User login
Fecal microbiota transplants: Finding new microbial markers for donor efficacy in UC
Donor microbiota stability and species evenness, along with presence of certain microbial species, may predict donor efficacy in fecal microbiota transplantation (FMT) for the treatment of patients with ulcerative colitis (UC), a new study suggests.
The authors noted that these markers of donor efficacy could be used to optimize selection of donors to treat patients with UC and improve outcomes.
The investigators hypothesized that “there are features beyond microbial richness, individual bacterial species, and specific metabolites that may aid in successful identification of effective donors.” They published these findings in Gut.
The LOTUS clinical trial explored the efficacy of lyophilized FMT in patients with UC, but was cut short because of the COVID-19 pandemic. The study investigators analyzed fecal samples from the two donors enrolled in the trial to identify functional and taxonomic differences within the donors’ microbiota that have clinical relevance to their efficacy in active UC. Donor 1’s samples showed 100% efficacy among patients with UC, while donor 2’s samples showed 36% efficacy.
In donor 1, the researchers observed “robust stability in species richness” during the sampling periods, whereas donor 2 exhibited larger fluctuations. Although the species richness was significantly greater in the donor 2, the researchers reported that donor 1 exhibited significantly greater diversity at the higher taxonomic level of phylum. According to the investigators, this was reflected by the detection of Euryarchaeota, Synergistetes, and Verrucomicrobia in donor 1 but not in the second donor.
Despite a higher species richness in donor 2, the researchers found that a higher rate of uniquely classified metagenome-assembled genomes was produced per sample in the donor 1, which indicates greater species evenness, the second marker of efficacy according to investigators.
Blautia wexlerae was a highly prevalent metagenome-assembled genome that was enriched in donor 1 compared with donor 2, and the researchers reported that “a taxon with high similarity (OTU215) showed evidence of engraftment in patients receiving donor 1.” In addition, B. wexlerae demonstrated a trend toward enrichment in donor 2 samples that were associated with positive outcomes in patients and demonstrated evidence of engraftment in patients who received donor 2.
Ninety bacterial species as well as one archaeon were differentially abundant between donors, including 44 donor samples which were greater than 0.1% in relative abundance. According to the researchers, 17 out of the 44 species were enriched in the effective donor, with 11 (64.7%) assembled into high-quality genomes highly prevalent in that donor and 6 that demonstrated evidence of engraftment in patients.
Lastly, the investigators sought to validate the observed associations between certain microbial taxa and donor clinical efficacy in an independent cohort. In this analysis, the investigators evaluated shotgun metagenomics data of donors used to treat patients with UC and examined relative abundances against patient outcomes. Species associated with treatment success included Ruminococcus bromii, B. wexlerae, Eubacterium hallii, Coprococcus catus, Fusicatenibacter saccharivorans, and Parabacteroides merdae.
“We identified microbiota stability and species evenness as markers of donor efficacy, as well as specific microbial species that could be employed to improve donor selection and build artificial microbial consortia to treat UC,” the investigators concluded.
Given that the study enrolled only two donors, the generalizability of the findings may be limited. The researchers wrote that another limitation of the data analysis was “the bias towards more relatively abundant taxa due to the inability to assemble genomes from low-abundance species.” The lack of prospective validation studies on the novel metrics is another limitation of the study.
Some investigators disclosed relationships with biomedical companies, such as Takeda and Janssen.
Donor microbiota stability and species evenness, along with presence of certain microbial species, may predict donor efficacy in fecal microbiota transplantation (FMT) for the treatment of patients with ulcerative colitis (UC), a new study suggests.
The authors noted that these markers of donor efficacy could be used to optimize selection of donors to treat patients with UC and improve outcomes.
The investigators hypothesized that “there are features beyond microbial richness, individual bacterial species, and specific metabolites that may aid in successful identification of effective donors.” They published these findings in Gut.
The LOTUS clinical trial explored the efficacy of lyophilized FMT in patients with UC, but was cut short because of the COVID-19 pandemic. The study investigators analyzed fecal samples from the two donors enrolled in the trial to identify functional and taxonomic differences within the donors’ microbiota that have clinical relevance to their efficacy in active UC. Donor 1’s samples showed 100% efficacy among patients with UC, while donor 2’s samples showed 36% efficacy.
In donor 1, the researchers observed “robust stability in species richness” during the sampling periods, whereas donor 2 exhibited larger fluctuations. Although the species richness was significantly greater in the donor 2, the researchers reported that donor 1 exhibited significantly greater diversity at the higher taxonomic level of phylum. According to the investigators, this was reflected by the detection of Euryarchaeota, Synergistetes, and Verrucomicrobia in donor 1 but not in the second donor.
Despite a higher species richness in donor 2, the researchers found that a higher rate of uniquely classified metagenome-assembled genomes was produced per sample in the donor 1, which indicates greater species evenness, the second marker of efficacy according to investigators.
Blautia wexlerae was a highly prevalent metagenome-assembled genome that was enriched in donor 1 compared with donor 2, and the researchers reported that “a taxon with high similarity (OTU215) showed evidence of engraftment in patients receiving donor 1.” In addition, B. wexlerae demonstrated a trend toward enrichment in donor 2 samples that were associated with positive outcomes in patients and demonstrated evidence of engraftment in patients who received donor 2.
Ninety bacterial species as well as one archaeon were differentially abundant between donors, including 44 donor samples which were greater than 0.1% in relative abundance. According to the researchers, 17 out of the 44 species were enriched in the effective donor, with 11 (64.7%) assembled into high-quality genomes highly prevalent in that donor and 6 that demonstrated evidence of engraftment in patients.
Lastly, the investigators sought to validate the observed associations between certain microbial taxa and donor clinical efficacy in an independent cohort. In this analysis, the investigators evaluated shotgun metagenomics data of donors used to treat patients with UC and examined relative abundances against patient outcomes. Species associated with treatment success included Ruminococcus bromii, B. wexlerae, Eubacterium hallii, Coprococcus catus, Fusicatenibacter saccharivorans, and Parabacteroides merdae.
“We identified microbiota stability and species evenness as markers of donor efficacy, as well as specific microbial species that could be employed to improve donor selection and build artificial microbial consortia to treat UC,” the investigators concluded.
Given that the study enrolled only two donors, the generalizability of the findings may be limited. The researchers wrote that another limitation of the data analysis was “the bias towards more relatively abundant taxa due to the inability to assemble genomes from low-abundance species.” The lack of prospective validation studies on the novel metrics is another limitation of the study.
Some investigators disclosed relationships with biomedical companies, such as Takeda and Janssen.
Donor microbiota stability and species evenness, along with presence of certain microbial species, may predict donor efficacy in fecal microbiota transplantation (FMT) for the treatment of patients with ulcerative colitis (UC), a new study suggests.
The authors noted that these markers of donor efficacy could be used to optimize selection of donors to treat patients with UC and improve outcomes.
The investigators hypothesized that “there are features beyond microbial richness, individual bacterial species, and specific metabolites that may aid in successful identification of effective donors.” They published these findings in Gut.
The LOTUS clinical trial explored the efficacy of lyophilized FMT in patients with UC, but was cut short because of the COVID-19 pandemic. The study investigators analyzed fecal samples from the two donors enrolled in the trial to identify functional and taxonomic differences within the donors’ microbiota that have clinical relevance to their efficacy in active UC. Donor 1’s samples showed 100% efficacy among patients with UC, while donor 2’s samples showed 36% efficacy.
In donor 1, the researchers observed “robust stability in species richness” during the sampling periods, whereas donor 2 exhibited larger fluctuations. Although the species richness was significantly greater in the donor 2, the researchers reported that donor 1 exhibited significantly greater diversity at the higher taxonomic level of phylum. According to the investigators, this was reflected by the detection of Euryarchaeota, Synergistetes, and Verrucomicrobia in donor 1 but not in the second donor.
Despite a higher species richness in donor 2, the researchers found that a higher rate of uniquely classified metagenome-assembled genomes was produced per sample in the donor 1, which indicates greater species evenness, the second marker of efficacy according to investigators.
Blautia wexlerae was a highly prevalent metagenome-assembled genome that was enriched in donor 1 compared with donor 2, and the researchers reported that “a taxon with high similarity (OTU215) showed evidence of engraftment in patients receiving donor 1.” In addition, B. wexlerae demonstrated a trend toward enrichment in donor 2 samples that were associated with positive outcomes in patients and demonstrated evidence of engraftment in patients who received donor 2.
Ninety bacterial species as well as one archaeon were differentially abundant between donors, including 44 donor samples which were greater than 0.1% in relative abundance. According to the researchers, 17 out of the 44 species were enriched in the effective donor, with 11 (64.7%) assembled into high-quality genomes highly prevalent in that donor and 6 that demonstrated evidence of engraftment in patients.
Lastly, the investigators sought to validate the observed associations between certain microbial taxa and donor clinical efficacy in an independent cohort. In this analysis, the investigators evaluated shotgun metagenomics data of donors used to treat patients with UC and examined relative abundances against patient outcomes. Species associated with treatment success included Ruminococcus bromii, B. wexlerae, Eubacterium hallii, Coprococcus catus, Fusicatenibacter saccharivorans, and Parabacteroides merdae.
“We identified microbiota stability and species evenness as markers of donor efficacy, as well as specific microbial species that could be employed to improve donor selection and build artificial microbial consortia to treat UC,” the investigators concluded.
Given that the study enrolled only two donors, the generalizability of the findings may be limited. The researchers wrote that another limitation of the data analysis was “the bias towards more relatively abundant taxa due to the inability to assemble genomes from low-abundance species.” The lack of prospective validation studies on the novel metrics is another limitation of the study.
Some investigators disclosed relationships with biomedical companies, such as Takeda and Janssen.
FROM GUT
Applications for the CUTIS 2023 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2023 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2023.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 28. The residents who are selected to write the column for the upcoming year will be notified by November 4.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2023 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2023.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 28. The residents who are selected to write the column for the upcoming year will be notified by November 4.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2023 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2023.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 28. The residents who are selected to write the column for the upcoming year will be notified by November 4.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Why exercise doesn’t help people with long COVID
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
Waking up at night could be your brain boosting your memory
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
FROM NATURE NEUROSCIENCE
Chronically low wages linked to subsequent memory decline
, new research suggests. In a new analysis of more than 3,000 participants in the Health and Retirement Study, those who sustained low wages in midlife showed significantly faster memory decline than their peers who never earned low wages.
The findings could have implications for future public policy and research initiatives, the investigators noted.
“Our findings, which suggest a pattern of sustained low-wage earning is harmful for cognitive health, [are] broadly applicable to researchers across numerous health disciplines,” said co-investigator Katrina Kezios, PhD, postdoctoral researcher, department of epidemiology, Mailman School of Public Health, Columbia University, New York.
The findings were presented at the 2022 Alzheimer’s Association International Conference.
Growing number of low-wage workers
Low-wage workers make up a growing share of the U.S. labor market. Yet little research has examined the long-term relationship between earning low wages and memory decline.
The current investigators assessed 1992-2016 data from the Health and Retirement Study, a longitudinal survey of nationally representative samples of Americans aged 50 years and older. Study participants are interviewed every 2 years and provide, among other things, information on work-related factors, including hourly wages.
Memory function was measured at each visit from 2004 to 2016 using a memory composite score. The score included immediate and delayed word recall memory assessments. For those who became too impaired to complete cognitive assessment, memory tests by proxy informants were utilized.
On average, participants completed 4.8 memory assessments over the course of the study.
Researchers defined “low wage” as an hourly wage lower than two-thirds of the federal median wage for the corresponding year. They categorized low-wage exposure history as “never” or “intermittent” or “sustained” on the basis of wages earned from 1992 to 2004.
The current analysis included 3,803 participants, 1,913 of whom were men. All participants were born from 1936 to 1941. In 2004, the average age was 65 years, and the mean memory score was 1.15 standard units.
The investigators adjusted for factors that could confound the relationship between wages and cognition, including the participant’s education, parental education, household wealth, and marital status. Later, whether the participants’ occupation type was of low skill or not was also included.
Cognitive harm
The confounder-adjusted annual rate of memory decline among workers who never earned low wages was –0.12 standard units (95% confidence interval, –0.14 to –0.10).
Compared with these workers, memory decline was significantly faster among participants with sustained low wage–earning during midlife (beta for interaction between time and exposure group, –0.012; 95% CI, –0.02 to 0.01), corresponding to an annual rate of –0.13 standard units.
Put another way, the cognitive aging experienced by workers earning low wages over a 10-year period was equivalent to what workers who never earned low wages would experience over 11 years.
Although similar associations were found for men and women, it was stronger in magnitude for men – a finding Dr. Kezios said was somewhat surprising. She noted that women are commonly more at risk for dementia than men.
However, she advises caution in interpreting this finding, as there were so few men in the sustained low-wage group. “Women disproportionately make up the group of workers earning low wages,” she said.
The negative low coefficient found for those who persistently earned low wages was also observed for those who intermittently earned low wages, but this was not statistically significant.
“We can speculate or hypothesize the cumulative effect of earning low wages at each exposure interval produces more cognitive harm than maybe earning low wages at some time points over that exposure period,” said Dr. Kezios.
A sensitivity analysis that examined wage earning at the same ages but in two different birth cohorts showed similar results for the two groups. When researchers removed self-employed workers from the study sample, the same association between sustained low wages and memory decline was found.
“Our findings held up, which gave us a little more reassurance that what we were seeing is at least signaling there might be something there,” said Dr. Kezios.
She described the study as a “first pass” for documenting the harmful cognitive effects of consistently earning low wages.
It would be interesting, she said, to now determine whether there’s a “dose effect” for having a low salary. However, other studies with different designs would be needed to determine at what income level cognitive health starts to be protected and the impact of raising the minimum wage, she added.
Unique study
Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said the study was unique. “I don’t think we have seen anything like this before,” said Dr. Snyder.
The study, which links sustained low-wage earning in midlife to later memory decline, “is looking beyond some of the other measures we’ve seen when we looked at socioeconomic status,” she noted.
The results “beg the question” of whether people who earn low wages have less access to health care, she added.
“We should think about how to ensure access and equity around health care and around potential ways that may address components of risk individuals have during their life course,” Dr. Snyder said.
She noted that the study provides a “start” at considering potential policies to address the impact of sustained low wages on overall health, particularly cognitive health, throughout life.
The study had no outside funding. Dr. Kezios has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In a new analysis of more than 3,000 participants in the Health and Retirement Study, those who sustained low wages in midlife showed significantly faster memory decline than their peers who never earned low wages.
The findings could have implications for future public policy and research initiatives, the investigators noted.
“Our findings, which suggest a pattern of sustained low-wage earning is harmful for cognitive health, [are] broadly applicable to researchers across numerous health disciplines,” said co-investigator Katrina Kezios, PhD, postdoctoral researcher, department of epidemiology, Mailman School of Public Health, Columbia University, New York.
The findings were presented at the 2022 Alzheimer’s Association International Conference.
Growing number of low-wage workers
Low-wage workers make up a growing share of the U.S. labor market. Yet little research has examined the long-term relationship between earning low wages and memory decline.
The current investigators assessed 1992-2016 data from the Health and Retirement Study, a longitudinal survey of nationally representative samples of Americans aged 50 years and older. Study participants are interviewed every 2 years and provide, among other things, information on work-related factors, including hourly wages.
Memory function was measured at each visit from 2004 to 2016 using a memory composite score. The score included immediate and delayed word recall memory assessments. For those who became too impaired to complete cognitive assessment, memory tests by proxy informants were utilized.
On average, participants completed 4.8 memory assessments over the course of the study.
Researchers defined “low wage” as an hourly wage lower than two-thirds of the federal median wage for the corresponding year. They categorized low-wage exposure history as “never” or “intermittent” or “sustained” on the basis of wages earned from 1992 to 2004.
The current analysis included 3,803 participants, 1,913 of whom were men. All participants were born from 1936 to 1941. In 2004, the average age was 65 years, and the mean memory score was 1.15 standard units.
The investigators adjusted for factors that could confound the relationship between wages and cognition, including the participant’s education, parental education, household wealth, and marital status. Later, whether the participants’ occupation type was of low skill or not was also included.
Cognitive harm
The confounder-adjusted annual rate of memory decline among workers who never earned low wages was –0.12 standard units (95% confidence interval, –0.14 to –0.10).
Compared with these workers, memory decline was significantly faster among participants with sustained low wage–earning during midlife (beta for interaction between time and exposure group, –0.012; 95% CI, –0.02 to 0.01), corresponding to an annual rate of –0.13 standard units.
Put another way, the cognitive aging experienced by workers earning low wages over a 10-year period was equivalent to what workers who never earned low wages would experience over 11 years.
Although similar associations were found for men and women, it was stronger in magnitude for men – a finding Dr. Kezios said was somewhat surprising. She noted that women are commonly more at risk for dementia than men.
However, she advises caution in interpreting this finding, as there were so few men in the sustained low-wage group. “Women disproportionately make up the group of workers earning low wages,” she said.
The negative low coefficient found for those who persistently earned low wages was also observed for those who intermittently earned low wages, but this was not statistically significant.
“We can speculate or hypothesize the cumulative effect of earning low wages at each exposure interval produces more cognitive harm than maybe earning low wages at some time points over that exposure period,” said Dr. Kezios.
A sensitivity analysis that examined wage earning at the same ages but in two different birth cohorts showed similar results for the two groups. When researchers removed self-employed workers from the study sample, the same association between sustained low wages and memory decline was found.
“Our findings held up, which gave us a little more reassurance that what we were seeing is at least signaling there might be something there,” said Dr. Kezios.
She described the study as a “first pass” for documenting the harmful cognitive effects of consistently earning low wages.
It would be interesting, she said, to now determine whether there’s a “dose effect” for having a low salary. However, other studies with different designs would be needed to determine at what income level cognitive health starts to be protected and the impact of raising the minimum wage, she added.
Unique study
Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said the study was unique. “I don’t think we have seen anything like this before,” said Dr. Snyder.
The study, which links sustained low-wage earning in midlife to later memory decline, “is looking beyond some of the other measures we’ve seen when we looked at socioeconomic status,” she noted.
The results “beg the question” of whether people who earn low wages have less access to health care, she added.
“We should think about how to ensure access and equity around health care and around potential ways that may address components of risk individuals have during their life course,” Dr. Snyder said.
She noted that the study provides a “start” at considering potential policies to address the impact of sustained low wages on overall health, particularly cognitive health, throughout life.
The study had no outside funding. Dr. Kezios has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In a new analysis of more than 3,000 participants in the Health and Retirement Study, those who sustained low wages in midlife showed significantly faster memory decline than their peers who never earned low wages.
The findings could have implications for future public policy and research initiatives, the investigators noted.
“Our findings, which suggest a pattern of sustained low-wage earning is harmful for cognitive health, [are] broadly applicable to researchers across numerous health disciplines,” said co-investigator Katrina Kezios, PhD, postdoctoral researcher, department of epidemiology, Mailman School of Public Health, Columbia University, New York.
The findings were presented at the 2022 Alzheimer’s Association International Conference.
Growing number of low-wage workers
Low-wage workers make up a growing share of the U.S. labor market. Yet little research has examined the long-term relationship between earning low wages and memory decline.
The current investigators assessed 1992-2016 data from the Health and Retirement Study, a longitudinal survey of nationally representative samples of Americans aged 50 years and older. Study participants are interviewed every 2 years and provide, among other things, information on work-related factors, including hourly wages.
Memory function was measured at each visit from 2004 to 2016 using a memory composite score. The score included immediate and delayed word recall memory assessments. For those who became too impaired to complete cognitive assessment, memory tests by proxy informants were utilized.
On average, participants completed 4.8 memory assessments over the course of the study.
Researchers defined “low wage” as an hourly wage lower than two-thirds of the federal median wage for the corresponding year. They categorized low-wage exposure history as “never” or “intermittent” or “sustained” on the basis of wages earned from 1992 to 2004.
The current analysis included 3,803 participants, 1,913 of whom were men. All participants were born from 1936 to 1941. In 2004, the average age was 65 years, and the mean memory score was 1.15 standard units.
The investigators adjusted for factors that could confound the relationship between wages and cognition, including the participant’s education, parental education, household wealth, and marital status. Later, whether the participants’ occupation type was of low skill or not was also included.
Cognitive harm
The confounder-adjusted annual rate of memory decline among workers who never earned low wages was –0.12 standard units (95% confidence interval, –0.14 to –0.10).
Compared with these workers, memory decline was significantly faster among participants with sustained low wage–earning during midlife (beta for interaction between time and exposure group, –0.012; 95% CI, –0.02 to 0.01), corresponding to an annual rate of –0.13 standard units.
Put another way, the cognitive aging experienced by workers earning low wages over a 10-year period was equivalent to what workers who never earned low wages would experience over 11 years.
Although similar associations were found for men and women, it was stronger in magnitude for men – a finding Dr. Kezios said was somewhat surprising. She noted that women are commonly more at risk for dementia than men.
However, she advises caution in interpreting this finding, as there were so few men in the sustained low-wage group. “Women disproportionately make up the group of workers earning low wages,” she said.
The negative low coefficient found for those who persistently earned low wages was also observed for those who intermittently earned low wages, but this was not statistically significant.
“We can speculate or hypothesize the cumulative effect of earning low wages at each exposure interval produces more cognitive harm than maybe earning low wages at some time points over that exposure period,” said Dr. Kezios.
A sensitivity analysis that examined wage earning at the same ages but in two different birth cohorts showed similar results for the two groups. When researchers removed self-employed workers from the study sample, the same association between sustained low wages and memory decline was found.
“Our findings held up, which gave us a little more reassurance that what we were seeing is at least signaling there might be something there,” said Dr. Kezios.
She described the study as a “first pass” for documenting the harmful cognitive effects of consistently earning low wages.
It would be interesting, she said, to now determine whether there’s a “dose effect” for having a low salary. However, other studies with different designs would be needed to determine at what income level cognitive health starts to be protected and the impact of raising the minimum wage, she added.
Unique study
Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said the study was unique. “I don’t think we have seen anything like this before,” said Dr. Snyder.
The study, which links sustained low-wage earning in midlife to later memory decline, “is looking beyond some of the other measures we’ve seen when we looked at socioeconomic status,” she noted.
The results “beg the question” of whether people who earn low wages have less access to health care, she added.
“We should think about how to ensure access and equity around health care and around potential ways that may address components of risk individuals have during their life course,” Dr. Snyder said.
She noted that the study provides a “start” at considering potential policies to address the impact of sustained low wages on overall health, particularly cognitive health, throughout life.
The study had no outside funding. Dr. Kezios has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAIC 2022
Individualized sensory care for older patients with dementia
Everyone gets by using environmental cues: For example, if you have to go to use the toilet in public, a bathroom sign prompts an immediate response. However, patients with dementia often struggle with environmental cues, which can complicate the already difficult task faced by their caregivers.
Individuals with dementia can lose awareness of such signs, and even colors, making it harder for them to interpret environmental cues.
The study, presented at the Alzheimer’s Association International Conference, recruited 30 pairs of patients and their caregivers. The approach is based on the Dunn model of sensory processing, which focuses on altering environments to maximize chances of success. It “explains that sensory processing is the information coming in, and then our ability to regulate and habituate to those sensations (creates) behavior,” Elizabeth Rhodus, MD, PhD, said during her talk. Dr. Rhodus is assistant professor of medicine at the University of Kentucky, Lexington.
Sensory-based interventions are not uncommon, but most are applied to pediatric populations and tend to focus on sensory processing disorders and autism spectrum disorder. The few programs that do focus on adults have varying methods and produce mixed results. Dr. Rhodus thinks that the key to success is individualization of therapy. “You’re going to like a certain sensation, and I might not like it. You can’t put us in the same room and expect the same results. We have to identify the preferences of how people interact with their environment, and what their brain does at a neuroscience level with that information,” she said.
Caregiving hacks
The program employs telehealth to work with caregivers so they can also create sensory environments within the home, using environment to trigger behavior.
For example, although individuals with dementia may have reduced response to color, the color red is unique. “Red is a cortical trigger. Red always stands out to people, so in our package that we send out as part of this intervention, we send out a roll of red duct tape,” said Dr. Rhodus.
An example of the use of red was a patient with dementia who had stopped drinking on his own, causing his caregiver daughter to be concerned that he would soon have to enter a nursing home. Examining the room, the occupational therapist realized that the water was kept out of sight, and suggested that the water glass be placed within the patient’s view, atop a square created with the red duct tape.
“These are just some of the simple concepts. They kind of seem easy. Some of my participants call them caregiving hacks, but it’s things that are grounded in neuroscience – how the brain processes the environment, and then how can we plug in supports and cues in whatever area is missing,” said Dr. Rhodus.
In the program, the caregiver fills out several online surveys, and an occupational therapist conducts an interview to identify specific challenges, such as bathing, or using the toilet, or going to church. Then an adult sensory profile reveals how the patient perceives his or her environment. “It’s taking those individual pieces, and then boiling it down to these mechanisms at the behavioral and neuroscience level,” said Dr. Rhodus. She said the entire setup process takes about an hour.
Impactful care
The individualized approach of the HARMONY (Helping Older Adults Create and Manage Occupations Successfully) method is promising, according to Monika Gross, executive director of the Poise Project, which uses the Alexander Technique to help people with chronic conditions such as Parkinson’s disease.
“Although it’s always a very simple idea that human beings need sensory processing aspects in their lives, from the time they’re infants through to the end of life, we don’t really focus on the end of life in a way that can bring meaning between the care partner and the person living with dementia. The other thing that was impressive is that this is in a rural community, where there often aren’t a lot of resources available, (such as) classes that the care partner can take their loved one to. So having something where the care partner has some confidence that they can really make an impact in that person that they are seeing decline, that they can see their behavior change [is good],” said Ms. Gross.
Empowering caregivers
The study included 30 pairs of patients and caregivers who were randomized to the individualized care (I), standardized care, or a control group. Adherence to weekly visits was high (I, 88%; S, 100%; C, 60%; P = .061). Retention was strong (I, 80%; S, 60%; C, 50%).
“It was feasible ... and at the end, we found a significant improvement in care partner satisfaction. We actually empowered these people to care for their loved ones, and in doing that, and helping them set up environmental cues, it allowed that person to perform at a more independent level,” said Dr. Rhodus.
The trial was only a proof of concept, so although the researchers saw signs of efficacy, it wasn’t powered to show that. They are currently enrolling additional patients and caregivers for larger studies to further test the approach.
Dr. Rhodus and Ms. Gross have no relevant financial disclosures.
Everyone gets by using environmental cues: For example, if you have to go to use the toilet in public, a bathroom sign prompts an immediate response. However, patients with dementia often struggle with environmental cues, which can complicate the already difficult task faced by their caregivers.
Individuals with dementia can lose awareness of such signs, and even colors, making it harder for them to interpret environmental cues.
The study, presented at the Alzheimer’s Association International Conference, recruited 30 pairs of patients and their caregivers. The approach is based on the Dunn model of sensory processing, which focuses on altering environments to maximize chances of success. It “explains that sensory processing is the information coming in, and then our ability to regulate and habituate to those sensations (creates) behavior,” Elizabeth Rhodus, MD, PhD, said during her talk. Dr. Rhodus is assistant professor of medicine at the University of Kentucky, Lexington.
Sensory-based interventions are not uncommon, but most are applied to pediatric populations and tend to focus on sensory processing disorders and autism spectrum disorder. The few programs that do focus on adults have varying methods and produce mixed results. Dr. Rhodus thinks that the key to success is individualization of therapy. “You’re going to like a certain sensation, and I might not like it. You can’t put us in the same room and expect the same results. We have to identify the preferences of how people interact with their environment, and what their brain does at a neuroscience level with that information,” she said.
Caregiving hacks
The program employs telehealth to work with caregivers so they can also create sensory environments within the home, using environment to trigger behavior.
For example, although individuals with dementia may have reduced response to color, the color red is unique. “Red is a cortical trigger. Red always stands out to people, so in our package that we send out as part of this intervention, we send out a roll of red duct tape,” said Dr. Rhodus.
An example of the use of red was a patient with dementia who had stopped drinking on his own, causing his caregiver daughter to be concerned that he would soon have to enter a nursing home. Examining the room, the occupational therapist realized that the water was kept out of sight, and suggested that the water glass be placed within the patient’s view, atop a square created with the red duct tape.
“These are just some of the simple concepts. They kind of seem easy. Some of my participants call them caregiving hacks, but it’s things that are grounded in neuroscience – how the brain processes the environment, and then how can we plug in supports and cues in whatever area is missing,” said Dr. Rhodus.
In the program, the caregiver fills out several online surveys, and an occupational therapist conducts an interview to identify specific challenges, such as bathing, or using the toilet, or going to church. Then an adult sensory profile reveals how the patient perceives his or her environment. “It’s taking those individual pieces, and then boiling it down to these mechanisms at the behavioral and neuroscience level,” said Dr. Rhodus. She said the entire setup process takes about an hour.
Impactful care
The individualized approach of the HARMONY (Helping Older Adults Create and Manage Occupations Successfully) method is promising, according to Monika Gross, executive director of the Poise Project, which uses the Alexander Technique to help people with chronic conditions such as Parkinson’s disease.
“Although it’s always a very simple idea that human beings need sensory processing aspects in their lives, from the time they’re infants through to the end of life, we don’t really focus on the end of life in a way that can bring meaning between the care partner and the person living with dementia. The other thing that was impressive is that this is in a rural community, where there often aren’t a lot of resources available, (such as) classes that the care partner can take their loved one to. So having something where the care partner has some confidence that they can really make an impact in that person that they are seeing decline, that they can see their behavior change [is good],” said Ms. Gross.
Empowering caregivers
The study included 30 pairs of patients and caregivers who were randomized to the individualized care (I), standardized care, or a control group. Adherence to weekly visits was high (I, 88%; S, 100%; C, 60%; P = .061). Retention was strong (I, 80%; S, 60%; C, 50%).
“It was feasible ... and at the end, we found a significant improvement in care partner satisfaction. We actually empowered these people to care for their loved ones, and in doing that, and helping them set up environmental cues, it allowed that person to perform at a more independent level,” said Dr. Rhodus.
The trial was only a proof of concept, so although the researchers saw signs of efficacy, it wasn’t powered to show that. They are currently enrolling additional patients and caregivers for larger studies to further test the approach.
Dr. Rhodus and Ms. Gross have no relevant financial disclosures.
Everyone gets by using environmental cues: For example, if you have to go to use the toilet in public, a bathroom sign prompts an immediate response. However, patients with dementia often struggle with environmental cues, which can complicate the already difficult task faced by their caregivers.
Individuals with dementia can lose awareness of such signs, and even colors, making it harder for them to interpret environmental cues.
The study, presented at the Alzheimer’s Association International Conference, recruited 30 pairs of patients and their caregivers. The approach is based on the Dunn model of sensory processing, which focuses on altering environments to maximize chances of success. It “explains that sensory processing is the information coming in, and then our ability to regulate and habituate to those sensations (creates) behavior,” Elizabeth Rhodus, MD, PhD, said during her talk. Dr. Rhodus is assistant professor of medicine at the University of Kentucky, Lexington.
Sensory-based interventions are not uncommon, but most are applied to pediatric populations and tend to focus on sensory processing disorders and autism spectrum disorder. The few programs that do focus on adults have varying methods and produce mixed results. Dr. Rhodus thinks that the key to success is individualization of therapy. “You’re going to like a certain sensation, and I might not like it. You can’t put us in the same room and expect the same results. We have to identify the preferences of how people interact with their environment, and what their brain does at a neuroscience level with that information,” she said.
Caregiving hacks
The program employs telehealth to work with caregivers so they can also create sensory environments within the home, using environment to trigger behavior.
For example, although individuals with dementia may have reduced response to color, the color red is unique. “Red is a cortical trigger. Red always stands out to people, so in our package that we send out as part of this intervention, we send out a roll of red duct tape,” said Dr. Rhodus.
An example of the use of red was a patient with dementia who had stopped drinking on his own, causing his caregiver daughter to be concerned that he would soon have to enter a nursing home. Examining the room, the occupational therapist realized that the water was kept out of sight, and suggested that the water glass be placed within the patient’s view, atop a square created with the red duct tape.
“These are just some of the simple concepts. They kind of seem easy. Some of my participants call them caregiving hacks, but it’s things that are grounded in neuroscience – how the brain processes the environment, and then how can we plug in supports and cues in whatever area is missing,” said Dr. Rhodus.
In the program, the caregiver fills out several online surveys, and an occupational therapist conducts an interview to identify specific challenges, such as bathing, or using the toilet, or going to church. Then an adult sensory profile reveals how the patient perceives his or her environment. “It’s taking those individual pieces, and then boiling it down to these mechanisms at the behavioral and neuroscience level,” said Dr. Rhodus. She said the entire setup process takes about an hour.
Impactful care
The individualized approach of the HARMONY (Helping Older Adults Create and Manage Occupations Successfully) method is promising, according to Monika Gross, executive director of the Poise Project, which uses the Alexander Technique to help people with chronic conditions such as Parkinson’s disease.
“Although it’s always a very simple idea that human beings need sensory processing aspects in their lives, from the time they’re infants through to the end of life, we don’t really focus on the end of life in a way that can bring meaning between the care partner and the person living with dementia. The other thing that was impressive is that this is in a rural community, where there often aren’t a lot of resources available, (such as) classes that the care partner can take their loved one to. So having something where the care partner has some confidence that they can really make an impact in that person that they are seeing decline, that they can see their behavior change [is good],” said Ms. Gross.
Empowering caregivers
The study included 30 pairs of patients and caregivers who were randomized to the individualized care (I), standardized care, or a control group. Adherence to weekly visits was high (I, 88%; S, 100%; C, 60%; P = .061). Retention was strong (I, 80%; S, 60%; C, 50%).
“It was feasible ... and at the end, we found a significant improvement in care partner satisfaction. We actually empowered these people to care for their loved ones, and in doing that, and helping them set up environmental cues, it allowed that person to perform at a more independent level,” said Dr. Rhodus.
The trial was only a proof of concept, so although the researchers saw signs of efficacy, it wasn’t powered to show that. They are currently enrolling additional patients and caregivers for larger studies to further test the approach.
Dr. Rhodus and Ms. Gross have no relevant financial disclosures.
FROM AAIC 2022
Linear leg rash

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846
The gut microbes have spoken: All fiber is good fiber
Finding a fiber of good moral fiber
If you’ve ever wandered into the supplement aisle at your local grocery store, you’ve probably noticed an overabundance of fiber supplements that claim to do this for you and benefit that. Since there’s no Food and Drug Administration regulation on fiber supplements, manufacturers are free to (and do) make whatever wild claims they like. And much like choosing which of 500 shows to watch on Netflix, when you’re spoiled for choice, it can be difficult to pick.
Enter a team of molecular geneticists and microbiologists from Duke University. They can’t tell you what show to watch next, but they can tell you which fiber to choose, thanks to their new study. And the answer? Yes.
Well that’s not very helpful, but let us explain. For their study, a group of 28 received three of the main fiber supplements (inulin, dextrin, and galactooligosaccharides) for a week each, followed by a week off of fibers for their gut to return to baseline until they’d received all three. Those who consumed the least fiber at baseline saw the greatest benefit from fiber supplementation, with no appreciable difference between the three types. It was the same story for study participants who already consumed enough fiber; because their guts already hosted a more-optimal microbiome, the type of supplement didn’t matter. The benefits were the same across the board.
In an additional study, the Duke researchers found that gut microbiomes reacted to new fiber within a day, being primed to consume fiber on the first dose and digesting it more quickly on the second fiber dose.
The results, the researchers pointed out, make sense, since the average American only consumes 20%-40% of their daily recommended supply of fiber. Our digestive systems aren’t picky; they just want more, so go out there and choose whatever fiber you’d like. Do that, and then feel free to eat as many double bacon cheeseburgers as you’d like. That is the pinnacle of diet right there. Dietitians literally could not complain about it.
Jarlsberg vs. Camembert: This time it’s skeletal
Fiber is fabulous, of course, but the road to dietary health and wellness fulfillment takes us to many other, equally wondrous places. Hey, look! This next exit is covered with cheese.
All the cheeses are here, from Abbaye de Belloc to Zwitser, and there, right between the jalapeno cheddar and the Jermi tortes you’ll find Jarlsberg, a mild, semisoft, nutty-flavored cheese that comes from Jarlsberg in eastern Norway. A recent study also suggests that Jarlsberg may help to prevent osteopenia and osteoporosis.
A group of Norwegian investigators gathered together 66 healthy women and gave them a daily portion of either Jarlsberg or Camembert for 6 weeks, at which point the Camembert group was switched to Jarlsberg for another 6 weeks.
The research team choose Camembert because of its similarity to Jarlsberg in fat and protein content. Jarlsberg, however, also is rich in vitamin K2, which is important for bone health, and a substance known as DHNA, which “might combat bone thinning and increase bone tissue formation,” they said in a Eurekalert release.
After the first 6 weeks, blood levels of osteocalcin; vitamin K2; and PINP, a peptide involved in bone turnover, were significantly higher in the Jarlsberg group only. All those measures rose significantly after the switch from Camembert to Jarlsberg, while levels of total and LDL cholesterol “fell significantly in the Camembert group after they switched to Jarlsberg,” the team added.
But wait! There’s more! HbA1c fell significantly among those initially eating the Jarlsberg but rose sharply in those eating Camembert. Do you see where this is going? After the Camembert group made the switch to Jarlsberg, their HbA1c levels fell significantly as well.
So it’s not just a cheese thing: The effects are specific to Jarlsberg. Can you guess what we’re having for lunch? Double bacon and fiber Jarlsbergers. Mmm, Jarlsburgers.
Luck be a lady: The mother of twins
It’s widely believed that women who have twins must be more fertile, giving birth to more than one child at a time. Some studies have supported the idea, but more recent work is refuting that claim. In actuality, it might just be more statistics and luck than fertility after all.
Those earlier studies supporting fertility didn’t specify whether the chances of twin births were based on the ability to produce more than one egg at a time or on the number of births that women had overall. Looking at 100,000 preindustrial European births, before contraception was available, researchers from Norway, Germany, France, and the United Kingdom found that the number of total births, twins included, makes all the difference.
“When a woman gives birth several times, the chances increase that at least one of these births will be a twin birth,” investigator Gine Roll Skjærvø of the Norwegian University of Science and Technology said in a written statement.
Since twins occur in 1%-3% of all births, the more births that a woman has, the better her chances of giving birth to twins. The researchers compared it to playing the lottery. You buy enough tickets, eventually your numbers are going to come up. Despite that, however, they found that women who give birth to twins give birth less often than those who don’t have twins. Which raises the idea of sheer luck.
The researchers said that there’s still a lot to uncover in twin births, noting that “uncritically comparing groups of women with and without twins can trick us into believing the opposite of what is really true. These groupings may either hide the effects of twinning and fertility genes where they exist, or vice versa, create the illusion of these if they do not exist.”
For now, this new research claims that it’s basically a lottery. And women who give birth to twins hit the jackpot.
Those with low wages may be earning future memory loss
Not only are low wages detrimental to our souls, hopes, and dreams, but a new study shows that low wages also are linked to quicker memory decline later in life. Sustained low wages not only cause stress and food insecurity in the lives of many, but they also can cause diseases such as depression, obesity, and high blood pressure, which are risk factors for cognitive aging.
The study was conducted using records from the Health and Retirement Study for the years 1992-2016 and focused on 2,879 adults born between 1936 and 1941. The participants were divided into three groups: those who never earned low wages, those who sometimes did, and those who always did.
The investigators found that workers who earned sustained low wages – defined as an hourly wage lower than two-thirds of the federal median wage for the corresponding year – “experienced significantly faster memory decline in older age” than did those who never earned low wages.
