User login
How best to approach these acute hand infections
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
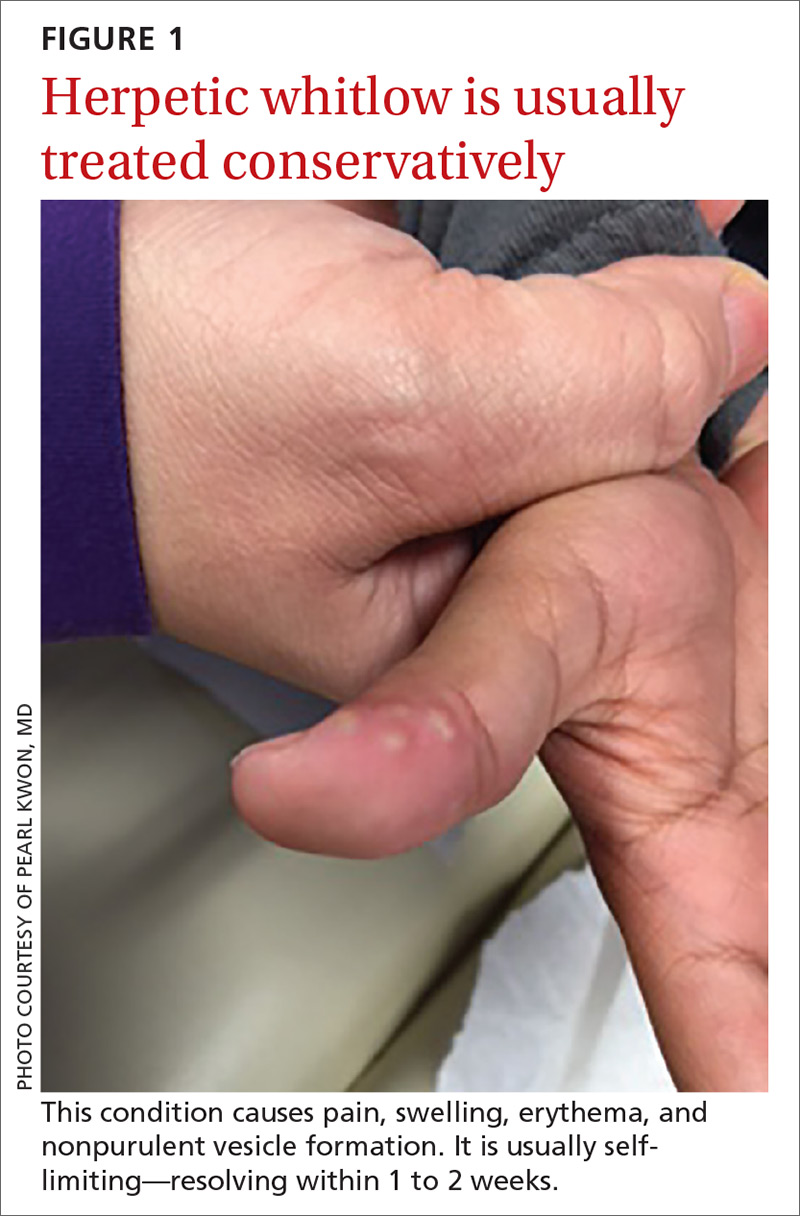
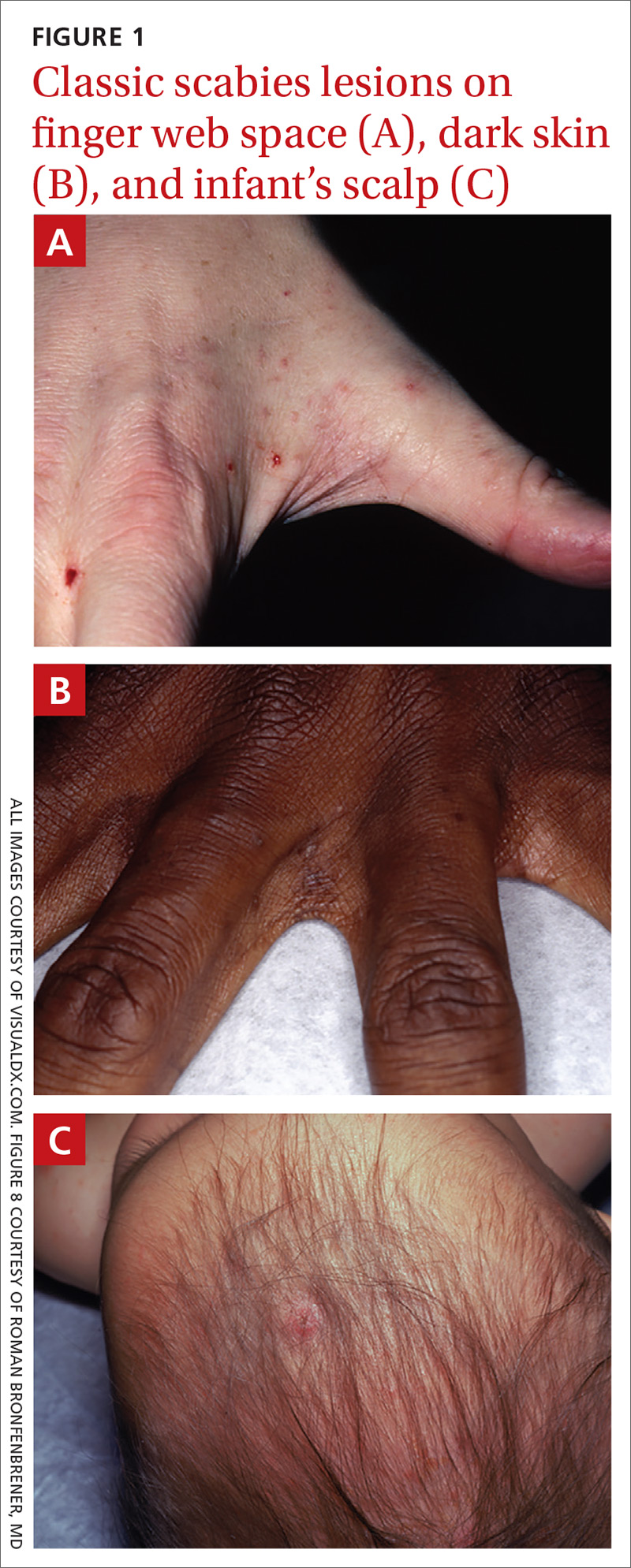
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2
Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
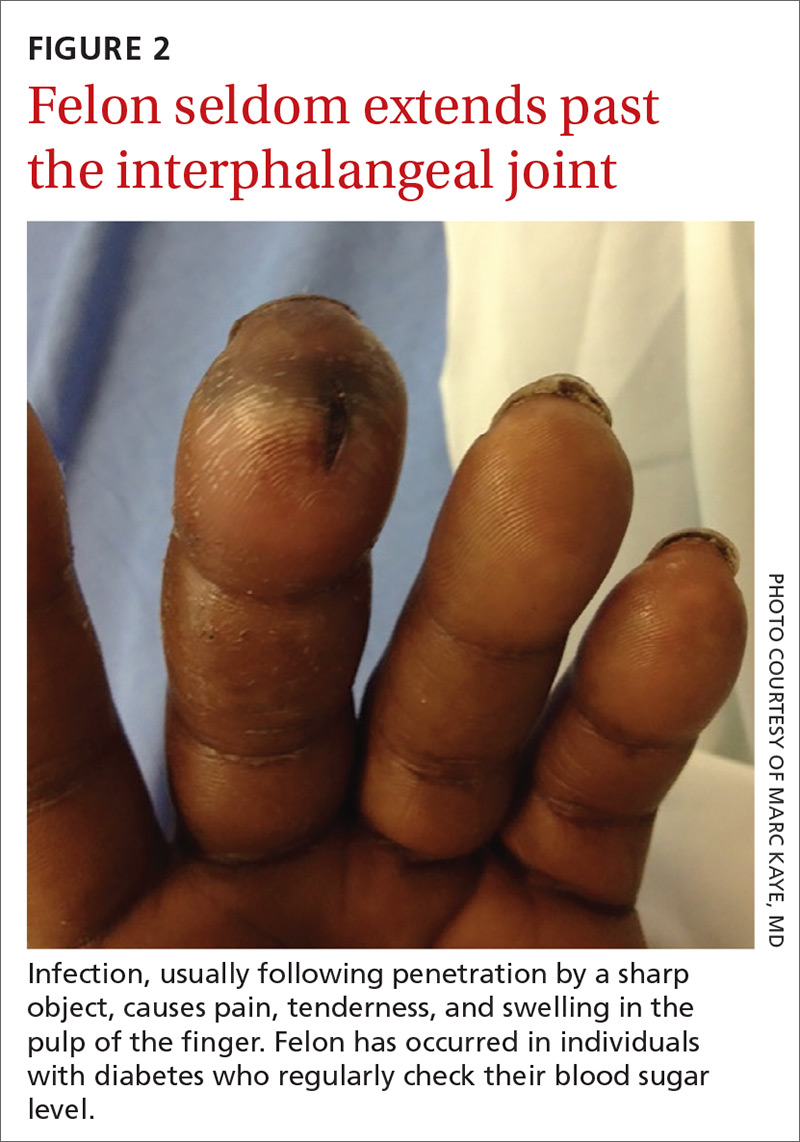
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8
Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
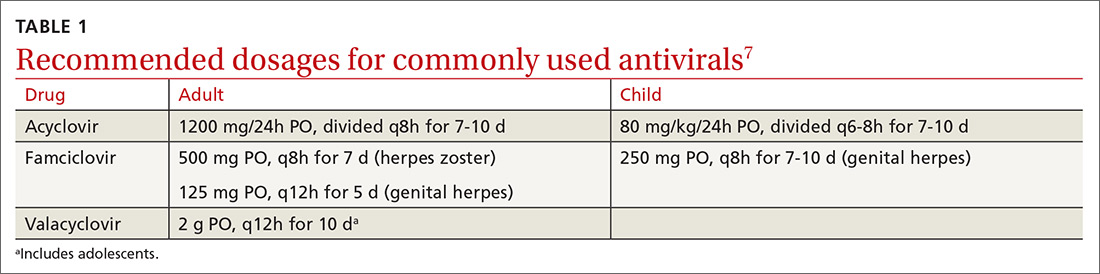
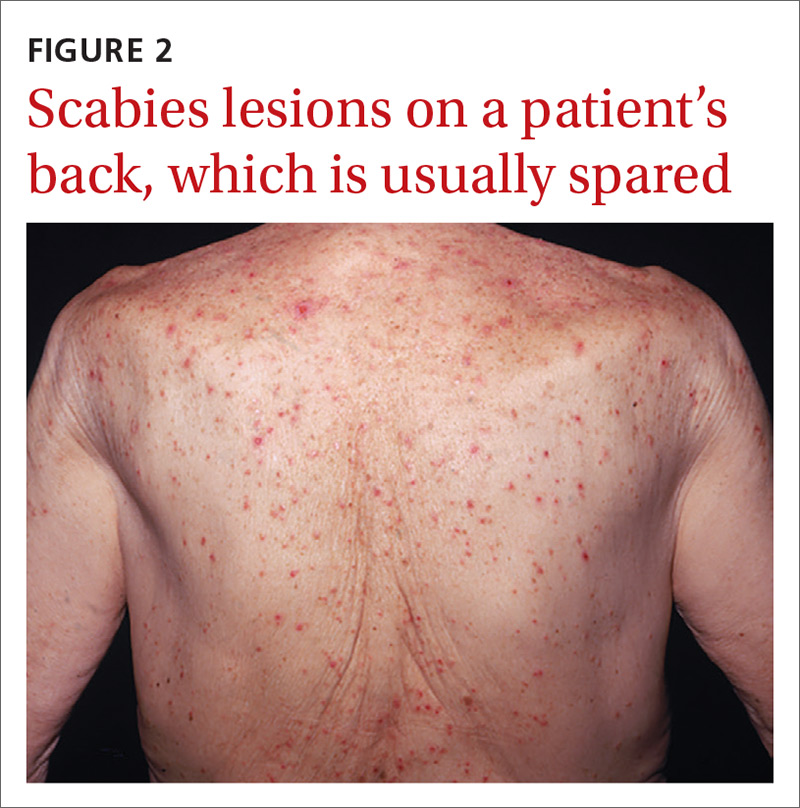
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11
How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
