User login
Nail Salon Safety: From Nail Dystrophy to Acrylate Contact Allergies
As residents, it is important to understand the steps of the manicuring process and be able to inform patients on how to maintain optimal nail health while continuing to go to nail salons. Most patients are not aware of the possible allergic, traumatic, and/or infectious complications of manicuring their nails. There are practical steps that can be taken to prevent nail issues, such as avoiding cutting one’s cuticles or using allergen-free nail polishes. These simple fixes can make a big difference in long-term nail health in our patients.
Nail Polish Application Process
The nails are first soaked in a warm soapy solution to soften the nail plate and cuticles.1 Then the nail tips and plates are filed and occasionally are smoothed with a drill. The cuticles are cut with a cuticle cutter. Nail polish—base coat, color enamel, and top coat—is then applied to the nail. Acrylic or sculptured nails and gel and dip manicures are composed of chemical monomers and polymers that harden either at room temperature or through UV or light-emitting diode (LED) exposure. The chemicals in these products can damage nails and cause allergic reactions.
Contact Dermatitis
Approximately 2% of individuals have been found to have allergic or irritant contact dermatitis to nail care products. The top 5 allergens implicated in nail products are (1) 2-hydroxyethyl methacrylate, (2) methyl methacrylate, (3) ethyl acrylate, (4) ethyl-2-cyanoacrylate, and (5) tosylamide.2 Methyl methacrylate was banned in 1974 by the US Food and Drug Administration due to reports of severe contact dermatitis, paronychia, and nail dystrophy.3 Due to their potent sensitizing effects, acrylates were named the contact allergen of the year in 2012 by the American Contact Dermatitis Society.3
Acrylates are plastic products formed by polymerization of acrylic or methacrylic acid.4 Artificial sculptured nails are created by mixing powdered polymethyl methacrylate polymers and liquid ethyl or isobutyl methacrylate monomers and then applying this mixture to the nail plate.5 Gel and powder nails employ a mixture that is similar to acrylic powders, which require UV or LED radiation to polymerize and harden on the nail plate.
Tosylamide, or tosylamide formaldehyde resin, is another potent allergen that promotes adhesion of the enamel to the nail.6 It is important to note that sensitization may develop months to years after using artificial nails.
Clinical features of contact allergy secondary to nail polish can vary. Some patients experience severe periungual dermatitis. Others can present with facial or eyelid dermatitis due to exposure to airborne particles of acrylates or from contact with fingertips bearing acrylic nails.6,7 If inhaled, acrylates also can cause wheezing asthma or allergic rhinoconjunctivitis.
Common Onychodystrophies
Damage to the natural nail plate is inevitable with continued wear of sculptured nails. With 2 to 4 months of consecutive wear, the natural nails turn yellow, brittle, and weak.5 One study noted that the thickness of an individual’s left thumb nail plate thinned from 0.059 cm to 0.03 cm after a gel manicure was removed from the nail.8 Nail injuries due to manicuring include keratin granulations, onycholysis, pincer nail deformities, pseudopsoriatic nails, lamellar onychoschizia, transverse leukonychia, and ingrown nails.6 One interesting nail dystrophy reported secondary to gel manicures is pterygium inversum unguis or a ventral pterygium that causes an abnormal painful adherence of the hyponychium to the ventral surface of the nail plate. Patients prone to developing pterygium inversum unguis can experience sensitivity, pain, or burning sensations during LED or UVA light exposure.9
Infections
In addition to contact allergies and nail dystrophies, each step of the manicuring process, such as cutting cuticles, presents opportunities for infectious agents to enter the nail fold. Acute or chronic paronychia, or inflammation of the nail fold, most commonly is caused by bacterial infections with Staphylococcus aureus. Green nail syndrome caused by Pseudomonas aeruginosa also is common.1 Onychomycosis due to Trichophyton rubrum is one of the most frequent fungal infections contracted at nail salons. Mycobacteria such as Mycobacterium fortuitum also have been implicated in infections from salons, as they can be found in the jets of pedicure spas, which are not sanitized regularly.10
Final Thoughts
Nail cosmetics are an integral part of many patients’ lives. Being able to educate yourself and your patients on the hazards of nail salons can help them avoid painful infections, contact allergies, and acute to chronic nail deformities. It is important for residents to be aware of the different dermatoses that can arise in men and women who frequent nail salons as the popularity of the nail beauty industry continues to rise.
- Reinecke JK, Hinshaw MA. Nail health in women. Int J Womens Dermatol. 2020;6:73-79. doi:10.1016/j.ijwd.2020.01.006
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermatitis. 2020;31:191-201. doi:10.1097/DER.0000000000000583
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society allergens of the year 2000 to 2020 [published online April 25, 2020]. Dermatol Clin. 2020;38:309-320. doi:10.1016/j.det.2020.02.011
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2587-2588.
- Iorizzo M, Piraccini BM, Tosti A. Nail cosmetics in nail disorders.J Cosmet Dermatol. 2007;6:53-58. doi:10.1111/j.1473-2165.2007.00290.x
- Maio P, Carvalho R, Amaro C, et al. Letter: allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J. 2012;18:13.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29. doi:10.1111/j.1473-2165.2011.00595.x
- Cervantes J, Sanchez M, Eber AE, et al. Pterygium inversum unguis secondary to gel polish [published online October 16, 2017]. J Eur Acad Dermatol Venereol. 2018;32:160-163. doi:10.1111/jdv.14603
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
As residents, it is important to understand the steps of the manicuring process and be able to inform patients on how to maintain optimal nail health while continuing to go to nail salons. Most patients are not aware of the possible allergic, traumatic, and/or infectious complications of manicuring their nails. There are practical steps that can be taken to prevent nail issues, such as avoiding cutting one’s cuticles or using allergen-free nail polishes. These simple fixes can make a big difference in long-term nail health in our patients.
Nail Polish Application Process
The nails are first soaked in a warm soapy solution to soften the nail plate and cuticles.1 Then the nail tips and plates are filed and occasionally are smoothed with a drill. The cuticles are cut with a cuticle cutter. Nail polish—base coat, color enamel, and top coat—is then applied to the nail. Acrylic or sculptured nails and gel and dip manicures are composed of chemical monomers and polymers that harden either at room temperature or through UV or light-emitting diode (LED) exposure. The chemicals in these products can damage nails and cause allergic reactions.
Contact Dermatitis
Approximately 2% of individuals have been found to have allergic or irritant contact dermatitis to nail care products. The top 5 allergens implicated in nail products are (1) 2-hydroxyethyl methacrylate, (2) methyl methacrylate, (3) ethyl acrylate, (4) ethyl-2-cyanoacrylate, and (5) tosylamide.2 Methyl methacrylate was banned in 1974 by the US Food and Drug Administration due to reports of severe contact dermatitis, paronychia, and nail dystrophy.3 Due to their potent sensitizing effects, acrylates were named the contact allergen of the year in 2012 by the American Contact Dermatitis Society.3
Acrylates are plastic products formed by polymerization of acrylic or methacrylic acid.4 Artificial sculptured nails are created by mixing powdered polymethyl methacrylate polymers and liquid ethyl or isobutyl methacrylate monomers and then applying this mixture to the nail plate.5 Gel and powder nails employ a mixture that is similar to acrylic powders, which require UV or LED radiation to polymerize and harden on the nail plate.
Tosylamide, or tosylamide formaldehyde resin, is another potent allergen that promotes adhesion of the enamel to the nail.6 It is important to note that sensitization may develop months to years after using artificial nails.
Clinical features of contact allergy secondary to nail polish can vary. Some patients experience severe periungual dermatitis. Others can present with facial or eyelid dermatitis due to exposure to airborne particles of acrylates or from contact with fingertips bearing acrylic nails.6,7 If inhaled, acrylates also can cause wheezing asthma or allergic rhinoconjunctivitis.
Common Onychodystrophies
Damage to the natural nail plate is inevitable with continued wear of sculptured nails. With 2 to 4 months of consecutive wear, the natural nails turn yellow, brittle, and weak.5 One study noted that the thickness of an individual’s left thumb nail plate thinned from 0.059 cm to 0.03 cm after a gel manicure was removed from the nail.8 Nail injuries due to manicuring include keratin granulations, onycholysis, pincer nail deformities, pseudopsoriatic nails, lamellar onychoschizia, transverse leukonychia, and ingrown nails.6 One interesting nail dystrophy reported secondary to gel manicures is pterygium inversum unguis or a ventral pterygium that causes an abnormal painful adherence of the hyponychium to the ventral surface of the nail plate. Patients prone to developing pterygium inversum unguis can experience sensitivity, pain, or burning sensations during LED or UVA light exposure.9
Infections
In addition to contact allergies and nail dystrophies, each step of the manicuring process, such as cutting cuticles, presents opportunities for infectious agents to enter the nail fold. Acute or chronic paronychia, or inflammation of the nail fold, most commonly is caused by bacterial infections with Staphylococcus aureus. Green nail syndrome caused by Pseudomonas aeruginosa also is common.1 Onychomycosis due to Trichophyton rubrum is one of the most frequent fungal infections contracted at nail salons. Mycobacteria such as Mycobacterium fortuitum also have been implicated in infections from salons, as they can be found in the jets of pedicure spas, which are not sanitized regularly.10
Final Thoughts
Nail cosmetics are an integral part of many patients’ lives. Being able to educate yourself and your patients on the hazards of nail salons can help them avoid painful infections, contact allergies, and acute to chronic nail deformities. It is important for residents to be aware of the different dermatoses that can arise in men and women who frequent nail salons as the popularity of the nail beauty industry continues to rise.
As residents, it is important to understand the steps of the manicuring process and be able to inform patients on how to maintain optimal nail health while continuing to go to nail salons. Most patients are not aware of the possible allergic, traumatic, and/or infectious complications of manicuring their nails. There are practical steps that can be taken to prevent nail issues, such as avoiding cutting one’s cuticles or using allergen-free nail polishes. These simple fixes can make a big difference in long-term nail health in our patients.
Nail Polish Application Process
The nails are first soaked in a warm soapy solution to soften the nail plate and cuticles.1 Then the nail tips and plates are filed and occasionally are smoothed with a drill. The cuticles are cut with a cuticle cutter. Nail polish—base coat, color enamel, and top coat—is then applied to the nail. Acrylic or sculptured nails and gel and dip manicures are composed of chemical monomers and polymers that harden either at room temperature or through UV or light-emitting diode (LED) exposure. The chemicals in these products can damage nails and cause allergic reactions.
