User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Lead pollutants as harmful to health as particulate matter
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
FROM THE LANCET PLANETARY HEALTH
Do new Alzheimer’s drugs get us closer to solving the Alzheimer’s disease riddle?
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Hyperbaric oxygen therapy for traumatic brain injury: Promising or wishful thinking?
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The surprising link between loneliness and Parkinson’s disease
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
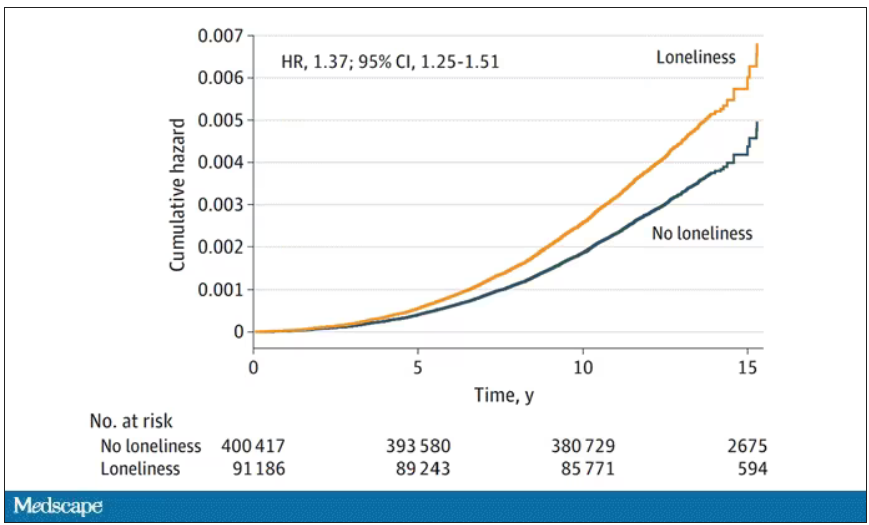
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
From scrubs to screens: Growing your patient base with social media
With physicians under increasing pressure to see more patients in shorter office visits, developing a social media presence may offer valuable opportunities to connect with patients, explain procedures, combat misinformation, talk through a published article, and even share a joke or meme.
But there are caveats for doctors posting on social media platforms. This news organization spoke to four doctors who successfully use social media.
Use social media for the right reasons
While you’re under no obligation to build a social media presence, if you’re going to do it, be sure your intentions are solid, said Don S. Dizon, MD, professor of medicine and professor of surgery at Brown University, Providence, R.I. Dr. Dizon, as @DoctorDon, has 44,700 TikTok followers and uses the platform to answer cancer-related questions.
“It should be your altruism that motivates you to post,” said Dr. Dizon, who is also associate director of community outreach and engagement at the Legorreta Cancer Center in Providence, R.I., and director of medical oncology at Rhode Island Hospital. “What we can do for society at large is to provide our input into issues, add informed opinions where there’s controversy, and address misinformation.”
If you don’t know where to start, consider seeking a digital mentor to talk through your options.
“You may never meet this person, but you should choose them if you like their style, their content, their delivery, and their perspective,” Dr. Dizon said. “Find another doctor out there on social media whom you feel you can emulate. Take your time, too. Soon enough, you’ll develop your own style and your own online persona.”
Post clear, accurate information
If you want to be lighthearted on social media, that’s your choice. But Jennifer Trachtenberg, a pediatrician with nearly 7,000 Instagram followers in New York who posts as @askdrjen, prefers to offer vaccine scheduling tips, alert parents about COVID-19 rates, and offer advice on cold and flu prevention.
“Right now, I’m mainly doing this to educate patients and make them aware of topics that I think are important and that I see my patients needing more information on,” she said. “We have to be clear: People take what we say seriously. So, while it’s important to be relatable, it’s even more important to share evidence-based information.”
Many patients get their information on social media
While patients once came to the doctor armed with information sourced via “Doctor Google,” today, just as many patients use social media to learn about their condition or the medications they’re taking.
Unfortunately, a recent Ohio State University, Columbus, study found that the majority of gynecologic cancer advice on TikTok, for example, was either misleading or inaccurate.
“This misinformation should be a motivator for physicians to explore the social media space,” Dr. Dizon said. “Our voices need to be on there.”
Break down barriers – and make connections
Mike Natter, MD, an endocrinologist in New York, has type 1 diabetes. This informs his work – and his life – and he’s passionate about sharing it with his 117,000 followers as @mike.natter on Instagram.
“A lot of type 1s follow me, so there’s an advocacy component to what I do,” he said. “I enjoy being able to raise awareness and keep people up to date on the newest research and treatment.”
But that’s not all: Dr. Natter is also an artist who went to art school before he went to medical school, and his account is rife with his cartoons and illustrations about everything from valvular disease to diabetic ketoacidosis.
“I found that I was drawing a lot of my notes in medical school,” he said. “When I drew my notes, I did quite well, and I think that using art and illustration is a great tool. It breaks down barriers and makes health information all the more accessible to everyone.”
Share your expertise as a doctor – and a person
As a mom and pediatrician, Krupa Playforth, MD, who practices in Vienna, Va., knows that what she posts carries weight. So, whether she’s writing about backpack safety tips, choking hazards, or separation anxiety, her followers can rest assured that she’s posting responsibly.
“Pediatricians often underestimate how smart parents are,” said Dr. Playforth, who has three kids, ages 8, 5, and 2, and has 137,000 followers on @thepediatricianmom, her Instagram account. “Their anxiety comes from an understandable place, which is why I see my role as that of a parent and pediatrician who can translate the knowledge pediatricians have into something parents can understand.”
Dr. Playforth, who jumped on social media during COVID-19 and experienced a positive response in her local community, said being on social media is imperative if you’re a pediatrician.
“This is the future of pediatric medicine in particular,” she said. “A lot of pediatricians don’t want to embrace social media, but I think that’s a mistake. After all, while parents think pediatricians have all the answers, when we think of our own children, most doctors are like other parents – we can’t think objectively about our kids. It’s helpful for me to share that and to help parents feel less alone.”
If you’re not yet using social media to the best of your physician abilities, you might take a shot at becoming widely recognizable. Pick a preferred platform, answer common patient questions, dispel medical myths, provide pertinent information, and let your personality shine.
A version of this article first appeared on Medscape.com.
With physicians under increasing pressure to see more patients in shorter office visits, developing a social media presence may offer valuable opportunities to connect with patients, explain procedures, combat misinformation, talk through a published article, and even share a joke or meme.
But there are caveats for doctors posting on social media platforms. This news organization spoke to four doctors who successfully use social media.
Use social media for the right reasons
While you’re under no obligation to build a social media presence, if you’re going to do it, be sure your intentions are solid, said Don S. Dizon, MD, professor of medicine and professor of surgery at Brown University, Providence, R.I. Dr. Dizon, as @DoctorDon, has 44,700 TikTok followers and uses the platform to answer cancer-related questions.
“It should be your altruism that motivates you to post,” said Dr. Dizon, who is also associate director of community outreach and engagement at the Legorreta Cancer Center in Providence, R.I., and director of medical oncology at Rhode Island Hospital. “What we can do for society at large is to provide our input into issues, add informed opinions where there’s controversy, and address misinformation.”
If you don’t know where to start, consider seeking a digital mentor to talk through your options.
