User login
Two Cystic Duct Stents Appear Better Than One
according to a retrospective multicenter study.
These findings suggest that endoscopists should prioritize dual stent placement when feasible, and consider adding a second stent in patients who previously received a single stent, James D. Haddad, MD, of the University of Texas Southwestern, Dallas, and colleagues reported.
The American Gastroenterological Association (AGA) has recognized the role of endoscopic drainage in managing acute cholecystitis in high-risk patients, but specific guidance on optimal technique and follow-up remains unclear, the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
“Despite accumulating data and increased interest in this technique, clear guidance on the ideal strategy for ETGBD is lacking,” Dr. Haddad and colleagues wrote. “For example, the optimal size, number, and follow-up of cystic duct stents for patients undergoing ETGBD has not been well established.”
To address this knowledge gap, the investigators analyzed data from 75 patients at five academic medical centers who had undergone ETGBD between June 2013 and October 2022. Patients were divided into two groups based on whether they received one or two cystic duct stents.
The primary outcome was clinical success, defined as symptom resolution without requiring another drainage procedure. Secondary outcomes included technical success (defined as successful stent placement), along with rates of adverse events and unplanned reinterventions.
Out of the 75 patients, 59 received a single stent, while 16 received dual stents. The median follow-up time was 407 days overall, with a longer follow-up in the single-stent group (433 days), compared with the double-stent group (118 days).
Clinical success was reported in 81.3% of cases, which technical success was achieved in 88.2% of cases.
Patients who received two stents had significantly lower rates of unplanned reintervention, compared with those who received a single stent (0% vs 25.4%; P = .02). The median time to unplanned reintervention in the single-stent group was 210 days.
Use of a 7 French stent was strongly associated with placement of two stents (odd ratio [OR], 15.5; P = .01). Similarly, patients with a prior percutaneous cholecystostomy tube were significantly more likely to have two stents placed (OR, 10.8; P = .001).
Adverse event rates were uncommon and not statistically different between groups, with an overall rate of 6.7%. Post-endoscopic retrograde cholangiopancreatography pancreatitis was the most common adverse event, occurring in two patients in the single-stent group and one patient in the double-stent group. There were no reported cases of cystic duct or gallbladder perforation.
“In conclusion,” the investigators wrote, “ETGBD with dual transpapillary gallbladder stenting is associated with a lower rate of unplanned reinterventions, compared with that with single stenting, and has a low rate of adverse events. Endoscopists performing ETGBD should consider planned exchange of solitary transpapillary gallbladder stents or interval ERCP for reattempted placement of a second stent if placement of two stents is not possible at the index ERCP.”
The investigators disclosed relationships with Boston Scientific, Motus GI, and ConMed.
according to a retrospective multicenter study.
These findings suggest that endoscopists should prioritize dual stent placement when feasible, and consider adding a second stent in patients who previously received a single stent, James D. Haddad, MD, of the University of Texas Southwestern, Dallas, and colleagues reported.
The American Gastroenterological Association (AGA) has recognized the role of endoscopic drainage in managing acute cholecystitis in high-risk patients, but specific guidance on optimal technique and follow-up remains unclear, the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
“Despite accumulating data and increased interest in this technique, clear guidance on the ideal strategy for ETGBD is lacking,” Dr. Haddad and colleagues wrote. “For example, the optimal size, number, and follow-up of cystic duct stents for patients undergoing ETGBD has not been well established.”
To address this knowledge gap, the investigators analyzed data from 75 patients at five academic medical centers who had undergone ETGBD between June 2013 and October 2022. Patients were divided into two groups based on whether they received one or two cystic duct stents.
The primary outcome was clinical success, defined as symptom resolution without requiring another drainage procedure. Secondary outcomes included technical success (defined as successful stent placement), along with rates of adverse events and unplanned reinterventions.
Out of the 75 patients, 59 received a single stent, while 16 received dual stents. The median follow-up time was 407 days overall, with a longer follow-up in the single-stent group (433 days), compared with the double-stent group (118 days).
Clinical success was reported in 81.3% of cases, which technical success was achieved in 88.2% of cases.
Patients who received two stents had significantly lower rates of unplanned reintervention, compared with those who received a single stent (0% vs 25.4%; P = .02). The median time to unplanned reintervention in the single-stent group was 210 days.
Use of a 7 French stent was strongly associated with placement of two stents (odd ratio [OR], 15.5; P = .01). Similarly, patients with a prior percutaneous cholecystostomy tube were significantly more likely to have two stents placed (OR, 10.8; P = .001).
Adverse event rates were uncommon and not statistically different between groups, with an overall rate of 6.7%. Post-endoscopic retrograde cholangiopancreatography pancreatitis was the most common adverse event, occurring in two patients in the single-stent group and one patient in the double-stent group. There were no reported cases of cystic duct or gallbladder perforation.
“In conclusion,” the investigators wrote, “ETGBD with dual transpapillary gallbladder stenting is associated with a lower rate of unplanned reinterventions, compared with that with single stenting, and has a low rate of adverse events. Endoscopists performing ETGBD should consider planned exchange of solitary transpapillary gallbladder stents or interval ERCP for reattempted placement of a second stent if placement of two stents is not possible at the index ERCP.”
The investigators disclosed relationships with Boston Scientific, Motus GI, and ConMed.
according to a retrospective multicenter study.
These findings suggest that endoscopists should prioritize dual stent placement when feasible, and consider adding a second stent in patients who previously received a single stent, James D. Haddad, MD, of the University of Texas Southwestern, Dallas, and colleagues reported.
The American Gastroenterological Association (AGA) has recognized the role of endoscopic drainage in managing acute cholecystitis in high-risk patients, but specific guidance on optimal technique and follow-up remains unclear, the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
“Despite accumulating data and increased interest in this technique, clear guidance on the ideal strategy for ETGBD is lacking,” Dr. Haddad and colleagues wrote. “For example, the optimal size, number, and follow-up of cystic duct stents for patients undergoing ETGBD has not been well established.”
To address this knowledge gap, the investigators analyzed data from 75 patients at five academic medical centers who had undergone ETGBD between June 2013 and October 2022. Patients were divided into two groups based on whether they received one or two cystic duct stents.
The primary outcome was clinical success, defined as symptom resolution without requiring another drainage procedure. Secondary outcomes included technical success (defined as successful stent placement), along with rates of adverse events and unplanned reinterventions.
Out of the 75 patients, 59 received a single stent, while 16 received dual stents. The median follow-up time was 407 days overall, with a longer follow-up in the single-stent group (433 days), compared with the double-stent group (118 days).
Clinical success was reported in 81.3% of cases, which technical success was achieved in 88.2% of cases.
Patients who received two stents had significantly lower rates of unplanned reintervention, compared with those who received a single stent (0% vs 25.4%; P = .02). The median time to unplanned reintervention in the single-stent group was 210 days.
Use of a 7 French stent was strongly associated with placement of two stents (odd ratio [OR], 15.5; P = .01). Similarly, patients with a prior percutaneous cholecystostomy tube were significantly more likely to have two stents placed (OR, 10.8; P = .001).
Adverse event rates were uncommon and not statistically different between groups, with an overall rate of 6.7%. Post-endoscopic retrograde cholangiopancreatography pancreatitis was the most common adverse event, occurring in two patients in the single-stent group and one patient in the double-stent group. There were no reported cases of cystic duct or gallbladder perforation.
“In conclusion,” the investigators wrote, “ETGBD with dual transpapillary gallbladder stenting is associated with a lower rate of unplanned reinterventions, compared with that with single stenting, and has a low rate of adverse events. Endoscopists performing ETGBD should consider planned exchange of solitary transpapillary gallbladder stents or interval ERCP for reattempted placement of a second stent if placement of two stents is not possible at the index ERCP.”
