User login
Match Day 2021: Psychiatry continues strong growth
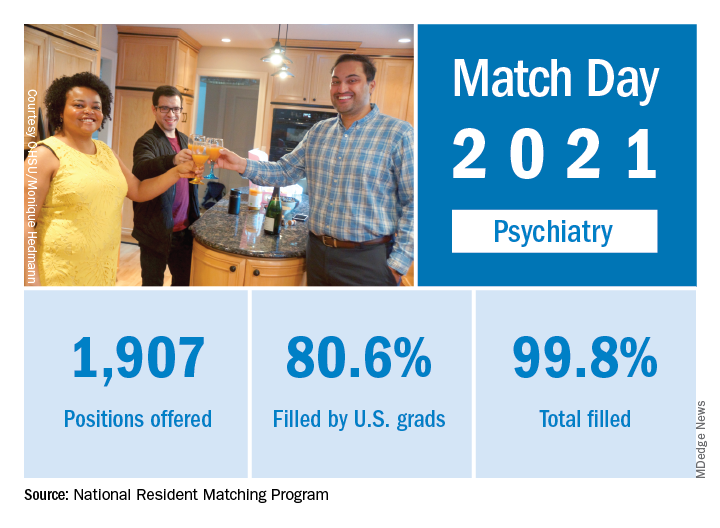
In a record year for the Match, psychiatry residencies filled 99.8% of their available positions in 2021, which were up 2.6% over last year, according to the National Resident Matching Program.
“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) more first-year (PGY-1) slots than ever before, for a fill rate of 94.8%, which was up from 94.6% the year before.
Psychiatry offered 1,907 positions in this year’s Match, up by 2.6% over 2020, and filled 1,904, for a 1-year increase of 3.6% and a fill rate of 99.8%. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
The number of positions offered in psychiatry residencies has increased by 412 (27.6%) since 2017, and such growth over time may “be a predictor of future physician workforce supply,” the NRMP suggested. Psychiatry also increased its share of all available residency positions from 5.1% in 2018 to 5.4% in 2021.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, BSN, president and CEO of the NRMP.
In a record year for the Match, psychiatry residencies filled 99.8% of their available positions in 2021, which were up 2.6% over last year, according to the National Resident Matching Program.

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) more first-year (PGY-1) slots than ever before, for a fill rate of 94.8%, which was up from 94.6% the year before.
Psychiatry offered 1,907 positions in this year’s Match, up by 2.6% over 2020, and filled 1,904, for a 1-year increase of 3.6% and a fill rate of 99.8%. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
The number of positions offered in psychiatry residencies has increased by 412 (27.6%) since 2017, and such growth over time may “be a predictor of future physician workforce supply,” the NRMP suggested. Psychiatry also increased its share of all available residency positions from 5.1% in 2018 to 5.4% in 2021.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, BSN, president and CEO of the NRMP.
In a record year for the Match, psychiatry residencies filled 99.8% of their available positions in 2021, which were up 2.6% over last year, according to the National Resident Matching Program.

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a written statement. Overall, the 2021 Main Residency Match offered (35,194) and filled (33,353) more first-year (PGY-1) slots than ever before, for a fill rate of 94.8%, which was up from 94.6% the year before.
Psychiatry offered 1,907 positions in this year’s Match, up by 2.6% over 2020, and filled 1,904, for a 1-year increase of 3.6% and a fill rate of 99.8%. The corresponding PGY-1 numbers for the Match as a whole were 70.4% U.S. and 21.1% international medical graduates, based on NRMP data.
The number of positions offered in psychiatry residencies has increased by 412 (27.6%) since 2017, and such growth over time may “be a predictor of future physician workforce supply,” the NRMP suggested. Psychiatry also increased its share of all available residency positions from 5.1% in 2018 to 5.4% in 2021.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized,” the NRMP noted, as “growth in registration was seen in every applicant group.” Compared with 2020, submissions of rank-order lists of programs were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen IMGs, and 15.0% for non–U.S.-citizen IMGs.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” said Donna L. Lamb, DHSc, MBA, BSN, president and CEO of the NRMP.
Diabetes prevention moves toward reality as studies published
Two newly published studies highlight recent success toward delaying the onset of type 1 diabetes in people at high risk and slowing progression in those with recent onset of the condition.
Both studies were initially presented in June 2020 at the annual scientific sessions of the American Diabetes Association and reported by this news organization at the time.
As yet, neither of the two strategies – preserving insulin-producing pancreatic beta-cell function soon after diagnosis or delaying type 1 diabetes onset in those at high risk – represent a cure or certain disease prevention.
However, both can potentially lead to better long-term glycemic control with less hypoglycemia and a lower risk for diabetes-related complications.
Combination treatment prolongs beta-cell function in new-onset disease
The first study, entitled, “Anti–interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes,” was published online March 1, 2021, in The Lancet Diabetes & Endocrinology by Matthias von Herrath, MD, of Novo Nordisk, Søborg, Denmark, and colleagues.
The randomized, placebo-controlled, double-blind, phase 2 combination treatment trial involved 308 individuals aged 18-45 years who had been diagnosed with type 1 diabetes in the previous 20 weeks and still had residual beta-cell function.
Patients were randomized with 77 per group to receive monoclonal anti-IL-21 plus liraglutide, anti-IL-21 alone, liraglutide alone, or placebo. The antibody was given intravenously every 6 weeks and liraglutide or matching placebo were self-administered by daily injections.
Compared with placebo (ratio to baseline, 0.61; 39% decrease), the decrease in mixed meal tolerance test stimulated C-peptide concentration from baseline to week 54 – the primary outcome – was significantly smaller with combination treatment (0.90, 10% decrease; estimated treatment ratio, 1.48; P = .0017), but not with anti-IL-21 alone (1.23; P = .093) or liraglutide alone (1.12; P = .38).
Despite greater insulin use in the placebo group, the decrease in hemoglobin A1c (a key secondary outcome) at week 54 was greater with all active treatments (–0.50 percentage points) than with placebo (–0.10 percentage points), although the differences versus placebo were not significant.
“The combination of anti-IL-21 and liraglutide could preserve beta-cell function in recently diagnosed type 1 diabetes,” the researchers said.
“These results suggest that this combination has the potential to offer a novel and valuable disease-modifying therapy for patients with recently diagnosed type 1 diabetes. However, the efficacy and safety need to be further investigated in a phase 3 program,” Dr. von Herrath and colleagues concluded.
Teplizumab: 3-year data continue to show benefit
The other study looked at delaying the onset of type 1 diabetes. Entitled, “Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals,” the article was published online March 3, 2021, in Science Translational Medicine by Emily K. Sims, MD, of the department of pediatrics, Indiana University, Indianapolis, and colleagues.
This trial of the anti-CD3 monoclonal antibody adds an additional year of follow-up to the “game-changer” 2-year data reported in 2019.
Among the 76 individuals aged 8-49 years who were positive for two or more type 1 diabetes–related autoantibodies, 50% of those randomized to a single 14-day infusion course of teplizumab remained diabetes free at a median follow-up of 923 days, compared with only 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01).
The teplizumab group had a greater average C-peptide area under the curve, compared with placebo, reflecting improved beta-cell function (1.96 vs 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“It is very encouraging to see that a single course of teplizumab delayed insulin dependence in this high-risk population for approximately 3 years versus placebo,” said Frank Martin, PhD, JDRF director of research at Provention Bio, which is developing teplizumab.
“These exciting results have been made possible by the unwavering efforts of TrialNet and Provention Bio. Teplizumab, if approved by the FDA, could positively change the course of disease development for people at risk of developing T1D and their standard of care,” he concluded.
The teplizumab study was funded by TrialNet. Dr. von Herrath is an employee of Novo Nordisk, which funded the study involving its drug liraglutide. Dr. Sims reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two newly published studies highlight recent success toward delaying the onset of type 1 diabetes in people at high risk and slowing progression in those with recent onset of the condition.
Both studies were initially presented in June 2020 at the annual scientific sessions of the American Diabetes Association and reported by this news organization at the time.
As yet, neither of the two strategies – preserving insulin-producing pancreatic beta-cell function soon after diagnosis or delaying type 1 diabetes onset in those at high risk – represent a cure or certain disease prevention.
However, both can potentially lead to better long-term glycemic control with less hypoglycemia and a lower risk for diabetes-related complications.
Combination treatment prolongs beta-cell function in new-onset disease
The first study, entitled, “Anti–interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes,” was published online March 1, 2021, in The Lancet Diabetes & Endocrinology by Matthias von Herrath, MD, of Novo Nordisk, Søborg, Denmark, and colleagues.
The randomized, placebo-controlled, double-blind, phase 2 combination treatment trial involved 308 individuals aged 18-45 years who had been diagnosed with type 1 diabetes in the previous 20 weeks and still had residual beta-cell function.
Patients were randomized with 77 per group to receive monoclonal anti-IL-21 plus liraglutide, anti-IL-21 alone, liraglutide alone, or placebo. The antibody was given intravenously every 6 weeks and liraglutide or matching placebo were self-administered by daily injections.
Compared with placebo (ratio to baseline, 0.61; 39% decrease), the decrease in mixed meal tolerance test stimulated C-peptide concentration from baseline to week 54 – the primary outcome – was significantly smaller with combination treatment (0.90, 10% decrease; estimated treatment ratio, 1.48; P = .0017), but not with anti-IL-21 alone (1.23; P = .093) or liraglutide alone (1.12; P = .38).
Despite greater insulin use in the placebo group, the decrease in hemoglobin A1c (a key secondary outcome) at week 54 was greater with all active treatments (–0.50 percentage points) than with placebo (–0.10 percentage points), although the differences versus placebo were not significant.
“The combination of anti-IL-21 and liraglutide could preserve beta-cell function in recently diagnosed type 1 diabetes,” the researchers said.