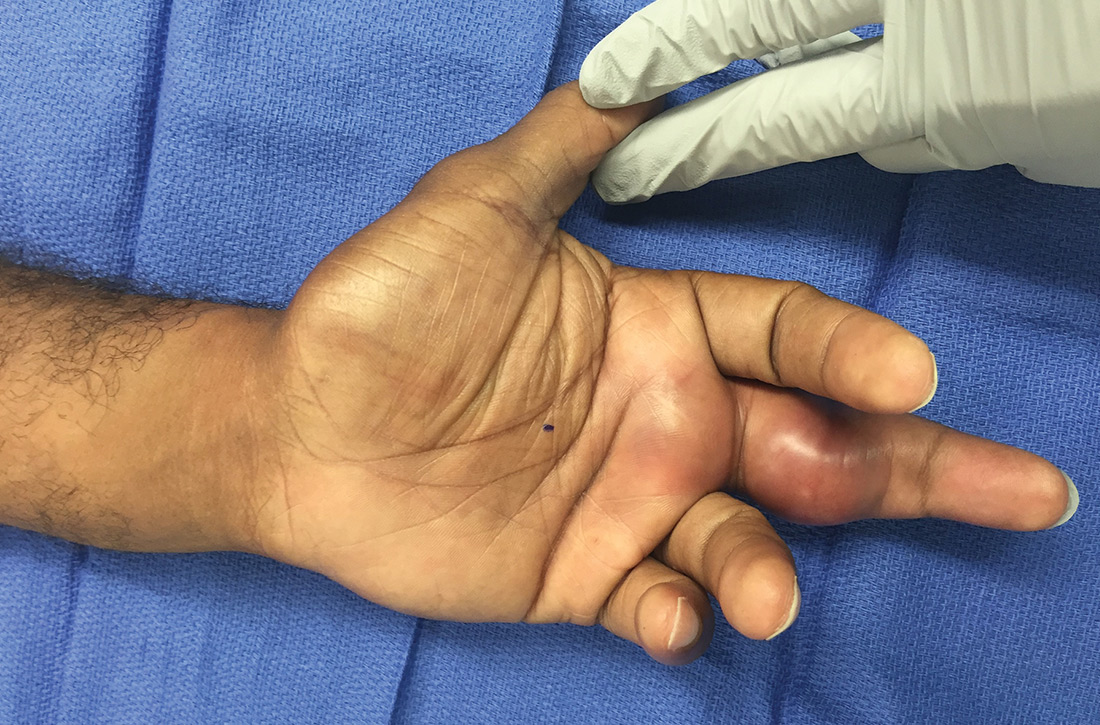
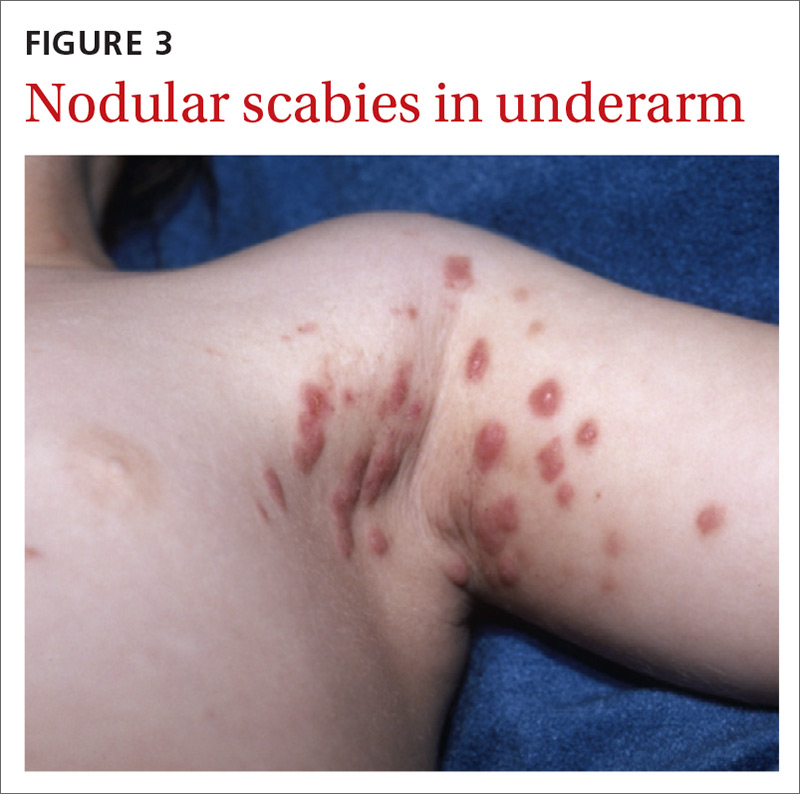
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13
How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22
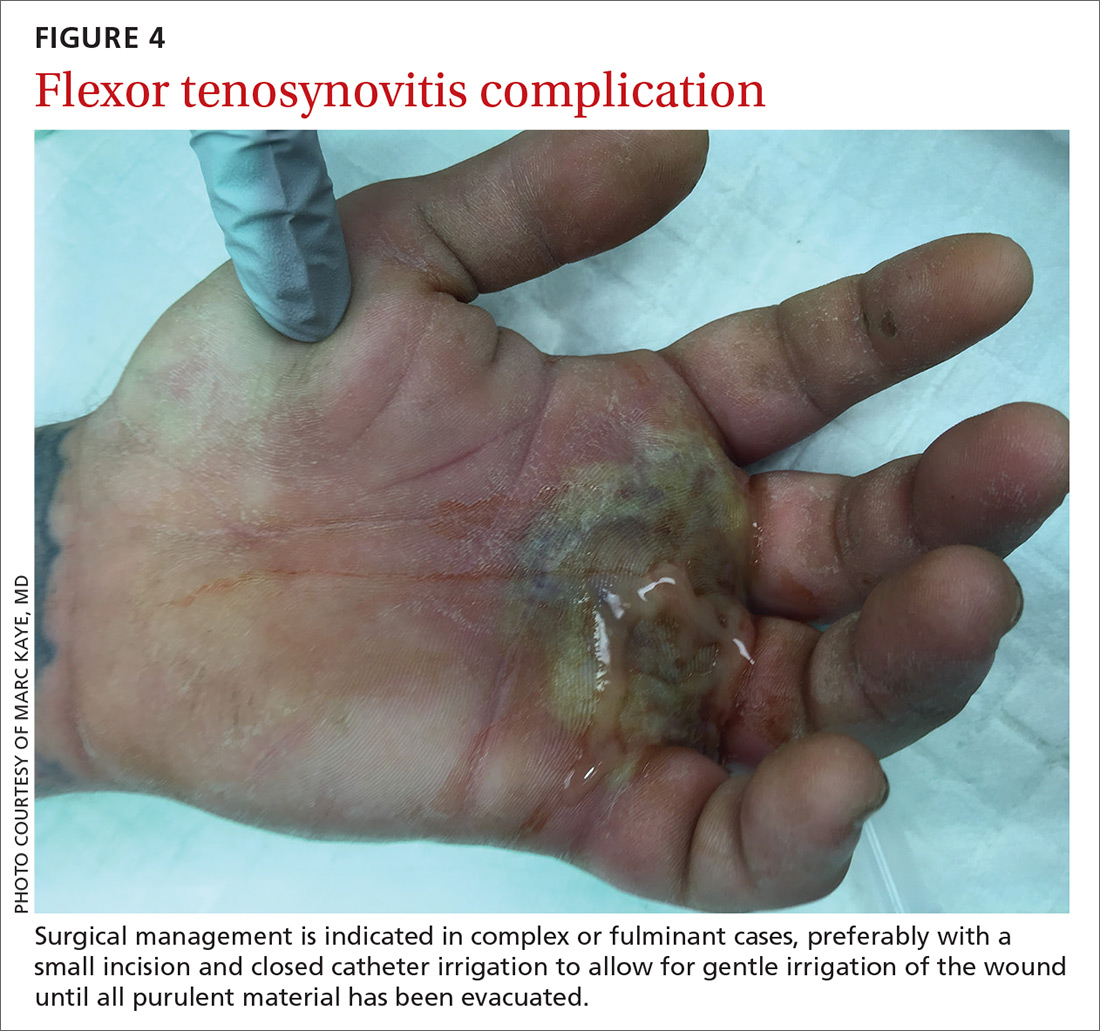
Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
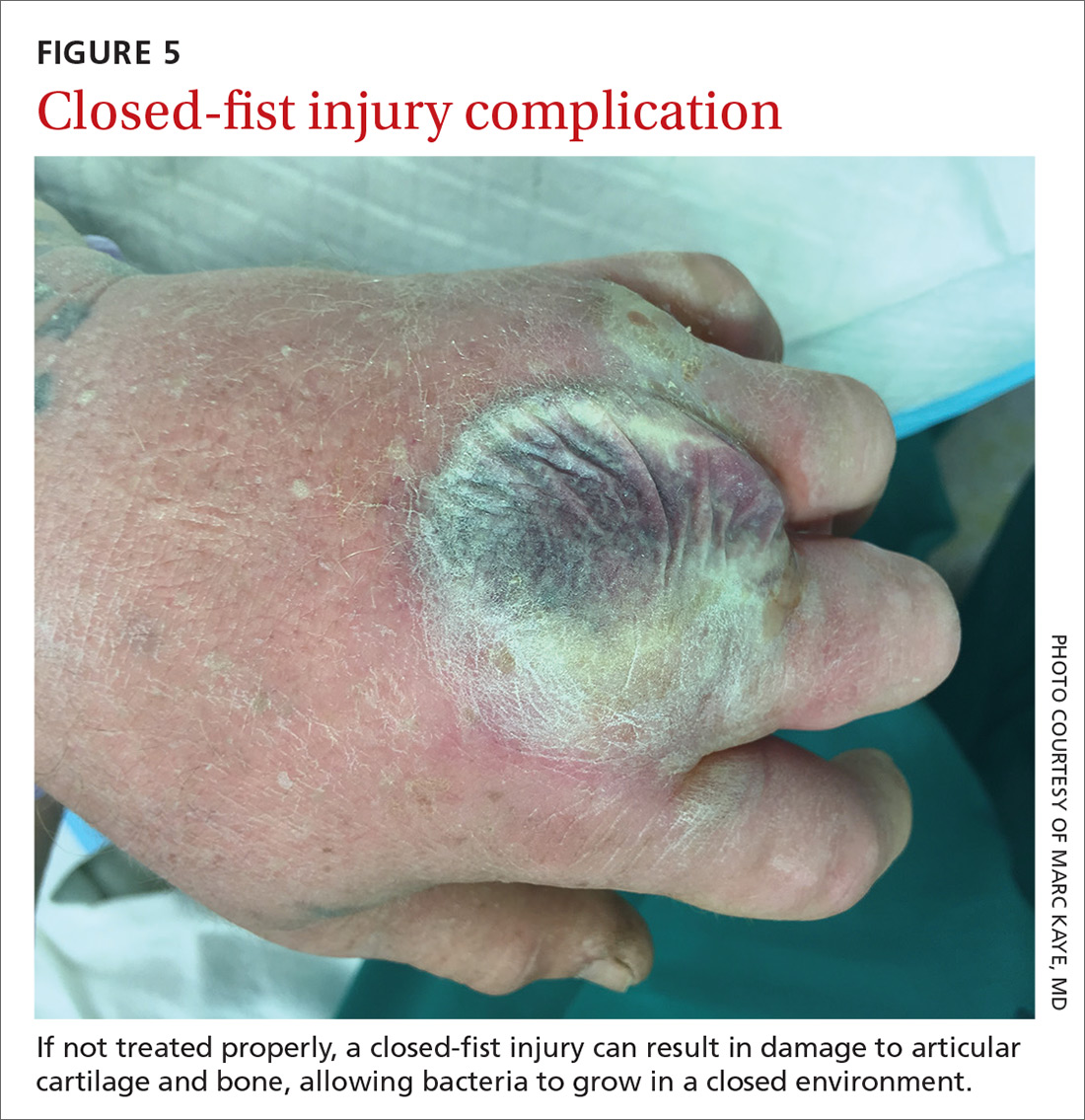
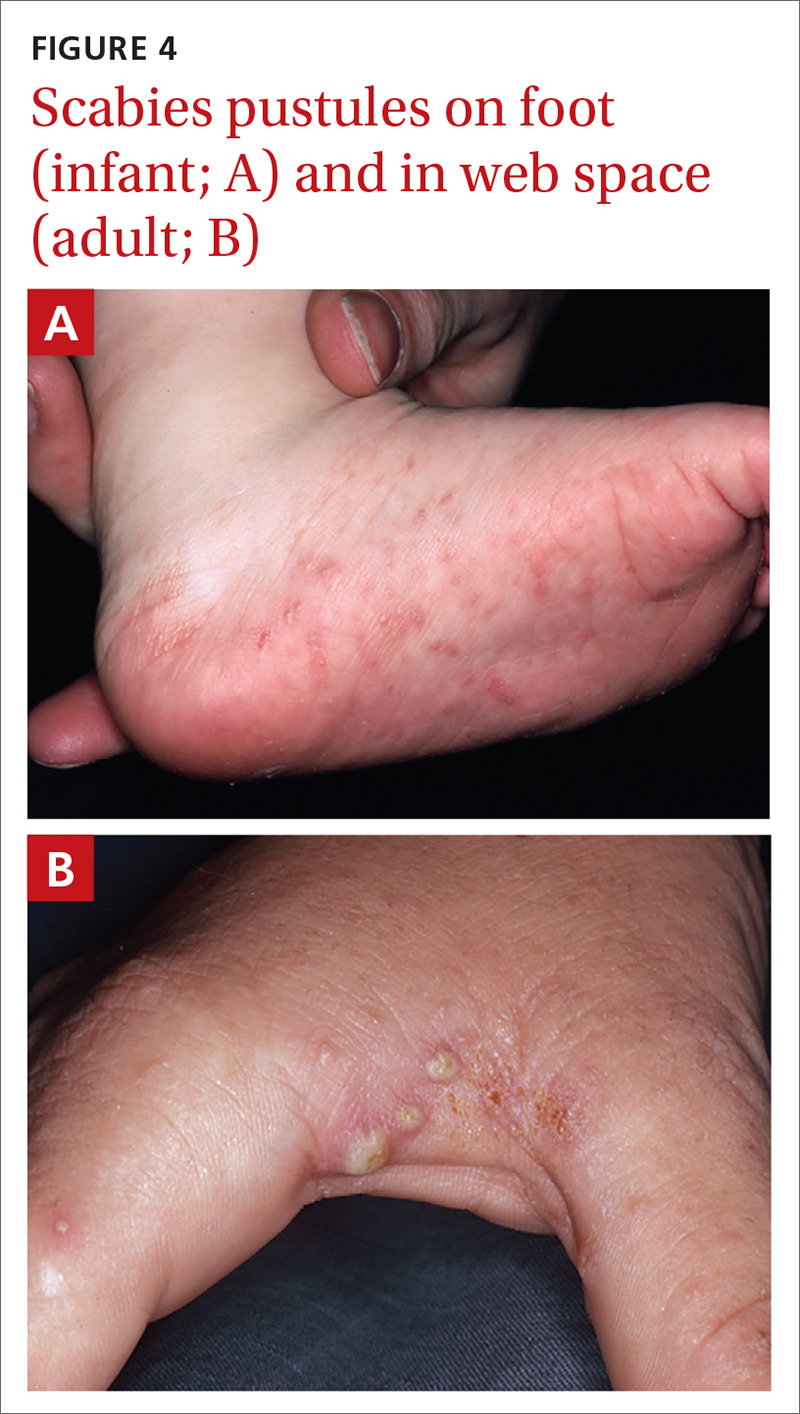
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24
Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
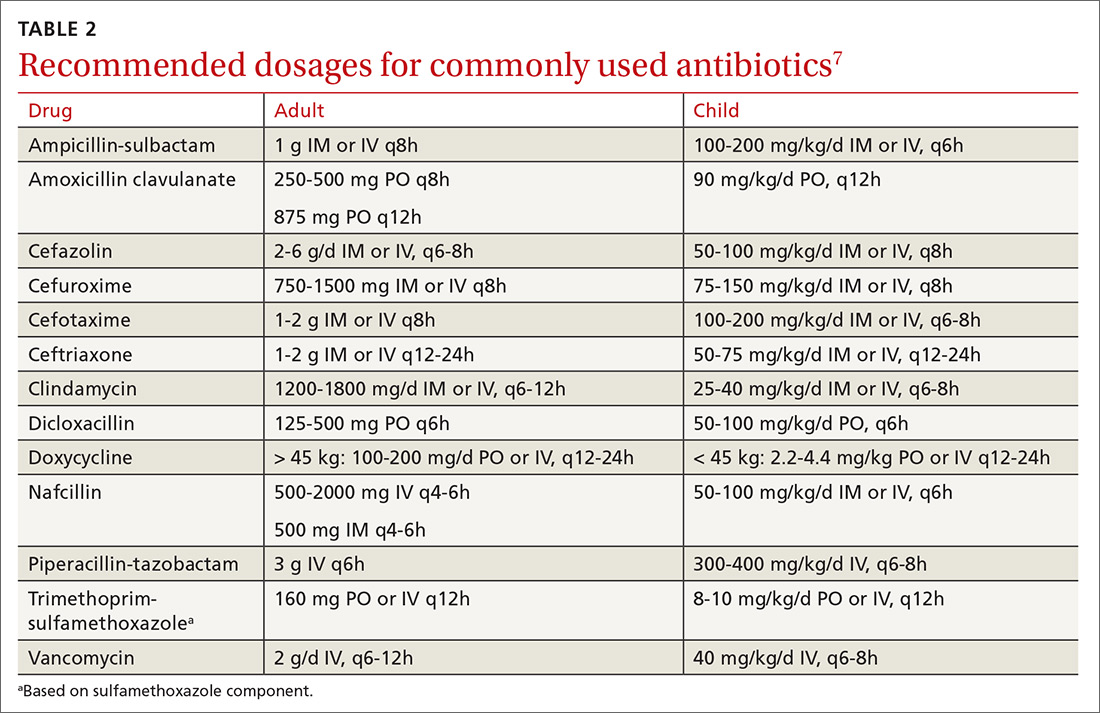
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; carlos.arango@jax.ufl.edu.