The investigators disclosed relationships with Boston Scientific, Motus GI, and ConMed.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
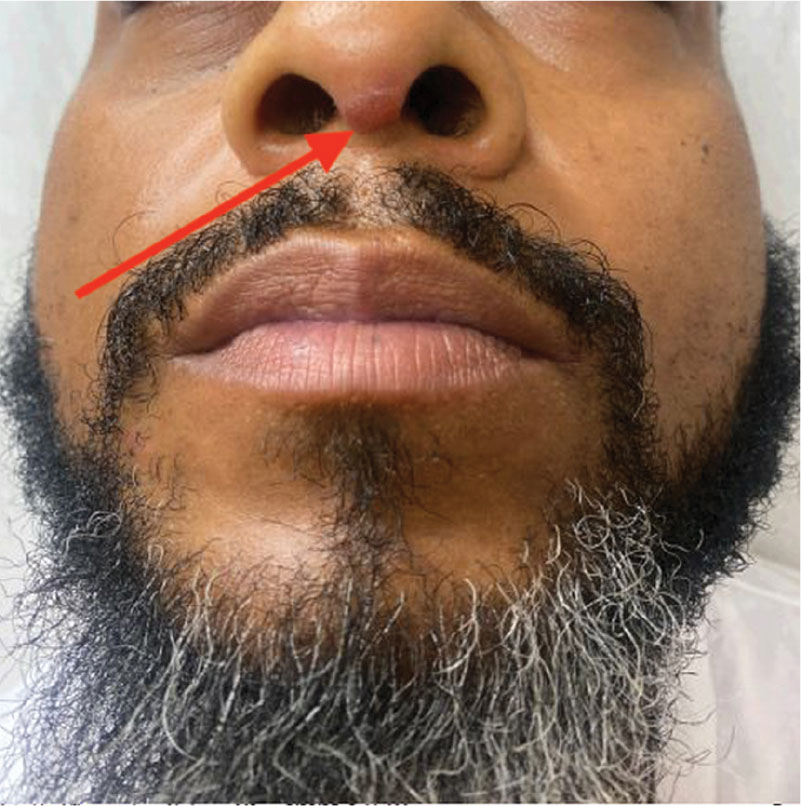
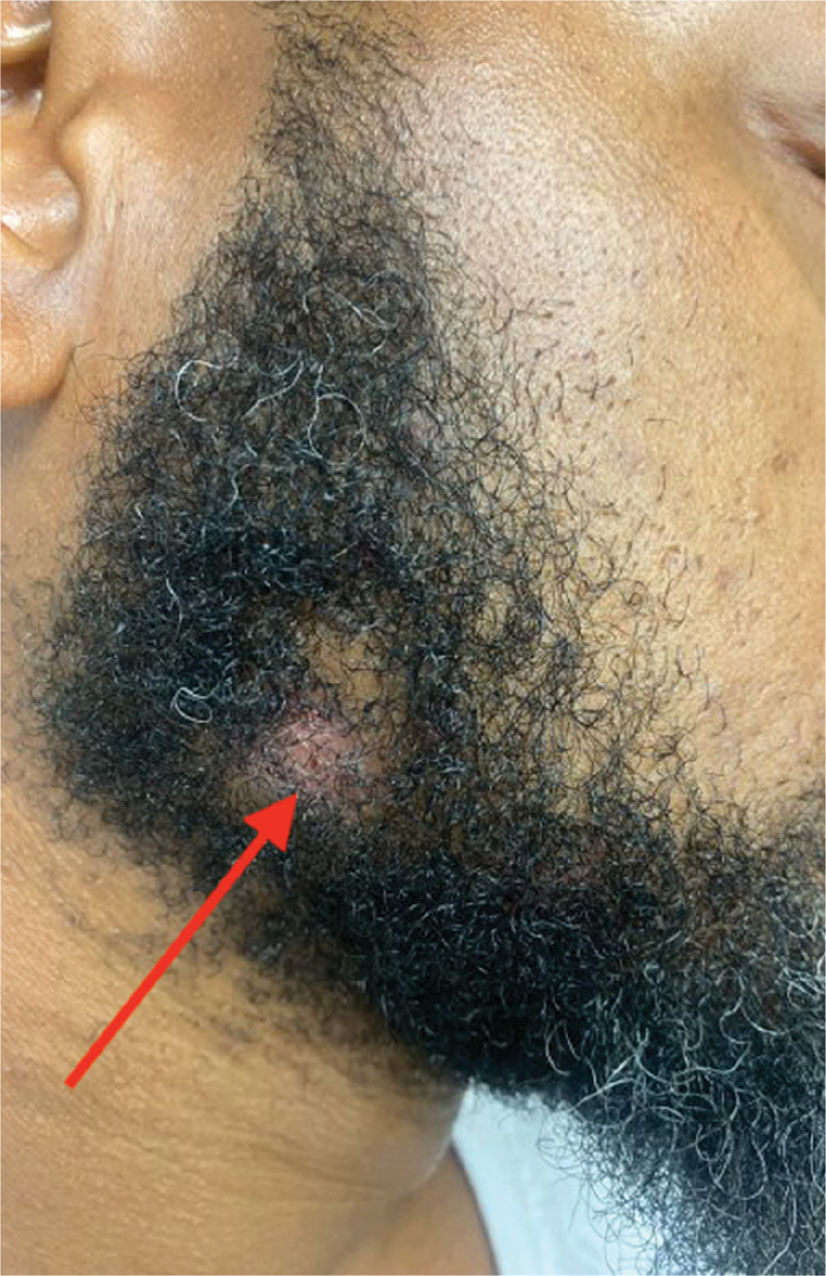
Violaceous Papules on Face
Violaceous Papules on Face
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
Violaceous Papules on Face
Violaceous Papules on Face
A 40-year-old man with no significant medical history or comorbidities presented with a violaceous papule involving his nasal tip and scaly, violaceous plaques with associated alopecia involving his beard (Figure). Skin biopsy confirmed granulomatous dermatitis. Additional workup was notable for hypercalcemia (10.5 mg/dL; reference range, 8.4-10.2 mg/dL), elevated chitotriosidase (317 nmol/h/mL; reference range, < 150 nmol/h/mL), and bibasilar opacities with left perihilar consolidation on chest X-ray. The patient had a prolonged PR interval (207 ms; reference range, 120-200 ms) on electrocardiogram. A cardiac positron emission tomography revealed low level fluorodeoxyglucose uptake in the left ventricle. No ocular involvement was noted on evaluation by ophthalmology. The patient’s pharmacotherapy included prednisone 10 mg daily, methotrexate 7.5 mg weekly, and hydroxychloroquine 200 mg daily.
Circulating Proteins Predict Crohn’s Disease Years in Advance
The 29-protein biosignature, which was validated across multiple independent cohorts, could potentially open doors to new preclinical interventions, lead author Olle Grännö, MD, of Örebro University in Sweden, and colleagues reported.
“Predictive biomarkers of future clinical onset of active inflammatory bowel disease could detect the disease during ‘a window of opportunity’ when the immune dysregulation is potentially reversible,” the investigators wrote in Gastroenterology.
Preclinical biomarker screening has proven effective in other immune-mediated diseases, such as type 1 diabetes, where risk stratification using autoantibodies enabled early intervention that delayed disease onset, they noted.
Previous studies suggested similar potential for inflammatory bowel disease (IBD) via predictive autoantibodies and serum proteins, although the accuracy of these markers was not validated in external cohorts. The present study aimed to fill this validation gap.
First, the investigators measured 178 plasma proteins in blood samples taken from 312 individuals before they were diagnosed with IBD. Using machine learning, Dr. Grännö and colleagues compared these findings with blood-matched controls who remained free of IBD through follow-up. This process revealed the 29-protein signature.
In the same discovery cohort, the panel of 29 proteins differentiated preclinical CD cases from controls with an area under the curve (AUC) of 0.85. The signature was then validated in an independent preclinical cohort of CD patients, with an AUC of 0.87.
While accuracy increased in proximity to clinical disease onset, the model was still highly predictive up to 16 years before CD diagnosis, at which time the AUC was 0.82. The panel showed perfect performance among newly diagnosed CD patients, with an AUC of 1.0, supporting clinical relevance.
Predictive power was statistically significant but less compelling among individuals with preclinical ulcerative colitis (UC). In this IBD subgroup, AUC for identification and validation cohorts was 0.77 and 0.67, respectively, while newly diagnosed patients had an AUC of 0.95.
“In preclinical samples, downregulated (but not upregulated) proteins related to gut barrier integrity and macrophage functionality correlated with time to diagnosis of CD,” Dr. Grännö and colleagues wrote. “Contrarily, all proteins associated with preclinical UC were upregulated, and only one protein marker correlated with the time to diagnosis.”
These findings suggest that disruptions in gut barrier integrity and macrophage function precede clinical CD onset, they explained, potentially serving as an early signal of inflammation-driven intestinal damage. In contrast, the preclinical UC signature primarily involved upregulated inflammatory markers.
Dr. Grännö and colleagues also examined the influence of genetic and environmental factors by comparing preclinical IBD signatures in unrelated and related twin pairs.
The CD biosignature had an AUC of 0.89 when comparing individuals with preclinical CD to matched external (unrelated) healthy twins. Predictive ability dropped significantly (AUC = 0.58) when comparing CD cases to their own healthy twin siblings, suggesting that genetic and shared environmental factors have a “predominant influence” on protein dysregulation.
In contrast, AUC among unrelated vs related twin controls was more similar for UC, at 0.76 and 0.64, respectively, indicating “a limited impact” of genetic and environmental factors on the protein signature.
Altogether, this study reinforces the concept of a long preclinical phase in CD, and highlights the potential for early detection and intervention, according to the investigators.
“The long preclinical period in CD endorses the adoption of early preventive strategies (e.g., diet alterations and medication) to potentially attenuate disease progression and improve the natural history of CD,” they concluded.
This study was funded by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Örebro University Hospital Research Foundation, and others. The investigators disclosed relationships with Pfizer, Janssen, AbbVie, and others.
Nowadays, preclinical biomarker discovery for inflammatory bowel diseases (IBD) is one of the key areas of study, aiming to identify the earliest stages of disease development and to find opportunities for early intervention. The study by Grännö and colleagues taps into this area and provides a significant advancement in the early detection of Crohn’s disease (CD) with a validated 29-plasma protein biomarker signature.
With an AUC of up to 0.87 in preclinical CD cases and even 0.82 as early as 16 years before diagnosis, these findings strongly support the notion that CD has a prolonged preclinical phase that is detectable up to many years before diagnosis. Importantly, their identified protein signatures also shed light on distinct pathophysiological mechanisms between CD and ulcerative colitis (UC), with CD characterized by early disruptions in gut barrier integrity and macrophage function, while UC was more marked by upregulated inflammatory markers.
For clinical practitioners, these findings have a strong transformative potential. Following further validation in larger cohorts and allowing clinical accessibility, preclinical biomarker screening could become a routine tool for risk stratification in at-risk individuals, such as those with a strong family history or genetic predisposition. This could enable implementation of early interventions, including dietary modifications and potentially prophylactic therapies, to delay or even prevent disease onset. Given that similar approaches have proven effective in type 1 diabetes, applying this strategy to IBD could significantly alter disease progression and patient outcomes.
Challenges remain before implementation in clinical practice could be realized. Standardized thresholds for risk assessment, cost-effectiveness analyses, and potential therapeutic strategies tailored to biomarker-positive individuals require further exploration. However, this study provides important data needed for a paradigm shift in IBD management — one that moves from reactive treatment to proactive prevention.
Arno R. Bourgonje, MD, PhD, is a postdoctoral fellow at the Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, and at the University Medical Center Groningen in Groningen, the Netherlands. He is involved in the European INTERCEPT consortium, which is focused on prediction and prevention of IBD. He reported no conflicts of interest.
Nowadays, preclinical biomarker discovery for inflammatory bowel diseases (IBD) is one of the key areas of study, aiming to identify the earliest stages of disease development and to find opportunities for early intervention. The study by Grännö and colleagues taps into this area and provides a significant advancement in the early detection of Crohn’s disease (CD) with a validated 29-plasma protein biomarker signature.
With an AUC of up to 0.87 in preclinical CD cases and even 0.82 as early as 16 years before diagnosis, these findings strongly support the notion that CD has a prolonged preclinical phase that is detectable up to many years before diagnosis. Importantly, their identified protein signatures also shed light on distinct pathophysiological mechanisms between CD and ulcerative colitis (UC), with CD characterized by early disruptions in gut barrier integrity and macrophage function, while UC was more marked by upregulated inflammatory markers.
