User login
An Update on JAK Inhibitors in Skin Disease
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Discoid Lupus
THE COMPARISON
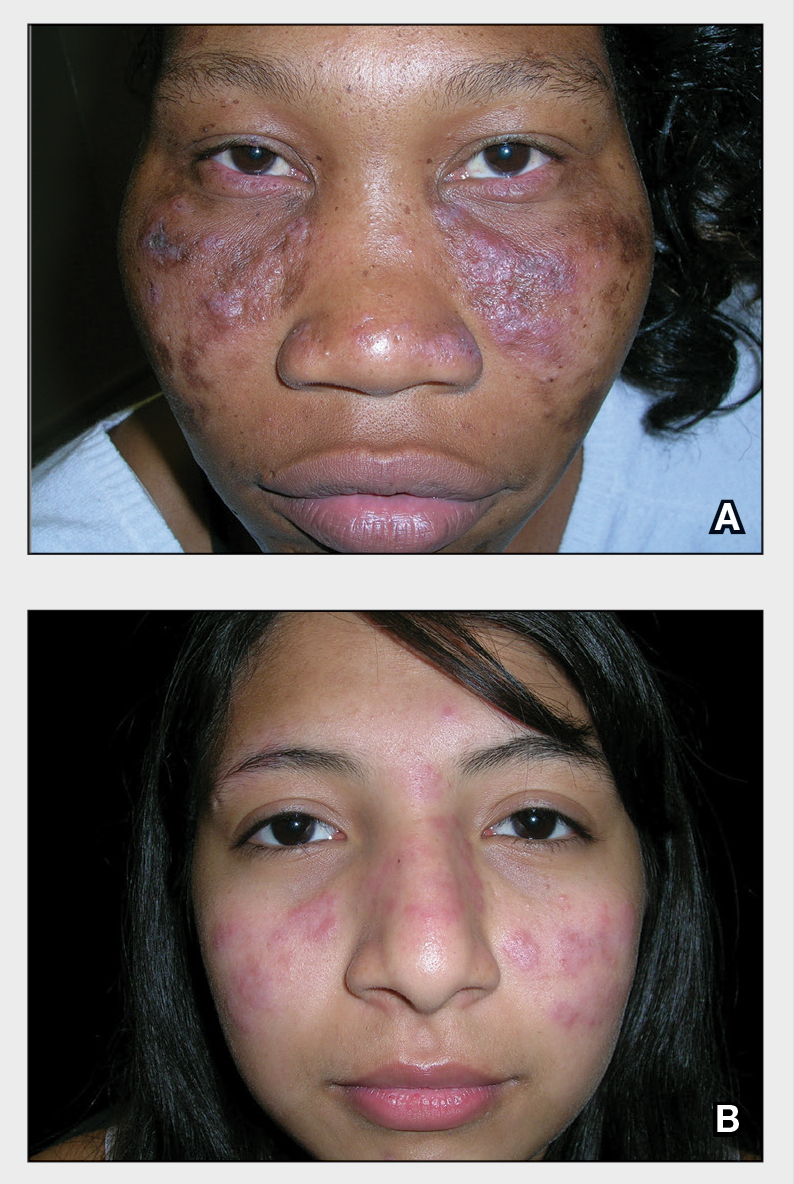
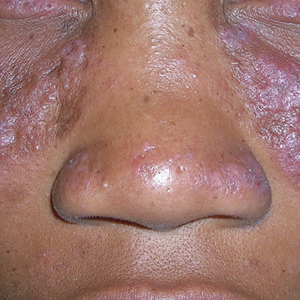
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
Discoid lupus erythematosus is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones:
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
Discoid lupus erythematosus lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. Discoid lupus erythematosus lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
- Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
- Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
- McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
- Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
Discoid lupus erythematosus is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones:
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
Discoid lupus erythematosus lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. Discoid lupus erythematosus lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
Discoid lupus erythematosus is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones:
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
Discoid lupus erythematosus lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. Discoid lupus erythematosus lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
- Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
- Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
- McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
- Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
- Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
- Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
- McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
- Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
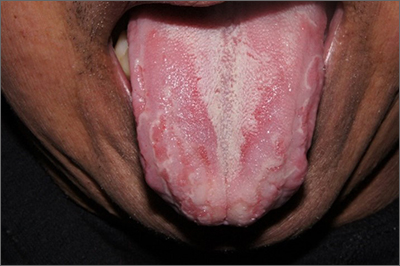
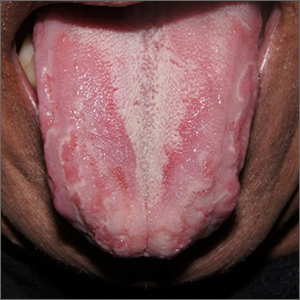
Unusual tongue markings
Well-demarcated, map-like tongue markings are consistent with migratory glossitis, also called geographic tongue, and can be recognized by its distinct clinical appearance. If performed, a biopsy would show psoriasiform mucositis.
Migratory glossitis is an uncommon condition found mostly in adults and occasionally in children. The prevalence may be as high as 2.5% globally and it may occur in conjunction with psoriasis, sharing some histologic features.1 (On close inspection, this patient was noted to have plaques on his elbows that were consistent with psoriasis.) While an immunogenic cause is suspected, the exact etiology is unknown.
Patients may develop these clinical findings quickly and just as quickly they may resolve. Discomfort and taste disturbances rarely occur. Hot, spicy, or acidic foods may be a contributing trigger. Tobacco-use appears to be protective. The presence of ulceration should prompt evaluation for a different diagnosis, such as erosive lichen planus, leukoplakia, candidiasis, or Behçet syndrome.
With minimal symptoms, treatment is rarely needed. Patients with any discomfort can be treated with topical lidocaine 2% swish and spit mouthwash, topical tacrolimus, or topical steroids.
The patient in this case was reassured that the diagnosis was not concerning and he was observed without active treatment. His psoriasis was treated with topical clobetasol ointment 0.05%. He has continued to have intermittent flares that he has yet to associate with any specific dietary causes.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Shareef S, Ettefagh L. Geographic tongue. StatPearls [Internet]. Updated August 3, 2021. Accessed February 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554466/
Well-demarcated, map-like tongue markings are consistent with migratory glossitis, also called geographic tongue, and can be recognized by its distinct clinical appearance. If performed, a biopsy would show psoriasiform mucositis.
Migratory glossitis is an uncommon condition found mostly in adults and occasionally in children. The prevalence may be as high as 2.5% globally and it may occur in conjunction with psoriasis, sharing some histologic features.1 (On close inspection, this patient was noted to have plaques on his elbows that were consistent with psoriasis.) While an immunogenic cause is suspected, the exact etiology is unknown.
Patients may develop these clinical findings quickly and just as quickly they may resolve. Discomfort and taste disturbances rarely occur. Hot, spicy, or acidic foods may be a contributing trigger. Tobacco-use appears to be protective. The presence of ulceration should prompt evaluation for a different diagnosis, such as erosive lichen planus, leukoplakia, candidiasis, or Behçet syndrome.
With minimal symptoms, treatment is rarely needed. Patients with any discomfort can be treated with topical lidocaine 2% swish and spit mouthwash, topical tacrolimus, or topical steroids.
The patient in this case was reassured that the diagnosis was not concerning and he was observed without active treatment. His psoriasis was treated with topical clobetasol ointment 0.05%. He has continued to have intermittent flares that he has yet to associate with any specific dietary causes.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Well-demarcated, map-like tongue markings are consistent with migratory glossitis, also called geographic tongue, and can be recognized by its distinct clinical appearance. If performed, a biopsy would show psoriasiform mucositis.
Migratory glossitis is an uncommon condition found mostly in adults and occasionally in children. The prevalence may be as high as 2.5% globally and it may occur in conjunction with psoriasis, sharing some histologic features.1 (On close inspection, this patient was noted to have plaques on his elbows that were consistent with psoriasis.) While an immunogenic cause is suspected, the exact etiology is unknown.
Patients may develop these clinical findings quickly and just as quickly they may resolve. Discomfort and taste disturbances rarely occur. Hot, spicy, or acidic foods may be a contributing trigger. Tobacco-use appears to be protective. The presence of ulceration should prompt evaluation for a different diagnosis, such as erosive lichen planus, leukoplakia, candidiasis, or Behçet syndrome.
With minimal symptoms, treatment is rarely needed. Patients with any discomfort can be treated with topical lidocaine 2% swish and spit mouthwash, topical tacrolimus, or topical steroids.
The patient in this case was reassured that the diagnosis was not concerning and he was observed without active treatment. His psoriasis was treated with topical clobetasol ointment 0.05%. He has continued to have intermittent flares that he has yet to associate with any specific dietary causes.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Shareef S, Ettefagh L. Geographic tongue. StatPearls [Internet]. Updated August 3, 2021. Accessed February 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554466/
1. Shareef S, Ettefagh L. Geographic tongue. StatPearls [Internet]. Updated August 3, 2021. Accessed February 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554466/
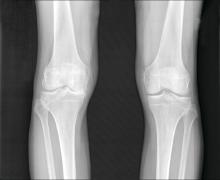
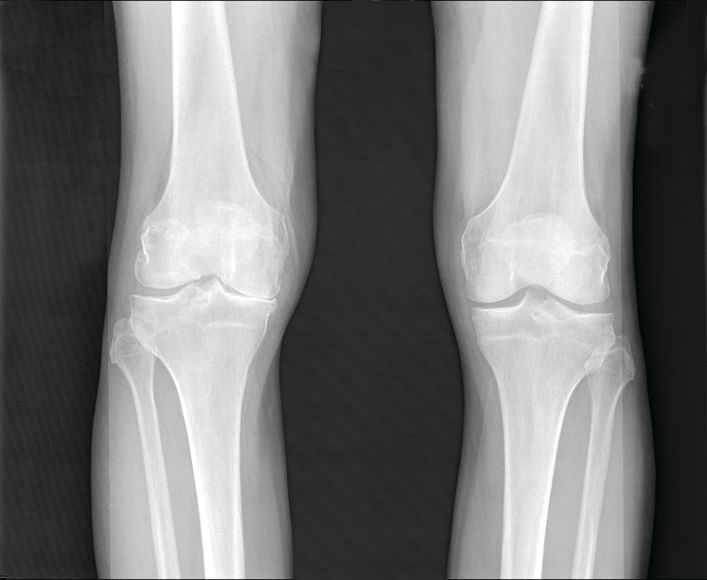
Osteoarthritis burden grows worldwide, Global Burden of Disease study finds
Prevalent cases of osteoarthritis increased significantly worldwide from 1990 to 2019, based on data from the Global Burden of Disease Study 2019.
OA remains a highly prevalent condition worldwide, with no nonsurgical interventions to prevent progression, wrote Huibin Long, MD, of Capital Medical University, Beijing, and colleagues.
Data from previous studies show that the prevalence of OA varies depending on the joints involved, with the knee being most frequently affected. However, site-specific data on OA trends and disease burden across regions or territories has not been well documented, they said.
In a study published in Arthritis & Rheumatology, the researchers analyzed data from the Global Burden of Disease Study, an ongoing project involving researchers in approximately 200 countries and territories to provide up-to-date information on the disease burdens of more than 350 types of diseases and injuries.
The Global Burden of Disease study for 2019 (GBD 2019) included data on age- and sex-specific incidence, prevalence, mortality, years of life lost, and disability-adjusted life-years for 369 diseases and injuries in 204 countries and territories. Countries were divided into five groups based on a composite sociodemographic index (SDI) of factors including fertility, income, and educational attainment; the SDI represents the quality and availability of health care, the researchers wrote.
OA was defined as radiologically confirmed Kellgren-Lawrence grade 2-4 and pain for at least 1 month during the past 12 months.
Overall, prevalent OA cases increased by 113.25% worldwide, from 247.51 million in 1990 to 527.81 million in 2019. China had the highest number of cases in 2019 (132.81 million), followed by India (62.36 million), and the United States (51.87 million). The percentage increases for these three countries from 1990 to 2019 were 156.58%, 165.75%, and 79.63%, respectively.