There are signs of inflation everywhere we look these days, but many people are not earning higher wages to compensate for the extra expenses. “Increasing the federal minimum wage, for example to $15 per hour, remains a gridlock issue in Congress,” lead author Katrina Kezios of the Columbia University Mailman School of Public Health, said in a statement released by the university.
If only salaries would rise instead of prices for once.
Finding a fiber of good moral fiber
If you’ve ever wandered into the supplement aisle at your local grocery store, you’ve probably noticed an overabundance of fiber supplements that claim to do this for you and benefit that. Since there’s no Food and Drug Administration regulation on fiber supplements, manufacturers are free to (and do) make whatever wild claims they like. And much like choosing which of 500 shows to watch on Netflix, when you’re spoiled for choice, it can be difficult to pick.
Enter a team of molecular geneticists and microbiologists from Duke University. They can’t tell you what show to watch next, but they can tell you which fiber to choose, thanks to their new study. And the answer? Yes.
Well that’s not very helpful, but let us explain. For their study, a group of 28 received three of the main fiber supplements (inulin, dextrin, and galactooligosaccharides) for a week each, followed by a week off of fibers for their gut to return to baseline until they’d received all three. Those who consumed the least fiber at baseline saw the greatest benefit from fiber supplementation, with no appreciable difference between the three types. It was the same story for study participants who already consumed enough fiber; because their guts already hosted a more-optimal microbiome, the type of supplement didn’t matter. The benefits were the same across the board.
In an additional study, the Duke researchers found that gut microbiomes reacted to new fiber within a day, being primed to consume fiber on the first dose and digesting it more quickly on the second fiber dose.
The results, the researchers pointed out, make sense, since the average American only consumes 20%-40% of their daily recommended supply of fiber. Our digestive systems aren’t picky; they just want more, so go out there and choose whatever fiber you’d like. Do that, and then feel free to eat as many double bacon cheeseburgers as you’d like. That is the pinnacle of diet right there. Dietitians literally could not complain about it.
Jarlsberg vs. Camembert: This time it’s skeletal
Fiber is fabulous, of course, but the road to dietary health and wellness fulfillment takes us to many other, equally wondrous places. Hey, look! This next exit is covered with cheese.
All the cheeses are here, from Abbaye de Belloc to Zwitser, and there, right between the jalapeno cheddar and the Jermi tortes you’ll find Jarlsberg, a mild, semisoft, nutty-flavored cheese that comes from Jarlsberg in eastern Norway. A recent study also suggests that Jarlsberg may help to prevent osteopenia and osteoporosis.
A group of Norwegian investigators gathered together 66 healthy women and gave them a daily portion of either Jarlsberg or Camembert for 6 weeks, at which point the Camembert group was switched to Jarlsberg for another 6 weeks.
The research team choose Camembert because of its similarity to Jarlsberg in fat and protein content. Jarlsberg, however, also is rich in vitamin K2, which is important for bone health, and a substance known as DHNA, which “might combat bone thinning and increase bone tissue formation,” they said in a Eurekalert release.
After the first 6 weeks, blood levels of osteocalcin; vitamin K2; and PINP, a peptide involved in bone turnover, were significantly higher in the Jarlsberg group only. All those measures rose significantly after the switch from Camembert to Jarlsberg, while levels of total and LDL cholesterol “fell significantly in the Camembert group after they switched to Jarlsberg,” the team added.
But wait! There’s more! HbA1c fell significantly among those initially eating the Jarlsberg but rose sharply in those eating Camembert. Do you see where this is going? After the Camembert group made the switch to Jarlsberg, their HbA1c levels fell significantly as well.
So it’s not just a cheese thing: The effects are specific to Jarlsberg. Can you guess what we’re having for lunch? Double bacon and fiber Jarlsbergers. Mmm, Jarlsburgers.
Luck be a lady: The mother of twins
It’s widely believed that women who have twins must be more fertile, giving birth to more than one child at a time. Some studies have supported the idea, but more recent work is refuting that claim. In actuality, it might just be more statistics and luck than fertility after all.
Those earlier studies supporting fertility didn’t specify whether the chances of twin births were based on the ability to produce more than one egg at a time or on the number of births that women had overall. Looking at 100,000 preindustrial European births, before contraception was available, researchers from Norway, Germany, France, and the United Kingdom found that the number of total births, twins included, makes all the difference.
“When a woman gives birth several times, the chances increase that at least one of these births will be a twin birth,” investigator Gine Roll Skjærvø of the Norwegian University of Science and Technology said in a written statement.
Since twins occur in 1%-3% of all births, the more births that a woman has, the better her chances of giving birth to twins. The researchers compared it to playing the lottery. You buy enough tickets, eventually your numbers are going to come up. Despite that, however, they found that women who give birth to twins give birth less often than those who don’t have twins. Which raises the idea of sheer luck.
The researchers said that there’s still a lot to uncover in twin births, noting that “uncritically comparing groups of women with and without twins can trick us into believing the opposite of what is really true. These groupings may either hide the effects of twinning and fertility genes where they exist, or vice versa, create the illusion of these if they do not exist.”
For now, this new research claims that it’s basically a lottery. And women who give birth to twins hit the jackpot.
Those with low wages may be earning future memory loss
Not only are low wages detrimental to our souls, hopes, and dreams, but a new study shows that low wages also are linked to quicker memory decline later in life. Sustained low wages not only cause stress and food insecurity in the lives of many, but they also can cause diseases such as depression, obesity, and high blood pressure, which are risk factors for cognitive aging.
The study was conducted using records from the Health and Retirement Study for the years 1992-2016 and focused on 2,879 adults born between 1936 and 1941. The participants were divided into three groups: those who never earned low wages, those who sometimes did, and those who always did.
The investigators found that workers who earned sustained low wages – defined as an hourly wage lower than two-thirds of the federal median wage for the corresponding year – “experienced significantly faster memory decline in older age” than did those who never earned low wages.
There are signs of inflation everywhere we look these days, but many people are not earning higher wages to compensate for the extra expenses. “Increasing the federal minimum wage, for example to $15 per hour, remains a gridlock issue in Congress,” lead author Katrina Kezios of the Columbia University Mailman School of Public Health, said in a statement released by the university.
If only salaries would rise instead of prices for once.
Finding a fiber of good moral fiber
If you’ve ever wandered into the supplement aisle at your local grocery store, you’ve probably noticed an overabundance of fiber supplements that claim to do this for you and benefit that. Since there’s no Food and Drug Administration regulation on fiber supplements, manufacturers are free to (and do) make whatever wild claims they like. And much like choosing which of 500 shows to watch on Netflix, when you’re spoiled for choice, it can be difficult to pick.
Enter a team of molecular geneticists and microbiologists from Duke University. They can’t tell you what show to watch next, but they can tell you which fiber to choose, thanks to their new study. And the answer? Yes.
Well that’s not very helpful, but let us explain. For their study, a group of 28 received three of the main fiber supplements (inulin, dextrin, and galactooligosaccharides) for a week each, followed by a week off of fibers for their gut to return to baseline until they’d received all three. Those who consumed the least fiber at baseline saw the greatest benefit from fiber supplementation, with no appreciable difference between the three types. It was the same story for study participants who already consumed enough fiber; because their guts already hosted a more-optimal microbiome, the type of supplement didn’t matter. The benefits were the same across the board.
In an additional study, the Duke researchers found that gut microbiomes reacted to new fiber within a day, being primed to consume fiber on the first dose and digesting it more quickly on the second fiber dose.
The results, the researchers pointed out, make sense, since the average American only consumes 20%-40% of their daily recommended supply of fiber. Our digestive systems aren’t picky; they just want more, so go out there and choose whatever fiber you’d like. Do that, and then feel free to eat as many double bacon cheeseburgers as you’d like. That is the pinnacle of diet right there. Dietitians literally could not complain about it.
Jarlsberg vs. Camembert: This time it’s skeletal
Fiber is fabulous, of course, but the road to dietary health and wellness fulfillment takes us to many other, equally wondrous places. Hey, look! This next exit is covered with cheese.
All the cheeses are here, from Abbaye de Belloc to Zwitser, and there, right between the jalapeno cheddar and the Jermi tortes you’ll find Jarlsberg, a mild, semisoft, nutty-flavored cheese that comes from Jarlsberg in eastern Norway. A recent study also suggests that Jarlsberg may help to prevent osteopenia and osteoporosis.
A group of Norwegian investigators gathered together 66 healthy women and gave them a daily portion of either Jarlsberg or Camembert for 6 weeks, at which point the Camembert group was switched to Jarlsberg for another 6 weeks.
The research team choose Camembert because of its similarity to Jarlsberg in fat and protein content. Jarlsberg, however, also is rich in vitamin K2, which is important for bone health, and a substance known as DHNA, which “might combat bone thinning and increase bone tissue formation,” they said in a Eurekalert release.
After the first 6 weeks, blood levels of osteocalcin; vitamin K2; and PINP, a peptide involved in bone turnover, were significantly higher in the Jarlsberg group only. All those measures rose significantly after the switch from Camembert to Jarlsberg, while levels of total and LDL cholesterol “fell significantly in the Camembert group after they switched to Jarlsberg,” the team added.
But wait! There’s more! HbA1c fell significantly among those initially eating the Jarlsberg but rose sharply in those eating Camembert. Do you see where this is going? After the Camembert group made the switch to Jarlsberg, their HbA1c levels fell significantly as well.
So it’s not just a cheese thing: The effects are specific to Jarlsberg. Can you guess what we’re having for lunch? Double bacon and fiber Jarlsbergers. Mmm, Jarlsburgers.
Luck be a lady: The mother of twins
It’s widely believed that women who have twins must be more fertile, giving birth to more than one child at a time. Some studies have supported the idea, but more recent work is refuting that claim. In actuality, it might just be more statistics and luck than fertility after all.
Those earlier studies supporting fertility didn’t specify whether the chances of twin births were based on the ability to produce more than one egg at a time or on the number of births that women had overall. Looking at 100,000 preindustrial European births, before contraception was available, researchers from Norway, Germany, France, and the United Kingdom found that the number of total births, twins included, makes all the difference.
“When a woman gives birth several times, the chances increase that at least one of these births will be a twin birth,” investigator Gine Roll Skjærvø of the Norwegian University of Science and Technology said in a written statement.
Since twins occur in 1%-3% of all births, the more births that a woman has, the better her chances of giving birth to twins. The researchers compared it to playing the lottery. You buy enough tickets, eventually your numbers are going to come up. Despite that, however, they found that women who give birth to twins give birth less often than those who don’t have twins. Which raises the idea of sheer luck.
The researchers said that there’s still a lot to uncover in twin births, noting that “uncritically comparing groups of women with and without twins can trick us into believing the opposite of what is really true. These groupings may either hide the effects of twinning and fertility genes where they exist, or vice versa, create the illusion of these if they do not exist.”
For now, this new research claims that it’s basically a lottery. And women who give birth to twins hit the jackpot.
Those with low wages may be earning future memory loss
Not only are low wages detrimental to our souls, hopes, and dreams, but a new study shows that low wages also are linked to quicker memory decline later in life. Sustained low wages not only cause stress and food insecurity in the lives of many, but they also can cause diseases such as depression, obesity, and high blood pressure, which are risk factors for cognitive aging.
The study was conducted using records from the Health and Retirement Study for the years 1992-2016 and focused on 2,879 adults born between 1936 and 1941. The participants were divided into three groups: those who never earned low wages, those who sometimes did, and those who always did.
The investigators found that workers who earned sustained low wages – defined as an hourly wage lower than two-thirds of the federal median wage for the corresponding year – “experienced significantly faster memory decline in older age” than did those who never earned low wages.
There are signs of inflation everywhere we look these days, but many people are not earning higher wages to compensate for the extra expenses. “Increasing the federal minimum wage, for example to $15 per hour, remains a gridlock issue in Congress,” lead author Katrina Kezios of the Columbia University Mailman School of Public Health, said in a statement released by the university.
If only salaries would rise instead of prices for once.
Perceptions of Community Service in Dermatology Residency Training Programs: A Survey-Based Study of Program Directors, Residents, and Recent Dermatology Residency Graduates
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.
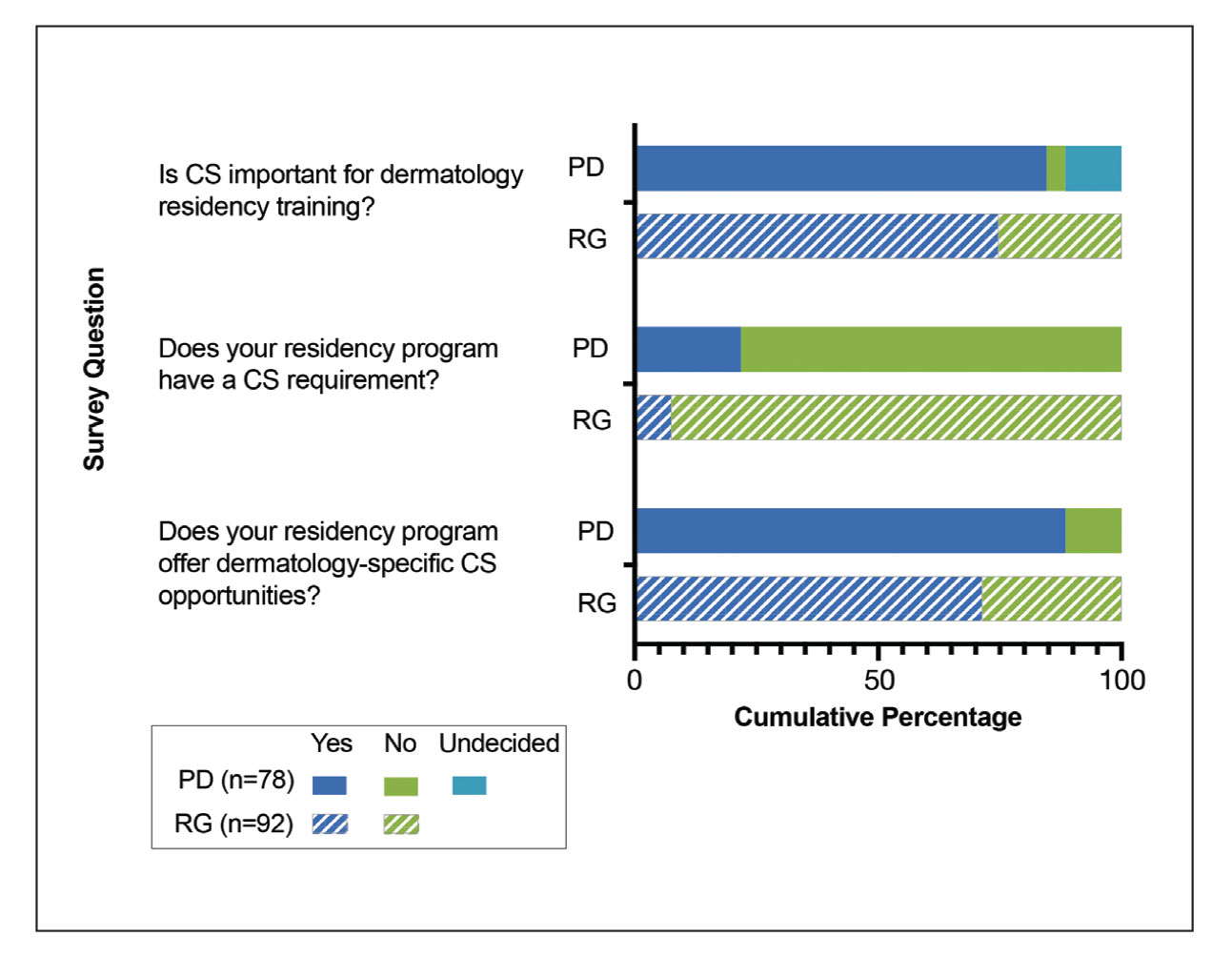
Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).
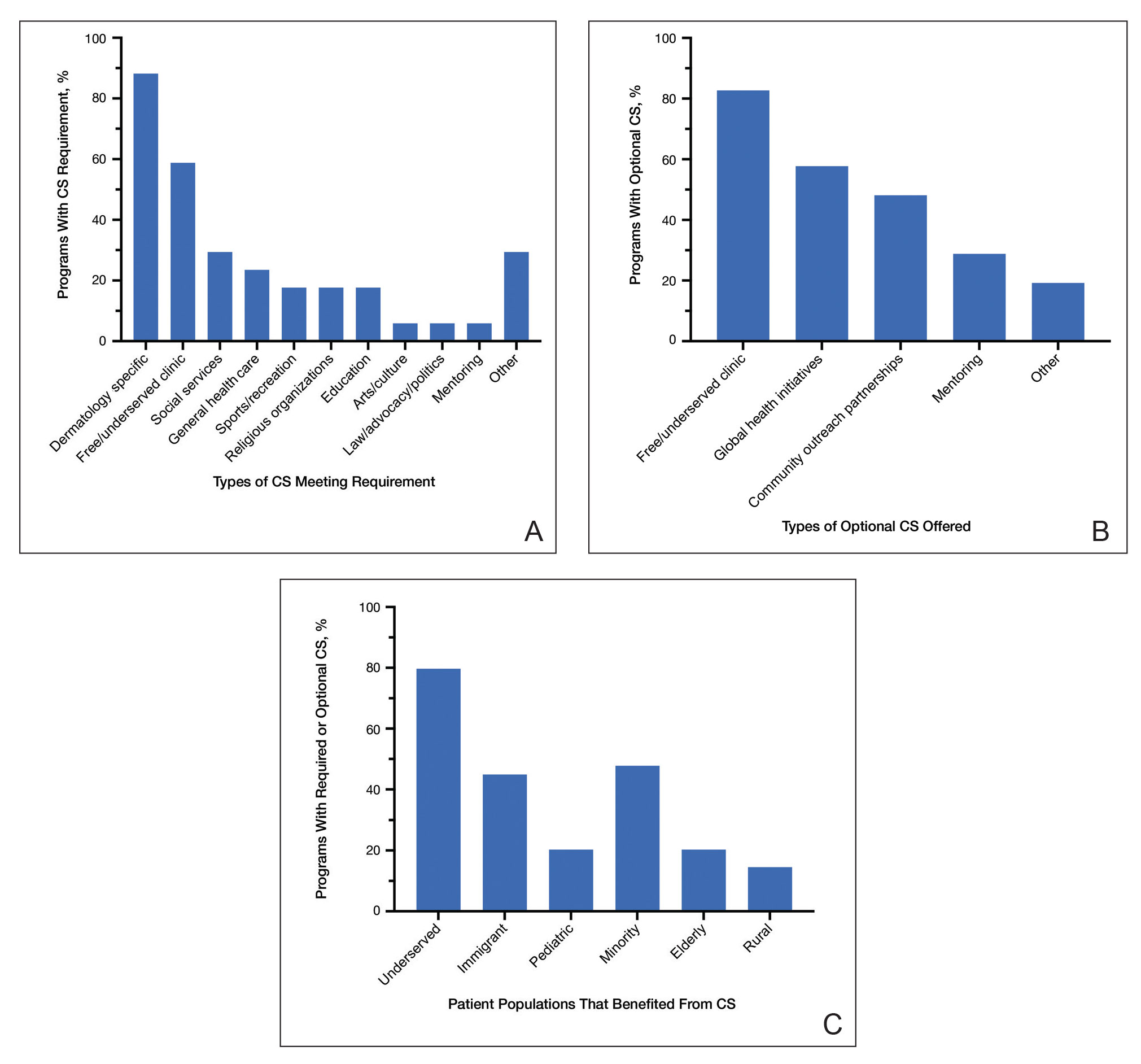
Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.