For clinical practitioners, these findings have a strong transformative potential. Following further validation in larger cohorts and allowing clinical accessibility, preclinical biomarker screening could become a routine tool for risk stratification in at-risk individuals, such as those with a strong family history or genetic predisposition. This could enable implementation of early interventions, including dietary modifications and potentially prophylactic therapies, to delay or even prevent disease onset. Given that similar approaches have proven effective in type 1 diabetes, applying this strategy to IBD could significantly alter disease progression and patient outcomes.
Challenges remain before implementation in clinical practice could be realized. Standardized thresholds for risk assessment, cost-effectiveness analyses, and potential therapeutic strategies tailored to biomarker-positive individuals require further exploration. However, this study provides important data needed for a paradigm shift in IBD management — one that moves from reactive treatment to proactive prevention.
Arno R. Bourgonje, MD, PhD, is a postdoctoral fellow at the Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, and at the University Medical Center Groningen in Groningen, the Netherlands. He is involved in the European INTERCEPT consortium, which is focused on prediction and prevention of IBD. He reported no conflicts of interest.
Nowadays, preclinical biomarker discovery for inflammatory bowel diseases (IBD) is one of the key areas of study, aiming to identify the earliest stages of disease development and to find opportunities for early intervention. The study by Grännö and colleagues taps into this area and provides a significant advancement in the early detection of Crohn’s disease (CD) with a validated 29-plasma protein biomarker signature.
With an AUC of up to 0.87 in preclinical CD cases and even 0.82 as early as 16 years before diagnosis, these findings strongly support the notion that CD has a prolonged preclinical phase that is detectable up to many years before diagnosis. Importantly, their identified protein signatures also shed light on distinct pathophysiological mechanisms between CD and ulcerative colitis (UC), with CD characterized by early disruptions in gut barrier integrity and macrophage function, while UC was more marked by upregulated inflammatory markers.
For clinical practitioners, these findings have a strong transformative potential. Following further validation in larger cohorts and allowing clinical accessibility, preclinical biomarker screening could become a routine tool for risk stratification in at-risk individuals, such as those with a strong family history or genetic predisposition. This could enable implementation of early interventions, including dietary modifications and potentially prophylactic therapies, to delay or even prevent disease onset. Given that similar approaches have proven effective in type 1 diabetes, applying this strategy to IBD could significantly alter disease progression and patient outcomes.
Challenges remain before implementation in clinical practice could be realized. Standardized thresholds for risk assessment, cost-effectiveness analyses, and potential therapeutic strategies tailored to biomarker-positive individuals require further exploration. However, this study provides important data needed for a paradigm shift in IBD management — one that moves from reactive treatment to proactive prevention.
Arno R. Bourgonje, MD, PhD, is a postdoctoral fellow at the Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, and at the University Medical Center Groningen in Groningen, the Netherlands. He is involved in the European INTERCEPT consortium, which is focused on prediction and prevention of IBD. He reported no conflicts of interest.
The 29-protein biosignature, which was validated across multiple independent cohorts, could potentially open doors to new preclinical interventions, lead author Olle Grännö, MD, of Örebro University in Sweden, and colleagues reported.
“Predictive biomarkers of future clinical onset of active inflammatory bowel disease could detect the disease during ‘a window of opportunity’ when the immune dysregulation is potentially reversible,” the investigators wrote in Gastroenterology.
Preclinical biomarker screening has proven effective in other immune-mediated diseases, such as type 1 diabetes, where risk stratification using autoantibodies enabled early intervention that delayed disease onset, they noted.
Previous studies suggested similar potential for inflammatory bowel disease (IBD) via predictive autoantibodies and serum proteins, although the accuracy of these markers was not validated in external cohorts. The present study aimed to fill this validation gap.
First, the investigators measured 178 plasma proteins in blood samples taken from 312 individuals before they were diagnosed with IBD. Using machine learning, Dr. Grännö and colleagues compared these findings with blood-matched controls who remained free of IBD through follow-up. This process revealed the 29-protein signature.
In the same discovery cohort, the panel of 29 proteins differentiated preclinical CD cases from controls with an area under the curve (AUC) of 0.85. The signature was then validated in an independent preclinical cohort of CD patients, with an AUC of 0.87.
While accuracy increased in proximity to clinical disease onset, the model was still highly predictive up to 16 years before CD diagnosis, at which time the AUC was 0.82. The panel showed perfect performance among newly diagnosed CD patients, with an AUC of 1.0, supporting clinical relevance.
Predictive power was statistically significant but less compelling among individuals with preclinical ulcerative colitis (UC). In this IBD subgroup, AUC for identification and validation cohorts was 0.77 and 0.67, respectively, while newly diagnosed patients had an AUC of 0.95.
“In preclinical samples, downregulated (but not upregulated) proteins related to gut barrier integrity and macrophage functionality correlated with time to diagnosis of CD,” Dr. Grännö and colleagues wrote. “Contrarily, all proteins associated with preclinical UC were upregulated, and only one protein marker correlated with the time to diagnosis.”
These findings suggest that disruptions in gut barrier integrity and macrophage function precede clinical CD onset, they explained, potentially serving as an early signal of inflammation-driven intestinal damage. In contrast, the preclinical UC signature primarily involved upregulated inflammatory markers.
Dr. Grännö and colleagues also examined the influence of genetic and environmental factors by comparing preclinical IBD signatures in unrelated and related twin pairs.
The CD biosignature had an AUC of 0.89 when comparing individuals with preclinical CD to matched external (unrelated) healthy twins. Predictive ability dropped significantly (AUC = 0.58) when comparing CD cases to their own healthy twin siblings, suggesting that genetic and shared environmental factors have a “predominant influence” on protein dysregulation.
In contrast, AUC among unrelated vs related twin controls was more similar for UC, at 0.76 and 0.64, respectively, indicating “a limited impact” of genetic and environmental factors on the protein signature.
Altogether, this study reinforces the concept of a long preclinical phase in CD, and highlights the potential for early detection and intervention, according to the investigators.
“The long preclinical period in CD endorses the adoption of early preventive strategies (e.g., diet alterations and medication) to potentially attenuate disease progression and improve the natural history of CD,” they concluded.
This study was funded by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Örebro University Hospital Research Foundation, and others. The investigators disclosed relationships with Pfizer, Janssen, AbbVie, and others.
The 29-protein biosignature, which was validated across multiple independent cohorts, could potentially open doors to new preclinical interventions, lead author Olle Grännö, MD, of Örebro University in Sweden, and colleagues reported.
“Predictive biomarkers of future clinical onset of active inflammatory bowel disease could detect the disease during ‘a window of opportunity’ when the immune dysregulation is potentially reversible,” the investigators wrote in Gastroenterology.
Preclinical biomarker screening has proven effective in other immune-mediated diseases, such as type 1 diabetes, where risk stratification using autoantibodies enabled early intervention that delayed disease onset, they noted.
Previous studies suggested similar potential for inflammatory bowel disease (IBD) via predictive autoantibodies and serum proteins, although the accuracy of these markers was not validated in external cohorts. The present study aimed to fill this validation gap.
First, the investigators measured 178 plasma proteins in blood samples taken from 312 individuals before they were diagnosed with IBD. Using machine learning, Dr. Grännö and colleagues compared these findings with blood-matched controls who remained free of IBD through follow-up. This process revealed the 29-protein signature.
In the same discovery cohort, the panel of 29 proteins differentiated preclinical CD cases from controls with an area under the curve (AUC) of 0.85. The signature was then validated in an independent preclinical cohort of CD patients, with an AUC of 0.87.
While accuracy increased in proximity to clinical disease onset, the model was still highly predictive up to 16 years before CD diagnosis, at which time the AUC was 0.82. The panel showed perfect performance among newly diagnosed CD patients, with an AUC of 1.0, supporting clinical relevance.
Predictive power was statistically significant but less compelling among individuals with preclinical ulcerative colitis (UC). In this IBD subgroup, AUC for identification and validation cohorts was 0.77 and 0.67, respectively, while newly diagnosed patients had an AUC of 0.95.
“In preclinical samples, downregulated (but not upregulated) proteins related to gut barrier integrity and macrophage functionality correlated with time to diagnosis of CD,” Dr. Grännö and colleagues wrote. “Contrarily, all proteins associated with preclinical UC were upregulated, and only one protein marker correlated with the time to diagnosis.”
These findings suggest that disruptions in gut barrier integrity and macrophage function precede clinical CD onset, they explained, potentially serving as an early signal of inflammation-driven intestinal damage. In contrast, the preclinical UC signature primarily involved upregulated inflammatory markers.
Dr. Grännö and colleagues also examined the influence of genetic and environmental factors by comparing preclinical IBD signatures in unrelated and related twin pairs.
The CD biosignature had an AUC of 0.89 when comparing individuals with preclinical CD to matched external (unrelated) healthy twins. Predictive ability dropped significantly (AUC = 0.58) when comparing CD cases to their own healthy twin siblings, suggesting that genetic and shared environmental factors have a “predominant influence” on protein dysregulation.
In contrast, AUC among unrelated vs related twin controls was more similar for UC, at 0.76 and 0.64, respectively, indicating “a limited impact” of genetic and environmental factors on the protein signature.
Altogether, this study reinforces the concept of a long preclinical phase in CD, and highlights the potential for early detection and intervention, according to the investigators.
“The long preclinical period in CD endorses the adoption of early preventive strategies (e.g., diet alterations and medication) to potentially attenuate disease progression and improve the natural history of CD,” they concluded.
This study was funded by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Örebro University Hospital Research Foundation, and others. The investigators disclosed relationships with Pfizer, Janssen, AbbVie, and others.
FROM GASTROENTEROLOGY
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
Where Are All the Nurses? Data Show That Some States Have a Far Higher Number of Nurses Per Capita Than Others
During their 12-hour shifts, registered nurses (RNs) in Arizona and Arkansas perform many of the same tasks as RNs in Wisconsin and Wyoming: Assessing patients, monitoring vital signs, administering medications, and charting records to provide the best patient care. The work might be similar, but there are vast differences in the number of RNs in each state.
In states like Idaho, Utah, and Nevada, which have the lowest number of nurses per capita, there are as few as 7 nurses per 1000 residents, compared with South Dakota and the District of Columbia, which have double the number of nurses than underserved states — giving them the highest number of nurses per capita.
Even states with the largest number of nurses per capita are not immune to the nursing shortage. The National Bureau of Labor Statistics estimates that there will be 195,400 job openings for RNs from 2021 to 2031.
So, what makes it easier for some states to recruit and retain RNs than others?
States With the Highest Number of Nurses Per Capita
South Dakota
RNs per 1000 residents: 15.79
Average wage: $67,030 or $32.23 per hour
Average rent in Sioux Falls: $1192 per month
The Midwestern state has more miles of shoreline than Florida, herds of wild buffalo, the highest summit east of the Rockies, and more nurses per capita than all other states . Healthcare is one of the major industries in the Mount Rushmore State.
Haifa Abou Samra, dean and professor at the University of South Dakota School of Health Sciences, Vermillion, isn’t surprised that RNs want to call the state home.
“South Dakota is a nice place to live,” Samra said. “[The] schools are wonderful. If people are growing families, there is support; neighbors support their neighbors, and it’s a relatively safe community.”
South Dakota has 19 approved nursing education programs that graduated 878 RNs in 2022. Scholarships and student loan forgiveness programs have helped attract qualified RNs, and collaborations between education and industry have been instrumental in addressing the nursing shortage, Samra told this news organization.
Even though RNs earn less than the median wage ($87,070 per year/41.38 per hour), South Dakota has a low cost of living and no individual income tax, which helps stretch those earnings.
District of Columbia
RNs per 1000 residents: 15.39
Average wage: $105,220 or $50.59 per hour
Average rent in Washington, DC: $2485 per month
After a shift at some of the top-ranking hospitals in the nation, RNs working in the compact capital region can explore museums, monuments, and cultural sites; walk along the banks of the Potomac River; or grab a bite at award-winning restaurants.
Washington, a top-ranking metro area because of its growth, high wages, and access to economic opportunities, is also home to several top-tier hospitals and some of the best healthcare in the nation, and RNs who want to pursue continuing education have access to top-tier universities.
Nurses in Washington, DC, might make some of the highest wages in the nation, but the region also has the second-highest cost of living in the United States, with average rents topping $2400 per month and an average home price of $594,337.
North Dakota
RNs per 1000 residents: 12.99
Average wage: $74,930 or $36.03 per hour
Average rent in Fargo: $1051 per month
North Dakota projects a 10.4% increase in employment for RNs, which is higher than the national average, and the state has implemented several strategies to address chronic nursing shortages. The Nurse Staffing Clearinghouse connects nursing school graduates with local employers and created a statewide nursing staffing pool for in-state recruitment of travel nurses.
But it’s not just plentiful job opportunities and a low cost of living that attract nurses to the Peace Garden State. The state and its largest cities, Bismarck and Fargo, hold several “best of” accolades, including nods for the safest places to live and among the Best Places to Raise a Family, giving it high marks for quality of life.
Sure, the winters are cold, but the outdoor recreation can’t be beaten. RNs can bundle up and see the bighorn sheep in the Badlands at Theodore Roosevelt National Park or explore expansive terrain for skiing, snowboarding, and snowmobile trails.
States With the Lowest Number of Nurses Per Capita
Nevada
RNs per 1000 residents: 7.92
Average wage: $96,201 or $46.25 per hour
Average rent in Las Vegas: $1478 per month
Despite a projected 23% job growth for RNs between 2020 and 2030, the state has struggled to fill open positions. It might be the higher-than-average cost of living (9.7% higher than the US average) or higher-than-average crime rates that make RNs reluctant to gamble on a job in the Silver State. But there are some big wins for nurses in the state.
Salaries are higher than the national average, there is no state income tax, and some of the lowest property taxes in the nation. Thanks to new legislation, RNs with student loan debt won’t have to bet on black at the casino to make their payments. The Health Equity and Loan Assistance Program is a new initiative that offers up to $120,000 in loan repayment assistance to providers, including RNs, who commit to working in underserved and rural areas across the state for 5 years.
The state also has incredible attractions, from the neon lights and over-the-top architecture in Las Vegas to iconic red rock canyons, stunning state parks, and landmarks like Hoover Dam and Lake Tahoe.
Utah
RNs per 1000 residents: 7.05
Average wage: $79,790 or $38.36 per hour
Average rent in Salt Lake City: $1611 per month
Healthcare is one of the biggest employers in Utah, and nurses are the most in-demand healthcare workers in the state. But below-average wages and a cost of living that is a whopping 28% higher than the national average could be some reasons that the Beehive State is struggling to attract nurses.
A high number of job vacancies mean higher patient-to-nurse ratios, creating additional stress for a workforce prone to burnout. Much of the state is rural, public transportation is inadequate, and poor air quality causes frequent haze and smog.
The challenges are offset by some big benefits: Utah has been ranked as the “best state” thanks to the strong economy, infrastructure, and quality education — and it doesn’t hurt that Utah is home to myriad outdoor recreation opportunities and the stunning scenery at landmarks like Bryce Canyon and Arches National Park.
Moreover, Utah is hustling to boost its RN workforce. The University of Utah, Salt Lake City, has increased enrollment by 25% and hired additional faculty to help boost the nursing workforce — and those who work in hospitals and health clinics across the state benefit from a flat 4.55% individual income tax rate.
Idaho
RNs per 1000 residents: 7.02
Average wage: $80,130 or $38.53 per hour
Average rent in Boise: $1646 per month
Although the nursing workforce in Idaho has increased, it still ranks as the lowest in the nation. Teresa Stanfill, DNP, RN, executive director for the Idaho Center for Nursing, said that the number of new nurses is too low to replace the number of retiring nurses.
The state introduced loan repayment programs that award up to $25,000 to cover student loan debt, and hospitals and health systems often offer sign-on bonuses and relocation packages to attract RNs. But long winters, an isolated location, and limited cultural options can make it harder to attract nurses to the state.
It’s easier to recruit RNs to suburban areas like Boise, Meridian, and Nampa, but rural parts of the state struggle, Stanfill added. The nursing shortage is among the reasons that 11 hospitals and emergency departments closed in 2024, and healthcare organizations slashed services across the state.
Idaho has a lot to offer RNs, from small-town charm, reasonable cost of living, and gorgeous landscapes that make it one of the top 10 fastest-growing states in the nation. Collaboration between industry leaders and nursing programs is focused on finding creative solutions to boost the nursing workforce in Idaho.
A version of this article first appeared on Medscape.com.
During their 12-hour shifts, registered nurses (RNs) in Arizona and Arkansas perform many of the same tasks as RNs in Wisconsin and Wyoming: Assessing patients, monitoring vital signs, administering medications, and charting records to provide the best patient care. The work might be similar, but there are vast differences in the number of RNs in each state.
In states like Idaho, Utah, and Nevada, which have the lowest number of nurses per capita, there are as few as 7 nurses per 1000 residents, compared with South Dakota and the District of Columbia, which have double the number of nurses than underserved states — giving them the highest number of nurses per capita.
Even states with the largest number of nurses per capita are not immune to the nursing shortage. The National Bureau of Labor Statistics estimates that there will be 195,400 job openings for RNs from 2021 to 2031.
So, what makes it easier for some states to recruit and retain RNs than others?
States With the Highest Number of Nurses Per Capita
South Dakota
RNs per 1000 residents: 15.79
Average wage: $67,030 or $32.23 per hour
Average rent in Sioux Falls: $1192 per month
The Midwestern state has more miles of shoreline than Florida, herds of wild buffalo, the highest summit east of the Rockies, and more nurses per capita than all other states . Healthcare is one of the major industries in the Mount Rushmore State.
Haifa Abou Samra, dean and professor at the University of South Dakota School of Health Sciences, Vermillion, isn’t surprised that RNs want to call the state home.
“South Dakota is a nice place to live,” Samra said. “[The] schools are wonderful. If people are growing families, there is support; neighbors support their neighbors, and it’s a relatively safe community.”
South Dakota has 19 approved nursing education programs that graduated 878 RNs in 2022. Scholarships and student loan forgiveness programs have helped attract qualified RNs, and collaborations between education and industry have been instrumental in addressing the nursing shortage, Samra told this news organization.
Even though RNs earn less than the median wage ($87,070 per year/41.38 per hour), South Dakota has a low cost of living and no individual income tax, which helps stretch those earnings.
District of Columbia
RNs per 1000 residents: 15.39
Average wage: $105,220 or $50.59 per hour
Average rent in Washington, DC: $2485 per month
After a shift at some of the top-ranking hospitals in the nation, RNs working in the compact capital region can explore museums, monuments, and cultural sites; walk along the banks of the Potomac River; or grab a bite at award-winning restaurants.
Washington, a top-ranking metro area because of its growth, high wages, and access to economic opportunities, is also home to several top-tier hospitals and some of the best healthcare in the nation, and RNs who want to pursue continuing education have access to top-tier universities.
Nurses in Washington, DC, might make some of the highest wages in the nation, but the region also has the second-highest cost of living in the United States, with average rents topping $2400 per month and an average home price of $594,337.
North Dakota
RNs per 1000 residents: 12.99
Average wage: $74,930 or $36.03 per hour
Average rent in Fargo: $1051 per month
North Dakota projects a 10.4% increase in employment for RNs, which is higher than the national average, and the state has implemented several strategies to address chronic nursing shortages. The Nurse Staffing Clearinghouse connects nursing school graduates with local employers and created a statewide nursing staffing pool for in-state recruitment of travel nurses.
But it’s not just plentiful job opportunities and a low cost of living that attract nurses to the Peace Garden State. The state and its largest cities, Bismarck and Fargo, hold several “best of” accolades, including nods for the safest places to live and among the Best Places to Raise a Family, giving it high marks for quality of life.
Sure, the winters are cold, but the outdoor recreation can’t be beaten. RNs can bundle up and see the bighorn sheep in the Badlands at Theodore Roosevelt National Park or explore expansive terrain for skiing, snowboarding, and snowmobile trails.
States With the Lowest Number of Nurses Per Capita
Nevada
RNs per 1000 residents: 7.92
Average wage: $96,201 or $46.25 per hour
Average rent in Las Vegas: $1478 per month
Despite a projected 23% job growth for RNs between 2020 and 2030, the state has struggled to fill open positions. It might be the higher-than-average cost of living (9.7% higher than the US average) or higher-than-average crime rates that make RNs reluctant to gamble on a job in the Silver State. But there are some big wins for nurses in the state.
Salaries are higher than the national average, there is no state income tax, and some of the lowest property taxes in the nation. Thanks to new legislation, RNs with student loan debt won’t have to bet on black at the casino to make their payments. The Health Equity and Loan Assistance Program is a new initiative that offers up to $120,000 in loan repayment assistance to providers, including RNs, who commit to working in underserved and rural areas across the state for 5 years.
The state also has incredible attractions, from the neon lights and over-the-top architecture in Las Vegas to iconic red rock canyons, stunning state parks, and landmarks like Hoover Dam and Lake Tahoe.
Utah
RNs per 1000 residents: 7.05
Average wage: $79,790 or $38.36 per hour
Average rent in Salt Lake City: $1611 per month
Healthcare is one of the biggest employers in Utah, and nurses are the most in-demand healthcare workers in the state. But below-average wages and a cost of living that is a whopping 28% higher than the national average could be some reasons that the Beehive State is struggling to attract nurses.
A high number of job vacancies mean higher patient-to-nurse ratios, creating additional stress for a workforce prone to burnout. Much of the state is rural, public transportation is inadequate, and poor air quality causes frequent haze and smog.
The challenges are offset by some big benefits: Utah has been ranked as the “best state” thanks to the strong economy, infrastructure, and quality education — and it doesn’t hurt that Utah is home to myriad outdoor recreation opportunities and the stunning scenery at landmarks like Bryce Canyon and Arches National Park.
Moreover, Utah is hustling to boost its RN workforce. The University of Utah, Salt Lake City, has increased enrollment by 25% and hired additional faculty to help boost the nursing workforce — and those who work in hospitals and health clinics across the state benefit from a flat 4.55% individual income tax rate.
Idaho
RNs per 1000 residents: 7.02
Average wage: $80,130 or $38.53 per hour
Average rent in Boise: $1646 per month
Although the nursing workforce in Idaho has increased, it still ranks as the lowest in the nation. Teresa Stanfill, DNP, RN, executive director for the Idaho Center for Nursing, said that the number of new nurses is too low to replace the number of retiring nurses.
The state introduced loan repayment programs that award up to $25,000 to cover student loan debt, and hospitals and health systems often offer sign-on bonuses and relocation packages to attract RNs. But long winters, an isolated location, and limited cultural options can make it harder to attract nurses to the state.
It’s easier to recruit RNs to suburban areas like Boise, Meridian, and Nampa, but rural parts of the state struggle, Stanfill added. The nursing shortage is among the reasons that 11 hospitals and emergency departments closed in 2024, and healthcare organizations slashed services across the state.
Idaho has a lot to offer RNs, from small-town charm, reasonable cost of living, and gorgeous landscapes that make it one of the top 10 fastest-growing states in the nation. Collaboration between industry leaders and nursing programs is focused on finding creative solutions to boost the nursing workforce in Idaho.
A version of this article first appeared on Medscape.com.
During their 12-hour shifts, registered nurses (RNs) in Arizona and Arkansas perform many of the same tasks as RNs in Wisconsin and Wyoming: Assessing patients, monitoring vital signs, administering medications, and charting records to provide the best patient care. The work might be similar, but there are vast differences in the number of RNs in each state.
In states like Idaho, Utah, and Nevada, which have the lowest number of nurses per capita, there are as few as 7 nurses per 1000 residents, compared with South Dakota and the District of Columbia, which have double the number of nurses than underserved states — giving them the highest number of nurses per capita.
Even states with the largest number of nurses per capita are not immune to the nursing shortage. The National Bureau of Labor Statistics estimates that there will be 195,400 job openings for RNs from 2021 to 2031.
So, what makes it easier for some states to recruit and retain RNs than others?
States With the Highest Number of Nurses Per Capita
South Dakota
RNs per 1000 residents: 15.79
Average wage: $67,030 or $32.23 per hour
Average rent in Sioux Falls: $1192 per month
The Midwestern state has more miles of shoreline than Florida, herds of wild buffalo, the highest summit east of the Rockies, and more nurses per capita than all other states . Healthcare is one of the major industries in the Mount Rushmore State.
Haifa Abou Samra, dean and professor at the University of South Dakota School of Health Sciences, Vermillion, isn’t surprised that RNs want to call the state home.
“South Dakota is a nice place to live,” Samra said. “[The] schools are wonderful. If people are growing families, there is support; neighbors support their neighbors, and it’s a relatively safe community.”
South Dakota has 19 approved nursing education programs that graduated 878 RNs in 2022. Scholarships and student loan forgiveness programs have helped attract qualified RNs, and collaborations between education and industry have been instrumental in addressing the nursing shortage, Samra told this news organization.
Even though RNs earn less than the median wage ($87,070 per year/41.38 per hour), South Dakota has a low cost of living and no individual income tax, which helps stretch those earnings.
District of Columbia
RNs per 1000 residents: 15.39
Average wage: $105,220 or $50.59 per hour
Average rent in Washington, DC: $2485 per month
After a shift at some of the top-ranking hospitals in the nation, RNs working in the compact capital region can explore museums, monuments, and cultural sites; walk along the banks of the Potomac River; or grab a bite at award-winning restaurants.
Washington, a top-ranking metro area because of its growth, high wages, and access to economic opportunities, is also home to several top-tier hospitals and some of the best healthcare in the nation, and RNs who want to pursue continuing education have access to top-tier universities.
Nurses in Washington, DC, might make some of the highest wages in the nation, but the region also has the second-highest cost of living in the United States, with average rents topping $2400 per month and an average home price of $594,337.
North Dakota
RNs per 1000 residents: 12.99
Average wage: $74,930 or $36.03 per hour
Average rent in Fargo: $1051 per month
North Dakota projects a 10.4% increase in employment for RNs, which is higher than the national average, and the state has implemented several strategies to address chronic nursing shortages. The Nurse Staffing Clearinghouse connects nursing school graduates with local employers and created a statewide nursing staffing pool for in-state recruitment of travel nurses.
But it’s not just plentiful job opportunities and a low cost of living that attract nurses to the Peace Garden State. The state and its largest cities, Bismarck and Fargo, hold several “best of” accolades, including nods for the safest places to live and among the Best Places to Raise a Family, giving it high marks for quality of life.
Sure, the winters are cold, but the outdoor recreation can’t be beaten. RNs can bundle up and see the bighorn sheep in the Badlands at Theodore Roosevelt National Park or explore expansive terrain for skiing, snowboarding, and snowmobile trails.
States With the Lowest Number of Nurses Per Capita
Nevada
RNs per 1000 residents: 7.92
Average wage: $96,201 or $46.25 per hour
Average rent in Las Vegas: $1478 per month
Despite a projected 23% job growth for RNs between 2020 and 2030, the state has struggled to fill open positions. It might be the higher-than-average cost of living (9.7% higher than the US average) or higher-than-average crime rates that make RNs reluctant to gamble on a job in the Silver State. But there are some big wins for nurses in the state.
Salaries are higher than the national average, there is no state income tax, and some of the lowest property taxes in the nation. Thanks to new legislation, RNs with student loan debt won’t have to bet on black at the casino to make their payments. The Health Equity and Loan Assistance Program is a new initiative that offers up to $120,000 in loan repayment assistance to providers, including RNs, who commit to working in underserved and rural areas across the state for 5 years.
The state also has incredible attractions, from the neon lights and over-the-top architecture in Las Vegas to iconic red rock canyons, stunning state parks, and landmarks like Hoover Dam and Lake Tahoe.
Utah
RNs per 1000 residents: 7.05
Average wage: $79,790 or $38.36 per hour
Average rent in Salt Lake City: $1611 per month
Healthcare is one of the biggest employers in Utah, and nurses are the most in-demand healthcare workers in the state. But below-average wages and a cost of living that is a whopping 28% higher than the national average could be some reasons that the Beehive State is struggling to attract nurses.
A high number of job vacancies mean higher patient-to-nurse ratios, creating additional stress for a workforce prone to burnout. Much of the state is rural, public transportation is inadequate, and poor air quality causes frequent haze and smog.
The challenges are offset by some big benefits: Utah has been ranked as the “best state” thanks to the strong economy, infrastructure, and quality education — and it doesn’t hurt that Utah is home to myriad outdoor recreation opportunities and the stunning scenery at landmarks like Bryce Canyon and Arches National Park.
Moreover, Utah is hustling to boost its RN workforce. The University of Utah, Salt Lake City, has increased enrollment by 25% and hired additional faculty to help boost the nursing workforce — and those who work in hospitals and health clinics across the state benefit from a flat 4.55% individual income tax rate.
Idaho
RNs per 1000 residents: 7.02
Average wage: $80,130 or $38.53 per hour
Average rent in Boise: $1646 per month
Although the nursing workforce in Idaho has increased, it still ranks as the lowest in the nation. Teresa Stanfill, DNP, RN, executive director for the Idaho Center for Nursing, said that the number of new nurses is too low to replace the number of retiring nurses.
The state introduced loan repayment programs that award up to $25,000 to cover student loan debt, and hospitals and health systems often offer sign-on bonuses and relocation packages to attract RNs. But long winters, an isolated location, and limited cultural options can make it harder to attract nurses to the state.
It’s easier to recruit RNs to suburban areas like Boise, Meridian, and Nampa, but rural parts of the state struggle, Stanfill added. The nursing shortage is among the reasons that 11 hospitals and emergency departments closed in 2024, and healthcare organizations slashed services across the state.
Idaho has a lot to offer RNs, from small-town charm, reasonable cost of living, and gorgeous landscapes that make it one of the top 10 fastest-growing states in the nation. Collaboration between industry leaders and nursing programs is focused on finding creative solutions to boost the nursing workforce in Idaho.
A version of this article first appeared on Medscape.com.
Bariatric Surgery: Nutrition’s Role in Patient Outcomes
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
How Many Patients in Early Cancer Trials Get Drugs Ultimately Approved by FDA?
TOPLINE:
One in six patients in phase 2 cancer trials received treatments that were eventually approved by the Food and Drug Administration (FDA), a new analysis found. This proportion increased to 1 in 5 when considering National Comprehensive Cancer Network (NCCN) off-label recommendations and decreased to about 1 in 11 for approved regimens considered to have a substantial clinical benefit.
METHODOLOGY:
- Patients enroll in phase 2 oncology trials seeking access to promising new treatments, but the risk-benefit assessments and the likelihood of receiving a therapy that ultimately gains FDA approval remain unclear. Previous research suggests that the odds are 1 in 83 patients for those enrolled in a phase 1 cancer trial.
- Researchers randomly selected 400 phase 2 cancer trials initiated between November 2012 and November 2015 (to give enough time for an approval to occur); these trials included more than 25,000 patients across 608 specific treatment cohorts testing 332 drugs.
- The primary endpoint was the proportion of patients enrolled in phase 2 trials who received a treatment regimen that later attained FDA approval — defined as the “therapeutic proportion.”
- A secondary endpoint was determining the therapeutic proportion based on the therapeutic value of drugs. The three benchmarks were FDA approval alone, FDA approval plus NCCN off-label recommendations, and FDA approval for drugs considered to have a substantial clinical benefit, based on the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS).
TAKEAWAY:
- A total of 4045 patients received a treatment regimen that advanced to FDA approval, corresponding to a therapeutic proportion of 16.2%.
- The therapeutic proportion increased to 19.4% when considering NCCN off-label recommendations and decreased to 9.3% for FDA-approved regimens considered to have a substantial clinical benefit, based on the ESMO-MCBS.
- The proportion of patients who participated in a trial in which the drug-indication pairing went on to phase 3 testing was 32.5%.
- Enrollment in a trial featuring biomarker enrichment, an immunotherapy drug, a large phase 2 cohort, and a nonrandomized, industry-sponsored trial all showed a trend toward a higher therapeutic proportion.
IN PRACTICE:
“By entering a phase 2 trial, a patient has a one in six chance of receiving a treatment that will later be approved for their condition,” the authors wrote. “The proportions described here, when juxtaposed with those estimated previously for phase 1 trials, suggest a striking improvement for a patient’s therapeutic prospects. This suggests that phase 1 trials do a good job at protecting patients downstream from unsafe and ineffective cancer treatments.”
In an editorial accompanying the study, Howard S. Hochster, MD, of the Rutgers Cancer Institute in New Brunswick, New Jersey, suggested that the 16.2% therapeutic proportion reported may be understated. For instance, “if using the criterion of drugs that were FDA approved in any indication and dose, the proportion of patient benefit in these trials rises to 38%, with a 51% benefit rate considering inclusion in NCCN guidelines,” he wrote.
SOURCE:
This study, led by Charlotte Ouimet, MSc, Department of Equity, Ethics and Policy, McGill University School of Population and Global Health, Montréal, Québec, Canada, was published online in Journal of the National Cancer Institute.
LIMITATIONS:
The longitudinal design of this study required using a historical cohort of phase 2 clinical trials, which may not reflect current drug development patterns. This study was underpowered to determine trial characteristics that predicted higher therapeutic proportions. Furthermore, the exclusion of cytotoxic drugs from the analysis resulted in a somewhat restricted view of overall drug development.
DISCLOSURES:
This study was supported by the Canadian Institutes of Health Research. The authors reported having no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
One in six patients in phase 2 cancer trials received treatments that were eventually approved by the Food and Drug Administration (FDA), a new analysis found. This proportion increased to 1 in 5 when considering National Comprehensive Cancer Network (NCCN) off-label recommendations and decreased to about 1 in 11 for approved regimens considered to have a substantial clinical benefit.
METHODOLOGY:
- Patients enroll in phase 2 oncology trials seeking access to promising new treatments, but the risk-benefit assessments and the likelihood of receiving a therapy that ultimately gains FDA approval remain unclear. Previous research suggests that the odds are 1 in 83 patients for those enrolled in a phase 1 cancer trial.
- Researchers randomly selected 400 phase 2 cancer trials initiated between November 2012 and November 2015 (to give enough time for an approval to occur); these trials included more than 25,000 patients across 608 specific treatment cohorts testing 332 drugs.
- The primary endpoint was the proportion of patients enrolled in phase 2 trials who received a treatment regimen that later attained FDA approval — defined as the “therapeutic proportion.”
- A secondary endpoint was determining the therapeutic proportion based on the therapeutic value of drugs. The three benchmarks were FDA approval alone, FDA approval plus NCCN off-label recommendations, and FDA approval for drugs considered to have a substantial clinical benefit, based on the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS).
TAKEAWAY:
- A total of 4045 patients received a treatment regimen that advanced to FDA approval, corresponding to a therapeutic proportion of 16.2%.
- The therapeutic proportion increased to 19.4% when considering NCCN off-label recommendations and decreased to 9.3% for FDA-approved regimens considered to have a substantial clinical benefit, based on the ESMO-MCBS.
- The proportion of patients who participated in a trial in which the drug-indication pairing went on to phase 3 testing was 32.5%.
- Enrollment in a trial featuring biomarker enrichment, an immunotherapy drug, a large phase 2 cohort, and a nonrandomized, industry-sponsored trial all showed a trend toward a higher therapeutic proportion.
IN PRACTICE:
“By entering a phase 2 trial, a patient has a one in six chance of receiving a treatment that will later be approved for their condition,” the authors wrote. “The proportions described here, when juxtaposed with those estimated previously for phase 1 trials, suggest a striking improvement for a patient’s therapeutic prospects. This suggests that phase 1 trials do a good job at protecting patients downstream from unsafe and ineffective cancer treatments.”
In an editorial accompanying the study, Howard S. Hochster, MD, of the Rutgers Cancer Institute in New Brunswick, New Jersey, suggested that the 16.2% therapeutic proportion reported may be understated. For instance, “if using the criterion of drugs that were FDA approved in any indication and dose, the proportion of patient benefit in these trials rises to 38%, with a 51% benefit rate considering inclusion in NCCN guidelines,” he wrote.
SOURCE:
This study, led by Charlotte Ouimet, MSc, Department of Equity, Ethics and Policy, McGill University School of Population and Global Health, Montréal, Québec, Canada, was published online in Journal of the National Cancer Institute.
LIMITATIONS:
The longitudinal design of this study required using a historical cohort of phase 2 clinical trials, which may not reflect current drug development patterns. This study was underpowered to determine trial characteristics that predicted higher therapeutic proportions. Furthermore, the exclusion of cytotoxic drugs from the analysis resulted in a somewhat restricted view of overall drug development.
DISCLOSURES:
This study was supported by the Canadian Institutes of Health Research. The authors reported having no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
One in six patients in phase 2 cancer trials received treatments that were eventually approved by the Food and Drug Administration (FDA), a new analysis found. This proportion increased to 1 in 5 when considering National Comprehensive Cancer Network (NCCN) off-label recommendations and decreased to about 1 in 11 for approved regimens considered to have a substantial clinical benefit.
METHODOLOGY:
- Patients enroll in phase 2 oncology trials seeking access to promising new treatments, but the risk-benefit assessments and the likelihood of receiving a therapy that ultimately gains FDA approval remain unclear. Previous research suggests that the odds are 1 in 83 patients for those enrolled in a phase 1 cancer trial.
- Researchers randomly selected 400 phase 2 cancer trials initiated between November 2012 and November 2015 (to give enough time for an approval to occur); these trials included more than 25,000 patients across 608 specific treatment cohorts testing 332 drugs.
- The primary endpoint was the proportion of patients enrolled in phase 2 trials who received a treatment regimen that later attained FDA approval — defined as the “therapeutic proportion.”
- A secondary endpoint was determining the therapeutic proportion based on the therapeutic value of drugs. The three benchmarks were FDA approval alone, FDA approval plus NCCN off-label recommendations, and FDA approval for drugs considered to have a substantial clinical benefit, based on the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS).
TAKEAWAY:
- A total of 4045 patients received a treatment regimen that advanced to FDA approval, corresponding to a therapeutic proportion of 16.2%.
- The therapeutic proportion increased to 19.4% when considering NCCN off-label recommendations and decreased to 9.3% for FDA-approved regimens considered to have a substantial clinical benefit, based on the ESMO-MCBS.
- The proportion of patients who participated in a trial in which the drug-indication pairing went on to phase 3 testing was 32.5%.
- Enrollment in a trial featuring biomarker enrichment, an immunotherapy drug, a large phase 2 cohort, and a nonrandomized, industry-sponsored trial all showed a trend toward a higher therapeutic proportion.
IN PRACTICE:
“By entering a phase 2 trial, a patient has a one in six chance of receiving a treatment that will later be approved for their condition,” the authors wrote. “The proportions described here, when juxtaposed with those estimated previously for phase 1 trials, suggest a striking improvement for a patient’s therapeutic prospects. This suggests that phase 1 trials do a good job at protecting patients downstream from unsafe and ineffective cancer treatments.”
In an editorial accompanying the study, Howard S. Hochster, MD, of the Rutgers Cancer Institute in New Brunswick, New Jersey, suggested that the 16.2% therapeutic proportion reported may be understated. For instance, “if using the criterion of drugs that were FDA approved in any indication and dose, the proportion of patient benefit in these trials rises to 38%, with a 51% benefit rate considering inclusion in NCCN guidelines,” he wrote.
SOURCE:
This study, led by Charlotte Ouimet, MSc, Department of Equity, Ethics and Policy, McGill University School of Population and Global Health, Montréal, Québec, Canada, was published online in Journal of the National Cancer Institute.
LIMITATIONS:
The longitudinal design of this study required using a historical cohort of phase 2 clinical trials, which may not reflect current drug development patterns. This study was underpowered to determine trial characteristics that predicted higher therapeutic proportions. Furthermore, the exclusion of cytotoxic drugs from the analysis resulted in a somewhat restricted view of overall drug development.
DISCLOSURES:
This study was supported by the Canadian Institutes of Health Research. The authors reported having no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Bariatric Surgery Lowers Risk for Long-Term Liver Complications in MASH-Related Cirrhosis
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM NATURE MEDICINE
Preventing Hepatitis B Reactivation: Updated Clinical Guidance From AGA
The document was published in Gastroenterology and replaces a previous guideline on prophylaxis for immunosuppressed patients issued in 2014.
Since then, many novel classes of immunosuppressives have been approved for various conditions, and potentially immunosuppressive therapies such as transcatheter arterial chemoembolization have been recognized as relevant to potential HBVr.
With reactivation a risk after immune-modulating exposures, such as to multiple drug classes and disease states, the update provides frontline clinicians with evidence-based advice for the management of HBVr in vulnerable individuals. And while antiviral prophylaxis is recommended for many, in select cases careful clinical monitoring may suffice for risk management.
“The risk of HBV reactivation depends on patient-, drug-, and disease-specific factors — and so it can range from very rare to more frequent,” said guideline coauthor Tracey G. Simon, MD, MPH, a hepatologist in the division of gastroenterology at Massachusetts General Hospital and an instructor at Harvard Medical School, both in Boston. “Not every at-risk individual needs pharmacologic treatment, but some certainly do, and this guideline was designed to try to better identify who needs treatment, based on those important drug- and virus-specific factors.”
Simon stressed the importance of creating this guideline to include many new therapies that carry varying degrees of reactivation risk. As to the strength of the evidence, she added, “for some of the questions, the panel was satisfied with the level of certainty. However, for other questions, the data are still very sparse, and so we have tried to ensure that these areas of uncertainty are highlighted clearly for providers and patients.”
Main Recommendations
AGA based its clinical recommendations on balancing desirable and undesirable effects, patient values and preferences, costs, and health equity considerations. It also provided a clinical decision support tool for making pharmacologic management decisions.
The panelists reviewed data on multiple immunosuppressive therapies from older agents such as anthracycline derivatives, corticosteroids, and anti–tumor necrosis factor (anti-TNF) drugs to chimeric antigen receptor T cells and recent biologics and inhibitors.
1. For individuals at high risk for HBVr, AGA recommended antiviral prophylaxis over monitoring alone. Strong recommendation, moderate-certainty evidence.
Implementation considerations: Use antivirals with a high barrier to resistance. Prophylaxis should be started before initiating medications that carry a risk for HBVr and should be continued for at least 6 months after discontinuation of risk-imposing therapy (at least 12 months for B cell–depleting agents).
2. For individuals at moderate risk for HBVr, antiviral prophylaxis was recommended over monitoring alone. Conditional recommendation, moderate-certainty evidence.
Implementation considerations: Use antivirals with a high barrier to resistance. Patients who place a higher value on avoiding long-term antiviral therapy and its associated cost and place a lower value on avoiding the small risk of reactivation (particularly those who are hepatitis B surface antigen [HBsAg]–negative) may reasonably select active monitoring over antiviral prophylaxis.
Careful consideration should be given to the feasibility and likelihood of adherence to long-term monitoring performed at 1- to 3-month intervals and including assessment of hepatitis B viral load and alanine aminotransferase.
3. For low-risk individuals, the AGA said monitoring alone may be used. Conditional recommendation, moderate-certainty evidence.
Implementation considerations: This recommendation assumes regular and sufficient follow-up with continued monitoring. Patients who place a higher value on avoiding the small risk of reactivation (particularly those on more than one low-risk immunosuppressive) and a lower value on the burden and cost of antiviral therapy may reasonably select antiviral therapy.
4. For individuals at risk for HBVr, the guideline recommended testing for hepatitis B. Strong recommendation, moderate-certainty evidence.
Implementation considerations: Given the Centers for Disease Control and Prevention’s universal screening guidance on hepatitis B for everyone aged 18 years or older by testing for HBsAg, anti-HBs, and total anti-hepatitis B core (HBc), the guideline said that stratifying screening practices by magnitude of HBVr risk is no longer needed.
It is reasonable to test initially for serologic markers alone (at minimum for HBsAg or anti-HBc) followed by viral load testing (HBV-DNA) if HBsAg and/or anti-HBc is positive.
Hepatitis C Virus (HCV) Coinfection With Direct-Acting Antiviral (DAA) Treatment
The panel identified 11 studies that provided data for the computation of baseline risk for HBVr in the HCV coinfection cohort undergoing DAA therapy.
In patients who were HBsAg-positive, the pooled baseline risk for HBVr was 240 per 1000, categorizing them to be at high risk for HBVr. The panel stated it is therefore reasonable to extend antiviral prophylaxis beyond the 12-24 weeks of DAA therapy to 6-12 months after cessation of DAA therapy, tailored by clinician judgment and patient preference.
A ‘Useful Clinical Tool’
Commenting on the guideline but not involved in it, Saikiran Kilaru, MD, a hepatologist at NYU Langone Health in New York City, said the update is “absolutely a useful clinical tool. Since the prior guidance was published, there has been a deluge of new medications and medication classes. Prior to the guidance, I was making recommendations based on the limited data available for hepatitis B reactivation risk for these new medications, using the 1%-10% moderate-risk category as guidance.”
In addition, Kilaru said, this guidance is driven by a higher level of evidence certainty than the mostly retrospective evidence that was previously available.
She cautioned that few downgraded risk categories are likely to cause consternation among physicians who have been operating without the benefit of larger meta-analyses of HBVr in new medication categories. “For example, the prior guidance had put anti-TNF as of moderate risk for hepatitis B core–positive-only patients and is now downgraded to low risk.” And other medications such as immune checkpoint inhibitors, which seemed to pose at least moderate risk based on smaller, retrospective studies are now considered to be in the low-risk category.
“It may take some time for these recommendations to be adopted, especially for physicians in the community who have seen fatal or severe reactivations in the past few years,” Kilaru said.
Kilaru pointed out that the guidance update does not clearly cover some standard immunosuppressive therapies used in autoimmune, rheumatologic, and posttransplant regimens, such as mycophenolate, tacrolimus, and cyclosporine. Nor does it address HBVr risk in some liver cancer treatments such as yttrium-90, which have been associated with reports of HBV reactivation.
The Future
According to Simon, more data are needed to better estimate HBVr risk in several important settings, including treatment with the most recently approved immunosuppressive drugs for which data are still limited, as well as combination treatments.
Kilaru noted that guideline updates such as this become increasingly relevant as cancer diagnoses rise and hepatitis B exposure and detection increase as well.
The AGA panel acknowledged that uncertainty remains in some patient risk categorizations. “As the armamentarium of immunotherapeutics evolves, it will be crucial to search for, use, and maintain studies that provide baseline HBV serologies; include a clear definition of HBVr; and enroll a large, nonselective cohort that can guide categorization of risk of HBVr,” the panelists wrote.
AGA provided all financial support for the development of this guideline. No funding from industry was offered or accepted to support the writing effort.
The authors reported no relevant competing interests, but one coauthor is an adviser for Gilead Sciences, and other authors disclosed various relationships with multiple private sector companies. Kilaru had no competing interests to disclose.
A version of this article appeared on Medscape.com.
The document was published in Gastroenterology and replaces a previous guideline on prophylaxis for immunosuppressed patients issued in 2014.
Since then, many novel classes of immunosuppressives have been approved for various conditions, and potentially immunosuppressive therapies such as transcatheter arterial chemoembolization have been recognized as relevant to potential HBVr.
With reactivation a risk after immune-modulating exposures, such as to multiple drug classes and disease states, the update provides frontline clinicians with evidence-based advice for the management of HBVr in vulnerable individuals. And while antiviral prophylaxis is recommended for many, in select cases careful clinical monitoring may suffice for risk management.
“The risk of HBV reactivation depends on patient-, drug-, and disease-specific factors — and so it can range from very rare to more frequent,” said guideline coauthor Tracey G. Simon, MD, MPH, a hepatologist in the division of gastroenterology at Massachusetts General Hospital and an instructor at Harvard Medical School, both in Boston. “Not every at-risk individual needs pharmacologic treatment, but some certainly do, and this guideline was designed to try to better identify who needs treatment, based on those important drug- and virus-specific factors.”
Simon stressed the importance of creating this guideline to include many new therapies that carry varying degrees of reactivation risk. As to the strength of the evidence, she added, “for some of the questions, the panel was satisfied with the level of certainty. However, for other questions, the data are still very sparse, and so we have tried to ensure that these areas of uncertainty are highlighted clearly for providers and patients.”
Main Recommendations
AGA based its clinical recommendations on balancing desirable and undesirable effects, patient values and preferences, costs, and health equity considerations. It also provided a clinical decision support tool for making pharmacologic management decisions.
The panelists reviewed data on multiple immunosuppressive therapies from older agents such as anthracycline derivatives, corticosteroids, and anti–tumor necrosis factor (anti-TNF) drugs to chimeric antigen receptor T cells and recent biologics and inhibitors.
1. For individuals at high risk for HBVr, AGA recommended antiviral prophylaxis over monitoring alone. Strong recommendation, moderate-certainty evidence.
Implementation considerations: Use antivirals with a high barrier to resistance. Prophylaxis should be started before initiating medications that carry a risk for HBVr and should be continued for at least 6 months after discontinuation of risk-imposing therapy (at least 12 months for B cell–depleting agents).
2. For individuals at moderate risk for HBVr, antiviral prophylaxis was recommended over monitoring alone. Conditional recommendation, moderate-certainty evidence.
Implementation considerations: Use antivirals with a high barrier to resistance. Patients who place a higher value on avoiding long-term antiviral therapy and its associated cost and place a lower value on avoiding the small risk of reactivation (particularly those who are hepatitis B surface antigen [HBsAg]–negative) may reasonably select active monitoring over antiviral prophylaxis.
Careful consideration should be given to the feasibility and likelihood of adherence to long-term monitoring performed at 1- to 3-month intervals and including assessment of hepatitis B viral load and alanine aminotransferase.
3. For low-risk individuals, the AGA said monitoring alone may be used. Conditional recommendation, moderate-certainty evidence.
Implementation considerations: This recommendation assumes regular and sufficient follow-up with continued monitoring. Patients who place a higher value on avoiding the small risk of reactivation (particularly those on more than one low-risk immunosuppressive) and a lower value on the burden and cost of antiviral therapy may reasonably select antiviral therapy.
4. For individuals at risk for HBVr, the guideline recommended testing for hepatitis B. Strong recommendation, moderate-certainty evidence.
Implementation considerations: Given the Centers for Disease Control and Prevention’s universal screening guidance on hepatitis B for everyone aged 18 years or older by testing for HBsAg, anti-HBs, and total anti-hepatitis B core (HBc), the guideline said that stratifying screening practices by magnitude of HBVr risk is no longer needed.
It is reasonable to test initially for serologic markers alone (at minimum for HBsAg or anti-HBc) followed by viral load testing (HBV-DNA) if HBsAg and/or anti-HBc is positive.
Hepatitis C Virus (HCV) Coinfection With Direct-Acting Antiviral (DAA) Treatment
The panel identified 11 studies that provided data for the computation of baseline risk for HBVr in the HCV coinfection cohort undergoing DAA therapy.
In patients who were HBsAg-positive, the pooled baseline risk for HBVr was 240 per 1000, categorizing them to be at high risk for HBVr. The panel stated it is therefore reasonable to extend antiviral prophylaxis beyond the 12-24 weeks of DAA therapy to 6-12 months after cessation of DAA therapy, tailored by clinician judgment and patient preference.
A ‘Useful Clinical Tool’
Commenting on the guideline but not involved in it, Saikiran Kilaru, MD, a hepatologist at NYU Langone Health in New York City, said the update is “absolutely a useful clinical tool. Since the prior guidance was published, there has been a deluge of new medications and medication classes. Prior to the guidance, I was making recommendations based on the limited data available for hepatitis B reactivation risk for these new medications, using the 1%-10% moderate-risk category as guidance.”
In addition, Kilaru said, this guidance is driven by a higher level of evidence certainty than the mostly retrospective evidence that was previously available.
She cautioned that few downgraded risk categories are likely to cause consternation among physicians who have been operating without the benefit of larger meta-analyses of HBVr in new medication categories. “For example, the prior guidance had put anti-TNF as of moderate risk for hepatitis B core–positive-only patients and is now downgraded to low risk.” And other medications such as immune checkpoint inhibitors, which seemed to pose at least moderate risk based on smaller, retrospective studies are now considered to be in the low-risk category.
“It may take some time for these recommendations to be adopted, especially for physicians in the community who have seen fatal or severe reactivations in the past few years,” Kilaru said.
Kilaru pointed out that the guidance update does not clearly cover some standard immunosuppressive therapies used in autoimmune, rheumatologic, and posttransplant regimens, such as mycophenolate, tacrolimus, and cyclosporine. Nor does it address HBVr risk in some liver cancer treatments such as yttrium-90, which have been associated with reports of HBV reactivation.
The Future
According to Simon, more data are needed to better estimate HBVr risk in several important settings, including treatment with the most recently approved immunosuppressive drugs for which data are still limited, as well as combination treatments.
Kilaru noted that guideline updates such as this become increasingly relevant as cancer diagnoses rise and hepatitis B exposure and detection increase as well.
The AGA panel acknowledged that uncertainty remains in some patient risk categorizations. “As the armamentarium of immunotherapeutics evolves, it will be crucial to search for, use, and maintain studies that provide baseline HBV serologies; include a clear definition of HBVr; and enroll a large, nonselective cohort that can guide categorization of risk of HBVr,” the panelists wrote.
AGA provided all financial support for the development of this guideline. No funding from industry was offered or accepted to support the writing effort.
The authors reported no relevant competing interests, but one coauthor is an adviser for Gilead Sciences, and other authors disclosed various relationships with multiple private sector companies. Kilaru had no competing interests to disclose.
A version of this article appeared on Medscape.com.
The document was published in Gastroenterology and replaces a previous guideline on prophylaxis for immunosuppressed patients issued in 2014.
Since then, many novel classes of immunosuppressives have been approved for various conditions, and potentially immunosuppressive therapies such as transcatheter arterial chemoembolization have been recognized as relevant to potential HBVr.
With reactivation a risk after immune-modulating exposures, such as to multiple drug classes and disease states, the update provides frontline clinicians with evidence-based advice for the management of HBVr in vulnerable individuals. And while antiviral prophylaxis is recommended for many, in select cases careful clinical monitoring may suffice for risk management.
“The risk of HBV reactivation depends on patient-, drug-, and disease-specific factors — and so it can range from very rare to more frequent,” said guideline coauthor Tracey G. Simon, MD, MPH, a hepatologist in the division of gastroenterology at Massachusetts General Hospital and an instructor at Harvard Medical School, both in Boston. “Not every at-risk individual needs pharmacologic treatment, but some certainly do, and this guideline was designed to try to better identify who needs treatment, based on those important drug- and virus-specific factors.”
Simon stressed the importance of creating this guideline to include many new therapies that carry varying degrees of reactivation risk. As to the strength of the evidence, she added, “for some of the questions, the panel was satisfied with the level of certainty. However, for other questions, the data are still very sparse, and so we have tried to ensure that these areas of uncertainty are highlighted clearly for providers and patients.”
Main Recommendations
AGA based its clinical recommendations on balancing desirable and undesirable effects, patient values and preferences, costs, and health equity considerations. It also provided a clinical decision support tool for making pharmacologic management decisions.
The panelists reviewed data on multiple immunosuppressive therapies from older agents such as anthracycline derivatives, corticosteroids, and anti–tumor necrosis factor (anti-TNF) drugs to chimeric antigen receptor T cells and recent biologics and inhibitors.
1. For individuals at high risk for HBVr, AGA recommended antiviral prophylaxis over monitoring alone. Strong recommendation, moderate-certainty evidence.
Implementation considerations: Use antivirals with a high barrier to resistance. Prophylaxis should be started before initiating medications that carry a risk for HBVr and should be continued for at least 6 months after discontinuation of risk-imposing therapy (at least 12 months for B cell–depleting agents).
2. For individuals at moderate risk for HBVr, antiviral prophylaxis was recommended over monitoring alone. Conditional recommendation, moderate-certainty evidence.
Implementation considerations: Use antivirals with a high barrier to resistance. Patients who place a higher value on avoiding long-term antiviral therapy and its associated cost and place a lower value on avoiding the small risk of reactivation (particularly those who are hepatitis B surface antigen [HBsAg]–negative) may reasonably select active monitoring over antiviral prophylaxis.
Careful consideration should be given to the feasibility and likelihood of adherence to long-term monitoring performed at 1- to 3-month intervals and including assessment of hepatitis B viral load and alanine aminotransferase.
3. For low-risk individuals, the AGA said monitoring alone may be used. Conditional recommendation, moderate-certainty evidence.
Implementation considerations: This recommendation assumes regular and sufficient follow-up with continued monitoring. Patients who place a higher value on avoiding the small risk of reactivation (particularly those on more than one low-risk immunosuppressive) and a lower value on the burden and cost of antiviral therapy may reasonably select antiviral therapy.
4. For individuals at risk for HBVr, the guideline recommended testing for hepatitis B. Strong recommendation, moderate-certainty evidence.
Implementation considerations: Given the Centers for Disease Control and Prevention’s universal screening guidance on hepatitis B for everyone aged 18 years or older by testing for HBsAg, anti-HBs, and total anti-hepatitis B core (HBc), the guideline said that stratifying screening practices by magnitude of HBVr risk is no longer needed.
It is reasonable to test initially for serologic markers alone (at minimum for HBsAg or anti-HBc) followed by viral load testing (HBV-DNA) if HBsAg and/or anti-HBc is positive.
Hepatitis C Virus (HCV) Coinfection With Direct-Acting Antiviral (DAA) Treatment
The panel identified 11 studies that provided data for the computation of baseline risk for HBVr in the HCV coinfection cohort undergoing DAA therapy.
In patients who were HBsAg-positive, the pooled baseline risk for HBVr was 240 per 1000, categorizing them to be at high risk for HBVr. The panel stated it is therefore reasonable to extend antiviral prophylaxis beyond the 12-24 weeks of DAA therapy to 6-12 months after cessation of DAA therapy, tailored by clinician judgment and patient preference.
A ‘Useful Clinical Tool’
Commenting on the guideline but not involved in it, Saikiran Kilaru, MD, a hepatologist at NYU Langone Health in New York City, said the update is “absolutely a useful clinical tool. Since the prior guidance was published, there has been a deluge of new medications and medication classes. Prior to the guidance, I was making recommendations based on the limited data available for hepatitis B reactivation risk for these new medications, using the 1%-10% moderate-risk category as guidance.”
In addition, Kilaru said, this guidance is driven by a higher level of evidence certainty than the mostly retrospective evidence that was previously available.
She cautioned that few downgraded risk categories are likely to cause consternation among physicians who have been operating without the benefit of larger meta-analyses of HBVr in new medication categories. “For example, the prior guidance had put anti-TNF as of moderate risk for hepatitis B core–positive-only patients and is now downgraded to low risk.” And other medications such as immune checkpoint inhibitors, which seemed to pose at least moderate risk based on smaller, retrospective studies are now considered to be in the low-risk category.
“It may take some time for these recommendations to be adopted, especially for physicians in the community who have seen fatal or severe reactivations in the past few years,” Kilaru said.
Kilaru pointed out that the guidance update does not clearly cover some standard immunosuppressive therapies used in autoimmune, rheumatologic, and posttransplant regimens, such as mycophenolate, tacrolimus, and cyclosporine. Nor does it address HBVr risk in some liver cancer treatments such as yttrium-90, which have been associated with reports of HBV reactivation.
The Future
According to Simon, more data are needed to better estimate HBVr risk in several important settings, including treatment with the most recently approved immunosuppressive drugs for which data are still limited, as well as combination treatments.
Kilaru noted that guideline updates such as this become increasingly relevant as cancer diagnoses rise and hepatitis B exposure and detection increase as well.
The AGA panel acknowledged that uncertainty remains in some patient risk categorizations. “As the armamentarium of immunotherapeutics evolves, it will be crucial to search for, use, and maintain studies that provide baseline HBV serologies; include a clear definition of HBVr; and enroll a large, nonselective cohort that can guide categorization of risk of HBVr,” the panelists wrote.
AGA provided all financial support for the development of this guideline. No funding from industry was offered or accepted to support the writing effort.
The authors reported no relevant competing interests, but one coauthor is an adviser for Gilead Sciences, and other authors disclosed various relationships with multiple private sector companies. Kilaru had no competing interests to disclose.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY





