User login
Question 1
Correct answer: A. Diphyllobothrium latum.
Rationale
This is likely a tapeworm infection with Diphyllobothrium latum. D. latum infection can be acquired from ingesting certain forms of freshwater fish, and those who consume raw fish, including sushi, are at increased risk. The classical manifestation of infection with D. latum is megaloblastic anemia due to vitamin B12 deficiency. D. latum has a unique affinity for vitamin B12 and therefore competes with the host for absorption. Humans become infected with Taenia by ingesting raw or undercooked infected meat containing cysticerci. Infection with Hymenolepis is common in children secondary to breaches in fecal-oral hygiene. Most infections are asymptomatic.
Reference
Webb C, Cabada MM. Curr Opin Infect Dis. 2017 Oct;30(5):504-10.
Correct answer: A. Diphyllobothrium latum.
Rationale
This is likely a tapeworm infection with Diphyllobothrium latum. D. latum infection can be acquired from ingesting certain forms of freshwater fish, and those who consume raw fish, including sushi, are at increased risk. The classical manifestation of infection with D. latum is megaloblastic anemia due to vitamin B12 deficiency. D. latum has a unique affinity for vitamin B12 and therefore competes with the host for absorption. Humans become infected with Taenia by ingesting raw or undercooked infected meat containing cysticerci. Infection with Hymenolepis is common in children secondary to breaches in fecal-oral hygiene. Most infections are asymptomatic.
Reference
Webb C, Cabada MM. Curr Opin Infect Dis. 2017 Oct;30(5):504-10.
Correct answer: A. Diphyllobothrium latum.
Rationale
This is likely a tapeworm infection with Diphyllobothrium latum. D. latum infection can be acquired from ingesting certain forms of freshwater fish, and those who consume raw fish, including sushi, are at increased risk. The classical manifestation of infection with D. latum is megaloblastic anemia due to vitamin B12 deficiency. D. latum has a unique affinity for vitamin B12 and therefore competes with the host for absorption. Humans become infected with Taenia by ingesting raw or undercooked infected meat containing cysticerci. Infection with Hymenolepis is common in children secondary to breaches in fecal-oral hygiene. Most infections are asymptomatic.
Reference
Webb C, Cabada MM. Curr Opin Infect Dis. 2017 Oct;30(5):504-10.
Q1. A 36-year-old man presents to the clinic with a history of diarrhea and significant fatigue for the last 2 months. He has no significant past medical history and works as a chef in a local sushi bar. He complains of six to seven watery stools daily with nocturnal symptoms. Diarrhea is associated with abdominal cramps, and he denies any passage of blood. His physical examination, including vital signs, is unremarkable. Laboratory investigation reveals 9.8 g/dL hemoglobin, with a mean corpuscular volume 110 fL. Peripheral eosinophilia is also noted. A stool sample is sent to the lab and is pending.
Pediatric insomnia: Assessment and diagnosis
FIRST OF 2 PARTS
A thorough evaluation can identify modifiable factors and guide treatment
Sleep problems are common among children and adolescents,1 with prevalence rates of 25% to 40%.2-4 Young children most commonly exhibit what is referred to as bedtime problems and night wakenings, whereas children in middle childhood (age 4 to 12) through adolescence (age 13 to 17) report insomnia. For many children, these problems persist.3 Insufficient sleep in children and adolescents worsens inattention, daytime fatigue, and cognitive and behavioral deficits.5 Assessment and treatment of sleep problems in children and adolescents is critical because poor sleep among youth increases the risk for depression, self-harm, and suicide,6,7 increases family stress, and decreases parental well-being.1
This 2-part article describes the assessment, diagnosis, and treatment of sleep problems among children and adolescents. In part 1, we focus on:
- sleep architecture (circadian rhythms, stages of sleep)
- sleep in healthy youth (age 6 to 17) and those with attention-deficit/hyperactivity disorder (ADHD), depressive disorders, and anxiety
- how to assess sleep, and the differential diagnosis of behavioral sleep problems in pediatric patients.
In Part 2, we will cover psychotherapeutic and psychopharmacologic interventions for youth with insomnia, and describe an effective approach to consultation with pediatric sleep medicine specialists.
How much sleep do children and adolescents need?
Throughout their development, children spend 40% to 50% of their time asleep. Sleep schedules are based on circadian rhythms, which are physical, mental, and behavioral changes that follow an approximately 24-hour cycle. Human circadian rhythm varies between 24 and 25 hours and is vital in determining our sleep patterns. Exposure to sunlight drives our circadian rhythm, sending signals to our bodies to “turn on” melatonin production at night (ie, 9
Box
Sleep architecture consists of 3 states: wake; non-rapid eye movement (NREM) sleep; and rapid eye movement (REM) sleep (“dreaming” sleep).2 These stages have distinct polysomnographic features of electroencephalographic EEG patterns, eye movements, and muscle tone.2 NREM sleep can be further divided into 3 stages: stage 1 (N1), stage 2 (N2), and stage 3 (N3). Stage 1 is the lightest stage and lasts for 30 seconds to 5 minutes; it is easy to wake up from stage 1 sleep. During stage 2 sleep, the body moves into a deeper sleep stage that is considered “true” sleep. This sleep stage is characterized by bursts of rhythmic rapid EEG activity known as spindles, as well as high-amplitude slow-wave spikes called K complexes.2 Stage 2 sleep lasts for 10 to 45 minutes. Stage 3, better known as “deep sleep,” slow-wave sleep, or delta sleep, is the most restorative sleep.2 Respiration is low and parasympathetic activity is high.2 It is difficult to be awakened during deep sleep, and if aroused, the person likely will feel confused or groggy. Deep sleep is followed by a return to lighter stage of sleep before the first REM sleep period begins.
REM sleep is the active stage of sleep. Breathing and heart rate become irregular, and the body experiences muscle atonia, or temporary paralysis, of arms and legs. When in REM sleep, individuals have the highest brain metabolic rates, and periodic bursts of eye movements.2 Most individuals move through stages of NREM and REM sleep in predicable ways, meaning they experience NREM sleep, return to a lighter stage of sleep after deep sleep, then move into REM sleep before the cycle repeats. It takes approximately 90 minutes for most adults to complete the NREM sleep cycle, and then REM sleep occurs before returning to NREM sleep.
In children, especially in infants and babies, sleep cycles are closer to 50 to 60 minutes. Newborns spend approximately 50% of their sleep in REM sleep, whereas adults spend 20% to 25% of their sleep in REM sleep. Children will spend more time in REM sleep until the third and fourth years of life, at which point REM gradually decreases to 20% to 25% by adulthood.
Sleep needs also change predictably throughout the lifespan. The National Sleep Foundation guidelines for sleep duration provide clinicians and parents with a range of recommended sleep for each stage of development. Infants require 14 to 17 hours of sleep, whereas adolescents need 8 to 10 hours by age 14 to 17.8 The key for clinicians is to determine if the child is within the recommended range, and how they are functioning on the number of hours of sleep they report. This allows for variation in how much sleep an individual child might need while acknowledging that some children within a specific age group might need more or less sleep than other children of the same age.
Sleep in healthy youth: Middle childhood
School-age children (age 6 to 12) typically need 9 to 10 hours of sleep over a 24-hour period.2 This developmental period is especially important for children to develop healthy sleep habits; however, developmentally appropriate cognitive and social/emotional factors might interfere with the quality and quantity of sleep. Middle childhood is a time when children can understand the dangers of the outside world (ie, violence, health problems) and resulting anxiety can disrupt sleep. Parents usually are less involved in bedtime as children approach adolescence, which leads to later bedtimes. At this stage, many children begin to take on more serious roles in their academics and extracurricular activities, peer relationships become more important, and use of electronics (eg, television, video games, internet, and handheld devices) increases—all of which compete with sleep.9 Frequent sleep issues during middle childhood include:
- irregular sleep-wake schedules
- later bedtimes
- decreased nighttime sleep
- increased caffeine intake
- reduced parental presence at bedtime
- daytime sleepiness.3
In school-age children, regular napping, falling asleep during short car rides, and daytime fatigue at school or home are cause for concern. When these symptoms are present, an evaluation is warranted.
Sleep in healthy youth: Adolescence
The National Sleep Foundation recommends adolescents obtain 8 to 10 hours of sleep per night; for some adolescents, as much as 11 hours of sleep per night might be appropriate.8 However, this contrasts with findings from the National Sleep Foundation’s Sleep in America Poll, which revealed that 75% of 12th graders report <8 hours of sleep nightly.10 Many adolescents experience delayed sleep phase syndrome or delayed sleep-wake phase disorder, which involves a persistent phase shift of >2 hours in the sleep-wake schedule that conflicts with the adolescent’s school, work, or lifestyle demands.11 Such circadian rhythm disorders typically result from a poor match between the sleep-wake schedule and the demands of the adolescent’s life, or a failure to synchronize their internal clock with a 24-hour circadian clock.12 Children typically become tired after sunset, but puberty is associated with reduced slow-wave sleep and changes in circadian rhythms. As a result, a 3-hour delay (delayed phase preference) is common among adolescents. At approximately age 20, people start to become tired after sunset and awaken earlier in the morning—a pattern driven by sunlight and the timing of melatonin release that will remain stable until the sixth decade of life.
Continue to: Effects of chronic sleep deprivation...
Effects of chronic sleep deprivation
Most older studies of sleep loss examined the impact of total sleep loss (sleep deprivation) rather than the effect of partial sleep loss or sleep restriction, a more commonly experienced phenomenon. More recent research shows that a cumulative sleep deficit could cause the body to override voluntary wakefulness and a sleep-deprived individual can experience brief “microsleeps” where they are unaware and lose attention/wakefulness for several seconds.2 This can be deadly if a sleep-deprived adolescent experiences microsleeps while driving.13
There is a well-studied correlation between chronic sleep deprivation and increased body mass index in children.14 This might be caused by reduction in physical activity as well as alterations in the “hunger hormones”—ghrelin and leptin—that have been observed with sleep deprivation.15-17 Other studies have noted decreased glucose tolerance, reduced insulin sensitivity, and catecholamine and cortisol secretion abnormalities, which place children at higher risk for metabolic syndrome and hypertension.13,18 Sleep deprivation also is associated with mood and anxiety disorders and is an independent risk factor for substance use and suicidal ideation among adolescents.19 Sleep deprivation increases impairments in impulse control, concentration, and attention, which could be especially problematic in school-age children.
How sleep is assessed
The sleep history is the first step in evaluating a child or adolescent for a sleep disorder. The sleep history includes exploring the chief complaint, sleep patterns and schedules, bedtime routines, and nocturnal and daytime behaviors (Table).

Chief complaint
Behavioral sleep specialists will assess the primary problem with everyone involved in the child’s bedtime.20 This might include parents (custodial and noncustodial), grandparents, or stepparents as well as the child/adolescent. This important step can reveal a sleep disorder or an inappropriately early bedtime relative to the child’s development. During this assessment, ask detailed questions about how long the sleep problem has persisted, the frequency of sleep problems, and any precipitating stressors. Parents and caregivers can review strategies they have tried, and for how long and to what extent interventions were implemented consistently to result in change.
Sleep patterns and schedules
Review the child/adolescent’s typical sleep patterns and behaviors. Ask parents and caregivers, as well as the patient, about general sleep schedules for the past few weeks or a typical 2-week time period.2 A behavioral assessment of sleep should include asking families about how the child/adolescent sleeps during the week and over the weekend, and if school-year sleep differs from summer or holiday sleep schedules. These questions can illuminate how long a sleep problem has been occurring and what sleep habits might be contributing to the problem. Bedtime
Determine if there is a set bedtime or if the child goes to bed when they wish. It is important to ascertain if the bedtime is age-appropriate, if weekday and weekend bedtimes differ, and to what extent extracurricular activities or school demands impact bedtime. Assess the consistency of the bedtime, the nature of bedtime routines (eg, is the child engaging in stimulating activities before bed), where the bedtime routine occurs (eg, sibling’s room, parents’ room, child’s room), and what role (if any) electronic devices play.2
Nocturnal behaviors
Assessment should include a series of questions and age-specific questionnaires to focus on what behaviors occur at night, including awakenings. Parents should be asked how frequent night awakenings occur, how long arousals last, and how the child signals for the parent (eg, calling out, climbing into parents’ bed).2 Additionally, ask how parents respond and what is required to help the child fall back asleep (eg, rocking, soothing, feeding). The presence of nightmares, night terrors, parasomnias, and sleep-related breathing disorders also must be assessed.20
Daytime behaviors
A sleep history should include assessment of daytime functioning, including daytime sleepiness, fatigue, morning waking, and functioning during school, extracurriculars, and homework. For children and teens, falling asleep in the car, while in school, or during passive activities (meals, conversation) suggests insufficient sleep, sleep disruption, or excessive daytime sleepiness.2
Continue to: Sleep disruption in youth with psychiatric disorders...
Sleep disruption in youth with psychiatric disorders
Disordered sleep is common across psychiatric disorders. The National Comorbidity Survey Adolescent Supplement—a nationally representative cross-sectional survey of adolescents (N = 10,123)—found that a later weeknight bedtime, shorter weeknight sleep duration, and greater weekend bedtime delay increased the risk of developing a mood, anxiety, or substance use (including nicotine) disorder, and suicidality. These risk factors also were associated with lower “perceived mental and physical health.”21 Clinicians should routinely obtain a sleep history in children and adolescents with these disorders. Consider using the sleep screening tool BEARS:
- Bedtime issues
- Excessive daytime sleepiness
- Awakenings
- Regularity and duration of sleep
- Snoring.
ADHD
Up to one-half of children and adolescents with ADHD experience sleep problems,22,23 including delayed sleep onset, bedtime resistance, daytime fatigue, and feeling groggy in the morning beyond what is typical (>20 minutes). Pharmacotherapy for ADHD contributes to sleep disturbances24,25 while sleep deprivation exacerbates inattention and hyperactivity. In youth with ADHD, restless leg syndrome, periodic limb movement disorder, and sleep-disordered breathing disorder are more common than in the general population.
