User login
Urinating multiple times per night
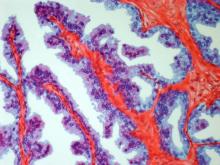
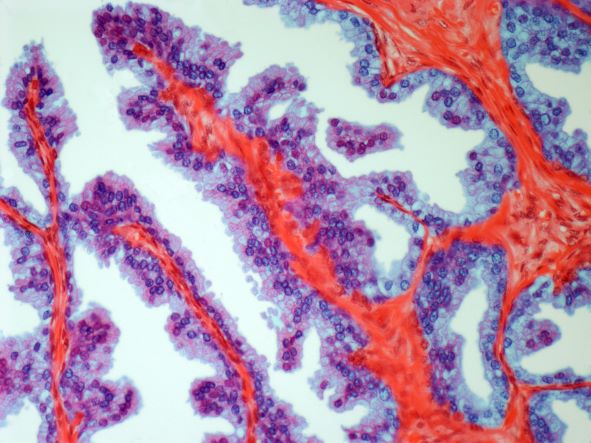
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 62-year-old man presents for routine prostate cancer screening. He notes that he has not been sleeping well as a result of getting up to urinate multiple times per night for the past few months. The patient underwent a prostate cancer screening about 26 months ago, and results were normal. On examination, digital rectal examination is normal, but prostate-specific antigen (PSA) levels are elevated at 10.2 ng/mL.
Safe supply programs aim to reduce drug overdose deaths
The Safer Alternatives for Emergency Response (SAFER) program provides a safe supply of substances to prevent drug overdose deaths, according to a new report.
The program has been operating in Vancouver, British Columbia, since April 2021. So far, the program has enrolled 58 participants who have reported benefits from having new options when other forms of treatment or harm reduction didn’t work. In addition, doctors who work with the program have reported increased medication adherence among the participants, as well as better chronic disease management.
Similar safe supply programs are being implemented or considered in other places across Canada. Since 2019, Health Canada has funded 18 safe supply pilot programs.
“When we look at the number of overdose deaths, it should be zero. These are preventable deaths,” author Christy Sutherland, MD, medical director at the PHS Community Services Society, Vancouver, which operates the SAFER program, told this news organization.
“As clinicians, we can see that the tools we have are working less because of prohibition. It drives the market to provide more potent and more dangerous options,” she said. “It’s critical that we disrupt the illicit market and provide medical solutions to keep people safe.”
The report was published in the Canadian Medical Association Journal.
Safe supply programs
Between January 2016 and June 2021, more than 24,000 people died from opioid toxicity in Canada, according to the authors. A key driver of the ongoing public health crisis has been the introduction of illicit fentanyl and other dangerous substances into the unregulated drug supply.
In recent years, several harm-reduction options and substance use disorder treatment programs have been introduced in Canada to stem overdose deaths. However, they haven’t been sufficient, and the number of deaths continues to rise.
“In 2010, methadone worked, but now even high doses don’t keep people out of withdrawal due to the infiltration of fentanyl,” Dr. Sutherland said. “It’s clinically not working anymore. People are now going through benzodiazepine withdrawal and opiate withdrawal at the same time.”
The changes have led doctors to call for programs that provide legal and regulated sources of psychoactive substances, also known as “safe supply” programs. In particular, low-barrier and flexible options are necessary to meet the needs of various people in the community.
In Vancouver, the SAFER program provides medications that are prescribed off-label as substitutes to the illicit drug supply. A multidisciplinary team oversees the program, including doctors, nurses, pharmacists, social workers, and people who have experience living with substance use.
The program’s approach is akin to the use of medications as treatments for substance use disorder, such as opioid-agonist therapy. However,
Enrolled participants can access medications, including opioids such as hydromorphone and fentanyl, as a substitute for the unregulated substances that they consume. A notable aspect of SAFER is the offer of fentanyl – with a known potency and without dangerous adulterants found in the local drug supply.
Promoting participant autonomy
Given the increasing rate of overdose deaths involving stimulants in Canada, the program also offers prescribed psychostimulants, such as methylphenidate and dextroamphetamine.
The program focuses on harm reduction and promoting participant autonomy. SAFER doesn’t have a predetermined schedule for medication access, which allows participants to return as they need.
“Creating this program has required patience to change our practices,” Dr. Sutherland said. “As you learn more and do more, you’re always growing because you care about your patients and want to help them, especially vulnerable people with a high risk for death.”
The SAFER program is integrated into health care and social services, and participants have access to on-site primary care from clinicians trained in addiction medicine. The program is located alongside a low-barrier prevention site, where supplies such as syringes, take-home naloxone kits, and drug-checking services are available.
The SAFER program will undergo a scientific evaluation, led by two of the co-authors, which will include about 200 participants. During a 2-year period, the evaluation will assess whether the program reduces the risk for overdose deaths and supports access to primary care, harm reduction, and substance use disorder treatment. In addition, the researchers will analyze other key outcomes, such as fatal versus nonfatal overdoses, medication adherence, and the qualitative lived experience of participants.
The end of prohibition?
“We’ve had the same challenges with people buying illegal drugs on the street for almost 30 years, but about 5 years ago, that all changed when fentanyl became a prominent drug, and overdose deaths skyrocketed,” Mark Tyndall, MD, a public health professor at the University of British Columbia, Vancouver, said in an interview.
Dr. Tyndall is also executive director of the British Columbia Centre for Disease Control and executive director of MySafe Society, a safe supply program in Canada for those with opioid addiction. He is not involved in the SAFER program.
SAFER and MySafe Society are positioned as low-barrier programs, he said, meaning that the public health response is primarily focused on preventing deaths and helping people to get access to medication that won’t kill them. The idea is to meet people where they are today.
However, these programs still face major barriers, such as limitations from federal regulators and stigmas around illicit drugs and harm-reduction programs.
“These beliefs are entrenched, and it takes a long time to help people understand that prohibition means that dangerous drugs are on the street,” he said. “I don’t think way more people are using than 10 years ago, but there was a supply of heroin that was stable in potency back then, and people weren’t dying.”
Ultimately, Dr. Tyndall said, drug policy experts would like to create a regulated supply, similar to the supply of cannabis. The political and regulatory process may take much longer to catch up, but he believes that it’s the most ethical way to reduce overdose deaths and the unregulated drug supply.
“The harshest critics of harm reduction often go to the liquor store every weekend,” he said. “It’s going to be a long process before people think this way, but having fentanyl and other dangerous drugs on the street has signaled the end stage of prohibition.”
The SAFER program is operated by PHS Community Services Society in partnership with Vancouver Coastal Health and funded through Health Canada’s Substance Use and Addiction Program. Dr. Tyndall reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The Safer Alternatives for Emergency Response (SAFER) program provides a safe supply of substances to prevent drug overdose deaths, according to a new report.
The program has been operating in Vancouver, British Columbia, since April 2021. So far, the program has enrolled 58 participants who have reported benefits from having new options when other forms of treatment or harm reduction didn’t work. In addition, doctors who work with the program have reported increased medication adherence among the participants, as well as better chronic disease management.
