User login
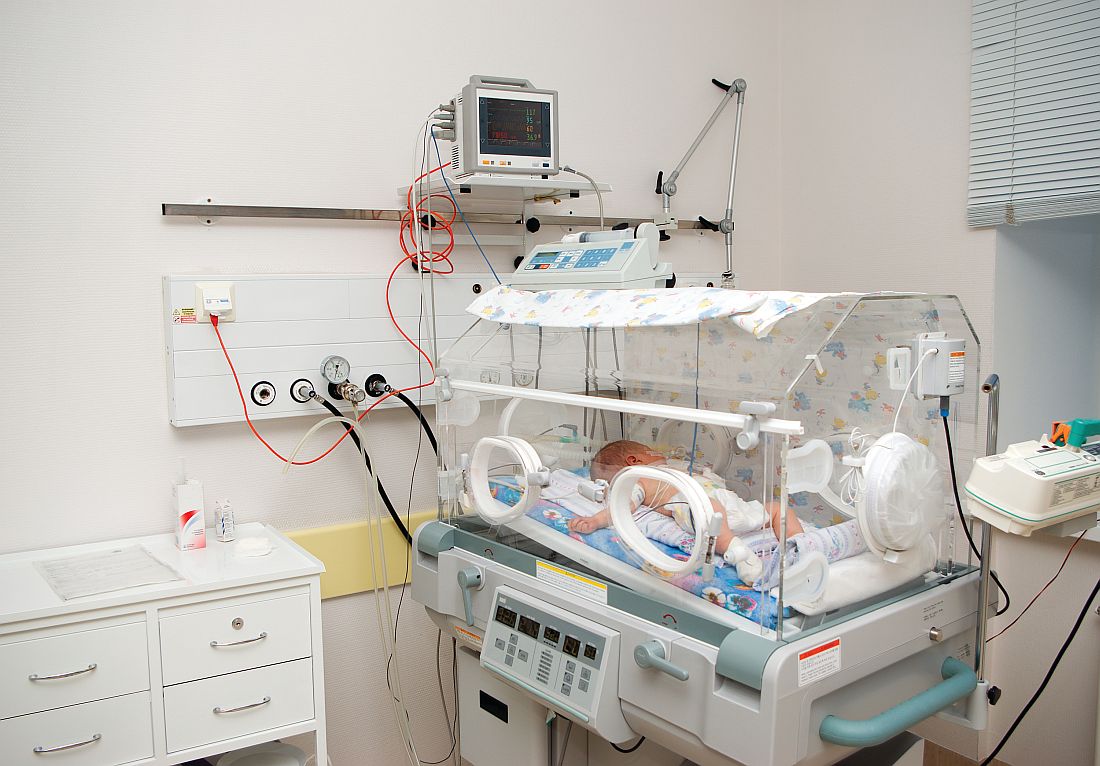
Interventions urged to stop rising NAS, stem Medicaid costs
The incidence of neonatal abstinence syndrome (NAS) and the corresponding costs to Medicaid are unlikely to decline unless interventions focus on stopping opioid use by low-income mothers, said Tyler N.A. Winkelman, MD, of Hennepin County Medical Center, Minneapolis, and his associates.
Dr. Winkelman and his associates conducted a serial cross-sectional analysis that used 9,115,457 birth discharge records from the 2004-2014 National Inpatient Sample (NIS), which were representative of 43.6 million weighted births. Overall, 3,991,336 infants were covered by Medicaid, which were representative of 19.1 million weighted births. There were 35,629 (0.89%) infants with a diagnosis of NAS, which were representative of 173,384 weighted births. Medicaid was the primary payer for 74% (95% confidence interval, 68.9%-77.9%) of NAS-related births in 2004 and 82% (95% CI, 80.5%-83.5%) of NAS-related births in 2014.
Infants with NAS who were enrolled in Medicaid were significantly more likely to be male, live in a rural county, and have comorbidities reflective of the syndrome than were infants without NAS who were enrolled in Medicaid, the researchers wrote in Pediatrics.
from 1.5 per 1,000 hospital births (95% CI, 1.2-1.9) to 8 per 1,000 hospital births (95% CI, 7.2-8.7), as the opioid epidemic worsened in the country.
Infants with NAS who were covered by Medicaid had a greater chance of being transferred to another hospital for care (9% vs. 7%; P = .02) and stay in the hospital longer (17 days vs. 15 days; P less than .001), compared with infants with NAS who were covered by private insurance.
NAS is costly. In the 2011-2014 era, mean hospital costs for a NAS infant covered by Medicaid were more than fivefold higher than for an infant without NAS ($19,340/birth vs. $3,700/birth; P less than .001). After adjustment for inflation, mean hospital costs for infants with NAS who were covered by Medicaid increased 26% between 2004-2006 and 2011-2014 ($15,350 vs. $19,340; P less than .001), the researchers reported. Annual hospital costs, which were adjusted for inflation to 2014 U.S. dollars, for all infants with NAS who were covered by Medicaid rose from $65.4 million in 2004 to $462 million in 2014.
“With the disproportionate impact of NAS on the Medicaid population, we suggest that NAS incidence rates are unlikely to improve without interventions targeted at low-income mothers and infants,” Dr. Winkelman and his associates concluded.
Nonpharmacologic interventions are available to help NAS infants. “Systematic implementation of policies that support rooming-in, breastfeeding, swaddling, on-demand feeding schedules, and minimization of sleep disruption may reduce symptoms of NAS and reduce the duration of, or even eliminate the need for, pharmacologic treatment of NAS,” the researchers said. “Pharmacologic treatment with buprenorphine, for example, has been shown to reduce hospital length of stay by 35%.”
Another intervention is medication-assisted treatment during pregnancy, which studies have shown “improves outcomes and reduces costs associated with NAS, compared with attempted abstinence,” Dr. Winkelman and his associates said. It does not necessarily reduce NAS incidence, but “it may prevent prolonged hospital stays due to preterm birth, reduce NICU [neonatal intensive care unit] admissions, decrease the severity of NAS symptoms, and improve birth outcomes for some infants.”
The researchers also endorsed screening, referral, and treatment for substance abuse and mental health disorders among reproductive age women, including adolescents, because these are risk factors for opioid abuse.
One author was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
SOURCE: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
The incidence of neonatal abstinence syndrome (NAS) and the corresponding costs to Medicaid are unlikely to decline unless interventions focus on stopping opioid use by low-income mothers, said Tyler N.A. Winkelman, MD, of Hennepin County Medical Center, Minneapolis, and his associates.
Dr. Winkelman and his associates conducted a serial cross-sectional analysis that used 9,115,457 birth discharge records from the 2004-2014 National Inpatient Sample (NIS), which were representative of 43.6 million weighted births. Overall, 3,991,336 infants were covered by Medicaid, which were representative of 19.1 million weighted births. There were 35,629 (0.89%) infants with a diagnosis of NAS, which were representative of 173,384 weighted births. Medicaid was the primary payer for 74% (95% confidence interval, 68.9%-77.9%) of NAS-related births in 2004 and 82% (95% CI, 80.5%-83.5%) of NAS-related births in 2014.
Infants with NAS who were enrolled in Medicaid were significantly more likely to be male, live in a rural county, and have comorbidities reflective of the syndrome than were infants without NAS who were enrolled in Medicaid, the researchers wrote in Pediatrics.
from 1.5 per 1,000 hospital births (95% CI, 1.2-1.9) to 8 per 1,000 hospital births (95% CI, 7.2-8.7), as the opioid epidemic worsened in the country.
Infants with NAS who were covered by Medicaid had a greater chance of being transferred to another hospital for care (9% vs. 7%; P = .02) and stay in the hospital longer (17 days vs. 15 days; P less than .001), compared with infants with NAS who were covered by private insurance.
NAS is costly. In the 2011-2014 era, mean hospital costs for a NAS infant covered by Medicaid were more than fivefold higher than for an infant without NAS ($19,340/birth vs. $3,700/birth; P less than .001). After adjustment for inflation, mean hospital costs for infants with NAS who were covered by Medicaid increased 26% between 2004-2006 and 2011-2014 ($15,350 vs. $19,340; P less than .001), the researchers reported. Annual hospital costs, which were adjusted for inflation to 2014 U.S. dollars, for all infants with NAS who were covered by Medicaid rose from $65.4 million in 2004 to $462 million in 2014.
“With the disproportionate impact of NAS on the Medicaid population, we suggest that NAS incidence rates are unlikely to improve without interventions targeted at low-income mothers and infants,” Dr. Winkelman and his associates concluded.
Nonpharmacologic interventions are available to help NAS infants. “Systematic implementation of policies that support rooming-in, breastfeeding, swaddling, on-demand feeding schedules, and minimization of sleep disruption may reduce symptoms of NAS and reduce the duration of, or even eliminate the need for, pharmacologic treatment of NAS,” the researchers said. “Pharmacologic treatment with buprenorphine, for example, has been shown to reduce hospital length of stay by 35%.”
Another intervention is medication-assisted treatment during pregnancy, which studies have shown “improves outcomes and reduces costs associated with NAS, compared with attempted abstinence,” Dr. Winkelman and his associates said. It does not necessarily reduce NAS incidence, but “it may prevent prolonged hospital stays due to preterm birth, reduce NICU [neonatal intensive care unit] admissions, decrease the severity of NAS symptoms, and improve birth outcomes for some infants.”
The researchers also endorsed screening, referral, and treatment for substance abuse and mental health disorders among reproductive age women, including adolescents, because these are risk factors for opioid abuse.
One author was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
SOURCE: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
The incidence of neonatal abstinence syndrome (NAS) and the corresponding costs to Medicaid are unlikely to decline unless interventions focus on stopping opioid use by low-income mothers, said Tyler N.A. Winkelman, MD, of Hennepin County Medical Center, Minneapolis, and his associates.
Dr. Winkelman and his associates conducted a serial cross-sectional analysis that used 9,115,457 birth discharge records from the 2004-2014 National Inpatient Sample (NIS), which were representative of 43.6 million weighted births. Overall, 3,991,336 infants were covered by Medicaid, which were representative of 19.1 million weighted births. There were 35,629 (0.89%) infants with a diagnosis of NAS, which were representative of 173,384 weighted births. Medicaid was the primary payer for 74% (95% confidence interval, 68.9%-77.9%) of NAS-related births in 2004 and 82% (95% CI, 80.5%-83.5%) of NAS-related births in 2014.
Infants with NAS who were enrolled in Medicaid were significantly more likely to be male, live in a rural county, and have comorbidities reflective of the syndrome than were infants without NAS who were enrolled in Medicaid, the researchers wrote in Pediatrics.
from 1.5 per 1,000 hospital births (95% CI, 1.2-1.9) to 8 per 1,000 hospital births (95% CI, 7.2-8.7), as the opioid epidemic worsened in the country.
Infants with NAS who were covered by Medicaid had a greater chance of being transferred to another hospital for care (9% vs. 7%; P = .02) and stay in the hospital longer (17 days vs. 15 days; P less than .001), compared with infants with NAS who were covered by private insurance.
NAS is costly. In the 2011-2014 era, mean hospital costs for a NAS infant covered by Medicaid were more than fivefold higher than for an infant without NAS ($19,340/birth vs. $3,700/birth; P less than .001). After adjustment for inflation, mean hospital costs for infants with NAS who were covered by Medicaid increased 26% between 2004-2006 and 2011-2014 ($15,350 vs. $19,340; P less than .001), the researchers reported. Annual hospital costs, which were adjusted for inflation to 2014 U.S. dollars, for all infants with NAS who were covered by Medicaid rose from $65.4 million in 2004 to $462 million in 2014.
“With the disproportionate impact of NAS on the Medicaid population, we suggest that NAS incidence rates are unlikely to improve without interventions targeted at low-income mothers and infants,” Dr. Winkelman and his associates concluded.
Nonpharmacologic interventions are available to help NAS infants. “Systematic implementation of policies that support rooming-in, breastfeeding, swaddling, on-demand feeding schedules, and minimization of sleep disruption may reduce symptoms of NAS and reduce the duration of, or even eliminate the need for, pharmacologic treatment of NAS,” the researchers said. “Pharmacologic treatment with buprenorphine, for example, has been shown to reduce hospital length of stay by 35%.”
Another intervention is medication-assisted treatment during pregnancy, which studies have shown “improves outcomes and reduces costs associated with NAS, compared with attempted abstinence,” Dr. Winkelman and his associates said. It does not necessarily reduce NAS incidence, but “it may prevent prolonged hospital stays due to preterm birth, reduce NICU [neonatal intensive care unit] admissions, decrease the severity of NAS symptoms, and improve birth outcomes for some infants.”
The researchers also endorsed screening, referral, and treatment for substance abuse and mental health disorders among reproductive age women, including adolescents, because these are risk factors for opioid abuse.
One author was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
SOURCE: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
FROM PEDIATRICS
Key clinical point:
Major finding: In 2011-2014, mean hospital costs for NAS infants covered by Medicaid were more than fivefold higher than for infants without NAS ($19,340/birth vs. $3,700/birth).
Study details: A serial cross-sectional analysis that usd data from the 2004-2014 National Inpatient Sample (NIS) of 9,115,457 birth discharge records.
Disclosures: Stephen W. Patrick, MD, was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
Source: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
Siponimod trial ‘first’ to show delayed disability in secondary progressive MS
Phase 3 data with siponimod, Novartis’ investigational treatment for multiple sclerosis, show a 21% reduction in the time to disease progression versus placebo in patients with the secondary progressive subtype of the disease.
A significantly lower percentage of patients with secondary progressive multiple sclerosis (SPMS) treated with siponimod met the primary endpoint of confirmed disease progression (CDP) at 3 months, compared with those who received placebo in the EXPAND trial (26% vs. 32% of patients; hazard ratio [HR], 0.79; 95% confidence interval [CI], 0.65–0.95; P = .013).
No significant difference between siponimod and placebo was seen in one of the two main secondary outcomes of the study – the time to 3-month confirmed worsening of the timed 25-foot walk test (T25FW). This result led Luanne Metz, MD, and Wei-Qiao Liu, MD, both of the department of clinical neurosciences and the Hotchkiss Brain Institute at the University of Calgary (Alta.), to take a cautionary view of the findings in an editorial about the trial’s results.
“Although siponimod seems to reduce the time to confirmed disability in SPMS, the treatment effect was small,” Dr. Metz and Dr. Liu argued.
“In our opinion, the reduction in the proportion of participants reaching the primary endpoint of only 6% and the absence of a significant difference for the key secondary clinical outcome [T25FW] are disappointing results and do not suggest that siponimod is an effective treatment for SPMS,” the two wrote.
“Confidence in the treatment benefit of siponimod in progressive MS will, in our opinion, require confirmation in a second trial. Trials of other novel treatments that target noninflammatory mechanisms are still needed,” they said.
EXPAND (Exploring the efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis) was an event- and exposure-driven double-blind trial that recruited patients with SPMS over a 2-year period starting in February 2013. Of 1,651 patients who were recruited, randomized, and actually received treatment, 1,099 were treated with oral siponimod, 2 mg once daily, and 546 were given a matching placebo.
Treatment was for up to 3 years or until 374 CDP events assessed via the Expanded Disability Status Scale (EDSS) had occurred. Patients who had CDP after 6 months in the double-blind trial could be re-consented and continue with double-blind treatment, switch to open-label siponimod, or stop study treatment and either remain on no treatment or receive another disease-modifying treatment.
On average, the patients had been diagnosed with SPMS for a mean of 3.8 years and had been first diagnosed with MS around 17 years prior to this.
The primary endpoint of the trial – CDP at 3 months – was first reported in 2016 at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). CDP was defined as a 0.5- to 1-point increase in EDSS depending on the baseline score.
A host of additional secondary endpoints were studied, with the risk of 6-month CDP being significantly reduced by siponimod versus placebo (HR, 0.74; 95% CI, 0.60-0.92; P = .0058).
The change from baseline in T2 lesion volume – the second of the two main secondary endpoints studied – showed a potential benefit of siponimod treatment over placebo, with a lower mean-adjusted increase in lesion volume over months 12 and 24 (183.9 mm3 vs. 879.2 mm3; P less than .0001).
More patients receiving siponimod than placebo were free from new or enlarging T2 lesions (57% vs. 37%) or T1 gadolinium-enhancing lesions (89% vs. 67%), and brain volume decreased at a lower rate with siponimod than with placebo.
Adverse events occurred at a higher rate with siponimod than with placebo (89% of patients vs. 82%), of which 18% and 15%, respectively, were defined as serious.
Adverse events seen more commonly in siponimod- than in placebo-treated patients were lymphopenia (1% vs. 0%), abnormal liver-related investigations (12% vs. 4%), hypertension (10% vs. 8%), and bradycardia (4% vs. 3%) and bradyarrhythmia (3% vs. 0.4%) at the start of treatment, which were mitigated by dose titration. Other adverse events that occurred less often but more frequently with siponimod included macular edema (2% vs. less than 1%), reactivation of varicella zoster (2% vs. 1%), and convulsions (2% vs. less than 1%).
“The safety profile of siponimod in EXPAND was generally aligned with that of other drugs in the class,” Dr. Kappos and his colleagues observed.
Siponimod, also known as BAF312, is a selective sphingosine 1-phosphate (S1P) receptor modulator that targets the targets the S1P 1 and 5 receptor subtypes; the other currently approved drug in its class is fingolimod (Gilenya), which is currently indicated for treating the relapsing-remitting form of multiple sclerosis (RRMS).
Phase 2 data have shown that siponimod could also be a promising treatment for RRMS, with reduced relapse rates and fewer brain lesions seen versus placebo (JAMA Neurol. 2016;73[9]:1089-98).
The EXPAND study was funded by Novartis. The lead author Dr. Kappos has received research and educational support funding via his institution from Novartis and multiple other pharmaceutical companies. Editorial author Dr. Metz has received grants from Roche and Biogen and personal fees from EMD Serono. The other editorial author, Dr. Liu, reported receiving personal fees from Novartis and personal fees and grants from EMD Serono. Dr. Liu was a back-up treating neurologist in the EXPAND trial.
SOURCE: Kappos L et al. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30475-6, and Metz L and Liu W. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30426-4.
Phase 3 data with siponimod, Novartis’ investigational treatment for multiple sclerosis, show a 21% reduction in the time to disease progression versus placebo in patients with the secondary progressive subtype of the disease.
A significantly lower percentage of patients with secondary progressive multiple sclerosis (SPMS) treated with siponimod met the primary endpoint of confirmed disease progression (CDP) at 3 months, compared with those who received placebo in the EXPAND trial (26% vs. 32% of patients; hazard ratio [HR], 0.79; 95% confidence interval [CI], 0.65–0.95; P = .013).
No significant difference between siponimod and placebo was seen in one of the two main secondary outcomes of the study – the time to 3-month confirmed worsening of the timed 25-foot walk test (T25FW). This result led Luanne Metz, MD, and Wei-Qiao Liu, MD, both of the department of clinical neurosciences and the Hotchkiss Brain Institute at the University of Calgary (Alta.), to take a cautionary view of the findings in an editorial about the trial’s results.
“Although siponimod seems to reduce the time to confirmed disability in SPMS, the treatment effect was small,” Dr. Metz and Dr. Liu argued.
“In our opinion, the reduction in the proportion of participants reaching the primary endpoint of only 6% and the absence of a significant difference for the key secondary clinical outcome [T25FW] are disappointing results and do not suggest that siponimod is an effective treatment for SPMS,” the two wrote.
“Confidence in the treatment benefit of siponimod in progressive MS will, in our opinion, require confirmation in a second trial. Trials of other novel treatments that target noninflammatory mechanisms are still needed,” they said.
EXPAND (Exploring the efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis) was an event- and exposure-driven double-blind trial that recruited patients with SPMS over a 2-year period starting in February 2013. Of 1,651 patients who were recruited, randomized, and actually received treatment, 1,099 were treated with oral siponimod, 2 mg once daily, and 546 were given a matching placebo.
Treatment was for up to 3 years or until 374 CDP events assessed via the Expanded Disability Status Scale (EDSS) had occurred. Patients who had CDP after 6 months in the double-blind trial could be re-consented and continue with double-blind treatment, switch to open-label siponimod, or stop study treatment and either remain on no treatment or receive another disease-modifying treatment.
On average, the patients had been diagnosed with SPMS for a mean of 3.8 years and had been first diagnosed with MS around 17 years prior to this.
The primary endpoint of the trial – CDP at 3 months – was first reported in 2016 at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). CDP was defined as a 0.5- to 1-point increase in EDSS depending on the baseline score.
A host of additional secondary endpoints were studied, with the risk of 6-month CDP being significantly reduced by siponimod versus placebo (HR, 0.74; 95% CI, 0.60-0.92; P = .0058).
The change from baseline in T2 lesion volume – the second of the two main secondary endpoints studied – showed a potential benefit of siponimod treatment over placebo, with a lower mean-adjusted increase in lesion volume over months 12 and 24 (183.9 mm3 vs. 879.2 mm3; P less than .0001).
More patients receiving siponimod than placebo were free from new or enlarging T2 lesions (57% vs. 37%) or T1 gadolinium-enhancing lesions (89% vs. 67%), and brain volume decreased at a lower rate with siponimod than with placebo.
Adverse events occurred at a higher rate with siponimod than with placebo (89% of patients vs. 82%), of which 18% and 15%, respectively, were defined as serious.
Adverse events seen more commonly in siponimod- than in placebo-treated patients were lymphopenia (1% vs. 0%), abnormal liver-related investigations (12% vs. 4%), hypertension (10% vs. 8%), and bradycardia (4% vs. 3%) and bradyarrhythmia (3% vs. 0.4%) at the start of treatment, which were mitigated by dose titration. Other adverse events that occurred less often but more frequently with siponimod included macular edema (2% vs. less than 1%), reactivation of varicella zoster (2% vs. 1%), and convulsions (2% vs. less than 1%).
“The safety profile of siponimod in EXPAND was generally aligned with that of other drugs in the class,” Dr. Kappos and his colleagues observed.
Siponimod, also known as BAF312, is a selective sphingosine 1-phosphate (S1P) receptor modulator that targets the targets the S1P 1 and 5 receptor subtypes; the other currently approved drug in its class is fingolimod (Gilenya), which is currently indicated for treating the relapsing-remitting form of multiple sclerosis (RRMS).
Phase 2 data have shown that siponimod could also be a promising treatment for RRMS, with reduced relapse rates and fewer brain lesions seen versus placebo (JAMA Neurol. 2016;73[9]:1089-98).
The EXPAND study was funded by Novartis. The lead author Dr. Kappos has received research and educational support funding via his institution from Novartis and multiple other pharmaceutical companies. Editorial author Dr. Metz has received grants from Roche and Biogen and personal fees from EMD Serono. The other editorial author, Dr. Liu, reported receiving personal fees from Novartis and personal fees and grants from EMD Serono. Dr. Liu was a back-up treating neurologist in the EXPAND trial.
SOURCE: Kappos L et al. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30475-6, and Metz L and Liu W. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30426-4.
Phase 3 data with siponimod, Novartis’ investigational treatment for multiple sclerosis, show a 21% reduction in the time to disease progression versus placebo in patients with the secondary progressive subtype of the disease.
A significantly lower percentage of patients with secondary progressive multiple sclerosis (SPMS) treated with siponimod met the primary endpoint of confirmed disease progression (CDP) at 3 months, compared with those who received placebo in the EXPAND trial (26% vs. 32% of patients; hazard ratio [HR], 0.79; 95% confidence interval [CI], 0.65–0.95; P = .013).
No significant difference between siponimod and placebo was seen in one of the two main secondary outcomes of the study – the time to 3-month confirmed worsening of the timed 25-foot walk test (T25FW). This result led Luanne Metz, MD, and Wei-Qiao Liu, MD, both of the department of clinical neurosciences and the Hotchkiss Brain Institute at the University of Calgary (Alta.), to take a cautionary view of the findings in an editorial about the trial’s results.
“Although siponimod seems to reduce the time to confirmed disability in SPMS, the treatment effect was small,” Dr. Metz and Dr. Liu argued.
“In our opinion, the reduction in the proportion of participants reaching the primary endpoint of only 6% and the absence of a significant difference for the key secondary clinical outcome [T25FW] are disappointing results and do not suggest that siponimod is an effective treatment for SPMS,” the two wrote.
“Confidence in the treatment benefit of siponimod in progressive MS will, in our opinion, require confirmation in a second trial. Trials of other novel treatments that target noninflammatory mechanisms are still needed,” they said.
EXPAND (Exploring the efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis) was an event- and exposure-driven double-blind trial that recruited patients with SPMS over a 2-year period starting in February 2013. Of 1,651 patients who were recruited, randomized, and actually received treatment, 1,099 were treated with oral siponimod, 2 mg once daily, and 546 were given a matching placebo.
Treatment was for up to 3 years or until 374 CDP events assessed via the Expanded Disability Status Scale (EDSS) had occurred. Patients who had CDP after 6 months in the double-blind trial could be re-consented and continue with double-blind treatment, switch to open-label siponimod, or stop study treatment and either remain on no treatment or receive another disease-modifying treatment.
On average, the patients had been diagnosed with SPMS for a mean of 3.8 years and had been first diagnosed with MS around 17 years prior to this.
The primary endpoint of the trial – CDP at 3 months – was first reported in 2016 at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). CDP was defined as a 0.5- to 1-point increase in EDSS depending on the baseline score.
A host of additional secondary endpoints were studied, with the risk of 6-month CDP being significantly reduced by siponimod versus placebo (HR, 0.74; 95% CI, 0.60-0.92; P = .0058).
The change from baseline in T2 lesion volume – the second of the two main secondary endpoints studied – showed a potential benefit of siponimod treatment over placebo, with a lower mean-adjusted increase in lesion volume over months 12 and 24 (183.9 mm3 vs. 879.2 mm3; P less than .0001).
More patients receiving siponimod than placebo were free from new or enlarging T2 lesions (57% vs. 37%) or T1 gadolinium-enhancing lesions (89% vs. 67%), and brain volume decreased at a lower rate with siponimod than with placebo.
Adverse events occurred at a higher rate with siponimod than with placebo (89% of patients vs. 82%), of which 18% and 15%, respectively, were defined as serious.
Adverse events seen more commonly in siponimod- than in placebo-treated patients were lymphopenia (1% vs. 0%), abnormal liver-related investigations (12% vs. 4%), hypertension (10% vs. 8%), and bradycardia (4% vs. 3%) and bradyarrhythmia (3% vs. 0.4%) at the start of treatment, which were mitigated by dose titration. Other adverse events that occurred less often but more frequently with siponimod included macular edema (2% vs. less than 1%), reactivation of varicella zoster (2% vs. 1%), and convulsions (2% vs. less than 1%).
“The safety profile of siponimod in EXPAND was generally aligned with that of other drugs in the class,” Dr. Kappos and his colleagues observed.
Siponimod, also known as BAF312, is a selective sphingosine 1-phosphate (S1P) receptor modulator that targets the targets the S1P 1 and 5 receptor subtypes; the other currently approved drug in its class is fingolimod (Gilenya), which is currently indicated for treating the relapsing-remitting form of multiple sclerosis (RRMS).
Phase 2 data have shown that siponimod could also be a promising treatment for RRMS, with reduced relapse rates and fewer brain lesions seen versus placebo (JAMA Neurol. 2016;73[9]:1089-98).
The EXPAND study was funded by Novartis. The lead author Dr. Kappos has received research and educational support funding via his institution from Novartis and multiple other pharmaceutical companies. Editorial author Dr. Metz has received grants from Roche and Biogen and personal fees from EMD Serono. The other editorial author, Dr. Liu, reported receiving personal fees from Novartis and personal fees and grants from EMD Serono. Dr. Liu was a back-up treating neurologist in the EXPAND trial.
SOURCE: Kappos L et al. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30475-6, and Metz L and Liu W. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30426-4.
FROM THE LANCET
Key clinical point:
Major finding: A significantly lower percentage of patients had confirmed disease progression at 3 months if treated with siponimod (26% vs. 32% with placebo; hazard ratio, 0.79; 95% confidence interval, 0.65–0.95; P = .013).
Study details: A double-blind, randomized, multicenter, placebo-controlled, phase 3 study of siponimod involving 1,651 patients with SPMS.
Disclosures: The EXPAND study was funded by Novartis. The lead author Dr. Kappos has received research and educational support funding via his institution from Novartis and multiple other pharmaceutical companies. Editorial author Dr. Metz has received grants from Roche and Biogen and personal fees from EMD Serono. The other editorial author, Dr. Liu, reported receiving personal fees from Novartis and personal fees and grants from EMD Serono. Dr. Liu was a back-up treating neurologist in the EXPAND trial.
Source: Kappos L et al. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30475-6, and Metz L and Liu W. Lancet. 2018 Mar 22. doi: 10.1016/S0140-6736(18)30426-4.
Adapting consumer technology into GI practice
BOSTON – You can command Alexa to order pizza and spool up your favorite flick, but accessing digital health data remains a struggle. Michael Docktor, MD, wants to change that.
A pediatric gastroenterologist and the clinical director of innovation at Boston Children’s Hospital, Dr. Docktor believes that it’s just a matter of time before consumer-driven digital technology fundamentally changes the way physicians and patients interact.
“In medicine, we are often in the habit of trying to recreate the wheel,” he said during the “Digital Health in GI Disease” session at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. “My hope and belief is that we can borrow from the best of the consumer technology world and apply that to our world in health care and GI specifically.”
Dr. Docktor shared some of the “tools and toys” that have come of his group’s program and also “exposed folks to things that they may not be thinking about traditionally in medicine.”
In particular, one area he focused on was how certain voice technologies are enhancing health care delivery, such as integration of Amazon’s Alexa. Consider Alexa a nurse on call.
he said. “We have placed a fairly large bet on voice in health care and built some skills for Alexa.”
He also highlighted a new virtual reality tool that was recently launched by Boston Children’s Hospital to help gastroenterologists better educate their patients.
HealthVoyager was developed in conjunction with Klick Health. The kid-friendly app lets children take a virtual ride through their GI tract. Clinicians draw in abnormal findings on a simplified template. The app then recreates those findings – lesions, polyps, or inflammatory changes – in the positions they actually occupy in the patient. It generates a QR code that’s given to the patient, allowing the child to access her imaging in a HIPAA-compliant manner.
It’s cool, sure, Dr. Docktor said. But does it bring any value to the physician-patient interaction?
“The challenge of digital health is to prove that there’s actual value, it’s not just a bunch of snazzy tech. Are patients really using it? Sharing it? Are they educating themselves and their family and their community? We want to study this clinically and validate whether or not it results in improved adherence and improved patient satisfaction.”
He covered other technologies, such as Chatbox and blockchain, and the roles they can play in health care.
In the not-too-distant future, Dr. Docktor envisions voice assistants integrated into daily medical practice. Amazon’s Alexa provides an aspirational goal, he said.
“We are seeing the rise of the voice assistant. By 2020, researchers predict that 50% of all Internet searches will happen just by voice. Voice interface, I believe, will be driving health care by interfacing with patients at home. I predict that over the next 5 years, most of us will have a medical encounter on a device like this. Technology is not a limiting factor in this scenario. It’s just red tape on the payer and provider side at this point.”
Dr. Docktor’s colleague, Carla E. Small, senior director of the Innovation & Digital Health Accelerator at Boston Children’s Hospital, provided another real-life example of his digital vision. The Innovation & Digital Health Accelerator is a division within the hospital devoted to identifying, nurturing, and implementing digital health care solutions.
“The world has moved to a technology-enabled health care environment, and we all have to be there along with it,” she said. “That also creates a great opportunity for those who have an interest in innovation. There is a lot of ground for changing the way we do things and really leveraging that creativity and innovation.”
One Accelerator product that’s up and running is Thermia. The online tool guides parents through the anxiety of managing a child’s fever.
Thermia quickly and easily allows concerned parents to interpret a child’s temperature and understand which steps they should consider taking. Parents enter their child’s age, temperature, weight, any associated symptoms like rash, sore throat, or GI upset, as well as comorbid medical conditions. An algorithm issues advice for treatment at home or, if the data suggest a risk or serious problem, suggests a visit to the pediatrician or the emergency room. Thermia also automatically calculates the dosage of over-the-counter antipyretic medications based on age and weight.
The Accelerator is investigating a host of other digital health products in different stages of concept, design, and execution. Health care simply has to embrace the digital trends that are changing the way people interact with their world.
The AGA Center for GI Innovation and Technology wants to hear the unique ways gastroenterologists are leveraging consumer technology in their practices. Send us an email at cgit@gastro.org.
gtwachtman@mdedge.com
msullivan@mdedge.com
BOSTON – You can command Alexa to order pizza and spool up your favorite flick, but accessing digital health data remains a struggle. Michael Docktor, MD, wants to change that.
A pediatric gastroenterologist and the clinical director of innovation at Boston Children’s Hospital, Dr. Docktor believes that it’s just a matter of time before consumer-driven digital technology fundamentally changes the way physicians and patients interact.
“In medicine, we are often in the habit of trying to recreate the wheel,” he said during the “Digital Health in GI Disease” session at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. “My hope and belief is that we can borrow from the best of the consumer technology world and apply that to our world in health care and GI specifically.”
Dr. Docktor shared some of the “tools and toys” that have come of his group’s program and also “exposed folks to things that they may not be thinking about traditionally in medicine.”
In particular, one area he focused on was how certain voice technologies are enhancing health care delivery, such as integration of Amazon’s Alexa. Consider Alexa a nurse on call.
he said. “We have placed a fairly large bet on voice in health care and built some skills for Alexa.”
He also highlighted a new virtual reality tool that was recently launched by Boston Children’s Hospital to help gastroenterologists better educate their patients.
HealthVoyager was developed in conjunction with Klick Health. The kid-friendly app lets children take a virtual ride through their GI tract. Clinicians draw in abnormal findings on a simplified template. The app then recreates those findings – lesions, polyps, or inflammatory changes – in the positions they actually occupy in the patient. It generates a QR code that’s given to the patient, allowing the child to access her imaging in a HIPAA-compliant manner.
It’s cool, sure, Dr. Docktor said. But does it bring any value to the physician-patient interaction?
“The challenge of digital health is to prove that there’s actual value, it’s not just a bunch of snazzy tech. Are patients really using it? Sharing it? Are they educating themselves and their family and their community? We want to study this clinically and validate whether or not it results in improved adherence and improved patient satisfaction.”
He covered other technologies, such as Chatbox and blockchain, and the roles they can play in health care.
In the not-too-distant future, Dr. Docktor envisions voice assistants integrated into daily medical practice. Amazon’s Alexa provides an aspirational goal, he said.
“We are seeing the rise of the voice assistant. By 2020, researchers predict that 50% of all Internet searches will happen just by voice. Voice interface, I believe, will be driving health care by interfacing with patients at home. I predict that over the next 5 years, most of us will have a medical encounter on a device like this. Technology is not a limiting factor in this scenario. It’s just red tape on the payer and provider side at this point.”
Dr. Docktor’s colleague, Carla E. Small, senior director of the Innovation & Digital Health Accelerator at Boston Children’s Hospital, provided another real-life example of his digital vision. The Innovation & Digital Health Accelerator is a division within the hospital devoted to identifying, nurturing, and implementing digital health care solutions.
“The world has moved to a technology-enabled health care environment, and we all have to be there along with it,” she said. “That also creates a great opportunity for those who have an interest in innovation. There is a lot of ground for changing the way we do things and really leveraging that creativity and innovation.”
One Accelerator product that’s up and running is Thermia. The online tool guides parents through the anxiety of managing a child’s fever.
Thermia quickly and easily allows concerned parents to interpret a child’s temperature and understand which steps they should consider taking. Parents enter their child’s age, temperature, weight, any associated symptoms like rash, sore throat, or GI upset, as well as comorbid medical conditions. An algorithm issues advice for treatment at home or, if the data suggest a risk or serious problem, suggests a visit to the pediatrician or the emergency room. Thermia also automatically calculates the dosage of over-the-counter antipyretic medications based on age and weight.
The Accelerator is investigating a host of other digital health products in different stages of concept, design, and execution. Health care simply has to embrace the digital trends that are changing the way people interact with their world.
The AGA Center for GI Innovation and Technology wants to hear the unique ways gastroenterologists are leveraging consumer technology in their practices. Send us an email at cgit@gastro.org.
gtwachtman@mdedge.com
msullivan@mdedge.com
BOSTON – You can command Alexa to order pizza and spool up your favorite flick, but accessing digital health data remains a struggle. Michael Docktor, MD, wants to change that.
A pediatric gastroenterologist and the clinical director of innovation at Boston Children’s Hospital, Dr. Docktor believes that it’s just a matter of time before consumer-driven digital technology fundamentally changes the way physicians and patients interact.
“In medicine, we are often in the habit of trying to recreate the wheel,” he said during the “Digital Health in GI Disease” session at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. “My hope and belief is that we can borrow from the best of the consumer technology world and apply that to our world in health care and GI specifically.”
Dr. Docktor shared some of the “tools and toys” that have come of his group’s program and also “exposed folks to things that they may not be thinking about traditionally in medicine.”
In particular, one area he focused on was how certain voice technologies are enhancing health care delivery, such as integration of Amazon’s Alexa. Consider Alexa a nurse on call.
he said. “We have placed a fairly large bet on voice in health care and built some skills for Alexa.”
He also highlighted a new virtual reality tool that was recently launched by Boston Children’s Hospital to help gastroenterologists better educate their patients.
HealthVoyager was developed in conjunction with Klick Health. The kid-friendly app lets children take a virtual ride through their GI tract. Clinicians draw in abnormal findings on a simplified template. The app then recreates those findings – lesions, polyps, or inflammatory changes – in the positions they actually occupy in the patient. It generates a QR code that’s given to the patient, allowing the child to access her imaging in a HIPAA-compliant manner.
It’s cool, sure, Dr. Docktor said. But does it bring any value to the physician-patient interaction?
“The challenge of digital health is to prove that there’s actual value, it’s not just a bunch of snazzy tech. Are patients really using it? Sharing it? Are they educating themselves and their family and their community? We want to study this clinically and validate whether or not it results in improved adherence and improved patient satisfaction.”
He covered other technologies, such as Chatbox and blockchain, and the roles they can play in health care.
In the not-too-distant future, Dr. Docktor envisions voice assistants integrated into daily medical practice. Amazon’s Alexa provides an aspirational goal, he said.
“We are seeing the rise of the voice assistant. By 2020, researchers predict that 50% of all Internet searches will happen just by voice. Voice interface, I believe, will be driving health care by interfacing with patients at home. I predict that over the next 5 years, most of us will have a medical encounter on a device like this. Technology is not a limiting factor in this scenario. It’s just red tape on the payer and provider side at this point.”
Dr. Docktor’s colleague, Carla E. Small, senior director of the Innovation & Digital Health Accelerator at Boston Children’s Hospital, provided another real-life example of his digital vision. The Innovation & Digital Health Accelerator is a division within the hospital devoted to identifying, nurturing, and implementing digital health care solutions.
“The world has moved to a technology-enabled health care environment, and we all have to be there along with it,” she said. “That also creates a great opportunity for those who have an interest in innovation. There is a lot of ground for changing the way we do things and really leveraging that creativity and innovation.”
One Accelerator product that’s up and running is Thermia. The online tool guides parents through the anxiety of managing a child’s fever.
Thermia quickly and easily allows concerned parents to interpret a child’s temperature and understand which steps they should consider taking. Parents enter their child’s age, temperature, weight, any associated symptoms like rash, sore throat, or GI upset, as well as comorbid medical conditions. An algorithm issues advice for treatment at home or, if the data suggest a risk or serious problem, suggests a visit to the pediatrician or the emergency room. Thermia also automatically calculates the dosage of over-the-counter antipyretic medications based on age and weight.
The Accelerator is investigating a host of other digital health products in different stages of concept, design, and execution. Health care simply has to embrace the digital trends that are changing the way people interact with their world.
The AGA Center for GI Innovation and Technology wants to hear the unique ways gastroenterologists are leveraging consumer technology in their practices. Send us an email at cgit@gastro.org.
gtwachtman@mdedge.com
msullivan@mdedge.com
REPORTING FROM THE 2018 AGA TECH SUMMIT
Cost, coverage concerns cited as barriers to acne medication adherence
Patients with acne who did not follow through with treatment cited high prices and insurance barriers as their main reasons for medication nonadherence, in a study published in JAMA Dermatology.
In the study, more than half of the 26 participants interviewed reported that they intended to fill the acne prescriptions but were not able to do so because of cost- or insurance-related concerns, reported Kira L. Ryskina, MD, of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, and her coauthors.
Of the initial 385 patients, 26 agreed to participate and met inclusion criteria. Most (58%) were aged 26-40 years, 19% were over aged 40, and 23% were younger than aged 26; 73% were female. Almost 40% had Medicaid coverage, 54% had commercial insurance, and the rest had “other.” Structured interviews were conducted via telephone between November 30, 2016, and January 31, 2017. Based on recorded interviews, five major themes were identified by investigator consensus: medication costs, poor understanding of prior authorization, physician-patient communication about costs, solutions and back-up plans offered by physicians, and reservations about treatment.
Lack of insurance coverage was a concern for 10 patients (38%), and high out-of-pocket costs were a concern for 11 patients (42%), the authors reported. Additionally, 5 participants (19%) said they had received inconsistent information from physicians, physician’s office staff, and pharmacy staff about the prior authorization process.
Participants reported having discussions with providers about treatment side effects and instructions for use – but not about price concerns. In addition, patients “did not expect physicians to know medication cost or insurance coverage,” the authors said. The patients also described various approaches recommended by providers to address problems obtaining medication, including asking patients to call the office back (7%), shopping around for medication (7%), suggesting alternatives to first-line treatment (7%), and offering coupons (4%). Backup plans (offered when the medication was not available) were received positively by 23% of the patients, as was a candid conversation about cost (19%), whereas shopping around and calling the office back were not, the authors said.
Overall, 10 patients (38%) reported reservations about treatment, which included concerns about possible adverse effects (15%), unwillingness to start a “strong” medication (8%), desire to try homeopathic treatment (3%), and belief that their acne was not serious enough to require medication (12%).
The results suggest that “physician-level interventions to improve primary adherence should incorporate discussion of medication costs and provide specific alternative plans in case the patient is unable to fill the prescription, rather than asking patients to call back,” the authors wrote. “Physicians who discuss medication costs and provide a concrete alternative plan may be able to improve primary adherence among their patients,” they concluded.
Limitations of the study include its qualitative nature and its limited generalizability because all participants were part of the same Philadelphia health system.
The study was funded by a Pfizer grant from the American Academy of Dermatology and was cosponsored by the Pennsylvania Academy of Dermatology and Dermatologic Surgery.
SOURCE: Ryskina K et al. JAMA Dermatol. 2018 Feb 28. doi: 10.1001/jamadermatol.2017.6144.
Patients with acne who did not follow through with treatment cited high prices and insurance barriers as their main reasons for medication nonadherence, in a study published in JAMA Dermatology.
In the study, more than half of the 26 participants interviewed reported that they intended to fill the acne prescriptions but were not able to do so because of cost- or insurance-related concerns, reported Kira L. Ryskina, MD, of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, and her coauthors.
Of the initial 385 patients, 26 agreed to participate and met inclusion criteria. Most (58%) were aged 26-40 years, 19% were over aged 40, and 23% were younger than aged 26; 73% were female. Almost 40% had Medicaid coverage, 54% had commercial insurance, and the rest had “other.” Structured interviews were conducted via telephone between November 30, 2016, and January 31, 2017. Based on recorded interviews, five major themes were identified by investigator consensus: medication costs, poor understanding of prior authorization, physician-patient communication about costs, solutions and back-up plans offered by physicians, and reservations about treatment.
Lack of insurance coverage was a concern for 10 patients (38%), and high out-of-pocket costs were a concern for 11 patients (42%), the authors reported. Additionally, 5 participants (19%) said they had received inconsistent information from physicians, physician’s office staff, and pharmacy staff about the prior authorization process.
Participants reported having discussions with providers about treatment side effects and instructions for use – but not about price concerns. In addition, patients “did not expect physicians to know medication cost or insurance coverage,” the authors said. The patients also described various approaches recommended by providers to address problems obtaining medication, including asking patients to call the office back (7%), shopping around for medication (7%), suggesting alternatives to first-line treatment (7%), and offering coupons (4%). Backup plans (offered when the medication was not available) were received positively by 23% of the patients, as was a candid conversation about cost (19%), whereas shopping around and calling the office back were not, the authors said.
Overall, 10 patients (38%) reported reservations about treatment, which included concerns about possible adverse effects (15%), unwillingness to start a “strong” medication (8%), desire to try homeopathic treatment (3%), and belief that their acne was not serious enough to require medication (12%).
The results suggest that “physician-level interventions to improve primary adherence should incorporate discussion of medication costs and provide specific alternative plans in case the patient is unable to fill the prescription, rather than asking patients to call back,” the authors wrote. “Physicians who discuss medication costs and provide a concrete alternative plan may be able to improve primary adherence among their patients,” they concluded.
Limitations of the study include its qualitative nature and its limited generalizability because all participants were part of the same Philadelphia health system.
The study was funded by a Pfizer grant from the American Academy of Dermatology and was cosponsored by the Pennsylvania Academy of Dermatology and Dermatologic Surgery.
SOURCE: Ryskina K et al. JAMA Dermatol. 2018 Feb 28. doi: 10.1001/jamadermatol.2017.6144.
Patients with acne who did not follow through with treatment cited high prices and insurance barriers as their main reasons for medication nonadherence, in a study published in JAMA Dermatology.
In the study, more than half of the 26 participants interviewed reported that they intended to fill the acne prescriptions but were not able to do so because of cost- or insurance-related concerns, reported Kira L. Ryskina, MD, of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, and her coauthors.
Of the initial 385 patients, 26 agreed to participate and met inclusion criteria. Most (58%) were aged 26-40 years, 19% were over aged 40, and 23% were younger than aged 26; 73% were female. Almost 40% had Medicaid coverage, 54% had commercial insurance, and the rest had “other.” Structured interviews were conducted via telephone between November 30, 2016, and January 31, 2017. Based on recorded interviews, five major themes were identified by investigator consensus: medication costs, poor understanding of prior authorization, physician-patient communication about costs, solutions and back-up plans offered by physicians, and reservations about treatment.
Lack of insurance coverage was a concern for 10 patients (38%), and high out-of-pocket costs were a concern for 11 patients (42%), the authors reported. Additionally, 5 participants (19%) said they had received inconsistent information from physicians, physician’s office staff, and pharmacy staff about the prior authorization process.
Participants reported having discussions with providers about treatment side effects and instructions for use – but not about price concerns. In addition, patients “did not expect physicians to know medication cost or insurance coverage,” the authors said. The patients also described various approaches recommended by providers to address problems obtaining medication, including asking patients to call the office back (7%), shopping around for medication (7%), suggesting alternatives to first-line treatment (7%), and offering coupons (4%). Backup plans (offered when the medication was not available) were received positively by 23% of the patients, as was a candid conversation about cost (19%), whereas shopping around and calling the office back were not, the authors said.
Overall, 10 patients (38%) reported reservations about treatment, which included concerns about possible adverse effects (15%), unwillingness to start a “strong” medication (8%), desire to try homeopathic treatment (3%), and belief that their acne was not serious enough to require medication (12%).
The results suggest that “physician-level interventions to improve primary adherence should incorporate discussion of medication costs and provide specific alternative plans in case the patient is unable to fill the prescription, rather than asking patients to call back,” the authors wrote. “Physicians who discuss medication costs and provide a concrete alternative plan may be able to improve primary adherence among their patients,” they concluded.
Limitations of the study include its qualitative nature and its limited generalizability because all participants were part of the same Philadelphia health system.
The study was funded by a Pfizer grant from the American Academy of Dermatology and was cosponsored by the Pennsylvania Academy of Dermatology and Dermatologic Surgery.
SOURCE: Ryskina K et al. JAMA Dermatol. 2018 Feb 28. doi: 10.1001/jamadermatol.2017.6144.
FROM JAMA DERMATOLOGY
Key clinical point: Most patients cited high prescription costs and insurance coverage concerns as reasons for medication nonadherence.
Major finding: Of the patients in the study, 65% reported being unable to fill prescriptions because of price or insurance coverage concerns.
Data source: A qualitative analysis of structured interviews with 26 acne patients in Philadelphia.
Disclosures: The study was funded by a Pfizer grant from the American Academy of Dermatology and was cosponsored by the Pennsylvania Academy of Dermatology and Dermatologic Surgery.
Source: Ryskina K et al. JAMA Dermatol. 2018 Feb 28. doi: 10.1001/jamadermatol.2017.6144.
FDA approves certolizumab label update for pregnancy, breastfeeding
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
What’s in the way of you and new tech?
BOSTON – Bringing new technology to your practice is not as simple as flipping a switch, as attendees of the Thursday afternoon AGA Tech Summit session “Physician Perspective on Barriers to Incorporating New Technology” learned. The Tech Summit is sponsored by the AGA Center for GI Innovation and Technology.
“As physicians think about being a part of taking on new technology, there are varying perspectives, including the perspective they have about their patients and the perspective they have for themselves,” Richard Rothstein, MD, chair of the department of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. “However, there are other perspectives as well, like the perspectives of the hospital or the ambulatory endoscopy center in which they work.”
He presented an intriguing historical example. Within months of the first demonstration of anesthetized surgery in 1846, the use of ether and the machine to deliver it were spreading rapidly through hospitals in large U.S. cities. European adoption soon followed.
However, decades passed before there was wide acceptance of Lister’s ideas on carbolic acid as a surgical antiseptic.
“Why was one technology adopted early and one later? Incentives to adopt both went in the same direction – improved patient outcomes. Both were based on ideas that violated prior beliefs. Both were technically complex. But one combatted a visible and immediate problem: pain. The other combatted an invisible and unproven problem: germs. Both made life better for the patient – but only one made life better for the surgeon. And that one, anesthesia, was the one that was quickly adopted.”
Even today, clinicians are the main drivers of the adoption of novel medical technology. They fall into two general categories, Dr. Rothstein said: early adopters, who want to be the first to offer an exciting new procedure, and late adopters, who wait for more information and want all the issues of that technology to be sorted out before diving in.
Each one stands in the same circle, however, forced to evaluate the issues that come along with adopting new tech, including training, credentialing and insurance, facility support, and how the new tool or procedure might affect the entire clinical team
Facilities have to tussle with these issues, too, Dr. Rothstein said.
Administrations wonder, “‘Will I get paid for this? Will it displace something else that’s equally effective that could be making more money? What resources do I need to implement it? Will it impact malpractice insurance rates for clinicians who work at my facility?’”
Patient choice also plays into the matter. Third-party payers may or may not have cutting-edge tech on their payment ledger. The specter of a self-pay procedure, no matter how potentially effective, is an enormous deterrent for patients, especially when figuring in the possibility of footing the bill for any associated complications. And of course, new technology and procedures lack the deep pool of efficacy and safety data that established ones lean upon – another potential sticking point for both clinicians and patients, Dr. Rothstein said.
“There are a lot of great ideas out there, and a lot of innovative devices, but without addressing the barriers to adoption, the technology will never get to the targeted goal of delivering better care to our patients,” he said.
BOSTON – Bringing new technology to your practice is not as simple as flipping a switch, as attendees of the Thursday afternoon AGA Tech Summit session “Physician Perspective on Barriers to Incorporating New Technology” learned. The Tech Summit is sponsored by the AGA Center for GI Innovation and Technology.
“As physicians think about being a part of taking on new technology, there are varying perspectives, including the perspective they have about their patients and the perspective they have for themselves,” Richard Rothstein, MD, chair of the department of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. “However, there are other perspectives as well, like the perspectives of the hospital or the ambulatory endoscopy center in which they work.”
He presented an intriguing historical example. Within months of the first demonstration of anesthetized surgery in 1846, the use of ether and the machine to deliver it were spreading rapidly through hospitals in large U.S. cities. European adoption soon followed.
However, decades passed before there was wide acceptance of Lister’s ideas on carbolic acid as a surgical antiseptic.
“Why was one technology adopted early and one later? Incentives to adopt both went in the same direction – improved patient outcomes. Both were based on ideas that violated prior beliefs. Both were technically complex. But one combatted a visible and immediate problem: pain. The other combatted an invisible and unproven problem: germs. Both made life better for the patient – but only one made life better for the surgeon. And that one, anesthesia, was the one that was quickly adopted.”
Even today, clinicians are the main drivers of the adoption of novel medical technology. They fall into two general categories, Dr. Rothstein said: early adopters, who want to be the first to offer an exciting new procedure, and late adopters, who wait for more information and want all the issues of that technology to be sorted out before diving in.
Each one stands in the same circle, however, forced to evaluate the issues that come along with adopting new tech, including training, credentialing and insurance, facility support, and how the new tool or procedure might affect the entire clinical team
Facilities have to tussle with these issues, too, Dr. Rothstein said.
Administrations wonder, “‘Will I get paid for this? Will it displace something else that’s equally effective that could be making more money? What resources do I need to implement it? Will it impact malpractice insurance rates for clinicians who work at my facility?’”
Patient choice also plays into the matter. Third-party payers may or may not have cutting-edge tech on their payment ledger. The specter of a self-pay procedure, no matter how potentially effective, is an enormous deterrent for patients, especially when figuring in the possibility of footing the bill for any associated complications. And of course, new technology and procedures lack the deep pool of efficacy and safety data that established ones lean upon – another potential sticking point for both clinicians and patients, Dr. Rothstein said.
“There are a lot of great ideas out there, and a lot of innovative devices, but without addressing the barriers to adoption, the technology will never get to the targeted goal of delivering better care to our patients,” he said.
BOSTON – Bringing new technology to your practice is not as simple as flipping a switch, as attendees of the Thursday afternoon AGA Tech Summit session “Physician Perspective on Barriers to Incorporating New Technology” learned. The Tech Summit is sponsored by the AGA Center for GI Innovation and Technology.
“As physicians think about being a part of taking on new technology, there are varying perspectives, including the perspective they have about their patients and the perspective they have for themselves,” Richard Rothstein, MD, chair of the department of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. “However, there are other perspectives as well, like the perspectives of the hospital or the ambulatory endoscopy center in which they work.”
He presented an intriguing historical example. Within months of the first demonstration of anesthetized surgery in 1846, the use of ether and the machine to deliver it were spreading rapidly through hospitals in large U.S. cities. European adoption soon followed.
However, decades passed before there was wide acceptance of Lister’s ideas on carbolic acid as a surgical antiseptic.
“Why was one technology adopted early and one later? Incentives to adopt both went in the same direction – improved patient outcomes. Both were based on ideas that violated prior beliefs. Both were technically complex. But one combatted a visible and immediate problem: pain. The other combatted an invisible and unproven problem: germs. Both made life better for the patient – but only one made life better for the surgeon. And that one, anesthesia, was the one that was quickly adopted.”
Even today, clinicians are the main drivers of the adoption of novel medical technology. They fall into two general categories, Dr. Rothstein said: early adopters, who want to be the first to offer an exciting new procedure, and late adopters, who wait for more information and want all the issues of that technology to be sorted out before diving in.
Each one stands in the same circle, however, forced to evaluate the issues that come along with adopting new tech, including training, credentialing and insurance, facility support, and how the new tool or procedure might affect the entire clinical team
Facilities have to tussle with these issues, too, Dr. Rothstein said.
Administrations wonder, “‘Will I get paid for this? Will it displace something else that’s equally effective that could be making more money? What resources do I need to implement it? Will it impact malpractice insurance rates for clinicians who work at my facility?’”
Patient choice also plays into the matter. Third-party payers may or may not have cutting-edge tech on their payment ledger. The specter of a self-pay procedure, no matter how potentially effective, is an enormous deterrent for patients, especially when figuring in the possibility of footing the bill for any associated complications. And of course, new technology and procedures lack the deep pool of efficacy and safety data that established ones lean upon – another potential sticking point for both clinicians and patients, Dr. Rothstein said.
“There are a lot of great ideas out there, and a lot of innovative devices, but without addressing the barriers to adoption, the technology will never get to the targeted goal of delivering better care to our patients,” he said.
REPORTING FROM 2018 AGA TECH SUMMIT
Experimental voxtalisib shows mixed results in phase 2 study
Voxtalisib, an investigational agent that targets both mTOR and multiple isoforms of PI3K, showed “promising” efficacy with acceptable safety in patients with relapsed or refractory follicular lymphoma (FL), results of a phase 2 trial indicate.
Among 46 patients with FL, the overall response rate was 41.3%, including five (10.9%) complete responses. The median progression-free survival in this group was 58 weeks, reported Jennifer R. Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and her colleagues.
“The observed activity of voxtalisib in relapsed or refractory follicular lymphoma, notable for inducing complete responses in 10.9% of patients, warrants further study,” the investigators wrote in a study published in the Lancet Haematology.
Efficacy of the drug was limited, however, against aggressive malignancies, including mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Voxtalisib (XL765) is a potent inhibitor of all four class I PI3Ks, as well as a less robust inhibitor of the mammalian target of rapamycin (mTOR). In contrast, idelalisib (Zydelig) – which is approved by the Food and Drug Administration for treatment of relapsed/refractory FL or for CLL, in combination with rituximab – inhibits only the delta isoform of PI3K, and does not have marked anti–mTOR properties.
The investigators conducted an open-label, nonrandomized trial of voxtalisib in 30 centers in the United States, Belgium, France, Germany, the Netherlands, and Australia.
Adults 18 years or older with relapsed or refractory MCL, FL, DLBCL or CLL/SLL with Eastern Cooperative Oncology Group performance status of 2 or less were enrolled. All patients received voxtalisib 50 mg orally twice daily in 28-day continuous dosing cycles until progression or unacceptable toxicity.
All patients who received more the 4 weeks of treatment and had both a baseline and one or more on-treatment tumor assessments were included in the efficacy analysis. Patients with lymphoma had received a median of three prior lines of therapy, and those with CLL had received a median of four prior lines.
The overall response rate in the entire study population was 18.3% (30 patients), including 22 partial and 8 complete responses. ORR rates were as follows:
- FL: 41.3% (19 of 46 patients).
- MCL: 11.9% (5 of 42 patients).
- DLBCL: 4.9% (2 of 41 patients).
- CLL/SLL: 11.4% (4 of 35 patients).
The safety analysis, which included all 167 patients enrolled, was consistent with that of previous studies of voxtalisib, the investigators said. The most frequently reported adverse events of any grade or type were diarrhea in 35% of patients, fatigue in 32%, nausea in 27%, pyrexia in 26%, cough 24%, and decreased appetite in 21%.
Grade 3 or greater adverse events include anemia in 12%, and pneumonia and thrombocytopenia in 8% each. Slightly more than half of all patients (58.1%) had a serious adverse event.
The investigators noted that voxtalisib’s short plasma half-life may explain the drug’s lack of efficacy against the aggressive lymphomas and CLL/SLL. Longer-acting formulations of the drug or more frequent dosing might address this problem, although the latter solution could be challenging for patients to follow, the investigators acknowledged.
In light of the results, no further studies of voxtalisib in CLL are planned, thought investigation of the drug alone or in combination with chemoimmunotherapy is warranted for patients with follicular lymphoma, the investigators wrote.
The study was funded by Sanofi. Dr. Brown reported consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
SOURCE: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
Voxtalisib, an investigational agent that targets both mTOR and multiple isoforms of PI3K, showed “promising” efficacy with acceptable safety in patients with relapsed or refractory follicular lymphoma (FL), results of a phase 2 trial indicate.
Among 46 patients with FL, the overall response rate was 41.3%, including five (10.9%) complete responses. The median progression-free survival in this group was 58 weeks, reported Jennifer R. Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and her colleagues.
“The observed activity of voxtalisib in relapsed or refractory follicular lymphoma, notable for inducing complete responses in 10.9% of patients, warrants further study,” the investigators wrote in a study published in the Lancet Haematology.
Efficacy of the drug was limited, however, against aggressive malignancies, including mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Voxtalisib (XL765) is a potent inhibitor of all four class I PI3Ks, as well as a less robust inhibitor of the mammalian target of rapamycin (mTOR). In contrast, idelalisib (Zydelig) – which is approved by the Food and Drug Administration for treatment of relapsed/refractory FL or for CLL, in combination with rituximab – inhibits only the delta isoform of PI3K, and does not have marked anti–mTOR properties.
The investigators conducted an open-label, nonrandomized trial of voxtalisib in 30 centers in the United States, Belgium, France, Germany, the Netherlands, and Australia.
Adults 18 years or older with relapsed or refractory MCL, FL, DLBCL or CLL/SLL with Eastern Cooperative Oncology Group performance status of 2 or less were enrolled. All patients received voxtalisib 50 mg orally twice daily in 28-day continuous dosing cycles until progression or unacceptable toxicity.
All patients who received more the 4 weeks of treatment and had both a baseline and one or more on-treatment tumor assessments were included in the efficacy analysis. Patients with lymphoma had received a median of three prior lines of therapy, and those with CLL had received a median of four prior lines.
The overall response rate in the entire study population was 18.3% (30 patients), including 22 partial and 8 complete responses. ORR rates were as follows:
- FL: 41.3% (19 of 46 patients).
- MCL: 11.9% (5 of 42 patients).
- DLBCL: 4.9% (2 of 41 patients).
- CLL/SLL: 11.4% (4 of 35 patients).
The safety analysis, which included all 167 patients enrolled, was consistent with that of previous studies of voxtalisib, the investigators said. The most frequently reported adverse events of any grade or type were diarrhea in 35% of patients, fatigue in 32%, nausea in 27%, pyrexia in 26%, cough 24%, and decreased appetite in 21%.
Grade 3 or greater adverse events include anemia in 12%, and pneumonia and thrombocytopenia in 8% each. Slightly more than half of all patients (58.1%) had a serious adverse event.
The investigators noted that voxtalisib’s short plasma half-life may explain the drug’s lack of efficacy against the aggressive lymphomas and CLL/SLL. Longer-acting formulations of the drug or more frequent dosing might address this problem, although the latter solution could be challenging for patients to follow, the investigators acknowledged.
In light of the results, no further studies of voxtalisib in CLL are planned, thought investigation of the drug alone or in combination with chemoimmunotherapy is warranted for patients with follicular lymphoma, the investigators wrote.
The study was funded by Sanofi. Dr. Brown reported consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
SOURCE: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
Voxtalisib, an investigational agent that targets both mTOR and multiple isoforms of PI3K, showed “promising” efficacy with acceptable safety in patients with relapsed or refractory follicular lymphoma (FL), results of a phase 2 trial indicate.
Among 46 patients with FL, the overall response rate was 41.3%, including five (10.9%) complete responses. The median progression-free survival in this group was 58 weeks, reported Jennifer R. Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and her colleagues.
“The observed activity of voxtalisib in relapsed or refractory follicular lymphoma, notable for inducing complete responses in 10.9% of patients, warrants further study,” the investigators wrote in a study published in the Lancet Haematology.
Efficacy of the drug was limited, however, against aggressive malignancies, including mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), or chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Voxtalisib (XL765) is a potent inhibitor of all four class I PI3Ks, as well as a less robust inhibitor of the mammalian target of rapamycin (mTOR). In contrast, idelalisib (Zydelig) – which is approved by the Food and Drug Administration for treatment of relapsed/refractory FL or for CLL, in combination with rituximab – inhibits only the delta isoform of PI3K, and does not have marked anti–mTOR properties.
The investigators conducted an open-label, nonrandomized trial of voxtalisib in 30 centers in the United States, Belgium, France, Germany, the Netherlands, and Australia.
Adults 18 years or older with relapsed or refractory MCL, FL, DLBCL or CLL/SLL with Eastern Cooperative Oncology Group performance status of 2 or less were enrolled. All patients received voxtalisib 50 mg orally twice daily in 28-day continuous dosing cycles until progression or unacceptable toxicity.
All patients who received more the 4 weeks of treatment and had both a baseline and one or more on-treatment tumor assessments were included in the efficacy analysis. Patients with lymphoma had received a median of three prior lines of therapy, and those with CLL had received a median of four prior lines.
The overall response rate in the entire study population was 18.3% (30 patients), including 22 partial and 8 complete responses. ORR rates were as follows:
- FL: 41.3% (19 of 46 patients).
- MCL: 11.9% (5 of 42 patients).
- DLBCL: 4.9% (2 of 41 patients).
- CLL/SLL: 11.4% (4 of 35 patients).
The safety analysis, which included all 167 patients enrolled, was consistent with that of previous studies of voxtalisib, the investigators said. The most frequently reported adverse events of any grade or type were diarrhea in 35% of patients, fatigue in 32%, nausea in 27%, pyrexia in 26%, cough 24%, and decreased appetite in 21%.
Grade 3 or greater adverse events include anemia in 12%, and pneumonia and thrombocytopenia in 8% each. Slightly more than half of all patients (58.1%) had a serious adverse event.
The investigators noted that voxtalisib’s short plasma half-life may explain the drug’s lack of efficacy against the aggressive lymphomas and CLL/SLL. Longer-acting formulations of the drug or more frequent dosing might address this problem, although the latter solution could be challenging for patients to follow, the investigators acknowledged.
In light of the results, no further studies of voxtalisib in CLL are planned, thought investigation of the drug alone or in combination with chemoimmunotherapy is warranted for patients with follicular lymphoma, the investigators wrote.
The study was funded by Sanofi. Dr. Brown reported consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
SOURCE: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The overall response rate in patients with relapsed/refractory FL was 41.3%.
Study details: Open-label, nonrandomized trial in 167 patients from 30 centers in six countries.
Disclosures: The study was funded by Sanofi. Dr. Brown disclosed consulting for Janssen, Gilead, Celgene, Sun BioPharma, Novartis, AbbVie, Pfizer, AstraZeneca, Astellas, RedX, Pharmacyclics, Genentech/Roche, Verastem, and TG Therapeutics, and grants from Gilead and Sun BioPharma.
Source: Brown J et al. Lancet Haematol. 2018 Mar 14. doi: 10.1016/S2352-3026(18)30030-9.
VIDEO: Digital health technologies are here to be embraced
BOSTON – One of the innovations in gastroenterology that is used on a day-to-day basis is digital technology, said Sri Komanduri, MD, in a video interview at the 2018 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Everything from Internet rate-your-doctor sites to bowel prep apps and EHRs qualify as digital health technology, said Dr. Komanduri, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago and vice chair of the AGA Center for GI Innovation and Technology.
Digital technology can facilitate endoscopy procedures, help patients communicate with their doctors about chronic conditions, and help patients better understand their illness. The role of the physician is to embrace those technologies that improve the quality of care.
BOSTON – One of the innovations in gastroenterology that is used on a day-to-day basis is digital technology, said Sri Komanduri, MD, in a video interview at the 2018 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Everything from Internet rate-your-doctor sites to bowel prep apps and EHRs qualify as digital health technology, said Dr. Komanduri, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago and vice chair of the AGA Center for GI Innovation and Technology.
Digital technology can facilitate endoscopy procedures, help patients communicate with their doctors about chronic conditions, and help patients better understand their illness. The role of the physician is to embrace those technologies that improve the quality of care.
BOSTON – One of the innovations in gastroenterology that is used on a day-to-day basis is digital technology, said Sri Komanduri, MD, in a video interview at the 2018 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Everything from Internet rate-your-doctor sites to bowel prep apps and EHRs qualify as digital health technology, said Dr. Komanduri, the medical director of the GI laboratory and director of interventional endoscopy at Northwestern University in Chicago and vice chair of the AGA Center for GI Innovation and Technology.
Digital technology can facilitate endoscopy procedures, help patients communicate with their doctors about chronic conditions, and help patients better understand their illness. The role of the physician is to embrace those technologies that improve the quality of care.
FROM THE 2018 AGA TECH SUMMIT
Update on AGA-Medtronic Research & Development Pilot Award in Technology
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research & Development Pilot Award in Technology by the AGA Research Foundation, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and its function. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington as a poster of distinction.
“Dr. Roland’s research is innovative and clinically relevant. It’s great to see the progress her team has made since receiving this grant from the AGA Research Foundation,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “I want to thank Medtronic for their partnership on this award and their shared commitment to funding innovative research projects.”
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiological mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using a wireless motility capsule (PillCam) – just the kind of technology fostered by the AGA GI Center for Innovation and Technology – to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with both IBS and SIBO have increased expression of proinflammatory markers, compared with those with IBS only; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries without understanding the etiology for this syndrome,” said Dr. Roland, the director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
To capture the data to be presented in the DDW poster, Dr. Roland’s team used the wireless motility capsule to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared with patients who have IBS without evidence of SIBO
“Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as tumor necrosis factor–alpha and interleukin 6, with those in patients who have only IBS. They will use their data to apply for future funding.
Since 2014, the AGA Research Foundation has partnered with medical technology companies such as Medtronic to provide a total of over $450,000 in research grants to six investigators working on novel and innovative technology projects. The AGA Research Foundation will begin accepting applications for the next round of research grants in summer 2018. Stay tuned to www.gastro.org/research-funding.
REPORTING FROM 2018 AGA TECH SUMMIT
‘Right to try’ bill passes House
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.