User login
In utero efavirenz, dolutegravir exposure linked to childhood neurologic problems
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
REPORTING FROM ID WEEK 2018
Soccer or Football Medicine? Global Sports Medicine for a Global Game
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
It’s time for universal CMV screening at birth
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
REPORTING FROM IDWEEK 2018
Opiate use tied to hepatitis C risk in youth
SAN FRANCISCO – A new study indicates young adults with opioid use disorder are seldom screened for hepatitis C virus infections; yet 11% of the subjects with opioid use disorder who were tested had been exposed to hepatitis C, and 6.8% had evidence of chronic hepatitis C infection.
Overall, 2.5% (6,812 subjects) of all subjects received hepatitis C testing and 122 (1.8%) tested positive. Based on health records, 23,345 had an ICD-9 code for any illicit drug use and 8.9% of those (2,090) were tested for HCV infection. Of the 933 subjects with an ICD-9 code for opioid use disorder, 35% were tested for HCV.
The results suggest that a group at significant risk of hepatitis C – those with opioid use disorder – is being overlooked in public health efforts to control the disease.
Clinicians may presume, “Oh, you just take opioids orally, you don’t inject drugs,” but oral opiate users can progress to intravenous drug use, Donna Futterman, MD, director of clinical pediatrics, Montefiore Medical Center, and professor of clinical pediatrics at Albert Einstein College of Medicine, both in New York, said during the press conference at the annual scientific meeting on infectious diseases.
Guidelines call for testing for hepatitis C only in individuals with known injected drug use, among other risk factors, but the research suggests that this significantly underestimates the population of teenagers and young adults who are at risk. Many who take opiates go on to use injectable drugs.
Another surprise finding in the study was that only 10.6% of those tested for hepatitis C had also been screened for human immunodeficiency virus (HIV).
The reasons for the low frequency of screening are likely complex, including lack of time, discomfort between the physician and patient, and concerns over privacy and stigma, according to Dr. Epstein, who emphasized the importance of communication to overcome such barriers.
“As a pediatrician, I try to be as open as possible with patients and let them know that anything they tell me is confidential. I start out discussing less private issues, things that are easier to talk about,” Dr. Epstein said.
But the results of the study also suggest that preconceived notions may be holding clinicians back from testing. “How can you test for hepatitis C and not think HIV?” Dr. Futterman said. “What is that differentiator in providers’ heads that makes them focus on one thing and not the other?”
SAN FRANCISCO – A new study indicates young adults with opioid use disorder are seldom screened for hepatitis C virus infections; yet 11% of the subjects with opioid use disorder who were tested had been exposed to hepatitis C, and 6.8% had evidence of chronic hepatitis C infection.
Overall, 2.5% (6,812 subjects) of all subjects received hepatitis C testing and 122 (1.8%) tested positive. Based on health records, 23,345 had an ICD-9 code for any illicit drug use and 8.9% of those (2,090) were tested for HCV infection. Of the 933 subjects with an ICD-9 code for opioid use disorder, 35% were tested for HCV.
The results suggest that a group at significant risk of hepatitis C – those with opioid use disorder – is being overlooked in public health efforts to control the disease.
Clinicians may presume, “Oh, you just take opioids orally, you don’t inject drugs,” but oral opiate users can progress to intravenous drug use, Donna Futterman, MD, director of clinical pediatrics, Montefiore Medical Center, and professor of clinical pediatrics at Albert Einstein College of Medicine, both in New York, said during the press conference at the annual scientific meeting on infectious diseases.
Guidelines call for testing for hepatitis C only in individuals with known injected drug use, among other risk factors, but the research suggests that this significantly underestimates the population of teenagers and young adults who are at risk. Many who take opiates go on to use injectable drugs.
Another surprise finding in the study was that only 10.6% of those tested for hepatitis C had also been screened for human immunodeficiency virus (HIV).
The reasons for the low frequency of screening are likely complex, including lack of time, discomfort between the physician and patient, and concerns over privacy and stigma, according to Dr. Epstein, who emphasized the importance of communication to overcome such barriers.
“As a pediatrician, I try to be as open as possible with patients and let them know that anything they tell me is confidential. I start out discussing less private issues, things that are easier to talk about,” Dr. Epstein said.
But the results of the study also suggest that preconceived notions may be holding clinicians back from testing. “How can you test for hepatitis C and not think HIV?” Dr. Futterman said. “What is that differentiator in providers’ heads that makes them focus on one thing and not the other?”
SAN FRANCISCO – A new study indicates young adults with opioid use disorder are seldom screened for hepatitis C virus infections; yet 11% of the subjects with opioid use disorder who were tested had been exposed to hepatitis C, and 6.8% had evidence of chronic hepatitis C infection.
Overall, 2.5% (6,812 subjects) of all subjects received hepatitis C testing and 122 (1.8%) tested positive. Based on health records, 23,345 had an ICD-9 code for any illicit drug use and 8.9% of those (2,090) were tested for HCV infection. Of the 933 subjects with an ICD-9 code for opioid use disorder, 35% were tested for HCV.
The results suggest that a group at significant risk of hepatitis C – those with opioid use disorder – is being overlooked in public health efforts to control the disease.
Clinicians may presume, “Oh, you just take opioids orally, you don’t inject drugs,” but oral opiate users can progress to intravenous drug use, Donna Futterman, MD, director of clinical pediatrics, Montefiore Medical Center, and professor of clinical pediatrics at Albert Einstein College of Medicine, both in New York, said during the press conference at the annual scientific meeting on infectious diseases.
Guidelines call for testing for hepatitis C only in individuals with known injected drug use, among other risk factors, but the research suggests that this significantly underestimates the population of teenagers and young adults who are at risk. Many who take opiates go on to use injectable drugs.
Another surprise finding in the study was that only 10.6% of those tested for hepatitis C had also been screened for human immunodeficiency virus (HIV).
The reasons for the low frequency of screening are likely complex, including lack of time, discomfort between the physician and patient, and concerns over privacy and stigma, according to Dr. Epstein, who emphasized the importance of communication to overcome such barriers.
“As a pediatrician, I try to be as open as possible with patients and let them know that anything they tell me is confidential. I start out discussing less private issues, things that are easier to talk about,” Dr. Epstein said.
But the results of the study also suggest that preconceived notions may be holding clinicians back from testing. “How can you test for hepatitis C and not think HIV?” Dr. Futterman said. “What is that differentiator in providers’ heads that makes them focus on one thing and not the other?”
REPORTING FROM ID WEEK 2018
Key clinical point: By focusing solely on injectable drug users, clinicians may miss many others who are at risk for hepatitis C infection.
Major finding: Among those with opiate use disorder, 11% tested positive for hepatitis C.
Study details: Survey of 269,124 teenagers and young adults visiting U.S. Federally Qualified Health Centers.
Disclosures: Dr. Epstein and Dr. Futterman have reported no conflicts of interest.
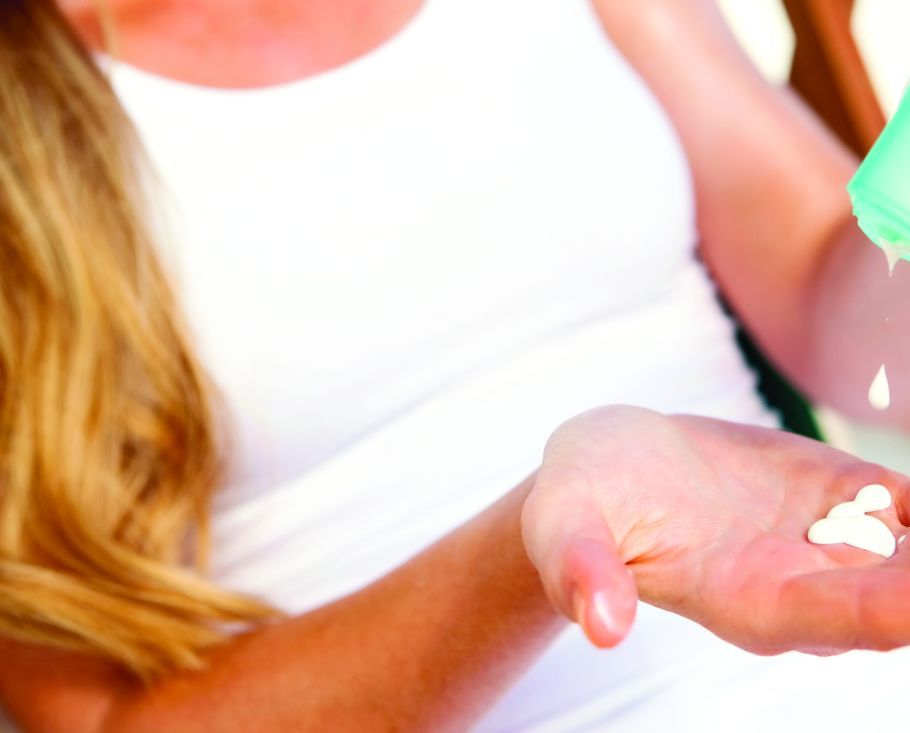
Sunscreens: Misleading labels, poor performance, and hype about their risks
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – Heads up! “Natural” mineral-based sunscreens don’t provide the protection of their rivals. Patients may get burned by scary hype about the supposed dangers of sunscreen. And sunscreen spray is great for the scalp of people whose hair is thinning.
In a presentation on sunscreens at the annual Coastal Dermatology Symposium, Vincent DeLeo, MD, of the University of Southern California, Los Angeles, offered the following tips on sunscreen and more.
Here’s a roundup of his pearls:
Sunscreens are getting better and are faring poorly, too.
Dr. DeLeo said. A 2013 comparison of sunscreens in 1997 and 2009 found that, among available sunscreens, the percentage of those with low SPF (under 15) fell from 27% to 6% during that time. (The Food and Drug Administration declared in 2011 that manufacturers must tell consumers that low SPF and/or non–broad spectrum sunscreens protect only against sunburn, not against skin cancer or skin aging.) The study also found that the percentage of sunscreens with UVA-1 (such as avobenzone or zinc oxide) filters grew from 5% to 70% (Photochem Photobiol Sci. 2013 Jan;12[1]:197-202).
But the label of sunscreens may not always be accurate. Earlier this year, Consumer Reports wrote that 36 of 73 sunscreens tested failed to correctly list their SPF protection level; 23 sunscreens missed their listed SPF levels by more than half. “Natural” or “mineral-only” sunscreens, which rely on such blockers as zinc oxide or titanium dioxide, performed the worst. Some patients prefer to use these sunscreens because they aren’t chemical based, and “may want to have a more natural sunscreen,” Dr. DeLeo said. “But they should be aware the sunscreens don’t always live up to the SPF level on the label.”
Beware of warnings about sunscreens.
Reports have warned Americans about supposed risks of sunscreen use such as low vitamin D levels from the lack of sun exposure, the exposure to titanium dioxide and zinc oxide nanoparticles, and the exposure to retinyl palmitate in sunscreen. Hawaiian officials, meanwhile, are banning some types of sunscreen chemicals in order to protect coral reefs.
Typical use of sunscreen will not dangerously lower vitamin D levels, Dr. DeLeo said, but people who use it every day may want to be cautious. He dismissed the concerns about nanoparticles and retinyl palmitate.
Dr. DeLeo said two sunscreen risks are real; sunscreens can trigger irritation, at a rate as high as 20%, and, rarely, allergic reactions, as well.
American sunscreens don’t stack up worldwide.
Simplicity often is a virtue. But, Dr. DeLeo said, it’s not helpful when it comes to the components of American sunscreens.
U.S. regulations only allow 16 ingredients in sunscreen while several more are allowed in Europe, he said. According to him, this helps explain why European sunscreens do a better job. European sunscreens “are much more absorbent, much better at absorbing radiation than the U.S. sunscreens,” he said. “It’s because we don’t have the same products as they have in Europe.”
The good news, he said, is that the FDA is considering expanding the number of ingredients allowed in sunscreen. The Sunscreen Innovation Act of 2014, a law passed by Congress, allows the FDA to use efficacy and safety data from Europe without requiring manufacturers to launch new, multimillion dollar tests, he said.
That’s good news for companies that want to improve U.S. sunscreens by selling a wider variety of types. “Sooner or later,” he said, “we will probably get these.”
Sunscreen sprays are tops at scalp protection.
Sunscreen sprays shouldn’t be applied to the face in children, Dr. DeLeo said, but they’re great for solo people because they facilitate protecting the back when there isn’t someone around to help them apply topical sunscreen.
How much spray should people use? A lot, he said. He added that sunscreen sprays are especially useful for the scalps of people with thinning hair.
Dr. DeLeo disclosed consulting work for Estée Lauder.
The meeting is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
REPORTING FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Some mutation testing can be useful at CLL diagnosis
CHICAGO – A number of mutation tests – including immunoglobulin heavy chain gene (IgVH), fluorescence in situ hybridization (FISH), and TP53 – provide useful prognostic information at the time of chronic lymphocytic leukemia (CLL) diagnosis, according to Paul M. Barr, MD.
“It’s understood that IgVH mutation status is certainly prognostic,” Dr. Barr, associate professor of hematology/oncology at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
The B-cell receptor of the CLL cells uses IgVH genes that may or may not have undergone somatic mutations, with unmutated being defined as 98% or more sequence homology to germline.
“This is indicative of stronger signaling through the B-cell receptor and, as we all know, predicts for an inferior prognosis,” he explained, citing a study that demonstrated superior survival rates with mutated IgVH genes (Blood. 1999;94[6]:1840-7).
“It’s also well understood and accepted that we should perform a FISH panel; we should look for interphase cytogenetics based on FISH in our patients,” Dr. Barr said. “Having said that, we, as medical oncologists, do not do a very good job of using this testing appropriately. Patterns of care studies have suggested that a significant number of patients don’t get FISH testing at diagnosis or before first-line therapy.”
In fact, a typical interphase FISH panel identifies cytogenetic lesions, including del(17p), del(11q), del(13q), and trisomy 12 in more than 80% of CLL cases, with del(13q) being the most common.
Another marker that can be assessed in CLL patients and has maintained prognostic value across multiple analyses is serum beta-2 microglobulin, Dr. Barr noted.
TP53 sequencing is valuable as well and has been associated with outcomes similar to those seen in patients with del(17p), he said, citing data from a study that found similarly poor outcomes with TP53 mutations or deletions and del(17p), even when minor subclones are identified using next-generation sequencing (Blood. 2014;123:2139-47).
“One of the primary reasons for this is that the two aberrations go together. Most often, if you have del(17p) you’re also going to find a TP53 mutation, but in about 30% of patients or so, only one allele is affected, so this is why it’s still important to test for TP53 mutations when you’re looking for a 17p deletion,” he said.
Numerous other recurrent mutations in CLL have been associated with poor overall survival and/or progression-free survival, including SF3B1, ATM, NOTCH1, POT1, BIRC3, and NFKBIE.
“The gut instinct is that maybe we should start testing for all of these mutations now, but I would caution everybody that we still need further validation before we can apply these to the majority of patients,” Dr. Barr said. “We still don’t know exactly what to do with all of these mutations – when and how often we should test for them, if the novel agents are truly better – so while, again, they can predict for inferior outcomes, I would say these are not yet standard of care to be tested in all patients.”
It is likely, though, that new prognostic systems will evolve as more is learned about how to use these molecular aberrations. Attempts are already being made to incorporate novel mutations into a prognostic system. Dr. Barr pointed to a report that looked at the integration of mutations and cytogenetic lesions to improve the accuracy of survival prediction in CLL (Blood. 2013;121:1403-12).
“But this still requires prospective testing, especially in patients getting the novel agents,” he said.
Conventional karyotyping also has potential, though a limited role in this setting, he said, noting that it can be reliably performed with stimulation of CLL cells.
“We also know additional aberrations are prognostic and that a complex karyotype predicts for a very poor outcome,” he said. The International Workshop on CLL (iwCLL) guidelines, which were recently updated for the first time in a decade, state that further validation is needed.
“I think it’s potentially useful in a very young patient you are considering taking to transplant, but again, I agree with the stance that this is not something that should be performed in every patient across the board,” he said.
The tests currently recommended by iwCLL before CLL treatment include IgVH mutation status; FISH for del(13q), del(11q), del(17p), and trisomy 12 in peripheral blood lymphocytes; and TP53.
“Some folks... don’t check a lot of these markers at diagnosis, but wait for patients to require therapy, and that’s a reasonable way to practice,” Dr. Barr said, noting, however, that he prefers knowing patients’ risk up front – especially for those patients he will see just once before they are “managed closer to home for the majority of their course.
“But if you [wait], then knowing what to repeat later is important,” he added. Namely, the FISH and TP53 tests are worth repeating as patients can acquire additional molecular aberrations over time.
Dr. Barr reported serving as a consultant for Pharmacyclics, AbbVie, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Novartis, and Seattle Genetics. He also reported receiving research funding from Pharmacyclics and AbbVie.
CHICAGO – A number of mutation tests – including immunoglobulin heavy chain gene (IgVH), fluorescence in situ hybridization (FISH), and TP53 – provide useful prognostic information at the time of chronic lymphocytic leukemia (CLL) diagnosis, according to Paul M. Barr, MD.
“It’s understood that IgVH mutation status is certainly prognostic,” Dr. Barr, associate professor of hematology/oncology at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
The B-cell receptor of the CLL cells uses IgVH genes that may or may not have undergone somatic mutations, with unmutated being defined as 98% or more sequence homology to germline.
“This is indicative of stronger signaling through the B-cell receptor and, as we all know, predicts for an inferior prognosis,” he explained, citing a study that demonstrated superior survival rates with mutated IgVH genes (Blood. 1999;94[6]:1840-7).
“It’s also well understood and accepted that we should perform a FISH panel; we should look for interphase cytogenetics based on FISH in our patients,” Dr. Barr said. “Having said that, we, as medical oncologists, do not do a very good job of using this testing appropriately. Patterns of care studies have suggested that a significant number of patients don’t get FISH testing at diagnosis or before first-line therapy.”
In fact, a typical interphase FISH panel identifies cytogenetic lesions, including del(17p), del(11q), del(13q), and trisomy 12 in more than 80% of CLL cases, with del(13q) being the most common.
Another marker that can be assessed in CLL patients and has maintained prognostic value across multiple analyses is serum beta-2 microglobulin, Dr. Barr noted.
TP53 sequencing is valuable as well and has been associated with outcomes similar to those seen in patients with del(17p), he said, citing data from a study that found similarly poor outcomes with TP53 mutations or deletions and del(17p), even when minor subclones are identified using next-generation sequencing (Blood. 2014;123:2139-47).
“One of the primary reasons for this is that the two aberrations go together. Most often, if you have del(17p) you’re also going to find a TP53 mutation, but in about 30% of patients or so, only one allele is affected, so this is why it’s still important to test for TP53 mutations when you’re looking for a 17p deletion,” he said.
Numerous other recurrent mutations in CLL have been associated with poor overall survival and/or progression-free survival, including SF3B1, ATM, NOTCH1, POT1, BIRC3, and NFKBIE.
“The gut instinct is that maybe we should start testing for all of these mutations now, but I would caution everybody that we still need further validation before we can apply these to the majority of patients,” Dr. Barr said. “We still don’t know exactly what to do with all of these mutations – when and how often we should test for them, if the novel agents are truly better – so while, again, they can predict for inferior outcomes, I would say these are not yet standard of care to be tested in all patients.”
It is likely, though, that new prognostic systems will evolve as more is learned about how to use these molecular aberrations. Attempts are already being made to incorporate novel mutations into a prognostic system. Dr. Barr pointed to a report that looked at the integration of mutations and cytogenetic lesions to improve the accuracy of survival prediction in CLL (Blood. 2013;121:1403-12).
“But this still requires prospective testing, especially in patients getting the novel agents,” he said.
Conventional karyotyping also has potential, though a limited role in this setting, he said, noting that it can be reliably performed with stimulation of CLL cells.
“We also know additional aberrations are prognostic and that a complex karyotype predicts for a very poor outcome,” he said. The International Workshop on CLL (iwCLL) guidelines, which were recently updated for the first time in a decade, state that further validation is needed.
“I think it’s potentially useful in a very young patient you are considering taking to transplant, but again, I agree with the stance that this is not something that should be performed in every patient across the board,” he said.
The tests currently recommended by iwCLL before CLL treatment include IgVH mutation status; FISH for del(13q), del(11q), del(17p), and trisomy 12 in peripheral blood lymphocytes; and TP53.
“Some folks... don’t check a lot of these markers at diagnosis, but wait for patients to require therapy, and that’s a reasonable way to practice,” Dr. Barr said, noting, however, that he prefers knowing patients’ risk up front – especially for those patients he will see just once before they are “managed closer to home for the majority of their course.
“But if you [wait], then knowing what to repeat later is important,” he added. Namely, the FISH and TP53 tests are worth repeating as patients can acquire additional molecular aberrations over time.
Dr. Barr reported serving as a consultant for Pharmacyclics, AbbVie, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Novartis, and Seattle Genetics. He also reported receiving research funding from Pharmacyclics and AbbVie.
CHICAGO – A number of mutation tests – including immunoglobulin heavy chain gene (IgVH), fluorescence in situ hybridization (FISH), and TP53 – provide useful prognostic information at the time of chronic lymphocytic leukemia (CLL) diagnosis, according to Paul M. Barr, MD.
“It’s understood that IgVH mutation status is certainly prognostic,” Dr. Barr, associate professor of hematology/oncology at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
The B-cell receptor of the CLL cells uses IgVH genes that may or may not have undergone somatic mutations, with unmutated being defined as 98% or more sequence homology to germline.
“This is indicative of stronger signaling through the B-cell receptor and, as we all know, predicts for an inferior prognosis,” he explained, citing a study that demonstrated superior survival rates with mutated IgVH genes (Blood. 1999;94[6]:1840-7).
“It’s also well understood and accepted that we should perform a FISH panel; we should look for interphase cytogenetics based on FISH in our patients,” Dr. Barr said. “Having said that, we, as medical oncologists, do not do a very good job of using this testing appropriately. Patterns of care studies have suggested that a significant number of patients don’t get FISH testing at diagnosis or before first-line therapy.”
In fact, a typical interphase FISH panel identifies cytogenetic lesions, including del(17p), del(11q), del(13q), and trisomy 12 in more than 80% of CLL cases, with del(13q) being the most common.
Another marker that can be assessed in CLL patients and has maintained prognostic value across multiple analyses is serum beta-2 microglobulin, Dr. Barr noted.
TP53 sequencing is valuable as well and has been associated with outcomes similar to those seen in patients with del(17p), he said, citing data from a study that found similarly poor outcomes with TP53 mutations or deletions and del(17p), even when minor subclones are identified using next-generation sequencing (Blood. 2014;123:2139-47).
“One of the primary reasons for this is that the two aberrations go together. Most often, if you have del(17p) you’re also going to find a TP53 mutation, but in about 30% of patients or so, only one allele is affected, so this is why it’s still important to test for TP53 mutations when you’re looking for a 17p deletion,” he said.
Numerous other recurrent mutations in CLL have been associated with poor overall survival and/or progression-free survival, including SF3B1, ATM, NOTCH1, POT1, BIRC3, and NFKBIE.
“The gut instinct is that maybe we should start testing for all of these mutations now, but I would caution everybody that we still need further validation before we can apply these to the majority of patients,” Dr. Barr said. “We still don’t know exactly what to do with all of these mutations – when and how often we should test for them, if the novel agents are truly better – so while, again, they can predict for inferior outcomes, I would say these are not yet standard of care to be tested in all patients.”
It is likely, though, that new prognostic systems will evolve as more is learned about how to use these molecular aberrations. Attempts are already being made to incorporate novel mutations into a prognostic system. Dr. Barr pointed to a report that looked at the integration of mutations and cytogenetic lesions to improve the accuracy of survival prediction in CLL (Blood. 2013;121:1403-12).
“But this still requires prospective testing, especially in patients getting the novel agents,” he said.
Conventional karyotyping also has potential, though a limited role in this setting, he said, noting that it can be reliably performed with stimulation of CLL cells.
“We also know additional aberrations are prognostic and that a complex karyotype predicts for a very poor outcome,” he said. The International Workshop on CLL (iwCLL) guidelines, which were recently updated for the first time in a decade, state that further validation is needed.
“I think it’s potentially useful in a very young patient you are considering taking to transplant, but again, I agree with the stance that this is not something that should be performed in every patient across the board,” he said.
The tests currently recommended by iwCLL before CLL treatment include IgVH mutation status; FISH for del(13q), del(11q), del(17p), and trisomy 12 in peripheral blood lymphocytes; and TP53.
“Some folks... don’t check a lot of these markers at diagnosis, but wait for patients to require therapy, and that’s a reasonable way to practice,” Dr. Barr said, noting, however, that he prefers knowing patients’ risk up front – especially for those patients he will see just once before they are “managed closer to home for the majority of their course.
“But if you [wait], then knowing what to repeat later is important,” he added. Namely, the FISH and TP53 tests are worth repeating as patients can acquire additional molecular aberrations over time.
Dr. Barr reported serving as a consultant for Pharmacyclics, AbbVie, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Novartis, and Seattle Genetics. He also reported receiving research funding from Pharmacyclics and AbbVie.
EXPERT ANALYSIS FROM MHM 2018
MELD sodium score tied to better transplant outcomes
Factoring hyponatremic status into liver graft allocations led to significant reductions in wait-list mortality, researchers reported in the November issue of Gastroenterology.
Hyponatremic patients with low MELD scores benefited significantly from allocation based on the end-stage liver disease–sodium (MELD-Na) score, while its survival benefit was less evident among patients with higher scores, said Shunji Nagai, MD, PhD, of Henry Ford Hospital, Detroit, and his associates. “Therefore, liver allocation rules such as Share 15 and Share 35 need to be revised to fulfill the Final Rule under the MELD-Na based allocation,” they wrote.
The Share 35 rule offers liver grafts locally and regionally to wait-listed patients with MELD-Na scores of at least 35. Under the Share 15 rule, livers are offered regionally or nationally before considering local candidates with MELD scores under 15. The traditional MELD scoring system excluded hyponatremia, which has since been found to independently predict death from cirrhosis. Therefore, in January 2016, a modified MELD-Na score was implemented for patients with traditional MELD scores of at least 12. The MELD-Na score assigns patients between 1 and 11 additional points, and patients with low MELD scores and severe hyponatremia receive the most points. To assess the impact of this change, Dr. Nagai and his associates compared wait-list and posttransplantation outcomes during the pre and post–MELD-Na eras and the survival benefit of liver transplantation during the MELD-Na period. The study included all adults wait-listed for livers from June 2013, when Share 35 was implemented, through September 2017.
Mortality within 90 days on the wait list fell significantly during the MELD-Na era (hazard ratio, 0.74; P less than .001). Transplantation conferred a “definitive” survival benefit when MELD-Na scores were 21-23 (HR versus wait list, 0.34; P less than .001). During the traditional MELD period, the equivalent cutoff was 15-17 (HR, 0.36; P less than .001). “As such, the current rules for liver allocation may be suboptimal under the MELD-Na–based allocation and the criteria for Share 15 may need to be revisited,” the researchers wrote. They recommended raising the cutoff to 21.
The study also confirmed mild hyponatremia (130-134 mmol/L), moderate hyponatremia (125-129 mmol/L), and severe hyponatremia (less than 125 mmol/L) as independent predictors of wait-list mortality during the traditional MELD era. Hazard ratios were 1.4, 1.8, and 1.7, respectively (all P less than .001). The implementation of MELD-Na significantly weakened these associations, with HRs of 1.1 (P = .3), 1.3 (P = .02), and 1.4 (P = .04), respectively).
The probability of transplantation also rose significantly during the MELD-Na era (HR, 1.2; P less than .001), possibly because of the opioid epidemic, the researchers said. Although greater availability of liver grafts might have improved wait-list outcomes, all score categories would have shown a positive impact if this was the only reason, they added. Instead, MELD-Na most benefited patients with lower scores.
Finally, posttransplantation outcomes worsened during the MELD-Na era, perhaps because of transplant population aging. However, the survival benefit of transplant shifted to higher score ranges during the MELD-Na era even after the researchers controlled for this effect. “According to this analysis,” they wrote, “the survival benefit of liver transplant was definitive in patients with score category of 21-23, which could further validate our proposal to revise Share 15 rule to ‘Share 21.’ ”
The investigators reported having no external funding sources or conflicts of interest.
SOURCE: Nagai S et al. Gastroenterology. 2018 Jul 26. doi: 10.1053/j.gastro.2018.07.025.
Factoring hyponatremic status into liver graft allocations led to significant reductions in wait-list mortality, researchers reported in the November issue of Gastroenterology.
Hyponatremic patients with low MELD scores benefited significantly from allocation based on the end-stage liver disease–sodium (MELD-Na) score, while its survival benefit was less evident among patients with higher scores, said Shunji Nagai, MD, PhD, of Henry Ford Hospital, Detroit, and his associates. “Therefore, liver allocation rules such as Share 15 and Share 35 need to be revised to fulfill the Final Rule under the MELD-Na based allocation,” they wrote.
The Share 35 rule offers liver grafts locally and regionally to wait-listed patients with MELD-Na scores of at least 35. Under the Share 15 rule, livers are offered regionally or nationally before considering local candidates with MELD scores under 15. The traditional MELD scoring system excluded hyponatremia, which has since been found to independently predict death from cirrhosis. Therefore, in January 2016, a modified MELD-Na score was implemented for patients with traditional MELD scores of at least 12. The MELD-Na score assigns patients between 1 and 11 additional points, and patients with low MELD scores and severe hyponatremia receive the most points. To assess the impact of this change, Dr. Nagai and his associates compared wait-list and posttransplantation outcomes during the pre and post–MELD-Na eras and the survival benefit of liver transplantation during the MELD-Na period. The study included all adults wait-listed for livers from June 2013, when Share 35 was implemented, through September 2017.
Mortality within 90 days on the wait list fell significantly during the MELD-Na era (hazard ratio, 0.74; P less than .001). Transplantation conferred a “definitive” survival benefit when MELD-Na scores were 21-23 (HR versus wait list, 0.34; P less than .001). During the traditional MELD period, the equivalent cutoff was 15-17 (HR, 0.36; P less than .001). “As such, the current rules for liver allocation may be suboptimal under the MELD-Na–based allocation and the criteria for Share 15 may need to be revisited,” the researchers wrote. They recommended raising the cutoff to 21.
The study also confirmed mild hyponatremia (130-134 mmol/L), moderate hyponatremia (125-129 mmol/L), and severe hyponatremia (less than 125 mmol/L) as independent predictors of wait-list mortality during the traditional MELD era. Hazard ratios were 1.4, 1.8, and 1.7, respectively (all P less than .001). The implementation of MELD-Na significantly weakened these associations, with HRs of 1.1 (P = .3), 1.3 (P = .02), and 1.4 (P = .04), respectively).
The probability of transplantation also rose significantly during the MELD-Na era (HR, 1.2; P less than .001), possibly because of the opioid epidemic, the researchers said. Although greater availability of liver grafts might have improved wait-list outcomes, all score categories would have shown a positive impact if this was the only reason, they added. Instead, MELD-Na most benefited patients with lower scores.
Finally, posttransplantation outcomes worsened during the MELD-Na era, perhaps because of transplant population aging. However, the survival benefit of transplant shifted to higher score ranges during the MELD-Na era even after the researchers controlled for this effect. “According to this analysis,” they wrote, “the survival benefit of liver transplant was definitive in patients with score category of 21-23, which could further validate our proposal to revise Share 15 rule to ‘Share 21.’ ”
The investigators reported having no external funding sources or conflicts of interest.
SOURCE: Nagai S et al. Gastroenterology. 2018 Jul 26. doi: 10.1053/j.gastro.2018.07.025.
Factoring hyponatremic status into liver graft allocations led to significant reductions in wait-list mortality, researchers reported in the November issue of Gastroenterology.
Hyponatremic patients with low MELD scores benefited significantly from allocation based on the end-stage liver disease–sodium (MELD-Na) score, while its survival benefit was less evident among patients with higher scores, said Shunji Nagai, MD, PhD, of Henry Ford Hospital, Detroit, and his associates. “Therefore, liver allocation rules such as Share 15 and Share 35 need to be revised to fulfill the Final Rule under the MELD-Na based allocation,” they wrote.
The Share 35 rule offers liver grafts locally and regionally to wait-listed patients with MELD-Na scores of at least 35. Under the Share 15 rule, livers are offered regionally or nationally before considering local candidates with MELD scores under 15. The traditional MELD scoring system excluded hyponatremia, which has since been found to independently predict death from cirrhosis. Therefore, in January 2016, a modified MELD-Na score was implemented for patients with traditional MELD scores of at least 12. The MELD-Na score assigns patients between 1 and 11 additional points, and patients with low MELD scores and severe hyponatremia receive the most points. To assess the impact of this change, Dr. Nagai and his associates compared wait-list and posttransplantation outcomes during the pre and post–MELD-Na eras and the survival benefit of liver transplantation during the MELD-Na period. The study included all adults wait-listed for livers from June 2013, when Share 35 was implemented, through September 2017.
Mortality within 90 days on the wait list fell significantly during the MELD-Na era (hazard ratio, 0.74; P less than .001). Transplantation conferred a “definitive” survival benefit when MELD-Na scores were 21-23 (HR versus wait list, 0.34; P less than .001). During the traditional MELD period, the equivalent cutoff was 15-17 (HR, 0.36; P less than .001). “As such, the current rules for liver allocation may be suboptimal under the MELD-Na–based allocation and the criteria for Share 15 may need to be revisited,” the researchers wrote. They recommended raising the cutoff to 21.
The study also confirmed mild hyponatremia (130-134 mmol/L), moderate hyponatremia (125-129 mmol/L), and severe hyponatremia (less than 125 mmol/L) as independent predictors of wait-list mortality during the traditional MELD era. Hazard ratios were 1.4, 1.8, and 1.7, respectively (all P less than .001). The implementation of MELD-Na significantly weakened these associations, with HRs of 1.1 (P = .3), 1.3 (P = .02), and 1.4 (P = .04), respectively).
The probability of transplantation also rose significantly during the MELD-Na era (HR, 1.2; P less than .001), possibly because of the opioid epidemic, the researchers said. Although greater availability of liver grafts might have improved wait-list outcomes, all score categories would have shown a positive impact if this was the only reason, they added. Instead, MELD-Na most benefited patients with lower scores.
Finally, posttransplantation outcomes worsened during the MELD-Na era, perhaps because of transplant population aging. However, the survival benefit of transplant shifted to higher score ranges during the MELD-Na era even after the researchers controlled for this effect. “According to this analysis,” they wrote, “the survival benefit of liver transplant was definitive in patients with score category of 21-23, which could further validate our proposal to revise Share 15 rule to ‘Share 21.’ ”
The investigators reported having no external funding sources or conflicts of interest.
SOURCE: Nagai S et al. Gastroenterology. 2018 Jul 26. doi: 10.1053/j.gastro.2018.07.025.
FROM GASTROENTEROLOGY
Key clinical point: The implementation of the MELD sodium (MELD-Na) score for liver allocation was associated with significantly improved outcomes for wait-listed patients.
Major finding: During the MELD-Na era, mortality within 90 days on the liver wait list dropped significantly (HR, 0.74; P less than .001) while the probability of transplant rose significantly (HR, 1.2; P less than .001).
Study details: Comparison of 18,850 adult transplant candidates during the traditional MELD era versus 14,512 candidates during the MELD-Na era.
Disclosures: The investigators had no external funding sources or conflicts of interest.
Source: Nagai S et al. Gastroenterology. 2018 Jul 26. doi: 10.1053/j.gastro.2018.07.025.
FDA expands CoolSculpting indication to submandibular area
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
FDA clearance of the expanded indication was based on results of a 22-week study in which patients achieved a mean 33% reduction in fat layer thickness after two treatments. In addition, 85% of patients reported satisfaction with their treatment in that and two other studies. The data were provided in Allergan’s press release announcing the clearance for the cryolipolysis device.
Adverse events associated with the CoolSculpting treatment include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, and skin sensitivity. People with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria should not receive CoolSculpting treatments, according to the company.
The FDA also expanded the CoolSculpting indication to people with a body mass index up to 46.2 kg/m2 when treating the submental and submandibular areas.
Frontline rituximab shows long-term success in indolent lymphoma
Advanced indolent lymphoma patients can be treated with a rituximab-containing regimen as first-line therapy and, in some cases, skip chemotherapy altogether, a study with 10 years of follow-up data suggests.
After a median of 10.6 years’ follow-up, almost three-quarters of patients (73%) in the study were alive, and 36% never required chemotherapy.
“This [overall survival] is at least as good as that observed in modern immunochemotherapy trials,” Sandra Lockmer, MD, of Karolinska University Hospital in Stockholm and her colleagues reported in the Journal of Clinical Oncology.
The study included 321 patients who were previously untreated and had been enrolled in two randomized clinical trials performed by the Nordic Lymphoma Group. The trials randomized patients to receive either rituximab monotherapy or rituximab combined with interferon alfa-2a. Neither trial used up-front chemotherapy.
Patients included in the follow-up analysis had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
The overall survival rate at 10 years after trial assignment was 75% and 66% after 15 years. Similarly, the lymphoma-specific survival rate was 81% at 10 years after trial assignment and 77% at 15 years, the researchers reported.
Overall, 117 patients did not require treatment with chemotherapy, but 24 patients were further treated with antibodies and/or radiation. Of the 93 patients who received no additional therapies after frontline treatment, 9 patients died from causes unrelated to their lymphoma.
Among the 237 patients who failed initial treatment, the median time to treatment failure was 1.5 years.
In terms of transformation to aggressive lymphoma, the rate was 2.4%/person-year overall. The cumulative risk of transformation was 20% at 10 years after trial assignment and 24% at 15 years.
The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
mschneider@mdedge.com
SOURCE: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
Advanced indolent lymphoma patients can be treated with a rituximab-containing regimen as first-line therapy and, in some cases, skip chemotherapy altogether, a study with 10 years of follow-up data suggests.
After a median of 10.6 years’ follow-up, almost three-quarters of patients (73%) in the study were alive, and 36% never required chemotherapy.
“This [overall survival] is at least as good as that observed in modern immunochemotherapy trials,” Sandra Lockmer, MD, of Karolinska University Hospital in Stockholm and her colleagues reported in the Journal of Clinical Oncology.
The study included 321 patients who were previously untreated and had been enrolled in two randomized clinical trials performed by the Nordic Lymphoma Group. The trials randomized patients to receive either rituximab monotherapy or rituximab combined with interferon alfa-2a. Neither trial used up-front chemotherapy.
Patients included in the follow-up analysis had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
The overall survival rate at 10 years after trial assignment was 75% and 66% after 15 years. Similarly, the lymphoma-specific survival rate was 81% at 10 years after trial assignment and 77% at 15 years, the researchers reported.
Overall, 117 patients did not require treatment with chemotherapy, but 24 patients were further treated with antibodies and/or radiation. Of the 93 patients who received no additional therapies after frontline treatment, 9 patients died from causes unrelated to their lymphoma.
Among the 237 patients who failed initial treatment, the median time to treatment failure was 1.5 years.
In terms of transformation to aggressive lymphoma, the rate was 2.4%/person-year overall. The cumulative risk of transformation was 20% at 10 years after trial assignment and 24% at 15 years.
The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
mschneider@mdedge.com
SOURCE: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
Advanced indolent lymphoma patients can be treated with a rituximab-containing regimen as first-line therapy and, in some cases, skip chemotherapy altogether, a study with 10 years of follow-up data suggests.
After a median of 10.6 years’ follow-up, almost three-quarters of patients (73%) in the study were alive, and 36% never required chemotherapy.
“This [overall survival] is at least as good as that observed in modern immunochemotherapy trials,” Sandra Lockmer, MD, of Karolinska University Hospital in Stockholm and her colleagues reported in the Journal of Clinical Oncology.
The study included 321 patients who were previously untreated and had been enrolled in two randomized clinical trials performed by the Nordic Lymphoma Group. The trials randomized patients to receive either rituximab monotherapy or rituximab combined with interferon alfa-2a. Neither trial used up-front chemotherapy.
Patients included in the follow-up analysis had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
The overall survival rate at 10 years after trial assignment was 75% and 66% after 15 years. Similarly, the lymphoma-specific survival rate was 81% at 10 years after trial assignment and 77% at 15 years, the researchers reported.
Overall, 117 patients did not require treatment with chemotherapy, but 24 patients were further treated with antibodies and/or radiation. Of the 93 patients who received no additional therapies after frontline treatment, 9 patients died from causes unrelated to their lymphoma.
Among the 237 patients who failed initial treatment, the median time to treatment failure was 1.5 years.
In terms of transformation to aggressive lymphoma, the rate was 2.4%/person-year overall. The cumulative risk of transformation was 20% at 10 years after trial assignment and 24% at 15 years.
The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
mschneider@mdedge.com
SOURCE: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: After a median of 10.6 years’ follow up, 73% of patients were alive, and 36% did not require chemotherapy.
Study details: Ten-year follow-up data from two trials on 321 previously untreated patients who had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
Disclosures: The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
Source: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
Studies reveal pregnancy trends in American women with MS
New evidence provides estimates of the pregnancy rates for American women with multiple sclerosis (MS), their complication rates, and the rates of relapse and disease-modifying drug treatment during different phases before and after pregnancy.
The two new studies, conducted by Maria K. Houtchens, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues involved retrospective mining of U.S. commercial health plan data in the IQVIA Real-World Data Adjudicated Claims–U.S. database between Jan. 1, 2006, and June 30, 2015.
The mean age of pregnant women in the nine annual cohorts during that period was just over 32 years for those with MS and just over 29 years for those without. The percentage of women without MS who had a pregnancy-related claim in the database declined from 8.83% in 2006 to 7.75% in 2014 after adjusting for age, region, payer, and Charlson Comorbidity Index score, whereas the percentage increased in women with MS during the same period, from 7.91% to 9.47%. The investigators matched 2,115 women with MS and 2,115 without MS who had live births for a variety of variables and found that women with MS had higher rates of premature labor (31.4% vs. 27.4%; P = .005), infection in pregnancy (13.3% vs. 10.9%; P = .016), maternal cardiovascular disease (3.0% vs. 1.9%; P = .028), anemia or acquired coagulation disorder (2.5% vs. 1.3%; P = .007), neurologic complications in pregnancy (1.6% vs. 0.6%; P = .005), and sexually transmitted diseases in pregnancy (0.4% vs. 0%; P = .045). During labor and delivery, women with MS who had a live birth more often had a claim for acquired damage to the fetus (27.8% vs. 23.5%; P = .002) and congenital fetal malformations (13.2% vs. 10.3%; P = .004) than did women without MS.
In the second study, Dr. Houtchens and two coauthors from the first study of the database reported on a set of 2,158 women who had a live birth during the study period and had 1 year of continuous insurance eligibility before and after pregnancy. The odds for having an MS relapse declined during pregnancy (odds ratio, 0.623; 95% confidence interval, 0.521-0.744), rose during the 6-week postpartum puerperium (OR, 1.710; 95% CI, 1.358-2.152), and leveled off during the last three postpartum quarters to remain at a higher level than before pregnancy (OR, 1.216; 95% CI, 1.052-1.406). Disease-modifying drug treatment followed the same pattern with 20% using it before pregnancy, dropping to about 2% in the second trimester, and peaking in about a quarter of all patients 9-12 months post partum.
SOURCES: Houtchens MK et al. Neurology. 2018 Sep 28. doi: 10.1212/WNL.0000000000006382; Houtchens MK et al. Neurology. 2018 Sep 28. doi: 10.1212/WNL.0000000000006384.
New evidence provides estimates of the pregnancy rates for American women with multiple sclerosis (MS), their complication rates, and the rates of relapse and disease-modifying drug treatment during different phases before and after pregnancy.
The two new studies, conducted by Maria K. Houtchens, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues involved retrospective mining of U.S. commercial health plan data in the IQVIA Real-World Data Adjudicated Claims–U.S. database between Jan. 1, 2006, and June 30, 2015.
The mean age of pregnant women in the nine annual cohorts during that period was just over 32 years for those with MS and just over 29 years for those without. The percentage of women without MS who had a pregnancy-related claim in the database declined from 8.83% in 2006 to 7.75% in 2014 after adjusting for age, region, payer, and Charlson Comorbidity Index score, whereas the percentage increased in women with MS during the same period, from 7.91% to 9.47%. The investigators matched 2,115 women with MS and 2,115 without MS who had live births for a variety of variables and found that women with MS had higher rates of premature labor (31.4% vs. 27.4%; P = .005), infection in pregnancy (13.3% vs. 10.9%; P = .016), maternal cardiovascular disease (3.0% vs. 1.9%; P = .028), anemia or acquired coagulation disorder (2.5% vs. 1.3%; P = .007), neurologic complications in pregnancy (1.6% vs. 0.6%; P = .005), and sexually transmitted diseases in pregnancy (0.4% vs. 0%; P = .045). During labor and delivery, women with MS who had a live birth more often had a claim for acquired damage to the fetus (27.8% vs. 23.5%; P = .002) and congenital fetal malformations (13.2% vs. 10.3%; P = .004) than did women without MS.
In the second study, Dr. Houtchens and two coauthors from the first study of the database reported on a set of 2,158 women who had a live birth during the study period and had 1 year of continuous insurance eligibility before and after pregnancy. The odds for having an MS relapse declined during pregnancy (odds ratio, 0.623; 95% confidence interval, 0.521-0.744), rose during the 6-week postpartum puerperium (OR, 1.710; 95% CI, 1.358-2.152), and leveled off during the last three postpartum quarters to remain at a higher level than before pregnancy (OR, 1.216; 95% CI, 1.052-1.406). Disease-modifying drug treatment followed the same pattern with 20% using it before pregnancy, dropping to about 2% in the second trimester, and peaking in about a quarter of all patients 9-12 months post partum.
SOURCES: Houtchens MK et al. Neurology. 2018 Sep 28. doi: 10.1212/WNL.0000000000006382; Houtchens MK et al. Neurology. 2018 Sep 28. doi: 10.1212/WNL.0000000000006384.
New evidence provides estimates of the pregnancy rates for American women with multiple sclerosis (MS), their complication rates, and the rates of relapse and disease-modifying drug treatment during different phases before and after pregnancy.
The two new studies, conducted by Maria K. Houtchens, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues involved retrospective mining of U.S. commercial health plan data in the IQVIA Real-World Data Adjudicated Claims–U.S. database between Jan. 1, 2006, and June 30, 2015.
The mean age of pregnant women in the nine annual cohorts during that period was just over 32 years for those with MS and just over 29 years for those without. The percentage of women without MS who had a pregnancy-related claim in the database declined from 8.83% in 2006 to 7.75% in 2014 after adjusting for age, region, payer, and Charlson Comorbidity Index score, whereas the percentage increased in women with MS during the same period, from 7.91% to 9.47%. The investigators matched 2,115 women with MS and 2,115 without MS who had live births for a variety of variables and found that women with MS had higher rates of premature labor (31.4% vs. 27.4%; P = .005), infection in pregnancy (13.3% vs. 10.9%; P = .016), maternal cardiovascular disease (3.0% vs. 1.9%; P = .028), anemia or acquired coagulation disorder (2.5% vs. 1.3%; P = .007), neurologic complications in pregnancy (1.6% vs. 0.6%; P = .005), and sexually transmitted diseases in pregnancy (0.4% vs. 0%; P = .045). During labor and delivery, women with MS who had a live birth more often had a claim for acquired damage to the fetus (27.8% vs. 23.5%; P = .002) and congenital fetal malformations (13.2% vs. 10.3%; P = .004) than did women without MS.
In the second study, Dr. Houtchens and two coauthors from the first study of the database reported on a set of 2,158 women who had a live birth during the study period and had 1 year of continuous insurance eligibility before and after pregnancy. The odds for having an MS relapse declined during pregnancy (odds ratio, 0.623; 95% confidence interval, 0.521-0.744), rose during the 6-week postpartum puerperium (OR, 1.710; 95% CI, 1.358-2.152), and leveled off during the last three postpartum quarters to remain at a higher level than before pregnancy (OR, 1.216; 95% CI, 1.052-1.406). Disease-modifying drug treatment followed the same pattern with 20% using it before pregnancy, dropping to about 2% in the second trimester, and peaking in about a quarter of all patients 9-12 months post partum.
SOURCES: Houtchens MK et al. Neurology. 2018 Sep 28. doi: 10.1212/WNL.0000000000006382; Houtchens MK et al. Neurology. 2018 Sep 28. doi: 10.1212/WNL.0000000000006384.
FROM NEUROLOGY