User login
For Gram-negative bacteremias, 7 days of antibiotics is enough
MADRID – Seven days of antibiotic therapy was just as effective as 14 days for patients with Gram-negative bacteremias.
The shorter course was associated with similar cure rates and a faster return to normal activities, Dafna Yahav, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“In patients hospitalized with Gram-negative bacteremia and sepsis, a course of 7 antibiotic days was not inferior to 14 days, and resulted in a more rapid return to baseline activity, “ said Dr. Yahav of the Rabin Medical Center, Petah Tikva, Israel. “This could lead to a change in accepted management algorithms and shortened antibiotic therapy. Potentially, though we did not show this in our trial, it may lead to reduced cost, reduced development of resistance, and fewer adverse events.”
During the past few years, a new dogma has emerged in antibiotic treatment paradigms, she said: Shorter is better. Brad Spellberg, MD, described this concept in his 2016 editorial in JAMA Internal Medicine, “The new antibiotic mantra” (Sep 1;176[9]:1254-5).
In it, Dr. Spellberg, of the University of Southern California, Los Angeles, addressed the long-held view that a full 10- or 14-day course of antibiotics was necessary to decrease the risk of creating a resistant strain, even if clinical symptoms were long resolved.
However, he noted, there is little evidence supporting the idea that longer courses suppress the rise of resistance – and, in fact, some data support the opposite.
“To the contrary, specifically for pneumonia, studies have shown that longer courses of therapy result in more emergence of antibiotic resistance, which is consistent with everything we know about natural selection, the driver of antibiotic resistance,” he noted. “In only a few types of infections does resistance emerge at the site of infection; rather, resistance typically emerges off target, among colonizing flora away from the site of infection. Thus, all that is achieved by treating an infection with antibiotics for longer than the patient has symptoms is increased selective pressure driving antibiotic resistance among our colonizing microbial flora.”
The European Union and Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America have all recently updated their antibiotic stewardship guidelines to include a strong recommendation for the shortest effective duration of antimicrobial therapy.
However, most of the supporting data were drawn from randomized, controlled studies of patients with lung, skin, and kidney infections. Short-course treatments have not been adequately studied in bacteremia patients, Dr. Yahav said.
The aim of her study, which was investigator initiated and received no external funding, was to demonstrate the noninferiority of 7 days of antibiotic therapy, compared with 14 days, in patients with bacteremia arising from Gram-negative infections.
The randomized, open-label study comprised 604 patients in three hospitals: two in Israel and one in Italy. Patients were eligible if they had an aerobic Gram-negative bacteremia of any infection source that was either community- or hospital acquired. The medication choice was left up to the treating physician. Patients were assessed at discharge, and at days 30 and 90.
The primary outcome was a composite 90-day endpoint of all-cause mortality, clinical failure (relapse, new local complications, or distant complications), and readmission or hospital stay longer than 14 days. There were a number of secondary outcomes, including new infection, emergence of antibiotic resistance, total hospital and total antibiotic days, time to return to baseline activity, and adverse events.
The cohort was a mean of 71 years old. About 60% were functionally independent, and the mean Charlson comorbidity score was 2. Most of the infections (90%) were nosocomial. The urinary tract was the largest source of infection (69%). Other sources were abdominal, respiratory, central venous catheter, and skin or soft tissue.
Escherichia coli was the most common infective organism (62%), followed by Klebsiella species and Enterobacteriaceae. A small number of patients had Acinetobacter and Pseudomonas infections.
In the intent-to-treat analysis, the primary composite outcome of all-cause mortality or extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different. The results were nearly identical in the per-protocol analysis (46% vs. 49.6%).
Likewise, none of the secondary outcomes posted a significant difference in favor of one treatment arm, including relapse (2.9% vs. 2.7%) and resistance development (10.8% vs. 9.7%).
Dr. Yahav pointed out that total antibiotic-use days were significantly less in the 7-day group, (5 days) than in the 14-day group (10 days). Patients in the short-duration group returned to their normal activities a day earlier than those in the longer-term group (2 days vs. 3 days), a difference that was statistically significant.
The total hospital stay from randomization to day 90 was only half a day shorter in the short-term group (mean, 3 days vs. 3.5 days). That was not a significant finding.
There were some differences in adverse events, although none was statistically significant. The short-duration arm had slightly more cases of kidney injury (0.5%), fewer cases of liver function abnormalities (–1.5%), and half as many rashes (two vs. four). There were two cases of Clostridium difficile in the short-use arm and one in the long-use arm, also not a significant difference.
A subgroup analysis looked at outcomes among the different sources of infection (urinary tract vs. other), whether empirical antibiotics were used, and whether the induced resistance was multdrug or non–multidrug. All of those differences hovered close to the null, but generally favored short antibiotic treatment, Dr. Yahav noted.
“I would conclude from these data that is generally safe to stop antibiotics after 7 days of covering antibiotics for Gram-negative bacteremia patients, if they are hemodynamically stable and nonneutropenic at 7 days, and have no uncontrolled source of infection,” she concluded.
The investigator-initiated study had no outside funding.
SOURCE: Yahav D et al. ECCMID 2018. Oral abstract O1120.
MADRID – Seven days of antibiotic therapy was just as effective as 14 days for patients with Gram-negative bacteremias.
The shorter course was associated with similar cure rates and a faster return to normal activities, Dafna Yahav, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“In patients hospitalized with Gram-negative bacteremia and sepsis, a course of 7 antibiotic days was not inferior to 14 days, and resulted in a more rapid return to baseline activity, “ said Dr. Yahav of the Rabin Medical Center, Petah Tikva, Israel. “This could lead to a change in accepted management algorithms and shortened antibiotic therapy. Potentially, though we did not show this in our trial, it may lead to reduced cost, reduced development of resistance, and fewer adverse events.”
During the past few years, a new dogma has emerged in antibiotic treatment paradigms, she said: Shorter is better. Brad Spellberg, MD, described this concept in his 2016 editorial in JAMA Internal Medicine, “The new antibiotic mantra” (Sep 1;176[9]:1254-5).
In it, Dr. Spellberg, of the University of Southern California, Los Angeles, addressed the long-held view that a full 10- or 14-day course of antibiotics was necessary to decrease the risk of creating a resistant strain, even if clinical symptoms were long resolved.
However, he noted, there is little evidence supporting the idea that longer courses suppress the rise of resistance – and, in fact, some data support the opposite.
“To the contrary, specifically for pneumonia, studies have shown that longer courses of therapy result in more emergence of antibiotic resistance, which is consistent with everything we know about natural selection, the driver of antibiotic resistance,” he noted. “In only a few types of infections does resistance emerge at the site of infection; rather, resistance typically emerges off target, among colonizing flora away from the site of infection. Thus, all that is achieved by treating an infection with antibiotics for longer than the patient has symptoms is increased selective pressure driving antibiotic resistance among our colonizing microbial flora.”
The European Union and Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America have all recently updated their antibiotic stewardship guidelines to include a strong recommendation for the shortest effective duration of antimicrobial therapy.
However, most of the supporting data were drawn from randomized, controlled studies of patients with lung, skin, and kidney infections. Short-course treatments have not been adequately studied in bacteremia patients, Dr. Yahav said.
The aim of her study, which was investigator initiated and received no external funding, was to demonstrate the noninferiority of 7 days of antibiotic therapy, compared with 14 days, in patients with bacteremia arising from Gram-negative infections.
The randomized, open-label study comprised 604 patients in three hospitals: two in Israel and one in Italy. Patients were eligible if they had an aerobic Gram-negative bacteremia of any infection source that was either community- or hospital acquired. The medication choice was left up to the treating physician. Patients were assessed at discharge, and at days 30 and 90.
The primary outcome was a composite 90-day endpoint of all-cause mortality, clinical failure (relapse, new local complications, or distant complications), and readmission or hospital stay longer than 14 days. There were a number of secondary outcomes, including new infection, emergence of antibiotic resistance, total hospital and total antibiotic days, time to return to baseline activity, and adverse events.
The cohort was a mean of 71 years old. About 60% were functionally independent, and the mean Charlson comorbidity score was 2. Most of the infections (90%) were nosocomial. The urinary tract was the largest source of infection (69%). Other sources were abdominal, respiratory, central venous catheter, and skin or soft tissue.
Escherichia coli was the most common infective organism (62%), followed by Klebsiella species and Enterobacteriaceae. A small number of patients had Acinetobacter and Pseudomonas infections.
In the intent-to-treat analysis, the primary composite outcome of all-cause mortality or extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different. The results were nearly identical in the per-protocol analysis (46% vs. 49.6%).
Likewise, none of the secondary outcomes posted a significant difference in favor of one treatment arm, including relapse (2.9% vs. 2.7%) and resistance development (10.8% vs. 9.7%).
Dr. Yahav pointed out that total antibiotic-use days were significantly less in the 7-day group, (5 days) than in the 14-day group (10 days). Patients in the short-duration group returned to their normal activities a day earlier than those in the longer-term group (2 days vs. 3 days), a difference that was statistically significant.
The total hospital stay from randomization to day 90 was only half a day shorter in the short-term group (mean, 3 days vs. 3.5 days). That was not a significant finding.
There were some differences in adverse events, although none was statistically significant. The short-duration arm had slightly more cases of kidney injury (0.5%), fewer cases of liver function abnormalities (–1.5%), and half as many rashes (two vs. four). There were two cases of Clostridium difficile in the short-use arm and one in the long-use arm, also not a significant difference.
A subgroup analysis looked at outcomes among the different sources of infection (urinary tract vs. other), whether empirical antibiotics were used, and whether the induced resistance was multdrug or non–multidrug. All of those differences hovered close to the null, but generally favored short antibiotic treatment, Dr. Yahav noted.
“I would conclude from these data that is generally safe to stop antibiotics after 7 days of covering antibiotics for Gram-negative bacteremia patients, if they are hemodynamically stable and nonneutropenic at 7 days, and have no uncontrolled source of infection,” she concluded.
The investigator-initiated study had no outside funding.
SOURCE: Yahav D et al. ECCMID 2018. Oral abstract O1120.
MADRID – Seven days of antibiotic therapy was just as effective as 14 days for patients with Gram-negative bacteremias.
The shorter course was associated with similar cure rates and a faster return to normal activities, Dafna Yahav, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“In patients hospitalized with Gram-negative bacteremia and sepsis, a course of 7 antibiotic days was not inferior to 14 days, and resulted in a more rapid return to baseline activity, “ said Dr. Yahav of the Rabin Medical Center, Petah Tikva, Israel. “This could lead to a change in accepted management algorithms and shortened antibiotic therapy. Potentially, though we did not show this in our trial, it may lead to reduced cost, reduced development of resistance, and fewer adverse events.”
During the past few years, a new dogma has emerged in antibiotic treatment paradigms, she said: Shorter is better. Brad Spellberg, MD, described this concept in his 2016 editorial in JAMA Internal Medicine, “The new antibiotic mantra” (Sep 1;176[9]:1254-5).
In it, Dr. Spellberg, of the University of Southern California, Los Angeles, addressed the long-held view that a full 10- or 14-day course of antibiotics was necessary to decrease the risk of creating a resistant strain, even if clinical symptoms were long resolved.
However, he noted, there is little evidence supporting the idea that longer courses suppress the rise of resistance – and, in fact, some data support the opposite.
“To the contrary, specifically for pneumonia, studies have shown that longer courses of therapy result in more emergence of antibiotic resistance, which is consistent with everything we know about natural selection, the driver of antibiotic resistance,” he noted. “In only a few types of infections does resistance emerge at the site of infection; rather, resistance typically emerges off target, among colonizing flora away from the site of infection. Thus, all that is achieved by treating an infection with antibiotics for longer than the patient has symptoms is increased selective pressure driving antibiotic resistance among our colonizing microbial flora.”
The European Union and Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America have all recently updated their antibiotic stewardship guidelines to include a strong recommendation for the shortest effective duration of antimicrobial therapy.
However, most of the supporting data were drawn from randomized, controlled studies of patients with lung, skin, and kidney infections. Short-course treatments have not been adequately studied in bacteremia patients, Dr. Yahav said.
The aim of her study, which was investigator initiated and received no external funding, was to demonstrate the noninferiority of 7 days of antibiotic therapy, compared with 14 days, in patients with bacteremia arising from Gram-negative infections.
The randomized, open-label study comprised 604 patients in three hospitals: two in Israel and one in Italy. Patients were eligible if they had an aerobic Gram-negative bacteremia of any infection source that was either community- or hospital acquired. The medication choice was left up to the treating physician. Patients were assessed at discharge, and at days 30 and 90.
The primary outcome was a composite 90-day endpoint of all-cause mortality, clinical failure (relapse, new local complications, or distant complications), and readmission or hospital stay longer than 14 days. There were a number of secondary outcomes, including new infection, emergence of antibiotic resistance, total hospital and total antibiotic days, time to return to baseline activity, and adverse events.
The cohort was a mean of 71 years old. About 60% were functionally independent, and the mean Charlson comorbidity score was 2. Most of the infections (90%) were nosocomial. The urinary tract was the largest source of infection (69%). Other sources were abdominal, respiratory, central venous catheter, and skin or soft tissue.
Escherichia coli was the most common infective organism (62%), followed by Klebsiella species and Enterobacteriaceae. A small number of patients had Acinetobacter and Pseudomonas infections.
In the intent-to-treat analysis, the primary composite outcome of all-cause mortality or extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different. The results were nearly identical in the per-protocol analysis (46% vs. 49.6%).
Likewise, none of the secondary outcomes posted a significant difference in favor of one treatment arm, including relapse (2.9% vs. 2.7%) and resistance development (10.8% vs. 9.7%).
Dr. Yahav pointed out that total antibiotic-use days were significantly less in the 7-day group, (5 days) than in the 14-day group (10 days). Patients in the short-duration group returned to their normal activities a day earlier than those in the longer-term group (2 days vs. 3 days), a difference that was statistically significant.
The total hospital stay from randomization to day 90 was only half a day shorter in the short-term group (mean, 3 days vs. 3.5 days). That was not a significant finding.
There were some differences in adverse events, although none was statistically significant. The short-duration arm had slightly more cases of kidney injury (0.5%), fewer cases of liver function abnormalities (–1.5%), and half as many rashes (two vs. four). There were two cases of Clostridium difficile in the short-use arm and one in the long-use arm, also not a significant difference.
A subgroup analysis looked at outcomes among the different sources of infection (urinary tract vs. other), whether empirical antibiotics were used, and whether the induced resistance was multdrug or non–multidrug. All of those differences hovered close to the null, but generally favored short antibiotic treatment, Dr. Yahav noted.
“I would conclude from these data that is generally safe to stop antibiotics after 7 days of covering antibiotics for Gram-negative bacteremia patients, if they are hemodynamically stable and nonneutropenic at 7 days, and have no uncontrolled source of infection,” she concluded.
The investigator-initiated study had no outside funding.
SOURCE: Yahav D et al. ECCMID 2018. Oral abstract O1120.
REPORTING FROM ECCMID 2018
Key clinical point: Two weeks of antibiotic treatment conferred no benefits over 7 days of treatment in patients with Gram-negative bacteremias.
Major finding: All-cause mortality and extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different.
Study details: The randomized, open-label trial comprised 604 patients.
Disclosures: The investigator-initiated study had no external funding. Dr. Yahav had no financial disclosures.
Source: Yahav D et al. ECCMID 2018. Oral Abstract O1120.
Blood type A linked to more-severe diarrhea
Diarrhea associated with enterotoxigenic Escherichia coli (ETEC) infection is more severe among individuals with blood type A, based on data from 106 adults exposed to the agent.
The reseachers speculate that the increased severity is related, in part, to the presence of an adhesin molecule known as EtpA, which binds to the surface of the cells and combines with blood group A glycans to promote a more severe infection.
“Our studies suggest that the many ETEC strains that produce the EtpA adhesin may be particularly well equipped to preferentially infect hosts who express A blood group antigen on mucosal surfaces,” wrote Pardeep Kumar, MD, of Washington University, Saint Louis, and his colleagues.
ETEC, associated with choleralike illness, remains a major cause of infectious diarrhea in developing countries. No current vaccine offers significant protection against it, but EtpA may be useful in vaccine development. “More recently discovered virulence factors and an improved understanding of ETEC microbial pathogenesis could offer additional avenues to protect those at highest risk for severe disease and uncover novel molecular targets for future vaccine development,” the researchers said.
In a study published in the Journal of Clinical Investigation, the researchers examined blood samples from 106 adults who had participated in previous human infection model studies. The volunteers were challenged with an ETEC strain known as H10407 that was originally isolated from a case of choleralike illness.
Diarrhea was more frequent among A blood type individuals, compared with other types. Individuals with B or O blood types were significantly more likely to have no diarrhea or mild diarrhea after the E. coli challenge, compared with type A individuals (44% vs. 19%).
Overall, significantly more individuals with A or AB blood types developed moderate to severe diarrhea, compared with those with B or O blood types (81% vs. 56%). In addition, significantly more individuals with blood type A needed early initiation of antibiotic therapy, compared with those with B or O blood types (72% vs. 43%).
The study findings were limited by several factors, including the use of higher levels of bacteria to cause the infection than a typical exposure in nature. Also, the volunteers were treated with antibiotics once they met the criteria for severe diarrhea.
The researchers also noted that other blood group A lectins in addition to EtpA may not have been identified.
“While our recent studies have potentially important clinical and [vaccinological] implications, further study of the relationship between blood group, disease severity, and antigen expression could guide and inform use of these antigens in vaccines,” they wrote.
The study was supported by funds from the Enteric Vaccine Initiative of PATH, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
SOURCE: Kumar P et al. J Clin Invest. 2018 May 17. doi: 10.1172/JCI97659.
Diarrhea associated with enterotoxigenic Escherichia coli (ETEC) infection is more severe among individuals with blood type A, based on data from 106 adults exposed to the agent.
The reseachers speculate that the increased severity is related, in part, to the presence of an adhesin molecule known as EtpA, which binds to the surface of the cells and combines with blood group A glycans to promote a more severe infection.
“Our studies suggest that the many ETEC strains that produce the EtpA adhesin may be particularly well equipped to preferentially infect hosts who express A blood group antigen on mucosal surfaces,” wrote Pardeep Kumar, MD, of Washington University, Saint Louis, and his colleagues.
ETEC, associated with choleralike illness, remains a major cause of infectious diarrhea in developing countries. No current vaccine offers significant protection against it, but EtpA may be useful in vaccine development. “More recently discovered virulence factors and an improved understanding of ETEC microbial pathogenesis could offer additional avenues to protect those at highest risk for severe disease and uncover novel molecular targets for future vaccine development,” the researchers said.
In a study published in the Journal of Clinical Investigation, the researchers examined blood samples from 106 adults who had participated in previous human infection model studies. The volunteers were challenged with an ETEC strain known as H10407 that was originally isolated from a case of choleralike illness.
Diarrhea was more frequent among A blood type individuals, compared with other types. Individuals with B or O blood types were significantly more likely to have no diarrhea or mild diarrhea after the E. coli challenge, compared with type A individuals (44% vs. 19%).
Overall, significantly more individuals with A or AB blood types developed moderate to severe diarrhea, compared with those with B or O blood types (81% vs. 56%). In addition, significantly more individuals with blood type A needed early initiation of antibiotic therapy, compared with those with B or O blood types (72% vs. 43%).
The study findings were limited by several factors, including the use of higher levels of bacteria to cause the infection than a typical exposure in nature. Also, the volunteers were treated with antibiotics once they met the criteria for severe diarrhea.
The researchers also noted that other blood group A lectins in addition to EtpA may not have been identified.
“While our recent studies have potentially important clinical and [vaccinological] implications, further study of the relationship between blood group, disease severity, and antigen expression could guide and inform use of these antigens in vaccines,” they wrote.
The study was supported by funds from the Enteric Vaccine Initiative of PATH, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
SOURCE: Kumar P et al. J Clin Invest. 2018 May 17. doi: 10.1172/JCI97659.
Diarrhea associated with enterotoxigenic Escherichia coli (ETEC) infection is more severe among individuals with blood type A, based on data from 106 adults exposed to the agent.
The reseachers speculate that the increased severity is related, in part, to the presence of an adhesin molecule known as EtpA, which binds to the surface of the cells and combines with blood group A glycans to promote a more severe infection.
“Our studies suggest that the many ETEC strains that produce the EtpA adhesin may be particularly well equipped to preferentially infect hosts who express A blood group antigen on mucosal surfaces,” wrote Pardeep Kumar, MD, of Washington University, Saint Louis, and his colleagues.
ETEC, associated with choleralike illness, remains a major cause of infectious diarrhea in developing countries. No current vaccine offers significant protection against it, but EtpA may be useful in vaccine development. “More recently discovered virulence factors and an improved understanding of ETEC microbial pathogenesis could offer additional avenues to protect those at highest risk for severe disease and uncover novel molecular targets for future vaccine development,” the researchers said.
In a study published in the Journal of Clinical Investigation, the researchers examined blood samples from 106 adults who had participated in previous human infection model studies. The volunteers were challenged with an ETEC strain known as H10407 that was originally isolated from a case of choleralike illness.
Diarrhea was more frequent among A blood type individuals, compared with other types. Individuals with B or O blood types were significantly more likely to have no diarrhea or mild diarrhea after the E. coli challenge, compared with type A individuals (44% vs. 19%).
Overall, significantly more individuals with A or AB blood types developed moderate to severe diarrhea, compared with those with B or O blood types (81% vs. 56%). In addition, significantly more individuals with blood type A needed early initiation of antibiotic therapy, compared with those with B or O blood types (72% vs. 43%).
The study findings were limited by several factors, including the use of higher levels of bacteria to cause the infection than a typical exposure in nature. Also, the volunteers were treated with antibiotics once they met the criteria for severe diarrhea.
The researchers also noted that other blood group A lectins in addition to EtpA may not have been identified.
“While our recent studies have potentially important clinical and [vaccinological] implications, further study of the relationship between blood group, disease severity, and antigen expression could guide and inform use of these antigens in vaccines,” they wrote.
The study was supported by funds from the Enteric Vaccine Initiative of PATH, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
SOURCE: Kumar P et al. J Clin Invest. 2018 May 17. doi: 10.1172/JCI97659.
FROM THE JOURNAL OF CLINICAL INVESTIGATION
Key clinical point: Severe diarrhea was significantly more frequent in adults with an A blood type, compared with B or O types.
Major finding: The overall relative risk of moderate to severe diarrhea was 1.44 for A blood groups, compared with non-A blood groups.
Study details: The data come from 106 adult volunteers who had participated in previous human infection model studies.
Disclosures: The study was supported by funds from the PATH Enteric Vaccine Initiative, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
Source: Kumar P et al. J Clin Invest. 2018 May 17; doi: 10.1172/JCI97659.
Canagliflozin linked to lower HbA1c levels in younger patients
BOSTON – The effects of canagliflozin on hemoglobin A1c levels were greater in participants under age 65 years in randomized clinical trials of the SGLT2 inhibitor, according to results of a prespecified subgroup analysis.
Some cardiovascular, renal, and mortality outcomes also varied by age group, according to the analysis of participants in the CANVAS (Canagliflozin Cardiovascular Assessment Study) program. Despite those differences, the effect of canagliflozin was generally consistent on other outcomes, such as body weight and blood pressure, in patient grouped into those aged less than 65 years and those aged 65 years and older, investigators reported at the annual meeting of the American Association of Clinical Endocrinologists.
Nonfatal MI, nonfatal stroke, and the composite of doubling of serum creatine, end-stage renal disease, or renal death were lower in study participants aged 65 and older on canagliflozin. Lower risks of cardiovascular death and all-cause mortality were seen in participants under age 65 years who were taking canagliflozin. However, “these results should be interpreted with caution given the large number of subgroup analyses performed,” wrote Fernando Ovalle, MD, director of the multidisciplinary comprehensive diabetes clinic at the University of Alabama at Birmingham, and his colleagues.
Canagliflozin was found to reduce risk of the composite endpoint of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke versus placebo in previously published results of the CANVAS study, which included individuals with type 2 diabetes mellitus who either had or were at high risk for having cardiovascular disease.
The two multicenter, randomized, controlled trials, CANVAS and CANVAS-R, included more than 10,000 patients. The subgroup analysis presented here at the AACE meeting included 5,578 patients aged less than 65 years (55%) and 4,564 patients aged 65 years or older (45%).
The subgroup analysis found that placebo-subtracted differences in HbA1c were greater in patients under 65 years than those in patients 65 or older (–0.7% and –0.5%, respectively; P less than .0001). However, there were no significant differences by age group in body weight changes, systolic blood pressure, or diastolic blood pressure.
Patients aged 65 years or older at baseline had a lower risk of nonfatal MI and nonfatal stroke with canagliflozin versus placebo (P for heterogeneity, .02 and.01, respectively), according to investigators. Older patients also had lower risk of the composite of serum creatinine doubling, end-stage kidney disease, or renal death with canagliflozin versus placebo (P = .01).
Patients aged less than 65 years at baseline had lower risk of cardiovascular death (P = .01) and all cause mortality (P = .01) for canagliflozin versus placebo.
Dr. Ovalle reported disclosures related to Merck, AstraZeneca, Sanofi Pasteur, Novo Nordisk, GlaxoSmithKline, Janssen, Eli Lilly, Medtronic, Pfizer, and GI Dynamics, along with the National Institutes of Health and Juvenile Diabetes Research Foundation.
SOURCE: Ovalle F et al. AACE 2018, Abstract 233.
BOSTON – The effects of canagliflozin on hemoglobin A1c levels were greater in participants under age 65 years in randomized clinical trials of the SGLT2 inhibitor, according to results of a prespecified subgroup analysis.
Some cardiovascular, renal, and mortality outcomes also varied by age group, according to the analysis of participants in the CANVAS (Canagliflozin Cardiovascular Assessment Study) program. Despite those differences, the effect of canagliflozin was generally consistent on other outcomes, such as body weight and blood pressure, in patient grouped into those aged less than 65 years and those aged 65 years and older, investigators reported at the annual meeting of the American Association of Clinical Endocrinologists.
Nonfatal MI, nonfatal stroke, and the composite of doubling of serum creatine, end-stage renal disease, or renal death were lower in study participants aged 65 and older on canagliflozin. Lower risks of cardiovascular death and all-cause mortality were seen in participants under age 65 years who were taking canagliflozin. However, “these results should be interpreted with caution given the large number of subgroup analyses performed,” wrote Fernando Ovalle, MD, director of the multidisciplinary comprehensive diabetes clinic at the University of Alabama at Birmingham, and his colleagues.
Canagliflozin was found to reduce risk of the composite endpoint of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke versus placebo in previously published results of the CANVAS study, which included individuals with type 2 diabetes mellitus who either had or were at high risk for having cardiovascular disease.
The two multicenter, randomized, controlled trials, CANVAS and CANVAS-R, included more than 10,000 patients. The subgroup analysis presented here at the AACE meeting included 5,578 patients aged less than 65 years (55%) and 4,564 patients aged 65 years or older (45%).
The subgroup analysis found that placebo-subtracted differences in HbA1c were greater in patients under 65 years than those in patients 65 or older (–0.7% and –0.5%, respectively; P less than .0001). However, there were no significant differences by age group in body weight changes, systolic blood pressure, or diastolic blood pressure.
Patients aged 65 years or older at baseline had a lower risk of nonfatal MI and nonfatal stroke with canagliflozin versus placebo (P for heterogeneity, .02 and.01, respectively), according to investigators. Older patients also had lower risk of the composite of serum creatinine doubling, end-stage kidney disease, or renal death with canagliflozin versus placebo (P = .01).
Patients aged less than 65 years at baseline had lower risk of cardiovascular death (P = .01) and all cause mortality (P = .01) for canagliflozin versus placebo.
Dr. Ovalle reported disclosures related to Merck, AstraZeneca, Sanofi Pasteur, Novo Nordisk, GlaxoSmithKline, Janssen, Eli Lilly, Medtronic, Pfizer, and GI Dynamics, along with the National Institutes of Health and Juvenile Diabetes Research Foundation.
SOURCE: Ovalle F et al. AACE 2018, Abstract 233.
BOSTON – The effects of canagliflozin on hemoglobin A1c levels were greater in participants under age 65 years in randomized clinical trials of the SGLT2 inhibitor, according to results of a prespecified subgroup analysis.
Some cardiovascular, renal, and mortality outcomes also varied by age group, according to the analysis of participants in the CANVAS (Canagliflozin Cardiovascular Assessment Study) program. Despite those differences, the effect of canagliflozin was generally consistent on other outcomes, such as body weight and blood pressure, in patient grouped into those aged less than 65 years and those aged 65 years and older, investigators reported at the annual meeting of the American Association of Clinical Endocrinologists.
Nonfatal MI, nonfatal stroke, and the composite of doubling of serum creatine, end-stage renal disease, or renal death were lower in study participants aged 65 and older on canagliflozin. Lower risks of cardiovascular death and all-cause mortality were seen in participants under age 65 years who were taking canagliflozin. However, “these results should be interpreted with caution given the large number of subgroup analyses performed,” wrote Fernando Ovalle, MD, director of the multidisciplinary comprehensive diabetes clinic at the University of Alabama at Birmingham, and his colleagues.
Canagliflozin was found to reduce risk of the composite endpoint of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke versus placebo in previously published results of the CANVAS study, which included individuals with type 2 diabetes mellitus who either had or were at high risk for having cardiovascular disease.
The two multicenter, randomized, controlled trials, CANVAS and CANVAS-R, included more than 10,000 patients. The subgroup analysis presented here at the AACE meeting included 5,578 patients aged less than 65 years (55%) and 4,564 patients aged 65 years or older (45%).
The subgroup analysis found that placebo-subtracted differences in HbA1c were greater in patients under 65 years than those in patients 65 or older (–0.7% and –0.5%, respectively; P less than .0001). However, there were no significant differences by age group in body weight changes, systolic blood pressure, or diastolic blood pressure.
Patients aged 65 years or older at baseline had a lower risk of nonfatal MI and nonfatal stroke with canagliflozin versus placebo (P for heterogeneity, .02 and.01, respectively), according to investigators. Older patients also had lower risk of the composite of serum creatinine doubling, end-stage kidney disease, or renal death with canagliflozin versus placebo (P = .01).
Patients aged less than 65 years at baseline had lower risk of cardiovascular death (P = .01) and all cause mortality (P = .01) for canagliflozin versus placebo.
Dr. Ovalle reported disclosures related to Merck, AstraZeneca, Sanofi Pasteur, Novo Nordisk, GlaxoSmithKline, Janssen, Eli Lilly, Medtronic, Pfizer, and GI Dynamics, along with the National Institutes of Health and Juvenile Diabetes Research Foundation.
SOURCE: Ovalle F et al. AACE 2018, Abstract 233.
REPORTING FROM AACE 2018
Key clinical point: Placebo-subtracted differences in HbA1c were greater in patients under age 65 years versus patients age 65 years and older, while some cardiovascular, renal, and mortality outcomes varied by age group.
Major finding: Placebo-subtracted differences in HbA1c were greater in patients under 65 years versus patients 65 or older (-0.7% and -0.5%, respectively; P less than 0.0001).
Study details: A prespecified subset analysis of the 2 multicenter randomized controlled trials in the CANVAS Program (CANVAS and CANVAS-R studies) including more than 10,000 individuals with type 2 diabetes mellitus and either established CV disease or high risk for CV disease.
Disclosures: Dr. Ovalle reported disclosures related to Merck, AstraZeneca, Sanofi Pasteur, Novo Nordisk, GlaxoSmithKline, Janssen, Eli Lilly, Medtronic, Pfizer, and GI Dynamics, along with the National Institutes of Health and Juvenile Diabetes Research Foundation.
Source: Ovalle F, et al. AACE 2018, Abstract 233
Most COPD patients on triple therapy can withdraw steroids
SAN DIEGO – In patients on long-term triple therapy and up to one exacerbation in the previous year, the withdrawal of inhaled corticosteroids (ICS) led to a small decrease in lung function that was not clinically important, with no associated difference in the rates of chronic obstructive pulmonary disease (COPD) exacerbations, dyspnea or as-needed bronchodilator use.
Those are key findings from the SUNSET trial, a 26-week, randomized, double-blind, parallel-group multicenter study to assess the efficacy and safety of the switch from long-term triple therapy to indacaterol/glycopyrronium (IND/GLY, 110/50 mcg once daily) or continuation of triple therapy with tiotropium 18 mcg once daily and salmeterol/fluticasone propionate fixed-dose combination 50/500 mcg b.i.d.
Dr. Chapman, director of the asthma and airways clinic at University Health Network, Toronto, noted that relatively few patients with COPD benefit from inhaled steroids. “Given the risk of adverse events (pneumonia, osteoporosis, etc.), we’d rather not give them when they’re not needed,” he said. “Inhaled steroids seem to play only one role in COPD: They tend to reduce exacerbations in the exacerbation-prone COPD patient. That’s about 20% of the COPD population. Despite this, a great many patients end up on triple therapy [long-acting bronchodilators/long-acting muscarinic antagonist (LABA/LAMA) and ICS] needlessly.”
For the study, Dr. Chapman and his associates enrolled 1,053 patients with moderate to severe COPD who’d had no more than one exacerbation in the previous year who had used triple therapy for at least 6 months prior to study inclusion. The primary endpoint of the study was noninferiority on change from baseline in postdose trough forced expiratory volume in 1 second (FEV1) (with a noninferiority margin of –50 mL) after 26 weeks. Exacerbations, health-related quality of life as measured by the St. George’s Respiratory Questionnaire (SGRQ-C), and breathlessness as measures by the Transition Dyspnea Index also were evaluated. Of the 1,053 patients, 527 were randomized to IND/GLY and 526 to triple therapy. Their mean age was 65 years and their mean postbronchodilator FEV1 was 1.6 L.
The researchers found that ICS withdrawal led to a mean reduction in trough FEV1 of –26 mL, which exceeded the noninferiority margin. This difference between treatments on trough FEV1 was driven by the subset of patients with high blood eosinophil counts at baseline (a mean of –68 mL for patients with at least 300 cells/mcL and a mean of –13 mL for patients with fewer than 300 cells/mcL). The two treatments showed similar annualized rates of moderate/severe COPD exacerbations (rate ratio, 1.08) and all (mild/moderate/severe) exacerbations (RR, 1.07). ICS withdrawal led to a small difference in SGRQ-C (1.4 U on week 26), but no differences in Transition Dyspnea Index or use of rescue medication over 26 weeks. Safety and tolerability were balanced across the two treatment groups.
“Although we found no overall increase in exacerbations with ‘de-escalation,’ there were, of course, exacerbations that occurred during the trial,” Dr. Chapman said. “We found that they tended to occur in the minority of patients who had elevated blood eosinophil counts, especially if the counts were elevated persistently (at screening and randomization). The relevant cut-point was blood eosinophil counts above 300/UL. If exacerbations did occur in this easily identifiable subpopulation, they tended to occur early, in the first month after de-escalation. This gives physicians a simply way to identify a population they might exercise caution and a period when careful monitoring is useful.”
He acknowledged certain limitations of the study, including its 6-month duration, which is shorter than most exacerbation studies. “But by recruiting at multiple sites in multiple countries and across seasons, we don’t think this was an importation limitation,” he said. “Of course, like most investigators, I can always think of things I wish I had tracked. My personal hunch is that FeNO [exhaled nitric oxide levels] might offer some useful information but that will be a hunch to explore in another study.”
SUNSET was sponsored by Novartis. Dr. Chapman disclosed that he has received fees for research, consulting and lectures from Novartis, as well as from several other pharmaceutical companies.
dbrunk@mdedge.com
SOURCE: Chapman K et al. ATS 2018 Abstract A1009.
SAN DIEGO – In patients on long-term triple therapy and up to one exacerbation in the previous year, the withdrawal of inhaled corticosteroids (ICS) led to a small decrease in lung function that was not clinically important, with no associated difference in the rates of chronic obstructive pulmonary disease (COPD) exacerbations, dyspnea or as-needed bronchodilator use.
Those are key findings from the SUNSET trial, a 26-week, randomized, double-blind, parallel-group multicenter study to assess the efficacy and safety of the switch from long-term triple therapy to indacaterol/glycopyrronium (IND/GLY, 110/50 mcg once daily) or continuation of triple therapy with tiotropium 18 mcg once daily and salmeterol/fluticasone propionate fixed-dose combination 50/500 mcg b.i.d.
Dr. Chapman, director of the asthma and airways clinic at University Health Network, Toronto, noted that relatively few patients with COPD benefit from inhaled steroids. “Given the risk of adverse events (pneumonia, osteoporosis, etc.), we’d rather not give them when they’re not needed,” he said. “Inhaled steroids seem to play only one role in COPD: They tend to reduce exacerbations in the exacerbation-prone COPD patient. That’s about 20% of the COPD population. Despite this, a great many patients end up on triple therapy [long-acting bronchodilators/long-acting muscarinic antagonist (LABA/LAMA) and ICS] needlessly.”
For the study, Dr. Chapman and his associates enrolled 1,053 patients with moderate to severe COPD who’d had no more than one exacerbation in the previous year who had used triple therapy for at least 6 months prior to study inclusion. The primary endpoint of the study was noninferiority on change from baseline in postdose trough forced expiratory volume in 1 second (FEV1) (with a noninferiority margin of –50 mL) after 26 weeks. Exacerbations, health-related quality of life as measured by the St. George’s Respiratory Questionnaire (SGRQ-C), and breathlessness as measures by the Transition Dyspnea Index also were evaluated. Of the 1,053 patients, 527 were randomized to IND/GLY and 526 to triple therapy. Their mean age was 65 years and their mean postbronchodilator FEV1 was 1.6 L.
The researchers found that ICS withdrawal led to a mean reduction in trough FEV1 of –26 mL, which exceeded the noninferiority margin. This difference between treatments on trough FEV1 was driven by the subset of patients with high blood eosinophil counts at baseline (a mean of –68 mL for patients with at least 300 cells/mcL and a mean of –13 mL for patients with fewer than 300 cells/mcL). The two treatments showed similar annualized rates of moderate/severe COPD exacerbations (rate ratio, 1.08) and all (mild/moderate/severe) exacerbations (RR, 1.07). ICS withdrawal led to a small difference in SGRQ-C (1.4 U on week 26), but no differences in Transition Dyspnea Index or use of rescue medication over 26 weeks. Safety and tolerability were balanced across the two treatment groups.
“Although we found no overall increase in exacerbations with ‘de-escalation,’ there were, of course, exacerbations that occurred during the trial,” Dr. Chapman said. “We found that they tended to occur in the minority of patients who had elevated blood eosinophil counts, especially if the counts were elevated persistently (at screening and randomization). The relevant cut-point was blood eosinophil counts above 300/UL. If exacerbations did occur in this easily identifiable subpopulation, they tended to occur early, in the first month after de-escalation. This gives physicians a simply way to identify a population they might exercise caution and a period when careful monitoring is useful.”
He acknowledged certain limitations of the study, including its 6-month duration, which is shorter than most exacerbation studies. “But by recruiting at multiple sites in multiple countries and across seasons, we don’t think this was an importation limitation,” he said. “Of course, like most investigators, I can always think of things I wish I had tracked. My personal hunch is that FeNO [exhaled nitric oxide levels] might offer some useful information but that will be a hunch to explore in another study.”
SUNSET was sponsored by Novartis. Dr. Chapman disclosed that he has received fees for research, consulting and lectures from Novartis, as well as from several other pharmaceutical companies.
dbrunk@mdedge.com
SOURCE: Chapman K et al. ATS 2018 Abstract A1009.
SAN DIEGO – In patients on long-term triple therapy and up to one exacerbation in the previous year, the withdrawal of inhaled corticosteroids (ICS) led to a small decrease in lung function that was not clinically important, with no associated difference in the rates of chronic obstructive pulmonary disease (COPD) exacerbations, dyspnea or as-needed bronchodilator use.
Those are key findings from the SUNSET trial, a 26-week, randomized, double-blind, parallel-group multicenter study to assess the efficacy and safety of the switch from long-term triple therapy to indacaterol/glycopyrronium (IND/GLY, 110/50 mcg once daily) or continuation of triple therapy with tiotropium 18 mcg once daily and salmeterol/fluticasone propionate fixed-dose combination 50/500 mcg b.i.d.
Dr. Chapman, director of the asthma and airways clinic at University Health Network, Toronto, noted that relatively few patients with COPD benefit from inhaled steroids. “Given the risk of adverse events (pneumonia, osteoporosis, etc.), we’d rather not give them when they’re not needed,” he said. “Inhaled steroids seem to play only one role in COPD: They tend to reduce exacerbations in the exacerbation-prone COPD patient. That’s about 20% of the COPD population. Despite this, a great many patients end up on triple therapy [long-acting bronchodilators/long-acting muscarinic antagonist (LABA/LAMA) and ICS] needlessly.”
For the study, Dr. Chapman and his associates enrolled 1,053 patients with moderate to severe COPD who’d had no more than one exacerbation in the previous year who had used triple therapy for at least 6 months prior to study inclusion. The primary endpoint of the study was noninferiority on change from baseline in postdose trough forced expiratory volume in 1 second (FEV1) (with a noninferiority margin of –50 mL) after 26 weeks. Exacerbations, health-related quality of life as measured by the St. George’s Respiratory Questionnaire (SGRQ-C), and breathlessness as measures by the Transition Dyspnea Index also were evaluated. Of the 1,053 patients, 527 were randomized to IND/GLY and 526 to triple therapy. Their mean age was 65 years and their mean postbronchodilator FEV1 was 1.6 L.
The researchers found that ICS withdrawal led to a mean reduction in trough FEV1 of –26 mL, which exceeded the noninferiority margin. This difference between treatments on trough FEV1 was driven by the subset of patients with high blood eosinophil counts at baseline (a mean of –68 mL for patients with at least 300 cells/mcL and a mean of –13 mL for patients with fewer than 300 cells/mcL). The two treatments showed similar annualized rates of moderate/severe COPD exacerbations (rate ratio, 1.08) and all (mild/moderate/severe) exacerbations (RR, 1.07). ICS withdrawal led to a small difference in SGRQ-C (1.4 U on week 26), but no differences in Transition Dyspnea Index or use of rescue medication over 26 weeks. Safety and tolerability were balanced across the two treatment groups.
“Although we found no overall increase in exacerbations with ‘de-escalation,’ there were, of course, exacerbations that occurred during the trial,” Dr. Chapman said. “We found that they tended to occur in the minority of patients who had elevated blood eosinophil counts, especially if the counts were elevated persistently (at screening and randomization). The relevant cut-point was blood eosinophil counts above 300/UL. If exacerbations did occur in this easily identifiable subpopulation, they tended to occur early, in the first month after de-escalation. This gives physicians a simply way to identify a population they might exercise caution and a period when careful monitoring is useful.”
He acknowledged certain limitations of the study, including its 6-month duration, which is shorter than most exacerbation studies. “But by recruiting at multiple sites in multiple countries and across seasons, we don’t think this was an importation limitation,” he said. “Of course, like most investigators, I can always think of things I wish I had tracked. My personal hunch is that FeNO [exhaled nitric oxide levels] might offer some useful information but that will be a hunch to explore in another study.”
SUNSET was sponsored by Novartis. Dr. Chapman disclosed that he has received fees for research, consulting and lectures from Novartis, as well as from several other pharmaceutical companies.
dbrunk@mdedge.com
SOURCE: Chapman K et al. ATS 2018 Abstract A1009.
REPORTING FROM ATS 2018
Key clinical point: Switching from triple therapy to indacaterol/glycopyrronium (IND/GLY) is effective in COPD patients and avoids long-term exposure to inhaled corticosteroids.
Major finding: ICS withdrawal led to a mean reduction in trough FEV1 of –26 mL, which exceeded the noninferiority margin.
Study details: A randomized trial of COPD patients in which 527 were randomized to IND/GLY and 526 to triple therapy.
Disclosures: SUNSET was sponsored by Novartis. Dr. Chapman disclosed that he has received fees for research, consulting, and lectures from Novartis, as well as from several other pharmaceutical companies.
Source: Chapman K et al. ATS 2018. Abstract A1009.
VIDEO: Canagliflozin’s HbA1c effect muted over time by placebo group effects
BOSTON – In a large, long-term study of canagliflozin versus placebo, an excess of discontinuations in the placebo group contributed to a dampening in the magnitude of improvement in hemoglobin A1c.
That’s according to investigators who reported the findings at the annual meeting of the American Association of Clinical Endocrinologists.
A higher rate of starting new antihyperglycemic agents in the placebo arm also likely contributed to the decrease in the difference in HbA1c after 52 weeks in CANVAS (Canagliflozin Cardiovascular Assessment Study), according to investigator Carol Wysham, MD, of the University of Washington, Spokane.
As previously reported, the two randomized trials in the CANVAS program showed that canagliflozin reduced risk of cardiovascular events in patients with type 2 diabetes at elevated risk of cardiovascular disease.
While the CANVAS program was not designed to assess glucose lowering – and, in fact, allowed adjustment of background antihyperglycemic agents at any time – canagliflozin patients were more likely than placebo-treated patients to achieve HbA1c less than 7.0%.
However, the mean placebo-subtracted reduction in HbA1c peaked at –0.64% at week 26 but shrank to –0.24% by week 338, the end of the study.
“In this case, [the analysis] is helping to understand why we might have seen a deterioration in A1c control over a very long period of time,” Dr. Wysham explained.
The analysis showed that, after week 52 of the study, more patients discontinued placebo, compared with those taking canagliflozin. Over the entire CANVAS program, investigators said, the rate of discontinuation was 118.0 per 1,000 patient-years in the placebo group and 94.1 per 1,000 patient-years for canagliflozin.
In addition, while the concomitant use of antihyperglycemic agents was well balanced at baseline, the number of participants initiating new antihyperglycemic agents over the course of the study was 27% for the placebo-treated patients and 17.8% for the canagliflozin-treated patients, investigators found.
All together, the CANVAS program has provided the longest-term experience to date regarding glycemic control with canagliflozin, demonstrating greater reductions in HbA1c, versus placebo, over about 6.5 years, Dr. Wysham and her coinvestigators said in their poster presentation.
The new analysis provides better clarity on why the durability in HbA1c benefit seemed somewhat attenuated over time, according to Dr. Wysham.
“Most of us think that, if you start an SGLT2 inhibitor, especially starting it relatively early in diagnosis of diabetes, it gives you better durability than what you might see with other agents,” she said. “In fact, that’s been seen in many of the clinical trials, compared to sulphonylurea or DPP-4 inhibitor.”
Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
SOURCE: Wysham C et al. AACE 2018, Abstract 262.
BOSTON – In a large, long-term study of canagliflozin versus placebo, an excess of discontinuations in the placebo group contributed to a dampening in the magnitude of improvement in hemoglobin A1c.
That’s according to investigators who reported the findings at the annual meeting of the American Association of Clinical Endocrinologists.
A higher rate of starting new antihyperglycemic agents in the placebo arm also likely contributed to the decrease in the difference in HbA1c after 52 weeks in CANVAS (Canagliflozin Cardiovascular Assessment Study), according to investigator Carol Wysham, MD, of the University of Washington, Spokane.
As previously reported, the two randomized trials in the CANVAS program showed that canagliflozin reduced risk of cardiovascular events in patients with type 2 diabetes at elevated risk of cardiovascular disease.
While the CANVAS program was not designed to assess glucose lowering – and, in fact, allowed adjustment of background antihyperglycemic agents at any time – canagliflozin patients were more likely than placebo-treated patients to achieve HbA1c less than 7.0%.
However, the mean placebo-subtracted reduction in HbA1c peaked at –0.64% at week 26 but shrank to –0.24% by week 338, the end of the study.
“In this case, [the analysis] is helping to understand why we might have seen a deterioration in A1c control over a very long period of time,” Dr. Wysham explained.
The analysis showed that, after week 52 of the study, more patients discontinued placebo, compared with those taking canagliflozin. Over the entire CANVAS program, investigators said, the rate of discontinuation was 118.0 per 1,000 patient-years in the placebo group and 94.1 per 1,000 patient-years for canagliflozin.
In addition, while the concomitant use of antihyperglycemic agents was well balanced at baseline, the number of participants initiating new antihyperglycemic agents over the course of the study was 27% for the placebo-treated patients and 17.8% for the canagliflozin-treated patients, investigators found.
All together, the CANVAS program has provided the longest-term experience to date regarding glycemic control with canagliflozin, demonstrating greater reductions in HbA1c, versus placebo, over about 6.5 years, Dr. Wysham and her coinvestigators said in their poster presentation.
The new analysis provides better clarity on why the durability in HbA1c benefit seemed somewhat attenuated over time, according to Dr. Wysham.
“Most of us think that, if you start an SGLT2 inhibitor, especially starting it relatively early in diagnosis of diabetes, it gives you better durability than what you might see with other agents,” she said. “In fact, that’s been seen in many of the clinical trials, compared to sulphonylurea or DPP-4 inhibitor.”
Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
SOURCE: Wysham C et al. AACE 2018, Abstract 262.
BOSTON – In a large, long-term study of canagliflozin versus placebo, an excess of discontinuations in the placebo group contributed to a dampening in the magnitude of improvement in hemoglobin A1c.
That’s according to investigators who reported the findings at the annual meeting of the American Association of Clinical Endocrinologists.
A higher rate of starting new antihyperglycemic agents in the placebo arm also likely contributed to the decrease in the difference in HbA1c after 52 weeks in CANVAS (Canagliflozin Cardiovascular Assessment Study), according to investigator Carol Wysham, MD, of the University of Washington, Spokane.
As previously reported, the two randomized trials in the CANVAS program showed that canagliflozin reduced risk of cardiovascular events in patients with type 2 diabetes at elevated risk of cardiovascular disease.
While the CANVAS program was not designed to assess glucose lowering – and, in fact, allowed adjustment of background antihyperglycemic agents at any time – canagliflozin patients were more likely than placebo-treated patients to achieve HbA1c less than 7.0%.
However, the mean placebo-subtracted reduction in HbA1c peaked at –0.64% at week 26 but shrank to –0.24% by week 338, the end of the study.
“In this case, [the analysis] is helping to understand why we might have seen a deterioration in A1c control over a very long period of time,” Dr. Wysham explained.
The analysis showed that, after week 52 of the study, more patients discontinued placebo, compared with those taking canagliflozin. Over the entire CANVAS program, investigators said, the rate of discontinuation was 118.0 per 1,000 patient-years in the placebo group and 94.1 per 1,000 patient-years for canagliflozin.
In addition, while the concomitant use of antihyperglycemic agents was well balanced at baseline, the number of participants initiating new antihyperglycemic agents over the course of the study was 27% for the placebo-treated patients and 17.8% for the canagliflozin-treated patients, investigators found.
All together, the CANVAS program has provided the longest-term experience to date regarding glycemic control with canagliflozin, demonstrating greater reductions in HbA1c, versus placebo, over about 6.5 years, Dr. Wysham and her coinvestigators said in their poster presentation.
The new analysis provides better clarity on why the durability in HbA1c benefit seemed somewhat attenuated over time, according to Dr. Wysham.
“Most of us think that, if you start an SGLT2 inhibitor, especially starting it relatively early in diagnosis of diabetes, it gives you better durability than what you might see with other agents,” she said. “In fact, that’s been seen in many of the clinical trials, compared to sulphonylurea or DPP-4 inhibitor.”
Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
SOURCE: Wysham C et al. AACE 2018, Abstract 262.
REPORTING FROM AACE 2018
Key clinical point: and a higher rate of starting new antihyperglycemic agents in the placebo arm.
Major finding: After week 52 of the study, more patients discontinued placebo versus canagliflozin. The number of participants initiating new antihyperglycemic agents over the course of the study was 17.8% for the canagliflozin arm and 27% for placebo.
Study details: An analysis of the effects of canagliflozin on HbA1c and changes in use of antihyperglycemic agents in the CANVAS study.
Disclosures: Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
Source: Wysham C et al. AACE 2018, Abstract 262.
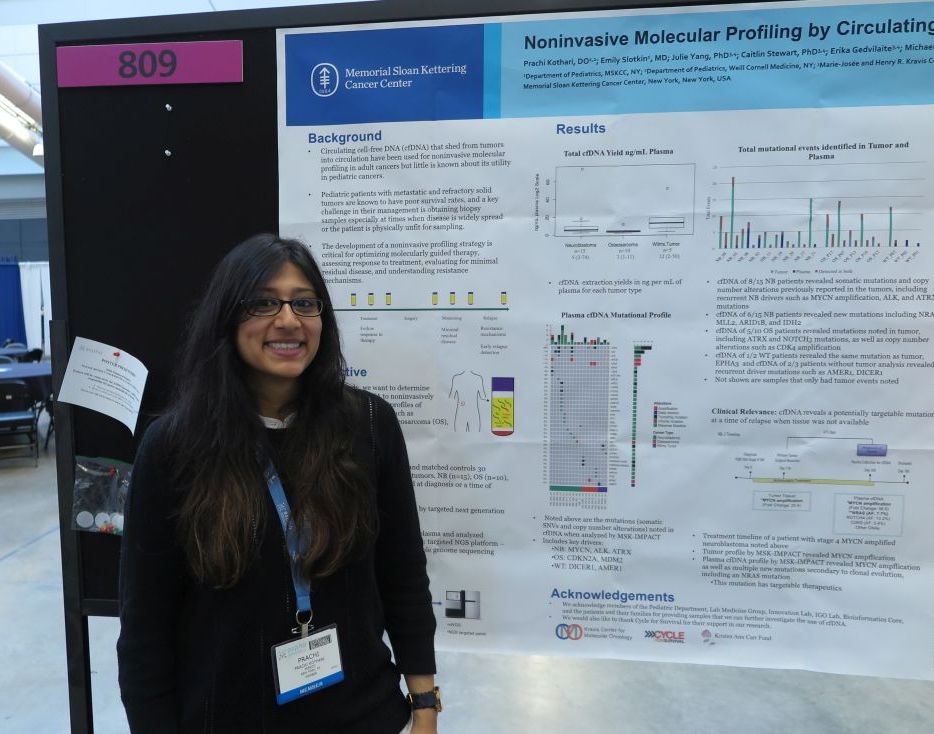
cfDNA reveals targetable mutations in pediatric neuroblastoma, sarcoma
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
REPORTING FROM ASPHO 2018
Key clinical point: Circulating free DNA analysis is a noninvasive method for detecting potential therapeutic targets.
Major finding: cfDNA revealed potentially targetable new mutations in 6 of 15 patients with neuroblastoma.
Study details: Retrospective analysis of tumor and plasma samples in 30 patients with neuroblastoma, osteosarcoma, or Wilms tumor.
Disclosures: The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
Source: Kothari P et al. ASPHO 2018. Abstract #809.
VIDEO: Acromegaly study reveals gender-specific differences
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
REPORTING FROM AACE 2018
Key clinical point: Men with acromegaly present at a younger age, have higher IGF-1 levels, and achieve lower biochemical control rates than do women with the disorder.
Major finding: Men were less likely than women to have surgical remissions or normal IGF-1 levels after adjuvant treatment (72% for men and 89% for women; P = .03).
Study details: A retrospective analysis of 112 patients (54 male, 58 female) operated on by one neurosurgeon during 1994-2016.
Disclosures: The presenter had no disclosures related to the presentation.
Source: Handa T et al. AACE 2018. Abstract #824.
VIDEO: Location of thyroid nodules may predict malignancy
BOSTON – according to the results of a retrospective, single-center study presented at the annual meeting of the American Association of Clinical Endocrinologists.
When nodules were located in the upper pole of the gland, the risk of malignancy was about 4 times higher than it was for nodules at other locations in the gland. Researchers confirmed the association using a multiple logistic regression model with adjustment for age, gender, body mass index, laterality, and number of nodules (odds ratio, 4.6; P = 0.03). The findings are believed to be the first to show an association between location of thyroid nodules on ultrasound and malignancy risk.
The results appear to elevate the value of ultrasound for predicting thyroid malignancy and should affect guidelines for ultrasound classification of thyroid nodules, researcher Fan Zhang, MD, PhD, a fellow at State University of New York, Brooklyn, said in a video interview. “In the future, I would recommend maybe we could consider including the location of thyroid nodules in the guidelines for better predictive value of malignancies,” she said.
Other ultrasound characteristics known to be associated with malignancy include findings of microcalcifications, increased vascularity, and nodules that are taller than they are wide, according to Dr. Zhang.
The retrospective review included data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017. Nearly 80% of the nodules in the review were located in the lower pole of the gland, about 10% were in the upper pole, 7% were in the middle pole, and about 2% were found in the isthmus.
Fourteen nodules, or 7.4%, were found to be malignant, Dr. Zhang and her coauthors said in their presentation. Of those 14 malignancies, 7 were among the 149 nodules in the lower pole, 4 were among the 18 in the upper pole, and 3 were among the 21 in the middle pole.
The anatomy of the thyroid gland may be a factor in why upper pole nodules would be more likely to be associated with malignancy, according to Dr. Zhang. “The veins in the upper lobe are more tortuous compared to in the lower lobe,” she said, noting that slow venous drainage may increase the possibility of developing malignancy.
Dr. Zhang had no relevant disclosures to report.
SOURCE: Zhang F et al. AACE 2018, Abstract 1204.
BOSTON – according to the results of a retrospective, single-center study presented at the annual meeting of the American Association of Clinical Endocrinologists.
When nodules were located in the upper pole of the gland, the risk of malignancy was about 4 times higher than it was for nodules at other locations in the gland. Researchers confirmed the association using a multiple logistic regression model with adjustment for age, gender, body mass index, laterality, and number of nodules (odds ratio, 4.6; P = 0.03). The findings are believed to be the first to show an association between location of thyroid nodules on ultrasound and malignancy risk.
The results appear to elevate the value of ultrasound for predicting thyroid malignancy and should affect guidelines for ultrasound classification of thyroid nodules, researcher Fan Zhang, MD, PhD, a fellow at State University of New York, Brooklyn, said in a video interview. “In the future, I would recommend maybe we could consider including the location of thyroid nodules in the guidelines for better predictive value of malignancies,” she said.
Other ultrasound characteristics known to be associated with malignancy include findings of microcalcifications, increased vascularity, and nodules that are taller than they are wide, according to Dr. Zhang.
The retrospective review included data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017. Nearly 80% of the nodules in the review were located in the lower pole of the gland, about 10% were in the upper pole, 7% were in the middle pole, and about 2% were found in the isthmus.
Fourteen nodules, or 7.4%, were found to be malignant, Dr. Zhang and her coauthors said in their presentation. Of those 14 malignancies, 7 were among the 149 nodules in the lower pole, 4 were among the 18 in the upper pole, and 3 were among the 21 in the middle pole.
The anatomy of the thyroid gland may be a factor in why upper pole nodules would be more likely to be associated with malignancy, according to Dr. Zhang. “The veins in the upper lobe are more tortuous compared to in the lower lobe,” she said, noting that slow venous drainage may increase the possibility of developing malignancy.
Dr. Zhang had no relevant disclosures to report.
SOURCE: Zhang F et al. AACE 2018, Abstract 1204.
BOSTON – according to the results of a retrospective, single-center study presented at the annual meeting of the American Association of Clinical Endocrinologists.
When nodules were located in the upper pole of the gland, the risk of malignancy was about 4 times higher than it was for nodules at other locations in the gland. Researchers confirmed the association using a multiple logistic regression model with adjustment for age, gender, body mass index, laterality, and number of nodules (odds ratio, 4.6; P = 0.03). The findings are believed to be the first to show an association between location of thyroid nodules on ultrasound and malignancy risk.
The results appear to elevate the value of ultrasound for predicting thyroid malignancy and should affect guidelines for ultrasound classification of thyroid nodules, researcher Fan Zhang, MD, PhD, a fellow at State University of New York, Brooklyn, said in a video interview. “In the future, I would recommend maybe we could consider including the location of thyroid nodules in the guidelines for better predictive value of malignancies,” she said.
Other ultrasound characteristics known to be associated with malignancy include findings of microcalcifications, increased vascularity, and nodules that are taller than they are wide, according to Dr. Zhang.
The retrospective review included data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017. Nearly 80% of the nodules in the review were located in the lower pole of the gland, about 10% were in the upper pole, 7% were in the middle pole, and about 2% were found in the isthmus.
Fourteen nodules, or 7.4%, were found to be malignant, Dr. Zhang and her coauthors said in their presentation. Of those 14 malignancies, 7 were among the 149 nodules in the lower pole, 4 were among the 18 in the upper pole, and 3 were among the 21 in the middle pole.
The anatomy of the thyroid gland may be a factor in why upper pole nodules would be more likely to be associated with malignancy, according to Dr. Zhang. “The veins in the upper lobe are more tortuous compared to in the lower lobe,” she said, noting that slow venous drainage may increase the possibility of developing malignancy.
Dr. Zhang had no relevant disclosures to report.
SOURCE: Zhang F et al. AACE 2018, Abstract 1204.
REPORTING FROM AACE 2018
Key clinical point: Thyroid nodules located in the upper pole may be considered a risk factor for malignancy.
Major finding: Assessment by location showed that 28.6% of nodules found in the upper pole were malignant, compared with 4.9% in the lower pole, 18.2% in the middle pole, and 14.3% in the isthmus (odds ratio, 5.8; P = 0.01).
Study details: A retrospective review including data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017.
Disclosures: Dr. Zhang had no relevant disclosures to report.
Source: Zhang F et al. AACE 2018, Abstract 1204.
VIDEO: Move beyond BMI to see obesity as a disease
BOSTON – , W. Timothy Garvey, MD, said.
“The term ‘obesity’ means so many things to different people,” Dr. Garvey explained in a video interview at the annual meeting of the American Association of Clinical Endocrinologists. “It doesn’t tell you what the impact is of excess adiposity on health.”
In fact, obesity meets the criteria needed to be defined as a disease, said Dr. Garvey, who coauthored a 2017 AACE position statement recommending a new diagnostic term for obesity: adiposity-based chronic disease, or ABCD.
“It’s not going to replace the general use of the term ‘obesity,’ of course; but for medical diagnosis, this term does tell you what we’re treating, and why we’re treating it,” noted Dr. Garvey, of the University of Alabama at Birmingham.
Instead of relying on BMI [body mass index], the ABCD model emphasizes a “complications-centric” approach that drives therapeutic decisions, which may include medication.
“A structured lifestyle intervention is the key to therapy, but if we add medications on to any lifestyle intervention, we’re going to get more bang for the buck,” Dr. Garvey explained.
“We’re going to get more weight loss and be able to keep it off for a longer period of time,” he added. “We want that in situations in particular where the patient really has complications. This could be diabetes, it could be prediabetes, it could be obstructive sleep apnea, symptomatic osteoarthritis in the knees, stress incontinence, hypertension – any one of a number of weight-related complications that are really impairing health.”
The five medications approved for chronic management of obesity all have been shown to be safe and effective in clinical trials. But they have different mechanisms of action, different side effect profiles, and different warnings and precautions, Dr. Garvey noted.
Understanding the pharmacology of all five drugs is important to help a specific patient achieve the best outcomes.
“There’s no drug that can be recommended, in a hierarchical sense, as being better than any others across the board in all patients,” Dr. Garvey explained. “We really need to individualize therapy based on their side effect profile and their types of complications that present with the patient.”
Endocrinologists can be particularly helpful in incorporating weight loss therapy into the overall therapeutic plan for refractory cases, he said, or in patients significantly burdened with metabolic complications, including dysglycemia, diabetes, hypertriglyceridemia, and nonalcoholic fatty liver disease.
Primary care physicians, advanced practice clinicians, dietitians, and others are needed on the team to engineer a successful lifestyle intervention for the obese patient. However, Dr. Garvey emphasized that the endocrinology subspecialty encompasses not only endocrinology and diabetes, but also metabolism.
“We need to take the lead here,” Dr. Garvey said. “Obesity is the most common metabolic disease on the planet.”
Dr. Garvey reported disclosures related to Janssen, Novo Nordisk, and Sanofi.
BOSTON – , W. Timothy Garvey, MD, said.
“The term ‘obesity’ means so many things to different people,” Dr. Garvey explained in a video interview at the annual meeting of the American Association of Clinical Endocrinologists. “It doesn’t tell you what the impact is of excess adiposity on health.”
In fact, obesity meets the criteria needed to be defined as a disease, said Dr. Garvey, who coauthored a 2017 AACE position statement recommending a new diagnostic term for obesity: adiposity-based chronic disease, or ABCD.
“It’s not going to replace the general use of the term ‘obesity,’ of course; but for medical diagnosis, this term does tell you what we’re treating, and why we’re treating it,” noted Dr. Garvey, of the University of Alabama at Birmingham.
Instead of relying on BMI [body mass index], the ABCD model emphasizes a “complications-centric” approach that drives therapeutic decisions, which may include medication.
“A structured lifestyle intervention is the key to therapy, but if we add medications on to any lifestyle intervention, we’re going to get more bang for the buck,” Dr. Garvey explained.
“We’re going to get more weight loss and be able to keep it off for a longer period of time,” he added. “We want that in situations in particular where the patient really has complications. This could be diabetes, it could be prediabetes, it could be obstructive sleep apnea, symptomatic osteoarthritis in the knees, stress incontinence, hypertension – any one of a number of weight-related complications that are really impairing health.”
The five medications approved for chronic management of obesity all have been shown to be safe and effective in clinical trials. But they have different mechanisms of action, different side effect profiles, and different warnings and precautions, Dr. Garvey noted.
Understanding the pharmacology of all five drugs is important to help a specific patient achieve the best outcomes.
“There’s no drug that can be recommended, in a hierarchical sense, as being better than any others across the board in all patients,” Dr. Garvey explained. “We really need to individualize therapy based on their side effect profile and their types of complications that present with the patient.”
Endocrinologists can be particularly helpful in incorporating weight loss therapy into the overall therapeutic plan for refractory cases, he said, or in patients significantly burdened with metabolic complications, including dysglycemia, diabetes, hypertriglyceridemia, and nonalcoholic fatty liver disease.
Primary care physicians, advanced practice clinicians, dietitians, and others are needed on the team to engineer a successful lifestyle intervention for the obese patient. However, Dr. Garvey emphasized that the endocrinology subspecialty encompasses not only endocrinology and diabetes, but also metabolism.
“We need to take the lead here,” Dr. Garvey said. “Obesity is the most common metabolic disease on the planet.”
Dr. Garvey reported disclosures related to Janssen, Novo Nordisk, and Sanofi.
BOSTON – , W. Timothy Garvey, MD, said.
“The term ‘obesity’ means so many things to different people,” Dr. Garvey explained in a video interview at the annual meeting of the American Association of Clinical Endocrinologists. “It doesn’t tell you what the impact is of excess adiposity on health.”
In fact, obesity meets the criteria needed to be defined as a disease, said Dr. Garvey, who coauthored a 2017 AACE position statement recommending a new diagnostic term for obesity: adiposity-based chronic disease, or ABCD.
“It’s not going to replace the general use of the term ‘obesity,’ of course; but for medical diagnosis, this term does tell you what we’re treating, and why we’re treating it,” noted Dr. Garvey, of the University of Alabama at Birmingham.
Instead of relying on BMI [body mass index], the ABCD model emphasizes a “complications-centric” approach that drives therapeutic decisions, which may include medication.
“A structured lifestyle intervention is the key to therapy, but if we add medications on to any lifestyle intervention, we’re going to get more bang for the buck,” Dr. Garvey explained.
“We’re going to get more weight loss and be able to keep it off for a longer period of time,” he added. “We want that in situations in particular where the patient really has complications. This could be diabetes, it could be prediabetes, it could be obstructive sleep apnea, symptomatic osteoarthritis in the knees, stress incontinence, hypertension – any one of a number of weight-related complications that are really impairing health.”
The five medications approved for chronic management of obesity all have been shown to be safe and effective in clinical trials. But they have different mechanisms of action, different side effect profiles, and different warnings and precautions, Dr. Garvey noted.
Understanding the pharmacology of all five drugs is important to help a specific patient achieve the best outcomes.
“There’s no drug that can be recommended, in a hierarchical sense, as being better than any others across the board in all patients,” Dr. Garvey explained. “We really need to individualize therapy based on their side effect profile and their types of complications that present with the patient.”
Endocrinologists can be particularly helpful in incorporating weight loss therapy into the overall therapeutic plan for refractory cases, he said, or in patients significantly burdened with metabolic complications, including dysglycemia, diabetes, hypertriglyceridemia, and nonalcoholic fatty liver disease.
Primary care physicians, advanced practice clinicians, dietitians, and others are needed on the team to engineer a successful lifestyle intervention for the obese patient. However, Dr. Garvey emphasized that the endocrinology subspecialty encompasses not only endocrinology and diabetes, but also metabolism.
“We need to take the lead here,” Dr. Garvey said. “Obesity is the most common metabolic disease on the planet.”
Dr. Garvey reported disclosures related to Janssen, Novo Nordisk, and Sanofi.
REPORTING FROM AACE 2018
Complete MUS mesh removal not linked to incontinence
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: There was no association between postsurgical incontinence risk and complete mesh removal.
Major finding: In patients without presurgical urodynamic incontinence, only obesity (OR, 4.74) and postmenopausal status (OR, 3.78) were linked to incontinence risk.
Study details: A retrospective analysis of 233 patients.
Disclosures: The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
Source: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.