User login
Mortality estimates put pancreatic cancer on the map
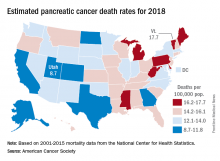
The alphabet may put Vermont right after Utah, but pancreatic cancer mortality has them on opposite sides of the country.
The expected number of deaths for 2018 and the current U.S. population estimate of nearly 326 million produce an expected death rate of 13.6 per 100,000 population. The Census Bureau estimates for the state populations, the deaths projected by the ACS, and a little math result in expected death rates of 8.7 per 100,000 for Utah and 17.7 for Vermont.
The incidence of pancreatic cancer increased by about 1% per year in whites and was stable in blacks from 2005 to 2014, although the rate of new cases in blacks is still about 25% higher than it is for whites. An estimated 55,440 new cases are expected in the United States in 2018, the ACS reported.
Although the survival rate for cancer of the pancreas has tripled since the mid-1970s, it is still much lower than for any other cancer. Five-year relative survival went from 3% in 1975-1977 to 9% in 2007-2013 for pancreatic cancer, while liver and intrahepatic bile duct cancer, which had the next-lowest survival rate, went from 3% to 19%, the ACS said.
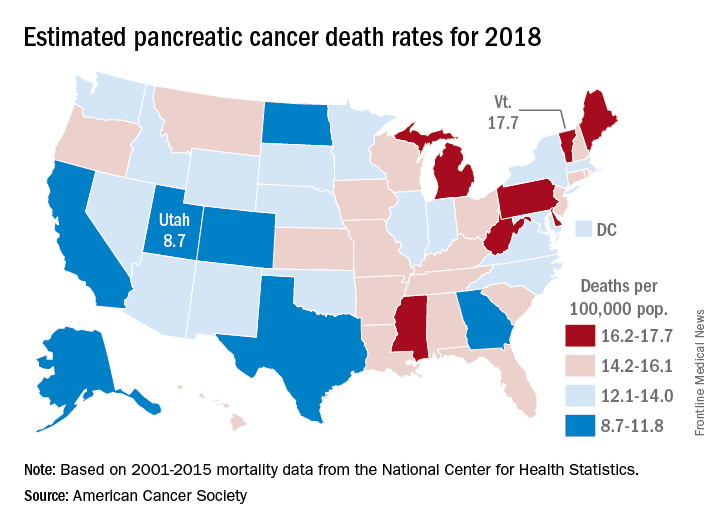
The alphabet may put Vermont right after Utah, but pancreatic cancer mortality has them on opposite sides of the country.
The expected number of deaths for 2018 and the current U.S. population estimate of nearly 326 million produce an expected death rate of 13.6 per 100,000 population. The Census Bureau estimates for the state populations, the deaths projected by the ACS, and a little math result in expected death rates of 8.7 per 100,000 for Utah and 17.7 for Vermont.
The incidence of pancreatic cancer increased by about 1% per year in whites and was stable in blacks from 2005 to 2014, although the rate of new cases in blacks is still about 25% higher than it is for whites. An estimated 55,440 new cases are expected in the United States in 2018, the ACS reported.
Although the survival rate for cancer of the pancreas has tripled since the mid-1970s, it is still much lower than for any other cancer. Five-year relative survival went from 3% in 1975-1977 to 9% in 2007-2013 for pancreatic cancer, while liver and intrahepatic bile duct cancer, which had the next-lowest survival rate, went from 3% to 19%, the ACS said.
The alphabet may put Vermont right after Utah, but pancreatic cancer mortality has them on opposite sides of the country.
The expected number of deaths for 2018 and the current U.S. population estimate of nearly 326 million produce an expected death rate of 13.6 per 100,000 population. The Census Bureau estimates for the state populations, the deaths projected by the ACS, and a little math result in expected death rates of 8.7 per 100,000 for Utah and 17.7 for Vermont.
The incidence of pancreatic cancer increased by about 1% per year in whites and was stable in blacks from 2005 to 2014, although the rate of new cases in blacks is still about 25% higher than it is for whites. An estimated 55,440 new cases are expected in the United States in 2018, the ACS reported.
Although the survival rate for cancer of the pancreas has tripled since the mid-1970s, it is still much lower than for any other cancer. Five-year relative survival went from 3% in 1975-1977 to 9% in 2007-2013 for pancreatic cancer, while liver and intrahepatic bile duct cancer, which had the next-lowest survival rate, went from 3% to 19%, the ACS said.
Legacy Society members sustain research
AGA Legacy Society members share a desire to guarantee long-term support for digestive disease research. Through their foresight and generosity, they help ensure the continued momentum of discovery and patient education that has characterized GI medicine in recent decades. Legacy Society member donations directly support young GI investigators as they establish independent research careers.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
AGA members support young researchers at a critical decision point in their lives – when many consider giving up their research careers due to a lack of funding. “I am honored to be a recipient of the Research Scholar Award. I would like to thank the foundation for their generous contribution that will fund a crucial transition in my career,” said Jose Saenz, MD, PhD, Washington University School of Medicine and 2017 AGA – Gastric Cancer Foundation Research Scholar Award recipient.
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. Gifts to the foundation support researchers working towards developing new treatments and diagnostics for patients with GI conditions.
“I am extremely grateful to be selected for this award. I would like to thank the foundation donors for their generous support. This award will me build a research program to better understand mechanisms that promote growth of cholangiocarcinoma,” remarks Silvia Affe, PhD, Columbia University, 2017 AGA Research Scholar recipient.
Donors who make gifts at the Legacy Society level before DDW will receive an invitation to the annual Benefactors’ Dinner at the Folger Shakespeare Library in Washington, DC. Individuals interested in learning more about Legacy Society membership may contact Stacey Hinton Tuneski, Senior Director of Development at stuneski@gastro.org or via phone (301) 222-4005. More information on the AGA Legacy Society including the current roster and acceptance form is available on the foundation’s website at www.gastro.org/legacysociety.
The makings of a grand celebration
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The Folger Shakespeare Library will be the location of the 2018 AGA Research Foundation Benefactors Dinner during DDW in Washington, DC. Just steps from the Capitol, the Great Hall and Pastor Reading room are a spectacular setting for an enjoyable evening with friends. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
AGA Legacy Society members share a desire to guarantee long-term support for digestive disease research. Through their foresight and generosity, they help ensure the continued momentum of discovery and patient education that has characterized GI medicine in recent decades. Legacy Society member donations directly support young GI investigators as they establish independent research careers.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
AGA members support young researchers at a critical decision point in their lives – when many consider giving up their research careers due to a lack of funding. “I am honored to be a recipient of the Research Scholar Award. I would like to thank the foundation for their generous contribution that will fund a crucial transition in my career,” said Jose Saenz, MD, PhD, Washington University School of Medicine and 2017 AGA – Gastric Cancer Foundation Research Scholar Award recipient.
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. Gifts to the foundation support researchers working towards developing new treatments and diagnostics for patients with GI conditions.
“I am extremely grateful to be selected for this award. I would like to thank the foundation donors for their generous support. This award will me build a research program to better understand mechanisms that promote growth of cholangiocarcinoma,” remarks Silvia Affe, PhD, Columbia University, 2017 AGA Research Scholar recipient.
Donors who make gifts at the Legacy Society level before DDW will receive an invitation to the annual Benefactors’ Dinner at the Folger Shakespeare Library in Washington, DC. Individuals interested in learning more about Legacy Society membership may contact Stacey Hinton Tuneski, Senior Director of Development at stuneski@gastro.org or via phone (301) 222-4005. More information on the AGA Legacy Society including the current roster and acceptance form is available on the foundation’s website at www.gastro.org/legacysociety.
The makings of a grand celebration
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The Folger Shakespeare Library will be the location of the 2018 AGA Research Foundation Benefactors Dinner during DDW in Washington, DC. Just steps from the Capitol, the Great Hall and Pastor Reading room are a spectacular setting for an enjoyable evening with friends. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
AGA Legacy Society members share a desire to guarantee long-term support for digestive disease research. Through their foresight and generosity, they help ensure the continued momentum of discovery and patient education that has characterized GI medicine in recent decades. Legacy Society member donations directly support young GI investigators as they establish independent research careers.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
AGA members support young researchers at a critical decision point in their lives – when many consider giving up their research careers due to a lack of funding. “I am honored to be a recipient of the Research Scholar Award. I would like to thank the foundation for their generous contribution that will fund a crucial transition in my career,” said Jose Saenz, MD, PhD, Washington University School of Medicine and 2017 AGA – Gastric Cancer Foundation Research Scholar Award recipient.
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. Gifts to the foundation support researchers working towards developing new treatments and diagnostics for patients with GI conditions.
“I am extremely grateful to be selected for this award. I would like to thank the foundation donors for their generous support. This award will me build a research program to better understand mechanisms that promote growth of cholangiocarcinoma,” remarks Silvia Affe, PhD, Columbia University, 2017 AGA Research Scholar recipient.
Donors who make gifts at the Legacy Society level before DDW will receive an invitation to the annual Benefactors’ Dinner at the Folger Shakespeare Library in Washington, DC. Individuals interested in learning more about Legacy Society membership may contact Stacey Hinton Tuneski, Senior Director of Development at stuneski@gastro.org or via phone (301) 222-4005. More information on the AGA Legacy Society including the current roster and acceptance form is available on the foundation’s website at www.gastro.org/legacysociety.
The makings of a grand celebration
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The Folger Shakespeare Library will be the location of the 2018 AGA Research Foundation Benefactors Dinner during DDW in Washington, DC. Just steps from the Capitol, the Great Hall and Pastor Reading room are a spectacular setting for an enjoyable evening with friends. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
MDedge Daily News: The Nonallergen of the Year
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Parabens are named “nonallergen” of the year, a gene test guides need for sentinel node biopsy in elderly melanoma patients, select atopic dermatitis patients need patch testing, and try these real solutions for delusional parasitosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Parabens are named “nonallergen” of the year, a gene test guides need for sentinel node biopsy in elderly melanoma patients, select atopic dermatitis patients need patch testing, and try these real solutions for delusional parasitosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Parabens are named “nonallergen” of the year, a gene test guides need for sentinel node biopsy in elderly melanoma patients, select atopic dermatitis patients need patch testing, and try these real solutions for delusional parasitosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
AGA Pres. Sheila Crowe Spends the Day on Capitol Hill
AGA President Sheila Crowe, MD, FRCPC, FACP, FACG, AGAF, recently spent the day on Capitol Hill meeting with lawmakers to advocate for AGA legislative priorities including increasing funding for NIH and biomedical research, support for the Removing Barriers to Colorectal Cancer Screening Act, and support for the Restoring the Patient’s Voice Act. Dr. Crowe met with eight congressional offices and received helpful feedback on the upcoming agenda in Congress and how it impacts AGA’s priorities.
NIH funding
Removing Barriers to Colorectal Cancer Screening Act
Fixing the current coinsurance problem for Medicare beneficiaries who undergo a screening colonoscopy that becomes therapeutic remains a top AGA priority. Most of the offices that Dr. Crowe met with were cosponsors of the legislation, the Removing Barriers to Colorectal Cancer Screening Act (HR 1017/S.479), that would waive coinsurance payment regardless of the screening outcome. Dr. Crowe shared her experience with patients and the financial burden this places on beneficiaries who need to be screened. Rep. Raul Ruiz, D-CA, and Rep. Scott Peters, D-CA, both members of the House Energy and Commerce Committee and supporters of the bill, will continue to advocate that the bill receive a hearing this year to help move it through Congress. The bill continues to have wide bipartisan support. Read more about the issue and how you can explain it to your patients.
Step therapy
More and more patients are being subject to step therapy protocols, also known as “fail first” under which they are required to try and fail sometimes two or three therapies before receiving coverage of the initial therapy recommended by their physician. With the emergence of new biologics to treat diseases like inflammatory bowel disease, more and more digestive disease patients are being subject to these protocols, which can have adverse effects on their health. Restoring the Patient’s Voice Act (HR 2077) would provide patients and providers with a fair and equitable appeals process when step therapy has been imposed and provides common sense exceptions for the provider to appeal. Dr. Crowe spoke of the impact this policy is having on digestive disease patients and the burden it puts on physician practices that have to take time away from patients to navigate the convoluted insurance appeals process. We are hopeful that many of the offices that we met with will support HR 2077. Read more about the issue.
Food is Medicine Working Group
Dr. Crowe also had a productive meeting with Rep. Jim McGovern’s, D-MA, office and learned more about the recently created Food is Medicine Working Group that he has initiated. McGovern is the Ranking Member of the Agriculture Committee’s Subcommittee on Nutrition which is responsible for our nation’s nutrition guidelines and the Supplemental Nutrition Assistance Program. The Working Group will focus on costs related to hunger and the importance of nutrition in treating chronic illness and disease. AGA looks forward to working with McGovern and members of the Working Group on this bipartisan initiative.
Capitol Hill needs to hear the voice of GI
In conjunction with Dr. Crowe’s visit, AGA launched a Virtual Advocacy Day to encourage members to contact their legislators in support of the issues that Dr. Crowe was advocating during her meetings. We thank those members who took time out of their schedules to take action.
AGA President Sheila Crowe, MD, FRCPC, FACP, FACG, AGAF, recently spent the day on Capitol Hill meeting with lawmakers to advocate for AGA legislative priorities including increasing funding for NIH and biomedical research, support for the Removing Barriers to Colorectal Cancer Screening Act, and support for the Restoring the Patient’s Voice Act. Dr. Crowe met with eight congressional offices and received helpful feedback on the upcoming agenda in Congress and how it impacts AGA’s priorities.
NIH funding
Removing Barriers to Colorectal Cancer Screening Act
Fixing the current coinsurance problem for Medicare beneficiaries who undergo a screening colonoscopy that becomes therapeutic remains a top AGA priority. Most of the offices that Dr. Crowe met with were cosponsors of the legislation, the Removing Barriers to Colorectal Cancer Screening Act (HR 1017/S.479), that would waive coinsurance payment regardless of the screening outcome. Dr. Crowe shared her experience with patients and the financial burden this places on beneficiaries who need to be screened. Rep. Raul Ruiz, D-CA, and Rep. Scott Peters, D-CA, both members of the House Energy and Commerce Committee and supporters of the bill, will continue to advocate that the bill receive a hearing this year to help move it through Congress. The bill continues to have wide bipartisan support. Read more about the issue and how you can explain it to your patients.
Step therapy
More and more patients are being subject to step therapy protocols, also known as “fail first” under which they are required to try and fail sometimes two or three therapies before receiving coverage of the initial therapy recommended by their physician. With the emergence of new biologics to treat diseases like inflammatory bowel disease, more and more digestive disease patients are being subject to these protocols, which can have adverse effects on their health. Restoring the Patient’s Voice Act (HR 2077) would provide patients and providers with a fair and equitable appeals process when step therapy has been imposed and provides common sense exceptions for the provider to appeal. Dr. Crowe spoke of the impact this policy is having on digestive disease patients and the burden it puts on physician practices that have to take time away from patients to navigate the convoluted insurance appeals process. We are hopeful that many of the offices that we met with will support HR 2077. Read more about the issue.
Food is Medicine Working Group
Dr. Crowe also had a productive meeting with Rep. Jim McGovern’s, D-MA, office and learned more about the recently created Food is Medicine Working Group that he has initiated. McGovern is the Ranking Member of the Agriculture Committee’s Subcommittee on Nutrition which is responsible for our nation’s nutrition guidelines and the Supplemental Nutrition Assistance Program. The Working Group will focus on costs related to hunger and the importance of nutrition in treating chronic illness and disease. AGA looks forward to working with McGovern and members of the Working Group on this bipartisan initiative.
Capitol Hill needs to hear the voice of GI
In conjunction with Dr. Crowe’s visit, AGA launched a Virtual Advocacy Day to encourage members to contact their legislators in support of the issues that Dr. Crowe was advocating during her meetings. We thank those members who took time out of their schedules to take action.
AGA President Sheila Crowe, MD, FRCPC, FACP, FACG, AGAF, recently spent the day on Capitol Hill meeting with lawmakers to advocate for AGA legislative priorities including increasing funding for NIH and biomedical research, support for the Removing Barriers to Colorectal Cancer Screening Act, and support for the Restoring the Patient’s Voice Act. Dr. Crowe met with eight congressional offices and received helpful feedback on the upcoming agenda in Congress and how it impacts AGA’s priorities.
NIH funding
Removing Barriers to Colorectal Cancer Screening Act
Fixing the current coinsurance problem for Medicare beneficiaries who undergo a screening colonoscopy that becomes therapeutic remains a top AGA priority. Most of the offices that Dr. Crowe met with were cosponsors of the legislation, the Removing Barriers to Colorectal Cancer Screening Act (HR 1017/S.479), that would waive coinsurance payment regardless of the screening outcome. Dr. Crowe shared her experience with patients and the financial burden this places on beneficiaries who need to be screened. Rep. Raul Ruiz, D-CA, and Rep. Scott Peters, D-CA, both members of the House Energy and Commerce Committee and supporters of the bill, will continue to advocate that the bill receive a hearing this year to help move it through Congress. The bill continues to have wide bipartisan support. Read more about the issue and how you can explain it to your patients.
Step therapy
More and more patients are being subject to step therapy protocols, also known as “fail first” under which they are required to try and fail sometimes two or three therapies before receiving coverage of the initial therapy recommended by their physician. With the emergence of new biologics to treat diseases like inflammatory bowel disease, more and more digestive disease patients are being subject to these protocols, which can have adverse effects on their health. Restoring the Patient’s Voice Act (HR 2077) would provide patients and providers with a fair and equitable appeals process when step therapy has been imposed and provides common sense exceptions for the provider to appeal. Dr. Crowe spoke of the impact this policy is having on digestive disease patients and the burden it puts on physician practices that have to take time away from patients to navigate the convoluted insurance appeals process. We are hopeful that many of the offices that we met with will support HR 2077. Read more about the issue.
Food is Medicine Working Group
Dr. Crowe also had a productive meeting with Rep. Jim McGovern’s, D-MA, office and learned more about the recently created Food is Medicine Working Group that he has initiated. McGovern is the Ranking Member of the Agriculture Committee’s Subcommittee on Nutrition which is responsible for our nation’s nutrition guidelines and the Supplemental Nutrition Assistance Program. The Working Group will focus on costs related to hunger and the importance of nutrition in treating chronic illness and disease. AGA looks forward to working with McGovern and members of the Working Group on this bipartisan initiative.
Capitol Hill needs to hear the voice of GI
In conjunction with Dr. Crowe’s visit, AGA launched a Virtual Advocacy Day to encourage members to contact their legislators in support of the issues that Dr. Crowe was advocating during her meetings. We thank those members who took time out of their schedules to take action.
Headlines from the 2018 Gastrointestinal Cancers Symposium
The 2018 Gastrointestinal Cancers Symposium took place Jan. 18-20, 2018, in San Francisco. During the meeting, investigators presented groundbreaking research designed to improve the diagnosis and treatment of GI cancers. Here are some of the most noteworthy headlines from the 2018 meeting.
Promising Results Using Liquid Biopsy to Improve CRC Early Detection
Researchers in Taiwan developed a screening test for early colorectal cancer (CRC) detection that requires a simple blood draw to assess for circulating tumor cells in the blood. The test demonstrates 88% accuracy to detect all stages of colorectal illness, including precancerous lesions. If validated and made commercially available, this test could be readily integrated into a patient’s routine physical exam, thereby increasing CRC screening compliance.
CELESTIAL Results May Lead to Cabozantinib Approval in Second-Line HCC
The phase III CELESTIAL trial met its primary endpoint by demonstrating a survival advantage with cabozantinib in patients with advanced hepatocellular carcinoma (HCC) that progressed following prior systemic therapy. Other outcomes included improvements in progression-free survival and objective response rate, as well as an acceptable safety profile, thus positioning cabozantinib for potential approval in the second-line setting in HCC.
RAINFALL Meets Primary Endpoint, But Ramucirumab Will Not Be Pursued for a First-Line Indication in G-GEJ Cancer
Results of the global, randomized, double-blind, placebo-controlled, phase III RAINFALL trial established the statistical benefit of ramucirumab, a monoclonal antibody targeting VEGFR-2, added to standard chemotherapy for patients with previously untreated metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. The findings revealed a significant 25% reduction in the risk of disease progression or death for the primary endpoint of progression-free survival (PFS). However, the reduction corresponded to only a 9-day improvement in median PFS, so the clinical benefit of frontline ramucirumab is debatable.
The Gastrointestinal Cancers Symposium is cosponsored by AGA, the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO).
More news from the 2018 Gastrointestinal Cancers Symposium is available at gicasym.org/daily-news.
The 2018 Gastrointestinal Cancers Symposium took place Jan. 18-20, 2018, in San Francisco. During the meeting, investigators presented groundbreaking research designed to improve the diagnosis and treatment of GI cancers. Here are some of the most noteworthy headlines from the 2018 meeting.
Promising Results Using Liquid Biopsy to Improve CRC Early Detection
Researchers in Taiwan developed a screening test for early colorectal cancer (CRC) detection that requires a simple blood draw to assess for circulating tumor cells in the blood. The test demonstrates 88% accuracy to detect all stages of colorectal illness, including precancerous lesions. If validated and made commercially available, this test could be readily integrated into a patient’s routine physical exam, thereby increasing CRC screening compliance.
CELESTIAL Results May Lead to Cabozantinib Approval in Second-Line HCC
The phase III CELESTIAL trial met its primary endpoint by demonstrating a survival advantage with cabozantinib in patients with advanced hepatocellular carcinoma (HCC) that progressed following prior systemic therapy. Other outcomes included improvements in progression-free survival and objective response rate, as well as an acceptable safety profile, thus positioning cabozantinib for potential approval in the second-line setting in HCC.
RAINFALL Meets Primary Endpoint, But Ramucirumab Will Not Be Pursued for a First-Line Indication in G-GEJ Cancer
Results of the global, randomized, double-blind, placebo-controlled, phase III RAINFALL trial established the statistical benefit of ramucirumab, a monoclonal antibody targeting VEGFR-2, added to standard chemotherapy for patients with previously untreated metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. The findings revealed a significant 25% reduction in the risk of disease progression or death for the primary endpoint of progression-free survival (PFS). However, the reduction corresponded to only a 9-day improvement in median PFS, so the clinical benefit of frontline ramucirumab is debatable.
The Gastrointestinal Cancers Symposium is cosponsored by AGA, the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO).
More news from the 2018 Gastrointestinal Cancers Symposium is available at gicasym.org/daily-news.
The 2018 Gastrointestinal Cancers Symposium took place Jan. 18-20, 2018, in San Francisco. During the meeting, investigators presented groundbreaking research designed to improve the diagnosis and treatment of GI cancers. Here are some of the most noteworthy headlines from the 2018 meeting.
Promising Results Using Liquid Biopsy to Improve CRC Early Detection
Researchers in Taiwan developed a screening test for early colorectal cancer (CRC) detection that requires a simple blood draw to assess for circulating tumor cells in the blood. The test demonstrates 88% accuracy to detect all stages of colorectal illness, including precancerous lesions. If validated and made commercially available, this test could be readily integrated into a patient’s routine physical exam, thereby increasing CRC screening compliance.
CELESTIAL Results May Lead to Cabozantinib Approval in Second-Line HCC
The phase III CELESTIAL trial met its primary endpoint by demonstrating a survival advantage with cabozantinib in patients with advanced hepatocellular carcinoma (HCC) that progressed following prior systemic therapy. Other outcomes included improvements in progression-free survival and objective response rate, as well as an acceptable safety profile, thus positioning cabozantinib for potential approval in the second-line setting in HCC.
RAINFALL Meets Primary Endpoint, But Ramucirumab Will Not Be Pursued for a First-Line Indication in G-GEJ Cancer
Results of the global, randomized, double-blind, placebo-controlled, phase III RAINFALL trial established the statistical benefit of ramucirumab, a monoclonal antibody targeting VEGFR-2, added to standard chemotherapy for patients with previously untreated metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. The findings revealed a significant 25% reduction in the risk of disease progression or death for the primary endpoint of progression-free survival (PFS). However, the reduction corresponded to only a 9-day improvement in median PFS, so the clinical benefit of frontline ramucirumab is debatable.
The Gastrointestinal Cancers Symposium is cosponsored by AGA, the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO).
More news from the 2018 Gastrointestinal Cancers Symposium is available at gicasym.org/daily-news.
Tamibarotene shows strong results in high-risk APL patients
ATLANTA – Maintenance therapy with the synthetic retinoid tamibarotene is more effective than all-trans retinoic acid (ATRA), for decreasing the relapse rate in patients with acute promyelocytic leukemia (APL) – a subtype of acute myeloid leukemia, according to 7-year findings from the JALSG-APL204 randomized controlled trial.
The relapse-free survival findings were particularly pronounced among high-risk patients with leukocyte counts of at least 10,000 per microliter, Akihiro Takeshita, MD, PhD, reported at the annual meeting of the American Society of Hematology.
“These results could lead to a new strategy for the treatment of high-risk patients, which is one of the recent priority issues in the treatment of APL,” said Dr. Takeshita of Hamamatsu (Japan) University.
Of 344 eligible patients aged 15-70 years with newly diagnosed APL and documented cytogenetic and/or molecular evidence of chromosomal translocation t(15;17) or PML/RAR-alpha gene expression, 269 entered the maintenance phase of the study after completing three courses of consolidation therapy and were assigned to receive ATRA or tamibarotene. At a mean follow-up of 7 years, the relapse-free survival rate was 84% in the 135 patients in the ATRA arm, compared with 93% among the 134 patients in the tamibarotene arm.
The difference between the groups was statistically significant, but an even greater difference was seen when the analysis was restricted to 52 high-risk patients with an initial leukocyte count of at least 10,000 per microliter (62% vs. 89%).
Both treatments were generally well tolerated, Dr. Takeshita reported.
Study subjects received ATRA at a daily dose of 45 mg/m2 for remission induction. Once complete remission was achieved, they received chemotherapy based on their initial leukocyte and blast count in the peripheral blood. Those who achieved molecular remission after consolidation chemotherapy were included in the current maintenance phase of the study. During this phase, ATRA was given at a daily dose of 45 mg/m2 divided into 3 doses for 14 days, and tamibarotene was given at a daily dose of 6 mg/m2 divided into 2 doses for 14 days. Each cycle of treatment was repeated every 3 months for 2 years.
Adverse events included secondary hematopoietic disorders in 12 cases, malignancies in 9 cases, and late cardiac complications of grade 3 or higher in 5 cases, but no significant difference in the rates of these events was seen between the two treatment groups, Dr. Takeshita noted.
Tamibarotene was studied in this trial because, compared with ATRA, it has been shown to have about a 10-fold increase in potency for inducing in vitro differentiation of NB-4 cells, enhanced chemical stability, and low affinity for cellular RA-binding protein.
“The clinical efficacy of tamibarotene for the treatment of APL has also been reported,” Dr. Takeshita added.
In the initial phases of the trial, no difference was seen between ATRA and tamibarotene with respect to 4-year relapse-free survival, but there did appear to be improved efficacy with tamibarotene in high-risk patients, which warranted further investigation, he said.
The current findings demonstrate the efficacy of tamibarotene vs. ATRA for decreasing the relapse rate at the 7-year observation point, and confirm the benefit in high-risk patients that was seen in earlier analyses, he concluded.
Dr. Takeshita reported receiving research funding from Chugai Pharmaceutical, Astellas Pharma, Pfizer Japan, and Takeda Pharmaceutical.
sworcester@frontlinemedcom.com
SOURCE: Takeshita A et al., ASH 2017, abstract 642.
ATLANTA – Maintenance therapy with the synthetic retinoid tamibarotene is more effective than all-trans retinoic acid (ATRA), for decreasing the relapse rate in patients with acute promyelocytic leukemia (APL) – a subtype of acute myeloid leukemia, according to 7-year findings from the JALSG-APL204 randomized controlled trial.
The relapse-free survival findings were particularly pronounced among high-risk patients with leukocyte counts of at least 10,000 per microliter, Akihiro Takeshita, MD, PhD, reported at the annual meeting of the American Society of Hematology.
“These results could lead to a new strategy for the treatment of high-risk patients, which is one of the recent priority issues in the treatment of APL,” said Dr. Takeshita of Hamamatsu (Japan) University.
Of 344 eligible patients aged 15-70 years with newly diagnosed APL and documented cytogenetic and/or molecular evidence of chromosomal translocation t(15;17) or PML/RAR-alpha gene expression, 269 entered the maintenance phase of the study after completing three courses of consolidation therapy and were assigned to receive ATRA or tamibarotene. At a mean follow-up of 7 years, the relapse-free survival rate was 84% in the 135 patients in the ATRA arm, compared with 93% among the 134 patients in the tamibarotene arm.
The difference between the groups was statistically significant, but an even greater difference was seen when the analysis was restricted to 52 high-risk patients with an initial leukocyte count of at least 10,000 per microliter (62% vs. 89%).
Both treatments were generally well tolerated, Dr. Takeshita reported.
Study subjects received ATRA at a daily dose of 45 mg/m2 for remission induction. Once complete remission was achieved, they received chemotherapy based on their initial leukocyte and blast count in the peripheral blood. Those who achieved molecular remission after consolidation chemotherapy were included in the current maintenance phase of the study. During this phase, ATRA was given at a daily dose of 45 mg/m2 divided into 3 doses for 14 days, and tamibarotene was given at a daily dose of 6 mg/m2 divided into 2 doses for 14 days. Each cycle of treatment was repeated every 3 months for 2 years.
Adverse events included secondary hematopoietic disorders in 12 cases, malignancies in 9 cases, and late cardiac complications of grade 3 or higher in 5 cases, but no significant difference in the rates of these events was seen between the two treatment groups, Dr. Takeshita noted.
Tamibarotene was studied in this trial because, compared with ATRA, it has been shown to have about a 10-fold increase in potency for inducing in vitro differentiation of NB-4 cells, enhanced chemical stability, and low affinity for cellular RA-binding protein.
“The clinical efficacy of tamibarotene for the treatment of APL has also been reported,” Dr. Takeshita added.
In the initial phases of the trial, no difference was seen between ATRA and tamibarotene with respect to 4-year relapse-free survival, but there did appear to be improved efficacy with tamibarotene in high-risk patients, which warranted further investigation, he said.
The current findings demonstrate the efficacy of tamibarotene vs. ATRA for decreasing the relapse rate at the 7-year observation point, and confirm the benefit in high-risk patients that was seen in earlier analyses, he concluded.
Dr. Takeshita reported receiving research funding from Chugai Pharmaceutical, Astellas Pharma, Pfizer Japan, and Takeda Pharmaceutical.
sworcester@frontlinemedcom.com
SOURCE: Takeshita A et al., ASH 2017, abstract 642.
ATLANTA – Maintenance therapy with the synthetic retinoid tamibarotene is more effective than all-trans retinoic acid (ATRA), for decreasing the relapse rate in patients with acute promyelocytic leukemia (APL) – a subtype of acute myeloid leukemia, according to 7-year findings from the JALSG-APL204 randomized controlled trial.
The relapse-free survival findings were particularly pronounced among high-risk patients with leukocyte counts of at least 10,000 per microliter, Akihiro Takeshita, MD, PhD, reported at the annual meeting of the American Society of Hematology.
“These results could lead to a new strategy for the treatment of high-risk patients, which is one of the recent priority issues in the treatment of APL,” said Dr. Takeshita of Hamamatsu (Japan) University.
Of 344 eligible patients aged 15-70 years with newly diagnosed APL and documented cytogenetic and/or molecular evidence of chromosomal translocation t(15;17) or PML/RAR-alpha gene expression, 269 entered the maintenance phase of the study after completing three courses of consolidation therapy and were assigned to receive ATRA or tamibarotene. At a mean follow-up of 7 years, the relapse-free survival rate was 84% in the 135 patients in the ATRA arm, compared with 93% among the 134 patients in the tamibarotene arm.
The difference between the groups was statistically significant, but an even greater difference was seen when the analysis was restricted to 52 high-risk patients with an initial leukocyte count of at least 10,000 per microliter (62% vs. 89%).
Both treatments were generally well tolerated, Dr. Takeshita reported.
Study subjects received ATRA at a daily dose of 45 mg/m2 for remission induction. Once complete remission was achieved, they received chemotherapy based on their initial leukocyte and blast count in the peripheral blood. Those who achieved molecular remission after consolidation chemotherapy were included in the current maintenance phase of the study. During this phase, ATRA was given at a daily dose of 45 mg/m2 divided into 3 doses for 14 days, and tamibarotene was given at a daily dose of 6 mg/m2 divided into 2 doses for 14 days. Each cycle of treatment was repeated every 3 months for 2 years.
Adverse events included secondary hematopoietic disorders in 12 cases, malignancies in 9 cases, and late cardiac complications of grade 3 or higher in 5 cases, but no significant difference in the rates of these events was seen between the two treatment groups, Dr. Takeshita noted.
Tamibarotene was studied in this trial because, compared with ATRA, it has been shown to have about a 10-fold increase in potency for inducing in vitro differentiation of NB-4 cells, enhanced chemical stability, and low affinity for cellular RA-binding protein.
“The clinical efficacy of tamibarotene for the treatment of APL has also been reported,” Dr. Takeshita added.
In the initial phases of the trial, no difference was seen between ATRA and tamibarotene with respect to 4-year relapse-free survival, but there did appear to be improved efficacy with tamibarotene in high-risk patients, which warranted further investigation, he said.
The current findings demonstrate the efficacy of tamibarotene vs. ATRA for decreasing the relapse rate at the 7-year observation point, and confirm the benefit in high-risk patients that was seen in earlier analyses, he concluded.
Dr. Takeshita reported receiving research funding from Chugai Pharmaceutical, Astellas Pharma, Pfizer Japan, and Takeda Pharmaceutical.
sworcester@frontlinemedcom.com
SOURCE: Takeshita A et al., ASH 2017, abstract 642.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: The 7-year relapse-free survival was 62% vs. 89% with ATRA vs. tamibarotene in high-risk patients.
Study details: Long-term maintenance results in 344 patients from a randomized controlled trial.
Disclosures: Dr. Takeshita reported receiving research funding from Chugai Pharmaceutical, Astellas Pharma, Pfizer Japan, and Takeda Pharmaceutical.
Source: Takeshita A et al. ASH 2017, abstract 642.
RF-positive polyarticular JIA looks like adult RA
New evidence suggests the rheumatoid factor–positive polyarticular subtype of juvenile idiopathic arthritis bears a close genetic resemblance to adult rheumatoid arthritis, lending support to the growing suspicion that in arthritis, biological underpinnings are more important than age of onset when it comes to characterizing and, potentially, choosing treatments.
Previous work had shown that rheumatoid factor (RF)–negative patients have genetic risks similar to those of adults with RF-negative disease. “If the RF-negative patients in adult and childhood are similar, then maybe the RF-positive patients are similar in their genetic background as well. That’s what this study was testing,” study coauthor Anne M. Stevens, MD, PhD, division chief of rheumatology at the University of Washington, Seattle, said in an interview. The study was published online Feb. 9 in Arthritis & Rheumatology.
There are seven recognized categories of juvenile idiopathic arthritis (JIA), and all are believed to have genetic risk factors. Previously, the researchers used the Immunochip custom microarray to map 186 autoimmune disease-associated loci from 11 autoimmune phenotypes, including adult rheumatoid arthritis (RA). In the current work, the researchers analyzed 340 RF-positive polyarticular JIA cases (292 females) and 14,412 controls (8,002 females) from the United States, United Kingdom, Germany, Canada, and Norway. RF-positive polyarticular disease accounts for about 5% of JIA cases, and its symptoms and presentations resemble adult RA.
The researchers found associations in the human leukocyte antigen (HLA) region. The most significant was found at rs3129769, near HLA-DRB1 (P = 5.51 x 10-31). This single nucleotide polymorphism (SNP) was in strong linkage disequilibrium (LD, r2 = 0.88) with the rs660895 HLA-DRB1 SNP that has been reported in adult RA (P = 2.14 x 10-29).
The researchers examined links between RF-positive polyarticular JIA and the 27 SNPs that had been identified in the previous study of oligoarticular/RF-negative polyarticular JIA. Just 6 of those 27 SNPs were significantly associated with RF-positive polyarticular JIA (P less than .05). On the other hand, of 44 SNPs most strongly associated with RA, 19 were associated with RF-positive polyarticular JIA (P less than .05).
That suggests that RF-positive polyarticular JIA cases are different from other JIA cases. “They’re more like adult patients than they’re like child patients,” said Dr. Stevens.
The researchers also compared the weighted genetic risk scores (wGRS) produced from the top RA loci to wGRS produced from the top oligoarticular/RF-negative polyarticular JIA loci. The wGRS from the top RA loci was a better predictor of RF-positive polyarticular JIA cases (area under the curve [AUC] = 0.71 versus AUC = 0.58; P = 8.26 x 10-33).
The wGRS from RA had similar success in predicting RF-positive polyarticular JIA and early-onset RA cases (AUC = 0.75; P = .25), but it fared worse in predicting late-onset RA (at 70 years or older, AUC = 0.62), compared with the wGRS from RF-positive polyarticular JIA (P = 1.65 x 10-5).
Those results suggest that RF-positive polyarticular JIA more closely resembles younger RA cases than older RA cases.
“If you consider early-onset RA patients, less than 29 years old when they develop RA, they look like JIA patients. But older RA patients, who are over 70 when they develop RA, they look like they totally have a different genetic background,” Dr. Stevens said.
The study could have clinical implications. The lead author, Anne Hinks, PhD, is a research fellow at the University of Manchester (England) and has led the charge to characterize JIA. The wGRS score she developed has the potential to help physicians diagnose classify and treat JIA patients. Currently, they must rely on the International League of Associations for Rheumatology criteria, which can take months to work through and may lead to misclassification diagnoses.
And in any case, the emerging genetic research suggests that the underlying genetics of JIA may be a better way to classify patients. “There’s a lot of overlap and risk of misclassifying patients with the current system. This weighted genetic risk score that Dr. Hinks developed could be used to classify patients with one DNA sample. This is the kind of clinical test we need,” Dr. Stevens said.
The study received funding from a range of government and private sources. Dr. Stevens has a patent licensed to Quest Diagnostics, is conducting research collaborations with Seattle Genetics and Kineta, and has received fellowship support from Pfizer.
SOURCE: Hinks A et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40443
New evidence suggests the rheumatoid factor–positive polyarticular subtype of juvenile idiopathic arthritis bears a close genetic resemblance to adult rheumatoid arthritis, lending support to the growing suspicion that in arthritis, biological underpinnings are more important than age of onset when it comes to characterizing and, potentially, choosing treatments.
Previous work had shown that rheumatoid factor (RF)–negative patients have genetic risks similar to those of adults with RF-negative disease. “If the RF-negative patients in adult and childhood are similar, then maybe the RF-positive patients are similar in their genetic background as well. That’s what this study was testing,” study coauthor Anne M. Stevens, MD, PhD, division chief of rheumatology at the University of Washington, Seattle, said in an interview. The study was published online Feb. 9 in Arthritis & Rheumatology.
There are seven recognized categories of juvenile idiopathic arthritis (JIA), and all are believed to have genetic risk factors. Previously, the researchers used the Immunochip custom microarray to map 186 autoimmune disease-associated loci from 11 autoimmune phenotypes, including adult rheumatoid arthritis (RA). In the current work, the researchers analyzed 340 RF-positive polyarticular JIA cases (292 females) and 14,412 controls (8,002 females) from the United States, United Kingdom, Germany, Canada, and Norway. RF-positive polyarticular disease accounts for about 5% of JIA cases, and its symptoms and presentations resemble adult RA.
The researchers found associations in the human leukocyte antigen (HLA) region. The most significant was found at rs3129769, near HLA-DRB1 (P = 5.51 x 10-31). This single nucleotide polymorphism (SNP) was in strong linkage disequilibrium (LD, r2 = 0.88) with the rs660895 HLA-DRB1 SNP that has been reported in adult RA (P = 2.14 x 10-29).
The researchers examined links between RF-positive polyarticular JIA and the 27 SNPs that had been identified in the previous study of oligoarticular/RF-negative polyarticular JIA. Just 6 of those 27 SNPs were significantly associated with RF-positive polyarticular JIA (P less than .05). On the other hand, of 44 SNPs most strongly associated with RA, 19 were associated with RF-positive polyarticular JIA (P less than .05).
That suggests that RF-positive polyarticular JIA cases are different from other JIA cases. “They’re more like adult patients than they’re like child patients,” said Dr. Stevens.
The researchers also compared the weighted genetic risk scores (wGRS) produced from the top RA loci to wGRS produced from the top oligoarticular/RF-negative polyarticular JIA loci. The wGRS from the top RA loci was a better predictor of RF-positive polyarticular JIA cases (area under the curve [AUC] = 0.71 versus AUC = 0.58; P = 8.26 x 10-33).
The wGRS from RA had similar success in predicting RF-positive polyarticular JIA and early-onset RA cases (AUC = 0.75; P = .25), but it fared worse in predicting late-onset RA (at 70 years or older, AUC = 0.62), compared with the wGRS from RF-positive polyarticular JIA (P = 1.65 x 10-5).
Those results suggest that RF-positive polyarticular JIA more closely resembles younger RA cases than older RA cases.
“If you consider early-onset RA patients, less than 29 years old when they develop RA, they look like JIA patients. But older RA patients, who are over 70 when they develop RA, they look like they totally have a different genetic background,” Dr. Stevens said.
The study could have clinical implications. The lead author, Anne Hinks, PhD, is a research fellow at the University of Manchester (England) and has led the charge to characterize JIA. The wGRS score she developed has the potential to help physicians diagnose classify and treat JIA patients. Currently, they must rely on the International League of Associations for Rheumatology criteria, which can take months to work through and may lead to misclassification diagnoses.
And in any case, the emerging genetic research suggests that the underlying genetics of JIA may be a better way to classify patients. “There’s a lot of overlap and risk of misclassifying patients with the current system. This weighted genetic risk score that Dr. Hinks developed could be used to classify patients with one DNA sample. This is the kind of clinical test we need,” Dr. Stevens said.
The study received funding from a range of government and private sources. Dr. Stevens has a patent licensed to Quest Diagnostics, is conducting research collaborations with Seattle Genetics and Kineta, and has received fellowship support from Pfizer.
SOURCE: Hinks A et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40443
New evidence suggests the rheumatoid factor–positive polyarticular subtype of juvenile idiopathic arthritis bears a close genetic resemblance to adult rheumatoid arthritis, lending support to the growing suspicion that in arthritis, biological underpinnings are more important than age of onset when it comes to characterizing and, potentially, choosing treatments.
Previous work had shown that rheumatoid factor (RF)–negative patients have genetic risks similar to those of adults with RF-negative disease. “If the RF-negative patients in adult and childhood are similar, then maybe the RF-positive patients are similar in their genetic background as well. That’s what this study was testing,” study coauthor Anne M. Stevens, MD, PhD, division chief of rheumatology at the University of Washington, Seattle, said in an interview. The study was published online Feb. 9 in Arthritis & Rheumatology.
There are seven recognized categories of juvenile idiopathic arthritis (JIA), and all are believed to have genetic risk factors. Previously, the researchers used the Immunochip custom microarray to map 186 autoimmune disease-associated loci from 11 autoimmune phenotypes, including adult rheumatoid arthritis (RA). In the current work, the researchers analyzed 340 RF-positive polyarticular JIA cases (292 females) and 14,412 controls (8,002 females) from the United States, United Kingdom, Germany, Canada, and Norway. RF-positive polyarticular disease accounts for about 5% of JIA cases, and its symptoms and presentations resemble adult RA.
The researchers found associations in the human leukocyte antigen (HLA) region. The most significant was found at rs3129769, near HLA-DRB1 (P = 5.51 x 10-31). This single nucleotide polymorphism (SNP) was in strong linkage disequilibrium (LD, r2 = 0.88) with the rs660895 HLA-DRB1 SNP that has been reported in adult RA (P = 2.14 x 10-29).
The researchers examined links between RF-positive polyarticular JIA and the 27 SNPs that had been identified in the previous study of oligoarticular/RF-negative polyarticular JIA. Just 6 of those 27 SNPs were significantly associated with RF-positive polyarticular JIA (P less than .05). On the other hand, of 44 SNPs most strongly associated with RA, 19 were associated with RF-positive polyarticular JIA (P less than .05).
That suggests that RF-positive polyarticular JIA cases are different from other JIA cases. “They’re more like adult patients than they’re like child patients,” said Dr. Stevens.
The researchers also compared the weighted genetic risk scores (wGRS) produced from the top RA loci to wGRS produced from the top oligoarticular/RF-negative polyarticular JIA loci. The wGRS from the top RA loci was a better predictor of RF-positive polyarticular JIA cases (area under the curve [AUC] = 0.71 versus AUC = 0.58; P = 8.26 x 10-33).
The wGRS from RA had similar success in predicting RF-positive polyarticular JIA and early-onset RA cases (AUC = 0.75; P = .25), but it fared worse in predicting late-onset RA (at 70 years or older, AUC = 0.62), compared with the wGRS from RF-positive polyarticular JIA (P = 1.65 x 10-5).
Those results suggest that RF-positive polyarticular JIA more closely resembles younger RA cases than older RA cases.
“If you consider early-onset RA patients, less than 29 years old when they develop RA, they look like JIA patients. But older RA patients, who are over 70 when they develop RA, they look like they totally have a different genetic background,” Dr. Stevens said.
The study could have clinical implications. The lead author, Anne Hinks, PhD, is a research fellow at the University of Manchester (England) and has led the charge to characterize JIA. The wGRS score she developed has the potential to help physicians diagnose classify and treat JIA patients. Currently, they must rely on the International League of Associations for Rheumatology criteria, which can take months to work through and may lead to misclassification diagnoses.
And in any case, the emerging genetic research suggests that the underlying genetics of JIA may be a better way to classify patients. “There’s a lot of overlap and risk of misclassifying patients with the current system. This weighted genetic risk score that Dr. Hinks developed could be used to classify patients with one DNA sample. This is the kind of clinical test we need,” Dr. Stevens said.
The study received funding from a range of government and private sources. Dr. Stevens has a patent licensed to Quest Diagnostics, is conducting research collaborations with Seattle Genetics and Kineta, and has received fellowship support from Pfizer.
SOURCE: Hinks A et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40443
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Genetics underlying arthritis may be more important than age of onset.
Major finding: The weighted genetic risk scores produced from the top RA loci was a better predictor of RF-positive polyarticular JIA than that generated from the top oligoarticular/RF-negative polyarticular JIA loci.
Data source: Case-control analysis of 340 cases and 14,412 controls.
Disclosures: The study received funding from a range of government and private sources. Dr. Stevens has a patent licensed to Quest Diagnostics, is conducting research collaborations with Seattle Genetics and Kineta, and has received fellowship support from Pfizer.
Source: Hinks A et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40443.
Topical imiquimod helps clear blurred lines in lentigo maligna excision
according to a study from the University of Utah.
Lentigo maligna is a subtype of melanoma in situ, usually occurring in the head and neck regions, the researchers said.
“Neoadjuvant topical imiquimod 5% cream applied 5 times weekly for 8 weeks was associated with decreased MDCs in LM treatment sites compared with the MDCs of negative control sites,” wrote Shadai Flores of the University of Utah, Salt Lake City, and her colleagues.
Previously, the ability to distinguish between the surgical border and surrounding background melanocytic hyperplasia was uncertain. Because of this uncertainty, LM removal required an average margin of 7.2 mm. Another study showed that topical imiquimod 5% cream enabled the removal of most LM tumors with 2-mm margins. This study “sought to evaluate MDCs in imiquimod-treated LM and negative control biopsy specimens to determine if there was a measurable difference in melanocyte density,” the researchers wrote in a research letter published in JAMA Dermatology.
The study prospectively followed 52 cases of LM treated with imiquimod 5% topical cream 5 days per week for 8 weeks followed by conservative staged excisions with 2-mm margins. Treatment with imiquimod 5% of LM was followed by a 2- to 4-month recuperation period before surgery could be performed. All patients in the study were treated by one Mohs surgeon at the Huntsman Cancer Institute at the university.
To establish an MDC baseline, a 10-mm long fusiform biopsy was taken as a negative control. The negative control sample site and the LM site were separated by approximately 6 cm, found on the same side of the body, and showed similar color changes. After a negative control was taken, an LM lesion was resected and subsequently quadrisected. The MDCs then were concurrently counted by the researchers and compared with the negative controls.
Of the 52 LM specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls. The median MDC from post–imiquimod-treated sites was 14.4, with a range of 0.5-26.6. This showed marked improvement over the negative controls, which had a median MDC of 20.0 (range of 9.0-36.7). A 2-tailed paired t test revealed that the results displayed statistical significance (P less than .001). Residual LM was seen in the central areas of 9 (17%) specimens, but 43 (83%) had no indication of residual LM.
“The decreased melanocytic hyperplasia in imiquimod-treated sites reduced ambiguity in making a distinction between the border of the excised LM and background melanocytic hyperplasia,” noted Ms. Flores and her colleagues.
The authors had no conflicts of interest.
SOURCE: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
according to a study from the University of Utah.
Lentigo maligna is a subtype of melanoma in situ, usually occurring in the head and neck regions, the researchers said.
“Neoadjuvant topical imiquimod 5% cream applied 5 times weekly for 8 weeks was associated with decreased MDCs in LM treatment sites compared with the MDCs of negative control sites,” wrote Shadai Flores of the University of Utah, Salt Lake City, and her colleagues.
Previously, the ability to distinguish between the surgical border and surrounding background melanocytic hyperplasia was uncertain. Because of this uncertainty, LM removal required an average margin of 7.2 mm. Another study showed that topical imiquimod 5% cream enabled the removal of most LM tumors with 2-mm margins. This study “sought to evaluate MDCs in imiquimod-treated LM and negative control biopsy specimens to determine if there was a measurable difference in melanocyte density,” the researchers wrote in a research letter published in JAMA Dermatology.
The study prospectively followed 52 cases of LM treated with imiquimod 5% topical cream 5 days per week for 8 weeks followed by conservative staged excisions with 2-mm margins. Treatment with imiquimod 5% of LM was followed by a 2- to 4-month recuperation period before surgery could be performed. All patients in the study were treated by one Mohs surgeon at the Huntsman Cancer Institute at the university.
To establish an MDC baseline, a 10-mm long fusiform biopsy was taken as a negative control. The negative control sample site and the LM site were separated by approximately 6 cm, found on the same side of the body, and showed similar color changes. After a negative control was taken, an LM lesion was resected and subsequently quadrisected. The MDCs then were concurrently counted by the researchers and compared with the negative controls.
Of the 52 LM specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls. The median MDC from post–imiquimod-treated sites was 14.4, with a range of 0.5-26.6. This showed marked improvement over the negative controls, which had a median MDC of 20.0 (range of 9.0-36.7). A 2-tailed paired t test revealed that the results displayed statistical significance (P less than .001). Residual LM was seen in the central areas of 9 (17%) specimens, but 43 (83%) had no indication of residual LM.
“The decreased melanocytic hyperplasia in imiquimod-treated sites reduced ambiguity in making a distinction between the border of the excised LM and background melanocytic hyperplasia,” noted Ms. Flores and her colleagues.
The authors had no conflicts of interest.
SOURCE: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
according to a study from the University of Utah.
Lentigo maligna is a subtype of melanoma in situ, usually occurring in the head and neck regions, the researchers said.
“Neoadjuvant topical imiquimod 5% cream applied 5 times weekly for 8 weeks was associated with decreased MDCs in LM treatment sites compared with the MDCs of negative control sites,” wrote Shadai Flores of the University of Utah, Salt Lake City, and her colleagues.
Previously, the ability to distinguish between the surgical border and surrounding background melanocytic hyperplasia was uncertain. Because of this uncertainty, LM removal required an average margin of 7.2 mm. Another study showed that topical imiquimod 5% cream enabled the removal of most LM tumors with 2-mm margins. This study “sought to evaluate MDCs in imiquimod-treated LM and negative control biopsy specimens to determine if there was a measurable difference in melanocyte density,” the researchers wrote in a research letter published in JAMA Dermatology.
The study prospectively followed 52 cases of LM treated with imiquimod 5% topical cream 5 days per week for 8 weeks followed by conservative staged excisions with 2-mm margins. Treatment with imiquimod 5% of LM was followed by a 2- to 4-month recuperation period before surgery could be performed. All patients in the study were treated by one Mohs surgeon at the Huntsman Cancer Institute at the university.
To establish an MDC baseline, a 10-mm long fusiform biopsy was taken as a negative control. The negative control sample site and the LM site were separated by approximately 6 cm, found on the same side of the body, and showed similar color changes. After a negative control was taken, an LM lesion was resected and subsequently quadrisected. The MDCs then were concurrently counted by the researchers and compared with the negative controls.
Of the 52 LM specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls. The median MDC from post–imiquimod-treated sites was 14.4, with a range of 0.5-26.6. This showed marked improvement over the negative controls, which had a median MDC of 20.0 (range of 9.0-36.7). A 2-tailed paired t test revealed that the results displayed statistical significance (P less than .001). Residual LM was seen in the central areas of 9 (17%) specimens, but 43 (83%) had no indication of residual LM.
“The decreased melanocytic hyperplasia in imiquimod-treated sites reduced ambiguity in making a distinction between the border of the excised LM and background melanocytic hyperplasia,” noted Ms. Flores and her colleagues.
The authors had no conflicts of interest.
SOURCE: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
FROM JAMA DERMATOLOGY
Key clinical point: Neoadjuvant, topical imiquimod 5% cream is associated with a decrease in melanocyte density counts (MDC).
Major finding: Of 52 patient specimens, 44 (85%) exhibited decreases in MDCs, compared with the negative controls.
Study details: A prospective study of 52 cases of lentigo maligna treated with imiquimod 5% topical cream 5 days per week for 8 weeks, followed by conservative staged excisions with 2-mm margins.
Disclosures: The authors had no conflicts of interest.
Source: Flores S et al. JAMA Dermatol. 2018 Feb 16. doi: 10.1001/jamadermatol.2017.5632.
How to advise adolescents ISO drugs on the ‘dark web’
There was a time, not so long ago, when in the popular imagination, a drug deal involved an aging hippie in a tie-dyed shirt and love beads, copping a joint at a Dead concert. Today, however, in the age of the Internet and smartphones, a teenager in his bedroom can select, order, and have delivered to his door illicit drugs via the marketplaces on the “dark web.”
As the name implies, the dark web is a subterranean layer of the Internet that is mysterious, ominous, and sometimes lawless. It lies “beneath” the surface web – the layer where grandmothers post on Facebook and purchase on Amazon.
The dark web largely was the brainchild of three mathematicians at the Naval Research Laboratory as a means of encrypting messages exchanged by the intelligence community. They dubbed their project “Tor,” for “The Onion Router,” as the system consists of layer after layer of random relays, permitting anonymity on the Internet with little risk of tracking or surveillance.
This online underworld first came to my attention several years ago. The father of a teen who was being treated for disruptive mood dysregulation, attention-deficit disorder, and alcohol and cannabis use disorder called to inform me that his son had been arrested at Lollapalooza with hundreds of Adderall tablets and Xanax bars. Several weeks later, in session, the young man disclosed to me that he had found simple instructions online about installing Tor, creating a VPN (a virtual private network), accessing the dark web, transacting with bitcoin, and identifying drug marketplaces. He also demonstrated a detailed knowledge of chemical manufacturing in China, pill pressing in Canada, and money laundering in Switzerland.
With the air of an insider sharing “trade secrets,” this young man described how dealers on the dark web avoid detection and ensure secure delivery of the goods: latex gloves, vacuum sealing, and bleach dipping to obviate fingerprints – human and chemical. He said that dealers will send “dummy” packages to throw off the authorities and that buyers often will use the address of a clueless or absent neighbor. In his case, however, the parcels were delivered to his doorstep.
. For one thing, acquiring drugs in this way can seem less risky, as there is no chance of being robbed at gunpoint in a sketchy neighborhood or being busted for possession during a routine traffic stop.
Crossing over to the world of the dark web also can give an adolescent a sense of being clever and rebellious and of pulling a “fast one” on the parents – and on us, the clinicians who are treating them. And a teen who is interested in using illicit substances and plays “Call of Duty” from the comfort of his family’s basement without actual injury or death might assume that he can attain illicit drugs that are safe and inexpensive.
There are counterarguments to misinformation about the dark web. For example, contrary to the notion that buying drugs on the dark web minimizes interdiction or arrest, clinicians should point out that since international law enforcement shut down Silk Road and incarcerated its founder, Ross Ulbricht (also known as “Dread Pirate Roberts”) in 2013, hundreds of other dark web marketplaces such as AlphaBay and Hansa have been silenced and their operators prosecuted.
Moreover, the U.S. Department of Justice recently launched the Joint Criminal Opioid Darknet Enforcement (J-CODE) group, and the U.S. Postal Service Inspection Service reportedly has been hiring cybercrime and dark web specialists to combat drug trafficking. It might come as a surprise to a teen that, in a state where recreational marijuana is legal, transport and delivery of cannabis by the postal service elevates purchase and possession to the level of a violation of federal law. Informing even the most oblivious or oppositional adolescent that a drug felony can disqualify him for college grants and loans, impede his search for gainful employment, or prohibit him from obtaining a professional license, might give him a moment’s pause.
Adolescents seeking to buy drugs on the dark web should brace themselves for another shock. Whether lulled by custom after years of shopping on Amazon or using PayPal, or simply dulled by addiction, they might have a blind trust that bitcoin tumblers and “dark” escrow accounts will secure their payments. It will be rude awakening when they learn that the transfer and holding of currency on the dark web is vulnerable to hackers and to operators of the marketplaces – who are known to simply abscond with funds.
Currently, the drug marketplaces on the dark web represent a thin slice of the total illicit drug trade. These marketplaces, however, are growing quickly and offer buyers a virtual smorgasbord: the leading prescription drugs bought are Xanax and OxyContin, whereas 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy and cannabis are the most commonly purchased controlled Schedule I substances, according to an article in The Economist. The estimated annual sales for 2016 ranged from $100 million to $200 million, but even more alarming is the percentage of substance abusers who have purchased drugs on the dark web: A recent article in The Independent reported that 13.2% of U.S. respondents self-reported making at least one purchase online, whereas in the United Kingdom, the self-reported percentage was 25.3 %, and in Finland, it was 41.4%.
Evidence abounds that drugs purchased on the dark web often are counterfeit and sometimes “dirty.” There is a report of “Viagra” containing cement dust, of “Ambien” containing haloperidol, and “Xanax” laced with fentanyl, the latter having been linked to several deaths and hospital admissions in the Bay Area in 2016. (Big Pharma, in fact, is engaged in surveillance and investigation of the sale of knockoffs online, The Telegraph reported in article about the proliferation of fake drugs available on the surface web).Unfortunately, attempting to reduce teen substance abuse using law enforcement measures directed at dark web marketplaces might be a game of whack-a-mole: As soon as one supply source is staunched, another surfaces. Indeed, addiction should be viewed not simply in terms of the biopsychosocial model, but also as an economic activity. Thus, it might be more beneficial for clinicians to concentrate our resources and efforts on curtailing the demand through education and treatment.
Dr. Marseille is a psychiatrist who works on the staff at a clinic in Winfield, Ill. His special interests include adolescent and addiction medicine, eating disorders, trauma, bipolar disorder, and the psychiatric manifestations of acute and chronic medical conditions.
There was a time, not so long ago, when in the popular imagination, a drug deal involved an aging hippie in a tie-dyed shirt and love beads, copping a joint at a Dead concert. Today, however, in the age of the Internet and smartphones, a teenager in his bedroom can select, order, and have delivered to his door illicit drugs via the marketplaces on the “dark web.”
As the name implies, the dark web is a subterranean layer of the Internet that is mysterious, ominous, and sometimes lawless. It lies “beneath” the surface web – the layer where grandmothers post on Facebook and purchase on Amazon.
The dark web largely was the brainchild of three mathematicians at the Naval Research Laboratory as a means of encrypting messages exchanged by the intelligence community. They dubbed their project “Tor,” for “The Onion Router,” as the system consists of layer after layer of random relays, permitting anonymity on the Internet with little risk of tracking or surveillance.
This online underworld first came to my attention several years ago. The father of a teen who was being treated for disruptive mood dysregulation, attention-deficit disorder, and alcohol and cannabis use disorder called to inform me that his son had been arrested at Lollapalooza with hundreds of Adderall tablets and Xanax bars. Several weeks later, in session, the young man disclosed to me that he had found simple instructions online about installing Tor, creating a VPN (a virtual private network), accessing the dark web, transacting with bitcoin, and identifying drug marketplaces. He also demonstrated a detailed knowledge of chemical manufacturing in China, pill pressing in Canada, and money laundering in Switzerland.
With the air of an insider sharing “trade secrets,” this young man described how dealers on the dark web avoid detection and ensure secure delivery of the goods: latex gloves, vacuum sealing, and bleach dipping to obviate fingerprints – human and chemical. He said that dealers will send “dummy” packages to throw off the authorities and that buyers often will use the address of a clueless or absent neighbor. In his case, however, the parcels were delivered to his doorstep.
. For one thing, acquiring drugs in this way can seem less risky, as there is no chance of being robbed at gunpoint in a sketchy neighborhood or being busted for possession during a routine traffic stop.
Crossing over to the world of the dark web also can give an adolescent a sense of being clever and rebellious and of pulling a “fast one” on the parents – and on us, the clinicians who are treating them. And a teen who is interested in using illicit substances and plays “Call of Duty” from the comfort of his family’s basement without actual injury or death might assume that he can attain illicit drugs that are safe and inexpensive.
There are counterarguments to misinformation about the dark web. For example, contrary to the notion that buying drugs on the dark web minimizes interdiction or arrest, clinicians should point out that since international law enforcement shut down Silk Road and incarcerated its founder, Ross Ulbricht (also known as “Dread Pirate Roberts”) in 2013, hundreds of other dark web marketplaces such as AlphaBay and Hansa have been silenced and their operators prosecuted.
Moreover, the U.S. Department of Justice recently launched the Joint Criminal Opioid Darknet Enforcement (J-CODE) group, and the U.S. Postal Service Inspection Service reportedly has been hiring cybercrime and dark web specialists to combat drug trafficking. It might come as a surprise to a teen that, in a state where recreational marijuana is legal, transport and delivery of cannabis by the postal service elevates purchase and possession to the level of a violation of federal law. Informing even the most oblivious or oppositional adolescent that a drug felony can disqualify him for college grants and loans, impede his search for gainful employment, or prohibit him from obtaining a professional license, might give him a moment’s pause.
Adolescents seeking to buy drugs on the dark web should brace themselves for another shock. Whether lulled by custom after years of shopping on Amazon or using PayPal, or simply dulled by addiction, they might have a blind trust that bitcoin tumblers and “dark” escrow accounts will secure their payments. It will be rude awakening when they learn that the transfer and holding of currency on the dark web is vulnerable to hackers and to operators of the marketplaces – who are known to simply abscond with funds.
Currently, the drug marketplaces on the dark web represent a thin slice of the total illicit drug trade. These marketplaces, however, are growing quickly and offer buyers a virtual smorgasbord: the leading prescription drugs bought are Xanax and OxyContin, whereas 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy and cannabis are the most commonly purchased controlled Schedule I substances, according to an article in The Economist. The estimated annual sales for 2016 ranged from $100 million to $200 million, but even more alarming is the percentage of substance abusers who have purchased drugs on the dark web: A recent article in The Independent reported that 13.2% of U.S. respondents self-reported making at least one purchase online, whereas in the United Kingdom, the self-reported percentage was 25.3 %, and in Finland, it was 41.4%.
Evidence abounds that drugs purchased on the dark web often are counterfeit and sometimes “dirty.” There is a report of “Viagra” containing cement dust, of “Ambien” containing haloperidol, and “Xanax” laced with fentanyl, the latter having been linked to several deaths and hospital admissions in the Bay Area in 2016. (Big Pharma, in fact, is engaged in surveillance and investigation of the sale of knockoffs online, The Telegraph reported in article about the proliferation of fake drugs available on the surface web).Unfortunately, attempting to reduce teen substance abuse using law enforcement measures directed at dark web marketplaces might be a game of whack-a-mole: As soon as one supply source is staunched, another surfaces. Indeed, addiction should be viewed not simply in terms of the biopsychosocial model, but also as an economic activity. Thus, it might be more beneficial for clinicians to concentrate our resources and efforts on curtailing the demand through education and treatment.
Dr. Marseille is a psychiatrist who works on the staff at a clinic in Winfield, Ill. His special interests include adolescent and addiction medicine, eating disorders, trauma, bipolar disorder, and the psychiatric manifestations of acute and chronic medical conditions.
There was a time, not so long ago, when in the popular imagination, a drug deal involved an aging hippie in a tie-dyed shirt and love beads, copping a joint at a Dead concert. Today, however, in the age of the Internet and smartphones, a teenager in his bedroom can select, order, and have delivered to his door illicit drugs via the marketplaces on the “dark web.”
As the name implies, the dark web is a subterranean layer of the Internet that is mysterious, ominous, and sometimes lawless. It lies “beneath” the surface web – the layer where grandmothers post on Facebook and purchase on Amazon.
The dark web largely was the brainchild of three mathematicians at the Naval Research Laboratory as a means of encrypting messages exchanged by the intelligence community. They dubbed their project “Tor,” for “The Onion Router,” as the system consists of layer after layer of random relays, permitting anonymity on the Internet with little risk of tracking or surveillance.
This online underworld first came to my attention several years ago. The father of a teen who was being treated for disruptive mood dysregulation, attention-deficit disorder, and alcohol and cannabis use disorder called to inform me that his son had been arrested at Lollapalooza with hundreds of Adderall tablets and Xanax bars. Several weeks later, in session, the young man disclosed to me that he had found simple instructions online about installing Tor, creating a VPN (a virtual private network), accessing the dark web, transacting with bitcoin, and identifying drug marketplaces. He also demonstrated a detailed knowledge of chemical manufacturing in China, pill pressing in Canada, and money laundering in Switzerland.
With the air of an insider sharing “trade secrets,” this young man described how dealers on the dark web avoid detection and ensure secure delivery of the goods: latex gloves, vacuum sealing, and bleach dipping to obviate fingerprints – human and chemical. He said that dealers will send “dummy” packages to throw off the authorities and that buyers often will use the address of a clueless or absent neighbor. In his case, however, the parcels were delivered to his doorstep.
. For one thing, acquiring drugs in this way can seem less risky, as there is no chance of being robbed at gunpoint in a sketchy neighborhood or being busted for possession during a routine traffic stop.
Crossing over to the world of the dark web also can give an adolescent a sense of being clever and rebellious and of pulling a “fast one” on the parents – and on us, the clinicians who are treating them. And a teen who is interested in using illicit substances and plays “Call of Duty” from the comfort of his family’s basement without actual injury or death might assume that he can attain illicit drugs that are safe and inexpensive.
There are counterarguments to misinformation about the dark web. For example, contrary to the notion that buying drugs on the dark web minimizes interdiction or arrest, clinicians should point out that since international law enforcement shut down Silk Road and incarcerated its founder, Ross Ulbricht (also known as “Dread Pirate Roberts”) in 2013, hundreds of other dark web marketplaces such as AlphaBay and Hansa have been silenced and their operators prosecuted.
Moreover, the U.S. Department of Justice recently launched the Joint Criminal Opioid Darknet Enforcement (J-CODE) group, and the U.S. Postal Service Inspection Service reportedly has been hiring cybercrime and dark web specialists to combat drug trafficking. It might come as a surprise to a teen that, in a state where recreational marijuana is legal, transport and delivery of cannabis by the postal service elevates purchase and possession to the level of a violation of federal law. Informing even the most oblivious or oppositional adolescent that a drug felony can disqualify him for college grants and loans, impede his search for gainful employment, or prohibit him from obtaining a professional license, might give him a moment’s pause.
Adolescents seeking to buy drugs on the dark web should brace themselves for another shock. Whether lulled by custom after years of shopping on Amazon or using PayPal, or simply dulled by addiction, they might have a blind trust that bitcoin tumblers and “dark” escrow accounts will secure their payments. It will be rude awakening when they learn that the transfer and holding of currency on the dark web is vulnerable to hackers and to operators of the marketplaces – who are known to simply abscond with funds.
Currently, the drug marketplaces on the dark web represent a thin slice of the total illicit drug trade. These marketplaces, however, are growing quickly and offer buyers a virtual smorgasbord: the leading prescription drugs bought are Xanax and OxyContin, whereas 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy and cannabis are the most commonly purchased controlled Schedule I substances, according to an article in The Economist. The estimated annual sales for 2016 ranged from $100 million to $200 million, but even more alarming is the percentage of substance abusers who have purchased drugs on the dark web: A recent article in The Independent reported that 13.2% of U.S. respondents self-reported making at least one purchase online, whereas in the United Kingdom, the self-reported percentage was 25.3 %, and in Finland, it was 41.4%.
Evidence abounds that drugs purchased on the dark web often are counterfeit and sometimes “dirty.” There is a report of “Viagra” containing cement dust, of “Ambien” containing haloperidol, and “Xanax” laced with fentanyl, the latter having been linked to several deaths and hospital admissions in the Bay Area in 2016. (Big Pharma, in fact, is engaged in surveillance and investigation of the sale of knockoffs online, The Telegraph reported in article about the proliferation of fake drugs available on the surface web).Unfortunately, attempting to reduce teen substance abuse using law enforcement measures directed at dark web marketplaces might be a game of whack-a-mole: As soon as one supply source is staunched, another surfaces. Indeed, addiction should be viewed not simply in terms of the biopsychosocial model, but also as an economic activity. Thus, it might be more beneficial for clinicians to concentrate our resources and efforts on curtailing the demand through education and treatment.
Dr. Marseille is a psychiatrist who works on the staff at a clinic in Winfield, Ill. His special interests include adolescent and addiction medicine, eating disorders, trauma, bipolar disorder, and the psychiatric manifestations of acute and chronic medical conditions.
The Clinicians’ Role in Building a System of Care: Army Behavioral Health Since 2001
The Military Health System has changed in countless ways during the era of the Global War on Terror, but no clinical area has been challenged as thoroughly and transformed as extensively as U.S. Army behavioral health care. Through 2011, the wars in Iraq and Afghanistan required soldiers to spend more than 1.5 million years in theater and contributed to significant increases in the incidence of posttraumatic stress disorder (PTSD), depression, and other behavioral health conditions in soldiers and family members.1,2
Unfortunately, the Army’s behavioral health treatment system, which had sufficiently met Army beneficiaries’ needs during peacetime, w
as not adequately resourced or structured to provide care on the scale that was required, and major problems in access, quality, continuity, and safety emerged.3 Army behavioral health and primary care providers experienced these challenges within their own practices, and many developed local solutions. Realizing that a near-complete system transformation was necessary, medical leaders turned to clinicians drawn from the field to develop a strategy to identify the best clinical practices and to build a standardized, cohesive system of care around them.
Although many challenges remain, the Army’s system of behavioral health care has substantially improved and now better supports clinicians in the field as they care for soldiers and their family members. Clinicians at the headquarters and local hospital levels played key roles in this transformation; however, very little literature on the topic exists. Clinicians engaging in current or planning for future health care system changes would benefit from a description of the process.
Unprecedented Challenges
As the wars in Iraq and Afghanistan intensified in the mid-2000s and the demand for behavioral health care grew, the full picture of the shortfalls in the Army’s system of behavioral health care came to the forefront. Provider shortages contributed to long waits for initial appointments, disrupted continuity, and reduced effectiveness of care.4 Even after an infusion of more than $1 billion in congressionally directed funding increased the number of staff and multiplied the clinical and nonclinical programs across the force, significant problems remained.5,6
Army hospitals divided behavioral health care between psychiatry, psychology, and social work fiefdoms, whose stovepipes often fractured communication between providers treating the same patient and prevented effective care coordination. Behavioral health clinics on each post offered different clinical services. Confused soldiers, leaders, and family members were forced to navigate various versions of behavioral health care each time they moved. Inaccurate, locally developed information systems produced little data on the effectiveness of local programs and hindered clinicians and leaders seeking to improve care. Without sufficient clinical capacity on the outpatient side, outpatient providers frequently admitted their patients to inpatient settings because it presented the only option to deliver needed services in a safe setting.
The turning point for improving care occurred in 2012 when senior medical leaders designated a behavioral health leadership team of clinicians, administrators, and analysts at the Army’s Office of the Surgeon General (OTSG) as its first service line. The move consolidated authority over behavioral health-related policy, programs, and funding and clearly communicated that Army Medicine was committed to improving behavioral health care. While behavioral health leaders had operated at OTSG for several years prior, they did not have the authority or resources to make widespread changes. Fortunately, the Army Surgeon General also adopted a command philosophy that emphasized the Operating Company Model, which sought to reduce variance by replicating successful practices across the enterprise. The Operating Company Model also limited each hospital commander’s authority to design their own unique clinical structure and shifted that responsibility to the clinicians in each service line at the headquarters level.
Forming a System of Care
The Behavioral Health Service Line partnered with the U.S. Army Public Health Command and systems engineers at the Massachusetts Institute of Technology to map the existing system of care and identify innovative clinical programs that represented best practices. In addition to 2 programs mandated by the DoD (Behavioral Health in Primary Care Medical Homes7 and Family Advocacy Programs),8 other programs (Embedded Behavioral Health, Multi-Disciplinary Behavioral Health clinics, Intensive Outpatient Program, inpatient care, residential care for substance use disorders, Connect Care, Tele-Behavioral Health, and the Child and Family Behavioral Health System) were selected for replication throughout the Army because they successfully demonstrated promising outcomes and filled a critical need.
To run the emerging standardized clinical programs, the Behavioral Health Service Line streamlined the leadership structure by eliminating all departments organized around provider discipline (ie, psychiatry, psychology, and social work) and created a single department of Behavioral Health at each Army hospital. For the first time, staff were organized into clinical teams based on the needs of the patients, not professional background. This change created new leadership opportunities across disciplines, reduced infighting, and eliminated a major source of confusion for patients and line leaders. The group of standard clinical programs, managed through integrated behavioral health departments in each Army hospital, was dubbed the Behavioral Health System of Care.
Change to department organizations and clinical programs created the need to reconfigure administrative data systems to provide accurate and timely information about system performance. Administrative teams revised Medical Expense Reporting and Performance System codes to provide visibility on important data, such as patient encounters and Revenue Value Units, down to the clinic and provider levels. To improve the reliability of existing data, OTSG staff led a multiyear effort to “clean” several data sources, such as those specifying provider type and work center.
Analysts used the refined workload information to build new tools, such as one to display individual provider productivity and one to predict the number and type of clinical staff required to meet the needs of the population at each Army installation. The administrative reorganization better informed clinical leaders at all levels by supplying meaningful data that enabled comparisons of performance within one or more Army hospitals.8
Translating System Changes Into Better Care
Despite transformative changes to Army behavioral health care, clinicians and leaders still had little insight into what mattered most: measuirng patient response to the treatment. To solve this issue, a team of Army clinical and information technology innovators developed the Behavioral Health Data Portal (BHDP), which used validated scales to collect input from patients at each outpatient visit about their current and recent symptoms. Clinicians use the information to complement their assessment, refine diagnoses, individualize treatment plans, and follow their patients’ progress.9
The BHDP also aggregates information on each patient’s progress into clinical outcome metrics that inform leaders about the effectiveness of the care their clinics provide. For the first time, analysts can establish correlations between specific actions at the clinic level and positive clinical outcomes. For example, the relationship between the use of evidence-based psychotherapies, regular follow-up, a strong therapeutic alliance, and provider use of BHDP have been clearly linked to more rapid resolution of PTSD symptoms. These insights provide opportunities to act on specific processes at the clinic level with high confidence that by doing so, clinicians are improving the effectiveness of the care delivered in their clinics.
Conclusion
The transformation of Army behavioral health care has encompassed all aspects of the treatment system, but it has been led by clinicians working at the local and headquarters levels (Figure). Although many challenges remain, today’s outpatient system is more efficient and effective. For example, Army medical facilities are now able to meet more of the total demand for outpatient behavioral health care of its beneficiaries; 77% in September 2017 compared with a low of 59% in January 2013, based on Army Strategic Management System data as of December 1, 2017. With
The Army continues to improve its system of care to better inform and enable clinicians to deliver evidence-based care. The clinician-led process that advanced this area of military medicine is applicable to others. A core group of dedicated clinical professionals committed to multiyear processes can implement large-scale changes if they are empowered and resourced by senior medical leaders. A standardized system of care built on clinical best practices and guided by clinicians using accurate data, including clinical outcomes, would benefit any component of the MHS.
1. Baiocchi D. Measuring Army deployments to Iraq and Afghanistan. https://www.rand.org/pubs/research_reports/RR145.html. Published 2013. Accessed December 1, 2017.
2. Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman D. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
3. Tanielian T, Jaycox LH (eds). Invisible wounds of war. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html. Published 2008. Accessed December 1, 2017.
4. Prine C. Army’s mental health programs swamped, understaffed. Pittsburgh Tribune-Review. http://triblive .com/x/pittsburghtrib/news/s_721604.html. Published February 7, 2011. Accessed December 1, 2017.
5. Weinick R, Beckjord E, Farmer C, et al. Programs addressing psychological health and traumatic brain injury among U.S. military servicemembers and their families. https://www.rand.org/pubs/technical_reports/TR950 .html. Published 2011. Accessed December 1, 2017.
6. Hoge CW, Ivany CG, Brusher EA, et al. Transformation of mental health care for U.S. soldiers and families during the Iraq and Afghanistan wars: where science and politics intersect. Am J Psychiatry. 2016;173(4):334-343.
7. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308-321.
8. Srinivasan J, Ivany CG, Sarmiento D, Woodson J. How the U.S. Army redesigned its mental health system. Harvard Business Review. https://hbr.org/2017/10/how-the-u-s-army-redesigned-its-mental-health-system. Published October 16, 2017. Accessed December 1, 2017.
9. Srinivasan J, Brown MD, Ivany CG, Woodson J. How the U.S. Army personalized its mental health care. Harvard Business Review. https://hbr.org/2016/12/how-the-u-s-army-personalized-its-mental-health-care. Published December 7, 2016. Accessed December 1, 2017.
The Military Health System has changed in countless ways during the era of the Global War on Terror, but no clinical area has been challenged as thoroughly and transformed as extensively as U.S. Army behavioral health care. Through 2011, the wars in Iraq and Afghanistan required soldiers to spend more than 1.5 million years in theater and contributed to significant increases in the incidence of posttraumatic stress disorder (PTSD), depression, and other behavioral health conditions in soldiers and family members.1,2
Unfortunately, the Army’s behavioral health treatment system, which had sufficiently met Army beneficiaries’ needs during peacetime, w
as not adequately resourced or structured to provide care on the scale that was required, and major problems in access, quality, continuity, and safety emerged.3 Army behavioral health and primary care providers experienced these challenges within their own practices, and many developed local solutions. Realizing that a near-complete system transformation was necessary, medical leaders turned to clinicians drawn from the field to develop a strategy to identify the best clinical practices and to build a standardized, cohesive system of care around them.
Although many challenges remain, the Army’s system of behavioral health care has substantially improved and now better supports clinicians in the field as they care for soldiers and their family members. Clinicians at the headquarters and local hospital levels played key roles in this transformation; however, very little literature on the topic exists. Clinicians engaging in current or planning for future health care system changes would benefit from a description of the process.
Unprecedented Challenges
As the wars in Iraq and Afghanistan intensified in the mid-2000s and the demand for behavioral health care grew, the full picture of the shortfalls in the Army’s system of behavioral health care came to the forefront. Provider shortages contributed to long waits for initial appointments, disrupted continuity, and reduced effectiveness of care.4 Even after an infusion of more than $1 billion in congressionally directed funding increased the number of staff and multiplied the clinical and nonclinical programs across the force, significant problems remained.5,6
Army hospitals divided behavioral health care between psychiatry, psychology, and social work fiefdoms, whose stovepipes often fractured communication between providers treating the same patient and prevented effective care coordination. Behavioral health clinics on each post offered different clinical services. Confused soldiers, leaders, and family members were forced to navigate various versions of behavioral health care each time they moved. Inaccurate, locally developed information systems produced little data on the effectiveness of local programs and hindered clinicians and leaders seeking to improve care. Without sufficient clinical capacity on the outpatient side, outpatient providers frequently admitted their patients to inpatient settings because it presented the only option to deliver needed services in a safe setting.
The turning point for improving care occurred in 2012 when senior medical leaders designated a behavioral health leadership team of clinicians, administrators, and analysts at the Army’s Office of the Surgeon General (OTSG) as its first service line. The move consolidated authority over behavioral health-related policy, programs, and funding and clearly communicated that Army Medicine was committed to improving behavioral health care. While behavioral health leaders had operated at OTSG for several years prior, they did not have the authority or resources to make widespread changes. Fortunately, the Army Surgeon General also adopted a command philosophy that emphasized the Operating Company Model, which sought to reduce variance by replicating successful practices across the enterprise. The Operating Company Model also limited each hospital commander’s authority to design their own unique clinical structure and shifted that responsibility to the clinicians in each service line at the headquarters level.
Forming a System of Care
The Behavioral Health Service Line partnered with the U.S. Army Public Health Command and systems engineers at the Massachusetts Institute of Technology to map the existing system of care and identify innovative clinical programs that represented best practices. In addition to 2 programs mandated by the DoD (Behavioral Health in Primary Care Medical Homes7 and Family Advocacy Programs),8 other programs (Embedded Behavioral Health, Multi-Disciplinary Behavioral Health clinics, Intensive Outpatient Program, inpatient care, residential care for substance use disorders, Connect Care, Tele-Behavioral Health, and the Child and Family Behavioral Health System) were selected for replication throughout the Army because they successfully demonstrated promising outcomes and filled a critical need.
To run the emerging standardized clinical programs, the Behavioral Health Service Line streamlined the leadership structure by eliminating all departments organized around provider discipline (ie, psychiatry, psychology, and social work) and created a single department of Behavioral Health at each Army hospital. For the first time, staff were organized into clinical teams based on the needs of the patients, not professional background. This change created new leadership opportunities across disciplines, reduced infighting, and eliminated a major source of confusion for patients and line leaders. The group of standard clinical programs, managed through integrated behavioral health departments in each Army hospital, was dubbed the Behavioral Health System of Care.
Change to department organizations and clinical programs created the need to reconfigure administrative data systems to provide accurate and timely information about system performance. Administrative teams revised Medical Expense Reporting and Performance System codes to provide visibility on important data, such as patient encounters and Revenue Value Units, down to the clinic and provider levels. To improve the reliability of existing data, OTSG staff led a multiyear effort to “clean” several data sources, such as those specifying provider type and work center.
Analysts used the refined workload information to build new tools, such as one to display individual provider productivity and one to predict the number and type of clinical staff required to meet the needs of the population at each Army installation. The administrative reorganization better informed clinical leaders at all levels by supplying meaningful data that enabled comparisons of performance within one or more Army hospitals.8
Translating System Changes Into Better Care
Despite transformative changes to Army behavioral health care, clinicians and leaders still had little insight into what mattered most: measuirng patient response to the treatment. To solve this issue, a team of Army clinical and information technology innovators developed the Behavioral Health Data Portal (BHDP), which used validated scales to collect input from patients at each outpatient visit about their current and recent symptoms. Clinicians use the information to complement their assessment, refine diagnoses, individualize treatment plans, and follow their patients’ progress.9
The BHDP also aggregates information on each patient’s progress into clinical outcome metrics that inform leaders about the effectiveness of the care their clinics provide. For the first time, analysts can establish correlations between specific actions at the clinic level and positive clinical outcomes. For example, the relationship between the use of evidence-based psychotherapies, regular follow-up, a strong therapeutic alliance, and provider use of BHDP have been clearly linked to more rapid resolution of PTSD symptoms. These insights provide opportunities to act on specific processes at the clinic level with high confidence that by doing so, clinicians are improving the effectiveness of the care delivered in their clinics.
Conclusion
The transformation of Army behavioral health care has encompassed all aspects of the treatment system, but it has been led by clinicians working at the local and headquarters levels (Figure). Although many challenges remain, today’s outpatient system is more efficient and effective. For example, Army medical facilities are now able to meet more of the total demand for outpatient behavioral health care of its beneficiaries; 77% in September 2017 compared with a low of 59% in January 2013, based on Army Strategic Management System data as of December 1, 2017. With
The Army continues to improve its system of care to better inform and enable clinicians to deliver evidence-based care. The clinician-led process that advanced this area of military medicine is applicable to others. A core group of dedicated clinical professionals committed to multiyear processes can implement large-scale changes if they are empowered and resourced by senior medical leaders. A standardized system of care built on clinical best practices and guided by clinicians using accurate data, including clinical outcomes, would benefit any component of the MHS.
The Military Health System has changed in countless ways during the era of the Global War on Terror, but no clinical area has been challenged as thoroughly and transformed as extensively as U.S. Army behavioral health care. Through 2011, the wars in Iraq and Afghanistan required soldiers to spend more than 1.5 million years in theater and contributed to significant increases in the incidence of posttraumatic stress disorder (PTSD), depression, and other behavioral health conditions in soldiers and family members.1,2
Unfortunately, the Army’s behavioral health treatment system, which had sufficiently met Army beneficiaries’ needs during peacetime, w
as not adequately resourced or structured to provide care on the scale that was required, and major problems in access, quality, continuity, and safety emerged.3 Army behavioral health and primary care providers experienced these challenges within their own practices, and many developed local solutions. Realizing that a near-complete system transformation was necessary, medical leaders turned to clinicians drawn from the field to develop a strategy to identify the best clinical practices and to build a standardized, cohesive system of care around them.
Although many challenges remain, the Army’s system of behavioral health care has substantially improved and now better supports clinicians in the field as they care for soldiers and their family members. Clinicians at the headquarters and local hospital levels played key roles in this transformation; however, very little literature on the topic exists. Clinicians engaging in current or planning for future health care system changes would benefit from a description of the process.
Unprecedented Challenges
As the wars in Iraq and Afghanistan intensified in the mid-2000s and the demand for behavioral health care grew, the full picture of the shortfalls in the Army’s system of behavioral health care came to the forefront. Provider shortages contributed to long waits for initial appointments, disrupted continuity, and reduced effectiveness of care.4 Even after an infusion of more than $1 billion in congressionally directed funding increased the number of staff and multiplied the clinical and nonclinical programs across the force, significant problems remained.5,6
Army hospitals divided behavioral health care between psychiatry, psychology, and social work fiefdoms, whose stovepipes often fractured communication between providers treating the same patient and prevented effective care coordination. Behavioral health clinics on each post offered different clinical services. Confused soldiers, leaders, and family members were forced to navigate various versions of behavioral health care each time they moved. Inaccurate, locally developed information systems produced little data on the effectiveness of local programs and hindered clinicians and leaders seeking to improve care. Without sufficient clinical capacity on the outpatient side, outpatient providers frequently admitted their patients to inpatient settings because it presented the only option to deliver needed services in a safe setting.
The turning point for improving care occurred in 2012 when senior medical leaders designated a behavioral health leadership team of clinicians, administrators, and analysts at the Army’s Office of the Surgeon General (OTSG) as its first service line. The move consolidated authority over behavioral health-related policy, programs, and funding and clearly communicated that Army Medicine was committed to improving behavioral health care. While behavioral health leaders had operated at OTSG for several years prior, they did not have the authority or resources to make widespread changes. Fortunately, the Army Surgeon General also adopted a command philosophy that emphasized the Operating Company Model, which sought to reduce variance by replicating successful practices across the enterprise. The Operating Company Model also limited each hospital commander’s authority to design their own unique clinical structure and shifted that responsibility to the clinicians in each service line at the headquarters level.
Forming a System of Care
The Behavioral Health Service Line partnered with the U.S. Army Public Health Command and systems engineers at the Massachusetts Institute of Technology to map the existing system of care and identify innovative clinical programs that represented best practices. In addition to 2 programs mandated by the DoD (Behavioral Health in Primary Care Medical Homes7 and Family Advocacy Programs),8 other programs (Embedded Behavioral Health, Multi-Disciplinary Behavioral Health clinics, Intensive Outpatient Program, inpatient care, residential care for substance use disorders, Connect Care, Tele-Behavioral Health, and the Child and Family Behavioral Health System) were selected for replication throughout the Army because they successfully demonstrated promising outcomes and filled a critical need.
To run the emerging standardized clinical programs, the Behavioral Health Service Line streamlined the leadership structure by eliminating all departments organized around provider discipline (ie, psychiatry, psychology, and social work) and created a single department of Behavioral Health at each Army hospital. For the first time, staff were organized into clinical teams based on the needs of the patients, not professional background. This change created new leadership opportunities across disciplines, reduced infighting, and eliminated a major source of confusion for patients and line leaders. The group of standard clinical programs, managed through integrated behavioral health departments in each Army hospital, was dubbed the Behavioral Health System of Care.
Change to department organizations and clinical programs created the need to reconfigure administrative data systems to provide accurate and timely information about system performance. Administrative teams revised Medical Expense Reporting and Performance System codes to provide visibility on important data, such as patient encounters and Revenue Value Units, down to the clinic and provider levels. To improve the reliability of existing data, OTSG staff led a multiyear effort to “clean” several data sources, such as those specifying provider type and work center.
Analysts used the refined workload information to build new tools, such as one to display individual provider productivity and one to predict the number and type of clinical staff required to meet the needs of the population at each Army installation. The administrative reorganization better informed clinical leaders at all levels by supplying meaningful data that enabled comparisons of performance within one or more Army hospitals.8
Translating System Changes Into Better Care
Despite transformative changes to Army behavioral health care, clinicians and leaders still had little insight into what mattered most: measuirng patient response to the treatment. To solve this issue, a team of Army clinical and information technology innovators developed the Behavioral Health Data Portal (BHDP), which used validated scales to collect input from patients at each outpatient visit about their current and recent symptoms. Clinicians use the information to complement their assessment, refine diagnoses, individualize treatment plans, and follow their patients’ progress.9
The BHDP also aggregates information on each patient’s progress into clinical outcome metrics that inform leaders about the effectiveness of the care their clinics provide. For the first time, analysts can establish correlations between specific actions at the clinic level and positive clinical outcomes. For example, the relationship between the use of evidence-based psychotherapies, regular follow-up, a strong therapeutic alliance, and provider use of BHDP have been clearly linked to more rapid resolution of PTSD symptoms. These insights provide opportunities to act on specific processes at the clinic level with high confidence that by doing so, clinicians are improving the effectiveness of the care delivered in their clinics.
Conclusion
The transformation of Army behavioral health care has encompassed all aspects of the treatment system, but it has been led by clinicians working at the local and headquarters levels (Figure). Although many challenges remain, today’s outpatient system is more efficient and effective. For example, Army medical facilities are now able to meet more of the total demand for outpatient behavioral health care of its beneficiaries; 77% in September 2017 compared with a low of 59% in January 2013, based on Army Strategic Management System data as of December 1, 2017. With
The Army continues to improve its system of care to better inform and enable clinicians to deliver evidence-based care. The clinician-led process that advanced this area of military medicine is applicable to others. A core group of dedicated clinical professionals committed to multiyear processes can implement large-scale changes if they are empowered and resourced by senior medical leaders. A standardized system of care built on clinical best practices and guided by clinicians using accurate data, including clinical outcomes, would benefit any component of the MHS.
1. Baiocchi D. Measuring Army deployments to Iraq and Afghanistan. https://www.rand.org/pubs/research_reports/RR145.html. Published 2013. Accessed December 1, 2017.
2. Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman D. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
3. Tanielian T, Jaycox LH (eds). Invisible wounds of war. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html. Published 2008. Accessed December 1, 2017.
4. Prine C. Army’s mental health programs swamped, understaffed. Pittsburgh Tribune-Review. http://triblive .com/x/pittsburghtrib/news/s_721604.html. Published February 7, 2011. Accessed December 1, 2017.
5. Weinick R, Beckjord E, Farmer C, et al. Programs addressing psychological health and traumatic brain injury among U.S. military servicemembers and their families. https://www.rand.org/pubs/technical_reports/TR950 .html. Published 2011. Accessed December 1, 2017.
6. Hoge CW, Ivany CG, Brusher EA, et al. Transformation of mental health care for U.S. soldiers and families during the Iraq and Afghanistan wars: where science and politics intersect. Am J Psychiatry. 2016;173(4):334-343.
7. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308-321.
8. Srinivasan J, Ivany CG, Sarmiento D, Woodson J. How the U.S. Army redesigned its mental health system. Harvard Business Review. https://hbr.org/2017/10/how-the-u-s-army-redesigned-its-mental-health-system. Published October 16, 2017. Accessed December 1, 2017.
9. Srinivasan J, Brown MD, Ivany CG, Woodson J. How the U.S. Army personalized its mental health care. Harvard Business Review. https://hbr.org/2016/12/how-the-u-s-army-personalized-its-mental-health-care. Published December 7, 2016. Accessed December 1, 2017.
1. Baiocchi D. Measuring Army deployments to Iraq and Afghanistan. https://www.rand.org/pubs/research_reports/RR145.html. Published 2013. Accessed December 1, 2017.
2. Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman D. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
3. Tanielian T, Jaycox LH (eds). Invisible wounds of war. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html. Published 2008. Accessed December 1, 2017.
4. Prine C. Army’s mental health programs swamped, understaffed. Pittsburgh Tribune-Review. http://triblive .com/x/pittsburghtrib/news/s_721604.html. Published February 7, 2011. Accessed December 1, 2017.
5. Weinick R, Beckjord E, Farmer C, et al. Programs addressing psychological health and traumatic brain injury among U.S. military servicemembers and their families. https://www.rand.org/pubs/technical_reports/TR950 .html. Published 2011. Accessed December 1, 2017.
6. Hoge CW, Ivany CG, Brusher EA, et al. Transformation of mental health care for U.S. soldiers and families during the Iraq and Afghanistan wars: where science and politics intersect. Am J Psychiatry. 2016;173(4):334-343.
7. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308-321.
8. Srinivasan J, Ivany CG, Sarmiento D, Woodson J. How the U.S. Army redesigned its mental health system. Harvard Business Review. https://hbr.org/2017/10/how-the-u-s-army-redesigned-its-mental-health-system. Published October 16, 2017. Accessed December 1, 2017.
9. Srinivasan J, Brown MD, Ivany CG, Woodson J. How the U.S. Army personalized its mental health care. Harvard Business Review. https://hbr.org/2016/12/how-the-u-s-army-personalized-its-mental-health-care. Published December 7, 2016. Accessed December 1, 2017.