User login
SCAI issues guidelines for PFO management, makes case for expansion
The first-ever guidelines for interventional cardiologists using percutaneous patent foramen ovale closure recommend expanding the use of the procedure beyond the Food and Drug Administration–approved indication following PFO-associated ischemic stroke, adding clarification about the use of PFO with anticoagulation and hedging against abuse and overuse of the procedure, said the chair of the guideline writing committee.
“The most important things surrounding these guidelines are to help clinicians and policymakers – third-party payers – to address PFO in patient subsets that were not included in the large randomized clinical trials that led to FDA approval,” said writing group chair Clifford J. Kavinsky, MD, PhD, chief of structural and interventional cardiology at Rush University Medical Center, Chicago.
The Society for Cardiovascular Angiography & Interventions issued the guidelines at its annual scientific sessions meeting in Atlanta and published them simultaneously in the society’s journal.
The guidelines issue strong and conditional recommendations. The former means clinicians should order the intervention for most patients; the latter means decisionmaking is more nuanced and should consider contributing factors.
The guidelines clarify patient selection for PFO closure outside the “pretty narrow” indication the FDA approved, Dr. Kavinsky said, which is for PFO-associated ischemic stroke in patients aged 18-60 years.
“So what about patients who are older than 60? What about patients who had their stroke 10 years ago?” Dr. Kavinsky asked. “Those are issues that were unanswered in the randomized clinical trials.”
The guidelines also refine recommendations about anticoagulation in these patients, including its use after PFO closure in selected patients, Dr. Kavinsky noted. “It’s the opinion of the panel that although anticoagulants may be effective, because of issues of noncompliance, because of issues of interruption of therapy by physicians for a variety of reasons, including surgery or noncompliance, that it is preferable to do a PFO device closure to giving anticoagulant therapy.”
Many of the recommendations cover PFO closure alongside antiplatelet or anticoagulation therapy. Key conditional recommendations for patients who haven’t had a PFO-related stroke are:
- Avoiding its routine use in patients with chronic migraines, prior decompression illness (DCI), thrombophilia, atrial septal aneurysm, transient ischemic attack (TIA), or deep vein thrombosis (DVT).
- Considering PFO closure in patients with platypnea-orthodeoxia syndrome (POS) with no other discernible cause of hypoxia or systemic embolism in whom other embolic causes have been ruled out.
In patients who’ve had a PFO-related stroke, the guidelines strongly recommend PFO closure versus antiplatelet therapy alone, but conditionally, not in patients with atrial fibrillation who’ve had an ischemic stroke. They also conditionally suggest PFO closure rather than long-term antiplatelet therapy alone in PFO stroke patients aged 60 and older, as well as those with thrombophilia already on antiplatelet therapy but not anticoagulation. However, the guidelines make no recommendation on PFO closure based on how much time has passed since the previous stroke.
“Furthermore,” Dr. Kavinsky said, “in patients who require lifelong anticoagulation because of recurrent DVT or recurrent pulmonary emboli or thrombopenia, if they’ve had a PFO-mediated stroke, then it’s our opinion that they should have their PFO closed in addition to taking lifelong anticoagulation because of the same issues of noncompliance and interruption of therapy.” Those are conditional recommendations.
The guideline also checks a box in the FDA labeling that mandated agreement between cardiology and neurology in patient selection. The American Academy of Neurology (AAN) issued its own guideline in 2020 for patients with stroke and PFO. In Europe, the European Society of Cardiology issued two position papers on expanded applications of PFO closure.
The recommendations on when PFO closure shouldn’t be done are noteworthy, Dr. Kavinsky said. “PFOs are present in 25% of the adult population, so the number of patients with PFO is huge and the indication for the FDA is really narrow: to reduce the risk of recurrent stroke in patients with PFO-mediated stroke. So, there’s the tremendous potential for abuse out there, of excessive procedures, of doing unnecessary procedures.”
The guidelines are a follow-up to the operator institutional requirements document SCAI issued in 2019 that set requirements for hospital offering and physicians performing PFO closure, Dr. Kavinsky added.
In an editorial accompanying the published guideline, Robert J. Sommer, MD, and Jamil A. Aboulhosn, MD, wrote that they support the recommendations “which help spotlight and clarify the growing list of potential indications for PFO closure.” They noted that the guidelines panel’s “strong” recommendations were for indications validated by randomized trials and that “conditional” recommendations were based on panelists’ experience and observational data.
“It is critical to recognize that most of these guidelines represent consensus opinion only,” wrote Dr. Sommer, who specializes in adult congenital and pediatric cardiology at Columbia University Irving Medical Center, New York, and Dr. Aboulhosn, an interventional cardiologist at Ronald Reagan University of California, Los Angeles, Medical Center. They emphasized the guidelines’ “heavy emphasis” on shared decisionmaking with patients.
Dr. Kavinsky is a principal investigator for Edwards Lifesciences, W.L. Gore and Associates, Medtronic, and Abbott. Dr. Sommer is a principal investigator and investigator in studies sponsored by W.L. Gore & Associates. Dr. Aboulhosn is a consultant to Abbott Medical.
The first-ever guidelines for interventional cardiologists using percutaneous patent foramen ovale closure recommend expanding the use of the procedure beyond the Food and Drug Administration–approved indication following PFO-associated ischemic stroke, adding clarification about the use of PFO with anticoagulation and hedging against abuse and overuse of the procedure, said the chair of the guideline writing committee.
“The most important things surrounding these guidelines are to help clinicians and policymakers – third-party payers – to address PFO in patient subsets that were not included in the large randomized clinical trials that led to FDA approval,” said writing group chair Clifford J. Kavinsky, MD, PhD, chief of structural and interventional cardiology at Rush University Medical Center, Chicago.
The Society for Cardiovascular Angiography & Interventions issued the guidelines at its annual scientific sessions meeting in Atlanta and published them simultaneously in the society’s journal.
The guidelines issue strong and conditional recommendations. The former means clinicians should order the intervention for most patients; the latter means decisionmaking is more nuanced and should consider contributing factors.
The guidelines clarify patient selection for PFO closure outside the “pretty narrow” indication the FDA approved, Dr. Kavinsky said, which is for PFO-associated ischemic stroke in patients aged 18-60 years.
“So what about patients who are older than 60? What about patients who had their stroke 10 years ago?” Dr. Kavinsky asked. “Those are issues that were unanswered in the randomized clinical trials.”
The guidelines also refine recommendations about anticoagulation in these patients, including its use after PFO closure in selected patients, Dr. Kavinsky noted. “It’s the opinion of the panel that although anticoagulants may be effective, because of issues of noncompliance, because of issues of interruption of therapy by physicians for a variety of reasons, including surgery or noncompliance, that it is preferable to do a PFO device closure to giving anticoagulant therapy.”
Many of the recommendations cover PFO closure alongside antiplatelet or anticoagulation therapy. Key conditional recommendations for patients who haven’t had a PFO-related stroke are:
- Avoiding its routine use in patients with chronic migraines, prior decompression illness (DCI), thrombophilia, atrial septal aneurysm, transient ischemic attack (TIA), or deep vein thrombosis (DVT).
- Considering PFO closure in patients with platypnea-orthodeoxia syndrome (POS) with no other discernible cause of hypoxia or systemic embolism in whom other embolic causes have been ruled out.
In patients who’ve had a PFO-related stroke, the guidelines strongly recommend PFO closure versus antiplatelet therapy alone, but conditionally, not in patients with atrial fibrillation who’ve had an ischemic stroke. They also conditionally suggest PFO closure rather than long-term antiplatelet therapy alone in PFO stroke patients aged 60 and older, as well as those with thrombophilia already on antiplatelet therapy but not anticoagulation. However, the guidelines make no recommendation on PFO closure based on how much time has passed since the previous stroke.
“Furthermore,” Dr. Kavinsky said, “in patients who require lifelong anticoagulation because of recurrent DVT or recurrent pulmonary emboli or thrombopenia, if they’ve had a PFO-mediated stroke, then it’s our opinion that they should have their PFO closed in addition to taking lifelong anticoagulation because of the same issues of noncompliance and interruption of therapy.” Those are conditional recommendations.
The guideline also checks a box in the FDA labeling that mandated agreement between cardiology and neurology in patient selection. The American Academy of Neurology (AAN) issued its own guideline in 2020 for patients with stroke and PFO. In Europe, the European Society of Cardiology issued two position papers on expanded applications of PFO closure.
The recommendations on when PFO closure shouldn’t be done are noteworthy, Dr. Kavinsky said. “PFOs are present in 25% of the adult population, so the number of patients with PFO is huge and the indication for the FDA is really narrow: to reduce the risk of recurrent stroke in patients with PFO-mediated stroke. So, there’s the tremendous potential for abuse out there, of excessive procedures, of doing unnecessary procedures.”
The guidelines are a follow-up to the operator institutional requirements document SCAI issued in 2019 that set requirements for hospital offering and physicians performing PFO closure, Dr. Kavinsky added.
In an editorial accompanying the published guideline, Robert J. Sommer, MD, and Jamil A. Aboulhosn, MD, wrote that they support the recommendations “which help spotlight and clarify the growing list of potential indications for PFO closure.” They noted that the guidelines panel’s “strong” recommendations were for indications validated by randomized trials and that “conditional” recommendations were based on panelists’ experience and observational data.
“It is critical to recognize that most of these guidelines represent consensus opinion only,” wrote Dr. Sommer, who specializes in adult congenital and pediatric cardiology at Columbia University Irving Medical Center, New York, and Dr. Aboulhosn, an interventional cardiologist at Ronald Reagan University of California, Los Angeles, Medical Center. They emphasized the guidelines’ “heavy emphasis” on shared decisionmaking with patients.
Dr. Kavinsky is a principal investigator for Edwards Lifesciences, W.L. Gore and Associates, Medtronic, and Abbott. Dr. Sommer is a principal investigator and investigator in studies sponsored by W.L. Gore & Associates. Dr. Aboulhosn is a consultant to Abbott Medical.
The first-ever guidelines for interventional cardiologists using percutaneous patent foramen ovale closure recommend expanding the use of the procedure beyond the Food and Drug Administration–approved indication following PFO-associated ischemic stroke, adding clarification about the use of PFO with anticoagulation and hedging against abuse and overuse of the procedure, said the chair of the guideline writing committee.
“The most important things surrounding these guidelines are to help clinicians and policymakers – third-party payers – to address PFO in patient subsets that were not included in the large randomized clinical trials that led to FDA approval,” said writing group chair Clifford J. Kavinsky, MD, PhD, chief of structural and interventional cardiology at Rush University Medical Center, Chicago.
The Society for Cardiovascular Angiography & Interventions issued the guidelines at its annual scientific sessions meeting in Atlanta and published them simultaneously in the society’s journal.
The guidelines issue strong and conditional recommendations. The former means clinicians should order the intervention for most patients; the latter means decisionmaking is more nuanced and should consider contributing factors.
The guidelines clarify patient selection for PFO closure outside the “pretty narrow” indication the FDA approved, Dr. Kavinsky said, which is for PFO-associated ischemic stroke in patients aged 18-60 years.
“So what about patients who are older than 60? What about patients who had their stroke 10 years ago?” Dr. Kavinsky asked. “Those are issues that were unanswered in the randomized clinical trials.”
The guidelines also refine recommendations about anticoagulation in these patients, including its use after PFO closure in selected patients, Dr. Kavinsky noted. “It’s the opinion of the panel that although anticoagulants may be effective, because of issues of noncompliance, because of issues of interruption of therapy by physicians for a variety of reasons, including surgery or noncompliance, that it is preferable to do a PFO device closure to giving anticoagulant therapy.”
Many of the recommendations cover PFO closure alongside antiplatelet or anticoagulation therapy. Key conditional recommendations for patients who haven’t had a PFO-related stroke are:
- Avoiding its routine use in patients with chronic migraines, prior decompression illness (DCI), thrombophilia, atrial septal aneurysm, transient ischemic attack (TIA), or deep vein thrombosis (DVT).
- Considering PFO closure in patients with platypnea-orthodeoxia syndrome (POS) with no other discernible cause of hypoxia or systemic embolism in whom other embolic causes have been ruled out.
In patients who’ve had a PFO-related stroke, the guidelines strongly recommend PFO closure versus antiplatelet therapy alone, but conditionally, not in patients with atrial fibrillation who’ve had an ischemic stroke. They also conditionally suggest PFO closure rather than long-term antiplatelet therapy alone in PFO stroke patients aged 60 and older, as well as those with thrombophilia already on antiplatelet therapy but not anticoagulation. However, the guidelines make no recommendation on PFO closure based on how much time has passed since the previous stroke.
“Furthermore,” Dr. Kavinsky said, “in patients who require lifelong anticoagulation because of recurrent DVT or recurrent pulmonary emboli or thrombopenia, if they’ve had a PFO-mediated stroke, then it’s our opinion that they should have their PFO closed in addition to taking lifelong anticoagulation because of the same issues of noncompliance and interruption of therapy.” Those are conditional recommendations.
The guideline also checks a box in the FDA labeling that mandated agreement between cardiology and neurology in patient selection. The American Academy of Neurology (AAN) issued its own guideline in 2020 for patients with stroke and PFO. In Europe, the European Society of Cardiology issued two position papers on expanded applications of PFO closure.
The recommendations on when PFO closure shouldn’t be done are noteworthy, Dr. Kavinsky said. “PFOs are present in 25% of the adult population, so the number of patients with PFO is huge and the indication for the FDA is really narrow: to reduce the risk of recurrent stroke in patients with PFO-mediated stroke. So, there’s the tremendous potential for abuse out there, of excessive procedures, of doing unnecessary procedures.”
The guidelines are a follow-up to the operator institutional requirements document SCAI issued in 2019 that set requirements for hospital offering and physicians performing PFO closure, Dr. Kavinsky added.
In an editorial accompanying the published guideline, Robert J. Sommer, MD, and Jamil A. Aboulhosn, MD, wrote that they support the recommendations “which help spotlight and clarify the growing list of potential indications for PFO closure.” They noted that the guidelines panel’s “strong” recommendations were for indications validated by randomized trials and that “conditional” recommendations were based on panelists’ experience and observational data.
“It is critical to recognize that most of these guidelines represent consensus opinion only,” wrote Dr. Sommer, who specializes in adult congenital and pediatric cardiology at Columbia University Irving Medical Center, New York, and Dr. Aboulhosn, an interventional cardiologist at Ronald Reagan University of California, Los Angeles, Medical Center. They emphasized the guidelines’ “heavy emphasis” on shared decisionmaking with patients.
Dr. Kavinsky is a principal investigator for Edwards Lifesciences, W.L. Gore and Associates, Medtronic, and Abbott. Dr. Sommer is a principal investigator and investigator in studies sponsored by W.L. Gore & Associates. Dr. Aboulhosn is a consultant to Abbott Medical.
FROM SCAI 2022
Advances In Neurologic Care
- Pharmacist Impact on Access to Care in an Epilepsy Clinic
- MRI Protocols for Veterans With Multiple Sclerosis
- Neuroimaging in the Era of Artificial Intelligence
- Autonomic Dysfunction in CADASIL Syndrome
- Pharmacist Impact on Access to Care in an Epilepsy Clinic
- MRI Protocols for Veterans With Multiple Sclerosis
- Neuroimaging in the Era of Artificial Intelligence
- Autonomic Dysfunction in CADASIL Syndrome
- Pharmacist Impact on Access to Care in an Epilepsy Clinic
- MRI Protocols for Veterans With Multiple Sclerosis
- Neuroimaging in the Era of Artificial Intelligence
- Autonomic Dysfunction in CADASIL Syndrome
Could new therapy for food ‘cues’ improve weight loss?
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Updated AHA/ASA guideline changes care for spontaneous intracerebral hemorrhage
Many strategies widely considered “standard care” for managing spontaneous intracerebral hemorrhage (ICH) are not as effective as previously thought and are no longer recommended in updated guidelines from the American Heart Association/American Stroke Association (ASA).
Compression stockings, antiseizure medication, and steroid treatment are among the treatments with uncertain effectiveness, the writing group says.
The 2022 Guideline for the Management of Patients With Spontaneous ICH was published online in Stroke. The 80-page document contains major changes and refinements to the 2015 guideline on ICH management.
“Advances have been made in an array of fields related to ICH, including the organization of regional health care systems, reversal of the negative effects of blood thinners, minimally invasive surgical procedures, and the underlying disease in small blood vessels,” Steven M. Greenberg, MD, PhD, chair of the guideline writing group with Harvard Medical School and Massachusetts General Hospital, both in Boston, said in a news release.
“We’ve updated sections across the board. There’s probably no area that went untouched with some tweaking and new evidence added that led to some changes in level of evidence or strength of a recommendation,” Dr. Greenberg added in an interview with this news organization.
“Each section comes with knowledge gaps, and it wasn’t hard to come up with knowledge gaps in every section,” Dr. Greenberg acknowledged.
Time-honored treatments no more?
Among the key updates are changes to some “time-honored” treatments that continue to be used with some “regularity” for patients with ICH, yet appear to confer either no benefit or harm, Dr. Greenberg said.
For example, for emergency or critical care treatment of ICH, prophylactic corticosteroids or continuous hyperosmolar therapy is not recommended, because it appears to have no benefit for outcome, while use of platelet transfusions outside the setting of emergency surgery or severe thrombocytopenia appears to worsen outcome, the authors say.
Use of graduated knee- or thigh-high compression stockings alone is not an effective prophylactic therapy for prevention of deep vein thrombosis (DVT). Instead, intermittent pneumatic compression (IPC) starting on the day of diagnosis is now recommended for DVT prophylaxis.
“This is an area where we still have a lot of exploration to do. It is unclear whether even specialized compression devices reduce the risks of deep vein thrombosis or improve the overall health of people with a brain bleed,” Dr. Greenberg said in the release.
The new guidance advises against use of antiseizure or antidepressant medications for ICH patients in whom there is no evidence of seizures or depression.
In clinical trials, antiseizure medication did not contribute to improvements in functionality or long-term seizure control, and the use of antidepressants increased the chance of bone fractures, the authors say.
The guideline also provides updated recommendations for acute reversal of anticoagulation after ICH. It highlights the use of protein complex concentrate for reversal of vitamin K antagonists, such as warfarin; idarucizumab for reversal of the thrombin inhibitor dabigatran; and andexanet alfa for reversal of factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
For acute blood pressure lowering after mild to moderate ICH, treatment regimens that limit blood pressure variability and achieve smooth, sustained blood pressure control appear to reduce hematoma expansion and yield better functional outcome, the guideline says.
It also notes that minimally invasive approaches for hematoma evacuation, compared with medical management alone‚ have been shown to reduce mortality.
For patients with cerebellar hemorrhage, indications for immediate surgical evacuation with or without an external ventricular drain to reduce mortality now include larger volume (> 15 mL) in addition to previously recommended indications of neurologic deterioration, brainstem compression, and hydrocephalus, the authors note.
However, a “major knowledge gap is whether we can improve functional outcome with hematoma evacuation,” Dr. Greenberg said.
Multidisciplinary care
For rehabilitation after ICH, the guideline reinforces the importance of having a multidisciplinary team to develop a comprehensive plan for recovery.
Starting rehabilitation activities such as stretching and functional task training may be considered 24 to 48 hours following mild or moderate ICH. However, early aggressive mobilization within the first 24 hours has been linked to an increased risk of death within 14 days after an ICH, the guideline says.
Knowledge gaps include how soon it’s safe to return to work, drive, and participate in other social engagements. Recommendations on sexual activity and exercise levels that are safe after a stroke are also needed.
“People need additional help with these lifestyle changes, whether it’s moving around more, curbing their alcohol use, or eating healthier foods. This all happens after they leave the hospital, and we need to be sure we are empowering families with the information they may need to be properly supportive,” Dr. Greenberg says in the release.
The guideline points to the patient’s home caregiver as a “key and sometimes overlooked” member of the care team. It recommends psychosocial education, practical support, and training for the caregiver to improve the patient’s balance, activity level, and overall quality of life.
Opportunity for prevention?
The guideline also suggests there may be an opportunity to prevent ICH in some people through neuroimaging markers.
While neuroimaging is not routinely performed as a part of risk stratification for primary ICH risk, damage to small blood vessels that is associated with ICH may be evident on MRI that could signal future ICH risk, the guideline says.
“We added to the guidelines for the first time a section on mostly imaging markers of risk for having a first-ever hemorrhage,” Dr. Greenberg said in an interview.
“We don’t make any recommendations as to how to act on these markers because there is a knowledge gap. The hope is that we’ll see growth in our ability to predict first-ever hemorrhage and be able to do things to prevent first-ever hemorrhage,” he said.
“We believe the wide range of knowledge set forth in the new guideline will translate into meaningful improvements in ICH care,” Dr. Greenberg adds in the release.
The updated guideline has been endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons, the Society of Vascular and Interventional Neurology, and the Neurocritical Care Society. The American Academy of Neurology has affirmed the value of this statement as an educational tool for neurologists.
This research had no commercial funding. Dr. Greenberg has disclosed no relevant financial relationships. A complete list of disclosures for the guideline group is available with the original article.
A version of this article first appeared on Medscape.com.
Many strategies widely considered “standard care” for managing spontaneous intracerebral hemorrhage (ICH) are not as effective as previously thought and are no longer recommended in updated guidelines from the American Heart Association/American Stroke Association (ASA).
Compression stockings, antiseizure medication, and steroid treatment are among the treatments with uncertain effectiveness, the writing group says.
The 2022 Guideline for the Management of Patients With Spontaneous ICH was published online in Stroke. The 80-page document contains major changes and refinements to the 2015 guideline on ICH management.
“Advances have been made in an array of fields related to ICH, including the organization of regional health care systems, reversal of the negative effects of blood thinners, minimally invasive surgical procedures, and the underlying disease in small blood vessels,” Steven M. Greenberg, MD, PhD, chair of the guideline writing group with Harvard Medical School and Massachusetts General Hospital, both in Boston, said in a news release.
“We’ve updated sections across the board. There’s probably no area that went untouched with some tweaking and new evidence added that led to some changes in level of evidence or strength of a recommendation,” Dr. Greenberg added in an interview with this news organization.
“Each section comes with knowledge gaps, and it wasn’t hard to come up with knowledge gaps in every section,” Dr. Greenberg acknowledged.
Time-honored treatments no more?
Among the key updates are changes to some “time-honored” treatments that continue to be used with some “regularity” for patients with ICH, yet appear to confer either no benefit or harm, Dr. Greenberg said.
For example, for emergency or critical care treatment of ICH, prophylactic corticosteroids or continuous hyperosmolar therapy is not recommended, because it appears to have no benefit for outcome, while use of platelet transfusions outside the setting of emergency surgery or severe thrombocytopenia appears to worsen outcome, the authors say.
Use of graduated knee- or thigh-high compression stockings alone is not an effective prophylactic therapy for prevention of deep vein thrombosis (DVT). Instead, intermittent pneumatic compression (IPC) starting on the day of diagnosis is now recommended for DVT prophylaxis.
“This is an area where we still have a lot of exploration to do. It is unclear whether even specialized compression devices reduce the risks of deep vein thrombosis or improve the overall health of people with a brain bleed,” Dr. Greenberg said in the release.
The new guidance advises against use of antiseizure or antidepressant medications for ICH patients in whom there is no evidence of seizures or depression.
In clinical trials, antiseizure medication did not contribute to improvements in functionality or long-term seizure control, and the use of antidepressants increased the chance of bone fractures, the authors say.
The guideline also provides updated recommendations for acute reversal of anticoagulation after ICH. It highlights the use of protein complex concentrate for reversal of vitamin K antagonists, such as warfarin; idarucizumab for reversal of the thrombin inhibitor dabigatran; and andexanet alfa for reversal of factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
For acute blood pressure lowering after mild to moderate ICH, treatment regimens that limit blood pressure variability and achieve smooth, sustained blood pressure control appear to reduce hematoma expansion and yield better functional outcome, the guideline says.
It also notes that minimally invasive approaches for hematoma evacuation, compared with medical management alone‚ have been shown to reduce mortality.
For patients with cerebellar hemorrhage, indications for immediate surgical evacuation with or without an external ventricular drain to reduce mortality now include larger volume (> 15 mL) in addition to previously recommended indications of neurologic deterioration, brainstem compression, and hydrocephalus, the authors note.
However, a “major knowledge gap is whether we can improve functional outcome with hematoma evacuation,” Dr. Greenberg said.
Multidisciplinary care
For rehabilitation after ICH, the guideline reinforces the importance of having a multidisciplinary team to develop a comprehensive plan for recovery.
Starting rehabilitation activities such as stretching and functional task training may be considered 24 to 48 hours following mild or moderate ICH. However, early aggressive mobilization within the first 24 hours has been linked to an increased risk of death within 14 days after an ICH, the guideline says.
Knowledge gaps include how soon it’s safe to return to work, drive, and participate in other social engagements. Recommendations on sexual activity and exercise levels that are safe after a stroke are also needed.
“People need additional help with these lifestyle changes, whether it’s moving around more, curbing their alcohol use, or eating healthier foods. This all happens after they leave the hospital, and we need to be sure we are empowering families with the information they may need to be properly supportive,” Dr. Greenberg says in the release.
The guideline points to the patient’s home caregiver as a “key and sometimes overlooked” member of the care team. It recommends psychosocial education, practical support, and training for the caregiver to improve the patient’s balance, activity level, and overall quality of life.
Opportunity for prevention?
The guideline also suggests there may be an opportunity to prevent ICH in some people through neuroimaging markers.
While neuroimaging is not routinely performed as a part of risk stratification for primary ICH risk, damage to small blood vessels that is associated with ICH may be evident on MRI that could signal future ICH risk, the guideline says.
“We added to the guidelines for the first time a section on mostly imaging markers of risk for having a first-ever hemorrhage,” Dr. Greenberg said in an interview.
“We don’t make any recommendations as to how to act on these markers because there is a knowledge gap. The hope is that we’ll see growth in our ability to predict first-ever hemorrhage and be able to do things to prevent first-ever hemorrhage,” he said.
“We believe the wide range of knowledge set forth in the new guideline will translate into meaningful improvements in ICH care,” Dr. Greenberg adds in the release.
The updated guideline has been endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons, the Society of Vascular and Interventional Neurology, and the Neurocritical Care Society. The American Academy of Neurology has affirmed the value of this statement as an educational tool for neurologists.
This research had no commercial funding. Dr. Greenberg has disclosed no relevant financial relationships. A complete list of disclosures for the guideline group is available with the original article.
A version of this article first appeared on Medscape.com.
Many strategies widely considered “standard care” for managing spontaneous intracerebral hemorrhage (ICH) are not as effective as previously thought and are no longer recommended in updated guidelines from the American Heart Association/American Stroke Association (ASA).
Compression stockings, antiseizure medication, and steroid treatment are among the treatments with uncertain effectiveness, the writing group says.
The 2022 Guideline for the Management of Patients With Spontaneous ICH was published online in Stroke. The 80-page document contains major changes and refinements to the 2015 guideline on ICH management.
“Advances have been made in an array of fields related to ICH, including the organization of regional health care systems, reversal of the negative effects of blood thinners, minimally invasive surgical procedures, and the underlying disease in small blood vessels,” Steven M. Greenberg, MD, PhD, chair of the guideline writing group with Harvard Medical School and Massachusetts General Hospital, both in Boston, said in a news release.
“We’ve updated sections across the board. There’s probably no area that went untouched with some tweaking and new evidence added that led to some changes in level of evidence or strength of a recommendation,” Dr. Greenberg added in an interview with this news organization.
“Each section comes with knowledge gaps, and it wasn’t hard to come up with knowledge gaps in every section,” Dr. Greenberg acknowledged.
Time-honored treatments no more?
Among the key updates are changes to some “time-honored” treatments that continue to be used with some “regularity” for patients with ICH, yet appear to confer either no benefit or harm, Dr. Greenberg said.
For example, for emergency or critical care treatment of ICH, prophylactic corticosteroids or continuous hyperosmolar therapy is not recommended, because it appears to have no benefit for outcome, while use of platelet transfusions outside the setting of emergency surgery or severe thrombocytopenia appears to worsen outcome, the authors say.
Use of graduated knee- or thigh-high compression stockings alone is not an effective prophylactic therapy for prevention of deep vein thrombosis (DVT). Instead, intermittent pneumatic compression (IPC) starting on the day of diagnosis is now recommended for DVT prophylaxis.
“This is an area where we still have a lot of exploration to do. It is unclear whether even specialized compression devices reduce the risks of deep vein thrombosis or improve the overall health of people with a brain bleed,” Dr. Greenberg said in the release.
The new guidance advises against use of antiseizure or antidepressant medications for ICH patients in whom there is no evidence of seizures or depression.
In clinical trials, antiseizure medication did not contribute to improvements in functionality or long-term seizure control, and the use of antidepressants increased the chance of bone fractures, the authors say.
The guideline also provides updated recommendations for acute reversal of anticoagulation after ICH. It highlights the use of protein complex concentrate for reversal of vitamin K antagonists, such as warfarin; idarucizumab for reversal of the thrombin inhibitor dabigatran; and andexanet alfa for reversal of factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
For acute blood pressure lowering after mild to moderate ICH, treatment regimens that limit blood pressure variability and achieve smooth, sustained blood pressure control appear to reduce hematoma expansion and yield better functional outcome, the guideline says.
It also notes that minimally invasive approaches for hematoma evacuation, compared with medical management alone‚ have been shown to reduce mortality.
For patients with cerebellar hemorrhage, indications for immediate surgical evacuation with or without an external ventricular drain to reduce mortality now include larger volume (> 15 mL) in addition to previously recommended indications of neurologic deterioration, brainstem compression, and hydrocephalus, the authors note.
However, a “major knowledge gap is whether we can improve functional outcome with hematoma evacuation,” Dr. Greenberg said.
Multidisciplinary care
For rehabilitation after ICH, the guideline reinforces the importance of having a multidisciplinary team to develop a comprehensive plan for recovery.
Starting rehabilitation activities such as stretching and functional task training may be considered 24 to 48 hours following mild or moderate ICH. However, early aggressive mobilization within the first 24 hours has been linked to an increased risk of death within 14 days after an ICH, the guideline says.
Knowledge gaps include how soon it’s safe to return to work, drive, and participate in other social engagements. Recommendations on sexual activity and exercise levels that are safe after a stroke are also needed.
“People need additional help with these lifestyle changes, whether it’s moving around more, curbing their alcohol use, or eating healthier foods. This all happens after they leave the hospital, and we need to be sure we are empowering families with the information they may need to be properly supportive,” Dr. Greenberg says in the release.
The guideline points to the patient’s home caregiver as a “key and sometimes overlooked” member of the care team. It recommends psychosocial education, practical support, and training for the caregiver to improve the patient’s balance, activity level, and overall quality of life.
Opportunity for prevention?
The guideline also suggests there may be an opportunity to prevent ICH in some people through neuroimaging markers.
While neuroimaging is not routinely performed as a part of risk stratification for primary ICH risk, damage to small blood vessels that is associated with ICH may be evident on MRI that could signal future ICH risk, the guideline says.
“We added to the guidelines for the first time a section on mostly imaging markers of risk for having a first-ever hemorrhage,” Dr. Greenberg said in an interview.
“We don’t make any recommendations as to how to act on these markers because there is a knowledge gap. The hope is that we’ll see growth in our ability to predict first-ever hemorrhage and be able to do things to prevent first-ever hemorrhage,” he said.
“We believe the wide range of knowledge set forth in the new guideline will translate into meaningful improvements in ICH care,” Dr. Greenberg adds in the release.
The updated guideline has been endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons, the Society of Vascular and Interventional Neurology, and the Neurocritical Care Society. The American Academy of Neurology has affirmed the value of this statement as an educational tool for neurologists.
This research had no commercial funding. Dr. Greenberg has disclosed no relevant financial relationships. A complete list of disclosures for the guideline group is available with the original article.
A version of this article first appeared on Medscape.com.
Cannabis vaping continues its rise in teens
More teenagers in the United States reported cannabis use with vaping in 2019, compared with 2017, while cannabis use without vaping declined, based on annual survey data from more than 50,000 teens.
“With vaping prevalence rising so quickly among teens, getting a clearer picture of how cannabis use is shifting helps inform prevention and cessation efforts,” corresponding author Noah T. Kreski, MPH, of Columbia University, New York, said in an interview.
“In just 2 years, the most common cannabis use pattern changed from ‘occasional use without vaping’ to ‘frequent use with vaping,’ said Mx. Kreski, who uses the honorific Mx. and the pronouns they/them. “Knowing that, as well as the high overlap of cannabis vaping with nicotine use and binge drinking, adds to the urgency of reducing adolescent vaping.”
To quantify the trends in cannabis vaping, the researchers reviewed data from Monitoring the Future, an annual survey of high school students across the United States. The study population included 51,052 individuals; approximately 49% were male and 49% were non-Hispanic White. The researchers examined frequency of cannabis use, trends across demographic groups, and concurrent use of cannabis and other substances such as alcohol and tobacco. The findings were published in the journal Addiction.
Frequent cannabis use was defined as six or more times in the past 30 days; occasional use was defined as one to five times in the past 30 days.
Frequent cannabis use with vaping increased from 2.1% in 2017 to 5.4% in 2019. Occasional cannabis use with vaping also increased, though less dramatically, from less than 2% in 2017 to approximately 3.5% in 2019.
By contrast, both frequent and occasional cannabis use without vaping declined from 2017 to 2019 (from 3.8% to 2.1% and from 6.9% to 4.4%, respectively).
Overall, the prevalence of any level of cannabis use increased from 13.9% in 2017 to 15.4% in 2019. Both males and females showed a similar increase in reported frequent cannabis use with vaping of approximately 3%.
The results document that vaping cannabis has become more common than smoking alone among U.S. teens across almost all demographic groups, and across sex, race, urbanicity, and level of parent education; however, the increased was especially marked among Hispanic/Latinx teens and those of lower socioeconomic status, the researchers wrote.
The researchers also examined the associations between cannabis use with and without vaping and concurrent nicotine and alcohol use. Overall, the strongest association was between smoking or vaping nicotine and vaping cannabis; teens who smoked or vaped nicotine were 42 times more likely than nonnicotine users to report vaping cannabis in the past 30 days (adjusted odds ratio, 42.28). In addition, more occasions of binge drinking were more strongly associated with cannabis use with vaping (up to 10 times more likely), compared with cannabis use without vaping, (aORs, 4.48-10.09).
The study findings were limited by several factors, including the lack of questions on tetrahydrocannabinol (THC) or cannabidiol content of the cannabis products used, although evidence suggests that the potency of cannabis products in the United States is increasing, the researchers noted. Other limitations included the cross-sectional design, which prevents making associations about causality, and lack of data on the quantity of cannabis used; only data on frequency of use were recorded.
However, the results reflect a rise in cannabis use with vaping among teens in the United States, along with an increased risk of tobacco use, e-cigarette use, and binge drinking, the researchers said.
As cannabis legalization expands across the United States, policies are needed to deter use among adolescents, the researchers wrote. “These policies should be crafted to reduce an emphasis on criminalization in preference for public health promotion given the history of unequal application of punitive consequences of drug use for racialized minorities in the United States. As products, delivery systems, potency, and marketing proliferate within a for-profit industry, increased attention to youth trends, including investment in sustained and evidence-based prevention and intervention, is increasingly necessary.”
The take-home message for clinicians is to ask whether your patients are vaping, because the prevalence is not only up, but fairly universal, Mx. Kreski said. “Have a discussion that covers a broad range of substance use topics and informs teens of the potential risks of vaping, while avoiding stigma.”
The message for parents is “to talk to your kids about the risks of vaping,” said Mx. Kreski. “Prioritize open communication rather than punishment, and work together with your teens to prevent or reduce vaping.” The message for teens: “Understand that vaping has risks. You should feel empowered to talk to your parents or doctor about those risks. While it may seem like everyone’s vaping, the majority don’t. Keeping communication open between parents/caregivers, teens, and health care providers is one of the best ways to address these trends in vaping.”
Beware more powerful cannabis products
“While drug use in general is declining in adolescents, marijuana use remains very common,” Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“There is growing evidence that marijuana is now the first drug used by adolescents – replacing alcohol and nicotine – and frequent use can lead to substance abuse,” said Dr. Curran, who specializes in adolescent medicine but was involved in the study. “Cannabis use patterns have evolved over time. As I frequently tell my patients and their families, new strains and hybrids of marijuana have higher potencies of THC. Many adolescents are eschewing smoking and in its place using marijuana concentrates (wax, oil, shatter) via vape, dab pen, or rig. Use of these methods puts adolescents at high risk of social and health complications such as [e-cigarette or vaping use-associated lung injury], cannabis hyperemesis syndrome, and psychosis – and understanding these patterns and associated drug use helps health care professionals and parents keep adolescents safe.”
The take-home message for clinicians is that marijuana use via vaping continues to rise and to become more common than “traditional” marijuana smoking, Dr. Curran said. “This increase is across genders, in nearly all race/ethnicities (especially in Latinx youth), and in youth from lower socioeconomic status.” Vaping marijuana is associated with other substance abuse, so health care professionals should include questions about different forms of marijuana use, such as vape, dab pen, or rig, when working with patients, and counsel patients and families about the risks associated with use of any of these products.
The study was supported by the National Center for Injury Prevention and Control and by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
More teenagers in the United States reported cannabis use with vaping in 2019, compared with 2017, while cannabis use without vaping declined, based on annual survey data from more than 50,000 teens.
“With vaping prevalence rising so quickly among teens, getting a clearer picture of how cannabis use is shifting helps inform prevention and cessation efforts,” corresponding author Noah T. Kreski, MPH, of Columbia University, New York, said in an interview.
“In just 2 years, the most common cannabis use pattern changed from ‘occasional use without vaping’ to ‘frequent use with vaping,’ said Mx. Kreski, who uses the honorific Mx. and the pronouns they/them. “Knowing that, as well as the high overlap of cannabis vaping with nicotine use and binge drinking, adds to the urgency of reducing adolescent vaping.”
To quantify the trends in cannabis vaping, the researchers reviewed data from Monitoring the Future, an annual survey of high school students across the United States. The study population included 51,052 individuals; approximately 49% were male and 49% were non-Hispanic White. The researchers examined frequency of cannabis use, trends across demographic groups, and concurrent use of cannabis and other substances such as alcohol and tobacco. The findings were published in the journal Addiction.
Frequent cannabis use was defined as six or more times in the past 30 days; occasional use was defined as one to five times in the past 30 days.
Frequent cannabis use with vaping increased from 2.1% in 2017 to 5.4% in 2019. Occasional cannabis use with vaping also increased, though less dramatically, from less than 2% in 2017 to approximately 3.5% in 2019.
By contrast, both frequent and occasional cannabis use without vaping declined from 2017 to 2019 (from 3.8% to 2.1% and from 6.9% to 4.4%, respectively).
Overall, the prevalence of any level of cannabis use increased from 13.9% in 2017 to 15.4% in 2019. Both males and females showed a similar increase in reported frequent cannabis use with vaping of approximately 3%.
The results document that vaping cannabis has become more common than smoking alone among U.S. teens across almost all demographic groups, and across sex, race, urbanicity, and level of parent education; however, the increased was especially marked among Hispanic/Latinx teens and those of lower socioeconomic status, the researchers wrote.
The researchers also examined the associations between cannabis use with and without vaping and concurrent nicotine and alcohol use. Overall, the strongest association was between smoking or vaping nicotine and vaping cannabis; teens who smoked or vaped nicotine were 42 times more likely than nonnicotine users to report vaping cannabis in the past 30 days (adjusted odds ratio, 42.28). In addition, more occasions of binge drinking were more strongly associated with cannabis use with vaping (up to 10 times more likely), compared with cannabis use without vaping, (aORs, 4.48-10.09).
The study findings were limited by several factors, including the lack of questions on tetrahydrocannabinol (THC) or cannabidiol content of the cannabis products used, although evidence suggests that the potency of cannabis products in the United States is increasing, the researchers noted. Other limitations included the cross-sectional design, which prevents making associations about causality, and lack of data on the quantity of cannabis used; only data on frequency of use were recorded.
However, the results reflect a rise in cannabis use with vaping among teens in the United States, along with an increased risk of tobacco use, e-cigarette use, and binge drinking, the researchers said.
As cannabis legalization expands across the United States, policies are needed to deter use among adolescents, the researchers wrote. “These policies should be crafted to reduce an emphasis on criminalization in preference for public health promotion given the history of unequal application of punitive consequences of drug use for racialized minorities in the United States. As products, delivery systems, potency, and marketing proliferate within a for-profit industry, increased attention to youth trends, including investment in sustained and evidence-based prevention and intervention, is increasingly necessary.”
The take-home message for clinicians is to ask whether your patients are vaping, because the prevalence is not only up, but fairly universal, Mx. Kreski said. “Have a discussion that covers a broad range of substance use topics and informs teens of the potential risks of vaping, while avoiding stigma.”
The message for parents is “to talk to your kids about the risks of vaping,” said Mx. Kreski. “Prioritize open communication rather than punishment, and work together with your teens to prevent or reduce vaping.” The message for teens: “Understand that vaping has risks. You should feel empowered to talk to your parents or doctor about those risks. While it may seem like everyone’s vaping, the majority don’t. Keeping communication open between parents/caregivers, teens, and health care providers is one of the best ways to address these trends in vaping.”
Beware more powerful cannabis products
“While drug use in general is declining in adolescents, marijuana use remains very common,” Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“There is growing evidence that marijuana is now the first drug used by adolescents – replacing alcohol and nicotine – and frequent use can lead to substance abuse,” said Dr. Curran, who specializes in adolescent medicine but was involved in the study. “Cannabis use patterns have evolved over time. As I frequently tell my patients and their families, new strains and hybrids of marijuana have higher potencies of THC. Many adolescents are eschewing smoking and in its place using marijuana concentrates (wax, oil, shatter) via vape, dab pen, or rig. Use of these methods puts adolescents at high risk of social and health complications such as [e-cigarette or vaping use-associated lung injury], cannabis hyperemesis syndrome, and psychosis – and understanding these patterns and associated drug use helps health care professionals and parents keep adolescents safe.”
The take-home message for clinicians is that marijuana use via vaping continues to rise and to become more common than “traditional” marijuana smoking, Dr. Curran said. “This increase is across genders, in nearly all race/ethnicities (especially in Latinx youth), and in youth from lower socioeconomic status.” Vaping marijuana is associated with other substance abuse, so health care professionals should include questions about different forms of marijuana use, such as vape, dab pen, or rig, when working with patients, and counsel patients and families about the risks associated with use of any of these products.
The study was supported by the National Center for Injury Prevention and Control and by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
More teenagers in the United States reported cannabis use with vaping in 2019, compared with 2017, while cannabis use without vaping declined, based on annual survey data from more than 50,000 teens.
“With vaping prevalence rising so quickly among teens, getting a clearer picture of how cannabis use is shifting helps inform prevention and cessation efforts,” corresponding author Noah T. Kreski, MPH, of Columbia University, New York, said in an interview.
“In just 2 years, the most common cannabis use pattern changed from ‘occasional use without vaping’ to ‘frequent use with vaping,’ said Mx. Kreski, who uses the honorific Mx. and the pronouns they/them. “Knowing that, as well as the high overlap of cannabis vaping with nicotine use and binge drinking, adds to the urgency of reducing adolescent vaping.”
To quantify the trends in cannabis vaping, the researchers reviewed data from Monitoring the Future, an annual survey of high school students across the United States. The study population included 51,052 individuals; approximately 49% were male and 49% were non-Hispanic White. The researchers examined frequency of cannabis use, trends across demographic groups, and concurrent use of cannabis and other substances such as alcohol and tobacco. The findings were published in the journal Addiction.
Frequent cannabis use was defined as six or more times in the past 30 days; occasional use was defined as one to five times in the past 30 days.
Frequent cannabis use with vaping increased from 2.1% in 2017 to 5.4% in 2019. Occasional cannabis use with vaping also increased, though less dramatically, from less than 2% in 2017 to approximately 3.5% in 2019.
By contrast, both frequent and occasional cannabis use without vaping declined from 2017 to 2019 (from 3.8% to 2.1% and from 6.9% to 4.4%, respectively).
Overall, the prevalence of any level of cannabis use increased from 13.9% in 2017 to 15.4% in 2019. Both males and females showed a similar increase in reported frequent cannabis use with vaping of approximately 3%.
The results document that vaping cannabis has become more common than smoking alone among U.S. teens across almost all demographic groups, and across sex, race, urbanicity, and level of parent education; however, the increased was especially marked among Hispanic/Latinx teens and those of lower socioeconomic status, the researchers wrote.
The researchers also examined the associations between cannabis use with and without vaping and concurrent nicotine and alcohol use. Overall, the strongest association was between smoking or vaping nicotine and vaping cannabis; teens who smoked or vaped nicotine were 42 times more likely than nonnicotine users to report vaping cannabis in the past 30 days (adjusted odds ratio, 42.28). In addition, more occasions of binge drinking were more strongly associated with cannabis use with vaping (up to 10 times more likely), compared with cannabis use without vaping, (aORs, 4.48-10.09).
The study findings were limited by several factors, including the lack of questions on tetrahydrocannabinol (THC) or cannabidiol content of the cannabis products used, although evidence suggests that the potency of cannabis products in the United States is increasing, the researchers noted. Other limitations included the cross-sectional design, which prevents making associations about causality, and lack of data on the quantity of cannabis used; only data on frequency of use were recorded.
However, the results reflect a rise in cannabis use with vaping among teens in the United States, along with an increased risk of tobacco use, e-cigarette use, and binge drinking, the researchers said.
As cannabis legalization expands across the United States, policies are needed to deter use among adolescents, the researchers wrote. “These policies should be crafted to reduce an emphasis on criminalization in preference for public health promotion given the history of unequal application of punitive consequences of drug use for racialized minorities in the United States. As products, delivery systems, potency, and marketing proliferate within a for-profit industry, increased attention to youth trends, including investment in sustained and evidence-based prevention and intervention, is increasingly necessary.”
The take-home message for clinicians is to ask whether your patients are vaping, because the prevalence is not only up, but fairly universal, Mx. Kreski said. “Have a discussion that covers a broad range of substance use topics and informs teens of the potential risks of vaping, while avoiding stigma.”
The message for parents is “to talk to your kids about the risks of vaping,” said Mx. Kreski. “Prioritize open communication rather than punishment, and work together with your teens to prevent or reduce vaping.” The message for teens: “Understand that vaping has risks. You should feel empowered to talk to your parents or doctor about those risks. While it may seem like everyone’s vaping, the majority don’t. Keeping communication open between parents/caregivers, teens, and health care providers is one of the best ways to address these trends in vaping.”
Beware more powerful cannabis products
“While drug use in general is declining in adolescents, marijuana use remains very common,” Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“There is growing evidence that marijuana is now the first drug used by adolescents – replacing alcohol and nicotine – and frequent use can lead to substance abuse,” said Dr. Curran, who specializes in adolescent medicine but was involved in the study. “Cannabis use patterns have evolved over time. As I frequently tell my patients and their families, new strains and hybrids of marijuana have higher potencies of THC. Many adolescents are eschewing smoking and in its place using marijuana concentrates (wax, oil, shatter) via vape, dab pen, or rig. Use of these methods puts adolescents at high risk of social and health complications such as [e-cigarette or vaping use-associated lung injury], cannabis hyperemesis syndrome, and psychosis – and understanding these patterns and associated drug use helps health care professionals and parents keep adolescents safe.”
The take-home message for clinicians is that marijuana use via vaping continues to rise and to become more common than “traditional” marijuana smoking, Dr. Curran said. “This increase is across genders, in nearly all race/ethnicities (especially in Latinx youth), and in youth from lower socioeconomic status.” Vaping marijuana is associated with other substance abuse, so health care professionals should include questions about different forms of marijuana use, such as vape, dab pen, or rig, when working with patients, and counsel patients and families about the risks associated with use of any of these products.
