User login
Trial supports less aggressive myeloma treatment
For patients with multiple myeloma that remains symptomatic within a year of starting therapy, neither a second autologous stem cell transplant nor more intensive consolidation therapy offered survival benefits superior to those seen with a single first autologous transplant and lenalidomide maintenance, reported investigators in a multicenter U.S. trial.
Among 758 patients with multiple myeloma (MM) who underwent standard induction therapy, followed by melphalan conditioning and autologous hematopoietic cell transplant (AHCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) between the three treatment arms, reported Edward A. Stadtmauer, MD, from the University of Pennsylvania, Philadelphia, and his colleagues.
Patients were randomized to either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide, bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant followed by lenalidomide maintenance.
“Single AHCT followed by len[alidomide] remains the standard of care. Greater than 80% of patients were alive at 38 months, which highlights excellent contemporary outcomes of patients with MM when treated with a standard approach of a multidrug induction followed by AHCT consolidation and maintenance,” they wrote in the Journal of Clinical Oncology.
The investigators hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and AHCT would improve survival, compared with a second AHCT.
To test this idea, they enrolled 758 patients from 54 U.S. centers and randomized them to one of three post-transplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and AHCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta-2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
The patients, who were a median age of 56 years old, had symptomatic multiple myeloma 12 months from the start of therapy without disease progression. They were randomly assigned to either AHCT followed by a second transplant and lenalidomide maintenance (247 patients), single transplant followed by RVD and lenalidomide maintenance (254), or single AHCT plus lenalidomide maintenance (257).
There were no significant differences between the groups in the primary endpoint of PFS at 38 months, with rates of 58.5% for the dual AHCT plus lenalidomide group, 57.8% for AHCT/RVD/lenalidomide, and 53.9% for AHCT/lenalidomide. Respective OS rates also did not differ significantly, at 81.8%, 85.4%, and 83.7%.
Complete response rates at 1 year were 50.5%, 58.4%, and 47.1%, respectively.
The three regimens also were similar in their toxicity profiles and in the risk of second malignancies.
The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
SOURCE: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
For patients with multiple myeloma that remains symptomatic within a year of starting therapy, neither a second autologous stem cell transplant nor more intensive consolidation therapy offered survival benefits superior to those seen with a single first autologous transplant and lenalidomide maintenance, reported investigators in a multicenter U.S. trial.
Among 758 patients with multiple myeloma (MM) who underwent standard induction therapy, followed by melphalan conditioning and autologous hematopoietic cell transplant (AHCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) between the three treatment arms, reported Edward A. Stadtmauer, MD, from the University of Pennsylvania, Philadelphia, and his colleagues.
Patients were randomized to either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide, bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant followed by lenalidomide maintenance.
“Single AHCT followed by len[alidomide] remains the standard of care. Greater than 80% of patients were alive at 38 months, which highlights excellent contemporary outcomes of patients with MM when treated with a standard approach of a multidrug induction followed by AHCT consolidation and maintenance,” they wrote in the Journal of Clinical Oncology.
The investigators hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and AHCT would improve survival, compared with a second AHCT.
To test this idea, they enrolled 758 patients from 54 U.S. centers and randomized them to one of three post-transplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and AHCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta-2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
The patients, who were a median age of 56 years old, had symptomatic multiple myeloma 12 months from the start of therapy without disease progression. They were randomly assigned to either AHCT followed by a second transplant and lenalidomide maintenance (247 patients), single transplant followed by RVD and lenalidomide maintenance (254), or single AHCT plus lenalidomide maintenance (257).
There were no significant differences between the groups in the primary endpoint of PFS at 38 months, with rates of 58.5% for the dual AHCT plus lenalidomide group, 57.8% for AHCT/RVD/lenalidomide, and 53.9% for AHCT/lenalidomide. Respective OS rates also did not differ significantly, at 81.8%, 85.4%, and 83.7%.
Complete response rates at 1 year were 50.5%, 58.4%, and 47.1%, respectively.
The three regimens also were similar in their toxicity profiles and in the risk of second malignancies.
The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
SOURCE: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
For patients with multiple myeloma that remains symptomatic within a year of starting therapy, neither a second autologous stem cell transplant nor more intensive consolidation therapy offered survival benefits superior to those seen with a single first autologous transplant and lenalidomide maintenance, reported investigators in a multicenter U.S. trial.
Among 758 patients with multiple myeloma (MM) who underwent standard induction therapy, followed by melphalan conditioning and autologous hematopoietic cell transplant (AHCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) between the three treatment arms, reported Edward A. Stadtmauer, MD, from the University of Pennsylvania, Philadelphia, and his colleagues.
Patients were randomized to either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide, bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant followed by lenalidomide maintenance.
“Single AHCT followed by len[alidomide] remains the standard of care. Greater than 80% of patients were alive at 38 months, which highlights excellent contemporary outcomes of patients with MM when treated with a standard approach of a multidrug induction followed by AHCT consolidation and maintenance,” they wrote in the Journal of Clinical Oncology.
The investigators hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and AHCT would improve survival, compared with a second AHCT.
To test this idea, they enrolled 758 patients from 54 U.S. centers and randomized them to one of three post-transplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and AHCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta-2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
The patients, who were a median age of 56 years old, had symptomatic multiple myeloma 12 months from the start of therapy without disease progression. They were randomly assigned to either AHCT followed by a second transplant and lenalidomide maintenance (247 patients), single transplant followed by RVD and lenalidomide maintenance (254), or single AHCT plus lenalidomide maintenance (257).
There were no significant differences between the groups in the primary endpoint of PFS at 38 months, with rates of 58.5% for the dual AHCT plus lenalidomide group, 57.8% for AHCT/RVD/lenalidomide, and 53.9% for AHCT/lenalidomide. Respective OS rates also did not differ significantly, at 81.8%, 85.4%, and 83.7%.
Complete response rates at 1 year were 50.5%, 58.4%, and 47.1%, respectively.
The three regimens also were similar in their toxicity profiles and in the risk of second malignancies.
The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
SOURCE: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: There were no differences in progression-free survival or overall survival among the three trial arms.
Study details: Randomized clinical trial with 758 patients with multiple myeloma.
Disclosures: The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
Source: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
Consider hysterectomy in patients with post-irradiated residual cervical cancer
according to a study of Bangladeshi patients with cervical cancer.
“Surgical intervention by either extrafascial or radical hysterectomy should be strongly considered for women with biopsy-confirmed residual disease after chemoradiation,” wrote lead authors Shahana Pervin, MBBS, and Farzana Islam Ruma, MBBS, of the National Institute of Cancer Research and Hospital and of the Railway General Hospital, respectively, in Dhaka, Bangladesh, and their associates. The study was published in the Journal of Global Oncology.
From 2009 to June 2013, this prospective longitudinal study collected data from 40 patients with biopsy-confirmed persistence of cervical cancer. The patients, who were being treated at one of two hospitals in Dhaka, Bangladesh, underwent either radical or extrafascial hysterectomy at least 12 weeks after initial radiation therapy.
At 5 years of follow-up, 36 (90%) had no evidence of disease. Of the 29 women who underwent extrafascial hysterectomy, 4 (14%) developed recurrent disease and 1 died. None of the 11 women who underwent radical hysterectomy had recurrences during the study period; that group, however, did suffer from “intraoperative, postoperative, and long-term complications.”
The investigators acknowledged the study’s several limitations, including a lack of standardized preoperative therapy, incomplete records of radiation dosing, and a limited number of patients. Along the same lines, another larger prospective trial to evaluate the two types of hysterectomy would “help guide what should be the standard of care for salvage therapy,” they wrote.
However, their findings emphasized the need for physicians in limited resource areas to have “a strong index of suspicion” when evaluating patients with locally advanced cervical cancer for residual disease. “Close clinical follow-up is crucial to identify these women in a timely manner,” the investigators added.
The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
SOURCE: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
according to a study of Bangladeshi patients with cervical cancer.
“Surgical intervention by either extrafascial or radical hysterectomy should be strongly considered for women with biopsy-confirmed residual disease after chemoradiation,” wrote lead authors Shahana Pervin, MBBS, and Farzana Islam Ruma, MBBS, of the National Institute of Cancer Research and Hospital and of the Railway General Hospital, respectively, in Dhaka, Bangladesh, and their associates. The study was published in the Journal of Global Oncology.
From 2009 to June 2013, this prospective longitudinal study collected data from 40 patients with biopsy-confirmed persistence of cervical cancer. The patients, who were being treated at one of two hospitals in Dhaka, Bangladesh, underwent either radical or extrafascial hysterectomy at least 12 weeks after initial radiation therapy.
At 5 years of follow-up, 36 (90%) had no evidence of disease. Of the 29 women who underwent extrafascial hysterectomy, 4 (14%) developed recurrent disease and 1 died. None of the 11 women who underwent radical hysterectomy had recurrences during the study period; that group, however, did suffer from “intraoperative, postoperative, and long-term complications.”
The investigators acknowledged the study’s several limitations, including a lack of standardized preoperative therapy, incomplete records of radiation dosing, and a limited number of patients. Along the same lines, another larger prospective trial to evaluate the two types of hysterectomy would “help guide what should be the standard of care for salvage therapy,” they wrote.
However, their findings emphasized the need for physicians in limited resource areas to have “a strong index of suspicion” when evaluating patients with locally advanced cervical cancer for residual disease. “Close clinical follow-up is crucial to identify these women in a timely manner,” the investigators added.
The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
SOURCE: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
according to a study of Bangladeshi patients with cervical cancer.
“Surgical intervention by either extrafascial or radical hysterectomy should be strongly considered for women with biopsy-confirmed residual disease after chemoradiation,” wrote lead authors Shahana Pervin, MBBS, and Farzana Islam Ruma, MBBS, of the National Institute of Cancer Research and Hospital and of the Railway General Hospital, respectively, in Dhaka, Bangladesh, and their associates. The study was published in the Journal of Global Oncology.
From 2009 to June 2013, this prospective longitudinal study collected data from 40 patients with biopsy-confirmed persistence of cervical cancer. The patients, who were being treated at one of two hospitals in Dhaka, Bangladesh, underwent either radical or extrafascial hysterectomy at least 12 weeks after initial radiation therapy.
At 5 years of follow-up, 36 (90%) had no evidence of disease. Of the 29 women who underwent extrafascial hysterectomy, 4 (14%) developed recurrent disease and 1 died. None of the 11 women who underwent radical hysterectomy had recurrences during the study period; that group, however, did suffer from “intraoperative, postoperative, and long-term complications.”
The investigators acknowledged the study’s several limitations, including a lack of standardized preoperative therapy, incomplete records of radiation dosing, and a limited number of patients. Along the same lines, another larger prospective trial to evaluate the two types of hysterectomy would “help guide what should be the standard of care for salvage therapy,” they wrote.
However, their findings emphasized the need for physicians in limited resource areas to have “a strong index of suspicion” when evaluating patients with locally advanced cervical cancer for residual disease. “Close clinical follow-up is crucial to identify these women in a timely manner,” the investigators added.
The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
SOURCE: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
Key clinical point: Hysterectomy should be strongly considered for women with biopsy-confirmed residual cervical cancer after radiation.
Major finding: At 5 years of follow-up, 90% of the patients who underwent hysterectomy had no evidence of disease.
Study details: A prospective longitudinal study of 40 patients with locally advanced cervical cancer who underwent hysterectomy after radiation at one of two hospitals in Dhaka, Bangladesh.
Disclosures: The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
Source: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
ADT harms likely limited to men with CV comorbidities
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Risk of cardiovascular death should be weighed against proven ADT benefits.
Major finding: ADT-related cardiovascular events appear limited to men with comorbid cardiovascular disease.
Study details: Review of clinical data on the cardiovascular consequences of ADT.
Disclosures: Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
Adolescence does not rule out bullous pemphigoid
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The course of adolescent bullous pemphigoid appears favorable, with long remission after the disease is controlled.
Major finding: The investigators found nine cases in children aged 10‐13 years, and five cases in children aged 14‐17 years.
Study details: A search in Medline detected 14 adolescents with a diagnosis of bullous pemphigoid.
Disclosures: No funding and no relevant financial disclosures were reported for the work.
Source: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Checkpoint inhibitors ‘viable treatment option’ in HIV-infected individuals
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
FROM JAMA ONCOLOGY
Key clinical point: Immune checkpoint inhibitors are a viable treatment option for HIV-infected patients, according to data supporting their safety and efficacy in this patient population.
Major finding: The treatment was well tolerated, with an 8.6% rate of grade 3 or greater immune-related adverse events, and no impact on HIV-related parameters.
Study details: A systematic review of 73 patients with HIV infection who had received treatment with a checkpoint inhibitor.
Disclosures: The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. One study author reported disclosures related to CARIS Life Science and AstraZeneca.
Source: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Combo appears to overcome aggressive L-NN-MCL
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Two patients with aggressive L-NN-MCL and P53 abnormalities who were treated with rituximab/ibrutinib and autologous stem cell transplantation remain free of disease 18 months later.
Study details: Two case reports.
Disclosures: The authors reported having no financial disclosures.
Source: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
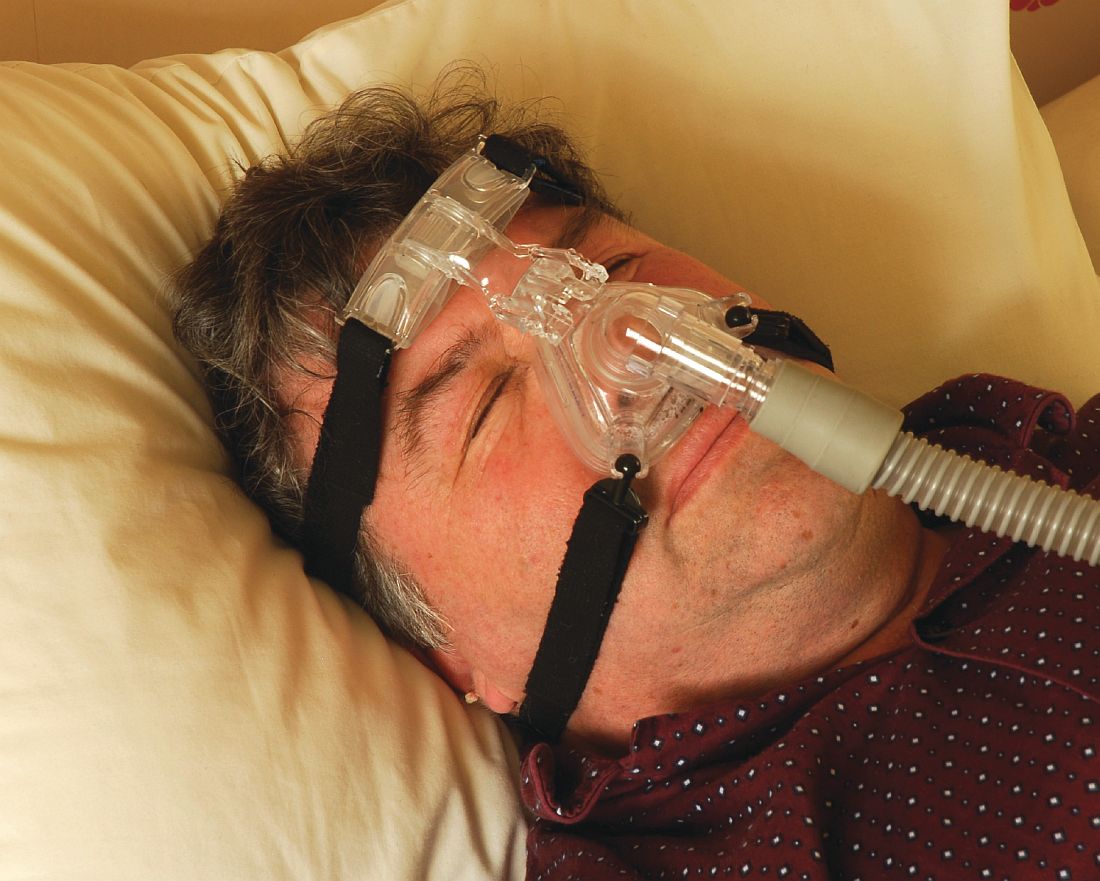
Socioeconomic status, race found to impact CPAP compliance
SAN DIEGO –
“CPAP is the gold standard treatment for OSA [obstructive sleep apnea] and is very effective, especially for those with severe disease,” researchers led by Philip S. LoSavio, MD, wrote in an abstract presented at the Triological Society’s Combined Sections Meeting. “However, CPAP is a significant challenge for patients for various reasons, with reports of only 46%-80% of OSA patients using CPAP for more than 4 consecutive hours on two out of three nights.”
In an effort to identify and define different factors associated with CPAP compliance, Dr. LoSavio and his colleagues collected data on 578 patients with OSA on CPAP who were treated at Rush University Medical Center, Chicago. The mean patient age was 58 years, 52% were female, 43% were African American, 40% were white, their mean body mass index was 36.91 kg/m2, and their mean apnea-hypopnea index was 37.25 events per hour. The researchers recorded CPAP use at office visits via CPAP module or card, and patients were considered CPAP compliant if their machines logged 4 consecutive hours of use for 70% or more of nights. During the office visits, patients completed a questionnaire asking if they were suffering from different otolaryngology-related diseases, including sinus headaches, gastroesophageal reflex, and enlarged tonsils. Dr. LoSavio, who heads the section of sleep surgery in the department of otorhinolaryngology at Rush University Medical Center, and his colleagues performed logistic regression to ascertain the effects of race and socioeconomic status on CPAP compliance while adjusting for OSA severity. They also analyzed the adjusted association of median income and self-reported symptoms of sinus headaches, GERD, and enlarged tonsils, on CPAP compliance.
They found that African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01). In addition, patients with mild OSA were less likely to be compliant compared with those who had severe disease (OR 0.57; P less than .03). Self-reported symptoms of sinus headaches, GERD, and enlarged tonsils were associated with significantly lower levels of compliance, while higher median income was positively associated with higher levels of compliance. When the researchers grouped incomes based on the 2018 federal tax classification brackets, they observed a significant association between compliance and median income (P less than .001), with a likelihood ratio of 20.4.
“Previous studies have shown that with increases in OSA disease severity, defined by higher [apnea-hypopnea index], comes increases in CPAP compliance, while other studies have alluded to the fact that lower socioeconomic status can affect CPAP compliance,” Dr. LoSavio and his associates wrote in their abstract. “A novel aspect of our study hoped to shed light on different otolaryngology-related diseases and how they might affect compliance. The patients with comorbid GERD, sinus headaches, and enlarged tonsils were less CPAP compliant in our study. These conditions are relatively easily treated and could therefore provide an avenue to increase CPAP compliance if addressed.” They acknowledged certain limitations of the study, including its single-center design and the self-reported nature of the patient questionnaire.
The researchers reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: LoSavio P et al. Triological CSM 2019, Abstracts.
SAN DIEGO –
“CPAP is the gold standard treatment for OSA [obstructive sleep apnea] and is very effective, especially for those with severe disease,” researchers led by Philip S. LoSavio, MD, wrote in an abstract presented at the Triological Society’s Combined Sections Meeting. “However, CPAP is a significant challenge for patients for various reasons, with reports of only 46%-80% of OSA patients using CPAP for more than 4 consecutive hours on two out of three nights.”
In an effort to identify and define different factors associated with CPAP compliance, Dr. LoSavio and his colleagues collected data on 578 patients with OSA on CPAP who were treated at Rush University Medical Center, Chicago. The mean patient age was 58 years, 52% were female, 43% were African American, 40% were white, their mean body mass index was 36.91 kg/m2, and their mean apnea-hypopnea index was 37.25 events per hour. The researchers recorded CPAP use at office visits via CPAP module or card, and patients were considered CPAP compliant if their machines logged 4 consecutive hours of use for 70% or more of nights. During the office visits, patients completed a questionnaire asking if they were suffering from different otolaryngology-related diseases, including sinus headaches, gastroesophageal reflex, and enlarged tonsils. Dr. LoSavio, who heads the section of sleep surgery in the department of otorhinolaryngology at Rush University Medical Center, and his colleagues performed logistic regression to ascertain the effects of race and socioeconomic status on CPAP compliance while adjusting for OSA severity. They also analyzed the adjusted association of median income and self-reported symptoms of sinus headaches, GERD, and enlarged tonsils, on CPAP compliance.
They found that African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01). In addition, patients with mild OSA were less likely to be compliant compared with those who had severe disease (OR 0.57; P less than .03). Self-reported symptoms of sinus headaches, GERD, and enlarged tonsils were associated with significantly lower levels of compliance, while higher median income was positively associated with higher levels of compliance. When the researchers grouped incomes based on the 2018 federal tax classification brackets, they observed a significant association between compliance and median income (P less than .001), with a likelihood ratio of 20.4.
“Previous studies have shown that with increases in OSA disease severity, defined by higher [apnea-hypopnea index], comes increases in CPAP compliance, while other studies have alluded to the fact that lower socioeconomic status can affect CPAP compliance,” Dr. LoSavio and his associates wrote in their abstract. “A novel aspect of our study hoped to shed light on different otolaryngology-related diseases and how they might affect compliance. The patients with comorbid GERD, sinus headaches, and enlarged tonsils were less CPAP compliant in our study. These conditions are relatively easily treated and could therefore provide an avenue to increase CPAP compliance if addressed.” They acknowledged certain limitations of the study, including its single-center design and the self-reported nature of the patient questionnaire.
The researchers reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: LoSavio P et al. Triological CSM 2019, Abstracts.
SAN DIEGO –
“CPAP is the gold standard treatment for OSA [obstructive sleep apnea] and is very effective, especially for those with severe disease,” researchers led by Philip S. LoSavio, MD, wrote in an abstract presented at the Triological Society’s Combined Sections Meeting. “However, CPAP is a significant challenge for patients for various reasons, with reports of only 46%-80% of OSA patients using CPAP for more than 4 consecutive hours on two out of three nights.”
In an effort to identify and define different factors associated with CPAP compliance, Dr. LoSavio and his colleagues collected data on 578 patients with OSA on CPAP who were treated at Rush University Medical Center, Chicago. The mean patient age was 58 years, 52% were female, 43% were African American, 40% were white, their mean body mass index was 36.91 kg/m2, and their mean apnea-hypopnea index was 37.25 events per hour. The researchers recorded CPAP use at office visits via CPAP module or card, and patients were considered CPAP compliant if their machines logged 4 consecutive hours of use for 70% or more of nights. During the office visits, patients completed a questionnaire asking if they were suffering from different otolaryngology-related diseases, including sinus headaches, gastroesophageal reflex, and enlarged tonsils. Dr. LoSavio, who heads the section of sleep surgery in the department of otorhinolaryngology at Rush University Medical Center, and his colleagues performed logistic regression to ascertain the effects of race and socioeconomic status on CPAP compliance while adjusting for OSA severity. They also analyzed the adjusted association of median income and self-reported symptoms of sinus headaches, GERD, and enlarged tonsils, on CPAP compliance.
They found that African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01). In addition, patients with mild OSA were less likely to be compliant compared with those who had severe disease (OR 0.57; P less than .03). Self-reported symptoms of sinus headaches, GERD, and enlarged tonsils were associated with significantly lower levels of compliance, while higher median income was positively associated with higher levels of compliance. When the researchers grouped incomes based on the 2018 federal tax classification brackets, they observed a significant association between compliance and median income (P less than .001), with a likelihood ratio of 20.4.
“Previous studies have shown that with increases in OSA disease severity, defined by higher [apnea-hypopnea index], comes increases in CPAP compliance, while other studies have alluded to the fact that lower socioeconomic status can affect CPAP compliance,” Dr. LoSavio and his associates wrote in their abstract. “A novel aspect of our study hoped to shed light on different otolaryngology-related diseases and how they might affect compliance. The patients with comorbid GERD, sinus headaches, and enlarged tonsils were less CPAP compliant in our study. These conditions are relatively easily treated and could therefore provide an avenue to increase CPAP compliance if addressed.” They acknowledged certain limitations of the study, including its single-center design and the self-reported nature of the patient questionnaire.
The researchers reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: LoSavio P et al. Triological CSM 2019, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: Compliance with continuous positive airway pressure is affected by patient socioeconomic status and race.
Major finding: African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01).
Study details: A retrospective study of 578 obstructive sleep apnea patients on CPAP.
Disclosures: The researchers reported having no financial disclosures.
Source: LoSavio P et al. Triological CSM 2019, Abstracts.
Trump administration salutes parade of generic drug approvals, but hundreds aren’t for sale
The Trump administration has been trumpeting a huge increase in Food and Drug Administration generic drug approvals during the past 2 years, the result of its actions to streamline a cumbersome process and combat anticompetitive practices. But nearly half of those newly approved drugs aren’t being sold in the United States, Kaiser Health News has found, meaning that many patients are deriving little practical benefit from the administration’s efforts.
The administration’s aggressive push to approve more generics is designed to spur more competition with expensive brand-name drugs, and drive prices lower, President Trump noted at a White House event in January 2019. The FDA has approved more than 1,600 generic drug applications since January 2017 – about a third more than it did during the last 2 years of the Obama administration.
But more than 700 generics, or about 43%, still weren’t on the market as of early January, a KHN data analysis of FDA and drug list price records shows. Even more noteworthy: 36% of generics that would be the first to compete against a branded drug are not yet for sale. That means thousands or even millions of patients have no option beyond buying branded drugs that can cost thousands of dollars per month.
“That’s shockingly high,” said former congressman Henry Waxman, who cosponsored the 1984 law that paved the way for the generic approval process as we know it today. He said he’d like to know more but suspects anticompetitive behavior is at least partly to blame and that revisions to the so-called Hatch-Waxman Act might be needed.
The approved generics that haven’t made it to American medicine cabinets include generic versions of expensive medicines like the blood thinner ticagrelor (Brilinta) and HIV medication emtricitabine/tenofovir disoproxil fumarate (Truvada). They also include six different generic versions of sodium nitroprusside (Nitropress), a heart failure drug, whose price spiked 310% in 2015.
Experts say a variety of factors are to blame. Generics sellers have fought for years against patent litigation and other delay tactics that protect brand-name drugs from competition. In recent years, vast industry consolidation has reduced the ranks of companies willing to purchase and distribute generics. And, in some cases, makers of generics obtain approvals and ultimately make a business decision to sit on them.
“It’s a real problem because we’re not getting all the expected competition,” FDA Commissioner Scott Gottlieb, MD, said in an interview, adding that it will be difficult to solve because it has so many causes. It takes five generics on the market to drive prices down to 33% of the original brand-name price, according to an FDA analysis.
Without generics to lower drug costs, branded manufacturers can continue to increase their prices, at a rate of roughly 10% a year, said Scott Knoer, PharmD, chief pharmacy officer at the Cleveland Clinic. “It makes health care costs go up across the board.”
Even if hospital patients don’t directly see high drug prices in their bills, the higher costs get passed to insurers, who pass them on as higher premiums, Dr. Knoer said. They also get passed to taxpayers, who pay for drugs covered by Medicare and Medicaid.
Consolidation on multiple tiers of the drug supply chain have changed the face of the generic drug market, warping supply and demand.
In some cases, key pharmaceutical ingredients are unavailable or a manufacturer doesn’t have the capacity to launch a product because it’s having difficulty meeting demand for existing products.
Manufacturing consolidation has dramatically reduced the production of injectable drugs, which are typically administered in a doctor’s office. This may be why 157 injectable generics that were approved in the past 2 years haven’t been brought to market.
Erin Fox, PharmD, a pharmacist at the University of Utah, Salt Lake City, who tracks drug shortages, said the KHN analysis of stalled generics “highlights that companies often have a lot of products ‘on the books’ but aren’t really making them.” A few generics on the list – like a 10% dextrose injection, to treat patients with low blood sugar – would have been helpful to combat shortages the past few years. “This comes up with shortages a lot – it looks like there are more suppliers than there really are,” Dr. Fox said.
A lot can change between the time a drugmaker files a generic application with the FDA and the time it’s approved.
Some drugmakers that applied for generic approval years ago switched their attention to more profitable products. Novartis, for instance, recently sold a generics division run by Sandoz so Sandoz could focus on other drugs, including biosimilars, which compete with expensive biologic drugs made from living organisms.
“Some of these [generic] drug applications have been sitting 6, 7, 8 years,” said Robert W. Pollock, a former acting deputy director of the FDA’s Office of Generic Drugs who now works for Lachman Consultants. By the time it’s approved, a generic can fall out of favor because patients taking the branded version reported new side effects or because a more-effective branded drug was approved.
For some generic manufacturers, there’s money to be made by waiting. Brand-name drugmakers will pay them to keep their products off the market as part of a tactic sometimes called “pay for delay.” The Federal Trade Commission estimates that such deals cost consumers and taxpayers $3.5 billion a year.
The number of these potentially anticompetitive settlements decreased from fiscal 2014 to fiscal 2015, according to the latest FTC report. Still, Dr. Gottlieb said he hopes to crack down on such tactics. The first generic to take on a branded drug is granted 180 days of exclusivity before the second and third generics can be approved, giving those products a clear advantage.
“We don’t like that companies are able to just park [a generic for] 180 days while they cut a deal not to come to market,” Dr. Gottlieb said, adding that with help from Congress he hopes to force companies to forfeit exclusivity if they don’t launch on time.
In some cases, according to Dr. Gottlieb, generic drugmakers wait until they’ve stockpiled a number of newly approved generics and have landed a contract with a purchaser before bringing their medicines to market.
These bundled contracts are secretive, so not much is known about them, but it means companies are filing generic applications just for the option of introducing generics, said health care economist Rena Conti of the Questrom School of Business at Boston University. They’ll wait until the most strategic time to launch, which could be after the competition shakes out, leaving them as “the last man standing,” Ms. Conti said. Then they can launch and hike the price.
To be sure, the FDA under Dr. Gottlieb’s leadership has taken steps to increase generic competition, from shaming brand-name drugmakers for blocking generics to publishing documents to help manufacturers win approval more easily. But approval doesn’t necessarily spur competition.
“We used to say it was all about getting in – once you got approval from the FDA, then you could go to market,” said Chip Davis, CEO of the Association for Accessible Medicines, the trade group for makers of generic drugs. The biggest challenges his members face is that there aren’t enough companies purchasing drugs, Mr. Davis said. Consolidation has led to three large buying groups covering 90% of the market, according to a Drug Channels Institute report. So, if you’re the fourth or fifth generic, you may have no one left to sell to.
Yet another barrier relates to how drug middlemen select the drugs they’ll cover under industry formularies, which determine what products insurance plans will cover. In some cases, middlemen known as “pharmacy benefit managers” have made it clear they don’t have room on their formularies for another generic. Or they do, but they give branded drugs preferential treatment with lower copays, hurting the generic’s market share.
Barriers to entry are lower under Gottlieb’s FDA than they’ve been in years past, Conti said, and regulations can help foster competition. But, she said, “they can only do so much.”
Methodology
To identify approved drugs that have not reached the market, Kaiser Health News used the FDA’s Orange Book database – as of Jan. 2 – to identify drug applications approved in 2017 or 2018. We then searched the FDA’s online National Drug Code directory for billing codes for the drugs associated with each application as of the same date. To account for a possible lag, we supplemented this list with a more complete billing code directory that we obtained via a Freedom of Information Act request. It includes codes with expected future launch dates that don’t appear in the online version.
According to experts, a billing code doesn’t necessarily mean a drug is on the market. However, every drug on the market needs a list price for reimbursement. We provided a list of application numbers and billing codes to information technology firm Connecture, which then told us whether each one was active, inactive, or had no list price as of Jan. 17.
If an application had at least one billing code with a list price attached, we counted it as on the market, even if other billing codes did not have list prices.
Sometimes, a single generic application can have multiple approval dates. If one of these approval dates occurred in the past 2 years, we included it in our analysis.
To determine whether a drug was a first generic, KHN used the FDA’s 2017 and 2018 lists of first generics as of Jan 2.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The Trump administration has been trumpeting a huge increase in Food and Drug Administration generic drug approvals during the past 2 years, the result of its actions to streamline a cumbersome process and combat anticompetitive practices. But nearly half of those newly approved drugs aren’t being sold in the United States, Kaiser Health News has found, meaning that many patients are deriving little practical benefit from the administration’s efforts.
The administration’s aggressive push to approve more generics is designed to spur more competition with expensive brand-name drugs, and drive prices lower, President Trump noted at a White House event in January 2019. The FDA has approved more than 1,600 generic drug applications since January 2017 – about a third more than it did during the last 2 years of the Obama administration.
But more than 700 generics, or about 43%, still weren’t on the market as of early January, a KHN data analysis of FDA and drug list price records shows. Even more noteworthy: 36% of generics that would be the first to compete against a branded drug are not yet for sale. That means thousands or even millions of patients have no option beyond buying branded drugs that can cost thousands of dollars per month.
“That’s shockingly high,” said former congressman Henry Waxman, who cosponsored the 1984 law that paved the way for the generic approval process as we know it today. He said he’d like to know more but suspects anticompetitive behavior is at least partly to blame and that revisions to the so-called Hatch-Waxman Act might be needed.
The approved generics that haven’t made it to American medicine cabinets include generic versions of expensive medicines like the blood thinner ticagrelor (Brilinta) and HIV medication emtricitabine/tenofovir disoproxil fumarate (Truvada). They also include six different generic versions of sodium nitroprusside (Nitropress), a heart failure drug, whose price spiked 310% in 2015.
Experts say a variety of factors are to blame. Generics sellers have fought for years against patent litigation and other delay tactics that protect brand-name drugs from competition. In recent years, vast industry consolidation has reduced the ranks of companies willing to purchase and distribute generics. And, in some cases, makers of generics obtain approvals and ultimately make a business decision to sit on them.
“It’s a real problem because we’re not getting all the expected competition,” FDA Commissioner Scott Gottlieb, MD, said in an interview, adding that it will be difficult to solve because it has so many causes. It takes five generics on the market to drive prices down to 33% of the original brand-name price, according to an FDA analysis.
Without generics to lower drug costs, branded manufacturers can continue to increase their prices, at a rate of roughly 10% a year, said Scott Knoer, PharmD, chief pharmacy officer at the Cleveland Clinic. “It makes health care costs go up across the board.”
Even if hospital patients don’t directly see high drug prices in their bills, the higher costs get passed to insurers, who pass them on as higher premiums, Dr. Knoer said. They also get passed to taxpayers, who pay for drugs covered by Medicare and Medicaid.
Consolidation on multiple tiers of the drug supply chain have changed the face of the generic drug market, warping supply and demand.
In some cases, key pharmaceutical ingredients are unavailable or a manufacturer doesn’t have the capacity to launch a product because it’s having difficulty meeting demand for existing products.
Manufacturing consolidation has dramatically reduced the production of injectable drugs, which are typically administered in a doctor’s office. This may be why 157 injectable generics that were approved in the past 2 years haven’t been brought to market.
Erin Fox, PharmD, a pharmacist at the University of Utah, Salt Lake City, who tracks drug shortages, said the KHN analysis of stalled generics “highlights that companies often have a lot of products ‘on the books’ but aren’t really making them.” A few generics on the list – like a 10% dextrose injection, to treat patients with low blood sugar – would have been helpful to combat shortages the past few years. “This comes up with shortages a lot – it looks like there are more suppliers than there really are,” Dr. Fox said.
A lot can change between the time a drugmaker files a generic application with the FDA and the time it’s approved.
Some drugmakers that applied for generic approval years ago switched their attention to more profitable products. Novartis, for instance, recently sold a generics division run by Sandoz so Sandoz could focus on other drugs, including biosimilars, which compete with expensive biologic drugs made from living organisms.
“Some of these [generic] drug applications have been sitting 6, 7, 8 years,” said Robert W. Pollock, a former acting deputy director of the FDA’s Office of Generic Drugs who now works for Lachman Consultants. By the time it’s approved, a generic can fall out of favor because patients taking the branded version reported new side effects or because a more-effective branded drug was approved.
For some generic manufacturers, there’s money to be made by waiting. Brand-name drugmakers will pay them to keep their products off the market as part of a tactic sometimes called “pay for delay.” The Federal Trade Commission estimates that such deals cost consumers and taxpayers $3.5 billion a year.
