User login
Improving health care with simulation
QI is for clinicians too
Simulation is commonly used in the education and training of health care professionals, but more recently it’s entering the quality improvement world.
“Instead of just thinking about training individuals and teams, people are starting to use simulation to look at the physical layout of resuscitation bays, to map work flows of a patient journey through hospitals, to identify latent safety threats,” said Victoria Brazil, MD, MBA, lead author of a study on the subject in BMJ Quality & Safety. “These are great things to do, but many of the people doing it didn’t have quality improvement skills or knowledge – that’s why we wrote this article.”
Dr. Brazil, a specialist in health care simulation at Gold Coast (Australia) Hospital and Health Service, explained that, “in terms of the top takeaways, for quality improvement teams – and I’m including everyday clinicians in this: Think about simulation as one of the tools that can be utilized when looking at the questions of how we make our performance better, whether that’s a team performance, environmental, investigational impacts, or one of my key interests, whether that’s about exploring and shaping culture in hospitals, which we’ve done a lot of work on using simulation.”
Quality improvement has become a very specialized field, she added, so hospitalists may think it’s outside their purview. “As clinicians, we don’t think about ourselves as being engaged in quality improvement. I think that’s a shame, because many of the things that we can do bit by bit to make our patient outcomes better, we need to be thinking about finding better ways to do those things. I suggest simulation is one way, and that doesn’t need to be a massive simulation center. It can be simulating the kind of things that are important to you, your teams, and your patients and using those to both explore improved performance.”
Dr. Brazil said that Gold Coast Hospital has used simulation as a way of getting people from different departments and different professions together to shape culture through understanding shared knowledge and goals around patient journeys.
“That’s been pretty successful for us, and I think it’s really important that quality improvement has that understanding of context and culture as well as the idea of having specific interventions – maybe like a simulation – to try and improve an outcome,” she said.
Reference
1. Brazil V et al.. Connecting simulation and quality improvement: How can healthcare simulation really improve patient care? BMJ Qual Saf. 2019 Jul 18. doi: 10.1136/bmjqs-2019-009767.
QI is for clinicians too
QI is for clinicians too
Simulation is commonly used in the education and training of health care professionals, but more recently it’s entering the quality improvement world.
“Instead of just thinking about training individuals and teams, people are starting to use simulation to look at the physical layout of resuscitation bays, to map work flows of a patient journey through hospitals, to identify latent safety threats,” said Victoria Brazil, MD, MBA, lead author of a study on the subject in BMJ Quality & Safety. “These are great things to do, but many of the people doing it didn’t have quality improvement skills or knowledge – that’s why we wrote this article.”
Dr. Brazil, a specialist in health care simulation at Gold Coast (Australia) Hospital and Health Service, explained that, “in terms of the top takeaways, for quality improvement teams – and I’m including everyday clinicians in this: Think about simulation as one of the tools that can be utilized when looking at the questions of how we make our performance better, whether that’s a team performance, environmental, investigational impacts, or one of my key interests, whether that’s about exploring and shaping culture in hospitals, which we’ve done a lot of work on using simulation.”
Quality improvement has become a very specialized field, she added, so hospitalists may think it’s outside their purview. “As clinicians, we don’t think about ourselves as being engaged in quality improvement. I think that’s a shame, because many of the things that we can do bit by bit to make our patient outcomes better, we need to be thinking about finding better ways to do those things. I suggest simulation is one way, and that doesn’t need to be a massive simulation center. It can be simulating the kind of things that are important to you, your teams, and your patients and using those to both explore improved performance.”
Dr. Brazil said that Gold Coast Hospital has used simulation as a way of getting people from different departments and different professions together to shape culture through understanding shared knowledge and goals around patient journeys.
“That’s been pretty successful for us, and I think it’s really important that quality improvement has that understanding of context and culture as well as the idea of having specific interventions – maybe like a simulation – to try and improve an outcome,” she said.
Reference
1. Brazil V et al.. Connecting simulation and quality improvement: How can healthcare simulation really improve patient care? BMJ Qual Saf. 2019 Jul 18. doi: 10.1136/bmjqs-2019-009767.
Simulation is commonly used in the education and training of health care professionals, but more recently it’s entering the quality improvement world.
“Instead of just thinking about training individuals and teams, people are starting to use simulation to look at the physical layout of resuscitation bays, to map work flows of a patient journey through hospitals, to identify latent safety threats,” said Victoria Brazil, MD, MBA, lead author of a study on the subject in BMJ Quality & Safety. “These are great things to do, but many of the people doing it didn’t have quality improvement skills or knowledge – that’s why we wrote this article.”
Dr. Brazil, a specialist in health care simulation at Gold Coast (Australia) Hospital and Health Service, explained that, “in terms of the top takeaways, for quality improvement teams – and I’m including everyday clinicians in this: Think about simulation as one of the tools that can be utilized when looking at the questions of how we make our performance better, whether that’s a team performance, environmental, investigational impacts, or one of my key interests, whether that’s about exploring and shaping culture in hospitals, which we’ve done a lot of work on using simulation.”
Quality improvement has become a very specialized field, she added, so hospitalists may think it’s outside their purview. “As clinicians, we don’t think about ourselves as being engaged in quality improvement. I think that’s a shame, because many of the things that we can do bit by bit to make our patient outcomes better, we need to be thinking about finding better ways to do those things. I suggest simulation is one way, and that doesn’t need to be a massive simulation center. It can be simulating the kind of things that are important to you, your teams, and your patients and using those to both explore improved performance.”
Dr. Brazil said that Gold Coast Hospital has used simulation as a way of getting people from different departments and different professions together to shape culture through understanding shared knowledge and goals around patient journeys.
“That’s been pretty successful for us, and I think it’s really important that quality improvement has that understanding of context and culture as well as the idea of having specific interventions – maybe like a simulation – to try and improve an outcome,” she said.
Reference
1. Brazil V et al.. Connecting simulation and quality improvement: How can healthcare simulation really improve patient care? BMJ Qual Saf. 2019 Jul 18. doi: 10.1136/bmjqs-2019-009767.
Do group visits improve HbA1c more than individual visits in patients with T2DM?
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
EVIDENCE-BASED ANSWER:
Yes. In patients with type 2 diabetes mellitus (T2DM), group visits led by health professionals or teams improved glycosylated hemoglobin (HbA1c) by 0.3% to 0.9% over usual care (strength of recommendation [SOR]: B, meta-analyses of randomized clinical trials [RCTs] with moderate to high risk of bias).
Patients taking oral antidiabetic agents alone appear to benefit more than patients on insulin. Peer-led group visits likely have no effect (SOR: B, subgroup analysis within a meta-analysis).
Treatment durations as long as 3 years are associated with larger decreases in HbA1c (by 0.25% per year) than treatment lasting less than a year (SOR: B, meta-analysis of RCTs involving patents with type 1 diabetes and T2DM).
Patients with T2DM should be offered group visits for diabetes education when available (SOR: C, expert opinion).
56-year-old woman • worsening pain in left upper arm • influenza vaccination in the arm a few days prior to pain onset • Dx?
