User login
All in the family
Six female doctors from two families share their journeys through medicine.
When Annie Uhing, MD, is stressed about work, she can call her mom. She and her mom are close, yes, but her mom is also a physician and understands the ups and downs of medical education and the unique challenges of being a woman in medicine.
“My mom and I were talking about this the other day – I don’t think we know any other mother-daughter pairs of doctors,” said Dr. Uhing.
In the United States, the number of female physicians has risen steadily since the mid- and late-20th century. As of 2019, women made up more than half of medical school classes across the country and 36.3% of the physician workforce.
Still, most female physicians are concentrated in a handful of specialties (such as pediatrics and obstetrics and gynecology) while the percentages of women in other areas remains extremely low (urology and orthopedic surgery). Many female physicians share anecdotal stories about not being taken seriously, like when a patient mistook them for a nurse, or preferred the advice of a male colleague to their own.
To celebrate International Women’s Day, this news organization talked to two families of female doctors about their experiences in medicine and how they inspire and support one another inside and outside the hospital.
Deborah, Charlene, and Annie
When Deborah Gaebler-Spira, MD, started medical school at the University of Illinois in 1975, women made up just 15% of her class. “For me, the idea that as a woman you could have a vocation that could be quite meaningful and self-directed – that was very important,” said Dr. Gaebler-Spira, now a pediatric rehabilitation physician at the Shirley Ryan Ability Lab and professor at Northwestern University in Chicago.
She blocked out a lot of discouragement along the way. In undergrad, the dean of the college warned Dr. Gaebler-Spira she’d never make it as a doctor. In medical school interviews, administrators could be hostile. “There was this feeling that you were taking a place of someone who really deserved it,” she said. When selecting a residency, Dr. Gaebler-Spira decided against a career in obstetrics because of the overt misogyny in the field at the time.
Instead, she went into pediatrics and physical medicine and rehabilitation, eventually working to become an expert in cerebral palsy. Along the way, Dr. Gaebler-Spira made lifelong friends with other female physicians and found strong female mentors, including Billie Adams, MD, and Helen Emery, MD.
When her sister, Charlene Gaebler-Uhing, MD, also decided to go into medicine, Dr. Gaebler-Spira said she “thought it was a sign of sanity as she was always much more competitive than I was! And if I could do it, no question she was able!”
Dr. Gaebler-Uhing, now an adolescent medicine specialist at Children’s Wisconsin in Milwaukee, followed her older sister’s footsteps to medical school in 1983, after first considering a career in social work.
While there were now more women going into medicine – her medical school class was about 25% women – problems persisted. During clinical rotations in residency, Dr. Gaebler-Uhing was often the only woman on a team and made the conscious decision to go professionally by her nickname, Charlie. “If a woman’s name was on the consult, her opinion and insights did not get the same value or respect as a male physician’s,” she said. “The only way they knew I was a woman was if they really knew me.”
The Gaebler sisters leaned on each other professionally and personally throughout their careers. When both sisters practiced in Chicago, they referred patients to one another. And Dr. Gaebler-Uhing said her older sister was a great role model for how to balance the dual roles of physician and parent, as few of the older female doctors who trained her were married or had a child.
Now Dr. Gaebler-Uhing’s daughter, Annie Uhing, MD, is entering medicine herself. She is currently pediatric resident at the University of Wisconsin American Family Hospital. She plans to do a chief year and then a pediatric endocrinology fellowship.
Growing up, Dr. Uhing wasn’t always sure she wanted to work as much as her parents, who are both doctors. But her mom provided a great example few of her friends had at home: “If you want to work, you should work and do what you want to do and it’s not wrong to want to have a really high-powered job as a woman,” said Dr. Uhing.
Kathryn, Susan, and Rita
The three sisters Kathryn Hudson, MD, Susan Schmidt, MD, and Rita Butler, MD, were inspired to go into medicine by their mother, Rita Watson, MD, who was one of the first female interventional cardiologists in the United States.
“I think we had a front row seat to what being a doctor was like,” said Dr. Hudson, a hematologist and oncologist and director of survivorship at Texas Oncology in Austin. Both parents were MDs – their dad was a pharmaceutical researcher at Merck – and they would excitedly discuss patient cases and drug development at the dinner table, said Dr. Butler, an interventional cardiology fellow at the Lankenau Heart Institute in Wynnewood, Pa.
All three sisters have vivid memories of ‘Take Your Daughter to Work Day’ at their mom’s hospital. “I remember going to Take Your Daughter to Work Day with her and watching her in action and thinking, oh my gosh, my mom is so cool and I want to be like her,” said Dr. Schmidt, a pediatric critical care specialist at St. Christopher’s Hospital for Children in Philadelphia. “I’ve always felt special that my mom was doing something really cool and really saving lives,” said Dr. Schmidt.
Their fourth sibling, John, isn’t a physician and “I honestly wonder if it’s because he never went to Take Your Daughter to Work Day!” said Dr. Butler.
Having a mother who had both a high-powered medical career and a family helped the three women know they could do the same. “It is a difficult journey, don’t get me wrong, but I never questioned that I could do it because my mom did it first,” said Dr. Hudson.
As adults, the sisters confide in one another as they navigate modern motherhood and careers, switching between discussing medical cases and parenting advice.
As hard as their mom worked while they were growing up, she didn’t have the pressure of living up to the “super mom” ideal we have now, said Dr. Butler. “Everyone wants women to work like they don’t have kids and everyone wants women to parent like they don’t have a job,” she said. Having two sisters who can provide reassurance and advice in that area goes a long way, she said.
“I think sharing that experience of navigating motherhood, a medical career, and marriage, and adult life with sisters who are going through all the same things is really special and I feel really fortunate for that,” said Dr. Schmidt.
*This story was updated on 3/8/2022.
Six female doctors from two families share their journeys through medicine.
Six female doctors from two families share their journeys through medicine.
When Annie Uhing, MD, is stressed about work, she can call her mom. She and her mom are close, yes, but her mom is also a physician and understands the ups and downs of medical education and the unique challenges of being a woman in medicine.
“My mom and I were talking about this the other day – I don’t think we know any other mother-daughter pairs of doctors,” said Dr. Uhing.
In the United States, the number of female physicians has risen steadily since the mid- and late-20th century. As of 2019, women made up more than half of medical school classes across the country and 36.3% of the physician workforce.
Still, most female physicians are concentrated in a handful of specialties (such as pediatrics and obstetrics and gynecology) while the percentages of women in other areas remains extremely low (urology and orthopedic surgery). Many female physicians share anecdotal stories about not being taken seriously, like when a patient mistook them for a nurse, or preferred the advice of a male colleague to their own.
To celebrate International Women’s Day, this news organization talked to two families of female doctors about their experiences in medicine and how they inspire and support one another inside and outside the hospital.
Deborah, Charlene, and Annie
When Deborah Gaebler-Spira, MD, started medical school at the University of Illinois in 1975, women made up just 15% of her class. “For me, the idea that as a woman you could have a vocation that could be quite meaningful and self-directed – that was very important,” said Dr. Gaebler-Spira, now a pediatric rehabilitation physician at the Shirley Ryan Ability Lab and professor at Northwestern University in Chicago.
She blocked out a lot of discouragement along the way. In undergrad, the dean of the college warned Dr. Gaebler-Spira she’d never make it as a doctor. In medical school interviews, administrators could be hostile. “There was this feeling that you were taking a place of someone who really deserved it,” she said. When selecting a residency, Dr. Gaebler-Spira decided against a career in obstetrics because of the overt misogyny in the field at the time.
Instead, she went into pediatrics and physical medicine and rehabilitation, eventually working to become an expert in cerebral palsy. Along the way, Dr. Gaebler-Spira made lifelong friends with other female physicians and found strong female mentors, including Billie Adams, MD, and Helen Emery, MD.
When her sister, Charlene Gaebler-Uhing, MD, also decided to go into medicine, Dr. Gaebler-Spira said she “thought it was a sign of sanity as she was always much more competitive than I was! And if I could do it, no question she was able!”
Dr. Gaebler-Uhing, now an adolescent medicine specialist at Children’s Wisconsin in Milwaukee, followed her older sister’s footsteps to medical school in 1983, after first considering a career in social work.
While there were now more women going into medicine – her medical school class was about 25% women – problems persisted. During clinical rotations in residency, Dr. Gaebler-Uhing was often the only woman on a team and made the conscious decision to go professionally by her nickname, Charlie. “If a woman’s name was on the consult, her opinion and insights did not get the same value or respect as a male physician’s,” she said. “The only way they knew I was a woman was if they really knew me.”
The Gaebler sisters leaned on each other professionally and personally throughout their careers. When both sisters practiced in Chicago, they referred patients to one another. And Dr. Gaebler-Uhing said her older sister was a great role model for how to balance the dual roles of physician and parent, as few of the older female doctors who trained her were married or had a child.
Now Dr. Gaebler-Uhing’s daughter, Annie Uhing, MD, is entering medicine herself. She is currently pediatric resident at the University of Wisconsin American Family Hospital. She plans to do a chief year and then a pediatric endocrinology fellowship.
Growing up, Dr. Uhing wasn’t always sure she wanted to work as much as her parents, who are both doctors. But her mom provided a great example few of her friends had at home: “If you want to work, you should work and do what you want to do and it’s not wrong to want to have a really high-powered job as a woman,” said Dr. Uhing.
Kathryn, Susan, and Rita
The three sisters Kathryn Hudson, MD, Susan Schmidt, MD, and Rita Butler, MD, were inspired to go into medicine by their mother, Rita Watson, MD, who was one of the first female interventional cardiologists in the United States.
“I think we had a front row seat to what being a doctor was like,” said Dr. Hudson, a hematologist and oncologist and director of survivorship at Texas Oncology in Austin. Both parents were MDs – their dad was a pharmaceutical researcher at Merck – and they would excitedly discuss patient cases and drug development at the dinner table, said Dr. Butler, an interventional cardiology fellow at the Lankenau Heart Institute in Wynnewood, Pa.
All three sisters have vivid memories of ‘Take Your Daughter to Work Day’ at their mom’s hospital. “I remember going to Take Your Daughter to Work Day with her and watching her in action and thinking, oh my gosh, my mom is so cool and I want to be like her,” said Dr. Schmidt, a pediatric critical care specialist at St. Christopher’s Hospital for Children in Philadelphia. “I’ve always felt special that my mom was doing something really cool and really saving lives,” said Dr. Schmidt.
Their fourth sibling, John, isn’t a physician and “I honestly wonder if it’s because he never went to Take Your Daughter to Work Day!” said Dr. Butler.
Having a mother who had both a high-powered medical career and a family helped the three women know they could do the same. “It is a difficult journey, don’t get me wrong, but I never questioned that I could do it because my mom did it first,” said Dr. Hudson.
As adults, the sisters confide in one another as they navigate modern motherhood and careers, switching between discussing medical cases and parenting advice.
As hard as their mom worked while they were growing up, she didn’t have the pressure of living up to the “super mom” ideal we have now, said Dr. Butler. “Everyone wants women to work like they don’t have kids and everyone wants women to parent like they don’t have a job,” she said. Having two sisters who can provide reassurance and advice in that area goes a long way, she said.
“I think sharing that experience of navigating motherhood, a medical career, and marriage, and adult life with sisters who are going through all the same things is really special and I feel really fortunate for that,” said Dr. Schmidt.
*This story was updated on 3/8/2022.
When Annie Uhing, MD, is stressed about work, she can call her mom. She and her mom are close, yes, but her mom is also a physician and understands the ups and downs of medical education and the unique challenges of being a woman in medicine.
“My mom and I were talking about this the other day – I don’t think we know any other mother-daughter pairs of doctors,” said Dr. Uhing.
In the United States, the number of female physicians has risen steadily since the mid- and late-20th century. As of 2019, women made up more than half of medical school classes across the country and 36.3% of the physician workforce.
Still, most female physicians are concentrated in a handful of specialties (such as pediatrics and obstetrics and gynecology) while the percentages of women in other areas remains extremely low (urology and orthopedic surgery). Many female physicians share anecdotal stories about not being taken seriously, like when a patient mistook them for a nurse, or preferred the advice of a male colleague to their own.
To celebrate International Women’s Day, this news organization talked to two families of female doctors about their experiences in medicine and how they inspire and support one another inside and outside the hospital.
Deborah, Charlene, and Annie
When Deborah Gaebler-Spira, MD, started medical school at the University of Illinois in 1975, women made up just 15% of her class. “For me, the idea that as a woman you could have a vocation that could be quite meaningful and self-directed – that was very important,” said Dr. Gaebler-Spira, now a pediatric rehabilitation physician at the Shirley Ryan Ability Lab and professor at Northwestern University in Chicago.
She blocked out a lot of discouragement along the way. In undergrad, the dean of the college warned Dr. Gaebler-Spira she’d never make it as a doctor. In medical school interviews, administrators could be hostile. “There was this feeling that you were taking a place of someone who really deserved it,” she said. When selecting a residency, Dr. Gaebler-Spira decided against a career in obstetrics because of the overt misogyny in the field at the time.
Instead, she went into pediatrics and physical medicine and rehabilitation, eventually working to become an expert in cerebral palsy. Along the way, Dr. Gaebler-Spira made lifelong friends with other female physicians and found strong female mentors, including Billie Adams, MD, and Helen Emery, MD.
When her sister, Charlene Gaebler-Uhing, MD, also decided to go into medicine, Dr. Gaebler-Spira said she “thought it was a sign of sanity as she was always much more competitive than I was! And if I could do it, no question she was able!”
Dr. Gaebler-Uhing, now an adolescent medicine specialist at Children’s Wisconsin in Milwaukee, followed her older sister’s footsteps to medical school in 1983, after first considering a career in social work.
While there were now more women going into medicine – her medical school class was about 25% women – problems persisted. During clinical rotations in residency, Dr. Gaebler-Uhing was often the only woman on a team and made the conscious decision to go professionally by her nickname, Charlie. “If a woman’s name was on the consult, her opinion and insights did not get the same value or respect as a male physician’s,” she said. “The only way they knew I was a woman was if they really knew me.”
The Gaebler sisters leaned on each other professionally and personally throughout their careers. When both sisters practiced in Chicago, they referred patients to one another. And Dr. Gaebler-Uhing said her older sister was a great role model for how to balance the dual roles of physician and parent, as few of the older female doctors who trained her were married or had a child.
Now Dr. Gaebler-Uhing’s daughter, Annie Uhing, MD, is entering medicine herself. She is currently pediatric resident at the University of Wisconsin American Family Hospital. She plans to do a chief year and then a pediatric endocrinology fellowship.
Growing up, Dr. Uhing wasn’t always sure she wanted to work as much as her parents, who are both doctors. But her mom provided a great example few of her friends had at home: “If you want to work, you should work and do what you want to do and it’s not wrong to want to have a really high-powered job as a woman,” said Dr. Uhing.
Kathryn, Susan, and Rita
The three sisters Kathryn Hudson, MD, Susan Schmidt, MD, and Rita Butler, MD, were inspired to go into medicine by their mother, Rita Watson, MD, who was one of the first female interventional cardiologists in the United States.
“I think we had a front row seat to what being a doctor was like,” said Dr. Hudson, a hematologist and oncologist and director of survivorship at Texas Oncology in Austin. Both parents were MDs – their dad was a pharmaceutical researcher at Merck – and they would excitedly discuss patient cases and drug development at the dinner table, said Dr. Butler, an interventional cardiology fellow at the Lankenau Heart Institute in Wynnewood, Pa.
All three sisters have vivid memories of ‘Take Your Daughter to Work Day’ at their mom’s hospital. “I remember going to Take Your Daughter to Work Day with her and watching her in action and thinking, oh my gosh, my mom is so cool and I want to be like her,” said Dr. Schmidt, a pediatric critical care specialist at St. Christopher’s Hospital for Children in Philadelphia. “I’ve always felt special that my mom was doing something really cool and really saving lives,” said Dr. Schmidt.
Their fourth sibling, John, isn’t a physician and “I honestly wonder if it’s because he never went to Take Your Daughter to Work Day!” said Dr. Butler.
Having a mother who had both a high-powered medical career and a family helped the three women know they could do the same. “It is a difficult journey, don’t get me wrong, but I never questioned that I could do it because my mom did it first,” said Dr. Hudson.
As adults, the sisters confide in one another as they navigate modern motherhood and careers, switching between discussing medical cases and parenting advice.
As hard as their mom worked while they were growing up, she didn’t have the pressure of living up to the “super mom” ideal we have now, said Dr. Butler. “Everyone wants women to work like they don’t have kids and everyone wants women to parent like they don’t have a job,” she said. Having two sisters who can provide reassurance and advice in that area goes a long way, she said.
“I think sharing that experience of navigating motherhood, a medical career, and marriage, and adult life with sisters who are going through all the same things is really special and I feel really fortunate for that,” said Dr. Schmidt.
*This story was updated on 3/8/2022.
Telescoping Stents to Maintain a 3-Way Patency of the Airway
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
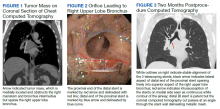
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
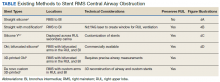
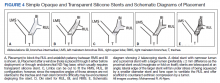
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
Impact of Lithium on Suicidality in the Veteran Population
Suicide is the tenth leading cause of death in the United States claiming nearly 48,000 individuals in 2019 and is the second leading cause of death among individuals aged 10 to 34 years.1 From 1999 to 2019, the suicide rate increased by 33%.1 In a retrospective study evaluating suicide risk in > 29,000 men, veterans had a greater risk for suicide in all age groups except for the oldest when compared with nonveterans.2 Another study of > 800,000 veterans found that younger veterans were most at risk for suicide.3 Veterans with completed suicides have a high incidence of affective disorders comorbid with substance use disorders, and therefore it is imperative to optimally treat these conditions to address suicidality.4 Additionally, a retrospective case-control study of veterans who died by suicide matched to controls identified that the cases had significantly higher rates of mental health conditions and suicidal ideation. Given that the veteran population is at higher risk of suicide, research of treatments to address suicidal ideation in veterans is needed.5
Lithium and Antisuicidal Properties
Lithium is the oldest treatment for bipolar disorder and is a long-standing first-line option due to its well-established efficacy as a mood stabilizer.6 Lithium’s antisuicidal properties separate it from the other pharmacologic options for bipolar disorder. A possible explanation for lithium’s unique antisuicidal properties is that these effects are mediated by its impact on aggression and impulsivity, which are both linked to an increased suicide risk.7,8 A meta-analysis by Baldessarini and colleagues demonstrated that patients with mood disorders who were prescribed lithium had a 5 times lower risk of suicide and attempts than did those not treated with lithium.9 Lithium’s current place in therapy is in the treatment of bipolar disorder and major depressive disorder augmentation.10-12Smith and colleagues found that in a cohort study of 21,194 veterans diagnosed with mental health conditions and initiated on lithium or valproate, there were no significant differences in associations with suicide observed between these agents over 365 days; however, there was a significant increased risk of suicide among patients discontinuing or modifying lithium within the first 180 days of treatment.13
Currently, lithium is thought to be underutilized at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, based on the number of prescriptions of lithium in the large population of veterans seen by mental health clinicians. MEDVAMC is a 538-bed academic teaching hospital serving approximately 130,000 veterans in southeast Texas. The Mental Health Care Line has 73 inpatient beds and an outpatient clinic serving > 12,000 patients annually. By retrospectively evaluating changes in suicidality in a sample of veterans prescribed lithium, we may be able to better understand the role that lithium plays in a population that has a higher suicide rate than does the general population. The primary objective of this study was to evaluate the change in number of suicide attempts from 3 months prior to lithium initiation to 3 months following a 6-month duration of lithium use. The secondary objective was to determine the change in suicidal ideation from the period prior to lithium use to the period following 6 months of lithium use.