Depressive disorders
Up to three-quarters of depressed children and 90% of depressed adolescents report sleep disturbances, including initial, middle, and terminal insomnia as well as hypersomnia.26 Disrupted sleep in pediatric patients with major depressive disorder could be moderated by the patient’s age, with depressive symptoms more common among adolescents (age 12 to 17) than among younger children (age 6 to 11).27 Successful treatment of depression fails to relieve dyssomnia in 10% of children. Sleep problems that persist after successfully treating a depressive episode could increase the risk of another depressive episode.28
Anxiety disorders
Sleep problems are common among children and adolescents with anxiety disorders.29 Longitudinal data from >900 children found that symptoms of sleep disturbance in early childhood were correlated with experiencing an anxiety disorder 20 years later.30 Fears related to the dark or monsters under the bed that are developmentally appropriate for younger children may interfere with sleep. However, in anxious children, fears might also be related to separation, sleeping alone, worry about the loss of a loved one, concerns about personal safety, fear of frightening dreams, or concerns about academics and social relationships. Anxious individuals ruminate about their worries, and this might be especially true for children at bedtime, when there are limited distractions from ruminative fears.31 Bedtime resistance, parental involvement in bedtime rituals, and cultural factors related to sleep also could play a role for children with anxiety symptoms and sleep problems.
Having an anxiety disorder is significantly associated with an increased risk of insomnia; however, 73% of the time anxiety symptoms precede an insomnia diagnosis.29 Sleep problems and anxiety symptoms might have a reciprocal influence on one another; tiredness that results from sleep problems could exacerbate anxiety, which further worsens sleep problems.
A bridge to treatment
A thorough assessment can help identify modifiable factors and guide treatment selections. In Part 2 of this article, we will describe healthy sleep practices, cognitive-behavioral therapy for insomnia, when pharmacotherapy might be indicated, and the evidence supporting several medications commonly used to treat pediatric insomnia. We also will discuss factors to consider when seeking consultation with a pediatric behavioral sleep specialist.
1. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932-948. doi:10.1093/jpepsy/jsu041
2. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569. doi:10.1016/j.pcl.2011.03.011
3. Meltzer LJ, Plaufcan MR, Thomas JH, et al. Sleep problems and sleep disorders in pediatric primary care: treatment recommendations, persistence, and health care utilization. J Clin Sleep Med. 2014;10(4):421-426. doi:10.5664/jcsm.3620
4. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569-581, viii. doi:10.1016/j.pop.2008.06.002
5. Williamson AA, Mindell JA, Hiscock H, et al. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J Child Psychol Psychiatry. 2020;61(10):1092-1103. doi:10.1111/jcpp.13303
6. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002; 53(1):561-569. doi:10.1016/s0022-3999(02)00446-4
7. Singareddy R, Krishnamurthy VB, Vgontzas AN, et al. Subjective and objective sleep and self-harm behaviors in young children: a general population study. Psychiatry Res. 2013;209(3):549-553. doi:10.1016/j.psychres.2013.03.036
8. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi:10.1016/j.sleh.2015.10.004
9. Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005-1010. doi:10.1542/peds.2008-3641
10. Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8(4):695-725.
11. Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):114-120. doi:10.1016/j.prrv.2008.01.001
12. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602-612. doi:10.1016/j.sleep.2006.12.002
13. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774-1786. doi:10.1542/peds.2005-0772
14. Kaczor M, Skalski M. Prevalence and consequences of insomnia in pediatric population. Psychiatr Pol. 2016;50(3):555-569. doi:10.12740/PP/61226
15. Gomes TN, Dos Santos FK, Santos D, et al. Correlates of sedentary time in children: a multilevel modelling approach. BMC Public Health. 2014;14:890. doi:10.1186/1471-2458-14-890
16. Stone MR, Stevens D, Faulkner GEJ. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56(2):112-117. doi:10.1016/j.ypmed.2012.11.015
17. Hart CN, Fava JL, Subak LL, et al. Time in bed is associated with decreased physical activity and higher BMI in women seeking weight loss treatment. ISRN Obes. 2012;2012:320157. doi:10.5402/2012/320157
18. Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. doi:10.1073/pnas.0706446105
19. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi:10.1016/j.smrv.2017.06.009
20. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Lippincott Williams & Wilkins; 2015.
21. Zhang J, Paksarian D, Lamers F, et al. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137-143. doi:10.1016/j.jpeds.2016.11.007
22. Gregory AM, Agnew-Blais JC, Matthews T, et al. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46(2):284-294. doi:10.1080/15374416.2016.1183499
23. Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: Defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811-828. doi:10.2165/11538990-000000000-00000
24. Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. J Sleep Res. 2010;19(2):366-373. doi:10.1111/j.1365-2869.2009.00795.x
25. Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395-404. doi:10.1097/DBP.0000000000000285
26. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148(1):66-71. doi:10.1016/j.jad.2012.11.049
27. Emslie GJ, Rush AJ, Weinberg WA, et al. Sleep EEG features of adolescents with major depression. Biol Psychiatry. 1994;36(9):573-581. doi:10.1016/0006-3223(94)90067-1
28. Alfano CA, Zakem AH, Costa NM, et al. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depress Anxiety. 2009;26(6):503-512. doi:10.1002/da.20443
29. Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224-232. doi:10.1097/01.chi.0000242233.06011.8e
30. Gregory AM, Caspi A, Eley TC, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157-163. doi: 10.1007/s10802-005-1824-0
31. Chorney DB, Detweiler MF, Morris TL, et al. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339-348. doi:10.1093/jpepsy/jsm105
32. Sadeh A. Stress, trauma, and sleep in children. Child Adolesc Psychiatr Clin N Am. 1996;5(3):685-700. doi:10.1016/S1056-4993(18)30356-0

33. Glod CA, Teicher MH, Hartman CR, et al. Increased nocturnal activity and impaired sleep maintenance in abused children. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1236-1243. doi:10.1097/00004583-199709000-00016
34. Strawn JR, Lu L, Peris TS, et al. Research review: pediatric anxiety disorders: what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114-139. doi:10.1111/jcpp.13262
35. Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi:10.1007/s11920-015-0591-z
36. Hamill Skoch S, Mills JA, Ramsey L, et al. Letter to editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder— a bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387-388. doi:10.1089/cap.2020.0169
FIRST OF 2 PARTS
A thorough evaluation can identify modifiable factors and guide treatment
Sleep problems are common among children and adolescents,1 with prevalence rates of 25% to 40%.2-4 Young children most commonly exhibit what is referred to as bedtime problems and night wakenings, whereas children in middle childhood (age 4 to 12) through adolescence (age 13 to 17) report insomnia. For many children, these problems persist.3 Insufficient sleep in children and adolescents worsens inattention, daytime fatigue, and cognitive and behavioral deficits.5 Assessment and treatment of sleep problems in children and adolescents is critical because poor sleep among youth increases the risk for depression, self-harm, and suicide,6,7 increases family stress, and decreases parental well-being.1
This 2-part article describes the assessment, diagnosis, and treatment of sleep problems among children and adolescents. In part 1, we focus on:
- sleep architecture (circadian rhythms, stages of sleep)
- sleep in healthy youth (age 6 to 17) and those with attention-deficit/hyperactivity disorder (ADHD), depressive disorders, and anxiety
- how to assess sleep, and the differential diagnosis of behavioral sleep problems in pediatric patients.
In Part 2, we will cover psychotherapeutic and psychopharmacologic interventions for youth with insomnia, and describe an effective approach to consultation with pediatric sleep medicine specialists.
How much sleep do children and adolescents need?
Throughout their development, children spend 40% to 50% of their time asleep. Sleep schedules are based on circadian rhythms, which are physical, mental, and behavioral changes that follow an approximately 24-hour cycle. Human circadian rhythm varies between 24 and 25 hours and is vital in determining our sleep patterns. Exposure to sunlight drives our circadian rhythm, sending signals to our bodies to “turn on” melatonin production at night (ie, 9
Box
Sleep architecture consists of 3 states: wake; non-rapid eye movement (NREM) sleep; and rapid eye movement (REM) sleep (“dreaming” sleep).2 These stages have distinct polysomnographic features of electroencephalographic EEG patterns, eye movements, and muscle tone.2 NREM sleep can be further divided into 3 stages: stage 1 (N1), stage 2 (N2), and stage 3 (N3). Stage 1 is the lightest stage and lasts for 30 seconds to 5 minutes; it is easy to wake up from stage 1 sleep. During stage 2 sleep, the body moves into a deeper sleep stage that is considered “true” sleep. This sleep stage is characterized by bursts of rhythmic rapid EEG activity known as spindles, as well as high-amplitude slow-wave spikes called K complexes.2 Stage 2 sleep lasts for 10 to 45 minutes. Stage 3, better known as “deep sleep,” slow-wave sleep, or delta sleep, is the most restorative sleep.2 Respiration is low and parasympathetic activity is high.2 It is difficult to be awakened during deep sleep, and if aroused, the person likely will feel confused or groggy. Deep sleep is followed by a return to lighter stage of sleep before the first REM sleep period begins.
REM sleep is the active stage of sleep. Breathing and heart rate become irregular, and the body experiences muscle atonia, or temporary paralysis, of arms and legs. When in REM sleep, individuals have the highest brain metabolic rates, and periodic bursts of eye movements.2 Most individuals move through stages of NREM and REM sleep in predicable ways, meaning they experience NREM sleep, return to a lighter stage of sleep after deep sleep, then move into REM sleep before the cycle repeats. It takes approximately 90 minutes for most adults to complete the NREM sleep cycle, and then REM sleep occurs before returning to NREM sleep.
In children, especially in infants and babies, sleep cycles are closer to 50 to 60 minutes. Newborns spend approximately 50% of their sleep in REM sleep, whereas adults spend 20% to 25% of their sleep in REM sleep. Children will spend more time in REM sleep until the third and fourth years of life, at which point REM gradually decreases to 20% to 25% by adulthood.
Sleep needs also change predictably throughout the lifespan. The National Sleep Foundation guidelines for sleep duration provide clinicians and parents with a range of recommended sleep for each stage of development. Infants require 14 to 17 hours of sleep, whereas adolescents need 8 to 10 hours by age 14 to 17.8 The key for clinicians is to determine if the child is within the recommended range, and how they are functioning on the number of hours of sleep they report. This allows for variation in how much sleep an individual child might need while acknowledging that some children within a specific age group might need more or less sleep than other children of the same age.
Sleep in healthy youth: Middle childhood
School-age children (age 6 to 12) typically need 9 to 10 hours of sleep over a 24-hour period.2 This developmental period is especially important for children to develop healthy sleep habits; however, developmentally appropriate cognitive and social/emotional factors might interfere with the quality and quantity of sleep. Middle childhood is a time when children can understand the dangers of the outside world (ie, violence, health problems) and resulting anxiety can disrupt sleep. Parents usually are less involved in bedtime as children approach adolescence, which leads to later bedtimes. At this stage, many children begin to take on more serious roles in their academics and extracurricular activities, peer relationships become more important, and use of electronics (eg, television, video games, internet, and handheld devices) increases—all of which compete with sleep.9 Frequent sleep issues during middle childhood include:
- irregular sleep-wake schedules
- later bedtimes
- decreased nighttime sleep
- increased caffeine intake
- reduced parental presence at bedtime
- daytime sleepiness.3
In school-age children, regular napping, falling asleep during short car rides, and daytime fatigue at school or home are cause for concern. When these symptoms are present, an evaluation is warranted.
Sleep in healthy youth: Adolescence
The National Sleep Foundation recommends adolescents obtain 8 to 10 hours of sleep per night; for some adolescents, as much as 11 hours of sleep per night might be appropriate.8 However, this contrasts with findings from the National Sleep Foundation’s Sleep in America Poll, which revealed that 75% of 12th graders report <8 hours of sleep nightly.10 Many adolescents experience delayed sleep phase syndrome or delayed sleep-wake phase disorder, which involves a persistent phase shift of >2 hours in the sleep-wake schedule that conflicts with the adolescent’s school, work, or lifestyle demands.11 Such circadian rhythm disorders typically result from a poor match between the sleep-wake schedule and the demands of the adolescent’s life, or a failure to synchronize their internal clock with a 24-hour circadian clock.12 Children typically become tired after sunset, but puberty is associated with reduced slow-wave sleep and changes in circadian rhythms. As a result, a 3-hour delay (delayed phase preference) is common among adolescents. At approximately age 20, people start to become tired after sunset and awaken earlier in the morning—a pattern driven by sunlight and the timing of melatonin release that will remain stable until the sixth decade of life.
Continue to: Effects of chronic sleep deprivation...
Effects of chronic sleep deprivation
Most older studies of sleep loss examined the impact of total sleep loss (sleep deprivation) rather than the effect of partial sleep loss or sleep restriction, a more commonly experienced phenomenon. More recent research shows that a cumulative sleep deficit could cause the body to override voluntary wakefulness and a sleep-deprived individual can experience brief “microsleeps” where they are unaware and lose attention/wakefulness for several seconds.2 This can be deadly if a sleep-deprived adolescent experiences microsleeps while driving.13
There is a well-studied correlation between chronic sleep deprivation and increased body mass index in children.14 This might be caused by reduction in physical activity as well as alterations in the “hunger hormones”—ghrelin and leptin—that have been observed with sleep deprivation.15-17 Other studies have noted decreased glucose tolerance, reduced insulin sensitivity, and catecholamine and cortisol secretion abnormalities, which place children at higher risk for metabolic syndrome and hypertension.13,18 Sleep deprivation also is associated with mood and anxiety disorders and is an independent risk factor for substance use and suicidal ideation among adolescents.19 Sleep deprivation increases impairments in impulse control, concentration, and attention, which could be especially problematic in school-age children.
How sleep is assessed
The sleep history is the first step in evaluating a child or adolescent for a sleep disorder. The sleep history includes exploring the chief complaint, sleep patterns and schedules, bedtime routines, and nocturnal and daytime behaviors (Table).

Chief complaint
Behavioral sleep specialists will assess the primary problem with everyone involved in the child’s bedtime.20 This might include parents (custodial and noncustodial), grandparents, or stepparents as well as the child/adolescent. This important step can reveal a sleep disorder or an inappropriately early bedtime relative to the child’s development. During this assessment, ask detailed questions about how long the sleep problem has persisted, the frequency of sleep problems, and any precipitating stressors. Parents and caregivers can review strategies they have tried, and for how long and to what extent interventions were implemented consistently to result in change.
Sleep patterns and schedules
Review the child/adolescent’s typical sleep patterns and behaviors. Ask parents and caregivers, as well as the patient, about general sleep schedules for the past few weeks or a typical 2-week time period.2 A behavioral assessment of sleep should include asking families about how the child/adolescent sleeps during the week and over the weekend, and if school-year sleep differs from summer or holiday sleep schedules. These questions can illuminate how long a sleep problem has been occurring and what sleep habits might be contributing to the problem. Bedtime
Determine if there is a set bedtime or if the child goes to bed when they wish. It is important to ascertain if the bedtime is age-appropriate, if weekday and weekend bedtimes differ, and to what extent extracurricular activities or school demands impact bedtime. Assess the consistency of the bedtime, the nature of bedtime routines (eg, is the child engaging in stimulating activities before bed), where the bedtime routine occurs (eg, sibling’s room, parents’ room, child’s room), and what role (if any) electronic devices play.2
Nocturnal behaviors
Assessment should include a series of questions and age-specific questionnaires to focus on what behaviors occur at night, including awakenings. Parents should be asked how frequent night awakenings occur, how long arousals last, and how the child signals for the parent (eg, calling out, climbing into parents’ bed).2 Additionally, ask how parents respond and what is required to help the child fall back asleep (eg, rocking, soothing, feeding). The presence of nightmares, night terrors, parasomnias, and sleep-related breathing disorders also must be assessed.20
Daytime behaviors
A sleep history should include assessment of daytime functioning, including daytime sleepiness, fatigue, morning waking, and functioning during school, extracurriculars, and homework. For children and teens, falling asleep in the car, while in school, or during passive activities (meals, conversation) suggests insufficient sleep, sleep disruption, or excessive daytime sleepiness.2
Continue to: Sleep disruption in youth with psychiatric disorders...
Sleep disruption in youth with psychiatric disorders
Disordered sleep is common across psychiatric disorders. The National Comorbidity Survey Adolescent Supplement—a nationally representative cross-sectional survey of adolescents (N = 10,123)—found that a later weeknight bedtime, shorter weeknight sleep duration, and greater weekend bedtime delay increased the risk of developing a mood, anxiety, or substance use (including nicotine) disorder, and suicidality. These risk factors also were associated with lower “perceived mental and physical health.”21 Clinicians should routinely obtain a sleep history in children and adolescents with these disorders. Consider using the sleep screening tool BEARS:
- Bedtime issues
- Excessive daytime sleepiness
- Awakenings
- Regularity and duration of sleep
- Snoring.
ADHD
Up to one-half of children and adolescents with ADHD experience sleep problems,22,23 including delayed sleep onset, bedtime resistance, daytime fatigue, and feeling groggy in the morning beyond what is typical (>20 minutes). Pharmacotherapy for ADHD contributes to sleep disturbances24,25 while sleep deprivation exacerbates inattention and hyperactivity. In youth with ADHD, restless leg syndrome, periodic limb movement disorder, and sleep-disordered breathing disorder are more common than in the general population.
Depressive disorders
Up to three-quarters of depressed children and 90% of depressed adolescents report sleep disturbances, including initial, middle, and terminal insomnia as well as hypersomnia.26 Disrupted sleep in pediatric patients with major depressive disorder could be moderated by the patient’s age, with depressive symptoms more common among adolescents (age 12 to 17) than among younger children (age 6 to 11).27 Successful treatment of depression fails to relieve dyssomnia in 10% of children. Sleep problems that persist after successfully treating a depressive episode could increase the risk of another depressive episode.28
Anxiety disorders
Sleep problems are common among children and adolescents with anxiety disorders.29 Longitudinal data from >900 children found that symptoms of sleep disturbance in early childhood were correlated with experiencing an anxiety disorder 20 years later.30 Fears related to the dark or monsters under the bed that are developmentally appropriate for younger children may interfere with sleep. However, in anxious children, fears might also be related to separation, sleeping alone, worry about the loss of a loved one, concerns about personal safety, fear of frightening dreams, or concerns about academics and social relationships. Anxious individuals ruminate about their worries, and this might be especially true for children at bedtime, when there are limited distractions from ruminative fears.31 Bedtime resistance, parental involvement in bedtime rituals, and cultural factors related to sleep also could play a role for children with anxiety symptoms and sleep problems.
Having an anxiety disorder is significantly associated with an increased risk of insomnia; however, 73% of the time anxiety symptoms precede an insomnia diagnosis.29 Sleep problems and anxiety symptoms might have a reciprocal influence on one another; tiredness that results from sleep problems could exacerbate anxiety, which further worsens sleep problems.
A bridge to treatment
A thorough assessment can help identify modifiable factors and guide treatment selections. In Part 2 of this article, we will describe healthy sleep practices, cognitive-behavioral therapy for insomnia, when pharmacotherapy might be indicated, and the evidence supporting several medications commonly used to treat pediatric insomnia. We also will discuss factors to consider when seeking consultation with a pediatric behavioral sleep specialist.
FIRST OF 2 PARTS
A thorough evaluation can identify modifiable factors and guide treatment
Sleep problems are common among children and adolescents,1 with prevalence rates of 25% to 40%.2-4 Young children most commonly exhibit what is referred to as bedtime problems and night wakenings, whereas children in middle childhood (age 4 to 12) through adolescence (age 13 to 17) report insomnia. For many children, these problems persist.3 Insufficient sleep in children and adolescents worsens inattention, daytime fatigue, and cognitive and behavioral deficits.5 Assessment and treatment of sleep problems in children and adolescents is critical because poor sleep among youth increases the risk for depression, self-harm, and suicide,6,7 increases family stress, and decreases parental well-being.1
This 2-part article describes the assessment, diagnosis, and treatment of sleep problems among children and adolescents. In part 1, we focus on:
- sleep architecture (circadian rhythms, stages of sleep)
- sleep in healthy youth (age 6 to 17) and those with attention-deficit/hyperactivity disorder (ADHD), depressive disorders, and anxiety
- how to assess sleep, and the differential diagnosis of behavioral sleep problems in pediatric patients.
In Part 2, we will cover psychotherapeutic and psychopharmacologic interventions for youth with insomnia, and describe an effective approach to consultation with pediatric sleep medicine specialists.
How much sleep do children and adolescents need?
Throughout their development, children spend 40% to 50% of their time asleep. Sleep schedules are based on circadian rhythms, which are physical, mental, and behavioral changes that follow an approximately 24-hour cycle. Human circadian rhythm varies between 24 and 25 hours and is vital in determining our sleep patterns. Exposure to sunlight drives our circadian rhythm, sending signals to our bodies to “turn on” melatonin production at night (ie, 9
Box
Sleep architecture consists of 3 states: wake; non-rapid eye movement (NREM) sleep; and rapid eye movement (REM) sleep (“dreaming” sleep).2 These stages have distinct polysomnographic features of electroencephalographic EEG patterns, eye movements, and muscle tone.2 NREM sleep can be further divided into 3 stages: stage 1 (N1), stage 2 (N2), and stage 3 (N3). Stage 1 is the lightest stage and lasts for 30 seconds to 5 minutes; it is easy to wake up from stage 1 sleep. During stage 2 sleep, the body moves into a deeper sleep stage that is considered “true” sleep. This sleep stage is characterized by bursts of rhythmic rapid EEG activity known as spindles, as well as high-amplitude slow-wave spikes called K complexes.2 Stage 2 sleep lasts for 10 to 45 minutes. Stage 3, better known as “deep sleep,” slow-wave sleep, or delta sleep, is the most restorative sleep.2 Respiration is low and parasympathetic activity is high.2 It is difficult to be awakened during deep sleep, and if aroused, the person likely will feel confused or groggy. Deep sleep is followed by a return to lighter stage of sleep before the first REM sleep period begins.
REM sleep is the active stage of sleep. Breathing and heart rate become irregular, and the body experiences muscle atonia, or temporary paralysis, of arms and legs. When in REM sleep, individuals have the highest brain metabolic rates, and periodic bursts of eye movements.2 Most individuals move through stages of NREM and REM sleep in predicable ways, meaning they experience NREM sleep, return to a lighter stage of sleep after deep sleep, then move into REM sleep before the cycle repeats. It takes approximately 90 minutes for most adults to complete the NREM sleep cycle, and then REM sleep occurs before returning to NREM sleep.
In children, especially in infants and babies, sleep cycles are closer to 50 to 60 minutes. Newborns spend approximately 50% of their sleep in REM sleep, whereas adults spend 20% to 25% of their sleep in REM sleep. Children will spend more time in REM sleep until the third and fourth years of life, at which point REM gradually decreases to 20% to 25% by adulthood.
Sleep needs also change predictably throughout the lifespan. The National Sleep Foundation guidelines for sleep duration provide clinicians and parents with a range of recommended sleep for each stage of development. Infants require 14 to 17 hours of sleep, whereas adolescents need 8 to 10 hours by age 14 to 17.8 The key for clinicians is to determine if the child is within the recommended range, and how they are functioning on the number of hours of sleep they report. This allows for variation in how much sleep an individual child might need while acknowledging that some children within a specific age group might need more or less sleep than other children of the same age.
Sleep in healthy youth: Middle childhood
School-age children (age 6 to 12) typically need 9 to 10 hours of sleep over a 24-hour period.2 This developmental period is especially important for children to develop healthy sleep habits; however, developmentally appropriate cognitive and social/emotional factors might interfere with the quality and quantity of sleep. Middle childhood is a time when children can understand the dangers of the outside world (ie, violence, health problems) and resulting anxiety can disrupt sleep. Parents usually are less involved in bedtime as children approach adolescence, which leads to later bedtimes. At this stage, many children begin to take on more serious roles in their academics and extracurricular activities, peer relationships become more important, and use of electronics (eg, television, video games, internet, and handheld devices) increases—all of which compete with sleep.9 Frequent sleep issues during middle childhood include:
- irregular sleep-wake schedules
- later bedtimes
- decreased nighttime sleep
- increased caffeine intake
- reduced parental presence at bedtime
- daytime sleepiness.3
In school-age children, regular napping, falling asleep during short car rides, and daytime fatigue at school or home are cause for concern. When these symptoms are present, an evaluation is warranted.
Sleep in healthy youth: Adolescence
The National Sleep Foundation recommends adolescents obtain 8 to 10 hours of sleep per night; for some adolescents, as much as 11 hours of sleep per night might be appropriate.8 However, this contrasts with findings from the National Sleep Foundation’s Sleep in America Poll, which revealed that 75% of 12th graders report <8 hours of sleep nightly.10 Many adolescents experience delayed sleep phase syndrome or delayed sleep-wake phase disorder, which involves a persistent phase shift of >2 hours in the sleep-wake schedule that conflicts with the adolescent’s school, work, or lifestyle demands.11 Such circadian rhythm disorders typically result from a poor match between the sleep-wake schedule and the demands of the adolescent’s life, or a failure to synchronize their internal clock with a 24-hour circadian clock.12 Children typically become tired after sunset, but puberty is associated with reduced slow-wave sleep and changes in circadian rhythms. As a result, a 3-hour delay (delayed phase preference) is common among adolescents. At approximately age 20, people start to become tired after sunset and awaken earlier in the morning—a pattern driven by sunlight and the timing of melatonin release that will remain stable until the sixth decade of life.
Continue to: Effects of chronic sleep deprivation...
Effects of chronic sleep deprivation
Most older studies of sleep loss examined the impact of total sleep loss (sleep deprivation) rather than the effect of partial sleep loss or sleep restriction, a more commonly experienced phenomenon. More recent research shows that a cumulative sleep deficit could cause the body to override voluntary wakefulness and a sleep-deprived individual can experience brief “microsleeps” where they are unaware and lose attention/wakefulness for several seconds.2 This can be deadly if a sleep-deprived adolescent experiences microsleeps while driving.13
There is a well-studied correlation between chronic sleep deprivation and increased body mass index in children.14 This might be caused by reduction in physical activity as well as alterations in the “hunger hormones”—ghrelin and leptin—that have been observed with sleep deprivation.15-17 Other studies have noted decreased glucose tolerance, reduced insulin sensitivity, and catecholamine and cortisol secretion abnormalities, which place children at higher risk for metabolic syndrome and hypertension.13,18 Sleep deprivation also is associated with mood and anxiety disorders and is an independent risk factor for substance use and suicidal ideation among adolescents.19 Sleep deprivation increases impairments in impulse control, concentration, and attention, which could be especially problematic in school-age children.
How sleep is assessed
The sleep history is the first step in evaluating a child or adolescent for a sleep disorder. The sleep history includes exploring the chief complaint, sleep patterns and schedules, bedtime routines, and nocturnal and daytime behaviors (Table).

Chief complaint
Behavioral sleep specialists will assess the primary problem with everyone involved in the child’s bedtime.20 This might include parents (custodial and noncustodial), grandparents, or stepparents as well as the child/adolescent. This important step can reveal a sleep disorder or an inappropriately early bedtime relative to the child’s development. During this assessment, ask detailed questions about how long the sleep problem has persisted, the frequency of sleep problems, and any precipitating stressors. Parents and caregivers can review strategies they have tried, and for how long and to what extent interventions were implemented consistently to result in change.
Sleep patterns and schedules
Review the child/adolescent’s typical sleep patterns and behaviors. Ask parents and caregivers, as well as the patient, about general sleep schedules for the past few weeks or a typical 2-week time period.2 A behavioral assessment of sleep should include asking families about how the child/adolescent sleeps during the week and over the weekend, and if school-year sleep differs from summer or holiday sleep schedules. These questions can illuminate how long a sleep problem has been occurring and what sleep habits might be contributing to the problem. Bedtime
Determine if there is a set bedtime or if the child goes to bed when they wish. It is important to ascertain if the bedtime is age-appropriate, if weekday and weekend bedtimes differ, and to what extent extracurricular activities or school demands impact bedtime. Assess the consistency of the bedtime, the nature of bedtime routines (eg, is the child engaging in stimulating activities before bed), where the bedtime routine occurs (eg, sibling’s room, parents’ room, child’s room), and what role (if any) electronic devices play.2
Nocturnal behaviors
Assessment should include a series of questions and age-specific questionnaires to focus on what behaviors occur at night, including awakenings. Parents should be asked how frequent night awakenings occur, how long arousals last, and how the child signals for the parent (eg, calling out, climbing into parents’ bed).2 Additionally, ask how parents respond and what is required to help the child fall back asleep (eg, rocking, soothing, feeding). The presence of nightmares, night terrors, parasomnias, and sleep-related breathing disorders also must be assessed.20
Daytime behaviors
A sleep history should include assessment of daytime functioning, including daytime sleepiness, fatigue, morning waking, and functioning during school, extracurriculars, and homework. For children and teens, falling asleep in the car, while in school, or during passive activities (meals, conversation) suggests insufficient sleep, sleep disruption, or excessive daytime sleepiness.2
Continue to: Sleep disruption in youth with psychiatric disorders...
Sleep disruption in youth with psychiatric disorders
Disordered sleep is common across psychiatric disorders. The National Comorbidity Survey Adolescent Supplement—a nationally representative cross-sectional survey of adolescents (N = 10,123)—found that a later weeknight bedtime, shorter weeknight sleep duration, and greater weekend bedtime delay increased the risk of developing a mood, anxiety, or substance use (including nicotine) disorder, and suicidality. These risk factors also were associated with lower “perceived mental and physical health.”21 Clinicians should routinely obtain a sleep history in children and adolescents with these disorders. Consider using the sleep screening tool BEARS:
- Bedtime issues
- Excessive daytime sleepiness
- Awakenings
- Regularity and duration of sleep
- Snoring.
ADHD
Up to one-half of children and adolescents with ADHD experience sleep problems,22,23 including delayed sleep onset, bedtime resistance, daytime fatigue, and feeling groggy in the morning beyond what is typical (>20 minutes). Pharmacotherapy for ADHD contributes to sleep disturbances24,25 while sleep deprivation exacerbates inattention and hyperactivity. In youth with ADHD, restless leg syndrome, periodic limb movement disorder, and sleep-disordered breathing disorder are more common than in the general population.
Depressive disorders
Up to three-quarters of depressed children and 90% of depressed adolescents report sleep disturbances, including initial, middle, and terminal insomnia as well as hypersomnia.26 Disrupted sleep in pediatric patients with major depressive disorder could be moderated by the patient’s age, with depressive symptoms more common among adolescents (age 12 to 17) than among younger children (age 6 to 11).27 Successful treatment of depression fails to relieve dyssomnia in 10% of children. Sleep problems that persist after successfully treating a depressive episode could increase the risk of another depressive episode.28
Anxiety disorders
Sleep problems are common among children and adolescents with anxiety disorders.29 Longitudinal data from >900 children found that symptoms of sleep disturbance in early childhood were correlated with experiencing an anxiety disorder 20 years later.30 Fears related to the dark or monsters under the bed that are developmentally appropriate for younger children may interfere with sleep. However, in anxious children, fears might also be related to separation, sleeping alone, worry about the loss of a loved one, concerns about personal safety, fear of frightening dreams, or concerns about academics and social relationships. Anxious individuals ruminate about their worries, and this might be especially true for children at bedtime, when there are limited distractions from ruminative fears.31 Bedtime resistance, parental involvement in bedtime rituals, and cultural factors related to sleep also could play a role for children with anxiety symptoms and sleep problems.
Having an anxiety disorder is significantly associated with an increased risk of insomnia; however, 73% of the time anxiety symptoms precede an insomnia diagnosis.29 Sleep problems and anxiety symptoms might have a reciprocal influence on one another; tiredness that results from sleep problems could exacerbate anxiety, which further worsens sleep problems.
A bridge to treatment
A thorough assessment can help identify modifiable factors and guide treatment selections. In Part 2 of this article, we will describe healthy sleep practices, cognitive-behavioral therapy for insomnia, when pharmacotherapy might be indicated, and the evidence supporting several medications commonly used to treat pediatric insomnia. We also will discuss factors to consider when seeking consultation with a pediatric behavioral sleep specialist.
1. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932-948. doi:10.1093/jpepsy/jsu041
2. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569. doi:10.1016/j.pcl.2011.03.011
3. Meltzer LJ, Plaufcan MR, Thomas JH, et al. Sleep problems and sleep disorders in pediatric primary care: treatment recommendations, persistence, and health care utilization. J Clin Sleep Med. 2014;10(4):421-426. doi:10.5664/jcsm.3620
4. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569-581, viii. doi:10.1016/j.pop.2008.06.002
5. Williamson AA, Mindell JA, Hiscock H, et al. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J Child Psychol Psychiatry. 2020;61(10):1092-1103. doi:10.1111/jcpp.13303
6. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002; 53(1):561-569. doi:10.1016/s0022-3999(02)00446-4
7. Singareddy R, Krishnamurthy VB, Vgontzas AN, et al. Subjective and objective sleep and self-harm behaviors in young children: a general population study. Psychiatry Res. 2013;209(3):549-553. doi:10.1016/j.psychres.2013.03.036
8. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi:10.1016/j.sleh.2015.10.004
9. Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005-1010. doi:10.1542/peds.2008-3641
10. Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8(4):695-725.
11. Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):114-120. doi:10.1016/j.prrv.2008.01.001
12. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602-612. doi:10.1016/j.sleep.2006.12.002
13. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774-1786. doi:10.1542/peds.2005-0772
14. Kaczor M, Skalski M. Prevalence and consequences of insomnia in pediatric population. Psychiatr Pol. 2016;50(3):555-569. doi:10.12740/PP/61226
15. Gomes TN, Dos Santos FK, Santos D, et al. Correlates of sedentary time in children: a multilevel modelling approach. BMC Public Health. 2014;14:890. doi:10.1186/1471-2458-14-890
16. Stone MR, Stevens D, Faulkner GEJ. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56(2):112-117. doi:10.1016/j.ypmed.2012.11.015
17. Hart CN, Fava JL, Subak LL, et al. Time in bed is associated with decreased physical activity and higher BMI in women seeking weight loss treatment. ISRN Obes. 2012;2012:320157. doi:10.5402/2012/320157
18. Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. doi:10.1073/pnas.0706446105
19. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi:10.1016/j.smrv.2017.06.009
20. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Lippincott Williams & Wilkins; 2015.
21. Zhang J, Paksarian D, Lamers F, et al. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137-143. doi:10.1016/j.jpeds.2016.11.007
22. Gregory AM, Agnew-Blais JC, Matthews T, et al. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46(2):284-294. doi:10.1080/15374416.2016.1183499
23. Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: Defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811-828. doi:10.2165/11538990-000000000-00000
24. Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. J Sleep Res. 2010;19(2):366-373. doi:10.1111/j.1365-2869.2009.00795.x
25. Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395-404. doi:10.1097/DBP.0000000000000285
26. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148(1):66-71. doi:10.1016/j.jad.2012.11.049
27. Emslie GJ, Rush AJ, Weinberg WA, et al. Sleep EEG features of adolescents with major depression. Biol Psychiatry. 1994;36(9):573-581. doi:10.1016/0006-3223(94)90067-1
28. Alfano CA, Zakem AH, Costa NM, et al. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depress Anxiety. 2009;26(6):503-512. doi:10.1002/da.20443
29. Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224-232. doi:10.1097/01.chi.0000242233.06011.8e
30. Gregory AM, Caspi A, Eley TC, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157-163. doi: 10.1007/s10802-005-1824-0
31. Chorney DB, Detweiler MF, Morris TL, et al. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339-348. doi:10.1093/jpepsy/jsm105
32. Sadeh A. Stress, trauma, and sleep in children. Child Adolesc Psychiatr Clin N Am. 1996;5(3):685-700. doi:10.1016/S1056-4993(18)30356-0

33. Glod CA, Teicher MH, Hartman CR, et al. Increased nocturnal activity and impaired sleep maintenance in abused children. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1236-1243. doi:10.1097/00004583-199709000-00016
34. Strawn JR, Lu L, Peris TS, et al. Research review: pediatric anxiety disorders: what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114-139. doi:10.1111/jcpp.13262
35. Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi:10.1007/s11920-015-0591-z
36. Hamill Skoch S, Mills JA, Ramsey L, et al. Letter to editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder— a bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387-388. doi:10.1089/cap.2020.0169
1. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932-948. doi:10.1093/jpepsy/jsu041
2. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569. doi:10.1016/j.pcl.2011.03.011
3. Meltzer LJ, Plaufcan MR, Thomas JH, et al. Sleep problems and sleep disorders in pediatric primary care: treatment recommendations, persistence, and health care utilization. J Clin Sleep Med. 2014;10(4):421-426. doi:10.5664/jcsm.3620
4. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569-581, viii. doi:10.1016/j.pop.2008.06.002
5. Williamson AA, Mindell JA, Hiscock H, et al. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J Child Psychol Psychiatry. 2020;61(10):1092-1103. doi:10.1111/jcpp.13303
6. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002; 53(1):561-569. doi:10.1016/s0022-3999(02)00446-4
7. Singareddy R, Krishnamurthy VB, Vgontzas AN, et al. Subjective and objective sleep and self-harm behaviors in young children: a general population study. Psychiatry Res. 2013;209(3):549-553. doi:10.1016/j.psychres.2013.03.036
8. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi:10.1016/j.sleh.2015.10.004
9. Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005-1010. doi:10.1542/peds.2008-3641
10. Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8(4):695-725.
11. Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):114-120. doi:10.1016/j.prrv.2008.01.001
12. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602-612. doi:10.1016/j.sleep.2006.12.002
13. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774-1786. doi:10.1542/peds.2005-0772
14. Kaczor M, Skalski M. Prevalence and consequences of insomnia in pediatric population. Psychiatr Pol. 2016;50(3):555-569. doi:10.12740/PP/61226
15. Gomes TN, Dos Santos FK, Santos D, et al. Correlates of sedentary time in children: a multilevel modelling approach. BMC Public Health. 2014;14:890. doi:10.1186/1471-2458-14-890
16. Stone MR, Stevens D, Faulkner GEJ. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56(2):112-117. doi:10.1016/j.ypmed.2012.11.015
17. Hart CN, Fava JL, Subak LL, et al. Time in bed is associated with decreased physical activity and higher BMI in women seeking weight loss treatment. ISRN Obes. 2012;2012:320157. doi:10.5402/2012/320157
18. Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. doi:10.1073/pnas.0706446105
19. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi:10.1016/j.smrv.2017.06.009
20. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Lippincott Williams & Wilkins; 2015.
21. Zhang J, Paksarian D, Lamers F, et al. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137-143. doi:10.1016/j.jpeds.2016.11.007
22. Gregory AM, Agnew-Blais JC, Matthews T, et al. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46(2):284-294. doi:10.1080/15374416.2016.1183499
23. Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: Defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811-828. doi:10.2165/11538990-000000000-00000
24. Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. J Sleep Res. 2010;19(2):366-373. doi:10.1111/j.1365-2869.2009.00795.x
25. Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395-404. doi:10.1097/DBP.0000000000000285
26. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148(1):66-71. doi:10.1016/j.jad.2012.11.049
27. Emslie GJ, Rush AJ, Weinberg WA, et al. Sleep EEG features of adolescents with major depression. Biol Psychiatry. 1994;36(9):573-581. doi:10.1016/0006-3223(94)90067-1
28. Alfano CA, Zakem AH, Costa NM, et al. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depress Anxiety. 2009;26(6):503-512. doi:10.1002/da.20443
29. Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224-232. doi:10.1097/01.chi.0000242233.06011.8e
30. Gregory AM, Caspi A, Eley TC, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157-163. doi: 10.1007/s10802-005-1824-0
31. Chorney DB, Detweiler MF, Morris TL, et al. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339-348. doi:10.1093/jpepsy/jsm105
32. Sadeh A. Stress, trauma, and sleep in children. Child Adolesc Psychiatr Clin N Am. 1996;5(3):685-700. doi:10.1016/S1056-4993(18)30356-0

33. Glod CA, Teicher MH, Hartman CR, et al. Increased nocturnal activity and impaired sleep maintenance in abused children. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1236-1243. doi:10.1097/00004583-199709000-00016
34. Strawn JR, Lu L, Peris TS, et al. Research review: pediatric anxiety disorders: what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114-139. doi:10.1111/jcpp.13262
35. Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi:10.1007/s11920-015-0591-z
36. Hamill Skoch S, Mills JA, Ramsey L, et al. Letter to editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder— a bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387-388. doi:10.1089/cap.2020.0169
Using measurement-based care to improve outcomes for patients with depression
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
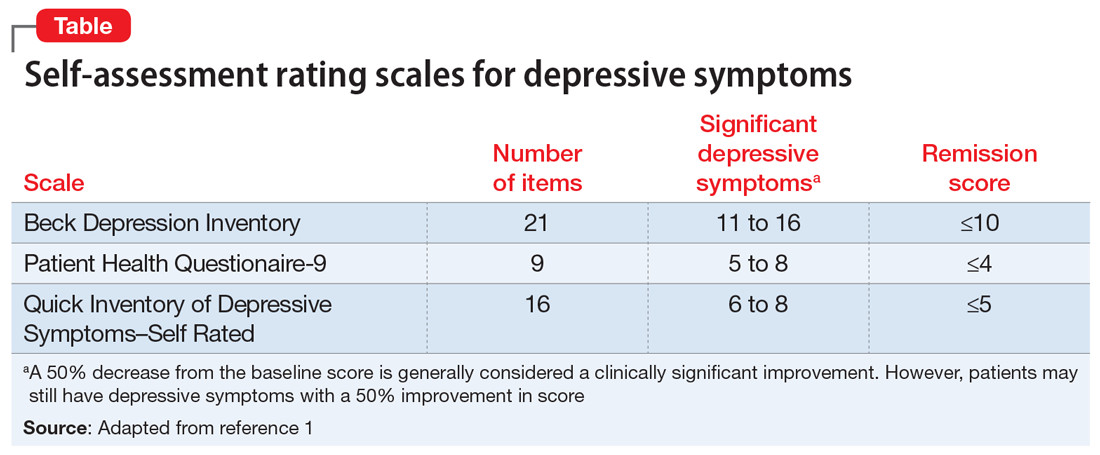
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.
The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Let’s talk about ‘chemsex’: Sexualized drug use among men who have sex with men
Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).
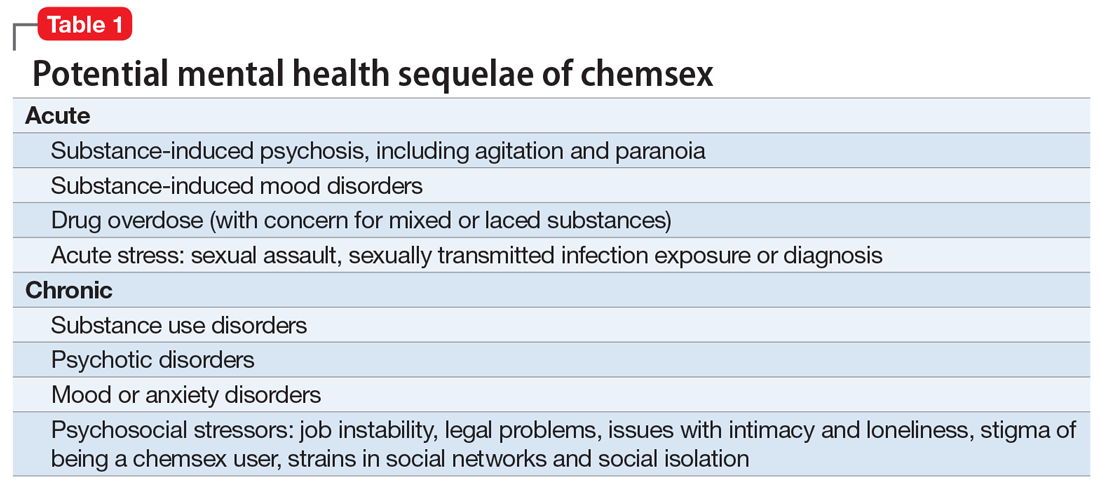
What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).

What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).

What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
Comments & Controversies
The perils of hubris
Dr. Nasrallah’s fascinating editorial on the psychiatric aspects of prominent individuals’ fall from grace (“From famous to infamous: Psychiatric aspects of the fall from grace,” From the Editor,
Perhaps fittingly, the phenomenon of self-destruction as a byproduct of success was most prominently “diagnosed” by business school professors, not physicians. The propensity for ethical failure at the apex of achievement was coined the “Bathsheba Syndrome,” in reference to the biblical tale of King David’s degenerative sequence of temptation, infidelity, deceit, and treachery while at the height of his power.2 David’s transgressions are enabled by the very success he has achieved.3
One of my valued mentors had an interesting, albeit unscientific, method of mitigating hubris. When he was a senior military lawyer, or judge advocate (JAG), and I was a junior one, my mentor took me to a briefing in which he provided a legal overview to newly minted colonels assuming command billets. One of the functions of JAGs is to provide counsel and advice to commanders. As Dr. Nasrallah noted in his editorial, military leaders are by no means immune from the proverbial fall from grace, and arguably particularly susceptible to it. In beginning his remarks, my mentor offered his heartfelt congratulations to the attendees on their promotion and then proceeded to hand out a pocket mirror for them to pass around. He asked each officer to look in the mirror and personally confirm for him that they were just as unattractive today as they were yesterday.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
The views expressed in this letter are those of the author and do not necessarily reflect those of any government agency.
1. Wolfe T. Bonfire of the vanities. Farrar, Straus and Giroux; 1987.
2. Ludwig DC, Longenecker CO. The Bathsheba syndrome: the ethical failure of successful leaders. J Bus Ethics. 1993;12:265-273.
3. 2 Samuel 11-12.
I enjoyed Dr. Nasrallah’s editorial and his discussion of the dangers of hubris. This brought to mind the role of the auriga in ancient Rome: "the auriga was a slave with gladiator status, whose duty it was to drive a biga, the light vehicle powered by two horses, to transport some important Romans, mainly duces (military commanders). An auriga was a sort of “chauffeur” for important men and was carefully selected from among trustworthy slaves only. It has been supposed also that this name was given to the slave who held a laurel crown, during Roman Triumphs, over the head of the dux, standing at his back but continuously whispering in his ears “Memento Mori” (“remember you are mortal”) to prevent the celebrated commander from losing his sense of proportion in the excesses of the celebrations.”1
Continue to: Mark S. Komrad, MD...
Mark S. Komrad, MD
Faculty of Psychiatry
Johns Hopkins Hospital
University of Maryland
Tulane University
Towson, Maryland
Reference
1. Auriga (slave). Accessed November 9, 2021. https://en.wikipedia.org/wiki/Auriga_(slave)
Barriers to care faced by African American patients
According to the US Department of Health and Human Services, the 5 domains of social determinants of health are Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1 Patients who are African American face many socioeconomic barriers to access to psychiatric care, including economic inequality, inadequate knowledge about mental health, and deficient social environments. These barriers have a significant impact on the accessibility of psychiatric health care within this community, and they need to be addressed.
Jegede et al2 discussed how financial woes and insecurity within the African American community contribute to health care inequalities and adverse health outcomes. According to the US Census Bureau,in 2020, compared to other ethnic groups, African American individuals had the lowest median income.3 Alang4 discussed how the stigma of mental health was a barrier among younger, college-educated individuals who are African American, and that those with higher education were more likely to minimize and report low treatment effectiveness. As clinicians, we often fail to discuss the effects the perceived social and cultural stigma of being diagnosed with a substance use or mental health disorder has on seeking care, treatment, and therapy by African American patients. The stigma of being judged by family members or the community and being seen as “weak” for seeking treatment has a detrimental impact on access to psychiatric care.2 It is our duty as clinicians to understand these kinds of stigmas and seek ways to mitigate them within this community.
Also, we must not underestimate the importance of patients having access to transportation to treatment. We know that social support is integral to treatment, recovery, and relapse prevention. Chronic cycles of treatment and relapse can occur due to inadequate social support. Having access to a reliable driver—especially one who is a family member or member of the community—can be vital to establishing social support. Jegede et al2 found that access to adequate transportation has proven therapeutic benefits and lessens the risk of relapse with decreased exposure to risky environments. We need to devise solutions to help patients find adequate and reliable transportation.
Clinicians should be culturally mindful and aware of the barriers to psychiatric care faced by patients who are African American. They should understand the importance of removing these barriers, and work to improve this population’s access to psychiatric care. Though this may be a daunting task that requires considerable time and resources, as health care providers, we can start the process by communicating and working with local politicians and community leaders. By working together, we can develop a plan to combat these socioeconomic barriers and provide access to psychiatric care within the African American community.
Craig Perry, MD
Elohor Otite, MD
Stacy Doumas, MD
Jersey Shore University Medical Center
Neptune, New Jersey
- Healthy People 2030, US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed November 9, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
2. Jegede O, Muvvala S, Katehis E, et al. Perceived barriers to access care, anticipated discrimination and structural vulnerability among African Americans with substance use disorders. Int J Soc Psychiatry. 2021;67(2):136-143.
3. Shrider EA, Kollar M, Chen F, et al. US Census Bureau, Current Population Reports, P60-273, Income and Poverty in the United States: 2020. US Government Publishing Office; 2021.
The perils of hubris
Dr. Nasrallah’s fascinating editorial on the psychiatric aspects of prominent individuals’ fall from grace (“From famous to infamous: Psychiatric aspects of the fall from grace,” From the Editor,
Perhaps fittingly, the phenomenon of self-destruction as a byproduct of success was most prominently “diagnosed” by business school professors, not physicians. The propensity for ethical failure at the apex of achievement was coined the “Bathsheba Syndrome,” in reference to the biblical tale of King David’s degenerative sequence of temptation, infidelity, deceit, and treachery while at the height of his power.2 David’s transgressions are enabled by the very success he has achieved.3
One of my valued mentors had an interesting, albeit unscientific, method of mitigating hubris. When he was a senior military lawyer, or judge advocate (JAG), and I was a junior one, my mentor took me to a briefing in which he provided a legal overview to newly minted colonels assuming command billets. One of the functions of JAGs is to provide counsel and advice to commanders. As Dr. Nasrallah noted in his editorial, military leaders are by no means immune from the proverbial fall from grace, and arguably particularly susceptible to it. In beginning his remarks, my mentor offered his heartfelt congratulations to the attendees on their promotion and then proceeded to hand out a pocket mirror for them to pass around. He asked each officer to look in the mirror and personally confirm for him that they were just as unattractive today as they were yesterday.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
The views expressed in this letter are those of the author and do not necessarily reflect those of any government agency.
1. Wolfe T. Bonfire of the vanities. Farrar, Straus and Giroux; 1987.
2. Ludwig DC, Longenecker CO. The Bathsheba syndrome: the ethical failure of successful leaders. J Bus Ethics. 1993;12:265-273.
3. 2 Samuel 11-12.
I enjoyed Dr. Nasrallah’s editorial and his discussion of the dangers of hubris. This brought to mind the role of the auriga in ancient Rome: "the auriga was a slave with gladiator status, whose duty it was to drive a biga, the light vehicle powered by two horses, to transport some important Romans, mainly duces (military commanders). An auriga was a sort of “chauffeur” for important men and was carefully selected from among trustworthy slaves only. It has been supposed also that this name was given to the slave who held a laurel crown, during Roman Triumphs, over the head of the dux, standing at his back but continuously whispering in his ears “Memento Mori” (“remember you are mortal”) to prevent the celebrated commander from losing his sense of proportion in the excesses of the celebrations.”1
Continue to: Mark S. Komrad, MD...
Mark S. Komrad, MD
Faculty of Psychiatry
Johns Hopkins Hospital
University of Maryland
Tulane University
Towson, Maryland
Reference
1. Auriga (slave). Accessed November 9, 2021. https://en.wikipedia.org/wiki/Auriga_(slave)
Barriers to care faced by African American patients
According to the US Department of Health and Human Services, the 5 domains of social determinants of health are Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1 Patients who are African American face many socioeconomic barriers to access to psychiatric care, including economic inequality, inadequate knowledge about mental health, and deficient social environments. These barriers have a significant impact on the accessibility of psychiatric health care within this community, and they need to be addressed.
Jegede et al2 discussed how financial woes and insecurity within the African American community contribute to health care inequalities and adverse health outcomes. According to the US Census Bureau,in 2020, compared to other ethnic groups, African American individuals had the lowest median income.3 Alang4 discussed how the stigma of mental health was a barrier among younger, college-educated individuals who are African American, and that those with higher education were more likely to minimize and report low treatment effectiveness. As clinicians, we often fail to discuss the effects the perceived social and cultural stigma of being diagnosed with a substance use or mental health disorder has on seeking care, treatment, and therapy by African American patients. The stigma of being judged by family members or the community and being seen as “weak” for seeking treatment has a detrimental impact on access to psychiatric care.2 It is our duty as clinicians to understand these kinds of stigmas and seek ways to mitigate them within this community.
Also, we must not underestimate the importance of patients having access to transportation to treatment. We know that social support is integral to treatment, recovery, and relapse prevention. Chronic cycles of treatment and relapse can occur due to inadequate social support. Having access to a reliable driver—especially one who is a family member or member of the community—can be vital to establishing social support. Jegede et al2 found that access to adequate transportation has proven therapeutic benefits and lessens the risk of relapse with decreased exposure to risky environments. We need to devise solutions to help patients find adequate and reliable transportation.
Clinicians should be culturally mindful and aware of the barriers to psychiatric care faced by patients who are African American. They should understand the importance of removing these barriers, and work to improve this population’s access to psychiatric care. Though this may be a daunting task that requires considerable time and resources, as health care providers, we can start the process by communicating and working with local politicians and community leaders. By working together, we can develop a plan to combat these socioeconomic barriers and provide access to psychiatric care within the African American community.
Craig Perry, MD
Elohor Otite, MD
Stacy Doumas, MD
Jersey Shore University Medical Center
Neptune, New Jersey
The perils of hubris
Dr. Nasrallah’s fascinating editorial on the psychiatric aspects of prominent individuals’ fall from grace (“From famous to infamous: Psychiatric aspects of the fall from grace,” From the Editor,
Perhaps fittingly, the phenomenon of self-destruction as a byproduct of success was most prominently “diagnosed” by business school professors, not physicians. The propensity for ethical failure at the apex of achievement was coined the “Bathsheba Syndrome,” in reference to the biblical tale of King David’s degenerative sequence of temptation, infidelity, deceit, and treachery while at the height of his power.2 David’s transgressions are enabled by the very success he has achieved.3
One of my valued mentors had an interesting, albeit unscientific, method of mitigating hubris. When he was a senior military lawyer, or judge advocate (JAG), and I was a junior one, my mentor took me to a briefing in which he provided a legal overview to newly minted colonels assuming command billets. One of the functions of JAGs is to provide counsel and advice to commanders. As Dr. Nasrallah noted in his editorial, military leaders are by no means immune from the proverbial fall from grace, and arguably particularly susceptible to it. In beginning his remarks, my mentor offered his heartfelt congratulations to the attendees on their promotion and then proceeded to hand out a pocket mirror for them to pass around. He asked each officer to look in the mirror and personally confirm for him that they were just as unattractive today as they were yesterday.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
The views expressed in this letter are those of the author and do not necessarily reflect those of any government agency.
1. Wolfe T. Bonfire of the vanities. Farrar, Straus and Giroux; 1987.
2. Ludwig DC, Longenecker CO. The Bathsheba syndrome: the ethical failure of successful leaders. J Bus Ethics. 1993;12:265-273.
3. 2 Samuel 11-12.
I enjoyed Dr. Nasrallah’s editorial and his discussion of the dangers of hubris. This brought to mind the role of the auriga in ancient Rome: "the auriga was a slave with gladiator status, whose duty it was to drive a biga, the light vehicle powered by two horses, to transport some important Romans, mainly duces (military commanders). An auriga was a sort of “chauffeur” for important men and was carefully selected from among trustworthy slaves only. It has been supposed also that this name was given to the slave who held a laurel crown, during Roman Triumphs, over the head of the dux, standing at his back but continuously whispering in his ears “Memento Mori” (“remember you are mortal”) to prevent the celebrated commander from losing his sense of proportion in the excesses of the celebrations.”1
Continue to: Mark S. Komrad, MD...
Mark S. Komrad, MD
Faculty of Psychiatry
Johns Hopkins Hospital
University of Maryland
Tulane University
Towson, Maryland
Reference
1. Auriga (slave). Accessed November 9, 2021. https://en.wikipedia.org/wiki/Auriga_(slave)
Barriers to care faced by African American patients
According to the US Department of Health and Human Services, the 5 domains of social determinants of health are Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1 Patients who are African American face many socioeconomic barriers to access to psychiatric care, including economic inequality, inadequate knowledge about mental health, and deficient social environments. These barriers have a significant impact on the accessibility of psychiatric health care within this community, and they need to be addressed.
Jegede et al2 discussed how financial woes and insecurity within the African American community contribute to health care inequalities and adverse health outcomes. According to the US Census Bureau,in 2020, compared to other ethnic groups, African American individuals had the lowest median income.3 Alang4 discussed how the stigma of mental health was a barrier among younger, college-educated individuals who are African American, and that those with higher education were more likely to minimize and report low treatment effectiveness. As clinicians, we often fail to discuss the effects the perceived social and cultural stigma of being diagnosed with a substance use or mental health disorder has on seeking care, treatment, and therapy by African American patients. The stigma of being judged by family members or the community and being seen as “weak” for seeking treatment has a detrimental impact on access to psychiatric care.2 It is our duty as clinicians to understand these kinds of stigmas and seek ways to mitigate them within this community.
Also, we must not underestimate the importance of patients having access to transportation to treatment. We know that social support is integral to treatment, recovery, and relapse prevention. Chronic cycles of treatment and relapse can occur due to inadequate social support. Having access to a reliable driver—especially one who is a family member or member of the community—can be vital to establishing social support. Jegede et al2 found that access to adequate transportation has proven therapeutic benefits and lessens the risk of relapse with decreased exposure to risky environments. We need to devise solutions to help patients find adequate and reliable transportation.
Clinicians should be culturally mindful and aware of the barriers to psychiatric care faced by patients who are African American. They should understand the importance of removing these barriers, and work to improve this population’s access to psychiatric care. Though this may be a daunting task that requires considerable time and resources, as health care providers, we can start the process by communicating and working with local politicians and community leaders. By working together, we can develop a plan to combat these socioeconomic barriers and provide access to psychiatric care within the African American community.
Craig Perry, MD
Elohor Otite, MD
Stacy Doumas, MD
Jersey Shore University Medical Center
Neptune, New Jersey
- Healthy People 2030, US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed November 9, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
2. Jegede O, Muvvala S, Katehis E, et al. Perceived barriers to access care, anticipated discrimination and structural vulnerability among African Americans with substance use disorders. Int J Soc Psychiatry. 2021;67(2):136-143.
3. Shrider EA, Kollar M, Chen F, et al. US Census Bureau, Current Population Reports, P60-273, Income and Poverty in the United States: 2020. US Government Publishing Office; 2021.
- Healthy People 2030, US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed November 9, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
2. Jegede O, Muvvala S, Katehis E, et al. Perceived barriers to access care, anticipated discrimination and structural vulnerability among African Americans with substance use disorders. Int J Soc Psychiatry. 2021;67(2):136-143.
3. Shrider EA, Kollar M, Chen F, et al. US Census Bureau, Current Population Reports, P60-273, Income and Poverty in the United States: 2020. US Government Publishing Office; 2021.
Sickle cell raises risk for stillbirth
Both sickle cell trait and sickle cell disease were significantly associated with an increased risk of stillbirth, based on data from more than 50,000 women.
Pregnant women with sickle cell disease (SCD) are at increased risk of complications, including stillbirth, but many women with the disease in the United States lack access to specialty care, Silvia P. Canelón, PhD, of the University of Pennsylvania, Philadelphia, and colleagues wrote. Sickle cell trait (SCT), defined as one abnormal allele of the hemoglobin gene, is not considered a disease state because many carriers are asymptomatic, and therefore even less likely to be assessed for potential complications. “However, it is possible for people with SCT to experience sickling of red blood cells under severe hypoxia, dehydration, and hyperthermia. This condition can lead to severe medical complications for sickle cell carriers, including fetal loss, splenic infarction, exercise-related sudden death, and others,” they noted.
In a study published in JAMA Network Open, the researchers reviewed data from 63,334 deliveries in 50,560 women between Jan. 1, 2010, and Aug. 15, 2017, at four quaternary academic medical centers in Pennsylvania. Of these, 1,904 had SCT but not SCD, and 164 had SCD. The mean age of the women was 29.5 years, and approximately 56% were single at the time of delivery. A majority (87%) of the study population was Rhesus-factor positive, 47.0% were Black or African American, 33.7% were White, and 45.2% had ABO blood type O.
Risk factors for stillbirth used in the analysis included SCD, numbers of pain crises and blood transfusions before delivery, delivery episode (to represent parity), history of cesarean delivery, multiple gestation, age, marital status, race and ethnicity, ABO blood type, Rhesus factor, and year of delivery.
Overall, the prevalence of stillbirth in women with SCT was 1.1%, compared with 0.8% in the general study population, and was significantly associated with increased risk of stillbirth after controlling for multiple risk factors. The adjusted odds ratio was 8.94 for stillbirth risk in women with SCT, compared with women without SCT (P = .045), although the risk was greater among women with SCD, compared with those without SCD (aOR, 26.40).
“In addition, the stratified analysis found Black or African American patients with SCD to be at higher risk of stillbirth, compared with Black or African American patients without SCD (aOR, 3.59),” but no significant association was noted between stillbirth and SCT, the researchers wrote. Stillbirth rates were 1.1% in Black or African American women overall, 2.7% in those with SCD, and 1.0% in those with SCT. Overall, multiple gestation was associated with an increased risk of stillbirth (aOR, 4.68), while a history of cesarean delivery and being married at the time of delivery were associated with decreased risk (aOR, 0.44 and 0.72, respectively).
The lack of association between stillbirth and SCT in Black or African American patients supports some previous research, but contradicts other studies, the researchers wrote. “Ultimately, it may be impossible to disentangle the risks due to the disease and those due to disparities associated with the disease that have resulted from longstanding inequity and stigma,” they said. The findings also suggest that biological mechanisms of SCT may contribute to severe clinical complications, and therefore “invite a more critical examination of the assumption that SCT is not a disease state.”
The study findings were limited by several factors including the lack of assessment of SCT independent of other comorbidities, such as hypertension, preeclampsia, diabetes, and obesity, and by the use of billing codes that could misclassify patients, the researchers noted.
However, the results support some findings from previous studies of the potential health complications for pregnant SCT patients. The large study population highlights the need to identify women’s SCT status during obstetric care, and to provide both pregnancy guidance for SCT patients and systemic support of comprehensive care for SCD and SCT patients, they concluded.
Disparities may drive stillbirth in sickle cell trait women
“There is a paucity of research evaluating sickle cell trait and the risk of adverse pregnancy outcomes such as stillbirth,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Prior studies evaluating the risk of stillbirth have yielded mixed results, and an increased risk of stillbirth in women with sickle cell trait has not been established. This study is unique in that it attempts to address how racial inequities and health disparities may contribute to risk of stillbirth in women with sickle cell trait.”
Although the study findings suggest an increased risk of stillbirth in women with sickle cell trait, an analysis stratified for Black or African American patients showed no association, Dr. Krishna said. “The prevalence of stillbirth was noted to be 1% among Black or African American patients with sickle cell trait compared to the prevalence of stillbirth of 1.1% among Black or African American women with no sickle cell trait or disease. Although, sickle cell trait or sickle cell disease can be found in any racial or ethnic group, it disproportionately affects Black or African Americans, with a sickle cell trait carrier rate of approximately 1 in 10. The mixed findings in this study amongst racial/ethnic groups further suggest that there is more research needed before an association between stillbirth and sickle cell trait can be supported.”
As for clinical implications, “it is well established that for women with sickle cell trait there is an increased risk of urinary tract infections in pregnancy,” said Dr. Krishna. “Women with sickle cell trait should have a urine culture performed at their first prenatal visit and each trimester. At this time, studies evaluating risk of stillbirth in women with sickle cell trait have yielded conflicting results, and current consensus is that women with sickle cell trait are not at increased risk. In comparison, women with sickle cell disease are at increased risk for stillbirth and adverse pregnancy outcomes. Women with sickle cell disease should be followed closely during pregnancy and fetal surveillance implemented at 32 weeks, if not sooner, to reduce risk of stillbirth.
“Prior studies evaluating risk of stillbirth in women with sickle cell trait consist of retrospective cohorts with small study populations,” Dr. Krishna added. Notably, the current study was limited by the inability to adjust for comorbidities including diabetes, hypertension, and obesity, that are not only associated with an increased risk for stillbirth, but also disproportionately common among Black women.
“More studies are needed evaluating the relationship between these comorbidities as well as studies specifically evaluating how race affects care and pregnancy outcomes,” Dr. Krisha emphasized.
The study was funded by the University of Pennsylvania department of biostatistics, epidemiology, and informatics. Lead author Dr. Canelón disclosed grants from the Centers for Disease Control and Prevention, Clinical and Translational Science Awards, and grants from the National Institutes of Health outside the submitted work. Dr. Krishna had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
Both sickle cell trait and sickle cell disease were significantly associated with an increased risk of stillbirth, based on data from more than 50,000 women.
Pregnant women with sickle cell disease (SCD) are at increased risk of complications, including stillbirth, but many women with the disease in the United States lack access to specialty care, Silvia P. Canelón, PhD, of the University of Pennsylvania, Philadelphia, and colleagues wrote. Sickle cell trait (SCT), defined as one abnormal allele of the hemoglobin gene, is not considered a disease state because many carriers are asymptomatic, and therefore even less likely to be assessed for potential complications. “However, it is possible for people with SCT to experience sickling of red blood cells under severe hypoxia, dehydration, and hyperthermia. This condition can lead to severe medical complications for sickle cell carriers, including fetal loss, splenic infarction, exercise-related sudden death, and others,” they noted.
In a study published in JAMA Network Open, the researchers reviewed data from 63,334 deliveries in 50,560 women between Jan. 1, 2010, and Aug. 15, 2017, at four quaternary academic medical centers in Pennsylvania. Of these, 1,904 had SCT but not SCD, and 164 had SCD. The mean age of the women was 29.5 years, and approximately 56% were single at the time of delivery. A majority (87%) of the study population was Rhesus-factor positive, 47.0% were Black or African American, 33.7% were White, and 45.2% had ABO blood type O.
Risk factors for stillbirth used in the analysis included SCD, numbers of pain crises and blood transfusions before delivery, delivery episode (to represent parity), history of cesarean delivery, multiple gestation, age, marital status, race and ethnicity, ABO blood type, Rhesus factor, and year of delivery.
Overall, the prevalence of stillbirth in women with SCT was 1.1%, compared with 0.8% in the general study population, and was significantly associated with increased risk of stillbirth after controlling for multiple risk factors. The adjusted odds ratio was 8.94 for stillbirth risk in women with SCT, compared with women without SCT (P = .045), although the risk was greater among women with SCD, compared with those without SCD (aOR, 26.40).
“In addition, the stratified analysis found Black or African American patients with SCD to be at higher risk of stillbirth, compared with Black or African American patients without SCD (aOR, 3.59),” but no significant association was noted between stillbirth and SCT, the researchers wrote. Stillbirth rates were 1.1% in Black or African American women overall, 2.7% in those with SCD, and 1.0% in those with SCT. Overall, multiple gestation was associated with an increased risk of stillbirth (aOR, 4.68), while a history of cesarean delivery and being married at the time of delivery were associated with decreased risk (aOR, 0.44 and 0.72, respectively).
The lack of association between stillbirth and SCT in Black or African American patients supports some previous research, but contradicts other studies, the researchers wrote. “Ultimately, it may be impossible to disentangle the risks due to the disease and those due to disparities associated with the disease that have resulted from longstanding inequity and stigma,” they said. The findings also suggest that biological mechanisms of SCT may contribute to severe clinical complications, and therefore “invite a more critical examination of the assumption that SCT is not a disease state.”
The study findings were limited by several factors including the lack of assessment of SCT independent of other comorbidities, such as hypertension, preeclampsia, diabetes, and obesity, and by the use of billing codes that could misclassify patients, the researchers noted.
However, the results support some findings from previous studies of the potential health complications for pregnant SCT patients. The large study population highlights the need to identify women’s SCT status during obstetric care, and to provide both pregnancy guidance for SCT patients and systemic support of comprehensive care for SCD and SCT patients, they concluded.
Disparities may drive stillbirth in sickle cell trait women
“There is a paucity of research evaluating sickle cell trait and the risk of adverse pregnancy outcomes such as stillbirth,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Prior studies evaluating the risk of stillbirth have yielded mixed results, and an increased risk of stillbirth in women with sickle cell trait has not been established. This study is unique in that it attempts to address how racial inequities and health disparities may contribute to risk of stillbirth in women with sickle cell trait.”
Although the study findings suggest an increased risk of stillbirth in women with sickle cell trait, an analysis stratified for Black or African American patients showed no association, Dr. Krishna said. “The prevalence of stillbirth was noted to be 1% among Black or African American patients with sickle cell trait compared to the prevalence of stillbirth of 1.1% among Black or African American women with no sickle cell trait or disease. Although, sickle cell trait or sickle cell disease can be found in any racial or ethnic group, it disproportionately affects Black or African Americans, with a sickle cell trait carrier rate of approximately 1 in 10. The mixed findings in this study amongst racial/ethnic groups further suggest that there is more research needed before an association between stillbirth and sickle cell trait can be supported.”
As for clinical implications, “it is well established that for women with sickle cell trait there is an increased risk of urinary tract infections in pregnancy,” said Dr. Krishna. “Women with sickle cell trait should have a urine culture performed at their first prenatal visit and each trimester. At this time, studies evaluating risk of stillbirth in women with sickle cell trait have yielded conflicting results, and current consensus is that women with sickle cell trait are not at increased risk. In comparison, women with sickle cell disease are at increased risk for stillbirth and adverse pregnancy outcomes. Women with sickle cell disease should be followed closely during pregnancy and fetal surveillance implemented at 32 weeks, if not sooner, to reduce risk of stillbirth.
“Prior studies evaluating risk of stillbirth in women with sickle cell trait consist of retrospective cohorts with small study populations,” Dr. Krishna added. Notably, the current study was limited by the inability to adjust for comorbidities including diabetes, hypertension, and obesity, that are not only associated with an increased risk for stillbirth, but also disproportionately common among Black women.
“More studies are needed evaluating the relationship between these comorbidities as well as studies specifically evaluating how race affects care and pregnancy outcomes,” Dr. Krisha emphasized.
The study was funded by the University of Pennsylvania department of biostatistics, epidemiology, and informatics. Lead author Dr. Canelón disclosed grants from the Centers for Disease Control and Prevention, Clinical and Translational Science Awards, and grants from the National Institutes of Health outside the submitted work. Dr. Krishna had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
Both sickle cell trait and sickle cell disease were significantly associated with an increased risk of stillbirth, based on data from more than 50,000 women.
Pregnant women with sickle cell disease (SCD) are at increased risk of complications, including stillbirth, but many women with the disease in the United States lack access to specialty care, Silvia P. Canelón, PhD, of the University of Pennsylvania, Philadelphia, and colleagues wrote. Sickle cell trait (SCT), defined as one abnormal allele of the hemoglobin gene, is not considered a disease state because many carriers are asymptomatic, and therefore even less likely to be assessed for potential complications. “However, it is possible for people with SCT to experience sickling of red blood cells under severe hypoxia, dehydration, and hyperthermia. This condition can lead to severe medical complications for sickle cell carriers, including fetal loss, splenic infarction, exercise-related sudden death, and others,” they noted.
In a study published in JAMA Network Open, the researchers reviewed data from 63,334 deliveries in 50,560 women between Jan. 1, 2010, and Aug. 15, 2017, at four quaternary academic medical centers in Pennsylvania. Of these, 1,904 had SCT but not SCD, and 164 had SCD. The mean age of the women was 29.5 years, and approximately 56% were single at the time of delivery. A majority (87%) of the study population was Rhesus-factor positive, 47.0% were Black or African American, 33.7% were White, and 45.2% had ABO blood type O.
Risk factors for stillbirth used in the analysis included SCD, numbers of pain crises and blood transfusions before delivery, delivery episode (to represent parity), history of cesarean delivery, multiple gestation, age, marital status, race and ethnicity, ABO blood type, Rhesus factor, and year of delivery.
Overall, the prevalence of stillbirth in women with SCT was 1.1%, compared with 0.8% in the general study population, and was significantly associated with increased risk of stillbirth after controlling for multiple risk factors. The adjusted odds ratio was 8.94 for stillbirth risk in women with SCT, compared with women without SCT (P = .045), although the risk was greater among women with SCD, compared with those without SCD (aOR, 26.40).
“In addition, the stratified analysis found Black or African American patients with SCD to be at higher risk of stillbirth, compared with Black or African American patients without SCD (aOR, 3.59),” but no significant association was noted between stillbirth and SCT, the researchers wrote. Stillbirth rates were 1.1% in Black or African American women overall, 2.7% in those with SCD, and 1.0% in those with SCT. Overall, multiple gestation was associated with an increased risk of stillbirth (aOR, 4.68), while a history of cesarean delivery and being married at the time of delivery were associated with decreased risk (aOR, 0.44 and 0.72, respectively).
The lack of association between stillbirth and SCT in Black or African American patients supports some previous research, but contradicts other studies, the researchers wrote. “Ultimately, it may be impossible to disentangle the risks due to the disease and those due to disparities associated with the disease that have resulted from longstanding inequity and stigma,” they said. The findings also suggest that biological mechanisms of SCT may contribute to severe clinical complications, and therefore “invite a more critical examination of the assumption that SCT is not a disease state.”
The study findings were limited by several factors including the lack of assessment of SCT independent of other comorbidities, such as hypertension, preeclampsia, diabetes, and obesity, and by the use of billing codes that could misclassify patients, the researchers noted.
However, the results support some findings from previous studies of the potential health complications for pregnant SCT patients. The large study population highlights the need to identify women’s SCT status during obstetric care, and to provide both pregnancy guidance for SCT patients and systemic support of comprehensive care for SCD and SCT patients, they concluded.
Disparities may drive stillbirth in sickle cell trait women
“There is a paucity of research evaluating sickle cell trait and the risk of adverse pregnancy outcomes such as stillbirth,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Prior studies evaluating the risk of stillbirth have yielded mixed results, and an increased risk of stillbirth in women with sickle cell trait has not been established. This study is unique in that it attempts to address how racial inequities and health disparities may contribute to risk of stillbirth in women with sickle cell trait.”
Although the study findings suggest an increased risk of stillbirth in women with sickle cell trait, an analysis stratified for Black or African American patients showed no association, Dr. Krishna said. “The prevalence of stillbirth was noted to be 1% among Black or African American patients with sickle cell trait compared to the prevalence of stillbirth of 1.1% among Black or African American women with no sickle cell trait or disease. Although, sickle cell trait or sickle cell disease can be found in any racial or ethnic group, it disproportionately affects Black or African Americans, with a sickle cell trait carrier rate of approximately 1 in 10. The mixed findings in this study amongst racial/ethnic groups further suggest that there is more research needed before an association between stillbirth and sickle cell trait can be supported.”
As for clinical implications, “it is well established that for women with sickle cell trait there is an increased risk of urinary tract infections in pregnancy,” said Dr. Krishna. “Women with sickle cell trait should have a urine culture performed at their first prenatal visit and each trimester. At this time, studies evaluating risk of stillbirth in women with sickle cell trait have yielded conflicting results, and current consensus is that women with sickle cell trait are not at increased risk. In comparison, women with sickle cell disease are at increased risk for stillbirth and adverse pregnancy outcomes. Women with sickle cell disease should be followed closely during pregnancy and fetal surveillance implemented at 32 weeks, if not sooner, to reduce risk of stillbirth.
“Prior studies evaluating risk of stillbirth in women with sickle cell trait consist of retrospective cohorts with small study populations,” Dr. Krishna added. Notably, the current study was limited by the inability to adjust for comorbidities including diabetes, hypertension, and obesity, that are not only associated with an increased risk for stillbirth, but also disproportionately common among Black women.
“More studies are needed evaluating the relationship between these comorbidities as well as studies specifically evaluating how race affects care and pregnancy outcomes,” Dr. Krisha emphasized.
The study was funded by the University of Pennsylvania department of biostatistics, epidemiology, and informatics. Lead author Dr. Canelón disclosed grants from the Centers for Disease Control and Prevention, Clinical and Translational Science Awards, and grants from the National Institutes of Health outside the submitted work. Dr. Krishna had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
FROM JAMA NETWORK OPEN
Geospatial maps show areas of Africa that need HIV services
Can geospatial mapping fill in the gaps in areas lagging behind in global efforts to end the HIV epidemic?
That’s what Diego Cuadros, PhD, assistant professor of health geography and disease modeling at the University of Cincinnati, set out to learn using geospatial data combined with prevalence data to identify the most underserved areas in Sub-Saharan Africa (SSA) for HIV services.
Study findings, which were published Nov. 24 in PLOS Global Public Health, highlight that as many as 1.5 million people living with HIV (PLHIV) in SSA have more than an hour’s motorized travel time both ways to access care, while roughly 3 million must set aside, at minimum, 30 minutes. When the only mode of transportation is walking, as much as 95.3% of underserved areas are faced with at least 30 minutes travel time.
This is simply the tip of the overall problem, Dr. Cuadros told this news organization.
“We are able to estimate how many people [whose] quality of life is being affected by HIV because they are not on treatment and most probably, HIV incidence is high in those areas. But [it’s not as simple as just] increasing the number of health care facilities,” he said. “We need to find strategies to be able to cover this population.”
Dr. Cuadros also noted that the problem goes both ways. “It’s hard for them to move, and it’s [also] hard to reach them,” he explained.
Mapping care, or lack thereof
Dr. Cuadros and team used two primary sources of data to generate high-resolution maps of underserved SSA areas: estimated number of PLHIV between the ages of 15 and 49 years in 47 SSA countries paired with population density and global map of travel time to the nearest health facility by motorized and nonmotorized (that is, walking) transportation. Combining these data allowed them to then detail the distance from access to care for every 5 km².
The mapping exercise showed that 90.5% of the total territory, in which about 7 million PLHIV resided, had more than 10 minutes motorized travel time to the nearest health care facility, while 74.6% were within 30 minutes, and 58.9% were within 60 minutes. Increases in threshold travel times (from 10 to 60 minutes) corresponded directly to declines in the average proportion of underserved areas (from 80.9% to 42.6%). However, in certain countries like Sudan and Mauritania, 99.4% of the areas were underserved at the 10 minute threshold, while more than 90% were underserved at the 60 minute threshold.
Corresponding rates for nonmotorized access to health services were similar: 88.7% (~17.6 million) PLHIV had 10 minutes walking time to health care services, while 57.8% (~11.5 million) had at least 30 minutes, and 33.0% (~6.6 million), at least 60 minutes. Likewise, as threshold times increased from 10 to 60 minutes, the percentage of affected PLHIV declined (to roughly 50% in two-thirds of the countries). But more than 70% of PLHIV resided in underserved areas in countries like Equatorial Guinea, Eritrea, South Sudan, and Sudan.
Geographical allocation of health service facilities underscores treatment gaps
“We think that most PLHIV live in urban areas or close to urban areas, and most of the health care facilities in Africa are concentrated in those areas. But [roughly 8 million people with HIV] are living in rural areas, and for most, movement is very difficult,” explained Dr. Cuadros, meaning that the majority are not on treatment despite the high incidence of HIV.
Inarguably, the pandemic has interrupted HIV services and treatment substantially on the African continent, further challenging any efforts to translate these study findings into actionable strategies.
“We’ve known for quite a while that distance and travel times and travel expenses are known risks for nonadherence, for lack of access to diagnostics, for people at risk for exposure,” Chris Beyrer, MD, MPH, Desmond M. Tutu Professor of Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview. (Dr. Beyrer was not involved in the study.)
“What’s new is the ability to really look at this across geographies and really home in on how many people face very long times and distances for travel. That’s a really important contribution,” he said.
Dr. Cuadros pointed out that these hard-to-reach populations are key to achieving the UNAID’s HIV elimination targets. “We’re going to have these pockets of transmission that are going to be really important for epidemic control,” he explained.
Toward that end, the onus appears to extend well beyond solutions that emphasize difficulty in reaching people from the provider perspective. “There’s quite a lot of what you might want to think of as blaming the victim for when people miss appointments, don’t appear to be adherent, [or] can’t stay reliably suppressed,” said Dr. Beyrer.
“It’s really important for providers in general to include in history and intake how far people have come, what their challenges are with travel, to really pay attention to those issues. Having this elegant analysis, this level of detail, is an important first step,” he added.
Dr. Cuadros has disclosed no relevant financial relationships. Dr. Beyrer reports a consulting agreement with Merck.
A version of this article first appeared on Medscape.com.
Can geospatial mapping fill in the gaps in areas lagging behind in global efforts to end the HIV epidemic?
That’s what Diego Cuadros, PhD, assistant professor of health geography and disease modeling at the University of Cincinnati, set out to learn using geospatial data combined with prevalence data to identify the most underserved areas in Sub-Saharan Africa (SSA) for HIV services.
Study findings, which were published Nov. 24 in PLOS Global Public Health, highlight that as many as 1.5 million people living with HIV (PLHIV) in SSA have more than an hour’s motorized travel time both ways to access care, while roughly 3 million must set aside, at minimum, 30 minutes. When the only mode of transportation is walking, as much as 95.3% of underserved areas are faced with at least 30 minutes travel time.
This is simply the tip of the overall problem, Dr. Cuadros told this news organization.
“We are able to estimate how many people [whose] quality of life is being affected by HIV because they are not on treatment and most probably, HIV incidence is high in those areas. But [it’s not as simple as just] increasing the number of health care facilities,” he said. “We need to find strategies to be able to cover this population.”
Dr. Cuadros also noted that the problem goes both ways. “It’s hard for them to move, and it’s [also] hard to reach them,” he explained.
Mapping care, or lack thereof
Dr. Cuadros and team used two primary sources of data to generate high-resolution maps of underserved SSA areas: estimated number of PLHIV between the ages of 15 and 49 years in 47 SSA countries paired with population density and global map of travel time to the nearest health facility by motorized and nonmotorized (that is, walking) transportation. Combining these data allowed them to then detail the distance from access to care for every 5 km².
The mapping exercise showed that 90.5% of the total territory, in which about 7 million PLHIV resided, had more than 10 minutes motorized travel time to the nearest health care facility, while 74.6% were within 30 minutes, and 58.9% were within 60 minutes. Increases in threshold travel times (from 10 to 60 minutes) corresponded directly to declines in the average proportion of underserved areas (from 80.9% to 42.6%). However, in certain countries like Sudan and Mauritania, 99.4% of the areas were underserved at the 10 minute threshold, while more than 90% were underserved at the 60 minute threshold.
Corresponding rates for nonmotorized access to health services were similar: 88.7% (~17.6 million) PLHIV had 10 minutes walking time to health care services, while 57.8% (~11.5 million) had at least 30 minutes, and 33.0% (~6.6 million), at least 60 minutes. Likewise, as threshold times increased from 10 to 60 minutes, the percentage of affected PLHIV declined (to roughly 50% in two-thirds of the countries). But more than 70% of PLHIV resided in underserved areas in countries like Equatorial Guinea, Eritrea, South Sudan, and Sudan.
Geographical allocation of health service facilities underscores treatment gaps
“We think that most PLHIV live in urban areas or close to urban areas, and most of the health care facilities in Africa are concentrated in those areas. But [roughly 8 million people with HIV] are living in rural areas, and for most, movement is very difficult,” explained Dr. Cuadros, meaning that the majority are not on treatment despite the high incidence of HIV.
Inarguably, the pandemic has interrupted HIV services and treatment substantially on the African continent, further challenging any efforts to translate these study findings into actionable strategies.
“We’ve known for quite a while that distance and travel times and travel expenses are known risks for nonadherence, for lack of access to diagnostics, for people at risk for exposure,” Chris Beyrer, MD, MPH, Desmond M. Tutu Professor of Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview. (Dr. Beyrer was not involved in the study.)
“What’s new is the ability to really look at this across geographies and really home in on how many people face very long times and distances for travel. That’s a really important contribution,” he said.
Dr. Cuadros pointed out that these hard-to-reach populations are key to achieving the UNAID’s HIV elimination targets. “We’re going to have these pockets of transmission that are going to be really important for epidemic control,” he explained.
Toward that end, the onus appears to extend well beyond solutions that emphasize difficulty in reaching people from the provider perspective. “There’s quite a lot of what you might want to think of as blaming the victim for when people miss appointments, don’t appear to be adherent, [or] can’t stay reliably suppressed,” said Dr. Beyrer.
“It’s really important for providers in general to include in history and intake how far people have come, what their challenges are with travel, to really pay attention to those issues. Having this elegant analysis, this level of detail, is an important first step,” he added.
Dr. Cuadros has disclosed no relevant financial relationships. Dr. Beyrer reports a consulting agreement with Merck.
A version of this article first appeared on Medscape.com.
Can geospatial mapping fill in the gaps in areas lagging behind in global efforts to end the HIV epidemic?
That’s what Diego Cuadros, PhD, assistant professor of health geography and disease modeling at the University of Cincinnati, set out to learn using geospatial data combined with prevalence data to identify the most underserved areas in Sub-Saharan Africa (SSA) for HIV services.
Study findings, which were published Nov. 24 in PLOS Global Public Health, highlight that as many as 1.5 million people living with HIV (PLHIV) in SSA have more than an hour’s motorized travel time both ways to access care, while roughly 3 million must set aside, at minimum, 30 minutes. When the only mode of transportation is walking, as much as 95.3% of underserved areas are faced with at least 30 minutes travel time.
This is simply the tip of the overall problem, Dr. Cuadros told this news organization.
“We are able to estimate how many people [whose] quality of life is being affected by HIV because they are not on treatment and most probably, HIV incidence is high in those areas. But [it’s not as simple as just] increasing the number of health care facilities,” he said. “We need to find strategies to be able to cover this population.”
Dr. Cuadros also noted that the problem goes both ways. “It’s hard for them to move, and it’s [also] hard to reach them,” he explained.
Mapping care, or lack thereof
Dr. Cuadros and team used two primary sources of data to generate high-resolution maps of underserved SSA areas: estimated number of PLHIV between the ages of 15 and 49 years in 47 SSA countries paired with population density and global map of travel time to the nearest health facility by motorized and nonmotorized (that is, walking) transportation. Combining these data allowed them to then detail the distance from access to care for every 5 km².
The mapping exercise showed that 90.5% of the total territory, in which about 7 million PLHIV resided, had more than 10 minutes motorized travel time to the nearest health care facility, while 74.6% were within 30 minutes, and 58.9% were within 60 minutes. Increases in threshold travel times (from 10 to 60 minutes) corresponded directly to declines in the average proportion of underserved areas (from 80.9% to 42.6%). However, in certain countries like Sudan and Mauritania, 99.4% of the areas were underserved at the 10 minute threshold, while more than 90% were underserved at the 60 minute threshold.
Corresponding rates for nonmotorized access to health services were similar: 88.7% (~17.6 million) PLHIV had 10 minutes walking time to health care services, while 57.8% (~11.5 million) had at least 30 minutes, and 33.0% (~6.6 million), at least 60 minutes. Likewise, as threshold times increased from 10 to 60 minutes, the percentage of affected PLHIV declined (to roughly 50% in two-thirds of the countries). But more than 70% of PLHIV resided in underserved areas in countries like Equatorial Guinea, Eritrea, South Sudan, and Sudan.
Geographical allocation of health service facilities underscores treatment gaps
“We think that most PLHIV live in urban areas or close to urban areas, and most of the health care facilities in Africa are concentrated in those areas. But [roughly 8 million people with HIV] are living in rural areas, and for most, movement is very difficult,” explained Dr. Cuadros, meaning that the majority are not on treatment despite the high incidence of HIV.
Inarguably, the pandemic has interrupted HIV services and treatment substantially on the African continent, further challenging any efforts to translate these study findings into actionable strategies.
“We’ve known for quite a while that distance and travel times and travel expenses are known risks for nonadherence, for lack of access to diagnostics, for people at risk for exposure,” Chris Beyrer, MD, MPH, Desmond M. Tutu Professor of Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview. (Dr. Beyrer was not involved in the study.)
“What’s new is the ability to really look at this across geographies and really home in on how many people face very long times and distances for travel. That’s a really important contribution,” he said.
Dr. Cuadros pointed out that these hard-to-reach populations are key to achieving the UNAID’s HIV elimination targets. “We’re going to have these pockets of transmission that are going to be really important for epidemic control,” he explained.
Toward that end, the onus appears to extend well beyond solutions that emphasize difficulty in reaching people from the provider perspective. “There’s quite a lot of what you might want to think of as blaming the victim for when people miss appointments, don’t appear to be adherent, [or] can’t stay reliably suppressed,” said Dr. Beyrer.
“It’s really important for providers in general to include in history and intake how far people have come, what their challenges are with travel, to really pay attention to those issues. Having this elegant analysis, this level of detail, is an important first step,” he added.
Dr. Cuadros has disclosed no relevant financial relationships. Dr. Beyrer reports a consulting agreement with Merck.
A version of this article first appeared on Medscape.com.
Two questions can help establish a diagnosis of hidradenitis suppurativa
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Lithium: An underutilized element
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.
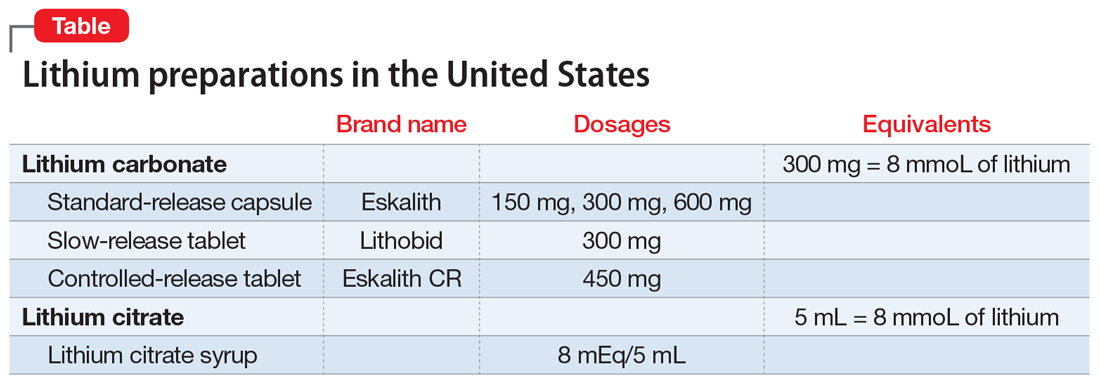
Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.

Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.

Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
3-year-history of difficulty walking
The patient has probably transitioned to the secondary progressive form of multiple sclerosis (MS). Four phenotypes have been identified in MS, with relapsing-remitting MS (RRMS) representing the most common and secondary progressive MS (SPMS) the second most common. RRMS is thought to begin as an inflammatory disease that over time becomes primarily neurodegenerative. The course of RRMS is marked by episodes of neurologic deficit followed by periods of remission which may be asymptomatic. When symptoms do not resolve — becoming fixed without remission — this is a sign of progression to SPMS. One in two RRMS patients will develop SPMS within 15 years of their diagnosis, leading to a progressive decrease of neurologic function and limitation of daily activities. Risk factors for developing SPMS include older age at onset of RRMS, longer duration of RRMS, and more cortical inflammatory lesions at baseline.
RRMS is diagnosed through clinical findings and laboratory results, the main approaches being MRI of the brain and spinal cord, and examination of cerebrospinal fluid. Neurologic symptoms must be consistent with those typically seen in MS, with deficit lasting for days to weeks. MRI is useful in monitoring disease progression (ie, new lesions that develop during relapses in RRMS). There are no universally accepted diagnostic criteria for SPMS, however. A patient usually can be diagnosed upon meeting these criteria: The patient was previously diagnosed with RRMS; the patient's symptoms are gradually worsening; this worsening is not tied to a relapse; and this worsening has been observed for 6 months or longer. Of note, SPMS' symptom-worsening characteristics can be subtle and difficult for patients to detect, and delays in diagnosis of up to several years are common.
Recognizing the onset of transition to SPMS is critical, as early initiation of therapy is thought to slow disease progression, the primary goal of treatment. In patients with SPMS, adhering to a holistic health program and managing comorbidities, especially vascular risk factors, can help preserve the health and functions of both the central nervous system and brain. Patients with SPMS who experience relapses or demonstrate new lesion formation as captured on MRI are thought to have active SPMS (aSPMS) and generally benefit from disease-modifying therapy (DMT). There is generally a transition period of about 5 years during which SPMS patients will still have a relapsing form of the disease, meaning that DMTs have proven to be effective in managing progressive MS should theoretically be beneficial for SPMS during this period. There are FDA-approved treatments for aSPMS, but off-label use is acceptable of those medications indicated for relapsing MS in those patients with evidence of relapses or new MRI activity.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
The patient has probably transitioned to the secondary progressive form of multiple sclerosis (MS). Four phenotypes have been identified in MS, with relapsing-remitting MS (RRMS) representing the most common and secondary progressive MS (SPMS) the second most common. RRMS is thought to begin as an inflammatory disease that over time becomes primarily neurodegenerative. The course of RRMS is marked by episodes of neurologic deficit followed by periods of remission which may be asymptomatic. When symptoms do not resolve — becoming fixed without remission — this is a sign of progression to SPMS. One in two RRMS patients will develop SPMS within 15 years of their diagnosis, leading to a progressive decrease of neurologic function and limitation of daily activities. Risk factors for developing SPMS include older age at onset of RRMS, longer duration of RRMS, and more cortical inflammatory lesions at baseline.
RRMS is diagnosed through clinical findings and laboratory results, the main approaches being MRI of the brain and spinal cord, and examination of cerebrospinal fluid. Neurologic symptoms must be consistent with those typically seen in MS, with deficit lasting for days to weeks. MRI is useful in monitoring disease progression (ie, new lesions that develop during relapses in RRMS). There are no universally accepted diagnostic criteria for SPMS, however. A patient usually can be diagnosed upon meeting these criteria: The patient was previously diagnosed with RRMS; the patient's symptoms are gradually worsening; this worsening is not tied to a relapse; and this worsening has been observed for 6 months or longer. Of note, SPMS' symptom-worsening characteristics can be subtle and difficult for patients to detect, and delays in diagnosis of up to several years are common.
Recognizing the onset of transition to SPMS is critical, as early initiation of therapy is thought to slow disease progression, the primary goal of treatment. In patients with SPMS, adhering to a holistic health program and managing comorbidities, especially vascular risk factors, can help preserve the health and functions of both the central nervous system and brain. Patients with SPMS who experience relapses or demonstrate new lesion formation as captured on MRI are thought to have active SPMS (aSPMS) and generally benefit from disease-modifying therapy (DMT). There is generally a transition period of about 5 years during which SPMS patients will still have a relapsing form of the disease, meaning that DMTs have proven to be effective in managing progressive MS should theoretically be beneficial for SPMS during this period. There are FDA-approved treatments for aSPMS, but off-label use is acceptable of those medications indicated for relapsing MS in those patients with evidence of relapses or new MRI activity.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
The patient has probably transitioned to the secondary progressive form of multiple sclerosis (MS). Four phenotypes have been identified in MS, with relapsing-remitting MS (RRMS) representing the most common and secondary progressive MS (SPMS) the second most common. RRMS is thought to begin as an inflammatory disease that over time becomes primarily neurodegenerative. The course of RRMS is marked by episodes of neurologic deficit followed by periods of remission which may be asymptomatic. When symptoms do not resolve — becoming fixed without remission — this is a sign of progression to SPMS. One in two RRMS patients will develop SPMS within 15 years of their diagnosis, leading to a progressive decrease of neurologic function and limitation of daily activities. Risk factors for developing SPMS include older age at onset of RRMS, longer duration of RRMS, and more cortical inflammatory lesions at baseline.
RRMS is diagnosed through clinical findings and laboratory results, the main approaches being MRI of the brain and spinal cord, and examination of cerebrospinal fluid. Neurologic symptoms must be consistent with those typically seen in MS, with deficit lasting for days to weeks. MRI is useful in monitoring disease progression (ie, new lesions that develop during relapses in RRMS). There are no universally accepted diagnostic criteria for SPMS, however. A patient usually can be diagnosed upon meeting these criteria: The patient was previously diagnosed with RRMS; the patient's symptoms are gradually worsening; this worsening is not tied to a relapse; and this worsening has been observed for 6 months or longer. Of note, SPMS' symptom-worsening characteristics can be subtle and difficult for patients to detect, and delays in diagnosis of up to several years are common.
Recognizing the onset of transition to SPMS is critical, as early initiation of therapy is thought to slow disease progression, the primary goal of treatment. In patients with SPMS, adhering to a holistic health program and managing comorbidities, especially vascular risk factors, can help preserve the health and functions of both the central nervous system and brain. Patients with SPMS who experience relapses or demonstrate new lesion formation as captured on MRI are thought to have active SPMS (aSPMS) and generally benefit from disease-modifying therapy (DMT). There is generally a transition period of about 5 years during which SPMS patients will still have a relapsing form of the disease, meaning that DMTs have proven to be effective in managing progressive MS should theoretically be beneficial for SPMS during this period. There are FDA-approved treatments for aSPMS, but off-label use is acceptable of those medications indicated for relapsing MS in those patients with evidence of relapses or new MRI activity.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
A 51-year-old woman presents with a 3-year history of difficulty walking. She says that it is difficult to pinpoint when her walking problems began but reports that it has been gradual. She recalls about 10 years back a history of numbness and tingling in her hands that improved over the course of a few weeks without any further workup. She also recalls blurry vision and loss of color perception in her left eye 5 years ago while traveling for work. Because the symptoms resolved on their own over 6-8 weeks, she never sought care. MRI shows plaques of demyelination.