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
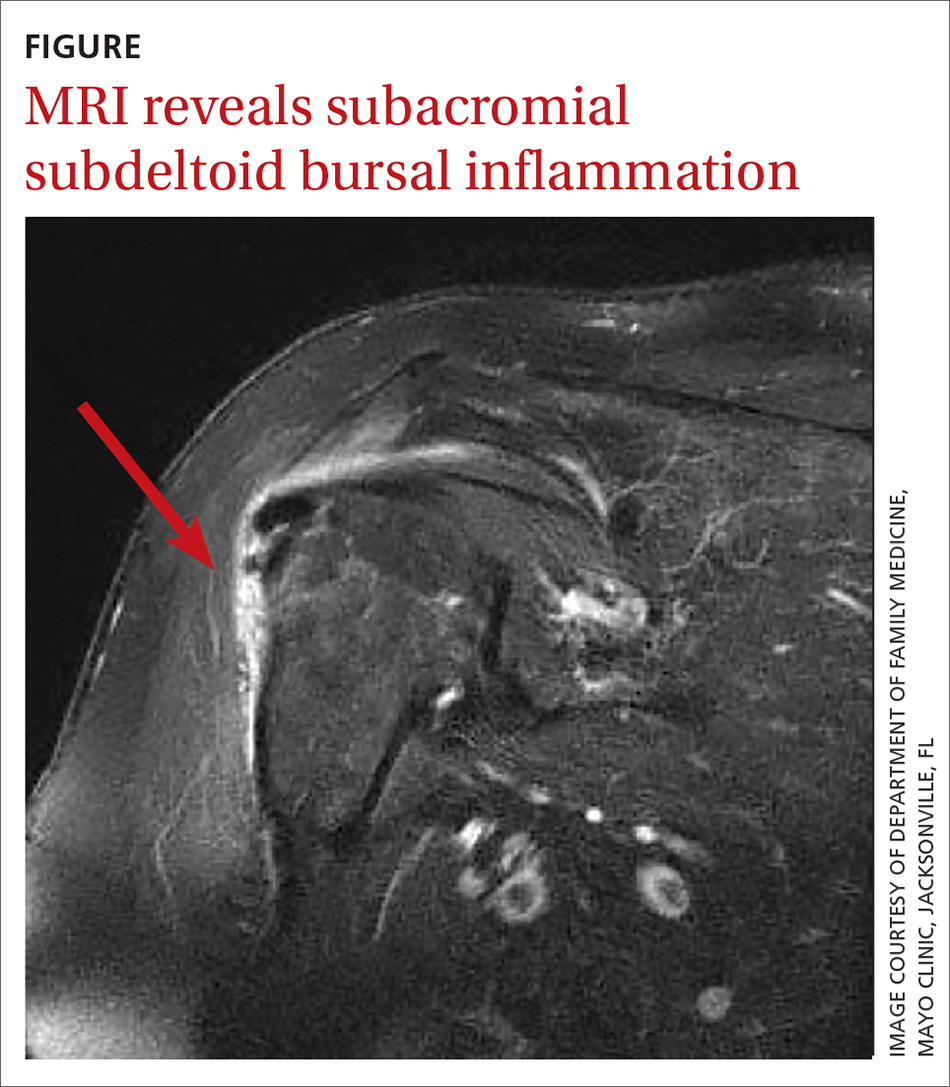
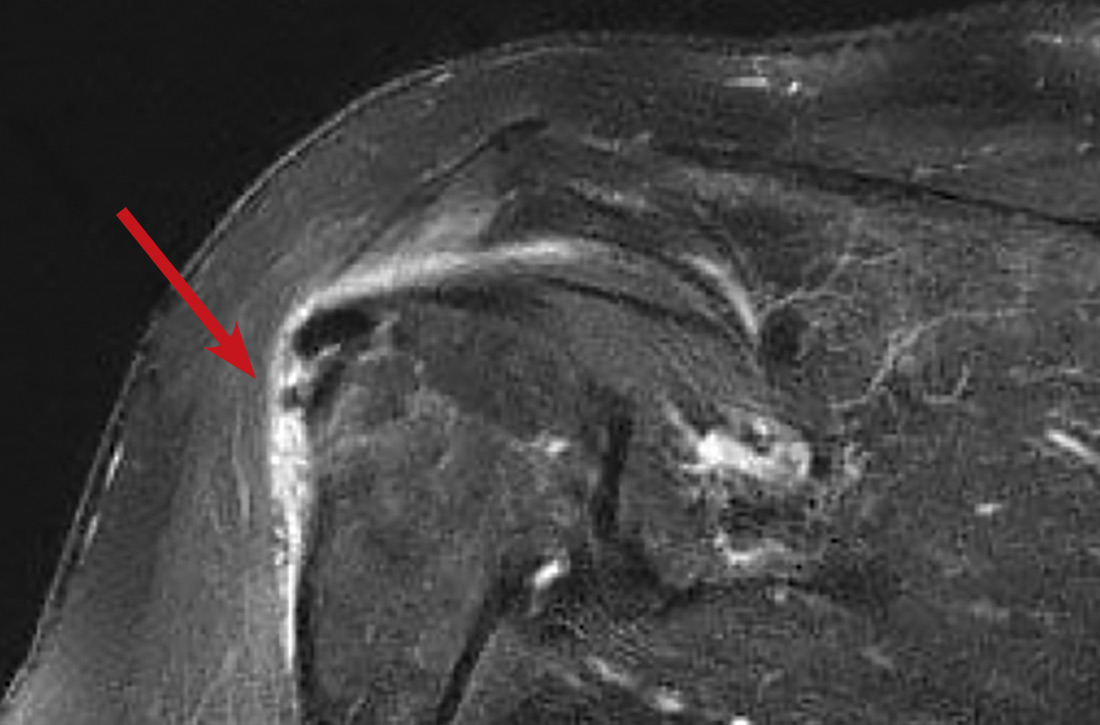
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; farford.bryan@mayo.edu
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; farford.bryan@mayo.edu
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; farford.bryan@mayo.edu
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
BP levels during endovascular stroke therapy affect neurologic outcomes
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
FROM JAMA NEUROLOGY
Echoes of SARS mark 2019 novel coronavirus outbreak
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
FROM THE LANCET
Right hip and pelvic pain
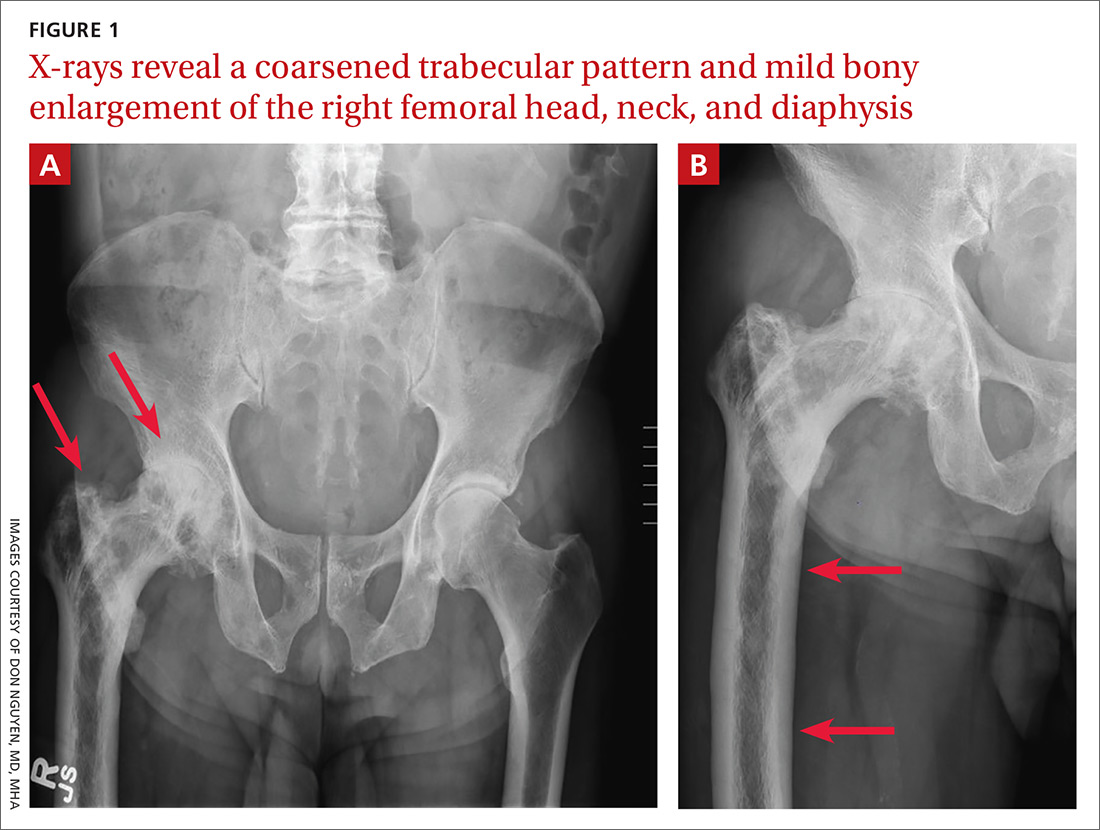
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
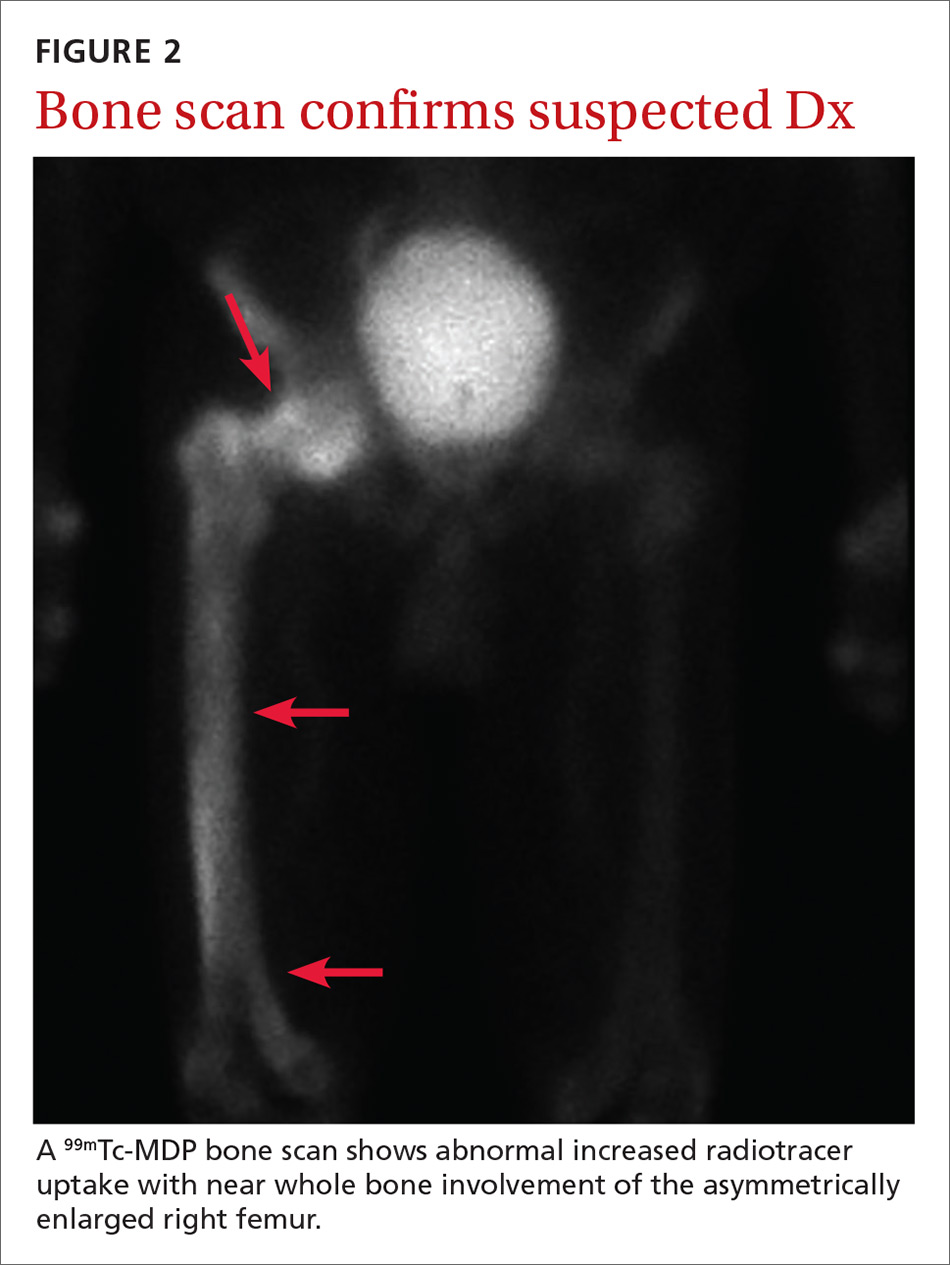
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; dnguyen42@bwh.harvard.edu
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; dnguyen42@bwh.harvard.edu
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; dnguyen42@bwh.harvard.edu
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
33-year-old man • flaccid paralysis in limbs • 30-lb weight loss • thyromegaly without nodules • Dx?