Contact Dermatitis
Approximately 2% of individuals have been found to have allergic or irritant contact dermatitis to nail care products. The top 5 allergens implicated in nail products are (1) 2-hydroxyethyl methacrylate, (2) methyl methacrylate, (3) ethyl acrylate, (4) ethyl-2-cyanoacrylate, and (5) tosylamide.2 Methyl methacrylate was banned in 1974 by the US Food and Drug Administration due to reports of severe contact dermatitis, paronychia, and nail dystrophy.3 Due to their potent sensitizing effects, acrylates were named the contact allergen of the year in 2012 by the American Contact Dermatitis Society.3
Acrylates are plastic products formed by polymerization of acrylic or methacrylic acid.4 Artificial sculptured nails are created by mixing powdered polymethyl methacrylate polymers and liquid ethyl or isobutyl methacrylate monomers and then applying this mixture to the nail plate.5 Gel and powder nails employ a mixture that is similar to acrylic powders, which require UV or LED radiation to polymerize and harden on the nail plate.
Tosylamide, or tosylamide formaldehyde resin, is another potent allergen that promotes adhesion of the enamel to the nail.6 It is important to note that sensitization may develop months to years after using artificial nails.
Clinical features of contact allergy secondary to nail polish can vary. Some patients experience severe periungual dermatitis. Others can present with facial or eyelid dermatitis due to exposure to airborne particles of acrylates or from contact with fingertips bearing acrylic nails.6,7 If inhaled, acrylates also can cause wheezing asthma or allergic rhinoconjunctivitis.
Common Onychodystrophies
Damage to the natural nail plate is inevitable with continued wear of sculptured nails. With 2 to 4 months of consecutive wear, the natural nails turn yellow, brittle, and weak.5 One study noted that the thickness of an individual’s left thumb nail plate thinned from 0.059 cm to 0.03 cm after a gel manicure was removed from the nail.8 Nail injuries due to manicuring include keratin granulations, onycholysis, pincer nail deformities, pseudopsoriatic nails, lamellar onychoschizia, transverse leukonychia, and ingrown nails.6 One interesting nail dystrophy reported secondary to gel manicures is pterygium inversum unguis or a ventral pterygium that causes an abnormal painful adherence of the hyponychium to the ventral surface of the nail plate. Patients prone to developing pterygium inversum unguis can experience sensitivity, pain, or burning sensations during LED or UVA light exposure.9
Infections
In addition to contact allergies and nail dystrophies, each step of the manicuring process, such as cutting cuticles, presents opportunities for infectious agents to enter the nail fold. Acute or chronic paronychia, or inflammation of the nail fold, most commonly is caused by bacterial infections with Staphylococcus aureus. Green nail syndrome caused by Pseudomonas aeruginosa also is common.1 Onychomycosis due to Trichophyton rubrum is one of the most frequent fungal infections contracted at nail salons. Mycobacteria such as Mycobacterium fortuitum also have been implicated in infections from salons, as they can be found in the jets of pedicure spas, which are not sanitized regularly.10
Final Thoughts
Nail cosmetics are an integral part of many patients’ lives. Being able to educate yourself and your patients on the hazards of nail salons can help them avoid painful infections, contact allergies, and acute to chronic nail deformities. It is important for residents to be aware of the different dermatoses that can arise in men and women who frequent nail salons as the popularity of the nail beauty industry continues to rise.
- Reinecke JK, Hinshaw MA. Nail health in women. Int J Womens Dermatol. 2020;6:73-79. doi:10.1016/j.ijwd.2020.01.006
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermatitis. 2020;31:191-201. doi:10.1097/DER.0000000000000583
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society allergens of the year 2000 to 2020 [published online April 25, 2020]. Dermatol Clin. 2020;38:309-320. doi:10.1016/j.det.2020.02.011
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2587-2588.
- Iorizzo M, Piraccini BM, Tosti A. Nail cosmetics in nail disorders.J Cosmet Dermatol. 2007;6:53-58. doi:10.1111/j.1473-2165.2007.00290.x
- Maio P, Carvalho R, Amaro C, et al. Letter: allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J. 2012;18:13.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29. doi:10.1111/j.1473-2165.2011.00595.x
- Cervantes J, Sanchez M, Eber AE, et al. Pterygium inversum unguis secondary to gel polish [published online October 16, 2017]. J Eur Acad Dermatol Venereol. 2018;32:160-163. doi:10.1111/jdv.14603
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Reinecke JK, Hinshaw MA. Nail health in women. Int J Womens Dermatol. 2020;6:73-79. doi:10.1016/j.ijwd.2020.01.006
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermatitis. 2020;31:191-201. doi:10.1097/DER.0000000000000583
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society allergens of the year 2000 to 2020 [published online April 25, 2020]. Dermatol Clin. 2020;38:309-320. doi:10.1016/j.det.2020.02.011
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2587-2588.
- Iorizzo M, Piraccini BM, Tosti A. Nail cosmetics in nail disorders.J Cosmet Dermatol. 2007;6:53-58. doi:10.1111/j.1473-2165.2007.00290.x
- Maio P, Carvalho R, Amaro C, et al. Letter: allergic contact dermatitis from sculptured acrylic nails: special presentation with a possible airborne pattern. Dermatol Online J. 2012;18:13.
- Chen AF, Chimento SM, Hu S, et al. Nail damage from gel polish manicure. J Cosmet Dermatol. 2012;11:27-29. doi:10.1111/j.1473-2165.2011.00595.x
- Cervantes J, Sanchez M, Eber AE, et al. Pterygium inversum unguis secondary to gel polish [published online October 16, 2017]. J Eur Acad Dermatol Venereol. 2018;32:160-163. doi:10.1111/jdv.14603
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
Resident Pearls
- Every step of the nail manicuring process presents opportunities for nail trauma, infections, and contact dermatitis.
- As residents, it is important to be aware of the hazards associated with nail salons and educate our patients accordingly.
- Nail health is essential to optimizing everyday work for our patients—whether it entails taking care of children, typing, or other hands-on activities.
COVID-19 and IPF: Fundamental similarities found
An AI-guided analysis of more than 1,000 human lung transcriptomic datasets found that COVID-19 resembles idiopathic pulmonary fibrosis (IPF) at a fundamental level, according to a study published in eBiomedicine, part of The Lancet Discovery Science.
In the aftermath of COVID-19, a significant number of patients develop a fibrotic lung disease, for which insights into pathogenesis, disease models, or treatment options are lacking, according to researchers Dr. Sinha and colleagues. This long-haul form of the disease culminates in a fibrotic type of interstitial lung disease (ILD). While the actual prevalence of post–COVID-19 ILD (PCLD) is still emerging, early analysis indicates that more than a third of COVID-19 survivors develop fibrotic abnormalities, according to the authors.
Previous research has shown that one of the important determinants for PCLD is the duration of disease. Among patients who developed fibrosis, approximately 4% of patients had a disease duration of less than 1 week; approximately 24% had a disease duration between 1 and 3 weeks; and around 61% had a disease duration longer than 3 weeks, the authors stated.
The lung transcriptomic datasets compared in their study were associated with various lung conditions. The researchers used two viral pandemic signatures (ViP and sViP) and one COVID lung-derived signature. They found that the resemblances included that COVID-19 recapitulates the gene expression patterns (ViP and IPF signatures), cytokine storm (IL15-centric), and the AT2 cytopathic changes, for example, injury, DNA damage, arrest in a transient, damage-induced progenitor state, and senescence-associated secretory phenotype (SASP).
In laboratory experiments, Dr. Sinha and colleagues were able to induce these same immunocytopathic features in preclinical COVID-19 models (human adult lung organoid and hamster) and to reverse them in the hamster model with effective anti–CoV-2 therapeutics.
PPI-network analyses pinpointed endoplasmic reticulum (ER) stress as one of the shared early triggers of both IPF and COVID-19, and immunohistochemistry studies validated the same in the lungs of deceased subjects with COVID-19 and the SARS-CoV-2–challenged hamster lungs. Additionally, lungs from transgenic mice, in which ER stress was induced specifically in the AT2 cells, faithfully recapitulated the host immune response and alveolar cytopathic changes that are induced by SARS-CoV-2.
stated corresponding author Pradipta Ghosh, MD, professor in the departments of medicine and cellular and molecular medicine, University of California, San Diego. “If proven in prospective studies, this biomarker could indicate who is at greatest risk for progressive fibrosis and may require lung transplantation,” she said in an interview.
Dr. Ghosh stated further, “When it comes to therapeutics in COVID lung or IPF, we also found that shared fundamental pathogenic mechanisms present excellent opportunities for developing therapeutics that can arrest the fibrogenic drivers in both diseases. One clue that emerged is a specific cytokine that is at the heart of the smoldering inflammation which is invariably associated with fibrosis. That is interleukin 15 [IL-15] and its receptor.” Dr. Ghosh observed that there are two Food and Drug Administration–approved drugs for IPF. “None are very effective in arresting this invariably fatal disease. Hence, finding better options to treat IPF is an urgent and an unmet need.”
Preclinical testing of hypotheses, Dr. Ghosh said, is next on the path to clinical trials. “We have the advantage of using human lung organoids (mini-lungs grown using stem cells) in a dish, adding additional cells to the system (like fibroblasts and immune cells), infecting them with the virus, or subjecting them to the IL-15 cytokine and monitoring lung fibrosis progression in a dish. Anti–IL-15 therapy can then be initiated to observe reversal of the fibrogenic cascade.” Hamsters have also been shown to provide appropriate models for mimicking lung fibrosis, Dr. Ghosh said.
“The report by Sinha and colleagues describes the fascinating similarities between drivers of post-COVID lung disease and idiopathic pulmonary fibrosis,” stated David Bowton, MD, professor emeritus, section on critical care, department of anesthesiology, Wake Forest University, Winston-Salem, N.C., in an interview. He added that, “Central to the mechanisms of induction of fibrosis in both disorders appears to be endoplasmic reticulum stress in alveolar type II cells (AT2). ER stress induces the unfolded protein response (UPR) that halts protein translation and promotes the degradation of misfolded proteins. Prolonged UPR can reprogram the cell or trigger apoptosis pathways. ER stress in the lung has been reported in a variety of cell lines including AT2 in IPF, bronchial and alveolar epithelial cells in asthma and [chronic obstructive pulmonary disease], and endothelial cells in pulmonary hypertension.”
Dr. Bowton commented further, including a caution, “Sinha and colleagues suggest that the identification of these gene signatures and mechanisms will be a fruitful avenue for developing effective therapeutics for IPF and other fibrotic lung diseases. I am hopeful that these data may offer clues that expedite this process. However, the redundancy of triggers for effector pathways in biologic systems argues that, even if successful, this will be [a] long and fraught process.”
The research study was supported by National Institutes of Health grants and funding from the Tobacco-Related Disease Research Program.
Dr. Sinha, Dr. Ghosh, and Dr. Bowton reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
An AI-guided analysis of more than 1,000 human lung transcriptomic datasets found that COVID-19 resembles idiopathic pulmonary fibrosis (IPF) at a fundamental level, according to a study published in eBiomedicine, part of The Lancet Discovery Science.
In the aftermath of COVID-19, a significant number of patients develop a fibrotic lung disease, for which insights into pathogenesis, disease models, or treatment options are lacking, according to researchers Dr. Sinha and colleagues. This long-haul form of the disease culminates in a fibrotic type of interstitial lung disease (ILD). While the actual prevalence of post–COVID-19 ILD (PCLD) is still emerging, early analysis indicates that more than a third of COVID-19 survivors develop fibrotic abnormalities, according to the authors.
Previous research has shown that one of the important determinants for PCLD is the duration of disease. Among patients who developed fibrosis, approximately 4% of patients had a disease duration of less than 1 week; approximately 24% had a disease duration between 1 and 3 weeks; and around 61% had a disease duration longer than 3 weeks, the authors stated.
The lung transcriptomic datasets compared in their study were associated with various lung conditions. The researchers used two viral pandemic signatures (ViP and sViP) and one COVID lung-derived signature. They found that the resemblances included that COVID-19 recapitulates the gene expression patterns (ViP and IPF signatures), cytokine storm (IL15-centric), and the AT2 cytopathic changes, for example, injury, DNA damage, arrest in a transient, damage-induced progenitor state, and senescence-associated secretory phenotype (SASP).
In laboratory experiments, Dr. Sinha and colleagues were able to induce these same immunocytopathic features in preclinical COVID-19 models (human adult lung organoid and hamster) and to reverse them in the hamster model with effective anti–CoV-2 therapeutics.
PPI-network analyses pinpointed endoplasmic reticulum (ER) stress as one of the shared early triggers of both IPF and COVID-19, and immunohistochemistry studies validated the same in the lungs of deceased subjects with COVID-19 and the SARS-CoV-2–challenged hamster lungs. Additionally, lungs from transgenic mice, in which ER stress was induced specifically in the AT2 cells, faithfully recapitulated the host immune response and alveolar cytopathic changes that are induced by SARS-CoV-2.
stated corresponding author Pradipta Ghosh, MD, professor in the departments of medicine and cellular and molecular medicine, University of California, San Diego. “If proven in prospective studies, this biomarker could indicate who is at greatest risk for progressive fibrosis and may require lung transplantation,” she said in an interview.
Dr. Ghosh stated further, “When it comes to therapeutics in COVID lung or IPF, we also found that shared fundamental pathogenic mechanisms present excellent opportunities for developing therapeutics that can arrest the fibrogenic drivers in both diseases. One clue that emerged is a specific cytokine that is at the heart of the smoldering inflammation which is invariably associated with fibrosis. That is interleukin 15 [IL-15] and its receptor.” Dr. Ghosh observed that there are two Food and Drug Administration–approved drugs for IPF. “None are very effective in arresting this invariably fatal disease. Hence, finding better options to treat IPF is an urgent and an unmet need.”
Preclinical testing of hypotheses, Dr. Ghosh said, is next on the path to clinical trials. “We have the advantage of using human lung organoids (mini-lungs grown using stem cells) in a dish, adding additional cells to the system (like fibroblasts and immune cells), infecting them with the virus, or subjecting them to the IL-15 cytokine and monitoring lung fibrosis progression in a dish. Anti–IL-15 therapy can then be initiated to observe reversal of the fibrogenic cascade.” Hamsters have also been shown to provide appropriate models for mimicking lung fibrosis, Dr. Ghosh said.
“The report by Sinha and colleagues describes the fascinating similarities between drivers of post-COVID lung disease and idiopathic pulmonary fibrosis,” stated David Bowton, MD, professor emeritus, section on critical care, department of anesthesiology, Wake Forest University, Winston-Salem, N.C., in an interview. He added that, “Central to the mechanisms of induction of fibrosis in both disorders appears to be endoplasmic reticulum stress in alveolar type II cells (AT2). ER stress induces the unfolded protein response (UPR) that halts protein translation and promotes the degradation of misfolded proteins. Prolonged UPR can reprogram the cell or trigger apoptosis pathways. ER stress in the lung has been reported in a variety of cell lines including AT2 in IPF, bronchial and alveolar epithelial cells in asthma and [chronic obstructive pulmonary disease], and endothelial cells in pulmonary hypertension.”
Dr. Bowton commented further, including a caution, “Sinha and colleagues suggest that the identification of these gene signatures and mechanisms will be a fruitful avenue for developing effective therapeutics for IPF and other fibrotic lung diseases. I am hopeful that these data may offer clues that expedite this process. However, the redundancy of triggers for effector pathways in biologic systems argues that, even if successful, this will be [a] long and fraught process.”
The research study was supported by National Institutes of Health grants and funding from the Tobacco-Related Disease Research Program.
Dr. Sinha, Dr. Ghosh, and Dr. Bowton reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
An AI-guided analysis of more than 1,000 human lung transcriptomic datasets found that COVID-19 resembles idiopathic pulmonary fibrosis (IPF) at a fundamental level, according to a study published in eBiomedicine, part of The Lancet Discovery Science.
In the aftermath of COVID-19, a significant number of patients develop a fibrotic lung disease, for which insights into pathogenesis, disease models, or treatment options are lacking, according to researchers Dr. Sinha and colleagues. This long-haul form of the disease culminates in a fibrotic type of interstitial lung disease (ILD). While the actual prevalence of post–COVID-19 ILD (PCLD) is still emerging, early analysis indicates that more than a third of COVID-19 survivors develop fibrotic abnormalities, according to the authors.
Previous research has shown that one of the important determinants for PCLD is the duration of disease. Among patients who developed fibrosis, approximately 4% of patients had a disease duration of less than 1 week; approximately 24% had a disease duration between 1 and 3 weeks; and around 61% had a disease duration longer than 3 weeks, the authors stated.
The lung transcriptomic datasets compared in their study were associated with various lung conditions. The researchers used two viral pandemic signatures (ViP and sViP) and one COVID lung-derived signature. They found that the resemblances included that COVID-19 recapitulates the gene expression patterns (ViP and IPF signatures), cytokine storm (IL15-centric), and the AT2 cytopathic changes, for example, injury, DNA damage, arrest in a transient, damage-induced progenitor state, and senescence-associated secretory phenotype (SASP).
In laboratory experiments, Dr. Sinha and colleagues were able to induce these same immunocytopathic features in preclinical COVID-19 models (human adult lung organoid and hamster) and to reverse them in the hamster model with effective anti–CoV-2 therapeutics.
PPI-network analyses pinpointed endoplasmic reticulum (ER) stress as one of the shared early triggers of both IPF and COVID-19, and immunohistochemistry studies validated the same in the lungs of deceased subjects with COVID-19 and the SARS-CoV-2–challenged hamster lungs. Additionally, lungs from transgenic mice, in which ER stress was induced specifically in the AT2 cells, faithfully recapitulated the host immune response and alveolar cytopathic changes that are induced by SARS-CoV-2.
stated corresponding author Pradipta Ghosh, MD, professor in the departments of medicine and cellular and molecular medicine, University of California, San Diego. “If proven in prospective studies, this biomarker could indicate who is at greatest risk for progressive fibrosis and may require lung transplantation,” she said in an interview.
Dr. Ghosh stated further, “When it comes to therapeutics in COVID lung or IPF, we also found that shared fundamental pathogenic mechanisms present excellent opportunities for developing therapeutics that can arrest the fibrogenic drivers in both diseases. One clue that emerged is a specific cytokine that is at the heart of the smoldering inflammation which is invariably associated with fibrosis. That is interleukin 15 [IL-15] and its receptor.” Dr. Ghosh observed that there are two Food and Drug Administration–approved drugs for IPF. “None are very effective in arresting this invariably fatal disease. Hence, finding better options to treat IPF is an urgent and an unmet need.”
Preclinical testing of hypotheses, Dr. Ghosh said, is next on the path to clinical trials. “We have the advantage of using human lung organoids (mini-lungs grown using stem cells) in a dish, adding additional cells to the system (like fibroblasts and immune cells), infecting them with the virus, or subjecting them to the IL-15 cytokine and monitoring lung fibrosis progression in a dish. Anti–IL-15 therapy can then be initiated to observe reversal of the fibrogenic cascade.” Hamsters have also been shown to provide appropriate models for mimicking lung fibrosis, Dr. Ghosh said.
“The report by Sinha and colleagues describes the fascinating similarities between drivers of post-COVID lung disease and idiopathic pulmonary fibrosis,” stated David Bowton, MD, professor emeritus, section on critical care, department of anesthesiology, Wake Forest University, Winston-Salem, N.C., in an interview. He added that, “Central to the mechanisms of induction of fibrosis in both disorders appears to be endoplasmic reticulum stress in alveolar type II cells (AT2). ER stress induces the unfolded protein response (UPR) that halts protein translation and promotes the degradation of misfolded proteins. Prolonged UPR can reprogram the cell or trigger apoptosis pathways. ER stress in the lung has been reported in a variety of cell lines including AT2 in IPF, bronchial and alveolar epithelial cells in asthma and [chronic obstructive pulmonary disease], and endothelial cells in pulmonary hypertension.”
Dr. Bowton commented further, including a caution, “Sinha and colleagues suggest that the identification of these gene signatures and mechanisms will be a fruitful avenue for developing effective therapeutics for IPF and other fibrotic lung diseases. I am hopeful that these data may offer clues that expedite this process. However, the redundancy of triggers for effector pathways in biologic systems argues that, even if successful, this will be [a] long and fraught process.”
The research study was supported by National Institutes of Health grants and funding from the Tobacco-Related Disease Research Program.
