User login
COVID vaccines’ protection dropped sharply over 6 months: Study
, a study of almost 800,000 veterans found.
The study, published in the journal Science ., says the three vaccines offered about the same protection against the virus in March, when the Delta variant was first detected in the United States, but that changed 6 months later.
The Moderna two-dose vaccine went from being 89% effective in March to 58% effective in September, according to a story about the study in theLos Angeles Times.
Meanwhile, the Pfizer/BioNTech vaccine went from being 87% effective to 45% effective over the same time period.
The Johnson & Johnson vaccine showed the biggest drop -- from 86% effectiveness to 13% over those 6 months.
“In summary, although vaccination remains protective against SARS-CoV-2 infection, protection waned as the Delta variant emerged in the U.S., and this decline did not differ by age,” the study said.
The three vaccines also lost effectiveness in the ability to protect against death in veterans 65 and over after only 3 months, the Los Angeles Times reported.
Compared to unvaccinated veterans in that age group, veterans who got the Moderna vaccine and had a breakthrough case were 76% less likely to die of COVID-19 by July.
The protection was 70% for Pfizer/BioNTech vaccine recipients and 52% for J&J vaccine recipients for the same age group, compared to unvaccinated veterans, according to the newspaper.
For veterans under 65, the protectiveness against a fatal case of COVID was 84% for Pfizer/BioNTech recipients, 82% for Moderna recipients, and 73% for J&J recipients, compared to unvaccinated veterans in that age group.
The study confirms the need for booster vaccines and protective measures such as vaccine passports, vaccine mandates, masking, hand-washing, and social distancing, the researchers said.
Of the veterans studied, about 500,000 were vaccinated and 300,000 were not. Researchers noted that the study population had 6 times as many men as women. About 48% of the study group was 65 or older, 29% was 50-64, while 24% was under 50.
Researchers from the Public Health Institute in Oakland, the Veterans Affairs Medical Center in San Francisco, and the University of Texas Health Science Center conducted the study.
A version of this article first appeared on WebMD.com.
, a study of almost 800,000 veterans found.
The study, published in the journal Science ., says the three vaccines offered about the same protection against the virus in March, when the Delta variant was first detected in the United States, but that changed 6 months later.
The Moderna two-dose vaccine went from being 89% effective in March to 58% effective in September, according to a story about the study in theLos Angeles Times.
Meanwhile, the Pfizer/BioNTech vaccine went from being 87% effective to 45% effective over the same time period.
The Johnson & Johnson vaccine showed the biggest drop -- from 86% effectiveness to 13% over those 6 months.
“In summary, although vaccination remains protective against SARS-CoV-2 infection, protection waned as the Delta variant emerged in the U.S., and this decline did not differ by age,” the study said.
The three vaccines also lost effectiveness in the ability to protect against death in veterans 65 and over after only 3 months, the Los Angeles Times reported.
Compared to unvaccinated veterans in that age group, veterans who got the Moderna vaccine and had a breakthrough case were 76% less likely to die of COVID-19 by July.
The protection was 70% for Pfizer/BioNTech vaccine recipients and 52% for J&J vaccine recipients for the same age group, compared to unvaccinated veterans, according to the newspaper.
For veterans under 65, the protectiveness against a fatal case of COVID was 84% for Pfizer/BioNTech recipients, 82% for Moderna recipients, and 73% for J&J recipients, compared to unvaccinated veterans in that age group.
The study confirms the need for booster vaccines and protective measures such as vaccine passports, vaccine mandates, masking, hand-washing, and social distancing, the researchers said.
Of the veterans studied, about 500,000 were vaccinated and 300,000 were not. Researchers noted that the study population had 6 times as many men as women. About 48% of the study group was 65 or older, 29% was 50-64, while 24% was under 50.
Researchers from the Public Health Institute in Oakland, the Veterans Affairs Medical Center in San Francisco, and the University of Texas Health Science Center conducted the study.
A version of this article first appeared on WebMD.com.
, a study of almost 800,000 veterans found.
The study, published in the journal Science ., says the three vaccines offered about the same protection against the virus in March, when the Delta variant was first detected in the United States, but that changed 6 months later.
The Moderna two-dose vaccine went from being 89% effective in March to 58% effective in September, according to a story about the study in theLos Angeles Times.
Meanwhile, the Pfizer/BioNTech vaccine went from being 87% effective to 45% effective over the same time period.
The Johnson & Johnson vaccine showed the biggest drop -- from 86% effectiveness to 13% over those 6 months.
“In summary, although vaccination remains protective against SARS-CoV-2 infection, protection waned as the Delta variant emerged in the U.S., and this decline did not differ by age,” the study said.
The three vaccines also lost effectiveness in the ability to protect against death in veterans 65 and over after only 3 months, the Los Angeles Times reported.
Compared to unvaccinated veterans in that age group, veterans who got the Moderna vaccine and had a breakthrough case were 76% less likely to die of COVID-19 by July.
The protection was 70% for Pfizer/BioNTech vaccine recipients and 52% for J&J vaccine recipients for the same age group, compared to unvaccinated veterans, according to the newspaper.
For veterans under 65, the protectiveness against a fatal case of COVID was 84% for Pfizer/BioNTech recipients, 82% for Moderna recipients, and 73% for J&J recipients, compared to unvaccinated veterans in that age group.
The study confirms the need for booster vaccines and protective measures such as vaccine passports, vaccine mandates, masking, hand-washing, and social distancing, the researchers said.
Of the veterans studied, about 500,000 were vaccinated and 300,000 were not. Researchers noted that the study population had 6 times as many men as women. About 48% of the study group was 65 or older, 29% was 50-64, while 24% was under 50.
Researchers from the Public Health Institute in Oakland, the Veterans Affairs Medical Center in San Francisco, and the University of Texas Health Science Center conducted the study.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
Severe COVID two times higher for cancer patients
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
FROM MEDRXIV
New transmission information should motivate hospitals to reexamine aerosol procedures, researchers say
Two studies published in Thorax have found that the use of continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO) to treat moderate to severe COVID-19 is not linked to a heightened risk of infection, as currently thought. Researchers say hospitals should use this information to re-examine aerosol procedures in regard to risk of transmission of SARS-CoV-2.
CPAP and HFNO have been thought to generate virus particles capable of contaminating the air and surfaces, necessitating additional infection control precautions such as segregating patients. However, this research demonstrates that both methods produced little measurable air or surface viral contamination. The amount of contamination was no more than with the use of supplemental oxygen and less than that produced when breathing, speaking, or coughing.
In one study, led by a team from the North Bristol NHS Trust, 25 healthy volunteers and eight hospitalized patients with COVID-19 were recruited and asked to breathe, speak, and cough in ultra-clean, laminar flow theaters followed by use of CPAP and HFNO. Aerosol emission was measured via two methodologies, simultaneously. Hospitalized patients with COVID-19 had cough recorded via the same methodology on the infectious diseases ward.
CPAP (with exhalation port filter) was found to produce less aerosol than breathing, speaking, and coughing, even with large > 50 L/min face mask leaks. Coughing was associated with the highest aerosol emissions of any recorded activity.
HFNO was associated with aerosol emission from the machine. Generated particles were small (< 1 mcm), passing from the machine through the patient and to the detector without coalescence with respiratory aerosol, and, consequently, would be unlikely to carry viral particles.
More aerosol was generated in cough from patients with COVID-19 (n = 8) than from volunteers.
In the second study, 30 hospitalized patients with COVID-19 requiring supplemental oxygen were prospectively enrolled. In this observational environmental sampling study, participants received either supplemental oxygen, CPAP, or HFNO (n = 10 in each group). A nasopharyngeal swab, three air, and three surface samples were collected from each participant and the clinical environment.
Overall, 21 of the 30 participants tested positive for SARS-CoV-2 RNA in the nasopharynx. In contrast, 4 out of 90 air samples and 6 of 90 surface samples tested positive for viral RNA, although there were an additional 10 suspected-positive samples in both air and surfaces samples.
Neither the use of CPAP nor HFNO nor coughing were associated with significantly more environmental contamination than supplemental oxygen use. Of the total positive or suspected-positive samples by viral PCR detection, only one nasopharynx sample from an HFNO patient was biologically viable in cell culture assay.
“Our findings show that the noninvasive breathing support methods do not pose a higher risk of transmitting infection, which has significant implications for the management of the patients,” said coauthor Danny McAuley, MD.
“If there isn’t a higher risk of infection transmission, current practices may be overcautious measures for certain settings, for example preventing relatives visiting the sickest patients, whilst underestimating the risk in other settings, such as coughing patients with early infection on general wards.”
Although both studies are small, the results do suggest that there is a need for an evidence-based reassessment of infection prevention and control measures for noninvasive respiratory support treatments that are currently considered aerosol generating procedures.
A version of this article first appeared on Univadis.com.
Two studies published in Thorax have found that the use of continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO) to treat moderate to severe COVID-19 is not linked to a heightened risk of infection, as currently thought. Researchers say hospitals should use this information to re-examine aerosol procedures in regard to risk of transmission of SARS-CoV-2.
CPAP and HFNO have been thought to generate virus particles capable of contaminating the air and surfaces, necessitating additional infection control precautions such as segregating patients. However, this research demonstrates that both methods produced little measurable air or surface viral contamination. The amount of contamination was no more than with the use of supplemental oxygen and less than that produced when breathing, speaking, or coughing.
In one study, led by a team from the North Bristol NHS Trust, 25 healthy volunteers and eight hospitalized patients with COVID-19 were recruited and asked to breathe, speak, and cough in ultra-clean, laminar flow theaters followed by use of CPAP and HFNO. Aerosol emission was measured via two methodologies, simultaneously. Hospitalized patients with COVID-19 had cough recorded via the same methodology on the infectious diseases ward.
CPAP (with exhalation port filter) was found to produce less aerosol than breathing, speaking, and coughing, even with large > 50 L/min face mask leaks. Coughing was associated with the highest aerosol emissions of any recorded activity.
HFNO was associated with aerosol emission from the machine. Generated particles were small (< 1 mcm), passing from the machine through the patient and to the detector without coalescence with respiratory aerosol, and, consequently, would be unlikely to carry viral particles.
More aerosol was generated in cough from patients with COVID-19 (n = 8) than from volunteers.
In the second study, 30 hospitalized patients with COVID-19 requiring supplemental oxygen were prospectively enrolled. In this observational environmental sampling study, participants received either supplemental oxygen, CPAP, or HFNO (n = 10 in each group). A nasopharyngeal swab, three air, and three surface samples were collected from each participant and the clinical environment.
Overall, 21 of the 30 participants tested positive for SARS-CoV-2 RNA in the nasopharynx. In contrast, 4 out of 90 air samples and 6 of 90 surface samples tested positive for viral RNA, although there were an additional 10 suspected-positive samples in both air and surfaces samples.
Neither the use of CPAP nor HFNO nor coughing were associated with significantly more environmental contamination than supplemental oxygen use. Of the total positive or suspected-positive samples by viral PCR detection, only one nasopharynx sample from an HFNO patient was biologically viable in cell culture assay.
“Our findings show that the noninvasive breathing support methods do not pose a higher risk of transmitting infection, which has significant implications for the management of the patients,” said coauthor Danny McAuley, MD.
“If there isn’t a higher risk of infection transmission, current practices may be overcautious measures for certain settings, for example preventing relatives visiting the sickest patients, whilst underestimating the risk in other settings, such as coughing patients with early infection on general wards.”
Although both studies are small, the results do suggest that there is a need for an evidence-based reassessment of infection prevention and control measures for noninvasive respiratory support treatments that are currently considered aerosol generating procedures.
A version of this article first appeared on Univadis.com.
Two studies published in Thorax have found that the use of continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO) to treat moderate to severe COVID-19 is not linked to a heightened risk of infection, as currently thought. Researchers say hospitals should use this information to re-examine aerosol procedures in regard to risk of transmission of SARS-CoV-2.
CPAP and HFNO have been thought to generate virus particles capable of contaminating the air and surfaces, necessitating additional infection control precautions such as segregating patients. However, this research demonstrates that both methods produced little measurable air or surface viral contamination. The amount of contamination was no more than with the use of supplemental oxygen and less than that produced when breathing, speaking, or coughing.
In one study, led by a team from the North Bristol NHS Trust, 25 healthy volunteers and eight hospitalized patients with COVID-19 were recruited and asked to breathe, speak, and cough in ultra-clean, laminar flow theaters followed by use of CPAP and HFNO. Aerosol emission was measured via two methodologies, simultaneously. Hospitalized patients with COVID-19 had cough recorded via the same methodology on the infectious diseases ward.
CPAP (with exhalation port filter) was found to produce less aerosol than breathing, speaking, and coughing, even with large > 50 L/min face mask leaks. Coughing was associated with the highest aerosol emissions of any recorded activity.
HFNO was associated with aerosol emission from the machine. Generated particles were small (< 1 mcm), passing from the machine through the patient and to the detector without coalescence with respiratory aerosol, and, consequently, would be unlikely to carry viral particles.
More aerosol was generated in cough from patients with COVID-19 (n = 8) than from volunteers.
In the second study, 30 hospitalized patients with COVID-19 requiring supplemental oxygen were prospectively enrolled. In this observational environmental sampling study, participants received either supplemental oxygen, CPAP, or HFNO (n = 10 in each group). A nasopharyngeal swab, three air, and three surface samples were collected from each participant and the clinical environment.
Overall, 21 of the 30 participants tested positive for SARS-CoV-2 RNA in the nasopharynx. In contrast, 4 out of 90 air samples and 6 of 90 surface samples tested positive for viral RNA, although there were an additional 10 suspected-positive samples in both air and surfaces samples.
Neither the use of CPAP nor HFNO nor coughing were associated with significantly more environmental contamination than supplemental oxygen use. Of the total positive or suspected-positive samples by viral PCR detection, only one nasopharynx sample from an HFNO patient was biologically viable in cell culture assay.
“Our findings show that the noninvasive breathing support methods do not pose a higher risk of transmitting infection, which has significant implications for the management of the patients,” said coauthor Danny McAuley, MD.
“If there isn’t a higher risk of infection transmission, current practices may be overcautious measures for certain settings, for example preventing relatives visiting the sickest patients, whilst underestimating the risk in other settings, such as coughing patients with early infection on general wards.”
Although both studies are small, the results do suggest that there is a need for an evidence-based reassessment of infection prevention and control measures for noninvasive respiratory support treatments that are currently considered aerosol generating procedures.
A version of this article first appeared on Univadis.com.
FROM THORAX
Decades spent searching for genes linked to rare blood cancer
Mary Lou McMaster, MD, has spent her entire career at the National Cancer Institute (NCI) searching for the genetic underpinnings that give rise to Waldenstrom's macroglobulinemia (WM).
After searching for decades, she has yet to uncover a "smoking gun," though a few tantalizing clues have emerged along the way.
"Our questions are pretty basic: Why are some people more susceptible to developing WM, and why does WM sometimes cluster in families?" she explained. It turns out that the answers are not at all simple.
Dr. McMaster described some of the clues that her team at the Clinical Genetics Branch of the NCI has unearthed in a presentation at the recent International Waldenstrom's Macroglobulinemia Foundation (IWMF) 2021 Virtual Educational Forum.
Commenting after the presentation, Steven Treon, MD, PhD, professor of medicine, Harvard Medical School, Boston, who is collaborating with Dr. McMaster on this work, said: "From these familial studies, we can learn how familial genomics may give us insights into disease prevention and treatment."
Identifying affected families
Work began in 2001 to identify families in which two or more family members had been diagnosed with WM or in which there was one patient with WM and at least one other relative with a related B-cell cancer, such as chronic lymphocytic leukemia.
For a frame of reference, they enrolled some families with only one member with WM and in which there was no known family history of the disease.
"Overall, we have learned that familial WM is a rare disease but not nearly as rare as we first thought," Dr. McMaster said.
For example, in a referral hospital setting, 5% of WM patients will report having a family member with the same disorder, and up to 20% of WM patients report having a family member with a related but different B-cell cancer, she noted.
NCI researchers also discovered that environmental factors contribute to the development of WM. Notable chemical or occupational exposures include exposures to pesticides, herbicides, and fertilizers. Infections and autoimmune disease are additional factors.
"This was not a surprise," Dr. McMaster commented regarding the role of occupational exposures. The research community has known for decades that a "lymphoma belt" cuts through the Midwest farming states.
Focusing on genetic susceptibility, Dr. McMaster and colleagues first tried to identify a rare germline variant that can be passed down to offspring and that might confer high risk for the disease.
"We used our high-risk families to study these types of changes, although they may be modified by other genes and environmental factors," Dr. McMaster explained.
Much to their collective disappointment, the research team has been unable to identify any rare germline variant that could account for WM in many families. What they did find were many small changes in genes that are known to be important in B-cell development and function, but all of those would lead to only a small increase in WM risk.
"What is holding us back is that, so far, we are not seeing the same gene affected in more than one family, so this suggests to us either that this is not the mechanism behind the development of WM in families, or we have an unfortunate situation where each family is going to have a genetic change that is private to that family and which is not found in other families," Dr. McMaster acknowledged.
Sheer difficulty
Given the difficulty of determining whether these small genetic changes had any detrimental functional effect in each and every family with a member who had WM, Dr. McMaster and colleagues have now turned their attention to genes that exert only a small effect on disease risk.
"Here, we focused on specific genes that we knew were important in the function of the immune system," she explained. "We did find a few genes that may contribute to risk, but those have not yet been confirmed by us or others, and we cannot say they are causative without that confirmation," she said.
The team has gone on to scan the highway of our genetic material so as to isolate genetic "mile markers." They then examine the area around a particular marker that they suspect contains genes that may be involved in WM.
One study they conducted involved a cohort of 217 patients with WM in which numerous family members had WM and so was enriched with susceptibility genes. A second cohort comprised 312 WM patients in which there were few WM cases among family members. Both of these cohorts were compared with a group of healthy control persons.
From these genome studies, "we found there are at least two regions of the genome that can contribute to WM susceptibility, the largest effect being on the short arm of chromosome 6, and the other on the long arm of chromosome 14," Dr. McMaster reported. Dr. McMaster feels that there are probably more regions on the genome that also contribute to WM, although they do not yet understand how these regions contribute to susceptibility.
"It's more evidence that WM likely results from a combination of events rather than one single gene variant," she observed. Dr. McMaster and colleagues are now collaborating with a large consortium of WM researchers to confirm and extend their findings. Plans are underway to analyze data from approximately 1,350 WM patients and more than 20,000 control persons within the next year.
"Our hope is that we will confirm our original findings and, because we now have a much larger sample, we will be able to discover additional regions of the genome that are contributing to susceptibility," Dr. McMaster said.
"A single gene is not likely to account for all WM, as we've looked carefully and others have looked too," she commented.
"So the risk for WM depends on a combination of genes and environmental exposures and possibly lifestyle factors as well, although we still estimate that approximately 25% of the heritability of WM can be attributed to these kinds of genetic changes," Dr. McMaster predicted.
Dr. McMaster has disclosed no relevant financial relationships. Dr. Treon has served as a director, officer, partner, employee, advisor, consultant, or trustee for Janssen, Pfizer, PCYC, and BioGene.
A version of this article first appeared on Medscape.com
Mary Lou McMaster, MD, has spent her entire career at the National Cancer Institute (NCI) searching for the genetic underpinnings that give rise to Waldenstrom's macroglobulinemia (WM).
After searching for decades, she has yet to uncover a "smoking gun," though a few tantalizing clues have emerged along the way.
"Our questions are pretty basic: Why are some people more susceptible to developing WM, and why does WM sometimes cluster in families?" she explained. It turns out that the answers are not at all simple.
Dr. McMaster described some of the clues that her team at the Clinical Genetics Branch of the NCI has unearthed in a presentation at the recent International Waldenstrom's Macroglobulinemia Foundation (IWMF) 2021 Virtual Educational Forum.
Commenting after the presentation, Steven Treon, MD, PhD, professor of medicine, Harvard Medical School, Boston, who is collaborating with Dr. McMaster on this work, said: "From these familial studies, we can learn how familial genomics may give us insights into disease prevention and treatment."
Identifying affected families
Work began in 2001 to identify families in which two or more family members had been diagnosed with WM or in which there was one patient with WM and at least one other relative with a related B-cell cancer, such as chronic lymphocytic leukemia.
For a frame of reference, they enrolled some families with only one member with WM and in which there was no known family history of the disease.
"Overall, we have learned that familial WM is a rare disease but not nearly as rare as we first thought," Dr. McMaster said.
For example, in a referral hospital setting, 5% of WM patients will report having a family member with the same disorder, and up to 20% of WM patients report having a family member with a related but different B-cell cancer, she noted.
NCI researchers also discovered that environmental factors contribute to the development of WM. Notable chemical or occupational exposures include exposures to pesticides, herbicides, and fertilizers. Infections and autoimmune disease are additional factors.
"This was not a surprise," Dr. McMaster commented regarding the role of occupational exposures. The research community has known for decades that a "lymphoma belt" cuts through the Midwest farming states.
Focusing on genetic susceptibility, Dr. McMaster and colleagues first tried to identify a rare germline variant that can be passed down to offspring and that might confer high risk for the disease.
"We used our high-risk families to study these types of changes, although they may be modified by other genes and environmental factors," Dr. McMaster explained.
Much to their collective disappointment, the research team has been unable to identify any rare germline variant that could account for WM in many families. What they did find were many small changes in genes that are known to be important in B-cell development and function, but all of those would lead to only a small increase in WM risk.
"What is holding us back is that, so far, we are not seeing the same gene affected in more than one family, so this suggests to us either that this is not the mechanism behind the development of WM in families, or we have an unfortunate situation where each family is going to have a genetic change that is private to that family and which is not found in other families," Dr. McMaster acknowledged.
Sheer difficulty
Given the difficulty of determining whether these small genetic changes had any detrimental functional effect in each and every family with a member who had WM, Dr. McMaster and colleagues have now turned their attention to genes that exert only a small effect on disease risk.
"Here, we focused on specific genes that we knew were important in the function of the immune system," she explained. "We did find a few genes that may contribute to risk, but those have not yet been confirmed by us or others, and we cannot say they are causative without that confirmation," she said.
The team has gone on to scan the highway of our genetic material so as to isolate genetic "mile markers." They then examine the area around a particular marker that they suspect contains genes that may be involved in WM.
One study they conducted involved a cohort of 217 patients with WM in which numerous family members had WM and so was enriched with susceptibility genes. A second cohort comprised 312 WM patients in which there were few WM cases among family members. Both of these cohorts were compared with a group of healthy control persons.
From these genome studies, "we found there are at least two regions of the genome that can contribute to WM susceptibility, the largest effect being on the short arm of chromosome 6, and the other on the long arm of chromosome 14," Dr. McMaster reported. Dr. McMaster feels that there are probably more regions on the genome that also contribute to WM, although they do not yet understand how these regions contribute to susceptibility.
"It's more evidence that WM likely results from a combination of events rather than one single gene variant," she observed. Dr. McMaster and colleagues are now collaborating with a large consortium of WM researchers to confirm and extend their findings. Plans are underway to analyze data from approximately 1,350 WM patients and more than 20,000 control persons within the next year.
"Our hope is that we will confirm our original findings and, because we now have a much larger sample, we will be able to discover additional regions of the genome that are contributing to susceptibility," Dr. McMaster said.
"A single gene is not likely to account for all WM, as we've looked carefully and others have looked too," she commented.
"So the risk for WM depends on a combination of genes and environmental exposures and possibly lifestyle factors as well, although we still estimate that approximately 25% of the heritability of WM can be attributed to these kinds of genetic changes," Dr. McMaster predicted.
Dr. McMaster has disclosed no relevant financial relationships. Dr. Treon has served as a director, officer, partner, employee, advisor, consultant, or trustee for Janssen, Pfizer, PCYC, and BioGene.
A version of this article first appeared on Medscape.com
Mary Lou McMaster, MD, has spent her entire career at the National Cancer Institute (NCI) searching for the genetic underpinnings that give rise to Waldenstrom's macroglobulinemia (WM).
After searching for decades, she has yet to uncover a "smoking gun," though a few tantalizing clues have emerged along the way.
"Our questions are pretty basic: Why are some people more susceptible to developing WM, and why does WM sometimes cluster in families?" she explained. It turns out that the answers are not at all simple.
Dr. McMaster described some of the clues that her team at the Clinical Genetics Branch of the NCI has unearthed in a presentation at the recent International Waldenstrom's Macroglobulinemia Foundation (IWMF) 2021 Virtual Educational Forum.
Commenting after the presentation, Steven Treon, MD, PhD, professor of medicine, Harvard Medical School, Boston, who is collaborating with Dr. McMaster on this work, said: "From these familial studies, we can learn how familial genomics may give us insights into disease prevention and treatment."
Identifying affected families
Work began in 2001 to identify families in which two or more family members had been diagnosed with WM or in which there was one patient with WM and at least one other relative with a related B-cell cancer, such as chronic lymphocytic leukemia.
For a frame of reference, they enrolled some families with only one member with WM and in which there was no known family history of the disease.
"Overall, we have learned that familial WM is a rare disease but not nearly as rare as we first thought," Dr. McMaster said.
For example, in a referral hospital setting, 5% of WM patients will report having a family member with the same disorder, and up to 20% of WM patients report having a family member with a related but different B-cell cancer, she noted.
NCI researchers also discovered that environmental factors contribute to the development of WM. Notable chemical or occupational exposures include exposures to pesticides, herbicides, and fertilizers. Infections and autoimmune disease are additional factors.
"This was not a surprise," Dr. McMaster commented regarding the role of occupational exposures. The research community has known for decades that a "lymphoma belt" cuts through the Midwest farming states.
Focusing on genetic susceptibility, Dr. McMaster and colleagues first tried to identify a rare germline variant that can be passed down to offspring and that might confer high risk for the disease.
"We used our high-risk families to study these types of changes, although they may be modified by other genes and environmental factors," Dr. McMaster explained.
Much to their collective disappointment, the research team has been unable to identify any rare germline variant that could account for WM in many families. What they did find were many small changes in genes that are known to be important in B-cell development and function, but all of those would lead to only a small increase in WM risk.
"What is holding us back is that, so far, we are not seeing the same gene affected in more than one family, so this suggests to us either that this is not the mechanism behind the development of WM in families, or we have an unfortunate situation where each family is going to have a genetic change that is private to that family and which is not found in other families," Dr. McMaster acknowledged.
Sheer difficulty
Given the difficulty of determining whether these small genetic changes had any detrimental functional effect in each and every family with a member who had WM, Dr. McMaster and colleagues have now turned their attention to genes that exert only a small effect on disease risk.
"Here, we focused on specific genes that we knew were important in the function of the immune system," she explained. "We did find a few genes that may contribute to risk, but those have not yet been confirmed by us or others, and we cannot say they are causative without that confirmation," she said.
The team has gone on to scan the highway of our genetic material so as to isolate genetic "mile markers." They then examine the area around a particular marker that they suspect contains genes that may be involved in WM.
One study they conducted involved a cohort of 217 patients with WM in which numerous family members had WM and so was enriched with susceptibility genes. A second cohort comprised 312 WM patients in which there were few WM cases among family members. Both of these cohorts were compared with a group of healthy control persons.
From these genome studies, "we found there are at least two regions of the genome that can contribute to WM susceptibility, the largest effect being on the short arm of chromosome 6, and the other on the long arm of chromosome 14," Dr. McMaster reported. Dr. McMaster feels that there are probably more regions on the genome that also contribute to WM, although they do not yet understand how these regions contribute to susceptibility.
"It's more evidence that WM likely results from a combination of events rather than one single gene variant," she observed. Dr. McMaster and colleagues are now collaborating with a large consortium of WM researchers to confirm and extend their findings. Plans are underway to analyze data from approximately 1,350 WM patients and more than 20,000 control persons within the next year.
"Our hope is that we will confirm our original findings and, because we now have a much larger sample, we will be able to discover additional regions of the genome that are contributing to susceptibility," Dr. McMaster said.
"A single gene is not likely to account for all WM, as we've looked carefully and others have looked too," she commented.
"So the risk for WM depends on a combination of genes and environmental exposures and possibly lifestyle factors as well, although we still estimate that approximately 25% of the heritability of WM can be attributed to these kinds of genetic changes," Dr. McMaster predicted.
Dr. McMaster has disclosed no relevant financial relationships. Dr. Treon has served as a director, officer, partner, employee, advisor, consultant, or trustee for Janssen, Pfizer, PCYC, and BioGene.
A version of this article first appeared on Medscape.com
Erratum (Cutis. 2021;108:181-184, 202)
Kowtoniuk RA, Liu YE, Jeter JP. Cutaneous cold weather injuries in the US Military. Cutis. 2021;108:181-184, 202. doi:10.12788/cutis.0363
In the article above from the October 2021 issue, an author’s name was spelled incorrectly. The correct byline appears below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Robert A. Kowtoniuk, DO; Yizhen E. Liu, MD; Jonathan P. Jeter, MD
Kowtoniuk RA, Liu YE, Jeter JP. Cutaneous cold weather injuries in the US Military. Cutis. 2021;108:181-184, 202. doi:10.12788/cutis.0363
In the article above from the October 2021 issue, an author’s name was spelled incorrectly. The correct byline appears below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Robert A. Kowtoniuk, DO; Yizhen E. Liu, MD; Jonathan P. Jeter, MD
Kowtoniuk RA, Liu YE, Jeter JP. Cutaneous cold weather injuries in the US Military. Cutis. 2021;108:181-184, 202. doi:10.12788/cutis.0363
In the article above from the October 2021 issue, an author’s name was spelled incorrectly. The correct byline appears below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Robert A. Kowtoniuk, DO; Yizhen E. Liu, MD; Jonathan P. Jeter, MD
Rituximab improves systemic sclerosis skin, lung symptoms
Rituximab effectively reduced skin sclerosis and appeared to have a beneficial effect on interstitial lung disease (ILD) for patients with systemic sclerosis (SSc) in a randomized, clinical trial.
At 24 weeks’ follow-up, there was significant improvement in total skin thickness scores among patients who received four once-weekly rituximab infusions, compared with patients who received placebo infusions. Among patients who received rituximab, there were also small but significant improvements in percentage of forced vital capacity (FVC). Among patients who received placebo, FVC worsened, reported Ayumi Yoshizaki, MD, of the University of Tokyo and colleagues.
“Systemic sclerosis is considered to have high unmet medical needs because of its poor prognosis and the lack of satisfactory and effective treatments,” he said at the virtual annual meeting of the American College of Rheumatology.
“Several clinical studies have suggested that B-cell depletion therapy with rituximab anti-CD20 antibody is effective in treating skin and lung fibrosis of SSc. However, no randomized, placebo-controlled trial has been able to confirm the efficacy of rituximab in SSc,” Dr. Yoshizaki said.
A rheumatologist who is currently conducting an investigator-initiated trial in which patients with SSC are undergoing treatment with rituximab followed by belimumab (Benlysta) said in an interview that he found the data to be “super interesting.”
“There are a lot of reasons to think that B cells might be important in systemic sclerosis, and actually that’s why our group had previously done an investigator-initiated trial with belimumab years ago,” said Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York.
Randomized trial
Dr. Yoshizaki and colleagues conducted the randomized, placebo-controlled DESIRES trial in four hospitals in Japan to evaluate the safety and efficacy of rituximab for the treatment of SSc.
In the investigator-initiated trial, patients aged 20-79 years who fulfilled ACR and European Alliance of Associations for Rheumatology classification criteria for systemic sclerosis and who had a modified Rodnan Skin Score (mRSS) of 10 or more and a life expectancy of at least 6 months were randomly assigned to receive infusions with either rituximab 375 mg/m2 or placebo once weekly for 4 weeks. Patients and clinicians were masked to treatment allocation.
The trial included 56 patients (51 women, 5 men). Of all patients enrolled, 27 of 28 who were allocated to receive rituximab and 22 of 28 who were allocated to receive placebo underwent at least one infusion and completed 24 weeks of follow-up.
The absolute change in mRSS at 24 weeks after the start of therapy, the primary endpoint, was –6.30 in the rituximab group, compared with +2.14 in the placebo group, a difference of –8.44 (P < .0001).
In a subgroup analysis, rituximab was superior to placebo regardless of disease duration, disease type (diffuse cutaneous or limited cutaneous SSc), prior receipt of systemic corticosteroids or immunosuppressants, or having C-reactive protein levels less than 0.3 mg/dL or at least 0.3 mg/dL.
However, there was no significant benefit with rituximab for patients with baseline mRSS of at least 20 or for those without ILD at baseline.
There was also evidence that rituximab reduced lung fibrosis. For patients assigned to the active drug, the absolute change in FVC at 24 weeks was +0.09% of the predicted value, compared with –3.56% for patients who received placebo (P = .044).
The researchers also observed radiographic evidence of lung improvement. The absolute change in the percentage of lung field occupied with interstitial shadows was –0.32% in the rituximab arm versus +2.39% in the placebo arm (P = .034). There was no significant between-group difference in the absolute change in diffusing capacity of lung for carbon monoxide, however.
Adverse events that occurred more frequently with rituximab included oral mucositis, diarrhea, and decreased neutrophil and white blood cell counts.
Convincing results
“What I thought the Japanese study did was to give a much more convincing proof of concept than has been out there,” Dr. Spiera said in an interview.
“There have been some preliminary experiences that have been encouraging with rituximab in scleroderma, most of which has been open label,” he said.
He also referred to a retrospective study by EUSTAR, the European Scleroderma Trials and Research group, which indicated that patients who had previously received rituximab seemed to have had better outcomes than patients who had been treated with other therapies.
Dr. Spiera added that, although he was glad to see the data from a randomized, placebo-controlled trial in this population, he was uncomfortable with the idea of leaving patients untreated for 6 months.
“From the standpoint of somebody wanting to know what strategies might be promising, this is great for us, but I would not have designed the trial that way,” he said.
The study results were previously published in the Lancet Rheumatology.
The study was supported by grants from the Japan Agency for Medical Research and Development and Zenyaku Kogyo. Dr. Yoshizaki disclosed no relevant financial relationships. Dr. Spiera has received grant/research support from and has consulted for Roche/Genentech, maker of rituximab, and has received compensation from other companies.
A version of this article first appeared on Medscape.com.
Rituximab effectively reduced skin sclerosis and appeared to have a beneficial effect on interstitial lung disease (ILD) for patients with systemic sclerosis (SSc) in a randomized, clinical trial.
At 24 weeks’ follow-up, there was significant improvement in total skin thickness scores among patients who received four once-weekly rituximab infusions, compared with patients who received placebo infusions. Among patients who received rituximab, there were also small but significant improvements in percentage of forced vital capacity (FVC). Among patients who received placebo, FVC worsened, reported Ayumi Yoshizaki, MD, of the University of Tokyo and colleagues.
“Systemic sclerosis is considered to have high unmet medical needs because of its poor prognosis and the lack of satisfactory and effective treatments,” he said at the virtual annual meeting of the American College of Rheumatology.
“Several clinical studies have suggested that B-cell depletion therapy with rituximab anti-CD20 antibody is effective in treating skin and lung fibrosis of SSc. However, no randomized, placebo-controlled trial has been able to confirm the efficacy of rituximab in SSc,” Dr. Yoshizaki said.
A rheumatologist who is currently conducting an investigator-initiated trial in which patients with SSC are undergoing treatment with rituximab followed by belimumab (Benlysta) said in an interview that he found the data to be “super interesting.”
“There are a lot of reasons to think that B cells might be important in systemic sclerosis, and actually that’s why our group had previously done an investigator-initiated trial with belimumab years ago,” said Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York.
Randomized trial
Dr. Yoshizaki and colleagues conducted the randomized, placebo-controlled DESIRES trial in four hospitals in Japan to evaluate the safety and efficacy of rituximab for the treatment of SSc.
In the investigator-initiated trial, patients aged 20-79 years who fulfilled ACR and European Alliance of Associations for Rheumatology classification criteria for systemic sclerosis and who had a modified Rodnan Skin Score (mRSS) of 10 or more and a life expectancy of at least 6 months were randomly assigned to receive infusions with either rituximab 375 mg/m2 or placebo once weekly for 4 weeks. Patients and clinicians were masked to treatment allocation.
The trial included 56 patients (51 women, 5 men). Of all patients enrolled, 27 of 28 who were allocated to receive rituximab and 22 of 28 who were allocated to receive placebo underwent at least one infusion and completed 24 weeks of follow-up.
The absolute change in mRSS at 24 weeks after the start of therapy, the primary endpoint, was –6.30 in the rituximab group, compared with +2.14 in the placebo group, a difference of –8.44 (P < .0001).
In a subgroup analysis, rituximab was superior to placebo regardless of disease duration, disease type (diffuse cutaneous or limited cutaneous SSc), prior receipt of systemic corticosteroids or immunosuppressants, or having C-reactive protein levels less than 0.3 mg/dL or at least 0.3 mg/dL.
However, there was no significant benefit with rituximab for patients with baseline mRSS of at least 20 or for those without ILD at baseline.
There was also evidence that rituximab reduced lung fibrosis. For patients assigned to the active drug, the absolute change in FVC at 24 weeks was +0.09% of the predicted value, compared with –3.56% for patients who received placebo (P = .044).
The researchers also observed radiographic evidence of lung improvement. The absolute change in the percentage of lung field occupied with interstitial shadows was –0.32% in the rituximab arm versus +2.39% in the placebo arm (P = .034). There was no significant between-group difference in the absolute change in diffusing capacity of lung for carbon monoxide, however.
Adverse events that occurred more frequently with rituximab included oral mucositis, diarrhea, and decreased neutrophil and white blood cell counts.
Convincing results
“What I thought the Japanese study did was to give a much more convincing proof of concept than has been out there,” Dr. Spiera said in an interview.
“There have been some preliminary experiences that have been encouraging with rituximab in scleroderma, most of which has been open label,” he said.
He also referred to a retrospective study by EUSTAR, the European Scleroderma Trials and Research group, which indicated that patients who had previously received rituximab seemed to have had better outcomes than patients who had been treated with other therapies.
Dr. Spiera added that, although he was glad to see the data from a randomized, placebo-controlled trial in this population, he was uncomfortable with the idea of leaving patients untreated for 6 months.
“From the standpoint of somebody wanting to know what strategies might be promising, this is great for us, but I would not have designed the trial that way,” he said.
The study results were previously published in the Lancet Rheumatology.
The study was supported by grants from the Japan Agency for Medical Research and Development and Zenyaku Kogyo. Dr. Yoshizaki disclosed no relevant financial relationships. Dr. Spiera has received grant/research support from and has consulted for Roche/Genentech, maker of rituximab, and has received compensation from other companies.
A version of this article first appeared on Medscape.com.
Rituximab effectively reduced skin sclerosis and appeared to have a beneficial effect on interstitial lung disease (ILD) for patients with systemic sclerosis (SSc) in a randomized, clinical trial.
At 24 weeks’ follow-up, there was significant improvement in total skin thickness scores among patients who received four once-weekly rituximab infusions, compared with patients who received placebo infusions. Among patients who received rituximab, there were also small but significant improvements in percentage of forced vital capacity (FVC). Among patients who received placebo, FVC worsened, reported Ayumi Yoshizaki, MD, of the University of Tokyo and colleagues.
“Systemic sclerosis is considered to have high unmet medical needs because of its poor prognosis and the lack of satisfactory and effective treatments,” he said at the virtual annual meeting of the American College of Rheumatology.
“Several clinical studies have suggested that B-cell depletion therapy with rituximab anti-CD20 antibody is effective in treating skin and lung fibrosis of SSc. However, no randomized, placebo-controlled trial has been able to confirm the efficacy of rituximab in SSc,” Dr. Yoshizaki said.
A rheumatologist who is currently conducting an investigator-initiated trial in which patients with SSC are undergoing treatment with rituximab followed by belimumab (Benlysta) said in an interview that he found the data to be “super interesting.”
“There are a lot of reasons to think that B cells might be important in systemic sclerosis, and actually that’s why our group had previously done an investigator-initiated trial with belimumab years ago,” said Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York.
Randomized trial
Dr. Yoshizaki and colleagues conducted the randomized, placebo-controlled DESIRES trial in four hospitals in Japan to evaluate the safety and efficacy of rituximab for the treatment of SSc.
In the investigator-initiated trial, patients aged 20-79 years who fulfilled ACR and European Alliance of Associations for Rheumatology classification criteria for systemic sclerosis and who had a modified Rodnan Skin Score (mRSS) of 10 or more and a life expectancy of at least 6 months were randomly assigned to receive infusions with either rituximab 375 mg/m2 or placebo once weekly for 4 weeks. Patients and clinicians were masked to treatment allocation.
The trial included 56 patients (51 women, 5 men). Of all patients enrolled, 27 of 28 who were allocated to receive rituximab and 22 of 28 who were allocated to receive placebo underwent at least one infusion and completed 24 weeks of follow-up.
The absolute change in mRSS at 24 weeks after the start of therapy, the primary endpoint, was –6.30 in the rituximab group, compared with +2.14 in the placebo group, a difference of –8.44 (P < .0001).
In a subgroup analysis, rituximab was superior to placebo regardless of disease duration, disease type (diffuse cutaneous or limited cutaneous SSc), prior receipt of systemic corticosteroids or immunosuppressants, or having C-reactive protein levels less than 0.3 mg/dL or at least 0.3 mg/dL.
However, there was no significant benefit with rituximab for patients with baseline mRSS of at least 20 or for those without ILD at baseline.
There was also evidence that rituximab reduced lung fibrosis. For patients assigned to the active drug, the absolute change in FVC at 24 weeks was +0.09% of the predicted value, compared with –3.56% for patients who received placebo (P = .044).
The researchers also observed radiographic evidence of lung improvement. The absolute change in the percentage of lung field occupied with interstitial shadows was –0.32% in the rituximab arm versus +2.39% in the placebo arm (P = .034). There was no significant between-group difference in the absolute change in diffusing capacity of lung for carbon monoxide, however.
Adverse events that occurred more frequently with rituximab included oral mucositis, diarrhea, and decreased neutrophil and white blood cell counts.
Convincing results
“What I thought the Japanese study did was to give a much more convincing proof of concept than has been out there,” Dr. Spiera said in an interview.
“There have been some preliminary experiences that have been encouraging with rituximab in scleroderma, most of which has been open label,” he said.
He also referred to a retrospective study by EUSTAR, the European Scleroderma Trials and Research group, which indicated that patients who had previously received rituximab seemed to have had better outcomes than patients who had been treated with other therapies.
Dr. Spiera added that, although he was glad to see the data from a randomized, placebo-controlled trial in this population, he was uncomfortable with the idea of leaving patients untreated for 6 months.
“From the standpoint of somebody wanting to know what strategies might be promising, this is great for us, but I would not have designed the trial that way,” he said.
The study results were previously published in the Lancet Rheumatology.
The study was supported by grants from the Japan Agency for Medical Research and Development and Zenyaku Kogyo. Dr. Yoshizaki disclosed no relevant financial relationships. Dr. Spiera has received grant/research support from and has consulted for Roche/Genentech, maker of rituximab, and has received compensation from other companies.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Seborrheic Dermatitis
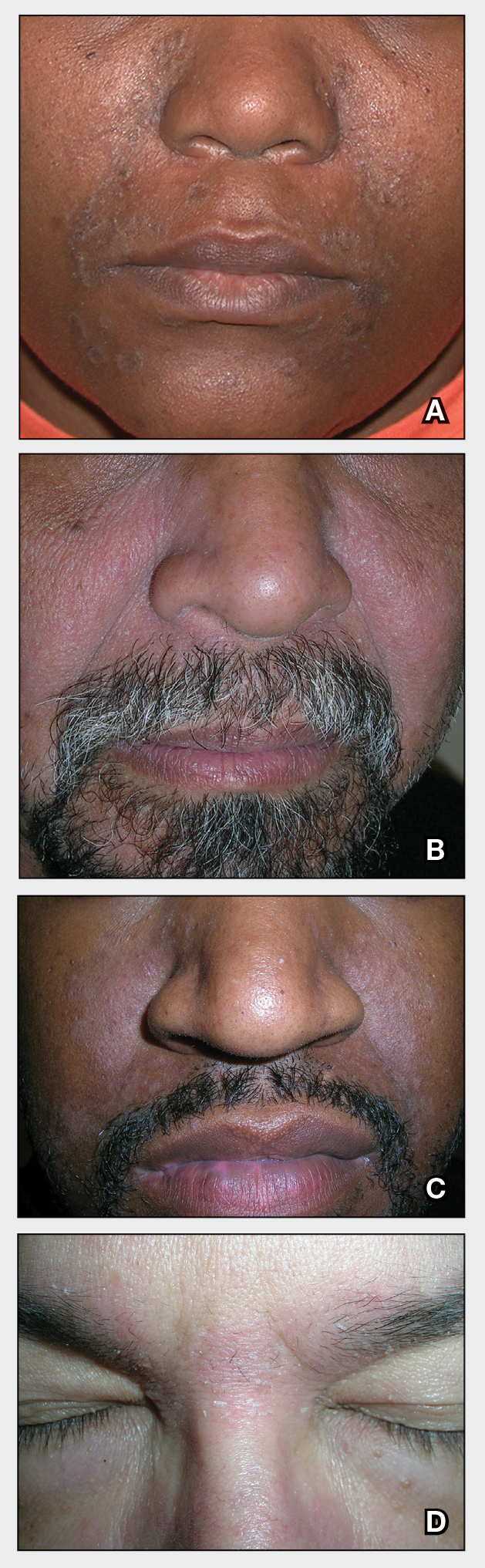
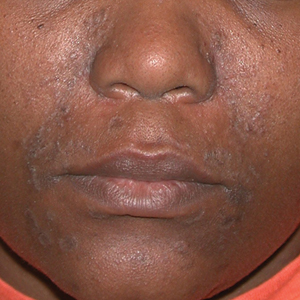
THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
Seborrheic dermatitis affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petal–like) plaques may be present (Figure A). Also, hypopigmented patches and plaques may be prominent (Figures B and C). The classic description includes thin pink patches and plaques with white greasy scale on the face (Figure D).
- The scalp may have diffuse scale or isolated scaly plaques.
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school-aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
Seborrheic dermatitis is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. Seborrheic dermatitis of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/pde.14525
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j .jaad.2010.05.037
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018; 17:1032-1036.
THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
Seborrheic dermatitis affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petal–like) plaques may be present (Figure A). Also, hypopigmented patches and plaques may be prominent (Figures B and C). The classic description includes thin pink patches and plaques with white greasy scale on the face (Figure D).
- The scalp may have diffuse scale or isolated scaly plaques.
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school-aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
Seborrheic dermatitis is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. Seborrheic dermatitis of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
Seborrheic dermatitis affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petal–like) plaques may be present (Figure A). Also, hypopigmented patches and plaques may be prominent (Figures B and C). The classic description includes thin pink patches and plaques with white greasy scale on the face (Figure D).
- The scalp may have diffuse scale or isolated scaly plaques.
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school-aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
Seborrheic dermatitis is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. Seborrheic dermatitis of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/pde.14525
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j .jaad.2010.05.037
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018; 17:1032-1036.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/pde.14525
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j .jaad.2010.05.037
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018; 17:1032-1036.
Psoriatic arthritis and axial spondyloarthritis patients succeed with reduced TNF inhibitor dosing
Reducing the dose of tumor necrosis factor inhibitors by approximately one-third did not increase disease activity in adults with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) in a stable low–disease activity state, according to findings from two parallel controlled retrospective cohort studies.
Disease activity–guided dose optimization (DAGDO) can reduce drug exposure in patients with PsA or axSpA who have low disease activity, but its impact on increased disease activity has not been as well studied as full-dose continuation, Celia A.J. Michielsens, MD, of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues wrote.
“DAGDO or discontinuation of bDMARDs [biologic disease-modifying antirheumatic drugs] as a standard of care in adults with stable axSpA is currently discouraged by” the American College of Rheumatology, the researchers said. However, guidelines from the European Alliance of Associations for Rheumatology allow for the slow tapering of bDMARDs in patients with sustained remission.
In a controlled, retrospective cohort study published in Rheumatology, the researchers analyzed data from their outpatient clinic, which initiated a specific TNF inhibitor DAGDO protocol in 2010 for patients with RA, PsA, and axSpA. Disease activity was measured using the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) for patients with PsA and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axSpA.
The study population included 153 patients with PsA who had a mean DAS28-CRP of 6.5 and 171 with axSpA who had a similar mean number of disease activity measurements (6.5 with DAS28-CRP and 6.4 with BASDAI). Median follow-up time was several months short of 4 years in each group. Treatment was divided into three periods: continuation of full TNF inhibitor dose, TNF inhibitor DAGDO, and a period with stable TNF inhibitor dose after DAGDO.
Overall, no significant differences appeared in mean DAS28-CRP and BASDAI over the course of the study between the period of the full TNF inhibitor dose continuation and both the TNF inhibitor DAGDO period and the stable TNF inhibitor dose period. Among PsA patients, the mean DAS28-CRP was 1.94 for the full-dose period, 2.0 in the TNF inhibitor DAGDO period, and 1.97 in the stable TNF inhibitor dose after DAGDO period. For axSpA patients, the mean BASDAI was 3.44, 3.47, and 3.48, respectively, for the three periods. Older age, longer disease duration, and longer follow-up were significantly associated with higher DAS28-CRP scores in patients with PsA, and older age and female gender were significantly associated with higher BASDAI scores in patients with axSpA.
The mean percentage of daily defined dose (%DDD) for patients with PsA was 108% during the full dose period, 62% in the TNF inhibitor DAGDO period, and 78% with stable TNF inhibitor after DAGDO, and nearly the same for patients with axSPA at 108%, 62%, and 72%, respectively.
The %DDD represents “a modest degree of tapering,” compared with studies in RA patients, the researchers noted. “Explanations for this difference could be that the full dose-reduction potential was not met due to suboptimal execution of the local protocol, whereas in prospective intervention trials, protocol adherence is likely higher.”
The study findings were limited by several factors including the open-label design and potential for nocebo effects, possible incorrect attribution, and information bias, as well as the use of DAS28-CRP and BASDAI rather than more modern measurement tools, the researchers noted.
However, the results were strengthened by the large sample size and real-world clinical setting, frequent assessment of disease activity, long-term follow-up, and the performance of DAGDO by rheumatologists familiar with the measuring tools, they said. The results suggest that DAGDO is safe and effective for patients with low disease activity in either condition, but randomized, prospective studies can provide more definitive evidence.
The study received no outside funding. One author disclosed relationships with multiple pharmaceutical companies.
Reducing the dose of tumor necrosis factor inhibitors by approximately one-third did not increase disease activity in adults with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) in a stable low–disease activity state, according to findings from two parallel controlled retrospective cohort studies.
Disease activity–guided dose optimization (DAGDO) can reduce drug exposure in patients with PsA or axSpA who have low disease activity, but its impact on increased disease activity has not been as well studied as full-dose continuation, Celia A.J. Michielsens, MD, of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues wrote.
“DAGDO or discontinuation of bDMARDs [biologic disease-modifying antirheumatic drugs] as a standard of care in adults with stable axSpA is currently discouraged by” the American College of Rheumatology, the researchers said. However, guidelines from the European Alliance of Associations for Rheumatology allow for the slow tapering of bDMARDs in patients with sustained remission.
In a controlled, retrospective cohort study published in Rheumatology, the researchers analyzed data from their outpatient clinic, which initiated a specific TNF inhibitor DAGDO protocol in 2010 for patients with RA, PsA, and axSpA. Disease activity was measured using the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) for patients with PsA and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axSpA.
The study population included 153 patients with PsA who had a mean DAS28-CRP of 6.5 and 171 with axSpA who had a similar mean number of disease activity measurements (6.5 with DAS28-CRP and 6.4 with BASDAI). Median follow-up time was several months short of 4 years in each group. Treatment was divided into three periods: continuation of full TNF inhibitor dose, TNF inhibitor DAGDO, and a period with stable TNF inhibitor dose after DAGDO.
Overall, no significant differences appeared in mean DAS28-CRP and BASDAI over the course of the study between the period of the full TNF inhibitor dose continuation and both the TNF inhibitor DAGDO period and the stable TNF inhibitor dose period. Among PsA patients, the mean DAS28-CRP was 1.94 for the full-dose period, 2.0 in the TNF inhibitor DAGDO period, and 1.97 in the stable TNF inhibitor dose after DAGDO period. For axSpA patients, the mean BASDAI was 3.44, 3.47, and 3.48, respectively, for the three periods. Older age, longer disease duration, and longer follow-up were significantly associated with higher DAS28-CRP scores in patients with PsA, and older age and female gender were significantly associated with higher BASDAI scores in patients with axSpA.
The mean percentage of daily defined dose (%DDD) for patients with PsA was 108% during the full dose period, 62% in the TNF inhibitor DAGDO period, and 78% with stable TNF inhibitor after DAGDO, and nearly the same for patients with axSPA at 108%, 62%, and 72%, respectively.
The %DDD represents “a modest degree of tapering,” compared with studies in RA patients, the researchers noted. “Explanations for this difference could be that the full dose-reduction potential was not met due to suboptimal execution of the local protocol, whereas in prospective intervention trials, protocol adherence is likely higher.”
The study findings were limited by several factors including the open-label design and potential for nocebo effects, possible incorrect attribution, and information bias, as well as the use of DAS28-CRP and BASDAI rather than more modern measurement tools, the researchers noted.
However, the results were strengthened by the large sample size and real-world clinical setting, frequent assessment of disease activity, long-term follow-up, and the performance of DAGDO by rheumatologists familiar with the measuring tools, they said. The results suggest that DAGDO is safe and effective for patients with low disease activity in either condition, but randomized, prospective studies can provide more definitive evidence.
The study received no outside funding. One author disclosed relationships with multiple pharmaceutical companies.
Reducing the dose of tumor necrosis factor inhibitors by approximately one-third did not increase disease activity in adults with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) in a stable low–disease activity state, according to findings from two parallel controlled retrospective cohort studies.
Disease activity–guided dose optimization (DAGDO) can reduce drug exposure in patients with PsA or axSpA who have low disease activity, but its impact on increased disease activity has not been as well studied as full-dose continuation, Celia A.J. Michielsens, MD, of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues wrote.
“DAGDO or discontinuation of bDMARDs [biologic disease-modifying antirheumatic drugs] as a standard of care in adults with stable axSpA is currently discouraged by” the American College of Rheumatology, the researchers said. However, guidelines from the European Alliance of Associations for Rheumatology allow for the slow tapering of bDMARDs in patients with sustained remission.
In a controlled, retrospective cohort study published in Rheumatology, the researchers analyzed data from their outpatient clinic, which initiated a specific TNF inhibitor DAGDO protocol in 2010 for patients with RA, PsA, and axSpA. Disease activity was measured using the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) for patients with PsA and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axSpA.
The study population included 153 patients with PsA who had a mean DAS28-CRP of 6.5 and 171 with axSpA who had a similar mean number of disease activity measurements (6.5 with DAS28-CRP and 6.4 with BASDAI). Median follow-up time was several months short of 4 years in each group. Treatment was divided into three periods: continuation of full TNF inhibitor dose, TNF inhibitor DAGDO, and a period with stable TNF inhibitor dose after DAGDO.
Overall, no significant differences appeared in mean DAS28-CRP and BASDAI over the course of the study between the period of the full TNF inhibitor dose continuation and both the TNF inhibitor DAGDO period and the stable TNF inhibitor dose period. Among PsA patients, the mean DAS28-CRP was 1.94 for the full-dose period, 2.0 in the TNF inhibitor DAGDO period, and 1.97 in the stable TNF inhibitor dose after DAGDO period. For axSpA patients, the mean BASDAI was 3.44, 3.47, and 3.48, respectively, for the three periods. Older age, longer disease duration, and longer follow-up were significantly associated with higher DAS28-CRP scores in patients with PsA, and older age and female gender were significantly associated with higher BASDAI scores in patients with axSpA.
The mean percentage of daily defined dose (%DDD) for patients with PsA was 108% during the full dose period, 62% in the TNF inhibitor DAGDO period, and 78% with stable TNF inhibitor after DAGDO, and nearly the same for patients with axSPA at 108%, 62%, and 72%, respectively.
The %DDD represents “a modest degree of tapering,” compared with studies in RA patients, the researchers noted. “Explanations for this difference could be that the full dose-reduction potential was not met due to suboptimal execution of the local protocol, whereas in prospective intervention trials, protocol adherence is likely higher.”
The study findings were limited by several factors including the open-label design and potential for nocebo effects, possible incorrect attribution, and information bias, as well as the use of DAS28-CRP and BASDAI rather than more modern measurement tools, the researchers noted.
However, the results were strengthened by the large sample size and real-world clinical setting, frequent assessment of disease activity, long-term follow-up, and the performance of DAGDO by rheumatologists familiar with the measuring tools, they said. The results suggest that DAGDO is safe and effective for patients with low disease activity in either condition, but randomized, prospective studies can provide more definitive evidence.
The study received no outside funding. One author disclosed relationships with multiple pharmaceutical companies.
FROM RHEUMATOLOGY
Risankizumab outperforms placebo at 6 months for psoriatic arthritis
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
FROM ACR 2021
Abatacept shows signal to delay onset of rheumatoid arthritis
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
FROM ACR 2021