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2
Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8
Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11
How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13
How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22
Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24
Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; carlos.arango@jax.ufl.edu.
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2
Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8
Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11
How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13
How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22
Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24
Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; carlos.arango@jax.ufl.edu.
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
PRACTICE RECOMMENDATIONS
› Obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli, and fungi. A
› Use antibiotics as an adjunct to elevation and splinting in flexor tenosynovitis to improve range-of-motion outcomes. A
› Notify your microbiology lab to enrich cultures with 10% CO2 to isolate Eikenella corrodens. A
› Consider prescribing acyclovir, famciclovir, or valacyclovir for herpetic whitlow. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
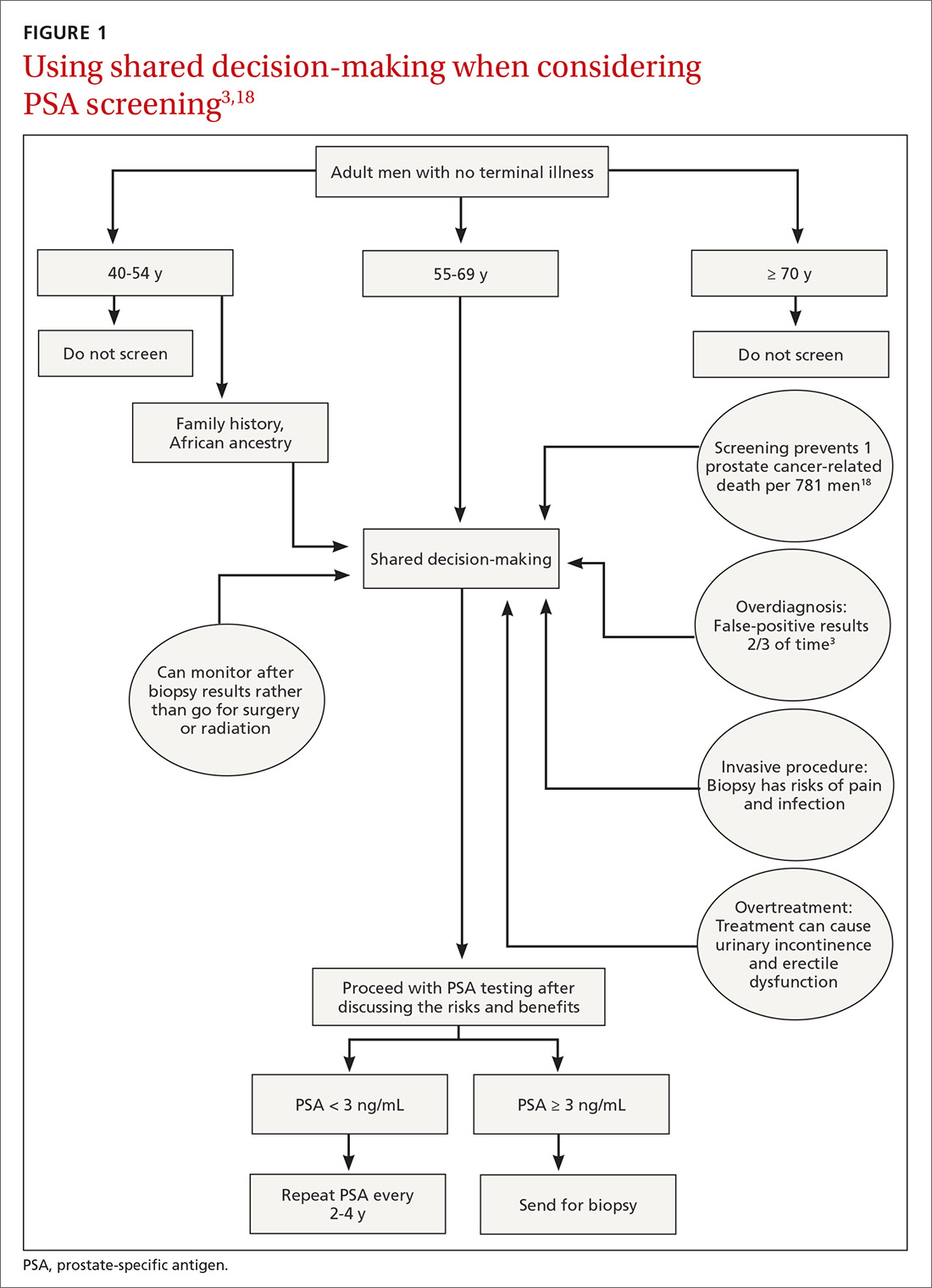
PSA cancer screening: A case for shared decision-making
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
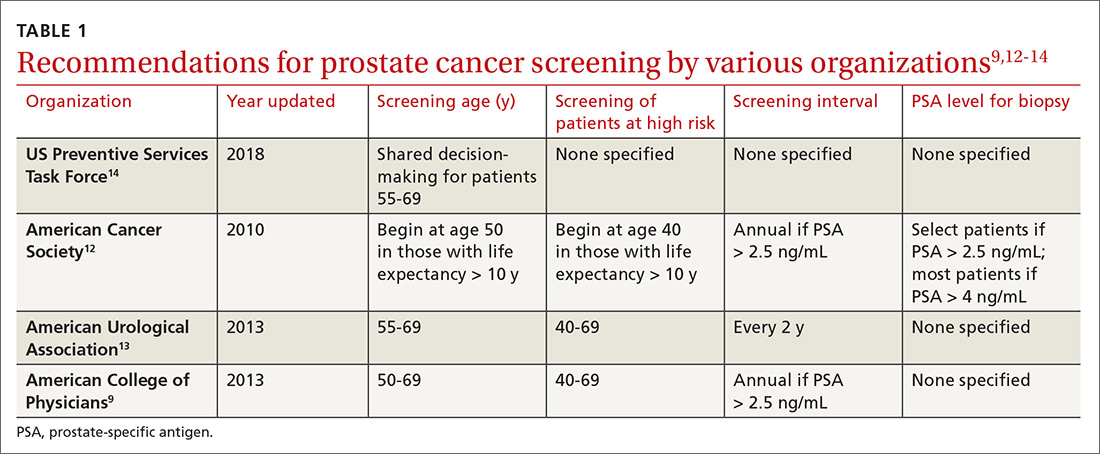
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
Does PSA-based screening improve patient-centered outcomes?
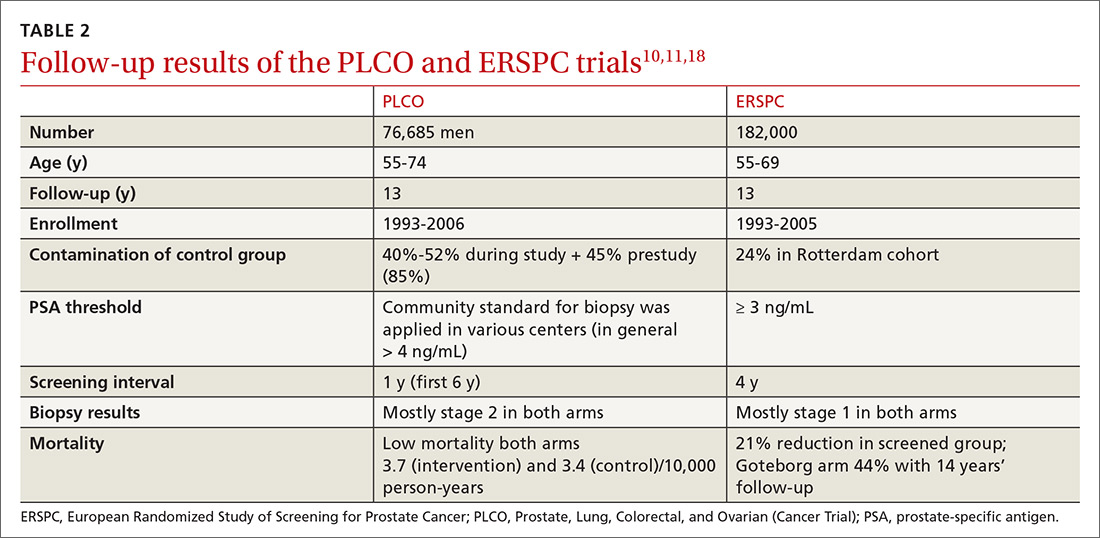
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
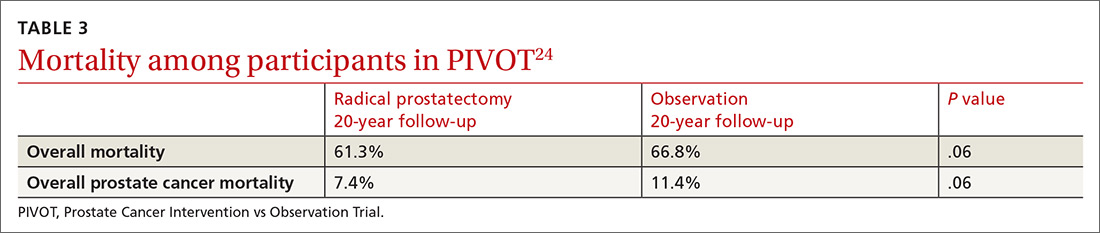
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
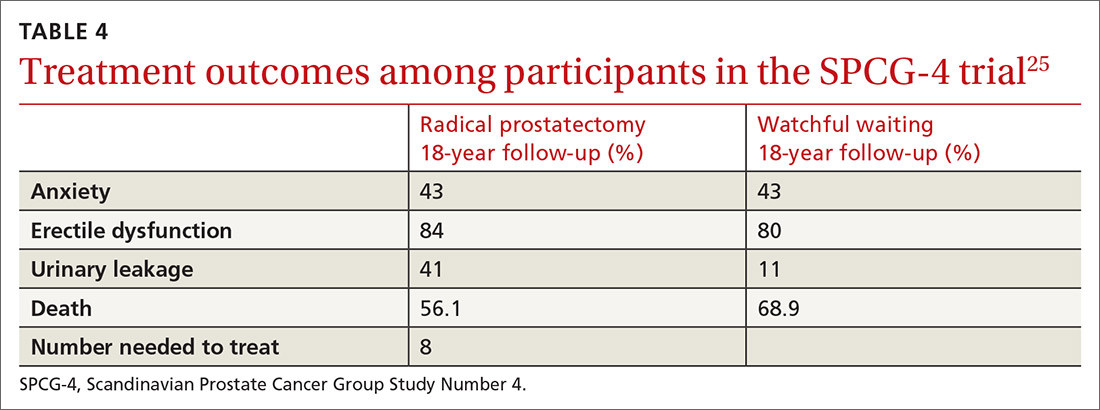
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
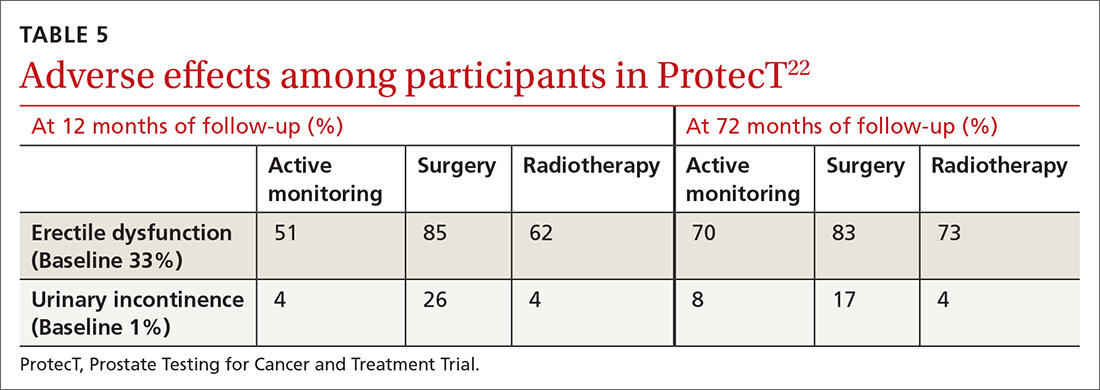
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; jxd114@case.edu.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; jxd114@case.edu.