Similar safe supply programs are being implemented or considered in other places across Canada. Since 2019, Health Canada has funded 18 safe supply pilot programs.
“When we look at the number of overdose deaths, it should be zero. These are preventable deaths,” author Christy Sutherland, MD, medical director at the PHS Community Services Society, Vancouver, which operates the SAFER program, told this news organization.
“As clinicians, we can see that the tools we have are working less because of prohibition. It drives the market to provide more potent and more dangerous options,” she said. “It’s critical that we disrupt the illicit market and provide medical solutions to keep people safe.”
The report was published in the Canadian Medical Association Journal.
Safe supply programs
Between January 2016 and June 2021, more than 24,000 people died from opioid toxicity in Canada, according to the authors. A key driver of the ongoing public health crisis has been the introduction of illicit fentanyl and other dangerous substances into the unregulated drug supply.
In recent years, several harm-reduction options and substance use disorder treatment programs have been introduced in Canada to stem overdose deaths. However, they haven’t been sufficient, and the number of deaths continues to rise.
“In 2010, methadone worked, but now even high doses don’t keep people out of withdrawal due to the infiltration of fentanyl,” Dr. Sutherland said. “It’s clinically not working anymore. People are now going through benzodiazepine withdrawal and opiate withdrawal at the same time.”
The changes have led doctors to call for programs that provide legal and regulated sources of psychoactive substances, also known as “safe supply” programs. In particular, low-barrier and flexible options are necessary to meet the needs of various people in the community.
In Vancouver, the SAFER program provides medications that are prescribed off-label as substitutes to the illicit drug supply. A multidisciplinary team oversees the program, including doctors, nurses, pharmacists, social workers, and people who have experience living with substance use.
The program’s approach is akin to the use of medications as treatments for substance use disorder, such as opioid-agonist therapy. However,
Enrolled participants can access medications, including opioids such as hydromorphone and fentanyl, as a substitute for the unregulated substances that they consume. A notable aspect of SAFER is the offer of fentanyl – with a known potency and without dangerous adulterants found in the local drug supply.
Promoting participant autonomy
Given the increasing rate of overdose deaths involving stimulants in Canada, the program also offers prescribed psychostimulants, such as methylphenidate and dextroamphetamine.
The program focuses on harm reduction and promoting participant autonomy. SAFER doesn’t have a predetermined schedule for medication access, which allows participants to return as they need.
“Creating this program has required patience to change our practices,” Dr. Sutherland said. “As you learn more and do more, you’re always growing because you care about your patients and want to help them, especially vulnerable people with a high risk for death.”
The SAFER program is integrated into health care and social services, and participants have access to on-site primary care from clinicians trained in addiction medicine. The program is located alongside a low-barrier prevention site, where supplies such as syringes, take-home naloxone kits, and drug-checking services are available.
The SAFER program will undergo a scientific evaluation, led by two of the co-authors, which will include about 200 participants. During a 2-year period, the evaluation will assess whether the program reduces the risk for overdose deaths and supports access to primary care, harm reduction, and substance use disorder treatment. In addition, the researchers will analyze other key outcomes, such as fatal versus nonfatal overdoses, medication adherence, and the qualitative lived experience of participants.
The end of prohibition?
“We’ve had the same challenges with people buying illegal drugs on the street for almost 30 years, but about 5 years ago, that all changed when fentanyl became a prominent drug, and overdose deaths skyrocketed,” Mark Tyndall, MD, a public health professor at the University of British Columbia, Vancouver, said in an interview.
Dr. Tyndall is also executive director of the British Columbia Centre for Disease Control and executive director of MySafe Society, a safe supply program in Canada for those with opioid addiction. He is not involved in the SAFER program.
SAFER and MySafe Society are positioned as low-barrier programs, he said, meaning that the public health response is primarily focused on preventing deaths and helping people to get access to medication that won’t kill them. The idea is to meet people where they are today.
However, these programs still face major barriers, such as limitations from federal regulators and stigmas around illicit drugs and harm-reduction programs.
“These beliefs are entrenched, and it takes a long time to help people understand that prohibition means that dangerous drugs are on the street,” he said. “I don’t think way more people are using than 10 years ago, but there was a supply of heroin that was stable in potency back then, and people weren’t dying.”
Ultimately, Dr. Tyndall said, drug policy experts would like to create a regulated supply, similar to the supply of cannabis. The political and regulatory process may take much longer to catch up, but he believes that it’s the most ethical way to reduce overdose deaths and the unregulated drug supply.
“The harshest critics of harm reduction often go to the liquor store every weekend,” he said. “It’s going to be a long process before people think this way, but having fentanyl and other dangerous drugs on the street has signaled the end stage of prohibition.”
The SAFER program is operated by PHS Community Services Society in partnership with Vancouver Coastal Health and funded through Health Canada’s Substance Use and Addiction Program. Dr. Tyndall reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The Safer Alternatives for Emergency Response (SAFER) program provides a safe supply of substances to prevent drug overdose deaths, according to a new report.
The program has been operating in Vancouver, British Columbia, since April 2021. So far, the program has enrolled 58 participants who have reported benefits from having new options when other forms of treatment or harm reduction didn’t work. In addition, doctors who work with the program have reported increased medication adherence among the participants, as well as better chronic disease management.
Similar safe supply programs are being implemented or considered in other places across Canada. Since 2019, Health Canada has funded 18 safe supply pilot programs.
“When we look at the number of overdose deaths, it should be zero. These are preventable deaths,” author Christy Sutherland, MD, medical director at the PHS Community Services Society, Vancouver, which operates the SAFER program, told this news organization.
“As clinicians, we can see that the tools we have are working less because of prohibition. It drives the market to provide more potent and more dangerous options,” she said. “It’s critical that we disrupt the illicit market and provide medical solutions to keep people safe.”
The report was published in the Canadian Medical Association Journal.
Safe supply programs
Between January 2016 and June 2021, more than 24,000 people died from opioid toxicity in Canada, according to the authors. A key driver of the ongoing public health crisis has been the introduction of illicit fentanyl and other dangerous substances into the unregulated drug supply.
In recent years, several harm-reduction options and substance use disorder treatment programs have been introduced in Canada to stem overdose deaths. However, they haven’t been sufficient, and the number of deaths continues to rise.
“In 2010, methadone worked, but now even high doses don’t keep people out of withdrawal due to the infiltration of fentanyl,” Dr. Sutherland said. “It’s clinically not working anymore. People are now going through benzodiazepine withdrawal and opiate withdrawal at the same time.”
The changes have led doctors to call for programs that provide legal and regulated sources of psychoactive substances, also known as “safe supply” programs. In particular, low-barrier and flexible options are necessary to meet the needs of various people in the community.
In Vancouver, the SAFER program provides medications that are prescribed off-label as substitutes to the illicit drug supply. A multidisciplinary team oversees the program, including doctors, nurses, pharmacists, social workers, and people who have experience living with substance use.
The program’s approach is akin to the use of medications as treatments for substance use disorder, such as opioid-agonist therapy. However,
Enrolled participants can access medications, including opioids such as hydromorphone and fentanyl, as a substitute for the unregulated substances that they consume. A notable aspect of SAFER is the offer of fentanyl – with a known potency and without dangerous adulterants found in the local drug supply.
Promoting participant autonomy
Given the increasing rate of overdose deaths involving stimulants in Canada, the program also offers prescribed psychostimulants, such as methylphenidate and dextroamphetamine.
The program focuses on harm reduction and promoting participant autonomy. SAFER doesn’t have a predetermined schedule for medication access, which allows participants to return as they need.
“Creating this program has required patience to change our practices,” Dr. Sutherland said. “As you learn more and do more, you’re always growing because you care about your patients and want to help them, especially vulnerable people with a high risk for death.”
The SAFER program is integrated into health care and social services, and participants have access to on-site primary care from clinicians trained in addiction medicine. The program is located alongside a low-barrier prevention site, where supplies such as syringes, take-home naloxone kits, and drug-checking services are available.
The SAFER program will undergo a scientific evaluation, led by two of the co-authors, which will include about 200 participants. During a 2-year period, the evaluation will assess whether the program reduces the risk for overdose deaths and supports access to primary care, harm reduction, and substance use disorder treatment. In addition, the researchers will analyze other key outcomes, such as fatal versus nonfatal overdoses, medication adherence, and the qualitative lived experience of participants.
The end of prohibition?
“We’ve had the same challenges with people buying illegal drugs on the street for almost 30 years, but about 5 years ago, that all changed when fentanyl became a prominent drug, and overdose deaths skyrocketed,” Mark Tyndall, MD, a public health professor at the University of British Columbia, Vancouver, said in an interview.
Dr. Tyndall is also executive director of the British Columbia Centre for Disease Control and executive director of MySafe Society, a safe supply program in Canada for those with opioid addiction. He is not involved in the SAFER program.
SAFER and MySafe Society are positioned as low-barrier programs, he said, meaning that the public health response is primarily focused on preventing deaths and helping people to get access to medication that won’t kill them. The idea is to meet people where they are today.
However, these programs still face major barriers, such as limitations from federal regulators and stigmas around illicit drugs and harm-reduction programs.
“These beliefs are entrenched, and it takes a long time to help people understand that prohibition means that dangerous drugs are on the street,” he said. “I don’t think way more people are using than 10 years ago, but there was a supply of heroin that was stable in potency back then, and people weren’t dying.”
Ultimately, Dr. Tyndall said, drug policy experts would like to create a regulated supply, similar to the supply of cannabis. The political and regulatory process may take much longer to catch up, but he believes that it’s the most ethical way to reduce overdose deaths and the unregulated drug supply.
“The harshest critics of harm reduction often go to the liquor store every weekend,” he said. “It’s going to be a long process before people think this way, but having fentanyl and other dangerous drugs on the street has signaled the end stage of prohibition.”
The SAFER program is operated by PHS Community Services Society in partnership with Vancouver Coastal Health and funded through Health Canada’s Substance Use and Addiction Program. Dr. Tyndall reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Path to parenthood in cardiology training fraught with obstacles
The first international survey of parental benefits and policies among cardiovascular training programs shows wide variability among institutions.
Although a majority of cardiology fellows became parents during training, the survey found that family benefits and policies were not uniformly available and that knowledge about the existence of such policies was low across all institutions.
The findings are published in the Journal of the American College of Cardiology.
Such variability highlights disparities in real-world experiences, say Estefania Oliveros, MD, Temple University Hospital, Philadelphia, and colleagues.
“There are no policies to protect cardiology trainees when they become parents that are uniform across the United States or even internationally, even though, according to our survey, 61.7% become parents during training,” Dr. Oliveros told this news organization.
Dr. Oliveros said she wanted to learn more about the status of institutional practices surrounding pregnant trainees during cardiovascular fellowship, not only in the U.S., but internationally: “I wanted to study this because of my own experience.”
“I was probably the first pregnant trainee at my institution, and there were no specific policies in place, so I had to find out on my own what to do about radiation safety, where I would breastfeed, schedule changes, how that would impact my graduation time, things like that,” Dr. Oliveros said. “It would be nice if you had the resources and your institution could accommodate your needs, instead of every time you have a pregnant person on your staff, you have to reinvent the wheel.”
Dr. Oliveros and colleagues conducted an online survey during August 2020-October 2020 that was distributed via social media. Responses were made anonymous to encourage unbiased feedback.
Among the 417 completed responses, 47 (11.3%) were from training program directors, 146 (35%) from current or former pregnant trainees, and 224 (53.7%) from current or former trainees who were not pregnant during cardiology training. Two-thirds of the respondents (67.1%) were parents.
Most survey respondents said they became pregnant during the third year of general cardiology (29.1%), followed by the first year of general cardiology (26.3%), and the second year of general cardiology (23.5%).
Only 13 of the 47 training program directors (27.7%) received guidance or training on how to accommodate pregnant trainees during fellowship.
Additionally, 26% of the trainees reported their institution had readily available breastfeeding and pumping policies, 39% responded that their institution had no such policies, and 34.9% said they did not know.
Nearly one-half of the programs offered rearrangement of schedules because of radiation concerns, 27.5% did not.
The amount of parental leave varied greatly worldwide. For Europe, Central and South America, Africa, and Australia, the average parental leave was more than 4 months; for Canada, it was more than 3 months; for the United States, it was 1 to 2 months; and for Asia, it was 3 to 4 weeks.
“There is no uniformity, no policies for things like breastfeeding or places where you can pump. None of that is installed, even though by law we’re supposed to have these things,” Dr. Oliveros said.
In all countries, paternity leave was uncommon (2.6% of respondents), even though 48.5% of the programs had paternity leave.
“I would like to see associations, program directors, even trainees helping each other in finding ways to accommodate parents to promote wellness and assure that trainees can have both good training and life balance,” she added.
In an accompanying editorial, Ileana L. Piña, MD, MPH, Thomas Jefferson Institute, Philadelphia, writes: “Enough has been said about our need for a greater percentage of women cardiologists. There is no need to further debate that fact. However, it is puzzling that despite > 50% of medical students being women, the cardiology specialty is fraught with recent survey reports of hostility in the workplace, concerns of long hours, exposure to radiation, and poor work-life balance that can compel trainees to choose delaying pregnancy or taking unpaid leave, which will, in turn, delay training. Therefore, it is not surprising that only 14.9% of cardiologist specialists and 21.9% of cardiology fellows are women.”
Dr. Piña notes that while the authors understand that it’s difficult to change national policies, they issue a “call to action” for organizations and program directors to demonstrate leadership by developing fair and balanced decisions regarding parental policies.
“Those decisions are so impactful that they can change career trajectories for the better or worse ... the current status is unacceptable and must change for the benefit of all trainees, their families, and the program directors. The problem is too important and pervasive,” she adds.
Dr. Piña concludes: “Perhaps if the women who are the subjects of, and often the unwitting party to, administrative decisions about their lives, choices, and welfare were invited to contribute to the changes, we would finally see an increase in the number of women in cardiology careers. After all, aren’t we about diversity and belonging?”
