User login
Don’t forget about OSHA
With the bewildering array of new bureaucracies that private practices are now forced to contend with, it is easy to forget about the older ones – especially the Occupational Health and Safety Administration (OSHA).
with all the applicable regulations. Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain site, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s Web site or order it at no charge by calling 800-321-OSHA.
Next, how old is your written exposure control plan for blood-borne pathogens? It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at your decision and why you feel that your current protocol is as good or better.
Review your list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” (My favorite in that category is liquid nitrogen; it’s hard to envision anything less hazardous, since it evaporates instantly if spilled, and cannot injure skin, or anything else, without purposeful, sustained exposure – and is great, incidentally, for extinguishing small fires.) For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
Check out your building’s exits. Everyone must be able to evacuate your office quickly in case of fire or other emergencies. At a minimum, you (or the owner of the building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars.
How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections, as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
With the bewildering array of new bureaucracies that private practices are now forced to contend with, it is easy to forget about the older ones – especially the Occupational Health and Safety Administration (OSHA).
with all the applicable regulations. Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain site, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s Web site or order it at no charge by calling 800-321-OSHA.
Next, how old is your written exposure control plan for blood-borne pathogens? It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at your decision and why you feel that your current protocol is as good or better.
Review your list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” (My favorite in that category is liquid nitrogen; it’s hard to envision anything less hazardous, since it evaporates instantly if spilled, and cannot injure skin, or anything else, without purposeful, sustained exposure – and is great, incidentally, for extinguishing small fires.) For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
Check out your building’s exits. Everyone must be able to evacuate your office quickly in case of fire or other emergencies. At a minimum, you (or the owner of the building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars.
How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections, as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
With the bewildering array of new bureaucracies that private practices are now forced to contend with, it is easy to forget about the older ones – especially the Occupational Health and Safety Administration (OSHA).
with all the applicable regulations. Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain site, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s Web site or order it at no charge by calling 800-321-OSHA.
Next, how old is your written exposure control plan for blood-borne pathogens? It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at your decision and why you feel that your current protocol is as good or better.
Review your list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” (My favorite in that category is liquid nitrogen; it’s hard to envision anything less hazardous, since it evaporates instantly if spilled, and cannot injure skin, or anything else, without purposeful, sustained exposure – and is great, incidentally, for extinguishing small fires.) For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
Check out your building’s exits. Everyone must be able to evacuate your office quickly in case of fire or other emergencies. At a minimum, you (or the owner of the building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars.
How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections, as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Review protocols, follow reprocessing guidelines to cut device-related HAIs
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
REPORTING FROM ICEID 2018
Key clinical point: Medical devices can be reservoirs and transmission vectors for health care–associated infections.
Major finding: Of 285 consultations, 48 involved medical devices or device reprocessing.
Study details: A review of records from 285 consultations
Disclosures: Dr. Benowitz reported having no disclosures
Source: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
Coronary CT angiography radiation dose fell 78% from 2007-2017
MUNICH – The median radiation dosage received by patients worldwide undergoing coronary CT angiography fell by 78% from 2007 to 2017, according to a prospective study with more than 4,500 patients.
This substantial drop in radiation occurred with a steady rate of nondiagnostic CT scans, less than 2% in both 2007 and 2017.
“Given the high diagnostic accuracy and the low radiation dose, coronary CT angiography should be considered as a first-line diagnostic test,” Jörg Hausleiter, MD, said at the annual congress of the European Society of Cardiology.
The results also showed a huge disparity in the range of radiation doses used worldwide, with a 37-fold intersite variability in the median dose. This finding “underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols,” said Dr. Hausleiter, professor of medicine at the University of Munich Clinic. He suggested updated imaging guidelines on radiation levels, more educational sessions on how to perform coronary CT angiography, and actions by vendors to adjust their standard imaging protocols.
The Prospective Multicenter Registry on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice in 2017 (PROTECTION-VI) study included 4,502 patients from a total of 61 sites in 32 countries. At each participating site, investigators enrolled consecutive adults during a randomly selected month in 2017, with a median of 51 patients enrolled at each site undergoing diagnostic coronary CT angiography. Comparison data for 2007 came from a similar study run by Dr. Hausleiter and his associates at that time, with 1,965 patients undergoing coronary CT angiography (JAMA. 2009 Feb 4;301[5]:500-7). In 2007, the median dose-length product of radiation for each scan was 885 mGy x cm, which corresponds to a radiation dose of about 12.4 mSv. In 2017, the median dose-length product was 195 mGy x cm, corresponding to a dose of about 2.7 mSv. By both measures the median dose dropped by roughly 78%.
A multivariate analysis identified three changes in the way clinicians obtained most of the CT scans during the two studied time periods that seemed to explain the drop in radiation dose. First, more scan protocols in 2017 used low tube potential; second, more protocols in 2017 used prospectively ECG-triggered axial high-pitch scans; and third, 2017 had increased use of iterative image reconstruction, Dr. Hausleiter said. Patient variables that had modest but significant links with increased radiation doses were higher body weight, higher heart rate, and no sinus rhythm.
Concurrently with Dr. Hausleiter’s talk at the congress, the results appeared in an article online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy546).
The results from the PROTECTION VI study show that the radiation doses used today for coronary CT angiography are very low. But the study is limited by looking only at the median doses used at 61 sites worldwide. I hope that the dose level seen in the study is what is now used at community hospitals across the United States, but for the time being we can’t be sure.
With today’s CT technology, as long as the dose-length product a patient receives is less than 200 mGy x cm, the facility is doing a good job of minimizing radiation exposure. As CT technology continues to improve, we can expect the median dose to fall even more in the future.
Todd C. Villines, MD , a cardiologist at Georgetown University in Washington and immediate past president of the Society of Cardiovascular CT, made these comments in an interview. He had no relevant disclosures.
The results from the PROTECTION VI study show that the radiation doses used today for coronary CT angiography are very low. But the study is limited by looking only at the median doses used at 61 sites worldwide. I hope that the dose level seen in the study is what is now used at community hospitals across the United States, but for the time being we can’t be sure.
With today’s CT technology, as long as the dose-length product a patient receives is less than 200 mGy x cm, the facility is doing a good job of minimizing radiation exposure. As CT technology continues to improve, we can expect the median dose to fall even more in the future.
Todd C. Villines, MD , a cardiologist at Georgetown University in Washington and immediate past president of the Society of Cardiovascular CT, made these comments in an interview. He had no relevant disclosures.
The results from the PROTECTION VI study show that the radiation doses used today for coronary CT angiography are very low. But the study is limited by looking only at the median doses used at 61 sites worldwide. I hope that the dose level seen in the study is what is now used at community hospitals across the United States, but for the time being we can’t be sure.
With today’s CT technology, as long as the dose-length product a patient receives is less than 200 mGy x cm, the facility is doing a good job of minimizing radiation exposure. As CT technology continues to improve, we can expect the median dose to fall even more in the future.
Todd C. Villines, MD , a cardiologist at Georgetown University in Washington and immediate past president of the Society of Cardiovascular CT, made these comments in an interview. He had no relevant disclosures.
MUNICH – The median radiation dosage received by patients worldwide undergoing coronary CT angiography fell by 78% from 2007 to 2017, according to a prospective study with more than 4,500 patients.
This substantial drop in radiation occurred with a steady rate of nondiagnostic CT scans, less than 2% in both 2007 and 2017.
“Given the high diagnostic accuracy and the low radiation dose, coronary CT angiography should be considered as a first-line diagnostic test,” Jörg Hausleiter, MD, said at the annual congress of the European Society of Cardiology.
The results also showed a huge disparity in the range of radiation doses used worldwide, with a 37-fold intersite variability in the median dose. This finding “underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols,” said Dr. Hausleiter, professor of medicine at the University of Munich Clinic. He suggested updated imaging guidelines on radiation levels, more educational sessions on how to perform coronary CT angiography, and actions by vendors to adjust their standard imaging protocols.
The Prospective Multicenter Registry on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice in 2017 (PROTECTION-VI) study included 4,502 patients from a total of 61 sites in 32 countries. At each participating site, investigators enrolled consecutive adults during a randomly selected month in 2017, with a median of 51 patients enrolled at each site undergoing diagnostic coronary CT angiography. Comparison data for 2007 came from a similar study run by Dr. Hausleiter and his associates at that time, with 1,965 patients undergoing coronary CT angiography (JAMA. 2009 Feb 4;301[5]:500-7). In 2007, the median dose-length product of radiation for each scan was 885 mGy x cm, which corresponds to a radiation dose of about 12.4 mSv. In 2017, the median dose-length product was 195 mGy x cm, corresponding to a dose of about 2.7 mSv. By both measures the median dose dropped by roughly 78%.
A multivariate analysis identified three changes in the way clinicians obtained most of the CT scans during the two studied time periods that seemed to explain the drop in radiation dose. First, more scan protocols in 2017 used low tube potential; second, more protocols in 2017 used prospectively ECG-triggered axial high-pitch scans; and third, 2017 had increased use of iterative image reconstruction, Dr. Hausleiter said. Patient variables that had modest but significant links with increased radiation doses were higher body weight, higher heart rate, and no sinus rhythm.
Concurrently with Dr. Hausleiter’s talk at the congress, the results appeared in an article online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy546).
MUNICH – The median radiation dosage received by patients worldwide undergoing coronary CT angiography fell by 78% from 2007 to 2017, according to a prospective study with more than 4,500 patients.
This substantial drop in radiation occurred with a steady rate of nondiagnostic CT scans, less than 2% in both 2007 and 2017.
“Given the high diagnostic accuracy and the low radiation dose, coronary CT angiography should be considered as a first-line diagnostic test,” Jörg Hausleiter, MD, said at the annual congress of the European Society of Cardiology.
The results also showed a huge disparity in the range of radiation doses used worldwide, with a 37-fold intersite variability in the median dose. This finding “underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols,” said Dr. Hausleiter, professor of medicine at the University of Munich Clinic. He suggested updated imaging guidelines on radiation levels, more educational sessions on how to perform coronary CT angiography, and actions by vendors to adjust their standard imaging protocols.
The Prospective Multicenter Registry on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice in 2017 (PROTECTION-VI) study included 4,502 patients from a total of 61 sites in 32 countries. At each participating site, investigators enrolled consecutive adults during a randomly selected month in 2017, with a median of 51 patients enrolled at each site undergoing diagnostic coronary CT angiography. Comparison data for 2007 came from a similar study run by Dr. Hausleiter and his associates at that time, with 1,965 patients undergoing coronary CT angiography (JAMA. 2009 Feb 4;301[5]:500-7). In 2007, the median dose-length product of radiation for each scan was 885 mGy x cm, which corresponds to a radiation dose of about 12.4 mSv. In 2017, the median dose-length product was 195 mGy x cm, corresponding to a dose of about 2.7 mSv. By both measures the median dose dropped by roughly 78%.
A multivariate analysis identified three changes in the way clinicians obtained most of the CT scans during the two studied time periods that seemed to explain the drop in radiation dose. First, more scan protocols in 2017 used low tube potential; second, more protocols in 2017 used prospectively ECG-triggered axial high-pitch scans; and third, 2017 had increased use of iterative image reconstruction, Dr. Hausleiter said. Patient variables that had modest but significant links with increased radiation doses were higher body weight, higher heart rate, and no sinus rhythm.
Concurrently with Dr. Hausleiter’s talk at the congress, the results appeared in an article online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy546).
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: The median radiation dose during coronary CT angiography fell from 2007 to 2017.
Major finding: The median dose-length product was 195 mGY x cm in 2017 and 885 mGy x cm in 2007.
Study details: PROTECTION VI, a prospective study run at 61 sites in 32 countries.
Disclosures: PROTECTION VI received no commercial funding. Dr. Hausleiter has received research funding from Abbott Vascular.
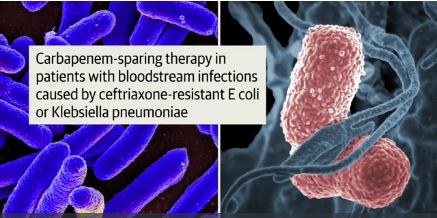
Piperacillin-tazobactam fails to outperform meropenem in bloodstream infections
A new study finds that piperacillin-tazobactam doesn’t improve mortality compared to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae. The findings were so striking that the study was ended early.

Courtesy: JAMA
“These findings do not support use of piperacillin-tazobactam in this setting,” wrote the authors. The report was published Sept. 11 in JAMA (2018;320[10]:984-94.)
According to the Centers for Disease Control and Prevention, an estimated 1,700 deaths in the United States in 2011 were caused by gram-negative bacteria that produce extended-spectrum beta-lactamase enzymes.
While carbapenems such as meropenem (Merrem) are “regarded as the treatment of choice for serious infections,” the MERINO trial (NCT02176122) authors wrote, their rising use could lead to drug resistance.
One alternate option is to embrace beta-lactam/beta-lactamase inhibitors such as piperacillin-tazobactam (Zosyn), the researchers noted, but research has produced conflicting results.
Piperacillin-tazobactam is an injected penicillin antibiotic used to treat conditions such as severe pneumonia, complicated urinary tract infections and complicated skin and soft tissue infections.
For the new study, researchers led by Patrick N. A. Harris, MBBS, of the University of Queensland, randomly assigned 188 patients to intravenous piperacillin-tazobactam (4.5 g every 6 hours) and 191 patients to meropenem (1 g every 8 hours) for 4-14 days, depending on clinician’s preference. (12 other patients did not continue with the study after initial randomization due to factors such as errors).
All patients were adults and had at least one blood test showing they were positive for E. coli or K. pneumoniae. They all had to be nonsusceptible to ceftriaxone (Rocephin) but susceptible to piperacillin-tazobactam.
The study was ceased prior to enrollment because of the risk of harm. Interim findings suggested the study was unlikely to show higher effectiveness for piperacillin-tazobactam
The primary analysis included 379 patients (mean age 67 years, 48% were women), and the primary outcome analysis included 378 patients.
A total of 23 (12.3%) of 187 patients in the piperacillin-tazobactam group died by 30 days compared to 7 (3.7%) of 191 in the meropenem group (risk difference: 8.6%, P = .90 for noninferiority).
By day 4, 68% of the piperacillin-tazobactam group and 75% of the meropenem group achieved clinical and microbiological resolution.
Serious adverse effects other than death were rare, occurring in around 3% of the piperacillin-tazobactam group and nearly 2% of the meropenem group.
The researchers note various limitations, including the unblinded nature of the study and the fact that it’s not known if extended or continuous infusions of piperacillin-tazobactam would boost the drug’s effectiveness. They also note that delays resulted in some patients initially receiving treatment with one of the study’s two drugs before being randomized to the other.
The study authors caution that it’s not clear if newer beta-lactam/beta-lactamase inhibitors agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be effective in this population.
The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures, including funding from drugmakers such as Pfizer, maker of Zosyn (through its subsidiary Wyeth) and Merrem.
SOURCE: Harris PNA et al. JAMA 2018 Sep 11;320[10]:984-94. doi: 10.1001/jama.2018.12163.
There may be no greater present-day antibiotic resistance threat than the prospect of nonsusceptibility developing among patients with blood poisoning who receive carbapenems for infections caused by E. coli or K. pneumonia. New antibiotics are being developed and researchers are taking a second look at existing drugs.
The new study aims to shed light on the effectiveness of piperacillin-tazobactam in this population compared to carbapenems. Surprisingly, the researchers failed to show a mortality benefit for the drug vs. meropenem. What now? Future research could shed light on newer beta-lactam/beta-lactamase inhibitors, and studies may also offer insight into alternatives such as short-term antibiotic therapy. The upcoming availability of electronic decision support tools may be helpful, and it remains important to prevent infections in the first place.
This commentary was taken from an editorial by Mary K. Hayden, MD, and Sarah Y. Won, MD, MPH, of Rush University Medical Center (JAMA 2018 Sep 11;320[10]:979-81). Dr. Hayden reports research funding from Colorox and serving as an investigator on research products that received product support from Sage Corporation, Molnlycke, Clorox, OpGen and Medline.
There may be no greater present-day antibiotic resistance threat than the prospect of nonsusceptibility developing among patients with blood poisoning who receive carbapenems for infections caused by E. coli or K. pneumonia. New antibiotics are being developed and researchers are taking a second look at existing drugs.
The new study aims to shed light on the effectiveness of piperacillin-tazobactam in this population compared to carbapenems. Surprisingly, the researchers failed to show a mortality benefit for the drug vs. meropenem. What now? Future research could shed light on newer beta-lactam/beta-lactamase inhibitors, and studies may also offer insight into alternatives such as short-term antibiotic therapy. The upcoming availability of electronic decision support tools may be helpful, and it remains important to prevent infections in the first place.
This commentary was taken from an editorial by Mary K. Hayden, MD, and Sarah Y. Won, MD, MPH, of Rush University Medical Center (JAMA 2018 Sep 11;320[10]:979-81). Dr. Hayden reports research funding from Colorox and serving as an investigator on research products that received product support from Sage Corporation, Molnlycke, Clorox, OpGen and Medline.
There may be no greater present-day antibiotic resistance threat than the prospect of nonsusceptibility developing among patients with blood poisoning who receive carbapenems for infections caused by E. coli or K. pneumonia. New antibiotics are being developed and researchers are taking a second look at existing drugs.
The new study aims to shed light on the effectiveness of piperacillin-tazobactam in this population compared to carbapenems. Surprisingly, the researchers failed to show a mortality benefit for the drug vs. meropenem. What now? Future research could shed light on newer beta-lactam/beta-lactamase inhibitors, and studies may also offer insight into alternatives such as short-term antibiotic therapy. The upcoming availability of electronic decision support tools may be helpful, and it remains important to prevent infections in the first place.
This commentary was taken from an editorial by Mary K. Hayden, MD, and Sarah Y. Won, MD, MPH, of Rush University Medical Center (JAMA 2018 Sep 11;320[10]:979-81). Dr. Hayden reports research funding from Colorox and serving as an investigator on research products that received product support from Sage Corporation, Molnlycke, Clorox, OpGen and Medline.
A new study finds that piperacillin-tazobactam doesn’t improve mortality compared to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae. The findings were so striking that the study was ended early.

Courtesy: JAMA
“These findings do not support use of piperacillin-tazobactam in this setting,” wrote the authors. The report was published Sept. 11 in JAMA (2018;320[10]:984-94.)
According to the Centers for Disease Control and Prevention, an estimated 1,700 deaths in the United States in 2011 were caused by gram-negative bacteria that produce extended-spectrum beta-lactamase enzymes.
While carbapenems such as meropenem (Merrem) are “regarded as the treatment of choice for serious infections,” the MERINO trial (NCT02176122) authors wrote, their rising use could lead to drug resistance.
One alternate option is to embrace beta-lactam/beta-lactamase inhibitors such as piperacillin-tazobactam (Zosyn), the researchers noted, but research has produced conflicting results.
Piperacillin-tazobactam is an injected penicillin antibiotic used to treat conditions such as severe pneumonia, complicated urinary tract infections and complicated skin and soft tissue infections.
For the new study, researchers led by Patrick N. A. Harris, MBBS, of the University of Queensland, randomly assigned 188 patients to intravenous piperacillin-tazobactam (4.5 g every 6 hours) and 191 patients to meropenem (1 g every 8 hours) for 4-14 days, depending on clinician’s preference. (12 other patients did not continue with the study after initial randomization due to factors such as errors).
All patients were adults and had at least one blood test showing they were positive for E. coli or K. pneumoniae. They all had to be nonsusceptible to ceftriaxone (Rocephin) but susceptible to piperacillin-tazobactam.
The study was ceased prior to enrollment because of the risk of harm. Interim findings suggested the study was unlikely to show higher effectiveness for piperacillin-tazobactam
The primary analysis included 379 patients (mean age 67 years, 48% were women), and the primary outcome analysis included 378 patients.
A total of 23 (12.3%) of 187 patients in the piperacillin-tazobactam group died by 30 days compared to 7 (3.7%) of 191 in the meropenem group (risk difference: 8.6%, P = .90 for noninferiority).
By day 4, 68% of the piperacillin-tazobactam group and 75% of the meropenem group achieved clinical and microbiological resolution.
Serious adverse effects other than death were rare, occurring in around 3% of the piperacillin-tazobactam group and nearly 2% of the meropenem group.
The researchers note various limitations, including the unblinded nature of the study and the fact that it’s not known if extended or continuous infusions of piperacillin-tazobactam would boost the drug’s effectiveness. They also note that delays resulted in some patients initially receiving treatment with one of the study’s two drugs before being randomized to the other.
The study authors caution that it’s not clear if newer beta-lactam/beta-lactamase inhibitors agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be effective in this population.
The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures, including funding from drugmakers such as Pfizer, maker of Zosyn (through its subsidiary Wyeth) and Merrem.
SOURCE: Harris PNA et al. JAMA 2018 Sep 11;320[10]:984-94. doi: 10.1001/jama.2018.12163.
A new study finds that piperacillin-tazobactam doesn’t improve mortality compared to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae. The findings were so striking that the study was ended early.

Courtesy: JAMA
“These findings do not support use of piperacillin-tazobactam in this setting,” wrote the authors. The report was published Sept. 11 in JAMA (2018;320[10]:984-94.)
According to the Centers for Disease Control and Prevention, an estimated 1,700 deaths in the United States in 2011 were caused by gram-negative bacteria that produce extended-spectrum beta-lactamase enzymes.
While carbapenems such as meropenem (Merrem) are “regarded as the treatment of choice for serious infections,” the MERINO trial (NCT02176122) authors wrote, their rising use could lead to drug resistance.
One alternate option is to embrace beta-lactam/beta-lactamase inhibitors such as piperacillin-tazobactam (Zosyn), the researchers noted, but research has produced conflicting results.
Piperacillin-tazobactam is an injected penicillin antibiotic used to treat conditions such as severe pneumonia, complicated urinary tract infections and complicated skin and soft tissue infections.
For the new study, researchers led by Patrick N. A. Harris, MBBS, of the University of Queensland, randomly assigned 188 patients to intravenous piperacillin-tazobactam (4.5 g every 6 hours) and 191 patients to meropenem (1 g every 8 hours) for 4-14 days, depending on clinician’s preference. (12 other patients did not continue with the study after initial randomization due to factors such as errors).
All patients were adults and had at least one blood test showing they were positive for E. coli or K. pneumoniae. They all had to be nonsusceptible to ceftriaxone (Rocephin) but susceptible to piperacillin-tazobactam.
The study was ceased prior to enrollment because of the risk of harm. Interim findings suggested the study was unlikely to show higher effectiveness for piperacillin-tazobactam
The primary analysis included 379 patients (mean age 67 years, 48% were women), and the primary outcome analysis included 378 patients.
A total of 23 (12.3%) of 187 patients in the piperacillin-tazobactam group died by 30 days compared to 7 (3.7%) of 191 in the meropenem group (risk difference: 8.6%, P = .90 for noninferiority).
By day 4, 68% of the piperacillin-tazobactam group and 75% of the meropenem group achieved clinical and microbiological resolution.
Serious adverse effects other than death were rare, occurring in around 3% of the piperacillin-tazobactam group and nearly 2% of the meropenem group.
The researchers note various limitations, including the unblinded nature of the study and the fact that it’s not known if extended or continuous infusions of piperacillin-tazobactam would boost the drug’s effectiveness. They also note that delays resulted in some patients initially receiving treatment with one of the study’s two drugs before being randomized to the other.
The study authors caution that it’s not clear if newer beta-lactam/beta-lactamase inhibitors agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be effective in this population.
The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures, including funding from drugmakers such as Pfizer, maker of Zosyn (through its subsidiary Wyeth) and Merrem.
SOURCE: Harris PNA et al. JAMA 2018 Sep 11;320[10]:984-94. doi: 10.1001/jama.2018.12163.
FROM JAMA
Key clinical point: Piperacillin-tazobactam isn’t a superior alternative to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae.
Major finding: By 30 days, 12% of patients in the piperacillin-tazobactam group died compared to 4% of the meropenem group.
Study details: Unblinded, randomized, noninferiority trial of 379 patients with bloodstream infection caused by ceftriaxone-nonsusceptible E. coli or K. pneumoniae who received piperacillin-tazobactam (n=188) or meropenem (n = 191).
Disclosures: The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures.
Source: Harris PNA et al. JAMA 2018 Sep 11. doi: 10.1001/jama.2018.12163.
Levofloxacin prophylaxis cuts bacteremia in pediatric acute leukemias
according to results of a multicenter, randomized phase 3 trial.
Risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children undergoing hematopoietic stem cell transplantation (HSCT), although a post hoc analysis accounting for time at risk did show a significant difference, according to results of this Children’s Oncology Group (COG) trial.
The reduction in risk for children with acute leukemias was similar to findings of adult studies showing the benefit of prophylactic antibiotics in patients with cancer-related neutropenia, said Sarah Alexander, MD, of the division of hematology/oncology, the Hospital for Sick Children, Toronto, and her coinvestigators.
Before this COG study, data on prophylactic antibiotics in children with cancer were limited to several small, single-group observational studies, Dr. Alexander and her coauthors wrote in JAMA.
Bacteremia was the primary outcome of the COG study, according to the investigators, because of its link to sepsis, increased health care utilization, and infection-related mortality. “Consequently, this outcome is meaningful to both clinicians and patients,” the investigators noted.
The multicenter, randomized, open-label phase 3 trial (ACCL0934) enrolled patients aged 6 months to 21 years, including 200 with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were to receive at least two intensive chemotherapy cycles, and 424 who were to receive a myeloablative autologous or allogeneic HSCT.
In the final analysis of the acute leukemias group, which included 195 patients, likelihood of bacteremia was 21.9% for those randomized to levofloxacin prophylaxis, versus 43.4% for no prophylaxis (P = 0.001).
In the final analysis of the HSCT group, which included 418 patients, likelihood of bacteremia was not significantly different, at 11.0% for levofloxacin prophylaxis, versus 17.3% for no prophylaxis (P = 0.06).
“Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia, but not among patients undergoing HSCT,” Dr. Armstrong and her coauthors said.
A post hoc analysis accounting for time at risk, however, showed a significant difference in favor of prophylaxis in both groups and a similar effect size between groups, according to investigators.
For the acute leukemias group, the rate of bacteremic episodes in that post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and no prophylaxis arms, respectively (P = 0.008). In the HSCT group, the rate was 5.3 versus 10.0 bacteremias per 1,000 patient-days in the prophylaxis and no prophylaxis arms (P = .02).
The similar effect size suggests that in the primary analysis, there was reduced power to detect a significant difference in the HSCT group because of fewer events, driven partly by a shorter duration of neutropenia in that group, Dr. Armstrong and her associates said.
“However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups,” they added.
Levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several Gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests that other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk, the investigators said.
Further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits, according to the investigators, who reported 23 serious adverse events in 8 patients, 11 of which were considered unrelated or unlikely to be related to levofloxacin.
“The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile-associated diarrhea, bacterial resistance, and musculoskeletal toxicities,” Dr. Armstrong and her colleagues noted.
The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
SOURCE: Alexander S, et al . JAMA. 2018;320(10):995-1004.
according to results of a multicenter, randomized phase 3 trial.
Risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children undergoing hematopoietic stem cell transplantation (HSCT), although a post hoc analysis accounting for time at risk did show a significant difference, according to results of this Children’s Oncology Group (COG) trial.
The reduction in risk for children with acute leukemias was similar to findings of adult studies showing the benefit of prophylactic antibiotics in patients with cancer-related neutropenia, said Sarah Alexander, MD, of the division of hematology/oncology, the Hospital for Sick Children, Toronto, and her coinvestigators.
Before this COG study, data on prophylactic antibiotics in children with cancer were limited to several small, single-group observational studies, Dr. Alexander and her coauthors wrote in JAMA.
Bacteremia was the primary outcome of the COG study, according to the investigators, because of its link to sepsis, increased health care utilization, and infection-related mortality. “Consequently, this outcome is meaningful to both clinicians and patients,” the investigators noted.
The multicenter, randomized, open-label phase 3 trial (ACCL0934) enrolled patients aged 6 months to 21 years, including 200 with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were to receive at least two intensive chemotherapy cycles, and 424 who were to receive a myeloablative autologous or allogeneic HSCT.
In the final analysis of the acute leukemias group, which included 195 patients, likelihood of bacteremia was 21.9% for those randomized to levofloxacin prophylaxis, versus 43.4% for no prophylaxis (P = 0.001).
In the final analysis of the HSCT group, which included 418 patients, likelihood of bacteremia was not significantly different, at 11.0% for levofloxacin prophylaxis, versus 17.3% for no prophylaxis (P = 0.06).
“Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia, but not among patients undergoing HSCT,” Dr. Armstrong and her coauthors said.
A post hoc analysis accounting for time at risk, however, showed a significant difference in favor of prophylaxis in both groups and a similar effect size between groups, according to investigators.
For the acute leukemias group, the rate of bacteremic episodes in that post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and no prophylaxis arms, respectively (P = 0.008). In the HSCT group, the rate was 5.3 versus 10.0 bacteremias per 1,000 patient-days in the prophylaxis and no prophylaxis arms (P = .02).
The similar effect size suggests that in the primary analysis, there was reduced power to detect a significant difference in the HSCT group because of fewer events, driven partly by a shorter duration of neutropenia in that group, Dr. Armstrong and her associates said.
“However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups,” they added.
Levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several Gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests that other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk, the investigators said.
Further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits, according to the investigators, who reported 23 serious adverse events in 8 patients, 11 of which were considered unrelated or unlikely to be related to levofloxacin.
“The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile-associated diarrhea, bacterial resistance, and musculoskeletal toxicities,” Dr. Armstrong and her colleagues noted.
The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
SOURCE: Alexander S, et al . JAMA. 2018;320(10):995-1004.
according to results of a multicenter, randomized phase 3 trial.
Risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children undergoing hematopoietic stem cell transplantation (HSCT), although a post hoc analysis accounting for time at risk did show a significant difference, according to results of this Children’s Oncology Group (COG) trial.
The reduction in risk for children with acute leukemias was similar to findings of adult studies showing the benefit of prophylactic antibiotics in patients with cancer-related neutropenia, said Sarah Alexander, MD, of the division of hematology/oncology, the Hospital for Sick Children, Toronto, and her coinvestigators.
Before this COG study, data on prophylactic antibiotics in children with cancer were limited to several small, single-group observational studies, Dr. Alexander and her coauthors wrote in JAMA.
Bacteremia was the primary outcome of the COG study, according to the investigators, because of its link to sepsis, increased health care utilization, and infection-related mortality. “Consequently, this outcome is meaningful to both clinicians and patients,” the investigators noted.
The multicenter, randomized, open-label phase 3 trial (ACCL0934) enrolled patients aged 6 months to 21 years, including 200 with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were to receive at least two intensive chemotherapy cycles, and 424 who were to receive a myeloablative autologous or allogeneic HSCT.
In the final analysis of the acute leukemias group, which included 195 patients, likelihood of bacteremia was 21.9% for those randomized to levofloxacin prophylaxis, versus 43.4% for no prophylaxis (P = 0.001).
In the final analysis of the HSCT group, which included 418 patients, likelihood of bacteremia was not significantly different, at 11.0% for levofloxacin prophylaxis, versus 17.3% for no prophylaxis (P = 0.06).
“Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia, but not among patients undergoing HSCT,” Dr. Armstrong and her coauthors said.
A post hoc analysis accounting for time at risk, however, showed a significant difference in favor of prophylaxis in both groups and a similar effect size between groups, according to investigators.
For the acute leukemias group, the rate of bacteremic episodes in that post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and no prophylaxis arms, respectively (P = 0.008). In the HSCT group, the rate was 5.3 versus 10.0 bacteremias per 1,000 patient-days in the prophylaxis and no prophylaxis arms (P = .02).
The similar effect size suggests that in the primary analysis, there was reduced power to detect a significant difference in the HSCT group because of fewer events, driven partly by a shorter duration of neutropenia in that group, Dr. Armstrong and her associates said.
“However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups,” they added.
Levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several Gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests that other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk, the investigators said.
Further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits, according to the investigators, who reported 23 serious adverse events in 8 patients, 11 of which were considered unrelated or unlikely to be related to levofloxacin.
“The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile-associated diarrhea, bacterial resistance, and musculoskeletal toxicities,” Dr. Armstrong and her colleagues noted.
The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
SOURCE: Alexander S, et al . JAMA. 2018;320(10):995-1004.
FROM JAMA
Key clinical point: Levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias undergoing intensive chemotherapy, but not in children undergoing hematopoietic stem cell transplantation (HSCT).
Major finding: Bacteremia likelihood was 21.9% versus 43.4% for prophylaxis and no prophylaxis, respectively, in the acute leukemias group (P = 0.001), and 11.0% versus 17.3% in the HSCT group (P = 0.06).
Study details: A randomized phase 3 clinical trial, including 200 patients with acute leukemias and 424 patients undergoing HSCT.
Disclosures: The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Study authors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
Source: Alexander S, et al. JAMA. 2018;320(10):995-1004.
Agminated Papules on the Neck
The Diagnosis: Pseudoxanthoma Elasticum
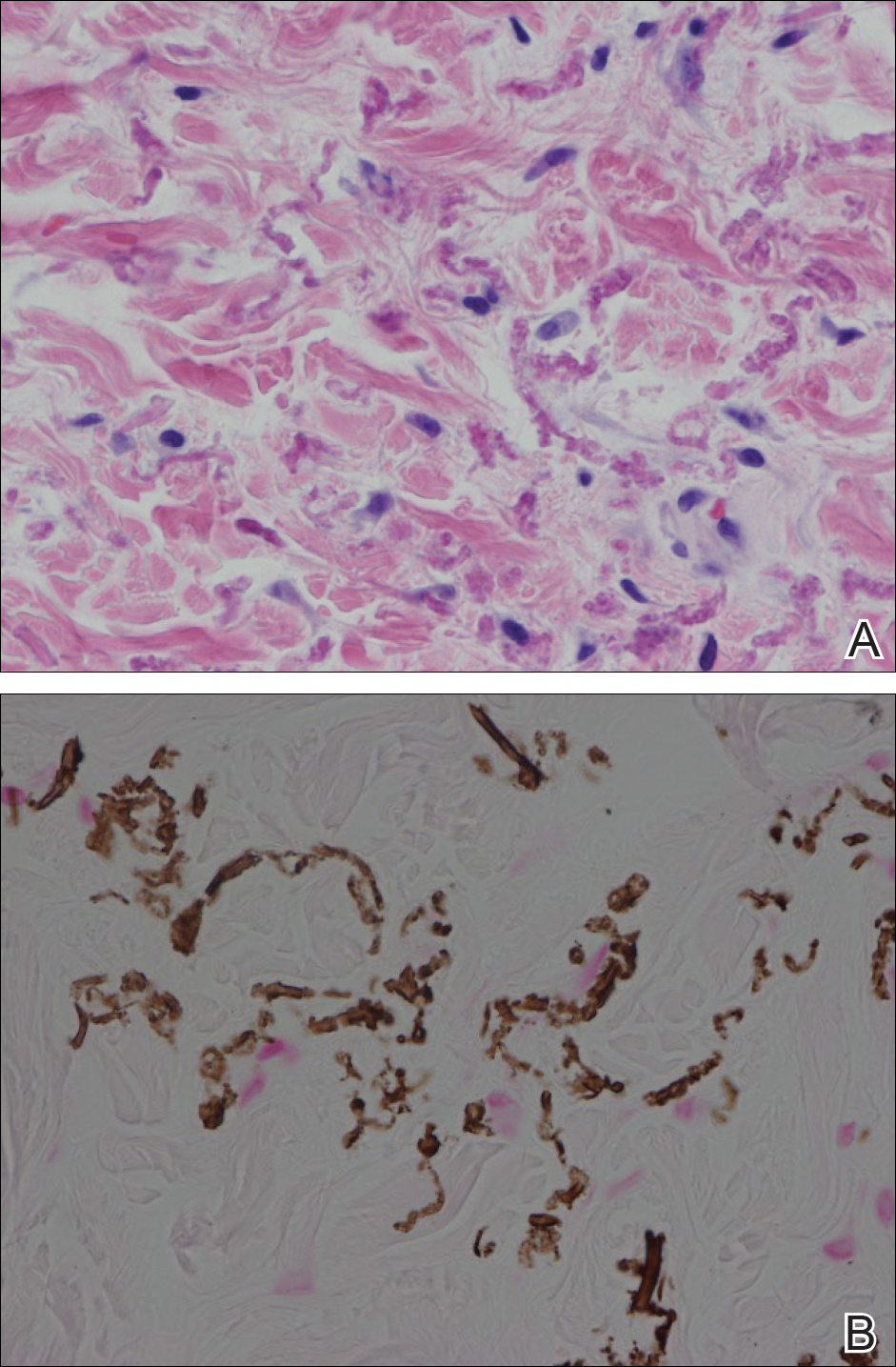
Histopathology showed abnormal curled frayed elastic fibers in the mid dermis (Figure, A); von Kossa stain was positive for calcified and fragmented elastic fibers (Figure, B). Based on clinical and histological findings, a diagnosis of pseudoxanthoma elasticum (PXE) was made.
Pseudoxanthoma elasticum is a rare multisystem heterogeneous genetic disorder that causes abnormal mineralization and fragmentation of tissue elastin fibers. Clinically, accumulation of mineralized elastin fibers leads to soft tissue calcification and late-onset pathology in the dermis, retinal Bruch membrane, and medial layers of large- and medium-sized arterial walls.
Pseudoxanthoma elasticum is an autosomal-recessive disease associated with more than 300 loss mutations in the ATP-binding cassette subfamily C member 6 gene, ABCC6.1,2 However, PXE clinically is characterized by wide variability in clinical progression and outcome as well as phenotypic overlap with other disorders such as generalized arterial calcification of infancy. Pseudoxanthoma elasticum affects an estimated 1 in 25,000 to 100,000 individuals with a female preponderance (2:1 ratio).1-3 Age of onset typically is in the second to third decades of life, with 80% of cases demonstrating skin manifestations before 20 years of age.2,3
The first and most benign finding often is the appearance of small soft asymptomatic yellow papules with a plucked chicken skin-like appearance that occur on the flexural areas such as the neck, axilla, antecubital, popliteal, inguinal, and periumbilical areas. These papules may progress to irregularly shaped, yellowish plaques with a leathery appearance; mucous membranes, often occurring on the inner aspect of the lower lips, also may be involved. More severe abdominal striae also may affect some but not all women with PXE. Histologic examination demonstrates swollen, clumped, and fragmented elastin fibers with calcium deposits in the mid dermis. Elastin-specific stains such as orcein and calcium-specific stains such as the von Kossa stain aid in the diagnosis.
Vision impairment subsequently develops in 50% to 70% of patients, with severe vision loss in 3% to 8% of patients.4,5 Ophthalmologic examination identifies characteristic angioid streaks (ie, gray lines radiating from the optic disk) and subretinal hemorrhages caused by brittle new vessel formation.
Bleeding complications, especially from the gastrointestinal tract, caused by arterial wall fragility may affect 10% of PXE patients.5 Although bleeding complications also may affect the genitourinary system, the risk for fetal loss or adverse reproductive outcomes is considered low.6 More insidiously, progressive arterial calcification and peripheral arterial disease contribute to accelerated atherosclerosis, causing earlier presentations of claudication, angina pectoris, myocardial infarction, and hypertension by the third and fourth decades of life.
Management of PXE is limited. Primary care providers should be attentive to cardiovascular screening for coronary and peripheral arterial disease. Patients should receive regular eye examinations, and choroidal neovascularization should be aggressively treated with photocoagulation, photodynamic therapy, and vascular endothelial growth factor inhibitors.1,3
Collagenous fibromas are slow-growing tumors but are histologically distinct, showing fibrous or myxoid connective tissue arising within adipose tissue. Cutaneous leiomyomas may be solitary or grouped, often painful papules composed histologically of bundles of smooth muscle. Cutaneous sclerosis in sclerosing mesenteritis is a rare cutaneous manifestation of an internal disorder and presents as asymptomatic indurated subcutaneous nodules but histologically is distinctive, demonstrating sclerosis with fat necrosis. Xanthoma disseminatum is a rare form of histiocytosis that commonly presents as hundreds of small yellowish brown or reddish brown papules symmetrically distributed on the face, trunk, and intertriginous areas.
On follow-up within a year after initial presentation, our patient was found to have early subtle angioid streaks on ophthalmologic examination with no vision loss. A transthoracic echocardiogram was performed and showed no cardiac abnormalities. Her pregnancy was complicated by intrauterine growth retardation in the third trimester; however, the patient delivered a healthy-appearing 2835 g neonate (10th percentile for gestational age) at 39 weeks of gestations via an uncomplicated cesarean delivery.
- Uitto J, Bercovitch L, Terry SF, et al. Pseudoxanthoma elasticum: progress in diagnostics and research towards treatment: summary of the 2010 PXE International Research Meeting. Am J Med Genet A. 2011;155A:1517-1526.
- Li Q, Jiang Q, Pfendner E, et al. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1-11.
- Finger RP, Charbel Issa P, Ladewig MS, et al. Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol. 2009;54:272-285.
- Li Y, Cui Y, Zhao H, et al. Pseudoxanthoma elasticum: a review of 86 cases in China. Intractable Rare Dis Res. 2014;3:75-78.
- Laube S, Moss C. Pseudoxanthoma elasticum. Arch Dis Child. 2005;90:754-756.
- Bercovitch L, Leroux T, Terry S, et al. Pregnancy and obstetrical outcomes in pseudoxanthoma elasticum. Br J Dermatol. 2004;151:1011-1018.
The Diagnosis: Pseudoxanthoma Elasticum
Histopathology showed abnormal curled frayed elastic fibers in the mid dermis (Figure, A); von Kossa stain was positive for calcified and fragmented elastic fibers (Figure, B). Based on clinical and histological findings, a diagnosis of pseudoxanthoma elasticum (PXE) was made.

Pseudoxanthoma elasticum is a rare multisystem heterogeneous genetic disorder that causes abnormal mineralization and fragmentation of tissue elastin fibers. Clinically, accumulation of mineralized elastin fibers leads to soft tissue calcification and late-onset pathology in the dermis, retinal Bruch membrane, and medial layers of large- and medium-sized arterial walls.
Pseudoxanthoma elasticum is an autosomal-recessive disease associated with more than 300 loss mutations in the ATP-binding cassette subfamily C member 6 gene, ABCC6.1,2 However, PXE clinically is characterized by wide variability in clinical progression and outcome as well as phenotypic overlap with other disorders such as generalized arterial calcification of infancy. Pseudoxanthoma elasticum affects an estimated 1 in 25,000 to 100,000 individuals with a female preponderance (2:1 ratio).1-3 Age of onset typically is in the second to third decades of life, with 80% of cases demonstrating skin manifestations before 20 years of age.2,3
The first and most benign finding often is the appearance of small soft asymptomatic yellow papules with a plucked chicken skin-like appearance that occur on the flexural areas such as the neck, axilla, antecubital, popliteal, inguinal, and periumbilical areas. These papules may progress to irregularly shaped, yellowish plaques with a leathery appearance; mucous membranes, often occurring on the inner aspect of the lower lips, also may be involved. More severe abdominal striae also may affect some but not all women with PXE. Histologic examination demonstrates swollen, clumped, and fragmented elastin fibers with calcium deposits in the mid dermis. Elastin-specific stains such as orcein and calcium-specific stains such as the von Kossa stain aid in the diagnosis.
Vision impairment subsequently develops in 50% to 70% of patients, with severe vision loss in 3% to 8% of patients.4,5 Ophthalmologic examination identifies characteristic angioid streaks (ie, gray lines radiating from the optic disk) and subretinal hemorrhages caused by brittle new vessel formation.
Bleeding complications, especially from the gastrointestinal tract, caused by arterial wall fragility may affect 10% of PXE patients.5 Although bleeding complications also may affect the genitourinary system, the risk for fetal loss or adverse reproductive outcomes is considered low.6 More insidiously, progressive arterial calcification and peripheral arterial disease contribute to accelerated atherosclerosis, causing earlier presentations of claudication, angina pectoris, myocardial infarction, and hypertension by the third and fourth decades of life.
Management of PXE is limited. Primary care providers should be attentive to cardiovascular screening for coronary and peripheral arterial disease. Patients should receive regular eye examinations, and choroidal neovascularization should be aggressively treated with photocoagulation, photodynamic therapy, and vascular endothelial growth factor inhibitors.1,3
Collagenous fibromas are slow-growing tumors but are histologically distinct, showing fibrous or myxoid connective tissue arising within adipose tissue. Cutaneous leiomyomas may be solitary or grouped, often painful papules composed histologically of bundles of smooth muscle. Cutaneous sclerosis in sclerosing mesenteritis is a rare cutaneous manifestation of an internal disorder and presents as asymptomatic indurated subcutaneous nodules but histologically is distinctive, demonstrating sclerosis with fat necrosis. Xanthoma disseminatum is a rare form of histiocytosis that commonly presents as hundreds of small yellowish brown or reddish brown papules symmetrically distributed on the face, trunk, and intertriginous areas.
On follow-up within a year after initial presentation, our patient was found to have early subtle angioid streaks on ophthalmologic examination with no vision loss. A transthoracic echocardiogram was performed and showed no cardiac abnormalities. Her pregnancy was complicated by intrauterine growth retardation in the third trimester; however, the patient delivered a healthy-appearing 2835 g neonate (10th percentile for gestational age) at 39 weeks of gestations via an uncomplicated cesarean delivery.
The Diagnosis: Pseudoxanthoma Elasticum
Histopathology showed abnormal curled frayed elastic fibers in the mid dermis (Figure, A); von Kossa stain was positive for calcified and fragmented elastic fibers (Figure, B). Based on clinical and histological findings, a diagnosis of pseudoxanthoma elasticum (PXE) was made.

Pseudoxanthoma elasticum is a rare multisystem heterogeneous genetic disorder that causes abnormal mineralization and fragmentation of tissue elastin fibers. Clinically, accumulation of mineralized elastin fibers leads to soft tissue calcification and late-onset pathology in the dermis, retinal Bruch membrane, and medial layers of large- and medium-sized arterial walls.
Pseudoxanthoma elasticum is an autosomal-recessive disease associated with more than 300 loss mutations in the ATP-binding cassette subfamily C member 6 gene, ABCC6.1,2 However, PXE clinically is characterized by wide variability in clinical progression and outcome as well as phenotypic overlap with other disorders such as generalized arterial calcification of infancy. Pseudoxanthoma elasticum affects an estimated 1 in 25,000 to 100,000 individuals with a female preponderance (2:1 ratio).1-3 Age of onset typically is in the second to third decades of life, with 80% of cases demonstrating skin manifestations before 20 years of age.2,3
The first and most benign finding often is the appearance of small soft asymptomatic yellow papules with a plucked chicken skin-like appearance that occur on the flexural areas such as the neck, axilla, antecubital, popliteal, inguinal, and periumbilical areas. These papules may progress to irregularly shaped, yellowish plaques with a leathery appearance; mucous membranes, often occurring on the inner aspect of the lower lips, also may be involved. More severe abdominal striae also may affect some but not all women with PXE. Histologic examination demonstrates swollen, clumped, and fragmented elastin fibers with calcium deposits in the mid dermis. Elastin-specific stains such as orcein and calcium-specific stains such as the von Kossa stain aid in the diagnosis.
Vision impairment subsequently develops in 50% to 70% of patients, with severe vision loss in 3% to 8% of patients.4,5 Ophthalmologic examination identifies characteristic angioid streaks (ie, gray lines radiating from the optic disk) and subretinal hemorrhages caused by brittle new vessel formation.
Bleeding complications, especially from the gastrointestinal tract, caused by arterial wall fragility may affect 10% of PXE patients.5 Although bleeding complications also may affect the genitourinary system, the risk for fetal loss or adverse reproductive outcomes is considered low.6 More insidiously, progressive arterial calcification and peripheral arterial disease contribute to accelerated atherosclerosis, causing earlier presentations of claudication, angina pectoris, myocardial infarction, and hypertension by the third and fourth decades of life.
Management of PXE is limited. Primary care providers should be attentive to cardiovascular screening for coronary and peripheral arterial disease. Patients should receive regular eye examinations, and choroidal neovascularization should be aggressively treated with photocoagulation, photodynamic therapy, and vascular endothelial growth factor inhibitors.1,3
Collagenous fibromas are slow-growing tumors but are histologically distinct, showing fibrous or myxoid connective tissue arising within adipose tissue. Cutaneous leiomyomas may be solitary or grouped, often painful papules composed histologically of bundles of smooth muscle. Cutaneous sclerosis in sclerosing mesenteritis is a rare cutaneous manifestation of an internal disorder and presents as asymptomatic indurated subcutaneous nodules but histologically is distinctive, demonstrating sclerosis with fat necrosis. Xanthoma disseminatum is a rare form of histiocytosis that commonly presents as hundreds of small yellowish brown or reddish brown papules symmetrically distributed on the face, trunk, and intertriginous areas.
On follow-up within a year after initial presentation, our patient was found to have early subtle angioid streaks on ophthalmologic examination with no vision loss. A transthoracic echocardiogram was performed and showed no cardiac abnormalities. Her pregnancy was complicated by intrauterine growth retardation in the third trimester; however, the patient delivered a healthy-appearing 2835 g neonate (10th percentile for gestational age) at 39 weeks of gestations via an uncomplicated cesarean delivery.
- Uitto J, Bercovitch L, Terry SF, et al. Pseudoxanthoma elasticum: progress in diagnostics and research towards treatment: summary of the 2010 PXE International Research Meeting. Am J Med Genet A. 2011;155A:1517-1526.
- Li Q, Jiang Q, Pfendner E, et al. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1-11.
- Finger RP, Charbel Issa P, Ladewig MS, et al. Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol. 2009;54:272-285.
- Li Y, Cui Y, Zhao H, et al. Pseudoxanthoma elasticum: a review of 86 cases in China. Intractable Rare Dis Res. 2014;3:75-78.
- Laube S, Moss C. Pseudoxanthoma elasticum. Arch Dis Child. 2005;90:754-756.
- Bercovitch L, Leroux T, Terry S, et al. Pregnancy and obstetrical outcomes in pseudoxanthoma elasticum. Br J Dermatol. 2004;151:1011-1018.
- Uitto J, Bercovitch L, Terry SF, et al. Pseudoxanthoma elasticum: progress in diagnostics and research towards treatment: summary of the 2010 PXE International Research Meeting. Am J Med Genet A. 2011;155A:1517-1526.
- Li Q, Jiang Q, Pfendner E, et al. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1-11.
- Finger RP, Charbel Issa P, Ladewig MS, et al. Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol. 2009;54:272-285.
- Li Y, Cui Y, Zhao H, et al. Pseudoxanthoma elasticum: a review of 86 cases in China. Intractable Rare Dis Res. 2014;3:75-78.
- Laube S, Moss C. Pseudoxanthoma elasticum. Arch Dis Child. 2005;90:754-756.
- Bercovitch L, Leroux T, Terry S, et al. Pregnancy and obstetrical outcomes in pseudoxanthoma elasticum. Br J Dermatol. 2004;151:1011-1018.
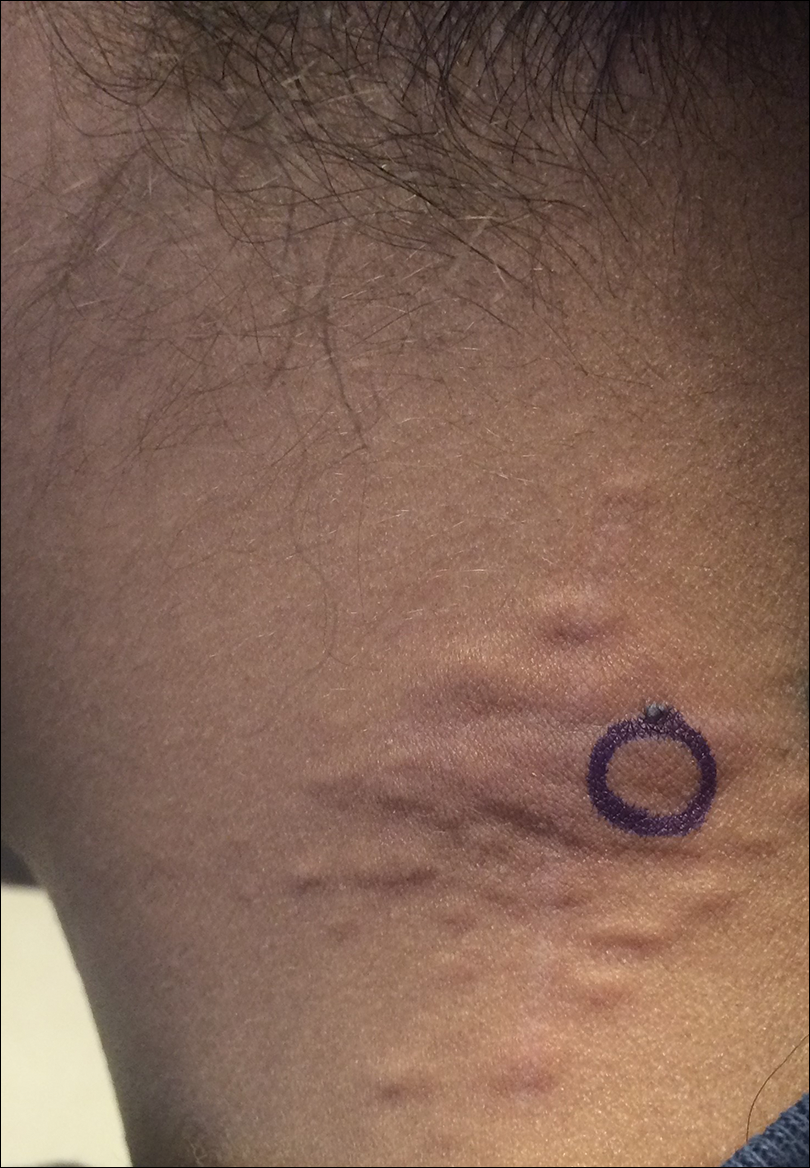
A 24-year-old woman presented with a lesion on the neck of 3 months' duration. She noted occasional mild pruritus at the site but no other symptoms or similar lesions elsewhere. At the time of presentation, she was at 17 weeks of gestation without any complications. Her medical history was notable for hypertension, unspecified chest pain with a normal electrocardiogram, and 2 spontaneous abortions. She denied a personal or family history of notable cardiovascular or gastrointestinal tract diseases. Examination of the skin showed indurated 3- to 5-mm papules coalescing into a 3- to 4-cm plaque on the left posterolateral neck.
Cutaneous lesions? Consider C. diphtheriae in those with foreign travel
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
REPORTING FROM ICEID 2018
Key clinical point: Corynebacterium diphtheriae should be considered in individuals who present with cutaneous lesions after traveling to diphtheria-endemic countries.
Major finding: Refer suspect C. diphtheriae isolates to state health departments.
Study details: A review of seven C. diphtheriae cases.
Disclosures: Ms. Griffith reported having no disclosures.
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
New IHS Website Addresses Opioid Crisis
The opioid crisis has taken a toll everywhere, but American Indians and Alaska Natives have been hardest hit. That group had the highest drug overdose death rates in 2015, and the largest percentage increase— > 500%—in the number of deaths between 1999 and 2015 compared with that of other racial and ethnic groups.
In February 2018, the IHS released the revised agency policy on chronic pain management. It also has now launched a website (www.ihs.gov/opioids) as another step in addressing the problem.
The website discusses the crisis response, funding opportunities, best practices, and proper pain management. It includes Community Opioid Action Plans, which inform the public about how indigenous planning using traditional practices and holistic, culturally appropriate approaches can help.
The website also provides resources for tribes, such as links to the Office of Tribal Affairs and Policy, the point of contact for tribal governments, tribal organizations and federal agencies on behavioral health issues that affect tribal communities; the Office of Indian Alcohol and Substance Abuse; and SAMHSA Tribal Training and Technical Assistance.
The opioid crisis has taken a toll everywhere, but American Indians and Alaska Natives have been hardest hit. That group had the highest drug overdose death rates in 2015, and the largest percentage increase— > 500%—in the number of deaths between 1999 and 2015 compared with that of other racial and ethnic groups.
In February 2018, the IHS released the revised agency policy on chronic pain management. It also has now launched a website (www.ihs.gov/opioids) as another step in addressing the problem.
The website discusses the crisis response, funding opportunities, best practices, and proper pain management. It includes Community Opioid Action Plans, which inform the public about how indigenous planning using traditional practices and holistic, culturally appropriate approaches can help.
The website also provides resources for tribes, such as links to the Office of Tribal Affairs and Policy, the point of contact for tribal governments, tribal organizations and federal agencies on behavioral health issues that affect tribal communities; the Office of Indian Alcohol and Substance Abuse; and SAMHSA Tribal Training and Technical Assistance.
The opioid crisis has taken a toll everywhere, but American Indians and Alaska Natives have been hardest hit. That group had the highest drug overdose death rates in 2015, and the largest percentage increase— > 500%—in the number of deaths between 1999 and 2015 compared with that of other racial and ethnic groups.
In February 2018, the IHS released the revised agency policy on chronic pain management. It also has now launched a website (www.ihs.gov/opioids) as another step in addressing the problem.
The website discusses the crisis response, funding opportunities, best practices, and proper pain management. It includes Community Opioid Action Plans, which inform the public about how indigenous planning using traditional practices and holistic, culturally appropriate approaches can help.
The website also provides resources for tribes, such as links to the Office of Tribal Affairs and Policy, the point of contact for tribal governments, tribal organizations and federal agencies on behavioral health issues that affect tribal communities; the Office of Indian Alcohol and Substance Abuse; and SAMHSA Tribal Training and Technical Assistance.
Hispanic ALL patients face higher treatment toxicity
Hispanic pediatric patients undergoing treatment for acute lymphoblastic leukemia (ALL) had a risk of methotrexate toxicity that was more than twice that of non-Hispanic whites, according to results of a prospective multicenter study.
Methotrexate toxicity often led to treatment modification or delays, which may have increased relapse risk in the Hispanic patients, according to investigator Michael E. Scheurer, PhD, MPH, of Baylor College of Medicine, Houston, and his colleagues.
“We had observed that our Hispanic patients tended to experience neurotoxicity more often than other groups, but we were surprised to see the magnitude of the difference,” Dr. Scheurer said in statement.
The study, described in Clinical Cancer Research, involved 280 patients with newly diagnosed ALL enrolled at one of three major U.S. pediatric cancer treatment centers. Nearly half of the patients (48.2%) were Hispanic, and approximately 86% had a diagnosis of pre B-cell leukemia.
The patients, who had a mean age of 8.4 years at diagnosis, were treated with modern ALL protocols and were followed from diagnosis to the start of maintenance/continuation therapy.
Methotrexate toxicity was seen in 39 patients at the time of the analysis. Of those patients, 29 (74.4%) were Hispanic, Dr. Scheurer and his coauthors reported.
Compared with non-Hispanic whites, Hispanics had a high risk of methotrexate neurotoxicity, even after the researchers accounted for age, sex, ALL risk stratification, and other factors (adjusted hazard ratio, 2.43; 95% confidence interval, 1.06-5.58).
Among nine patients who experienced a second neurotoxic event, all were Hispanic.
Patients who had neurotoxicity received an average of 2.25 fewer doses of intrathecal methotrexate, and slightly lower intravenous methotrexate doses. About three-quarters of the patients experiencing methotrexate toxicity received leucovorin after intrathecal methotrexate, according to the investigators, who noted that leucovorin may interact with methotrexate and reduce efficacy.
“These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL,” wrote Dr. Scheurer and his coauthors.
Relapse occurred in 15.4% of patients with neurotoxicity (6 of 39 patients), and in 2.1% of patients with no neurotoxicity (13 of 241 patients).
Taken together, the findings add to the growing body of evidence that Hispanics and other minority pediatric patients with ALL experience “significant disparities” in treatment outcomes, according to the investigators.
That body of evidence includes several recent cases series that suggest Hispanic patients with ALL have a high prevalence of methotrexate neurotoxicity.
It remains unclear why Hispanic patients would have a higher risk of methotrexate toxicity, and that must be explored in future studies, the investigators said.
The research team is currently investigating biomarkers that may help identify patients at risk of methotrexate toxicity up front. “If we can identify these at-risk patients, we can potentially employ strategies to either fully prevent or mitigate these toxicities,” Dr. Scheurer said in a statement.
The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The researchers reported having no potential conflicts of interest.
SOURCE: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
Hispanic pediatric patients undergoing treatment for acute lymphoblastic leukemia (ALL) had a risk of methotrexate toxicity that was more than twice that of non-Hispanic whites, according to results of a prospective multicenter study.
Methotrexate toxicity often led to treatment modification or delays, which may have increased relapse risk in the Hispanic patients, according to investigator Michael E. Scheurer, PhD, MPH, of Baylor College of Medicine, Houston, and his colleagues.
“We had observed that our Hispanic patients tended to experience neurotoxicity more often than other groups, but we were surprised to see the magnitude of the difference,” Dr. Scheurer said in statement.
The study, described in Clinical Cancer Research, involved 280 patients with newly diagnosed ALL enrolled at one of three major U.S. pediatric cancer treatment centers. Nearly half of the patients (48.2%) were Hispanic, and approximately 86% had a diagnosis of pre B-cell leukemia.
The patients, who had a mean age of 8.4 years at diagnosis, were treated with modern ALL protocols and were followed from diagnosis to the start of maintenance/continuation therapy.
Methotrexate toxicity was seen in 39 patients at the time of the analysis. Of those patients, 29 (74.4%) were Hispanic, Dr. Scheurer and his coauthors reported.
Compared with non-Hispanic whites, Hispanics had a high risk of methotrexate neurotoxicity, even after the researchers accounted for age, sex, ALL risk stratification, and other factors (adjusted hazard ratio, 2.43; 95% confidence interval, 1.06-5.58).
Among nine patients who experienced a second neurotoxic event, all were Hispanic.
Patients who had neurotoxicity received an average of 2.25 fewer doses of intrathecal methotrexate, and slightly lower intravenous methotrexate doses. About three-quarters of the patients experiencing methotrexate toxicity received leucovorin after intrathecal methotrexate, according to the investigators, who noted that leucovorin may interact with methotrexate and reduce efficacy.
“These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL,” wrote Dr. Scheurer and his coauthors.
Relapse occurred in 15.4% of patients with neurotoxicity (6 of 39 patients), and in 2.1% of patients with no neurotoxicity (13 of 241 patients).
Taken together, the findings add to the growing body of evidence that Hispanics and other minority pediatric patients with ALL experience “significant disparities” in treatment outcomes, according to the investigators.
That body of evidence includes several recent cases series that suggest Hispanic patients with ALL have a high prevalence of methotrexate neurotoxicity.
It remains unclear why Hispanic patients would have a higher risk of methotrexate toxicity, and that must be explored in future studies, the investigators said.
The research team is currently investigating biomarkers that may help identify patients at risk of methotrexate toxicity up front. “If we can identify these at-risk patients, we can potentially employ strategies to either fully prevent or mitigate these toxicities,” Dr. Scheurer said in a statement.
The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The researchers reported having no potential conflicts of interest.
SOURCE: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
Hispanic pediatric patients undergoing treatment for acute lymphoblastic leukemia (ALL) had a risk of methotrexate toxicity that was more than twice that of non-Hispanic whites, according to results of a prospective multicenter study.
Methotrexate toxicity often led to treatment modification or delays, which may have increased relapse risk in the Hispanic patients, according to investigator Michael E. Scheurer, PhD, MPH, of Baylor College of Medicine, Houston, and his colleagues.
“We had observed that our Hispanic patients tended to experience neurotoxicity more often than other groups, but we were surprised to see the magnitude of the difference,” Dr. Scheurer said in statement.
The study, described in Clinical Cancer Research, involved 280 patients with newly diagnosed ALL enrolled at one of three major U.S. pediatric cancer treatment centers. Nearly half of the patients (48.2%) were Hispanic, and approximately 86% had a diagnosis of pre B-cell leukemia.
The patients, who had a mean age of 8.4 years at diagnosis, were treated with modern ALL protocols and were followed from diagnosis to the start of maintenance/continuation therapy.
Methotrexate toxicity was seen in 39 patients at the time of the analysis. Of those patients, 29 (74.4%) were Hispanic, Dr. Scheurer and his coauthors reported.
Compared with non-Hispanic whites, Hispanics had a high risk of methotrexate neurotoxicity, even after the researchers accounted for age, sex, ALL risk stratification, and other factors (adjusted hazard ratio, 2.43; 95% confidence interval, 1.06-5.58).
Among nine patients who experienced a second neurotoxic event, all were Hispanic.
Patients who had neurotoxicity received an average of 2.25 fewer doses of intrathecal methotrexate, and slightly lower intravenous methotrexate doses. About three-quarters of the patients experiencing methotrexate toxicity received leucovorin after intrathecal methotrexate, according to the investigators, who noted that leucovorin may interact with methotrexate and reduce efficacy.
“These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL,” wrote Dr. Scheurer and his coauthors.
Relapse occurred in 15.4% of patients with neurotoxicity (6 of 39 patients), and in 2.1% of patients with no neurotoxicity (13 of 241 patients).
Taken together, the findings add to the growing body of evidence that Hispanics and other minority pediatric patients with ALL experience “significant disparities” in treatment outcomes, according to the investigators.
That body of evidence includes several recent cases series that suggest Hispanic patients with ALL have a high prevalence of methotrexate neurotoxicity.
It remains unclear why Hispanic patients would have a higher risk of methotrexate toxicity, and that must be explored in future studies, the investigators said.
The research team is currently investigating biomarkers that may help identify patients at risk of methotrexate toxicity up front. “If we can identify these at-risk patients, we can potentially employ strategies to either fully prevent or mitigate these toxicities,” Dr. Scheurer said in a statement.
The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The researchers reported having no potential conflicts of interest.
SOURCE: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.
FROM CLINICAL CANCER RESEARCH
Key clinical point:
Major finding: After researchers accounted for age, sex, ALL risk stratification, and other factors, the adjusted hazard ratio was 2.43 (95% CI, 1.06-5.58).
Study details: A prospective multicenter study of 280 patients with newly diagnosed ALL, nearly half of whom were Hispanic.
Disclosures: The research was supported by the National Institutes of Health and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, a St. Baldrick’s Foundation Consortium Research Grant. The study authors reported having no potential conflicts of interest.
Source: Taylor OA et al. Clin Cancer Res. 2018 Sep 11. doi: 10.1158/1078-0432.CCR-18-0939.