User login
AGA Clinical Practice Update: P-CABs Can Help When PPI Therapy Fails
, according to a recent clinical practice update from the American Gastroenterological Association (AGA).
However, P-CABs are recommended in place of proton pump inhibitors (PPIs) for most patients with Helicobacter pylori and other conditions where patients haven’t responded to PPIs.
“P-CABs are a newer medication class now available in the US, associated with more rapid, potent, and prolonged gastric acid inhibition than PPI formulations,” said lead author Amit Patel, MD, a gastroenterologist at the Duke University School of Medicine and Durham Veterans Affairs Medical Center, Durham, North Carolina.
“P-CABs have potentially significant clinical benefits in the management of Helicobacter pylori infection and GERD, particularly more severe erosive esophagitis,” he said. “Emerging data are affording additional insights into the clinical benefits of P-CABs in settings such as on-demand therapy for reflux-associated symptoms, bleeding gastroduodenal ulcers, and endoscopic eradication therapy for Barrett’s esophagus.”
The update was published in Gastroenterology .
P-CAB Developments
For most patients, PPIs and histamine-2 receptor antagonists remain the primary way to inhibit gastric acid secretion for common upper gastrointestinal conditions, the authors wrote. However, P-CABs such as vonoprazan and tegoprazan may provide relief when PPIs have limitations.
Unlike PPIs, P-CABs are considered acid-stable, don’t require premeal dosing, aren’t prodrugs, and don’t require conversion to an active form to provide pharmacologic effects. They tend to have longer half-lives and more rapid onset. Serum gastrin levels typically remain higher with P-CABs.
In terms of safety, randomized trial data indicate that P-CABs are generally well tolerated and have short-term and medium-term safety similar to PPIs. Due to potent acid suppression, enteric infection risks remain higher, though long-term safety data is needed, the authors wrote.
Overall, P-CABs appear to be equally as potent or more potent than PPIs, though more potent acid inhibition isn’t necessarily associated with better outcomes, the authors wrote. For most foregut acid-related disorders — such as heartburn and prevention of nonsteroidal anti-inflammatory drug–associated ulcers — P-CABs can help when patients fail PPI therapy.
In general, though, nonclinical factors related to cost, barriers to obtaining medication, and limited long-term safety data may outweigh the advantages of P-CABs, especially if clinical superiority isn’t yet known, the authors wrote.
For GERD, clinicians generally shouldn’t use P-CABs as first-line therapy for patients with uninvestigated heartburn symptoms or nonerosive reflux disease. However, P-CABs should be used for those with documented acid-related reflux who fail therapy with twice-daily PPIs. They may also be appropriate for on-demand heartburn therapy, although more evidence is needed.
For erosive esophagitis, P-CABs generally shouldn’t be used for milder cases but can be considered for patients with more severe cases that haven’t responded to PPIs, including refractory esophagitis.
For H pylori, P-CABs should be used in place of PPIs for eradication regimens, including among patients with clarithromycin-resistant strains. In contrast with most of the other indications in the update, the short-term duration of H pylori treatment reduced the authors’ concerns about P-CAB costs and safety.
For peptic ulcer disease, P-CABs generally shouldn’t be used as first-line treatment or prophylaxis. However, the rapid onset and potent acid inhibition could be useful for patients with bleeding gastroduodenal ulcers and high-risk stigmata.
“Emerging data will allow refinements in the populations and clinical settings for which P-CABs at various doses may be considered and advised — and may reveal more clinical scenarios in which they can provide meaningful benefit,” Patel said. “Further investigations, including additional populations and novel indicators, as well as evaluating long-term safety data and cost-effectiveness, are warranted, as P-CABs are incorporated more broadly into clinical practice worldwide.”
The authors received no specific funding for this update. Patel reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a recent clinical practice update from the American Gastroenterological Association (AGA).
However, P-CABs are recommended in place of proton pump inhibitors (PPIs) for most patients with Helicobacter pylori and other conditions where patients haven’t responded to PPIs.
“P-CABs are a newer medication class now available in the US, associated with more rapid, potent, and prolonged gastric acid inhibition than PPI formulations,” said lead author Amit Patel, MD, a gastroenterologist at the Duke University School of Medicine and Durham Veterans Affairs Medical Center, Durham, North Carolina.
“P-CABs have potentially significant clinical benefits in the management of Helicobacter pylori infection and GERD, particularly more severe erosive esophagitis,” he said. “Emerging data are affording additional insights into the clinical benefits of P-CABs in settings such as on-demand therapy for reflux-associated symptoms, bleeding gastroduodenal ulcers, and endoscopic eradication therapy for Barrett’s esophagus.”
The update was published in Gastroenterology .
P-CAB Developments
For most patients, PPIs and histamine-2 receptor antagonists remain the primary way to inhibit gastric acid secretion for common upper gastrointestinal conditions, the authors wrote. However, P-CABs such as vonoprazan and tegoprazan may provide relief when PPIs have limitations.
Unlike PPIs, P-CABs are considered acid-stable, don’t require premeal dosing, aren’t prodrugs, and don’t require conversion to an active form to provide pharmacologic effects. They tend to have longer half-lives and more rapid onset. Serum gastrin levels typically remain higher with P-CABs.
In terms of safety, randomized trial data indicate that P-CABs are generally well tolerated and have short-term and medium-term safety similar to PPIs. Due to potent acid suppression, enteric infection risks remain higher, though long-term safety data is needed, the authors wrote.
Overall, P-CABs appear to be equally as potent or more potent than PPIs, though more potent acid inhibition isn’t necessarily associated with better outcomes, the authors wrote. For most foregut acid-related disorders — such as heartburn and prevention of nonsteroidal anti-inflammatory drug–associated ulcers — P-CABs can help when patients fail PPI therapy.
In general, though, nonclinical factors related to cost, barriers to obtaining medication, and limited long-term safety data may outweigh the advantages of P-CABs, especially if clinical superiority isn’t yet known, the authors wrote.
For GERD, clinicians generally shouldn’t use P-CABs as first-line therapy for patients with uninvestigated heartburn symptoms or nonerosive reflux disease. However, P-CABs should be used for those with documented acid-related reflux who fail therapy with twice-daily PPIs. They may also be appropriate for on-demand heartburn therapy, although more evidence is needed.
For erosive esophagitis, P-CABs generally shouldn’t be used for milder cases but can be considered for patients with more severe cases that haven’t responded to PPIs, including refractory esophagitis.
For H pylori, P-CABs should be used in place of PPIs for eradication regimens, including among patients with clarithromycin-resistant strains. In contrast with most of the other indications in the update, the short-term duration of H pylori treatment reduced the authors’ concerns about P-CAB costs and safety.
For peptic ulcer disease, P-CABs generally shouldn’t be used as first-line treatment or prophylaxis. However, the rapid onset and potent acid inhibition could be useful for patients with bleeding gastroduodenal ulcers and high-risk stigmata.
“Emerging data will allow refinements in the populations and clinical settings for which P-CABs at various doses may be considered and advised — and may reveal more clinical scenarios in which they can provide meaningful benefit,” Patel said. “Further investigations, including additional populations and novel indicators, as well as evaluating long-term safety data and cost-effectiveness, are warranted, as P-CABs are incorporated more broadly into clinical practice worldwide.”
The authors received no specific funding for this update. Patel reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a recent clinical practice update from the American Gastroenterological Association (AGA).
However, P-CABs are recommended in place of proton pump inhibitors (PPIs) for most patients with Helicobacter pylori and other conditions where patients haven’t responded to PPIs.
“P-CABs are a newer medication class now available in the US, associated with more rapid, potent, and prolonged gastric acid inhibition than PPI formulations,” said lead author Amit Patel, MD, a gastroenterologist at the Duke University School of Medicine and Durham Veterans Affairs Medical Center, Durham, North Carolina.
“P-CABs have potentially significant clinical benefits in the management of Helicobacter pylori infection and GERD, particularly more severe erosive esophagitis,” he said. “Emerging data are affording additional insights into the clinical benefits of P-CABs in settings such as on-demand therapy for reflux-associated symptoms, bleeding gastroduodenal ulcers, and endoscopic eradication therapy for Barrett’s esophagus.”
The update was published in Gastroenterology .
P-CAB Developments
For most patients, PPIs and histamine-2 receptor antagonists remain the primary way to inhibit gastric acid secretion for common upper gastrointestinal conditions, the authors wrote. However, P-CABs such as vonoprazan and tegoprazan may provide relief when PPIs have limitations.
Unlike PPIs, P-CABs are considered acid-stable, don’t require premeal dosing, aren’t prodrugs, and don’t require conversion to an active form to provide pharmacologic effects. They tend to have longer half-lives and more rapid onset. Serum gastrin levels typically remain higher with P-CABs.
In terms of safety, randomized trial data indicate that P-CABs are generally well tolerated and have short-term and medium-term safety similar to PPIs. Due to potent acid suppression, enteric infection risks remain higher, though long-term safety data is needed, the authors wrote.
Overall, P-CABs appear to be equally as potent or more potent than PPIs, though more potent acid inhibition isn’t necessarily associated with better outcomes, the authors wrote. For most foregut acid-related disorders — such as heartburn and prevention of nonsteroidal anti-inflammatory drug–associated ulcers — P-CABs can help when patients fail PPI therapy.
In general, though, nonclinical factors related to cost, barriers to obtaining medication, and limited long-term safety data may outweigh the advantages of P-CABs, especially if clinical superiority isn’t yet known, the authors wrote.
For GERD, clinicians generally shouldn’t use P-CABs as first-line therapy for patients with uninvestigated heartburn symptoms or nonerosive reflux disease. However, P-CABs should be used for those with documented acid-related reflux who fail therapy with twice-daily PPIs. They may also be appropriate for on-demand heartburn therapy, although more evidence is needed.
For erosive esophagitis, P-CABs generally shouldn’t be used for milder cases but can be considered for patients with more severe cases that haven’t responded to PPIs, including refractory esophagitis.
For H pylori, P-CABs should be used in place of PPIs for eradication regimens, including among patients with clarithromycin-resistant strains. In contrast with most of the other indications in the update, the short-term duration of H pylori treatment reduced the authors’ concerns about P-CAB costs and safety.
For peptic ulcer disease, P-CABs generally shouldn’t be used as first-line treatment or prophylaxis. However, the rapid onset and potent acid inhibition could be useful for patients with bleeding gastroduodenal ulcers and high-risk stigmata.
“Emerging data will allow refinements in the populations and clinical settings for which P-CABs at various doses may be considered and advised — and may reveal more clinical scenarios in which they can provide meaningful benefit,” Patel said. “Further investigations, including additional populations and novel indicators, as well as evaluating long-term safety data and cost-effectiveness, are warranted, as P-CABs are incorporated more broadly into clinical practice worldwide.”
The authors received no specific funding for this update. Patel reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Liver Stiffness Measurement Predicts Long-Term Outcomes In Pediatric Biliary Atresia
according to investigators.
These findings suggest that LSM may serve as a noninvasive tool for risk stratification and treatment planning in this population, reported lead author Jean P. Molleston, MD, of Indiana University School of Medicine, Indianapolis, and colleagues.
“Biliary atresia is frequently complicated by hepatic fibrosis with progression to cirrhosis and portal hypertension manifested by ascites, hepatopulmonary syndrome, and variceal bleeding,” the investigators wrote in Gastroenterology. “The ability to predict these outcomes can inform clinical decision-making.”
To this end, VCTE has been gaining increasing support in the pediatric setting.
“Advantages of VCTE over liver biopsy include convenience, cost, sampling bias, and risk,” the investigators wrote. “VCTE potentially allows (1) fibrosis estimation, (2) prediction of portal hypertension complications/survival, and (3) ability to noninvasively monitor liver stiffness as a fibrosis surrogate.”
The present multicenter study aimed to gauge the prognostic utility of VCTE among 254 patients, aged 21 years or younger, with biliary atresia. All patients had a valid baseline LSM, plus longitudinal clinical and laboratory data drawn from studies by the Childhood Liver Disease Research Network (ChiLDReN). Liver stiffness was assessed noninvasively with FibroScan devices, adhering to protocols that required at least 10 valid measurements and a variability of less than 30%.
The primary outcomes were survival with native liver (SNL), defined as the time to liver transplantation or death, and a composite measure of liver-related events, including the first occurrence of transplantation, death, ascites, variceal bleeding, or hepatopulmonary syndrome. Secondary outcomes focused on the trajectory of platelet decline, a marker of disease progression. The study also explored the relationship between baseline LSM and conventional biomarkers, including platelet count, albumin, and bilirubin.
LSM was a strong predictor of long-term outcomes. Specifically, Kaplan-Meier analysis showed significant differences in 5-year SNL across LSM strata (P < .001). Children with LSM values less than 10 kPa had excellent 5-year SNL rates (LSM 10 to < 15 kPa, 88.9%; 95% CI, 75.1-95.3%), while those with LSM of at least 15 kPa exhibited substantially lower 5-year SNL (58.9%; 95% CI, 46.0-69.7%).
Similarly, event-free survival (EFS) rates declined as LSM values increased (P < .001). Participants with LSM less than 10 kPa had a 5-year EFS rate of 92.2% versus with 61.2% for those with LSM of at least 15 kPa.
LSM also predicted platelet decline. For every twofold increase in baseline LSM, platelet counts declined by an additional 4,000/mm3 per year (P < .001). This association was illustrated through predicted trajectories for participants with LSM values of 4, 7, 12, 18, and 42 kPa, corresponding to different percentiles of disease severity.
Cox proportional hazards analysis indicated that a two-fold increase in LSM was associated with a hazard ratio of 3.3 (P < .001) for liver transplant or death. While LSM had good discrimination on its own (C statistic = 0.83), it did not significantly improve predictive accuracy when added to models based on platelet count, albumin, and bilirubin.
“This noninvasive measurement could potentially be used to predict natural history, stratify patients for clinical trials, plan interventions, and provide anticipatory guidance,” Molleston and colleagues concluded. This study was supported by grants from the National Institute of Diabetes, Digestive and Kidney Diseases; National Institutes of Health; Childhood Liver Disease Research Network; and others. The investigators disclosed no conflicts of interest.
Grading liver stiffness using elastography is a widely utilized tool in adult populations, and its application is expanding in pediatric hepatology clinics. Clinicians incorporate liver stiffness measurements (LSM) alongside clinical findings and biochemical markers to noninvasively assess the degree of hepatic fibrosis and cirrhosis. Molleston and colleagues leveraged the robust data from the National Institute of Diabetes and Digestive and Kidney Diseases–supported network ChiLDReN and found that LSM in children with biliary atresia (BA) correlate with the progression to complications associated with portal hypertension and liver transplantation. While these findings are not unexpected, this compelling investigation accomplishes the important function of validating the utility of elastography in this cohort.
Prognosticating the timeline of complications stemming from biliary atresia is a central tenet of pediatric hepatology. Helping families understand what the future may hold for their child is critical in fostering long-term relationships between clinicians and caregivers. Furthermore, establishing clear expectations regarding follow-up care and monitoring is beneficial for both providers and patients. Of particular importance is minimizing the need for invasive procedures, such as liver biopsy, which, while relatively safe, remains burdensome and is rarely used to assess fibrosis in BA.
Pediatric hepatologists already consider multiple factors — including age at hepatoportoenterostomy, subsequent clearance of cholestasis, exam findings such as splenomegaly, and platelet count — to predict the clinical course of infants with BA. The addition of a data-driven approach to interpreting liver stiffness measurements represents a valuable new tool in this expanding repertoire, offering an encouraging prospect for both providers and families navigating the complexities of pediatric liver disease.
Aaron Bennett, MD, is a fellow in the Division of Gastroenterology, Hepatology and Nutrition at Children’s Hospital of Philadelphia in Pennsylvania. Elizabeth B. Rand, MD, is the medical director of the Liver Transplant Program, director of the Gastroenterology Fellowship Program, and director of the Advanced Transplant Hepatology Program at Children’s Hospital of Philadelphia.
Grading liver stiffness using elastography is a widely utilized tool in adult populations, and its application is expanding in pediatric hepatology clinics. Clinicians incorporate liver stiffness measurements (LSM) alongside clinical findings and biochemical markers to noninvasively assess the degree of hepatic fibrosis and cirrhosis. Molleston and colleagues leveraged the robust data from the National Institute of Diabetes and Digestive and Kidney Diseases–supported network ChiLDReN and found that LSM in children with biliary atresia (BA) correlate with the progression to complications associated with portal hypertension and liver transplantation. While these findings are not unexpected, this compelling investigation accomplishes the important function of validating the utility of elastography in this cohort.
Prognosticating the timeline of complications stemming from biliary atresia is a central tenet of pediatric hepatology. Helping families understand what the future may hold for their child is critical in fostering long-term relationships between clinicians and caregivers. Furthermore, establishing clear expectations regarding follow-up care and monitoring is beneficial for both providers and patients. Of particular importance is minimizing the need for invasive procedures, such as liver biopsy, which, while relatively safe, remains burdensome and is rarely used to assess fibrosis in BA.
Pediatric hepatologists already consider multiple factors — including age at hepatoportoenterostomy, subsequent clearance of cholestasis, exam findings such as splenomegaly, and platelet count — to predict the clinical course of infants with BA. The addition of a data-driven approach to interpreting liver stiffness measurements represents a valuable new tool in this expanding repertoire, offering an encouraging prospect for both providers and families navigating the complexities of pediatric liver disease.
Aaron Bennett, MD, is a fellow in the Division of Gastroenterology, Hepatology and Nutrition at Children’s Hospital of Philadelphia in Pennsylvania. Elizabeth B. Rand, MD, is the medical director of the Liver Transplant Program, director of the Gastroenterology Fellowship Program, and director of the Advanced Transplant Hepatology Program at Children’s Hospital of Philadelphia.
Grading liver stiffness using elastography is a widely utilized tool in adult populations, and its application is expanding in pediatric hepatology clinics. Clinicians incorporate liver stiffness measurements (LSM) alongside clinical findings and biochemical markers to noninvasively assess the degree of hepatic fibrosis and cirrhosis. Molleston and colleagues leveraged the robust data from the National Institute of Diabetes and Digestive and Kidney Diseases–supported network ChiLDReN and found that LSM in children with biliary atresia (BA) correlate with the progression to complications associated with portal hypertension and liver transplantation. While these findings are not unexpected, this compelling investigation accomplishes the important function of validating the utility of elastography in this cohort.
Prognosticating the timeline of complications stemming from biliary atresia is a central tenet of pediatric hepatology. Helping families understand what the future may hold for their child is critical in fostering long-term relationships between clinicians and caregivers. Furthermore, establishing clear expectations regarding follow-up care and monitoring is beneficial for both providers and patients. Of particular importance is minimizing the need for invasive procedures, such as liver biopsy, which, while relatively safe, remains burdensome and is rarely used to assess fibrosis in BA.
Pediatric hepatologists already consider multiple factors — including age at hepatoportoenterostomy, subsequent clearance of cholestasis, exam findings such as splenomegaly, and platelet count — to predict the clinical course of infants with BA. The addition of a data-driven approach to interpreting liver stiffness measurements represents a valuable new tool in this expanding repertoire, offering an encouraging prospect for both providers and families navigating the complexities of pediatric liver disease.
Aaron Bennett, MD, is a fellow in the Division of Gastroenterology, Hepatology and Nutrition at Children’s Hospital of Philadelphia in Pennsylvania. Elizabeth B. Rand, MD, is the medical director of the Liver Transplant Program, director of the Gastroenterology Fellowship Program, and director of the Advanced Transplant Hepatology Program at Children’s Hospital of Philadelphia.
according to investigators.
These findings suggest that LSM may serve as a noninvasive tool for risk stratification and treatment planning in this population, reported lead author Jean P. Molleston, MD, of Indiana University School of Medicine, Indianapolis, and colleagues.
“Biliary atresia is frequently complicated by hepatic fibrosis with progression to cirrhosis and portal hypertension manifested by ascites, hepatopulmonary syndrome, and variceal bleeding,” the investigators wrote in Gastroenterology. “The ability to predict these outcomes can inform clinical decision-making.”
To this end, VCTE has been gaining increasing support in the pediatric setting.
“Advantages of VCTE over liver biopsy include convenience, cost, sampling bias, and risk,” the investigators wrote. “VCTE potentially allows (1) fibrosis estimation, (2) prediction of portal hypertension complications/survival, and (3) ability to noninvasively monitor liver stiffness as a fibrosis surrogate.”
The present multicenter study aimed to gauge the prognostic utility of VCTE among 254 patients, aged 21 years or younger, with biliary atresia. All patients had a valid baseline LSM, plus longitudinal clinical and laboratory data drawn from studies by the Childhood Liver Disease Research Network (ChiLDReN). Liver stiffness was assessed noninvasively with FibroScan devices, adhering to protocols that required at least 10 valid measurements and a variability of less than 30%.
The primary outcomes were survival with native liver (SNL), defined as the time to liver transplantation or death, and a composite measure of liver-related events, including the first occurrence of transplantation, death, ascites, variceal bleeding, or hepatopulmonary syndrome. Secondary outcomes focused on the trajectory of platelet decline, a marker of disease progression. The study also explored the relationship between baseline LSM and conventional biomarkers, including platelet count, albumin, and bilirubin.
LSM was a strong predictor of long-term outcomes. Specifically, Kaplan-Meier analysis showed significant differences in 5-year SNL across LSM strata (P < .001). Children with LSM values less than 10 kPa had excellent 5-year SNL rates (LSM 10 to < 15 kPa, 88.9%; 95% CI, 75.1-95.3%), while those with LSM of at least 15 kPa exhibited substantially lower 5-year SNL (58.9%; 95% CI, 46.0-69.7%).
Similarly, event-free survival (EFS) rates declined as LSM values increased (P < .001). Participants with LSM less than 10 kPa had a 5-year EFS rate of 92.2% versus with 61.2% for those with LSM of at least 15 kPa.
LSM also predicted platelet decline. For every twofold increase in baseline LSM, platelet counts declined by an additional 4,000/mm3 per year (P < .001). This association was illustrated through predicted trajectories for participants with LSM values of 4, 7, 12, 18, and 42 kPa, corresponding to different percentiles of disease severity.
Cox proportional hazards analysis indicated that a two-fold increase in LSM was associated with a hazard ratio of 3.3 (P < .001) for liver transplant or death. While LSM had good discrimination on its own (C statistic = 0.83), it did not significantly improve predictive accuracy when added to models based on platelet count, albumin, and bilirubin.
“This noninvasive measurement could potentially be used to predict natural history, stratify patients for clinical trials, plan interventions, and provide anticipatory guidance,” Molleston and colleagues concluded. This study was supported by grants from the National Institute of Diabetes, Digestive and Kidney Diseases; National Institutes of Health; Childhood Liver Disease Research Network; and others. The investigators disclosed no conflicts of interest.
according to investigators.
These findings suggest that LSM may serve as a noninvasive tool for risk stratification and treatment planning in this population, reported lead author Jean P. Molleston, MD, of Indiana University School of Medicine, Indianapolis, and colleagues.
“Biliary atresia is frequently complicated by hepatic fibrosis with progression to cirrhosis and portal hypertension manifested by ascites, hepatopulmonary syndrome, and variceal bleeding,” the investigators wrote in Gastroenterology. “The ability to predict these outcomes can inform clinical decision-making.”
To this end, VCTE has been gaining increasing support in the pediatric setting.
“Advantages of VCTE over liver biopsy include convenience, cost, sampling bias, and risk,” the investigators wrote. “VCTE potentially allows (1) fibrosis estimation, (2) prediction of portal hypertension complications/survival, and (3) ability to noninvasively monitor liver stiffness as a fibrosis surrogate.”
The present multicenter study aimed to gauge the prognostic utility of VCTE among 254 patients, aged 21 years or younger, with biliary atresia. All patients had a valid baseline LSM, plus longitudinal clinical and laboratory data drawn from studies by the Childhood Liver Disease Research Network (ChiLDReN). Liver stiffness was assessed noninvasively with FibroScan devices, adhering to protocols that required at least 10 valid measurements and a variability of less than 30%.
The primary outcomes were survival with native liver (SNL), defined as the time to liver transplantation or death, and a composite measure of liver-related events, including the first occurrence of transplantation, death, ascites, variceal bleeding, or hepatopulmonary syndrome. Secondary outcomes focused on the trajectory of platelet decline, a marker of disease progression. The study also explored the relationship between baseline LSM and conventional biomarkers, including platelet count, albumin, and bilirubin.
LSM was a strong predictor of long-term outcomes. Specifically, Kaplan-Meier analysis showed significant differences in 5-year SNL across LSM strata (P < .001). Children with LSM values less than 10 kPa had excellent 5-year SNL rates (LSM 10 to < 15 kPa, 88.9%; 95% CI, 75.1-95.3%), while those with LSM of at least 15 kPa exhibited substantially lower 5-year SNL (58.9%; 95% CI, 46.0-69.7%).
Similarly, event-free survival (EFS) rates declined as LSM values increased (P < .001). Participants with LSM less than 10 kPa had a 5-year EFS rate of 92.2% versus with 61.2% for those with LSM of at least 15 kPa.
LSM also predicted platelet decline. For every twofold increase in baseline LSM, platelet counts declined by an additional 4,000/mm3 per year (P < .001). This association was illustrated through predicted trajectories for participants with LSM values of 4, 7, 12, 18, and 42 kPa, corresponding to different percentiles of disease severity.
Cox proportional hazards analysis indicated that a two-fold increase in LSM was associated with a hazard ratio of 3.3 (P < .001) for liver transplant or death. While LSM had good discrimination on its own (C statistic = 0.83), it did not significantly improve predictive accuracy when added to models based on platelet count, albumin, and bilirubin.
“This noninvasive measurement could potentially be used to predict natural history, stratify patients for clinical trials, plan interventions, and provide anticipatory guidance,” Molleston and colleagues concluded. This study was supported by grants from the National Institute of Diabetes, Digestive and Kidney Diseases; National Institutes of Health; Childhood Liver Disease Research Network; and others. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Lipophilic Statins May Protect Against HCC In Select Liver Disease Patients
according to investigators.
These findings also pave the way for new research into targeted therapies, personalized prevention strategies, and broader applications in high-risk populations, Erik Almazan, MD, and Raymond T. Chung, MD, of Harvard Medical School, Boston, Massachusetts, reported.
“Statins, metformin, and aspirin are low-cost medications often prescribed for the management of diseases associated with metabolic syndrome that have been associated with reduced HCC risk, the investigators wrote in Gastro Hep Advances. “Despite these findings, few studies have focused on populations in the US or without hepatitis B virus (HBV) or hepatitis C virus (HCV).”
To address this knowledge gap, Almazan and Chung retrospectively analyzed data from 3,677 patients with hepatic fibrosis and cirrhosis, drawn from the All of Us Controlled Tier Dataset v7, which spans May 2018 to July 2022.
Within this population, 94 patients had HCC, while 3,583 served as controls. Lipophilic statin use was compared with hydrophilic statins, metformin, and aspirin. Multivariable logistic regression controlled for confounders including age, sex, race, and the presence of HBV or HCV.
Participants in the HCC cohort were older (mean age, 64 vs 58 years), more likely to be male (64.1% vs 50.0%), and had higher rates of chronic HBV (9.6% vs 2.5%) and chronic HCV (36.2% vs. 20.5%) compared to controls (P ≤ .01).
As a class, lipophilic statins were associated with a 36% reduced risk of HCC (odds ratio [OR], 0.64; 95% CI, 0.41-1.00; P < .05). Specifically, atorvastatin was associated with a 41% reduced risk (OR, 0.59; 95% CI, 0.37-0.93; P = .02), while simvastatin was associated with a 54% reduced risk (OR, 0.46; 95% CI, 0.22-0.97; P = .04).
In contrast, hydrophilic statins, such as pravastatin and rosuvastatin, showed no significant association with HCC risk. Similarly, no protective association was observed for metformin or aspirin.
These findings suggest that lipophilic statins could provide a practical and cost-effective strategy for HCC prevention, particularly in patients with metabolic syndrome or alcohol-related liver disease, according to Almazan and Chung. These high-risk groups often lack accessible and noninvasive prevention options, further highlighting the clinical relevance of these results.
The investigators proposed that the chemopreventive effects of lipophilic statins may be linked to their ability to passively diffuse into cells and modulate pathways involved in cancer development, such as the mevalonate pathway. These potential mechanisms remain poorly understood.
Almazan and Chung also pointed out several study limitations, including lack of granular data on statin doses and treatment duration, absence of serologic and imaging confirmation of hepatic fibrosis and cirrhosis, and a study cohort drawn from populations historically underrepresented in medical research, potentially limiting generalizability to the broader US population.
“Nevertheless, we believe that our study adds valuable information to the literature on statin use and its association with HCC with data from a US-based sample inclusive of individuals with risk factors other than HBV and HCV,” the investigators wrote. “These results provide further support for trials (such as NCT05028829) evaluating the utility of lipophilic statins for chemoprevention in HCC for persons at risk.”This study was supported by various National Institutes of Health grants. The investigators disclosed no conflicts of interest.
Hepatocellular carcinoma (HCC) incidence continues to increase in the United States. Because of its poor prognosis and limited treatment options, prevention strategies are critically needed, yet there are no Food and Drug Administration–approved treatments for HCC prevention. In the United States, metabolic syndrome has a high prevalence and is a significant contributor to HCC burden. Many individuals with metabolic syndrome are eligible for statin therapy, which has been associated with HCC chemoprevention. Evidence suggests that lipophilic statins may be more effective chemopreventive agents than hydrophilic statins. However, previous studies have largely focused on populations with hepatitis C virus, making it unclear whether these findings are generalizable to individuals with other liver disease etiologies.
Our findings support the chemopreventive potential of lipophilic statins in patients with hepatic fibrosis and cirrhosis, regardless of the underlying cause. If lipophilic statins are confirmed as effective chemopreventive agents, HCC prevention could begin in the primary care setting. For example, primary care providers treating patients with metabolic syndrome and an indication for statin therapy could select treatment with lipophilic statins over hydrophilic statins. This approach would be cost-effective, relatively simple to implement, and benefit many patients, including those from lower socioeconomic backgrounds who are at higher risk.
Large-scale clinical trials and basic science studies are necessary to confirm the role of lipophilic statins in HCC prevention. Supporting precision medicine initiatives like the All of Us Research Program could help identify individuals most likely to benefit and address gaps in current HCC prevention strategies.
Erik Almazan, MD, is a resident physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts. Raymond T. Chung, MD, is director of the Hepatology and Liver Center at Massachusetts General Hospital and Harvard Medical School, Boston. They have no conflicts to disclose.
Hepatocellular carcinoma (HCC) incidence continues to increase in the United States. Because of its poor prognosis and limited treatment options, prevention strategies are critically needed, yet there are no Food and Drug Administration–approved treatments for HCC prevention. In the United States, metabolic syndrome has a high prevalence and is a significant contributor to HCC burden. Many individuals with metabolic syndrome are eligible for statin therapy, which has been associated with HCC chemoprevention. Evidence suggests that lipophilic statins may be more effective chemopreventive agents than hydrophilic statins. However, previous studies have largely focused on populations with hepatitis C virus, making it unclear whether these findings are generalizable to individuals with other liver disease etiologies.
Our findings support the chemopreventive potential of lipophilic statins in patients with hepatic fibrosis and cirrhosis, regardless of the underlying cause. If lipophilic statins are confirmed as effective chemopreventive agents, HCC prevention could begin in the primary care setting. For example, primary care providers treating patients with metabolic syndrome and an indication for statin therapy could select treatment with lipophilic statins over hydrophilic statins. This approach would be cost-effective, relatively simple to implement, and benefit many patients, including those from lower socioeconomic backgrounds who are at higher risk.
Large-scale clinical trials and basic science studies are necessary to confirm the role of lipophilic statins in HCC prevention. Supporting precision medicine initiatives like the All of Us Research Program could help identify individuals most likely to benefit and address gaps in current HCC prevention strategies.
Erik Almazan, MD, is a resident physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts. Raymond T. Chung, MD, is director of the Hepatology and Liver Center at Massachusetts General Hospital and Harvard Medical School, Boston. They have no conflicts to disclose.
Hepatocellular carcinoma (HCC) incidence continues to increase in the United States. Because of its poor prognosis and limited treatment options, prevention strategies are critically needed, yet there are no Food and Drug Administration–approved treatments for HCC prevention. In the United States, metabolic syndrome has a high prevalence and is a significant contributor to HCC burden. Many individuals with metabolic syndrome are eligible for statin therapy, which has been associated with HCC chemoprevention. Evidence suggests that lipophilic statins may be more effective chemopreventive agents than hydrophilic statins. However, previous studies have largely focused on populations with hepatitis C virus, making it unclear whether these findings are generalizable to individuals with other liver disease etiologies.
Our findings support the chemopreventive potential of lipophilic statins in patients with hepatic fibrosis and cirrhosis, regardless of the underlying cause. If lipophilic statins are confirmed as effective chemopreventive agents, HCC prevention could begin in the primary care setting. For example, primary care providers treating patients with metabolic syndrome and an indication for statin therapy could select treatment with lipophilic statins over hydrophilic statins. This approach would be cost-effective, relatively simple to implement, and benefit many patients, including those from lower socioeconomic backgrounds who are at higher risk.
Large-scale clinical trials and basic science studies are necessary to confirm the role of lipophilic statins in HCC prevention. Supporting precision medicine initiatives like the All of Us Research Program could help identify individuals most likely to benefit and address gaps in current HCC prevention strategies.
Erik Almazan, MD, is a resident physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts. Raymond T. Chung, MD, is director of the Hepatology and Liver Center at Massachusetts General Hospital and Harvard Medical School, Boston. They have no conflicts to disclose.
according to investigators.
These findings also pave the way for new research into targeted therapies, personalized prevention strategies, and broader applications in high-risk populations, Erik Almazan, MD, and Raymond T. Chung, MD, of Harvard Medical School, Boston, Massachusetts, reported.
“Statins, metformin, and aspirin are low-cost medications often prescribed for the management of diseases associated with metabolic syndrome that have been associated with reduced HCC risk, the investigators wrote in Gastro Hep Advances. “Despite these findings, few studies have focused on populations in the US or without hepatitis B virus (HBV) or hepatitis C virus (HCV).”
To address this knowledge gap, Almazan and Chung retrospectively analyzed data from 3,677 patients with hepatic fibrosis and cirrhosis, drawn from the All of Us Controlled Tier Dataset v7, which spans May 2018 to July 2022.
Within this population, 94 patients had HCC, while 3,583 served as controls. Lipophilic statin use was compared with hydrophilic statins, metformin, and aspirin. Multivariable logistic regression controlled for confounders including age, sex, race, and the presence of HBV or HCV.
Participants in the HCC cohort were older (mean age, 64 vs 58 years), more likely to be male (64.1% vs 50.0%), and had higher rates of chronic HBV (9.6% vs 2.5%) and chronic HCV (36.2% vs. 20.5%) compared to controls (P ≤ .01).
As a class, lipophilic statins were associated with a 36% reduced risk of HCC (odds ratio [OR], 0.64; 95% CI, 0.41-1.00; P < .05). Specifically, atorvastatin was associated with a 41% reduced risk (OR, 0.59; 95% CI, 0.37-0.93; P = .02), while simvastatin was associated with a 54% reduced risk (OR, 0.46; 95% CI, 0.22-0.97; P = .04).
In contrast, hydrophilic statins, such as pravastatin and rosuvastatin, showed no significant association with HCC risk. Similarly, no protective association was observed for metformin or aspirin.
These findings suggest that lipophilic statins could provide a practical and cost-effective strategy for HCC prevention, particularly in patients with metabolic syndrome or alcohol-related liver disease, according to Almazan and Chung. These high-risk groups often lack accessible and noninvasive prevention options, further highlighting the clinical relevance of these results.
The investigators proposed that the chemopreventive effects of lipophilic statins may be linked to their ability to passively diffuse into cells and modulate pathways involved in cancer development, such as the mevalonate pathway. These potential mechanisms remain poorly understood.
Almazan and Chung also pointed out several study limitations, including lack of granular data on statin doses and treatment duration, absence of serologic and imaging confirmation of hepatic fibrosis and cirrhosis, and a study cohort drawn from populations historically underrepresented in medical research, potentially limiting generalizability to the broader US population.
“Nevertheless, we believe that our study adds valuable information to the literature on statin use and its association with HCC with data from a US-based sample inclusive of individuals with risk factors other than HBV and HCV,” the investigators wrote. “These results provide further support for trials (such as NCT05028829) evaluating the utility of lipophilic statins for chemoprevention in HCC for persons at risk.”This study was supported by various National Institutes of Health grants. The investigators disclosed no conflicts of interest.
according to investigators.
These findings also pave the way for new research into targeted therapies, personalized prevention strategies, and broader applications in high-risk populations, Erik Almazan, MD, and Raymond T. Chung, MD, of Harvard Medical School, Boston, Massachusetts, reported.
“Statins, metformin, and aspirin are low-cost medications often prescribed for the management of diseases associated with metabolic syndrome that have been associated with reduced HCC risk, the investigators wrote in Gastro Hep Advances. “Despite these findings, few studies have focused on populations in the US or without hepatitis B virus (HBV) or hepatitis C virus (HCV).”
To address this knowledge gap, Almazan and Chung retrospectively analyzed data from 3,677 patients with hepatic fibrosis and cirrhosis, drawn from the All of Us Controlled Tier Dataset v7, which spans May 2018 to July 2022.
Within this population, 94 patients had HCC, while 3,583 served as controls. Lipophilic statin use was compared with hydrophilic statins, metformin, and aspirin. Multivariable logistic regression controlled for confounders including age, sex, race, and the presence of HBV or HCV.
Participants in the HCC cohort were older (mean age, 64 vs 58 years), more likely to be male (64.1% vs 50.0%), and had higher rates of chronic HBV (9.6% vs 2.5%) and chronic HCV (36.2% vs. 20.5%) compared to controls (P ≤ .01).
As a class, lipophilic statins were associated with a 36% reduced risk of HCC (odds ratio [OR], 0.64; 95% CI, 0.41-1.00; P < .05). Specifically, atorvastatin was associated with a 41% reduced risk (OR, 0.59; 95% CI, 0.37-0.93; P = .02), while simvastatin was associated with a 54% reduced risk (OR, 0.46; 95% CI, 0.22-0.97; P = .04).
In contrast, hydrophilic statins, such as pravastatin and rosuvastatin, showed no significant association with HCC risk. Similarly, no protective association was observed for metformin or aspirin.
These findings suggest that lipophilic statins could provide a practical and cost-effective strategy for HCC prevention, particularly in patients with metabolic syndrome or alcohol-related liver disease, according to Almazan and Chung. These high-risk groups often lack accessible and noninvasive prevention options, further highlighting the clinical relevance of these results.
The investigators proposed that the chemopreventive effects of lipophilic statins may be linked to their ability to passively diffuse into cells and modulate pathways involved in cancer development, such as the mevalonate pathway. These potential mechanisms remain poorly understood.
Almazan and Chung also pointed out several study limitations, including lack of granular data on statin doses and treatment duration, absence of serologic and imaging confirmation of hepatic fibrosis and cirrhosis, and a study cohort drawn from populations historically underrepresented in medical research, potentially limiting generalizability to the broader US population.
“Nevertheless, we believe that our study adds valuable information to the literature on statin use and its association with HCC with data from a US-based sample inclusive of individuals with risk factors other than HBV and HCV,” the investigators wrote. “These results provide further support for trials (such as NCT05028829) evaluating the utility of lipophilic statins for chemoprevention in HCC for persons at risk.”This study was supported by various National Institutes of Health grants. The investigators disclosed no conflicts of interest.
FROM GASTRO HEP ADVANCES
Areas of Hope Offered in 2024 VA Suicide Report
Suicide was the 12th-leading cause of death for veterans in 2022. However, fewer veterans died by suicide in 2022 than in 12 of the previous 14 years, according to the 2024 National Veteran Suicide Prevention Annual Report released by the US Department of Veterans Affairs (VA).
The review is the most comprehensive national report on veteran suicide and is based on verified data from the Centers for Disease Control and US Department of Defense from 2001-2022, or the most recent years the VA has data.
The report states that 6407 veterans died by suicide in 2022, 3 more than the year before. For comparison, 41,484 nonveteran US adults died by suicide in 2022, 1476 more than 2021. It is important to assess suicide mortality rates in the context of population changes, the report cautions. From 2001-2022, the veteran population dropped from 25.8 million to 18.5 million, a 28.4% decrease. During that same period, the nonveteran US adult population increased from 186.5 million to 242.4 million, a 30.0% jump.
On average, 131 US adults died by suicide each day in 2022: 18 veterans and 114 nonveterans. Among all US adults, including veterans, the average number of suicides per day rose from 81 per day in 2001 to 131 per day in 2022. The average number of veteran suicides per day rose from 16.5 in 2001 to 17.6 in 2022.
“Hope serves an important role within suicide prevention efforts,” the VA said. “Within the challenges faced in 2022, key areas of hope emerged.”
Among those key findings are a 24.1% decrease in age-adjusted suicide rates, a 37% suicide rate reduction among individuals who received VA homeless program services, 3.8% suicide rate decrease in veterans aged 18 to 34 years, and considerable drops in suicide rates for veterans with Veterans Health Administration mental health diagnoses of anxiety (36.1%), depression (34.5%), posttraumatic stress disorder (31.6%), and alcohol use disorder (13.7%).
Eliminating veteran suicide is VA’s top clinical priority and a critical aspect of the strategy for reducing military and veteran suicide. Since 2022, VA has worked aggressively to expand support, including offering no-cost health care to veterans in suicidal crisis; launching the 988 (then press 1) hotline, qualified responders through the Veterans Crisis Line; expanding firearm suicide prevention efforts; and encouraging veterans to reach out for help through a national veteran suicide prevention awareness campaign.
“There is nothing more important to VA than ending veteran suicide,“ said Secretary of Veterans Affairs Denis McDonough. “We will learn from this report to better serve veterans and save lives.”
Suicide was the 12th-leading cause of death for veterans in 2022. However, fewer veterans died by suicide in 2022 than in 12 of the previous 14 years, according to the 2024 National Veteran Suicide Prevention Annual Report released by the US Department of Veterans Affairs (VA).
The review is the most comprehensive national report on veteran suicide and is based on verified data from the Centers for Disease Control and US Department of Defense from 2001-2022, or the most recent years the VA has data.
The report states that 6407 veterans died by suicide in 2022, 3 more than the year before. For comparison, 41,484 nonveteran US adults died by suicide in 2022, 1476 more than 2021. It is important to assess suicide mortality rates in the context of population changes, the report cautions. From 2001-2022, the veteran population dropped from 25.8 million to 18.5 million, a 28.4% decrease. During that same period, the nonveteran US adult population increased from 186.5 million to 242.4 million, a 30.0% jump.
On average, 131 US adults died by suicide each day in 2022: 18 veterans and 114 nonveterans. Among all US adults, including veterans, the average number of suicides per day rose from 81 per day in 2001 to 131 per day in 2022. The average number of veteran suicides per day rose from 16.5 in 2001 to 17.6 in 2022.
“Hope serves an important role within suicide prevention efforts,” the VA said. “Within the challenges faced in 2022, key areas of hope emerged.”
Among those key findings are a 24.1% decrease in age-adjusted suicide rates, a 37% suicide rate reduction among individuals who received VA homeless program services, 3.8% suicide rate decrease in veterans aged 18 to 34 years, and considerable drops in suicide rates for veterans with Veterans Health Administration mental health diagnoses of anxiety (36.1%), depression (34.5%), posttraumatic stress disorder (31.6%), and alcohol use disorder (13.7%).
Eliminating veteran suicide is VA’s top clinical priority and a critical aspect of the strategy for reducing military and veteran suicide. Since 2022, VA has worked aggressively to expand support, including offering no-cost health care to veterans in suicidal crisis; launching the 988 (then press 1) hotline, qualified responders through the Veterans Crisis Line; expanding firearm suicide prevention efforts; and encouraging veterans to reach out for help through a national veteran suicide prevention awareness campaign.
“There is nothing more important to VA than ending veteran suicide,“ said Secretary of Veterans Affairs Denis McDonough. “We will learn from this report to better serve veterans and save lives.”
Suicide was the 12th-leading cause of death for veterans in 2022. However, fewer veterans died by suicide in 2022 than in 12 of the previous 14 years, according to the 2024 National Veteran Suicide Prevention Annual Report released by the US Department of Veterans Affairs (VA).
The review is the most comprehensive national report on veteran suicide and is based on verified data from the Centers for Disease Control and US Department of Defense from 2001-2022, or the most recent years the VA has data.
The report states that 6407 veterans died by suicide in 2022, 3 more than the year before. For comparison, 41,484 nonveteran US adults died by suicide in 2022, 1476 more than 2021. It is important to assess suicide mortality rates in the context of population changes, the report cautions. From 2001-2022, the veteran population dropped from 25.8 million to 18.5 million, a 28.4% decrease. During that same period, the nonveteran US adult population increased from 186.5 million to 242.4 million, a 30.0% jump.
On average, 131 US adults died by suicide each day in 2022: 18 veterans and 114 nonveterans. Among all US adults, including veterans, the average number of suicides per day rose from 81 per day in 2001 to 131 per day in 2022. The average number of veteran suicides per day rose from 16.5 in 2001 to 17.6 in 2022.
“Hope serves an important role within suicide prevention efforts,” the VA said. “Within the challenges faced in 2022, key areas of hope emerged.”
Among those key findings are a 24.1% decrease in age-adjusted suicide rates, a 37% suicide rate reduction among individuals who received VA homeless program services, 3.8% suicide rate decrease in veterans aged 18 to 34 years, and considerable drops in suicide rates for veterans with Veterans Health Administration mental health diagnoses of anxiety (36.1%), depression (34.5%), posttraumatic stress disorder (31.6%), and alcohol use disorder (13.7%).
Eliminating veteran suicide is VA’s top clinical priority and a critical aspect of the strategy for reducing military and veteran suicide. Since 2022, VA has worked aggressively to expand support, including offering no-cost health care to veterans in suicidal crisis; launching the 988 (then press 1) hotline, qualified responders through the Veterans Crisis Line; expanding firearm suicide prevention efforts; and encouraging veterans to reach out for help through a national veteran suicide prevention awareness campaign.
“There is nothing more important to VA than ending veteran suicide,“ said Secretary of Veterans Affairs Denis McDonough. “We will learn from this report to better serve veterans and save lives.”
AGA Legacy Society Members Sustain GI Research
Research creates successful practices. Patients benefit from GI research daily in practices. Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
AGA Legacy Society members have answered this call for support. They recognize the value that research has had in their profession, both in academic medicine and in private practice, and are showing their appreciation by giving back.
“I donated to the AGA Research Foundation to ensure the vitality of our specialty, and to fund the research of future generations of gastroenterologists,” said Michael Camilleri, MD, AGAF, of Mayo Clinic, Rochester, Minn., and an AGA Legacy Society member who currently serves as AGA Research Foundation Chair. “Funding from organizations like the AGA Research Foundation is crucial for young scientists and gastroenterologists to launch their careers. At the start of my career, I received two AGA research awards. As a grateful recipient of such funding, I felt it was my turn to support the mission of the organization that I regard as my academic home away from home institution.”
AGA members who make gifts at the AGA Legacy Society level any time before Digestive Disease Week® (DDW) 2025 will receive an invitation to the AGA Research Foundation Benefactor’s Event in San Diego, California. Interested in learning more about the AGA Legacy Society membership? Contact foundation@gastro.org or visit https://foundation.gastro.org/our-donors/aga-legacy-society/ for more information about the AGA Legacy Society.
Research creates successful practices. Patients benefit from GI research daily in practices. Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
AGA Legacy Society members have answered this call for support. They recognize the value that research has had in their profession, both in academic medicine and in private practice, and are showing their appreciation by giving back.
“I donated to the AGA Research Foundation to ensure the vitality of our specialty, and to fund the research of future generations of gastroenterologists,” said Michael Camilleri, MD, AGAF, of Mayo Clinic, Rochester, Minn., and an AGA Legacy Society member who currently serves as AGA Research Foundation Chair. “Funding from organizations like the AGA Research Foundation is crucial for young scientists and gastroenterologists to launch their careers. At the start of my career, I received two AGA research awards. As a grateful recipient of such funding, I felt it was my turn to support the mission of the organization that I regard as my academic home away from home institution.”
AGA members who make gifts at the AGA Legacy Society level any time before Digestive Disease Week® (DDW) 2025 will receive an invitation to the AGA Research Foundation Benefactor’s Event in San Diego, California. Interested in learning more about the AGA Legacy Society membership? Contact foundation@gastro.org or visit https://foundation.gastro.org/our-donors/aga-legacy-society/ for more information about the AGA Legacy Society.
Research creates successful practices. Patients benefit from GI research daily in practices. Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
AGA Legacy Society members have answered this call for support. They recognize the value that research has had in their profession, both in academic medicine and in private practice, and are showing their appreciation by giving back.
“I donated to the AGA Research Foundation to ensure the vitality of our specialty, and to fund the research of future generations of gastroenterologists,” said Michael Camilleri, MD, AGAF, of Mayo Clinic, Rochester, Minn., and an AGA Legacy Society member who currently serves as AGA Research Foundation Chair. “Funding from organizations like the AGA Research Foundation is crucial for young scientists and gastroenterologists to launch their careers. At the start of my career, I received two AGA research awards. As a grateful recipient of such funding, I felt it was my turn to support the mission of the organization that I regard as my academic home away from home institution.”
AGA members who make gifts at the AGA Legacy Society level any time before Digestive Disease Week® (DDW) 2025 will receive an invitation to the AGA Research Foundation Benefactor’s Event in San Diego, California. Interested in learning more about the AGA Legacy Society membership? Contact foundation@gastro.org or visit https://foundation.gastro.org/our-donors/aga-legacy-society/ for more information about the AGA Legacy Society.
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Integrative medicine or complementary alternative medicine (IM/CAM) is increasingly being recognized as an integral part of optimal health and healing. IM/CAM “reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines.”1 IM/CAM encompasses a wide range of therapies, conceptual frameworks, and health care-related professions, such as acupuncture, massage, dietary supplements, mindfulness, yoga, meditation and guided imagery.1 Research has found that 30% to 98% of patients with chronic conditions seek IM/CAM therapies.1-3
Despite the high prevalence of patients utilizing IM/CAM therapies and the National Institutes of Health grants for IM/CAM education, implementation of IM/CAM instruction in graduate medical education programs remains inconsistent.1 Barriers cited by programs include a lack of IM/CAM experts in the program, faculty training, competing financial resources, and an already full resident education schedule.4 As a result, many physicians have limited or no training in IM/CAM.1,5
The US Department of Veterans Affairs (VA) offers IM/CAM health programs to veterans and caregivers as part of its whole health care initiative.6 Several VA health care systems have adopted whole health and IM/CAM through programs for mental health integration into primary care; women’s health; integrative pain care; geriatrics, through adoption of Age-Friendly Health Systems standards; and nutrition and physical activity.7-13 The VA provides training to more medical students than any other health system: > 95% of US medical schools are affiliated with a VA medical center (VAMC).14 As part of the training mission, VA seeks to encourage students of diverse professions to consider careers in the VA.14
Residency is a time for newly licensed physicians to acquire additional experience and training to translate knowledge and skills acquired during medical school directly to patient care.15 However, residency curricula have limited time to incorporate IM/CAM training. Residency training is also physically and psychosocially demanding, often resulting in inadequate self-care, poor work-life balance, and disrupted sleep.16-18 Resident wellness is at a historic low, resulting in high rates of burnout during training.4,15
Residency programs are required to provide wellness education; however, most programs include minimal content.19 Despite high rates of burnout, formal curricula on the topic have not been established. 20 IM/CAM education also can provide a path for residents to learn about and engage in mindfulness-based training or cognitive stress reduction for self-care.
INTEGRATIVE WHOLE HEALTH ROTATION
In 2017, the Baltimore Geriatric Research Education and Clinical Center (GRECC) established an IM/whole health residency rotation and created a structured curriculum incorporating self-assessment, active reflection, and self-care to complement training in specific IM/CAM modalities for residents in family medicine. The curriculum evaluated how this training improved residents’ perceptions of IM/CAM and how it personally and professionally impacted the practice of self-care as a strategy to decrease burnout. We hypothesized that this structured experience would increase IM/CAM knowledge among clinicians while promoting the importance and practice of self-care to reduce burnout.
The 2-week IM/CAM curriculum was developed by University of Maryland School of Medicine faculty in partnership with the Baltimore GRECC and staff at the VA Maryland Health Care System. The curriculum was designed to expose residents to the 8 components of the whole health Circle of Health (moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind) in addition to IM/ CAM modalities the VA is mandated to offer to veterans (acupuncture, chiropractic, meditation, massage therapy, biofeedback, clinical hypnosis, guided imagery, yoga, and tai chi).21 Twelve residents (1 preventive medicine and 11 third-year family medicine residents) rotated individually throughout the year as part of their behavioral health block rotation. All residents completed the 2-week curriculum as their schedules allowed. The curriculum consisted of didactics sessions and activities at the Baltimore, Loch Raven, and Perry Point VAMCs. Residents completed evaluations before and after the rotation. The experience described in this article by the residents and the survey data were collected from the 2018/2019 training year. A rotation syllabus, competencies adapted from Locke and colleagues and skills residents obtain during this rotation that support these competencies, as well as a resident sample schedule were developed (eAppendix is available at doi:10.12788/fp.0544).1

Rotation Overview
for each resident were built around instructional opportunities, which included 1-on-1 didactics, direct observation of treatment modalities, and personal reflection of the residents’ self-care practices. While each resident’s rotation schedule varied slightly due to their schedules, the foundational instruction elements were the same. Didactic session themes included an overview of IM/CAM, nutrition, narrative medicine, pain psychology, music therapy, chaplain services, motor-cognitive training, and exercise guidelines. Assigned readings, including peer-reviewed literature on IM/CAM therapies, complemented all sessions. Residents created an evidence-supported integrative treatment plan for a patient with a condition of interest to them.
Residents observed clinician-led veteran group sessions on IM/CAM treatment modalities, including guided meditation, mindfulness and relaxation, self-awareness, living well with chronic pain, tai chi, drumming for health and balance, anger management, recovery group, acceptance and commitment therapy, and Gerofit exercise. The group classes allowed residents to actively participate in the activity or discussion. Residents also shadowed VA clinicians in sleep, pain, nutrition, acupuncture, and mental health clinics.
Residents were encouraged to practice self-care during the 2-week rotation. The rotation schedule built in free time, including a 1-hour daily lunch period, for residents to consider their own health habits, complete a personal health inventory, and try self-care activities outlined on the syllabus with links to resources. These resources also served as educational materials that residents could share with patients. All materials, including didactic lectures, journal articles and self-care resources, were provided to each resident through a free online course to ensure residents had access throughout and following completion of the rotation. This content, including the rotation evaluation metrics, is available upon request from the corresponding author.
Evaluations
Residents completed a survey before and after the rotation to measure IM/CAM knowledge and application and self-care/ burnout perceptions. Residents were asked to evaluate rotation sessions and comment on whether this rotation benefited them personally and professionally (Table 1). Descriptive statistics were analyzed using Microsoft Excel. Given the small sample size and lack of statistical power, only mean survey results are reported in this article. Because this opportunity is specific to the University of Maryland School of Medicine and the proposed project was part of ordinary educational practice, the study was deemed not human subject research by the University of Maryland Institutional Review Board (HP-00089256).

Perceptions and attitudes toward IM/CAM were assessed using a survey designed by the University of Minnesota Academic Health Center. It included 18 items scored on a 5-point semantic rating scale (1, strongly disagree; 5, strongly agree).22 Residents rated their level of agreement with statements reflecting both positive (eg, clinical care should integrate the best of conventional and CAM practices) and negative (eg, CAM is a threat to public health) views. Three questions adapted from the NHIS Adult Complementary Health Questionnaire and UC Irvine Survey of Health Care Use and Practice assessed the use of IM/CAM resources.23,24
Resident knowledge and application of IM/CAM were measured using a case study designed by the course faculty. The case listed a chief complaint of nerve pain, with a history of chronic pain, neuropathic pain, anxiety, chronic fatigue, depression, insomnia, posttraumatic stress disorder, history of present illness, past surgical history, medication list, review of symptoms, laboratory values, and physical examination. The residents completed an assessment before and after the rotation. Residents rated their confidence in the diagnosis and treatment of 8 medical conditions using a 5-point semantic rating scale (Table 2). Self-care importance and selfcare frequency were measured by a variety of means, including 3 survey questions, the Five Facet Mindfulness Questionnaire, 2 prompts on a 7-point semantic scale, and a slightly modified version of the validated Perceived Stress Scale.25-28
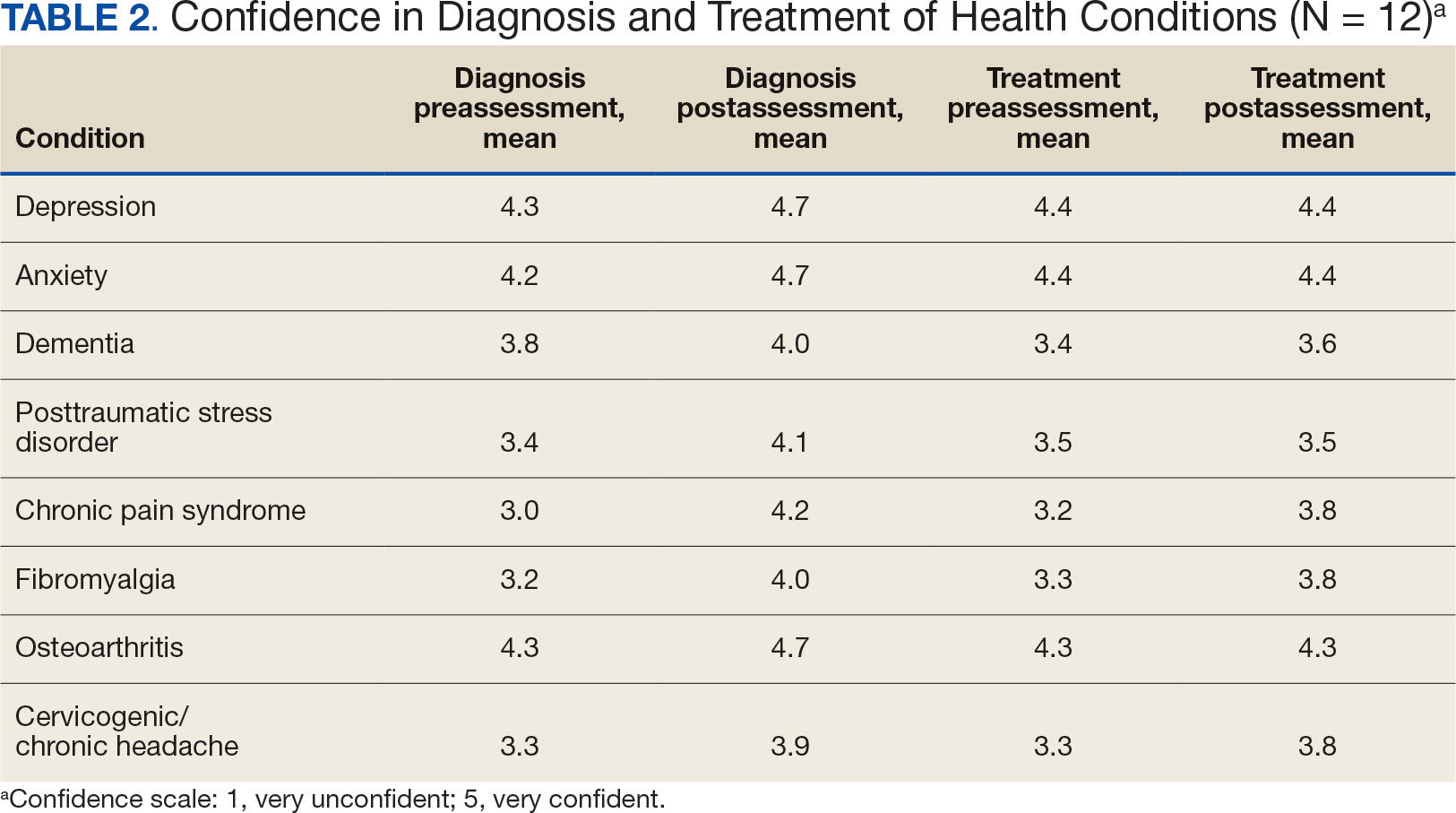
Survey Results
Residents gave the rotation positive feedback with a mean score of 8.5 out of 10. They reported the beneficial impact of seeing the nontraditional and nonpharmacological practices in treating patients, chronic pain management team approaches, and enjoyed being able to participate in group classes with patients. Many residents expressed a desire for a longer rotation to have more time to experience the behavioral health-focused sessions. Residents also requested additional information on nutritional supplements/natural medicines, battlefield acupuncture training and osteopathic manipulative therapy practices. All residents reported the rotation personally and/or professionally benefited them (Appendix).
Given the sample of 12 residents, values are presented as prerotation to postrotation comparisons without statistical analysis. There was a trend towards an increase in the reported use and recommendation of 26 modalities of nonconventional therapies following the rotation. There was also a slight increase in resource knowledge and use of these resources, and residents reported accessing more types of resources. Mean scores of the case study to gauge knowledge and application of IM increased from 7.5 at baseline to 11.0 after the rotation. Resident confidence in diagnosis increased for all 8 conditions, but confidence in treatment only increased for 4 conditions.
Results of self-care importance, self-care frequency and mindfulness were consistent baseline to postrotation. The mean time residents spent regularly practicing self-care during a work week increased slightly while feelings of burnout decreased. The perceived stress scale average score decreased from 13.4 at baseline to 10.5 after rotation.
DISCUSSION
The implementation of an IM residency rotation that incorporates whole health and interprofessional practices demonstrated improved perception and increased use of IM/CAM resources and knowledge among a small sample of third-year residents. Residents reported they had a positive experience participating in the rotation and gained knowledge, resources, and skills they felt confident discussing with their patients.
Many studies reported favorable attitudes and perceptions of IM/CAM use among physicians, but few have assessed these measures while implementing a training curriculum.3,4,22 Gardiner and colleagues reported on the perception and use of IM resources among family medicine residents.4 The study found that while 58% of all residents reported IM/CAM as an important part of their training, only 60% reported they received it or had specific learning objectives in their curriculum. 4 The program outlined in this study and previous research illustrate that physicians recognize the importance of IM/CAM education in training programs, but most were unaware of the resources available or did not feel comfortable counseling patients about most IM/CAM applications.
Residents in this program slightly increased their use of IM/CAM to diagnose and treat medical conditions after the rotation. A study by Wahner-Roedler and colleagues assessed physician knowledge regarding common IM/CAM therapies.3 On average, physicians only felt knowledgeable and comfortable counseling patients for 3 of 13 listed treatments/techniques and few natural herbal treatments. The study also found that most physicians had difficulty accessing IM/CAM information at their institution despite having free access to electronic databases. However, this study only assessed physician attitudes of IM/CAM and did not include an educational component to increase their knowledge of the modalities.3 This evaluation supports the need for interventions like the program described in this article that provide physicians with access to evidence-based resources combined with the applied experiences to increase their comfort within this growing field.
Though the sample size in this study was small, its results support existing research indicating that clinicians view selfcare as important. Many residents were already using a self-care plan at baseline, but there was slight increase in the practice of self-care during the rotation and a slight decrease in burnout. Previous research reflects high rates of burnout and relatively poor quality of life among primary care physicians.15 Burnout is associated with lower quality of care, lower patient satisfaction and contributes to medical errors. Studies suggest as many as 60% of primary care physicians report symptoms of burnout, which negatively affected the quality of patient care they provide.15
Despite the profound effects burnout has on physicians and patient care, a standardized wellness education or self-care tool kit is not currently available. The University of Massachusetts recently introduced a pilot program to promote resident wellness that demonstrated favorable results.15 A meta-analysis of physicians and medical trainees found decreases in anxiety and symptoms of anxiety as well as a decrease in burnout among participants in cognitive, behavioral and mindfulness interventions.29 However, unlike our program, these programs focused solely on the well-being of medical trainees, residents, and physicians and didn’t focus on the patient-clinician interactions. Given the impact on patient care, there is a need to develop and implement additional programs like our residency rotation that promote health and wellness among physicians while also evaluating how physicians may translate these skills to patient education.
While this program st i l l exists for third-year residents at Baltimore GRECC, it has significantly changed since the COVID-19 pandemic. For about the first 6 months of the pandemic, when physical distancing requirements were in place, family medicine trainees were not able to rotate. Upon return to the facility, many group classes were cancelled and some clinicians no longer offered the sessions. The rotation has evolved to a hybrid format, where many group classes for veteran patients are offered virtually, and residents observe a mix of virtual and in-person shadowing opportunities. Our formal evaluation included administering the survey and occurred from July 2018 to July 2019 but wasn’t implemented upon return to post-COVID activities due to the inconsistent experiences offered to residents over the past few years. Future research should evaluate the impact of this hybrid program on the clinicians and explore dissemination to other VAMCs and their academic affiliates.
Limitations
Project recruitment was limited to 11 family medicine and 1 preventive medicine resident. Perceptions, use of IM/CAM, and knowledge about IM/CAM could be considerably different in different departments with varying schedules, hours worked, and patient volumes. Secondly, the survey was conducted 2 weeks apart. Indications of self-care and burnout may not reflect long-term effects, adoption, or maintenance. Future research should include longer follow up to examine how this type of educational activity may impact burnout rates of physicians following the completion of residency, as well as changes in perspectives of IM/CAM while practicing as a physician. Trainees were exposed to a wide range of health care professions, but additional research is needed regarding medical resident perceptions of the roles of specific professions in a collaborative health care team.30,31
CONCLUSIONS
The residency rotation program illustrates the benefits of establishing a standardized IM/CAM rotation that includes self-care resources in family medicine programs to adequately train clinicians to practice wellness and promote it to their patients. The results of this project suggest this type of training will help residents assess the literature to better counsel patients on IM/CAM options while also providing strategies for maintaining optimal health and well-being for health care professionals. Broadening and shifting the scope of medicine from treatment to prevention, personal wellness, and optimal healing should be a top priority.
- Locke AB, Gordon A, Guerrera MP, Gardiner P, Lebensohn P. Recommended integrative medicine competencies for family medicine residents. Explore (NY). 2013;9(5):308-313. doi:10.1016/j.explore.2013.06.005
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. doi:10.1001/jama.280.18.1569
- Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501. doi:10.1093/ecam/nel036
- Gardiner P, Filippelli AC, Lebensohn P, Bonakdar R. Family medicine residency program directors attitudes and knowledge of family medicine CAM competencies. Explore (NY). 2013;9(5):299-307. doi:10.1016/j.explore.2013.06.002
- Sierpina V, Levine R, Astin J, Tan A. Use of mind-body therapies in psychiatry and family medicine faculty and residents: attitudes, barriers, and gender differences. Explore (NY). 2007;3(2):129-135. doi:10.1016/j.explore.2006.12.001
- Krist AH, South-Paul J, Meisnere M, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023.
- Bokhour BG, DeFaccio R, Gaj L, et al. Changes in patientreported outcomes associated with receiving whole health in the Veteran Health Administration (VHA)’s National Demonstration Project. J Gen Intern Med. 2024;39(1):84-94. doi:10.1007/s11606-023-08376-0
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial- spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Gabrielian S, Jones AL, Hoge AE, et al. Enhancing primary care experiences for homeless patients with serious mental illness: results from a national survey. J Prim Care Community Health. 2021;12:2150132721993654. doi:10.1177/2150132721993654
- Matthieu MM, Church KA, Taylor LD, et al. Integrating the age-friendly health systems movement in Veterans Health Administration: national advance care planning via group visits and the 4Ms framework. Health Soc Work. 2023;48(4):277-280. doi:10.1093/hsw/hlad022
- Meisler AW, Gianoli MO, Na PJ, Pietrzak RH. Functional disability in US military veterans: the importance of integrated whole health initiatives. Prim Care Companion CNS Disord. 2023;25(4):22m03461. doi:10.4088/PCC.22m03461
- Ortmeyer HK, Giffuni J, Etchberger D, Katzel L. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13(19):3047. doi:10.3390/ani13193047
- Sullivan MB, Hill K, Ballengee LA, et al. Remotely delivered psychologically informed mindful movement physical therapy for pain care: a framework for operationalization. Glob Adv Integr Med Health. 2023;12:27536130231209751. doi:10.1177/27536130231209751
- (OAA) OoAA. 75th Anniversary: Passion to learn. Power to heal. Washington DC.: US Department of Veterans Affairs; 2021. https://content.yudu.com/web/448fx/0A448g9/75thAnniversary2021/html/index.html?page=24&origin=reader
- Runyan C, Savageau JA, Potts S, Weinreb L. Impact of a family medicine resident wellness curriculum: a feasibility study. Med Educ Online. 2016;21:30648. doi:10.3402/meo.v21.30648
- Lafreniere JP, Rios R, Packer H, Ghazarian S, Wright SM, Levine RB. Burned out at the bedside: patient perceptions of physician burnout in an internal medicine resident continuity clinic. J Gen Intern Med. 2016;31(2):203-208. doi:10.1007/s11606-015-3503-3
- Freedy JR, Staley C, Mims LD, et al. Social, individual, and environmental characteristics of family medicine resident burnout: a CERA study. Fam Med. 2022;54(4):270-276. doi:10.22454/FamMed.2022.526799
- Alrishan MA, Alshammari SA. Prevalence of sleep deprivation and its effect on the performance of family medicine residents in Riyadh, Saudi Arabia. J Family Community Med. 2020;27(2):125-130. doi:10.4103/jfcm.JFCM_9_20
- ACGME. ACGME Program Requirements for Graduate Medical Education in Family Medicine. https://www.acgme.org/globalassets/pfassets/programrequirements/120_familymedicine_2024.pdf
- Nene Y, Tadi P. Resident Burnout. In: StatPearls; 2023.
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the veterans affairs to a whole health system of care: time for action and research. Med Care. 2020;58(4):295-300. doi:10.1097/MLF.0000000000001316
- Kreitzer MJ, Mitten D, Harris I, Shandeling J. Attitudes toward CAM among medical, nursing, and pharmacy faculty and students: a comparative analysis. Altern Ther Health Med. 2002;8(6):44-53.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
- Nguyen J, Liu MA, Patel RJ, Tahara K, Nguyen AL. Use and interest in complementary and alternative medicine among college students seeking healthcare at a university campus student health center. Complement Ther Clin Pract. 2016;24:103-108. doi:10.1016/j.ctcp.2016.06.001
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi:10.1177/1073191105283504
- Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi:10.1177/1073191107313003
- West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318- 1321. doi:10.1007/s11606-009-1129-z
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
- Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202(5):353-359. doi:10.1097/NMD.0000000000000130
- Visser CLF, Ket JCF, Croiset G, Kusurkar RA. Perceptions of residents, medical and nursing students about interprofessional education: a systematic review of the quantitative and qualitative literature. BMC Med Educ. 2017;17(1):77. doi:10.1186/s12909-017-0909-0
- Lingard L, Espin S, Evans C, Hawryluck L. The rules of the game: interprofessional collaboration on the intensive care unit team. Crit Care. 2004;8(6):R403-408. doi:10.1186/cc2958
Integrative medicine or complementary alternative medicine (IM/CAM) is increasingly being recognized as an integral part of optimal health and healing. IM/CAM “reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines.”1 IM/CAM encompasses a wide range of therapies, conceptual frameworks, and health care-related professions, such as acupuncture, massage, dietary supplements, mindfulness, yoga, meditation and guided imagery.1 Research has found that 30% to 98% of patients with chronic conditions seek IM/CAM therapies.1-3
Despite the high prevalence of patients utilizing IM/CAM therapies and the National Institutes of Health grants for IM/CAM education, implementation of IM/CAM instruction in graduate medical education programs remains inconsistent.1 Barriers cited by programs include a lack of IM/CAM experts in the program, faculty training, competing financial resources, and an already full resident education schedule.4 As a result, many physicians have limited or no training in IM/CAM.1,5
The US Department of Veterans Affairs (VA) offers IM/CAM health programs to veterans and caregivers as part of its whole health care initiative.6 Several VA health care systems have adopted whole health and IM/CAM through programs for mental health integration into primary care; women’s health; integrative pain care; geriatrics, through adoption of Age-Friendly Health Systems standards; and nutrition and physical activity.7-13 The VA provides training to more medical students than any other health system: > 95% of US medical schools are affiliated with a VA medical center (VAMC).14 As part of the training mission, VA seeks to encourage students of diverse professions to consider careers in the VA.14
Residency is a time for newly licensed physicians to acquire additional experience and training to translate knowledge and skills acquired during medical school directly to patient care.15 However, residency curricula have limited time to incorporate IM/CAM training. Residency training is also physically and psychosocially demanding, often resulting in inadequate self-care, poor work-life balance, and disrupted sleep.16-18 Resident wellness is at a historic low, resulting in high rates of burnout during training.4,15
Residency programs are required to provide wellness education; however, most programs include minimal content.19 Despite high rates of burnout, formal curricula on the topic have not been established. 20 IM/CAM education also can provide a path for residents to learn about and engage in mindfulness-based training or cognitive stress reduction for self-care.
INTEGRATIVE WHOLE HEALTH ROTATION
In 2017, the Baltimore Geriatric Research Education and Clinical Center (GRECC) established an IM/whole health residency rotation and created a structured curriculum incorporating self-assessment, active reflection, and self-care to complement training in specific IM/CAM modalities for residents in family medicine. The curriculum evaluated how this training improved residents’ perceptions of IM/CAM and how it personally and professionally impacted the practice of self-care as a strategy to decrease burnout. We hypothesized that this structured experience would increase IM/CAM knowledge among clinicians while promoting the importance and practice of self-care to reduce burnout.
The 2-week IM/CAM curriculum was developed by University of Maryland School of Medicine faculty in partnership with the Baltimore GRECC and staff at the VA Maryland Health Care System. The curriculum was designed to expose residents to the 8 components of the whole health Circle of Health (moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind) in addition to IM/ CAM modalities the VA is mandated to offer to veterans (acupuncture, chiropractic, meditation, massage therapy, biofeedback, clinical hypnosis, guided imagery, yoga, and tai chi).21 Twelve residents (1 preventive medicine and 11 third-year family medicine residents) rotated individually throughout the year as part of their behavioral health block rotation. All residents completed the 2-week curriculum as their schedules allowed. The curriculum consisted of didactics sessions and activities at the Baltimore, Loch Raven, and Perry Point VAMCs. Residents completed evaluations before and after the rotation. The experience described in this article by the residents and the survey data were collected from the 2018/2019 training year. A rotation syllabus, competencies adapted from Locke and colleagues and skills residents obtain during this rotation that support these competencies, as well as a resident sample schedule were developed (eAppendix is available at doi:10.12788/fp.0544).1

Rotation Overview
for each resident were built around instructional opportunities, which included 1-on-1 didactics, direct observation of treatment modalities, and personal reflection of the residents’ self-care practices. While each resident’s rotation schedule varied slightly due to their schedules, the foundational instruction elements were the same. Didactic session themes included an overview of IM/CAM, nutrition, narrative medicine, pain psychology, music therapy, chaplain services, motor-cognitive training, and exercise guidelines. Assigned readings, including peer-reviewed literature on IM/CAM therapies, complemented all sessions. Residents created an evidence-supported integrative treatment plan for a patient with a condition of interest to them.
Residents observed clinician-led veteran group sessions on IM/CAM treatment modalities, including guided meditation, mindfulness and relaxation, self-awareness, living well with chronic pain, tai chi, drumming for health and balance, anger management, recovery group, acceptance and commitment therapy, and Gerofit exercise. The group classes allowed residents to actively participate in the activity or discussion. Residents also shadowed VA clinicians in sleep, pain, nutrition, acupuncture, and mental health clinics.
Residents were encouraged to practice self-care during the 2-week rotation. The rotation schedule built in free time, including a 1-hour daily lunch period, for residents to consider their own health habits, complete a personal health inventory, and try self-care activities outlined on the syllabus with links to resources. These resources also served as educational materials that residents could share with patients. All materials, including didactic lectures, journal articles and self-care resources, were provided to each resident through a free online course to ensure residents had access throughout and following completion of the rotation. This content, including the rotation evaluation metrics, is available upon request from the corresponding author.
Evaluations
Residents completed a survey before and after the rotation to measure IM/CAM knowledge and application and self-care/ burnout perceptions. Residents were asked to evaluate rotation sessions and comment on whether this rotation benefited them personally and professionally (Table 1). Descriptive statistics were analyzed using Microsoft Excel. Given the small sample size and lack of statistical power, only mean survey results are reported in this article. Because this opportunity is specific to the University of Maryland School of Medicine and the proposed project was part of ordinary educational practice, the study was deemed not human subject research by the University of Maryland Institutional Review Board (HP-00089256).

Perceptions and attitudes toward IM/CAM were assessed using a survey designed by the University of Minnesota Academic Health Center. It included 18 items scored on a 5-point semantic rating scale (1, strongly disagree; 5, strongly agree).22 Residents rated their level of agreement with statements reflecting both positive (eg, clinical care should integrate the best of conventional and CAM practices) and negative (eg, CAM is a threat to public health) views. Three questions adapted from the NHIS Adult Complementary Health Questionnaire and UC Irvine Survey of Health Care Use and Practice assessed the use of IM/CAM resources.23,24
Resident knowledge and application of IM/CAM were measured using a case study designed by the course faculty. The case listed a chief complaint of nerve pain, with a history of chronic pain, neuropathic pain, anxiety, chronic fatigue, depression, insomnia, posttraumatic stress disorder, history of present illness, past surgical history, medication list, review of symptoms, laboratory values, and physical examination. The residents completed an assessment before and after the rotation. Residents rated their confidence in the diagnosis and treatment of 8 medical conditions using a 5-point semantic rating scale (Table 2). Self-care importance and selfcare frequency were measured by a variety of means, including 3 survey questions, the Five Facet Mindfulness Questionnaire, 2 prompts on a 7-point semantic scale, and a slightly modified version of the validated Perceived Stress Scale.25-28

Survey Results
Residents gave the rotation positive feedback with a mean score of 8.5 out of 10. They reported the beneficial impact of seeing the nontraditional and nonpharmacological practices in treating patients, chronic pain management team approaches, and enjoyed being able to participate in group classes with patients. Many residents expressed a desire for a longer rotation to have more time to experience the behavioral health-focused sessions. Residents also requested additional information on nutritional supplements/natural medicines, battlefield acupuncture training and osteopathic manipulative therapy practices. All residents reported the rotation personally and/or professionally benefited them (Appendix).
Given the sample of 12 residents, values are presented as prerotation to postrotation comparisons without statistical analysis. There was a trend towards an increase in the reported use and recommendation of 26 modalities of nonconventional therapies following the rotation. There was also a slight increase in resource knowledge and use of these resources, and residents reported accessing more types of resources. Mean scores of the case study to gauge knowledge and application of IM increased from 7.5 at baseline to 11.0 after the rotation. Resident confidence in diagnosis increased for all 8 conditions, but confidence in treatment only increased for 4 conditions.
Results of self-care importance, self-care frequency and mindfulness were consistent baseline to postrotation. The mean time residents spent regularly practicing self-care during a work week increased slightly while feelings of burnout decreased. The perceived stress scale average score decreased from 13.4 at baseline to 10.5 after rotation.
DISCUSSION
The implementation of an IM residency rotation that incorporates whole health and interprofessional practices demonstrated improved perception and increased use of IM/CAM resources and knowledge among a small sample of third-year residents. Residents reported they had a positive experience participating in the rotation and gained knowledge, resources, and skills they felt confident discussing with their patients.
Many studies reported favorable attitudes and perceptions of IM/CAM use among physicians, but few have assessed these measures while implementing a training curriculum.3,4,22 Gardiner and colleagues reported on the perception and use of IM resources among family medicine residents.4 The study found that while 58% of all residents reported IM/CAM as an important part of their training, only 60% reported they received it or had specific learning objectives in their curriculum. 4 The program outlined in this study and previous research illustrate that physicians recognize the importance of IM/CAM education in training programs, but most were unaware of the resources available or did not feel comfortable counseling patients about most IM/CAM applications.
Residents in this program slightly increased their use of IM/CAM to diagnose and treat medical conditions after the rotation. A study by Wahner-Roedler and colleagues assessed physician knowledge regarding common IM/CAM therapies.3 On average, physicians only felt knowledgeable and comfortable counseling patients for 3 of 13 listed treatments/techniques and few natural herbal treatments. The study also found that most physicians had difficulty accessing IM/CAM information at their institution despite having free access to electronic databases. However, this study only assessed physician attitudes of IM/CAM and did not include an educational component to increase their knowledge of the modalities.3 This evaluation supports the need for interventions like the program described in this article that provide physicians with access to evidence-based resources combined with the applied experiences to increase their comfort within this growing field.
Though the sample size in this study was small, its results support existing research indicating that clinicians view selfcare as important. Many residents were already using a self-care plan at baseline, but there was slight increase in the practice of self-care during the rotation and a slight decrease in burnout. Previous research reflects high rates of burnout and relatively poor quality of life among primary care physicians.15 Burnout is associated with lower quality of care, lower patient satisfaction and contributes to medical errors. Studies suggest as many as 60% of primary care physicians report symptoms of burnout, which negatively affected the quality of patient care they provide.15
Despite the profound effects burnout has on physicians and patient care, a standardized wellness education or self-care tool kit is not currently available. The University of Massachusetts recently introduced a pilot program to promote resident wellness that demonstrated favorable results.15 A meta-analysis of physicians and medical trainees found decreases in anxiety and symptoms of anxiety as well as a decrease in burnout among participants in cognitive, behavioral and mindfulness interventions.29 However, unlike our program, these programs focused solely on the well-being of medical trainees, residents, and physicians and didn’t focus on the patient-clinician interactions. Given the impact on patient care, there is a need to develop and implement additional programs like our residency rotation that promote health and wellness among physicians while also evaluating how physicians may translate these skills to patient education.
While this program st i l l exists for third-year residents at Baltimore GRECC, it has significantly changed since the COVID-19 pandemic. For about the first 6 months of the pandemic, when physical distancing requirements were in place, family medicine trainees were not able to rotate. Upon return to the facility, many group classes were cancelled and some clinicians no longer offered the sessions. The rotation has evolved to a hybrid format, where many group classes for veteran patients are offered virtually, and residents observe a mix of virtual and in-person shadowing opportunities. Our formal evaluation included administering the survey and occurred from July 2018 to July 2019 but wasn’t implemented upon return to post-COVID activities due to the inconsistent experiences offered to residents over the past few years. Future research should evaluate the impact of this hybrid program on the clinicians and explore dissemination to other VAMCs and their academic affiliates.
Limitations
Project recruitment was limited to 11 family medicine and 1 preventive medicine resident. Perceptions, use of IM/CAM, and knowledge about IM/CAM could be considerably different in different departments with varying schedules, hours worked, and patient volumes. Secondly, the survey was conducted 2 weeks apart. Indications of self-care and burnout may not reflect long-term effects, adoption, or maintenance. Future research should include longer follow up to examine how this type of educational activity may impact burnout rates of physicians following the completion of residency, as well as changes in perspectives of IM/CAM while practicing as a physician. Trainees were exposed to a wide range of health care professions, but additional research is needed regarding medical resident perceptions of the roles of specific professions in a collaborative health care team.30,31
CONCLUSIONS
The residency rotation program illustrates the benefits of establishing a standardized IM/CAM rotation that includes self-care resources in family medicine programs to adequately train clinicians to practice wellness and promote it to their patients. The results of this project suggest this type of training will help residents assess the literature to better counsel patients on IM/CAM options while also providing strategies for maintaining optimal health and well-being for health care professionals. Broadening and shifting the scope of medicine from treatment to prevention, personal wellness, and optimal healing should be a top priority.
Integrative medicine or complementary alternative medicine (IM/CAM) is increasingly being recognized as an integral part of optimal health and healing. IM/CAM “reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines.”1 IM/CAM encompasses a wide range of therapies, conceptual frameworks, and health care-related professions, such as acupuncture, massage, dietary supplements, mindfulness, yoga, meditation and guided imagery.1 Research has found that 30% to 98% of patients with chronic conditions seek IM/CAM therapies.1-3
Despite the high prevalence of patients utilizing IM/CAM therapies and the National Institutes of Health grants for IM/CAM education, implementation of IM/CAM instruction in graduate medical education programs remains inconsistent.1 Barriers cited by programs include a lack of IM/CAM experts in the program, faculty training, competing financial resources, and an already full resident education schedule.4 As a result, many physicians have limited or no training in IM/CAM.1,5
The US Department of Veterans Affairs (VA) offers IM/CAM health programs to veterans and caregivers as part of its whole health care initiative.6 Several VA health care systems have adopted whole health and IM/CAM through programs for mental health integration into primary care; women’s health; integrative pain care; geriatrics, through adoption of Age-Friendly Health Systems standards; and nutrition and physical activity.7-13 The VA provides training to more medical students than any other health system: > 95% of US medical schools are affiliated with a VA medical center (VAMC).14 As part of the training mission, VA seeks to encourage students of diverse professions to consider careers in the VA.14
Residency is a time for newly licensed physicians to acquire additional experience and training to translate knowledge and skills acquired during medical school directly to patient care.15 However, residency curricula have limited time to incorporate IM/CAM training. Residency training is also physically and psychosocially demanding, often resulting in inadequate self-care, poor work-life balance, and disrupted sleep.16-18 Resident wellness is at a historic low, resulting in high rates of burnout during training.4,15
Residency programs are required to provide wellness education; however, most programs include minimal content.19 Despite high rates of burnout, formal curricula on the topic have not been established. 20 IM/CAM education also can provide a path for residents to learn about and engage in mindfulness-based training or cognitive stress reduction for self-care.
INTEGRATIVE WHOLE HEALTH ROTATION
In 2017, the Baltimore Geriatric Research Education and Clinical Center (GRECC) established an IM/whole health residency rotation and created a structured curriculum incorporating self-assessment, active reflection, and self-care to complement training in specific IM/CAM modalities for residents in family medicine. The curriculum evaluated how this training improved residents’ perceptions of IM/CAM and how it personally and professionally impacted the practice of self-care as a strategy to decrease burnout. We hypothesized that this structured experience would increase IM/CAM knowledge among clinicians while promoting the importance and practice of self-care to reduce burnout.
The 2-week IM/CAM curriculum was developed by University of Maryland School of Medicine faculty in partnership with the Baltimore GRECC and staff at the VA Maryland Health Care System. The curriculum was designed to expose residents to the 8 components of the whole health Circle of Health (moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind) in addition to IM/ CAM modalities the VA is mandated to offer to veterans (acupuncture, chiropractic, meditation, massage therapy, biofeedback, clinical hypnosis, guided imagery, yoga, and tai chi).21 Twelve residents (1 preventive medicine and 11 third-year family medicine residents) rotated individually throughout the year as part of their behavioral health block rotation. All residents completed the 2-week curriculum as their schedules allowed. The curriculum consisted of didactics sessions and activities at the Baltimore, Loch Raven, and Perry Point VAMCs. Residents completed evaluations before and after the rotation. The experience described in this article by the residents and the survey data were collected from the 2018/2019 training year. A rotation syllabus, competencies adapted from Locke and colleagues and skills residents obtain during this rotation that support these competencies, as well as a resident sample schedule were developed (eAppendix is available at doi:10.12788/fp.0544).1

Rotation Overview
for each resident were built around instructional opportunities, which included 1-on-1 didactics, direct observation of treatment modalities, and personal reflection of the residents’ self-care practices. While each resident’s rotation schedule varied slightly due to their schedules, the foundational instruction elements were the same. Didactic session themes included an overview of IM/CAM, nutrition, narrative medicine, pain psychology, music therapy, chaplain services, motor-cognitive training, and exercise guidelines. Assigned readings, including peer-reviewed literature on IM/CAM therapies, complemented all sessions. Residents created an evidence-supported integrative treatment plan for a patient with a condition of interest to them.
Residents observed clinician-led veteran group sessions on IM/CAM treatment modalities, including guided meditation, mindfulness and relaxation, self-awareness, living well with chronic pain, tai chi, drumming for health and balance, anger management, recovery group, acceptance and commitment therapy, and Gerofit exercise. The group classes allowed residents to actively participate in the activity or discussion. Residents also shadowed VA clinicians in sleep, pain, nutrition, acupuncture, and mental health clinics.
Residents were encouraged to practice self-care during the 2-week rotation. The rotation schedule built in free time, including a 1-hour daily lunch period, for residents to consider their own health habits, complete a personal health inventory, and try self-care activities outlined on the syllabus with links to resources. These resources also served as educational materials that residents could share with patients. All materials, including didactic lectures, journal articles and self-care resources, were provided to each resident through a free online course to ensure residents had access throughout and following completion of the rotation. This content, including the rotation evaluation metrics, is available upon request from the corresponding author.
Evaluations
Residents completed a survey before and after the rotation to measure IM/CAM knowledge and application and self-care/ burnout perceptions. Residents were asked to evaluate rotation sessions and comment on whether this rotation benefited them personally and professionally (Table 1). Descriptive statistics were analyzed using Microsoft Excel. Given the small sample size and lack of statistical power, only mean survey results are reported in this article. Because this opportunity is specific to the University of Maryland School of Medicine and the proposed project was part of ordinary educational practice, the study was deemed not human subject research by the University of Maryland Institutional Review Board (HP-00089256).

Perceptions and attitudes toward IM/CAM were assessed using a survey designed by the University of Minnesota Academic Health Center. It included 18 items scored on a 5-point semantic rating scale (1, strongly disagree; 5, strongly agree).22 Residents rated their level of agreement with statements reflecting both positive (eg, clinical care should integrate the best of conventional and CAM practices) and negative (eg, CAM is a threat to public health) views. Three questions adapted from the NHIS Adult Complementary Health Questionnaire and UC Irvine Survey of Health Care Use and Practice assessed the use of IM/CAM resources.23,24
Resident knowledge and application of IM/CAM were measured using a case study designed by the course faculty. The case listed a chief complaint of nerve pain, with a history of chronic pain, neuropathic pain, anxiety, chronic fatigue, depression, insomnia, posttraumatic stress disorder, history of present illness, past surgical history, medication list, review of symptoms, laboratory values, and physical examination. The residents completed an assessment before and after the rotation. Residents rated their confidence in the diagnosis and treatment of 8 medical conditions using a 5-point semantic rating scale (Table 2). Self-care importance and selfcare frequency were measured by a variety of means, including 3 survey questions, the Five Facet Mindfulness Questionnaire, 2 prompts on a 7-point semantic scale, and a slightly modified version of the validated Perceived Stress Scale.25-28

Survey Results
Residents gave the rotation positive feedback with a mean score of 8.5 out of 10. They reported the beneficial impact of seeing the nontraditional and nonpharmacological practices in treating patients, chronic pain management team approaches, and enjoyed being able to participate in group classes with patients. Many residents expressed a desire for a longer rotation to have more time to experience the behavioral health-focused sessions. Residents also requested additional information on nutritional supplements/natural medicines, battlefield acupuncture training and osteopathic manipulative therapy practices. All residents reported the rotation personally and/or professionally benefited them (Appendix).
Given the sample of 12 residents, values are presented as prerotation to postrotation comparisons without statistical analysis. There was a trend towards an increase in the reported use and recommendation of 26 modalities of nonconventional therapies following the rotation. There was also a slight increase in resource knowledge and use of these resources, and residents reported accessing more types of resources. Mean scores of the case study to gauge knowledge and application of IM increased from 7.5 at baseline to 11.0 after the rotation. Resident confidence in diagnosis increased for all 8 conditions, but confidence in treatment only increased for 4 conditions.
Results of self-care importance, self-care frequency and mindfulness were consistent baseline to postrotation. The mean time residents spent regularly practicing self-care during a work week increased slightly while feelings of burnout decreased. The perceived stress scale average score decreased from 13.4 at baseline to 10.5 after rotation.
DISCUSSION
The implementation of an IM residency rotation that incorporates whole health and interprofessional practices demonstrated improved perception and increased use of IM/CAM resources and knowledge among a small sample of third-year residents. Residents reported they had a positive experience participating in the rotation and gained knowledge, resources, and skills they felt confident discussing with their patients.
Many studies reported favorable attitudes and perceptions of IM/CAM use among physicians, but few have assessed these measures while implementing a training curriculum.3,4,22 Gardiner and colleagues reported on the perception and use of IM resources among family medicine residents.4 The study found that while 58% of all residents reported IM/CAM as an important part of their training, only 60% reported they received it or had specific learning objectives in their curriculum. 4 The program outlined in this study and previous research illustrate that physicians recognize the importance of IM/CAM education in training programs, but most were unaware of the resources available or did not feel comfortable counseling patients about most IM/CAM applications.
Residents in this program slightly increased their use of IM/CAM to diagnose and treat medical conditions after the rotation. A study by Wahner-Roedler and colleagues assessed physician knowledge regarding common IM/CAM therapies.3 On average, physicians only felt knowledgeable and comfortable counseling patients for 3 of 13 listed treatments/techniques and few natural herbal treatments. The study also found that most physicians had difficulty accessing IM/CAM information at their institution despite having free access to electronic databases. However, this study only assessed physician attitudes of IM/CAM and did not include an educational component to increase their knowledge of the modalities.3 This evaluation supports the need for interventions like the program described in this article that provide physicians with access to evidence-based resources combined with the applied experiences to increase their comfort within this growing field.
Though the sample size in this study was small, its results support existing research indicating that clinicians view selfcare as important. Many residents were already using a self-care plan at baseline, but there was slight increase in the practice of self-care during the rotation and a slight decrease in burnout. Previous research reflects high rates of burnout and relatively poor quality of life among primary care physicians.15 Burnout is associated with lower quality of care, lower patient satisfaction and contributes to medical errors. Studies suggest as many as 60% of primary care physicians report symptoms of burnout, which negatively affected the quality of patient care they provide.15
Despite the profound effects burnout has on physicians and patient care, a standardized wellness education or self-care tool kit is not currently available. The University of Massachusetts recently introduced a pilot program to promote resident wellness that demonstrated favorable results.15 A meta-analysis of physicians and medical trainees found decreases in anxiety and symptoms of anxiety as well as a decrease in burnout among participants in cognitive, behavioral and mindfulness interventions.29 However, unlike our program, these programs focused solely on the well-being of medical trainees, residents, and physicians and didn’t focus on the patient-clinician interactions. Given the impact on patient care, there is a need to develop and implement additional programs like our residency rotation that promote health and wellness among physicians while also evaluating how physicians may translate these skills to patient education.
While this program st i l l exists for third-year residents at Baltimore GRECC, it has significantly changed since the COVID-19 pandemic. For about the first 6 months of the pandemic, when physical distancing requirements were in place, family medicine trainees were not able to rotate. Upon return to the facility, many group classes were cancelled and some clinicians no longer offered the sessions. The rotation has evolved to a hybrid format, where many group classes for veteran patients are offered virtually, and residents observe a mix of virtual and in-person shadowing opportunities. Our formal evaluation included administering the survey and occurred from July 2018 to July 2019 but wasn’t implemented upon return to post-COVID activities due to the inconsistent experiences offered to residents over the past few years. Future research should evaluate the impact of this hybrid program on the clinicians and explore dissemination to other VAMCs and their academic affiliates.
Limitations
Project recruitment was limited to 11 family medicine and 1 preventive medicine resident. Perceptions, use of IM/CAM, and knowledge about IM/CAM could be considerably different in different departments with varying schedules, hours worked, and patient volumes. Secondly, the survey was conducted 2 weeks apart. Indications of self-care and burnout may not reflect long-term effects, adoption, or maintenance. Future research should include longer follow up to examine how this type of educational activity may impact burnout rates of physicians following the completion of residency, as well as changes in perspectives of IM/CAM while practicing as a physician. Trainees were exposed to a wide range of health care professions, but additional research is needed regarding medical resident perceptions of the roles of specific professions in a collaborative health care team.30,31
CONCLUSIONS
The residency rotation program illustrates the benefits of establishing a standardized IM/CAM rotation that includes self-care resources in family medicine programs to adequately train clinicians to practice wellness and promote it to their patients. The results of this project suggest this type of training will help residents assess the literature to better counsel patients on IM/CAM options while also providing strategies for maintaining optimal health and well-being for health care professionals. Broadening and shifting the scope of medicine from treatment to prevention, personal wellness, and optimal healing should be a top priority.
- Locke AB, Gordon A, Guerrera MP, Gardiner P, Lebensohn P. Recommended integrative medicine competencies for family medicine residents. Explore (NY). 2013;9(5):308-313. doi:10.1016/j.explore.2013.06.005
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. doi:10.1001/jama.280.18.1569
- Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501. doi:10.1093/ecam/nel036
- Gardiner P, Filippelli AC, Lebensohn P, Bonakdar R. Family medicine residency program directors attitudes and knowledge of family medicine CAM competencies. Explore (NY). 2013;9(5):299-307. doi:10.1016/j.explore.2013.06.002
- Sierpina V, Levine R, Astin J, Tan A. Use of mind-body therapies in psychiatry and family medicine faculty and residents: attitudes, barriers, and gender differences. Explore (NY). 2007;3(2):129-135. doi:10.1016/j.explore.2006.12.001
- Krist AH, South-Paul J, Meisnere M, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023.
- Bokhour BG, DeFaccio R, Gaj L, et al. Changes in patientreported outcomes associated with receiving whole health in the Veteran Health Administration (VHA)’s National Demonstration Project. J Gen Intern Med. 2024;39(1):84-94. doi:10.1007/s11606-023-08376-0
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial- spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Gabrielian S, Jones AL, Hoge AE, et al. Enhancing primary care experiences for homeless patients with serious mental illness: results from a national survey. J Prim Care Community Health. 2021;12:2150132721993654. doi:10.1177/2150132721993654
- Matthieu MM, Church KA, Taylor LD, et al. Integrating the age-friendly health systems movement in Veterans Health Administration: national advance care planning via group visits and the 4Ms framework. Health Soc Work. 2023;48(4):277-280. doi:10.1093/hsw/hlad022
- Meisler AW, Gianoli MO, Na PJ, Pietrzak RH. Functional disability in US military veterans: the importance of integrated whole health initiatives. Prim Care Companion CNS Disord. 2023;25(4):22m03461. doi:10.4088/PCC.22m03461
- Ortmeyer HK, Giffuni J, Etchberger D, Katzel L. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13(19):3047. doi:10.3390/ani13193047
- Sullivan MB, Hill K, Ballengee LA, et al. Remotely delivered psychologically informed mindful movement physical therapy for pain care: a framework for operationalization. Glob Adv Integr Med Health. 2023;12:27536130231209751. doi:10.1177/27536130231209751
- (OAA) OoAA. 75th Anniversary: Passion to learn. Power to heal. Washington DC.: US Department of Veterans Affairs; 2021. https://content.yudu.com/web/448fx/0A448g9/75thAnniversary2021/html/index.html?page=24&origin=reader
- Runyan C, Savageau JA, Potts S, Weinreb L. Impact of a family medicine resident wellness curriculum: a feasibility study. Med Educ Online. 2016;21:30648. doi:10.3402/meo.v21.30648
- Lafreniere JP, Rios R, Packer H, Ghazarian S, Wright SM, Levine RB. Burned out at the bedside: patient perceptions of physician burnout in an internal medicine resident continuity clinic. J Gen Intern Med. 2016;31(2):203-208. doi:10.1007/s11606-015-3503-3
- Freedy JR, Staley C, Mims LD, et al. Social, individual, and environmental characteristics of family medicine resident burnout: a CERA study. Fam Med. 2022;54(4):270-276. doi:10.22454/FamMed.2022.526799
- Alrishan MA, Alshammari SA. Prevalence of sleep deprivation and its effect on the performance of family medicine residents in Riyadh, Saudi Arabia. J Family Community Med. 2020;27(2):125-130. doi:10.4103/jfcm.JFCM_9_20
- ACGME. ACGME Program Requirements for Graduate Medical Education in Family Medicine. https://www.acgme.org/globalassets/pfassets/programrequirements/120_familymedicine_2024.pdf
- Nene Y, Tadi P. Resident Burnout. In: StatPearls; 2023.
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the veterans affairs to a whole health system of care: time for action and research. Med Care. 2020;58(4):295-300. doi:10.1097/MLF.0000000000001316
- Kreitzer MJ, Mitten D, Harris I, Shandeling J. Attitudes toward CAM among medical, nursing, and pharmacy faculty and students: a comparative analysis. Altern Ther Health Med. 2002;8(6):44-53.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
- Nguyen J, Liu MA, Patel RJ, Tahara K, Nguyen AL. Use and interest in complementary and alternative medicine among college students seeking healthcare at a university campus student health center. Complement Ther Clin Pract. 2016;24:103-108. doi:10.1016/j.ctcp.2016.06.001
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi:10.1177/1073191105283504
- Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi:10.1177/1073191107313003
- West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318- 1321. doi:10.1007/s11606-009-1129-z
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
- Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202(5):353-359. doi:10.1097/NMD.0000000000000130
- Visser CLF, Ket JCF, Croiset G, Kusurkar RA. Perceptions of residents, medical and nursing students about interprofessional education: a systematic review of the quantitative and qualitative literature. BMC Med Educ. 2017;17(1):77. doi:10.1186/s12909-017-0909-0
- Lingard L, Espin S, Evans C, Hawryluck L. The rules of the game: interprofessional collaboration on the intensive care unit team. Crit Care. 2004;8(6):R403-408. doi:10.1186/cc2958
- Locke AB, Gordon A, Guerrera MP, Gardiner P, Lebensohn P. Recommended integrative medicine competencies for family medicine residents. Explore (NY). 2013;9(5):308-313. doi:10.1016/j.explore.2013.06.005
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. doi:10.1001/jama.280.18.1569
- Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501. doi:10.1093/ecam/nel036
- Gardiner P, Filippelli AC, Lebensohn P, Bonakdar R. Family medicine residency program directors attitudes and knowledge of family medicine CAM competencies. Explore (NY). 2013;9(5):299-307. doi:10.1016/j.explore.2013.06.002
- Sierpina V, Levine R, Astin J, Tan A. Use of mind-body therapies in psychiatry and family medicine faculty and residents: attitudes, barriers, and gender differences. Explore (NY). 2007;3(2):129-135. doi:10.1016/j.explore.2006.12.001
- Krist AH, South-Paul J, Meisnere M, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023.
- Bokhour BG, DeFaccio R, Gaj L, et al. Changes in patientreported outcomes associated with receiving whole health in the Veteran Health Administration (VHA)’s National Demonstration Project. J Gen Intern Med. 2024;39(1):84-94. doi:10.1007/s11606-023-08376-0
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial- spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Gabrielian S, Jones AL, Hoge AE, et al. Enhancing primary care experiences for homeless patients with serious mental illness: results from a national survey. J Prim Care Community Health. 2021;12:2150132721993654. doi:10.1177/2150132721993654
- Matthieu MM, Church KA, Taylor LD, et al. Integrating the age-friendly health systems movement in Veterans Health Administration: national advance care planning via group visits and the 4Ms framework. Health Soc Work. 2023;48(4):277-280. doi:10.1093/hsw/hlad022
- Meisler AW, Gianoli MO, Na PJ, Pietrzak RH. Functional disability in US military veterans: the importance of integrated whole health initiatives. Prim Care Companion CNS Disord. 2023;25(4):22m03461. doi:10.4088/PCC.22m03461
- Ortmeyer HK, Giffuni J, Etchberger D, Katzel L. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13(19):3047. doi:10.3390/ani13193047
- Sullivan MB, Hill K, Ballengee LA, et al. Remotely delivered psychologically informed mindful movement physical therapy for pain care: a framework for operationalization. Glob Adv Integr Med Health. 2023;12:27536130231209751. doi:10.1177/27536130231209751
- (OAA) OoAA. 75th Anniversary: Passion to learn. Power to heal. Washington DC.: US Department of Veterans Affairs; 2021. https://content.yudu.com/web/448fx/0A448g9/75thAnniversary2021/html/index.html?page=24&origin=reader
- Runyan C, Savageau JA, Potts S, Weinreb L. Impact of a family medicine resident wellness curriculum: a feasibility study. Med Educ Online. 2016;21:30648. doi:10.3402/meo.v21.30648
- Lafreniere JP, Rios R, Packer H, Ghazarian S, Wright SM, Levine RB. Burned out at the bedside: patient perceptions of physician burnout in an internal medicine resident continuity clinic. J Gen Intern Med. 2016;31(2):203-208. doi:10.1007/s11606-015-3503-3
- Freedy JR, Staley C, Mims LD, et al. Social, individual, and environmental characteristics of family medicine resident burnout: a CERA study. Fam Med. 2022;54(4):270-276. doi:10.22454/FamMed.2022.526799
- Alrishan MA, Alshammari SA. Prevalence of sleep deprivation and its effect on the performance of family medicine residents in Riyadh, Saudi Arabia. J Family Community Med. 2020;27(2):125-130. doi:10.4103/jfcm.JFCM_9_20
- ACGME. ACGME Program Requirements for Graduate Medical Education in Family Medicine. https://www.acgme.org/globalassets/pfassets/programrequirements/120_familymedicine_2024.pdf
- Nene Y, Tadi P. Resident Burnout. In: StatPearls; 2023.
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the veterans affairs to a whole health system of care: time for action and research. Med Care. 2020;58(4):295-300. doi:10.1097/MLF.0000000000001316
- Kreitzer MJ, Mitten D, Harris I, Shandeling J. Attitudes toward CAM among medical, nursing, and pharmacy faculty and students: a comparative analysis. Altern Ther Health Med. 2002;8(6):44-53.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
- Nguyen J, Liu MA, Patel RJ, Tahara K, Nguyen AL. Use and interest in complementary and alternative medicine among college students seeking healthcare at a university campus student health center. Complement Ther Clin Pract. 2016;24:103-108. doi:10.1016/j.ctcp.2016.06.001
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi:10.1177/1073191105283504
- Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi:10.1177/1073191107313003
- West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318- 1321. doi:10.1007/s11606-009-1129-z
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
- Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202(5):353-359. doi:10.1097/NMD.0000000000000130
- Visser CLF, Ket JCF, Croiset G, Kusurkar RA. Perceptions of residents, medical and nursing students about interprofessional education: a systematic review of the quantitative and qualitative literature. BMC Med Educ. 2017;17(1):77. doi:10.1186/s12909-017-0909-0
- Lingard L, Espin S, Evans C, Hawryluck L. The rules of the game: interprofessional collaboration on the intensive care unit team. Crit Care. 2004;8(6):R403-408. doi:10.1186/cc2958
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.
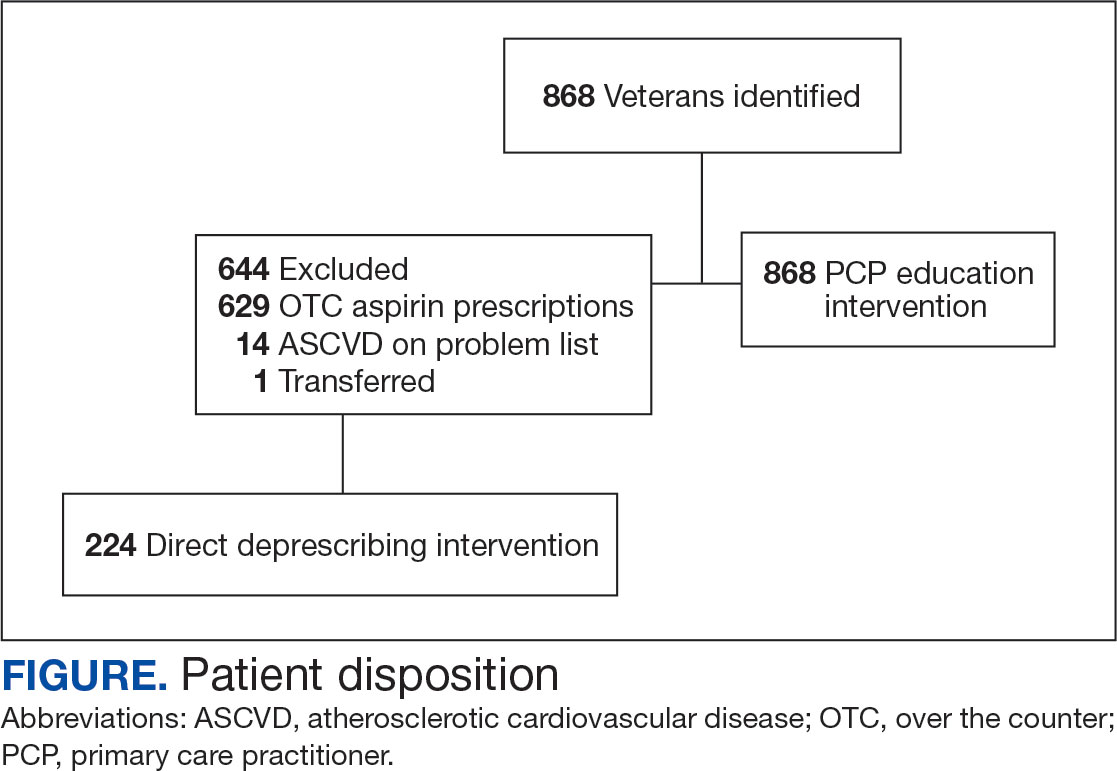
Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).
The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).
The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.

Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans








