User login
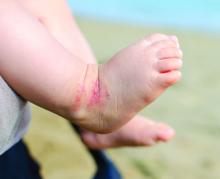
Very preterm birth is linked to reduced risk of eczema
according to data from a meta-analysis of 18 studies.
Previous research suggests that low birth weight is protective against the development of atopic dermatitis, said Tingting Zhu, PhD, of West China Second University Hospital, Chengdu, and colleagues.
Preterm birth (before 37 completed weeks’ gestation) was divided into subgroups of extremely preterm (less than 28 weeks’ gestation), very preterm (28 weeks’ to less than 32 weeks’ gestation), and moderate/late preterm (32 weeks’ gestation to less than 37 weeks’ gestation).
In an analysis based on gestational age, children had a significantly reduced risk of eczema if they were very preterm (relative risk, 0.77; 95% confidence interval, 0.70-0.84, P less than .01; adjusted RR, 0.73; 95% CI, 0.64-0.82; P less than 0.01), compared with children born full term. The association between eczema and preterm birth was no longer significant among children born moderately preterm, Dr. Zhu and associates reported.
The reasons for the impact of very preterm birth on eczema are unclear, but maturation of the stratum corneum at 29-37 weeks’ gestational age could play a role, the researchers noted. Also, limited microflora in very preterm infants could affect acquiring immune tolerance and lead to reduced risk of eczema. The study was limited by several factors, including variations in gestational age and inconsistent assessments of eczema among the studies.
However, the large sample size lends strength to the results, and further studies are needed to explore how the environment, nutrition, immune system development, and skin barrier function impact the risk of eczema in very preterm infants, Dr. Zhu and associates said.
The researchers had no relevant financial disclosures. The researchers had no financial conflicts to disclose. The study was funded in part by the National Science Foundation of China, the Ministry of Health of China, and various other grants.
SOURCE: Zhu T et al. J Amer Dermatol. 2018. doi: 10.1016/j.jaad.2017.12.015.
according to data from a meta-analysis of 18 studies.
Previous research suggests that low birth weight is protective against the development of atopic dermatitis, said Tingting Zhu, PhD, of West China Second University Hospital, Chengdu, and colleagues.
Preterm birth (before 37 completed weeks’ gestation) was divided into subgroups of extremely preterm (less than 28 weeks’ gestation), very preterm (28 weeks’ to less than 32 weeks’ gestation), and moderate/late preterm (32 weeks’ gestation to less than 37 weeks’ gestation).
In an analysis based on gestational age, children had a significantly reduced risk of eczema if they were very preterm (relative risk, 0.77; 95% confidence interval, 0.70-0.84, P less than .01; adjusted RR, 0.73; 95% CI, 0.64-0.82; P less than 0.01), compared with children born full term. The association between eczema and preterm birth was no longer significant among children born moderately preterm, Dr. Zhu and associates reported.
The reasons for the impact of very preterm birth on eczema are unclear, but maturation of the stratum corneum at 29-37 weeks’ gestational age could play a role, the researchers noted. Also, limited microflora in very preterm infants could affect acquiring immune tolerance and lead to reduced risk of eczema. The study was limited by several factors, including variations in gestational age and inconsistent assessments of eczema among the studies.
However, the large sample size lends strength to the results, and further studies are needed to explore how the environment, nutrition, immune system development, and skin barrier function impact the risk of eczema in very preterm infants, Dr. Zhu and associates said.
The researchers had no relevant financial disclosures. The researchers had no financial conflicts to disclose. The study was funded in part by the National Science Foundation of China, the Ministry of Health of China, and various other grants.
SOURCE: Zhu T et al. J Amer Dermatol. 2018. doi: 10.1016/j.jaad.2017.12.015.
according to data from a meta-analysis of 18 studies.
Previous research suggests that low birth weight is protective against the development of atopic dermatitis, said Tingting Zhu, PhD, of West China Second University Hospital, Chengdu, and colleagues.
Preterm birth (before 37 completed weeks’ gestation) was divided into subgroups of extremely preterm (less than 28 weeks’ gestation), very preterm (28 weeks’ to less than 32 weeks’ gestation), and moderate/late preterm (32 weeks’ gestation to less than 37 weeks’ gestation).
In an analysis based on gestational age, children had a significantly reduced risk of eczema if they were very preterm (relative risk, 0.77; 95% confidence interval, 0.70-0.84, P less than .01; adjusted RR, 0.73; 95% CI, 0.64-0.82; P less than 0.01), compared with children born full term. The association between eczema and preterm birth was no longer significant among children born moderately preterm, Dr. Zhu and associates reported.
The reasons for the impact of very preterm birth on eczema are unclear, but maturation of the stratum corneum at 29-37 weeks’ gestational age could play a role, the researchers noted. Also, limited microflora in very preterm infants could affect acquiring immune tolerance and lead to reduced risk of eczema. The study was limited by several factors, including variations in gestational age and inconsistent assessments of eczema among the studies.
However, the large sample size lends strength to the results, and further studies are needed to explore how the environment, nutrition, immune system development, and skin barrier function impact the risk of eczema in very preterm infants, Dr. Zhu and associates said.
The researchers had no relevant financial disclosures. The researchers had no financial conflicts to disclose. The study was funded in part by the National Science Foundation of China, the Ministry of Health of China, and various other grants.
SOURCE: Zhu T et al. J Amer Dermatol. 2018. doi: 10.1016/j.jaad.2017.12.015.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Very preterm birth was associated with a significantly reduced risk of eczema, compared with full-term birth, but no difference in risk appeared between moderate preterm and full-term birth.
Major finding: Children had a significantly reduced risk of eczema if they were very preterm (RR, 0.77; 95% CI, 0.70-0.84; P less than .01; aRR 0.73, 95% CI, 0.64-0.82; P less than .01), compared with children born full term.
Data source: The data come from a meta-analysis of 18 studies.
Disclosures: The researchers had no financial conflicts to disclose. The study was funded in part by the National Science Foundation of China, the Ministry of Health of China, and various other grants.
Source: Zhu T et al. J Amer Dermatol. 2018. doi: 10.1016/j.jaad.2017.12.015.
The price of protection
It’s very likely that you have at least one or two female patients who play lacrosse. The sport has been reported to be the fastest-growing high school sport in the United States. (“Lacrosse is Actually America’s Fastest-Growing Sport,” by John Templon, BuzzFeed News, June 30, 2014). When I played in college, most of my teammates were products of prep schools in the Northeast or one of the few local hotbeds in Baltimore, Long Island, or the Finger Lakes Region of New York. But pickings were slim, and there was room for walk-ons like me looking to learn a new sport and stay in shape for football. Now hundreds of high schools in all parts of the country offer the sport for both boys and girls.
With growing awareness of the long-term effects of repeated head trauma, there has been a call from some parents and organizers of women’s lacrosse to require helmets on all players (“As Concussion Worries Rise, Girls’ Lacrosse Turns to Headgear,” by Bill Pennington, The New York Times, Nov 23, 2017). To those of us who have committed our professional lives to the health of children, the inclusion of helmets to the standard equipment for a female lacrosse player sounds like a good idea.
However, the proposed mandate has its critics, including several college coaches. Karen Corbett, women’s lacrosse coach at the University of Pennsylvania, has said that, players “will start to lead with their head because they feel protected, and that causes more injuries. We’ll become a more physical sport and a very different sport than we are today.”
Although I’m afraid that there are few data to support the validity of Dr. Hanley’s prediction, any observer of college hockey over the last 3 or 4 decades will tell you that he was unfortunately correct. There have been certainly fewer lacerations and eye injuries since face masks were introduced, but the game has become far more violent, and head, neck, and spine injuries have become more frequent. I think part of the problem is that game officials have been duped by the same false assumption as the players that more protection would make the game safer, and enforcement of the rules has not kept up with the technological changes.
There will always be injuries in any sport, but before we as physicians lend our support to a proposed change in protective equipment, we should step back and look at the broader picture. While the loss of an eye for an individual player is a tragedy, did we put several dozen more players at greater risk for spinal injury in college hockey with more protective gear? If adding headgear protects female lacrosse players from concussions, what might be the result if play becomes more physical? Protection can come with a price.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
It’s very likely that you have at least one or two female patients who play lacrosse. The sport has been reported to be the fastest-growing high school sport in the United States. (“Lacrosse is Actually America’s Fastest-Growing Sport,” by John Templon, BuzzFeed News, June 30, 2014). When I played in college, most of my teammates were products of prep schools in the Northeast or one of the few local hotbeds in Baltimore, Long Island, or the Finger Lakes Region of New York. But pickings were slim, and there was room for walk-ons like me looking to learn a new sport and stay in shape for football. Now hundreds of high schools in all parts of the country offer the sport for both boys and girls.
With growing awareness of the long-term effects of repeated head trauma, there has been a call from some parents and organizers of women’s lacrosse to require helmets on all players (“As Concussion Worries Rise, Girls’ Lacrosse Turns to Headgear,” by Bill Pennington, The New York Times, Nov 23, 2017). To those of us who have committed our professional lives to the health of children, the inclusion of helmets to the standard equipment for a female lacrosse player sounds like a good idea.
However, the proposed mandate has its critics, including several college coaches. Karen Corbett, women’s lacrosse coach at the University of Pennsylvania, has said that, players “will start to lead with their head because they feel protected, and that causes more injuries. We’ll become a more physical sport and a very different sport than we are today.”
Although I’m afraid that there are few data to support the validity of Dr. Hanley’s prediction, any observer of college hockey over the last 3 or 4 decades will tell you that he was unfortunately correct. There have been certainly fewer lacerations and eye injuries since face masks were introduced, but the game has become far more violent, and head, neck, and spine injuries have become more frequent. I think part of the problem is that game officials have been duped by the same false assumption as the players that more protection would make the game safer, and enforcement of the rules has not kept up with the technological changes.
There will always be injuries in any sport, but before we as physicians lend our support to a proposed change in protective equipment, we should step back and look at the broader picture. While the loss of an eye for an individual player is a tragedy, did we put several dozen more players at greater risk for spinal injury in college hockey with more protective gear? If adding headgear protects female lacrosse players from concussions, what might be the result if play becomes more physical? Protection can come with a price.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
It’s very likely that you have at least one or two female patients who play lacrosse. The sport has been reported to be the fastest-growing high school sport in the United States. (“Lacrosse is Actually America’s Fastest-Growing Sport,” by John Templon, BuzzFeed News, June 30, 2014). When I played in college, most of my teammates were products of prep schools in the Northeast or one of the few local hotbeds in Baltimore, Long Island, or the Finger Lakes Region of New York. But pickings were slim, and there was room for walk-ons like me looking to learn a new sport and stay in shape for football. Now hundreds of high schools in all parts of the country offer the sport for both boys and girls.
With growing awareness of the long-term effects of repeated head trauma, there has been a call from some parents and organizers of women’s lacrosse to require helmets on all players (“As Concussion Worries Rise, Girls’ Lacrosse Turns to Headgear,” by Bill Pennington, The New York Times, Nov 23, 2017). To those of us who have committed our professional lives to the health of children, the inclusion of helmets to the standard equipment for a female lacrosse player sounds like a good idea.
However, the proposed mandate has its critics, including several college coaches. Karen Corbett, women’s lacrosse coach at the University of Pennsylvania, has said that, players “will start to lead with their head because they feel protected, and that causes more injuries. We’ll become a more physical sport and a very different sport than we are today.”
Although I’m afraid that there are few data to support the validity of Dr. Hanley’s prediction, any observer of college hockey over the last 3 or 4 decades will tell you that he was unfortunately correct. There have been certainly fewer lacerations and eye injuries since face masks were introduced, but the game has become far more violent, and head, neck, and spine injuries have become more frequent. I think part of the problem is that game officials have been duped by the same false assumption as the players that more protection would make the game safer, and enforcement of the rules has not kept up with the technological changes.
There will always be injuries in any sport, but before we as physicians lend our support to a proposed change in protective equipment, we should step back and look at the broader picture. While the loss of an eye for an individual player is a tragedy, did we put several dozen more players at greater risk for spinal injury in college hockey with more protective gear? If adding headgear protects female lacrosse players from concussions, what might be the result if play becomes more physical? Protection can come with a price.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
Early Hip Fracture Surgery Is Associated with Lower 30-Day Mortality
Study Overview
Objective. To determine the association between wait times for hip fracture surgery and outcomes after surgery and to identify the optimal time window for conducting hip fracture surgery.
Design. Observational cohort study.
Setting and participants. The study was conducted using population-based health administrative databases in Ontario, Canada. The databases collected information on health care services, physician and hospital information, and demographic characteristics in Ontario. The investigators used the databases to identify adults undergoing hip fracture surgery between April 2009 and March 2014. Excluded were adults who are non-Ontario residents, those with elective hospital admissions, those with prior hip fractures, and patients without hospital arrival time data. Other exclusion criteria include age younger than 45 years, those with delay in surgery longer than 10 days, surgery performed by a nonorthopedic surgeon, and those at hospitals with fewer than 5 hip fracture surgeries during the study period.
The primary independent variable was wait time for surgery, calculated from time from emergency department arrival until surgery and rounded in hours. Other covariates included in the analysis were patient characteristics including age, sex and comorbid conditions using the Deyo-Charlson comorbidity index, the Johns Hopkins Collapsed Aggregated Diagnosis Groups, and other validated algorithms. In addition, other conditions associated with hip fracture were included—osteomyelitis, bone cancer, other fractures, history of total hip arthroplasty, and multiple trauma. Additional covariates included median neighborhood household income quintile as a proxy for socioeconomic status, patient’s discharge disposition, and rural status. Characteristics of the procedure including procedure type, duration and timing (working vs. after hours) were assessed. Surgeon- and hospital-related factors included years since orthopedic certification as a proxy for surgeon experience and number of hip fracture procedures performed in the year preceding the event for surgeon and hospital. Other hospital characteristics included academic or community-based hospital, hospital size, and hospital’s capacity for performing nonelective surgery.
Main outcome measures. The main outcome measure was mortality within 30 days of being admitted for hip fracture surgery. Other secondary outcomes included mortality at 90 and 365 days after admission, medical complications within 30, 90, and 365 days, and a composite of mortality and any complications at these timeframes. Complications included myocardial infarction, deep vein thrombosis, pulmonary embolism and pneumonia. Statistical analysis include modeling for the probability of complications according to the time elapsed from emergency department arrival to surgery using risk adjusted spline analyses. The association between surgical wait time and mortality was graphically represented to visualize an inflection point when complications begin to rise. The area under the receiver operating characteristic curve was calculated at time thresholds around the area of inflection and the time producing the maximum area under the curve was selected as the threshold to classify patients
as receiving early or delayed surgery. Early and delayed patients were matched using propensity score with 1:1 matching without replacement. Outcomes were compared between early and delayed groups after matching and absolute risk differences were calculated using generalized estimating equations.
Main results. A total of 42,230 adults were included, with a mean age of 80.1 (SD 10.7) years; 70.5% were women. The average time from arrival to emergency room to surgery was 38.8 (SD 28.8) hours. The spline models identified an area of inflection at 24 hours when the risk of complications begins to rise. The investigators used 24 hours as a time point to classify patients into early or delayed surgery group. 33.6% of patients received early surgery and 66.4% had delayed surgery. Propensity score matching yielded a sample of 13,731 in each group. Patients with delayed surgery compared with early surgery had higher 30-day mortality (6.5% vs. 5.8%, absolute risk difference 0.79%), rate of pulmonary embolism (1.2% vs. 0.7%, absolute risk difference 0.51%), rate of myocardial infarction (1.2% vs. 0.8%, absolute risk difference 0.39%), and rate of pneumonia (4.6% vs. 3.7%, absolute risk difference 0.95%). For the composite outcome, 12.1% vs. 10.1% had mortality or complications in the delayed group and the early group respectively with an absolute difference of 2.16%. Outcomes at 90 days and 365 days were similar and remained significant. In subgroups of patients without comorbidity and those receiving surgery within 36 hours the results remained similar.
Conclusion. Early hip fracture surgery, defined as within 24 hours after arrival to emergency room, is associated with lower mortality and complications when compared to delayed surgery.
Commentary
Hip fracture affects predominantly older adults and leads to potential devastating consequences. Older adults who experience hip fracture have increased risk of functional decline, institutionalization, and death [1]. As hip fracture care often include surgical repair, many studies have examined the impact of timing of surgery on hip fracture outcomes, as the timing of surgery is a potentially modifiable factor that could impact patient outcomes [2]. Prior smaller cohort studies have demonstrated that delayed surgery may impact outcomes but the reasons for the delay, such as medical complexity, may also play a role in increasing the risk of adverse outcomes [3]. The current study adds to the previous literature by examining a large population-based cohort, thereby allowing for analysis that takes into account medical comorbidities using matching methods and sensitivity analyses that examined a sample without comorbidities. The study also employs a different approach to defining early vs. delayed surgery by using analytical methods to determine when risk of complications begins to rise. The results indicate that early surgery is associated with better outcomes at 30 days and beyond and that delaying surgery beyond 24 hours is associated with poorer patient outcomes.
Patients with hip fracture require care from multiple disciplines and care across multiple settings. These care components may also have an impact on patient outcomes, particularly outcomes at 90 and 365 days; some examples include anesthesia care during hip fracture surgery [4], pain control, early mobilization, and delirium prevention [1,5]. A limitation of utilizing administrative databases is that some of these potentially important factors that may affect outcome may not be included and thus cannot be controlled for. It is conceivable that early surgery may be associated with care characteristics that may also be favorable to outcomes. Another limitation is that it is still difficult to tease out the effect of medical complexity at the
time of hip fracture presentation, which may impact both timing of surgery and patient outcomes, despite sensitivity analyses that limit the sample to those who had surgery within 36 hours and also those without medical comorbidities according to the administrative data, and adjusting for antiplatelet or anticoagulant medications. It is also important to note that a randomized controlled trial may further elucidate the causal relationship between timing of surgery and patient outcomes. Despite the limitations of the study, the results make a strong case for limiting surgical wait time to within 24 hours from the time when the patient arrives in the emergency room.
Applications for Clinical Practice
Similar to how hospitals organize their care for patients with acute myocardial infarction for early reperfusion, and for patients with acute ischemic stroke with early thombolytic therapy, hip fracture care may need to be organized and coordinated in order to reduce surgical wait time to within 24 hours. Timely assessments by an orthopedic surgeon, anesthesiologist, and medical consultants to prepare patients for surgery and making available operating room and staff for hip fracture patients are necessary steps to reach the goal of reducing surgical wait time.
—William W. Hung, MD, MPH
1. Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA 2012;307:2185–94.
2. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004;291:1738–43.
3. Vidán MT, Sánchez E, Gracia Y, et al. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med 2011;155:226–33.
4. Neuman MD, Silber JH, Elkassabany NM, et al. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012;117: 72–92.
5. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014;28:e49–55.
Study Overview
Objective. To determine the association between wait times for hip fracture surgery and outcomes after surgery and to identify the optimal time window for conducting hip fracture surgery.
Design. Observational cohort study.
Setting and participants. The study was conducted using population-based health administrative databases in Ontario, Canada. The databases collected information on health care services, physician and hospital information, and demographic characteristics in Ontario. The investigators used the databases to identify adults undergoing hip fracture surgery between April 2009 and March 2014. Excluded were adults who are non-Ontario residents, those with elective hospital admissions, those with prior hip fractures, and patients without hospital arrival time data. Other exclusion criteria include age younger than 45 years, those with delay in surgery longer than 10 days, surgery performed by a nonorthopedic surgeon, and those at hospitals with fewer than 5 hip fracture surgeries during the study period.
The primary independent variable was wait time for surgery, calculated from time from emergency department arrival until surgery and rounded in hours. Other covariates included in the analysis were patient characteristics including age, sex and comorbid conditions using the Deyo-Charlson comorbidity index, the Johns Hopkins Collapsed Aggregated Diagnosis Groups, and other validated algorithms. In addition, other conditions associated with hip fracture were included—osteomyelitis, bone cancer, other fractures, history of total hip arthroplasty, and multiple trauma. Additional covariates included median neighborhood household income quintile as a proxy for socioeconomic status, patient’s discharge disposition, and rural status. Characteristics of the procedure including procedure type, duration and timing (working vs. after hours) were assessed. Surgeon- and hospital-related factors included years since orthopedic certification as a proxy for surgeon experience and number of hip fracture procedures performed in the year preceding the event for surgeon and hospital. Other hospital characteristics included academic or community-based hospital, hospital size, and hospital’s capacity for performing nonelective surgery.
Main outcome measures. The main outcome measure was mortality within 30 days of being admitted for hip fracture surgery. Other secondary outcomes included mortality at 90 and 365 days after admission, medical complications within 30, 90, and 365 days, and a composite of mortality and any complications at these timeframes. Complications included myocardial infarction, deep vein thrombosis, pulmonary embolism and pneumonia. Statistical analysis include modeling for the probability of complications according to the time elapsed from emergency department arrival to surgery using risk adjusted spline analyses. The association between surgical wait time and mortality was graphically represented to visualize an inflection point when complications begin to rise. The area under the receiver operating characteristic curve was calculated at time thresholds around the area of inflection and the time producing the maximum area under the curve was selected as the threshold to classify patients
as receiving early or delayed surgery. Early and delayed patients were matched using propensity score with 1:1 matching without replacement. Outcomes were compared between early and delayed groups after matching and absolute risk differences were calculated using generalized estimating equations.
Main results. A total of 42,230 adults were included, with a mean age of 80.1 (SD 10.7) years; 70.5% were women. The average time from arrival to emergency room to surgery was 38.8 (SD 28.8) hours. The spline models identified an area of inflection at 24 hours when the risk of complications begins to rise. The investigators used 24 hours as a time point to classify patients into early or delayed surgery group. 33.6% of patients received early surgery and 66.4% had delayed surgery. Propensity score matching yielded a sample of 13,731 in each group. Patients with delayed surgery compared with early surgery had higher 30-day mortality (6.5% vs. 5.8%, absolute risk difference 0.79%), rate of pulmonary embolism (1.2% vs. 0.7%, absolute risk difference 0.51%), rate of myocardial infarction (1.2% vs. 0.8%, absolute risk difference 0.39%), and rate of pneumonia (4.6% vs. 3.7%, absolute risk difference 0.95%). For the composite outcome, 12.1% vs. 10.1% had mortality or complications in the delayed group and the early group respectively with an absolute difference of 2.16%. Outcomes at 90 days and 365 days were similar and remained significant. In subgroups of patients without comorbidity and those receiving surgery within 36 hours the results remained similar.
Conclusion. Early hip fracture surgery, defined as within 24 hours after arrival to emergency room, is associated with lower mortality and complications when compared to delayed surgery.
Commentary
Hip fracture affects predominantly older adults and leads to potential devastating consequences. Older adults who experience hip fracture have increased risk of functional decline, institutionalization, and death [1]. As hip fracture care often include surgical repair, many studies have examined the impact of timing of surgery on hip fracture outcomes, as the timing of surgery is a potentially modifiable factor that could impact patient outcomes [2]. Prior smaller cohort studies have demonstrated that delayed surgery may impact outcomes but the reasons for the delay, such as medical complexity, may also play a role in increasing the risk of adverse outcomes [3]. The current study adds to the previous literature by examining a large population-based cohort, thereby allowing for analysis that takes into account medical comorbidities using matching methods and sensitivity analyses that examined a sample without comorbidities. The study also employs a different approach to defining early vs. delayed surgery by using analytical methods to determine when risk of complications begins to rise. The results indicate that early surgery is associated with better outcomes at 30 days and beyond and that delaying surgery beyond 24 hours is associated with poorer patient outcomes.
Patients with hip fracture require care from multiple disciplines and care across multiple settings. These care components may also have an impact on patient outcomes, particularly outcomes at 90 and 365 days; some examples include anesthesia care during hip fracture surgery [4], pain control, early mobilization, and delirium prevention [1,5]. A limitation of utilizing administrative databases is that some of these potentially important factors that may affect outcome may not be included and thus cannot be controlled for. It is conceivable that early surgery may be associated with care characteristics that may also be favorable to outcomes. Another limitation is that it is still difficult to tease out the effect of medical complexity at the
time of hip fracture presentation, which may impact both timing of surgery and patient outcomes, despite sensitivity analyses that limit the sample to those who had surgery within 36 hours and also those without medical comorbidities according to the administrative data, and adjusting for antiplatelet or anticoagulant medications. It is also important to note that a randomized controlled trial may further elucidate the causal relationship between timing of surgery and patient outcomes. Despite the limitations of the study, the results make a strong case for limiting surgical wait time to within 24 hours from the time when the patient arrives in the emergency room.
Applications for Clinical Practice
Similar to how hospitals organize their care for patients with acute myocardial infarction for early reperfusion, and for patients with acute ischemic stroke with early thombolytic therapy, hip fracture care may need to be organized and coordinated in order to reduce surgical wait time to within 24 hours. Timely assessments by an orthopedic surgeon, anesthesiologist, and medical consultants to prepare patients for surgery and making available operating room and staff for hip fracture patients are necessary steps to reach the goal of reducing surgical wait time.
—William W. Hung, MD, MPH
Study Overview
Objective. To determine the association between wait times for hip fracture surgery and outcomes after surgery and to identify the optimal time window for conducting hip fracture surgery.
Design. Observational cohort study.
Setting and participants. The study was conducted using population-based health administrative databases in Ontario, Canada. The databases collected information on health care services, physician and hospital information, and demographic characteristics in Ontario. The investigators used the databases to identify adults undergoing hip fracture surgery between April 2009 and March 2014. Excluded were adults who are non-Ontario residents, those with elective hospital admissions, those with prior hip fractures, and patients without hospital arrival time data. Other exclusion criteria include age younger than 45 years, those with delay in surgery longer than 10 days, surgery performed by a nonorthopedic surgeon, and those at hospitals with fewer than 5 hip fracture surgeries during the study period.
The primary independent variable was wait time for surgery, calculated from time from emergency department arrival until surgery and rounded in hours. Other covariates included in the analysis were patient characteristics including age, sex and comorbid conditions using the Deyo-Charlson comorbidity index, the Johns Hopkins Collapsed Aggregated Diagnosis Groups, and other validated algorithms. In addition, other conditions associated with hip fracture were included—osteomyelitis, bone cancer, other fractures, history of total hip arthroplasty, and multiple trauma. Additional covariates included median neighborhood household income quintile as a proxy for socioeconomic status, patient’s discharge disposition, and rural status. Characteristics of the procedure including procedure type, duration and timing (working vs. after hours) were assessed. Surgeon- and hospital-related factors included years since orthopedic certification as a proxy for surgeon experience and number of hip fracture procedures performed in the year preceding the event for surgeon and hospital. Other hospital characteristics included academic or community-based hospital, hospital size, and hospital’s capacity for performing nonelective surgery.
Main outcome measures. The main outcome measure was mortality within 30 days of being admitted for hip fracture surgery. Other secondary outcomes included mortality at 90 and 365 days after admission, medical complications within 30, 90, and 365 days, and a composite of mortality and any complications at these timeframes. Complications included myocardial infarction, deep vein thrombosis, pulmonary embolism and pneumonia. Statistical analysis include modeling for the probability of complications according to the time elapsed from emergency department arrival to surgery using risk adjusted spline analyses. The association between surgical wait time and mortality was graphically represented to visualize an inflection point when complications begin to rise. The area under the receiver operating characteristic curve was calculated at time thresholds around the area of inflection and the time producing the maximum area under the curve was selected as the threshold to classify patients
as receiving early or delayed surgery. Early and delayed patients were matched using propensity score with 1:1 matching without replacement. Outcomes were compared between early and delayed groups after matching and absolute risk differences were calculated using generalized estimating equations.
Main results. A total of 42,230 adults were included, with a mean age of 80.1 (SD 10.7) years; 70.5% were women. The average time from arrival to emergency room to surgery was 38.8 (SD 28.8) hours. The spline models identified an area of inflection at 24 hours when the risk of complications begins to rise. The investigators used 24 hours as a time point to classify patients into early or delayed surgery group. 33.6% of patients received early surgery and 66.4% had delayed surgery. Propensity score matching yielded a sample of 13,731 in each group. Patients with delayed surgery compared with early surgery had higher 30-day mortality (6.5% vs. 5.8%, absolute risk difference 0.79%), rate of pulmonary embolism (1.2% vs. 0.7%, absolute risk difference 0.51%), rate of myocardial infarction (1.2% vs. 0.8%, absolute risk difference 0.39%), and rate of pneumonia (4.6% vs. 3.7%, absolute risk difference 0.95%). For the composite outcome, 12.1% vs. 10.1% had mortality or complications in the delayed group and the early group respectively with an absolute difference of 2.16%. Outcomes at 90 days and 365 days were similar and remained significant. In subgroups of patients without comorbidity and those receiving surgery within 36 hours the results remained similar.
Conclusion. Early hip fracture surgery, defined as within 24 hours after arrival to emergency room, is associated with lower mortality and complications when compared to delayed surgery.
Commentary
Hip fracture affects predominantly older adults and leads to potential devastating consequences. Older adults who experience hip fracture have increased risk of functional decline, institutionalization, and death [1]. As hip fracture care often include surgical repair, many studies have examined the impact of timing of surgery on hip fracture outcomes, as the timing of surgery is a potentially modifiable factor that could impact patient outcomes [2]. Prior smaller cohort studies have demonstrated that delayed surgery may impact outcomes but the reasons for the delay, such as medical complexity, may also play a role in increasing the risk of adverse outcomes [3]. The current study adds to the previous literature by examining a large population-based cohort, thereby allowing for analysis that takes into account medical comorbidities using matching methods and sensitivity analyses that examined a sample without comorbidities. The study also employs a different approach to defining early vs. delayed surgery by using analytical methods to determine when risk of complications begins to rise. The results indicate that early surgery is associated with better outcomes at 30 days and beyond and that delaying surgery beyond 24 hours is associated with poorer patient outcomes.
Patients with hip fracture require care from multiple disciplines and care across multiple settings. These care components may also have an impact on patient outcomes, particularly outcomes at 90 and 365 days; some examples include anesthesia care during hip fracture surgery [4], pain control, early mobilization, and delirium prevention [1,5]. A limitation of utilizing administrative databases is that some of these potentially important factors that may affect outcome may not be included and thus cannot be controlled for. It is conceivable that early surgery may be associated with care characteristics that may also be favorable to outcomes. Another limitation is that it is still difficult to tease out the effect of medical complexity at the
time of hip fracture presentation, which may impact both timing of surgery and patient outcomes, despite sensitivity analyses that limit the sample to those who had surgery within 36 hours and also those without medical comorbidities according to the administrative data, and adjusting for antiplatelet or anticoagulant medications. It is also important to note that a randomized controlled trial may further elucidate the causal relationship between timing of surgery and patient outcomes. Despite the limitations of the study, the results make a strong case for limiting surgical wait time to within 24 hours from the time when the patient arrives in the emergency room.
Applications for Clinical Practice
Similar to how hospitals organize their care for patients with acute myocardial infarction for early reperfusion, and for patients with acute ischemic stroke with early thombolytic therapy, hip fracture care may need to be organized and coordinated in order to reduce surgical wait time to within 24 hours. Timely assessments by an orthopedic surgeon, anesthesiologist, and medical consultants to prepare patients for surgery and making available operating room and staff for hip fracture patients are necessary steps to reach the goal of reducing surgical wait time.
—William W. Hung, MD, MPH
1. Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA 2012;307:2185–94.
2. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004;291:1738–43.
3. Vidán MT, Sánchez E, Gracia Y, et al. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med 2011;155:226–33.
4. Neuman MD, Silber JH, Elkassabany NM, et al. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012;117: 72–92.
5. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014;28:e49–55.
1. Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA 2012;307:2185–94.
2. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004;291:1738–43.
3. Vidán MT, Sánchez E, Gracia Y, et al. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med 2011;155:226–33.
4. Neuman MD, Silber JH, Elkassabany NM, et al. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012;117: 72–92.
5. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014;28:e49–55.
Low caffeine in blood could be marker of early Parkinson’s
Low serum caffeine and caffeine metabolite levels after an overnight fast may be a sensitive way to detect the presence of Parkinson’s disease, according to the results of a new case-control study.
Levels of caffeine and its metabolites were also lower in Parkinson’s disease (PD) patients who had motor dysfunction, compared with those without motor dysfunction, but no differences in serum levels of caffeine metabolites could be detected between patients with mild to more severe stages of PD, reported Motoki Fujimaki, MD, of Juntendo University, Tokyo, and colleagues. The report was published online Jan. 3 in Neurology.
To test that idea, Dr. Fujimaki and associates recruited 31 healthy controls (18 women) and 108 patients with PD but no dementia (50 women). The control group’s mean caffeine intake of 115.81 mg/day (standard deviation, 69.22) was similar to PD patients’ intake of 107.50 mg/day (SD, 67.27).
Serum caffeine levels measured after an overnight fast showed that a cutoff of 33.04 pmol/10 mcL identified PD with an area under the curve (AUC) of 0.78 (sensitivity 76.9%, specificity 74.2%). Inclusion of the primary caffeine metabolites theophylline, theobromine, and paraxanthine improved the AUC to 0.87. When the researchers included all 11 measurable metabolites, the AUC jumped to 0.98.
Genetic analyses found no significant differences in the frequencies of caffeine metabolism–associated genetic variants between PD patients and controls.
The study was limited by the fact that it was conducted at a single university hospital, and the patient population did not include many severe cases. The algorithm should also be studied in other PD patient populations.
The study was funded by grants from several Japanese government agencies. Some of the authors have financial relationships with the pharmaceutical industry.
SOURCE: Fujimaki M et al. Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004888
A key question is what is causing the decrease in serum concentration found in patients with Parkinson’s disease? Nearly all of the patients were receiving treatment, which could have affected serum levels.
The researchers addressed this by looking for an association between serum caffeine metabolite levels and levodopa equivalent doses, and they found none.
Still, the validity of the study depends on whether caffeine metabolism may be affected by treatment. To demonstrate the utility of caffeine metabolites unequivocally, a future study will have to reproduce these results in patients with untreated PD or subjects at high risk of PD, such as those with prodromal signs of PD.
David G. Munoz, MD, is in the department of laboratory medicine and pathobiology at the University of Toronto. Shinsuke Fujioka, MD is in the department of neurology at Fukuoka (Japan) University. Dr. Munoz and Dr. Fujioka reported having no financial disclosures. Their comments are derived from an editorial accompanying the study by Dr. Fujimaki and colleagues (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004898).
A key question is what is causing the decrease in serum concentration found in patients with Parkinson’s disease? Nearly all of the patients were receiving treatment, which could have affected serum levels.
The researchers addressed this by looking for an association between serum caffeine metabolite levels and levodopa equivalent doses, and they found none.
Still, the validity of the study depends on whether caffeine metabolism may be affected by treatment. To demonstrate the utility of caffeine metabolites unequivocally, a future study will have to reproduce these results in patients with untreated PD or subjects at high risk of PD, such as those with prodromal signs of PD.
David G. Munoz, MD, is in the department of laboratory medicine and pathobiology at the University of Toronto. Shinsuke Fujioka, MD is in the department of neurology at Fukuoka (Japan) University. Dr. Munoz and Dr. Fujioka reported having no financial disclosures. Their comments are derived from an editorial accompanying the study by Dr. Fujimaki and colleagues (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004898).
A key question is what is causing the decrease in serum concentration found in patients with Parkinson’s disease? Nearly all of the patients were receiving treatment, which could have affected serum levels.
The researchers addressed this by looking for an association between serum caffeine metabolite levels and levodopa equivalent doses, and they found none.
Still, the validity of the study depends on whether caffeine metabolism may be affected by treatment. To demonstrate the utility of caffeine metabolites unequivocally, a future study will have to reproduce these results in patients with untreated PD or subjects at high risk of PD, such as those with prodromal signs of PD.
David G. Munoz, MD, is in the department of laboratory medicine and pathobiology at the University of Toronto. Shinsuke Fujioka, MD is in the department of neurology at Fukuoka (Japan) University. Dr. Munoz and Dr. Fujioka reported having no financial disclosures. Their comments are derived from an editorial accompanying the study by Dr. Fujimaki and colleagues (Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004898).
Low serum caffeine and caffeine metabolite levels after an overnight fast may be a sensitive way to detect the presence of Parkinson’s disease, according to the results of a new case-control study.
Levels of caffeine and its metabolites were also lower in Parkinson’s disease (PD) patients who had motor dysfunction, compared with those without motor dysfunction, but no differences in serum levels of caffeine metabolites could be detected between patients with mild to more severe stages of PD, reported Motoki Fujimaki, MD, of Juntendo University, Tokyo, and colleagues. The report was published online Jan. 3 in Neurology.
To test that idea, Dr. Fujimaki and associates recruited 31 healthy controls (18 women) and 108 patients with PD but no dementia (50 women). The control group’s mean caffeine intake of 115.81 mg/day (standard deviation, 69.22) was similar to PD patients’ intake of 107.50 mg/day (SD, 67.27).
Serum caffeine levels measured after an overnight fast showed that a cutoff of 33.04 pmol/10 mcL identified PD with an area under the curve (AUC) of 0.78 (sensitivity 76.9%, specificity 74.2%). Inclusion of the primary caffeine metabolites theophylline, theobromine, and paraxanthine improved the AUC to 0.87. When the researchers included all 11 measurable metabolites, the AUC jumped to 0.98.
Genetic analyses found no significant differences in the frequencies of caffeine metabolism–associated genetic variants between PD patients and controls.
The study was limited by the fact that it was conducted at a single university hospital, and the patient population did not include many severe cases. The algorithm should also be studied in other PD patient populations.
The study was funded by grants from several Japanese government agencies. Some of the authors have financial relationships with the pharmaceutical industry.
SOURCE: Fujimaki M et al. Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004888
Low serum caffeine and caffeine metabolite levels after an overnight fast may be a sensitive way to detect the presence of Parkinson’s disease, according to the results of a new case-control study.
Levels of caffeine and its metabolites were also lower in Parkinson’s disease (PD) patients who had motor dysfunction, compared with those without motor dysfunction, but no differences in serum levels of caffeine metabolites could be detected between patients with mild to more severe stages of PD, reported Motoki Fujimaki, MD, of Juntendo University, Tokyo, and colleagues. The report was published online Jan. 3 in Neurology.
To test that idea, Dr. Fujimaki and associates recruited 31 healthy controls (18 women) and 108 patients with PD but no dementia (50 women). The control group’s mean caffeine intake of 115.81 mg/day (standard deviation, 69.22) was similar to PD patients’ intake of 107.50 mg/day (SD, 67.27).
Serum caffeine levels measured after an overnight fast showed that a cutoff of 33.04 pmol/10 mcL identified PD with an area under the curve (AUC) of 0.78 (sensitivity 76.9%, specificity 74.2%). Inclusion of the primary caffeine metabolites theophylline, theobromine, and paraxanthine improved the AUC to 0.87. When the researchers included all 11 measurable metabolites, the AUC jumped to 0.98.
Genetic analyses found no significant differences in the frequencies of caffeine metabolism–associated genetic variants between PD patients and controls.
The study was limited by the fact that it was conducted at a single university hospital, and the patient population did not include many severe cases. The algorithm should also be studied in other PD patient populations.
The study was funded by grants from several Japanese government agencies. Some of the authors have financial relationships with the pharmaceutical industry.
SOURCE: Fujimaki M et al. Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004888
FROM NEUROLOGY
Key clinical point:
Major finding: Combining serum levels of caffeine and nine related metabolites identified individuals with PD with an AUC of 0.98.
Data source: Analysis of 108 Parkinson’s patients and 31 healthy controls.
Disclosures: The study was funded by grants from several Japanese government agencies. Some of the authors have financial relationships with the pharmaceutical industry.
Source: Fujimaki M et al., Neurology. 2018 Jan 3. doi: 10.1212/WNL.0000000000004888
Improving Strength and Balance for Long-Term Care Residents At Risk for Falling: Suggestions for Practice
From the Geriatric Education and Research in Aging Sciences Centre, McMaster University Hamilton, ON (Dr. McArthur) and the University of Waterloo and Research Institute for Aging, Waterloo, ON (Dr. Giangregorio), Canada
Abstract
- Objective: To synthesize the available literature on exercise and falls reduction interventions in long-term care (LTC) and provide practical information for clinicians and other decision makers.
- Methods: Review of positive trials included in systematic reviews.
- Results: Falls are a major concern for residents, families, clinicians, and decision-makers in LTC. Exercise is recommended as part of a multifactorial falls prevention program for residents in LTC. Strength and balance exercises should be incorporated into the multifactorial falls prevention program. They should be challenging and progressed as the residents’ abilities improve. Evidence suggests that exercises should be completed 2 to 3 times per week for a period longer than 6 months. Exercise programs in LTC should be resident-centered and should consider residents’ potential physical and cognitive impairments. Exercises in standing should be prioritized where appropriate.
- Conclusion: Appropriately challenging and progressive strength and balance exercises should be included in a multifactorial falls prevention program for residents in LTC.
Key words: long-term care; nursing homes; falls reduction; exercise.
Falls are common in long-term care (LTC) homes: the estimated falls rate is 1.5 falls per bed per year, which is 3 times greater than that for older adults living in the community [1]. Falls can have significant consequences for residents in LTC, including functional disability, fractures, pain, reduced quality of life, and death [1–6]. Indeed, 25% of residents who are hospitalized after a fall die within 1 year [3]. Consequently, falls prevention programs are important to help in reducing falls and averting the associated negative consequences.
Exercise may address the circumstances and physical deconditioning that often contribute to falls in LTC residents. Weight shifting [7], walking, and transferring [8–10], are common activities that precede falls, suggesting that balance, gait, and functional mobility training may be possible targets for prevention. Additionally, it is estimated that LTC residents spend three quarters of their waking time in sedentary activities [11,12] and have a high prevalence of sarcopenia [13–16]. Challenging balance training and resistance exercise are well-known intervention for reducing falls [17] and improving muscle strength for community-dwelling older adults [18]. However, evidence around balance and strength training for preventing falls in LTC is mixed [17,19,20], and careful planning and modification of exercises is necessary to meet the needs of LTC residents.
Residents in LTC are often medically complex, with multiple comorbidities [21] that can affect their ability to meaningfully participate in exercise. In Canada, 56.3% of residents have a diagnosis of Alzheimer’s or other dementias, 25.0% have diabetes, 14.4% have chronic obstructive pulmonary disease, and 21.2% have experienced a stroke [21]. Residents also often have significant functional impairments. For example, 97% of residents require assistance with basic activities of daily living [21]. Therefore, the lack of effect of exercise as a single falls prevention strategy observed in previous studies may be because the often complex, multimorbid LTC population likely requires a multifactorial approach to fall prevention [17]. Additionally, organizational aspects of LTC homes (eg, specific funds dedicated to employing exercise professionals and to support exercise programming) can affect residents’ engagement in exercise [22,23]. Subsequently, prescribing exercises in the LTC context must consider both resident characteristics and organizational features of the LTC home (eg, professionals available to support exercise programming).
A comprehensive exercise prescription describes the elements of an appropriate exercise program to facilitate implementation of that program. The exercise prescription should include a description of the type (eg, balance, strength) and intensity of exercises (eg, subjective or objective measurement of how hard the resident is working) included in the program [24]. The prescription should also include a description of the dose of exercise: frequency of exercise participation (eg, 2 days per week), duration of individual exercise sessions (eg, 30-minute sessions), and duration of exercise program (eg, 12-week program) [24]. Lastly, the prescription should describe the setting of the exercise program (eg, group or individual basis) and the professional delivering the program (eg, physiotherapist, fitness instructor) [24].
Therefore, the objectives of this article are to (1) synthesize studies demonstrating a positive effect of exercise on reducing falls for residents in LTC; (2) provide an overview of the principles of balance and strength training to guide clinicians in designing appropriate exercise prescription; and (3) make suggestions for clinical practice regarding an appropriate strength and balance exercise protocol by considering the influence of the LTC context.
Methods
To provide clinicians and other policy-makers with a description of which balance and strength exercises may be effective for preventing falls, we synthesized trials that demonstrated a positive effect on reducing falls or falls risk for residents in LTC. Studies were identified through a database search for systematic reviews in PubMed, Ovid, and Google Scholar using the keywords falls, long-term care, nursing homes, exercise, strength, balance, and systematic reviews. Our purpose was to provide practical information on what works to prevent falls through balance and strength training for residents in LTC rather than to evaluate the available evidence. Therefore, only positive trials from systematic reviews were discussed, as we wanted to present exercises that seem to have a positive effect on decreasing falls. Positive trials were defined as those included in identified systematic reviews with a risk or rate ratio and confidence intervals below 1.0.
We first provide an overview of the conclusions of the systematic reviews found in our search. Next, for each positive trial we describe the following elements of the exercise component of the intervention: frequency, time of sessions, length of program, intensity, type of exercise including a description of the specific exercises performed, whether the intervention was delivered in a group or on an individual basis, the professional delivering the intervention, and any other features of the intervention aside from the exercise component. We used the ProFaNE taxonomy definitions [25] to identify and describe each element of the exercise interventions. Frequency is the number of times per week that residents engage in sessions, time of sessions is the amount allocated to each exercise session, duration of program is how long the resident participates in the exercise program, and intensity is the subjective or objective report of how hard the resident is working [25]. The types of exercises described were those targeting balance defined as “...the efficient transfer of bodyweight from one part of the body to another or challenges specific aspects of the balance systems (eg, vestibular system)” [25], and strength defined as “...contracting the muscles against a resistance to ‘overload’ and bring about a training effect in the muscular system” [25]. Strength could be either an external resistance (eg, dumbbell) or using body weight against gravity (eg, squat) [25].
Results
We found 3 systematic reviews that include exercise programs to reduce falls in LTC homes [17,19,20]. Overall, evidence suggests that exercise should be included as part of a multifactorial falls prevention program for residents in LTC. There is limited evidence that exercise as a single intervention prevents falls, and some trials, albeit underpowered, even demonstrate an increased risk of falling in the exercise group compared to control [19]. With regards to specific exercise programs, the Cochrane review found that gait, balance, and functional training decrease the rate of falls but not the risk of falling [26–28], and the 2013 review by Silva et al [20] concluded that combined exercise programs (ie, multiple types of exercise) that include balance tasks, are completed frequently (2–3 times per week), and over a long term (greater than 6 months) were most effective at preventing falls [20].
