User login
FDA approves point-of-care COVID-19 antigen test

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.
Gender gaps persist in academic rheumatology
They’re less likely to hold a higher-level professorship position, feature as a senior author on a paper, or receive a federal grant. Two recent studies underscore progress for and barriers to career advancement.
One cross-sectional analysis of practicing U.S. rheumatologists found that fewer women are professors compared with men (12.6% vs. 36.8%) or associate professors (17.5% vs. 28%). A larger proportion of women serve as assistant professors (55.5% vs. 31.5%). From a leadership perspective, women are making progress. Their odds are similar to men as far as holding a fellowship or division director position in a rheumatology division.
For this study, published in Arthritis & Rheumatology on Aug. 16, April Jorge, MD, and her colleagues at Massachusetts General Hospital and Harvard Medical School, both in Boston, identified 6,125 rheumatologists from a database of all licensed physicians and used multivariate logistic regression to assess gender differences in academic advancement. They arrived at their results after accounting for variables such as age, research and academic appointments, publications, achievements, and years since residency graduation.
Women rheumatologists are younger, completing their residency more recently than their male colleagues. Their numbers in academic rheumatology have gradually increased over the last few decades, recently outpacing men. In 2015, the American College of Rheumatology reported that women made up 41% of the workforce and 66% of rheumatology fellows. Dr. Jorge and associates stressed the importance of fostering women in leadership positions and ensuring gender equity in academic career advancement.
Women also had fewer publications and grants from the National Institutes of Health. Several factors could account for this, such as time spent in the workforce or on parental leave, work-life balance, and mentorship. “However, gender differences in academic promotion remained after adjusting for each of these typical promotion criteria, indicating that other unidentified factors also contribute to the gap in promotion for women academic rheumatologists,” the investigators noted.
The authors weren’t able to assess how parental leave and work effort affected results or why pay differences existed between men and women. They also weren’t able to determine how many physicians left academic practice. “If greater numbers of women than men left the academic rheumatology workforce – for one of many reasons, including that they were not promoted – our findings could underestimate sex differences in academic rank,” they acknowledged.
Lower authorship rate examined
Fewer women in full or associate professor positions might explain why female authorship on research papers is underrepresented, according to another study published Aug. 18 in Arthritis & Rheumatology. Ekta Bagga and colleagues at the University of Auckland (New Zealand) examined 7,651 original research articles from high-impact rheumatology and general medical journals published during 2015-2019 and reported that women were much less likely to achieve first or senior author positions in reports of randomized, controlled trials. This was especially true for studies initiated and funded by industry, compared with other research designs.
More gender parity existed for first authorship than senior authorship – women first authors and senior authors appeared in 51.5% and 35.3% of the papers, respectively. For all geographical regions, the proportion of women senior authors fell below 40%. Representation was especially low in regions other than Europe and North America. These observations likely reflect gender disparities in the medical workforce, Nicola Dalbeth, MD, the study’s senior author, said in an interview.
“We know that, although women make up almost half the rheumatology workforce in many countries around the world, we are less likely to be in positions of senior academic leadership,” added Dr. Dalbeth, a rheumatologist and professor at the University of Auckland’s Bone and Joint Research Group. Institutions and industry should take steps to ensure that women rheumatologists get equal representation, particularly in clinical trial development, she added.
The study had its limitations, one of which was that the researchers didn’t analyze individual author names. This means that one person may have authored multiple articles. “Given the relatively low number of women in academic rheumatology leadership positions, our method of analysis may have overrepresented the number of women authors of rheumatology publications, particularly in senior positions,” stated Dr. Dalbeth and colleagues.
Implicit bias in academia
The articles by Jorge et al. and Bagga et al. suggest that implicit bias is as prevalent in medicine as it is in general society, Jason Kolfenbach, MD, said in an interview. Dr. Kolfenbach is an associate professor of medicine and rheumatology and director of the rheumatology fellowship program at University of Colorado at Denver, Aurora.
“The study by Jorge et al. is eye opening because it demonstrates that academic promotion is lower among women even after adjustment for some of these measures of academic productivity,” Dr. Kolfenbach said. It’s likely that bias plays some role “since there is a human element behind promotions committees, as well as committees selecting faculty for the creation of guidelines and speaker panels at national conferences.”
The study by Bagga et al. “matches my personal perception of industry-sponsored studies and pharmaceutical-sponsored speakers bureaus, namely that they are overrepresented by male faculty,” Dr. Kolfenbach continued.
Prior to COVID-19, the department of medicine at the University of Colorado had begun participating in a formal program called the Bias Reduction in Internal Medicine Initiative, a National Institutes of Health–sponsored study. “I’m hopeful programs such as this can lead to a more equitable situation than described by the findings in these two studies,” he added.
Article type, country of origin play a role
Other research corroborates the findings in these two papers. Giovanni Adami, MD, and coauthors examined 366 rheumatology guidelines and recommendations and determined that only 32% featured a female first author. However, authorship did increase for women over time, achieving parity in 2017.
There are several points to consider when exploring gender disparity, Dr. Adami said in an interview. “Original articles, industry-sponsored articles, and recommendation articles explore different disparities,” he offered. Recommendations and industry-sponsored articles are usually authored by international experts such as division directors or full professors. Original articles, in comparison, aren’t as affected by the “opinion leader” effect, he added.
Country of origin is also a crucial aspect, Dr. Adami said. In his own search of guidelines and articles published by Japanese or Chinese researchers, he noticed that males made up the vast majority of authors. “The cultural aspects of the country where research develops is a vital thing to consider when analyzing gender disparity.”
Dr. Adami’s homeland of Italy is a case in point: most of the division chiefs and professors are male. “Here in Italy, there’s a common belief that a woman cannot pursue an academic career or aim for a leadership position,” he noted.
Italy’s public university system has seen some improvements in gender parity, he continued. “For example, in 2009 there were 61,000 new medical students in Italy, and the majority [57%] were female. Nonetheless, we still have more male professors of medicine and more male PhD candidates.”
Gender gap narrows for conference speakers
In another study, rheumatologists Jean Liew, MD, of the University of Washington, Seattle, and Kanika Monga, MD, of the University of Texas Health Science Center at Houston, found notable gender gaps in speakers at ACR conferences. Women represented under 50% of speakers at these meetings over a 2-year period. “Although the gender gap at recent ACR meetings was narrower as compared with other conferences, we must remain cognizant of its presence and continue to work towards equal representation,” the authors wrote in a correspondence letter in Annals of the Rheumatic Diseases.
Dr. Monga said she was excited to see so many studies on the topic of gender disparities in rheumatology. The Jorge et al. and Bagga et al. papers “delve deeper into quantifying the gender gap in rheumatology. These studies allow us to better identify where the discrepancies may be,” she said in an interview.
“I found it very interesting that women were less likely to be promoted in academic rank but as likely as men to hold leadership positions,” Dr. Monga said. She agreed with the authors that criteria for academic promotion should be reassessed to ensure that it values the diversity of scholarly work that rheumatologists pursue.
Men may still outnumber women speakers at ACR meetings, but the Liew and Monga study did report a 4.2% increase in female speaker representation from 2017 to 2018. “We were happy to note that that continued to be the case at The American College of Rheumatology’s Annual Meeting in 2019. I hope that this reflects a positive change in our specialty,” she said.
Dr. Dalbeth’s study received support from a University of Auckland Summer Studentship Award. She has received consulting fees, speaker fees, or grants from AstraZeneca, Horizon, Amgen, Janssen, and other companies outside of the submitted work. The other authors declared no competing interests.
Dr. Jorge receives funds from the Rheumatology Research Foundation. The senior author on her study receives funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
They’re less likely to hold a higher-level professorship position, feature as a senior author on a paper, or receive a federal grant. Two recent studies underscore progress for and barriers to career advancement.
One cross-sectional analysis of practicing U.S. rheumatologists found that fewer women are professors compared with men (12.6% vs. 36.8%) or associate professors (17.5% vs. 28%). A larger proportion of women serve as assistant professors (55.5% vs. 31.5%). From a leadership perspective, women are making progress. Their odds are similar to men as far as holding a fellowship or division director position in a rheumatology division.
For this study, published in Arthritis & Rheumatology on Aug. 16, April Jorge, MD, and her colleagues at Massachusetts General Hospital and Harvard Medical School, both in Boston, identified 6,125 rheumatologists from a database of all licensed physicians and used multivariate logistic regression to assess gender differences in academic advancement. They arrived at their results after accounting for variables such as age, research and academic appointments, publications, achievements, and years since residency graduation.
Women rheumatologists are younger, completing their residency more recently than their male colleagues. Their numbers in academic rheumatology have gradually increased over the last few decades, recently outpacing men. In 2015, the American College of Rheumatology reported that women made up 41% of the workforce and 66% of rheumatology fellows. Dr. Jorge and associates stressed the importance of fostering women in leadership positions and ensuring gender equity in academic career advancement.
Women also had fewer publications and grants from the National Institutes of Health. Several factors could account for this, such as time spent in the workforce or on parental leave, work-life balance, and mentorship. “However, gender differences in academic promotion remained after adjusting for each of these typical promotion criteria, indicating that other unidentified factors also contribute to the gap in promotion for women academic rheumatologists,” the investigators noted.
The authors weren’t able to assess how parental leave and work effort affected results or why pay differences existed between men and women. They also weren’t able to determine how many physicians left academic practice. “If greater numbers of women than men left the academic rheumatology workforce – for one of many reasons, including that they were not promoted – our findings could underestimate sex differences in academic rank,” they acknowledged.
Lower authorship rate examined
Fewer women in full or associate professor positions might explain why female authorship on research papers is underrepresented, according to another study published Aug. 18 in Arthritis & Rheumatology. Ekta Bagga and colleagues at the University of Auckland (New Zealand) examined 7,651 original research articles from high-impact rheumatology and general medical journals published during 2015-2019 and reported that women were much less likely to achieve first or senior author positions in reports of randomized, controlled trials. This was especially true for studies initiated and funded by industry, compared with other research designs.
More gender parity existed for first authorship than senior authorship – women first authors and senior authors appeared in 51.5% and 35.3% of the papers, respectively. For all geographical regions, the proportion of women senior authors fell below 40%. Representation was especially low in regions other than Europe and North America. These observations likely reflect gender disparities in the medical workforce, Nicola Dalbeth, MD, the study’s senior author, said in an interview.
“We know that, although women make up almost half the rheumatology workforce in many countries around the world, we are less likely to be in positions of senior academic leadership,” added Dr. Dalbeth, a rheumatologist and professor at the University of Auckland’s Bone and Joint Research Group. Institutions and industry should take steps to ensure that women rheumatologists get equal representation, particularly in clinical trial development, she added.
The study had its limitations, one of which was that the researchers didn’t analyze individual author names. This means that one person may have authored multiple articles. “Given the relatively low number of women in academic rheumatology leadership positions, our method of analysis may have overrepresented the number of women authors of rheumatology publications, particularly in senior positions,” stated Dr. Dalbeth and colleagues.
Implicit bias in academia
The articles by Jorge et al. and Bagga et al. suggest that implicit bias is as prevalent in medicine as it is in general society, Jason Kolfenbach, MD, said in an interview. Dr. Kolfenbach is an associate professor of medicine and rheumatology and director of the rheumatology fellowship program at University of Colorado at Denver, Aurora.
“The study by Jorge et al. is eye opening because it demonstrates that academic promotion is lower among women even after adjustment for some of these measures of academic productivity,” Dr. Kolfenbach said. It’s likely that bias plays some role “since there is a human element behind promotions committees, as well as committees selecting faculty for the creation of guidelines and speaker panels at national conferences.”
The study by Bagga et al. “matches my personal perception of industry-sponsored studies and pharmaceutical-sponsored speakers bureaus, namely that they are overrepresented by male faculty,” Dr. Kolfenbach continued.
Prior to COVID-19, the department of medicine at the University of Colorado had begun participating in a formal program called the Bias Reduction in Internal Medicine Initiative, a National Institutes of Health–sponsored study. “I’m hopeful programs such as this can lead to a more equitable situation than described by the findings in these two studies,” he added.
Article type, country of origin play a role
Other research corroborates the findings in these two papers. Giovanni Adami, MD, and coauthors examined 366 rheumatology guidelines and recommendations and determined that only 32% featured a female first author. However, authorship did increase for women over time, achieving parity in 2017.
There are several points to consider when exploring gender disparity, Dr. Adami said in an interview. “Original articles, industry-sponsored articles, and recommendation articles explore different disparities,” he offered. Recommendations and industry-sponsored articles are usually authored by international experts such as division directors or full professors. Original articles, in comparison, aren’t as affected by the “opinion leader” effect, he added.
Country of origin is also a crucial aspect, Dr. Adami said. In his own search of guidelines and articles published by Japanese or Chinese researchers, he noticed that males made up the vast majority of authors. “The cultural aspects of the country where research develops is a vital thing to consider when analyzing gender disparity.”
Dr. Adami’s homeland of Italy is a case in point: most of the division chiefs and professors are male. “Here in Italy, there’s a common belief that a woman cannot pursue an academic career or aim for a leadership position,” he noted.
Italy’s public university system has seen some improvements in gender parity, he continued. “For example, in 2009 there were 61,000 new medical students in Italy, and the majority [57%] were female. Nonetheless, we still have more male professors of medicine and more male PhD candidates.”
Gender gap narrows for conference speakers
In another study, rheumatologists Jean Liew, MD, of the University of Washington, Seattle, and Kanika Monga, MD, of the University of Texas Health Science Center at Houston, found notable gender gaps in speakers at ACR conferences. Women represented under 50% of speakers at these meetings over a 2-year period. “Although the gender gap at recent ACR meetings was narrower as compared with other conferences, we must remain cognizant of its presence and continue to work towards equal representation,” the authors wrote in a correspondence letter in Annals of the Rheumatic Diseases.
Dr. Monga said she was excited to see so many studies on the topic of gender disparities in rheumatology. The Jorge et al. and Bagga et al. papers “delve deeper into quantifying the gender gap in rheumatology. These studies allow us to better identify where the discrepancies may be,” she said in an interview.
“I found it very interesting that women were less likely to be promoted in academic rank but as likely as men to hold leadership positions,” Dr. Monga said. She agreed with the authors that criteria for academic promotion should be reassessed to ensure that it values the diversity of scholarly work that rheumatologists pursue.
Men may still outnumber women speakers at ACR meetings, but the Liew and Monga study did report a 4.2% increase in female speaker representation from 2017 to 2018. “We were happy to note that that continued to be the case at The American College of Rheumatology’s Annual Meeting in 2019. I hope that this reflects a positive change in our specialty,” she said.
Dr. Dalbeth’s study received support from a University of Auckland Summer Studentship Award. She has received consulting fees, speaker fees, or grants from AstraZeneca, Horizon, Amgen, Janssen, and other companies outside of the submitted work. The other authors declared no competing interests.
Dr. Jorge receives funds from the Rheumatology Research Foundation. The senior author on her study receives funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
They’re less likely to hold a higher-level professorship position, feature as a senior author on a paper, or receive a federal grant. Two recent studies underscore progress for and barriers to career advancement.
One cross-sectional analysis of practicing U.S. rheumatologists found that fewer women are professors compared with men (12.6% vs. 36.8%) or associate professors (17.5% vs. 28%). A larger proportion of women serve as assistant professors (55.5% vs. 31.5%). From a leadership perspective, women are making progress. Their odds are similar to men as far as holding a fellowship or division director position in a rheumatology division.
For this study, published in Arthritis & Rheumatology on Aug. 16, April Jorge, MD, and her colleagues at Massachusetts General Hospital and Harvard Medical School, both in Boston, identified 6,125 rheumatologists from a database of all licensed physicians and used multivariate logistic regression to assess gender differences in academic advancement. They arrived at their results after accounting for variables such as age, research and academic appointments, publications, achievements, and years since residency graduation.
Women rheumatologists are younger, completing their residency more recently than their male colleagues. Their numbers in academic rheumatology have gradually increased over the last few decades, recently outpacing men. In 2015, the American College of Rheumatology reported that women made up 41% of the workforce and 66% of rheumatology fellows. Dr. Jorge and associates stressed the importance of fostering women in leadership positions and ensuring gender equity in academic career advancement.
Women also had fewer publications and grants from the National Institutes of Health. Several factors could account for this, such as time spent in the workforce or on parental leave, work-life balance, and mentorship. “However, gender differences in academic promotion remained after adjusting for each of these typical promotion criteria, indicating that other unidentified factors also contribute to the gap in promotion for women academic rheumatologists,” the investigators noted.
The authors weren’t able to assess how parental leave and work effort affected results or why pay differences existed between men and women. They also weren’t able to determine how many physicians left academic practice. “If greater numbers of women than men left the academic rheumatology workforce – for one of many reasons, including that they were not promoted – our findings could underestimate sex differences in academic rank,” they acknowledged.
Lower authorship rate examined
Fewer women in full or associate professor positions might explain why female authorship on research papers is underrepresented, according to another study published Aug. 18 in Arthritis & Rheumatology. Ekta Bagga and colleagues at the University of Auckland (New Zealand) examined 7,651 original research articles from high-impact rheumatology and general medical journals published during 2015-2019 and reported that women were much less likely to achieve first or senior author positions in reports of randomized, controlled trials. This was especially true for studies initiated and funded by industry, compared with other research designs.
More gender parity existed for first authorship than senior authorship – women first authors and senior authors appeared in 51.5% and 35.3% of the papers, respectively. For all geographical regions, the proportion of women senior authors fell below 40%. Representation was especially low in regions other than Europe and North America. These observations likely reflect gender disparities in the medical workforce, Nicola Dalbeth, MD, the study’s senior author, said in an interview.
“We know that, although women make up almost half the rheumatology workforce in many countries around the world, we are less likely to be in positions of senior academic leadership,” added Dr. Dalbeth, a rheumatologist and professor at the University of Auckland’s Bone and Joint Research Group. Institutions and industry should take steps to ensure that women rheumatologists get equal representation, particularly in clinical trial development, she added.
The study had its limitations, one of which was that the researchers didn’t analyze individual author names. This means that one person may have authored multiple articles. “Given the relatively low number of women in academic rheumatology leadership positions, our method of analysis may have overrepresented the number of women authors of rheumatology publications, particularly in senior positions,” stated Dr. Dalbeth and colleagues.
Implicit bias in academia
The articles by Jorge et al. and Bagga et al. suggest that implicit bias is as prevalent in medicine as it is in general society, Jason Kolfenbach, MD, said in an interview. Dr. Kolfenbach is an associate professor of medicine and rheumatology and director of the rheumatology fellowship program at University of Colorado at Denver, Aurora.
“The study by Jorge et al. is eye opening because it demonstrates that academic promotion is lower among women even after adjustment for some of these measures of academic productivity,” Dr. Kolfenbach said. It’s likely that bias plays some role “since there is a human element behind promotions committees, as well as committees selecting faculty for the creation of guidelines and speaker panels at national conferences.”
The study by Bagga et al. “matches my personal perception of industry-sponsored studies and pharmaceutical-sponsored speakers bureaus, namely that they are overrepresented by male faculty,” Dr. Kolfenbach continued.
Prior to COVID-19, the department of medicine at the University of Colorado had begun participating in a formal program called the Bias Reduction in Internal Medicine Initiative, a National Institutes of Health–sponsored study. “I’m hopeful programs such as this can lead to a more equitable situation than described by the findings in these two studies,” he added.
Article type, country of origin play a role
Other research corroborates the findings in these two papers. Giovanni Adami, MD, and coauthors examined 366 rheumatology guidelines and recommendations and determined that only 32% featured a female first author. However, authorship did increase for women over time, achieving parity in 2017.
There are several points to consider when exploring gender disparity, Dr. Adami said in an interview. “Original articles, industry-sponsored articles, and recommendation articles explore different disparities,” he offered. Recommendations and industry-sponsored articles are usually authored by international experts such as division directors or full professors. Original articles, in comparison, aren’t as affected by the “opinion leader” effect, he added.
Country of origin is also a crucial aspect, Dr. Adami said. In his own search of guidelines and articles published by Japanese or Chinese researchers, he noticed that males made up the vast majority of authors. “The cultural aspects of the country where research develops is a vital thing to consider when analyzing gender disparity.”
Dr. Adami’s homeland of Italy is a case in point: most of the division chiefs and professors are male. “Here in Italy, there’s a common belief that a woman cannot pursue an academic career or aim for a leadership position,” he noted.
Italy’s public university system has seen some improvements in gender parity, he continued. “For example, in 2009 there were 61,000 new medical students in Italy, and the majority [57%] were female. Nonetheless, we still have more male professors of medicine and more male PhD candidates.”
Gender gap narrows for conference speakers
In another study, rheumatologists Jean Liew, MD, of the University of Washington, Seattle, and Kanika Monga, MD, of the University of Texas Health Science Center at Houston, found notable gender gaps in speakers at ACR conferences. Women represented under 50% of speakers at these meetings over a 2-year period. “Although the gender gap at recent ACR meetings was narrower as compared with other conferences, we must remain cognizant of its presence and continue to work towards equal representation,” the authors wrote in a correspondence letter in Annals of the Rheumatic Diseases.
Dr. Monga said she was excited to see so many studies on the topic of gender disparities in rheumatology. The Jorge et al. and Bagga et al. papers “delve deeper into quantifying the gender gap in rheumatology. These studies allow us to better identify where the discrepancies may be,” she said in an interview.
“I found it very interesting that women were less likely to be promoted in academic rank but as likely as men to hold leadership positions,” Dr. Monga said. She agreed with the authors that criteria for academic promotion should be reassessed to ensure that it values the diversity of scholarly work that rheumatologists pursue.
Men may still outnumber women speakers at ACR meetings, but the Liew and Monga study did report a 4.2% increase in female speaker representation from 2017 to 2018. “We were happy to note that that continued to be the case at The American College of Rheumatology’s Annual Meeting in 2019. I hope that this reflects a positive change in our specialty,” she said.
Dr. Dalbeth’s study received support from a University of Auckland Summer Studentship Award. She has received consulting fees, speaker fees, or grants from AstraZeneca, Horizon, Amgen, Janssen, and other companies outside of the submitted work. The other authors declared no competing interests.
Dr. Jorge receives funds from the Rheumatology Research Foundation. The senior author on her study receives funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
New AGA guidance on virus testing patients before endoscopy
A new evidence-based review published in Gastroenterology helps you answer the question: Should my endoscopy center test asymptomatic patients for SARS-CoV-2 prior to endoscopy?
Key guidance for GIs
1. Endoscopy centers in areas with an intermediate prevalence of SARS-CoV-2 infection should consider testing patients for the virus before endoscopy. Several important factors contribute to this decision including testing feasibility, personal protective equipment (PPE) availability, and risk aversion threshold of endoscopists and staff.
2. Endoscopy centers in both low- and high-prevalence areas may not benefit from a pre-testing strategy.
- Rationale for low-prevalence areas: Diagnostic tests have a high proportion of false positives with significant downstream consequences, such as patient burden (quarantining and out of work for 14 days), unnecessarily delayed cases, and over-utilization of testing which may already be limited in availability. Therefore, PPE availability may drive decision-making for case triage instead. If PPE is not limited, then the majority of endoscopists and staff may reasonably select to use N95/N99 respirators or PAPRs.
- Rationale for high-prevalence areas: Highest available PPE (such as N95/N99 respirators or PAPRs) would be used universally, as available. Additionally, testing is often limited because of a high demand for a potential surge of cases.
AGA created an online tool to help endoscopy centers make decisions about their pre-endoscopy testing strategy. This tool combines local prevalence with diagnostic test performance data to calculate the proportion of true versus false positives and negatives to help endoscopy centers understand the downstream consequences of implementing a pre-procedure testing strategy.
To access the Rapid Review and online tool, visit www.gastro.org/COVID.
A new evidence-based review published in Gastroenterology helps you answer the question: Should my endoscopy center test asymptomatic patients for SARS-CoV-2 prior to endoscopy?
Key guidance for GIs
1. Endoscopy centers in areas with an intermediate prevalence of SARS-CoV-2 infection should consider testing patients for the virus before endoscopy. Several important factors contribute to this decision including testing feasibility, personal protective equipment (PPE) availability, and risk aversion threshold of endoscopists and staff.
2. Endoscopy centers in both low- and high-prevalence areas may not benefit from a pre-testing strategy.
- Rationale for low-prevalence areas: Diagnostic tests have a high proportion of false positives with significant downstream consequences, such as patient burden (quarantining and out of work for 14 days), unnecessarily delayed cases, and over-utilization of testing which may already be limited in availability. Therefore, PPE availability may drive decision-making for case triage instead. If PPE is not limited, then the majority of endoscopists and staff may reasonably select to use N95/N99 respirators or PAPRs.
- Rationale for high-prevalence areas: Highest available PPE (such as N95/N99 respirators or PAPRs) would be used universally, as available. Additionally, testing is often limited because of a high demand for a potential surge of cases.
AGA created an online tool to help endoscopy centers make decisions about their pre-endoscopy testing strategy. This tool combines local prevalence with diagnostic test performance data to calculate the proportion of true versus false positives and negatives to help endoscopy centers understand the downstream consequences of implementing a pre-procedure testing strategy.
To access the Rapid Review and online tool, visit www.gastro.org/COVID.
A new evidence-based review published in Gastroenterology helps you answer the question: Should my endoscopy center test asymptomatic patients for SARS-CoV-2 prior to endoscopy?
Key guidance for GIs
1. Endoscopy centers in areas with an intermediate prevalence of SARS-CoV-2 infection should consider testing patients for the virus before endoscopy. Several important factors contribute to this decision including testing feasibility, personal protective equipment (PPE) availability, and risk aversion threshold of endoscopists and staff.
2. Endoscopy centers in both low- and high-prevalence areas may not benefit from a pre-testing strategy.
- Rationale for low-prevalence areas: Diagnostic tests have a high proportion of false positives with significant downstream consequences, such as patient burden (quarantining and out of work for 14 days), unnecessarily delayed cases, and over-utilization of testing which may already be limited in availability. Therefore, PPE availability may drive decision-making for case triage instead. If PPE is not limited, then the majority of endoscopists and staff may reasonably select to use N95/N99 respirators or PAPRs.
- Rationale for high-prevalence areas: Highest available PPE (such as N95/N99 respirators or PAPRs) would be used universally, as available. Additionally, testing is often limited because of a high demand for a potential surge of cases.
AGA created an online tool to help endoscopy centers make decisions about their pre-endoscopy testing strategy. This tool combines local prevalence with diagnostic test performance data to calculate the proportion of true versus false positives and negatives to help endoscopy centers understand the downstream consequences of implementing a pre-procedure testing strategy.
To access the Rapid Review and online tool, visit www.gastro.org/COVID.
How we’re combatting racism, health disparities
The AGA Equity Project advisory board has released a new commentary in Gastroenterology: “From Intention to Action: Operationalizing AGA Diversity Policy to Combat Racism and Health Disparities in Gastroenterology.”
The commentary provides a transparent self-examination of AGA’s recent racial and ethnic demographic data of its members, volunteer leaders, and staff compared with U.S. population data. It also assesses AGA’s previous initiatives focused on diversity, equity, and inclusion. It then looks ahead by detailing AGA’s plans to further operationalize the goals enumerated in the AGA Diversity Policy.
For more information, read the full commentary at www.gastro.org/diversitycommentary.
The AGA Equity Project advisory board has released a new commentary in Gastroenterology: “From Intention to Action: Operationalizing AGA Diversity Policy to Combat Racism and Health Disparities in Gastroenterology.”
The commentary provides a transparent self-examination of AGA’s recent racial and ethnic demographic data of its members, volunteer leaders, and staff compared with U.S. population data. It also assesses AGA’s previous initiatives focused on diversity, equity, and inclusion. It then looks ahead by detailing AGA’s plans to further operationalize the goals enumerated in the AGA Diversity Policy.
For more information, read the full commentary at www.gastro.org/diversitycommentary.
The AGA Equity Project advisory board has released a new commentary in Gastroenterology: “From Intention to Action: Operationalizing AGA Diversity Policy to Combat Racism and Health Disparities in Gastroenterology.”
The commentary provides a transparent self-examination of AGA’s recent racial and ethnic demographic data of its members, volunteer leaders, and staff compared with U.S. population data. It also assesses AGA’s previous initiatives focused on diversity, equity, and inclusion. It then looks ahead by detailing AGA’s plans to further operationalize the goals enumerated in the AGA Diversity Policy.
For more information, read the full commentary at www.gastro.org/diversitycommentary.
AGA launches new virtual series on COVID-19 findings
Join us for our new GI Forging Forward virtual symposia series, a practical educational training program covering timely topics for GIs through the lens of COVID-19. Experts in the field will present the latest COVID-19 findings, share proven strategies to communicate and manage disaster and crisis situations, and educate participants on evidence-based recommendations to meet today’s evolving needs. Upcoming topics will cover keeping you, your staff, and patients safe, new approaches and training in research, leading in times of crisis, and rapid-response guideline development.
Registration for this month’s virtual webinars are now open:
Demystifying publishing in AGA journals: Perspectives from our authors and editors: Sept. 3, 2020, 5:30 p.m. EDT
Flexing your communications skills during a time of crisis: Sept. 17, 2020, 5:30 p.m. EDT
For more information, visit www.gastro.org/GIForgingForward.
Join us for our new GI Forging Forward virtual symposia series, a practical educational training program covering timely topics for GIs through the lens of COVID-19. Experts in the field will present the latest COVID-19 findings, share proven strategies to communicate and manage disaster and crisis situations, and educate participants on evidence-based recommendations to meet today’s evolving needs. Upcoming topics will cover keeping you, your staff, and patients safe, new approaches and training in research, leading in times of crisis, and rapid-response guideline development.
