User login
Multiple Lesions With Recurrent Bleeding
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
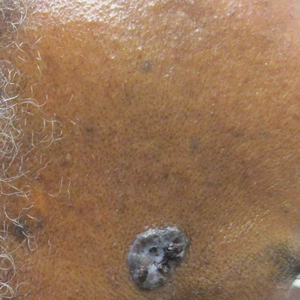
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
A 63-year-old man with frontal bossing and a history of jaw cysts presented with numerous lesions on the scalp, trunk, and legs with recurrent bleeding. Both of his siblings had similar findings. Many lesions had been present for at least 40 years. Physical examination revealed a large, irregular, atrophic, hyperpigmented plaque on the central scalp with multiple translucent hyperpigmented papules at the periphery (top). Similar papules and plaques were present at the temples, around the waist, and on the distal lower extremities, leading to surgical excision of the largest leg lesions. In addition, there were many pinpoint pits on both palms (bottom). A biopsy was submitted for review, which confirmed basal cell carcinomas on the scalp.
U.S. obesity rates soar in early adulthood
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.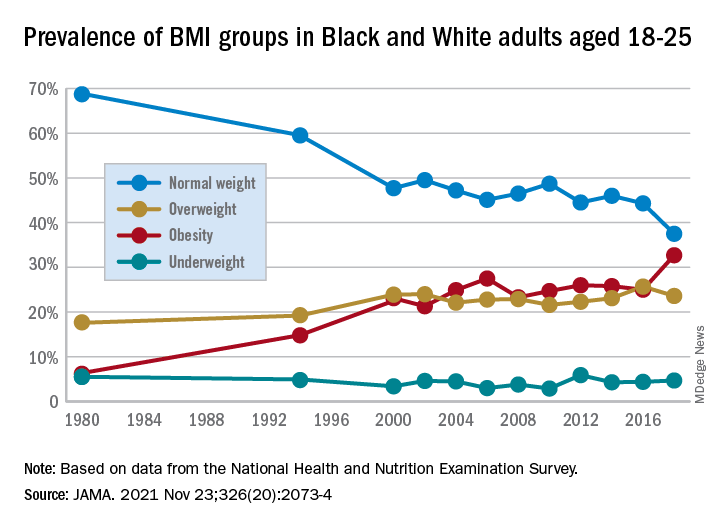
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Malpractice case: What really killed this patient? Experts disagree
A patient with many comorbidities undergoing surgery presents a number of challenges to the healthcare team. This case highlights why solid preparation for the pre-and post-op care of such patients is so important.
A 56-year-old morbidly obese man with a history of hypertension, diabetes, sleep apnea, and elevated cholesterol presented to an ambulatory surgery center for knee arthroscopy. Following a brief pre-op assessment, his airway was rated a III using both the American Society of Anesthesiologists (ASA) and Mallampati classification systems. It was decided to use a laryngeal mask airway (LMA) with 100 µg of fentanyl and 2 mgmidazolam, followed by inhalation anesthesia.
After the procedure, the LMA was removed and the patient was moved to the post-anesthesia care unit (PACU). The patient was unresponsive for about 20 minutes and exhibited signs of respiratory distress. Efforts were made to open the airway with jaw thrusts and nasal trumpet. The anesthesiologist determined that the patient was suffering from congestive heart failure, aspiration, or pulmonary edema.
The anesthesiologist administered 40 µg of naloxone. The patient began to awaken but had oxygen saturation readings in the high 70s. The patient was encouraged to take slow, deep breaths. Rhonchi were heard, and the patient complained of shortness of breath. The ECG reading was unchanged from the pre-op test.
Thirty minutes after the first dose, a second dose of 40 µg naloxone was administered with no improvement. Oxygen saturation remained between 79% and 88%. Albuterol was given with little effect. The patient’s respiration rate was 44.
The patient was reintubated. Copious pink, frothy fluid was suctioned from the endotracheal tube. The patient received propofol, urosemide, and paralytic agents with the code team present to assist. The patient’s heart rate continued to decline to about 45 beats/min. The patient was transferred to a hospital emergency department.
Upon arrival in the emergency department, the patient was in asystolic arrest. Attempts to place a transvenous pacer were unsuccessful. The nasogastric tube returned 400 cc of brown coffee-grounds gastric fluid. After 30 minutes of CPR, the patient was pronounced dead.
The autopsy report noted no apparent airway obstruction, so the pathologist determined that the cause of death was flash pulmonary edema. Negative pressure pulmonary edema is a form of flash pulmonary edema caused by forceful inspiratory efforts made against a blocked airway. Toxic levels of ropivacaine were found in the patient’s blood. The pathologist noted hypertrophic cardiomyopathy and a grossly enlarged heart.
The patient’s family filed a claim after his death. The plaintiffs argued that the LMA was removed too soon for a patient with sleep apnea and a class III Mallampati score. They raised questions about the high levels of ropivacaine and wondered whether it contributed to bradycardia. They claimed that the reintubation took too long, resulting in high end-tidal CO2. They also noted inconsistent documentation between PACU nurses and the anesthesiologist.
Some defense experts were supportive of the care, stating that the cause of death was probably from a fatal arrhythmia due to hypotension and an enlarged heart. The defense experts questioned whether undiagnosed pulmonary hypertension would explain the failure to respond to furosemide. It was noted that both of the patient’s parents had died suddenly following surgeries. The assumed cause of their deaths was coronary artery disease. This case settled.
How the claim may have been prevented: Dr. Feldman’s tips
Prevent adverse events by managing clinical decisions based on the individual patient’s needs. The history of sleep apnea and a rating of a Mallampati class III airway in this ASA III patient indicated a high risk for a difficult intubation. Consideration should have been given to performing the procedure in a hospital rather than in an ambulatory surgery center. The overall goal is to maintain a secure airway until the patient is able to maintain it on their own.
Preclude malpractice claims by having good communication with patients. Unfortunately, anesthesiologists don’t typically have an opportunity to develop a relationship with patients, but for patients at high risk, like this one, mandatory visits or calls to an anesthesiology-run pre-op clinic or ambulatory surgery center would give the anesthesiologist the opportunity to have a lengthy and informative discussion about risks, benefits, and alternatives. In addition, it would give the anesthesiologist time to discuss risks with both the surgeon and the patient.
Prevail in lawsuits by fully documenting the preoperative anesthesia assessment. There were questions about inconsistencies in documentation between the PACU nurses and anesthesiologists. Frequent huddles between the PACU staff (including nurses and physicians) may lead not only to more coordinated care but also to more consistent documentation, which will show that the care team acted together in caring for the patient.
A version of this article first appeared on Medscape.com.
A patient with many comorbidities undergoing surgery presents a number of challenges to the healthcare team. This case highlights why solid preparation for the pre-and post-op care of such patients is so important.
A 56-year-old morbidly obese man with a history of hypertension, diabetes, sleep apnea, and elevated cholesterol presented to an ambulatory surgery center for knee arthroscopy. Following a brief pre-op assessment, his airway was rated a III using both the American Society of Anesthesiologists (ASA) and Mallampati classification systems. It was decided to use a laryngeal mask airway (LMA) with 100 µg of fentanyl and 2 mgmidazolam, followed by inhalation anesthesia.
After the procedure, the LMA was removed and the patient was moved to the post-anesthesia care unit (PACU). The patient was unresponsive for about 20 minutes and exhibited signs of respiratory distress. Efforts were made to open the airway with jaw thrusts and nasal trumpet. The anesthesiologist determined that the patient was suffering from congestive heart failure, aspiration, or pulmonary edema.
The anesthesiologist administered 40 µg of naloxone. The patient began to awaken but had oxygen saturation readings in the high 70s. The patient was encouraged to take slow, deep breaths. Rhonchi were heard, and the patient complained of shortness of breath. The ECG reading was unchanged from the pre-op test.
Thirty minutes after the first dose, a second dose of 40 µg naloxone was administered with no improvement. Oxygen saturation remained between 79% and 88%. Albuterol was given with little effect. The patient’s respiration rate was 44.
The patient was reintubated. Copious pink, frothy fluid was suctioned from the endotracheal tube. The patient received propofol, urosemide, and paralytic agents with the code team present to assist. The patient’s heart rate continued to decline to about 45 beats/min. The patient was transferred to a hospital emergency department.
Upon arrival in the emergency department, the patient was in asystolic arrest. Attempts to place a transvenous pacer were unsuccessful. The nasogastric tube returned 400 cc of brown coffee-grounds gastric fluid. After 30 minutes of CPR, the patient was pronounced dead.
The autopsy report noted no apparent airway obstruction, so the pathologist determined that the cause of death was flash pulmonary edema. Negative pressure pulmonary edema is a form of flash pulmonary edema caused by forceful inspiratory efforts made against a blocked airway. Toxic levels of ropivacaine were found in the patient’s blood. The pathologist noted hypertrophic cardiomyopathy and a grossly enlarged heart.
The patient’s family filed a claim after his death. The plaintiffs argued that the LMA was removed too soon for a patient with sleep apnea and a class III Mallampati score. They raised questions about the high levels of ropivacaine and wondered whether it contributed to bradycardia. They claimed that the reintubation took too long, resulting in high end-tidal CO2. They also noted inconsistent documentation between PACU nurses and the anesthesiologist.
Some defense experts were supportive of the care, stating that the cause of death was probably from a fatal arrhythmia due to hypotension and an enlarged heart. The defense experts questioned whether undiagnosed pulmonary hypertension would explain the failure to respond to furosemide. It was noted that both of the patient’s parents had died suddenly following surgeries. The assumed cause of their deaths was coronary artery disease. This case settled.
How the claim may have been prevented: Dr. Feldman’s tips
Prevent adverse events by managing clinical decisions based on the individual patient’s needs. The history of sleep apnea and a rating of a Mallampati class III airway in this ASA III patient indicated a high risk for a difficult intubation. Consideration should have been given to performing the procedure in a hospital rather than in an ambulatory surgery center. The overall goal is to maintain a secure airway until the patient is able to maintain it on their own.
Preclude malpractice claims by having good communication with patients. Unfortunately, anesthesiologists don’t typically have an opportunity to develop a relationship with patients, but for patients at high risk, like this one, mandatory visits or calls to an anesthesiology-run pre-op clinic or ambulatory surgery center would give the anesthesiologist the opportunity to have a lengthy and informative discussion about risks, benefits, and alternatives. In addition, it would give the anesthesiologist time to discuss risks with both the surgeon and the patient.
Prevail in lawsuits by fully documenting the preoperative anesthesia assessment. There were questions about inconsistencies in documentation between the PACU nurses and anesthesiologists. Frequent huddles between the PACU staff (including nurses and physicians) may lead not only to more coordinated care but also to more consistent documentation, which will show that the care team acted together in caring for the patient.
A version of this article first appeared on Medscape.com.
A patient with many comorbidities undergoing surgery presents a number of challenges to the healthcare team. This case highlights why solid preparation for the pre-and post-op care of such patients is so important.
A 56-year-old morbidly obese man with a history of hypertension, diabetes, sleep apnea, and elevated cholesterol presented to an ambulatory surgery center for knee arthroscopy. Following a brief pre-op assessment, his airway was rated a III using both the American Society of Anesthesiologists (ASA) and Mallampati classification systems. It was decided to use a laryngeal mask airway (LMA) with 100 µg of fentanyl and 2 mgmidazolam, followed by inhalation anesthesia.
After the procedure, the LMA was removed and the patient was moved to the post-anesthesia care unit (PACU). The patient was unresponsive for about 20 minutes and exhibited signs of respiratory distress. Efforts were made to open the airway with jaw thrusts and nasal trumpet. The anesthesiologist determined that the patient was suffering from congestive heart failure, aspiration, or pulmonary edema.
The anesthesiologist administered 40 µg of naloxone. The patient began to awaken but had oxygen saturation readings in the high 70s. The patient was encouraged to take slow, deep breaths. Rhonchi were heard, and the patient complained of shortness of breath. The ECG reading was unchanged from the pre-op test.
Thirty minutes after the first dose, a second dose of 40 µg naloxone was administered with no improvement. Oxygen saturation remained between 79% and 88%. Albuterol was given with little effect. The patient’s respiration rate was 44.
The patient was reintubated. Copious pink, frothy fluid was suctioned from the endotracheal tube. The patient received propofol, urosemide, and paralytic agents with the code team present to assist. The patient’s heart rate continued to decline to about 45 beats/min. The patient was transferred to a hospital emergency department.
Upon arrival in the emergency department, the patient was in asystolic arrest. Attempts to place a transvenous pacer were unsuccessful. The nasogastric tube returned 400 cc of brown coffee-grounds gastric fluid. After 30 minutes of CPR, the patient was pronounced dead.
The autopsy report noted no apparent airway obstruction, so the pathologist determined that the cause of death was flash pulmonary edema. Negative pressure pulmonary edema is a form of flash pulmonary edema caused by forceful inspiratory efforts made against a blocked airway. Toxic levels of ropivacaine were found in the patient’s blood. The pathologist noted hypertrophic cardiomyopathy and a grossly enlarged heart.
The patient’s family filed a claim after his death. The plaintiffs argued that the LMA was removed too soon for a patient with sleep apnea and a class III Mallampati score. They raised questions about the high levels of ropivacaine and wondered whether it contributed to bradycardia. They claimed that the reintubation took too long, resulting in high end-tidal CO2. They also noted inconsistent documentation between PACU nurses and the anesthesiologist.
Some defense experts were supportive of the care, stating that the cause of death was probably from a fatal arrhythmia due to hypotension and an enlarged heart. The defense experts questioned whether undiagnosed pulmonary hypertension would explain the failure to respond to furosemide. It was noted that both of the patient’s parents had died suddenly following surgeries. The assumed cause of their deaths was coronary artery disease. This case settled.
How the claim may have been prevented: Dr. Feldman’s tips
Prevent adverse events by managing clinical decisions based on the individual patient’s needs. The history of sleep apnea and a rating of a Mallampati class III airway in this ASA III patient indicated a high risk for a difficult intubation. Consideration should have been given to performing the procedure in a hospital rather than in an ambulatory surgery center. The overall goal is to maintain a secure airway until the patient is able to maintain it on their own.
Preclude malpractice claims by having good communication with patients. Unfortunately, anesthesiologists don’t typically have an opportunity to develop a relationship with patients, but for patients at high risk, like this one, mandatory visits or calls to an anesthesiology-run pre-op clinic or ambulatory surgery center would give the anesthesiologist the opportunity to have a lengthy and informative discussion about risks, benefits, and alternatives. In addition, it would give the anesthesiologist time to discuss risks with both the surgeon and the patient.
Prevail in lawsuits by fully documenting the preoperative anesthesia assessment. There were questions about inconsistencies in documentation between the PACU nurses and anesthesiologists. Frequent huddles between the PACU staff (including nurses and physicians) may lead not only to more coordinated care but also to more consistent documentation, which will show that the care team acted together in caring for the patient.
A version of this article first appeared on Medscape.com.
Adolescents, THC, and the risk of psychosis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact letters@currentpsychiatry.com.
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact letters@currentpsychiatry.com.
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact letters@currentpsychiatry.com.
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
Opioids for headache?
Some believe that the medications, though risky, can be a useful tool in the neurologist’s treatment arsenal, while others argue that opioids are just too risky when there are other, safer alternatives available.
Those were the cruxes of arguments put forward by Paul Rizzoli, MD, and Christopher H. Gottschalk, MD, who conducted individual talks at the 2021 Scottsdale Headache Symposium. Dr. Rizzoli, associate professor of neurology at Harvard Medical School, Boston, argued in favor of the use of opioids and butalbital-containing medications. Dr. Gottschalk, assistant professor of neurology at Yale University, New Haven, Conn., argued against their use.
In certain situations opioids are worth the risk
Whether or not to use opioids in the treatment of headache is “a reasonable question, because these medications can clearly be seen as having risk. So perhaps another way to frame this question is as a risk-benefit issue. Are these medications worth the risk? How useful is the benefit of opioids, if the consequence is dependence or addiction?” Dr. Rizzoli began.
Although reviews show effectiveness of opioids in treating migraine, a three-part review in 2012 found greater efficacy of dihydroergotamine (DHE), ketorolac, and chlorpromazine. That’s not surprising, said Dr. Rizzoli, since those competing drugs are migraine-specific.
Dr. Rizzoli quoted a 2014 review indicating that there were incomplete data on the relative efficacy of opioids versus other analgesics, and for some patients opioids would likely be the optimal treatment, such as those who have contraindications to ergot-type medications or neuroleptic medications, pregnant women, or patients who don’t respond to other medications.
Dr. Rizzoli noted that The International Association for the Study of Pain has concluded that no other oral medications provide immediate and effective pain relief, and that short-term use rarely leads to addiction.
“So, to me, the answer is not to avoid opioids or outlaw them but instead to use them judiciously and infrequently, and in a short term or rescue fashion,” said Dr. Rizzoli.
He pointed out that physicians accept risks of other medications, and act to mitigate those risks. He said that risk mitigation with opioids can take the form of avoiding prescriptions in some situations, like when patients have a personal or family history of substance abuse, or in cases of some behavioral or emotional disorders.
Dr. Rizzoli went on to discuss the use of butalbital, which acts as a CNS depressant and has a variety of effects, including sedation, anxiolytic, hypnotic, and antiepileptic effects, but it is only a weak analgesic, but it nevertheless works in headache, said Dr. Rizzoli, citing patient reports and personal experience.
“It’s difficult to appreciate this theme of efficacy behind all the hype in the literature and in the press against butalbital, and the fact that it has not been adequately studied. But I would submit that the fact that we are even having this discussion is support enough for the use of butalbital. If butalbital either didn’t work or was simply a drug of abuse, it would likely have faded away by now,” said Dr. Rizzoli. He conceded that butalbital can be overused and may lead episodic headache to become chronic daily headache, but he noted that Seymour Solomon, MD, professor emeritus at Albert Einstein College of Medicine, New York, has estimated that removal of butalbital from the market would reduce chronic headache in the general population by only a small fraction of one percent.
Butalbital also has another interesting effect, which is that patients may quickly return to normal functioning after the headache resolves. “Maybe this is all due to management of anxiety, the presumed mechanism of action of barbiturates. So, instead of lobbying for its removal, I would propose that we should take a closer look at what’s going on here, and what the mechanism of action of this fairly interesting compound might be,” said Dr. Rizzoli.
Dr. Rizzoli also said there is some evidence that migraine-specific drugs also affect the tolerance to opioid drugs. “Somehow, they seem to interact with the opioid pain system. If that’s true, the implication is that you probably cannot escape the opioid receptors in the management of migraine,” said Dr. Rizzoli.
Ultimately, he supports the judicious use of opioids and butalbital containing-medications for headache relief. “My argument is that it is just too simplistic to cease use of these meds. Yes, they should be used in a restricted and careful way, but not abandoned,” said Dr. Rizzoli.
Opiates should be avoided
Following Dr. Rizzoli’s presentation, Dr. Gottschalk presented an argument against the use of opioids in the treatment of headache.
He began by quoting the ABIM Choosing Wisely Campaign of 2012, which concluded that fioricet and narcotics should be avoided in headache unless the patient is desperate. “As a headache specialist, I can tell you that I have not faced situations sufficiently desperate to use any of these. The American Headache Society in a series of evidence assessments has concluded similarly, that they are of no use,” said Dr. Gottschalk.
Opiates and barbiturates may also increase risk of migraine chronification. One study found that triptans are associated with low rates of chronification, at just a few percent when used fewer than 4 days a month, and about 20% per year when used 10-14 days per month. Opiate use showed a broadly similar pattern, while barbiturates showed a particularly alarming pattern: “Every level of use was associated with astronomically high rates and measurably higher at the highest level of use. For opiates, the odds ratio was about 2 – statistically significant. For barbiturates it was clearly greater than 2, whereas with triptans, the odds ratio showed a nonsignificant, slight increase in risk. And for NSAIDs, the odds ratio was, if anything, less than 1,” said Dr. Gottschalk.
He also discussed aspects of behavioral pharmacology, in which positive reinforcement associated with decreased headache may encourage repeated use of the drug. “Given these, it should be no surprise to anyone that emergency room treatment with opiates for acute migraine is clearly associated with increased recidivism for patients given those drugs,” said Dr. Gottschalk.
Opiate use is associated with increased pain sensitivity, and in the case of migraine, it may interfere with the activity of other treatments.
As for butalbital-containing compounds, they are positive-reinforcing drugs, and they are not indicated for migraine, only tension headache. There is no evidence of benefit in migraine, but butalbital is anxiolytic, which could lead an individual to increase its use.
A recent meta-analysis of therapies for episodic migraine found that hydromorphone and meperidine are less effective than standard therapies such as prochlorperazine or metoclopramide. Another study suggested that opioid use may interfere with the efficacy of NSAIDs in the emergency room environment, while a post hoc analysis of rizatriptan clinical trials found that recent opiate use was associated with a lower response rate, and the effect was more pronounced in women.
Among patients with chronic migraine, a 2004 study found that opiates were the most commonly used medication, and other studies found that chronic migraine does not arise in nonmigraine patients treated with opiates, “suggesting that migraine is specifically prone to opiate-induced hyperalgesia of migraine itself,” said Dr. Gottschalk.
Even under careful monitoring, misuse occurs in more than 50% of patients, “suggesting that even under the best circumstances, it is difficult to use this class of drugs safely in long term,” said Dr. Gottschalk.
He pointed out that the risk of drug addiction rises with various clinical and socioeconomic factors, including living in impoverished environments, adverse childhood experiences, low socioeconomic status, exposure to pollutants, and stressors. “In other words, all features associated with systemic racism are clearly associated with an increased risk of addiction,” said Dr. Gottschalk. Other factors include availability of the drug, such as whether or not a physician prescribes it, and repeated use.
These concerns, combined with positive-reinforcing properties of opiates and association with migraine progression and refractoriness, and the lack of progression risk found with use of NSAIDs and triptans, and the fact that effective acute therapy is associated with a lower risk of progression, argue against the use of opiates, said Dr. Gottschalk.
There is even a potential risk that the experience of migraine and its relief due to self-administration may become a rewarding experience that propagates the problem. It’s possible that anticipatory anxiety related to fear stressors could lead to migraine, or to physical sensations interpreted as migraine prodrome. “[It] raises the question of whether or not positive reinforcement by drugs makes migraine itself a rewarding experience and therefore more likely to occur as a cue for drug self-administration. The question I pose is: Is there any reason to test this theory in drugs of no proven benefit in the treatment of migraine? I would say very clearly, No,” said Dr. Gottschalk.
Clarifying the finer points of the debate
In the Q&A session after the talk, Dr. Rizzoli said that he doesn’t advocate for long-term use of opiates, except in rare cases where the diagnosis gets changed to a chronic pain syndrome. “We’re talking about intermittent use for treatment of an acute event. Do we put limits on them? I think the answer is clearly Yes, and the limits are more strict than those for triptans. My own sense as a clinician is I want all of the available tools. From a clinical perspective, there are a large number of people who do just fine with intermittent use of these medicines, and so I wouldn’t restrict them,” said Dr. Rizzoli.
Dr. Gottschalk agreed that opiates may make sense for some patients, but expressed concerns about any and all physicians prescribing them. “The part about the tools is partly a question of: Who gets to use them? In the hands of a headache specialist in those isolated cases with careful restrictions, sure. But what I’m making is a slippery slope argument: What we know is that in emergency rooms, these are used routinely, and that [those] patients are precisely the ones who are at higher risk of addiction. So in some sense, I’m just saying I think we need to have much clearer boundaries,” he said.
Dr. Rizzoli has no relevant financial disclosures. Dr. Gottschalk has been on the advisory boards of Alder, AbbVie, Amgen/Novartis, Biohaven, Theranica, Upsher-Smith, Axsome, Vorso, Currax, and Impel. He has been a consultant for Alder, Alexion, and Spherix Global Insights. He has received research support from Relivion.
Some believe that the medications, though risky, can be a useful tool in the neurologist’s treatment arsenal, while others argue that opioids are just too risky when there are other, safer alternatives available.
Those were the cruxes of arguments put forward by Paul Rizzoli, MD, and Christopher H. Gottschalk, MD, who conducted individual talks at the 2021 Scottsdale Headache Symposium. Dr. Rizzoli, associate professor of neurology at Harvard Medical School, Boston, argued in favor of the use of opioids and butalbital-containing medications. Dr. Gottschalk, assistant professor of neurology at Yale University, New Haven, Conn., argued against their use.
In certain situations opioids are worth the risk
Whether or not to use opioids in the treatment of headache is “a reasonable question, because these medications can clearly be seen as having risk. So perhaps another way to frame this question is as a risk-benefit issue. Are these medications worth the risk? How useful is the benefit of opioids, if the consequence is dependence or addiction?” Dr. Rizzoli began.
Although reviews show effectiveness of opioids in treating migraine, a three-part review in 2012 found greater efficacy of dihydroergotamine (DHE), ketorolac, and chlorpromazine. That’s not surprising, said Dr. Rizzoli, since those competing drugs are migraine-specific.
Dr. Rizzoli quoted a 2014 review indicating that there were incomplete data on the relative efficacy of opioids versus other analgesics, and for some patients opioids would likely be the optimal treatment, such as those who have contraindications to ergot-type medications or neuroleptic medications, pregnant women, or patients who don’t respond to other medications.
Dr. Rizzoli noted that The International Association for the Study of Pain has concluded that no other oral medications provide immediate and effective pain relief, and that short-term use rarely leads to addiction.
“So, to me, the answer is not to avoid opioids or outlaw them but instead to use them judiciously and infrequently, and in a short term or rescue fashion,” said Dr. Rizzoli.
He pointed out that physicians accept risks of other medications, and act to mitigate those risks. He said that risk mitigation with opioids can take the form of avoiding prescriptions in some situations, like when patients have a personal or family history of substance abuse, or in cases of some behavioral or emotional disorders.
Dr. Rizzoli went on to discuss the use of butalbital, which acts as a CNS depressant and has a variety of effects, including sedation, anxiolytic, hypnotic, and antiepileptic effects, but it is only a weak analgesic, but it nevertheless works in headache, said Dr. Rizzoli, citing patient reports and personal experience.
“It’s difficult to appreciate this theme of efficacy behind all the hype in the literature and in the press against butalbital, and the fact that it has not been adequately studied. But I would submit that the fact that we are even having this discussion is support enough for the use of butalbital. If butalbital either didn’t work or was simply a drug of abuse, it would likely have faded away by now,” said Dr. Rizzoli. He conceded that butalbital can be overused and may lead episodic headache to become chronic daily headache, but he noted that Seymour Solomon, MD, professor emeritus at Albert Einstein College of Medicine, New York, has estimated that removal of butalbital from the market would reduce chronic headache in the general population by only a small fraction of one percent.
Butalbital also has another interesting effect, which is that patients may quickly return to normal functioning after the headache resolves. “Maybe this is all due to management of anxiety, the presumed mechanism of action of barbiturates. So, instead of lobbying for its removal, I would propose that we should take a closer look at what’s going on here, and what the mechanism of action of this fairly interesting compound might be,” said Dr. Rizzoli.
Dr. Rizzoli also said there is some evidence that migraine-specific drugs also affect the tolerance to opioid drugs. “Somehow, they seem to interact with the opioid pain system. If that’s true, the implication is that you probably cannot escape the opioid receptors in the management of migraine,” said Dr. Rizzoli.
Ultimately, he supports the judicious use of opioids and butalbital containing-medications for headache relief. “My argument is that it is just too simplistic to cease use of these meds. Yes, they should be used in a restricted and careful way, but not abandoned,” said Dr. Rizzoli.
Opiates should be avoided
Following Dr. Rizzoli’s presentation, Dr. Gottschalk presented an argument against the use of opioids in the treatment of headache.
He began by quoting the ABIM Choosing Wisely Campaign of 2012, which concluded that fioricet and narcotics should be avoided in headache unless the patient is desperate. “As a headache specialist, I can tell you that I have not faced situations sufficiently desperate to use any of these. The American Headache Society in a series of evidence assessments has concluded similarly, that they are of no use,” said Dr. Gottschalk.
Opiates and barbiturates may also increase risk of migraine chronification. One study found that triptans are associated with low rates of chronification, at just a few percent when used fewer than 4 days a month, and about 20% per year when used 10-14 days per month. Opiate use showed a broadly similar pattern, while barbiturates showed a particularly alarming pattern: “Every level of use was associated with astronomically high rates and measurably higher at the highest level of use. For opiates, the odds ratio was about 2 – statistically significant. For barbiturates it was clearly greater than 2, whereas with triptans, the odds ratio showed a nonsignificant, slight increase in risk. And for NSAIDs, the odds ratio was, if anything, less than 1,” said Dr. Gottschalk.
He also discussed aspects of behavioral pharmacology, in which positive reinforcement associated with decreased headache may encourage repeated use of the drug. “Given these, it should be no surprise to anyone that emergency room treatment with opiates for acute migraine is clearly associated with increased recidivism for patients given those drugs,” said Dr. Gottschalk.
Opiate use is associated with increased pain sensitivity, and in the case of migraine, it may interfere with the activity of other treatments.
As for butalbital-containing compounds, they are positive-reinforcing drugs, and they are not indicated for migraine, only tension headache. There is no evidence of benefit in migraine, but butalbital is anxiolytic, which could lead an individual to increase its use.
A recent meta-analysis of therapies for episodic migraine found that hydromorphone and meperidine are less effective than standard therapies such as prochlorperazine or metoclopramide. Another study suggested that opioid use may interfere with the efficacy of NSAIDs in the emergency room environment, while a post hoc analysis of rizatriptan clinical trials found that recent opiate use was associated with a lower response rate, and the effect was more pronounced in women.
Among patients with chronic migraine, a 2004 study found that opiates were the most commonly used medication, and other studies found that chronic migraine does not arise in nonmigraine patients treated with opiates, “suggesting that migraine is specifically prone to opiate-induced hyperalgesia of migraine itself,” said Dr. Gottschalk.
Even under careful monitoring, misuse occurs in more than 50% of patients, “suggesting that even under the best circumstances, it is difficult to use this class of drugs safely in long term,” said Dr. Gottschalk.
He pointed out that the risk of drug addiction rises with various clinical and socioeconomic factors, including living in impoverished environments, adverse childhood experiences, low socioeconomic status, exposure to pollutants, and stressors. “In other words, all features associated with systemic racism are clearly associated with an increased risk of addiction,” said Dr. Gottschalk. Other factors include availability of the drug, such as whether or not a physician prescribes it, and repeated use.
These concerns, combined with positive-reinforcing properties of opiates and association with migraine progression and refractoriness, and the lack of progression risk found with use of NSAIDs and triptans, and the fact that effective acute therapy is associated with a lower risk of progression, argue against the use of opiates, said Dr. Gottschalk.
There is even a potential risk that the experience of migraine and its relief due to self-administration may become a rewarding experience that propagates the problem. It’s possible that anticipatory anxiety related to fear stressors could lead to migraine, or to physical sensations interpreted as migraine prodrome. “[It] raises the question of whether or not positive reinforcement by drugs makes migraine itself a rewarding experience and therefore more likely to occur as a cue for drug self-administration. The question I pose is: Is there any reason to test this theory in drugs of no proven benefit in the treatment of migraine? I would say very clearly, No,” said Dr. Gottschalk.
Clarifying the finer points of the debate
In the Q&A session after the talk, Dr. Rizzoli said that he doesn’t advocate for long-term use of opiates, except in rare cases where the diagnosis gets changed to a chronic pain syndrome. “We’re talking about intermittent use for treatment of an acute event. Do we put limits on them? I think the answer is clearly Yes, and the limits are more strict than those for triptans. My own sense as a clinician is I want all of the available tools. From a clinical perspective, there are a large number of people who do just fine with intermittent use of these medicines, and so I wouldn’t restrict them,” said Dr. Rizzoli.
Dr. Gottschalk agreed that opiates may make sense for some patients, but expressed concerns about any and all physicians prescribing them. “The part about the tools is partly a question of: Who gets to use them? In the hands of a headache specialist in those isolated cases with careful restrictions, sure. But what I’m making is a slippery slope argument: What we know is that in emergency rooms, these are used routinely, and that [those] patients are precisely the ones who are at higher risk of addiction. So in some sense, I’m just saying I think we need to have much clearer boundaries,” he said.
Dr. Rizzoli has no relevant financial disclosures. Dr. Gottschalk has been on the advisory boards of Alder, AbbVie, Amgen/Novartis, Biohaven, Theranica, Upsher-Smith, Axsome, Vorso, Currax, and Impel. He has been a consultant for Alder, Alexion, and Spherix Global Insights. He has received research support from Relivion.
Some believe that the medications, though risky, can be a useful tool in the neurologist’s treatment arsenal, while others argue that opioids are just too risky when there are other, safer alternatives available.
Those were the cruxes of arguments put forward by Paul Rizzoli, MD, and Christopher H. Gottschalk, MD, who conducted individual talks at the 2021 Scottsdale Headache Symposium. Dr. Rizzoli, associate professor of neurology at Harvard Medical School, Boston, argued in favor of the use of opioids and butalbital-containing medications. Dr. Gottschalk, assistant professor of neurology at Yale University, New Haven, Conn., argued against their use.
In certain situations opioids are worth the risk
Whether or not to use opioids in the treatment of headache is “a reasonable question, because these medications can clearly be seen as having risk. So perhaps another way to frame this question is as a risk-benefit issue. Are these medications worth the risk? How useful is the benefit of opioids, if the consequence is dependence or addiction?” Dr. Rizzoli began.
Although reviews show effectiveness of opioids in treating migraine, a three-part review in 2012 found greater efficacy of dihydroergotamine (DHE), ketorolac, and chlorpromazine. That’s not surprising, said Dr. Rizzoli, since those competing drugs are migraine-specific.
Dr. Rizzoli quoted a 2014 review indicating that there were incomplete data on the relative efficacy of opioids versus other analgesics, and for some patients opioids would likely be the optimal treatment, such as those who have contraindications to ergot-type medications or neuroleptic medications, pregnant women, or patients who don’t respond to other medications.
Dr. Rizzoli noted that The International Association for the Study of Pain has concluded that no other oral medications provide immediate and effective pain relief, and that short-term use rarely leads to addiction.
“So, to me, the answer is not to avoid opioids or outlaw them but instead to use them judiciously and infrequently, and in a short term or rescue fashion,” said Dr. Rizzoli.
He pointed out that physicians accept risks of other medications, and act to mitigate those risks. He said that risk mitigation with opioids can take the form of avoiding prescriptions in some situations, like when patients have a personal or family history of substance abuse, or in cases of some behavioral or emotional disorders.
Dr. Rizzoli went on to discuss the use of butalbital, which acts as a CNS depressant and has a variety of effects, including sedation, anxiolytic, hypnotic, and antiepileptic effects, but it is only a weak analgesic, but it nevertheless works in headache, said Dr. Rizzoli, citing patient reports and personal experience.
“It’s difficult to appreciate this theme of efficacy behind all the hype in the literature and in the press against butalbital, and the fact that it has not been adequately studied. But I would submit that the fact that we are even having this discussion is support enough for the use of butalbital. If butalbital either didn’t work or was simply a drug of abuse, it would likely have faded away by now,” said Dr. Rizzoli. He conceded that butalbital can be overused and may lead episodic headache to become chronic daily headache, but he noted that Seymour Solomon, MD, professor emeritus at Albert Einstein College of Medicine, New York, has estimated that removal of butalbital from the market would reduce chronic headache in the general population by only a small fraction of one percent.
Butalbital also has another interesting effect, which is that patients may quickly return to normal functioning after the headache resolves. “Maybe this is all due to management of anxiety, the presumed mechanism of action of barbiturates. So, instead of lobbying for its removal, I would propose that we should take a closer look at what’s going on here, and what the mechanism of action of this fairly interesting compound might be,” said Dr. Rizzoli.
Dr. Rizzoli also said there is some evidence that migraine-specific drugs also affect the tolerance to opioid drugs. “Somehow, they seem to interact with the opioid pain system. If that’s true, the implication is that you probably cannot escape the opioid receptors in the management of migraine,” said Dr. Rizzoli.
Ultimately, he supports the judicious use of opioids and butalbital containing-medications for headache relief. “My argument is that it is just too simplistic to cease use of these meds. Yes, they should be used in a restricted and careful way, but not abandoned,” said Dr. Rizzoli.
Opiates should be avoided
Following Dr. Rizzoli’s presentation, Dr. Gottschalk presented an argument against the use of opioids in the treatment of headache.
He began by quoting the ABIM Choosing Wisely Campaign of 2012, which concluded that fioricet and narcotics should be avoided in headache unless the patient is desperate. “As a headache specialist, I can tell you that I have not faced situations sufficiently desperate to use any of these. The American Headache Society in a series of evidence assessments has concluded similarly, that they are of no use,” said Dr. Gottschalk.
Opiates and barbiturates may also increase risk of migraine chronification. One study found that triptans are associated with low rates of chronification, at just a few percent when used fewer than 4 days a month, and about 20% per year when used 10-14 days per month. Opiate use showed a broadly similar pattern, while barbiturates showed a particularly alarming pattern: “Every level of use was associated with astronomically high rates and measurably higher at the highest level of use. For opiates, the odds ratio was about 2 – statistically significant. For barbiturates it was clearly greater than 2, whereas with triptans, the odds ratio showed a nonsignificant, slight increase in risk. And for NSAIDs, the odds ratio was, if anything, less than 1,” said Dr. Gottschalk.
He also discussed aspects of behavioral pharmacology, in which positive reinforcement associated with decreased headache may encourage repeated use of the drug. “Given these, it should be no surprise to anyone that emergency room treatment with opiates for acute migraine is clearly associated with increased recidivism for patients given those drugs,” said Dr. Gottschalk.
Opiate use is associated with increased pain sensitivity, and in the case of migraine, it may interfere with the activity of other treatments.
As for butalbital-containing compounds, they are positive-reinforcing drugs, and they are not indicated for migraine, only tension headache. There is no evidence of benefit in migraine, but butalbital is anxiolytic, which could lead an individual to increase its use.
A recent meta-analysis of therapies for episodic migraine found that hydromorphone and meperidine are less effective than standard therapies such as prochlorperazine or metoclopramide. Another study suggested that opioid use may interfere with the efficacy of NSAIDs in the emergency room environment, while a post hoc analysis of rizatriptan clinical trials found that recent opiate use was associated with a lower response rate, and the effect was more pronounced in women.
Among patients with chronic migraine, a 2004 study found that opiates were the most commonly used medication, and other studies found that chronic migraine does not arise in nonmigraine patients treated with opiates, “suggesting that migraine is specifically prone to opiate-induced hyperalgesia of migraine itself,” said Dr. Gottschalk.
Even under careful monitoring, misuse occurs in more than 50% of patients, “suggesting that even under the best circumstances, it is difficult to use this class of drugs safely in long term,” said Dr. Gottschalk.
He pointed out that the risk of drug addiction rises with various clinical and socioeconomic factors, including living in impoverished environments, adverse childhood experiences, low socioeconomic status, exposure to pollutants, and stressors. “In other words, all features associated with systemic racism are clearly associated with an increased risk of addiction,” said Dr. Gottschalk. Other factors include availability of the drug, such as whether or not a physician prescribes it, and repeated use.
These concerns, combined with positive-reinforcing properties of opiates and association with migraine progression and refractoriness, and the lack of progression risk found with use of NSAIDs and triptans, and the fact that effective acute therapy is associated with a lower risk of progression, argue against the use of opiates, said Dr. Gottschalk.
There is even a potential risk that the experience of migraine and its relief due to self-administration may become a rewarding experience that propagates the problem. It’s possible that anticipatory anxiety related to fear stressors could lead to migraine, or to physical sensations interpreted as migraine prodrome. “[It] raises the question of whether or not positive reinforcement by drugs makes migraine itself a rewarding experience and therefore more likely to occur as a cue for drug self-administration. The question I pose is: Is there any reason to test this theory in drugs of no proven benefit in the treatment of migraine? I would say very clearly, No,” said Dr. Gottschalk.
Clarifying the finer points of the debate
In the Q&A session after the talk, Dr. Rizzoli said that he doesn’t advocate for long-term use of opiates, except in rare cases where the diagnosis gets changed to a chronic pain syndrome. “We’re talking about intermittent use for treatment of an acute event. Do we put limits on them? I think the answer is clearly Yes, and the limits are more strict than those for triptans. My own sense as a clinician is I want all of the available tools. From a clinical perspective, there are a large number of people who do just fine with intermittent use of these medicines, and so I wouldn’t restrict them,” said Dr. Rizzoli.
Dr. Gottschalk agreed that opiates may make sense for some patients, but expressed concerns about any and all physicians prescribing them. “The part about the tools is partly a question of: Who gets to use them? In the hands of a headache specialist in those isolated cases with careful restrictions, sure. But what I’m making is a slippery slope argument: What we know is that in emergency rooms, these are used routinely, and that [those] patients are precisely the ones who are at higher risk of addiction. So in some sense, I’m just saying I think we need to have much clearer boundaries,” he said.
Dr. Rizzoli has no relevant financial disclosures. Dr. Gottschalk has been on the advisory boards of Alder, AbbVie, Amgen/Novartis, Biohaven, Theranica, Upsher-Smith, Axsome, Vorso, Currax, and Impel. He has been a consultant for Alder, Alexion, and Spherix Global Insights. He has received research support from Relivion.
FROM 2021 SCOTTSDALE HEADACHE SYMPOSIUM
Clinical Edge Journal Scan Commentary: Prostate Cancer December 2021
Racial and ethnic disparities in prostate cancer diagnosis and treatment are well recognized. Prostate magnetic resonance imaging (MRI) as part of a diagnostic approach for people with elevated PSA may decrease unnecessary biopsies or better direct biopsies based on results, and its use is increasing significantly. Abashidze et al examined Medicare claims between 2011 and 2017 to determine if racial disparities were present with respect to the use of prostate MRI. Compared with White men, Black, Asian, and Hispanic men were less likely to have a prostate MRI within 180 days after an elevated PSA (results stratified at 2.5, 4, or 10 ng/mL). The differences were most pronounced for patients with a PSA at 4 ng/mL or higher. The results suggest that providers should be aware of potential system and individual factors that influence racial and ethnic disparities in the use of prostate MRI.
Studies designed to evaluate germline and somatic genomic abnormalities in prostate cancer have come to the forefront in the last decade. Lynch syndrome is an inherited cancer syndrome of abnormalities in at least one of the commonly recognized mismatch repair genes: MLH1, MSH2, MSH6, or PMS2. While testing for mismatch repair deficiency is recommended in the advanced setting where systemic therapy is being considered, such testing outside of known family history is unclear in the setting of screening for prostate cancer. Bancroft et al compared PSA levels (and prostate cancer incidence) as part of baseline screening between carriers of one of mismatch repair genes (MLH1, MSH2, MSH6) and non-carrier controls. Carriers of MLH1 and MSH6 variants had a higher incidence of prostate cancer compared with controls. While not yet recommended for standard practice, further studies will be needed to verify these compelling results.
Racial and ethnic disparities in prostate cancer diagnosis and treatment are well recognized. Prostate magnetic resonance imaging (MRI) as part of a diagnostic approach for people with elevated PSA may decrease unnecessary biopsies or better direct biopsies based on results, and its use is increasing significantly. Abashidze et al examined Medicare claims between 2011 and 2017 to determine if racial disparities were present with respect to the use of prostate MRI. Compared with White men, Black, Asian, and Hispanic men were less likely to have a prostate MRI within 180 days after an elevated PSA (results stratified at 2.5, 4, or 10 ng/mL). The differences were most pronounced for patients with a PSA at 4 ng/mL or higher. The results suggest that providers should be aware of potential system and individual factors that influence racial and ethnic disparities in the use of prostate MRI.
Studies designed to evaluate germline and somatic genomic abnormalities in prostate cancer have come to the forefront in the last decade. Lynch syndrome is an inherited cancer syndrome of abnormalities in at least one of the commonly recognized mismatch repair genes: MLH1, MSH2, MSH6, or PMS2. While testing for mismatch repair deficiency is recommended in the advanced setting where systemic therapy is being considered, such testing outside of known family history is unclear in the setting of screening for prostate cancer. Bancroft et al compared PSA levels (and prostate cancer incidence) as part of baseline screening between carriers of one of mismatch repair genes (MLH1, MSH2, MSH6) and non-carrier controls. Carriers of MLH1 and MSH6 variants had a higher incidence of prostate cancer compared with controls. While not yet recommended for standard practice, further studies will be needed to verify these compelling results.
Racial and ethnic disparities in prostate cancer diagnosis and treatment are well recognized. Prostate magnetic resonance imaging (MRI) as part of a diagnostic approach for people with elevated PSA may decrease unnecessary biopsies or better direct biopsies based on results, and its use is increasing significantly. Abashidze et al examined Medicare claims between 2011 and 2017 to determine if racial disparities were present with respect to the use of prostate MRI. Compared with White men, Black, Asian, and Hispanic men were less likely to have a prostate MRI within 180 days after an elevated PSA (results stratified at 2.5, 4, or 10 ng/mL). The differences were most pronounced for patients with a PSA at 4 ng/mL or higher. The results suggest that providers should be aware of potential system and individual factors that influence racial and ethnic disparities in the use of prostate MRI.
Studies designed to evaluate germline and somatic genomic abnormalities in prostate cancer have come to the forefront in the last decade. Lynch syndrome is an inherited cancer syndrome of abnormalities in at least one of the commonly recognized mismatch repair genes: MLH1, MSH2, MSH6, or PMS2. While testing for mismatch repair deficiency is recommended in the advanced setting where systemic therapy is being considered, such testing outside of known family history is unclear in the setting of screening for prostate cancer. Bancroft et al compared PSA levels (and prostate cancer incidence) as part of baseline screening between carriers of one of mismatch repair genes (MLH1, MSH2, MSH6) and non-carrier controls. Carriers of MLH1 and MSH6 variants had a higher incidence of prostate cancer compared with controls. While not yet recommended for standard practice, further studies will be needed to verify these compelling results.
Vaping: Understand the risks
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
Intranasal vs. intramuscular naloxone in reversing opioid overdose
Background: Naloxone is an opioid antagonist that works to treat opioid overdose. Few randomized trials have assessed the efficacy of intranasal administration, whereas more data have been published supporting use of intramuscular naloxone. This prospective trial examines the ability of the same dose (800 mcg per 1 mL solution) of intranasal naloxone vs. intramuscular naloxone at managing opioid overdose.
Study design: Double-blind double-dummy randomized clinical trial.
Setting: Single supervised injection center in Sydney.
Synopsis: In this study, 197 participants with opioid overdose were randomized to intramuscular or intranasal naloxone. If the patient did not respond to either (GSC score less than 13, RR less than 10, or oxygen saturation less than 95%), a rescue dose of intramuscular naloxone was given. Participants who received the intramuscular naloxone were less likely to need the rescue dose (8.6% vs. 23.1%; odds ratio, 0.35; P = .002). The time to achieve an RR greater than 10 (15 vs. 8 minutes) and GSC score greater than 13 (17 vs. 8 minutes) was longer in the intranasal than the intramuscular group. Limitations include the setting of a controlled environment. Also, this protocol called for an initial 5 minutes of ventilation prior to randomization, which selected for more severe overdose cases in the overall study population. More studies are needed to assess efficacy in the field, needlestick injuries, and larger intranasal doses.
Bottom line: Intranasal naloxone effectively reverses opioid overdose but not as effectively as intramuscular naloxone at the same dose.
Citation: Dietze P et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: A randomized clinical trial. JAMA Netw Open. 2019;2:e1914977. doi: 1
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Naloxone is an opioid antagonist that works to treat opioid overdose. Few randomized trials have assessed the efficacy of intranasal administration, whereas more data have been published supporting use of intramuscular naloxone. This prospective trial examines the ability of the same dose (800 mcg per 1 mL solution) of intranasal naloxone vs. intramuscular naloxone at managing opioid overdose.
Study design: Double-blind double-dummy randomized clinical trial.
Setting: Single supervised injection center in Sydney.
Synopsis: In this study, 197 participants with opioid overdose were randomized to intramuscular or intranasal naloxone. If the patient did not respond to either (GSC score less than 13, RR less than 10, or oxygen saturation less than 95%), a rescue dose of intramuscular naloxone was given. Participants who received the intramuscular naloxone were less likely to need the rescue dose (8.6% vs. 23.1%; odds ratio, 0.35; P = .002). The time to achieve an RR greater than 10 (15 vs. 8 minutes) and GSC score greater than 13 (17 vs. 8 minutes) was longer in the intranasal than the intramuscular group. Limitations include the setting of a controlled environment. Also, this protocol called for an initial 5 minutes of ventilation prior to randomization, which selected for more severe overdose cases in the overall study population. More studies are needed to assess efficacy in the field, needlestick injuries, and larger intranasal doses.
Bottom line: Intranasal naloxone effectively reverses opioid overdose but not as effectively as intramuscular naloxone at the same dose.
Citation: Dietze P et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: A randomized clinical trial. JAMA Netw Open. 2019;2:e1914977. doi: 1
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Naloxone is an opioid antagonist that works to treat opioid overdose. Few randomized trials have assessed the efficacy of intranasal administration, whereas more data have been published supporting use of intramuscular naloxone. This prospective trial examines the ability of the same dose (800 mcg per 1 mL solution) of intranasal naloxone vs. intramuscular naloxone at managing opioid overdose.
Study design: Double-blind double-dummy randomized clinical trial.
Setting: Single supervised injection center in Sydney.
Synopsis: In this study, 197 participants with opioid overdose were randomized to intramuscular or intranasal naloxone. If the patient did not respond to either (GSC score less than 13, RR less than 10, or oxygen saturation less than 95%), a rescue dose of intramuscular naloxone was given. Participants who received the intramuscular naloxone were less likely to need the rescue dose (8.6% vs. 23.1%; odds ratio, 0.35; P = .002). The time to achieve an RR greater than 10 (15 vs. 8 minutes) and GSC score greater than 13 (17 vs. 8 minutes) was longer in the intranasal than the intramuscular group. Limitations include the setting of a controlled environment. Also, this protocol called for an initial 5 minutes of ventilation prior to randomization, which selected for more severe overdose cases in the overall study population. More studies are needed to assess efficacy in the field, needlestick injuries, and larger intranasal doses.
Bottom line: Intranasal naloxone effectively reverses opioid overdose but not as effectively as intramuscular naloxone at the same dose.
Citation: Dietze P et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: A randomized clinical trial. JAMA Netw Open. 2019;2:e1914977. doi: 1
Dr. Welter is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Big drop in U.S. cervical cancer rates, mortality in younger women
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Three drugs go head-to-head in advanced lung cancer study
The findings were reported in JAMA Network Open.
“Until recently, chemotherapy with platinum doublet was the standard first-line option for most patients with advanced NSCLC who did not have these genetic drivers or were not tested for them and remains the first choice in many parts of the world,” wrote the authors of the study which was led by Sreeram Ramagopalan, PhD, of F. Hoffmann-La Roche in Switzerland which funded the study.
Atezolizumab (Tecentriq, Genentech), which was approved in October by the U.S. Food and Drug Administration, is a monoclonal antibody that targets programmed cell death ligand 1 (PD-L1). It is also approved as monotherapy for patients with advanced NSCLC whose disease progressed despite treatment with platinum-based chemotherapy.
This is the first-known analysis that compares atezolizumab, nivolumab (Opdivo, Bristol Myers Squibb), and docetaxel (Taxotere, Sanofi) in patients outside of clinical trials, said Vivek Subbiah, MD, of MD Anderson Cancer Center and the study’s first author. “We have several new immune checkpoint inhibitors approved for treatment for NSCLC. Head-to-head comparison of the effectiveness of these agents in the real world are lacking,” he said.
Treatment with immune checkpoint inhibitors has shown improvement in the survival of patients with advanced NSCLC who failed chemotherapy treatment.
This study included 3,336 patients (mean age 67 years, 54.6% men) with advanced NSCLC who were treated with platinum-based chemotherapy. Data were collected from more than 1,000 clinics in the United States. Of the patients, 206 received atezolizumab, 500 received docetaxel, and 2,630 received nivolumab.
Patients were followed between May 2011 and March 2020. Atezolizumab and nivolumab showed a similar overall survival in these patients, but atezolizumab showed a longer overall survival, compared with docetaxel.
“Compared with docetaxel, atezolizumab was associated with significantly longer survival in the overall population and across all subgroups analyzed,” including patients with stage IIIB or IV cancer at diagnosis and nonsquamous NSCLC, the authors wrote. “Atezolizumab was associated with longer overall survival compared with docetaxel and was on par with nivolumab, supporting current clinical guidelines for systemic therapy for patients with advanced NSCLC in the U.S.”
Limitations of the study included its observational design and a small number of patients receiving atezolizumab. The authors suggested that studies using larger sample sizes are needed.
This study was funded by F. Hoffmann-La Roche. Genentech is a subsidiary of F. Hoffmann-La Roche.
The findings were reported in JAMA Network Open.
“Until recently, chemotherapy with platinum doublet was the standard first-line option for most patients with advanced NSCLC who did not have these genetic drivers or were not tested for them and remains the first choice in many parts of the world,” wrote the authors of the study which was led by Sreeram Ramagopalan, PhD, of F. Hoffmann-La Roche in Switzerland which funded the study.
Atezolizumab (Tecentriq, Genentech), which was approved in October by the U.S. Food and Drug Administration, is a monoclonal antibody that targets programmed cell death ligand 1 (PD-L1). It is also approved as monotherapy for patients with advanced NSCLC whose disease progressed despite treatment with platinum-based chemotherapy.
This is the first-known analysis that compares atezolizumab, nivolumab (Opdivo, Bristol Myers Squibb), and docetaxel (Taxotere, Sanofi) in patients outside of clinical trials, said Vivek Subbiah, MD, of MD Anderson Cancer Center and the study’s first author. “We have several new immune checkpoint inhibitors approved for treatment for NSCLC. Head-to-head comparison of the effectiveness of these agents in the real world are lacking,” he said.
Treatment with immune checkpoint inhibitors has shown improvement in the survival of patients with advanced NSCLC who failed chemotherapy treatment.
This study included 3,336 patients (mean age 67 years, 54.6% men) with advanced NSCLC who were treated with platinum-based chemotherapy. Data were collected from more than 1,000 clinics in the United States. Of the patients, 206 received atezolizumab, 500 received docetaxel, and 2,630 received nivolumab.
Patients were followed between May 2011 and March 2020. Atezolizumab and nivolumab showed a similar overall survival in these patients, but atezolizumab showed a longer overall survival, compared with docetaxel.
“Compared with docetaxel, atezolizumab was associated with significantly longer survival in the overall population and across all subgroups analyzed,” including patients with stage IIIB or IV cancer at diagnosis and nonsquamous NSCLC, the authors wrote. “Atezolizumab was associated with longer overall survival compared with docetaxel and was on par with nivolumab, supporting current clinical guidelines for systemic therapy for patients with advanced NSCLC in the U.S.”
Limitations of the study included its observational design and a small number of patients receiving atezolizumab. The authors suggested that studies using larger sample sizes are needed.
This study was funded by F. Hoffmann-La Roche. Genentech is a subsidiary of F. Hoffmann-La Roche.
The findings were reported in JAMA Network Open.
“Until recently, chemotherapy with platinum doublet was the standard first-line option for most patients with advanced NSCLC who did not have these genetic drivers or were not tested for them and remains the first choice in many parts of the world,” wrote the authors of the study which was led by Sreeram Ramagopalan, PhD, of F. Hoffmann-La Roche in Switzerland which funded the study.
Atezolizumab (Tecentriq, Genentech), which was approved in October by the U.S. Food and Drug Administration, is a monoclonal antibody that targets programmed cell death ligand 1 (PD-L1). It is also approved as monotherapy for patients with advanced NSCLC whose disease progressed despite treatment with platinum-based chemotherapy.
This is the first-known analysis that compares atezolizumab, nivolumab (Opdivo, Bristol Myers Squibb), and docetaxel (Taxotere, Sanofi) in patients outside of clinical trials, said Vivek Subbiah, MD, of MD Anderson Cancer Center and the study’s first author. “We have several new immune checkpoint inhibitors approved for treatment for NSCLC. Head-to-head comparison of the effectiveness of these agents in the real world are lacking,” he said.
Treatment with immune checkpoint inhibitors has shown improvement in the survival of patients with advanced NSCLC who failed chemotherapy treatment.
This study included 3,336 patients (mean age 67 years, 54.6% men) with advanced NSCLC who were treated with platinum-based chemotherapy. Data were collected from more than 1,000 clinics in the United States. Of the patients, 206 received atezolizumab, 500 received docetaxel, and 2,630 received nivolumab.
Patients were followed between May 2011 and March 2020. Atezolizumab and nivolumab showed a similar overall survival in these patients, but atezolizumab showed a longer overall survival, compared with docetaxel.
“Compared with docetaxel, atezolizumab was associated with significantly longer survival in the overall population and across all subgroups analyzed,” including patients with stage IIIB or IV cancer at diagnosis and nonsquamous NSCLC, the authors wrote. “Atezolizumab was associated with longer overall survival compared with docetaxel and was on par with nivolumab, supporting current clinical guidelines for systemic therapy for patients with advanced NSCLC in the U.S.”
Limitations of the study included its observational design and a small number of patients receiving atezolizumab. The authors suggested that studies using larger sample sizes are needed.
This study was funded by F. Hoffmann-La Roche. Genentech is a subsidiary of F. Hoffmann-La Roche.
FROM JAMA NETWORK OPEN