User login
Sexual aids not available to cancer survivors despite recommendations
ORLANDO – Therapeutic aids for sexual rehabilitation were not available at most major cancer centers, according to results of a structured telephone survey presented at the Cancer Survivorship Symposium.
Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no such aids for women, said lead study author Sharon Bober, PhD, a psychologist at the Dana-Farber Cancer Institute in Boston, Massachusetts.
“I think the scarcity of all of these products really underscores the cultural taboos around sexual dysfunction, as did some of the discomfort of the staff responding to our calls,” Dr. Bober said in a press conference at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
Cancer treatment guidelines from the National Comprehensive Cancer Network (NCCN) recommend therapeutic aids for sexual health rehabilitation including vaginal dilators, moisturizers, and vacuum erection devices, Dr. Bober said.
Dr. Bober and her colleagues surveyed 25 NCI-designated Cancer Centers/National Comprehensive Cancer Network–member institutions about on-site availability of sexual aids and resources for cancer survivors.
After conducting internet searches and phone calls designed to identify potential sources of sexual aids at each center, study staff posed as relatives of patients and used a structured script to query cancer center staff about on-site availability of sexual aids.
Separate calls were conducted to query on availability of men and women’s sexual aids.
Of 23 centers that responded about men, 87% reported having no sexual aids, and of 22 centers that responded about women, 72% reported having no sexual aids, Dr. Bober reported at the symposium.
The lack of sexual aids was particularly notable given the wide availability of wigs, prosthetics, sunscreen, and other cancer care products at leading cancer centers, she added.
“Only one center of the 25 had an extensive list of products and resources for both men and women, which may well serve as a model when we think about the needs for cancer survivors in general,” said Dr. Bober.
These results suggest that leading cancer centers are not meeting the needs of cancer survivors in terms of recommended sexual therapeutic aids and informational resources, according to Timothy Gilligan, MD, an American Society of Clinical Oncology expert and member of the Cancer Survivorship news planning team.
“You sort of wonder where a cancer patient’s supposed to go to get this information if not at the Cancer Center,” said Dr. Gilligan, who moderated the press conference. “We’re really kind of leaving them shortchanged here, and the good news is I think we could easily do better if we just decide that we want to.”
The study was funded by Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
SOURCE: Bober S. et al. Cancer Survivorship Symposium Abstract #134
ORLANDO – Therapeutic aids for sexual rehabilitation were not available at most major cancer centers, according to results of a structured telephone survey presented at the Cancer Survivorship Symposium.
Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no such aids for women, said lead study author Sharon Bober, PhD, a psychologist at the Dana-Farber Cancer Institute in Boston, Massachusetts.
“I think the scarcity of all of these products really underscores the cultural taboos around sexual dysfunction, as did some of the discomfort of the staff responding to our calls,” Dr. Bober said in a press conference at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
Cancer treatment guidelines from the National Comprehensive Cancer Network (NCCN) recommend therapeutic aids for sexual health rehabilitation including vaginal dilators, moisturizers, and vacuum erection devices, Dr. Bober said.
Dr. Bober and her colleagues surveyed 25 NCI-designated Cancer Centers/National Comprehensive Cancer Network–member institutions about on-site availability of sexual aids and resources for cancer survivors.
After conducting internet searches and phone calls designed to identify potential sources of sexual aids at each center, study staff posed as relatives of patients and used a structured script to query cancer center staff about on-site availability of sexual aids.
Separate calls were conducted to query on availability of men and women’s sexual aids.
Of 23 centers that responded about men, 87% reported having no sexual aids, and of 22 centers that responded about women, 72% reported having no sexual aids, Dr. Bober reported at the symposium.
The lack of sexual aids was particularly notable given the wide availability of wigs, prosthetics, sunscreen, and other cancer care products at leading cancer centers, she added.
“Only one center of the 25 had an extensive list of products and resources for both men and women, which may well serve as a model when we think about the needs for cancer survivors in general,” said Dr. Bober.
These results suggest that leading cancer centers are not meeting the needs of cancer survivors in terms of recommended sexual therapeutic aids and informational resources, according to Timothy Gilligan, MD, an American Society of Clinical Oncology expert and member of the Cancer Survivorship news planning team.
“You sort of wonder where a cancer patient’s supposed to go to get this information if not at the Cancer Center,” said Dr. Gilligan, who moderated the press conference. “We’re really kind of leaving them shortchanged here, and the good news is I think we could easily do better if we just decide that we want to.”
The study was funded by Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
SOURCE: Bober S. et al. Cancer Survivorship Symposium Abstract #134
ORLANDO – Therapeutic aids for sexual rehabilitation were not available at most major cancer centers, according to results of a structured telephone survey presented at the Cancer Survivorship Symposium.
Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no such aids for women, said lead study author Sharon Bober, PhD, a psychologist at the Dana-Farber Cancer Institute in Boston, Massachusetts.
“I think the scarcity of all of these products really underscores the cultural taboos around sexual dysfunction, as did some of the discomfort of the staff responding to our calls,” Dr. Bober said in a press conference at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
Cancer treatment guidelines from the National Comprehensive Cancer Network (NCCN) recommend therapeutic aids for sexual health rehabilitation including vaginal dilators, moisturizers, and vacuum erection devices, Dr. Bober said.
Dr. Bober and her colleagues surveyed 25 NCI-designated Cancer Centers/National Comprehensive Cancer Network–member institutions about on-site availability of sexual aids and resources for cancer survivors.
After conducting internet searches and phone calls designed to identify potential sources of sexual aids at each center, study staff posed as relatives of patients and used a structured script to query cancer center staff about on-site availability of sexual aids.
Separate calls were conducted to query on availability of men and women’s sexual aids.
Of 23 centers that responded about men, 87% reported having no sexual aids, and of 22 centers that responded about women, 72% reported having no sexual aids, Dr. Bober reported at the symposium.
The lack of sexual aids was particularly notable given the wide availability of wigs, prosthetics, sunscreen, and other cancer care products at leading cancer centers, she added.
“Only one center of the 25 had an extensive list of products and resources for both men and women, which may well serve as a model when we think about the needs for cancer survivors in general,” said Dr. Bober.
These results suggest that leading cancer centers are not meeting the needs of cancer survivors in terms of recommended sexual therapeutic aids and informational resources, according to Timothy Gilligan, MD, an American Society of Clinical Oncology expert and member of the Cancer Survivorship news planning team.
“You sort of wonder where a cancer patient’s supposed to go to get this information if not at the Cancer Center,” said Dr. Gilligan, who moderated the press conference. “We’re really kind of leaving them shortchanged here, and the good news is I think we could easily do better if we just decide that we want to.”
The study was funded by Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
SOURCE: Bober S. et al. Cancer Survivorship Symposium Abstract #134
FROM THE CSC 2018
Key clinical point: Therapeutic aids for sexual health rehabilitation were not available at most leading cancer centers, despite clinical practice guidelines recommending their use.
Major finding: Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no aids for women.
Data source: Analysis of responses from cancer center staff at 25 NCI-designated cancer centers to telephone queries that used a structured script.
Disclosures: Study funding came from Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
Source: Bober S. et al. Cancer Survivorship Symposium, Abstract #134.
Salpingectomy at cesarean feasible, but adds to operative time
DALLAS – Salpingectomy – which can reduce the risk for later ovarian cancer – was completed successfully in about two-thirds of women who desired permanent contraception at cesarean delivery and were randomized to receive this procedure rather than simple tubal ligation.
In a study of 80 patients, operative times were longer by 15 minutes for those who received salpingectomy, and neither group had adverse outcomes or serious complications, according to Akila Subramaniam, MD, who presented the findings of the single-site Salpingectomy at Cesarean Delivery for Ovarian Cancer Reduction (SCORE) trial at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The strategy to perform salpingectomy during cesarean delivery may be one way to reduce the incidence of ovarian cancer, “the most lethal gynecologic malignancy in the United States,” said Dr. Subramaniam, a maternal-fetal medicine specialist at the University of Alabama at Birmingham. “Primary prevention is the focus to reduce the ovarian cancer burden.”
However, Dr. Subramaniam said, there had been limited prospective data on salpingectomy performed at the time of cesarean delivery. One theoretical concern is an increased risk of bleeding; another is the additional operative time required for a potentially difficult dissection of the entire fallopian tube.
Dr. Subramaniam and her colleagues constructed a clinical trial that asked patients who were receiving cesarean delivery and who desired surgical sterilization to agree to randomization to complete salpingectomy or standard tubal ligation. Patient allocation was determined by computer-generated numbers, placed in a sealed envelope, and revealed to the surgeon only at the time of the opening incision for the cesarean procedure. Patients were unaware of the allocation until hospital discharge.
The single-center trial enrolled women undergoing a planned cesarean delivery at 35 or more weeks’ gestation, including those who had previous cesareans and women with multiple gestations or fetal malpresentations. Women who went on to cesarean after a trial of labor after prior cesarean were also eligible.
Patients younger than 25 years and patients with known fetal anomalies or fetal demise were excluded, as were women with previous tubal surgery and those who were anticoagulated or had immunodeficiency. The study did not enroll women who were known to carry the BRCA mutation.
Patients who were randomized to the intervention arm received a complete salpingectomy involving excision from the fimbriae to within 1 cm of the cornua, when technically feasible. The control arm participants received a standard bilateral tubal ligation using either the modified Pomeroy technique or the Parkland technique.
All study participants received routine pre- and postoperative care and instructions, and had study follow-up visits at 1 and 6 weeks post partum.
The study had two primary endpoints: rate of bilateral completion of the randomized procedure, and mean total operative time measured from skin incision to closure. Secondary outcomes included assessments of blood loss and surgical complications, followed through the 6-week postpartum visit.
The study just met the predetermined statistical power needed to detect a 10-minute difference in operative time, enrolling 80 patients and randomizing 40 to each arm.
Of the 40 patients randomized to salpingectomy, 27 (67.5%) received the intended complete salpingectomy. Three had a unilateral salpingectomy, with a tubal ligation contralaterally. Eight patients received bilateral tubal ligations, and in two patients, the surgeon was unable to perform any sterilization procedure at all.
In the tubal ligation arm, 38 patients (95%) received bilateral tubal ligation, 1 patient received a unilateral tubal ligation and a unilateral salpingectomy, and 1 patient received no procedure. The difference in success of completing the intended procedures between the two study arms was statistically significant (P = .002); in both groups, adhesions and scarring were the primary impediments to successful completion of the intended procedure, Dr. Subramaniam said.
Operative times were longer by about 15 minutes in the salpingectomy arm, with the difference accounted for by the longer duration of the sterilization procedure. No significant differences were seen in estimated blood loss or decrease in hematocrit between the two groups, and pain scores were similar.
The study, which successfully recruited 80 women from 221 approached, “may be underpowered for safety outcomes,” said Dr. Subramaniam. She also pointed out that there was no assessment of ovarian reserve, and that it’s not possible to assess the true risk reduction of salpingectomy in this study design.
Still, “It’s reasonable to consider this surgical sterilization method during cesarean as an ovarian cancer risk-reducing strategy.” One impediment to study recruitment, she said, was that after receiving education about salpingectomy, many patients desired salpingectomy and were not willing to risk randomization to simple tubal ligation.
Dr. Subramaniam reported receiving research funding for the study from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
SOURCE: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
DALLAS – Salpingectomy – which can reduce the risk for later ovarian cancer – was completed successfully in about two-thirds of women who desired permanent contraception at cesarean delivery and were randomized to receive this procedure rather than simple tubal ligation.
In a study of 80 patients, operative times were longer by 15 minutes for those who received salpingectomy, and neither group had adverse outcomes or serious complications, according to Akila Subramaniam, MD, who presented the findings of the single-site Salpingectomy at Cesarean Delivery for Ovarian Cancer Reduction (SCORE) trial at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The strategy to perform salpingectomy during cesarean delivery may be one way to reduce the incidence of ovarian cancer, “the most lethal gynecologic malignancy in the United States,” said Dr. Subramaniam, a maternal-fetal medicine specialist at the University of Alabama at Birmingham. “Primary prevention is the focus to reduce the ovarian cancer burden.”
However, Dr. Subramaniam said, there had been limited prospective data on salpingectomy performed at the time of cesarean delivery. One theoretical concern is an increased risk of bleeding; another is the additional operative time required for a potentially difficult dissection of the entire fallopian tube.
Dr. Subramaniam and her colleagues constructed a clinical trial that asked patients who were receiving cesarean delivery and who desired surgical sterilization to agree to randomization to complete salpingectomy or standard tubal ligation. Patient allocation was determined by computer-generated numbers, placed in a sealed envelope, and revealed to the surgeon only at the time of the opening incision for the cesarean procedure. Patients were unaware of the allocation until hospital discharge.
The single-center trial enrolled women undergoing a planned cesarean delivery at 35 or more weeks’ gestation, including those who had previous cesareans and women with multiple gestations or fetal malpresentations. Women who went on to cesarean after a trial of labor after prior cesarean were also eligible.
Patients younger than 25 years and patients with known fetal anomalies or fetal demise were excluded, as were women with previous tubal surgery and those who were anticoagulated or had immunodeficiency. The study did not enroll women who were known to carry the BRCA mutation.
Patients who were randomized to the intervention arm received a complete salpingectomy involving excision from the fimbriae to within 1 cm of the cornua, when technically feasible. The control arm participants received a standard bilateral tubal ligation using either the modified Pomeroy technique or the Parkland technique.
All study participants received routine pre- and postoperative care and instructions, and had study follow-up visits at 1 and 6 weeks post partum.
The study had two primary endpoints: rate of bilateral completion of the randomized procedure, and mean total operative time measured from skin incision to closure. Secondary outcomes included assessments of blood loss and surgical complications, followed through the 6-week postpartum visit.
The study just met the predetermined statistical power needed to detect a 10-minute difference in operative time, enrolling 80 patients and randomizing 40 to each arm.
Of the 40 patients randomized to salpingectomy, 27 (67.5%) received the intended complete salpingectomy. Three had a unilateral salpingectomy, with a tubal ligation contralaterally. Eight patients received bilateral tubal ligations, and in two patients, the surgeon was unable to perform any sterilization procedure at all.
In the tubal ligation arm, 38 patients (95%) received bilateral tubal ligation, 1 patient received a unilateral tubal ligation and a unilateral salpingectomy, and 1 patient received no procedure. The difference in success of completing the intended procedures between the two study arms was statistically significant (P = .002); in both groups, adhesions and scarring were the primary impediments to successful completion of the intended procedure, Dr. Subramaniam said.
Operative times were longer by about 15 minutes in the salpingectomy arm, with the difference accounted for by the longer duration of the sterilization procedure. No significant differences were seen in estimated blood loss or decrease in hematocrit between the two groups, and pain scores were similar.
The study, which successfully recruited 80 women from 221 approached, “may be underpowered for safety outcomes,” said Dr. Subramaniam. She also pointed out that there was no assessment of ovarian reserve, and that it’s not possible to assess the true risk reduction of salpingectomy in this study design.
Still, “It’s reasonable to consider this surgical sterilization method during cesarean as an ovarian cancer risk-reducing strategy.” One impediment to study recruitment, she said, was that after receiving education about salpingectomy, many patients desired salpingectomy and were not willing to risk randomization to simple tubal ligation.
Dr. Subramaniam reported receiving research funding for the study from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
SOURCE: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
DALLAS – Salpingectomy – which can reduce the risk for later ovarian cancer – was completed successfully in about two-thirds of women who desired permanent contraception at cesarean delivery and were randomized to receive this procedure rather than simple tubal ligation.
In a study of 80 patients, operative times were longer by 15 minutes for those who received salpingectomy, and neither group had adverse outcomes or serious complications, according to Akila Subramaniam, MD, who presented the findings of the single-site Salpingectomy at Cesarean Delivery for Ovarian Cancer Reduction (SCORE) trial at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The strategy to perform salpingectomy during cesarean delivery may be one way to reduce the incidence of ovarian cancer, “the most lethal gynecologic malignancy in the United States,” said Dr. Subramaniam, a maternal-fetal medicine specialist at the University of Alabama at Birmingham. “Primary prevention is the focus to reduce the ovarian cancer burden.”
However, Dr. Subramaniam said, there had been limited prospective data on salpingectomy performed at the time of cesarean delivery. One theoretical concern is an increased risk of bleeding; another is the additional operative time required for a potentially difficult dissection of the entire fallopian tube.
Dr. Subramaniam and her colleagues constructed a clinical trial that asked patients who were receiving cesarean delivery and who desired surgical sterilization to agree to randomization to complete salpingectomy or standard tubal ligation. Patient allocation was determined by computer-generated numbers, placed in a sealed envelope, and revealed to the surgeon only at the time of the opening incision for the cesarean procedure. Patients were unaware of the allocation until hospital discharge.
The single-center trial enrolled women undergoing a planned cesarean delivery at 35 or more weeks’ gestation, including those who had previous cesareans and women with multiple gestations or fetal malpresentations. Women who went on to cesarean after a trial of labor after prior cesarean were also eligible.
Patients younger than 25 years and patients with known fetal anomalies or fetal demise were excluded, as were women with previous tubal surgery and those who were anticoagulated or had immunodeficiency. The study did not enroll women who were known to carry the BRCA mutation.
Patients who were randomized to the intervention arm received a complete salpingectomy involving excision from the fimbriae to within 1 cm of the cornua, when technically feasible. The control arm participants received a standard bilateral tubal ligation using either the modified Pomeroy technique or the Parkland technique.
All study participants received routine pre- and postoperative care and instructions, and had study follow-up visits at 1 and 6 weeks post partum.
The study had two primary endpoints: rate of bilateral completion of the randomized procedure, and mean total operative time measured from skin incision to closure. Secondary outcomes included assessments of blood loss and surgical complications, followed through the 6-week postpartum visit.
The study just met the predetermined statistical power needed to detect a 10-minute difference in operative time, enrolling 80 patients and randomizing 40 to each arm.
Of the 40 patients randomized to salpingectomy, 27 (67.5%) received the intended complete salpingectomy. Three had a unilateral salpingectomy, with a tubal ligation contralaterally. Eight patients received bilateral tubal ligations, and in two patients, the surgeon was unable to perform any sterilization procedure at all.
In the tubal ligation arm, 38 patients (95%) received bilateral tubal ligation, 1 patient received a unilateral tubal ligation and a unilateral salpingectomy, and 1 patient received no procedure. The difference in success of completing the intended procedures between the two study arms was statistically significant (P = .002); in both groups, adhesions and scarring were the primary impediments to successful completion of the intended procedure, Dr. Subramaniam said.
Operative times were longer by about 15 minutes in the salpingectomy arm, with the difference accounted for by the longer duration of the sterilization procedure. No significant differences were seen in estimated blood loss or decrease in hematocrit between the two groups, and pain scores were similar.
The study, which successfully recruited 80 women from 221 approached, “may be underpowered for safety outcomes,” said Dr. Subramaniam. She also pointed out that there was no assessment of ovarian reserve, and that it’s not possible to assess the true risk reduction of salpingectomy in this study design.
Still, “It’s reasonable to consider this surgical sterilization method during cesarean as an ovarian cancer risk-reducing strategy.” One impediment to study recruitment, she said, was that after receiving education about salpingectomy, many patients desired salpingectomy and were not willing to risk randomization to simple tubal ligation.
Dr. Subramaniam reported receiving research funding for the study from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
SOURCE: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Salpingectomy was successful in two-thirds of patients but added 15 minutes to operative time.
Major finding: The intended procedure was successful in 27 of 40 salpingectomy patients (67.5%) and 38 of 40 tubal ligation patients (95%; P = .02).
Study details: Randomized controlled trial of 80 women receiving bilateral salpingectomy or tubal ligation at cesarean delivery.
Disclosures: Study funding was received from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
Source: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
Flu increase may be slowing
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)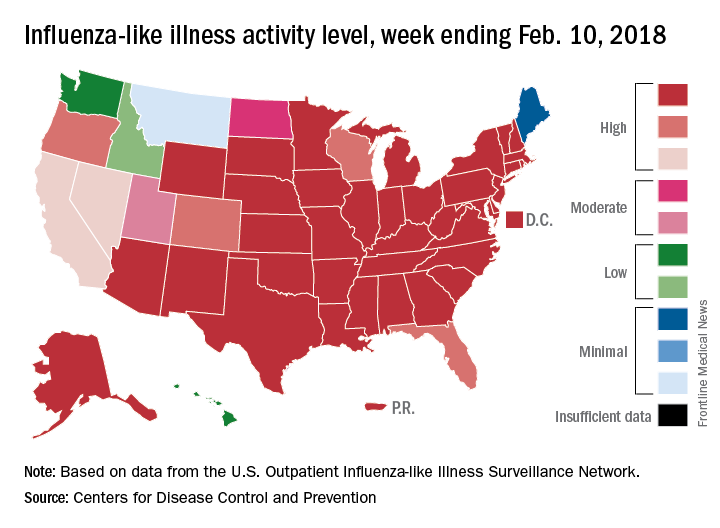
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
FROM THE CDC WEEKLY U.S. INFLUENZA SURVEILLANCE REPORT
Inaccurate depictions of inpatient psychiatry foster stigma
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
FDA approves new injection product to reduce preterm birth risk
Makena, a progestin injection for the prevention of preterm birth that has been approved for use since 2011, has received U.S. Food and Drug Administration approval for a subcutaneous auto-injection product to supplant its intramuscular injection formulation, announced its manufacturer, AMAG Pharmaceuticals, on Feb. 14.
The intramuscular injection’s 7-year orphan drug exclusivity expired earlier in February. AMAG plans to offer both products priced at parity, according to the company’s press release. The newer product has a smaller, thinner needle. Both products are intended for use in a woman who has a singleton pregnancy of less than 37 weeks’ gestation and who has had a prior spontaneous preterm singleton delivery. The intramuscular injection is available in both single-dose and multidose vials.
Makena revenues in 2017 were nearly $400 million. The drug was the subject of a price controversy in 2011 under its maker at the time, KV Pharmaceutical, which subsequently cut the price by more than half.
The injection has several contraindications, including blood clots and hormone-sensitive cancers. Its efficacy is based on an improved number of women who used it and were delivered at more than 37 weeks’ gestation rather than on a directly demonstrated clinical benefit in neonatal morbidity or mortality.
Read more in AMAG’s press release.
Makena, a progestin injection for the prevention of preterm birth that has been approved for use since 2011, has received U.S. Food and Drug Administration approval for a subcutaneous auto-injection product to supplant its intramuscular injection formulation, announced its manufacturer, AMAG Pharmaceuticals, on Feb. 14.
The intramuscular injection’s 7-year orphan drug exclusivity expired earlier in February. AMAG plans to offer both products priced at parity, according to the company’s press release. The newer product has a smaller, thinner needle. Both products are intended for use in a woman who has a singleton pregnancy of less than 37 weeks’ gestation and who has had a prior spontaneous preterm singleton delivery. The intramuscular injection is available in both single-dose and multidose vials.
Makena revenues in 2017 were nearly $400 million. The drug was the subject of a price controversy in 2011 under its maker at the time, KV Pharmaceutical, which subsequently cut the price by more than half.
The injection has several contraindications, including blood clots and hormone-sensitive cancers. Its efficacy is based on an improved number of women who used it and were delivered at more than 37 weeks’ gestation rather than on a directly demonstrated clinical benefit in neonatal morbidity or mortality.
Read more in AMAG’s press release.
Makena, a progestin injection for the prevention of preterm birth that has been approved for use since 2011, has received U.S. Food and Drug Administration approval for a subcutaneous auto-injection product to supplant its intramuscular injection formulation, announced its manufacturer, AMAG Pharmaceuticals, on Feb. 14.
The intramuscular injection’s 7-year orphan drug exclusivity expired earlier in February. AMAG plans to offer both products priced at parity, according to the company’s press release. The newer product has a smaller, thinner needle. Both products are intended for use in a woman who has a singleton pregnancy of less than 37 weeks’ gestation and who has had a prior spontaneous preterm singleton delivery. The intramuscular injection is available in both single-dose and multidose vials.
Makena revenues in 2017 were nearly $400 million. The drug was the subject of a price controversy in 2011 under its maker at the time, KV Pharmaceutical, which subsequently cut the price by more than half.
The injection has several contraindications, including blood clots and hormone-sensitive cancers. Its efficacy is based on an improved number of women who used it and were delivered at more than 37 weeks’ gestation rather than on a directly demonstrated clinical benefit in neonatal morbidity or mortality.
Read more in AMAG’s press release.
Screening for adolescent idiopathic scoliosis
The United States Preventive Services Task Force (USPSTF) has issued recommendations on screening for idiopathic scoliosis in asymptomatic children and adolescents aged 10-18 years.1 This recommendation concluded that the current evidence on the benefits and harms of screening is insufficient (I statement) and updated its 2004 recommendation against routine screening, in which it had concluded that the harms of screening exceeded the potential benefits (D recommendation).
Importance
Screening methods
The USPSTF concluded that currently available screening tests can accurately detect adolescent idiopathic scoliosis. Screening methods include visual inspection using the forward bend test, use of scoliometer measurement of the angle of trunk rotation during forward bend test with a rotation of 5 degrees–7 degrees recommended to be referred for radiography, and Moiré topography that enumerates asymmetric contour lines on the back (values greater than 2 are referred to radiography).
The USPSTF reviewed seven fair-quality observational studies (n = 447,243) and concluded that screening with a combination of forward bend test, scoliometer measurement and that Moiré topography had the highest sensitivity (93.8%) and specificity (99.2%), a low false-negative rate (6.2%), the lowest false-positive rate (0.8%), and the highest positive predictive value (81%). Sensitivity was lower when screening programs used only one or two screening tests, and single screening tests were associated with highest false-positive rates.
In general, the potential harms associated with false-positive results include psychological harm, chest radiation exposure, and other unnecessary treatment, but the USPSTF did not find evidence on the direct harms of screening.
Effectiveness of intervention or treatment
Bracing: The USPSTF found five studies (n = 651) that evaluated the effectiveness of treatment with three different types of braces. The average ages of participants ranged from 12 to 13 years, and their curvature severity varied from Cobb angle of 20 degrees to 30 degrees. The largest study (n = 242) was a good-quality, international, controlled clinical trial known as the Bracing in Adolescent Idiopathic Scoliosis Trial; it demonstrated significant benefit and quality-of-life outcomes associated with bracing for 18 hours/day. In this study, the rate of treatment success in the as-treated analysis was 72% in the intervention group and 48% in the control group. The rate of treatment success in the intention-to-treat analysis was 75% in the intervention group and 42% in the control group. The number needed to treat was three to prevent one case of curvature progression past 50%.
Exercise: The USPSTF found just two trials (n = 184) that evaluated the effectiveness of tailored physiotherapeutic, scoliosis-specific exercise treatments. The participants were older than 10 years and had Cobb angles ranging from 10 degrees to 25 degrees. At the 12-month follow-up, the studies showed significant improvement, including those in quality-of-life measures. In one of the trials, the intervention group had a Cobb angle reduction of 4.9 degrees while the control group had an increase of 2.8 degrees.
Harms: Only one good-quality study (n = 242) reported harms of bracing, which include skin problems, body pain, physical limitations, anxiety, and depression. The USPSTF did not find any studies that assessed the harms of treatment with exercise or surgery.
Association between spinal curvature severity and adult health outcomes
The USPSTF did not find any studies that directly addressed whether changes in the severity of spinal curvature in adolescence resulted in changes in adult health outcomes. The USPSTF did review two fair-quality retrospective, observational, long-term, follow-up analyses (n = 339) of adults diagnosed with idiopathic scoliosis in adolescence and treated with either bracing or surgery. Quality of life measurements, pulmonary consequences, and pregnancy outcomes were not significantly different between the two treatment groups or between those treated and those simply observed. However, those treated with bracing did report more negative treatment experience and body distortion.
Recommendation of others
The Scoliosis Research Society, American Academy of Orthopedic Surgeons, Pediatric Orthopedic Society of North America, and American Academy of Pediatrics issued a joint position statement in September 2015 recommending that screening examinations for scoliosis should be performed for females at ages 10 and 12 years and for males at either 13 or 14 years.2
Their rationale, articulated in the statement and in an editorial in JAMA accompanying the publication of the USPSTF statement, is primarily based on findings in the Bracing in Adolescent Idiopathic Scoliosis Trial that showed a 56% decrease in the rate of progression of moderate curves to greater than 50 degrees. The evidence that intervention works – along with concerns about costs, family burden, loss of school time, risks of surgical complications, and the 22% need for long-term revision surgery – makes avoidance of progression of curves in scoliosis a high-value issue. In addition, they reasoned, the screening trials from which the false-positive values were derived were primarily school-based screening and not done in physician offices.
The Bottom Line
Although the joint statement made by pediatric orthopedic societies and the American Academy of Pediatrics had recommended screening examinations, the USPSTF concluded that the current evidence is insufficient and that the balance of benefits and harms of screening for adolescent (aged 10-18 years) idiopathic scoliosis (Cobb angle greater than 10 degrees) cannot be determined, giving an “I” recommendation.
Dr. Aarisha Shrestha is a first-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
1. US Preventive Services Task Force. JAMA. 2018;319(2):165–72.
2. HreskoMT et al. SRS/POSNA/AAOS/AAP position statement: Screening for the early detection for idiopathic scoliosis in adolescents. 2015. Accessed December 8, 2017.
The United States Preventive Services Task Force (USPSTF) has issued recommendations on screening for idiopathic scoliosis in asymptomatic children and adolescents aged 10-18 years.1 This recommendation concluded that the current evidence on the benefits and harms of screening is insufficient (I statement) and updated its 2004 recommendation against routine screening, in which it had concluded that the harms of screening exceeded the potential benefits (D recommendation).
Importance
Screening methods
The USPSTF concluded that currently available screening tests can accurately detect adolescent idiopathic scoliosis. Screening methods include visual inspection using the forward bend test, use of scoliometer measurement of the angle of trunk rotation during forward bend test with a rotation of 5 degrees–7 degrees recommended to be referred for radiography, and Moiré topography that enumerates asymmetric contour lines on the back (values greater than 2 are referred to radiography).
The USPSTF reviewed seven fair-quality observational studies (n = 447,243) and concluded that screening with a combination of forward bend test, scoliometer measurement and that Moiré topography had the highest sensitivity (93.8%) and specificity (99.2%), a low false-negative rate (6.2%), the lowest false-positive rate (0.8%), and the highest positive predictive value (81%). Sensitivity was lower when screening programs used only one or two screening tests, and single screening tests were associated with highest false-positive rates.
In general, the potential harms associated with false-positive results include psychological harm, chest radiation exposure, and other unnecessary treatment, but the USPSTF did not find evidence on the direct harms of screening.
Effectiveness of intervention or treatment
Bracing: The USPSTF found five studies (n = 651) that evaluated the effectiveness of treatment with three different types of braces. The average ages of participants ranged from 12 to 13 years, and their curvature severity varied from Cobb angle of 20 degrees to 30 degrees. The largest study (n = 242) was a good-quality, international, controlled clinical trial known as the Bracing in Adolescent Idiopathic Scoliosis Trial; it demonstrated significant benefit and quality-of-life outcomes associated with bracing for 18 hours/day. In this study, the rate of treatment success in the as-treated analysis was 72% in the intervention group and 48% in the control group. The rate of treatment success in the intention-to-treat analysis was 75% in the intervention group and 42% in the control group. The number needed to treat was three to prevent one case of curvature progression past 50%.
Exercise: The USPSTF found just two trials (n = 184) that evaluated the effectiveness of tailored physiotherapeutic, scoliosis-specific exercise treatments. The participants were older than 10 years and had Cobb angles ranging from 10 degrees to 25 degrees. At the 12-month follow-up, the studies showed significant improvement, including those in quality-of-life measures. In one of the trials, the intervention group had a Cobb angle reduction of 4.9 degrees while the control group had an increase of 2.8 degrees.
Harms: Only one good-quality study (n = 242) reported harms of bracing, which include skin problems, body pain, physical limitations, anxiety, and depression. The USPSTF did not find any studies that assessed the harms of treatment with exercise or surgery.
Association between spinal curvature severity and adult health outcomes
The USPSTF did not find any studies that directly addressed whether changes in the severity of spinal curvature in adolescence resulted in changes in adult health outcomes. The USPSTF did review two fair-quality retrospective, observational, long-term, follow-up analyses (n = 339) of adults diagnosed with idiopathic scoliosis in adolescence and treated with either bracing or surgery. Quality of life measurements, pulmonary consequences, and pregnancy outcomes were not significantly different between the two treatment groups or between those treated and those simply observed. However, those treated with bracing did report more negative treatment experience and body distortion.
Recommendation of others
The Scoliosis Research Society, American Academy of Orthopedic Surgeons, Pediatric Orthopedic Society of North America, and American Academy of Pediatrics issued a joint position statement in September 2015 recommending that screening examinations for scoliosis should be performed for females at ages 10 and 12 years and for males at either 13 or 14 years.2
Their rationale, articulated in the statement and in an editorial in JAMA accompanying the publication of the USPSTF statement, is primarily based on findings in the Bracing in Adolescent Idiopathic Scoliosis Trial that showed a 56% decrease in the rate of progression of moderate curves to greater than 50 degrees. The evidence that intervention works – along with concerns about costs, family burden, loss of school time, risks of surgical complications, and the 22% need for long-term revision surgery – makes avoidance of progression of curves in scoliosis a high-value issue. In addition, they reasoned, the screening trials from which the false-positive values were derived were primarily school-based screening and not done in physician offices.
The Bottom Line
Although the joint statement made by pediatric orthopedic societies and the American Academy of Pediatrics had recommended screening examinations, the USPSTF concluded that the current evidence is insufficient and that the balance of benefits and harms of screening for adolescent (aged 10-18 years) idiopathic scoliosis (Cobb angle greater than 10 degrees) cannot be determined, giving an “I” recommendation.
Dr. Aarisha Shrestha is a first-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
1. US Preventive Services Task Force. JAMA. 2018;319(2):165–72.
2. HreskoMT et al. SRS/POSNA/AAOS/AAP position statement: Screening for the early detection for idiopathic scoliosis in adolescents. 2015. Accessed December 8, 2017.
The United States Preventive Services Task Force (USPSTF) has issued recommendations on screening for idiopathic scoliosis in asymptomatic children and adolescents aged 10-18 years.1 This recommendation concluded that the current evidence on the benefits and harms of screening is insufficient (I statement) and updated its 2004 recommendation against routine screening, in which it had concluded that the harms of screening exceeded the potential benefits (D recommendation).
Importance
Screening methods
The USPSTF concluded that currently available screening tests can accurately detect adolescent idiopathic scoliosis. Screening methods include visual inspection using the forward bend test, use of scoliometer measurement of the angle of trunk rotation during forward bend test with a rotation of 5 degrees–7 degrees recommended to be referred for radiography, and Moiré topography that enumerates asymmetric contour lines on the back (values greater than 2 are referred to radiography).
The USPSTF reviewed seven fair-quality observational studies (n = 447,243) and concluded that screening with a combination of forward bend test, scoliometer measurement and that Moiré topography had the highest sensitivity (93.8%) and specificity (99.2%), a low false-negative rate (6.2%), the lowest false-positive rate (0.8%), and the highest positive predictive value (81%). Sensitivity was lower when screening programs used only one or two screening tests, and single screening tests were associated with highest false-positive rates.
In general, the potential harms associated with false-positive results include psychological harm, chest radiation exposure, and other unnecessary treatment, but the USPSTF did not find evidence on the direct harms of screening.
Effectiveness of intervention or treatment
Bracing: The USPSTF found five studies (n = 651) that evaluated the effectiveness of treatment with three different types of braces. The average ages of participants ranged from 12 to 13 years, and their curvature severity varied from Cobb angle of 20 degrees to 30 degrees. The largest study (n = 242) was a good-quality, international, controlled clinical trial known as the Bracing in Adolescent Idiopathic Scoliosis Trial; it demonstrated significant benefit and quality-of-life outcomes associated with bracing for 18 hours/day. In this study, the rate of treatment success in the as-treated analysis was 72% in the intervention group and 48% in the control group. The rate of treatment success in the intention-to-treat analysis was 75% in the intervention group and 42% in the control group. The number needed to treat was three to prevent one case of curvature progression past 50%.
Exercise: The USPSTF found just two trials (n = 184) that evaluated the effectiveness of tailored physiotherapeutic, scoliosis-specific exercise treatments. The participants were older than 10 years and had Cobb angles ranging from 10 degrees to 25 degrees. At the 12-month follow-up, the studies showed significant improvement, including those in quality-of-life measures. In one of the trials, the intervention group had a Cobb angle reduction of 4.9 degrees while the control group had an increase of 2.8 degrees.
Harms: Only one good-quality study (n = 242) reported harms of bracing, which include skin problems, body pain, physical limitations, anxiety, and depression. The USPSTF did not find any studies that assessed the harms of treatment with exercise or surgery.
Association between spinal curvature severity and adult health outcomes
The USPSTF did not find any studies that directly addressed whether changes in the severity of spinal curvature in adolescence resulted in changes in adult health outcomes. The USPSTF did review two fair-quality retrospective, observational, long-term, follow-up analyses (n = 339) of adults diagnosed with idiopathic scoliosis in adolescence and treated with either bracing or surgery. Quality of life measurements, pulmonary consequences, and pregnancy outcomes were not significantly different between the two treatment groups or between those treated and those simply observed. However, those treated with bracing did report more negative treatment experience and body distortion.
Recommendation of others
The Scoliosis Research Society, American Academy of Orthopedic Surgeons, Pediatric Orthopedic Society of North America, and American Academy of Pediatrics issued a joint position statement in September 2015 recommending that screening examinations for scoliosis should be performed for females at ages 10 and 12 years and for males at either 13 or 14 years.2
Their rationale, articulated in the statement and in an editorial in JAMA accompanying the publication of the USPSTF statement, is primarily based on findings in the Bracing in Adolescent Idiopathic Scoliosis Trial that showed a 56% decrease in the rate of progression of moderate curves to greater than 50 degrees. The evidence that intervention works – along with concerns about costs, family burden, loss of school time, risks of surgical complications, and the 22% need for long-term revision surgery – makes avoidance of progression of curves in scoliosis a high-value issue. In addition, they reasoned, the screening trials from which the false-positive values were derived were primarily school-based screening and not done in physician offices.
The Bottom Line
Although the joint statement made by pediatric orthopedic societies and the American Academy of Pediatrics had recommended screening examinations, the USPSTF concluded that the current evidence is insufficient and that the balance of benefits and harms of screening for adolescent (aged 10-18 years) idiopathic scoliosis (Cobb angle greater than 10 degrees) cannot be determined, giving an “I” recommendation.
Dr. Aarisha Shrestha is a first-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
1. US Preventive Services Task Force. JAMA. 2018;319(2):165–72.
2. HreskoMT et al. SRS/POSNA/AAOS/AAP position statement: Screening for the early detection for idiopathic scoliosis in adolescents. 2015. Accessed December 8, 2017.
Denosumab on par with zoledronic acid for multiple myeloma bone disease
Denosumab was noninferior to zoledronic acid at delaying skeletal-related events in patients with newly diagnosed multiple myeloma and one or more lytic bone lesions, according to findings from an international randomized trial.
In a phase 3 double-blind, double-dummy, controlled trial, patients were randomly assigned to receive either subcutaneous denosumab or intravenous zoledronic acid, plus the investigator’s choice of first-line antimyeloma therapy. The primary endpoint of noninferiority of denosumab at preventing time to first skeletal-related event, compared with zoledronic acid, was met, reported Noopur Raje, MD, of the Massachusetts General Hospital Cancer Center, Boston, and colleagues.
Median progression-free survival, an exploratory endpoint, was significantly longer with denosumab – 46.1 months vs. 35.4 months – translating into a hazard ratio of 0.82 (P = .036) for progression on denosumab. There was no difference in overall survival, however.
Denosumab is a monoclonal antibody that binds to and inactivates receptor activator of nuclear factor kappa-B ligand, a promoter of osteoclast formation, activation, and survival. Zoledronic acid is a bisphosphonate that may have antimyeloma effects, the investigators noted.
“The greater progression-free survival with denosumab than with zoledronic acid is compelling in view of the previous preclinical and clinical evidence supporting an anti-RANKL[receptor factor kappa-B ligand]–mediated, antimyeloma effect. These results, in combination with the improved renal adverse event profile, support denosumab as an additional option to the standard of care for patients with multiple myeloma,” the investigators wrote in The Lancet Oncology.
The trial included 1,718 patients age 18 and older treated at 259 centers in 29 countries. All patients had newly diagnosed multiple myeloma with at least one documented lytic bone lesion. The patients were randomly assigned to denosumab or zoledronic acid, and were stratified by intent to undergo autologous stem cell transplant, antimyeloma therapy regimen, stage according to the International Staging System, previous skeletal-related events, and region.
As noted, the trial met the primary endpoint of noninferiority of denosumab, with a hazard ratio for time to first skeletal-related event vs. zoledronic acid of 0.98 (P = .010).
The safety analysis, which included all patients who were randomized and received at least one dose of study medication (850 on denosumab and 852 on zoledronic acid) showed that the agents were associated with similar incidences of neutropenia, thrombocytopenia, anemia, febrile neutropenia, and pneumonia. The incidence of renal toxicity, however, was lower with denosumab than with zoledronic acid (10% vs. 17%, respectively), whereas hypocalcemia was higher with denosumab (17% vs. 12%). There were no significant differences in the incidence of osteonecrosis of the jaw, a common problem with osteoclast inhibitors.
There was one treatment-related death, a case of cardiac arrest in a patient treated with zoledronic acid.
The investigators noted that the study was limited by a lack of response data, and by the fact that patients with creatinine clearance less than 30 mL/minute were not enrolled because of study blinding and the product label of zoledronic acid.
The study was sponsored by Amgen. Dr. Raje and multiple coauthors disclosed personal fees from Amgen and other companies. Three of the coauthors are current or former Amgen employees.
SOURCE: Raje NS et al. Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30072-X.
The study by Dr. Raje and colleagues is the largest placebo-controlled study ever done in patients with myeloma, but there are still many questions about how to move forward with this treatment. For instance, since only patients with myeloma-related bone disease at diagnosis were enrolled, the benefit of denosumab in patients without bone disease is uncertain. Additionally, the study did not show a survival benefit for denosumab over zoledronic acid.
Cost is also a factor, as denosumab is a higher priced treatment and requires continuous therapy. New treatment options, such as anti-CD38 monoclonal antibodies, are also available. It’s unclear how denosumab adds value in the context of these new therapies.
Meletios A. Dimopoulos, MD, and Efstathios Kastritis, MD, are with the department of clinical therapeutics at the National and Kapodistrian University of Athens, Greece. Dr. Dimopoulos had received honoraria from Celgene, Amgen, Takeda, Janssen, and Novartis. Dr. Kastritis has received honoraria from Janssen, Amgen, Prothena, Takeda, and Genesis Pharma. Their remarks are adapted from an accompanying editorial (Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30075-5).
The study by Dr. Raje and colleagues is the largest placebo-controlled study ever done in patients with myeloma, but there are still many questions about how to move forward with this treatment. For instance, since only patients with myeloma-related bone disease at diagnosis were enrolled, the benefit of denosumab in patients without bone disease is uncertain. Additionally, the study did not show a survival benefit for denosumab over zoledronic acid.
Cost is also a factor, as denosumab is a higher priced treatment and requires continuous therapy. New treatment options, such as anti-CD38 monoclonal antibodies, are also available. It’s unclear how denosumab adds value in the context of these new therapies.
Meletios A. Dimopoulos, MD, and Efstathios Kastritis, MD, are with the department of clinical therapeutics at the National and Kapodistrian University of Athens, Greece. Dr. Dimopoulos had received honoraria from Celgene, Amgen, Takeda, Janssen, and Novartis. Dr. Kastritis has received honoraria from Janssen, Amgen, Prothena, Takeda, and Genesis Pharma. Their remarks are adapted from an accompanying editorial (Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30075-5).
The study by Dr. Raje and colleagues is the largest placebo-controlled study ever done in patients with myeloma, but there are still many questions about how to move forward with this treatment. For instance, since only patients with myeloma-related bone disease at diagnosis were enrolled, the benefit of denosumab in patients without bone disease is uncertain. Additionally, the study did not show a survival benefit for denosumab over zoledronic acid.
Cost is also a factor, as denosumab is a higher priced treatment and requires continuous therapy. New treatment options, such as anti-CD38 monoclonal antibodies, are also available. It’s unclear how denosumab adds value in the context of these new therapies.
Meletios A. Dimopoulos, MD, and Efstathios Kastritis, MD, are with the department of clinical therapeutics at the National and Kapodistrian University of Athens, Greece. Dr. Dimopoulos had received honoraria from Celgene, Amgen, Takeda, Janssen, and Novartis. Dr. Kastritis has received honoraria from Janssen, Amgen, Prothena, Takeda, and Genesis Pharma. Their remarks are adapted from an accompanying editorial (Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30075-5).
Denosumab was noninferior to zoledronic acid at delaying skeletal-related events in patients with newly diagnosed multiple myeloma and one or more lytic bone lesions, according to findings from an international randomized trial.
In a phase 3 double-blind, double-dummy, controlled trial, patients were randomly assigned to receive either subcutaneous denosumab or intravenous zoledronic acid, plus the investigator’s choice of first-line antimyeloma therapy. The primary endpoint of noninferiority of denosumab at preventing time to first skeletal-related event, compared with zoledronic acid, was met, reported Noopur Raje, MD, of the Massachusetts General Hospital Cancer Center, Boston, and colleagues.
Median progression-free survival, an exploratory endpoint, was significantly longer with denosumab – 46.1 months vs. 35.4 months – translating into a hazard ratio of 0.82 (P = .036) for progression on denosumab. There was no difference in overall survival, however.
Denosumab is a monoclonal antibody that binds to and inactivates receptor activator of nuclear factor kappa-B ligand, a promoter of osteoclast formation, activation, and survival. Zoledronic acid is a bisphosphonate that may have antimyeloma effects, the investigators noted.
“The greater progression-free survival with denosumab than with zoledronic acid is compelling in view of the previous preclinical and clinical evidence supporting an anti-RANKL[receptor factor kappa-B ligand]–mediated, antimyeloma effect. These results, in combination with the improved renal adverse event profile, support denosumab as an additional option to the standard of care for patients with multiple myeloma,” the investigators wrote in The Lancet Oncology.
The trial included 1,718 patients age 18 and older treated at 259 centers in 29 countries. All patients had newly diagnosed multiple myeloma with at least one documented lytic bone lesion. The patients were randomly assigned to denosumab or zoledronic acid, and were stratified by intent to undergo autologous stem cell transplant, antimyeloma therapy regimen, stage according to the International Staging System, previous skeletal-related events, and region.
As noted, the trial met the primary endpoint of noninferiority of denosumab, with a hazard ratio for time to first skeletal-related event vs. zoledronic acid of 0.98 (P = .010).
The safety analysis, which included all patients who were randomized and received at least one dose of study medication (850 on denosumab and 852 on zoledronic acid) showed that the agents were associated with similar incidences of neutropenia, thrombocytopenia, anemia, febrile neutropenia, and pneumonia. The incidence of renal toxicity, however, was lower with denosumab than with zoledronic acid (10% vs. 17%, respectively), whereas hypocalcemia was higher with denosumab (17% vs. 12%). There were no significant differences in the incidence of osteonecrosis of the jaw, a common problem with osteoclast inhibitors.
There was one treatment-related death, a case of cardiac arrest in a patient treated with zoledronic acid.
The investigators noted that the study was limited by a lack of response data, and by the fact that patients with creatinine clearance less than 30 mL/minute were not enrolled because of study blinding and the product label of zoledronic acid.
The study was sponsored by Amgen. Dr. Raje and multiple coauthors disclosed personal fees from Amgen and other companies. Three of the coauthors are current or former Amgen employees.
SOURCE: Raje NS et al. Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30072-X.
Denosumab was noninferior to zoledronic acid at delaying skeletal-related events in patients with newly diagnosed multiple myeloma and one or more lytic bone lesions, according to findings from an international randomized trial.
In a phase 3 double-blind, double-dummy, controlled trial, patients were randomly assigned to receive either subcutaneous denosumab or intravenous zoledronic acid, plus the investigator’s choice of first-line antimyeloma therapy. The primary endpoint of noninferiority of denosumab at preventing time to first skeletal-related event, compared with zoledronic acid, was met, reported Noopur Raje, MD, of the Massachusetts General Hospital Cancer Center, Boston, and colleagues.
Median progression-free survival, an exploratory endpoint, was significantly longer with denosumab – 46.1 months vs. 35.4 months – translating into a hazard ratio of 0.82 (P = .036) for progression on denosumab. There was no difference in overall survival, however.
Denosumab is a monoclonal antibody that binds to and inactivates receptor activator of nuclear factor kappa-B ligand, a promoter of osteoclast formation, activation, and survival. Zoledronic acid is a bisphosphonate that may have antimyeloma effects, the investigators noted.
“The greater progression-free survival with denosumab than with zoledronic acid is compelling in view of the previous preclinical and clinical evidence supporting an anti-RANKL[receptor factor kappa-B ligand]–mediated, antimyeloma effect. These results, in combination with the improved renal adverse event profile, support denosumab as an additional option to the standard of care for patients with multiple myeloma,” the investigators wrote in The Lancet Oncology.