“These results suggest that this combination has the potential to offer a novel and valuable disease-modifying therapy for patients with recently diagnosed type 1 diabetes. However, the efficacy and safety need to be further investigated in a phase 3 program,” Dr. von Herrath and colleagues concluded.
Teplizumab: 3-year data continue to show benefit
The other study looked at delaying the onset of type 1 diabetes. Entitled, “Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals,” the article was published online March 3, 2021, in Science Translational Medicine by Emily K. Sims, MD, of the department of pediatrics, Indiana University, Indianapolis, and colleagues.
This trial of the anti-CD3 monoclonal antibody adds an additional year of follow-up to the “game-changer” 2-year data reported in 2019.
Among the 76 individuals aged 8-49 years who were positive for two or more type 1 diabetes–related autoantibodies, 50% of those randomized to a single 14-day infusion course of teplizumab remained diabetes free at a median follow-up of 923 days, compared with only 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01).
The teplizumab group had a greater average C-peptide area under the curve, compared with placebo, reflecting improved beta-cell function (1.96 vs 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“It is very encouraging to see that a single course of teplizumab delayed insulin dependence in this high-risk population for approximately 3 years versus placebo,” said Frank Martin, PhD, JDRF director of research at Provention Bio, which is developing teplizumab.
“These exciting results have been made possible by the unwavering efforts of TrialNet and Provention Bio. Teplizumab, if approved by the FDA, could positively change the course of disease development for people at risk of developing T1D and their standard of care,” he concluded.
The teplizumab study was funded by TrialNet. Dr. von Herrath is an employee of Novo Nordisk, which funded the study involving its drug liraglutide. Dr. Sims reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two newly published studies highlight recent success toward delaying the onset of type 1 diabetes in people at high risk and slowing progression in those with recent onset of the condition.
Both studies were initially presented in June 2020 at the annual scientific sessions of the American Diabetes Association and reported by this news organization at the time.
As yet, neither of the two strategies – preserving insulin-producing pancreatic beta-cell function soon after diagnosis or delaying type 1 diabetes onset in those at high risk – represent a cure or certain disease prevention.
However, both can potentially lead to better long-term glycemic control with less hypoglycemia and a lower risk for diabetes-related complications.
Combination treatment prolongs beta-cell function in new-onset disease
The first study, entitled, “Anti–interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes,” was published online March 1, 2021, in The Lancet Diabetes & Endocrinology by Matthias von Herrath, MD, of Novo Nordisk, Søborg, Denmark, and colleagues.
The randomized, placebo-controlled, double-blind, phase 2 combination treatment trial involved 308 individuals aged 18-45 years who had been diagnosed with type 1 diabetes in the previous 20 weeks and still had residual beta-cell function.
Patients were randomized with 77 per group to receive monoclonal anti-IL-21 plus liraglutide, anti-IL-21 alone, liraglutide alone, or placebo. The antibody was given intravenously every 6 weeks and liraglutide or matching placebo were self-administered by daily injections.
Compared with placebo (ratio to baseline, 0.61; 39% decrease), the decrease in mixed meal tolerance test stimulated C-peptide concentration from baseline to week 54 – the primary outcome – was significantly smaller with combination treatment (0.90, 10% decrease; estimated treatment ratio, 1.48; P = .0017), but not with anti-IL-21 alone (1.23; P = .093) or liraglutide alone (1.12; P = .38).
Despite greater insulin use in the placebo group, the decrease in hemoglobin A1c (a key secondary outcome) at week 54 was greater with all active treatments (–0.50 percentage points) than with placebo (–0.10 percentage points), although the differences versus placebo were not significant.
“The combination of anti-IL-21 and liraglutide could preserve beta-cell function in recently diagnosed type 1 diabetes,” the researchers said.
“These results suggest that this combination has the potential to offer a novel and valuable disease-modifying therapy for patients with recently diagnosed type 1 diabetes. However, the efficacy and safety need to be further investigated in a phase 3 program,” Dr. von Herrath and colleagues concluded.
Teplizumab: 3-year data continue to show benefit
The other study looked at delaying the onset of type 1 diabetes. Entitled, “Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals,” the article was published online March 3, 2021, in Science Translational Medicine by Emily K. Sims, MD, of the department of pediatrics, Indiana University, Indianapolis, and colleagues.
This trial of the anti-CD3 monoclonal antibody adds an additional year of follow-up to the “game-changer” 2-year data reported in 2019.
Among the 76 individuals aged 8-49 years who were positive for two or more type 1 diabetes–related autoantibodies, 50% of those randomized to a single 14-day infusion course of teplizumab remained diabetes free at a median follow-up of 923 days, compared with only 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01).
The teplizumab group had a greater average C-peptide area under the curve, compared with placebo, reflecting improved beta-cell function (1.96 vs 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“It is very encouraging to see that a single course of teplizumab delayed insulin dependence in this high-risk population for approximately 3 years versus placebo,” said Frank Martin, PhD, JDRF director of research at Provention Bio, which is developing teplizumab.
“These exciting results have been made possible by the unwavering efforts of TrialNet and Provention Bio. Teplizumab, if approved by the FDA, could positively change the course of disease development for people at risk of developing T1D and their standard of care,” he concluded.
The teplizumab study was funded by TrialNet. Dr. von Herrath is an employee of Novo Nordisk, which funded the study involving its drug liraglutide. Dr. Sims reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultraprocessed foods, many marketed as healthy, raise CVD risk
Eating ultraprocessed foods poses a significant risk to cardiovascular and coronary heart health, according to prospective data from about 3,000 people in the Framingham Offspring Cohort, the second generation of participants in the Framingham Heart Study.
Each regular, daily serving of ultraprocessed food was linked with significant elevations of 5%-9% in the relative rates of “hard” cardiovascular disease (CVD) events, hard coronary heart disease (CHD) events, overall CVD events, and CVD death, after adjustments for numerous potential confounders including energy intake, body mass index, waist circumference, and blood pressure, Filippa Juul, PhD, and associates wrote in a report published in the Journal of the American College of Cardiology.
“Consumption of ultraprocessed foods makes up over half of the daily calories in the average American diet and are increasingly consumed worldwide. As poor diet is a major modifiable risk factor for heart disease, it represents a critical target in prevention efforts,” said Dr. Juul, a nutritional epidemiologist at New York University, in a statement released by the American College of Cardiology.
“Our findings add to a growing body of evidence suggesting cardiovascular benefits of limiting ultraprocessed foods. Ultraprocessed foods are ubiquitous and include many foods that are marketed as healthy, such as protein bars, breakfast cereals, and most industrially produced breads,” she added. Other commonplace members of the ultraprocessed food group include carbonated soft drinks, packaged snacks, candies, sausages, margarines, and energy drinks. The concept of ultraprocessed foods as a distinct, wide-ranging, and dangerous food category first appeared in 2010, and then received an update from a United Nations panel in 2019 as what’s now called the NOVA classification system.
Ultraprocessed foods fly under the radar
“Although cardiovascular guidelines emphasize consuming minimally processed foods, such as fruits, vegetables, whole grains, and nuts, they give less attention to the importance of minimizing ultraprocessed food,” wrote Robert J. Ostfeld, MD, and Kathleen E. Allen, MS, in an editorial that accompanied the new report. This reduced attention may be because of a “paucity of studies examining the association cardiovascular outcomes and ultraprocessed foods.”
The new evidence demands new policies, educational efforts, and labeling changes, suggested Dr. Ostfeld, director of preventive cardiology at Montefiore Health System in New York, and Ms. Allen, a dietitian at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “The goal should be to make the unhealthy choice the hard choice and the healthy choice the easy choice.”
The new analysis used data collected from people enrolled the Framingham Offspring Cohort, with their clinical metrics and diet information collected during 1991-1995 serving as their baseline. After excluding participants with prevalent CVD at baseline and those with incomplete follow-up of CVD events, the researchers had a cohort of 3,003 adults with an average follow-up of 18 years. At baseline, the cohort averaged 54 years of age; 55% were women, their average body mass index was 27.3 kg/m2, and about 6% had diabetes. They reported eating, on average, 7.5 servings of ultraprocessed food daily.
During follow-up, the cohort tallied 648 incident CVD events, including 251 hard CVD events (coronary death, MI, or stroke) and 163 hard CHD events (coronary death or MI), and 713 total deaths including 108 CVD deaths. Other CVD events recorded but not considered hard included heart failure, intermittent claudication, and transient ischemic attack.
In a multivariate-adjusted analysis, each average daily portion of ultraprocessed food was linked with an significant 7% relative increase in the incidence of a hard CVD event, compared with participants who ate fewer ultraprocessed food portions, and a 9% relative increase in the rate of hard CHD events, the study’s two prespecified primary outcomes. The researchers also found that each ultraprocessed serving significantly was associated with a 5% relative increased rate of total CVD events, and a 9% relative rise in CVD deaths. The analysis showed no significant association between total mortality and ultraprocessed food intake. (Average follow-up for the mortality analyses was 20 years.)
The authors also reported endpoint associations with intake of specific types of ultraprocessed foods, and found significantly increased associations specifically for portions of bread, ultraprocessed meat, salty snacks, and low-calorie soft drinks.
Convenient, omnipresent, and affordable
The authors acknowledged that the associations they found need examination in ethnically diverse populations, but nonetheless the findings “suggest the need for increased efforts to implement population-wide strategies” to lower consumption of ultraprocessed foods. “Given the convenience, omnipresence, and affordability of ultraprocessed foods, careful nutrition counseling is needed to design individualized, patient-centered, heart-healthy diets,” they concluded.
“Population-wide strategies such as taxation on sugar-sweetened beverages and other ultraprocessed foods and recommendations regarding processing levels in national dietary guidelines are needed to reduce the intake of ultraprocessed foods,” added Dr. Juul in her statement. “Of course, we must also implement policies that increase the availability, accessibility, and affordability of nutritious, minimally processed foods, especially in disadvantaged populations. At the clinical level, there is a need for increased commitment to individualized nutrition counseling for adopting sustainable heart-healthy diets.”
The study had no commercial funding. Dr. Juul and coauthors, Dr. Ostfeld, and Ms. Allen had no disclosures.
Eating ultraprocessed foods poses a significant risk to cardiovascular and coronary heart health, according to prospective data from about 3,000 people in the Framingham Offspring Cohort, the second generation of participants in the Framingham Heart Study.
Each regular, daily serving of ultraprocessed food was linked with significant elevations of 5%-9% in the relative rates of “hard” cardiovascular disease (CVD) events, hard coronary heart disease (CHD) events, overall CVD events, and CVD death, after adjustments for numerous potential confounders including energy intake, body mass index, waist circumference, and blood pressure, Filippa Juul, PhD, and associates wrote in a report published in the Journal of the American College of Cardiology.
“Consumption of ultraprocessed foods makes up over half of the daily calories in the average American diet and are increasingly consumed worldwide. As poor diet is a major modifiable risk factor for heart disease, it represents a critical target in prevention efforts,” said Dr. Juul, a nutritional epidemiologist at New York University, in a statement released by the American College of Cardiology.
“Our findings add to a growing body of evidence suggesting cardiovascular benefits of limiting ultraprocessed foods. Ultraprocessed foods are ubiquitous and include many foods that are marketed as healthy, such as protein bars, breakfast cereals, and most industrially produced breads,” she added. Other commonplace members of the ultraprocessed food group include carbonated soft drinks, packaged snacks, candies, sausages, margarines, and energy drinks. The concept of ultraprocessed foods as a distinct, wide-ranging, and dangerous food category first appeared in 2010, and then received an update from a United Nations panel in 2019 as what’s now called the NOVA classification system.
Ultraprocessed foods fly under the radar
“Although cardiovascular guidelines emphasize consuming minimally processed foods, such as fruits, vegetables, whole grains, and nuts, they give less attention to the importance of minimizing ultraprocessed food,” wrote Robert J. Ostfeld, MD, and Kathleen E. Allen, MS, in an editorial that accompanied the new report. This reduced attention may be because of a “paucity of studies examining the association cardiovascular outcomes and ultraprocessed foods.”
The new evidence demands new policies, educational efforts, and labeling changes, suggested Dr. Ostfeld, director of preventive cardiology at Montefiore Health System in New York, and Ms. Allen, a dietitian at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “The goal should be to make the unhealthy choice the hard choice and the healthy choice the easy choice.”
The new analysis used data collected from people enrolled the Framingham Offspring Cohort, with their clinical metrics and diet information collected during 1991-1995 serving as their baseline. After excluding participants with prevalent CVD at baseline and those with incomplete follow-up of CVD events, the researchers had a cohort of 3,003 adults with an average follow-up of 18 years. At baseline, the cohort averaged 54 years of age; 55% were women, their average body mass index was 27.3 kg/m2, and about 6% had diabetes. They reported eating, on average, 7.5 servings of ultraprocessed food daily.
During follow-up, the cohort tallied 648 incident CVD events, including 251 hard CVD events (coronary death, MI, or stroke) and 163 hard CHD events (coronary death or MI), and 713 total deaths including 108 CVD deaths. Other CVD events recorded but not considered hard included heart failure, intermittent claudication, and transient ischemic attack.
In a multivariate-adjusted analysis, each average daily portion of ultraprocessed food was linked with an significant 7% relative increase in the incidence of a hard CVD event, compared with participants who ate fewer ultraprocessed food portions, and a 9% relative increase in the rate of hard CHD events, the study’s two prespecified primary outcomes. The researchers also found that each ultraprocessed serving significantly was associated with a 5% relative increased rate of total CVD events, and a 9% relative rise in CVD deaths. The analysis showed no significant association between total mortality and ultraprocessed food intake. (Average follow-up for the mortality analyses was 20 years.)
The authors also reported endpoint associations with intake of specific types of ultraprocessed foods, and found significantly increased associations specifically for portions of bread, ultraprocessed meat, salty snacks, and low-calorie soft drinks.
Convenient, omnipresent, and affordable
The authors acknowledged that the associations they found need examination in ethnically diverse populations, but nonetheless the findings “suggest the need for increased efforts to implement population-wide strategies” to lower consumption of ultraprocessed foods. “Given the convenience, omnipresence, and affordability of ultraprocessed foods, careful nutrition counseling is needed to design individualized, patient-centered, heart-healthy diets,” they concluded.
“Population-wide strategies such as taxation on sugar-sweetened beverages and other ultraprocessed foods and recommendations regarding processing levels in national dietary guidelines are needed to reduce the intake of ultraprocessed foods,” added Dr. Juul in her statement. “Of course, we must also implement policies that increase the availability, accessibility, and affordability of nutritious, minimally processed foods, especially in disadvantaged populations. At the clinical level, there is a need for increased commitment to individualized nutrition counseling for adopting sustainable heart-healthy diets.”
The study had no commercial funding. Dr. Juul and coauthors, Dr. Ostfeld, and Ms. Allen had no disclosures.
Eating ultraprocessed foods poses a significant risk to cardiovascular and coronary heart health, according to prospective data from about 3,000 people in the Framingham Offspring Cohort, the second generation of participants in the Framingham Heart Study.
Each regular, daily serving of ultraprocessed food was linked with significant elevations of 5%-9% in the relative rates of “hard” cardiovascular disease (CVD) events, hard coronary heart disease (CHD) events, overall CVD events, and CVD death, after adjustments for numerous potential confounders including energy intake, body mass index, waist circumference, and blood pressure, Filippa Juul, PhD, and associates wrote in a report published in the Journal of the American College of Cardiology.
“Consumption of ultraprocessed foods makes up over half of the daily calories in the average American diet and are increasingly consumed worldwide. As poor diet is a major modifiable risk factor for heart disease, it represents a critical target in prevention efforts,” said Dr. Juul, a nutritional epidemiologist at New York University, in a statement released by the American College of Cardiology.
“Our findings add to a growing body of evidence suggesting cardiovascular benefits of limiting ultraprocessed foods. Ultraprocessed foods are ubiquitous and include many foods that are marketed as healthy, such as protein bars, breakfast cereals, and most industrially produced breads,” she added. Other commonplace members of the ultraprocessed food group include carbonated soft drinks, packaged snacks, candies, sausages, margarines, and energy drinks. The concept of ultraprocessed foods as a distinct, wide-ranging, and dangerous food category first appeared in 2010, and then received an update from a United Nations panel in 2019 as what’s now called the NOVA classification system.
Ultraprocessed foods fly under the radar
“Although cardiovascular guidelines emphasize consuming minimally processed foods, such as fruits, vegetables, whole grains, and nuts, they give less attention to the importance of minimizing ultraprocessed food,” wrote Robert J. Ostfeld, MD, and Kathleen E. Allen, MS, in an editorial that accompanied the new report. This reduced attention may be because of a “paucity of studies examining the association cardiovascular outcomes and ultraprocessed foods.”
The new evidence demands new policies, educational efforts, and labeling changes, suggested Dr. Ostfeld, director of preventive cardiology at Montefiore Health System in New York, and Ms. Allen, a dietitian at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “The goal should be to make the unhealthy choice the hard choice and the healthy choice the easy choice.”
The new analysis used data collected from people enrolled the Framingham Offspring Cohort, with their clinical metrics and diet information collected during 1991-1995 serving as their baseline. After excluding participants with prevalent CVD at baseline and those with incomplete follow-up of CVD events, the researchers had a cohort of 3,003 adults with an average follow-up of 18 years. At baseline, the cohort averaged 54 years of age; 55% were women, their average body mass index was 27.3 kg/m2, and about 6% had diabetes. They reported eating, on average, 7.5 servings of ultraprocessed food daily.
During follow-up, the cohort tallied 648 incident CVD events, including 251 hard CVD events (coronary death, MI, or stroke) and 163 hard CHD events (coronary death or MI), and 713 total deaths including 108 CVD deaths. Other CVD events recorded but not considered hard included heart failure, intermittent claudication, and transient ischemic attack.
In a multivariate-adjusted analysis, each average daily portion of ultraprocessed food was linked with an significant 7% relative increase in the incidence of a hard CVD event, compared with participants who ate fewer ultraprocessed food portions, and a 9% relative increase in the rate of hard CHD events, the study’s two prespecified primary outcomes. The researchers also found that each ultraprocessed serving significantly was associated with a 5% relative increased rate of total CVD events, and a 9% relative rise in CVD deaths. The analysis showed no significant association between total mortality and ultraprocessed food intake. (Average follow-up for the mortality analyses was 20 years.)
The authors also reported endpoint associations with intake of specific types of ultraprocessed foods, and found significantly increased associations specifically for portions of bread, ultraprocessed meat, salty snacks, and low-calorie soft drinks.
Convenient, omnipresent, and affordable
The authors acknowledged that the associations they found need examination in ethnically diverse populations, but nonetheless the findings “suggest the need for increased efforts to implement population-wide strategies” to lower consumption of ultraprocessed foods. “Given the convenience, omnipresence, and affordability of ultraprocessed foods, careful nutrition counseling is needed to design individualized, patient-centered, heart-healthy diets,” they concluded.
“Population-wide strategies such as taxation on sugar-sweetened beverages and other ultraprocessed foods and recommendations regarding processing levels in national dietary guidelines are needed to reduce the intake of ultraprocessed foods,” added Dr. Juul in her statement. “Of course, we must also implement policies that increase the availability, accessibility, and affordability of nutritious, minimally processed foods, especially in disadvantaged populations. At the clinical level, there is a need for increased commitment to individualized nutrition counseling for adopting sustainable heart-healthy diets.”
The study had no commercial funding. Dr. Juul and coauthors, Dr. Ostfeld, and Ms. Allen had no disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Omidubicel improves on umbilical cord blood transplants
Omidubicel, an investigational enriched umbilical cord blood product being developed by Gamida Cell for transplantation in patients with blood cancers, appears to have some advantages over standard umbilical cord blood.
The results come from a global phase 3 trial (NCT02730299) presented at the annual meeting of the European Society for Blood and Bone Marrow Transplantation.
“Transplantation with omidubicel, compared to standard cord blood transplantation, results in faster hematopoietic recovery, fewer infections, and fewer days in hospital,” said coinvestigator Guillermo F. Sanz, MD, PhD, from the Hospital Universitari i Politècnic la Fe in Valencia, Spain.
“Omidubicel should be considered as the new standard of care for patients eligible for umbilical cord blood transplantation,” Dr. Sanz concluded.
Zachariah DeFilipp, MD, from Mass General Cancer Center in Boston, a hematopoietic stem cell transplantation specialist who was not involved in the study, said in an interview that “omidubicel significantly improves the engraftment after transplant, as compared to standard cord blood transplant. For patients that lack an HLA-matched donor, this approach can help overcome the prolonged cytopenias that occur with standard cord blood transplants in adults.”
Gamida Cell plans to submit these data for approval of omidubicel by the Food and Drug Administration in the fourth quarter of 2021.
Omidubicel is also being evaluated in a phase 1/2 clinical study in patients with severe aplastic anemia (NCT03173937).
Expanding possibilities
Although umbilical cord blood stem cell grafts come from a readily available source and show greater tolerance across HLA barriers than other sources (such as bone marrow), the relatively low dose of stem cells in each unit results in delayed hematopoietic recovery, increased transplant-related morbidity and mortality, and longer hospitalizations, Dr. Sanz said.
Omidubicel consists of two cryopreserved fractions from a single cord blood unit. The product contains both noncultured CD133-negative cells, including T cells, and CD133-positive cells that are then expanded ex vivo for 21 days in the presence of nicotinamide.
“Nicotinamide increases stem and progenitor cells, inhibits differentiation and increases migration, bone marrow homing, and engraftment efficiency while preserving cellular functionality and phenotype,” Dr. Sanz explained during his presentation.
In an earlier phase 1/2 trial in 36 patients with high-risk hematologic malignancies, omidubicel was associated with hematopoietic engraftment lasting at least 10 years.
Details of phase 3 trial results
The global phase 3 trial was conducted in 125 patients (aged 13-65 years) with high-risk malignancies, including acute myeloid and lymphoblastic leukemias, myelodysplastic syndrome, chronic myeloid leukemia, lymphomas, and rare leukemias. These patients were all eligible for allogeneic stem cell transplantation but did not have matched donors.
Patients were randomly assigned to receive hematopoietic reconstitution with either omidubicel (n = 52) or standard cord blood (n = 58).
At 42 days of follow-up, the median time to neutrophil engraftment in the intention-to-treat (ITT) population, the primary endpoint, was 12 days with omidubicel versus 22 days with standard cord blood (P < .001).
In the as-treated population – the 108 patients who actually received omidubicel or standard cord blood – median time to engraftment was 10.0 versus 20.5 days, respectively (P < .001).
Rates of neutrophil engraftment at 42 days were 96% with omidubicel versus 89% with standard cord blood.
The secondary endpoint of time-to-platelet engraftment in the ITT population also favored omidubicel, with a cumulative day 42 incidence rate of 55%, compared with 35% with standard cord blood (P = .028).
In the as-treated population, median times to platelet engraftment were 37 days and 50 days, respectively (P = .023). The cumulative rates of platelet engraftment at 100 days of follow-up were 83% and 73%, respectively.
The incidence of grade 2 or 3 bacterial or invasive fungal infections by day 100 in the ITT population was 37% among patients who received omidubicel, compared with 57% for patients who received standard cord blood (P = .027). Viral infections occurred in 10% versus 26% of patients, respectively.
The incidence of acute graft versus host disease at day 100 was similar between treatment groups, and there was no significant difference at 1 year.
Relapse and nonrelapse mortality rates, as well as disease-free and overall survival rates also did not differ between groups.
In the first 100 days post transplant, patients who received omidubicel were alive and out of the hospital for a median of 60.5 days, compared with 48 days for patients who received standard cord blood (P = .005).
The study was funded by Gamida Cell. Dr. Sanz reported receiving research funding from the company and several others, and consulting fees, honoraria, speakers bureau activity, and travel expenses from other companies. Dr. DeFilipp reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omidubicel, an investigational enriched umbilical cord blood product being developed by Gamida Cell for transplantation in patients with blood cancers, appears to have some advantages over standard umbilical cord blood.
The results come from a global phase 3 trial (NCT02730299) presented at the annual meeting of the European Society for Blood and Bone Marrow Transplantation.
“Transplantation with omidubicel, compared to standard cord blood transplantation, results in faster hematopoietic recovery, fewer infections, and fewer days in hospital,” said coinvestigator Guillermo F. Sanz, MD, PhD, from the Hospital Universitari i Politècnic la Fe in Valencia, Spain.
“Omidubicel should be considered as the new standard of care for patients eligible for umbilical cord blood transplantation,” Dr. Sanz concluded.
Zachariah DeFilipp, MD, from Mass General Cancer Center in Boston, a hematopoietic stem cell transplantation specialist who was not involved in the study, said in an interview that “omidubicel significantly improves the engraftment after transplant, as compared to standard cord blood transplant. For patients that lack an HLA-matched donor, this approach can help overcome the prolonged cytopenias that occur with standard cord blood transplants in adults.”
Gamida Cell plans to submit these data for approval of omidubicel by the Food and Drug Administration in the fourth quarter of 2021.
Omidubicel is also being evaluated in a phase 1/2 clinical study in patients with severe aplastic anemia (NCT03173937).
Expanding possibilities
Although umbilical cord blood stem cell grafts come from a readily available source and show greater tolerance across HLA barriers than other sources (such as bone marrow), the relatively low dose of stem cells in each unit results in delayed hematopoietic recovery, increased transplant-related morbidity and mortality, and longer hospitalizations, Dr. Sanz said.
Omidubicel consists of two cryopreserved fractions from a single cord blood unit. The product contains both noncultured CD133-negative cells, including T cells, and CD133-positive cells that are then expanded ex vivo for 21 days in the presence of nicotinamide.
“Nicotinamide increases stem and progenitor cells, inhibits differentiation and increases migration, bone marrow homing, and engraftment efficiency while preserving cellular functionality and phenotype,” Dr. Sanz explained during his presentation.
In an earlier phase 1/2 trial in 36 patients with high-risk hematologic malignancies, omidubicel was associated with hematopoietic engraftment lasting at least 10 years.
Details of phase 3 trial results
The global phase 3 trial was conducted in 125 patients (aged 13-65 years) with high-risk malignancies, including acute myeloid and lymphoblastic leukemias, myelodysplastic syndrome, chronic myeloid leukemia, lymphomas, and rare leukemias. These patients were all eligible for allogeneic stem cell transplantation but did not have matched donors.
Patients were randomly assigned to receive hematopoietic reconstitution with either omidubicel (n = 52) or standard cord blood (n = 58).
At 42 days of follow-up, the median time to neutrophil engraftment in the intention-to-treat (ITT) population, the primary endpoint, was 12 days with omidubicel versus 22 days with standard cord blood (P < .001).
In the as-treated population – the 108 patients who actually received omidubicel or standard cord blood – median time to engraftment was 10.0 versus 20.5 days, respectively (P < .001).
Rates of neutrophil engraftment at 42 days were 96% with omidubicel versus 89% with standard cord blood.
The secondary endpoint of time-to-platelet engraftment in the ITT population also favored omidubicel, with a cumulative day 42 incidence rate of 55%, compared with 35% with standard cord blood (P = .028).
In the as-treated population, median times to platelet engraftment were 37 days and 50 days, respectively (P = .023). The cumulative rates of platelet engraftment at 100 days of follow-up were 83% and 73%, respectively.
The incidence of grade 2 or 3 bacterial or invasive fungal infections by day 100 in the ITT population was 37% among patients who received omidubicel, compared with 57% for patients who received standard cord blood (P = .027). Viral infections occurred in 10% versus 26% of patients, respectively.
The incidence of acute graft versus host disease at day 100 was similar between treatment groups, and there was no significant difference at 1 year.
Relapse and nonrelapse mortality rates, as well as disease-free and overall survival rates also did not differ between groups.
In the first 100 days post transplant, patients who received omidubicel were alive and out of the hospital for a median of 60.5 days, compared with 48 days for patients who received standard cord blood (P = .005).
The study was funded by Gamida Cell. Dr. Sanz reported receiving research funding from the company and several others, and consulting fees, honoraria, speakers bureau activity, and travel expenses from other companies. Dr. DeFilipp reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omidubicel, an investigational enriched umbilical cord blood product being developed by Gamida Cell for transplantation in patients with blood cancers, appears to have some advantages over standard umbilical cord blood.
The results come from a global phase 3 trial (NCT02730299) presented at the annual meeting of the European Society for Blood and Bone Marrow Transplantation.
“Transplantation with omidubicel, compared to standard cord blood transplantation, results in faster hematopoietic recovery, fewer infections, and fewer days in hospital,” said coinvestigator Guillermo F. Sanz, MD, PhD, from the Hospital Universitari i Politècnic la Fe in Valencia, Spain.
“Omidubicel should be considered as the new standard of care for patients eligible for umbilical cord blood transplantation,” Dr. Sanz concluded.
Zachariah DeFilipp, MD, from Mass General Cancer Center in Boston, a hematopoietic stem cell transplantation specialist who was not involved in the study, said in an interview that “omidubicel significantly improves the engraftment after transplant, as compared to standard cord blood transplant. For patients that lack an HLA-matched donor, this approach can help overcome the prolonged cytopenias that occur with standard cord blood transplants in adults.”
Gamida Cell plans to submit these data for approval of omidubicel by the Food and Drug Administration in the fourth quarter of 2021.
Omidubicel is also being evaluated in a phase 1/2 clinical study in patients with severe aplastic anemia (NCT03173937).
Expanding possibilities
Although umbilical cord blood stem cell grafts come from a readily available source and show greater tolerance across HLA barriers than other sources (such as bone marrow), the relatively low dose of stem cells in each unit results in delayed hematopoietic recovery, increased transplant-related morbidity and mortality, and longer hospitalizations, Dr. Sanz said.
Omidubicel consists of two cryopreserved fractions from a single cord blood unit. The product contains both noncultured CD133-negative cells, including T cells, and CD133-positive cells that are then expanded ex vivo for 21 days in the presence of nicotinamide.
“Nicotinamide increases stem and progenitor cells, inhibits differentiation and increases migration, bone marrow homing, and engraftment efficiency while preserving cellular functionality and phenotype,” Dr. Sanz explained during his presentation.
In an earlier phase 1/2 trial in 36 patients with high-risk hematologic malignancies, omidubicel was associated with hematopoietic engraftment lasting at least 10 years.
Details of phase 3 trial results
The global phase 3 trial was conducted in 125 patients (aged 13-65 years) with high-risk malignancies, including acute myeloid and lymphoblastic leukemias, myelodysplastic syndrome, chronic myeloid leukemia, lymphomas, and rare leukemias. These patients were all eligible for allogeneic stem cell transplantation but did not have matched donors.
Patients were randomly assigned to receive hematopoietic reconstitution with either omidubicel (n = 52) or standard cord blood (n = 58).
At 42 days of follow-up, the median time to neutrophil engraftment in the intention-to-treat (ITT) population, the primary endpoint, was 12 days with omidubicel versus 22 days with standard cord blood (P < .001).
In the as-treated population – the 108 patients who actually received omidubicel or standard cord blood – median time to engraftment was 10.0 versus 20.5 days, respectively (P < .001).
Rates of neutrophil engraftment at 42 days were 96% with omidubicel versus 89% with standard cord blood.
The secondary endpoint of time-to-platelet engraftment in the ITT population also favored omidubicel, with a cumulative day 42 incidence rate of 55%, compared with 35% with standard cord blood (P = .028).
In the as-treated population, median times to platelet engraftment were 37 days and 50 days, respectively (P = .023). The cumulative rates of platelet engraftment at 100 days of follow-up were 83% and 73%, respectively.
The incidence of grade 2 or 3 bacterial or invasive fungal infections by day 100 in the ITT population was 37% among patients who received omidubicel, compared with 57% for patients who received standard cord blood (P = .027). Viral infections occurred in 10% versus 26% of patients, respectively.
The incidence of acute graft versus host disease at day 100 was similar between treatment groups, and there was no significant difference at 1 year.
Relapse and nonrelapse mortality rates, as well as disease-free and overall survival rates also did not differ between groups.
In the first 100 days post transplant, patients who received omidubicel were alive and out of the hospital for a median of 60.5 days, compared with 48 days for patients who received standard cord blood (P = .005).
The study was funded by Gamida Cell. Dr. Sanz reported receiving research funding from the company and several others, and consulting fees, honoraria, speakers bureau activity, and travel expenses from other companies. Dr. DeFilipp reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
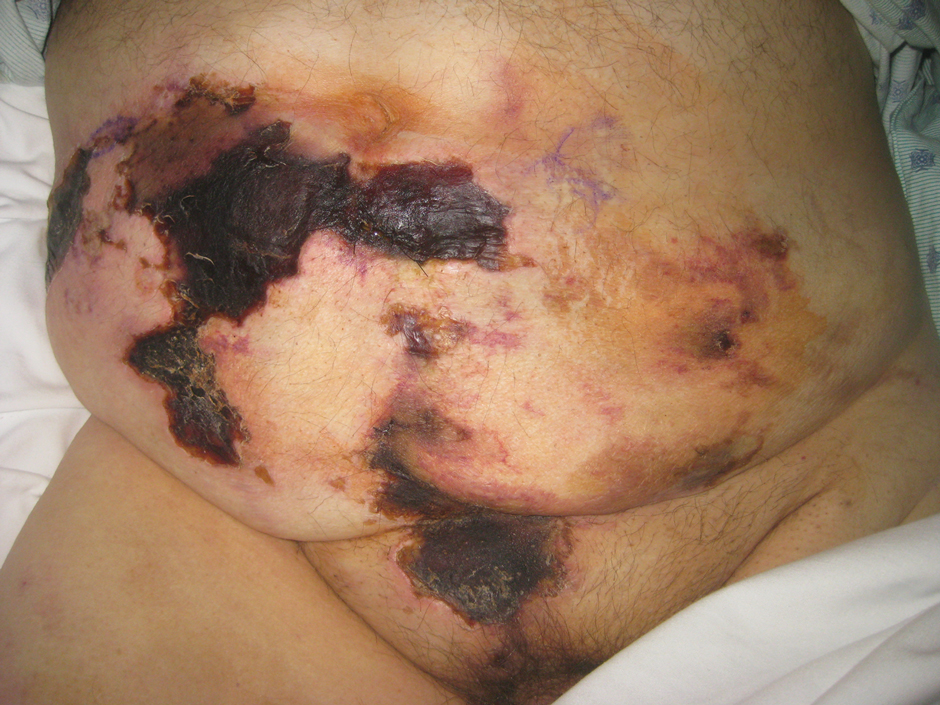
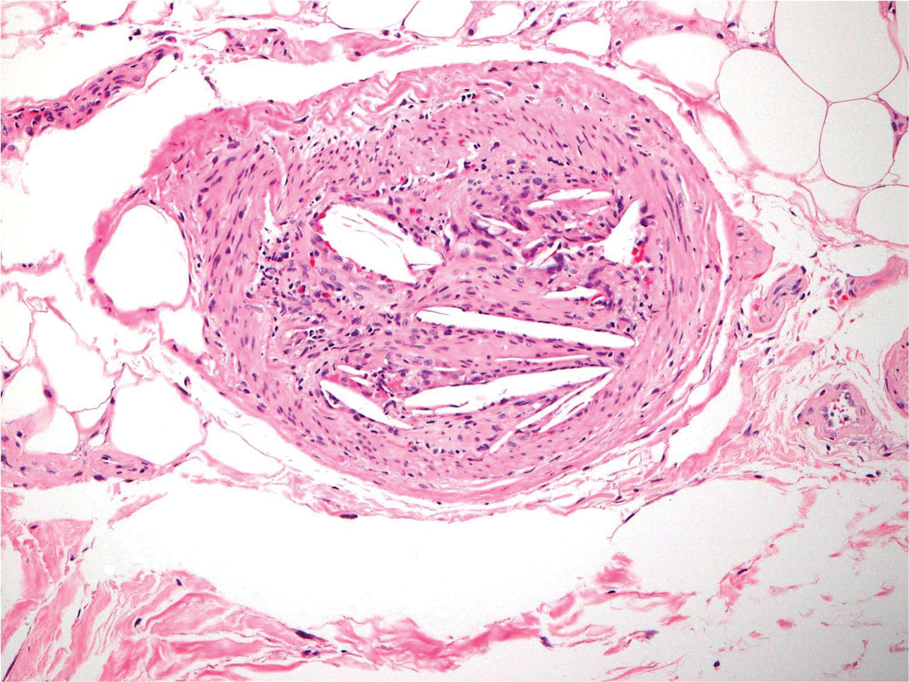
Abdominal aortic calcification may further raise known fracture risk
A new study has found that older men with high levels of abdominal aortic calcification (AAC) and a prevalent vertebral fracture – both of which can be assessed via lateral spine radiographs – are at increased risk of hip, clinical vertebral, and major osteoporotic fractures.
“The results of this study and others suggest that it may be appropriate to expand lateral spine imaging to include those with a significant pre-test probability of higher AAC being present,” wrote John T. Schousboe, MD, of the Park Nicollet Clinic and HealthPartners Institute in Bloomington, Minn. The study was published in the Journal of Bone and Mineral Research.
To determine the impact of prevalent vertebral fractures and AAC on fracture risk, the researchers assessed the lateral spine radiographs of 5,365 men who were enrolled in the Osteoporotic Fractures in Men (MrOS) study. All participants were 65 years or older, community dwelling, able to walk without assistance, and without bilateral hip arthroplasties. They split patients’ 24-point AAC (ACC-24) scores at the baseline visit into four levels: 0-1, 2-4, 5-8, and greater than 9. Self-reports of fractures were solicited from the cohort every 4 months.
Of all participants, 7.6% (n = 407) had a prevalent vertebral fracture at baseline. They were, on average, 1.5 years older than participants without a fracture; they were also more likely to be white and to have a prior nonspine fracture, along with having a lower femoral neck BMD (0.718 g/cm2, compared with 0.787 g/cm2; P < .001). In addition, significantly more men with a prevalent vertebral fracture had an AAC score greater than 9 (27% vs. 21.2%).
After an average follow-up period of 12.4 years (standard deviation, 5.2), 634 men had a major osteoporotic fracture, 283 had a hip fracture, 206 had a clinical vertebral fracture, and 2,626 died without having any of the three. After adjustment for risk factors such as age, prior nonspine fracture, and prevalent vertebral fracture, men with higher AAC-24 scores had a higher risk of major osteoporotic fracture, compared with men who had scores of 0-1: a hazard ratio of 1.38 (95% confidence interval, 1.10-1.73; P < .001) for scores 2-4, a HR of 1.45 (95% CI, 1.14-1.84; P < .001) for scores 5-8, and a HR of 1.65 (95% CI, 1.29-2.10; P < .001) for scores greater than 9.
Similar findings were reported regarding risk of hip fractures: a HR of 1.54 (95% CI, 1.07-2.20; P < .001) for men with AAC-24 scores 2-4, a HR of 1.40 (95% CI, 0.96-2.06; P < .001) for scores 5-8, and a HR of 2.17 (95% CI, 1.50-3.13; P < .001) for scores greater than 9. AAC-24 score severity was not associated with a higher risk of clinical vertebral fractures.
After adjustment for risk factors and AAC-24 score, men with prevalent vertebral fractures had an increased risk of all three fracture outcomes, compared with men without any fractures at baseline: a HR of 1.56 (95% CI, 1.12-2.16; P < .001) for hip fracture, a HR of 1.85 (95% CI, 1.48-2.31; P < .001) for major osteoporotic fracture, and a HR of 2.76 (95% CI, 1.94-3.91; P < .001) for clinical vertebral fracture.
Adjusting for competing mortality produced similar results: men with higher levels of AAC had increased risk of major osteoporotic fracture and hip fracture, although AAC-24 score was not associated with higher risk of clinical vertebral fractures. Prevalent vertebral fractures were also still associated with higher risk of hip (subdistribution HR, 1.42; 95% CI, 1.01-2.00; P = .004), major osteoporotic fracture (SHR, 1.71; 95% CI, 1.36-2.14; P < .001), and clinical vertebral fracture (SHR, 2.46; 95% CI, 1.72-3.52; P < .001).
Fracture risk assessment proves to be “a nice proof of concept”
“It’s well known that prevalent fractures predict future fractures,” said Thomas M. Link, MD, PhD, chief of the musculoskeletal imaging section in the department of radiology and biomedical imaging at the University of California, San Francisco, in an interview. “The new finding is that aortic calcifications combined with prevalent fractures perform better in predicting major osteoporotic fractures. Traditionally on radiographs, we note that patients who have more calcifications in vessels have less density or calcium in the bone, so this is a nice proof of concept.”
“While the study shows excellent reproducibility, it is not clear how the AAC-24 score was validated,” he added. “Theoretically, abdominal CT could be used for this.”
Along with validation of the AAC-24 score on lateral spine radiographs, he expressed a desire that future research would be “clearer regarding how this would potentially impact patient management. Prevalent fractures already are an indication to treat patients with osteoporosis-specific drugs. How would the results of this study impact management beyond that?”
The authors acknowledged their study’s other potential limitations, including limits in their ability to estimate absolute and relative hip fracture risk in men with low AAC scores but a prevalent vertebral fracture. In addition, they noted that their cohort was “mostly white, healthy, community-dwelling older men” and therefore may not be generalizable to other populations.
The study was supported by the National Institutes of Health, including grants from the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and the NIH Roadmap for Medical Research. One author reported being supported by a National Heart Foundation of Australia Future Leader Fellowship. The others disclosed no potential conflicts of interest.
A new study has found that older men with high levels of abdominal aortic calcification (AAC) and a prevalent vertebral fracture – both of which can be assessed via lateral spine radiographs – are at increased risk of hip, clinical vertebral, and major osteoporotic fractures.
“The results of this study and others suggest that it may be appropriate to expand lateral spine imaging to include those with a significant pre-test probability of higher AAC being present,” wrote John T. Schousboe, MD, of the Park Nicollet Clinic and HealthPartners Institute in Bloomington, Minn. The study was published in the Journal of Bone and Mineral Research.
To determine the impact of prevalent vertebral fractures and AAC on fracture risk, the researchers assessed the lateral spine radiographs of 5,365 men who were enrolled in the Osteoporotic Fractures in Men (MrOS) study. All participants were 65 years or older, community dwelling, able to walk without assistance, and without bilateral hip arthroplasties. They split patients’ 24-point AAC (ACC-24) scores at the baseline visit into four levels: 0-1, 2-4, 5-8, and greater than 9. Self-reports of fractures were solicited from the cohort every 4 months.
Of all participants, 7.6% (n = 407) had a prevalent vertebral fracture at baseline. They were, on average, 1.5 years older than participants without a fracture; they were also more likely to be white and to have a prior nonspine fracture, along with having a lower femoral neck BMD (0.718 g/cm2, compared with 0.787 g/cm2; P < .001). In addition, significantly more men with a prevalent vertebral fracture had an AAC score greater than 9 (27% vs. 21.2%).
After an average follow-up period of 12.4 years (standard deviation, 5.2), 634 men had a major osteoporotic fracture, 283 had a hip fracture, 206 had a clinical vertebral fracture, and 2,626 died without having any of the three. After adjustment for risk factors such as age, prior nonspine fracture, and prevalent vertebral fracture, men with higher AAC-24 scores had a higher risk of major osteoporotic fracture, compared with men who had scores of 0-1: a hazard ratio of 1.38 (95% confidence interval, 1.10-1.73; P < .001) for scores 2-4, a HR of 1.45 (95% CI, 1.14-1.84; P < .001) for scores 5-8, and a HR of 1.65 (95% CI, 1.29-2.10; P < .001) for scores greater than 9.
Similar findings were reported regarding risk of hip fractures: a HR of 1.54 (95% CI, 1.07-2.20; P < .001) for men with AAC-24 scores 2-4, a HR of 1.40 (95% CI, 0.96-2.06; P < .001) for scores 5-8, and a HR of 2.17 (95% CI, 1.50-3.13; P < .001) for scores greater than 9. AAC-24 score severity was not associated with a higher risk of clinical vertebral fractures.
After adjustment for risk factors and AAC-24 score, men with prevalent vertebral fractures had an increased risk of all three fracture outcomes, compared with men without any fractures at baseline: a HR of 1.56 (95% CI, 1.12-2.16; P < .001) for hip fracture, a HR of 1.85 (95% CI, 1.48-2.31; P < .001) for major osteoporotic fracture, and a HR of 2.76 (95% CI, 1.94-3.91; P < .001) for clinical vertebral fracture.
Adjusting for competing mortality produced similar results: men with higher levels of AAC had increased risk of major osteoporotic fracture and hip fracture, although AAC-24 score was not associated with higher risk of clinical vertebral fractures. Prevalent vertebral fractures were also still associated with higher risk of hip (subdistribution HR, 1.42; 95% CI, 1.01-2.00; P = .004), major osteoporotic fracture (SHR, 1.71; 95% CI, 1.36-2.14; P < .001), and clinical vertebral fracture (SHR, 2.46; 95% CI, 1.72-3.52; P < .001).
Fracture risk assessment proves to be “a nice proof of concept”
“It’s well known that prevalent fractures predict future fractures,” said Thomas M. Link, MD, PhD, chief of the musculoskeletal imaging section in the department of radiology and biomedical imaging at the University of California, San Francisco, in an interview. “The new finding is that aortic calcifications combined with prevalent fractures perform better in predicting major osteoporotic fractures. Traditionally on radiographs, we note that patients who have more calcifications in vessels have less density or calcium in the bone, so this is a nice proof of concept.”
“While the study shows excellent reproducibility, it is not clear how the AAC-24 score was validated,” he added. “Theoretically, abdominal CT could be used for this.”
Along with validation of the AAC-24 score on lateral spine radiographs, he expressed a desire that future research would be “clearer regarding how this would potentially impact patient management. Prevalent fractures already are an indication to treat patients with osteoporosis-specific drugs. How would the results of this study impact management beyond that?”
The authors acknowledged their study’s other potential limitations, including limits in their ability to estimate absolute and relative hip fracture risk in men with low AAC scores but a prevalent vertebral fracture. In addition, they noted that their cohort was “mostly white, healthy, community-dwelling older men” and therefore may not be generalizable to other populations.
The study was supported by the National Institutes of Health, including grants from the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and the NIH Roadmap for Medical Research. One author reported being supported by a National Heart Foundation of Australia Future Leader Fellowship. The others disclosed no potential conflicts of interest.
A new study has found that older men with high levels of abdominal aortic calcification (AAC) and a prevalent vertebral fracture – both of which can be assessed via lateral spine radiographs – are at increased risk of hip, clinical vertebral, and major osteoporotic fractures.
“The results of this study and others suggest that it may be appropriate to expand lateral spine imaging to include those with a significant pre-test probability of higher AAC being present,” wrote John T. Schousboe, MD, of the Park Nicollet Clinic and HealthPartners Institute in Bloomington, Minn. The study was published in the Journal of Bone and Mineral Research.
To determine the impact of prevalent vertebral fractures and AAC on fracture risk, the researchers assessed the lateral spine radiographs of 5,365 men who were enrolled in the Osteoporotic Fractures in Men (MrOS) study. All participants were 65 years or older, community dwelling, able to walk without assistance, and without bilateral hip arthroplasties. They split patients’ 24-point AAC (ACC-24) scores at the baseline visit into four levels: 0-1, 2-4, 5-8, and greater than 9. Self-reports of fractures were solicited from the cohort every 4 months.
Of all participants, 7.6% (n = 407) had a prevalent vertebral fracture at baseline. They were, on average, 1.5 years older than participants without a fracture; they were also more likely to be white and to have a prior nonspine fracture, along with having a lower femoral neck BMD (0.718 g/cm2, compared with 0.787 g/cm2; P < .001). In addition, significantly more men with a prevalent vertebral fracture had an AAC score greater than 9 (27% vs. 21.2%).
