User login
Terra Firma-Forme Dermatosis Mimicking Livedo Racemosa
To the Editor:
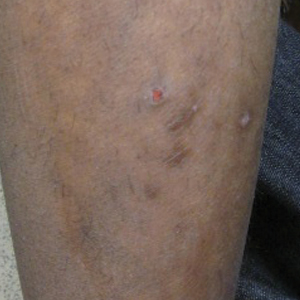
A 17-year-old adolescent boy presented with dark spots on the legs and back of 2 months’ duration. He was not taking any medications and the spots could not be washed away by scrubbing with soap and water. He denied symptoms, except occasional itching. Family history revealed a maternal uncle with protein C deficiency and a maternal grandmother with systemic lupus erythematosus. Review of systems was negative; the patient denied joint pain and contact with heating pads or laptop computers. Based on the initial presentation, an underlying systemic condition was suspected. Physical examination revealed reticulate, nonblanching, brown patches on the bilateral arms, legs, and back in an apparent livedoid pattern (Figure). The patient’s history and physical examination suggested terra firma-forme dermatosis, livedo racemosa, or another vasculopathic process. However, gentle rubbing of the skin with an alcohol swab removed the discoloration completely, leading to the diagnosis of terra firma-forme dermatosis.
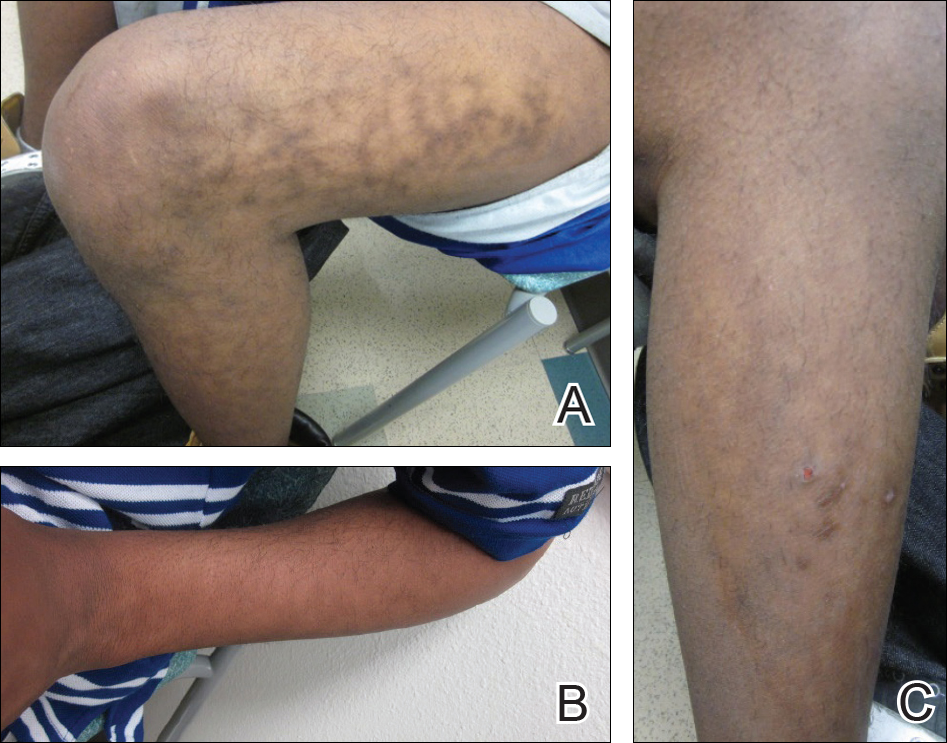
Livedo racemosa appears as an irregular, focal, reticulated discoloration of the skin.1 The reticulated pattern of livedo racemosa has a branched or broken-up appearance.2 Livedo racemosa indicates a disruption in the vasculature due to inflammation or occlusion.1 The change is pathologic and does not blanch or resolve with warming.1,2 The condition can progress to pigmentation and ulceration.1 Livedo racemosa is a cutaneous manifestation of underlying vascular pathology. Due to a variety of causes, skin biopsy is nondiagnostic. Livedo racemosa can be caused by conditions such as systemic lupus erythematosus, syphilis, tuberculosis, polycythemia rubra vera, and Sneddon syndrome, among others.3-5
Terra firma-forme dermatosis was reported in 1987 by Duncan et al.6 The condition classically presents with an exasperated mother who is unable to clean the “dirt” off her child’s skin despite multiple vigorous scrubbing attempts. The condition most commonly occurs in the summer months on the neck, face, and ankles.7,8 Duncan et al6 reported that when the affected area was prepared for a biopsy, clean skin was revealed after wiping with an alcohol swab. No other cleansing agent has been reported to effectively remove the discoloration of terra firma-forme dermatosis. Hoping to elucidate a cause, Duncan et al6 performed both bacteriologic and fungal studies. The bacterial skin culture grew only normal flora, and fungal culture grew only normal contaminants consistent with the potassium hydroxide preparation of skin scraping. Histopathologic examination showed hyperkeratosis and orthokeratosis but not parakeratosis. Staining revealed melanin in the hyperkeratotic areas.6 Although the cause of this condition largely is unknown, it is thought that the epidermis in the affected areas could undergo altered maturation, resulting in trapping melanin that causes the skin to appear hyperkeratotic and hyperpigmented.1 In our case, wiping the skin revealed the unsuspected diagnosis of terra firma-forme dermatosis displaying an unusual pseudolivedoid pattern. With apparently hyperpigmented processes, rubbing the skin with alcohol may help avoid unnecessary aggressive workup.
- Parsi K, Partsch H, Rabe E, et al. Reticulate eruptions: part 2. historical perspectives, morphology, terminology and classification. Australas J Dermatol. 2011;52:237-244.
- Ehrmann S. A new vascular symptom in syphilis [in German]. Wien Med Wochenschr. 1907;57:777-782.
- Sneddon IB. Cerebrovascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Golden RL. Livedo reticularis in systemic lupus erythematosus. Arch Dermatol. 1963;87:299-301.
- Lyell A, Church R. The cutaneous manifestations of polyarteritis nodosa. Br J Dermatol. 1954;66:335-343.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;23:297-300.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
To the Editor:
A 17-year-old adolescent boy presented with dark spots on the legs and back of 2 months’ duration. He was not taking any medications and the spots could not be washed away by scrubbing with soap and water. He denied symptoms, except occasional itching. Family history revealed a maternal uncle with protein C deficiency and a maternal grandmother with systemic lupus erythematosus. Review of systems was negative; the patient denied joint pain and contact with heating pads or laptop computers. Based on the initial presentation, an underlying systemic condition was suspected. Physical examination revealed reticulate, nonblanching, brown patches on the bilateral arms, legs, and back in an apparent livedoid pattern (Figure). The patient’s history and physical examination suggested terra firma-forme dermatosis, livedo racemosa, or another vasculopathic process. However, gentle rubbing of the skin with an alcohol swab removed the discoloration completely, leading to the diagnosis of terra firma-forme dermatosis.

Livedo racemosa appears as an irregular, focal, reticulated discoloration of the skin.1 The reticulated pattern of livedo racemosa has a branched or broken-up appearance.2 Livedo racemosa indicates a disruption in the vasculature due to inflammation or occlusion.1 The change is pathologic and does not blanch or resolve with warming.1,2 The condition can progress to pigmentation and ulceration.1 Livedo racemosa is a cutaneous manifestation of underlying vascular pathology. Due to a variety of causes, skin biopsy is nondiagnostic. Livedo racemosa can be caused by conditions such as systemic lupus erythematosus, syphilis, tuberculosis, polycythemia rubra vera, and Sneddon syndrome, among others.3-5
Terra firma-forme dermatosis was reported in 1987 by Duncan et al.6 The condition classically presents with an exasperated mother who is unable to clean the “dirt” off her child’s skin despite multiple vigorous scrubbing attempts. The condition most commonly occurs in the summer months on the neck, face, and ankles.7,8 Duncan et al6 reported that when the affected area was prepared for a biopsy, clean skin was revealed after wiping with an alcohol swab. No other cleansing agent has been reported to effectively remove the discoloration of terra firma-forme dermatosis. Hoping to elucidate a cause, Duncan et al6 performed both bacteriologic and fungal studies. The bacterial skin culture grew only normal flora, and fungal culture grew only normal contaminants consistent with the potassium hydroxide preparation of skin scraping. Histopathologic examination showed hyperkeratosis and orthokeratosis but not parakeratosis. Staining revealed melanin in the hyperkeratotic areas.6 Although the cause of this condition largely is unknown, it is thought that the epidermis in the affected areas could undergo altered maturation, resulting in trapping melanin that causes the skin to appear hyperkeratotic and hyperpigmented.1 In our case, wiping the skin revealed the unsuspected diagnosis of terra firma-forme dermatosis displaying an unusual pseudolivedoid pattern. With apparently hyperpigmented processes, rubbing the skin with alcohol may help avoid unnecessary aggressive workup.
To the Editor:
A 17-year-old adolescent boy presented with dark spots on the legs and back of 2 months’ duration. He was not taking any medications and the spots could not be washed away by scrubbing with soap and water. He denied symptoms, except occasional itching. Family history revealed a maternal uncle with protein C deficiency and a maternal grandmother with systemic lupus erythematosus. Review of systems was negative; the patient denied joint pain and contact with heating pads or laptop computers. Based on the initial presentation, an underlying systemic condition was suspected. Physical examination revealed reticulate, nonblanching, brown patches on the bilateral arms, legs, and back in an apparent livedoid pattern (Figure). The patient’s history and physical examination suggested terra firma-forme dermatosis, livedo racemosa, or another vasculopathic process. However, gentle rubbing of the skin with an alcohol swab removed the discoloration completely, leading to the diagnosis of terra firma-forme dermatosis.

Livedo racemosa appears as an irregular, focal, reticulated discoloration of the skin.1 The reticulated pattern of livedo racemosa has a branched or broken-up appearance.2 Livedo racemosa indicates a disruption in the vasculature due to inflammation or occlusion.1 The change is pathologic and does not blanch or resolve with warming.1,2 The condition can progress to pigmentation and ulceration.1 Livedo racemosa is a cutaneous manifestation of underlying vascular pathology. Due to a variety of causes, skin biopsy is nondiagnostic. Livedo racemosa can be caused by conditions such as systemic lupus erythematosus, syphilis, tuberculosis, polycythemia rubra vera, and Sneddon syndrome, among others.3-5
Terra firma-forme dermatosis was reported in 1987 by Duncan et al.6 The condition classically presents with an exasperated mother who is unable to clean the “dirt” off her child’s skin despite multiple vigorous scrubbing attempts. The condition most commonly occurs in the summer months on the neck, face, and ankles.7,8 Duncan et al6 reported that when the affected area was prepared for a biopsy, clean skin was revealed after wiping with an alcohol swab. No other cleansing agent has been reported to effectively remove the discoloration of terra firma-forme dermatosis. Hoping to elucidate a cause, Duncan et al6 performed both bacteriologic and fungal studies. The bacterial skin culture grew only normal flora, and fungal culture grew only normal contaminants consistent with the potassium hydroxide preparation of skin scraping. Histopathologic examination showed hyperkeratosis and orthokeratosis but not parakeratosis. Staining revealed melanin in the hyperkeratotic areas.6 Although the cause of this condition largely is unknown, it is thought that the epidermis in the affected areas could undergo altered maturation, resulting in trapping melanin that causes the skin to appear hyperkeratotic and hyperpigmented.1 In our case, wiping the skin revealed the unsuspected diagnosis of terra firma-forme dermatosis displaying an unusual pseudolivedoid pattern. With apparently hyperpigmented processes, rubbing the skin with alcohol may help avoid unnecessary aggressive workup.
- Parsi K, Partsch H, Rabe E, et al. Reticulate eruptions: part 2. historical perspectives, morphology, terminology and classification. Australas J Dermatol. 2011;52:237-244.
- Ehrmann S. A new vascular symptom in syphilis [in German]. Wien Med Wochenschr. 1907;57:777-782.
- Sneddon IB. Cerebrovascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Golden RL. Livedo reticularis in systemic lupus erythematosus. Arch Dermatol. 1963;87:299-301.
- Lyell A, Church R. The cutaneous manifestations of polyarteritis nodosa. Br J Dermatol. 1954;66:335-343.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;23:297-300.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Parsi K, Partsch H, Rabe E, et al. Reticulate eruptions: part 2. historical perspectives, morphology, terminology and classification. Australas J Dermatol. 2011;52:237-244.
- Ehrmann S. A new vascular symptom in syphilis [in German]. Wien Med Wochenschr. 1907;57:777-782.
- Sneddon IB. Cerebrovascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Golden RL. Livedo reticularis in systemic lupus erythematosus. Arch Dermatol. 1963;87:299-301.
- Lyell A, Church R. The cutaneous manifestations of polyarteritis nodosa. Br J Dermatol. 1954;66:335-343.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;23:297-300.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
Practice Points
- Clinicians should include terra firma-forme dermatosis in the differential diagnosis of any hyperpigmented condition, regardless of pattern of presentation.
- Clean the skin with an alcohol wipe to rule out a diagnosis of terra firma-forme dermatosis.
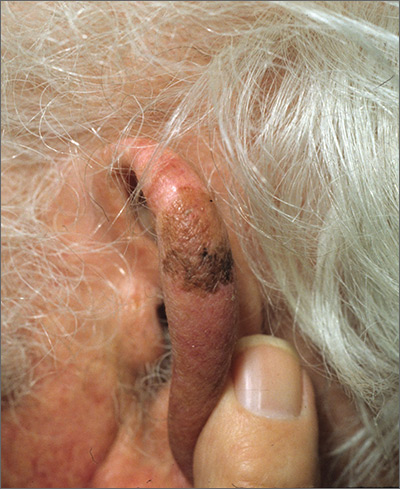
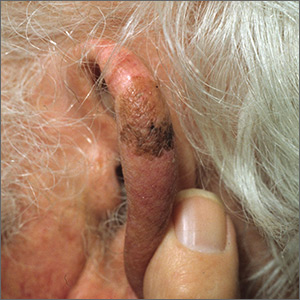
Brown spot on ear
The FP explained to the patient that this could be a skin cancer—specifically, a melanoma.
The FP performed a broad shave biopsy, being careful not to cut into the cartilage. (See the Watch & Learn video on “Shave biopsy.”) The FP did his best to include most of the pigmented area involved, but the convex surface made it difficult to biopsy the whole lesion. He was especially careful to include the darker area because it looked most atypical. The diagnosis came back as lentigo maligna.
The patient was referred for Mohs surgery for complete excision and repair. (Mohs surgery is recommended to spare tissue and maximize cure.) After complete excision, the patient learned that the melanoma was not invasive, but in situ. This suggested a very good prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP explained to the patient that this could be a skin cancer—specifically, a melanoma.
The FP performed a broad shave biopsy, being careful not to cut into the cartilage. (See the Watch & Learn video on “Shave biopsy.”) The FP did his best to include most of the pigmented area involved, but the convex surface made it difficult to biopsy the whole lesion. He was especially careful to include the darker area because it looked most atypical. The diagnosis came back as lentigo maligna.
The patient was referred for Mohs surgery for complete excision and repair. (Mohs surgery is recommended to spare tissue and maximize cure.) After complete excision, the patient learned that the melanoma was not invasive, but in situ. This suggested a very good prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP explained to the patient that this could be a skin cancer—specifically, a melanoma.
The FP performed a broad shave biopsy, being careful not to cut into the cartilage. (See the Watch & Learn video on “Shave biopsy.”) The FP did his best to include most of the pigmented area involved, but the convex surface made it difficult to biopsy the whole lesion. He was especially careful to include the darker area because it looked most atypical. The diagnosis came back as lentigo maligna.
The patient was referred for Mohs surgery for complete excision and repair. (Mohs surgery is recommended to spare tissue and maximize cure.) After complete excision, the patient learned that the melanoma was not invasive, but in situ. This suggested a very good prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Insights could change treatment, classification of MPAL
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
New U.S. cancer cases may exceed 2.3 million by 2035
The American Association for Cancer Research (AACR) has released its annual Cancer Progress Report, detailing recent advances in the fight against cancer and calling on elected officials to address the challenges that remain.
The AACR Cancer Progress Report 2018 lists the 22 new approvals for cancer treatments that have occurred during the last 12 months, including 12 therapies approved to treat hematologic malignancies.
However, the report also notes that cancer continues to pose immense public health challenges in the United States.
The estimated number of new cancer cases for 2018 is 1,735,350, and the estimated number of cancer deaths is 609,640.
The number of new cancer cases is predicted to increase to 2,387,304 in 2035. This is due, in large part, to the rising number of people age 65 and older, according to the report.
With this in mind, the AACR is calling on elected officials to:
Maintain “robust, sustained, and predictable growth” of the National Institutes of Health (NIH) budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
Make sure the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives—including the National Cancer Moonshot—“is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion—a $308 million increase above its FY 2018 level—to secure support for regulatory science and speed the development of medical products that are safe and effective.
Provide the Centers for Disease Control and Prevention’s Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
The American Association for Cancer Research (AACR) has released its annual Cancer Progress Report, detailing recent advances in the fight against cancer and calling on elected officials to address the challenges that remain.
The AACR Cancer Progress Report 2018 lists the 22 new approvals for cancer treatments that have occurred during the last 12 months, including 12 therapies approved to treat hematologic malignancies.
However, the report also notes that cancer continues to pose immense public health challenges in the United States.
The estimated number of new cancer cases for 2018 is 1,735,350, and the estimated number of cancer deaths is 609,640.
The number of new cancer cases is predicted to increase to 2,387,304 in 2035. This is due, in large part, to the rising number of people age 65 and older, according to the report.
With this in mind, the AACR is calling on elected officials to:
Maintain “robust, sustained, and predictable growth” of the National Institutes of Health (NIH) budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
Make sure the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives—including the National Cancer Moonshot—“is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion—a $308 million increase above its FY 2018 level—to secure support for regulatory science and speed the development of medical products that are safe and effective.
Provide the Centers for Disease Control and Prevention’s Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
The American Association for Cancer Research (AACR) has released its annual Cancer Progress Report, detailing recent advances in the fight against cancer and calling on elected officials to address the challenges that remain.
The AACR Cancer Progress Report 2018 lists the 22 new approvals for cancer treatments that have occurred during the last 12 months, including 12 therapies approved to treat hematologic malignancies.
However, the report also notes that cancer continues to pose immense public health challenges in the United States.
The estimated number of new cancer cases for 2018 is 1,735,350, and the estimated number of cancer deaths is 609,640.
The number of new cancer cases is predicted to increase to 2,387,304 in 2035. This is due, in large part, to the rising number of people age 65 and older, according to the report.
With this in mind, the AACR is calling on elected officials to:
Maintain “robust, sustained, and predictable growth” of the National Institutes of Health (NIH) budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
Make sure the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives—including the National Cancer Moonshot—“is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion—a $308 million increase above its FY 2018 level—to secure support for regulatory science and speed the development of medical products that are safe and effective.
Provide the Centers for Disease Control and Prevention’s Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
Prophylaxis reduces bacteremia in some kids
In a phase 3 study, levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias who received intensive chemotherapy.
However, the risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children who underwent hematopoietic stem cell transplant (HSCT).
Sarah Alexander, MD, of the Hospital for Sick Children in Toronto, Ontario, Canada, and her colleagues reported these findings in JAMA.
This multicenter, randomized trial (ACCL0934) enrolled patients aged 6 months to 21 years.
There were 200 patients with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were set to receive chemotherapy and 424 patients who were to receive a myeloablative autologous or allogeneic HSCT.
The acute leukemia patients were randomized to receive no prophylaxis (n=100) or levofloxacin prophylaxis (n=100) for two consecutive cycles of chemotherapy.
The HSCT recipients were randomized to receive no prophylaxis (n=214) or levofloxacin prophylaxis (n=210) during one HSCT procedure.
Results
In the primary analysis of the acute leukemia group (n=195), the incidence of bacteremia was 21.9% for those randomized to levofloxacin and 43.4% for those who did not receive prophylaxis (P=0.001).
In the primary analysis of the HSCT group (n=418), the incidence of bacteremia was 11.0% in the levofloxacin arm and 17.3% in the control arm (P=0.06).
However, a post hoc analysis accounting for time at risk showed a significant difference in favor of prophylaxis in both the acute leukemia and HSCT groups and a similar effect size between groups.
For the acute leukemia group, the rate of bacteremic episodes in the post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.008).
In the HSCT group, the rate of bacteremic episodes was 5.3 versus 10.0 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.02).
The researchers said it is possible that the effect of prophylaxis was similar between the HSCT and acute leukemia groups, but there was reduced power to detect a significant difference because of fewer events among HSCT recipients.
However, the differences between the HSCT and acute leukemia groups in the primary analysis might also be explained by differences in supportive care measures or infections with pathogens that had differential sensitivity to levofloxacin.
The researchers noted that levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk.
Dr. Alexander and her colleagues also said further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits.
In the current study, there were 23 serious adverse events reported in 8 patients. Twelve of these events, occurring in two patients, may have been related to levofloxacin.
This research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
In a phase 3 study, levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias who received intensive chemotherapy.
However, the risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children who underwent hematopoietic stem cell transplant (HSCT).
Sarah Alexander, MD, of the Hospital for Sick Children in Toronto, Ontario, Canada, and her colleagues reported these findings in JAMA.
This multicenter, randomized trial (ACCL0934) enrolled patients aged 6 months to 21 years.
There were 200 patients with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were set to receive chemotherapy and 424 patients who were to receive a myeloablative autologous or allogeneic HSCT.
The acute leukemia patients were randomized to receive no prophylaxis (n=100) or levofloxacin prophylaxis (n=100) for two consecutive cycles of chemotherapy.
The HSCT recipients were randomized to receive no prophylaxis (n=214) or levofloxacin prophylaxis (n=210) during one HSCT procedure.
Results
In the primary analysis of the acute leukemia group (n=195), the incidence of bacteremia was 21.9% for those randomized to levofloxacin and 43.4% for those who did not receive prophylaxis (P=0.001).
In the primary analysis of the HSCT group (n=418), the incidence of bacteremia was 11.0% in the levofloxacin arm and 17.3% in the control arm (P=0.06).
However, a post hoc analysis accounting for time at risk showed a significant difference in favor of prophylaxis in both the acute leukemia and HSCT groups and a similar effect size between groups.
For the acute leukemia group, the rate of bacteremic episodes in the post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.008).
In the HSCT group, the rate of bacteremic episodes was 5.3 versus 10.0 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.02).
The researchers said it is possible that the effect of prophylaxis was similar between the HSCT and acute leukemia groups, but there was reduced power to detect a significant difference because of fewer events among HSCT recipients.
However, the differences between the HSCT and acute leukemia groups in the primary analysis might also be explained by differences in supportive care measures or infections with pathogens that had differential sensitivity to levofloxacin.
The researchers noted that levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk.
Dr. Alexander and her colleagues also said further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits.
In the current study, there were 23 serious adverse events reported in 8 patients. Twelve of these events, occurring in two patients, may have been related to levofloxacin.
This research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
In a phase 3 study, levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias who received intensive chemotherapy.
However, the risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children who underwent hematopoietic stem cell transplant (HSCT).
Sarah Alexander, MD, of the Hospital for Sick Children in Toronto, Ontario, Canada, and her colleagues reported these findings in JAMA.
This multicenter, randomized trial (ACCL0934) enrolled patients aged 6 months to 21 years.
There were 200 patients with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were set to receive chemotherapy and 424 patients who were to receive a myeloablative autologous or allogeneic HSCT.
The acute leukemia patients were randomized to receive no prophylaxis (n=100) or levofloxacin prophylaxis (n=100) for two consecutive cycles of chemotherapy.
The HSCT recipients were randomized to receive no prophylaxis (n=214) or levofloxacin prophylaxis (n=210) during one HSCT procedure.
Results
In the primary analysis of the acute leukemia group (n=195), the incidence of bacteremia was 21.9% for those randomized to levofloxacin and 43.4% for those who did not receive prophylaxis (P=0.001).
In the primary analysis of the HSCT group (n=418), the incidence of bacteremia was 11.0% in the levofloxacin arm and 17.3% in the control arm (P=0.06).
However, a post hoc analysis accounting for time at risk showed a significant difference in favor of prophylaxis in both the acute leukemia and HSCT groups and a similar effect size between groups.
For the acute leukemia group, the rate of bacteremic episodes in the post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.008).
In the HSCT group, the rate of bacteremic episodes was 5.3 versus 10.0 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.02).
The researchers said it is possible that the effect of prophylaxis was similar between the HSCT and acute leukemia groups, but there was reduced power to detect a significant difference because of fewer events among HSCT recipients.
However, the differences between the HSCT and acute leukemia groups in the primary analysis might also be explained by differences in supportive care measures or infections with pathogens that had differential sensitivity to levofloxacin.
The researchers noted that levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk.
Dr. Alexander and her colleagues also said further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits.
In the current study, there were 23 serious adverse events reported in 8 patients. Twelve of these events, occurring in two patients, may have been related to levofloxacin.
This research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
Screen all infants exposed to Zika for eye abnormalities, study suggests
CNS abnormalities associated with antenatal Zika virus infection correlate strongly with opthalmic abnormalities, but there were cases of eye abnormalities in the absence of CNS abnormalities, which suggests a need for universal eye screening in endemic areas, according to a study published in Pediatrics.
Irena Tsui, MD, of the University of California, Los Angeles, and her associates examined 224 infants suspected of antenatal Zika virus infection for eye abnormalities between Jan. 2, 2016, and Feb. 28, 2017. They found that 40% had CNS abnormalities and 25% of all infants had eye abnormalities; of those 90 infants with CNS abnormalities, 54% had eye abnormalities, which makes for an odds ratio of 14.9 (P less than .0001). However, among the 134 infants without CNS abnormalities, 4% had eye abnormalities.
The study also investigated the existence of eye abnormalities among infants did not laboratory-confirmed diagnosis of Zika virus infection. To do so, they performed reverse transcriptase polymerase chain reaction (RT-PCR) testing on 189 infants. They found eye abnormalities among 22% of the 156 RT-PCR–positive infants and 38% of the 68 RT-PCR–unconfirmed infants. Among the 52% of infants with eye abnormalities who were reexamined, there were no signs of worsening, ongoing activity, or regression in their lesions.
The guidelines in Brazil, where the study was performed, recommend eye examinations only for infants with microcephaly, and the United States currently recommends it only at the discretion of the health care provider. The study investigators think that
“The early identification of eye abnormalities enables low-vision interventions to improve visual function with important repercussions for neurocognitive development,” they concluded.
SOURCE: Tsui I et al. Pediatrics. 2018 Oct;142(4):e20181104.
CNS abnormalities associated with antenatal Zika virus infection correlate strongly with opthalmic abnormalities, but there were cases of eye abnormalities in the absence of CNS abnormalities, which suggests a need for universal eye screening in endemic areas, according to a study published in Pediatrics.
Irena Tsui, MD, of the University of California, Los Angeles, and her associates examined 224 infants suspected of antenatal Zika virus infection for eye abnormalities between Jan. 2, 2016, and Feb. 28, 2017. They found that 40% had CNS abnormalities and 25% of all infants had eye abnormalities; of those 90 infants with CNS abnormalities, 54% had eye abnormalities, which makes for an odds ratio of 14.9 (P less than .0001). However, among the 134 infants without CNS abnormalities, 4% had eye abnormalities.
The study also investigated the existence of eye abnormalities among infants did not laboratory-confirmed diagnosis of Zika virus infection. To do so, they performed reverse transcriptase polymerase chain reaction (RT-PCR) testing on 189 infants. They found eye abnormalities among 22% of the 156 RT-PCR–positive infants and 38% of the 68 RT-PCR–unconfirmed infants. Among the 52% of infants with eye abnormalities who were reexamined, there were no signs of worsening, ongoing activity, or regression in their lesions.
The guidelines in Brazil, where the study was performed, recommend eye examinations only for infants with microcephaly, and the United States currently recommends it only at the discretion of the health care provider. The study investigators think that
“The early identification of eye abnormalities enables low-vision interventions to improve visual function with important repercussions for neurocognitive development,” they concluded.
SOURCE: Tsui I et al. Pediatrics. 2018 Oct;142(4):e20181104.
CNS abnormalities associated with antenatal Zika virus infection correlate strongly with opthalmic abnormalities, but there were cases of eye abnormalities in the absence of CNS abnormalities, which suggests a need for universal eye screening in endemic areas, according to a study published in Pediatrics.
Irena Tsui, MD, of the University of California, Los Angeles, and her associates examined 224 infants suspected of antenatal Zika virus infection for eye abnormalities between Jan. 2, 2016, and Feb. 28, 2017. They found that 40% had CNS abnormalities and 25% of all infants had eye abnormalities; of those 90 infants with CNS abnormalities, 54% had eye abnormalities, which makes for an odds ratio of 14.9 (P less than .0001). However, among the 134 infants without CNS abnormalities, 4% had eye abnormalities.
The study also investigated the existence of eye abnormalities among infants did not laboratory-confirmed diagnosis of Zika virus infection. To do so, they performed reverse transcriptase polymerase chain reaction (RT-PCR) testing on 189 infants. They found eye abnormalities among 22% of the 156 RT-PCR–positive infants and 38% of the 68 RT-PCR–unconfirmed infants. Among the 52% of infants with eye abnormalities who were reexamined, there were no signs of worsening, ongoing activity, or regression in their lesions.
The guidelines in Brazil, where the study was performed, recommend eye examinations only for infants with microcephaly, and the United States currently recommends it only at the discretion of the health care provider. The study investigators think that
“The early identification of eye abnormalities enables low-vision interventions to improve visual function with important repercussions for neurocognitive development,” they concluded.
SOURCE: Tsui I et al. Pediatrics. 2018 Oct;142(4):e20181104.
FROM PEDIATRICS
Think DEB, not BMS, with high bleeding risk
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
REPORTING FROM EUROPCR 2018
Key clinical point:
Major finding: The 9-month MACE rate was 1.9% in the drug-eluting balloon group versus 12.4% with a bare-metal stent.
Study details: This prospective, multicenter, single-blind trial randomized 208 high–bleeding risk patients with de novo lesions in large coronary vessels to PCI with a drug-eluting balloon-only or a bare-metal stent.
Disclosures: The presenter reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
Psoriatic arthritis activity spikes briefly postpartum
Disease activity for women with psoriatic arthritis seeking pregnancy was relatively stable through 1 year after delivery, but there was a significant jump at 6 months postpartum follow-up, based on data from a prospective study of more than 100 patients.
Previous research has described rheumatoid arthritis remission during pregnancy, but the symptoms of psoriatic arthritis (PsA) before, during, and after pregnancy have not been well studied, wrote Kristin Ursin, MD, of Trondheim (Norway) University Hospital, and her colleagues.
In a study published in Arthritis Care & Research, the investigators reviewed data from 108 pregnancies in 103 women with PsA who were diagnosed between January 2006 and October 2017.
The participants were enrolled in a Norwegian nationwide registry that followed women with inflammatory diseases from preconception through 1 year after delivery. Disease activity was measured using the DAS28-CRP (28-Joint Disease Activity Score with C-reactive Protein). Participants were assessed at seven time points: before pregnancy, during each trimester, and at 6 weeks, 6 months, and 12 months after delivery.
Although approximately 75% of the women had stable disease activity throughout the study period, activity spiked at 6 months after delivery; DAS28-CRP scores at 6 months post partum were significantly higher than scores at 6 weeks post partum (2.71 vs. 2.45, respectively; P = .016).
The researchers conducted an additional analysis of the potential role of tumor necrosis factor inhibitor use and found that women taking a TNFi had significantly lower disease activity during pregnancy, compared with women not taking a TNFi; mean DAS28-CRP scores at 6 months post partum for these groups were 2.22 and 2.72, respectively (P = .043).
The study was limited by the use of DAS28-CRP as the main measure of disease activity; the index does not include potentially affected distal interphalangeal joints. In addition, not all the participants were assessed at each of the seven time points. However, the results suggest that most pregnant women with PsA experience low levels of disease activity, the researchers said. “Future research on pregnancy in women with PsA should include extended joint count (66/68 joints), and assessment of dactylitis, entheses, axial skeleton, and psoriasis,” they added.
The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
SOURCE: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
Disease activity for women with psoriatic arthritis seeking pregnancy was relatively stable through 1 year after delivery, but there was a significant jump at 6 months postpartum follow-up, based on data from a prospective study of more than 100 patients.
Previous research has described rheumatoid arthritis remission during pregnancy, but the symptoms of psoriatic arthritis (PsA) before, during, and after pregnancy have not been well studied, wrote Kristin Ursin, MD, of Trondheim (Norway) University Hospital, and her colleagues.
In a study published in Arthritis Care & Research, the investigators reviewed data from 108 pregnancies in 103 women with PsA who were diagnosed between January 2006 and October 2017.
The participants were enrolled in a Norwegian nationwide registry that followed women with inflammatory diseases from preconception through 1 year after delivery. Disease activity was measured using the DAS28-CRP (28-Joint Disease Activity Score with C-reactive Protein). Participants were assessed at seven time points: before pregnancy, during each trimester, and at 6 weeks, 6 months, and 12 months after delivery.
Although approximately 75% of the women had stable disease activity throughout the study period, activity spiked at 6 months after delivery; DAS28-CRP scores at 6 months post partum were significantly higher than scores at 6 weeks post partum (2.71 vs. 2.45, respectively; P = .016).
The researchers conducted an additional analysis of the potential role of tumor necrosis factor inhibitor use and found that women taking a TNFi had significantly lower disease activity during pregnancy, compared with women not taking a TNFi; mean DAS28-CRP scores at 6 months post partum for these groups were 2.22 and 2.72, respectively (P = .043).
The study was limited by the use of DAS28-CRP as the main measure of disease activity; the index does not include potentially affected distal interphalangeal joints. In addition, not all the participants were assessed at each of the seven time points. However, the results suggest that most pregnant women with PsA experience low levels of disease activity, the researchers said. “Future research on pregnancy in women with PsA should include extended joint count (66/68 joints), and assessment of dactylitis, entheses, axial skeleton, and psoriasis,” they added.
The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
SOURCE: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
Disease activity for women with psoriatic arthritis seeking pregnancy was relatively stable through 1 year after delivery, but there was a significant jump at 6 months postpartum follow-up, based on data from a prospective study of more than 100 patients.
Previous research has described rheumatoid arthritis remission during pregnancy, but the symptoms of psoriatic arthritis (PsA) before, during, and after pregnancy have not been well studied, wrote Kristin Ursin, MD, of Trondheim (Norway) University Hospital, and her colleagues.
In a study published in Arthritis Care & Research, the investigators reviewed data from 108 pregnancies in 103 women with PsA who were diagnosed between January 2006 and October 2017.
The participants were enrolled in a Norwegian nationwide registry that followed women with inflammatory diseases from preconception through 1 year after delivery. Disease activity was measured using the DAS28-CRP (28-Joint Disease Activity Score with C-reactive Protein). Participants were assessed at seven time points: before pregnancy, during each trimester, and at 6 weeks, 6 months, and 12 months after delivery.
Although approximately 75% of the women had stable disease activity throughout the study period, activity spiked at 6 months after delivery; DAS28-CRP scores at 6 months post partum were significantly higher than scores at 6 weeks post partum (2.71 vs. 2.45, respectively; P = .016).
The researchers conducted an additional analysis of the potential role of tumor necrosis factor inhibitor use and found that women taking a TNFi had significantly lower disease activity during pregnancy, compared with women not taking a TNFi; mean DAS28-CRP scores at 6 months post partum for these groups were 2.22 and 2.72, respectively (P = .043).
The study was limited by the use of DAS28-CRP as the main measure of disease activity; the index does not include potentially affected distal interphalangeal joints. In addition, not all the participants were assessed at each of the seven time points. However, the results suggest that most pregnant women with PsA experience low levels of disease activity, the researchers said. “Future research on pregnancy in women with PsA should include extended joint count (66/68 joints), and assessment of dactylitis, entheses, axial skeleton, and psoriasis,” they added.
The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
SOURCE: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Disease activity for pregnant women with PsA decreased during pregnancy, increased significantly by 6 months post partum, and returned to baseline by 12 months post partum.
Major finding: The average DAS28-CRP was 2.71 at 6 months vs. 2.45 at 6 weeks (P = .016).
Study details: The data come from 108 women with 103 pregnancies who were part of a national registry in Norway.
Disclosures: The researchers had no financial conflicts to disclose. The study was funded by the department of rheumatology at Trondheim University Hospital and the Research Fund of the Norwegian organization for people with rheumatic disease.
Source: Ursin K et al. Arthritis Care Res. 2018 Sep 7. doi: 10.1002/acr.23747.
Novel AKT inhibitor active against MM cells
A novel inhibitor of AKT pathway signaling showed significant cytotoxic activity in mouse models and in human cells isolated from patients with primary or relapsed multiple myeloma (MM), investigators reported.
The experimental agent, labeled HS1793, is a derivative of the naturally occurring antioxidant compound resveratrol. In preclinical studies, HS1793 was shown to offer “great promise in eliminating MM cells and improving therapeutic responses in primary and relapsed/refractory MM patients,” according to Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues.
In a series of experiments, described in the journal Cancer Letters, the investigators demonstrated that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in multiple myeloma cells, and was cytotoxic and specific for myeloma cells in a mouse model of human metastatic myeloma, and in samples of human multiple myeloma cells.
When activated, AKT promotes oncogenesis by in turn activating other downstream pathways involved in proliferation or survival of malignant cells.
“AKT is frequently activated in MM cells and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They first screened 400 compounds, and narrowed in on resveratrol analogs, eventually choosing HS1793 as the most promising candidate.
This first experiment found evidence that suggested that the compound inhibits AKT activation by interfering with the interaction between AKT and its promoter HSP90.
They then showed in human MM cell lines that the antimyeloma action of HS1793 appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In a separate series of experiments, they found that the inhibition by HS1793 of AKT/HSP90 interaction results in cell death by suppressing nuclear factor kappa–B (NF-KB) pathway signaling. The investigators had previously reported that a different compound, an inhibitor of spindle protein kinesin, induced MM cell death via inhibition of NF-KB signaling.
Next, the investigators showed that HS1793-induced cell death was caused by the direct inhibition of AKT that in turn suppressed NF-KB activation.
Finally, they showed in a mouse model of multiple myeloma metastatic to bone that HS1793 “dramatically decreased” lytic skull and femur lesions in treated mice, compared with mice treated with a vehicle placebo, and increased survival of the mice that received the AKT inhibitor.
They also showed that HS1793 was cytotoxic to multiple myeloma cells but not to normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” they wrote.
The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
SOURCE: Song IS et al. Cancer Lett. 2018;432:205-15.
A novel inhibitor of AKT pathway signaling showed significant cytotoxic activity in mouse models and in human cells isolated from patients with primary or relapsed multiple myeloma (MM), investigators reported.
The experimental agent, labeled HS1793, is a derivative of the naturally occurring antioxidant compound resveratrol. In preclinical studies, HS1793 was shown to offer “great promise in eliminating MM cells and improving therapeutic responses in primary and relapsed/refractory MM patients,” according to Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues.
In a series of experiments, described in the journal Cancer Letters, the investigators demonstrated that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in multiple myeloma cells, and was cytotoxic and specific for myeloma cells in a mouse model of human metastatic myeloma, and in samples of human multiple myeloma cells.
When activated, AKT promotes oncogenesis by in turn activating other downstream pathways involved in proliferation or survival of malignant cells.
“AKT is frequently activated in MM cells and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They first screened 400 compounds, and narrowed in on resveratrol analogs, eventually choosing HS1793 as the most promising candidate.
This first experiment found evidence that suggested that the compound inhibits AKT activation by interfering with the interaction between AKT and its promoter HSP90.
They then showed in human MM cell lines that the antimyeloma action of HS1793 appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In a separate series of experiments, they found that the inhibition by HS1793 of AKT/HSP90 interaction results in cell death by suppressing nuclear factor kappa–B (NF-KB) pathway signaling. The investigators had previously reported that a different compound, an inhibitor of spindle protein kinesin, induced MM cell death via inhibition of NF-KB signaling.
Next, the investigators showed that HS1793-induced cell death was caused by the direct inhibition of AKT that in turn suppressed NF-KB activation.
Finally, they showed in a mouse model of multiple myeloma metastatic to bone that HS1793 “dramatically decreased” lytic skull and femur lesions in treated mice, compared with mice treated with a vehicle placebo, and increased survival of the mice that received the AKT inhibitor.
They also showed that HS1793 was cytotoxic to multiple myeloma cells but not to normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” they wrote.
The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
SOURCE: Song IS et al. Cancer Lett. 2018;432:205-15.
A novel inhibitor of AKT pathway signaling showed significant cytotoxic activity in mouse models and in human cells isolated from patients with primary or relapsed multiple myeloma (MM), investigators reported.
The experimental agent, labeled HS1793, is a derivative of the naturally occurring antioxidant compound resveratrol. In preclinical studies, HS1793 was shown to offer “great promise in eliminating MM cells and improving therapeutic responses in primary and relapsed/refractory MM patients,” according to Jin Han, MD, PhD, of Inje University in Busan, South Korea, and colleagues.
In a series of experiments, described in the journal Cancer Letters, the investigators demonstrated that HS1793 decreased AKT signaling to induce mitochondria-mediated cell death in multiple myeloma cells, and was cytotoxic and specific for myeloma cells in a mouse model of human metastatic myeloma, and in samples of human multiple myeloma cells.
When activated, AKT promotes oncogenesis by in turn activating other downstream pathways involved in proliferation or survival of malignant cells.
“AKT is frequently activated in MM cells and the incidence of AKT activation correlates positively with disease activity,” the authors noted.
They first screened 400 compounds, and narrowed in on resveratrol analogs, eventually choosing HS1793 as the most promising candidate.
This first experiment found evidence that suggested that the compound inhibits AKT activation by interfering with the interaction between AKT and its promoter HSP90.
They then showed in human MM cell lines that the antimyeloma action of HS1793 appeared to be from a dose-dependent effect that allowed for mitochondria-mediated programmed cell death.
In a separate series of experiments, they found that the inhibition by HS1793 of AKT/HSP90 interaction results in cell death by suppressing nuclear factor kappa–B (NF-KB) pathway signaling. The investigators had previously reported that a different compound, an inhibitor of spindle protein kinesin, induced MM cell death via inhibition of NF-KB signaling.
Next, the investigators showed that HS1793-induced cell death was caused by the direct inhibition of AKT that in turn suppressed NF-KB activation.
Finally, they showed in a mouse model of multiple myeloma metastatic to bone that HS1793 “dramatically decreased” lytic skull and femur lesions in treated mice, compared with mice treated with a vehicle placebo, and increased survival of the mice that received the AKT inhibitor.
They also showed that HS1793 was cytotoxic to multiple myeloma cells but not to normal plasma cells isolated from patients with MM.
“Given that HS1793 treatment specifically induced the death of primary and relapsed MM cells, HS1793 offers excellent translational potential as a novel MM therapy,” they wrote.
The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
SOURCE: Song IS et al. Cancer Lett. 2018;432:205-15.
FROM CANCER LETTERS
Key clinical point:
Major finding: HS1793 showed significant multiple myeloma cytotoxicity in mouse models and in human cells isolated from patients with primary or relapsed/refractory myeloma.
Study details: Preclinical investigations in cell lines, murine models, and isolated human multiple myeloma cells.
Disclosures: The study was supported by grants from the Korean government. The researchers reported having no potential conflicts of interest.
Source: Song IS et al. Cancer Lett. 2018:432:205-15.
MDS posttransplant gene sequencing prognostic for progression
For patients with myelodysplastic syndrome, gene sequencing of bone marrow samples early after bone marrow transplant with curative intent may provide important prognostic information.
Among 86 patients with myelodysplastic syndrome (MDS), higher maximum variant allele frequency of residual disease–associated mutations at 30 days posttransplantation was significantly associated with disease progression and lower rates of progression-free survival (PFS) at 1 year, reported Eric J. Duncavage, MD, from Washington University in St. Louis, and his colleagues.
“Although this exploratory study has limitations, our results suggest that sequencing-based detection of tumor cells and measurable residual disease after allogeneic hematopoietic stem cell transplantation has prognostic significance for patients with MDS,” they wrote in the New England Journal of Medicine.
Risk of progression was significantly higher among patients who had undergone reduced-intensity conditioning prior to hematopoietic stem cell transplants (HSCT) than among patients who had undergone myeloablative conditioning regimens.
To get a better handle on the significance of molecular predictors of disease progression after HSCT, the authors used enhanced exome sequencing to evaluate paired samples of bone marrow and control DNA from normal skin, and error-corrected sequencing to identify somatic single-nucleotide variant mutations in posttransplant samples.
They detected at least one validated somatic mutation in the pretransplant samples from 86 of 90 patients. Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after transplantation. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the authors explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not have progression (P less than .001).
In all, 53.1% of patients with one or more mutations with a variant allele frequency of at least 0.5% at 30 days had disease progression within a year, compared with 13% of patients who did not have the mutations, even after adjustment for the type of conditioning regimen. The hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P less than .001).
The association between the presence of one or more mutations with a variant allele frequency of at least 0.5% with increased risk of disease progression was also seen at 100 days, even after adjustment for conditioning regimen (66.7% vs. 0%; HR, 6.52; P less than .001). In multivariable analysis controlling for prognostic scores, maximum variant allele frequency at 30 days, TP53 mutation status and conditioning regimen, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than fourfold risk of progression, including when the revised International Prognostic Scoring System score and conditioning regimen were considered as covariates. (HR, 4.48; P less than .001),
A separate multivariable analysis of PFS controlling for maximum variant allele frequency at day 30, conditioning regimen, age at transplantation, and type of MDS showed that mutations were associated with a more than twofold risk of progression or death (HR, 2.39; P = .002).
This analysis also showed that secondary acute myeloid leukemia was associated with worse PFS, compared with primary MDS (HR, 2.24; P = .001).
The investigators acknowledged that the high-coverage exome sequencing technique used for the study is not routinely available in the clinic. To control for this, they also looked at their data using a subset of genes that are usually included in gene sequencing panels for MDS and AML.
“Although we identified fewer patients with mutations with the use of this approach than with enhanced exome sequencing, the prognostic value of detection of measurable residual disease was still highly clinically significant,” they wrote.
The study was supported by grants from the Leukemia and Lymphoma Society, Edward P. Evans Foundation, National Cancer Institute, National Institutes of Health, Gabrielle’s Angel Foundation, and the Lottie Caroline Hardy Trust. Dr. Duncavage disclosed personal fees from AbbVie and Cofactor Genomics. The majority of coauthors reported nothing to disclose.
SOURCE: Duncavage EJ et al. N Engl J Med 2018;379:1028-41.
For patients with myelodysplastic syndrome, gene sequencing of bone marrow samples early after bone marrow transplant with curative intent may provide important prognostic information.
Among 86 patients with myelodysplastic syndrome (MDS), higher maximum variant allele frequency of residual disease–associated mutations at 30 days posttransplantation was significantly associated with disease progression and lower rates of progression-free survival (PFS) at 1 year, reported Eric J. Duncavage, MD, from Washington University in St. Louis, and his colleagues.
“Although this exploratory study has limitations, our results suggest that sequencing-based detection of tumor cells and measurable residual disease after allogeneic hematopoietic stem cell transplantation has prognostic significance for patients with MDS,” they wrote in the New England Journal of Medicine.
Risk of progression was significantly higher among patients who had undergone reduced-intensity conditioning prior to hematopoietic stem cell transplants (HSCT) than among patients who had undergone myeloablative conditioning regimens.
To get a better handle on the significance of molecular predictors of disease progression after HSCT, the authors used enhanced exome sequencing to evaluate paired samples of bone marrow and control DNA from normal skin, and error-corrected sequencing to identify somatic single-nucleotide variant mutations in posttransplant samples.
They detected at least one validated somatic mutation in the pretransplant samples from 86 of 90 patients. Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after transplantation. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the authors explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not have progression (P less than .001).
In all, 53.1% of patients with one or more mutations with a variant allele frequency of at least 0.5% at 30 days had disease progression within a year, compared with 13% of patients who did not have the mutations, even after adjustment for the type of conditioning regimen. The hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P less than .001).
The association between the presence of one or more mutations with a variant allele frequency of at least 0.5% with increased risk of disease progression was also seen at 100 days, even after adjustment for conditioning regimen (66.7% vs. 0%; HR, 6.52; P less than .001). In multivariable analysis controlling for prognostic scores, maximum variant allele frequency at 30 days, TP53 mutation status and conditioning regimen, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than fourfold risk of progression, including when the revised International Prognostic Scoring System score and conditioning regimen were considered as covariates. (HR, 4.48; P less than .001),
A separate multivariable analysis of PFS controlling for maximum variant allele frequency at day 30, conditioning regimen, age at transplantation, and type of MDS showed that mutations were associated with a more than twofold risk of progression or death (HR, 2.39; P = .002).
This analysis also showed that secondary acute myeloid leukemia was associated with worse PFS, compared with primary MDS (HR, 2.24; P = .001).
The investigators acknowledged that the high-coverage exome sequencing technique used for the study is not routinely available in the clinic. To control for this, they also looked at their data using a subset of genes that are usually included in gene sequencing panels for MDS and AML.
“Although we identified fewer patients with mutations with the use of this approach than with enhanced exome sequencing, the prognostic value of detection of measurable residual disease was still highly clinically significant,” they wrote.
The study was supported by grants from the Leukemia and Lymphoma Society, Edward P. Evans Foundation, National Cancer Institute, National Institutes of Health, Gabrielle’s Angel Foundation, and the Lottie Caroline Hardy Trust. Dr. Duncavage disclosed personal fees from AbbVie and Cofactor Genomics. The majority of coauthors reported nothing to disclose.
SOURCE: Duncavage EJ et al. N Engl J Med 2018;379:1028-41.
For patients with myelodysplastic syndrome, gene sequencing of bone marrow samples early after bone marrow transplant with curative intent may provide important prognostic information.
Among 86 patients with myelodysplastic syndrome (MDS), higher maximum variant allele frequency of residual disease–associated mutations at 30 days posttransplantation was significantly associated with disease progression and lower rates of progression-free survival (PFS) at 1 year, reported Eric J. Duncavage, MD, from Washington University in St. Louis, and his colleagues.
“Although this exploratory study has limitations, our results suggest that sequencing-based detection of tumor cells and measurable residual disease after allogeneic hematopoietic stem cell transplantation has prognostic significance for patients with MDS,” they wrote in the New England Journal of Medicine.
Risk of progression was significantly higher among patients who had undergone reduced-intensity conditioning prior to hematopoietic stem cell transplants (HSCT) than among patients who had undergone myeloablative conditioning regimens.
To get a better handle on the significance of molecular predictors of disease progression after HSCT, the authors used enhanced exome sequencing to evaluate paired samples of bone marrow and control DNA from normal skin, and error-corrected sequencing to identify somatic single-nucleotide variant mutations in posttransplant samples.
They detected at least one validated somatic mutation in the pretransplant samples from 86 of 90 patients. Of the 86 patients, 32 had at least one mutation with a maximum variant allele frequency of at least 0.5% detected 30 days after transplantation. The frequency is equivalent to 1 heterozygous mutant cell per 100 cells, the authors explained.
Patients who experienced disease progression had mutations with a median maximum variant allele frequency of 0.9%, compared with 0% for patients who did not have progression (P less than .001).
In all, 53.1% of patients with one or more mutations with a variant allele frequency of at least 0.5% at 30 days had disease progression within a year, compared with 13% of patients who did not have the mutations, even after adjustment for the type of conditioning regimen. The hazard ratio (HR) for disease progression in the patients with mutations was 3.86 (P less than .001).
The association between the presence of one or more mutations with a variant allele frequency of at least 0.5% with increased risk of disease progression was also seen at 100 days, even after adjustment for conditioning regimen (66.7% vs. 0%; HR, 6.52; P less than .001). In multivariable analysis controlling for prognostic scores, maximum variant allele frequency at 30 days, TP53 mutation status and conditioning regimen, the presence of a mutation with at least 0.5% variant allele frequency was associated with a more than fourfold risk of progression, including when the revised International Prognostic Scoring System score and conditioning regimen were considered as covariates. (HR, 4.48; P less than .001),
A separate multivariable analysis of PFS controlling for maximum variant allele frequency at day 30, conditioning regimen, age at transplantation, and type of MDS showed that mutations were associated with a more than twofold risk of progression or death (HR, 2.39; P = .002).
This analysis also showed that secondary acute myeloid leukemia was associated with worse PFS, compared with primary MDS (HR, 2.24; P = .001).
The investigators acknowledged that the high-coverage exome sequencing technique used for the study is not routinely available in the clinic. To control for this, they also looked at their data using a subset of genes that are usually included in gene sequencing panels for MDS and AML.
“Although we identified fewer patients with mutations with the use of this approach than with enhanced exome sequencing, the prognostic value of detection of measurable residual disease was still highly clinically significant,” they wrote.
The study was supported by grants from the Leukemia and Lymphoma Society, Edward P. Evans Foundation, National Cancer Institute, National Institutes of Health, Gabrielle’s Angel Foundation, and the Lottie Caroline Hardy Trust. Dr. Duncavage disclosed personal fees from AbbVie and Cofactor Genomics. The majority of coauthors reported nothing to disclose.
SOURCE: Duncavage EJ et al. N Engl J Med 2018;379:1028-41.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Myelodysplastic syndrome (MDS)–associated mutations present 30 days after stem cell transplant may be predict disease progression and survival.
Major finding: Higher maximum variant allele frequency of residual disease–associated mutations at 30 days posttransplantation was significantly associated with disease progression and lower rates of progression-free survival at 1 year.
Study details: Exploratory study of mutations pre- and posttransplant in 90 patients with primary or therapy-related MDS or secondary acute myeloid leukemia.
Disclosures: The study was supported by grants from the Leukemia and Lymphoma Society, Edward P. Evans Foundation, National Cancer Institute, National Institutes of Health, Gabrielle’s Angel Foundation, and the Lottie Caroline Hardy Trust. Dr. Duncavage disclosed personal fees from AbbVie and Cofactor Genomics. The majority of the coauthors reported nothing to disclose.
Source: Duncavage EJ et al. N Engl J Med 2018;379:1028-41.