The study was supported by the National Center for Injury Prevention and Control and by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM ADDICTION
Man with distal flexion deformities
On the basis of history and presentation, this patient's psoriatic disease has probably evolved to psoriatic arthritis mutilans (PAM). PAM is considered the most severe form of psoriatic arthritis (PsA), causing joint destruction and functional disability. It is estimated to affect about 5% of patients with PsA, with an equal sex distribution. Psoriatic nail dystrophy, a hallmark of PsA, appears to be a clinical biomarker of PAM development. Patients with PAM are generally younger at diagnosis than those with less severe forms of disease. Disease-modifying antirheumatic drugs and anti-TNF therapy do not appear to prevent the development of PAM, as evidenced by the present case.
In general, clinical presentation of PsA is heterogeneous and can be similar to that of other rheumatic diseases such as rheumatoid arthritis or osteoarthritis, complicating the differential diagnosis. The Classification Criteria for Psoriatic Arthritis (CASPAR) are considered the most sensitive diagnostic criteria, encompassing evidence of psoriasis; nail dystrophy; lab findings of typical autoantibodies (negative rheumatoid factor); and phenomena that are characteristic of PsA, like dactylitis.
Workup for PAM often includes radiography, ultrasound, and MRI or CT. With no established consensus, classification systems for the condition vary clinically and radiographically. Radiographic features suggestive of PAM include osteolysis or extended bone resorption; pencil-in-cup changes; joint subluxation; and, less often, ankylosis. Osteolysis has been defined as bone resorption with more than 50% loss of joint surface on both sides of the joint. Clinically, dissolution of the joint causes redundant, overlying skin with a telescoping motion of the digit. Other clinical features of PAM include digital shortening and flail joints. Of note, involvement of one small joint in the hands or feet is diagnostic of PAM.
In the setting of PsA, multiple genetic factors have been described, including presence of HLA-B27 and HLA-DRB1, but none are considered defining factors for the disease. A recent population-based study shows that presence of HLA-B27 was significantly increased among patients with PAM (45%) compared with patients with less severe PsA (13%) and healthy controls (13%).
According to the American College of Rheumatology guidelines, first-line therapy in adult patients who have active PsA and are treatment-naive is a TNFi biologic agent. For the patient in this case, who has active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic may be appropriate; however, switching to an interleukin-17 inhibitor may also be considered because this patient has severe disease. Data on the comparative efficacy of different biological agents for treatment of PAM are not yet available.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of history and presentation, this patient's psoriatic disease has probably evolved to psoriatic arthritis mutilans (PAM). PAM is considered the most severe form of psoriatic arthritis (PsA), causing joint destruction and functional disability. It is estimated to affect about 5% of patients with PsA, with an equal sex distribution. Psoriatic nail dystrophy, a hallmark of PsA, appears to be a clinical biomarker of PAM development. Patients with PAM are generally younger at diagnosis than those with less severe forms of disease. Disease-modifying antirheumatic drugs and anti-TNF therapy do not appear to prevent the development of PAM, as evidenced by the present case.
In general, clinical presentation of PsA is heterogeneous and can be similar to that of other rheumatic diseases such as rheumatoid arthritis or osteoarthritis, complicating the differential diagnosis. The Classification Criteria for Psoriatic Arthritis (CASPAR) are considered the most sensitive diagnostic criteria, encompassing evidence of psoriasis; nail dystrophy; lab findings of typical autoantibodies (negative rheumatoid factor); and phenomena that are characteristic of PsA, like dactylitis.
Workup for PAM often includes radiography, ultrasound, and MRI or CT. With no established consensus, classification systems for the condition vary clinically and radiographically. Radiographic features suggestive of PAM include osteolysis or extended bone resorption; pencil-in-cup changes; joint subluxation; and, less often, ankylosis. Osteolysis has been defined as bone resorption with more than 50% loss of joint surface on both sides of the joint. Clinically, dissolution of the joint causes redundant, overlying skin with a telescoping motion of the digit. Other clinical features of PAM include digital shortening and flail joints. Of note, involvement of one small joint in the hands or feet is diagnostic of PAM.
In the setting of PsA, multiple genetic factors have been described, including presence of HLA-B27 and HLA-DRB1, but none are considered defining factors for the disease. A recent population-based study shows that presence of HLA-B27 was significantly increased among patients with PAM (45%) compared with patients with less severe PsA (13%) and healthy controls (13%).
According to the American College of Rheumatology guidelines, first-line therapy in adult patients who have active PsA and are treatment-naive is a TNFi biologic agent. For the patient in this case, who has active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic may be appropriate; however, switching to an interleukin-17 inhibitor may also be considered because this patient has severe disease. Data on the comparative efficacy of different biological agents for treatment of PAM are not yet available.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of history and presentation, this patient's psoriatic disease has probably evolved to psoriatic arthritis mutilans (PAM). PAM is considered the most severe form of psoriatic arthritis (PsA), causing joint destruction and functional disability. It is estimated to affect about 5% of patients with PsA, with an equal sex distribution. Psoriatic nail dystrophy, a hallmark of PsA, appears to be a clinical biomarker of PAM development. Patients with PAM are generally younger at diagnosis than those with less severe forms of disease. Disease-modifying antirheumatic drugs and anti-TNF therapy do not appear to prevent the development of PAM, as evidenced by the present case.
In general, clinical presentation of PsA is heterogeneous and can be similar to that of other rheumatic diseases such as rheumatoid arthritis or osteoarthritis, complicating the differential diagnosis. The Classification Criteria for Psoriatic Arthritis (CASPAR) are considered the most sensitive diagnostic criteria, encompassing evidence of psoriasis; nail dystrophy; lab findings of typical autoantibodies (negative rheumatoid factor); and phenomena that are characteristic of PsA, like dactylitis.
Workup for PAM often includes radiography, ultrasound, and MRI or CT. With no established consensus, classification systems for the condition vary clinically and radiographically. Radiographic features suggestive of PAM include osteolysis or extended bone resorption; pencil-in-cup changes; joint subluxation; and, less often, ankylosis. Osteolysis has been defined as bone resorption with more than 50% loss of joint surface on both sides of the joint. Clinically, dissolution of the joint causes redundant, overlying skin with a telescoping motion of the digit. Other clinical features of PAM include digital shortening and flail joints. Of note, involvement of one small joint in the hands or feet is diagnostic of PAM.
In the setting of PsA, multiple genetic factors have been described, including presence of HLA-B27 and HLA-DRB1, but none are considered defining factors for the disease. A recent population-based study shows that presence of HLA-B27 was significantly increased among patients with PAM (45%) compared with patients with less severe PsA (13%) and healthy controls (13%).
According to the American College of Rheumatology guidelines, first-line therapy in adult patients who have active PsA and are treatment-naive is a TNFi biologic agent. For the patient in this case, who has active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic may be appropriate; however, switching to an interleukin-17 inhibitor may also be considered because this patient has severe disease. Data on the comparative efficacy of different biological agents for treatment of PAM are not yet available.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 43-year-old man presents with distal flexion deformities and telescoping of the digits. The patient was diagnosed with psoriasis at age 31 and he has several immediate family members who previously received the same diagnosis. He has been treated intermittently with tumor necrosis factor inhibitor (TNFi) biologic monotherapy but admits to nonadherence when disease activity seems to quiet down. Radiography shows osteolysis and dissolution of the joint.
Hormones account for 10% of lipid changes after menopause
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Improved cancer survival in states with ACA Medicaid expansion
compared with patients in states that did not adopt the expansion.
The finding comes from an American Cancer Society study of more than 2 million patients with newly diagnosed cancer, published online in the Journal of the National Cancer Institute.
The analysis also showed that the evidence was strongest for malignancies with poor prognosis such as lung, pancreatic, and liver cancer, and also for colorectal cancer.
Importantly, improvements in survival were larger in non-Hispanic Black patients and individuals residing in rural areas, suggesting there was a narrowing of disparities in cancer survival by race and rurality.
“Our findings provide further evidence of the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health care disruptions caused by the COVID-19 pandemic,” said lead author Xuesong Han, PhD, scientific director of health services research at the American Cancer Society, in a statement. “What’s encouraging is the American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to increase eligibility.”
The ACA provided states with incentives to expand Medicaid eligibility to all low-income adults under 138% federal poverty level, regardless of parental status.
As of last month, just 12 states have not yet opted for Medicaid expansion, even though the American Rescue Plan Act of 2021 provides new incentives for those remaining jurisdictions. But to date, none of the remaining states have taken advantage of these new incentives.
An interactive map showing the status of Medicare expansion by state is available here. The 12 states that have not adopted Medicare expansion (as of April) are Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, South Dakota, Tennessee, Texas, Wisconsin, and Wyoming.
The benefit of Medicaid expansion on cancer outcomes has already been observed in other studies. The first study to show a survival benefit was presented at the 2020 American Society of Clinical Oncology annual meeting. That analysis showed that cancer mortality declined by 29% in states that expanded Medicaid and by 25% in those that did not. The authors also noted that the greatest mortality benefit was observed in Hispanic patients.
Improved survival with expansion
In the current paper, Dr. Han and colleagues used population-based cancer registries from 42 states and compared data on patients aged 18-62 years who were diagnosed with cancer in a period of 2 years before (2010-2012) and after (2014-2016) ACA Medicaid expansion. They were followed through Sept. 30, 2013, and Dec. 31, 2017, respectively.
The analysis involved a total of 2.5 million patients, of whom 1.52 million lived in states that adopted Medicaid expansion and compared with 1 million patients were in states that did not.
Patients with grouped by sex, race and ethnicity, census tract-level poverty, and rurality. The authors note that non-Hispanic Black patients and those from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states.
During the 2-year follow-up period, a total of 453,487 deaths occurred (257,950 in expansion states and 195,537 in nonexpansion states).
Overall, patients in expansion states generally had better survival versus those in nonexpansion states, the authors comment. However, for most cancer types, overall survival improved after the ACA for both groups of states.
The 2-year overall survival increased from 80.6% before the ACA to 82.2% post ACA in expansion states and from 78.7% to 80% in nonexpansion states.
This extrapolated to net increase of 0.44 percentage points in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer, lung cancer, non-Hodgkin’s lymphoma, pancreatic cancer, and liver cancer.
For Hispanic patients, 2-year survival also increased but was similar in expansion and nonexpansion states, and little net change was associated with Medicaid expansion.
“Our study shows that the increase was largely driven by improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments,” said Dr. Han. “It adds to accumulating evidence of the multiple benefits of Medicaid expansion.”
A version of this article first appeared on Medscape.com.
compared with patients in states that did not adopt the expansion.
The finding comes from an American Cancer Society study of more than 2 million patients with newly diagnosed cancer, published online in the Journal of the National Cancer Institute.
The analysis also showed that the evidence was strongest for malignancies with poor prognosis such as lung, pancreatic, and liver cancer, and also for colorectal cancer.
Importantly, improvements in survival were larger in non-Hispanic Black patients and individuals residing in rural areas, suggesting there was a narrowing of disparities in cancer survival by race and rurality.
“Our findings provide further evidence of the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health care disruptions caused by the COVID-19 pandemic,” said lead author Xuesong Han, PhD, scientific director of health services research at the American Cancer Society, in a statement. “What’s encouraging is the American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to increase eligibility.”
The ACA provided states with incentives to expand Medicaid eligibility to all low-income adults under 138% federal poverty level, regardless of parental status.
As of last month, just 12 states have not yet opted for Medicaid expansion, even though the American Rescue Plan Act of 2021 provides new incentives for those remaining jurisdictions. But to date, none of the remaining states have taken advantage of these new incentives.
An interactive map showing the status of Medicare expansion by state is available here. The 12 states that have not adopted Medicare expansion (as of April) are Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, South Dakota, Tennessee, Texas, Wisconsin, and Wyoming.
The benefit of Medicaid expansion on cancer outcomes has already been observed in other studies. The first study to show a survival benefit was presented at the 2020 American Society of Clinical Oncology annual meeting. That analysis showed that cancer mortality declined by 29% in states that expanded Medicaid and by 25% in those that did not. The authors also noted that the greatest mortality benefit was observed in Hispanic patients.
Improved survival with expansion
In the current paper, Dr. Han and colleagues used population-based cancer registries from 42 states and compared data on patients aged 18-62 years who were diagnosed with cancer in a period of 2 years before (2010-2012) and after (2014-2016) ACA Medicaid expansion. They were followed through Sept. 30, 2013, and Dec. 31, 2017, respectively.
The analysis involved a total of 2.5 million patients, of whom 1.52 million lived in states that adopted Medicaid expansion and compared with 1 million patients were in states that did not.
Patients with grouped by sex, race and ethnicity, census tract-level poverty, and rurality. The authors note that non-Hispanic Black patients and those from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states.
During the 2-year follow-up period, a total of 453,487 deaths occurred (257,950 in expansion states and 195,537 in nonexpansion states).
Overall, patients in expansion states generally had better survival versus those in nonexpansion states, the authors comment. However, for most cancer types, overall survival improved after the ACA for both groups of states.
The 2-year overall survival increased from 80.6% before the ACA to 82.2% post ACA in expansion states and from 78.7% to 80% in nonexpansion states.
This extrapolated to net increase of 0.44 percentage points in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer, lung cancer, non-Hodgkin’s lymphoma, pancreatic cancer, and liver cancer.
For Hispanic patients, 2-year survival also increased but was similar in expansion and nonexpansion states, and little net change was associated with Medicaid expansion.
“Our study shows that the increase was largely driven by improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments,” said Dr. Han. “It adds to accumulating evidence of the multiple benefits of Medicaid expansion.”
A version of this article first appeared on Medscape.com.
compared with patients in states that did not adopt the expansion.
The finding comes from an American Cancer Society study of more than 2 million patients with newly diagnosed cancer, published online in the Journal of the National Cancer Institute.
The analysis also showed that the evidence was strongest for malignancies with poor prognosis such as lung, pancreatic, and liver cancer, and also for colorectal cancer.
Importantly, improvements in survival were larger in non-Hispanic Black patients and individuals residing in rural areas, suggesting there was a narrowing of disparities in cancer survival by race and rurality.
“Our findings provide further evidence of the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health care disruptions caused by the COVID-19 pandemic,” said lead author Xuesong Han, PhD, scientific director of health services research at the American Cancer Society, in a statement. “What’s encouraging is the American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to increase eligibility.”
The ACA provided states with incentives to expand Medicaid eligibility to all low-income adults under 138% federal poverty level, regardless of parental status.
As of last month, just 12 states have not yet opted for Medicaid expansion, even though the American Rescue Plan Act of 2021 provides new incentives for those remaining jurisdictions. But to date, none of the remaining states have taken advantage of these new incentives.
An interactive map showing the status of Medicare expansion by state is available here. The 12 states that have not adopted Medicare expansion (as of April) are Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, South Dakota, Tennessee, Texas, Wisconsin, and Wyoming.
The benefit of Medicaid expansion on cancer outcomes has already been observed in other studies. The first study to show a survival benefit was presented at the 2020 American Society of Clinical Oncology annual meeting. That analysis showed that cancer mortality declined by 29% in states that expanded Medicaid and by 25% in those that did not. The authors also noted that the greatest mortality benefit was observed in Hispanic patients.
Improved survival with expansion
In the current paper, Dr. Han and colleagues used population-based cancer registries from 42 states and compared data on patients aged 18-62 years who were diagnosed with cancer in a period of 2 years before (2010-2012) and after (2014-2016) ACA Medicaid expansion. They were followed through Sept. 30, 2013, and Dec. 31, 2017, respectively.
The analysis involved a total of 2.5 million patients, of whom 1.52 million lived in states that adopted Medicaid expansion and compared with 1 million patients were in states that did not.
Patients with grouped by sex, race and ethnicity, census tract-level poverty, and rurality. The authors note that non-Hispanic Black patients and those from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states.
During the 2-year follow-up period, a total of 453,487 deaths occurred (257,950 in expansion states and 195,537 in nonexpansion states).
Overall, patients in expansion states generally had better survival versus those in nonexpansion states, the authors comment. However, for most cancer types, overall survival improved after the ACA for both groups of states.
The 2-year overall survival increased from 80.6% before the ACA to 82.2% post ACA in expansion states and from 78.7% to 80% in nonexpansion states.
This extrapolated to net increase of 0.44 percentage points in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer, lung cancer, non-Hodgkin’s lymphoma, pancreatic cancer, and liver cancer.
For Hispanic patients, 2-year survival also increased but was similar in expansion and nonexpansion states, and little net change was associated with Medicaid expansion.
“Our study shows that the increase was largely driven by improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments,” said Dr. Han. “It adds to accumulating evidence of the multiple benefits of Medicaid expansion.”
A version of this article first appeared on Medscape.com.
Steroid use associated with higher relapse risk in some UC
Patients who have histologically active ulcerative colitis (UC) with a Mayo endoscopic subscore (MES) of 1 and a history of steroid use may be at increased risk for clinical relapse, according to a new single-center, retrospective analysis.
In recent years, treat-to-target approaches in UC have incorporated clinician and patient-reported outcomes, along with endoscopic remission, defined as MES 1 or less. However, additional studies showed a higher risk of relapse with MES 1, and the STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) guidelines released in 2021 suggest that only MES 0 indicates endoscopic healing.
Nevertheless, there is concern that striving to achieve MES 0 could lead to overtreatment, since MES values are somewhat subjective, and patients with MES 1 sometimes report few or no symptoms. This led to the current study by Gyeol Seong, MD, and colleagues, published in Inflammatory Bowel Diseases.
Commenting on the study, Miguel Regueiro, MD, chair for the Digestive Disease and Surgery Institute and a professor of medicine at the Cleveland Clinic said “the question has always been, if the patient is in endoscopic remission and clinically feels well, how important is it to have histologically normal mucosa?”
In their retrospective study, Dr. Seong and colleagues analyzed data from 492 patients. The median age was 48, and 51.6% were male. The median duration of disease was 78 months. During a median follow-up of 549 days, 18.7% experienced a relapse, defined as “change or escalation of medication, hospitalization due to the aggravation of UC, or total colectomy.” Of these patients, 39.4% had used steroids and 51.4% had a MES score of 0 at the index endoscopy, and 70.5% had histologic improvement, as defined by a Geboes score of less than 3.1.
A Geboes score of 3.1 or higher and a history of steroid use was associated with an increased cumulative incidence of clinical relapse, compared with a Geboes score lower than 3.1 and no steroid use (P = .001). In a multivariate analysis, histologic activity alone was not a predictor of relapse. Among patients with an MES score of 1, a history of steroid use predicted risk of relapse (adjusted hazard ratio, 2.102; P = .006).
Although Dr. Regueiro said the new findings would not change his current practice, he does incorporate histopathology in clinical decision-making. In cases where a patient is in endoscopic remission but has histologic activity, he will consider adjusting the current medication rather than changing to a different therapeutic. “I wouldn’t recommend that we switch patients off one therapy to another just because of histologic activity.”
That’s because patients often improve dramatically after effective therapy. Dr. Regueiro said: “The one concern is whether the next medicine works as well as what the patient is already on? I’m also worried that we burn through our biologics too quickly. We go from one to another and another, sometimes I think we just need to optimize the one they’re on.”
The study does have the potential to influence surveillance for dysplasia, according to Dr. Regueiro, noting that a recent retrospective analysis showed an increased risk of dysplasia in UC with persistent histologic activity. “This suggests the need for a colonoscopy in a patient who has had ulcerative colitis for a number of years, even if there’s improvement but, on biopsy, there’s evidence of inflammation.”
According to Dr. Regueiro, such patients might benefit from a fecal calprotectin measurement in 3-6 months to monitor for colonic inflammation. Increased levels might be a sign that the patient is skipping medication doses, although other factors, like respiratory infections, can also explain the results. If the patient’s medication adherence is good, another therapy can be added or the dose increased, and the next endoscopy can be pushed up.
A key limitation to the study by Dr. Seong and colleagues is that it was based in South Korea. “Would this be the same in different countries and different populations? That is still a question,” said Dr. Regueiro.
Dr. Regueiro has received unrestricted educational grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, Bristol-Myers Squibb, and Lilly. He has been on advisory boards or consulted for numerous companies. He also has relationships with the following CME companies: CME Outfitters, Imedex, GI Health Foundation, Cornerstones, Remedy, MJH Life Sciences, Medscape, MDEducation, WebMD, and HMPGlobal.
Patients who have histologically active ulcerative colitis (UC) with a Mayo endoscopic subscore (MES) of 1 and a history of steroid use may be at increased risk for clinical relapse, according to a new single-center, retrospective analysis.
In recent years, treat-to-target approaches in UC have incorporated clinician and patient-reported outcomes, along with endoscopic remission, defined as MES 1 or less. However, additional studies showed a higher risk of relapse with MES 1, and the STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) guidelines released in 2021 suggest that only MES 0 indicates endoscopic healing.
Nevertheless, there is concern that striving to achieve MES 0 could lead to overtreatment, since MES values are somewhat subjective, and patients with MES 1 sometimes report few or no symptoms. This led to the current study by Gyeol Seong, MD, and colleagues, published in Inflammatory Bowel Diseases.
Commenting on the study, Miguel Regueiro, MD, chair for the Digestive Disease and Surgery Institute and a professor of medicine at the Cleveland Clinic said “the question has always been, if the patient is in endoscopic remission and clinically feels well, how important is it to have histologically normal mucosa?”
In their retrospective study, Dr. Seong and colleagues analyzed data from 492 patients. The median age was 48, and 51.6% were male. The median duration of disease was 78 months. During a median follow-up of 549 days, 18.7% experienced a relapse, defined as “change or escalation of medication, hospitalization due to the aggravation of UC, or total colectomy.” Of these patients, 39.4% had used steroids and 51.4% had a MES score of 0 at the index endoscopy, and 70.5% had histologic improvement, as defined by a Geboes score of less than 3.1.
A Geboes score of 3.1 or higher and a history of steroid use was associated with an increased cumulative incidence of clinical relapse, compared with a Geboes score lower than 3.1 and no steroid use (P = .001). In a multivariate analysis, histologic activity alone was not a predictor of relapse. Among patients with an MES score of 1, a history of steroid use predicted risk of relapse (adjusted hazard ratio, 2.102; P = .006).
Although Dr. Regueiro said the new findings would not change his current practice, he does incorporate histopathology in clinical decision-making. In cases where a patient is in endoscopic remission but has histologic activity, he will consider adjusting the current medication rather than changing to a different therapeutic. “I wouldn’t recommend that we switch patients off one therapy to another just because of histologic activity.”
That’s because patients often improve dramatically after effective therapy. Dr. Regueiro said: “The one concern is whether the next medicine works as well as what the patient is already on? I’m also worried that we burn through our biologics too quickly. We go from one to another and another, sometimes I think we just need to optimize the one they’re on.”
The study does have the potential to influence surveillance for dysplasia, according to Dr. Regueiro, noting that a recent retrospective analysis showed an increased risk of dysplasia in UC with persistent histologic activity. “This suggests the need for a colonoscopy in a patient who has had ulcerative colitis for a number of years, even if there’s improvement but, on biopsy, there’s evidence of inflammation.”
According to Dr. Regueiro, such patients might benefit from a fecal calprotectin measurement in 3-6 months to monitor for colonic inflammation. Increased levels might be a sign that the patient is skipping medication doses, although other factors, like respiratory infections, can also explain the results. If the patient’s medication adherence is good, another therapy can be added or the dose increased, and the next endoscopy can be pushed up.
A key limitation to the study by Dr. Seong and colleagues is that it was based in South Korea. “Would this be the same in different countries and different populations? That is still a question,” said Dr. Regueiro.
Dr. Regueiro has received unrestricted educational grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, Bristol-Myers Squibb, and Lilly. He has been on advisory boards or consulted for numerous companies. He also has relationships with the following CME companies: CME Outfitters, Imedex, GI Health Foundation, Cornerstones, Remedy, MJH Life Sciences, Medscape, MDEducation, WebMD, and HMPGlobal.
Patients who have histologically active ulcerative colitis (UC) with a Mayo endoscopic subscore (MES) of 1 and a history of steroid use may be at increased risk for clinical relapse, according to a new single-center, retrospective analysis.
In recent years, treat-to-target approaches in UC have incorporated clinician and patient-reported outcomes, along with endoscopic remission, defined as MES 1 or less. However, additional studies showed a higher risk of relapse with MES 1, and the STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) guidelines released in 2021 suggest that only MES 0 indicates endoscopic healing.
Nevertheless, there is concern that striving to achieve MES 0 could lead to overtreatment, since MES values are somewhat subjective, and patients with MES 1 sometimes report few or no symptoms. This led to the current study by Gyeol Seong, MD, and colleagues, published in Inflammatory Bowel Diseases.
Commenting on the study, Miguel Regueiro, MD, chair for the Digestive Disease and Surgery Institute and a professor of medicine at the Cleveland Clinic said “the question has always been, if the patient is in endoscopic remission and clinically feels well, how important is it to have histologically normal mucosa?”
In their retrospective study, Dr. Seong and colleagues analyzed data from 492 patients. The median age was 48, and 51.6% were male. The median duration of disease was 78 months. During a median follow-up of 549 days, 18.7% experienced a relapse, defined as “change or escalation of medication, hospitalization due to the aggravation of UC, or total colectomy.” Of these patients, 39.4% had used steroids and 51.4% had a MES score of 0 at the index endoscopy, and 70.5% had histologic improvement, as defined by a Geboes score of less than 3.1.
A Geboes score of 3.1 or higher and a history of steroid use was associated with an increased cumulative incidence of clinical relapse, compared with a Geboes score lower than 3.1 and no steroid use (P = .001). In a multivariate analysis, histologic activity alone was not a predictor of relapse. Among patients with an MES score of 1, a history of steroid use predicted risk of relapse (adjusted hazard ratio, 2.102; P = .006).
Although Dr. Regueiro said the new findings would not change his current practice, he does incorporate histopathology in clinical decision-making. In cases where a patient is in endoscopic remission but has histologic activity, he will consider adjusting the current medication rather than changing to a different therapeutic. “I wouldn’t recommend that we switch patients off one therapy to another just because of histologic activity.”
That’s because patients often improve dramatically after effective therapy. Dr. Regueiro said: “The one concern is whether the next medicine works as well as what the patient is already on? I’m also worried that we burn through our biologics too quickly. We go from one to another and another, sometimes I think we just need to optimize the one they’re on.”
The study does have the potential to influence surveillance for dysplasia, according to Dr. Regueiro, noting that a recent retrospective analysis showed an increased risk of dysplasia in UC with persistent histologic activity. “This suggests the need for a colonoscopy in a patient who has had ulcerative colitis for a number of years, even if there’s improvement but, on biopsy, there’s evidence of inflammation.”
According to Dr. Regueiro, such patients might benefit from a fecal calprotectin measurement in 3-6 months to monitor for colonic inflammation. Increased levels might be a sign that the patient is skipping medication doses, although other factors, like respiratory infections, can also explain the results. If the patient’s medication adherence is good, another therapy can be added or the dose increased, and the next endoscopy can be pushed up.
A key limitation to the study by Dr. Seong and colleagues is that it was based in South Korea. “Would this be the same in different countries and different populations? That is still a question,” said Dr. Regueiro.
Dr. Regueiro has received unrestricted educational grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, Bristol-Myers Squibb, and Lilly. He has been on advisory boards or consulted for numerous companies. He also has relationships with the following CME companies: CME Outfitters, Imedex, GI Health Foundation, Cornerstones, Remedy, MJH Life Sciences, Medscape, MDEducation, WebMD, and HMPGlobal.
FROM INFLAMMATORY BOWEL DISEASES
NAVIGATOR steers uncontrolled asthma toward calmer seas
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.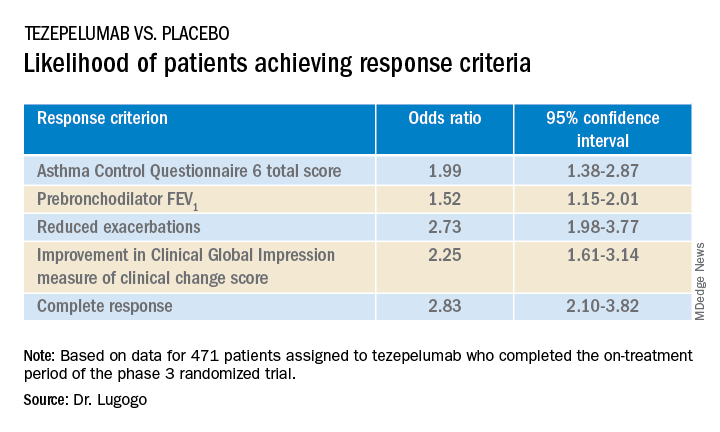
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
AT ATS 2022