“You may never meet this person, but you should choose them if you like their style, their content, their delivery, and their perspective,” Dr. Dizon said. “Find another doctor out there on social media whom you feel you can emulate. Take your time, too. Soon enough, you’ll develop your own style and your own online persona.”
Post clear, accurate information
If you want to be lighthearted on social media, that’s your choice. But Jennifer Trachtenberg, a pediatrician with nearly 7,000 Instagram followers in New York who posts as @askdrjen, prefers to offer vaccine scheduling tips, alert parents about COVID-19 rates, and offer advice on cold and flu prevention.
“Right now, I’m mainly doing this to educate patients and make them aware of topics that I think are important and that I see my patients needing more information on,” she said. “We have to be clear: People take what we say seriously. So, while it’s important to be relatable, it’s even more important to share evidence-based information.”
Many patients get their information on social media
While patients once came to the doctor armed with information sourced via “Doctor Google,” today, just as many patients use social media to learn about their condition or the medications they’re taking.
Unfortunately, a recent Ohio State University, Columbus, study found that the majority of gynecologic cancer advice on TikTok, for example, was either misleading or inaccurate.
“This misinformation should be a motivator for physicians to explore the social media space,” Dr. Dizon said. “Our voices need to be on there.”
Break down barriers – and make connections
Mike Natter, MD, an endocrinologist in New York, has type 1 diabetes. This informs his work – and his life – and he’s passionate about sharing it with his 117,000 followers as @mike.natter on Instagram.
“A lot of type 1s follow me, so there’s an advocacy component to what I do,” he said. “I enjoy being able to raise awareness and keep people up to date on the newest research and treatment.”
But that’s not all: Dr. Natter is also an artist who went to art school before he went to medical school, and his account is rife with his cartoons and illustrations about everything from valvular disease to diabetic ketoacidosis.
“I found that I was drawing a lot of my notes in medical school,” he said. “When I drew my notes, I did quite well, and I think that using art and illustration is a great tool. It breaks down barriers and makes health information all the more accessible to everyone.”
Share your expertise as a doctor – and a person
As a mom and pediatrician, Krupa Playforth, MD, who practices in Vienna, Va., knows that what she posts carries weight. So, whether she’s writing about backpack safety tips, choking hazards, or separation anxiety, her followers can rest assured that she’s posting responsibly.
“Pediatricians often underestimate how smart parents are,” said Dr. Playforth, who has three kids, ages 8, 5, and 2, and has 137,000 followers on @thepediatricianmom, her Instagram account. “Their anxiety comes from an understandable place, which is why I see my role as that of a parent and pediatrician who can translate the knowledge pediatricians have into something parents can understand.”
Dr. Playforth, who jumped on social media during COVID-19 and experienced a positive response in her local community, said being on social media is imperative if you’re a pediatrician.
“This is the future of pediatric medicine in particular,” she said. “A lot of pediatricians don’t want to embrace social media, but I think that’s a mistake. After all, while parents think pediatricians have all the answers, when we think of our own children, most doctors are like other parents – we can’t think objectively about our kids. It’s helpful for me to share that and to help parents feel less alone.”
If you’re not yet using social media to the best of your physician abilities, you might take a shot at becoming widely recognizable. Pick a preferred platform, answer common patient questions, dispel medical myths, provide pertinent information, and let your personality shine.
A version of this article first appeared on Medscape.com.
With physicians under increasing pressure to see more patients in shorter office visits, developing a social media presence may offer valuable opportunities to connect with patients, explain procedures, combat misinformation, talk through a published article, and even share a joke or meme.
But there are caveats for doctors posting on social media platforms. This news organization spoke to four doctors who successfully use social media.
Use social media for the right reasons
While you’re under no obligation to build a social media presence, if you’re going to do it, be sure your intentions are solid, said Don S. Dizon, MD, professor of medicine and professor of surgery at Brown University, Providence, R.I. Dr. Dizon, as @DoctorDon, has 44,700 TikTok followers and uses the platform to answer cancer-related questions.
“It should be your altruism that motivates you to post,” said Dr. Dizon, who is also associate director of community outreach and engagement at the Legorreta Cancer Center in Providence, R.I., and director of medical oncology at Rhode Island Hospital. “What we can do for society at large is to provide our input into issues, add informed opinions where there’s controversy, and address misinformation.”
If you don’t know where to start, consider seeking a digital mentor to talk through your options.
“You may never meet this person, but you should choose them if you like their style, their content, their delivery, and their perspective,” Dr. Dizon said. “Find another doctor out there on social media whom you feel you can emulate. Take your time, too. Soon enough, you’ll develop your own style and your own online persona.”
Post clear, accurate information
If you want to be lighthearted on social media, that’s your choice. But Jennifer Trachtenberg, a pediatrician with nearly 7,000 Instagram followers in New York who posts as @askdrjen, prefers to offer vaccine scheduling tips, alert parents about COVID-19 rates, and offer advice on cold and flu prevention.
“Right now, I’m mainly doing this to educate patients and make them aware of topics that I think are important and that I see my patients needing more information on,” she said. “We have to be clear: People take what we say seriously. So, while it’s important to be relatable, it’s even more important to share evidence-based information.”
Many patients get their information on social media
While patients once came to the doctor armed with information sourced via “Doctor Google,” today, just as many patients use social media to learn about their condition or the medications they’re taking.
Unfortunately, a recent Ohio State University, Columbus, study found that the majority of gynecologic cancer advice on TikTok, for example, was either misleading or inaccurate.
“This misinformation should be a motivator for physicians to explore the social media space,” Dr. Dizon said. “Our voices need to be on there.”
Break down barriers – and make connections
Mike Natter, MD, an endocrinologist in New York, has type 1 diabetes. This informs his work – and his life – and he’s passionate about sharing it with his 117,000 followers as @mike.natter on Instagram.
“A lot of type 1s follow me, so there’s an advocacy component to what I do,” he said. “I enjoy being able to raise awareness and keep people up to date on the newest research and treatment.”
But that’s not all: Dr. Natter is also an artist who went to art school before he went to medical school, and his account is rife with his cartoons and illustrations about everything from valvular disease to diabetic ketoacidosis.
“I found that I was drawing a lot of my notes in medical school,” he said. “When I drew my notes, I did quite well, and I think that using art and illustration is a great tool. It breaks down barriers and makes health information all the more accessible to everyone.”
Share your expertise as a doctor – and a person
As a mom and pediatrician, Krupa Playforth, MD, who practices in Vienna, Va., knows that what she posts carries weight. So, whether she’s writing about backpack safety tips, choking hazards, or separation anxiety, her followers can rest assured that she’s posting responsibly.
“Pediatricians often underestimate how smart parents are,” said Dr. Playforth, who has three kids, ages 8, 5, and 2, and has 137,000 followers on @thepediatricianmom, her Instagram account. “Their anxiety comes from an understandable place, which is why I see my role as that of a parent and pediatrician who can translate the knowledge pediatricians have into something parents can understand.”
Dr. Playforth, who jumped on social media during COVID-19 and experienced a positive response in her local community, said being on social media is imperative if you’re a pediatrician.
“This is the future of pediatric medicine in particular,” she said. “A lot of pediatricians don’t want to embrace social media, but I think that’s a mistake. After all, while parents think pediatricians have all the answers, when we think of our own children, most doctors are like other parents – we can’t think objectively about our kids. It’s helpful for me to share that and to help parents feel less alone.”
If you’re not yet using social media to the best of your physician abilities, you might take a shot at becoming widely recognizable. Pick a preferred platform, answer common patient questions, dispel medical myths, provide pertinent information, and let your personality shine.
A version of this article first appeared on Medscape.com.
CBT linked to reduced pain, less catastrophizing in fibromyalgia
TOPLINE:
In patients with fibromyalgia, cognitive behavior therapy (CBT) can reduce pain through its effect on pain-related catastrophizing, which involves intensified cognitive and emotional responses to things like intrusive thoughts, a new study suggests.
METHODOLOGY:
- The study included 98 female patients with fibromyalgia (FM), mean age about 42 years, who underwent a baseline neuroimaging assessment and were randomly assigned to CBT (where patients learned to identify negative thoughts and use cognitive restructuring to diminish pain-related distress) or a matched educational intervention (where patients learned about fibromyalgia and chronic pain); both groups had eight weekly individual 60- to 75-minute visits.
- The primary outcome was the pain interference subscale of the Brief Pain Inventory (BPI); secondary outcomes included the BPI pain severity subscale, the Fibromyalgia Impact Questionnaire–Revised (FIQR), and the Pain Catastrophizing Scale (PCS), which includes subscales of rumination, magnification, and helplessness.
- Researchers used functional magnetic resonance imaging (fMRI)-adapted task to investigate the neural circuitry supporting pain catastrophizing.
TAKEAWAY:
- After controlling for baseline values, BPI pain interference scores were significantly reduced, with a larger reduction in the CBT group, compared with the education group (P = .03), which was also the case for FIQR scores (P = .05) and pain catastrophizing (P = .04).
- There were larger reductions in pain-related symptomatology in the CBT group, but they did not reach statistical significance.
- Following CBT treatment, the study showed reduced connectivity between regions of the brain associated with self-awareness, pain, and emotional processing.
IN PRACTICE:
The results “highlight the important role of targeting pain catastrophizing with psychotherapy, particularly for patients reporting high levels of catastrophizing cognitions” write the authors, adding that altered network connectivity identified by the study “may emerge as a valuable biomarker of catastrophizing-related cognitive and affective processes.”
SOURCE:
The study was carried out by Jeungchan Lee, PhD, department of radiology, center for biomedical imaging, Massachusetts General Hospital, Boston, and the Discovery Center for Recovery from Chronic Pain, Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, and colleagues. It was published in Arthritis & Rheumatology.
LIMITATIONS:
Findings were limited to female participants. CBT for chronic pain includes different therapeutic modules, and the study can’t draw definitive conclusions regarding which CBT skills were most beneficial to patients in reducing catastrophizing. Baseline symptom severity was higher for the CBT group, which may complicate interpretation of the findings.
DISCLOSURES:
The study received support from the National Institutes of Health: National Center for Complementary and Integrative Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Center for Research Resources. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with fibromyalgia, cognitive behavior therapy (CBT) can reduce pain through its effect on pain-related catastrophizing, which involves intensified cognitive and emotional responses to things like intrusive thoughts, a new study suggests.
METHODOLOGY:
- The study included 98 female patients with fibromyalgia (FM), mean age about 42 years, who underwent a baseline neuroimaging assessment and were randomly assigned to CBT (where patients learned to identify negative thoughts and use cognitive restructuring to diminish pain-related distress) or a matched educational intervention (where patients learned about fibromyalgia and chronic pain); both groups had eight weekly individual 60- to 75-minute visits.
- The primary outcome was the pain interference subscale of the Brief Pain Inventory (BPI); secondary outcomes included the BPI pain severity subscale, the Fibromyalgia Impact Questionnaire–Revised (FIQR), and the Pain Catastrophizing Scale (PCS), which includes subscales of rumination, magnification, and helplessness.
- Researchers used functional magnetic resonance imaging (fMRI)-adapted task to investigate the neural circuitry supporting pain catastrophizing.
TAKEAWAY:
- After controlling for baseline values, BPI pain interference scores were significantly reduced, with a larger reduction in the CBT group, compared with the education group (P = .03), which was also the case for FIQR scores (P = .05) and pain catastrophizing (P = .04).
- There were larger reductions in pain-related symptomatology in the CBT group, but they did not reach statistical significance.
- Following CBT treatment, the study showed reduced connectivity between regions of the brain associated with self-awareness, pain, and emotional processing.
IN PRACTICE:
The results “highlight the important role of targeting pain catastrophizing with psychotherapy, particularly for patients reporting high levels of catastrophizing cognitions” write the authors, adding that altered network connectivity identified by the study “may emerge as a valuable biomarker of catastrophizing-related cognitive and affective processes.”
SOURCE:
The study was carried out by Jeungchan Lee, PhD, department of radiology, center for biomedical imaging, Massachusetts General Hospital, Boston, and the Discovery Center for Recovery from Chronic Pain, Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, and colleagues. It was published in Arthritis & Rheumatology.
LIMITATIONS:
Findings were limited to female participants. CBT for chronic pain includes different therapeutic modules, and the study can’t draw definitive conclusions regarding which CBT skills were most beneficial to patients in reducing catastrophizing. Baseline symptom severity was higher for the CBT group, which may complicate interpretation of the findings.
DISCLOSURES:
The study received support from the National Institutes of Health: National Center for Complementary and Integrative Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Center for Research Resources. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with fibromyalgia, cognitive behavior therapy (CBT) can reduce pain through its effect on pain-related catastrophizing, which involves intensified cognitive and emotional responses to things like intrusive thoughts, a new study suggests.
METHODOLOGY:
- The study included 98 female patients with fibromyalgia (FM), mean age about 42 years, who underwent a baseline neuroimaging assessment and were randomly assigned to CBT (where patients learned to identify negative thoughts and use cognitive restructuring to diminish pain-related distress) or a matched educational intervention (where patients learned about fibromyalgia and chronic pain); both groups had eight weekly individual 60- to 75-minute visits.
- The primary outcome was the pain interference subscale of the Brief Pain Inventory (BPI); secondary outcomes included the BPI pain severity subscale, the Fibromyalgia Impact Questionnaire–Revised (FIQR), and the Pain Catastrophizing Scale (PCS), which includes subscales of rumination, magnification, and helplessness.
- Researchers used functional magnetic resonance imaging (fMRI)-adapted task to investigate the neural circuitry supporting pain catastrophizing.
TAKEAWAY:
- After controlling for baseline values, BPI pain interference scores were significantly reduced, with a larger reduction in the CBT group, compared with the education group (P = .03), which was also the case for FIQR scores (P = .05) and pain catastrophizing (P = .04).
- There were larger reductions in pain-related symptomatology in the CBT group, but they did not reach statistical significance.
- Following CBT treatment, the study showed reduced connectivity between regions of the brain associated with self-awareness, pain, and emotional processing.
IN PRACTICE:
The results “highlight the important role of targeting pain catastrophizing with psychotherapy, particularly for patients reporting high levels of catastrophizing cognitions” write the authors, adding that altered network connectivity identified by the study “may emerge as a valuable biomarker of catastrophizing-related cognitive and affective processes.”
SOURCE:
The study was carried out by Jeungchan Lee, PhD, department of radiology, center for biomedical imaging, Massachusetts General Hospital, Boston, and the Discovery Center for Recovery from Chronic Pain, Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, and colleagues. It was published in Arthritis & Rheumatology.
LIMITATIONS:
Findings were limited to female participants. CBT for chronic pain includes different therapeutic modules, and the study can’t draw definitive conclusions regarding which CBT skills were most beneficial to patients in reducing catastrophizing. Baseline symptom severity was higher for the CBT group, which may complicate interpretation of the findings.
DISCLOSURES:
The study received support from the National Institutes of Health: National Center for Complementary and Integrative Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Center for Research Resources. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
What’s right and wrong for doctors on social media
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
Multivitamins and dementia: Untangling the COSMOS study web
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I’ll make a note of that
I’ve worked hard to get rid of paper, or at least minimize it.
I use e-fax for sending and receiving as much as possible. I send scripts and order digitally when I can.
But, 23 years into a paperless practice, the stuff isn’t going away soon. Nor I do I want it to.
For many applications paper is just easier (at least to me) to use. When I have a meeting and know I’ll need to read from notes, I’d much rather have them on paper than a screen, so I print them up. Even a grocery list is easier to scribble down on something and look at as I wander the aisles, rather than navigate to an app every 2 minutes. Paper isn’t susceptible to the whims of battery power, signal strength, being dropped, or software glitches.
I’m also not particularly good at taking notes on a computer. I’m sure most of the current generation of physicians is (or they just use a scribe), but I’m old school. Since day one I’ve had a note pad on my desk, jotting points and observations down on the fly (I use a pencil, too, if anyone remembers what that is). Then, when I have time, I type up my notes from the paper.
I also still have patients who, for whatever reason, want a handwritten prescription. Or sometimes need the legendary “doctor’s note” for work or school. Or need me to fill out forms.
Having grown up with paper, and been through school and residency with paper, it’s not easy to give it up entirely. There’s something reassuring about the tactile nature of flipping pages as opposed to scrolling up and down.
I’m not complaining about its decreased use, though. A digital world is, for the most part, much, much easier. Even now paper is just a transient medium for me. It’s either going to be scanned or shredded (or scanned, then shredded) when I’m done. I don’t want the hassle of paper charts as my repository of information. Currently I have 23 years of charts sitting on a Mac-Mini, and accessible from wherever I am on Earth (as long as I have a decent signal). You definitely can’t do that with paper.
On a larger scale paper has other, more significant, drawbacks: deforestation, pollution, freshwater and petroleum usage, and others. I’m aware of this, use only scratch paper for my scribbles and lists, and buy recycled paper products as much as possible.
Certainly I wish we had less use of it. For one thing, I’d love to be rid of all the junk mail that comes to my house, which far outnumbers anything of importance. I always send it straight to recycling, but it would be far better if it had never been created in the first place.
Realistically, though, it’s still a key part of medical practice and everyday life. I don’t see that changing anytime soon, nor do I really want it to. I’ll leave it to a future generation of doctors to make that break.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve worked hard to get rid of paper, or at least minimize it.
I use e-fax for sending and receiving as much as possible. I send scripts and order digitally when I can.
But, 23 years into a paperless practice, the stuff isn’t going away soon. Nor I do I want it to.
For many applications paper is just easier (at least to me) to use. When I have a meeting and know I’ll need to read from notes, I’d much rather have them on paper than a screen, so I print them up. Even a grocery list is easier to scribble down on something and look at as I wander the aisles, rather than navigate to an app every 2 minutes. Paper isn’t susceptible to the whims of battery power, signal strength, being dropped, or software glitches.
I’m also not particularly good at taking notes on a computer. I’m sure most of the current generation of physicians is (or they just use a scribe), but I’m old school. Since day one I’ve had a note pad on my desk, jotting points and observations down on the fly (I use a pencil, too, if anyone remembers what that is). Then, when I have time, I type up my notes from the paper.
I also still have patients who, for whatever reason, want a handwritten prescription. Or sometimes need the legendary “doctor’s note” for work or school. Or need me to fill out forms.
Having grown up with paper, and been through school and residency with paper, it’s not easy to give it up entirely. There’s something reassuring about the tactile nature of flipping pages as opposed to scrolling up and down.
I’m not complaining about its decreased use, though. A digital world is, for the most part, much, much easier. Even now paper is just a transient medium for me. It’s either going to be scanned or shredded (or scanned, then shredded) when I’m done. I don’t want the hassle of paper charts as my repository of information. Currently I have 23 years of charts sitting on a Mac-Mini, and accessible from wherever I am on Earth (as long as I have a decent signal). You definitely can’t do that with paper.
On a larger scale paper has other, more significant, drawbacks: deforestation, pollution, freshwater and petroleum usage, and others. I’m aware of this, use only scratch paper for my scribbles and lists, and buy recycled paper products as much as possible.
Certainly I wish we had less use of it. For one thing, I’d love to be rid of all the junk mail that comes to my house, which far outnumbers anything of importance. I always send it straight to recycling, but it would be far better if it had never been created in the first place.
Realistically, though, it’s still a key part of medical practice and everyday life. I don’t see that changing anytime soon, nor do I really want it to. I’ll leave it to a future generation of doctors to make that break.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve worked hard to get rid of paper, or at least minimize it.
I use e-fax for sending and receiving as much as possible. I send scripts and order digitally when I can.
But, 23 years into a paperless practice, the stuff isn’t going away soon. Nor I do I want it to.
For many applications paper is just easier (at least to me) to use. When I have a meeting and know I’ll need to read from notes, I’d much rather have them on paper than a screen, so I print them up. Even a grocery list is easier to scribble down on something and look at as I wander the aisles, rather than navigate to an app every 2 minutes. Paper isn’t susceptible to the whims of battery power, signal strength, being dropped, or software glitches.
I’m also not particularly good at taking notes on a computer. I’m sure most of the current generation of physicians is (or they just use a scribe), but I’m old school. Since day one I’ve had a note pad on my desk, jotting points and observations down on the fly (I use a pencil, too, if anyone remembers what that is). Then, when I have time, I type up my notes from the paper.
I also still have patients who, for whatever reason, want a handwritten prescription. Or sometimes need the legendary “doctor’s note” for work or school. Or need me to fill out forms.
Having grown up with paper, and been through school and residency with paper, it’s not easy to give it up entirely. There’s something reassuring about the tactile nature of flipping pages as opposed to scrolling up and down.
I’m not complaining about its decreased use, though. A digital world is, for the most part, much, much easier. Even now paper is just a transient medium for me. It’s either going to be scanned or shredded (or scanned, then shredded) when I’m done. I don’t want the hassle of paper charts as my repository of information. Currently I have 23 years of charts sitting on a Mac-Mini, and accessible from wherever I am on Earth (as long as I have a decent signal). You definitely can’t do that with paper.
On a larger scale paper has other, more significant, drawbacks: deforestation, pollution, freshwater and petroleum usage, and others. I’m aware of this, use only scratch paper for my scribbles and lists, and buy recycled paper products as much as possible.
Certainly I wish we had less use of it. For one thing, I’d love to be rid of all the junk mail that comes to my house, which far outnumbers anything of importance. I always send it straight to recycling, but it would be far better if it had never been created in the first place.
Realistically, though, it’s still a key part of medical practice and everyday life. I don’t see that changing anytime soon, nor do I really want it to. I’ll leave it to a future generation of doctors to make that break.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Are cellular therapies the future of autoimmune disease?
