User login
Primary Sclerosing Cholangitis (PSC) and Its Importance in Clinical Practice
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
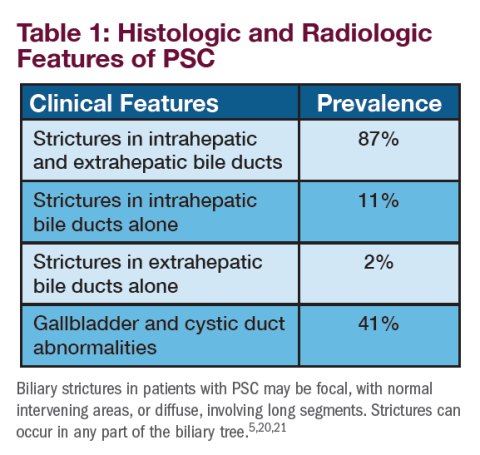
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Integrated Artificial Intelligence Screening to Optimize Patient Identification for Bronchoscopic Lung Volume Reduction Therapy: Redefining Patient Selection with SeleCT™ Screening
See how pilot programs at an academic center and a community hospital demonstrated how an AI-powered screening tool better identifies candidates for bronchoscopic lung volume reduction (BLVR). The result is improved access and accelerated time to intervention for patients with severe emphysema, as well as lowered burdens on healthcare systems.
Click here to read more
See how pilot programs at an academic center and a community hospital demonstrated how an AI-powered screening tool better identifies candidates for bronchoscopic lung volume reduction (BLVR). The result is improved access and accelerated time to intervention for patients with severe emphysema, as well as lowered burdens on healthcare systems.
Click here to read more
See how pilot programs at an academic center and a community hospital demonstrated how an AI-powered screening tool better identifies candidates for bronchoscopic lung volume reduction (BLVR). The result is improved access and accelerated time to intervention for patients with severe emphysema, as well as lowered burdens on healthcare systems.
Click here to read more
AGA Guidelines Endorse Earlier Use of High-Efficacy Drugs for Ulcerative Colitis
In a rapidly expanding therapeutic landscape,
“These are the first living guidelines published by a GI society, highlighting the interest and need to provide timely guidance to all stakeholders in a rapidly evolving field,” first author Siddharth Singh, MD, of the Division of Gastroenterology in the Department of Medicine at University of California, San Diego, said in an interview. Living guidance allows for ongoing revision of individual recommendations as new data emerge. Nearly 2 million Americans have UC.
Issued in Gastroenterology and updating the last guidance in 2020, the recommendations suggest more efficacious drugs should be used sooner. “Early use of advanced therapies including biologics and small-molecule drugs are more effective than 5-aminosalicylates [5-ASAs] or thiopurines and methotrexate for most patients with moderate to severe UC and those with poor prognostic factors,” coauthor and gastroenterologist Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“We provide a practical guidance based on best-available evidence to make it easy for the treating clinician to make informed choices from the multiplicity of available treatments for UC,” added guidelines coauthor Ashwin Ananthakrishnan, MBBS, MPH, AGAF, a gastroenterologist at Massachusetts General Hospital in Boston.
The comprehensive, patient-centered document comes with this caveat from the AGA panel: “These guidelines are meant to be broad recommendations for management of patients with moderate to severe UC and are not intended to address the intricacies of individual patients,” they wrote. “Provider experience and patient values and preferences can inform treating providers and patients to reasonably choose alternative treatment options.”
One gastroenterologist who has been eagerly awaiting these guidelines but not involved in the panel is James D. Lewis, MD, MSCE, AGAF, a professor of medicine and epidemiology at Perelman School of Medicine at the University of Pennsylvania, Philadelphia. “The choice of medications for moderately to severely active UC has expanded tremendously in the past few years,” he said in an interview. “This resulted in the dismantling of the historical therapeutic pyramid.” And while there are many more treatment options, knowing which medication to use for which patient and in which sequence has become much more complicated.
“These guidelines will be extremely helpful for clinicians trying to navigate this new era of UC care,” he said.
The guidelines also outline implementation considerations for optimal use in different scenarios. “Key considerations include patient-related factors such as age, frailty, other health conditions, consideration for pregnancy, patient preferences, and access to healthcare,” Agrawal said.
Specifics
Overall, the guidance recommends advanced or immunomodulatory therapy after failure of 5-ASAs rather than a step-up approach. Moderate to severe disease is defined as a Mayo endoscopic severity subscore of 2 or 3.
The recommendation may also apply to mild disease in the presence of a high burden of inflammation and a poor prognosis or steroid dependence or resistance.
The AGA guideline panelists took account of differences in treatment efficacy between drugs within the same therapeutic class and made their recommendations by specific drugs rather than therapy class.
Based on varying degrees of evidence certainty, the AGA recommends or suggests the following management specifics in adult outpatients with moderate to severe disease:
- Any of the following is recommended over no treatment: infliximab (Remicade), golimumab (Simponi), vedolizumab (Entyvio), tofacitinib (Xeljanz), upadacitinib (Rinvoq), ustekinumab (Stelara), ozanimod (Zeposia), etrasimod (Velsipity), risankizumab (Skyrizi), and guselkumab (Tremfya).
- Adalimumab (Humira), filgotinib (Jyseleca), and mirikizumab (Omvoh) are suggested over no treatment.
- Biosimilars to infliximab, adalimumab, and ustekinumab can be considered of equivalent efficacy to their originator drugs.
- For patients naive to advanced therapies, the AGA panel proposes using a higher-efficacy medication (eg, infliximab, vedolizumab, ozanimod, etrasimod, upadacitinib, risankizumab, and guselkumab) or an intermediate-efficacy medication (golimumab, ustekinumab, tofacitinib, filgotinib, and mirikizumab) rather than a lower-efficacy medication such as adalimumab.
- In patients previously exposed to advanced therapy, particularly tumor necrosis factor (TNF)–alpha antagonists, the panel suggests using a higher-efficacy medication (tofacitinib, upadacitinib, and ustekinumab) or an intermediate-efficacy agent (filgotinib, mirikizumab, risankizumab, and guselkumab) over a lower-efficacy medication (adalimumab, vedolizumab, ozanimod, and etrasimod).
- The panel suggests against the use of thiopurine monotherapy for inducing remission but suggests thiopurine monotherapy over no treatment for maintenance of (typically corticosteroid-induced) remission.
- The panel suggests against the use of methotrexate monotherapy for induction or maintenance of remission.
- Infliximab, adalimumab, and golimumab in combination with an immunomodulator are suggested over monotherapy.
- The panel makes no recommendation for or against non-TNF antagonist biologics in combination with an immunomodulator over non-TNF biologics alone.
- For patients in corticosteroid-free clinical remission for at least 6 months on combination therapy with TNF antagonists and immunomodulators, the panel suggests against withdrawing TNF antagonists but makes no recommendation for or against withdrawing immunomodulators.
- For those who have failed 5-ASAs and have escalated to immunomodulators or advanced therapies, the panel suggests stopping these agents. It suggests the early use of advanced therapies and/or immunomodulator therapy rather than gradual step-up after failure of 5-ASAs.
According to Lewis, the guidance will be useful to both community physicians and highly specialized gastroenterologists. “While few practicing physicians will be able to commit the entirety of the classifications in this guideline to memory, the tool is a quick reference resource to help providers and patients to choose between the many options,” he said.
However, he noted that not all patients and providers may have the same priorities as the guidelines. “There are a few nuances to the methods of the AGA guidelines. For example, the panel prioritized efficacy over safety because the incidence of serious adverse events secondary to medications is relatively rare.”
Lewis also noted that the way the panel classified higher-, intermediate-, and lower-efficacy medications sometimes produced surprising results. “For example, among patients naive to advanced therapies, the IL [interleukin]–23 inhibitors risankizumab and guselkumab were classified as higher efficacy, while the IL-12/23 inhibitor ustekinumab was considered intermediate efficacy,” he said. “These were reversed for patients with prior exposure to advanced therapies, where ustekinumab was considered higher efficacy and all three IL-23 inhibitors were considered intermediate efficacy.”
The Future
The panel identified several knowledge gaps that future studies should address. These include a paucity of head-to-head comparison trials, including active comparators to accurately inform positioning of different treatments and therapeutic mechanisms.
The panelists also noted a literature gap on the efficacy of different therapies in the setting of failure or intolerance to non-TNF antagonist advanced therapy, which could be relevant to drugs that may have a greater overlap in their therapeutic mechanisms — for instance, anti-trafficking agents.
They pointed to a paucity of data on how predictive models can inform future treatment selection in the real-world setting. “There is clearly a need for identifying biomarkers predictive of response to individual therapies, to facilitate optimal choice of therapies,” they wrote.
The panel also recognized that novel therapeutic strategies may soon be in use, including combination advanced therapy or episodic use of nonimmunogenic advanced therapies such as small molecules. “Further primary data are required to accurately inform the positioning of such strategies,” they wrote.