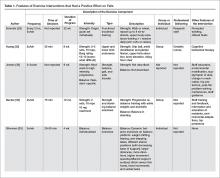
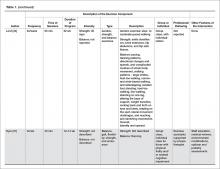
A more recent systematic review and meta-analysis [17] also concluded that there was no evidence that exercise as a single intervention can prevent falls for residents in LTC. Table 1 provides a description of the exercise component of the seven positive trials [29–35] that were included in the 3 systematic reviews we identified in our search.
Type of Exercise
Balance Exercises
There were 4 positive trials that included balance exercises in their intervention [31,33–35]. Trials that had a positive effect on reducing falls and included balance training employed mostly dynamic balance exercises in standing (Table 1). However, only 2 of the 7 trials provided a detailed description of their balance exercises (Table 1) [26,34]. Jensen et al [30] and Dyer et al [31] did not include a description of the balance training performed but stated that balance was part of the multicomponent exercise program. Becker et al [36] stated that participants performed standing balance exercises, while Schnelle et al [39] and Huang et al [32] did not include balance training in their trial.
Strength Exercises
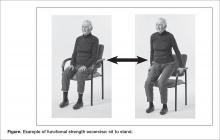
Of the 7 positive trials included in this review, 6 included strength exercises [29–32,34,35]. The strength activities used in trials where exercise had a positive effect on decreasing falls included functional activities [29,31] and progressive resistance training [31,36] (Table 1). Functional activities are those that replicate what a resident might be required to do in their everyday life, such as performing sit-to-stands out of a chair (Figure)
Frequency, Time of Sessions, Duration of Program
In our description of positive trials, exercise was performed on 2 to 3 days per week for 20 to 75 minutes per session, for periods ranging from 4 to 52 weeks (Table 1).
Intensity
For the trials including balance exercises, one trial described the intensity as resident-specific [37] and another as individualized [33]. Two studies did not describe the intensity of their balance exercises [31,34]. The intensity of strength exercises included in the positive trials was individualized for one of the trial [29]. Two trials had participants complete 2 to 3 sets of 10 repetitions [32,35], with one indicating an intensity of 12–13 or “somewhat difficult” on the Borg Rating of Perceived Exertion Scale [32] and the other using a 10-rep max [35]. Two studies described their strength exercises as progressive [31,37], and one at a moderate to high intensity [30]. Lord et al prescribed 30 repetitions of each strength exercise [34].
Delivery of Intervention
Exercise was delivered in a group setting for 4 of the trials [31,32,34,36], individually for 2 of the trials [26,29], and the setting was not described for one of the trials (Table 1) [30]. Finally, only 3 of the 7 articles reported the professional delivering the intervention: one was research staff [29], one was geriatric nurses [32], and one was exercise assistants supported by a physiotherapist [31].
Discussion
There is limited evidence to support the use of strength and balance exercise as a single intervention to prevent falls in LTC. However, exercise should be included as part of a multifactorial falls prevention program. Trials that had a positive effect on decreasing falls training used dynamic balance exercises in standing, functional training, and progressive resistance training on 2 to 3 days per week, for 20 to 75 minutes per session, over 4 to 52 weeks. The intensity of balance exercises was individualized, and strength exercises were described as somewhat difficult or performed at a moderate to high intensity. Exercise was performed in a group or individually, and was delivered by research staff, geriatric nurses, exercise assistants supervised by physiotherapists, or more frequently, it was not reported who delivered the intervention.
Balance Training
Our work suggests that standing, dynamic balance exercises may be best to decrease falls. Example balance exercises include reducing the base of support (eg, standing with feet together instead of apart, or tandem with one foot in front), moving the center of gravity and control body position while standing (eg, reaching, weight shifting, stepping up or down), and standing without using arms for support or reducing reliance on the upper limbs for support (eg, use one hand on a handrail instead of two, or two fingers instead of the whole hand) [17]. It is well established that balance training programs, especially those including challenging exercises, can prevent falls in community-dwelling older adults [17]. However, the relationship is not as clear in LTC.
Strength Training
Reduced muscle strength has been identified as an important risk factor for falls [38]. There are also many psychological and metabolic benefits to strength training [39]. To induce change in muscular strength, resistance exercises need to be challenging and progressive. Our work suggests that strength training that is effective at decreasing falls is functional and progressive, and is completed at a moderate to high intensity. A resident should be able to do a strength exercise for one to two sets of 6 to 8 repetitions before being fatigued [40]. Once the resident can complete two sets of 13 to 15 repetitions easily the exercise should be progressed. Residents who are particularly deconditioned may need to begin with lower intensity strength exercises (eg, only do one set, with a lower resistance and progress to a higher resistance) [40]. Residents should perform resistance exercises for all major muscle groups [40]. Progression could include increasing the number of sets (eg, increase from one to two sets), the resistance (eg, holding dumbbells while squatting), or the intensity of the exercise (eg, squat lower or faster) [41].
Implementing Exercise Programs in LTC
Implementation of exercise programs into LTC homes should consider the dose of exercise (eg, time and frequency of sessions, duration of program), if they are delivered in a group or individual setting, and who is delivering them. First, trials included in this paper suggest that strength and balance exercises to prevent falls were delivered 2 to 3 times per week, for 20 to 75 minutes per session, over 4 to 52 weeks. Second, previous work has established that exercise programs delivered on 2 to 3 days per week over a period of more than 6 months are most effective at reducing falls in LTC [20]. Finally, a recent task force report from an international group of clinician researchers in LTC recommends twice weekly exercise sessions lasting 35 to 45 minutes each [40]. Therefore, strength and balance exercises to prevent falls in LTC should be delivered at least twice per week, for at least 20 minutes, for greater than 6 weeks’ duration.
Whether exercise should be performed in a group or individual setting remains unclear. Two of the 6 positive trials in this paper were completed individually, while 3 were in a group. The aforementioned task force also recommended that every resident who does not have contraindications to exercise must have an individualized exercise program as part of their health care plan [40]. However, whether the exercise program is provided on an individual basis or in a group setting was not delineated. Indeed, there are currently no recommendations concerning prioritizing group or individual exercise programs. Therefore, exercise programs being implemented into LTC homes should consider the residents’ preferences, the social benefits of group exercise, and the feasibility of individualizing exercises for the complex needs of residents in LTC in large group settings.
Finally, which professionals should deliver the exercise program is also uncertain. Only 3 of the positive trials in this paper described the professional delivering the intervention, with one being research staff, one geriatric nurses, and one exercise assistants supported by a physiotherapist. We suggest that professionals delivering an exercise program should be trained in exercise planning, delivery, and progression, be familiar with the principles of balance and strength training, and have training in working with older adults in LTC.
Modifications for Physical Impairments
Residents in LTC often have complex health needs, with multiple comorbidities (eg, stroke, Parkinson’s disease, multiple sclerosis) [21]. Modifications of strength and balance exercises may be required to accommodate for physical impairments (eg, hemiplegia, drop foot, freezing gait). For example, if a resident has hemiplegia and cannot fully activate the muscles of one arm, one can do resistance exercises with a dumbbell on the functioning side and active assisted range of motion (ie, the exercise provider assists the resident to achieve full range of motion against gravity) on the hemiparetic side. A resident with Parkinson’s disease who has freezing gait may need visual or rhythmical verbal cues to be able to accomplish standing balance tasks such as altered walking patterns (eg, wide or narrow stepping) [42].
Modifications for Cognitive Impairments
More than 80% of residents in LTC have some degree of cognitive impairment [21]. Cognitive impairment may be the result of stroke, depression, traumatic injuries, medications, and degenerative diseases such as Parkinson’s and Alzheimer’s disease [43]. A common misconception is that residents with cognitive impairment cannot benefit from exercise because they cannot learn new skills and have difficulty following directions. On the contrary, evidence suggests that exercise can improve functional mobility for residents with cognitive impairment [44,45].
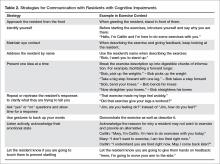
Residents with cognitive impairment may require a different approach to facilitate participation in the desired exercises because of difficulty following multi-step directions, responsive behaviors, or increased distractibility [46]. Clear communication is key in improving the quality of interaction for residents with cognitive impairment. The Alzheimer Society of Ontario suggests 10 strategies for communicating with people with dementia [47], and we have provided suggestions of how to apply these communication strategies to the exercise context in LTC (Table 2). Other suggestions for engaging residents with cognitive impairment in strength and balance training include making the exercises functional (eg, ask them to pick something up of the floor to perform a squat, or reach a point on the wall to do calf raises) and playful (eg, toss a ball back and forth or sing a song about rowing to promote weight shifting) [48].
Standing versus Seated Exercises
Residents may not be able to participate in standing exercises for several reasons: perhaps the resident cannot stand or has severe balance impairments and a high falls risk; the resident may have poor insight into which exercises are safe to perform in standing versus sitting; or there may be limited supervision of a large group exercise class where the risk of falls is a concern. If balance impairments are a concern, where the risk of injury or falling while completing exercises in standing outweighs the benefit of doing the exercises, then seated exercises are appropriate. However, when residents are able, we recommend encouraging some or all exercises in standing, to facilitate carry over of strength gains into functional tasks such as being able to rise from a chair and walking. A recent study, comparing standing versus seated exercises for community dwelling older adults, saw greater functional gains for those who completed the standing exercises [49]. Therefore, strength and balance exercises should be performed in standing, where appropriate.
Resident-Centered Exercise for Falls Prevention
Putting the resident at the center of falls prevention is important. Previous work has found that older adults have expressed a strong preference for care that transcends traditional biomedical care and that values efficiency, consistency, and hierarchical decision making [50]. On the contrary, resident-centered care emphasizes well-being and quality of life as defined by the resident, values giving residents greater control over the nature of services they receive, and respects their rights to be involved in every day decision making [51,52]. Indeed, residents may choose to engage in risky behaviors that increase their risk of falls but also increases their quality of life. Previous work has found disconnects between residents’ perceived frailty and the potential ability of protective devices to prevent adverse events, such as falls and fractures [53]. Additionally, one study identified that older residents feared being labelled, so instead hid impairments and chose to refuse assistance and assistive devices [54]. For example, a resident with impaired balance and gait may choose to walk independently when they have been deemed as requiring a gait aid (eg, rollator walker). However, they may value walking without a gait aid and accept the increased risk of falling. Therefore, it is essential to find the delicate balance between respecting a resident’s right to make their own decisions and preventing adverse events, such as falls [52]. An example of this would be respecting a resident’s right to refuse to attend exercise programming even though the team may think they can benefit from strength and balance training.
There is limited evidence around falls prevention and resident-centered care. A recent systematic review [55] revealed that resident-centered care may increase falls rates [56,57]. However, the authors of the review attributed the increase in falls to differences in frailty between the control and intervention group [56], and to environmental factors (eg, slippery flooring material, lack of handrails) [57]. Additionally, these trials did not include an exercise program as part of the resident-centered care program. On the other hand, resident-centered care has been associated with reduction of boredom, helplessness, and depression [58,59]. Most studies included in the review were quasi-experimental, which significantly limits the evidence quality [55]. At this point in time, the evidence suggests that resident-centered care is important for mood and quality of life but may have a negative or no effect on reducing falls.
Multifactorial Falls Prevention Programs
While there are mixed results about the effect of exercise as a single intervention for reducing falls for residents in LTC, the literature clearly supports exercise as part of a multifactorial falls prevention program [17,20,60–62]. A 2015 umbrella review [62] of meta-analyses of randomized controlled trials of falls prevention interventions in LTC concluded that multifactorial interventions were the most effective at preventing falls in LTC. Additionally, recently developed recommendations for fracture prevention in LTC [61] suggest that balance, strength, and functional training should be included for residents who are not at high risk of fracture, while for those at high risk, exercise should be provided as part of a multifactorial falls prevention intervention. Clinicians must therefore incorporate elements aside from exercise into their falls prevention strategies. Interventions that have shown positive effects on reducing falls when delivered as part of multifactorial interventions include: staff and resident education [31,35,37], environmental modifications [31,35], supply/repair/provision of assistive devices [30], falls problem-solving conferences [30], urinary incontinence management [29], medication review [30], optician review [31], and cognitive behavioral therapy [32].
Conclusion and Suggestions for Clinical Practice
We suggest incorporating strength and balance exercises as part of a multifactorial falls prevention program for residents in LTC. Balance exercises should be challenging and dynamic (eg, weight shifting). Strength exercises should be of a moderate to high intensity (eg, can complete one to sets of 6 to 8 repetitions) and need to be progressed as the residents’ abilities improve. Residents should participate in strength and balance training on 2 to 3 days per week, for 30- to 45-minute sessions, for at least 6 months. Exercises in standing should be prioritized where appropriate. Exercise could be delivered in a group or individual format, but should consider the residents’ preferences, the social benefits of group exercise, and the feasibility of individualizing exercises for the complex needs of residents in LTC in large group settings. Professionals delivering an exercise program should be trained in exercise planning, delivery, and progression, be familiar with the principles of balance and strength training, and have training in working with older adults in LTC. Exercise programs in LTC should be resident-centered and consider residents’ potential physical and cognitive impairments.
Funding/support: Dr. Giangregorio was supported by grants from the Canadian Frailty Network and Canadian Institutes of Health Research.
1. Harris IA, Yong S, McEvoy L, Thorn L. A prospective study of the effect of nursing home residency on mortality following hip fracture. ANZ J Surg 2010;80:447–50.
2. Ooms ME, Vlasman P, Lips P, et al. The incidence of hip fractures in independent and institutionalized elderly people. Osteoporos Int 1994;4:6–10.
3. Ayoung-Chee P, McIntyre L, Ebel BE, et al. Long-term outcomes of ground-level falls in the elderly. J Trauma Acute Care Surg 2014;76:498–503.
4. Heinrich S, Rapp K, Rissmann U, et al. Cost of falls in old age: a systematic review. Osteoporos Int 2010;21: 891–902.
5. Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med 1994;121:442–51.
6. Hartholt KA, van Beeck EF, Polinder S, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long-term reduced quality of life. J Trauma
2011;71:748–53.
7. Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet 2013;381:
47–54.
8. Rapp K, Becker C, Cameron ID, et al. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of bavarian nursing homes. J Am Med Dir Assoc 2012;13:187.
9. Büchele G, Becker C, Cameron ID, et al. Predictors of serious consequences of falls in residential aged care: analysis of more than 70,000 falls from residents of Bavarian nursing homes. J Am Med Dir Assoc 2014;15:559–63.
10. McArthur C, Gonzalez DA, Roy E, Giangregorio L. What are the circumstances of falls and fractures in long-term care? Can J Aging / La Rev Can du Vieil 2016;35:491–8.
11. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.
12. Ikezoe T, Asakawa Y, Shima H, et al. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr 2013;57:221–5.
13. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015;82:418–23.
14. Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc 2014;15:267–72.
15. Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–6.
16. Yalcin A, Aras S, Atmis V, et al. Sarcopenia prevalence and factors associated with sarcopenia in older people living in a nursing home in Ankara Turkey. Geriatr Gerontol Int
2016;16:903–10.
17. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med October 2016.
18. Liu C, Latham NK. Progressive resistance strength training for improving physical function in older adults. In: Liu C, ed. Cochrane Database Syst Rev;2009:CD002759.
19. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev;2012:CD005465.
20. Silva RB, Eslick GD, Duque G. Exercise for falls and fracture prevention in long term care facilities: a systematic review and meta-analysis. J Am Med Dir Assoc 2013;14:685–9.
21. Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Can J Aging 2011;30: 371–90.
22. Benjamin K, Edwards N, Guitard P, et al. Factors that influence physical activity in long-term care: Perspectives of residents, staff, and significant others. Can J Aging 2011;30:247–58.
23. Benjamin K, Edwards N, Ploeg J, Legault F. Barriers to physical activity and restorative care for residents in long-term care: A review of the literature. J Aging Phys Act 2014;22:154–65.
24. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed. American College of Sports Medicine; 2013.
25. Prevention of Falls Network Europe. Prevention of Falls Network Europe. Accessed 27 Nov 2017 at www.profane.eu.org/.
26. Sihvonen SE, Sipilä S, Era PA. Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology 2004;50:87–95.
27. Sakamoto K, Nakamura T, Hagino H, et al. Effects of unipedal standing balance exercise on the prevention of falls and hip fracture among clinically defined high-risk elderly individuals: a randomized controlled trial. J Orthop Sci 2006;11:467–72.
28. Shimada H, Obuchi S, Furuna T, Suzuki T. New intervention program for preventing falls among frail elderly people: the effects of perturbed walking exercise using a bilateral separated treadmill. Am J Phys Med Rehabil 2004;83:493–9.
29. Schnelle JF, Kapur K, Alessi C, et al. Does an exercise and incontinence intervention save healthcare costs in a nursing home population? J Am Geriatr Soc 2003;51:161–8.
30. Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities: A cluster randomized trial. Ann Intern Med 2002;136:733–41.
31. Dyer CAE. Falls prevention in residential care homes: a randomised controlled trial. Age Ageing 2004;33:596–602.
32. Huang T-T, Chung M-L, Chen F-R, Chin Y-F, Wang B-H. Evaluation of a combined cognitive-behavioural and exercise intervention to manage fear of falling among elderly residents in nursing homes. Aging Ment Health 2016;20:2–12.
33. Sihvonen S, Sipilä S, Taskinen S, Era P. Fall incidence in frail older women after individualized visual feedback-based balance training. Gerontology 2004;50:411–6.
34. Lord SR, Castell S, Corcoran J, et al. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: a randomized, controlled trial. J Am Geriatr Soc 2003;51:1685–92.
35. Becker C, Kron M, Lindemann U, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. J Am Geriatr Soc 2003;51:306–13.
36. Becker C, Kron M, Lindemann U, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. J Am Geriatr Soc 2003;51:306–13.
37. Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities. A cluster randomized trial. Ann Intern Med 2002;136:733–41.
38. Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2004;52: 1121–9.
39. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sport Exerc 2009;41:1510–30.
40. de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc 2016;17:381–92.
41. American College of Sports Medicine. Progression models in resistance training for healthy adults. Med Sci Sport Exerc 2009;41:687–708.
42. Fietzek UM, Schroeteler FE, Ziegler K, et al. Randomized cross-over trial to investigate the efficacy of a two-week physiotherapy programme with repetitive exercises of cueing to reduce the severity of freezing of gait in patients with Parkinson’s disease. Clin Rehabil 2014;28:902–11.
43. Patterson C, Feightner J, Garcia A, MacKnight C. General risk factors for dementia: A systematic evidence review. Alzheimer Dement 2007;3:341–7.
44. Roach KE, Tappen RM, Kirk-Sanchez N, et al. A randomized controlled trial of an activity specific exercise program for individuals with alzheimer disease in long-term care settings. J Geriatr Phys Ther 2011;34:50–6.
45. Christofoletti G, Oliani MM, Gobbi S, et al. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil 2008;22:618–26.
46. van Alphen HJM, Hortobágyi T, van Heuvelen MJG. Barriers, motivators, and facilitators of physical activity in dementia patients: A systematic review. Arch Gerontol Geriatr 2016;66:109–18.
47. Alzheimer Society of Ontario. Rethink Dementia. Accessed 18 Sep 2017 at http://rethinkdementia.ca/.
48. Roach KE, Tappen RM, Kirk-Sanchez N, et al. A randomized controlled trial of an activity specific exercise program for individuals with Alzheimer disease in long-term care settings. J Geriatr Phys Ther 2011;34:50–6.
49. Brach JS, Perera S, Gilmore S, et al. Effectiveness of a timing and coordination group exercise program to improve mobility in community-dwelling older adults. JAMA Intern Med August 2017.
50. Rosher RB, Robinson S. Impact of the Eden alternative on family satisfaction. J Am Med Dir Assoc 2005;6:189–93.
51. Crandall LG, White DL, Schuldheis S, Talerico KA. Initiating person-centered care practices in long-term care facilities. J Gerontol Nurs 2007;33:47–56.
52. Sims-Gould J, McKay HA, Feldman F, et al. Autonomy, choice, patient-centered care, and hip protectors: the experience of residents and staff in long-term care. J Appl Gerontol 2014;33:690–709.
53. Robinovitch SN, Cronin T. Perception of postural limits in elderly nursing home and day care participants. J Gerontol A Biol Sci Med Sci 1999;54:B124-30.
54. Perkins MM, Ball MM, Whittington FJ, Hollingsworth C. Relational autonomy in assisted living: a focus on diverse care settings for older adults. J Aging Stud 2012;26:214–25.
55. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging 2013;8:1–10.
56. Coleman MT, Looney S, O’Brien J, et al. The Eden Alternative: findings after 1 year of implementation. J Gerontol A Biol Sci Med Sci 2002;57:M422–7.
57. Chenoweth L, King MT, Jeon Y-H, et al. Caring for Aged Dementia Care Resident Study (CADRES) of personcentred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8: 317–25.
58. Bergman-Evans B. Beyond the basics. Effects of the Eden Alternative model on quality of life issues. J Gerontol Nurs 2004;30:27–34.
59. Robinson SB, Rosher RB. Tangling with the barriers to culture change: creating a resident-centered nursing home environment. J Gerontol Nurs 2006;32:19–25.
60. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 2012;12.
61. Papaioannou A, Santesso N, Morin SN, et al. Recommendations for preventing fracture in long-term care. Can Med Assoc J 2015;187:1135–44.
62. Stubbs B, Denkinger MD, Brefka S, Dallmeier D. What works to prevent falls in older adults dwelling in long term care facilities and hospitals? An umbrella review of meta-analyses of randomised controlled trials. Maturitas 2015;81:335–42.
From the Geriatric Education and Research in Aging Sciences Centre, McMaster University Hamilton, ON (Dr. McArthur) and the University of Waterloo and Research Institute for Aging, Waterloo, ON (Dr. Giangregorio), Canada
Abstract
- Objective: To synthesize the available literature on exercise and falls reduction interventions in long-term care (LTC) and provide practical information for clinicians and other decision makers.
- Methods: Review of positive trials included in systematic reviews.
- Results: Falls are a major concern for residents, families, clinicians, and decision-makers in LTC. Exercise is recommended as part of a multifactorial falls prevention program for residents in LTC. Strength and balance exercises should be incorporated into the multifactorial falls prevention program. They should be challenging and progressed as the residents’ abilities improve. Evidence suggests that exercises should be completed 2 to 3 times per week for a period longer than 6 months. Exercise programs in LTC should be resident-centered and should consider residents’ potential physical and cognitive impairments. Exercises in standing should be prioritized where appropriate.
- Conclusion: Appropriately challenging and progressive strength and balance exercises should be included in a multifactorial falls prevention program for residents in LTC.
Key words: long-term care; nursing homes; falls reduction; exercise.
Falls are common in long-term care (LTC) homes: the estimated falls rate is 1.5 falls per bed per year, which is 3 times greater than that for older adults living in the community [1]. Falls can have significant consequences for residents in LTC, including functional disability, fractures, pain, reduced quality of life, and death [1–6]. Indeed, 25% of residents who are hospitalized after a fall die within 1 year [3]. Consequently, falls prevention programs are important to help in reducing falls and averting the associated negative consequences.
Exercise may address the circumstances and physical deconditioning that often contribute to falls in LTC residents. Weight shifting [7], walking, and transferring [8–10], are common activities that precede falls, suggesting that balance, gait, and functional mobility training may be possible targets for prevention. Additionally, it is estimated that LTC residents spend three quarters of their waking time in sedentary activities [11,12] and have a high prevalence of sarcopenia [13–16]. Challenging balance training and resistance exercise are well-known intervention for reducing falls [17] and improving muscle strength for community-dwelling older adults [18]. However, evidence around balance and strength training for preventing falls in LTC is mixed [17,19,20], and careful planning and modification of exercises is necessary to meet the needs of LTC residents.
Residents in LTC are often medically complex, with multiple comorbidities [21] that can affect their ability to meaningfully participate in exercise. In Canada, 56.3% of residents have a diagnosis of Alzheimer’s or other dementias, 25.0% have diabetes, 14.4% have chronic obstructive pulmonary disease, and 21.2% have experienced a stroke [21]. Residents also often have significant functional impairments. For example, 97% of residents require assistance with basic activities of daily living [21]. Therefore, the lack of effect of exercise as a single falls prevention strategy observed in previous studies may be because the often complex, multimorbid LTC population likely requires a multifactorial approach to fall prevention [17]. Additionally, organizational aspects of LTC homes (eg, specific funds dedicated to employing exercise professionals and to support exercise programming) can affect residents’ engagement in exercise [22,23]. Subsequently, prescribing exercises in the LTC context must consider both resident characteristics and organizational features of the LTC home (eg, professionals available to support exercise programming).
A comprehensive exercise prescription describes the elements of an appropriate exercise program to facilitate implementation of that program. The exercise prescription should include a description of the type (eg, balance, strength) and intensity of exercises (eg, subjective or objective measurement of how hard the resident is working) included in the program [24]. The prescription should also include a description of the dose of exercise: frequency of exercise participation (eg, 2 days per week), duration of individual exercise sessions (eg, 30-minute sessions), and duration of exercise program (eg, 12-week program) [24]. Lastly, the prescription should describe the setting of the exercise program (eg, group or individual basis) and the professional delivering the program (eg, physiotherapist, fitness instructor) [24].
Therefore, the objectives of this article are to (1) synthesize studies demonstrating a positive effect of exercise on reducing falls for residents in LTC; (2) provide an overview of the principles of balance and strength training to guide clinicians in designing appropriate exercise prescription; and (3) make suggestions for clinical practice regarding an appropriate strength and balance exercise protocol by considering the influence of the LTC context.
Methods
To provide clinicians and other policy-makers with a description of which balance and strength exercises may be effective for preventing falls, we synthesized trials that demonstrated a positive effect on reducing falls or falls risk for residents in LTC. Studies were identified through a database search for systematic reviews in PubMed, Ovid, and Google Scholar using the keywords falls, long-term care, nursing homes, exercise, strength, balance, and systematic reviews. Our purpose was to provide practical information on what works to prevent falls through balance and strength training for residents in LTC rather than to evaluate the available evidence. Therefore, only positive trials from systematic reviews were discussed, as we wanted to present exercises that seem to have a positive effect on decreasing falls. Positive trials were defined as those included in identified systematic reviews with a risk or rate ratio and confidence intervals below 1.0.
We first provide an overview of the conclusions of the systematic reviews found in our search. Next, for each positive trial we describe the following elements of the exercise component of the intervention: frequency, time of sessions, length of program, intensity, type of exercise including a description of the specific exercises performed, whether the intervention was delivered in a group or on an individual basis, the professional delivering the intervention, and any other features of the intervention aside from the exercise component. We used the ProFaNE taxonomy definitions [25] to identify and describe each element of the exercise interventions. Frequency is the number of times per week that residents engage in sessions, time of sessions is the amount allocated to each exercise session, duration of program is how long the resident participates in the exercise program, and intensity is the subjective or objective report of how hard the resident is working [25]. The types of exercises described were those targeting balance defined as “...the efficient transfer of bodyweight from one part of the body to another or challenges specific aspects of the balance systems (eg, vestibular system)” [25], and strength defined as “...contracting the muscles against a resistance to ‘overload’ and bring about a training effect in the muscular system” [25]. Strength could be either an external resistance (eg, dumbbell) or using body weight against gravity (eg, squat) [25].
Results
We found 3 systematic reviews that include exercise programs to reduce falls in LTC homes [17,19,20]. Overall, evidence suggests that exercise should be included as part of a multifactorial falls prevention program for residents in LTC. There is limited evidence that exercise as a single intervention prevents falls, and some trials, albeit underpowered, even demonstrate an increased risk of falling in the exercise group compared to control [19]. With regards to specific exercise programs, the Cochrane review found that gait, balance, and functional training decrease the rate of falls but not the risk of falling [26–28], and the 2013 review by Silva et al [20] concluded that combined exercise programs (ie, multiple types of exercise) that include balance tasks, are completed frequently (2–3 times per week), and over a long term (greater than 6 months) were most effective at preventing falls [20].
A more recent systematic review and meta-analysis [17] also concluded that there was no evidence that exercise as a single intervention can prevent falls for residents in LTC. Table 1 provides a description of the exercise component of the seven positive trials [29–35] that were included in the 3 systematic reviews we identified in our search.
Type of Exercise
Balance Exercises
There were 4 positive trials that included balance exercises in their intervention [31,33–35]. Trials that had a positive effect on reducing falls and included balance training employed mostly dynamic balance exercises in standing (Table 1). However, only 2 of the 7 trials provided a detailed description of their balance exercises (Table 1) [26,34]. Jensen et al [30] and Dyer et al [31] did not include a description of the balance training performed but stated that balance was part of the multicomponent exercise program. Becker et al [36] stated that participants performed standing balance exercises, while Schnelle et al [39] and Huang et al [32] did not include balance training in their trial.
Strength Exercises
Of the 7 positive trials included in this review, 6 included strength exercises [29–32,34,35]. The strength activities used in trials where exercise had a positive effect on decreasing falls included functional activities [29,31] and progressive resistance training [31,36] (Table 1). Functional activities are those that replicate what a resident might be required to do in their everyday life, such as performing sit-to-stands out of a chair (Figure)
Frequency, Time of Sessions, Duration of Program
In our description of positive trials, exercise was performed on 2 to 3 days per week for 20 to 75 minutes per session, for periods ranging from 4 to 52 weeks (Table 1).
Intensity
For the trials including balance exercises, one trial described the intensity as resident-specific [37] and another as individualized [33]. Two studies did not describe the intensity of their balance exercises [31,34]. The intensity of strength exercises included in the positive trials was individualized for one of the trial [29]. Two trials had participants complete 2 to 3 sets of 10 repetitions [32,35], with one indicating an intensity of 12–13 or “somewhat difficult” on the Borg Rating of Perceived Exertion Scale [32] and the other using a 10-rep max [35]. Two studies described their strength exercises as progressive [31,37], and one at a moderate to high intensity [30]. Lord et al prescribed 30 repetitions of each strength exercise [34].
Delivery of Intervention
Exercise was delivered in a group setting for 4 of the trials [31,32,34,36], individually for 2 of the trials [26,29], and the setting was not described for one of the trials (Table 1) [30]. Finally, only 3 of the 7 articles reported the professional delivering the intervention: one was research staff [29], one was geriatric nurses [32], and one was exercise assistants supported by a physiotherapist [31].
Discussion
There is limited evidence to support the use of strength and balance exercise as a single intervention to prevent falls in LTC. However, exercise should be included as part of a multifactorial falls prevention program. Trials that had a positive effect on decreasing falls training used dynamic balance exercises in standing, functional training, and progressive resistance training on 2 to 3 days per week, for 20 to 75 minutes per session, over 4 to 52 weeks. The intensity of balance exercises was individualized, and strength exercises were described as somewhat difficult or performed at a moderate to high intensity. Exercise was performed in a group or individually, and was delivered by research staff, geriatric nurses, exercise assistants supervised by physiotherapists, or more frequently, it was not reported who delivered the intervention.
Balance Training
Our work suggests that standing, dynamic balance exercises may be best to decrease falls. Example balance exercises include reducing the base of support (eg, standing with feet together instead of apart, or tandem with one foot in front), moving the center of gravity and control body position while standing (eg, reaching, weight shifting, stepping up or down), and standing without using arms for support or reducing reliance on the upper limbs for support (eg, use one hand on a handrail instead of two, or two fingers instead of the whole hand) [17]. It is well established that balance training programs, especially those including challenging exercises, can prevent falls in community-dwelling older adults [17]. However, the relationship is not as clear in LTC.
Strength Training
Reduced muscle strength has been identified as an important risk factor for falls [38]. There are also many psychological and metabolic benefits to strength training [39]. To induce change in muscular strength, resistance exercises need to be challenging and progressive. Our work suggests that strength training that is effective at decreasing falls is functional and progressive, and is completed at a moderate to high intensity. A resident should be able to do a strength exercise for one to two sets of 6 to 8 repetitions before being fatigued [40]. Once the resident can complete two sets of 13 to 15 repetitions easily the exercise should be progressed. Residents who are particularly deconditioned may need to begin with lower intensity strength exercises (eg, only do one set, with a lower resistance and progress to a higher resistance) [40]. Residents should perform resistance exercises for all major muscle groups [40]. Progression could include increasing the number of sets (eg, increase from one to two sets), the resistance (eg, holding dumbbells while squatting), or the intensity of the exercise (eg, squat lower or faster) [41].
Implementing Exercise Programs in LTC
Implementation of exercise programs into LTC homes should consider the dose of exercise (eg, time and frequency of sessions, duration of program), if they are delivered in a group or individual setting, and who is delivering them. First, trials included in this paper suggest that strength and balance exercises to prevent falls were delivered 2 to 3 times per week, for 20 to 75 minutes per session, over 4 to 52 weeks. Second, previous work has established that exercise programs delivered on 2 to 3 days per week over a period of more than 6 months are most effective at reducing falls in LTC [20]. Finally, a recent task force report from an international group of clinician researchers in LTC recommends twice weekly exercise sessions lasting 35 to 45 minutes each [40]. Therefore, strength and balance exercises to prevent falls in LTC should be delivered at least twice per week, for at least 20 minutes, for greater than 6 weeks’ duration.
Whether exercise should be performed in a group or individual setting remains unclear. Two of the 6 positive trials in this paper were completed individually, while 3 were in a group. The aforementioned task force also recommended that every resident who does not have contraindications to exercise must have an individualized exercise program as part of their health care plan [40]. However, whether the exercise program is provided on an individual basis or in a group setting was not delineated. Indeed, there are currently no recommendations concerning prioritizing group or individual exercise programs. Therefore, exercise programs being implemented into LTC homes should consider the residents’ preferences, the social benefits of group exercise, and the feasibility of individualizing exercises for the complex needs of residents in LTC in large group settings.
Finally, which professionals should deliver the exercise program is also uncertain. Only 3 of the positive trials in this paper described the professional delivering the intervention, with one being research staff, one geriatric nurses, and one exercise assistants supported by a physiotherapist. We suggest that professionals delivering an exercise program should be trained in exercise planning, delivery, and progression, be familiar with the principles of balance and strength training, and have training in working with older adults in LTC.
Modifications for Physical Impairments
Residents in LTC often have complex health needs, with multiple comorbidities (eg, stroke, Parkinson’s disease, multiple sclerosis) [21]. Modifications of strength and balance exercises may be required to accommodate for physical impairments (eg, hemiplegia, drop foot, freezing gait). For example, if a resident has hemiplegia and cannot fully activate the muscles of one arm, one can do resistance exercises with a dumbbell on the functioning side and active assisted range of motion (ie, the exercise provider assists the resident to achieve full range of motion against gravity) on the hemiparetic side. A resident with Parkinson’s disease who has freezing gait may need visual or rhythmical verbal cues to be able to accomplish standing balance tasks such as altered walking patterns (eg, wide or narrow stepping) [42].
Modifications for Cognitive Impairments
More than 80% of residents in LTC have some degree of cognitive impairment [21]. Cognitive impairment may be the result of stroke, depression, traumatic injuries, medications, and degenerative diseases such as Parkinson’s and Alzheimer’s disease [43]. A common misconception is that residents with cognitive impairment cannot benefit from exercise because they cannot learn new skills and have difficulty following directions. On the contrary, evidence suggests that exercise can improve functional mobility for residents with cognitive impairment [44,45].
Residents with cognitive impairment may require a different approach to facilitate participation in the desired exercises because of difficulty following multi-step directions, responsive behaviors, or increased distractibility [46]. Clear communication is key in improving the quality of interaction for residents with cognitive impairment. The Alzheimer Society of Ontario suggests 10 strategies for communicating with people with dementia [47], and we have provided suggestions of how to apply these communication strategies to the exercise context in LTC (Table 2). Other suggestions for engaging residents with cognitive impairment in strength and balance training include making the exercises functional (eg, ask them to pick something up of the floor to perform a squat, or reach a point on the wall to do calf raises) and playful (eg, toss a ball back and forth or sing a song about rowing to promote weight shifting) [48].
Standing versus Seated Exercises
Residents may not be able to participate in standing exercises for several reasons: perhaps the resident cannot stand or has severe balance impairments and a high falls risk; the resident may have poor insight into which exercises are safe to perform in standing versus sitting; or there may be limited supervision of a large group exercise class where the risk of falls is a concern. If balance impairments are a concern, where the risk of injury or falling while completing exercises in standing outweighs the benefit of doing the exercises, then seated exercises are appropriate. However, when residents are able, we recommend encouraging some or all exercises in standing, to facilitate carry over of strength gains into functional tasks such as being able to rise from a chair and walking. A recent study, comparing standing versus seated exercises for community dwelling older adults, saw greater functional gains for those who completed the standing exercises [49]. Therefore, strength and balance exercises should be performed in standing, where appropriate.
Resident-Centered Exercise for Falls Prevention
Putting the resident at the center of falls prevention is important. Previous work has found that older adults have expressed a strong preference for care that transcends traditional biomedical care and that values efficiency, consistency, and hierarchical decision making [50]. On the contrary, resident-centered care emphasizes well-being and quality of life as defined by the resident, values giving residents greater control over the nature of services they receive, and respects their rights to be involved in every day decision making [51,52]. Indeed, residents may choose to engage in risky behaviors that increase their risk of falls but also increases their quality of life. Previous work has found disconnects between residents’ perceived frailty and the potential ability of protective devices to prevent adverse events, such as falls and fractures [53]. Additionally, one study identified that older residents feared being labelled, so instead hid impairments and chose to refuse assistance and assistive devices [54]. For example, a resident with impaired balance and gait may choose to walk independently when they have been deemed as requiring a gait aid (eg, rollator walker). However, they may value walking without a gait aid and accept the increased risk of falling. Therefore, it is essential to find the delicate balance between respecting a resident’s right to make their own decisions and preventing adverse events, such as falls [52]. An example of this would be respecting a resident’s right to refuse to attend exercise programming even though the team may think they can benefit from strength and balance training.
There is limited evidence around falls prevention and resident-centered care. A recent systematic review [55] revealed that resident-centered care may increase falls rates [56,57]. However, the authors of the review attributed the increase in falls to differences in frailty between the control and intervention group [56], and to environmental factors (eg, slippery flooring material, lack of handrails) [57]. Additionally, these trials did not include an exercise program as part of the resident-centered care program. On the other hand, resident-centered care has been associated with reduction of boredom, helplessness, and depression [58,59]. Most studies included in the review were quasi-experimental, which significantly limits the evidence quality [55]. At this point in time, the evidence suggests that resident-centered care is important for mood and quality of life but may have a negative or no effect on reducing falls.
Multifactorial Falls Prevention Programs
While there are mixed results about the effect of exercise as a single intervention for reducing falls for residents in LTC, the literature clearly supports exercise as part of a multifactorial falls prevention program [17,20,60–62]. A 2015 umbrella review [62] of meta-analyses of randomized controlled trials of falls prevention interventions in LTC concluded that multifactorial interventions were the most effective at preventing falls in LTC. Additionally, recently developed recommendations for fracture prevention in LTC [61] suggest that balance, strength, and functional training should be included for residents who are not at high risk of fracture, while for those at high risk, exercise should be provided as part of a multifactorial falls prevention intervention. Clinicians must therefore incorporate elements aside from exercise into their falls prevention strategies. Interventions that have shown positive effects on reducing falls when delivered as part of multifactorial interventions include: staff and resident education [31,35,37], environmental modifications [31,35], supply/repair/provision of assistive devices [30], falls problem-solving conferences [30], urinary incontinence management [29], medication review [30], optician review [31], and cognitive behavioral therapy [32].
Conclusion and Suggestions for Clinical Practice
We suggest incorporating strength and balance exercises as part of a multifactorial falls prevention program for residents in LTC. Balance exercises should be challenging and dynamic (eg, weight shifting). Strength exercises should be of a moderate to high intensity (eg, can complete one to sets of 6 to 8 repetitions) and need to be progressed as the residents’ abilities improve. Residents should participate in strength and balance training on 2 to 3 days per week, for 30- to 45-minute sessions, for at least 6 months. Exercises in standing should be prioritized where appropriate. Exercise could be delivered in a group or individual format, but should consider the residents’ preferences, the social benefits of group exercise, and the feasibility of individualizing exercises for the complex needs of residents in LTC in large group settings. Professionals delivering an exercise program should be trained in exercise planning, delivery, and progression, be familiar with the principles of balance and strength training, and have training in working with older adults in LTC. Exercise programs in LTC should be resident-centered and consider residents’ potential physical and cognitive impairments.
Funding/support: Dr. Giangregorio was supported by grants from the Canadian Frailty Network and Canadian Institutes of Health Research.
From the Geriatric Education and Research in Aging Sciences Centre, McMaster University Hamilton, ON (Dr. McArthur) and the University of Waterloo and Research Institute for Aging, Waterloo, ON (Dr. Giangregorio), Canada
Abstract
- Objective: To synthesize the available literature on exercise and falls reduction interventions in long-term care (LTC) and provide practical information for clinicians and other decision makers.
- Methods: Review of positive trials included in systematic reviews.
- Results: Falls are a major concern for residents, families, clinicians, and decision-makers in LTC. Exercise is recommended as part of a multifactorial falls prevention program for residents in LTC. Strength and balance exercises should be incorporated into the multifactorial falls prevention program. They should be challenging and progressed as the residents’ abilities improve. Evidence suggests that exercises should be completed 2 to 3 times per week for a period longer than 6 months. Exercise programs in LTC should be resident-centered and should consider residents’ potential physical and cognitive impairments. Exercises in standing should be prioritized where appropriate.
- Conclusion: Appropriately challenging and progressive strength and balance exercises should be included in a multifactorial falls prevention program for residents in LTC.
Key words: long-term care; nursing homes; falls reduction; exercise.
Falls are common in long-term care (LTC) homes: the estimated falls rate is 1.5 falls per bed per year, which is 3 times greater than that for older adults living in the community [1]. Falls can have significant consequences for residents in LTC, including functional disability, fractures, pain, reduced quality of life, and death [1–6]. Indeed, 25% of residents who are hospitalized after a fall die within 1 year [3]. Consequently, falls prevention programs are important to help in reducing falls and averting the associated negative consequences.
Exercise may address the circumstances and physical deconditioning that often contribute to falls in LTC residents. Weight shifting [7], walking, and transferring [8–10], are common activities that precede falls, suggesting that balance, gait, and functional mobility training may be possible targets for prevention. Additionally, it is estimated that LTC residents spend three quarters of their waking time in sedentary activities [11,12] and have a high prevalence of sarcopenia [13–16]. Challenging balance training and resistance exercise are well-known intervention for reducing falls [17] and improving muscle strength for community-dwelling older adults [18]. However, evidence around balance and strength training for preventing falls in LTC is mixed [17,19,20], and careful planning and modification of exercises is necessary to meet the needs of LTC residents.
Residents in LTC are often medically complex, with multiple comorbidities [21] that can affect their ability to meaningfully participate in exercise. In Canada, 56.3% of residents have a diagnosis of Alzheimer’s or other dementias, 25.0% have diabetes, 14.4% have chronic obstructive pulmonary disease, and 21.2% have experienced a stroke [21]. Residents also often have significant functional impairments. For example, 97% of residents require assistance with basic activities of daily living [21]. Therefore, the lack of effect of exercise as a single falls prevention strategy observed in previous studies may be because the often complex, multimorbid LTC population likely requires a multifactorial approach to fall prevention [17]. Additionally, organizational aspects of LTC homes (eg, specific funds dedicated to employing exercise professionals and to support exercise programming) can affect residents’ engagement in exercise [22,23]. Subsequently, prescribing exercises in the LTC context must consider both resident characteristics and organizational features of the LTC home (eg, professionals available to support exercise programming).
A comprehensive exercise prescription describes the elements of an appropriate exercise program to facilitate implementation of that program. The exercise prescription should include a description of the type (eg, balance, strength) and intensity of exercises (eg, subjective or objective measurement of how hard the resident is working) included in the program [24]. The prescription should also include a description of the dose of exercise: frequency of exercise participation (eg, 2 days per week), duration of individual exercise sessions (eg, 30-minute sessions), and duration of exercise program (eg, 12-week program) [24]. Lastly, the prescription should describe the setting of the exercise program (eg, group or individual basis) and the professional delivering the program (eg, physiotherapist, fitness instructor) [24].
Therefore, the objectives of this article are to (1) synthesize studies demonstrating a positive effect of exercise on reducing falls for residents in LTC; (2) provide an overview of the principles of balance and strength training to guide clinicians in designing appropriate exercise prescription; and (3) make suggestions for clinical practice regarding an appropriate strength and balance exercise protocol by considering the influence of the LTC context.
Methods
To provide clinicians and other policy-makers with a description of which balance and strength exercises may be effective for preventing falls, we synthesized trials that demonstrated a positive effect on reducing falls or falls risk for residents in LTC. Studies were identified through a database search for systematic reviews in PubMed, Ovid, and Google Scholar using the keywords falls, long-term care, nursing homes, exercise, strength, balance, and systematic reviews. Our purpose was to provide practical information on what works to prevent falls through balance and strength training for residents in LTC rather than to evaluate the available evidence. Therefore, only positive trials from systematic reviews were discussed, as we wanted to present exercises that seem to have a positive effect on decreasing falls. Positive trials were defined as those included in identified systematic reviews with a risk or rate ratio and confidence intervals below 1.0.
We first provide an overview of the conclusions of the systematic reviews found in our search. Next, for each positive trial we describe the following elements of the exercise component of the intervention: frequency, time of sessions, length of program, intensity, type of exercise including a description of the specific exercises performed, whether the intervention was delivered in a group or on an individual basis, the professional delivering the intervention, and any other features of the intervention aside from the exercise component. We used the ProFaNE taxonomy definitions [25] to identify and describe each element of the exercise interventions. Frequency is the number of times per week that residents engage in sessions, time of sessions is the amount allocated to each exercise session, duration of program is how long the resident participates in the exercise program, and intensity is the subjective or objective report of how hard the resident is working [25]. The types of exercises described were those targeting balance defined as “...the efficient transfer of bodyweight from one part of the body to another or challenges specific aspects of the balance systems (eg, vestibular system)” [25], and strength defined as “...contracting the muscles against a resistance to ‘overload’ and bring about a training effect in the muscular system” [25]. Strength could be either an external resistance (eg, dumbbell) or using body weight against gravity (eg, squat) [25].
Results
We found 3 systematic reviews that include exercise programs to reduce falls in LTC homes [17,19,20]. Overall, evidence suggests that exercise should be included as part of a multifactorial falls prevention program for residents in LTC. There is limited evidence that exercise as a single intervention prevents falls, and some trials, albeit underpowered, even demonstrate an increased risk of falling in the exercise group compared to control [19]. With regards to specific exercise programs, the Cochrane review found that gait, balance, and functional training decrease the rate of falls but not the risk of falling [26–28], and the 2013 review by Silva et al [20] concluded that combined exercise programs (ie, multiple types of exercise) that include balance tasks, are completed frequently (2–3 times per week), and over a long term (greater than 6 months) were most effective at preventing falls [20].
A more recent systematic review and meta-analysis [17] also concluded that there was no evidence that exercise as a single intervention can prevent falls for residents in LTC. Table 1 provides a description of the exercise component of the seven positive trials [29–35] that were included in the 3 systematic reviews we identified in our search.
Type of Exercise
Balance Exercises
There were 4 positive trials that included balance exercises in their intervention [31,33–35]. Trials that had a positive effect on reducing falls and included balance training employed mostly dynamic balance exercises in standing (Table 1). However, only 2 of the 7 trials provided a detailed description of their balance exercises (Table 1) [26,34]. Jensen et al [30] and Dyer et al [31] did not include a description of the balance training performed but stated that balance was part of the multicomponent exercise program. Becker et al [36] stated that participants performed standing balance exercises, while Schnelle et al [39] and Huang et al [32] did not include balance training in their trial.
Strength Exercises
Of the 7 positive trials included in this review, 6 included strength exercises [29–32,34,35]. The strength activities used in trials where exercise had a positive effect on decreasing falls included functional activities [29,31] and progressive resistance training [31,36] (Table 1). Functional activities are those that replicate what a resident might be required to do in their everyday life, such as performing sit-to-stands out of a chair (Figure)
Frequency, Time of Sessions, Duration of Program
In our description of positive trials, exercise was performed on 2 to 3 days per week for 20 to 75 minutes per session, for periods ranging from 4 to 52 weeks (Table 1).
Intensity
For the trials including balance exercises, one trial described the intensity as resident-specific [37] and another as individualized [33]. Two studies did not describe the intensity of their balance exercises [31,34]. The intensity of strength exercises included in the positive trials was individualized for one of the trial [29]. Two trials had participants complete 2 to 3 sets of 10 repetitions [32,35], with one indicating an intensity of 12–13 or “somewhat difficult” on the Borg Rating of Perceived Exertion Scale [32] and the other using a 10-rep max [35]. Two studies described their strength exercises as progressive [31,37], and one at a moderate to high intensity [30]. Lord et al prescribed 30 repetitions of each strength exercise [34].
Delivery of Intervention
Exercise was delivered in a group setting for 4 of the trials [31,32,34,36], individually for 2 of the trials [26,29], and the setting was not described for one of the trials (Table 1) [30]. Finally, only 3 of the 7 articles reported the professional delivering the intervention: one was research staff [29], one was geriatric nurses [32], and one was exercise assistants supported by a physiotherapist [31].
Discussion
There is limited evidence to support the use of strength and balance exercise as a single intervention to prevent falls in LTC. However, exercise should be included as part of a multifactorial falls prevention program. Trials that had a positive effect on decreasing falls training used dynamic balance exercises in standing, functional training, and progressive resistance training on 2 to 3 days per week, for 20 to 75 minutes per session, over 4 to 52 weeks. The intensity of balance exercises was individualized, and strength exercises were described as somewhat difficult or performed at a moderate to high intensity. Exercise was performed in a group or individually, and was delivered by research staff, geriatric nurses, exercise assistants supervised by physiotherapists, or more frequently, it was not reported who delivered the intervention.
Balance Training
Our work suggests that standing, dynamic balance exercises may be best to decrease falls. Example balance exercises include reducing the base of support (eg, standing with feet together instead of apart, or tandem with one foot in front), moving the center of gravity and control body position while standing (eg, reaching, weight shifting, stepping up or down), and standing without using arms for support or reducing reliance on the upper limbs for support (eg, use one hand on a handrail instead of two, or two fingers instead of the whole hand) [17]. It is well established that balance training programs, especially those including challenging exercises, can prevent falls in community-dwelling older adults [17]. However, the relationship is not as clear in LTC.
Strength Training
Reduced muscle strength has been identified as an important risk factor for falls [38]. There are also many psychological and metabolic benefits to strength training [39]. To induce change in muscular strength, resistance exercises need to be challenging and progressive. Our work suggests that strength training that is effective at decreasing falls is functional and progressive, and is completed at a moderate to high intensity. A resident should be able to do a strength exercise for one to two sets of 6 to 8 repetitions before being fatigued [40]. Once the resident can complete two sets of 13 to 15 repetitions easily the exercise should be progressed. Residents who are particularly deconditioned may need to begin with lower intensity strength exercises (eg, only do one set, with a lower resistance and progress to a higher resistance) [40]. Residents should perform resistance exercises for all major muscle groups [40]. Progression could include increasing the number of sets (eg, increase from one to two sets), the resistance (eg, holding dumbbells while squatting), or the intensity of the exercise (eg, squat lower or faster) [41].
Implementing Exercise Programs in LTC
Implementation of exercise programs into LTC homes should consider the dose of exercise (eg, time and frequency of sessions, duration of program), if they are delivered in a group or individual setting, and who is delivering them. First, trials included in this paper suggest that strength and balance exercises to prevent falls were delivered 2 to 3 times per week, for 20 to 75 minutes per session, over 4 to 52 weeks. Second, previous work has established that exercise programs delivered on 2 to 3 days per week over a period of more than 6 months are most effective at reducing falls in LTC [20]. Finally, a recent task force report from an international group of clinician researchers in LTC recommends twice weekly exercise sessions lasting 35 to 45 minutes each [40]. Therefore, strength and balance exercises to prevent falls in LTC should be delivered at least twice per week, for at least 20 minutes, for greater than 6 weeks’ duration.
Whether exercise should be performed in a group or individual setting remains unclear. Two of the 6 positive trials in this paper were completed individually, while 3 were in a group. The aforementioned task force also recommended that every resident who does not have contraindications to exercise must have an individualized exercise program as part of their health care plan [40]. However, whether the exercise program is provided on an individual basis or in a group setting was not delineated. Indeed, there are currently no recommendations concerning prioritizing group or individual exercise programs. Therefore, exercise programs being implemented into LTC homes should consider the residents’ preferences, the social benefits of group exercise, and the feasibility of individualizing exercises for the complex needs of residents in LTC in large group settings.
Finally, which professionals should deliver the exercise program is also uncertain. Only 3 of the positive trials in this paper described the professional delivering the intervention, with one being research staff, one geriatric nurses, and one exercise assistants supported by a physiotherapist. We suggest that professionals delivering an exercise program should be trained in exercise planning, delivery, and progression, be familiar with the principles of balance and strength training, and have training in working with older adults in LTC.
Modifications for Physical Impairments
Residents in LTC often have complex health needs, with multiple comorbidities (eg, stroke, Parkinson’s disease, multiple sclerosis) [21]. Modifications of strength and balance exercises may be required to accommodate for physical impairments (eg, hemiplegia, drop foot, freezing gait). For example, if a resident has hemiplegia and cannot fully activate the muscles of one arm, one can do resistance exercises with a dumbbell on the functioning side and active assisted range of motion (ie, the exercise provider assists the resident to achieve full range of motion against gravity) on the hemiparetic side. A resident with Parkinson’s disease who has freezing gait may need visual or rhythmical verbal cues to be able to accomplish standing balance tasks such as altered walking patterns (eg, wide or narrow stepping) [42].
Modifications for Cognitive Impairments
More than 80% of residents in LTC have some degree of cognitive impairment [21]. Cognitive impairment may be the result of stroke, depression, traumatic injuries, medications, and degenerative diseases such as Parkinson’s and Alzheimer’s disease [43]. A common misconception is that residents with cognitive impairment cannot benefit from exercise because they cannot learn new skills and have difficulty following directions. On the contrary, evidence suggests that exercise can improve functional mobility for residents with cognitive impairment [44,45].
Residents with cognitive impairment may require a different approach to facilitate participation in the desired exercises because of difficulty following multi-step directions, responsive behaviors, or increased distractibility [46]. Clear communication is key in improving the quality of interaction for residents with cognitive impairment. The Alzheimer Society of Ontario suggests 10 strategies for communicating with people with dementia [47], and we have provided suggestions of how to apply these communication strategies to the exercise context in LTC (Table 2). Other suggestions for engaging residents with cognitive impairment in strength and balance training include making the exercises functional (eg, ask them to pick something up of the floor to perform a squat, or reach a point on the wall to do calf raises) and playful (eg, toss a ball back and forth or sing a song about rowing to promote weight shifting) [48].
Standing versus Seated Exercises
Residents may not be able to participate in standing exercises for several reasons: perhaps the resident cannot stand or has severe balance impairments and a high falls risk; the resident may have poor insight into which exercises are safe to perform in standing versus sitting; or there may be limited supervision of a large group exercise class where the risk of falls is a concern. If balance impairments are a concern, where the risk of injury or falling while completing exercises in standing outweighs the benefit of doing the exercises, then seated exercises are appropriate. However, when residents are able, we recommend encouraging some or all exercises in standing, to facilitate carry over of strength gains into functional tasks such as being able to rise from a chair and walking. A recent study, comparing standing versus seated exercises for community dwelling older adults, saw greater functional gains for those who completed the standing exercises [49]. Therefore, strength and balance exercises should be performed in standing, where appropriate.
Resident-Centered Exercise for Falls Prevention
Putting the resident at the center of falls prevention is important. Previous work has found that older adults have expressed a strong preference for care that transcends traditional biomedical care and that values efficiency, consistency, and hierarchical decision making [50]. On the contrary, resident-centered care emphasizes well-being and quality of life as defined by the resident, values giving residents greater control over the nature of services they receive, and respects their rights to be involved in every day decision making [51,52]. Indeed, residents may choose to engage in risky behaviors that increase their risk of falls but also increases their quality of life. Previous work has found disconnects between residents’ perceived frailty and the potential ability of protective devices to prevent adverse events, such as falls and fractures [53]. Additionally, one study identified that older residents feared being labelled, so instead hid impairments and chose to refuse assistance and assistive devices [54]. For example, a resident with impaired balance and gait may choose to walk independently when they have been deemed as requiring a gait aid (eg, rollator walker). However, they may value walking without a gait aid and accept the increased risk of falling. Therefore, it is essential to find the delicate balance between respecting a resident’s right to make their own decisions and preventing adverse events, such as falls [52]. An example of this would be respecting a resident’s right to refuse to attend exercise programming even though the team may think they can benefit from strength and balance training.
There is limited evidence around falls prevention and resident-centered care. A recent systematic review [55] revealed that resident-centered care may increase falls rates [56,57]. However, the authors of the review attributed the increase in falls to differences in frailty between the control and intervention group [56], and to environmental factors (eg, slippery flooring material, lack of handrails) [57]. Additionally, these trials did not include an exercise program as part of the resident-centered care program. On the other hand, resident-centered care has been associated with reduction of boredom, helplessness, and depression [58,59]. Most studies included in the review were quasi-experimental, which significantly limits the evidence quality [55]. At this point in time, the evidence suggests that resident-centered care is important for mood and quality of life but may have a negative or no effect on reducing falls.
Multifactorial Falls Prevention Programs
While there are mixed results about the effect of exercise as a single intervention for reducing falls for residents in LTC, the literature clearly supports exercise as part of a multifactorial falls prevention program [17,20,60–62]. A 2015 umbrella review [62] of meta-analyses of randomized controlled trials of falls prevention interventions in LTC concluded that multifactorial interventions were the most effective at preventing falls in LTC. Additionally, recently developed recommendations for fracture prevention in LTC [61] suggest that balance, strength, and functional training should be included for residents who are not at high risk of fracture, while for those at high risk, exercise should be provided as part of a multifactorial falls prevention intervention. Clinicians must therefore incorporate elements aside from exercise into their falls prevention strategies. Interventions that have shown positive effects on reducing falls when delivered as part of multifactorial interventions include: staff and resident education [31,35,37], environmental modifications [31,35], supply/repair/provision of assistive devices [30], falls problem-solving conferences [30], urinary incontinence management [29], medication review [30], optician review [31], and cognitive behavioral therapy [32].
Conclusion and Suggestions for Clinical Practice
We suggest incorporating strength and balance exercises as part of a multifactorial falls prevention program for residents in LTC. Balance exercises should be challenging and dynamic (eg, weight shifting). Strength exercises should be of a moderate to high intensity (eg, can complete one to sets of 6 to 8 repetitions) and need to be progressed as the residents’ abilities improve. Residents should participate in strength and balance training on 2 to 3 days per week, for 30- to 45-minute sessions, for at least 6 months. Exercises in standing should be prioritized where appropriate. Exercise could be delivered in a group or individual format, but should consider the residents’ preferences, the social benefits of group exercise, and the feasibility of individualizing exercises for the complex needs of residents in LTC in large group settings. Professionals delivering an exercise program should be trained in exercise planning, delivery, and progression, be familiar with the principles of balance and strength training, and have training in working with older adults in LTC. Exercise programs in LTC should be resident-centered and consider residents’ potential physical and cognitive impairments.
Funding/support: Dr. Giangregorio was supported by grants from the Canadian Frailty Network and Canadian Institutes of Health Research.
1. Harris IA, Yong S, McEvoy L, Thorn L. A prospective study of the effect of nursing home residency on mortality following hip fracture. ANZ J Surg 2010;80:447–50.
2. Ooms ME, Vlasman P, Lips P, et al. The incidence of hip fractures in independent and institutionalized elderly people. Osteoporos Int 1994;4:6–10.
3. Ayoung-Chee P, McIntyre L, Ebel BE, et al. Long-term outcomes of ground-level falls in the elderly. J Trauma Acute Care Surg 2014;76:498–503.
4. Heinrich S, Rapp K, Rissmann U, et al. Cost of falls in old age: a systematic review. Osteoporos Int 2010;21: 891–902.
5. Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med 1994;121:442–51.
6. Hartholt KA, van Beeck EF, Polinder S, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long-term reduced quality of life. J Trauma
2011;71:748–53.
7. Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet 2013;381:
47–54.
8. Rapp K, Becker C, Cameron ID, et al. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of bavarian nursing homes. J Am Med Dir Assoc 2012;13:187.
9. Büchele G, Becker C, Cameron ID, et al. Predictors of serious consequences of falls in residential aged care: analysis of more than 70,000 falls from residents of Bavarian nursing homes. J Am Med Dir Assoc 2014;15:559–63.
10. McArthur C, Gonzalez DA, Roy E, Giangregorio L. What are the circumstances of falls and fractures in long-term care? Can J Aging / La Rev Can du Vieil 2016;35:491–8.
11. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.
12. Ikezoe T, Asakawa Y, Shima H, et al. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr 2013;57:221–5.
13. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015;82:418–23.
14. Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc 2014;15:267–72.
15. Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–6.
16. Yalcin A, Aras S, Atmis V, et al. Sarcopenia prevalence and factors associated with sarcopenia in older people living in a nursing home in Ankara Turkey. Geriatr Gerontol Int
2016;16:903–10.
17. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med October 2016.
18. Liu C, Latham NK. Progressive resistance strength training for improving physical function in older adults. In: Liu C, ed. Cochrane Database Syst Rev;2009:CD002759.
19. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev;2012:CD005465.
20. Silva RB, Eslick GD, Duque G. Exercise for falls and fracture prevention in long term care facilities: a systematic review and meta-analysis. J Am Med Dir Assoc 2013;14:685–9.
21. Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Can J Aging 2011;30: 371–90.
22. Benjamin K, Edwards N, Guitard P, et al. Factors that influence physical activity in long-term care: Perspectives of residents, staff, and significant others. Can J Aging 2011;30:247–58.
23. Benjamin K, Edwards N, Ploeg J, Legault F. Barriers to physical activity and restorative care for residents in long-term care: A review of the literature. J Aging Phys Act 2014;22:154–65.
24. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed. American College of Sports Medicine; 2013.
25. Prevention of Falls Network Europe. Prevention of Falls Network Europe. Accessed 27 Nov 2017 at www.profane.eu.org/.
26. Sihvonen SE, Sipilä S, Era PA. Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology 2004;50:87–95.
27. Sakamoto K, Nakamura T, Hagino H, et al. Effects of unipedal standing balance exercise on the prevention of falls and hip fracture among clinically defined high-risk elderly individuals: a randomized controlled trial. J Orthop Sci 2006;11:467–72.
28. Shimada H, Obuchi S, Furuna T, Suzuki T. New intervention program for preventing falls among frail elderly people: the effects of perturbed walking exercise using a bilateral separated treadmill. Am J Phys Med Rehabil 2004;83:493–9.
29. Schnelle JF, Kapur K, Alessi C, et al. Does an exercise and incontinence intervention save healthcare costs in a nursing home population? J Am Geriatr Soc 2003;51:161–8.
30. Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities: A cluster randomized trial. Ann Intern Med 2002;136:733–41.
31. Dyer CAE. Falls prevention in residential care homes: a randomised controlled trial. Age Ageing 2004;33:596–602.
32. Huang T-T, Chung M-L, Chen F-R, Chin Y-F, Wang B-H. Evaluation of a combined cognitive-behavioural and exercise intervention to manage fear of falling among elderly residents in nursing homes. Aging Ment Health 2016;20:2–12.
33. Sihvonen S, Sipilä S, Taskinen S, Era P. Fall incidence in frail older women after individualized visual feedback-based balance training. Gerontology 2004;50:411–6.
34. Lord SR, Castell S, Corcoran J, et al. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: a randomized, controlled trial. J Am Geriatr Soc 2003;51:1685–92.
35. Becker C, Kron M, Lindemann U, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. J Am Geriatr Soc 2003;51:306–13.
36. Becker C, Kron M, Lindemann U, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. J Am Geriatr Soc 2003;51:306–13.
37. Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities. A cluster randomized trial. Ann Intern Med 2002;136:733–41.
38. Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2004;52: 1121–9.
39. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sport Exerc 2009;41:1510–30.
40. de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc 2016;17:381–92.
41. American College of Sports Medicine. Progression models in resistance training for healthy adults. Med Sci Sport Exerc 2009;41:687–708.
42. Fietzek UM, Schroeteler FE, Ziegler K, et al. Randomized cross-over trial to investigate the efficacy of a two-week physiotherapy programme with repetitive exercises of cueing to reduce the severity of freezing of gait in patients with Parkinson’s disease. Clin Rehabil 2014;28:902–11.
43. Patterson C, Feightner J, Garcia A, MacKnight C. General risk factors for dementia: A systematic evidence review. Alzheimer Dement 2007;3:341–7.
44. Roach KE, Tappen RM, Kirk-Sanchez N, et al. A randomized controlled trial of an activity specific exercise program for individuals with alzheimer disease in long-term care settings. J Geriatr Phys Ther 2011;34:50–6.
45. Christofoletti G, Oliani MM, Gobbi S, et al. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil 2008;22:618–26.
46. van Alphen HJM, Hortobágyi T, van Heuvelen MJG. Barriers, motivators, and facilitators of physical activity in dementia patients: A systematic review. Arch Gerontol Geriatr 2016;66:109–18.
47. Alzheimer Society of Ontario. Rethink Dementia. Accessed 18 Sep 2017 at http://rethinkdementia.ca/.
48. Roach KE, Tappen RM, Kirk-Sanchez N, et al. A randomized controlled trial of an activity specific exercise program for individuals with Alzheimer disease in long-term care settings. J Geriatr Phys Ther 2011;34:50–6.
49. Brach JS, Perera S, Gilmore S, et al. Effectiveness of a timing and coordination group exercise program to improve mobility in community-dwelling older adults. JAMA Intern Med August 2017.
50. Rosher RB, Robinson S. Impact of the Eden alternative on family satisfaction. J Am Med Dir Assoc 2005;6:189–93.
51. Crandall LG, White DL, Schuldheis S, Talerico KA. Initiating person-centered care practices in long-term care facilities. J Gerontol Nurs 2007;33:47–56.
52. Sims-Gould J, McKay HA, Feldman F, et al. Autonomy, choice, patient-centered care, and hip protectors: the experience of residents and staff in long-term care. J Appl Gerontol 2014;33:690–709.
53. Robinovitch SN, Cronin T. Perception of postural limits in elderly nursing home and day care participants. J Gerontol A Biol Sci Med Sci 1999;54:B124-30.
54. Perkins MM, Ball MM, Whittington FJ, Hollingsworth C. Relational autonomy in assisted living: a focus on diverse care settings for older adults. J Aging Stud 2012;26:214–25.
55. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging 2013;8:1–10.
56. Coleman MT, Looney S, O’Brien J, et al. The Eden Alternative: findings after 1 year of implementation. J Gerontol A Biol Sci Med Sci 2002;57:M422–7.
57. Chenoweth L, King MT, Jeon Y-H, et al. Caring for Aged Dementia Care Resident Study (CADRES) of personcentred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8: 317–25.
58. Bergman-Evans B. Beyond the basics. Effects of the Eden Alternative model on quality of life issues. J Gerontol Nurs 2004;30:27–34.
59. Robinson SB, Rosher RB. Tangling with the barriers to culture change: creating a resident-centered nursing home environment. J Gerontol Nurs 2006;32:19–25.
60. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 2012;12.
61. Papaioannou A, Santesso N, Morin SN, et al. Recommendations for preventing fracture in long-term care. Can Med Assoc J 2015;187:1135–44.
62. Stubbs B, Denkinger MD, Brefka S, Dallmeier D. What works to prevent falls in older adults dwelling in long term care facilities and hospitals? An umbrella review of meta-analyses of randomised controlled trials. Maturitas 2015;81:335–42.
1. Harris IA, Yong S, McEvoy L, Thorn L. A prospective study of the effect of nursing home residency on mortality following hip fracture. ANZ J Surg 2010;80:447–50.
2. Ooms ME, Vlasman P, Lips P, et al. The incidence of hip fractures in independent and institutionalized elderly people. Osteoporos Int 1994;4:6–10.
3. Ayoung-Chee P, McIntyre L, Ebel BE, et al. Long-term outcomes of ground-level falls in the elderly. J Trauma Acute Care Surg 2014;76:498–503.
4. Heinrich S, Rapp K, Rissmann U, et al. Cost of falls in old age: a systematic review. Osteoporos Int 2010;21: 891–902.
5. Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med 1994;121:442–51.
6. Hartholt KA, van Beeck EF, Polinder S, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long-term reduced quality of life. J Trauma
2011;71:748–53.
7. Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet 2013;381:
47–54.
8. Rapp K, Becker C, Cameron ID, et al. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of bavarian nursing homes. J Am Med Dir Assoc 2012;13:187.
9. Büchele G, Becker C, Cameron ID, et al. Predictors of serious consequences of falls in residential aged care: analysis of more than 70,000 falls from residents of Bavarian nursing homes. J Am Med Dir Assoc 2014;15:559–63.
10. McArthur C, Gonzalez DA, Roy E, Giangregorio L. What are the circumstances of falls and fractures in long-term care? Can J Aging / La Rev Can du Vieil 2016;35:491–8.
11. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.
12. Ikezoe T, Asakawa Y, Shima H, et al. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr 2013;57:221–5.
13. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015;82:418–23.
14. Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc 2014;15:267–72.
15. Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–6.
16. Yalcin A, Aras S, Atmis V, et al. Sarcopenia prevalence and factors associated with sarcopenia in older people living in a nursing home in Ankara Turkey. Geriatr Gerontol Int
2016;16:903–10.
17. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med October 2016.
18. Liu C, Latham NK. Progressive resistance strength training for improving physical function in older adults. In: Liu C, ed. Cochrane Database Syst Rev;2009:CD002759.
19. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev;2012:CD005465.
20. Silva RB, Eslick GD, Duque G. Exercise for falls and fracture prevention in long term care facilities: a systematic review and meta-analysis. J Am Med Dir Assoc 2013;14:685–9.
21. Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Can J Aging 2011;30: 371–90.
22. Benjamin K, Edwards N, Guitard P, et al. Factors that influence physical activity in long-term care: Perspectives of residents, staff, and significant others. Can J Aging 2011;30:247–58.
23. Benjamin K, Edwards N, Ploeg J, Legault F. Barriers to physical activity and restorative care for residents in long-term care: A review of the literature. J Aging Phys Act 2014;22:154–65.
24. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed. American College of Sports Medicine; 2013.
25. Prevention of Falls Network Europe. Prevention of Falls Network Europe. Accessed 27 Nov 2017 at www.profane.eu.org/.
26. Sihvonen SE, Sipilä S, Era PA. Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology 2004;50:87–95.
27. Sakamoto K, Nakamura T, Hagino H, et al. Effects of unipedal standing balance exercise on the prevention of falls and hip fracture among clinically defined high-risk elderly individuals: a randomized controlled trial. J Orthop Sci 2006;11:467–72.
28. Shimada H, Obuchi S, Furuna T, Suzuki T. New intervention program for preventing falls among frail elderly people: the effects of perturbed walking exercise using a bilateral separated treadmill. Am J Phys Med Rehabil 2004;83:493–9.
29. Schnelle JF, Kapur K, Alessi C, et al. Does an exercise and incontinence intervention save healthcare costs in a nursing home population? J Am Geriatr Soc 2003;51:161–8.
30. Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities: A cluster randomized trial. Ann Intern Med 2002;136:733–41.
31. Dyer CAE. Falls prevention in residential care homes: a randomised controlled trial. Age Ageing 2004;33:596–602.
32. Huang T-T, Chung M-L, Chen F-R, Chin Y-F, Wang B-H. Evaluation of a combined cognitive-behavioural and exercise intervention to manage fear of falling among elderly residents in nursing homes. Aging Ment Health 2016;20:2–12.
33. Sihvonen S, Sipilä S, Taskinen S, Era P. Fall incidence in frail older women after individualized visual feedback-based balance training. Gerontology 2004;50:411–6.
34. Lord SR, Castell S, Corcoran J, et al. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: a randomized, controlled trial. J Am Geriatr Soc 2003;51:1685–92.
35. Becker C, Kron M, Lindemann U, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. J Am Geriatr Soc 2003;51:306–13.
36. Becker C, Kron M, Lindemann U, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. J Am Geriatr Soc 2003;51:306–13.
37. Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities. A cluster randomized trial. Ann Intern Med 2002;136:733–41.
38. Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2004;52: 1121–9.
39. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sport Exerc 2009;41:1510–30.
40. de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc 2016;17:381–92.
41. American College of Sports Medicine. Progression models in resistance training for healthy adults. Med Sci Sport Exerc 2009;41:687–708.
42. Fietzek UM, Schroeteler FE, Ziegler K, et al. Randomized cross-over trial to investigate the efficacy of a two-week physiotherapy programme with repetitive exercises of cueing to reduce the severity of freezing of gait in patients with Parkinson’s disease. Clin Rehabil 2014;28:902–11.
43. Patterson C, Feightner J, Garcia A, MacKnight C. General risk factors for dementia: A systematic evidence review. Alzheimer Dement 2007;3:341–7.
44. Roach KE, Tappen RM, Kirk-Sanchez N, et al. A randomized controlled trial of an activity specific exercise program for individuals with alzheimer disease in long-term care settings. J Geriatr Phys Ther 2011;34:50–6.
45. Christofoletti G, Oliani MM, Gobbi S, et al. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil 2008;22:618–26.
46. van Alphen HJM, Hortobágyi T, van Heuvelen MJG. Barriers, motivators, and facilitators of physical activity in dementia patients: A systematic review. Arch Gerontol Geriatr 2016;66:109–18.
47. Alzheimer Society of Ontario. Rethink Dementia. Accessed 18 Sep 2017 at http://rethinkdementia.ca/.
48. Roach KE, Tappen RM, Kirk-Sanchez N, et al. A randomized controlled trial of an activity specific exercise program for individuals with Alzheimer disease in long-term care settings. J Geriatr Phys Ther 2011;34:50–6.
49. Brach JS, Perera S, Gilmore S, et al. Effectiveness of a timing and coordination group exercise program to improve mobility in community-dwelling older adults. JAMA Intern Med August 2017.
50. Rosher RB, Robinson S. Impact of the Eden alternative on family satisfaction. J Am Med Dir Assoc 2005;6:189–93.
51. Crandall LG, White DL, Schuldheis S, Talerico KA. Initiating person-centered care practices in long-term care facilities. J Gerontol Nurs 2007;33:47–56.
52. Sims-Gould J, McKay HA, Feldman F, et al. Autonomy, choice, patient-centered care, and hip protectors: the experience of residents and staff in long-term care. J Appl Gerontol 2014;33:690–709.
53. Robinovitch SN, Cronin T. Perception of postural limits in elderly nursing home and day care participants. J Gerontol A Biol Sci Med Sci 1999;54:B124-30.
54. Perkins MM, Ball MM, Whittington FJ, Hollingsworth C. Relational autonomy in assisted living: a focus on diverse care settings for older adults. J Aging Stud 2012;26:214–25.
55. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging 2013;8:1–10.
56. Coleman MT, Looney S, O’Brien J, et al. The Eden Alternative: findings after 1 year of implementation. J Gerontol A Biol Sci Med Sci 2002;57:M422–7.
57. Chenoweth L, King MT, Jeon Y-H, et al. Caring for Aged Dementia Care Resident Study (CADRES) of personcentred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8: 317–25.
58. Bergman-Evans B. Beyond the basics. Effects of the Eden Alternative model on quality of life issues. J Gerontol Nurs 2004;30:27–34.
59. Robinson SB, Rosher RB. Tangling with the barriers to culture change: creating a resident-centered nursing home environment. J Gerontol Nurs 2006;32:19–25.
60. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 2012;12.
61. Papaioannou A, Santesso N, Morin SN, et al. Recommendations for preventing fracture in long-term care. Can Med Assoc J 2015;187:1135–44.
62. Stubbs B, Denkinger MD, Brefka S, Dallmeier D. What works to prevent falls in older adults dwelling in long term care facilities and hospitals? An umbrella review of meta-analyses of randomised controlled trials. Maturitas 2015;81:335–42.
Screening for Metabolic Syndrome in People with Severe Mental Illness
From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].
Metabolic Monitoring
Given the increased risk of metabolic syndrome among people with SMI, and the association of metabolic syndrome with increased morbidity and all-cause mortality, there has been a growing awareness of the importance of screening for metabolic syndrome among people with SMI. Metabolic monitoring involves routine screening for metabolic parameters and assessment of metabolic risk factors among people with SMI who are prescribed antipsychotic medications. Various practice guidelines have been developed in the United States and internationally to assess for metabolic risk factors in people prescribed antipsychotic medications [26]. Current metabolic monitoring guidelines in the United States stem from 2004 consensus recommendations of the American Diabetes Association and American Psychiatric Association along with the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity for metabolic monitoring among people prescribed SGAs [23]. These recommendations include a time line for routine monitoring of weight/body mass index, waist circumference, blood pressure, fasting blood glucose or hemoglobin A1c, and fasting lipids (Table 2). Guidelines recommend screening at baseline, more frequently within the first 3 months, and then annually [23].
Though guidelines recommend measurement of waist circumference as a marker for metabolic health, body mass index is often used alone as a measure of obesity [27,28]. This may be due to the relative ease of obtaining weight over waist circumference. For example, weight is more likely to be part of clinic workflows and many providers may not be accustomed to measuring waist circumference. However, waist circumference does provide additional information regarding metabolic health [29], as central adiposity is a marker of cardiometabolic risk and related to insulin resistance [21]. Further modifications of the guidelines have included ethnicity-specific waist measurements [30].
There is evidence that non-fasting lipids may be substituted for fasting lipid panels, particularly for patients who may have difficulty adhering to fasting due to cognitive difficulties. Vanderlip and colleagues argue that fasting serum cholesterol panels are not necessary for screening for dyslipidemia given that non-HDL cholesterol is calculated based on total cholesterol and HDL, which do not substantially differ between fasting and non-fasting values [31]. Hemoglobin A1c is recommended as a screening test for blood glucose abnormalities given that it does not require a fasting state and can therefore be more easily obtained for many patients. The choice to obtain a fasting blood glucose versus hemoglobin A1c may depend on multiple factors, including that a person can adhere to fasting and the cost of the laboratory test.
Routine monitoring of metabolic parameters is an integral step in targeting interventions to treat metabolic syndrome. These interventions include lifestyle modifications and evidence-based treatment guidelines for management of associated dyslipidemia, hypertension, and type 2 diabetes mellitus.
Current Metabolic Screening Practices
Despite the presence of defined guidelines, estimates show persistently low rates of metabolic monitoring among adults prescribed SGAs [32]. One study of 3 state Medicaid programs showed little to no improvement in screening rates for glucose and lipids post dissemination of the 2004 APA/ADA guidelines [33]. They noted a nonsignificant change in rates of glucose testing from 27% to 30% and small change in lipid testing from 10% to 11% among patients prescribed SGAs between 2002–2005 [33]. Examining screening rates among Medicaid recipients in Missouri between 2010–2012, Morrato and colleagues found glucose testing rates of 80% with lipid testing remaining at 41% [34]. A retrospective study of adult Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 showed rates of screening for lipids and glucose to increase over time; glucose monitoring increased from 56.6% to 72.6% and lipids from 38.3% to 41.2% [35]. A review by Mangurian and colleagues suggested rates of glucose (fasting blood glucose or hemoglobin A1c) and lipid screening as low as 30% among people prescribed antipsychotic medications [14]. Furthermore, they underscore the impact of low screening rates, stating that if 20% of adults with SMI have diabetes and 70% remain unscreened, then approximately 2 million adults with SMI and diabetes in the United States would not be identified within our current system [14].
Higher rates of screening have been shown for Medicaid populations than commercially insured populations [36]. Haupt et al compared lipid and glucose testing pre- and post- ADA/APA guideline implementation among commercially insured patients. They found an increase from 8.4% to 10.5% post guideline implementation for baseline lipid testing and from 6.8% to 9.0% for lipid testing at 12 weeks post-antipsychotic initiation [36]. Baseline glucose testing increased from 17.3% to 21.8% and from 14.1% to 17.9 % at 12-week post antipsychotic initiation. In alignment with findings from other studies, testing rates were particularly low for children [36].
Low screening rates have been found among children and adolescents prescribed SGAs [37] despite evidence that youth may be at risk of developing more significant metabolic sequelae from SGAs [19]. Edelsohn and colleagues found an increase from 30% to 50% for glucose screening and from 19% to 28% for lipid screening among youth Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 [35]. Connolly and colleagues reported on metabolic screening rates for children and adolescents prescribed SGAs over the 8 years following announcement of the 2004 ADA/APA guidelines. Using insurance claims data, they found screening rates for fasting blood glucose and hemoglobin A1c temporarily increased following guideline dissemination, then dropped during the period 2004–2008, and again increased slightly [38].
Barriers to Screening
Barriers to screening exist at the level of the individual patient and provider as well as at the clinic and larger systems levels. Lack of provider awareness of evidence-based guidelines for metabolic monitoring despite the presence of the 2004 ADA/APA guidelines has been cited by researchers as an impediment to screening. In a survey of primary care clinicians in San Francisco, Mangurian et al found that 40% of primary care providers did not know about the ADA/APA consensus guidelines for metabolic monitoring. The same survey of primary care providers identified additional impediments to screening, including obstacles to collaboration with psychiatric providers and to scheduling patients for psychiatric follow-up [39]. Another clinician survey conducted by Parameswaran et al found that psychiatrists viewed psychiatric illness severity, lack of staff time, and lack of clinician time as significant barriers to metabolic screening. In addition, clinicians identified factors related to the complexity of coordinating care across systems as obstacles; these included barriers to coordinating follow-up with medical providers, long wait times for patients to see medical providers, and difficulty collaborating with medical providers [40].
Other systems-level barriers include lack of a population-based approach to screening (eg, registries) and lack of electronic record integration, which impedes the ability of primary care and psychiatry providers to share information related to the ordering of metabolic screening tests and prescribing of medications [41]. Mangurian calls for integration of electronic medical record systems between primary care and psychiatry, a population-based approach to metabolic monitoring utilizing registries and other elements of collaborative care models, and primary care consultation to aid in the treatment of metabolic abnormalities [41]. Amiel et al point to systems-level factors “including but not limited to … poor access to general medical services, inadequate medical record-keeping infrastructure, lack of in-system compliance incentives and lack of centralized oversight” [26].
Based on their experience implementing a computer-based intervention for metabolic monitoring, Lai et al propose that the following factors may influence providers’ engagement in metabolic monitoring: lack of apparent symptoms to suggest metabolic syndrome, patients’ lack of engagement in care, and poor access to care. They identify additional factors at the clinician level to include under-recognition of the need for metabolic monitoring, lack of familiarity with screening guidelines, lack of agreement with guidelines, and the potential for individual clinicians to forget to order tests [42]. At the systems-level, they identify the absence of ongoing training as a potential reason why sustained testing was not observed in their intervention [42].
In a 2011 survey of providers prescribing antipsychotic medication to Medicaid beneficiaries in Missouri, Morrato and colleagues found that factors limiting frequency of health care utilization were closely linked to lack of metabolic testing. They also noted disparities in screening guidelines may lead to lack of routine metabolic monitoring; providers may screen based on prescribed medication, diagnosis, or other risk factor based stratification depending on the guidelines followed [34].
Current Unmet Needs
Vulnerable Populations
Though rates of metabolic screening remain low for all groups prescribed antipsychotic medications, studies have consistently shown low rates of screening among children and adolescents [35,36]. Edelsohn and colleagues hypothesize that the cause of these low rates is multifactorial, including that guardians may be reluctant to have young people undergo blood draws [35]. Morrato and colleagues suggest that policymakers should focus initiatives on younger, healthier adults, who they found to have lower rates of screening [37].
Racial and ethnic minorities with SMI constitute another particularly vulnerable population, with some studies showing an increased risk of metabolic sequelae and lower likelihood of treatment for diabetes and other metabolic derangements among African American and Latino populations with SMI [14,43,44].
Integration of Care
Lack of widespread integration of care between mental health and primary care remains another unmet need [41]. Hasnain and colleagues recommend improved communication between mental health and primary care clinicians to coordinate care to improve rates of monitoring, facilitate early follow-up of metabolic abnormalities, and avoid duplication of monitoring efforts [45]. Morrato and colleagues recommend that efforts to increase rates of metabolic monitoring be targeted not only to providers practicing in community mental health centers, but also to other practice settings including primary care. They found that for 75% of people prescribed antipsychotic medications, the prescriptions were started by prescribing providers who practiced outside of a community mental health center [34] and recommend that educational initiatives and performance improvement interventions broaden to include primary care and other care settings [34].
Potential Interventions for Improvement
Early interventions to improve metabolic screening rates have included educational initiatives to teach providers about consensus guidelines. However, initiatives to educate clinicians on metabolic monitoring have shown to be inadequate to significantly improve rates of screening [33]. Therefore, subsequent initiatives have sought to influence screening rates by targeting behavior of individual clinicians with point-of-care tools, electronic reminders, or through systems-level reorganization towards population-based care [27,42,46].
A variety of clinical interventions focus on technologies that remind clinicians to order metabolic monitoring tests according to screening guidelines. One public mental health service in Queensland, Australia, created a standardized metabolic monitoring form to be uploaded to the electronic medical record. In their implementation study examining the efficacy of the metabolic monitoring form, they found that only 36% of the forms contained data. When data were recorded, there were significantly higher rates of documentation of measurements (weight, body mass index, blood pressure) rather than laboratory tests (including lipids and fasting blood glucose) [27].
Computerized reminder systems for metabolic monitoring have been studied in both outpatient and inpatient settings. Lai and colleagues studied the impact of a computerized reminder system on lab monitoring for metabolic parameters among outpatients with schizophrenia prescribed SGAs [42]. This intervention also included an educational component with discussion of metabolic monitoring for people prescribed SGAs at meetings with attending psychiatrists. Computer reminders were displayed when a provider failed to order fasting plasma glucose or lipids (cholesterol, triglyceride) for patients prescribed clozapine, olanzapine, quetiapine, or risperidone. The study found a statistically significant improvement in laboratory metabolic screening for patients prescribed SGAs after implementation, with the greatest impact 6-months post-intervention, though with subsequent decline in screening rates [42].
Psychiatric inpatient hospitalizations provide an opportunity to obtain testing at the time of treatment initiation and also for ongoing monitoring in a location where fasting laboratory tests may be more easily obtained given onsite phlebotomy. One intervention targeting psychiatric inpatients utilized a computerized physician order entry system with the goal to improve metabolic screening among patients prescribed SGAs. Set in a large academic medical setting, the study found inpatient metabolic monitoring rates did not change significantly after implementation of these pop-up computer alerts, comparing rates immediately and 4 years after implementation [46].
There has been increasing focus on integrating mental health and medical care in an effort to improve the health of people with mental illness [47]. Mangurian and colleagues found that the likelihood of diabetes mellitus screening doubled for people with severe mental illness who were seen for at least one primary care visit in addition to mental health treatment [48]. Haupt similarly found higher rates of metabolic screening among patients who had greater than one primary care visit [36]. Models of integration include both integration of medical services into mental health treatment as well as incorporation of mental health services into primary care. For people with SMI, integration efforts have largely focused on integrating primary care services into community mental health settings [49]. The Substance Abuse and Mental Health Service Administration’s (SAMHSA) Primary and Behavioral Health Care Integration (PBHCI) grants program and the Affordable Care Act’s Health Home Initiative are examples of federal incentive programs for improved integration between behavioral health and primary care [49]. In their evaluation of the PBHCI grant program, Scharf and colleagues presented findings that patients at 3 matched clinics with PCBHI grants showed improvement in some lipids, diastolic blood pressure, and fasting blood glucose, though not smoking or body mass index [50].
Conclusion
Several risk factors contribute to an increase in cardiometabolic risk for people with severe mental illness, including poor nutrition, sedentary lifestyle, social determinants of health, and prescribed antipsychotic medications. Metabolic monitoring aims to address these health disparities by screening for metabolic parameters and identifying abnormalities in order to target appropriate health interventions. Screening rates for metabolic parameters remain low for children, adolescents, and adults prescribed second-generation antipsychotics despite published guidelines and clinical interventions to improve screening. More system-wide interventions to improve collaboration between mental health and primary care are needed to enhance screening and prevent cardiovascular disease risk in this vulnerable population.
Corresponding author: Carrie Cunningham, MD, MPH, Zuckerberg San Francisco General Hospital, 1001 Potrero Ave, Suite 7M, San Francisco, CA 94110, carrie.cunningham@ucsf.edu.
Funding/support: Dr. Cunningham was supported by the UCSF-Zuckerberg San Francisco General Public Psychiatry Fellowship. Mr. Riano was supported by the NIH Center Grant from the National Institute of Diabetes and Digestive and Kidney Diseases for The Health Delivery Systems-Center for Diabetes Translational Research (CDTR) (P30DK092924) and by the UCSF-San Francisco General Hospital Public Psychiatry Fellowship. Dr. Mangurian received support from a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03 DK101857), as well as NIH Career Development Award (K23MH093689).
1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014.
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
3. American Heart Association. What is metabolic syndrome? 2015.
4. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339–47.
5. Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Rev Bras Psiquiatria 2013;35:88–93.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32.
7. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015:1–10.
8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–31.
9. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41.
10. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chron Dis 2006;3:A42.
11. Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’–a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17:594.
12. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–96.
13. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naïve and treated patients. J Pharmacol Pharmacother 2013;4:176–86.
14. Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med 2016:1–9.
15. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005;19(Suppl 1):1–93.
16. Baptista T, De Mendoza S, Beaulieu S, et al. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord 2004;2:290–307.
17. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52–77.
18. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247–59.
19. Kryzhanovskaya LA, Xu W, Millen BA, et al. Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adol Psychopharmacol 2012;22:157–65.
20. Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35.
21. Zhang Z-J, YAO Z-J, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2004;184:58–62.
22. Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatr Invest 2015;12:242–8.
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 596–601.
24. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33:985–94.
25. Henderson DC. Atypical antipsychotic-induced diabetes mellitus. CNS Drugs 2002;16:77–89.
26. Amiel JM, Mangurian CV, Ganguli R, Newcomer JW. Addressing cardiometabolic risk during treatment with antipsychotic medications. Curr Opin Psychiatry 2008;21:613–8.
27. Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service–a retrospective file audit. BMC Psychiatry 2016;16:109.
28. Rosenbaum S, Nijjar S, Watkins A, et al. Nurse‐assessed metabolic monitoring: A file audit of risk factor prevalence and impact of an intervention to enhance measurement of waist circumference. Int J Ment Health Nurs 2014;23:252–6.
29. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007;15:1061–7.
30. Tan C-E, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182–6.
31. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv 2014;65:573–6.
32. Mitchell A, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012;42:125–47.
33. Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17–24.
34. Morrato EH, Campagna EJ, Brewer SE, et al. Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016;73:721–30.
35. Edelsohn GA, Parthasarathy M, Terhorst L, et al. Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. J Manage Care Specialty Pharm 2015;21:769–77.
36. Haupt DW, Rosenblatt LC, Kim E, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 2009;166:345–53.
37. Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51.
38. Connolly JG, Toomey TJ, Schneeweiss MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003–2011. Psychiatr Serv 2015;66:604–9.
39. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv 2013;64:597–9.
40. Parameswaran SG, Chang C, Swenson AK, et al. Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophren Res 2013;143:395–6.