Dr. Sinha, Dr. Ghosh, and Dr. Bowton reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM eBIOMEDICINE
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
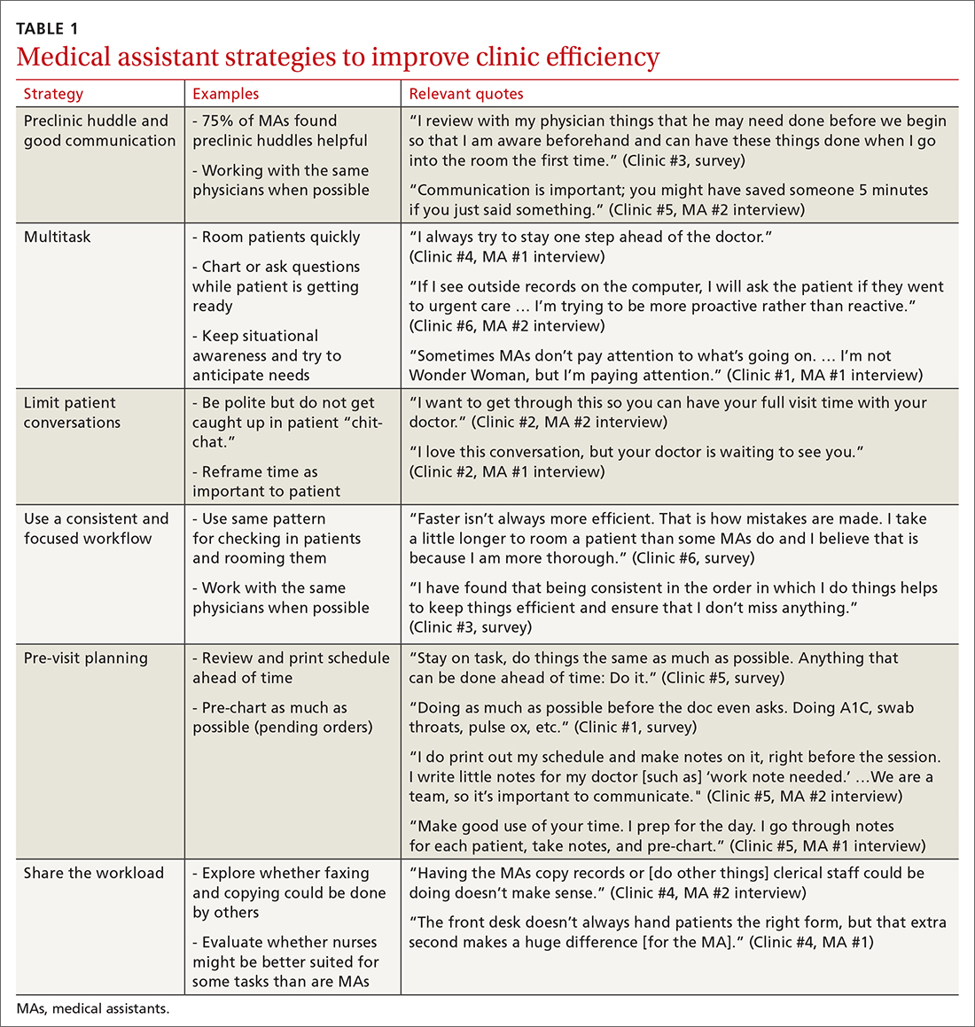
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
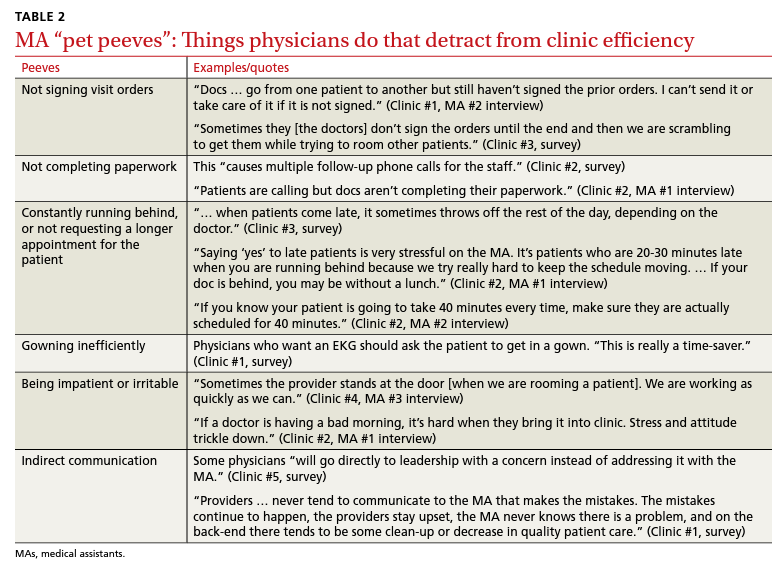
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; ktgold@umich.edu
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; ktgold@umich.edu
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; ktgold@umich.edu
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Haven’t had COVID yet? Wanna bet?
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
Long COVID comes in three forms: Study
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
Death risk doubles for Black infants with bronchopulmonary dysplasia
Infants with bronchopulmonary dysplasia (BPD) who were born to Black mothers were significantly more likely to die or to have a longer hospital stay than infants of other ethnicities, based on data from more than 800 infants.
The overall incidence of BPD is rising, in part because of improved survival for extremely preterm infants, wrote Tamorah R. Lewis, MD, of the University of Missouri, Kansas City, and colleagues.
Previous studies suggest that racial disparities may affect outcomes for preterm infants with a range of neonatal morbidities during neonatal ICU (NICU) hospitalization, including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis. However, the association of racial disparities with outcomes for preterm infants with BPD remains unclear, they said.
In a study published in JAMA Pediatrics, the researchers, on behalf of the Bronchopulmonary Dysplasia Collaborative, reviewed data from 834 preterm infants enrolled in the BPD Collaborative registry from Jan. 1, 2015, to July 19, 2021, at eight centers in the United States.
The study infants were born at less than 32 weeks’ gestation and were diagnosed with severe BPD according to the 2001 National Institutes of Health Consensus Criteria. The study population included 276 Black infants and 558 white infants. The median gestational age was 24 weeks, and 41% of the infants were female.
The primary outcomes were infant death and length of hospital stay.
Although death was infrequent (4% overall), Black maternal race was significantly associated with an increased risk of death from BPD (adjusted odds ratio, 2.1). Black maternal race also was significantly associated with a longer hospital stay for the infants, with an adjusted between-group difference of 10 days.
Infants of Black mothers also were more likely than those with White mothers to receive invasive respiratory support at the time of delivery. Black infants were more likely than White infants to have lower gestational age, lower birth weight and length, and smaller head circumference.
However, the proportions of cesarean deliveries, gender distribution, and infants small for gestational age were similar between Black and White infant groups. Medication exposure at 36 weeks postmenstrual age (PMA) also was similar for Black and White infants, and 50% of patients overall were treated with nasal continuous positive airway pressure at 36 weeks’ PMA. Awareness of the increased risk of death and longer hospital stay for Black infants is critical, “given the highly variable outcomes for patients with BPD and the uncertainty regarding demographic factors that contribute to late respiratory morbidity in severe BPD,” the researchers wrote.
The study findings were limited by several factors including variations among study centers in the identification and recording of maternal race, lack of data on paternal race, and the focus specifically on Black maternal race and not other ethnicities. Given the documented health disparities for Black individuals in the United States, “we restricted our cohort to only those patients born to Black or White mothers to estimate the association of Black maternal race and adverse in-hospital outcomes in infants with severe BPD,” the researchers wrote
Other limitations include the lack of data surrounding infant death and inability to adjust for all potential modifiers of BPD pathogenesis and progression, such as BPD comorbidities.
Prospective studies are needed to identify the sociodemographic mechanisms that may contribute to health outcome disparities for Black infants with severe BPD, the researchers emphasized.
In the meantime, the results highlight the need for more attention to variations in care for infants with BPD of different races, and approaches to family-centered care should consider “the precise needs of high-risk, structurally disadvantaged families while informing the design of prospective trials that improve outcomes for high-risk subgroups of children with severe BPD,” they concluded.
Data raise questions about the origin of disparities
The current study findings contribute to the knowledge and awareness of disparities in the high-risk NICU population, Nicolas A. Bamat, MD, and colleagues wrote in an accompanying editorial. “Further, their findings oppose the central tendency in the literature: that infants of Black mothers have less severe lung disease of prematurity during the birth hospitalization.”
The editorial authors noted that the study’s inclusion of racial characteristics as confounding variables to assess the effect of race on health “can imply questionable assumptions about where in a causal pathway racism begins to exert an effect,” whether after a diagnosis of BPD, during pregnancy in response to inequitable obstetric care, or “centuries ago, propagating forward through the shared experience of communities oppressed by the legacy of racism and its ongoing contemporary manifestations.”
The editorial authors added that, “in lung disease of prematurity, few variables are reliable antecedents to race as an exposure. Complex adjustment is necessary to reduce bias in targeted research questions.” However, the current study findings highlight the need to move toward more equitable neonatal care, and to prioritize interventions to reduce racial health disparities at the level of the NICU as well as at the hospital and government policy levels.
Consider range of contributing factors and confounders
The current study is important because “it is imperative to measure racial outcomes in health care in order to highlight and address disparities and biases,” Tim Joos, MD, said in an interview. However, “it can be difficult to determine how much race is a factor in itself versus a proxy for other important characteristics, such as socioeconomic status and level of education, that can confound the results.”
In the current study, the twofold-increased death rate in the premature infants of Black mothers is concerning and deserves further attention, Dr. Joos said. “The 10-day longer length of stay for infants of Black mothers seems quite shocking at first glance, but because of the long hospital stays for these extremely premature infants in general, it is about 7% longer than the infants born to White mothers.”
The take-home message is that this difference is still significant, and can reflect many factors including disease severity and complications, need for feeding assistance, teaching, and setting up home supports, said Dr. Joos.
As for additional research, “it would be useful for hospitals to break down why the differences exist, although I worry a provider or institution will feel they need to discharge Black families sooner to avoid being biased. Family preference and comfort level should be given high priority,” he emphasized.
The study received no outside funding, but lead author Dr. Lewis was supported by the National Institute on Child Health and Development and the Robert Wood Johnson Foundation. Several coauthors were supported by other grants from the National Institutes of Health. Dr. Barnat and one coauthor were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Joos had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Infants with bronchopulmonary dysplasia (BPD) who were born to Black mothers were significantly more likely to die or to have a longer hospital stay than infants of other ethnicities, based on data from more than 800 infants.
The overall incidence of BPD is rising, in part because of improved survival for extremely preterm infants, wrote Tamorah R. Lewis, MD, of the University of Missouri, Kansas City, and colleagues.
Previous studies suggest that racial disparities may affect outcomes for preterm infants with a range of neonatal morbidities during neonatal ICU (NICU) hospitalization, including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis. However, the association of racial disparities with outcomes for preterm infants with BPD remains unclear, they said.
In a study published in JAMA Pediatrics, the researchers, on behalf of the Bronchopulmonary Dysplasia Collaborative, reviewed data from 834 preterm infants enrolled in the BPD Collaborative registry from Jan. 1, 2015, to July 19, 2021, at eight centers in the United States.
The study infants were born at less than 32 weeks’ gestation and were diagnosed with severe BPD according to the 2001 National Institutes of Health Consensus Criteria. The study population included 276 Black infants and 558 white infants. The median gestational age was 24 weeks, and 41% of the infants were female.
