User login
Same-day discharge for hysterectomy
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
FDA approves IL-23 antagonist for plaque psoriasis
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
Think methotrexate for granulomatous mastitis
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM RWCS 2018
Key clinical point:
Major finding: At 3 months, 17 of 19 patients showed improvement on methotrexate at 15 mg/week, and at 12 months, 12 of 15 had experienced disease resolution on 20 mg/week.
Study details: A single-center retrospective review of 19 patients with granulomatous mastitis.
Disclosures: The presenter reported having no financial conflicts of interest regarding her presentation.
Tardive dyskinesia is theme of awards competition for early career psychiatrists
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at dianne@mhaus.org. Winners will be announced by Aug. 10, 2018. For additional information, write to dianne@mhaus.org or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at dianne@mhaus.org. Winners will be announced by Aug. 10, 2018. For additional information, write to dianne@mhaus.org or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
Important advances in neuroscience and clinical psychiatry have been achieved in recent years, but there are significant gaps in knowledge and much that we don’t understand about the brain and behavior. Further advances depend on cultivating and supporting a new generation of dedicated basic science and clinical investigators. While there is a compelling need to attract, recruit, and encourage talented individuals to pursue scholarly interests, competing life and career demands often prove daunting.
The theme of the competition this year concerning tardive dyskinesia is timely and consistent with the mission of NMSIS to promote knowledge on neurologic side effects of antipsychotic drugs. Tardive dyskinesia can have a negative impact on the social, psychological, and physical well-being of patients; it remains a legacy of past treatment with antipsychotics; it is an increasing concern among an ever widening population of patients receiving even newer antipsychotics; and there are now two Food and Drug Administration–approved treatments for the disorder. Early career psychiatrists may have had limited instruction on tardive dyskinesia, which has not received prominent attention in curricular programs in recent years. Thus, in addition to supporting scholarly work and research experience, the 2018 Promising Scholars Award Program aims to promote knowledge and skills in managing patients with tardive dyskinesia.
Specific learning objectives are:
- Participants will learn the steps necessary to prepare a scientific manuscript for publication.
- Participants will review comments by expert referees and learn to incorporate and respond to the peer review process.
- Participants will review the evidence related to the diagnosis and treatment of tardive dyskinesia.
- Participants will be introduced to the spectrum of educational and networking opportunities at the Institute for Psychiatric Services conference.
In the past, this program was very popular and gained national recognition among psychiatric trainees. Numerous submitted papers were accepted for publication in peer-reviewed journals after the competition was completed.
Instructions for manuscript preparation are:
- First author must be a student, resident, or fellow.
- Papers should address specific issues related to the theme of tardive dyskinesia and be no longer than 15 double-spaced typed pages in length (excluding references and illustrations).
- Literature reviews, case reports, or studies that are original and newly developed or recently published are acceptable.
- Reviews and feedback will be provided by a panel of academic psychiatrists.
- Papers will be judged on relevance to tardive dyskinesia, originality, scholarship, scientific rigor, valid methodology, clinical significance, and organization.
To participate, papers and curriculum vitae of the first author must be submitted by July 1, 2018, to Dianne Daugherty by email at dianne@mhaus.org. Winners will be announced by Aug. 10, 2018. For additional information, write to dianne@mhaus.org or visit www.mhaus.org/nmsis/about-us/what-is-nmsis.
Dr. Caroff, professor of psychiatry, Corporal Michael J. Crescenz VA Medical Center and at the University of Pennsylvania, both in Philadelphia, is director of the NMSIS. He served as consultant to Neurocrine Biosciences and Teva Pharmaceutical Industries, and receives research grant funding from Neurocrine Biosciences.
A Recalcitrant Case of Toxic Epidermal Necrolysis
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
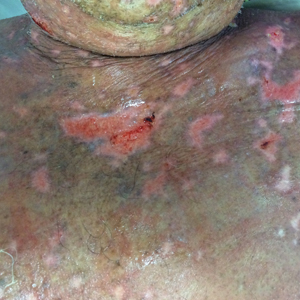
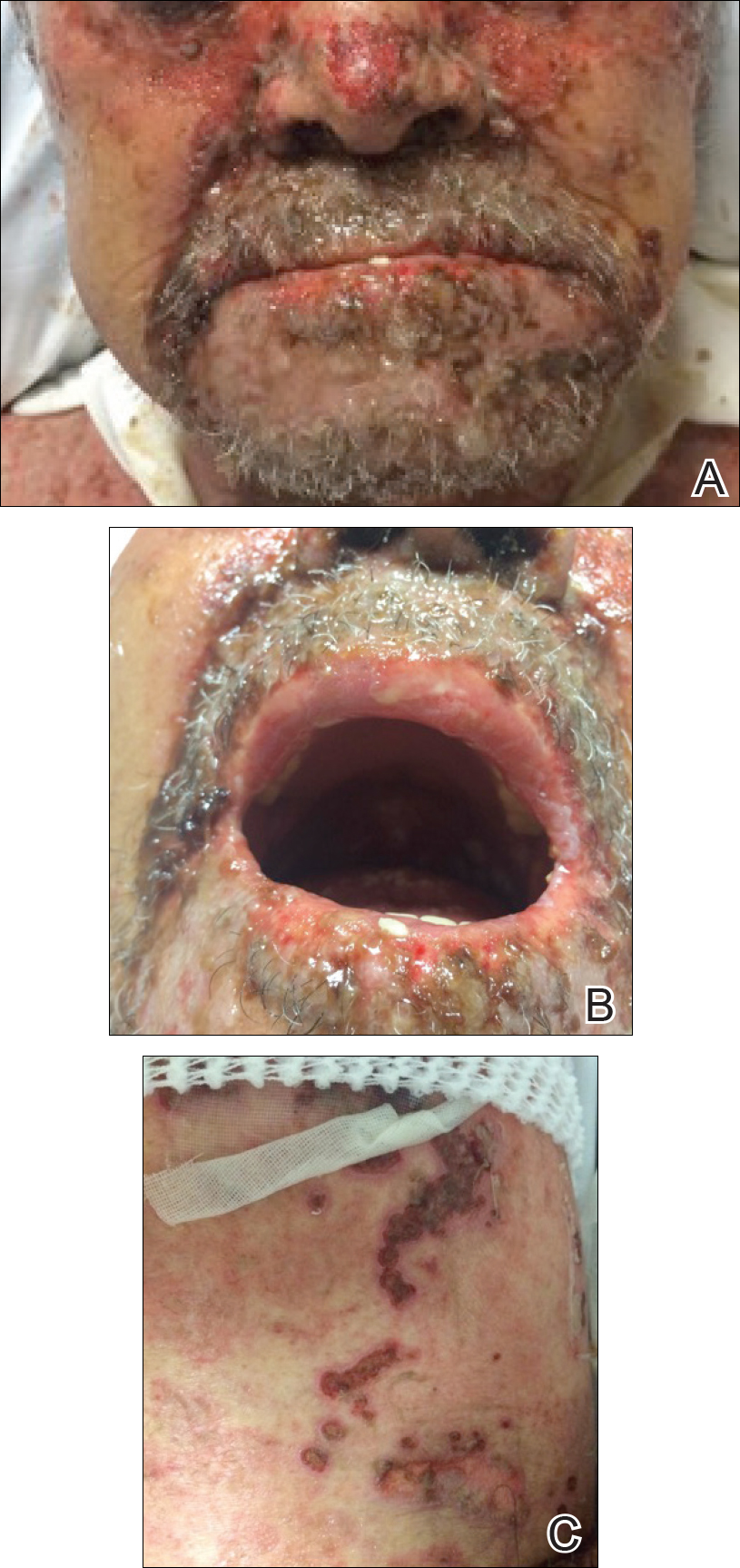
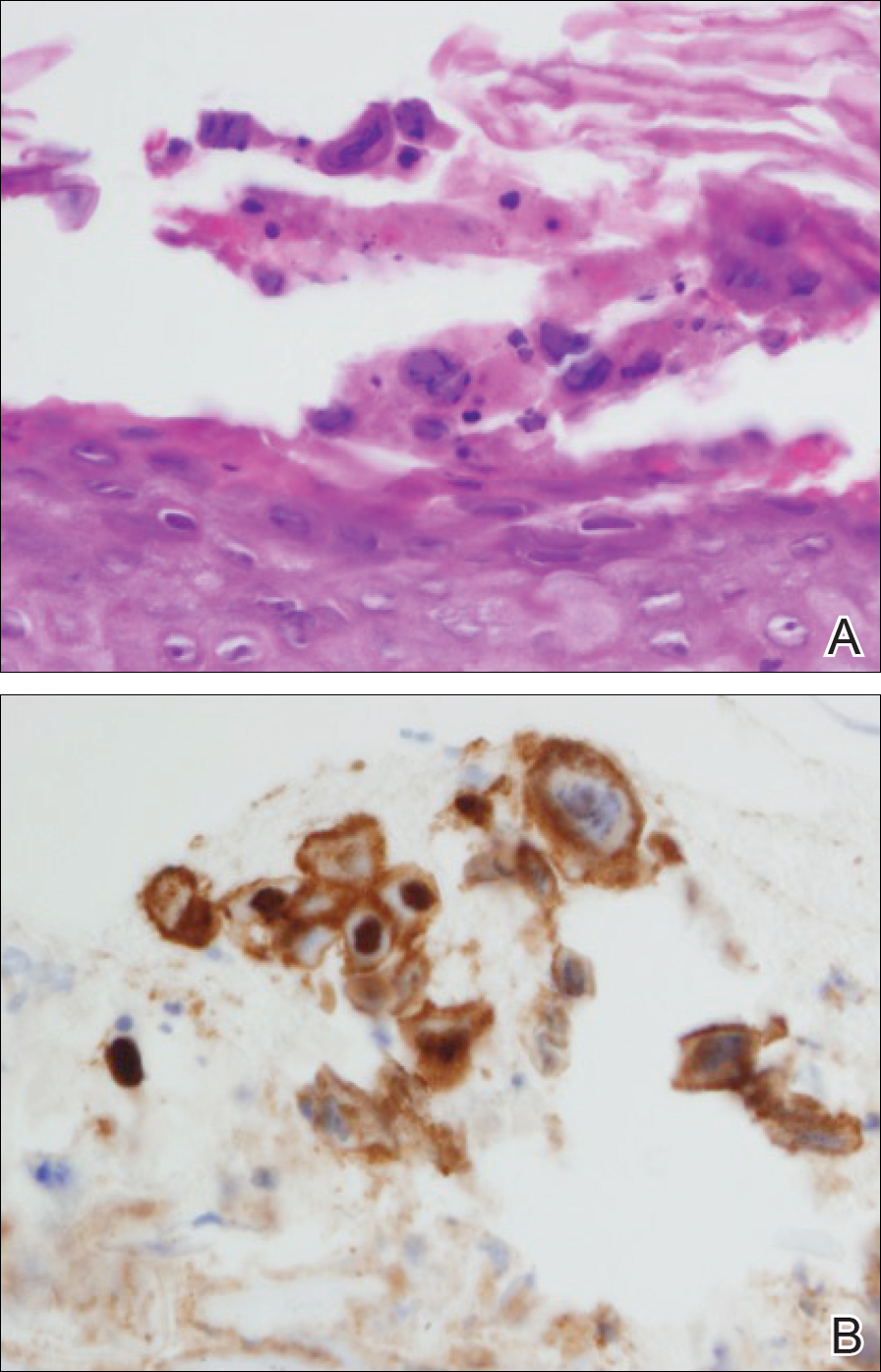
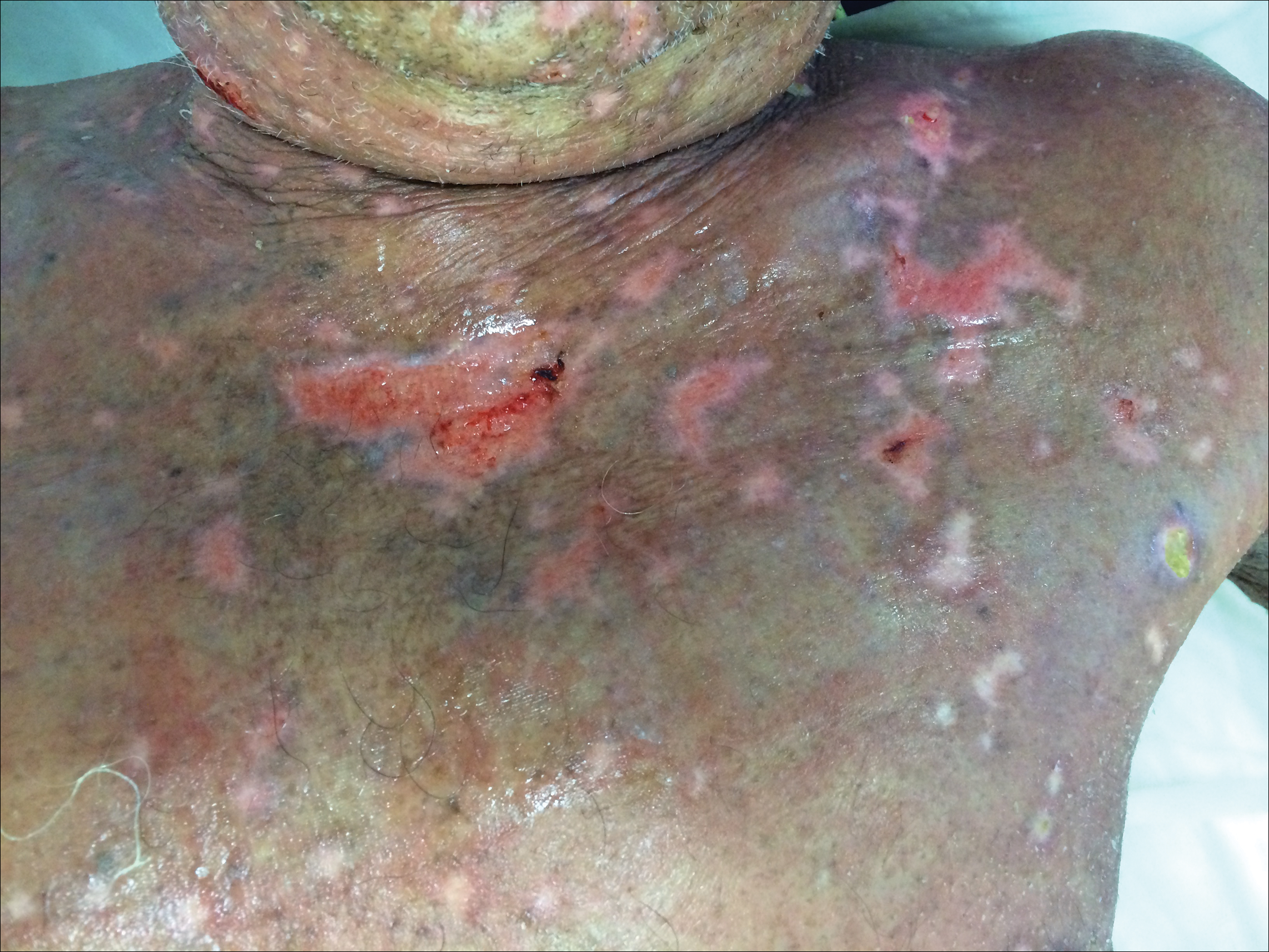
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.
Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
Practice Points
- Toxic epidermal necrolysis can be difficult to diagnose and treat.
- Patients who are refractory to treatment should prompt further management considerations.
The retirement horizon creeps up
My lease is up later this year, after 5 1/2 years. It doesn’t seem that long. Some days it feels like I just moved in.
As a result, I had an email exchange recently with the building’s manager and we hashed out an agreement on a new 10-year contract. In the process, I realized that sort of time frame might (and, again, I say might) take me into retirement.
And now I’m starting to think about retiring and the career endgame.
Granted, it’s still 10 years away, and knowing me I’ll probably want to work another 5 years or so beyond that if I can. I like what I do and would probably go stir crazy without this job. Besides, given the current anti-doctor financial climate, I may not be able to retire in 10 years, even if I want to.
But still, it’s an odd realization to think that, after all those applications, and classes, and tests, and rotations, and all the other things you went through ... that your career is closer to wrapping up than it is to the beginning.
How did that happen?
And I’ll continue to try. Even after the halfway point I still get up each morning wanting to help people. The same sentiments I expressed in a personal statement so long ago are still there, and hopefully always will be. When they’re gone, it’s time to leave. Hopefully, they’ll be with me until I’m ready to sign off.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My lease is up later this year, after 5 1/2 years. It doesn’t seem that long. Some days it feels like I just moved in.
As a result, I had an email exchange recently with the building’s manager and we hashed out an agreement on a new 10-year contract. In the process, I realized that sort of time frame might (and, again, I say might) take me into retirement.
And now I’m starting to think about retiring and the career endgame.
Granted, it’s still 10 years away, and knowing me I’ll probably want to work another 5 years or so beyond that if I can. I like what I do and would probably go stir crazy without this job. Besides, given the current anti-doctor financial climate, I may not be able to retire in 10 years, even if I want to.
But still, it’s an odd realization to think that, after all those applications, and classes, and tests, and rotations, and all the other things you went through ... that your career is closer to wrapping up than it is to the beginning.
How did that happen?
And I’ll continue to try. Even after the halfway point I still get up each morning wanting to help people. The same sentiments I expressed in a personal statement so long ago are still there, and hopefully always will be. When they’re gone, it’s time to leave. Hopefully, they’ll be with me until I’m ready to sign off.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My lease is up later this year, after 5 1/2 years. It doesn’t seem that long. Some days it feels like I just moved in.
As a result, I had an email exchange recently with the building’s manager and we hashed out an agreement on a new 10-year contract. In the process, I realized that sort of time frame might (and, again, I say might) take me into retirement.
And now I’m starting to think about retiring and the career endgame.
Granted, it’s still 10 years away, and knowing me I’ll probably want to work another 5 years or so beyond that if I can. I like what I do and would probably go stir crazy without this job. Besides, given the current anti-doctor financial climate, I may not be able to retire in 10 years, even if I want to.
But still, it’s an odd realization to think that, after all those applications, and classes, and tests, and rotations, and all the other things you went through ... that your career is closer to wrapping up than it is to the beginning.
How did that happen?
And I’ll continue to try. Even after the halfway point I still get up each morning wanting to help people. The same sentiments I expressed in a personal statement so long ago are still there, and hopefully always will be. When they’re gone, it’s time to leave. Hopefully, they’ll be with me until I’m ready to sign off.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
FDA updates breast implant–associated lymphoma cases, risk
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
Serial entrepreneur examines the risk-to-reward ratio balance in GI innovation
BOSTON – Just out of fellowship, Christopher C. Thompson, MD, director of therapeutic endoscopy, Brigham and Women’s Hospital, Boston, adapted an antireflux suturing device for use in a bariatric procedure. It worked so well he began using it routinely and taught others the technique. That was the first step in a journey that has taken him from consulting with industry to a founder of start-ups.
“The device company heard about what we were doing and were interested,” Dr. Thompson recounted during his How-I-Did-It lecture at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. In the end, he served as a consultant in the development of a new suturing device specific for the bariatric procedure. This included helping secure a patent and learning first-hand what steps are needed to get a device to market.
“I do not have any regrets. It was good for my career and fun to be involved, but there was not much financial gain for me or for my department,” Dr. Thompson said.
His subsequent experience with licensing was an incremental step forward. In one example, he worked on developing an endoscopic simulator, an important unmet need both for teaching and evaluating skills in diagnostic colonoscopy, including a kinematic analysis that helped identify techniques that are associated with high levels of skill.
“We developed the technology in-house through a series of grants. The risks were low, but the rewards were better because the money helped fund activities in our department,” he said.
That device, too, has been very successful, but Dr. Thompson said it is important to recognize how far innovation can go when the work stays in the academic setting and the goal is licensing the technology. More recently, he took a nonsurgical anastomosis device through preclinical testing, but he was then unable to attract a device company for the next steps of development.
“With no one interested, we created a start-up,” Dr. Thompson said. The company, GI Windows, has now taken this product, a magnetic endo-luminal anastomosis bypass device for the treatment of diabetes mellitus, into advanced stages of clinical testing. Relative to licensing arrangements, this involved a different level of participation.
“A start-up means creating a board, raising money, and being involved in details that can involve a lot of heavy lifting,” Dr. Thompson said. “It is basically a second job.”
The ongoing clinical studies in patients with diabetes have been very encouraging. Dr. Thompson reported that a large proportion of patients with diabetes fitted with the device have been able to reduce or discontinue their antidiabetic medications, and high rates of excess weight loss have been documented.
GI Windows was created for the sole purpose of developing the anastomosis device, but Dr. Thompson was also involved in creating another company, now sold, that started without a specific device in mind.
“The products we developed were just from brainstorming on unmet needs, and we had several successes. That was a chance to learn new areas of the business, including building a sales force and learning how to get involved in international distribution, which were separate from trying simply to produce a viable clinical tool,” he said.
Creating companies, rather than licensing ideas, trades higher risk for greater reward, but Dr. Thompson emphasized that these rewards are not just financial.
“It is exciting to develop a team you trust, get a successful company off the ground, and watch it grow,” Dr. Thompson said. He indicated that the risk-to-reward calculation should not be undertaken independent of the value of the learning experience.
BOSTON – Just out of fellowship, Christopher C. Thompson, MD, director of therapeutic endoscopy, Brigham and Women’s Hospital, Boston, adapted an antireflux suturing device for use in a bariatric procedure. It worked so well he began using it routinely and taught others the technique. That was the first step in a journey that has taken him from consulting with industry to a founder of start-ups.
“The device company heard about what we were doing and were interested,” Dr. Thompson recounted during his How-I-Did-It lecture at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. In the end, he served as a consultant in the development of a new suturing device specific for the bariatric procedure. This included helping secure a patent and learning first-hand what steps are needed to get a device to market.
“I do not have any regrets. It was good for my career and fun to be involved, but there was not much financial gain for me or for my department,” Dr. Thompson said.
His subsequent experience with licensing was an incremental step forward. In one example, he worked on developing an endoscopic simulator, an important unmet need both for teaching and evaluating skills in diagnostic colonoscopy, including a kinematic analysis that helped identify techniques that are associated with high levels of skill.
“We developed the technology in-house through a series of grants. The risks were low, but the rewards were better because the money helped fund activities in our department,” he said.
That device, too, has been very successful, but Dr. Thompson said it is important to recognize how far innovation can go when the work stays in the academic setting and the goal is licensing the technology. More recently, he took a nonsurgical anastomosis device through preclinical testing, but he was then unable to attract a device company for the next steps of development.
“With no one interested, we created a start-up,” Dr. Thompson said. The company, GI Windows, has now taken this product, a magnetic endo-luminal anastomosis bypass device for the treatment of diabetes mellitus, into advanced stages of clinical testing. Relative to licensing arrangements, this involved a different level of participation.
“A start-up means creating a board, raising money, and being involved in details that can involve a lot of heavy lifting,” Dr. Thompson said. “It is basically a second job.”
The ongoing clinical studies in patients with diabetes have been very encouraging. Dr. Thompson reported that a large proportion of patients with diabetes fitted with the device have been able to reduce or discontinue their antidiabetic medications, and high rates of excess weight loss have been documented.
GI Windows was created for the sole purpose of developing the anastomosis device, but Dr. Thompson was also involved in creating another company, now sold, that started without a specific device in mind.
“The products we developed were just from brainstorming on unmet needs, and we had several successes. That was a chance to learn new areas of the business, including building a sales force and learning how to get involved in international distribution, which were separate from trying simply to produce a viable clinical tool,” he said.
Creating companies, rather than licensing ideas, trades higher risk for greater reward, but Dr. Thompson emphasized that these rewards are not just financial.
“It is exciting to develop a team you trust, get a successful company off the ground, and watch it grow,” Dr. Thompson said. He indicated that the risk-to-reward calculation should not be undertaken independent of the value of the learning experience.
BOSTON – Just out of fellowship, Christopher C. Thompson, MD, director of therapeutic endoscopy, Brigham and Women’s Hospital, Boston, adapted an antireflux suturing device for use in a bariatric procedure. It worked so well he began using it routinely and taught others the technique. That was the first step in a journey that has taken him from consulting with industry to a founder of start-ups.
“The device company heard about what we were doing and were interested,” Dr. Thompson recounted during his How-I-Did-It lecture at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. In the end, he served as a consultant in the development of a new suturing device specific for the bariatric procedure. This included helping secure a patent and learning first-hand what steps are needed to get a device to market.
“I do not have any regrets. It was good for my career and fun to be involved, but there was not much financial gain for me or for my department,” Dr. Thompson said.
His subsequent experience with licensing was an incremental step forward. In one example, he worked on developing an endoscopic simulator, an important unmet need both for teaching and evaluating skills in diagnostic colonoscopy, including a kinematic analysis that helped identify techniques that are associated with high levels of skill.
“We developed the technology in-house through a series of grants. The risks were low, but the rewards were better because the money helped fund activities in our department,” he said.
That device, too, has been very successful, but Dr. Thompson said it is important to recognize how far innovation can go when the work stays in the academic setting and the goal is licensing the technology. More recently, he took a nonsurgical anastomosis device through preclinical testing, but he was then unable to attract a device company for the next steps of development.
“With no one interested, we created a start-up,” Dr. Thompson said. The company, GI Windows, has now taken this product, a magnetic endo-luminal anastomosis bypass device for the treatment of diabetes mellitus, into advanced stages of clinical testing. Relative to licensing arrangements, this involved a different level of participation.
“A start-up means creating a board, raising money, and being involved in details that can involve a lot of heavy lifting,” Dr. Thompson said. “It is basically a second job.”
The ongoing clinical studies in patients with diabetes have been very encouraging. Dr. Thompson reported that a large proportion of patients with diabetes fitted with the device have been able to reduce or discontinue their antidiabetic medications, and high rates of excess weight loss have been documented.
GI Windows was created for the sole purpose of developing the anastomosis device, but Dr. Thompson was also involved in creating another company, now sold, that started without a specific device in mind.
“The products we developed were just from brainstorming on unmet needs, and we had several successes. That was a chance to learn new areas of the business, including building a sales force and learning how to get involved in international distribution, which were separate from trying simply to produce a viable clinical tool,” he said.
Creating companies, rather than licensing ideas, trades higher risk for greater reward, but Dr. Thompson emphasized that these rewards are not just financial.
“It is exciting to develop a team you trust, get a successful company off the ground, and watch it grow,” Dr. Thompson said. He indicated that the risk-to-reward calculation should not be undertaken independent of the value of the learning experience.
REPORTING FROM 2018 AGA TECH SUMMIT
Study links RA flares after joint replacement to disease activity, not medications
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Sixty-five percent of RA patients developed flares after joint replacement surgery, and it was more common in those with higher baseline RA activity (odds ratio, 2.11; P = .015).
Study details: Prospective study of 120 patients with RA who underwent hip replacement (44%) or knee replacement (56%).
Disclosures: The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. The lead author disclosed receiving research funding from Novartis and Roche.
Source: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366.
Low microbiota diversity linked to poor survival after transplant
SALT LAKE CITY – A multicenter study confirmed that diversity of gut microbiota is associated with better survival after allogeneic hematopoietic cell transplantation (HCT), while low diversity and the predominance of pathogenic bacteria are linked to graft versus host disease (GVHD).
Lower calorie intake and exposure to broad-spectrum antibiotics were both associated with lower diversity, the study found.
“One of the striking findings early on was this association between diversity in the gut and overall survival,” said Jonathan Peled, MD, PhD, noting that his research group also saw that high gut diversity was associated with lower rates of GVHD-related mortality.
“The first question that I want to ask today is ‘Are the patterns of microbiota injury that have been described in single-center studies and their association with clinical outcomes consistent across geography?’” Dr. Peled said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
To answer this, Dr. Peled and his associates at Memorial Sloan Kettering Cancer Center (MSKCC), New York, teamed up with a research group at Duke University, Durham, N.C., and with investigators in Regensburg, Germany. The international group devised a study that would use centralized sequencing and analysis to examine patient fecal samples from all three centers.
In all, 5,310 samples were obtained from 1,034 HCT patients. MSKCC contributed most of the samples (n = 908, 87.8%), with Regensburg contributing 79 (7.6%) and Duke contributing 47 (4.5%).
The most common malignancies treated were acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. The balance of graft sources and conditioning intensity varied between centers, but overall, more than three-quarters of grafts were from peripheral blood stem cells and just over half of patients received myeloablative conditioning.
The centralized microbiota profiling involved extracting bacterial DNA, and then using polymerase chain reaction to amplify 16sRNA for sequencing and subsequent taxonomic identification.
“Samples can be segregated into clusters according to microbiota composition,” said Dr. Peled, a medical oncologist at MSKCC. The investigators used an algorithm called t-distributed stochastic neighbor embedding, or tSNE, to help detect patterns in microbiota composition and diversity before and throughout the HCT process. Visualizations using tSNE allow for two-dimensional representations of complicated associations and interrelatedness in data.
“Color-coded by diversity and time, we see that these early samples tend to be more diverse,” in the tSNE analyses, Dr. Peled said. The later clusters, he said, show evidence of lower diversity and injury.
Individual samples can also be coded in a way that shows clusters by abundance of various bacterial taxa, Dr. Peled said. “The early, diverse cluster tends to be dominated, or filled, by anaerobic commensals such as Firmicutes and Clostridia, which we and others have found are associated with good outcomes after transplant.”
The lower-diversity states seen later, after transplant, tend to be dominated by a variety of pathogenic bacteria, Dr. Peled said. These include Enterococcus and Proteobacteria, a phylum that includes Klebsiella and Escherichia coli species. This predominance has been associated with subsequent bacteremia, he said.
“Patients tend to enter transplant with a relatively diverse flora, and a frequent event in the posttransplant samples is domination by these pathobiomes,” Dr. Peled said. “In some cases, almost the entire composition of the gut is [composed] of a single species.” This loss of diversity and single-species domination was seen across the three geographically diverse research sites, he said.
This decimation of diversity is linked to poor transplant outcomes. In particular, Dr. Peled said, an enterococcus-dominated gut had previously been associated with higher risk for acute GVHD and with gastrointestinal GVHD.
Here, the multisite data showed that at Regensburg, higher enterococcus abundance on days 7-14 post HCT was associated with increased risk of GI GVHD. At MSKCC, enterococcus domination was associated with a hazard ratio of 1.4 for acute GVHD (P = .008). The MSKCC group used data from 503 patients, defining domination as at least 30% relative abundance in any sample from post-HCT days 7-21.
Patients at both MSKCC and Regensburg had a better chance of overall survival if they had high intestinal microbial diversity around the period of neutrophil engraftment, as seen in a sample collected within 7 days of post-HCT day 14. At MSKCC, data for 651 patients showed a statistically significant association (P = .006); this finding was reproduced at Regensburg, which also saw a significant association (P = .015) for the 59 patients studied, Dr. Peled reported.
Increased treatment-related mortality was seen for patients who had low microbial diversity following neutrophil engraftment as well. Of 372 MSKCC patients who had samples available 7-50 days after engraftment, high diversity was associated with better overall survival, and with lower treatment-related mortality (P = .03 for both).
Dr. Peled and his collaborators also divided patients into quartiles by amount of biodiversity. They found that comparing the highest to the lowest biodiversity quartile showed significantly overall survival benefits for the highest-diversity group (P = .007).
The problem starts before transplant, Dr. Peled explained. The researchers found that compared with healthy controls at MSKCC and data from the Human Microbiome Project, HCT patients entered their transplant with significantly less gut biodiversity.
The second question to be addressed is “What are the key environmental determinants of intestinal microbiota composition?” said Dr. Peled.
“Peri-HCT exposure to broad-spectrum antibiotics is associated with lower intestinal microbial diversity,” he said. For 5,936 samples taken from 976 patients receiving allogeneic HCT, the most significant difference in diversity between those with and without broad-spectrum antibiotic exposure was seen at day 15 post transplant (P = .008).
Higher calorie intake was also associated with greater diversity (P less than .001). Higher dietary fiber intake was associated with higher abundance of Blautia, a genus considered to be a healthy commensal microorganism, Dr. Peled said.
“Conditioning intensity is associated with the magnitude of diversity loss, and with distinct microbiome configurations,” said Dr. Peled. Using 4,311 samples from 908 patients, a myeloablative conditioning regimen (n = 508) was associated with significantly less diversity when compared with reduced intensity (n = 316) and nonmyeloablative regimens (n = 84; P =.002 and P less than .001, respectively).
To answer a third question – What is the natural history of recovery from microbiota injury after HCT? – the investigators looked at trends over time for 28 allogeneic HCT recipients. With a total of 294 samples for analysis, Dr. Peled and his group found that “diversity increases, but often to a configuration distinct from the pre-HCT state.” It took some patients nearly a year to return to their pretransplant level of diversity.
Patients in the subset of those who go on to develop lower gastrointestinal GVHD have an intestinal microbiota composition that is distinct from those patients whose GVHD exclusively involved the upper gastrointestinal tract, the skin, or the liver (P = .019), Dr. Peled said.
He and his team are currently enrolling patients for a phase 2 randomized clinical trial (NCT03078010) that will explore strategies to deescalate the use of broad-spectrum antibiotics for febrile neutropenia in patients with allogeneic HCT. The trial will randomize patients to receive either piperacillin-tazobactam, the current standard of care at MSKCC, or cefepime with deescalation to aztreonam with vancomycin, the microbiota-sparing strategy. The trial will examine the abundance of Clostridiales and Blautia species, gut biodiversity, the rate of GVHD, bacteremia, and survival rates.
The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics
SOURCE: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
SALT LAKE CITY – A multicenter study confirmed that diversity of gut microbiota is associated with better survival after allogeneic hematopoietic cell transplantation (HCT), while low diversity and the predominance of pathogenic bacteria are linked to graft versus host disease (GVHD).
Lower calorie intake and exposure to broad-spectrum antibiotics were both associated with lower diversity, the study found.
“One of the striking findings early on was this association between diversity in the gut and overall survival,” said Jonathan Peled, MD, PhD, noting that his research group also saw that high gut diversity was associated with lower rates of GVHD-related mortality.
“The first question that I want to ask today is ‘Are the patterns of microbiota injury that have been described in single-center studies and their association with clinical outcomes consistent across geography?’” Dr. Peled said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
To answer this, Dr. Peled and his associates at Memorial Sloan Kettering Cancer Center (MSKCC), New York, teamed up with a research group at Duke University, Durham, N.C., and with investigators in Regensburg, Germany. The international group devised a study that would use centralized sequencing and analysis to examine patient fecal samples from all three centers.
In all, 5,310 samples were obtained from 1,034 HCT patients. MSKCC contributed most of the samples (n = 908, 87.8%), with Regensburg contributing 79 (7.6%) and Duke contributing 47 (4.5%).
The most common malignancies treated were acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. The balance of graft sources and conditioning intensity varied between centers, but overall, more than three-quarters of grafts were from peripheral blood stem cells and just over half of patients received myeloablative conditioning.
The centralized microbiota profiling involved extracting bacterial DNA, and then using polymerase chain reaction to amplify 16sRNA for sequencing and subsequent taxonomic identification.
“Samples can be segregated into clusters according to microbiota composition,” said Dr. Peled, a medical oncologist at MSKCC. The investigators used an algorithm called t-distributed stochastic neighbor embedding, or tSNE, to help detect patterns in microbiota composition and diversity before and throughout the HCT process. Visualizations using tSNE allow for two-dimensional representations of complicated associations and interrelatedness in data.
“Color-coded by diversity and time, we see that these early samples tend to be more diverse,” in the tSNE analyses, Dr. Peled said. The later clusters, he said, show evidence of lower diversity and injury.
Individual samples can also be coded in a way that shows clusters by abundance of various bacterial taxa, Dr. Peled said. “The early, diverse cluster tends to be dominated, or filled, by anaerobic commensals such as Firmicutes and Clostridia, which we and others have found are associated with good outcomes after transplant.”
The lower-diversity states seen later, after transplant, tend to be dominated by a variety of pathogenic bacteria, Dr. Peled said. These include Enterococcus and Proteobacteria, a phylum that includes Klebsiella and Escherichia coli species. This predominance has been associated with subsequent bacteremia, he said.
“Patients tend to enter transplant with a relatively diverse flora, and a frequent event in the posttransplant samples is domination by these pathobiomes,” Dr. Peled said. “In some cases, almost the entire composition of the gut is [composed] of a single species.” This loss of diversity and single-species domination was seen across the three geographically diverse research sites, he said.
This decimation of diversity is linked to poor transplant outcomes. In particular, Dr. Peled said, an enterococcus-dominated gut had previously been associated with higher risk for acute GVHD and with gastrointestinal GVHD.
Here, the multisite data showed that at Regensburg, higher enterococcus abundance on days 7-14 post HCT was associated with increased risk of GI GVHD. At MSKCC, enterococcus domination was associated with a hazard ratio of 1.4 for acute GVHD (P = .008). The MSKCC group used data from 503 patients, defining domination as at least 30% relative abundance in any sample from post-HCT days 7-21.
Patients at both MSKCC and Regensburg had a better chance of overall survival if they had high intestinal microbial diversity around the period of neutrophil engraftment, as seen in a sample collected within 7 days of post-HCT day 14. At MSKCC, data for 651 patients showed a statistically significant association (P = .006); this finding was reproduced at Regensburg, which also saw a significant association (P = .015) for the 59 patients studied, Dr. Peled reported.
Increased treatment-related mortality was seen for patients who had low microbial diversity following neutrophil engraftment as well. Of 372 MSKCC patients who had samples available 7-50 days after engraftment, high diversity was associated with better overall survival, and with lower treatment-related mortality (P = .03 for both).
Dr. Peled and his collaborators also divided patients into quartiles by amount of biodiversity. They found that comparing the highest to the lowest biodiversity quartile showed significantly overall survival benefits for the highest-diversity group (P = .007).
The problem starts before transplant, Dr. Peled explained. The researchers found that compared with healthy controls at MSKCC and data from the Human Microbiome Project, HCT patients entered their transplant with significantly less gut biodiversity.
The second question to be addressed is “What are the key environmental determinants of intestinal microbiota composition?” said Dr. Peled.
“Peri-HCT exposure to broad-spectrum antibiotics is associated with lower intestinal microbial diversity,” he said. For 5,936 samples taken from 976 patients receiving allogeneic HCT, the most significant difference in diversity between those with and without broad-spectrum antibiotic exposure was seen at day 15 post transplant (P = .008).
Higher calorie intake was also associated with greater diversity (P less than .001). Higher dietary fiber intake was associated with higher abundance of Blautia, a genus considered to be a healthy commensal microorganism, Dr. Peled said.
“Conditioning intensity is associated with the magnitude of diversity loss, and with distinct microbiome configurations,” said Dr. Peled. Using 4,311 samples from 908 patients, a myeloablative conditioning regimen (n = 508) was associated with significantly less diversity when compared with reduced intensity (n = 316) and nonmyeloablative regimens (n = 84; P =.002 and P less than .001, respectively).
To answer a third question – What is the natural history of recovery from microbiota injury after HCT? – the investigators looked at trends over time for 28 allogeneic HCT recipients. With a total of 294 samples for analysis, Dr. Peled and his group found that “diversity increases, but often to a configuration distinct from the pre-HCT state.” It took some patients nearly a year to return to their pretransplant level of diversity.
Patients in the subset of those who go on to develop lower gastrointestinal GVHD have an intestinal microbiota composition that is distinct from those patients whose GVHD exclusively involved the upper gastrointestinal tract, the skin, or the liver (P = .019), Dr. Peled said.
He and his team are currently enrolling patients for a phase 2 randomized clinical trial (NCT03078010) that will explore strategies to deescalate the use of broad-spectrum antibiotics for febrile neutropenia in patients with allogeneic HCT. The trial will randomize patients to receive either piperacillin-tazobactam, the current standard of care at MSKCC, or cefepime with deescalation to aztreonam with vancomycin, the microbiota-sparing strategy. The trial will examine the abundance of Clostridiales and Blautia species, gut biodiversity, the rate of GVHD, bacteremia, and survival rates.
The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics
SOURCE: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
SALT LAKE CITY – A multicenter study confirmed that diversity of gut microbiota is associated with better survival after allogeneic hematopoietic cell transplantation (HCT), while low diversity and the predominance of pathogenic bacteria are linked to graft versus host disease (GVHD).
Lower calorie intake and exposure to broad-spectrum antibiotics were both associated with lower diversity, the study found.
“One of the striking findings early on was this association between diversity in the gut and overall survival,” said Jonathan Peled, MD, PhD, noting that his research group also saw that high gut diversity was associated with lower rates of GVHD-related mortality.
“The first question that I want to ask today is ‘Are the patterns of microbiota injury that have been described in single-center studies and their association with clinical outcomes consistent across geography?’” Dr. Peled said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
To answer this, Dr. Peled and his associates at Memorial Sloan Kettering Cancer Center (MSKCC), New York, teamed up with a research group at Duke University, Durham, N.C., and with investigators in Regensburg, Germany. The international group devised a study that would use centralized sequencing and analysis to examine patient fecal samples from all three centers.
In all, 5,310 samples were obtained from 1,034 HCT patients. MSKCC contributed most of the samples (n = 908, 87.8%), with Regensburg contributing 79 (7.6%) and Duke contributing 47 (4.5%).
The most common malignancies treated were acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. The balance of graft sources and conditioning intensity varied between centers, but overall, more than three-quarters of grafts were from peripheral blood stem cells and just over half of patients received myeloablative conditioning.
The centralized microbiota profiling involved extracting bacterial DNA, and then using polymerase chain reaction to amplify 16sRNA for sequencing and subsequent taxonomic identification.
“Samples can be segregated into clusters according to microbiota composition,” said Dr. Peled, a medical oncologist at MSKCC. The investigators used an algorithm called t-distributed stochastic neighbor embedding, or tSNE, to help detect patterns in microbiota composition and diversity before and throughout the HCT process. Visualizations using tSNE allow for two-dimensional representations of complicated associations and interrelatedness in data.
“Color-coded by diversity and time, we see that these early samples tend to be more diverse,” in the tSNE analyses, Dr. Peled said. The later clusters, he said, show evidence of lower diversity and injury.
Individual samples can also be coded in a way that shows clusters by abundance of various bacterial taxa, Dr. Peled said. “The early, diverse cluster tends to be dominated, or filled, by anaerobic commensals such as Firmicutes and Clostridia, which we and others have found are associated with good outcomes after transplant.”
The lower-diversity states seen later, after transplant, tend to be dominated by a variety of pathogenic bacteria, Dr. Peled said. These include Enterococcus and Proteobacteria, a phylum that includes Klebsiella and Escherichia coli species. This predominance has been associated with subsequent bacteremia, he said.
“Patients tend to enter transplant with a relatively diverse flora, and a frequent event in the posttransplant samples is domination by these pathobiomes,” Dr. Peled said. “In some cases, almost the entire composition of the gut is [composed] of a single species.” This loss of diversity and single-species domination was seen across the three geographically diverse research sites, he said.
This decimation of diversity is linked to poor transplant outcomes. In particular, Dr. Peled said, an enterococcus-dominated gut had previously been associated with higher risk for acute GVHD and with gastrointestinal GVHD.
Here, the multisite data showed that at Regensburg, higher enterococcus abundance on days 7-14 post HCT was associated with increased risk of GI GVHD. At MSKCC, enterococcus domination was associated with a hazard ratio of 1.4 for acute GVHD (P = .008). The MSKCC group used data from 503 patients, defining domination as at least 30% relative abundance in any sample from post-HCT days 7-21.
Patients at both MSKCC and Regensburg had a better chance of overall survival if they had high intestinal microbial diversity around the period of neutrophil engraftment, as seen in a sample collected within 7 days of post-HCT day 14. At MSKCC, data for 651 patients showed a statistically significant association (P = .006); this finding was reproduced at Regensburg, which also saw a significant association (P = .015) for the 59 patients studied, Dr. Peled reported.
Increased treatment-related mortality was seen for patients who had low microbial diversity following neutrophil engraftment as well. Of 372 MSKCC patients who had samples available 7-50 days after engraftment, high diversity was associated with better overall survival, and with lower treatment-related mortality (P = .03 for both).
Dr. Peled and his collaborators also divided patients into quartiles by amount of biodiversity. They found that comparing the highest to the lowest biodiversity quartile showed significantly overall survival benefits for the highest-diversity group (P = .007).
The problem starts before transplant, Dr. Peled explained. The researchers found that compared with healthy controls at MSKCC and data from the Human Microbiome Project, HCT patients entered their transplant with significantly less gut biodiversity.
The second question to be addressed is “What are the key environmental determinants of intestinal microbiota composition?” said Dr. Peled.
“Peri-HCT exposure to broad-spectrum antibiotics is associated with lower intestinal microbial diversity,” he said. For 5,936 samples taken from 976 patients receiving allogeneic HCT, the most significant difference in diversity between those with and without broad-spectrum antibiotic exposure was seen at day 15 post transplant (P = .008).
Higher calorie intake was also associated with greater diversity (P less than .001). Higher dietary fiber intake was associated with higher abundance of Blautia, a genus considered to be a healthy commensal microorganism, Dr. Peled said.
“Conditioning intensity is associated with the magnitude of diversity loss, and with distinct microbiome configurations,” said Dr. Peled. Using 4,311 samples from 908 patients, a myeloablative conditioning regimen (n = 508) was associated with significantly less diversity when compared with reduced intensity (n = 316) and nonmyeloablative regimens (n = 84; P =.002 and P less than .001, respectively).
To answer a third question – What is the natural history of recovery from microbiota injury after HCT? – the investigators looked at trends over time for 28 allogeneic HCT recipients. With a total of 294 samples for analysis, Dr. Peled and his group found that “diversity increases, but often to a configuration distinct from the pre-HCT state.” It took some patients nearly a year to return to their pretransplant level of diversity.
Patients in the subset of those who go on to develop lower gastrointestinal GVHD have an intestinal microbiota composition that is distinct from those patients whose GVHD exclusively involved the upper gastrointestinal tract, the skin, or the liver (P = .019), Dr. Peled said.
He and his team are currently enrolling patients for a phase 2 randomized clinical trial (NCT03078010) that will explore strategies to deescalate the use of broad-spectrum antibiotics for febrile neutropenia in patients with allogeneic HCT. The trial will randomize patients to receive either piperacillin-tazobactam, the current standard of care at MSKCC, or cefepime with deescalation to aztreonam with vancomycin, the microbiota-sparing strategy. The trial will examine the abundance of Clostridiales and Blautia species, gut biodiversity, the rate of GVHD, bacteremia, and survival rates.
The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics
SOURCE: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: High microbiota diversity post transplant was associated with better overall survival at two sites (P = .006 and P = .015).
Study details: Multicenter study of 5,310 fecal samples obtained from 1,034 hematopoietic cell transplant recipients.
Disclosures: The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics.
Source: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.