User login
Dual e-cigarette and combustible tobacco use compound respiratory disease risk
according to recent longitudinal analysis published in the American Journal of Preventive Medicine.
E-cigarettes have been promoted as a safer alternative to combustible tobacco, and until recently, there has been little and conflicting evidence by which to test this hypothesis. This study conducted by Dharma N. Bhatta, PhD, and Stanton A. Glantz, PhD, of the Center for Tobacco Control Research and Education at the University of California, San Francisco, is one of the first longitudinal examinations of e-cigarette use and controlling for combustible tobacco use.
Dr. Bhatta and Dr. Glantz performed a multivariable, logistic regression analysis of adults enrolled in the nationally representative, population-based, longitudinal Population Assessment of Tobacco and Health study. The researchers analyzed the tobacco use of adults in the study in three waves, following them through wave 1 (September 2013 to December 2014), wave 2 (October 2014 to October 2015), and wave 3 (October 2015 to October 2016), analyzing the data between 2018 and 2019. Overall, wave 1 began with 32,320 participants, and 15.1% of adults reported respiratory disease at baseline.
Lung or respiratory disease was assessed by asking participants whether they had been told by a health professional that they had chronic obstructive pulmonary disease, chronic bronchitis, emphysema, or asthma. The researchers defined e-cigarette and combustible tobacco use as participants who never, currently, or formerly used e-cigarettes or smoked combustible tobacco. Participants who indicated they used e-cigarettes or combustible tobacco frequently or infrequently were placed in the current-user group, while past users were those participants who said they used to, but no longer use e-cigarettes or combustible tobacco.
The results showed former e-cigarette use (adjusted odds ratio, 1.34; 95% confidence interval, 1.23-1.46) and current e-cigarette use (aOR, 1.32; 95% CI, 1.17-1.49) were associated with an increased risk of having incident respiratory disease.
The data showed a not unexpected statistically significant association between former combustible tobacco use (aOR, 1.29; 95% CI, 1.14-1.47) as well as current combustible tobacco use (aOR, 1.61; 95% CI, 1.42-1.82) and incident respiratory disease risk.
There was a statistically significant association between respiratory disease and former or current e-cigarette use for adults who did not have respiratory disease at baseline, after adjusting for factors such as current combustible tobacco use, clinical variables, and demographic differences. Participants in wave 1 who reported former (aOR, 1.31; 95% CI, 1.07-1.60) or current e-cigarette use (aOR, 1.29; 95% CI, 1.03-1.61) had a significantly higher risk of developing incident respiratory disease in subsequent waves. There was also a statistically significant association between use of combustible tobacco and subsequent respiratory disease in later waves of the study (aOR, 2.56; 95% CI, 1.92-3.41), which the researchers noted was independent of the usual risks associated with combustible tobacco.
The investigators also looked at the link between dual use of e-cigarettes and combustible tobacco and respiratory disease risk. “The much more common pattern is dual use, in which an e-cigarette user continues to smoke combusted tobacco products at the same time (93.7% of e-cigarette users at wave 2 and 91.2% at wave 3 also used combustible tobacco; 73.3% of e-cigarette users at wave 2 and 64.9% at wave 3 also smoked cigarettes),” they wrote.
The odds of developing respiratory disease for participants who used both e-cigarettes and combustible tobacco were 3.30, compared with a participant who never used e-cigarettes, with similar results seen when comparing e-cigarettes and cigarettes.
“Although switching from combustible tobacco, including cigarettes, to e-cigarettes theoretically could reduce the risk of developing respiratory disease, current evidence indicates a high prevalence of dual use, which is associated with in-creased risk beyond combustible tobacco use,” the investigators wrote.
Harold J. Farber, MD, FCCP, professor of pediatrics in the pulmonary section at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, said in an interview that the increased respiratory risk among dual users, who are likely using e-cigarettes and combustible tobacco together as a way to quit smoking, is particularly concerning.
“There is substantial reason to be concerned about efficacy of electronic cigarette products. Real-world observational studies have shown that, on average, tobacco smokers who use electronic cigarettes are less likely to stop smoking than those who do not use electronic cigarettes,” he said. “People who have stopped tobacco smoking but use electronic cigarettes are more likely to relapse to tobacco smoking than those who do not use electronic cigarettes.”
Dr. Farber noted that there are other Food and Drug Administration–approved medications for treating tobacco addiction. In addition, the World Health Organization, American Medical Association, Centers for Disease Control and Prevention, and FDA have all advised that e-cigarettes should not be used as smoking cessation aids, he said, especially in light of current outbreak of life-threatening e-cigarette and vaping lung injuries currently being investigated by the CDC and FDA.
“These study results suggest that the CDC reports of e-cigarette, or vaping, product use–associated lung injury are likely to be just the tip of the iceberg,” he said. “Although the CDC has identified vitamin E acetate–containing products as an important culprit, it is unlikely to be the only one. There are many substances in the emissions of e-cigarettes that have known irritant and/or toxic effects on the airways.”
Dr. Bhatta and Dr. Glantz acknowledged several limitations in their analysis, including the possibility of recall bias, not distinguishing between nondaily and daily e-cigarette or combustible tobacco use, and combining respiratory conditions together to achieve adequate power. The study shows an association, but the mechanism by which e-cigarettes may contribute to the development of lung disease remains under investigation.
This study was supported by grants from the National Institute on Drug Abuse; the National Cancer Institute; the FDA Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center Global Cancer Program. Dr. Bhatta and Dr. Glantz reported no relevant conflicts of interest.
SOURCE: Bhatta DN, Glantz SA. Am J Prev Med. 2019 Dec 16. doi: 10.1016/j.amepre.2019.07.028.
according to recent longitudinal analysis published in the American Journal of Preventive Medicine.
E-cigarettes have been promoted as a safer alternative to combustible tobacco, and until recently, there has been little and conflicting evidence by which to test this hypothesis. This study conducted by Dharma N. Bhatta, PhD, and Stanton A. Glantz, PhD, of the Center for Tobacco Control Research and Education at the University of California, San Francisco, is one of the first longitudinal examinations of e-cigarette use and controlling for combustible tobacco use.
Dr. Bhatta and Dr. Glantz performed a multivariable, logistic regression analysis of adults enrolled in the nationally representative, population-based, longitudinal Population Assessment of Tobacco and Health study. The researchers analyzed the tobacco use of adults in the study in three waves, following them through wave 1 (September 2013 to December 2014), wave 2 (October 2014 to October 2015), and wave 3 (October 2015 to October 2016), analyzing the data between 2018 and 2019. Overall, wave 1 began with 32,320 participants, and 15.1% of adults reported respiratory disease at baseline.
Lung or respiratory disease was assessed by asking participants whether they had been told by a health professional that they had chronic obstructive pulmonary disease, chronic bronchitis, emphysema, or asthma. The researchers defined e-cigarette and combustible tobacco use as participants who never, currently, or formerly used e-cigarettes or smoked combustible tobacco. Participants who indicated they used e-cigarettes or combustible tobacco frequently or infrequently were placed in the current-user group, while past users were those participants who said they used to, but no longer use e-cigarettes or combustible tobacco.
The results showed former e-cigarette use (adjusted odds ratio, 1.34; 95% confidence interval, 1.23-1.46) and current e-cigarette use (aOR, 1.32; 95% CI, 1.17-1.49) were associated with an increased risk of having incident respiratory disease.
The data showed a not unexpected statistically significant association between former combustible tobacco use (aOR, 1.29; 95% CI, 1.14-1.47) as well as current combustible tobacco use (aOR, 1.61; 95% CI, 1.42-1.82) and incident respiratory disease risk.
There was a statistically significant association between respiratory disease and former or current e-cigarette use for adults who did not have respiratory disease at baseline, after adjusting for factors such as current combustible tobacco use, clinical variables, and demographic differences. Participants in wave 1 who reported former (aOR, 1.31; 95% CI, 1.07-1.60) or current e-cigarette use (aOR, 1.29; 95% CI, 1.03-1.61) had a significantly higher risk of developing incident respiratory disease in subsequent waves. There was also a statistically significant association between use of combustible tobacco and subsequent respiratory disease in later waves of the study (aOR, 2.56; 95% CI, 1.92-3.41), which the researchers noted was independent of the usual risks associated with combustible tobacco.
The investigators also looked at the link between dual use of e-cigarettes and combustible tobacco and respiratory disease risk. “The much more common pattern is dual use, in which an e-cigarette user continues to smoke combusted tobacco products at the same time (93.7% of e-cigarette users at wave 2 and 91.2% at wave 3 also used combustible tobacco; 73.3% of e-cigarette users at wave 2 and 64.9% at wave 3 also smoked cigarettes),” they wrote.
The odds of developing respiratory disease for participants who used both e-cigarettes and combustible tobacco were 3.30, compared with a participant who never used e-cigarettes, with similar results seen when comparing e-cigarettes and cigarettes.