THE CASE
A 33-year-old Hispanic man with no significant past medical history presented to the emergency department with generalized flaccid paralysis in both arms and legs. Two days before, he had been working on a construction site in hot weather. The following day, he woke up with very little energy or strength to perform his daily activities, and he had pain in the inguinal area and both calves. He denied taking any medications or supplements.
The patient had complete muscle weakness and was unable to move his arms and legs. He reported dysphagia and an unintentional weight loss of 30 lb during the previous month.
On physical examination, the patient’s vital signs were within the normal range, and mild thyromegaly without nodules was present. Neurologic examination revealed decreased deep tendon reflexes with intact sensation. Muscle strength in his arms and legs was 0/5.
Initial laboratory test results included a potassium level of 2.2 mEq/L (normal range, 3.5–5 mEq/L) and normal acid-basic status that was confirmed by an arterial blood gas measurement. Serum magnesium was 1.6 mg/dL (normal range, 1.6–2.5 mg/dL); phosphorus, 1.9 mg/dL (normal range, 2.7–4.5 mg/dL); and random urinary potassium, 16 mEq/L (normal range, 25–125 mEq/L). An initial chest x-ray was normal, and an electrocardiogram showed a prolonged QT interval, flattening of the T wave, and a prominent U wave consistent with hypokalemia.
THE DIAGNOSIS
The initial clinical diagnosis was hypokalemic paralysis. The patient was treated with intravenous (IV) potassium chloride 40 mEq
Evaluation of the patient’s hypokalemia revealed the following: thyroid-stimulating hormone (TSH) level, < 0.01 microIU/mL (normal range, 0.27–4.2 microIU/mL); free T4 (thyroxine) level, 4.47 ng/dL (normal range, 0.08–1.70 ng/dL); total T3 (triiodothyronine) level, 17.5 ng/dL (normal range, 2.6–4.4 ng/dL).
The patient was diagnosed with hypokalemic periodic paralysis (HPP) secondary to thyrotoxicosis, also known as thyrotoxicosis periodic paralysis (TPP). His hyperthyroidism was treated with oral atenolol 25 mg/d and oral methimazole 10 mg tid.
Continue to: Within a few hours...
Within a few hours of this treatment, the patient experienced significant improvement in muscle strength and complete resolution of weakness in his arms and legs. Serial measurements of potassium levels normalized.
Further workup revealed that the patient’s thyroid-stimulating immunoglobulin (TSI) was 4.2 on the TSI index (normal, ≤ 1.3) and his thyroid peroxidase (TPO) antibody level was 133.4 IU/mL (normal, < 34 IU/mL). Ultrasonography showed decreased echogenicity of the thyroid gland, consistent with the acute phase of Hashimoto thyroiditis or Graves disease.
The patient was unaware that he had any thyroid disorder previously. He was a private-pay, undocumented immigrant and did not have a regular primary care physician. On discharge, he was referred to a local primary care physician as well as an endocrinologist. He was discharged on atenolol and methimazole.
DISCUSSION
A rare neuromuscular disorder known as periodic paralysis can be precipitated by a hypokalemic or hyperkalemic state; HPP is more common and can be either familial (a defect in the gene) or acquired (secondary to thyrotoxicosis; TPP).1,2 In both forms of periodic paralysis, patients present with hypokalemia and paralysis. Physicians need to look closely at thyroid lab test results so as not to miss the cause of the paralysis.
TPP is most commonly seen in Asian populations, and 95% of cases reported occur in males, despite the higher incidence of hyperthyroidism in females.3 TPP can be precipitated by emotional stress, steroid use, beta-adrenergic bronchodilators, heavy exercise, fasting, or high-carbohydrate meals.2-4 In our patient, heavy exercise and fasting likely were the triggers.
Continue to: The pathophysiology for the hypokalemia...
The pathophysiology for the hypokalemia in TPP is thought to involve the sodium/potassium–adenosine triphosphatase (Na+/K+–ATPase) pump. This pump activity is increased in skeletal muscle and platelets in patients with TPP vs patients with thyrotoxicosis alone.3,5
The role of Hashimoto thyrotoxicosis. Most acquired cases of TPP are mainly secondary to Graves disease with elevated levels of TSI and mildly elevated or normal levels of TPO. In this case, the patient was in the acute phase of Hashimoto thyrotoxicosis (“hashitoxicosis”) with elevated levels of TPO and only mildly elevated TSI.Imaging studies to support the diagnosis, such as a thyroid uptake scan or ultrasonography, are not necessary to determine the cause of thyrotoxicosis. In the absence of test results for TPO and TSI antibodies, however, a scan can be helpful.6,7
Treatment of TPP consists of early recognition and supportive management by correcting the potassium deficit; failure to do so could cause severe complications, such as respiratory failure and psychosis.8 Because of the risk for rebound hyperkalemia, serial potassium levels must be measured until a stable potassium level in the normal range is achieved.
Nonselective beta-blockers, such as propranolol (3 mg/kg) 4 times per day, have been reported to ameliorate the periodic paralysis and prevent rebound hyperkalemia.9 Finally, restoring a euthyroid state will prevent the patient from experiencing future attacks.
THE TAKEAWAY
Few medical conditions result in complete muscle paralysis in a matter of hours. Clinicians should consider the possibility of TPP in any patient who presents with acute onset of paralysis.
CORRESPONDENCE
Jorge Luis Chavez, MD; 8405 E. San Pedro Drive, Scottsdale, AZ 85258; jorgeluischavezmd@yahoo.com.
1. Fontaine B. Periodic paralysis. Adv Genet. 2008;63:3-23.
2. Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore).1992;71:109-120.
3. Lin YF, Wu CC, Pei D, et al. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003;21:339-342.
4. Yu TS, Tseng CF, Chuang YY, et al. Potassium chloride supplementation alone may not improve hypokalemia in thyrotoxic hypokalemic periodic paralysis. J Emerg Med. 2007;32:263-265.
5. Chan A, Shinde R, Chow CC, et al. In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ. 1991;303:1096-1099.
6. Harsch IA, Hahn EG, Strobel D. Hashitoxicosis—three cases and a review of the literature. Eur Endocrinol. 2008;4:70-72. 7. Pou Ucha JL. Imaging in hyperthyroidism. In: Díaz-Soto G, ed. Thyroid Disorders: Focus on Hyperthyroidism. InTechOpen; 2014. www.intechopen.com/books/thyroid-disorders-focus-on-hyperthyroidism/imaging-in-hyperthyroidism. Accessed January 14, 2020.
8. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci. 2010;340:147-153.
9. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620-623.
THE CASE
A 33-year-old Hispanic man with no significant past medical history presented to the emergency department with generalized flaccid paralysis in both arms and legs. Two days before, he had been working on a construction site in hot weather. The following day, he woke up with very little energy or strength to perform his daily activities, and he had pain in the inguinal area and both calves. He denied taking any medications or supplements.
The patient had complete muscle weakness and was unable to move his arms and legs. He reported dysphagia and an unintentional weight loss of 30 lb during the previous month.
On physical examination, the patient’s vital signs were within the normal range, and mild thyromegaly without nodules was present. Neurologic examination revealed decreased deep tendon reflexes with intact sensation. Muscle strength in his arms and legs was 0/5.
Initial laboratory test results included a potassium level of 2.2 mEq/L (normal range, 3.5–5 mEq/L) and normal acid-basic status that was confirmed by an arterial blood gas measurement. Serum magnesium was 1.6 mg/dL (normal range, 1.6–2.5 mg/dL); phosphorus, 1.9 mg/dL (normal range, 2.7–4.5 mg/dL); and random urinary potassium, 16 mEq/L (normal range, 25–125 mEq/L). An initial chest x-ray was normal, and an electrocardiogram showed a prolonged QT interval, flattening of the T wave, and a prominent U wave consistent with hypokalemia.
THE DIAGNOSIS
The initial clinical diagnosis was hypokalemic paralysis. The patient was treated with intravenous (IV) potassium chloride 40 mEq
Evaluation of the patient’s hypokalemia revealed the following: thyroid-stimulating hormone (TSH) level, < 0.01 microIU/mL (normal range, 0.27–4.2 microIU/mL); free T4 (thyroxine) level, 4.47 ng/dL (normal range, 0.08–1.70 ng/dL); total T3 (triiodothyronine) level, 17.5 ng/dL (normal range, 2.6–4.4 ng/dL).
The patient was diagnosed with hypokalemic periodic paralysis (HPP) secondary to thyrotoxicosis, also known as thyrotoxicosis periodic paralysis (TPP). His hyperthyroidism was treated with oral atenolol 25 mg/d and oral methimazole 10 mg tid.
Continue to: Within a few hours...
Within a few hours of this treatment, the patient experienced significant improvement in muscle strength and complete resolution of weakness in his arms and legs. Serial measurements of potassium levels normalized.
Further workup revealed that the patient’s thyroid-stimulating immunoglobulin (TSI) was 4.2 on the TSI index (normal, ≤ 1.3) and his thyroid peroxidase (TPO) antibody level was 133.4 IU/mL (normal, < 34 IU/mL). Ultrasonography showed decreased echogenicity of the thyroid gland, consistent with the acute phase of Hashimoto thyroiditis or Graves disease.
The patient was unaware that he had any thyroid disorder previously. He was a private-pay, undocumented immigrant and did not have a regular primary care physician. On discharge, he was referred to a local primary care physician as well as an endocrinologist. He was discharged on atenolol and methimazole.
DISCUSSION
A rare neuromuscular disorder known as periodic paralysis can be precipitated by a hypokalemic or hyperkalemic state; HPP is more common and can be either familial (a defect in the gene) or acquired (secondary to thyrotoxicosis; TPP).1,2 In both forms of periodic paralysis, patients present with hypokalemia and paralysis. Physicians need to look closely at thyroid lab test results so as not to miss the cause of the paralysis.
TPP is most commonly seen in Asian populations, and 95% of cases reported occur in males, despite the higher incidence of hyperthyroidism in females.3 TPP can be precipitated by emotional stress, steroid use, beta-adrenergic bronchodilators, heavy exercise, fasting, or high-carbohydrate meals.2-4 In our patient, heavy exercise and fasting likely were the triggers.
Continue to: The pathophysiology for the hypokalemia...
The pathophysiology for the hypokalemia in TPP is thought to involve the sodium/potassium–adenosine triphosphatase (Na+/K+–ATPase) pump. This pump activity is increased in skeletal muscle and platelets in patients with TPP vs patients with thyrotoxicosis alone.3,5
The role of Hashimoto thyrotoxicosis. Most acquired cases of TPP are mainly secondary to Graves disease with elevated levels of TSI and mildly elevated or normal levels of TPO. In this case, the patient was in the acute phase of Hashimoto thyrotoxicosis (“hashitoxicosis”) with elevated levels of TPO and only mildly elevated TSI.Imaging studies to support the diagnosis, such as a thyroid uptake scan or ultrasonography, are not necessary to determine the cause of thyrotoxicosis. In the absence of test results for TPO and TSI antibodies, however, a scan can be helpful.6,7
Treatment of TPP consists of early recognition and supportive management by correcting the potassium deficit; failure to do so could cause severe complications, such as respiratory failure and psychosis.8 Because of the risk for rebound hyperkalemia, serial potassium levels must be measured until a stable potassium level in the normal range is achieved.
Nonselective beta-blockers, such as propranolol (3 mg/kg) 4 times per day, have been reported to ameliorate the periodic paralysis and prevent rebound hyperkalemia.9 Finally, restoring a euthyroid state will prevent the patient from experiencing future attacks.
THE TAKEAWAY
Few medical conditions result in complete muscle paralysis in a matter of hours. Clinicians should consider the possibility of TPP in any patient who presents with acute onset of paralysis.
CORRESPONDENCE
Jorge Luis Chavez, MD; 8405 E. San Pedro Drive, Scottsdale, AZ 85258; jorgeluischavezmd@yahoo.com.
THE CASE
A 33-year-old Hispanic man with no significant past medical history presented to the emergency department with generalized flaccid paralysis in both arms and legs. Two days before, he had been working on a construction site in hot weather. The following day, he woke up with very little energy or strength to perform his daily activities, and he had pain in the inguinal area and both calves. He denied taking any medications or supplements.
The patient had complete muscle weakness and was unable to move his arms and legs. He reported dysphagia and an unintentional weight loss of 30 lb during the previous month.
On physical examination, the patient’s vital signs were within the normal range, and mild thyromegaly without nodules was present. Neurologic examination revealed decreased deep tendon reflexes with intact sensation. Muscle strength in his arms and legs was 0/5.
Initial laboratory test results included a potassium level of 2.2 mEq/L (normal range, 3.5–5 mEq/L) and normal acid-basic status that was confirmed by an arterial blood gas measurement. Serum magnesium was 1.6 mg/dL (normal range, 1.6–2.5 mg/dL); phosphorus, 1.9 mg/dL (normal range, 2.7–4.5 mg/dL); and random urinary potassium, 16 mEq/L (normal range, 25–125 mEq/L). An initial chest x-ray was normal, and an electrocardiogram showed a prolonged QT interval, flattening of the T wave, and a prominent U wave consistent with hypokalemia.
THE DIAGNOSIS
The initial clinical diagnosis was hypokalemic paralysis. The patient was treated with intravenous (IV) potassium chloride 40 mEq
Evaluation of the patient’s hypokalemia revealed the following: thyroid-stimulating hormone (TSH) level, < 0.01 microIU/mL (normal range, 0.27–4.2 microIU/mL); free T4 (thyroxine) level, 4.47 ng/dL (normal range, 0.08–1.70 ng/dL); total T3 (triiodothyronine) level, 17.5 ng/dL (normal range, 2.6–4.4 ng/dL).
The patient was diagnosed with hypokalemic periodic paralysis (HPP) secondary to thyrotoxicosis, also known as thyrotoxicosis periodic paralysis (TPP). His hyperthyroidism was treated with oral atenolol 25 mg/d and oral methimazole 10 mg tid.
Continue to: Within a few hours...
Within a few hours of this treatment, the patient experienced significant improvement in muscle strength and complete resolution of weakness in his arms and legs. Serial measurements of potassium levels normalized.
Further workup revealed that the patient’s thyroid-stimulating immunoglobulin (TSI) was 4.2 on the TSI index (normal, ≤ 1.3) and his thyroid peroxidase (TPO) antibody level was 133.4 IU/mL (normal, < 34 IU/mL). Ultrasonography showed decreased echogenicity of the thyroid gland, consistent with the acute phase of Hashimoto thyroiditis or Graves disease.
The patient was unaware that he had any thyroid disorder previously. He was a private-pay, undocumented immigrant and did not have a regular primary care physician. On discharge, he was referred to a local primary care physician as well as an endocrinologist. He was discharged on atenolol and methimazole.
DISCUSSION
A rare neuromuscular disorder known as periodic paralysis can be precipitated by a hypokalemic or hyperkalemic state; HPP is more common and can be either familial (a defect in the gene) or acquired (secondary to thyrotoxicosis; TPP).1,2 In both forms of periodic paralysis, patients present with hypokalemia and paralysis. Physicians need to look closely at thyroid lab test results so as not to miss the cause of the paralysis.
TPP is most commonly seen in Asian populations, and 95% of cases reported occur in males, despite the higher incidence of hyperthyroidism in females.3 TPP can be precipitated by emotional stress, steroid use, beta-adrenergic bronchodilators, heavy exercise, fasting, or high-carbohydrate meals.2-4 In our patient, heavy exercise and fasting likely were the triggers.
Continue to: The pathophysiology for the hypokalemia...
The pathophysiology for the hypokalemia in TPP is thought to involve the sodium/potassium–adenosine triphosphatase (Na+/K+–ATPase) pump. This pump activity is increased in skeletal muscle and platelets in patients with TPP vs patients with thyrotoxicosis alone.3,5
The role of Hashimoto thyrotoxicosis. Most acquired cases of TPP are mainly secondary to Graves disease with elevated levels of TSI and mildly elevated or normal levels of TPO. In this case, the patient was in the acute phase of Hashimoto thyrotoxicosis (“hashitoxicosis”) with elevated levels of TPO and only mildly elevated TSI.Imaging studies to support the diagnosis, such as a thyroid uptake scan or ultrasonography, are not necessary to determine the cause of thyrotoxicosis. In the absence of test results for TPO and TSI antibodies, however, a scan can be helpful.6,7
Treatment of TPP consists of early recognition and supportive management by correcting the potassium deficit; failure to do so could cause severe complications, such as respiratory failure and psychosis.8 Because of the risk for rebound hyperkalemia, serial potassium levels must be measured until a stable potassium level in the normal range is achieved.
Nonselective beta-blockers, such as propranolol (3 mg/kg) 4 times per day, have been reported to ameliorate the periodic paralysis and prevent rebound hyperkalemia.9 Finally, restoring a euthyroid state will prevent the patient from experiencing future attacks.
THE TAKEAWAY
Few medical conditions result in complete muscle paralysis in a matter of hours. Clinicians should consider the possibility of TPP in any patient who presents with acute onset of paralysis.
CORRESPONDENCE
Jorge Luis Chavez, MD; 8405 E. San Pedro Drive, Scottsdale, AZ 85258; jorgeluischavezmd@yahoo.com.
1. Fontaine B. Periodic paralysis. Adv Genet. 2008;63:3-23.
2. Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore).1992;71:109-120.
3. Lin YF, Wu CC, Pei D, et al. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003;21:339-342.
4. Yu TS, Tseng CF, Chuang YY, et al. Potassium chloride supplementation alone may not improve hypokalemia in thyrotoxic hypokalemic periodic paralysis. J Emerg Med. 2007;32:263-265.
5. Chan A, Shinde R, Chow CC, et al. In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ. 1991;303:1096-1099.
6. Harsch IA, Hahn EG, Strobel D. Hashitoxicosis—three cases and a review of the literature. Eur Endocrinol. 2008;4:70-72. 7. Pou Ucha JL. Imaging in hyperthyroidism. In: Díaz-Soto G, ed. Thyroid Disorders: Focus on Hyperthyroidism. InTechOpen; 2014. www.intechopen.com/books/thyroid-disorders-focus-on-hyperthyroidism/imaging-in-hyperthyroidism. Accessed January 14, 2020.
8. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci. 2010;340:147-153.
9. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620-623.
1. Fontaine B. Periodic paralysis. Adv Genet. 2008;63:3-23.
2. Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore).1992;71:109-120.
3. Lin YF, Wu CC, Pei D, et al. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003;21:339-342.
4. Yu TS, Tseng CF, Chuang YY, et al. Potassium chloride supplementation alone may not improve hypokalemia in thyrotoxic hypokalemic periodic paralysis. J Emerg Med. 2007;32:263-265.
5. Chan A, Shinde R, Chow CC, et al. In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ. 1991;303:1096-1099.
6. Harsch IA, Hahn EG, Strobel D. Hashitoxicosis—three cases and a review of the literature. Eur Endocrinol. 2008;4:70-72. 7. Pou Ucha JL. Imaging in hyperthyroidism. In: Díaz-Soto G, ed. Thyroid Disorders: Focus on Hyperthyroidism. InTechOpen; 2014. www.intechopen.com/books/thyroid-disorders-focus-on-hyperthyroidism/imaging-in-hyperthyroidism. Accessed January 14, 2020.
8. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci. 2010;340:147-153.
9. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620-623.
A better approach to preventing active TB?
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
PRACTICE CHANGER
Use 4 months of rifampin instead of 9 months of isoniazid to treat adults with latent tuberculosis; rifampin is associated with fewer adverse events and higher completion rates.
STRENGTH OF RECOMMENDATION
A: Based on a randomized controlled trial and a previous Cochrane review.
Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
A practical guide to the management of phobias
THE CASE
Joe S* is a 25-year-old white man who lives with his mother and has a 5-year history of worsening hypertension. He recently presented to the clinic with heart palpitations, shortness of breath, abdominal distress, and dizziness. He said that it was difficult for him to leave his home due to the intense fear he experiences. He said that these symptoms did not occur at home, nor when he visited specific “safe” locations, such as his girlfriend’s apartment. He reported that his fear had increased over the previous 2 years, and that he had progressively limited the distance he traveled from home. He also reported difficulty being in crowds and said, “The idea of going to the movies is torture.”
●
*The patient’s name has been changed to protect his identity.
The most prevalent psychiatric maladies in primary care are anxiety and mood disorders.1,2 Anxiety disorders are patterns of maladaptive behaviors in conjunction with or response to excessive fear or anxiety.3 The most prevalent anxiety disorder in the United States is specific phobia, the fear of a particular object or situation, with a 12-month prevalence rate of 12.1%.2
Other phobias diagnosed separately in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), include social phobia and agoraphobia, which are, respectively, the fear of being negatively evaluated in social situations and the fear of being trapped in public/open spaces. Social phobia and agoraphobia have diagnostic criteria nearly identical to those of simple phobias regarding the fear response, with the primary differences being the specific phobic situations or stimuli.
Unfortunately, these phobias are likely to be undiagnosed and untreated in primary care partly because patients may not seek treatment.4-6 The ease of avoiding some phobic situations contributes to a lack of treatment seeking.5 Furthermore, commonly used brief measures for psychiatric conditions generally identify depression and anxiety but not phobias. However, family physicians do have resources not only to diagnose these disorders, but also to work with patients to ameliorate them. Collaboration with behavioral health providers is key, as patients with phobias generally benefit from cognitive behavioral therapy (CBT), while those with comorbid psychiatric conditions may benefit from a combination of CBT and medication.
Phobic response vs adaptive fear and anxiety
The terms anxiety and fear often overlap when used to describe a negative emotional state of arousal. However, fear is a response to an actual (or perceived) imminent threat, whereas anxiety is the response to a perceived future threat.3 Fear, although unpleasant, serves an adaptive function in responding to immediate danger.7 Anxiety, in turn, may represent an adaptive function for future activities associated with fear. For example, a cave dweller having seen a bear enter a cave in the past (fear-evoking stimulus) may experience anxiety when exploring a different cave (anxiety that a bear may be present). In this situation, the cave dweller’s fear and anxiety responses are important for survival.
Continue to: With phobias...
With phobias, the fear and anxiety responses become maladaptive.3 Specifically, they involve inaccurate beliefs about a specific type of stimulus that could be an object (snake), environment (ocean), or situation (crowded room). Accompanying the maladaptive thoughts are correspondingly exaggerated emotions, physiologic effects, and behavioral responses in alignment with one another.8 The development of this response and the etiology of phobias is complex and is still being debated.7,9,10 Research points to 4 primary pathways: direct psychological conditioning, modeling (watching others), instruction/information, and nonassociative (innate) acquisition.7,10 While the first 3 pathways involve learned responses, the last results from biological predispositions.
DIAGNOSING PHOBIAS: WHAT TO ZERO IN ON
DSM-5 provides diagnostic criteria for specific phobia, agoraphobia, and social phobia, with each diagnosis requiring that symptoms be present for at least 6 months (TABLE 1).3 Diagnosis of phobias should include evaluation of 4 components of a patient’s functioning: subjective fears, cognitions, physiologic responses, and behaviors.8
- Subjective fears: the patient’s described level of distress/agitation to a specific stimulus.
- Cognitions: the patient’s thoughts/beliefs regarding the stimulus.
- Physiologic responses: changes in heart rate, respiration, blood pressure, and other sympathetic nervous system responses with exposure to stimuli.
- Behavioral responses: the most common response is avoidance, with displays of anger, irritability, or apprehension when avoidance of the stimulus is impossible.
Evaluating these 4 components can be accomplished with structured interviews, behavioral observations, or collateral reports from family members or the patient’s peers.8 Thorough questioning and evaluation (TABLE 28) can enable accurate differentiation between phobias unique to specific stimuli and other DSM-5 disorders that might cause similar symptoms. For example, a patient diagnosed with post-traumatic stress disorder (PTSD) might have a fear response even when triggering stimuli are not present. Identification of a clear, life-threatening incident could help with a differential between phobias and PTSD. However, a patient could be diagnosed with both disorders, as the 2 conditions are not mutually exclusive.
The physiologic and behavioral response symptoms of phobia can also mimic purely medical conditions. Hypertension or tachycardia observed during a medical visit could be due to the fear associated with agoraphobia or with a medically related specific phobia. Blood pressure elevated during testing at the medical appointment could be normal with at-home monitoring by the patient. Thus, blood pressure and heart rate screenings performed at home instead of in public places may help to rule out whether potentially elevated numbers are related to a fear response. Fear and avoidance-like symptoms can also be due to substance abuse, and appropriate drug screening can provide information for an accurate diagnosis.
HOW BEST TO TREAT PHOBIAS
Although research demonstrates that a variety of psychotherapeutic and pharmacologic treatments are efficacious for phobias, in some instances the true utility of an intervention to meaningfully improve a patient’s life is questionable. The issue is that the research evaluating treatment often evaluates only one component of a phobic response (eg,
Continue to: Psychotherapeutic interventions...
Psychotherapeutic interventions for phobias have shown substantial benefit. CBT is helpful, with the most efficacious technique being exposure therapy.5,6,8,11,12 CBT can begin during the initial primary care visit with the family physician educating the patient about phobias and available treatments.
With exposure therapy, patients are introduced to the source of anxiety over time, whereby they learn to manage the distress
Pharmacologic interventions—specifically selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SNRIs)—have been effective in treating social phobia and agoraphobia.6 However, treatment of specific phobias via pharmacologic interventions is not supported due to limited efficacy and few studies for SSRIs and SNRIs.5,6
Benzodiazepines, although effective in alleviating some phobic symptoms, are not recommended per current guidelines due to adverse effects and potential exacerbation of the phobic response once discontinued.5,6 This poor result with benzodiazepines may be due to the absence of simultaneous emotional exposure to the feared stimuli. Unfortunately, little research has been done on the long-term effects of pharmacologic intervention once the treatment has been discontinued.11 So, for medication, the question of how long treatment effect lasts after discontinuation remains unanswered.
THE CASE
Mr. S’s family physician diagnosed his condition as agoraphobia with panic attacks. He was prescribed sertraline for his panic attacks and referred for CBT with a psychologist. CBT focused on cognitive restructuring as well as gradual exposure where he would travel with increasing distances to various locations. After 10 months of treatment, Mr. S was able to overcome the agoraphobia and took an “awesome” vacation. He also reported a significant decrease in panic symptoms.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu.
1. Simon G, Ormel J, VonKorff M, et al. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995; 152:352-357.
2. Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012; 21:169-184.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013:189-233.
4. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015; 17:327-335.
5. Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007; 27:266-286.
6. Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017; 19:93-107.
7. Poulton R, Menzies RG. Non-associative fear acquisition: a review of the evidence from retrospective and longitudinal research. Behav Res Ther. 2002; 40:127-149.
8. Davis III TE, Ollendick TH. Empirically supported treatments for specific phobia in children: Do efficacious treatments address the components of a phobic response? Clin Psychol Sci Pract. 2005; 12:144-160.
9. Field AP. Is conditioning a useful framework for understanding the development and treatment of phobias? Clin Psychol Rev. 2006; 26:857-875.
10. King NJ, Eleonora G, Ollendick TH. Etiology of childhood phobias: current status of Rachman’s three pathways theory. Behav Res Ther. 1998; 36:297-309.
11. Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: a meta-analysis. J Clin Psychopharmacol. 2001; 21:311-324.
12. Wolitzky-Taylor KB, Horowitz JD, Powers MB, et al. Psychological approaches in the treatment of specific phobias: a meta-analysis. Clin Psychol Rev. 2008; 28:1021-1037.
13. Zlomke K, Davis III TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008; 39:207-223.
THE CASE
Joe S* is a 25-year-old white man who lives with his mother and has a 5-year history of worsening hypertension. He recently presented to the clinic with heart palpitations, shortness of breath, abdominal distress, and dizziness. He said that it was difficult for him to leave his home due to the intense fear he experiences. He said that these symptoms did not occur at home, nor when he visited specific “safe” locations, such as his girlfriend’s apartment. He reported that his fear had increased over the previous 2 years, and that he had progressively limited the distance he traveled from home. He also reported difficulty being in crowds and said, “The idea of going to the movies is torture.”
●
*The patient’s name has been changed to protect his identity.
The most prevalent psychiatric maladies in primary care are anxiety and mood disorders.1,2 Anxiety disorders are patterns of maladaptive behaviors in conjunction with or response to excessive fear or anxiety.3 The most prevalent anxiety disorder in the United States is specific phobia, the fear of a particular object or situation, with a 12-month prevalence rate of 12.1%.2
Other phobias diagnosed separately in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), include social phobia and agoraphobia, which are, respectively, the fear of being negatively evaluated in social situations and the fear of being trapped in public/open spaces. Social phobia and agoraphobia have diagnostic criteria nearly identical to those of simple phobias regarding the fear response, with the primary differences being the specific phobic situations or stimuli.
Unfortunately, these phobias are likely to be undiagnosed and untreated in primary care partly because patients may not seek treatment.4-6 The ease of avoiding some phobic situations contributes to a lack of treatment seeking.5 Furthermore, commonly used brief measures for psychiatric conditions generally identify depression and anxiety but not phobias. However, family physicians do have resources not only to diagnose these disorders, but also to work with patients to ameliorate them. Collaboration with behavioral health providers is key, as patients with phobias generally benefit from cognitive behavioral therapy (CBT), while those with comorbid psychiatric conditions may benefit from a combination of CBT and medication.
Phobic response vs adaptive fear and anxiety
The terms anxiety and fear often overlap when used to describe a negative emotional state of arousal. However, fear is a response to an actual (or perceived) imminent threat, whereas anxiety is the response to a perceived future threat.3 Fear, although unpleasant, serves an adaptive function in responding to immediate danger.7 Anxiety, in turn, may represent an adaptive function for future activities associated with fear. For example, a cave dweller having seen a bear enter a cave in the past (fear-evoking stimulus) may experience anxiety when exploring a different cave (anxiety that a bear may be present). In this situation, the cave dweller’s fear and anxiety responses are important for survival.
Continue to: With phobias...
With phobias, the fear and anxiety responses become maladaptive.3 Specifically, they involve inaccurate beliefs about a specific type of stimulus that could be an object (snake), environment (ocean), or situation (crowded room). Accompanying the maladaptive thoughts are correspondingly exaggerated emotions, physiologic effects, and behavioral responses in alignment with one another.8 The development of this response and the etiology of phobias is complex and is still being debated.7,9,10 Research points to 4 primary pathways: direct psychological conditioning, modeling (watching others), instruction/information, and nonassociative (innate) acquisition.7,10 While the first 3 pathways involve learned responses, the last results from biological predispositions.
DIAGNOSING PHOBIAS: WHAT TO ZERO IN ON
DSM-5 provides diagnostic criteria for specific phobia, agoraphobia, and social phobia, with each diagnosis requiring that symptoms be present for at least 6 months (TABLE 1).3 Diagnosis of phobias should include evaluation of 4 components of a patient’s functioning: subjective fears, cognitions, physiologic responses, and behaviors.8
- Subjective fears: the patient’s described level of distress/agitation to a specific stimulus.
- Cognitions: the patient’s thoughts/beliefs regarding the stimulus.
- Physiologic responses: changes in heart rate, respiration, blood pressure, and other sympathetic nervous system responses with exposure to stimuli.
- Behavioral responses: the most common response is avoidance, with displays of anger, irritability, or apprehension when avoidance of the stimulus is impossible.
Evaluating these 4 components can be accomplished with structured interviews, behavioral observations, or collateral reports from family members or the patient’s peers.8 Thorough questioning and evaluation (TABLE 28) can enable accurate differentiation between phobias unique to specific stimuli and other DSM-5 disorders that might cause similar symptoms. For example, a patient diagnosed with post-traumatic stress disorder (PTSD) might have a fear response even when triggering stimuli are not present. Identification of a clear, life-threatening incident could help with a differential between phobias and PTSD. However, a patient could be diagnosed with both disorders, as the 2 conditions are not mutually exclusive.
The physiologic and behavioral response symptoms of phobia can also mimic purely medical conditions. Hypertension or tachycardia observed during a medical visit could be due to the fear associated with agoraphobia or with a medically related specific phobia. Blood pressure elevated during testing at the medical appointment could be normal with at-home monitoring by the patient. Thus, blood pressure and heart rate screenings performed at home instead of in public places may help to rule out whether potentially elevated numbers are related to a fear response. Fear and avoidance-like symptoms can also be due to substance abuse, and appropriate drug screening can provide information for an accurate diagnosis.
HOW BEST TO TREAT PHOBIAS
Although research demonstrates that a variety of psychotherapeutic and pharmacologic treatments are efficacious for phobias, in some instances the true utility of an intervention to meaningfully improve a patient’s life is questionable. The issue is that the research evaluating treatment often evaluates only one component of a phobic response (eg,
Continue to: Psychotherapeutic interventions...
Psychotherapeutic interventions for phobias have shown substantial benefit. CBT is helpful, with the most efficacious technique being exposure therapy.5,6,8,11,12 CBT can begin during the initial primary care visit with the family physician educating the patient about phobias and available treatments.
With exposure therapy, patients are introduced to the source of anxiety over time, whereby they learn to manage the distress
Pharmacologic interventions—specifically selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SNRIs)—have been effective in treating social phobia and agoraphobia.6 However, treatment of specific phobias via pharmacologic interventions is not supported due to limited efficacy and few studies for SSRIs and SNRIs.5,6
Benzodiazepines, although effective in alleviating some phobic symptoms, are not recommended per current guidelines due to adverse effects and potential exacerbation of the phobic response once discontinued.5,6 This poor result with benzodiazepines may be due to the absence of simultaneous emotional exposure to the feared stimuli. Unfortunately, little research has been done on the long-term effects of pharmacologic intervention once the treatment has been discontinued.11 So, for medication, the question of how long treatment effect lasts after discontinuation remains unanswered.
THE CASE
Mr. S’s family physician diagnosed his condition as agoraphobia with panic attacks. He was prescribed sertraline for his panic attacks and referred for CBT with a psychologist. CBT focused on cognitive restructuring as well as gradual exposure where he would travel with increasing distances to various locations. After 10 months of treatment, Mr. S was able to overcome the agoraphobia and took an “awesome” vacation. He also reported a significant decrease in panic symptoms.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu.
THE CASE
Joe S* is a 25-year-old white man who lives with his mother and has a 5-year history of worsening hypertension. He recently presented to the clinic with heart palpitations, shortness of breath, abdominal distress, and dizziness. He said that it was difficult for him to leave his home due to the intense fear he experiences. He said that these symptoms did not occur at home, nor when he visited specific “safe” locations, such as his girlfriend’s apartment. He reported that his fear had increased over the previous 2 years, and that he had progressively limited the distance he traveled from home. He also reported difficulty being in crowds and said, “The idea of going to the movies is torture.”
●
*The patient’s name has been changed to protect his identity.
The most prevalent psychiatric maladies in primary care are anxiety and mood disorders.1,2 Anxiety disorders are patterns of maladaptive behaviors in conjunction with or response to excessive fear or anxiety.3 The most prevalent anxiety disorder in the United States is specific phobia, the fear of a particular object or situation, with a 12-month prevalence rate of 12.1%.2
Other phobias diagnosed separately in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), include social phobia and agoraphobia, which are, respectively, the fear of being negatively evaluated in social situations and the fear of being trapped in public/open spaces. Social phobia and agoraphobia have diagnostic criteria nearly identical to those of simple phobias regarding the fear response, with the primary differences being the specific phobic situations or stimuli.
Unfortunately, these phobias are likely to be undiagnosed and untreated in primary care partly because patients may not seek treatment.4-6 The ease of avoiding some phobic situations contributes to a lack of treatment seeking.5 Furthermore, commonly used brief measures for psychiatric conditions generally identify depression and anxiety but not phobias. However, family physicians do have resources not only to diagnose these disorders, but also to work with patients to ameliorate them. Collaboration with behavioral health providers is key, as patients with phobias generally benefit from cognitive behavioral therapy (CBT), while those with comorbid psychiatric conditions may benefit from a combination of CBT and medication.
Phobic response vs adaptive fear and anxiety
The terms anxiety and fear often overlap when used to describe a negative emotional state of arousal. However, fear is a response to an actual (or perceived) imminent threat, whereas anxiety is the response to a perceived future threat.3 Fear, although unpleasant, serves an adaptive function in responding to immediate danger.7 Anxiety, in turn, may represent an adaptive function for future activities associated with fear. For example, a cave dweller having seen a bear enter a cave in the past (fear-evoking stimulus) may experience anxiety when exploring a different cave (anxiety that a bear may be present). In this situation, the cave dweller’s fear and anxiety responses are important for survival.
Continue to: With phobias...
With phobias, the fear and anxiety responses become maladaptive.3 Specifically, they involve inaccurate beliefs about a specific type of stimulus that could be an object (snake), environment (ocean), or situation (crowded room). Accompanying the maladaptive thoughts are correspondingly exaggerated emotions, physiologic effects, and behavioral responses in alignment with one another.8 The development of this response and the etiology of phobias is complex and is still being debated.7,9,10 Research points to 4 primary pathways: direct psychological conditioning, modeling (watching others), instruction/information, and nonassociative (innate) acquisition.7,10 While the first 3 pathways involve learned responses, the last results from biological predispositions.
DIAGNOSING PHOBIAS: WHAT TO ZERO IN ON
DSM-5 provides diagnostic criteria for specific phobia, agoraphobia, and social phobia, with each diagnosis requiring that symptoms be present for at least 6 months (TABLE 1).3 Diagnosis of phobias should include evaluation of 4 components of a patient’s functioning: subjective fears, cognitions, physiologic responses, and behaviors.8
- Subjective fears: the patient’s described level of distress/agitation to a specific stimulus.
- Cognitions: the patient’s thoughts/beliefs regarding the stimulus.
- Physiologic responses: changes in heart rate, respiration, blood pressure, and other sympathetic nervous system responses with exposure to stimuli.
- Behavioral responses: the most common response is avoidance, with displays of anger, irritability, or apprehension when avoidance of the stimulus is impossible.
Evaluating these 4 components can be accomplished with structured interviews, behavioral observations, or collateral reports from family members or the patient’s peers.8 Thorough questioning and evaluation (TABLE 28) can enable accurate differentiation between phobias unique to specific stimuli and other DSM-5 disorders that might cause similar symptoms. For example, a patient diagnosed with post-traumatic stress disorder (PTSD) might have a fear response even when triggering stimuli are not present. Identification of a clear, life-threatening incident could help with a differential between phobias and PTSD. However, a patient could be diagnosed with both disorders, as the 2 conditions are not mutually exclusive.
The physiologic and behavioral response symptoms of phobia can also mimic purely medical conditions. Hypertension or tachycardia observed during a medical visit could be due to the fear associated with agoraphobia or with a medically related specific phobia. Blood pressure elevated during testing at the medical appointment could be normal with at-home monitoring by the patient. Thus, blood pressure and heart rate screenings performed at home instead of in public places may help to rule out whether potentially elevated numbers are related to a fear response. Fear and avoidance-like symptoms can also be due to substance abuse, and appropriate drug screening can provide information for an accurate diagnosis.
HOW BEST TO TREAT PHOBIAS
Although research demonstrates that a variety of psychotherapeutic and pharmacologic treatments are efficacious for phobias, in some instances the true utility of an intervention to meaningfully improve a patient’s life is questionable. The issue is that the research evaluating treatment often evaluates only one component of a phobic response (eg,
Continue to: Psychotherapeutic interventions...
Psychotherapeutic interventions for phobias have shown substantial benefit. CBT is helpful, with the most efficacious technique being exposure therapy.5,6,8,11,12 CBT can begin during the initial primary care visit with the family physician educating the patient about phobias and available treatments.
With exposure therapy, patients are introduced to the source of anxiety over time, whereby they learn to manage the distress
Pharmacologic interventions—specifically selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SNRIs)—have been effective in treating social phobia and agoraphobia.6 However, treatment of specific phobias via pharmacologic interventions is not supported due to limited efficacy and few studies for SSRIs and SNRIs.5,6
Benzodiazepines, although effective in alleviating some phobic symptoms, are not recommended per current guidelines due to adverse effects and potential exacerbation of the phobic response once discontinued.5,6 This poor result with benzodiazepines may be due to the absence of simultaneous emotional exposure to the feared stimuli. Unfortunately, little research has been done on the long-term effects of pharmacologic intervention once the treatment has been discontinued.11 So, for medication, the question of how long treatment effect lasts after discontinuation remains unanswered.
THE CASE
Mr. S’s family physician diagnosed his condition as agoraphobia with panic attacks. He was prescribed sertraline for his panic attacks and referred for CBT with a psychologist. CBT focused on cognitive restructuring as well as gradual exposure where he would travel with increasing distances to various locations. After 10 months of treatment, Mr. S was able to overcome the agoraphobia and took an “awesome” vacation. He also reported a significant decrease in panic symptoms.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu.
1. Simon G, Ormel J, VonKorff M, et al. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995; 152:352-357.
2. Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012; 21:169-184.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013:189-233.
4. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015; 17:327-335.
5. Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007; 27:266-286.
6. Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017; 19:93-107.
7. Poulton R, Menzies RG. Non-associative fear acquisition: a review of the evidence from retrospective and longitudinal research. Behav Res Ther. 2002; 40:127-149.
8. Davis III TE, Ollendick TH. Empirically supported treatments for specific phobia in children: Do efficacious treatments address the components of a phobic response? Clin Psychol Sci Pract. 2005; 12:144-160.
9. Field AP. Is conditioning a useful framework for understanding the development and treatment of phobias? Clin Psychol Rev. 2006; 26:857-875.
10. King NJ, Eleonora G, Ollendick TH. Etiology of childhood phobias: current status of Rachman’s three pathways theory. Behav Res Ther. 1998; 36:297-309.
11. Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: a meta-analysis. J Clin Psychopharmacol. 2001; 21:311-324.
12. Wolitzky-Taylor KB, Horowitz JD, Powers MB, et al. Psychological approaches in the treatment of specific phobias: a meta-analysis. Clin Psychol Rev. 2008; 28:1021-1037.
13. Zlomke K, Davis III TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008; 39:207-223.
1. Simon G, Ormel J, VonKorff M, et al. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995; 152:352-357.
2. Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012; 21:169-184.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013:189-233.
4. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015; 17:327-335.
5. Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007; 27:266-286.
6. Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017; 19:93-107.
7. Poulton R, Menzies RG. Non-associative fear acquisition: a review of the evidence from retrospective and longitudinal research. Behav Res Ther. 2002; 40:127-149.
8. Davis III TE, Ollendick TH. Empirically supported treatments for specific phobia in children: Do efficacious treatments address the components of a phobic response? Clin Psychol Sci Pract. 2005; 12:144-160.
9. Field AP. Is conditioning a useful framework for understanding the development and treatment of phobias? Clin Psychol Rev. 2006; 26:857-875.
10. King NJ, Eleonora G, Ollendick TH. Etiology of childhood phobias: current status of Rachman’s three pathways theory. Behav Res Ther. 1998; 36:297-309.
11. Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: a meta-analysis. J Clin Psychopharmacol. 2001; 21:311-324.
12. Wolitzky-Taylor KB, Horowitz JD, Powers MB, et al. Psychological approaches in the treatment of specific phobias: a meta-analysis. Clin Psychol Rev. 2008; 28:1021-1037.
13. Zlomke K, Davis III TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008; 39:207-223.
Resecting primary tumors fails to boost survival in metastatic CRC
SAN FRANCISCO – The common practice of resecting asymptomatic primary tumors in patients with unresectable metastases of colorectal cancer does not improve survival but does increase morbidity, and it should therefore be discontinued, according to the phase 3 iPACS trial.
About 80% of patients with stage IV colorectal cancer at diagnosis have metastases that cannot be resected. “For asymptomatic patients, there is no consensus regarding resection of the primary tumor. There have been no randomized controlled trials comparing upfront chemotherapy with primary tumor resection,” said lead investigator Yukihide Kanemitsu, MD, department of colorectal surgery, National Cancer Center Hospital, Tokyo.
He and coinvestigators with the Japan Clinical Oncology Group enrolled in the randomized controlled trial 160 patients with untreated stage IV colorectal cancer who did not have any symptoms from their primary and had up to three unresectable sites of metastases (liver, lungs, distant lymph nodes, and/or peritoneum).
The trial was stopped early for futility, Dr. Kanemitsu reported at the 2020 GI Cancers Symposium. Median overall survival was slightly more than 2 years regardless of whether patients immediately received chemotherapy or underwent primary tumor resection before receiving chemotherapy.
However, 4% of the patients who underwent resection died in the postoperative period from complications. And the resection group had more frequent and more severe chemotherapy-related morbidity, with a higher rate of grade 3 and 4 adverse events (49% vs. 36%).
“Chemotherapy remains the standard therapy for asymptomatic unresectable stage IV colorectal cancer patients,” Dr. Kanemitsu concluded. “Primary tumor resection is not recommended for these patients.”
RCTs should remain the gold standard
“As someone who has built my career on health services research over the last decade working with real-world data, I have become alarmed in recent years at what appears to be a conversation in our community about leaving the randomized controlled trial behind and just using real-world evidence,” said invited discussant Christopher M. Booth, MD, professor, departments of oncology and medicine, Queen’s University, Kingston, Ont., and Canada Research Chair in Population Cancer Care. “Fundamentally, I think this is a really bad idea. The randomized controlled trial should remain the gold standard for efficacy of new cancer therapies.”
The high level of use of primary tumor resection has largely been driven by a meta-analysis that found a dramatic reduction in risk of death with this surgery (Ann Surg Oncol. 2014;21:3900-8), he noted. However, a subsequent retrospective cohort study showed that the apparent benefit disappeared with application of rigorous statistical methods that compensated for biases in the data (Cancer. 2017;123:1124-33).
“I completely support the conclusions presented today,” Dr. Booth asserted, citing the lack of efficacy and greater morbidity and postoperative mortality of primary tumor resection in iPACS, along with the totality of research evidence. “While we can learn a lot from real-world data, it cannot replace randomized controlled trials. In our patients with an asymptomatic primary tumor in the context of incurable colorectal cancer, I believe there is no longer a role for primary tumor resection.”
Study details
In the iPACS trial, patients in the primary tumor resection group underwent open or laparoscopic colectomy/high anterior resection with a D1-D3 lymph node dissection. Concurrent resection of involved organs was not permitted, except for the mesentery, small intestine, greater omentum, and ovary.
In the resection group, the time between surgery and start of chemotherapy was 8 to 56 days, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
With a median follow-up of 22.0 months, median overall survival was 25.9 months with primary tumor resection followed by chemotherapy and 26.7 months with chemotherapy alone (hazard ratio for death, 1.10; P = .69). Findings were similar in subgroup analyses. Median progression-free survival was 10.4 and 12.1 months, respectively (hazard ratio for progression or death, 1.08).
Patients in the primary tumor resection group had higher rates of chemotherapy-related grade 3 and 4 paresthesia (5% vs. 1%), hypertension (17% vs. 8%), diarrhea (5% vs. 1%), and neuropathy (14% vs. 9%), Dr. Kanemitsu reported.
In terms of secondary surgeries, the R0 (curative) resection rate was 3% in the primary tumor resection group and 5% in the chemotherapy group. Thirteen percent of patients in the chemotherapy group underwent palliative surgery for symptoms caused by their primary tumor.
Dr. Kanemitsu disclosed that he receives honoraria from Chugai Pharma, Covidien, Ethicon, and Intuitive Surgical, and that he has a consulting or advisory role with Covidien. The study was funded by a Health and Labor Sciences Research Grant for Clinical Cancer Research. Dr. Booth disclosed that he has no conflicts of interest.
SOURCE: Kanemitsu Y et al. 2020 GI Cancers Symposium. Abstract 7.
SAN FRANCISCO – The common practice of resecting asymptomatic primary tumors in patients with unresectable metastases of colorectal cancer does not improve survival but does increase morbidity, and it should therefore be discontinued, according to the phase 3 iPACS trial.
About 80% of patients with stage IV colorectal cancer at diagnosis have metastases that cannot be resected. “For asymptomatic patients, there is no consensus regarding resection of the primary tumor. There have been no randomized controlled trials comparing upfront chemotherapy with primary tumor resection,” said lead investigator Yukihide Kanemitsu, MD, department of colorectal surgery, National Cancer Center Hospital, Tokyo.
He and coinvestigators with the Japan Clinical Oncology Group enrolled in the randomized controlled trial 160 patients with untreated stage IV colorectal cancer who did not have any symptoms from their primary and had up to three unresectable sites of metastases (liver, lungs, distant lymph nodes, and/or peritoneum).
The trial was stopped early for futility, Dr. Kanemitsu reported at the 2020 GI Cancers Symposium. Median overall survival was slightly more than 2 years regardless of whether patients immediately received chemotherapy or underwent primary tumor resection before receiving chemotherapy.
However, 4% of the patients who underwent resection died in the postoperative period from complications. And the resection group had more frequent and more severe chemotherapy-related morbidity, with a higher rate of grade 3 and 4 adverse events (49% vs. 36%).
“Chemotherapy remains the standard therapy for asymptomatic unresectable stage IV colorectal cancer patients,” Dr. Kanemitsu concluded. “Primary tumor resection is not recommended for these patients.”
RCTs should remain the gold standard
“As someone who has built my career on health services research over the last decade working with real-world data, I have become alarmed in recent years at what appears to be a conversation in our community about leaving the randomized controlled trial behind and just using real-world evidence,” said invited discussant Christopher M. Booth, MD, professor, departments of oncology and medicine, Queen’s University, Kingston, Ont., and Canada Research Chair in Population Cancer Care. “Fundamentally, I think this is a really bad idea. The randomized controlled trial should remain the gold standard for efficacy of new cancer therapies.”
The high level of use of primary tumor resection has largely been driven by a meta-analysis that found a dramatic reduction in risk of death with this surgery (Ann Surg Oncol. 2014;21:3900-8), he noted. However, a subsequent retrospective cohort study showed that the apparent benefit disappeared with application of rigorous statistical methods that compensated for biases in the data (Cancer. 2017;123:1124-33).
“I completely support the conclusions presented today,” Dr. Booth asserted, citing the lack of efficacy and greater morbidity and postoperative mortality of primary tumor resection in iPACS, along with the totality of research evidence. “While we can learn a lot from real-world data, it cannot replace randomized controlled trials. In our patients with an asymptomatic primary tumor in the context of incurable colorectal cancer, I believe there is no longer a role for primary tumor resection.”
Study details
In the iPACS trial, patients in the primary tumor resection group underwent open or laparoscopic colectomy/high anterior resection with a D1-D3 lymph node dissection. Concurrent resection of involved organs was not permitted, except for the mesentery, small intestine, greater omentum, and ovary.
In the resection group, the time between surgery and start of chemotherapy was 8 to 56 days, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
With a median follow-up of 22.0 months, median overall survival was 25.9 months with primary tumor resection followed by chemotherapy and 26.7 months with chemotherapy alone (hazard ratio for death, 1.10; P = .69). Findings were similar in subgroup analyses. Median progression-free survival was 10.4 and 12.1 months, respectively (hazard ratio for progression or death, 1.08).
Patients in the primary tumor resection group had higher rates of chemotherapy-related grade 3 and 4 paresthesia (5% vs. 1%), hypertension (17% vs. 8%), diarrhea (5% vs. 1%), and neuropathy (14% vs. 9%), Dr. Kanemitsu reported.
In terms of secondary surgeries, the R0 (curative) resection rate was 3% in the primary tumor resection group and 5% in the chemotherapy group. Thirteen percent of patients in the chemotherapy group underwent palliative surgery for symptoms caused by their primary tumor.
Dr. Kanemitsu disclosed that he receives honoraria from Chugai Pharma, Covidien, Ethicon, and Intuitive Surgical, and that he has a consulting or advisory role with Covidien. The study was funded by a Health and Labor Sciences Research Grant for Clinical Cancer Research. Dr. Booth disclosed that he has no conflicts of interest.
SOURCE: Kanemitsu Y et al. 2020 GI Cancers Symposium. Abstract 7.
SAN FRANCISCO – The common practice of resecting asymptomatic primary tumors in patients with unresectable metastases of colorectal cancer does not improve survival but does increase morbidity, and it should therefore be discontinued, according to the phase 3 iPACS trial.
About 80% of patients with stage IV colorectal cancer at diagnosis have metastases that cannot be resected. “For asymptomatic patients, there is no consensus regarding resection of the primary tumor. There have been no randomized controlled trials comparing upfront chemotherapy with primary tumor resection,” said lead investigator Yukihide Kanemitsu, MD, department of colorectal surgery, National Cancer Center Hospital, Tokyo.
He and coinvestigators with the Japan Clinical Oncology Group enrolled in the randomized controlled trial 160 patients with untreated stage IV colorectal cancer who did not have any symptoms from their primary and had up to three unresectable sites of metastases (liver, lungs, distant lymph nodes, and/or peritoneum).
The trial was stopped early for futility, Dr. Kanemitsu reported at the 2020 GI Cancers Symposium. Median overall survival was slightly more than 2 years regardless of whether patients immediately received chemotherapy or underwent primary tumor resection before receiving chemotherapy.
However, 4% of the patients who underwent resection died in the postoperative period from complications. And the resection group had more frequent and more severe chemotherapy-related morbidity, with a higher rate of grade 3 and 4 adverse events (49% vs. 36%).
“Chemotherapy remains the standard therapy for asymptomatic unresectable stage IV colorectal cancer patients,” Dr. Kanemitsu concluded. “Primary tumor resection is not recommended for these patients.”
RCTs should remain the gold standard
“As someone who has built my career on health services research over the last decade working with real-world data, I have become alarmed in recent years at what appears to be a conversation in our community about leaving the randomized controlled trial behind and just using real-world evidence,” said invited discussant Christopher M. Booth, MD, professor, departments of oncology and medicine, Queen’s University, Kingston, Ont., and Canada Research Chair in Population Cancer Care. “Fundamentally, I think this is a really bad idea. The randomized controlled trial should remain the gold standard for efficacy of new cancer therapies.”
The high level of use of primary tumor resection has largely been driven by a meta-analysis that found a dramatic reduction in risk of death with this surgery (Ann Surg Oncol. 2014;21:3900-8), he noted. However, a subsequent retrospective cohort study showed that the apparent benefit disappeared with application of rigorous statistical methods that compensated for biases in the data (Cancer. 2017;123:1124-33).
“I completely support the conclusions presented today,” Dr. Booth asserted, citing the lack of efficacy and greater morbidity and postoperative mortality of primary tumor resection in iPACS, along with the totality of research evidence. “While we can learn a lot from real-world data, it cannot replace randomized controlled trials. In our patients with an asymptomatic primary tumor in the context of incurable colorectal cancer, I believe there is no longer a role for primary tumor resection.”
Study details
In the iPACS trial, patients in the primary tumor resection group underwent open or laparoscopic colectomy/high anterior resection with a D1-D3 lymph node dissection. Concurrent resection of involved organs was not permitted, except for the mesentery, small intestine, greater omentum, and ovary.
In the resection group, the time between surgery and start of chemotherapy was 8 to 56 days, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
With a median follow-up of 22.0 months, median overall survival was 25.9 months with primary tumor resection followed by chemotherapy and 26.7 months with chemotherapy alone (hazard ratio for death, 1.10; P = .69). Findings were similar in subgroup analyses. Median progression-free survival was 10.4 and 12.1 months, respectively (hazard ratio for progression or death, 1.08).
Patients in the primary tumor resection group had higher rates of chemotherapy-related grade 3 and 4 paresthesia (5% vs. 1%), hypertension (17% vs. 8%), diarrhea (5% vs. 1%), and neuropathy (14% vs. 9%), Dr. Kanemitsu reported.
In terms of secondary surgeries, the R0 (curative) resection rate was 3% in the primary tumor resection group and 5% in the chemotherapy group. Thirteen percent of patients in the chemotherapy group underwent palliative surgery for symptoms caused by their primary tumor.
Dr. Kanemitsu disclosed that he receives honoraria from Chugai Pharma, Covidien, Ethicon, and Intuitive Surgical, and that he has a consulting or advisory role with Covidien. The study was funded by a Health and Labor Sciences Research Grant for Clinical Cancer Research. Dr. Booth disclosed that he has no conflicts of interest.
SOURCE: Kanemitsu Y et al. 2020 GI Cancers Symposium. Abstract 7.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM