User login
FDA releases updates on BIA-ALCL
The US Food and Drug Administration (FDA) has released new information on breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The agency said it is now aware of 414 cases of BIA-ALCL, which includes 9 patients who died.
In addition, the medical literature suggests that patients with textured breast implants have a lifetime risk of developing BIA-ALCL that ranges from 1 in 3817 to 1 in 30,000.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health.
“We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants. As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
Reports to FDA
Most of the BIA-ALCL cases reported to the FDA occurred in patients with textured implants (n=242), but 30 occurred in patients with smooth implants. In the remaining 173 cases, the implant surface was not specified.
There were more silicone implants (n=234) than saline implants (n=179), and there was 1 case in which the implant filling was not specified.
The patients’ median age was 53 (range, 24-90), and the median time from last implant to BIA-ALCL diagnosis was 8 years (range, 0-44).
Cases of BIA-ALCL were ALK-negative (n=124) or did not have ALK status specified (n=290). And they were CD30-positive (n=126) or did not have CD30 status specified (n=288).
The most common clinical presentation was seroma (n=203), followed by breast swelling/pain (n=101), peri-implant mass/lump (n=45), and capsular contracture (n=42). In some cases, more than one clinical presentation was listed, and there were 141 cases where clinical presentation was unspecified/uncertain.
Medical literature
The FDA said a “significant body of medical literature” on BIA-ALCL has been published since the agency’s 2011 report on this malignancy.
For the aforementioned lifetime risk estimates—1 case of BIA-ALCL in 3817 to 30,000 individuals with textured implants—the FDA cited 3 sources:
- BIA-ALCL Resources : By the numbers, and what they mean
- Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk
- Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast.
Recommendations, more updates
The FDA said this updated information does not change its recommendations regarding breast implants. The agency said the decision to obtain breast implants should be made based on individual needs and with the most complete information about risks and benefits.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL,” Dr Ashar said. “At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue.”
The FDA is also updating the content and format of the webpage for the agency’s breast implant post-approval studies to make current information about these studies easier for patients to read and understand.
The US Food and Drug Administration (FDA) has released new information on breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The agency said it is now aware of 414 cases of BIA-ALCL, which includes 9 patients who died.
In addition, the medical literature suggests that patients with textured breast implants have a lifetime risk of developing BIA-ALCL that ranges from 1 in 3817 to 1 in 30,000.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health.
“We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants. As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
Reports to FDA
Most of the BIA-ALCL cases reported to the FDA occurred in patients with textured implants (n=242), but 30 occurred in patients with smooth implants. In the remaining 173 cases, the implant surface was not specified.
There were more silicone implants (n=234) than saline implants (n=179), and there was 1 case in which the implant filling was not specified.
The patients’ median age was 53 (range, 24-90), and the median time from last implant to BIA-ALCL diagnosis was 8 years (range, 0-44).
Cases of BIA-ALCL were ALK-negative (n=124) or did not have ALK status specified (n=290). And they were CD30-positive (n=126) or did not have CD30 status specified (n=288).
The most common clinical presentation was seroma (n=203), followed by breast swelling/pain (n=101), peri-implant mass/lump (n=45), and capsular contracture (n=42). In some cases, more than one clinical presentation was listed, and there were 141 cases where clinical presentation was unspecified/uncertain.
Medical literature
The FDA said a “significant body of medical literature” on BIA-ALCL has been published since the agency’s 2011 report on this malignancy.
For the aforementioned lifetime risk estimates—1 case of BIA-ALCL in 3817 to 30,000 individuals with textured implants—the FDA cited 3 sources:
- BIA-ALCL Resources : By the numbers, and what they mean
- Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk
- Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast.
Recommendations, more updates
The FDA said this updated information does not change its recommendations regarding breast implants. The agency said the decision to obtain breast implants should be made based on individual needs and with the most complete information about risks and benefits.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL,” Dr Ashar said. “At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue.”
The FDA is also updating the content and format of the webpage for the agency’s breast implant post-approval studies to make current information about these studies easier for patients to read and understand.
The US Food and Drug Administration (FDA) has released new information on breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The agency said it is now aware of 414 cases of BIA-ALCL, which includes 9 patients who died.
In addition, the medical literature suggests that patients with textured breast implants have a lifetime risk of developing BIA-ALCL that ranges from 1 in 3817 to 1 in 30,000.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health.
“We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants. As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
Reports to FDA
Most of the BIA-ALCL cases reported to the FDA occurred in patients with textured implants (n=242), but 30 occurred in patients with smooth implants. In the remaining 173 cases, the implant surface was not specified.
There were more silicone implants (n=234) than saline implants (n=179), and there was 1 case in which the implant filling was not specified.
The patients’ median age was 53 (range, 24-90), and the median time from last implant to BIA-ALCL diagnosis was 8 years (range, 0-44).
Cases of BIA-ALCL were ALK-negative (n=124) or did not have ALK status specified (n=290). And they were CD30-positive (n=126) or did not have CD30 status specified (n=288).
The most common clinical presentation was seroma (n=203), followed by breast swelling/pain (n=101), peri-implant mass/lump (n=45), and capsular contracture (n=42). In some cases, more than one clinical presentation was listed, and there were 141 cases where clinical presentation was unspecified/uncertain.
Medical literature
The FDA said a “significant body of medical literature” on BIA-ALCL has been published since the agency’s 2011 report on this malignancy.
For the aforementioned lifetime risk estimates—1 case of BIA-ALCL in 3817 to 30,000 individuals with textured implants—the FDA cited 3 sources:
- BIA-ALCL Resources : By the numbers, and what they mean
- Breast Implant–Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk
- Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast.
Recommendations, more updates
The FDA said this updated information does not change its recommendations regarding breast implants. The agency said the decision to obtain breast implants should be made based on individual needs and with the most complete information about risks and benefits.
“We hope that this information prompts providers and patients to have important, informed conversations about breast implants and the risk of BIA-ALCL,” Dr Ashar said. “At the same time, we remain committed to working in partnership with all stakeholders to continue to study, understand, and provide updates about this important public health issue.”
The FDA is also updating the content and format of the webpage for the agency’s breast implant post-approval studies to make current information about these studies easier for patients to read and understand.
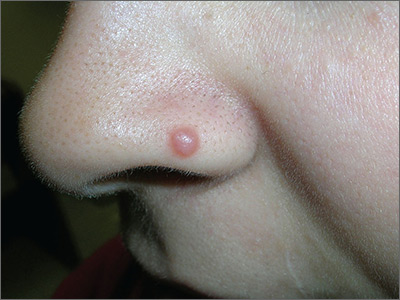
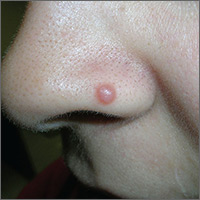
Growth on nose
The FP considered several possibilities as part of the differential diagnosis: compound nevus, Spitz nevus, dysplastic nevus, fibrous papule, angiokeratoma, and amelanotic melanoma. A shave biopsy identified the lesion as a Spitz nevus.
Spitz nevi are uncommon solitary pink to black colored dome-shaped papules that usually appear in the first 2 decades of life. They have features that are histologically similar to melanoma and some may, in fact, be Spitzoid melanomas. When a Spitz nevus is suspected, the lesion should be biopsied for histopathologic diagnosis. If the diagnosis is confirmed, the typical treatment is to perform a complete excision with clear margins.
In this case, the pathologist noted that the deep margin was positive and recommended a conservative re-excision. The patient was sent to a dermatologist who fully excised the lesion with no complications.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP considered several possibilities as part of the differential diagnosis: compound nevus, Spitz nevus, dysplastic nevus, fibrous papule, angiokeratoma, and amelanotic melanoma. A shave biopsy identified the lesion as a Spitz nevus.
Spitz nevi are uncommon solitary pink to black colored dome-shaped papules that usually appear in the first 2 decades of life. They have features that are histologically similar to melanoma and some may, in fact, be Spitzoid melanomas. When a Spitz nevus is suspected, the lesion should be biopsied for histopathologic diagnosis. If the diagnosis is confirmed, the typical treatment is to perform a complete excision with clear margins.
In this case, the pathologist noted that the deep margin was positive and recommended a conservative re-excision. The patient was sent to a dermatologist who fully excised the lesion with no complications.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP considered several possibilities as part of the differential diagnosis: compound nevus, Spitz nevus, dysplastic nevus, fibrous papule, angiokeratoma, and amelanotic melanoma. A shave biopsy identified the lesion as a Spitz nevus.
Spitz nevi are uncommon solitary pink to black colored dome-shaped papules that usually appear in the first 2 decades of life. They have features that are histologically similar to melanoma and some may, in fact, be Spitzoid melanomas. When a Spitz nevus is suspected, the lesion should be biopsied for histopathologic diagnosis. If the diagnosis is confirmed, the typical treatment is to perform a complete excision with clear margins.
In this case, the pathologist noted that the deep margin was positive and recommended a conservative re-excision. The patient was sent to a dermatologist who fully excised the lesion with no complications.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
DPP-4 inhibitors increase IBD risk in diabetes
a study has found.
Researchers reported the results of an observational cohort study of 141,170 patients with type 2 diabetes newly treated with noninsulin antidiabetic drugs, with 552,413 person years of follow-up. Of these, 30,488 patients (21.6%) received at least one prescription for a dipeptidyl peptidase-4 inhibitor, and median duration of use was 1.6 years.
The report was published March 21 in the BMJ.
The researchers found that dipeptidyl peptidase-4 (DPP-4) inhibitors were associated with a 75% increased risk of IBD, compared with other antidiabetic drugs (53.4 vs. 34.5 per 100,000 per year, 95% confidence interval, 1.22-2.49).
The risk increased with longer duration of use, peaking at a nearly threefold increase in the risk of IBD after 3-4 years of taking DPP-4 inhibitors (hazard ratio 2.9, 95% CI, 1.31-6.41), and declining to a 45% increase in risk with 4 years of use.
“Although the absolute risk is low, physicians should be aware of this possible association and perhaps refrain from prescribing dipeptidyl peptidase-4 inhibitors for people at high risk (that is, those with a family history of disease or with known autoimmune conditions),” wrote Devin Abrahami of McGill University, Montreal, and coauthors. “Moreover, patients presenting with persistent gastrointestinal symptoms such as abdominal pain or diarrhoea should be closely monitored for worsening of symptoms.”
The same pattern was seen with years since initiation of medication, with a peak in the risk of IBD seen at 3-4 years after initiation followed by a decline.
“This gradual increase in the risk is consistent with the hypothesis of a possible delayed effect of the use of dipeptidyl peptidase-4 inhibitors on the incidence of inflammatory bowel disease,” the authors wrote.
When compared directly with insulin, the use of DPP-4 inhibitors was associated with an over twofold increase in the risk of IBD (HR, 2.28, 95% CI, 1.07-4.85).
The use of DPP-4 inhibitors was also associated with a greater than twofold increase in the risk of ulcerative colitis but no significant effect was seen for Crohn’s disease. However, the authors noted that this result was based on relatively few events and should be interpreted with caution.
The research did not find any difference in risk across different DPP-4 inhibitor drugs.
The DPP-4 enzyme is known to be expressed on the surface of cell types involved in immune response, and patients with IBD have been found to have lower serum DPP-4 enzyme concentrations than healthy controls.
Yet the authors said this was the first study to their knowledge that specifically investigated the effect of DPP-4 inhibitor use on the incidence of IBD.
One previous observational study actually found a decreased risk of a composite outcome of several autoimmune disorders – including IBD – with the use of DPP-4 inhibitors, but it did not report on IBD specifically. The authors also noted that DPP-4 may have a different biological function in IBD.
The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
SOURCE: Abrahami D et al. BMJ. 2018;360:k872.
a study has found.
Researchers reported the results of an observational cohort study of 141,170 patients with type 2 diabetes newly treated with noninsulin antidiabetic drugs, with 552,413 person years of follow-up. Of these, 30,488 patients (21.6%) received at least one prescription for a dipeptidyl peptidase-4 inhibitor, and median duration of use was 1.6 years.
The report was published March 21 in the BMJ.
The researchers found that dipeptidyl peptidase-4 (DPP-4) inhibitors were associated with a 75% increased risk of IBD, compared with other antidiabetic drugs (53.4 vs. 34.5 per 100,000 per year, 95% confidence interval, 1.22-2.49).
The risk increased with longer duration of use, peaking at a nearly threefold increase in the risk of IBD after 3-4 years of taking DPP-4 inhibitors (hazard ratio 2.9, 95% CI, 1.31-6.41), and declining to a 45% increase in risk with 4 years of use.
“Although the absolute risk is low, physicians should be aware of this possible association and perhaps refrain from prescribing dipeptidyl peptidase-4 inhibitors for people at high risk (that is, those with a family history of disease or with known autoimmune conditions),” wrote Devin Abrahami of McGill University, Montreal, and coauthors. “Moreover, patients presenting with persistent gastrointestinal symptoms such as abdominal pain or diarrhoea should be closely monitored for worsening of symptoms.”
The same pattern was seen with years since initiation of medication, with a peak in the risk of IBD seen at 3-4 years after initiation followed by a decline.
“This gradual increase in the risk is consistent with the hypothesis of a possible delayed effect of the use of dipeptidyl peptidase-4 inhibitors on the incidence of inflammatory bowel disease,” the authors wrote.
When compared directly with insulin, the use of DPP-4 inhibitors was associated with an over twofold increase in the risk of IBD (HR, 2.28, 95% CI, 1.07-4.85).
The use of DPP-4 inhibitors was also associated with a greater than twofold increase in the risk of ulcerative colitis but no significant effect was seen for Crohn’s disease. However, the authors noted that this result was based on relatively few events and should be interpreted with caution.
The research did not find any difference in risk across different DPP-4 inhibitor drugs.
The DPP-4 enzyme is known to be expressed on the surface of cell types involved in immune response, and patients with IBD have been found to have lower serum DPP-4 enzyme concentrations than healthy controls.
Yet the authors said this was the first study to their knowledge that specifically investigated the effect of DPP-4 inhibitor use on the incidence of IBD.
One previous observational study actually found a decreased risk of a composite outcome of several autoimmune disorders – including IBD – with the use of DPP-4 inhibitors, but it did not report on IBD specifically. The authors also noted that DPP-4 may have a different biological function in IBD.
The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
SOURCE: Abrahami D et al. BMJ. 2018;360:k872.
a study has found.
Researchers reported the results of an observational cohort study of 141,170 patients with type 2 diabetes newly treated with noninsulin antidiabetic drugs, with 552,413 person years of follow-up. Of these, 30,488 patients (21.6%) received at least one prescription for a dipeptidyl peptidase-4 inhibitor, and median duration of use was 1.6 years.
The report was published March 21 in the BMJ.
The researchers found that dipeptidyl peptidase-4 (DPP-4) inhibitors were associated with a 75% increased risk of IBD, compared with other antidiabetic drugs (53.4 vs. 34.5 per 100,000 per year, 95% confidence interval, 1.22-2.49).
The risk increased with longer duration of use, peaking at a nearly threefold increase in the risk of IBD after 3-4 years of taking DPP-4 inhibitors (hazard ratio 2.9, 95% CI, 1.31-6.41), and declining to a 45% increase in risk with 4 years of use.
“Although the absolute risk is low, physicians should be aware of this possible association and perhaps refrain from prescribing dipeptidyl peptidase-4 inhibitors for people at high risk (that is, those with a family history of disease or with known autoimmune conditions),” wrote Devin Abrahami of McGill University, Montreal, and coauthors. “Moreover, patients presenting with persistent gastrointestinal symptoms such as abdominal pain or diarrhoea should be closely monitored for worsening of symptoms.”
The same pattern was seen with years since initiation of medication, with a peak in the risk of IBD seen at 3-4 years after initiation followed by a decline.
“This gradual increase in the risk is consistent with the hypothesis of a possible delayed effect of the use of dipeptidyl peptidase-4 inhibitors on the incidence of inflammatory bowel disease,” the authors wrote.
When compared directly with insulin, the use of DPP-4 inhibitors was associated with an over twofold increase in the risk of IBD (HR, 2.28, 95% CI, 1.07-4.85).
The use of DPP-4 inhibitors was also associated with a greater than twofold increase in the risk of ulcerative colitis but no significant effect was seen for Crohn’s disease. However, the authors noted that this result was based on relatively few events and should be interpreted with caution.
The research did not find any difference in risk across different DPP-4 inhibitor drugs.
The DPP-4 enzyme is known to be expressed on the surface of cell types involved in immune response, and patients with IBD have been found to have lower serum DPP-4 enzyme concentrations than healthy controls.
Yet the authors said this was the first study to their knowledge that specifically investigated the effect of DPP-4 inhibitor use on the incidence of IBD.
One previous observational study actually found a decreased risk of a composite outcome of several autoimmune disorders – including IBD – with the use of DPP-4 inhibitors, but it did not report on IBD specifically. The authors also noted that DPP-4 may have a different biological function in IBD.
The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
SOURCE: Abrahami D et al. BMJ. 2018;360:k872.
FROM THE BMJ
Key clinical point: Use of DPP-4 inhibitors may put patients with type 2 diabetes at increased risk of developing IBD.
Major finding: Use of DPP-4 inhibitors linked to 75% increase in IBD risk in type 2 diabetes.
Study details: An observational cohort study of 141,170 patients with type 2 diabetes.
Disclosures: The Canadian Institutes of Health Research funded the study. No conflicts of interest were declared.
Source: Abrahami D et al. BMJ. 2018;360:k872.
VIDEO: Case for rivaroxaban & aspirin for PAD gets stronger
ORLANDO – Combination treatment with aspirin and a low dosage of the anticoagulant rivaroxaban had a broader benefit for reducing adverse events in patients with peripheral artery disease than initially reported from the COMPASS trial, which included more than 7,000 patients whose primary diagnosis at study entry was stable PAD.
Secondary analysis of data from the PAD patients enrolled in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial showed that, compared with aspirin alone, treatment with 100 mg aspirin daily plus a low dosage of the anticoagulant rivaroxaban (2.5 mg b.i.d.) resulted in a statistically significant, 24% relative cut in the combined rate of vascular interventions: acute or chronic limb ischemia, vascular amputations, peripheral vascular bypass or percutaneous intervention, and vascular hospitalizations, Sonia S. Anand, MD, said at the annual meeting of the American College of Cardiology.
This finding plus the results in a prior report from COMPASS that rivaroxaban plus aspirin cut major adverse limb events (MALE) by 33%, compared with aspirin alone (Lancet. 2018 Jan 20;391:219-9), together show that rivaroxaban plus aspirin represent a new, effective regimen for a majority of PAD patients (in addition to treating with a statin and an ACE inhibitor) to cut adverse outcomes in a high-risk but historically undertreated patient population, Dr. Anand said in a video interview.
Additional analysis that Dr. Anand reported in her talk showed that patients with PAD who had an index MALE during follow-up had a very high rate of subsequent events. Among the 128 PAD patients in COMPASS who had an index MALE (major vascular amputation or severe limb ischemia that resulted in an intervention) and, compared with the PAD patients who did not have a MALE, the rate of subsequent death was more than three times higher, the rate of hospitalization increased more than 7-fold, and the rate of amputation increased nearly 200-fold.
Concurrently with Dr. Anand’s report at the meeting the results appeared in an article online in the Journal of the American College of Cardiology.
The analysis of post-MALE outcomes, as well as the expanded vascular-outcomes analysis, focused on 6,391 of the COMPASS patients who had lower-extremity PAD and were randomized to either 100 mg aspirin daily or the low-dosage rivaroxaban plus aspirin regimen. COMPASS also randomized patients to a higher-dosage rivaroxaban-only regimen administered at 5 mg b.i.d., but this arm did not perform as well as the lower-dosage regimen, with an efficacy that equaled aspirin only and with more bleeding. The primary efficacy and safety data from COMPASS for all 27,395 enrolled patients, which included many patients with stable coronary artery disease and without diagnosed PAD, appeared in 2017 (N Engl J Med. Oct 5;377[14]:1319-30).
Dr. Anand also reported on a comparison of the clinical and demographic profiles of the 128 PAD patients who developed MALE during follow-up and the 6,263 who did not, a 2% incidence during almost 2 years.
Multivariate analysis identified four significant factors that closely linked with MALE incidence: a history of peripheral surgery or angioplasty, prior limb amputation, baseline Fontaine classification of stage III or IV, and treatment in COMPASS with aspirin alone and not with rivaroxaban plus aspirin.
The new, additional analyses Dr. Anand reported also showed total peripheral vascular outcomes during follow-up in COMPASS in 8.0% of patients on aspirin only and 6.2% of patients on aspirin plus low-dosage rivaroxaban, a 24% relative risk reduction, and vascular interventions in 7.1% of aspirin-only patients and in 5.5% of the combined-regimen patients, also a 24% relative risk reduction. MALE occurred in 2.6% of the aspirin-only patients and in 1.5% of patients on both drugs, a 33% relative risk reduction. All three of these relative risk reductions were statistically significant, said Dr. Anand, a cardiologist and professor of medicine at McMaster University in Hamilton, Ont.
She estimated that about two-thirds of the PAD patients she sees in routine practice would qualify for treatment with aspirin plus low-dosage rivaroxaban once the 2.5 mg formulation becomes approved by regulators. The companies that jointly market rivaroxaban (Xarelto)have an application pending with the Food and Drug Administration to market a 2.5-mg pill based on the COMPASS results. Patients with stable PAD who are not good candidates for the COMPASS regimen are those with a history of a major bleed, those who require full-dose anticoagulation for a comorbidity such as atrial fibrillation or a mechanical heart valve, and patients with newly diagnosed, stable PAD without concurrent coronary artery disease who might receive adequate protection from aspirin alone, Dr. Anand said.
COMPASS was sponsored by Bayer, the company that along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
SOURCE: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
ORLANDO – Combination treatment with aspirin and a low dosage of the anticoagulant rivaroxaban had a broader benefit for reducing adverse events in patients with peripheral artery disease than initially reported from the COMPASS trial, which included more than 7,000 patients whose primary diagnosis at study entry was stable PAD.
Secondary analysis of data from the PAD patients enrolled in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial showed that, compared with aspirin alone, treatment with 100 mg aspirin daily plus a low dosage of the anticoagulant rivaroxaban (2.5 mg b.i.d.) resulted in a statistically significant, 24% relative cut in the combined rate of vascular interventions: acute or chronic limb ischemia, vascular amputations, peripheral vascular bypass or percutaneous intervention, and vascular hospitalizations, Sonia S. Anand, MD, said at the annual meeting of the American College of Cardiology.
This finding plus the results in a prior report from COMPASS that rivaroxaban plus aspirin cut major adverse limb events (MALE) by 33%, compared with aspirin alone (Lancet. 2018 Jan 20;391:219-9), together show that rivaroxaban plus aspirin represent a new, effective regimen for a majority of PAD patients (in addition to treating with a statin and an ACE inhibitor) to cut adverse outcomes in a high-risk but historically undertreated patient population, Dr. Anand said in a video interview.
Additional analysis that Dr. Anand reported in her talk showed that patients with PAD who had an index MALE during follow-up had a very high rate of subsequent events. Among the 128 PAD patients in COMPASS who had an index MALE (major vascular amputation or severe limb ischemia that resulted in an intervention) and, compared with the PAD patients who did not have a MALE, the rate of subsequent death was more than three times higher, the rate of hospitalization increased more than 7-fold, and the rate of amputation increased nearly 200-fold.
Concurrently with Dr. Anand’s report at the meeting the results appeared in an article online in the Journal of the American College of Cardiology.
The analysis of post-MALE outcomes, as well as the expanded vascular-outcomes analysis, focused on 6,391 of the COMPASS patients who had lower-extremity PAD and were randomized to either 100 mg aspirin daily or the low-dosage rivaroxaban plus aspirin regimen. COMPASS also randomized patients to a higher-dosage rivaroxaban-only regimen administered at 5 mg b.i.d., but this arm did not perform as well as the lower-dosage regimen, with an efficacy that equaled aspirin only and with more bleeding. The primary efficacy and safety data from COMPASS for all 27,395 enrolled patients, which included many patients with stable coronary artery disease and without diagnosed PAD, appeared in 2017 (N Engl J Med. Oct 5;377[14]:1319-30).
Dr. Anand also reported on a comparison of the clinical and demographic profiles of the 128 PAD patients who developed MALE during follow-up and the 6,263 who did not, a 2% incidence during almost 2 years.
Multivariate analysis identified four significant factors that closely linked with MALE incidence: a history of peripheral surgery or angioplasty, prior limb amputation, baseline Fontaine classification of stage III or IV, and treatment in COMPASS with aspirin alone and not with rivaroxaban plus aspirin.
The new, additional analyses Dr. Anand reported also showed total peripheral vascular outcomes during follow-up in COMPASS in 8.0% of patients on aspirin only and 6.2% of patients on aspirin plus low-dosage rivaroxaban, a 24% relative risk reduction, and vascular interventions in 7.1% of aspirin-only patients and in 5.5% of the combined-regimen patients, also a 24% relative risk reduction. MALE occurred in 2.6% of the aspirin-only patients and in 1.5% of patients on both drugs, a 33% relative risk reduction. All three of these relative risk reductions were statistically significant, said Dr. Anand, a cardiologist and professor of medicine at McMaster University in Hamilton, Ont.
She estimated that about two-thirds of the PAD patients she sees in routine practice would qualify for treatment with aspirin plus low-dosage rivaroxaban once the 2.5 mg formulation becomes approved by regulators. The companies that jointly market rivaroxaban (Xarelto)have an application pending with the Food and Drug Administration to market a 2.5-mg pill based on the COMPASS results. Patients with stable PAD who are not good candidates for the COMPASS regimen are those with a history of a major bleed, those who require full-dose anticoagulation for a comorbidity such as atrial fibrillation or a mechanical heart valve, and patients with newly diagnosed, stable PAD without concurrent coronary artery disease who might receive adequate protection from aspirin alone, Dr. Anand said.
COMPASS was sponsored by Bayer, the company that along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
SOURCE: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
ORLANDO – Combination treatment with aspirin and a low dosage of the anticoagulant rivaroxaban had a broader benefit for reducing adverse events in patients with peripheral artery disease than initially reported from the COMPASS trial, which included more than 7,000 patients whose primary diagnosis at study entry was stable PAD.
Secondary analysis of data from the PAD patients enrolled in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial showed that, compared with aspirin alone, treatment with 100 mg aspirin daily plus a low dosage of the anticoagulant rivaroxaban (2.5 mg b.i.d.) resulted in a statistically significant, 24% relative cut in the combined rate of vascular interventions: acute or chronic limb ischemia, vascular amputations, peripheral vascular bypass or percutaneous intervention, and vascular hospitalizations, Sonia S. Anand, MD, said at the annual meeting of the American College of Cardiology.
This finding plus the results in a prior report from COMPASS that rivaroxaban plus aspirin cut major adverse limb events (MALE) by 33%, compared with aspirin alone (Lancet. 2018 Jan 20;391:219-9), together show that rivaroxaban plus aspirin represent a new, effective regimen for a majority of PAD patients (in addition to treating with a statin and an ACE inhibitor) to cut adverse outcomes in a high-risk but historically undertreated patient population, Dr. Anand said in a video interview.
Additional analysis that Dr. Anand reported in her talk showed that patients with PAD who had an index MALE during follow-up had a very high rate of subsequent events. Among the 128 PAD patients in COMPASS who had an index MALE (major vascular amputation or severe limb ischemia that resulted in an intervention) and, compared with the PAD patients who did not have a MALE, the rate of subsequent death was more than three times higher, the rate of hospitalization increased more than 7-fold, and the rate of amputation increased nearly 200-fold.
Concurrently with Dr. Anand’s report at the meeting the results appeared in an article online in the Journal of the American College of Cardiology.
The analysis of post-MALE outcomes, as well as the expanded vascular-outcomes analysis, focused on 6,391 of the COMPASS patients who had lower-extremity PAD and were randomized to either 100 mg aspirin daily or the low-dosage rivaroxaban plus aspirin regimen. COMPASS also randomized patients to a higher-dosage rivaroxaban-only regimen administered at 5 mg b.i.d., but this arm did not perform as well as the lower-dosage regimen, with an efficacy that equaled aspirin only and with more bleeding. The primary efficacy and safety data from COMPASS for all 27,395 enrolled patients, which included many patients with stable coronary artery disease and without diagnosed PAD, appeared in 2017 (N Engl J Med. Oct 5;377[14]:1319-30).
Dr. Anand also reported on a comparison of the clinical and demographic profiles of the 128 PAD patients who developed MALE during follow-up and the 6,263 who did not, a 2% incidence during almost 2 years.
Multivariate analysis identified four significant factors that closely linked with MALE incidence: a history of peripheral surgery or angioplasty, prior limb amputation, baseline Fontaine classification of stage III or IV, and treatment in COMPASS with aspirin alone and not with rivaroxaban plus aspirin.
The new, additional analyses Dr. Anand reported also showed total peripheral vascular outcomes during follow-up in COMPASS in 8.0% of patients on aspirin only and 6.2% of patients on aspirin plus low-dosage rivaroxaban, a 24% relative risk reduction, and vascular interventions in 7.1% of aspirin-only patients and in 5.5% of the combined-regimen patients, also a 24% relative risk reduction. MALE occurred in 2.6% of the aspirin-only patients and in 1.5% of patients on both drugs, a 33% relative risk reduction. All three of these relative risk reductions were statistically significant, said Dr. Anand, a cardiologist and professor of medicine at McMaster University in Hamilton, Ont.
She estimated that about two-thirds of the PAD patients she sees in routine practice would qualify for treatment with aspirin plus low-dosage rivaroxaban once the 2.5 mg formulation becomes approved by regulators. The companies that jointly market rivaroxaban (Xarelto)have an application pending with the Food and Drug Administration to market a 2.5-mg pill based on the COMPASS results. Patients with stable PAD who are not good candidates for the COMPASS regimen are those with a history of a major bleed, those who require full-dose anticoagulation for a comorbidity such as atrial fibrillation or a mechanical heart valve, and patients with newly diagnosed, stable PAD without concurrent coronary artery disease who might receive adequate protection from aspirin alone, Dr. Anand said.
COMPASS was sponsored by Bayer, the company that along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
SOURCE: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
REPORTING FROM ACC 18
Key clinical point: PAD patients on rivaroxaban plus aspirin had significantly fewer adverse peripheral vascular outcomes.
Major finding: Dual therapy cut total adverse peripheral vascular outcomes by a relative 24%, compared with aspirin alone, during 23-month follow-up.
Study details: Secondary analysis of data from COMPASS, a multicenter, randomized trial with 6,391 patients in the new analysis.
Disclosures: COMPASS was sponsored by Bayer, the company that, along with Janssen markets rivaroxaban. Dr. Anand has been a consultant to Bayer and Novartis. Dr. Beckman has been a consultant to Janssen and other pharmaceutical companies.
Source: Anand SS et al. J Amer Coll Cardiol. 2018 Mar 11. doi: 10.1016/j.jacc.2018.03.008.
Parents surveyed about underage drinking
Most parents of children aged 10-17 years have talked to them about alcohol consumption, but many do not consider brain health to be an important reason to avoid underage drinking, according to a recent survey of 1,000 parents.
The 76% of parents who reported having at least one conversation about alcohol was up by 7% from a survey conducted in 2003, the Foundation for Advancing Alcohol Responsibility said.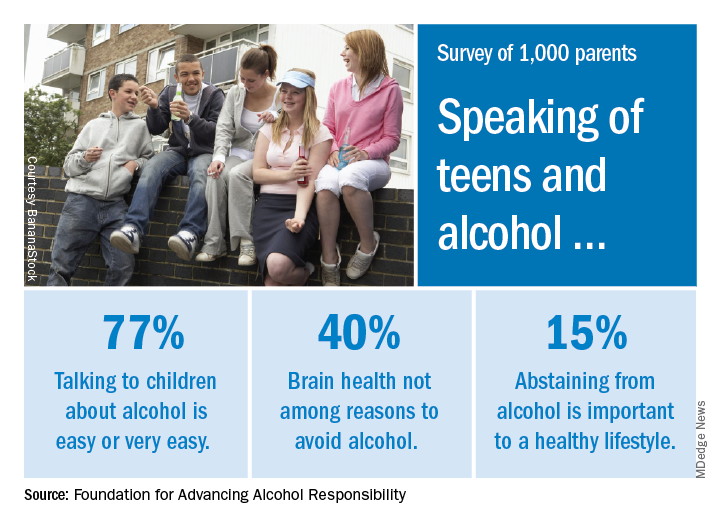
As for the most important reasons to avoid alcohol, 79% said that it interferes with judgment and the ability to make good decisions, and 77% mentioned the unintended consequences of consuming too much. Slightly more than 40% did not include its effects on brain development, the report said.
“Adolescence includes critical phases in brain development. The area of the brain that controls reasoning – helps us think before we act – matures later in the third decade of life. The sooner that parents speak with their children about the dangers of drinking alcohol underage, the better,” said Deborah Gilboa, MD, a Pittsburgh family physician who serves on the foundation’s education advisory board.
The survey was conducted online by GfK between Nov. 10 and 12, 2017, among adults aged 18 years and over with at least one child aged 10-17 years. The margin of error is plus or minus 3 percentage points for the full sample.
Most parents of children aged 10-17 years have talked to them about alcohol consumption, but many do not consider brain health to be an important reason to avoid underage drinking, according to a recent survey of 1,000 parents.
The 76% of parents who reported having at least one conversation about alcohol was up by 7% from a survey conducted in 2003, the Foundation for Advancing Alcohol Responsibility said.
As for the most important reasons to avoid alcohol, 79% said that it interferes with judgment and the ability to make good decisions, and 77% mentioned the unintended consequences of consuming too much. Slightly more than 40% did not include its effects on brain development, the report said.
“Adolescence includes critical phases in brain development. The area of the brain that controls reasoning – helps us think before we act – matures later in the third decade of life. The sooner that parents speak with their children about the dangers of drinking alcohol underage, the better,” said Deborah Gilboa, MD, a Pittsburgh family physician who serves on the foundation’s education advisory board.
The survey was conducted online by GfK between Nov. 10 and 12, 2017, among adults aged 18 years and over with at least one child aged 10-17 years. The margin of error is plus or minus 3 percentage points for the full sample.
Most parents of children aged 10-17 years have talked to them about alcohol consumption, but many do not consider brain health to be an important reason to avoid underage drinking, according to a recent survey of 1,000 parents.
The 76% of parents who reported having at least one conversation about alcohol was up by 7% from a survey conducted in 2003, the Foundation for Advancing Alcohol Responsibility said.
As for the most important reasons to avoid alcohol, 79% said that it interferes with judgment and the ability to make good decisions, and 77% mentioned the unintended consequences of consuming too much. Slightly more than 40% did not include its effects on brain development, the report said.
“Adolescence includes critical phases in brain development. The area of the brain that controls reasoning – helps us think before we act – matures later in the third decade of life. The sooner that parents speak with their children about the dangers of drinking alcohol underage, the better,” said Deborah Gilboa, MD, a Pittsburgh family physician who serves on the foundation’s education advisory board.
The survey was conducted online by GfK between Nov. 10 and 12, 2017, among adults aged 18 years and over with at least one child aged 10-17 years. The margin of error is plus or minus 3 percentage points for the full sample.
Bleeding episodes more common in boys with VWD
SAN DIEGO – Among children with types 1 and 2 von Willebrand disease (VWD), a higher proportion of boys than girls reported ever having a bleeding episode and using more treatment products. But the trend did not continue among children with type 3 disease.
Those are some of the key findings from a never-before-published analysis of surveillance data from the Centers for Disease Control and Prevention presented by Karon Abe, PhD, during a poster session at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The researchers retrieved data from the UDC (Universal Data Collection System), a federally funded surveillance system of people with hemophilia and other bleeding disorders treated at 130 U.S. Hemophilia Treatment Centers (HTCs) during 1998-2011. Although UDC data collection ended in 2011, a current CDC bleeding surveillance project called Community Counts continues and expands on the work of the UDC.
Between 1998 and 2011, data were collected on 2,413 children with VWD aged 2-12 years. Of these, 2,070 had type 1, 224 had type 2, and 119 had type 3 VWD. The researchers used chi-square analysis and Wilcoxon rank sum tests to assess differences in bleeding characteristics by sex and by type of VWD. Next, they used a multivariate regression model to examine the association between demographic and clinical characteristics and a history of ever having had a bleeding episode among type 1 VWD patients.
Nearly two-thirds of children (65%) were non-Hispanic, 17% were Hispanic, 8% were black, and the remainder were from other ethnicities. In addition, 40% of the children had no family history of a bleeding disorder.
The median age of first bleed was lower among children with type 3 VWD, compared with other VWD types, and was lower among boys than girls with type 1 VWD (36 months vs. 48 months, respectively; P less than .001) and type 3 VWD (9 months vs. 12 months; P = .04), Dr. Abe reported.
A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P = .01) and type 2 VWD (90% vs. 75%; P = .01), but not among children with type 3 VWD (97% vs. 96%; P = .77).
A higher prevalence of treatment-product use was reported among children with type 3 VWD, compared with those with the other VWD types (a mean of 95% vs. 79% and 71% among types 2 and 1, respectively). A significantly higher prevalence of the use of treatment product was seen among boys than girls with type 1 VWD (73% vs. 68%, P = .03) and type 2 VWD (87% vs. 72%, P =.01), but not type 3 VWD (94% vs. 96%, P = .87).
The most common sites of the first bleed among all patients regardless of gender or VWD type were epistaxis and oral cavity bleeding.
“To our surprise, the boys were showing more bleeding and were receiving more product than the females,” Dr. Abe said in an interview. “This is a fairly large population.”
Multivariate regression analysis revealed independent associations between the following patient characteristics and ever having a bleed among children with type 1 VWD: male gender (adjusted odds ratio, 1.23); being aged 7-9 years at registration, compared with being aged 2-6 years (aOR, 1.5); being black (aOR, 1.7); being Asian, Native Hawaiian or Pacific Islander (aOR, 2.4), being Hispanic (aOR, 2.8), and being some other race/ethnicity (aOR, 1.8). However, family history of a bleeding disorder was protective (aOR, 0.721).
Dr. Abe said she hopes that the findings will raise awareness and help physicians to educate families about bleeding symptoms and intervene to treat bleeding episodes appropriately. She and her associates are planning to compare the data with Community Counts, “so it’s more up to date,” she said.
Dr. Abe reported having no financial disclosures.
SOURCE: Abe K et al. THSNA 2018, Poster 145.
SAN DIEGO – Among children with types 1 and 2 von Willebrand disease (VWD), a higher proportion of boys than girls reported ever having a bleeding episode and using more treatment products. But the trend did not continue among children with type 3 disease.
Those are some of the key findings from a never-before-published analysis of surveillance data from the Centers for Disease Control and Prevention presented by Karon Abe, PhD, during a poster session at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The researchers retrieved data from the UDC (Universal Data Collection System), a federally funded surveillance system of people with hemophilia and other bleeding disorders treated at 130 U.S. Hemophilia Treatment Centers (HTCs) during 1998-2011. Although UDC data collection ended in 2011, a current CDC bleeding surveillance project called Community Counts continues and expands on the work of the UDC.
Between 1998 and 2011, data were collected on 2,413 children with VWD aged 2-12 years. Of these, 2,070 had type 1, 224 had type 2, and 119 had type 3 VWD. The researchers used chi-square analysis and Wilcoxon rank sum tests to assess differences in bleeding characteristics by sex and by type of VWD. Next, they used a multivariate regression model to examine the association between demographic and clinical characteristics and a history of ever having had a bleeding episode among type 1 VWD patients.
Nearly two-thirds of children (65%) were non-Hispanic, 17% were Hispanic, 8% were black, and the remainder were from other ethnicities. In addition, 40% of the children had no family history of a bleeding disorder.
The median age of first bleed was lower among children with type 3 VWD, compared with other VWD types, and was lower among boys than girls with type 1 VWD (36 months vs. 48 months, respectively; P less than .001) and type 3 VWD (9 months vs. 12 months; P = .04), Dr. Abe reported.
A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P = .01) and type 2 VWD (90% vs. 75%; P = .01), but not among children with type 3 VWD (97% vs. 96%; P = .77).
A higher prevalence of treatment-product use was reported among children with type 3 VWD, compared with those with the other VWD types (a mean of 95% vs. 79% and 71% among types 2 and 1, respectively). A significantly higher prevalence of the use of treatment product was seen among boys than girls with type 1 VWD (73% vs. 68%, P = .03) and type 2 VWD (87% vs. 72%, P =.01), but not type 3 VWD (94% vs. 96%, P = .87).
The most common sites of the first bleed among all patients regardless of gender or VWD type were epistaxis and oral cavity bleeding.
“To our surprise, the boys were showing more bleeding and were receiving more product than the females,” Dr. Abe said in an interview. “This is a fairly large population.”
Multivariate regression analysis revealed independent associations between the following patient characteristics and ever having a bleed among children with type 1 VWD: male gender (adjusted odds ratio, 1.23); being aged 7-9 years at registration, compared with being aged 2-6 years (aOR, 1.5); being black (aOR, 1.7); being Asian, Native Hawaiian or Pacific Islander (aOR, 2.4), being Hispanic (aOR, 2.8), and being some other race/ethnicity (aOR, 1.8). However, family history of a bleeding disorder was protective (aOR, 0.721).
Dr. Abe said she hopes that the findings will raise awareness and help physicians to educate families about bleeding symptoms and intervene to treat bleeding episodes appropriately. She and her associates are planning to compare the data with Community Counts, “so it’s more up to date,” she said.
Dr. Abe reported having no financial disclosures.
SOURCE: Abe K et al. THSNA 2018, Poster 145.
SAN DIEGO – Among children with types 1 and 2 von Willebrand disease (VWD), a higher proportion of boys than girls reported ever having a bleeding episode and using more treatment products. But the trend did not continue among children with type 3 disease.
Those are some of the key findings from a never-before-published analysis of surveillance data from the Centers for Disease Control and Prevention presented by Karon Abe, PhD, during a poster session at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The researchers retrieved data from the UDC (Universal Data Collection System), a federally funded surveillance system of people with hemophilia and other bleeding disorders treated at 130 U.S. Hemophilia Treatment Centers (HTCs) during 1998-2011. Although UDC data collection ended in 2011, a current CDC bleeding surveillance project called Community Counts continues and expands on the work of the UDC.
Between 1998 and 2011, data were collected on 2,413 children with VWD aged 2-12 years. Of these, 2,070 had type 1, 224 had type 2, and 119 had type 3 VWD. The researchers used chi-square analysis and Wilcoxon rank sum tests to assess differences in bleeding characteristics by sex and by type of VWD. Next, they used a multivariate regression model to examine the association between demographic and clinical characteristics and a history of ever having had a bleeding episode among type 1 VWD patients.
Nearly two-thirds of children (65%) were non-Hispanic, 17% were Hispanic, 8% were black, and the remainder were from other ethnicities. In addition, 40% of the children had no family history of a bleeding disorder.
The median age of first bleed was lower among children with type 3 VWD, compared with other VWD types, and was lower among boys than girls with type 1 VWD (36 months vs. 48 months, respectively; P less than .001) and type 3 VWD (9 months vs. 12 months; P = .04), Dr. Abe reported.
A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P = .01) and type 2 VWD (90% vs. 75%; P = .01), but not among children with type 3 VWD (97% vs. 96%; P = .77).
A higher prevalence of treatment-product use was reported among children with type 3 VWD, compared with those with the other VWD types (a mean of 95% vs. 79% and 71% among types 2 and 1, respectively). A significantly higher prevalence of the use of treatment product was seen among boys than girls with type 1 VWD (73% vs. 68%, P = .03) and type 2 VWD (87% vs. 72%, P =.01), but not type 3 VWD (94% vs. 96%, P = .87).
The most common sites of the first bleed among all patients regardless of gender or VWD type were epistaxis and oral cavity bleeding.
“To our surprise, the boys were showing more bleeding and were receiving more product than the females,” Dr. Abe said in an interview. “This is a fairly large population.”
Multivariate regression analysis revealed independent associations between the following patient characteristics and ever having a bleed among children with type 1 VWD: male gender (adjusted odds ratio, 1.23); being aged 7-9 years at registration, compared with being aged 2-6 years (aOR, 1.5); being black (aOR, 1.7); being Asian, Native Hawaiian or Pacific Islander (aOR, 2.4), being Hispanic (aOR, 2.8), and being some other race/ethnicity (aOR, 1.8). However, family history of a bleeding disorder was protective (aOR, 0.721).
Dr. Abe said she hopes that the findings will raise awareness and help physicians to educate families about bleeding symptoms and intervene to treat bleeding episodes appropriately. She and her associates are planning to compare the data with Community Counts, “so it’s more up to date,” she said.
Dr. Abe reported having no financial disclosures.
SOURCE: Abe K et al. THSNA 2018, Poster 145.
REPORTING FROM THSNA 2018
Key clinical point:
Major finding: A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P= .01) and type 2 VWD (90% vs. 75%; P= .01), but not among children with type 3 VWD (97% vs. 96%; P= .77).
Study details: An analysis of 2,413 children with VWD aged 2-12 years.
Disclosures: Dr. Abe reported having no financial disclosures.
Source: Abe K et al. THSNA 2018, Poster 145.
Alternative oxygen therapy reduces treatment failure in bronchiolitis
High-flow oxygen therapy outside the ICU boosts the likelihood that infants with bronchiolitis will avoid treatment failure and an escalation of treatment, a study finds.
“High flow can be safely used in general emergency wards and general pediatric ward settings in regional and metropolitan hospitals that have no immediate direct access to dedicated pediatric intensive care facilities,” study coauthor Andreas Schibler, MD, of University of Queensland in Australia, said in an interview. The findings were published March 22 in the New England Journal of Medicine.
“The typical treatment for bronchiolitis is supportive therapy, providing nutrition, fluids, and if needed respiratory support including provision of oxygen,” Dr. Schibler said.
The prognosis is generally goods thanks to improvements in intensive care, he said, which some infants need because the standard oxygen therapy provided in general pediatric wards is insufficient. The new study examines whether high-flow oxygen therapy through a cannula – which he said has become more common – reduces the risk of treatment failure in non-ICU therapy, compared with standard oxygen treatment.
Dr. Schibler and his colleagues tracked 1,472 patients under 12 months with bronchiolitis and a need for oxygen treatment who were randomly assigned to high-flow or standard oxygen therapy to maintain their oxygen saturation at 92%-98% or 94%-98%, depending on policy at the hospital. The subjects were patients at 17 hospitals in Australia and New Zealand.
A total of 739 infants received high-flow treatment that provided heated and humidified oxygen at a rate of 2 liters per kilogram of body weight per minute. The other 733 infants received standard oxygen therapy up to a maximum 2 liters per minute.
The treatment failed, requiring an escalation of care, in 87 of 739 patients (12%) in the high-flow group and 167 of 733 (23%) in the standard-therapy group. (risk difference = –11% points; 95% confidence interval, –15 to –7; P less than .001).
“The ease to use and simplicity of high flow made us recognize and think that this level of respiratory care can be provided outside intensive care,” Dr. Schibler said. “This was further supported by the observational fact that most of these infants with bronchiolitis showed a dramatically improved respiratory condition once on high flow.”
Dr. Schibler said there haven’t been any signs of adverse effects from high-flow oxygen therapy. As for the cost of the treatment, he said it is “likely offset by a reduced need for intensive care therapy or costs associated with transferring to a children’s hospital.”
What should physicians and hospitals take from the study findings? “If a hospital explores the option to use high flow in bronchiolitis, then start the therapy early in the disease process or once an oxygen requirement is recognized,” Dr. Schibler said. “Implementation of a solid and structured training program with a clear hospital guideline based on the evidence will ensure the staff who care for these patients will be empowered and comfortable to adjust the oxygen levels given by the high-flow equipment. The greater the confidence and comfort level for the nursing and respiratory technician staff the better for these infants, as they will sooner observe those infants who are not responding well and may require a higher level of care such as intensive care or they will recognize the infant who responds well.”
The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment and consumables and travel/accommodation support. Study authors reported various grants and other support.
SOURCE: Franklin D et al. N Engl J Med. 2018;378(12):1112-31.
High-flow oxygen therapy outside the ICU boosts the likelihood that infants with bronchiolitis will avoid treatment failure and an escalation of treatment, a study finds.
“High flow can be safely used in general emergency wards and general pediatric ward settings in regional and metropolitan hospitals that have no immediate direct access to dedicated pediatric intensive care facilities,” study coauthor Andreas Schibler, MD, of University of Queensland in Australia, said in an interview. The findings were published March 22 in the New England Journal of Medicine.
“The typical treatment for bronchiolitis is supportive therapy, providing nutrition, fluids, and if needed respiratory support including provision of oxygen,” Dr. Schibler said.
The prognosis is generally goods thanks to improvements in intensive care, he said, which some infants need because the standard oxygen therapy provided in general pediatric wards is insufficient. The new study examines whether high-flow oxygen therapy through a cannula – which he said has become more common – reduces the risk of treatment failure in non-ICU therapy, compared with standard oxygen treatment.
Dr. Schibler and his colleagues tracked 1,472 patients under 12 months with bronchiolitis and a need for oxygen treatment who were randomly assigned to high-flow or standard oxygen therapy to maintain their oxygen saturation at 92%-98% or 94%-98%, depending on policy at the hospital. The subjects were patients at 17 hospitals in Australia and New Zealand.
A total of 739 infants received high-flow treatment that provided heated and humidified oxygen at a rate of 2 liters per kilogram of body weight per minute. The other 733 infants received standard oxygen therapy up to a maximum 2 liters per minute.
The treatment failed, requiring an escalation of care, in 87 of 739 patients (12%) in the high-flow group and 167 of 733 (23%) in the standard-therapy group. (risk difference = –11% points; 95% confidence interval, –15 to –7; P less than .001).
“The ease to use and simplicity of high flow made us recognize and think that this level of respiratory care can be provided outside intensive care,” Dr. Schibler said. “This was further supported by the observational fact that most of these infants with bronchiolitis showed a dramatically improved respiratory condition once on high flow.”
Dr. Schibler said there haven’t been any signs of adverse effects from high-flow oxygen therapy. As for the cost of the treatment, he said it is “likely offset by a reduced need for intensive care therapy or costs associated with transferring to a children’s hospital.”
What should physicians and hospitals take from the study findings? “If a hospital explores the option to use high flow in bronchiolitis, then start the therapy early in the disease process or once an oxygen requirement is recognized,” Dr. Schibler said. “Implementation of a solid and structured training program with a clear hospital guideline based on the evidence will ensure the staff who care for these patients will be empowered and comfortable to adjust the oxygen levels given by the high-flow equipment. The greater the confidence and comfort level for the nursing and respiratory technician staff the better for these infants, as they will sooner observe those infants who are not responding well and may require a higher level of care such as intensive care or they will recognize the infant who responds well.”
The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment and consumables and travel/accommodation support. Study authors reported various grants and other support.
SOURCE: Franklin D et al. N Engl J Med. 2018;378(12):1112-31.
High-flow oxygen therapy outside the ICU boosts the likelihood that infants with bronchiolitis will avoid treatment failure and an escalation of treatment, a study finds.
“High flow can be safely used in general emergency wards and general pediatric ward settings in regional and metropolitan hospitals that have no immediate direct access to dedicated pediatric intensive care facilities,” study coauthor Andreas Schibler, MD, of University of Queensland in Australia, said in an interview. The findings were published March 22 in the New England Journal of Medicine.
“The typical treatment for bronchiolitis is supportive therapy, providing nutrition, fluids, and if needed respiratory support including provision of oxygen,” Dr. Schibler said.
The prognosis is generally goods thanks to improvements in intensive care, he said, which some infants need because the standard oxygen therapy provided in general pediatric wards is insufficient. The new study examines whether high-flow oxygen therapy through a cannula – which he said has become more common – reduces the risk of treatment failure in non-ICU therapy, compared with standard oxygen treatment.
Dr. Schibler and his colleagues tracked 1,472 patients under 12 months with bronchiolitis and a need for oxygen treatment who were randomly assigned to high-flow or standard oxygen therapy to maintain their oxygen saturation at 92%-98% or 94%-98%, depending on policy at the hospital. The subjects were patients at 17 hospitals in Australia and New Zealand.
A total of 739 infants received high-flow treatment that provided heated and humidified oxygen at a rate of 2 liters per kilogram of body weight per minute. The other 733 infants received standard oxygen therapy up to a maximum 2 liters per minute.
The treatment failed, requiring an escalation of care, in 87 of 739 patients (12%) in the high-flow group and 167 of 733 (23%) in the standard-therapy group. (risk difference = –11% points; 95% confidence interval, –15 to –7; P less than .001).
“The ease to use and simplicity of high flow made us recognize and think that this level of respiratory care can be provided outside intensive care,” Dr. Schibler said. “This was further supported by the observational fact that most of these infants with bronchiolitis showed a dramatically improved respiratory condition once on high flow.”
Dr. Schibler said there haven’t been any signs of adverse effects from high-flow oxygen therapy. As for the cost of the treatment, he said it is “likely offset by a reduced need for intensive care therapy or costs associated with transferring to a children’s hospital.”
What should physicians and hospitals take from the study findings? “If a hospital explores the option to use high flow in bronchiolitis, then start the therapy early in the disease process or once an oxygen requirement is recognized,” Dr. Schibler said. “Implementation of a solid and structured training program with a clear hospital guideline based on the evidence will ensure the staff who care for these patients will be empowered and comfortable to adjust the oxygen levels given by the high-flow equipment. The greater the confidence and comfort level for the nursing and respiratory technician staff the better for these infants, as they will sooner observe those infants who are not responding well and may require a higher level of care such as intensive care or they will recognize the infant who responds well.”
The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment and consumables and travel/accommodation support. Study authors reported various grants and other support.
SOURCE: Franklin D et al. N Engl J Med. 2018;378(12):1112-31.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In non-ICUs, infants under 12 months with bronchiolitis are less likely to fail treatment if they are given high-flow oxygen therapy instead of standard oxygen therapy.
Major finding: Treatment failure occurred in 8 of 739 (12%) patients in the high-flow oxygen therapy group and 167 of 733 (23%) in the standard-therapy group.
Study details: Multicenter, randomized, controlled trial of 1,472 infants.
Disclosures: The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment/consumables and travel/accommodation support. Study authors reported various grants and other support.
Source: Franklin D et al. N Engl J Med 2018;378(12):1112-31.
H. pylori eradication cuts new gastric cancers by half
Treatment for Helicobacter pylori infection cut the incidence of new gastric cancers in half among patients undergoing endoscopic resection of early gastric cancer, according to results of a recent randomized, placebo-controlled study.
Patients receiving H. pylori treatment also had greater improvement from baseline in grade of gastric corpus atrophy, compared with patients receiving placebo, according to the study. The results were published in the New England Journal of Medicine.
“We speculate that persistent inflammation of gastric mucosa with H. pylori infection promotes carcinogenesis and also increases tumor growth or invasiveness,” said Il Ju Choi, MD, PhD, of the Center for Gastric Cancer, National Cancer Center, Goyang, South Korea, and coauthors.
Patients with early gastric cancers not at risk for lymph node metastasis may benefit from endoscopic resection. However, these patients are at high risk of developing new gastric cancer, and usually experience glandular atrophy, or advanced loss of mucosal glandular tissue, the authors said.
One nonrandomized study suggested H. pylori eradication could prevent development of subsequent cancers after endoscopic resection, according to the authors, but subsequent open-label trials were inconsistent on whether the treatment reduced cancer incidence.
Accordingly, Dr. Choi and colleagues conducted a prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Of 396 patients included in an intention-to-treat analysis, 194 were randomized to receive antibiotics for H. pylori eradication, and 202 received placebo.
Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (hazard ratio, 0.50; 95% confidence interval, 0.26-0.94; P = .03).
Histologic analysis, performed in 327 patients, showed that 48.4% of patients in the treatment group had improvement in atrophy grade at the gastric corpus lesser curvature, compared to just 15.0% of the placebo group (P less than .001), the investigators reported.
Mild adverse events were more frequent in the treatment arm (42.0% versus 10.2%; P less than .001), and there were no serious adverse events, they added.
Despite the approximate 50% reduction in incidence of new gastric cancers and histologic improvements, the researchers said that further study would be required to optimize treatment approaches for patients undergoing endoscopic resection for high-grade adenoma or early gastric cancer.
“H. pylori eradication reduces, but cannot completely abolish, the risk of metachronous gastric cancer,” wrote Dr. Choi and colleagues. “Thus, molecular markers, including aberrant methylation at specific genes, might help to identify high-risk patients even after successful eradication.”
The researchers reported that they had nothing to disclose related to the study.
SOURCE: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
The study by Choi and colleagues suggests Helicobacter pylori eradication is effective at stopping the carcinogenic process in patients with severe chronic atrophic gastritis, an advanced precursor lesion to gastric cancer, according to Peter Malfertheiner, MD.
“It is a striking finding that H. pylori eradication may still be effective at this stage, since such therapy decreased the development of gastric cancer by 50% in this trial,” Dr. Malfertheiner wrote in an editorial.
In the randomized, placebo-controlled trial, H. pylori eradication after endoscopic removal of early stage disease effectively prevented metachronous gastric cancers (i.e., those detected on endoscopy at 1-year follow-up or thereafter) with a hazard ratio of 0.50, Dr. Malfertheiner noted.
The results confirm and strengthen previous findings by showing a significant improvement in atrophic gastritis, he added.
“In this endoscopic procedure, removal of early gastric cancer or high-grade adenoma leaves the stomach largely conserved but with the atrophic gastric mucosa remaining in a preneoplastic ‘alarm state,’ ” he noted.
However, the potential link between cancer recurrence and atrophic gastritis was not explored in this particular study report, Dr. Malfertheiner said. Thus, it is unclear whether gastric cancer recurrence was prevented specifically in the subset of patients with atrophic gastritis.
It could be that eradication of H. pylori directly arrests carcinogenic mechanisms directly by ending persistent inflammation, he speculated.
“The beneficial effect may also be mediated by an alteration in the composition of the gastric microbiota because of improvement in the grade of gastric atrophy and a return toward normal gastric acid production,” he added.
Dr. Malfertheiner is with the Clinic of Gastroenterology, Otto von Guericke University, Magdeburg, Germany. These comments are derived from his editorial (N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMe1800147). Dr. Malfertheiner reported personal fees from Allergan, Biohit, and Infai outside the submitted editorial.
The study by Choi and colleagues suggests Helicobacter pylori eradication is effective at stopping the carcinogenic process in patients with severe chronic atrophic gastritis, an advanced precursor lesion to gastric cancer, according to Peter Malfertheiner, MD.
“It is a striking finding that H. pylori eradication may still be effective at this stage, since such therapy decreased the development of gastric cancer by 50% in this trial,” Dr. Malfertheiner wrote in an editorial.
In the randomized, placebo-controlled trial, H. pylori eradication after endoscopic removal of early stage disease effectively prevented metachronous gastric cancers (i.e., those detected on endoscopy at 1-year follow-up or thereafter) with a hazard ratio of 0.50, Dr. Malfertheiner noted.
The results confirm and strengthen previous findings by showing a significant improvement in atrophic gastritis, he added.
“In this endoscopic procedure, removal of early gastric cancer or high-grade adenoma leaves the stomach largely conserved but with the atrophic gastric mucosa remaining in a preneoplastic ‘alarm state,’ ” he noted.
However, the potential link between cancer recurrence and atrophic gastritis was not explored in this particular study report, Dr. Malfertheiner said. Thus, it is unclear whether gastric cancer recurrence was prevented specifically in the subset of patients with atrophic gastritis.
It could be that eradication of H. pylori directly arrests carcinogenic mechanisms directly by ending persistent inflammation, he speculated.
“The beneficial effect may also be mediated by an alteration in the composition of the gastric microbiota because of improvement in the grade of gastric atrophy and a return toward normal gastric acid production,” he added.
Dr. Malfertheiner is with the Clinic of Gastroenterology, Otto von Guericke University, Magdeburg, Germany. These comments are derived from his editorial (N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMe1800147). Dr. Malfertheiner reported personal fees from Allergan, Biohit, and Infai outside the submitted editorial.
The study by Choi and colleagues suggests Helicobacter pylori eradication is effective at stopping the carcinogenic process in patients with severe chronic atrophic gastritis, an advanced precursor lesion to gastric cancer, according to Peter Malfertheiner, MD.
“It is a striking finding that H. pylori eradication may still be effective at this stage, since such therapy decreased the development of gastric cancer by 50% in this trial,” Dr. Malfertheiner wrote in an editorial.
In the randomized, placebo-controlled trial, H. pylori eradication after endoscopic removal of early stage disease effectively prevented metachronous gastric cancers (i.e., those detected on endoscopy at 1-year follow-up or thereafter) with a hazard ratio of 0.50, Dr. Malfertheiner noted.
The results confirm and strengthen previous findings by showing a significant improvement in atrophic gastritis, he added.
“In this endoscopic procedure, removal of early gastric cancer or high-grade adenoma leaves the stomach largely conserved but with the atrophic gastric mucosa remaining in a preneoplastic ‘alarm state,’ ” he noted.
However, the potential link between cancer recurrence and atrophic gastritis was not explored in this particular study report, Dr. Malfertheiner said. Thus, it is unclear whether gastric cancer recurrence was prevented specifically in the subset of patients with atrophic gastritis.
It could be that eradication of H. pylori directly arrests carcinogenic mechanisms directly by ending persistent inflammation, he speculated.
“The beneficial effect may also be mediated by an alteration in the composition of the gastric microbiota because of improvement in the grade of gastric atrophy and a return toward normal gastric acid production,” he added.
Dr. Malfertheiner is with the Clinic of Gastroenterology, Otto von Guericke University, Magdeburg, Germany. These comments are derived from his editorial (N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMe1800147). Dr. Malfertheiner reported personal fees from Allergan, Biohit, and Infai outside the submitted editorial.
Treatment for Helicobacter pylori infection cut the incidence of new gastric cancers in half among patients undergoing endoscopic resection of early gastric cancer, according to results of a recent randomized, placebo-controlled study.
Patients receiving H. pylori treatment also had greater improvement from baseline in grade of gastric corpus atrophy, compared with patients receiving placebo, according to the study. The results were published in the New England Journal of Medicine.
“We speculate that persistent inflammation of gastric mucosa with H. pylori infection promotes carcinogenesis and also increases tumor growth or invasiveness,” said Il Ju Choi, MD, PhD, of the Center for Gastric Cancer, National Cancer Center, Goyang, South Korea, and coauthors.
Patients with early gastric cancers not at risk for lymph node metastasis may benefit from endoscopic resection. However, these patients are at high risk of developing new gastric cancer, and usually experience glandular atrophy, or advanced loss of mucosal glandular tissue, the authors said.
One nonrandomized study suggested H. pylori eradication could prevent development of subsequent cancers after endoscopic resection, according to the authors, but subsequent open-label trials were inconsistent on whether the treatment reduced cancer incidence.
Accordingly, Dr. Choi and colleagues conducted a prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Of 396 patients included in an intention-to-treat analysis, 194 were randomized to receive antibiotics for H. pylori eradication, and 202 received placebo.
Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (hazard ratio, 0.50; 95% confidence interval, 0.26-0.94; P = .03).
Histologic analysis, performed in 327 patients, showed that 48.4% of patients in the treatment group had improvement in atrophy grade at the gastric corpus lesser curvature, compared to just 15.0% of the placebo group (P less than .001), the investigators reported.
Mild adverse events were more frequent in the treatment arm (42.0% versus 10.2%; P less than .001), and there were no serious adverse events, they added.
Despite the approximate 50% reduction in incidence of new gastric cancers and histologic improvements, the researchers said that further study would be required to optimize treatment approaches for patients undergoing endoscopic resection for high-grade adenoma or early gastric cancer.
“H. pylori eradication reduces, but cannot completely abolish, the risk of metachronous gastric cancer,” wrote Dr. Choi and colleagues. “Thus, molecular markers, including aberrant methylation at specific genes, might help to identify high-risk patients even after successful eradication.”
The researchers reported that they had nothing to disclose related to the study.
SOURCE: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
Treatment for Helicobacter pylori infection cut the incidence of new gastric cancers in half among patients undergoing endoscopic resection of early gastric cancer, according to results of a recent randomized, placebo-controlled study.
Patients receiving H. pylori treatment also had greater improvement from baseline in grade of gastric corpus atrophy, compared with patients receiving placebo, according to the study. The results were published in the New England Journal of Medicine.
“We speculate that persistent inflammation of gastric mucosa with H. pylori infection promotes carcinogenesis and also increases tumor growth or invasiveness,” said Il Ju Choi, MD, PhD, of the Center for Gastric Cancer, National Cancer Center, Goyang, South Korea, and coauthors.
Patients with early gastric cancers not at risk for lymph node metastasis may benefit from endoscopic resection. However, these patients are at high risk of developing new gastric cancer, and usually experience glandular atrophy, or advanced loss of mucosal glandular tissue, the authors said.
One nonrandomized study suggested H. pylori eradication could prevent development of subsequent cancers after endoscopic resection, according to the authors, but subsequent open-label trials were inconsistent on whether the treatment reduced cancer incidence.
Accordingly, Dr. Choi and colleagues conducted a prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Of 396 patients included in an intention-to-treat analysis, 194 were randomized to receive antibiotics for H. pylori eradication, and 202 received placebo.
Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (hazard ratio, 0.50; 95% confidence interval, 0.26-0.94; P = .03).
Histologic analysis, performed in 327 patients, showed that 48.4% of patients in the treatment group had improvement in atrophy grade at the gastric corpus lesser curvature, compared to just 15.0% of the placebo group (P less than .001), the investigators reported.
Mild adverse events were more frequent in the treatment arm (42.0% versus 10.2%; P less than .001), and there were no serious adverse events, they added.
Despite the approximate 50% reduction in incidence of new gastric cancers and histologic improvements, the researchers said that further study would be required to optimize treatment approaches for patients undergoing endoscopic resection for high-grade adenoma or early gastric cancer.
“H. pylori eradication reduces, but cannot completely abolish, the risk of metachronous gastric cancer,” wrote Dr. Choi and colleagues. “Thus, molecular markers, including aberrant methylation at specific genes, might help to identify high-risk patients even after successful eradication.”
The researchers reported that they had nothing to disclose related to the study.
SOURCE: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Among patients undergoing endoscopic resection of early gastric cancer, incidence of new gastric cancers was approximately 50% lower for those who received treatment for Helicobacter pylori infection.
Major finding: Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (HR, 0.50; 95% CI, 0.26-0.94; P = .03).
Study details: A prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Disclosures: The authors reported that they had nothing to disclose related to the study.
Source: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
Report: Abortion in U.S. is safe and effective
, according to a consensus study report by the National Academies of Science, Engineering, and Medicine.
Six private foundations commissioned a comprehensive report from the National Academies, which focused on eight questions related to the safety and quality of U.S. abortion care. The resulting Committee on Reproductive Health Services limited itself to these questions and did not make specific policy or clinical recommendations, though they did note that “state regulations have created barriers to optimizing each dimension of quality care.” The report was released on March 16.
The committee focused on the four legal abortion methods in the United States – medication, aspiration, dilation and evacuation (D&E), and induction – and concluded that all four are safe, but that induction is so rare that there is a lack of quality research on the procedure’s risks in women with prior cesarean deliveries. D&E, though less painful, costly, and time consuming than induction, is banned in Mississippi and West Virginia (with exceptions for emergencies) and limited elsewhere in the country by a lack of physicians trained to perform the procedure.
In attempting to assess the physical and mental health risks of abortion procedures, the committee found that “much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research.” Surveying research that they considered high quality, the committee concluded that there is no increased risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation, preterm birth, breast cancer, or mental health disorders such as depression, anxiety, or posttraumatic stress disorder associated with a woman having an abortion.
The committee was not able to find high-quality research to evaluate the risk of ectopic pregnancy, miscarriage or stillbirth, or long-term mortality associated with abortion. However, it did find an increased risk of preterm birth before 28 weeks’ gestation in a nulliparous women who had had two or more aspiration abortions, compared with women with no abortion history. The risk of preterm birth is greater in women if the interval between their abortion and their next conception is less than 6 months. The same risk exists for short intervals between pregnancy in general, the committee noted.
Overall, they wrote, “serious complications are rare and occur much less frequently than during childbirth.”
The committee identified several kinds of state-level regulations that are obstacles to effective abortion care in the United States. Such regulations “may limit the number of available providers, misinform women of the risks of the procedures they are considering, overrule women’s and clinicians’ medical decision making, or require medically unnecessary services and delays in care.” Some laws “prohibit the abortion method that is most effective for a particular clinical circumstance” (for example, D&E).
Access to care varies widely geographically, and 17% of women must travel more than 50 miles to obtain an abortion. Regulations that required counseling, whether the woman desires counseling or not, are cited as an example of inferior patient-centered care.
The committee delineated safeguards that are necessary to manage emergencies that might arise from an abortion procedure, such as resuscitation and monitoring equipment to be located in a facility for procedures involving moderate and deep sedation. However, they did not find any evidence that it is necessary for clinicians performing abortions to have hospital admitting privileges. In the Whole Woman’s Health v. Hellerstedt decision in 2016, the U.S. Supreme Court ruled that the admitting privileges requirement in Texas was an undue burden on access to care. The committee wrote that it is sufficient for the facility to have an emergency transfer plan in place.
The committee also identified areas for further evaluation. First, whether the Food and Drug Administration should expand the distribution of mifepristone, the only drug currently approved for medication abortions, but which can only be dispensed to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. The committee also called for examining more effective methods of pain management, whether advanced practice clinicians can be trained to perform D&Es, and ways to provide more social and psychological supports for lower-income women or who are at risk of intimate partner or other forms of violence.
On March 16, the National Academies of Science, Engineering, and Medicine released a comprehensive report finding that abortion is safe and effective but inaccessible to many women.
A high-quality foundation of evidence, contributed to by many U.S. family planning researchers, provided the studies on which the conclusions of the National Academies are based. We are fortunate that the Society of Family Planning provides research funding and a forum for family planning researchers to continue to produce the high-quality evidence used by policy makers to improve access to and quality of abortion care.
Eve Espey, MD, MPH, is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. She reported having no relevant financial disclosures.
On March 16, the National Academies of Science, Engineering, and Medicine released a comprehensive report finding that abortion is safe and effective but inaccessible to many women.
A high-quality foundation of evidence, contributed to by many U.S. family planning researchers, provided the studies on which the conclusions of the National Academies are based. We are fortunate that the Society of Family Planning provides research funding and a forum for family planning researchers to continue to produce the high-quality evidence used by policy makers to improve access to and quality of abortion care.
Eve Espey, MD, MPH, is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. She reported having no relevant financial disclosures.
On March 16, the National Academies of Science, Engineering, and Medicine released a comprehensive report finding that abortion is safe and effective but inaccessible to many women.
A high-quality foundation of evidence, contributed to by many U.S. family planning researchers, provided the studies on which the conclusions of the National Academies are based. We are fortunate that the Society of Family Planning provides research funding and a forum for family planning researchers to continue to produce the high-quality evidence used by policy makers to improve access to and quality of abortion care.
Eve Espey, MD, MPH, is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. She reported having no relevant financial disclosures.
, according to a consensus study report by the National Academies of Science, Engineering, and Medicine.
Six private foundations commissioned a comprehensive report from the National Academies, which focused on eight questions related to the safety and quality of U.S. abortion care. The resulting Committee on Reproductive Health Services limited itself to these questions and did not make specific policy or clinical recommendations, though they did note that “state regulations have created barriers to optimizing each dimension of quality care.” The report was released on March 16.
The committee focused on the four legal abortion methods in the United States – medication, aspiration, dilation and evacuation (D&E), and induction – and concluded that all four are safe, but that induction is so rare that there is a lack of quality research on the procedure’s risks in women with prior cesarean deliveries. D&E, though less painful, costly, and time consuming than induction, is banned in Mississippi and West Virginia (with exceptions for emergencies) and limited elsewhere in the country by a lack of physicians trained to perform the procedure.
In attempting to assess the physical and mental health risks of abortion procedures, the committee found that “much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research.” Surveying research that they considered high quality, the committee concluded that there is no increased risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation, preterm birth, breast cancer, or mental health disorders such as depression, anxiety, or posttraumatic stress disorder associated with a woman having an abortion.
The committee was not able to find high-quality research to evaluate the risk of ectopic pregnancy, miscarriage or stillbirth, or long-term mortality associated with abortion. However, it did find an increased risk of preterm birth before 28 weeks’ gestation in a nulliparous women who had had two or more aspiration abortions, compared with women with no abortion history. The risk of preterm birth is greater in women if the interval between their abortion and their next conception is less than 6 months. The same risk exists for short intervals between pregnancy in general, the committee noted.
Overall, they wrote, “serious complications are rare and occur much less frequently than during childbirth.”
The committee identified several kinds of state-level regulations that are obstacles to effective abortion care in the United States. Such regulations “may limit the number of available providers, misinform women of the risks of the procedures they are considering, overrule women’s and clinicians’ medical decision making, or require medically unnecessary services and delays in care.” Some laws “prohibit the abortion method that is most effective for a particular clinical circumstance” (for example, D&E).
Access to care varies widely geographically, and 17% of women must travel more than 50 miles to obtain an abortion. Regulations that required counseling, whether the woman desires counseling or not, are cited as an example of inferior patient-centered care.
The committee delineated safeguards that are necessary to manage emergencies that might arise from an abortion procedure, such as resuscitation and monitoring equipment to be located in a facility for procedures involving moderate and deep sedation. However, they did not find any evidence that it is necessary for clinicians performing abortions to have hospital admitting privileges. In the Whole Woman’s Health v. Hellerstedt decision in 2016, the U.S. Supreme Court ruled that the admitting privileges requirement in Texas was an undue burden on access to care. The committee wrote that it is sufficient for the facility to have an emergency transfer plan in place.
The committee also identified areas for further evaluation. First, whether the Food and Drug Administration should expand the distribution of mifepristone, the only drug currently approved for medication abortions, but which can only be dispensed to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. The committee also called for examining more effective methods of pain management, whether advanced practice clinicians can be trained to perform D&Es, and ways to provide more social and psychological supports for lower-income women or who are at risk of intimate partner or other forms of violence.
, according to a consensus study report by the National Academies of Science, Engineering, and Medicine.
Six private foundations commissioned a comprehensive report from the National Academies, which focused on eight questions related to the safety and quality of U.S. abortion care. The resulting Committee on Reproductive Health Services limited itself to these questions and did not make specific policy or clinical recommendations, though they did note that “state regulations have created barriers to optimizing each dimension of quality care.” The report was released on March 16.
The committee focused on the four legal abortion methods in the United States – medication, aspiration, dilation and evacuation (D&E), and induction – and concluded that all four are safe, but that induction is so rare that there is a lack of quality research on the procedure’s risks in women with prior cesarean deliveries. D&E, though less painful, costly, and time consuming than induction, is banned in Mississippi and West Virginia (with exceptions for emergencies) and limited elsewhere in the country by a lack of physicians trained to perform the procedure.
In attempting to assess the physical and mental health risks of abortion procedures, the committee found that “much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research.” Surveying research that they considered high quality, the committee concluded that there is no increased risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation, preterm birth, breast cancer, or mental health disorders such as depression, anxiety, or posttraumatic stress disorder associated with a woman having an abortion.
The committee was not able to find high-quality research to evaluate the risk of ectopic pregnancy, miscarriage or stillbirth, or long-term mortality associated with abortion. However, it did find an increased risk of preterm birth before 28 weeks’ gestation in a nulliparous women who had had two or more aspiration abortions, compared with women with no abortion history. The risk of preterm birth is greater in women if the interval between their abortion and their next conception is less than 6 months. The same risk exists for short intervals between pregnancy in general, the committee noted.
Overall, they wrote, “serious complications are rare and occur much less frequently than during childbirth.”
The committee identified several kinds of state-level regulations that are obstacles to effective abortion care in the United States. Such regulations “may limit the number of available providers, misinform women of the risks of the procedures they are considering, overrule women’s and clinicians’ medical decision making, or require medically unnecessary services and delays in care.” Some laws “prohibit the abortion method that is most effective for a particular clinical circumstance” (for example, D&E).
Access to care varies widely geographically, and 17% of women must travel more than 50 miles to obtain an abortion. Regulations that required counseling, whether the woman desires counseling or not, are cited as an example of inferior patient-centered care.
The committee delineated safeguards that are necessary to manage emergencies that might arise from an abortion procedure, such as resuscitation and monitoring equipment to be located in a facility for procedures involving moderate and deep sedation. However, they did not find any evidence that it is necessary for clinicians performing abortions to have hospital admitting privileges. In the Whole Woman’s Health v. Hellerstedt decision in 2016, the U.S. Supreme Court ruled that the admitting privileges requirement in Texas was an undue burden on access to care. The committee wrote that it is sufficient for the facility to have an emergency transfer plan in place.
The committee also identified areas for further evaluation. First, whether the Food and Drug Administration should expand the distribution of mifepristone, the only drug currently approved for medication abortions, but which can only be dispensed to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. The committee also called for examining more effective methods of pain management, whether advanced practice clinicians can be trained to perform D&Es, and ways to provide more social and psychological supports for lower-income women or who are at risk of intimate partner or other forms of violence.
Genetic Pap tests catch more cancers
A Pap test combined with assays for gene mutations showed an 81% and 33% sensitivity or identifying endometrial and ovarian cancer, respectively.
In a study published in Science Translational Medicine, Yuxuan Wang, MD, of Johns Hopkins University, Baltimore, and her colleagues used a polymerase chain reaction–based test known as PapSEEK to examine Pap brush samples from 382 women with endometrial cancer, 245 women with ovarian cancer, and 714 women without cancer.
Overall, 81% of the endometrial cancer patients and 29% of the ovarian cancer patients had detectable mutations on Pap smears taken with a Pap brush. Among endometrial cancer patients, the genetic test identified mutations in 78% of patients with early-stage disease and 89% of those with late-stage disease. Among ovarian cancer patients, the genetic test identified 28% of patients with early-stage disease and 30% of those with late-stage disease.
The investigators also used the genetic test on Tao brush samples from the intrauterine cavity from a subset of 123 patients with endometrial cancer, 51 with ovarian cancer, and 125 controls and identified genetic mutations in 93% and 45% of endometrial and ovarian cancers, respectively.
The results demonstrate the potential of mutation-based diagnostic testing, and samples taken with the Tao brush may be especially helpful for ovarian cancers, especially in samples combining Pap and plasma sample data, the researchers said.
The study was limited by several factors including its retrospective design and having a study population confined to women with known cancers, the researchers noted. More research is needed, but results suggest the potential for DNA analysis to catch cancers early in a screening setting, they said. “Our study lays the foundation for evaluating PapSEEK in a large prospective study,” ideally including patients at high risk for gynecologic cancers, they added.
The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
SOURCE: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
A Pap test combined with assays for gene mutations showed an 81% and 33% sensitivity or identifying endometrial and ovarian cancer, respectively.
In a study published in Science Translational Medicine, Yuxuan Wang, MD, of Johns Hopkins University, Baltimore, and her colleagues used a polymerase chain reaction–based test known as PapSEEK to examine Pap brush samples from 382 women with endometrial cancer, 245 women with ovarian cancer, and 714 women without cancer.
Overall, 81% of the endometrial cancer patients and 29% of the ovarian cancer patients had detectable mutations on Pap smears taken with a Pap brush. Among endometrial cancer patients, the genetic test identified mutations in 78% of patients with early-stage disease and 89% of those with late-stage disease. Among ovarian cancer patients, the genetic test identified 28% of patients with early-stage disease and 30% of those with late-stage disease.
The investigators also used the genetic test on Tao brush samples from the intrauterine cavity from a subset of 123 patients with endometrial cancer, 51 with ovarian cancer, and 125 controls and identified genetic mutations in 93% and 45% of endometrial and ovarian cancers, respectively.
The results demonstrate the potential of mutation-based diagnostic testing, and samples taken with the Tao brush may be especially helpful for ovarian cancers, especially in samples combining Pap and plasma sample data, the researchers said.
The study was limited by several factors including its retrospective design and having a study population confined to women with known cancers, the researchers noted. More research is needed, but results suggest the potential for DNA analysis to catch cancers early in a screening setting, they said. “Our study lays the foundation for evaluating PapSEEK in a large prospective study,” ideally including patients at high risk for gynecologic cancers, they added.
The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
SOURCE: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
A Pap test combined with assays for gene mutations showed an 81% and 33% sensitivity or identifying endometrial and ovarian cancer, respectively.
In a study published in Science Translational Medicine, Yuxuan Wang, MD, of Johns Hopkins University, Baltimore, and her colleagues used a polymerase chain reaction–based test known as PapSEEK to examine Pap brush samples from 382 women with endometrial cancer, 245 women with ovarian cancer, and 714 women without cancer.
Overall, 81% of the endometrial cancer patients and 29% of the ovarian cancer patients had detectable mutations on Pap smears taken with a Pap brush. Among endometrial cancer patients, the genetic test identified mutations in 78% of patients with early-stage disease and 89% of those with late-stage disease. Among ovarian cancer patients, the genetic test identified 28% of patients with early-stage disease and 30% of those with late-stage disease.
The investigators also used the genetic test on Tao brush samples from the intrauterine cavity from a subset of 123 patients with endometrial cancer, 51 with ovarian cancer, and 125 controls and identified genetic mutations in 93% and 45% of endometrial and ovarian cancers, respectively.
The results demonstrate the potential of mutation-based diagnostic testing, and samples taken with the Tao brush may be especially helpful for ovarian cancers, especially in samples combining Pap and plasma sample data, the researchers said.
The study was limited by several factors including its retrospective design and having a study population confined to women with known cancers, the researchers noted. More research is needed, but results suggest the potential for DNA analysis to catch cancers early in a screening setting, they said. “Our study lays the foundation for evaluating PapSEEK in a large prospective study,” ideally including patients at high risk for gynecologic cancers, they added.
The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
SOURCE: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Genetics-based Pap test identified endometrial and ovarian cancers.
Major finding: Pap brush samples identified mutations in 81% of women with endometrial cancer and 29% of women with ovarian cancer.
Study details: Analysis of 1,915 Pap samples from 1,658 women; 1,002 were healthy controls, while 656 had gynecologic cancer.
Disclosures: The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
Source: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.