The primary outcomes were infant death and length of hospital stay.
Although death was infrequent (4% overall), Black maternal race was significantly associated with an increased risk of death from BPD (adjusted odds ratio, 2.1). Black maternal race also was significantly associated with a longer hospital stay for the infants, with an adjusted between-group difference of 10 days.
Infants of Black mothers also were more likely than those with White mothers to receive invasive respiratory support at the time of delivery. Black infants were more likely than White infants to have lower gestational age, lower birth weight and length, and smaller head circumference.
However, the proportions of cesarean deliveries, gender distribution, and infants small for gestational age were similar between Black and White infant groups. Medication exposure at 36 weeks postmenstrual age (PMA) also was similar for Black and White infants, and 50% of patients overall were treated with nasal continuous positive airway pressure at 36 weeks’ PMA. Awareness of the increased risk of death and longer hospital stay for Black infants is critical, “given the highly variable outcomes for patients with BPD and the uncertainty regarding demographic factors that contribute to late respiratory morbidity in severe BPD,” the researchers wrote.
The study findings were limited by several factors including variations among study centers in the identification and recording of maternal race, lack of data on paternal race, and the focus specifically on Black maternal race and not other ethnicities. Given the documented health disparities for Black individuals in the United States, “we restricted our cohort to only those patients born to Black or White mothers to estimate the association of Black maternal race and adverse in-hospital outcomes in infants with severe BPD,” the researchers wrote
Other limitations include the lack of data surrounding infant death and inability to adjust for all potential modifiers of BPD pathogenesis and progression, such as BPD comorbidities.
Prospective studies are needed to identify the sociodemographic mechanisms that may contribute to health outcome disparities for Black infants with severe BPD, the researchers emphasized.
In the meantime, the results highlight the need for more attention to variations in care for infants with BPD of different races, and approaches to family-centered care should consider “the precise needs of high-risk, structurally disadvantaged families while informing the design of prospective trials that improve outcomes for high-risk subgroups of children with severe BPD,” they concluded.
Data raise questions about the origin of disparities
The current study findings contribute to the knowledge and awareness of disparities in the high-risk NICU population, Nicolas A. Bamat, MD, and colleagues wrote in an accompanying editorial. “Further, their findings oppose the central tendency in the literature: that infants of Black mothers have less severe lung disease of prematurity during the birth hospitalization.”
The editorial authors noted that the study’s inclusion of racial characteristics as confounding variables to assess the effect of race on health “can imply questionable assumptions about where in a causal pathway racism begins to exert an effect,” whether after a diagnosis of BPD, during pregnancy in response to inequitable obstetric care, or “centuries ago, propagating forward through the shared experience of communities oppressed by the legacy of racism and its ongoing contemporary manifestations.”
The editorial authors added that, “in lung disease of prematurity, few variables are reliable antecedents to race as an exposure. Complex adjustment is necessary to reduce bias in targeted research questions.” However, the current study findings highlight the need to move toward more equitable neonatal care, and to prioritize interventions to reduce racial health disparities at the level of the NICU as well as at the hospital and government policy levels.
Consider range of contributing factors and confounders
The current study is important because “it is imperative to measure racial outcomes in health care in order to highlight and address disparities and biases,” Tim Joos, MD, said in an interview. However, “it can be difficult to determine how much race is a factor in itself versus a proxy for other important characteristics, such as socioeconomic status and level of education, that can confound the results.”
In the current study, the twofold-increased death rate in the premature infants of Black mothers is concerning and deserves further attention, Dr. Joos said. “The 10-day longer length of stay for infants of Black mothers seems quite shocking at first glance, but because of the long hospital stays for these extremely premature infants in general, it is about 7% longer than the infants born to White mothers.”
The take-home message is that this difference is still significant, and can reflect many factors including disease severity and complications, need for feeding assistance, teaching, and setting up home supports, said Dr. Joos.
As for additional research, “it would be useful for hospitals to break down why the differences exist, although I worry a provider or institution will feel they need to discharge Black families sooner to avoid being biased. Family preference and comfort level should be given high priority,” he emphasized.
The study received no outside funding, but lead author Dr. Lewis was supported by the National Institute on Child Health and Development and the Robert Wood Johnson Foundation. Several coauthors were supported by other grants from the National Institutes of Health. Dr. Barnat and one coauthor were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Joos had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Infants with bronchopulmonary dysplasia (BPD) who were born to Black mothers were significantly more likely to die or to have a longer hospital stay than infants of other ethnicities, based on data from more than 800 infants.
The overall incidence of BPD is rising, in part because of improved survival for extremely preterm infants, wrote Tamorah R. Lewis, MD, of the University of Missouri, Kansas City, and colleagues.
Previous studies suggest that racial disparities may affect outcomes for preterm infants with a range of neonatal morbidities during neonatal ICU (NICU) hospitalization, including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis. However, the association of racial disparities with outcomes for preterm infants with BPD remains unclear, they said.
In a study published in JAMA Pediatrics, the researchers, on behalf of the Bronchopulmonary Dysplasia Collaborative, reviewed data from 834 preterm infants enrolled in the BPD Collaborative registry from Jan. 1, 2015, to July 19, 2021, at eight centers in the United States.
The study infants were born at less than 32 weeks’ gestation and were diagnosed with severe BPD according to the 2001 National Institutes of Health Consensus Criteria. The study population included 276 Black infants and 558 white infants. The median gestational age was 24 weeks, and 41% of the infants were female.
The primary outcomes were infant death and length of hospital stay.
Although death was infrequent (4% overall), Black maternal race was significantly associated with an increased risk of death from BPD (adjusted odds ratio, 2.1). Black maternal race also was significantly associated with a longer hospital stay for the infants, with an adjusted between-group difference of 10 days.
Infants of Black mothers also were more likely than those with White mothers to receive invasive respiratory support at the time of delivery. Black infants were more likely than White infants to have lower gestational age, lower birth weight and length, and smaller head circumference.
However, the proportions of cesarean deliveries, gender distribution, and infants small for gestational age were similar between Black and White infant groups. Medication exposure at 36 weeks postmenstrual age (PMA) also was similar for Black and White infants, and 50% of patients overall were treated with nasal continuous positive airway pressure at 36 weeks’ PMA. Awareness of the increased risk of death and longer hospital stay for Black infants is critical, “given the highly variable outcomes for patients with BPD and the uncertainty regarding demographic factors that contribute to late respiratory morbidity in severe BPD,” the researchers wrote.
The study findings were limited by several factors including variations among study centers in the identification and recording of maternal race, lack of data on paternal race, and the focus specifically on Black maternal race and not other ethnicities. Given the documented health disparities for Black individuals in the United States, “we restricted our cohort to only those patients born to Black or White mothers to estimate the association of Black maternal race and adverse in-hospital outcomes in infants with severe BPD,” the researchers wrote
Other limitations include the lack of data surrounding infant death and inability to adjust for all potential modifiers of BPD pathogenesis and progression, such as BPD comorbidities.
Prospective studies are needed to identify the sociodemographic mechanisms that may contribute to health outcome disparities for Black infants with severe BPD, the researchers emphasized.
In the meantime, the results highlight the need for more attention to variations in care for infants with BPD of different races, and approaches to family-centered care should consider “the precise needs of high-risk, structurally disadvantaged families while informing the design of prospective trials that improve outcomes for high-risk subgroups of children with severe BPD,” they concluded.
Data raise questions about the origin of disparities
The current study findings contribute to the knowledge and awareness of disparities in the high-risk NICU population, Nicolas A. Bamat, MD, and colleagues wrote in an accompanying editorial. “Further, their findings oppose the central tendency in the literature: that infants of Black mothers have less severe lung disease of prematurity during the birth hospitalization.”
The editorial authors noted that the study’s inclusion of racial characteristics as confounding variables to assess the effect of race on health “can imply questionable assumptions about where in a causal pathway racism begins to exert an effect,” whether after a diagnosis of BPD, during pregnancy in response to inequitable obstetric care, or “centuries ago, propagating forward through the shared experience of communities oppressed by the legacy of racism and its ongoing contemporary manifestations.”
The editorial authors added that, “in lung disease of prematurity, few variables are reliable antecedents to race as an exposure. Complex adjustment is necessary to reduce bias in targeted research questions.” However, the current study findings highlight the need to move toward more equitable neonatal care, and to prioritize interventions to reduce racial health disparities at the level of the NICU as well as at the hospital and government policy levels.
Consider range of contributing factors and confounders
The current study is important because “it is imperative to measure racial outcomes in health care in order to highlight and address disparities and biases,” Tim Joos, MD, said in an interview. However, “it can be difficult to determine how much race is a factor in itself versus a proxy for other important characteristics, such as socioeconomic status and level of education, that can confound the results.”
In the current study, the twofold-increased death rate in the premature infants of Black mothers is concerning and deserves further attention, Dr. Joos said. “The 10-day longer length of stay for infants of Black mothers seems quite shocking at first glance, but because of the long hospital stays for these extremely premature infants in general, it is about 7% longer than the infants born to White mothers.”
The take-home message is that this difference is still significant, and can reflect many factors including disease severity and complications, need for feeding assistance, teaching, and setting up home supports, said Dr. Joos.
As for additional research, “it would be useful for hospitals to break down why the differences exist, although I worry a provider or institution will feel they need to discharge Black families sooner to avoid being biased. Family preference and comfort level should be given high priority,” he emphasized.
The study received no outside funding, but lead author Dr. Lewis was supported by the National Institute on Child Health and Development and the Robert Wood Johnson Foundation. Several coauthors were supported by other grants from the National Institutes of Health. Dr. Barnat and one coauthor were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Joos had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Resection of five-centimeter cesarean scar ectopic pregnancy and isthmocele repair using vascular clamps
Fraud
News reports this week indicate that the U.S. Department of Justice is investigating Cassava Sciences over the investigational Alzheimer’s disease agent simufilam. An article in Science alleged that the company’s research included altered or duplicated brain images.
Cassava, not surprisingly, denies this. And I’m not going to take sides. Maybe they’ll be exonerated, maybe not.
But the bigger point here is the importance of checking such things. Alzheimer’s disease, beyond being a horrible neurological disease, is also big money. REALLY big money. If a company were to develop a truly effective treatment for it, they’d be poised to reap a worldwide financial windfall.
I’m not criticizing that, either. If such a drug were to be developed, with all of the time and money that goes into such things, they’d have earned every penny.
But the financial incentives certainly do increase the risk of less-than-ethical behavior. This isn’t just in Alzheimer’s disease, but across the board in medicine. The main plot line of the 1993 Harrison Ford flick “The Fugitive” was based on a drug company using falsified data, bribes, and other criminal activities (like murder) to bring a potentially dangerous (but high-profit) drug to market.
Less-than-ethical behavior is not new in research either. In 1926 Paul Kammerer’s attempt to prove Lamarckian evolution was shown to be a fraud. Cover-ups of potentially dangerous drugs have also occurred, or been alleged, and resulted in some being withdrawn from the market.
I’m not sure this is any worse than the multitude of over-the-counter products I see in the store saying they promote brain health, joint health, immune health, whatever ... then, in tiny letters, adding “these statements have not been authorized by the FDA. This drug is not intended to cure, prevent, or treat any disease.” This is no different than guys selling snake oil and other worthless elixirs out of a horse-drawn wagon. Why they aren’t regulated in the same way Pfizer or Lilly are is beyond me.
Even beyond the old method of making up figures, data can still be iffy. We use the phrase “numbers don’t lie” – and generally they don’t – but the ability to “spin” them to suit any narrative has become an art form. If you can’t change the data, make them fit into a better scenario. Somehow.
Which brings me back to why it’s critically important that such studies be open to review by people who don’t have a conflict of interest in the success or failure of the drugs. And there are many: from shareholders, from executives, even from the knowledge that a bad outcome may mean they’re out of a job.
Fraud is nothing new in medicine. I also don’t see it going away anytime in the future. It’s not the nature of medicine, but it is the nature of some people. And a few of them increase the need for legitimacy in everyone else.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
News reports this week indicate that the U.S. Department of Justice is investigating Cassava Sciences over the investigational Alzheimer’s disease agent simufilam. An article in Science alleged that the company’s research included altered or duplicated brain images.
Cassava, not surprisingly, denies this. And I’m not going to take sides. Maybe they’ll be exonerated, maybe not.
But the bigger point here is the importance of checking such things. Alzheimer’s disease, beyond being a horrible neurological disease, is also big money. REALLY big money. If a company were to develop a truly effective treatment for it, they’d be poised to reap a worldwide financial windfall.
I’m not criticizing that, either. If such a drug were to be developed, with all of the time and money that goes into such things, they’d have earned every penny.
But the financial incentives certainly do increase the risk of less-than-ethical behavior. This isn’t just in Alzheimer’s disease, but across the board in medicine. The main plot line of the 1993 Harrison Ford flick “The Fugitive” was based on a drug company using falsified data, bribes, and other criminal activities (like murder) to bring a potentially dangerous (but high-profit) drug to market.
Less-than-ethical behavior is not new in research either. In 1926 Paul Kammerer’s attempt to prove Lamarckian evolution was shown to be a fraud. Cover-ups of potentially dangerous drugs have also occurred, or been alleged, and resulted in some being withdrawn from the market.
I’m not sure this is any worse than the multitude of over-the-counter products I see in the store saying they promote brain health, joint health, immune health, whatever ... then, in tiny letters, adding “these statements have not been authorized by the FDA. This drug is not intended to cure, prevent, or treat any disease.” This is no different than guys selling snake oil and other worthless elixirs out of a horse-drawn wagon. Why they aren’t regulated in the same way Pfizer or Lilly are is beyond me.
Even beyond the old method of making up figures, data can still be iffy. We use the phrase “numbers don’t lie” – and generally they don’t – but the ability to “spin” them to suit any narrative has become an art form. If you can’t change the data, make them fit into a better scenario. Somehow.
Which brings me back to why it’s critically important that such studies be open to review by people who don’t have a conflict of interest in the success or failure of the drugs. And there are many: from shareholders, from executives, even from the knowledge that a bad outcome may mean they’re out of a job.
Fraud is nothing new in medicine. I also don’t see it going away anytime in the future. It’s not the nature of medicine, but it is the nature of some people. And a few of them increase the need for legitimacy in everyone else.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
News reports this week indicate that the U.S. Department of Justice is investigating Cassava Sciences over the investigational Alzheimer’s disease agent simufilam. An article in Science alleged that the company’s research included altered or duplicated brain images.
Cassava, not surprisingly, denies this. And I’m not going to take sides. Maybe they’ll be exonerated, maybe not.
But the bigger point here is the importance of checking such things. Alzheimer’s disease, beyond being a horrible neurological disease, is also big money. REALLY big money. If a company were to develop a truly effective treatment for it, they’d be poised to reap a worldwide financial windfall.
I’m not criticizing that, either. If such a drug were to be developed, with all of the time and money that goes into such things, they’d have earned every penny.
But the financial incentives certainly do increase the risk of less-than-ethical behavior. This isn’t just in Alzheimer’s disease, but across the board in medicine. The main plot line of the 1993 Harrison Ford flick “The Fugitive” was based on a drug company using falsified data, bribes, and other criminal activities (like murder) to bring a potentially dangerous (but high-profit) drug to market.
Less-than-ethical behavior is not new in research either. In 1926 Paul Kammerer’s attempt to prove Lamarckian evolution was shown to be a fraud. Cover-ups of potentially dangerous drugs have also occurred, or been alleged, and resulted in some being withdrawn from the market.
I’m not sure this is any worse than the multitude of over-the-counter products I see in the store saying they promote brain health, joint health, immune health, whatever ... then, in tiny letters, adding “these statements have not been authorized by the FDA. This drug is not intended to cure, prevent, or treat any disease.” This is no different than guys selling snake oil and other worthless elixirs out of a horse-drawn wagon. Why they aren’t regulated in the same way Pfizer or Lilly are is beyond me.
Even beyond the old method of making up figures, data can still be iffy. We use the phrase “numbers don’t lie” – and generally they don’t – but the ability to “spin” them to suit any narrative has become an art form. If you can’t change the data, make them fit into a better scenario. Somehow.
Which brings me back to why it’s critically important that such studies be open to review by people who don’t have a conflict of interest in the success or failure of the drugs. And there are many: from shareholders, from executives, even from the knowledge that a bad outcome may mean they’re out of a job.
Fraud is nothing new in medicine. I also don’t see it going away anytime in the future. It’s not the nature of medicine, but it is the nature of some people. And a few of them increase the need for legitimacy in everyone else.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
More evidence that ultraprocessed foods are detrimental for the brain
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included participants aged 35 and older, showed that higher intake of UPFs was significantly associated with a faster rate of decline in both executive and global cognitive function.
“Based on these findings, doctors might counsel patients to prefer cooking at home [and] choosing fresher ingredients instead of buying ready-made meals and snacks,” said coinvestigator Natalia Gonçalves, PhD, University of São Paulo, Brazil.
Presented at the Alzheimer’s Association International Conference, the findings align with those from a recent study in Neurology. That study linked a diet high in UPFs to an increased risk for dementia.
Increasing worldwide consumption
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs include soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, fries, and many more.
Over the past 30 years, there has been a steady increase in consumption of UPFs worldwide. They are thought to induce systemic inflammation and oxidative stress and have been linked to a variety of ailments, such as overweight/obesity, cardiovascular disease, and cancer.
UPFs may also be a risk factor for cognitive decline, although data are scarce as to their effects on the brain.
To investigate, Dr. Gonçalves and colleagues evaluated longitudinal data on 10,775 adults (mean age, 50.6 years; 56% women; 55% White) who participated in the ELSA-Brasil study. They were evaluated in three waves (2008-2010, 2012-2014, and 2017-2019).
Information on diet was obtained via food frequency questionnaires and included information regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
Significant decline
Using linear mixed effects models that were adjusted for sociodemographic, lifestyle, and clinical variables, the investigators assessed the association of dietary UPFs as a percentage of total daily calories with cognitive performance over time.
During a median follow-up of 8 years, UPF intake in quartiles 2 to 4 (vs. quartile 1) was associated with a significant decline in global cognition (P = .003) and executive function (P = .015).
“Participants who reported consumption of more than 20% of daily calories from ultraprocessed foods had a 28% faster rate of global cognitive decline and a 25% faster decrease of the executive function compared to those who reported eating less than 20% of daily calories from ultraprocessed foods,” Dr. Gonçalves reported.
“Considering a person who eats a total of 2,000 kcal per day, 20% of daily calories from ultraprocessed foods are about two 1.5-ounce bars of KitKat, or five slices of bread, or about a third of an 8.5-ounce package of chips,” she explained.
Dr. Gonçalves noted that the reasons UPFs may harm the brain remain a “very relevant but not yet well-studied topic.”
Hypotheses include secondary effects from cerebrovascular lesions or chronic inflammation processes. More studies are needed to investigate the possible mechanisms that might explain the harm of UPFs to the brain, she said.
‘Troubling but not surprising’
Commenting on the study, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association, said there is “growing evidence that what we eat can impact our brains as we age.”
He added that many previous studies have suggested it is best for the brain for one to eat a heart-healthy, balanced diet that is low in processed foods and high in whole, nutritional foods, such as vegetables and fruits.
“These new data from the Alzheimer’s Association International Conference suggest eating a large amount of ultraprocessed food can significantly accelerate cognitive decline,” said Dr. Griffin, who was not involved with the research.
He noted that an increase in the availability and consumption of fast foods, processed foods, and UPFs is due to a number of socioeconomic factors, including low access to healthy foods, less time to prepare foods from scratch, and an inability to afford whole foods.
“Ultraprocessed foods make up more than half of American diets. It’s troubling but not surprising to see new data suggesting these foods can significantly accelerate cognitive decline,” Dr. Griffin said.
“The good news is there are steps we can take to reduce risk of cognitive decline as we age. These include eating a balanced diet, exercising regularly, getting good sleep, staying cognitively engaged, protecting from head injury, not smoking, and managing heart health,” he added.
Past research has suggested that the greatest benefit is from engaging in combinations of these lifestyle changes and that they are beneficial at any age, he noted.
“Even if you begin with one or two healthful actions, you’re moving in the right direction. It’s never too early or too late to incorporate these habits into your life,” Dr. Griffin said.
The study had no specific funding. Dr. Gonçalves and Dr. Griffin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included participants aged 35 and older, showed that higher intake of UPFs was significantly associated with a faster rate of decline in both executive and global cognitive function.
“Based on these findings, doctors might counsel patients to prefer cooking at home [and] choosing fresher ingredients instead of buying ready-made meals and snacks,” said coinvestigator Natalia Gonçalves, PhD, University of São Paulo, Brazil.
Presented at the Alzheimer’s Association International Conference, the findings align with those from a recent study in Neurology. That study linked a diet high in UPFs to an increased risk for dementia.
Increasing worldwide consumption
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs include soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, fries, and many more.
Over the past 30 years, there has been a steady increase in consumption of UPFs worldwide. They are thought to induce systemic inflammation and oxidative stress and have been linked to a variety of ailments, such as overweight/obesity, cardiovascular disease, and cancer.