The number of these potentially anticompetitive settlements decreased from fiscal 2014 to fiscal 2015, according to the latest FTC report. Still, Dr. Gottlieb said he hopes to crack down on such tactics. The first generic to take on a branded drug is granted 180 days of exclusivity before the second and third generics can be approved, giving those products a clear advantage.
“We don’t like that companies are able to just park [a generic for] 180 days while they cut a deal not to come to market,” Dr. Gottlieb said, adding that with help from Congress he hopes to force companies to forfeit exclusivity if they don’t launch on time.
In some cases, according to Dr. Gottlieb, generic drugmakers wait until they’ve stockpiled a number of newly approved generics and have landed a contract with a purchaser before bringing their medicines to market.
These bundled contracts are secretive, so not much is known about them, but it means companies are filing generic applications just for the option of introducing generics, said health care economist Rena Conti of the Questrom School of Business at Boston University. They’ll wait until the most strategic time to launch, which could be after the competition shakes out, leaving them as “the last man standing,” Ms. Conti said. Then they can launch and hike the price.
To be sure, the FDA under Dr. Gottlieb’s leadership has taken steps to increase generic competition, from shaming brand-name drugmakers for blocking generics to publishing documents to help manufacturers win approval more easily. But approval doesn’t necessarily spur competition.
“We used to say it was all about getting in – once you got approval from the FDA, then you could go to market,” said Chip Davis, CEO of the Association for Accessible Medicines, the trade group for makers of generic drugs. The biggest challenges his members face is that there aren’t enough companies purchasing drugs, Mr. Davis said. Consolidation has led to three large buying groups covering 90% of the market, according to a Drug Channels Institute report. So, if you’re the fourth or fifth generic, you may have no one left to sell to.
Yet another barrier relates to how drug middlemen select the drugs they’ll cover under industry formularies, which determine what products insurance plans will cover. In some cases, middlemen known as “pharmacy benefit managers” have made it clear they don’t have room on their formularies for another generic. Or they do, but they give branded drugs preferential treatment with lower copays, hurting the generic’s market share.
Barriers to entry are lower under Gottlieb’s FDA than they’ve been in years past, Conti said, and regulations can help foster competition. But, she said, “they can only do so much.”
Methodology
To identify approved drugs that have not reached the market, Kaiser Health News used the FDA’s Orange Book database – as of Jan. 2 – to identify drug applications approved in 2017 or 2018. We then searched the FDA’s online National Drug Code directory for billing codes for the drugs associated with each application as of the same date. To account for a possible lag, we supplemented this list with a more complete billing code directory that we obtained via a Freedom of Information Act request. It includes codes with expected future launch dates that don’t appear in the online version.
According to experts, a billing code doesn’t necessarily mean a drug is on the market. However, every drug on the market needs a list price for reimbursement. We provided a list of application numbers and billing codes to information technology firm Connecture, which then told us whether each one was active, inactive, or had no list price as of Jan. 17.
If an application had at least one billing code with a list price attached, we counted it as on the market, even if other billing codes did not have list prices.
Sometimes, a single generic application can have multiple approval dates. If one of these approval dates occurred in the past 2 years, we included it in our analysis.
To determine whether a drug was a first generic, KHN used the FDA’s 2017 and 2018 lists of first generics as of Jan 2.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The Trump administration has been trumpeting a huge increase in Food and Drug Administration generic drug approvals during the past 2 years, the result of its actions to streamline a cumbersome process and combat anticompetitive practices. But nearly half of those newly approved drugs aren’t being sold in the United States, Kaiser Health News has found, meaning that many patients are deriving little practical benefit from the administration’s efforts.
The administration’s aggressive push to approve more generics is designed to spur more competition with expensive brand-name drugs, and drive prices lower, President Trump noted at a White House event in January 2019. The FDA has approved more than 1,600 generic drug applications since January 2017 – about a third more than it did during the last 2 years of the Obama administration.
But more than 700 generics, or about 43%, still weren’t on the market as of early January, a KHN data analysis of FDA and drug list price records shows. Even more noteworthy: 36% of generics that would be the first to compete against a branded drug are not yet for sale. That means thousands or even millions of patients have no option beyond buying branded drugs that can cost thousands of dollars per month.
“That’s shockingly high,” said former congressman Henry Waxman, who cosponsored the 1984 law that paved the way for the generic approval process as we know it today. He said he’d like to know more but suspects anticompetitive behavior is at least partly to blame and that revisions to the so-called Hatch-Waxman Act might be needed.
The approved generics that haven’t made it to American medicine cabinets include generic versions of expensive medicines like the blood thinner ticagrelor (Brilinta) and HIV medication emtricitabine/tenofovir disoproxil fumarate (Truvada). They also include six different generic versions of sodium nitroprusside (Nitropress), a heart failure drug, whose price spiked 310% in 2015.
Experts say a variety of factors are to blame. Generics sellers have fought for years against patent litigation and other delay tactics that protect brand-name drugs from competition. In recent years, vast industry consolidation has reduced the ranks of companies willing to purchase and distribute generics. And, in some cases, makers of generics obtain approvals and ultimately make a business decision to sit on them.
“It’s a real problem because we’re not getting all the expected competition,” FDA Commissioner Scott Gottlieb, MD, said in an interview, adding that it will be difficult to solve because it has so many causes. It takes five generics on the market to drive prices down to 33% of the original brand-name price, according to an FDA analysis.
Without generics to lower drug costs, branded manufacturers can continue to increase their prices, at a rate of roughly 10% a year, said Scott Knoer, PharmD, chief pharmacy officer at the Cleveland Clinic. “It makes health care costs go up across the board.”
Even if hospital patients don’t directly see high drug prices in their bills, the higher costs get passed to insurers, who pass them on as higher premiums, Dr. Knoer said. They also get passed to taxpayers, who pay for drugs covered by Medicare and Medicaid.
Consolidation on multiple tiers of the drug supply chain have changed the face of the generic drug market, warping supply and demand.
In some cases, key pharmaceutical ingredients are unavailable or a manufacturer doesn’t have the capacity to launch a product because it’s having difficulty meeting demand for existing products.
Manufacturing consolidation has dramatically reduced the production of injectable drugs, which are typically administered in a doctor’s office. This may be why 157 injectable generics that were approved in the past 2 years haven’t been brought to market.
Erin Fox, PharmD, a pharmacist at the University of Utah, Salt Lake City, who tracks drug shortages, said the KHN analysis of stalled generics “highlights that companies often have a lot of products ‘on the books’ but aren’t really making them.” A few generics on the list – like a 10% dextrose injection, to treat patients with low blood sugar – would have been helpful to combat shortages the past few years. “This comes up with shortages a lot – it looks like there are more suppliers than there really are,” Dr. Fox said.
A lot can change between the time a drugmaker files a generic application with the FDA and the time it’s approved.
Some drugmakers that applied for generic approval years ago switched their attention to more profitable products. Novartis, for instance, recently sold a generics division run by Sandoz so Sandoz could focus on other drugs, including biosimilars, which compete with expensive biologic drugs made from living organisms.
“Some of these [generic] drug applications have been sitting 6, 7, 8 years,” said Robert W. Pollock, a former acting deputy director of the FDA’s Office of Generic Drugs who now works for Lachman Consultants. By the time it’s approved, a generic can fall out of favor because patients taking the branded version reported new side effects or because a more-effective branded drug was approved.
For some generic manufacturers, there’s money to be made by waiting. Brand-name drugmakers will pay them to keep their products off the market as part of a tactic sometimes called “pay for delay.” The Federal Trade Commission estimates that such deals cost consumers and taxpayers $3.5 billion a year.
The number of these potentially anticompetitive settlements decreased from fiscal 2014 to fiscal 2015, according to the latest FTC report. Still, Dr. Gottlieb said he hopes to crack down on such tactics. The first generic to take on a branded drug is granted 180 days of exclusivity before the second and third generics can be approved, giving those products a clear advantage.
“We don’t like that companies are able to just park [a generic for] 180 days while they cut a deal not to come to market,” Dr. Gottlieb said, adding that with help from Congress he hopes to force companies to forfeit exclusivity if they don’t launch on time.
In some cases, according to Dr. Gottlieb, generic drugmakers wait until they’ve stockpiled a number of newly approved generics and have landed a contract with a purchaser before bringing their medicines to market.
These bundled contracts are secretive, so not much is known about them, but it means companies are filing generic applications just for the option of introducing generics, said health care economist Rena Conti of the Questrom School of Business at Boston University. They’ll wait until the most strategic time to launch, which could be after the competition shakes out, leaving them as “the last man standing,” Ms. Conti said. Then they can launch and hike the price.
To be sure, the FDA under Dr. Gottlieb’s leadership has taken steps to increase generic competition, from shaming brand-name drugmakers for blocking generics to publishing documents to help manufacturers win approval more easily. But approval doesn’t necessarily spur competition.
“We used to say it was all about getting in – once you got approval from the FDA, then you could go to market,” said Chip Davis, CEO of the Association for Accessible Medicines, the trade group for makers of generic drugs. The biggest challenges his members face is that there aren’t enough companies purchasing drugs, Mr. Davis said. Consolidation has led to three large buying groups covering 90% of the market, according to a Drug Channels Institute report. So, if you’re the fourth or fifth generic, you may have no one left to sell to.
Yet another barrier relates to how drug middlemen select the drugs they’ll cover under industry formularies, which determine what products insurance plans will cover. In some cases, middlemen known as “pharmacy benefit managers” have made it clear they don’t have room on their formularies for another generic. Or they do, but they give branded drugs preferential treatment with lower copays, hurting the generic’s market share.
Barriers to entry are lower under Gottlieb’s FDA than they’ve been in years past, Conti said, and regulations can help foster competition. But, she said, “they can only do so much.”
Methodology
To identify approved drugs that have not reached the market, Kaiser Health News used the FDA’s Orange Book database – as of Jan. 2 – to identify drug applications approved in 2017 or 2018. We then searched the FDA’s online National Drug Code directory for billing codes for the drugs associated with each application as of the same date. To account for a possible lag, we supplemented this list with a more complete billing code directory that we obtained via a Freedom of Information Act request. It includes codes with expected future launch dates that don’t appear in the online version.
According to experts, a billing code doesn’t necessarily mean a drug is on the market. However, every drug on the market needs a list price for reimbursement. We provided a list of application numbers and billing codes to information technology firm Connecture, which then told us whether each one was active, inactive, or had no list price as of Jan. 17.
If an application had at least one billing code with a list price attached, we counted it as on the market, even if other billing codes did not have list prices.
Sometimes, a single generic application can have multiple approval dates. If one of these approval dates occurred in the past 2 years, we included it in our analysis.
To determine whether a drug was a first generic, KHN used the FDA’s 2017 and 2018 lists of first generics as of Jan 2.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Increased risk of second cancers in mycosis fungoides
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: In a cohort of MF patients from the SEER database, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Study details: Retrospective study of 6,196 MF patients from the SEER database, and a single-center cohort of 172 MF patients who were matched to 172 patients with seborrheic dermatitis.
Disclosures: This research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures.
500 Women in Medicine: Part I
Ms. Gerull and Ms. Loe are third-year medical students at Washington University School of Medicine in St. Louis.
Their aim is to create a network of support and advancement for women in medicine. 500 Women in Medicine is a pod of the organization 500 Women Scientists.
In this episode, Nick Andrews speaks with the two innovators about their motivation to found this organization.
Correction, 3/12/19: An earlier version of this article misstated Kate Gerull's name.
Ms. Gerull and Ms. Loe are third-year medical students at Washington University School of Medicine in St. Louis.
Their aim is to create a network of support and advancement for women in medicine. 500 Women in Medicine is a pod of the organization 500 Women Scientists.
In this episode, Nick Andrews speaks with the two innovators about their motivation to found this organization.
Correction, 3/12/19: An earlier version of this article misstated Kate Gerull's name.
Ms. Gerull and Ms. Loe are third-year medical students at Washington University School of Medicine in St. Louis.
Their aim is to create a network of support and advancement for women in medicine. 500 Women in Medicine is a pod of the organization 500 Women Scientists.
In this episode, Nick Andrews speaks with the two innovators about their motivation to found this organization.
Correction, 3/12/19: An earlier version of this article misstated Kate Gerull's name.