41. Mangurian C. Patient-centered medical care in community mental health settings. Psychiatr Serv 2017;68:213-.
42. Lai C-L, Chan H-Y, Pan Y-J, Chen C-H. The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015;48:25–9.
43. Lambert BL, Chou C-H, Chang K-Y, et al. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005;14:417–25.
44. Ramaswamy K, Kozma CM, Nasrallah H. Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 2007;30:589–99.
45. Hasnain M, Vieweg WVR, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diab 2009;3:5–15.
46. Lee J, Dalack G, Casher M, et al. Persistence of metabolic monitoring for psychiatry inpatients treated with second‐generation antipsychotics utilizing a computer‐based intervention. J Clin Pharm Therap 2016;41:209–13.
47. Katz MH. Improving the health of persons with serious mental illness. JAMA Intern Med 2015;175:1979–80.
48. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015;175:1977–9.
49. Gerrity M. Integrating primary care into behavioral health settings: What works. New York: Milbank Memorial Fund; 2014.
50. Scharf DM EN, Hackbarth NS, Horvitz-Lennon M, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). 2014.
From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].
Metabolic Monitoring
Given the increased risk of metabolic syndrome among people with SMI, and the association of metabolic syndrome with increased morbidity and all-cause mortality, there has been a growing awareness of the importance of screening for metabolic syndrome among people with SMI. Metabolic monitoring involves routine screening for metabolic parameters and assessment of metabolic risk factors among people with SMI who are prescribed antipsychotic medications. Various practice guidelines have been developed in the United States and internationally to assess for metabolic risk factors in people prescribed antipsychotic medications [26]. Current metabolic monitoring guidelines in the United States stem from 2004 consensus recommendations of the American Diabetes Association and American Psychiatric Association along with the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity for metabolic monitoring among people prescribed SGAs [23]. These recommendations include a time line for routine monitoring of weight/body mass index, waist circumference, blood pressure, fasting blood glucose or hemoglobin A1c, and fasting lipids (Table 2). Guidelines recommend screening at baseline, more frequently within the first 3 months, and then annually [23].
Though guidelines recommend measurement of waist circumference as a marker for metabolic health, body mass index is often used alone as a measure of obesity [27,28]. This may be due to the relative ease of obtaining weight over waist circumference. For example, weight is more likely to be part of clinic workflows and many providers may not be accustomed to measuring waist circumference. However, waist circumference does provide additional information regarding metabolic health [29], as central adiposity is a marker of cardiometabolic risk and related to insulin resistance [21]. Further modifications of the guidelines have included ethnicity-specific waist measurements [30].
There is evidence that non-fasting lipids may be substituted for fasting lipid panels, particularly for patients who may have difficulty adhering to fasting due to cognitive difficulties. Vanderlip and colleagues argue that fasting serum cholesterol panels are not necessary for screening for dyslipidemia given that non-HDL cholesterol is calculated based on total cholesterol and HDL, which do not substantially differ between fasting and non-fasting values [31]. Hemoglobin A1c is recommended as a screening test for blood glucose abnormalities given that it does not require a fasting state and can therefore be more easily obtained for many patients. The choice to obtain a fasting blood glucose versus hemoglobin A1c may depend on multiple factors, including that a person can adhere to fasting and the cost of the laboratory test.
Routine monitoring of metabolic parameters is an integral step in targeting interventions to treat metabolic syndrome. These interventions include lifestyle modifications and evidence-based treatment guidelines for management of associated dyslipidemia, hypertension, and type 2 diabetes mellitus.
Current Metabolic Screening Practices
Despite the presence of defined guidelines, estimates show persistently low rates of metabolic monitoring among adults prescribed SGAs [32]. One study of 3 state Medicaid programs showed little to no improvement in screening rates for glucose and lipids post dissemination of the 2004 APA/ADA guidelines [33]. They noted a nonsignificant change in rates of glucose testing from 27% to 30% and small change in lipid testing from 10% to 11% among patients prescribed SGAs between 2002–2005 [33]. Examining screening rates among Medicaid recipients in Missouri between 2010–2012, Morrato and colleagues found glucose testing rates of 80% with lipid testing remaining at 41% [34]. A retrospective study of adult Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 showed rates of screening for lipids and glucose to increase over time; glucose monitoring increased from 56.6% to 72.6% and lipids from 38.3% to 41.2% [35]. A review by Mangurian and colleagues suggested rates of glucose (fasting blood glucose or hemoglobin A1c) and lipid screening as low as 30% among people prescribed antipsychotic medications [14]. Furthermore, they underscore the impact of low screening rates, stating that if 20% of adults with SMI have diabetes and 70% remain unscreened, then approximately 2 million adults with SMI and diabetes in the United States would not be identified within our current system [14].
Higher rates of screening have been shown for Medicaid populations than commercially insured populations [36]. Haupt et al compared lipid and glucose testing pre- and post- ADA/APA guideline implementation among commercially insured patients. They found an increase from 8.4% to 10.5% post guideline implementation for baseline lipid testing and from 6.8% to 9.0% for lipid testing at 12 weeks post-antipsychotic initiation [36]. Baseline glucose testing increased from 17.3% to 21.8% and from 14.1% to 17.9 % at 12-week post antipsychotic initiation. In alignment with findings from other studies, testing rates were particularly low for children [36].
Low screening rates have been found among children and adolescents prescribed SGAs [37] despite evidence that youth may be at risk of developing more significant metabolic sequelae from SGAs [19]. Edelsohn and colleagues found an increase from 30% to 50% for glucose screening and from 19% to 28% for lipid screening among youth Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 [35]. Connolly and colleagues reported on metabolic screening rates for children and adolescents prescribed SGAs over the 8 years following announcement of the 2004 ADA/APA guidelines. Using insurance claims data, they found screening rates for fasting blood glucose and hemoglobin A1c temporarily increased following guideline dissemination, then dropped during the period 2004–2008, and again increased slightly [38].
Barriers to Screening
Barriers to screening exist at the level of the individual patient and provider as well as at the clinic and larger systems levels. Lack of provider awareness of evidence-based guidelines for metabolic monitoring despite the presence of the 2004 ADA/APA guidelines has been cited by researchers as an impediment to screening. In a survey of primary care clinicians in San Francisco, Mangurian et al found that 40% of primary care providers did not know about the ADA/APA consensus guidelines for metabolic monitoring. The same survey of primary care providers identified additional impediments to screening, including obstacles to collaboration with psychiatric providers and to scheduling patients for psychiatric follow-up [39]. Another clinician survey conducted by Parameswaran et al found that psychiatrists viewed psychiatric illness severity, lack of staff time, and lack of clinician time as significant barriers to metabolic screening. In addition, clinicians identified factors related to the complexity of coordinating care across systems as obstacles; these included barriers to coordinating follow-up with medical providers, long wait times for patients to see medical providers, and difficulty collaborating with medical providers [40].
Other systems-level barriers include lack of a population-based approach to screening (eg, registries) and lack of electronic record integration, which impedes the ability of primary care and psychiatry providers to share information related to the ordering of metabolic screening tests and prescribing of medications [41]. Mangurian calls for integration of electronic medical record systems between primary care and psychiatry, a population-based approach to metabolic monitoring utilizing registries and other elements of collaborative care models, and primary care consultation to aid in the treatment of metabolic abnormalities [41]. Amiel et al point to systems-level factors “including but not limited to … poor access to general medical services, inadequate medical record-keeping infrastructure, lack of in-system compliance incentives and lack of centralized oversight” [26].
Based on their experience implementing a computer-based intervention for metabolic monitoring, Lai et al propose that the following factors may influence providers’ engagement in metabolic monitoring: lack of apparent symptoms to suggest metabolic syndrome, patients’ lack of engagement in care, and poor access to care. They identify additional factors at the clinician level to include under-recognition of the need for metabolic monitoring, lack of familiarity with screening guidelines, lack of agreement with guidelines, and the potential for individual clinicians to forget to order tests [42]. At the systems-level, they identify the absence of ongoing training as a potential reason why sustained testing was not observed in their intervention [42].
In a 2011 survey of providers prescribing antipsychotic medication to Medicaid beneficiaries in Missouri, Morrato and colleagues found that factors limiting frequency of health care utilization were closely linked to lack of metabolic testing. They also noted disparities in screening guidelines may lead to lack of routine metabolic monitoring; providers may screen based on prescribed medication, diagnosis, or other risk factor based stratification depending on the guidelines followed [34].
Current Unmet Needs
Vulnerable Populations
Though rates of metabolic screening remain low for all groups prescribed antipsychotic medications, studies have consistently shown low rates of screening among children and adolescents [35,36]. Edelsohn and colleagues hypothesize that the cause of these low rates is multifactorial, including that guardians may be reluctant to have young people undergo blood draws [35]. Morrato and colleagues suggest that policymakers should focus initiatives on younger, healthier adults, who they found to have lower rates of screening [37].
Racial and ethnic minorities with SMI constitute another particularly vulnerable population, with some studies showing an increased risk of metabolic sequelae and lower likelihood of treatment for diabetes and other metabolic derangements among African American and Latino populations with SMI [14,43,44].
Integration of Care
Lack of widespread integration of care between mental health and primary care remains another unmet need [41]. Hasnain and colleagues recommend improved communication between mental health and primary care clinicians to coordinate care to improve rates of monitoring, facilitate early follow-up of metabolic abnormalities, and avoid duplication of monitoring efforts [45]. Morrato and colleagues recommend that efforts to increase rates of metabolic monitoring be targeted not only to providers practicing in community mental health centers, but also to other practice settings including primary care. They found that for 75% of people prescribed antipsychotic medications, the prescriptions were started by prescribing providers who practiced outside of a community mental health center [34] and recommend that educational initiatives and performance improvement interventions broaden to include primary care and other care settings [34].
Potential Interventions for Improvement
Early interventions to improve metabolic screening rates have included educational initiatives to teach providers about consensus guidelines. However, initiatives to educate clinicians on metabolic monitoring have shown to be inadequate to significantly improve rates of screening [33]. Therefore, subsequent initiatives have sought to influence screening rates by targeting behavior of individual clinicians with point-of-care tools, electronic reminders, or through systems-level reorganization towards population-based care [27,42,46].
A variety of clinical interventions focus on technologies that remind clinicians to order metabolic monitoring tests according to screening guidelines. One public mental health service in Queensland, Australia, created a standardized metabolic monitoring form to be uploaded to the electronic medical record. In their implementation study examining the efficacy of the metabolic monitoring form, they found that only 36% of the forms contained data. When data were recorded, there were significantly higher rates of documentation of measurements (weight, body mass index, blood pressure) rather than laboratory tests (including lipids and fasting blood glucose) [27].
Computerized reminder systems for metabolic monitoring have been studied in both outpatient and inpatient settings. Lai and colleagues studied the impact of a computerized reminder system on lab monitoring for metabolic parameters among outpatients with schizophrenia prescribed SGAs [42]. This intervention also included an educational component with discussion of metabolic monitoring for people prescribed SGAs at meetings with attending psychiatrists. Computer reminders were displayed when a provider failed to order fasting plasma glucose or lipids (cholesterol, triglyceride) for patients prescribed clozapine, olanzapine, quetiapine, or risperidone. The study found a statistically significant improvement in laboratory metabolic screening for patients prescribed SGAs after implementation, with the greatest impact 6-months post-intervention, though with subsequent decline in screening rates [42].
Psychiatric inpatient hospitalizations provide an opportunity to obtain testing at the time of treatment initiation and also for ongoing monitoring in a location where fasting laboratory tests may be more easily obtained given onsite phlebotomy. One intervention targeting psychiatric inpatients utilized a computerized physician order entry system with the goal to improve metabolic screening among patients prescribed SGAs. Set in a large academic medical setting, the study found inpatient metabolic monitoring rates did not change significantly after implementation of these pop-up computer alerts, comparing rates immediately and 4 years after implementation [46].
There has been increasing focus on integrating mental health and medical care in an effort to improve the health of people with mental illness [47]. Mangurian and colleagues found that the likelihood of diabetes mellitus screening doubled for people with severe mental illness who were seen for at least one primary care visit in addition to mental health treatment [48]. Haupt similarly found higher rates of metabolic screening among patients who had greater than one primary care visit [36]. Models of integration include both integration of medical services into mental health treatment as well as incorporation of mental health services into primary care. For people with SMI, integration efforts have largely focused on integrating primary care services into community mental health settings [49]. The Substance Abuse and Mental Health Service Administration’s (SAMHSA) Primary and Behavioral Health Care Integration (PBHCI) grants program and the Affordable Care Act’s Health Home Initiative are examples of federal incentive programs for improved integration between behavioral health and primary care [49]. In their evaluation of the PBHCI grant program, Scharf and colleagues presented findings that patients at 3 matched clinics with PCBHI grants showed improvement in some lipids, diastolic blood pressure, and fasting blood glucose, though not smoking or body mass index [50].
Conclusion
Several risk factors contribute to an increase in cardiometabolic risk for people with severe mental illness, including poor nutrition, sedentary lifestyle, social determinants of health, and prescribed antipsychotic medications. Metabolic monitoring aims to address these health disparities by screening for metabolic parameters and identifying abnormalities in order to target appropriate health interventions. Screening rates for metabolic parameters remain low for children, adolescents, and adults prescribed second-generation antipsychotics despite published guidelines and clinical interventions to improve screening. More system-wide interventions to improve collaboration between mental health and primary care are needed to enhance screening and prevent cardiovascular disease risk in this vulnerable population.
Corresponding author: Carrie Cunningham, MD, MPH, Zuckerberg San Francisco General Hospital, 1001 Potrero Ave, Suite 7M, San Francisco, CA 94110, carrie.cunningham@ucsf.edu.
Funding/support: Dr. Cunningham was supported by the UCSF-Zuckerberg San Francisco General Public Psychiatry Fellowship. Mr. Riano was supported by the NIH Center Grant from the National Institute of Diabetes and Digestive and Kidney Diseases for The Health Delivery Systems-Center for Diabetes Translational Research (CDTR) (P30DK092924) and by the UCSF-San Francisco General Hospital Public Psychiatry Fellowship. Dr. Mangurian received support from a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03 DK101857), as well as NIH Career Development Award (K23MH093689).
From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].
Metabolic Monitoring
Given the increased risk of metabolic syndrome among people with SMI, and the association of metabolic syndrome with increased morbidity and all-cause mortality, there has been a growing awareness of the importance of screening for metabolic syndrome among people with SMI. Metabolic monitoring involves routine screening for metabolic parameters and assessment of metabolic risk factors among people with SMI who are prescribed antipsychotic medications. Various practice guidelines have been developed in the United States and internationally to assess for metabolic risk factors in people prescribed antipsychotic medications [26]. Current metabolic monitoring guidelines in the United States stem from 2004 consensus recommendations of the American Diabetes Association and American Psychiatric Association along with the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity for metabolic monitoring among people prescribed SGAs [23]. These recommendations include a time line for routine monitoring of weight/body mass index, waist circumference, blood pressure, fasting blood glucose or hemoglobin A1c, and fasting lipids (Table 2). Guidelines recommend screening at baseline, more frequently within the first 3 months, and then annually [23].
Though guidelines recommend measurement of waist circumference as a marker for metabolic health, body mass index is often used alone as a measure of obesity [27,28]. This may be due to the relative ease of obtaining weight over waist circumference. For example, weight is more likely to be part of clinic workflows and many providers may not be accustomed to measuring waist circumference. However, waist circumference does provide additional information regarding metabolic health [29], as central adiposity is a marker of cardiometabolic risk and related to insulin resistance [21]. Further modifications of the guidelines have included ethnicity-specific waist measurements [30].
There is evidence that non-fasting lipids may be substituted for fasting lipid panels, particularly for patients who may have difficulty adhering to fasting due to cognitive difficulties. Vanderlip and colleagues argue that fasting serum cholesterol panels are not necessary for screening for dyslipidemia given that non-HDL cholesterol is calculated based on total cholesterol and HDL, which do not substantially differ between fasting and non-fasting values [31]. Hemoglobin A1c is recommended as a screening test for blood glucose abnormalities given that it does not require a fasting state and can therefore be more easily obtained for many patients. The choice to obtain a fasting blood glucose versus hemoglobin A1c may depend on multiple factors, including that a person can adhere to fasting and the cost of the laboratory test.
Routine monitoring of metabolic parameters is an integral step in targeting interventions to treat metabolic syndrome. These interventions include lifestyle modifications and evidence-based treatment guidelines for management of associated dyslipidemia, hypertension, and type 2 diabetes mellitus.
Current Metabolic Screening Practices
Despite the presence of defined guidelines, estimates show persistently low rates of metabolic monitoring among adults prescribed SGAs [32]. One study of 3 state Medicaid programs showed little to no improvement in screening rates for glucose and lipids post dissemination of the 2004 APA/ADA guidelines [33]. They noted a nonsignificant change in rates of glucose testing from 27% to 30% and small change in lipid testing from 10% to 11% among patients prescribed SGAs between 2002–2005 [33]. Examining screening rates among Medicaid recipients in Missouri between 2010–2012, Morrato and colleagues found glucose testing rates of 80% with lipid testing remaining at 41% [34]. A retrospective study of adult Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 showed rates of screening for lipids and glucose to increase over time; glucose monitoring increased from 56.6% to 72.6% and lipids from 38.3% to 41.2% [35]. A review by Mangurian and colleagues suggested rates of glucose (fasting blood glucose or hemoglobin A1c) and lipid screening as low as 30% among people prescribed antipsychotic medications [14]. Furthermore, they underscore the impact of low screening rates, stating that if 20% of adults with SMI have diabetes and 70% remain unscreened, then approximately 2 million adults with SMI and diabetes in the United States would not be identified within our current system [14].
Higher rates of screening have been shown for Medicaid populations than commercially insured populations [36]. Haupt et al compared lipid and glucose testing pre- and post- ADA/APA guideline implementation among commercially insured patients. They found an increase from 8.4% to 10.5% post guideline implementation for baseline lipid testing and from 6.8% to 9.0% for lipid testing at 12 weeks post-antipsychotic initiation [36]. Baseline glucose testing increased from 17.3% to 21.8% and from 14.1% to 17.9 % at 12-week post antipsychotic initiation. In alignment with findings from other studies, testing rates were particularly low for children [36].
Low screening rates have been found among children and adolescents prescribed SGAs [37] despite evidence that youth may be at risk of developing more significant metabolic sequelae from SGAs [19]. Edelsohn and colleagues found an increase from 30% to 50% for glucose screening and from 19% to 28% for lipid screening among youth Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 [35]. Connolly and colleagues reported on metabolic screening rates for children and adolescents prescribed SGAs over the 8 years following announcement of the 2004 ADA/APA guidelines. Using insurance claims data, they found screening rates for fasting blood glucose and hemoglobin A1c temporarily increased following guideline dissemination, then dropped during the period 2004–2008, and again increased slightly [38].
Barriers to Screening
Barriers to screening exist at the level of the individual patient and provider as well as at the clinic and larger systems levels. Lack of provider awareness of evidence-based guidelines for metabolic monitoring despite the presence of the 2004 ADA/APA guidelines has been cited by researchers as an impediment to screening. In a survey of primary care clinicians in San Francisco, Mangurian et al found that 40% of primary care providers did not know about the ADA/APA consensus guidelines for metabolic monitoring. The same survey of primary care providers identified additional impediments to screening, including obstacles to collaboration with psychiatric providers and to scheduling patients for psychiatric follow-up [39]. Another clinician survey conducted by Parameswaran et al found that psychiatrists viewed psychiatric illness severity, lack of staff time, and lack of clinician time as significant barriers to metabolic screening. In addition, clinicians identified factors related to the complexity of coordinating care across systems as obstacles; these included barriers to coordinating follow-up with medical providers, long wait times for patients to see medical providers, and difficulty collaborating with medical providers [40].
Other systems-level barriers include lack of a population-based approach to screening (eg, registries) and lack of electronic record integration, which impedes the ability of primary care and psychiatry providers to share information related to the ordering of metabolic screening tests and prescribing of medications [41]. Mangurian calls for integration of electronic medical record systems between primary care and psychiatry, a population-based approach to metabolic monitoring utilizing registries and other elements of collaborative care models, and primary care consultation to aid in the treatment of metabolic abnormalities [41]. Amiel et al point to systems-level factors “including but not limited to … poor access to general medical services, inadequate medical record-keeping infrastructure, lack of in-system compliance incentives and lack of centralized oversight” [26].
Based on their experience implementing a computer-based intervention for metabolic monitoring, Lai et al propose that the following factors may influence providers’ engagement in metabolic monitoring: lack of apparent symptoms to suggest metabolic syndrome, patients’ lack of engagement in care, and poor access to care. They identify additional factors at the clinician level to include under-recognition of the need for metabolic monitoring, lack of familiarity with screening guidelines, lack of agreement with guidelines, and the potential for individual clinicians to forget to order tests [42]. At the systems-level, they identify the absence of ongoing training as a potential reason why sustained testing was not observed in their intervention [42].
In a 2011 survey of providers prescribing antipsychotic medication to Medicaid beneficiaries in Missouri, Morrato and colleagues found that factors limiting frequency of health care utilization were closely linked to lack of metabolic testing. They also noted disparities in screening guidelines may lead to lack of routine metabolic monitoring; providers may screen based on prescribed medication, diagnosis, or other risk factor based stratification depending on the guidelines followed [34].
Current Unmet Needs
Vulnerable Populations
Though rates of metabolic screening remain low for all groups prescribed antipsychotic medications, studies have consistently shown low rates of screening among children and adolescents [35,36]. Edelsohn and colleagues hypothesize that the cause of these low rates is multifactorial, including that guardians may be reluctant to have young people undergo blood draws [35]. Morrato and colleagues suggest that policymakers should focus initiatives on younger, healthier adults, who they found to have lower rates of screening [37].
Racial and ethnic minorities with SMI constitute another particularly vulnerable population, with some studies showing an increased risk of metabolic sequelae and lower likelihood of treatment for diabetes and other metabolic derangements among African American and Latino populations with SMI [14,43,44].
Integration of Care
Lack of widespread integration of care between mental health and primary care remains another unmet need [41]. Hasnain and colleagues recommend improved communication between mental health and primary care clinicians to coordinate care to improve rates of monitoring, facilitate early follow-up of metabolic abnormalities, and avoid duplication of monitoring efforts [45]. Morrato and colleagues recommend that efforts to increase rates of metabolic monitoring be targeted not only to providers practicing in community mental health centers, but also to other practice settings including primary care. They found that for 75% of people prescribed antipsychotic medications, the prescriptions were started by prescribing providers who practiced outside of a community mental health center [34] and recommend that educational initiatives and performance improvement interventions broaden to include primary care and other care settings [34].
Potential Interventions for Improvement
Early interventions to improve metabolic screening rates have included educational initiatives to teach providers about consensus guidelines. However, initiatives to educate clinicians on metabolic monitoring have shown to be inadequate to significantly improve rates of screening [33]. Therefore, subsequent initiatives have sought to influence screening rates by targeting behavior of individual clinicians with point-of-care tools, electronic reminders, or through systems-level reorganization towards population-based care [27,42,46].