To further calculate trends in OA, the researchers used age-standardized prevalence rates (ASRs). The overall ASRs increased from 6,173.38 per 100,000 individuals in 1990 to 6,348.25 per 100,000 individuals in 2019, for an estimated annual percentage change of 0.12%. The ASR of OA varied substantially across countries in 2019, with the highest level observed in the United States (9,960.88 per 100,000) and the lowest in Timor-Leste (3,768.44 per 100,000). The prevalence of OA was higher in countries with higher SDI levels, such as the United States and the Republic of Korea, and increased life expectancy may play a role, they said.
OA prevalence increased with age; the prevalence of OA among adults peaked at 60-64 years in both 1990 and 2019. The absolute number of cases rose most sharply among individuals aged 95 years and older, increasing nearly fourfold during the 30-year period. The ASR of OA was also highest for people aged 95 years or older.
As for site-specific prevalence in 2019, OA of the knee was the most common site worldwide (60.6% of cases), followed by OA of the hand (23.7%), other joint sites (10.2%), and the hip (5.5%).
The ASR of OA increased for knee, hip, and other joints, with estimated annual percentage changes of 0.32%, 0.28%, and 0.18%, respectively, but decreased by 0.36% for the hand.
OA in large joints, such as the knee and hip, is often associated with higher disease burden, the researchers said. However, this held true for only knee OA because in this study, “globally as well as in most regions and countries, joints with the main disease burden were the knee, followed by the hand, [and] other joints except spine, while OA [of the] hip contributed the least,” they noted.
The study findings were limited by several factors including the adjustments from individual studies in the GBD and the exclusion of spinal symptoms, which might have contributed to an underestimation of disease burden, the researchers noted. Other limitations included the lack of assessment of the effect of health systems as part of the SDI, they said.
Overall, the results support a trend of increasing OA worldwide that is expected to continue in part because of the aging global population and the ongoing epidemic of obesity, the researchers said.
“Public awareness of the modifiable risk factors, and potential education programs of prevention of disease occurrence are essential to alleviate the enormous burden of OA,” they concluded.
The study was supported by the Beijing Postdoctoral Research Foundation and National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Prevalent cases of osteoarthritis increased significantly worldwide from 1990 to 2019, based on data from the Global Burden of Disease Study 2019.
OA remains a highly prevalent condition worldwide, with no nonsurgical interventions to prevent progression, wrote Huibin Long, MD, of Capital Medical University, Beijing, and colleagues.
Data from previous studies show that the prevalence of OA varies depending on the joints involved, with the knee being most frequently affected. However, site-specific data on OA trends and disease burden across regions or territories has not been well documented, they said.
In a study published in Arthritis & Rheumatology, the researchers analyzed data from the Global Burden of Disease Study, an ongoing project involving researchers in approximately 200 countries and territories to provide up-to-date information on the disease burdens of more than 350 types of diseases and injuries.
The Global Burden of Disease study for 2019 (GBD 2019) included data on age- and sex-specific incidence, prevalence, mortality, years of life lost, and disability-adjusted life-years for 369 diseases and injuries in 204 countries and territories. Countries were divided into five groups based on a composite sociodemographic index (SDI) of factors including fertility, income, and educational attainment; the SDI represents the quality and availability of health care, the researchers wrote.
OA was defined as radiologically confirmed Kellgren-Lawrence grade 2-4 and pain for at least 1 month during the past 12 months.
Overall, prevalent OA cases increased by 113.25% worldwide, from 247.51 million in 1990 to 527.81 million in 2019. China had the highest number of cases in 2019 (132.81 million), followed by India (62.36 million), and the United States (51.87 million). The percentage increases for these three countries from 1990 to 2019 were 156.58%, 165.75%, and 79.63%, respectively.
To further calculate trends in OA, the researchers used age-standardized prevalence rates (ASRs). The overall ASRs increased from 6,173.38 per 100,000 individuals in 1990 to 6,348.25 per 100,000 individuals in 2019, for an estimated annual percentage change of 0.12%. The ASR of OA varied substantially across countries in 2019, with the highest level observed in the United States (9,960.88 per 100,000) and the lowest in Timor-Leste (3,768.44 per 100,000). The prevalence of OA was higher in countries with higher SDI levels, such as the United States and the Republic of Korea, and increased life expectancy may play a role, they said.
OA prevalence increased with age; the prevalence of OA among adults peaked at 60-64 years in both 1990 and 2019. The absolute number of cases rose most sharply among individuals aged 95 years and older, increasing nearly fourfold during the 30-year period. The ASR of OA was also highest for people aged 95 years or older.
As for site-specific prevalence in 2019, OA of the knee was the most common site worldwide (60.6% of cases), followed by OA of the hand (23.7%), other joint sites (10.2%), and the hip (5.5%).
The ASR of OA increased for knee, hip, and other joints, with estimated annual percentage changes of 0.32%, 0.28%, and 0.18%, respectively, but decreased by 0.36% for the hand.
OA in large joints, such as the knee and hip, is often associated with higher disease burden, the researchers said. However, this held true for only knee OA because in this study, “globally as well as in most regions and countries, joints with the main disease burden were the knee, followed by the hand, [and] other joints except spine, while OA [of the] hip contributed the least,” they noted.
The study findings were limited by several factors including the adjustments from individual studies in the GBD and the exclusion of spinal symptoms, which might have contributed to an underestimation of disease burden, the researchers noted. Other limitations included the lack of assessment of the effect of health systems as part of the SDI, they said.
Overall, the results support a trend of increasing OA worldwide that is expected to continue in part because of the aging global population and the ongoing epidemic of obesity, the researchers said.
“Public awareness of the modifiable risk factors, and potential education programs of prevention of disease occurrence are essential to alleviate the enormous burden of OA,” they concluded.
The study was supported by the Beijing Postdoctoral Research Foundation and National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Prevalent cases of osteoarthritis increased significantly worldwide from 1990 to 2019, based on data from the Global Burden of Disease Study 2019.
OA remains a highly prevalent condition worldwide, with no nonsurgical interventions to prevent progression, wrote Huibin Long, MD, of Capital Medical University, Beijing, and colleagues.
Data from previous studies show that the prevalence of OA varies depending on the joints involved, with the knee being most frequently affected. However, site-specific data on OA trends and disease burden across regions or territories has not been well documented, they said.
In a study published in Arthritis & Rheumatology, the researchers analyzed data from the Global Burden of Disease Study, an ongoing project involving researchers in approximately 200 countries and territories to provide up-to-date information on the disease burdens of more than 350 types of diseases and injuries.
The Global Burden of Disease study for 2019 (GBD 2019) included data on age- and sex-specific incidence, prevalence, mortality, years of life lost, and disability-adjusted life-years for 369 diseases and injuries in 204 countries and territories. Countries were divided into five groups based on a composite sociodemographic index (SDI) of factors including fertility, income, and educational attainment; the SDI represents the quality and availability of health care, the researchers wrote.
OA was defined as radiologically confirmed Kellgren-Lawrence grade 2-4 and pain for at least 1 month during the past 12 months.
Overall, prevalent OA cases increased by 113.25% worldwide, from 247.51 million in 1990 to 527.81 million in 2019. China had the highest number of cases in 2019 (132.81 million), followed by India (62.36 million), and the United States (51.87 million). The percentage increases for these three countries from 1990 to 2019 were 156.58%, 165.75%, and 79.63%, respectively.
To further calculate trends in OA, the researchers used age-standardized prevalence rates (ASRs). The overall ASRs increased from 6,173.38 per 100,000 individuals in 1990 to 6,348.25 per 100,000 individuals in 2019, for an estimated annual percentage change of 0.12%. The ASR of OA varied substantially across countries in 2019, with the highest level observed in the United States (9,960.88 per 100,000) and the lowest in Timor-Leste (3,768.44 per 100,000). The prevalence of OA was higher in countries with higher SDI levels, such as the United States and the Republic of Korea, and increased life expectancy may play a role, they said.
OA prevalence increased with age; the prevalence of OA among adults peaked at 60-64 years in both 1990 and 2019. The absolute number of cases rose most sharply among individuals aged 95 years and older, increasing nearly fourfold during the 30-year period. The ASR of OA was also highest for people aged 95 years or older.
As for site-specific prevalence in 2019, OA of the knee was the most common site worldwide (60.6% of cases), followed by OA of the hand (23.7%), other joint sites (10.2%), and the hip (5.5%).
The ASR of OA increased for knee, hip, and other joints, with estimated annual percentage changes of 0.32%, 0.28%, and 0.18%, respectively, but decreased by 0.36% for the hand.
OA in large joints, such as the knee and hip, is often associated with higher disease burden, the researchers said. However, this held true for only knee OA because in this study, “globally as well as in most regions and countries, joints with the main disease burden were the knee, followed by the hand, [and] other joints except spine, while OA [of the] hip contributed the least,” they noted.
The study findings were limited by several factors including the adjustments from individual studies in the GBD and the exclusion of spinal symptoms, which might have contributed to an underestimation of disease burden, the researchers noted. Other limitations included the lack of assessment of the effect of health systems as part of the SDI, they said.
Overall, the results support a trend of increasing OA worldwide that is expected to continue in part because of the aging global population and the ongoing epidemic of obesity, the researchers said.
“Public awareness of the modifiable risk factors, and potential education programs of prevention of disease occurrence are essential to alleviate the enormous burden of OA,” they concluded.
The study was supported by the Beijing Postdoctoral Research Foundation and National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS & RHEUMATOLOGY
Hernia recurrence has improved only slightly
, according to a new research letter published March 1 in JAMA. Patients who underwent minimally invasive hernia repair had a higher incidence of reoperation than those who underwent open repairs.
In the United States, surgeons perform more than 1 million hernia repairs each year, according to the U.S. Food and Drug Administration. Despite hernias being such a common condition, it is “not at the forefront of many research agendas,” senior author Dana Telem, MD, an associate professor and section chief of general surgery at University of Michigan Health in Ann Arbor, said in an interview
While many surgical outcomes are measured within 30 days of operation, recurrences generally happen within 2 to 5 years after repair, she said. The last study that looked at reoperations for hernia repair at 10 years was published in 2003 and found that about 20% of patients needed surgery for reoccurrence over a decade. “We don’t really have a good understanding of what happened after these operations,” she explained. “Without knowing that piece, it is hard to go back retrospectively and understand what is the right operation for the right person at the right time.”
To understand rates of reoperation for hernia reoccurrence in today’s U.S. population of older adults, Dr. Telem and colleagues sorted through Medicare claims data to find adult patients who had undergone ventral or incisional and umbilical hernia repair from January 1, 2007 through December 31, 2018. They identified a total of 175,735 patients, 162,292 that underwent ventral or incisional hernia repair and 13,443 that underwent umbilical hernia repair. The average age of patients was 68.9 years and 39.2% were men. Most patients were White (87.2%), 8.1% were Black, 1.9% were Hispanic, and 0.5% were Asian. Median follow-up was 5.3 years.
