User login
NSAIDs’ short-term safety, IVUS beats angio, and more
, NSAIDs show cardiovascular and renal safety in the short term, a registry analysis supports use of direct oral anticoagulants over warfarin, and IVUS-guided stent placement bests angiography-led placement.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon
Alexa
Apple Podcasts
Google Podcasts
, NSAIDs show cardiovascular and renal safety in the short term, a registry analysis supports use of direct oral anticoagulants over warfarin, and IVUS-guided stent placement bests angiography-led placement.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon
Alexa
Apple Podcasts
Google Podcasts
, NSAIDs show cardiovascular and renal safety in the short term, a registry analysis supports use of direct oral anticoagulants over warfarin, and IVUS-guided stent placement bests angiography-led placement.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon
Alexa
Apple Podcasts
Google Podcasts
Patient Preferences in Office-Based Orthopedic Care: A Prospective Evaluation
ABSTRACT
Patient satisfaction has become a topic of interest within orthopedics as the landscape of provider reimbursement continues to evolve to reward value of care. Online internet physician rating sites are becoming increasingly popular ways for patients to subjectively express their provider experience. Understanding what patients value during their episode of care is important in the modern healthcare environment. The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. A prospective survey was electronically administered to 212 patients in an adult reconstruction clinic. One hundred ninety-six patients (92.5%) completed the survey. Demographic questions regarding age, sex, ethnicity, and prior adult reconstruction surgical history were obtained. When patients were asked how much time they would like the doctor to spend with them on a routine visit, the most common answer was 10 to 15 minutes (41.3%), with only 10.2% patients desiring >20 minutes. The majority of patients (83.1%) believe ≥30 minutes is too long to wait to see their surgeon. Less than half of patients (41.8%) stated that they would feel as though they were receiving below average care if seen only by a nurse practitioner or physician’s assistant at a postoperative visit. Patients reported no significant age, gender, or ethnicity preferences for their physician. Recommendations from friends or other physicians was the most common (66.4%) way for patients to find their physicians, while 12.2% utilized online rating sites during their search. Optimizing patient experiences in the office may include keeping wait times to <30 minutes and educating patients on the roles of physician extenders. More work needs to be done to further elucidate variables influencing the subjective patient experience with their orthopedic care.
Continue to: Patient satisfaction...
Patient satisfaction has become an important focus in the rapidly changing healthcare environment due to the significant impact it has on healthcare delivery, healthcare economics, assessment of the quality of care, development of patient-care models, and quality improvement initiatives.1-4 Historically, the quality of care was measured by objective metrics such as complication rates, range-of-motion, and the provider’s expert opinion on the outcome. While those metrics are still impactful variables when defining a successful outcome, the medical community is now increasingly recognizing the importance of patients’ perspectives when defining successful treatments. Patient satisfaction is now highly regarded by clinicians and the government when considering outcomes and is even being incorporated into determining the value of care. Under the Affordable Care Act, patients assumed a more active role in clinical decision-making as well as in creating quality and efficiency initiatives.5,6 By 2017, 2% of the United States government’s Medicare payments will be redistributed among hospitals and physicians based on their quality and efficiency metrics, which are largely determined by patients’ evaluations of care.7 As a result, there has been significant interest in identifying variables influencing patient satisfaction and subjective outcomes.8,9
Patient satisfaction is related to both the outcomes of care and the process of care. As first described by Donabedian,10patients may be satisfied with the successful outcome of their care, but dissatisfied with how they received their care. The process of care is complex and considers many aspects of healthcare delivery, including time, cost, healthcare provider interactions, and burdens faced. While patient satisfaction with outcomes and process of care are heavily related, they should be regarded separately. It is essential that providers understand what variables are important to patients with regards to how they experience healthcare and choose their provider, especially surrounding elective procedures such as hip and knee arthroplasty.11,12
Within orthopedic surgery, patient satisfaction scores are beginning to be incorporated as part of the standard-of-care quality metrics obtained along with patient-reported outcome measures (PROMs) at defined time points postoperatively. Furthermore, PROMs and patient satisfaction data are becoming an increasingly important component of medical decision-making.13-16 Several authors have reported that increased patient satisfaction is correlated with increased compliance, improved treatment outcomes across numerous medical settings, including orthopedics, decreased risk of litigation, and higher patient ratings of the quality of care.17,18 Various factors, including meeting of expectations, staff politeness, the communication skills of the surgeon, and waiting times, have been suggested to influence eventual patient satisfaction within the surgical literature.19-21 However, within orthopedic surgery there is a paucity of investigations evaluating how patients determine preferences and satisfaction with the process of care.
The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. The authors hypothesize that the majority of patients find their physicians through online rating sites or recommendations from family and friends. The authors believe that patients expect to be seen in <30 minutes and will be unsatisfied overall with the amount of time that they spend with their physician.
Continue to: METHODS...
METHODS
The senior author (BRL) and a research team created a 15-question survey to evaluate patient preferences regarding the demographic characteristics (eg, age, gender, ethnicity) of their physician, wait times in a waiting room, time spent with the physician, care received from physician extenders (eg, nurse practitioners, physician assistants), and how they learned of their physician (Appendix). An a priori power analysis was conducted to determine that approximately 200 patients were needed for inclusion.11,22 Following Institutional Review Board approval (ORA 15051104), the survey was administered to 212 patients in a single-surgeon, adult reconstruction clinic. The survey was digitally administered on a touch-screen tablet using an electronic independent third party survey center (SurveyMonkey Inc) devoid of any identifying data. The survey was offered to all patients >21 years of age who were English-speaking and in the common area as patients waiting to be seen, from June 2015 to March 2016. A research assistant approached patients in the waiting room and asked if they would like to participate in a short survey regarding what factors influence the patient-physician relationship from the patient’s perspective.
Appendix 1
- Do you wish to partake in this 3-minute survey?
- Have you had a prior knee or hip replacement?
- What is your age?
- 30-40 years
- 40-50 years
- 50-60 years
- 60-70 years
- 70-80 years
- 80+ years
- What is your gender?
- Which of the following best represents your racial or ethnic heritage?
- African American
- How much time would you like the doctor to spend talking to you on a routine visit?
- 0-5 minutes
- 5-10 minutes
- 10-15 minutes
- 15-20 minutes
- 20-30 minutes
- >30 minutes
- How long is too long to wait to see the doctor?
- 10 minutes
- 20 minutes
- 30 minutes
- 40 minutes
- 50 minutes
- An hour or more
- If you were to only see a physician’s assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care?
- Overall I am satisfied with the time the doctor spends with me.
- If you were to need a major surgery, would you want the physician to tell you what he or she would do if they were in your shoes?
- Would you prefer your doctor to be the same race/ethnicity as you?
- No
- No Preference
- Would you feel more comfortable with a male as opposed to a female orthopedic surgeon?
- Would you feel more comfortable with a female as opposed to a male orthopedic surgeon?
- What age would you like your physician to be?
- 25-35 years old
- 35-45 years old
- 45-55 years old
- 55-65 years old
- 65 years and older
- No preference
- How do you usually find your physician?
- Friends’ recommendations
- Healthcare provider’s recommendations
- Insurance plans
- Online research/ratings
- Other
Descriptive statistics were used to analyze subject demographics and survey responses. Chi-square analyses and multinomial logistic regressions were utilized to compare responses. All statistical analyses were conducted using SPSS version 24.0 software (SPSS Inc). Statistical significance was set at P < 0.05.
RESULTS
Of the 212 patients who were invited to participate, 196 patients (92.4%) agreed and completed the survey. Demographic and surgical history information can be found in Table 1. The majority of patients were female (62%) and above the age of 50 years (92.4%). Almost half (48.5%) of patients had a prior hip or knee replacement.
Table 1. Survey Respondent Demographics
| Number | Percent |
Age Range | ||
30-40 years | 4 | 2.0% |
40-50 years | 11 | 5.6% |
50-60 years | 47 | 24.0% |
60-70 years | 84 | 42.9% |
70-80 years | 41 | 20.9% |
>80 years | 9 | 4.6% |
Gender | ||
Male | 74 | 37.8% |
Female | 122 | 62.2% |
Ethnicity | ||
African American | 39 | 19.9% |
Asian | 3 | 1.5% |
Caucasian | 140 | 71.4% |
Hispanic | 10 | 5.1% |
Other | 4 | 2.0% |
Prior knee or hip replacement | ||
Yes | 95 | 48.5% |
No | 55 | 28.1% |
No Response | 46 | 23.5% |
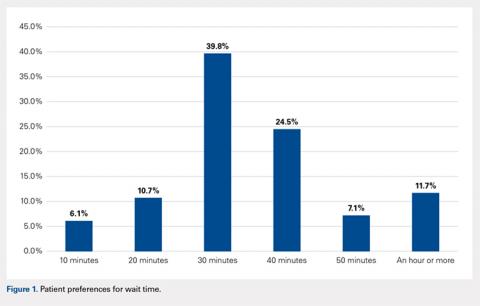
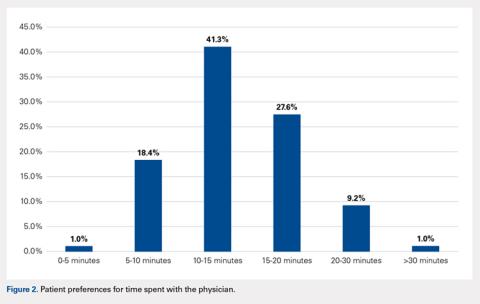
When asked how long is too long to wait to see the doctor, 30 minutes (39.8%) was most commonly selected, followed by 40 minutes (24.5%) (Figure 1). When asked how much time patients would like the doctor to spend with them during an office visit, the majority (68.9%) selected either 10 to 15 minutes (41.3%) or 15 to 20 minutes (27.6%) (Figure 2). The majority of patients (92.3%) were satisfied with the amount of time the doctor spent with them. In addition, 94.9% of respondents would want their doctor to tell them what they would do if they were in the patient’s shoes when making decisions regarding their medical care (Table 2). Less than half of respondents (41.8%) believe that seeing a physician extender (eg, nurse practitioner or physician assistant) at a postoperative visit would result in a lower quality of care (Table 2).
Table 2. Responses to Survey Questions
If you were to only see a physician's assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care? | ||
Answer choices | Number | Percent |
No | 114 | 58.2% |
Yes | 82 | 41.8% |
If you were to need a major surgery would you want the physician to tell you what he or she would do if they were in your shoes? | ||
Answer choices | Number | Percent |
No | 10 | 5.1% |
Yes | 186 | 94.9% |
Would you prefer your doctor to be the same race/ethnicity as you? | ||
Answer choices | Number | Percent |
No | 29 | 14.8% |
Yes | 3 | 1.5% |
No Preference | 164 | 83.7% |
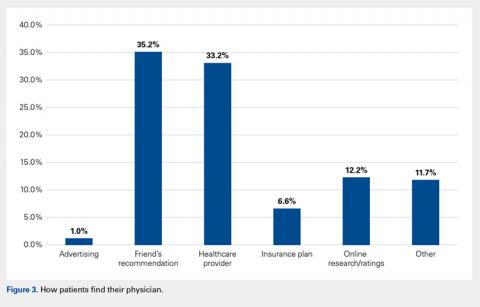
When asked if patients preferred a doctor of the same race/ethnicity, the vast majority (83.7%) had no preference (Table 2). There was no significant difference found between male and female respondents when asked if they would feel more comfortable with a male as opposed to a female orthopedic surgeon (P = .118) and vice versa (P = .604) (Table 3). Most patients preferred a physician between the ages of 45 and 55 years (39.3%), followed by 35 and 45 years (23.0%); however, this preference was not statistically significant (Table 4). Older patients were more likely to prefer younger physicians (odds ratio, 4.612 for 25-35 years of age; odds ratio, 1.328 for 35-45 years of age). Only 12.2% of patients reported online research/rating sites as the main resource utilized when selecting their physician (Figure 3). The majority (68.4%) reported that recommendations from either friends (35.2%) or healthcare providers (33.2%) were the main avenues through which they found their physicians.
Table 3. Overall Responses to Questions Regarding Male and Female Orthopedic Surgeonsa
Would you feel more comfortable with a male as opposed to a female orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 164 | 83.7% | 106 (86.9%) | 58 (78.4%) | 0.118 |
Yes | 32 | 16.3% | 16 (13.1%) | 16 (21.6%) |
|
Would you feel more comfortable with a female as opposed to a male orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 186 | 94.9% | 115 (94.3%) | 71 (95.9%) | 0.604 |
Yes | 10 | 5.1% | 7 (5.7%) | 3 (4.1%) |
|
aResponses were broken down by gender and compared utilizing a 2 x 2 chi-square analysis to test for significant differences in respondents’ gender preferences for their orthopedic surgeon.
Table 4. Patient Preferences Regarding Physician Age
What age would you like your physician to be? |
| 95% Confidence Interval | ||||
Answer Choices | Number or Responses | Percent | P value | Exp(β) | Lower Bound | Upper Bound |
25-35 years | 1 | 0.5% | 0.217 | 4.612 | 0.407 | 52.283 |
35-45 years | 45 | 23.0% | 0.161 | 1.328 | 0.893 | 1.975 |
45-55 years | 77 | 39.3% | 0.159 | 1.276 | 0.909 | 1.791 |
55-65 years | 9 | 4.6% | 0.483 | 1.302 | 0.624 | 2.717 |
≥65 years | 2 | 1.0% | 0.272 | 0.491 | 0.138 | 1.748 |
No preferencea | 62 | 31.6% | Reference | |||
aNo preference was used as the reference category for the answer choices, while the age bracket “>80 years” was used as the reference for the age of respondent variable.
Continue to: DISCUSSION...
DISCUSSION
The results of this study demonstrate that patients have several expectations and preferences with regards to the care they receive from physicians in the office. Patients prefer to wait <30 minutes before seeing their provider and desire only 10 to 20 minutes with their doctor. Patients do not have specific preferences with regards to the gender or ethnicity of their physician but would prefer a physician in the middle of their career, aged 45 to 55 years. Ultimately, patients do believe that seeing a physician at a postoperative visit is important, as just under half of patients thought that seeing a physician extender alone at a postoperative visit resulted in a lower quality of care.
While these results were obtained in a population specifically seeking the care of an orthopedic adult reconstruction surgeon, the results demonstrate that patients do not necessarily desire an unreasonable amount of time with their doctor. Patients simply want to be seen in a timely fashion and receive the full undivided attention of their doctor for approximately 20 minutes. Similarly, Patterson and colleagues22 found, in their series of 182 patients who presented to an orthopedic surgeon, that there was a significant correlation between time spent with the surgeon and overall patient satisfaction. Interestingly, the authors reported that patient satisfaction was not correlated with education level, sex, marital status, whether the patients were evaluated by a resident physician before seeing the attending surgeon, self-reported mental status, tobacco usage, the type of clinic visit, or the waiting time to see the surgeon (average, about 40 minutes for this cohort).22 Similarly, Teunis and colleagues23 reported an average 32-minute wait time in 81 patients presenting for care at an orthopedic hand clinic and demonstrated that a longer wait time was associated with decreased patient satisfaction. These results corroborate the findings of this study that a short wait time is important to patients when evaluating the process of care. Additionally, patients do not have unreasonable expectations with regards to the amount of time they would like to spend with the physician. A physician who has a clinic for 9 hours a day would thus be able to see 54 patients and still spend at least 10 minutes with each patient. The quality of the physician-patient interaction is likely more important than the actual amount of time spent; however, based on this study, patients do have certain expectations about how much time physicians should spend with them.
There were no significant sex, age, or ethnicity preferences in our specific patient cohort. However, a sizable percentage of respondents, 41.8%, believed that they were receiving inferior care if they only saw a physician extender at a routine follow-up visit. Many orthopedic surgeons rely on the care provided by physician extenders to enable them to see additional patients. Physician extenders are well trained to provide high-quality care, including at routine postoperative visits. The results of this study, that many patients believe physician extenders provide lower-quality care, may be a result of inadequate patient education regarding the extensive training and education physician extenders undergo. Physician extenders are qualified, licensed healthcare professionals who are playing increasingly important roles within orthopedics and medicine as a whole. As the demand for orthopedic surgeons to see more patients increases, so does the role of physician extenders. Future research is warranted into educating the public regarding the importance of these healthcare providers and the adequacy of their training.
While many practices now routinely obtain patient satisfaction scores, another modality through which patients can express their satisfaction and experiences with healthcare providers is through online internet physician rating sites (IPRS). These sites have exploded in number and popularity in recent years and, according to some studies, have a very real effect on provider selection.24 Interestingly, a low percentage of patients in this study utilized IPRS reviews to find their doctors. In a recent prospective survey study of 1000 consecutive patients presenting for care at the Mayo Clinic, Burkle and Keegan24 reported that 27% of patients would choose not to see a physician based on a negative IPRS review. Interestingly, only 1.0% of patients reported finding their doctor through advertising. Numerous authors have recently addressed advertising in orthopedic surgery, specifically direct-to-consumer marking, including the influence of physician self-promotion on patients.25,26 Specifically, Halawi and Barsoum26 discussed how direct-to-consumer marketing is commonly disseminated to the public through television and print advertisements, which are modalities more commonly utilized by older generations. However, many advertising agencies are moving toward internet-based advertising, especially through orthopedic group and individual surgeon websites for self-promoting advertisement, as approximately 75% of Americans use the internet for health-related information.25,27 The fact that many patients in this study did not utilize IPRS reviews or advertising (much of which is electronic) may be a result of the older, less internet-centric demographic that is often seen in an adult reconstruction clinic. Future research is warranted to determine what demographic of patients value IPRS reviews and how those reviews influence physician selection and the patient experience.
There are several limitations to this study. First, the majority of the surveyed population was Caucasian, and our results may not be equally reflective of diverse ethnic backgrounds. Second, the cohort size, while based on previous studies conducted in a similar fashion, may be underpowered to detect significant differences for 1 or more of these questions. In addition, having a question regarding the patient’s medical background or experiences may have provided further insight as to why patients selected the answers that they did. Furthermore, questions regarding the patient’s education level, religious background, and income brackets may have provided further context in which to evaluate their responses. These questions were omitted in an effort to keep the questionnaire at a length that would maximize enrollment and prevent survey fatigue. Future research is warranted to determine what patient-specific, injury/symptom-specific, and treatment-specific variables influence the subjective patient experience.
CONCLUSION
The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them. Patients reported no significant gender- or ethnicity-based preferences for their doctor. The majority of patients believe that a wait time exceeding 30 minutes is too long. A greater effort needs to be made to educate patients and the public about the significant and effective roles nurse practitioners and physician assistants can play within the healthcare system. While this cohort did not report notable utilization of IPRS reviews, it remains essential to understand what factors influence patients’ subjective experiences with their providers to ensure that patients achieve their desired outcomes, and report as such on these websites as they continue to gain popularity. Diminishing clinic wait times and understanding patient preferences may lead to a greater percentage of “satisfied” patients. While the majority of focus has been and will likely continue to be on improving patients’ satisfaction with their outcomes, more work needs to be done focusing specifically on the process through which outcomes are achieved.
1. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84–A(9):1560-1572.
2. Carr-Hill RA. The measurement of patient satisfaction. J Public Health Med. 1992;14(3):236-249.
3. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. Med Care. 1995;33(4):392-406. doi:10.1097/00005650-199504000-00006.
4. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219-248. doi:10.1177/107755879305000205.
5. Bozic KJ. Orthopaedic healthcare worldwide: shared medical decision making in orthopaedics. Clin Orthop Relat Res. 2013;471(5):1412-1414. doi:10.1007/s11999-013-2838-5.
6. Youm J, Chenok KE, Belkora J, Chiu V, Bozic KJ. The emerging case for shared decision making in orthopaedics. Instr Course Lect. 2013;62:587-594. doi:10.2106/00004623-201210170-00011.
7. Blumenthal D, Abrams M, Nuzum R. The affordable CARE Act at 5 years. N Engl J Med. 2015;373(16):1580. doi:10.1056/NEJMc1510015.
8. Shirley ED, Sanders JO. Patient satisfaction: implications and predictors of success. J Bone Joint Surg Am. 2013;95(10):e69. doi:10.2106/JBJS.L.01048.
9. Morris BJ, Jahangir AA, Sethi MK. Patient satisfaction: an emerging health policy issue. AAOS Now Web site. http://www.aaos.org/AAOSNow/2013/Jun/advocacy/advocacy5/?ssopc=1. Published June 2013. Accessed November 19, 2016.
10. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. doi:10.1001/jama.260.12.1743.
11. Bozic KJ, Kaufman D, Chan VC, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clin Orthop Relat Res. 2013;471(6):1865-1872. doi:10.1007/s11999-012-2640-9.
12. Davies AR, Ware JE Jr. Involving consumers in quality of care assessment. Health Aff (Millwood). 1988;7(1):33-48.
13. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25(5):1103-1112. doi:10.1007/s11136-015-1168-3.
14. Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429-439. doi:10.1016/j.ygyno.2014.11.071.
15. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754-775. doi:10.1111/1468-0009.12091.
16. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104-109. doi:10.2106/JBJS.N.00328.
17. Williams B. Patient satisfaction: a valid concept? Soc Sci Med. 1994;38(4):509-516. doi:10.1016/0277-9536(94)90247-X.
18. Hickson GB, Clayton EW, Entman SS, et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583-1587. doi:10.1001/jama.1994.03520200039032.
19. Larsson BW, Larsson G, Chantereau MW, von Holstein KS. International comparisons of patients' views on quality of care. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18(1):62-73. doi:10.1108/09526860510576974.
20. McLafferty RB, Williams RG, Lambert AD, Dunnington GL. Surgeon communication behaviors that lead patients to not recommend the surgeon to family members or friends: analysis and impact. Surgery. 2006;140(4):616-624. doi:https://doi.org/10.1016/j.surg.2006.06.021.
21. Mira JJ, Tomás O, Virtudes-Pérez M, Nebot C, Rodríguez-Marín J. Predictors of patient satisfaction in surgery. Surgery. 2009;145(5):536-541. doi:10.1016/j.surg.2009.01.012.
22. Patterson BM, Eskildsen SM, Clement RC, et al. Patient satisfaction is associated with time with provider but not clinic wait time among orthopedic patients. Orthopedics. 2017;40(1):43-48. doi:10.3928/01477447-20161013-05.
23. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated With patient satisfaction. Clin Orthop Relat Res. 2015;473(7):2362-2368. doi:10.1007/s11999-014-4090-z.
24. Burkle CM, Keegan MT. Popularity of internet physician rating sites and their apparent influence on patients' choices of physicians. BMC Health Serv Res. 2015;15:416. doi:10.1186/s12913-015-1099-2.
25. Mohney S, Lee DJ, Elfar JC. The effect of orthopedic advertising and self-promotion on a naive population. Am J Orthop. 2016;45(4):E227-E232.
26. Halawi MJ, Barsoum WK. Direct-to-consumer marketing: implications for patient care and orthopedic education. Am J Orthop. 2016;45(6):E335-E336.
27. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156. doi:10.1007/s11606-010-1425-7.
ABSTRACT
Patient satisfaction has become a topic of interest within orthopedics as the landscape of provider reimbursement continues to evolve to reward value of care. Online internet physician rating sites are becoming increasingly popular ways for patients to subjectively express their provider experience. Understanding what patients value during their episode of care is important in the modern healthcare environment. The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. A prospective survey was electronically administered to 212 patients in an adult reconstruction clinic. One hundred ninety-six patients (92.5%) completed the survey. Demographic questions regarding age, sex, ethnicity, and prior adult reconstruction surgical history were obtained. When patients were asked how much time they would like the doctor to spend with them on a routine visit, the most common answer was 10 to 15 minutes (41.3%), with only 10.2% patients desiring >20 minutes. The majority of patients (83.1%) believe ≥30 minutes is too long to wait to see their surgeon. Less than half of patients (41.8%) stated that they would feel as though they were receiving below average care if seen only by a nurse practitioner or physician’s assistant at a postoperative visit. Patients reported no significant age, gender, or ethnicity preferences for their physician. Recommendations from friends or other physicians was the most common (66.4%) way for patients to find their physicians, while 12.2% utilized online rating sites during their search. Optimizing patient experiences in the office may include keeping wait times to <30 minutes and educating patients on the roles of physician extenders. More work needs to be done to further elucidate variables influencing the subjective patient experience with their orthopedic care.
Continue to: Patient satisfaction...
Patient satisfaction has become an important focus in the rapidly changing healthcare environment due to the significant impact it has on healthcare delivery, healthcare economics, assessment of the quality of care, development of patient-care models, and quality improvement initiatives.1-4 Historically, the quality of care was measured by objective metrics such as complication rates, range-of-motion, and the provider’s expert opinion on the outcome. While those metrics are still impactful variables when defining a successful outcome, the medical community is now increasingly recognizing the importance of patients’ perspectives when defining successful treatments. Patient satisfaction is now highly regarded by clinicians and the government when considering outcomes and is even being incorporated into determining the value of care. Under the Affordable Care Act, patients assumed a more active role in clinical decision-making as well as in creating quality and efficiency initiatives.5,6 By 2017, 2% of the United States government’s Medicare payments will be redistributed among hospitals and physicians based on their quality and efficiency metrics, which are largely determined by patients’ evaluations of care.7 As a result, there has been significant interest in identifying variables influencing patient satisfaction and subjective outcomes.8,9
Patient satisfaction is related to both the outcomes of care and the process of care. As first described by Donabedian,10patients may be satisfied with the successful outcome of their care, but dissatisfied with how they received their care. The process of care is complex and considers many aspects of healthcare delivery, including time, cost, healthcare provider interactions, and burdens faced. While patient satisfaction with outcomes and process of care are heavily related, they should be regarded separately. It is essential that providers understand what variables are important to patients with regards to how they experience healthcare and choose their provider, especially surrounding elective procedures such as hip and knee arthroplasty.11,12
Within orthopedic surgery, patient satisfaction scores are beginning to be incorporated as part of the standard-of-care quality metrics obtained along with patient-reported outcome measures (PROMs) at defined time points postoperatively. Furthermore, PROMs and patient satisfaction data are becoming an increasingly important component of medical decision-making.13-16 Several authors have reported that increased patient satisfaction is correlated with increased compliance, improved treatment outcomes across numerous medical settings, including orthopedics, decreased risk of litigation, and higher patient ratings of the quality of care.17,18 Various factors, including meeting of expectations, staff politeness, the communication skills of the surgeon, and waiting times, have been suggested to influence eventual patient satisfaction within the surgical literature.19-21 However, within orthopedic surgery there is a paucity of investigations evaluating how patients determine preferences and satisfaction with the process of care.
The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. The authors hypothesize that the majority of patients find their physicians through online rating sites or recommendations from family and friends. The authors believe that patients expect to be seen in <30 minutes and will be unsatisfied overall with the amount of time that they spend with their physician.
Continue to: METHODS...
METHODS
The senior author (BRL) and a research team created a 15-question survey to evaluate patient preferences regarding the demographic characteristics (eg, age, gender, ethnicity) of their physician, wait times in a waiting room, time spent with the physician, care received from physician extenders (eg, nurse practitioners, physician assistants), and how they learned of their physician (Appendix). An a priori power analysis was conducted to determine that approximately 200 patients were needed for inclusion.11,22 Following Institutional Review Board approval (ORA 15051104), the survey was administered to 212 patients in a single-surgeon, adult reconstruction clinic. The survey was digitally administered on a touch-screen tablet using an electronic independent third party survey center (SurveyMonkey Inc) devoid of any identifying data. The survey was offered to all patients >21 years of age who were English-speaking and in the common area as patients waiting to be seen, from June 2015 to March 2016. A research assistant approached patients in the waiting room and asked if they would like to participate in a short survey regarding what factors influence the patient-physician relationship from the patient’s perspective.
Appendix 1
- Do you wish to partake in this 3-minute survey?
- Have you had a prior knee or hip replacement?
- What is your age?
- 30-40 years
- 40-50 years
- 50-60 years
- 60-70 years
- 70-80 years
- 80+ years
- What is your gender?
- Which of the following best represents your racial or ethnic heritage?
- African American
- How much time would you like the doctor to spend talking to you on a routine visit?
- 0-5 minutes
- 5-10 minutes
- 10-15 minutes
- 15-20 minutes
- 20-30 minutes
- >30 minutes
- How long is too long to wait to see the doctor?
- 10 minutes
- 20 minutes
- 30 minutes
- 40 minutes
- 50 minutes
- An hour or more
- If you were to only see a physician’s assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care?
- Overall I am satisfied with the time the doctor spends with me.
- If you were to need a major surgery, would you want the physician to tell you what he or she would do if they were in your shoes?
- Would you prefer your doctor to be the same race/ethnicity as you?
- No
- No Preference
- Would you feel more comfortable with a male as opposed to a female orthopedic surgeon?
- Would you feel more comfortable with a female as opposed to a male orthopedic surgeon?
- What age would you like your physician to be?
- 25-35 years old
- 35-45 years old
- 45-55 years old
- 55-65 years old
- 65 years and older
- No preference
- How do you usually find your physician?
- Friends’ recommendations
- Healthcare provider’s recommendations
- Insurance plans
- Online research/ratings
- Other
Descriptive statistics were used to analyze subject demographics and survey responses. Chi-square analyses and multinomial logistic regressions were utilized to compare responses. All statistical analyses were conducted using SPSS version 24.0 software (SPSS Inc). Statistical significance was set at P < 0.05.
RESULTS
Of the 212 patients who were invited to participate, 196 patients (92.4%) agreed and completed the survey. Demographic and surgical history information can be found in Table 1. The majority of patients were female (62%) and above the age of 50 years (92.4%). Almost half (48.5%) of patients had a prior hip or knee replacement.
Table 1. Survey Respondent Demographics
| Number | Percent |
Age Range | ||
30-40 years | 4 | 2.0% |
40-50 years | 11 | 5.6% |
50-60 years | 47 | 24.0% |
60-70 years | 84 | 42.9% |
70-80 years | 41 | 20.9% |
>80 years | 9 | 4.6% |
Gender | ||
Male | 74 | 37.8% |
Female | 122 | 62.2% |
Ethnicity | ||
African American | 39 | 19.9% |
Asian | 3 | 1.5% |
Caucasian | 140 | 71.4% |
Hispanic | 10 | 5.1% |
Other | 4 | 2.0% |
Prior knee or hip replacement | ||
Yes | 95 | 48.5% |
No | 55 | 28.1% |
No Response | 46 | 23.5% |
When asked how long is too long to wait to see the doctor, 30 minutes (39.8%) was most commonly selected, followed by 40 minutes (24.5%) (Figure 1). When asked how much time patients would like the doctor to spend with them during an office visit, the majority (68.9%) selected either 10 to 15 minutes (41.3%) or 15 to 20 minutes (27.6%) (Figure 2). The majority of patients (92.3%) were satisfied with the amount of time the doctor spent with them. In addition, 94.9% of respondents would want their doctor to tell them what they would do if they were in the patient’s shoes when making decisions regarding their medical care (Table 2). Less than half of respondents (41.8%) believe that seeing a physician extender (eg, nurse practitioner or physician assistant) at a postoperative visit would result in a lower quality of care (Table 2).
Table 2. Responses to Survey Questions
If you were to only see a physician's assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care? | ||
Answer choices | Number | Percent |
No | 114 | 58.2% |
Yes | 82 | 41.8% |
If you were to need a major surgery would you want the physician to tell you what he or she would do if they were in your shoes? | ||
Answer choices | Number | Percent |
No | 10 | 5.1% |
Yes | 186 | 94.9% |
Would you prefer your doctor to be the same race/ethnicity as you? | ||
Answer choices | Number | Percent |
No | 29 | 14.8% |
Yes | 3 | 1.5% |
No Preference | 164 | 83.7% |
When asked if patients preferred a doctor of the same race/ethnicity, the vast majority (83.7%) had no preference (Table 2). There was no significant difference found between male and female respondents when asked if they would feel more comfortable with a male as opposed to a female orthopedic surgeon (P = .118) and vice versa (P = .604) (Table 3). Most patients preferred a physician between the ages of 45 and 55 years (39.3%), followed by 35 and 45 years (23.0%); however, this preference was not statistically significant (Table 4). Older patients were more likely to prefer younger physicians (odds ratio, 4.612 for 25-35 years of age; odds ratio, 1.328 for 35-45 years of age). Only 12.2% of patients reported online research/rating sites as the main resource utilized when selecting their physician (Figure 3). The majority (68.4%) reported that recommendations from either friends (35.2%) or healthcare providers (33.2%) were the main avenues through which they found their physicians.
Table 3. Overall Responses to Questions Regarding Male and Female Orthopedic Surgeonsa
Would you feel more comfortable with a male as opposed to a female orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 164 | 83.7% | 106 (86.9%) | 58 (78.4%) | 0.118 |
Yes | 32 | 16.3% | 16 (13.1%) | 16 (21.6%) |
|
Would you feel more comfortable with a female as opposed to a male orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 186 | 94.9% | 115 (94.3%) | 71 (95.9%) | 0.604 |
Yes | 10 | 5.1% | 7 (5.7%) | 3 (4.1%) |
|
aResponses were broken down by gender and compared utilizing a 2 x 2 chi-square analysis to test for significant differences in respondents’ gender preferences for their orthopedic surgeon.
Table 4. Patient Preferences Regarding Physician Age
What age would you like your physician to be? |
| 95% Confidence Interval | ||||
Answer Choices | Number or Responses | Percent | P value | Exp(β) | Lower Bound | Upper Bound |
25-35 years | 1 | 0.5% | 0.217 | 4.612 | 0.407 | 52.283 |
35-45 years | 45 | 23.0% | 0.161 | 1.328 | 0.893 | 1.975 |
45-55 years | 77 | 39.3% | 0.159 | 1.276 | 0.909 | 1.791 |
55-65 years | 9 | 4.6% | 0.483 | 1.302 | 0.624 | 2.717 |
≥65 years | 2 | 1.0% | 0.272 | 0.491 | 0.138 | 1.748 |
No preferencea | 62 | 31.6% | Reference | |||
aNo preference was used as the reference category for the answer choices, while the age bracket “>80 years” was used as the reference for the age of respondent variable.
Continue to: DISCUSSION...
DISCUSSION
The results of this study demonstrate that patients have several expectations and preferences with regards to the care they receive from physicians in the office. Patients prefer to wait <30 minutes before seeing their provider and desire only 10 to 20 minutes with their doctor. Patients do not have specific preferences with regards to the gender or ethnicity of their physician but would prefer a physician in the middle of their career, aged 45 to 55 years. Ultimately, patients do believe that seeing a physician at a postoperative visit is important, as just under half of patients thought that seeing a physician extender alone at a postoperative visit resulted in a lower quality of care.
While these results were obtained in a population specifically seeking the care of an orthopedic adult reconstruction surgeon, the results demonstrate that patients do not necessarily desire an unreasonable amount of time with their doctor. Patients simply want to be seen in a timely fashion and receive the full undivided attention of their doctor for approximately 20 minutes. Similarly, Patterson and colleagues22 found, in their series of 182 patients who presented to an orthopedic surgeon, that there was a significant correlation between time spent with the surgeon and overall patient satisfaction. Interestingly, the authors reported that patient satisfaction was not correlated with education level, sex, marital status, whether the patients were evaluated by a resident physician before seeing the attending surgeon, self-reported mental status, tobacco usage, the type of clinic visit, or the waiting time to see the surgeon (average, about 40 minutes for this cohort).22 Similarly, Teunis and colleagues23 reported an average 32-minute wait time in 81 patients presenting for care at an orthopedic hand clinic and demonstrated that a longer wait time was associated with decreased patient satisfaction. These results corroborate the findings of this study that a short wait time is important to patients when evaluating the process of care. Additionally, patients do not have unreasonable expectations with regards to the amount of time they would like to spend with the physician. A physician who has a clinic for 9 hours a day would thus be able to see 54 patients and still spend at least 10 minutes with each patient. The quality of the physician-patient interaction is likely more important than the actual amount of time spent; however, based on this study, patients do have certain expectations about how much time physicians should spend with them.
There were no significant sex, age, or ethnicity preferences in our specific patient cohort. However, a sizable percentage of respondents, 41.8%, believed that they were receiving inferior care if they only saw a physician extender at a routine follow-up visit. Many orthopedic surgeons rely on the care provided by physician extenders to enable them to see additional patients. Physician extenders are well trained to provide high-quality care, including at routine postoperative visits. The results of this study, that many patients believe physician extenders provide lower-quality care, may be a result of inadequate patient education regarding the extensive training and education physician extenders undergo. Physician extenders are qualified, licensed healthcare professionals who are playing increasingly important roles within orthopedics and medicine as a whole. As the demand for orthopedic surgeons to see more patients increases, so does the role of physician extenders. Future research is warranted into educating the public regarding the importance of these healthcare providers and the adequacy of their training.
While many practices now routinely obtain patient satisfaction scores, another modality through which patients can express their satisfaction and experiences with healthcare providers is through online internet physician rating sites (IPRS). These sites have exploded in number and popularity in recent years and, according to some studies, have a very real effect on provider selection.24 Interestingly, a low percentage of patients in this study utilized IPRS reviews to find their doctors. In a recent prospective survey study of 1000 consecutive patients presenting for care at the Mayo Clinic, Burkle and Keegan24 reported that 27% of patients would choose not to see a physician based on a negative IPRS review. Interestingly, only 1.0% of patients reported finding their doctor through advertising. Numerous authors have recently addressed advertising in orthopedic surgery, specifically direct-to-consumer marking, including the influence of physician self-promotion on patients.25,26 Specifically, Halawi and Barsoum26 discussed how direct-to-consumer marketing is commonly disseminated to the public through television and print advertisements, which are modalities more commonly utilized by older generations. However, many advertising agencies are moving toward internet-based advertising, especially through orthopedic group and individual surgeon websites for self-promoting advertisement, as approximately 75% of Americans use the internet for health-related information.25,27 The fact that many patients in this study did not utilize IPRS reviews or advertising (much of which is electronic) may be a result of the older, less internet-centric demographic that is often seen in an adult reconstruction clinic. Future research is warranted to determine what demographic of patients value IPRS reviews and how those reviews influence physician selection and the patient experience.
There are several limitations to this study. First, the majority of the surveyed population was Caucasian, and our results may not be equally reflective of diverse ethnic backgrounds. Second, the cohort size, while based on previous studies conducted in a similar fashion, may be underpowered to detect significant differences for 1 or more of these questions. In addition, having a question regarding the patient’s medical background or experiences may have provided further insight as to why patients selected the answers that they did. Furthermore, questions regarding the patient’s education level, religious background, and income brackets may have provided further context in which to evaluate their responses. These questions were omitted in an effort to keep the questionnaire at a length that would maximize enrollment and prevent survey fatigue. Future research is warranted to determine what patient-specific, injury/symptom-specific, and treatment-specific variables influence the subjective patient experience.
CONCLUSION
The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them. Patients reported no significant gender- or ethnicity-based preferences for their doctor. The majority of patients believe that a wait time exceeding 30 minutes is too long. A greater effort needs to be made to educate patients and the public about the significant and effective roles nurse practitioners and physician assistants can play within the healthcare system. While this cohort did not report notable utilization of IPRS reviews, it remains essential to understand what factors influence patients’ subjective experiences with their providers to ensure that patients achieve their desired outcomes, and report as such on these websites as they continue to gain popularity. Diminishing clinic wait times and understanding patient preferences may lead to a greater percentage of “satisfied” patients. While the majority of focus has been and will likely continue to be on improving patients’ satisfaction with their outcomes, more work needs to be done focusing specifically on the process through which outcomes are achieved.
ABSTRACT
Patient satisfaction has become a topic of interest within orthopedics as the landscape of provider reimbursement continues to evolve to reward value of care. Online internet physician rating sites are becoming increasingly popular ways for patients to subjectively express their provider experience. Understanding what patients value during their episode of care is important in the modern healthcare environment. The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. A prospective survey was electronically administered to 212 patients in an adult reconstruction clinic. One hundred ninety-six patients (92.5%) completed the survey. Demographic questions regarding age, sex, ethnicity, and prior adult reconstruction surgical history were obtained. When patients were asked how much time they would like the doctor to spend with them on a routine visit, the most common answer was 10 to 15 minutes (41.3%), with only 10.2% patients desiring >20 minutes. The majority of patients (83.1%) believe ≥30 minutes is too long to wait to see their surgeon. Less than half of patients (41.8%) stated that they would feel as though they were receiving below average care if seen only by a nurse practitioner or physician’s assistant at a postoperative visit. Patients reported no significant age, gender, or ethnicity preferences for their physician. Recommendations from friends or other physicians was the most common (66.4%) way for patients to find their physicians, while 12.2% utilized online rating sites during their search. Optimizing patient experiences in the office may include keeping wait times to <30 minutes and educating patients on the roles of physician extenders. More work needs to be done to further elucidate variables influencing the subjective patient experience with their orthopedic care.
Continue to: Patient satisfaction...
Patient satisfaction has become an important focus in the rapidly changing healthcare environment due to the significant impact it has on healthcare delivery, healthcare economics, assessment of the quality of care, development of patient-care models, and quality improvement initiatives.1-4 Historically, the quality of care was measured by objective metrics such as complication rates, range-of-motion, and the provider’s expert opinion on the outcome. While those metrics are still impactful variables when defining a successful outcome, the medical community is now increasingly recognizing the importance of patients’ perspectives when defining successful treatments. Patient satisfaction is now highly regarded by clinicians and the government when considering outcomes and is even being incorporated into determining the value of care. Under the Affordable Care Act, patients assumed a more active role in clinical decision-making as well as in creating quality and efficiency initiatives.5,6 By 2017, 2% of the United States government’s Medicare payments will be redistributed among hospitals and physicians based on their quality and efficiency metrics, which are largely determined by patients’ evaluations of care.7 As a result, there has been significant interest in identifying variables influencing patient satisfaction and subjective outcomes.8,9
Patient satisfaction is related to both the outcomes of care and the process of care. As first described by Donabedian,10patients may be satisfied with the successful outcome of their care, but dissatisfied with how they received their care. The process of care is complex and considers many aspects of healthcare delivery, including time, cost, healthcare provider interactions, and burdens faced. While patient satisfaction with outcomes and process of care are heavily related, they should be regarded separately. It is essential that providers understand what variables are important to patients with regards to how they experience healthcare and choose their provider, especially surrounding elective procedures such as hip and knee arthroplasty.11,12
Within orthopedic surgery, patient satisfaction scores are beginning to be incorporated as part of the standard-of-care quality metrics obtained along with patient-reported outcome measures (PROMs) at defined time points postoperatively. Furthermore, PROMs and patient satisfaction data are becoming an increasingly important component of medical decision-making.13-16 Several authors have reported that increased patient satisfaction is correlated with increased compliance, improved treatment outcomes across numerous medical settings, including orthopedics, decreased risk of litigation, and higher patient ratings of the quality of care.17,18 Various factors, including meeting of expectations, staff politeness, the communication skills of the surgeon, and waiting times, have been suggested to influence eventual patient satisfaction within the surgical literature.19-21 However, within orthopedic surgery there is a paucity of investigations evaluating how patients determine preferences and satisfaction with the process of care.
The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. The authors hypothesize that the majority of patients find their physicians through online rating sites or recommendations from family and friends. The authors believe that patients expect to be seen in <30 minutes and will be unsatisfied overall with the amount of time that they spend with their physician.
Continue to: METHODS...
METHODS
The senior author (BRL) and a research team created a 15-question survey to evaluate patient preferences regarding the demographic characteristics (eg, age, gender, ethnicity) of their physician, wait times in a waiting room, time spent with the physician, care received from physician extenders (eg, nurse practitioners, physician assistants), and how they learned of their physician (Appendix). An a priori power analysis was conducted to determine that approximately 200 patients were needed for inclusion.11,22 Following Institutional Review Board approval (ORA 15051104), the survey was administered to 212 patients in a single-surgeon, adult reconstruction clinic. The survey was digitally administered on a touch-screen tablet using an electronic independent third party survey center (SurveyMonkey Inc) devoid of any identifying data. The survey was offered to all patients >21 years of age who were English-speaking and in the common area as patients waiting to be seen, from June 2015 to March 2016. A research assistant approached patients in the waiting room and asked if they would like to participate in a short survey regarding what factors influence the patient-physician relationship from the patient’s perspective.
Appendix 1
- Do you wish to partake in this 3-minute survey?
- Have you had a prior knee or hip replacement?
- What is your age?
- 30-40 years
- 40-50 years
- 50-60 years
- 60-70 years
- 70-80 years
- 80+ years
- What is your gender?
- Which of the following best represents your racial or ethnic heritage?
- African American
- How much time would you like the doctor to spend talking to you on a routine visit?
- 0-5 minutes
- 5-10 minutes
- 10-15 minutes
- 15-20 minutes
- 20-30 minutes
- >30 minutes
- How long is too long to wait to see the doctor?
- 10 minutes
- 20 minutes
- 30 minutes
- 40 minutes
- 50 minutes
- An hour or more
- If you were to only see a physician’s assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care?
- Overall I am satisfied with the time the doctor spends with me.
- If you were to need a major surgery, would you want the physician to tell you what he or she would do if they were in your shoes?
- Would you prefer your doctor to be the same race/ethnicity as you?
- No
- No Preference
- Would you feel more comfortable with a male as opposed to a female orthopedic surgeon?
- Would you feel more comfortable with a female as opposed to a male orthopedic surgeon?
- What age would you like your physician to be?
- 25-35 years old
- 35-45 years old
- 45-55 years old
- 55-65 years old
- 65 years and older
- No preference
- How do you usually find your physician?
- Friends’ recommendations
- Healthcare provider’s recommendations
- Insurance plans
- Online research/ratings
- Other
Descriptive statistics were used to analyze subject demographics and survey responses. Chi-square analyses and multinomial logistic regressions were utilized to compare responses. All statistical analyses were conducted using SPSS version 24.0 software (SPSS Inc). Statistical significance was set at P < 0.05.
RESULTS
Of the 212 patients who were invited to participate, 196 patients (92.4%) agreed and completed the survey. Demographic and surgical history information can be found in Table 1. The majority of patients were female (62%) and above the age of 50 years (92.4%). Almost half (48.5%) of patients had a prior hip or knee replacement.
Table 1. Survey Respondent Demographics
| Number | Percent |
Age Range | ||
30-40 years | 4 | 2.0% |
40-50 years | 11 | 5.6% |
50-60 years | 47 | 24.0% |
60-70 years | 84 | 42.9% |
70-80 years | 41 | 20.9% |
>80 years | 9 | 4.6% |
Gender | ||
Male | 74 | 37.8% |
Female | 122 | 62.2% |
Ethnicity | ||
African American | 39 | 19.9% |
Asian | 3 | 1.5% |
Caucasian | 140 | 71.4% |
Hispanic | 10 | 5.1% |
Other | 4 | 2.0% |
Prior knee or hip replacement | ||
Yes | 95 | 48.5% |
No | 55 | 28.1% |
No Response | 46 | 23.5% |
When asked how long is too long to wait to see the doctor, 30 minutes (39.8%) was most commonly selected, followed by 40 minutes (24.5%) (Figure 1). When asked how much time patients would like the doctor to spend with them during an office visit, the majority (68.9%) selected either 10 to 15 minutes (41.3%) or 15 to 20 minutes (27.6%) (Figure 2). The majority of patients (92.3%) were satisfied with the amount of time the doctor spent with them. In addition, 94.9% of respondents would want their doctor to tell them what they would do if they were in the patient’s shoes when making decisions regarding their medical care (Table 2). Less than half of respondents (41.8%) believe that seeing a physician extender (eg, nurse practitioner or physician assistant) at a postoperative visit would result in a lower quality of care (Table 2).
Table 2. Responses to Survey Questions
If you were to only see a physician's assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care? | ||
Answer choices | Number | Percent |
No | 114 | 58.2% |
Yes | 82 | 41.8% |
If you were to need a major surgery would you want the physician to tell you what he or she would do if they were in your shoes? | ||
Answer choices | Number | Percent |
No | 10 | 5.1% |
Yes | 186 | 94.9% |
Would you prefer your doctor to be the same race/ethnicity as you? | ||
Answer choices | Number | Percent |
No | 29 | 14.8% |
Yes | 3 | 1.5% |
No Preference | 164 | 83.7% |
When asked if patients preferred a doctor of the same race/ethnicity, the vast majority (83.7%) had no preference (Table 2). There was no significant difference found between male and female respondents when asked if they would feel more comfortable with a male as opposed to a female orthopedic surgeon (P = .118) and vice versa (P = .604) (Table 3). Most patients preferred a physician between the ages of 45 and 55 years (39.3%), followed by 35 and 45 years (23.0%); however, this preference was not statistically significant (Table 4). Older patients were more likely to prefer younger physicians (odds ratio, 4.612 for 25-35 years of age; odds ratio, 1.328 for 35-45 years of age). Only 12.2% of patients reported online research/rating sites as the main resource utilized when selecting their physician (Figure 3). The majority (68.4%) reported that recommendations from either friends (35.2%) or healthcare providers (33.2%) were the main avenues through which they found their physicians.
Table 3. Overall Responses to Questions Regarding Male and Female Orthopedic Surgeonsa
Would you feel more comfortable with a male as opposed to a female orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 164 | 83.7% | 106 (86.9%) | 58 (78.4%) | 0.118 |
Yes | 32 | 16.3% | 16 (13.1%) | 16 (21.6%) |
|
Would you feel more comfortable with a female as opposed to a male orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 186 | 94.9% | 115 (94.3%) | 71 (95.9%) | 0.604 |
Yes | 10 | 5.1% | 7 (5.7%) | 3 (4.1%) |
|
aResponses were broken down by gender and compared utilizing a 2 x 2 chi-square analysis to test for significant differences in respondents’ gender preferences for their orthopedic surgeon.
Table 4. Patient Preferences Regarding Physician Age
What age would you like your physician to be? |
| 95% Confidence Interval | ||||
Answer Choices | Number or Responses | Percent | P value | Exp(β) | Lower Bound | Upper Bound |
25-35 years | 1 | 0.5% | 0.217 | 4.612 | 0.407 | 52.283 |
35-45 years | 45 | 23.0% | 0.161 | 1.328 | 0.893 | 1.975 |
45-55 years | 77 | 39.3% | 0.159 | 1.276 | 0.909 | 1.791 |
55-65 years | 9 | 4.6% | 0.483 | 1.302 | 0.624 | 2.717 |
≥65 years | 2 | 1.0% | 0.272 | 0.491 | 0.138 | 1.748 |
No preferencea | 62 | 31.6% | Reference | |||
aNo preference was used as the reference category for the answer choices, while the age bracket “>80 years” was used as the reference for the age of respondent variable.
Continue to: DISCUSSION...
DISCUSSION
The results of this study demonstrate that patients have several expectations and preferences with regards to the care they receive from physicians in the office. Patients prefer to wait <30 minutes before seeing their provider and desire only 10 to 20 minutes with their doctor. Patients do not have specific preferences with regards to the gender or ethnicity of their physician but would prefer a physician in the middle of their career, aged 45 to 55 years. Ultimately, patients do believe that seeing a physician at a postoperative visit is important, as just under half of patients thought that seeing a physician extender alone at a postoperative visit resulted in a lower quality of care.
While these results were obtained in a population specifically seeking the care of an orthopedic adult reconstruction surgeon, the results demonstrate that patients do not necessarily desire an unreasonable amount of time with their doctor. Patients simply want to be seen in a timely fashion and receive the full undivided attention of their doctor for approximately 20 minutes. Similarly, Patterson and colleagues22 found, in their series of 182 patients who presented to an orthopedic surgeon, that there was a significant correlation between time spent with the surgeon and overall patient satisfaction. Interestingly, the authors reported that patient satisfaction was not correlated with education level, sex, marital status, whether the patients were evaluated by a resident physician before seeing the attending surgeon, self-reported mental status, tobacco usage, the type of clinic visit, or the waiting time to see the surgeon (average, about 40 minutes for this cohort).22 Similarly, Teunis and colleagues23 reported an average 32-minute wait time in 81 patients presenting for care at an orthopedic hand clinic and demonstrated that a longer wait time was associated with decreased patient satisfaction. These results corroborate the findings of this study that a short wait time is important to patients when evaluating the process of care. Additionally, patients do not have unreasonable expectations with regards to the amount of time they would like to spend with the physician. A physician who has a clinic for 9 hours a day would thus be able to see 54 patients and still spend at least 10 minutes with each patient. The quality of the physician-patient interaction is likely more important than the actual amount of time spent; however, based on this study, patients do have certain expectations about how much time physicians should spend with them.
There were no significant sex, age, or ethnicity preferences in our specific patient cohort. However, a sizable percentage of respondents, 41.8%, believed that they were receiving inferior care if they only saw a physician extender at a routine follow-up visit. Many orthopedic surgeons rely on the care provided by physician extenders to enable them to see additional patients. Physician extenders are well trained to provide high-quality care, including at routine postoperative visits. The results of this study, that many patients believe physician extenders provide lower-quality care, may be a result of inadequate patient education regarding the extensive training and education physician extenders undergo. Physician extenders are qualified, licensed healthcare professionals who are playing increasingly important roles within orthopedics and medicine as a whole. As the demand for orthopedic surgeons to see more patients increases, so does the role of physician extenders. Future research is warranted into educating the public regarding the importance of these healthcare providers and the adequacy of their training.
While many practices now routinely obtain patient satisfaction scores, another modality through which patients can express their satisfaction and experiences with healthcare providers is through online internet physician rating sites (IPRS). These sites have exploded in number and popularity in recent years and, according to some studies, have a very real effect on provider selection.24 Interestingly, a low percentage of patients in this study utilized IPRS reviews to find their doctors. In a recent prospective survey study of 1000 consecutive patients presenting for care at the Mayo Clinic, Burkle and Keegan24 reported that 27% of patients would choose not to see a physician based on a negative IPRS review. Interestingly, only 1.0% of patients reported finding their doctor through advertising. Numerous authors have recently addressed advertising in orthopedic surgery, specifically direct-to-consumer marking, including the influence of physician self-promotion on patients.25,26 Specifically, Halawi and Barsoum26 discussed how direct-to-consumer marketing is commonly disseminated to the public through television and print advertisements, which are modalities more commonly utilized by older generations. However, many advertising agencies are moving toward internet-based advertising, especially through orthopedic group and individual surgeon websites for self-promoting advertisement, as approximately 75% of Americans use the internet for health-related information.25,27 The fact that many patients in this study did not utilize IPRS reviews or advertising (much of which is electronic) may be a result of the older, less internet-centric demographic that is often seen in an adult reconstruction clinic. Future research is warranted to determine what demographic of patients value IPRS reviews and how those reviews influence physician selection and the patient experience.
There are several limitations to this study. First, the majority of the surveyed population was Caucasian, and our results may not be equally reflective of diverse ethnic backgrounds. Second, the cohort size, while based on previous studies conducted in a similar fashion, may be underpowered to detect significant differences for 1 or more of these questions. In addition, having a question regarding the patient’s medical background or experiences may have provided further insight as to why patients selected the answers that they did. Furthermore, questions regarding the patient’s education level, religious background, and income brackets may have provided further context in which to evaluate their responses. These questions were omitted in an effort to keep the questionnaire at a length that would maximize enrollment and prevent survey fatigue. Future research is warranted to determine what patient-specific, injury/symptom-specific, and treatment-specific variables influence the subjective patient experience.
CONCLUSION
The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them. Patients reported no significant gender- or ethnicity-based preferences for their doctor. The majority of patients believe that a wait time exceeding 30 minutes is too long. A greater effort needs to be made to educate patients and the public about the significant and effective roles nurse practitioners and physician assistants can play within the healthcare system. While this cohort did not report notable utilization of IPRS reviews, it remains essential to understand what factors influence patients’ subjective experiences with their providers to ensure that patients achieve their desired outcomes, and report as such on these websites as they continue to gain popularity. Diminishing clinic wait times and understanding patient preferences may lead to a greater percentage of “satisfied” patients. While the majority of focus has been and will likely continue to be on improving patients’ satisfaction with their outcomes, more work needs to be done focusing specifically on the process through which outcomes are achieved.
1. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84–A(9):1560-1572.
2. Carr-Hill RA. The measurement of patient satisfaction. J Public Health Med. 1992;14(3):236-249.
3. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. Med Care. 1995;33(4):392-406. doi:10.1097/00005650-199504000-00006.
4. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219-248. doi:10.1177/107755879305000205.
5. Bozic KJ. Orthopaedic healthcare worldwide: shared medical decision making in orthopaedics. Clin Orthop Relat Res. 2013;471(5):1412-1414. doi:10.1007/s11999-013-2838-5.
6. Youm J, Chenok KE, Belkora J, Chiu V, Bozic KJ. The emerging case for shared decision making in orthopaedics. Instr Course Lect. 2013;62:587-594. doi:10.2106/00004623-201210170-00011.
7. Blumenthal D, Abrams M, Nuzum R. The affordable CARE Act at 5 years. N Engl J Med. 2015;373(16):1580. doi:10.1056/NEJMc1510015.
8. Shirley ED, Sanders JO. Patient satisfaction: implications and predictors of success. J Bone Joint Surg Am. 2013;95(10):e69. doi:10.2106/JBJS.L.01048.
9. Morris BJ, Jahangir AA, Sethi MK. Patient satisfaction: an emerging health policy issue. AAOS Now Web site. http://www.aaos.org/AAOSNow/2013/Jun/advocacy/advocacy5/?ssopc=1. Published June 2013. Accessed November 19, 2016.
10. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. doi:10.1001/jama.260.12.1743.
11. Bozic KJ, Kaufman D, Chan VC, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clin Orthop Relat Res. 2013;471(6):1865-1872. doi:10.1007/s11999-012-2640-9.
12. Davies AR, Ware JE Jr. Involving consumers in quality of care assessment. Health Aff (Millwood). 1988;7(1):33-48.
13. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25(5):1103-1112. doi:10.1007/s11136-015-1168-3.
14. Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429-439. doi:10.1016/j.ygyno.2014.11.071.
15. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754-775. doi:10.1111/1468-0009.12091.
16. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104-109. doi:10.2106/JBJS.N.00328.
17. Williams B. Patient satisfaction: a valid concept? Soc Sci Med. 1994;38(4):509-516. doi:10.1016/0277-9536(94)90247-X.
18. Hickson GB, Clayton EW, Entman SS, et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583-1587. doi:10.1001/jama.1994.03520200039032.
19. Larsson BW, Larsson G, Chantereau MW, von Holstein KS. International comparisons of patients' views on quality of care. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18(1):62-73. doi:10.1108/09526860510576974.
20. McLafferty RB, Williams RG, Lambert AD, Dunnington GL. Surgeon communication behaviors that lead patients to not recommend the surgeon to family members or friends: analysis and impact. Surgery. 2006;140(4):616-624. doi:https://doi.org/10.1016/j.surg.2006.06.021.
21. Mira JJ, Tomás O, Virtudes-Pérez M, Nebot C, Rodríguez-Marín J. Predictors of patient satisfaction in surgery. Surgery. 2009;145(5):536-541. doi:10.1016/j.surg.2009.01.012.
22. Patterson BM, Eskildsen SM, Clement RC, et al. Patient satisfaction is associated with time with provider but not clinic wait time among orthopedic patients. Orthopedics. 2017;40(1):43-48. doi:10.3928/01477447-20161013-05.
23. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated With patient satisfaction. Clin Orthop Relat Res. 2015;473(7):2362-2368. doi:10.1007/s11999-014-4090-z.
24. Burkle CM, Keegan MT. Popularity of internet physician rating sites and their apparent influence on patients' choices of physicians. BMC Health Serv Res. 2015;15:416. doi:10.1186/s12913-015-1099-2.
25. Mohney S, Lee DJ, Elfar JC. The effect of orthopedic advertising and self-promotion on a naive population. Am J Orthop. 2016;45(4):E227-E232.
26. Halawi MJ, Barsoum WK. Direct-to-consumer marketing: implications for patient care and orthopedic education. Am J Orthop. 2016;45(6):E335-E336.
27. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156. doi:10.1007/s11606-010-1425-7.
1. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84–A(9):1560-1572.
2. Carr-Hill RA. The measurement of patient satisfaction. J Public Health Med. 1992;14(3):236-249.
3. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. Med Care. 1995;33(4):392-406. doi:10.1097/00005650-199504000-00006.
4. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219-248. doi:10.1177/107755879305000205.
5. Bozic KJ. Orthopaedic healthcare worldwide: shared medical decision making in orthopaedics. Clin Orthop Relat Res. 2013;471(5):1412-1414. doi:10.1007/s11999-013-2838-5.
6. Youm J, Chenok KE, Belkora J, Chiu V, Bozic KJ. The emerging case for shared decision making in orthopaedics. Instr Course Lect. 2013;62:587-594. doi:10.2106/00004623-201210170-00011.
7. Blumenthal D, Abrams M, Nuzum R. The affordable CARE Act at 5 years. N Engl J Med. 2015;373(16):1580. doi:10.1056/NEJMc1510015.
8. Shirley ED, Sanders JO. Patient satisfaction: implications and predictors of success. J Bone Joint Surg Am. 2013;95(10):e69. doi:10.2106/JBJS.L.01048.
9. Morris BJ, Jahangir AA, Sethi MK. Patient satisfaction: an emerging health policy issue. AAOS Now Web site. http://www.aaos.org/AAOSNow/2013/Jun/advocacy/advocacy5/?ssopc=1. Published June 2013. Accessed November 19, 2016.
10. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. doi:10.1001/jama.260.12.1743.
11. Bozic KJ, Kaufman D, Chan VC, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clin Orthop Relat Res. 2013;471(6):1865-1872. doi:10.1007/s11999-012-2640-9.
12. Davies AR, Ware JE Jr. Involving consumers in quality of care assessment. Health Aff (Millwood). 1988;7(1):33-48.
13. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25(5):1103-1112. doi:10.1007/s11136-015-1168-3.
14. Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429-439. doi:10.1016/j.ygyno.2014.11.071.
15. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754-775. doi:10.1111/1468-0009.12091.
16. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104-109. doi:10.2106/JBJS.N.00328.
17. Williams B. Patient satisfaction: a valid concept? Soc Sci Med. 1994;38(4):509-516. doi:10.1016/0277-9536(94)90247-X.
18. Hickson GB, Clayton EW, Entman SS, et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583-1587. doi:10.1001/jama.1994.03520200039032.
19. Larsson BW, Larsson G, Chantereau MW, von Holstein KS. International comparisons of patients' views on quality of care. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18(1):62-73. doi:10.1108/09526860510576974.
20. McLafferty RB, Williams RG, Lambert AD, Dunnington GL. Surgeon communication behaviors that lead patients to not recommend the surgeon to family members or friends: analysis and impact. Surgery. 2006;140(4):616-624. doi:https://doi.org/10.1016/j.surg.2006.06.021.
21. Mira JJ, Tomás O, Virtudes-Pérez M, Nebot C, Rodríguez-Marín J. Predictors of patient satisfaction in surgery. Surgery. 2009;145(5):536-541. doi:10.1016/j.surg.2009.01.012.
22. Patterson BM, Eskildsen SM, Clement RC, et al. Patient satisfaction is associated with time with provider but not clinic wait time among orthopedic patients. Orthopedics. 2017;40(1):43-48. doi:10.3928/01477447-20161013-05.
23. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated With patient satisfaction. Clin Orthop Relat Res. 2015;473(7):2362-2368. doi:10.1007/s11999-014-4090-z.
24. Burkle CM, Keegan MT. Popularity of internet physician rating sites and their apparent influence on patients' choices of physicians. BMC Health Serv Res. 2015;15:416. doi:10.1186/s12913-015-1099-2.
25. Mohney S, Lee DJ, Elfar JC. The effect of orthopedic advertising and self-promotion on a naive population. Am J Orthop. 2016;45(4):E227-E232.
26. Halawi MJ, Barsoum WK. Direct-to-consumer marketing: implications for patient care and orthopedic education. Am J Orthop. 2016;45(6):E335-E336.
27. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156. doi:10.1007/s11606-010-1425-7.
TAKE-HOME POINTS
- The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them.
- Patients reported no significant gender- or ethnicity-based preferences for their doctor.
- The majority of patients believe that a wait time exceeding 30 minutes is too long.
- Nearly 42% of respondents felt they would be receiving below average medical care if seen only by a nurse practitioner or physician’s assistant at a postoperative appointment.
- Recommendations from friends is the most common way patients find their physicians.
Hip and Core Muscle Injuries in Soccer
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
TAKE-HOME POINTS
- Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need surgical intervention.
- Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain.
- Acute groin pain in soccer players is most commonly caused by muscle strain involving the adductor longus, the iliopsoas or the rectus femoris.
- Inguinal-related groin pain is a common cause of chronic groin pain and typically is the most challenging to treat with a complex pathophysiology and a high association with femoroacetabular impingement.
- Hip-related groin pain (femoroacetabular impingement, labral tears, and chondral injuries) usually respond well to surgical intervention and has high rates of return to sport.
Genes found more important than diet in hyperuricemia
CHICAGO – Diet plays a significantly less important role than genes in the development of high serum urate levels that typically precede gout, research from New Zealand has revealed.
These findings stem from a first-ever systematic analysis of a large data set to “determine the relative contributions of inherited genetic variants and overall diet to variance in serum urate concentrations,” Tanya J. Major, PhD, of the University of Otago in Dunedin, New Zealand, and her colleagues first reported in The BMJ and then less than 2 weeks later at the annual meeting of the American College of Rheumatology.
Prior studies have estimated that genetic factors explain 25%-60% of the variability in serum urate levels, but diet also has long been considered a risk factor for gout, with certain diets such as the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet shown in studies to reduce serum urate levels and the risk of gout, the researchers noted.
The new study found that common genetic factors accounted for nearly 24% of the variation in serum urate levels, whereas diet accounted for less than 1%.
Before these findings, the impact of diet on hyperuricemia was known to be limited, but that doesn’t mean that diet is meaningless, Michael H. Pillinger, MD, who was not involved in the study, said in an interview at the ACR annual meeting.
“What this work does from a scientific point of view is underline the genetic basis [of hyperuricemia]. What it does from a practical and clinical point of view is tell us that diet is a meaningful thing to adjust. It will provide other health benefits, but it isn’t the solution, and we know that it rarely is,” said Dr. Pillinger, professor of medicine and biochemistry and molecular pharmacology at New York University. “We shouldn’t expect a really large impact from diet, and we shouldn’t make people feel guilty about their diets, and optimally you want to manage a diet that a patient can tolerate and not one that will be impossible to sustain.”
Dr. Major had a similar message in an interview at the meeting: “By focusing on diet and treating urate levels through diet, you start to kind of get this blame element going on that the reason you’ve got gout is you’re eating the wrong foods, and so then it makes people less likely to want to go to see their doctor to start with. And then when they do try to follow their doctor’s advice about their diet, and nothing changes, then it starts to get frustrating for them as well.”
The research team analyzed dietary survey data from five U.S. cohort studies (Atherosclerosis Risk in Communities, Coronary Artery Risk Development in [Young] Adults, Cardiovascular Heart Study, Framingham Heart Study, and Third National Health and Nutrition Examination Survey [NHANES III]) that altogether included 16,760 individuals (8,414 men and 8,346 women) of European ancestry.
Participants were aged 18 years or older, did not have gout or kidney disease, and were not taking urate-lowering or diuretic drugs. Serum urate measurements, dietary survey data, and confounders such as gender, age, body mass index, smoking status, and average daily calorie intake were all recorded.
The results showed that 15 foods were significantly associated with serum urate levels. Six were established urate-modifying foods: beer, liquor, wine, soft drinks, skim milk, and meat (beef, pork, or lamb). The remaining nine foods included two less established urate-modifying foods (cheese and noncitrus fruit) and seven food items without established associations: poultry, potatoes, brown bread, peanuts, margarine, cold cereal, and eggs.
Beer and liquor had the strongest urate-raising effect, associated with a 1.38 micromol/L increase in serum urate per serving per week, equating to a 9.66 micromol/L (0.16 mg/dL) increase per daily serving.
The authors noted that the associations seen with known and confirmed serum urate–influencing foods were consistent with previously reported associations in terms of effect and magnitude. Nevertheless, they noted that “each of these established foods explained less than 1% of variation in serum urate levels within the full cohort.”
When the researchers analyzed the data according to three diet scores based on healthy diet guidelines, they found that diet explained “very little variance in serum urate levels (0.28% for the DASH diet, 0.15% for the Healthy Eating diet, 0.06% for the Mediterranean diet, and 0.16% for the data-driven diet pattern).”
However, they noted that even though their findings, along with previous research, suggest that a clinically relevant decrease in serum urate levels could be achieved with the DASH diet, the implementation of such a diet might not be “straightforward” because “the barriers to implementing this diet both at a population level and in a primary care setting are yet to be overcome.”
In contrast, when the research team looked at genes in the cohort they found that common genetic factors explained 23.9% of variation in serum urate levels in the full cohort (excluding the NHANES III study because of a lack of genotype data).
Baseline visit diet information for these cohort studies ranged from the mid-1980s to the early to mid-2000s, making it hard to account for changes in food composition over time. Dr. Major said that food compositions overall were likely to be more processed over time and contain more urate-raising foods. However, any effects of food composition changes on urate levels over time would be more likely be felt at the level of individual food products, not on diets overall, she said.
The variability in the diet scores that the investigators noted across individuals and regions in these U.S. cohort studies did not appear to affect the results, Dr. Major noted. Furthermore, adjustment within models for these diet scores did not affect the degree of variation in urate levels that genetics accounted for, whereas adjustment for genetics lowered the amount of variation that diet contributed to urate levels.
The findings also don’t have any say on the relative contribution of diet versus genetics on gout flares, which is a separate issue. Many patients would agree that they suspect certain foods trigger gout flares even if they don’t affect baseline urate levels very much, Dr. Pillinger said.
The next steps for Dr. Major and her coinvestigators may be to see if diet is influencing urate levels to the same degree in people with gout as this study shows for the general population, she said.The investigators noted that the use of different food questionnaires between the five studies is one limitation of the analysis, and there should be some caution in generalizing the results to people with gout or those of non-European ancestry.
The study was supported by the Health Research Council of New Zealand and the University of Otago. One author reported receiving consulting and speaker fees or grants from several pharmaceutical companies that manufacture urate-lowering drugs.
This article was updated 10/25/18.
SOURCE: Major TJ et al. BMJ. 2018. doi: 10.1136/bmj.k3951.
People with gout are often stigmatized by society because of the common misconception that the condition is caused by an unhealthy diet and lifestyle. This well-established and harmful view, which also exists among health professionals and the media, can stop people from seeking help for their condition, which means opportunities to initiate treatment can be missed.
The study by Major et al. provides valuable evidence that a considerable part of a person’s predisposition to high serum urate levels, and therefore gout, is not modifiable. It should be noted, however, that the findings do not provide evidence to support a change in current guidelines, which recommend that people with gout adopt a diet that avoids excessive consumption of high-risk foods.
Nevertheless, the findings have broad implications for both people with gout and the health professionals who care for them. They provide a welcome opportunity to address these serious barriers to treatment and reduce the burden associated with what is a common and easily treatable condition.
While the authors caution against extending their findings to people with clinically diagnosed gout, it is unlikely that the causes of hyperuricemia in this patient population are different from those studied in patients with gout.
Lorraine Watson and Edward Roddy, DM, are affiliated with Arthritis Research UK Primary Care Centre at Keele University, Staffordshire, England. These comments are adapted from their accompanying editorial (BMJ. 2018. doi: 10.1136/bmj.k4140). They declared no relevant conflicts of interest.
People with gout are often stigmatized by society because of the common misconception that the condition is caused by an unhealthy diet and lifestyle. This well-established and harmful view, which also exists among health professionals and the media, can stop people from seeking help for their condition, which means opportunities to initiate treatment can be missed.
The study by Major et al. provides valuable evidence that a considerable part of a person’s predisposition to high serum urate levels, and therefore gout, is not modifiable. It should be noted, however, that the findings do not provide evidence to support a change in current guidelines, which recommend that people with gout adopt a diet that avoids excessive consumption of high-risk foods.
Nevertheless, the findings have broad implications for both people with gout and the health professionals who care for them. They provide a welcome opportunity to address these serious barriers to treatment and reduce the burden associated with what is a common and easily treatable condition.
While the authors caution against extending their findings to people with clinically diagnosed gout, it is unlikely that the causes of hyperuricemia in this patient population are different from those studied in patients with gout.
Lorraine Watson and Edward Roddy, DM, are affiliated with Arthritis Research UK Primary Care Centre at Keele University, Staffordshire, England. These comments are adapted from their accompanying editorial (BMJ. 2018. doi: 10.1136/bmj.k4140). They declared no relevant conflicts of interest.
People with gout are often stigmatized by society because of the common misconception that the condition is caused by an unhealthy diet and lifestyle. This well-established and harmful view, which also exists among health professionals and the media, can stop people from seeking help for their condition, which means opportunities to initiate treatment can be missed.
The study by Major et al. provides valuable evidence that a considerable part of a person’s predisposition to high serum urate levels, and therefore gout, is not modifiable. It should be noted, however, that the findings do not provide evidence to support a change in current guidelines, which recommend that people with gout adopt a diet that avoids excessive consumption of high-risk foods.
Nevertheless, the findings have broad implications for both people with gout and the health professionals who care for them. They provide a welcome opportunity to address these serious barriers to treatment and reduce the burden associated with what is a common and easily treatable condition.
While the authors caution against extending their findings to people with clinically diagnosed gout, it is unlikely that the causes of hyperuricemia in this patient population are different from those studied in patients with gout.
Lorraine Watson and Edward Roddy, DM, are affiliated with Arthritis Research UK Primary Care Centre at Keele University, Staffordshire, England. These comments are adapted from their accompanying editorial (BMJ. 2018. doi: 10.1136/bmj.k4140). They declared no relevant conflicts of interest.
CHICAGO – Diet plays a significantly less important role than genes in the development of high serum urate levels that typically precede gout, research from New Zealand has revealed.
These findings stem from a first-ever systematic analysis of a large data set to “determine the relative contributions of inherited genetic variants and overall diet to variance in serum urate concentrations,” Tanya J. Major, PhD, of the University of Otago in Dunedin, New Zealand, and her colleagues first reported in The BMJ and then less than 2 weeks later at the annual meeting of the American College of Rheumatology.
Prior studies have estimated that genetic factors explain 25%-60% of the variability in serum urate levels, but diet also has long been considered a risk factor for gout, with certain diets such as the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet shown in studies to reduce serum urate levels and the risk of gout, the researchers noted.
The new study found that common genetic factors accounted for nearly 24% of the variation in serum urate levels, whereas diet accounted for less than 1%.
Before these findings, the impact of diet on hyperuricemia was known to be limited, but that doesn’t mean that diet is meaningless, Michael H. Pillinger, MD, who was not involved in the study, said in an interview at the ACR annual meeting.
“What this work does from a scientific point of view is underline the genetic basis [of hyperuricemia]. What it does from a practical and clinical point of view is tell us that diet is a meaningful thing to adjust. It will provide other health benefits, but it isn’t the solution, and we know that it rarely is,” said Dr. Pillinger, professor of medicine and biochemistry and molecular pharmacology at New York University. “We shouldn’t expect a really large impact from diet, and we shouldn’t make people feel guilty about their diets, and optimally you want to manage a diet that a patient can tolerate and not one that will be impossible to sustain.”
Dr. Major had a similar message in an interview at the meeting: “By focusing on diet and treating urate levels through diet, you start to kind of get this blame element going on that the reason you’ve got gout is you’re eating the wrong foods, and so then it makes people less likely to want to go to see their doctor to start with. And then when they do try to follow their doctor’s advice about their diet, and nothing changes, then it starts to get frustrating for them as well.”
The research team analyzed dietary survey data from five U.S. cohort studies (Atherosclerosis Risk in Communities, Coronary Artery Risk Development in [Young] Adults, Cardiovascular Heart Study, Framingham Heart Study, and Third National Health and Nutrition Examination Survey [NHANES III]) that altogether included 16,760 individuals (8,414 men and 8,346 women) of European ancestry.
Participants were aged 18 years or older, did not have gout or kidney disease, and were not taking urate-lowering or diuretic drugs. Serum urate measurements, dietary survey data, and confounders such as gender, age, body mass index, smoking status, and average daily calorie intake were all recorded.
The results showed that 15 foods were significantly associated with serum urate levels. Six were established urate-modifying foods: beer, liquor, wine, soft drinks, skim milk, and meat (beef, pork, or lamb). The remaining nine foods included two less established urate-modifying foods (cheese and noncitrus fruit) and seven food items without established associations: poultry, potatoes, brown bread, peanuts, margarine, cold cereal, and eggs.
Beer and liquor had the strongest urate-raising effect, associated with a 1.38 micromol/L increase in serum urate per serving per week, equating to a 9.66 micromol/L (0.16 mg/dL) increase per daily serving.
The authors noted that the associations seen with known and confirmed serum urate–influencing foods were consistent with previously reported associations in terms of effect and magnitude. Nevertheless, they noted that “each of these established foods explained less than 1% of variation in serum urate levels within the full cohort.”
When the researchers analyzed the data according to three diet scores based on healthy diet guidelines, they found that diet explained “very little variance in serum urate levels (0.28% for the DASH diet, 0.15% for the Healthy Eating diet, 0.06% for the Mediterranean diet, and 0.16% for the data-driven diet pattern).”
However, they noted that even though their findings, along with previous research, suggest that a clinically relevant decrease in serum urate levels could be achieved with the DASH diet, the implementation of such a diet might not be “straightforward” because “the barriers to implementing this diet both at a population level and in a primary care setting are yet to be overcome.”
In contrast, when the research team looked at genes in the cohort they found that common genetic factors explained 23.9% of variation in serum urate levels in the full cohort (excluding the NHANES III study because of a lack of genotype data).
Baseline visit diet information for these cohort studies ranged from the mid-1980s to the early to mid-2000s, making it hard to account for changes in food composition over time. Dr. Major said that food compositions overall were likely to be more processed over time and contain more urate-raising foods. However, any effects of food composition changes on urate levels over time would be more likely be felt at the level of individual food products, not on diets overall, she said.
The variability in the diet scores that the investigators noted across individuals and regions in these U.S. cohort studies did not appear to affect the results, Dr. Major noted. Furthermore, adjustment within models for these diet scores did not affect the degree of variation in urate levels that genetics accounted for, whereas adjustment for genetics lowered the amount of variation that diet contributed to urate levels.
The findings also don’t have any say on the relative contribution of diet versus genetics on gout flares, which is a separate issue. Many patients would agree that they suspect certain foods trigger gout flares even if they don’t affect baseline urate levels very much, Dr. Pillinger said.
The next steps for Dr. Major and her coinvestigators may be to see if diet is influencing urate levels to the same degree in people with gout as this study shows for the general population, she said.The investigators noted that the use of different food questionnaires between the five studies is one limitation of the analysis, and there should be some caution in generalizing the results to people with gout or those of non-European ancestry.
The study was supported by the Health Research Council of New Zealand and the University of Otago. One author reported receiving consulting and speaker fees or grants from several pharmaceutical companies that manufacture urate-lowering drugs.
This article was updated 10/25/18.
SOURCE: Major TJ et al. BMJ. 2018. doi: 10.1136/bmj.k3951.
CHICAGO – Diet plays a significantly less important role than genes in the development of high serum urate levels that typically precede gout, research from New Zealand has revealed.
These findings stem from a first-ever systematic analysis of a large data set to “determine the relative contributions of inherited genetic variants and overall diet to variance in serum urate concentrations,” Tanya J. Major, PhD, of the University of Otago in Dunedin, New Zealand, and her colleagues first reported in The BMJ and then less than 2 weeks later at the annual meeting of the American College of Rheumatology.
Prior studies have estimated that genetic factors explain 25%-60% of the variability in serum urate levels, but diet also has long been considered a risk factor for gout, with certain diets such as the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet shown in studies to reduce serum urate levels and the risk of gout, the researchers noted.
The new study found that common genetic factors accounted for nearly 24% of the variation in serum urate levels, whereas diet accounted for less than 1%.
Before these findings, the impact of diet on hyperuricemia was known to be limited, but that doesn’t mean that diet is meaningless, Michael H. Pillinger, MD, who was not involved in the study, said in an interview at the ACR annual meeting.
“What this work does from a scientific point of view is underline the genetic basis [of hyperuricemia]. What it does from a practical and clinical point of view is tell us that diet is a meaningful thing to adjust. It will provide other health benefits, but it isn’t the solution, and we know that it rarely is,” said Dr. Pillinger, professor of medicine and biochemistry and molecular pharmacology at New York University. “We shouldn’t expect a really large impact from diet, and we shouldn’t make people feel guilty about their diets, and optimally you want to manage a diet that a patient can tolerate and not one that will be impossible to sustain.”
Dr. Major had a similar message in an interview at the meeting: “By focusing on diet and treating urate levels through diet, you start to kind of get this blame element going on that the reason you’ve got gout is you’re eating the wrong foods, and so then it makes people less likely to want to go to see their doctor to start with. And then when they do try to follow their doctor’s advice about their diet, and nothing changes, then it starts to get frustrating for them as well.”
The research team analyzed dietary survey data from five U.S. cohort studies (Atherosclerosis Risk in Communities, Coronary Artery Risk Development in [Young] Adults, Cardiovascular Heart Study, Framingham Heart Study, and Third National Health and Nutrition Examination Survey [NHANES III]) that altogether included 16,760 individuals (8,414 men and 8,346 women) of European ancestry.
Participants were aged 18 years or older, did not have gout or kidney disease, and were not taking urate-lowering or diuretic drugs. Serum urate measurements, dietary survey data, and confounders such as gender, age, body mass index, smoking status, and average daily calorie intake were all recorded.
The results showed that 15 foods were significantly associated with serum urate levels. Six were established urate-modifying foods: beer, liquor, wine, soft drinks, skim milk, and meat (beef, pork, or lamb). The remaining nine foods included two less established urate-modifying foods (cheese and noncitrus fruit) and seven food items without established associations: poultry, potatoes, brown bread, peanuts, margarine, cold cereal, and eggs.
Beer and liquor had the strongest urate-raising effect, associated with a 1.38 micromol/L increase in serum urate per serving per week, equating to a 9.66 micromol/L (0.16 mg/dL) increase per daily serving.
The authors noted that the associations seen with known and confirmed serum urate–influencing foods were consistent with previously reported associations in terms of effect and magnitude. Nevertheless, they noted that “each of these established foods explained less than 1% of variation in serum urate levels within the full cohort.”
When the researchers analyzed the data according to three diet scores based on healthy diet guidelines, they found that diet explained “very little variance in serum urate levels (0.28% for the DASH diet, 0.15% for the Healthy Eating diet, 0.06% for the Mediterranean diet, and 0.16% for the data-driven diet pattern).”
However, they noted that even though their findings, along with previous research, suggest that a clinically relevant decrease in serum urate levels could be achieved with the DASH diet, the implementation of such a diet might not be “straightforward” because “the barriers to implementing this diet both at a population level and in a primary care setting are yet to be overcome.”
In contrast, when the research team looked at genes in the cohort they found that common genetic factors explained 23.9% of variation in serum urate levels in the full cohort (excluding the NHANES III study because of a lack of genotype data).
Baseline visit diet information for these cohort studies ranged from the mid-1980s to the early to mid-2000s, making it hard to account for changes in food composition over time. Dr. Major said that food compositions overall were likely to be more processed over time and contain more urate-raising foods. However, any effects of food composition changes on urate levels over time would be more likely be felt at the level of individual food products, not on diets overall, she said.
The variability in the diet scores that the investigators noted across individuals and regions in these U.S. cohort studies did not appear to affect the results, Dr. Major noted. Furthermore, adjustment within models for these diet scores did not affect the degree of variation in urate levels that genetics accounted for, whereas adjustment for genetics lowered the amount of variation that diet contributed to urate levels.
The findings also don’t have any say on the relative contribution of diet versus genetics on gout flares, which is a separate issue. Many patients would agree that they suspect certain foods trigger gout flares even if they don’t affect baseline urate levels very much, Dr. Pillinger said.
The next steps for Dr. Major and her coinvestigators may be to see if diet is influencing urate levels to the same degree in people with gout as this study shows for the general population, she said.The investigators noted that the use of different food questionnaires between the five studies is one limitation of the analysis, and there should be some caution in generalizing the results to people with gout or those of non-European ancestry.
The study was supported by the Health Research Council of New Zealand and the University of Otago. One author reported receiving consulting and speaker fees or grants from several pharmaceutical companies that manufacture urate-lowering drugs.
This article was updated 10/25/18.
SOURCE: Major TJ et al. BMJ. 2018. doi: 10.1136/bmj.k3951.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Diet explained less than 1% of variation in urate levels, whereas common genetic factors explained 23.9% of the variation in urate levels.
Study details: A meta-analysis of five U.S. cohort studies that included dietary survey data from 16,760 people of European ancestry.
Disclosures: The study was supported by the Health Research Council of New Zealand and the University of Otago. One author reported receiving consulting and speaker fees or grants from several pharmaceutical companies that manufacture urate-lowering drugs.
Source: Major T et al. BMJ. 2018. doi: 10.1136/bmj.k3951.
Updated analysis from JAVELIN Renal 101 to be presented at ESMO 2018
An updated analysis of interim results for JAVELIN Renal 101 will be presented at a Presidential Symposium during the annual congress of the European Society for Medical Oncology (ESMO 2018), to be held Oct. 19-23 in Munich.
The phase 3 trial compared avelumab (Bavencio) in combination with axitinib (Inlyta) to sunitinib monotherapy as first-line treatment, in patients with advanced renal cell carcinoma (RCC). Interim analysis results announced by the company in a September press release indicated the combination of immunotherapy and a tyrosine kinase inhibitor showed “a statistically significant improvement in progression-free survival by central review for patients treated with the combination whose tumors had programmed death ligand-1‒positive (PD-L1+) expression greater than 1% (primary objective), as well as in the entire study population regardless of PD-L1 tumor expression (secondary objective).”
An updated analysis of progression-free survival and overall response rates will be presented at ESMO 2018 by Robert J. Motzer, MD of Memorial Sloan Kettering Cancer Center, New York.
A phase 1b study (JAVELIN Renal 100), published in Lancet Oncology, found the safety profile of the combination to be similar to either drug alone.
For the phase 3 trial, more than 800 patients with advanced RCC were randomized to first-line treatment with the combination of avelumab (10 mg/kg IV every 2 weeks) plus axitinib (5 mg orally, twice daily) or monotherapy with sunitinib (50 mg orally once daily, 4 weeks on/2 weeks off). The overall survival results will be presented at the Presidential Symposium 2 on Oct. 21.
This article was updated on 10/15/18 to reflect the fact that interim results will be presented.
An updated analysis of interim results for JAVELIN Renal 101 will be presented at a Presidential Symposium during the annual congress of the European Society for Medical Oncology (ESMO 2018), to be held Oct. 19-23 in Munich.
The phase 3 trial compared avelumab (Bavencio) in combination with axitinib (Inlyta) to sunitinib monotherapy as first-line treatment, in patients with advanced renal cell carcinoma (RCC). Interim analysis results announced by the company in a September press release indicated the combination of immunotherapy and a tyrosine kinase inhibitor showed “a statistically significant improvement in progression-free survival by central review for patients treated with the combination whose tumors had programmed death ligand-1‒positive (PD-L1+) expression greater than 1% (primary objective), as well as in the entire study population regardless of PD-L1 tumor expression (secondary objective).”
An updated analysis of progression-free survival and overall response rates will be presented at ESMO 2018 by Robert J. Motzer, MD of Memorial Sloan Kettering Cancer Center, New York.
A phase 1b study (JAVELIN Renal 100), published in Lancet Oncology, found the safety profile of the combination to be similar to either drug alone.
For the phase 3 trial, more than 800 patients with advanced RCC were randomized to first-line treatment with the combination of avelumab (10 mg/kg IV every 2 weeks) plus axitinib (5 mg orally, twice daily) or monotherapy with sunitinib (50 mg orally once daily, 4 weeks on/2 weeks off). The overall survival results will be presented at the Presidential Symposium 2 on Oct. 21.
This article was updated on 10/15/18 to reflect the fact that interim results will be presented.
An updated analysis of interim results for JAVELIN Renal 101 will be presented at a Presidential Symposium during the annual congress of the European Society for Medical Oncology (ESMO 2018), to be held Oct. 19-23 in Munich.
The phase 3 trial compared avelumab (Bavencio) in combination with axitinib (Inlyta) to sunitinib monotherapy as first-line treatment, in patients with advanced renal cell carcinoma (RCC). Interim analysis results announced by the company in a September press release indicated the combination of immunotherapy and a tyrosine kinase inhibitor showed “a statistically significant improvement in progression-free survival by central review for patients treated with the combination whose tumors had programmed death ligand-1‒positive (PD-L1+) expression greater than 1% (primary objective), as well as in the entire study population regardless of PD-L1 tumor expression (secondary objective).”
An updated analysis of progression-free survival and overall response rates will be presented at ESMO 2018 by Robert J. Motzer, MD of Memorial Sloan Kettering Cancer Center, New York.
A phase 1b study (JAVELIN Renal 100), published in Lancet Oncology, found the safety profile of the combination to be similar to either drug alone.
For the phase 3 trial, more than 800 patients with advanced RCC were randomized to first-line treatment with the combination of avelumab (10 mg/kg IV every 2 weeks) plus axitinib (5 mg orally, twice daily) or monotherapy with sunitinib (50 mg orally once daily, 4 weeks on/2 weeks off). The overall survival results will be presented at the Presidential Symposium 2 on Oct. 21.
This article was updated on 10/15/18 to reflect the fact that interim results will be presented.
Entospletinib falls short in relapsed/refractory DLBCL
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The rate of progression-free survival at 16 weeks was 3.6% with a median PFS of 1.5 months.
Study details: An analysis of 43 relapsed/refractory DLBCL patients who received single-agent entospletinib.
Disclosures: The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
Source: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Level of Serum Neurofilament Light Enables Treatment Monitoring
The biomarker indicates disease severity and predicts clinical and imaging outcomes.
BERLIN—Serum neurofilament light chain (sNfL) levels are clinically relevant, and data suggest cut points to enable disease severity stratification and treatment monitoring in patients with relapsing-remitting multiple sclerosis (MS), according to research described at ECTRIMS 2018.
Various investigations have indicated that sNfL is associated with disease activity and predicts long-term clinical and imaging outcomes in patients with relapsing-remitting MS. Disease-modifying treatments (DMTs) reduce sNfL levels in these patients. “The integration of sNfL into clinical practice will require a standardized, validated assay and defined, clinically meaningful cut points validated with real-world data,” said Peter Calabresi, MD, Director of the Division of Neuroimmunology at Johns Hopkins Medicine in Baltimore, and colleagues.
Dr. Calabresi and colleagues conducted a study to define the sNfL levels relevant to disease severity stratification and treatment monitoring in patients with relapsing-remitting MS using samples and data from phase III clinical studies supported by Biogen. They measured sNfL with a single-molecule array Advantage kit or laboratory method in serial samples from more than 1,000 patients enrolled in four studies.
In the ADVANCE trial (which examined peginterferon beta-1a in 594 patients with relapsing-remitting MS), sNfL was measured at baseline, every three months until Year 2, and then every six months until Year 4. In the CHAMPS trial (which analyzed interferon beta 1a in 319 patients with clinically isolated syndrome), sNfL was measured at baseline and Week 48. In the MSCRG study (which examined interferon beta 1a in 164 patients with relapsing-remitting MS), sNfL was measured at Years 3 and 4. In SENTINEL (which examined natalizumab in 122 patients with relapsing-remitting MS), sNfL was measured at baseline and Week 96. Statistical analyses included Spearman correlation, analysis of variance, chi-squared test, Kaplan-Meier analysis, and multivariate logistic regression.
Baseline sNfL levels were associated with the number of enhancing lesions and accumulation of new T2 lesions over time. Patients with no evident disease activity had consistently low sNfL levels, but those with active disease, especially with high brain atrophy rates, had elevated sNfL levels. Dr. Calabresi’s group found that an sNfL level greater than 16 pg/mL indicated a high probability of disease activity over the following year (positive predictive values of 92% and 95% in test and verification cohorts, respectively). Using the average of sNfL levels at baseline and Months 3 and 6 further improved the positive predictive value. Similarly, an sNfL level greater than 16 pg/mL was associated long-term with worse clinical and imaging outcomes, including progression of Expanded Disability Status Scale score (12 years), increase in T2 lesion volume (10 years), and brain atrophy (five years). DMTs lowered sNfL levels. Natalizumab reduced sNfL below 16 pg/mL in 96% of patients.
The study was sponsored by Biogen.
The biomarker indicates disease severity and predicts clinical and imaging outcomes.
The biomarker indicates disease severity and predicts clinical and imaging outcomes.
BERLIN—Serum neurofilament light chain (sNfL) levels are clinically relevant, and data suggest cut points to enable disease severity stratification and treatment monitoring in patients with relapsing-remitting multiple sclerosis (MS), according to research described at ECTRIMS 2018.
Various investigations have indicated that sNfL is associated with disease activity and predicts long-term clinical and imaging outcomes in patients with relapsing-remitting MS. Disease-modifying treatments (DMTs) reduce sNfL levels in these patients. “The integration of sNfL into clinical practice will require a standardized, validated assay and defined, clinically meaningful cut points validated with real-world data,” said Peter Calabresi, MD, Director of the Division of Neuroimmunology at Johns Hopkins Medicine in Baltimore, and colleagues.
Dr. Calabresi and colleagues conducted a study to define the sNfL levels relevant to disease severity stratification and treatment monitoring in patients with relapsing-remitting MS using samples and data from phase III clinical studies supported by Biogen. They measured sNfL with a single-molecule array Advantage kit or laboratory method in serial samples from more than 1,000 patients enrolled in four studies.
In the ADVANCE trial (which examined peginterferon beta-1a in 594 patients with relapsing-remitting MS), sNfL was measured at baseline, every three months until Year 2, and then every six months until Year 4. In the CHAMPS trial (which analyzed interferon beta 1a in 319 patients with clinically isolated syndrome), sNfL was measured at baseline and Week 48. In the MSCRG study (which examined interferon beta 1a in 164 patients with relapsing-remitting MS), sNfL was measured at Years 3 and 4. In SENTINEL (which examined natalizumab in 122 patients with relapsing-remitting MS), sNfL was measured at baseline and Week 96. Statistical analyses included Spearman correlation, analysis of variance, chi-squared test, Kaplan-Meier analysis, and multivariate logistic regression.
Baseline sNfL levels were associated with the number of enhancing lesions and accumulation of new T2 lesions over time. Patients with no evident disease activity had consistently low sNfL levels, but those with active disease, especially with high brain atrophy rates, had elevated sNfL levels. Dr. Calabresi’s group found that an sNfL level greater than 16 pg/mL indicated a high probability of disease activity over the following year (positive predictive values of 92% and 95% in test and verification cohorts, respectively). Using the average of sNfL levels at baseline and Months 3 and 6 further improved the positive predictive value. Similarly, an sNfL level greater than 16 pg/mL was associated long-term with worse clinical and imaging outcomes, including progression of Expanded Disability Status Scale score (12 years), increase in T2 lesion volume (10 years), and brain atrophy (five years). DMTs lowered sNfL levels. Natalizumab reduced sNfL below 16 pg/mL in 96% of patients.
The study was sponsored by Biogen.
BERLIN—Serum neurofilament light chain (sNfL) levels are clinically relevant, and data suggest cut points to enable disease severity stratification and treatment monitoring in patients with relapsing-remitting multiple sclerosis (MS), according to research described at ECTRIMS 2018.
Various investigations have indicated that sNfL is associated with disease activity and predicts long-term clinical and imaging outcomes in patients with relapsing-remitting MS. Disease-modifying treatments (DMTs) reduce sNfL levels in these patients. “The integration of sNfL into clinical practice will require a standardized, validated assay and defined, clinically meaningful cut points validated with real-world data,” said Peter Calabresi, MD, Director of the Division of Neuroimmunology at Johns Hopkins Medicine in Baltimore, and colleagues.
Dr. Calabresi and colleagues conducted a study to define the sNfL levels relevant to disease severity stratification and treatment monitoring in patients with relapsing-remitting MS using samples and data from phase III clinical studies supported by Biogen. They measured sNfL with a single-molecule array Advantage kit or laboratory method in serial samples from more than 1,000 patients enrolled in four studies.
In the ADVANCE trial (which examined peginterferon beta-1a in 594 patients with relapsing-remitting MS), sNfL was measured at baseline, every three months until Year 2, and then every six months until Year 4. In the CHAMPS trial (which analyzed interferon beta 1a in 319 patients with clinically isolated syndrome), sNfL was measured at baseline and Week 48. In the MSCRG study (which examined interferon beta 1a in 164 patients with relapsing-remitting MS), sNfL was measured at Years 3 and 4. In SENTINEL (which examined natalizumab in 122 patients with relapsing-remitting MS), sNfL was measured at baseline and Week 96. Statistical analyses included Spearman correlation, analysis of variance, chi-squared test, Kaplan-Meier analysis, and multivariate logistic regression.
Baseline sNfL levels were associated with the number of enhancing lesions and accumulation of new T2 lesions over time. Patients with no evident disease activity had consistently low sNfL levels, but those with active disease, especially with high brain atrophy rates, had elevated sNfL levels. Dr. Calabresi’s group found that an sNfL level greater than 16 pg/mL indicated a high probability of disease activity over the following year (positive predictive values of 92% and 95% in test and verification cohorts, respectively). Using the average of sNfL levels at baseline and Months 3 and 6 further improved the positive predictive value. Similarly, an sNfL level greater than 16 pg/mL was associated long-term with worse clinical and imaging outcomes, including progression of Expanded Disability Status Scale score (12 years), increase in T2 lesion volume (10 years), and brain atrophy (five years). DMTs lowered sNfL levels. Natalizumab reduced sNfL below 16 pg/mL in 96% of patients.
The study was sponsored by Biogen.
COPD: Triple trumps dual therapy regardless of baseline reversibility
SAN ANTONIO – Regardless of COPD patients’ bronchodilator reversibility at baseline, versus dual therapies, according to a recent retrospective analysis of a randomized, double-blind study.
FF/UMEC/VI, a triple-therapy combination of an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist (ICS/LAMA/LABA), was superior to both LAMA/LABA and ICS/LABA combinations in reducing the rate of moderate to severe exacerbation and lung function, the analysis showed.
The ICS/LAMA/LABA combination, compared with LAMA/LABA, also significantly reduced the rate of severe exacerbations and time to first moderate to severe exacerbations in both reversible and nonreversible patients, Robert Wise, MD, FCCP, of Johns Hopkins University, Baltimore, said at the annual meeting of the American College of Chest Physicians.
The analysis was based on data from IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
“The results across both reversibility subgroups are consistent with those observed in the intention-to-treat or overall study population and show a similar benefit-to-risk profile of the triple therapy across different subtypes based on bronchodilator reversibility,” Dr. Wise told attendees in a podium presentation.
Reversibility was defined as a difference between pre- and postalbuterol assessment of FEV1 of equal to or greater than 12% and equal to or greater than 200 mL at screening, Dr. Wise said.
Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction, according to data reported in the meeting abstract.
Severe exacerbation rates dropped by 44% and 31%, respectively, in the reversible and nonreversible patients for triple versus dual therapy, he added.
Triple therapy reduced time to first moderate to severe exacerbation versus dual therapy by 25.6% in reversible and 13.6% in nonreversible COPD patients, the data showed.
The FF/UMEC/VI combination also demonstrated improvements versus UMEC/VI in time to first severe exacerbation for both the reversible and nonreversible groups, as well as improved quality of life in both groups as measured by the St. George Respiratory Questionnaire (SGRQ) in both groups.
Results were somewhat different when comparing the FF/UMEC/VI combination with the FF/VI – the ICS/LABA combination – in this post hoc analysis.
Triple therapy did reduce moderate to severe exacerbations and improved lung function regardless of baseline reversibility. However, for the reversible patients, ICS/LAMA/LABA versus ICS/LABA did not significantly reduce risk specifically of severe exacerbations, time to first moderate to severe exacerbation, or increase odds of being an SGRQ responder, Dr. Wise said.
Nonetheless, these findings taken together imply that this ICS/LAMA/LABA combination provides clinically relevant improvements versus dual therapy across a range of important outcomes regardless of baseline reversibility, according to Dr. Wise and colleagues.
Dr. Wise and coinvestigators provided disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
SOURCE: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j.chest.2018.08.662
SAN ANTONIO – Regardless of COPD patients’ bronchodilator reversibility at baseline, versus dual therapies, according to a recent retrospective analysis of a randomized, double-blind study.
FF/UMEC/VI, a triple-therapy combination of an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist (ICS/LAMA/LABA), was superior to both LAMA/LABA and ICS/LABA combinations in reducing the rate of moderate to severe exacerbation and lung function, the analysis showed.
The ICS/LAMA/LABA combination, compared with LAMA/LABA, also significantly reduced the rate of severe exacerbations and time to first moderate to severe exacerbations in both reversible and nonreversible patients, Robert Wise, MD, FCCP, of Johns Hopkins University, Baltimore, said at the annual meeting of the American College of Chest Physicians.
The analysis was based on data from IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
“The results across both reversibility subgroups are consistent with those observed in the intention-to-treat or overall study population and show a similar benefit-to-risk profile of the triple therapy across different subtypes based on bronchodilator reversibility,” Dr. Wise told attendees in a podium presentation.
Reversibility was defined as a difference between pre- and postalbuterol assessment of FEV1 of equal to or greater than 12% and equal to or greater than 200 mL at screening, Dr. Wise said.
Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction, according to data reported in the meeting abstract.
Severe exacerbation rates dropped by 44% and 31%, respectively, in the reversible and nonreversible patients for triple versus dual therapy, he added.
Triple therapy reduced time to first moderate to severe exacerbation versus dual therapy by 25.6% in reversible and 13.6% in nonreversible COPD patients, the data showed.
The FF/UMEC/VI combination also demonstrated improvements versus UMEC/VI in time to first severe exacerbation for both the reversible and nonreversible groups, as well as improved quality of life in both groups as measured by the St. George Respiratory Questionnaire (SGRQ) in both groups.
Results were somewhat different when comparing the FF/UMEC/VI combination with the FF/VI – the ICS/LABA combination – in this post hoc analysis.
Triple therapy did reduce moderate to severe exacerbations and improved lung function regardless of baseline reversibility. However, for the reversible patients, ICS/LAMA/LABA versus ICS/LABA did not significantly reduce risk specifically of severe exacerbations, time to first moderate to severe exacerbation, or increase odds of being an SGRQ responder, Dr. Wise said.
Nonetheless, these findings taken together imply that this ICS/LAMA/LABA combination provides clinically relevant improvements versus dual therapy across a range of important outcomes regardless of baseline reversibility, according to Dr. Wise and colleagues.
Dr. Wise and coinvestigators provided disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
SOURCE: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j.chest.2018.08.662
SAN ANTONIO – Regardless of COPD patients’ bronchodilator reversibility at baseline, versus dual therapies, according to a recent retrospective analysis of a randomized, double-blind study.
FF/UMEC/VI, a triple-therapy combination of an inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist (ICS/LAMA/LABA), was superior to both LAMA/LABA and ICS/LABA combinations in reducing the rate of moderate to severe exacerbation and lung function, the analysis showed.
The ICS/LAMA/LABA combination, compared with LAMA/LABA, also significantly reduced the rate of severe exacerbations and time to first moderate to severe exacerbations in both reversible and nonreversible patients, Robert Wise, MD, FCCP, of Johns Hopkins University, Baltimore, said at the annual meeting of the American College of Chest Physicians.
The analysis was based on data from IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
“The results across both reversibility subgroups are consistent with those observed in the intention-to-treat or overall study population and show a similar benefit-to-risk profile of the triple therapy across different subtypes based on bronchodilator reversibility,” Dr. Wise told attendees in a podium presentation.
Reversibility was defined as a difference between pre- and postalbuterol assessment of FEV1 of equal to or greater than 12% and equal to or greater than 200 mL at screening, Dr. Wise said.
Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction, according to data reported in the meeting abstract.
Severe exacerbation rates dropped by 44% and 31%, respectively, in the reversible and nonreversible patients for triple versus dual therapy, he added.
Triple therapy reduced time to first moderate to severe exacerbation versus dual therapy by 25.6% in reversible and 13.6% in nonreversible COPD patients, the data showed.
The FF/UMEC/VI combination also demonstrated improvements versus UMEC/VI in time to first severe exacerbation for both the reversible and nonreversible groups, as well as improved quality of life in both groups as measured by the St. George Respiratory Questionnaire (SGRQ) in both groups.
Results were somewhat different when comparing the FF/UMEC/VI combination with the FF/VI – the ICS/LABA combination – in this post hoc analysis.
Triple therapy did reduce moderate to severe exacerbations and improved lung function regardless of baseline reversibility. However, for the reversible patients, ICS/LAMA/LABA versus ICS/LABA did not significantly reduce risk specifically of severe exacerbations, time to first moderate to severe exacerbation, or increase odds of being an SGRQ responder, Dr. Wise said.
Nonetheless, these findings taken together imply that this ICS/LAMA/LABA combination provides clinically relevant improvements versus dual therapy across a range of important outcomes regardless of baseline reversibility, according to Dr. Wise and colleagues.
Dr. Wise and coinvestigators provided disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
SOURCE: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j.chest.2018.08.662
REPORTING FROM CHEST 2018
Key clinical point: Triple therapy with fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) is superior to UMEC/VI in COPD patients regardless of baseline bronchodilator reversibility.
Major finding: Reversible patients had a 40% reduction in the rate of moderate to severe exacerbations for FF/UMEC/VI versus UMEC/VI, while nonreversible patients had a 21% reduction.
Study details: Retrospective analysis of IMPACT, an international, randomized, 52-week study that included more than 10,000 patients with symptomatic COPD, of whom 18% demonstrated reversibility at screening.
Disclosures: Study authors reported disclosures related to Boehringer Ingelheim, BTG, Chiesi, GlaxoSmithKline, Mereo, Novartis, PneumRx, Prometic, and Pulmonx.
Source: Wise R et al. Chest. 2018 Oct. doi: 10.1016/j/chest.2018.08.662.
Should return to fertility be a concern for nulliparous patients using an IUD?
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1
“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
ICU infections: Chlorhexidine wipes tame MRSA, CRE
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
REPORTING FROM ID WEEK 2018
Key clinical point: For high rates of MRSA and CRE in the ICU, consider decolonization instead of contact precautions.
Major finding: Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6; infection rates fell from 3.9 isolates to 2 per 10,000 patient-days.
Study details: Review of ICU quality improvement initiative
Disclosures: There was no funding for the work, and the investigators had no disclosures.
Source: Moss J et al. ID Week 2018, Abstract 32.