User login
Etravirine Lowers Risk of Hospitalization for Patients With HIV
When all 3 original classes of antiretroviral drugs no longer suppress viral load in a patient with HIV, the next step may be a new drug like etravirine (ETR), a nonnucleoside reverse transcriptase inhibitor (NNRTI). And, according to a French study, that could be a good way of keeping patients out of the hospital.
Using data from the French Hospital Database on HIV (FHDH), researchers analyzed hospitalization rates among heavily treated HIV-1 infected patients on failing regimens between 2005 (etravirine became available in France in 2006) and 2011. They compared 2 groups of patients: those who had received ETR plus a ritonavir-boosted protease inhibitor (PI) and those who had not. The primary endpoint of the study was hospitalization, divided by AIDS-defining cause and non–AIDS-defining cause.
Of 3,884 patients who had been exposed to at least 2 nucleoside reverse transcriptase inhibitors (NRTI), 2 PIs, and 1 NNRTI, 838 received ETR + PI.
During 13,986 person-years of follow-up, there were 2,484 hospitalizations among 956 patients: 617 were from an AIDS-defining cause in 301 patients, and 1,867 from a non–AIDS-defining cause in 828 patients.
The ETR + PI was associated with a 20% reduction in the hospitalization rate, mainly due to the reduction in AIDS hospitalizations. The researchers suggest that the clinical benefit of ETR could be explained by a high rate of virologic suppression (62% at month 6) and excellent tolerability. The FHDH did not include adherence data, but adherence is “unlikely” to explain the better outcome, the researchers say, given that ETR is a twice-daily drug, which may lead to slightly lower adherence than does a once-daily regimen.
The is the first study, to their knowledge, the researchers say, to focus on the risk of hospitalizations in current clinical practice and to show a positive effect.
Source:
Potard V, Goujard C, Valantin MA, et al. BMC Infect Dis. 2018;8:326.
doi: 10.1186/s12879-018-3231-5.
When all 3 original classes of antiretroviral drugs no longer suppress viral load in a patient with HIV, the next step may be a new drug like etravirine (ETR), a nonnucleoside reverse transcriptase inhibitor (NNRTI). And, according to a French study, that could be a good way of keeping patients out of the hospital.
Using data from the French Hospital Database on HIV (FHDH), researchers analyzed hospitalization rates among heavily treated HIV-1 infected patients on failing regimens between 2005 (etravirine became available in France in 2006) and 2011. They compared 2 groups of patients: those who had received ETR plus a ritonavir-boosted protease inhibitor (PI) and those who had not. The primary endpoint of the study was hospitalization, divided by AIDS-defining cause and non–AIDS-defining cause.
Of 3,884 patients who had been exposed to at least 2 nucleoside reverse transcriptase inhibitors (NRTI), 2 PIs, and 1 NNRTI, 838 received ETR + PI.
During 13,986 person-years of follow-up, there were 2,484 hospitalizations among 956 patients: 617 were from an AIDS-defining cause in 301 patients, and 1,867 from a non–AIDS-defining cause in 828 patients.
The ETR + PI was associated with a 20% reduction in the hospitalization rate, mainly due to the reduction in AIDS hospitalizations. The researchers suggest that the clinical benefit of ETR could be explained by a high rate of virologic suppression (62% at month 6) and excellent tolerability. The FHDH did not include adherence data, but adherence is “unlikely” to explain the better outcome, the researchers say, given that ETR is a twice-daily drug, which may lead to slightly lower adherence than does a once-daily regimen.
The is the first study, to their knowledge, the researchers say, to focus on the risk of hospitalizations in current clinical practice and to show a positive effect.
Source:
Potard V, Goujard C, Valantin MA, et al. BMC Infect Dis. 2018;8:326.
doi: 10.1186/s12879-018-3231-5.
When all 3 original classes of antiretroviral drugs no longer suppress viral load in a patient with HIV, the next step may be a new drug like etravirine (ETR), a nonnucleoside reverse transcriptase inhibitor (NNRTI). And, according to a French study, that could be a good way of keeping patients out of the hospital.
Using data from the French Hospital Database on HIV (FHDH), researchers analyzed hospitalization rates among heavily treated HIV-1 infected patients on failing regimens between 2005 (etravirine became available in France in 2006) and 2011. They compared 2 groups of patients: those who had received ETR plus a ritonavir-boosted protease inhibitor (PI) and those who had not. The primary endpoint of the study was hospitalization, divided by AIDS-defining cause and non–AIDS-defining cause.
Of 3,884 patients who had been exposed to at least 2 nucleoside reverse transcriptase inhibitors (NRTI), 2 PIs, and 1 NNRTI, 838 received ETR + PI.
During 13,986 person-years of follow-up, there were 2,484 hospitalizations among 956 patients: 617 were from an AIDS-defining cause in 301 patients, and 1,867 from a non–AIDS-defining cause in 828 patients.
The ETR + PI was associated with a 20% reduction in the hospitalization rate, mainly due to the reduction in AIDS hospitalizations. The researchers suggest that the clinical benefit of ETR could be explained by a high rate of virologic suppression (62% at month 6) and excellent tolerability. The FHDH did not include adherence data, but adherence is “unlikely” to explain the better outcome, the researchers say, given that ETR is a twice-daily drug, which may lead to slightly lower adherence than does a once-daily regimen.
The is the first study, to their knowledge, the researchers say, to focus on the risk of hospitalizations in current clinical practice and to show a positive effect.
Source:
Potard V, Goujard C, Valantin MA, et al. BMC Infect Dis. 2018;8:326.
doi: 10.1186/s12879-018-3231-5.
Study reveals ‘complete mental health’ among cancer survivors
New research suggests cancer survivors are just as likely as people without a history of cancer to have complete mental health (CMH), which is defined as “optimal functioning” and the “absence of psychopathology.”
In a study of nearly 11,000 Canadians, 77.5% of cancer survivors and 76.8% of people with no cancer history had CMH.
As for patients who were battling cancer at the time of the study, 66.1% had CMH.
Esme Fuller-Thomson, PhD, and Keri West, both of the University of Toronto in Canada, conducted this research and reported the findings in Aging & Mental Health.
“Cancer patients were doing much better than we had expected,” Dr. Fuller-Thomson said. “Two-thirds met our very stringent criteria for complete mental health . . . . The news for cancer survivors was even better, with three-quarters living in complete mental health, which is a prevalence comparable to that of individuals with no cancer history.”
This study included a nationally representative sample of Canadian community dwellers age 50 and older. Subjects had current cancer (n=438), previous cancer (n=1174), or no cancer history (n=9279).
Data were obtained from Statistics Canada’s 2012 Canadian Community Health Survey-Mental Health.
To meet criteria for CMH, subjects had to have all of the following:
- Absence of mental illness, addictions, and suicidal thoughts in the past year
- Almost daily happiness or life satisfaction in the past month
- Psychosocial well-being.
The prevalence of CMH was 77.5% in cancer survivors and 76.8% in subjects who had never had cancer. Both were significantly higher than the 66.1% prevalence of CMH in current cancer patients (P<0.001).
In a multivariable model adjusted for demographics, current cancer patients had 45% lower odds of CMH compared to subjects with no cancer history (odds ratio [OR]=0.55). The odds of CMH were comparable for cancer survivors and those without a history of cancer (OR=0.98).
The researchers also conducted a multivariable analysis in which they adjusted for “all relevant factors,” which included demographics as well as adverse childhood events, socioeconomic status, health variables, lifetime mental illness, etc.
In this analysis, current cancer patients had 37% lower odds of CMH than subjects with no cancer history (OR=0.63). And cancer survivors had comparable odds of CMH as those with no cancer history (OR=1.06).
The researchers identified several factors that were associated with CMH in the population affected by cancer.
“Among those with former or current cancer, the odds of complete mental health were higher for women, white, married, and older respondents, as well as those with higher income and those who did not have disabling pain nor functional limitations,” West said.
“We found that earlier difficulties cast a long shadow. Those who had been physically abused during their childhood and those who had ever had depression or anxiety disorders were less likely to be in complete mental health.”
West and Dr Fuller-Thomson emphasized that these results are only correlational, and it is impossible to determine causality due to the cross-sectional and observational nature of the study.
The pair also said future longitudinal research is needed to improve understanding of what pathways improve resilience and recovery among cancer patients.
New research suggests cancer survivors are just as likely as people without a history of cancer to have complete mental health (CMH), which is defined as “optimal functioning” and the “absence of psychopathology.”
In a study of nearly 11,000 Canadians, 77.5% of cancer survivors and 76.8% of people with no cancer history had CMH.
As for patients who were battling cancer at the time of the study, 66.1% had CMH.
Esme Fuller-Thomson, PhD, and Keri West, both of the University of Toronto in Canada, conducted this research and reported the findings in Aging & Mental Health.
“Cancer patients were doing much better than we had expected,” Dr. Fuller-Thomson said. “Two-thirds met our very stringent criteria for complete mental health . . . . The news for cancer survivors was even better, with three-quarters living in complete mental health, which is a prevalence comparable to that of individuals with no cancer history.”
This study included a nationally representative sample of Canadian community dwellers age 50 and older. Subjects had current cancer (n=438), previous cancer (n=1174), or no cancer history (n=9279).
Data were obtained from Statistics Canada’s 2012 Canadian Community Health Survey-Mental Health.
To meet criteria for CMH, subjects had to have all of the following:
- Absence of mental illness, addictions, and suicidal thoughts in the past year
- Almost daily happiness or life satisfaction in the past month
- Psychosocial well-being.
The prevalence of CMH was 77.5% in cancer survivors and 76.8% in subjects who had never had cancer. Both were significantly higher than the 66.1% prevalence of CMH in current cancer patients (P<0.001).
In a multivariable model adjusted for demographics, current cancer patients had 45% lower odds of CMH compared to subjects with no cancer history (odds ratio [OR]=0.55). The odds of CMH were comparable for cancer survivors and those without a history of cancer (OR=0.98).
The researchers also conducted a multivariable analysis in which they adjusted for “all relevant factors,” which included demographics as well as adverse childhood events, socioeconomic status, health variables, lifetime mental illness, etc.
In this analysis, current cancer patients had 37% lower odds of CMH than subjects with no cancer history (OR=0.63). And cancer survivors had comparable odds of CMH as those with no cancer history (OR=1.06).
The researchers identified several factors that were associated with CMH in the population affected by cancer.
“Among those with former or current cancer, the odds of complete mental health were higher for women, white, married, and older respondents, as well as those with higher income and those who did not have disabling pain nor functional limitations,” West said.
“We found that earlier difficulties cast a long shadow. Those who had been physically abused during their childhood and those who had ever had depression or anxiety disorders were less likely to be in complete mental health.”
West and Dr Fuller-Thomson emphasized that these results are only correlational, and it is impossible to determine causality due to the cross-sectional and observational nature of the study.
The pair also said future longitudinal research is needed to improve understanding of what pathways improve resilience and recovery among cancer patients.
New research suggests cancer survivors are just as likely as people without a history of cancer to have complete mental health (CMH), which is defined as “optimal functioning” and the “absence of psychopathology.”
In a study of nearly 11,000 Canadians, 77.5% of cancer survivors and 76.8% of people with no cancer history had CMH.
As for patients who were battling cancer at the time of the study, 66.1% had CMH.
Esme Fuller-Thomson, PhD, and Keri West, both of the University of Toronto in Canada, conducted this research and reported the findings in Aging & Mental Health.
“Cancer patients were doing much better than we had expected,” Dr. Fuller-Thomson said. “Two-thirds met our very stringent criteria for complete mental health . . . . The news for cancer survivors was even better, with three-quarters living in complete mental health, which is a prevalence comparable to that of individuals with no cancer history.”
This study included a nationally representative sample of Canadian community dwellers age 50 and older. Subjects had current cancer (n=438), previous cancer (n=1174), or no cancer history (n=9279).
Data were obtained from Statistics Canada’s 2012 Canadian Community Health Survey-Mental Health.
To meet criteria for CMH, subjects had to have all of the following:
- Absence of mental illness, addictions, and suicidal thoughts in the past year
- Almost daily happiness or life satisfaction in the past month
- Psychosocial well-being.
The prevalence of CMH was 77.5% in cancer survivors and 76.8% in subjects who had never had cancer. Both were significantly higher than the 66.1% prevalence of CMH in current cancer patients (P<0.001).
In a multivariable model adjusted for demographics, current cancer patients had 45% lower odds of CMH compared to subjects with no cancer history (odds ratio [OR]=0.55). The odds of CMH were comparable for cancer survivors and those without a history of cancer (OR=0.98).
The researchers also conducted a multivariable analysis in which they adjusted for “all relevant factors,” which included demographics as well as adverse childhood events, socioeconomic status, health variables, lifetime mental illness, etc.
In this analysis, current cancer patients had 37% lower odds of CMH than subjects with no cancer history (OR=0.63). And cancer survivors had comparable odds of CMH as those with no cancer history (OR=1.06).
The researchers identified several factors that were associated with CMH in the population affected by cancer.
“Among those with former or current cancer, the odds of complete mental health were higher for women, white, married, and older respondents, as well as those with higher income and those who did not have disabling pain nor functional limitations,” West said.
“We found that earlier difficulties cast a long shadow. Those who had been physically abused during their childhood and those who had ever had depression or anxiety disorders were less likely to be in complete mental health.”
West and Dr Fuller-Thomson emphasized that these results are only correlational, and it is impossible to determine causality due to the cross-sectional and observational nature of the study.
The pair also said future longitudinal research is needed to improve understanding of what pathways improve resilience and recovery among cancer patients.
Combo produces high response rate in CLL
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Drug receives priority review for second MM indication
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for elotuzumab (Empliciti).
With this sBLA, Bristol-Myers Squibb Company is seeking approval for elotuzumab in combination with pomalidomide and low-dose dexamethasone to treat patients with relapsed/refractory multiple myeloma (MM) who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
The FDA expects to make a decision on the sBLA for elotuzumab by December 27, 2018.
Elotuzumab is already FDA-approved for use in combination with lenalidomide and dexamethasone to treat MM patients who have received one to three prior therapies.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities.
Supporting trial
The sBLA for elotuzumab is supported by data from ELOQUENT-3, a randomized, phase 2 study in which researchers evaluated the addition of elotuzumab to pomalidomide and low-dose dexamethasone in patients with relapsed/refractory MM.
Data from this study were presented at the 23rd Congress of the European Hematology Association in June.
The trial randomized 117 MM patients who received two or more prior therapies and were either refractory or relapsed and refractory to lenalidomide and a proteasome inhibitor.
The patients were randomized to receive either elotuzumab, pomalidomide, and low-dose dexamethasone (EPd; n=60) or pomalidomide and low-dose dexamethasone (Pd; n=57) in 28-day cycles until disease progression or unacceptable toxicity.
The overall response rate was 53% in the EPd arm and 26% in the Pd arm (odds ratio=3.25; P=0.0029).
The median progression-free survival was 10.3 months in the EPd arm and 4.7 months in the Pd arm (hazard ratio=0.54, P=0.0078).
Overall survival data were not mature at last follow-up, but there was a trend favoring EPd over Pd (hazard ratio=0.62).
The researchers said adverse events (AEs) in the EPd arm were consistent with expectations based on previous results with elotuzumab and pomalidomide regimens.
Grade 3-4 nonhematologic AEs (in the EPd and Pd arms, respectively) included constipation (2% and 0%), hyperglycemia (8% and 7%), bone pain (3% and 0%), dyspnea (3% and 2%), fatigue (0% and 4%), respiratory tract infection (0% and 2%), and upper respiratory tract infection (0% and 2%).
Grade 3-4 hematologic AEs (in the EPd and Pd arms, respectively) included anemia (10% and 20%), neutropenia (13% and 27%), thrombocytopenia (8% and 5%), and lymphopenia (8% and 2%).
Grade 3-4 AEs of special interest (in the EPd and Pd arms, respectively) included infections (13% and 22%), vascular disorders (3% and 0%), cardiac disorders (7% and 4%), and neoplasms (2% and 11%).
In the EPd arm, grade 5 AEs included infection (n=3), cardiac failure (n=1), and general physical health deterioration (n=1).
In the Pd arm, grade 5 AEs included malignant neoplasm progression (n=4), infection (n=1), multiple organ failure and infection (n=1), myocardial infarction (n=1), and plasma cell myeloma (n=1).
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for elotuzumab (Empliciti).
With this sBLA, Bristol-Myers Squibb Company is seeking approval for elotuzumab in combination with pomalidomide and low-dose dexamethasone to treat patients with relapsed/refractory multiple myeloma (MM) who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
The FDA expects to make a decision on the sBLA for elotuzumab by December 27, 2018.
Elotuzumab is already FDA-approved for use in combination with lenalidomide and dexamethasone to treat MM patients who have received one to three prior therapies.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities.
Supporting trial
The sBLA for elotuzumab is supported by data from ELOQUENT-3, a randomized, phase 2 study in which researchers evaluated the addition of elotuzumab to pomalidomide and low-dose dexamethasone in patients with relapsed/refractory MM.
Data from this study were presented at the 23rd Congress of the European Hematology Association in June.
The trial randomized 117 MM patients who received two or more prior therapies and were either refractory or relapsed and refractory to lenalidomide and a proteasome inhibitor.
The patients were randomized to receive either elotuzumab, pomalidomide, and low-dose dexamethasone (EPd; n=60) or pomalidomide and low-dose dexamethasone (Pd; n=57) in 28-day cycles until disease progression or unacceptable toxicity.
The overall response rate was 53% in the EPd arm and 26% in the Pd arm (odds ratio=3.25; P=0.0029).
The median progression-free survival was 10.3 months in the EPd arm and 4.7 months in the Pd arm (hazard ratio=0.54, P=0.0078).
Overall survival data were not mature at last follow-up, but there was a trend favoring EPd over Pd (hazard ratio=0.62).
The researchers said adverse events (AEs) in the EPd arm were consistent with expectations based on previous results with elotuzumab and pomalidomide regimens.
Grade 3-4 nonhematologic AEs (in the EPd and Pd arms, respectively) included constipation (2% and 0%), hyperglycemia (8% and 7%), bone pain (3% and 0%), dyspnea (3% and 2%), fatigue (0% and 4%), respiratory tract infection (0% and 2%), and upper respiratory tract infection (0% and 2%).
Grade 3-4 hematologic AEs (in the EPd and Pd arms, respectively) included anemia (10% and 20%), neutropenia (13% and 27%), thrombocytopenia (8% and 5%), and lymphopenia (8% and 2%).
Grade 3-4 AEs of special interest (in the EPd and Pd arms, respectively) included infections (13% and 22%), vascular disorders (3% and 0%), cardiac disorders (7% and 4%), and neoplasms (2% and 11%).
In the EPd arm, grade 5 AEs included infection (n=3), cardiac failure (n=1), and general physical health deterioration (n=1).
In the Pd arm, grade 5 AEs included malignant neoplasm progression (n=4), infection (n=1), multiple organ failure and infection (n=1), myocardial infarction (n=1), and plasma cell myeloma (n=1).
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for elotuzumab (Empliciti).
With this sBLA, Bristol-Myers Squibb Company is seeking approval for elotuzumab in combination with pomalidomide and low-dose dexamethasone to treat patients with relapsed/refractory multiple myeloma (MM) who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
The FDA expects to make a decision on the sBLA for elotuzumab by December 27, 2018.
Elotuzumab is already FDA-approved for use in combination with lenalidomide and dexamethasone to treat MM patients who have received one to three prior therapies.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities.
Supporting trial
The sBLA for elotuzumab is supported by data from ELOQUENT-3, a randomized, phase 2 study in which researchers evaluated the addition of elotuzumab to pomalidomide and low-dose dexamethasone in patients with relapsed/refractory MM.
Data from this study were presented at the 23rd Congress of the European Hematology Association in June.
The trial randomized 117 MM patients who received two or more prior therapies and were either refractory or relapsed and refractory to lenalidomide and a proteasome inhibitor.
The patients were randomized to receive either elotuzumab, pomalidomide, and low-dose dexamethasone (EPd; n=60) or pomalidomide and low-dose dexamethasone (Pd; n=57) in 28-day cycles until disease progression or unacceptable toxicity.
The overall response rate was 53% in the EPd arm and 26% in the Pd arm (odds ratio=3.25; P=0.0029).
The median progression-free survival was 10.3 months in the EPd arm and 4.7 months in the Pd arm (hazard ratio=0.54, P=0.0078).
Overall survival data were not mature at last follow-up, but there was a trend favoring EPd over Pd (hazard ratio=0.62).
The researchers said adverse events (AEs) in the EPd arm were consistent with expectations based on previous results with elotuzumab and pomalidomide regimens.
Grade 3-4 nonhematologic AEs (in the EPd and Pd arms, respectively) included constipation (2% and 0%), hyperglycemia (8% and 7%), bone pain (3% and 0%), dyspnea (3% and 2%), fatigue (0% and 4%), respiratory tract infection (0% and 2%), and upper respiratory tract infection (0% and 2%).
Grade 3-4 hematologic AEs (in the EPd and Pd arms, respectively) included anemia (10% and 20%), neutropenia (13% and 27%), thrombocytopenia (8% and 5%), and lymphopenia (8% and 2%).
Grade 3-4 AEs of special interest (in the EPd and Pd arms, respectively) included infections (13% and 22%), vascular disorders (3% and 0%), cardiac disorders (7% and 4%), and neoplasms (2% and 11%).
In the EPd arm, grade 5 AEs included infection (n=3), cardiac failure (n=1), and general physical health deterioration (n=1).
In the Pd arm, grade 5 AEs included malignant neoplasm progression (n=4), infection (n=1), multiple organ failure and infection (n=1), myocardial infarction (n=1), and plasma cell myeloma (n=1).
Study examines the world of alcohol use
Considerable variations were seen in alcohol consumption. In 2016, males overall consumed more than twice as many standard drinks per day as females: 1.70 versus 0.73. Alcohol consumption in those aged 15-95 years was highest in the top quintile of countries according to sociodemographic development for both males (2.9 drinks per day) and females (1.9) and lowest in the bottom quintile of countries for males (1.4) and the second-lowest quintile for females (0.3), Max G. Griswold, MA, of the University of Washington, Seattle, and his associates said in the Lancet.
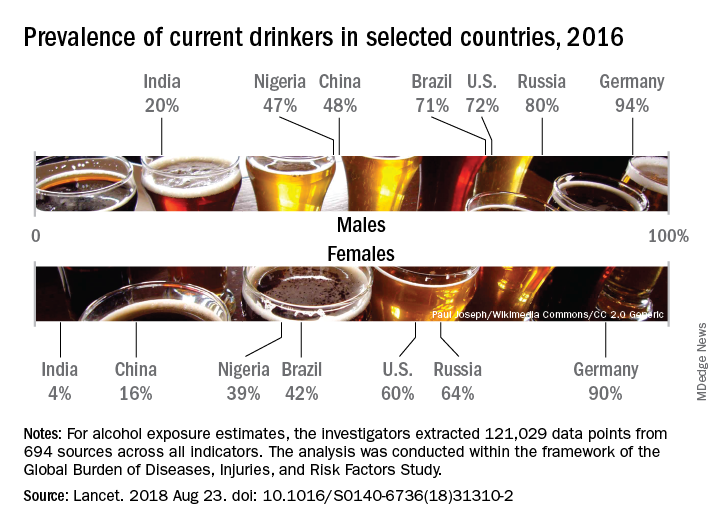
Denmark had the highest prevalence of current drinkers of any country for both males (97%) and females (95%) in 2016; Pakistan was lowest for males (0.8%) and Bangladesh was lowest for females (0.3%). The United States had a prevalence of 72% for males and 60% for females, along with consumption rates of 3.2 drinks per day for males and 1.9 for females. Alcohol-related diseases caused 6.7% of male deaths and 2.3% of female deaths in the United States, both close to the global numbers of 6.8% for males and 2.2% for females, the investigators said.
The analysis, conducted within the framework of the Global Burden of Diseases, Injuries, and Risk Factors Study, showed that even a single alcoholic drink a day increases the risk of developing 1 of the 23 alcohol-related health problems by 0.5% a year for people aged 15-95 years, which translates into a rate of 918 per 100,000 population, compared with 914 per 100,000 for nondrinkers. Consuming two drinks a day raises the risk to 7%, which would be an incidence of 977 per 100,000, and those who have five drinks a day increase their risk by 37%, which works out to 1,252 people per 100,000 who would develop an alcohol-related disease, Mr. Griswold and his associates said.
In an editorial comment, Robyn Burton, PhD, of King’s College London and Nick Sheron, MD, of the University of Southampton (England), wrote that “the conclusions of the study are clear and unambiguous: Alcohol is a colossal global health issue and small reductions in health-related harms at low levels of alcohol intake are outweighed by the increased risk of other health-related harms, including cancer. … These diseases of unhealthy behaviors, facilitated by unhealthy environments and fueled by commercial interests putting shareholder value ahead of the tragic human consequences, are the dominant health issue of the 21st century. The solutions are straightforward: Increasing taxation creates income for hard-pressed health ministries, and reducing the exposure of children to alcohol marketing has no downsides.”
The study was funded by the Bill and Melinda Gates Foundation. Mr. Griswold did not have any conflicts to disclose, but six of his several hundred coauthors did make such disclosures.
SOURCE: Griswold MG et al. Lancet. 2018 Aug 23. doi: 10.1016/S0140-6736(18)31310-2.

Considerable variations were seen in alcohol consumption. In 2016, males overall consumed more than twice as many standard drinks per day as females: 1.70 versus 0.73. Alcohol consumption in those aged 15-95 years was highest in the top quintile of countries according to sociodemographic development for both males (2.9 drinks per day) and females (1.9) and lowest in the bottom quintile of countries for males (1.4) and the second-lowest quintile for females (0.3), Max G. Griswold, MA, of the University of Washington, Seattle, and his associates said in the Lancet.
Denmark had the highest prevalence of current drinkers of any country for both males (97%) and females (95%) in 2016; Pakistan was lowest for males (0.8%) and Bangladesh was lowest for females (0.3%). The United States had a prevalence of 72% for males and 60% for females, along with consumption rates of 3.2 drinks per day for males and 1.9 for females. Alcohol-related diseases caused 6.7% of male deaths and 2.3% of female deaths in the United States, both close to the global numbers of 6.8% for males and 2.2% for females, the investigators said.
The analysis, conducted within the framework of the Global Burden of Diseases, Injuries, and Risk Factors Study, showed that even a single alcoholic drink a day increases the risk of developing 1 of the 23 alcohol-related health problems by 0.5% a year for people aged 15-95 years, which translates into a rate of 918 per 100,000 population, compared with 914 per 100,000 for nondrinkers. Consuming two drinks a day raises the risk to 7%, which would be an incidence of 977 per 100,000, and those who have five drinks a day increase their risk by 37%, which works out to 1,252 people per 100,000 who would develop an alcohol-related disease, Mr. Griswold and his associates said.
In an editorial comment, Robyn Burton, PhD, of King’s College London and Nick Sheron, MD, of the University of Southampton (England), wrote that “the conclusions of the study are clear and unambiguous: Alcohol is a colossal global health issue and small reductions in health-related harms at low levels of alcohol intake are outweighed by the increased risk of other health-related harms, including cancer. … These diseases of unhealthy behaviors, facilitated by unhealthy environments and fueled by commercial interests putting shareholder value ahead of the tragic human consequences, are the dominant health issue of the 21st century. The solutions are straightforward: Increasing taxation creates income for hard-pressed health ministries, and reducing the exposure of children to alcohol marketing has no downsides.”
The study was funded by the Bill and Melinda Gates Foundation. Mr. Griswold did not have any conflicts to disclose, but six of his several hundred coauthors did make such disclosures.
SOURCE: Griswold MG et al. Lancet. 2018 Aug 23. doi: 10.1016/S0140-6736(18)31310-2.

Considerable variations were seen in alcohol consumption. In 2016, males overall consumed more than twice as many standard drinks per day as females: 1.70 versus 0.73. Alcohol consumption in those aged 15-95 years was highest in the top quintile of countries according to sociodemographic development for both males (2.9 drinks per day) and females (1.9) and lowest in the bottom quintile of countries for males (1.4) and the second-lowest quintile for females (0.3), Max G. Griswold, MA, of the University of Washington, Seattle, and his associates said in the Lancet.
Denmark had the highest prevalence of current drinkers of any country for both males (97%) and females (95%) in 2016; Pakistan was lowest for males (0.8%) and Bangladesh was lowest for females (0.3%). The United States had a prevalence of 72% for males and 60% for females, along with consumption rates of 3.2 drinks per day for males and 1.9 for females. Alcohol-related diseases caused 6.7% of male deaths and 2.3% of female deaths in the United States, both close to the global numbers of 6.8% for males and 2.2% for females, the investigators said.
The analysis, conducted within the framework of the Global Burden of Diseases, Injuries, and Risk Factors Study, showed that even a single alcoholic drink a day increases the risk of developing 1 of the 23 alcohol-related health problems by 0.5% a year for people aged 15-95 years, which translates into a rate of 918 per 100,000 population, compared with 914 per 100,000 for nondrinkers. Consuming two drinks a day raises the risk to 7%, which would be an incidence of 977 per 100,000, and those who have five drinks a day increase their risk by 37%, which works out to 1,252 people per 100,000 who would develop an alcohol-related disease, Mr. Griswold and his associates said.
In an editorial comment, Robyn Burton, PhD, of King’s College London and Nick Sheron, MD, of the University of Southampton (England), wrote that “the conclusions of the study are clear and unambiguous: Alcohol is a colossal global health issue and small reductions in health-related harms at low levels of alcohol intake are outweighed by the increased risk of other health-related harms, including cancer. … These diseases of unhealthy behaviors, facilitated by unhealthy environments and fueled by commercial interests putting shareholder value ahead of the tragic human consequences, are the dominant health issue of the 21st century. The solutions are straightforward: Increasing taxation creates income for hard-pressed health ministries, and reducing the exposure of children to alcohol marketing has no downsides.”
The study was funded by the Bill and Melinda Gates Foundation. Mr. Griswold did not have any conflicts to disclose, but six of his several hundred coauthors did make such disclosures.
SOURCE: Griswold MG et al. Lancet. 2018 Aug 23. doi: 10.1016/S0140-6736(18)31310-2.
FROM THE LANCET
Combo treatment yields MRD-negative remissions in CLL
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: The overall response rate for obinutuzumab plus venetoclax in CLL was 95%.
Study details: An ongoing, phase 2, open-label trial in 66 patients with chronic lymphocytic leukemia.
Disclosures: The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Source: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The impact of tuition-free medical education
On Aug.16, the New York University School of Medicine announced it was offering full-tuition scholarships to all current and future students in its MD Program, regardless of need or merit – and that this policy also would apply to all matriculating students for the remainder of their medical school education at NYU.
This bold initiative, they stated, was being instituted to simultaneously address the rising costs of medical education while still attracting the best and brightest to careers in medicine. In doing so, NYU School of Medicine (at which I hold a faculty appointment) became the first Top 10–ranked medical school in the nation to do so.
The symbolism of this announcement was noticeable: It was made at the medical school’s annual white coat ceremony, when each new student is presented with a white lab coat to mark the beginning of their medical education and training.
I count myself among many medical professionals and, indeed, others outside of medicine who have long advocated for free tuition for medical education, at the very least for those who have little or no means to pay. This particularly painful burden of debt often serves as a deterrent to many individuals who are considering a career in medicine or medical research.
According to the Association of American Medical Colleges,
What might develop as a result of NYU’s decision and that of several other schools who have adopted debt-reduction policies?
First, that these programs might have a ripple effect at other medical schools, and create a movement for more students to earn a medical degree without incurring a crushing financial burden. Some other schools, like Columbia University, already have taken steps, such as replacing all student loans with scholarships creating a “debt-free” medical school. It would, indeed, be a powerful message if other schools developed similar creative solutions to this problem.
Second, there is hope that debt relief will encourage more medical school graduates to pursue careers in such specialties as family medicine, psychiatry, pediatrics, and geriatrics – because they will not have the additional financial pressure to pursue careers in more lucrative specialties in order to pay off debt. While many medical school graduates point to other issues like complex reimbursement as a greater deterrent to a specific specialty choice, I certainly hope that debt relief will have a positive effect in shifting the subspecialty paradigm.
Third, these actions might incentivize the federal government to establish an AmeriCorps-type program, in which the cost of a medical education is covered in return for a commitment to practice medicine for a period of time in underserved areas of the country. Such an approach also might motivate more medical school graduates to pursue careers in primary care specialties and help address some of the ongoing concerns related to the uneven distribution of physicians in the United States.
Another issue that often comes up is the impact of debt burden on “burnout” among medical students. This is a complex subject – and one that actually affects physicians beyond medical school and into residency training and medical practice.
There is no doubt that debt weighs heavily on the minds of medical students – and many enter medical school having sustained significant debt already from previous education in colleges and universities. However, the causes and influences on burnout in training are multifactorial. Earlier in medical school, the impact of debt obligation may be less apparent because of the other challenges students face when beginning medical school.
However, once trainees begin residency and fellowship training – and especially during early career years when many are beginning to have families – concerns about increasing financial strain become even more prominent. For many young physicians, already stressed by other extenuating factors, it would be a tremendous relief not to have that debt pursuing them.
As someone who teaches and mentors medical students and residents, I firmly believe that most students pursue a career in medicine for altruistic reasons: to help cure illness and take care of patients, to make new scientific discoveries, and to train the next generation of physicians who will follow them into the medical profession. Unfortunately, outside economic influences – such as increased competition among health care systems, shrinking reimbursements, loss of joy and meaning in medicine, increasing isolation of the caregiver from the patient and the significant cost of a medical education – lead many physicians to burn out prematurely and, for some, to leave the profession altogether.
By eliminating medical school debt, we can remove one of these constraints and make the practice of medicine as rewarding and gratifying as it has been in the past, and more accessible to those who truly wish to care for others.
Dr. Bernstein is a professor in the departments of psychiatry and neurology at New York University and a past president of the American Psychiatric Association.
On Aug.16, the New York University School of Medicine announced it was offering full-tuition scholarships to all current and future students in its MD Program, regardless of need or merit – and that this policy also would apply to all matriculating students for the remainder of their medical school education at NYU.
This bold initiative, they stated, was being instituted to simultaneously address the rising costs of medical education while still attracting the best and brightest to careers in medicine. In doing so, NYU School of Medicine (at which I hold a faculty appointment) became the first Top 10–ranked medical school in the nation to do so.
The symbolism of this announcement was noticeable: It was made at the medical school’s annual white coat ceremony, when each new student is presented with a white lab coat to mark the beginning of their medical education and training.
I count myself among many medical professionals and, indeed, others outside of medicine who have long advocated for free tuition for medical education, at the very least for those who have little or no means to pay. This particularly painful burden of debt often serves as a deterrent to many individuals who are considering a career in medicine or medical research.
According to the Association of American Medical Colleges,
What might develop as a result of NYU’s decision and that of several other schools who have adopted debt-reduction policies?
First, that these programs might have a ripple effect at other medical schools, and create a movement for more students to earn a medical degree without incurring a crushing financial burden. Some other schools, like Columbia University, already have taken steps, such as replacing all student loans with scholarships creating a “debt-free” medical school. It would, indeed, be a powerful message if other schools developed similar creative solutions to this problem.
Second, there is hope that debt relief will encourage more medical school graduates to pursue careers in such specialties as family medicine, psychiatry, pediatrics, and geriatrics – because they will not have the additional financial pressure to pursue careers in more lucrative specialties in order to pay off debt. While many medical school graduates point to other issues like complex reimbursement as a greater deterrent to a specific specialty choice, I certainly hope that debt relief will have a positive effect in shifting the subspecialty paradigm.
Third, these actions might incentivize the federal government to establish an AmeriCorps-type program, in which the cost of a medical education is covered in return for a commitment to practice medicine for a period of time in underserved areas of the country. Such an approach also might motivate more medical school graduates to pursue careers in primary care specialties and help address some of the ongoing concerns related to the uneven distribution of physicians in the United States.
Another issue that often comes up is the impact of debt burden on “burnout” among medical students. This is a complex subject – and one that actually affects physicians beyond medical school and into residency training and medical practice.
There is no doubt that debt weighs heavily on the minds of medical students – and many enter medical school having sustained significant debt already from previous education in colleges and universities. However, the causes and influences on burnout in training are multifactorial. Earlier in medical school, the impact of debt obligation may be less apparent because of the other challenges students face when beginning medical school.
However, once trainees begin residency and fellowship training – and especially during early career years when many are beginning to have families – concerns about increasing financial strain become even more prominent. For many young physicians, already stressed by other extenuating factors, it would be a tremendous relief not to have that debt pursuing them.
As someone who teaches and mentors medical students and residents, I firmly believe that most students pursue a career in medicine for altruistic reasons: to help cure illness and take care of patients, to make new scientific discoveries, and to train the next generation of physicians who will follow them into the medical profession. Unfortunately, outside economic influences – such as increased competition among health care systems, shrinking reimbursements, loss of joy and meaning in medicine, increasing isolation of the caregiver from the patient and the significant cost of a medical education – lead many physicians to burn out prematurely and, for some, to leave the profession altogether.
By eliminating medical school debt, we can remove one of these constraints and make the practice of medicine as rewarding and gratifying as it has been in the past, and more accessible to those who truly wish to care for others.
Dr. Bernstein is a professor in the departments of psychiatry and neurology at New York University and a past president of the American Psychiatric Association.
On Aug.16, the New York University School of Medicine announced it was offering full-tuition scholarships to all current and future students in its MD Program, regardless of need or merit – and that this policy also would apply to all matriculating students for the remainder of their medical school education at NYU.
This bold initiative, they stated, was being instituted to simultaneously address the rising costs of medical education while still attracting the best and brightest to careers in medicine. In doing so, NYU School of Medicine (at which I hold a faculty appointment) became the first Top 10–ranked medical school in the nation to do so.
The symbolism of this announcement was noticeable: It was made at the medical school’s annual white coat ceremony, when each new student is presented with a white lab coat to mark the beginning of their medical education and training.
I count myself among many medical professionals and, indeed, others outside of medicine who have long advocated for free tuition for medical education, at the very least for those who have little or no means to pay. This particularly painful burden of debt often serves as a deterrent to many individuals who are considering a career in medicine or medical research.
According to the Association of American Medical Colleges,
What might develop as a result of NYU’s decision and that of several other schools who have adopted debt-reduction policies?
First, that these programs might have a ripple effect at other medical schools, and create a movement for more students to earn a medical degree without incurring a crushing financial burden. Some other schools, like Columbia University, already have taken steps, such as replacing all student loans with scholarships creating a “debt-free” medical school. It would, indeed, be a powerful message if other schools developed similar creative solutions to this problem.
Second, there is hope that debt relief will encourage more medical school graduates to pursue careers in such specialties as family medicine, psychiatry, pediatrics, and geriatrics – because they will not have the additional financial pressure to pursue careers in more lucrative specialties in order to pay off debt. While many medical school graduates point to other issues like complex reimbursement as a greater deterrent to a specific specialty choice, I certainly hope that debt relief will have a positive effect in shifting the subspecialty paradigm.
Third, these actions might incentivize the federal government to establish an AmeriCorps-type program, in which the cost of a medical education is covered in return for a commitment to practice medicine for a period of time in underserved areas of the country. Such an approach also might motivate more medical school graduates to pursue careers in primary care specialties and help address some of the ongoing concerns related to the uneven distribution of physicians in the United States.
Another issue that often comes up is the impact of debt burden on “burnout” among medical students. This is a complex subject – and one that actually affects physicians beyond medical school and into residency training and medical practice.
There is no doubt that debt weighs heavily on the minds of medical students – and many enter medical school having sustained significant debt already from previous education in colleges and universities. However, the causes and influences on burnout in training are multifactorial. Earlier in medical school, the impact of debt obligation may be less apparent because of the other challenges students face when beginning medical school.
However, once trainees begin residency and fellowship training – and especially during early career years when many are beginning to have families – concerns about increasing financial strain become even more prominent. For many young physicians, already stressed by other extenuating factors, it would be a tremendous relief not to have that debt pursuing them.
As someone who teaches and mentors medical students and residents, I firmly believe that most students pursue a career in medicine for altruistic reasons: to help cure illness and take care of patients, to make new scientific discoveries, and to train the next generation of physicians who will follow them into the medical profession. Unfortunately, outside economic influences – such as increased competition among health care systems, shrinking reimbursements, loss of joy and meaning in medicine, increasing isolation of the caregiver from the patient and the significant cost of a medical education – lead many physicians to burn out prematurely and, for some, to leave the profession altogether.
By eliminating medical school debt, we can remove one of these constraints and make the practice of medicine as rewarding and gratifying as it has been in the past, and more accessible to those who truly wish to care for others.
Dr. Bernstein is a professor in the departments of psychiatry and neurology at New York University and a past president of the American Psychiatric Association.
Barriers loom for HCV care in young people who inject drugs
Young adults who inject drugs and are infected with hepatitis C virus “face unique barriers to HCV testing, counseling, and treatment,” according to Margie R. Skeer, ScD, of Tufts University, Boston, and her fellow researchers.
Dr. Skeer and her colleagues found five themes in 24 in-depth interviews with people aged 22-30 years who inject drugs and have HCV infection. At the time of the interviews, none of the patients had received the newer HCV treatment regimens (Drug Alcohol Depend. 2018 Sep 1;190:246-54).
These themes captured the knowledge of and experience of HCV along the continuum of care:
1. Deservingness of HCV treatment and stigma.
2. Dissatisfaction with provider interactions.
3. Perceived lack of referral to treatment and care continuity.
4. Disincentives around HCV treatment for PWID.
5. Perceived need for treatment.
The interviewees were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after their diagnosis. They also tended to affirm the belief that they did not deserve treatment. They felt stigmatized by insurance companies and clinicians, thereby reducing their engagement in the care continuum. And, at the time, insurance companies enforced “sobriety” restrictions dictating the length of time patients had to be off drugs before qualifying for HCV treatment. In the words of one interviewee: “[Caregivers] have a big stigma when it comes to addicts. ... Their whole demeanor changes. They rush you, they slam things, they are very impatient with you, and it is very saddening to see.”
Interviewees reported no or incomplete referrals or being given pamphlets and flyers. They reported little follow-up as to whether they sought additional care, and experienced a lack of confidence from medical professionals that they could be counted on to adhere to an HCV treatment regimen.
Interviewees stated that injection drug use and HCV are inevitably linked, and that IV drug users will eventually contract HCV infections.
“Hep C’s no big deal, Hep C’s like the common cold for the junkie. ... It might take 5 years away from your, you know, life but, you know, we’re not even gonna live that long anyways, so who cares about it anyway,” remarked a 28-year old woman, who was not currently injecting drugs.
The study authors said there is an increased need to provide patient-oriented care for young injection drug users and described the potential benefits of some insurance companies reducing their sobriety and disease severity restrictions.
“Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention,” Dr. Skeer and her colleagues concluded.
The authors reported that they had no conflicts of interest.
AGA patient materials can help your patients better understand and manage living with hepatitis C. Learn more at patient.gastro.org.
Young adults who inject drugs and are infected with hepatitis C virus “face unique barriers to HCV testing, counseling, and treatment,” according to Margie R. Skeer, ScD, of Tufts University, Boston, and her fellow researchers.
Dr. Skeer and her colleagues found five themes in 24 in-depth interviews with people aged 22-30 years who inject drugs and have HCV infection. At the time of the interviews, none of the patients had received the newer HCV treatment regimens (Drug Alcohol Depend. 2018 Sep 1;190:246-54).
These themes captured the knowledge of and experience of HCV along the continuum of care:
1. Deservingness of HCV treatment and stigma.
2. Dissatisfaction with provider interactions.
3. Perceived lack of referral to treatment and care continuity.
4. Disincentives around HCV treatment for PWID.
5. Perceived need for treatment.
The interviewees were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after their diagnosis. They also tended to affirm the belief that they did not deserve treatment. They felt stigmatized by insurance companies and clinicians, thereby reducing their engagement in the care continuum. And, at the time, insurance companies enforced “sobriety” restrictions dictating the length of time patients had to be off drugs before qualifying for HCV treatment. In the words of one interviewee: “[Caregivers] have a big stigma when it comes to addicts. ... Their whole demeanor changes. They rush you, they slam things, they are very impatient with you, and it is very saddening to see.”
Interviewees reported no or incomplete referrals or being given pamphlets and flyers. They reported little follow-up as to whether they sought additional care, and experienced a lack of confidence from medical professionals that they could be counted on to adhere to an HCV treatment regimen.
Interviewees stated that injection drug use and HCV are inevitably linked, and that IV drug users will eventually contract HCV infections.
“Hep C’s no big deal, Hep C’s like the common cold for the junkie. ... It might take 5 years away from your, you know, life but, you know, we’re not even gonna live that long anyways, so who cares about it anyway,” remarked a 28-year old woman, who was not currently injecting drugs.
The study authors said there is an increased need to provide patient-oriented care for young injection drug users and described the potential benefits of some insurance companies reducing their sobriety and disease severity restrictions.
“Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention,” Dr. Skeer and her colleagues concluded.
The authors reported that they had no conflicts of interest.
AGA patient materials can help your patients better understand and manage living with hepatitis C. Learn more at patient.gastro.org.
Young adults who inject drugs and are infected with hepatitis C virus “face unique barriers to HCV testing, counseling, and treatment,” according to Margie R. Skeer, ScD, of Tufts University, Boston, and her fellow researchers.
Dr. Skeer and her colleagues found five themes in 24 in-depth interviews with people aged 22-30 years who inject drugs and have HCV infection. At the time of the interviews, none of the patients had received the newer HCV treatment regimens (Drug Alcohol Depend. 2018 Sep 1;190:246-54).
These themes captured the knowledge of and experience of HCV along the continuum of care:
1. Deservingness of HCV treatment and stigma.
2. Dissatisfaction with provider interactions.
3. Perceived lack of referral to treatment and care continuity.
4. Disincentives around HCV treatment for PWID.
5. Perceived need for treatment.
The interviewees were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after their diagnosis. They also tended to affirm the belief that they did not deserve treatment. They felt stigmatized by insurance companies and clinicians, thereby reducing their engagement in the care continuum. And, at the time, insurance companies enforced “sobriety” restrictions dictating the length of time patients had to be off drugs before qualifying for HCV treatment. In the words of one interviewee: “[Caregivers] have a big stigma when it comes to addicts. ... Their whole demeanor changes. They rush you, they slam things, they are very impatient with you, and it is very saddening to see.”
Interviewees reported no or incomplete referrals or being given pamphlets and flyers. They reported little follow-up as to whether they sought additional care, and experienced a lack of confidence from medical professionals that they could be counted on to adhere to an HCV treatment regimen.
Interviewees stated that injection drug use and HCV are inevitably linked, and that IV drug users will eventually contract HCV infections.
“Hep C’s no big deal, Hep C’s like the common cold for the junkie. ... It might take 5 years away from your, you know, life but, you know, we’re not even gonna live that long anyways, so who cares about it anyway,” remarked a 28-year old woman, who was not currently injecting drugs.
The study authors said there is an increased need to provide patient-oriented care for young injection drug users and described the potential benefits of some insurance companies reducing their sobriety and disease severity restrictions.
“Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention,” Dr. Skeer and her colleagues concluded.
The authors reported that they had no conflicts of interest.
AGA patient materials can help your patients better understand and manage living with hepatitis C. Learn more at patient.gastro.org.
FROM DRUG AND ALCOHOL DEPENDENCE
Options for treatment of bipolar disorder during pregnancy
The management of bipolar disorder during pregnancy is a critical clinical situation demanding great attention to issues such as reproductive safety of psychiatric medications used by women with bipolar disorder to maintain emotional well-being, compared with the established risk of relapse if patients stopped those medications.
Treatment of bipolar disorder frequently includes mainstay treatment with mood stabilizers such as sodium valproate, lithium, lamotrigine, and second-generation atypical antipsychotics. While we have robust information regarding the reproductive safety of sodium valproate, it is a teratogen with a very high risk for neural tube defects. In contrast, data over the 15 years have been very supportive of the reproductive safety of lamotrigine. The last decade has seen growing use of second-generation antipsychotics, so-called atypical antipsychotics. There has been growing interest in the reproductive safety of these medicines given their use both for acute mania and for prophylaxis of bipolar disorder; they also are used as an adjunct to treat patients with major depression. Atypical antipsychotics are widely used off-label to treat obsessive compulsive disorder, other anxiety disorders, and a spectrum of psychiatric illness.
Until relatively recently, data on the reproductive safety of second-generation atypical antipsychotics has been relatively sparse, with the small number of prospective studies yielding a small total number of patients. Over the same period of time, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) at Massachusetts General Hospital (MGH) was established, modeled after the North American Antiepileptic Drug Registry as a prospective registry of women with histories of first trimester exposure to atypical antipsychotics.
Over the last several years, the MGH NPRAA has accumulated very rigorous, prospectively ascertained data on outcomes following first trimester exposure to the atypical antipsychotics. Given the high prevalence of the use of this class of medications in reproductive-age women, data on the reproductive safety of atypical antipsychotics has been anxiously awaited and also has been relatively reassuring based on sources such as the NPRAA and also analyses of data from large administrative databases. For example, a recent paper published in JAMA Psychiatry by KF Huybrechts and her colleagues of 1,360,101 pregnant women who were enrolled in the Medicaid Analytic Extract database found an adjusted relative risk of 1.05 for congenital malformations in births for patients exposed to atypical antipsychotics (2016;73[9]:938-46).
Patients most often present with questions not about the reproductive safety of a class of medications, but about the safety of a particular medicine. A recent paper from our own group published in the American Journal of Psychiatry using data from the MGH NPRAA–described outcome of fetal exposure to quetiapine with a total of 152 women exposed to quetiapine and 205 unexposed patients. These patients were prospectively followed and compared with controls not exposed to the atypical antipsychotic but having a history of psychiatric morbidity. There was a 1.29% risk of major malformations in women exposed to quetiapine vs. 1.43% in the unexposed population (2018 Aug 16. doi: 10.1176/appi.ajp.2018.18010098).
The positive features of the MGH NPRAA include the careful rigorous assessments of patients over time as well as review of their obstetric, neonatal, and pediatric records up to 6 months, with blinded adjudication of outcome. The limitation of the small sample size remains with findings including relatively wide confidence intervals. With that being said, included in the paper in the discussion section is a pooled analysis of prospective data regarding quetiapine from the world’s literature that supports the findings of even this small prospective study in our registry, namely flat risk or absence of data suggesting that quetiapine is a major teratogen (pooled risk ratio, 1.03; 95% confidence interval, 0.89-1.19).
The delineation of risk for atypical antipsychotics is an extremely important area of research from a clinical point of view because it may help inform choices made by women with bipolar disorder who are well and maintained on these medicines as they wrestle with risk of relapse when agents are discontinued on one hand and reproductive safety concerns on the other.
Although not as widely used as perhaps a decade ago, data on the reproductive safety of lithium only continue to grow and become more refined. Use of lithium, a known teratogen with studies dating back to the 1970s, has an increased risk for cardiovascular malformations with the classic reference being to the small heightened risk of Ebstein’s anomaly (0.05%-0.1%). More recent studies from large administrative databases have been published with new data regarding risk of fetal exposure to lithium.
Two recent studies on lithium help to clarify some lingering questions about lithium use during pregnancy and risk for cardiovascular malformations. In one study published in the New England Journal of Medicine, researchers have demonstrated a small increased risk for cardiac malformations associated with using lithium during the first trimester (2017;376:2245-54). After researchers controlled for potential confounding factors, the adjusted risk ratio for cardiac malformations among infants exposed to lithium was 1.65 (95% CI, 1.02-2.68), compared with nonexposed infants. In a second study published in Lancet Psychiatry (2018 Aug;5[8]:644-52), a primary data meta-analysis of pregnant women and their children from six international cohorts in Denmark, Sweden, and Canada, there was no significant difference in major cardiac malformations between the lithium-exposed group, 2.1% (0.5%-3.7%), and the reference group, 1.6% (1.0%-2.1%).
Women with particularly brittle bipolar disorder or with histories of response to lithium may, in consultation with their doctors, consider use of lithium during pregnancy given the almost 50-year history of data accumulation on its reproductive safety, compared with some of the other mood stabilizers for which there is either confirmed teratogenicity (sodium valproate) or still incomplete data. Moreover, given the high risk for postpartum relapse of mood disorder in women who suffer from bipolar disorder, it is important to remember that the most robust data on prophylactic benefit of mood stabilizer during the peripartum period are with lithium.
Reproductive age women with bipolar disorder have for decades been caught between a teratologic rock and a clinical hard place. More recent data that have emerged from rigorously conducted registries and carefully analyzed administrative databases allow for more effective collaboration between patient and doctor as together they make personal decisions that match individual clinical situations with personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
The management of bipolar disorder during pregnancy is a critical clinical situation demanding great attention to issues such as reproductive safety of psychiatric medications used by women with bipolar disorder to maintain emotional well-being, compared with the established risk of relapse if patients stopped those medications.
Treatment of bipolar disorder frequently includes mainstay treatment with mood stabilizers such as sodium valproate, lithium, lamotrigine, and second-generation atypical antipsychotics. While we have robust information regarding the reproductive safety of sodium valproate, it is a teratogen with a very high risk for neural tube defects. In contrast, data over the 15 years have been very supportive of the reproductive safety of lamotrigine. The last decade has seen growing use of second-generation antipsychotics, so-called atypical antipsychotics. There has been growing interest in the reproductive safety of these medicines given their use both for acute mania and for prophylaxis of bipolar disorder; they also are used as an adjunct to treat patients with major depression. Atypical antipsychotics are widely used off-label to treat obsessive compulsive disorder, other anxiety disorders, and a spectrum of psychiatric illness.
Until relatively recently, data on the reproductive safety of second-generation atypical antipsychotics has been relatively sparse, with the small number of prospective studies yielding a small total number of patients. Over the same period of time, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) at Massachusetts General Hospital (MGH) was established, modeled after the North American Antiepileptic Drug Registry as a prospective registry of women with histories of first trimester exposure to atypical antipsychotics.
Over the last several years, the MGH NPRAA has accumulated very rigorous, prospectively ascertained data on outcomes following first trimester exposure to the atypical antipsychotics. Given the high prevalence of the use of this class of medications in reproductive-age women, data on the reproductive safety of atypical antipsychotics has been anxiously awaited and also has been relatively reassuring based on sources such as the NPRAA and also analyses of data from large administrative databases. For example, a recent paper published in JAMA Psychiatry by KF Huybrechts and her colleagues of 1,360,101 pregnant women who were enrolled in the Medicaid Analytic Extract database found an adjusted relative risk of 1.05 for congenital malformations in births for patients exposed to atypical antipsychotics (2016;73[9]:938-46).
Patients most often present with questions not about the reproductive safety of a class of medications, but about the safety of a particular medicine. A recent paper from our own group published in the American Journal of Psychiatry using data from the MGH NPRAA–described outcome of fetal exposure to quetiapine with a total of 152 women exposed to quetiapine and 205 unexposed patients. These patients were prospectively followed and compared with controls not exposed to the atypical antipsychotic but having a history of psychiatric morbidity. There was a 1.29% risk of major malformations in women exposed to quetiapine vs. 1.43% in the unexposed population (2018 Aug 16. doi: 10.1176/appi.ajp.2018.18010098).
The positive features of the MGH NPRAA include the careful rigorous assessments of patients over time as well as review of their obstetric, neonatal, and pediatric records up to 6 months, with blinded adjudication of outcome. The limitation of the small sample size remains with findings including relatively wide confidence intervals. With that being said, included in the paper in the discussion section is a pooled analysis of prospective data regarding quetiapine from the world’s literature that supports the findings of even this small prospective study in our registry, namely flat risk or absence of data suggesting that quetiapine is a major teratogen (pooled risk ratio, 1.03; 95% confidence interval, 0.89-1.19).
The delineation of risk for atypical antipsychotics is an extremely important area of research from a clinical point of view because it may help inform choices made by women with bipolar disorder who are well and maintained on these medicines as they wrestle with risk of relapse when agents are discontinued on one hand and reproductive safety concerns on the other.
Although not as widely used as perhaps a decade ago, data on the reproductive safety of lithium only continue to grow and become more refined. Use of lithium, a known teratogen with studies dating back to the 1970s, has an increased risk for cardiovascular malformations with the classic reference being to the small heightened risk of Ebstein’s anomaly (0.05%-0.1%). More recent studies from large administrative databases have been published with new data regarding risk of fetal exposure to lithium.
Two recent studies on lithium help to clarify some lingering questions about lithium use during pregnancy and risk for cardiovascular malformations. In one study published in the New England Journal of Medicine, researchers have demonstrated a small increased risk for cardiac malformations associated with using lithium during the first trimester (2017;376:2245-54). After researchers controlled for potential confounding factors, the adjusted risk ratio for cardiac malformations among infants exposed to lithium was 1.65 (95% CI, 1.02-2.68), compared with nonexposed infants. In a second study published in Lancet Psychiatry (2018 Aug;5[8]:644-52), a primary data meta-analysis of pregnant women and their children from six international cohorts in Denmark, Sweden, and Canada, there was no significant difference in major cardiac malformations between the lithium-exposed group, 2.1% (0.5%-3.7%), and the reference group, 1.6% (1.0%-2.1%).
Women with particularly brittle bipolar disorder or with histories of response to lithium may, in consultation with their doctors, consider use of lithium during pregnancy given the almost 50-year history of data accumulation on its reproductive safety, compared with some of the other mood stabilizers for which there is either confirmed teratogenicity (sodium valproate) or still incomplete data. Moreover, given the high risk for postpartum relapse of mood disorder in women who suffer from bipolar disorder, it is important to remember that the most robust data on prophylactic benefit of mood stabilizer during the peripartum period are with lithium.
Reproductive age women with bipolar disorder have for decades been caught between a teratologic rock and a clinical hard place. More recent data that have emerged from rigorously conducted registries and carefully analyzed administrative databases allow for more effective collaboration between patient and doctor as together they make personal decisions that match individual clinical situations with personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
The management of bipolar disorder during pregnancy is a critical clinical situation demanding great attention to issues such as reproductive safety of psychiatric medications used by women with bipolar disorder to maintain emotional well-being, compared with the established risk of relapse if patients stopped those medications.
Treatment of bipolar disorder frequently includes mainstay treatment with mood stabilizers such as sodium valproate, lithium, lamotrigine, and second-generation atypical antipsychotics. While we have robust information regarding the reproductive safety of sodium valproate, it is a teratogen with a very high risk for neural tube defects. In contrast, data over the 15 years have been very supportive of the reproductive safety of lamotrigine. The last decade has seen growing use of second-generation antipsychotics, so-called atypical antipsychotics. There has been growing interest in the reproductive safety of these medicines given their use both for acute mania and for prophylaxis of bipolar disorder; they also are used as an adjunct to treat patients with major depression. Atypical antipsychotics are widely used off-label to treat obsessive compulsive disorder, other anxiety disorders, and a spectrum of psychiatric illness.
Until relatively recently, data on the reproductive safety of second-generation atypical antipsychotics has been relatively sparse, with the small number of prospective studies yielding a small total number of patients. Over the same period of time, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) at Massachusetts General Hospital (MGH) was established, modeled after the North American Antiepileptic Drug Registry as a prospective registry of women with histories of first trimester exposure to atypical antipsychotics.
Over the last several years, the MGH NPRAA has accumulated very rigorous, prospectively ascertained data on outcomes following first trimester exposure to the atypical antipsychotics. Given the high prevalence of the use of this class of medications in reproductive-age women, data on the reproductive safety of atypical antipsychotics has been anxiously awaited and also has been relatively reassuring based on sources such as the NPRAA and also analyses of data from large administrative databases. For example, a recent paper published in JAMA Psychiatry by KF Huybrechts and her colleagues of 1,360,101 pregnant women who were enrolled in the Medicaid Analytic Extract database found an adjusted relative risk of 1.05 for congenital malformations in births for patients exposed to atypical antipsychotics (2016;73[9]:938-46).
Patients most often present with questions not about the reproductive safety of a class of medications, but about the safety of a particular medicine. A recent paper from our own group published in the American Journal of Psychiatry using data from the MGH NPRAA–described outcome of fetal exposure to quetiapine with a total of 152 women exposed to quetiapine and 205 unexposed patients. These patients were prospectively followed and compared with controls not exposed to the atypical antipsychotic but having a history of psychiatric morbidity. There was a 1.29% risk of major malformations in women exposed to quetiapine vs. 1.43% in the unexposed population (2018 Aug 16. doi: 10.1176/appi.ajp.2018.18010098).
The positive features of the MGH NPRAA include the careful rigorous assessments of patients over time as well as review of their obstetric, neonatal, and pediatric records up to 6 months, with blinded adjudication of outcome. The limitation of the small sample size remains with findings including relatively wide confidence intervals. With that being said, included in the paper in the discussion section is a pooled analysis of prospective data regarding quetiapine from the world’s literature that supports the findings of even this small prospective study in our registry, namely flat risk or absence of data suggesting that quetiapine is a major teratogen (pooled risk ratio, 1.03; 95% confidence interval, 0.89-1.19).
The delineation of risk for atypical antipsychotics is an extremely important area of research from a clinical point of view because it may help inform choices made by women with bipolar disorder who are well and maintained on these medicines as they wrestle with risk of relapse when agents are discontinued on one hand and reproductive safety concerns on the other.
Although not as widely used as perhaps a decade ago, data on the reproductive safety of lithium only continue to grow and become more refined. Use of lithium, a known teratogen with studies dating back to the 1970s, has an increased risk for cardiovascular malformations with the classic reference being to the small heightened risk of Ebstein’s anomaly (0.05%-0.1%). More recent studies from large administrative databases have been published with new data regarding risk of fetal exposure to lithium.
Two recent studies on lithium help to clarify some lingering questions about lithium use during pregnancy and risk for cardiovascular malformations. In one study published in the New England Journal of Medicine, researchers have demonstrated a small increased risk for cardiac malformations associated with using lithium during the first trimester (2017;376:2245-54). After researchers controlled for potential confounding factors, the adjusted risk ratio for cardiac malformations among infants exposed to lithium was 1.65 (95% CI, 1.02-2.68), compared with nonexposed infants. In a second study published in Lancet Psychiatry (2018 Aug;5[8]:644-52), a primary data meta-analysis of pregnant women and their children from six international cohorts in Denmark, Sweden, and Canada, there was no significant difference in major cardiac malformations between the lithium-exposed group, 2.1% (0.5%-3.7%), and the reference group, 1.6% (1.0%-2.1%).
Women with particularly brittle bipolar disorder or with histories of response to lithium may, in consultation with their doctors, consider use of lithium during pregnancy given the almost 50-year history of data accumulation on its reproductive safety, compared with some of the other mood stabilizers for which there is either confirmed teratogenicity (sodium valproate) or still incomplete data. Moreover, given the high risk for postpartum relapse of mood disorder in women who suffer from bipolar disorder, it is important to remember that the most robust data on prophylactic benefit of mood stabilizer during the peripartum period are with lithium.
Reproductive age women with bipolar disorder have for decades been caught between a teratologic rock and a clinical hard place. More recent data that have emerged from rigorously conducted registries and carefully analyzed administrative databases allow for more effective collaboration between patient and doctor as together they make personal decisions that match individual clinical situations with personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
Prealbumin level predicts outcomes for HCC resection
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
FROM HPB
Key clinical point: Preoperative prealbumin levels independently predicted survival after curative liver resection for HCC, while preoperative albumin levels did not.
Major finding: The hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
Study details: Retrospective analysis that included 1,483 patients with HCC newly diagnosed at one of six institutions in China during 2001-2014.
Disclosures: The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. The study authors had no conflicts of interest to disclose.
Source: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.