“We need to normalize pregnancy and parental leave across the globe,” Laxmi S. Mehta, MD, Ohio State University Weiner Medical Center, Columbus, said in an interview.
As previously reported, Dr. Mehta recently led a study that surveyed 323 women cardiologists who were working while they were pregnant. Her study found that 75% of these women experienced discriminatory maternity leave practices, some of which were likely violations of the federal Family and Medical Leave Act.
“If we want more women to pursue a career in cardiology, then employers and health systems need to adequately support parenthood, including allowing people to spend uninterrupted time with their newborns without the fear of discrimination, retaliation, or financial burden,” Dr. Mehta said.
Limitations of the study are the small sample size, potential for bias associated with social media distribution, and the fact that 75% of respondents were women, Dr. Oliveros and colleagues write.
Dr. Oliveros, Dr. Piña, and Dr. Mehta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The first international survey of parental benefits and policies among cardiovascular training programs shows wide variability among institutions.
Although a majority of cardiology fellows became parents during training, the survey found that family benefits and policies were not uniformly available and that knowledge about the existence of such policies was low across all institutions.
The findings are published in the Journal of the American College of Cardiology.
Such variability highlights disparities in real-world experiences, say Estefania Oliveros, MD, Temple University Hospital, Philadelphia, and colleagues.
“There are no policies to protect cardiology trainees when they become parents that are uniform across the United States or even internationally, even though, according to our survey, 61.7% become parents during training,” Dr. Oliveros told this news organization.
Dr. Oliveros said she wanted to learn more about the status of institutional practices surrounding pregnant trainees during cardiovascular fellowship, not only in the U.S., but internationally: “I wanted to study this because of my own experience.”
“I was probably the first pregnant trainee at my institution, and there were no specific policies in place, so I had to find out on my own what to do about radiation safety, where I would breastfeed, schedule changes, how that would impact my graduation time, things like that,” Dr. Oliveros said. “It would be nice if you had the resources and your institution could accommodate your needs, instead of every time you have a pregnant person on your staff, you have to reinvent the wheel.”
Dr. Oliveros and colleagues conducted an online survey during August 2020-October 2020 that was distributed via social media. Responses were made anonymous to encourage unbiased feedback.
Among the 417 completed responses, 47 (11.3%) were from training program directors, 146 (35%) from current or former pregnant trainees, and 224 (53.7%) from current or former trainees who were not pregnant during cardiology training. Two-thirds of the respondents (67.1%) were parents.
Most survey respondents said they became pregnant during the third year of general cardiology (29.1%), followed by the first year of general cardiology (26.3%), and the second year of general cardiology (23.5%).
Only 13 of the 47 training program directors (27.7%) received guidance or training on how to accommodate pregnant trainees during fellowship.
Additionally, 26% of the trainees reported their institution had readily available breastfeeding and pumping policies, 39% responded that their institution had no such policies, and 34.9% said they did not know.
Nearly one-half of the programs offered rearrangement of schedules because of radiation concerns, 27.5% did not.
The amount of parental leave varied greatly worldwide. For Europe, Central and South America, Africa, and Australia, the average parental leave was more than 4 months; for Canada, it was more than 3 months; for the United States, it was 1 to 2 months; and for Asia, it was 3 to 4 weeks.
“There is no uniformity, no policies for things like breastfeeding or places where you can pump. None of that is installed, even though by law we’re supposed to have these things,” Dr. Oliveros said.
In all countries, paternity leave was uncommon (2.6% of respondents), even though 48.5% of the programs had paternity leave.
“I would like to see associations, program directors, even trainees helping each other in finding ways to accommodate parents to promote wellness and assure that trainees can have both good training and life balance,” she added.
In an accompanying editorial, Ileana L. Piña, MD, MPH, Thomas Jefferson Institute, Philadelphia, writes: “Enough has been said about our need for a greater percentage of women cardiologists. There is no need to further debate that fact. However, it is puzzling that despite > 50% of medical students being women, the cardiology specialty is fraught with recent survey reports of hostility in the workplace, concerns of long hours, exposure to radiation, and poor work-life balance that can compel trainees to choose delaying pregnancy or taking unpaid leave, which will, in turn, delay training. Therefore, it is not surprising that only 14.9% of cardiologist specialists and 21.9% of cardiology fellows are women.”
Dr. Piña notes that while the authors understand that it’s difficult to change national policies, they issue a “call to action” for organizations and program directors to demonstrate leadership by developing fair and balanced decisions regarding parental policies.
“Those decisions are so impactful that they can change career trajectories for the better or worse ... the current status is unacceptable and must change for the benefit of all trainees, their families, and the program directors. The problem is too important and pervasive,” she adds.
Dr. Piña concludes: “Perhaps if the women who are the subjects of, and often the unwitting party to, administrative decisions about their lives, choices, and welfare were invited to contribute to the changes, we would finally see an increase in the number of women in cardiology careers. After all, aren’t we about diversity and belonging?”
“We need to normalize pregnancy and parental leave across the globe,” Laxmi S. Mehta, MD, Ohio State University Weiner Medical Center, Columbus, said in an interview.
As previously reported, Dr. Mehta recently led a study that surveyed 323 women cardiologists who were working while they were pregnant. Her study found that 75% of these women experienced discriminatory maternity leave practices, some of which were likely violations of the federal Family and Medical Leave Act.
“If we want more women to pursue a career in cardiology, then employers and health systems need to adequately support parenthood, including allowing people to spend uninterrupted time with their newborns without the fear of discrimination, retaliation, or financial burden,” Dr. Mehta said.
Limitations of the study are the small sample size, potential for bias associated with social media distribution, and the fact that 75% of respondents were women, Dr. Oliveros and colleagues write.
Dr. Oliveros, Dr. Piña, and Dr. Mehta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The first international survey of parental benefits and policies among cardiovascular training programs shows wide variability among institutions.
Although a majority of cardiology fellows became parents during training, the survey found that family benefits and policies were not uniformly available and that knowledge about the existence of such policies was low across all institutions.
The findings are published in the Journal of the American College of Cardiology.
Such variability highlights disparities in real-world experiences, say Estefania Oliveros, MD, Temple University Hospital, Philadelphia, and colleagues.
“There are no policies to protect cardiology trainees when they become parents that are uniform across the United States or even internationally, even though, according to our survey, 61.7% become parents during training,” Dr. Oliveros told this news organization.
Dr. Oliveros said she wanted to learn more about the status of institutional practices surrounding pregnant trainees during cardiovascular fellowship, not only in the U.S., but internationally: “I wanted to study this because of my own experience.”
“I was probably the first pregnant trainee at my institution, and there were no specific policies in place, so I had to find out on my own what to do about radiation safety, where I would breastfeed, schedule changes, how that would impact my graduation time, things like that,” Dr. Oliveros said. “It would be nice if you had the resources and your institution could accommodate your needs, instead of every time you have a pregnant person on your staff, you have to reinvent the wheel.”
Dr. Oliveros and colleagues conducted an online survey during August 2020-October 2020 that was distributed via social media. Responses were made anonymous to encourage unbiased feedback.
Among the 417 completed responses, 47 (11.3%) were from training program directors, 146 (35%) from current or former pregnant trainees, and 224 (53.7%) from current or former trainees who were not pregnant during cardiology training. Two-thirds of the respondents (67.1%) were parents.
Most survey respondents said they became pregnant during the third year of general cardiology (29.1%), followed by the first year of general cardiology (26.3%), and the second year of general cardiology (23.5%).
Only 13 of the 47 training program directors (27.7%) received guidance or training on how to accommodate pregnant trainees during fellowship.
Additionally, 26% of the trainees reported their institution had readily available breastfeeding and pumping policies, 39% responded that their institution had no such policies, and 34.9% said they did not know.
Nearly one-half of the programs offered rearrangement of schedules because of radiation concerns, 27.5% did not.
The amount of parental leave varied greatly worldwide. For Europe, Central and South America, Africa, and Australia, the average parental leave was more than 4 months; for Canada, it was more than 3 months; for the United States, it was 1 to 2 months; and for Asia, it was 3 to 4 weeks.
“There is no uniformity, no policies for things like breastfeeding or places where you can pump. None of that is installed, even though by law we’re supposed to have these things,” Dr. Oliveros said.
In all countries, paternity leave was uncommon (2.6% of respondents), even though 48.5% of the programs had paternity leave.
“I would like to see associations, program directors, even trainees helping each other in finding ways to accommodate parents to promote wellness and assure that trainees can have both good training and life balance,” she added.
In an accompanying editorial, Ileana L. Piña, MD, MPH, Thomas Jefferson Institute, Philadelphia, writes: “Enough has been said about our need for a greater percentage of women cardiologists. There is no need to further debate that fact. However, it is puzzling that despite > 50% of medical students being women, the cardiology specialty is fraught with recent survey reports of hostility in the workplace, concerns of long hours, exposure to radiation, and poor work-life balance that can compel trainees to choose delaying pregnancy or taking unpaid leave, which will, in turn, delay training. Therefore, it is not surprising that only 14.9% of cardiologist specialists and 21.9% of cardiology fellows are women.”
Dr. Piña notes that while the authors understand that it’s difficult to change national policies, they issue a “call to action” for organizations and program directors to demonstrate leadership by developing fair and balanced decisions regarding parental policies.
“Those decisions are so impactful that they can change career trajectories for the better or worse ... the current status is unacceptable and must change for the benefit of all trainees, their families, and the program directors. The problem is too important and pervasive,” she adds.
Dr. Piña concludes: “Perhaps if the women who are the subjects of, and often the unwitting party to, administrative decisions about their lives, choices, and welfare were invited to contribute to the changes, we would finally see an increase in the number of women in cardiology careers. After all, aren’t we about diversity and belonging?”
“We need to normalize pregnancy and parental leave across the globe,” Laxmi S. Mehta, MD, Ohio State University Weiner Medical Center, Columbus, said in an interview.
As previously reported, Dr. Mehta recently led a study that surveyed 323 women cardiologists who were working while they were pregnant. Her study found that 75% of these women experienced discriminatory maternity leave practices, some of which were likely violations of the federal Family and Medical Leave Act.
“If we want more women to pursue a career in cardiology, then employers and health systems need to adequately support parenthood, including allowing people to spend uninterrupted time with their newborns without the fear of discrimination, retaliation, or financial burden,” Dr. Mehta said.
Limitations of the study are the small sample size, potential for bias associated with social media distribution, and the fact that 75% of respondents were women, Dr. Oliveros and colleagues write.
Dr. Oliveros, Dr. Piña, and Dr. Mehta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sugar-sweetened beverage and sugar consumption tied with incidence of and mortality from proximal colon cancer
Key clinical point: High sugar-sweetened beverage (SSB) and total fructose intake was associated with increased incidence of and mortality from proximal colon cancer, especially during the later stages of colorectal tumorigenesis.
Major finding: SSB and total fructose consumption was associated with a significant increase in the incidence of (hazard ratio [HR] per 1-serving/d increment 1.18 and HR per 25-g/d increment 1.18, respectively; both Ptrend = .02) and mortality from (HR 1.39; Ptrend = .002 and HR 1.42; Ptrend = .003, respectively) proximal colon cancer.
Study details: This large-scale study included 121,111 adult health professionals from two US prospective cohorts, the Nurses’ Health Study and Health Professionals Follow-Up Study.
Disclosures: The study was sponsored by grants from the US National Institutes of Health, American Cancer Society, and American Institute for Cancer Research. Some authors declared consulting and advisory board participation for and receiving research funds from various sources.
Source: Yuan C et al. Sugar-sweetened beverage and sugar consumption and colorectal cancer incidence and mortality according to anatomic subsite. Am J Clin Nutr. 2022 (Apr 25). Doi: 10.1093/ajcn/nqac040
Key clinical point: High sugar-sweetened beverage (SSB) and total fructose intake was associated with increased incidence of and mortality from proximal colon cancer, especially during the later stages of colorectal tumorigenesis.
Major finding: SSB and total fructose consumption was associated with a significant increase in the incidence of (hazard ratio [HR] per 1-serving/d increment 1.18 and HR per 25-g/d increment 1.18, respectively; both Ptrend = .02) and mortality from (HR 1.39; Ptrend = .002 and HR 1.42; Ptrend = .003, respectively) proximal colon cancer.
Study details: This large-scale study included 121,111 adult health professionals from two US prospective cohorts, the Nurses’ Health Study and Health Professionals Follow-Up Study.
Disclosures: The study was sponsored by grants from the US National Institutes of Health, American Cancer Society, and American Institute for Cancer Research. Some authors declared consulting and advisory board participation for and receiving research funds from various sources.
Source: Yuan C et al. Sugar-sweetened beverage and sugar consumption and colorectal cancer incidence and mortality according to anatomic subsite. Am J Clin Nutr. 2022 (Apr 25). Doi: 10.1093/ajcn/nqac040
Key clinical point: High sugar-sweetened beverage (SSB) and total fructose intake was associated with increased incidence of and mortality from proximal colon cancer, especially during the later stages of colorectal tumorigenesis.
Major finding: SSB and total fructose consumption was associated with a significant increase in the incidence of (hazard ratio [HR] per 1-serving/d increment 1.18 and HR per 25-g/d increment 1.18, respectively; both Ptrend = .02) and mortality from (HR 1.39; Ptrend = .002 and HR 1.42; Ptrend = .003, respectively) proximal colon cancer.
Study details: This large-scale study included 121,111 adult health professionals from two US prospective cohorts, the Nurses’ Health Study and Health Professionals Follow-Up Study.
Disclosures: The study was sponsored by grants from the US National Institutes of Health, American Cancer Society, and American Institute for Cancer Research. Some authors declared consulting and advisory board participation for and receiving research funds from various sources.
Source: Yuan C et al. Sugar-sweetened beverage and sugar consumption and colorectal cancer incidence and mortality according to anatomic subsite. Am J Clin Nutr. 2022 (Apr 25). Doi: 10.1093/ajcn/nqac040
ctDNA: Strong prognostic biomarker but lacks true clinical utility in mCRC
Key clinical point: Circulating tumor DNA (ctDNA) has substantiated its role as a strong prognostic biomarker in patients with metastatic colorectal cancer (mCRC). However, uncovering its true clinical value for these patients calls for prospective clinical trials with standardized methodologies.
Major finding: High baseline ctDNA levels were associated with a shorter progression-free survival (PFS; hazard ratio [HR] 2.2; 95% CI 1.8-2.8) and overall survival (OS; HR 2.4; 95% CI 1.9-3.1), with a small or no early decline in ctDNA levels with treatment being associated with a shorter PFS (HR 3.0; 95% CI 2.2-4.2) and OS (HR 2.8; 95% CI 2.1-3.9). Clonal evolution and lead-time results were inconsistent, with most studies having a high bias risk in ≥1 domain.
Study details: Findings are from a meta-analysis of 71 studies that included 6930 patients with mCRC.
Disclosures: The study was supported by the Danish Cancer Society. The authors declared no conflicts of interest.
Source: Callesen LB et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Br J Cancer. 2022 (Apr 19). Doi: 10.1038/s41416-022-01816-4
Key clinical point: Circulating tumor DNA (ctDNA) has substantiated its role as a strong prognostic biomarker in patients with metastatic colorectal cancer (mCRC). However, uncovering its true clinical value for these patients calls for prospective clinical trials with standardized methodologies.
Major finding: High baseline ctDNA levels were associated with a shorter progression-free survival (PFS; hazard ratio [HR] 2.2; 95% CI 1.8-2.8) and overall survival (OS; HR 2.4; 95% CI 1.9-3.1), with a small or no early decline in ctDNA levels with treatment being associated with a shorter PFS (HR 3.0; 95% CI 2.2-4.2) and OS (HR 2.8; 95% CI 2.1-3.9). Clonal evolution and lead-time results were inconsistent, with most studies having a high bias risk in ≥1 domain.
Study details: Findings are from a meta-analysis of 71 studies that included 6930 patients with mCRC.
Disclosures: The study was supported by the Danish Cancer Society. The authors declared no conflicts of interest.
Source: Callesen LB et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Br J Cancer. 2022 (Apr 19). Doi: 10.1038/s41416-022-01816-4
Key clinical point: Circulating tumor DNA (ctDNA) has substantiated its role as a strong prognostic biomarker in patients with metastatic colorectal cancer (mCRC). However, uncovering its true clinical value for these patients calls for prospective clinical trials with standardized methodologies.
Major finding: High baseline ctDNA levels were associated with a shorter progression-free survival (PFS; hazard ratio [HR] 2.2; 95% CI 1.8-2.8) and overall survival (OS; HR 2.4; 95% CI 1.9-3.1), with a small or no early decline in ctDNA levels with treatment being associated with a shorter PFS (HR 3.0; 95% CI 2.2-4.2) and OS (HR 2.8; 95% CI 2.1-3.9). Clonal evolution and lead-time results were inconsistent, with most studies having a high bias risk in ≥1 domain.
Study details: Findings are from a meta-analysis of 71 studies that included 6930 patients with mCRC.
Disclosures: The study was supported by the Danish Cancer Society. The authors declared no conflicts of interest.
Source: Callesen LB et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Br J Cancer. 2022 (Apr 19). Doi: 10.1038/s41416-022-01816-4
ctDNA: Strong prognostic biomarker but lacks true clinical utility in mCRC
Key clinical point: Circulating tumor DNA (ctDNA) has substantiated its role as a strong prognostic biomarker in patients with metastatic colorectal cancer (mCRC). However, uncovering its true clinical value for these patients calls for prospective clinical trials with standardized methodologies.
Major finding: High baseline ctDNA levels were associated with a shorter progression-free survival (PFS; hazard ratio [HR] 2.2; 95% CI 1.8-2.8) and overall survival (OS; HR 2.4; 95% CI 1.9-3.1), with a small or no early decline in ctDNA levels with treatment being associated with a shorter PFS (HR 3.0; 95% CI 2.2-4.2) and OS (HR 2.8; 95% CI 2.1-3.9). Clonal evolution and lead-time results were inconsistent, with most studies having a high bias risk in ≥1 domain.
Study details: Findings are from a meta-analysis of 71 studies that included 6930 patients with mCRC.
Disclosures: The study was supported by the Danish Cancer Society. The authors declared no conflicts of interest.
Source: Callesen LB et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Br J Cancer. 2022 (Apr 19). Doi: 10.1038/s41416-022-01816-4
Key clinical point: Circulating tumor DNA (ctDNA) has substantiated its role as a strong prognostic biomarker in patients with metastatic colorectal cancer (mCRC). However, uncovering its true clinical value for these patients calls for prospective clinical trials with standardized methodologies.
Major finding: High baseline ctDNA levels were associated with a shorter progression-free survival (PFS; hazard ratio [HR] 2.2; 95% CI 1.8-2.8) and overall survival (OS; HR 2.4; 95% CI 1.9-3.1), with a small or no early decline in ctDNA levels with treatment being associated with a shorter PFS (HR 3.0; 95% CI 2.2-4.2) and OS (HR 2.8; 95% CI 2.1-3.9). Clonal evolution and lead-time results were inconsistent, with most studies having a high bias risk in ≥1 domain.
Study details: Findings are from a meta-analysis of 71 studies that included 6930 patients with mCRC.
Disclosures: The study was supported by the Danish Cancer Society. The authors declared no conflicts of interest.
Source: Callesen LB et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Br J Cancer. 2022 (Apr 19). Doi: 10.1038/s41416-022-01816-4
Key clinical point: Circulating tumor DNA (ctDNA) has substantiated its role as a strong prognostic biomarker in patients with metastatic colorectal cancer (mCRC). However, uncovering its true clinical value for these patients calls for prospective clinical trials with standardized methodologies.
Major finding: High baseline ctDNA levels were associated with a shorter progression-free survival (PFS; hazard ratio [HR] 2.2; 95% CI 1.8-2.8) and overall survival (OS; HR 2.4; 95% CI 1.9-3.1), with a small or no early decline in ctDNA levels with treatment being associated with a shorter PFS (HR 3.0; 95% CI 2.2-4.2) and OS (HR 2.8; 95% CI 2.1-3.9). Clonal evolution and lead-time results were inconsistent, with most studies having a high bias risk in ≥1 domain.
Study details: Findings are from a meta-analysis of 71 studies that included 6930 patients with mCRC.
Disclosures: The study was supported by the Danish Cancer Society. The authors declared no conflicts of interest.
Source: Callesen LB et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Br J Cancer. 2022 (Apr 19). Doi: 10.1038/s41416-022-01816-4
KRAS p.G12C mutations may have prognostic implications in mCRC
Key clinical point: Patients with KRAS p.G12C-mutant metastatic colorectal cancer (mCRC) show poor treatment outcomes, which are numerically worse than those in patients without this mutation or with KRAS non-p.G12C mutations, thus highlighting the prognostic value of KRAS p.G12C mutations.
Major finding: After the first-line therapy, the KRAS p.G12C, KRAS non-p.G12C, and non-KRAS (RAS/BRAF wild-type) mutation cohorts and the overall mCRC cohort had a median overall survival (95% CI) of 16.1 (13.0-19.0), 18.3 (17.2-19.3), 23.4 (21.9-24.9), and 19.2 (18.5-19.8) months and a median real-world progression-free survival (95% CI) of 7.4 (6.3-9.5), 9.0 (8.2-9.7), 10.6 (9.8-11.6), and 9.2 (8.6-9.7) months, respectively.
Study details: This retrospective real-world study included 6477 adult patients with mCRC and genomic sequencing data, of which 238, 2947, and 2249 had KRAS p.G12C, KRAS non-p.G12C, and non-KRAS mutations, respectively.
Disclosures: The study was funded by Amgen Inc. Some authors reported serving as consultants or advisors for and receiving honoraria or research funds from various sources, including Amgen. The other authors are employees of Amgen.
Source: Fakih M et al. Real-world study of characteristics and treatment outcomes among patients with KRAS p.G12C-mutated or other KRAS mutated metastatic colorectal cancer. Oncologist. 2022 (Apr 26). Doi: 10.1093/oncolo/oyac077
Key clinical point: Patients with KRAS p.G12C-mutant metastatic colorectal cancer (mCRC) show poor treatment outcomes, which are numerically worse than those in patients without this mutation or with KRAS non-p.G12C mutations, thus highlighting the prognostic value of KRAS p.G12C mutations.
Major finding: After the first-line therapy, the KRAS p.G12C, KRAS non-p.G12C, and non-KRAS (RAS/BRAF wild-type) mutation cohorts and the overall mCRC cohort had a median overall survival (95% CI) of 16.1 (13.0-19.0), 18.3 (17.2-19.3), 23.4 (21.9-24.9), and 19.2 (18.5-19.8) months and a median real-world progression-free survival (95% CI) of 7.4 (6.3-9.5), 9.0 (8.2-9.7), 10.6 (9.8-11.6), and 9.2 (8.6-9.7) months, respectively.
Study details: This retrospective real-world study included 6477 adult patients with mCRC and genomic sequencing data, of which 238, 2947, and 2249 had KRAS p.G12C, KRAS non-p.G12C, and non-KRAS mutations, respectively.
Disclosures: The study was funded by Amgen Inc. Some authors reported serving as consultants or advisors for and receiving honoraria or research funds from various sources, including Amgen. The other authors are employees of Amgen.
Source: Fakih M et al. Real-world study of characteristics and treatment outcomes among patients with KRAS p.G12C-mutated or other KRAS mutated metastatic colorectal cancer. Oncologist. 2022 (Apr 26). Doi: 10.1093/oncolo/oyac077
Key clinical point: Patients with KRAS p.G12C-mutant metastatic colorectal cancer (mCRC) show poor treatment outcomes, which are numerically worse than those in patients without this mutation or with KRAS non-p.G12C mutations, thus highlighting the prognostic value of KRAS p.G12C mutations.
Major finding: After the first-line therapy, the KRAS p.G12C, KRAS non-p.G12C, and non-KRAS (RAS/BRAF wild-type) mutation cohorts and the overall mCRC cohort had a median overall survival (95% CI) of 16.1 (13.0-19.0), 18.3 (17.2-19.3), 23.4 (21.9-24.9), and 19.2 (18.5-19.8) months and a median real-world progression-free survival (95% CI) of 7.4 (6.3-9.5), 9.0 (8.2-9.7), 10.6 (9.8-11.6), and 9.2 (8.6-9.7) months, respectively.
Study details: This retrospective real-world study included 6477 adult patients with mCRC and genomic sequencing data, of which 238, 2947, and 2249 had KRAS p.G12C, KRAS non-p.G12C, and non-KRAS mutations, respectively.
Disclosures: The study was funded by Amgen Inc. Some authors reported serving as consultants or advisors for and receiving honoraria or research funds from various sources, including Amgen. The other authors are employees of Amgen.
Source: Fakih M et al. Real-world study of characteristics and treatment outcomes among patients with KRAS p.G12C-mutated or other KRAS mutated metastatic colorectal cancer. Oncologist. 2022 (Apr 26). Doi: 10.1093/oncolo/oyac077
Evidence supporting initiation of CRC screening before 50 years of age in women
Key clinical point: Colorectal cancer (CRC) screening before 50 years of age was associated with a reduced risk for CRC among US women, including CRC diagnosis before 55 years of age.
Major finding: Compared with no endoscopy, initiating endoscopy at the age of <45 (adjusted hazard ratio [aHR] 0.37; 95% CI 0.26-0.53), 45-49 (aHR 0.43; 95% CI 0.29-0.62), 50-54 (aHR 0.47; 95% CI 0.35-0.62), and ≥55 (aHR 0.46; 95% CI 0.30-0.69) years was associated with a significantly lower CRC risk, with initiating endoscopy before 50 years of age being associated with a decreased risk for CRC diagnosis before 55 years of age (<45 years: aHR 0.45, 95% CI 0.29-0.70; 45-49 years: aHR 0.43, 95% CI, 0.24-0.76).
Study details: This prospective cohort study enrolled 111,801 female health professionals aged 26-46 years with no history of cancer from the Nurses’ Health Study II.
Disclosures: The study was supported by the US National Institutes of Health (NIH). Some authors reported serving as consultants for or receiving research grants or personal fees from various organizations, including NIH.
Source: Ma W et al. Age at initiation of lower gastrointestinal endoscopy and colorectal cancer risk among US women. JAMA Oncol. 2022 (May 5). Doi: 10.1001/jamaoncol.2022.0883
Key clinical point: Colorectal cancer (CRC) screening before 50 years of age was associated with a reduced risk for CRC among US women, including CRC diagnosis before 55 years of age.
Major finding: Compared with no endoscopy, initiating endoscopy at the age of <45 (adjusted hazard ratio [aHR] 0.37; 95% CI 0.26-0.53), 45-49 (aHR 0.43; 95% CI 0.29-0.62), 50-54 (aHR 0.47; 95% CI 0.35-0.62), and ≥55 (aHR 0.46; 95% CI 0.30-0.69) years was associated with a significantly lower CRC risk, with initiating endoscopy before 50 years of age being associated with a decreased risk for CRC diagnosis before 55 years of age (<45 years: aHR 0.45, 95% CI 0.29-0.70; 45-49 years: aHR 0.43, 95% CI, 0.24-0.76).
Study details: This prospective cohort study enrolled 111,801 female health professionals aged 26-46 years with no history of cancer from the Nurses’ Health Study II.
Disclosures: The study was supported by the US National Institutes of Health (NIH). Some authors reported serving as consultants for or receiving research grants or personal fees from various organizations, including NIH.
Source: Ma W et al. Age at initiation of lower gastrointestinal endoscopy and colorectal cancer risk among US women. JAMA Oncol. 2022 (May 5). Doi: 10.1001/jamaoncol.2022.0883
Key clinical point: Colorectal cancer (CRC) screening before 50 years of age was associated with a reduced risk for CRC among US women, including CRC diagnosis before 55 years of age.
Major finding: Compared with no endoscopy, initiating endoscopy at the age of <45 (adjusted hazard ratio [aHR] 0.37; 95% CI 0.26-0.53), 45-49 (aHR 0.43; 95% CI 0.29-0.62), 50-54 (aHR 0.47; 95% CI 0.35-0.62), and ≥55 (aHR 0.46; 95% CI 0.30-0.69) years was associated with a significantly lower CRC risk, with initiating endoscopy before 50 years of age being associated with a decreased risk for CRC diagnosis before 55 years of age (<45 years: aHR 0.45, 95% CI 0.29-0.70; 45-49 years: aHR 0.43, 95% CI, 0.24-0.76).
Study details: This prospective cohort study enrolled 111,801 female health professionals aged 26-46 years with no history of cancer from the Nurses’ Health Study II.
Disclosures: The study was supported by the US National Institutes of Health (NIH). Some authors reported serving as consultants for or receiving research grants or personal fees from various organizations, including NIH.
Source: Ma W et al. Age at initiation of lower gastrointestinal endoscopy and colorectal cancer risk among US women. JAMA Oncol. 2022 (May 5). Doi: 10.1001/jamaoncol.2022.0883
Can proximal serrated polyp detection rate serve as an indicator for interval post-colonoscopy CRC?
Key clinical point: The proximal serrated polyp (PSP) detection rate (DR) of an endoscopist is inversely associated with the incidence of interval post-colonoscopy colorectal cancer (CRC) and should be universally adopted as a separate quality indicator alongside adenoma DR (ADR) to accelerate CRC prevention.
Major finding: With each percentage point increase in PSP DR, the adjusted interval post-colonoscopy CRC rate reduced by 7% (adjusted hazard ratio 0.93; P < .0001).
Study details: This was a population-based study including patients aged 55-76 years with a positive fecal immunochemical test who underwent a colonoscopy; the data of 277,555 colonoscopies were included in the PSP DR calculations.
Disclosures: The study did not receive any funding. A few authors declared serving as speakers or advisory board members or receiving consulting fees or research grants from various sources.
Source: van Toledo DEFWM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022 (May 9). Doi: 10.1016/S2468-1253(22)00090-5
Key clinical point: The proximal serrated polyp (PSP) detection rate (DR) of an endoscopist is inversely associated with the incidence of interval post-colonoscopy colorectal cancer (CRC) and should be universally adopted as a separate quality indicator alongside adenoma DR (ADR) to accelerate CRC prevention.
Major finding: With each percentage point increase in PSP DR, the adjusted interval post-colonoscopy CRC rate reduced by 7% (adjusted hazard ratio 0.93; P < .0001).
Study details: This was a population-based study including patients aged 55-76 years with a positive fecal immunochemical test who underwent a colonoscopy; the data of 277,555 colonoscopies were included in the PSP DR calculations.
Disclosures: The study did not receive any funding. A few authors declared serving as speakers or advisory board members or receiving consulting fees or research grants from various sources.
Source: van Toledo DEFWM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022 (May 9). Doi: 10.1016/S2468-1253(22)00090-5
Key clinical point: The proximal serrated polyp (PSP) detection rate (DR) of an endoscopist is inversely associated with the incidence of interval post-colonoscopy colorectal cancer (CRC) and should be universally adopted as a separate quality indicator alongside adenoma DR (ADR) to accelerate CRC prevention.
Major finding: With each percentage point increase in PSP DR, the adjusted interval post-colonoscopy CRC rate reduced by 7% (adjusted hazard ratio 0.93; P < .0001).
Study details: This was a population-based study including patients aged 55-76 years with a positive fecal immunochemical test who underwent a colonoscopy; the data of 277,555 colonoscopies were included in the PSP DR calculations.
Disclosures: The study did not receive any funding. A few authors declared serving as speakers or advisory board members or receiving consulting fees or research grants from various sources.
Source: van Toledo DEFWM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022 (May 9). Doi: 10.1016/S2468-1253(22)00090-5
Can proximal serrated polyp detection rate serve as an indicator for interval post-colonoscopy CRC?
Key clinical point: The proximal serrated polyp (PSP) detection rate (DR) of an endoscopist is inversely associated with the incidence of interval post-colonoscopy colorectal cancer (CRC) and should be universally adopted as a separate quality indicator alongside adenoma DR (ADR) to accelerate CRC prevention.
Major finding: With each percentage point increase in PSP DR, the adjusted interval post-colonoscopy CRC rate reduced by 7% (adjusted hazard ratio 0.93; P < .0001).
Study details: This was a population-based study including patients aged 55-76 years with a positive fecal immunochemical test who underwent a colonoscopy; the data of 277,555 colonoscopies were included in the PSP DR calculations.
Disclosures: The study did not receive any funding. A few authors declared serving as speakers or advisory board members or receiving consulting fees or research grants from various sources.
Source: van Toledo DEFWM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022 (May 9). Doi: 10.1016/S2468-1253(22)00090-5
Key clinical point: The proximal serrated polyp (PSP) detection rate (DR) of an endoscopist is inversely associated with the incidence of interval post-colonoscopy colorectal cancer (CRC) and should be universally adopted as a separate quality indicator alongside adenoma DR (ADR) to accelerate CRC prevention.
Major finding: With each percentage point increase in PSP DR, the adjusted interval post-colonoscopy CRC rate reduced by 7% (adjusted hazard ratio 0.93; P < .0001).
Study details: This was a population-based study including patients aged 55-76 years with a positive fecal immunochemical test who underwent a colonoscopy; the data of 277,555 colonoscopies were included in the PSP DR calculations.
Disclosures: The study did not receive any funding. A few authors declared serving as speakers or advisory board members or receiving consulting fees or research grants from various sources.
Source: van Toledo DEFWM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022 (May 9). Doi: 10.1016/S2468-1253(22)00090-5
Key clinical point: The proximal serrated polyp (PSP) detection rate (DR) of an endoscopist is inversely associated with the incidence of interval post-colonoscopy colorectal cancer (CRC) and should be universally adopted as a separate quality indicator alongside adenoma DR (ADR) to accelerate CRC prevention.
Major finding: With each percentage point increase in PSP DR, the adjusted interval post-colonoscopy CRC rate reduced by 7% (adjusted hazard ratio 0.93; P < .0001).
Study details: This was a population-based study including patients aged 55-76 years with a positive fecal immunochemical test who underwent a colonoscopy; the data of 277,555 colonoscopies were included in the PSP DR calculations.
Disclosures: The study did not receive any funding. A few authors declared serving as speakers or advisory board members or receiving consulting fees or research grants from various sources.
Source: van Toledo DEFWM et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022 (May 9). Doi: 10.1016/S2468-1253(22)00090-5