A revolutionary treatment for cancers may also be able to treat and reset the immune system to provide long-term remission or possibly even cure certain autoimmune diseases.
Chimeric antigen receptor (CAR) T-cell therapy has offered a novel approach to treating hematologic cancers since 2017, but there are early signs that these cellular immunotherapies could be repurposed for B-cell mediated autoimmune diseases.
In September of last year, researchers in Germany reported that five patients with refractory systemic lupus erythematosus (SLE) treated with CAR T-cell therapy all achieved drug-free remission. At the time of publication, no patients had relapsed for up to 17 months after treatment. The authors described seroconversion of antinuclear antibodies in two patients with the longest follow-up, “indicating that abrogation of autoimmune B-cell clones may lead to a more widespread correction of autoimmunity,” the researchers write.
In another case study published in June, researchers used CD-19 targeted CAR-T cells to treat a 41-year-old man with refractory antisynthetase syndrome with progressive myositis and interstitial lung disease. Six months after treatment, there were no signs of myositis on MRI and a chest CT scan showed full regression of alveolitis.
Since then, two biotechnology companies – Cabaletta Bio in Philadelphia and Kyverna Therapeutics in Emeryville, Calif. – have already been granted fast-track designations from the U.S. Food and Drug Administration for CAR T-cell therapy for SLE and lupus nephritis. Bristol-Myers Squibb is also conducting a phase 1 trial in patients with severe, refractory SLE. Several biotechnology companies and hospitals in China are also conducting clinical trials for SLE. But this is only the tip of the iceberg regarding cellular therapies for autoimmune disease, said Max Konig, MD, PhD, an assistant professor of medicine in the division of rheumatology at Johns Hopkins University, Baltimore.
“It’s an incredibly exciting time. It’s unprecedented in the history of autoimmunity,” he noted.
A ‘reboot’ for the immune system
B-cell targeted therapies have been around since the early 2000s with drugs like rituximab, a monoclonal antibody medication that targets CD20, an antigen expressed on the surface of B cells. The CAR T cells currently available target another surface antigen, CD19, and are a much more potent therapy. Both are effective at depleting B cells in blood, but these engineered CD19-targeted T cells can reach B cells sitting in tissues in a way that antibody therapies cannot, Dr. Konig explained.
“If you have a patient with myositis, for example, where autoreactive B cells are sitting in the inflamed muscle, or a patient with rheumatoid arthritis, where you have disease-relevant B cells in hard-to-reach tissues like the synovium, those cells are much harder to deplete with an antibody, compared to a T cell that evolved to surveil and effectively kill in all tissues,” he explained.
In this process, T cells are collected from patients via leukapheresis and then re-engineered to express chimeric antigen receptors. A few days before these modified T cells are infused back into the patient, the patients are given a low-dose chemotherapy (lymphodepletion) regimen to help increase the effectiveness of the therapy. The one-time infusion is generally given on an inpatient basis, and patients are then monitored in hospital for side effects.
Once B cells are depleted, disease symptoms improve. But in the case studies published to date, once B cells re-emerge, they are naïve and no longer producing autoreactive B cells.
“Maybe it’s like a tabula rasa: You wipe [the B cells] out and start with a clean slate. Then, the immune system reboots, and now it’s working, whereas before it was messed up,” said Carl June, MD, who directs the Center for Cellular Immunotherapies at the at the University of Pennsylvania, Philadelphia. Dr. June and his research team led the development of CAR T-cell therapies for blood cancers.
The findings suggest that autoantibodies “might not be hardwired into the immune system,” he said.
But Dr. Konig stressed that we are still in the early days of clinical trials, and more research is necessary to understand the safety and efficacy of these therapies.
“There’s an incredible buzz around CAR T cells at the moment in rheumatology, which is great because I think that’s where the future is,” he said. “But we still need to learn how to appropriately apply these therapies in randomized, controlled trials.”
So far, the evidence behind CD19 CAR T-cell therapies in autoimmune disease is from case studies and phase 1 trials in a very small number of selected patients. (The upcoming Cabaletta and Kyverna trials in lupus will also be small, consisting of 12 patients each.)
Risks of intensive therapy
But while these therapies show promise, the process is very intensive. The lymphodepleting regimen increases the risk for infection and patients are commonly hospitalized for a week or more following infusion for toxicity monitoring. Serious adverse events such as cytokine release syndrome (CRS) can occur days to weeks after CAR T-cell infusion. In the five-patient case series reported in 2022, patients were hospitalized for 10 days following treatment.
The patient with antisynthetase syndrome, as well as three of five patients in the SLE case series study experienced mild CRS following infusion. Patients are also at a high risk for infection, as the engineered T cells target all B cells, not just the autoreactive immune cells.
The inability to differentiate between disease-causing and protective immune cells is an issue for all currently available drugs treating autoimmune disease, Dr. Konig said. But scientists are already working on how to make these potent cellular therapies safer and more precise.
Alternatives to standard CAR T-cell therapies
Engineering T cells with RNA is a new approach to limit the side effects and toxicity of CAR T-cell therapy, said Chris Jewell, PhD, the chief scientific officer at Cartesian Therapeutics, a biotechnology company based in Gaithersburg, Md. The company’s RNA CAR T-cell (rCAR-T) therapy – called DESCARTES-08 – is in phase 2 clinical trials for treatment of myasthenia gravis. Once these rCAR-T cells are infused in patients, as they divide, the RNAnaturally decays, he explained, meaning that after a certain point, the CAR is no longer expressed.
DESCARTES-08 targets B-cell maturation antigen (BCMA), which is primarily expressed on plasma cells, rather than all B cells, Dr. Jewell said.
“Targeting BCMA, we actually have a more selective profile,” he explained. “We are targeting the cells primarily responsible for the pathogenicity; many plasma cells – such as long-lived plasma cells – also take a long time to repopulate.”
This therapy also does not require lymphodepletion prior to infusion and can be done in an outpatient setting. The therapy is given in multiple infusions, once per week.
In the most recent clinical trial, patients with myasthenia gravis received six infusions over 6 weeks and experienced notable decreases in myasthenia gravis severity scale at up to 9 months of follow-up.
While standard CAR T-cell therapies under clinical investigational up to now all use effector T cells, regulatory T cells (Tregs) can also be engineered to target autoimmune disease. Abata Therapeutics, based in Boston, is using this approach for therapies for progressive multiple sclerosis and type 1 diabetes. These engineered Tregs express a T-cell receptor (TCR) that recognizes tissue-specific antigens and suppress inflammation at the site of the disease. “Treg-based cell therapies are really harnessing the natural power of regulatory cells to reset immune tolerance and recalibrate the immune system,” said their chief medical officer, Leonard Dragone, MD, PhD.
These therapies are derived from terminally differentiated cells that have limited capacity to produce pro-inflammatory cytokines including interleukin-2 or interferon gamma, Dr. Dragone explained. “CRS is difficult to envision from engineered Treg products and hasn’t been observed in any clinical experience with polyclonal Tregs,” he said.
This approach also does not require lymphodepletion prior to treatment. The company’s Treg cellular therapy for progressive MS is currently in investigational new drug-enabling studies, and they aim to dose their first patients in 2024.
Precision immunotherapy
For B-cell driven autoimmune diseases where the autoantibody is known, researchers have begun to re-engineer T cells to recognize only autoreactive B cells. While CD19 CAR T cells act more like a sledgehammer, these precision cellular immunotherapies are “like a razor’s strike,” Dr. June said.