Methods
This was a single-site, retrospective chart review conducted between October 2017 and April 2018. Prior to data collection, the MEDVAMC Research and Development committee approved the study as quality assurance research. Patients with an active lithium prescription were identified using the VA Lithium Lab Monitoring Dashboard, which includes all patients on lithium, their lithium level, and other data such as upcoming appointments.
Inclusion criteria consisted of adults who were aged ≥ 18 years, had an active lithium prescription on the date of data extraction, and had an active lithium prescription for at least 6 months. Patients were excluded if they had < 3 months of data before and/or after lithium was used for 6 months, and if they were initiated on lithium outside MEDVAMC. Cumulatively, patients had to have at least 12 months of data: 3 months prior to lithium use, at least 6 months of lithium use, and 9 months after lithium initiation.
Suicide Attempt and Suicidal Ideation Identification
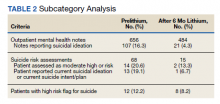
When determining the number of suicide attempts, we recorded 4 data points: Veterans Crisis Line notes documenting suicide attempts, hospital admissions for suicide attempts, suicide behavior reports within the indicated time frame, and mental health progress notes documenting suicide attempts. Suicidal ideation was measured in 4 ways. First, we looked at the percentage of outpatient mental health progress notes documenting suicidal ideation. Second, using the Patient Health Questionnaire-9 (PHQ-9) depression assessments, we looked at the percentage of patients that indicated several days, more than half the days, or nearly every day to the question, “Thoughts that you would be better off dead or of hurting yourself in some way.”14 Third, we recorded the percentage of suicide risk assessments that patients responded yes to both questions on current preoccupation with suicidal thoughts and serious intent and plan to commit suicide with access to guns, stashed pills, or other means. Finally, we noted the percentage of suicide risk assessments where the assessment of risk was moderate or high.
A retrospective electronic health record (EHR) review was performed and the following information was obtained: patient demographics, lithium refill history, concomitant psychotropic medications and psychotherapy, lithium levels, comorbidities at lithium initiation, presence of a high-risk suicide flag in the EHR, suicide risk assessments, suicide behavior reports, Veteran Crisis Line notes, PHQ-9 assessments, and hospital admission and mental health outpatient notes. The lithium therapeutic range of 0.6-1.2 mmol/L is indicated for bipolar disorder and not other indications where the dose is typically titrated to effect rather than level. Medication possession ratio (MPR) was also calculated for lithium (sum of days’ supply for all fills in period ÷ number of days in period). A high-risk suicide flag alerts clinicians and staff that a mental health professional considers the veteran at risk for suicide.15 Statistical analysis was performed using the paired t test for means to assess proportional differences between variables for the primary and secondary outcomes. Descriptive statistics were used to describe the baseline characteristics.
Results
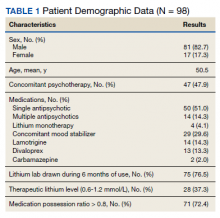
A total of 214 patients with an active prescription for lithium were identified on the Lithium Lab Monitoring Dashboard on October 31, 2017. After exclusion criteria were applied, 98 patients were included in the study (Figure 1). The 2 most common reasons for exclusion were due to patients not being on lithium for at least 6 months and being initiated on lithium at an outside facility. One patient was enrolled in a lithium research study (the medication ordered was lithium/placebo) and another patient refused all psychotropic medications according to the progress notes.
Most of the 98 patients (82.7%) were male with average age 50.5 years (Table 1). Almost half the patients (n = 47) were concomitantly participating in psychotherapy, and 50 (51.0%) patients received at least 1 antipsychotic medication. Twenty-nine patients had an active prescription for an additional mood stabilizer, and only 4 (4.1%) patients received lithium as monotherapy. Only 75 (76.5%) patients had a lithium level drawn during the 6 months of therapy, with 28 (37.3%) patients having a therapeutic lithium level (0.6 - 1.2 mmol/L). Seventy-one patients (72.4% ) were adherent to lithium therapy with a MPR > 0.8.16 Participants had 13 different psychiatric diagnoses at the time of lithium initiation; the most common were bipolar spectrum disorder (n = 38; 38.8%), depressive disorder (n = 27; 27.6%), and posttraumatic stress disorder (PTSD) (n = 26; 26.5%). Of note, 5 patients had a diagnosis of only PTSD without a concomitant mood disorder.
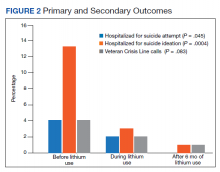
For the primary outcome, hospitalization for a suspected suicide attempt decreased from 4 (4.1%) before lithium use with a mean (SD) 0.04 (0.20) attempts per person to none within 3 months after lithium use for 6 months (t(97) = 2.03, P = .045) (Figure 2). The secondary outcome of hospitalization for suicidal ideations also decreased from 13 (13.3%) before lithium use with a mean (SD) 0.1 (0.3) ideations per person to 1 (1.0%) within 3 months after lithium use for 6 months with a mean (SD) 0.01 (0.1) ideations per person (t(97) = 3.68, P = .0004). Veteran Crisis Line calls also decreased from 4 (4.1%) with a mean (SD) 0.04 (0.2) calls per person to 1 (1.0%) within 3 months after 6 months of lithium with a mean (SD) 0.01 (0.1) calls per person (t(97) = 1.75, P = .08). The comparison of metrics from 3 months before lithium initiation and within 3 months after use saw decreases in all categories (Table 2). Outpatient notes documenting suicidal ideation decreased, as did the number of patients with a high-risk suicide flag.
Discussion
The results of this study suggest lithium may have a role in reducing suicidality in a veteran population. There was a statistically significant reduction in hospitalizations for suicide attempt and suicidal ideation after at least 6 months of lithium use. These results are comparable with a previously published study that observed significant decreases in suicidal behavior and/or hospitalization risks among veterans taking lithium compared with those not taking lithium.17 Our study was similar in respect to the reduced hospitalizations among a veteran population; however, the previous study did not report a difference in suicide attempts and lithium use. This could be related to the longer follow-up time in the previous study (3 years) vs our study (9 months).
Our study identified a significant reduction in Veteran Crisis Line calls after at least 6 months of lithium use. While a reduction in suicidal ideations could be implicated in the decrease in crisis line calls, there may be a confounding variable. It is possible that after lithium initiation, veterans had more frequent contact with health care practitioners due to laboratory test monitoring and follow-up visits and thus had concerns/crises addressed during these interactions ultimately leading to a decreased utilization of the crisis line. Interestingly, there was a reduction in mental health outpatient notes from the prelithium period to the 3-month period that followed 6 months of lithium therapy. However, our study did not report on the number of mental health outpatient notes or visits during the 6-month lithium duration. Additionally, time of year/season could have an impact on suicidality, but this relationship was not evaluated in this study.
The presence of high-risk suicide flags also decreased from the prelithium period to the period 3 months following 6 months of lithium use. High-risk flags are reviewed by the suicide prevention coordinators and mental health professionals every 90 days; therefore, the patients with flags had multiple opportunities for review and thus renewal or discontinuation during the study period. A similar rationale can be applied to the high-risk flag as with the Veteran Crisis Line reduction, although this change could also be representative of a decrease in suicidality. Our study is different from other lithium studies because it included patients with a multitude of psychiatric diagnoses rather than just mood disorders. Five of the patients had a diagnosis of only PTSD and no documented mood disorder at the time of lithium initiation. Additional research is needed on the impact of lithium on suicidality in veterans with PTSD and psychiatric conditions other than mood disorders.
Underutilization of Lithium
Despite widespread knowledge of lithium’s antisuicidal effects, it is underutilized as a mood stabilizer in the US.18 There are various modifiable barriers that impact the prescribing as well as use of lithium. Clinicians may not be fully aware of lithium’s antisuicidal properties and may also have a low level of confidence in patients’ likelihood of adherence to laboratory monitoring.18,19 Due to the narrow therapeutic index of lithium, the consequences of nonadherence to monitoring can be dangerous, which may deter mental health professionals from prescribing this antisuicidal agent. At MEDVAMC, only 72.4% of patients with a lithium prescription had a lithium level drawn within a 6-month period. This could be attributed to patient nonadherence (eg, the test was ordered but the patient did not go) or clinician nonadherence (eg, test was not ordered).
With increased clinician education as well as clinics dedicated to lithium management that allow for closer follow-up, facilities may see an increased level of comfort with lithium use. Lithium management clinics that provide close follow-up may also help address patient-related concerns about adverse effects and allow for close monitoring. To facilitate lithium monitoring at MEDVAMC, mental health practitioners and pharmacists developed a lithium test monitoring menu that serves as a “one-stop shop” for lithium baseline and ongoing test results.
In the future, we may study the impact of this test monitoring menu on lithium prescribing. One may also consider whether lithium levels need to be monitored at different frequencies (eg, less frequently for depression than bipolar disorder) depending on the diagnoses. A better understanding of the necessity for therapeutic monitoring may potentially reduce barriers to prescribing for patients who do not have indications that have a recommended therapeutic range (eg, bipolar disorder).
Lithium Adherence
A primary patient-related concern for low lithium utilization is poor adherence. In this sample, 71 patients (72.4%) were considered fully adherent. This was higher than the rate of 54.1% reported by Sajatovic and colleagues in a study evaluating adherence to lithium and other anticonvulsants in veterans with bipolar disorder.20 Patients’ beliefs about medications and overall health as well as knowledge of the illness and treatment may impact adherence.21 The literature indicates that strategies such as cognitive behavioral therapy (CBT) and didactic lectures positively impact patients’ attitudes about lithium, which ultimately influences adherence.21-23 Involving a family member or significant other in psychotherapy may also improve lithium adherence.21 Specifically in the VA, to address knowledge deficits and improve overall adherence, the Lithium Lab Monitoring Dashboard could be used to identify and invite new lithium starts to educational groups about lithium. These groups could also serve as lithium management clinics.
Limitations
There were several limitations to this study. This was a single-site, retrospective chart review with a small sample size. We studied a cross-section of veterans with only active prescriptions, which limited the sample size. The results should be interpreted cautiously because < 40% of patients who had a level drawn were in the therapeutic range. Patients whose lithium levels were outside of the therapeutic range may have not been fully adherent to the medication. Further analysis based on reason for lithium prescription (eg, bipolar disorder vs depression vs aggression/impulsivity in PTSD) may be helpful in better understanding the results.
Additionally, while we collected data on concomitant mood stabilizers and antipsychotics, we did not collect data on concurrent antidepressant therapy and only 4% of patients were on lithium monotherapy. Data regarding veterans undergoing concurrent CBT during their lithium trial were not assessed in this study and could be considered a confounding factor for future studies. We included any Veteran Crisis Line call in our results regardless of the reason for the call, which could have led to overreporting of this suicidality marker.
Given its small sample size, this study should be considered as hypothesis-generating. Further studies are needed to address lithium’s antisuicidal effects in specific diagnoses (eg, PTSD, anxiety, schizoaffective disorder) to better understand its place in therapy. Studies evaluating the relationship between dosing and suicidality may help provide insight into whether the antisuicidal effect of lithium is dose-dependent and whether a specific dose range rather than a therapeutic level should be targeted for antisuicidal purposes.
Conclusions
People treated for an affective disorder have a 30-times greater risk of suicide than do those in the general population; however, as lithium can reduce the risk of suicide and self-harm, it should continue to have an important role in clinical practice.24 At MEDVAMC, we observed a statistically significant reduction in hospitalization for suicide attempts and suicidal ideation in veterans prescribed lithium following nonfatal suicide behavior and suicidal ideation. Prospective randomized placebo-controlled studies are needed to better understand lithium’s antisuicidal effects.
1. Centers for Disease Control and Prevention. Preventing Suicide Fact Sheet. Updated April 2021. Accessed February 16, 2022. https://www.cdc.gov/suicide/pdf/preventing-suicide-factsheet-2021-508.pdf
2. Kaplan MS, McFarland BH, Huguet N, Valenstein M. Suicide risk and precipitating circumstances among young, middle-aged, and older male veterans. Am J Public Health. 2012;102 Suppl 1(Suppl 1):S131-S137. doi:10.2105/AJPH.2011.300445
3. Zivin K, Kim HM, McCarthy JF, et al. Suicide mortality among individuals receiving treatment for depression in the Veterans Affairs health system: associations with patient and treatment setting characteristics. Am J Public Health. 2007;97(12):2193-2198. doi:10.2105/AJPH.2007.115477
4. Lehmann L, McCormick RA, McCracken L. Suicidal behavior among patients in the VA health care system. Psychiatr Serv. 1995;46(10):1069-1071. doi:10.1176/ps.46.10.1069
5. Dobscha SK, Denneson LM, Kovas AE, et al. Correlates of suicide among veterans treated in primary care: case-control study of a nationally representative sample. J Gen Intern Med. 2014;29(suppl 4):853-860. doi:10.1007/s11606-014-3028-1
6. Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
7. Kovacsics CE, Gottesman II, Gould TD. Lithium’s antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175-198. doi:10.1146/annurev.pharmtox.011008.145557
8. Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181-189. doi:10.1176/ajp.156.2.181
9. Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review [published correction appears in Bipolar Disord. 2007 May;9(3):314]. Bipolar Disord. 2006;8(5 Pt 2):625-639. doi:10.1111/j.1399-5618.2006.00344.x
10. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. doi:10.1111/bdi.12609
11. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640. doi:10.1192/bjp.162.5.634
12. Bauer M, Bschor T, Kunz D, Berghöfer A, Ströhle A, Müller-Oerlinghausen B. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157(9):1429-1435. doi:10.1176/appi.ajp.157.9.1429
13. Smith EG, Austin KL, Kim HM, et al. Suicide risk in Veterans Health Administration patients with mental health diagnoses initiating lithium or valproate: a historical prospective cohort study. BMC Psychiatry. 2014;14:357. Published 2014 Dec 17. doi:10.1186/s12888-014-0357-x
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
15. US Department of Veterans Affairs, Veterans Health Administration. Use of patient record flags to identify patients at high risk for suicide. VHA Directive 2008-036. Published July 18, 2008. Accessed February 7, 2022. www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1719
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Medication adherence in a comparative effectiveness trial for bipolar disorder. Acta Psychiatr Scand. 2014;129(5):359-365. doi:10.1111/acps.12202
17. Yerevanian BI, Koek RJ, Mintz J. Bipolar pharmacotherapy and suicidal behavior. Part I: Lithium, divalproex and carbamazepine. J Affect Disord. 2007;103(1-3):5-11. doi:10.1016/j.jad.2007.05.019
18. Post RM. The New News about Lithium: An Underutilized Treatment in the United States. Neuropsychopharmacology. 2018;43(5):1174-1179. doi:10.1038/npp.2017.238
19. Öhlund L, Ott M, Oja S, et al. Reasons for lithium discontinuation in men and women with bipolar disorder: a retrospective cohort study [published correction appears in BMC Psychiatry. 2018 Oct 3;18(1):322]. BMC Psychiatry. 2018;18(1):37. Published 2018 Feb 7. doi:10.1186/s12888-018-1622-1
20. Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58(6):855-863. doi:10.1176/ps.2007.58.6.855
21. Chakrabarti S. Treatment-adherence in bipolar disorder: A patient-centred approach. World J Psychiatry. 2016;6(4):399-409. Published 2016 Dec 22. doi:10.5498/wjp.v6.i4.399
22. Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in bipolar disorder: a review of current psychosocial treatment efficacy and recommendations for future treatment development. Behav Modif. 2008;32(3):267-301. doi:10.1177/0145445507309023
23. Peet M, Harvey NS. Lithium maintenance: 1. A standard education programme for patients. Br J Psychiatry. 1991;158:197-200. doi:10.1192/bjp.158.2.197
24. Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi:10.1136/bmj.f3646
Suicide is the tenth leading cause of death in the United States claiming nearly 48,000 individuals in 2019 and is the second leading cause of death among individuals aged 10 to 34 years.1 From 1999 to 2019, the suicide rate increased by 33%.1 In a retrospective study evaluating suicide risk in > 29,000 men, veterans had a greater risk for suicide in all age groups except for the oldest when compared with nonveterans.2 Another study of > 800,000 veterans found that younger veterans were most at risk for suicide.3 Veterans with completed suicides have a high incidence of affective disorders comorbid with substance use disorders, and therefore it is imperative to optimally treat these conditions to address suicidality.4 Additionally, a retrospective case-control study of veterans who died by suicide matched to controls identified that the cases had significantly higher rates of mental health conditions and suicidal ideation. Given that the veteran population is at higher risk of suicide, research of treatments to address suicidal ideation in veterans is needed.5
Lithium and Antisuicidal Properties
Lithium is the oldest treatment for bipolar disorder and is a long-standing first-line option due to its well-established efficacy as a mood stabilizer.6 Lithium’s antisuicidal properties separate it from the other pharmacologic options for bipolar disorder. A possible explanation for lithium’s unique antisuicidal properties is that these effects are mediated by its impact on aggression and impulsivity, which are both linked to an increased suicide risk.7,8 A meta-analysis by Baldessarini and colleagues demonstrated that patients with mood disorders who were prescribed lithium had a 5 times lower risk of suicide and attempts than did those not treated with lithium.9 Lithium’s current place in therapy is in the treatment of bipolar disorder and major depressive disorder augmentation.10-12Smith and colleagues found that in a cohort study of 21,194 veterans diagnosed with mental health conditions and initiated on lithium or valproate, there were no significant differences in associations with suicide observed between these agents over 365 days; however, there was a significant increased risk of suicide among patients discontinuing or modifying lithium within the first 180 days of treatment.13
Currently, lithium is thought to be underutilized at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, based on the number of prescriptions of lithium in the large population of veterans seen by mental health clinicians. MEDVAMC is a 538-bed academic teaching hospital serving approximately 130,000 veterans in southeast Texas. The Mental Health Care Line has 73 inpatient beds and an outpatient clinic serving > 12,000 patients annually. By retrospectively evaluating changes in suicidality in a sample of veterans prescribed lithium, we may be able to better understand the role that lithium plays in a population that has a higher suicide rate than does the general population. The primary objective of this study was to evaluate the change in number of suicide attempts from 3 months prior to lithium initiation to 3 months following a 6-month duration of lithium use. The secondary objective was to determine the change in suicidal ideation from the period prior to lithium use to the period following 6 months of lithium use.
Methods
This was a single-site, retrospective chart review conducted between October 2017 and April 2018. Prior to data collection, the MEDVAMC Research and Development committee approved the study as quality assurance research. Patients with an active lithium prescription were identified using the VA Lithium Lab Monitoring Dashboard, which includes all patients on lithium, their lithium level, and other data such as upcoming appointments.
Inclusion criteria consisted of adults who were aged ≥ 18 years, had an active lithium prescription on the date of data extraction, and had an active lithium prescription for at least 6 months. Patients were excluded if they had < 3 months of data before and/or after lithium was used for 6 months, and if they were initiated on lithium outside MEDVAMC. Cumulatively, patients had to have at least 12 months of data: 3 months prior to lithium use, at least 6 months of lithium use, and 9 months after lithium initiation.
Suicide Attempt and Suicidal Ideation Identification
When determining the number of suicide attempts, we recorded 4 data points: Veterans Crisis Line notes documenting suicide attempts, hospital admissions for suicide attempts, suicide behavior reports within the indicated time frame, and mental health progress notes documenting suicide attempts. Suicidal ideation was measured in 4 ways. First, we looked at the percentage of outpatient mental health progress notes documenting suicidal ideation. Second, using the Patient Health Questionnaire-9 (PHQ-9) depression assessments, we looked at the percentage of patients that indicated several days, more than half the days, or nearly every day to the question, “Thoughts that you would be better off dead or of hurting yourself in some way.”14 Third, we recorded the percentage of suicide risk assessments that patients responded yes to both questions on current preoccupation with suicidal thoughts and serious intent and plan to commit suicide with access to guns, stashed pills, or other means. Finally, we noted the percentage of suicide risk assessments where the assessment of risk was moderate or high.
A retrospective electronic health record (EHR) review was performed and the following information was obtained: patient demographics, lithium refill history, concomitant psychotropic medications and psychotherapy, lithium levels, comorbidities at lithium initiation, presence of a high-risk suicide flag in the EHR, suicide risk assessments, suicide behavior reports, Veteran Crisis Line notes, PHQ-9 assessments, and hospital admission and mental health outpatient notes. The lithium therapeutic range of 0.6-1.2 mmol/L is indicated for bipolar disorder and not other indications where the dose is typically titrated to effect rather than level. Medication possession ratio (MPR) was also calculated for lithium (sum of days’ supply for all fills in period ÷ number of days in period). A high-risk suicide flag alerts clinicians and staff that a mental health professional considers the veteran at risk for suicide.15 Statistical analysis was performed using the paired t test for means to assess proportional differences between variables for the primary and secondary outcomes. Descriptive statistics were used to describe the baseline characteristics.
Results
A total of 214 patients with an active prescription for lithium were identified on the Lithium Lab Monitoring Dashboard on October 31, 2017. After exclusion criteria were applied, 98 patients were included in the study (Figure 1). The 2 most common reasons for exclusion were due to patients not being on lithium for at least 6 months and being initiated on lithium at an outside facility. One patient was enrolled in a lithium research study (the medication ordered was lithium/placebo) and another patient refused all psychotropic medications according to the progress notes.
Most of the 98 patients (82.7%) were male with average age 50.5 years (Table 1). Almost half the patients (n = 47) were concomitantly participating in psychotherapy, and 50 (51.0%) patients received at least 1 antipsychotic medication. Twenty-nine patients had an active prescription for an additional mood stabilizer, and only 4 (4.1%) patients received lithium as monotherapy. Only 75 (76.5%) patients had a lithium level drawn during the 6 months of therapy, with 28 (37.3%) patients having a therapeutic lithium level (0.6 - 1.2 mmol/L). Seventy-one patients (72.4% ) were adherent to lithium therapy with a MPR > 0.8.16 Participants had 13 different psychiatric diagnoses at the time of lithium initiation; the most common were bipolar spectrum disorder (n = 38; 38.8%), depressive disorder (n = 27; 27.6%), and posttraumatic stress disorder (PTSD) (n = 26; 26.5%). Of note, 5 patients had a diagnosis of only PTSD without a concomitant mood disorder.
For the primary outcome, hospitalization for a suspected suicide attempt decreased from 4 (4.1%) before lithium use with a mean (SD) 0.04 (0.20) attempts per person to none within 3 months after lithium use for 6 months (t(97) = 2.03, P = .045) (Figure 2). The secondary outcome of hospitalization for suicidal ideations also decreased from 13 (13.3%) before lithium use with a mean (SD) 0.1 (0.3) ideations per person to 1 (1.0%) within 3 months after lithium use for 6 months with a mean (SD) 0.01 (0.1) ideations per person (t(97) = 3.68, P = .0004). Veteran Crisis Line calls also decreased from 4 (4.1%) with a mean (SD) 0.04 (0.2) calls per person to 1 (1.0%) within 3 months after 6 months of lithium with a mean (SD) 0.01 (0.1) calls per person (t(97) = 1.75, P = .08). The comparison of metrics from 3 months before lithium initiation and within 3 months after use saw decreases in all categories (Table 2). Outpatient notes documenting suicidal ideation decreased, as did the number of patients with a high-risk suicide flag.
Discussion
The results of this study suggest lithium may have a role in reducing suicidality in a veteran population. There was a statistically significant reduction in hospitalizations for suicide attempt and suicidal ideation after at least 6 months of lithium use. These results are comparable with a previously published study that observed significant decreases in suicidal behavior and/or hospitalization risks among veterans taking lithium compared with those not taking lithium.17 Our study was similar in respect to the reduced hospitalizations among a veteran population; however, the previous study did not report a difference in suicide attempts and lithium use. This could be related to the longer follow-up time in the previous study (3 years) vs our study (9 months).
Our study identified a significant reduction in Veteran Crisis Line calls after at least 6 months of lithium use. While a reduction in suicidal ideations could be implicated in the decrease in crisis line calls, there may be a confounding variable. It is possible that after lithium initiation, veterans had more frequent contact with health care practitioners due to laboratory test monitoring and follow-up visits and thus had concerns/crises addressed during these interactions ultimately leading to a decreased utilization of the crisis line. Interestingly, there was a reduction in mental health outpatient notes from the prelithium period to the 3-month period that followed 6 months of lithium therapy. However, our study did not report on the number of mental health outpatient notes or visits during the 6-month lithium duration. Additionally, time of year/season could have an impact on suicidality, but this relationship was not evaluated in this study.
The presence of high-risk suicide flags also decreased from the prelithium period to the period 3 months following 6 months of lithium use. High-risk flags are reviewed by the suicide prevention coordinators and mental health professionals every 90 days; therefore, the patients with flags had multiple opportunities for review and thus renewal or discontinuation during the study period. A similar rationale can be applied to the high-risk flag as with the Veteran Crisis Line reduction, although this change could also be representative of a decrease in suicidality. Our study is different from other lithium studies because it included patients with a multitude of psychiatric diagnoses rather than just mood disorders. Five of the patients had a diagnosis of only PTSD and no documented mood disorder at the time of lithium initiation. Additional research is needed on the impact of lithium on suicidality in veterans with PTSD and psychiatric conditions other than mood disorders.
Underutilization of Lithium
Despite widespread knowledge of lithium’s antisuicidal effects, it is underutilized as a mood stabilizer in the US.18 There are various modifiable barriers that impact the prescribing as well as use of lithium. Clinicians may not be fully aware of lithium’s antisuicidal properties and may also have a low level of confidence in patients’ likelihood of adherence to laboratory monitoring.18,19 Due to the narrow therapeutic index of lithium, the consequences of nonadherence to monitoring can be dangerous, which may deter mental health professionals from prescribing this antisuicidal agent. At MEDVAMC, only 72.4% of patients with a lithium prescription had a lithium level drawn within a 6-month period. This could be attributed to patient nonadherence (eg, the test was ordered but the patient did not go) or clinician nonadherence (eg, test was not ordered).
With increased clinician education as well as clinics dedicated to lithium management that allow for closer follow-up, facilities may see an increased level of comfort with lithium use. Lithium management clinics that provide close follow-up may also help address patient-related concerns about adverse effects and allow for close monitoring. To facilitate lithium monitoring at MEDVAMC, mental health practitioners and pharmacists developed a lithium test monitoring menu that serves as a “one-stop shop” for lithium baseline and ongoing test results.
In the future, we may study the impact of this test monitoring menu on lithium prescribing. One may also consider whether lithium levels need to be monitored at different frequencies (eg, less frequently for depression than bipolar disorder) depending on the diagnoses. A better understanding of the necessity for therapeutic monitoring may potentially reduce barriers to prescribing for patients who do not have indications that have a recommended therapeutic range (eg, bipolar disorder).
Lithium Adherence
A primary patient-related concern for low lithium utilization is poor adherence. In this sample, 71 patients (72.4%) were considered fully adherent. This was higher than the rate of 54.1% reported by Sajatovic and colleagues in a study evaluating adherence to lithium and other anticonvulsants in veterans with bipolar disorder.20 Patients’ beliefs about medications and overall health as well as knowledge of the illness and treatment may impact adherence.21 The literature indicates that strategies such as cognitive behavioral therapy (CBT) and didactic lectures positively impact patients’ attitudes about lithium, which ultimately influences adherence.21-23 Involving a family member or significant other in psychotherapy may also improve lithium adherence.21 Specifically in the VA, to address knowledge deficits and improve overall adherence, the Lithium Lab Monitoring Dashboard could be used to identify and invite new lithium starts to educational groups about lithium. These groups could also serve as lithium management clinics.
Limitations
There were several limitations to this study. This was a single-site, retrospective chart review with a small sample size. We studied a cross-section of veterans with only active prescriptions, which limited the sample size. The results should be interpreted cautiously because < 40% of patients who had a level drawn were in the therapeutic range. Patients whose lithium levels were outside of the therapeutic range may have not been fully adherent to the medication. Further analysis based on reason for lithium prescription (eg, bipolar disorder vs depression vs aggression/impulsivity in PTSD) may be helpful in better understanding the results.
Additionally, while we collected data on concomitant mood stabilizers and antipsychotics, we did not collect data on concurrent antidepressant therapy and only 4% of patients were on lithium monotherapy. Data regarding veterans undergoing concurrent CBT during their lithium trial were not assessed in this study and could be considered a confounding factor for future studies. We included any Veteran Crisis Line call in our results regardless of the reason for the call, which could have led to overreporting of this suicidality marker.
Given its small sample size, this study should be considered as hypothesis-generating. Further studies are needed to address lithium’s antisuicidal effects in specific diagnoses (eg, PTSD, anxiety, schizoaffective disorder) to better understand its place in therapy. Studies evaluating the relationship between dosing and suicidality may help provide insight into whether the antisuicidal effect of lithium is dose-dependent and whether a specific dose range rather than a therapeutic level should be targeted for antisuicidal purposes.
Conclusions
People treated for an affective disorder have a 30-times greater risk of suicide than do those in the general population; however, as lithium can reduce the risk of suicide and self-harm, it should continue to have an important role in clinical practice.24 At MEDVAMC, we observed a statistically significant reduction in hospitalization for suicide attempts and suicidal ideation in veterans prescribed lithium following nonfatal suicide behavior and suicidal ideation. Prospective randomized placebo-controlled studies are needed to better understand lithium’s antisuicidal effects.
Suicide is the tenth leading cause of death in the United States claiming nearly 48,000 individuals in 2019 and is the second leading cause of death among individuals aged 10 to 34 years.1 From 1999 to 2019, the suicide rate increased by 33%.1 In a retrospective study evaluating suicide risk in > 29,000 men, veterans had a greater risk for suicide in all age groups except for the oldest when compared with nonveterans.2 Another study of > 800,000 veterans found that younger veterans were most at risk for suicide.3 Veterans with completed suicides have a high incidence of affective disorders comorbid with substance use disorders, and therefore it is imperative to optimally treat these conditions to address suicidality.4 Additionally, a retrospective case-control study of veterans who died by suicide matched to controls identified that the cases had significantly higher rates of mental health conditions and suicidal ideation. Given that the veteran population is at higher risk of suicide, research of treatments to address suicidal ideation in veterans is needed.5
Lithium and Antisuicidal Properties
Lithium is the oldest treatment for bipolar disorder and is a long-standing first-line option due to its well-established efficacy as a mood stabilizer.6 Lithium’s antisuicidal properties separate it from the other pharmacologic options for bipolar disorder. A possible explanation for lithium’s unique antisuicidal properties is that these effects are mediated by its impact on aggression and impulsivity, which are both linked to an increased suicide risk.7,8 A meta-analysis by Baldessarini and colleagues demonstrated that patients with mood disorders who were prescribed lithium had a 5 times lower risk of suicide and attempts than did those not treated with lithium.9 Lithium’s current place in therapy is in the treatment of bipolar disorder and major depressive disorder augmentation.10-12Smith and colleagues found that in a cohort study of 21,194 veterans diagnosed with mental health conditions and initiated on lithium or valproate, there were no significant differences in associations with suicide observed between these agents over 365 days; however, there was a significant increased risk of suicide among patients discontinuing or modifying lithium within the first 180 days of treatment.13
Currently, lithium is thought to be underutilized at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, based on the number of prescriptions of lithium in the large population of veterans seen by mental health clinicians. MEDVAMC is a 538-bed academic teaching hospital serving approximately 130,000 veterans in southeast Texas. The Mental Health Care Line has 73 inpatient beds and an outpatient clinic serving > 12,000 patients annually. By retrospectively evaluating changes in suicidality in a sample of veterans prescribed lithium, we may be able to better understand the role that lithium plays in a population that has a higher suicide rate than does the general population. The primary objective of this study was to evaluate the change in number of suicide attempts from 3 months prior to lithium initiation to 3 months following a 6-month duration of lithium use. The secondary objective was to determine the change in suicidal ideation from the period prior to lithium use to the period following 6 months of lithium use.
Methods
This was a single-site, retrospective chart review conducted between October 2017 and April 2018. Prior to data collection, the MEDVAMC Research and Development committee approved the study as quality assurance research. Patients with an active lithium prescription were identified using the VA Lithium Lab Monitoring Dashboard, which includes all patients on lithium, their lithium level, and other data such as upcoming appointments.
Inclusion criteria consisted of adults who were aged ≥ 18 years, had an active lithium prescription on the date of data extraction, and had an active lithium prescription for at least 6 months. Patients were excluded if they had < 3 months of data before and/or after lithium was used for 6 months, and if they were initiated on lithium outside MEDVAMC. Cumulatively, patients had to have at least 12 months of data: 3 months prior to lithium use, at least 6 months of lithium use, and 9 months after lithium initiation.
Suicide Attempt and Suicidal Ideation Identification
When determining the number of suicide attempts, we recorded 4 data points: Veterans Crisis Line notes documenting suicide attempts, hospital admissions for suicide attempts, suicide behavior reports within the indicated time frame, and mental health progress notes documenting suicide attempts. Suicidal ideation was measured in 4 ways. First, we looked at the percentage of outpatient mental health progress notes documenting suicidal ideation. Second, using the Patient Health Questionnaire-9 (PHQ-9) depression assessments, we looked at the percentage of patients that indicated several days, more than half the days, or nearly every day to the question, “Thoughts that you would be better off dead or of hurting yourself in some way.”14 Third, we recorded the percentage of suicide risk assessments that patients responded yes to both questions on current preoccupation with suicidal thoughts and serious intent and plan to commit suicide with access to guns, stashed pills, or other means. Finally, we noted the percentage of suicide risk assessments where the assessment of risk was moderate or high.
A retrospective electronic health record (EHR) review was performed and the following information was obtained: patient demographics, lithium refill history, concomitant psychotropic medications and psychotherapy, lithium levels, comorbidities at lithium initiation, presence of a high-risk suicide flag in the EHR, suicide risk assessments, suicide behavior reports, Veteran Crisis Line notes, PHQ-9 assessments, and hospital admission and mental health outpatient notes. The lithium therapeutic range of 0.6-1.2 mmol/L is indicated for bipolar disorder and not other indications where the dose is typically titrated to effect rather than level. Medication possession ratio (MPR) was also calculated for lithium (sum of days’ supply for all fills in period ÷ number of days in period). A high-risk suicide flag alerts clinicians and staff that a mental health professional considers the veteran at risk for suicide.15 Statistical analysis was performed using the paired t test for means to assess proportional differences between variables for the primary and secondary outcomes. Descriptive statistics were used to describe the baseline characteristics.
Results
A total of 214 patients with an active prescription for lithium were identified on the Lithium Lab Monitoring Dashboard on October 31, 2017. After exclusion criteria were applied, 98 patients were included in the study (Figure 1). The 2 most common reasons for exclusion were due to patients not being on lithium for at least 6 months and being initiated on lithium at an outside facility. One patient was enrolled in a lithium research study (the medication ordered was lithium/placebo) and another patient refused all psychotropic medications according to the progress notes.
Most of the 98 patients (82.7%) were male with average age 50.5 years (Table 1). Almost half the patients (n = 47) were concomitantly participating in psychotherapy, and 50 (51.0%) patients received at least 1 antipsychotic medication. Twenty-nine patients had an active prescription for an additional mood stabilizer, and only 4 (4.1%) patients received lithium as monotherapy. Only 75 (76.5%) patients had a lithium level drawn during the 6 months of therapy, with 28 (37.3%) patients having a therapeutic lithium level (0.6 - 1.2 mmol/L). Seventy-one patients (72.4% ) were adherent to lithium therapy with a MPR > 0.8.16 Participants had 13 different psychiatric diagnoses at the time of lithium initiation; the most common were bipolar spectrum disorder (n = 38; 38.8%), depressive disorder (n = 27; 27.6%), and posttraumatic stress disorder (PTSD) (n = 26; 26.5%). Of note, 5 patients had a diagnosis of only PTSD without a concomitant mood disorder.
For the primary outcome, hospitalization for a suspected suicide attempt decreased from 4 (4.1%) before lithium use with a mean (SD) 0.04 (0.20) attempts per person to none within 3 months after lithium use for 6 months (t(97) = 2.03, P = .045) (Figure 2). The secondary outcome of hospitalization for suicidal ideations also decreased from 13 (13.3%) before lithium use with a mean (SD) 0.1 (0.3) ideations per person to 1 (1.0%) within 3 months after lithium use for 6 months with a mean (SD) 0.01 (0.1) ideations per person (t(97) = 3.68, P = .0004). Veteran Crisis Line calls also decreased from 4 (4.1%) with a mean (SD) 0.04 (0.2) calls per person to 1 (1.0%) within 3 months after 6 months of lithium with a mean (SD) 0.01 (0.1) calls per person (t(97) = 1.75, P = .08). The comparison of metrics from 3 months before lithium initiation and within 3 months after use saw decreases in all categories (Table 2). Outpatient notes documenting suicidal ideation decreased, as did the number of patients with a high-risk suicide flag.
Discussion
The results of this study suggest lithium may have a role in reducing suicidality in a veteran population. There was a statistically significant reduction in hospitalizations for suicide attempt and suicidal ideation after at least 6 months of lithium use. These results are comparable with a previously published study that observed significant decreases in suicidal behavior and/or hospitalization risks among veterans taking lithium compared with those not taking lithium.17 Our study was similar in respect to the reduced hospitalizations among a veteran population; however, the previous study did not report a difference in suicide attempts and lithium use. This could be related to the longer follow-up time in the previous study (3 years) vs our study (9 months).
Our study identified a significant reduction in Veteran Crisis Line calls after at least 6 months of lithium use. While a reduction in suicidal ideations could be implicated in the decrease in crisis line calls, there may be a confounding variable. It is possible that after lithium initiation, veterans had more frequent contact with health care practitioners due to laboratory test monitoring and follow-up visits and thus had concerns/crises addressed during these interactions ultimately leading to a decreased utilization of the crisis line. Interestingly, there was a reduction in mental health outpatient notes from the prelithium period to the 3-month period that followed 6 months of lithium therapy. However, our study did not report on the number of mental health outpatient notes or visits during the 6-month lithium duration. Additionally, time of year/season could have an impact on suicidality, but this relationship was not evaluated in this study.
The presence of high-risk suicide flags also decreased from the prelithium period to the period 3 months following 6 months of lithium use. High-risk flags are reviewed by the suicide prevention coordinators and mental health professionals every 90 days; therefore, the patients with flags had multiple opportunities for review and thus renewal or discontinuation during the study period. A similar rationale can be applied to the high-risk flag as with the Veteran Crisis Line reduction, although this change could also be representative of a decrease in suicidality. Our study is different from other lithium studies because it included patients with a multitude of psychiatric diagnoses rather than just mood disorders. Five of the patients had a diagnosis of only PTSD and no documented mood disorder at the time of lithium initiation. Additional research is needed on the impact of lithium on suicidality in veterans with PTSD and psychiatric conditions other than mood disorders.
Underutilization of Lithium
Despite widespread knowledge of lithium’s antisuicidal effects, it is underutilized as a mood stabilizer in the US.18 There are various modifiable barriers that impact the prescribing as well as use of lithium. Clinicians may not be fully aware of lithium’s antisuicidal properties and may also have a low level of confidence in patients’ likelihood of adherence to laboratory monitoring.18,19 Due to the narrow therapeutic index of lithium, the consequences of nonadherence to monitoring can be dangerous, which may deter mental health professionals from prescribing this antisuicidal agent. At MEDVAMC, only 72.4% of patients with a lithium prescription had a lithium level drawn within a 6-month period. This could be attributed to patient nonadherence (eg, the test was ordered but the patient did not go) or clinician nonadherence (eg, test was not ordered).
With increased clinician education as well as clinics dedicated to lithium management that allow for closer follow-up, facilities may see an increased level of comfort with lithium use. Lithium management clinics that provide close follow-up may also help address patient-related concerns about adverse effects and allow for close monitoring. To facilitate lithium monitoring at MEDVAMC, mental health practitioners and pharmacists developed a lithium test monitoring menu that serves as a “one-stop shop” for lithium baseline and ongoing test results.
In the future, we may study the impact of this test monitoring menu on lithium prescribing. One may also consider whether lithium levels need to be monitored at different frequencies (eg, less frequently for depression than bipolar disorder) depending on the diagnoses. A better understanding of the necessity for therapeutic monitoring may potentially reduce barriers to prescribing for patients who do not have indications that have a recommended therapeutic range (eg, bipolar disorder).
Lithium Adherence
A primary patient-related concern for low lithium utilization is poor adherence. In this sample, 71 patients (72.4%) were considered fully adherent. This was higher than the rate of 54.1% reported by Sajatovic and colleagues in a study evaluating adherence to lithium and other anticonvulsants in veterans with bipolar disorder.20 Patients’ beliefs about medications and overall health as well as knowledge of the illness and treatment may impact adherence.21 The literature indicates that strategies such as cognitive behavioral therapy (CBT) and didactic lectures positively impact patients’ attitudes about lithium, which ultimately influences adherence.21-23 Involving a family member or significant other in psychotherapy may also improve lithium adherence.21 Specifically in the VA, to address knowledge deficits and improve overall adherence, the Lithium Lab Monitoring Dashboard could be used to identify and invite new lithium starts to educational groups about lithium. These groups could also serve as lithium management clinics.
Limitations
There were several limitations to this study. This was a single-site, retrospective chart review with a small sample size. We studied a cross-section of veterans with only active prescriptions, which limited the sample size. The results should be interpreted cautiously because < 40% of patients who had a level drawn were in the therapeutic range. Patients whose lithium levels were outside of the therapeutic range may have not been fully adherent to the medication. Further analysis based on reason for lithium prescription (eg, bipolar disorder vs depression vs aggression/impulsivity in PTSD) may be helpful in better understanding the results.
Additionally, while we collected data on concomitant mood stabilizers and antipsychotics, we did not collect data on concurrent antidepressant therapy and only 4% of patients were on lithium monotherapy. Data regarding veterans undergoing concurrent CBT during their lithium trial were not assessed in this study and could be considered a confounding factor for future studies. We included any Veteran Crisis Line call in our results regardless of the reason for the call, which could have led to overreporting of this suicidality marker.
Given its small sample size, this study should be considered as hypothesis-generating. Further studies are needed to address lithium’s antisuicidal effects in specific diagnoses (eg, PTSD, anxiety, schizoaffective disorder) to better understand its place in therapy. Studies evaluating the relationship between dosing and suicidality may help provide insight into whether the antisuicidal effect of lithium is dose-dependent and whether a specific dose range rather than a therapeutic level should be targeted for antisuicidal purposes.
Conclusions
People treated for an affective disorder have a 30-times greater risk of suicide than do those in the general population; however, as lithium can reduce the risk of suicide and self-harm, it should continue to have an important role in clinical practice.24 At MEDVAMC, we observed a statistically significant reduction in hospitalization for suicide attempts and suicidal ideation in veterans prescribed lithium following nonfatal suicide behavior and suicidal ideation. Prospective randomized placebo-controlled studies are needed to better understand lithium’s antisuicidal effects.
1. Centers for Disease Control and Prevention. Preventing Suicide Fact Sheet. Updated April 2021. Accessed February 16, 2022. https://www.cdc.gov/suicide/pdf/preventing-suicide-factsheet-2021-508.pdf
2. Kaplan MS, McFarland BH, Huguet N, Valenstein M. Suicide risk and precipitating circumstances among young, middle-aged, and older male veterans. Am J Public Health. 2012;102 Suppl 1(Suppl 1):S131-S137. doi:10.2105/AJPH.2011.300445
3. Zivin K, Kim HM, McCarthy JF, et al. Suicide mortality among individuals receiving treatment for depression in the Veterans Affairs health system: associations with patient and treatment setting characteristics. Am J Public Health. 2007;97(12):2193-2198. doi:10.2105/AJPH.2007.115477
4. Lehmann L, McCormick RA, McCracken L. Suicidal behavior among patients in the VA health care system. Psychiatr Serv. 1995;46(10):1069-1071. doi:10.1176/ps.46.10.1069
5. Dobscha SK, Denneson LM, Kovas AE, et al. Correlates of suicide among veterans treated in primary care: case-control study of a nationally representative sample. J Gen Intern Med. 2014;29(suppl 4):853-860. doi:10.1007/s11606-014-3028-1
6. Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
7. Kovacsics CE, Gottesman II, Gould TD. Lithium’s antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175-198. doi:10.1146/annurev.pharmtox.011008.145557
8. Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181-189. doi:10.1176/ajp.156.2.181
9. Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review [published correction appears in Bipolar Disord. 2007 May;9(3):314]. Bipolar Disord. 2006;8(5 Pt 2):625-639. doi:10.1111/j.1399-5618.2006.00344.x
10. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. doi:10.1111/bdi.12609
11. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640. doi:10.1192/bjp.162.5.634
12. Bauer M, Bschor T, Kunz D, Berghöfer A, Ströhle A, Müller-Oerlinghausen B. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157(9):1429-1435. doi:10.1176/appi.ajp.157.9.1429
13. Smith EG, Austin KL, Kim HM, et al. Suicide risk in Veterans Health Administration patients with mental health diagnoses initiating lithium or valproate: a historical prospective cohort study. BMC Psychiatry. 2014;14:357. Published 2014 Dec 17. doi:10.1186/s12888-014-0357-x
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
15. US Department of Veterans Affairs, Veterans Health Administration. Use of patient record flags to identify patients at high risk for suicide. VHA Directive 2008-036. Published July 18, 2008. Accessed February 7, 2022. www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1719
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Medication adherence in a comparative effectiveness trial for bipolar disorder. Acta Psychiatr Scand. 2014;129(5):359-365. doi:10.1111/acps.12202
17. Yerevanian BI, Koek RJ, Mintz J. Bipolar pharmacotherapy and suicidal behavior. Part I: Lithium, divalproex and carbamazepine. J Affect Disord. 2007;103(1-3):5-11. doi:10.1016/j.jad.2007.05.019
18. Post RM. The New News about Lithium: An Underutilized Treatment in the United States. Neuropsychopharmacology. 2018;43(5):1174-1179. doi:10.1038/npp.2017.238
19. Öhlund L, Ott M, Oja S, et al. Reasons for lithium discontinuation in men and women with bipolar disorder: a retrospective cohort study [published correction appears in BMC Psychiatry. 2018 Oct 3;18(1):322]. BMC Psychiatry. 2018;18(1):37. Published 2018 Feb 7. doi:10.1186/s12888-018-1622-1
20. Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58(6):855-863. doi:10.1176/ps.2007.58.6.855
21. Chakrabarti S. Treatment-adherence in bipolar disorder: A patient-centred approach. World J Psychiatry. 2016;6(4):399-409. Published 2016 Dec 22. doi:10.5498/wjp.v6.i4.399
22. Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in bipolar disorder: a review of current psychosocial treatment efficacy and recommendations for future treatment development. Behav Modif. 2008;32(3):267-301. doi:10.1177/0145445507309023
23. Peet M, Harvey NS. Lithium maintenance: 1. A standard education programme for patients. Br J Psychiatry. 1991;158:197-200. doi:10.1192/bjp.158.2.197
24. Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi:10.1136/bmj.f3646
1. Centers for Disease Control and Prevention. Preventing Suicide Fact Sheet. Updated April 2021. Accessed February 16, 2022. https://www.cdc.gov/suicide/pdf/preventing-suicide-factsheet-2021-508.pdf
2. Kaplan MS, McFarland BH, Huguet N, Valenstein M. Suicide risk and precipitating circumstances among young, middle-aged, and older male veterans. Am J Public Health. 2012;102 Suppl 1(Suppl 1):S131-S137. doi:10.2105/AJPH.2011.300445
3. Zivin K, Kim HM, McCarthy JF, et al. Suicide mortality among individuals receiving treatment for depression in the Veterans Affairs health system: associations with patient and treatment setting characteristics. Am J Public Health. 2007;97(12):2193-2198. doi:10.2105/AJPH.2007.115477
4. Lehmann L, McCormick RA, McCracken L. Suicidal behavior among patients in the VA health care system. Psychiatr Serv. 1995;46(10):1069-1071. doi:10.1176/ps.46.10.1069
5. Dobscha SK, Denneson LM, Kovas AE, et al. Correlates of suicide among veterans treated in primary care: case-control study of a nationally representative sample. J Gen Intern Med. 2014;29(suppl 4):853-860. doi:10.1007/s11606-014-3028-1
6. Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
7. Kovacsics CE, Gottesman II, Gould TD. Lithium’s antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175-198. doi:10.1146/annurev.pharmtox.011008.145557
8. Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181-189. doi:10.1176/ajp.156.2.181
9. Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review [published correction appears in Bipolar Disord. 2007 May;9(3):314]. Bipolar Disord. 2006;8(5 Pt 2):625-639. doi:10.1111/j.1399-5618.2006.00344.x
10. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. doi:10.1111/bdi.12609
11. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640. doi:10.1192/bjp.162.5.634
12. Bauer M, Bschor T, Kunz D, Berghöfer A, Ströhle A, Müller-Oerlinghausen B. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157(9):1429-1435. doi:10.1176/appi.ajp.157.9.1429
13. Smith EG, Austin KL, Kim HM, et al. Suicide risk in Veterans Health Administration patients with mental health diagnoses initiating lithium or valproate: a historical prospective cohort study. BMC Psychiatry. 2014;14:357. Published 2014 Dec 17. doi:10.1186/s12888-014-0357-x
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
15. US Department of Veterans Affairs, Veterans Health Administration. Use of patient record flags to identify patients at high risk for suicide. VHA Directive 2008-036. Published July 18, 2008. Accessed February 7, 2022. www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1719
16. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Medication adherence in a comparative effectiveness trial for bipolar disorder. Acta Psychiatr Scand. 2014;129(5):359-365. doi:10.1111/acps.12202
17. Yerevanian BI, Koek RJ, Mintz J. Bipolar pharmacotherapy and suicidal behavior. Part I: Lithium, divalproex and carbamazepine. J Affect Disord. 2007;103(1-3):5-11. doi:10.1016/j.jad.2007.05.019
18. Post RM. The New News about Lithium: An Underutilized Treatment in the United States. Neuropsychopharmacology. 2018;43(5):1174-1179. doi:10.1038/npp.2017.238
19. Öhlund L, Ott M, Oja S, et al. Reasons for lithium discontinuation in men and women with bipolar disorder: a retrospective cohort study [published correction appears in BMC Psychiatry. 2018 Oct 3;18(1):322]. BMC Psychiatry. 2018;18(1):37. Published 2018 Feb 7. doi:10.1186/s12888-018-1622-1
20. Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58(6):855-863. doi:10.1176/ps.2007.58.6.855
21. Chakrabarti S. Treatment-adherence in bipolar disorder: A patient-centred approach. World J Psychiatry. 2016;6(4):399-409. Published 2016 Dec 22. doi:10.5498/wjp.v6.i4.399
22. Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in bipolar disorder: a review of current psychosocial treatment efficacy and recommendations for future treatment development. Behav Modif. 2008;32(3):267-301. doi:10.1177/0145445507309023
23. Peet M, Harvey NS. Lithium maintenance: 1. A standard education programme for patients. Br J Psychiatry. 1991;158:197-200. doi:10.1192/bjp.158.2.197
24. Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi:10.1136/bmj.f3646
A Pioneer in Women’s Federal Practice
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
Long COVID patients may develop nerve damage: Study
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
FROM NEUROLOGY: NEUROIMMUNOLOGY & NEUROINFLAMMATION
New gene signals for asthma-COPD overlap identified
A large-scale analysis of the genetic underpinnings of the overlap between asthma and chronic obstructive pulmonary disease (COPD) has identified eight novel signals that may be linked to inflammation and worse asthma outcomes, according to an international team of investigators.
They conducted a genome-wide association study (GWAS) of 8,068 individuals with asthma-COPD overlap (ACO) and 40,360 controls with either asthma or COPD alone and identified genetic variants that could predispose patients to ACO.
The findings by Catherine John, MBBChir, from the University of Leicester, England, and colleagues from Europe, the United States, and Canada were published online in Chest.
“Our study contributes to understanding the genetic landscape of asthma and COPD at the population level,” explained coauthor Lystra P. Hayden, MD, from Brigham and Women’s Hospital and Harvard Medical School, Boston, in an email.
“It provides evidence for shared genetic influence ranging from variants implicated in asthma to those implicated in fixed airflow obstruction and specifically those which influence an intermediate phenotype with features of both, which was the focus of this investigation,” she wrote.
Although the study does not provide sufficient evidence to support patient-level genetic testing for these variants, “it does suggest potential common biological mechanisms underpinning both asthma and COPD, which in the future could inform treatments of people displaying features of both conditions,” Dr. Hayden said.
Asthma and COPD are distinct conditions but share certain features, such as airflow obstruction, inflammation, and cytokine profiles. Recent studies have suggested that patients with features of both conditions – ACO – have worse outcomes than those with either condition alone.
The authors note that recent guidelines from the Global Initiative for Chronic Obstructive Lung Disease “emphasize that asthma and COPD are different conditions but may coexist in the same patient.”
“One of the things that genetics can do is to see if there is a genetic basis to the etiology of both of these conditions,” said coauthor Sina A. Gharib, MD, professor of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle.
“Are these both just the same disease manifesting differently, or are they two really different diseases? That’s an ongoing controversy, and I don’t think this paper or any other paper that I’ve seen really settles this,” he said in an interview.
He noted that most genetic studies of asthma tend to exclude patients with COPD, and vice versa, because the complexity of the respective phenotypes can make it difficult to draw strong conclusions about genetic contributions to disease etiology.
Dr. Gharib’s group contributed data for the validation portion of the study.
Genetic study
The authors first drew data on the patients with ACO and controls from the UK Biobank and conducted a GWAS using a cutoff P value of less than 5x10-6 to identify “promising” signals that remained significant in analyses comparing patients with ACO with those with asthma alone and with COPD alone.
They then analyzed the variants in 12 independent cohorts and identified 31 variants they deemed to be worth a closer look.
“These signals suggest a spectrum of shared genetic influences, some predominantly influencing asthma (FAM105A, GLB1, PHB, TSLP), others predominantly influencing fixed airflow obstruction (IL17RD, C5orf56, HLA-DQB1). One intergenic signal on chromosome 5 had not been previously associated with asthma, COPD or lung function,” they write.
The investigators also performed subgroup analyses stratified by age of asthma onset and by smoking status.
“We found that for the 31 ACO signals of interest examined, the effect sizes amongst cases with childhood-onset asthma were highly correlated to those with adult onset. Effect sizes in ever- and never-smokers were also closely correlated. Our study suggests that ACO is not due solely to smoking in people with asthma,” Dr. Hayden said.
In addition, they tested variants across a wide range of phenotypes and found that traits of eosinophil counts, atopy, and asthma were prominent.
“Our findings suggest a spectrum of shared genetic influences, from variants predominantly influencing asthma to those predominantly influencing fixed airflow obstruction. We focus on variants that tend towards an intermediate phenotype with features of both asthma and fixed airflow obstruction with pathways implicating innate and adaptive immunity and potentially bone development and signals for which the biology remains unclear,” they write.
While it’s still unclear whether ACO is a distinct entity unto itself, “we do seem to find some signals that seem to be uniquely displayed in this population that has diagnosis of asthma but also has a reduction in lung function that’s not reversible, which is what we see in COPD,” Dr. Gharib said.
Further exploration of the biology of ACO could lead to development of methods to prevent fixed airflow obstruction in patients with asthma, the authors conclude.
The study was supported by BREATHE, the Health Data Research Hub for Respiratory Health in partnership with SAIL Databank. Dr. Hayden and Dr. Gharib reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale analysis of the genetic underpinnings of the overlap between asthma and chronic obstructive pulmonary disease (COPD) has identified eight novel signals that may be linked to inflammation and worse asthma outcomes, according to an international team of investigators.
They conducted a genome-wide association study (GWAS) of 8,068 individuals with asthma-COPD overlap (ACO) and 40,360 controls with either asthma or COPD alone and identified genetic variants that could predispose patients to ACO.
The findings by Catherine John, MBBChir, from the University of Leicester, England, and colleagues from Europe, the United States, and Canada were published online in Chest.
“Our study contributes to understanding the genetic landscape of asthma and COPD at the population level,” explained coauthor Lystra P. Hayden, MD, from Brigham and Women’s Hospital and Harvard Medical School, Boston, in an email.
“It provides evidence for shared genetic influence ranging from variants implicated in asthma to those implicated in fixed airflow obstruction and specifically those which influence an intermediate phenotype with features of both, which was the focus of this investigation,” she wrote.
Although the study does not provide sufficient evidence to support patient-level genetic testing for these variants, “it does suggest potential common biological mechanisms underpinning both asthma and COPD, which in the future could inform treatments of people displaying features of both conditions,” Dr. Hayden said.
Asthma and COPD are distinct conditions but share certain features, such as airflow obstruction, inflammation, and cytokine profiles. Recent studies have suggested that patients with features of both conditions – ACO – have worse outcomes than those with either condition alone.
The authors note that recent guidelines from the Global Initiative for Chronic Obstructive Lung Disease “emphasize that asthma and COPD are different conditions but may coexist in the same patient.”
“One of the things that genetics can do is to see if there is a genetic basis to the etiology of both of these conditions,” said coauthor Sina A. Gharib, MD, professor of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle.
“Are these both just the same disease manifesting differently, or are they two really different diseases? That’s an ongoing controversy, and I don’t think this paper or any other paper that I’ve seen really settles this,” he said in an interview.
He noted that most genetic studies of asthma tend to exclude patients with COPD, and vice versa, because the complexity of the respective phenotypes can make it difficult to draw strong conclusions about genetic contributions to disease etiology.
Dr. Gharib’s group contributed data for the validation portion of the study.
Genetic study
The authors first drew data on the patients with ACO and controls from the UK Biobank and conducted a GWAS using a cutoff P value of less than 5x10-6 to identify “promising” signals that remained significant in analyses comparing patients with ACO with those with asthma alone and with COPD alone.
They then analyzed the variants in 12 independent cohorts and identified 31 variants they deemed to be worth a closer look.
“These signals suggest a spectrum of shared genetic influences, some predominantly influencing asthma (FAM105A, GLB1, PHB, TSLP), others predominantly influencing fixed airflow obstruction (IL17RD, C5orf56, HLA-DQB1). One intergenic signal on chromosome 5 had not been previously associated with asthma, COPD or lung function,” they write.
The investigators also performed subgroup analyses stratified by age of asthma onset and by smoking status.
“We found that for the 31 ACO signals of interest examined, the effect sizes amongst cases with childhood-onset asthma were highly correlated to those with adult onset. Effect sizes in ever- and never-smokers were also closely correlated. Our study suggests that ACO is not due solely to smoking in people with asthma,” Dr. Hayden said.
In addition, they tested variants across a wide range of phenotypes and found that traits of eosinophil counts, atopy, and asthma were prominent.
“Our findings suggest a spectrum of shared genetic influences, from variants predominantly influencing asthma to those predominantly influencing fixed airflow obstruction. We focus on variants that tend towards an intermediate phenotype with features of both asthma and fixed airflow obstruction with pathways implicating innate and adaptive immunity and potentially bone development and signals for which the biology remains unclear,” they write.
While it’s still unclear whether ACO is a distinct entity unto itself, “we do seem to find some signals that seem to be uniquely displayed in this population that has diagnosis of asthma but also has a reduction in lung function that’s not reversible, which is what we see in COPD,” Dr. Gharib said.
Further exploration of the biology of ACO could lead to development of methods to prevent fixed airflow obstruction in patients with asthma, the authors conclude.
The study was supported by BREATHE, the Health Data Research Hub for Respiratory Health in partnership with SAIL Databank. Dr. Hayden and Dr. Gharib reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale analysis of the genetic underpinnings of the overlap between asthma and chronic obstructive pulmonary disease (COPD) has identified eight novel signals that may be linked to inflammation and worse asthma outcomes, according to an international team of investigators.
They conducted a genome-wide association study (GWAS) of 8,068 individuals with asthma-COPD overlap (ACO) and 40,360 controls with either asthma or COPD alone and identified genetic variants that could predispose patients to ACO.
The findings by Catherine John, MBBChir, from the University of Leicester, England, and colleagues from Europe, the United States, and Canada were published online in Chest.
“Our study contributes to understanding the genetic landscape of asthma and COPD at the population level,” explained coauthor Lystra P. Hayden, MD, from Brigham and Women’s Hospital and Harvard Medical School, Boston, in an email.
“It provides evidence for shared genetic influence ranging from variants implicated in asthma to those implicated in fixed airflow obstruction and specifically those which influence an intermediate phenotype with features of both, which was the focus of this investigation,” she wrote.
Although the study does not provide sufficient evidence to support patient-level genetic testing for these variants, “it does suggest potential common biological mechanisms underpinning both asthma and COPD, which in the future could inform treatments of people displaying features of both conditions,” Dr. Hayden said.
Asthma and COPD are distinct conditions but share certain features, such as airflow obstruction, inflammation, and cytokine profiles. Recent studies have suggested that patients with features of both conditions – ACO – have worse outcomes than those with either condition alone.
The authors note that recent guidelines from the Global Initiative for Chronic Obstructive Lung Disease “emphasize that asthma and COPD are different conditions but may coexist in the same patient.”
“One of the things that genetics can do is to see if there is a genetic basis to the etiology of both of these conditions,” said coauthor Sina A. Gharib, MD, professor of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle.
“Are these both just the same disease manifesting differently, or are they two really different diseases? That’s an ongoing controversy, and I don’t think this paper or any other paper that I’ve seen really settles this,” he said in an interview.
He noted that most genetic studies of asthma tend to exclude patients with COPD, and vice versa, because the complexity of the respective phenotypes can make it difficult to draw strong conclusions about genetic contributions to disease etiology.
Dr. Gharib’s group contributed data for the validation portion of the study.
Genetic study
The authors first drew data on the patients with ACO and controls from the UK Biobank and conducted a GWAS using a cutoff P value of less than 5x10-6 to identify “promising” signals that remained significant in analyses comparing patients with ACO with those with asthma alone and with COPD alone.
They then analyzed the variants in 12 independent cohorts and identified 31 variants they deemed to be worth a closer look.
“These signals suggest a spectrum of shared genetic influences, some predominantly influencing asthma (FAM105A, GLB1, PHB, TSLP), others predominantly influencing fixed airflow obstruction (IL17RD, C5orf56, HLA-DQB1). One intergenic signal on chromosome 5 had not been previously associated with asthma, COPD or lung function,” they write.
The investigators also performed subgroup analyses stratified by age of asthma onset and by smoking status.
“We found that for the 31 ACO signals of interest examined, the effect sizes amongst cases with childhood-onset asthma were highly correlated to those with adult onset. Effect sizes in ever- and never-smokers were also closely correlated. Our study suggests that ACO is not due solely to smoking in people with asthma,” Dr. Hayden said.
In addition, they tested variants across a wide range of phenotypes and found that traits of eosinophil counts, atopy, and asthma were prominent.
“Our findings suggest a spectrum of shared genetic influences, from variants predominantly influencing asthma to those predominantly influencing fixed airflow obstruction. We focus on variants that tend towards an intermediate phenotype with features of both asthma and fixed airflow obstruction with pathways implicating innate and adaptive immunity and potentially bone development and signals for which the biology remains unclear,” they write.
While it’s still unclear whether ACO is a distinct entity unto itself, “we do seem to find some signals that seem to be uniquely displayed in this population that has diagnosis of asthma but also has a reduction in lung function that’s not reversible, which is what we see in COPD,” Dr. Gharib said.
Further exploration of the biology of ACO could lead to development of methods to prevent fixed airflow obstruction in patients with asthma, the authors conclude.
The study was supported by BREATHE, the Health Data Research Hub for Respiratory Health in partnership with SAIL Databank. Dr. Hayden and Dr. Gharib reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mindfulness intervention curbs opioid misuse, chronic pain
In a randomized clinical trial, 250 adults with both opioid misuse and chronic pain received either the intervention, called mindfulness-oriented recovery enhancement (MORE), or supportive psychotherapy.
Results showed the first group was twice as likely to reduce opioid misuse after 9 months than the latter group.
The intervention was developed by Eric Garland, PhD, director of the Center on Mindfulness and Integrative Health Intervention Development (C-MIIND), University of Utah, Salt Lake City. “As the largest and longest-term clinical trial of MORE ever conducted, this study definitively establishes the efficacy of MORE as a treatment for chronic pain and opioid misuse,” he told this news organization.
The findings were published online Feb. 28 in JAMA Internal Medicine.
Self-regulation
Study participants included 250 adults (64% women; mean age, 51.8 years) with co-occurring opioid misuse and chronic pain who were randomly allocated to receive MORE or supportive psychotherapy, which served as a control group.
Both interventions were delivered by trained clinical social workers in six primary care clinics in Utah to groups of 6-12 participants across 8 weekly 2-hour sessions.
The MORE intervention, detailed on Dr. Garland’s website, provides sequenced training in mindfulness, reappraisal, and savoring skills.
Mindfulness consisted of meditation on breathing and body sensations to strengthen self-regulation of compulsive opioid use and to mitigate pain and opioid craving by reinterpreting these experiences as innocuous sensory information.
Reappraisal consisted of reframing maladaptive thoughts to decrease negative emotions and engender meaning in life.
Savoring consisted of training in focusing awareness on pleasurable events and sensations to amplify positive emotions and reward.
Fewer depressive symptoms
Through 9 months of follow-up, the MORE group had about a twofold greater likelihood than the supportive psychotherapy group for reduction in opioid misuse (odds ratio [OR], 2.06; 95% confidence interval, 1.17-3.61; P = .01)
“MORE reduced opioid misuse by 45% 9 months after the end of treatment, more than doubling the effect of standard supportive psychotherapy and exceeding the effect size of other therapies for opioid misuse among people with chronic pain,” Dr. Garland said.
Members of the MORE group experienced greater reduction in pain severity and pain-related functional interference compared with members of the control group.
“MORE’s effect size on chronic pain symptoms was greater than that observed for CBT, the current gold standard psychological treatment for chronic pain,” Dr. Garland noted.
Compared with supportive psychotherapy, MORE decreased emotional distress, depressive symptoms, and real-time reports of opioid craving in daily life.
“Although nearly 70% of participants met criteria for depression at the beginning of the trial, on average, patients in MORE no longer exhibited symptoms consistent with major depressive disorder by the end of the study,” Dr. Garland said.
The current study builds on prior studies of MORE showing similar results, as reported previously by this news organization.
MORE can be successfully delivered in routine primary care, Dr. Garland noted. “In this trial, we delivered MORE in conference rooms, break rooms, and lunch rooms at community primary care clinics,” he added.
‘Powerful program’
To date, Dr. Garland has trained more than 450 physicians, nurses, social workers, and psychologists in health care systems across the country to implement MORE as an insurance-reimbursable group visit for patients in need.
One of them is Nancy Sudak, MD, chief well-being officer and director of integrative health, Essentia Health, Duluth, Minn.
“MORE is a very powerful program that teaches patients how to turn down the volume of their pain. I’ve been quite impressed by the power of MORE,” Dr. Sudak told this news organization
She noted that “buy-in” from patients is key – and the more a clinician knows a patient, the easier the buy-in.
“I recruited most of the patients in my groups from my own practice, so I already knew the patients quite well and there wasn’t really a need to sell it,” Dr. Sudak said.
“We have tried to operationalize it through our system and find that, as long as our recruitment techniques are robust enough, it’s not that hard to find patients to fill the groups, especially because chronic pain is just so common,” she added.
Dr. Sudak has found that patients who participate in MORE “bond and learn with each other and support each other. Patients love it, providers love it, and it’s a way to address isolation and loneliness” that can come with certain conditions.
“There are really only upsides to the group visit model and I think we’ll be seeing quite a bit more of it in the future,” she added.
Evidence-based data
Anna Parisi, PhD, is also delivering MORE to patients. She told this news organization, she was “really drawn” to the MORE program because oftentimes patients who require the most sophisticated therapies receive the ones with the least evidence.
This is often “what folks in the community are getting when they’re struggling with substance use,” added Dr. Parisi, a postdoctoral research associate working with Dr. Garland at the University of Utah. Dr. Parisi was not a coauthor on the current study.
“With MORE, all of the strategies and techniques are tied to mechanistic studies of their efficacy, so you know that what you’re delivering has a rationale behind it,” she said.
Like Dr. Sudak, Dr. Parisi said her patients, for the most part, have been receptive to the program. Although at first some were skeptical about mindfulness – with one patient using the term “tree-hugging” – they found immediate benefit even after the first session.
“That really helps them stay motivated to finish the program,” Dr. Parisi said.
This work was supported by a grant from the National Institute on Drug Abuse. Dr. Garland serves as director of the Center on Mindfulness and Integrative Health Intervention Development, which provides MORE, mindfulness-based therapy, and CBT in the context of research trials for no cost to research participants. He receives honoraria and payment for delivering seminars, lectures, and teaching engagements related to training clinicians in MORE and mindfulness and receives royalties from BehaVR and from the sales of books related to MORE outside the submitted work. Dr. Sudak and Dr. Parisi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial, 250 adults with both opioid misuse and chronic pain received either the intervention, called mindfulness-oriented recovery enhancement (MORE), or supportive psychotherapy.
Results showed the first group was twice as likely to reduce opioid misuse after 9 months than the latter group.
The intervention was developed by Eric Garland, PhD, director of the Center on Mindfulness and Integrative Health Intervention Development (C-MIIND), University of Utah, Salt Lake City. “As the largest and longest-term clinical trial of MORE ever conducted, this study definitively establishes the efficacy of MORE as a treatment for chronic pain and opioid misuse,” he told this news organization.
The findings were published online Feb. 28 in JAMA Internal Medicine.
Self-regulation
Study participants included 250 adults (64% women; mean age, 51.8 years) with co-occurring opioid misuse and chronic pain who were randomly allocated to receive MORE or supportive psychotherapy, which served as a control group.
Both interventions were delivered by trained clinical social workers in six primary care clinics in Utah to groups of 6-12 participants across 8 weekly 2-hour sessions.
The MORE intervention, detailed on Dr. Garland’s website, provides sequenced training in mindfulness, reappraisal, and savoring skills.
Mindfulness consisted of meditation on breathing and body sensations to strengthen self-regulation of compulsive opioid use and to mitigate pain and opioid craving by reinterpreting these experiences as innocuous sensory information.
Reappraisal consisted of reframing maladaptive thoughts to decrease negative emotions and engender meaning in life.
Savoring consisted of training in focusing awareness on pleasurable events and sensations to amplify positive emotions and reward.
Fewer depressive symptoms
Through 9 months of follow-up, the MORE group had about a twofold greater likelihood than the supportive psychotherapy group for reduction in opioid misuse (odds ratio [OR], 2.06; 95% confidence interval, 1.17-3.61; P = .01)
“MORE reduced opioid misuse by 45% 9 months after the end of treatment, more than doubling the effect of standard supportive psychotherapy and exceeding the effect size of other therapies for opioid misuse among people with chronic pain,” Dr. Garland said.
Members of the MORE group experienced greater reduction in pain severity and pain-related functional interference compared with members of the control group.
“MORE’s effect size on chronic pain symptoms was greater than that observed for CBT, the current gold standard psychological treatment for chronic pain,” Dr. Garland noted.
Compared with supportive psychotherapy, MORE decreased emotional distress, depressive symptoms, and real-time reports of opioid craving in daily life.
“Although nearly 70% of participants met criteria for depression at the beginning of the trial, on average, patients in MORE no longer exhibited symptoms consistent with major depressive disorder by the end of the study,” Dr. Garland said.
The current study builds on prior studies of MORE showing similar results, as reported previously by this news organization.
MORE can be successfully delivered in routine primary care, Dr. Garland noted. “In this trial, we delivered MORE in conference rooms, break rooms, and lunch rooms at community primary care clinics,” he added.
‘Powerful program’
To date, Dr. Garland has trained more than 450 physicians, nurses, social workers, and psychologists in health care systems across the country to implement MORE as an insurance-reimbursable group visit for patients in need.
One of them is Nancy Sudak, MD, chief well-being officer and director of integrative health, Essentia Health, Duluth, Minn.
“MORE is a very powerful program that teaches patients how to turn down the volume of their pain. I’ve been quite impressed by the power of MORE,” Dr. Sudak told this news organization
She noted that “buy-in” from patients is key – and the more a clinician knows a patient, the easier the buy-in.
“I recruited most of the patients in my groups from my own practice, so I already knew the patients quite well and there wasn’t really a need to sell it,” Dr. Sudak said.
“We have tried to operationalize it through our system and find that, as long as our recruitment techniques are robust enough, it’s not that hard to find patients to fill the groups, especially because chronic pain is just so common,” she added.
Dr. Sudak has found that patients who participate in MORE “bond and learn with each other and support each other. Patients love it, providers love it, and it’s a way to address isolation and loneliness” that can come with certain conditions.
“There are really only upsides to the group visit model and I think we’ll be seeing quite a bit more of it in the future,” she added.
Evidence-based data
Anna Parisi, PhD, is also delivering MORE to patients. She told this news organization, she was “really drawn” to the MORE program because oftentimes patients who require the most sophisticated therapies receive the ones with the least evidence.
This is often “what folks in the community are getting when they’re struggling with substance use,” added Dr. Parisi, a postdoctoral research associate working with Dr. Garland at the University of Utah. Dr. Parisi was not a coauthor on the current study.
“With MORE, all of the strategies and techniques are tied to mechanistic studies of their efficacy, so you know that what you’re delivering has a rationale behind it,” she said.
Like Dr. Sudak, Dr. Parisi said her patients, for the most part, have been receptive to the program. Although at first some were skeptical about mindfulness – with one patient using the term “tree-hugging” – they found immediate benefit even after the first session.
“That really helps them stay motivated to finish the program,” Dr. Parisi said.
This work was supported by a grant from the National Institute on Drug Abuse. Dr. Garland serves as director of the Center on Mindfulness and Integrative Health Intervention Development, which provides MORE, mindfulness-based therapy, and CBT in the context of research trials for no cost to research participants. He receives honoraria and payment for delivering seminars, lectures, and teaching engagements related to training clinicians in MORE and mindfulness and receives royalties from BehaVR and from the sales of books related to MORE outside the submitted work. Dr. Sudak and Dr. Parisi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial, 250 adults with both opioid misuse and chronic pain received either the intervention, called mindfulness-oriented recovery enhancement (MORE), or supportive psychotherapy.
Results showed the first group was twice as likely to reduce opioid misuse after 9 months than the latter group.
The intervention was developed by Eric Garland, PhD, director of the Center on Mindfulness and Integrative Health Intervention Development (C-MIIND), University of Utah, Salt Lake City. “As the largest and longest-term clinical trial of MORE ever conducted, this study definitively establishes the efficacy of MORE as a treatment for chronic pain and opioid misuse,” he told this news organization.
The findings were published online Feb. 28 in JAMA Internal Medicine.
Self-regulation
Study participants included 250 adults (64% women; mean age, 51.8 years) with co-occurring opioid misuse and chronic pain who were randomly allocated to receive MORE or supportive psychotherapy, which served as a control group.
Both interventions were delivered by trained clinical social workers in six primary care clinics in Utah to groups of 6-12 participants across 8 weekly 2-hour sessions.
The MORE intervention, detailed on Dr. Garland’s website, provides sequenced training in mindfulness, reappraisal, and savoring skills.
Mindfulness consisted of meditation on breathing and body sensations to strengthen self-regulation of compulsive opioid use and to mitigate pain and opioid craving by reinterpreting these experiences as innocuous sensory information.
Reappraisal consisted of reframing maladaptive thoughts to decrease negative emotions and engender meaning in life.
Savoring consisted of training in focusing awareness on pleasurable events and sensations to amplify positive emotions and reward.
Fewer depressive symptoms
Through 9 months of follow-up, the MORE group had about a twofold greater likelihood than the supportive psychotherapy group for reduction in opioid misuse (odds ratio [OR], 2.06; 95% confidence interval, 1.17-3.61; P = .01)
“MORE reduced opioid misuse by 45% 9 months after the end of treatment, more than doubling the effect of standard supportive psychotherapy and exceeding the effect size of other therapies for opioid misuse among people with chronic pain,” Dr. Garland said.
Members of the MORE group experienced greater reduction in pain severity and pain-related functional interference compared with members of the control group.
“MORE’s effect size on chronic pain symptoms was greater than that observed for CBT, the current gold standard psychological treatment for chronic pain,” Dr. Garland noted.
Compared with supportive psychotherapy, MORE decreased emotional distress, depressive symptoms, and real-time reports of opioid craving in daily life.
“Although nearly 70% of participants met criteria for depression at the beginning of the trial, on average, patients in MORE no longer exhibited symptoms consistent with major depressive disorder by the end of the study,” Dr. Garland said.
The current study builds on prior studies of MORE showing similar results, as reported previously by this news organization.
MORE can be successfully delivered in routine primary care, Dr. Garland noted. “In this trial, we delivered MORE in conference rooms, break rooms, and lunch rooms at community primary care clinics,” he added.
‘Powerful program’
To date, Dr. Garland has trained more than 450 physicians, nurses, social workers, and psychologists in health care systems across the country to implement MORE as an insurance-reimbursable group visit for patients in need.
One of them is Nancy Sudak, MD, chief well-being officer and director of integrative health, Essentia Health, Duluth, Minn.
“MORE is a very powerful program that teaches patients how to turn down the volume of their pain. I’ve been quite impressed by the power of MORE,” Dr. Sudak told this news organization
She noted that “buy-in” from patients is key – and the more a clinician knows a patient, the easier the buy-in.
“I recruited most of the patients in my groups from my own practice, so I already knew the patients quite well and there wasn’t really a need to sell it,” Dr. Sudak said.
“We have tried to operationalize it through our system and find that, as long as our recruitment techniques are robust enough, it’s not that hard to find patients to fill the groups, especially because chronic pain is just so common,” she added.
Dr. Sudak has found that patients who participate in MORE “bond and learn with each other and support each other. Patients love it, providers love it, and it’s a way to address isolation and loneliness” that can come with certain conditions.
“There are really only upsides to the group visit model and I think we’ll be seeing quite a bit more of it in the future,” she added.
Evidence-based data
Anna Parisi, PhD, is also delivering MORE to patients. She told this news organization, she was “really drawn” to the MORE program because oftentimes patients who require the most sophisticated therapies receive the ones with the least evidence.
This is often “what folks in the community are getting when they’re struggling with substance use,” added Dr. Parisi, a postdoctoral research associate working with Dr. Garland at the University of Utah. Dr. Parisi was not a coauthor on the current study.
“With MORE, all of the strategies and techniques are tied to mechanistic studies of their efficacy, so you know that what you’re delivering has a rationale behind it,” she said.
Like Dr. Sudak, Dr. Parisi said her patients, for the most part, have been receptive to the program. Although at first some were skeptical about mindfulness – with one patient using the term “tree-hugging” – they found immediate benefit even after the first session.
“That really helps them stay motivated to finish the program,” Dr. Parisi said.
This work was supported by a grant from the National Institute on Drug Abuse. Dr. Garland serves as director of the Center on Mindfulness and Integrative Health Intervention Development, which provides MORE, mindfulness-based therapy, and CBT in the context of research trials for no cost to research participants. He receives honoraria and payment for delivering seminars, lectures, and teaching engagements related to training clinicians in MORE and mindfulness and receives royalties from BehaVR and from the sales of books related to MORE outside the submitted work. Dr. Sudak and Dr. Parisi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ozanimod shows long-term safety, despite a pandemic
The study began in 2020 and also captured data on COVID-19 infections, and found that most were mild and resembled the profile of COVID-19 infections in the broader MS population.
Ozanimod is approved for the treatment of relapsing MS (RMS) and moderately to severe ulcerative colitis.
The DAYBREAK trial revealed a safety profile that broadly matched what was seen in the pivotal studies, with the exception that one case of progressive multifocal leukoencephalopathy (PML) emerged in the study population.
“So now we do know that ozanimod can cause PML, just as fingolimod can cause PML. I think some of us were hoping that perhaps the extent of immune suppression was going to be somewhat different in ozanimod and that PML might not occur. It’s a rare complication, but one that we now know can occur with this drug,” Bruce Cree, MD, PhD, said in an interview.
Ozanimod is a more selective drug than fingolimod. It affects only cell surface expression of the S1P1 and S1P5 receptors, and not other known S1P receptors. Ozanimod does not require first-dose observation and cardiac monitoring in most patients, and it can be taken at home.
“The two products have not been compared head-to-head. This is all comparison of data from different studies, and one has to take those considerations in mind as important caveats. But generally speaking, the safety profile and tolerability profile of ozanimod seems to be a little bit better, in my opinion, compared to that of fingolimod,” said Dr. Cree, who presented the results of the study at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). He is professor of neurology at the University of California, San Francisco.
Stable efficacy and no worsening of COVID-19 outcomes
Among 2,181 participants in DAYBREAK who were at risk of COVID-19, 8.7% had confirmed or suspected cases of COVID-19 during the study period. All were unvaccinated. Fourteen cases were considered serious, and there were two COVID-19–related deaths, and a third death caused by a pulmonary abscess related to an earlier COVID-19 infection. “When you look at this data and compare it to other datasets, this is not too dissimilar from rates of mortality that we would expect or serious infection that we see in other MS cohorts. So there doesn’t seem to be a striking worsening of COVID outcomes with ozanimod,” said Dr. Cree.
The benefit of the drug appeared to remain stable over multiple years. The annualized relapse rate was low and the relapse rate appeared to decline further over time. “It’s not an absolutely flat line, there is some curvature to it. So that that’s good news as well. And then the objective observation of lesion formation also is attenuated over time. We see a therapeutic effect on new radiographic lesions as well, and very low rates of disability worsening in ozanimod patients,” said Dr. Cree.
Overall, the study included 2,494 patients who entered the open-label extension study of the phase 1-3 trials. The study began in November 2019, and the current data extend through May 10, 2021. A total of 736 patients started out with interferon beta-1a and later switched to 0.92 mg ozanimod, 877 patients started at 0.46 mg ozanimod and switched to 0.92 mg ozanimod, and 881 were on a continuous dose of 0.92 mg ozanimod.
Three-quarters of the patients were relapse free at 36 months, 71% at 48 months. Among those who were on 0.92 mg ozanimod continuously, 64% were relapse-free through 60 months of treatment.
Among the cohort, 7.6% experienced severe treatment-emergent adverse events (TEAEs), 11.9% experienced serious TEAEs, and 3.0% discontinued ozanimod because of TEAEs. Common TEAEs included nasopharyngitis (59.3%), headache (46.1%), upper respiratory tract infection (31.5%), lymphopenia (29.4%), decreased absolute lymphocyte count (ALC, 24.5%), back pain (22.7%), and hypertension (20.7%).
Furthermore, 1.4% of patients developed treatment-emergent malignancies, 0.4% developed macular edema, 2.8% had cardiac TEAEs, and 9.8% had ALC levels below 0.2 x 109/L.
Encouraging data
The COVID-19 data were encouraging, according to Patricia Coyle, MD, who was asked to comment on the study. “190 individuals out of 2,181 seems quite reasonable, and they had three deaths. It certainly didn’t look like any excessive numbers of COVID, or excessive numbers of deaths,” said Dr. Coyle, professor of neurology and director of Stony Brook (N.Y.) MS Comprehensive Care Center.
She noted that other database studies have shown an association between increased risk and anti-CD20 agents, but they haven’t really seen that with the other disease-modifying therapies. “I think this is some long-term data that says that ozanimod appears to be well tolerated without having any surprising late toxicity,” said Dr. Coyle.
The study was funded by Celgene International II. Dr. Cree has consulted for Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Novartis, Sanofi, TG Therapeutics, and Therini, and received grant support from Genentech. Dr. Coyle has consulted or received speaker fees from Accordant, Alexion, Biogen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Horizon Therapeutics, Janssen, Mylan, Novartis, Sanofi Genzyme, TG Therapeutics, and Viela Bio. Dr. Coyle has received research funding from Actelion, Alkermes, Celgene, CorEvitas LLC, Genentech/Roche, MedDay, Novartis, and Sanofi Genzyme.
The study began in 2020 and also captured data on COVID-19 infections, and found that most were mild and resembled the profile of COVID-19 infections in the broader MS population.
Ozanimod is approved for the treatment of relapsing MS (RMS) and moderately to severe ulcerative colitis.
The DAYBREAK trial revealed a safety profile that broadly matched what was seen in the pivotal studies, with the exception that one case of progressive multifocal leukoencephalopathy (PML) emerged in the study population.
“So now we do know that ozanimod can cause PML, just as fingolimod can cause PML. I think some of us were hoping that perhaps the extent of immune suppression was going to be somewhat different in ozanimod and that PML might not occur. It’s a rare complication, but one that we now know can occur with this drug,” Bruce Cree, MD, PhD, said in an interview.
Ozanimod is a more selective drug than fingolimod. It affects only cell surface expression of the S1P1 and S1P5 receptors, and not other known S1P receptors. Ozanimod does not require first-dose observation and cardiac monitoring in most patients, and it can be taken at home.
“The two products have not been compared head-to-head. This is all comparison of data from different studies, and one has to take those considerations in mind as important caveats. But generally speaking, the safety profile and tolerability profile of ozanimod seems to be a little bit better, in my opinion, compared to that of fingolimod,” said Dr. Cree, who presented the results of the study at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). He is professor of neurology at the University of California, San Francisco.
Stable efficacy and no worsening of COVID-19 outcomes
Among 2,181 participants in DAYBREAK who were at risk of COVID-19, 8.7% had confirmed or suspected cases of COVID-19 during the study period. All were unvaccinated. Fourteen cases were considered serious, and there were two COVID-19–related deaths, and a third death caused by a pulmonary abscess related to an earlier COVID-19 infection. “When you look at this data and compare it to other datasets, this is not too dissimilar from rates of mortality that we would expect or serious infection that we see in other MS cohorts. So there doesn’t seem to be a striking worsening of COVID outcomes with ozanimod,” said Dr. Cree.
The benefit of the drug appeared to remain stable over multiple years. The annualized relapse rate was low and the relapse rate appeared to decline further over time. “It’s not an absolutely flat line, there is some curvature to it. So that that’s good news as well. And then the objective observation of lesion formation also is attenuated over time. We see a therapeutic effect on new radiographic lesions as well, and very low rates of disability worsening in ozanimod patients,” said Dr. Cree.
Overall, the study included 2,494 patients who entered the open-label extension study of the phase 1-3 trials. The study began in November 2019, and the current data extend through May 10, 2021. A total of 736 patients started out with interferon beta-1a and later switched to 0.92 mg ozanimod, 877 patients started at 0.46 mg ozanimod and switched to 0.92 mg ozanimod, and 881 were on a continuous dose of 0.92 mg ozanimod.
Three-quarters of the patients were relapse free at 36 months, 71% at 48 months. Among those who were on 0.92 mg ozanimod continuously, 64% were relapse-free through 60 months of treatment.
Among the cohort, 7.6% experienced severe treatment-emergent adverse events (TEAEs), 11.9% experienced serious TEAEs, and 3.0% discontinued ozanimod because of TEAEs. Common TEAEs included nasopharyngitis (59.3%), headache (46.1%), upper respiratory tract infection (31.5%), lymphopenia (29.4%), decreased absolute lymphocyte count (ALC, 24.5%), back pain (22.7%), and hypertension (20.7%).
Furthermore, 1.4% of patients developed treatment-emergent malignancies, 0.4% developed macular edema, 2.8% had cardiac TEAEs, and 9.8% had ALC levels below 0.2 x 109/L.
Encouraging data
The COVID-19 data were encouraging, according to Patricia Coyle, MD, who was asked to comment on the study. “190 individuals out of 2,181 seems quite reasonable, and they had three deaths. It certainly didn’t look like any excessive numbers of COVID, or excessive numbers of deaths,” said Dr. Coyle, professor of neurology and director of Stony Brook (N.Y.) MS Comprehensive Care Center.
She noted that other database studies have shown an association between increased risk and anti-CD20 agents, but they haven’t really seen that with the other disease-modifying therapies. “I think this is some long-term data that says that ozanimod appears to be well tolerated without having any surprising late toxicity,” said Dr. Coyle.
The study was funded by Celgene International II. Dr. Cree has consulted for Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Novartis, Sanofi, TG Therapeutics, and Therini, and received grant support from Genentech. Dr. Coyle has consulted or received speaker fees from Accordant, Alexion, Biogen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Horizon Therapeutics, Janssen, Mylan, Novartis, Sanofi Genzyme, TG Therapeutics, and Viela Bio. Dr. Coyle has received research funding from Actelion, Alkermes, Celgene, CorEvitas LLC, Genentech/Roche, MedDay, Novartis, and Sanofi Genzyme.
The study began in 2020 and also captured data on COVID-19 infections, and found that most were mild and resembled the profile of COVID-19 infections in the broader MS population.
Ozanimod is approved for the treatment of relapsing MS (RMS) and moderately to severe ulcerative colitis.
The DAYBREAK trial revealed a safety profile that broadly matched what was seen in the pivotal studies, with the exception that one case of progressive multifocal leukoencephalopathy (PML) emerged in the study population.
“So now we do know that ozanimod can cause PML, just as fingolimod can cause PML. I think some of us were hoping that perhaps the extent of immune suppression was going to be somewhat different in ozanimod and that PML might not occur. It’s a rare complication, but one that we now know can occur with this drug,” Bruce Cree, MD, PhD, said in an interview.
Ozanimod is a more selective drug than fingolimod. It affects only cell surface expression of the S1P1 and S1P5 receptors, and not other known S1P receptors. Ozanimod does not require first-dose observation and cardiac monitoring in most patients, and it can be taken at home.
“The two products have not been compared head-to-head. This is all comparison of data from different studies, and one has to take those considerations in mind as important caveats. But generally speaking, the safety profile and tolerability profile of ozanimod seems to be a little bit better, in my opinion, compared to that of fingolimod,” said Dr. Cree, who presented the results of the study at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). He is professor of neurology at the University of California, San Francisco.
Stable efficacy and no worsening of COVID-19 outcomes
Among 2,181 participants in DAYBREAK who were at risk of COVID-19, 8.7% had confirmed or suspected cases of COVID-19 during the study period. All were unvaccinated. Fourteen cases were considered serious, and there were two COVID-19–related deaths, and a third death caused by a pulmonary abscess related to an earlier COVID-19 infection. “When you look at this data and compare it to other datasets, this is not too dissimilar from rates of mortality that we would expect or serious infection that we see in other MS cohorts. So there doesn’t seem to be a striking worsening of COVID outcomes with ozanimod,” said Dr. Cree.
The benefit of the drug appeared to remain stable over multiple years. The annualized relapse rate was low and the relapse rate appeared to decline further over time. “It’s not an absolutely flat line, there is some curvature to it. So that that’s good news as well. And then the objective observation of lesion formation also is attenuated over time. We see a therapeutic effect on new radiographic lesions as well, and very low rates of disability worsening in ozanimod patients,” said Dr. Cree.
Overall, the study included 2,494 patients who entered the open-label extension study of the phase 1-3 trials. The study began in November 2019, and the current data extend through May 10, 2021. A total of 736 patients started out with interferon beta-1a and later switched to 0.92 mg ozanimod, 877 patients started at 0.46 mg ozanimod and switched to 0.92 mg ozanimod, and 881 were on a continuous dose of 0.92 mg ozanimod.
Three-quarters of the patients were relapse free at 36 months, 71% at 48 months. Among those who were on 0.92 mg ozanimod continuously, 64% were relapse-free through 60 months of treatment.
Among the cohort, 7.6% experienced severe treatment-emergent adverse events (TEAEs), 11.9% experienced serious TEAEs, and 3.0% discontinued ozanimod because of TEAEs. Common TEAEs included nasopharyngitis (59.3%), headache (46.1%), upper respiratory tract infection (31.5%), lymphopenia (29.4%), decreased absolute lymphocyte count (ALC, 24.5%), back pain (22.7%), and hypertension (20.7%).
Furthermore, 1.4% of patients developed treatment-emergent malignancies, 0.4% developed macular edema, 2.8% had cardiac TEAEs, and 9.8% had ALC levels below 0.2 x 109/L.
Encouraging data
The COVID-19 data were encouraging, according to Patricia Coyle, MD, who was asked to comment on the study. “190 individuals out of 2,181 seems quite reasonable, and they had three deaths. It certainly didn’t look like any excessive numbers of COVID, or excessive numbers of deaths,” said Dr. Coyle, professor of neurology and director of Stony Brook (N.Y.) MS Comprehensive Care Center.
She noted that other database studies have shown an association between increased risk and anti-CD20 agents, but they haven’t really seen that with the other disease-modifying therapies. “I think this is some long-term data that says that ozanimod appears to be well tolerated without having any surprising late toxicity,” said Dr. Coyle.
The study was funded by Celgene International II. Dr. Cree has consulted for Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Novartis, Sanofi, TG Therapeutics, and Therini, and received grant support from Genentech. Dr. Coyle has consulted or received speaker fees from Accordant, Alexion, Biogen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Horizon Therapeutics, Janssen, Mylan, Novartis, Sanofi Genzyme, TG Therapeutics, and Viela Bio. Dr. Coyle has received research funding from Actelion, Alkermes, Celgene, CorEvitas LLC, Genentech/Roche, MedDay, Novartis, and Sanofi Genzyme.
FROM ACTRIMS FORUM 2022
Is a progression-free survival benefit alone really worth $10,000 a month?
In the field of lung cancer, and more broadly in oncology, many of our biggest advances in 2021 have come as clinically meaningful improvements in surrogate endpoints – disease-free survival, progression-free survival, and sometimes even pathologic complete response rate.
I have historically been most compelled to consider new findings to be practice-changing when they improve overall survival or quality of life – the endpoints that translate to direct benefits for patients. However, I also feel it is appropriate to call surrogate endpoints practice-changing when they can predict improvements in overall survival or quality of life.
Take the PACIFIC trial, which assessed maintenance durvalumab after concurrent chemoradiation for unresectable stage III non–small cell lung cancer (NSCLC).
Back in 2017, I was initially unconvinced by the interim phase 3 data that were presented in a press release that highlighted the disease-free survival benefit. However, after examining additional data more closely, I saw the dramatic improvement in time to distant relapse or death was overwhelmingly likely to predict an improvement in overall survival – a benefit that the data subsequently bore out.
More recently, the disease-free survival results for adjuvant osimertinib in resected endothelial growth factor receptor mutation–positive NSCLC and adjuvant atezolizumab in resected programmed death-ligand 1–positive stage II-IIIA NSCLC have led to excitement about Food and Drug Administration approvals for these therapies. Although there is reason to be cautious about the likelihood of an overall survival benefit with either therapy – particularly for patients with low programmed death-ligand 1 who receive atezolizumab – I think that the results are promising enough to discuss these treatment options with appropriate patients.
Some argue, however, that overall survival is not necessarily a critical goal and that certain surrogate endpoints are inherently beneficial. Patients and oncologists may, for instance, view delaying disease progression as a win, even if overall survival remains the same.
I appreciate the view that favorable scan results are an achievement, even without a survival benefit. Patients appreciate the good news, and it is gratifying for us to deliver it. However, what remains unspoken is whether the benefit can be provided at a reasonable value given the financial costs associated with the new treatment.
In the United States, we consider the physician-patient relationship to be autonomous and even revered, but we conveniently ignore the fact that both are deciding on treatments that are funded by people who are not represented in the room. And in a health care system that fails to cover basic cancer care needs as well as other critical, high-value interventions for both the uninsured and underinsured, we should acknowledge that our decisions redirect limited resources from others.
Is it the best use of $10,000 per month for a new drug that improves disease-free survival but not overall survival?
At the same time, we also have to remain vigilant and reflect on whether we are echoing the marketing messages of the companies selling these treatments. Having recently watched the excellent Hulu series Dopesick, which realistically portrays the medical community’s egregious overuse of Oxycontin at the behest of Purdue Pharmaceuticals, it is striking to see how effectively the pharmaceutical industry can co-opt stakeholders. Very few physicians or patients have expertise in health care policy with broad societal perspective, yet subspecialists offer edicts as if society should dedicate unlimited resources first and foremost to our career focus or personal cause.
I certainly appreciate the appeal of surrogate endpoints in a world in which we hope to offer novel therapies to patients in a timely fashion. In the next few years, some of our most promising data in oncology will demand that we consider whether surrogate endpoints are practice-changing. We are facing a fundamental question: Are we using these surrogate endpoints to predict overall survival or quality of life or do these endpoints stand on their own as practice-changing metrics?
We need to acknowledge that our primary clinical focus is not the only one that deserves our attention, particularly when our treatment decisions are, in fact, spending other people’s money. We should be asking not whether we prefer to deliver good news after a scan, but whether that alone is enough to justify the high cost of a new treatment without an overall survival benefit.
Dr. West disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, and Merck; serving as a speaker or a member of a speakers bureau for Ariad/Takeda, AstraZeneca, and Genentech/Roche; and receiving income from Eli Lilly. A version of this article first appeared on Medscape.com.
In the field of lung cancer, and more broadly in oncology, many of our biggest advances in 2021 have come as clinically meaningful improvements in surrogate endpoints – disease-free survival, progression-free survival, and sometimes even pathologic complete response rate.
I have historically been most compelled to consider new findings to be practice-changing when they improve overall survival or quality of life – the endpoints that translate to direct benefits for patients. However, I also feel it is appropriate to call surrogate endpoints practice-changing when they can predict improvements in overall survival or quality of life.
Take the PACIFIC trial, which assessed maintenance durvalumab after concurrent chemoradiation for unresectable stage III non–small cell lung cancer (NSCLC).
Back in 2017, I was initially unconvinced by the interim phase 3 data that were presented in a press release that highlighted the disease-free survival benefit. However, after examining additional data more closely, I saw the dramatic improvement in time to distant relapse or death was overwhelmingly likely to predict an improvement in overall survival – a benefit that the data subsequently bore out.
More recently, the disease-free survival results for adjuvant osimertinib in resected endothelial growth factor receptor mutation–positive NSCLC and adjuvant atezolizumab in resected programmed death-ligand 1–positive stage II-IIIA NSCLC have led to excitement about Food and Drug Administration approvals for these therapies. Although there is reason to be cautious about the likelihood of an overall survival benefit with either therapy – particularly for patients with low programmed death-ligand 1 who receive atezolizumab – I think that the results are promising enough to discuss these treatment options with appropriate patients.
Some argue, however, that overall survival is not necessarily a critical goal and that certain surrogate endpoints are inherently beneficial. Patients and oncologists may, for instance, view delaying disease progression as a win, even if overall survival remains the same.
I appreciate the view that favorable scan results are an achievement, even without a survival benefit. Patients appreciate the good news, and it is gratifying for us to deliver it. However, what remains unspoken is whether the benefit can be provided at a reasonable value given the financial costs associated with the new treatment.
In the United States, we consider the physician-patient relationship to be autonomous and even revered, but we conveniently ignore the fact that both are deciding on treatments that are funded by people who are not represented in the room. And in a health care system that fails to cover basic cancer care needs as well as other critical, high-value interventions for both the uninsured and underinsured, we should acknowledge that our decisions redirect limited resources from others.
Is it the best use of $10,000 per month for a new drug that improves disease-free survival but not overall survival?
At the same time, we also have to remain vigilant and reflect on whether we are echoing the marketing messages of the companies selling these treatments. Having recently watched the excellent Hulu series Dopesick, which realistically portrays the medical community’s egregious overuse of Oxycontin at the behest of Purdue Pharmaceuticals, it is striking to see how effectively the pharmaceutical industry can co-opt stakeholders. Very few physicians or patients have expertise in health care policy with broad societal perspective, yet subspecialists offer edicts as if society should dedicate unlimited resources first and foremost to our career focus or personal cause.
I certainly appreciate the appeal of surrogate endpoints in a world in which we hope to offer novel therapies to patients in a timely fashion. In the next few years, some of our most promising data in oncology will demand that we consider whether surrogate endpoints are practice-changing. We are facing a fundamental question: Are we using these surrogate endpoints to predict overall survival or quality of life or do these endpoints stand on their own as practice-changing metrics?
We need to acknowledge that our primary clinical focus is not the only one that deserves our attention, particularly when our treatment decisions are, in fact, spending other people’s money. We should be asking not whether we prefer to deliver good news after a scan, but whether that alone is enough to justify the high cost of a new treatment without an overall survival benefit.
Dr. West disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, and Merck; serving as a speaker or a member of a speakers bureau for Ariad/Takeda, AstraZeneca, and Genentech/Roche; and receiving income from Eli Lilly. A version of this article first appeared on Medscape.com.
In the field of lung cancer, and more broadly in oncology, many of our biggest advances in 2021 have come as clinically meaningful improvements in surrogate endpoints – disease-free survival, progression-free survival, and sometimes even pathologic complete response rate.
I have historically been most compelled to consider new findings to be practice-changing when they improve overall survival or quality of life – the endpoints that translate to direct benefits for patients. However, I also feel it is appropriate to call surrogate endpoints practice-changing when they can predict improvements in overall survival or quality of life.
Take the PACIFIC trial, which assessed maintenance durvalumab after concurrent chemoradiation for unresectable stage III non–small cell lung cancer (NSCLC).
Back in 2017, I was initially unconvinced by the interim phase 3 data that were presented in a press release that highlighted the disease-free survival benefit. However, after examining additional data more closely, I saw the dramatic improvement in time to distant relapse or death was overwhelmingly likely to predict an improvement in overall survival – a benefit that the data subsequently bore out.
More recently, the disease-free survival results for adjuvant osimertinib in resected endothelial growth factor receptor mutation–positive NSCLC and adjuvant atezolizumab in resected programmed death-ligand 1–positive stage II-IIIA NSCLC have led to excitement about Food and Drug Administration approvals for these therapies. Although there is reason to be cautious about the likelihood of an overall survival benefit with either therapy – particularly for patients with low programmed death-ligand 1 who receive atezolizumab – I think that the results are promising enough to discuss these treatment options with appropriate patients.
Some argue, however, that overall survival is not necessarily a critical goal and that certain surrogate endpoints are inherently beneficial. Patients and oncologists may, for instance, view delaying disease progression as a win, even if overall survival remains the same.
I appreciate the view that favorable scan results are an achievement, even without a survival benefit. Patients appreciate the good news, and it is gratifying for us to deliver it. However, what remains unspoken is whether the benefit can be provided at a reasonable value given the financial costs associated with the new treatment.
In the United States, we consider the physician-patient relationship to be autonomous and even revered, but we conveniently ignore the fact that both are deciding on treatments that are funded by people who are not represented in the room. And in a health care system that fails to cover basic cancer care needs as well as other critical, high-value interventions for both the uninsured and underinsured, we should acknowledge that our decisions redirect limited resources from others.
Is it the best use of $10,000 per month for a new drug that improves disease-free survival but not overall survival?
At the same time, we also have to remain vigilant and reflect on whether we are echoing the marketing messages of the companies selling these treatments. Having recently watched the excellent Hulu series Dopesick, which realistically portrays the medical community’s egregious overuse of Oxycontin at the behest of Purdue Pharmaceuticals, it is striking to see how effectively the pharmaceutical industry can co-opt stakeholders. Very few physicians or patients have expertise in health care policy with broad societal perspective, yet subspecialists offer edicts as if society should dedicate unlimited resources first and foremost to our career focus or personal cause.
I certainly appreciate the appeal of surrogate endpoints in a world in which we hope to offer novel therapies to patients in a timely fashion. In the next few years, some of our most promising data in oncology will demand that we consider whether surrogate endpoints are practice-changing. We are facing a fundamental question: Are we using these surrogate endpoints to predict overall survival or quality of life or do these endpoints stand on their own as practice-changing metrics?
We need to acknowledge that our primary clinical focus is not the only one that deserves our attention, particularly when our treatment decisions are, in fact, spending other people’s money. We should be asking not whether we prefer to deliver good news after a scan, but whether that alone is enough to justify the high cost of a new treatment without an overall survival benefit.
Dr. West disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, and Merck; serving as a speaker or a member of a speakers bureau for Ariad/Takeda, AstraZeneca, and Genentech/Roche; and receiving income from Eli Lilly. A version of this article first appeared on Medscape.com.
Debate heats up on how best to treat gender-questioning kids
The past week has seen heated debate about the complex issue of how to best treat children with gender dysphoria, with further developments in a number of U.S. states and in Sweden.
In the U.S., more states have moved to prevent the use of any medical treatment, such as puberty blockers or cross-sex hormones, in kids younger than the age of 18, most recently in a state Senate vote in Alabama last week, and in Texas, where Governor Greg Abbott is said to have ordered state agencies to investigate reports of gender-transition procedures on children as “child abuse.”
At least one parent has, because of this, established a crowdfunding page to try to raise money to move away from Texas, fearful of being accused of child abuse if their child with gender dysphoria receives hormone therapy. And a countersuit has been filed there by the ACLU of Texas and Lambda Legal, a civil rights organization, on behalf of one parent said to be under investigation.
But on the flip side, parents living in more liberal states – where children under the age of 18 can often get hormones to transition without parental consent – are considering moving out of them to protect their children. These parents are concerned that their kids do not know enough about the side effects of puberty blockers, or lifetime use of cross-sex hormones and its implications, to be able to make properly informed decisions at such a young age.
Meanwhile, Sweden has further tightened its restrictions on medical therapy to treat gender-questioning kids, with a recent announcement from its National Board of Health and Welfare (NBHW), on Feb. 22, urging restraint in hormone treatment of minors with gender dysphoria following a review by the agency there that assesses health technologies, the SBU.
Based on the review results, the NBHW’s overall conclusion is that the risks of puberty blocking and cross-sex hormone treatment for those under 18 currently outweigh the possible benefits for the group as a whole. The agency now says hormone treatment should only be offered in exceptional cases outside the framework of research, and principally, only in adolescents with childhood-onset gender dysphoria, as opposed to those who develop it during puberty, or in their teens, as is the case with most teenagers currently presenting.
At the same time, gender-affirming hormone treatment for adolescents who identify as transgender or nonbinary is associated with changes in depression and suicidality, according to a new U.S. survey published Feb. 25 in JAMA Network Health.
However, experts who spoke to this news organization were critical of the study, noting it was small, conducted in just 104 youth who were an average age of 15.8 years and of whom only 63% completed the survey at the final timepoint, just 1 year after starting therapy. In addition, there was no control group, among other limitations.
“The most worrying thing is that they haven’t described the pros and cons of the treatment that they are researching. We know that there are risks inherent in using gender-affirming medicine, as with all medications,” Anna Hutchinson, DClinPsych, of the Integrated Psychology Clinic, London, told this news organization.
“For example, when people with gender dysphoria use cross-sex hormones, there is a burden of treatment that can last a lifetime, both for those who benefit from the treatment and those who detransition or regret later on,” said Dr. Hutchinson, who has extensive experience of working with young people with issues related to sexuality or gender.
“This isn’t mentioned at all, which makes the paper appear quite biased towards using one approach for managing gender dysphoria and related distress, whilst not acknowledging any risks of doing so or considering alternatives,” she noted.
Why were some treated with hormones while others weren’t?
The newly published survey is by PhD student Diana M. Tordoff, MPH, of the Department of Epidemiology, University of Washington, Seattle, and colleagues. Published alongside was an invited commentary by Brett Dolotina, BS, of Massachusetts General Hospital, Boston, and Jack L. Turban, MD, MHS, of Stanford (Calif.) University.
The study was conducted at an urban multidisciplinary gender clinic in Seattle among transgender and nonbinary adolescents and young adults seeking gender-affirming care from August 2017 to June 2018. Data were analyzed from August 2020 to November 2021.
Participating in the study were 104 youths aged 13-20 years (mean age, 15.8 years), 63 transmasculine (born female) individuals (60.6%), 27 transfeminine (born male) individuals (26.0%), 10 nonbinary or gender-fluid individuals (9.6%), and four youths who responded, “I don’t know,” or did not respond to the gender-identity question (3.8%).
At baseline, 59 individuals (56.7%) had moderate-to-severe depression, 52 individuals (50.0%) had moderate-to-severe anxiety, and 45 individuals (43.3%) reported self-harm or suicidal thoughts.
By the end of the study, 69 youths (66.3%) had received puberty blockers, cross-sex hormones (testosterone for girls transitioning to male and estrogen for boys transitioning to female), or both interventions, while 35 youths had not received either intervention (33.7%).
After adjustment for temporal trends and potential confounders, there were a 60% lower odds of depression (adjusted odds ratio, 0.40; 95% confidence interval, 0.17-0.95) and 73% lower odds of suicidality (aOR, 0.27; 95% CI, 0.11-0.65) among youths who had initiated puberty blockers or cross-sex hormones.
There was no association between these treatments and anxiety, however (aOR, 1.01; 95% CI, 0.41-2.51).
Dr. Hutchinson points out that nonbinary and gender fluid “are not diagnostic or clinical terms,” adding, “there is no information about how the groups were chosen or if any of them met the criteria for gender dysphoria. It seems strange to not have measured gender dysphoria, both before and after interventions, in a group of children presenting with gender dysphoria.”
She adds: “I am questioning why ‘gender-affirming’ medicine appears to be being used here as a specific intervention for depression and suicidality? [That] wouldn’t usually be the first reason to commence these particular treatments. Why didn’t they provide therapy or antidepressant medication to this group of young people, as is routine for managing mood and/or suicidality in all other patient cohorts?”
In their commentary, Mr. Dolotina and Dr. Turban observe: “The rate of suicidality among the Tordoff et al. sample after receiving gender-affirming care was still much higher than national rates of suicidality among youth in the U.S., denoting that ... other mental health determinants must be addressed ... including gender minority stress.”
Small study, no control group, large loss to follow-up
Dr. Hutchinson also criticizes the small sample size of just 104 youth and “large loss to follow-up, whereby only 65 of those 104 [youth] completed the final survey in a short time [1 year].” This could indicate “that only the most satisfied kids stayed the course,” she suggests.
And importantly, the findings on depression and suicidality rely on the experience of only five people in the no-treatment group at 12 months, she points out.
Also, as the authors themselves acknowledge, they didn’t control for other psychiatric medicines that the participants might have been taking at baseline.
“It’s important to know more about all of this in order to draw accurate conclusions about what works, or does not, for whom,” noted Dr. Hutchinson.
Most patients, too, she notes, were females-to-males taking testosterone. Therefore, the finding that they experienced a reduction in depression might simply reflect the widely reported antidepressant effects of testosterone.
Also expressing concern about the small sample size and “lack of a control group” is Michelle Mackness, MC, a Canadian counselor in private practice who has experience working with gender-questioning individuals, detransitioners, and those experiencing complications related to their transition.
“Tordoff et al.’s assertion that there is a ‘robust evidence base’ supporting pediatric transition seems out-of-step with recent global developments in care policies and protocols for gender-questioning youth,” she points out.
“Neither the study authors or commentators acknowledge, let alone address, the fact that Finland, the U.K., and Sweden have recently determined that the evidence allegedly supporting medical interventions for pediatric transition is ‘inconclusive’,” she adds.
Asked to respond, Ms. Tordoff did not directly address this question. Rather, she reiterated to this news organization: “We found that receipt of puberty blockers and gender-affirming hormones was associated with a 60% lower odds of depression and a 73% lower odds of suicidal thoughts by the end of our study follow-up. We conducted extensive sensitivity analyses, which support the robustness of our study findings.”
She added: “These results are consistent with other recently published prospective cohort studies (please see citations provided within the manuscript).”
Parents may move states
It is this concern about the lifetime burden of treatment involved with transitioning that gives some parents of children with gender dysphoria pause for thought, especially those who live in more liberal U.S. states.
Indeed, two of America’s leading psychologists who work in this field, including one who is transgender herself, told this news organization in November they are now concerned about a lack of adequate psychological evaluations of youth with gender dysphoria before any medical treatment is considered.
So while one parent, Violet A., last week established a GoFundMe page for her child, entitled, “Help Us Move Isa to Safety,” stating she needed to move from Texas due to Governor Abbott’s pronouncements there “to a state that won’t consider me an abuser when I seek medical care for my trans child and potentially remove her from my custody,” some parents feel the need instead to move from more liberal states.
Some tell their stories anonymously, as they don’t want to risk causing their gender-questioning children further distress, as detailed on the Genspect website.
A version of this article first appeared on Medscape.com.
The past week has seen heated debate about the complex issue of how to best treat children with gender dysphoria, with further developments in a number of U.S. states and in Sweden.
In the U.S., more states have moved to prevent the use of any medical treatment, such as puberty blockers or cross-sex hormones, in kids younger than the age of 18, most recently in a state Senate vote in Alabama last week, and in Texas, where Governor Greg Abbott is said to have ordered state agencies to investigate reports of gender-transition procedures on children as “child abuse.”
At least one parent has, because of this, established a crowdfunding page to try to raise money to move away from Texas, fearful of being accused of child abuse if their child with gender dysphoria receives hormone therapy. And a countersuit has been filed there by the ACLU of Texas and Lambda Legal, a civil rights organization, on behalf of one parent said to be under investigation.
But on the flip side, parents living in more liberal states – where children under the age of 18 can often get hormones to transition without parental consent – are considering moving out of them to protect their children. These parents are concerned that their kids do not know enough about the side effects of puberty blockers, or lifetime use of cross-sex hormones and its implications, to be able to make properly informed decisions at such a young age.
Meanwhile, Sweden has further tightened its restrictions on medical therapy to treat gender-questioning kids, with a recent announcement from its National Board of Health and Welfare (NBHW), on Feb. 22, urging restraint in hormone treatment of minors with gender dysphoria following a review by the agency there that assesses health technologies, the SBU.
Based on the review results, the NBHW’s overall conclusion is that the risks of puberty blocking and cross-sex hormone treatment for those under 18 currently outweigh the possible benefits for the group as a whole. The agency now says hormone treatment should only be offered in exceptional cases outside the framework of research, and principally, only in adolescents with childhood-onset gender dysphoria, as opposed to those who develop it during puberty, or in their teens, as is the case with most teenagers currently presenting.
At the same time, gender-affirming hormone treatment for adolescents who identify as transgender or nonbinary is associated with changes in depression and suicidality, according to a new U.S. survey published Feb. 25 in JAMA Network Health.
However, experts who spoke to this news organization were critical of the study, noting it was small, conducted in just 104 youth who were an average age of 15.8 years and of whom only 63% completed the survey at the final timepoint, just 1 year after starting therapy. In addition, there was no control group, among other limitations.
“The most worrying thing is that they haven’t described the pros and cons of the treatment that they are researching. We know that there are risks inherent in using gender-affirming medicine, as with all medications,” Anna Hutchinson, DClinPsych, of the Integrated Psychology Clinic, London, told this news organization.
“For example, when people with gender dysphoria use cross-sex hormones, there is a burden of treatment that can last a lifetime, both for those who benefit from the treatment and those who detransition or regret later on,” said Dr. Hutchinson, who has extensive experience of working with young people with issues related to sexuality or gender.
“This isn’t mentioned at all, which makes the paper appear quite biased towards using one approach for managing gender dysphoria and related distress, whilst not acknowledging any risks of doing so or considering alternatives,” she noted.
Why were some treated with hormones while others weren’t?
The newly published survey is by PhD student Diana M. Tordoff, MPH, of the Department of Epidemiology, University of Washington, Seattle, and colleagues. Published alongside was an invited commentary by Brett Dolotina, BS, of Massachusetts General Hospital, Boston, and Jack L. Turban, MD, MHS, of Stanford (Calif.) University.
The study was conducted at an urban multidisciplinary gender clinic in Seattle among transgender and nonbinary adolescents and young adults seeking gender-affirming care from August 2017 to June 2018. Data were analyzed from August 2020 to November 2021.
Participating in the study were 104 youths aged 13-20 years (mean age, 15.8 years), 63 transmasculine (born female) individuals (60.6%), 27 transfeminine (born male) individuals (26.0%), 10 nonbinary or gender-fluid individuals (9.6%), and four youths who responded, “I don’t know,” or did not respond to the gender-identity question (3.8%).
At baseline, 59 individuals (56.7%) had moderate-to-severe depression, 52 individuals (50.0%) had moderate-to-severe anxiety, and 45 individuals (43.3%) reported self-harm or suicidal thoughts.
By the end of the study, 69 youths (66.3%) had received puberty blockers, cross-sex hormones (testosterone for girls transitioning to male and estrogen for boys transitioning to female), or both interventions, while 35 youths had not received either intervention (33.7%).
After adjustment for temporal trends and potential confounders, there were a 60% lower odds of depression (adjusted odds ratio, 0.40; 95% confidence interval, 0.17-0.95) and 73% lower odds of suicidality (aOR, 0.27; 95% CI, 0.11-0.65) among youths who had initiated puberty blockers or cross-sex hormones.
There was no association between these treatments and anxiety, however (aOR, 1.01; 95% CI, 0.41-2.51).
Dr. Hutchinson points out that nonbinary and gender fluid “are not diagnostic or clinical terms,” adding, “there is no information about how the groups were chosen or if any of them met the criteria for gender dysphoria. It seems strange to not have measured gender dysphoria, both before and after interventions, in a group of children presenting with gender dysphoria.”
She adds: “I am questioning why ‘gender-affirming’ medicine appears to be being used here as a specific intervention for depression and suicidality? [That] wouldn’t usually be the first reason to commence these particular treatments. Why didn’t they provide therapy or antidepressant medication to this group of young people, as is routine for managing mood and/or suicidality in all other patient cohorts?”
In their commentary, Mr. Dolotina and Dr. Turban observe: “The rate of suicidality among the Tordoff et al. sample after receiving gender-affirming care was still much higher than national rates of suicidality among youth in the U.S., denoting that ... other mental health determinants must be addressed ... including gender minority stress.”
Small study, no control group, large loss to follow-up
Dr. Hutchinson also criticizes the small sample size of just 104 youth and “large loss to follow-up, whereby only 65 of those 104 [youth] completed the final survey in a short time [1 year].” This could indicate “that only the most satisfied kids stayed the course,” she suggests.
And importantly, the findings on depression and suicidality rely on the experience of only five people in the no-treatment group at 12 months, she points out.
Also, as the authors themselves acknowledge, they didn’t control for other psychiatric medicines that the participants might have been taking at baseline.
“It’s important to know more about all of this in order to draw accurate conclusions about what works, or does not, for whom,” noted Dr. Hutchinson.
Most patients, too, she notes, were females-to-males taking testosterone. Therefore, the finding that they experienced a reduction in depression might simply reflect the widely reported antidepressant effects of testosterone.
Also expressing concern about the small sample size and “lack of a control group” is Michelle Mackness, MC, a Canadian counselor in private practice who has experience working with gender-questioning individuals, detransitioners, and those experiencing complications related to their transition.
“Tordoff et al.’s assertion that there is a ‘robust evidence base’ supporting pediatric transition seems out-of-step with recent global developments in care policies and protocols for gender-questioning youth,” she points out.
“Neither the study authors or commentators acknowledge, let alone address, the fact that Finland, the U.K., and Sweden have recently determined that the evidence allegedly supporting medical interventions for pediatric transition is ‘inconclusive’,” she adds.
Asked to respond, Ms. Tordoff did not directly address this question. Rather, she reiterated to this news organization: “We found that receipt of puberty blockers and gender-affirming hormones was associated with a 60% lower odds of depression and a 73% lower odds of suicidal thoughts by the end of our study follow-up. We conducted extensive sensitivity analyses, which support the robustness of our study findings.”
She added: “These results are consistent with other recently published prospective cohort studies (please see citations provided within the manuscript).”
Parents may move states
It is this concern about the lifetime burden of treatment involved with transitioning that gives some parents of children with gender dysphoria pause for thought, especially those who live in more liberal U.S. states.
Indeed, two of America’s leading psychologists who work in this field, including one who is transgender herself, told this news organization in November they are now concerned about a lack of adequate psychological evaluations of youth with gender dysphoria before any medical treatment is considered.
So while one parent, Violet A., last week established a GoFundMe page for her child, entitled, “Help Us Move Isa to Safety,” stating she needed to move from Texas due to Governor Abbott’s pronouncements there “to a state that won’t consider me an abuser when I seek medical care for my trans child and potentially remove her from my custody,” some parents feel the need instead to move from more liberal states.
Some tell their stories anonymously, as they don’t want to risk causing their gender-questioning children further distress, as detailed on the Genspect website.
A version of this article first appeared on Medscape.com.
The past week has seen heated debate about the complex issue of how to best treat children with gender dysphoria, with further developments in a number of U.S. states and in Sweden.
In the U.S., more states have moved to prevent the use of any medical treatment, such as puberty blockers or cross-sex hormones, in kids younger than the age of 18, most recently in a state Senate vote in Alabama last week, and in Texas, where Governor Greg Abbott is said to have ordered state agencies to investigate reports of gender-transition procedures on children as “child abuse.”
At least one parent has, because of this, established a crowdfunding page to try to raise money to move away from Texas, fearful of being accused of child abuse if their child with gender dysphoria receives hormone therapy. And a countersuit has been filed there by the ACLU of Texas and Lambda Legal, a civil rights organization, on behalf of one parent said to be under investigation.
But on the flip side, parents living in more liberal states – where children under the age of 18 can often get hormones to transition without parental consent – are considering moving out of them to protect their children. These parents are concerned that their kids do not know enough about the side effects of puberty blockers, or lifetime use of cross-sex hormones and its implications, to be able to make properly informed decisions at such a young age.
Meanwhile, Sweden has further tightened its restrictions on medical therapy to treat gender-questioning kids, with a recent announcement from its National Board of Health and Welfare (NBHW), on Feb. 22, urging restraint in hormone treatment of minors with gender dysphoria following a review by the agency there that assesses health technologies, the SBU.
Based on the review results, the NBHW’s overall conclusion is that the risks of puberty blocking and cross-sex hormone treatment for those under 18 currently outweigh the possible benefits for the group as a whole. The agency now says hormone treatment should only be offered in exceptional cases outside the framework of research, and principally, only in adolescents with childhood-onset gender dysphoria, as opposed to those who develop it during puberty, or in their teens, as is the case with most teenagers currently presenting.
At the same time, gender-affirming hormone treatment for adolescents who identify as transgender or nonbinary is associated with changes in depression and suicidality, according to a new U.S. survey published Feb. 25 in JAMA Network Health.
However, experts who spoke to this news organization were critical of the study, noting it was small, conducted in just 104 youth who were an average age of 15.8 years and of whom only 63% completed the survey at the final timepoint, just 1 year after starting therapy. In addition, there was no control group, among other limitations.
“The most worrying thing is that they haven’t described the pros and cons of the treatment that they are researching. We know that there are risks inherent in using gender-affirming medicine, as with all medications,” Anna Hutchinson, DClinPsych, of the Integrated Psychology Clinic, London, told this news organization.
“For example, when people with gender dysphoria use cross-sex hormones, there is a burden of treatment that can last a lifetime, both for those who benefit from the treatment and those who detransition or regret later on,” said Dr. Hutchinson, who has extensive experience of working with young people with issues related to sexuality or gender.
“This isn’t mentioned at all, which makes the paper appear quite biased towards using one approach for managing gender dysphoria and related distress, whilst not acknowledging any risks of doing so or considering alternatives,” she noted.
Why were some treated with hormones while others weren’t?
The newly published survey is by PhD student Diana M. Tordoff, MPH, of the Department of Epidemiology, University of Washington, Seattle, and colleagues. Published alongside was an invited commentary by Brett Dolotina, BS, of Massachusetts General Hospital, Boston, and Jack L. Turban, MD, MHS, of Stanford (Calif.) University.
The study was conducted at an urban multidisciplinary gender clinic in Seattle among transgender and nonbinary adolescents and young adults seeking gender-affirming care from August 2017 to June 2018. Data were analyzed from August 2020 to November 2021.
Participating in the study were 104 youths aged 13-20 years (mean age, 15.8 years), 63 transmasculine (born female) individuals (60.6%), 27 transfeminine (born male) individuals (26.0%), 10 nonbinary or gender-fluid individuals (9.6%), and four youths who responded, “I don’t know,” or did not respond to the gender-identity question (3.8%).
At baseline, 59 individuals (56.7%) had moderate-to-severe depression, 52 individuals (50.0%) had moderate-to-severe anxiety, and 45 individuals (43.3%) reported self-harm or suicidal thoughts.
By the end of the study, 69 youths (66.3%) had received puberty blockers, cross-sex hormones (testosterone for girls transitioning to male and estrogen for boys transitioning to female), or both interventions, while 35 youths had not received either intervention (33.7%).
After adjustment for temporal trends and potential confounders, there were a 60% lower odds of depression (adjusted odds ratio, 0.40; 95% confidence interval, 0.17-0.95) and 73% lower odds of suicidality (aOR, 0.27; 95% CI, 0.11-0.65) among youths who had initiated puberty blockers or cross-sex hormones.
There was no association between these treatments and anxiety, however (aOR, 1.01; 95% CI, 0.41-2.51).
Dr. Hutchinson points out that nonbinary and gender fluid “are not diagnostic or clinical terms,” adding, “there is no information about how the groups were chosen or if any of them met the criteria for gender dysphoria. It seems strange to not have measured gender dysphoria, both before and after interventions, in a group of children presenting with gender dysphoria.”
She adds: “I am questioning why ‘gender-affirming’ medicine appears to be being used here as a specific intervention for depression and suicidality? [That] wouldn’t usually be the first reason to commence these particular treatments. Why didn’t they provide therapy or antidepressant medication to this group of young people, as is routine for managing mood and/or suicidality in all other patient cohorts?”
In their commentary, Mr. Dolotina and Dr. Turban observe: “The rate of suicidality among the Tordoff et al. sample after receiving gender-affirming care was still much higher than national rates of suicidality among youth in the U.S., denoting that ... other mental health determinants must be addressed ... including gender minority stress.”
Small study, no control group, large loss to follow-up
Dr. Hutchinson also criticizes the small sample size of just 104 youth and “large loss to follow-up, whereby only 65 of those 104 [youth] completed the final survey in a short time [1 year].” This could indicate “that only the most satisfied kids stayed the course,” she suggests.
And importantly, the findings on depression and suicidality rely on the experience of only five people in the no-treatment group at 12 months, she points out.
Also, as the authors themselves acknowledge, they didn’t control for other psychiatric medicines that the participants might have been taking at baseline.
“It’s important to know more about all of this in order to draw accurate conclusions about what works, or does not, for whom,” noted Dr. Hutchinson.
Most patients, too, she notes, were females-to-males taking testosterone. Therefore, the finding that they experienced a reduction in depression might simply reflect the widely reported antidepressant effects of testosterone.
Also expressing concern about the small sample size and “lack of a control group” is Michelle Mackness, MC, a Canadian counselor in private practice who has experience working with gender-questioning individuals, detransitioners, and those experiencing complications related to their transition.
“Tordoff et al.’s assertion that there is a ‘robust evidence base’ supporting pediatric transition seems out-of-step with recent global developments in care policies and protocols for gender-questioning youth,” she points out.
“Neither the study authors or commentators acknowledge, let alone address, the fact that Finland, the U.K., and Sweden have recently determined that the evidence allegedly supporting medical interventions for pediatric transition is ‘inconclusive’,” she adds.
Asked to respond, Ms. Tordoff did not directly address this question. Rather, she reiterated to this news organization: “We found that receipt of puberty blockers and gender-affirming hormones was associated with a 60% lower odds of depression and a 73% lower odds of suicidal thoughts by the end of our study follow-up. We conducted extensive sensitivity analyses, which support the robustness of our study findings.”
She added: “These results are consistent with other recently published prospective cohort studies (please see citations provided within the manuscript).”
Parents may move states
It is this concern about the lifetime burden of treatment involved with transitioning that gives some parents of children with gender dysphoria pause for thought, especially those who live in more liberal U.S. states.
Indeed, two of America’s leading psychologists who work in this field, including one who is transgender herself, told this news organization in November they are now concerned about a lack of adequate psychological evaluations of youth with gender dysphoria before any medical treatment is considered.
So while one parent, Violet A., last week established a GoFundMe page for her child, entitled, “Help Us Move Isa to Safety,” stating she needed to move from Texas due to Governor Abbott’s pronouncements there “to a state that won’t consider me an abuser when I seek medical care for my trans child and potentially remove her from my custody,” some parents feel the need instead to move from more liberal states.
Some tell their stories anonymously, as they don’t want to risk causing their gender-questioning children further distress, as detailed on the Genspect website.
A version of this article first appeared on Medscape.com.