User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
New international dermatology registry tracks monkeypox cases
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
Dermatology and monkeypox: What you need to know
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
Children and COVID: ED visits and new admissions change course
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).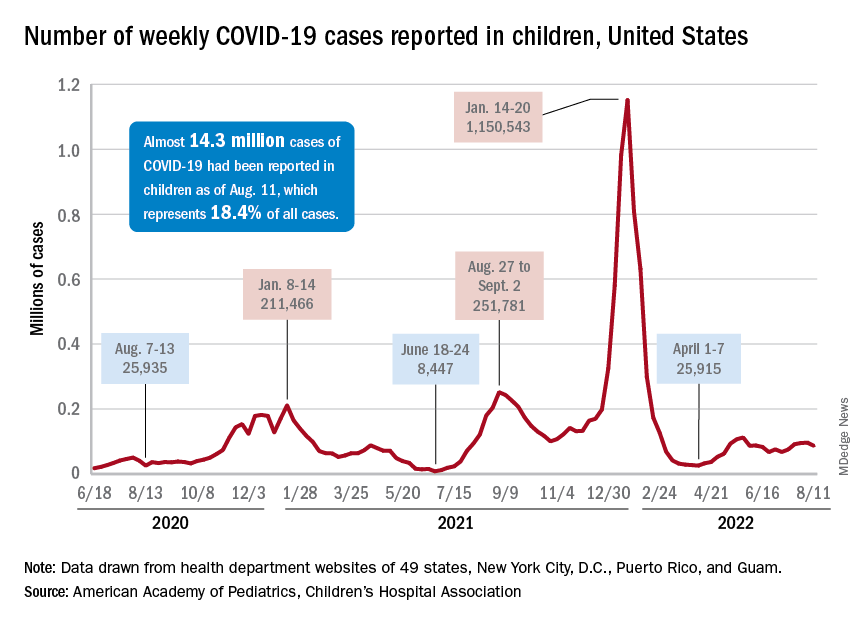
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.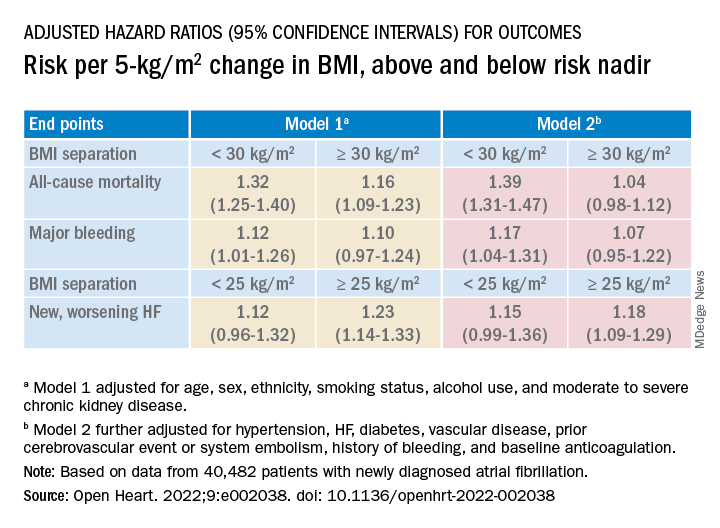
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Dermatologists skeptical of calamine lotion TikTok trend
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Patients who won’t pay: What’s your recourse?
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Guidelines: Convalescent plasma not recommended for most hospitalized with COVID
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
In summarizing the practice statement, the authors write, “CCP is most effective when transfused with high neutralizing titers early after symptom onset.”
The five guidelines, were published in Annals of Internal Medicine. The guidelines and strength of recommendations are:
- Nonhospitalized patients at high risk for disease progression should have CCP transfusion in addition to usual standard of care. (weak)
- CCP transfusion should not be done for unselected hospitalized patients with moderate or severe disease. This does not apply to immunosuppressed patients or those who lack antibodies against SARS-CoV-2. (strong)
- CCP transfusion is suggested in addition to the usual standard of care for hospitalized patients with COVID-19 who do not have SARS-CoV-2 antibodies at admission. (weak)
- Prophylactic CCP transfusion is not recommended for uninfected people with close contact exposure to someone with COVID-19. (weak)
- The AABB suggests CCP transfusion along with standard of care for hospitalized patients with COVID-19 and preexisting immunosuppression. (weak)
Multiple guidelines for use of CCP are similar
In an accompanying editorial, Jason V. Baker, MD, MS, and H. Clifford Lane, MD, who are part of the National Institutes of Health Treatment Guidelines Panel, say guidelines from that organization around CCP generally align with those of the AABB and the Infectious Diseases Society of America.
They all note CCP’s potential for helping immunocompromised patients and they recommend against CCP in unselected, hospitalized patients.
The main difference is that the AABB also “suggests” using CCP in combination with other standard treatments for outpatients at high risk for disease progression, regardless of their immune status, write Dr. Baker, who is with Hennepin Healthcare and the department of medicine at the University of Minnesota in Minneapolis, and Dr. Lane, who is with the National Institutes of Health.
The precise circumstance for recommending CCP remains unclear, Dr. Baker and Dr. Lane write. That’s because most available evidence has come in the absence of vaccines and antiviral agents, including nirmatrelvir–ritonavir (Paxlovid), they explain.
“At this point in the pandemic, it seems that the patient most likely to benefit from passive antibody therapy is the immunocompromised host with COVID-19 who cannot mount their own antibody response to vaccine or prior infection,” they write.
“In that setting, and in the absence of other antiviral treatments or progression despite receipt of standard treatments, high-titer CCP from a recently recovered donor is a reasonable approach,” they conclude.
Eileen Barrett, MD, MPH, an assistant professor in the division of hospital medicine at the University of New Mexico in Albuquerque, said in an interview that “clinical guidelines like this really help practicing physicians as we navigate the explosion of research findings since the start of the pandemic.”
One strong recommendation
Dr. Barrett pointed out that four of the five recommendations are rated “weak.”
“The weak recommendations for convalescent plasma in most situations is very humbling,” she said, “particularly as we recall the earliest days of the pandemic when many hospitalized patients received this treatment when little was known about what could help.”
She highlighted the paper’s only strong recommendation, which was against convalescent plasma use for the vast majority of hospitalized patients with COVID.
“That clinical bottom line is what most clinicians will look for,” she said.
“Similarly,” she said, “the accompanying editorial is so helpful in reminding the reader that, despite some possible benefit to convalescent plasma in a smaller subgroup of patients, variant-appropriate monoclonal antibodies and antivirals are better options.”
The disclosures for lead author of the guidelines, Lise J. Estcourt, MB BChir, DPhil, with the National Health Service Blood and Transplant Department and Radcliffe department of medicine at the University of Oxford (England) and her colleagues are available at https://rmed.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1079. The editorialists and Dr. Barrett declare no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Risk factors in children linked to stroke as soon as 30s, 40s
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
How nonadherence complicates cardiology, in two trials
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Each study adds new twist
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Polio virus found in NYC sewer system
Polio virus has been discovered in New York City’s sewers, suggesting that the virus is circulating in the city, New York’s health authorities said Aug. 12.
“For every one case of paralytic polio identified, hundreds more may be undetected,” Dr. Bassett said. “The best way to keep adults and children polio-free is through safe and effective immunization.”
Polio can cause permanent paralysis of limbs and even death in some cases. Before this outbreak, the last case of polio in the United States was in 2013.
The announcement came after a man in Rockland County, New York, north of the city, was stricken with polio at the end of July and paralyzed.
Now, health officials fear that the detection of polio in NYC wastewater could bring other cases of paralytic polio.
“It is not surprising, since this is something already seen with Rockland County,” Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, told this news organization. “This is solely the result of under-vaccination in the area. I think it’s likely that we will see a few paralytic cases but not a high number.”
Vaccinations declined in pandemic
Among the worries is that vaccination rates across New York City dipped during the pandemic because pediatrician visits were postponed.
In New York City, the overall rate of polio vaccination among children aged 5 years or younger is 86%. Still, in some city ZIP codes, fewer than two-thirds of children in that age group have received the full dosage, which worries health officials.
However, most adults were vaccinated against polio as children.
Across New York state, nearly 80% of people have been vaccinated, according to data from the state public health department. Those who are unvaccinated are at risk, but the polio vaccine is nearly 100% effective in people who are fully immunized.
New York health authorities are calling on those who are unvaccinated to get their shots immediately.
“The risk to New Yorkers is real, but the defense is so simple – get vaccinated against polio,” New York City Health Commissioner Ashwin Vasan, MD, PhD, said in a statement. “Polio is entirely preventable, and its reappearance should be a call for all of us.”
Though many of those who are infected have no symptoms, about 4% will get viral meningitis “and about 1 in 200 will become paralyzed,” according to a news release.
Symptoms can be flu-like
Symptoms can include those similar to the flu, such as sore throat, fever, fatigue, nausea, and stomach ache. There is no cure for the disease.
The city’s health department has given no details about where exactly polio had been found in NYC’s wastewater nor did they give dates the virus was detected.
Health authorities urged parents of children who are not yet fully vaccinated to bring them to their pediatricians.
In 1916, polio killed 6,000 people in the United States and left at least another 21,000 – most of them children – permanently disabled.
An outbreak in 1952 caused paralysis in more than 20,000 people and left many children on iron lungs. The first effective vaccine emerged just a few years later and the virus began to wane.
A version of this article first appeared on Medscape.com.
Polio virus has been discovered in New York City’s sewers, suggesting that the virus is circulating in the city, New York’s health authorities said Aug. 12.
“For every one case of paralytic polio identified, hundreds more may be undetected,” Dr. Bassett said. “The best way to keep adults and children polio-free is through safe and effective immunization.”
Polio can cause permanent paralysis of limbs and even death in some cases. Before this outbreak, the last case of polio in the United States was in 2013.
The announcement came after a man in Rockland County, New York, north of the city, was stricken with polio at the end of July and paralyzed.
Now, health officials fear that the detection of polio in NYC wastewater could bring other cases of paralytic polio.
“It is not surprising, since this is something already seen with Rockland County,” Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, told this news organization. “This is solely the result of under-vaccination in the area. I think it’s likely that we will see a few paralytic cases but not a high number.”
Vaccinations declined in pandemic
Among the worries is that vaccination rates across New York City dipped during the pandemic because pediatrician visits were postponed.
In New York City, the overall rate of polio vaccination among children aged 5 years or younger is 86%. Still, in some city ZIP codes, fewer than two-thirds of children in that age group have received the full dosage, which worries health officials.
However, most adults were vaccinated against polio as children.
Across New York state, nearly 80% of people have been vaccinated, according to data from the state public health department. Those who are unvaccinated are at risk, but the polio vaccine is nearly 100% effective in people who are fully immunized.
New York health authorities are calling on those who are unvaccinated to get their shots immediately.
“The risk to New Yorkers is real, but the defense is so simple – get vaccinated against polio,” New York City Health Commissioner Ashwin Vasan, MD, PhD, said in a statement. “Polio is entirely preventable, and its reappearance should be a call for all of us.”
Though many of those who are infected have no symptoms, about 4% will get viral meningitis “and about 1 in 200 will become paralyzed,” according to a news release.
Symptoms can be flu-like
Symptoms can include those similar to the flu, such as sore throat, fever, fatigue, nausea, and stomach ache. There is no cure for the disease.
The city’s health department has given no details about where exactly polio had been found in NYC’s wastewater nor did they give dates the virus was detected.
Health authorities urged parents of children who are not yet fully vaccinated to bring them to their pediatricians.
In 1916, polio killed 6,000 people in the United States and left at least another 21,000 – most of them children – permanently disabled.
An outbreak in 1952 caused paralysis in more than 20,000 people and left many children on iron lungs. The first effective vaccine emerged just a few years later and the virus began to wane.
A version of this article first appeared on Medscape.com.
Polio virus has been discovered in New York City’s sewers, suggesting that the virus is circulating in the city, New York’s health authorities said Aug. 12.
“For every one case of paralytic polio identified, hundreds more may be undetected,” Dr. Bassett said. “The best way to keep adults and children polio-free is through safe and effective immunization.”
Polio can cause permanent paralysis of limbs and even death in some cases. Before this outbreak, the last case of polio in the United States was in 2013.
The announcement came after a man in Rockland County, New York, north of the city, was stricken with polio at the end of July and paralyzed.
Now, health officials fear that the detection of polio in NYC wastewater could bring other cases of paralytic polio.
“It is not surprising, since this is something already seen with Rockland County,” Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, told this news organization. “This is solely the result of under-vaccination in the area. I think it’s likely that we will see a few paralytic cases but not a high number.”
Vaccinations declined in pandemic
Among the worries is that vaccination rates across New York City dipped during the pandemic because pediatrician visits were postponed.
In New York City, the overall rate of polio vaccination among children aged 5 years or younger is 86%. Still, in some city ZIP codes, fewer than two-thirds of children in that age group have received the full dosage, which worries health officials.
However, most adults were vaccinated against polio as children.
Across New York state, nearly 80% of people have been vaccinated, according to data from the state public health department. Those who are unvaccinated are at risk, but the polio vaccine is nearly 100% effective in people who are fully immunized.
New York health authorities are calling on those who are unvaccinated to get their shots immediately.
“The risk to New Yorkers is real, but the defense is so simple – get vaccinated against polio,” New York City Health Commissioner Ashwin Vasan, MD, PhD, said in a statement. “Polio is entirely preventable, and its reappearance should be a call for all of us.”
Though many of those who are infected have no symptoms, about 4% will get viral meningitis “and about 1 in 200 will become paralyzed,” according to a news release.
Symptoms can be flu-like
Symptoms can include those similar to the flu, such as sore throat, fever, fatigue, nausea, and stomach ache. There is no cure for the disease.
The city’s health department has given no details about where exactly polio had been found in NYC’s wastewater nor did they give dates the virus was detected.
Health authorities urged parents of children who are not yet fully vaccinated to bring them to their pediatricians.
In 1916, polio killed 6,000 people in the United States and left at least another 21,000 – most of them children – permanently disabled.
An outbreak in 1952 caused paralysis in more than 20,000 people and left many children on iron lungs. The first effective vaccine emerged just a few years later and the virus began to wane.
A version of this article first appeared on Medscape.com.