“Although switching from combustible tobacco, including cigarettes, to e-cigarettes theoretically could reduce the risk of developing respiratory disease, current evidence indicates a high prevalence of dual use, which is associated with in-creased risk beyond combustible tobacco use,” the investigators wrote.
Harold J. Farber, MD, FCCP, professor of pediatrics in the pulmonary section at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, said in an interview that the increased respiratory risk among dual users, who are likely using e-cigarettes and combustible tobacco together as a way to quit smoking, is particularly concerning.
“There is substantial reason to be concerned about efficacy of electronic cigarette products. Real-world observational studies have shown that, on average, tobacco smokers who use electronic cigarettes are less likely to stop smoking than those who do not use electronic cigarettes,” he said. “People who have stopped tobacco smoking but use electronic cigarettes are more likely to relapse to tobacco smoking than those who do not use electronic cigarettes.”
Dr. Farber noted that there are other Food and Drug Administration–approved medications for treating tobacco addiction. In addition, the World Health Organization, American Medical Association, Centers for Disease Control and Prevention, and FDA have all advised that e-cigarettes should not be used as smoking cessation aids, he said, especially in light of current outbreak of life-threatening e-cigarette and vaping lung injuries currently being investigated by the CDC and FDA.
“These study results suggest that the CDC reports of e-cigarette, or vaping, product use–associated lung injury are likely to be just the tip of the iceberg,” he said. “Although the CDC has identified vitamin E acetate–containing products as an important culprit, it is unlikely to be the only one. There are many substances in the emissions of e-cigarettes that have known irritant and/or toxic effects on the airways.”
Dr. Bhatta and Dr. Glantz acknowledged several limitations in their analysis, including the possibility of recall bias, not distinguishing between nondaily and daily e-cigarette or combustible tobacco use, and combining respiratory conditions together to achieve adequate power. The study shows an association, but the mechanism by which e-cigarettes may contribute to the development of lung disease remains under investigation.
This study was supported by grants from the National Institute on Drug Abuse; the National Cancer Institute; the FDA Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center Global Cancer Program. Dr. Bhatta and Dr. Glantz reported no relevant conflicts of interest.
SOURCE: Bhatta DN, Glantz SA. Am J Prev Med. 2019 Dec 16. doi: 10.1016/j.amepre.2019.07.028.
according to recent longitudinal analysis published in the American Journal of Preventive Medicine.
E-cigarettes have been promoted as a safer alternative to combustible tobacco, and until recently, there has been little and conflicting evidence by which to test this hypothesis. This study conducted by Dharma N. Bhatta, PhD, and Stanton A. Glantz, PhD, of the Center for Tobacco Control Research and Education at the University of California, San Francisco, is one of the first longitudinal examinations of e-cigarette use and controlling for combustible tobacco use.
Dr. Bhatta and Dr. Glantz performed a multivariable, logistic regression analysis of adults enrolled in the nationally representative, population-based, longitudinal Population Assessment of Tobacco and Health study. The researchers analyzed the tobacco use of adults in the study in three waves, following them through wave 1 (September 2013 to December 2014), wave 2 (October 2014 to October 2015), and wave 3 (October 2015 to October 2016), analyzing the data between 2018 and 2019. Overall, wave 1 began with 32,320 participants, and 15.1% of adults reported respiratory disease at baseline.
Lung or respiratory disease was assessed by asking participants whether they had been told by a health professional that they had chronic obstructive pulmonary disease, chronic bronchitis, emphysema, or asthma. The researchers defined e-cigarette and combustible tobacco use as participants who never, currently, or formerly used e-cigarettes or smoked combustible tobacco. Participants who indicated they used e-cigarettes or combustible tobacco frequently or infrequently were placed in the current-user group, while past users were those participants who said they used to, but no longer use e-cigarettes or combustible tobacco.
The results showed former e-cigarette use (adjusted odds ratio, 1.34; 95% confidence interval, 1.23-1.46) and current e-cigarette use (aOR, 1.32; 95% CI, 1.17-1.49) were associated with an increased risk of having incident respiratory disease.
The data showed a not unexpected statistically significant association between former combustible tobacco use (aOR, 1.29; 95% CI, 1.14-1.47) as well as current combustible tobacco use (aOR, 1.61; 95% CI, 1.42-1.82) and incident respiratory disease risk.
There was a statistically significant association between respiratory disease and former or current e-cigarette use for adults who did not have respiratory disease at baseline, after adjusting for factors such as current combustible tobacco use, clinical variables, and demographic differences. Participants in wave 1 who reported former (aOR, 1.31; 95% CI, 1.07-1.60) or current e-cigarette use (aOR, 1.29; 95% CI, 1.03-1.61) had a significantly higher risk of developing incident respiratory disease in subsequent waves. There was also a statistically significant association between use of combustible tobacco and subsequent respiratory disease in later waves of the study (aOR, 2.56; 95% CI, 1.92-3.41), which the researchers noted was independent of the usual risks associated with combustible tobacco.
The investigators also looked at the link between dual use of e-cigarettes and combustible tobacco and respiratory disease risk. “The much more common pattern is dual use, in which an e-cigarette user continues to smoke combusted tobacco products at the same time (93.7% of e-cigarette users at wave 2 and 91.2% at wave 3 also used combustible tobacco; 73.3% of e-cigarette users at wave 2 and 64.9% at wave 3 also smoked cigarettes),” they wrote.
The odds of developing respiratory disease for participants who used both e-cigarettes and combustible tobacco were 3.30, compared with a participant who never used e-cigarettes, with similar results seen when comparing e-cigarettes and cigarettes.
“Although switching from combustible tobacco, including cigarettes, to e-cigarettes theoretically could reduce the risk of developing respiratory disease, current evidence indicates a high prevalence of dual use, which is associated with in-creased risk beyond combustible tobacco use,” the investigators wrote.
Harold J. Farber, MD, FCCP, professor of pediatrics in the pulmonary section at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, said in an interview that the increased respiratory risk among dual users, who are likely using e-cigarettes and combustible tobacco together as a way to quit smoking, is particularly concerning.
“There is substantial reason to be concerned about efficacy of electronic cigarette products. Real-world observational studies have shown that, on average, tobacco smokers who use electronic cigarettes are less likely to stop smoking than those who do not use electronic cigarettes,” he said. “People who have stopped tobacco smoking but use electronic cigarettes are more likely to relapse to tobacco smoking than those who do not use electronic cigarettes.”
Dr. Farber noted that there are other Food and Drug Administration–approved medications for treating tobacco addiction. In addition, the World Health Organization, American Medical Association, Centers for Disease Control and Prevention, and FDA have all advised that e-cigarettes should not be used as smoking cessation aids, he said, especially in light of current outbreak of life-threatening e-cigarette and vaping lung injuries currently being investigated by the CDC and FDA.
“These study results suggest that the CDC reports of e-cigarette, or vaping, product use–associated lung injury are likely to be just the tip of the iceberg,” he said. “Although the CDC has identified vitamin E acetate–containing products as an important culprit, it is unlikely to be the only one. There are many substances in the emissions of e-cigarettes that have known irritant and/or toxic effects on the airways.”
Dr. Bhatta and Dr. Glantz acknowledged several limitations in their analysis, including the possibility of recall bias, not distinguishing between nondaily and daily e-cigarette or combustible tobacco use, and combining respiratory conditions together to achieve adequate power. The study shows an association, but the mechanism by which e-cigarettes may contribute to the development of lung disease remains under investigation.
This study was supported by grants from the National Institute on Drug Abuse; the National Cancer Institute; the FDA Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center Global Cancer Program. Dr. Bhatta and Dr. Glantz reported no relevant conflicts of interest.
SOURCE: Bhatta DN, Glantz SA. Am J Prev Med. 2019 Dec 16. doi: 10.1016/j.amepre.2019.07.028.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Moffitt announces new chief digital innovation officer
Edmondo Robinson, MD, is the new senior vice president and chief digital innovation officer at Moffitt Cancer Center in Tampa, Fla. In this newly created position, Dr. Robinson will “oversee Moffitt’s portfolio of digital innovation, including the development and commercialization of health products, tools, and technology.”
Dr. Robinson is also associate professor of medicine at Thomas Jefferson University’s Sidney Kimmel Medical College in Philadelphia. He was previously the chief transformation officer and senior vice-president of consumerism at ChristianaCare, a health system based in Wilmington, Del. Dr. Robinson’s research is focused on health services, particularly care transitions and how technology impacts care delivery.
In other news, Elizabeth Fox, MD, has been named senior vice president of clinical trials research at St. Jude Children’s Research Hospital in Memphis, Tenn. She will also serve as the associate director for clinical research in the St. Jude Comprehensive Cancer Center. Dr. Fox will take on these roles in January 2020.
Dr. Fox was previously director of developmental therapeutics in oncology and professor of pediatrics at Children’s Hospital of Philadelphia. According to St. Jude, Dr. Fox is an expert in integrating clinical and preclinical pharmacology in clinical trial design.
The International Society of Gastrointestinal Oncology has appointed Weijing Sun, MD, as its president-elect. After his 2-year term as president-elect, Dr. Sun will take over as president from Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Sun is director of the division of medical oncology and associate director for clinical research at the University of Kansas Cancer Center in Kansas City. He is a gastrointestinal medical oncologist with a research focus on the development of new treatments for pancreatic, gastroesophageal, hepatobiliary, and colorectal cancers.
Finally, Elizabeth Plimack, MD, has been elected to the American Society of Clinical Oncology’s board of directors for a term of 4 years. She will begin this appointment in June 2020.
Dr. Plimack is a professor and chief of the division of genitourinary medical oncology at Fox Chase Cancer Center in Philadelphia. Dr. Plimack’s clinical practice is focused on the treatment of kidney, bladder, prostate, and testicular cancer. Her research is focused on developing new therapies for bladder and kidney cancers.
Edmondo Robinson, MD, is the new senior vice president and chief digital innovation officer at Moffitt Cancer Center in Tampa, Fla. In this newly created position, Dr. Robinson will “oversee Moffitt’s portfolio of digital innovation, including the development and commercialization of health products, tools, and technology.”
Dr. Robinson is also associate professor of medicine at Thomas Jefferson University’s Sidney Kimmel Medical College in Philadelphia. He was previously the chief transformation officer and senior vice-president of consumerism at ChristianaCare, a health system based in Wilmington, Del. Dr. Robinson’s research is focused on health services, particularly care transitions and how technology impacts care delivery.
In other news, Elizabeth Fox, MD, has been named senior vice president of clinical trials research at St. Jude Children’s Research Hospital in Memphis, Tenn. She will also serve as the associate director for clinical research in the St. Jude Comprehensive Cancer Center. Dr. Fox will take on these roles in January 2020.
Dr. Fox was previously director of developmental therapeutics in oncology and professor of pediatrics at Children’s Hospital of Philadelphia. According to St. Jude, Dr. Fox is an expert in integrating clinical and preclinical pharmacology in clinical trial design.
The International Society of Gastrointestinal Oncology has appointed Weijing Sun, MD, as its president-elect. After his 2-year term as president-elect, Dr. Sun will take over as president from Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Sun is director of the division of medical oncology and associate director for clinical research at the University of Kansas Cancer Center in Kansas City. He is a gastrointestinal medical oncologist with a research focus on the development of new treatments for pancreatic, gastroesophageal, hepatobiliary, and colorectal cancers.
Finally, Elizabeth Plimack, MD, has been elected to the American Society of Clinical Oncology’s board of directors for a term of 4 years. She will begin this appointment in June 2020.
Dr. Plimack is a professor and chief of the division of genitourinary medical oncology at Fox Chase Cancer Center in Philadelphia. Dr. Plimack’s clinical practice is focused on the treatment of kidney, bladder, prostate, and testicular cancer. Her research is focused on developing new therapies for bladder and kidney cancers.
Edmondo Robinson, MD, is the new senior vice president and chief digital innovation officer at Moffitt Cancer Center in Tampa, Fla. In this newly created position, Dr. Robinson will “oversee Moffitt’s portfolio of digital innovation, including the development and commercialization of health products, tools, and technology.”
Dr. Robinson is also associate professor of medicine at Thomas Jefferson University’s Sidney Kimmel Medical College in Philadelphia. He was previously the chief transformation officer and senior vice-president of consumerism at ChristianaCare, a health system based in Wilmington, Del. Dr. Robinson’s research is focused on health services, particularly care transitions and how technology impacts care delivery.
In other news, Elizabeth Fox, MD, has been named senior vice president of clinical trials research at St. Jude Children’s Research Hospital in Memphis, Tenn. She will also serve as the associate director for clinical research in the St. Jude Comprehensive Cancer Center. Dr. Fox will take on these roles in January 2020.
Dr. Fox was previously director of developmental therapeutics in oncology and professor of pediatrics at Children’s Hospital of Philadelphia. According to St. Jude, Dr. Fox is an expert in integrating clinical and preclinical pharmacology in clinical trial design.
The International Society of Gastrointestinal Oncology has appointed Weijing Sun, MD, as its president-elect. After his 2-year term as president-elect, Dr. Sun will take over as president from Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Sun is director of the division of medical oncology and associate director for clinical research at the University of Kansas Cancer Center in Kansas City. He is a gastrointestinal medical oncologist with a research focus on the development of new treatments for pancreatic, gastroesophageal, hepatobiliary, and colorectal cancers.
Finally, Elizabeth Plimack, MD, has been elected to the American Society of Clinical Oncology’s board of directors for a term of 4 years. She will begin this appointment in June 2020.
Dr. Plimack is a professor and chief of the division of genitourinary medical oncology at Fox Chase Cancer Center in Philadelphia. Dr. Plimack’s clinical practice is focused on the treatment of kidney, bladder, prostate, and testicular cancer. Her research is focused on developing new therapies for bladder and kidney cancers.
Withdrawal of candidacy for APA President-Elect
To the readers of Current Psychiatry,
The American Psychiatric Association (APA) informed me on 12-27-19 that my editorial in the December issue about my candidacy for APA President-Elect was unfair to the other candidates because they should have been invited to publish their own statements side-by-side with mine. I was not aware of this because the APA election rules allow a candidate to blog or write on all social media or to send a mass mailing unilaterally. I take full responsibility for my mistake and decided to inform the APA Board of Trustees that I am withdrawing my candidacy for APA President-Elect. I hope the elections will go smoothly and wish the APA well.
Please note that my loyalty to the APA is very strong. That’s why my January 2020 editorial strongly urges all psychiatrists to join (or rejoin) the APA because unity will make it more possible for us to advocate for our patients, increase access to mental health, eliminate stigma, achieve true parity, and raise the profile of psychiatry as a medical discipline.
As you may have read in my campaign statement, one of my major goals as a candidate was to change the name of the APA to the American Psychiatric Physicians Association, or APPA. This name change is critical so that the public knows our medical identity. It also will differentiate us from the other APA (American Psychological Association), which is the first to appear when anyone enters APA on Google or other search engines. I will lobby vigorously with the current APA president, the APA CEO, and whoever becomes President-Elect to get this name change approved by the Board of Trustees. I am very sure that the vast majority of psychiatrists will support such a name change.
Thank you and I hope 2020 will be a happy and healthy year for all of you, and for all our psychiatric patients.
To the readers of Current Psychiatry,
The American Psychiatric Association (APA) informed me on 12-27-19 that my editorial in the December issue about my candidacy for APA President-Elect was unfair to the other candidates because they should have been invited to publish their own statements side-by-side with mine. I was not aware of this because the APA election rules allow a candidate to blog or write on all social media or to send a mass mailing unilaterally. I take full responsibility for my mistake and decided to inform the APA Board of Trustees that I am withdrawing my candidacy for APA President-Elect. I hope the elections will go smoothly and wish the APA well.
Please note that my loyalty to the APA is very strong. That’s why my January 2020 editorial strongly urges all psychiatrists to join (or rejoin) the APA because unity will make it more possible for us to advocate for our patients, increase access to mental health, eliminate stigma, achieve true parity, and raise the profile of psychiatry as a medical discipline.
As you may have read in my campaign statement, one of my major goals as a candidate was to change the name of the APA to the American Psychiatric Physicians Association, or APPA. This name change is critical so that the public knows our medical identity. It also will differentiate us from the other APA (American Psychological Association), which is the first to appear when anyone enters APA on Google or other search engines. I will lobby vigorously with the current APA president, the APA CEO, and whoever becomes President-Elect to get this name change approved by the Board of Trustees. I am very sure that the vast majority of psychiatrists will support such a name change.
Thank you and I hope 2020 will be a happy and healthy year for all of you, and for all our psychiatric patients.
To the readers of Current Psychiatry,
The American Psychiatric Association (APA) informed me on 12-27-19 that my editorial in the December issue about my candidacy for APA President-Elect was unfair to the other candidates because they should have been invited to publish their own statements side-by-side with mine. I was not aware of this because the APA election rules allow a candidate to blog or write on all social media or to send a mass mailing unilaterally. I take full responsibility for my mistake and decided to inform the APA Board of Trustees that I am withdrawing my candidacy for APA President-Elect. I hope the elections will go smoothly and wish the APA well.
Please note that my loyalty to the APA is very strong. That’s why my January 2020 editorial strongly urges all psychiatrists to join (or rejoin) the APA because unity will make it more possible for us to advocate for our patients, increase access to mental health, eliminate stigma, achieve true parity, and raise the profile of psychiatry as a medical discipline.
As you may have read in my campaign statement, one of my major goals as a candidate was to change the name of the APA to the American Psychiatric Physicians Association, or APPA. This name change is critical so that the public knows our medical identity. It also will differentiate us from the other APA (American Psychological Association), which is the first to appear when anyone enters APA on Google or other search engines. I will lobby vigorously with the current APA president, the APA CEO, and whoever becomes President-Elect to get this name change approved by the Board of Trustees. I am very sure that the vast majority of psychiatrists will support such a name change.
Thank you and I hope 2020 will be a happy and healthy year for all of you, and for all our psychiatric patients.
Early increase in flu activity shows no signs of slowing
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.
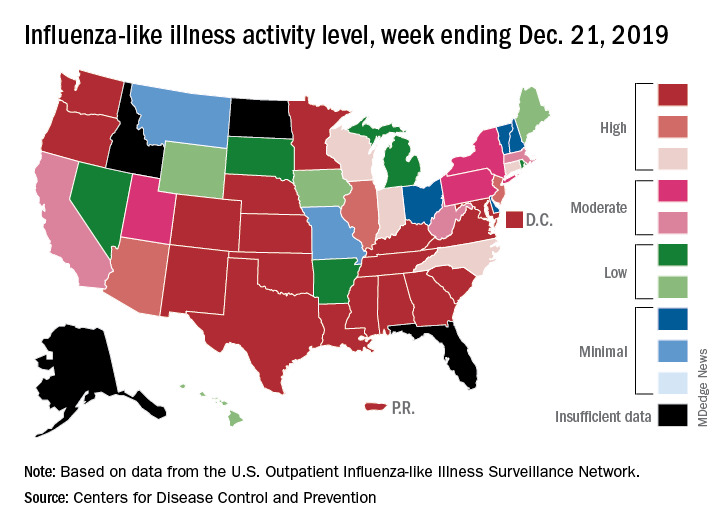
reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
Calif. woman poisoned by methylmercury-containing skin cream
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
FROM MMWR
Replacement meals boost nutrient intake by pregnant women with obesity
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
REPORTING FROM OBESITY WEEK 2019
New cystic fibrosis therapy raises hopes among specialists and patients
A newly approved triple-combination modulator to treat cystic fibrosis (CF) has raised expectations of a treatment turning point among patients and specialists. If the early results are sustained, elexacaftor/ivacaftor/tezacaftor (Trikafta) could prove to be the rare case of a much-touted new medicine that meets high expectations.
“CF even in infants causes inflammation, so we know that lung damage can start early and progress,” said Susan Millard, MD, FCCP, of Helen DeVos Children’s Hospital in Grand Rapids, Mich., and the local clinical research director for the pediatric pulmonary and sleep medicine section. “This oral drug therapy is actually treating the underlying problem, as opposed to many of the therapies we have that take hours to nebulize and only work locally in the airways.”
Dr. Millard is the recent past pediatric editor for Chest Physician and has been a local principal investigator at Helen DeVos Children’s Hospital for many Vertex-sponsored clinical studies.
The pivotal studies
The Food and Drug Administration approval of Trikafta rested on two pivotal phase 3, placebo-controlled studies, one in patients with two copies of the most common CF mutations, F508del, and the second in patients with one copy of F508del and a second mutation that was called a “minimal-function” mutation. The findings have ignited the hopes of many people with CF and their physicians. The drug was approved in October 2019 for patients aged 12 years and older who have at least one F508del mutation of the cystic fibrosis transmembrane conductance regulator gene. About 90% of patients in the United States have at least one copy of F508del. In the study looking at patients with one copy of F508del, the mean predicted forced expiratory volume in 1 second increased 13.8% in patients taking the drug versus placebo (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMoa1908639). The number of pulmonary exacerbations decreased by 63% in the Trikafta group, compared with placebo. Pulmonary exacerbations were described as a change in specific symptoms that required treatment with a new oral, intravenous, or inhaled antibiotic. Serious adverse drug reactions that occurred more frequently in patients receiving Trikafta, compared with placebo, were rash and influenza events.
In the study that included patients with two copies of F508del, on average, the lung function increased 10% versus patients on ivacaftor/tezacaftor at 4 weeks. In addition, there was a 45.1 mmol/L on average decrease in the sweat chloride level in the Trikafta group, compared with ivacaftor/tezacaftor.
A hopeful start
Robert Giusti, MD, a pediatric pulmonologist at New York University Langone Health, is also hopeful. “This could be the kind of treatment that will make a revolution in terms of [cystic fibrosis] care if it can be started very early in life shortly after diagnosis. We anticipate that patients will be disease free for a longer period of time.”
The Cystic Fibrosis Foundation’s (CFF) “venture philanthropy” initiative played an important role in the development of the drug by Vertex Pharmaceuticals. The CFF has invested many millions of dollars in research by drug companies since the 1980s and was an early backer of Vertex. According to a statement on the CFF website, the Foundation sold its royalty rights for treatments developed by Vertex for $3.3 billion in 2014. The drug has a list price of about $311,000 a year. Payment issues may arise in the future, but for now, Vertex has stated that insurers and some Medicaid programs have begun paying claims for Trikafta
Specialists who treat CF now are watching to see how well patients tolerate this highly anticipated drug – and how well it meets expectations. The Therapeutic Development Network, the clinical research division of the CFF, is enrolling patients taking Trikafta in an observational study to follow for long-term follow-up.
Meeting expectations
“[Long-term efficacy is] something that we’re always concerned about. When the drug comes to market, is it going to be as effective as we thought it might be?” said Ryan Thomas, MD, director of the Cystic Fibrosis Center at Michigan State University, East Lansing. The MSU Cystic Fibrosis Center receives funding from the Cystic Fibrosis Foundation.
Almost one in five patients could not tolerate treatment with Orkambi, most often because of adverse breathing events, according to a French study published in the American Journal of Respiratory and Critical Care Medicine. The investigators wrote: “Among the 845 patients (292 adolescents, 553 adults) who initiated lumacaftor/ivacaftor, 18.2% (154 patients) discontinued treatment, often due to respiratory (48.1%, 74 patients) or nonrespiratory (27.9%, 43 patients) adverse events” and that the discontinuation rate was considerably higher than previously reported in clinical trials.
“We thought [Orkambi] was going to be something that could have a big effect,” Dr. Thomas said. “It turned out that it was harder for people to tolerate than we thought and the improvements weren’t as sustained as we thought they might be. I really don’t think this will end up being the case with Trikafta.”
Longer-term data are starting to emerge, which may ease some of the concerns inherent in working with a newer medicine. “These [data] suggest that this is going to be a game changer,” Dr. Thomas said. “If Trikafta is this efficacious, well, we’re talking about having people with CF who will live full lifespans without a lung transplant, and that is so rare.”
The decrease in hospitalizations, improved CT scans, and lower rates of lung function decline suggest it could be “the Holy Grail,” Dr. Thomas said.
A different disease
Trikafta is the latest in a series of improvements of CF treatment in recent decades, recalled Dr. Giusti, who has been in this field for about 3 decades. “It used to be that I attended many funerals for children with CF. Now with patients living longer and healthier lives I am invited to attend their weddings and even their children’s baptisms and bris ceremonies. It is a very different disease than it used to be.”
The promise of Trikafta leaves behind the minority of patients for whom the drug won’t work. This is for the 10% of patients that have rare mutations. That can lead to difficult conversations with parents about why this new option is not a choice for their child, Dr. Millard said. “It just crushes you, but the Cystic Fibrosis Foundation is committing a lot of new research in that direction. Their mantra is ‘until it is done.’ ”
Realistic expectations
William (Randy) Hunt, MD, FAAP, FACP, assistant professor of medicine in the Division of Pulmonary, Allergy, Critical Care and Sleep, Emory University School of Medicine, Atlanta, agrees that Trikafta is an exciting development in CF treatment. He noted, “Starting this medication early in life may very well significantly attenuate the disease, but it is not a cure. For individuals who already have significant disease, we may not see the same level of improvements in lung function as what we saw in the studies. The studies generally excluded individuals with ppFEV1 < 40%. Nevertheless, I remain optimistic and have been prescribing it to nearly everyone that qualifies after a discussion.”
Dr. Hunt added, “Patients are asking if they can stop their current chronic CF therapies once they start Trikafta. The answer is “no, at least not right now.” While all the relatively short-term data around Trikafta are very promising, we do not yet know how sustained the long-term benefits will be. Still, safely removing therapeutic burden from our patient population is a real interest. There are plans underway by the CFF and other institutions to systematically research whether discontinuing chronic CF therapies is safe in the setting of Trikafta.”
He concluded that 10% of individuals with CF mutations still do not respond to the modulators currently available. “We will not leave that population behind, but treating these remaining mutations is going to take continued efforts and likely modulators that are therapeutically differently from the mechanism of actions of those that are currently available,” he said.
Therese Borden contributed to this article.
1/2/2020 - This story was updated.
A newly approved triple-combination modulator to treat cystic fibrosis (CF) has raised expectations of a treatment turning point among patients and specialists. If the early results are sustained, elexacaftor/ivacaftor/tezacaftor (Trikafta) could prove to be the rare case of a much-touted new medicine that meets high expectations.
“CF even in infants causes inflammation, so we know that lung damage can start early and progress,” said Susan Millard, MD, FCCP, of Helen DeVos Children’s Hospital in Grand Rapids, Mich., and the local clinical research director for the pediatric pulmonary and sleep medicine section. “This oral drug therapy is actually treating the underlying problem, as opposed to many of the therapies we have that take hours to nebulize and only work locally in the airways.”
Dr. Millard is the recent past pediatric editor for Chest Physician and has been a local principal investigator at Helen DeVos Children’s Hospital for many Vertex-sponsored clinical studies.
The pivotal studies
The Food and Drug Administration approval of Trikafta rested on two pivotal phase 3, placebo-controlled studies, one in patients with two copies of the most common CF mutations, F508del, and the second in patients with one copy of F508del and a second mutation that was called a “minimal-function” mutation. The findings have ignited the hopes of many people with CF and their physicians. The drug was approved in October 2019 for patients aged 12 years and older who have at least one F508del mutation of the cystic fibrosis transmembrane conductance regulator gene. About 90% of patients in the United States have at least one copy of F508del. In the study looking at patients with one copy of F508del, the mean predicted forced expiratory volume in 1 second increased 13.8% in patients taking the drug versus placebo (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMoa1908639). The number of pulmonary exacerbations decreased by 63% in the Trikafta group, compared with placebo. Pulmonary exacerbations were described as a change in specific symptoms that required treatment with a new oral, intravenous, or inhaled antibiotic. Serious adverse drug reactions that occurred more frequently in patients receiving Trikafta, compared with placebo, were rash and influenza events.
In the study that included patients with two copies of F508del, on average, the lung function increased 10% versus patients on ivacaftor/tezacaftor at 4 weeks. In addition, there was a 45.1 mmol/L on average decrease in the sweat chloride level in the Trikafta group, compared with ivacaftor/tezacaftor.
A hopeful start
Robert Giusti, MD, a pediatric pulmonologist at New York University Langone Health, is also hopeful. “This could be the kind of treatment that will make a revolution in terms of [cystic fibrosis] care if it can be started very early in life shortly after diagnosis. We anticipate that patients will be disease free for a longer period of time.”
The Cystic Fibrosis Foundation’s (CFF) “venture philanthropy” initiative played an important role in the development of the drug by Vertex Pharmaceuticals. The CFF has invested many millions of dollars in research by drug companies since the 1980s and was an early backer of Vertex. According to a statement on the CFF website, the Foundation sold its royalty rights for treatments developed by Vertex for $3.3 billion in 2014. The drug has a list price of about $311,000 a year. Payment issues may arise in the future, but for now, Vertex has stated that insurers and some Medicaid programs have begun paying claims for Trikafta
Specialists who treat CF now are watching to see how well patients tolerate this highly anticipated drug – and how well it meets expectations. The Therapeutic Development Network, the clinical research division of the CFF, is enrolling patients taking Trikafta in an observational study to follow for long-term follow-up.
Meeting expectations
“[Long-term efficacy is] something that we’re always concerned about. When the drug comes to market, is it going to be as effective as we thought it might be?” said Ryan Thomas, MD, director of the Cystic Fibrosis Center at Michigan State University, East Lansing. The MSU Cystic Fibrosis Center receives funding from the Cystic Fibrosis Foundation.
Almost one in five patients could not tolerate treatment with Orkambi, most often because of adverse breathing events, according to a French study published in the American Journal of Respiratory and Critical Care Medicine. The investigators wrote: “Among the 845 patients (292 adolescents, 553 adults) who initiated lumacaftor/ivacaftor, 18.2% (154 patients) discontinued treatment, often due to respiratory (48.1%, 74 patients) or nonrespiratory (27.9%, 43 patients) adverse events” and that the discontinuation rate was considerably higher than previously reported in clinical trials.
“We thought [Orkambi] was going to be something that could have a big effect,” Dr. Thomas said. “It turned out that it was harder for people to tolerate than we thought and the improvements weren’t as sustained as we thought they might be. I really don’t think this will end up being the case with Trikafta.”
Longer-term data are starting to emerge, which may ease some of the concerns inherent in working with a newer medicine. “These [data] suggest that this is going to be a game changer,” Dr. Thomas said. “If Trikafta is this efficacious, well, we’re talking about having people with CF who will live full lifespans without a lung transplant, and that is so rare.”
The decrease in hospitalizations, improved CT scans, and lower rates of lung function decline suggest it could be “the Holy Grail,” Dr. Thomas said.
A different disease
Trikafta is the latest in a series of improvements of CF treatment in recent decades, recalled Dr. Giusti, who has been in this field for about 3 decades. “It used to be that I attended many funerals for children with CF. Now with patients living longer and healthier lives I am invited to attend their weddings and even their children’s baptisms and bris ceremonies. It is a very different disease than it used to be.”
The promise of Trikafta leaves behind the minority of patients for whom the drug won’t work. This is for the 10% of patients that have rare mutations. That can lead to difficult conversations with parents about why this new option is not a choice for their child, Dr. Millard said. “It just crushes you, but the Cystic Fibrosis Foundation is committing a lot of new research in that direction. Their mantra is ‘until it is done.’ ”
Realistic expectations
William (Randy) Hunt, MD, FAAP, FACP, assistant professor of medicine in the Division of Pulmonary, Allergy, Critical Care and Sleep, Emory University School of Medicine, Atlanta, agrees that Trikafta is an exciting development in CF treatment. He noted, “Starting this medication early in life may very well significantly attenuate the disease, but it is not a cure. For individuals who already have significant disease, we may not see the same level of improvements in lung function as what we saw in the studies. The studies generally excluded individuals with ppFEV1 < 40%. Nevertheless, I remain optimistic and have been prescribing it to nearly everyone that qualifies after a discussion.”
Dr. Hunt added, “Patients are asking if they can stop their current chronic CF therapies once they start Trikafta. The answer is “no, at least not right now.” While all the relatively short-term data around Trikafta are very promising, we do not yet know how sustained the long-term benefits will be. Still, safely removing therapeutic burden from our patient population is a real interest. There are plans underway by the CFF and other institutions to systematically research whether discontinuing chronic CF therapies is safe in the setting of Trikafta.”
He concluded that 10% of individuals with CF mutations still do not respond to the modulators currently available. “We will not leave that population behind, but treating these remaining mutations is going to take continued efforts and likely modulators that are therapeutically differently from the mechanism of actions of those that are currently available,” he said.
Therese Borden contributed to this article.
1/2/2020 - This story was updated.
A newly approved triple-combination modulator to treat cystic fibrosis (CF) has raised expectations of a treatment turning point among patients and specialists. If the early results are sustained, elexacaftor/ivacaftor/tezacaftor (Trikafta) could prove to be the rare case of a much-touted new medicine that meets high expectations.
“CF even in infants causes inflammation, so we know that lung damage can start early and progress,” said Susan Millard, MD, FCCP, of Helen DeVos Children’s Hospital in Grand Rapids, Mich., and the local clinical research director for the pediatric pulmonary and sleep medicine section. “This oral drug therapy is actually treating the underlying problem, as opposed to many of the therapies we have that take hours to nebulize and only work locally in the airways.”
Dr. Millard is the recent past pediatric editor for Chest Physician and has been a local principal investigator at Helen DeVos Children’s Hospital for many Vertex-sponsored clinical studies.
The pivotal studies
The Food and Drug Administration approval of Trikafta rested on two pivotal phase 3, placebo-controlled studies, one in patients with two copies of the most common CF mutations, F508del, and the second in patients with one copy of F508del and a second mutation that was called a “minimal-function” mutation. The findings have ignited the hopes of many people with CF and their physicians. The drug was approved in October 2019 for patients aged 12 years and older who have at least one F508del mutation of the cystic fibrosis transmembrane conductance regulator gene. About 90% of patients in the United States have at least one copy of F508del. In the study looking at patients with one copy of F508del, the mean predicted forced expiratory volume in 1 second increased 13.8% in patients taking the drug versus placebo (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMoa1908639). The number of pulmonary exacerbations decreased by 63% in the Trikafta group, compared with placebo. Pulmonary exacerbations were described as a change in specific symptoms that required treatment with a new oral, intravenous, or inhaled antibiotic. Serious adverse drug reactions that occurred more frequently in patients receiving Trikafta, compared with placebo, were rash and influenza events.
In the study that included patients with two copies of F508del, on average, the lung function increased 10% versus patients on ivacaftor/tezacaftor at 4 weeks. In addition, there was a 45.1 mmol/L on average decrease in the sweat chloride level in the Trikafta group, compared with ivacaftor/tezacaftor.
A hopeful start
Robert Giusti, MD, a pediatric pulmonologist at New York University Langone Health, is also hopeful. “This could be the kind of treatment that will make a revolution in terms of [cystic fibrosis] care if it can be started very early in life shortly after diagnosis. We anticipate that patients will be disease free for a longer period of time.”
The Cystic Fibrosis Foundation’s (CFF) “venture philanthropy” initiative played an important role in the development of the drug by Vertex Pharmaceuticals. The CFF has invested many millions of dollars in research by drug companies since the 1980s and was an early backer of Vertex. According to a statement on the CFF website, the Foundation sold its royalty rights for treatments developed by Vertex for $3.3 billion in 2014. The drug has a list price of about $311,000 a year. Payment issues may arise in the future, but for now, Vertex has stated that insurers and some Medicaid programs have begun paying claims for Trikafta
Specialists who treat CF now are watching to see how well patients tolerate this highly anticipated drug – and how well it meets expectations. The Therapeutic Development Network, the clinical research division of the CFF, is enrolling patients taking Trikafta in an observational study to follow for long-term follow-up.
Meeting expectations
“[Long-term efficacy is] something that we’re always concerned about. When the drug comes to market, is it going to be as effective as we thought it might be?” said Ryan Thomas, MD, director of the Cystic Fibrosis Center at Michigan State University, East Lansing. The MSU Cystic Fibrosis Center receives funding from the Cystic Fibrosis Foundation.
Almost one in five patients could not tolerate treatment with Orkambi, most often because of adverse breathing events, according to a French study published in the American Journal of Respiratory and Critical Care Medicine. The investigators wrote: “Among the 845 patients (292 adolescents, 553 adults) who initiated lumacaftor/ivacaftor, 18.2% (154 patients) discontinued treatment, often due to respiratory (48.1%, 74 patients) or nonrespiratory (27.9%, 43 patients) adverse events” and that the discontinuation rate was considerably higher than previously reported in clinical trials.
“We thought [Orkambi] was going to be something that could have a big effect,” Dr. Thomas said. “It turned out that it was harder for people to tolerate than we thought and the improvements weren’t as sustained as we thought they might be. I really don’t think this will end up being the case with Trikafta.”
Longer-term data are starting to emerge, which may ease some of the concerns inherent in working with a newer medicine. “These [data] suggest that this is going to be a game changer,” Dr. Thomas said. “If Trikafta is this efficacious, well, we’re talking about having people with CF who will live full lifespans without a lung transplant, and that is so rare.”
The decrease in hospitalizations, improved CT scans, and lower rates of lung function decline suggest it could be “the Holy Grail,” Dr. Thomas said.
A different disease
Trikafta is the latest in a series of improvements of CF treatment in recent decades, recalled Dr. Giusti, who has been in this field for about 3 decades. “It used to be that I attended many funerals for children with CF. Now with patients living longer and healthier lives I am invited to attend their weddings and even their children’s baptisms and bris ceremonies. It is a very different disease than it used to be.”
The promise of Trikafta leaves behind the minority of patients for whom the drug won’t work. This is for the 10% of patients that have rare mutations. That can lead to difficult conversations with parents about why this new option is not a choice for their child, Dr. Millard said. “It just crushes you, but the Cystic Fibrosis Foundation is committing a lot of new research in that direction. Their mantra is ‘until it is done.’ ”
Realistic expectations
William (Randy) Hunt, MD, FAAP, FACP, assistant professor of medicine in the Division of Pulmonary, Allergy, Critical Care and Sleep, Emory University School of Medicine, Atlanta, agrees that Trikafta is an exciting development in CF treatment. He noted, “Starting this medication early in life may very well significantly attenuate the disease, but it is not a cure. For individuals who already have significant disease, we may not see the same level of improvements in lung function as what we saw in the studies. The studies generally excluded individuals with ppFEV1 < 40%. Nevertheless, I remain optimistic and have been prescribing it to nearly everyone that qualifies after a discussion.”
Dr. Hunt added, “Patients are asking if they can stop their current chronic CF therapies once they start Trikafta. The answer is “no, at least not right now.” While all the relatively short-term data around Trikafta are very promising, we do not yet know how sustained the long-term benefits will be. Still, safely removing therapeutic burden from our patient population is a real interest. There are plans underway by the CFF and other institutions to systematically research whether discontinuing chronic CF therapies is safe in the setting of Trikafta.”
He concluded that 10% of individuals with CF mutations still do not respond to the modulators currently available. “We will not leave that population behind, but treating these remaining mutations is going to take continued efforts and likely modulators that are therapeutically differently from the mechanism of actions of those that are currently available,” he said.
Therese Borden contributed to this article.
1/2/2020 - This story was updated.
Dual HER2 therapy added to CRT for esophageal cancer is tolerable, active
Adding dual HER2-targeted therapy to neoadjuvant chemoradiotherapy is a well tolerated and efficacious treatment strategy in patients with resectable esophageal adenocarcinoma positive for this receptor, a multicenter phase 2 TRAP trial suggests.
Survival of esophageal cancer with neoadjuvant chemoradiotherapy alone remains poor, note senior investigator Hanneke W. M. van Laarhoven, MD, PhD, Amsterdam University Medical Center, University of Amsterdam, Cancer Center Amsterdam, and coinvestigators. But 15%-43% of tumors are positive for HER2, raising the possibility that targeted therapy could improve outcomes for some.
The investigators enrolled in the TRAP trial 40 patients with resectable HER2-positive adenocarcinoma of the esophagus or gastroesophageal junction. Among the patients, 78% were men, and the median age was 63 years. Planned treatment for all patients consisted of neoadjuvant chemoradiotherapy with carboplatin and paclitaxel in weeks 1 through 5, plus both trastuzumab (Herceptin) and pertuzumab (Perjeta) in weeks 2 through 13, then surgery in week 14.
Trial results reported in the Journal of Clinical Oncology showed that 83% of the patients completed treatment with trastuzumab and pertuzumab, meeting the predefined 80% threshold for feasibility of this treatment.
There were no unexpected safety events. Some 48% of patients had grade 3 or worse adverse events (mainly diarrhea and dysphagia), and 28% had serious adverse events (mainly vomiting, nausea, and gastrointestinal hemorrhage).
Of the 38 patients who underwent surgery, all achieved a complete (R0) resection and 34% achieved a pathologic complete response.
With a median follow-up of 32.1 months, the 3-year rates of progression-free survival and overall survival were 72% and 71%, respectively.
Compared with a cohort of similar patients from the Netherlands Cancer Registry who received conventional neoadjuvant chemoradiotherapy and were matched on propensity score (but not HER2 status), the trial patients had a significantly lower risk of death (hazard ratio, 0.58; 95% confidence interval, 0.34-0.97).
Neither pharmacokinetic measures nor tumor activity on [18F]fluorodeoxyglucose positron emission tomography predicted outcomes. But patients whose tumors had 3+ immunohistochemical HER2 expression had better overall survival (P = .007), and patients whose tumors were positive for growth factor receptor-bound protein 7 (Grb7) had a better treatment response (P = .016).
“This is the first study to our knowledge demonstrating the feasibility of the addition of both trastuzumab and pertuzumab to standard neoadjuvant chemoradiotherapy with carboplatin and paclitaxel in patients with resectable HER2-positive esophageal adenocarcinoma ... ” Dr. van Laarhoven and coinvestigators wrote.
“[T]he addition of trastuzumab and pertuzumab to neoadjuvant chemoradiotherapy is safe and tolerable for patients with HER2-positive esophageal adenocarcinoma, and preliminary efficacy results seem promising,” they concluded. “Because data on an HER2-positive control group are lacking, a randomized phase III study is warranted to demonstrate the superiority of the addition of trastuzumab and pertuzumab.”
Dr. van Laarhoven disclosed honoraria from Lilly/ImClone; a consulting or advisory role with Lilly/ImClone, Nordic Group, Bristol-Myers Squibb, and Servier; research funding to her institution from numerous pharmaceutical companies; and travel, accommodations, and/or expenses from AstraZeneca. The study was supported by the Academic Medical Center, Amsterdam, and by an unrestricted research grant from Hoffmann-LaRoche, Basel, Switzerland.
SOURCE: Stroes CI et al. J Clin Oncol. 2019 Dec 6. doi: 10.1200/JCO.19.01814.
Adding dual HER2-targeted therapy to neoadjuvant chemoradiotherapy is a well tolerated and efficacious treatment strategy in patients with resectable esophageal adenocarcinoma positive for this receptor, a multicenter phase 2 TRAP trial suggests.
Survival of esophageal cancer with neoadjuvant chemoradiotherapy alone remains poor, note senior investigator Hanneke W. M. van Laarhoven, MD, PhD, Amsterdam University Medical Center, University of Amsterdam, Cancer Center Amsterdam, and coinvestigators. But 15%-43% of tumors are positive for HER2, raising the possibility that targeted therapy could improve outcomes for some.
