User login
VIDEO: Real-world findings on hybrid closed-loop insulin system
BOSTON – Real-world experience with the Medtronic MiniMed 670G, a hybrid closed-loop insulin delivery system, showed the device was associated with improved average glucose readings and more time in euglycemia in 26 patients with type 1 diabetes.
The findings go beyond the safety data from the clinical trial of the MiniMed 670G system, Kathryn Weaver, MD, of the University of Washington, Seattle, and her colleagues reported in a poster presented at the annual meeting of the American Association of Clinical Endocrinologists.

The clinical trial included a 2-week run-in period during which the system was used in manual mode before it was switched to automated mode. Mean sensor glucose readings for participants went from 150.2 mg/dL during run-in to 150.8 mg/dL at the end of 3 months, which was not a statistically significant difference (JAMA. 2016;316[13]:1407-8).
In the real-world study, average sensor glucose readings dropped from a mean 169.46 mg/dL at baseline to 157.08 mg/dL at the end of the 3-month study period (P = .05). Also, the time spent with blood glucose levels greater than 180 mg/dL fell from 26.5% to 20% (P = .007), while the amount of time with glucose readings between 70 and 180 mg/dL increased from 61.7% to 71.1% (P = .02). Periods of hypoglycemia and severe hypoglycemia were already low at baseline and did not change, Dr. Weaver said.
“It is important to note that the initial pivotal trial was a study designed to evaluate safety not a study designed to evaluate effectiveness. And the [trial] group did demonstrate safety; they had a very significant reduction in the amount of hypoglycemia” with the pump, said Dr. Weaver. “We did not show a significant reduction in hypoglycemia in our [real-world] group, likely because we had a very low rate of hypoglycemia going into the study.”
Two of the study coauthors are employees of Medtronic, which manufactures the MiniMed 670G insulin pump/continuous glucose monitor. Medtronic did not provide funding support for the study or provide the closed-loop systems, and Dr. Weaver reported that she had no relevant financial disclosures.
SOURCE: Weaver K et al. AACE 2018, Abstract 210.
BOSTON – Real-world experience with the Medtronic MiniMed 670G, a hybrid closed-loop insulin delivery system, showed the device was associated with improved average glucose readings and more time in euglycemia in 26 patients with type 1 diabetes.
The findings go beyond the safety data from the clinical trial of the MiniMed 670G system, Kathryn Weaver, MD, of the University of Washington, Seattle, and her colleagues reported in a poster presented at the annual meeting of the American Association of Clinical Endocrinologists.

The clinical trial included a 2-week run-in period during which the system was used in manual mode before it was switched to automated mode. Mean sensor glucose readings for participants went from 150.2 mg/dL during run-in to 150.8 mg/dL at the end of 3 months, which was not a statistically significant difference (JAMA. 2016;316[13]:1407-8).
In the real-world study, average sensor glucose readings dropped from a mean 169.46 mg/dL at baseline to 157.08 mg/dL at the end of the 3-month study period (P = .05). Also, the time spent with blood glucose levels greater than 180 mg/dL fell from 26.5% to 20% (P = .007), while the amount of time with glucose readings between 70 and 180 mg/dL increased from 61.7% to 71.1% (P = .02). Periods of hypoglycemia and severe hypoglycemia were already low at baseline and did not change, Dr. Weaver said.
“It is important to note that the initial pivotal trial was a study designed to evaluate safety not a study designed to evaluate effectiveness. And the [trial] group did demonstrate safety; they had a very significant reduction in the amount of hypoglycemia” with the pump, said Dr. Weaver. “We did not show a significant reduction in hypoglycemia in our [real-world] group, likely because we had a very low rate of hypoglycemia going into the study.”
Two of the study coauthors are employees of Medtronic, which manufactures the MiniMed 670G insulin pump/continuous glucose monitor. Medtronic did not provide funding support for the study or provide the closed-loop systems, and Dr. Weaver reported that she had no relevant financial disclosures.
SOURCE: Weaver K et al. AACE 2018, Abstract 210.
BOSTON – Real-world experience with the Medtronic MiniMed 670G, a hybrid closed-loop insulin delivery system, showed the device was associated with improved average glucose readings and more time in euglycemia in 26 patients with type 1 diabetes.
The findings go beyond the safety data from the clinical trial of the MiniMed 670G system, Kathryn Weaver, MD, of the University of Washington, Seattle, and her colleagues reported in a poster presented at the annual meeting of the American Association of Clinical Endocrinologists.

The clinical trial included a 2-week run-in period during which the system was used in manual mode before it was switched to automated mode. Mean sensor glucose readings for participants went from 150.2 mg/dL during run-in to 150.8 mg/dL at the end of 3 months, which was not a statistically significant difference (JAMA. 2016;316[13]:1407-8).
In the real-world study, average sensor glucose readings dropped from a mean 169.46 mg/dL at baseline to 157.08 mg/dL at the end of the 3-month study period (P = .05). Also, the time spent with blood glucose levels greater than 180 mg/dL fell from 26.5% to 20% (P = .007), while the amount of time with glucose readings between 70 and 180 mg/dL increased from 61.7% to 71.1% (P = .02). Periods of hypoglycemia and severe hypoglycemia were already low at baseline and did not change, Dr. Weaver said.
“It is important to note that the initial pivotal trial was a study designed to evaluate safety not a study designed to evaluate effectiveness. And the [trial] group did demonstrate safety; they had a very significant reduction in the amount of hypoglycemia” with the pump, said Dr. Weaver. “We did not show a significant reduction in hypoglycemia in our [real-world] group, likely because we had a very low rate of hypoglycemia going into the study.”
Two of the study coauthors are employees of Medtronic, which manufactures the MiniMed 670G insulin pump/continuous glucose monitor. Medtronic did not provide funding support for the study or provide the closed-loop systems, and Dr. Weaver reported that she had no relevant financial disclosures.
SOURCE: Weaver K et al. AACE 2018, Abstract 210.
REPORTING FROM AACE 2018
Vestibular/oculomotor component of concussion warrants more attention
NEW ORLEANS – Vestibular and oculomotor impairment is increasingly recognized as a common, underappreciated, and yet treatable aspect of sports concussions, Gary W. Dorshimer, MD, said at the annual meeting of the American College of Physicians.
A major advance in the diagnosis and treatment of this form of impairment has been achieved by researchers at the University of Pittsburgh Medical Center sports medicine concussion program.
“The Pitt group has come up with a nice exam to assess this part of the concussion injury, which doesn’t affect your memory, it doesn’t affect your cognition, it affects what I’ve found to be the thing that takes the longest to get better: the oculomotor/vestibular mechanism,” explained Dr. Dorshimer, chief of general internal medicine at Penn Medicine, Philadelphia, and team physician for the Philadelphia Flyers professional ice hockey team.
The exam, which the Pitt group has described in full detail (Am J Sports Med. 2014;42(10):2479-86), is known as the Vestibular/Ocular Motor Screening assessment, or VOMS. The tool has filled an unmet need in sports medicine, he said. It takes only a few minutes for a physician to perform. The rating scale assesses visual motion sensitivity, smooth eye pursuits, horizontal and vertical saccades, the vestibular ocular reflex, and convergence. Positive findings warrant specialized referral for targeted rehabilitation using visual-ocular and vestibular therapies.
The symptoms of sports concussion–related oculomotor/vestibular impairment may include nausea, vertigo, dizziness, blurred or double vision, difficulty tracking a moving target, and discomfort in busy environments. These symptoms often translate to difficulty reading and academic problems, which historically often were misinterpreted as cognitive impairments.
It’s estimated that oculomotor/vestibular impairment occurs in roughly 60% of sports concussions. These vestibular and/or vision symptoms are associated with protracted recovery. And preliminary evidence demonstrates that targeted physical therapies are effective in speeding recovery.
“It’s so important to be able to find this [impairment] because it’s something you can do something about. We find that when these things are off and people work on them, they get better. That’s why so many people in the field are now saying that if a patient works hard, does the rehabilitation, the majority of them are going to get better. And they won’t get better unless they press forward,” the internist said.
The VOMS screen is simple to perform. It entails tasks such as convergence testing, in which the physician moves a finger or pen steadily closer to the patient’s face; if the patient reports that the single object has turned into two at a distance of more than 6 cm, that’s a positive result indicative of convergence insufficiency.
In another task, the physician hold his two index fingers apart and has the patients move their eyes from finger to finger while holding their heads still.
“I’m not that interested in whether they’re catching the tips of fingers, I’m interested in if they can go fast, and can they go faster if I challenge them, or do they stop doing it? When people with ocular vestibular dysfunction start doing this task, they’re going to slow down. They can’t keep it up because it’s so unpleasant. It really bothers them a lot,” Dr. Dorshimer observed.
In his experience, another key element in a smooth and successful recovery from sports concussions, in addition to getting skilled help for vestibular/oculomotor impairment, if present, is to encourage a positive attitude.
“If you think you’re going to get CTE [chronic traumatic encephalopathy] when you get older because you got waffled a bit in sport, that’s just such a negative attitude. I mean, you can’t lie to them: We don’t know. But I take care of a ton of retired athletes who don’t have CTE. Maybe they’re going to have some tangles in their brains, but they don’t have it clinically. So you want them to keep a positive attitude,” he emphasized.
“CTE was around when your parents and grandparents were jocks. They went out on the playground and pummeled each other every day after school. There are probably all kinds of factors involved in CTE: the number of concussions, hereditary factors, alcohol, drugs. No one really knows yet,” he said.
Dr. Dorshimer reported having no financial conflicts regarding his presentation on the athlete as patient.
NEW ORLEANS – Vestibular and oculomotor impairment is increasingly recognized as a common, underappreciated, and yet treatable aspect of sports concussions, Gary W. Dorshimer, MD, said at the annual meeting of the American College of Physicians.
A major advance in the diagnosis and treatment of this form of impairment has been achieved by researchers at the University of Pittsburgh Medical Center sports medicine concussion program.
“The Pitt group has come up with a nice exam to assess this part of the concussion injury, which doesn’t affect your memory, it doesn’t affect your cognition, it affects what I’ve found to be the thing that takes the longest to get better: the oculomotor/vestibular mechanism,” explained Dr. Dorshimer, chief of general internal medicine at Penn Medicine, Philadelphia, and team physician for the Philadelphia Flyers professional ice hockey team.
The exam, which the Pitt group has described in full detail (Am J Sports Med. 2014;42(10):2479-86), is known as the Vestibular/Ocular Motor Screening assessment, or VOMS. The tool has filled an unmet need in sports medicine, he said. It takes only a few minutes for a physician to perform. The rating scale assesses visual motion sensitivity, smooth eye pursuits, horizontal and vertical saccades, the vestibular ocular reflex, and convergence. Positive findings warrant specialized referral for targeted rehabilitation using visual-ocular and vestibular therapies.
The symptoms of sports concussion–related oculomotor/vestibular impairment may include nausea, vertigo, dizziness, blurred or double vision, difficulty tracking a moving target, and discomfort in busy environments. These symptoms often translate to difficulty reading and academic problems, which historically often were misinterpreted as cognitive impairments.
It’s estimated that oculomotor/vestibular impairment occurs in roughly 60% of sports concussions. These vestibular and/or vision symptoms are associated with protracted recovery. And preliminary evidence demonstrates that targeted physical therapies are effective in speeding recovery.
“It’s so important to be able to find this [impairment] because it’s something you can do something about. We find that when these things are off and people work on them, they get better. That’s why so many people in the field are now saying that if a patient works hard, does the rehabilitation, the majority of them are going to get better. And they won’t get better unless they press forward,” the internist said.
The VOMS screen is simple to perform. It entails tasks such as convergence testing, in which the physician moves a finger or pen steadily closer to the patient’s face; if the patient reports that the single object has turned into two at a distance of more than 6 cm, that’s a positive result indicative of convergence insufficiency.
In another task, the physician hold his two index fingers apart and has the patients move their eyes from finger to finger while holding their heads still.
“I’m not that interested in whether they’re catching the tips of fingers, I’m interested in if they can go fast, and can they go faster if I challenge them, or do they stop doing it? When people with ocular vestibular dysfunction start doing this task, they’re going to slow down. They can’t keep it up because it’s so unpleasant. It really bothers them a lot,” Dr. Dorshimer observed.
In his experience, another key element in a smooth and successful recovery from sports concussions, in addition to getting skilled help for vestibular/oculomotor impairment, if present, is to encourage a positive attitude.
“If you think you’re going to get CTE [chronic traumatic encephalopathy] when you get older because you got waffled a bit in sport, that’s just such a negative attitude. I mean, you can’t lie to them: We don’t know. But I take care of a ton of retired athletes who don’t have CTE. Maybe they’re going to have some tangles in their brains, but they don’t have it clinically. So you want them to keep a positive attitude,” he emphasized.
“CTE was around when your parents and grandparents were jocks. They went out on the playground and pummeled each other every day after school. There are probably all kinds of factors involved in CTE: the number of concussions, hereditary factors, alcohol, drugs. No one really knows yet,” he said.
Dr. Dorshimer reported having no financial conflicts regarding his presentation on the athlete as patient.
NEW ORLEANS – Vestibular and oculomotor impairment is increasingly recognized as a common, underappreciated, and yet treatable aspect of sports concussions, Gary W. Dorshimer, MD, said at the annual meeting of the American College of Physicians.
A major advance in the diagnosis and treatment of this form of impairment has been achieved by researchers at the University of Pittsburgh Medical Center sports medicine concussion program.
“The Pitt group has come up with a nice exam to assess this part of the concussion injury, which doesn’t affect your memory, it doesn’t affect your cognition, it affects what I’ve found to be the thing that takes the longest to get better: the oculomotor/vestibular mechanism,” explained Dr. Dorshimer, chief of general internal medicine at Penn Medicine, Philadelphia, and team physician for the Philadelphia Flyers professional ice hockey team.
The exam, which the Pitt group has described in full detail (Am J Sports Med. 2014;42(10):2479-86), is known as the Vestibular/Ocular Motor Screening assessment, or VOMS. The tool has filled an unmet need in sports medicine, he said. It takes only a few minutes for a physician to perform. The rating scale assesses visual motion sensitivity, smooth eye pursuits, horizontal and vertical saccades, the vestibular ocular reflex, and convergence. Positive findings warrant specialized referral for targeted rehabilitation using visual-ocular and vestibular therapies.
The symptoms of sports concussion–related oculomotor/vestibular impairment may include nausea, vertigo, dizziness, blurred or double vision, difficulty tracking a moving target, and discomfort in busy environments. These symptoms often translate to difficulty reading and academic problems, which historically often were misinterpreted as cognitive impairments.
It’s estimated that oculomotor/vestibular impairment occurs in roughly 60% of sports concussions. These vestibular and/or vision symptoms are associated with protracted recovery. And preliminary evidence demonstrates that targeted physical therapies are effective in speeding recovery.
“It’s so important to be able to find this [impairment] because it’s something you can do something about. We find that when these things are off and people work on them, they get better. That’s why so many people in the field are now saying that if a patient works hard, does the rehabilitation, the majority of them are going to get better. And they won’t get better unless they press forward,” the internist said.
The VOMS screen is simple to perform. It entails tasks such as convergence testing, in which the physician moves a finger or pen steadily closer to the patient’s face; if the patient reports that the single object has turned into two at a distance of more than 6 cm, that’s a positive result indicative of convergence insufficiency.
In another task, the physician hold his two index fingers apart and has the patients move their eyes from finger to finger while holding their heads still.
“I’m not that interested in whether they’re catching the tips of fingers, I’m interested in if they can go fast, and can they go faster if I challenge them, or do they stop doing it? When people with ocular vestibular dysfunction start doing this task, they’re going to slow down. They can’t keep it up because it’s so unpleasant. It really bothers them a lot,” Dr. Dorshimer observed.
In his experience, another key element in a smooth and successful recovery from sports concussions, in addition to getting skilled help for vestibular/oculomotor impairment, if present, is to encourage a positive attitude.
“If you think you’re going to get CTE [chronic traumatic encephalopathy] when you get older because you got waffled a bit in sport, that’s just such a negative attitude. I mean, you can’t lie to them: We don’t know. But I take care of a ton of retired athletes who don’t have CTE. Maybe they’re going to have some tangles in their brains, but they don’t have it clinically. So you want them to keep a positive attitude,” he emphasized.
“CTE was around when your parents and grandparents were jocks. They went out on the playground and pummeled each other every day after school. There are probably all kinds of factors involved in CTE: the number of concussions, hereditary factors, alcohol, drugs. No one really knows yet,” he said.
Dr. Dorshimer reported having no financial conflicts regarding his presentation on the athlete as patient.
REPORTING FROM ACP INTERNAL MEDICINE
HBV birth dose predicts vaccine adherence
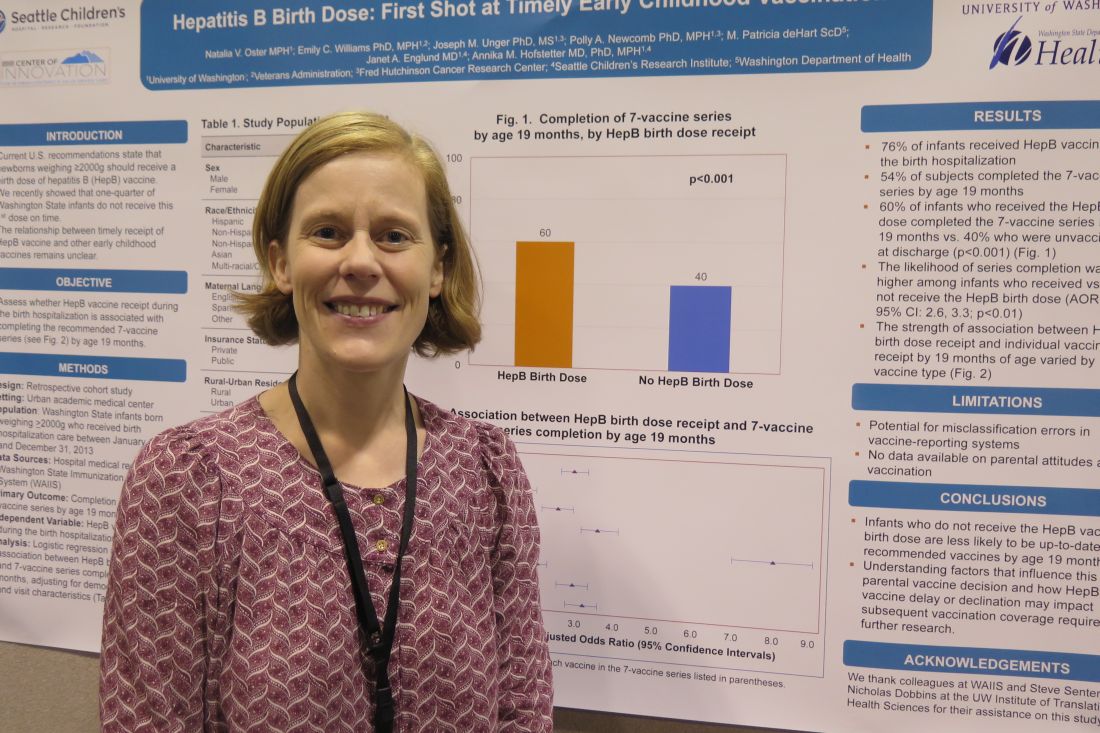
TORONTO – Infants who do not receive the hepatitis B vaccine birth dose are less likely to be up-to-date recipients of recommended vaccines by 19 months, based on results from a retrospective study of more than 9,000 infants.
“As pediatricians, we should be mindful of that when we are meeting families after the birth hospitalization and start a conversation at that point around vaccines,” one of the study authors, Annika M. Hofstetter, MD, PhD, said in an interview at the Pediatric Academic Societies meeting.
Of the 9,080 infants, 51% were male, 49% were non-Hispanic white, 56% were covered by public health insurance, and 47% stayed in the hospital for 48 hours or longer. The researchers reported that 76% infants received the HBV during the birth hospitalization, and 54% of subjects completed the seven-vaccine series by age 19 months. They also found that (P less than .001). Infants who received the HBV birth dose were 2.9 times more likely to complete the seven-vaccine series by age 19 months, compared with those who did not receive the HBV birth dose.
“Parents are making their first vaccine decision during that birth hospitalization,” said Dr. Hofstetter, who also conducts immunization research studies at Seattle Children’s Research Institute. “It’s unclear what underlies this decision, such as specific parent concerns or the way in which we as providers in the hospital are communicating vaccine information to the families. It’s telling, and it will be interesting to further explore the factors that are determining whether a family gets the vaccine during the birth hospitalization or not, and how we as a pediatric community can start having effective vaccine conversations earlier.”
She acknowledged certain limitations of the study, including the potential for misclassification errors in vaccine reporting systems and the fact that no data were available on parental attitudes about vaccination. The researchers reported having no financial disclosures.
TORONTO – Infants who do not receive the hepatitis B vaccine birth dose are less likely to be up-to-date recipients of recommended vaccines by 19 months, based on results from a retrospective study of more than 9,000 infants.
“As pediatricians, we should be mindful of that when we are meeting families after the birth hospitalization and start a conversation at that point around vaccines,” one of the study authors, Annika M. Hofstetter, MD, PhD, said in an interview at the Pediatric Academic Societies meeting.
Of the 9,080 infants, 51% were male, 49% were non-Hispanic white, 56% were covered by public health insurance, and 47% stayed in the hospital for 48 hours or longer. The researchers reported that 76% infants received the HBV during the birth hospitalization, and 54% of subjects completed the seven-vaccine series by age 19 months. They also found that (P less than .001). Infants who received the HBV birth dose were 2.9 times more likely to complete the seven-vaccine series by age 19 months, compared with those who did not receive the HBV birth dose.
“Parents are making their first vaccine decision during that birth hospitalization,” said Dr. Hofstetter, who also conducts immunization research studies at Seattle Children’s Research Institute. “It’s unclear what underlies this decision, such as specific parent concerns or the way in which we as providers in the hospital are communicating vaccine information to the families. It’s telling, and it will be interesting to further explore the factors that are determining whether a family gets the vaccine during the birth hospitalization or not, and how we as a pediatric community can start having effective vaccine conversations earlier.”
She acknowledged certain limitations of the study, including the potential for misclassification errors in vaccine reporting systems and the fact that no data were available on parental attitudes about vaccination. The researchers reported having no financial disclosures.
TORONTO – Infants who do not receive the hepatitis B vaccine birth dose are less likely to be up-to-date recipients of recommended vaccines by 19 months, based on results from a retrospective study of more than 9,000 infants.
“As pediatricians, we should be mindful of that when we are meeting families after the birth hospitalization and start a conversation at that point around vaccines,” one of the study authors, Annika M. Hofstetter, MD, PhD, said in an interview at the Pediatric Academic Societies meeting.
Of the 9,080 infants, 51% were male, 49% were non-Hispanic white, 56% were covered by public health insurance, and 47% stayed in the hospital for 48 hours or longer. The researchers reported that 76% infants received the HBV during the birth hospitalization, and 54% of subjects completed the seven-vaccine series by age 19 months. They also found that (P less than .001). Infants who received the HBV birth dose were 2.9 times more likely to complete the seven-vaccine series by age 19 months, compared with those who did not receive the HBV birth dose.
“Parents are making their first vaccine decision during that birth hospitalization,” said Dr. Hofstetter, who also conducts immunization research studies at Seattle Children’s Research Institute. “It’s unclear what underlies this decision, such as specific parent concerns or the way in which we as providers in the hospital are communicating vaccine information to the families. It’s telling, and it will be interesting to further explore the factors that are determining whether a family gets the vaccine during the birth hospitalization or not, and how we as a pediatric community can start having effective vaccine conversations earlier.”
She acknowledged certain limitations of the study, including the potential for misclassification errors in vaccine reporting systems and the fact that no data were available on parental attitudes about vaccination. The researchers reported having no financial disclosures.
AT PAS 2018
Key clinical point: Likelihood of completing the 7-vaccine series at 19 months was higher among infants who received the HBV birth dose.
Major finding: Infants who received the HBV birth dose were 2.9 times more likely to complete the 7-vaccine series by age 19 months, compared with those who did not receive the HBV birth dose.
Study details: A retrospective review of 9,080 infants born weighing at least 2,000 grams who received hospitalization care between January 1, 2008 and December 31, 2013.
Disclosures: The researchers reported having no financial disclosures.
Behavioral sleep intervention linked to sleep improvement in infants
(BSI) in a real-world setting, Sarah M. Honaker, PhD, of Indiana University, Indianapolis, and her associates, reported in the Journal of Pediatrics.
In a study of 652 parents who participated, parents started BSI when their infants were as young as less than 1 month of age and as late as 18 months of age. Most parents started BSI at 3-5 months.
Crying generally was greatest the first night, occurring in 45% of cases when all BSI approaches were considered. It lasted a mean 43 minutes, which dropped significantly after 1 week to a mean 9 minutes (P less than .001). Crying was considered most intense (on a 1-5 scale) on the initial night of BSI, a mean 4.42, and this “was equally true for all of the BSI approaches,” Dr. Honaker and her colleagues wrote.
In most cases, the parents’ first attempt at BSI worked (83%). Success varied by BSI approach, with the highest first attempt success rate in the unmodified extinction group (90%), followed by parental presence without support (83%), modified extinction (81%), and parental presence with support (65%). Eventually, 27% of parents were successful with a different approach than the one with which they started. Most commonly, they changed from modified extinction to unmodified extinction (66% of those who changed approaches).
“The majority of parents report successfully implementing BSI at a variety of ages across infancy, primarily using extinction-based approaches,” the researchers concluded. “Few significant differences were found between approaches, suggesting that health providers should offer parents options for BSI implementation.”
SOURCE: Honaker SM et al., J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.04.009.
(BSI) in a real-world setting, Sarah M. Honaker, PhD, of Indiana University, Indianapolis, and her associates, reported in the Journal of Pediatrics.
In a study of 652 parents who participated, parents started BSI when their infants were as young as less than 1 month of age and as late as 18 months of age. Most parents started BSI at 3-5 months.
Crying generally was greatest the first night, occurring in 45% of cases when all BSI approaches were considered. It lasted a mean 43 minutes, which dropped significantly after 1 week to a mean 9 minutes (P less than .001). Crying was considered most intense (on a 1-5 scale) on the initial night of BSI, a mean 4.42, and this “was equally true for all of the BSI approaches,” Dr. Honaker and her colleagues wrote.
In most cases, the parents’ first attempt at BSI worked (83%). Success varied by BSI approach, with the highest first attempt success rate in the unmodified extinction group (90%), followed by parental presence without support (83%), modified extinction (81%), and parental presence with support (65%). Eventually, 27% of parents were successful with a different approach than the one with which they started. Most commonly, they changed from modified extinction to unmodified extinction (66% of those who changed approaches).
“The majority of parents report successfully implementing BSI at a variety of ages across infancy, primarily using extinction-based approaches,” the researchers concluded. “Few significant differences were found between approaches, suggesting that health providers should offer parents options for BSI implementation.”
SOURCE: Honaker SM et al., J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.04.009.
(BSI) in a real-world setting, Sarah M. Honaker, PhD, of Indiana University, Indianapolis, and her associates, reported in the Journal of Pediatrics.
In a study of 652 parents who participated, parents started BSI when their infants were as young as less than 1 month of age and as late as 18 months of age. Most parents started BSI at 3-5 months.
Crying generally was greatest the first night, occurring in 45% of cases when all BSI approaches were considered. It lasted a mean 43 minutes, which dropped significantly after 1 week to a mean 9 minutes (P less than .001). Crying was considered most intense (on a 1-5 scale) on the initial night of BSI, a mean 4.42, and this “was equally true for all of the BSI approaches,” Dr. Honaker and her colleagues wrote.
In most cases, the parents’ first attempt at BSI worked (83%). Success varied by BSI approach, with the highest first attempt success rate in the unmodified extinction group (90%), followed by parental presence without support (83%), modified extinction (81%), and parental presence with support (65%). Eventually, 27% of parents were successful with a different approach than the one with which they started. Most commonly, they changed from modified extinction to unmodified extinction (66% of those who changed approaches).
“The majority of parents report successfully implementing BSI at a variety of ages across infancy, primarily using extinction-based approaches,” the researchers concluded. “Few significant differences were found between approaches, suggesting that health providers should offer parents options for BSI implementation.”
SOURCE: Honaker SM et al., J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.04.009.
FROM THE JOURNAL OF PEDIATRICS
VIDEO: Let clinical scenario, not imaging, guide sarcoidosis treatment
SANDESTIN, FLA. – Don’t be a slave to imaging when evaluating the patient with sarcoidosis.
“Sometimes, the worst-looking patients [on imaging] have the best prognosis,” Daniel Culver, DO, said at the annual Congress of Clinical Rheumatology. Patients with Löfgren’s syndrome are a very good example of this tenet, he said in an interview. Scans can look alarming, with multiple widespread granulomas. But Löfgren’s is generally a benign condition, despite its threatening mien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Instead of imaging, “Let two things drive your decision to treat: danger to an organ, and quality of life,” said Dr. Culver, a pulmonologist and director of the Sarcoidosis Center of Excellence at the Cleveland Clinic in Ohio; he is also president of the World Association for Sarcoidosis.
He agrees with a decision schema published in 2015 (Clin Chest Med. 2015;36[4]:751-67).
Six factors weigh in favor of treatment:
- Symptomatic disease.
- Impaired organ function.
- Disease endangering an organ.
- Progressive disease.
- Clear-cut disease activity.
- Low likelihood of remission.
These must be balanced – with patient input as the fulcrum – against five factors that favor conservative management:
- Minimal symptoms.
- Good organ function.
- Low risk of danger to organs.
- Inactive disease.
- Higher likelihood of remission.
The decision to embark on a treatment program, usually starting with a steroid-based regimen, can’t be taken lightly, Dr. Culver said. A 2017 study showed that steroids pose a cumulative risk of toxicities for sarcoidosis patients (Respir Med. 2017 Nov;132:9-14). Patients who started steroids faced more than a doubling in the risk of a toxic side effect by 96 months when compared with those who didn’t. But even short-term steroid use increased the risk of a toxicity, Dr. Culver said. The study noted that problems can begin to occur in as little as 1 month, at a cumulative dose as low as 1 g.
For patients who fall onto the “treat” side of the risk teeter-totter, Dr. Culver recommended starting with an initial course of prednisone at 20-30 mg daily for no more than 4 weeks. Responders can taper to less than 10 mg/day. Those who continue to do well can maintain low-dose prednisone for up to 12 months and then complete the taper. Patients who relapse can add an immune modulator (methotrexate, azathioprine, leflunomide, or mycophenolate).
Those who have an inadequate response to the initial prednisone course should then get an immune modulator. If they do well, that can be maintained; a second modulator can be brought on board if necessary.
For those who don’t respond at all to the initial prednisone course, it’s necessary to proceed immediately to an immunosuppressive regimen to prevent irreversible fibrosis.
Dr. Culver noted associations with multiple pharmaceutical companies, but said none were relevant to his talk.
SOURCE: Culver D. CCR 2018.
SANDESTIN, FLA. – Don’t be a slave to imaging when evaluating the patient with sarcoidosis.
“Sometimes, the worst-looking patients [on imaging] have the best prognosis,” Daniel Culver, DO, said at the annual Congress of Clinical Rheumatology. Patients with Löfgren’s syndrome are a very good example of this tenet, he said in an interview. Scans can look alarming, with multiple widespread granulomas. But Löfgren’s is generally a benign condition, despite its threatening mien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Instead of imaging, “Let two things drive your decision to treat: danger to an organ, and quality of life,” said Dr. Culver, a pulmonologist and director of the Sarcoidosis Center of Excellence at the Cleveland Clinic in Ohio; he is also president of the World Association for Sarcoidosis.
He agrees with a decision schema published in 2015 (Clin Chest Med. 2015;36[4]:751-67).
Six factors weigh in favor of treatment:
- Symptomatic disease.
- Impaired organ function.
- Disease endangering an organ.
- Progressive disease.
- Clear-cut disease activity.
- Low likelihood of remission.
These must be balanced – with patient input as the fulcrum – against five factors that favor conservative management:
- Minimal symptoms.
- Good organ function.
- Low risk of danger to organs.
- Inactive disease.
- Higher likelihood of remission.
The decision to embark on a treatment program, usually starting with a steroid-based regimen, can’t be taken lightly, Dr. Culver said. A 2017 study showed that steroids pose a cumulative risk of toxicities for sarcoidosis patients (Respir Med. 2017 Nov;132:9-14). Patients who started steroids faced more than a doubling in the risk of a toxic side effect by 96 months when compared with those who didn’t. But even short-term steroid use increased the risk of a toxicity, Dr. Culver said. The study noted that problems can begin to occur in as little as 1 month, at a cumulative dose as low as 1 g.
For patients who fall onto the “treat” side of the risk teeter-totter, Dr. Culver recommended starting with an initial course of prednisone at 20-30 mg daily for no more than 4 weeks. Responders can taper to less than 10 mg/day. Those who continue to do well can maintain low-dose prednisone for up to 12 months and then complete the taper. Patients who relapse can add an immune modulator (methotrexate, azathioprine, leflunomide, or mycophenolate).
Those who have an inadequate response to the initial prednisone course should then get an immune modulator. If they do well, that can be maintained; a second modulator can be brought on board if necessary.
For those who don’t respond at all to the initial prednisone course, it’s necessary to proceed immediately to an immunosuppressive regimen to prevent irreversible fibrosis.
Dr. Culver noted associations with multiple pharmaceutical companies, but said none were relevant to his talk.
SOURCE: Culver D. CCR 2018.
SANDESTIN, FLA. – Don’t be a slave to imaging when evaluating the patient with sarcoidosis.
“Sometimes, the worst-looking patients [on imaging] have the best prognosis,” Daniel Culver, DO, said at the annual Congress of Clinical Rheumatology. Patients with Löfgren’s syndrome are a very good example of this tenet, he said in an interview. Scans can look alarming, with multiple widespread granulomas. But Löfgren’s is generally a benign condition, despite its threatening mien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Instead of imaging, “Let two things drive your decision to treat: danger to an organ, and quality of life,” said Dr. Culver, a pulmonologist and director of the Sarcoidosis Center of Excellence at the Cleveland Clinic in Ohio; he is also president of the World Association for Sarcoidosis.
He agrees with a decision schema published in 2015 (Clin Chest Med. 2015;36[4]:751-67).
Six factors weigh in favor of treatment:
- Symptomatic disease.
- Impaired organ function.
- Disease endangering an organ.
- Progressive disease.
- Clear-cut disease activity.
- Low likelihood of remission.
These must be balanced – with patient input as the fulcrum – against five factors that favor conservative management:
- Minimal symptoms.
- Good organ function.
- Low risk of danger to organs.
- Inactive disease.
- Higher likelihood of remission.
The decision to embark on a treatment program, usually starting with a steroid-based regimen, can’t be taken lightly, Dr. Culver said. A 2017 study showed that steroids pose a cumulative risk of toxicities for sarcoidosis patients (Respir Med. 2017 Nov;132:9-14). Patients who started steroids faced more than a doubling in the risk of a toxic side effect by 96 months when compared with those who didn’t. But even short-term steroid use increased the risk of a toxicity, Dr. Culver said. The study noted that problems can begin to occur in as little as 1 month, at a cumulative dose as low as 1 g.
For patients who fall onto the “treat” side of the risk teeter-totter, Dr. Culver recommended starting with an initial course of prednisone at 20-30 mg daily for no more than 4 weeks. Responders can taper to less than 10 mg/day. Those who continue to do well can maintain low-dose prednisone for up to 12 months and then complete the taper. Patients who relapse can add an immune modulator (methotrexate, azathioprine, leflunomide, or mycophenolate).
Those who have an inadequate response to the initial prednisone course should then get an immune modulator. If they do well, that can be maintained; a second modulator can be brought on board if necessary.
For those who don’t respond at all to the initial prednisone course, it’s necessary to proceed immediately to an immunosuppressive regimen to prevent irreversible fibrosis.
Dr. Culver noted associations with multiple pharmaceutical companies, but said none were relevant to his talk.
SOURCE: Culver D. CCR 2018.
REPORTING FROM CCR 18
Drug-related deaths continue to rise in United States
TORONTO – Drug-related deaths in America are rising faster than ever.
Rear Adm. Wanda D. Barfield, MD shared recent data from the U.S. National Center for Health Statistics on people aged 15 years and older at the Pediatric Academic Societies annual meeting. Between 1999 and 2016, for example, the number of drug overdose deaths rose more than threefold, from 6.1/100,000 standard population in 1999 to 19.8/100,000 in 2016. For males, the rate increased from 8.2/100,000 in 1999 to 26.2/100,000 in 2016. For females, the rate increased from 3.9/100,000 in 1999 to 13.4/100,000 in 2016.
Dr. Barfield, director of the division of reproductive health at the Centers for Disease Control and Prevention, said that in 2016, the NCHS also found that 22 states and the District of Columbia had drug overdoses that were significantly higher than the national average. The states with the highest number of drug overdose deaths were the District of Columbia, New Hampshire, Pennsylvania, and West Virginia while the states with the lowest observed rates were Nebraska, North Dakota, South Dakota, and Texas.
“Many of these drug overdose deaths are linked to opioids, but not exclusively,” Dr. Barfield said. “In the past, the overall opioid-related overdose deaths were mainly attributed to commonly prescribed opioid medications. However, in recent years, we’re seeing more deaths due to illicit drugs such as heroin and fentanyl.”
The NCHS found that the age-adjusted rate for drug overdose deaths involving synthetic opioids other than methadone doubled from 2015 to 2016, and that drug overdose deaths involving synthetic opioids other than methadone increased from 0.3/100,000 in 1999 to 6.2/100,000 in 2016. The rate increased an average of 18% per year from 1999 to 2006, remained steady from 2006 to 2013, but increased by 88% per year from 2013 to 2016. At the same time, drug overdose deaths involving heroin increased from 0.7/100,000 in 1999 to 1/100,000 in 2010, to 4.9/100,000 in 2016.
According to Dr. Barfield, the spike in opioid use since 1999 stems directly from increased prescribing rates. “In 2015, the number of opioids prescribed was enough so that every American could be medicated around the clock for 3 weeks,” she said. “In addition to the number of prescriptions, the average day’s supply of prescription opioids increased from 2006 to 2015, from 13.3 days in 2006 to 17.7 days in 2015.” What’s more, a recent CDC Vital Signs found that the amount of opioids prescribed per person varied widely among U.S. counties in 2015. “The wide variation among counties suggests a lack of consistency among providers when prescribing opioids,” Dr. Barfield said. “It’s concerning, as higher opioid prescribing puts patients at risk for addiction.”
At the same time, opioid overdose ED visits continue to rise. Data from the CDC’s National Syndromic Surveillance Program found that from July 2016 to September 2017, opioid overdose ED visits increased by 30% for men, by 24% for women, and for all adult age groups (31% among those aged 25-34 years, 36% among those aged 35-54 years, and 32% among those aged 55 years and older).
There’s a problem of prescription opioid use among pregnant women. Published estimates indicate that 14%-22% of women filled an opioid prescription during pregnancy, Dr. Barfield said. Among pregnant women, the prevalence of maternal opioid use or dependence during hospitalization for delivery has increased 127%, from 1.7 /1,000 delivery admissions in 1998 to 3.9/1,000 delivery admissions in 2011 (Anesthesiology 2014;121[6]:1158-65). There also has been a significant increase in neonatal abstinence syndrome (NAS), which is most commonly attributed to opioid exposure during pregnancy, from 1.2/1,000 U.S. hospital births in 2000 to 8/1,000 U.S. hospital births in 2014. “NAS is still on the rise,” Dr. Barfield said. “In 2012, we saw one baby with NAS born every 25 minutes. In 2014, that number jumped to one baby born with NAS every 15 minutes. That means about 96 infants with NAS are born daily,” she said. “Where do you think we’re going to be when we look at 2018 data?”
Role for pediatricians
Dr. Barfield closed her presentation by underscoring the role pediatricians play in counseling patients about opioid abuse or dependence during pregnancy. “We know that providers have a tremendous impact on patients and their families,” she said. “We also know that issues leading to a newborn having NAS are complex, so adopting a public health approach focused on prevention, expansion of treatment, and improvements in child welfare systems is vital.” Specifically, she said, health care providers can “bridge the gap” between clinical care and public health; lead in their communities, not just within their hospital or practice; work as a team member with colleagues in other fields of medicine such as obstetrics, family medicine, and addiction care when caring for infants with NAS, and by considering the social determinants of health.
“One way to adopt a public health perspective is to remember that the health of the fetus and baby rely on more than just prenatal care,” Dr. Barfield said. “We’re all part of a larger whole, surrounded by our families, communities, regions, state, and even our countries of origin. What’s going on with the mom, her family, and the larger community impacts the baby’s health. In other words, the social determinants of health matter, and are an important part of the conversation on NAS.”
She reported having no financial disclosures.
TORONTO – Drug-related deaths in America are rising faster than ever.
Rear Adm. Wanda D. Barfield, MD shared recent data from the U.S. National Center for Health Statistics on people aged 15 years and older at the Pediatric Academic Societies annual meeting. Between 1999 and 2016, for example, the number of drug overdose deaths rose more than threefold, from 6.1/100,000 standard population in 1999 to 19.8/100,000 in 2016. For males, the rate increased from 8.2/100,000 in 1999 to 26.2/100,000 in 2016. For females, the rate increased from 3.9/100,000 in 1999 to 13.4/100,000 in 2016.
Dr. Barfield, director of the division of reproductive health at the Centers for Disease Control and Prevention, said that in 2016, the NCHS also found that 22 states and the District of Columbia had drug overdoses that were significantly higher than the national average. The states with the highest number of drug overdose deaths were the District of Columbia, New Hampshire, Pennsylvania, and West Virginia while the states with the lowest observed rates were Nebraska, North Dakota, South Dakota, and Texas.
“Many of these drug overdose deaths are linked to opioids, but not exclusively,” Dr. Barfield said. “In the past, the overall opioid-related overdose deaths were mainly attributed to commonly prescribed opioid medications. However, in recent years, we’re seeing more deaths due to illicit drugs such as heroin and fentanyl.”
The NCHS found that the age-adjusted rate for drug overdose deaths involving synthetic opioids other than methadone doubled from 2015 to 2016, and that drug overdose deaths involving synthetic opioids other than methadone increased from 0.3/100,000 in 1999 to 6.2/100,000 in 2016. The rate increased an average of 18% per year from 1999 to 2006, remained steady from 2006 to 2013, but increased by 88% per year from 2013 to 2016. At the same time, drug overdose deaths involving heroin increased from 0.7/100,000 in 1999 to 1/100,000 in 2010, to 4.9/100,000 in 2016.
According to Dr. Barfield, the spike in opioid use since 1999 stems directly from increased prescribing rates. “In 2015, the number of opioids prescribed was enough so that every American could be medicated around the clock for 3 weeks,” she said. “In addition to the number of prescriptions, the average day’s supply of prescription opioids increased from 2006 to 2015, from 13.3 days in 2006 to 17.7 days in 2015.” What’s more, a recent CDC Vital Signs found that the amount of opioids prescribed per person varied widely among U.S. counties in 2015. “The wide variation among counties suggests a lack of consistency among providers when prescribing opioids,” Dr. Barfield said. “It’s concerning, as higher opioid prescribing puts patients at risk for addiction.”
At the same time, opioid overdose ED visits continue to rise. Data from the CDC’s National Syndromic Surveillance Program found that from July 2016 to September 2017, opioid overdose ED visits increased by 30% for men, by 24% for women, and for all adult age groups (31% among those aged 25-34 years, 36% among those aged 35-54 years, and 32% among those aged 55 years and older).
There’s a problem of prescription opioid use among pregnant women. Published estimates indicate that 14%-22% of women filled an opioid prescription during pregnancy, Dr. Barfield said. Among pregnant women, the prevalence of maternal opioid use or dependence during hospitalization for delivery has increased 127%, from 1.7 /1,000 delivery admissions in 1998 to 3.9/1,000 delivery admissions in 2011 (Anesthesiology 2014;121[6]:1158-65). There also has been a significant increase in neonatal abstinence syndrome (NAS), which is most commonly attributed to opioid exposure during pregnancy, from 1.2/1,000 U.S. hospital births in 2000 to 8/1,000 U.S. hospital births in 2014. “NAS is still on the rise,” Dr. Barfield said. “In 2012, we saw one baby with NAS born every 25 minutes. In 2014, that number jumped to one baby born with NAS every 15 minutes. That means about 96 infants with NAS are born daily,” she said. “Where do you think we’re going to be when we look at 2018 data?”
Role for pediatricians
Dr. Barfield closed her presentation by underscoring the role pediatricians play in counseling patients about opioid abuse or dependence during pregnancy. “We know that providers have a tremendous impact on patients and their families,” she said. “We also know that issues leading to a newborn having NAS are complex, so adopting a public health approach focused on prevention, expansion of treatment, and improvements in child welfare systems is vital.” Specifically, she said, health care providers can “bridge the gap” between clinical care and public health; lead in their communities, not just within their hospital or practice; work as a team member with colleagues in other fields of medicine such as obstetrics, family medicine, and addiction care when caring for infants with NAS, and by considering the social determinants of health.
“One way to adopt a public health perspective is to remember that the health of the fetus and baby rely on more than just prenatal care,” Dr. Barfield said. “We’re all part of a larger whole, surrounded by our families, communities, regions, state, and even our countries of origin. What’s going on with the mom, her family, and the larger community impacts the baby’s health. In other words, the social determinants of health matter, and are an important part of the conversation on NAS.”
She reported having no financial disclosures.
TORONTO – Drug-related deaths in America are rising faster than ever.
Rear Adm. Wanda D. Barfield, MD shared recent data from the U.S. National Center for Health Statistics on people aged 15 years and older at the Pediatric Academic Societies annual meeting. Between 1999 and 2016, for example, the number of drug overdose deaths rose more than threefold, from 6.1/100,000 standard population in 1999 to 19.8/100,000 in 2016. For males, the rate increased from 8.2/100,000 in 1999 to 26.2/100,000 in 2016. For females, the rate increased from 3.9/100,000 in 1999 to 13.4/100,000 in 2016.
Dr. Barfield, director of the division of reproductive health at the Centers for Disease Control and Prevention, said that in 2016, the NCHS also found that 22 states and the District of Columbia had drug overdoses that were significantly higher than the national average. The states with the highest number of drug overdose deaths were the District of Columbia, New Hampshire, Pennsylvania, and West Virginia while the states with the lowest observed rates were Nebraska, North Dakota, South Dakota, and Texas.
“Many of these drug overdose deaths are linked to opioids, but not exclusively,” Dr. Barfield said. “In the past, the overall opioid-related overdose deaths were mainly attributed to commonly prescribed opioid medications. However, in recent years, we’re seeing more deaths due to illicit drugs such as heroin and fentanyl.”
The NCHS found that the age-adjusted rate for drug overdose deaths involving synthetic opioids other than methadone doubled from 2015 to 2016, and that drug overdose deaths involving synthetic opioids other than methadone increased from 0.3/100,000 in 1999 to 6.2/100,000 in 2016. The rate increased an average of 18% per year from 1999 to 2006, remained steady from 2006 to 2013, but increased by 88% per year from 2013 to 2016. At the same time, drug overdose deaths involving heroin increased from 0.7/100,000 in 1999 to 1/100,000 in 2010, to 4.9/100,000 in 2016.
According to Dr. Barfield, the spike in opioid use since 1999 stems directly from increased prescribing rates. “In 2015, the number of opioids prescribed was enough so that every American could be medicated around the clock for 3 weeks,” she said. “In addition to the number of prescriptions, the average day’s supply of prescription opioids increased from 2006 to 2015, from 13.3 days in 2006 to 17.7 days in 2015.” What’s more, a recent CDC Vital Signs found that the amount of opioids prescribed per person varied widely among U.S. counties in 2015. “The wide variation among counties suggests a lack of consistency among providers when prescribing opioids,” Dr. Barfield said. “It’s concerning, as higher opioid prescribing puts patients at risk for addiction.”
At the same time, opioid overdose ED visits continue to rise. Data from the CDC’s National Syndromic Surveillance Program found that from July 2016 to September 2017, opioid overdose ED visits increased by 30% for men, by 24% for women, and for all adult age groups (31% among those aged 25-34 years, 36% among those aged 35-54 years, and 32% among those aged 55 years and older).
There’s a problem of prescription opioid use among pregnant women. Published estimates indicate that 14%-22% of women filled an opioid prescription during pregnancy, Dr. Barfield said. Among pregnant women, the prevalence of maternal opioid use or dependence during hospitalization for delivery has increased 127%, from 1.7 /1,000 delivery admissions in 1998 to 3.9/1,000 delivery admissions in 2011 (Anesthesiology 2014;121[6]:1158-65). There also has been a significant increase in neonatal abstinence syndrome (NAS), which is most commonly attributed to opioid exposure during pregnancy, from 1.2/1,000 U.S. hospital births in 2000 to 8/1,000 U.S. hospital births in 2014. “NAS is still on the rise,” Dr. Barfield said. “In 2012, we saw one baby with NAS born every 25 minutes. In 2014, that number jumped to one baby born with NAS every 15 minutes. That means about 96 infants with NAS are born daily,” she said. “Where do you think we’re going to be when we look at 2018 data?”
Role for pediatricians
Dr. Barfield closed her presentation by underscoring the role pediatricians play in counseling patients about opioid abuse or dependence during pregnancy. “We know that providers have a tremendous impact on patients and their families,” she said. “We also know that issues leading to a newborn having NAS are complex, so adopting a public health approach focused on prevention, expansion of treatment, and improvements in child welfare systems is vital.” Specifically, she said, health care providers can “bridge the gap” between clinical care and public health; lead in their communities, not just within their hospital or practice; work as a team member with colleagues in other fields of medicine such as obstetrics, family medicine, and addiction care when caring for infants with NAS, and by considering the social determinants of health.
“One way to adopt a public health perspective is to remember that the health of the fetus and baby rely on more than just prenatal care,” Dr. Barfield said. “We’re all part of a larger whole, surrounded by our families, communities, regions, state, and even our countries of origin. What’s going on with the mom, her family, and the larger community impacts the baby’s health. In other words, the social determinants of health matter, and are an important part of the conversation on NAS.”
She reported having no financial disclosures.
EXPERT ANALYSIS FROM PAS 2018
FDA approves Aimovig for migraine prevention
The Food and Drug Administration has approved Aimovig (erenumab-aooe), delivered once a month via injection, for the prevention of migraines in adults.
The final clinical trial included 667 chronic migraine patients, and over a 3-month period, those treated with Aimovig had, on average, 2.5 fewer migraine days per month, compared with those who received placebo.
The most commonly reported adverse events were injection-site reactions and constipation.
“Aimovig provides patients with a novel option for reducing the number of days with migraine. We need new treatments for this painful and often debilitating condition,” Eric Bastings, MD, deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has approved Aimovig (erenumab-aooe), delivered once a month via injection, for the prevention of migraines in adults.
The final clinical trial included 667 chronic migraine patients, and over a 3-month period, those treated with Aimovig had, on average, 2.5 fewer migraine days per month, compared with those who received placebo.
The most commonly reported adverse events were injection-site reactions and constipation.
“Aimovig provides patients with a novel option for reducing the number of days with migraine. We need new treatments for this painful and often debilitating condition,” Eric Bastings, MD, deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has approved Aimovig (erenumab-aooe), delivered once a month via injection, for the prevention of migraines in adults.
The final clinical trial included 667 chronic migraine patients, and over a 3-month period, those treated with Aimovig had, on average, 2.5 fewer migraine days per month, compared with those who received placebo.
The most commonly reported adverse events were injection-site reactions and constipation.
“Aimovig provides patients with a novel option for reducing the number of days with migraine. We need new treatments for this painful and often debilitating condition,” Eric Bastings, MD, deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
FDA warns of birth defect risks from dolutegravir
The Food and Drug Administration has issued a Drug Safety Communication alert that serious cases of neural tube birth defects have been reported in babies born to women treated with dolutegravir for human immunodeficiency virus. Reported defects have involved the brain, spine, and spinal cord.
To date, in this observational study, there are no reported cases of babies born with neural tube defects to women starting dolutegravir later in pregnancy, according to the FDA. “We are investigating this new safety issue and will update the public when we have more information,” the alert stated.
Dolutegravir is an FDA-approved antiretroviral used to treat HIV. The drug is available as a single ingredient under the brand name Tivicay and as a combination tablet with other HIV medicines under the brand names Juluca and Triumeq.
Medwatch also released an alert for medical practitioners to report adverse events related to this issue.
The Food and Drug Administration has issued a Drug Safety Communication alert that serious cases of neural tube birth defects have been reported in babies born to women treated with dolutegravir for human immunodeficiency virus. Reported defects have involved the brain, spine, and spinal cord.
To date, in this observational study, there are no reported cases of babies born with neural tube defects to women starting dolutegravir later in pregnancy, according to the FDA. “We are investigating this new safety issue and will update the public when we have more information,” the alert stated.
Dolutegravir is an FDA-approved antiretroviral used to treat HIV. The drug is available as a single ingredient under the brand name Tivicay and as a combination tablet with other HIV medicines under the brand names Juluca and Triumeq.
Medwatch also released an alert for medical practitioners to report adverse events related to this issue.
The Food and Drug Administration has issued a Drug Safety Communication alert that serious cases of neural tube birth defects have been reported in babies born to women treated with dolutegravir for human immunodeficiency virus. Reported defects have involved the brain, spine, and spinal cord.
To date, in this observational study, there are no reported cases of babies born with neural tube defects to women starting dolutegravir later in pregnancy, according to the FDA. “We are investigating this new safety issue and will update the public when we have more information,” the alert stated.
Dolutegravir is an FDA-approved antiretroviral used to treat HIV. The drug is available as a single ingredient under the brand name Tivicay and as a combination tablet with other HIV medicines under the brand names Juluca and Triumeq.
Medwatch also released an alert for medical practitioners to report adverse events related to this issue.
Legalization of marijuana debate moving away from medical need
NEW YORK – The risk curve of societal harm from marijuana when graphed from a policy of complete prohibition to one of unregulated access is U shaped, according to a panel of experts who participated in a workshop on the harms and benefits of cannabis at the annual meeting of the American Psychiatric Association.
Based on more than 80 years of experience, it is clear that prohibition supports criminal activity but does not eliminate use, said Jason Hershberger, MD, chair of the department of psychiatry at Brookdale University Hospital Medical Center, New York. With progressive degrees of decriminalization, the harms to society associated with criminal distribution and the criminalization of use decreases, but they are replaced with increasing societal risks imposed by the availability of an intoxicating substance.
Theoretically, one argument is that the inflection point occurs where cannabis has been decriminalized for medical indications but prohibited for recreational use. But this is a tenuous position. Clinicians, including psychiatrists, might support the use of cannabis to reduce stress, but it is well known that the pleasant intoxication provided by cannabis is relaxing – whether or not this is characterized as stress relief.
reported Stephan M. Carlson, MD, who also is affiliated with Brookdale University Hospital Medical Center. Based on his belief that marijuana, after alcohol and tobacco, may soon be the third legal drug where it isn’t already, “physicians have just a temporary role” in mediating the debate about medical legalization from a legal or political standpoint. Why? Because this part of the debate is growing irrelevant.
This does not mean medical indications or medical harms are not valid topics of discussion, Dr. Hershberger and Dr. Carlson said. The problem is that recreational use is supplanting medical use as the central issue in making this drug available.
The road toward U.S. legalization started in 1996, when California voters approved the use of marijuana for medical indications. Other states followed. Then, in 2012, Colorado and Washington became the first states to approve the drug for recreational use. In total, 29 states now permit some form of legal use of marijuana. In addition, marijuana possession has been decriminalized in several states where it remains illegal.
“From the federal perspective, marijuana is still a Schedule I drug. This means that it is easier to conduct research on cocaine, which is a Schedule II drug, than it is on marijuana,” Dr. Hershberger said. While the current presidential administration has adopted a tougher stance on distribution and use of marijuana than the previous, more than 60% of Americans in surveys support decriminalization of marijuana, according to Dr. Hershberger. He suggested that the current acceptability of marijuana is the reason that a recent survey indicated that more U.S. high school seniors than ever before have tried marijuana.
In light of the perceptions that the marijuana ban was ineffective, that marijuana is acceptable, and that profits from marijuana sales and distributions have been going to criminals, depriving government of tax dollars, continued decriminalization appears to be inevitable, Dr. Hershberger said. However, he believes that complete deregulation poses important risk, including an inevitable increase in accidents produced by motor impairment. It is also reasonable to anticipate more substance use in vulnerable populations. The risk to the development of children and adolescents who are likely to gain easier access to cannabis is unknown.
For psychiatrists and other medical specialists who wish to participate in the debate about the degree to which marijuana is decriminalized, both Dr. Hershberger and Dr. Carlson argued that a clear and realistic view of the landscape is essential.
“One of the reasons we are in this state we are in is that many people have declared the war on drugs a failure,” Dr. Hershberger said. Although he acknowledged that recognizing the limited efficacy of a marijuana ban is a reasonable starting point for discussion, he also expressed concern about large corporations being permitted to market cannabis in a way that obscures its risks or encourages use by those at greatest risk of harm, such as minors.
“We have to ask ourselves, is America ready for a cannabis commercial during the Super Bowl?” Dr. Hershberger said. He believes that an important trade-off exists for risks regardless of the degree to which marijuana is legalized.
“We have to approach this in the context of our current reality,” Dr. Carlson agreed. He, like Dr. Hershberger, cautioned that there are no simple answers.
Dr. Hershberger and Dr. Carlson reported no potential conflicts of interest.
NEW YORK – The risk curve of societal harm from marijuana when graphed from a policy of complete prohibition to one of unregulated access is U shaped, according to a panel of experts who participated in a workshop on the harms and benefits of cannabis at the annual meeting of the American Psychiatric Association.
Based on more than 80 years of experience, it is clear that prohibition supports criminal activity but does not eliminate use, said Jason Hershberger, MD, chair of the department of psychiatry at Brookdale University Hospital Medical Center, New York. With progressive degrees of decriminalization, the harms to society associated with criminal distribution and the criminalization of use decreases, but they are replaced with increasing societal risks imposed by the availability of an intoxicating substance.
Theoretically, one argument is that the inflection point occurs where cannabis has been decriminalized for medical indications but prohibited for recreational use. But this is a tenuous position. Clinicians, including psychiatrists, might support the use of cannabis to reduce stress, but it is well known that the pleasant intoxication provided by cannabis is relaxing – whether or not this is characterized as stress relief.
reported Stephan M. Carlson, MD, who also is affiliated with Brookdale University Hospital Medical Center. Based on his belief that marijuana, after alcohol and tobacco, may soon be the third legal drug where it isn’t already, “physicians have just a temporary role” in mediating the debate about medical legalization from a legal or political standpoint. Why? Because this part of the debate is growing irrelevant.
This does not mean medical indications or medical harms are not valid topics of discussion, Dr. Hershberger and Dr. Carlson said. The problem is that recreational use is supplanting medical use as the central issue in making this drug available.
The road toward U.S. legalization started in 1996, when California voters approved the use of marijuana for medical indications. Other states followed. Then, in 2012, Colorado and Washington became the first states to approve the drug for recreational use. In total, 29 states now permit some form of legal use of marijuana. In addition, marijuana possession has been decriminalized in several states where it remains illegal.
“From the federal perspective, marijuana is still a Schedule I drug. This means that it is easier to conduct research on cocaine, which is a Schedule II drug, than it is on marijuana,” Dr. Hershberger said. While the current presidential administration has adopted a tougher stance on distribution and use of marijuana than the previous, more than 60% of Americans in surveys support decriminalization of marijuana, according to Dr. Hershberger. He suggested that the current acceptability of marijuana is the reason that a recent survey indicated that more U.S. high school seniors than ever before have tried marijuana.
In light of the perceptions that the marijuana ban was ineffective, that marijuana is acceptable, and that profits from marijuana sales and distributions have been going to criminals, depriving government of tax dollars, continued decriminalization appears to be inevitable, Dr. Hershberger said. However, he believes that complete deregulation poses important risk, including an inevitable increase in accidents produced by motor impairment. It is also reasonable to anticipate more substance use in vulnerable populations. The risk to the development of children and adolescents who are likely to gain easier access to cannabis is unknown.
For psychiatrists and other medical specialists who wish to participate in the debate about the degree to which marijuana is decriminalized, both Dr. Hershberger and Dr. Carlson argued that a clear and realistic view of the landscape is essential.
“One of the reasons we are in this state we are in is that many people have declared the war on drugs a failure,” Dr. Hershberger said. Although he acknowledged that recognizing the limited efficacy of a marijuana ban is a reasonable starting point for discussion, he also expressed concern about large corporations being permitted to market cannabis in a way that obscures its risks or encourages use by those at greatest risk of harm, such as minors.
“We have to ask ourselves, is America ready for a cannabis commercial during the Super Bowl?” Dr. Hershberger said. He believes that an important trade-off exists for risks regardless of the degree to which marijuana is legalized.
“We have to approach this in the context of our current reality,” Dr. Carlson agreed. He, like Dr. Hershberger, cautioned that there are no simple answers.
Dr. Hershberger and Dr. Carlson reported no potential conflicts of interest.
NEW YORK – The risk curve of societal harm from marijuana when graphed from a policy of complete prohibition to one of unregulated access is U shaped, according to a panel of experts who participated in a workshop on the harms and benefits of cannabis at the annual meeting of the American Psychiatric Association.
Based on more than 80 years of experience, it is clear that prohibition supports criminal activity but does not eliminate use, said Jason Hershberger, MD, chair of the department of psychiatry at Brookdale University Hospital Medical Center, New York. With progressive degrees of decriminalization, the harms to society associated with criminal distribution and the criminalization of use decreases, but they are replaced with increasing societal risks imposed by the availability of an intoxicating substance.
Theoretically, one argument is that the inflection point occurs where cannabis has been decriminalized for medical indications but prohibited for recreational use. But this is a tenuous position. Clinicians, including psychiatrists, might support the use of cannabis to reduce stress, but it is well known that the pleasant intoxication provided by cannabis is relaxing – whether or not this is characterized as stress relief.
reported Stephan M. Carlson, MD, who also is affiliated with Brookdale University Hospital Medical Center. Based on his belief that marijuana, after alcohol and tobacco, may soon be the third legal drug where it isn’t already, “physicians have just a temporary role” in mediating the debate about medical legalization from a legal or political standpoint. Why? Because this part of the debate is growing irrelevant.
This does not mean medical indications or medical harms are not valid topics of discussion, Dr. Hershberger and Dr. Carlson said. The problem is that recreational use is supplanting medical use as the central issue in making this drug available.
The road toward U.S. legalization started in 1996, when California voters approved the use of marijuana for medical indications. Other states followed. Then, in 2012, Colorado and Washington became the first states to approve the drug for recreational use. In total, 29 states now permit some form of legal use of marijuana. In addition, marijuana possession has been decriminalized in several states where it remains illegal.
“From the federal perspective, marijuana is still a Schedule I drug. This means that it is easier to conduct research on cocaine, which is a Schedule II drug, than it is on marijuana,” Dr. Hershberger said. While the current presidential administration has adopted a tougher stance on distribution and use of marijuana than the previous, more than 60% of Americans in surveys support decriminalization of marijuana, according to Dr. Hershberger. He suggested that the current acceptability of marijuana is the reason that a recent survey indicated that more U.S. high school seniors than ever before have tried marijuana.
In light of the perceptions that the marijuana ban was ineffective, that marijuana is acceptable, and that profits from marijuana sales and distributions have been going to criminals, depriving government of tax dollars, continued decriminalization appears to be inevitable, Dr. Hershberger said. However, he believes that complete deregulation poses important risk, including an inevitable increase in accidents produced by motor impairment. It is also reasonable to anticipate more substance use in vulnerable populations. The risk to the development of children and adolescents who are likely to gain easier access to cannabis is unknown.
For psychiatrists and other medical specialists who wish to participate in the debate about the degree to which marijuana is decriminalized, both Dr. Hershberger and Dr. Carlson argued that a clear and realistic view of the landscape is essential.
“One of the reasons we are in this state we are in is that many people have declared the war on drugs a failure,” Dr. Hershberger said. Although he acknowledged that recognizing the limited efficacy of a marijuana ban is a reasonable starting point for discussion, he also expressed concern about large corporations being permitted to market cannabis in a way that obscures its risks or encourages use by those at greatest risk of harm, such as minors.
“We have to ask ourselves, is America ready for a cannabis commercial during the Super Bowl?” Dr. Hershberger said. He believes that an important trade-off exists for risks regardless of the degree to which marijuana is legalized.
“We have to approach this in the context of our current reality,” Dr. Carlson agreed. He, like Dr. Hershberger, cautioned that there are no simple answers.
Dr. Hershberger and Dr. Carlson reported no potential conflicts of interest.
EXPERT ANALYSIS FROM APA
Physical inactivity linked to lymphoma risk
A lifetime of physical inactivity could significantly increase the risk of developing both Hodgkin and non-Hodgkin lymphoma, according to a case-control study.
Researchers examined self-reported lifetime physical activity in 87 patients with Hodgkin lymphoma and 236 patients with non-Hodgkin lymphoma, as well as 1,300 cancer-free controls.
Researchers also found a nearly threefold higher risk of Hodgkin lymphoma among overweight and obese individuals who were physically inactive, compared with those who were active (odds ratio, 2.79; P = .01). Similarly, physically inactive individuals who had never smoked had a greater than threefold increase in risk, compared with never-smokers who were active (OR, 3.30; P less than .001). But despite these significant associations for smoking and weight, the small samples sizes meant they were not significant in multivariable-adjusted models, the researchers noted. For non-Hodgkin lymphoma, the associations between obesity/overweight and smoking status were also not statistically significant in multivariable-adjusted models.
Previous studies looking at the role of physical activity in Hodgkin and non-Hodgkin lymphoma had yielded mixed and inconclusive results. Since then, researchers have begun to specifically consider the role of physical inactivity, rather than physical activity, as the exposure of interest.
“An additional advantage of identifying inactivity as the exposure of interest is that a body of literature suggests that those who are at the lower end of the physical activity continuum are less likely to overreport physical activity than those who engage in greater levels of physical activity,” the researchers wrote.
They acknowledged that relying on self-reported levels of physical activity was a limitation of their study. However, they also pointed out that previous research suggested that simplified physical activity questionnaires that took a dichotomous approach to activity/inactivity were effective at identifying the most physically inactivity individuals in a population.
“Continued evidence for adverse associations between physical inactivity and cancer endpoints substantiate a powerful public health message that any amount of regular activity appears to associate with decreased cancer risk,” they wrote.
One researcher was supported by the New York State Department of Health. No conflicts of interest were reported.
SOURCE: Etter JL et al. Leuk Res. 2018 Mar 27;69:7-11.
A lifetime of physical inactivity could significantly increase the risk of developing both Hodgkin and non-Hodgkin lymphoma, according to a case-control study.
Researchers examined self-reported lifetime physical activity in 87 patients with Hodgkin lymphoma and 236 patients with non-Hodgkin lymphoma, as well as 1,300 cancer-free controls.
Researchers also found a nearly threefold higher risk of Hodgkin lymphoma among overweight and obese individuals who were physically inactive, compared with those who were active (odds ratio, 2.79; P = .01). Similarly, physically inactive individuals who had never smoked had a greater than threefold increase in risk, compared with never-smokers who were active (OR, 3.30; P less than .001). But despite these significant associations for smoking and weight, the small samples sizes meant they were not significant in multivariable-adjusted models, the researchers noted. For non-Hodgkin lymphoma, the associations between obesity/overweight and smoking status were also not statistically significant in multivariable-adjusted models.
Previous studies looking at the role of physical activity in Hodgkin and non-Hodgkin lymphoma had yielded mixed and inconclusive results. Since then, researchers have begun to specifically consider the role of physical inactivity, rather than physical activity, as the exposure of interest.
“An additional advantage of identifying inactivity as the exposure of interest is that a body of literature suggests that those who are at the lower end of the physical activity continuum are less likely to overreport physical activity than those who engage in greater levels of physical activity,” the researchers wrote.
They acknowledged that relying on self-reported levels of physical activity was a limitation of their study. However, they also pointed out that previous research suggested that simplified physical activity questionnaires that took a dichotomous approach to activity/inactivity were effective at identifying the most physically inactivity individuals in a population.
“Continued evidence for adverse associations between physical inactivity and cancer endpoints substantiate a powerful public health message that any amount of regular activity appears to associate with decreased cancer risk,” they wrote.
One researcher was supported by the New York State Department of Health. No conflicts of interest were reported.
SOURCE: Etter JL et al. Leuk Res. 2018 Mar 27;69:7-11.
A lifetime of physical inactivity could significantly increase the risk of developing both Hodgkin and non-Hodgkin lymphoma, according to a case-control study.
Researchers examined self-reported lifetime physical activity in 87 patients with Hodgkin lymphoma and 236 patients with non-Hodgkin lymphoma, as well as 1,300 cancer-free controls.
Researchers also found a nearly threefold higher risk of Hodgkin lymphoma among overweight and obese individuals who were physically inactive, compared with those who were active (odds ratio, 2.79; P = .01). Similarly, physically inactive individuals who had never smoked had a greater than threefold increase in risk, compared with never-smokers who were active (OR, 3.30; P less than .001). But despite these significant associations for smoking and weight, the small samples sizes meant they were not significant in multivariable-adjusted models, the researchers noted. For non-Hodgkin lymphoma, the associations between obesity/overweight and smoking status were also not statistically significant in multivariable-adjusted models.
Previous studies looking at the role of physical activity in Hodgkin and non-Hodgkin lymphoma had yielded mixed and inconclusive results. Since then, researchers have begun to specifically consider the role of physical inactivity, rather than physical activity, as the exposure of interest.
“An additional advantage of identifying inactivity as the exposure of interest is that a body of literature suggests that those who are at the lower end of the physical activity continuum are less likely to overreport physical activity than those who engage in greater levels of physical activity,” the researchers wrote.
They acknowledged that relying on self-reported levels of physical activity was a limitation of their study. However, they also pointed out that previous research suggested that simplified physical activity questionnaires that took a dichotomous approach to activity/inactivity were effective at identifying the most physically inactivity individuals in a population.
“Continued evidence for adverse associations between physical inactivity and cancer endpoints substantiate a powerful public health message that any amount of regular activity appears to associate with decreased cancer risk,” they wrote.
One researcher was supported by the New York State Department of Health. No conflicts of interest were reported.
SOURCE: Etter JL et al. Leuk Res. 2018 Mar 27;69:7-11.
FROM LEUKEMIA RESEARCH
Key clinical point:
Major finding: A lifetime of physical inactivity is associated with a 90% increased risk of Hodgkin lymphoma and a 35% increased risk of non-Hodgkin lymphoma.Study details: A case-control study in 323 patients with Hodgkin or non-Hodgkin lymphoma and 1,300 cancer-free controls.
Disclosures: One researcher was supported by the New York State Department of Health. No conflicts of interest were declared.
Source: Etter JL et al. Leuk Res. 2018 Mar 27;69:7-11.