User login
Effect of Alirocumab Monotherapy vs Ezetimibe Plus Statin Therapy on LDL-C Lowering in Veterans With History of ASCVD
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).
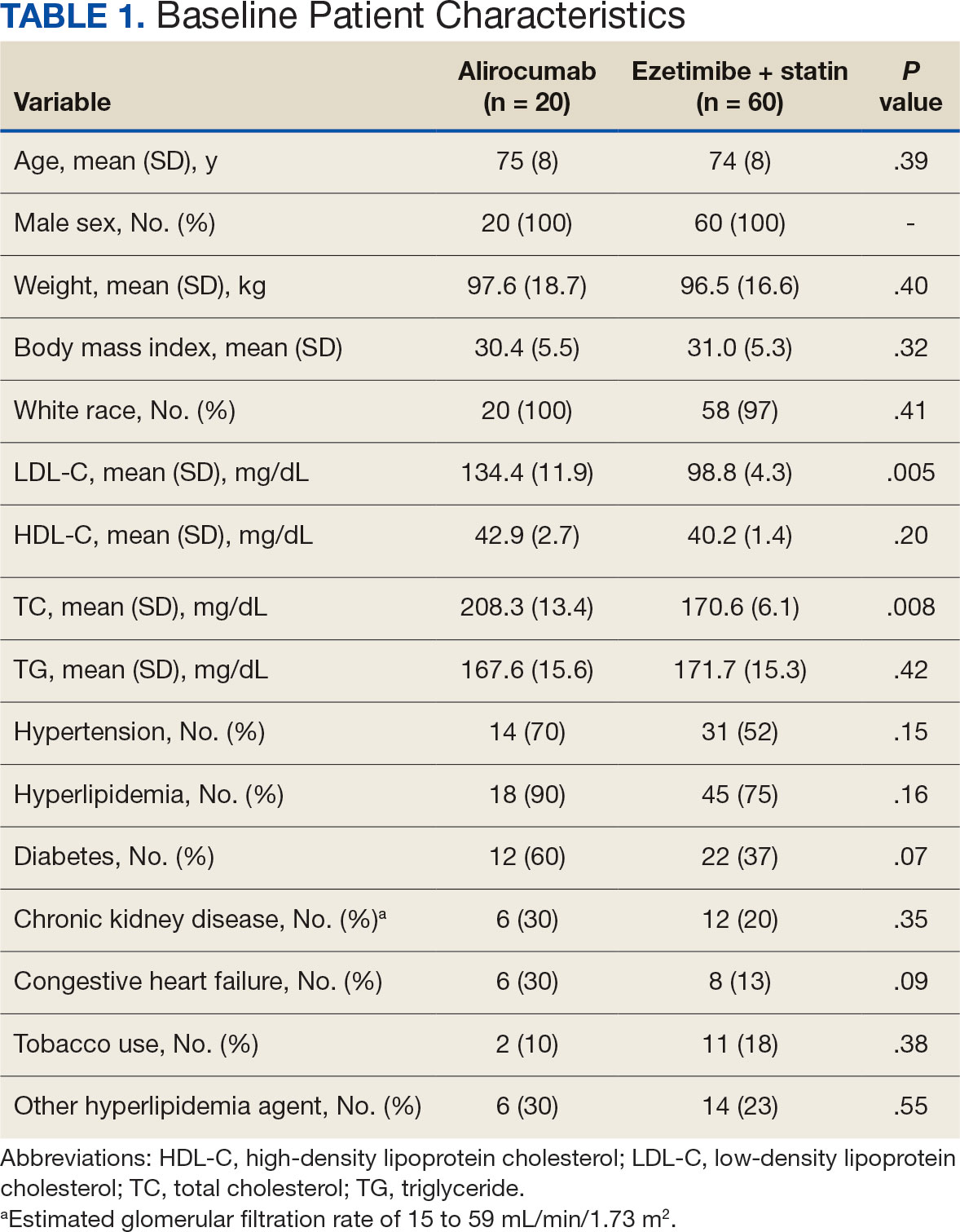
Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
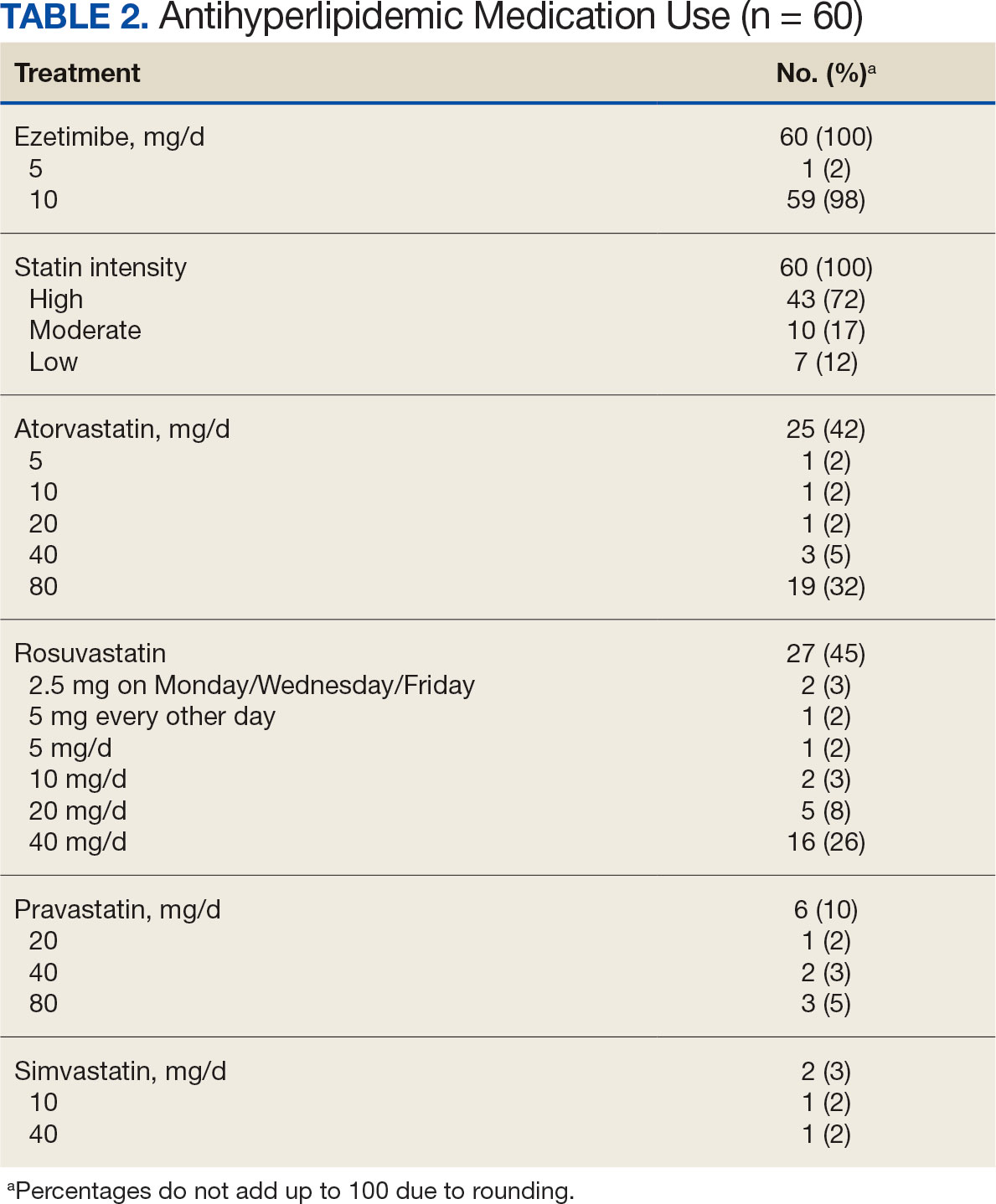
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).
Primary Endpoint
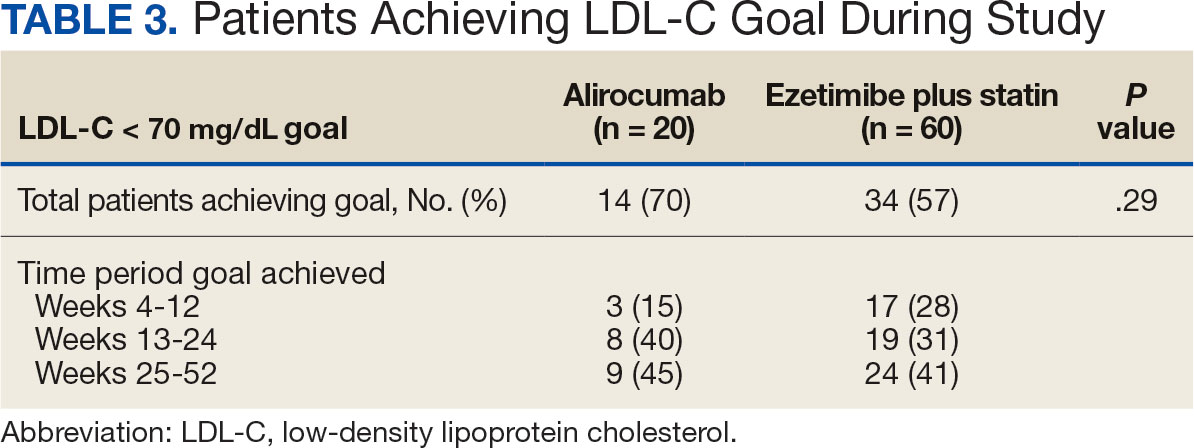
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).
Secondary Endpoints
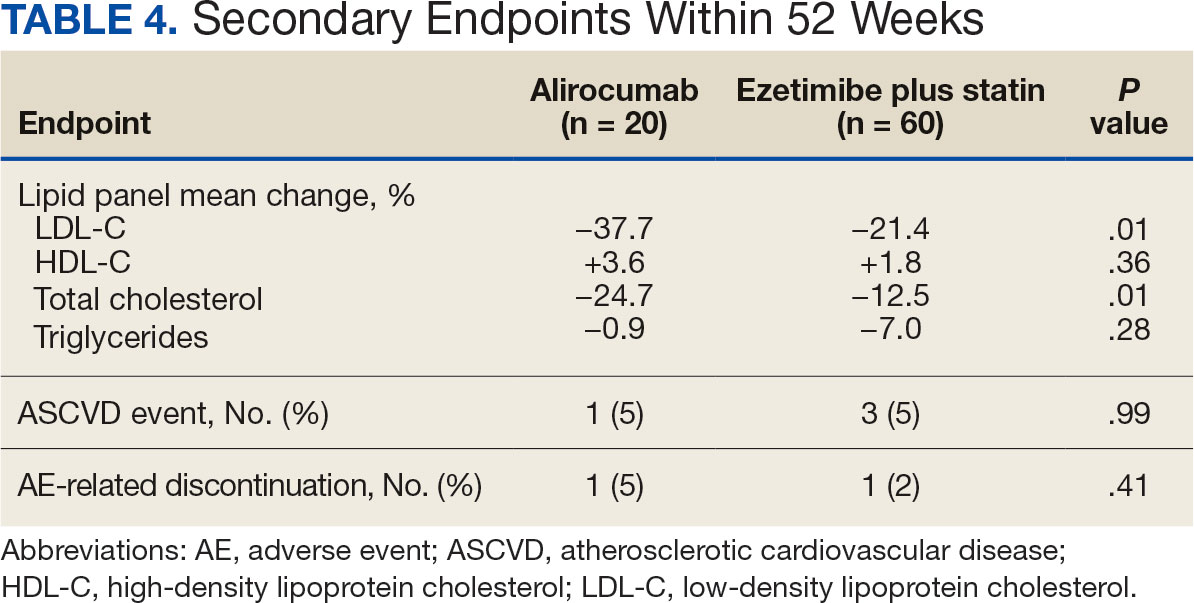
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.
DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).

Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).

Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).

Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.

DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).

Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).

Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).

Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.

DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Alcohol Use Disorder Therapy Remains Underutilized in Alcohol-Associated Liver Disease
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Endoscopic Sleeve Gastroplasty Yields Durable Weight Loss at 10 Years
PHILADELPHIA —
“The procedure is dependable and safe and should be considered among individuals who have not attained their desired results through lifestyle medications and those who are not eligible for or choose not to undergo bariatric procedures,” said Ali Lahooti, with the Department of Gastroenterology and Hepatology, Weill Cornell Medical College, New York City. He presented his research at the annual meeting of the American College of Gastroenterology (ACG).
Obesity is a growing global health challenge. Lifestyle modification as a standalone therapy has limited effectiveness achieving weight loss. Pharmacotherapies are more efficacious, but they’re also associated with higher costs of and risk for side effects, leading to lower rates of compliance, Lahooti explained.
Bariatric surgery remains the most effective therapy for management of obesity and improvement of comorbid conditions, yet < 1% of candidates undergo a surgical intervention either because of access, cost, or fear of the procedure.
“Endoscopic treatments for obesity, such as ESG, can potentially fill this gap by combining durable weight loss with lower risk and costs,” Lahooti said.
He and his colleagues assessed outcomes out to 10 years in 404 patients (mean age, 45 years; 76% women; mean body mass index, 37.3) who underwent ESG between 2013 and 2024 at a single large tertiary hospital.
Out of the 404 patients, 397, 335, 249, and 110 patients were eligible for 1-, 3-, 5-, and 10-year follow-up, with complete follow-up rates of 85%, 66%, 79%, and 62%, respectively.
The primary outcome was weight loss at 10 years after ESG reported at percent total body weight loss (%TBWL).
At 10 years, mean %TBWL (the primary outcome) was 10.5% — with 53% of patients maintaining at least 5% TBWL and 42% maintaining at least 10% weight loss, Lahooti reported.
ESG had a favorable safety profile; 20% of patients experienced mild abdominal pain, constipation, heartburn, and nausea after the procedure that typically resolved within 2 weeks of the procedure.
“There were a total of three moderate adverse events — two perigastric leaks, one repaired endoscopically, and another that only required antibiotics,” Lahooti reported. There were no severe or fatal adverse events.
About 11% of patients had endoscopic revision via retightening or resuturing at 10 years, the study team noted in their conference abstract.
Bariatric Surgery Remains Gold Standard
Lahooti shared that in his experience, some patients will need a revision at “about 40 months,” but at the same time, he’s seen some patients at 10 years “and their sutures are still in place.”
Session comoderator Shivangi Kothari, MD, with the Center for Advanced Therapeutic Endoscopy, University of Rochester Medical Center in New York, congratulated Lahooti for providing “robust” long-term data on ESG and said, “there is a need for more studies like this.”
In an interview, Ann M. Rogers, MD, president of the American Society for Metabolic and Bariatric Surgery, noted that bariatric surgery remains the “gold standard for weight loss and metabolic improvements,” with studies showing “around 30%” TWBL at 10 years, compared with about 10% at 10 years in this study.
Another key caveat, said Rogers, is that there are practical barriers to ESG; insurance typically does not cover the procedure because they view it as “cosmetic.”
The study had no commercial funding. Lahooti and Rogers had no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA —
“The procedure is dependable and safe and should be considered among individuals who have not attained their desired results through lifestyle medications and those who are not eligible for or choose not to undergo bariatric procedures,” said Ali Lahooti, with the Department of Gastroenterology and Hepatology, Weill Cornell Medical College, New York City. He presented his research at the annual meeting of the American College of Gastroenterology (ACG).
Obesity is a growing global health challenge. Lifestyle modification as a standalone therapy has limited effectiveness achieving weight loss. Pharmacotherapies are more efficacious, but they’re also associated with higher costs of and risk for side effects, leading to lower rates of compliance, Lahooti explained.
Bariatric surgery remains the most effective therapy for management of obesity and improvement of comorbid conditions, yet < 1% of candidates undergo a surgical intervention either because of access, cost, or fear of the procedure.
“Endoscopic treatments for obesity, such as ESG, can potentially fill this gap by combining durable weight loss with lower risk and costs,” Lahooti said.
He and his colleagues assessed outcomes out to 10 years in 404 patients (mean age, 45 years; 76% women; mean body mass index, 37.3) who underwent ESG between 2013 and 2024 at a single large tertiary hospital.
Out of the 404 patients, 397, 335, 249, and 110 patients were eligible for 1-, 3-, 5-, and 10-year follow-up, with complete follow-up rates of 85%, 66%, 79%, and 62%, respectively.
The primary outcome was weight loss at 10 years after ESG reported at percent total body weight loss (%TBWL).
At 10 years, mean %TBWL (the primary outcome) was 10.5% — with 53% of patients maintaining at least 5% TBWL and 42% maintaining at least 10% weight loss, Lahooti reported.
ESG had a favorable safety profile; 20% of patients experienced mild abdominal pain, constipation, heartburn, and nausea after the procedure that typically resolved within 2 weeks of the procedure.
“There were a total of three moderate adverse events — two perigastric leaks, one repaired endoscopically, and another that only required antibiotics,” Lahooti reported. There were no severe or fatal adverse events.
About 11% of patients had endoscopic revision via retightening or resuturing at 10 years, the study team noted in their conference abstract.
Bariatric Surgery Remains Gold Standard
Lahooti shared that in his experience, some patients will need a revision at “about 40 months,” but at the same time, he’s seen some patients at 10 years “and their sutures are still in place.”
Session comoderator Shivangi Kothari, MD, with the Center for Advanced Therapeutic Endoscopy, University of Rochester Medical Center in New York, congratulated Lahooti for providing “robust” long-term data on ESG and said, “there is a need for more studies like this.”
In an interview, Ann M. Rogers, MD, president of the American Society for Metabolic and Bariatric Surgery, noted that bariatric surgery remains the “gold standard for weight loss and metabolic improvements,” with studies showing “around 30%” TWBL at 10 years, compared with about 10% at 10 years in this study.
Another key caveat, said Rogers, is that there are practical barriers to ESG; insurance typically does not cover the procedure because they view it as “cosmetic.”
The study had no commercial funding. Lahooti and Rogers had no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA —
“The procedure is dependable and safe and should be considered among individuals who have not attained their desired results through lifestyle medications and those who are not eligible for or choose not to undergo bariatric procedures,” said Ali Lahooti, with the Department of Gastroenterology and Hepatology, Weill Cornell Medical College, New York City. He presented his research at the annual meeting of the American College of Gastroenterology (ACG).
Obesity is a growing global health challenge. Lifestyle modification as a standalone therapy has limited effectiveness achieving weight loss. Pharmacotherapies are more efficacious, but they’re also associated with higher costs of and risk for side effects, leading to lower rates of compliance, Lahooti explained.
Bariatric surgery remains the most effective therapy for management of obesity and improvement of comorbid conditions, yet < 1% of candidates undergo a surgical intervention either because of access, cost, or fear of the procedure.
“Endoscopic treatments for obesity, such as ESG, can potentially fill this gap by combining durable weight loss with lower risk and costs,” Lahooti said.
He and his colleagues assessed outcomes out to 10 years in 404 patients (mean age, 45 years; 76% women; mean body mass index, 37.3) who underwent ESG between 2013 and 2024 at a single large tertiary hospital.
Out of the 404 patients, 397, 335, 249, and 110 patients were eligible for 1-, 3-, 5-, and 10-year follow-up, with complete follow-up rates of 85%, 66%, 79%, and 62%, respectively.
The primary outcome was weight loss at 10 years after ESG reported at percent total body weight loss (%TBWL).
At 10 years, mean %TBWL (the primary outcome) was 10.5% — with 53% of patients maintaining at least 5% TBWL and 42% maintaining at least 10% weight loss, Lahooti reported.
ESG had a favorable safety profile; 20% of patients experienced mild abdominal pain, constipation, heartburn, and nausea after the procedure that typically resolved within 2 weeks of the procedure.
“There were a total of three moderate adverse events — two perigastric leaks, one repaired endoscopically, and another that only required antibiotics,” Lahooti reported. There were no severe or fatal adverse events.
About 11% of patients had endoscopic revision via retightening or resuturing at 10 years, the study team noted in their conference abstract.
Bariatric Surgery Remains Gold Standard
Lahooti shared that in his experience, some patients will need a revision at “about 40 months,” but at the same time, he’s seen some patients at 10 years “and their sutures are still in place.”
Session comoderator Shivangi Kothari, MD, with the Center for Advanced Therapeutic Endoscopy, University of Rochester Medical Center in New York, congratulated Lahooti for providing “robust” long-term data on ESG and said, “there is a need for more studies like this.”
In an interview, Ann M. Rogers, MD, president of the American Society for Metabolic and Bariatric Surgery, noted that bariatric surgery remains the “gold standard for weight loss and metabolic improvements,” with studies showing “around 30%” TWBL at 10 years, compared with about 10% at 10 years in this study.
Another key caveat, said Rogers, is that there are practical barriers to ESG; insurance typically does not cover the procedure because they view it as “cosmetic.”
The study had no commercial funding. Lahooti and Rogers had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Social Determinants of Health: The Impact on Pediatric Health and Well-Being
Case vignette: A 16-year-old Nepali-born English-speaking adolescent presents for a well-child visit and notes concerns for anxiety, depression, and a history of trauma. She resides with her parents who work in hospitality with limited time off, and thus she presented for the initial office visit with a neighbor. Parents were not readily available to discuss treatment recommendations, including medication options. The teen shares a number of challenges that makes coming to appointments difficult. You also notice that the patient currently is not enrolled in insurance, though she appears eligible.
The above vignette highlights various social issues and concerns that impact access to healthcare and overall health/well-being. Social determinants of health (SDOH) and factors centered on mental health are now widely known to impact pediatric health and wellbeing. The Office of Disease Prevention and Health Promotion defines SDOH as “conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.” SDOH can be grouped into five domains: Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1
Additionally, when considering determinants that impact the mental health of children, it is prudent to consider parental psychosocial factors and adverse childhood experiences (ACEs), such as witnessing interpersonal violence, child abuse, parental substance use, and parental depression.2 All these factors have been shown to impact an individual’s mental and physical health not only contemporaneously but also later in life.3
Screening Tool for Pediatric Social Histories
developed by Kenyon et al,4 with further derivations from Colvin et al.5 Utilizing this tool can assist providers with identifying social needs.
The tool begins with a framing statement — “Let me ask you some questions I ask every family” — then proceeds to discuss relevant topics as shared below:
I: Income; Insurance
- Do you have any concerns about making ends meet?
- Do you have any concerns about your child’s health insurance?
H: Hunger, Housing Conditions; Homeless
- Do you have any concerns about having enough food?
- Have you ever been worried whether your food would run out before you got money to buy more?
- Within the past year has the food you bought ever not lasted, and you didn’t have money to get more?
- Do you have any concerns about poor housing conditions like mice, mold, or cockroaches?
- Do you have any concerns about being evicted or not being able to pay the rent?
- Do you have any concerns about not being able to pay your mortgage?
E: Education; Ensuring Safety (Violence)
- Do you have any concerns about your child’s educational needs?
- [DO NOT ASK IN FRONT OF CHILD 3 OR OLDER OR IN FRONT OF OTHER PARTNER] “From speaking to families, I have learned that violence in the home is common and now I ask all families about violence in the home. Do you have any concerns about violence in your home?”
L: Legal status (Immigration)
- What hospital was your child born in?
- If not in the United States: “Are you aware that your child may be eligible for benefits even though they were not born in the US? If you would like, I can have a social worker come talk to you about some possible benefits your child may be eligible for. Would you like me to do that?”
P: Power of Attorney; Guardianship
- Are you the biological mother or father of this child?
- [If not] “Can you show me the power of attorney or guardianship document you have?”
- **PATIENTS >17+ with Mental Incapacity: Ask for Guardianship.
This tool can help with identifying families with significant social needs so that one can attain further historical information and subsequently share resources to assist with any challenges.
Consider the Role of Adverse Childhood Experiences
Additionally, as noted, ACEs often play an important role in overall health and well-being; they include experiencing childhood abuse, neglect, and/or household dysfunction. The impact of these early exposures can lead to toxic stress that can negatively alter the brain and the body’s response to stress over time.3 There are various tools readily available online that can assist with identifying ACEs and interpreting their prevalence. The American Academy of Pediatrics has an updated page of commonly used screening tools. Early identification and intervention can help mitigate the impact of these experiences on long-term outcomes.
Important Considerations Regarding Screening for SDOH and/or ACEs:
- Please consider if screening is helpful in your space, recognizing that there are benefits and potential ethical considerations to screen or not. Ensure an interdisciplinary approach if screening is implemented to ensure that the patient’s experience and well-being is prioritized.
- Try to be intentional in your communication with parents. The patient and family are our teachers and know best what they need.
- Consider what is available in your community and what can be offered to ensure that parents and families are appropriate and eligible for a particular resource.
- Encourage continuous collaboration and partnership with community providers who offer resources that a family may benefit from to ensure that the resource continues to be available.
Returning to the Vignette
Administering the IHELP tool has led to identifying that the adolescent’s insurance has lapsed, but she remains eligible, and the family seeks support to re-enroll. The family shares concerns regarding educational needs, as the child has not attended school for the past year and is not on track to graduate. The IHELP tool also helps you identify inconsistent transportation availability. Ultimately, a social work consultation is placed which assists with re-enrolling in insurance for the child and obtaining a bus pass for in-person visits. The patient is also supported in enrolling in the use of a videoconferencing platform for virtual visits. You and your team reach out to the school, which provides valuable information regarding the child’s status and how best to support re-engagement. On follow-up, she is now readily engaged in appointments and shares she is no longer worrying about transportation, which has been helpful. She has started initial conversations with the school and has a condensed schedule for reintegration.
Dr. Abdul-Karim, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Office of Disease Prevention and Health Promotion, US Department of Health & Human Services. Social Determinants of Health. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
2. Cotton N and Shim R. J Am Acad Child Adolesc Psychiatry. 2022 Nov;61(11):1385-1389. doi: 10.1016/j.jaac.2022.04.020.
3. US Centers for Disease Control and Prevention. Adverse Childhood Experiences (ACEs): Preventing Early Trauma to Improve Adult Health. https://www.cdc.gov/vitalsigns/aces/index.html.
4. Kenyon C et al. Pediatrics. 2007 Sep;120(3):e734-e738. doi: 10.1542/peds.2006-2495.
5. Colvin JD et al. Acad Pediatr. 2016 Mar;16(2):168-174. doi: 10.1016/j.acap.2015.06.001.
Case vignette: A 16-year-old Nepali-born English-speaking adolescent presents for a well-child visit and notes concerns for anxiety, depression, and a history of trauma. She resides with her parents who work in hospitality with limited time off, and thus she presented for the initial office visit with a neighbor. Parents were not readily available to discuss treatment recommendations, including medication options. The teen shares a number of challenges that makes coming to appointments difficult. You also notice that the patient currently is not enrolled in insurance, though she appears eligible.
The above vignette highlights various social issues and concerns that impact access to healthcare and overall health/well-being. Social determinants of health (SDOH) and factors centered on mental health are now widely known to impact pediatric health and wellbeing. The Office of Disease Prevention and Health Promotion defines SDOH as “conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.” SDOH can be grouped into five domains: Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1
Additionally, when considering determinants that impact the mental health of children, it is prudent to consider parental psychosocial factors and adverse childhood experiences (ACEs), such as witnessing interpersonal violence, child abuse, parental substance use, and parental depression.2 All these factors have been shown to impact an individual’s mental and physical health not only contemporaneously but also later in life.3
Screening Tool for Pediatric Social Histories
developed by Kenyon et al,4 with further derivations from Colvin et al.5 Utilizing this tool can assist providers with identifying social needs.
The tool begins with a framing statement — “Let me ask you some questions I ask every family” — then proceeds to discuss relevant topics as shared below:
I: Income; Insurance
- Do you have any concerns about making ends meet?
- Do you have any concerns about your child’s health insurance?
H: Hunger, Housing Conditions; Homeless
- Do you have any concerns about having enough food?
- Have you ever been worried whether your food would run out before you got money to buy more?
- Within the past year has the food you bought ever not lasted, and you didn’t have money to get more?
- Do you have any concerns about poor housing conditions like mice, mold, or cockroaches?
- Do you have any concerns about being evicted or not being able to pay the rent?
- Do you have any concerns about not being able to pay your mortgage?
E: Education; Ensuring Safety (Violence)
- Do you have any concerns about your child’s educational needs?
- [DO NOT ASK IN FRONT OF CHILD 3 OR OLDER OR IN FRONT OF OTHER PARTNER] “From speaking to families, I have learned that violence in the home is common and now I ask all families about violence in the home. Do you have any concerns about violence in your home?”
L: Legal status (Immigration)
- What hospital was your child born in?
- If not in the United States: “Are you aware that your child may be eligible for benefits even though they were not born in the US? If you would like, I can have a social worker come talk to you about some possible benefits your child may be eligible for. Would you like me to do that?”
P: Power of Attorney; Guardianship
- Are you the biological mother or father of this child?
- [If not] “Can you show me the power of attorney or guardianship document you have?”
- **PATIENTS >17+ with Mental Incapacity: Ask for Guardianship.
This tool can help with identifying families with significant social needs so that one can attain further historical information and subsequently share resources to assist with any challenges.
Consider the Role of Adverse Childhood Experiences
Additionally, as noted, ACEs often play an important role in overall health and well-being; they include experiencing childhood abuse, neglect, and/or household dysfunction. The impact of these early exposures can lead to toxic stress that can negatively alter the brain and the body’s response to stress over time.3 There are various tools readily available online that can assist with identifying ACEs and interpreting their prevalence. The American Academy of Pediatrics has an updated page of commonly used screening tools. Early identification and intervention can help mitigate the impact of these experiences on long-term outcomes.
Important Considerations Regarding Screening for SDOH and/or ACEs:
- Please consider if screening is helpful in your space, recognizing that there are benefits and potential ethical considerations to screen or not. Ensure an interdisciplinary approach if screening is implemented to ensure that the patient’s experience and well-being is prioritized.
- Try to be intentional in your communication with parents. The patient and family are our teachers and know best what they need.
- Consider what is available in your community and what can be offered to ensure that parents and families are appropriate and eligible for a particular resource.
- Encourage continuous collaboration and partnership with community providers who offer resources that a family may benefit from to ensure that the resource continues to be available.
Returning to the Vignette
Administering the IHELP tool has led to identifying that the adolescent’s insurance has lapsed, but she remains eligible, and the family seeks support to re-enroll. The family shares concerns regarding educational needs, as the child has not attended school for the past year and is not on track to graduate. The IHELP tool also helps you identify inconsistent transportation availability. Ultimately, a social work consultation is placed which assists with re-enrolling in insurance for the child and obtaining a bus pass for in-person visits. The patient is also supported in enrolling in the use of a videoconferencing platform for virtual visits. You and your team reach out to the school, which provides valuable information regarding the child’s status and how best to support re-engagement. On follow-up, she is now readily engaged in appointments and shares she is no longer worrying about transportation, which has been helpful. She has started initial conversations with the school and has a condensed schedule for reintegration.
Dr. Abdul-Karim, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Office of Disease Prevention and Health Promotion, US Department of Health & Human Services. Social Determinants of Health. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
2. Cotton N and Shim R. J Am Acad Child Adolesc Psychiatry. 2022 Nov;61(11):1385-1389. doi: 10.1016/j.jaac.2022.04.020.
3. US Centers for Disease Control and Prevention. Adverse Childhood Experiences (ACEs): Preventing Early Trauma to Improve Adult Health. https://www.cdc.gov/vitalsigns/aces/index.html.
4. Kenyon C et al. Pediatrics. 2007 Sep;120(3):e734-e738. doi: 10.1542/peds.2006-2495.
5. Colvin JD et al. Acad Pediatr. 2016 Mar;16(2):168-174. doi: 10.1016/j.acap.2015.06.001.
Case vignette: A 16-year-old Nepali-born English-speaking adolescent presents for a well-child visit and notes concerns for anxiety, depression, and a history of trauma. She resides with her parents who work in hospitality with limited time off, and thus she presented for the initial office visit with a neighbor. Parents were not readily available to discuss treatment recommendations, including medication options. The teen shares a number of challenges that makes coming to appointments difficult. You also notice that the patient currently is not enrolled in insurance, though she appears eligible.
The above vignette highlights various social issues and concerns that impact access to healthcare and overall health/well-being. Social determinants of health (SDOH) and factors centered on mental health are now widely known to impact pediatric health and wellbeing. The Office of Disease Prevention and Health Promotion defines SDOH as “conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.” SDOH can be grouped into five domains: Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1
Additionally, when considering determinants that impact the mental health of children, it is prudent to consider parental psychosocial factors and adverse childhood experiences (ACEs), such as witnessing interpersonal violence, child abuse, parental substance use, and parental depression.2 All these factors have been shown to impact an individual’s mental and physical health not only contemporaneously but also later in life.3
Screening Tool for Pediatric Social Histories
developed by Kenyon et al,4 with further derivations from Colvin et al.5 Utilizing this tool can assist providers with identifying social needs.
The tool begins with a framing statement — “Let me ask you some questions I ask every family” — then proceeds to discuss relevant topics as shared below:
I: Income; Insurance
- Do you have any concerns about making ends meet?
- Do you have any concerns about your child’s health insurance?
H: Hunger, Housing Conditions; Homeless
- Do you have any concerns about having enough food?
- Have you ever been worried whether your food would run out before you got money to buy more?
- Within the past year has the food you bought ever not lasted, and you didn’t have money to get more?
- Do you have any concerns about poor housing conditions like mice, mold, or cockroaches?
- Do you have any concerns about being evicted or not being able to pay the rent?
- Do you have any concerns about not being able to pay your mortgage?
E: Education; Ensuring Safety (Violence)
- Do you have any concerns about your child’s educational needs?
- [DO NOT ASK IN FRONT OF CHILD 3 OR OLDER OR IN FRONT OF OTHER PARTNER] “From speaking to families, I have learned that violence in the home is common and now I ask all families about violence in the home. Do you have any concerns about violence in your home?”
L: Legal status (Immigration)
- What hospital was your child born in?
- If not in the United States: “Are you aware that your child may be eligible for benefits even though they were not born in the US? If you would like, I can have a social worker come talk to you about some possible benefits your child may be eligible for. Would you like me to do that?”
P: Power of Attorney; Guardianship
- Are you the biological mother or father of this child?
- [If not] “Can you show me the power of attorney or guardianship document you have?”
- **PATIENTS >17+ with Mental Incapacity: Ask for Guardianship.
This tool can help with identifying families with significant social needs so that one can attain further historical information and subsequently share resources to assist with any challenges.
Consider the Role of Adverse Childhood Experiences
Additionally, as noted, ACEs often play an important role in overall health and well-being; they include experiencing childhood abuse, neglect, and/or household dysfunction. The impact of these early exposures can lead to toxic stress that can negatively alter the brain and the body’s response to stress over time.3 There are various tools readily available online that can assist with identifying ACEs and interpreting their prevalence. The American Academy of Pediatrics has an updated page of commonly used screening tools. Early identification and intervention can help mitigate the impact of these experiences on long-term outcomes.
Important Considerations Regarding Screening for SDOH and/or ACEs:
- Please consider if screening is helpful in your space, recognizing that there are benefits and potential ethical considerations to screen or not. Ensure an interdisciplinary approach if screening is implemented to ensure that the patient’s experience and well-being is prioritized.
- Try to be intentional in your communication with parents. The patient and family are our teachers and know best what they need.
- Consider what is available in your community and what can be offered to ensure that parents and families are appropriate and eligible for a particular resource.
- Encourage continuous collaboration and partnership with community providers who offer resources that a family may benefit from to ensure that the resource continues to be available.
Returning to the Vignette
Administering the IHELP tool has led to identifying that the adolescent’s insurance has lapsed, but she remains eligible, and the family seeks support to re-enroll. The family shares concerns regarding educational needs, as the child has not attended school for the past year and is not on track to graduate. The IHELP tool also helps you identify inconsistent transportation availability. Ultimately, a social work consultation is placed which assists with re-enrolling in insurance for the child and obtaining a bus pass for in-person visits. The patient is also supported in enrolling in the use of a videoconferencing platform for virtual visits. You and your team reach out to the school, which provides valuable information regarding the child’s status and how best to support re-engagement. On follow-up, she is now readily engaged in appointments and shares she is no longer worrying about transportation, which has been helpful. She has started initial conversations with the school and has a condensed schedule for reintegration.
Dr. Abdul-Karim, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Office of Disease Prevention and Health Promotion, US Department of Health & Human Services. Social Determinants of Health. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
2. Cotton N and Shim R. J Am Acad Child Adolesc Psychiatry. 2022 Nov;61(11):1385-1389. doi: 10.1016/j.jaac.2022.04.020.
3. US Centers for Disease Control and Prevention. Adverse Childhood Experiences (ACEs): Preventing Early Trauma to Improve Adult Health. https://www.cdc.gov/vitalsigns/aces/index.html.
4. Kenyon C et al. Pediatrics. 2007 Sep;120(3):e734-e738. doi: 10.1542/peds.2006-2495.
5. Colvin JD et al. Acad Pediatr. 2016 Mar;16(2):168-174. doi: 10.1016/j.acap.2015.06.001.
Faster Brain Atrophy Linked to MCI
While some brain atrophy is expected in aging, high levels of atrophy in the white matter and high enlargement in the ventricles are associated with earlier progression from normal cognition to MCI, the study found. The researchers also identified diabetes and atypical levels of amyloid beta protein in the cerebrospinal fluid as risk factors for brain atrophy and MCI.
For their research, published online on JAMA Network Open, Yuto Uchida, MD, PhD, and his colleagues at the Johns Hopkins University School of Medicine in Baltimore, Maryland, looked at data for 185 individuals (mean age, 55.4 years; 63% women) who were cognitively normal at baseline and followed for a median of 20 years.
All had been enrolled in a longitudinal cohort study on biomarkers of cognitive decline conducted at Johns Hopkins. Each participant underwent a median of five structural MRI studies during the follow-up period as well as annual cognitive testing. Altogether 60 individuals developed MCI, with eight of them progressing to dementia.
“We hypothesized that annual rates of change of segmental brain volumes would be associated with vascular risk factors among middle-aged and older adults and that these trends would be associated with the progression from normal cognition to MCI,” Uchida and colleagues wrote.
Uniquely Long Follow-Up
Most longitudinal studies using structural MRI count a decade or less of follow-up, the study authors noted. This makes it difficult to discern whether the annual rates of change of brain volumes are affected by vascular risk factors or are useful in predicting MCI, they said. Individual differences in brain aging make population-based studies less informative.
This study’s long timeframe allowed for tracking of brain changes “on an individual basis, which facilitates the differentiation between interindividual and intraindividual variations and leads to more accurate estimations of rates of brain atrophy,” Uchida and colleagues wrote.
People with high levels of atrophy in the white matter and enlargement in the ventricles saw earlier progression to MCI (hazard ratio [HR], 1.86; 95% CI, 1.24-2.49; P = .001). Diabetes mellitus was associated with progression to MCI (HR, 1.41; 95% CI, 1.06-1.76; P = .04), as was a low CSF Abeta42:Abeta40 ratio (HR, 1.48; 95% CI, 1.09-1.88; P = .04).
People with both diabetes and an abnormal amyloid profile were even more vulnerable to developing MCI (HR, 1.55; 95% CI, 1.13-1.98; P = .03). This indicated “a synergic association of diabetes and amyloid pathology with MCI progression,” Uchida and colleagues wrote, noting that insulin resistance has been shown to promote the formation of amyloid plaques, a hallmark of Alzheimer’s disease.
The findings also underscore that “white matter volume changes are closely associated with cognitive function in aging, suggesting that white matter degeneration may play a crucial role in cognitive decline,” the authors noted.
Uchida and colleagues acknowledged the modest size and imbalanced sex ratio of their study cohort as potential weaknesses, as well as the fact that the imaging technologies had changed over the course of the study. Most of the participants were White with family histories of dementia.
Findings May Lead to Targeted Interventions
In an editorial comment accompanying Uchida and colleagues’ study, Shohei Fujita, MD, PhD, of Massachusetts General Hospital, Boston, said that, while a more diverse population sample would be desirable and should be sought for future studies, the results nonetheless highlight “the potential of long-term longitudinal brain MRI datasets in elucidating the interplay of risk factors underlying cognitive decline and the potential benefits of controlling diabetes to reduce the risk of progression” along the Alzheimer’s disease continuum.
The findings may prove informative, Fujita said, in developing “targeted interventions for those most susceptible to progressive brain changes, potentially combining lifestyle modifications and pharmacological treatments.”
Uchida and colleagues’ study was funded by the Alzheimer’s Association, the National Alzheimer’s Coordinating Center, and the National Institutes of Health. The study’s corresponding author, Kenichi Oishi, disclosed funding from the Richman Family Foundation, Richman, the Sharp Family Foundation, and others. Uchida and Fujita reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
While some brain atrophy is expected in aging, high levels of atrophy in the white matter and high enlargement in the ventricles are associated with earlier progression from normal cognition to MCI, the study found. The researchers also identified diabetes and atypical levels of amyloid beta protein in the cerebrospinal fluid as risk factors for brain atrophy and MCI.
For their research, published online on JAMA Network Open, Yuto Uchida, MD, PhD, and his colleagues at the Johns Hopkins University School of Medicine in Baltimore, Maryland, looked at data for 185 individuals (mean age, 55.4 years; 63% women) who were cognitively normal at baseline and followed for a median of 20 years.
All had been enrolled in a longitudinal cohort study on biomarkers of cognitive decline conducted at Johns Hopkins. Each participant underwent a median of five structural MRI studies during the follow-up period as well as annual cognitive testing. Altogether 60 individuals developed MCI, with eight of them progressing to dementia.
“We hypothesized that annual rates of change of segmental brain volumes would be associated with vascular risk factors among middle-aged and older adults and that these trends would be associated with the progression from normal cognition to MCI,” Uchida and colleagues wrote.
Uniquely Long Follow-Up
Most longitudinal studies using structural MRI count a decade or less of follow-up, the study authors noted. This makes it difficult to discern whether the annual rates of change of brain volumes are affected by vascular risk factors or are useful in predicting MCI, they said. Individual differences in brain aging make population-based studies less informative.
This study’s long timeframe allowed for tracking of brain changes “on an individual basis, which facilitates the differentiation between interindividual and intraindividual variations and leads to more accurate estimations of rates of brain atrophy,” Uchida and colleagues wrote.
People with high levels of atrophy in the white matter and enlargement in the ventricles saw earlier progression to MCI (hazard ratio [HR], 1.86; 95% CI, 1.24-2.49; P = .001). Diabetes mellitus was associated with progression to MCI (HR, 1.41; 95% CI, 1.06-1.76; P = .04), as was a low CSF Abeta42:Abeta40 ratio (HR, 1.48; 95% CI, 1.09-1.88; P = .04).
People with both diabetes and an abnormal amyloid profile were even more vulnerable to developing MCI (HR, 1.55; 95% CI, 1.13-1.98; P = .03). This indicated “a synergic association of diabetes and amyloid pathology with MCI progression,” Uchida and colleagues wrote, noting that insulin resistance has been shown to promote the formation of amyloid plaques, a hallmark of Alzheimer’s disease.
The findings also underscore that “white matter volume changes are closely associated with cognitive function in aging, suggesting that white matter degeneration may play a crucial role in cognitive decline,” the authors noted.
Uchida and colleagues acknowledged the modest size and imbalanced sex ratio of their study cohort as potential weaknesses, as well as the fact that the imaging technologies had changed over the course of the study. Most of the participants were White with family histories of dementia.
Findings May Lead to Targeted Interventions
In an editorial comment accompanying Uchida and colleagues’ study, Shohei Fujita, MD, PhD, of Massachusetts General Hospital, Boston, said that, while a more diverse population sample would be desirable and should be sought for future studies, the results nonetheless highlight “the potential of long-term longitudinal brain MRI datasets in elucidating the interplay of risk factors underlying cognitive decline and the potential benefits of controlling diabetes to reduce the risk of progression” along the Alzheimer’s disease continuum.
The findings may prove informative, Fujita said, in developing “targeted interventions for those most susceptible to progressive brain changes, potentially combining lifestyle modifications and pharmacological treatments.”
Uchida and colleagues’ study was funded by the Alzheimer’s Association, the National Alzheimer’s Coordinating Center, and the National Institutes of Health. The study’s corresponding author, Kenichi Oishi, disclosed funding from the Richman Family Foundation, Richman, the Sharp Family Foundation, and others. Uchida and Fujita reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
While some brain atrophy is expected in aging, high levels of atrophy in the white matter and high enlargement in the ventricles are associated with earlier progression from normal cognition to MCI, the study found. The researchers also identified diabetes and atypical levels of amyloid beta protein in the cerebrospinal fluid as risk factors for brain atrophy and MCI.
For their research, published online on JAMA Network Open, Yuto Uchida, MD, PhD, and his colleagues at the Johns Hopkins University School of Medicine in Baltimore, Maryland, looked at data for 185 individuals (mean age, 55.4 years; 63% women) who were cognitively normal at baseline and followed for a median of 20 years.
All had been enrolled in a longitudinal cohort study on biomarkers of cognitive decline conducted at Johns Hopkins. Each participant underwent a median of five structural MRI studies during the follow-up period as well as annual cognitive testing. Altogether 60 individuals developed MCI, with eight of them progressing to dementia.
“We hypothesized that annual rates of change of segmental brain volumes would be associated with vascular risk factors among middle-aged and older adults and that these trends would be associated with the progression from normal cognition to MCI,” Uchida and colleagues wrote.
Uniquely Long Follow-Up
Most longitudinal studies using structural MRI count a decade or less of follow-up, the study authors noted. This makes it difficult to discern whether the annual rates of change of brain volumes are affected by vascular risk factors or are useful in predicting MCI, they said. Individual differences in brain aging make population-based studies less informative.
This study’s long timeframe allowed for tracking of brain changes “on an individual basis, which facilitates the differentiation between interindividual and intraindividual variations and leads to more accurate estimations of rates of brain atrophy,” Uchida and colleagues wrote.
People with high levels of atrophy in the white matter and enlargement in the ventricles saw earlier progression to MCI (hazard ratio [HR], 1.86; 95% CI, 1.24-2.49; P = .001). Diabetes mellitus was associated with progression to MCI (HR, 1.41; 95% CI, 1.06-1.76; P = .04), as was a low CSF Abeta42:Abeta40 ratio (HR, 1.48; 95% CI, 1.09-1.88; P = .04).
People with both diabetes and an abnormal amyloid profile were even more vulnerable to developing MCI (HR, 1.55; 95% CI, 1.13-1.98; P = .03). This indicated “a synergic association of diabetes and amyloid pathology with MCI progression,” Uchida and colleagues wrote, noting that insulin resistance has been shown to promote the formation of amyloid plaques, a hallmark of Alzheimer’s disease.
The findings also underscore that “white matter volume changes are closely associated with cognitive function in aging, suggesting that white matter degeneration may play a crucial role in cognitive decline,” the authors noted.
Uchida and colleagues acknowledged the modest size and imbalanced sex ratio of their study cohort as potential weaknesses, as well as the fact that the imaging technologies had changed over the course of the study. Most of the participants were White with family histories of dementia.
Findings May Lead to Targeted Interventions
In an editorial comment accompanying Uchida and colleagues’ study, Shohei Fujita, MD, PhD, of Massachusetts General Hospital, Boston, said that, while a more diverse population sample would be desirable and should be sought for future studies, the results nonetheless highlight “the potential of long-term longitudinal brain MRI datasets in elucidating the interplay of risk factors underlying cognitive decline and the potential benefits of controlling diabetes to reduce the risk of progression” along the Alzheimer’s disease continuum.
The findings may prove informative, Fujita said, in developing “targeted interventions for those most susceptible to progressive brain changes, potentially combining lifestyle modifications and pharmacological treatments.”
Uchida and colleagues’ study was funded by the Alzheimer’s Association, the National Alzheimer’s Coordinating Center, and the National Institutes of Health. The study’s corresponding author, Kenichi Oishi, disclosed funding from the Richman Family Foundation, Richman, the Sharp Family Foundation, and others. Uchida and Fujita reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Survey Study Shows How to Reduce Family Physician Burnout
Family physician burnout rates are among the highest in medicine. More than half (51%) reported burnout in a Medscape report from January 2024; only emergency physicians (63%) and obstetricians/gynecologists and oncologists (both 53%) had higher rates.
In a recent study, researchers examined what’s driving the burnout through a serial cross-sectional survey of family physicians. Authors conclude that reducing burnout may be most effective with a focus on two factors: Decreasing time spent at home on electronic health record (EHR) tasks and building stronger nurse-physician teams.
Findings by Lisa S. Rotenstein, MD, MBA, MSc, Division of Clinical Informatics, Department of Medicine, University of California, San Francisco, and colleagues were published in JAMA Network Open. The findings debunk some longstanding assumptions, Christine A. Sinsky, MD, vice president of professional satisfaction with the American Medical Association, wrote in an editorial.
“This study advances our understanding that addressing physician burnout is not about more EHR training and not specifically about moving to paying for value; rather, it is about developing stronger nurse-physician core teams. These are novel and important findings with actionable lessons for physician and health system leaders,” Sinsky wrote.
More Than 10,000 Physicians; 100% Response Rate
The study included 10,315 physicians who answered questions related to burnout on the American Board of Family Medicine’s Continuous Certification Questionnaire between 2017 and 2023. Researchers achieved a 100% response rate by requiring diplomates to complete the survey before submitting their exam.
The median age of respondents was 50 years. More than half (57.8%) were employees, 11.3% were full owners of their practices, and 3.2% were contractors. Responses indicated that 10% practiced as solo physicians, 20.4% were in a practice with more than 20 physicians, and the rest were in a practice with 2-19 physicians. More than three fourths of the physicians practiced in an urban/suburban setting, and 13.5% practiced in a rural setting.
Physicians’ perceptions that EHR use at home was appropriate were associated with 0.58 times the odds of burnout (95% CI, 0.53-0.64; P < .001), and high team efficiency was associated with 0.61 times the odds of burnout (95% CI, 0.56-0.67).
Physician collaboration with a registered nurse was associated with greater odds of high team efficiency (odds ratio [OR], 1.35; 95% CI, 1.22-1.50). Collaboration with a physician assistant was associated with greater odds of appropriate home EHR time (OR, 1.13; 95% CI, 1.03-1.24).
Numbers Needed to Treat
“When translated to a number needed to treat, these ORs suggest that eight additional physicians perceiving appropriate home EHR time would result in prevention of one additional case of burnout, and nine additional physicians perceiving high team efficiency would result in prevention of one case of burnout,” the authors wrote.
The authors also noted that EHR proficiency was not associated with burnout (OR, 0.93; 95% CI, 0.85-1.02; P = .12). Self-reported EHR proficiency remained high and steady over the study period.
“It is time to lay to rest the myth of the technology-resistant physician,” Sinsky wrote in the editorial. “The problem is not the end user.”
Sinsky said the findings also show that value-based compensation “is not a panacea” and, in fact, participation in such payment programs was associated with both more time working on the EHR at home and lower team efficiency.
Fee-for-service models are often painted as the culprit, she noted.
“The key in either compensation model is to direct sufficient financial resources to primary care to cover the costs of optimal team size, skill level, and stability. In my experience, this is a minimum of two clinical assistants (including at least one nurse) per 1.0 clinical full-time equivalent physician, with the same team of individuals working together on a daily basis to develop trust, reliance, and efficiencies.”
Medical Assistants (MAs) Replacing Nurses on Core Teams
In many cases, nurses have been replaced on core clinical teams by MAs, who, with a narrower scope of practice, put work back on the physician’s plate, Sinsky noted, and the MAs also often work in pools rather than with one physician.
“The result is that nurses in many settings are sequestered in a room with a computer and a telephone, with limited direct interactions with their patients or physicians, and physicians spend more time each day on tasks that do not require their medical training,” Sinsky wrote.
Strengths of the study include the large sample size, a 100% response rate to the survey, and consistency of findings over the 6 years.
Steven Waldren, MD, MS, chief medical informatics officer for the American Academy of Family Physicians, said the results of the study confirm what the organization knows to be true through various analyses and talks with doctors: “Even if you can just focus on documentation and improve that, it gives docs hope that other things can happen and actually improve. We saw a decrease in burnout in just solving that one problem.”
Team-based care also allows physicians to talk through challenges and off-load tasks, which allows them to focus on patient care, he said.
Waldren added that some technology upgrades can help reduce burnout without adding staff. He pointed to promising technology in managing EHR inbox messages and in artificial intelligence (AI) solutions for developing a visit summary and patient instructions that can then be reviewed by a physician.
He gave an example of ambient documentation. “We’ve seen that it reduces the amount of documentation time by 60%-70%,” he said. The products in this space record the physician-patient conversation and generate a summary to be reviewed by the physician for accuracy.
“These tools now are highly accurate,” he said. They are also able to remove clinically irrelevant details. He said, for example, if a patient talks about her recent golf outing on a trip to Ireland, the program will record only that she recently had an international trip to Ireland and remove the golf details. The technology has been available for many months, he said.
Sonia Rivera-Martinez, DO, an associate professor of family medicine at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, New York, said AI solutions are impressive but expensive, which is why her practice has not upgraded to AI-generated visit summaries.
She said even in her academic setting where there is less pressure to see several patients per hour, after-hours EHR work is a reality for her and her colleagues as seeing patients is paired with the demands of teaching students. Her practice is also part of an accountable care organization, which adds its own set of documentation demands.
Nearly 30 Hours a Week of EHR Work at Home
Rivera-Martinez estimated that she spends 20-30 hours each week doing EHR tasks at home and said the study authors have highlighted an important problem.
She said she has also seen the value of strong nurse-physician teams in her practice. The two nurses in her practice, for instance, know they have permission to administer flu shots and do other routine tasks without the physicians having to place the order. “But I can’t say it eliminates having to do work outside (of work hours).”
She said before current EHR documentation demands, “I used to be able to finish a progress note in less than 5 minutes.” Now, she said, with her medically complex patient population, it takes her 20-30 minutes to complete a patient’s progress note.
The findings of the study have particular significance with the rising prevalence of burnout among family physicians, the authors wrote. “Clinical leaders and policymakers seeking to develop care delivery models that enable sustainable primary care practice should focus on ensuring adequate team support and acceptable EHR workloads for physicians.”
This study was funded by the United States Office of the National Coordinator for Health Information Technology and Department of Health and Human Services. Additionally, Rotenstein’s time was funded by The Physicians Foundation. Rotenstein reported personal fees from Phreesia; stock grants from serving on the advisory board of Augmedix; and grants from the Agency for Healthcare Research and Quality, American Medical Association, The Physicians Foundation, and Association of Chiefs and Leaders of General Internal Medicine outside the submitted work. Nathaniel Hendrix reported grants from the Office of the National Coordinator for Health Information Technology during the conduct of the study. One coauthor reported a cooperative agreement from the Office of the National Coordinator for Health Information Technology (now Assistant Secretary for Technology Policy/Office of the National Coordinator for Health Information Technology). Another coauthor reported that the University of California, San Francisco, has received funding from the Office of the National Coordinator for Health Information Technology to partner with the American Board of Family Medicine to revise the survey over time to better capture interoperability. Sinsky, Rivera-Martinez, and Waldren reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Family physician burnout rates are among the highest in medicine. More than half (51%) reported burnout in a Medscape report from January 2024; only emergency physicians (63%) and obstetricians/gynecologists and oncologists (both 53%) had higher rates.
In a recent study, researchers examined what’s driving the burnout through a serial cross-sectional survey of family physicians. Authors conclude that reducing burnout may be most effective with a focus on two factors: Decreasing time spent at home on electronic health record (EHR) tasks and building stronger nurse-physician teams.
Findings by Lisa S. Rotenstein, MD, MBA, MSc, Division of Clinical Informatics, Department of Medicine, University of California, San Francisco, and colleagues were published in JAMA Network Open. The findings debunk some longstanding assumptions, Christine A. Sinsky, MD, vice president of professional satisfaction with the American Medical Association, wrote in an editorial.
“This study advances our understanding that addressing physician burnout is not about more EHR training and not specifically about moving to paying for value; rather, it is about developing stronger nurse-physician core teams. These are novel and important findings with actionable lessons for physician and health system leaders,” Sinsky wrote.
More Than 10,000 Physicians; 100% Response Rate
The study included 10,315 physicians who answered questions related to burnout on the American Board of Family Medicine’s Continuous Certification Questionnaire between 2017 and 2023. Researchers achieved a 100% response rate by requiring diplomates to complete the survey before submitting their exam.
The median age of respondents was 50 years. More than half (57.8%) were employees, 11.3% were full owners of their practices, and 3.2% were contractors. Responses indicated that 10% practiced as solo physicians, 20.4% were in a practice with more than 20 physicians, and the rest were in a practice with 2-19 physicians. More than three fourths of the physicians practiced in an urban/suburban setting, and 13.5% practiced in a rural setting.
Physicians’ perceptions that EHR use at home was appropriate were associated with 0.58 times the odds of burnout (95% CI, 0.53-0.64; P < .001), and high team efficiency was associated with 0.61 times the odds of burnout (95% CI, 0.56-0.67).
Physician collaboration with a registered nurse was associated with greater odds of high team efficiency (odds ratio [OR], 1.35; 95% CI, 1.22-1.50). Collaboration with a physician assistant was associated with greater odds of appropriate home EHR time (OR, 1.13; 95% CI, 1.03-1.24).
Numbers Needed to Treat
“When translated to a number needed to treat, these ORs suggest that eight additional physicians perceiving appropriate home EHR time would result in prevention of one additional case of burnout, and nine additional physicians perceiving high team efficiency would result in prevention of one case of burnout,” the authors wrote.
The authors also noted that EHR proficiency was not associated with burnout (OR, 0.93; 95% CI, 0.85-1.02; P = .12). Self-reported EHR proficiency remained high and steady over the study period.
“It is time to lay to rest the myth of the technology-resistant physician,” Sinsky wrote in the editorial. “The problem is not the end user.”
Sinsky said the findings also show that value-based compensation “is not a panacea” and, in fact, participation in such payment programs was associated with both more time working on the EHR at home and lower team efficiency.
Fee-for-service models are often painted as the culprit, she noted.
“The key in either compensation model is to direct sufficient financial resources to primary care to cover the costs of optimal team size, skill level, and stability. In my experience, this is a minimum of two clinical assistants (including at least one nurse) per 1.0 clinical full-time equivalent physician, with the same team of individuals working together on a daily basis to develop trust, reliance, and efficiencies.”
Medical Assistants (MAs) Replacing Nurses on Core Teams
In many cases, nurses have been replaced on core clinical teams by MAs, who, with a narrower scope of practice, put work back on the physician’s plate, Sinsky noted, and the MAs also often work in pools rather than with one physician.
“The result is that nurses in many settings are sequestered in a room with a computer and a telephone, with limited direct interactions with their patients or physicians, and physicians spend more time each day on tasks that do not require their medical training,” Sinsky wrote.
Strengths of the study include the large sample size, a 100% response rate to the survey, and consistency of findings over the 6 years.
Steven Waldren, MD, MS, chief medical informatics officer for the American Academy of Family Physicians, said the results of the study confirm what the organization knows to be true through various analyses and talks with doctors: “Even if you can just focus on documentation and improve that, it gives docs hope that other things can happen and actually improve. We saw a decrease in burnout in just solving that one problem.”
Team-based care also allows physicians to talk through challenges and off-load tasks, which allows them to focus on patient care, he said.
Waldren added that some technology upgrades can help reduce burnout without adding staff. He pointed to promising technology in managing EHR inbox messages and in artificial intelligence (AI) solutions for developing a visit summary and patient instructions that can then be reviewed by a physician.
He gave an example of ambient documentation. “We’ve seen that it reduces the amount of documentation time by 60%-70%,” he said. The products in this space record the physician-patient conversation and generate a summary to be reviewed by the physician for accuracy.
“These tools now are highly accurate,” he said. They are also able to remove clinically irrelevant details. He said, for example, if a patient talks about her recent golf outing on a trip to Ireland, the program will record only that she recently had an international trip to Ireland and remove the golf details. The technology has been available for many months, he said.
Sonia Rivera-Martinez, DO, an associate professor of family medicine at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, New York, said AI solutions are impressive but expensive, which is why her practice has not upgraded to AI-generated visit summaries.
She said even in her academic setting where there is less pressure to see several patients per hour, after-hours EHR work is a reality for her and her colleagues as seeing patients is paired with the demands of teaching students. Her practice is also part of an accountable care organization, which adds its own set of documentation demands.
Nearly 30 Hours a Week of EHR Work at Home
Rivera-Martinez estimated that she spends 20-30 hours each week doing EHR tasks at home and said the study authors have highlighted an important problem.
She said she has also seen the value of strong nurse-physician teams in her practice. The two nurses in her practice, for instance, know they have permission to administer flu shots and do other routine tasks without the physicians having to place the order. “But I can’t say it eliminates having to do work outside (of work hours).”
She said before current EHR documentation demands, “I used to be able to finish a progress note in less than 5 minutes.” Now, she said, with her medically complex patient population, it takes her 20-30 minutes to complete a patient’s progress note.
The findings of the study have particular significance with the rising prevalence of burnout among family physicians, the authors wrote. “Clinical leaders and policymakers seeking to develop care delivery models that enable sustainable primary care practice should focus on ensuring adequate team support and acceptable EHR workloads for physicians.”
This study was funded by the United States Office of the National Coordinator for Health Information Technology and Department of Health and Human Services. Additionally, Rotenstein’s time was funded by The Physicians Foundation. Rotenstein reported personal fees from Phreesia; stock grants from serving on the advisory board of Augmedix; and grants from the Agency for Healthcare Research and Quality, American Medical Association, The Physicians Foundation, and Association of Chiefs and Leaders of General Internal Medicine outside the submitted work. Nathaniel Hendrix reported grants from the Office of the National Coordinator for Health Information Technology during the conduct of the study. One coauthor reported a cooperative agreement from the Office of the National Coordinator for Health Information Technology (now Assistant Secretary for Technology Policy/Office of the National Coordinator for Health Information Technology). Another coauthor reported that the University of California, San Francisco, has received funding from the Office of the National Coordinator for Health Information Technology to partner with the American Board of Family Medicine to revise the survey over time to better capture interoperability. Sinsky, Rivera-Martinez, and Waldren reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Family physician burnout rates are among the highest in medicine. More than half (51%) reported burnout in a Medscape report from January 2024; only emergency physicians (63%) and obstetricians/gynecologists and oncologists (both 53%) had higher rates.
In a recent study, researchers examined what’s driving the burnout through a serial cross-sectional survey of family physicians. Authors conclude that reducing burnout may be most effective with a focus on two factors: Decreasing time spent at home on electronic health record (EHR) tasks and building stronger nurse-physician teams.
Findings by Lisa S. Rotenstein, MD, MBA, MSc, Division of Clinical Informatics, Department of Medicine, University of California, San Francisco, and colleagues were published in JAMA Network Open. The findings debunk some longstanding assumptions, Christine A. Sinsky, MD, vice president of professional satisfaction with the American Medical Association, wrote in an editorial.
“This study advances our understanding that addressing physician burnout is not about more EHR training and not specifically about moving to paying for value; rather, it is about developing stronger nurse-physician core teams. These are novel and important findings with actionable lessons for physician and health system leaders,” Sinsky wrote.
More Than 10,000 Physicians; 100% Response Rate
The study included 10,315 physicians who answered questions related to burnout on the American Board of Family Medicine’s Continuous Certification Questionnaire between 2017 and 2023. Researchers achieved a 100% response rate by requiring diplomates to complete the survey before submitting their exam.
The median age of respondents was 50 years. More than half (57.8%) were employees, 11.3% were full owners of their practices, and 3.2% were contractors. Responses indicated that 10% practiced as solo physicians, 20.4% were in a practice with more than 20 physicians, and the rest were in a practice with 2-19 physicians. More than three fourths of the physicians practiced in an urban/suburban setting, and 13.5% practiced in a rural setting.
Physicians’ perceptions that EHR use at home was appropriate were associated with 0.58 times the odds of burnout (95% CI, 0.53-0.64; P < .001), and high team efficiency was associated with 0.61 times the odds of burnout (95% CI, 0.56-0.67).
Physician collaboration with a registered nurse was associated with greater odds of high team efficiency (odds ratio [OR], 1.35; 95% CI, 1.22-1.50). Collaboration with a physician assistant was associated with greater odds of appropriate home EHR time (OR, 1.13; 95% CI, 1.03-1.24).
Numbers Needed to Treat
“When translated to a number needed to treat, these ORs suggest that eight additional physicians perceiving appropriate home EHR time would result in prevention of one additional case of burnout, and nine additional physicians perceiving high team efficiency would result in prevention of one case of burnout,” the authors wrote.
The authors also noted that EHR proficiency was not associated with burnout (OR, 0.93; 95% CI, 0.85-1.02; P = .12). Self-reported EHR proficiency remained high and steady over the study period.
“It is time to lay to rest the myth of the technology-resistant physician,” Sinsky wrote in the editorial. “The problem is not the end user.”
Sinsky said the findings also show that value-based compensation “is not a panacea” and, in fact, participation in such payment programs was associated with both more time working on the EHR at home and lower team efficiency.
Fee-for-service models are often painted as the culprit, she noted.
“The key in either compensation model is to direct sufficient financial resources to primary care to cover the costs of optimal team size, skill level, and stability. In my experience, this is a minimum of two clinical assistants (including at least one nurse) per 1.0 clinical full-time equivalent physician, with the same team of individuals working together on a daily basis to develop trust, reliance, and efficiencies.”
Medical Assistants (MAs) Replacing Nurses on Core Teams
In many cases, nurses have been replaced on core clinical teams by MAs, who, with a narrower scope of practice, put work back on the physician’s plate, Sinsky noted, and the MAs also often work in pools rather than with one physician.
“The result is that nurses in many settings are sequestered in a room with a computer and a telephone, with limited direct interactions with their patients or physicians, and physicians spend more time each day on tasks that do not require their medical training,” Sinsky wrote.
Strengths of the study include the large sample size, a 100% response rate to the survey, and consistency of findings over the 6 years.
Steven Waldren, MD, MS, chief medical informatics officer for the American Academy of Family Physicians, said the results of the study confirm what the organization knows to be true through various analyses and talks with doctors: “Even if you can just focus on documentation and improve that, it gives docs hope that other things can happen and actually improve. We saw a decrease in burnout in just solving that one problem.”
Team-based care also allows physicians to talk through challenges and off-load tasks, which allows them to focus on patient care, he said.
Waldren added that some technology upgrades can help reduce burnout without adding staff. He pointed to promising technology in managing EHR inbox messages and in artificial intelligence (AI) solutions for developing a visit summary and patient instructions that can then be reviewed by a physician.
He gave an example of ambient documentation. “We’ve seen that it reduces the amount of documentation time by 60%-70%,” he said. The products in this space record the physician-patient conversation and generate a summary to be reviewed by the physician for accuracy.
“These tools now are highly accurate,” he said. They are also able to remove clinically irrelevant details. He said, for example, if a patient talks about her recent golf outing on a trip to Ireland, the program will record only that she recently had an international trip to Ireland and remove the golf details. The technology has been available for many months, he said.
Sonia Rivera-Martinez, DO, an associate professor of family medicine at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, New York, said AI solutions are impressive but expensive, which is why her practice has not upgraded to AI-generated visit summaries.
She said even in her academic setting where there is less pressure to see several patients per hour, after-hours EHR work is a reality for her and her colleagues as seeing patients is paired with the demands of teaching students. Her practice is also part of an accountable care organization, which adds its own set of documentation demands.
Nearly 30 Hours a Week of EHR Work at Home
Rivera-Martinez estimated that she spends 20-30 hours each week doing EHR tasks at home and said the study authors have highlighted an important problem.
She said she has also seen the value of strong nurse-physician teams in her practice. The two nurses in her practice, for instance, know they have permission to administer flu shots and do other routine tasks without the physicians having to place the order. “But I can’t say it eliminates having to do work outside (of work hours).”
She said before current EHR documentation demands, “I used to be able to finish a progress note in less than 5 minutes.” Now, she said, with her medically complex patient population, it takes her 20-30 minutes to complete a patient’s progress note.
The findings of the study have particular significance with the rising prevalence of burnout among family physicians, the authors wrote. “Clinical leaders and policymakers seeking to develop care delivery models that enable sustainable primary care practice should focus on ensuring adequate team support and acceptable EHR workloads for physicians.”
This study was funded by the United States Office of the National Coordinator for Health Information Technology and Department of Health and Human Services. Additionally, Rotenstein’s time was funded by The Physicians Foundation. Rotenstein reported personal fees from Phreesia; stock grants from serving on the advisory board of Augmedix; and grants from the Agency for Healthcare Research and Quality, American Medical Association, The Physicians Foundation, and Association of Chiefs and Leaders of General Internal Medicine outside the submitted work. Nathaniel Hendrix reported grants from the Office of the National Coordinator for Health Information Technology during the conduct of the study. One coauthor reported a cooperative agreement from the Office of the National Coordinator for Health Information Technology (now Assistant Secretary for Technology Policy/Office of the National Coordinator for Health Information Technology). Another coauthor reported that the University of California, San Francisco, has received funding from the Office of the National Coordinator for Health Information Technology to partner with the American Board of Family Medicine to revise the survey over time to better capture interoperability. Sinsky, Rivera-Martinez, and Waldren reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
How to Manage Patients on GLP-1s Before Surgery
, as does the US Food and Drug Administration’s (FDA’s) labeling for the drugs. The changes can be challenging to keep up with, and endocrinologists seem to be making their own decisions based on clinical experience and their interpretations of the potential impact and value of the emerging information.
The latest FDA label change warns about the risk for pulmonary aspiration but notes “insufficient” data to inform recommendations to mitigate the risk in vulnerable patients. Yet, the latest multi-society guidance, led by the American Society of Anesthesiologists (ASA) and based on consensus, not evidence, has nuanced advice for managing patients at risk.
Does the FDA’s label change make a difference regarding the multi-society guidance, which was published earlier? “The answer is no,” Girish Joshi, MD, vice chair, ASA Committee on Practice Parameters, told this news organization. “The concern of increased pulmonary aspiration in patients who are on GLP-1 receptor agonists has been known, and that concern still exists. So, we started with not an assumption but the premise that patients on GLP-1 receptor agonists are at a higher risk of aspiration during sedation, analgesia, and/or general anesthesia. The FDA basically confirms what we say in the guidance.”
Joshi, professor in the Anesthesiology and Pain Management Department at UT Southwestern Medical Center, Dallas, aimed to make the guidance, which was published simultaneously in several society journals, more implementable with a letter to the editor of Anesthesiology. The key, he said, is to identify patients at higher risk for aspiration; all others would follow treatment as usual.
The letter highlights three overarching recommendations and then expands upon them: Standardized preoperative assessment for risk for delayed gastric emptying (yes/no); selective preoperative care plan based on delayed gastric emptying assessment and shared decision-making; and on the day of the procedure, reassess for delayed gastric emptying and mitigate risk if there is clinical concern.
But it seems as though, for now, endocrinologists are managing these patients as they see fit, within the parameters of any institutional guidance requirements. Here is what they said about their practice:
Amy E. Rothberg, MD, DABOM, director of the Weight Management Program & Rewind at the University of Michigan, Ann Arbor, Michigan, said, “I think it makes sense to inform our patients of the labeling and rare but potential adverse effects if they intend to undergo anesthesia for a scheduled procedure/surgery. There is never no risk of aspiration during anesthesia.”
“I find it a bit curious that ASA implies that those who experience GI side effects are more likely than those who do not to have this potential risk. I doubt there is evidence that those without GI side effects are necessarily ‘safer’ and a study to determine that is unlikely to take be conducted.”
“My institution does require a 1-week pause on GLP-1s for those undergoing anesthesia for surgery,” she added. “That’s not evidence-based either, but probably reduces the risk of aspiration during anesthesia — but I don’t know what the actual denominator is for aspiration in those who continued vs those who took a pause from GLP-1s. Pausing does certainly (anecdotally) increase the traffic of communications between physicians and their patients about what to do in the interval.”
Anne Peters, MD, a professor of clinical medicine and a clinical scholar at the Keck School of Medicine of the University of Southern California, Los Angeles, said, “The FDA label change is a warning that really doesn’t say exactly who on GLP-1 RAs is at highest risk or what to do, and if any intervention has been shown to help. The ASA recommendations seem much more nuanced and practical, including point-of-care gastric ultrasound to see if there is retained food/fluid prior to surgery.”
“In my practice, I individualize what I say, depending on the person and the circumstance,” she said. “Mostly, I have people hold one dose before planned surgery, so they have been 10 days at least without a dose. But if worried about gastrointestinal symptoms or gastroparesis, I have them do a clear liquid diet for 24 hours presurgery. Or at least avoid heavy fat meals the day before.”
“There is a risk of aspiration with anything that slows gastric emptying — maybe even in patients with gastroparesis at baseline due to physiologic, not pharmacological, reasons — and anesthesiologists should be aware of the need to assess patients individually.”
Michael A. Weintraub, MD, of NYU Langone Health Diabetes & Endocrine Associates in New York City, observed, “The risk of a pulmonary aspiration event with GLP-1 medication is quite rare, but not zero. On the other hand, stopping the GLP-1 can cause hyperglycemia or rebound weight gain. Furthermore, it can become complicated to restart GLP1 dosing, particularly given the existing medication shortages.”
“In most cases, stopping a weekly GLP-1 medication 1 week prior to the procedure minimizes the risks of pulmonary aspiration and prevents any worsening hyperglycemia or weight gain,” he said. However, taking the drug 7 days prior to the procedure is optimal. “That way, they would be due for the next dose on the day of the procedure, and taking it the day following procedure minimizes disruption in their once-weekly regimen.”
Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, advised that physicians weigh the risk of stopping the medication (which can cause a glycemic spike) vs risk for aspiration.
“In my opinion, all patients should follow a strict liquid diet or NPO status prior to a surgery to further decrease the risk of aspiration,” she said. “I generally hold the GLP-1 RA for a week before a surgery. If additional glycemic control is necessary, I will add to or adjust one of the patient’s other diabetes medications.”
Jaime Almandoz, MD, associate professor of medicine and medical director of the Weight Wellness Program in Dallas, said, “As endocrinologists, we typically rely on our anesthesia colleagues for guidance on perioperative management. In light of emerging guidelines for holding GLP-1 medications, we also recommend patients adopt a liquid diet 24 hours prior to surgery, along with the fasting protocol.”
“For those managing diabetes with GLP-1 therapies, it is crucial to establish a blood sugar management plan while off these medications, especially during fasting or postoperative periods, which can be further influenced by many factors, including nausea, pain medications, and antibiotics after the procedure.”
Joshi added that at Parkland Hospital in Dallas, “we do a huge number of cases using the same information. We identify patients who are at risk, and then we tell our proceduralists and our surgeons if they’re in the escalating phase of the dosing or if they have GI symptoms; don’t even schedule them as an elective case; wait till the escalation phase is over and then schedule them.”
“That way,” he said, “it becomes logistically easy to manage because the recommendation from the group is that patients who are at higher risk should receive a 24-hour liquid diet — the same as colonoscopy. But sometimes it can be challenging to do so.”
Joshi has received honoraria for consultation from Merck Sharp & Dohme, Vertex Pharmaceuticals, and Haisco-USA Pharmaceuticals. Gupta is on the speakers bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie. Almandoz serves on advisory boards for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. The other experts declared no relevant relationships.
A version of this article first appeared on Medscape.com.
, as does the US Food and Drug Administration’s (FDA’s) labeling for the drugs. The changes can be challenging to keep up with, and endocrinologists seem to be making their own decisions based on clinical experience and their interpretations of the potential impact and value of the emerging information.
The latest FDA label change warns about the risk for pulmonary aspiration but notes “insufficient” data to inform recommendations to mitigate the risk in vulnerable patients. Yet, the latest multi-society guidance, led by the American Society of Anesthesiologists (ASA) and based on consensus, not evidence, has nuanced advice for managing patients at risk.
Does the FDA’s label change make a difference regarding the multi-society guidance, which was published earlier? “The answer is no,” Girish Joshi, MD, vice chair, ASA Committee on Practice Parameters, told this news organization. “The concern of increased pulmonary aspiration in patients who are on GLP-1 receptor agonists has been known, and that concern still exists. So, we started with not an assumption but the premise that patients on GLP-1 receptor agonists are at a higher risk of aspiration during sedation, analgesia, and/or general anesthesia. The FDA basically confirms what we say in the guidance.”
Joshi, professor in the Anesthesiology and Pain Management Department at UT Southwestern Medical Center, Dallas, aimed to make the guidance, which was published simultaneously in several society journals, more implementable with a letter to the editor of Anesthesiology. The key, he said, is to identify patients at higher risk for aspiration; all others would follow treatment as usual.
The letter highlights three overarching recommendations and then expands upon them: Standardized preoperative assessment for risk for delayed gastric emptying (yes/no); selective preoperative care plan based on delayed gastric emptying assessment and shared decision-making; and on the day of the procedure, reassess for delayed gastric emptying and mitigate risk if there is clinical concern.
But it seems as though, for now, endocrinologists are managing these patients as they see fit, within the parameters of any institutional guidance requirements. Here is what they said about their practice:
Amy E. Rothberg, MD, DABOM, director of the Weight Management Program & Rewind at the University of Michigan, Ann Arbor, Michigan, said, “I think it makes sense to inform our patients of the labeling and rare but potential adverse effects if they intend to undergo anesthesia for a scheduled procedure/surgery. There is never no risk of aspiration during anesthesia.”
“I find it a bit curious that ASA implies that those who experience GI side effects are more likely than those who do not to have this potential risk. I doubt there is evidence that those without GI side effects are necessarily ‘safer’ and a study to determine that is unlikely to take be conducted.”
“My institution does require a 1-week pause on GLP-1s for those undergoing anesthesia for surgery,” she added. “That’s not evidence-based either, but probably reduces the risk of aspiration during anesthesia — but I don’t know what the actual denominator is for aspiration in those who continued vs those who took a pause from GLP-1s. Pausing does certainly (anecdotally) increase the traffic of communications between physicians and their patients about what to do in the interval.”
Anne Peters, MD, a professor of clinical medicine and a clinical scholar at the Keck School of Medicine of the University of Southern California, Los Angeles, said, “The FDA label change is a warning that really doesn’t say exactly who on GLP-1 RAs is at highest risk or what to do, and if any intervention has been shown to help. The ASA recommendations seem much more nuanced and practical, including point-of-care gastric ultrasound to see if there is retained food/fluid prior to surgery.”
“In my practice, I individualize what I say, depending on the person and the circumstance,” she said. “Mostly, I have people hold one dose before planned surgery, so they have been 10 days at least without a dose. But if worried about gastrointestinal symptoms or gastroparesis, I have them do a clear liquid diet for 24 hours presurgery. Or at least avoid heavy fat meals the day before.”
“There is a risk of aspiration with anything that slows gastric emptying — maybe even in patients with gastroparesis at baseline due to physiologic, not pharmacological, reasons — and anesthesiologists should be aware of the need to assess patients individually.”
Michael A. Weintraub, MD, of NYU Langone Health Diabetes & Endocrine Associates in New York City, observed, “The risk of a pulmonary aspiration event with GLP-1 medication is quite rare, but not zero. On the other hand, stopping the GLP-1 can cause hyperglycemia or rebound weight gain. Furthermore, it can become complicated to restart GLP1 dosing, particularly given the existing medication shortages.”
“In most cases, stopping a weekly GLP-1 medication 1 week prior to the procedure minimizes the risks of pulmonary aspiration and prevents any worsening hyperglycemia or weight gain,” he said. However, taking the drug 7 days prior to the procedure is optimal. “That way, they would be due for the next dose on the day of the procedure, and taking it the day following procedure minimizes disruption in their once-weekly regimen.”
Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, advised that physicians weigh the risk of stopping the medication (which can cause a glycemic spike) vs risk for aspiration.
“In my opinion, all patients should follow a strict liquid diet or NPO status prior to a surgery to further decrease the risk of aspiration,” she said. “I generally hold the GLP-1 RA for a week before a surgery. If additional glycemic control is necessary, I will add to or adjust one of the patient’s other diabetes medications.”
Jaime Almandoz, MD, associate professor of medicine and medical director of the Weight Wellness Program in Dallas, said, “As endocrinologists, we typically rely on our anesthesia colleagues for guidance on perioperative management. In light of emerging guidelines for holding GLP-1 medications, we also recommend patients adopt a liquid diet 24 hours prior to surgery, along with the fasting protocol.”
“For those managing diabetes with GLP-1 therapies, it is crucial to establish a blood sugar management plan while off these medications, especially during fasting or postoperative periods, which can be further influenced by many factors, including nausea, pain medications, and antibiotics after the procedure.”
Joshi added that at Parkland Hospital in Dallas, “we do a huge number of cases using the same information. We identify patients who are at risk, and then we tell our proceduralists and our surgeons if they’re in the escalating phase of the dosing or if they have GI symptoms; don’t even schedule them as an elective case; wait till the escalation phase is over and then schedule them.”
“That way,” he said, “it becomes logistically easy to manage because the recommendation from the group is that patients who are at higher risk should receive a 24-hour liquid diet — the same as colonoscopy. But sometimes it can be challenging to do so.”
Joshi has received honoraria for consultation from Merck Sharp & Dohme, Vertex Pharmaceuticals, and Haisco-USA Pharmaceuticals. Gupta is on the speakers bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie. Almandoz serves on advisory boards for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. The other experts declared no relevant relationships.
A version of this article first appeared on Medscape.com.
, as does the US Food and Drug Administration’s (FDA’s) labeling for the drugs. The changes can be challenging to keep up with, and endocrinologists seem to be making their own decisions based on clinical experience and their interpretations of the potential impact and value of the emerging information.
The latest FDA label change warns about the risk for pulmonary aspiration but notes “insufficient” data to inform recommendations to mitigate the risk in vulnerable patients. Yet, the latest multi-society guidance, led by the American Society of Anesthesiologists (ASA) and based on consensus, not evidence, has nuanced advice for managing patients at risk.
Does the FDA’s label change make a difference regarding the multi-society guidance, which was published earlier? “The answer is no,” Girish Joshi, MD, vice chair, ASA Committee on Practice Parameters, told this news organization. “The concern of increased pulmonary aspiration in patients who are on GLP-1 receptor agonists has been known, and that concern still exists. So, we started with not an assumption but the premise that patients on GLP-1 receptor agonists are at a higher risk of aspiration during sedation, analgesia, and/or general anesthesia. The FDA basically confirms what we say in the guidance.”
Joshi, professor in the Anesthesiology and Pain Management Department at UT Southwestern Medical Center, Dallas, aimed to make the guidance, which was published simultaneously in several society journals, more implementable with a letter to the editor of Anesthesiology. The key, he said, is to identify patients at higher risk for aspiration; all others would follow treatment as usual.
The letter highlights three overarching recommendations and then expands upon them: Standardized preoperative assessment for risk for delayed gastric emptying (yes/no); selective preoperative care plan based on delayed gastric emptying assessment and shared decision-making; and on the day of the procedure, reassess for delayed gastric emptying and mitigate risk if there is clinical concern.
But it seems as though, for now, endocrinologists are managing these patients as they see fit, within the parameters of any institutional guidance requirements. Here is what they said about their practice:
Amy E. Rothberg, MD, DABOM, director of the Weight Management Program & Rewind at the University of Michigan, Ann Arbor, Michigan, said, “I think it makes sense to inform our patients of the labeling and rare but potential adverse effects if they intend to undergo anesthesia for a scheduled procedure/surgery. There is never no risk of aspiration during anesthesia.”
“I find it a bit curious that ASA implies that those who experience GI side effects are more likely than those who do not to have this potential risk. I doubt there is evidence that those without GI side effects are necessarily ‘safer’ and a study to determine that is unlikely to take be conducted.”
“My institution does require a 1-week pause on GLP-1s for those undergoing anesthesia for surgery,” she added. “That’s not evidence-based either, but probably reduces the risk of aspiration during anesthesia — but I don’t know what the actual denominator is for aspiration in those who continued vs those who took a pause from GLP-1s. Pausing does certainly (anecdotally) increase the traffic of communications between physicians and their patients about what to do in the interval.”
Anne Peters, MD, a professor of clinical medicine and a clinical scholar at the Keck School of Medicine of the University of Southern California, Los Angeles, said, “The FDA label change is a warning that really doesn’t say exactly who on GLP-1 RAs is at highest risk or what to do, and if any intervention has been shown to help. The ASA recommendations seem much more nuanced and practical, including point-of-care gastric ultrasound to see if there is retained food/fluid prior to surgery.”
“In my practice, I individualize what I say, depending on the person and the circumstance,” she said. “Mostly, I have people hold one dose before planned surgery, so they have been 10 days at least without a dose. But if worried about gastrointestinal symptoms or gastroparesis, I have them do a clear liquid diet for 24 hours presurgery. Or at least avoid heavy fat meals the day before.”
“There is a risk of aspiration with anything that slows gastric emptying — maybe even in patients with gastroparesis at baseline due to physiologic, not pharmacological, reasons — and anesthesiologists should be aware of the need to assess patients individually.”
Michael A. Weintraub, MD, of NYU Langone Health Diabetes & Endocrine Associates in New York City, observed, “The risk of a pulmonary aspiration event with GLP-1 medication is quite rare, but not zero. On the other hand, stopping the GLP-1 can cause hyperglycemia or rebound weight gain. Furthermore, it can become complicated to restart GLP1 dosing, particularly given the existing medication shortages.”
“In most cases, stopping a weekly GLP-1 medication 1 week prior to the procedure minimizes the risks of pulmonary aspiration and prevents any worsening hyperglycemia or weight gain,” he said. However, taking the drug 7 days prior to the procedure is optimal. “That way, they would be due for the next dose on the day of the procedure, and taking it the day following procedure minimizes disruption in their once-weekly regimen.”
Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, advised that physicians weigh the risk of stopping the medication (which can cause a glycemic spike) vs risk for aspiration.
“In my opinion, all patients should follow a strict liquid diet or NPO status prior to a surgery to further decrease the risk of aspiration,” she said. “I generally hold the GLP-1 RA for a week before a surgery. If additional glycemic control is necessary, I will add to or adjust one of the patient’s other diabetes medications.”
Jaime Almandoz, MD, associate professor of medicine and medical director of the Weight Wellness Program in Dallas, said, “As endocrinologists, we typically rely on our anesthesia colleagues for guidance on perioperative management. In light of emerging guidelines for holding GLP-1 medications, we also recommend patients adopt a liquid diet 24 hours prior to surgery, along with the fasting protocol.”
“For those managing diabetes with GLP-1 therapies, it is crucial to establish a blood sugar management plan while off these medications, especially during fasting or postoperative periods, which can be further influenced by many factors, including nausea, pain medications, and antibiotics after the procedure.”
Joshi added that at Parkland Hospital in Dallas, “we do a huge number of cases using the same information. We identify patients who are at risk, and then we tell our proceduralists and our surgeons if they’re in the escalating phase of the dosing or if they have GI symptoms; don’t even schedule them as an elective case; wait till the escalation phase is over and then schedule them.”
“That way,” he said, “it becomes logistically easy to manage because the recommendation from the group is that patients who are at higher risk should receive a 24-hour liquid diet — the same as colonoscopy. But sometimes it can be challenging to do so.”
Joshi has received honoraria for consultation from Merck Sharp & Dohme, Vertex Pharmaceuticals, and Haisco-USA Pharmaceuticals. Gupta is on the speakers bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie. Almandoz serves on advisory boards for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. The other experts declared no relevant relationships.
A version of this article first appeared on Medscape.com.
Continuous Glucose Monitors for All? Opinions Remain Mixed
The recent US Food and Drug Administration (FDA) clearance of two over-the-counter (OTC) continuous glucose monitors (CGMs) — Dexcom’s Stelo and Abbott’s Lingo — has sparked interest in potentially expanding their use to those without diabetes or prediabetes.
There are several valid questions about how the general population might benefit from CGMs. Can they motivate those struggling with overweight to shed pounds? Would they prompt users to follow more healthful eating patterns? Can they act as a canary in the coal mine, alerting users to prediabetes?
The short answer to these questions is, we don’t know.
“Glucose levels fluctuate in everyone in response to meals, exercise, stress, etc, but there has been no credible research to support CGM use by most people who do not have diabetes,” Jill Crandall, MD, chief of endocrinology at Albert Einstein College of Medicine and Montefiore Health System in New York City, said in an interview.
“The utility of CGM for people without diabetes hasn’t been established and the drive to market CGM as an OTC device seems largely driven by financial considerations,” Crandall said. She advocates instead for a strategy directed at more meaningful objectives.
“For now, efforts should be focused on making CGMs available to patients who will clearly benefit — ie, people with diabetes, especially those who are using insulin and those who are struggling to achieve desired levels of glucose control.”
Nicole Spartano, PhD, assistant professor of medicine in endocrinology, diabetes, nutrition and weight management at Boston University’s Chobanian & Avedisian School of Medicine in Massachusetts, agreed with this assessment.
“It is definitely too early to make recommendations for patients without diabetes based on their CGM data,” said Spartano, who also serves as the director of the Glucose Monitoring Station at the Framingham Heart Study in Framingham, Massachusetts. “We simply do not have enough follow-up data to tell us which CGM metrics are associated with higher risk for disease.”
Spartano served as the lead author of a recent study showing time spent in various CGM ranges in a large cohort of individuals without diabetes using the Dexcom G6 Pro model. In the future, she said the data may be used to establish reference ranges for clinicians and individuals.
“We are working on another paper surveying diabetologists and CGM experts about how they interpret CGM reports from individuals without diabetes,” she said in an interview. Although the data are not yet published, Spartano said, “we are finding that clinicians are currently very discordant in how they interpret these reports.”
Potential Benefits Right Now
Satish Garg, MD, director of the Adult Clinic at the Barbara Davis Center for Diabetes at the University of Colorado Anschutz Medical Campus, Aurora, and editor-in-chief of Diabetes Technology & Therapeutics, is convinced that glucose should be considered another vital sign, like blood pressure, pulse rate, respiration rate, and body temperature. Therefore, he sees the use of a CGM in people without diabetes as a way to build awareness and perhaps prompt behavior modification.
“Someone with an A1c of 4.9 on a normal day may notice that they’ve gained a little bit of weight, and if they use an OTC CGM and start seeing changes, it might help them to modulate their diet themselves, whether they see a dietitian or not,” Garg said.
He gave the example of “a natural behavioral change” occurring when someone using a CGM declines to eat a post-meal dessert after seeing their blood glucose had already risen to 170.
Wearing a CGM also has the potential to alert the user to high blood glucose, leading them to an earlier diagnosis of prediabetes or diabetes, Shichun Bao, MD, PhD, Diabetes Technology Program Leader at the Vanderbilt Eskind Diabetes Clinic of Vanderbilt University in Nashville, Tennessee, said in an interview. She has had cases where a family member of someone with diabetes used the patient’s fingerstick meter, found that their glucose was 280, and self-diagnosed with diabetes.
“It’s the same thing with the CGM,” she said. “If they somehow did not know they have diabetes and they wear a CGM and it shows their sugar is high, that will help them to know to see their provider to get a diagnosis, get treated, and track progression.”
Given the shortage of endocrinologists and long waits for appointments in the United States and elsewhere, it is very likely that primary care physicians will be the ones fielding questions from individuals without diabetes interested in purchasing an OTC CGM. Internist Douglas Paauw, MD, a professor at the University of Washington School of Medicine, Seattle, said in an interview that, for his practice, “the benefits outweigh some of the limitations.”
“I don’t really think somebody who doesn’t have diabetes needs to be using a CGM all the time or long term,” he said. “But I have used it in a few people without diabetes, and I think if someone can afford to use it for 2-4 weeks, especially if they’ve been gaining weight, then they can really recognize what happens to their bodies when they eat certain foods.”
Paauw added that CGMs are a more effective means of teaching his patients than them receiving a lecture from him on healthy eating. “There’s nothing like immediate feedback on what happens to your body to change behavior.”
Similarly, William Golden, medical director at Arkansas Medicaid and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview that “it is difficult to justify coverage for CGMs on demand — but if people want to invest in their own devices and the technology motivates them to eat better and/or lose weight, then there are benefits to be had.”
Potential Downsides
Although it may seem simple to use an OTC CGM to measure blood glucose on the fly, in the real world it can take patients time to understand these devices, “especially the first day or so, when users are going to get false lows,” Bao said. “Clinicians need to tell them if you don’t feel like your sugar is low and the device says it’s low, whether they do or don’t have diabetes, they should do a fingerstick glucose test to confirm the low before rushing to take in sugar. On the other hand, if they drink a lot of juice, their sugar will go high. So, it can create problems and false results either way.”
Many factors affect glucose, she said. “When you’re sick, glucose can go high, and when you’re very sick, in the ICU, sometimes it can be low. It depends on the situation.” Bao noted that certain vitamins and drugs can also interfere with readings.
Bao doesn’t see value in having people without diabetes monitor their glucose continuously. “If they want to see what foods or exercise do to their body, they will probably benefit from a short trial to gain some insight; otherwise, they’re wasting money,” she said.
Another potential downside is that there’s no head-to-head comparison data with the approved devices, Garg said. “But it’s clear to us that Stelo’s range is very narrow, 70 to 200, whereas the Lingo ranges are pretty much full, from 40 to 400 or 55 to 400. So, we don’t know the accuracy of these sensors.”
Golden observed that for certain patients, CGMs may lead to psychological distress rather than providing a sense of control over their blood glucose levels.
“I have had a nondiabetic patient or two that obsessed about their blood sugars and a device would only magnify their anxiety/neurosis,” he said. “The bottom line is that it’s a tool for a balanced approach to health management, but the daily results must be kept in perspective!”
Educate Patients, Primary Care Physicians
To maximize potential benefits for patients without diabetes, clinicians need to be well trained in the use and interpretation of results from the devices, Bao said. They can then better educate their patients, including discussing with them possible pitfalls surrounding their use.
“For example, a patient may see that their blood glucose, as measured by a fingerstick, is 95, whereas the CGM says 140, and ask, ‘Which one do I trust?’ ”
This is where the patient can be educated about the difference between interstitial glucose, as measured by the CGM, and blood glucose, as measured by the fingerstick. Because it takes about 15 minutes for blood glucose to get to the interstitial tissue, there’s lag time, and the two measurements will differ.
“A discrepancy of 20% is totally acceptable for that reason,” Bao said.
She has also seen several examples where patients were misled by their CGM when its censor became dislodged.
“Sometimes when a sensor has moved, the patient may push it back in because they don’t want to throw it away. But it doesn’t work that way, and they end up with inaccurate readings.”
At a minimum, Bao added, clinicians and patients should read the package insert but also be aware that it doesn’t list everything that might go wrong or interfere with the device’s accuracy.
Manufacturers of OTC devices should be training primary care and family practice doctors in their use, given the expected “huge” influx of patients wanting to use them, according to Garg.
“If you are expecting endos or diabetes specialists to see these people, that’s never going to happen,” he said. “We have a big shortage of these specialists, so industry has to train these doctors. Patients will bring their doctor’s data, and the clinicians need to learn the basics of how to interpret the glucose values they see. Then they can treat these patients rather than shipping all of them to endos who likely are not available.”
Paauw agreed that CGM training should be directed largely toward primary care professionals, who can help their under-resourced endocrinologist colleagues from seeing an uptick in “the worried well.”
“The bottom line is that primary care professionals do need to understand the CGM,” he said. “They do need to get comfortable with it. They do need to come up with opinions on how to use it. The public’s going to be using it, and we need to be competent in it and use our subspecialists appropriately.”
Spartano received funding for an investigator-initiated research grant from Novo Nordisk unrelated to the cited CGM studies. Garg , Bao, Paauw, Golden, and Crandall declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The recent US Food and Drug Administration (FDA) clearance of two over-the-counter (OTC) continuous glucose monitors (CGMs) — Dexcom’s Stelo and Abbott’s Lingo — has sparked interest in potentially expanding their use to those without diabetes or prediabetes.
There are several valid questions about how the general population might benefit from CGMs. Can they motivate those struggling with overweight to shed pounds? Would they prompt users to follow more healthful eating patterns? Can they act as a canary in the coal mine, alerting users to prediabetes?
The short answer to these questions is, we don’t know.
“Glucose levels fluctuate in everyone in response to meals, exercise, stress, etc, but there has been no credible research to support CGM use by most people who do not have diabetes,” Jill Crandall, MD, chief of endocrinology at Albert Einstein College of Medicine and Montefiore Health System in New York City, said in an interview.
“The utility of CGM for people without diabetes hasn’t been established and the drive to market CGM as an OTC device seems largely driven by financial considerations,” Crandall said. She advocates instead for a strategy directed at more meaningful objectives.
“For now, efforts should be focused on making CGMs available to patients who will clearly benefit — ie, people with diabetes, especially those who are using insulin and those who are struggling to achieve desired levels of glucose control.”
Nicole Spartano, PhD, assistant professor of medicine in endocrinology, diabetes, nutrition and weight management at Boston University’s Chobanian & Avedisian School of Medicine in Massachusetts, agreed with this assessment.
“It is definitely too early to make recommendations for patients without diabetes based on their CGM data,” said Spartano, who also serves as the director of the Glucose Monitoring Station at the Framingham Heart Study in Framingham, Massachusetts. “We simply do not have enough follow-up data to tell us which CGM metrics are associated with higher risk for disease.”
Spartano served as the lead author of a recent study showing time spent in various CGM ranges in a large cohort of individuals without diabetes using the Dexcom G6 Pro model. In the future, she said the data may be used to establish reference ranges for clinicians and individuals.
“We are working on another paper surveying diabetologists and CGM experts about how they interpret CGM reports from individuals without diabetes,” she said in an interview. Although the data are not yet published, Spartano said, “we are finding that clinicians are currently very discordant in how they interpret these reports.”
Potential Benefits Right Now
Satish Garg, MD, director of the Adult Clinic at the Barbara Davis Center for Diabetes at the University of Colorado Anschutz Medical Campus, Aurora, and editor-in-chief of Diabetes Technology & Therapeutics, is convinced that glucose should be considered another vital sign, like blood pressure, pulse rate, respiration rate, and body temperature. Therefore, he sees the use of a CGM in people without diabetes as a way to build awareness and perhaps prompt behavior modification.
“Someone with an A1c of 4.9 on a normal day may notice that they’ve gained a little bit of weight, and if they use an OTC CGM and start seeing changes, it might help them to modulate their diet themselves, whether they see a dietitian or not,” Garg said.
He gave the example of “a natural behavioral change” occurring when someone using a CGM declines to eat a post-meal dessert after seeing their blood glucose had already risen to 170.
Wearing a CGM also has the potential to alert the user to high blood glucose, leading them to an earlier diagnosis of prediabetes or diabetes, Shichun Bao, MD, PhD, Diabetes Technology Program Leader at the Vanderbilt Eskind Diabetes Clinic of Vanderbilt University in Nashville, Tennessee, said in an interview. She has had cases where a family member of someone with diabetes used the patient’s fingerstick meter, found that their glucose was 280, and self-diagnosed with diabetes.
“It’s the same thing with the CGM,” she said. “If they somehow did not know they have diabetes and they wear a CGM and it shows their sugar is high, that will help them to know to see their provider to get a diagnosis, get treated, and track progression.”
Given the shortage of endocrinologists and long waits for appointments in the United States and elsewhere, it is very likely that primary care physicians will be the ones fielding questions from individuals without diabetes interested in purchasing an OTC CGM. Internist Douglas Paauw, MD, a professor at the University of Washington School of Medicine, Seattle, said in an interview that, for his practice, “the benefits outweigh some of the limitations.”
“I don’t really think somebody who doesn’t have diabetes needs to be using a CGM all the time or long term,” he said. “But I have used it in a few people without diabetes, and I think if someone can afford to use it for 2-4 weeks, especially if they’ve been gaining weight, then they can really recognize what happens to their bodies when they eat certain foods.”
Paauw added that CGMs are a more effective means of teaching his patients than them receiving a lecture from him on healthy eating. “There’s nothing like immediate feedback on what happens to your body to change behavior.”
Similarly, William Golden, medical director at Arkansas Medicaid and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview that “it is difficult to justify coverage for CGMs on demand — but if people want to invest in their own devices and the technology motivates them to eat better and/or lose weight, then there are benefits to be had.”
Potential Downsides
Although it may seem simple to use an OTC CGM to measure blood glucose on the fly, in the real world it can take patients time to understand these devices, “especially the first day or so, when users are going to get false lows,” Bao said. “Clinicians need to tell them if you don’t feel like your sugar is low and the device says it’s low, whether they do or don’t have diabetes, they should do a fingerstick glucose test to confirm the low before rushing to take in sugar. On the other hand, if they drink a lot of juice, their sugar will go high. So, it can create problems and false results either way.”
Many factors affect glucose, she said. “When you’re sick, glucose can go high, and when you’re very sick, in the ICU, sometimes it can be low. It depends on the situation.” Bao noted that certain vitamins and drugs can also interfere with readings.
Bao doesn’t see value in having people without diabetes monitor their glucose continuously. “If they want to see what foods or exercise do to their body, they will probably benefit from a short trial to gain some insight; otherwise, they’re wasting money,” she said.
Another potential downside is that there’s no head-to-head comparison data with the approved devices, Garg said. “But it’s clear to us that Stelo’s range is very narrow, 70 to 200, whereas the Lingo ranges are pretty much full, from 40 to 400 or 55 to 400. So, we don’t know the accuracy of these sensors.”
Golden observed that for certain patients, CGMs may lead to psychological distress rather than providing a sense of control over their blood glucose levels.
“I have had a nondiabetic patient or two that obsessed about their blood sugars and a device would only magnify their anxiety/neurosis,” he said. “The bottom line is that it’s a tool for a balanced approach to health management, but the daily results must be kept in perspective!”
Educate Patients, Primary Care Physicians
To maximize potential benefits for patients without diabetes, clinicians need to be well trained in the use and interpretation of results from the devices, Bao said. They can then better educate their patients, including discussing with them possible pitfalls surrounding their use.
“For example, a patient may see that their blood glucose, as measured by a fingerstick, is 95, whereas the CGM says 140, and ask, ‘Which one do I trust?’ ”
This is where the patient can be educated about the difference between interstitial glucose, as measured by the CGM, and blood glucose, as measured by the fingerstick. Because it takes about 15 minutes for blood glucose to get to the interstitial tissue, there’s lag time, and the two measurements will differ.
“A discrepancy of 20% is totally acceptable for that reason,” Bao said.
She has also seen several examples where patients were misled by their CGM when its censor became dislodged.
“Sometimes when a sensor has moved, the patient may push it back in because they don’t want to throw it away. But it doesn’t work that way, and they end up with inaccurate readings.”
At a minimum, Bao added, clinicians and patients should read the package insert but also be aware that it doesn’t list everything that might go wrong or interfere with the device’s accuracy.
Manufacturers of OTC devices should be training primary care and family practice doctors in their use, given the expected “huge” influx of patients wanting to use them, according to Garg.
“If you are expecting endos or diabetes specialists to see these people, that’s never going to happen,” he said. “We have a big shortage of these specialists, so industry has to train these doctors. Patients will bring their doctor’s data, and the clinicians need to learn the basics of how to interpret the glucose values they see. Then they can treat these patients rather than shipping all of them to endos who likely are not available.”
Paauw agreed that CGM training should be directed largely toward primary care professionals, who can help their under-resourced endocrinologist colleagues from seeing an uptick in “the worried well.”
“The bottom line is that primary care professionals do need to understand the CGM,” he said. “They do need to get comfortable with it. They do need to come up with opinions on how to use it. The public’s going to be using it, and we need to be competent in it and use our subspecialists appropriately.”
Spartano received funding for an investigator-initiated research grant from Novo Nordisk unrelated to the cited CGM studies. Garg , Bao, Paauw, Golden, and Crandall declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The recent US Food and Drug Administration (FDA) clearance of two over-the-counter (OTC) continuous glucose monitors (CGMs) — Dexcom’s Stelo and Abbott’s Lingo — has sparked interest in potentially expanding their use to those without diabetes or prediabetes.
There are several valid questions about how the general population might benefit from CGMs. Can they motivate those struggling with overweight to shed pounds? Would they prompt users to follow more healthful eating patterns? Can they act as a canary in the coal mine, alerting users to prediabetes?
The short answer to these questions is, we don’t know.
“Glucose levels fluctuate in everyone in response to meals, exercise, stress, etc, but there has been no credible research to support CGM use by most people who do not have diabetes,” Jill Crandall, MD, chief of endocrinology at Albert Einstein College of Medicine and Montefiore Health System in New York City, said in an interview.
“The utility of CGM for people without diabetes hasn’t been established and the drive to market CGM as an OTC device seems largely driven by financial considerations,” Crandall said. She advocates instead for a strategy directed at more meaningful objectives.
“For now, efforts should be focused on making CGMs available to patients who will clearly benefit — ie, people with diabetes, especially those who are using insulin and those who are struggling to achieve desired levels of glucose control.”
Nicole Spartano, PhD, assistant professor of medicine in endocrinology, diabetes, nutrition and weight management at Boston University’s Chobanian & Avedisian School of Medicine in Massachusetts, agreed with this assessment.
“It is definitely too early to make recommendations for patients without diabetes based on their CGM data,” said Spartano, who also serves as the director of the Glucose Monitoring Station at the Framingham Heart Study in Framingham, Massachusetts. “We simply do not have enough follow-up data to tell us which CGM metrics are associated with higher risk for disease.”
Spartano served as the lead author of a recent study showing time spent in various CGM ranges in a large cohort of individuals without diabetes using the Dexcom G6 Pro model. In the future, she said the data may be used to establish reference ranges for clinicians and individuals.
“We are working on another paper surveying diabetologists and CGM experts about how they interpret CGM reports from individuals without diabetes,” she said in an interview. Although the data are not yet published, Spartano said, “we are finding that clinicians are currently very discordant in how they interpret these reports.”
Potential Benefits Right Now
Satish Garg, MD, director of the Adult Clinic at the Barbara Davis Center for Diabetes at the University of Colorado Anschutz Medical Campus, Aurora, and editor-in-chief of Diabetes Technology & Therapeutics, is convinced that glucose should be considered another vital sign, like blood pressure, pulse rate, respiration rate, and body temperature. Therefore, he sees the use of a CGM in people without diabetes as a way to build awareness and perhaps prompt behavior modification.
“Someone with an A1c of 4.9 on a normal day may notice that they’ve gained a little bit of weight, and if they use an OTC CGM and start seeing changes, it might help them to modulate their diet themselves, whether they see a dietitian or not,” Garg said.
He gave the example of “a natural behavioral change” occurring when someone using a CGM declines to eat a post-meal dessert after seeing their blood glucose had already risen to 170.
Wearing a CGM also has the potential to alert the user to high blood glucose, leading them to an earlier diagnosis of prediabetes or diabetes, Shichun Bao, MD, PhD, Diabetes Technology Program Leader at the Vanderbilt Eskind Diabetes Clinic of Vanderbilt University in Nashville, Tennessee, said in an interview. She has had cases where a family member of someone with diabetes used the patient’s fingerstick meter, found that their glucose was 280, and self-diagnosed with diabetes.
“It’s the same thing with the CGM,” she said. “If they somehow did not know they have diabetes and they wear a CGM and it shows their sugar is high, that will help them to know to see their provider to get a diagnosis, get treated, and track progression.”
Given the shortage of endocrinologists and long waits for appointments in the United States and elsewhere, it is very likely that primary care physicians will be the ones fielding questions from individuals without diabetes interested in purchasing an OTC CGM. Internist Douglas Paauw, MD, a professor at the University of Washington School of Medicine, Seattle, said in an interview that, for his practice, “the benefits outweigh some of the limitations.”
“I don’t really think somebody who doesn’t have diabetes needs to be using a CGM all the time or long term,” he said. “But I have used it in a few people without diabetes, and I think if someone can afford to use it for 2-4 weeks, especially if they’ve been gaining weight, then they can really recognize what happens to their bodies when they eat certain foods.”
Paauw added that CGMs are a more effective means of teaching his patients than them receiving a lecture from him on healthy eating. “There’s nothing like immediate feedback on what happens to your body to change behavior.”
Similarly, William Golden, medical director at Arkansas Medicaid and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview that “it is difficult to justify coverage for CGMs on demand — but if people want to invest in their own devices and the technology motivates them to eat better and/or lose weight, then there are benefits to be had.”
Potential Downsides
Although it may seem simple to use an OTC CGM to measure blood glucose on the fly, in the real world it can take patients time to understand these devices, “especially the first day or so, when users are going to get false lows,” Bao said. “Clinicians need to tell them if you don’t feel like your sugar is low and the device says it’s low, whether they do or don’t have diabetes, they should do a fingerstick glucose test to confirm the low before rushing to take in sugar. On the other hand, if they drink a lot of juice, their sugar will go high. So, it can create problems and false results either way.”
Many factors affect glucose, she said. “When you’re sick, glucose can go high, and when you’re very sick, in the ICU, sometimes it can be low. It depends on the situation.” Bao noted that certain vitamins and drugs can also interfere with readings.
Bao doesn’t see value in having people without diabetes monitor their glucose continuously. “If they want to see what foods or exercise do to their body, they will probably benefit from a short trial to gain some insight; otherwise, they’re wasting money,” she said.
Another potential downside is that there’s no head-to-head comparison data with the approved devices, Garg said. “But it’s clear to us that Stelo’s range is very narrow, 70 to 200, whereas the Lingo ranges are pretty much full, from 40 to 400 or 55 to 400. So, we don’t know the accuracy of these sensors.”
Golden observed that for certain patients, CGMs may lead to psychological distress rather than providing a sense of control over their blood glucose levels.
“I have had a nondiabetic patient or two that obsessed about their blood sugars and a device would only magnify their anxiety/neurosis,” he said. “The bottom line is that it’s a tool for a balanced approach to health management, but the daily results must be kept in perspective!”
Educate Patients, Primary Care Physicians
To maximize potential benefits for patients without diabetes, clinicians need to be well trained in the use and interpretation of results from the devices, Bao said. They can then better educate their patients, including discussing with them possible pitfalls surrounding their use.
“For example, a patient may see that their blood glucose, as measured by a fingerstick, is 95, whereas the CGM says 140, and ask, ‘Which one do I trust?’ ”
This is where the patient can be educated about the difference between interstitial glucose, as measured by the CGM, and blood glucose, as measured by the fingerstick. Because it takes about 15 minutes for blood glucose to get to the interstitial tissue, there’s lag time, and the two measurements will differ.
“A discrepancy of 20% is totally acceptable for that reason,” Bao said.
She has also seen several examples where patients were misled by their CGM when its censor became dislodged.
“Sometimes when a sensor has moved, the patient may push it back in because they don’t want to throw it away. But it doesn’t work that way, and they end up with inaccurate readings.”
At a minimum, Bao added, clinicians and patients should read the package insert but also be aware that it doesn’t list everything that might go wrong or interfere with the device’s accuracy.
Manufacturers of OTC devices should be training primary care and family practice doctors in their use, given the expected “huge” influx of patients wanting to use them, according to Garg.
“If you are expecting endos or diabetes specialists to see these people, that’s never going to happen,” he said. “We have a big shortage of these specialists, so industry has to train these doctors. Patients will bring their doctor’s data, and the clinicians need to learn the basics of how to interpret the glucose values they see. Then they can treat these patients rather than shipping all of them to endos who likely are not available.”
Paauw agreed that CGM training should be directed largely toward primary care professionals, who can help their under-resourced endocrinologist colleagues from seeing an uptick in “the worried well.”
“The bottom line is that primary care professionals do need to understand the CGM,” he said. “They do need to get comfortable with it. They do need to come up with opinions on how to use it. The public’s going to be using it, and we need to be competent in it and use our subspecialists appropriately.”
Spartano received funding for an investigator-initiated research grant from Novo Nordisk unrelated to the cited CGM studies. Garg , Bao, Paauw, Golden, and Crandall declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.