User login
VIDEO: Painful skin conditions need pain management by dermatologists
Patients with painful skin conditions need pain management that is provided by their dermatologists, Robert G. Micheletti, MD, contended in a presentation at the annual meeting of the American Academy of Dermatology.
Dermatologists are the experts when it comes to treating painful skin conditions like pyoderma gangrenosum, hidradenitis suppurativa, calciphylaxis, and vasculopathies. “We should be willing to treat the pain that goes with (these conditions), at least within our scope of practice,” said Dr. Micheletti, co-director of the Inpatient Dermatology Consult Service at the University of Pennsylvania, Philadelphia. “At the same time, we know opioids should be prescribed only when necessary, at the lowest effective dose, and for the shortest possible duration.”
In our exclusive video interview, Dr. Micheletti outlined the keys to successful care of patients with painful skin disease. He described patient characteristics that influence prescribing choices and tips for accurately assessing pain needs with a preference for a conservative regimen that utilizes non-opioids and avoids over-reliance on narcotics.
Source: Micheletti, R., Session F013
Patients with painful skin conditions need pain management that is provided by their dermatologists, Robert G. Micheletti, MD, contended in a presentation at the annual meeting of the American Academy of Dermatology.
Dermatologists are the experts when it comes to treating painful skin conditions like pyoderma gangrenosum, hidradenitis suppurativa, calciphylaxis, and vasculopathies. “We should be willing to treat the pain that goes with (these conditions), at least within our scope of practice,” said Dr. Micheletti, co-director of the Inpatient Dermatology Consult Service at the University of Pennsylvania, Philadelphia. “At the same time, we know opioids should be prescribed only when necessary, at the lowest effective dose, and for the shortest possible duration.”
In our exclusive video interview, Dr. Micheletti outlined the keys to successful care of patients with painful skin disease. He described patient characteristics that influence prescribing choices and tips for accurately assessing pain needs with a preference for a conservative regimen that utilizes non-opioids and avoids over-reliance on narcotics.
Source: Micheletti, R., Session F013
Patients with painful skin conditions need pain management that is provided by their dermatologists, Robert G. Micheletti, MD, contended in a presentation at the annual meeting of the American Academy of Dermatology.
Dermatologists are the experts when it comes to treating painful skin conditions like pyoderma gangrenosum, hidradenitis suppurativa, calciphylaxis, and vasculopathies. “We should be willing to treat the pain that goes with (these conditions), at least within our scope of practice,” said Dr. Micheletti, co-director of the Inpatient Dermatology Consult Service at the University of Pennsylvania, Philadelphia. “At the same time, we know opioids should be prescribed only when necessary, at the lowest effective dose, and for the shortest possible duration.”
In our exclusive video interview, Dr. Micheletti outlined the keys to successful care of patients with painful skin disease. He described patient characteristics that influence prescribing choices and tips for accurately assessing pain needs with a preference for a conservative regimen that utilizes non-opioids and avoids over-reliance on narcotics.
Source: Micheletti, R., Session F013
VIDEO: Delusional parasitosis? Try these real solutions
SAN DIEGO – The path to successful treatment of patients with imagined skin disorders is paved with compassion, according to John Koo, MD, a dermatologist and psychiatrist with the University of California at San Francisco.
When a patient presents with delusional parasitosis -- horror stories about imagined infestations of parasites or bugs – the key to successful treatment is a positive attitude and validation, not denial, Dr. Koo said in a presentation at the annual meeting of the American Academy of Dermatology.
"I cannot afford to go in (the exam room) with a long face," he said. "If I go in and I’m not looking happy, things can deteriorate quickly. So I make sure I go in with the biggest smile on my face like I'm meeting my favorite Hollywood star."
"When I say something like 'It's like a living hell, isn't it,' patients are really touched, he said. The patient’s response is typically 'You're the first dermatologist to understand what I'm going through.' You cannot endorse their delusion, but you can endorse their suffering."
In our video interview, Dr. Koo delved into techniques for the successful work-up and evaluation of patients with delusional parasitosis, the varying degrees of the condition, medications used for treatment, and the prospects for eventual drug-free relief.
Dr. Koo reports no relevant financial disclosures.
SAN DIEGO – The path to successful treatment of patients with imagined skin disorders is paved with compassion, according to John Koo, MD, a dermatologist and psychiatrist with the University of California at San Francisco.
When a patient presents with delusional parasitosis -- horror stories about imagined infestations of parasites or bugs – the key to successful treatment is a positive attitude and validation, not denial, Dr. Koo said in a presentation at the annual meeting of the American Academy of Dermatology.
"I cannot afford to go in (the exam room) with a long face," he said. "If I go in and I’m not looking happy, things can deteriorate quickly. So I make sure I go in with the biggest smile on my face like I'm meeting my favorite Hollywood star."
"When I say something like 'It's like a living hell, isn't it,' patients are really touched, he said. The patient’s response is typically 'You're the first dermatologist to understand what I'm going through.' You cannot endorse their delusion, but you can endorse their suffering."
In our video interview, Dr. Koo delved into techniques for the successful work-up and evaluation of patients with delusional parasitosis, the varying degrees of the condition, medications used for treatment, and the prospects for eventual drug-free relief.
Dr. Koo reports no relevant financial disclosures.
SAN DIEGO – The path to successful treatment of patients with imagined skin disorders is paved with compassion, according to John Koo, MD, a dermatologist and psychiatrist with the University of California at San Francisco.
When a patient presents with delusional parasitosis -- horror stories about imagined infestations of parasites or bugs – the key to successful treatment is a positive attitude and validation, not denial, Dr. Koo said in a presentation at the annual meeting of the American Academy of Dermatology.
"I cannot afford to go in (the exam room) with a long face," he said. "If I go in and I’m not looking happy, things can deteriorate quickly. So I make sure I go in with the biggest smile on my face like I'm meeting my favorite Hollywood star."
"When I say something like 'It's like a living hell, isn't it,' patients are really touched, he said. The patient’s response is typically 'You're the first dermatologist to understand what I'm going through.' You cannot endorse their delusion, but you can endorse their suffering."
In our video interview, Dr. Koo delved into techniques for the successful work-up and evaluation of patients with delusional parasitosis, the varying degrees of the condition, medications used for treatment, and the prospects for eventual drug-free relief.
Dr. Koo reports no relevant financial disclosures.
REPORTING FROM AAD 18
Balancing quality and cost of care with patient well-being
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
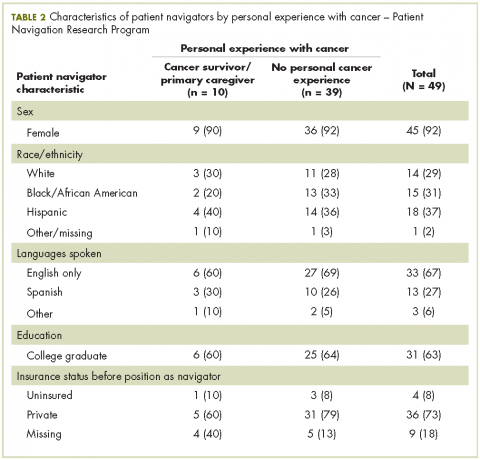
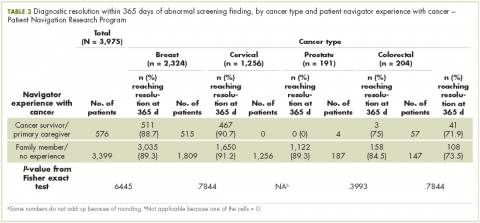
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Topical anticholinergic improved hyperhidrosis in children
SAN DIEGO – A topical anticholinergic drug, glycopyrronium tosylate, was as safe and effective for treating hyperhidrosis in children 9-16 years old as it was in adults in two phase 3 trials that included 25 treated children, raising the prospect it could become the first drug to gain Food and Drug Administration approval for treating pediatric hyperhidrosis.
“Topical glycopyrronium tosylate treatment may provide a much needed treatment option for those with primary axillary hyperhidrosis, including pediatric patients,” Adelaide A. Hebert, MD, said at the annual meeting of the American Academy of Dermatology.
The data she reported from a post hoc analysis included 25 children. 9-16 years old, who received a daily, topical application of glycopyrronium tosylate to their underarms for 4 weeks and 19 children treated with vehicle only. The children were enrolled in either of a pair of phase 3 pivotal trials that together randomized 697 patients. In November 2017, Dermira, the company developing this drug, submitted an application to the FDA for marketing approval of the agent for adults and children at least 9 years old. A statement from the company said an FDA decision is expected by mid-2018.
Getting approval from the FDA for an effective pediatric hyperhidrosis treatment would be an important advance because nothing now exists in that space, said Dr. Hebert, professor of dermatology and pediatrics and director of pediatric dermatology at the University of Texas Health Sciences Center at Houston.
Based on past FDA actions, safety data from 25 children should be adequate to support pediatric labeling, she said in an interview, though she added that confirmatory safety data from a phase 4 study in children would be a welcome future addition. Hyperhydrosis in adolescents is “underappreciated, underdiagnosed, and is very impactful,” and currently has limited treatment options that are readily available for children, especially effective options for more severe hyperhidrosis.
The pediatric data came from the phase 3, randomized, double-blind, vehicle-controlled ATMOS-1 (DRM04 in Subjects With Axillary Hyperhidrosis) trial. The trial ran at several U.S. and German centers, although only the U.S. centers enrolled pediatric patients.
The two trials enrolled patients with “intolerable or barely tolerable” primary, axillary hyperhidrosis of at least 6 months' duration. After 4 weeks, patients treated with glycopyrronium tosylate had improvements in their daily diary account of axillary sweating and in sweat production. Dr. Hebert and her associates reported overall results from the two trials at various prior dermatology meetings, and the company reported some of the results in a press release, but the results have not yet been published in a journal.
The new, pediatric analysis that Dr. Hebert reported showed that the responder rate based on a 4 point or greater improvement in daily sweat diary assessments occurred in 60% of the actively treated children and in 13% of the controls. A 50% or greater reduction in sweat production occurred in 80% of the treated children and in 55% of controls. Quality of life, measured using the Children’s Dermatology Life Quality Index improved by an average of 8 points among the treated children, compared with an average 2-point improvement among the controls. This level of improvement among the glycopyrronium-treated patients would have been enough to move patients from the moderate-effect category at baseline to a no- or small-effect category.
The treatment was generally well tolerated, with no serious adverse effects reported and with treatment effects that were primarily as expected from an anticholinergic agent, including dry mouth, pupil dilation, and blurred vision. One of the 25 treated children withdrew because of these effects, which then resolved. Blood testing showed no systemic absorption of the drug, Dr. Hebert said.
The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an advisor to the editorial board of Dermatology News.
SOURCE: Hebert A et al. Annual meeting of the American Academy of Dermatology Abstract 6659.
SAN DIEGO – A topical anticholinergic drug, glycopyrronium tosylate, was as safe and effective for treating hyperhidrosis in children 9-16 years old as it was in adults in two phase 3 trials that included 25 treated children, raising the prospect it could become the first drug to gain Food and Drug Administration approval for treating pediatric hyperhidrosis.
“Topical glycopyrronium tosylate treatment may provide a much needed treatment option for those with primary axillary hyperhidrosis, including pediatric patients,” Adelaide A. Hebert, MD, said at the annual meeting of the American Academy of Dermatology.
The data she reported from a post hoc analysis included 25 children. 9-16 years old, who received a daily, topical application of glycopyrronium tosylate to their underarms for 4 weeks and 19 children treated with vehicle only. The children were enrolled in either of a pair of phase 3 pivotal trials that together randomized 697 patients. In November 2017, Dermira, the company developing this drug, submitted an application to the FDA for marketing approval of the agent for adults and children at least 9 years old. A statement from the company said an FDA decision is expected by mid-2018.
Getting approval from the FDA for an effective pediatric hyperhidrosis treatment would be an important advance because nothing now exists in that space, said Dr. Hebert, professor of dermatology and pediatrics and director of pediatric dermatology at the University of Texas Health Sciences Center at Houston.
Based on past FDA actions, safety data from 25 children should be adequate to support pediatric labeling, she said in an interview, though she added that confirmatory safety data from a phase 4 study in children would be a welcome future addition. Hyperhydrosis in adolescents is “underappreciated, underdiagnosed, and is very impactful,” and currently has limited treatment options that are readily available for children, especially effective options for more severe hyperhidrosis.
The pediatric data came from the phase 3, randomized, double-blind, vehicle-controlled ATMOS-1 (DRM04 in Subjects With Axillary Hyperhidrosis) trial. The trial ran at several U.S. and German centers, although only the U.S. centers enrolled pediatric patients.
The two trials enrolled patients with “intolerable or barely tolerable” primary, axillary hyperhidrosis of at least 6 months' duration. After 4 weeks, patients treated with glycopyrronium tosylate had improvements in their daily diary account of axillary sweating and in sweat production. Dr. Hebert and her associates reported overall results from the two trials at various prior dermatology meetings, and the company reported some of the results in a press release, but the results have not yet been published in a journal.
The new, pediatric analysis that Dr. Hebert reported showed that the responder rate based on a 4 point or greater improvement in daily sweat diary assessments occurred in 60% of the actively treated children and in 13% of the controls. A 50% or greater reduction in sweat production occurred in 80% of the treated children and in 55% of controls. Quality of life, measured using the Children’s Dermatology Life Quality Index improved by an average of 8 points among the treated children, compared with an average 2-point improvement among the controls. This level of improvement among the glycopyrronium-treated patients would have been enough to move patients from the moderate-effect category at baseline to a no- or small-effect category.
The treatment was generally well tolerated, with no serious adverse effects reported and with treatment effects that were primarily as expected from an anticholinergic agent, including dry mouth, pupil dilation, and blurred vision. One of the 25 treated children withdrew because of these effects, which then resolved. Blood testing showed no systemic absorption of the drug, Dr. Hebert said.
The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an advisor to the editorial board of Dermatology News.
SOURCE: Hebert A et al. Annual meeting of the American Academy of Dermatology Abstract 6659.
SAN DIEGO – A topical anticholinergic drug, glycopyrronium tosylate, was as safe and effective for treating hyperhidrosis in children 9-16 years old as it was in adults in two phase 3 trials that included 25 treated children, raising the prospect it could become the first drug to gain Food and Drug Administration approval for treating pediatric hyperhidrosis.
“Topical glycopyrronium tosylate treatment may provide a much needed treatment option for those with primary axillary hyperhidrosis, including pediatric patients,” Adelaide A. Hebert, MD, said at the annual meeting of the American Academy of Dermatology.
The data she reported from a post hoc analysis included 25 children. 9-16 years old, who received a daily, topical application of glycopyrronium tosylate to their underarms for 4 weeks and 19 children treated with vehicle only. The children were enrolled in either of a pair of phase 3 pivotal trials that together randomized 697 patients. In November 2017, Dermira, the company developing this drug, submitted an application to the FDA for marketing approval of the agent for adults and children at least 9 years old. A statement from the company said an FDA decision is expected by mid-2018.
Getting approval from the FDA for an effective pediatric hyperhidrosis treatment would be an important advance because nothing now exists in that space, said Dr. Hebert, professor of dermatology and pediatrics and director of pediatric dermatology at the University of Texas Health Sciences Center at Houston.
Based on past FDA actions, safety data from 25 children should be adequate to support pediatric labeling, she said in an interview, though she added that confirmatory safety data from a phase 4 study in children would be a welcome future addition. Hyperhydrosis in adolescents is “underappreciated, underdiagnosed, and is very impactful,” and currently has limited treatment options that are readily available for children, especially effective options for more severe hyperhidrosis.
The pediatric data came from the phase 3, randomized, double-blind, vehicle-controlled ATMOS-1 (DRM04 in Subjects With Axillary Hyperhidrosis) trial. The trial ran at several U.S. and German centers, although only the U.S. centers enrolled pediatric patients.
The two trials enrolled patients with “intolerable or barely tolerable” primary, axillary hyperhidrosis of at least 6 months' duration. After 4 weeks, patients treated with glycopyrronium tosylate had improvements in their daily diary account of axillary sweating and in sweat production. Dr. Hebert and her associates reported overall results from the two trials at various prior dermatology meetings, and the company reported some of the results in a press release, but the results have not yet been published in a journal.
The new, pediatric analysis that Dr. Hebert reported showed that the responder rate based on a 4 point or greater improvement in daily sweat diary assessments occurred in 60% of the actively treated children and in 13% of the controls. A 50% or greater reduction in sweat production occurred in 80% of the treated children and in 55% of controls. Quality of life, measured using the Children’s Dermatology Life Quality Index improved by an average of 8 points among the treated children, compared with an average 2-point improvement among the controls. This level of improvement among the glycopyrronium-treated patients would have been enough to move patients from the moderate-effect category at baseline to a no- or small-effect category.
The treatment was generally well tolerated, with no serious adverse effects reported and with treatment effects that were primarily as expected from an anticholinergic agent, including dry mouth, pupil dilation, and blurred vision. One of the 25 treated children withdrew because of these effects, which then resolved. Blood testing showed no systemic absorption of the drug, Dr. Hebert said.
The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an advisor to the editorial board of Dermatology News.
SOURCE: Hebert A et al. Annual meeting of the American Academy of Dermatology Abstract 6659.
REPORTING FROM AAD 18
Key clinical point: Max. Daily, topical glycopyrronium tosylate safely controlled pediatric hyperhidrosis.
Major finding: At least a 4-point improvement in the axillary sweating daily diary score occurred in 60% of treated patients and in 13% of controls.
Study details: Post hoc analysis of data from 44 children enrolled in either of two pivotal trials, ATMOS-1 and ATMOS-2.
Disclosures: The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an adviser to the editorial board of Dermatology News.
Source: Hebert A et al. AAD 2018, Abstract 6659.
VIDEO: Gene test guides need for sentinel node biopsy in elderly melanoma patients
SAN DIEGO – The results of a gene expression test, along with tumor thickness and patient age, can guide the need for sentinel lymph node biopsy, based on results from more than1,400 consecutively tested patients from 26 U.S. surgical oncology, medical oncology and dermatologic practices.
The findings, presented by John Vetto, MD, at the annual meeting of the American Academy of Dermatology, indicate the DecisionDx test correctly identified patients aged 65 and older with T1-T2 tumors whose risk of sentinel node metastasis was lower than 5%. The most recent melanoma guidelines from the National Comprehensive Cancer Network recommend that clinicians “discuss and offer” sentinel node biopsy if a patient has a greater than 10% likelihood of a positive node. If the likelihood is 5%-10%, the recommendation is to “discuss and consider” the procedure. But if the likelihood of a positive node is less than 5%, the guidelines recommend against a biopsy.
“Sentinel node biopsy (has) risks, especially in medically compromised older patients,” Dr. Vetto, professor of surgery at the Oregon Health and Sciences University, Portland, said in an interview, in which he discussed clinical use of the test. “This test offers us a good way to assess the risk/benefit ratio so we can better care for patients, and follow the newest guidelines about sentinel node biopsy.”
The DecisionDx Melanoma, developed by Castle Biosciences, tests for the expression of 28 genes know to play a role in melanoma metastasis, and three control genes. Tumors are stratified either as Class 1, with a 3% chance of spreading within 5 years, or Class 2, with a 69% risk of metastasis. There are two subclasses: 1A, which has an extremely low risk of progression, and 2b, which has an extremely high risk of progression.
For patients with T1-T2 tumors who had a Class 1A test result (lowest risk of recurrence), SLN positivity was 4.6% for all ages, 2.8% in patients 55 years and older, and 1.6% in patients 65 years and older. For patients with T1-T2 tumors who had a Class 2B test result (highest risk of recurrence), SLN positivity was 18.8% for all ages, 16.4% in patients 55 years and older and 11.9% in patients 65 years and older.
Dr. Vetto is a paid speaker for Castle Biosciences.
SOURCE: Vetto et al. AAD 2018 late-breaking research, Abstract 6805
SAN DIEGO – The results of a gene expression test, along with tumor thickness and patient age, can guide the need for sentinel lymph node biopsy, based on results from more than1,400 consecutively tested patients from 26 U.S. surgical oncology, medical oncology and dermatologic practices.
The findings, presented by John Vetto, MD, at the annual meeting of the American Academy of Dermatology, indicate the DecisionDx test correctly identified patients aged 65 and older with T1-T2 tumors whose risk of sentinel node metastasis was lower than 5%. The most recent melanoma guidelines from the National Comprehensive Cancer Network recommend that clinicians “discuss and offer” sentinel node biopsy if a patient has a greater than 10% likelihood of a positive node. If the likelihood is 5%-10%, the recommendation is to “discuss and consider” the procedure. But if the likelihood of a positive node is less than 5%, the guidelines recommend against a biopsy.
“Sentinel node biopsy (has) risks, especially in medically compromised older patients,” Dr. Vetto, professor of surgery at the Oregon Health and Sciences University, Portland, said in an interview, in which he discussed clinical use of the test. “This test offers us a good way to assess the risk/benefit ratio so we can better care for patients, and follow the newest guidelines about sentinel node biopsy.”
The DecisionDx Melanoma, developed by Castle Biosciences, tests for the expression of 28 genes know to play a role in melanoma metastasis, and three control genes. Tumors are stratified either as Class 1, with a 3% chance of spreading within 5 years, or Class 2, with a 69% risk of metastasis. There are two subclasses: 1A, which has an extremely low risk of progression, and 2b, which has an extremely high risk of progression.
For patients with T1-T2 tumors who had a Class 1A test result (lowest risk of recurrence), SLN positivity was 4.6% for all ages, 2.8% in patients 55 years and older, and 1.6% in patients 65 years and older. For patients with T1-T2 tumors who had a Class 2B test result (highest risk of recurrence), SLN positivity was 18.8% for all ages, 16.4% in patients 55 years and older and 11.9% in patients 65 years and older.
Dr. Vetto is a paid speaker for Castle Biosciences.
SOURCE: Vetto et al. AAD 2018 late-breaking research, Abstract 6805
SAN DIEGO – The results of a gene expression test, along with tumor thickness and patient age, can guide the need for sentinel lymph node biopsy, based on results from more than1,400 consecutively tested patients from 26 U.S. surgical oncology, medical oncology and dermatologic practices.
The findings, presented by John Vetto, MD, at the annual meeting of the American Academy of Dermatology, indicate the DecisionDx test correctly identified patients aged 65 and older with T1-T2 tumors whose risk of sentinel node metastasis was lower than 5%. The most recent melanoma guidelines from the National Comprehensive Cancer Network recommend that clinicians “discuss and offer” sentinel node biopsy if a patient has a greater than 10% likelihood of a positive node. If the likelihood is 5%-10%, the recommendation is to “discuss and consider” the procedure. But if the likelihood of a positive node is less than 5%, the guidelines recommend against a biopsy.
“Sentinel node biopsy (has) risks, especially in medically compromised older patients,” Dr. Vetto, professor of surgery at the Oregon Health and Sciences University, Portland, said in an interview, in which he discussed clinical use of the test. “This test offers us a good way to assess the risk/benefit ratio so we can better care for patients, and follow the newest guidelines about sentinel node biopsy.”
The DecisionDx Melanoma, developed by Castle Biosciences, tests for the expression of 28 genes know to play a role in melanoma metastasis, and three control genes. Tumors are stratified either as Class 1, with a 3% chance of spreading within 5 years, or Class 2, with a 69% risk of metastasis. There are two subclasses: 1A, which has an extremely low risk of progression, and 2b, which has an extremely high risk of progression.
For patients with T1-T2 tumors who had a Class 1A test result (lowest risk of recurrence), SLN positivity was 4.6% for all ages, 2.8% in patients 55 years and older, and 1.6% in patients 65 years and older. For patients with T1-T2 tumors who had a Class 2B test result (highest risk of recurrence), SLN positivity was 18.8% for all ages, 16.4% in patients 55 years and older and 11.9% in patients 65 years and older.
Dr. Vetto is a paid speaker for Castle Biosciences.
SOURCE: Vetto et al. AAD 2018 late-breaking research, Abstract 6805
REPORTING FROM AAD 18
Massive liver metastasis from colon adenocarcinoma causing cardiac tamponade
Colorectal cancer is the third most commonly diagnosed cancer in the United States.1 About 5% of Americans will be diagnosed with colorectal cancer in their lifetime, of which 20% will present with distant metastasis.2 The most common sites of metastasis are regional lymph nodes, liver, lung and peritoneum, and patients may present with signs or symptoms related to disease burden at any of these organs.
Case presentation and summary
A 55-year-old man had presented to an outside hospital in August of 2014 with 6 months of hematochezia and a 40-lb weight loss. He was found to be severely anemic on admission (hemoglobin, 4.9 g/dL [normal, 13-17 g/dL], hematocrit, 16% [normal, 35%-45%]). A computed-tomography (CT) scan of the abdomen and pelvis with contrast revealed a mass of 6.9 x 4.7 x 6.3 cm in the rectosigmoid colon and a mass of 10.0 x 12.0 x 10.7 cm in the right hepatic lobe consistent with metastatic disease. The patient was taken to the operating room where the rectosigmoid mass was resected completely. The liver mass was deemed unresectable because of its large size, and surgically directed therapy could not be performed. Pathology was consistent with a T3N1 moderately differentiated adenocarcinoma 11 cm from the anal verge. Further molecular tumor studies revealed wild type KRAS and NRAS, as well as a BRAF mutation.
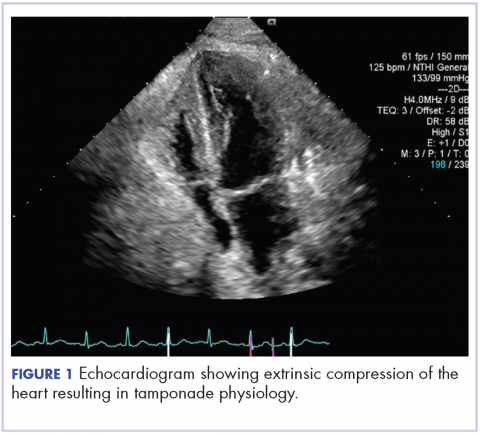
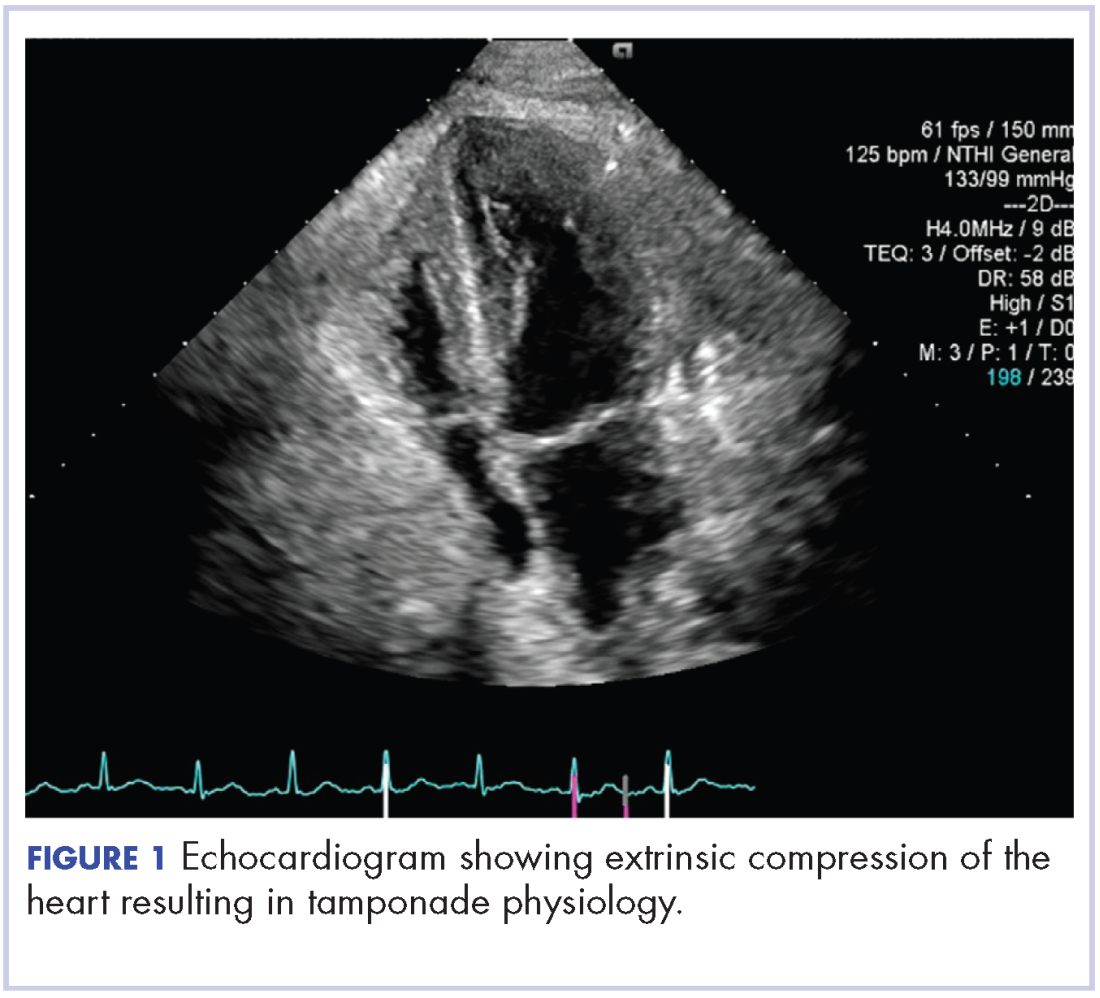
About 4 weeks after the surgery, the patient was seen at our institution for an initial consultation and was noted to have significant anasarca, including 4+ pitting lower extremity edema and scrotal edema. He complained of dyspnea on exertion, which he attributed to deconditioning. His resting heart rate was found to be 123 beats per minute (normal, 60-100 bpm). Jugular venous distention was present. The patient was sent for an urgent echocardiogram, which showed external compression of the right atrium and ventricle by his liver metastasis resulting in tamponade physiology without the presence of any pericardial effusion (Figure 1).
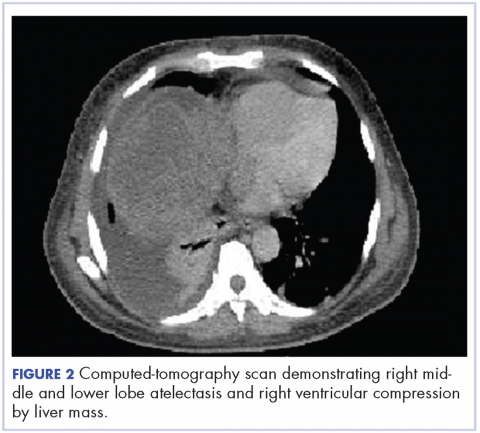
A CT of the abdomen and pelvis at that time showed that the liver mass had increased to 17.6 x 12.1 x 16.1 cm, exerting pressure on the heart and causing atelectasis of the right middle and lower lung lobes (Figure 2).
Treatment plan
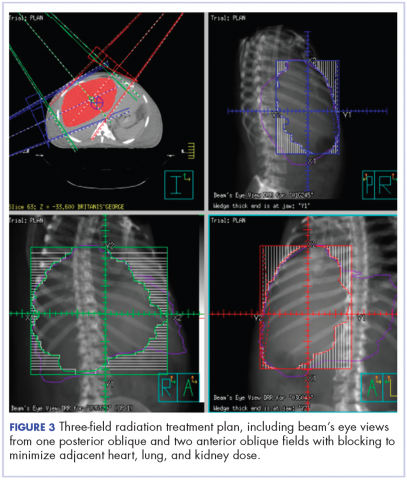
The patient was evaluated by surgical oncology for resection, but his cardiovascular status placed him at high risk for perioperative complications, so such surgery was not pursued. Radioembolization was considered but not pursued because the process needed to evaluate, plan, and treat was not considered sufficiently timely. We consulted with our radiation oncology colleagues about external beam radiotherapy (EBRT) for rapid palliation. They evaluated the patient and recommended the EBRT, and the patient signed consent for treatment. We performed a CT-based simulation and generated an external beam, linear-accelerator–based treatment plan. The plan consisted of three 15-megavoltage photon fields delivering 3,000 cGy in 10 fractions to the whole liver, with appropriate multileaf collimation blocking to minimize dose to adjacent heart, right lung, and bilateral kidneys (Figure 3).
Before initiation of the EBRT, the patient received systemic chemotherapy with a dose-adjusted FOLFOX regimen (5-FU bolus 200 mg/m2, leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, with infusional 5-FU 2,400 mg/m2 over 46 hours). After completing 1 dose of modified FOLFOX, he completed 10 fractions of whole liver radiotherapy with the aforementioned plan. He tolerated the initial treatment well and his subjective symptoms improved. The patient then proceeded to further systemic therapy. After recent data demonstrated improved median progression-free survival and response rates with FOLFOXIRI plus bevacizumab (infusional 5-FU 3200 mg/m2, leucovorin 200 mg/m2, irinotecan 165 mg/m2, and oxaliplatin 85 mg/m2, bevacizumab 5 mg/kg) versus FOLFIRI plus bevacizumab,3 we decided to modify his systemic therapy to FOLFOXIRI with bevacizumab to induce a better response.
Treatment response
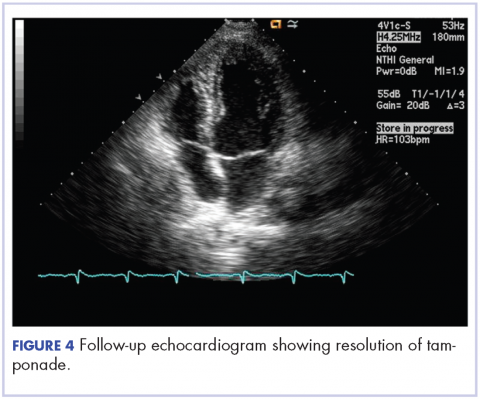
After 2 doses of chemotherapy and completion of radiotherapy, the edema and shortness of breath improved. A follow-up echocardiogram performed a month after completion of EBRT, 1 dose of FOLFOX, and 1 dose of FOLFOXIRI showed resolution of the cardiac compression (Figure 4).
A CT scan of the abdomen and pelvis obtained after 3 cycles of FOLFOXIRI showed marked decrease in the size of the right lobe hepatic mass from 17.6 x 12.1 cm to 12.0 x 8.0 cm. Given the survival benefit of VEGF inhibition in colon cancer, bevacizumab (5 mg/kg) was added to the FOLFOXIRI regimen with cycle 4. Unfortunately, after the 5th cycle, a CT scan of the abdomen showed an increase in size of the hepatic lesions. At this time, FOLFOXIRI and bevacizumab were stopped, and given the tumor’s KRAS/NRAS wild type status, systemic therapy was changed to panitumumab (6 mg/kg). The patient initially tolerated treatment well, but after 9 cycles, the total bilirubin started to increase. CT abdomen at this point was consistent with progression of disease. The patient was not eligible for a clinical trial targeting BRAF mutation given the elevated bilirubin. Regorafanib (80 mg daily for 3 weeks on and 1 week off) was started. After the first cycle, the total bilirubin increased further and the regorafanib was dose reduced to 40 mg daily. Unfortunately, a repeat CT scan of the abdomen demonstrated progression of disease, and given that he developed a progressive transaminitis and hyperbilirubinemia, hospice care was recommended. The patient died shortly thereafter, about 15 months after his initial diagnosis.
Discussion
Massive liver metastasis in the setting of disseminated cancer is not an uncommon manifestation of advanced cancer that can have life-threatening consequences. In te present case, a bulky liver metastasis caused extrinsic compression of the right atrium, resulting in obvious clinical and echocardiogram-proven cardiac tamponade physiology. To our knowledge, this is the first reported case of the treatment of a bulky hepatic metastasis causing cardiac tamponade. In this patient’s case, both radiotherapy and chemotherapy were given safely in rapid sequence resulting in quick resolution of the patient’s symptoms and echocardiogram findings. The presence of a BRAF mutation conferred a poor prognosis and poor response to systemic chemotherapy. Nevertheless, the patient showed good response to a FOLFOXIRI regimen, chosen in this emergent situation given its significantly higher response rates compared with the standard FOLFIRI regimen, which was tolerated well with minimal adverse effects.
Findings from randomized controlled trials examining the role of palliative radiotherapy for metastatic liver disease have suggested that dose escalation above 30 Gy to the whole liver may lead to unacceptably high rates of radiation-induced liver disease, which typically leads to mortality.4-8 Two prospective trials comparing twice daily with daily fractionation have shown no benefit to hyperfractionation, with possibly increased rates of acute toxicity in the setting of hepatocellular carcinoma.9,10 There is emerging evidence that partial liver irradiation, in the appropriate setting in the form of boost after whole-liver RT or stereotactic body radiotherapy, may allow for further dose escalation while avoiding clinical hepatitis.11 Although there is no clear consensus about optimal RT dose and fractionation, the aforementioned studies show that dose and fractionation schemes ranging between 21 Gy and 30 Gy in 1.5 Gy to 3 Gy daily fractions likely provide the best therapeutic ratio for whole-liver irradiation.
In conclusion, this case demonstrates the resolution of cardiac tamponade from a massive liver colorectal metastasis after chemoradiation and illustrates the potential utility of adding radiotherapy to chemotherapy in an urgent scenario where the former might not typically be considered.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Published 2015. Accessed October 10, 2017.
2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
3. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
4. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117-123.
5. Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA. 1975;232(6):625-628.
6. Sherman DM, Weichselbaum R, Order SE, Cloud L, Trey C, Piro AJ. Palliation of hepatic metastasis. Cancer. 1978;41(5):2013-2017.
7. Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys. 1977;2:129-132.
8. Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
9. Raju PI, Maruyama Y, DeSimone P, MacDonald J. Treatment of liver metastases with a combination of chemotherapy and hyperfractionated external radiation therapy. Am J Clin Oncol. 1987;10(1):41-43.
10. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223-1229.
11. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14(3):722-728.
Colorectal cancer is the third most commonly diagnosed cancer in the United States.1 About 5% of Americans will be diagnosed with colorectal cancer in their lifetime, of which 20% will present with distant metastasis.2 The most common sites of metastasis are regional lymph nodes, liver, lung and peritoneum, and patients may present with signs or symptoms related to disease burden at any of these organs.
Case presentation and summary
A 55-year-old man had presented to an outside hospital in August of 2014 with 6 months of hematochezia and a 40-lb weight loss. He was found to be severely anemic on admission (hemoglobin, 4.9 g/dL [normal, 13-17 g/dL], hematocrit, 16% [normal, 35%-45%]). A computed-tomography (CT) scan of the abdomen and pelvis with contrast revealed a mass of 6.9 x 4.7 x 6.3 cm in the rectosigmoid colon and a mass of 10.0 x 12.0 x 10.7 cm in the right hepatic lobe consistent with metastatic disease. The patient was taken to the operating room where the rectosigmoid mass was resected completely. The liver mass was deemed unresectable because of its large size, and surgically directed therapy could not be performed. Pathology was consistent with a T3N1 moderately differentiated adenocarcinoma 11 cm from the anal verge. Further molecular tumor studies revealed wild type KRAS and NRAS, as well as a BRAF mutation.
About 4 weeks after the surgery, the patient was seen at our institution for an initial consultation and was noted to have significant anasarca, including 4+ pitting lower extremity edema and scrotal edema. He complained of dyspnea on exertion, which he attributed to deconditioning. His resting heart rate was found to be 123 beats per minute (normal, 60-100 bpm). Jugular venous distention was present. The patient was sent for an urgent echocardiogram, which showed external compression of the right atrium and ventricle by his liver metastasis resulting in tamponade physiology without the presence of any pericardial effusion (Figure 1).
A CT of the abdomen and pelvis at that time showed that the liver mass had increased to 17.6 x 12.1 x 16.1 cm, exerting pressure on the heart and causing atelectasis of the right middle and lower lung lobes (Figure 2).
Treatment plan
The patient was evaluated by surgical oncology for resection, but his cardiovascular status placed him at high risk for perioperative complications, so such surgery was not pursued. Radioembolization was considered but not pursued because the process needed to evaluate, plan, and treat was not considered sufficiently timely. We consulted with our radiation oncology colleagues about external beam radiotherapy (EBRT) for rapid palliation. They evaluated the patient and recommended the EBRT, and the patient signed consent for treatment. We performed a CT-based simulation and generated an external beam, linear-accelerator–based treatment plan. The plan consisted of three 15-megavoltage photon fields delivering 3,000 cGy in 10 fractions to the whole liver, with appropriate multileaf collimation blocking to minimize dose to adjacent heart, right lung, and bilateral kidneys (Figure 3).
Before initiation of the EBRT, the patient received systemic chemotherapy with a dose-adjusted FOLFOX regimen (5-FU bolus 200 mg/m2, leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, with infusional 5-FU 2,400 mg/m2 over 46 hours). After completing 1 dose of modified FOLFOX, he completed 10 fractions of whole liver radiotherapy with the aforementioned plan. He tolerated the initial treatment well and his subjective symptoms improved. The patient then proceeded to further systemic therapy. After recent data demonstrated improved median progression-free survival and response rates with FOLFOXIRI plus bevacizumab (infusional 5-FU 3200 mg/m2, leucovorin 200 mg/m2, irinotecan 165 mg/m2, and oxaliplatin 85 mg/m2, bevacizumab 5 mg/kg) versus FOLFIRI plus bevacizumab,3 we decided to modify his systemic therapy to FOLFOXIRI with bevacizumab to induce a better response.
Treatment response
After 2 doses of chemotherapy and completion of radiotherapy, the edema and shortness of breath improved. A follow-up echocardiogram performed a month after completion of EBRT, 1 dose of FOLFOX, and 1 dose of FOLFOXIRI showed resolution of the cardiac compression (Figure 4).
A CT scan of the abdomen and pelvis obtained after 3 cycles of FOLFOXIRI showed marked decrease in the size of the right lobe hepatic mass from 17.6 x 12.1 cm to 12.0 x 8.0 cm. Given the survival benefit of VEGF inhibition in colon cancer, bevacizumab (5 mg/kg) was added to the FOLFOXIRI regimen with cycle 4. Unfortunately, after the 5th cycle, a CT scan of the abdomen showed an increase in size of the hepatic lesions. At this time, FOLFOXIRI and bevacizumab were stopped, and given the tumor’s KRAS/NRAS wild type status, systemic therapy was changed to panitumumab (6 mg/kg). The patient initially tolerated treatment well, but after 9 cycles, the total bilirubin started to increase. CT abdomen at this point was consistent with progression of disease. The patient was not eligible for a clinical trial targeting BRAF mutation given the elevated bilirubin. Regorafanib (80 mg daily for 3 weeks on and 1 week off) was started. After the first cycle, the total bilirubin increased further and the regorafanib was dose reduced to 40 mg daily. Unfortunately, a repeat CT scan of the abdomen demonstrated progression of disease, and given that he developed a progressive transaminitis and hyperbilirubinemia, hospice care was recommended. The patient died shortly thereafter, about 15 months after his initial diagnosis.
Discussion
Massive liver metastasis in the setting of disseminated cancer is not an uncommon manifestation of advanced cancer that can have life-threatening consequences. In te present case, a bulky liver metastasis caused extrinsic compression of the right atrium, resulting in obvious clinical and echocardiogram-proven cardiac tamponade physiology. To our knowledge, this is the first reported case of the treatment of a bulky hepatic metastasis causing cardiac tamponade. In this patient’s case, both radiotherapy and chemotherapy were given safely in rapid sequence resulting in quick resolution of the patient’s symptoms and echocardiogram findings. The presence of a BRAF mutation conferred a poor prognosis and poor response to systemic chemotherapy. Nevertheless, the patient showed good response to a FOLFOXIRI regimen, chosen in this emergent situation given its significantly higher response rates compared with the standard FOLFIRI regimen, which was tolerated well with minimal adverse effects.
Findings from randomized controlled trials examining the role of palliative radiotherapy for metastatic liver disease have suggested that dose escalation above 30 Gy to the whole liver may lead to unacceptably high rates of radiation-induced liver disease, which typically leads to mortality.4-8 Two prospective trials comparing twice daily with daily fractionation have shown no benefit to hyperfractionation, with possibly increased rates of acute toxicity in the setting of hepatocellular carcinoma.9,10 There is emerging evidence that partial liver irradiation, in the appropriate setting in the form of boost after whole-liver RT or stereotactic body radiotherapy, may allow for further dose escalation while avoiding clinical hepatitis.11 Although there is no clear consensus about optimal RT dose and fractionation, the aforementioned studies show that dose and fractionation schemes ranging between 21 Gy and 30 Gy in 1.5 Gy to 3 Gy daily fractions likely provide the best therapeutic ratio for whole-liver irradiation.
In conclusion, this case demonstrates the resolution of cardiac tamponade from a massive liver colorectal metastasis after chemoradiation and illustrates the potential utility of adding radiotherapy to chemotherapy in an urgent scenario where the former might not typically be considered.
Colorectal cancer is the third most commonly diagnosed cancer in the United States.1 About 5% of Americans will be diagnosed with colorectal cancer in their lifetime, of which 20% will present with distant metastasis.2 The most common sites of metastasis are regional lymph nodes, liver, lung and peritoneum, and patients may present with signs or symptoms related to disease burden at any of these organs.
Case presentation and summary
A 55-year-old man had presented to an outside hospital in August of 2014 with 6 months of hematochezia and a 40-lb weight loss. He was found to be severely anemic on admission (hemoglobin, 4.9 g/dL [normal, 13-17 g/dL], hematocrit, 16% [normal, 35%-45%]). A computed-tomography (CT) scan of the abdomen and pelvis with contrast revealed a mass of 6.9 x 4.7 x 6.3 cm in the rectosigmoid colon and a mass of 10.0 x 12.0 x 10.7 cm in the right hepatic lobe consistent with metastatic disease. The patient was taken to the operating room where the rectosigmoid mass was resected completely. The liver mass was deemed unresectable because of its large size, and surgically directed therapy could not be performed. Pathology was consistent with a T3N1 moderately differentiated adenocarcinoma 11 cm from the anal verge. Further molecular tumor studies revealed wild type KRAS and NRAS, as well as a BRAF mutation.
About 4 weeks after the surgery, the patient was seen at our institution for an initial consultation and was noted to have significant anasarca, including 4+ pitting lower extremity edema and scrotal edema. He complained of dyspnea on exertion, which he attributed to deconditioning. His resting heart rate was found to be 123 beats per minute (normal, 60-100 bpm). Jugular venous distention was present. The patient was sent for an urgent echocardiogram, which showed external compression of the right atrium and ventricle by his liver metastasis resulting in tamponade physiology without the presence of any pericardial effusion (Figure 1).
A CT of the abdomen and pelvis at that time showed that the liver mass had increased to 17.6 x 12.1 x 16.1 cm, exerting pressure on the heart and causing atelectasis of the right middle and lower lung lobes (Figure 2).
Treatment plan
The patient was evaluated by surgical oncology for resection, but his cardiovascular status placed him at high risk for perioperative complications, so such surgery was not pursued. Radioembolization was considered but not pursued because the process needed to evaluate, plan, and treat was not considered sufficiently timely. We consulted with our radiation oncology colleagues about external beam radiotherapy (EBRT) for rapid palliation. They evaluated the patient and recommended the EBRT, and the patient signed consent for treatment. We performed a CT-based simulation and generated an external beam, linear-accelerator–based treatment plan. The plan consisted of three 15-megavoltage photon fields delivering 3,000 cGy in 10 fractions to the whole liver, with appropriate multileaf collimation blocking to minimize dose to adjacent heart, right lung, and bilateral kidneys (Figure 3).
Before initiation of the EBRT, the patient received systemic chemotherapy with a dose-adjusted FOLFOX regimen (5-FU bolus 200 mg/m2, leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, with infusional 5-FU 2,400 mg/m2 over 46 hours). After completing 1 dose of modified FOLFOX, he completed 10 fractions of whole liver radiotherapy with the aforementioned plan. He tolerated the initial treatment well and his subjective symptoms improved. The patient then proceeded to further systemic therapy. After recent data demonstrated improved median progression-free survival and response rates with FOLFOXIRI plus bevacizumab (infusional 5-FU 3200 mg/m2, leucovorin 200 mg/m2, irinotecan 165 mg/m2, and oxaliplatin 85 mg/m2, bevacizumab 5 mg/kg) versus FOLFIRI plus bevacizumab,3 we decided to modify his systemic therapy to FOLFOXIRI with bevacizumab to induce a better response.
Treatment response
After 2 doses of chemotherapy and completion of radiotherapy, the edema and shortness of breath improved. A follow-up echocardiogram performed a month after completion of EBRT, 1 dose of FOLFOX, and 1 dose of FOLFOXIRI showed resolution of the cardiac compression (Figure 4).
A CT scan of the abdomen and pelvis obtained after 3 cycles of FOLFOXIRI showed marked decrease in the size of the right lobe hepatic mass from 17.6 x 12.1 cm to 12.0 x 8.0 cm. Given the survival benefit of VEGF inhibition in colon cancer, bevacizumab (5 mg/kg) was added to the FOLFOXIRI regimen with cycle 4. Unfortunately, after the 5th cycle, a CT scan of the abdomen showed an increase in size of the hepatic lesions. At this time, FOLFOXIRI and bevacizumab were stopped, and given the tumor’s KRAS/NRAS wild type status, systemic therapy was changed to panitumumab (6 mg/kg). The patient initially tolerated treatment well, but after 9 cycles, the total bilirubin started to increase. CT abdomen at this point was consistent with progression of disease. The patient was not eligible for a clinical trial targeting BRAF mutation given the elevated bilirubin. Regorafanib (80 mg daily for 3 weeks on and 1 week off) was started. After the first cycle, the total bilirubin increased further and the regorafanib was dose reduced to 40 mg daily. Unfortunately, a repeat CT scan of the abdomen demonstrated progression of disease, and given that he developed a progressive transaminitis and hyperbilirubinemia, hospice care was recommended. The patient died shortly thereafter, about 15 months after his initial diagnosis.
Discussion
Massive liver metastasis in the setting of disseminated cancer is not an uncommon manifestation of advanced cancer that can have life-threatening consequences. In te present case, a bulky liver metastasis caused extrinsic compression of the right atrium, resulting in obvious clinical and echocardiogram-proven cardiac tamponade physiology. To our knowledge, this is the first reported case of the treatment of a bulky hepatic metastasis causing cardiac tamponade. In this patient’s case, both radiotherapy and chemotherapy were given safely in rapid sequence resulting in quick resolution of the patient’s symptoms and echocardiogram findings. The presence of a BRAF mutation conferred a poor prognosis and poor response to systemic chemotherapy. Nevertheless, the patient showed good response to a FOLFOXIRI regimen, chosen in this emergent situation given its significantly higher response rates compared with the standard FOLFIRI regimen, which was tolerated well with minimal adverse effects.
Findings from randomized controlled trials examining the role of palliative radiotherapy for metastatic liver disease have suggested that dose escalation above 30 Gy to the whole liver may lead to unacceptably high rates of radiation-induced liver disease, which typically leads to mortality.4-8 Two prospective trials comparing twice daily with daily fractionation have shown no benefit to hyperfractionation, with possibly increased rates of acute toxicity in the setting of hepatocellular carcinoma.9,10 There is emerging evidence that partial liver irradiation, in the appropriate setting in the form of boost after whole-liver RT or stereotactic body radiotherapy, may allow for further dose escalation while avoiding clinical hepatitis.11 Although there is no clear consensus about optimal RT dose and fractionation, the aforementioned studies show that dose and fractionation schemes ranging between 21 Gy and 30 Gy in 1.5 Gy to 3 Gy daily fractions likely provide the best therapeutic ratio for whole-liver irradiation.
In conclusion, this case demonstrates the resolution of cardiac tamponade from a massive liver colorectal metastasis after chemoradiation and illustrates the potential utility of adding radiotherapy to chemotherapy in an urgent scenario where the former might not typically be considered.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Published 2015. Accessed October 10, 2017.
2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
3. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
4. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117-123.
5. Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA. 1975;232(6):625-628.
6. Sherman DM, Weichselbaum R, Order SE, Cloud L, Trey C, Piro AJ. Palliation of hepatic metastasis. Cancer. 1978;41(5):2013-2017.
7. Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys. 1977;2:129-132.
8. Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
9. Raju PI, Maruyama Y, DeSimone P, MacDonald J. Treatment of liver metastases with a combination of chemotherapy and hyperfractionated external radiation therapy. Am J Clin Oncol. 1987;10(1):41-43.
10. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223-1229.
11. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14(3):722-728.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Published 2015. Accessed October 10, 2017.
2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
3. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
4. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117-123.
5. Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA. 1975;232(6):625-628.
6. Sherman DM, Weichselbaum R, Order SE, Cloud L, Trey C, Piro AJ. Palliation of hepatic metastasis. Cancer. 1978;41(5):2013-2017.
7. Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys. 1977;2:129-132.
8. Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
9. Raju PI, Maruyama Y, DeSimone P, MacDonald J. Treatment of liver metastases with a combination of chemotherapy and hyperfractionated external radiation therapy. Am J Clin Oncol. 1987;10(1):41-43.
10. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223-1229.
11. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14(3):722-728.
Cardiac pleomorphic sarcoma after placement of Dacron graft
Primary cardiac tumors, either benign or malignant, are very rare. The combined incidence is 0.002% on pooled autopsy series.1 The benign tumors account for 63% of primary cardiac tumors and include myxoma, the most common, and followed by papillary fibroelastoma, fibroma, and hemangioma. The remaining 37% are malignant tumors, essentially predominated by sarcomas.1
Although myxoma is the most common tumor arising in the left atrium, we present a case that shows that sarcoma can also arise from the same chamber. In fact, sarcomas could mimic cardiac myxoma.2 The cardiac sarcomas can have similar clinical presentation and more importantly can share similar histopathological features. Sarcomas may have myxoid features.2 Cases diagnosed as cardiac myxomas should be diligently worked up to rule out the presence of sarcomas with myxoid features. In addition, foreign bodies have been found to induce sarcomas in experimental animals.3,4 In particular, 2 case reports have described sarcomas arising in association with Dacron vascular prostheses in humans.5,6 We present here the case of a patient who was diagnosed with cardiac pleomorphic sarcoma 8 years after the placement of a Dacron graft.
Case presentation and summary
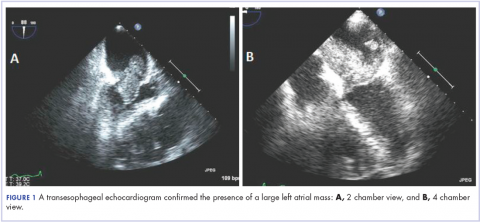
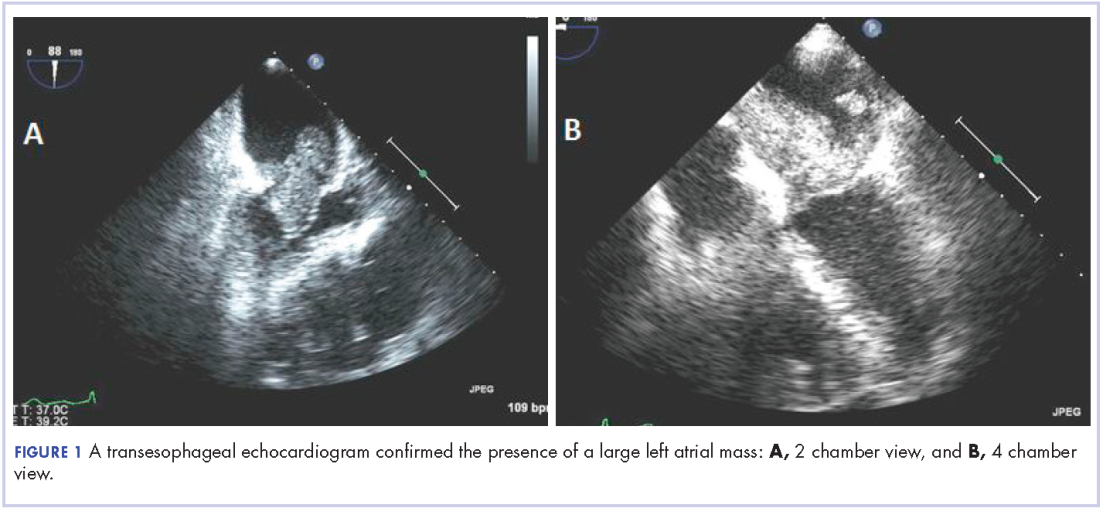
A 56-year-old woman with history of left atrial myxoma status after resection in 2005 and placement of a Dacron graft, morbid obesity, hypertension, and asthma presented to the emergency department with progressively worsening shortness of breath and blurry vision over period of 2 months. Acute coronary syndrome was ruled out by electrocardiogram and serial biomarkers. A computed-tomography angiogram was pursued because of her history of left atrial myxoma, and the results suggested the presence of a left atrial tumor. She underwent a transesophageal echocardiogram, which confirmed the presence of a large left atrial mass that likely was attached to the interatrial septum prolapsing across the mitral valve and was suggestive for recurrent left atrial myxoma (Figure 1). The results of a cardiac catheterization showed normal coronaries.
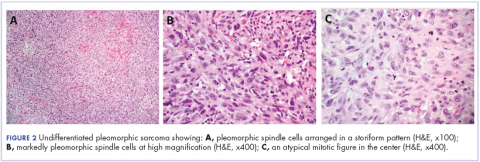
The patient subsequently underwent an excision of the left atrial tumor with profound internal and external myocardial cooling using antegrade blood cardioplegia under mildly hypothermic cardiopulmonary bypass. Frozen sections showed high-grade malignancy in favor of sarcoma. The hematoxylin and eosin stained permanent sections showed sheets of malignant pleomorphic spindle cells focally arranged in a storiform pattern. There were areas of necrosis and abundant mitotic activity. By immunohistochemical (IHC) stains, the tumor cells were diffusely positive for vimentin, and negative for pan-cytokeratin antibody (AE1/AE3), S-100 protein, Melan-A antibody, HMB45, CD34, CD31, myogenin, and MYOD1. IHC stains for CK-OSCAR, desmin, and smooth muscle actin were focally positive, and a ki-67 stain showed a proliferation index of about 80%. The histologic and IHC findings were consistent with a final diagnosis of high-grade undifferentiated pleomorphic sarcoma (Figure 2).
A positron emission tomography scan performed November 2013 did not show any other activity. The patient was scheduled for chemotherapy with adriamycin and ifosfamide with a plan for total of 6 cycles. Before her admission for the chemotherapy, the patient was admitted to the hospital for atrial fibrillation with rapid ventricular response and had multiple complications requiring prolonged hospitalization and rehabilitation. Repeat imaging 2 months later showed diffuse metastatic disease. However, her performance status had declined and she was not eligible for chemotherapy. She was placed under hospice care.
Discussion
This case demonstrates development of a cardiac pleomorphic sarcoma, a rare tumor, after placement of a Dacron graft. Given that foreign bodies have been found to induce sarcomas in experimental animals,3,4 and a few case reports have described sarcomas arising in association with Dacron vascular prostheses, 5-10 it seems that an exuberant host response around the foreign body might represent an important intermediate step in the development of the sarcoma.
There is no clearly defined pathogenesis that explains the link between a Dacron graft and sarcomas. In 1950s, Oppenheimer and colleagues described the formation of malignant tumors by various types of plastics, including Dacron, that were embedded in rats. 3,4 Most of the tumors were some form of sarcomas. It was inferred that physical properties of the plastics may have some role in tumor development. Plastics in sheet form or film that remained in situ for more than 6 months induced significant number of tumors compared with other forms such as sponges, films with holes, or powders.3,4 The 3-dimensional polymeric structure of the Dacron graft seems to play a role in induction of sarcoma as well. A pore diameter of less than 0.4 mm may increase tumorigenicity.11 The removal of the material before the 6-months mark does not lead to malignant tumors, which further supports the link between Dacron graft and formation of tumor. A pocket is formed around the foreign material after a certain period, as has been shown in histologic studies as the site of tumor origin.9,10
At the molecular level, the MDM-2/p53 pathway has been cited as possible mechanism for pathogenesis of intimal sarcoma.12,13 It has been suggested that endothelial dysplasia occurs as a precursor lesion in these sarcomas.14 The Dacron graft may cause a dysplastic effect on the endothelium leading to this precursor lesion and in certain cases transforming into sarcoma. Further definitive studies are required.
The primary treatment for cardiac sarcoma is surgical removal, although it is not always feasible. Findings in a Mayo clinic study showed that the median survival was 17 months for patients who underwent complete surgical excision, compared with 6 months for those who complete resection was not possible.15 In addition, a 10% survival rate at 1 year has been reported in primary cardiac sarcomas that are treated without any type of surgery.16
There is no clear-cut evidence supporting or refuting adjuvant chemotherapy for cardiac sarcoma. Some have inferred a potential benefit of adjuvant chemotherapy although definitive conclusions cannot be drawn. The median survival was 16.5 months in a case series of patients who received adjuvant chemotherapy, compared with 9 months and 11 months in 2 other case series.17,18,19 Multiple chemotherapy regimens have been used in the past for treatment. A retrospective s
Radiation showed some benefit in progression-free survival in a French retrospective study.21 Radiation therapies have been tried in other cases, as well in addition to chemotherapy. However, there is not enough data to support or refute it at this time.15,17,20 Several sporadic cases reported show benefit of cardiac transplantation.21,22
Conclusion
In consideration of the placement of the Dacron graft 8 years before the tumor occurrence, the anatomic proximity of the tumor to the Dacron graft, and the association between sarcoma with Dacron in medical literature, it seems logical to infer that this unusual malignancy in our patient is associated with the Dacron prosthesis.
1. Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11(4):E12.
2. Awamleh P, Alberca MT, Gamallo C, Enrech S, Sarraj A. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol. 2007;30(6):306-308.
3. Oppenheimer BS, Oppenheimer ET, Stout AP, Danishefsky I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305-306.
4. Oppenheimer BS, Oppenheimer ET, Stout AP, Willhite M, Danishefski, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 1958;11(1):204-213.
5. Almeida NJ, Hoang P, Biddle P, Arouni A, Esterbrooks D. Primary cardiac angiosarcoma: in a patient with a Dacron aortic prosthesis. Tex Heart Inst J. 2011;38(1):61-65; discussion 65.
6. Stewart B, Manglik N, Zhao B, et al. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22(5):351-356.
7. Umscheid TW, Rouhani G, Morlang T, et al. Hemangiosarcoma after endovascular aortic aneurysm repair. J Endovasc Ther. 2007;14(1):101-105.
8. Ben-Izhak O, Vlodavsky E, Ofer A, Engel A, Nitecky S, Hoffman A. Epithelioid angiosarcoma associated with a Dacron vascular graft. Am J Surg Pathol. 1999;23(11):1418-1422.
9. Fyfe BS, Quintana CS, Kaneko M, Griepp RB. Aortic sarcoma four years after Dacron graft insertion. Ann Thorac Surg. 1994;58(6):1752-1754.
10. O’Connell TX, Fee HJ, Golding A. Sarcoma associated with Dacron prosthetic material: case report and review of the literature. J Thorac Cardiovasc Surg. 1976;72(1):94-96.
11. Karp RD, Johnson KH, Buoen LC, et al. Tumorogenesis by millipore filters in mice: histology and ultastructure of tissue reactions, as related to pore size. J Natl Cancer Inst. 1973;51:1275-1285.
12. Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
13. Zeitz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Surg Pathol. 1998;153:1425-1433.
14. Haber LM, Truong L. Immunohistochemical demonstration of the endothelialnature of aortic intimal sarcoma. Am J Surg Pathol. 1988 Oct;12(10):798-802. PubMed PMID: 3138923.
15. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112(11):2440-2446.
16. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261-262.
17. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34(4):295-304.
18. Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DC. Primary cardiac sarcomas. Ann Thorac Surg. 1990; 51; 906-910.
19. Murphy WR, Sweeney MS, Putnam JB et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49;612-618.
20. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624-1628.
21. Isambert N, Ray-Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128-136.
22. Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928-938.
Primary cardiac tumors, either benign or malignant, are very rare. The combined incidence is 0.002% on pooled autopsy series.1 The benign tumors account for 63% of primary cardiac tumors and include myxoma, the most common, and followed by papillary fibroelastoma, fibroma, and hemangioma. The remaining 37% are malignant tumors, essentially predominated by sarcomas.1
Although myxoma is the most common tumor arising in the left atrium, we present a case that shows that sarcoma can also arise from the same chamber. In fact, sarcomas could mimic cardiac myxoma.2 The cardiac sarcomas can have similar clinical presentation and more importantly can share similar histopathological features. Sarcomas may have myxoid features.2 Cases diagnosed as cardiac myxomas should be diligently worked up to rule out the presence of sarcomas with myxoid features. In addition, foreign bodies have been found to induce sarcomas in experimental animals.3,4 In particular, 2 case reports have described sarcomas arising in association with Dacron vascular prostheses in humans.5,6 We present here the case of a patient who was diagnosed with cardiac pleomorphic sarcoma 8 years after the placement of a Dacron graft.
Case presentation and summary
A 56-year-old woman with history of left atrial myxoma status after resection in 2005 and placement of a Dacron graft, morbid obesity, hypertension, and asthma presented to the emergency department with progressively worsening shortness of breath and blurry vision over period of 2 months. Acute coronary syndrome was ruled out by electrocardiogram and serial biomarkers. A computed-tomography angiogram was pursued because of her history of left atrial myxoma, and the results suggested the presence of a left atrial tumor. She underwent a transesophageal echocardiogram, which confirmed the presence of a large left atrial mass that likely was attached to the interatrial septum prolapsing across the mitral valve and was suggestive for recurrent left atrial myxoma (Figure 1). The results of a cardiac catheterization showed normal coronaries.
The patient subsequently underwent an excision of the left atrial tumor with profound internal and external myocardial cooling using antegrade blood cardioplegia under mildly hypothermic cardiopulmonary bypass. Frozen sections showed high-grade malignancy in favor of sarcoma. The hematoxylin and eosin stained permanent sections showed sheets of malignant pleomorphic spindle cells focally arranged in a storiform pattern. There were areas of necrosis and abundant mitotic activity. By immunohistochemical (IHC) stains, the tumor cells were diffusely positive for vimentin, and negative for pan-cytokeratin antibody (AE1/AE3), S-100 protein, Melan-A antibody, HMB45, CD34, CD31, myogenin, and MYOD1. IHC stains for CK-OSCAR, desmin, and smooth muscle actin were focally positive, and a ki-67 stain showed a proliferation index of about 80%. The histologic and IHC findings were consistent with a final diagnosis of high-grade undifferentiated pleomorphic sarcoma (Figure 2).
A positron emission tomography scan performed November 2013 did not show any other activity. The patient was scheduled for chemotherapy with adriamycin and ifosfamide with a plan for total of 6 cycles. Before her admission for the chemotherapy, the patient was admitted to the hospital for atrial fibrillation with rapid ventricular response and had multiple complications requiring prolonged hospitalization and rehabilitation. Repeat imaging 2 months later showed diffuse metastatic disease. However, her performance status had declined and she was not eligible for chemotherapy. She was placed under hospice care.
Discussion
This case demonstrates development of a cardiac pleomorphic sarcoma, a rare tumor, after placement of a Dacron graft. Given that foreign bodies have been found to induce sarcomas in experimental animals,3,4 and a few case reports have described sarcomas arising in association with Dacron vascular prostheses, 5-10 it seems that an exuberant host response around the foreign body might represent an important intermediate step in the development of the sarcoma.
There is no clearly defined pathogenesis that explains the link between a Dacron graft and sarcomas. In 1950s, Oppenheimer and colleagues described the formation of malignant tumors by various types of plastics, including Dacron, that were embedded in rats. 3,4 Most of the tumors were some form of sarcomas. It was inferred that physical properties of the plastics may have some role in tumor development. Plastics in sheet form or film that remained in situ for more than 6 months induced significant number of tumors compared with other forms such as sponges, films with holes, or powders.3,4 The 3-dimensional polymeric structure of the Dacron graft seems to play a role in induction of sarcoma as well. A pore diameter of less than 0.4 mm may increase tumorigenicity.11 The removal of the material before the 6-months mark does not lead to malignant tumors, which further supports the link between Dacron graft and formation of tumor. A pocket is formed around the foreign material after a certain period, as has been shown in histologic studies as the site of tumor origin.9,10
At the molecular level, the MDM-2/p53 pathway has been cited as possible mechanism for pathogenesis of intimal sarcoma.12,13 It has been suggested that endothelial dysplasia occurs as a precursor lesion in these sarcomas.14 The Dacron graft may cause a dysplastic effect on the endothelium leading to this precursor lesion and in certain cases transforming into sarcoma. Further definitive studies are required.
The primary treatment for cardiac sarcoma is surgical removal, although it is not always feasible. Findings in a Mayo clinic study showed that the median survival was 17 months for patients who underwent complete surgical excision, compared with 6 months for those who complete resection was not possible.15 In addition, a 10% survival rate at 1 year has been reported in primary cardiac sarcomas that are treated without any type of surgery.16
There is no clear-cut evidence supporting or refuting adjuvant chemotherapy for cardiac sarcoma. Some have inferred a potential benefit of adjuvant chemotherapy although definitive conclusions cannot be drawn. The median survival was 16.5 months in a case series of patients who received adjuvant chemotherapy, compared with 9 months and 11 months in 2 other case series.17,18,19 Multiple chemotherapy regimens have been used in the past for treatment. A retrospective s
Radiation showed some benefit in progression-free survival in a French retrospective study.21 Radiation therapies have been tried in other cases, as well in addition to chemotherapy. However, there is not enough data to support or refute it at this time.15,17,20 Several sporadic cases reported show benefit of cardiac transplantation.21,22
Conclusion
In consideration of the placement of the Dacron graft 8 years before the tumor occurrence, the anatomic proximity of the tumor to the Dacron graft, and the association between sarcoma with Dacron in medical literature, it seems logical to infer that this unusual malignancy in our patient is associated with the Dacron prosthesis.
Primary cardiac tumors, either benign or malignant, are very rare. The combined incidence is 0.002% on pooled autopsy series.1 The benign tumors account for 63% of primary cardiac tumors and include myxoma, the most common, and followed by papillary fibroelastoma, fibroma, and hemangioma. The remaining 37% are malignant tumors, essentially predominated by sarcomas.1
Although myxoma is the most common tumor arising in the left atrium, we present a case that shows that sarcoma can also arise from the same chamber. In fact, sarcomas could mimic cardiac myxoma.2 The cardiac sarcomas can have similar clinical presentation and more importantly can share similar histopathological features. Sarcomas may have myxoid features.2 Cases diagnosed as cardiac myxomas should be diligently worked up to rule out the presence of sarcomas with myxoid features. In addition, foreign bodies have been found to induce sarcomas in experimental animals.3,4 In particular, 2 case reports have described sarcomas arising in association with Dacron vascular prostheses in humans.5,6 We present here the case of a patient who was diagnosed with cardiac pleomorphic sarcoma 8 years after the placement of a Dacron graft.
Case presentation and summary
A 56-year-old woman with history of left atrial myxoma status after resection in 2005 and placement of a Dacron graft, morbid obesity, hypertension, and asthma presented to the emergency department with progressively worsening shortness of breath and blurry vision over period of 2 months. Acute coronary syndrome was ruled out by electrocardiogram and serial biomarkers. A computed-tomography angiogram was pursued because of her history of left atrial myxoma, and the results suggested the presence of a left atrial tumor. She underwent a transesophageal echocardiogram, which confirmed the presence of a large left atrial mass that likely was attached to the interatrial septum prolapsing across the mitral valve and was suggestive for recurrent left atrial myxoma (Figure 1). The results of a cardiac catheterization showed normal coronaries.
The patient subsequently underwent an excision of the left atrial tumor with profound internal and external myocardial cooling using antegrade blood cardioplegia under mildly hypothermic cardiopulmonary bypass. Frozen sections showed high-grade malignancy in favor of sarcoma. The hematoxylin and eosin stained permanent sections showed sheets of malignant pleomorphic spindle cells focally arranged in a storiform pattern. There were areas of necrosis and abundant mitotic activity. By immunohistochemical (IHC) stains, the tumor cells were diffusely positive for vimentin, and negative for pan-cytokeratin antibody (AE1/AE3), S-100 protein, Melan-A antibody, HMB45, CD34, CD31, myogenin, and MYOD1. IHC stains for CK-OSCAR, desmin, and smooth muscle actin were focally positive, and a ki-67 stain showed a proliferation index of about 80%. The histologic and IHC findings were consistent with a final diagnosis of high-grade undifferentiated pleomorphic sarcoma (Figure 2).
A positron emission tomography scan performed November 2013 did not show any other activity. The patient was scheduled for chemotherapy with adriamycin and ifosfamide with a plan for total of 6 cycles. Before her admission for the chemotherapy, the patient was admitted to the hospital for atrial fibrillation with rapid ventricular response and had multiple complications requiring prolonged hospitalization and rehabilitation. Repeat imaging 2 months later showed diffuse metastatic disease. However, her performance status had declined and she was not eligible for chemotherapy. She was placed under hospice care.
Discussion
This case demonstrates development of a cardiac pleomorphic sarcoma, a rare tumor, after placement of a Dacron graft. Given that foreign bodies have been found to induce sarcomas in experimental animals,3,4 and a few case reports have described sarcomas arising in association with Dacron vascular prostheses, 5-10 it seems that an exuberant host response around the foreign body might represent an important intermediate step in the development of the sarcoma.
There is no clearly defined pathogenesis that explains the link between a Dacron graft and sarcomas. In 1950s, Oppenheimer and colleagues described the formation of malignant tumors by various types of plastics, including Dacron, that were embedded in rats. 3,4 Most of the tumors were some form of sarcomas. It was inferred that physical properties of the plastics may have some role in tumor development. Plastics in sheet form or film that remained in situ for more than 6 months induced significant number of tumors compared with other forms such as sponges, films with holes, or powders.3,4 The 3-dimensional polymeric structure of the Dacron graft seems to play a role in induction of sarcoma as well. A pore diameter of less than 0.4 mm may increase tumorigenicity.11 The removal of the material before the 6-months mark does not lead to malignant tumors, which further supports the link between Dacron graft and formation of tumor. A pocket is formed around the foreign material after a certain period, as has been shown in histologic studies as the site of tumor origin.9,10
At the molecular level, the MDM-2/p53 pathway has been cited as possible mechanism for pathogenesis of intimal sarcoma.12,13 It has been suggested that endothelial dysplasia occurs as a precursor lesion in these sarcomas.14 The Dacron graft may cause a dysplastic effect on the endothelium leading to this precursor lesion and in certain cases transforming into sarcoma. Further definitive studies are required.
The primary treatment for cardiac sarcoma is surgical removal, although it is not always feasible. Findings in a Mayo clinic study showed that the median survival was 17 months for patients who underwent complete surgical excision, compared with 6 months for those who complete resection was not possible.15 In addition, a 10% survival rate at 1 year has been reported in primary cardiac sarcomas that are treated without any type of surgery.16
There is no clear-cut evidence supporting or refuting adjuvant chemotherapy for cardiac sarcoma. Some have inferred a potential benefit of adjuvant chemotherapy although definitive conclusions cannot be drawn. The median survival was 16.5 months in a case series of patients who received adjuvant chemotherapy, compared with 9 months and 11 months in 2 other case series.17,18,19 Multiple chemotherapy regimens have been used in the past for treatment. A retrospective s
Radiation showed some benefit in progression-free survival in a French retrospective study.21 Radiation therapies have been tried in other cases, as well in addition to chemotherapy. However, there is not enough data to support or refute it at this time.15,17,20 Several sporadic cases reported show benefit of cardiac transplantation.21,22
Conclusion
In consideration of the placement of the Dacron graft 8 years before the tumor occurrence, the anatomic proximity of the tumor to the Dacron graft, and the association between sarcoma with Dacron in medical literature, it seems logical to infer that this unusual malignancy in our patient is associated with the Dacron prosthesis.
1. Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11(4):E12.
2. Awamleh P, Alberca MT, Gamallo C, Enrech S, Sarraj A. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol. 2007;30(6):306-308.
3. Oppenheimer BS, Oppenheimer ET, Stout AP, Danishefsky I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305-306.
4. Oppenheimer BS, Oppenheimer ET, Stout AP, Willhite M, Danishefski, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 1958;11(1):204-213.
5. Almeida NJ, Hoang P, Biddle P, Arouni A, Esterbrooks D. Primary cardiac angiosarcoma: in a patient with a Dacron aortic prosthesis. Tex Heart Inst J. 2011;38(1):61-65; discussion 65.
6. Stewart B, Manglik N, Zhao B, et al. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22(5):351-356.
7. Umscheid TW, Rouhani G, Morlang T, et al. Hemangiosarcoma after endovascular aortic aneurysm repair. J Endovasc Ther. 2007;14(1):101-105.
8. Ben-Izhak O, Vlodavsky E, Ofer A, Engel A, Nitecky S, Hoffman A. Epithelioid angiosarcoma associated with a Dacron vascular graft. Am J Surg Pathol. 1999;23(11):1418-1422.
9. Fyfe BS, Quintana CS, Kaneko M, Griepp RB. Aortic sarcoma four years after Dacron graft insertion. Ann Thorac Surg. 1994;58(6):1752-1754.
10. O’Connell TX, Fee HJ, Golding A. Sarcoma associated with Dacron prosthetic material: case report and review of the literature. J Thorac Cardiovasc Surg. 1976;72(1):94-96.
11. Karp RD, Johnson KH, Buoen LC, et al. Tumorogenesis by millipore filters in mice: histology and ultastructure of tissue reactions, as related to pore size. J Natl Cancer Inst. 1973;51:1275-1285.
12. Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
13. Zeitz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Surg Pathol. 1998;153:1425-1433.
14. Haber LM, Truong L. Immunohistochemical demonstration of the endothelialnature of aortic intimal sarcoma. Am J Surg Pathol. 1988 Oct;12(10):798-802. PubMed PMID: 3138923.
15. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112(11):2440-2446.
16. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261-262.
17. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34(4):295-304.
18. Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DC. Primary cardiac sarcomas. Ann Thorac Surg. 1990; 51; 906-910.
19. Murphy WR, Sweeney MS, Putnam JB et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49;612-618.
20. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624-1628.
21. Isambert N, Ray-Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128-136.
22. Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928-938.
1. Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11(4):E12.
2. Awamleh P, Alberca MT, Gamallo C, Enrech S, Sarraj A. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol. 2007;30(6):306-308.
3. Oppenheimer BS, Oppenheimer ET, Stout AP, Danishefsky I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305-306.
4. Oppenheimer BS, Oppenheimer ET, Stout AP, Willhite M, Danishefski, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 1958;11(1):204-213.
5. Almeida NJ, Hoang P, Biddle P, Arouni A, Esterbrooks D. Primary cardiac angiosarcoma: in a patient with a Dacron aortic prosthesis. Tex Heart Inst J. 2011;38(1):61-65; discussion 65.
6. Stewart B, Manglik N, Zhao B, et al. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22(5):351-356.
7. Umscheid TW, Rouhani G, Morlang T, et al. Hemangiosarcoma after endovascular aortic aneurysm repair. J Endovasc Ther. 2007;14(1):101-105.
8. Ben-Izhak O, Vlodavsky E, Ofer A, Engel A, Nitecky S, Hoffman A. Epithelioid angiosarcoma associated with a Dacron vascular graft. Am J Surg Pathol. 1999;23(11):1418-1422.
9. Fyfe BS, Quintana CS, Kaneko M, Griepp RB. Aortic sarcoma four years after Dacron graft insertion. Ann Thorac Surg. 1994;58(6):1752-1754.
10. O’Connell TX, Fee HJ, Golding A. Sarcoma associated with Dacron prosthetic material: case report and review of the literature. J Thorac Cardiovasc Surg. 1976;72(1):94-96.
11. Karp RD, Johnson KH, Buoen LC, et al. Tumorogenesis by millipore filters in mice: histology and ultastructure of tissue reactions, as related to pore size. J Natl Cancer Inst. 1973;51:1275-1285.
12. Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
13. Zeitz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Surg Pathol. 1998;153:1425-1433.
14. Haber LM, Truong L. Immunohistochemical demonstration of the endothelialnature of aortic intimal sarcoma. Am J Surg Pathol. 1988 Oct;12(10):798-802. PubMed PMID: 3138923.
15. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112(11):2440-2446.
16. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261-262.
17. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34(4):295-304.
18. Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DC. Primary cardiac sarcomas. Ann Thorac Surg. 1990; 51; 906-910.
19. Murphy WR, Sweeney MS, Putnam JB et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49;612-618.
20. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624-1628.
21. Isambert N, Ray-Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128-136.
22. Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928-938.
Study reveals lack of sexual aids for cancer survivors
ORLANDO—A new study suggests many US cancer centers do not have therapeutic aids for patients who experience sexual dysfunction after cancer treatment.
Of 25 cancer centers polled, 80% said they had no sexual aids available on site for men, and 64% said they had no such aids for women.
Sharon Bober, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues presented this research at the 2018 Cancer Survivorship Symposium (abstract 134*).
“[P]roviding sexual aids is one step toward treating sexual health like any other aspect of survivorship care,” Dr Bober said.
“It should be no different than providing wigs and head coverings to women who have lost their hair due to chemotherapy. It’s important to give patients the message that regaining sexual health is a perfectly valid and life-affirming aspect of regaining overall quality of life.”
Dr Bober and her colleagues conducted this study to determine the availability of sexual aids at 25 National Cancer Institute-designated cancer centers.
The researchers called these centers posing as a spouse, adult child, or sibling of a patient. The team made separate calls to ask about sexual aids for women and those for men.
Women’s sexual aids
Twenty-four percent of cancer centers (n=6) said they had sexual aids for women, 64% (n=16) did not, and 12% of centers were unreachable (n=3).
The most common aids were personal lubrication, vaginal moisturizer, and vaginal dilators—all of which were available at 5 centers.
Three centers had vibrators, 2 had books/pamphlets, 2 had pelvic floor exercisers, and 2 had product lists.
Men’s sexual aids
Twelve percent of cancer centers (n=3) said they had sexual aids for men, 80% (n=20) did not, and 8% (n=2) were unreachable.
Two centers said they had personal lubrication available for men, 2 had penile support rings, 1 had vacuum erection devices, and 1 had books/pamphlets.
Next steps
Now, Dr Bober and her colleagues hope to query the other 44 National Cancer Institute-designated cancer centers to see what products they are selling and perhaps conduct patient surveys to find out what types of resources are most useful for cancer survivors.
“What we really need to do is go to the centers that are successfully providing sexual health products and find out how they promote and provide resources to their patients,” Dr Bober said.
“We can’t keep the conversation at the 10,000-foot level. We need to talk concretely about how to partner with providers to make sexual health resources, including sexual health aids, available so cancer survivors can get the help that they need.”
*Information presented differs from the abstract.
ORLANDO—A new study suggests many US cancer centers do not have therapeutic aids for patients who experience sexual dysfunction after cancer treatment.
Of 25 cancer centers polled, 80% said they had no sexual aids available on site for men, and 64% said they had no such aids for women.
Sharon Bober, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues presented this research at the 2018 Cancer Survivorship Symposium (abstract 134*).
“[P]roviding sexual aids is one step toward treating sexual health like any other aspect of survivorship care,” Dr Bober said.
“It should be no different than providing wigs and head coverings to women who have lost their hair due to chemotherapy. It’s important to give patients the message that regaining sexual health is a perfectly valid and life-affirming aspect of regaining overall quality of life.”
Dr Bober and her colleagues conducted this study to determine the availability of sexual aids at 25 National Cancer Institute-designated cancer centers.
The researchers called these centers posing as a spouse, adult child, or sibling of a patient. The team made separate calls to ask about sexual aids for women and those for men.
Women’s sexual aids
Twenty-four percent of cancer centers (n=6) said they had sexual aids for women, 64% (n=16) did not, and 12% of centers were unreachable (n=3).
The most common aids were personal lubrication, vaginal moisturizer, and vaginal dilators—all of which were available at 5 centers.
Three centers had vibrators, 2 had books/pamphlets, 2 had pelvic floor exercisers, and 2 had product lists.
Men’s sexual aids
Twelve percent of cancer centers (n=3) said they had sexual aids for men, 80% (n=20) did not, and 8% (n=2) were unreachable.
Two centers said they had personal lubrication available for men, 2 had penile support rings, 1 had vacuum erection devices, and 1 had books/pamphlets.
Next steps
Now, Dr Bober and her colleagues hope to query the other 44 National Cancer Institute-designated cancer centers to see what products they are selling and perhaps conduct patient surveys to find out what types of resources are most useful for cancer survivors.
“What we really need to do is go to the centers that are successfully providing sexual health products and find out how they promote and provide resources to their patients,” Dr Bober said.
“We can’t keep the conversation at the 10,000-foot level. We need to talk concretely about how to partner with providers to make sexual health resources, including sexual health aids, available so cancer survivors can get the help that they need.”
*Information presented differs from the abstract.
ORLANDO—A new study suggests many US cancer centers do not have therapeutic aids for patients who experience sexual dysfunction after cancer treatment.
Of 25 cancer centers polled, 80% said they had no sexual aids available on site for men, and 64% said they had no such aids for women.
Sharon Bober, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues presented this research at the 2018 Cancer Survivorship Symposium (abstract 134*).
“[P]roviding sexual aids is one step toward treating sexual health like any other aspect of survivorship care,” Dr Bober said.
“It should be no different than providing wigs and head coverings to women who have lost their hair due to chemotherapy. It’s important to give patients the message that regaining sexual health is a perfectly valid and life-affirming aspect of regaining overall quality of life.”
Dr Bober and her colleagues conducted this study to determine the availability of sexual aids at 25 National Cancer Institute-designated cancer centers.
The researchers called these centers posing as a spouse, adult child, or sibling of a patient. The team made separate calls to ask about sexual aids for women and those for men.
Women’s sexual aids
Twenty-four percent of cancer centers (n=6) said they had sexual aids for women, 64% (n=16) did not, and 12% of centers were unreachable (n=3).
The most common aids were personal lubrication, vaginal moisturizer, and vaginal dilators—all of which were available at 5 centers.
Three centers had vibrators, 2 had books/pamphlets, 2 had pelvic floor exercisers, and 2 had product lists.
Men’s sexual aids
Twelve percent of cancer centers (n=3) said they had sexual aids for men, 80% (n=20) did not, and 8% (n=2) were unreachable.
Two centers said they had personal lubrication available for men, 2 had penile support rings, 1 had vacuum erection devices, and 1 had books/pamphlets.
Next steps
Now, Dr Bober and her colleagues hope to query the other 44 National Cancer Institute-designated cancer centers to see what products they are selling and perhaps conduct patient surveys to find out what types of resources are most useful for cancer survivors.
“What we really need to do is go to the centers that are successfully providing sexual health products and find out how they promote and provide resources to their patients,” Dr Bober said.
“We can’t keep the conversation at the 10,000-foot level. We need to talk concretely about how to partner with providers to make sexual health resources, including sexual health aids, available so cancer survivors can get the help that they need.”
*Information presented differs from the abstract.
Patient navigators’ personal experiences with cancer: does it have an impact on treatment?
Patient navigation has emerged in the past decade as a strategy to decrease cancer disparities among low-income, minority populations. Patient navigators help individuals who face personal and systemtic barriers to gaining access to care.1 Their role is to help patients find their way through a complex health care system,2,3 including logistic support of rescheduling appointments, assistance with transportation, and child care needs. They provide personal support, including coaching patients on their clinical visits, educating them about the cancer treatment process, and addressing their fears of diagnosis and treatment. Patient navigation has shown improvement in cancer screening rates, time to diagnostic resolution for those patients who have abnormal cancer screening tests, and quality of cancer care.4,5
In hiring patient navigators, it is not clear which professional training and skill sets and what personal experiences are most useful to becoming an effective navigator. Personal cancer experience can include a personal diagnosis, the experience of serving as a primary caregiver for a patient during treatment, or having a family member or close friend with cancer. Several current support programs specifically recruit cancer survivors on the assumption that their cancer treatment experience can provide helpful insights to a current patient for both emotional and logistical support.6 In this paper, we sought to address whether patient navigation promotes more timely diagnostic care if the navigator has experience with cancer.
Methods
This is a secondary analysis of the patients with abnormal cancer screening in the navigation arm of the national Patient Navigation Research Program (PNRP) study,1, 5 a collaborative effort across 10 centers to investigate the efficacy of patient navigation on improving patient-level outcomes for those who have abnormal results from a breast, cervical, colorectal, or prostate cancer screening test. The study demonstrated that patient navigation was effective in reducing delays in diagnosis and treatment5 and resulting in a higher quality of care,4 especially among vulnerable populations.7 The Institutional Review Board of each respective institution approved the research.
All of the patient navigators were paid employees with a minimum high-school diploma or equivalent. Navigators’ activities were standardized across centers through a national training program.8 Navigators used the care management model to identify and address barriers to care and to track participants throughout the course of their diagnostic evaluation,9 with the primary aim of timely diagnostic resolution. Most navigation programs were embedded within the clinical care system and interacted with patients through mail, by phone, and face-to-face contact.1
Data collection
Each center used agreed-upon inclusion and exclusion criteria and collected and coded the same patient-level data. Medical records were abstracted for pertinent clinical data on patients. Demographic data were collected through a patient survey or extracted from medical record registration. The central data coordinating center collected navigator information including demographic characteristics and experience with cancer.
We created a new variable, Personal experience with cancer. Personal experience with cancer was based on three questions asked of navigators: whether they were a cancer survivor; whether they were the primary caregiver to a family member or close friend with cancer; and whether they had a family member with cancer. Because of the small sample size, responses from navigators who were cancer survivors (n = 6) or primary caregivers to a family member with cancer (n = 4) were collapsed into a single category, referred to as personal experience with cancer, to compare with navigators who had no personal experience with cancer, which included those who reported a family member with cancer but who were not serving as a primary caregiver.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Each clinical center received approved from their institution’s human subjects review board. Informed consent was obtained from all patient navigator participants included in the study. Participating patients completed informed consent at some centers. At other centers where the study design was an implementation of a system intervention, a waiver of informed consent was approved by the Institutional Review Board.
Data analysis
The primary outcome variable was time to diagnostic resolution. We included only participants supported by a single navigator. A Fisher exact test by cancer type was used to compare the two groups (personal experience vs none) in the proportion of patients who achieved diagnostic resolution by 365 days. We reviewed the percentage of patients resolved for the total population as well as stratified by cancer site (breast, cervical, prostate, and colorectal), owing to the known mean differences in time to diagnostic resolution by type of cancer.
Cox proportional hazard models and adjusted hazard ratios were developed and calculated to examine the impact of navigator’s personal experience with cancer on time to resolution, controlling for patient gender, race, age, and cancer type in the models. The analysis controlled for the individual effect of navigators through clustering. We used P < .05 as the cut-off for significance, and used Stata 10.1 (StataCorp, College Station Texas 77845) for all analyses.
Results
Our analytic sample included the 3,975 patients with only 1 navigator over the course of the study, 79% of the navigation (n = 5,063) arm. Most of the patients were women (93%), and most were from racial and ethnic minority communities. Most patients spoke English (60%), with Spanish (33%) as the next most common language. Most patients were publically insured (38%) or uninsured (40%) (Table 1).
Of the total 49 navigators, 6 were cancer survivors and 4 were primary caregivers to a family member with cancer; an additional 19 reported that they had family members with cancer (Table 2). Most of the navigators were women. The racial/ethnic distribution mirrored the populations they served: white (29%); black or African American (31%); and Hispanic (37%). English was the only spoken language of 67% of the navigators; 27% spoke Spanish, and 6% reported speaking another language. Most had a college degree (63%).
The unadjusted bivariate comparison of patients who achieved diagnostic resolution within 365 days, by navigator experience with cancer, are shown in Table 3. We found no difference in time to diagnostic resolution for those patients for whom navigators had personal experience with cancer compared with those whose navigators had no experience. When stratified by type of cancer screening abnormality (breast, cervical, prostate, or colorectal), the results also did not reveal a significant difference in the proportion of patients achieving diagnostic resolution by 365 days by navigator experience with cancer.
In the Cox proportional hazard model adjusting for patient gender, age, race/ethnicity, cancer type, and adjusting for navigator using clustering, there was no difference between patients whose navigators had experience with cancer care, and those who did not (adjusted hazard ratio, 1.03; 95% confidence interval, .83-1.3). The level of education of navigators was not significantly associated with time to diagnostic resolution for patients.
Discussion
Although several cancer support programs have explicitly used cancer survivors as patient navigators or other supports for patients in active cancer care, there are scant data on whether this expertise improves care. Our study was not able to identify that navigators with previous experience with cancer care, either as a patient or as the primary caregiver, was associated with improved time to diagnostic resolution.
As patient navigation has become the standard of cancer diagnostic and treatment practices, there is a need to develop competencies and standards for hiring and training navigators. Part of this hiring process is to determine what past experience and training are relevant for effective navigation. There is little previous research on relevant skills of navigators, with only one study having demonstrated that language and racial/ ethnic concordance between patients and navigators was associated with more timely care. The national PNRP program hired mostly lay navigators with minimal medical experience, but with affiliations to the communities of the patients receiving care. Our program has demonstrated that lay individuals can be trained in the logistic aspects of navigation.5 Although it may seem intuitive that the experience of being a cancer survivor may make a navigator more empathetic, it is also possible that being too close to the experience of survivorship can also pose challenges to a navigator. Alternatively, navigation may be equally effective with proper training regardless of previous experience with cancer.
Our study is limited to addressing the outcome of timely resolution in the diagnostic phase of care after abnormal cancer screening. It is possible that past experience with cancer care will be beneficial when providing navigation for cancer care. While this study represents one of the largest groups of navigators who have been studied, the small sample may have limited our ability to detect differences. Our study has the benefit of a diverse group of navigators from a nationally representative, multi-site study. We suggest that prior experience with cancer care is not a prerequisite to supporting diagnostic care after abnormal cancer screening. Providing appropriate training to navigators may be sufficient to ensure effective and appropriate care is provided by patient navigators.
Acknowledgments
The authors acknowledge the contributions of the following members of the Patient Navigation Research Program:Clinical centers Boston Medical Center and Boston University: Karen M Freund (principal investigator [PI]), Tracy A Battaglia (co-PI); Denver Health and Hospital Authority: Peter Raich (PI), Elizabeth Whitley (co-PI); George Washington University Cancer Institute: Steven R Patierno (PI), Lisa M Alexander, Paul H Levine, Heather A Young, Heather J Hoffman, Nancy L LaVerda; H Lee Moffitt Cancer Center and Research Institute: Richard G Roetzheim (PI), Cathy Meade, Kristen J Wells; Northwest Portland Area Indian Health Board: Victoria Warren-Mears (PI); Northwestern University Robert H Lurie Comprehensive Cancer Center: Steven Rosen (PI), Melissa Simon; The Ohio State University Comprehensive Cancer Center: Electra Paskett (PI); University of Illinois at Chicago and Access Community Health Center: Elizabeth Calhoun (PI), Julie Darnell. University of Rochester: Kevin Fiscella (PI), Samantha Hendren; University of Texas Health Science Center at San Antonio Cancer Therapy and Research Center: Donald Dudley (PI), Kevin Hall, Anand Karnard, Amelie Ramirez. Program office National Cancer Institute, Center to Reduce Cancer Health Disparities: Martha Hare, Mollie Howerton, Ken Chu, Emmanuel Taylor, Mary Ann Van Dyun. Evaluation contractor NOVA Research Company: Paul Young, Frederick Snyder
6. Macvean ML, White VM, Sanson-Fisher R. One-to-one volunteer support programs for people with cancer: a review of the literature. Patient Educ Couns. 2008;70:10-24.
7. Rodday AM, Parsons SK, Snyder F, et al. The impact of patient navigation in eliminating economic disparities in cancer care. Cancer. 2015;121(22):4025-4034.
10. Charlot M, Santana MC, Chen CA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477-1483.
Patient navigation has emerged in the past decade as a strategy to decrease cancer disparities among low-income, minority populations. Patient navigators help individuals who face personal and systemtic barriers to gaining access to care.1 Their role is to help patients find their way through a complex health care system,2,3 including logistic support of rescheduling appointments, assistance with transportation, and child care needs. They provide personal support, including coaching patients on their clinical visits, educating them about the cancer treatment process, and addressing their fears of diagnosis and treatment. Patient navigation has shown improvement in cancer screening rates, time to diagnostic resolution for those patients who have abnormal cancer screening tests, and quality of cancer care.4,5
In hiring patient navigators, it is not clear which professional training and skill sets and what personal experiences are most useful to becoming an effective navigator. Personal cancer experience can include a personal diagnosis, the experience of serving as a primary caregiver for a patient during treatment, or having a family member or close friend with cancer. Several current support programs specifically recruit cancer survivors on the assumption that their cancer treatment experience can provide helpful insights to a current patient for both emotional and logistical support.6 In this paper, we sought to address whether patient navigation promotes more timely diagnostic care if the navigator has experience with cancer.
Methods
This is a secondary analysis of the patients with abnormal cancer screening in the navigation arm of the national Patient Navigation Research Program (PNRP) study,1, 5 a collaborative effort across 10 centers to investigate the efficacy of patient navigation on improving patient-level outcomes for those who have abnormal results from a breast, cervical, colorectal, or prostate cancer screening test. The study demonstrated that patient navigation was effective in reducing delays in diagnosis and treatment5 and resulting in a higher quality of care,4 especially among vulnerable populations.7 The Institutional Review Board of each respective institution approved the research.
All of the patient navigators were paid employees with a minimum high-school diploma or equivalent. Navigators’ activities were standardized across centers through a national training program.8 Navigators used the care management model to identify and address barriers to care and to track participants throughout the course of their diagnostic evaluation,9 with the primary aim of timely diagnostic resolution. Most navigation programs were embedded within the clinical care system and interacted with patients through mail, by phone, and face-to-face contact.1
Data collection
Each center used agreed-upon inclusion and exclusion criteria and collected and coded the same patient-level data. Medical records were abstracted for pertinent clinical data on patients. Demographic data were collected through a patient survey or extracted from medical record registration. The central data coordinating center collected navigator information including demographic characteristics and experience with cancer.
We created a new variable, Personal experience with cancer. Personal experience with cancer was based on three questions asked of navigators: whether they were a cancer survivor; whether they were the primary caregiver to a family member or close friend with cancer; and whether they had a family member with cancer. Because of the small sample size, responses from navigators who were cancer survivors (n = 6) or primary caregivers to a family member with cancer (n = 4) were collapsed into a single category, referred to as personal experience with cancer, to compare with navigators who had no personal experience with cancer, which included those who reported a family member with cancer but who were not serving as a primary caregiver.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Each clinical center received approved from their institution’s human subjects review board. Informed consent was obtained from all patient navigator participants included in the study. Participating patients completed informed consent at some centers. At other centers where the study design was an implementation of a system intervention, a waiver of informed consent was approved by the Institutional Review Board.
Data analysis
The primary outcome variable was time to diagnostic resolution. We included only participants supported by a single navigator. A Fisher exact test by cancer type was used to compare the two groups (personal experience vs none) in the proportion of patients who achieved diagnostic resolution by 365 days. We reviewed the percentage of patients resolved for the total population as well as stratified by cancer site (breast, cervical, prostate, and colorectal), owing to the known mean differences in time to diagnostic resolution by type of cancer.
Cox proportional hazard models and adjusted hazard ratios were developed and calculated to examine the impact of navigator’s personal experience with cancer on time to resolution, controlling for patient gender, race, age, and cancer type in the models. The analysis controlled for the individual effect of navigators through clustering. We used P < .05 as the cut-off for significance, and used Stata 10.1 (StataCorp, College Station Texas 77845) for all analyses.
Results
Our analytic sample included the 3,975 patients with only 1 navigator over the course of the study, 79% of the navigation (n = 5,063) arm. Most of the patients were women (93%), and most were from racial and ethnic minority communities. Most patients spoke English (60%), with Spanish (33%) as the next most common language. Most patients were publically insured (38%) or uninsured (40%) (Table 1).
Of the total 49 navigators, 6 were cancer survivors and 4 were primary caregivers to a family member with cancer; an additional 19 reported that they had family members with cancer (Table 2). Most of the navigators were women. The racial/ethnic distribution mirrored the populations they served: white (29%); black or African American (31%); and Hispanic (37%). English was the only spoken language of 67% of the navigators; 27% spoke Spanish, and 6% reported speaking another language. Most had a college degree (63%).
The unadjusted bivariate comparison of patients who achieved diagnostic resolution within 365 days, by navigator experience with cancer, are shown in Table 3. We found no difference in time to diagnostic resolution for those patients for whom navigators had personal experience with cancer compared with those whose navigators had no experience. When stratified by type of cancer screening abnormality (breast, cervical, prostate, or colorectal), the results also did not reveal a significant difference in the proportion of patients achieving diagnostic resolution by 365 days by navigator experience with cancer.
In the Cox proportional hazard model adjusting for patient gender, age, race/ethnicity, cancer type, and adjusting for navigator using clustering, there was no difference between patients whose navigators had experience with cancer care, and those who did not (adjusted hazard ratio, 1.03; 95% confidence interval, .83-1.3). The level of education of navigators was not significantly associated with time to diagnostic resolution for patients.
Discussion
Although several cancer support programs have explicitly used cancer survivors as patient navigators or other supports for patients in active cancer care, there are scant data on whether this expertise improves care. Our study was not able to identify that navigators with previous experience with cancer care, either as a patient or as the primary caregiver, was associated with improved time to diagnostic resolution.
As patient navigation has become the standard of cancer diagnostic and treatment practices, there is a need to develop competencies and standards for hiring and training navigators. Part of this hiring process is to determine what past experience and training are relevant for effective navigation. There is little previous research on relevant skills of navigators, with only one study having demonstrated that language and racial/ ethnic concordance between patients and navigators was associated with more timely care. The national PNRP program hired mostly lay navigators with minimal medical experience, but with affiliations to the communities of the patients receiving care. Our program has demonstrated that lay individuals can be trained in the logistic aspects of navigation.5 Although it may seem intuitive that the experience of being a cancer survivor may make a navigator more empathetic, it is also possible that being too close to the experience of survivorship can also pose challenges to a navigator. Alternatively, navigation may be equally effective with proper training regardless of previous experience with cancer.
Our study is limited to addressing the outcome of timely resolution in the diagnostic phase of care after abnormal cancer screening. It is possible that past experience with cancer care will be beneficial when providing navigation for cancer care. While this study represents one of the largest groups of navigators who have been studied, the small sample may have limited our ability to detect differences. Our study has the benefit of a diverse group of navigators from a nationally representative, multi-site study. We suggest that prior experience with cancer care is not a prerequisite to supporting diagnostic care after abnormal cancer screening. Providing appropriate training to navigators may be sufficient to ensure effective and appropriate care is provided by patient navigators.
Acknowledgments
The authors acknowledge the contributions of the following members of the Patient Navigation Research Program:Clinical centers Boston Medical Center and Boston University: Karen M Freund (principal investigator [PI]), Tracy A Battaglia (co-PI); Denver Health and Hospital Authority: Peter Raich (PI), Elizabeth Whitley (co-PI); George Washington University Cancer Institute: Steven R Patierno (PI), Lisa M Alexander, Paul H Levine, Heather A Young, Heather J Hoffman, Nancy L LaVerda; H Lee Moffitt Cancer Center and Research Institute: Richard G Roetzheim (PI), Cathy Meade, Kristen J Wells; Northwest Portland Area Indian Health Board: Victoria Warren-Mears (PI); Northwestern University Robert H Lurie Comprehensive Cancer Center: Steven Rosen (PI), Melissa Simon; The Ohio State University Comprehensive Cancer Center: Electra Paskett (PI); University of Illinois at Chicago and Access Community Health Center: Elizabeth Calhoun (PI), Julie Darnell. University of Rochester: Kevin Fiscella (PI), Samantha Hendren; University of Texas Health Science Center at San Antonio Cancer Therapy and Research Center: Donald Dudley (PI), Kevin Hall, Anand Karnard, Amelie Ramirez. Program office National Cancer Institute, Center to Reduce Cancer Health Disparities: Martha Hare, Mollie Howerton, Ken Chu, Emmanuel Taylor, Mary Ann Van Dyun. Evaluation contractor NOVA Research Company: Paul Young, Frederick Snyder
Patient navigation has emerged in the past decade as a strategy to decrease cancer disparities among low-income, minority populations. Patient navigators help individuals who face personal and systemtic barriers to gaining access to care.1 Their role is to help patients find their way through a complex health care system,2,3 including logistic support of rescheduling appointments, assistance with transportation, and child care needs. They provide personal support, including coaching patients on their clinical visits, educating them about the cancer treatment process, and addressing their fears of diagnosis and treatment. Patient navigation has shown improvement in cancer screening rates, time to diagnostic resolution for those patients who have abnormal cancer screening tests, and quality of cancer care.4,5
In hiring patient navigators, it is not clear which professional training and skill sets and what personal experiences are most useful to becoming an effective navigator. Personal cancer experience can include a personal diagnosis, the experience of serving as a primary caregiver for a patient during treatment, or having a family member or close friend with cancer. Several current support programs specifically recruit cancer survivors on the assumption that their cancer treatment experience can provide helpful insights to a current patient for both emotional and logistical support.6 In this paper, we sought to address whether patient navigation promotes more timely diagnostic care if the navigator has experience with cancer.
Methods
This is a secondary analysis of the patients with abnormal cancer screening in the navigation arm of the national Patient Navigation Research Program (PNRP) study,1, 5 a collaborative effort across 10 centers to investigate the efficacy of patient navigation on improving patient-level outcomes for those who have abnormal results from a breast, cervical, colorectal, or prostate cancer screening test. The study demonstrated that patient navigation was effective in reducing delays in diagnosis and treatment5 and resulting in a higher quality of care,4 especially among vulnerable populations.7 The Institutional Review Board of each respective institution approved the research.
All of the patient navigators were paid employees with a minimum high-school diploma or equivalent. Navigators’ activities were standardized across centers through a national training program.8 Navigators used the care management model to identify and address barriers to care and to track participants throughout the course of their diagnostic evaluation,9 with the primary aim of timely diagnostic resolution. Most navigation programs were embedded within the clinical care system and interacted with patients through mail, by phone, and face-to-face contact.1
Data collection
Each center used agreed-upon inclusion and exclusion criteria and collected and coded the same patient-level data. Medical records were abstracted for pertinent clinical data on patients. Demographic data were collected through a patient survey or extracted from medical record registration. The central data coordinating center collected navigator information including demographic characteristics and experience with cancer.
We created a new variable, Personal experience with cancer. Personal experience with cancer was based on three questions asked of navigators: whether they were a cancer survivor; whether they were the primary caregiver to a family member or close friend with cancer; and whether they had a family member with cancer. Because of the small sample size, responses from navigators who were cancer survivors (n = 6) or primary caregivers to a family member with cancer (n = 4) were collapsed into a single category, referred to as personal experience with cancer, to compare with navigators who had no personal experience with cancer, which included those who reported a family member with cancer but who were not serving as a primary caregiver.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Each clinical center received approved from their institution’s human subjects review board. Informed consent was obtained from all patient navigator participants included in the study. Participating patients completed informed consent at some centers. At other centers where the study design was an implementation of a system intervention, a waiver of informed consent was approved by the Institutional Review Board.
Data analysis
The primary outcome variable was time to diagnostic resolution. We included only participants supported by a single navigator. A Fisher exact test by cancer type was used to compare the two groups (personal experience vs none) in the proportion of patients who achieved diagnostic resolution by 365 days. We reviewed the percentage of patients resolved for the total population as well as stratified by cancer site (breast, cervical, prostate, and colorectal), owing to the known mean differences in time to diagnostic resolution by type of cancer.
Cox proportional hazard models and adjusted hazard ratios were developed and calculated to examine the impact of navigator’s personal experience with cancer on time to resolution, controlling for patient gender, race, age, and cancer type in the models. The analysis controlled for the individual effect of navigators through clustering. We used P < .05 as the cut-off for significance, and used Stata 10.1 (StataCorp, College Station Texas 77845) for all analyses.
Results
Our analytic sample included the 3,975 patients with only 1 navigator over the course of the study, 79% of the navigation (n = 5,063) arm. Most of the patients were women (93%), and most were from racial and ethnic minority communities. Most patients spoke English (60%), with Spanish (33%) as the next most common language. Most patients were publically insured (38%) or uninsured (40%) (Table 1).
Of the total 49 navigators, 6 were cancer survivors and 4 were primary caregivers to a family member with cancer; an additional 19 reported that they had family members with cancer (Table 2). Most of the navigators were women. The racial/ethnic distribution mirrored the populations they served: white (29%); black or African American (31%); and Hispanic (37%). English was the only spoken language of 67% of the navigators; 27% spoke Spanish, and 6% reported speaking another language. Most had a college degree (63%).
The unadjusted bivariate comparison of patients who achieved diagnostic resolution within 365 days, by navigator experience with cancer, are shown in Table 3. We found no difference in time to diagnostic resolution for those patients for whom navigators had personal experience with cancer compared with those whose navigators had no experience. When stratified by type of cancer screening abnormality (breast, cervical, prostate, or colorectal), the results also did not reveal a significant difference in the proportion of patients achieving diagnostic resolution by 365 days by navigator experience with cancer.
In the Cox proportional hazard model adjusting for patient gender, age, race/ethnicity, cancer type, and adjusting for navigator using clustering, there was no difference between patients whose navigators had experience with cancer care, and those who did not (adjusted hazard ratio, 1.03; 95% confidence interval, .83-1.3). The level of education of navigators was not significantly associated with time to diagnostic resolution for patients.
Discussion
Although several cancer support programs have explicitly used cancer survivors as patient navigators or other supports for patients in active cancer care, there are scant data on whether this expertise improves care. Our study was not able to identify that navigators with previous experience with cancer care, either as a patient or as the primary caregiver, was associated with improved time to diagnostic resolution.
As patient navigation has become the standard of cancer diagnostic and treatment practices, there is a need to develop competencies and standards for hiring and training navigators. Part of this hiring process is to determine what past experience and training are relevant for effective navigation. There is little previous research on relevant skills of navigators, with only one study having demonstrated that language and racial/ ethnic concordance between patients and navigators was associated with more timely care. The national PNRP program hired mostly lay navigators with minimal medical experience, but with affiliations to the communities of the patients receiving care. Our program has demonstrated that lay individuals can be trained in the logistic aspects of navigation.5 Although it may seem intuitive that the experience of being a cancer survivor may make a navigator more empathetic, it is also possible that being too close to the experience of survivorship can also pose challenges to a navigator. Alternatively, navigation may be equally effective with proper training regardless of previous experience with cancer.
Our study is limited to addressing the outcome of timely resolution in the diagnostic phase of care after abnormal cancer screening. It is possible that past experience with cancer care will be beneficial when providing navigation for cancer care. While this study represents one of the largest groups of navigators who have been studied, the small sample may have limited our ability to detect differences. Our study has the benefit of a diverse group of navigators from a nationally representative, multi-site study. We suggest that prior experience with cancer care is not a prerequisite to supporting diagnostic care after abnormal cancer screening. Providing appropriate training to navigators may be sufficient to ensure effective and appropriate care is provided by patient navigators.
Acknowledgments
The authors acknowledge the contributions of the following members of the Patient Navigation Research Program:Clinical centers Boston Medical Center and Boston University: Karen M Freund (principal investigator [PI]), Tracy A Battaglia (co-PI); Denver Health and Hospital Authority: Peter Raich (PI), Elizabeth Whitley (co-PI); George Washington University Cancer Institute: Steven R Patierno (PI), Lisa M Alexander, Paul H Levine, Heather A Young, Heather J Hoffman, Nancy L LaVerda; H Lee Moffitt Cancer Center and Research Institute: Richard G Roetzheim (PI), Cathy Meade, Kristen J Wells; Northwest Portland Area Indian Health Board: Victoria Warren-Mears (PI); Northwestern University Robert H Lurie Comprehensive Cancer Center: Steven Rosen (PI), Melissa Simon; The Ohio State University Comprehensive Cancer Center: Electra Paskett (PI); University of Illinois at Chicago and Access Community Health Center: Elizabeth Calhoun (PI), Julie Darnell. University of Rochester: Kevin Fiscella (PI), Samantha Hendren; University of Texas Health Science Center at San Antonio Cancer Therapy and Research Center: Donald Dudley (PI), Kevin Hall, Anand Karnard, Amelie Ramirez. Program office National Cancer Institute, Center to Reduce Cancer Health Disparities: Martha Hare, Mollie Howerton, Ken Chu, Emmanuel Taylor, Mary Ann Van Dyun. Evaluation contractor NOVA Research Company: Paul Young, Frederick Snyder
6. Macvean ML, White VM, Sanson-Fisher R. One-to-one volunteer support programs for people with cancer: a review of the literature. Patient Educ Couns. 2008;70:10-24.
7. Rodday AM, Parsons SK, Snyder F, et al. The impact of patient navigation in eliminating economic disparities in cancer care. Cancer. 2015;121(22):4025-4034.
10. Charlot M, Santana MC, Chen CA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477-1483.
6. Macvean ML, White VM, Sanson-Fisher R. One-to-one volunteer support programs for people with cancer: a review of the literature. Patient Educ Couns. 2008;70:10-24.
7. Rodday AM, Parsons SK, Snyder F, et al. The impact of patient navigation in eliminating economic disparities in cancer care. Cancer. 2015;121(22):4025-4034.
10. Charlot M, Santana MC, Chen CA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477-1483.
Imfinzi approved for stage III unresectable NSCLC
Durvalumab (Imfinzi) has been approved as the first treatment for patients with stage III unresectable non-small cell lung cancer (NSCLC) that has not progressed after chemotherapy and radiation, the Food and Drug Administration announced on Feb. 16.
Chemoradiation had been the only option for such patients. “Although a small number of patients may be cured with the chemoradiation, the cancer may eventually progress. Patients now have an approved therapy that has been shown to keep the cancer from progressing for a longer time after chemoradiation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
Durvalumab, which targets the PD-1/PD-L1 pathway, was previously granted accelerated approval in 2017 for the treatment of certain patients with locally advanced or metastatic bladder cancer.
The approval for the treatment of stage III, unresectable NSCLC was based on the results of the randomized PACIFIC trial of 713 patients whose cancer had not progressed after completing chemotherapy and radiation. The trial measured progression-free survival with durvalumab or a placebo, and found a median progression-free survival of 16.8 months for patients taking durvalumab and 5.6 months for patients receiving a placebo. The drug's sponsor, AstraZeneca, has agreed to provide data on overall survival in the study.
Common side effects of durvalumab in patients with stage III unresectable NSCLC include cough, fatigue, inflammation in the lungs (pneumonitis/radiation pneumonitis), upper respiratory tract infections, difficulty breathing (dyspnea) and rash.
Serious risks of durvalumab include pneumonitis, hepatitis, colitis, endocrinopathies, and nephritis. Other serious side effects include infection and infusion-related reactions. Durvalumab can cause harm to a developing fetus; women should be advised of the potential risk to the fetus and to use effective contraception.
Durvalumab (Imfinzi) has been approved as the first treatment for patients with stage III unresectable non-small cell lung cancer (NSCLC) that has not progressed after chemotherapy and radiation, the Food and Drug Administration announced on Feb. 16.
Chemoradiation had been the only option for such patients. “Although a small number of patients may be cured with the chemoradiation, the cancer may eventually progress. Patients now have an approved therapy that has been shown to keep the cancer from progressing for a longer time after chemoradiation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
Durvalumab, which targets the PD-1/PD-L1 pathway, was previously granted accelerated approval in 2017 for the treatment of certain patients with locally advanced or metastatic bladder cancer.
The approval for the treatment of stage III, unresectable NSCLC was based on the results of the randomized PACIFIC trial of 713 patients whose cancer had not progressed after completing chemotherapy and radiation. The trial measured progression-free survival with durvalumab or a placebo, and found a median progression-free survival of 16.8 months for patients taking durvalumab and 5.6 months for patients receiving a placebo. The drug's sponsor, AstraZeneca, has agreed to provide data on overall survival in the study.
Common side effects of durvalumab in patients with stage III unresectable NSCLC include cough, fatigue, inflammation in the lungs (pneumonitis/radiation pneumonitis), upper respiratory tract infections, difficulty breathing (dyspnea) and rash.
Serious risks of durvalumab include pneumonitis, hepatitis, colitis, endocrinopathies, and nephritis. Other serious side effects include infection and infusion-related reactions. Durvalumab can cause harm to a developing fetus; women should be advised of the potential risk to the fetus and to use effective contraception.
Durvalumab (Imfinzi) has been approved as the first treatment for patients with stage III unresectable non-small cell lung cancer (NSCLC) that has not progressed after chemotherapy and radiation, the Food and Drug Administration announced on Feb. 16.
Chemoradiation had been the only option for such patients. “Although a small number of patients may be cured with the chemoradiation, the cancer may eventually progress. Patients now have an approved therapy that has been shown to keep the cancer from progressing for a longer time after chemoradiation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
Durvalumab, which targets the PD-1/PD-L1 pathway, was previously granted accelerated approval in 2017 for the treatment of certain patients with locally advanced or metastatic bladder cancer.
The approval for the treatment of stage III, unresectable NSCLC was based on the results of the randomized PACIFIC trial of 713 patients whose cancer had not progressed after completing chemotherapy and radiation. The trial measured progression-free survival with durvalumab or a placebo, and found a median progression-free survival of 16.8 months for patients taking durvalumab and 5.6 months for patients receiving a placebo. The drug's sponsor, AstraZeneca, has agreed to provide data on overall survival in the study.
Common side effects of durvalumab in patients with stage III unresectable NSCLC include cough, fatigue, inflammation in the lungs (pneumonitis/radiation pneumonitis), upper respiratory tract infections, difficulty breathing (dyspnea) and rash.
Serious risks of durvalumab include pneumonitis, hepatitis, colitis, endocrinopathies, and nephritis. Other serious side effects include infection and infusion-related reactions. Durvalumab can cause harm to a developing fetus; women should be advised of the potential risk to the fetus and to use effective contraception.