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; jxd114@case.edu.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
PRACTICE RECOMMENDATIONS
› Recommend individualized decision-making to men ages 55 to 69 years after discussing the potential benefits and risks of prostate-specific antigen (PSA)-based screening. B
› Do not use a PSA-based screening method for prostate cancer in men ages < 50 years or > 70 years or men with a life expectancy < 10 years. C
› Do not routinely recommend PSA-based screening to men with a family history of prostate cancer or to men who are African American. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Scabies: Refine your exam, avoid these diagnostic pitfalls
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).
The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8
Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.
Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.
Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12
Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).
Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.
Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.
Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.
Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; usatine@uthscsa.edu.
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; art_papier@urmc.rochester.edu.
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).
The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8
Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.
Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.
Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12
Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).
Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.
Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.
Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.
Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; usatine@uthscsa.edu.
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; art_papier@urmc.rochester.edu.
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).
The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8
Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.
Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.
Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12
Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).
Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.
Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.
Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.
Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; usatine@uthscsa.edu.
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; art_papier@urmc.rochester.edu.
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
VIDEO shows scabies mite in motion
PRACTICE RECOMMENDATIONS
› Consider scabies with any severe pruritic eruption. Conduct a thorough physical exam, preferably with a dermatoscope, for burrows in the webs and sides of fingers, proximal palm, and wrists. A
› Consider scabies in all patients—especially the immunocompromised—who have distal white or yellow thick, scaly, or crusted plaques. C
› Include scabies in the differential when patients present with smooth nodules of the genitals or pruritic smooth papules and plaques in other locations. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
10 proven strategies to help patients maintain weight loss
New studies show that many people who lose weight can maintain that loss for longer than a few months by utilizing strategies that can be undertaken upon your recommendation and with your ongoing support. In this article, I review the evidence that supports the effectiveness of those interventions and activities for helping patients keep off the weight they’ve lost.
Prolonging the duration of weight maintenance
Until recently, most studies that focused on weight maintenance after weight loss followed subjects for only a few months or a year after the goal was achieved. With that limited window of follow-up, the belief arose in weight-loss medicine that most people gain back lost weight within 2 years. Findings that are emerging from recent studies with longer follow-up, however, suggest that weight loss can be maintained for as long as 8 years.1
The National Health and Nutrition Examination Survey2 and the Action in Health for Diabetes (Look AHEAD) trial3,4 reported that, among adults who lost 10% or more of body weight, approximately 60% maintained that weight loss at 1 year. Look AHEAD had a much longer duration: 42% of participants who lost at least 10% of body weight by the end of Year 1 maintained at least that 10% loss by the end of Year 4.5 In addition, Look AHEAD demonstrated that extended provision of maintenance interventions after weight loss can facilitate clinically meaningful weight loss for as long as 8 years—2 or 3 times longer than what was reported in earlier randomized trials.4
We have evidence-based guidance for achieving long-term weight maintenance and good reason to believe that success is achievable for patients. The 10 strategies that follow can help you to guide patients to become successful “maintainers.”
1. Emphasize more weight loss in the first 3 months of a program
Losing more weight initially seems to point to more success in relation to maintenance. This suggests that more intensive help, such as more frequent visits with a physician and a dietitian during the first 3 months might be an important step to help patients lose and maintain weight.
Much of our information on successful maintainers comes from the National Weight Control Registry (NWCR) at the Warren Alpert Medical School at Brown University.1 This research study has gathered information from more than 10,000 people who successfully lost ≥ 30 lb (average, 60 lb) and kept it off for at least 1 year. To challenge the widespread belief that only a few people who attempt weight loss succeed long term, the NWCR identifies and investigates the characteristics of individuals who have succeeded.
A new and encouraging finding is from a small study that showed that people can maintain weight loss brought about by either medical or surgical means: Those who lost > 15% of their starting weight and were followed closely by health care professionals maintained their weight loss at 1 year.5
Continue to: 2. Advise patients to consume fewer calories and eat more nonglycemic fruits and vegetables
2.
When a person loses weight, their basal metabolic rate drops; to maintain their new weight, they need to consume fewer calories. That person must continue to have a calorie deficit, which varies individually but is often about 500 kcal/d. There is no formula for this; at our clinic, when a patient achieves goal weight, we have them increase intake by 100 kcal/d/wk in nutritious food until they start to gain weight. When they start to gain weight, we have them decrease intake by 100 kcal/d until they do not gain any longer.
Many patients complain of hunger after they lose weight because of an increase in the body’s level of ghrelin, the hunger hormone, and a decrease in the level of leptin, which is associated with satiety. Many achieve a lower calorie count and fight hunger by increasing fiber intake.
In a 24-year study that looked at weight change, researchers noted a strong inverse association between increased intake of higher-fiber, lower-glycemic fruits and vegetables and weight change.6 Lower-glycemic vegetables include most vegetables (exceptions are corn, potatoes, and peas, which are associated with weight gain). Benefit was strongest with berries, apples, pears, tofu or soy, cauliflower, and cruciferous and green leafy vegetables.6 Adding 1 serving a day of nonstarchy fruits and vegetables was associated with less weight gain over time.
The order in which food is eaten might be important, as evidenced by a small study7 that focused on patients with diabetes. Investigators found that subjects who ate vegetables first, protein second, and grain third had fewer fluctuations in blood glucose level than those who ate carbohydrates first—suggesting that this order might be a good way for patients to eat at least some of their meals. The reduced insulin excursions observed in this experimental setting suggest that the vegetable–protein–grain meal pattern can improve insulin sensitivity and help with blood glucose control.
3.
In a small, randomized controlled study8 in 2019 at the National Institutes of Health, 20 inpatients were fed an ultraprocessed diet that was matched, in calories and macronutrients, in an unprocessed diet fed to controls. Subjects in the ultraprocessed food group ate, on average, 500 kcal/d more and gained 2 lbs in 2 weeks. An ultraprocessed breakfast might consist of a bagel with cream cheese and turkey bacon; the unprocessed breakfast was oatmeal with bananas, walnuts, and skim milk. Notably, the ultraprocessed diet was cheaper; nonprocessed foods cost 50% more.
Continue to: A retrospective review...
A retrospective review of a sample of US adults’ caloric and nutritional intake determined that eating at a full-service restaurant is not associated with consumption of fewer calories than eating at a fast food restaurant: Eating at either type of restaurant was associated with excess (approximately 200 kcal/meal) caloric intake.9
4. Emphasize the importance of eating breakfast and increasing protein intake
Increased protein throughout the day, particularly at breakfast, has been suggested to help with weight maintenance. In a large European study,10 even slightly increased protein intake (approximately 1.2 g/kg of body weight and of low glycemic index food) was associated with weight maintenance. In another review,11 researchers concluded that 25 to 30 g of protein at each meal can provide improvement in appetite and weight management, although they cautioned that further research is needed. A study that looked at increasing intake of protein at breakfast to 35 g in adolescent females resulted in less snacking later in the day.12
In the NWCR, successful maintainers had breakfast daily, a lower fat diet, and fewer calories (approximately 1500 kcal/d)— routines that were all associated with greater success.1 Therefore, eating protein at approximately 1.2 g/kg of body weight (possibly, even more [35 g] at breakfast) and ingesting less fat and fewer calories all contributed to successful maintenance. Eating nuts and legume-based proteins, such as beans and tofu, should be encouraged.
Only a few studies have looked at dairy protein intake and weight maintenance. In one study, consumption of dairy proteins was not associated with a change in body weight or other metabolic risk markers during weight maintenance.13 Yogurt, because of its probiotic content, might be good for weight maintenance, but this has not been studied well, and studies that have been conducted are inconclusive.14
Another study looked at consumption of protein supplements. It found no improvement in body composition over a 24-week period when protein intake was increased to 1.45 g/kg when compared to 1.16 g/kg in controls. Although subjects felt less hungry, this was not reflected in a reduction in caloric intake.15
Continue to: Most patients do need counseling...
Most patients do need counseling on whole grain intake: Explaining that a bagel is the same as 4 servings of toast and that a cup (ie, a fistful) of cooked pasta is 3 servings of grains is helpful. Patients should aim for 1 serving of grain at each meal; when shopping for grains, they should choose those that have the “whole” first on the list of ingredients because whole grain, rather than refined grain, intake is associated with less diabetes and colon cancer.16
5.
Self-monitoring is key to weight maintenance. This can mean weighing oneself or tracking one’s food intake (or both). Daily weighing is important: A study showed that patients who decrease how often they weigh themselves were likely to eat more and thus gain weight.17
Monitoring intake is also important. Recommended online calorie counters (eg, myfitnesspal.com, loseit.com), tools such as a Fitbit, or even keeping a food diary to help patients track intake. In a review of technology-based interventions to maintain weight loss, the use of apps was variable and effectiveness of devices was mixed. The authors recommended that physicians complement Web-based applications with personal contact.18
6. Encourage patients to spend more time exercising
After weight loss is achieved, maintaining a high level of activity is important. Recommendations focus on moving about 1 hr/d or 200 to 300 min/wk.19 A program of several daily “bouts,” or episodes of moderate-to-vigorous physical activity, is recommended in the new Centers for Disease Control and Prevention guidelines19 and might be preferable, or equivalent, to a concentrated expenditure of energy. Patients might consider, for example, a 10-minute session, 4 times a day, 5 days a week, instead of a single, 40-minute session, 5 days a week.19 Furthermore, to sustain weight loss, moderate exercise might be more effective than exercise of vigorous intensity or extended duration.19 Most patients in the NWCR report that walking is their principal form of activity.1
Resistance training, which improves muscle strength and endurance, with or without diet restriction, has not been shown to be effective for weight loss but might help with weight maintenance and might improve a patient’s lipid profile, insulin resistance, and blood pressure. In obese adolescents, resistance weight training led to positive changes in body composition, such as decreased waist circumference.20 Resistance training likely enhances weight maintenance and should be encouraged because of its effect on increasing lean muscle mass, the most important factor in determining basal metabolic rate.
Continue to: 7. Work with patients to ensure sound sleep hygiene
7. Work with patients to ensure sound sleep hygiene
Short sleep duration (< 6 hours a night) is associated with obesity. There are few studies on weight maintenance and sleep; a study that was reviewed by the NWCR found that people who are highly successful at both weight loss and long-term maintenance are more likely to (1) be categorized as a “morning-type” chronotype (ie, getting up early), and (2) report longer sleep duration and better sleep quality, compared to treatment-seeking overweight and obese subjects. Furthermore, these NWCR subjects were more likely to report shorter sleep latency (time required to fall asleep) and were less likely to report short sleep, defined as < 6 to 7 hours a night.21
Patients should strive for 7 to 8 hours of sleep a night; sleep apnea should be addressed as necessary.17 It is important for doctors to encourage patients to go to bed and get up at the same times every day (eg, 10 pm to 6 am daily).
8. Start a trial of medical therapy
Weight-loss medicines are beyond the scope of this article but worth discussing. In accordance with obesity guidelines, if a patient responds well to a weight-loss medication and loses ≥ 5% of body weight after 3 months, continue prescribing the medication. If the medication is ineffective or the patient experiences adverse effects, stop the prescription and consider an alternative medication or approach to maintenance.
The US Food and Drug Administration has approved 5 medications for long-term use in weight maintenance: the 2 combination formulations bupropion–naltrexone and phentermine–topiramate, as well as liraglutide, lorcaserin, and orlistat. A review of the use of these drugs over 1 year showed that they provide a modest favorable effect on cardiometabolic outcomes that vary by drug class.22 In particular, liraglutide has been shown to reduce the risk of cardiovascular disease outcomes in patients with diabetes who have a history of atherosclerotic disease or heart attack and stroke.23 Further research is needed to evaluate the long-term impact of these drugs on cardiovascular risk.
9.
Certain personal traits and behaviors appear to help people lose weight: In a study, maintainers were more likely to be characterized as being good problem-solvers, having hope, and having a more positive mood.24,25
Continue to: Addressing mental health issues...
Addressing mental health issues, especially depression, is paramount in patients with obesity. Treating patients with depression and hopelessness, as well as helping them with problem-solving, should be the focus of weight-management care.
Choosing an antidepressant not associated with the adverse effect of weight gain is important. Almost all selective serotonin reuptake inhibitors and tricyclic antidepressants are associated with weight gain; bupropion is weight neutral and should be first-line treatment for patients who are overweight and obese.26 If using an antidepressant associated with weight gain, initiate weight monitoring if the patient gains 3% of body weight in the first month of therapy. When caring for a patient who takes an antipsychotic, consider consulting with their mental health professional to determine the value of prescribing metformin, which has been shown to decrease weight gain associated with antipsychotics.27
Because depression, anxiety, and attention deficit-hyperactivity disorder are all associated with obesity, it is important to work with obese patients’ mental health care providers to design ways to improve their care.
10.
Focusing on establishing good habits can be helpful. In Great Britain, a program focused on forming new healthy habits and breaking old unhealthy habits by restructuring daily routines and increasing mindfulness was successful in keeping weight off in 65% of participants—an impressive degree of success.24 Successful maintainers were sent daily text messages requesting that they interrupt their routines, which shifted their attention away from eating. Some of these distracting tasks included driving to work by a different route or volunteering for a charity. Such small changes helped improve weight maintenance.
Intuitive eating and mindfulness can help. New concepts that focus on healthy eating without caloric restriction are also emerging; one such approach is intuitive eating, which promotes eating that is based not only on cues connected to hunger and fullness, but also on enjoyment of food, such as eating slowly and savoring every bite.28 Techniques such as sipping water or resting the fork between bites of food has been helpful with some patients. More research in the area of intuitive eating is needed.
Continue to: Recognizing true physiologic hunger...
Recognizing true physiologic hunger distinct from emotional hunger can improve with mindfulness training. This practice might provide a better way to help the so-called yo-yo dieter and binge eater to reach metabolic health. Interestingly, successful weight maintainers who allow themselves less restriction on weekends maintain weight better than those who try to restrict diet every day, according to a recent study.29
Recommend a support group. Accountability is important: Group therapy in obesity treatment may be more effective than individual treatment.30
The support of a group and the regular attendance at group meetings are connected to further significant weight loss in the weight-loss period and, later, during maintenance. Monthly meetings seem to help patients with weight maintenance but more research is needed to determine what interval of support-group meeting attendance is most effective.
CORRESPONDENCE
Marijane Hynes, MD, The George Washington University Medical Faculty Associates, Fifth Floor/Internal Medicine, 2150 Pennsylvania Avenue NW, Washington, DC 20037; mhynes@mfa.gwu.edu.
1. Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17-23.
2. Weiss EC, Galuska DA, Kettel Khan LK, et al. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34-40.
3. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374-1383.
4. Kulovitz MG, Kolkmeyer D, Conn CA, et al. Medical weight loss versus bariatric surgery: does method affect body composition and weight maintenance after 15% reduction in body weight? Nutrition. 2014;30:49-54.
5. Look AHEAD Research Group. Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring). 2014;22:5-13.
6. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878.
7. Shukla AP, Iliescu RG, Thomas CE, et al. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98-e99.
8. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3.
9. An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70:97-103.
10. Larsen TM, Dalskov S-M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113.
11. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S.
12. Leidy HJ, Ortinau LC, Douglas SM, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677-688.
13. Bendtsen LQ, Lorenzen JK, Larsen TM, et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br J Nutr. 2014;111:944-953.
14. Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. 2014;99(5 suppl):1229S-1234S.
15. Kjølbæk L, Sørensen LB, Søndertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684-697.
16. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445.
17. Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md). 2007;15:3091-3096.
18. Lee S, Lindquist R. A review of technology-based interventions to maintain weight loss. Telemed J E Health. 2015;21:217-232.
19. 2018 Physical Activity Guidelines Advisory Committee, US Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 13, 2020.
20. Shultz SP, Byrne NM, Dahiya MR, et al. Resistance weight training affects body composition in obese adolescents: a pilot study (Abstract 2932; Board #231). Med Sci Sports Exerc. 2011;43:834.
21. Ross KM, Graham Thomas JG, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465-471.
22. Khera R, Pandey A, Chandar AK, et al. Association of weight loss medications with cardiometabolic risk factors: systematic review and network meta-analysis. Circulation. 2016;134(suppl 1):A16413.
23. Verma RS, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without a history of myocardial infarction or stroke: post hoc analysis from the LEADER trial. Circulation. 2018;138:2884-2894.
24. Cleo G, Glasziou P, Beller E, et al. Habit-based interventions for weight loss maintenance in adults with overweight and obesity: a randomized controlled trial. Int J Obes (Lond). 2019;43:374-383.
25. Robertson S, Davies M, Winefield H. Positive psychological correlates of successful weight maintenance in Australia. Clinical Psychologist. 2017;21:236-244.
26. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951.
27. Agarwal SM, Ahsan Z, Lockwood J, et al. A systematic review and meta-analysis of pharmacological interventions for reduction or prevention of weight gain in schizophrenia (poster). Schizophr Bull. 2018;44:S413.
28. Van Dyke N, Drinkwater Eric J. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757-1766.
29. Jorge R, Santos I, Teixeira VH, et al. Does diet strictness level during weekends and holiday periods influence 1-year follow-up weight loss maintenance? Evidence from the Portuguese Weight Control Registry. Nutr J. 2019;18:3.
30. Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717-721.
New studies show that many people who lose weight can maintain that loss for longer than a few months by utilizing strategies that can be undertaken upon your recommendation and with your ongoing support. In this article, I review the evidence that supports the effectiveness of those interventions and activities for helping patients keep off the weight they’ve lost.
Prolonging the duration of weight maintenance
Until recently, most studies that focused on weight maintenance after weight loss followed subjects for only a few months or a year after the goal was achieved. With that limited window of follow-up, the belief arose in weight-loss medicine that most people gain back lost weight within 2 years. Findings that are emerging from recent studies with longer follow-up, however, suggest that weight loss can be maintained for as long as 8 years.1
The National Health and Nutrition Examination Survey2 and the Action in Health for Diabetes (Look AHEAD) trial3,4 reported that, among adults who lost 10% or more of body weight, approximately 60% maintained that weight loss at 1 year. Look AHEAD had a much longer duration: 42% of participants who lost at least 10% of body weight by the end of Year 1 maintained at least that 10% loss by the end of Year 4.5 In addition, Look AHEAD demonstrated that extended provision of maintenance interventions after weight loss can facilitate clinically meaningful weight loss for as long as 8 years—2 or 3 times longer than what was reported in earlier randomized trials.4
We have evidence-based guidance for achieving long-term weight maintenance and good reason to believe that success is achievable for patients. The 10 strategies that follow can help you to guide patients to become successful “maintainers.”
1. Emphasize more weight loss in the first 3 months of a program
Losing more weight initially seems to point to more success in relation to maintenance. This suggests that more intensive help, such as more frequent visits with a physician and a dietitian during the first 3 months might be an important step to help patients lose and maintain weight.
Much of our information on successful maintainers comes from the National Weight Control Registry (NWCR) at the Warren Alpert Medical School at Brown University.1 This research study has gathered information from more than 10,000 people who successfully lost ≥ 30 lb (average, 60 lb) and kept it off for at least 1 year. To challenge the widespread belief that only a few people who attempt weight loss succeed long term, the NWCR identifies and investigates the characteristics of individuals who have succeeded.
A new and encouraging finding is from a small study that showed that people can maintain weight loss brought about by either medical or surgical means: Those who lost > 15% of their starting weight and were followed closely by health care professionals maintained their weight loss at 1 year.5
Continue to: 2. Advise patients to consume fewer calories and eat more nonglycemic fruits and vegetables
2.
When a person loses weight, their basal metabolic rate drops; to maintain their new weight, they need to consume fewer calories. That person must continue to have a calorie deficit, which varies individually but is often about 500 kcal/d. There is no formula for this; at our clinic, when a patient achieves goal weight, we have them increase intake by 100 kcal/d/wk in nutritious food until they start to gain weight. When they start to gain weight, we have them decrease intake by 100 kcal/d until they do not gain any longer.
Many patients complain of hunger after they lose weight because of an increase in the body’s level of ghrelin, the hunger hormone, and a decrease in the level of leptin, which is associated with satiety. Many achieve a lower calorie count and fight hunger by increasing fiber intake.
In a 24-year study that looked at weight change, researchers noted a strong inverse association between increased intake of higher-fiber, lower-glycemic fruits and vegetables and weight change.6 Lower-glycemic vegetables include most vegetables (exceptions are corn, potatoes, and peas, which are associated with weight gain). Benefit was strongest with berries, apples, pears, tofu or soy, cauliflower, and cruciferous and green leafy vegetables.6 Adding 1 serving a day of nonstarchy fruits and vegetables was associated with less weight gain over time.
The order in which food is eaten might be important, as evidenced by a small study7 that focused on patients with diabetes. Investigators found that subjects who ate vegetables first, protein second, and grain third had fewer fluctuations in blood glucose level than those who ate carbohydrates first—suggesting that this order might be a good way for patients to eat at least some of their meals. The reduced insulin excursions observed in this experimental setting suggest that the vegetable–protein–grain meal pattern can improve insulin sensitivity and help with blood glucose control.
3.
In a small, randomized controlled study8 in 2019 at the National Institutes of Health, 20 inpatients were fed an ultraprocessed diet that was matched, in calories and macronutrients, in an unprocessed diet fed to controls. Subjects in the ultraprocessed food group ate, on average, 500 kcal/d more and gained 2 lbs in 2 weeks. An ultraprocessed breakfast might consist of a bagel with cream cheese and turkey bacon; the unprocessed breakfast was oatmeal with bananas, walnuts, and skim milk. Notably, the ultraprocessed diet was cheaper; nonprocessed foods cost 50% more.
Continue to: A retrospective review...
A retrospective review of a sample of US adults’ caloric and nutritional intake determined that eating at a full-service restaurant is not associated with consumption of fewer calories than eating at a fast food restaurant: Eating at either type of restaurant was associated with excess (approximately 200 kcal/meal) caloric intake.9
4. Emphasize the importance of eating breakfast and increasing protein intake
Increased protein throughout the day, particularly at breakfast, has been suggested to help with weight maintenance. In a large European study,10 even slightly increased protein intake (approximately 1.2 g/kg of body weight and of low glycemic index food) was associated with weight maintenance. In another review,11 researchers concluded that 25 to 30 g of protein at each meal can provide improvement in appetite and weight management, although they cautioned that further research is needed. A study that looked at increasing intake of protein at breakfast to 35 g in adolescent females resulted in less snacking later in the day.12
In the NWCR, successful maintainers had breakfast daily, a lower fat diet, and fewer calories (approximately 1500 kcal/d)— routines that were all associated with greater success.1 Therefore, eating protein at approximately 1.2 g/kg of body weight (possibly, even more [35 g] at breakfast) and ingesting less fat and fewer calories all contributed to successful maintenance. Eating nuts and legume-based proteins, such as beans and tofu, should be encouraged.
Only a few studies have looked at dairy protein intake and weight maintenance. In one study, consumption of dairy proteins was not associated with a change in body weight or other metabolic risk markers during weight maintenance.13 Yogurt, because of its probiotic content, might be good for weight maintenance, but this has not been studied well, and studies that have been conducted are inconclusive.14
Another study looked at consumption of protein supplements. It found no improvement in body composition over a 24-week period when protein intake was increased to 1.45 g/kg when compared to 1.16 g/kg in controls. Although subjects felt less hungry, this was not reflected in a reduction in caloric intake.15
Continue to: Most patients do need counseling...
Most patients do need counseling on whole grain intake: Explaining that a bagel is the same as 4 servings of toast and that a cup (ie, a fistful) of cooked pasta is 3 servings of grains is helpful. Patients should aim for 1 serving of grain at each meal; when shopping for grains, they should choose those that have the “whole” first on the list of ingredients because whole grain, rather than refined grain, intake is associated with less diabetes and colon cancer.16
5.
Self-monitoring is key to weight maintenance. This can mean weighing oneself or tracking one’s food intake (or both). Daily weighing is important: A study showed that patients who decrease how often they weigh themselves were likely to eat more and thus gain weight.17
Monitoring intake is also important. Recommended online calorie counters (eg, myfitnesspal.com, loseit.com), tools such as a Fitbit, or even keeping a food diary to help patients track intake. In a review of technology-based interventions to maintain weight loss, the use of apps was variable and effectiveness of devices was mixed. The authors recommended that physicians complement Web-based applications with personal contact.18
6. Encourage patients to spend more time exercising
After weight loss is achieved, maintaining a high level of activity is important. Recommendations focus on moving about 1 hr/d or 200 to 300 min/wk.19 A program of several daily “bouts,” or episodes of moderate-to-vigorous physical activity, is recommended in the new Centers for Disease Control and Prevention guidelines19 and might be preferable, or equivalent, to a concentrated expenditure of energy. Patients might consider, for example, a 10-minute session, 4 times a day, 5 days a week, instead of a single, 40-minute session, 5 days a week.19 Furthermore, to sustain weight loss, moderate exercise might be more effective than exercise of vigorous intensity or extended duration.19 Most patients in the NWCR report that walking is their principal form of activity.1
Resistance training, which improves muscle strength and endurance, with or without diet restriction, has not been shown to be effective for weight loss but might help with weight maintenance and might improve a patient’s lipid profile, insulin resistance, and blood pressure. In obese adolescents, resistance weight training led to positive changes in body composition, such as decreased waist circumference.20 Resistance training likely enhances weight maintenance and should be encouraged because of its effect on increasing lean muscle mass, the most important factor in determining basal metabolic rate.
Continue to: 7. Work with patients to ensure sound sleep hygiene
7. Work with patients to ensure sound sleep hygiene
Short sleep duration (< 6 hours a night) is associated with obesity. There are few studies on weight maintenance and sleep; a study that was reviewed by the NWCR found that people who are highly successful at both weight loss and long-term maintenance are more likely to (1) be categorized as a “morning-type” chronotype (ie, getting up early), and (2) report longer sleep duration and better sleep quality, compared to treatment-seeking overweight and obese subjects. Furthermore, these NWCR subjects were more likely to report shorter sleep latency (time required to fall asleep) and were less likely to report short sleep, defined as < 6 to 7 hours a night.21
Patients should strive for 7 to 8 hours of sleep a night; sleep apnea should be addressed as necessary.17 It is important for doctors to encourage patients to go to bed and get up at the same times every day (eg, 10 pm to 6 am daily).
8. Start a trial of medical therapy
Weight-loss medicines are beyond the scope of this article but worth discussing. In accordance with obesity guidelines, if a patient responds well to a weight-loss medication and loses ≥ 5% of body weight after 3 months, continue prescribing the medication. If the medication is ineffective or the patient experiences adverse effects, stop the prescription and consider an alternative medication or approach to maintenance.
The US Food and Drug Administration has approved 5 medications for long-term use in weight maintenance: the 2 combination formulations bupropion–naltrexone and phentermine–topiramate, as well as liraglutide, lorcaserin, and orlistat. A review of the use of these drugs over 1 year showed that they provide a modest favorable effect on cardiometabolic outcomes that vary by drug class.22 In particular, liraglutide has been shown to reduce the risk of cardiovascular disease outcomes in patients with diabetes who have a history of atherosclerotic disease or heart attack and stroke.23 Further research is needed to evaluate the long-term impact of these drugs on cardiovascular risk.
9.
Certain personal traits and behaviors appear to help people lose weight: In a study, maintainers were more likely to be characterized as being good problem-solvers, having hope, and having a more positive mood.24,25
Continue to: Addressing mental health issues...
Addressing mental health issues, especially depression, is paramount in patients with obesity. Treating patients with depression and hopelessness, as well as helping them with problem-solving, should be the focus of weight-management care.
Choosing an antidepressant not associated with the adverse effect of weight gain is important. Almost all selective serotonin reuptake inhibitors and tricyclic antidepressants are associated with weight gain; bupropion is weight neutral and should be first-line treatment for patients who are overweight and obese.26 If using an antidepressant associated with weight gain, initiate weight monitoring if the patient gains 3% of body weight in the first month of therapy. When caring for a patient who takes an antipsychotic, consider consulting with their mental health professional to determine the value of prescribing metformin, which has been shown to decrease weight gain associated with antipsychotics.27
Because depression, anxiety, and attention deficit-hyperactivity disorder are all associated with obesity, it is important to work with obese patients’ mental health care providers to design ways to improve their care.
10.
Focusing on establishing good habits can be helpful. In Great Britain, a program focused on forming new healthy habits and breaking old unhealthy habits by restructuring daily routines and increasing mindfulness was successful in keeping weight off in 65% of participants—an impressive degree of success.24 Successful maintainers were sent daily text messages requesting that they interrupt their routines, which shifted their attention away from eating. Some of these distracting tasks included driving to work by a different route or volunteering for a charity. Such small changes helped improve weight maintenance.
Intuitive eating and mindfulness can help. New concepts that focus on healthy eating without caloric restriction are also emerging; one such approach is intuitive eating, which promotes eating that is based not only on cues connected to hunger and fullness, but also on enjoyment of food, such as eating slowly and savoring every bite.28 Techniques such as sipping water or resting the fork between bites of food has been helpful with some patients. More research in the area of intuitive eating is needed.
Continue to: Recognizing true physiologic hunger...
Recognizing true physiologic hunger distinct from emotional hunger can improve with mindfulness training. This practice might provide a better way to help the so-called yo-yo dieter and binge eater to reach metabolic health. Interestingly, successful weight maintainers who allow themselves less restriction on weekends maintain weight better than those who try to restrict diet every day, according to a recent study.29
Recommend a support group. Accountability is important: Group therapy in obesity treatment may be more effective than individual treatment.30
The support of a group and the regular attendance at group meetings are connected to further significant weight loss in the weight-loss period and, later, during maintenance. Monthly meetings seem to help patients with weight maintenance but more research is needed to determine what interval of support-group meeting attendance is most effective.
CORRESPONDENCE
Marijane Hynes, MD, The George Washington University Medical Faculty Associates, Fifth Floor/Internal Medicine, 2150 Pennsylvania Avenue NW, Washington, DC 20037; mhynes@mfa.gwu.edu.
New studies show that many people who lose weight can maintain that loss for longer than a few months by utilizing strategies that can be undertaken upon your recommendation and with your ongoing support. In this article, I review the evidence that supports the effectiveness of those interventions and activities for helping patients keep off the weight they’ve lost.
Prolonging the duration of weight maintenance
Until recently, most studies that focused on weight maintenance after weight loss followed subjects for only a few months or a year after the goal was achieved. With that limited window of follow-up, the belief arose in weight-loss medicine that most people gain back lost weight within 2 years. Findings that are emerging from recent studies with longer follow-up, however, suggest that weight loss can be maintained for as long as 8 years.1
The National Health and Nutrition Examination Survey2 and the Action in Health for Diabetes (Look AHEAD) trial3,4 reported that, among adults who lost 10% or more of body weight, approximately 60% maintained that weight loss at 1 year. Look AHEAD had a much longer duration: 42% of participants who lost at least 10% of body weight by the end of Year 1 maintained at least that 10% loss by the end of Year 4.5 In addition, Look AHEAD demonstrated that extended provision of maintenance interventions after weight loss can facilitate clinically meaningful weight loss for as long as 8 years—2 or 3 times longer than what was reported in earlier randomized trials.4
We have evidence-based guidance for achieving long-term weight maintenance and good reason to believe that success is achievable for patients. The 10 strategies that follow can help you to guide patients to become successful “maintainers.”
1. Emphasize more weight loss in the first 3 months of a program
Losing more weight initially seems to point to more success in relation to maintenance. This suggests that more intensive help, such as more frequent visits with a physician and a dietitian during the first 3 months might be an important step to help patients lose and maintain weight.
Much of our information on successful maintainers comes from the National Weight Control Registry (NWCR) at the Warren Alpert Medical School at Brown University.1 This research study has gathered information from more than 10,000 people who successfully lost ≥ 30 lb (average, 60 lb) and kept it off for at least 1 year. To challenge the widespread belief that only a few people who attempt weight loss succeed long term, the NWCR identifies and investigates the characteristics of individuals who have succeeded.
A new and encouraging finding is from a small study that showed that people can maintain weight loss brought about by either medical or surgical means: Those who lost > 15% of their starting weight and were followed closely by health care professionals maintained their weight loss at 1 year.5
Continue to: 2. Advise patients to consume fewer calories and eat more nonglycemic fruits and vegetables
2.
When a person loses weight, their basal metabolic rate drops; to maintain their new weight, they need to consume fewer calories. That person must continue to have a calorie deficit, which varies individually but is often about 500 kcal/d. There is no formula for this; at our clinic, when a patient achieves goal weight, we have them increase intake by 100 kcal/d/wk in nutritious food until they start to gain weight. When they start to gain weight, we have them decrease intake by 100 kcal/d until they do not gain any longer.
Many patients complain of hunger after they lose weight because of an increase in the body’s level of ghrelin, the hunger hormone, and a decrease in the level of leptin, which is associated with satiety. Many achieve a lower calorie count and fight hunger by increasing fiber intake.
In a 24-year study that looked at weight change, researchers noted a strong inverse association between increased intake of higher-fiber, lower-glycemic fruits and vegetables and weight change.6 Lower-glycemic vegetables include most vegetables (exceptions are corn, potatoes, and peas, which are associated with weight gain). Benefit was strongest with berries, apples, pears, tofu or soy, cauliflower, and cruciferous and green leafy vegetables.6 Adding 1 serving a day of nonstarchy fruits and vegetables was associated with less weight gain over time.
The order in which food is eaten might be important, as evidenced by a small study7 that focused on patients with diabetes. Investigators found that subjects who ate vegetables first, protein second, and grain third had fewer fluctuations in blood glucose level than those who ate carbohydrates first—suggesting that this order might be a good way for patients to eat at least some of their meals. The reduced insulin excursions observed in this experimental setting suggest that the vegetable–protein–grain meal pattern can improve insulin sensitivity and help with blood glucose control.
3.
In a small, randomized controlled study8 in 2019 at the National Institutes of Health, 20 inpatients were fed an ultraprocessed diet that was matched, in calories and macronutrients, in an unprocessed diet fed to controls. Subjects in the ultraprocessed food group ate, on average, 500 kcal/d more and gained 2 lbs in 2 weeks. An ultraprocessed breakfast might consist of a bagel with cream cheese and turkey bacon; the unprocessed breakfast was oatmeal with bananas, walnuts, and skim milk. Notably, the ultraprocessed diet was cheaper; nonprocessed foods cost 50% more.
Continue to: A retrospective review...
A retrospective review of a sample of US adults’ caloric and nutritional intake determined that eating at a full-service restaurant is not associated with consumption of fewer calories than eating at a fast food restaurant: Eating at either type of restaurant was associated with excess (approximately 200 kcal/meal) caloric intake.9
4. Emphasize the importance of eating breakfast and increasing protein intake
Increased protein throughout the day, particularly at breakfast, has been suggested to help with weight maintenance. In a large European study,10 even slightly increased protein intake (approximately 1.2 g/kg of body weight and of low glycemic index food) was associated with weight maintenance. In another review,11 researchers concluded that 25 to 30 g of protein at each meal can provide improvement in appetite and weight management, although they cautioned that further research is needed. A study that looked at increasing intake of protein at breakfast to 35 g in adolescent females resulted in less snacking later in the day.12
In the NWCR, successful maintainers had breakfast daily, a lower fat diet, and fewer calories (approximately 1500 kcal/d)— routines that were all associated with greater success.1 Therefore, eating protein at approximately 1.2 g/kg of body weight (possibly, even more [35 g] at breakfast) and ingesting less fat and fewer calories all contributed to successful maintenance. Eating nuts and legume-based proteins, such as beans and tofu, should be encouraged.
Only a few studies have looked at dairy protein intake and weight maintenance. In one study, consumption of dairy proteins was not associated with a change in body weight or other metabolic risk markers during weight maintenance.13 Yogurt, because of its probiotic content, might be good for weight maintenance, but this has not been studied well, and studies that have been conducted are inconclusive.14
Another study looked at consumption of protein supplements. It found no improvement in body composition over a 24-week period when protein intake was increased to 1.45 g/kg when compared to 1.16 g/kg in controls. Although subjects felt less hungry, this was not reflected in a reduction in caloric intake.15
Continue to: Most patients do need counseling...
Most patients do need counseling on whole grain intake: Explaining that a bagel is the same as 4 servings of toast and that a cup (ie, a fistful) of cooked pasta is 3 servings of grains is helpful. Patients should aim for 1 serving of grain at each meal; when shopping for grains, they should choose those that have the “whole” first on the list of ingredients because whole grain, rather than refined grain, intake is associated with less diabetes and colon cancer.16
5.
Self-monitoring is key to weight maintenance. This can mean weighing oneself or tracking one’s food intake (or both). Daily weighing is important: A study showed that patients who decrease how often they weigh themselves were likely to eat more and thus gain weight.17
Monitoring intake is also important. Recommended online calorie counters (eg, myfitnesspal.com, loseit.com), tools such as a Fitbit, or even keeping a food diary to help patients track intake. In a review of technology-based interventions to maintain weight loss, the use of apps was variable and effectiveness of devices was mixed. The authors recommended that physicians complement Web-based applications with personal contact.18
6. Encourage patients to spend more time exercising
After weight loss is achieved, maintaining a high level of activity is important. Recommendations focus on moving about 1 hr/d or 200 to 300 min/wk.19 A program of several daily “bouts,” or episodes of moderate-to-vigorous physical activity, is recommended in the new Centers for Disease Control and Prevention guidelines19 and might be preferable, or equivalent, to a concentrated expenditure of energy. Patients might consider, for example, a 10-minute session, 4 times a day, 5 days a week, instead of a single, 40-minute session, 5 days a week.19 Furthermore, to sustain weight loss, moderate exercise might be more effective than exercise of vigorous intensity or extended duration.19 Most patients in the NWCR report that walking is their principal form of activity.1
Resistance training, which improves muscle strength and endurance, with or without diet restriction, has not been shown to be effective for weight loss but might help with weight maintenance and might improve a patient’s lipid profile, insulin resistance, and blood pressure. In obese adolescents, resistance weight training led to positive changes in body composition, such as decreased waist circumference.20 Resistance training likely enhances weight maintenance and should be encouraged because of its effect on increasing lean muscle mass, the most important factor in determining basal metabolic rate.
Continue to: 7. Work with patients to ensure sound sleep hygiene
7. Work with patients to ensure sound sleep hygiene
Short sleep duration (< 6 hours a night) is associated with obesity. There are few studies on weight maintenance and sleep; a study that was reviewed by the NWCR found that people who are highly successful at both weight loss and long-term maintenance are more likely to (1) be categorized as a “morning-type” chronotype (ie, getting up early), and (2) report longer sleep duration and better sleep quality, compared to treatment-seeking overweight and obese subjects. Furthermore, these NWCR subjects were more likely to report shorter sleep latency (time required to fall asleep) and were less likely to report short sleep, defined as < 6 to 7 hours a night.21
Patients should strive for 7 to 8 hours of sleep a night; sleep apnea should be addressed as necessary.17 It is important for doctors to encourage patients to go to bed and get up at the same times every day (eg, 10 pm to 6 am daily).
8. Start a trial of medical therapy
Weight-loss medicines are beyond the scope of this article but worth discussing. In accordance with obesity guidelines, if a patient responds well to a weight-loss medication and loses ≥ 5% of body weight after 3 months, continue prescribing the medication. If the medication is ineffective or the patient experiences adverse effects, stop the prescription and consider an alternative medication or approach to maintenance.
The US Food and Drug Administration has approved 5 medications for long-term use in weight maintenance: the 2 combination formulations bupropion–naltrexone and phentermine–topiramate, as well as liraglutide, lorcaserin, and orlistat. A review of the use of these drugs over 1 year showed that they provide a modest favorable effect on cardiometabolic outcomes that vary by drug class.22 In particular, liraglutide has been shown to reduce the risk of cardiovascular disease outcomes in patients with diabetes who have a history of atherosclerotic disease or heart attack and stroke.23 Further research is needed to evaluate the long-term impact of these drugs on cardiovascular risk.
9.
Certain personal traits and behaviors appear to help people lose weight: In a study, maintainers were more likely to be characterized as being good problem-solvers, having hope, and having a more positive mood.24,25
Continue to: Addressing mental health issues...
Addressing mental health issues, especially depression, is paramount in patients with obesity. Treating patients with depression and hopelessness, as well as helping them with problem-solving, should be the focus of weight-management care.
Choosing an antidepressant not associated with the adverse effect of weight gain is important. Almost all selective serotonin reuptake inhibitors and tricyclic antidepressants are associated with weight gain; bupropion is weight neutral and should be first-line treatment for patients who are overweight and obese.26 If using an antidepressant associated with weight gain, initiate weight monitoring if the patient gains 3% of body weight in the first month of therapy. When caring for a patient who takes an antipsychotic, consider consulting with their mental health professional to determine the value of prescribing metformin, which has been shown to decrease weight gain associated with antipsychotics.27
Because depression, anxiety, and attention deficit-hyperactivity disorder are all associated with obesity, it is important to work with obese patients’ mental health care providers to design ways to improve their care.
10.
Focusing on establishing good habits can be helpful. In Great Britain, a program focused on forming new healthy habits and breaking old unhealthy habits by restructuring daily routines and increasing mindfulness was successful in keeping weight off in 65% of participants—an impressive degree of success.24 Successful maintainers were sent daily text messages requesting that they interrupt their routines, which shifted their attention away from eating. Some of these distracting tasks included driving to work by a different route or volunteering for a charity. Such small changes helped improve weight maintenance.
Intuitive eating and mindfulness can help. New concepts that focus on healthy eating without caloric restriction are also emerging; one such approach is intuitive eating, which promotes eating that is based not only on cues connected to hunger and fullness, but also on enjoyment of food, such as eating slowly and savoring every bite.28 Techniques such as sipping water or resting the fork between bites of food has been helpful with some patients. More research in the area of intuitive eating is needed.
Continue to: Recognizing true physiologic hunger...
Recognizing true physiologic hunger distinct from emotional hunger can improve with mindfulness training. This practice might provide a better way to help the so-called yo-yo dieter and binge eater to reach metabolic health. Interestingly, successful weight maintainers who allow themselves less restriction on weekends maintain weight better than those who try to restrict diet every day, according to a recent study.29
Recommend a support group. Accountability is important: Group therapy in obesity treatment may be more effective than individual treatment.30
The support of a group and the regular attendance at group meetings are connected to further significant weight loss in the weight-loss period and, later, during maintenance. Monthly meetings seem to help patients with weight maintenance but more research is needed to determine what interval of support-group meeting attendance is most effective.
CORRESPONDENCE
Marijane Hynes, MD, The George Washington University Medical Faculty Associates, Fifth Floor/Internal Medicine, 2150 Pennsylvania Avenue NW, Washington, DC 20037; mhynes@mfa.gwu.edu.
1. Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17-23.
2. Weiss EC, Galuska DA, Kettel Khan LK, et al. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34-40.
3. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374-1383.
4. Kulovitz MG, Kolkmeyer D, Conn CA, et al. Medical weight loss versus bariatric surgery: does method affect body composition and weight maintenance after 15% reduction in body weight? Nutrition. 2014;30:49-54.
5. Look AHEAD Research Group. Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring). 2014;22:5-13.
6. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878.
7. Shukla AP, Iliescu RG, Thomas CE, et al. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98-e99.
8. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3.
9. An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70:97-103.
10. Larsen TM, Dalskov S-M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113.
11. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S.
12. Leidy HJ, Ortinau LC, Douglas SM, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677-688.
13. Bendtsen LQ, Lorenzen JK, Larsen TM, et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br J Nutr. 2014;111:944-953.
14. Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. 2014;99(5 suppl):1229S-1234S.
15. Kjølbæk L, Sørensen LB, Søndertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684-697.
16. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445.
17. Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md). 2007;15:3091-3096.
18. Lee S, Lindquist R. A review of technology-based interventions to maintain weight loss. Telemed J E Health. 2015;21:217-232.
19. 2018 Physical Activity Guidelines Advisory Committee, US Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 13, 2020.
20. Shultz SP, Byrne NM, Dahiya MR, et al. Resistance weight training affects body composition in obese adolescents: a pilot study (Abstract 2932; Board #231). Med Sci Sports Exerc. 2011;43:834.
21. Ross KM, Graham Thomas JG, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465-471.
22. Khera R, Pandey A, Chandar AK, et al. Association of weight loss medications with cardiometabolic risk factors: systematic review and network meta-analysis. Circulation. 2016;134(suppl 1):A16413.
23. Verma RS, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without a history of myocardial infarction or stroke: post hoc analysis from the LEADER trial. Circulation. 2018;138:2884-2894.
24. Cleo G, Glasziou P, Beller E, et al. Habit-based interventions for weight loss maintenance in adults with overweight and obesity: a randomized controlled trial. Int J Obes (Lond). 2019;43:374-383.
25. Robertson S, Davies M, Winefield H. Positive psychological correlates of successful weight maintenance in Australia. Clinical Psychologist. 2017;21:236-244.
26. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951.
27. Agarwal SM, Ahsan Z, Lockwood J, et al. A systematic review and meta-analysis of pharmacological interventions for reduction or prevention of weight gain in schizophrenia (poster). Schizophr Bull. 2018;44:S413.
28. Van Dyke N, Drinkwater Eric J. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757-1766.
29. Jorge R, Santos I, Teixeira VH, et al. Does diet strictness level during weekends and holiday periods influence 1-year follow-up weight loss maintenance? Evidence from the Portuguese Weight Control Registry. Nutr J. 2019;18:3.
30. Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717-721.
1. Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17-23.
2. Weiss EC, Galuska DA, Kettel Khan LK, et al. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34-40.
3. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374-1383.
4. Kulovitz MG, Kolkmeyer D, Conn CA, et al. Medical weight loss versus bariatric surgery: does method affect body composition and weight maintenance after 15% reduction in body weight? Nutrition. 2014;30:49-54.
5. Look AHEAD Research Group. Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring). 2014;22:5-13.
6. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878.
7. Shukla AP, Iliescu RG, Thomas CE, et al. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98-e99.
8. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3.
9. An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70:97-103.
10. Larsen TM, Dalskov S-M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113.
11. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S.
12. Leidy HJ, Ortinau LC, Douglas SM, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677-688.
13. Bendtsen LQ, Lorenzen JK, Larsen TM, et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br J Nutr. 2014;111:944-953.
14. Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. 2014;99(5 suppl):1229S-1234S.
15. Kjølbæk L, Sørensen LB, Søndertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684-697.
16. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445.
17. Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md). 2007;15:3091-3096.
18. Lee S, Lindquist R. A review of technology-based interventions to maintain weight loss. Telemed J E Health. 2015;21:217-232.
19. 2018 Physical Activity Guidelines Advisory Committee, US Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 13, 2020.
20. Shultz SP, Byrne NM, Dahiya MR, et al. Resistance weight training affects body composition in obese adolescents: a pilot study (Abstract 2932; Board #231). Med Sci Sports Exerc. 2011;43:834.
21. Ross KM, Graham Thomas JG, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465-471.
22. Khera R, Pandey A, Chandar AK, et al. Association of weight loss medications with cardiometabolic risk factors: systematic review and network meta-analysis. Circulation. 2016;134(suppl 1):A16413.
23. Verma RS, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without a history of myocardial infarction or stroke: post hoc analysis from the LEADER trial. Circulation. 2018;138:2884-2894.
24. Cleo G, Glasziou P, Beller E, et al. Habit-based interventions for weight loss maintenance in adults with overweight and obesity: a randomized controlled trial. Int J Obes (Lond). 2019;43:374-383.
25. Robertson S, Davies M, Winefield H. Positive psychological correlates of successful weight maintenance in Australia. Clinical Psychologist. 2017;21:236-244.
26. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951.
27. Agarwal SM, Ahsan Z, Lockwood J, et al. A systematic review and meta-analysis of pharmacological interventions for reduction or prevention of weight gain in schizophrenia (poster). Schizophr Bull. 2018;44:S413.
28. Van Dyke N, Drinkwater Eric J. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757-1766.
29. Jorge R, Santos I, Teixeira VH, et al. Does diet strictness level during weekends and holiday periods influence 1-year follow-up weight loss maintenance? Evidence from the Portuguese Weight Control Registry. Nutr J. 2019;18:3.
30. Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717-721.
PRACTICE RECOMMENDATIONS
› Encourage patients to lose more weight early in their effort, which is predictive of successful long-term maintenance. B
› Support patients’ efforts to maintain weight loss by encouraging them to consume fewer calories and eat more nonglycemic fruits and vegetables A, eat at home and avoid processed foods B, work with you in addressing mental health concerns B, and increase time spent exercising A.
› Consider the potential value of prescribing a US Food and Drug Administration–indicated medication for weight maintenance. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Infographic: Applications for the Ketogenic Diet in Dermatology
This infographic is available in the PDF above.
This infographic is available in the PDF above.
This infographic is available in the PDF above.
Routine heart disease screening not recommended
BNP testing improves outcomes in evaluation of dyspnea
Data on treating bronchiolitis severity limited
Depression after miscarriage: Follow-up care is key
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
Dermatologists are uniquely suited to help sexual-, gender-minority patients
ORLANDO – There is a these patients, Angelo Landriscina, MD, said at the ODAC Dermatology, Aesthetic, & Clinical Conference.
“Our knowledge lagging behind is really to our own detriment,” said Dr. Landriscina, chief resident of dermatology at George Washington University, Washington. “The unique comorbidities and lived experiences of queer patients can impact their dermatologic disease. In addition to that, we are in a really unique position to provide life-changing care for these patients.”
The topic of dermatologic care for SGM patients has been discussed with interest in the dermatology community, but it has not been well studied. In one of two papers recently published in Pediatric Dermatology, Markus D. Boos, MD, PhD, from the University of Washington, Seattle, and Seattle Children’s Hospital, and coauthors noted that there is a particular knowledge gap in how to care for SGM pediatric and adolescent patients (Pediatr Dermatol. 2019 Sep;36[5]:581-6; 587-93).
In his presentation, Dr. Landriscina outlined the differences between sexual orientation – an emotional, romantic, or sexual attraction to others – and gender identity, or how one perceives their own gender. Lesbian, gay, transgender, and queer/questioning are classical definitions, but SGM patients may also self-identify in any number of other ways. Some SGM patients may identify as gender-fluid or nonbinary, while genderqueer is an umbrella term for individuals who don’t identify with typical gender roles. On the topic of pronouns, asking SGM patients how they want to be referred to is ideal, but the singular they is considered a gender-neutral term that should work for most situations.
There are also terms to avoid: “Homosexual” may be acceptable to some SGM patients, but is from an era when same-sex attraction was pathologized; “sexual preference” characterizes sexuality as a choice; the term “lifestyle” perpetuates the idea that all SGM patients are the same; and referring to transgender patients as "pre-op" or "post-op" is problematic as these terms imply that transgender identity is defined by a medical transition. Using transgender or gay as a noun, or the word transsexual, is offensive and shouldn’t be used at all, according to Dr. Landriscina.
As these terms continue to change and be redefined, dermatologists are likely to encounter terms they are unfamiliar with in the clinic, but he emphasized that attendees need not know every term to care for these patients. “The easiest way to be right in all of these situations is to let your patients define themselves,” he said, but noted that “assumptions are your worst enemy. You’re going to have to ask the hard questions.”
Updating understanding of SGM patients
Much of the medical community’s understanding of SGM patients hasn’t been updated in decades, Dr. Landriscina said. For example, medical school students typically learn about SGM risk factors that only apply to men who have sex with men (MSM), but there is new interest in caring for transgender patients within dermatology. The focus on classical notions for treating MSM can be reductive, Dr. Landriscina explained. “It boils a whole community of people down to one disease process. I think that we need to expand the thought that there are other associations here. There are other risks these patients face.”
In contrast, dermatologists who strive to understand their patients can better see them as a whole person, can engage in a better differential diagnosis, are aware of the current comorbidities SGM patients face that affect dermatologic disease, and can even work with the patient toward preventative care.
“It’s been decades since we’ve had a paradigm shift about this, and I think it’s time,” he said.
Overall, SGM patients have a higher likelihood of suffering from mental illness and suicidal ideation, with 10%-20% of lesbian, gay, and bisexual patients attempting suicide. Gender-minority patients have a significantly higher rate of attempting suicide at 40%. SGM patients are also more likely to be homeless and uninsured, and have the highest rates of tobacco, alcohol, and illicit drug use. In the SGM population, there is a higher likelihood of being victimized, and discriminated against, with this risk being much higher in transgender patients.
For MSM, there is a high risk of HIV and other STIs such as herpes simplex virus type 2 (HSV2), human papillomavirus (HPV), gonorrhea, and chlamydia, Dr. Landriscina said. They are also at risk for hepatitis A, B, and C; clusters of meningococcal meningitis; and human herpes virus 8. While MSM are more likely to use sunscreen, they also are more likely to use tanning beds and not wear protective clothing outdoors, and are at a greater risk of skin cancer. The risks of body dysmorphia and eating disorders are also increased for MSM.
While there is not as much research on risk factors for women who have sex with women (WSW), they are still at risk for HIV, HSV, and HPV and are less likely to engage in safe sex practices. For women who have sex with both men and other women, there is an even greater risk of STIs. While WSW are more likely to perceive less need for screening, they should be given the same screening as all other women, and dermatologists can help by ensuring these patients are connected with primary care providers, he said.
Dermatologic sequelae for transgender patients
For patients who transition from male to female, there is little information on their sexual risk from studies, but their care should be managed similarly to MSM, Dr. Landriscina said. When seeing transgender patients, dermatologists should be aware of issues of gender dysphoria, but not offer any intervention without first having a conversation about the patient’s hopes and goals. “Not every patient will have the means or desire to have everything that you can offer,” he said.
For patients who choose to undergo a female-to-male transition, dermatological sequelae may include classical manifestations of androgen excess from hormone therapy such as acne and androgenic alopecia; acne, miliaria, tinea corporis, contact dermatitis from chest binding; and surgical scars and keloids. For acne, isotretinoin is an option if a case is severe, but dermatologists should be aware these patients may still be able to become pregnant. There is no consensus on treatment for androgenic alopecia, but use of finasteride might block wanted secondary sex characteristics, Dr. Landriscina noted.
Patients who undergo a male-to-female transition may develop melasma or asteatotic eczema while receiving estrogen therapy; unwanted facial or body hair; and complications from illicit “filler” injections that may cause foreign-body granulomas, bacterial or atypical mycobacterial infection, lymphedema, and scarring.
Transgender patients may choose to undergo aesthetic treatments that can affirm their gender and decrease their gender dysphoria, augment the effects of hormone therapy and gender-confirmation surgery, and improve their quality of life. “While this has classically fallen under the purview of plastic surgery, I feel like we’re uniquely positioned to provide really life-changing aesthetic services to these patients,” Dr. Landriscina said.
Creating an inclusive environment is key to successfully caring for SGM patients. Any practice policies should have SGM-inclusive language, employees should receive mandatory LGBTQ+ focused training, and a point person should oversee LGBTQ+ matters, according to the Joint Commission’s LGBT Guide. Practices should also begin collecting data on sexual orientation and gender identity, which may help patients who are reluctant to vocally disclose their sexual orientation and gender identity and expand understanding of SGM patients. “Before you even walk into that visit, you know what the patient’s identity is, how they want to be addressed,” said Dr. Landriscina. “It also shows patients that you value what their identities are and that you’re competent in taking care of them.”
Dr. Landriscina encouraged attendees to take the information they learned in the session and “run with it.”
“Go for it. Keep learning,” he said. “There’s more about this topic than I even had the chance to include. Your patients are going to appreciate your dedication to them.”
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University and medical director of ODAC, acknowledged the large gaps in care for SGM patients and the unique role dermatologists can play in their care, both in terms of medical and surgical procedures.
“There are specific considerations that we as dermatologists need to think about in terms of just quality of life, potentially mental disease, homelessness, access to care. I think if we consider the whole picture, we can not only provide dermatologic care, but maybe serve as a pivot point to direct them to other specialists, and other physicians, and even nonphysicians who play a role in all facets of life to really get these individuals all the care, in broader senses, that they need,” he said.
Dr. Landriscina reported no relevant conflicts of interest.
ORLANDO – There is a these patients, Angelo Landriscina, MD, said at the ODAC Dermatology, Aesthetic, & Clinical Conference.
“Our knowledge lagging behind is really to our own detriment,” said Dr. Landriscina, chief resident of dermatology at George Washington University, Washington. “The unique comorbidities and lived experiences of queer patients can impact their dermatologic disease. In addition to that, we are in a really unique position to provide life-changing care for these patients.”
The topic of dermatologic care for SGM patients has been discussed with interest in the dermatology community, but it has not been well studied. In one of two papers recently published in Pediatric Dermatology, Markus D. Boos, MD, PhD, from the University of Washington, Seattle, and Seattle Children’s Hospital, and coauthors noted that there is a particular knowledge gap in how to care for SGM pediatric and adolescent patients (Pediatr Dermatol. 2019 Sep;36[5]:581-6; 587-93).
In his presentation, Dr. Landriscina outlined the differences between sexual orientation – an emotional, romantic, or sexual attraction to others – and gender identity, or how one perceives their own gender. Lesbian, gay, transgender, and queer/questioning are classical definitions, but SGM patients may also self-identify in any number of other ways. Some SGM patients may identify as gender-fluid or nonbinary, while genderqueer is an umbrella term for individuals who don’t identify with typical gender roles. On the topic of pronouns, asking SGM patients how they want to be referred to is ideal, but the singular they is considered a gender-neutral term that should work for most situations.
There are also terms to avoid: “Homosexual” may be acceptable to some SGM patients, but is from an era when same-sex attraction was pathologized; “sexual preference” characterizes sexuality as a choice; the term “lifestyle” perpetuates the idea that all SGM patients are the same; and referring to transgender patients as "pre-op" or "post-op" is problematic as these terms imply that transgender identity is defined by a medical transition. Using transgender or gay as a noun, or the word transsexual, is offensive and shouldn’t be used at all, according to Dr. Landriscina.
As these terms continue to change and be redefined, dermatologists are likely to encounter terms they are unfamiliar with in the clinic, but he emphasized that attendees need not know every term to care for these patients. “The easiest way to be right in all of these situations is to let your patients define themselves,” he said, but noted that “assumptions are your worst enemy. You’re going to have to ask the hard questions.”
Updating understanding of SGM patients
Much of the medical community’s understanding of SGM patients hasn’t been updated in decades, Dr. Landriscina said. For example, medical school students typically learn about SGM risk factors that only apply to men who have sex with men (MSM), but there is new interest in caring for transgender patients within dermatology. The focus on classical notions for treating MSM can be reductive, Dr. Landriscina explained. “It boils a whole community of people down to one disease process. I think that we need to expand the thought that there are other associations here. There are other risks these patients face.”
In contrast, dermatologists who strive to understand their patients can better see them as a whole person, can engage in a better differential diagnosis, are aware of the current comorbidities SGM patients face that affect dermatologic disease, and can even work with the patient toward preventative care.
“It’s been decades since we’ve had a paradigm shift about this, and I think it’s time,” he said.
Overall, SGM patients have a higher likelihood of suffering from mental illness and suicidal ideation, with 10%-20% of lesbian, gay, and bisexual patients attempting suicide. Gender-minority patients have a significantly higher rate of attempting suicide at 40%. SGM patients are also more likely to be homeless and uninsured, and have the highest rates of tobacco, alcohol, and illicit drug use. In the SGM population, there is a higher likelihood of being victimized, and discriminated against, with this risk being much higher in transgender patients.
For MSM, there is a high risk of HIV and other STIs such as herpes simplex virus type 2 (HSV2), human papillomavirus (HPV), gonorrhea, and chlamydia, Dr. Landriscina said. They are also at risk for hepatitis A, B, and C; clusters of meningococcal meningitis; and human herpes virus 8. While MSM are more likely to use sunscreen, they also are more likely to use tanning beds and not wear protective clothing outdoors, and are at a greater risk of skin cancer. The risks of body dysmorphia and eating disorders are also increased for MSM.
While there is not as much research on risk factors for women who have sex with women (WSW), they are still at risk for HIV, HSV, and HPV and are less likely to engage in safe sex practices. For women who have sex with both men and other women, there is an even greater risk of STIs. While WSW are more likely to perceive less need for screening, they should be given the same screening as all other women, and dermatologists can help by ensuring these patients are connected with primary care providers, he said.
Dermatologic sequelae for transgender patients
For patients who transition from male to female, there is little information on their sexual risk from studies, but their care should be managed similarly to MSM, Dr. Landriscina said. When seeing transgender patients, dermatologists should be aware of issues of gender dysphoria, but not offer any intervention without first having a conversation about the patient’s hopes and goals. “Not every patient will have the means or desire to have everything that you can offer,” he said.
For patients who choose to undergo a female-to-male transition, dermatological sequelae may include classical manifestations of androgen excess from hormone therapy such as acne and androgenic alopecia; acne, miliaria, tinea corporis, contact dermatitis from chest binding; and surgical scars and keloids. For acne, isotretinoin is an option if a case is severe, but dermatologists should be aware these patients may still be able to become pregnant. There is no consensus on treatment for androgenic alopecia, but use of finasteride might block wanted secondary sex characteristics, Dr. Landriscina noted.
Patients who undergo a male-to-female transition may develop melasma or asteatotic eczema while receiving estrogen therapy; unwanted facial or body hair; and complications from illicit “filler” injections that may cause foreign-body granulomas, bacterial or atypical mycobacterial infection, lymphedema, and scarring.
Transgender patients may choose to undergo aesthetic treatments that can affirm their gender and decrease their gender dysphoria, augment the effects of hormone therapy and gender-confirmation surgery, and improve their quality of life. “While this has classically fallen under the purview of plastic surgery, I feel like we’re uniquely positioned to provide really life-changing aesthetic services to these patients,” Dr. Landriscina said.
Creating an inclusive environment is key to successfully caring for SGM patients. Any practice policies should have SGM-inclusive language, employees should receive mandatory LGBTQ+ focused training, and a point person should oversee LGBTQ+ matters, according to the Joint Commission’s LGBT Guide. Practices should also begin collecting data on sexual orientation and gender identity, which may help patients who are reluctant to vocally disclose their sexual orientation and gender identity and expand understanding of SGM patients. “Before you even walk into that visit, you know what the patient’s identity is, how they want to be addressed,” said Dr. Landriscina. “It also shows patients that you value what their identities are and that you’re competent in taking care of them.”
Dr. Landriscina encouraged attendees to take the information they learned in the session and “run with it.”
“Go for it. Keep learning,” he said. “There’s more about this topic than I even had the chance to include. Your patients are going to appreciate your dedication to them.”
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University and medical director of ODAC, acknowledged the large gaps in care for SGM patients and the unique role dermatologists can play in their care, both in terms of medical and surgical procedures.
“There are specific considerations that we as dermatologists need to think about in terms of just quality of life, potentially mental disease, homelessness, access to care. I think if we consider the whole picture, we can not only provide dermatologic care, but maybe serve as a pivot point to direct them to other specialists, and other physicians, and even nonphysicians who play a role in all facets of life to really get these individuals all the care, in broader senses, that they need,” he said.
Dr. Landriscina reported no relevant conflicts of interest.
ORLANDO – There is a these patients, Angelo Landriscina, MD, said at the ODAC Dermatology, Aesthetic, & Clinical Conference.
“Our knowledge lagging behind is really to our own detriment,” said Dr. Landriscina, chief resident of dermatology at George Washington University, Washington. “The unique comorbidities and lived experiences of queer patients can impact their dermatologic disease. In addition to that, we are in a really unique position to provide life-changing care for these patients.”
The topic of dermatologic care for SGM patients has been discussed with interest in the dermatology community, but it has not been well studied. In one of two papers recently published in Pediatric Dermatology, Markus D. Boos, MD, PhD, from the University of Washington, Seattle, and Seattle Children’s Hospital, and coauthors noted that there is a particular knowledge gap in how to care for SGM pediatric and adolescent patients (Pediatr Dermatol. 2019 Sep;36[5]:581-6; 587-93).
In his presentation, Dr. Landriscina outlined the differences between sexual orientation – an emotional, romantic, or sexual attraction to others – and gender identity, or how one perceives their own gender. Lesbian, gay, transgender, and queer/questioning are classical definitions, but SGM patients may also self-identify in any number of other ways. Some SGM patients may identify as gender-fluid or nonbinary, while genderqueer is an umbrella term for individuals who don’t identify with typical gender roles. On the topic of pronouns, asking SGM patients how they want to be referred to is ideal, but the singular they is considered a gender-neutral term that should work for most situations.
There are also terms to avoid: “Homosexual” may be acceptable to some SGM patients, but is from an era when same-sex attraction was pathologized; “sexual preference” characterizes sexuality as a choice; the term “lifestyle” perpetuates the idea that all SGM patients are the same; and referring to transgender patients as "pre-op" or "post-op" is problematic as these terms imply that transgender identity is defined by a medical transition. Using transgender or gay as a noun, or the word transsexual, is offensive and shouldn’t be used at all, according to Dr. Landriscina.
As these terms continue to change and be redefined, dermatologists are likely to encounter terms they are unfamiliar with in the clinic, but he emphasized that attendees need not know every term to care for these patients. “The easiest way to be right in all of these situations is to let your patients define themselves,” he said, but noted that “assumptions are your worst enemy. You’re going to have to ask the hard questions.”
Updating understanding of SGM patients
Much of the medical community’s understanding of SGM patients hasn’t been updated in decades, Dr. Landriscina said. For example, medical school students typically learn about SGM risk factors that only apply to men who have sex with men (MSM), but there is new interest in caring for transgender patients within dermatology. The focus on classical notions for treating MSM can be reductive, Dr. Landriscina explained. “It boils a whole community of people down to one disease process. I think that we need to expand the thought that there are other associations here. There are other risks these patients face.”
In contrast, dermatologists who strive to understand their patients can better see them as a whole person, can engage in a better differential diagnosis, are aware of the current comorbidities SGM patients face that affect dermatologic disease, and can even work with the patient toward preventative care.
“It’s been decades since we’ve had a paradigm shift about this, and I think it’s time,” he said.
Overall, SGM patients have a higher likelihood of suffering from mental illness and suicidal ideation, with 10%-20% of lesbian, gay, and bisexual patients attempting suicide. Gender-minority patients have a significantly higher rate of attempting suicide at 40%. SGM patients are also more likely to be homeless and uninsured, and have the highest rates of tobacco, alcohol, and illicit drug use. In the SGM population, there is a higher likelihood of being victimized, and discriminated against, with this risk being much higher in transgender patients.
For MSM, there is a high risk of HIV and other STIs such as herpes simplex virus type 2 (HSV2), human papillomavirus (HPV), gonorrhea, and chlamydia, Dr. Landriscina said. They are also at risk for hepatitis A, B, and C; clusters of meningococcal meningitis; and human herpes virus 8. While MSM are more likely to use sunscreen, they also are more likely to use tanning beds and not wear protective clothing outdoors, and are at a greater risk of skin cancer. The risks of body dysmorphia and eating disorders are also increased for MSM.
While there is not as much research on risk factors for women who have sex with women (WSW), they are still at risk for HIV, HSV, and HPV and are less likely to engage in safe sex practices. For women who have sex with both men and other women, there is an even greater risk of STIs. While WSW are more likely to perceive less need for screening, they should be given the same screening as all other women, and dermatologists can help by ensuring these patients are connected with primary care providers, he said.
Dermatologic sequelae for transgender patients
For patients who transition from male to female, there is little information on their sexual risk from studies, but their care should be managed similarly to MSM, Dr. Landriscina said. When seeing transgender patients, dermatologists should be aware of issues of gender dysphoria, but not offer any intervention without first having a conversation about the patient’s hopes and goals. “Not every patient will have the means or desire to have everything that you can offer,” he said.
For patients who choose to undergo a female-to-male transition, dermatological sequelae may include classical manifestations of androgen excess from hormone therapy such as acne and androgenic alopecia; acne, miliaria, tinea corporis, contact dermatitis from chest binding; and surgical scars and keloids. For acne, isotretinoin is an option if a case is severe, but dermatologists should be aware these patients may still be able to become pregnant. There is no consensus on treatment for androgenic alopecia, but use of finasteride might block wanted secondary sex characteristics, Dr. Landriscina noted.
Patients who undergo a male-to-female transition may develop melasma or asteatotic eczema while receiving estrogen therapy; unwanted facial or body hair; and complications from illicit “filler” injections that may cause foreign-body granulomas, bacterial or atypical mycobacterial infection, lymphedema, and scarring.
Transgender patients may choose to undergo aesthetic treatments that can affirm their gender and decrease their gender dysphoria, augment the effects of hormone therapy and gender-confirmation surgery, and improve their quality of life. “While this has classically fallen under the purview of plastic surgery, I feel like we’re uniquely positioned to provide really life-changing aesthetic services to these patients,” Dr. Landriscina said.
Creating an inclusive environment is key to successfully caring for SGM patients. Any practice policies should have SGM-inclusive language, employees should receive mandatory LGBTQ+ focused training, and a point person should oversee LGBTQ+ matters, according to the Joint Commission’s LGBT Guide. Practices should also begin collecting data on sexual orientation and gender identity, which may help patients who are reluctant to vocally disclose their sexual orientation and gender identity and expand understanding of SGM patients. “Before you even walk into that visit, you know what the patient’s identity is, how they want to be addressed,” said Dr. Landriscina. “It also shows patients that you value what their identities are and that you’re competent in taking care of them.”
Dr. Landriscina encouraged attendees to take the information they learned in the session and “run with it.”
“Go for it. Keep learning,” he said. “There’s more about this topic than I even had the chance to include. Your patients are going to appreciate your dedication to them.”
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University and medical director of ODAC, acknowledged the large gaps in care for SGM patients and the unique role dermatologists can play in their care, both in terms of medical and surgical procedures.
“There are specific considerations that we as dermatologists need to think about in terms of just quality of life, potentially mental disease, homelessness, access to care. I think if we consider the whole picture, we can not only provide dermatologic care, but maybe serve as a pivot point to direct them to other specialists, and other physicians, and even nonphysicians who play a role in all facets of life to really get these individuals all the care, in broader senses, that they need,” he said.
Dr. Landriscina reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2020