The trial included 1,718 patients age 18 and older treated at 259 centers in 29 countries. All patients had newly diagnosed multiple myeloma with at least one documented lytic bone lesion. The patients were randomly assigned to denosumab or zoledronic acid, and were stratified by intent to undergo autologous stem cell transplant, antimyeloma therapy regimen, stage according to the International Staging System, previous skeletal-related events, and region.
As noted, the trial met the primary endpoint of noninferiority of denosumab, with a hazard ratio for time to first skeletal-related event vs. zoledronic acid of 0.98 (P = .010).
The safety analysis, which included all patients who were randomized and received at least one dose of study medication (850 on denosumab and 852 on zoledronic acid) showed that the agents were associated with similar incidences of neutropenia, thrombocytopenia, anemia, febrile neutropenia, and pneumonia. The incidence of renal toxicity, however, was lower with denosumab than with zoledronic acid (10% vs. 17%, respectively), whereas hypocalcemia was higher with denosumab (17% vs. 12%). There were no significant differences in the incidence of osteonecrosis of the jaw, a common problem with osteoclast inhibitors.
There was one treatment-related death, a case of cardiac arrest in a patient treated with zoledronic acid.
The investigators noted that the study was limited by a lack of response data, and by the fact that patients with creatinine clearance less than 30 mL/minute were not enrolled because of study blinding and the product label of zoledronic acid.
The study was sponsored by Amgen. Dr. Raje and multiple coauthors disclosed personal fees from Amgen and other companies. Three of the coauthors are current or former Amgen employees.
SOURCE: Raje NS et al. Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30072-X.
FROM LANCET ONCOLOGY
Key clinical point: Denosumab was noninferior to zoledronic acid for time to skeletal-related events in patients with multiple myeloma with bone involvement.
Major finding: The hazard ratio for noninferiority of denosumab was 0.98 (P = .010).
Data source: A phase 3 randomized double-blind, double-dummy, controlled trial in 1,718 patients with newly diagnosed multiple myeloma with one or more lytic bone lesions.
Disclosures: The study was sponsored by Amgen. Dr. Raje and multiple coauthors disclosed personal fees from Amgen and other companies. Three of the coauthors are current or former Amgen employees.
Source: Raje NS et al. Lancet Oncol. 2018 Feb 8. doi: 10.1016/S1470-2045(18)30072-X.
Set realistic expectations prior to perioral rejuvenation procedures
MIAMI – The success of perioral rejuvenation depends in large part on setting realistic expectations. But there are also tips and tricks to individualizing the technique for each patient that can lead to better outcomes and greater satisfaction – whether patients receive injections into the fine lines above the lip, full-field erbium laser resurfacing, neuromodulator treatment, or a combination approach, according to Joel L. Cohen, MD.
When a patient presents with major lines in the perioral area, an “orange peel” texture, and/or elastotic changes, laser resurfacing can be an appropriate option. “Full field erbium laser resurfacing can give patients a nice improvement of upper lip lines and even a nice contraction of oral commissure,” Dr. Cohen said at the Orlando Dermatology Aesthetic and Clinical Conference.
Although each treatment is individualized to the patient, “I tend to do full field erbium resurfacing around the mouth and eyes, and fractional ablative resurfacing around the rest of the face,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in metropolitan Denver.
More downtime is associated with laser resurfacing compared with fillers or neuromodulator injections, but long-term patient satisfaction, even with improvement in quality of life for some patients (who become less anxious about these lines and more self-confident), can make this approach worthwhile. During his presentation, Dr. Cohen showed photos of many of his treated patients, including one woman whose grandchildren he said had been commenting about the “orange peel texture of her upper lip,” until she completed the resurfacing treatment.
Keep expectations realistic
Dr. Cohen recommended counseling patients about the potential benefits – and the caveats – associated with full-field erbium heavier resurfacing. “Make sure people understand they will look terrible for several days after heavy resurfacing, usually taking about 10-12 days to re-epithelialize,” he said. “We need to tell patients that the perioral area typically manifests more lines than other areas, so we need treat this area differently than just ablative fractional resurfacing in many cases.”
He explained that with heavier resurfacing procedures, it helps to show patients what is expected over the days to weeks in the healing process. They need to understand and see photos that show that the full-field erbium areas will have a yellow fibrinous healing response for the first week or so, which looks very different from the fractional ablative-treated areas (which are more typically red, weepy, and swollen).
He encourages these patients to come back a few days after the procedure to check their healing and review wound-care instructions, especially for patients who have deeper full-field perioral erbium resurfacing (those who are treated with 450-700 microns). Another tip he provided is to have these postresurfacing patients enter/exit through a separate entrance and also sit in a separate cosmetic waiting room at off-hours.
Re-epithelialization generally takes about 10-12 days for most people, with a maximal improvement at approximately 3 months, Dr. Cohen said. “Some patients can see not only significant improvement of upper lip lines, but often a nice contraction of the oral commissure even before fillers are performed to buttress the marionette area and oral commissure,” he said.
With full-field ablative resurfacing in specific areas, rather than simply fractional ablative resurfacing, it is also important to educate patients that some postinflammatory erythema is expected, which, in some cases, may persist for a few months. “In my experience, topical vasoconstrictors don’t seem to help minimize prolonged redness in the full-field erbium areas, but potent topical steroids can be beneficial,” Dr. Cohen said.
More tips for success
Injected local anesthesia is warranted prior to heavier laser resurfacing to keep patients as comfortable as possible. An infraorbital block with an added submucosal/sulcus block with plain lidocaine can be a good approach, he noted. Different perioral and facial areas have different degrees of lines, requiring different laser settings. He prefers to use plain lidocaine perioral blocks, “so that I can theoretically best see the endpoint pinpoint bleeding,” he said, adding that “significant pinpoint bleeding is a good place to stop.”
Typically, he uses a neuromodulator a week or two before full-field perioral erbium resurfacing. “I choose not to give a neuromodulator on the same day as I am concerned about swelling or manipulation of the skin causing unwanted spread to adjacent musculature,” Dr. Cohen said.
Another tip is to take photos with more than one device. “Standardized photos may lose detail of etched lines; we take both iPad and standardized camera system photos,” he said, adding that it is important that clinic staff are proficient at taking proper before-and-after photos, making sure, for example, that the patient does not have confounding makeup or lipstick on for photos, and patient positioning is consistent.
He said it is imperative to emphasize the importance of diligent sun protection for several months after the laser procedure. “Every patient reassures us they use sunscreen, but we often don’t know what sunscreen they are using or how frequently they are using it,” Dr. Cohen said. “If they don’t follow our specific instructions to use a physical block sunscreen, they will significantly increase their risk of developing postinflammatory hyperpigmentation. This caveat applies all year round, and isn’t just for those that go to the beach or play golf, but is also especially important for those patients that ski or hike at higher altitudes.”
Depending on the degree of etched-in lines in the perioral area, one perioral full-field laser resurfacing treatment is generally sufficient for most patients to see significant improvement. For those with more severe etched lines and/or bigger goals for improvement, additional treatments can be performed – but he generally waits about 3 months to see the overall effect of the initial treatment session.
If patients have just a few discrete etched-lines on each side of the upper lip, fillers can be helpful. But, the number and caliber of fine lines on the cutaneous lip limit how much a dermatologist can realistically treat. “So for people with many, many etched-in lines on the cutaneous lip, I explain that fillers are not the right tool for the job – and that they need heavier laser resurfacing.” And those patients really concerned about downtime need to understand that the bruising that can occur with fillers for several days can lead to some degree of social downtime as well.
Options to treat perioral lines not ‘etched in’
Sometimes younger patients, those in their late 20s to early 40s, present with concerns about their perioral appearance. Although they do not have lines at rest yet, they can be unhappy with the muscle columns that appear above their lips with animation that begin to cause lines at rest imprinted in the skin. And many of these women complain that their lipstick bleeds into this area,” Dr. Cohen said. “These patients without etched lines can be treated with a neuromodulator alone to soften the mechanical action of the orbicularis oris muscle,” he pointed out.
Dr. Cohen disclosed having participated in clinical trials and/or having served as a consultant for Merz, Allergan, Galderma, Suneva, Sciton, and Lutronic.
MIAMI – The success of perioral rejuvenation depends in large part on setting realistic expectations. But there are also tips and tricks to individualizing the technique for each patient that can lead to better outcomes and greater satisfaction – whether patients receive injections into the fine lines above the lip, full-field erbium laser resurfacing, neuromodulator treatment, or a combination approach, according to Joel L. Cohen, MD.
When a patient presents with major lines in the perioral area, an “orange peel” texture, and/or elastotic changes, laser resurfacing can be an appropriate option. “Full field erbium laser resurfacing can give patients a nice improvement of upper lip lines and even a nice contraction of oral commissure,” Dr. Cohen said at the Orlando Dermatology Aesthetic and Clinical Conference.
Although each treatment is individualized to the patient, “I tend to do full field erbium resurfacing around the mouth and eyes, and fractional ablative resurfacing around the rest of the face,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in metropolitan Denver.
More downtime is associated with laser resurfacing compared with fillers or neuromodulator injections, but long-term patient satisfaction, even with improvement in quality of life for some patients (who become less anxious about these lines and more self-confident), can make this approach worthwhile. During his presentation, Dr. Cohen showed photos of many of his treated patients, including one woman whose grandchildren he said had been commenting about the “orange peel texture of her upper lip,” until she completed the resurfacing treatment.
Keep expectations realistic
Dr. Cohen recommended counseling patients about the potential benefits – and the caveats – associated with full-field erbium heavier resurfacing. “Make sure people understand they will look terrible for several days after heavy resurfacing, usually taking about 10-12 days to re-epithelialize,” he said. “We need to tell patients that the perioral area typically manifests more lines than other areas, so we need treat this area differently than just ablative fractional resurfacing in many cases.”
He explained that with heavier resurfacing procedures, it helps to show patients what is expected over the days to weeks in the healing process. They need to understand and see photos that show that the full-field erbium areas will have a yellow fibrinous healing response for the first week or so, which looks very different from the fractional ablative-treated areas (which are more typically red, weepy, and swollen).
He encourages these patients to come back a few days after the procedure to check their healing and review wound-care instructions, especially for patients who have deeper full-field perioral erbium resurfacing (those who are treated with 450-700 microns). Another tip he provided is to have these postresurfacing patients enter/exit through a separate entrance and also sit in a separate cosmetic waiting room at off-hours.
Re-epithelialization generally takes about 10-12 days for most people, with a maximal improvement at approximately 3 months, Dr. Cohen said. “Some patients can see not only significant improvement of upper lip lines, but often a nice contraction of the oral commissure even before fillers are performed to buttress the marionette area and oral commissure,” he said.
With full-field ablative resurfacing in specific areas, rather than simply fractional ablative resurfacing, it is also important to educate patients that some postinflammatory erythema is expected, which, in some cases, may persist for a few months. “In my experience, topical vasoconstrictors don’t seem to help minimize prolonged redness in the full-field erbium areas, but potent topical steroids can be beneficial,” Dr. Cohen said.
More tips for success
Injected local anesthesia is warranted prior to heavier laser resurfacing to keep patients as comfortable as possible. An infraorbital block with an added submucosal/sulcus block with plain lidocaine can be a good approach, he noted. Different perioral and facial areas have different degrees of lines, requiring different laser settings. He prefers to use plain lidocaine perioral blocks, “so that I can theoretically best see the endpoint pinpoint bleeding,” he said, adding that “significant pinpoint bleeding is a good place to stop.”
Typically, he uses a neuromodulator a week or two before full-field perioral erbium resurfacing. “I choose not to give a neuromodulator on the same day as I am concerned about swelling or manipulation of the skin causing unwanted spread to adjacent musculature,” Dr. Cohen said.
Another tip is to take photos with more than one device. “Standardized photos may lose detail of etched lines; we take both iPad and standardized camera system photos,” he said, adding that it is important that clinic staff are proficient at taking proper before-and-after photos, making sure, for example, that the patient does not have confounding makeup or lipstick on for photos, and patient positioning is consistent.
He said it is imperative to emphasize the importance of diligent sun protection for several months after the laser procedure. “Every patient reassures us they use sunscreen, but we often don’t know what sunscreen they are using or how frequently they are using it,” Dr. Cohen said. “If they don’t follow our specific instructions to use a physical block sunscreen, they will significantly increase their risk of developing postinflammatory hyperpigmentation. This caveat applies all year round, and isn’t just for those that go to the beach or play golf, but is also especially important for those patients that ski or hike at higher altitudes.”
Depending on the degree of etched-in lines in the perioral area, one perioral full-field laser resurfacing treatment is generally sufficient for most patients to see significant improvement. For those with more severe etched lines and/or bigger goals for improvement, additional treatments can be performed – but he generally waits about 3 months to see the overall effect of the initial treatment session.
If patients have just a few discrete etched-lines on each side of the upper lip, fillers can be helpful. But, the number and caliber of fine lines on the cutaneous lip limit how much a dermatologist can realistically treat. “So for people with many, many etched-in lines on the cutaneous lip, I explain that fillers are not the right tool for the job – and that they need heavier laser resurfacing.” And those patients really concerned about downtime need to understand that the bruising that can occur with fillers for several days can lead to some degree of social downtime as well.
Options to treat perioral lines not ‘etched in’
Sometimes younger patients, those in their late 20s to early 40s, present with concerns about their perioral appearance. Although they do not have lines at rest yet, they can be unhappy with the muscle columns that appear above their lips with animation that begin to cause lines at rest imprinted in the skin. And many of these women complain that their lipstick bleeds into this area,” Dr. Cohen said. “These patients without etched lines can be treated with a neuromodulator alone to soften the mechanical action of the orbicularis oris muscle,” he pointed out.
Dr. Cohen disclosed having participated in clinical trials and/or having served as a consultant for Merz, Allergan, Galderma, Suneva, Sciton, and Lutronic.
MIAMI – The success of perioral rejuvenation depends in large part on setting realistic expectations. But there are also tips and tricks to individualizing the technique for each patient that can lead to better outcomes and greater satisfaction – whether patients receive injections into the fine lines above the lip, full-field erbium laser resurfacing, neuromodulator treatment, or a combination approach, according to Joel L. Cohen, MD.
When a patient presents with major lines in the perioral area, an “orange peel” texture, and/or elastotic changes, laser resurfacing can be an appropriate option. “Full field erbium laser resurfacing can give patients a nice improvement of upper lip lines and even a nice contraction of oral commissure,” Dr. Cohen said at the Orlando Dermatology Aesthetic and Clinical Conference.
Although each treatment is individualized to the patient, “I tend to do full field erbium resurfacing around the mouth and eyes, and fractional ablative resurfacing around the rest of the face,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in metropolitan Denver.
More downtime is associated with laser resurfacing compared with fillers or neuromodulator injections, but long-term patient satisfaction, even with improvement in quality of life for some patients (who become less anxious about these lines and more self-confident), can make this approach worthwhile. During his presentation, Dr. Cohen showed photos of many of his treated patients, including one woman whose grandchildren he said had been commenting about the “orange peel texture of her upper lip,” until she completed the resurfacing treatment.
Keep expectations realistic
Dr. Cohen recommended counseling patients about the potential benefits – and the caveats – associated with full-field erbium heavier resurfacing. “Make sure people understand they will look terrible for several days after heavy resurfacing, usually taking about 10-12 days to re-epithelialize,” he said. “We need to tell patients that the perioral area typically manifests more lines than other areas, so we need treat this area differently than just ablative fractional resurfacing in many cases.”
He explained that with heavier resurfacing procedures, it helps to show patients what is expected over the days to weeks in the healing process. They need to understand and see photos that show that the full-field erbium areas will have a yellow fibrinous healing response for the first week or so, which looks very different from the fractional ablative-treated areas (which are more typically red, weepy, and swollen).
He encourages these patients to come back a few days after the procedure to check their healing and review wound-care instructions, especially for patients who have deeper full-field perioral erbium resurfacing (those who are treated with 450-700 microns). Another tip he provided is to have these postresurfacing patients enter/exit through a separate entrance and also sit in a separate cosmetic waiting room at off-hours.
Re-epithelialization generally takes about 10-12 days for most people, with a maximal improvement at approximately 3 months, Dr. Cohen said. “Some patients can see not only significant improvement of upper lip lines, but often a nice contraction of the oral commissure even before fillers are performed to buttress the marionette area and oral commissure,” he said.
With full-field ablative resurfacing in specific areas, rather than simply fractional ablative resurfacing, it is also important to educate patients that some postinflammatory erythema is expected, which, in some cases, may persist for a few months. “In my experience, topical vasoconstrictors don’t seem to help minimize prolonged redness in the full-field erbium areas, but potent topical steroids can be beneficial,” Dr. Cohen said.
More tips for success
Injected local anesthesia is warranted prior to heavier laser resurfacing to keep patients as comfortable as possible. An infraorbital block with an added submucosal/sulcus block with plain lidocaine can be a good approach, he noted. Different perioral and facial areas have different degrees of lines, requiring different laser settings. He prefers to use plain lidocaine perioral blocks, “so that I can theoretically best see the endpoint pinpoint bleeding,” he said, adding that “significant pinpoint bleeding is a good place to stop.”
Typically, he uses a neuromodulator a week or two before full-field perioral erbium resurfacing. “I choose not to give a neuromodulator on the same day as I am concerned about swelling or manipulation of the skin causing unwanted spread to adjacent musculature,” Dr. Cohen said.
Another tip is to take photos with more than one device. “Standardized photos may lose detail of etched lines; we take both iPad and standardized camera system photos,” he said, adding that it is important that clinic staff are proficient at taking proper before-and-after photos, making sure, for example, that the patient does not have confounding makeup or lipstick on for photos, and patient positioning is consistent.
He said it is imperative to emphasize the importance of diligent sun protection for several months after the laser procedure. “Every patient reassures us they use sunscreen, but we often don’t know what sunscreen they are using or how frequently they are using it,” Dr. Cohen said. “If they don’t follow our specific instructions to use a physical block sunscreen, they will significantly increase their risk of developing postinflammatory hyperpigmentation. This caveat applies all year round, and isn’t just for those that go to the beach or play golf, but is also especially important for those patients that ski or hike at higher altitudes.”
Depending on the degree of etched-in lines in the perioral area, one perioral full-field laser resurfacing treatment is generally sufficient for most patients to see significant improvement. For those with more severe etched lines and/or bigger goals for improvement, additional treatments can be performed – but he generally waits about 3 months to see the overall effect of the initial treatment session.
If patients have just a few discrete etched-lines on each side of the upper lip, fillers can be helpful. But, the number and caliber of fine lines on the cutaneous lip limit how much a dermatologist can realistically treat. “So for people with many, many etched-in lines on the cutaneous lip, I explain that fillers are not the right tool for the job – and that they need heavier laser resurfacing.” And those patients really concerned about downtime need to understand that the bruising that can occur with fillers for several days can lead to some degree of social downtime as well.
Options to treat perioral lines not ‘etched in’
Sometimes younger patients, those in their late 20s to early 40s, present with concerns about their perioral appearance. Although they do not have lines at rest yet, they can be unhappy with the muscle columns that appear above their lips with animation that begin to cause lines at rest imprinted in the skin. And many of these women complain that their lipstick bleeds into this area,” Dr. Cohen said. “These patients without etched lines can be treated with a neuromodulator alone to soften the mechanical action of the orbicularis oris muscle,” he pointed out.
Dr. Cohen disclosed having participated in clinical trials and/or having served as a consultant for Merz, Allergan, Galderma, Suneva, Sciton, and Lutronic.
EXPERT ANALYSIS FROM ODAC 2018
Anticoagulation use in new-onset secondary atrial fibrillation
Background: Data on the efficacy of anticoagulation to reduce stroke risk in patients with new-onset atrial fibrillation due to acute coronary syndrome (ACS), acute pulmonary disease (APD), and sepsis are limited.
Study design: Retrospective cohort study.
Setting: All hospitals in Quebec.
Synopsis: Authors included 2,304 patients aged 65 and older with new atrial fibrillation secondary to ACS, APD, and sepsis. Anticoagulation was started for 38.4%, 34.1%, and 27.7% of these patients and the incidence of stroke was 5.4%, 3.9%, and 5.8% in the ACS, APD, and sepsis populations, respectively. After 3 years, anticoagulation use was not associated with a lower risk of ischemic stroke in any cohort. In a multivariate analysis adjusted for the HAS-BLED score, anticoagulation was associated with a higher risk of bleeding in patients with APD (odds ratio, 1.72; 95% confidence interval, 1.23-2.39) but not in ACS or sepsis.
The major limitation of this study was the reliance on administrative data alone, making it difficult to confirm and capture all patients with transient atrial fibrillation.
Bottom line: Anticoagulation use in patients with secondary atrial fibrillation may not be associated with a reduction in ischemic strokes, but may be associated with an increased bleeding risk in patients with atrial fibrillation secondary to acute pulmonary disease.
Citation: Quon MJ et al. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC: Clinical Electrophysiology. Article in Press.
Dr. Gala is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: Data on the efficacy of anticoagulation to reduce stroke risk in patients with new-onset atrial fibrillation due to acute coronary syndrome (ACS), acute pulmonary disease (APD), and sepsis are limited.
Study design: Retrospective cohort study.
Setting: All hospitals in Quebec.
Synopsis: Authors included 2,304 patients aged 65 and older with new atrial fibrillation secondary to ACS, APD, and sepsis. Anticoagulation was started for 38.4%, 34.1%, and 27.7% of these patients and the incidence of stroke was 5.4%, 3.9%, and 5.8% in the ACS, APD, and sepsis populations, respectively. After 3 years, anticoagulation use was not associated with a lower risk of ischemic stroke in any cohort. In a multivariate analysis adjusted for the HAS-BLED score, anticoagulation was associated with a higher risk of bleeding in patients with APD (odds ratio, 1.72; 95% confidence interval, 1.23-2.39) but not in ACS or sepsis.
The major limitation of this study was the reliance on administrative data alone, making it difficult to confirm and capture all patients with transient atrial fibrillation.
Bottom line: Anticoagulation use in patients with secondary atrial fibrillation may not be associated with a reduction in ischemic strokes, but may be associated with an increased bleeding risk in patients with atrial fibrillation secondary to acute pulmonary disease.
Citation: Quon MJ et al. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC: Clinical Electrophysiology. Article in Press.
Dr. Gala is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: Data on the efficacy of anticoagulation to reduce stroke risk in patients with new-onset atrial fibrillation due to acute coronary syndrome (ACS), acute pulmonary disease (APD), and sepsis are limited.
Study design: Retrospective cohort study.
Setting: All hospitals in Quebec.
Synopsis: Authors included 2,304 patients aged 65 and older with new atrial fibrillation secondary to ACS, APD, and sepsis. Anticoagulation was started for 38.4%, 34.1%, and 27.7% of these patients and the incidence of stroke was 5.4%, 3.9%, and 5.8% in the ACS, APD, and sepsis populations, respectively. After 3 years, anticoagulation use was not associated with a lower risk of ischemic stroke in any cohort. In a multivariate analysis adjusted for the HAS-BLED score, anticoagulation was associated with a higher risk of bleeding in patients with APD (odds ratio, 1.72; 95% confidence interval, 1.23-2.39) but not in ACS or sepsis.
The major limitation of this study was the reliance on administrative data alone, making it difficult to confirm and capture all patients with transient atrial fibrillation.
Bottom line: Anticoagulation use in patients with secondary atrial fibrillation may not be associated with a reduction in ischemic strokes, but may be associated with an increased bleeding risk in patients with atrial fibrillation secondary to acute pulmonary disease.
Citation: Quon MJ et al. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC: Clinical Electrophysiology. Article in Press.
Dr. Gala is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Exercise during chemotherapy may yield long-term physical benefits
ORLANDO – Physical exercise during adjuvant chemotherapy may lead to improved physical activity and decreased fatigue years after the treatment is completed, results of a recent analysis suggest.
Four years after participating in an exercise program that took place during cancer treatment, patients reported more moderate-to-vigorous activity and less fatigue, compared with patients who did not participate in the program, according to long-term follow-up results presented at the Cancer Survivorship Symposium.
“We think that offering exercise during cancer treatment, including chemotherapy, is recommended and has beneficial short- and long-term effects on health,” said Anne M. May, PhD, of University Medical Center, Utrecht, the Netherlands.
Speaking in a press conference at the symposium, Dr. May described results of the analysis, which included 128 patients who had previously participated in PACT, a 237-patient randomized controlled trial evaluating a supervised exercise program versus usual care in patients undergoing adjuvant treatment for breast or colon cancer.
The 18-week exercise program included moderate- to high-intensity aerobic and strength training under physical therapist supervision for 60 minutes twice weekly, plus home-based physical activity for 30 minutes three times weekly.
At 4 years’ follow-up, patients in the exercise group reported on average 90 minutes of moderate-to-vigorous exercise per week, compared to an average of 70 minutes per week in the usual care group (P less than .05), Dr. May reported at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
There was also a trend toward decreased fatigue reported in the exercise vs. usual care group, though the finding did not reach statistical significance, she said.
It is “encouraging” to see that this exercise program had a long-term impact on patients’ physical activity levels, said ASCO expert Timothy Gilligan, MD, MSc.
“I think the public sometimes gets jaded because the nutritional recommendations seem to change every year, but if you look at the research on exercise in health … it’s interesting how consistent the data is that exercise really is good for us – if we can only get people to do it,” said Dr. Gilligan, who moderated the press conference.
Dr. May said she had no disclosures to report for the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
SOURCE: May AM et al. Cancer Survivorship Symposium, Abstract 99.
ORLANDO – Physical exercise during adjuvant chemotherapy may lead to improved physical activity and decreased fatigue years after the treatment is completed, results of a recent analysis suggest.
Four years after participating in an exercise program that took place during cancer treatment, patients reported more moderate-to-vigorous activity and less fatigue, compared with patients who did not participate in the program, according to long-term follow-up results presented at the Cancer Survivorship Symposium.
“We think that offering exercise during cancer treatment, including chemotherapy, is recommended and has beneficial short- and long-term effects on health,” said Anne M. May, PhD, of University Medical Center, Utrecht, the Netherlands.
Speaking in a press conference at the symposium, Dr. May described results of the analysis, which included 128 patients who had previously participated in PACT, a 237-patient randomized controlled trial evaluating a supervised exercise program versus usual care in patients undergoing adjuvant treatment for breast or colon cancer.
The 18-week exercise program included moderate- to high-intensity aerobic and strength training under physical therapist supervision for 60 minutes twice weekly, plus home-based physical activity for 30 minutes three times weekly.
At 4 years’ follow-up, patients in the exercise group reported on average 90 minutes of moderate-to-vigorous exercise per week, compared to an average of 70 minutes per week in the usual care group (P less than .05), Dr. May reported at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
There was also a trend toward decreased fatigue reported in the exercise vs. usual care group, though the finding did not reach statistical significance, she said.
It is “encouraging” to see that this exercise program had a long-term impact on patients’ physical activity levels, said ASCO expert Timothy Gilligan, MD, MSc.
“I think the public sometimes gets jaded because the nutritional recommendations seem to change every year, but if you look at the research on exercise in health … it’s interesting how consistent the data is that exercise really is good for us – if we can only get people to do it,” said Dr. Gilligan, who moderated the press conference.
Dr. May said she had no disclosures to report for the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
SOURCE: May AM et al. Cancer Survivorship Symposium, Abstract 99.
ORLANDO – Physical exercise during adjuvant chemotherapy may lead to improved physical activity and decreased fatigue years after the treatment is completed, results of a recent analysis suggest.
Four years after participating in an exercise program that took place during cancer treatment, patients reported more moderate-to-vigorous activity and less fatigue, compared with patients who did not participate in the program, according to long-term follow-up results presented at the Cancer Survivorship Symposium.
“We think that offering exercise during cancer treatment, including chemotherapy, is recommended and has beneficial short- and long-term effects on health,” said Anne M. May, PhD, of University Medical Center, Utrecht, the Netherlands.
Speaking in a press conference at the symposium, Dr. May described results of the analysis, which included 128 patients who had previously participated in PACT, a 237-patient randomized controlled trial evaluating a supervised exercise program versus usual care in patients undergoing adjuvant treatment for breast or colon cancer.
The 18-week exercise program included moderate- to high-intensity aerobic and strength training under physical therapist supervision for 60 minutes twice weekly, plus home-based physical activity for 30 minutes three times weekly.
At 4 years’ follow-up, patients in the exercise group reported on average 90 minutes of moderate-to-vigorous exercise per week, compared to an average of 70 minutes per week in the usual care group (P less than .05), Dr. May reported at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
There was also a trend toward decreased fatigue reported in the exercise vs. usual care group, though the finding did not reach statistical significance, she said.
It is “encouraging” to see that this exercise program had a long-term impact on patients’ physical activity levels, said ASCO expert Timothy Gilligan, MD, MSc.
“I think the public sometimes gets jaded because the nutritional recommendations seem to change every year, but if you look at the research on exercise in health … it’s interesting how consistent the data is that exercise really is good for us – if we can only get people to do it,” said Dr. Gilligan, who moderated the press conference.
Dr. May said she had no disclosures to report for the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
SOURCE: May AM et al. Cancer Survivorship Symposium, Abstract 99.
REPORTING FROM CSC 2018
Key clinical point: Exercise during adjuvant chemotherapy may lead to lower levels of fatigue and higher levels of physical activity years after treatment is completed.
Major finding: Four years after participating in an 18-week exercise program during cancer treatment, patients reported an average of 90 minutes of moderate-to-vigorous physical activity per day, compared with 70 minutes for patients who did not participate (P less than .05).
Study details: Long-term follow-up of 128 patients who had participated in the PACT study, a randomized controlled trial of a supervised exercise program versus usual care in breast and colon cancer patients undergoing adjuvant treatment including chemotherapy.
Disclosures: The authors reported no disclosures relevant to the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
Source: May AM et al. Cancer Survivorship Symposium, Abstract 99.