Over the 10-year study period, 25,061 patients required reoperation for hernia recurrence with an adjusted cumulative incidence of 16.1% (95% CI, 16.1% - 16.2%). Patients who underwent open repair had a lower incidence of recurrence over 10 years than those who underwent minimally invasive repair for all hernia types (Table 1).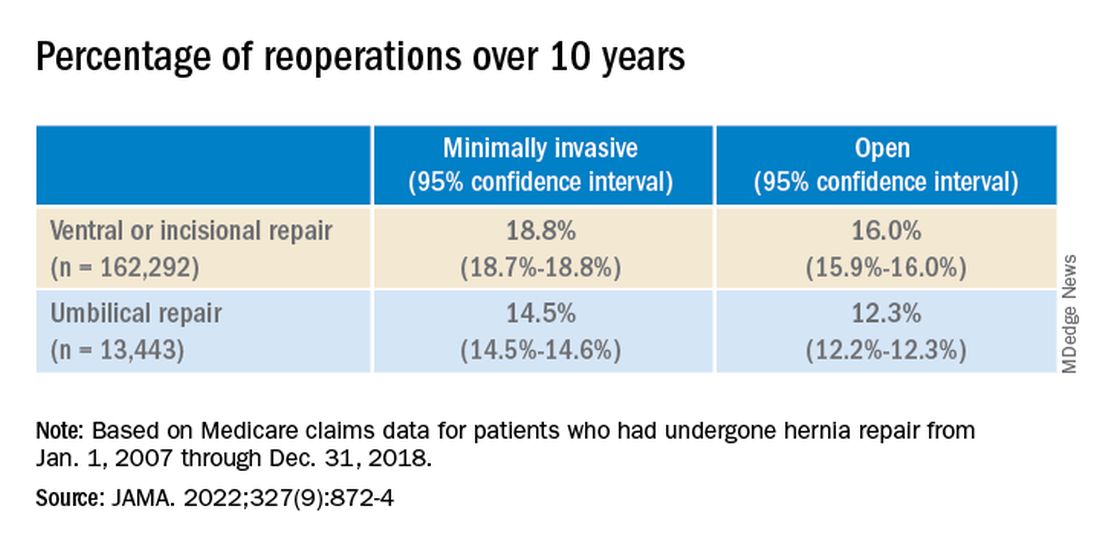
While it appears that hernia recurrence and reoperation have only marginally improved from 2003 to today, Vedra Augenstein, MD, an associate professor of surgery at the Atrium Health General & Complex Abdominal Surgery facility in Charlotte, N.C., suspects there is more to the story. “I think the reason it hasn’t gotten a whole lot better is just because we are operating on much tougher cases than we used to,” she said in an interview. “The way we are fixing hernias has changed and patients are being optimized differently.” Dr. Augenstein was not involved with the research.
To better understand how recurrence has changed over time, there needs to be more data about the comorbidities of patients, the techniques employed, and the meshes used in these surgeries, she said. Those numbers are not available in the published JAMA research letter, but Dr. Telem and colleagues will be submitting an article about this work with greater details.
Dr. Augenstein was also surprised that minimally invasive surgeries had higher incidences of reoperation for recurrence compared to open hernia surgeries. “I would think that patients who had minimally invasive repairs would actually have a lower chance of having postoperative complications because of wound issues,” she said. “Literature has shown that the recurrence rate is lower [in minimally invasive surgeries] because of fewer surgical site infections.”
While Dr. Telem also considers this research letter to be the first step in understanding modern hernia surgery outcomes, it is also a reminder that there is room for improvement in hernia repair surgeries. This includes advising patients on risk factors that may make them more likely to have a hernia recurrence, such as obesity, smoking, and diabetes, she added. “If we know it’s not a perfect science, then we have to do everything that we can upfront to help those numbers.”
Dr. Telem has reported receiving grants from the Agency for Healthcare Research and Quality and consulting fees from Medtronic. Dr. Augenstein has reported consulting for Intuitive Surgical, Medtronic, Allergan, Acelity, Vicarious Surgical, and Bard Pharmaceuticals and has received honoraria for speaking from Medtronic, Allergan, Intuitive Surgical, Acelity, and WL Gore.
A version of this article first appeared on Medscape.com.
, according to a new research letter published March 1 in JAMA. Patients who underwent minimally invasive hernia repair had a higher incidence of reoperation than those who underwent open repairs.
In the United States, surgeons perform more than 1 million hernia repairs each year, according to the U.S. Food and Drug Administration. Despite hernias being such a common condition, it is “not at the forefront of many research agendas,” senior author Dana Telem, MD, an associate professor and section chief of general surgery at University of Michigan Health in Ann Arbor, said in an interview
While many surgical outcomes are measured within 30 days of operation, recurrences generally happen within 2 to 5 years after repair, she said. The last study that looked at reoperations for hernia repair at 10 years was published in 2003 and found that about 20% of patients needed surgery for reoccurrence over a decade. “We don’t really have a good understanding of what happened after these operations,” she explained. “Without knowing that piece, it is hard to go back retrospectively and understand what is the right operation for the right person at the right time.”
To understand rates of reoperation for hernia reoccurrence in today’s U.S. population of older adults, Dr. Telem and colleagues sorted through Medicare claims data to find adult patients who had undergone ventral or incisional and umbilical hernia repair from January 1, 2007 through December 31, 2018. They identified a total of 175,735 patients, 162,292 that underwent ventral or incisional hernia repair and 13,443 that underwent umbilical hernia repair. The average age of patients was 68.9 years and 39.2% were men. Most patients were White (87.2%), 8.1% were Black, 1.9% were Hispanic, and 0.5% were Asian. Median follow-up was 5.3 years.
Over the 10-year study period, 25,061 patients required reoperation for hernia recurrence with an adjusted cumulative incidence of 16.1% (95% CI, 16.1% - 16.2%). Patients who underwent open repair had a lower incidence of recurrence over 10 years than those who underwent minimally invasive repair for all hernia types (Table 1).
While it appears that hernia recurrence and reoperation have only marginally improved from 2003 to today, Vedra Augenstein, MD, an associate professor of surgery at the Atrium Health General & Complex Abdominal Surgery facility in Charlotte, N.C., suspects there is more to the story. “I think the reason it hasn’t gotten a whole lot better is just because we are operating on much tougher cases than we used to,” she said in an interview. “The way we are fixing hernias has changed and patients are being optimized differently.” Dr. Augenstein was not involved with the research.
To better understand how recurrence has changed over time, there needs to be more data about the comorbidities of patients, the techniques employed, and the meshes used in these surgeries, she said. Those numbers are not available in the published JAMA research letter, but Dr. Telem and colleagues will be submitting an article about this work with greater details.
Dr. Augenstein was also surprised that minimally invasive surgeries had higher incidences of reoperation for recurrence compared to open hernia surgeries. “I would think that patients who had minimally invasive repairs would actually have a lower chance of having postoperative complications because of wound issues,” she said. “Literature has shown that the recurrence rate is lower [in minimally invasive surgeries] because of fewer surgical site infections.”
While Dr. Telem also considers this research letter to be the first step in understanding modern hernia surgery outcomes, it is also a reminder that there is room for improvement in hernia repair surgeries. This includes advising patients on risk factors that may make them more likely to have a hernia recurrence, such as obesity, smoking, and diabetes, she added. “If we know it’s not a perfect science, then we have to do everything that we can upfront to help those numbers.”
Dr. Telem has reported receiving grants from the Agency for Healthcare Research and Quality and consulting fees from Medtronic. Dr. Augenstein has reported consulting for Intuitive Surgical, Medtronic, Allergan, Acelity, Vicarious Surgical, and Bard Pharmaceuticals and has received honoraria for speaking from Medtronic, Allergan, Intuitive Surgical, Acelity, and WL Gore.
A version of this article first appeared on Medscape.com.
, according to a new research letter published March 1 in JAMA. Patients who underwent minimally invasive hernia repair had a higher incidence of reoperation than those who underwent open repairs.
In the United States, surgeons perform more than 1 million hernia repairs each year, according to the U.S. Food and Drug Administration. Despite hernias being such a common condition, it is “not at the forefront of many research agendas,” senior author Dana Telem, MD, an associate professor and section chief of general surgery at University of Michigan Health in Ann Arbor, said in an interview
While many surgical outcomes are measured within 30 days of operation, recurrences generally happen within 2 to 5 years after repair, she said. The last study that looked at reoperations for hernia repair at 10 years was published in 2003 and found that about 20% of patients needed surgery for reoccurrence over a decade. “We don’t really have a good understanding of what happened after these operations,” she explained. “Without knowing that piece, it is hard to go back retrospectively and understand what is the right operation for the right person at the right time.”
To understand rates of reoperation for hernia reoccurrence in today’s U.S. population of older adults, Dr. Telem and colleagues sorted through Medicare claims data to find adult patients who had undergone ventral or incisional and umbilical hernia repair from January 1, 2007 through December 31, 2018. They identified a total of 175,735 patients, 162,292 that underwent ventral or incisional hernia repair and 13,443 that underwent umbilical hernia repair. The average age of patients was 68.9 years and 39.2% were men. Most patients were White (87.2%), 8.1% were Black, 1.9% were Hispanic, and 0.5% were Asian. Median follow-up was 5.3 years.
Over the 10-year study period, 25,061 patients required reoperation for hernia recurrence with an adjusted cumulative incidence of 16.1% (95% CI, 16.1% - 16.2%). Patients who underwent open repair had a lower incidence of recurrence over 10 years than those who underwent minimally invasive repair for all hernia types (Table 1).
While it appears that hernia recurrence and reoperation have only marginally improved from 2003 to today, Vedra Augenstein, MD, an associate professor of surgery at the Atrium Health General & Complex Abdominal Surgery facility in Charlotte, N.C., suspects there is more to the story. “I think the reason it hasn’t gotten a whole lot better is just because we are operating on much tougher cases than we used to,” she said in an interview. “The way we are fixing hernias has changed and patients are being optimized differently.” Dr. Augenstein was not involved with the research.
To better understand how recurrence has changed over time, there needs to be more data about the comorbidities of patients, the techniques employed, and the meshes used in these surgeries, she said. Those numbers are not available in the published JAMA research letter, but Dr. Telem and colleagues will be submitting an article about this work with greater details.
Dr. Augenstein was also surprised that minimally invasive surgeries had higher incidences of reoperation for recurrence compared to open hernia surgeries. “I would think that patients who had minimally invasive repairs would actually have a lower chance of having postoperative complications because of wound issues,” she said. “Literature has shown that the recurrence rate is lower [in minimally invasive surgeries] because of fewer surgical site infections.”
While Dr. Telem also considers this research letter to be the first step in understanding modern hernia surgery outcomes, it is also a reminder that there is room for improvement in hernia repair surgeries. This includes advising patients on risk factors that may make them more likely to have a hernia recurrence, such as obesity, smoking, and diabetes, she added. “If we know it’s not a perfect science, then we have to do everything that we can upfront to help those numbers.”
Dr. Telem has reported receiving grants from the Agency for Healthcare Research and Quality and consulting fees from Medtronic. Dr. Augenstein has reported consulting for Intuitive Surgical, Medtronic, Allergan, Acelity, Vicarious Surgical, and Bard Pharmaceuticals and has received honoraria for speaking from Medtronic, Allergan, Intuitive Surgical, Acelity, and WL Gore.
A version of this article first appeared on Medscape.com.
FROM JAMA
Artificial intelligence aids assessment of UC activity, remission
Not only are artificial intelligence (AI) systems potentially highly accurate for assessment of disease activity and remission of ulcerative colitis (UC), but they can mitigate some limits of human assessment, according to presentations at the 17th congress of the European Crohn’s and Colitis Organisation.
Importantly, AI systems have the potential to supplement the services of expert histopathologists and endoscopists rather than replace them, several experts asserted at the meeting.
“We will always need pathologists,” reassured inflammatory bowel disease (IBD) specialist Laurent Peyrin-Biroulet, MD, PhD, of Nancy (France) University Hospital, who presented about the use of an AI-driven scoring system to measure histological disease activity in UC.
Dr. Peyrin-Biroulet, who is the president of ECCO and acts as the scientific secretary of the International Organization for the Study of IBD, added that the use of AI systems could mean that pathologists have more time to do other tasks. Not only that, but it’s also not always possible to have IBD pathologist in every center, everywhere in the world.
“If we can get something that will automatically evaluate the disease activity, I think it will be something fantastic,” Dr. Peyrin-Biroulet said, “and it’s the reason why we were thinking that there is a need for an automated method to measure histological activity in UC.”
Old concept enhancing current practice
The idea of using AI systems to aid diagnostics is not new but now makes even more sense in the post–COVID-19 era, suggested Aaron F. Pollett, MD, MSc, FRCPC, codirector of the division of diagnostic medical genetics at Mount Sinai Hospital in Toronto and a pathologist with a specialty interest in gastrointestinal pathology.
“When we talk about artificial intelligence and histology, there’s actually a very long history, it goes back over 30 years,” Dr. Pollett said, from assessing cervical samples to its use in breast screening.
What seems to be sudden flurry of activity in the world of AI and pathology in recent years comes down to having a higher capacity for looking at large images, having access to large data sets, and having a high amount of computing power, Dr. Pollet inferred. Moreover, “the capacity and the need for whole slide imaging has really grown especially in the last few years as the pandemic has forced centers to adopt.” The need to work remotely and flexibly across centers and the number of available pathologists have also played a role.
AI systems that use image-based retrieval systems are making good headway in IBD, particularly in the diagnosis of UC where “some of the initial research is showing it can be quite good,” said Dr. Pollett. The “patchiness that Crohn’s can have in comparison to UC” means that it’s still an emerging area, but can perhaps be useful for more questionable cases in which “having that degree of certainty can certainly help because there is a discrepancy between specialist and nonspecialist pathologists in the likelihood that what they predict on the biopsy will be the underlying disease.”
AI systems in IBD – do they work?
Histopathology is becoming increasingly integrated into IBD clinical trial design at the behest of the Food and Drug Administration and European associations such as ECCO. This can be a tedious procedure that can be prone to error and disagreement between scorers.
The AI-driven scoring system that Dr. Peyrin-Biroulet and associates have been working on aims to fix all that by using machine learning and image processing to set up a reproducible system. Their system, which is based on the Nancy histological index for UC, shows high correlation (87%) with histopathologists’ assessment and was 100% accurate in identifying images with high (grade 4) or no (grade 0) inflammatory activity. The accuracy decreased, however, when trying to distinguish between more moderate activity, with a 75% accuracy for identifying grade 3 and 82% accuracy for grades 1 or 2.
“I’m actually very fascinated to see how we can be supported by the AI work in our practice,” observed Francesca Rosini, a histopathologist working at S. Orsola–Malpighi University Hospital in Bologna, Italy.
Dr. Rosini, who chaired the digital oral presentation session in which Dr. Peyrin-Biroulet had presented also noted that “obviously for us as well [as AI systems] no activity or severe activity is the easiest part but when it’s in between that’s where the problems come.”
Simplifying histological scoring
Simplifying scoring for use in AI systems could be the key to their future success, as Tommaso Lorenzo Parigi, MD, from Humanitas University in Milan, and a research fellow at the University of Birmingham (England), suggested.
“Histology is particularly important to distinguish between mild activity and remission,” Dr. Parigi said. “More than 30 histological scores that have been proposed, but their adoption in clinical practice remains limited.”
Dr. Parigi has been part of an international team that has developed a simplified histological score based on “the presence of absence of neutrophils, regardless of their number,” since these are “key determinants of disease activity”.
The score, known as the Paddington International Virtual Chromoendoscopy Scre (PICaSSO) Histologic Remission Index (PHRI), has been shown to correlate well with endoscopic outcomes and thus a good measure to include in AI systems. The results of this work were published online in Gut to coincide with the ECCO congress.
“We are getting close to a world where we could screen biopsies with this kind of systems and consider skipping the pathologists result if AI detected activity,” Dr. Parigi provocatively suggested. “Of course, we need to increase and improve our sensitivity, and we are currently working on that to reduce false negatives, as well as training our model to use and apply other histological scores.”
Assessing the gut in real time
Perhaps one of the most exciting developments it to be able to use these AI technologies to examine the gut in real time.
“Virtual chromoendoscopy will give you the opportunity to distinguish very carefully all the details of mucosal vascular pattern,” said Marietta Iacucci MD, PhD, FASGE, AGAF, an associate professor and gastroenterology consultant at the Birmingham (England) University Hospitals.
“So AI can give you, in real time, the score but at the same time it can help to target, to do biopsies for healing,” Dr. Iacucci added when reporting the results of a study evaluating the performance of the first virtual chromoendoscopy AI system to detect endoscopic and histologic remission in UC.
The system was proven to predict endoscopic remission very accurately (94% using PICaSSO and 87% using the UC endoscopic index of severity) when compared with a human endoscopist. Rates of predicting histological remission were also high, at around 83%-85%, depending on the score used.
“For the future, this AI tool can expediate, support, and standardize the endoscopic evaluation of UC mucosal healing in clinical practice and in clinical trials,” Dr. Iacucci said.
The next steps are to combine virtual chromoendoscopy with the PHRI and to validate the tool in a multicenter, international PICaSSO-AI study.
The AI-driven scoring system presented by Dr. Peyrin-Biroulet was supported by Takeda. Dr. Peryin-Biroulet acknowledged the receipt of personal fees and grants from Takeda along with multiple other Pharma companies and owning stock options from CTMA. Dr. Iacucci has received research grants from Pentax, AbbVie, Olympus, and Fujifilm and personal fees from Pentax, AbbVie and Janssen. Dr. Pollett, Dr. Rosini, and Dr. Parigi had no financial conflicts of interest to disclose.
Not only are artificial intelligence (AI) systems potentially highly accurate for assessment of disease activity and remission of ulcerative colitis (UC), but they can mitigate some limits of human assessment, according to presentations at the 17th congress of the European Crohn’s and Colitis Organisation.
Importantly, AI systems have the potential to supplement the services of expert histopathologists and endoscopists rather than replace them, several experts asserted at the meeting.
“We will always need pathologists,” reassured inflammatory bowel disease (IBD) specialist Laurent Peyrin-Biroulet, MD, PhD, of Nancy (France) University Hospital, who presented about the use of an AI-driven scoring system to measure histological disease activity in UC.
Dr. Peyrin-Biroulet, who is the president of ECCO and acts as the scientific secretary of the International Organization for the Study of IBD, added that the use of AI systems could mean that pathologists have more time to do other tasks. Not only that, but it’s also not always possible to have IBD pathologist in every center, everywhere in the world.
“If we can get something that will automatically evaluate the disease activity, I think it will be something fantastic,” Dr. Peyrin-Biroulet said, “and it’s the reason why we were thinking that there is a need for an automated method to measure histological activity in UC.”
Old concept enhancing current practice
The idea of using AI systems to aid diagnostics is not new but now makes even more sense in the post–COVID-19 era, suggested Aaron F. Pollett, MD, MSc, FRCPC, codirector of the division of diagnostic medical genetics at Mount Sinai Hospital in Toronto and a pathologist with a specialty interest in gastrointestinal pathology.
“When we talk about artificial intelligence and histology, there’s actually a very long history, it goes back over 30 years,” Dr. Pollett said, from assessing cervical samples to its use in breast screening.
What seems to be sudden flurry of activity in the world of AI and pathology in recent years comes down to having a higher capacity for looking at large images, having access to large data sets, and having a high amount of computing power, Dr. Pollet inferred. Moreover, “the capacity and the need for whole slide imaging has really grown especially in the last few years as the pandemic has forced centers to adopt.” The need to work remotely and flexibly across centers and the number of available pathologists have also played a role.
AI systems that use image-based retrieval systems are making good headway in IBD, particularly in the diagnosis of UC where “some of the initial research is showing it can be quite good,” said Dr. Pollett. The “patchiness that Crohn’s can have in comparison to UC” means that it’s still an emerging area, but can perhaps be useful for more questionable cases in which “having that degree of certainty can certainly help because there is a discrepancy between specialist and nonspecialist pathologists in the likelihood that what they predict on the biopsy will be the underlying disease.”
AI systems in IBD – do they work?
Histopathology is becoming increasingly integrated into IBD clinical trial design at the behest of the Food and Drug Administration and European associations such as ECCO. This can be a tedious procedure that can be prone to error and disagreement between scorers.
The AI-driven scoring system that Dr. Peyrin-Biroulet and associates have been working on aims to fix all that by using machine learning and image processing to set up a reproducible system. Their system, which is based on the Nancy histological index for UC, shows high correlation (87%) with histopathologists’ assessment and was 100% accurate in identifying images with high (grade 4) or no (grade 0) inflammatory activity. The accuracy decreased, however, when trying to distinguish between more moderate activity, with a 75% accuracy for identifying grade 3 and 82% accuracy for grades 1 or 2.
“I’m actually very fascinated to see how we can be supported by the AI work in our practice,” observed Francesca Rosini, a histopathologist working at S. Orsola–Malpighi University Hospital in Bologna, Italy.
Dr. Rosini, who chaired the digital oral presentation session in which Dr. Peyrin-Biroulet had presented also noted that “obviously for us as well [as AI systems] no activity or severe activity is the easiest part but when it’s in between that’s where the problems come.”
Simplifying histological scoring
Simplifying scoring for use in AI systems could be the key to their future success, as Tommaso Lorenzo Parigi, MD, from Humanitas University in Milan, and a research fellow at the University of Birmingham (England), suggested.
“Histology is particularly important to distinguish between mild activity and remission,” Dr. Parigi said. “More than 30 histological scores that have been proposed, but their adoption in clinical practice remains limited.”
Dr. Parigi has been part of an international team that has developed a simplified histological score based on “the presence of absence of neutrophils, regardless of their number,” since these are “key determinants of disease activity”.
The score, known as the Paddington International Virtual Chromoendoscopy Scre (PICaSSO) Histologic Remission Index (PHRI), has been shown to correlate well with endoscopic outcomes and thus a good measure to include in AI systems. The results of this work were published online in Gut to coincide with the ECCO congress.
“We are getting close to a world where we could screen biopsies with this kind of systems and consider skipping the pathologists result if AI detected activity,” Dr. Parigi provocatively suggested. “Of course, we need to increase and improve our sensitivity, and we are currently working on that to reduce false negatives, as well as training our model to use and apply other histological scores.”
Assessing the gut in real time
Perhaps one of the most exciting developments it to be able to use these AI technologies to examine the gut in real time.
“Virtual chromoendoscopy will give you the opportunity to distinguish very carefully all the details of mucosal vascular pattern,” said Marietta Iacucci MD, PhD, FASGE, AGAF, an associate professor and gastroenterology consultant at the Birmingham (England) University Hospitals.
“So AI can give you, in real time, the score but at the same time it can help to target, to do biopsies for healing,” Dr. Iacucci added when reporting the results of a study evaluating the performance of the first virtual chromoendoscopy AI system to detect endoscopic and histologic remission in UC.
The system was proven to predict endoscopic remission very accurately (94% using PICaSSO and 87% using the UC endoscopic index of severity) when compared with a human endoscopist. Rates of predicting histological remission were also high, at around 83%-85%, depending on the score used.
“For the future, this AI tool can expediate, support, and standardize the endoscopic evaluation of UC mucosal healing in clinical practice and in clinical trials,” Dr. Iacucci said.
The next steps are to combine virtual chromoendoscopy with the PHRI and to validate the tool in a multicenter, international PICaSSO-AI study.
The AI-driven scoring system presented by Dr. Peyrin-Biroulet was supported by Takeda. Dr. Peryin-Biroulet acknowledged the receipt of personal fees and grants from Takeda along with multiple other Pharma companies and owning stock options from CTMA. Dr. Iacucci has received research grants from Pentax, AbbVie, Olympus, and Fujifilm and personal fees from Pentax, AbbVie and Janssen. Dr. Pollett, Dr. Rosini, and Dr. Parigi had no financial conflicts of interest to disclose.
Not only are artificial intelligence (AI) systems potentially highly accurate for assessment of disease activity and remission of ulcerative colitis (UC), but they can mitigate some limits of human assessment, according to presentations at the 17th congress of the European Crohn’s and Colitis Organisation.
Importantly, AI systems have the potential to supplement the services of expert histopathologists and endoscopists rather than replace them, several experts asserted at the meeting.
“We will always need pathologists,” reassured inflammatory bowel disease (IBD) specialist Laurent Peyrin-Biroulet, MD, PhD, of Nancy (France) University Hospital, who presented about the use of an AI-driven scoring system to measure histological disease activity in UC.
Dr. Peyrin-Biroulet, who is the president of ECCO and acts as the scientific secretary of the International Organization for the Study of IBD, added that the use of AI systems could mean that pathologists have more time to do other tasks. Not only that, but it’s also not always possible to have IBD pathologist in every center, everywhere in the world.
“If we can get something that will automatically evaluate the disease activity, I think it will be something fantastic,” Dr. Peyrin-Biroulet said, “and it’s the reason why we were thinking that there is a need for an automated method to measure histological activity in UC.”
Old concept enhancing current practice
The idea of using AI systems to aid diagnostics is not new but now makes even more sense in the post–COVID-19 era, suggested Aaron F. Pollett, MD, MSc, FRCPC, codirector of the division of diagnostic medical genetics at Mount Sinai Hospital in Toronto and a pathologist with a specialty interest in gastrointestinal pathology.
“When we talk about artificial intelligence and histology, there’s actually a very long history, it goes back over 30 years,” Dr. Pollett said, from assessing cervical samples to its use in breast screening.
What seems to be sudden flurry of activity in the world of AI and pathology in recent years comes down to having a higher capacity for looking at large images, having access to large data sets, and having a high amount of computing power, Dr. Pollet inferred. Moreover, “the capacity and the need for whole slide imaging has really grown especially in the last few years as the pandemic has forced centers to adopt.” The need to work remotely and flexibly across centers and the number of available pathologists have also played a role.
AI systems that use image-based retrieval systems are making good headway in IBD, particularly in the diagnosis of UC where “some of the initial research is showing it can be quite good,” said Dr. Pollett. The “patchiness that Crohn’s can have in comparison to UC” means that it’s still an emerging area, but can perhaps be useful for more questionable cases in which “having that degree of certainty can certainly help because there is a discrepancy between specialist and nonspecialist pathologists in the likelihood that what they predict on the biopsy will be the underlying disease.”
AI systems in IBD – do they work?
Histopathology is becoming increasingly integrated into IBD clinical trial design at the behest of the Food and Drug Administration and European associations such as ECCO. This can be a tedious procedure that can be prone to error and disagreement between scorers.
The AI-driven scoring system that Dr. Peyrin-Biroulet and associates have been working on aims to fix all that by using machine learning and image processing to set up a reproducible system. Their system, which is based on the Nancy histological index for UC, shows high correlation (87%) with histopathologists’ assessment and was 100% accurate in identifying images with high (grade 4) or no (grade 0) inflammatory activity. The accuracy decreased, however, when trying to distinguish between more moderate activity, with a 75% accuracy for identifying grade 3 and 82% accuracy for grades 1 or 2.
“I’m actually very fascinated to see how we can be supported by the AI work in our practice,” observed Francesca Rosini, a histopathologist working at S. Orsola–Malpighi University Hospital in Bologna, Italy.
Dr. Rosini, who chaired the digital oral presentation session in which Dr. Peyrin-Biroulet had presented also noted that “obviously for us as well [as AI systems] no activity or severe activity is the easiest part but when it’s in between that’s where the problems come.”
Simplifying histological scoring
Simplifying scoring for use in AI systems could be the key to their future success, as Tommaso Lorenzo Parigi, MD, from Humanitas University in Milan, and a research fellow at the University of Birmingham (England), suggested.
“Histology is particularly important to distinguish between mild activity and remission,” Dr. Parigi said. “More than 30 histological scores that have been proposed, but their adoption in clinical practice remains limited.”
Dr. Parigi has been part of an international team that has developed a simplified histological score based on “the presence of absence of neutrophils, regardless of their number,” since these are “key determinants of disease activity”.
The score, known as the Paddington International Virtual Chromoendoscopy Scre (PICaSSO) Histologic Remission Index (PHRI), has been shown to correlate well with endoscopic outcomes and thus a good measure to include in AI systems. The results of this work were published online in Gut to coincide with the ECCO congress.
“We are getting close to a world where we could screen biopsies with this kind of systems and consider skipping the pathologists result if AI detected activity,” Dr. Parigi provocatively suggested. “Of course, we need to increase and improve our sensitivity, and we are currently working on that to reduce false negatives, as well as training our model to use and apply other histological scores.”
Assessing the gut in real time
Perhaps one of the most exciting developments it to be able to use these AI technologies to examine the gut in real time.
“Virtual chromoendoscopy will give you the opportunity to distinguish very carefully all the details of mucosal vascular pattern,” said Marietta Iacucci MD, PhD, FASGE, AGAF, an associate professor and gastroenterology consultant at the Birmingham (England) University Hospitals.
“So AI can give you, in real time, the score but at the same time it can help to target, to do biopsies for healing,” Dr. Iacucci added when reporting the results of a study evaluating the performance of the first virtual chromoendoscopy AI system to detect endoscopic and histologic remission in UC.
The system was proven to predict endoscopic remission very accurately (94% using PICaSSO and 87% using the UC endoscopic index of severity) when compared with a human endoscopist. Rates of predicting histological remission were also high, at around 83%-85%, depending on the score used.
“For the future, this AI tool can expediate, support, and standardize the endoscopic evaluation of UC mucosal healing in clinical practice and in clinical trials,” Dr. Iacucci said.
The next steps are to combine virtual chromoendoscopy with the PHRI and to validate the tool in a multicenter, international PICaSSO-AI study.
The AI-driven scoring system presented by Dr. Peyrin-Biroulet was supported by Takeda. Dr. Peryin-Biroulet acknowledged the receipt of personal fees and grants from Takeda along with multiple other Pharma companies and owning stock options from CTMA. Dr. Iacucci has received research grants from Pentax, AbbVie, Olympus, and Fujifilm and personal fees from Pentax, AbbVie and Janssen. Dr. Pollett, Dr. Rosini, and Dr. Parigi had no financial conflicts of interest to disclose.
FROM ECCO 2022
Endoscopic healing of Crohn’s disease could differ by biologic
Greater endoscopic healing at 1 year might be achieved in people with Crohn’s disease if they are treated with anti–tumor necrosis factor (TNF) drugs than if they are treated with certain other biologics it appears.
In a pooled analysis of data from four different clinical trial programs, which altogether included 344 patients with Crohn’s disease, both an infliximab biosimilar and adalimumab were associated with better endoscopic healing rates of both the ileum and colon than were either vedolizumab or ustekinumab.
The difference disappeared for ileal not colonic involvement, however, if patients had been biologic naive before receiving any of the four drugs that were compared.
“Recent studies have suggested that the ileum and colon differ with regards to their ability to achieve healing in Crohn’s disease,” Neeraj Narula, MD, said in reporting the analysis at the 17th congress of the European Crohn’s and Colitis Organisation.
“Our group has shown that larger ulcers in the ileum and rectum in particular do not heal as well as other areas of the colon when using infliximab therapies,” added Dr. Narula, who is an assistant professor of medicine at McMaster University, Hamilton, Ont., and the director of the IBD clinic and staff gastroenterologist at Hamilton Health Sciences.
Whether there are differences in how the ileal and colonic regions heal in response to biologic therapy is not known, which is why Dr. Narula and colleagues carried out their analysis.
Pooling pivotal trial program data
“Our primary aim was to evaluate the efficacy of four approved biologic therapies for Crohn’s disease with regards to their ability to achieve endoscopic healing after continuous use for 1 year,” he noted.
For their analysis, original data from the EXTEND, UNITI, VERSIFY, and infliximab biosimilar CT-P13 clinical trial programs were obtained and pooled.
The extent of mucosal inflammation and thereby healing were determined using a modified version of the Simple Endoscopic Score for Crohn’s disease (SES-CD), which is a measure often used in clinical trials.
At inclusion, patients had to have had an SES-CD score of 3 or more in at least one segment of the ileum or colon and confirmed ulceration. The primary endpoint was endoscopic healing defined as an SES-CD score of 0 after 1 year’s continuous treatment.
Multivariate logistic regression was used, and adjustments were made for potential confounding factors, such as how long people had had Crohn’s disease, the use of steroids, and if there had been prior anti-TNF failure.
Main results
Overall, 299 patients were in the final analysis; most (n = 141) had been treated with the infliximab biosimilar, with 61 treated with adalimumab, 56 vedolizumab, and 41 ustekinumab.
The highest rate of endoscopic healing at 1 year for ileal involvement was seen with the infliximab biosimilar (36.7% of patients) and the lowest rate with vedolizumab (18.6%), with rates of 30% and 22.7% for adalimumab and ustekinumab, respectively. Only the comparison between the infliximab biosimilar and vedolizumab was statistically significant (P = .038).
As for ileal ulcers, there were fewer seen with both anti-TNF treatments than with either ustekinumab or vedolizumab, at 40.8% for the infliximab biosimilar, 30% for adalimumab, 17.7% for ustekinumab, and 8.7% for vedolizumab. Rates of ileal ulcer absence in biologic-naive patients were a respective 36.7%, 37.5%, 40%, and 21.9%.
In terms of colonic involvement, the lowest rate of endoscopic healing occurred in patients treated with ustekinumab, at 29%, and the highest for adalimumab (62.5%), followed by the infliximab biosimilar (52.4%) and then vedolizumab (31.3%).
Absence of colonic ulcers was similarly low for ustekinumab (29.6%) and higher for the other three groups (64.9%, 70.5%, and 41.2%, respectively). When considering biologic-naive patients, there was a significant difference in the absence of colonic ulcers comparing adalimumab (66.7; P = .004) and the infliximab biosimilar (52.4%; P = .022), but not vedolizumab (37.1%) versus ustekinumab (29.4%).
Lots of questions and limitations
Dr. Narula’s presentation garnered a lot of questions, with viewers noting that the number of patients was too small or methodologically too flawed to be able to draw any sound conclusions.
“We acknowledge that our study cannot substitute for head-to-head trials of biologics in Crohn’s disease since we cannot account for all confounding variables,” said Dr. Narula.
“We did try to account for this limitation by performing some subgroup analyses to account for biologic-naive patients only,” he added, alongside the multivariate analyses.
Also, there might be a difference in the duration of treatment needed before endoscopic healing is seen, as the biologics studied all have a different duration of onset. The dosages used may also be important, and Dr. Narula conceded that their analyses were done assuming standard doses, which may not have been optimized.
There were several demographic differences between the infliximab arm and the other treatments. Of note, 76% of patients had been given immunomodulators at the same time, which is known to enhance the effects of infliximab.
Dr. Narula pointed out, however, that baseline characteristic were pretty similar in the other three study arms, and adalimumab still showed superiority in the analyses that were performed.
So are anti-TNFs the best choice?
“Ultimately, we always factor in the therapeutic index of therapy, trying to weigh benefit versus risk,” Dr. Narula said in answering a question from the chair of the session on the risks associated with anti-TNFs.
“We didn’t compare risk within this clinical trial, but certainly risk can be compared, and there’s things like number needed to treat versus number needed to harm to ultimately come at a best answer for the patient,” he added.
Dr. Narula disclosed receiving grants from Takeda and Pfizer; personal fees from AbbVie, Janssen, Takeda, Pfizer, Merck, Amgen, and Sandoz; and nonfinancial support from AbbVie, Janssen, Takeda, Pfizer, Ferring, and Lupin. The data used in the analysis were obtained through YODA Project #2021-4778 which has an agreement with Janssen Research & Developmen and via Vivli, which has access to data from AbbVie and Takeda. Data were also obtained with permission from Celltrion.
Greater endoscopic healing at 1 year might be achieved in people with Crohn’s disease if they are treated with anti–tumor necrosis factor (TNF) drugs than if they are treated with certain other biologics it appears.
In a pooled analysis of data from four different clinical trial programs, which altogether included 344 patients with Crohn’s disease, both an infliximab biosimilar and adalimumab were associated with better endoscopic healing rates of both the ileum and colon than were either vedolizumab or ustekinumab.
The difference disappeared for ileal not colonic involvement, however, if patients had been biologic naive before receiving any of the four drugs that were compared.
“Recent studies have suggested that the ileum and colon differ with regards to their ability to achieve healing in Crohn’s disease,” Neeraj Narula, MD, said in reporting the analysis at the 17th congress of the European Crohn’s and Colitis Organisation.
“Our group has shown that larger ulcers in the ileum and rectum in particular do not heal as well as other areas of the colon when using infliximab therapies,” added Dr. Narula, who is an assistant professor of medicine at McMaster University, Hamilton, Ont., and the director of the IBD clinic and staff gastroenterologist at Hamilton Health Sciences.
Whether there are differences in how the ileal and colonic regions heal in response to biologic therapy is not known, which is why Dr. Narula and colleagues carried out their analysis.
Pooling pivotal trial program data
“Our primary aim was to evaluate the efficacy of four approved biologic therapies for Crohn’s disease with regards to their ability to achieve endoscopic healing after continuous use for 1 year,” he noted.
For their analysis, original data from the EXTEND, UNITI, VERSIFY, and infliximab biosimilar CT-P13 clinical trial programs were obtained and pooled.
The extent of mucosal inflammation and thereby healing were determined using a modified version of the Simple Endoscopic Score for Crohn’s disease (SES-CD), which is a measure often used in clinical trials.
At inclusion, patients had to have had an SES-CD score of 3 or more in at least one segment of the ileum or colon and confirmed ulceration. The primary endpoint was endoscopic healing defined as an SES-CD score of 0 after 1 year’s continuous treatment.
Multivariate logistic regression was used, and adjustments were made for potential confounding factors, such as how long people had had Crohn’s disease, the use of steroids, and if there had been prior anti-TNF failure.
Main results
Overall, 299 patients were in the final analysis; most (n = 141) had been treated with the infliximab biosimilar, with 61 treated with adalimumab, 56 vedolizumab, and 41 ustekinumab.
The highest rate of endoscopic healing at 1 year for ileal involvement was seen with the infliximab biosimilar (36.7% of patients) and the lowest rate with vedolizumab (18.6%), with rates of 30% and 22.7% for adalimumab and ustekinumab, respectively. Only the comparison between the infliximab biosimilar and vedolizumab was statistically significant (P = .038).
As for ileal ulcers, there were fewer seen with both anti-TNF treatments than with either ustekinumab or vedolizumab, at 40.8% for the infliximab biosimilar, 30% for adalimumab, 17.7% for ustekinumab, and 8.7% for vedolizumab. Rates of ileal ulcer absence in biologic-naive patients were a respective 36.7%, 37.5%, 40%, and 21.9%.
In terms of colonic involvement, the lowest rate of endoscopic healing occurred in patients treated with ustekinumab, at 29%, and the highest for adalimumab (62.5%), followed by the infliximab biosimilar (52.4%) and then vedolizumab (31.3%).
Absence of colonic ulcers was similarly low for ustekinumab (29.6%) and higher for the other three groups (64.9%, 70.5%, and 41.2%, respectively). When considering biologic-naive patients, there was a significant difference in the absence of colonic ulcers comparing adalimumab (66.7; P = .004) and the infliximab biosimilar (52.4%; P = .022), but not vedolizumab (37.1%) versus ustekinumab (29.4%).
Lots of questions and limitations
Dr. Narula’s presentation garnered a lot of questions, with viewers noting that the number of patients was too small or methodologically too flawed to be able to draw any sound conclusions.
“We acknowledge that our study cannot substitute for head-to-head trials of biologics in Crohn’s disease since we cannot account for all confounding variables,” said Dr. Narula.
“We did try to account for this limitation by performing some subgroup analyses to account for biologic-naive patients only,” he added, alongside the multivariate analyses.
Also, there might be a difference in the duration of treatment needed before endoscopic healing is seen, as the biologics studied all have a different duration of onset. The dosages used may also be important, and Dr. Narula conceded that their analyses were done assuming standard doses, which may not have been optimized.
There were several demographic differences between the infliximab arm and the other treatments. Of note, 76% of patients had been given immunomodulators at the same time, which is known to enhance the effects of infliximab.
Dr. Narula pointed out, however, that baseline characteristic were pretty similar in the other three study arms, and adalimumab still showed superiority in the analyses that were performed.
So are anti-TNFs the best choice?
“Ultimately, we always factor in the therapeutic index of therapy, trying to weigh benefit versus risk,” Dr. Narula said in answering a question from the chair of the session on the risks associated with anti-TNFs.
“We didn’t compare risk within this clinical trial, but certainly risk can be compared, and there’s things like number needed to treat versus number needed to harm to ultimately come at a best answer for the patient,” he added.
Dr. Narula disclosed receiving grants from Takeda and Pfizer; personal fees from AbbVie, Janssen, Takeda, Pfizer, Merck, Amgen, and Sandoz; and nonfinancial support from AbbVie, Janssen, Takeda, Pfizer, Ferring, and Lupin. The data used in the analysis were obtained through YODA Project #2021-4778 which has an agreement with Janssen Research & Developmen and via Vivli, which has access to data from AbbVie and Takeda. Data were also obtained with permission from Celltrion.
Greater endoscopic healing at 1 year might be achieved in people with Crohn’s disease if they are treated with anti–tumor necrosis factor (TNF) drugs than if they are treated with certain other biologics it appears.
In a pooled analysis of data from four different clinical trial programs, which altogether included 344 patients with Crohn’s disease, both an infliximab biosimilar and adalimumab were associated with better endoscopic healing rates of both the ileum and colon than were either vedolizumab or ustekinumab.
The difference disappeared for ileal not colonic involvement, however, if patients had been biologic naive before receiving any of the four drugs that were compared.
“Recent studies have suggested that the ileum and colon differ with regards to their ability to achieve healing in Crohn’s disease,” Neeraj Narula, MD, said in reporting the analysis at the 17th congress of the European Crohn’s and Colitis Organisation.
“Our group has shown that larger ulcers in the ileum and rectum in particular do not heal as well as other areas of the colon when using infliximab therapies,” added Dr. Narula, who is an assistant professor of medicine at McMaster University, Hamilton, Ont., and the director of the IBD clinic and staff gastroenterologist at Hamilton Health Sciences.
Whether there are differences in how the ileal and colonic regions heal in response to biologic therapy is not known, which is why Dr. Narula and colleagues carried out their analysis.
Pooling pivotal trial program data
“Our primary aim was to evaluate the efficacy of four approved biologic therapies for Crohn’s disease with regards to their ability to achieve endoscopic healing after continuous use for 1 year,” he noted.
For their analysis, original data from the EXTEND, UNITI, VERSIFY, and infliximab biosimilar CT-P13 clinical trial programs were obtained and pooled.
The extent of mucosal inflammation and thereby healing were determined using a modified version of the Simple Endoscopic Score for Crohn’s disease (SES-CD), which is a measure often used in clinical trials.
At inclusion, patients had to have had an SES-CD score of 3 or more in at least one segment of the ileum or colon and confirmed ulceration. The primary endpoint was endoscopic healing defined as an SES-CD score of 0 after 1 year’s continuous treatment.
Multivariate logistic regression was used, and adjustments were made for potential confounding factors, such as how long people had had Crohn’s disease, the use of steroids, and if there had been prior anti-TNF failure.
Main results
Overall, 299 patients were in the final analysis; most (n = 141) had been treated with the infliximab biosimilar, with 61 treated with adalimumab, 56 vedolizumab, and 41 ustekinumab.
The highest rate of endoscopic healing at 1 year for ileal involvement was seen with the infliximab biosimilar (36.7% of patients) and the lowest rate with vedolizumab (18.6%), with rates of 30% and 22.7% for adalimumab and ustekinumab, respectively. Only the comparison between the infliximab biosimilar and vedolizumab was statistically significant (P = .038).
As for ileal ulcers, there were fewer seen with both anti-TNF treatments than with either ustekinumab or vedolizumab, at 40.8% for the infliximab biosimilar, 30% for adalimumab, 17.7% for ustekinumab, and 8.7% for vedolizumab. Rates of ileal ulcer absence in biologic-naive patients were a respective 36.7%, 37.5%, 40%, and 21.9%.
In terms of colonic involvement, the lowest rate of endoscopic healing occurred in patients treated with ustekinumab, at 29%, and the highest for adalimumab (62.5%), followed by the infliximab biosimilar (52.4%) and then vedolizumab (31.3%).
Absence of colonic ulcers was similarly low for ustekinumab (29.6%) and higher for the other three groups (64.9%, 70.5%, and 41.2%, respectively). When considering biologic-naive patients, there was a significant difference in the absence of colonic ulcers comparing adalimumab (66.7; P = .004) and the infliximab biosimilar (52.4%; P = .022), but not vedolizumab (37.1%) versus ustekinumab (29.4%).
Lots of questions and limitations
Dr. Narula’s presentation garnered a lot of questions, with viewers noting that the number of patients was too small or methodologically too flawed to be able to draw any sound conclusions.
“We acknowledge that our study cannot substitute for head-to-head trials of biologics in Crohn’s disease since we cannot account for all confounding variables,” said Dr. Narula.
“We did try to account for this limitation by performing some subgroup analyses to account for biologic-naive patients only,” he added, alongside the multivariate analyses.
Also, there might be a difference in the duration of treatment needed before endoscopic healing is seen, as the biologics studied all have a different duration of onset. The dosages used may also be important, and Dr. Narula conceded that their analyses were done assuming standard doses, which may not have been optimized.
There were several demographic differences between the infliximab arm and the other treatments. Of note, 76% of patients had been given immunomodulators at the same time, which is known to enhance the effects of infliximab.
Dr. Narula pointed out, however, that baseline characteristic were pretty similar in the other three study arms, and adalimumab still showed superiority in the analyses that were performed.
So are anti-TNFs the best choice?
“Ultimately, we always factor in the therapeutic index of therapy, trying to weigh benefit versus risk,” Dr. Narula said in answering a question from the chair of the session on the risks associated with anti-TNFs.
“We didn’t compare risk within this clinical trial, but certainly risk can be compared, and there’s things like number needed to treat versus number needed to harm to ultimately come at a best answer for the patient,” he added.
Dr. Narula disclosed receiving grants from Takeda and Pfizer; personal fees from AbbVie, Janssen, Takeda, Pfizer, Merck, Amgen, and Sandoz; and nonfinancial support from AbbVie, Janssen, Takeda, Pfizer, Ferring, and Lupin. The data used in the analysis were obtained through YODA Project #2021-4778 which has an agreement with Janssen Research & Developmen and via Vivli, which has access to data from AbbVie and Takeda. Data were also obtained with permission from Celltrion.
FROM ECCO 2022
Azithromycin doesn’t prevent recurrent wheezing after acute infant RSV
Azithromycin administered for severe early-life respiratory syncytial virus (RSV) bronchiolitis did not prevent recurrent wheezing in affected children over the next 2-4 years, a randomized, single-center study found.
Antibiotics are frequently given to patients with RSV bronchiolitis, although this practice is not supported by American Academy of Pediatrics clinical guidelines. Many doctors will prescribe them anyway if they see redness in the ears or other signs of infection, lead author Avraham Beigelman, MD, a pediatric allergist and immunologist at Washington University in St. Louis, said in an interview.
The double-blind, placebo-controlled trial, presented at the 2022 meeting of the American Academy of Allergy, Asthma & Immunology in Phoenix, was simultaneously published online Feb. 27, 2022, in the New England Journal of Medicine–Evidence.
Since azithromycin has shown anti-inflammatory benefit in chronic lung diseases and is a mainstay of care in cystic fibrosis and had shown previous effects in RSV patients, this trial examined its potential for preventing future recurrent wheezing in infants hospitalized with RSV who are at risk for developing asthma later. About half of children admitted to the hospital for RSV will develop asthma by age 7, Dr. Beigelman said.
“We were very surprised that azithromycin didn’t help in this trial given our previous findings,” Dr. Beigelman said.
And while those given azithromycin versus those given a placebo showed no significant decrease in recurrent wheezing, there was a slight suggestion that treatment with antibiotics of any kind may increase the risk of later wheezing in infants hospitalized with the virus.
“The study was not designed to tease at the effects of different antibiotics or combinations of antibiotics, so we have to be very cautious about this trend,” Dr. Beigelman said. “There may be short-term effects and long-term effects. Certain antibiotics may affect the infant microbiome in other parts of the body, such as the gut, [in] a way that may predispose to asthma. But all these associations suggest that early-life antibiotics for viral infections are not good for you.”
He pointed to the longstanding question among clinicians whether it is the antibiotic that’s increasing the risk of the harm or the condition for which the antibiotic is prescribed. These exploratory data, however, suggest that antibiotics for RSV may be causing harm.
In pursuit of that hypothesis, his group has collected airway microbiome samples from these infants and plan to investigate whether bacteria colonizing the airway may interact with the antibiotics to increase wheezing. The researchers will analyze stool samples from the babies to see whether the gut microbiome may also play a role in wheezing and the subsequent risk of developing childhood asthma.
Study details
The trial prospectively enrolled 200 otherwise healthy babies aged 1-18 months who were hospitalized at St. Louis Children’s Hospital for acute RSV bronchiolitis. Although RSV is a very common pediatric virus, only bout 3% of babies will require hospitalization in order to receive oxygen, Dr. Beigelman said.
Babies were randomly assigned to receive placebo or oral azithromycin at 10 mg/kg daily for 7 days, followed by 5 mg/kg daily for 7 days. Randomization was stratified by recent open-label antibiotic use. The primary outcome was recurrent wheeze, defined as a third episode of post-RSV wheeze over the following 2-4 years.
The biologic activity of azithromycin was clear since nasal-wash interleukin at day 14 after randomization was lower in azithromycin-treated infants. But despite evidence of activity, the risk of post-RSV recurrent wheeze was similar in both arms: 47% in the azithromycin group versus 36% in the placebo group, for an adjusted hazard ratio of 1.45 (95% confidence interval, 0.92-2.29; P = .11).
Nor did azithromycin lower the risk of recurrent wheeze in babies already receiving other antibiotics at the time of enrollment (HR, 0.94; 95% CI, 0.43-2.07). As for antibiotic-naive participants receiving azithromycin, there was a slight signal of potential increased risk of developing recurrent wheezing (HR, 1.79; 95% CI, 1.03-3.1).
The bottom line? The findings support current clinical guidelines recommending against the use of antibiotics for RSV. “At the very least, azithromycin and antibiotics in general have no benefit in preventing recurrent wheeze, and there is a possibility they may be harmful,” Dr. Beigelman said.
This trial is funded by the National Heart, Lung, and Blood Institute. Dr. Beigelman reported relationships with AstraZeneca, Novartis, and Sanofi. Two study coauthors disclosed various ties to industry.
Azithromycin administered for severe early-life respiratory syncytial virus (RSV) bronchiolitis did not prevent recurrent wheezing in affected children over the next 2-4 years, a randomized, single-center study found.
Antibiotics are frequently given to patients with RSV bronchiolitis, although this practice is not supported by American Academy of Pediatrics clinical guidelines. Many doctors will prescribe them anyway if they see redness in the ears or other signs of infection, lead author Avraham Beigelman, MD, a pediatric allergist and immunologist at Washington University in St. Louis, said in an interview.
The double-blind, placebo-controlled trial, presented at the 2022 meeting of the American Academy of Allergy, Asthma & Immunology in Phoenix, was simultaneously published online Feb. 27, 2022, in the New England Journal of Medicine–Evidence.
Since azithromycin has shown anti-inflammatory benefit in chronic lung diseases and is a mainstay of care in cystic fibrosis and had shown previous effects in RSV patients, this trial examined its potential for preventing future recurrent wheezing in infants hospitalized with RSV who are at risk for developing asthma later. About half of children admitted to the hospital for RSV will develop asthma by age 7, Dr. Beigelman said.
“We were very surprised that azithromycin didn’t help in this trial given our previous findings,” Dr. Beigelman said.
And while those given azithromycin versus those given a placebo showed no significant decrease in recurrent wheezing, there was a slight suggestion that treatment with antibiotics of any kind may increase the risk of later wheezing in infants hospitalized with the virus.
“The study was not designed to tease at the effects of different antibiotics or combinations of antibiotics, so we have to be very cautious about this trend,” Dr. Beigelman said. “There may be short-term effects and long-term effects. Certain antibiotics may affect the infant microbiome in other parts of the body, such as the gut, [in] a way that may predispose to asthma. But all these associations suggest that early-life antibiotics for viral infections are not good for you.”
He pointed to the longstanding question among clinicians whether it is the antibiotic that’s increasing the risk of the harm or the condition for which the antibiotic is prescribed. These exploratory data, however, suggest that antibiotics for RSV may be causing harm.
In pursuit of that hypothesis, his group has collected airway microbiome samples from these infants and plan to investigate whether bacteria colonizing the airway may interact with the antibiotics to increase wheezing. The researchers will analyze stool samples from the babies to see whether the gut microbiome may also play a role in wheezing and the subsequent risk of developing childhood asthma.
Study details
The trial prospectively enrolled 200 otherwise healthy babies aged 1-18 months who were hospitalized at St. Louis Children’s Hospital for acute RSV bronchiolitis. Although RSV is a very common pediatric virus, only bout 3% of babies will require hospitalization in order to receive oxygen, Dr. Beigelman said.
Babies were randomly assigned to receive placebo or oral azithromycin at 10 mg/kg daily for 7 days, followed by 5 mg/kg daily for 7 days. Randomization was stratified by recent open-label antibiotic use. The primary outcome was recurrent wheeze, defined as a third episode of post-RSV wheeze over the following 2-4 years.
The biologic activity of azithromycin was clear since nasal-wash interleukin at day 14 after randomization was lower in azithromycin-treated infants. But despite evidence of activity, the risk of post-RSV recurrent wheeze was similar in both arms: 47% in the azithromycin group versus 36% in the placebo group, for an adjusted hazard ratio of 1.45 (95% confidence interval, 0.92-2.29; P = .11).
Nor did azithromycin lower the risk of recurrent wheeze in babies already receiving other antibiotics at the time of enrollment (HR, 0.94; 95% CI, 0.43-2.07). As for antibiotic-naive participants receiving azithromycin, there was a slight signal of potential increased risk of developing recurrent wheezing (HR, 1.79; 95% CI, 1.03-3.1).
The bottom line? The findings support current clinical guidelines recommending against the use of antibiotics for RSV. “At the very least, azithromycin and antibiotics in general have no benefit in preventing recurrent wheeze, and there is a possibility they may be harmful,” Dr. Beigelman said.
This trial is funded by the National Heart, Lung, and Blood Institute. Dr. Beigelman reported relationships with AstraZeneca, Novartis, and Sanofi. Two study coauthors disclosed various ties to industry.
Azithromycin administered for severe early-life respiratory syncytial virus (RSV) bronchiolitis did not prevent recurrent wheezing in affected children over the next 2-4 years, a randomized, single-center study found.
Antibiotics are frequently given to patients with RSV bronchiolitis, although this practice is not supported by American Academy of Pediatrics clinical guidelines. Many doctors will prescribe them anyway if they see redness in the ears or other signs of infection, lead author Avraham Beigelman, MD, a pediatric allergist and immunologist at Washington University in St. Louis, said in an interview.
The double-blind, placebo-controlled trial, presented at the 2022 meeting of the American Academy of Allergy, Asthma & Immunology in Phoenix, was simultaneously published online Feb. 27, 2022, in the New England Journal of Medicine–Evidence.
Since azithromycin has shown anti-inflammatory benefit in chronic lung diseases and is a mainstay of care in cystic fibrosis and had shown previous effects in RSV patients, this trial examined its potential for preventing future recurrent wheezing in infants hospitalized with RSV who are at risk for developing asthma later. About half of children admitted to the hospital for RSV will develop asthma by age 7, Dr. Beigelman said.
“We were very surprised that azithromycin didn’t help in this trial given our previous findings,” Dr. Beigelman said.
And while those given azithromycin versus those given a placebo showed no significant decrease in recurrent wheezing, there was a slight suggestion that treatment with antibiotics of any kind may increase the risk of later wheezing in infants hospitalized with the virus.
“The study was not designed to tease at the effects of different antibiotics or combinations of antibiotics, so we have to be very cautious about this trend,” Dr. Beigelman said. “There may be short-term effects and long-term effects. Certain antibiotics may affect the infant microbiome in other parts of the body, such as the gut, [in] a way that may predispose to asthma. But all these associations suggest that early-life antibiotics for viral infections are not good for you.”
He pointed to the longstanding question among clinicians whether it is the antibiotic that’s increasing the risk of the harm or the condition for which the antibiotic is prescribed. These exploratory data, however, suggest that antibiotics for RSV may be causing harm.
In pursuit of that hypothesis, his group has collected airway microbiome samples from these infants and plan to investigate whether bacteria colonizing the airway may interact with the antibiotics to increase wheezing. The researchers will analyze stool samples from the babies to see whether the gut microbiome may also play a role in wheezing and the subsequent risk of developing childhood asthma.
Study details
The trial prospectively enrolled 200 otherwise healthy babies aged 1-18 months who were hospitalized at St. Louis Children’s Hospital for acute RSV bronchiolitis. Although RSV is a very common pediatric virus, only bout 3% of babies will require hospitalization in order to receive oxygen, Dr. Beigelman said.
Babies were randomly assigned to receive placebo or oral azithromycin at 10 mg/kg daily for 7 days, followed by 5 mg/kg daily for 7 days. Randomization was stratified by recent open-label antibiotic use. The primary outcome was recurrent wheeze, defined as a third episode of post-RSV wheeze over the following 2-4 years.
The biologic activity of azithromycin was clear since nasal-wash interleukin at day 14 after randomization was lower in azithromycin-treated infants. But despite evidence of activity, the risk of post-RSV recurrent wheeze was similar in both arms: 47% in the azithromycin group versus 36% in the placebo group, for an adjusted hazard ratio of 1.45 (95% confidence interval, 0.92-2.29; P = .11).
Nor did azithromycin lower the risk of recurrent wheeze in babies already receiving other antibiotics at the time of enrollment (HR, 0.94; 95% CI, 0.43-2.07). As for antibiotic-naive participants receiving azithromycin, there was a slight signal of potential increased risk of developing recurrent wheezing (HR, 1.79; 95% CI, 1.03-3.1).
The bottom line? The findings support current clinical guidelines recommending against the use of antibiotics for RSV. “At the very least, azithromycin and antibiotics in general have no benefit in preventing recurrent wheeze, and there is a possibility they may be harmful,” Dr. Beigelman said.
This trial is funded by the National Heart, Lung, and Blood Institute. Dr. Beigelman reported relationships with AstraZeneca, Novartis, and Sanofi. Two study coauthors disclosed various ties to industry.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE–EVIDENCE
Oncology care model reduces cost of supportive care meds
The Oncology Care Model (OCM), launched by the Centers for Medicare & Medicaid Services (CMS) with the goal of reducing spending for Medicare beneficiaries, was “associated with meaningful changes in the use of supportive care medications during chemotherapy treatment episodes,” according to new findings.
The OCM led to a statistically significant reduction in the use of denosumab – a pricier bone-modifying drug – by patients with bone metastases without changing the overall use of bone-modifying medications. The OCM also prompted more rapid adoption of a less expensive white blood cell growth factor agent – the biosimilar filgrastim – and more selective use of costly antiemetics as primary prophylaxis for chemotherapy-induced nausea.
study author Gabriel A. Brooks, MD, MPH, of the Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine, Lebanon, N.H., and colleagues write.
The study was published online Feb. 25 in the Journal of Clinical Oncology.
Since the OCM was launched in 2016, several studies have evaluated whether the alternative payment model reached its goal of reducing spending while improving or maintaining the quality of cancer care.
The results have been decidedly mixed.
As previously reported by this news organization, one study found that after 4 years, the OCM led to a $155 million net loss to Medicare. During that time, physician participation in the program also declined, with the number of practices dropping almost 30% between 2016 and 2020.
Other studies, however, have highlighted more positive results.
One large community practice reported saving Medicare $3 million over the course of 1 year. Another analysis found that among community practices that adopted the OCM, in the first year of the program, there was less physician-administered drug use by patients with prostate cancer, lower drug costs by patients with lung and prostate cancer, fewer visits by patients with breast or colon cancer, and lower office-based costs in all cancers analyzed. However, these savings were largely offset by the costs of these programs.
In the current study, DR. Brooks and colleagues compared the use of supportive care medications – bone-modifying drugs as well as prophylactic white blood cell (WBC) growth factors and antiemetics – in practices that adopted the OCM and those that didn’t.
More specifically, the authors zeroed in on the bone-modifying agent denosumab for patients with breast, lung, or prostate cancer and the WBC growth factor biosimilar filgrastim for those receiving chemotherapy for breast, lung, or colorectal cancer. Prophylactic use of higher-cost neurokinin-1 (NK1) antagonists and long-acting serotonin antagonists for patients receiving chemotherapy for any type of cancer was also evaluated.
The authors evaluated chemotherapy episodes assigned to OCM (n = 201) and comparison practices (n = 534) using Medicare claims from 2013-2019.
There was a total of 255,638 treatment episodes for bone metastases. The authors found that the OCM led to relative reductions in the use of denosumab but not in the overall use of bone-modifying medications, which included the less costly options zoledronic acid and pamidronate. The use of denosumab was similar for OCM and comparison practices during the baseline period, but during the intervention period, there were statistically significant relative reductions in the use of denosumab at OCM practices for breast (-5.0%), prostate (-4.0%), and lung cancer (-4.1%).
For WBC growth factors, 164,310 episodes were included in analyses. The OCM did not affect the use of prophylactic WBC growth factors during breast cancer chemotherapy for those at high risk of febrile neutropenia but did lead to a relative decrease during intermediate-risk chemotherapy (-7.6%). The authors observed no OCM impact on the use of prophylactic WBC growth factors among intermediate-risk lung or colorectal cancer patients. But, during the intervention period, OCM practices did demonstrate an increased use of originator or biosimilar filgrastim (57.3%) compared to other practices (47.6%), and the quarterly rate of increase in the use of the biosimilar grew 2.6 percentage points faster in OCM practices.
The authors report that there were 414,792 treatment episodes involving the use of prophylactic antiemetics. Overall, among patients receiving chemotherapy with high or moderate emetic risk, the OCM led to reductions in the prophylactic use of NK1 antagonists and long-acting serotonin antagonists. The authors report a 6.0 percentage point reduction in the use of NK1 antagonists during high-emetic-risk chemotherapy.
“We found that OCM was associated with meaningful changes in the use of supportive care medications during chemotherapy treatment episodes consistent with value-based care redesign,” the authors conclude. “These impacts on supportive care medication use align with previously reported spending reductions attributable to OCM and suggest that alternative payment models have potential to drive value-based changes in supportive care during cancer treatment.”
The study was supported by CMS. Several of the coauthors have reported relationships with industry, as noted in the article.
A version of this article first appeared on Medscape.com.
The Oncology Care Model (OCM), launched by the Centers for Medicare & Medicaid Services (CMS) with the goal of reducing spending for Medicare beneficiaries, was “associated with meaningful changes in the use of supportive care medications during chemotherapy treatment episodes,” according to new findings.
The OCM led to a statistically significant reduction in the use of denosumab – a pricier bone-modifying drug – by patients with bone metastases without changing the overall use of bone-modifying medications. The OCM also prompted more rapid adoption of a less expensive white blood cell growth factor agent – the biosimilar filgrastim – and more selective use of costly antiemetics as primary prophylaxis for chemotherapy-induced nausea.
study author Gabriel A. Brooks, MD, MPH, of the Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine, Lebanon, N.H., and colleagues write.
The study was published online Feb. 25 in the Journal of Clinical Oncology.
Since the OCM was launched in 2016, several studies have evaluated whether the alternative payment model reached its goal of reducing spending while improving or maintaining the quality of cancer care.
The results have been decidedly mixed.
As previously reported by this news organization, one study found that after 4 years, the OCM led to a $155 million net loss to Medicare. During that time, physician participation in the program also declined, with the number of practices dropping almost 30% between 2016 and 2020.
Other studies, however, have highlighted more positive results.
One large community practice reported saving Medicare $3 million over the course of 1 year. Another analysis found that among community practices that adopted the OCM, in the first year of the program, there was less physician-administered drug use by patients with prostate cancer, lower drug costs by patients with lung and prostate cancer, fewer visits by patients with breast or colon cancer, and lower office-based costs in all cancers analyzed. However, these savings were largely offset by the costs of these programs.
In the current study, DR. Brooks and colleagues compared the use of supportive care medications – bone-modifying drugs as well as prophylactic white blood cell (WBC) growth factors and antiemetics – in practices that adopted the OCM and those that didn’t.
More specifically, the authors zeroed in on the bone-modifying agent denosumab for patients with breast, lung, or prostate cancer and the WBC growth factor biosimilar filgrastim for those receiving chemotherapy for breast, lung, or colorectal cancer. Prophylactic use of higher-cost neurokinin-1 (NK1) antagonists and long-acting serotonin antagonists for patients receiving chemotherapy for any type of cancer was also evaluated.
The authors evaluated chemotherapy episodes assigned to OCM (n = 201) and comparison practices (n = 534) using Medicare claims from 2013-2019.
There was a total of 255,638 treatment episodes for bone metastases. The authors found that the OCM led to relative reductions in the use of denosumab but not in the overall use of bone-modifying medications, which included the less costly options zoledronic acid and pamidronate. The use of denosumab was similar for OCM and comparison practices during the baseline period, but during the intervention period, there were statistically significant relative reductions in the use of denosumab at OCM practices for breast (-5.0%), prostate (-4.0%), and lung cancer (-4.1%).
For WBC growth factors, 164,310 episodes were included in analyses. The OCM did not affect the use of prophylactic WBC growth factors during breast cancer chemotherapy for those at high risk of febrile neutropenia but did lead to a relative decrease during intermediate-risk chemotherapy (-7.6%). The authors observed no OCM impact on the use of prophylactic WBC growth factors among intermediate-risk lung or colorectal cancer patients. But, during the intervention period, OCM practices did demonstrate an increased use of originator or biosimilar filgrastim (57.3%) compared to other practices (47.6%), and the quarterly rate of increase in the use of the biosimilar grew 2.6 percentage points faster in OCM practices.
The authors report that there were 414,792 treatment episodes involving the use of prophylactic antiemetics. Overall, among patients receiving chemotherapy with high or moderate emetic risk, the OCM led to reductions in the prophylactic use of NK1 antagonists and long-acting serotonin antagonists. The authors report a 6.0 percentage point reduction in the use of NK1 antagonists during high-emetic-risk chemotherapy.
“We found that OCM was associated with meaningful changes in the use of supportive care medications during chemotherapy treatment episodes consistent with value-based care redesign,” the authors conclude. “These impacts on supportive care medication use align with previously reported spending reductions attributable to OCM and suggest that alternative payment models have potential to drive value-based changes in supportive care during cancer treatment.”
The study was supported by CMS. Several of the coauthors have reported relationships with industry, as noted in the article.
A version of this article first appeared on Medscape.com.
The Oncology Care Model (OCM), launched by the Centers for Medicare & Medicaid Services (CMS) with the goal of reducing spending for Medicare beneficiaries, was “associated with meaningful changes in the use of supportive care medications during chemotherapy treatment episodes,” according to new findings.
The OCM led to a statistically significant reduction in the use of denosumab – a pricier bone-modifying drug – by patients with bone metastases without changing the overall use of bone-modifying medications. The OCM also prompted more rapid adoption of a less expensive white blood cell growth factor agent – the biosimilar filgrastim – and more selective use of costly antiemetics as primary prophylaxis for chemotherapy-induced nausea.
study author Gabriel A. Brooks, MD, MPH, of the Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine, Lebanon, N.H., and colleagues write.
The study was published online Feb. 25 in the Journal of Clinical Oncology.
Since the OCM was launched in 2016, several studies have evaluated whether the alternative payment model reached its goal of reducing spending while improving or maintaining the quality of cancer care.
The results have been decidedly mixed.
As previously reported by this news organization, one study found that after 4 years, the OCM led to a $155 million net loss to Medicare. During that time, physician participation in the program also declined, with the number of practices dropping almost 30% between 2016 and 2020.
Other studies, however, have highlighted more positive results.
One large community practice reported saving Medicare $3 million over the course of 1 year. Another analysis found that among community practices that adopted the OCM, in the first year of the program, there was less physician-administered drug use by patients with prostate cancer, lower drug costs by patients with lung and prostate cancer, fewer visits by patients with breast or colon cancer, and lower office-based costs in all cancers analyzed. However, these savings were largely offset by the costs of these programs.
In the current study, DR. Brooks and colleagues compared the use of supportive care medications – bone-modifying drugs as well as prophylactic white blood cell (WBC) growth factors and antiemetics – in practices that adopted the OCM and those that didn’t.
More specifically, the authors zeroed in on the bone-modifying agent denosumab for patients with breast, lung, or prostate cancer and the WBC growth factor biosimilar filgrastim for those receiving chemotherapy for breast, lung, or colorectal cancer. Prophylactic use of higher-cost neurokinin-1 (NK1) antagonists and long-acting serotonin antagonists for patients receiving chemotherapy for any type of cancer was also evaluated.
The authors evaluated chemotherapy episodes assigned to OCM (n = 201) and comparison practices (n = 534) using Medicare claims from 2013-2019.
There was a total of 255,638 treatment episodes for bone metastases. The authors found that the OCM led to relative reductions in the use of denosumab but not in the overall use of bone-modifying medications, which included the less costly options zoledronic acid and pamidronate. The use of denosumab was similar for OCM and comparison practices during the baseline period, but during the intervention period, there were statistically significant relative reductions in the use of denosumab at OCM practices for breast (-5.0%), prostate (-4.0%), and lung cancer (-4.1%).
For WBC growth factors, 164,310 episodes were included in analyses. The OCM did not affect the use of prophylactic WBC growth factors during breast cancer chemotherapy for those at high risk of febrile neutropenia but did lead to a relative decrease during intermediate-risk chemotherapy (-7.6%). The authors observed no OCM impact on the use of prophylactic WBC growth factors among intermediate-risk lung or colorectal cancer patients. But, during the intervention period, OCM practices did demonstrate an increased use of originator or biosimilar filgrastim (57.3%) compared to other practices (47.6%), and the quarterly rate of increase in the use of the biosimilar grew 2.6 percentage points faster in OCM practices.
The authors report that there were 414,792 treatment episodes involving the use of prophylactic antiemetics. Overall, among patients receiving chemotherapy with high or moderate emetic risk, the OCM led to reductions in the prophylactic use of NK1 antagonists and long-acting serotonin antagonists. The authors report a 6.0 percentage point reduction in the use of NK1 antagonists during high-emetic-risk chemotherapy.
“We found that OCM was associated with meaningful changes in the use of supportive care medications during chemotherapy treatment episodes consistent with value-based care redesign,” the authors conclude. “These impacts on supportive care medication use align with previously reported spending reductions attributable to OCM and suggest that alternative payment models have potential to drive value-based changes in supportive care during cancer treatment.”
The study was supported by CMS. Several of the coauthors have reported relationships with industry, as noted in the article.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ONCOLOGY
Practicing across state lines: A challenge for telemental health
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.