User login
Few new cancer drugs replace current standards of care
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Abdominal rash
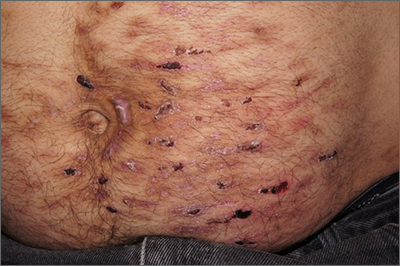
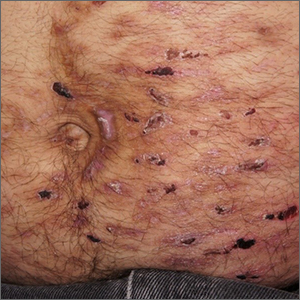
Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006

Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006
Boring is good. Boring is right. Boring is … interesting
Can you keep it down? I’m trying to be boring
He chides his friends for not looking both ways before crossing the road. He is never questioned by the police because they fall asleep listening to him talk. He has won the office’s coveted perfect attendance award 10 years running. Look out, Dos Equis guy, you’ve got some new competition. That’s right, it’s the most boring man in the world.
For this boring study (sorry, study on boredom) conducted by English researchers and published in Personality and Social Psychology Bulletin, people were surveyed on various jobs and hobbies, ranking them by how exciting or boring they are, as well as how competent someone with those jobs/hobbies would be, their willingness to avoid someone with those jobs/hobbies, and how much they’d need to be paid to spend time with someone who had an undesirable job/hobby.
According to the British public, the most boring person in the world is a religious data analyst who likes to sleep and lives in a small town. In fact, spending time with this person is almost a full-time job on its own: To make it worth their while, survey subjects wanted 35 pounds a day. The boring person also was viewed as less competent, as is anyone with a boring job.
Now, there probably aren’t a lot of religious data analysts out there, but don’t worry, there are plenty of other boring jobs – accounting, tax/insurance, cleaning, and banking rounded out the top five (apparently people don’t like finances) – and hobbies – watching TV, observing animals, and mathematics filled out the top five. In case you’re curious, performing artists, scientists, journalists, health professionals, and teachers were viewed as having exciting jobs; exciting hobbies included gaming, reading, domestic tasks (really?), gardening, and writing.
Lead researcher Wijnand Van Tilburg, PhD, made an excellent point about people with boring jobs: They “have power in society – perhaps we should try not to upset them and stereotype them as boring!”
We think they should lean into it and make The Most Boring Man in the World ads: “When I drive a car off the lot, its value increases because I used the correct lending association. Batman trusts me with his Batmobile insurance. I can make those Cuban cigars tax exempt. Stay financially solvent, my friends.”
Fungi, but make it fashion
Fashion is an expensive and costly industry to sustain. Cotton production takes a toll on the environment, leather production comes with environmental and ethical/moral conundrums, and thanks to fast fashion, about 85% of textiles are being thrown away in the United States.
Researchers at the University of Borås in Sweden, however, have found a newish solution to create leather, cotton, and other textiles. And as with so many of the finer things, it starts with unsold bread from the grocery store.
Akram Zamani, PhD, and her team take that bread and turn it into breadcrumbs, then combine it with water and Rhizopus delemar, a fungus typically found in decaying food. After a couple of days of feasting on the bread, the fungus produces natural fibers made of chitin and chitosan that accumulate in the cell walls. After proteins, lipids, and other byproducts are removed, the team is left with a jelly-like substance made of those fibrous cell walls that can be spun into a fabric.
The researchers started small with very thin nonpliable sheets, but with a little layering by using tree tannins for softness and alkali for strength, their fungal leather is more like real leather than competing fungal leathers. Not to mention its being able to be produced in a fraction of the time.
This new fungal leather is fast to produce, it’s biodegradable, and it uses only natural ingredients to treat the materials. It’s the ultimate environmental fashion statement.
Who’s afraid of cancer? Not C. elegans
And now, we bring you part 2 of our ongoing series: Creatures that can diagnose cancer. Last week, we discovered that ants are well on their way to replacing dogs in our medical labs and in our hearts. This week, we present the even-more-lovable nematode.
The soil-dwelling nematode Caenorhabditis elegans, which is less than 1 mm long, is known to be “attracted or repelled by certain odors, so we came up with an idea that the roundworm could be used to detect lung cancer,” Shin Sik Choi, PhD, of Myongji University in South Korea, who is the project’s principal investigator, said in a statement on Eurekalert.
Dr. Choi’s team created a “worm-on-a-chip” that allowed the nematodes to choose between a drop of culture media from lung cancer cells and media from normal lung fibroblasts. An hour after being placed in the chip’s central chamber, more nematodes had crawled toward the lung cancer media than the normal-cell sample.
The investigators estimate that the device is about 70% effective at detecting cancer cells, but “they hope to increase both the accuracy and sensitivity of the method by using worms that were previously exposed to cancer cell media and therefore have a ‘memory’ of cancer-specific odor molecules,” according to the statement from the American Chemical Society.
Since C. elegans is easy to grow in a lab and, apparently, easy to train, the researchers hope that the worm-on-a-chip can become a quick, easy, economical, and noninvasive cancer screen.
So watch out cancer, because we never bet against the creepy crawlies.
Mosquitoes have us figured out
We are nearing mosquito season; quite possibly the most annoying and itchy time of the year. We stock up on bottles of bug spray, but somehow we still get bite after bite. It appears that mosquitoes are basically able to ignore our bug sprays, which explains why we’re still covered in bites after the Fourth of July fireworks. It turns out mosquitoes are more complex than we thought for such tiny creatures.
There’s plenty of research on the best ways to keep mosquitoes away, because not only are they incredibly annoying, but they also carry potentially harmful diseases. In a recent experiment, researchers used mosquitoes that were genetically modified to have an excessive amount of an odor receptor called AgOR2, which responds to the smell of humans.
“AgOR2 overexpression threw a wrench in the whole system by inactivating olfactory receptors in these mosquitoes,” Christopher Potter, PhD, associate professor of neuroscience at Johns Hopkins University, said in a written statement.
After testing how these genetically modified mosquitoes reacted to some of the common smells of bug spray such as lemongrass, they discovered that it’s easy for the mosquitoes to ignore the smell. We wish it were that easy for us to ignore that chemically fruity smell.
Researchers continue to work hard to figure out how to repel mosquitoes and we’re rooting for them as summer approaches, despite the mosquito’s status as a creepy crawly.
Can you keep it down? I’m trying to be boring
He chides his friends for not looking both ways before crossing the road. He is never questioned by the police because they fall asleep listening to him talk. He has won the office’s coveted perfect attendance award 10 years running. Look out, Dos Equis guy, you’ve got some new competition. That’s right, it’s the most boring man in the world.
For this boring study (sorry, study on boredom) conducted by English researchers and published in Personality and Social Psychology Bulletin, people were surveyed on various jobs and hobbies, ranking them by how exciting or boring they are, as well as how competent someone with those jobs/hobbies would be, their willingness to avoid someone with those jobs/hobbies, and how much they’d need to be paid to spend time with someone who had an undesirable job/hobby.
According to the British public, the most boring person in the world is a religious data analyst who likes to sleep and lives in a small town. In fact, spending time with this person is almost a full-time job on its own: To make it worth their while, survey subjects wanted 35 pounds a day. The boring person also was viewed as less competent, as is anyone with a boring job.
Now, there probably aren’t a lot of religious data analysts out there, but don’t worry, there are plenty of other boring jobs – accounting, tax/insurance, cleaning, and banking rounded out the top five (apparently people don’t like finances) – and hobbies – watching TV, observing animals, and mathematics filled out the top five. In case you’re curious, performing artists, scientists, journalists, health professionals, and teachers were viewed as having exciting jobs; exciting hobbies included gaming, reading, domestic tasks (really?), gardening, and writing.
Lead researcher Wijnand Van Tilburg, PhD, made an excellent point about people with boring jobs: They “have power in society – perhaps we should try not to upset them and stereotype them as boring!”
We think they should lean into it and make The Most Boring Man in the World ads: “When I drive a car off the lot, its value increases because I used the correct lending association. Batman trusts me with his Batmobile insurance. I can make those Cuban cigars tax exempt. Stay financially solvent, my friends.”
Fungi, but make it fashion
Fashion is an expensive and costly industry to sustain. Cotton production takes a toll on the environment, leather production comes with environmental and ethical/moral conundrums, and thanks to fast fashion, about 85% of textiles are being thrown away in the United States.
Researchers at the University of Borås in Sweden, however, have found a newish solution to create leather, cotton, and other textiles. And as with so many of the finer things, it starts with unsold bread from the grocery store.
Akram Zamani, PhD, and her team take that bread and turn it into breadcrumbs, then combine it with water and Rhizopus delemar, a fungus typically found in decaying food. After a couple of days of feasting on the bread, the fungus produces natural fibers made of chitin and chitosan that accumulate in the cell walls. After proteins, lipids, and other byproducts are removed, the team is left with a jelly-like substance made of those fibrous cell walls that can be spun into a fabric.
The researchers started small with very thin nonpliable sheets, but with a little layering by using tree tannins for softness and alkali for strength, their fungal leather is more like real leather than competing fungal leathers. Not to mention its being able to be produced in a fraction of the time.
This new fungal leather is fast to produce, it’s biodegradable, and it uses only natural ingredients to treat the materials. It’s the ultimate environmental fashion statement.
Who’s afraid of cancer? Not C. elegans
And now, we bring you part 2 of our ongoing series: Creatures that can diagnose cancer. Last week, we discovered that ants are well on their way to replacing dogs in our medical labs and in our hearts. This week, we present the even-more-lovable nematode.
The soil-dwelling nematode Caenorhabditis elegans, which is less than 1 mm long, is known to be “attracted or repelled by certain odors, so we came up with an idea that the roundworm could be used to detect lung cancer,” Shin Sik Choi, PhD, of Myongji University in South Korea, who is the project’s principal investigator, said in a statement on Eurekalert.
Dr. Choi’s team created a “worm-on-a-chip” that allowed the nematodes to choose between a drop of culture media from lung cancer cells and media from normal lung fibroblasts. An hour after being placed in the chip’s central chamber, more nematodes had crawled toward the lung cancer media than the normal-cell sample.
The investigators estimate that the device is about 70% effective at detecting cancer cells, but “they hope to increase both the accuracy and sensitivity of the method by using worms that were previously exposed to cancer cell media and therefore have a ‘memory’ of cancer-specific odor molecules,” according to the statement from the American Chemical Society.
Since C. elegans is easy to grow in a lab and, apparently, easy to train, the researchers hope that the worm-on-a-chip can become a quick, easy, economical, and noninvasive cancer screen.
So watch out cancer, because we never bet against the creepy crawlies.
Mosquitoes have us figured out
We are nearing mosquito season; quite possibly the most annoying and itchy time of the year. We stock up on bottles of bug spray, but somehow we still get bite after bite. It appears that mosquitoes are basically able to ignore our bug sprays, which explains why we’re still covered in bites after the Fourth of July fireworks. It turns out mosquitoes are more complex than we thought for such tiny creatures.
There’s plenty of research on the best ways to keep mosquitoes away, because not only are they incredibly annoying, but they also carry potentially harmful diseases. In a recent experiment, researchers used mosquitoes that were genetically modified to have an excessive amount of an odor receptor called AgOR2, which responds to the smell of humans.
“AgOR2 overexpression threw a wrench in the whole system by inactivating olfactory receptors in these mosquitoes,” Christopher Potter, PhD, associate professor of neuroscience at Johns Hopkins University, said in a written statement.
After testing how these genetically modified mosquitoes reacted to some of the common smells of bug spray such as lemongrass, they discovered that it’s easy for the mosquitoes to ignore the smell. We wish it were that easy for us to ignore that chemically fruity smell.
Researchers continue to work hard to figure out how to repel mosquitoes and we’re rooting for them as summer approaches, despite the mosquito’s status as a creepy crawly.
Can you keep it down? I’m trying to be boring
He chides his friends for not looking both ways before crossing the road. He is never questioned by the police because they fall asleep listening to him talk. He has won the office’s coveted perfect attendance award 10 years running. Look out, Dos Equis guy, you’ve got some new competition. That’s right, it’s the most boring man in the world.
For this boring study (sorry, study on boredom) conducted by English researchers and published in Personality and Social Psychology Bulletin, people were surveyed on various jobs and hobbies, ranking them by how exciting or boring they are, as well as how competent someone with those jobs/hobbies would be, their willingness to avoid someone with those jobs/hobbies, and how much they’d need to be paid to spend time with someone who had an undesirable job/hobby.
According to the British public, the most boring person in the world is a religious data analyst who likes to sleep and lives in a small town. In fact, spending time with this person is almost a full-time job on its own: To make it worth their while, survey subjects wanted 35 pounds a day. The boring person also was viewed as less competent, as is anyone with a boring job.
Now, there probably aren’t a lot of religious data analysts out there, but don’t worry, there are plenty of other boring jobs – accounting, tax/insurance, cleaning, and banking rounded out the top five (apparently people don’t like finances) – and hobbies – watching TV, observing animals, and mathematics filled out the top five. In case you’re curious, performing artists, scientists, journalists, health professionals, and teachers were viewed as having exciting jobs; exciting hobbies included gaming, reading, domestic tasks (really?), gardening, and writing.
Lead researcher Wijnand Van Tilburg, PhD, made an excellent point about people with boring jobs: They “have power in society – perhaps we should try not to upset them and stereotype them as boring!”
We think they should lean into it and make The Most Boring Man in the World ads: “When I drive a car off the lot, its value increases because I used the correct lending association. Batman trusts me with his Batmobile insurance. I can make those Cuban cigars tax exempt. Stay financially solvent, my friends.”
Fungi, but make it fashion
Fashion is an expensive and costly industry to sustain. Cotton production takes a toll on the environment, leather production comes with environmental and ethical/moral conundrums, and thanks to fast fashion, about 85% of textiles are being thrown away in the United States.
Researchers at the University of Borås in Sweden, however, have found a newish solution to create leather, cotton, and other textiles. And as with so many of the finer things, it starts with unsold bread from the grocery store.
Akram Zamani, PhD, and her team take that bread and turn it into breadcrumbs, then combine it with water and Rhizopus delemar, a fungus typically found in decaying food. After a couple of days of feasting on the bread, the fungus produces natural fibers made of chitin and chitosan that accumulate in the cell walls. After proteins, lipids, and other byproducts are removed, the team is left with a jelly-like substance made of those fibrous cell walls that can be spun into a fabric.
The researchers started small with very thin nonpliable sheets, but with a little layering by using tree tannins for softness and alkali for strength, their fungal leather is more like real leather than competing fungal leathers. Not to mention its being able to be produced in a fraction of the time.
This new fungal leather is fast to produce, it’s biodegradable, and it uses only natural ingredients to treat the materials. It’s the ultimate environmental fashion statement.
Who’s afraid of cancer? Not C. elegans
And now, we bring you part 2 of our ongoing series: Creatures that can diagnose cancer. Last week, we discovered that ants are well on their way to replacing dogs in our medical labs and in our hearts. This week, we present the even-more-lovable nematode.
The soil-dwelling nematode Caenorhabditis elegans, which is less than 1 mm long, is known to be “attracted or repelled by certain odors, so we came up with an idea that the roundworm could be used to detect lung cancer,” Shin Sik Choi, PhD, of Myongji University in South Korea, who is the project’s principal investigator, said in a statement on Eurekalert.
Dr. Choi’s team created a “worm-on-a-chip” that allowed the nematodes to choose between a drop of culture media from lung cancer cells and media from normal lung fibroblasts. An hour after being placed in the chip’s central chamber, more nematodes had crawled toward the lung cancer media than the normal-cell sample.
The investigators estimate that the device is about 70% effective at detecting cancer cells, but “they hope to increase both the accuracy and sensitivity of the method by using worms that were previously exposed to cancer cell media and therefore have a ‘memory’ of cancer-specific odor molecules,” according to the statement from the American Chemical Society.
Since C. elegans is easy to grow in a lab and, apparently, easy to train, the researchers hope that the worm-on-a-chip can become a quick, easy, economical, and noninvasive cancer screen.
So watch out cancer, because we never bet against the creepy crawlies.
Mosquitoes have us figured out
We are nearing mosquito season; quite possibly the most annoying and itchy time of the year. We stock up on bottles of bug spray, but somehow we still get bite after bite. It appears that mosquitoes are basically able to ignore our bug sprays, which explains why we’re still covered in bites after the Fourth of July fireworks. It turns out mosquitoes are more complex than we thought for such tiny creatures.
There’s plenty of research on the best ways to keep mosquitoes away, because not only are they incredibly annoying, but they also carry potentially harmful diseases. In a recent experiment, researchers used mosquitoes that were genetically modified to have an excessive amount of an odor receptor called AgOR2, which responds to the smell of humans.
“AgOR2 overexpression threw a wrench in the whole system by inactivating olfactory receptors in these mosquitoes,” Christopher Potter, PhD, associate professor of neuroscience at Johns Hopkins University, said in a written statement.
After testing how these genetically modified mosquitoes reacted to some of the common smells of bug spray such as lemongrass, they discovered that it’s easy for the mosquitoes to ignore the smell. We wish it were that easy for us to ignore that chemically fruity smell.
Researchers continue to work hard to figure out how to repel mosquitoes and we’re rooting for them as summer approaches, despite the mosquito’s status as a creepy crawly.
Curcumin supplementation may improve metabolic, inflammatory, and obesity markers in women with RA
Key clinical point: Curcumin consumption for 8 weeks as a part of an integrated approach could help modulate metabolic factors, inflammation, and adiposity in women with rheumatoid arthritis (RA).
Major finding: After 8 weeks, insulin resistance, erythrocyte sedimentation rate, serum levels of high-sensitivity C-reactive protein, and triglycerides improved significantly in the curcumin supplementation vs. placebo group (all P < .05). Moreover, curcumin supplementation significantly decreased mean weight, body mass index, and waist circumference by 0.45% (P < .001), 0.57% (P = .003), and 0.23% (P = .008), respectively, vs. no significant changes observed in placebo group.
Study details: The findings come from a randomized, double-blind, placebo-controlled clinical trial including 44 women with RA randomly assigned to either curcumin supplementation (500 mg/day; n = 22) or placebo (n = 22) for 8 weeks.
Disclosures: This study was supported by Research Vice-Chancellor and Nutrition Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. The authors declared no conflicts of interest.
Source: Pourhabibi-Zarandi F et al. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2022 (Feb 17). Doi: 10.1002/ptr.7422
Key clinical point: Curcumin consumption for 8 weeks as a part of an integrated approach could help modulate metabolic factors, inflammation, and adiposity in women with rheumatoid arthritis (RA).
Major finding: After 8 weeks, insulin resistance, erythrocyte sedimentation rate, serum levels of high-sensitivity C-reactive protein, and triglycerides improved significantly in the curcumin supplementation vs. placebo group (all P < .05). Moreover, curcumin supplementation significantly decreased mean weight, body mass index, and waist circumference by 0.45% (P < .001), 0.57% (P = .003), and 0.23% (P = .008), respectively, vs. no significant changes observed in placebo group.
Study details: The findings come from a randomized, double-blind, placebo-controlled clinical trial including 44 women with RA randomly assigned to either curcumin supplementation (500 mg/day; n = 22) or placebo (n = 22) for 8 weeks.
Disclosures: This study was supported by Research Vice-Chancellor and Nutrition Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. The authors declared no conflicts of interest.
Source: Pourhabibi-Zarandi F et al. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2022 (Feb 17). Doi: 10.1002/ptr.7422
Key clinical point: Curcumin consumption for 8 weeks as a part of an integrated approach could help modulate metabolic factors, inflammation, and adiposity in women with rheumatoid arthritis (RA).
Major finding: After 8 weeks, insulin resistance, erythrocyte sedimentation rate, serum levels of high-sensitivity C-reactive protein, and triglycerides improved significantly in the curcumin supplementation vs. placebo group (all P < .05). Moreover, curcumin supplementation significantly decreased mean weight, body mass index, and waist circumference by 0.45% (P < .001), 0.57% (P = .003), and 0.23% (P = .008), respectively, vs. no significant changes observed in placebo group.
Study details: The findings come from a randomized, double-blind, placebo-controlled clinical trial including 44 women with RA randomly assigned to either curcumin supplementation (500 mg/day; n = 22) or placebo (n = 22) for 8 weeks.
Disclosures: This study was supported by Research Vice-Chancellor and Nutrition Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. The authors declared no conflicts of interest.
Source: Pourhabibi-Zarandi F et al. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2022 (Feb 17). Doi: 10.1002/ptr.7422
Long-term use of fostamatinib may increase the risk for malignant neoplasm in RA
Key clinical point: Fostamatinib use in patients with rheumatoid arthritis (RA) was not associated with an overall increased risk of developing neoplasms compared with placebo. However, prolonged use may raise the risk for malignant neoplasms.
Major finding: Fostamatinib vs. placebo was not associated with an increased risk for total neoplasms (Peto odds ratio [OR] 2.62; 95% CI 0.97-7.10), malignant neoplasms (Peto OR 3.08; 95% CI 0.96-9.91), or benign neoplasms (Peto OR 1.71; 95% CI 0.26-11.36). However, long-term fostamatinib treatment vs. placebo was associated with a higher risk for malignant neoplasms at 52 weeks (Peto OR 4.49; 95% CI 1.03-19.60).
Study details: This was a meta-analysis of seven trials including 4,971 patients with RA treated with fostamatinib.
Disclosures: This study was supported by Post-doctoral Research and Development Fund of West China Hospital of Sichuan University and others. The authors declared no conflict of interests.
Source: Chan Y et al. Neoplasm risk in patients with rheumatoid arthritis treated with fostamatinib: A systematic review and meta-analysis. Front Pharmacol. 2022;13:768980 (Mar 2). Doi: 10.3389/fphar.2022.768980
Key clinical point: Fostamatinib use in patients with rheumatoid arthritis (RA) was not associated with an overall increased risk of developing neoplasms compared with placebo. However, prolonged use may raise the risk for malignant neoplasms.
Major finding: Fostamatinib vs. placebo was not associated with an increased risk for total neoplasms (Peto odds ratio [OR] 2.62; 95% CI 0.97-7.10), malignant neoplasms (Peto OR 3.08; 95% CI 0.96-9.91), or benign neoplasms (Peto OR 1.71; 95% CI 0.26-11.36). However, long-term fostamatinib treatment vs. placebo was associated with a higher risk for malignant neoplasms at 52 weeks (Peto OR 4.49; 95% CI 1.03-19.60).
Study details: This was a meta-analysis of seven trials including 4,971 patients with RA treated with fostamatinib.
Disclosures: This study was supported by Post-doctoral Research and Development Fund of West China Hospital of Sichuan University and others. The authors declared no conflict of interests.
Source: Chan Y et al. Neoplasm risk in patients with rheumatoid arthritis treated with fostamatinib: A systematic review and meta-analysis. Front Pharmacol. 2022;13:768980 (Mar 2). Doi: 10.3389/fphar.2022.768980
Key clinical point: Fostamatinib use in patients with rheumatoid arthritis (RA) was not associated with an overall increased risk of developing neoplasms compared with placebo. However, prolonged use may raise the risk for malignant neoplasms.
Major finding: Fostamatinib vs. placebo was not associated with an increased risk for total neoplasms (Peto odds ratio [OR] 2.62; 95% CI 0.97-7.10), malignant neoplasms (Peto OR 3.08; 95% CI 0.96-9.91), or benign neoplasms (Peto OR 1.71; 95% CI 0.26-11.36). However, long-term fostamatinib treatment vs. placebo was associated with a higher risk for malignant neoplasms at 52 weeks (Peto OR 4.49; 95% CI 1.03-19.60).
Study details: This was a meta-analysis of seven trials including 4,971 patients with RA treated with fostamatinib.
Disclosures: This study was supported by Post-doctoral Research and Development Fund of West China Hospital of Sichuan University and others. The authors declared no conflict of interests.
Source: Chan Y et al. Neoplasm risk in patients with rheumatoid arthritis treated with fostamatinib: A systematic review and meta-analysis. Front Pharmacol. 2022;13:768980 (Mar 2). Doi: 10.3389/fphar.2022.768980
Higher intake of coffee and decaffeinated coffee linked to risk of developing RA
Key clinical point: Higher intake of coffee and decaffeinated coffee was associated with a higher risk of developing rheumatoid arthritis (RA).
Major finding: Each additional cup of coffee and decaffeinated coffee consumed per day increased the risk of developing RA by 6% (relative risk [RR] 1.06; 95% CI 1.02-1.10) and 11% (RR 1.11; 95% CI 1.05-1.18), respectively. However, no such association was observed between consumption of caffeinated coffee, caffeine, or tea and risk for RA.
Study details: This was a dose-response meta-analysis of five prospective cohort studies involving 266,985 participants.
Disclosures: No information on funding was reported. The authors declared no potential conflicts of interest.
Source: Asoudeh F et al. Caffeine, coffee, tea and risk of rheumatoid arthritis: Systematic review and dose-response meta-analysis of prospective cohort studies. Front Nutr. 2022;9:822557 (Feb 10). Doi: 10.3389/fnut.2022.822557
Key clinical point: Higher intake of coffee and decaffeinated coffee was associated with a higher risk of developing rheumatoid arthritis (RA).
Major finding: Each additional cup of coffee and decaffeinated coffee consumed per day increased the risk of developing RA by 6% (relative risk [RR] 1.06; 95% CI 1.02-1.10) and 11% (RR 1.11; 95% CI 1.05-1.18), respectively. However, no such association was observed between consumption of caffeinated coffee, caffeine, or tea and risk for RA.
Study details: This was a dose-response meta-analysis of five prospective cohort studies involving 266,985 participants.
Disclosures: No information on funding was reported. The authors declared no potential conflicts of interest.
Source: Asoudeh F et al. Caffeine, coffee, tea and risk of rheumatoid arthritis: Systematic review and dose-response meta-analysis of prospective cohort studies. Front Nutr. 2022;9:822557 (Feb 10). Doi: 10.3389/fnut.2022.822557
Key clinical point: Higher intake of coffee and decaffeinated coffee was associated with a higher risk of developing rheumatoid arthritis (RA).
Major finding: Each additional cup of coffee and decaffeinated coffee consumed per day increased the risk of developing RA by 6% (relative risk [RR] 1.06; 95% CI 1.02-1.10) and 11% (RR 1.11; 95% CI 1.05-1.18), respectively. However, no such association was observed between consumption of caffeinated coffee, caffeine, or tea and risk for RA.
Study details: This was a dose-response meta-analysis of five prospective cohort studies involving 266,985 participants.
Disclosures: No information on funding was reported. The authors declared no potential conflicts of interest.
Source: Asoudeh F et al. Caffeine, coffee, tea and risk of rheumatoid arthritis: Systematic review and dose-response meta-analysis of prospective cohort studies. Front Nutr. 2022;9:822557 (Feb 10). Doi: 10.3389/fnut.2022.822557
Real-world study finds increased remission with fewer corticosteroids and more biologics in RA
Key clinical point: Modern management of rheumatoid arthritis (RA) led to a larger proportion of patients achieving remission with fewer corticosteroids and more biologics over a 7-year follow-up period.
Major finding: Twice as many patients with RA were in remission at 7-year follow-up vs. at 1 year. Patients achieving remission at 7 years were more likely to be using biological disease-modifying antirheumatic drugs (DMARD) and conventional DMARD (P = .02), whereas corticosteroids (P = .02) at higher doses (P = .013) were mostly prescribed to patients not achieving remission at 7 years.
Study details: The data come from a retrospective study of 215 patients with RA whose clinical and biological data were analyzed at 1 year (2009) and 7 years (2015).
Disclosures: The authors declared no source of funding or conflicts of interest.
Source: Larid G et al. Increased remission with fewer corticosteroids and more biologics in rheumatoid arthritis at 7-year follow-up in real-life conditions. Sci Rep. 2022;12:2563 (Feb 15). Doi: 10.1038/s41598-022-06584-y
Key clinical point: Modern management of rheumatoid arthritis (RA) led to a larger proportion of patients achieving remission with fewer corticosteroids and more biologics over a 7-year follow-up period.
Major finding: Twice as many patients with RA were in remission at 7-year follow-up vs. at 1 year. Patients achieving remission at 7 years were more likely to be using biological disease-modifying antirheumatic drugs (DMARD) and conventional DMARD (P = .02), whereas corticosteroids (P = .02) at higher doses (P = .013) were mostly prescribed to patients not achieving remission at 7 years.
Study details: The data come from a retrospective study of 215 patients with RA whose clinical and biological data were analyzed at 1 year (2009) and 7 years (2015).
Disclosures: The authors declared no source of funding or conflicts of interest.
Source: Larid G et al. Increased remission with fewer corticosteroids and more biologics in rheumatoid arthritis at 7-year follow-up in real-life conditions. Sci Rep. 2022;12:2563 (Feb 15). Doi: 10.1038/s41598-022-06584-y
Key clinical point: Modern management of rheumatoid arthritis (RA) led to a larger proportion of patients achieving remission with fewer corticosteroids and more biologics over a 7-year follow-up period.
Major finding: Twice as many patients with RA were in remission at 7-year follow-up vs. at 1 year. Patients achieving remission at 7 years were more likely to be using biological disease-modifying antirheumatic drugs (DMARD) and conventional DMARD (P = .02), whereas corticosteroids (P = .02) at higher doses (P = .013) were mostly prescribed to patients not achieving remission at 7 years.
Study details: The data come from a retrospective study of 215 patients with RA whose clinical and biological data were analyzed at 1 year (2009) and 7 years (2015).
Disclosures: The authors declared no source of funding or conflicts of interest.
Source: Larid G et al. Increased remission with fewer corticosteroids and more biologics in rheumatoid arthritis at 7-year follow-up in real-life conditions. Sci Rep. 2022;12:2563 (Feb 15). Doi: 10.1038/s41598-022-06584-y
Temporary methotrexate withdrawal boosts anti-SARS-CoV-2 immunogenicity in RA
Key clinical point: A 2-week methotrexate withdrawal after each dose of Sinovac-CoronaVac vaccine may improve anti-SARS-CoV-2 immunogenicity in patients with rheumatoid arthritis (RA) with low disease activity (LDA) or remission. However, this may increase flare rates, thereby requiring close surveillance of disease activity.
Major finding: At 6 weeks after the second vaccine dose, patients who withheld vs. maintained methotrexate after both vaccine shots had a significantly higher seroconversion (P = .019) with a parallel increase in geometric mean titer (P = .006). However, the flare rate (Clinical Disease Activity Index >10) was higher in the group that withheld vs. continued methotrexate (P = .011).
Study details: Findings are from the single-center, prospective CoronavRheum study that included 138 patients with RA with LDA/remission at first vaccine dose who were randomly assigned to maintain (n = 69) methotrexate use or institute a 2-week withdrawal (n = 60) of methotrexate after each dose of Sinovac-CoronaVac vaccine.
Disclosures: This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and B3 - Bolsa de Valores do Brasil. No conflicts of interest were declared.
Source: Renner Araujo CS et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: A randomised clinical trial. Ann Rheum Dis. 2022 (Feb 22). Doi: 10.1136/annrheumdis-2021-221916
Key clinical point: A 2-week methotrexate withdrawal after each dose of Sinovac-CoronaVac vaccine may improve anti-SARS-CoV-2 immunogenicity in patients with rheumatoid arthritis (RA) with low disease activity (LDA) or remission. However, this may increase flare rates, thereby requiring close surveillance of disease activity.
Major finding: At 6 weeks after the second vaccine dose, patients who withheld vs. maintained methotrexate after both vaccine shots had a significantly higher seroconversion (P = .019) with a parallel increase in geometric mean titer (P = .006). However, the flare rate (Clinical Disease Activity Index >10) was higher in the group that withheld vs. continued methotrexate (P = .011).
Study details: Findings are from the single-center, prospective CoronavRheum study that included 138 patients with RA with LDA/remission at first vaccine dose who were randomly assigned to maintain (n = 69) methotrexate use or institute a 2-week withdrawal (n = 60) of methotrexate after each dose of Sinovac-CoronaVac vaccine.
Disclosures: This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and B3 - Bolsa de Valores do Brasil. No conflicts of interest were declared.
Source: Renner Araujo CS et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: A randomised clinical trial. Ann Rheum Dis. 2022 (Feb 22). Doi: 10.1136/annrheumdis-2021-221916
Key clinical point: A 2-week methotrexate withdrawal after each dose of Sinovac-CoronaVac vaccine may improve anti-SARS-CoV-2 immunogenicity in patients with rheumatoid arthritis (RA) with low disease activity (LDA) or remission. However, this may increase flare rates, thereby requiring close surveillance of disease activity.
Major finding: At 6 weeks after the second vaccine dose, patients who withheld vs. maintained methotrexate after both vaccine shots had a significantly higher seroconversion (P = .019) with a parallel increase in geometric mean titer (P = .006). However, the flare rate (Clinical Disease Activity Index >10) was higher in the group that withheld vs. continued methotrexate (P = .011).
Study details: Findings are from the single-center, prospective CoronavRheum study that included 138 patients with RA with LDA/remission at first vaccine dose who were randomly assigned to maintain (n = 69) methotrexate use or institute a 2-week withdrawal (n = 60) of methotrexate after each dose of Sinovac-CoronaVac vaccine.
Disclosures: This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and B3 - Bolsa de Valores do Brasil. No conflicts of interest were declared.
Source: Renner Araujo CS et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: A randomised clinical trial. Ann Rheum Dis. 2022 (Feb 22). Doi: 10.1136/annrheumdis-2021-221916
Predictors of flare following remission and treatment withdrawal in early RA
Key clinical point: Patient physical function and magnetic resonance imaging (MRI) measures of inflammation and damage at the time of treatment withdrawal (tWD) were independent predictors of flare at 6 and 12 months after tWD in patients with early rheumatoid arthritis (RA).
Major finding: At 6 and 12 months after tWD, a Health Assessment Questionnaire-Disability Index score of >0.5 (adjusted odds ratio [aOR] 3.97; P = .0060 and aOR 5.09; P = .0048, respectively) and an MRI erosion score of >2 (aOR 2.81; P = .0176 and aOR 2.38; P = .0495, respectively) were independently associated with flare.
Study details: This was a post hoc analysis of phase 3b AVERT study involving 172 patients with early RA who achieved remission with methotrexate or abatacept at 1 year and entered a 12-month tWD period.
Disclosures: The study was supported by Bristol Myers Squibb. The authors reported receiving grant/research funding and speaking and consulting fees from various sources, including Bristol Myers Squibb. Dr. Ahmad and Dr. Banerjee reported being employees or shareholders of Bristol Myers Squibb.
Source: Ahmad HA et al. Prediction of flare following remission and treatment withdrawal in early rheumatoid arthritis: Post hoc analysis of a phase IIIb trial with abatacept. Arthritis Res Ther. 2022;24:47 (Feb 16). Doi: 10.1186/s13075-022-02735-8
Key clinical point: Patient physical function and magnetic resonance imaging (MRI) measures of inflammation and damage at the time of treatment withdrawal (tWD) were independent predictors of flare at 6 and 12 months after tWD in patients with early rheumatoid arthritis (RA).
Major finding: At 6 and 12 months after tWD, a Health Assessment Questionnaire-Disability Index score of >0.5 (adjusted odds ratio [aOR] 3.97; P = .0060 and aOR 5.09; P = .0048, respectively) and an MRI erosion score of >2 (aOR 2.81; P = .0176 and aOR 2.38; P = .0495, respectively) were independently associated with flare.
Study details: This was a post hoc analysis of phase 3b AVERT study involving 172 patients with early RA who achieved remission with methotrexate or abatacept at 1 year and entered a 12-month tWD period.
Disclosures: The study was supported by Bristol Myers Squibb. The authors reported receiving grant/research funding and speaking and consulting fees from various sources, including Bristol Myers Squibb. Dr. Ahmad and Dr. Banerjee reported being employees or shareholders of Bristol Myers Squibb.
Source: Ahmad HA et al. Prediction of flare following remission and treatment withdrawal in early rheumatoid arthritis: Post hoc analysis of a phase IIIb trial with abatacept. Arthritis Res Ther. 2022;24:47 (Feb 16). Doi: 10.1186/s13075-022-02735-8
Key clinical point: Patient physical function and magnetic resonance imaging (MRI) measures of inflammation and damage at the time of treatment withdrawal (tWD) were independent predictors of flare at 6 and 12 months after tWD in patients with early rheumatoid arthritis (RA).
Major finding: At 6 and 12 months after tWD, a Health Assessment Questionnaire-Disability Index score of >0.5 (adjusted odds ratio [aOR] 3.97; P = .0060 and aOR 5.09; P = .0048, respectively) and an MRI erosion score of >2 (aOR 2.81; P = .0176 and aOR 2.38; P = .0495, respectively) were independently associated with flare.
Study details: This was a post hoc analysis of phase 3b AVERT study involving 172 patients with early RA who achieved remission with methotrexate or abatacept at 1 year and entered a 12-month tWD period.
Disclosures: The study was supported by Bristol Myers Squibb. The authors reported receiving grant/research funding and speaking and consulting fees from various sources, including Bristol Myers Squibb. Dr. Ahmad and Dr. Banerjee reported being employees or shareholders of Bristol Myers Squibb.
Source: Ahmad HA et al. Prediction of flare following remission and treatment withdrawal in early rheumatoid arthritis: Post hoc analysis of a phase IIIb trial with abatacept. Arthritis Res Ther. 2022;24:47 (Feb 16). Doi: 10.1186/s13075-022-02735-8
Satisfactory immune response to PCV-13 in RA patients receiving upadacitinib
Key clinical point: Approximately two-thirds of patients with rheumatoid arthritis (RA) receiving 15 mg upadacitinib and methotrexate attained a satisfactory humoral response to pneumococcal 13-valent conjugate vaccine (PCV-13) through 12 weeks postvaccination.
Major finding: At 4 weeks, 67.5% (95% CI 57.4%-77.5%) of patients receiving 15 mg upadacitinib and methotrexate attained a satisfactory humoral response to PCV-13, which was sustained until week 12 (64.6%; 95% CI 54.0%-75.1%), with response being similar irrespective of concomitant corticosteroids. Adverse events were mostly mild in severity.
Study details: Findings are from a phase 2 open-label extension trial, Balance-Extend, including 111 patients with RA receiving a stable dose of 15 mg upadacitinib (n = 87) or 30 mg (n = 24) once daily with background methotrexate who were administered PCV-13.
Disclosures: This study was supported by AbbVie. A Friedman, B Hendrickson, Y Li, and J Klaff reported being employees or shareholders of AbbVie, and others declared serving as editorial board members or receiving grants, consulting fees, or honoraria from various sources, including AbbVie.
Source: Winthrop K et al. Evaluation of response to 13-valent conjugated pneumococcal vaccination in patients with rheumatoid arthritis receiving upadacitinib: Results from a phase 2 open-label extension study. RMD Open. 2022;8:e002110 (Mar 4). Doi: 10.1136/rmdopen-2021-002110
Key clinical point: Approximately two-thirds of patients with rheumatoid arthritis (RA) receiving 15 mg upadacitinib and methotrexate attained a satisfactory humoral response to pneumococcal 13-valent conjugate vaccine (PCV-13) through 12 weeks postvaccination.
Major finding: At 4 weeks, 67.5% (95% CI 57.4%-77.5%) of patients receiving 15 mg upadacitinib and methotrexate attained a satisfactory humoral response to PCV-13, which was sustained until week 12 (64.6%; 95% CI 54.0%-75.1%), with response being similar irrespective of concomitant corticosteroids. Adverse events were mostly mild in severity.
Study details: Findings are from a phase 2 open-label extension trial, Balance-Extend, including 111 patients with RA receiving a stable dose of 15 mg upadacitinib (n = 87) or 30 mg (n = 24) once daily with background methotrexate who were administered PCV-13.
Disclosures: This study was supported by AbbVie. A Friedman, B Hendrickson, Y Li, and J Klaff reported being employees or shareholders of AbbVie, and others declared serving as editorial board members or receiving grants, consulting fees, or honoraria from various sources, including AbbVie.
Source: Winthrop K et al. Evaluation of response to 13-valent conjugated pneumococcal vaccination in patients with rheumatoid arthritis receiving upadacitinib: Results from a phase 2 open-label extension study. RMD Open. 2022;8:e002110 (Mar 4). Doi: 10.1136/rmdopen-2021-002110
Key clinical point: Approximately two-thirds of patients with rheumatoid arthritis (RA) receiving 15 mg upadacitinib and methotrexate attained a satisfactory humoral response to pneumococcal 13-valent conjugate vaccine (PCV-13) through 12 weeks postvaccination.
Major finding: At 4 weeks, 67.5% (95% CI 57.4%-77.5%) of patients receiving 15 mg upadacitinib and methotrexate attained a satisfactory humoral response to PCV-13, which was sustained until week 12 (64.6%; 95% CI 54.0%-75.1%), with response being similar irrespective of concomitant corticosteroids. Adverse events were mostly mild in severity.
Study details: Findings are from a phase 2 open-label extension trial, Balance-Extend, including 111 patients with RA receiving a stable dose of 15 mg upadacitinib (n = 87) or 30 mg (n = 24) once daily with background methotrexate who were administered PCV-13.
Disclosures: This study was supported by AbbVie. A Friedman, B Hendrickson, Y Li, and J Klaff reported being employees or shareholders of AbbVie, and others declared serving as editorial board members or receiving grants, consulting fees, or honoraria from various sources, including AbbVie.
Source: Winthrop K et al. Evaluation of response to 13-valent conjugated pneumococcal vaccination in patients with rheumatoid arthritis receiving upadacitinib: Results from a phase 2 open-label extension study. RMD Open. 2022;8:e002110 (Mar 4). Doi: 10.1136/rmdopen-2021-002110