User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Mistake: Doc does vasectomy instead of circumcision; patient sues; more
, according to a story reported in the Des Moines Register, among other news sites.
In 2015, an immigrant from Myanmar named Zaw Zaw was referred by his primary care physician (PCP) to The Iowa Clinic, in West Des Moines, for a circumcision.
Because Mr. Zaw didn’t speak English, a language translation company provided him with an interpreter for the procedure, as well as for any necessary follow-up appointments. The procedure would be performed by urologist Kevin Birusingh, MD, who at the time was employed by the clinic. Prior to the surgery, however, a miscommunication occurred, leading the urologist to perform a vasectomy instead of a circumcision on his patient. It was not until a later follow-up visit that the error was discovered, along with Mr. Zaw’s realization that he was now medically sterile and unable to father more children.
Mr. Zaw sued both Dr. Birusingh and the clinic, which in turn sued the translation company. Against Dr. Birusingh, Mr. Zaw made two claims: one, that the doctor hadn’t obtained the proper informed consent, and two, that he had engaged in “negligent” communications with several key individuals, including other clinic staff members and Dr. Zaw’s PCP.
In 2019, a trial jury found the clinic liable, awarding Mr. Zaw more than $1.4 million in damages. The same jury found no liability on the part of the translation company, however.
Following the verdict, the clinic appealed to the Iowa Court of Appeals. Late last month, the appeals court sent the case back to the lower court for a new trial.
In its ruling, written by Judge Sharon Soorholtz Greer, the appeals court said it could find no evidence that, in failing to communicate personally with his colleagues or Mr. Zaw’s PCP, Dr. Birusingh had violated an established standard of care. For this reason, Judge Greer said, the claim of negligent communication should have been dismissed before it went to the jury. Because it hadn’t been, however, she concluded there was no way of determining to what extent, if at all, it affected the jury verdict. She ordered a new trial that would exclude the negligent communication claim.
In its appeal, The Iowa Clinic also sought to have the first claim dismissed – the one involving informed consent. Contrary to the testimony of a defense expert witness, the clinic argued, Iowa malpractice law doesn’t automatically fault a doctor whose patient misunderstands the procedure he or she is about to receive – as long as, that is, the doctor has made a “reasonable effort” to inform the patient beforehand.
Judge Greer agreed on this point of general law but still permitted the retrial to go forward. Why? She did so because, as the decision made clear, no expert testimony is needed to establish medical malpractice if the lack of care is so obvious that it’s within the comprehension of a layperson.
Mr. Zaw and his attorney, Ben Novotny, have petitioned the Iowa Supreme Court to review the appeals decision.
If the high court refuses that petition and the trial court schedules a new trial on the informed consent issue alone, Mr. Novotny is optimistic: “However it’s determined, whether it’s here [district court] or at the Supreme Court, we’ll live with the court’s decision, we’ll retry the case, and we’ll ask for more money.”
Jury exceeds state cap in infant head-trauma case
In what’s being called the state’s largest medical malpractice judgment to date, a Nebraska jury has handed down a multimillion-dollar award to a couple whose daughter was improperly discharged from the hospital after suffering a fall-related seizure, a story in the Omaha World-Herald reports.
The fall occurred in 2017 at a day care center, where then 11-month-old Vivianne Marousek hit her head while playing and began experiencing a seizure. Taken to an Omaha hospital, the infant was first treated by an emergency department doctor and then placed in the care of a hospital pediatrician. (The ED doctor wasn’t a party to the subsequent suit.)
According to the plaintiffs, after examining and observing the child, the pediatrician concluded that her seizures wouldn’t persist and that she should be discharged from the hospital. Within 48 hours after returning home, however, Vivianne suffered severe seizures, resulting in debilitating brain damage. Healthy before her fall, the now 6-year-old is blind, in a wheelchair, has a form of cerebral palsy, and can’t communicate beyond rudimentary responses to her parents’ voices.
After a 10-hour deliberation, the trial jury found both the hospital and the pediatrician liable for the child’s injuries. It awarded $21.5 million in damages for Vivianne’s ongoing medical care and $4.6 million in noneconomic damages to her parents.
An attorney for the hospital and pediatrician is expected to contest the award. Specifically, he’s expected to ask that the trial judge impose Nebraska’s $2.25 million cap on medical malpractice verdicts, thereby reducing the total award to $4.5 million, to be split evenly between Vivianne and her parents.
If that happens, the attorney for the plaintiffs has promised to contest the request, arguing that the state’s cap is unconstitutional and that the child’s lifetime medical bills will far exceed it.
University’s negligence caused them unnecessary suffering, women claim
A group of seven women has sued Yale University Medical School, in New Haven, Conn., for failing to safeguard the pain medication normally used during in vitro fertilization treatments, reports a story on Eyewitness News3 and other news sites.
The women’s suit follows a March 2021 guilty plea by a Yale staff nurse who was addicted to pain meds. In her plea, the nurse admitted to using a syringe to extract fentanyl from vials and then refilling those same vials with saline. Federal prosecutors say that at least 175 vials – some containing only saline and others with trace amounts of fentanyl – were tampered with in this manner.
As a result of Yale’s failure to guard against such actions, the women claim, they were subjected to unnecessary trauma and stress during their IVF treatments, which experts say can be unpleasant and take a physiologic toll on the body without the proper pain control.
The current suit won’t be the last, says the attorney representing the group of seven women. “We have somewhere on the line of 40-50 women who’ve been affected who contacted us,” he says.
A spokesperson for Yale declined to comment on the pending litigation.
A version of this article first appeared on Medscape.com.
, according to a story reported in the Des Moines Register, among other news sites.
In 2015, an immigrant from Myanmar named Zaw Zaw was referred by his primary care physician (PCP) to The Iowa Clinic, in West Des Moines, for a circumcision.
Because Mr. Zaw didn’t speak English, a language translation company provided him with an interpreter for the procedure, as well as for any necessary follow-up appointments. The procedure would be performed by urologist Kevin Birusingh, MD, who at the time was employed by the clinic. Prior to the surgery, however, a miscommunication occurred, leading the urologist to perform a vasectomy instead of a circumcision on his patient. It was not until a later follow-up visit that the error was discovered, along with Mr. Zaw’s realization that he was now medically sterile and unable to father more children.
Mr. Zaw sued both Dr. Birusingh and the clinic, which in turn sued the translation company. Against Dr. Birusingh, Mr. Zaw made two claims: one, that the doctor hadn’t obtained the proper informed consent, and two, that he had engaged in “negligent” communications with several key individuals, including other clinic staff members and Dr. Zaw’s PCP.
In 2019, a trial jury found the clinic liable, awarding Mr. Zaw more than $1.4 million in damages. The same jury found no liability on the part of the translation company, however.
Following the verdict, the clinic appealed to the Iowa Court of Appeals. Late last month, the appeals court sent the case back to the lower court for a new trial.
In its ruling, written by Judge Sharon Soorholtz Greer, the appeals court said it could find no evidence that, in failing to communicate personally with his colleagues or Mr. Zaw’s PCP, Dr. Birusingh had violated an established standard of care. For this reason, Judge Greer said, the claim of negligent communication should have been dismissed before it went to the jury. Because it hadn’t been, however, she concluded there was no way of determining to what extent, if at all, it affected the jury verdict. She ordered a new trial that would exclude the negligent communication claim.
In its appeal, The Iowa Clinic also sought to have the first claim dismissed – the one involving informed consent. Contrary to the testimony of a defense expert witness, the clinic argued, Iowa malpractice law doesn’t automatically fault a doctor whose patient misunderstands the procedure he or she is about to receive – as long as, that is, the doctor has made a “reasonable effort” to inform the patient beforehand.
Judge Greer agreed on this point of general law but still permitted the retrial to go forward. Why? She did so because, as the decision made clear, no expert testimony is needed to establish medical malpractice if the lack of care is so obvious that it’s within the comprehension of a layperson.
Mr. Zaw and his attorney, Ben Novotny, have petitioned the Iowa Supreme Court to review the appeals decision.
If the high court refuses that petition and the trial court schedules a new trial on the informed consent issue alone, Mr. Novotny is optimistic: “However it’s determined, whether it’s here [district court] or at the Supreme Court, we’ll live with the court’s decision, we’ll retry the case, and we’ll ask for more money.”
Jury exceeds state cap in infant head-trauma case
In what’s being called the state’s largest medical malpractice judgment to date, a Nebraska jury has handed down a multimillion-dollar award to a couple whose daughter was improperly discharged from the hospital after suffering a fall-related seizure, a story in the Omaha World-Herald reports.
The fall occurred in 2017 at a day care center, where then 11-month-old Vivianne Marousek hit her head while playing and began experiencing a seizure. Taken to an Omaha hospital, the infant was first treated by an emergency department doctor and then placed in the care of a hospital pediatrician. (The ED doctor wasn’t a party to the subsequent suit.)
According to the plaintiffs, after examining and observing the child, the pediatrician concluded that her seizures wouldn’t persist and that she should be discharged from the hospital. Within 48 hours after returning home, however, Vivianne suffered severe seizures, resulting in debilitating brain damage. Healthy before her fall, the now 6-year-old is blind, in a wheelchair, has a form of cerebral palsy, and can’t communicate beyond rudimentary responses to her parents’ voices.
After a 10-hour deliberation, the trial jury found both the hospital and the pediatrician liable for the child’s injuries. It awarded $21.5 million in damages for Vivianne’s ongoing medical care and $4.6 million in noneconomic damages to her parents.
An attorney for the hospital and pediatrician is expected to contest the award. Specifically, he’s expected to ask that the trial judge impose Nebraska’s $2.25 million cap on medical malpractice verdicts, thereby reducing the total award to $4.5 million, to be split evenly between Vivianne and her parents.
If that happens, the attorney for the plaintiffs has promised to contest the request, arguing that the state’s cap is unconstitutional and that the child’s lifetime medical bills will far exceed it.
University’s negligence caused them unnecessary suffering, women claim
A group of seven women has sued Yale University Medical School, in New Haven, Conn., for failing to safeguard the pain medication normally used during in vitro fertilization treatments, reports a story on Eyewitness News3 and other news sites.
The women’s suit follows a March 2021 guilty plea by a Yale staff nurse who was addicted to pain meds. In her plea, the nurse admitted to using a syringe to extract fentanyl from vials and then refilling those same vials with saline. Federal prosecutors say that at least 175 vials – some containing only saline and others with trace amounts of fentanyl – were tampered with in this manner.
As a result of Yale’s failure to guard against such actions, the women claim, they were subjected to unnecessary trauma and stress during their IVF treatments, which experts say can be unpleasant and take a physiologic toll on the body without the proper pain control.
The current suit won’t be the last, says the attorney representing the group of seven women. “We have somewhere on the line of 40-50 women who’ve been affected who contacted us,” he says.
A spokesperson for Yale declined to comment on the pending litigation.
A version of this article first appeared on Medscape.com.
, according to a story reported in the Des Moines Register, among other news sites.
In 2015, an immigrant from Myanmar named Zaw Zaw was referred by his primary care physician (PCP) to The Iowa Clinic, in West Des Moines, for a circumcision.
Because Mr. Zaw didn’t speak English, a language translation company provided him with an interpreter for the procedure, as well as for any necessary follow-up appointments. The procedure would be performed by urologist Kevin Birusingh, MD, who at the time was employed by the clinic. Prior to the surgery, however, a miscommunication occurred, leading the urologist to perform a vasectomy instead of a circumcision on his patient. It was not until a later follow-up visit that the error was discovered, along with Mr. Zaw’s realization that he was now medically sterile and unable to father more children.
Mr. Zaw sued both Dr. Birusingh and the clinic, which in turn sued the translation company. Against Dr. Birusingh, Mr. Zaw made two claims: one, that the doctor hadn’t obtained the proper informed consent, and two, that he had engaged in “negligent” communications with several key individuals, including other clinic staff members and Dr. Zaw’s PCP.
In 2019, a trial jury found the clinic liable, awarding Mr. Zaw more than $1.4 million in damages. The same jury found no liability on the part of the translation company, however.
Following the verdict, the clinic appealed to the Iowa Court of Appeals. Late last month, the appeals court sent the case back to the lower court for a new trial.
In its ruling, written by Judge Sharon Soorholtz Greer, the appeals court said it could find no evidence that, in failing to communicate personally with his colleagues or Mr. Zaw’s PCP, Dr. Birusingh had violated an established standard of care. For this reason, Judge Greer said, the claim of negligent communication should have been dismissed before it went to the jury. Because it hadn’t been, however, she concluded there was no way of determining to what extent, if at all, it affected the jury verdict. She ordered a new trial that would exclude the negligent communication claim.
In its appeal, The Iowa Clinic also sought to have the first claim dismissed – the one involving informed consent. Contrary to the testimony of a defense expert witness, the clinic argued, Iowa malpractice law doesn’t automatically fault a doctor whose patient misunderstands the procedure he or she is about to receive – as long as, that is, the doctor has made a “reasonable effort” to inform the patient beforehand.
Judge Greer agreed on this point of general law but still permitted the retrial to go forward. Why? She did so because, as the decision made clear, no expert testimony is needed to establish medical malpractice if the lack of care is so obvious that it’s within the comprehension of a layperson.
Mr. Zaw and his attorney, Ben Novotny, have petitioned the Iowa Supreme Court to review the appeals decision.
If the high court refuses that petition and the trial court schedules a new trial on the informed consent issue alone, Mr. Novotny is optimistic: “However it’s determined, whether it’s here [district court] or at the Supreme Court, we’ll live with the court’s decision, we’ll retry the case, and we’ll ask for more money.”
Jury exceeds state cap in infant head-trauma case
In what’s being called the state’s largest medical malpractice judgment to date, a Nebraska jury has handed down a multimillion-dollar award to a couple whose daughter was improperly discharged from the hospital after suffering a fall-related seizure, a story in the Omaha World-Herald reports.
The fall occurred in 2017 at a day care center, where then 11-month-old Vivianne Marousek hit her head while playing and began experiencing a seizure. Taken to an Omaha hospital, the infant was first treated by an emergency department doctor and then placed in the care of a hospital pediatrician. (The ED doctor wasn’t a party to the subsequent suit.)
According to the plaintiffs, after examining and observing the child, the pediatrician concluded that her seizures wouldn’t persist and that she should be discharged from the hospital. Within 48 hours after returning home, however, Vivianne suffered severe seizures, resulting in debilitating brain damage. Healthy before her fall, the now 6-year-old is blind, in a wheelchair, has a form of cerebral palsy, and can’t communicate beyond rudimentary responses to her parents’ voices.
After a 10-hour deliberation, the trial jury found both the hospital and the pediatrician liable for the child’s injuries. It awarded $21.5 million in damages for Vivianne’s ongoing medical care and $4.6 million in noneconomic damages to her parents.
An attorney for the hospital and pediatrician is expected to contest the award. Specifically, he’s expected to ask that the trial judge impose Nebraska’s $2.25 million cap on medical malpractice verdicts, thereby reducing the total award to $4.5 million, to be split evenly between Vivianne and her parents.
If that happens, the attorney for the plaintiffs has promised to contest the request, arguing that the state’s cap is unconstitutional and that the child’s lifetime medical bills will far exceed it.
University’s negligence caused them unnecessary suffering, women claim
A group of seven women has sued Yale University Medical School, in New Haven, Conn., for failing to safeguard the pain medication normally used during in vitro fertilization treatments, reports a story on Eyewitness News3 and other news sites.
The women’s suit follows a March 2021 guilty plea by a Yale staff nurse who was addicted to pain meds. In her plea, the nurse admitted to using a syringe to extract fentanyl from vials and then refilling those same vials with saline. Federal prosecutors say that at least 175 vials – some containing only saline and others with trace amounts of fentanyl – were tampered with in this manner.
As a result of Yale’s failure to guard against such actions, the women claim, they were subjected to unnecessary trauma and stress during their IVF treatments, which experts say can be unpleasant and take a physiologic toll on the body without the proper pain control.
The current suit won’t be the last, says the attorney representing the group of seven women. “We have somewhere on the line of 40-50 women who’ve been affected who contacted us,” he says.
A spokesperson for Yale declined to comment on the pending litigation.
A version of this article first appeared on Medscape.com.
More Americans skipping medical care because of cost, survey says
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
Appendicitis: Up-front antibiotics OK in select patients
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
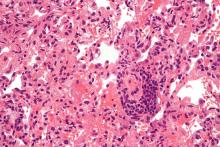
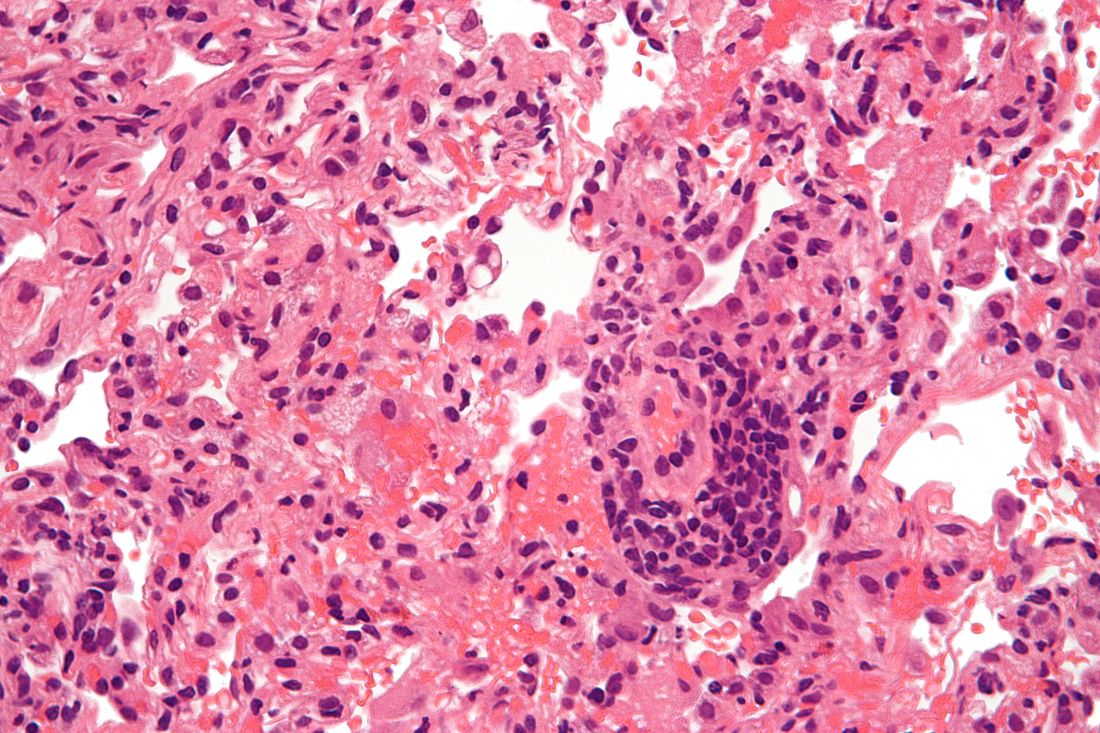
Lung transplantation in the era of COVID-19: New issues and paradigms
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Filler complications involving vascular necrosis, vision changes on the rise
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM ASDS 2021
A very strange place to find a tooth
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
Female patients fare worse with male surgeons, study finds
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
New data on rare myocarditis after COVID-19 vaccination
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
AHA challenges diet doctor’s study alleging COVID vax risks
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
In metastatic breast cancer, primary resections on the decline
Retrospective studies have suggested a possible benefit to resection, according to Sasha Douglas, MD, who presented the study (abstract PD7-06) at the 2021 San Antonio Breast Cancer Symposium. “Intuitively, you would think that you would want to get the primary tumor out even if it’s metastasized, so that it couldn’t metastasize more,” said Dr. Douglas, who is a surgical resident at the University of California, San Diego.
However, clinical trials have yielded mixed results, and the picture is complicated by the various molecular subtypes of breast cancer, metastatic sites, and other factors. “Different studies, whether it’s retrospective, and a really large database that has lots of numbers of patients, can give you a different answer than a smaller prospective randomized, controlled study in a different cohort of patients. So, we just thought it would be really interesting to look at all the trends over time at Commission on Cancer–accredited hospitals. Do they seem to be following what the latest literature is showing?” Dr. Douglas said in an interview.
The researchers used data from 87,331 cases from the National Cancer Database (NCDB) and examined rates of primary surgery as well as palliative care in women with metastatic breast cancer who had responded well to systemic therapy.
Between 2004 and 2009, the frequency of primary tumor resection remained near 35% (with a peak of 37% in 2009), then began a steady descent to 18% by 2017. The researchers found similar trends in estrogen receptor–positive/progesterone receptor–positive, HER2-negative (ER/PR+HERer2–); HER2-positive; and triple-negative subtypes.
In 2004, 48% of patients received only systemic therapy, while 37% received some combination of surgery and radiation to the primary tumor. By 2019, 69% received only systemic therapy and 20% received locoregional therapy (P < .001). “It seems that surgeons and providers and medical oncologists are becoming more selective about who they’re going to offer surgery to, and I think that’s very appropriate,” said Dr. Douglas.
But another finding suggests room for improvement: Just 21% of patients received palliative care. “I think that everybody with a major systemic illness like this would benefit from palliative care, just on a supportive basis. The palliative care team can really help people with quality of life, but I think it still has that stigma, and I think that’s what we’ve seen from our study,” said Dr. Douglas.
“We’re just postulating, [but] a lot of that could be from the stigma of thinking that palliative care means giving up. It doesn’t necessarily mean that. It means you’re dealing with a difficult chronic illness, and [palliative care] can be very, very helpful for patients,” said Dr. Douglas.
The study is limited by its retrospective nature, and palliative care might be underreported in the NCDB.
The study was funded by the National Cancer Institute and the University of California, San Diego. Dr. Douglas has no financial disclosures.
Retrospective studies have suggested a possible benefit to resection, according to Sasha Douglas, MD, who presented the study (abstract PD7-06) at the 2021 San Antonio Breast Cancer Symposium. “Intuitively, you would think that you would want to get the primary tumor out even if it’s metastasized, so that it couldn’t metastasize more,” said Dr. Douglas, who is a surgical resident at the University of California, San Diego.
However, clinical trials have yielded mixed results, and the picture is complicated by the various molecular subtypes of breast cancer, metastatic sites, and other factors. “Different studies, whether it’s retrospective, and a really large database that has lots of numbers of patients, can give you a different answer than a smaller prospective randomized, controlled study in a different cohort of patients. So, we just thought it would be really interesting to look at all the trends over time at Commission on Cancer–accredited hospitals. Do they seem to be following what the latest literature is showing?” Dr. Douglas said in an interview.
The researchers used data from 87,331 cases from the National Cancer Database (NCDB) and examined rates of primary surgery as well as palliative care in women with metastatic breast cancer who had responded well to systemic therapy.
Between 2004 and 2009, the frequency of primary tumor resection remained near 35% (with a peak of 37% in 2009), then began a steady descent to 18% by 2017. The researchers found similar trends in estrogen receptor–positive/progesterone receptor–positive, HER2-negative (ER/PR+HERer2–); HER2-positive; and triple-negative subtypes.
In 2004, 48% of patients received only systemic therapy, while 37% received some combination of surgery and radiation to the primary tumor. By 2019, 69% received only systemic therapy and 20% received locoregional therapy (P < .001). “It seems that surgeons and providers and medical oncologists are becoming more selective about who they’re going to offer surgery to, and I think that’s very appropriate,” said Dr. Douglas.
But another finding suggests room for improvement: Just 21% of patients received palliative care. “I think that everybody with a major systemic illness like this would benefit from palliative care, just on a supportive basis. The palliative care team can really help people with quality of life, but I think it still has that stigma, and I think that’s what we’ve seen from our study,” said Dr. Douglas.
“We’re just postulating, [but] a lot of that could be from the stigma of thinking that palliative care means giving up. It doesn’t necessarily mean that. It means you’re dealing with a difficult chronic illness, and [palliative care] can be very, very helpful for patients,” said Dr. Douglas.
The study is limited by its retrospective nature, and palliative care might be underreported in the NCDB.
The study was funded by the National Cancer Institute and the University of California, San Diego. Dr. Douglas has no financial disclosures.
Retrospective studies have suggested a possible benefit to resection, according to Sasha Douglas, MD, who presented the study (abstract PD7-06) at the 2021 San Antonio Breast Cancer Symposium. “Intuitively, you would think that you would want to get the primary tumor out even if it’s metastasized, so that it couldn’t metastasize more,” said Dr. Douglas, who is a surgical resident at the University of California, San Diego.
However, clinical trials have yielded mixed results, and the picture is complicated by the various molecular subtypes of breast cancer, metastatic sites, and other factors. “Different studies, whether it’s retrospective, and a really large database that has lots of numbers of patients, can give you a different answer than a smaller prospective randomized, controlled study in a different cohort of patients. So, we just thought it would be really interesting to look at all the trends over time at Commission on Cancer–accredited hospitals. Do they seem to be following what the latest literature is showing?” Dr. Douglas said in an interview.
The researchers used data from 87,331 cases from the National Cancer Database (NCDB) and examined rates of primary surgery as well as palliative care in women with metastatic breast cancer who had responded well to systemic therapy.
Between 2004 and 2009, the frequency of primary tumor resection remained near 35% (with a peak of 37% in 2009), then began a steady descent to 18% by 2017. The researchers found similar trends in estrogen receptor–positive/progesterone receptor–positive, HER2-negative (ER/PR+HERer2–); HER2-positive; and triple-negative subtypes.
In 2004, 48% of patients received only systemic therapy, while 37% received some combination of surgery and radiation to the primary tumor. By 2019, 69% received only systemic therapy and 20% received locoregional therapy (P < .001). “It seems that surgeons and providers and medical oncologists are becoming more selective about who they’re going to offer surgery to, and I think that’s very appropriate,” said Dr. Douglas.
But another finding suggests room for improvement: Just 21% of patients received palliative care. “I think that everybody with a major systemic illness like this would benefit from palliative care, just on a supportive basis. The palliative care team can really help people with quality of life, but I think it still has that stigma, and I think that’s what we’ve seen from our study,” said Dr. Douglas.
“We’re just postulating, [but] a lot of that could be from the stigma of thinking that palliative care means giving up. It doesn’t necessarily mean that. It means you’re dealing with a difficult chronic illness, and [palliative care] can be very, very helpful for patients,” said Dr. Douglas.
The study is limited by its retrospective nature, and palliative care might be underreported in the NCDB.
The study was funded by the National Cancer Institute and the University of California, San Diego. Dr. Douglas has no financial disclosures.
FROM SABCS 2021