Registration for this month’s virtual webinars are now open:
Demystifying publishing in AGA journals: Perspectives from our authors and editors: Sept. 3, 2020, 5:30 p.m. EDT
Flexing your communications skills during a time of crisis: Sept. 17, 2020, 5:30 p.m. EDT
For more information, visit www.gastro.org/GIForgingForward.
Join us for our new GI Forging Forward virtual symposia series, a practical educational training program covering timely topics for GIs through the lens of COVID-19. Experts in the field will present the latest COVID-19 findings, share proven strategies to communicate and manage disaster and crisis situations, and educate participants on evidence-based recommendations to meet today’s evolving needs. Upcoming topics will cover keeping you, your staff, and patients safe, new approaches and training in research, leading in times of crisis, and rapid-response guideline development.
Registration for this month’s virtual webinars are now open:
Demystifying publishing in AGA journals: Perspectives from our authors and editors: Sept. 3, 2020, 5:30 p.m. EDT
Flexing your communications skills during a time of crisis: Sept. 17, 2020, 5:30 p.m. EDT
For more information, visit www.gastro.org/GIForgingForward.
Field Cancerization With Multiple Keratoacanthomas Successfully Treated With Topical and Intralesional 5-Fluorouracil
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
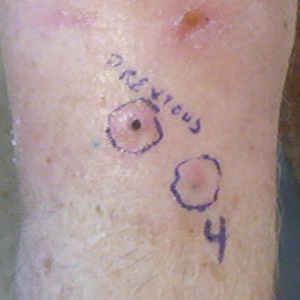
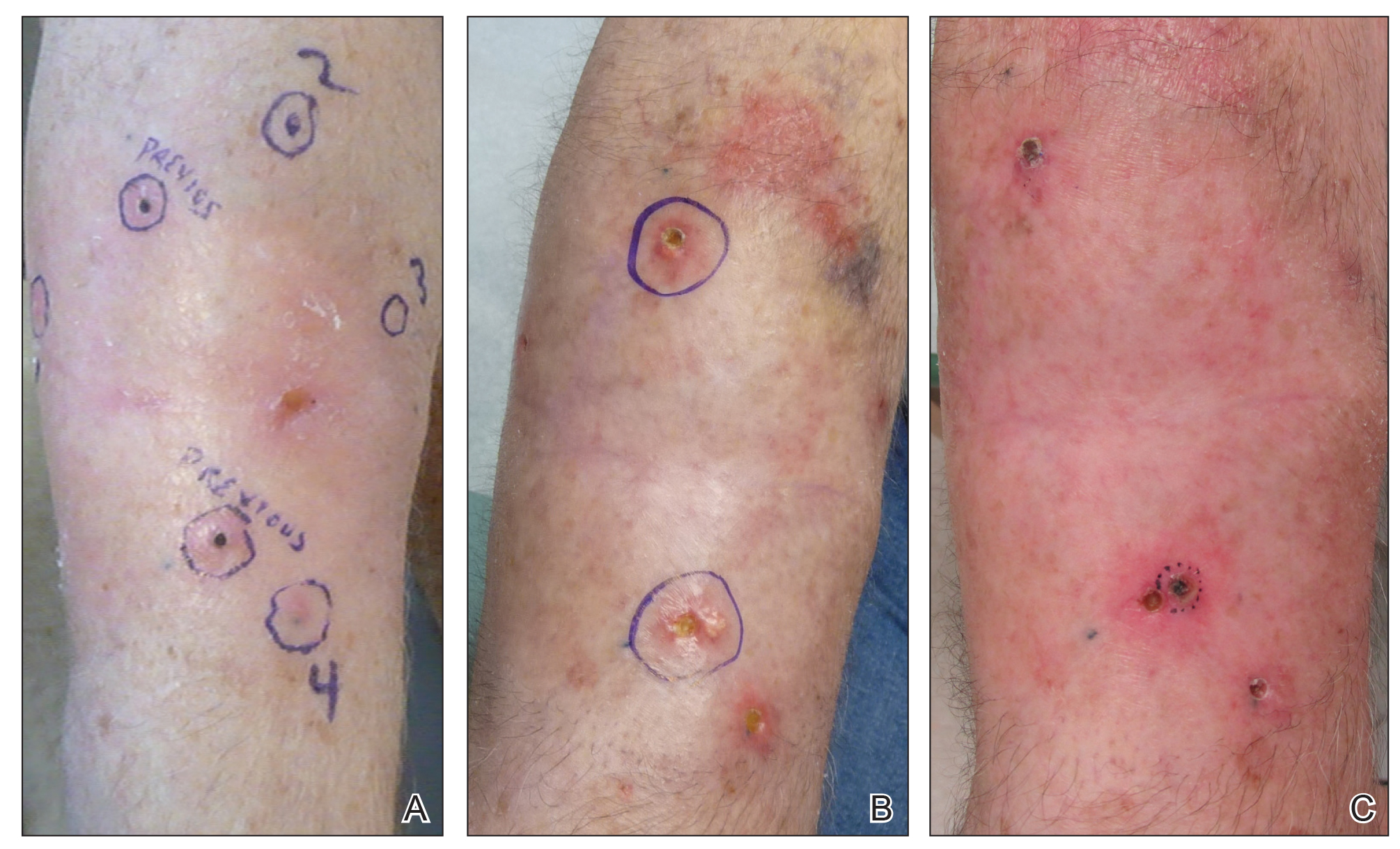
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
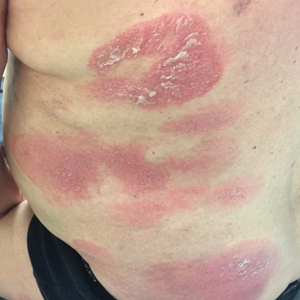
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.
Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.

Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.

Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
Product update: Cervical dilator, hair rejuvenation cap, pelvic floor SUI treatment
NEW CERVICAL DILATOR
Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
NEW CERVICAL DILATOR

Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
NEW CERVICAL DILATOR

Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
Long-standing Dermatitis Treated With Dupilumab With Subsequent Progression to Cutaneous T-cell Lymphoma
Dupilumab is a novel medication that is approved by the US Food and Drug Administration to treat moderate to severe atopic dermatitis (AD) in patients 6 years and older. Dupilumab is an injectable fully human monoclonal antibody. It provides a giant leap toward a better quality of life for patients with AD. Dupilumab works by binding to the shared α subunit of the IL-4 receptor (IL-4R), thus inhibiting IL-4 and IL-13 from using that signaling pathway. The documented side-effect profile includes injection-site reaction, keratitis, nasopharyngitis, and headache.1
We initiated off-label treatment with dupilumab in 3 adult patients who had a history of long-standing adult-onset dermatitis confirmed by histopathology. The 3 patients received a loading dose of 600 mg subcutaneously, followed by 300 mg every other week. Following treatment, the patients had expansion of their disease, with features consistent with cutaneous T-cell lymphoma (CTCL) on subsequent biopsies. These 3 cases demonstrate the well-known adage that the diagnosis of CTCL often requires multiple biopsies performed over time. Although dupilumab has proved efficacious and safe for treating AD, dermatologists should be cautious before starting this medication in an adult who has new-onset dermatitis and no history of atopy.
Case Reports
Patient 1
A 61-year-old man presented to dermatology after being lost to follow-up for several years and was started on dupilumab for long-standing nonspecific eczematous dermatitis based on histopathology. He had a pruritic rash of 10 years’ duration that had been biopsied multiple times and was found to be consistent with dermatitis and lichen simplex chronicus (Figure 1). He had been treated with triamcinolone ointment 0.1% and narrowband UVB as often as 3 times weekly over many years. The patient also had a history of idiopathic CD4 lymphopenia with consistently negative tests for human immunodeficiency virus.
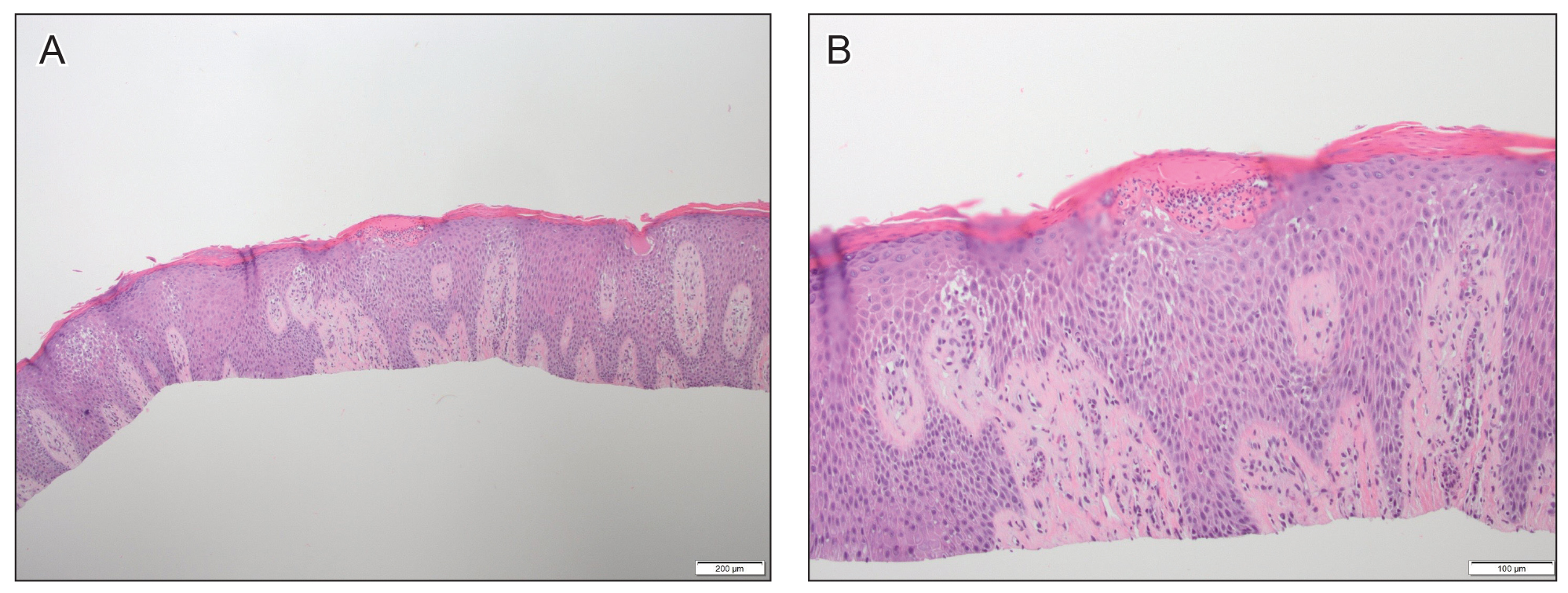
At approximately the same time as dupilumab was initiated, he was started on 60 mg daily of prednisone by his pulmonologist because of a history of restrictive lung disease of unknown cause. While taking prednisone, he experienced notable improvement in his skin condition; however, as he was slowly tapered off prednisone, he noted remarkable worsening of the dermatitis. Dupilumab was discontinued. Two more biopsies were performed; findings on both were consistent with mycosis fungoides (MF)(Figure 2).
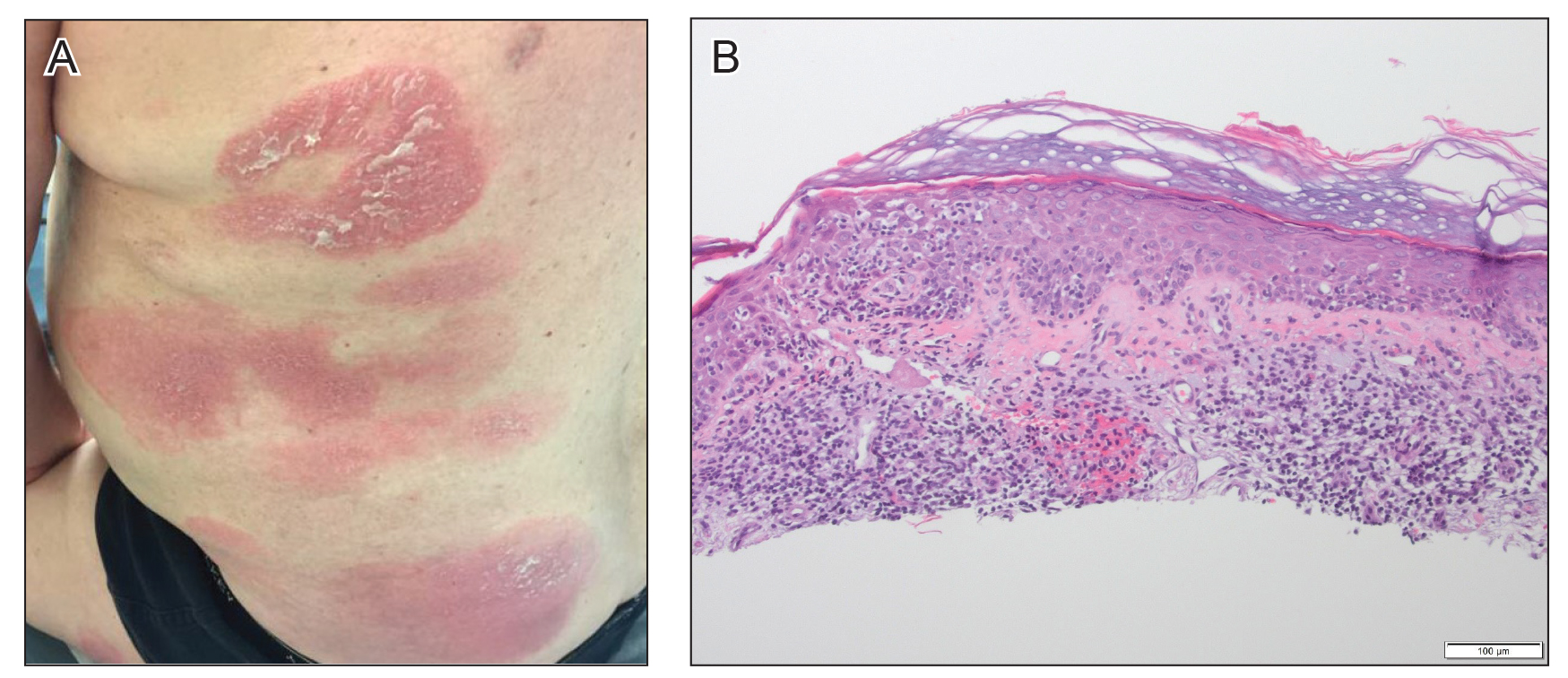
Patient 2
A 52-year-old man presented with indurated, red, scaly plaques on the legs and arms. Initial biopsy was consistent with psoriasiform dermatitis that was thought to be due to a primarily eczematous process. Because of the clinical suspicion of psoriasis, the patient was at first treated with topical betamethasone and eventually was transitioned to multiple injectable biologics without improvement. There was no response to multiple psoriasis treatments, and the original pathology report was re-reviewed. The report noted a substantial eczematous component; therefore, a decision was made to transition him to dupilumab. He also was at first provided with a prednisone taper due to the severity of the cutaneous disease.
Initially, the patient noted 15% to 20% improvement; however, after 6 injections, dupilumab appeared to lose efficacy. Due to a lack of response to multiple biologic medications as well as dupilumab, another biopsy was performed. Findings were consistent with MF.
Patient 3
A 60-year-old woman with diffuse, pruritic, and erythematous dermatitis of 3 years’ duration was referred from an outside dermatology group. Prior biopsies were consistent with eczematous dermatitis. However, because 1 isolated plaque demonstrated findings consistent with psoriasis, she was started on guselkumab, which was discontinued after 12 weeks of therapy for lack of efficacy. The patient also had been treated with a short course of narrowband UVB and topical corticosteroids without benefit.
Upon initial evaluation in our clinic, there was concern for Sézary syndrome; however, peripheral blood studies were normal, and there was no monoclonal spike or irregularity in the patient’s Sézary flow cytometry panel. A biopsy demonstrated lichenoid dermatitis, possibly consistent with drug eruption. All supplements and likely medication culprits were discontinued without improvement.
Prior to follow-up in our clinic, the patient was again evaluated by an outside dermatologist and started on dupilumab. After 3 doses, she discontinued the medication because there was no improvement in the cutaneous symptoms. Findings on repeat biopsy following dupilumab treatment were consistent with MF.
Comment
Mycosis fungoides is a rare chronic T-cell lymphoma that can smolder for decades as nonspecific dermatitis before declaring itself fully on skin biopsy.2 In many cases, MF masquerades as eczema, psoriasis, contact dermatitis, or other dermatitides, and it often responds to the same medications, making diagnosis even more challenging. Treatment options include topical steroids, narrowband UVB, topical nitrogen mustard, topical carmustine, and bexarotene gel for early-stage disease.3 Although it cannot be determined which patients will progress, some do, and therapies must then be upgraded.
We reported 3 patients with adult-onset dermatitis and multiple biopsies demonstrating nondiagnostic findings, which, in retrospect, likely represented early smoldering CTCL. Each of these patients was treated with dupilumab because multiple biopsies demonstrated findings consistent with nondiagnostic dermatitis, along with a lack of response to standard therapies. In all 3 cases, however, the patients had no history of eczema or atopy. After starting dupilumab, each patient had an acute exacerbation of dermatitis; immediately thereafter, biopsies were consistent with CTCL.
These patients most likely had smoldering CTCL that expressed itself fully after dupilumab was started. Biologic medications and their effects on the immune system have been shown to have multiple unanticipated effects on the skin.4-6 We are not insinuating that dupilumab was the cause of our patients having developed CTCL, but we do propose that the underlying interplay of dupilumab with the immune system might have accelerated progression of underlying CTCL, resulting in the lymphoma presenting itself clinically and histopathologically. We also must mention that all 3 cases could represent a “true, true, and unrelated” phenomenon.
A proposed mechanism for how dupilumab might hasten progression of CTCL is based on a functional increase of IL-13 available for binding at the IL-13 receptor (IL-13R) α2 site following blockade of the IL-13Rα1 site by dupilumab (Figure 3). The pathway that is blocked by dupilumab provides improvement in AD by blocking the α subunit of the IL-4R, making it a receptor antagonist for both IL-4 and IL-13. The IL-4R forms a heterodimer with both γ c and separately with IL-13Rα1. As a result, IL-4 and IL-13 cannot bind to their respective targets; thus, downstream signaling that is required for AD is halted.7 IL-13, in addition to IL-4R, also binds to an IL-13Rα2. IL-13 and both of its receptors are upregulated in CTCL, particularly IL-13Rα2.8
One of the principal ways that CTCL survives is through autocrine signaling, inducing more IL-13 and more IL-13Rα2, which is not seen in normal skin.8 Autocrine signaling plays a critical role in cancer activation and in providing self-sustaining growth signals to tumors.9 In addition, it has been documented that IL-13Rα2 has a higher affinity for IL-13 than the affinity of IL-13Rα1.10 As such, when the dupilumab receptor is blocked, our proposed mechanism of acceleration of CTCL is based on a functional increase in IL-13 available for binding at the IL-13Rα2 site, following indirect blockade of the α1 receptor with dupilumab, which effectively increases available IL-13 to be shunted down the tumorigenic pathway.
We recognize that this proposed mechanism is a theory; additionally, it should be noted that dupilumab is approved only for the treatment of AD and asthma. In our 3 cases, we used dupilumab off label in patients who did not have a clear case of AD or a childhood history of the disease.
When screening patients for the use of dupilumab, it is important to treat only those who have a classic history of moderate to severe AD, including itch, family history, and rash in the classic atopic distribution. We propose that these cases represent potential exacerbation of extant CTCL following exposure to dupilumab.
The manufacturer of dupilumab has reported 1 case of stage IV MF in a 57-year-old man 48 days after the first dose of dupilumab, leading to permanent discontinuation. The patient had ongoing disease at the time of the report, and the manufacturer stated that use of dupilumab was unrelated to disease.11 Studies are needed to explore any potential immunologic link between dupilumab and progression of CTCL.
- Raedler LA. Dupixent (dupilumab) first biologic drug approved for patients with moderate-to-severe atopic dermatitis. Am Health Drug Benefits. 2018;11:58-60.
- Skov AG, Gniadecki R. Delay in the histopathologic diagnosis of mycosis fungoides. Acta Derm Venereol. 2015;95:472-475.
- Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1031-1056.
- Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic [published online December 18, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.12.018.
- Tierney E, Kirthi S, Ramsay B, et al. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271-273.
- Orrell KA, Murphrey M, Kelm RC, et al. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors secukinumab and ixekizumab: postmarketing analysis from the RADAR (“Research on Adverse Drug events And Reports”) program. J Am Acad Dermatol. 2018;79:777-778.
- Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139-150.
- Geskin LJ, Viragova S, Stolz DB, et al. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015;125:2798-2805.
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780-2790.
- Andrews A-L, Holloway JW, Puddicombe SM, et al. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073-46078.
- Data on file. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
Dupilumab is a novel medication that is approved by the US Food and Drug Administration to treat moderate to severe atopic dermatitis (AD) in patients 6 years and older. Dupilumab is an injectable fully human monoclonal antibody. It provides a giant leap toward a better quality of life for patients with AD. Dupilumab works by binding to the shared α subunit of the IL-4 receptor (IL-4R), thus inhibiting IL-4 and IL-13 from using that signaling pathway. The documented side-effect profile includes injection-site reaction, keratitis, nasopharyngitis, and headache.1
We initiated off-label treatment with dupilumab in 3 adult patients who had a history of long-standing adult-onset dermatitis confirmed by histopathology. The 3 patients received a loading dose of 600 mg subcutaneously, followed by 300 mg every other week. Following treatment, the patients had expansion of their disease, with features consistent with cutaneous T-cell lymphoma (CTCL) on subsequent biopsies. These 3 cases demonstrate the well-known adage that the diagnosis of CTCL often requires multiple biopsies performed over time. Although dupilumab has proved efficacious and safe for treating AD, dermatologists should be cautious before starting this medication in an adult who has new-onset dermatitis and no history of atopy.
Case Reports
Patient 1
A 61-year-old man presented to dermatology after being lost to follow-up for several years and was started on dupilumab for long-standing nonspecific eczematous dermatitis based on histopathology. He had a pruritic rash of 10 years’ duration that had been biopsied multiple times and was found to be consistent with dermatitis and lichen simplex chronicus (Figure 1). He had been treated with triamcinolone ointment 0.1% and narrowband UVB as often as 3 times weekly over many years. The patient also had a history of idiopathic CD4 lymphopenia with consistently negative tests for human immunodeficiency virus.

At approximately the same time as dupilumab was initiated, he was started on 60 mg daily of prednisone by his pulmonologist because of a history of restrictive lung disease of unknown cause. While taking prednisone, he experienced notable improvement in his skin condition; however, as he was slowly tapered off prednisone, he noted remarkable worsening of the dermatitis. Dupilumab was discontinued. Two more biopsies were performed; findings on both were consistent with mycosis fungoides (MF)(Figure 2).

Patient 2
A 52-year-old man presented with indurated, red, scaly plaques on the legs and arms. Initial biopsy was consistent with psoriasiform dermatitis that was thought to be due to a primarily eczematous process. Because of the clinical suspicion of psoriasis, the patient was at first treated with topical betamethasone and eventually was transitioned to multiple injectable biologics without improvement. There was no response to multiple psoriasis treatments, and the original pathology report was re-reviewed. The report noted a substantial eczematous component; therefore, a decision was made to transition him to dupilumab. He also was at first provided with a prednisone taper due to the severity of the cutaneous disease.
Initially, the patient noted 15% to 20% improvement; however, after 6 injections, dupilumab appeared to lose efficacy. Due to a lack of response to multiple biologic medications as well as dupilumab, another biopsy was performed. Findings were consistent with MF.
Patient 3
A 60-year-old woman with diffuse, pruritic, and erythematous dermatitis of 3 years’ duration was referred from an outside dermatology group. Prior biopsies were consistent with eczematous dermatitis. However, because 1 isolated plaque demonstrated findings consistent with psoriasis, she was started on guselkumab, which was discontinued after 12 weeks of therapy for lack of efficacy. The patient also had been treated with a short course of narrowband UVB and topical corticosteroids without benefit.
Upon initial evaluation in our clinic, there was concern for Sézary syndrome; however, peripheral blood studies were normal, and there was no monoclonal spike or irregularity in the patient’s Sézary flow cytometry panel. A biopsy demonstrated lichenoid dermatitis, possibly consistent with drug eruption. All supplements and likely medication culprits were discontinued without improvement.
Prior to follow-up in our clinic, the patient was again evaluated by an outside dermatologist and started on dupilumab. After 3 doses, she discontinued the medication because there was no improvement in the cutaneous symptoms. Findings on repeat biopsy following dupilumab treatment were consistent with MF.
Comment
Mycosis fungoides is a rare chronic T-cell lymphoma that can smolder for decades as nonspecific dermatitis before declaring itself fully on skin biopsy.2 In many cases, MF masquerades as eczema, psoriasis, contact dermatitis, or other dermatitides, and it often responds to the same medications, making diagnosis even more challenging. Treatment options include topical steroids, narrowband UVB, topical nitrogen mustard, topical carmustine, and bexarotene gel for early-stage disease.3 Although it cannot be determined which patients will progress, some do, and therapies must then be upgraded.
We reported 3 patients with adult-onset dermatitis and multiple biopsies demonstrating nondiagnostic findings, which, in retrospect, likely represented early smoldering CTCL. Each of these patients was treated with dupilumab because multiple biopsies demonstrated findings consistent with nondiagnostic dermatitis, along with a lack of response to standard therapies. In all 3 cases, however, the patients had no history of eczema or atopy. After starting dupilumab, each patient had an acute exacerbation of dermatitis; immediately thereafter, biopsies were consistent with CTCL.
These patients most likely had smoldering CTCL that expressed itself fully after dupilumab was started. Biologic medications and their effects on the immune system have been shown to have multiple unanticipated effects on the skin.4-6 We are not insinuating that dupilumab was the cause of our patients having developed CTCL, but we do propose that the underlying interplay of dupilumab with the immune system might have accelerated progression of underlying CTCL, resulting in the lymphoma presenting itself clinically and histopathologically. We also must mention that all 3 cases could represent a “true, true, and unrelated” phenomenon.
A proposed mechanism for how dupilumab might hasten progression of CTCL is based on a functional increase of IL-13 available for binding at the IL-13 receptor (IL-13R) α2 site following blockade of the IL-13Rα1 site by dupilumab (Figure 3). The pathway that is blocked by dupilumab provides improvement in AD by blocking the α subunit of the IL-4R, making it a receptor antagonist for both IL-4 and IL-13. The IL-4R forms a heterodimer with both γ c and separately with IL-13Rα1. As a result, IL-4 and IL-13 cannot bind to their respective targets; thus, downstream signaling that is required for AD is halted.7 IL-13, in addition to IL-4R, also binds to an IL-13Rα2. IL-13 and both of its receptors are upregulated in CTCL, particularly IL-13Rα2.8

One of the principal ways that CTCL survives is through autocrine signaling, inducing more IL-13 and more IL-13Rα2, which is not seen in normal skin.8 Autocrine signaling plays a critical role in cancer activation and in providing self-sustaining growth signals to tumors.9 In addition, it has been documented that IL-13Rα2 has a higher affinity for IL-13 than the affinity of IL-13Rα1.10 As such, when the dupilumab receptor is blocked, our proposed mechanism of acceleration of CTCL is based on a functional increase in IL-13 available for binding at the IL-13Rα2 site, following indirect blockade of the α1 receptor with dupilumab, which effectively increases available IL-13 to be shunted down the tumorigenic pathway.
We recognize that this proposed mechanism is a theory; additionally, it should be noted that dupilumab is approved only for the treatment of AD and asthma. In our 3 cases, we used dupilumab off label in patients who did not have a clear case of AD or a childhood history of the disease.
When screening patients for the use of dupilumab, it is important to treat only those who have a classic history of moderate to severe AD, including itch, family history, and rash in the classic atopic distribution. We propose that these cases represent potential exacerbation of extant CTCL following exposure to dupilumab.
The manufacturer of dupilumab has reported 1 case of stage IV MF in a 57-year-old man 48 days after the first dose of dupilumab, leading to permanent discontinuation. The patient had ongoing disease at the time of the report, and the manufacturer stated that use of dupilumab was unrelated to disease.11 Studies are needed to explore any potential immunologic link between dupilumab and progression of CTCL.
Dupilumab is a novel medication that is approved by the US Food and Drug Administration to treat moderate to severe atopic dermatitis (AD) in patients 6 years and older. Dupilumab is an injectable fully human monoclonal antibody. It provides a giant leap toward a better quality of life for patients with AD. Dupilumab works by binding to the shared α subunit of the IL-4 receptor (IL-4R), thus inhibiting IL-4 and IL-13 from using that signaling pathway. The documented side-effect profile includes injection-site reaction, keratitis, nasopharyngitis, and headache.1
We initiated off-label treatment with dupilumab in 3 adult patients who had a history of long-standing adult-onset dermatitis confirmed by histopathology. The 3 patients received a loading dose of 600 mg subcutaneously, followed by 300 mg every other week. Following treatment, the patients had expansion of their disease, with features consistent with cutaneous T-cell lymphoma (CTCL) on subsequent biopsies. These 3 cases demonstrate the well-known adage that the diagnosis of CTCL often requires multiple biopsies performed over time. Although dupilumab has proved efficacious and safe for treating AD, dermatologists should be cautious before starting this medication in an adult who has new-onset dermatitis and no history of atopy.
Case Reports
Patient 1
A 61-year-old man presented to dermatology after being lost to follow-up for several years and was started on dupilumab for long-standing nonspecific eczematous dermatitis based on histopathology. He had a pruritic rash of 10 years’ duration that had been biopsied multiple times and was found to be consistent with dermatitis and lichen simplex chronicus (Figure 1). He had been treated with triamcinolone ointment 0.1% and narrowband UVB as often as 3 times weekly over many years. The patient also had a history of idiopathic CD4 lymphopenia with consistently negative tests for human immunodeficiency virus.

At approximately the same time as dupilumab was initiated, he was started on 60 mg daily of prednisone by his pulmonologist because of a history of restrictive lung disease of unknown cause. While taking prednisone, he experienced notable improvement in his skin condition; however, as he was slowly tapered off prednisone, he noted remarkable worsening of the dermatitis. Dupilumab was discontinued. Two more biopsies were performed; findings on both were consistent with mycosis fungoides (MF)(Figure 2).

Patient 2
A 52-year-old man presented with indurated, red, scaly plaques on the legs and arms. Initial biopsy was consistent with psoriasiform dermatitis that was thought to be due to a primarily eczematous process. Because of the clinical suspicion of psoriasis, the patient was at first treated with topical betamethasone and eventually was transitioned to multiple injectable biologics without improvement. There was no response to multiple psoriasis treatments, and the original pathology report was re-reviewed. The report noted a substantial eczematous component; therefore, a decision was made to transition him to dupilumab. He also was at first provided with a prednisone taper due to the severity of the cutaneous disease.
Initially, the patient noted 15% to 20% improvement; however, after 6 injections, dupilumab appeared to lose efficacy. Due to a lack of response to multiple biologic medications as well as dupilumab, another biopsy was performed. Findings were consistent with MF.
Patient 3
A 60-year-old woman with diffuse, pruritic, and erythematous dermatitis of 3 years’ duration was referred from an outside dermatology group. Prior biopsies were consistent with eczematous dermatitis. However, because 1 isolated plaque demonstrated findings consistent with psoriasis, she was started on guselkumab, which was discontinued after 12 weeks of therapy for lack of efficacy. The patient also had been treated with a short course of narrowband UVB and topical corticosteroids without benefit.
Upon initial evaluation in our clinic, there was concern for Sézary syndrome; however, peripheral blood studies were normal, and there was no monoclonal spike or irregularity in the patient’s Sézary flow cytometry panel. A biopsy demonstrated lichenoid dermatitis, possibly consistent with drug eruption. All supplements and likely medication culprits were discontinued without improvement.
Prior to follow-up in our clinic, the patient was again evaluated by an outside dermatologist and started on dupilumab. After 3 doses, she discontinued the medication because there was no improvement in the cutaneous symptoms. Findings on repeat biopsy following dupilumab treatment were consistent with MF.
Comment
Mycosis fungoides is a rare chronic T-cell lymphoma that can smolder for decades as nonspecific dermatitis before declaring itself fully on skin biopsy.2 In many cases, MF masquerades as eczema, psoriasis, contact dermatitis, or other dermatitides, and it often responds to the same medications, making diagnosis even more challenging. Treatment options include topical steroids, narrowband UVB, topical nitrogen mustard, topical carmustine, and bexarotene gel for early-stage disease.3 Although it cannot be determined which patients will progress, some do, and therapies must then be upgraded.
We reported 3 patients with adult-onset dermatitis and multiple biopsies demonstrating nondiagnostic findings, which, in retrospect, likely represented early smoldering CTCL. Each of these patients was treated with dupilumab because multiple biopsies demonstrated findings consistent with nondiagnostic dermatitis, along with a lack of response to standard therapies. In all 3 cases, however, the patients had no history of eczema or atopy. After starting dupilumab, each patient had an acute exacerbation of dermatitis; immediately thereafter, biopsies were consistent with CTCL.
These patients most likely had smoldering CTCL that expressed itself fully after dupilumab was started. Biologic medications and their effects on the immune system have been shown to have multiple unanticipated effects on the skin.4-6 We are not insinuating that dupilumab was the cause of our patients having developed CTCL, but we do propose that the underlying interplay of dupilumab with the immune system might have accelerated progression of underlying CTCL, resulting in the lymphoma presenting itself clinically and histopathologically. We also must mention that all 3 cases could represent a “true, true, and unrelated” phenomenon.
A proposed mechanism for how dupilumab might hasten progression of CTCL is based on a functional increase of IL-13 available for binding at the IL-13 receptor (IL-13R) α2 site following blockade of the IL-13Rα1 site by dupilumab (Figure 3). The pathway that is blocked by dupilumab provides improvement in AD by blocking the α subunit of the IL-4R, making it a receptor antagonist for both IL-4 and IL-13. The IL-4R forms a heterodimer with both γ c and separately with IL-13Rα1. As a result, IL-4 and IL-13 cannot bind to their respective targets; thus, downstream signaling that is required for AD is halted.7 IL-13, in addition to IL-4R, also binds to an IL-13Rα2. IL-13 and both of its receptors are upregulated in CTCL, particularly IL-13Rα2.8

One of the principal ways that CTCL survives is through autocrine signaling, inducing more IL-13 and more IL-13Rα2, which is not seen in normal skin.8 Autocrine signaling plays a critical role in cancer activation and in providing self-sustaining growth signals to tumors.9 In addition, it has been documented that IL-13Rα2 has a higher affinity for IL-13 than the affinity of IL-13Rα1.10 As such, when the dupilumab receptor is blocked, our proposed mechanism of acceleration of CTCL is based on a functional increase in IL-13 available for binding at the IL-13Rα2 site, following indirect blockade of the α1 receptor with dupilumab, which effectively increases available IL-13 to be shunted down the tumorigenic pathway.
We recognize that this proposed mechanism is a theory; additionally, it should be noted that dupilumab is approved only for the treatment of AD and asthma. In our 3 cases, we used dupilumab off label in patients who did not have a clear case of AD or a childhood history of the disease.
When screening patients for the use of dupilumab, it is important to treat only those who have a classic history of moderate to severe AD, including itch, family history, and rash in the classic atopic distribution. We propose that these cases represent potential exacerbation of extant CTCL following exposure to dupilumab.
The manufacturer of dupilumab has reported 1 case of stage IV MF in a 57-year-old man 48 days after the first dose of dupilumab, leading to permanent discontinuation. The patient had ongoing disease at the time of the report, and the manufacturer stated that use of dupilumab was unrelated to disease.11 Studies are needed to explore any potential immunologic link between dupilumab and progression of CTCL.
- Raedler LA. Dupixent (dupilumab) first biologic drug approved for patients with moderate-to-severe atopic dermatitis. Am Health Drug Benefits. 2018;11:58-60.
- Skov AG, Gniadecki R. Delay in the histopathologic diagnosis of mycosis fungoides. Acta Derm Venereol. 2015;95:472-475.
- Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1031-1056.
- Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic [published online December 18, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.12.018.
- Tierney E, Kirthi S, Ramsay B, et al. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271-273.
- Orrell KA, Murphrey M, Kelm RC, et al. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors secukinumab and ixekizumab: postmarketing analysis from the RADAR (“Research on Adverse Drug events And Reports”) program. J Am Acad Dermatol. 2018;79:777-778.
- Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139-150.
- Geskin LJ, Viragova S, Stolz DB, et al. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015;125:2798-2805.
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780-2790.
- Andrews A-L, Holloway JW, Puddicombe SM, et al. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073-46078.
- Data on file. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Raedler LA. Dupixent (dupilumab) first biologic drug approved for patients with moderate-to-severe atopic dermatitis. Am Health Drug Benefits. 2018;11:58-60.
- Skov AG, Gniadecki R. Delay in the histopathologic diagnosis of mycosis fungoides. Acta Derm Venereol. 2015;95:472-475.
- Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1031-1056.
- Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic [published online December 18, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.12.018.
- Tierney E, Kirthi S, Ramsay B, et al. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271-273.
- Orrell KA, Murphrey M, Kelm RC, et al. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors secukinumab and ixekizumab: postmarketing analysis from the RADAR (“Research on Adverse Drug events And Reports”) program. J Am Acad Dermatol. 2018;79:777-778.
- Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139-150.
- Geskin LJ, Viragova S, Stolz DB, et al. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015;125:2798-2805.
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780-2790.
- Andrews A-L, Holloway JW, Puddicombe SM, et al. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073-46078.
- Data on file. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
Practice Points
- Dupilumab is a safe and effective treatment for atopic dermatitis (AD) in both children and adults.
- Prior to starting treatment for presumed adult-onset AD, consider smoldering cutaneous T-cell lymphoma (CTCL).
- Dupilumab may interact with the cutaneous immune system, leading to an expedited presentation of CTCL in patients with chronic adult-onset AD.
Report touts PFO closure in divers; experts disagree
A new report recommends the surgical closure of patent foramen ovale (PFO) in high-risk divers, but physicians in the United States urged caution about widespread use of the procedure in this population.
“PFO closure is recommended in divers with a high-grade PFO, with a history of unprovoked decompression sickness [DCS], or at the diver’s preference. Besides protection from DCS, PFO closure also offers the diver life-long protection from PFO-associated stroke,” write the authors of an analysis of the DIVE-PFO Registry.
The investigators, led by Jakub Honêk, MD, PhD, of Motol
Over a mean of about 7 years, the divers who underwent catheter-based PFO closure had no unprovoked decompression sickness (DCS), while the condition occurred in 11% of those who hadn’t had the procedure, according to the report, a research letter published online in the Journal of the American College of Cardiology.Decompression sickness, also known as the bends, can occur as gas bubbles pass through the circulatory system as divers ascend. In divers with PFO, which affects about 25% of the population, the bubbles can bypass filtration in the lungs and cause strokes, said neurologist David Thaler, MD, PhD, of Tufts Medical Center, Boston.
PFO closure via surgery is one option for divers with PFO, but there’s debate over whether the procedure should be widespread. For the new research letter, researchers prospectively tracked 748 divers in the DIVE-PFO (Decompression Illness Prevention in Divers with a Patent Foramen Ovale) registry during 2006-2018. Twenty-two percent had high-grade PFO.
In divers with PFO of grade 3 or above, procedures were performed if patients had a history of DCS or if they couldn’t adapt to conservative diving recommendations. The researchers said this population included commercial divers.
The groups that did or didn’t undergo surgery were similar in age (40.0 and 37.3 years, respectively, P = 0.079), and sex (78.2% and 79.6% male, respectively, P = 0.893), but differed in number of new dives (30,684 vs. 25,328, respectively, P < 0.001,), ). They were tracked for a mean of 7.1 years and 6.5 years, respectively.
It’s not clear whether the divers who underwent the closure procedure had fewer DCSs because they were more cautious about dive safety than the other diver group. The research letter doesn’t mention whether strokes occurred in divers in the two groups.
The study authors write that the results are consistent with previous findings that “PFO closure eliminates arterial gas emboli, “PFO is a major risk factor for unprovoked DCS,” and “PFO closure is a safe procedure with a very low complication rate.”
In interviews, physicians who are familiar with diver safety questioned the value of the findings and said medical professionals shouldn’t change practice.
Not so fast, experts say
Dr. Thaler, the Tufts Medical Center neurologist, questioned why the report explored a link between PFO and DCS. Overall, he said, the findings are too incomplete to inform practice. Anesthesiologist Richard Moon, MD, of Duke University, Durham, N.C., also questioned the study’s examination of DCS. “Most DCS cases are uncorrelated with PFO. It is only serious cases, a minority, that could conceivably be related to PFO, and even then, many serious cases that occur in divers with PFO are unrelated to it.” He added that “numerous divers with mild DCS ... have been mistakenly evaluated for PFO. Such practice is unsubstantiated by data.”
Should more closures be performed in this population? “I would be hesitant to make the recommended closures in divers,” said cardiologist David C. Peritz, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H. “There are probably other ways that you can decrease your chances of getting decompression illness and make your dives more safe.”
Cardiologist Clifford J. Kavinsky, MD, PhD, of Rush University Medical Center, Chicago, said the closure procedure is “relatively safe” when performed by experienced surgeons. He noted that it is “only approved to prevent recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have experienced a cortical stroke presumed to be of embolic nature and for which no obvious cause can be found.”
As for high-risk divers, he said PFO closures “can be considered, but the data as yet are not strong enough to strongly recommend it.”
The Czech Republic’s Ministry of Health funded the study. The authors report no relevant disclosures. Dr. Thaler, Dr. Kavinsky, Dr. Moon and Dr. Peritz report no relevant disclosures.
SOURCE: Honěk J et al. J Am Coll Cardiol. 2020 Sep 1;76(9):1149-50.
A new report recommends the surgical closure of patent foramen ovale (PFO) in high-risk divers, but physicians in the United States urged caution about widespread use of the procedure in this population.
“PFO closure is recommended in divers with a high-grade PFO, with a history of unprovoked decompression sickness [DCS], or at the diver’s preference. Besides protection from DCS, PFO closure also offers the diver life-long protection from PFO-associated stroke,” write the authors of an analysis of the DIVE-PFO Registry.
The investigators, led by Jakub Honêk, MD, PhD, of Motol
Over a mean of about 7 years, the divers who underwent catheter-based PFO closure had no unprovoked decompression sickness (DCS), while the condition occurred in 11% of those who hadn’t had the procedure, according to the report, a research letter published online in the Journal of the American College of Cardiology.Decompression sickness, also known as the bends, can occur as gas bubbles pass through the circulatory system as divers ascend. In divers with PFO, which affects about 25% of the population, the bubbles can bypass filtration in the lungs and cause strokes, said neurologist David Thaler, MD, PhD, of Tufts Medical Center, Boston.
PFO closure via surgery is one option for divers with PFO, but there’s debate over whether the procedure should be widespread. For the new research letter, researchers prospectively tracked 748 divers in the DIVE-PFO (Decompression Illness Prevention in Divers with a Patent Foramen Ovale) registry during 2006-2018. Twenty-two percent had high-grade PFO.
In divers with PFO of grade 3 or above, procedures were performed if patients had a history of DCS or if they couldn’t adapt to conservative diving recommendations. The researchers said this population included commercial divers.
The groups that did or didn’t undergo surgery were similar in age (40.0 and 37.3 years, respectively, P = 0.079), and sex (78.2% and 79.6% male, respectively, P = 0.893), but differed in number of new dives (30,684 vs. 25,328, respectively, P < 0.001,), ). They were tracked for a mean of 7.1 years and 6.5 years, respectively.
It’s not clear whether the divers who underwent the closure procedure had fewer DCSs because they were more cautious about dive safety than the other diver group. The research letter doesn’t mention whether strokes occurred in divers in the two groups.
The study authors write that the results are consistent with previous findings that “PFO closure eliminates arterial gas emboli, “PFO is a major risk factor for unprovoked DCS,” and “PFO closure is a safe procedure with a very low complication rate.”
In interviews, physicians who are familiar with diver safety questioned the value of the findings and said medical professionals shouldn’t change practice.
Not so fast, experts say
Dr. Thaler, the Tufts Medical Center neurologist, questioned why the report explored a link between PFO and DCS. Overall, he said, the findings are too incomplete to inform practice. Anesthesiologist Richard Moon, MD, of Duke University, Durham, N.C., also questioned the study’s examination of DCS. “Most DCS cases are uncorrelated with PFO. It is only serious cases, a minority, that could conceivably be related to PFO, and even then, many serious cases that occur in divers with PFO are unrelated to it.” He added that “numerous divers with mild DCS ... have been mistakenly evaluated for PFO. Such practice is unsubstantiated by data.”
Should more closures be performed in this population? “I would be hesitant to make the recommended closures in divers,” said cardiologist David C. Peritz, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H. “There are probably other ways that you can decrease your chances of getting decompression illness and make your dives more safe.”
Cardiologist Clifford J. Kavinsky, MD, PhD, of Rush University Medical Center, Chicago, said the closure procedure is “relatively safe” when performed by experienced surgeons. He noted that it is “only approved to prevent recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have experienced a cortical stroke presumed to be of embolic nature and for which no obvious cause can be found.”
As for high-risk divers, he said PFO closures “can be considered, but the data as yet are not strong enough to strongly recommend it.”
The Czech Republic’s Ministry of Health funded the study. The authors report no relevant disclosures. Dr. Thaler, Dr. Kavinsky, Dr. Moon and Dr. Peritz report no relevant disclosures.
SOURCE: Honěk J et al. J Am Coll Cardiol. 2020 Sep 1;76(9):1149-50.
A new report recommends the surgical closure of patent foramen ovale (PFO) in high-risk divers, but physicians in the United States urged caution about widespread use of the procedure in this population.
“PFO closure is recommended in divers with a high-grade PFO, with a history of unprovoked decompression sickness [DCS], or at the diver’s preference. Besides protection from DCS, PFO closure also offers the diver life-long protection from PFO-associated stroke,” write the authors of an analysis of the DIVE-PFO Registry.
The investigators, led by Jakub Honêk, MD, PhD, of Motol
Over a mean of about 7 years, the divers who underwent catheter-based PFO closure had no unprovoked decompression sickness (DCS), while the condition occurred in 11% of those who hadn’t had the procedure, according to the report, a research letter published online in the Journal of the American College of Cardiology.Decompression sickness, also known as the bends, can occur as gas bubbles pass through the circulatory system as divers ascend. In divers with PFO, which affects about 25% of the population, the bubbles can bypass filtration in the lungs and cause strokes, said neurologist David Thaler, MD, PhD, of Tufts Medical Center, Boston.
PFO closure via surgery is one option for divers with PFO, but there’s debate over whether the procedure should be widespread. For the new research letter, researchers prospectively tracked 748 divers in the DIVE-PFO (Decompression Illness Prevention in Divers with a Patent Foramen Ovale) registry during 2006-2018. Twenty-two percent had high-grade PFO.
In divers with PFO of grade 3 or above, procedures were performed if patients had a history of DCS or if they couldn’t adapt to conservative diving recommendations. The researchers said this population included commercial divers.
The groups that did or didn’t undergo surgery were similar in age (40.0 and 37.3 years, respectively, P = 0.079), and sex (78.2% and 79.6% male, respectively, P = 0.893), but differed in number of new dives (30,684 vs. 25,328, respectively, P < 0.001,), ). They were tracked for a mean of 7.1 years and 6.5 years, respectively.
It’s not clear whether the divers who underwent the closure procedure had fewer DCSs because they were more cautious about dive safety than the other diver group. The research letter doesn’t mention whether strokes occurred in divers in the two groups.
The study authors write that the results are consistent with previous findings that “PFO closure eliminates arterial gas emboli, “PFO is a major risk factor for unprovoked DCS,” and “PFO closure is a safe procedure with a very low complication rate.”
In interviews, physicians who are familiar with diver safety questioned the value of the findings and said medical professionals shouldn’t change practice.
Not so fast, experts say
Dr. Thaler, the Tufts Medical Center neurologist, questioned why the report explored a link between PFO and DCS. Overall, he said, the findings are too incomplete to inform practice. Anesthesiologist Richard Moon, MD, of Duke University, Durham, N.C., also questioned the study’s examination of DCS. “Most DCS cases are uncorrelated with PFO. It is only serious cases, a minority, that could conceivably be related to PFO, and even then, many serious cases that occur in divers with PFO are unrelated to it.” He added that “numerous divers with mild DCS ... have been mistakenly evaluated for PFO. Such practice is unsubstantiated by data.”
Should more closures be performed in this population? “I would be hesitant to make the recommended closures in divers,” said cardiologist David C. Peritz, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H. “There are probably other ways that you can decrease your chances of getting decompression illness and make your dives more safe.”
Cardiologist Clifford J. Kavinsky, MD, PhD, of Rush University Medical Center, Chicago, said the closure procedure is “relatively safe” when performed by experienced surgeons. He noted that it is “only approved to prevent recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have experienced a cortical stroke presumed to be of embolic nature and for which no obvious cause can be found.”
As for high-risk divers, he said PFO closures “can be considered, but the data as yet are not strong enough to strongly recommend it.”
The Czech Republic’s Ministry of Health funded the study. The authors report no relevant disclosures. Dr. Thaler, Dr. Kavinsky, Dr. Moon and Dr. Peritz report no relevant disclosures.
SOURCE: Honěk J et al. J Am Coll Cardiol. 2020 Sep 1;76(9):1149-50.
FROM JACC