The investigators enrolled in the TRAP trial 40 patients with resectable HER2-positive adenocarcinoma of the esophagus or gastroesophageal junction. Among the patients, 78% were men, and the median age was 63 years. Planned treatment for all patients consisted of neoadjuvant chemoradiotherapy with carboplatin and paclitaxel in weeks 1 through 5, plus both trastuzumab (Herceptin) and pertuzumab (Perjeta) in weeks 2 through 13, then surgery in week 14.
Trial results reported in the Journal of Clinical Oncology showed that 83% of the patients completed treatment with trastuzumab and pertuzumab, meeting the predefined 80% threshold for feasibility of this treatment.
There were no unexpected safety events. Some 48% of patients had grade 3 or worse adverse events (mainly diarrhea and dysphagia), and 28% had serious adverse events (mainly vomiting, nausea, and gastrointestinal hemorrhage).
Of the 38 patients who underwent surgery, all achieved a complete (R0) resection and 34% achieved a pathologic complete response.
With a median follow-up of 32.1 months, the 3-year rates of progression-free survival and overall survival were 72% and 71%, respectively.
Compared with a cohort of similar patients from the Netherlands Cancer Registry who received conventional neoadjuvant chemoradiotherapy and were matched on propensity score (but not HER2 status), the trial patients had a significantly lower risk of death (hazard ratio, 0.58; 95% confidence interval, 0.34-0.97).
Neither pharmacokinetic measures nor tumor activity on [18F]fluorodeoxyglucose positron emission tomography predicted outcomes. But patients whose tumors had 3+ immunohistochemical HER2 expression had better overall survival (P = .007), and patients whose tumors were positive for growth factor receptor-bound protein 7 (Grb7) had a better treatment response (P = .016).
“This is the first study to our knowledge demonstrating the feasibility of the addition of both trastuzumab and pertuzumab to standard neoadjuvant chemoradiotherapy with carboplatin and paclitaxel in patients with resectable HER2-positive esophageal adenocarcinoma ... ” Dr. van Laarhoven and coinvestigators wrote.
“[T]he addition of trastuzumab and pertuzumab to neoadjuvant chemoradiotherapy is safe and tolerable for patients with HER2-positive esophageal adenocarcinoma, and preliminary efficacy results seem promising,” they concluded. “Because data on an HER2-positive control group are lacking, a randomized phase III study is warranted to demonstrate the superiority of the addition of trastuzumab and pertuzumab.”
Dr. van Laarhoven disclosed honoraria from Lilly/ImClone; a consulting or advisory role with Lilly/ImClone, Nordic Group, Bristol-Myers Squibb, and Servier; research funding to her institution from numerous pharmaceutical companies; and travel, accommodations, and/or expenses from AstraZeneca. The study was supported by the Academic Medical Center, Amsterdam, and by an unrestricted research grant from Hoffmann-LaRoche, Basel, Switzerland.
SOURCE: Stroes CI et al. J Clin Oncol. 2019 Dec 6. doi: 10.1200/JCO.19.01814.
Adding dual HER2-targeted therapy to neoadjuvant chemoradiotherapy is a well tolerated and efficacious treatment strategy in patients with resectable esophageal adenocarcinoma positive for this receptor, a multicenter phase 2 TRAP trial suggests.
Survival of esophageal cancer with neoadjuvant chemoradiotherapy alone remains poor, note senior investigator Hanneke W. M. van Laarhoven, MD, PhD, Amsterdam University Medical Center, University of Amsterdam, Cancer Center Amsterdam, and coinvestigators. But 15%-43% of tumors are positive for HER2, raising the possibility that targeted therapy could improve outcomes for some.
The investigators enrolled in the TRAP trial 40 patients with resectable HER2-positive adenocarcinoma of the esophagus or gastroesophageal junction. Among the patients, 78% were men, and the median age was 63 years. Planned treatment for all patients consisted of neoadjuvant chemoradiotherapy with carboplatin and paclitaxel in weeks 1 through 5, plus both trastuzumab (Herceptin) and pertuzumab (Perjeta) in weeks 2 through 13, then surgery in week 14.
Trial results reported in the Journal of Clinical Oncology showed that 83% of the patients completed treatment with trastuzumab and pertuzumab, meeting the predefined 80% threshold for feasibility of this treatment.
There were no unexpected safety events. Some 48% of patients had grade 3 or worse adverse events (mainly diarrhea and dysphagia), and 28% had serious adverse events (mainly vomiting, nausea, and gastrointestinal hemorrhage).
Of the 38 patients who underwent surgery, all achieved a complete (R0) resection and 34% achieved a pathologic complete response.
With a median follow-up of 32.1 months, the 3-year rates of progression-free survival and overall survival were 72% and 71%, respectively.
Compared with a cohort of similar patients from the Netherlands Cancer Registry who received conventional neoadjuvant chemoradiotherapy and were matched on propensity score (but not HER2 status), the trial patients had a significantly lower risk of death (hazard ratio, 0.58; 95% confidence interval, 0.34-0.97).
Neither pharmacokinetic measures nor tumor activity on [18F]fluorodeoxyglucose positron emission tomography predicted outcomes. But patients whose tumors had 3+ immunohistochemical HER2 expression had better overall survival (P = .007), and patients whose tumors were positive for growth factor receptor-bound protein 7 (Grb7) had a better treatment response (P = .016).
“This is the first study to our knowledge demonstrating the feasibility of the addition of both trastuzumab and pertuzumab to standard neoadjuvant chemoradiotherapy with carboplatin and paclitaxel in patients with resectable HER2-positive esophageal adenocarcinoma ... ” Dr. van Laarhoven and coinvestigators wrote.
“[T]he addition of trastuzumab and pertuzumab to neoadjuvant chemoradiotherapy is safe and tolerable for patients with HER2-positive esophageal adenocarcinoma, and preliminary efficacy results seem promising,” they concluded. “Because data on an HER2-positive control group are lacking, a randomized phase III study is warranted to demonstrate the superiority of the addition of trastuzumab and pertuzumab.”
Dr. van Laarhoven disclosed honoraria from Lilly/ImClone; a consulting or advisory role with Lilly/ImClone, Nordic Group, Bristol-Myers Squibb, and Servier; research funding to her institution from numerous pharmaceutical companies; and travel, accommodations, and/or expenses from AstraZeneca. The study was supported by the Academic Medical Center, Amsterdam, and by an unrestricted research grant from Hoffmann-LaRoche, Basel, Switzerland.
SOURCE: Stroes CI et al. J Clin Oncol. 2019 Dec 6. doi: 10.1200/JCO.19.01814.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Data support maintenance in metastatic colorectal cancer
Results from a review and meta-analysis support the use of maintenance after first-line therapy in patients with metastatic colorectal cancer.
The data showed that continuous induction did not confer a survival benefit over observation or maintenance therapy; however, maintenance improved progression-free survival (PFS), but not overall survival (OS), compared with observation. Furthermore, maintenance including a fluoropyrimidine, with or without bevacizumab, was most likely to improve survival.
These findings suggest maintenance may be appropriate for most patients with metastatic colorectal cancer, according to Mohamad Bassam Sonbol, MD, of Mayo Clinic in Phoenix and coauthors. Dr. Sonbol is the lead author of the review and meta-analysis, which was published in JAMA Oncology.
“For most patients, we are recommending a period of maintenance treatment,” Dr. Sonbol said in a JAMA Oncology podcast. “With the fluoropyrimidine, in most circumstances, we’re using capecitabine. Then, if cost is not an issue and toxicity is not an issue, we’re adding bevacizumab to this combination.”
Dr. Sonbol noted that certain patients, particularly those who have experienced a lot of toxicity, may do better with observation.
To reach these conclusions, Dr. Sonbol and colleagues analyzed data from 12 randomized phase 2 and 3 trials. Data from all 12 trials were included in the qualitative synthesis, but only 11 of the trials were included in the meta-analysis because of a lack of PFS and OS data from one trial.
In all, the data included 5,540 patients with previously untreated metastatic colorectal cancer. They ranged in age from 23 to 85 years, and most of them were male (64.4%).
For induction, patients received cytotoxic chemotherapy, with or without a biologic. For 10 trials, the induction regimen included oxaliplatin with either capecitabine or fluorouracil and leucovorin. Two trials included irinotecan-based induction.
In one trial, patients were managed with either continuous induction or observation. Continuous induction did not provide a PFS benefit (hazard ratio, 0.71; 95% confidence interval, 0.46-1.09) or an OS benefit (HR, 0.95; 95% CI, 0.85-1.07). In three trials, continuous induction was compared with maintenance. There was no PFS benefit (HR, 1.22; 95% CI, 0.84-1.78) or OS benefit (HR, 1.04; 95% CI, 0.92-1.17) with continuous induction over maintenance. There were six trials comparing maintenance to observation. Maintenance did provide a PFS benefit over observation (HR, 0.58; 95% CI, 0.43-0.77), but there was no OS benefit with maintenance (HR, 0.91; 95% CI, 0.83-1.01).
To compare maintenance regimens to one another, the researchers used surface under the cumulative ranking (SUCRA) probabilities. Higher SUCRA scores reflected greater efficacy.
Maintenance including a fluoropyrimidine, with or without bevacizumab, ranked higher than bevacizumab alone for both PFS and OS. The SUCRA values for PFS were 36.5% for bevacizumab, 67.1% for fluoropyrimidine, and 99.8% for fluoropyrimidine plus bevacizumab. The SUCRA values for OS were 32.6%, 81.3%, and 73.2%, respectively.
Dr. Sonbol and colleagues noted that this research is limited by the nature of meta-analyses (as compared with a prospective trial), a lack of blinding in some of the trials evaluated, and the use of study-level data rather than individual patient data.
Still, the researchers are recommending maintenance for most patients with metastatic colorectal cancer who achieve at least stable disease after induction. The team acknowledged that some patients may fare better with observation, and factors such as toxicity, cost, and patient preference should be considered when deciding on maintenance.
Dr. Sonbol did not disclose any conflicts of interest. Other authors disclosed relationships with multiple pharmaceutical companies.
SOURCE: Sonbol MB et al. JAMA Oncol. 2019 Dec 19. doi: 10.1001/jamaoncol.2019.4489.
Results from a review and meta-analysis support the use of maintenance after first-line therapy in patients with metastatic colorectal cancer.
The data showed that continuous induction did not confer a survival benefit over observation or maintenance therapy; however, maintenance improved progression-free survival (PFS), but not overall survival (OS), compared with observation. Furthermore, maintenance including a fluoropyrimidine, with or without bevacizumab, was most likely to improve survival.
These findings suggest maintenance may be appropriate for most patients with metastatic colorectal cancer, according to Mohamad Bassam Sonbol, MD, of Mayo Clinic in Phoenix and coauthors. Dr. Sonbol is the lead author of the review and meta-analysis, which was published in JAMA Oncology.
“For most patients, we are recommending a period of maintenance treatment,” Dr. Sonbol said in a JAMA Oncology podcast. “With the fluoropyrimidine, in most circumstances, we’re using capecitabine. Then, if cost is not an issue and toxicity is not an issue, we’re adding bevacizumab to this combination.”
Dr. Sonbol noted that certain patients, particularly those who have experienced a lot of toxicity, may do better with observation.
To reach these conclusions, Dr. Sonbol and colleagues analyzed data from 12 randomized phase 2 and 3 trials. Data from all 12 trials were included in the qualitative synthesis, but only 11 of the trials were included in the meta-analysis because of a lack of PFS and OS data from one trial.
In all, the data included 5,540 patients with previously untreated metastatic colorectal cancer. They ranged in age from 23 to 85 years, and most of them were male (64.4%).
For induction, patients received cytotoxic chemotherapy, with or without a biologic. For 10 trials, the induction regimen included oxaliplatin with either capecitabine or fluorouracil and leucovorin. Two trials included irinotecan-based induction.
In one trial, patients were managed with either continuous induction or observation. Continuous induction did not provide a PFS benefit (hazard ratio, 0.71; 95% confidence interval, 0.46-1.09) or an OS benefit (HR, 0.95; 95% CI, 0.85-1.07). In three trials, continuous induction was compared with maintenance. There was no PFS benefit (HR, 1.22; 95% CI, 0.84-1.78) or OS benefit (HR, 1.04; 95% CI, 0.92-1.17) with continuous induction over maintenance. There were six trials comparing maintenance to observation. Maintenance did provide a PFS benefit over observation (HR, 0.58; 95% CI, 0.43-0.77), but there was no OS benefit with maintenance (HR, 0.91; 95% CI, 0.83-1.01).
To compare maintenance regimens to one another, the researchers used surface under the cumulative ranking (SUCRA) probabilities. Higher SUCRA scores reflected greater efficacy.
Maintenance including a fluoropyrimidine, with or without bevacizumab, ranked higher than bevacizumab alone for both PFS and OS. The SUCRA values for PFS were 36.5% for bevacizumab, 67.1% for fluoropyrimidine, and 99.8% for fluoropyrimidine plus bevacizumab. The SUCRA values for OS were 32.6%, 81.3%, and 73.2%, respectively.
Dr. Sonbol and colleagues noted that this research is limited by the nature of meta-analyses (as compared with a prospective trial), a lack of blinding in some of the trials evaluated, and the use of study-level data rather than individual patient data.
Still, the researchers are recommending maintenance for most patients with metastatic colorectal cancer who achieve at least stable disease after induction. The team acknowledged that some patients may fare better with observation, and factors such as toxicity, cost, and patient preference should be considered when deciding on maintenance.
Dr. Sonbol did not disclose any conflicts of interest. Other authors disclosed relationships with multiple pharmaceutical companies.
SOURCE: Sonbol MB et al. JAMA Oncol. 2019 Dec 19. doi: 10.1001/jamaoncol.2019.4489.
Results from a review and meta-analysis support the use of maintenance after first-line therapy in patients with metastatic colorectal cancer.
The data showed that continuous induction did not confer a survival benefit over observation or maintenance therapy; however, maintenance improved progression-free survival (PFS), but not overall survival (OS), compared with observation. Furthermore, maintenance including a fluoropyrimidine, with or without bevacizumab, was most likely to improve survival.
These findings suggest maintenance may be appropriate for most patients with metastatic colorectal cancer, according to Mohamad Bassam Sonbol, MD, of Mayo Clinic in Phoenix and coauthors. Dr. Sonbol is the lead author of the review and meta-analysis, which was published in JAMA Oncology.
“For most patients, we are recommending a period of maintenance treatment,” Dr. Sonbol said in a JAMA Oncology podcast. “With the fluoropyrimidine, in most circumstances, we’re using capecitabine. Then, if cost is not an issue and toxicity is not an issue, we’re adding bevacizumab to this combination.”
Dr. Sonbol noted that certain patients, particularly those who have experienced a lot of toxicity, may do better with observation.
To reach these conclusions, Dr. Sonbol and colleagues analyzed data from 12 randomized phase 2 and 3 trials. Data from all 12 trials were included in the qualitative synthesis, but only 11 of the trials were included in the meta-analysis because of a lack of PFS and OS data from one trial.
In all, the data included 5,540 patients with previously untreated metastatic colorectal cancer. They ranged in age from 23 to 85 years, and most of them were male (64.4%).
For induction, patients received cytotoxic chemotherapy, with or without a biologic. For 10 trials, the induction regimen included oxaliplatin with either capecitabine or fluorouracil and leucovorin. Two trials included irinotecan-based induction.
In one trial, patients were managed with either continuous induction or observation. Continuous induction did not provide a PFS benefit (hazard ratio, 0.71; 95% confidence interval, 0.46-1.09) or an OS benefit (HR, 0.95; 95% CI, 0.85-1.07). In three trials, continuous induction was compared with maintenance. There was no PFS benefit (HR, 1.22; 95% CI, 0.84-1.78) or OS benefit (HR, 1.04; 95% CI, 0.92-1.17) with continuous induction over maintenance. There were six trials comparing maintenance to observation. Maintenance did provide a PFS benefit over observation (HR, 0.58; 95% CI, 0.43-0.77), but there was no OS benefit with maintenance (HR, 0.91; 95% CI, 0.83-1.01).
To compare maintenance regimens to one another, the researchers used surface under the cumulative ranking (SUCRA) probabilities. Higher SUCRA scores reflected greater efficacy.
Maintenance including a fluoropyrimidine, with or without bevacizumab, ranked higher than bevacizumab alone for both PFS and OS. The SUCRA values for PFS were 36.5% for bevacizumab, 67.1% for fluoropyrimidine, and 99.8% for fluoropyrimidine plus bevacizumab. The SUCRA values for OS were 32.6%, 81.3%, and 73.2%, respectively.
Dr. Sonbol and colleagues noted that this research is limited by the nature of meta-analyses (as compared with a prospective trial), a lack of blinding in some of the trials evaluated, and the use of study-level data rather than individual patient data.
Still, the researchers are recommending maintenance for most patients with metastatic colorectal cancer who achieve at least stable disease after induction. The team acknowledged that some patients may fare better with observation, and factors such as toxicity, cost, and patient preference should be considered when deciding on maintenance.
Dr. Sonbol did not disclose any conflicts of interest. Other authors disclosed relationships with multiple pharmaceutical companies.
SOURCE: Sonbol MB et al. JAMA Oncol. 2019 Dec 19. doi: 10.1001/jamaoncol.2019.4489.
FROM JAMA ONCOLOGY
The measles comeback of 2019
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.