After an average follow-up period of 12.4 years (standard deviation, 5.2), 634 men had a major osteoporotic fracture, 283 had a hip fracture, 206 had a clinical vertebral fracture, and 2,626 died without having any of the three. After adjustment for risk factors such as age, prior nonspine fracture, and prevalent vertebral fracture, men with higher AAC-24 scores had a higher risk of major osteoporotic fracture, compared with men who had scores of 0-1: a hazard ratio of 1.38 (95% confidence interval, 1.10-1.73; P < .001) for scores 2-4, a HR of 1.45 (95% CI, 1.14-1.84; P < .001) for scores 5-8, and a HR of 1.65 (95% CI, 1.29-2.10; P < .001) for scores greater than 9.
Similar findings were reported regarding risk of hip fractures: a HR of 1.54 (95% CI, 1.07-2.20; P < .001) for men with AAC-24 scores 2-4, a HR of 1.40 (95% CI, 0.96-2.06; P < .001) for scores 5-8, and a HR of 2.17 (95% CI, 1.50-3.13; P < .001) for scores greater than 9. AAC-24 score severity was not associated with a higher risk of clinical vertebral fractures.
After adjustment for risk factors and AAC-24 score, men with prevalent vertebral fractures had an increased risk of all three fracture outcomes, compared with men without any fractures at baseline: a HR of 1.56 (95% CI, 1.12-2.16; P < .001) for hip fracture, a HR of 1.85 (95% CI, 1.48-2.31; P < .001) for major osteoporotic fracture, and a HR of 2.76 (95% CI, 1.94-3.91; P < .001) for clinical vertebral fracture.
Adjusting for competing mortality produced similar results: men with higher levels of AAC had increased risk of major osteoporotic fracture and hip fracture, although AAC-24 score was not associated with higher risk of clinical vertebral fractures. Prevalent vertebral fractures were also still associated with higher risk of hip (subdistribution HR, 1.42; 95% CI, 1.01-2.00; P = .004), major osteoporotic fracture (SHR, 1.71; 95% CI, 1.36-2.14; P < .001), and clinical vertebral fracture (SHR, 2.46; 95% CI, 1.72-3.52; P < .001).
Fracture risk assessment proves to be “a nice proof of concept”
“It’s well known that prevalent fractures predict future fractures,” said Thomas M. Link, MD, PhD, chief of the musculoskeletal imaging section in the department of radiology and biomedical imaging at the University of California, San Francisco, in an interview. “The new finding is that aortic calcifications combined with prevalent fractures perform better in predicting major osteoporotic fractures. Traditionally on radiographs, we note that patients who have more calcifications in vessels have less density or calcium in the bone, so this is a nice proof of concept.”
“While the study shows excellent reproducibility, it is not clear how the AAC-24 score was validated,” he added. “Theoretically, abdominal CT could be used for this.”
Along with validation of the AAC-24 score on lateral spine radiographs, he expressed a desire that future research would be “clearer regarding how this would potentially impact patient management. Prevalent fractures already are an indication to treat patients with osteoporosis-specific drugs. How would the results of this study impact management beyond that?”
The authors acknowledged their study’s other potential limitations, including limits in their ability to estimate absolute and relative hip fracture risk in men with low AAC scores but a prevalent vertebral fracture. In addition, they noted that their cohort was “mostly white, healthy, community-dwelling older men” and therefore may not be generalizable to other populations.
The study was supported by the National Institutes of Health, including grants from the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and the NIH Roadmap for Medical Research. One author reported being supported by a National Heart Foundation of Australia Future Leader Fellowship. The others disclosed no potential conflicts of interest.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
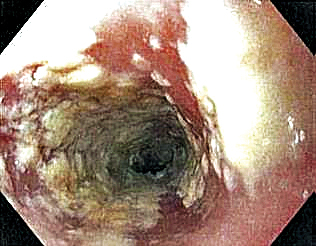
Emergent ERCP in acute cholangitis linked with better outcomes
Background: Acute cholangitis (AC) in its most severe form is associated with a high mortality rate. Most patients respond to medical management involving intravenous hydration and antibiotics, though a sizable portion require biliary drainage. Current guidelines advocate for urgent drainage depending on the severity of AC, though do not specify optimal timing. Existing literature is conflicting on when ERCP should ideally be done for AC.
Study design: Systematic review and meta-analysis.
Setting: Literature search involving PubMed, Medline, and Embase databases.
Synopsis: Nine studies with 7,534 patients were included in the final meta-analysis. Emergent ERCP was associated with a lower in-hospital mortality (IHM; odds ratio, 0.52; 95% confidence interval, 0.28-0.98) and shorter length of stay (LOS; mean difference, –2.87 days; 95% CI, –1.55 to –4.18), compared to urgent ERCP. The IHM mortality difference was true for both patients with severe AC (as defined by evidence of end-organ dysfunction) and mild-moderate AC. There was a trend toward lower 30-day mortality in patients who underwent emergent ERCP, though it did not reach statistical significance.
The studies included in the analysis were observational studies, so no causal relationship can be established. Only two of the nine studies reported outcome differences stratified by severity of presentation. Etiology of the AC was inconsistently reported amongst studies.
Bottom line: Emergent ERCP appears to be associated with reduced mortality and LOS in patients presenting with AC, though larger randomized controlled trials are needed to better delineate the optimal timing for biliary drainage in these patients.
Citation: Iqbal U et al. Emergent versus urgent ERCP in acute cholangitis: A systematic review and meta-analysis. Gastrointes Endosc. 2019 Oct 16. doi: 10.1016/j.gie.2019.09.040.
Dr. Babbel is a hospitalist and assistant professor of medicine at the University of Utah, Salt Lake City.
Background: Acute cholangitis (AC) in its most severe form is associated with a high mortality rate. Most patients respond to medical management involving intravenous hydration and antibiotics, though a sizable portion require biliary drainage. Current guidelines advocate for urgent drainage depending on the severity of AC, though do not specify optimal timing. Existing literature is conflicting on when ERCP should ideally be done for AC.
Study design: Systematic review and meta-analysis.
Setting: Literature search involving PubMed, Medline, and Embase databases.
Synopsis: Nine studies with 7,534 patients were included in the final meta-analysis. Emergent ERCP was associated with a lower in-hospital mortality (IHM; odds ratio, 0.52; 95% confidence interval, 0.28-0.98) and shorter length of stay (LOS; mean difference, –2.87 days; 95% CI, –1.55 to –4.18), compared to urgent ERCP. The IHM mortality difference was true for both patients with severe AC (as defined by evidence of end-organ dysfunction) and mild-moderate AC. There was a trend toward lower 30-day mortality in patients who underwent emergent ERCP, though it did not reach statistical significance.
The studies included in the analysis were observational studies, so no causal relationship can be established. Only two of the nine studies reported outcome differences stratified by severity of presentation. Etiology of the AC was inconsistently reported amongst studies.
Bottom line: Emergent ERCP appears to be associated with reduced mortality and LOS in patients presenting with AC, though larger randomized controlled trials are needed to better delineate the optimal timing for biliary drainage in these patients.
Citation: Iqbal U et al. Emergent versus urgent ERCP in acute cholangitis: A systematic review and meta-analysis. Gastrointes Endosc. 2019 Oct 16. doi: 10.1016/j.gie.2019.09.040.
Dr. Babbel is a hospitalist and assistant professor of medicine at the University of Utah, Salt Lake City.
Background: Acute cholangitis (AC) in its most severe form is associated with a high mortality rate. Most patients respond to medical management involving intravenous hydration and antibiotics, though a sizable portion require biliary drainage. Current guidelines advocate for urgent drainage depending on the severity of AC, though do not specify optimal timing. Existing literature is conflicting on when ERCP should ideally be done for AC.
Study design: Systematic review and meta-analysis.
Setting: Literature search involving PubMed, Medline, and Embase databases.
Synopsis: Nine studies with 7,534 patients were included in the final meta-analysis. Emergent ERCP was associated with a lower in-hospital mortality (IHM; odds ratio, 0.52; 95% confidence interval, 0.28-0.98) and shorter length of stay (LOS; mean difference, –2.87 days; 95% CI, –1.55 to –4.18), compared to urgent ERCP. The IHM mortality difference was true for both patients with severe AC (as defined by evidence of end-organ dysfunction) and mild-moderate AC. There was a trend toward lower 30-day mortality in patients who underwent emergent ERCP, though it did not reach statistical significance.
The studies included in the analysis were observational studies, so no causal relationship can be established. Only two of the nine studies reported outcome differences stratified by severity of presentation. Etiology of the AC was inconsistently reported amongst studies.
Bottom line: Emergent ERCP appears to be associated with reduced mortality and LOS in patients presenting with AC, though larger randomized controlled trials are needed to better delineate the optimal timing for biliary drainage in these patients.
Citation: Iqbal U et al. Emergent versus urgent ERCP in acute cholangitis: A systematic review and meta-analysis. Gastrointes Endosc. 2019 Oct 16. doi: 10.1016/j.gie.2019.09.040.
Dr. Babbel is a hospitalist and assistant professor of medicine at the University of Utah, Salt Lake City.
How to talk to patients reluctant to get a COVID-19 vaccine
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Cutaneous Cholesterol Embolization to the Lower Trunk: An Underrecognized Presentation
To the Editor:
A 65-year-old man with severe atherosclerotic disease developed multiple painful eschars on the lower abdomen, thighs, sacrum, and perineum. He initially presented with myocardial ischemia and claudication and underwent 3 cardiac catheterizations as well as stenting of the superficial femoral artery. Within 2 weeks, he developed exquisitely tender nodules on the lower abdomen, clinically presumed to be sites of enoxaparin injections. These lesions gradually expanded and ulcerated to involve the sacrum, buttock, perineum, and upper thighs (Figure 1). Two punch biopsies from ulcerated skin taken 10 days apart demonstrated necrosis of skin and subcutaneous fat without evidence of vasculitis, vasculopathy, emboli, or notable inflammation despite examination of multiple levels of all submitted tissue. A definitive cause for the ulcerations remained elusive with development of new lesions. A third incisional biopsy of a newly developed, nonulcerated, subcutaneous nodule performed 8 weeks after presentation revealed multiple cholesterol emboli (Figure 2). He was treated with warfarin and clopidogrel bisulfate as well as local wound care. The lesions slowly resolved over the next 4 to 6 months.
Cholesterol embolization syndrome occurs when disrupted atherosclerotic plaques embolize from large proximal arteries to more distal arterioles, resulting in ischemic damage to 1 or more organ systems.1 It can occur spontaneously but often is a consequence of thrombolytic therapy, anticoagulation, and angioinvasive procedures.2,3 Cutaneous manifestations include livedo reticularis, retiform purpura, nodules, and gangrene. Although livedo reticularis may extend from the legs to the trunk, gangrenous lesions predominantly involve the distal digits.
This case illustrates the challenge in diagnosis of cholesterol emboli, both clinically and histologically. Cutaneous lesions are morphologically variable and often occur with systemic manifestations, mimicking numerous conditions.1 Lower extremity involvement is a well-known occurrence in cholesterol embolization (ie, blue toe syndrome); however, periumbilical and lumbosacral lesions have not been emphasized in the dermatologic or peripheral vascular literature. Our patient’s initial diagnosis was enoxaparin necrosis at abdominal injection sites; however, this unusual distribution of lesions was ultimately determined to be the consequence of cholesterol embolization from the inferior epigastric and superficial external pudendal arteries at the time of stenting of the superficial femoral artery. Proximal truncal involvement should be recognized as an atypical but important cutaneous manifestation to facilitate timely diagnosis and treatment.4,5
Our patient’s course also highlights the potential need for multiple biopsies. Although the gold standard for diagnosis is histologic confirmation, a negative biopsy does not always exclude cholesterol emboli, and one should have a low threshold to perform additional biopsies in the appropriate clinical setting.
- Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: a review of 221 cases in the English literature. Angiology. 1987;38:769-784.
- Fukumoto Y, Tsutsui H, Tsuchihashi M, et al. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211-216.
- Karalis DG, Chandrasekaran K, Victor MF, et al. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991;17:73.
- Zaytsev P, Miller K, Pellettiere EV. Cutaneous cholesterol emboli with infarction clinically mimicking heparin necrosis—a case report. Angiology. 1986;37:471-476.
- Erdim M, Tezel E, Biskin N. A case of skin necrosis as a result of cholesterol crystal embolisation. J Plast Reconstr Aesthet Surg. 2006;59:429-432.
To the Editor:
A 65-year-old man with severe atherosclerotic disease developed multiple painful eschars on the lower abdomen, thighs, sacrum, and perineum. He initially presented with myocardial ischemia and claudication and underwent 3 cardiac catheterizations as well as stenting of the superficial femoral artery. Within 2 weeks, he developed exquisitely tender nodules on the lower abdomen, clinically presumed to be sites of enoxaparin injections. These lesions gradually expanded and ulcerated to involve the sacrum, buttock, perineum, and upper thighs (Figure 1). Two punch biopsies from ulcerated skin taken 10 days apart demonstrated necrosis of skin and subcutaneous fat without evidence of vasculitis, vasculopathy, emboli, or notable inflammation despite examination of multiple levels of all submitted tissue. A definitive cause for the ulcerations remained elusive with development of new lesions. A third incisional biopsy of a newly developed, nonulcerated, subcutaneous nodule performed 8 weeks after presentation revealed multiple cholesterol emboli (Figure 2). He was treated with warfarin and clopidogrel bisulfate as well as local wound care. The lesions slowly resolved over the next 4 to 6 months.
Cholesterol embolization syndrome occurs when disrupted atherosclerotic plaques embolize from large proximal arteries to more distal arterioles, resulting in ischemic damage to 1 or more organ systems.1 It can occur spontaneously but often is a consequence of thrombolytic therapy, anticoagulation, and angioinvasive procedures.2,3 Cutaneous manifestations include livedo reticularis, retiform purpura, nodules, and gangrene. Although livedo reticularis may extend from the legs to the trunk, gangrenous lesions predominantly involve the distal digits.
This case illustrates the challenge in diagnosis of cholesterol emboli, both clinically and histologically. Cutaneous lesions are morphologically variable and often occur with systemic manifestations, mimicking numerous conditions.1 Lower extremity involvement is a well-known occurrence in cholesterol embolization (ie, blue toe syndrome); however, periumbilical and lumbosacral lesions have not been emphasized in the dermatologic or peripheral vascular literature. Our patient’s initial diagnosis was enoxaparin necrosis at abdominal injection sites; however, this unusual distribution of lesions was ultimately determined to be the consequence of cholesterol embolization from the inferior epigastric and superficial external pudendal arteries at the time of stenting of the superficial femoral artery. Proximal truncal involvement should be recognized as an atypical but important cutaneous manifestation to facilitate timely diagnosis and treatment.4,5
Our patient’s course also highlights the potential need for multiple biopsies. Although the gold standard for diagnosis is histologic confirmation, a negative biopsy does not always exclude cholesterol emboli, and one should have a low threshold to perform additional biopsies in the appropriate clinical setting.
To the Editor:
A 65-year-old man with severe atherosclerotic disease developed multiple painful eschars on the lower abdomen, thighs, sacrum, and perineum. He initially presented with myocardial ischemia and claudication and underwent 3 cardiac catheterizations as well as stenting of the superficial femoral artery. Within 2 weeks, he developed exquisitely tender nodules on the lower abdomen, clinically presumed to be sites of enoxaparin injections. These lesions gradually expanded and ulcerated to involve the sacrum, buttock, perineum, and upper thighs (Figure 1). Two punch biopsies from ulcerated skin taken 10 days apart demonstrated necrosis of skin and subcutaneous fat without evidence of vasculitis, vasculopathy, emboli, or notable inflammation despite examination of multiple levels of all submitted tissue. A definitive cause for the ulcerations remained elusive with development of new lesions. A third incisional biopsy of a newly developed, nonulcerated, subcutaneous nodule performed 8 weeks after presentation revealed multiple cholesterol emboli (Figure 2). He was treated with warfarin and clopidogrel bisulfate as well as local wound care. The lesions slowly resolved over the next 4 to 6 months.
Cholesterol embolization syndrome occurs when disrupted atherosclerotic plaques embolize from large proximal arteries to more distal arterioles, resulting in ischemic damage to 1 or more organ systems.1 It can occur spontaneously but often is a consequence of thrombolytic therapy, anticoagulation, and angioinvasive procedures.2,3 Cutaneous manifestations include livedo reticularis, retiform purpura, nodules, and gangrene. Although livedo reticularis may extend from the legs to the trunk, gangrenous lesions predominantly involve the distal digits.
This case illustrates the challenge in diagnosis of cholesterol emboli, both clinically and histologically. Cutaneous lesions are morphologically variable and often occur with systemic manifestations, mimicking numerous conditions.1 Lower extremity involvement is a well-known occurrence in cholesterol embolization (ie, blue toe syndrome); however, periumbilical and lumbosacral lesions have not been emphasized in the dermatologic or peripheral vascular literature. Our patient’s initial diagnosis was enoxaparin necrosis at abdominal injection sites; however, this unusual distribution of lesions was ultimately determined to be the consequence of cholesterol embolization from the inferior epigastric and superficial external pudendal arteries at the time of stenting of the superficial femoral artery. Proximal truncal involvement should be recognized as an atypical but important cutaneous manifestation to facilitate timely diagnosis and treatment.4,5
Our patient’s course also highlights the potential need for multiple biopsies. Although the gold standard for diagnosis is histologic confirmation, a negative biopsy does not always exclude cholesterol emboli, and one should have a low threshold to perform additional biopsies in the appropriate clinical setting.
- Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: a review of 221 cases in the English literature. Angiology. 1987;38:769-784.
- Fukumoto Y, Tsutsui H, Tsuchihashi M, et al. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211-216.
- Karalis DG, Chandrasekaran K, Victor MF, et al. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991;17:73.
- Zaytsev P, Miller K, Pellettiere EV. Cutaneous cholesterol emboli with infarction clinically mimicking heparin necrosis—a case report. Angiology. 1986;37:471-476.
- Erdim M, Tezel E, Biskin N. A case of skin necrosis as a result of cholesterol crystal embolisation. J Plast Reconstr Aesthet Surg. 2006;59:429-432.
- Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: a review of 221 cases in the English literature. Angiology. 1987;38:769-784.
- Fukumoto Y, Tsutsui H, Tsuchihashi M, et al. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211-216.
- Karalis DG, Chandrasekaran K, Victor MF, et al. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991;17:73.
- Zaytsev P, Miller K, Pellettiere EV. Cutaneous cholesterol emboli with infarction clinically mimicking heparin necrosis—a case report. Angiology. 1986;37:471-476.
- Erdim M, Tezel E, Biskin N. A case of skin necrosis as a result of cholesterol crystal embolisation. J Plast Reconstr Aesthet Surg. 2006;59:429-432.
Practice Points
- Cholesterol embolization may occur in proximal locations, and index of suspicion should be high in patients who are at risk.
- Several biopsies may be necessary to make a diagnosis of cholesterol emboli.
Candida Esophagitis Associated With Adalimumab for Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
Practice Points
- Adalimumab is an effective treatment for patients with hidradenitis suppurativa.
- There is risk for opportunistic infections with adalimumab, and patients should be monitored closely.
New data on worldwide mental health impact of COVID-19
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.