These guidelines were fully funded by the AGA Institute. Singh and Agrawal are supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), and Ananthakrishnan is supported by the NIDDK, as well as by the Leona M. and Harry B. Helmsley Charitable Trust and the Chleck Family Foundation. Singh disclosed Institutional research grants from Pfizer. Agrawal reported consulting for Douglas Pharmaceuticals. Several coauthors disclosed receiving consulting fees and/or research support from various private companies in the healthcare field. One author reported stock ownership stock in Exact Sciences. Lewis reported consulting, advisory board service, or data monitoring for Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen Pharmaceuticals, Merck, Pfizer, Protagonist Therapeutics, and Sanofi. He received research funding or in-kind support from Nestle Health Science, Takeda, Janssen Pharmaceuticals, AbbVie, and Eli Lilly and has had educational grants from Janssen.
A version of this article appeared on Medscape.com.
In a rapidly expanding therapeutic landscape,
“These are the first living guidelines published by a GI society, highlighting the interest and need to provide timely guidance to all stakeholders in a rapidly evolving field,” first author Siddharth Singh, MD, of the Division of Gastroenterology in the Department of Medicine at University of California, San Diego, said in an interview. Living guidance allows for ongoing revision of individual recommendations as new data emerge. Nearly 2 million Americans have UC.
Issued in Gastroenterology and updating the last guidance in 2020, the recommendations suggest more efficacious drugs should be used sooner. “Early use of advanced therapies including biologics and small-molecule drugs are more effective than 5-aminosalicylates [5-ASAs] or thiopurines and methotrexate for most patients with moderate to severe UC and those with poor prognostic factors,” coauthor and gastroenterologist Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“We provide a practical guidance based on best-available evidence to make it easy for the treating clinician to make informed choices from the multiplicity of available treatments for UC,” added guidelines coauthor Ashwin Ananthakrishnan, MBBS, MPH, AGAF, a gastroenterologist at Massachusetts General Hospital in Boston.
The comprehensive, patient-centered document comes with this caveat from the AGA panel: “These guidelines are meant to be broad recommendations for management of patients with moderate to severe UC and are not intended to address the intricacies of individual patients,” they wrote. “Provider experience and patient values and preferences can inform treating providers and patients to reasonably choose alternative treatment options.”
One gastroenterologist who has been eagerly awaiting these guidelines but not involved in the panel is James D. Lewis, MD, MSCE, AGAF, a professor of medicine and epidemiology at Perelman School of Medicine at the University of Pennsylvania, Philadelphia. “The choice of medications for moderately to severely active UC has expanded tremendously in the past few years,” he said in an interview. “This resulted in the dismantling of the historical therapeutic pyramid.” And while there are many more treatment options, knowing which medication to use for which patient and in which sequence has become much more complicated.
“These guidelines will be extremely helpful for clinicians trying to navigate this new era of UC care,” he said.
The guidelines also outline implementation considerations for optimal use in different scenarios. “Key considerations include patient-related factors such as age, frailty, other health conditions, consideration for pregnancy, patient preferences, and access to healthcare,” Agrawal said.
Specifics
Overall, the guidance recommends advanced or immunomodulatory therapy after failure of 5-ASAs rather than a step-up approach. Moderate to severe disease is defined as a Mayo endoscopic severity subscore of 2 or 3.
The recommendation may also apply to mild disease in the presence of a high burden of inflammation and a poor prognosis or steroid dependence or resistance.
The AGA guideline panelists took account of differences in treatment efficacy between drugs within the same therapeutic class and made their recommendations by specific drugs rather than therapy class.
Based on varying degrees of evidence certainty, the AGA recommends or suggests the following management specifics in adult outpatients with moderate to severe disease:
- Any of the following is recommended over no treatment: infliximab (Remicade), golimumab (Simponi), vedolizumab (Entyvio), tofacitinib (Xeljanz), upadacitinib (Rinvoq), ustekinumab (Stelara), ozanimod (Zeposia), etrasimod (Velsipity), risankizumab (Skyrizi), and guselkumab (Tremfya).
- Adalimumab (Humira), filgotinib (Jyseleca), and mirikizumab (Omvoh) are suggested over no treatment.
- Biosimilars to infliximab, adalimumab, and ustekinumab can be considered of equivalent efficacy to their originator drugs.
- For patients naive to advanced therapies, the AGA panel proposes using a higher-efficacy medication (eg, infliximab, vedolizumab, ozanimod, etrasimod, upadacitinib, risankizumab, and guselkumab) or an intermediate-efficacy medication (golimumab, ustekinumab, tofacitinib, filgotinib, and mirikizumab) rather than a lower-efficacy medication such as adalimumab.
- In patients previously exposed to advanced therapy, particularly tumor necrosis factor (TNF)–alpha antagonists, the panel suggests using a higher-efficacy medication (tofacitinib, upadacitinib, and ustekinumab) or an intermediate-efficacy agent (filgotinib, mirikizumab, risankizumab, and guselkumab) over a lower-efficacy medication (adalimumab, vedolizumab, ozanimod, and etrasimod).
- The panel suggests against the use of thiopurine monotherapy for inducing remission but suggests thiopurine monotherapy over no treatment for maintenance of (typically corticosteroid-induced) remission.
- The panel suggests against the use of methotrexate monotherapy for induction or maintenance of remission.
- Infliximab, adalimumab, and golimumab in combination with an immunomodulator are suggested over monotherapy.
- The panel makes no recommendation for or against non-TNF antagonist biologics in combination with an immunomodulator over non-TNF biologics alone.
- For patients in corticosteroid-free clinical remission for at least 6 months on combination therapy with TNF antagonists and immunomodulators, the panel suggests against withdrawing TNF antagonists but makes no recommendation for or against withdrawing immunomodulators.
- For those who have failed 5-ASAs and have escalated to immunomodulators or advanced therapies, the panel suggests stopping these agents. It suggests the early use of advanced therapies and/or immunomodulator therapy rather than gradual step-up after failure of 5-ASAs.
According to Lewis, the guidance will be useful to both community physicians and highly specialized gastroenterologists. “While few practicing physicians will be able to commit the entirety of the classifications in this guideline to memory, the tool is a quick reference resource to help providers and patients to choose between the many options,” he said.
However, he noted that not all patients and providers may have the same priorities as the guidelines. “There are a few nuances to the methods of the AGA guidelines. For example, the panel prioritized efficacy over safety because the incidence of serious adverse events secondary to medications is relatively rare.”
Lewis also noted that the way the panel classified higher-, intermediate-, and lower-efficacy medications sometimes produced surprising results. “For example, among patients naive to advanced therapies, the IL [interleukin]–23 inhibitors risankizumab and guselkumab were classified as higher efficacy, while the IL-12/23 inhibitor ustekinumab was considered intermediate efficacy,” he said. “These were reversed for patients with prior exposure to advanced therapies, where ustekinumab was considered higher efficacy and all three IL-23 inhibitors were considered intermediate efficacy.”
The Future
The panel identified several knowledge gaps that future studies should address. These include a paucity of head-to-head comparison trials, including active comparators to accurately inform positioning of different treatments and therapeutic mechanisms.
The panelists also noted a literature gap on the efficacy of different therapies in the setting of failure or intolerance to non-TNF antagonist advanced therapy, which could be relevant to drugs that may have a greater overlap in their therapeutic mechanisms — for instance, anti-trafficking agents.
They pointed to a paucity of data on how predictive models can inform future treatment selection in the real-world setting. “There is clearly a need for identifying biomarkers predictive of response to individual therapies, to facilitate optimal choice of therapies,” they wrote.
The panel also recognized that novel therapeutic strategies may soon be in use, including combination advanced therapy or episodic use of nonimmunogenic advanced therapies such as small molecules. “Further primary data are required to accurately inform the positioning of such strategies,” they wrote.
These guidelines were fully funded by the AGA Institute. Singh and Agrawal are supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), and Ananthakrishnan is supported by the NIDDK, as well as by the Leona M. and Harry B. Helmsley Charitable Trust and the Chleck Family Foundation. Singh disclosed Institutional research grants from Pfizer. Agrawal reported consulting for Douglas Pharmaceuticals. Several coauthors disclosed receiving consulting fees and/or research support from various private companies in the healthcare field. One author reported stock ownership stock in Exact Sciences. Lewis reported consulting, advisory board service, or data monitoring for Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen Pharmaceuticals, Merck, Pfizer, Protagonist Therapeutics, and Sanofi. He received research funding or in-kind support from Nestle Health Science, Takeda, Janssen Pharmaceuticals, AbbVie, and Eli Lilly and has had educational grants from Janssen.
A version of this article appeared on Medscape.com.
In a rapidly expanding therapeutic landscape,
“These are the first living guidelines published by a GI society, highlighting the interest and need to provide timely guidance to all stakeholders in a rapidly evolving field,” first author Siddharth Singh, MD, of the Division of Gastroenterology in the Department of Medicine at University of California, San Diego, said in an interview. Living guidance allows for ongoing revision of individual recommendations as new data emerge. Nearly 2 million Americans have UC.
Issued in Gastroenterology and updating the last guidance in 2020, the recommendations suggest more efficacious drugs should be used sooner. “Early use of advanced therapies including biologics and small-molecule drugs are more effective than 5-aminosalicylates [5-ASAs] or thiopurines and methotrexate for most patients with moderate to severe UC and those with poor prognostic factors,” coauthor and gastroenterologist Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“We provide a practical guidance based on best-available evidence to make it easy for the treating clinician to make informed choices from the multiplicity of available treatments for UC,” added guidelines coauthor Ashwin Ananthakrishnan, MBBS, MPH, AGAF, a gastroenterologist at Massachusetts General Hospital in Boston.
The comprehensive, patient-centered document comes with this caveat from the AGA panel: “These guidelines are meant to be broad recommendations for management of patients with moderate to severe UC and are not intended to address the intricacies of individual patients,” they wrote. “Provider experience and patient values and preferences can inform treating providers and patients to reasonably choose alternative treatment options.”
One gastroenterologist who has been eagerly awaiting these guidelines but not involved in the panel is James D. Lewis, MD, MSCE, AGAF, a professor of medicine and epidemiology at Perelman School of Medicine at the University of Pennsylvania, Philadelphia. “The choice of medications for moderately to severely active UC has expanded tremendously in the past few years,” he said in an interview. “This resulted in the dismantling of the historical therapeutic pyramid.” And while there are many more treatment options, knowing which medication to use for which patient and in which sequence has become much more complicated.
“These guidelines will be extremely helpful for clinicians trying to navigate this new era of UC care,” he said.
The guidelines also outline implementation considerations for optimal use in different scenarios. “Key considerations include patient-related factors such as age, frailty, other health conditions, consideration for pregnancy, patient preferences, and access to healthcare,” Agrawal said.
Specifics
Overall, the guidance recommends advanced or immunomodulatory therapy after failure of 5-ASAs rather than a step-up approach. Moderate to severe disease is defined as a Mayo endoscopic severity subscore of 2 or 3.
The recommendation may also apply to mild disease in the presence of a high burden of inflammation and a poor prognosis or steroid dependence or resistance.
The AGA guideline panelists took account of differences in treatment efficacy between drugs within the same therapeutic class and made their recommendations by specific drugs rather than therapy class.
Based on varying degrees of evidence certainty, the AGA recommends or suggests the following management specifics in adult outpatients with moderate to severe disease:
- Any of the following is recommended over no treatment: infliximab (Remicade), golimumab (Simponi), vedolizumab (Entyvio), tofacitinib (Xeljanz), upadacitinib (Rinvoq), ustekinumab (Stelara), ozanimod (Zeposia), etrasimod (Velsipity), risankizumab (Skyrizi), and guselkumab (Tremfya).
- Adalimumab (Humira), filgotinib (Jyseleca), and mirikizumab (Omvoh) are suggested over no treatment.
- Biosimilars to infliximab, adalimumab, and ustekinumab can be considered of equivalent efficacy to their originator drugs.
- For patients naive to advanced therapies, the AGA panel proposes using a higher-efficacy medication (eg, infliximab, vedolizumab, ozanimod, etrasimod, upadacitinib, risankizumab, and guselkumab) or an intermediate-efficacy medication (golimumab, ustekinumab, tofacitinib, filgotinib, and mirikizumab) rather than a lower-efficacy medication such as adalimumab.
- In patients previously exposed to advanced therapy, particularly tumor necrosis factor (TNF)–alpha antagonists, the panel suggests using a higher-efficacy medication (tofacitinib, upadacitinib, and ustekinumab) or an intermediate-efficacy agent (filgotinib, mirikizumab, risankizumab, and guselkumab) over a lower-efficacy medication (adalimumab, vedolizumab, ozanimod, and etrasimod).
- The panel suggests against the use of thiopurine monotherapy for inducing remission but suggests thiopurine monotherapy over no treatment for maintenance of (typically corticosteroid-induced) remission.
- The panel suggests against the use of methotrexate monotherapy for induction or maintenance of remission.
- Infliximab, adalimumab, and golimumab in combination with an immunomodulator are suggested over monotherapy.
- The panel makes no recommendation for or against non-TNF antagonist biologics in combination with an immunomodulator over non-TNF biologics alone.
- For patients in corticosteroid-free clinical remission for at least 6 months on combination therapy with TNF antagonists and immunomodulators, the panel suggests against withdrawing TNF antagonists but makes no recommendation for or against withdrawing immunomodulators.
- For those who have failed 5-ASAs and have escalated to immunomodulators or advanced therapies, the panel suggests stopping these agents. It suggests the early use of advanced therapies and/or immunomodulator therapy rather than gradual step-up after failure of 5-ASAs.
According to Lewis, the guidance will be useful to both community physicians and highly specialized gastroenterologists. “While few practicing physicians will be able to commit the entirety of the classifications in this guideline to memory, the tool is a quick reference resource to help providers and patients to choose between the many options,” he said.
However, he noted that not all patients and providers may have the same priorities as the guidelines. “There are a few nuances to the methods of the AGA guidelines. For example, the panel prioritized efficacy over safety because the incidence of serious adverse events secondary to medications is relatively rare.”
Lewis also noted that the way the panel classified higher-, intermediate-, and lower-efficacy medications sometimes produced surprising results. “For example, among patients naive to advanced therapies, the IL [interleukin]–23 inhibitors risankizumab and guselkumab were classified as higher efficacy, while the IL-12/23 inhibitor ustekinumab was considered intermediate efficacy,” he said. “These were reversed for patients with prior exposure to advanced therapies, where ustekinumab was considered higher efficacy and all three IL-23 inhibitors were considered intermediate efficacy.”
The Future
The panel identified several knowledge gaps that future studies should address. These include a paucity of head-to-head comparison trials, including active comparators to accurately inform positioning of different treatments and therapeutic mechanisms.
The panelists also noted a literature gap on the efficacy of different therapies in the setting of failure or intolerance to non-TNF antagonist advanced therapy, which could be relevant to drugs that may have a greater overlap in their therapeutic mechanisms — for instance, anti-trafficking agents.
They pointed to a paucity of data on how predictive models can inform future treatment selection in the real-world setting. “There is clearly a need for identifying biomarkers predictive of response to individual therapies, to facilitate optimal choice of therapies,” they wrote.
The panel also recognized that novel therapeutic strategies may soon be in use, including combination advanced therapy or episodic use of nonimmunogenic advanced therapies such as small molecules. “Further primary data are required to accurately inform the positioning of such strategies,” they wrote.
These guidelines were fully funded by the AGA Institute. Singh and Agrawal are supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), and Ananthakrishnan is supported by the NIDDK, as well as by the Leona M. and Harry B. Helmsley Charitable Trust and the Chleck Family Foundation. Singh disclosed Institutional research grants from Pfizer. Agrawal reported consulting for Douglas Pharmaceuticals. Several coauthors disclosed receiving consulting fees and/or research support from various private companies in the healthcare field. One author reported stock ownership stock in Exact Sciences. Lewis reported consulting, advisory board service, or data monitoring for Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen Pharmaceuticals, Merck, Pfizer, Protagonist Therapeutics, and Sanofi. He received research funding or in-kind support from Nestle Health Science, Takeda, Janssen Pharmaceuticals, AbbVie, and Eli Lilly and has had educational grants from Janssen.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Pharmacist Advocates for Early Adoption of Quadruple Therapy in HFrEF Treatment
SAN DIEGO — An Air Force pharmacist urged colleagues in the military to advocate for the gold standard of quadruple therapy in patients with heart failure with reduced ejection fraction (HFrEF). “When possible, initiate and optimize quadruple therapy before discharge; don’t leave it for a primary care manager (PCM) to handle,” said Maj. Elizabeth Tesch, PharmD, of Maxwell Air Force Base, Montgomery, Ala., in a presentation here at the Joint Federal Pharmacy Seminar. Tesch also cautioned colleagues about the proper use of IV inotropes and vasodilators in congestive heart failure and warned of the dangers of polypharmacy.
“It’s just as important to use medications that provide a mortality benefit in these patients as it is to remove things that are either harmful or lack trial benefit data,” Tesch said.
In patients with acute heart failure and systolic blood pressure < 90 mmHg, guidelines recommend using both an inotrope and a vasopressor. “There tends to be better data about 2 of them together vs just cranking up a vasoconstrictor, which we tend to sometimes to do when a patient’s blood pressure is bottoming out,” Tesch explained. “But in these patients specifically, that tends to lead to increased afterload, difficulty with cardiac output, and then increased risk of ischemia. So it tends to be better to use both.”
Ideally, Tesch said, patients stabilize within a couple days. In cases of HFrEF, this is when quadruple therapy can enter the picture.
Quadruple therapy consists of the “4 pillars”: a sodium-glucose co-transporter 2 inhibitor (SGLT2i), a β blocker, a mineralocorticoid receptor antagonist (MRA), and either an angiotensin receptor neprilysin inhibitor (ARNI), an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB).
Tesch noted that the need for titration varies by drug. β blockers typically will need the most up-titration, often in several steps, followed by ARNIs. MRAs may require only one titration or even not at all, and SGLT2 inhibitors do not require titration.
“[Clinicians] are most comfortable giving ACE inhibitors, ARBs, and β blockers to patients, she said. But new research suggests there is a 10.3% jump in mortality risk (absolute risk difference) compared to ACEi/ β blocker/ARB therapy. Additionally, a 2022 systematic review linked quadruple therapy to a gain of 5 years of life (ranging from 2.5 to7.5 years) for 70-year-old patients compared to no therapy.
“I don't know how many times I've had a conversation along the lines of, ‘Hey, can we go ahead and start an SGLT2 on this patient?’ only to hear, ‘We'll give that to the PCM [primary care manager]. That sounds like a PCM thing. You just want to get them out of here, it’s a PCM problem.’”
But quick initiation of treatment is crucial. “We're seeing very real mortality benefit data very quickly in these patients,” Tesch said.
As for polypharmacy, Tesch highlighted the importance of reducing mediation load when possible. “If they have nothing else wrong, these patients will walk out the door on quadruple therapy and perhaps a diuretic, but they probably have a lot more going on,” she said. “All of us in this room are fully aware of what polypharmacy can do to these patients: increased drug interactions, side effects, higher cost, and decreased patient compliance. This is a problem for the heart failure population that really translates into readmissions and increased mortality. We've got to be able to peel off things that are either harmful or not helping.”
Statins, for example, have questionable benefit in HFrEF without coronary artery disease or hyperlipidemia, she said. Oral iron and vitamin D supplementation also have uncertain benefits in the HFrEF population.
Tesch highlighted a pair of reports – one from 2024 and the other from 2022 – that recommended certain therapies in heart failure, including the antidepressant citalopram (Celexa), the hypertension/urinary retention drug doxazosin (Cardura), and DPP-4 inhibitors (eg, diabetes/weight-loss drugs such as liraglutide [Saxenda]).
Tesch has no disclosures.
SAN DIEGO — An Air Force pharmacist urged colleagues in the military to advocate for the gold standard of quadruple therapy in patients with heart failure with reduced ejection fraction (HFrEF). “When possible, initiate and optimize quadruple therapy before discharge; don’t leave it for a primary care manager (PCM) to handle,” said Maj. Elizabeth Tesch, PharmD, of Maxwell Air Force Base, Montgomery, Ala., in a presentation here at the Joint Federal Pharmacy Seminar. Tesch also cautioned colleagues about the proper use of IV inotropes and vasodilators in congestive heart failure and warned of the dangers of polypharmacy.
“It’s just as important to use medications that provide a mortality benefit in these patients as it is to remove things that are either harmful or lack trial benefit data,” Tesch said.
In patients with acute heart failure and systolic blood pressure < 90 mmHg, guidelines recommend using both an inotrope and a vasopressor. “There tends to be better data about 2 of them together vs just cranking up a vasoconstrictor, which we tend to sometimes to do when a patient’s blood pressure is bottoming out,” Tesch explained. “But in these patients specifically, that tends to lead to increased afterload, difficulty with cardiac output, and then increased risk of ischemia. So it tends to be better to use both.”
Ideally, Tesch said, patients stabilize within a couple days. In cases of HFrEF, this is when quadruple therapy can enter the picture.
Quadruple therapy consists of the “4 pillars”: a sodium-glucose co-transporter 2 inhibitor (SGLT2i), a β blocker, a mineralocorticoid receptor antagonist (MRA), and either an angiotensin receptor neprilysin inhibitor (ARNI), an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB).
Tesch noted that the need for titration varies by drug. β blockers typically will need the most up-titration, often in several steps, followed by ARNIs. MRAs may require only one titration or even not at all, and SGLT2 inhibitors do not require titration.
“[Clinicians] are most comfortable giving ACE inhibitors, ARBs, and β blockers to patients, she said. But new research suggests there is a 10.3% jump in mortality risk (absolute risk difference) compared to ACEi/ β blocker/ARB therapy. Additionally, a 2022 systematic review linked quadruple therapy to a gain of 5 years of life (ranging from 2.5 to7.5 years) for 70-year-old patients compared to no therapy.
“I don't know how many times I've had a conversation along the lines of, ‘Hey, can we go ahead and start an SGLT2 on this patient?’ only to hear, ‘We'll give that to the PCM [primary care manager]. That sounds like a PCM thing. You just want to get them out of here, it’s a PCM problem.’”
But quick initiation of treatment is crucial. “We're seeing very real mortality benefit data very quickly in these patients,” Tesch said.
As for polypharmacy, Tesch highlighted the importance of reducing mediation load when possible. “If they have nothing else wrong, these patients will walk out the door on quadruple therapy and perhaps a diuretic, but they probably have a lot more going on,” she said. “All of us in this room are fully aware of what polypharmacy can do to these patients: increased drug interactions, side effects, higher cost, and decreased patient compliance. This is a problem for the heart failure population that really translates into readmissions and increased mortality. We've got to be able to peel off things that are either harmful or not helping.”
Statins, for example, have questionable benefit in HFrEF without coronary artery disease or hyperlipidemia, she said. Oral iron and vitamin D supplementation also have uncertain benefits in the HFrEF population.
Tesch highlighted a pair of reports – one from 2024 and the other from 2022 – that recommended certain therapies in heart failure, including the antidepressant citalopram (Celexa), the hypertension/urinary retention drug doxazosin (Cardura), and DPP-4 inhibitors (eg, diabetes/weight-loss drugs such as liraglutide [Saxenda]).
Tesch has no disclosures.
SAN DIEGO — An Air Force pharmacist urged colleagues in the military to advocate for the gold standard of quadruple therapy in patients with heart failure with reduced ejection fraction (HFrEF). “When possible, initiate and optimize quadruple therapy before discharge; don’t leave it for a primary care manager (PCM) to handle,” said Maj. Elizabeth Tesch, PharmD, of Maxwell Air Force Base, Montgomery, Ala., in a presentation here at the Joint Federal Pharmacy Seminar. Tesch also cautioned colleagues about the proper use of IV inotropes and vasodilators in congestive heart failure and warned of the dangers of polypharmacy.
“It’s just as important to use medications that provide a mortality benefit in these patients as it is to remove things that are either harmful or lack trial benefit data,” Tesch said.
In patients with acute heart failure and systolic blood pressure < 90 mmHg, guidelines recommend using both an inotrope and a vasopressor. “There tends to be better data about 2 of them together vs just cranking up a vasoconstrictor, which we tend to sometimes to do when a patient’s blood pressure is bottoming out,” Tesch explained. “But in these patients specifically, that tends to lead to increased afterload, difficulty with cardiac output, and then increased risk of ischemia. So it tends to be better to use both.”
Ideally, Tesch said, patients stabilize within a couple days. In cases of HFrEF, this is when quadruple therapy can enter the picture.
Quadruple therapy consists of the “4 pillars”: a sodium-glucose co-transporter 2 inhibitor (SGLT2i), a β blocker, a mineralocorticoid receptor antagonist (MRA), and either an angiotensin receptor neprilysin inhibitor (ARNI), an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB).
Tesch noted that the need for titration varies by drug. β blockers typically will need the most up-titration, often in several steps, followed by ARNIs. MRAs may require only one titration or even not at all, and SGLT2 inhibitors do not require titration.
“[Clinicians] are most comfortable giving ACE inhibitors, ARBs, and β blockers to patients, she said. But new research suggests there is a 10.3% jump in mortality risk (absolute risk difference) compared to ACEi/ β blocker/ARB therapy. Additionally, a 2022 systematic review linked quadruple therapy to a gain of 5 years of life (ranging from 2.5 to7.5 years) for 70-year-old patients compared to no therapy.
“I don't know how many times I've had a conversation along the lines of, ‘Hey, can we go ahead and start an SGLT2 on this patient?’ only to hear, ‘We'll give that to the PCM [primary care manager]. That sounds like a PCM thing. You just want to get them out of here, it’s a PCM problem.’”
But quick initiation of treatment is crucial. “We're seeing very real mortality benefit data very quickly in these patients,” Tesch said.
As for polypharmacy, Tesch highlighted the importance of reducing mediation load when possible. “If they have nothing else wrong, these patients will walk out the door on quadruple therapy and perhaps a diuretic, but they probably have a lot more going on,” she said. “All of us in this room are fully aware of what polypharmacy can do to these patients: increased drug interactions, side effects, higher cost, and decreased patient compliance. This is a problem for the heart failure population that really translates into readmissions and increased mortality. We've got to be able to peel off things that are either harmful or not helping.”
Statins, for example, have questionable benefit in HFrEF without coronary artery disease or hyperlipidemia, she said. Oral iron and vitamin D supplementation also have uncertain benefits in the HFrEF population.
Tesch highlighted a pair of reports – one from 2024 and the other from 2022 – that recommended certain therapies in heart failure, including the antidepressant citalopram (Celexa), the hypertension/urinary retention drug doxazosin (Cardura), and DPP-4 inhibitors (eg, diabetes/weight-loss drugs such as liraglutide [Saxenda]).
Tesch has no disclosures.
AI Tool Identifies Undiagnosed Early-Stage MASLD
SAN DIEGO — according to new research.
Among the patients identified by the algorithm as meeting the criteria for MASLD, only a small percentage had an MASLD-associated diagnostic code.
“A significant portion of patients who meet criteria for MASLD go undiagnosed, which can lead to delays in care and progression to advanced liver disease,” said lead author Ariana Stuart, MD, an internal medicine resident at the University of Washington, Seattle, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“However, people shouldn’t interpret our findings as a lack of primary care training or management,” she said. “Instead, this study indicates that AI can complement physician workflow and address the limitations of traditional clinical practice.”
Developing an MASLD Algorithm
Typically, the identification of MASLD has relied on clinician recognition and descriptions in chart notes, Stuart said. Early-stage disease often goes unnoticed, particularly if patients remain asymptomatic, until cirrhosis develops.
To address this, Stuart and colleagues created a machine learning, natural language processing AI algorithm on the basis of MASLD criteria from AASLD: Hepatic steatosis on imaging and at least one metabolic factor (elevated body mass index, hypertension, prediabetes or diabetes, or dyslipidemia). The model was validated by two physicians, who manually reviewed monthly cohorts generated by the algorithm.
Between December 2023 and May 2024, the researchers used the algorithm to analyze an MASLD cohort from medical centers in the Seattle area. The mean age was 51 years, 44% were women, and 68% were White. Those with alcohol-associated liver disease, metastatic malignancy, and autoimmune, genetic, and infectious causes of liver disease were excluded.
The algorithm identified 957 patients with imaging that matched MASLD criteria.
Among those, 137 patients (17%) identified by the algorithm had an MASLD-associated diagnostic code. For these patients, the mean time from initial imaging with steatosis to diagnosis was 33 days, according to patient records.
An additional 26 patients received an MASLD diagnosis during the study period, with a mean time to diagnosis of 56.2 days.
In terms of patient management, 245 patients (26%) had contact with a gastroenterologist or hepatologist based on documentation of a letter, phone call, or office visit. In addition, 546 patients (57%) were screened for hepatitis C.
After adjusting for an over-inclusion error rate of 12.8% and an overdiagnosis rate of 0.02%, the research team found 697 patients (83%) lacked a relevant diagnosis. After multiple iterations, the algorithm achieved an accuracy of about 88%, Stuart said.
Considering Future AI Use
Stuart and colleagues are now testing the algorithm in larger groups and across longer periods.
After that, they intend to implement a quality improvement program to increase awareness for clinicians and primary care providers, as well as train users on how to interpret and move forward with findings of hepatic steatosis in patient records.
For instance, future AI models could flag patients for additional testing, improve chart review, and aid in research efforts around cardiometabolic comorbidities associated with MASLD, she said.
Looking ahead, AI tools such as these represent what’s possible for advancements in research, patient care, and clinical workflows, said Ashley Spann, MD, assistant professor and transplant hepatologist at Vanderbilt University, Nashville, Tennessee, and director of clinical research informatics for Vanderbilt’s Gastroenterology Division.
“AI, in my view, is actually augmented intelligence,” she added. “We need to think about the people and processes involved.”
Spann, who spoke about the use of AI tools in medicine in general, stressed the need for transparency in AI use, careful validation of input-output data, frameworks for machine learning models in medicine, and standardization across institutions.
“What we ultimately need is an infrastructure that supports the simultaneous deployment and evaluation of these models,” she said. “We all need to be on the same page and make sure our models work in multiple settings and make adjustments based on algorithmovigilance afterward.”
Stuart reported no relevant disclosures. Spann serves on Epic’s hepatology steering board, which has focused on how to use AI tools in electronic medical records.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to new research.
Among the patients identified by the algorithm as meeting the criteria for MASLD, only a small percentage had an MASLD-associated diagnostic code.
“A significant portion of patients who meet criteria for MASLD go undiagnosed, which can lead to delays in care and progression to advanced liver disease,” said lead author Ariana Stuart, MD, an internal medicine resident at the University of Washington, Seattle, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“However, people shouldn’t interpret our findings as a lack of primary care training or management,” she said. “Instead, this study indicates that AI can complement physician workflow and address the limitations of traditional clinical practice.”
Developing an MASLD Algorithm
Typically, the identification of MASLD has relied on clinician recognition and descriptions in chart notes, Stuart said. Early-stage disease often goes unnoticed, particularly if patients remain asymptomatic, until cirrhosis develops.
To address this, Stuart and colleagues created a machine learning, natural language processing AI algorithm on the basis of MASLD criteria from AASLD: Hepatic steatosis on imaging and at least one metabolic factor (elevated body mass index, hypertension, prediabetes or diabetes, or dyslipidemia). The model was validated by two physicians, who manually reviewed monthly cohorts generated by the algorithm.
Between December 2023 and May 2024, the researchers used the algorithm to analyze an MASLD cohort from medical centers in the Seattle area. The mean age was 51 years, 44% were women, and 68% were White. Those with alcohol-associated liver disease, metastatic malignancy, and autoimmune, genetic, and infectious causes of liver disease were excluded.
The algorithm identified 957 patients with imaging that matched MASLD criteria.
Among those, 137 patients (17%) identified by the algorithm had an MASLD-associated diagnostic code. For these patients, the mean time from initial imaging with steatosis to diagnosis was 33 days, according to patient records.
An additional 26 patients received an MASLD diagnosis during the study period, with a mean time to diagnosis of 56.2 days.
In terms of patient management, 245 patients (26%) had contact with a gastroenterologist or hepatologist based on documentation of a letter, phone call, or office visit. In addition, 546 patients (57%) were screened for hepatitis C.
After adjusting for an over-inclusion error rate of 12.8% and an overdiagnosis rate of 0.02%, the research team found 697 patients (83%) lacked a relevant diagnosis. After multiple iterations, the algorithm achieved an accuracy of about 88%, Stuart said.
Considering Future AI Use
Stuart and colleagues are now testing the algorithm in larger groups and across longer periods.
After that, they intend to implement a quality improvement program to increase awareness for clinicians and primary care providers, as well as train users on how to interpret and move forward with findings of hepatic steatosis in patient records.
For instance, future AI models could flag patients for additional testing, improve chart review, and aid in research efforts around cardiometabolic comorbidities associated with MASLD, she said.
Looking ahead, AI tools such as these represent what’s possible for advancements in research, patient care, and clinical workflows, said Ashley Spann, MD, assistant professor and transplant hepatologist at Vanderbilt University, Nashville, Tennessee, and director of clinical research informatics for Vanderbilt’s Gastroenterology Division.
“AI, in my view, is actually augmented intelligence,” she added. “We need to think about the people and processes involved.”
Spann, who spoke about the use of AI tools in medicine in general, stressed the need for transparency in AI use, careful validation of input-output data, frameworks for machine learning models in medicine, and standardization across institutions.
“What we ultimately need is an infrastructure that supports the simultaneous deployment and evaluation of these models,” she said. “We all need to be on the same page and make sure our models work in multiple settings and make adjustments based on algorithmovigilance afterward.”
Stuart reported no relevant disclosures. Spann serves on Epic’s hepatology steering board, which has focused on how to use AI tools in electronic medical records.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to new research.
Among the patients identified by the algorithm as meeting the criteria for MASLD, only a small percentage had an MASLD-associated diagnostic code.
“A significant portion of patients who meet criteria for MASLD go undiagnosed, which can lead to delays in care and progression to advanced liver disease,” said lead author Ariana Stuart, MD, an internal medicine resident at the University of Washington, Seattle, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“However, people shouldn’t interpret our findings as a lack of primary care training or management,” she said. “Instead, this study indicates that AI can complement physician workflow and address the limitations of traditional clinical practice.”
Developing an MASLD Algorithm
Typically, the identification of MASLD has relied on clinician recognition and descriptions in chart notes, Stuart said. Early-stage disease often goes unnoticed, particularly if patients remain asymptomatic, until cirrhosis develops.
To address this, Stuart and colleagues created a machine learning, natural language processing AI algorithm on the basis of MASLD criteria from AASLD: Hepatic steatosis on imaging and at least one metabolic factor (elevated body mass index, hypertension, prediabetes or diabetes, or dyslipidemia). The model was validated by two physicians, who manually reviewed monthly cohorts generated by the algorithm.
Between December 2023 and May 2024, the researchers used the algorithm to analyze an MASLD cohort from medical centers in the Seattle area. The mean age was 51 years, 44% were women, and 68% were White. Those with alcohol-associated liver disease, metastatic malignancy, and autoimmune, genetic, and infectious causes of liver disease were excluded.
The algorithm identified 957 patients with imaging that matched MASLD criteria.
Among those, 137 patients (17%) identified by the algorithm had an MASLD-associated diagnostic code. For these patients, the mean time from initial imaging with steatosis to diagnosis was 33 days, according to patient records.
An additional 26 patients received an MASLD diagnosis during the study period, with a mean time to diagnosis of 56.2 days.
In terms of patient management, 245 patients (26%) had contact with a gastroenterologist or hepatologist based on documentation of a letter, phone call, or office visit. In addition, 546 patients (57%) were screened for hepatitis C.
After adjusting for an over-inclusion error rate of 12.8% and an overdiagnosis rate of 0.02%, the research team found 697 patients (83%) lacked a relevant diagnosis. After multiple iterations, the algorithm achieved an accuracy of about 88%, Stuart said.
Considering Future AI Use
Stuart and colleagues are now testing the algorithm in larger groups and across longer periods.
After that, they intend to implement a quality improvement program to increase awareness for clinicians and primary care providers, as well as train users on how to interpret and move forward with findings of hepatic steatosis in patient records.
For instance, future AI models could flag patients for additional testing, improve chart review, and aid in research efforts around cardiometabolic comorbidities associated with MASLD, she said.
Looking ahead, AI tools such as these represent what’s possible for advancements in research, patient care, and clinical workflows, said Ashley Spann, MD, assistant professor and transplant hepatologist at Vanderbilt University, Nashville, Tennessee, and director of clinical research informatics for Vanderbilt’s Gastroenterology Division.
“AI, in my view, is actually augmented intelligence,” she added. “We need to think about the people and processes involved.”
Spann, who spoke about the use of AI tools in medicine in general, stressed the need for transparency in AI use, careful validation of input-output data, frameworks for machine learning models in medicine, and standardization across institutions.
“What we ultimately need is an infrastructure that supports the simultaneous deployment and evaluation of these models,” she said. “We all need to be on the same page and make sure our models work in multiple settings and make adjustments based on algorithmovigilance afterward.”
Stuart reported no relevant disclosures. Spann serves on Epic’s hepatology steering board, which has focused on how to use AI tools in electronic medical records.
A version of this article appeared on Medscape.com.
FROM AASLD 24
‘Watershed Moment’: Semaglutide Shown to Be Effective in MASH
SAN DIEGO — according to interim results from a phase 3 trial.
At 72 weeks, a 2.4-mg once-weekly subcutaneous dose of semaglutide demonstrated superiority, compared with placebo, for the two primary endpoints: Resolution of steatohepatitis with no worsening of fibrosis and improvement in liver fibrosis with no worsening of steatohepatitis.
“It’s been a long journey. I’ve been working with GLP-1s for 16 years, and it’s great to be able to report the first GLP-1 receptor agonist to demonstrate efficacy in a phase 3 trial for MASH,” said lead author Philip Newsome, MD, PhD, director of the Roger Williams Institute of Liver Studies at King’s College London in England.
“There were also improvements in a slew of other noninvasive markers,” said Newsome, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Although already seen in a broader context, “it’s nice to see a demonstration of the cardiometabolic benefits in the context of MASH and a reassuring safety profile,” he added.
Interim ESSENCE Trial Analysis
ESSENCE (NCT04822181) is an ongoing multicenter, phase 3 randomized, double-blind, placebo-controlled outcome trial studying semaglutide for the potential treatment of MASH.
The trial includes 1200 participants with biopsy-defined MASH and fibrosis, stages F2 and F3, who were randomized 2:1 to a once-weekly subcutaneous injection of 2.4 mg of semaglutide or placebo for 240 weeks. After initiation, the semaglutide dosage was increased every 4 weeks up to 16 weeks when the full dose (2.4 mg) was reached.
In a planned interim analysis, the trial investigators evaluated the primary endpoints at week 72 for the first 800 participants, with biopsies taken at weeks 1 and 72.
A total of 534 people were randomized to the semaglutide group, including 169 with F2 fibrosis and 365 with F3 fibrosis. Among the 266 participants randomized to placebo, 81 had F2 fibrosis and 185 had F3 fibrosis.
At baseline, the patient characteristics were similar between the groups (mean age, 56 years; body mass index, 34.6). A majority of participants also were White (67.5%), women (57.1%), had type 2 diabetes (55.9%), F3 fibrosis (68.8%), and enhanced liver fibrosis (ELF) scores around 10 (55.5%).
For the first primary endpoint, 62.9% of those in the semaglutide group and 34.1% of those in the placebo group reached resolution of steatohepatitis with no worsening of fibrosis. This represented an estimated difference in responder proportions (EDP) of 28.9%.
In addition, 37% of those in the semaglutide group and 22.5% of those in the placebo group met the second primary endpoint of improvement in liver fibrosis with no worsening of steatohepatitis (EDP, 14.4%).
Among the secondary endpoints, combined resolution of steatohepatitis with a one-stage improvement in liver fibrosis occurred in 32.8% of the semaglutide group and 16.2% of the placebo group (EDP, 16.6%).
In additional analyses, Newsome and colleagues found 20%-40% improvements in liver enzymes and noninvasive fibrosis markers, such as ELF and vibration-controlled transient elastography liver stiffness.
Weight loss was also significant, with a 10.5% reduction in the semaglutide group compared with a 2% reduction in the placebo group.
Cardiometabolic risk factors improved as well, with changes in blood pressure measurements, hemoglobin A1c scores, and cholesterol values.
Although not considered statistically significant, patients in the semaglutide group also reported greater reductions in body pain.
In a safety analysis of 1195 participants at 96 weeks, adverse events, severe adverse events, and discontinuations were similar in both groups. Not surprisingly, gastrointestinal side effects were more commonly reported in the semaglutide group, Newsome said.
Highly Anticipated Results
After Newsome’s presentation, attendees applauded.
Rohit Loomba, MD, a gastroenterologist at the University of California, San Diego, who was not involved with the study, called the results the “highlight of the meeting.”
This sentiment was echoed by Naga Chalasani, MD, AGAF, a gastroenterologist at Indiana University Medical Center, Indianapolis, who called the results a “watershed moment in the MASH field” with “terrific data.”
Based on questions after the presentation, Newsome indicated that future ESSENCE reports would look at certain aspects of the results, such as the 10% weight loss among those in the semaglutide group, as well as the mechanisms of histological and fibrosis improvement.
“We know from other GLP-1 trials that more weight loss occurs in those who don’t have type 2 diabetes, and we’re still running those analyses,” he said. “Weight loss is clearly a major contributor to MASH improvement, but there seem to be some weight-independent effects here, which are likely linked to insulin sensitivity or inflammation. We look forward to presenting those analyses in due course.”
In a comment, Kimberly Ann Brown, MD, AGAF, chief of gastroenterology and hepatology at Henry Ford Health System in Detroit, Michigan, AASLD Foundation chair, and comoderator of the late-breaking abstract session, spoke about the highly anticipated presentation.
“This study was really the pinnacle of this meeting. We’ve all been waiting for this data, in large part because many of our patients are already using these medications,” Brown said. “Seeing the benefit for the liver, as well as lipids and other cardiovascular measures, is so important. Having this confirmatory study will hopefully lead to the availability of the medication for this indication among our patients.”
Newsome reported numerous disclosures, including consultant relationships with pharmaceutical companies, such as Novo Nordisk, Boehringer Ingelheim, and Madrigal Pharmaceuticals. Loomba has research grant relationships with numerous companies, including Hanmi, Gilead, Galmed Pharmaceuticals, Galectin Therapeutics, Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim. Chalasani has consultant relationships with Ipsen, Pfizer, Merck, Altimmune, GSK, Madrigal Pharmaceuticals, and Zydus. Brown reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to interim results from a phase 3 trial.
At 72 weeks, a 2.4-mg once-weekly subcutaneous dose of semaglutide demonstrated superiority, compared with placebo, for the two primary endpoints: Resolution of steatohepatitis with no worsening of fibrosis and improvement in liver fibrosis with no worsening of steatohepatitis.
“It’s been a long journey. I’ve been working with GLP-1s for 16 years, and it’s great to be able to report the first GLP-1 receptor agonist to demonstrate efficacy in a phase 3 trial for MASH,” said lead author Philip Newsome, MD, PhD, director of the Roger Williams Institute of Liver Studies at King’s College London in England.
“There were also improvements in a slew of other noninvasive markers,” said Newsome, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Although already seen in a broader context, “it’s nice to see a demonstration of the cardiometabolic benefits in the context of MASH and a reassuring safety profile,” he added.
Interim ESSENCE Trial Analysis
ESSENCE (NCT04822181) is an ongoing multicenter, phase 3 randomized, double-blind, placebo-controlled outcome trial studying semaglutide for the potential treatment of MASH.
The trial includes 1200 participants with biopsy-defined MASH and fibrosis, stages F2 and F3, who were randomized 2:1 to a once-weekly subcutaneous injection of 2.4 mg of semaglutide or placebo for 240 weeks. After initiation, the semaglutide dosage was increased every 4 weeks up to 16 weeks when the full dose (2.4 mg) was reached.
In a planned interim analysis, the trial investigators evaluated the primary endpoints at week 72 for the first 800 participants, with biopsies taken at weeks 1 and 72.
A total of 534 people were randomized to the semaglutide group, including 169 with F2 fibrosis and 365 with F3 fibrosis. Among the 266 participants randomized to placebo, 81 had F2 fibrosis and 185 had F3 fibrosis.
At baseline, the patient characteristics were similar between the groups (mean age, 56 years; body mass index, 34.6). A majority of participants also were White (67.5%), women (57.1%), had type 2 diabetes (55.9%), F3 fibrosis (68.8%), and enhanced liver fibrosis (ELF) scores around 10 (55.5%).
For the first primary endpoint, 62.9% of those in the semaglutide group and 34.1% of those in the placebo group reached resolution of steatohepatitis with no worsening of fibrosis. This represented an estimated difference in responder proportions (EDP) of 28.9%.
In addition, 37% of those in the semaglutide group and 22.5% of those in the placebo group met the second primary endpoint of improvement in liver fibrosis with no worsening of steatohepatitis (EDP, 14.4%).
Among the secondary endpoints, combined resolution of steatohepatitis with a one-stage improvement in liver fibrosis occurred in 32.8% of the semaglutide group and 16.2% of the placebo group (EDP, 16.6%).
In additional analyses, Newsome and colleagues found 20%-40% improvements in liver enzymes and noninvasive fibrosis markers, such as ELF and vibration-controlled transient elastography liver stiffness.
Weight loss was also significant, with a 10.5% reduction in the semaglutide group compared with a 2% reduction in the placebo group.
Cardiometabolic risk factors improved as well, with changes in blood pressure measurements, hemoglobin A1c scores, and cholesterol values.
Although not considered statistically significant, patients in the semaglutide group also reported greater reductions in body pain.
In a safety analysis of 1195 participants at 96 weeks, adverse events, severe adverse events, and discontinuations were similar in both groups. Not surprisingly, gastrointestinal side effects were more commonly reported in the semaglutide group, Newsome said.
Highly Anticipated Results
After Newsome’s presentation, attendees applauded.
Rohit Loomba, MD, a gastroenterologist at the University of California, San Diego, who was not involved with the study, called the results the “highlight of the meeting.”
This sentiment was echoed by Naga Chalasani, MD, AGAF, a gastroenterologist at Indiana University Medical Center, Indianapolis, who called the results a “watershed moment in the MASH field” with “terrific data.”
Based on questions after the presentation, Newsome indicated that future ESSENCE reports would look at certain aspects of the results, such as the 10% weight loss among those in the semaglutide group, as well as the mechanisms of histological and fibrosis improvement.
“We know from other GLP-1 trials that more weight loss occurs in those who don’t have type 2 diabetes, and we’re still running those analyses,” he said. “Weight loss is clearly a major contributor to MASH improvement, but there seem to be some weight-independent effects here, which are likely linked to insulin sensitivity or inflammation. We look forward to presenting those analyses in due course.”
In a comment, Kimberly Ann Brown, MD, AGAF, chief of gastroenterology and hepatology at Henry Ford Health System in Detroit, Michigan, AASLD Foundation chair, and comoderator of the late-breaking abstract session, spoke about the highly anticipated presentation.
“This study was really the pinnacle of this meeting. We’ve all been waiting for this data, in large part because many of our patients are already using these medications,” Brown said. “Seeing the benefit for the liver, as well as lipids and other cardiovascular measures, is so important. Having this confirmatory study will hopefully lead to the availability of the medication for this indication among our patients.”
Newsome reported numerous disclosures, including consultant relationships with pharmaceutical companies, such as Novo Nordisk, Boehringer Ingelheim, and Madrigal Pharmaceuticals. Loomba has research grant relationships with numerous companies, including Hanmi, Gilead, Galmed Pharmaceuticals, Galectin Therapeutics, Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim. Chalasani has consultant relationships with Ipsen, Pfizer, Merck, Altimmune, GSK, Madrigal Pharmaceuticals, and Zydus. Brown reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to interim results from a phase 3 trial.
At 72 weeks, a 2.4-mg once-weekly subcutaneous dose of semaglutide demonstrated superiority, compared with placebo, for the two primary endpoints: Resolution of steatohepatitis with no worsening of fibrosis and improvement in liver fibrosis with no worsening of steatohepatitis.
“It’s been a long journey. I’ve been working with GLP-1s for 16 years, and it’s great to be able to report the first GLP-1 receptor agonist to demonstrate efficacy in a phase 3 trial for MASH,” said lead author Philip Newsome, MD, PhD, director of the Roger Williams Institute of Liver Studies at King’s College London in England.
“There were also improvements in a slew of other noninvasive markers,” said Newsome, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Although already seen in a broader context, “it’s nice to see a demonstration of the cardiometabolic benefits in the context of MASH and a reassuring safety profile,” he added.
Interim ESSENCE Trial Analysis
ESSENCE (NCT04822181) is an ongoing multicenter, phase 3 randomized, double-blind, placebo-controlled outcome trial studying semaglutide for the potential treatment of MASH.
The trial includes 1200 participants with biopsy-defined MASH and fibrosis, stages F2 and F3, who were randomized 2:1 to a once-weekly subcutaneous injection of 2.4 mg of semaglutide or placebo for 240 weeks. After initiation, the semaglutide dosage was increased every 4 weeks up to 16 weeks when the full dose (2.4 mg) was reached.
In a planned interim analysis, the trial investigators evaluated the primary endpoints at week 72 for the first 800 participants, with biopsies taken at weeks 1 and 72.
A total of 534 people were randomized to the semaglutide group, including 169 with F2 fibrosis and 365 with F3 fibrosis. Among the 266 participants randomized to placebo, 81 had F2 fibrosis and 185 had F3 fibrosis.
At baseline, the patient characteristics were similar between the groups (mean age, 56 years; body mass index, 34.6). A majority of participants also were White (67.5%), women (57.1%), had type 2 diabetes (55.9%), F3 fibrosis (68.8%), and enhanced liver fibrosis (ELF) scores around 10 (55.5%).
For the first primary endpoint, 62.9% of those in the semaglutide group and 34.1% of those in the placebo group reached resolution of steatohepatitis with no worsening of fibrosis. This represented an estimated difference in responder proportions (EDP) of 28.9%.
In addition, 37% of those in the semaglutide group and 22.5% of those in the placebo group met the second primary endpoint of improvement in liver fibrosis with no worsening of steatohepatitis (EDP, 14.4%).
Among the secondary endpoints, combined resolution of steatohepatitis with a one-stage improvement in liver fibrosis occurred in 32.8% of the semaglutide group and 16.2% of the placebo group (EDP, 16.6%).
In additional analyses, Newsome and colleagues found 20%-40% improvements in liver enzymes and noninvasive fibrosis markers, such as ELF and vibration-controlled transient elastography liver stiffness.
Weight loss was also significant, with a 10.5% reduction in the semaglutide group compared with a 2% reduction in the placebo group.
Cardiometabolic risk factors improved as well, with changes in blood pressure measurements, hemoglobin A1c scores, and cholesterol values.
Although not considered statistically significant, patients in the semaglutide group also reported greater reductions in body pain.
In a safety analysis of 1195 participants at 96 weeks, adverse events, severe adverse events, and discontinuations were similar in both groups. Not surprisingly, gastrointestinal side effects were more commonly reported in the semaglutide group, Newsome said.
Highly Anticipated Results
After Newsome’s presentation, attendees applauded.
Rohit Loomba, MD, a gastroenterologist at the University of California, San Diego, who was not involved with the study, called the results the “highlight of the meeting.”
This sentiment was echoed by Naga Chalasani, MD, AGAF, a gastroenterologist at Indiana University Medical Center, Indianapolis, who called the results a “watershed moment in the MASH field” with “terrific data.”
Based on questions after the presentation, Newsome indicated that future ESSENCE reports would look at certain aspects of the results, such as the 10% weight loss among those in the semaglutide group, as well as the mechanisms of histological and fibrosis improvement.
“We know from other GLP-1 trials that more weight loss occurs in those who don’t have type 2 diabetes, and we’re still running those analyses,” he said. “Weight loss is clearly a major contributor to MASH improvement, but there seem to be some weight-independent effects here, which are likely linked to insulin sensitivity or inflammation. We look forward to presenting those analyses in due course.”
In a comment, Kimberly Ann Brown, MD, AGAF, chief of gastroenterology and hepatology at Henry Ford Health System in Detroit, Michigan, AASLD Foundation chair, and comoderator of the late-breaking abstract session, spoke about the highly anticipated presentation.
“This study was really the pinnacle of this meeting. We’ve all been waiting for this data, in large part because many of our patients are already using these medications,” Brown said. “Seeing the benefit for the liver, as well as lipids and other cardiovascular measures, is so important. Having this confirmatory study will hopefully lead to the availability of the medication for this indication among our patients.”
Newsome reported numerous disclosures, including consultant relationships with pharmaceutical companies, such as Novo Nordisk, Boehringer Ingelheim, and Madrigal Pharmaceuticals. Loomba has research grant relationships with numerous companies, including Hanmi, Gilead, Galmed Pharmaceuticals, Galectin Therapeutics, Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim. Chalasani has consultant relationships with Ipsen, Pfizer, Merck, Altimmune, GSK, Madrigal Pharmaceuticals, and Zydus. Brown reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AASLD 24
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).
The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).
In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).
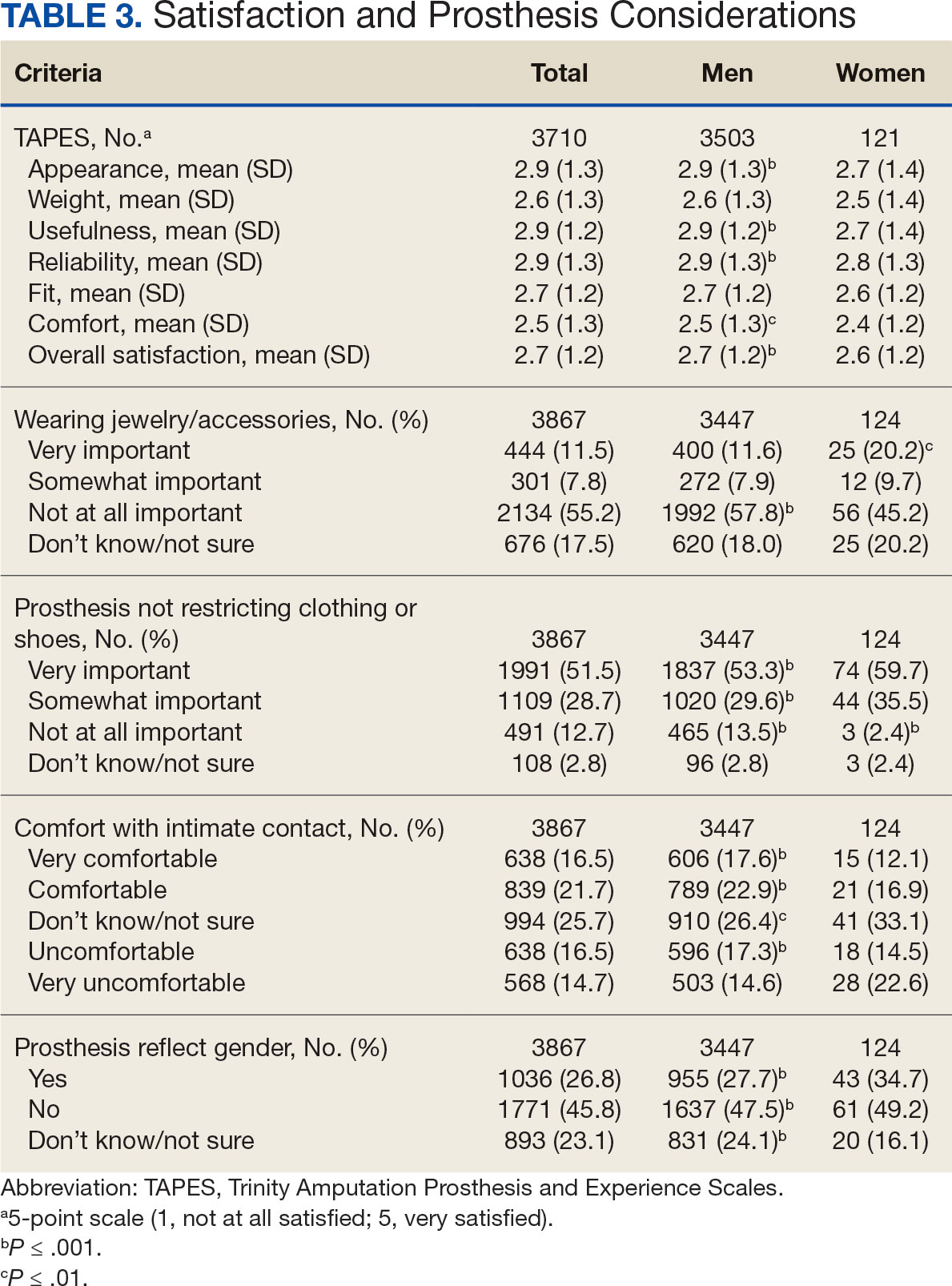
Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.
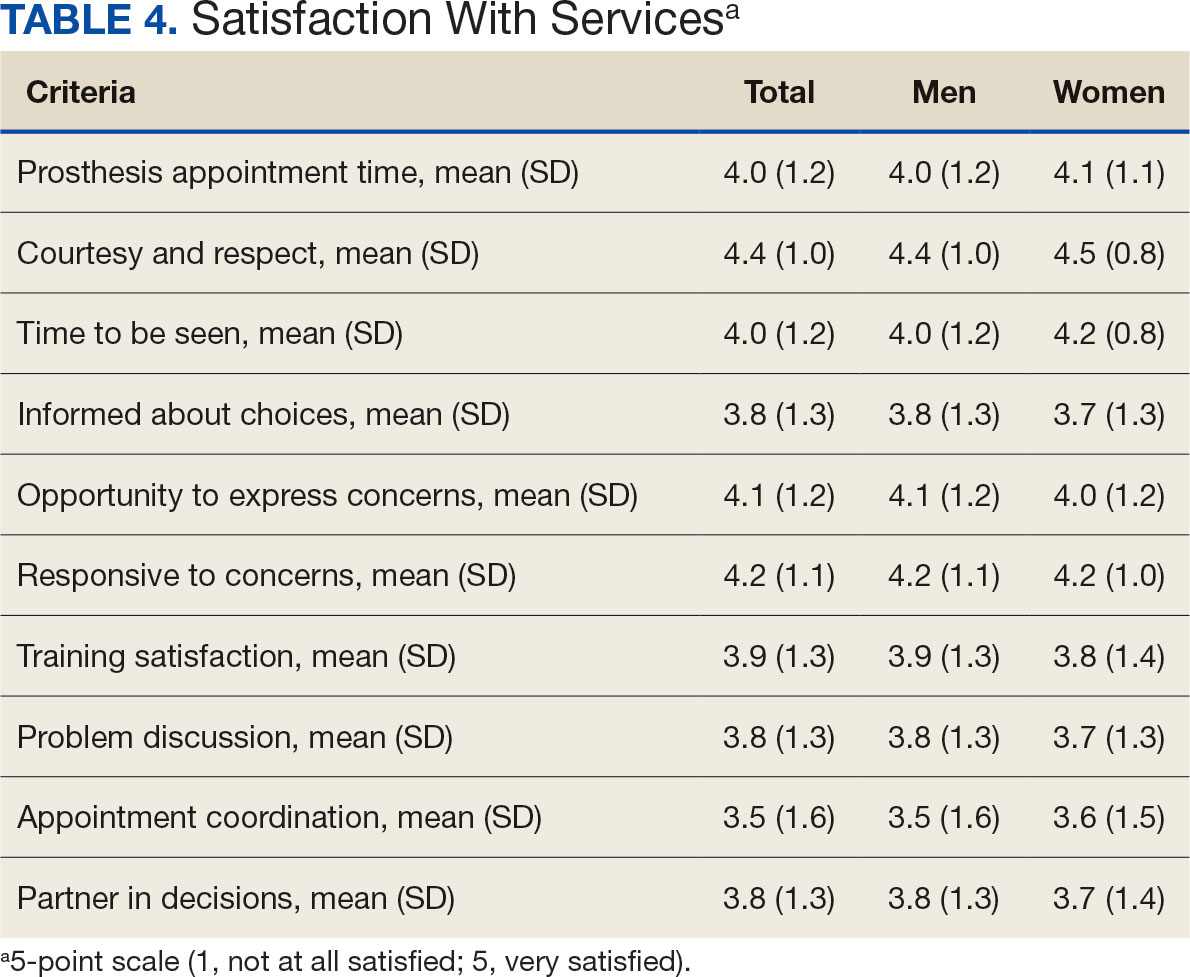
Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans