User login
FDA advises against azithromycin use in allo-HSCT recipients
The US Food and Drug Administration (FDA) is warning against long-term use of azithromycin (Zithromax, Zmax) in patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT).
Azithromycin has been used off-label as prophylaxis for bronchiolitis obliterans syndrome in these patients.
However, a trial published in JAMA last year suggested that long-term azithromycin use increases the risk of relapse and death in patients undergoing allo-HSCT as treatment for hematologic malignancies.
The FDA said it is reviewing additional data and will communicate its conclusions and recommendations when the review is complete.
In the meantime, the agency said healthcare professionals should not prescribe long-term azithromycin to allo-HSCT recipients for prophylaxis of bronchiolitis obliterans syndrome. However, patients should not stop taking azithromycin without first consulting their healthcare professionals.
Healthcare professionals and patients can report adverse events related to azithromycin to the FDA’s MedWatch program.
Pfizer, which markets Zithromax, has issued a Dear Healthcare Provider letter warning about the risks of relapse and death associated with long-term azithromycin use in allo-HSCT recipients.
The company said there is no evidence to suggest increased risks in other patient populations or when azithromycin is used for FDA-approved indications.
It isn’t clear why allo-HSCT recipients may have an increased risk of relapse/death with long-term azithromycin use. However, Pfizer said the available evidence raises questions about the safety of prophylactic azithromycin in this patient population, suggesting the risks outweigh the benefits.
The evidence comes from the ALLOZITHRO trial, which was published in JAMA in August 2017.
The trial included 480 patients who had undergone allo-HSCT for a hematologic malignancy. They were randomized to receive 250 mg of azithromycin (n=243) or placebo (n=237) 3 times a week for 2 years, beginning at the start of conditioning.
The trial was stopped about 13 months after enrollment was completed because there was an unexpected increase in the rate of relapse and death in patients taking azithromycin.
The 2-year cumulative incidence of relapse was 33.5% in the azithromycin group and 22.3% in the placebo group (unadjusted cause-specific hazard ratio [HR]=1.7, P=0.002).
The 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (adjusted HR=1.5, P=0.02).
The 2-year airflow decline-free survival rate was 32.8% in the azithromycin group and 41.3% in the placebo group (unadjusted HR=1.3, P=0.03).
And the incidence of bronchiolitis obliterans syndrome was 6% in the azithromycin group and 3% in the placebo group (P=0.08).
The US Food and Drug Administration (FDA) is warning against long-term use of azithromycin (Zithromax, Zmax) in patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT).
Azithromycin has been used off-label as prophylaxis for bronchiolitis obliterans syndrome in these patients.
However, a trial published in JAMA last year suggested that long-term azithromycin use increases the risk of relapse and death in patients undergoing allo-HSCT as treatment for hematologic malignancies.
The FDA said it is reviewing additional data and will communicate its conclusions and recommendations when the review is complete.
In the meantime, the agency said healthcare professionals should not prescribe long-term azithromycin to allo-HSCT recipients for prophylaxis of bronchiolitis obliterans syndrome. However, patients should not stop taking azithromycin without first consulting their healthcare professionals.
Healthcare professionals and patients can report adverse events related to azithromycin to the FDA’s MedWatch program.
Pfizer, which markets Zithromax, has issued a Dear Healthcare Provider letter warning about the risks of relapse and death associated with long-term azithromycin use in allo-HSCT recipients.
The company said there is no evidence to suggest increased risks in other patient populations or when azithromycin is used for FDA-approved indications.
It isn’t clear why allo-HSCT recipients may have an increased risk of relapse/death with long-term azithromycin use. However, Pfizer said the available evidence raises questions about the safety of prophylactic azithromycin in this patient population, suggesting the risks outweigh the benefits.
The evidence comes from the ALLOZITHRO trial, which was published in JAMA in August 2017.
The trial included 480 patients who had undergone allo-HSCT for a hematologic malignancy. They were randomized to receive 250 mg of azithromycin (n=243) or placebo (n=237) 3 times a week for 2 years, beginning at the start of conditioning.
The trial was stopped about 13 months after enrollment was completed because there was an unexpected increase in the rate of relapse and death in patients taking azithromycin.
The 2-year cumulative incidence of relapse was 33.5% in the azithromycin group and 22.3% in the placebo group (unadjusted cause-specific hazard ratio [HR]=1.7, P=0.002).
The 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (adjusted HR=1.5, P=0.02).
The 2-year airflow decline-free survival rate was 32.8% in the azithromycin group and 41.3% in the placebo group (unadjusted HR=1.3, P=0.03).
And the incidence of bronchiolitis obliterans syndrome was 6% in the azithromycin group and 3% in the placebo group (P=0.08).
The US Food and Drug Administration (FDA) is warning against long-term use of azithromycin (Zithromax, Zmax) in patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT).
Azithromycin has been used off-label as prophylaxis for bronchiolitis obliterans syndrome in these patients.
However, a trial published in JAMA last year suggested that long-term azithromycin use increases the risk of relapse and death in patients undergoing allo-HSCT as treatment for hematologic malignancies.
The FDA said it is reviewing additional data and will communicate its conclusions and recommendations when the review is complete.
In the meantime, the agency said healthcare professionals should not prescribe long-term azithromycin to allo-HSCT recipients for prophylaxis of bronchiolitis obliterans syndrome. However, patients should not stop taking azithromycin without first consulting their healthcare professionals.
Healthcare professionals and patients can report adverse events related to azithromycin to the FDA’s MedWatch program.
Pfizer, which markets Zithromax, has issued a Dear Healthcare Provider letter warning about the risks of relapse and death associated with long-term azithromycin use in allo-HSCT recipients.
The company said there is no evidence to suggest increased risks in other patient populations or when azithromycin is used for FDA-approved indications.
It isn’t clear why allo-HSCT recipients may have an increased risk of relapse/death with long-term azithromycin use. However, Pfizer said the available evidence raises questions about the safety of prophylactic azithromycin in this patient population, suggesting the risks outweigh the benefits.
The evidence comes from the ALLOZITHRO trial, which was published in JAMA in August 2017.
The trial included 480 patients who had undergone allo-HSCT for a hematologic malignancy. They were randomized to receive 250 mg of azithromycin (n=243) or placebo (n=237) 3 times a week for 2 years, beginning at the start of conditioning.
The trial was stopped about 13 months after enrollment was completed because there was an unexpected increase in the rate of relapse and death in patients taking azithromycin.
The 2-year cumulative incidence of relapse was 33.5% in the azithromycin group and 22.3% in the placebo group (unadjusted cause-specific hazard ratio [HR]=1.7, P=0.002).
The 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (adjusted HR=1.5, P=0.02).
The 2-year airflow decline-free survival rate was 32.8% in the azithromycin group and 41.3% in the placebo group (unadjusted HR=1.3, P=0.03).
And the incidence of bronchiolitis obliterans syndrome was 6% in the azithromycin group and 3% in the placebo group (P=0.08).
Everything’s Fine … Except His Spine
ANSWER
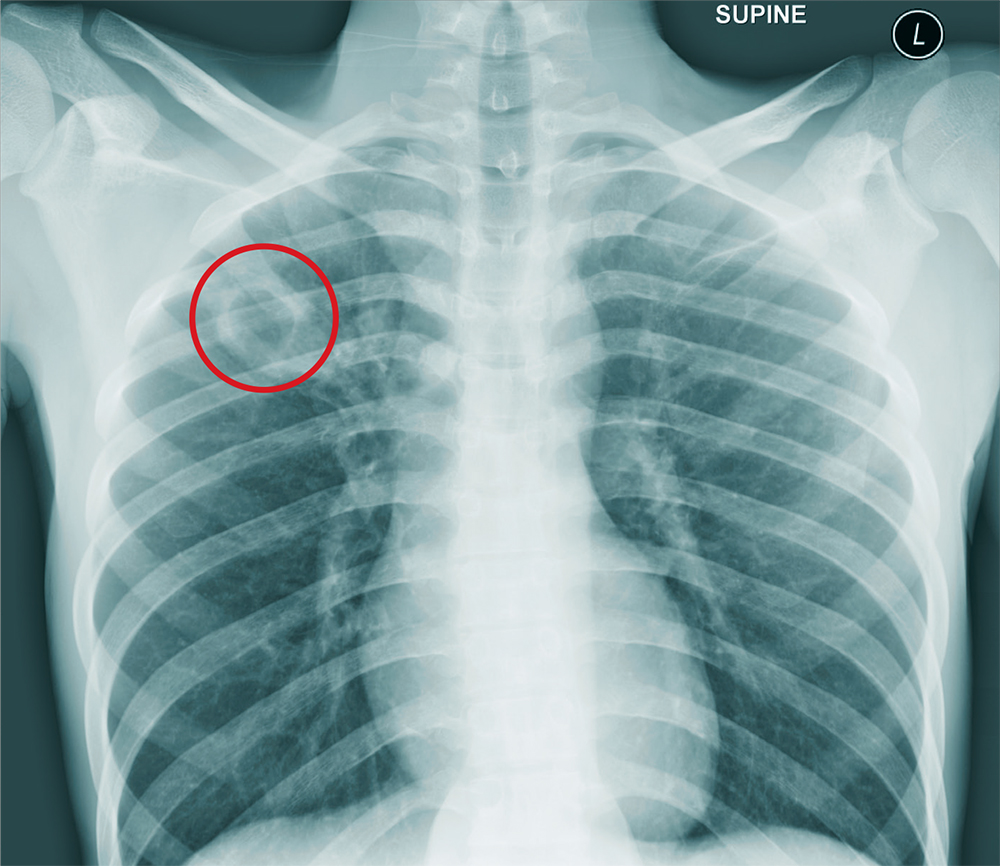
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
A 25-year-old man is admitted to your facility for a possible infection in his spine. He reports a two-week history of severe back pain with no history of injury or trauma. Imaging performed at an outside facility suggested compression and erosion of his vertebral bodies at the thoracolumbar junction, and the radiologist raised concern for possible osteomyelitis and diskitis.
The patient is otherwise healthy and denies any medical problems. He denies drug use of any form. Review of systems is significant for a three-month history of anorexia and night sweats but no fever.
Physical exam reveals a healthy-appearing male with normal vital signs. His heart and lung sounds are normal.
A chest radiograph is obtained (shown). What is your impression?
Treating immunotherapy-related AEs in the emergency department
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
Low disease activity in SLE compares favorably with clinical remission as an acceptable goal
Canadian researchers report.
The findings endorse the endpoint used to define low disease activity in the study – Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of 2 or less – as a meaningful treat-to-target outcome in SLE and also help to fill in missing information about the long-term longitudinal status of patients with recent-onset disease who enter either low disease activity or clinical remission status, first author Konstantinos Tselios, MD, PhD, and coauthors at the Centre for Prognosis Studies in the Rheumatic Diseases at the University Health Network’s Toronto Lupus Clinic, wrote in Arthritis Care & Research.
The concept of low disease activity (LDA) has emerged because it is rare for SLE patients to achieve sustained complete remission, the authors said. Existing studies on the outcomes of LDA and clinical remission have demonstrated comparable outcomes between the two in terms of damage accrual after 2 years, but they enrolled patients with prevalent disease, many of whom were in late stages.
“Given that disease duration has a significant impact on disease activity, the prevalence and characteristics of complete remission and LDA might have been affected,” they wrote.
The investigators therefore conducted the current study to assess damage accrual, medications used, flare rate, and mortality in inception patients who sustained complete remission and LDA status for 10 years.
The study involved 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up and no time interval between clinic visits exceeding 18 months. All patients fulfilled the revised American College of Rheumatology criteria for SLE classification or had three criteria and a supportive biopsy. The patients all had either LDA or clinical remission (SLEDAI-2K = 0, excluding serology) within the first 5 years since enrollment and maintained that status for 10 consecutive years, regardless of therapy.
A total of 10.1% of patients (n = 27) achieved prolonged clinical remission. Clinical manifestations at baseline included mucocutaneous disease in 70.4%, arthritis in 59.3%, cytopenias in 55.6%, nephritis in 25.9%, and central nervous involvement in 25.9%. Nephritis was class II in three patients, class III in one, and class IV in three.
For the 18% of patients (n = 48) who achieved sustained LDA, clinical manifestations included mucocutaneous disease in 81.3%, cytopenias in 66.7%, arthritis in 60.4%, serositis in 29.2%, nephritis in 22.9%, and central nervous system disease in 8.3%. The nephritis was class II in two patients, class III in four, class IV in two, and class VI in one. Patients in both groups were similar at baseline, but mean prednisone dose was higher in the remission group and more patients in the LDA group were taking antimalarials.
Time to remission and time to LDA were 1.2 years in both groups. After 10 years of follow-up, the mean SLEDAI was 1.2 in the remission group and 1.6 in the LDA group (P = .31) and 3.7 among patients not achieving either goal (n = 192; P less than .001).
Moreover, serology (complement C3/C4 and anti-double stranded DNA positivity) and antiextractable nuclear antigen antibody profile were similar in both groups.
No differences in flare rate or mortality emerged. Disease flares were observed in 25.9% of the remission group and 31.2% of the LDA group (P = .63) and flare rates were 0.038/patient-year and 0.065/patient-year, respectively (P = .23). Mortality after 10 years was 11.1% in the remission group and 10.4% in the LDA group (P = .93).
“Maintenance of this state could be an acceptable target for the long-term management of SLE. The concept of LDA is relatively new in SLE. Since complete remission has not been strictly defined yet and is rare, a more realistic target, such as LDA, may be of value in daily clinical practice as well as in clinical trials,” the investigators wrote.
The authors noted that the study was limited by the relatively low number of patients.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
SOURCE: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
Canadian researchers report.
The findings endorse the endpoint used to define low disease activity in the study – Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of 2 or less – as a meaningful treat-to-target outcome in SLE and also help to fill in missing information about the long-term longitudinal status of patients with recent-onset disease who enter either low disease activity or clinical remission status, first author Konstantinos Tselios, MD, PhD, and coauthors at the Centre for Prognosis Studies in the Rheumatic Diseases at the University Health Network’s Toronto Lupus Clinic, wrote in Arthritis Care & Research.
The concept of low disease activity (LDA) has emerged because it is rare for SLE patients to achieve sustained complete remission, the authors said. Existing studies on the outcomes of LDA and clinical remission have demonstrated comparable outcomes between the two in terms of damage accrual after 2 years, but they enrolled patients with prevalent disease, many of whom were in late stages.
“Given that disease duration has a significant impact on disease activity, the prevalence and characteristics of complete remission and LDA might have been affected,” they wrote.
The investigators therefore conducted the current study to assess damage accrual, medications used, flare rate, and mortality in inception patients who sustained complete remission and LDA status for 10 years.
The study involved 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up and no time interval between clinic visits exceeding 18 months. All patients fulfilled the revised American College of Rheumatology criteria for SLE classification or had three criteria and a supportive biopsy. The patients all had either LDA or clinical remission (SLEDAI-2K = 0, excluding serology) within the first 5 years since enrollment and maintained that status for 10 consecutive years, regardless of therapy.
A total of 10.1% of patients (n = 27) achieved prolonged clinical remission. Clinical manifestations at baseline included mucocutaneous disease in 70.4%, arthritis in 59.3%, cytopenias in 55.6%, nephritis in 25.9%, and central nervous involvement in 25.9%. Nephritis was class II in three patients, class III in one, and class IV in three.
For the 18% of patients (n = 48) who achieved sustained LDA, clinical manifestations included mucocutaneous disease in 81.3%, cytopenias in 66.7%, arthritis in 60.4%, serositis in 29.2%, nephritis in 22.9%, and central nervous system disease in 8.3%. The nephritis was class II in two patients, class III in four, class IV in two, and class VI in one. Patients in both groups were similar at baseline, but mean prednisone dose was higher in the remission group and more patients in the LDA group were taking antimalarials.
Time to remission and time to LDA were 1.2 years in both groups. After 10 years of follow-up, the mean SLEDAI was 1.2 in the remission group and 1.6 in the LDA group (P = .31) and 3.7 among patients not achieving either goal (n = 192; P less than .001).
Moreover, serology (complement C3/C4 and anti-double stranded DNA positivity) and antiextractable nuclear antigen antibody profile were similar in both groups.
No differences in flare rate or mortality emerged. Disease flares were observed in 25.9% of the remission group and 31.2% of the LDA group (P = .63) and flare rates were 0.038/patient-year and 0.065/patient-year, respectively (P = .23). Mortality after 10 years was 11.1% in the remission group and 10.4% in the LDA group (P = .93).
“Maintenance of this state could be an acceptable target for the long-term management of SLE. The concept of LDA is relatively new in SLE. Since complete remission has not been strictly defined yet and is rare, a more realistic target, such as LDA, may be of value in daily clinical practice as well as in clinical trials,” the investigators wrote.
The authors noted that the study was limited by the relatively low number of patients.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
SOURCE: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
Canadian researchers report.
The findings endorse the endpoint used to define low disease activity in the study – Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of 2 or less – as a meaningful treat-to-target outcome in SLE and also help to fill in missing information about the long-term longitudinal status of patients with recent-onset disease who enter either low disease activity or clinical remission status, first author Konstantinos Tselios, MD, PhD, and coauthors at the Centre for Prognosis Studies in the Rheumatic Diseases at the University Health Network’s Toronto Lupus Clinic, wrote in Arthritis Care & Research.
The concept of low disease activity (LDA) has emerged because it is rare for SLE patients to achieve sustained complete remission, the authors said. Existing studies on the outcomes of LDA and clinical remission have demonstrated comparable outcomes between the two in terms of damage accrual after 2 years, but they enrolled patients with prevalent disease, many of whom were in late stages.
“Given that disease duration has a significant impact on disease activity, the prevalence and characteristics of complete remission and LDA might have been affected,” they wrote.
The investigators therefore conducted the current study to assess damage accrual, medications used, flare rate, and mortality in inception patients who sustained complete remission and LDA status for 10 years.
The study involved 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up and no time interval between clinic visits exceeding 18 months. All patients fulfilled the revised American College of Rheumatology criteria for SLE classification or had three criteria and a supportive biopsy. The patients all had either LDA or clinical remission (SLEDAI-2K = 0, excluding serology) within the first 5 years since enrollment and maintained that status for 10 consecutive years, regardless of therapy.
A total of 10.1% of patients (n = 27) achieved prolonged clinical remission. Clinical manifestations at baseline included mucocutaneous disease in 70.4%, arthritis in 59.3%, cytopenias in 55.6%, nephritis in 25.9%, and central nervous involvement in 25.9%. Nephritis was class II in three patients, class III in one, and class IV in three.
For the 18% of patients (n = 48) who achieved sustained LDA, clinical manifestations included mucocutaneous disease in 81.3%, cytopenias in 66.7%, arthritis in 60.4%, serositis in 29.2%, nephritis in 22.9%, and central nervous system disease in 8.3%. The nephritis was class II in two patients, class III in four, class IV in two, and class VI in one. Patients in both groups were similar at baseline, but mean prednisone dose was higher in the remission group and more patients in the LDA group were taking antimalarials.
Time to remission and time to LDA were 1.2 years in both groups. After 10 years of follow-up, the mean SLEDAI was 1.2 in the remission group and 1.6 in the LDA group (P = .31) and 3.7 among patients not achieving either goal (n = 192; P less than .001).
Moreover, serology (complement C3/C4 and anti-double stranded DNA positivity) and antiextractable nuclear antigen antibody profile were similar in both groups.
No differences in flare rate or mortality emerged. Disease flares were observed in 25.9% of the remission group and 31.2% of the LDA group (P = .63) and flare rates were 0.038/patient-year and 0.065/patient-year, respectively (P = .23). Mortality after 10 years was 11.1% in the remission group and 10.4% in the LDA group (P = .93).
“Maintenance of this state could be an acceptable target for the long-term management of SLE. The concept of LDA is relatively new in SLE. Since complete remission has not been strictly defined yet and is rare, a more realistic target, such as LDA, may be of value in daily clinical practice as well as in clinical trials,” the investigators wrote.
The authors noted that the study was limited by the relatively low number of patients.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
SOURCE: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Low disease activity, as defined by a SLE Disease Activity Index 2000 score of 2 or less, appears to be a meaningful treat-to-target outcome in SLE.
Major finding: SLE patients with either prolonged clinical remission or prolonged low disease activity had comparable damage accrual, flare rate, and mortality in a 10-year period after enrollment.
Study details: 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up.
Disclosures: The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
Source: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
Children, adolescents of divorcing parents want more say
Parents who are in the midst of a divorce might want to protect their children from details tied to the breakup, such as parenting arrangements. But they might be doing more harm than good.
A survey of 61 children and adolescents who recently had gone through their parents’ divorce suggested that many want a say in how the divorce will affect them, according to a report broadcast by the Australian Broadcasting Corporation (ABC).
The parents’ actions are benevolent in their intention, but they “may be associated with the experience of harm on the part of children and young people where their agency and capacity to participate in decision making that affects them isn’t acknowledged and accommodated,” said study lead Rachel Carson, PhD, of the Australian Institute of Family Studies, in an interview.
To conduct the survey, the research team conducted interviews with the children and adolescents, aged 10-17 years. They also interviewed 47 parents of those children.
Most of the children and adolescents interviewed said they felt isolated from their parents’ divorce, with little or no influence on the process. The ABC report included several quotations from the interviewees, including “Phoebe,” who was 15 years old at the time of the survey.
“When a kid goes through a divorce, a lot of the time the adults become very immature, so the children grow up a lot quicker,” Phoebe said.
The survey concluded that a “child-inclusive approach be adopted incorporating the features of effective professional practice. ...A commitment to this approach would importantly be a step towards meeting the loud and clear calls from participating children and young people to ‘give children a bigger voice, more of the time.’ ”
Click here to listen to the ABC story.
Parents who are in the midst of a divorce might want to protect their children from details tied to the breakup, such as parenting arrangements. But they might be doing more harm than good.
A survey of 61 children and adolescents who recently had gone through their parents’ divorce suggested that many want a say in how the divorce will affect them, according to a report broadcast by the Australian Broadcasting Corporation (ABC).
The parents’ actions are benevolent in their intention, but they “may be associated with the experience of harm on the part of children and young people where their agency and capacity to participate in decision making that affects them isn’t acknowledged and accommodated,” said study lead Rachel Carson, PhD, of the Australian Institute of Family Studies, in an interview.
To conduct the survey, the research team conducted interviews with the children and adolescents, aged 10-17 years. They also interviewed 47 parents of those children.
Most of the children and adolescents interviewed said they felt isolated from their parents’ divorce, with little or no influence on the process. The ABC report included several quotations from the interviewees, including “Phoebe,” who was 15 years old at the time of the survey.
“When a kid goes through a divorce, a lot of the time the adults become very immature, so the children grow up a lot quicker,” Phoebe said.
The survey concluded that a “child-inclusive approach be adopted incorporating the features of effective professional practice. ...A commitment to this approach would importantly be a step towards meeting the loud and clear calls from participating children and young people to ‘give children a bigger voice, more of the time.’ ”
Click here to listen to the ABC story.
Parents who are in the midst of a divorce might want to protect their children from details tied to the breakup, such as parenting arrangements. But they might be doing more harm than good.
A survey of 61 children and adolescents who recently had gone through their parents’ divorce suggested that many want a say in how the divorce will affect them, according to a report broadcast by the Australian Broadcasting Corporation (ABC).
The parents’ actions are benevolent in their intention, but they “may be associated with the experience of harm on the part of children and young people where their agency and capacity to participate in decision making that affects them isn’t acknowledged and accommodated,” said study lead Rachel Carson, PhD, of the Australian Institute of Family Studies, in an interview.
To conduct the survey, the research team conducted interviews with the children and adolescents, aged 10-17 years. They also interviewed 47 parents of those children.
Most of the children and adolescents interviewed said they felt isolated from their parents’ divorce, with little or no influence on the process. The ABC report included several quotations from the interviewees, including “Phoebe,” who was 15 years old at the time of the survey.
“When a kid goes through a divorce, a lot of the time the adults become very immature, so the children grow up a lot quicker,” Phoebe said.
The survey concluded that a “child-inclusive approach be adopted incorporating the features of effective professional practice. ...A commitment to this approach would importantly be a step towards meeting the loud and clear calls from participating children and young people to ‘give children a bigger voice, more of the time.’ ”
Click here to listen to the ABC story.
Company is giving drug users a second chance
An electric wire plant in Richmond, Ind., is bucking the practice of rejecting job applicants who fail drug tests.
Instead, the century-old Belden, a St. Louis-based company that also makes cable products, Internet routers, and other high-tech equipment, is offering Richmond plant applicants a job – if they complete a free 1- to 4-month drug treatment program.
The pilot program – called Pathways to Employment – aims to address two problems: the opioid crisis gripping the country and the worker shortage faced by many U.S. companies. Belden works with several local partners. The initiative was conceived with Mitchell S. Rosenthal, MD, a psychiatrist who serves as president of the Rosenthal Center for Addiction Studies and was founder of Phoenix House, the nonprofit drug treatment group. The program is thought to be the first of its kind.
in hiring the folks we needed,” said Doug Brenneke, Belden’s vice president of research and development, in an interview with National Public Radio.
Belden also is offering the program to the broader community – not just people who are part of the plant’s workforce. “We think part of the solution is offering basically a path out,” Mr. Brenneke told NPR. “It’s not a silver bullet, but it is part of an overall solution, we believe, to the epidemic.”
Click here to hear the NPR story.
An electric wire plant in Richmond, Ind., is bucking the practice of rejecting job applicants who fail drug tests.
Instead, the century-old Belden, a St. Louis-based company that also makes cable products, Internet routers, and other high-tech equipment, is offering Richmond plant applicants a job – if they complete a free 1- to 4-month drug treatment program.
The pilot program – called Pathways to Employment – aims to address two problems: the opioid crisis gripping the country and the worker shortage faced by many U.S. companies. Belden works with several local partners. The initiative was conceived with Mitchell S. Rosenthal, MD, a psychiatrist who serves as president of the Rosenthal Center for Addiction Studies and was founder of Phoenix House, the nonprofit drug treatment group. The program is thought to be the first of its kind.
in hiring the folks we needed,” said Doug Brenneke, Belden’s vice president of research and development, in an interview with National Public Radio.
Belden also is offering the program to the broader community – not just people who are part of the plant’s workforce. “We think part of the solution is offering basically a path out,” Mr. Brenneke told NPR. “It’s not a silver bullet, but it is part of an overall solution, we believe, to the epidemic.”
Click here to hear the NPR story.
An electric wire plant in Richmond, Ind., is bucking the practice of rejecting job applicants who fail drug tests.
Instead, the century-old Belden, a St. Louis-based company that also makes cable products, Internet routers, and other high-tech equipment, is offering Richmond plant applicants a job – if they complete a free 1- to 4-month drug treatment program.
The pilot program – called Pathways to Employment – aims to address two problems: the opioid crisis gripping the country and the worker shortage faced by many U.S. companies. Belden works with several local partners. The initiative was conceived with Mitchell S. Rosenthal, MD, a psychiatrist who serves as president of the Rosenthal Center for Addiction Studies and was founder of Phoenix House, the nonprofit drug treatment group. The program is thought to be the first of its kind.
in hiring the folks we needed,” said Doug Brenneke, Belden’s vice president of research and development, in an interview with National Public Radio.
Belden also is offering the program to the broader community – not just people who are part of the plant’s workforce. “We think part of the solution is offering basically a path out,” Mr. Brenneke told NPR. “It’s not a silver bullet, but it is part of an overall solution, we believe, to the epidemic.”
Click here to hear the NPR story.
Mothers’ parenting decisions and social norms
Leaving children to fend for themselves used to be viewed as a positive aspect of parenting. Now, in an age of fear, a parent, particularly a mother, can be judged as negligent – to the point of criminal prosecution – for actions like leaving a child to play in a park.
In the New York Times, writer Kim Brooks recounted her own story of leaving her 4-year-old son locked in a car on what she calls a “cool March day” so that she could run a quick errand. That fateful decision led to a series of encounters between Ms. Brooks and the criminal justice system, including a charge of “contributing to the delinquency of a minor.”
Ms. Brooks examined the challenges that mothers face in a world quick to pass judgment on their parenting decisions. She called those moral judgments “mother-shaming” and said that the judgments and risk assessments are intertwined.
“People don’t only think that leaving children alone is dangerous and therefore immoral. They also think it is immoral and therefore dangerous. said Barbara W. Sarnecka, PhD, a cognitive science professor at the University of California, Irvine, told Ms. Brooks. Dr. Sarnecka went on to say that, although children do not have the same rights as adults, “they have some rights, and not just to safety. They have the right to some freedom, to some independence, and to a little bit of danger.”
Click here to read the Time article.
Leaving children to fend for themselves used to be viewed as a positive aspect of parenting. Now, in an age of fear, a parent, particularly a mother, can be judged as negligent – to the point of criminal prosecution – for actions like leaving a child to play in a park.
In the New York Times, writer Kim Brooks recounted her own story of leaving her 4-year-old son locked in a car on what she calls a “cool March day” so that she could run a quick errand. That fateful decision led to a series of encounters between Ms. Brooks and the criminal justice system, including a charge of “contributing to the delinquency of a minor.”
Ms. Brooks examined the challenges that mothers face in a world quick to pass judgment on their parenting decisions. She called those moral judgments “mother-shaming” and said that the judgments and risk assessments are intertwined.
“People don’t only think that leaving children alone is dangerous and therefore immoral. They also think it is immoral and therefore dangerous. said Barbara W. Sarnecka, PhD, a cognitive science professor at the University of California, Irvine, told Ms. Brooks. Dr. Sarnecka went on to say that, although children do not have the same rights as adults, “they have some rights, and not just to safety. They have the right to some freedom, to some independence, and to a little bit of danger.”
Click here to read the Time article.
Leaving children to fend for themselves used to be viewed as a positive aspect of parenting. Now, in an age of fear, a parent, particularly a mother, can be judged as negligent – to the point of criminal prosecution – for actions like leaving a child to play in a park.
In the New York Times, writer Kim Brooks recounted her own story of leaving her 4-year-old son locked in a car on what she calls a “cool March day” so that she could run a quick errand. That fateful decision led to a series of encounters between Ms. Brooks and the criminal justice system, including a charge of “contributing to the delinquency of a minor.”
Ms. Brooks examined the challenges that mothers face in a world quick to pass judgment on their parenting decisions. She called those moral judgments “mother-shaming” and said that the judgments and risk assessments are intertwined.
“People don’t only think that leaving children alone is dangerous and therefore immoral. They also think it is immoral and therefore dangerous. said Barbara W. Sarnecka, PhD, a cognitive science professor at the University of California, Irvine, told Ms. Brooks. Dr. Sarnecka went on to say that, although children do not have the same rights as adults, “they have some rights, and not just to safety. They have the right to some freedom, to some independence, and to a little bit of danger.”
Click here to read the Time article.
Native American writer explores identity
The debut novel “There There” by Tommy Orange explores what it means to be Native American in contemporary urban America.
In an interview with the PBS Newshour, Mr. Orange, a member of the Cheyenne and Arapaho tribes who grew up in Oakland, Calif., describes the ways in which living in an urban environment in an ethnically diverse American city can be isolating and lonely for Native Americans.
“We know the sound of the freeway better than we do rivers, the howl of distant trains better than wolf howls,” Mr. Orange said, reading from his book. “We know the smell of gas and freshly wet concrete and burned rubber better than we do the smell of cedar or sage or even frybread. We ride buses, trains, and cars across, over, and under concrete plains. Being Indian has never been about returning to the land. The land is everywhere or nowhere.”
For Mr. Orange, whose mother is white and father is Native American, identity in Oakland has been a fluid concept. “If I’m in the Fruitvale [area of Oakland], people will speak Spanish to me first,” he said in the interview.
Establishing identity can be a difficult task for Native Americans, Mr. Orange said, because parents often do not share stories about history – “sometimes because of pain,” he said.
To watch the PBS Newshour interview with Mr. Orange, click here.
The debut novel “There There” by Tommy Orange explores what it means to be Native American in contemporary urban America.
In an interview with the PBS Newshour, Mr. Orange, a member of the Cheyenne and Arapaho tribes who grew up in Oakland, Calif., describes the ways in which living in an urban environment in an ethnically diverse American city can be isolating and lonely for Native Americans.
“We know the sound of the freeway better than we do rivers, the howl of distant trains better than wolf howls,” Mr. Orange said, reading from his book. “We know the smell of gas and freshly wet concrete and burned rubber better than we do the smell of cedar or sage or even frybread. We ride buses, trains, and cars across, over, and under concrete plains. Being Indian has never been about returning to the land. The land is everywhere or nowhere.”
For Mr. Orange, whose mother is white and father is Native American, identity in Oakland has been a fluid concept. “If I’m in the Fruitvale [area of Oakland], people will speak Spanish to me first,” he said in the interview.
Establishing identity can be a difficult task for Native Americans, Mr. Orange said, because parents often do not share stories about history – “sometimes because of pain,” he said.
To watch the PBS Newshour interview with Mr. Orange, click here.
The debut novel “There There” by Tommy Orange explores what it means to be Native American in contemporary urban America.
In an interview with the PBS Newshour, Mr. Orange, a member of the Cheyenne and Arapaho tribes who grew up in Oakland, Calif., describes the ways in which living in an urban environment in an ethnically diverse American city can be isolating and lonely for Native Americans.
“We know the sound of the freeway better than we do rivers, the howl of distant trains better than wolf howls,” Mr. Orange said, reading from his book. “We know the smell of gas and freshly wet concrete and burned rubber better than we do the smell of cedar or sage or even frybread. We ride buses, trains, and cars across, over, and under concrete plains. Being Indian has never been about returning to the land. The land is everywhere or nowhere.”
For Mr. Orange, whose mother is white and father is Native American, identity in Oakland has been a fluid concept. “If I’m in the Fruitvale [area of Oakland], people will speak Spanish to me first,” he said in the interview.
Establishing identity can be a difficult task for Native Americans, Mr. Orange said, because parents often do not share stories about history – “sometimes because of pain,” he said.
To watch the PBS Newshour interview with Mr. Orange, click here.
Herceptin linked to doubling of HF risk in women with breast cancer
Adding more evidence to an ongoing debate, a large new study suggests that patients with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of developing heart failure, with older women at highest risk.
The study also found that most patients who took trastuzumab didn’t receive recommended cardiac screening.
The researchers said their findings are unique because they tracked both younger and older patients. “By examining the rates of both cardiac monitoring and cardiotoxicity, we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients,” wrote Mariana Chavez-MacGregor, MD, MSC, of the University of Texas MD Anderson Cancer Center, and her associates. The report was published in JACC: Cardiovascular Imaging.
While Trastuzumab has boosted breast cancer survival rates for patients with HER2-positive tumors, it’s also raised concerns about cardiotoxicity that could be an indicator of subsequent congestive heart failure (Cochrane Database Syst Rev. 2014 Jun 12;(6):CD006242).
According to the new study, the risk of trastuzumab risk is linked to damage to cardiac myocytes that can cause reversible cardiotoxicity.
The prescribing information for trastuzumab advises patients to undergo cardiac monitoring before treatment with trastuzumab and every 3 months during treatment. Recommendations by medical organizations have varied.
Now, as a 2016 report put it, it’s “increasingly unclear” whether frequent routine monitoring is appropriate for all patients (J Clin Oncol. 2016 Apr 1;34[10]:1030-3).
For the current study, Dr. Chavez-MacGregor and her associates identified 16,456 adult women in the United States who were diagnosed with nonmetastatic invasive breast cancer from 2009 to 2014. Researchers tracked the group, with a median age of 56, through as late as 2015.
The women were treated with chemotherapy within 6 months of diagnosis, and 4,325 received trastuzumab.
Of all the subjects, 692 patients (4.2%) developed heart failure following chemotherapy. The rate among patients treated with trastuzumab was higher, at 8.3%, compared with 2.7% for those not treated with trastuzumab (P less than .001).
The researchers also looked at anthracycline users and found that they were slightly more likely to develop HF (4.6% vs. 4.0% among nonusers, P = .048).
Increased age boosted the risk of HF in the trastuzumab-treated patients, and the risk was highest in those treated with both anthracyclines and trastuzumab. Other factors linked to more risk were comorbidities, hypertension, and valve disease.
After adjusting for confounders, the researchers estimated that those treated with trastuzumab were 2.01 times more likely to develop HF (HR, 2.01; 95% confidence interval, 1.72-2.36), and those who took anthracycline were 1.53 times more likely (HR, 1.53; 95% CI, 1.30-1.80)
The researchers also examined medical records for evidence that subjects underwent cardiac screening at least once every 4 months, not 3 months, as the prescribing information recommends. The study team chose to focus on 4-month intervals “to compensate for differences in scheduling, resources, or levels of accessibility to medical care.”
Medical records suggest that 73.5% of patients who took trastuzumab underwent cardiac screening at the beginning of therapy, but only 46.2% continued to do so at least every 4 months.
An adjusted model linked more screening to the use of anthracyclines and taxanes, radiation treatment, and living in the Northeast vs. the West.
“HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% compared with 6.5%, respectively; P less than.001), suggesting that, as more patients are screened, more patients are likely to be found having HF,” the researchers reported.
However, they added that “our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.”
The researchers also noted that the findings suggest that screening has become more common in recent years.
“The number of cancer survivors is expected to increase over time, and we will continue to see patients develop treatment-related cardiotoxicity,” the researchers wrote. “Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment–related cardiotoxicity are needed.”
The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors reported grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors reported no disclosures.
SOURCE: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging. 2018 Aug;11[8]1084-93.
While trastuzumab clearly benefits patients with HER2-positive breast cancer at various stages of progression, concerns about heart failure persist. Studies have suggested that the drug doesn’t boost the risk of late cardiac events, but it’s not clear if this is due to mandated screening in these trials. The new study provides more evidence that adherence to screening guidelines is limited, and recent trials offer evidence that the general cardiac risk may be overblown. Future studies could be designed to offer insight into the wisdom of adjusting screening regimens based on stratification of risk. A meta-analysis could also be helpful, and the upcoming results of the SAFE-HEART study will provide information about the safety of anti-HER2 antibody therapy in patients with low but asymptomatic left-ventricular ejection fraction.
These comments are excerpted from a commentary by Chau T. Dang, MD, of Memorial Sloan Kettering Cancer Center, New York, and her associates (JACC: Cardiovasc Imaging. 2018 Aug;11[8]:1094-7). Most of the commentary authors report various disclosures.
While trastuzumab clearly benefits patients with HER2-positive breast cancer at various stages of progression, concerns about heart failure persist. Studies have suggested that the drug doesn’t boost the risk of late cardiac events, but it’s not clear if this is due to mandated screening in these trials. The new study provides more evidence that adherence to screening guidelines is limited, and recent trials offer evidence that the general cardiac risk may be overblown. Future studies could be designed to offer insight into the wisdom of adjusting screening regimens based on stratification of risk. A meta-analysis could also be helpful, and the upcoming results of the SAFE-HEART study will provide information about the safety of anti-HER2 antibody therapy in patients with low but asymptomatic left-ventricular ejection fraction.
These comments are excerpted from a commentary by Chau T. Dang, MD, of Memorial Sloan Kettering Cancer Center, New York, and her associates (JACC: Cardiovasc Imaging. 2018 Aug;11[8]:1094-7). Most of the commentary authors report various disclosures.
While trastuzumab clearly benefits patients with HER2-positive breast cancer at various stages of progression, concerns about heart failure persist. Studies have suggested that the drug doesn’t boost the risk of late cardiac events, but it’s not clear if this is due to mandated screening in these trials. The new study provides more evidence that adherence to screening guidelines is limited, and recent trials offer evidence that the general cardiac risk may be overblown. Future studies could be designed to offer insight into the wisdom of adjusting screening regimens based on stratification of risk. A meta-analysis could also be helpful, and the upcoming results of the SAFE-HEART study will provide information about the safety of anti-HER2 antibody therapy in patients with low but asymptomatic left-ventricular ejection fraction.
These comments are excerpted from a commentary by Chau T. Dang, MD, of Memorial Sloan Kettering Cancer Center, New York, and her associates (JACC: Cardiovasc Imaging. 2018 Aug;11[8]:1094-7). Most of the commentary authors report various disclosures.
Adding more evidence to an ongoing debate, a large new study suggests that patients with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of developing heart failure, with older women at highest risk.
The study also found that most patients who took trastuzumab didn’t receive recommended cardiac screening.
The researchers said their findings are unique because they tracked both younger and older patients. “By examining the rates of both cardiac monitoring and cardiotoxicity, we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients,” wrote Mariana Chavez-MacGregor, MD, MSC, of the University of Texas MD Anderson Cancer Center, and her associates. The report was published in JACC: Cardiovascular Imaging.
While Trastuzumab has boosted breast cancer survival rates for patients with HER2-positive tumors, it’s also raised concerns about cardiotoxicity that could be an indicator of subsequent congestive heart failure (Cochrane Database Syst Rev. 2014 Jun 12;(6):CD006242).
According to the new study, the risk of trastuzumab risk is linked to damage to cardiac myocytes that can cause reversible cardiotoxicity.
The prescribing information for trastuzumab advises patients to undergo cardiac monitoring before treatment with trastuzumab and every 3 months during treatment. Recommendations by medical organizations have varied.
Now, as a 2016 report put it, it’s “increasingly unclear” whether frequent routine monitoring is appropriate for all patients (J Clin Oncol. 2016 Apr 1;34[10]:1030-3).
For the current study, Dr. Chavez-MacGregor and her associates identified 16,456 adult women in the United States who were diagnosed with nonmetastatic invasive breast cancer from 2009 to 2014. Researchers tracked the group, with a median age of 56, through as late as 2015.
The women were treated with chemotherapy within 6 months of diagnosis, and 4,325 received trastuzumab.
Of all the subjects, 692 patients (4.2%) developed heart failure following chemotherapy. The rate among patients treated with trastuzumab was higher, at 8.3%, compared with 2.7% for those not treated with trastuzumab (P less than .001).
The researchers also looked at anthracycline users and found that they were slightly more likely to develop HF (4.6% vs. 4.0% among nonusers, P = .048).
Increased age boosted the risk of HF in the trastuzumab-treated patients, and the risk was highest in those treated with both anthracyclines and trastuzumab. Other factors linked to more risk were comorbidities, hypertension, and valve disease.
After adjusting for confounders, the researchers estimated that those treated with trastuzumab were 2.01 times more likely to develop HF (HR, 2.01; 95% confidence interval, 1.72-2.36), and those who took anthracycline were 1.53 times more likely (HR, 1.53; 95% CI, 1.30-1.80)
The researchers also examined medical records for evidence that subjects underwent cardiac screening at least once every 4 months, not 3 months, as the prescribing information recommends. The study team chose to focus on 4-month intervals “to compensate for differences in scheduling, resources, or levels of accessibility to medical care.”
Medical records suggest that 73.5% of patients who took trastuzumab underwent cardiac screening at the beginning of therapy, but only 46.2% continued to do so at least every 4 months.
An adjusted model linked more screening to the use of anthracyclines and taxanes, radiation treatment, and living in the Northeast vs. the West.
“HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% compared with 6.5%, respectively; P less than.001), suggesting that, as more patients are screened, more patients are likely to be found having HF,” the researchers reported.
However, they added that “our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.”
The researchers also noted that the findings suggest that screening has become more common in recent years.
“The number of cancer survivors is expected to increase over time, and we will continue to see patients develop treatment-related cardiotoxicity,” the researchers wrote. “Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment–related cardiotoxicity are needed.”
The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors reported grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors reported no disclosures.
SOURCE: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging. 2018 Aug;11[8]1084-93.
Adding more evidence to an ongoing debate, a large new study suggests that patients with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of developing heart failure, with older women at highest risk.
The study also found that most patients who took trastuzumab didn’t receive recommended cardiac screening.
The researchers said their findings are unique because they tracked both younger and older patients. “By examining the rates of both cardiac monitoring and cardiotoxicity, we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients,” wrote Mariana Chavez-MacGregor, MD, MSC, of the University of Texas MD Anderson Cancer Center, and her associates. The report was published in JACC: Cardiovascular Imaging.
While Trastuzumab has boosted breast cancer survival rates for patients with HER2-positive tumors, it’s also raised concerns about cardiotoxicity that could be an indicator of subsequent congestive heart failure (Cochrane Database Syst Rev. 2014 Jun 12;(6):CD006242).
According to the new study, the risk of trastuzumab risk is linked to damage to cardiac myocytes that can cause reversible cardiotoxicity.
The prescribing information for trastuzumab advises patients to undergo cardiac monitoring before treatment with trastuzumab and every 3 months during treatment. Recommendations by medical organizations have varied.
Now, as a 2016 report put it, it’s “increasingly unclear” whether frequent routine monitoring is appropriate for all patients (J Clin Oncol. 2016 Apr 1;34[10]:1030-3).
For the current study, Dr. Chavez-MacGregor and her associates identified 16,456 adult women in the United States who were diagnosed with nonmetastatic invasive breast cancer from 2009 to 2014. Researchers tracked the group, with a median age of 56, through as late as 2015.
The women were treated with chemotherapy within 6 months of diagnosis, and 4,325 received trastuzumab.
Of all the subjects, 692 patients (4.2%) developed heart failure following chemotherapy. The rate among patients treated with trastuzumab was higher, at 8.3%, compared with 2.7% for those not treated with trastuzumab (P less than .001).
The researchers also looked at anthracycline users and found that they were slightly more likely to develop HF (4.6% vs. 4.0% among nonusers, P = .048).
Increased age boosted the risk of HF in the trastuzumab-treated patients, and the risk was highest in those treated with both anthracyclines and trastuzumab. Other factors linked to more risk were comorbidities, hypertension, and valve disease.
After adjusting for confounders, the researchers estimated that those treated with trastuzumab were 2.01 times more likely to develop HF (HR, 2.01; 95% confidence interval, 1.72-2.36), and those who took anthracycline were 1.53 times more likely (HR, 1.53; 95% CI, 1.30-1.80)
The researchers also examined medical records for evidence that subjects underwent cardiac screening at least once every 4 months, not 3 months, as the prescribing information recommends. The study team chose to focus on 4-month intervals “to compensate for differences in scheduling, resources, or levels of accessibility to medical care.”
Medical records suggest that 73.5% of patients who took trastuzumab underwent cardiac screening at the beginning of therapy, but only 46.2% continued to do so at least every 4 months.
An adjusted model linked more screening to the use of anthracyclines and taxanes, radiation treatment, and living in the Northeast vs. the West.
“HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% compared with 6.5%, respectively; P less than.001), suggesting that, as more patients are screened, more patients are likely to be found having HF,” the researchers reported.
However, they added that “our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.”
The researchers also noted that the findings suggest that screening has become more common in recent years.
“The number of cancer survivors is expected to increase over time, and we will continue to see patients develop treatment-related cardiotoxicity,” the researchers wrote. “Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment–related cardiotoxicity are needed.”
The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors reported grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors reported no disclosures.
SOURCE: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging. 2018 Aug;11[8]1084-93.
FROM JACC: CARDIOVASCULAR IMAGING
Key clinical point: Women with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of heart failure, but most aren’t screened frequently.
Major finding: Patients who took trastuzumab were 2.01 times more likely to develop HF (HR, 95% CI, 1.72-2.36) than were those who didn’t. Of all patients who took the drug, fewer than half received recommended frequency of screening.
Study details: Analysis of 16,456 U.S. adult women with nonmetastatic breast cancer diagnosed from 2009 to 2014 and tracked through 2015. Of those, 4.2% developed HF.
Disclosures: The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors report grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors report no disclosures.
Source: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging 2018 Aug;11[8]1084-93.
FDA warning shines light on vaginal rejuvenation
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.