ’s fraudulent billing claim.
Dr. Talbot faces a maximum penalty of 10 years for conspiracy to commit health care fraud and 20 years each for the other counts, if convicted.
Dr. Talbot was candidate for local coroner
In February 2015, Dr. Talbot announced his candidacy for coroner for St. Tammany Parish, about an hour’s drive south of New Orleans, reported the Times Picayune. The seat was open because the previous coroner had resigned and ultimately pleaded guilty to a federal corruption charge.
The Times Picayune reported at the time that Dr. Talbot was a U.S. Navy veteran, in addition to serving as medical director and a primary care physician at the Medical Care Center in Slidell. Among the services provided to his patients were evaluations and treatment for substance use and mental health disorders, according to a press release issued by Dr. Talbot’s campaign.
Dr. Talbot’s medical license was issued in 1999 and inactive as of 2017, per the Louisiana State Board of Medical Examiners.
Louisiana expects $325M in multistate settlement with opioid companies
Louisiana is a party to a multistate and multijurisdictional lawsuit where the state is expected to receive more than $325 million in a settlement reached with drug distributors Cardinal, McKesson, and AmerisourceBergen, and drug manufacturer Johnson & Johnson, reported the Louisiana Illuminator in July. The total settlement may reach $26 billion dollars.
The Associated Press reported in July that there have been at least $40 billion in completed or proposed settlements, penalties, and fines between governments as a result of the opioid epidemic since 2007.
That total doesn’t include a proposed settlement involving members of the Sackler family, who own Purdue Pharmaceuticals, which manufactured and marketed the opioid painkiller OxyContin. The Sackler family have agreed to pay approximately $4.3 billion and surrender ownership of their bankrupt company, reported NPR. The family’s proposed settlement is part of a deal involving Purdue Pharmaceuticals worth more than $10 billion, reported Reuters.
In 2020, there were more than 81,000 drug overdose deaths, the highest number recorded in a 12-month period, per the U.S. Centers for Disease Control and Prevention. Fentanyl, an illicitly manufactured synthetic opioid, was the lead driver of those deaths.
A version of this article first appeared on Medscape.com.
A according to the U.S. Department of Justice. The substances include oxycodone and morphine.
Adrian Dexter Talbot, MD, 55, of Slidell, La., is also charged with maintaining a medical clinic for the purpose of illegally distributing controlled substances, per the indictment.
Because the opioid prescriptions were filled using beneficiaries’ health insurance, Dr. Talbot is also charged with defrauding Medicare, Medicaid, and Blue Cross and Blue Shield of Louisiana of more than $5.1 million.
When contacted by this news organization for comment on the case via Twitter, Dr. Talbot or a representative responded with a link to his self-published book on Amazon. In his author bio, Dr. Talbot refers to himself as “a former assassin,” “retired military commander,” and “leader of the Medellin Cartel’s New York operations at the age of 16.” The Medellin Cartel is a notorious drug distribution empire.
Dr. Talbot is listed as the author of another book on Google Books detailing his time as a “former teenage assassin” and leader of the cartel, told as he struggles with early onset Alzheimer’s.
Dr. Talbot could spend decades in prison
According to the National Institute on Drug Abuse, 444 residents of the Bayou State lost their lives because of an opioid-related drug overdose in 2018. During that year, the state’s health care providers wrote more than 79.4 opioid prescriptions for every 100 persons, which puts the state in the top five in the United States in 2018, when the average U.S. rate was 51.4 prescriptions per 100 persons.
Charged with one count each of conspiracy to unlawfully distribute and dispense controlled substances and maintaining drug-involved premises and conspiracy to commit health care fraud, Dr. Talbot is also charged with four counts of unlawfully distributing and dispensing controlled substances. He is scheduled for a federal court appearance on September 10.
In addition to presigning prescriptions for individuals he didn’t meet or examine, federal officials allege Dr. Talbot hired another health care provider to similarly presign prescriptions for people who weren’t examined at a medical practice in Slidell, where Dr. Talbot was employed. The DOJ says Dr. Talbot took a full-time job in Pineville, La., and presigned prescriptions while no longer physically present at the Slidell clinic.
A speaker’s bio for Dr. Talbot indicates he worked as chief of medical services for the Alexandria Veterans Affairs Health Care System in Pineville.
According to the DOJ’s indictment, Dr. Talbot was aware that patients were filling the prescriptions that were provided outside the scope of professional practice and not for a legitimate medical purpose. This is what triggered the DOJ’s fraudulent billing claim.
Dr. Talbot faces a maximum penalty of 10 years for conspiracy to commit health care fraud and 20 years each for the other counts, if convicted.
Dr. Talbot was candidate for local coroner
In February 2015, Dr. Talbot announced his candidacy for coroner for St. Tammany Parish, about an hour’s drive south of New Orleans, reported the Times Picayune. The seat was open because the previous coroner had resigned and ultimately pleaded guilty to a federal corruption charge.
The Times Picayune reported at the time that Dr. Talbot was a U.S. Navy veteran, in addition to serving as medical director and a primary care physician at the Medical Care Center in Slidell. Among the services provided to his patients were evaluations and treatment for substance use and mental health disorders, according to a press release issued by Dr. Talbot’s campaign.
Dr. Talbot’s medical license was issued in 1999 and inactive as of 2017, per the Louisiana State Board of Medical Examiners.
Louisiana expects $325M in multistate settlement with opioid companies
Louisiana is a party to a multistate and multijurisdictional lawsuit where the state is expected to receive more than $325 million in a settlement reached with drug distributors Cardinal, McKesson, and AmerisourceBergen, and drug manufacturer Johnson & Johnson, reported the Louisiana Illuminator in July. The total settlement may reach $26 billion dollars.
The Associated Press reported in July that there have been at least $40 billion in completed or proposed settlements, penalties, and fines between governments as a result of the opioid epidemic since 2007.
That total doesn’t include a proposed settlement involving members of the Sackler family, who own Purdue Pharmaceuticals, which manufactured and marketed the opioid painkiller OxyContin. The Sackler family have agreed to pay approximately $4.3 billion and surrender ownership of their bankrupt company, reported NPR. The family’s proposed settlement is part of a deal involving Purdue Pharmaceuticals worth more than $10 billion, reported Reuters.
In 2020, there were more than 81,000 drug overdose deaths, the highest number recorded in a 12-month period, per the U.S. Centers for Disease Control and Prevention. Fentanyl, an illicitly manufactured synthetic opioid, was the lead driver of those deaths.
A version of this article first appeared on Medscape.com.
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
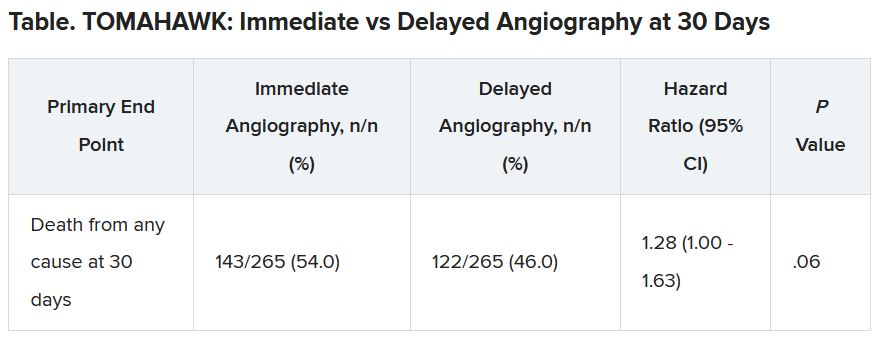
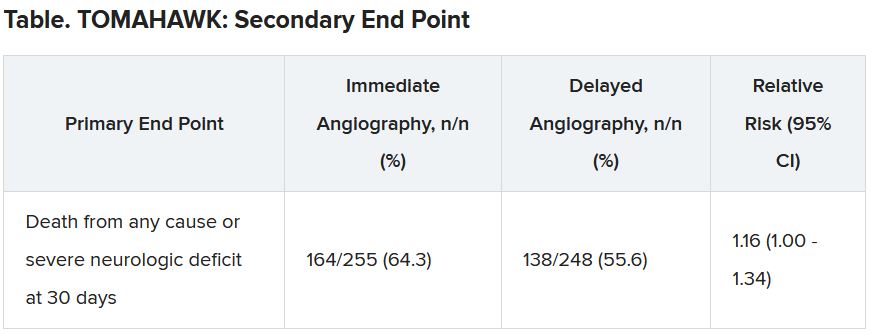
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
CDC panel unanimously backs Pfizer vax, fortifying FDA approval
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
Children’s upper airways primed to combat SARS-CoV-2 infection
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The other epidemic: Violence against health care workers
After working two busy evening hospital shifts, I was eating breakfast with my children when I started reading about physicians confronted and verbally abused during school board meetings for advocating for face masks in school. The pandemic changed course with the Delta variant increasing hospitalizations, and it seems to me the public response to physicians and health care workers also changed.
During the first wave of the pandemic, public support accompanied health care workers’ sacrifices. Nightly applause rang through New York City, there were donations of food, and murals reflected public backing.
We as a nation rallied. We masked up and locked down. We produced vaccines. COVID cases decreased, and by spring, a hint of normalcy bloomed.
Then the virus changed, and the Delta variant spread. Pandemic fatigue set in. Health care workers asked for help with continued masking and increased vaccinations and instead were met with threats. The summer, already made difficult, makes the prospect of winter even more daunting.
This kind of abuse is persistent
Violence against health care workers is not a new dilemma. Stories abound of patients or family members physically attacking, verbally abusing, or harassing health care workers. A 2014 survey reported almost 80% of nurses attacked during their career. Data from the Bureau of Labor Statistics also reveals health care workers experience more nonfatal workplace violence, as compared with other professions.
Nurses, who often spend the most face-to-face time with patients, receive a litany of abuse. A 2019 nursing survey reported 59% of respondents experiencing verbal abuse from patients and more than 43% experiencing verbal abuse from patients’ families. Even more concerning is 23% of survey respondents reporting physical abuse, an increase from 20% in 2018.
Physicians, likewise, are not immune from the same maltreatment. A 2014 physician survey reported more than 71% of physicians in the United States have experienced at least one incident of workplace violence in their careers. Of the physician specialties, the highest rates of violence are in the emergency department and against less experienced physicians. This is likely caused by the higher rates of patient frustration in EDs as a result of long wait times, overcrowding, and boarding while awaiting an inpatient room.
These statistics are disheartening. However, what I find most discouraging is the almost submissive acceptance of this abuse in the health care field as almost 73% of health care workers feel that the abuse is part of the job.
COVID and the increase in violence against health care workers
As the Delta variant spreads, hospitals’ capacity to handle both COVID and non-COVID issues is further strained. Compounding this stress is the public’s pandemic fatigue and the ongoing battles with masking and vaccinations.
In San Antonio, health care workers faced verbal and physical abuse as they enforced masking and visitation restrictions for COVID patients. Online, health care workers, who advocate for masking or vaccination, are often subject to death threats, threats to family members, and verbal abuse on social media. Veiled threats of “we know who you are” and “we will find you” follow physicians who advocate for masking in schools.
This problem is not isolated to the United States. In Italy, a COVID patient spat at health care workers who asked them to wait, resulting in closure of an entire hospital ward. In the United Kingdom, health care workers were subject to the same abuse as those in San Antonio when trying to enforce masking in the hospital. In India, Pakistan, and Spain, a stigma exists against health care workers for being sources of contagion.
The presence of a growing divide between health care workers and those we serve threatens to undermine not only delivery of care but also our response to the pandemic. This is in addition to the mental health burden and compassion fatigue suffered by many health care workers who find their efforts in doubt. An already strained medical system will find it difficult to withstand the loss of its essential workforce.
Standing united against health care worker abuse
Despite the level of discord surrounding COVID-19, it is important that health care workers remain united. An effective response to the increase in violence toward health care workers will greatly depend on how we address the following.
First, we must actively work to combat the spread of misinformation that erodes the public trust in science and medicine. Transparency is paramount. Policy changes and plans for implementation should be open and free of political influence. This remains a challenge due to the CDC’s standing as both a federal and scientific institution. A steadfast and explicit presentation of scientific evidence by the CDC is a vital first step in repairing this trust.
In addition, we must become our own advocates. The passage of HR 1195, the Workplace Violence Prevention for Health Care and Social Service Workers Act, in the House of Representatives with bipartisan support is an indication that the time is ripe for sweeping change. Its supporters include the American Nurses Association, American Psychiatric Nurses Association, National Nurses United, and the American College of Emergency Physicians. Active opposition includes the American Hospital Association, which cites prohibitive cost as a source of objection.
HR 1195 now waits in the U.S. Senate for approval. We should alert local, state, and health system leadership to the violence against health care workers. We should demand increased protection for our most vulnerable colleagues in EDs and hospitals. Our advocacy will produce a paradigm shift away from the acceptance of this abuse.
Lastly, we must be mindful of compassion fatigue and health care worker burnout. Cynicism threatens to take away our greatest strengths of empathy and humanity. In our work environment, we must lift each other up and increase our awareness of when our colleagues need help. Self-care and creative outlets are encouraged. Admittedly, I am blogging as a personal safeguard against compassion fatigue and burnout.
The pandemic will have enduring implications both positive and negative. It is my hope that support for health care workers not only endures but is also enhanced long after the pandemic ends.
Giancarlo Toledanes, DO, is an assistant professor of pediatrics and a pediatric hospitalist at Texas Children’s Hospital and Baylor College of Medicine, both in Houston. He has no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
After working two busy evening hospital shifts, I was eating breakfast with my children when I started reading about physicians confronted and verbally abused during school board meetings for advocating for face masks in school. The pandemic changed course with the Delta variant increasing hospitalizations, and it seems to me the public response to physicians and health care workers also changed.
During the first wave of the pandemic, public support accompanied health care workers’ sacrifices. Nightly applause rang through New York City, there were donations of food, and murals reflected public backing.
We as a nation rallied. We masked up and locked down. We produced vaccines. COVID cases decreased, and by spring, a hint of normalcy bloomed.
Then the virus changed, and the Delta variant spread. Pandemic fatigue set in. Health care workers asked for help with continued masking and increased vaccinations and instead were met with threats. The summer, already made difficult, makes the prospect of winter even more daunting.
This kind of abuse is persistent
Violence against health care workers is not a new dilemma. Stories abound of patients or family members physically attacking, verbally abusing, or harassing health care workers. A 2014 survey reported almost 80% of nurses attacked during their career. Data from the Bureau of Labor Statistics also reveals health care workers experience more nonfatal workplace violence, as compared with other professions.
Nurses, who often spend the most face-to-face time with patients, receive a litany of abuse. A 2019 nursing survey reported 59% of respondents experiencing verbal abuse from patients and more than 43% experiencing verbal abuse from patients’ families. Even more concerning is 23% of survey respondents reporting physical abuse, an increase from 20% in 2018.
Physicians, likewise, are not immune from the same maltreatment. A 2014 physician survey reported more than 71% of physicians in the United States have experienced at least one incident of workplace violence in their careers. Of the physician specialties, the highest rates of violence are in the emergency department and against less experienced physicians. This is likely caused by the higher rates of patient frustration in EDs as a result of long wait times, overcrowding, and boarding while awaiting an inpatient room.
These statistics are disheartening. However, what I find most discouraging is the almost submissive acceptance of this abuse in the health care field as almost 73% of health care workers feel that the abuse is part of the job.
COVID and the increase in violence against health care workers
As the Delta variant spreads, hospitals’ capacity to handle both COVID and non-COVID issues is further strained. Compounding this stress is the public’s pandemic fatigue and the ongoing battles with masking and vaccinations.
In San Antonio, health care workers faced verbal and physical abuse as they enforced masking and visitation restrictions for COVID patients. Online, health care workers, who advocate for masking or vaccination, are often subject to death threats, threats to family members, and verbal abuse on social media. Veiled threats of “we know who you are” and “we will find you” follow physicians who advocate for masking in schools.
This problem is not isolated to the United States. In Italy, a COVID patient spat at health care workers who asked them to wait, resulting in closure of an entire hospital ward. In the United Kingdom, health care workers were subject to the same abuse as those in San Antonio when trying to enforce masking in the hospital. In India, Pakistan, and Spain, a stigma exists against health care workers for being sources of contagion.
The presence of a growing divide between health care workers and those we serve threatens to undermine not only delivery of care but also our response to the pandemic. This is in addition to the mental health burden and compassion fatigue suffered by many health care workers who find their efforts in doubt. An already strained medical system will find it difficult to withstand the loss of its essential workforce.
Standing united against health care worker abuse
Despite the level of discord surrounding COVID-19, it is important that health care workers remain united. An effective response to the increase in violence toward health care workers will greatly depend on how we address the following.
First, we must actively work to combat the spread of misinformation that erodes the public trust in science and medicine. Transparency is paramount. Policy changes and plans for implementation should be open and free of political influence. This remains a challenge due to the CDC’s standing as both a federal and scientific institution. A steadfast and explicit presentation of scientific evidence by the CDC is a vital first step in repairing this trust.
In addition, we must become our own advocates. The passage of HR 1195, the Workplace Violence Prevention for Health Care and Social Service Workers Act, in the House of Representatives with bipartisan support is an indication that the time is ripe for sweeping change. Its supporters include the American Nurses Association, American Psychiatric Nurses Association, National Nurses United, and the American College of Emergency Physicians. Active opposition includes the American Hospital Association, which cites prohibitive cost as a source of objection.
HR 1195 now waits in the U.S. Senate for approval. We should alert local, state, and health system leadership to the violence against health care workers. We should demand increased protection for our most vulnerable colleagues in EDs and hospitals. Our advocacy will produce a paradigm shift away from the acceptance of this abuse.
Lastly, we must be mindful of compassion fatigue and health care worker burnout. Cynicism threatens to take away our greatest strengths of empathy and humanity. In our work environment, we must lift each other up and increase our awareness of when our colleagues need help. Self-care and creative outlets are encouraged. Admittedly, I am blogging as a personal safeguard against compassion fatigue and burnout.
The pandemic will have enduring implications both positive and negative. It is my hope that support for health care workers not only endures but is also enhanced long after the pandemic ends.
Giancarlo Toledanes, DO, is an assistant professor of pediatrics and a pediatric hospitalist at Texas Children’s Hospital and Baylor College of Medicine, both in Houston. He has no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
After working two busy evening hospital shifts, I was eating breakfast with my children when I started reading about physicians confronted and verbally abused during school board meetings for advocating for face masks in school. The pandemic changed course with the Delta variant increasing hospitalizations, and it seems to me the public response to physicians and health care workers also changed.
During the first wave of the pandemic, public support accompanied health care workers’ sacrifices. Nightly applause rang through New York City, there were donations of food, and murals reflected public backing.
We as a nation rallied. We masked up and locked down. We produced vaccines. COVID cases decreased, and by spring, a hint of normalcy bloomed.
Then the virus changed, and the Delta variant spread. Pandemic fatigue set in. Health care workers asked for help with continued masking and increased vaccinations and instead were met with threats. The summer, already made difficult, makes the prospect of winter even more daunting.
This kind of abuse is persistent
Violence against health care workers is not a new dilemma. Stories abound of patients or family members physically attacking, verbally abusing, or harassing health care workers. A 2014 survey reported almost 80% of nurses attacked during their career. Data from the Bureau of Labor Statistics also reveals health care workers experience more nonfatal workplace violence, as compared with other professions.
Nurses, who often spend the most face-to-face time with patients, receive a litany of abuse. A 2019 nursing survey reported 59% of respondents experiencing verbal abuse from patients and more than 43% experiencing verbal abuse from patients’ families. Even more concerning is 23% of survey respondents reporting physical abuse, an increase from 20% in 2018.
Physicians, likewise, are not immune from the same maltreatment. A 2014 physician survey reported more than 71% of physicians in the United States have experienced at least one incident of workplace violence in their careers. Of the physician specialties, the highest rates of violence are in the emergency department and against less experienced physicians. This is likely caused by the higher rates of patient frustration in EDs as a result of long wait times, overcrowding, and boarding while awaiting an inpatient room.
These statistics are disheartening. However, what I find most discouraging is the almost submissive acceptance of this abuse in the health care field as almost 73% of health care workers feel that the abuse is part of the job.
COVID and the increase in violence against health care workers
As the Delta variant spreads, hospitals’ capacity to handle both COVID and non-COVID issues is further strained. Compounding this stress is the public’s pandemic fatigue and the ongoing battles with masking and vaccinations.
In San Antonio, health care workers faced verbal and physical abuse as they enforced masking and visitation restrictions for COVID patients. Online, health care workers, who advocate for masking or vaccination, are often subject to death threats, threats to family members, and verbal abuse on social media. Veiled threats of “we know who you are” and “we will find you” follow physicians who advocate for masking in schools.
This problem is not isolated to the United States. In Italy, a COVID patient spat at health care workers who asked them to wait, resulting in closure of an entire hospital ward. In the United Kingdom, health care workers were subject to the same abuse as those in San Antonio when trying to enforce masking in the hospital. In India, Pakistan, and Spain, a stigma exists against health care workers for being sources of contagion.
The presence of a growing divide between health care workers and those we serve threatens to undermine not only delivery of care but also our response to the pandemic. This is in addition to the mental health burden and compassion fatigue suffered by many health care workers who find their efforts in doubt. An already strained medical system will find it difficult to withstand the loss of its essential workforce.
Standing united against health care worker abuse
Despite the level of discord surrounding COVID-19, it is important that health care workers remain united. An effective response to the increase in violence toward health care workers will greatly depend on how we address the following.
First, we must actively work to combat the spread of misinformation that erodes the public trust in science and medicine. Transparency is paramount. Policy changes and plans for implementation should be open and free of political influence. This remains a challenge due to the CDC’s standing as both a federal and scientific institution. A steadfast and explicit presentation of scientific evidence by the CDC is a vital first step in repairing this trust.
In addition, we must become our own advocates. The passage of HR 1195, the Workplace Violence Prevention for Health Care and Social Service Workers Act, in the House of Representatives with bipartisan support is an indication that the time is ripe for sweeping change. Its supporters include the American Nurses Association, American Psychiatric Nurses Association, National Nurses United, and the American College of Emergency Physicians. Active opposition includes the American Hospital Association, which cites prohibitive cost as a source of objection.
HR 1195 now waits in the U.S. Senate for approval. We should alert local, state, and health system leadership to the violence against health care workers. We should demand increased protection for our most vulnerable colleagues in EDs and hospitals. Our advocacy will produce a paradigm shift away from the acceptance of this abuse.
Lastly, we must be mindful of compassion fatigue and health care worker burnout. Cynicism threatens to take away our greatest strengths of empathy and humanity. In our work environment, we must lift each other up and increase our awareness of when our colleagues need help. Self-care and creative outlets are encouraged. Admittedly, I am blogging as a personal safeguard against compassion fatigue and burnout.
The pandemic will have enduring implications both positive and negative. It is my hope that support for health care workers not only endures but is also enhanced long after the pandemic ends.
Giancarlo Toledanes, DO, is an assistant professor of pediatrics and a pediatric hospitalist at Texas Children’s Hospital and Baylor College of Medicine, both in Houston. He has no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
PA gets prison time for knowingly prescribing unneeded addictive drugs
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
The secret I’ll take to my grave: Doc reveals
An internist will never forget the dark secret his patient revealed during a routine visit – or the grim aftermath.
The patient, who had a progressive, incurable neurological condition, confided that he planned to kill himself. The patient intended to conceal the true manner and make the death look natural.
“[He planned to do it] very carefully at home so no one would know,” said the internist, who remains anonymous. “[He shared] the methods he would use.”
Perhaps more shocking than the patient’s confession was the physician’s response.
“He did not require my help to do what he planned, and I did not try to stop him,” said the internist. “I reported his death as ‘natural causes’ and never told anyone.”
An ob.gyn., for instance, wrote about struggling with whether to tell a father that his newborn baby was not his genetic child. The newborn had a blood type that made it impossible for the father to be biologically related to the infant, the anonymous doctor wrote.
“I told the wife who then informed me she had a lover,” the ob.gyn. said. “I never told the husband.”
It’s uncertain whether carrying the burden of such hidden knowledge affected the physicians involved in these cases. However, in general, secrets can weigh heavily on the minds of those who keep them and can contribute to stress, said Malia Mason, PhD, a psychologist and dean of research at Columbia Business School in New York. Holding onto secrets can cause depression and anxiety, research shows. The more often people think about the secret, the greater the impact, according to a recent study coauthored by Dr. Mason.
“Keeping a secret diminishes well-being,” Dr. Mason said. “It makes people feel socially distant. It lowers relationship satisfaction, and it leads people to feel inauthentic. The reason that secrets do this is because people think about them all the time. The more you think about it, the more you see these consequences.”
Feelings that stem from a secret depend on the content. The more immoral a secret is thought to be, the more people feel ashamed, according to a 2021 analysis of thousands of secrets, reported by Michael L. Slepian, PhD, and Alex Koch, PhD. However, secrets more related to a person’s profession are often internalized differently, the study found. The more a secret fell higher on the profession/goal-oriented dimension, the more people felt they had insight into the secret, according to the analysis. For example, having clear thinking about the secret and/or knowing how to handle it.
“The more shame participants felt from their secret, the more they indicated the secret hurt their well-being,” Dr. Slepian and Dr. Koch wrote in the study. “The more insight participants felt they had into their secret, the less they indicated the secret hurt their well-being.”
Suspicious deaths exposed after investigations
The internist’s account of keeping his patient’s suicide a secret raises many questions, such as how the patient masked his manner of death. The internist did not share any more details about the incident.
Suicides are among the most challenging manners of deaths to certify, according to James Gill, MD, a pathologist and president of the National Association of Medical Examiners. Death investigators must demonstrate intent, meaning the individuals caused the injury to intentionally harm themselves. Fewer than half of people who die by suicide leave a note, Dr. Gill said, so investigators can’t rely on the absence or the presence of a note in making their determination.
A decedent who had cancer or a severe neurological disorder presents further challenges, said Dr. Gill, who serves as chief medical examiner for the state of Connecticut.
“These [deaths] may not be unexpected and may not be reported to the medical examiner/coroner,” Dr. Gill said. “If there is no suspicion and the treating doctor is willing to sign the death certificate, the death will not come under the jurisdiction of the medical examiner.”
Dr. Gill recalled a death his colleague once investigated that appeared to be natural but emerged as something else after a deeper look.
A woman with metastatic breast cancer was about to be discharged from a hospital into hospice the next morning. The night before, she had a “going away” party with friends who came to visit her in the hospital. Shortly after the friends left, the woman was found dead. Because of her condition, she could have died at any time, Dr. Gill said, but she also had a history of depression and hospital staff were suspicious. The death was reported to the medical examiner’s office.
Toxicology testing found markedly elevated concentrations of phenytoin and pentobarbital, neither of which were prescribed during her hospital stay. Dr. Gill said it turned out that the woman and her friends worked at a veterinarian’s office, and the medication they used to euthanize dogs was a combination of phenytoin and pentobarbital.
“The death was certified as a homicide because of the direct actions of another, but a reasonable argument could be made for suicide,” Dr. Gill said.
In a similar case reported in the journal Science & Justice, a 64-year-old cardiologist was found lifeless by his wife after he collapsed near the stairs of his home. Next to his body was a bottle of whiskey and two cups, one that appeared to be used for the alcohol and one with a yellowish liquid smelling of honey. The wife reported that her husband always drank whiskey with honey before bed. The death was initially classified as natural, but after vehement protest by the family, a forensic autopsy was performed.
Prior to the autopsy, death investigators learned the decedent, who was a well-known and successful practitioner in his community, had Parkinson’s disease. At times, he could not sign his prescriptions because of the increasing tremor in his hands, according to the case study. Investigators learned the patient’s mother had also suffered from Parkinson’s, and that her son had witnessed her decline.
The autopsy revealed only nonspecific lesions such as acute stasis of the viscera, moderate pulmonary and cerebral edema, and moderate generalized atheromatosis. Histological examinations did not yield any unusual findings.
An analysis of the beverage containers detected pentobarbital in the yellowish syrup residue of the second cup. Testing of the doctor’s peripheral blood revealed the presence of a metabolite of pentobarbital, ethanol, and traces of phenobarbital. In addition, a urine analysis showed the presence of venlafaxine, an antidepressant, as well as the benzophenone of lorazepam, a sedating benzodiazepine, and metoclopramide, an antiemetic.
Lead author C. Brandt-Casadevall, MD, and colleagues wrote that the levels were clearly compatible with a scenario of a pentobarbital overdose with a lethal outcome.
“... It is obvious that the victim attempted to hide his suicide from his family circle,” Dr. Brandt-Casadevall and colleagues wrote. “Thus, we obtained no evidence indicating that he might have spoken at any point of putting an end to his life. There was no written note. The victim did not wait to be alone at home. Instead, he committed his act in a routine situation: his wife was watching television late at night and he was upstairs, presumably going to sleep. Thus, he had one to two hours at his disposal, and he ingested a very fast-acting drug which would make any attempt at reanimation impossible, even after a brief period of time. This may have induced the physician in charge to believe that the cause of death was cardiac origin, a likely hypothesis given the age of the victim.”
What to do when a terminally ill patient talks suicide
When a terminally ill patient expresses the desire to end his or her life, it’s important to understand that desire is often a result of existential suffering, a sense of hopelessness, and lack of social support, said Lynn A. Jansen, PhD, a bioethicist at the University of Arizona in Tucson.
“The duty of beneficence requires that physicians attempt to provide the support and care that is needed,” said Dr. Jansen. “Here, interdisciplinary teamwork is important and should be utilized. Physicians should refer patients to professionals, such as social workers, pastoral care, psychologists, etc., who are better able to address these issues.”
The rate of desire for a hastened death among terminally ill patients ranges from 17% to 45%, depending on the population studied and how the desire is evaluated, according to an analysis in the Primary Care Companion to the Journal of Clinical Psychiatry. In one study, 14% of about 130 palliative care patients with cancer had a strong desire to quicken the dying process.
In addition, patients with neurologic disorders have a significantly higher suicide rate than that of those without neurologic disorders, a recent JAMA study found. About 1 in 150 patients diagnosed with a neurological disorder dies by suicide, the analysis determined.
A tricky point to remember is that a desire by a terminally ill patient to hasten his or her death by suicide should not be taken by itself to indicate depression, Dr. Jansen noted.
“In principle, such patients can make an autonomous decision to end their lives,” she said. “However, the expression of such a desire is very often associated with depression and forms of suffering that can be effectively addressed by the health care team.”
Physicians can also explore other avenues with the patient such as palliative care or making sure adequate pain relief is available, added Robert Klitzman, MD, professor of psychiatry and academic director of the master of science in bioethics program at Columbia University, New York.
“If they are saying it’s because they are distressed, ethically, a doctor can and should find ways to decrease their distress,” he said.
Of course, those who practice in the U.S. jurisdictions that have physician-assisted-dying laws have different legal and ethical elements to consider. Physicians in these areas have no ethical duty to participate in the process, Dr. Jansen said, but they have a duty to refer patients who express a desire to pursue physician aid-in-dying to another provider who can assist them.
Physician aid-in-dying laws vary somewhat so it’s important that physicians in these areas be aware of their specific statute, Dr. Klitzman said. California, Colorado, Hawaii, Maine, New Jersey, New Mexico, Oregon, Vermont, Washington, and the District of Columbia have these laws.
“In these states, if a terminally ill patient says they don’t want to live anymore, a physician would first decide if this is a result of depression or if it’s a request for physician aid-in-dying,” he said. “Even then, in most cases, the patient would be evaluated by not one, but two different health professionals at two different points. We want to see if it is a consistent decision that the person has made that they want physician aid-in-dying, and not just that they’ve had a bad day or a setback in their treatment.”
In the case of the internist who told no one of his patient’s suicide plan, Dr. Klitzman said he would have dug deeper into the patient’s mindset.
“Not knowing anything about the patient or the doctor, I would have responded differently,” he said. “I think a physician should address why a patient feels that way. They may feel their pain is unbearable, and we potentially offer more pain relief. Maybe the patient shows evidence of having depression, which may be treatable [with medication]. The patient would then feel better and be able to spend quality time with family and loved ones, make sure their affairs are in order, and have a chance to say goodbye.”
A version of this article first appeared on Medscape.com.
An internist will never forget the dark secret his patient revealed during a routine visit – or the grim aftermath.
The patient, who had a progressive, incurable neurological condition, confided that he planned to kill himself. The patient intended to conceal the true manner and make the death look natural.
“[He planned to do it] very carefully at home so no one would know,” said the internist, who remains anonymous. “[He shared] the methods he would use.”
Perhaps more shocking than the patient’s confession was the physician’s response.
“He did not require my help to do what he planned, and I did not try to stop him,” said the internist. “I reported his death as ‘natural causes’ and never told anyone.”
An ob.gyn., for instance, wrote about struggling with whether to tell a father that his newborn baby was not his genetic child. The newborn had a blood type that made it impossible for the father to be biologically related to the infant, the anonymous doctor wrote.
“I told the wife who then informed me she had a lover,” the ob.gyn. said. “I never told the husband.”
It’s uncertain whether carrying the burden of such hidden knowledge affected the physicians involved in these cases. However, in general, secrets can weigh heavily on the minds of those who keep them and can contribute to stress, said Malia Mason, PhD, a psychologist and dean of research at Columbia Business School in New York. Holding onto secrets can cause depression and anxiety, research shows. The more often people think about the secret, the greater the impact, according to a recent study coauthored by Dr. Mason.
“Keeping a secret diminishes well-being,” Dr. Mason said. “It makes people feel socially distant. It lowers relationship satisfaction, and it leads people to feel inauthentic. The reason that secrets do this is because people think about them all the time. The more you think about it, the more you see these consequences.”
Feelings that stem from a secret depend on the content. The more immoral a secret is thought to be, the more people feel ashamed, according to a 2021 analysis of thousands of secrets, reported by Michael L. Slepian, PhD, and Alex Koch, PhD. However, secrets more related to a person’s profession are often internalized differently, the study found. The more a secret fell higher on the profession/goal-oriented dimension, the more people felt they had insight into the secret, according to the analysis. For example, having clear thinking about the secret and/or knowing how to handle it.
“The more shame participants felt from their secret, the more they indicated the secret hurt their well-being,” Dr. Slepian and Dr. Koch wrote in the study. “The more insight participants felt they had into their secret, the less they indicated the secret hurt their well-being.”
Suspicious deaths exposed after investigations
The internist’s account of keeping his patient’s suicide a secret raises many questions, such as how the patient masked his manner of death. The internist did not share any more details about the incident.
Suicides are among the most challenging manners of deaths to certify, according to James Gill, MD, a pathologist and president of the National Association of Medical Examiners. Death investigators must demonstrate intent, meaning the individuals caused the injury to intentionally harm themselves. Fewer than half of people who die by suicide leave a note, Dr. Gill said, so investigators can’t rely on the absence or the presence of a note in making their determination.
A decedent who had cancer or a severe neurological disorder presents further challenges, said Dr. Gill, who serves as chief medical examiner for the state of Connecticut.
“These [deaths] may not be unexpected and may not be reported to the medical examiner/coroner,” Dr. Gill said. “If there is no suspicion and the treating doctor is willing to sign the death certificate, the death will not come under the jurisdiction of the medical examiner.”
Dr. Gill recalled a death his colleague once investigated that appeared to be natural but emerged as something else after a deeper look.
A woman with metastatic breast cancer was about to be discharged from a hospital into hospice the next morning. The night before, she had a “going away” party with friends who came to visit her in the hospital. Shortly after the friends left, the woman was found dead. Because of her condition, she could have died at any time, Dr. Gill said, but she also had a history of depression and hospital staff were suspicious. The death was reported to the medical examiner’s office.
Toxicology testing found markedly elevated concentrations of phenytoin and pentobarbital, neither of which were prescribed during her hospital stay. Dr. Gill said it turned out that the woman and her friends worked at a veterinarian’s office, and the medication they used to euthanize dogs was a combination of phenytoin and pentobarbital.
“The death was certified as a homicide because of the direct actions of another, but a reasonable argument could be made for suicide,” Dr. Gill said.
In a similar case reported in the journal Science & Justice, a 64-year-old cardiologist was found lifeless by his wife after he collapsed near the stairs of his home. Next to his body was a bottle of whiskey and two cups, one that appeared to be used for the alcohol and one with a yellowish liquid smelling of honey. The wife reported that her husband always drank whiskey with honey before bed. The death was initially classified as natural, but after vehement protest by the family, a forensic autopsy was performed.
Prior to the autopsy, death investigators learned the decedent, who was a well-known and successful practitioner in his community, had Parkinson’s disease. At times, he could not sign his prescriptions because of the increasing tremor in his hands, according to the case study. Investigators learned the patient’s mother had also suffered from Parkinson’s, and that her son had witnessed her decline.
The autopsy revealed only nonspecific lesions such as acute stasis of the viscera, moderate pulmonary and cerebral edema, and moderate generalized atheromatosis. Histological examinations did not yield any unusual findings.
An analysis of the beverage containers detected pentobarbital in the yellowish syrup residue of the second cup. Testing of the doctor’s peripheral blood revealed the presence of a metabolite of pentobarbital, ethanol, and traces of phenobarbital. In addition, a urine analysis showed the presence of venlafaxine, an antidepressant, as well as the benzophenone of lorazepam, a sedating benzodiazepine, and metoclopramide, an antiemetic.
Lead author C. Brandt-Casadevall, MD, and colleagues wrote that the levels were clearly compatible with a scenario of a pentobarbital overdose with a lethal outcome.
“... It is obvious that the victim attempted to hide his suicide from his family circle,” Dr. Brandt-Casadevall and colleagues wrote. “Thus, we obtained no evidence indicating that he might have spoken at any point of putting an end to his life. There was no written note. The victim did not wait to be alone at home. Instead, he committed his act in a routine situation: his wife was watching television late at night and he was upstairs, presumably going to sleep. Thus, he had one to two hours at his disposal, and he ingested a very fast-acting drug which would make any attempt at reanimation impossible, even after a brief period of time. This may have induced the physician in charge to believe that the cause of death was cardiac origin, a likely hypothesis given the age of the victim.”
What to do when a terminally ill patient talks suicide
When a terminally ill patient expresses the desire to end his or her life, it’s important to understand that desire is often a result of existential suffering, a sense of hopelessness, and lack of social support, said Lynn A. Jansen, PhD, a bioethicist at the University of Arizona in Tucson.
“The duty of beneficence requires that physicians attempt to provide the support and care that is needed,” said Dr. Jansen. “Here, interdisciplinary teamwork is important and should be utilized. Physicians should refer patients to professionals, such as social workers, pastoral care, psychologists, etc., who are better able to address these issues.”
The rate of desire for a hastened death among terminally ill patients ranges from 17% to 45%, depending on the population studied and how the desire is evaluated, according to an analysis in the Primary Care Companion to the Journal of Clinical Psychiatry. In one study, 14% of about 130 palliative care patients with cancer had a strong desire to quicken the dying process.
In addition, patients with neurologic disorders have a significantly higher suicide rate than that of those without neurologic disorders, a recent JAMA study found. About 1 in 150 patients diagnosed with a neurological disorder dies by suicide, the analysis determined.
A tricky point to remember is that a desire by a terminally ill patient to hasten his or her death by suicide should not be taken by itself to indicate depression, Dr. Jansen noted.
“In principle, such patients can make an autonomous decision to end their lives,” she said. “However, the expression of such a desire is very often associated with depression and forms of suffering that can be effectively addressed by the health care team.”
Physicians can also explore other avenues with the patient such as palliative care or making sure adequate pain relief is available, added Robert Klitzman, MD, professor of psychiatry and academic director of the master of science in bioethics program at Columbia University, New York.
“If they are saying it’s because they are distressed, ethically, a doctor can and should find ways to decrease their distress,” he said.
Of course, those who practice in the U.S. jurisdictions that have physician-assisted-dying laws have different legal and ethical elements to consider. Physicians in these areas have no ethical duty to participate in the process, Dr. Jansen said, but they have a duty to refer patients who express a desire to pursue physician aid-in-dying to another provider who can assist them.
Physician aid-in-dying laws vary somewhat so it’s important that physicians in these areas be aware of their specific statute, Dr. Klitzman said. California, Colorado, Hawaii, Maine, New Jersey, New Mexico, Oregon, Vermont, Washington, and the District of Columbia have these laws.
“In these states, if a terminally ill patient says they don’t want to live anymore, a physician would first decide if this is a result of depression or if it’s a request for physician aid-in-dying,” he said. “Even then, in most cases, the patient would be evaluated by not one, but two different health professionals at two different points. We want to see if it is a consistent decision that the person has made that they want physician aid-in-dying, and not just that they’ve had a bad day or a setback in their treatment.”
In the case of the internist who told no one of his patient’s suicide plan, Dr. Klitzman said he would have dug deeper into the patient’s mindset.
“Not knowing anything about the patient or the doctor, I would have responded differently,” he said. “I think a physician should address why a patient feels that way. They may feel their pain is unbearable, and we potentially offer more pain relief. Maybe the patient shows evidence of having depression, which may be treatable [with medication]. The patient would then feel better and be able to spend quality time with family and loved ones, make sure their affairs are in order, and have a chance to say goodbye.”
A version of this article first appeared on Medscape.com.
An internist will never forget the dark secret his patient revealed during a routine visit – or the grim aftermath.
The patient, who had a progressive, incurable neurological condition, confided that he planned to kill himself. The patient intended to conceal the true manner and make the death look natural.
“[He planned to do it] very carefully at home so no one would know,” said the internist, who remains anonymous. “[He shared] the methods he would use.”
Perhaps more shocking than the patient’s confession was the physician’s response.
“He did not require my help to do what he planned, and I did not try to stop him,” said the internist. “I reported his death as ‘natural causes’ and never told anyone.”
An ob.gyn., for instance, wrote about struggling with whether to tell a father that his newborn baby was not his genetic child. The newborn had a blood type that made it impossible for the father to be biologically related to the infant, the anonymous doctor wrote.
“I told the wife who then informed me she had a lover,” the ob.gyn. said. “I never told the husband.”
It’s uncertain whether carrying the burden of such hidden knowledge affected the physicians involved in these cases. However, in general, secrets can weigh heavily on the minds of those who keep them and can contribute to stress, said Malia Mason, PhD, a psychologist and dean of research at Columbia Business School in New York. Holding onto secrets can cause depression and anxiety, research shows. The more often people think about the secret, the greater the impact, according to a recent study coauthored by Dr. Mason.
“Keeping a secret diminishes well-being,” Dr. Mason said. “It makes people feel socially distant. It lowers relationship satisfaction, and it leads people to feel inauthentic. The reason that secrets do this is because people think about them all the time. The more you think about it, the more you see these consequences.”
Feelings that stem from a secret depend on the content. The more immoral a secret is thought to be, the more people feel ashamed, according to a 2021 analysis of thousands of secrets, reported by Michael L. Slepian, PhD, and Alex Koch, PhD. However, secrets more related to a person’s profession are often internalized differently, the study found. The more a secret fell higher on the profession/goal-oriented dimension, the more people felt they had insight into the secret, according to the analysis. For example, having clear thinking about the secret and/or knowing how to handle it.
“The more shame participants felt from their secret, the more they indicated the secret hurt their well-being,” Dr. Slepian and Dr. Koch wrote in the study. “The more insight participants felt they had into their secret, the less they indicated the secret hurt their well-being.”
Suspicious deaths exposed after investigations
The internist’s account of keeping his patient’s suicide a secret raises many questions, such as how the patient masked his manner of death. The internist did not share any more details about the incident.
Suicides are among the most challenging manners of deaths to certify, according to James Gill, MD, a pathologist and president of the National Association of Medical Examiners. Death investigators must demonstrate intent, meaning the individuals caused the injury to intentionally harm themselves. Fewer than half of people who die by suicide leave a note, Dr. Gill said, so investigators can’t rely on the absence or the presence of a note in making their determination.
A decedent who had cancer or a severe neurological disorder presents further challenges, said Dr. Gill, who serves as chief medical examiner for the state of Connecticut.
“These [deaths] may not be unexpected and may not be reported to the medical examiner/coroner,” Dr. Gill said. “If there is no suspicion and the treating doctor is willing to sign the death certificate, the death will not come under the jurisdiction of the medical examiner.”
Dr. Gill recalled a death his colleague once investigated that appeared to be natural but emerged as something else after a deeper look.
A woman with metastatic breast cancer was about to be discharged from a hospital into hospice the next morning. The night before, she had a “going away” party with friends who came to visit her in the hospital. Shortly after the friends left, the woman was found dead. Because of her condition, she could have died at any time, Dr. Gill said, but she also had a history of depression and hospital staff were suspicious. The death was reported to the medical examiner’s office.
Toxicology testing found markedly elevated concentrations of phenytoin and pentobarbital, neither of which were prescribed during her hospital stay. Dr. Gill said it turned out that the woman and her friends worked at a veterinarian’s office, and the medication they used to euthanize dogs was a combination of phenytoin and pentobarbital.
“The death was certified as a homicide because of the direct actions of another, but a reasonable argument could be made for suicide,” Dr. Gill said.
In a similar case reported in the journal Science & Justice, a 64-year-old cardiologist was found lifeless by his wife after he collapsed near the stairs of his home. Next to his body was a bottle of whiskey and two cups, one that appeared to be used for the alcohol and one with a yellowish liquid smelling of honey. The wife reported that her husband always drank whiskey with honey before bed. The death was initially classified as natural, but after vehement protest by the family, a forensic autopsy was performed.
Prior to the autopsy, death investigators learned the decedent, who was a well-known and successful practitioner in his community, had Parkinson’s disease. At times, he could not sign his prescriptions because of the increasing tremor in his hands, according to the case study. Investigators learned the patient’s mother had also suffered from Parkinson’s, and that her son had witnessed her decline.
The autopsy revealed only nonspecific lesions such as acute stasis of the viscera, moderate pulmonary and cerebral edema, and moderate generalized atheromatosis. Histological examinations did not yield any unusual findings.
An analysis of the beverage containers detected pentobarbital in the yellowish syrup residue of the second cup. Testing of the doctor’s peripheral blood revealed the presence of a metabolite of pentobarbital, ethanol, and traces of phenobarbital. In addition, a urine analysis showed the presence of venlafaxine, an antidepressant, as well as the benzophenone of lorazepam, a sedating benzodiazepine, and metoclopramide, an antiemetic.
Lead author C. Brandt-Casadevall, MD, and colleagues wrote that the levels were clearly compatible with a scenario of a pentobarbital overdose with a lethal outcome.
“... It is obvious that the victim attempted to hide his suicide from his family circle,” Dr. Brandt-Casadevall and colleagues wrote. “Thus, we obtained no evidence indicating that he might have spoken at any point of putting an end to his life. There was no written note. The victim did not wait to be alone at home. Instead, he committed his act in a routine situation: his wife was watching television late at night and he was upstairs, presumably going to sleep. Thus, he had one to two hours at his disposal, and he ingested a very fast-acting drug which would make any attempt at reanimation impossible, even after a brief period of time. This may have induced the physician in charge to believe that the cause of death was cardiac origin, a likely hypothesis given the age of the victim.”
What to do when a terminally ill patient talks suicide
When a terminally ill patient expresses the desire to end his or her life, it’s important to understand that desire is often a result of existential suffering, a sense of hopelessness, and lack of social support, said Lynn A. Jansen, PhD, a bioethicist at the University of Arizona in Tucson.
“The duty of beneficence requires that physicians attempt to provide the support and care that is needed,” said Dr. Jansen. “Here, interdisciplinary teamwork is important and should be utilized. Physicians should refer patients to professionals, such as social workers, pastoral care, psychologists, etc., who are better able to address these issues.”
The rate of desire for a hastened death among terminally ill patients ranges from 17% to 45%, depending on the population studied and how the desire is evaluated, according to an analysis in the Primary Care Companion to the Journal of Clinical Psychiatry. In one study, 14% of about 130 palliative care patients with cancer had a strong desire to quicken the dying process.
In addition, patients with neurologic disorders have a significantly higher suicide rate than that of those without neurologic disorders, a recent JAMA study found. About 1 in 150 patients diagnosed with a neurological disorder dies by suicide, the analysis determined.
A tricky point to remember is that a desire by a terminally ill patient to hasten his or her death by suicide should not be taken by itself to indicate depression, Dr. Jansen noted.
“In principle, such patients can make an autonomous decision to end their lives,” she said. “However, the expression of such a desire is very often associated with depression and forms of suffering that can be effectively addressed by the health care team.”
Physicians can also explore other avenues with the patient such as palliative care or making sure adequate pain relief is available, added Robert Klitzman, MD, professor of psychiatry and academic director of the master of science in bioethics program at Columbia University, New York.
“If they are saying it’s because they are distressed, ethically, a doctor can and should find ways to decrease their distress,” he said.
Of course, those who practice in the U.S. jurisdictions that have physician-assisted-dying laws have different legal and ethical elements to consider. Physicians in these areas have no ethical duty to participate in the process, Dr. Jansen said, but they have a duty to refer patients who express a desire to pursue physician aid-in-dying to another provider who can assist them.
Physician aid-in-dying laws vary somewhat so it’s important that physicians in these areas be aware of their specific statute, Dr. Klitzman said. California, Colorado, Hawaii, Maine, New Jersey, New Mexico, Oregon, Vermont, Washington, and the District of Columbia have these laws.
“In these states, if a terminally ill patient says they don’t want to live anymore, a physician would first decide if this is a result of depression or if it’s a request for physician aid-in-dying,” he said. “Even then, in most cases, the patient would be evaluated by not one, but two different health professionals at two different points. We want to see if it is a consistent decision that the person has made that they want physician aid-in-dying, and not just that they’ve had a bad day or a setback in their treatment.”
In the case of the internist who told no one of his patient’s suicide plan, Dr. Klitzman said he would have dug deeper into the patient’s mindset.
“Not knowing anything about the patient or the doctor, I would have responded differently,” he said. “I think a physician should address why a patient feels that way. They may feel their pain is unbearable, and we potentially offer more pain relief. Maybe the patient shows evidence of having depression, which may be treatable [with medication]. The patient would then feel better and be able to spend quality time with family and loved ones, make sure their affairs are in order, and have a chance to say goodbye.”
A version of this article first appeared on Medscape.com.
Walnuts lowered LDL cholesterol in healthy seniors: WAHA study
Benefit sustained over 2 years
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
Benefit sustained over 2 years
Benefit sustained over 2 years
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
FROM CIRCULATION
LOOP trial undercuts value of long-term continuous ECG screening for AFib
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.