“The chimeric autoantibody receptor (CAAR) approach targets autoantibodies that are expressed only on the surface of autoimmune B cells and are not expressed on normal B cells, which ideally should lead to precision targeting of just the cells that cause autoimmune disease,” explained Aimee Payne, MD, PhD, professor of dermatology and director of the Penn Clinical Autoimmunity Center of Excellence at the University of Pennsylvania, Philadelphia.
She and her research team used this approach to develop a treatment for mucosal pemphigus vulgaris, an autoimmune blistering disease of mucous membranes driven by autoantibodies against desmoglein 3.
“The current standard of care for pemphigus is to treat with steroids and rituximab, an infusion therapy that results in global, but temporary, B-cell depletion,” she said. “By expressing desmoglein 3 (DSG3) on the surface of the CAAR T-cell therapy, we target just the anti-DSG3 B cells that cause disease in mucosal pemphigus vulgaris and spare the healthy B cells.”
The therapy – called DSG3-CAART – is being developed by Cabaletta Bio and is now in phase 1 clinical trials. The approach is also being investigated to treat certain types of myasthenia gravis and membranous nephropathy.
Dr. Konig’s lab at Johns Hopkins developed and is now exploring a new precision cellular immunotherapy approach, chimeric autoantigen-T cell receptor (CATCR) T-cell therapy, to treat antiphospholipid syndrome, which is in preclinical stages. In this approach, Dr. Konig and his team are “re-engineering the natural T-cell receptor to selectively kill disease-causing B cells that drive antiphospholipid syndrome,” he explained.
He anticipates the CD19 CAR T-cell therapies currently in clinical trials will help to pave the way for this new generation of precision cellular therapies. The ultimate goal of these therapies, he said, is to uncouple therapeutic potency from infection risk.
“That’s really the holy grail in the treatment of autoimmune diseases. It’s tantalizingly close, but we’re not there yet.”
Dr. June is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. Dr. June is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune SA, Alaunos, BlueSphere Bio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng Capital, Poseida, Verismo, and WIRB-Copernicus Group. Dr. Konig is a consultant for argenx and Revel and is listed as inventor for patent applications filed by John Hopkins University. Dr. Payne holds equity, grants, payments, and patent licensing from Cabaletta Bio and consults for Janssen.
A version of this article first appeared on Medscape.com.
A revolutionary treatment for cancers may also be able to treat and reset the immune system to provide long-term remission or possibly even cure certain autoimmune diseases.
Chimeric antigen receptor (CAR) T-cell therapy has offered a novel approach to treating hematologic cancers since 2017, but there are early signs that these cellular immunotherapies could be repurposed for B-cell mediated autoimmune diseases.
In September of last year, researchers in Germany reported that five patients with refractory systemic lupus erythematosus (SLE) treated with CAR T-cell therapy all achieved drug-free remission. At the time of publication, no patients had relapsed for up to 17 months after treatment. The authors described seroconversion of antinuclear antibodies in two patients with the longest follow-up, “indicating that abrogation of autoimmune B-cell clones may lead to a more widespread correction of autoimmunity,” the researchers write.
In another case study published in June, researchers used CD-19 targeted CAR-T cells to treat a 41-year-old man with refractory antisynthetase syndrome with progressive myositis and interstitial lung disease. Six months after treatment, there were no signs of myositis on MRI and a chest CT scan showed full regression of alveolitis.
Since then, two biotechnology companies – Cabaletta Bio in Philadelphia and Kyverna Therapeutics in Emeryville, Calif. – have already been granted fast-track designations from the U.S. Food and Drug Administration for CAR T-cell therapy for SLE and lupus nephritis. Bristol-Myers Squibb is also conducting a phase 1 trial in patients with severe, refractory SLE. Several biotechnology companies and hospitals in China are also conducting clinical trials for SLE. But this is only the tip of the iceberg regarding cellular therapies for autoimmune disease, said Max Konig, MD, PhD, an assistant professor of medicine in the division of rheumatology at Johns Hopkins University, Baltimore.
“It’s an incredibly exciting time. It’s unprecedented in the history of autoimmunity,” he noted.
A ‘reboot’ for the immune system
B-cell targeted therapies have been around since the early 2000s with drugs like rituximab, a monoclonal antibody medication that targets CD20, an antigen expressed on the surface of B cells. The CAR T cells currently available target another surface antigen, CD19, and are a much more potent therapy. Both are effective at depleting B cells in blood, but these engineered CD19-targeted T cells can reach B cells sitting in tissues in a way that antibody therapies cannot, Dr. Konig explained.
“If you have a patient with myositis, for example, where autoreactive B cells are sitting in the inflamed muscle, or a patient with rheumatoid arthritis, where you have disease-relevant B cells in hard-to-reach tissues like the synovium, those cells are much harder to deplete with an antibody, compared to a T cell that evolved to surveil and effectively kill in all tissues,” he explained.
In this process, T cells are collected from patients via leukapheresis and then re-engineered to express chimeric antigen receptors. A few days before these modified T cells are infused back into the patient, the patients are given a low-dose chemotherapy (lymphodepletion) regimen to help increase the effectiveness of the therapy. The one-time infusion is generally given on an inpatient basis, and patients are then monitored in hospital for side effects.
Once B cells are depleted, disease symptoms improve. But in the case studies published to date, once B cells re-emerge, they are naïve and no longer producing autoreactive B cells.
“Maybe it’s like a tabula rasa: You wipe [the B cells] out and start with a clean slate. Then, the immune system reboots, and now it’s working, whereas before it was messed up,” said Carl June, MD, who directs the Center for Cellular Immunotherapies at the at the University of Pennsylvania, Philadelphia. Dr. June and his research team led the development of CAR T-cell therapies for blood cancers.
The findings suggest that autoantibodies “might not be hardwired into the immune system,” he said.
But Dr. Konig stressed that we are still in the early days of clinical trials, and more research is necessary to understand the safety and efficacy of these therapies.
“There’s an incredible buzz around CAR T cells at the moment in rheumatology, which is great because I think that’s where the future is,” he said. “But we still need to learn how to appropriately apply these therapies in randomized, controlled trials.”
So far, the evidence behind CD19 CAR T-cell therapies in autoimmune disease is from case studies and phase 1 trials in a very small number of selected patients. (The upcoming Cabaletta and Kyverna trials in lupus will also be small, consisting of 12 patients each.)
Risks of intensive therapy
But while these therapies show promise, the process is very intensive. The lymphodepleting regimen increases the risk for infection and patients are commonly hospitalized for a week or more following infusion for toxicity monitoring. Serious adverse events such as cytokine release syndrome (CRS) can occur days to weeks after CAR T-cell infusion. In the five-patient case series reported in 2022, patients were hospitalized for 10 days following treatment.
The patient with antisynthetase syndrome, as well as three of five patients in the SLE case series study experienced mild CRS following infusion. Patients are also at a high risk for infection, as the engineered T cells target all B cells, not just the autoreactive immune cells.
The inability to differentiate between disease-causing and protective immune cells is an issue for all currently available drugs treating autoimmune disease, Dr. Konig said. But scientists are already working on how to make these potent cellular therapies safer and more precise.
Alternatives to standard CAR T-cell therapies
Engineering T cells with RNA is a new approach to limit the side effects and toxicity of CAR T-cell therapy, said Chris Jewell, PhD, the chief scientific officer at Cartesian Therapeutics, a biotechnology company based in Gaithersburg, Md. The company’s RNA CAR T-cell (rCAR-T) therapy – called DESCARTES-08 – is in phase 2 clinical trials for treatment of myasthenia gravis. Once these rCAR-T cells are infused in patients, as they divide, the RNAnaturally decays, he explained, meaning that after a certain point, the CAR is no longer expressed.
DESCARTES-08 targets B-cell maturation antigen (BCMA), which is primarily expressed on plasma cells, rather than all B cells, Dr. Jewell said.
“Targeting BCMA, we actually have a more selective profile,” he explained. “We are targeting the cells primarily responsible for the pathogenicity; many plasma cells – such as long-lived plasma cells – also take a long time to repopulate.”
This therapy also does not require lymphodepletion prior to infusion and can be done in an outpatient setting. The therapy is given in multiple infusions, once per week.
In the most recent clinical trial, patients with myasthenia gravis received six infusions over 6 weeks and experienced notable decreases in myasthenia gravis severity scale at up to 9 months of follow-up.
While standard CAR T-cell therapies under clinical investigational up to now all use effector T cells, regulatory T cells (Tregs) can also be engineered to target autoimmune disease. Abata Therapeutics, based in Boston, is using this approach for therapies for progressive multiple sclerosis and type 1 diabetes. These engineered Tregs express a T-cell receptor (TCR) that recognizes tissue-specific antigens and suppress inflammation at the site of the disease. “Treg-based cell therapies are really harnessing the natural power of regulatory cells to reset immune tolerance and recalibrate the immune system,” said their chief medical officer, Leonard Dragone, MD, PhD.
These therapies are derived from terminally differentiated cells that have limited capacity to produce pro-inflammatory cytokines including interleukin-2 or interferon gamma, Dr. Dragone explained. “CRS is difficult to envision from engineered Treg products and hasn’t been observed in any clinical experience with polyclonal Tregs,” he said.
This approach also does not require lymphodepletion prior to treatment. The company’s Treg cellular therapy for progressive MS is currently in investigational new drug-enabling studies, and they aim to dose their first patients in 2024.
Precision immunotherapy
For B-cell driven autoimmune diseases where the autoantibody is known, researchers have begun to re-engineer T cells to recognize only autoreactive B cells. While CD19 CAR T cells act more like a sledgehammer, these precision cellular immunotherapies are “like a razor’s strike,” Dr. June said.
“The chimeric autoantibody receptor (CAAR) approach targets autoantibodies that are expressed only on the surface of autoimmune B cells and are not expressed on normal B cells, which ideally should lead to precision targeting of just the cells that cause autoimmune disease,” explained Aimee Payne, MD, PhD, professor of dermatology and director of the Penn Clinical Autoimmunity Center of Excellence at the University of Pennsylvania, Philadelphia.
She and her research team used this approach to develop a treatment for mucosal pemphigus vulgaris, an autoimmune blistering disease of mucous membranes driven by autoantibodies against desmoglein 3.
“The current standard of care for pemphigus is to treat with steroids and rituximab, an infusion therapy that results in global, but temporary, B-cell depletion,” she said. “By expressing desmoglein 3 (DSG3) on the surface of the CAAR T-cell therapy, we target just the anti-DSG3 B cells that cause disease in mucosal pemphigus vulgaris and spare the healthy B cells.”
The therapy – called DSG3-CAART – is being developed by Cabaletta Bio and is now in phase 1 clinical trials. The approach is also being investigated to treat certain types of myasthenia gravis and membranous nephropathy.
Dr. Konig’s lab at Johns Hopkins developed and is now exploring a new precision cellular immunotherapy approach, chimeric autoantigen-T cell receptor (CATCR) T-cell therapy, to treat antiphospholipid syndrome, which is in preclinical stages. In this approach, Dr. Konig and his team are “re-engineering the natural T-cell receptor to selectively kill disease-causing B cells that drive antiphospholipid syndrome,” he explained.
He anticipates the CD19 CAR T-cell therapies currently in clinical trials will help to pave the way for this new generation of precision cellular therapies. The ultimate goal of these therapies, he said, is to uncouple therapeutic potency from infection risk.
“That’s really the holy grail in the treatment of autoimmune diseases. It’s tantalizingly close, but we’re not there yet.”
Dr. June is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. Dr. June is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune SA, Alaunos, BlueSphere Bio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng Capital, Poseida, Verismo, and WIRB-Copernicus Group. Dr. Konig is a consultant for argenx and Revel and is listed as inventor for patent applications filed by John Hopkins University. Dr. Payne holds equity, grants, payments, and patent licensing from Cabaletta Bio and consults for Janssen.
A version of this article first appeared on Medscape.com.
A revolutionary treatment for cancers may also be able to treat and reset the immune system to provide long-term remission or possibly even cure certain autoimmune diseases.
Chimeric antigen receptor (CAR) T-cell therapy has offered a novel approach to treating hematologic cancers since 2017, but there are early signs that these cellular immunotherapies could be repurposed for B-cell mediated autoimmune diseases.
In September of last year, researchers in Germany reported that five patients with refractory systemic lupus erythematosus (SLE) treated with CAR T-cell therapy all achieved drug-free remission. At the time of publication, no patients had relapsed for up to 17 months after treatment. The authors described seroconversion of antinuclear antibodies in two patients with the longest follow-up, “indicating that abrogation of autoimmune B-cell clones may lead to a more widespread correction of autoimmunity,” the researchers write.
In another case study published in June, researchers used CD-19 targeted CAR-T cells to treat a 41-year-old man with refractory antisynthetase syndrome with progressive myositis and interstitial lung disease. Six months after treatment, there were no signs of myositis on MRI and a chest CT scan showed full regression of alveolitis.
Since then, two biotechnology companies – Cabaletta Bio in Philadelphia and Kyverna Therapeutics in Emeryville, Calif. – have already been granted fast-track designations from the U.S. Food and Drug Administration for CAR T-cell therapy for SLE and lupus nephritis. Bristol-Myers Squibb is also conducting a phase 1 trial in patients with severe, refractory SLE. Several biotechnology companies and hospitals in China are also conducting clinical trials for SLE. But this is only the tip of the iceberg regarding cellular therapies for autoimmune disease, said Max Konig, MD, PhD, an assistant professor of medicine in the division of rheumatology at Johns Hopkins University, Baltimore.
“It’s an incredibly exciting time. It’s unprecedented in the history of autoimmunity,” he noted.
A ‘reboot’ for the immune system
B-cell targeted therapies have been around since the early 2000s with drugs like rituximab, a monoclonal antibody medication that targets CD20, an antigen expressed on the surface of B cells. The CAR T cells currently available target another surface antigen, CD19, and are a much more potent therapy. Both are effective at depleting B cells in blood, but these engineered CD19-targeted T cells can reach B cells sitting in tissues in a way that antibody therapies cannot, Dr. Konig explained.
“If you have a patient with myositis, for example, where autoreactive B cells are sitting in the inflamed muscle, or a patient with rheumatoid arthritis, where you have disease-relevant B cells in hard-to-reach tissues like the synovium, those cells are much harder to deplete with an antibody, compared to a T cell that evolved to surveil and effectively kill in all tissues,” he explained.
In this process, T cells are collected from patients via leukapheresis and then re-engineered to express chimeric antigen receptors. A few days before these modified T cells are infused back into the patient, the patients are given a low-dose chemotherapy (lymphodepletion) regimen to help increase the effectiveness of the therapy. The one-time infusion is generally given on an inpatient basis, and patients are then monitored in hospital for side effects.
Once B cells are depleted, disease symptoms improve. But in the case studies published to date, once B cells re-emerge, they are naïve and no longer producing autoreactive B cells.
“Maybe it’s like a tabula rasa: You wipe [the B cells] out and start with a clean slate. Then, the immune system reboots, and now it’s working, whereas before it was messed up,” said Carl June, MD, who directs the Center for Cellular Immunotherapies at the at the University of Pennsylvania, Philadelphia. Dr. June and his research team led the development of CAR T-cell therapies for blood cancers.
The findings suggest that autoantibodies “might not be hardwired into the immune system,” he said.
But Dr. Konig stressed that we are still in the early days of clinical trials, and more research is necessary to understand the safety and efficacy of these therapies.
“There’s an incredible buzz around CAR T cells at the moment in rheumatology, which is great because I think that’s where the future is,” he said. “But we still need to learn how to appropriately apply these therapies in randomized, controlled trials.”
So far, the evidence behind CD19 CAR T-cell therapies in autoimmune disease is from case studies and phase 1 trials in a very small number of selected patients. (The upcoming Cabaletta and Kyverna trials in lupus will also be small, consisting of 12 patients each.)
Risks of intensive therapy
But while these therapies show promise, the process is very intensive. The lymphodepleting regimen increases the risk for infection and patients are commonly hospitalized for a week or more following infusion for toxicity monitoring. Serious adverse events such as cytokine release syndrome (CRS) can occur days to weeks after CAR T-cell infusion. In the five-patient case series reported in 2022, patients were hospitalized for 10 days following treatment.
The patient with antisynthetase syndrome, as well as three of five patients in the SLE case series study experienced mild CRS following infusion. Patients are also at a high risk for infection, as the engineered T cells target all B cells, not just the autoreactive immune cells.
The inability to differentiate between disease-causing and protective immune cells is an issue for all currently available drugs treating autoimmune disease, Dr. Konig said. But scientists are already working on how to make these potent cellular therapies safer and more precise.
Alternatives to standard CAR T-cell therapies
Engineering T cells with RNA is a new approach to limit the side effects and toxicity of CAR T-cell therapy, said Chris Jewell, PhD, the chief scientific officer at Cartesian Therapeutics, a biotechnology company based in Gaithersburg, Md. The company’s RNA CAR T-cell (rCAR-T) therapy – called DESCARTES-08 – is in phase 2 clinical trials for treatment of myasthenia gravis. Once these rCAR-T cells are infused in patients, as they divide, the RNAnaturally decays, he explained, meaning that after a certain point, the CAR is no longer expressed.
DESCARTES-08 targets B-cell maturation antigen (BCMA), which is primarily expressed on plasma cells, rather than all B cells, Dr. Jewell said.
“Targeting BCMA, we actually have a more selective profile,” he explained. “We are targeting the cells primarily responsible for the pathogenicity; many plasma cells – such as long-lived plasma cells – also take a long time to repopulate.”
This therapy also does not require lymphodepletion prior to infusion and can be done in an outpatient setting. The therapy is given in multiple infusions, once per week.
In the most recent clinical trial, patients with myasthenia gravis received six infusions over 6 weeks and experienced notable decreases in myasthenia gravis severity scale at up to 9 months of follow-up.
While standard CAR T-cell therapies under clinical investigational up to now all use effector T cells, regulatory T cells (Tregs) can also be engineered to target autoimmune disease. Abata Therapeutics, based in Boston, is using this approach for therapies for progressive multiple sclerosis and type 1 diabetes. These engineered Tregs express a T-cell receptor (TCR) that recognizes tissue-specific antigens and suppress inflammation at the site of the disease. “Treg-based cell therapies are really harnessing the natural power of regulatory cells to reset immune tolerance and recalibrate the immune system,” said their chief medical officer, Leonard Dragone, MD, PhD.
These therapies are derived from terminally differentiated cells that have limited capacity to produce pro-inflammatory cytokines including interleukin-2 or interferon gamma, Dr. Dragone explained. “CRS is difficult to envision from engineered Treg products and hasn’t been observed in any clinical experience with polyclonal Tregs,” he said.
This approach also does not require lymphodepletion prior to treatment. The company’s Treg cellular therapy for progressive MS is currently in investigational new drug-enabling studies, and they aim to dose their first patients in 2024.
Precision immunotherapy
For B-cell driven autoimmune diseases where the autoantibody is known, researchers have begun to re-engineer T cells to recognize only autoreactive B cells. While CD19 CAR T cells act more like a sledgehammer, these precision cellular immunotherapies are “like a razor’s strike,” Dr. June said.
“The chimeric autoantibody receptor (CAAR) approach targets autoantibodies that are expressed only on the surface of autoimmune B cells and are not expressed on normal B cells, which ideally should lead to precision targeting of just the cells that cause autoimmune disease,” explained Aimee Payne, MD, PhD, professor of dermatology and director of the Penn Clinical Autoimmunity Center of Excellence at the University of Pennsylvania, Philadelphia.
She and her research team used this approach to develop a treatment for mucosal pemphigus vulgaris, an autoimmune blistering disease of mucous membranes driven by autoantibodies against desmoglein 3.
“The current standard of care for pemphigus is to treat with steroids and rituximab, an infusion therapy that results in global, but temporary, B-cell depletion,” she said. “By expressing desmoglein 3 (DSG3) on the surface of the CAAR T-cell therapy, we target just the anti-DSG3 B cells that cause disease in mucosal pemphigus vulgaris and spare the healthy B cells.”
The therapy – called DSG3-CAART – is being developed by Cabaletta Bio and is now in phase 1 clinical trials. The approach is also being investigated to treat certain types of myasthenia gravis and membranous nephropathy.
Dr. Konig’s lab at Johns Hopkins developed and is now exploring a new precision cellular immunotherapy approach, chimeric autoantigen-T cell receptor (CATCR) T-cell therapy, to treat antiphospholipid syndrome, which is in preclinical stages. In this approach, Dr. Konig and his team are “re-engineering the natural T-cell receptor to selectively kill disease-causing B cells that drive antiphospholipid syndrome,” he explained.
He anticipates the CD19 CAR T-cell therapies currently in clinical trials will help to pave the way for this new generation of precision cellular therapies. The ultimate goal of these therapies, he said, is to uncouple therapeutic potency from infection risk.
“That’s really the holy grail in the treatment of autoimmune diseases. It’s tantalizingly close, but we’re not there yet.”
Dr. June is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives license revenue from such licenses. Dr. June is a scientific founder of Tmunity Therapeutics and Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune SA, Alaunos, BlueSphere Bio, Cabaletta, Carisma, Cartography Biosciences, Cellares, Celldex, Decheng Capital, Poseida, Verismo, and WIRB-Copernicus Group. Dr. Konig is a consultant for argenx and Revel and is listed as inventor for patent applications filed by John Hopkins University. Dr. Payne holds equity, grants, payments, and patent licensing from Cabaletta Bio and consults for Janssen.
A version of this article first appeared on Medscape.com.