UPFs may also be a risk factor for cognitive decline, although data are scarce as to their effects on the brain.
To investigate, Dr. Gonçalves and colleagues evaluated longitudinal data on 10,775 adults (mean age, 50.6 years; 56% women; 55% White) who participated in the ELSA-Brasil study. They were evaluated in three waves (2008-2010, 2012-2014, and 2017-2019).
Information on diet was obtained via food frequency questionnaires and included information regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
Significant decline
Using linear mixed effects models that were adjusted for sociodemographic, lifestyle, and clinical variables, the investigators assessed the association of dietary UPFs as a percentage of total daily calories with cognitive performance over time.
During a median follow-up of 8 years, UPF intake in quartiles 2 to 4 (vs. quartile 1) was associated with a significant decline in global cognition (P = .003) and executive function (P = .015).
“Participants who reported consumption of more than 20% of daily calories from ultraprocessed foods had a 28% faster rate of global cognitive decline and a 25% faster decrease of the executive function compared to those who reported eating less than 20% of daily calories from ultraprocessed foods,” Dr. Gonçalves reported.
“Considering a person who eats a total of 2,000 kcal per day, 20% of daily calories from ultraprocessed foods are about two 1.5-ounce bars of KitKat, or five slices of bread, or about a third of an 8.5-ounce package of chips,” she explained.
Dr. Gonçalves noted that the reasons UPFs may harm the brain remain a “very relevant but not yet well-studied topic.”
Hypotheses include secondary effects from cerebrovascular lesions or chronic inflammation processes. More studies are needed to investigate the possible mechanisms that might explain the harm of UPFs to the brain, she said.
‘Troubling but not surprising’
Commenting on the study, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association, said there is “growing evidence that what we eat can impact our brains as we age.”
He added that many previous studies have suggested it is best for the brain for one to eat a heart-healthy, balanced diet that is low in processed foods and high in whole, nutritional foods, such as vegetables and fruits.
“These new data from the Alzheimer’s Association International Conference suggest eating a large amount of ultraprocessed food can significantly accelerate cognitive decline,” said Dr. Griffin, who was not involved with the research.
He noted that an increase in the availability and consumption of fast foods, processed foods, and UPFs is due to a number of socioeconomic factors, including low access to healthy foods, less time to prepare foods from scratch, and an inability to afford whole foods.
“Ultraprocessed foods make up more than half of American diets. It’s troubling but not surprising to see new data suggesting these foods can significantly accelerate cognitive decline,” Dr. Griffin said.
“The good news is there are steps we can take to reduce risk of cognitive decline as we age. These include eating a balanced diet, exercising regularly, getting good sleep, staying cognitively engaged, protecting from head injury, not smoking, and managing heart health,” he added.
Past research has suggested that the greatest benefit is from engaging in combinations of these lifestyle changes and that they are beneficial at any age, he noted.
“Even if you begin with one or two healthful actions, you’re moving in the right direction. It’s never too early or too late to incorporate these habits into your life,” Dr. Griffin said.
The study had no specific funding. Dr. Gonçalves and Dr. Griffin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included participants aged 35 and older, showed that higher intake of UPFs was significantly associated with a faster rate of decline in both executive and global cognitive function.
“Based on these findings, doctors might counsel patients to prefer cooking at home [and] choosing fresher ingredients instead of buying ready-made meals and snacks,” said coinvestigator Natalia Gonçalves, PhD, University of São Paulo, Brazil.
Presented at the Alzheimer’s Association International Conference, the findings align with those from a recent study in Neurology. That study linked a diet high in UPFs to an increased risk for dementia.
Increasing worldwide consumption
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs include soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, fries, and many more.
Over the past 30 years, there has been a steady increase in consumption of UPFs worldwide. They are thought to induce systemic inflammation and oxidative stress and have been linked to a variety of ailments, such as overweight/obesity, cardiovascular disease, and cancer.
UPFs may also be a risk factor for cognitive decline, although data are scarce as to their effects on the brain.
To investigate, Dr. Gonçalves and colleagues evaluated longitudinal data on 10,775 adults (mean age, 50.6 years; 56% women; 55% White) who participated in the ELSA-Brasil study. They were evaluated in three waves (2008-2010, 2012-2014, and 2017-2019).
Information on diet was obtained via food frequency questionnaires and included information regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
Significant decline
Using linear mixed effects models that were adjusted for sociodemographic, lifestyle, and clinical variables, the investigators assessed the association of dietary UPFs as a percentage of total daily calories with cognitive performance over time.
During a median follow-up of 8 years, UPF intake in quartiles 2 to 4 (vs. quartile 1) was associated with a significant decline in global cognition (P = .003) and executive function (P = .015).
“Participants who reported consumption of more than 20% of daily calories from ultraprocessed foods had a 28% faster rate of global cognitive decline and a 25% faster decrease of the executive function compared to those who reported eating less than 20% of daily calories from ultraprocessed foods,” Dr. Gonçalves reported.
“Considering a person who eats a total of 2,000 kcal per day, 20% of daily calories from ultraprocessed foods are about two 1.5-ounce bars of KitKat, or five slices of bread, or about a third of an 8.5-ounce package of chips,” she explained.
Dr. Gonçalves noted that the reasons UPFs may harm the brain remain a “very relevant but not yet well-studied topic.”
Hypotheses include secondary effects from cerebrovascular lesions or chronic inflammation processes. More studies are needed to investigate the possible mechanisms that might explain the harm of UPFs to the brain, she said.
‘Troubling but not surprising’
Commenting on the study, Percy Griffin, PhD, director of scientific engagement for the Alzheimer’s Association, said there is “growing evidence that what we eat can impact our brains as we age.”
He added that many previous studies have suggested it is best for the brain for one to eat a heart-healthy, balanced diet that is low in processed foods and high in whole, nutritional foods, such as vegetables and fruits.
“These new data from the Alzheimer’s Association International Conference suggest eating a large amount of ultraprocessed food can significantly accelerate cognitive decline,” said Dr. Griffin, who was not involved with the research.
He noted that an increase in the availability and consumption of fast foods, processed foods, and UPFs is due to a number of socioeconomic factors, including low access to healthy foods, less time to prepare foods from scratch, and an inability to afford whole foods.
“Ultraprocessed foods make up more than half of American diets. It’s troubling but not surprising to see new data suggesting these foods can significantly accelerate cognitive decline,” Dr. Griffin said.
“The good news is there are steps we can take to reduce risk of cognitive decline as we age. These include eating a balanced diet, exercising regularly, getting good sleep, staying cognitively engaged, protecting from head injury, not smoking, and managing heart health,” he added.
Past research has suggested that the greatest benefit is from engaging in combinations of these lifestyle changes and that they are beneficial at any age, he noted.
“Even if you begin with one or two healthful actions, you’re moving in the right direction. It’s never too early or too late to incorporate these habits into your life,” Dr. Griffin said.
The study had no specific funding. Dr. Gonçalves and Dr. Griffin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAIC 2022
Racism tied to cognition in middle-aged, elderly
It is generally understood that racism, whether structural or personal, harms the well-being of the individual who experiences it. It has harmful health effects, and it contributes to ethnic inequality.
That was the fundamental message behind two studies presented at a press conference at the Alzheimer’s Association International Conference.
“We know that there are communities like black African Americans and Hispanic Latinos who are at greater risk for developing Alzheimer’s or another dementia,” said Carl Hill, PhD, who served as a moderator during the press conference. He pointed out that the genetic and lifestyle factors linked to dementia tell only part of the story. “It’s important that the science also examines the unique experiences of those at greater risk for dementia in our society,” said Dr. Hill, who is Alzheimer’s Association Chief Diversity Equity and Inclusion Officer.
Racism, memory, and cognition in middle-aged patients
Jennifer J. Manly, PhD, professor of neuropsychology at Columbia University, New York, presented a study of experience of racism and memory scores among a highly diverse, middle-aged cohort.
“There’s little understanding of how the multiple levels of racism – including intrapersonal, institutional, and structural racism – influence cognitive aging and dementia risk,” Dr. Manly said during the press conference.
Among 1,095 participants, 19.5% were non-Latinx White (61% female, mean age 57), 26.0% were non-Latinx Black (63% female, mean age 56), 32.3% were English-speaking Latinx (66% female, mean age 50), and 21.2% were Spanish-speaking Latinx (68% female, mean age 58).
The researchers used the Everyday Discrimination (ED) scale to measure experience of individual racism, the Major Discrimination (MD) scale to measure experience of institutional racism, and residential segregation of the census block group for an individual’s parents to measure residential segregation. Outcome measures included the Digit Span to assess attention and working memory, and the Selective Reminding Test to assess episodic memory.
The study found a clear association between racism and cognition. “The association of interpersonal racism to memory corresponds to 3 years of chronological age, and was driven by non-Hispanic black participants. Next, there was a reliable relationship between institutional racism and memory scores among non-Hispanic black participants, such that each reported civil rights violation corresponded to the effect of about 4.5 years of age on memory,” said Dr. Manly.
“The bottom line is that our results suggest that exposure to racism is a substantial driver of later life memory function, even in middle age, and especially for Black people,” Dr. Manly added.
The results should alert physicians to the complexities of racism and its impact. “Health providers need to be aware that many accumulated risks are historical and structural, and not controlled by the individual. Maybe more importantly, the medical system itself may perpetuate discriminatory experiences that contribute to worse health,” said Dr. Manly.
Latinx concerns
Also at the press conference, Adriana Perez, PhD, emphasized the challenges that Spanish-speaking Latinxs have with health care. Just 5%-7% of nurses are Latinx. “The same could be said for physicians, for clinical psychologists ... as you look at the really critical positions to address brain health equity, we are not represented there,” said Dr. Perez, an assistant professor and senior fellow at the University of Pennsylvania School of Nursing in Philadelphia.
She also pointed out that Latinx representation in clinical trials is very low, even though surveys performed by the Alzheimer’s Association show that this population values medical science and is willing to participate. In fact, 85% said they would participate if invited. The trouble is that many clinical trial announcements state that participants must speak English. Even the many Latinos who are bilingual may be put off by that wording: “That is a message that you’re not invited. That’s how it’s perceived,” said Dr. Perez.
Racism and cognition in the elderly
At the press conference, Kristen George, PhD, presented results from a study of individuals over age 90. “Racial disparities in dementia have been well characterized, particularly among those people who are aged 65 and older, but we don’t know very much about the oldest old individuals who are aged 90 and older. This group is one of the fastest growing segments of the population, and it’s becoming increasingly diverse,” said Dr. George, assistant professor of epidemiology at the University of California, Davis.
The group enrolled 445 Asian, Black, Latinx, White, and multiracial individuals who were members of Kaiser Permanente Northern California, with a mean age of 92.7 years. They used the Major Experiences of Discrimination Scale to assess discrimination.
The researchers divided them into three groups based on gender, race, and responses to the 10-item scale. Class 1 included largely White men who had reported workplace discrimination, with an average of two major discrimination experiences. Class 2 was made up of White women and non-Whites who reported little or no discrimination, with an average of 0 experiences. Class 3 included all non-White participants, and they reported a mean of four discrimination experiences.
Using class 2 as a reference, executive function was better among class 1 individuals (beta = 0.28; 95% CI, 0.03-0.52) but there was no significant difference between class 3 and class 2. Class 1 had better baseline semantic memory than class 2 (beta = 0.33; 95% CI, 0.07-0.58), and those in class 3 performed significantly worse than class 2 (beta = –0.24; 95% CI, –0.48 to –0.00). There were no between-group differences in baseline verbal or episodic memory.
Dr. Perez, Dr. Manly, Dr. George, and Dr. Hill have no relevant financial disclosures.
It is generally understood that racism, whether structural or personal, harms the well-being of the individual who experiences it. It has harmful health effects, and it contributes to ethnic inequality.
That was the fundamental message behind two studies presented at a press conference at the Alzheimer’s Association International Conference.
“We know that there are communities like black African Americans and Hispanic Latinos who are at greater risk for developing Alzheimer’s or another dementia,” said Carl Hill, PhD, who served as a moderator during the press conference. He pointed out that the genetic and lifestyle factors linked to dementia tell only part of the story. “It’s important that the science also examines the unique experiences of those at greater risk for dementia in our society,” said Dr. Hill, who is Alzheimer’s Association Chief Diversity Equity and Inclusion Officer.
Racism, memory, and cognition in middle-aged patients
Jennifer J. Manly, PhD, professor of neuropsychology at Columbia University, New York, presented a study of experience of racism and memory scores among a highly diverse, middle-aged cohort.
“There’s little understanding of how the multiple levels of racism – including intrapersonal, institutional, and structural racism – influence cognitive aging and dementia risk,” Dr. Manly said during the press conference.
Among 1,095 participants, 19.5% were non-Latinx White (61% female, mean age 57), 26.0% were non-Latinx Black (63% female, mean age 56), 32.3% were English-speaking Latinx (66% female, mean age 50), and 21.2% were Spanish-speaking Latinx (68% female, mean age 58).
The researchers used the Everyday Discrimination (ED) scale to measure experience of individual racism, the Major Discrimination (MD) scale to measure experience of institutional racism, and residential segregation of the census block group for an individual’s parents to measure residential segregation. Outcome measures included the Digit Span to assess attention and working memory, and the Selective Reminding Test to assess episodic memory.
The study found a clear association between racism and cognition. “The association of interpersonal racism to memory corresponds to 3 years of chronological age, and was driven by non-Hispanic black participants. Next, there was a reliable relationship between institutional racism and memory scores among non-Hispanic black participants, such that each reported civil rights violation corresponded to the effect of about 4.5 years of age on memory,” said Dr. Manly.
“The bottom line is that our results suggest that exposure to racism is a substantial driver of later life memory function, even in middle age, and especially for Black people,” Dr. Manly added.
The results should alert physicians to the complexities of racism and its impact. “Health providers need to be aware that many accumulated risks are historical and structural, and not controlled by the individual. Maybe more importantly, the medical system itself may perpetuate discriminatory experiences that contribute to worse health,” said Dr. Manly.
Latinx concerns
Also at the press conference, Adriana Perez, PhD, emphasized the challenges that Spanish-speaking Latinxs have with health care. Just 5%-7% of nurses are Latinx. “The same could be said for physicians, for clinical psychologists ... as you look at the really critical positions to address brain health equity, we are not represented there,” said Dr. Perez, an assistant professor and senior fellow at the University of Pennsylvania School of Nursing in Philadelphia.
She also pointed out that Latinx representation in clinical trials is very low, even though surveys performed by the Alzheimer’s Association show that this population values medical science and is willing to participate. In fact, 85% said they would participate if invited. The trouble is that many clinical trial announcements state that participants must speak English. Even the many Latinos who are bilingual may be put off by that wording: “That is a message that you’re not invited. That’s how it’s perceived,” said Dr. Perez.
Racism and cognition in the elderly
At the press conference, Kristen George, PhD, presented results from a study of individuals over age 90. “Racial disparities in dementia have been well characterized, particularly among those people who are aged 65 and older, but we don’t know very much about the oldest old individuals who are aged 90 and older. This group is one of the fastest growing segments of the population, and it’s becoming increasingly diverse,” said Dr. George, assistant professor of epidemiology at the University of California, Davis.
The group enrolled 445 Asian, Black, Latinx, White, and multiracial individuals who were members of Kaiser Permanente Northern California, with a mean age of 92.7 years. They used the Major Experiences of Discrimination Scale to assess discrimination.
The researchers divided them into three groups based on gender, race, and responses to the 10-item scale. Class 1 included largely White men who had reported workplace discrimination, with an average of two major discrimination experiences. Class 2 was made up of White women and non-Whites who reported little or no discrimination, with an average of 0 experiences. Class 3 included all non-White participants, and they reported a mean of four discrimination experiences.
Using class 2 as a reference, executive function was better among class 1 individuals (beta = 0.28; 95% CI, 0.03-0.52) but there was no significant difference between class 3 and class 2. Class 1 had better baseline semantic memory than class 2 (beta = 0.33; 95% CI, 0.07-0.58), and those in class 3 performed significantly worse than class 2 (beta = –0.24; 95% CI, –0.48 to –0.00). There were no between-group differences in baseline verbal or episodic memory.
Dr. Perez, Dr. Manly, Dr. George, and Dr. Hill have no relevant financial disclosures.
It is generally understood that racism, whether structural or personal, harms the well-being of the individual who experiences it. It has harmful health effects, and it contributes to ethnic inequality.
That was the fundamental message behind two studies presented at a press conference at the Alzheimer’s Association International Conference.
“We know that there are communities like black African Americans and Hispanic Latinos who are at greater risk for developing Alzheimer’s or another dementia,” said Carl Hill, PhD, who served as a moderator during the press conference. He pointed out that the genetic and lifestyle factors linked to dementia tell only part of the story. “It’s important that the science also examines the unique experiences of those at greater risk for dementia in our society,” said Dr. Hill, who is Alzheimer’s Association Chief Diversity Equity and Inclusion Officer.
Racism, memory, and cognition in middle-aged patients
Jennifer J. Manly, PhD, professor of neuropsychology at Columbia University, New York, presented a study of experience of racism and memory scores among a highly diverse, middle-aged cohort.
“There’s little understanding of how the multiple levels of racism – including intrapersonal, institutional, and structural racism – influence cognitive aging and dementia risk,” Dr. Manly said during the press conference.
Among 1,095 participants, 19.5% were non-Latinx White (61% female, mean age 57), 26.0% were non-Latinx Black (63% female, mean age 56), 32.3% were English-speaking Latinx (66% female, mean age 50), and 21.2% were Spanish-speaking Latinx (68% female, mean age 58).
The researchers used the Everyday Discrimination (ED) scale to measure experience of individual racism, the Major Discrimination (MD) scale to measure experience of institutional racism, and residential segregation of the census block group for an individual’s parents to measure residential segregation. Outcome measures included the Digit Span to assess attention and working memory, and the Selective Reminding Test to assess episodic memory.
The study found a clear association between racism and cognition. “The association of interpersonal racism to memory corresponds to 3 years of chronological age, and was driven by non-Hispanic black participants. Next, there was a reliable relationship between institutional racism and memory scores among non-Hispanic black participants, such that each reported civil rights violation corresponded to the effect of about 4.5 years of age on memory,” said Dr. Manly.
“The bottom line is that our results suggest that exposure to racism is a substantial driver of later life memory function, even in middle age, and especially for Black people,” Dr. Manly added.
The results should alert physicians to the complexities of racism and its impact. “Health providers need to be aware that many accumulated risks are historical and structural, and not controlled by the individual. Maybe more importantly, the medical system itself may perpetuate discriminatory experiences that contribute to worse health,” said Dr. Manly.
Latinx concerns
Also at the press conference, Adriana Perez, PhD, emphasized the challenges that Spanish-speaking Latinxs have with health care. Just 5%-7% of nurses are Latinx. “The same could be said for physicians, for clinical psychologists ... as you look at the really critical positions to address brain health equity, we are not represented there,” said Dr. Perez, an assistant professor and senior fellow at the University of Pennsylvania School of Nursing in Philadelphia.
She also pointed out that Latinx representation in clinical trials is very low, even though surveys performed by the Alzheimer’s Association show that this population values medical science and is willing to participate. In fact, 85% said they would participate if invited. The trouble is that many clinical trial announcements state that participants must speak English. Even the many Latinos who are bilingual may be put off by that wording: “That is a message that you’re not invited. That’s how it’s perceived,” said Dr. Perez.
Racism and cognition in the elderly
At the press conference, Kristen George, PhD, presented results from a study of individuals over age 90. “Racial disparities in dementia have been well characterized, particularly among those people who are aged 65 and older, but we don’t know very much about the oldest old individuals who are aged 90 and older. This group is one of the fastest growing segments of the population, and it’s becoming increasingly diverse,” said Dr. George, assistant professor of epidemiology at the University of California, Davis.
The group enrolled 445 Asian, Black, Latinx, White, and multiracial individuals who were members of Kaiser Permanente Northern California, with a mean age of 92.7 years. They used the Major Experiences of Discrimination Scale to assess discrimination.
The researchers divided them into three groups based on gender, race, and responses to the 10-item scale. Class 1 included largely White men who had reported workplace discrimination, with an average of two major discrimination experiences. Class 2 was made up of White women and non-Whites who reported little or no discrimination, with an average of 0 experiences. Class 3 included all non-White participants, and they reported a mean of four discrimination experiences.
Using class 2 as a reference, executive function was better among class 1 individuals (beta = 0.28; 95% CI, 0.03-0.52) but there was no significant difference between class 3 and class 2. Class 1 had better baseline semantic memory than class 2 (beta = 0.33; 95% CI, 0.07-0.58), and those in class 3 performed significantly worse than class 2 (beta = –0.24; 95% CI, –0.48 to –0.00). There were no between-group differences in baseline verbal or episodic memory.
Dr. Perez, Dr. Manly, Dr. George, and Dr. Hill have no relevant financial disclosures.
FROM AAIC 2022