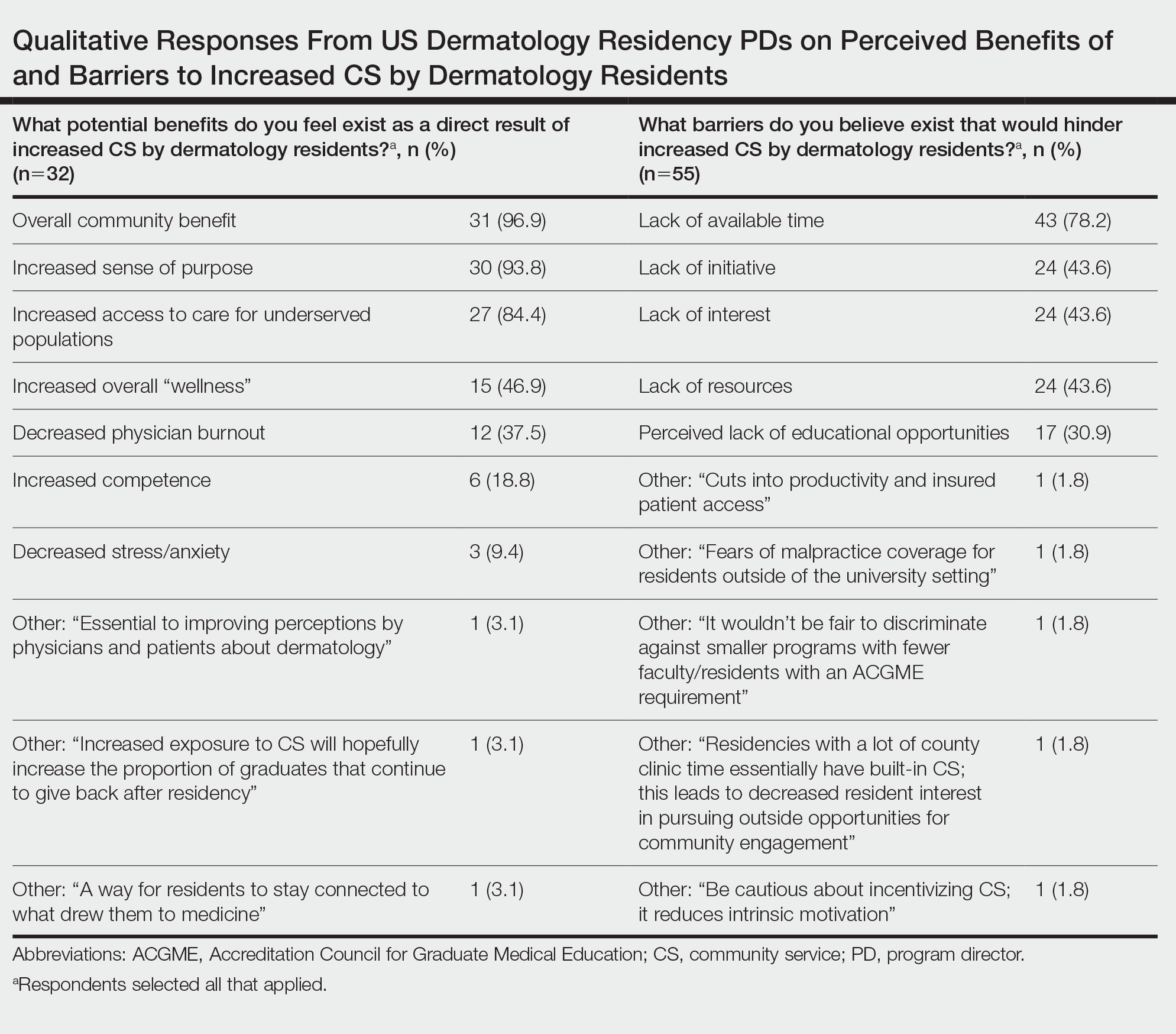
Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Participation of dermatology residents in service-learning experiences increases awareness of health disparities and social factors impacting dermatologic care and promotes a lifelong commitment to serving vulnerable populations.
- Integrating service learning into the dermatology residency program curriculum enhances trainees’ cultural sensitivity and encourages the prioritization of skin health equity.
- Service learning will help bridge the gap in access to dermatologic care for patients in medically marginalized communities.
Nail Salon Safety: From Nail Dystrophy to Acrylate Contact Allergies
As residents, it is important to understand the steps of the manicuring process and be able to inform patients on how to maintain optimal nail health while continuing to go to nail salons. Most patients are not aware of the possible allergic, traumatic, and/or infectious complications of manicuring their nails. There are practical steps that can be taken to prevent nail issues, such as avoiding cutting one’s cuticles or using allergen-free nail polishes. These simple fixes can make a big difference in long-term nail health in our patients.
Nail Polish Application Process
The nails are first soaked in a warm soapy solution to soften the nail plate and cuticles.1 Then the nail tips and plates are filed and occasionally are smoothed with a drill. The cuticles are cut with a cuticle cutter. Nail polish—base coat, color enamel, and top coat—is then applied to the nail. Acrylic or sculptured nails and gel and dip manicures are composed of chemical monomers and polymers that harden either at room temperature or through UV or light-emitting diode (LED) exposure. The chemicals in these products can damage nails and cause allergic reactions.
Contact Dermatitis
Approximately 2% of individuals have been found to have allergic or irritant contact dermatitis to nail care products. The top 5 allergens implicated in nail products are (1) 2-hydroxyethyl methacrylate, (2) methyl methacrylate, (3) ethyl acrylate, (4) ethyl-2-cyanoacrylate, and (5) tosylamide.2 Methyl methacrylate was banned in 1974 by the US Food and Drug Administration due to reports of severe contact dermatitis, paronychia, and nail dystrophy.3 Due to their potent sensitizing effects, acrylates were named the contact allergen of the year in 2012 by the American Contact Dermatitis Society.3
Acrylates are plastic products formed by polymerization of acrylic or methacrylic acid.4 Artificial sculptured nails are created by mixing powdered polymethyl methacrylate polymers and liquid ethyl or isobutyl methacrylate monomers and then applying this mixture to the nail plate.5 Gel and powder nails employ a mixture that is similar to acrylic powders, which require UV or LED radiation to polymerize and harden on the nail plate.
Tosylamide, or tosylamide formaldehyde resin, is another potent allergen that promotes adhesion of the enamel to the nail.6 It is important to note that sensitization may develop months to years after using artificial nails.
Clinical features of contact allergy secondary to nail polish can vary. Some patients experience severe periungual dermatitis. Others can present with facial or eyelid dermatitis due to exposure to airborne particles of acrylates or from contact with fingertips bearing acrylic nails.6,7 If inhaled, acrylates also can cause wheezing asthma or allergic rhinoconjunctivitis.
Common Onychodystrophies
Damage to the natural nail plate is inevitable with continued wear of sculptured nails. With 2 to 4 months of consecutive wear, the natural nails turn yellow, brittle, and weak.5 One study noted that the thickness of an individual’s left thumb nail plate thinned from 0.059 cm to 0.03 cm after a gel manicure was removed from the nail.8 Nail injuries due to manicuring include keratin granulations, onycholysis, pincer nail deformities, pseudopsoriatic nails, lamellar onychoschizia, transverse leukonychia, and ingrown nails.6 One interesting nail dystrophy reported secondary to gel manicures is pterygium inversum unguis or a ventral pterygium that causes an abnormal painful adherence of the hyponychium to the ventral surface of the nail plate. Patients prone to developing pterygium inversum unguis can experience sensitivity, pain, or burning sensations during LED or UVA light exposure.9
Infections
In addition to contact allergies and nail dystrophies, each step of the manicuring process, such as cutting cuticles, presents opportunities for infectious agents to enter the nail fold. Acute or chronic paronychia, or inflammation of the nail fold, most commonly is caused by bacterial infections with Staphylococcus aureus. Green nail syndrome caused by Pseudomonas aeruginosa also is common.1 Onychomycosis due to Trichophyton rubrum is one of the most frequent fungal infections contracted at nail salons. Mycobacteria such as Mycobacterium fortuitum also have been implicated in infections from salons, as they can be found in the jets of pedicure spas, which are not sanitized regularly.10
Final Thoughts
Nail cosmetics are an integral part of many patients’ lives. Being able to educate yourself and your patients on the hazards of nail salons can help them avoid painful infections, contact allergies, and acute to chronic nail deformities. It is important for residents to be aware of the different dermatoses that can arise in men and women who frequent nail salons as the popularity of the nail beauty industry continues to rise.
- Reinecke JK, Hinshaw MA. Nail health in women. Int J Womens Dermatol. 2020;6:73-79. doi:10.1016/j.ijwd.2020.01.006
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermatitis. 2020;31:191-201. doi:10.1097/DER.0000000000000583
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society allergens of the year 2000 to 2020 [published online April 25, 2020]. Dermatol Clin. 2020;38:309-320. doi:10.1016/j.det.2020.02.011
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2587-2588.
- Iorizzo M, Piraccini BM, Tosti A. Nail cosmetics in nail disorders.J Cosmet Dermatol. 2007;6:53-58. doi:10.1111/j.1473-2165.2007.00290.x
- Maio P, Carvalho R, Amaro C, et al. Letter: allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J. 2012;18:13.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29. doi:10.1111/j.1473-2165.2011.00595.x
- Cervantes J, Sanchez M, Eber AE, et al. Pterygium inversum unguis secondary to gel polish [published online October 16, 2017]. J Eur Acad Dermatol Venereol. 2018;32:160-163. doi:10.1111/jdv.14603
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
As residents, it is important to understand the steps of the manicuring process and be able to inform patients on how to maintain optimal nail health while continuing to go to nail salons. Most patients are not aware of the possible allergic, traumatic, and/or infectious complications of manicuring their nails. There are practical steps that can be taken to prevent nail issues, such as avoiding cutting one’s cuticles or using allergen-free nail polishes. These simple fixes can make a big difference in long-term nail health in our patients.
Nail Polish Application Process
The nails are first soaked in a warm soapy solution to soften the nail plate and cuticles.1 Then the nail tips and plates are filed and occasionally are smoothed with a drill. The cuticles are cut with a cuticle cutter. Nail polish—base coat, color enamel, and top coat—is then applied to the nail. Acrylic or sculptured nails and gel and dip manicures are composed of chemical monomers and polymers that harden either at room temperature or through UV or light-emitting diode (LED) exposure. The chemicals in these products can damage nails and cause allergic reactions.
Contact Dermatitis
Approximately 2% of individuals have been found to have allergic or irritant contact dermatitis to nail care products. The top 5 allergens implicated in nail products are (1) 2-hydroxyethyl methacrylate, (2) methyl methacrylate, (3) ethyl acrylate, (4) ethyl-2-cyanoacrylate, and (5) tosylamide.2 Methyl methacrylate was banned in 1974 by the US Food and Drug Administration due to reports of severe contact dermatitis, paronychia, and nail dystrophy.3 Due to their potent sensitizing effects, acrylates were named the contact allergen of the year in 2012 by the American Contact Dermatitis Society.3
Acrylates are plastic products formed by polymerization of acrylic or methacrylic acid.4 Artificial sculptured nails are created by mixing powdered polymethyl methacrylate polymers and liquid ethyl or isobutyl methacrylate monomers and then applying this mixture to the nail plate.5 Gel and powder nails employ a mixture that is similar to acrylic powders, which require UV or LED radiation to polymerize and harden on the nail plate.
Tosylamide, or tosylamide formaldehyde resin, is another potent allergen that promotes adhesion of the enamel to the nail.6 It is important to note that sensitization may develop months to years after using artificial nails.
Clinical features of contact allergy secondary to nail polish can vary. Some patients experience severe periungual dermatitis. Others can present with facial or eyelid dermatitis due to exposure to airborne particles of acrylates or from contact with fingertips bearing acrylic nails.6,7 If inhaled, acrylates also can cause wheezing asthma or allergic rhinoconjunctivitis.
Common Onychodystrophies
Damage to the natural nail plate is inevitable with continued wear of sculptured nails. With 2 to 4 months of consecutive wear, the natural nails turn yellow, brittle, and weak.5 One study noted that the thickness of an individual’s left thumb nail plate thinned from 0.059 cm to 0.03 cm after a gel manicure was removed from the nail.8 Nail injuries due to manicuring include keratin granulations, onycholysis, pincer nail deformities, pseudopsoriatic nails, lamellar onychoschizia, transverse leukonychia, and ingrown nails.6 One interesting nail dystrophy reported secondary to gel manicures is pterygium inversum unguis or a ventral pterygium that causes an abnormal painful adherence of the hyponychium to the ventral surface of the nail plate. Patients prone to developing pterygium inversum unguis can experience sensitivity, pain, or burning sensations during LED or UVA light exposure.9
Infections
In addition to contact allergies and nail dystrophies, each step of the manicuring process, such as cutting cuticles, presents opportunities for infectious agents to enter the nail fold. Acute or chronic paronychia, or inflammation of the nail fold, most commonly is caused by bacterial infections with Staphylococcus aureus. Green nail syndrome caused by Pseudomonas aeruginosa also is common.1 Onychomycosis due to Trichophyton rubrum is one of the most frequent fungal infections contracted at nail salons. Mycobacteria such as Mycobacterium fortuitum also have been implicated in infections from salons, as they can be found in the jets of pedicure spas, which are not sanitized regularly.10
Final Thoughts
Nail cosmetics are an integral part of many patients’ lives. Being able to educate yourself and your patients on the hazards of nail salons can help them avoid painful infections, contact allergies, and acute to chronic nail deformities. It is important for residents to be aware of the different dermatoses that can arise in men and women who frequent nail salons as the popularity of the nail beauty industry continues to rise.
As residents, it is important to understand the steps of the manicuring process and be able to inform patients on how to maintain optimal nail health while continuing to go to nail salons. Most patients are not aware of the possible allergic, traumatic, and/or infectious complications of manicuring their nails. There are practical steps that can be taken to prevent nail issues, such as avoiding cutting one’s cuticles or using allergen-free nail polishes. These simple fixes can make a big difference in long-term nail health in our patients.
Nail Polish Application Process
The nails are first soaked in a warm soapy solution to soften the nail plate and cuticles.1 Then the nail tips and plates are filed and occasionally are smoothed with a drill. The cuticles are cut with a cuticle cutter. Nail polish—base coat, color enamel, and top coat—is then applied to the nail. Acrylic or sculptured nails and gel and dip manicures are composed of chemical monomers and polymers that harden either at room temperature or through UV or light-emitting diode (LED) exposure. The chemicals in these products can damage nails and cause allergic reactions.
Contact Dermatitis
Approximately 2% of individuals have been found to have allergic or irritant contact dermatitis to nail care products. The top 5 allergens implicated in nail products are (1) 2-hydroxyethyl methacrylate, (2) methyl methacrylate, (3) ethyl acrylate, (4) ethyl-2-cyanoacrylate, and (5) tosylamide.2 Methyl methacrylate was banned in 1974 by the US Food and Drug Administration due to reports of severe contact dermatitis, paronychia, and nail dystrophy.3 Due to their potent sensitizing effects, acrylates were named the contact allergen of the year in 2012 by the American Contact Dermatitis Society.3
Acrylates are plastic products formed by polymerization of acrylic or methacrylic acid.4 Artificial sculptured nails are created by mixing powdered polymethyl methacrylate polymers and liquid ethyl or isobutyl methacrylate monomers and then applying this mixture to the nail plate.5 Gel and powder nails employ a mixture that is similar to acrylic powders, which require UV or LED radiation to polymerize and harden on the nail plate.
Tosylamide, or tosylamide formaldehyde resin, is another potent allergen that promotes adhesion of the enamel to the nail.6 It is important to note that sensitization may develop months to years after using artificial nails.
Clinical features of contact allergy secondary to nail polish can vary. Some patients experience severe periungual dermatitis. Others can present with facial or eyelid dermatitis due to exposure to airborne particles of acrylates or from contact with fingertips bearing acrylic nails.6,7 If inhaled, acrylates also can cause wheezing asthma or allergic rhinoconjunctivitis.
Common Onychodystrophies
Damage to the natural nail plate is inevitable with continued wear of sculptured nails. With 2 to 4 months of consecutive wear, the natural nails turn yellow, brittle, and weak.5 One study noted that the thickness of an individual’s left thumb nail plate thinned from 0.059 cm to 0.03 cm after a gel manicure was removed from the nail.8 Nail injuries due to manicuring include keratin granulations, onycholysis, pincer nail deformities, pseudopsoriatic nails, lamellar onychoschizia, transverse leukonychia, and ingrown nails.6 One interesting nail dystrophy reported secondary to gel manicures is pterygium inversum unguis or a ventral pterygium that causes an abnormal painful adherence of the hyponychium to the ventral surface of the nail plate. Patients prone to developing pterygium inversum unguis can experience sensitivity, pain, or burning sensations during LED or UVA light exposure.9
Infections
In addition to contact allergies and nail dystrophies, each step of the manicuring process, such as cutting cuticles, presents opportunities for infectious agents to enter the nail fold. Acute or chronic paronychia, or inflammation of the nail fold, most commonly is caused by bacterial infections with Staphylococcus aureus. Green nail syndrome caused by Pseudomonas aeruginosa also is common.1 Onychomycosis due to Trichophyton rubrum is one of the most frequent fungal infections contracted at nail salons. Mycobacteria such as Mycobacterium fortuitum also have been implicated in infections from salons, as they can be found in the jets of pedicure spas, which are not sanitized regularly.10
Final Thoughts
Nail cosmetics are an integral part of many patients’ lives. Being able to educate yourself and your patients on the hazards of nail salons can help them avoid painful infections, contact allergies, and acute to chronic nail deformities. It is important for residents to be aware of the different dermatoses that can arise in men and women who frequent nail salons as the popularity of the nail beauty industry continues to rise.
- Reinecke JK, Hinshaw MA. Nail health in women. Int J Womens Dermatol. 2020;6:73-79. doi:10.1016/j.ijwd.2020.01.006
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermatitis. 2020;31:191-201. doi:10.1097/DER.0000000000000583
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society allergens of the year 2000 to 2020 [published online April 25, 2020]. Dermatol Clin. 2020;38:309-320. doi:10.1016/j.det.2020.02.011
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2587-2588.
- Iorizzo M, Piraccini BM, Tosti A. Nail cosmetics in nail disorders.J Cosmet Dermatol. 2007;6:53-58. doi:10.1111/j.1473-2165.2007.00290.x
- Maio P, Carvalho R, Amaro C, et al. Letter: allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J. 2012;18:13.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29. doi:10.1111/j.1473-2165.2011.00595.x
- Cervantes J, Sanchez M, Eber AE, et al. Pterygium inversum unguis secondary to gel polish [published online October 16, 2017]. J Eur Acad Dermatol Venereol. 2018;32:160-163. doi:10.1111/jdv.14603
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Reinecke JK, Hinshaw MA. Nail health in women. Int J Womens Dermatol. 2020;6:73-79. doi:10.1016/j.ijwd.2020.01.006
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermatitis. 2020;31:191-201. doi:10.1097/DER.0000000000000583
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society allergens of the year 2000 to 2020 [published online April 25, 2020]. Dermatol Clin. 2020;38:309-320. doi:10.1016/j.det.2020.02.011
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2587-2588.
- Iorizzo M, Piraccini BM, Tosti A. Nail cosmetics in nail disorders.J Cosmet Dermatol. 2007;6:53-58. doi:10.1111/j.1473-2165.2007.00290.x
- Maio P, Carvalho R, Amaro C, et al. Letter: allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J. 2012;18:13.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29. doi:10.1111/j.1473-2165.2011.00595.x
- Cervantes J, Sanchez M, Eber AE, et al. Pterygium inversum unguis secondary to gel polish [published online October 16, 2017]. J Eur Acad Dermatol Venereol. 2018;32:160-163. doi:10.1111/jdv.14603
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
Resident Pearls
- Every step of the nail manicuring process presents opportunities for nail trauma, infections, and contact dermatitis.
- As residents, it is important to be aware of the hazards associated with nail salons and educate our patients accordingly.
- Nail health is essential to optimizing everyday work for our patients—whether it entails taking care of children, typing, or other hands-on activities.