A variety of clinical interventions focus on technologies that remind clinicians to order metabolic monitoring tests according to screening guidelines. One public mental health service in Queensland, Australia, created a standardized metabolic monitoring form to be uploaded to the electronic medical record. In their implementation study examining the efficacy of the metabolic monitoring form, they found that only 36% of the forms contained data. When data were recorded, there were significantly higher rates of documentation of measurements (weight, body mass index, blood pressure) rather than laboratory tests (including lipids and fasting blood glucose) [27].
Computerized reminder systems for metabolic monitoring have been studied in both outpatient and inpatient settings. Lai and colleagues studied the impact of a computerized reminder system on lab monitoring for metabolic parameters among outpatients with schizophrenia prescribed SGAs [42]. This intervention also included an educational component with discussion of metabolic monitoring for people prescribed SGAs at meetings with attending psychiatrists. Computer reminders were displayed when a provider failed to order fasting plasma glucose or lipids (cholesterol, triglyceride) for patients prescribed clozapine, olanzapine, quetiapine, or risperidone. The study found a statistically significant improvement in laboratory metabolic screening for patients prescribed SGAs after implementation, with the greatest impact 6-months post-intervention, though with subsequent decline in screening rates [42].
Psychiatric inpatient hospitalizations provide an opportunity to obtain testing at the time of treatment initiation and also for ongoing monitoring in a location where fasting laboratory tests may be more easily obtained given onsite phlebotomy. One intervention targeting psychiatric inpatients utilized a computerized physician order entry system with the goal to improve metabolic screening among patients prescribed SGAs. Set in a large academic medical setting, the study found inpatient metabolic monitoring rates did not change significantly after implementation of these pop-up computer alerts, comparing rates immediately and 4 years after implementation [46].
There has been increasing focus on integrating mental health and medical care in an effort to improve the health of people with mental illness [47]. Mangurian and colleagues found that the likelihood of diabetes mellitus screening doubled for people with severe mental illness who were seen for at least one primary care visit in addition to mental health treatment [48]. Haupt similarly found higher rates of metabolic screening among patients who had greater than one primary care visit [36]. Models of integration include both integration of medical services into mental health treatment as well as incorporation of mental health services into primary care. For people with SMI, integration efforts have largely focused on integrating primary care services into community mental health settings [49]. The Substance Abuse and Mental Health Service Administration’s (SAMHSA) Primary and Behavioral Health Care Integration (PBHCI) grants program and the Affordable Care Act’s Health Home Initiative are examples of federal incentive programs for improved integration between behavioral health and primary care [49]. In their evaluation of the PBHCI grant program, Scharf and colleagues presented findings that patients at 3 matched clinics with PCBHI grants showed improvement in some lipids, diastolic blood pressure, and fasting blood glucose, though not smoking or body mass index [50].
Conclusion
Several risk factors contribute to an increase in cardiometabolic risk for people with severe mental illness, including poor nutrition, sedentary lifestyle, social determinants of health, and prescribed antipsychotic medications. Metabolic monitoring aims to address these health disparities by screening for metabolic parameters and identifying abnormalities in order to target appropriate health interventions. Screening rates for metabolic parameters remain low for children, adolescents, and adults prescribed second-generation antipsychotics despite published guidelines and clinical interventions to improve screening. More system-wide interventions to improve collaboration between mental health and primary care are needed to enhance screening and prevent cardiovascular disease risk in this vulnerable population.
Corresponding author: Carrie Cunningham, MD, MPH, Zuckerberg San Francisco General Hospital, 1001 Potrero Ave, Suite 7M, San Francisco, CA 94110, carrie.cunningham@ucsf.edu.
Funding/support: Dr. Cunningham was supported by the UCSF-Zuckerberg San Francisco General Public Psychiatry Fellowship. Mr. Riano was supported by the NIH Center Grant from the National Institute of Diabetes and Digestive and Kidney Diseases for The Health Delivery Systems-Center for Diabetes Translational Research (CDTR) (P30DK092924) and by the UCSF-San Francisco General Hospital Public Psychiatry Fellowship. Dr. Mangurian received support from a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03 DK101857), as well as NIH Career Development Award (K23MH093689).
1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014.
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
3. American Heart Association. What is metabolic syndrome? 2015.
4. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339–47.
5. Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Rev Bras Psiquiatria 2013;35:88–93.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32.
7. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015:1–10.
8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–31.
9. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41.
10. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chron Dis 2006;3:A42.
11. Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’–a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17:594.
12. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–96.
13. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naïve and treated patients. J Pharmacol Pharmacother 2013;4:176–86.
14. Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med 2016:1–9.
15. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005;19(Suppl 1):1–93.
16. Baptista T, De Mendoza S, Beaulieu S, et al. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord 2004;2:290–307.
17. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52–77.
18. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247–59.
19. Kryzhanovskaya LA, Xu W, Millen BA, et al. Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adol Psychopharmacol 2012;22:157–65.
20. Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35.
21. Zhang Z-J, YAO Z-J, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2004;184:58–62.
22. Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatr Invest 2015;12:242–8.
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 596–601.
24. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33:985–94.
25. Henderson DC. Atypical antipsychotic-induced diabetes mellitus. CNS Drugs 2002;16:77–89.
26. Amiel JM, Mangurian CV, Ganguli R, Newcomer JW. Addressing cardiometabolic risk during treatment with antipsychotic medications. Curr Opin Psychiatry 2008;21:613–8.
27. Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service–a retrospective file audit. BMC Psychiatry 2016;16:109.
28. Rosenbaum S, Nijjar S, Watkins A, et al. Nurse‐assessed metabolic monitoring: A file audit of risk factor prevalence and impact of an intervention to enhance measurement of waist circumference. Int J Ment Health Nurs 2014;23:252–6.
29. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007;15:1061–7.
30. Tan C-E, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182–6.
31. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv 2014;65:573–6.
32. Mitchell A, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012;42:125–47.
33. Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17–24.
34. Morrato EH, Campagna EJ, Brewer SE, et al. Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016;73:721–30.
35. Edelsohn GA, Parthasarathy M, Terhorst L, et al. Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. J Manage Care Specialty Pharm 2015;21:769–77.
36. Haupt DW, Rosenblatt LC, Kim E, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 2009;166:345–53.
37. Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51.
38. Connolly JG, Toomey TJ, Schneeweiss MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003–2011. Psychiatr Serv 2015;66:604–9.
39. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv 2013;64:597–9.
40. Parameswaran SG, Chang C, Swenson AK, et al. Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophren Res 2013;143:395–6.
41. Mangurian C. Patient-centered medical care in community mental health settings. Psychiatr Serv 2017;68:213-.
42. Lai C-L, Chan H-Y, Pan Y-J, Chen C-H. The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015;48:25–9.
43. Lambert BL, Chou C-H, Chang K-Y, et al. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005;14:417–25.
44. Ramaswamy K, Kozma CM, Nasrallah H. Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 2007;30:589–99.
45. Hasnain M, Vieweg WVR, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diab 2009;3:5–15.
46. Lee J, Dalack G, Casher M, et al. Persistence of metabolic monitoring for psychiatry inpatients treated with second‐generation antipsychotics utilizing a computer‐based intervention. J Clin Pharm Therap 2016;41:209–13.
47. Katz MH. Improving the health of persons with serious mental illness. JAMA Intern Med 2015;175:1979–80.
48. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015;175:1977–9.
49. Gerrity M. Integrating primary care into behavioral health settings: What works. New York: Milbank Memorial Fund; 2014.
50. Scharf DM EN, Hackbarth NS, Horvitz-Lennon M, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). 2014.
1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014.
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
3. American Heart Association. What is metabolic syndrome? 2015.
4. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339–47.
5. Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Rev Bras Psiquiatria 2013;35:88–93.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32.
7. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015:1–10.
8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–31.
9. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41.
10. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chron Dis 2006;3:A42.
11. Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’–a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17:594.
12. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–96.
13. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naïve and treated patients. J Pharmacol Pharmacother 2013;4:176–86.
14. Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med 2016:1–9.
15. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005;19(Suppl 1):1–93.
16. Baptista T, De Mendoza S, Beaulieu S, et al. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord 2004;2:290–307.
17. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52–77.
18. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247–59.
19. Kryzhanovskaya LA, Xu W, Millen BA, et al. Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adol Psychopharmacol 2012;22:157–65.
20. Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35.
21. Zhang Z-J, YAO Z-J, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2004;184:58–62.
22. Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatr Invest 2015;12:242–8.
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 596–601.
24. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33:985–94.
25. Henderson DC. Atypical antipsychotic-induced diabetes mellitus. CNS Drugs 2002;16:77–89.
26. Amiel JM, Mangurian CV, Ganguli R, Newcomer JW. Addressing cardiometabolic risk during treatment with antipsychotic medications. Curr Opin Psychiatry 2008;21:613–8.
27. Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service–a retrospective file audit. BMC Psychiatry 2016;16:109.
28. Rosenbaum S, Nijjar S, Watkins A, et al. Nurse‐assessed metabolic monitoring: A file audit of risk factor prevalence and impact of an intervention to enhance measurement of waist circumference. Int J Ment Health Nurs 2014;23:252–6.
29. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007;15:1061–7.
30. Tan C-E, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182–6.
31. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv 2014;65:573–6.
32. Mitchell A, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012;42:125–47.
33. Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17–24.
34. Morrato EH, Campagna EJ, Brewer SE, et al. Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016;73:721–30.
35. Edelsohn GA, Parthasarathy M, Terhorst L, et al. Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. J Manage Care Specialty Pharm 2015;21:769–77.
36. Haupt DW, Rosenblatt LC, Kim E, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 2009;166:345–53.
37. Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51.
38. Connolly JG, Toomey TJ, Schneeweiss MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003–2011. Psychiatr Serv 2015;66:604–9.
39. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv 2013;64:597–9.
40. Parameswaran SG, Chang C, Swenson AK, et al. Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophren Res 2013;143:395–6.
41. Mangurian C. Patient-centered medical care in community mental health settings. Psychiatr Serv 2017;68:213-.
42. Lai C-L, Chan H-Y, Pan Y-J, Chen C-H. The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015;48:25–9.
43. Lambert BL, Chou C-H, Chang K-Y, et al. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005;14:417–25.
44. Ramaswamy K, Kozma CM, Nasrallah H. Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 2007;30:589–99.
45. Hasnain M, Vieweg WVR, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diab 2009;3:5–15.
46. Lee J, Dalack G, Casher M, et al. Persistence of metabolic monitoring for psychiatry inpatients treated with second‐generation antipsychotics utilizing a computer‐based intervention. J Clin Pharm Therap 2016;41:209–13.
47. Katz MH. Improving the health of persons with serious mental illness. JAMA Intern Med 2015;175:1979–80.
48. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015;175:1977–9.
49. Gerrity M. Integrating primary care into behavioral health settings: What works. New York: Milbank Memorial Fund; 2014.
50. Scharf DM EN, Hackbarth NS, Horvitz-Lennon M, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). 2014.
Denosumab indication now includes multiple myeloma, Amgen announces
The Food and Drug Administration has expanded the indications for denosumab (Xgeva), previously indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors, to include patients with multiple myeloma, according to a press release from Amgen, the manufacturer of Xgeva.
“Up to 40% of [multiple myeloma] patients remain untreated for the prevention of bone complications, and the percentage is highest among patients with renal impairment at the time of diagnosis. Denosumab, which is not cleared through the kidneys, offers multiple myeloma patients bone protection with a convenient subcutaneous administration, providing patients with a novel treatment option,” Dr. Noopur Raje, director of the Center for Multiple Myeloma, Massachusetts General Hospital Cancer Center, Boston, said in the press release.
Adverse events in multiple myeloma patients were broadly similar to the known safety profile of denosumab. The most common adverse events were diarrhea, nausea, anemia, back pain, thrombocytopenia, peripheral edema, hypocalcemia, upper respiratory tract infection, rash, and headache. The most common adverse event resulting in discontinuation of treatment was osteonecrosis of the jaw.
Find the full press release on the Amgen website.
The Food and Drug Administration has expanded the indications for denosumab (Xgeva), previously indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors, to include patients with multiple myeloma, according to a press release from Amgen, the manufacturer of Xgeva.
“Up to 40% of [multiple myeloma] patients remain untreated for the prevention of bone complications, and the percentage is highest among patients with renal impairment at the time of diagnosis. Denosumab, which is not cleared through the kidneys, offers multiple myeloma patients bone protection with a convenient subcutaneous administration, providing patients with a novel treatment option,” Dr. Noopur Raje, director of the Center for Multiple Myeloma, Massachusetts General Hospital Cancer Center, Boston, said in the press release.
Adverse events in multiple myeloma patients were broadly similar to the known safety profile of denosumab. The most common adverse events were diarrhea, nausea, anemia, back pain, thrombocytopenia, peripheral edema, hypocalcemia, upper respiratory tract infection, rash, and headache. The most common adverse event resulting in discontinuation of treatment was osteonecrosis of the jaw.
Find the full press release on the Amgen website.
The Food and Drug Administration has expanded the indications for denosumab (Xgeva), previously indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors, to include patients with multiple myeloma, according to a press release from Amgen, the manufacturer of Xgeva.
“Up to 40% of [multiple myeloma] patients remain untreated for the prevention of bone complications, and the percentage is highest among patients with renal impairment at the time of diagnosis. Denosumab, which is not cleared through the kidneys, offers multiple myeloma patients bone protection with a convenient subcutaneous administration, providing patients with a novel treatment option,” Dr. Noopur Raje, director of the Center for Multiple Myeloma, Massachusetts General Hospital Cancer Center, Boston, said in the press release.
Adverse events in multiple myeloma patients were broadly similar to the known safety profile of denosumab. The most common adverse events were diarrhea, nausea, anemia, back pain, thrombocytopenia, peripheral edema, hypocalcemia, upper respiratory tract infection, rash, and headache. The most common adverse event resulting in discontinuation of treatment was osteonecrosis of the jaw.
Find the full press release on the Amgen website.
Home Monitoring of Cystic Fibrosis
Study Overview
Objective. To determine if an intervention directed toward early detection of pulmonary exacerbations using electronic home monitoring of spirometry and symptoms would result in slower decline in lung function.
Design. Multicenter, randomized, nonblinded 2-arm clinical trial.
Setting and participants. The study was conducted at 14 cystic fibrosis centers in the United States between 2011 and 2015. Cystic fibrosis patients (stable at baseline, FEV1 > 25% predicted) at least 14 years old (adolescent and adults) were included and randomized 1:1 to either an early intervention arm or usual care arm.
Intervention. The intervention arm used home-based spirometers and patient-reported respiratory symptoms using the Cystic Fibrosis Respiratory Symptoms Diary (CFRSD), which was to be completed twice weekly and collected by the central AM2 system. This AM2 system alerted sites to contact patients for an acute pulmonary exacerbation evaluation when FEV1 values fell by greater than 10% from baseline or CFRSD worsened from baseline in two or more of eight respiratory symptoms. The usual care arm patients had quarterly CF visits and/or acute visits based on their need.
Main outcome measures. The primary outcome variable was the 52-week change in FEV1 volume in liters. Secondary outcome variables were changes in CFQ-R (Cystic Fibrosis Questionnaire, revised), CFRSD, FEV1 % predicted, FVC in liters, FEF25-75%, time to first acute pulmonary exacerbation, time from first pulmonary exacerbation to subsequent pulmonary exacerbation, number of hospitalization days, number of hospitalizations, percent change in prevalence of Pseudomonas or Staphylococcus aureus and global assessment of protocol burden score.
Main results. A total of 267 patients were randomized. The results were analyzed using intention-to-treat analysis. There was no significant difference between study arms in 52-week mean change in FEV1 slope (mean slope difference, 0.00 L, 95% confidence interval, –0.07 to 0.07; P = 0.99). The early intervention arm subjects detected exacerbations sooner and more frequently than usual care arm subjects (time to first exacerbation hazard ratio, 1.45; 94% confidence interval, 1.09 to 1.93; P = 0.01). Adverse events were not significantly different between treatment arms.
Conclusion. An intervention of electronic home monitoring of patients with CF was able to detect more exacerbations than usual care, but this did not result in slower decline in lung function.
Commentary
Establishing efficacy and safety of home monitoring is a popular research topic in the current era of information technology. Most data to date has come from chronic adult disease such as heart failure, diabetes, or COPD [1]. While relatively rare, CF is a chronic lung disease that could potentially benefit from home monitoring. This is supported by previous evidence suggesting that up to a quarter of pulmonary exacerbations in CF patients result in worsened baseline lung function [2]. Close monitoring of symptoms and FEV1 using home monitoring was hypothesized to improve management and long-term function in this population. Indeed, in children with CF, electronic home monitoring of symptoms and lung function was able to detect pulmonary exacerbations early [3]. Frequency of monitoring is widely variable between centers, and some suggest aggressive monitoring of CF provides better clinical outcomes [4]. Current CF guidelines do not make specific recommendations regarding frequency of monitoring.
In this study, Lechtzin et al attempted to determine if the early detection of acute pulmonary exacerbations in CF patients by home monitoring and treatment would prevent progressive decline in lung function. This multicenter randomized trial was conducted at large CF centers in the US with a total cohort of 267 patients. The study had a mean follow-up time of 46.8 weeks per participant in the intervention arm and a mean follow-up time of 50.9 weeks per participant in the usual care arm. Given the predefined follow-up length (52 weeks) the primary outcome of FEV1 in liters was deemed sensitive enough to detect a decline of lung function. However the discrepancy between follow-up times with the intervention group having a 4.1-week shorter mean follow-up than the usual care could have influenced the interpretation of the results. Additionally, a large percentage of these patients were clinically stable at initial enrollment, with an average FEV1 % predicted of 79.5%. The stability of initial participants raises questions as to the efficacy of home monitoring in CF patient with moderate to severe lung disease. Mostly importantly, due to the nature of intervention the study could not be blinded, which could have substantially increased anxiety and self-awareness of patients in reporting their symptoms in the intervention arm.
Currently, an established consensus definition of pulmonary exacerbations of CF is lacking. Previous studies have proposed several different criteria of acute pulmonary exacerbations. Most proposed definitions depend on symptom changes such as cough, sputum, chest pain, shortness of breath, fatigue and weight-loss, making the definition less specific or objective.
The number of acute visits in the intervention arm was significantly higher than that in the usual care arm (153 vs 64). Despite a higher number of visits with intervention group, a significant number of these visits did not lead to a diagnosis of acute pulmonary exacerbation. Reportedly, 108 acute visits met protocol-defined pulmonary exacerbation and 29 acute visits did not meet protocol-defined pulmonary exacerbation in the intervention arm compared to 44 and 12 respectively in the usual care arm of the study. Given that the groups had similar baseline demographics and were randomized appropriately, one would expect that the number of acute visits severe enough to meet protocol-defined criteria as a pulmonary exacerbation would be similar in both groups. However, the absolute number of protocol-defined pulmonary exacerbations was far greater in the intervention group. Therefore, one could question the clinical significance of what was defined as acute pulmonary exacerbation. Potentially, the elevation of the absolute number of protocol-defined pulmonary exacerbations in the intervention group was simply due to increased surveillance. If the former were correct, one would expect the lack of identification/treatment of a significant number of pulmonary exacerbations in the usual care group would have led to a larger decline in FEV1 after 52 weeks than was seen in the results when compared to the intervention group. Given that the results of the study indicate no significant difference in change in FEV1 between study arms, perhaps the studied parameters in the intervention group were overly sensitive.
Of note, the usual care arm did have a statistically significant higher rate of hospitalizations and IV antibiotic use, suggesting that early identification of acute visits can identify patients earlier in the course of an acute pulmonary exacerbation and prevent higher level of care, though at the expense of more acute event “false positives,” or over-diagnosis. This trade-off may not result in cost saving, though this was not a consideration of this study. Additionally, there was likely difference in treatment, as treatment was not standardized, with potential implications for the validity of results.
The early intervention protocol was not only shown to lead to increased visits with no benefit in lung function decline, but as one may expect, also proved to be remarkably burdensome to many patients compared to the usual care protocol. Entering data on a weekly basis (or perhaps even monthly) was found to be burdensome in many remote-monitoring trials [5]. This may be especially apparent in a younger age group: in this study the average age of the study population was between 18 and 30 years of age. It can be hypothesized that this age group may not have enough responsibility, time, or enthusiasm to participate in home monitoring. Home monitoring maybe more effective in a disease condition where the average age is older or in a pediatric population in whom the parents oversee the care of the patient or have more time and receive subjective benefit from home monitoring services.
Less may be sufficient. The current study suggests that the home monitoring in CF may increase medical expense and unnecessary antibiotic use with no improvement in lung function. It is difficult to assess from this study the impact that the burden of home monitoring would have on clinical outcomes, however, previous meta-analysis of data studying COPD populations using home monitoring system, interestingly, also had increased health service usage and even led to increase in mortality in the intervention group compared with usual care group [1,6].
Perhaps the negative result of current study is due to the oftentimes variable definitions of and management algorithms for pulmonary exacerbations rather than the home monitoring system itself. Limited evidence exists for optimal threshold identification [7]. Aggregated, large amounts of data gathered by telemonitoring have not been proven to be used effectively. Moreover, as mentioned, a clear definition and management guidelines for pulmonary exacerbation are lacking. As a next step, studies are ongoing to evaluate how to use the collected data without increasing harm or cost. This could utilize machine learning or developing a more specific model defining and predicting pulmonary exacerbations as well as standardized indications for antibiotic therapy and hospitalization.
Applications for Clinical Practice
CF patients suffer from frequent pulmonary exacerbations and close monitoring and appropriate treatment is necessary to prevent progressive decline of lung function. This study has shown no benefit of electronic home monitoring in CF patients based on symptoms and spirometry over usual care. However, this negative outcome may be due to the limitation of the current definition of pulmonary exacerbation and lack of a consensus management algorithm. Optimizing the definition of pulmonary exacerbation and protocoling management based on severity may improve future evaluations of electronic home monitoring. Electronic home monitoring may help identify patients requiring evaluation; however, clinicians should continue to manage CF patients with conventional tools including regular follow-up visits, thorough history taking, and appropriate use of antibiotics based on their clinical acumen.
—Minkyung Kwon, MD, Joel Roberson, MD, Drew Willey, MD, and Neal Patel, MD (Mayo Clinic Florida, Jacksonville, FL, except for Dr. Roberson, of Oakland University/ Beaumont Health, Royal Oak, MI)
1. Polisena J, Tran K, Cimon K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2010;16 :120–7.
2. Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627–32.
3. van Horck M, Winkens B, Wesseling G, et al. Early detection of pulmonary exacerbations in children with Cystic Fibrosis by electronic home monitoring of symptoms and lung function. Sci Rep 2017;7:12350.
4. Johnson C, Butler SM, Konstan MW, et al. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest 2003;123:20–7.
5. Ding H, Karunanithi M, Kanagasingam Y, et al. A pilot study of a mobile-phone-based home monitoring system to assist in remote interventions in cases of acute exacerbation of COPD. J Telemed Telecare 2014;20:128–34.
6. Kargiannakis M, Fitzsimmons DA, Bentley CL, Mountain GA. Does telehealth monitoring identify exacerbations of chronic obstructive pulmonary disease and reduce hospitalisations? an analysis of system data. JMIR Med Inform 2017;5:e8.
7. Finkelstein J, Jeong IC. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann N Y Acad Sci 2017;1387:153–65.
Study Overview
Objective. To determine if an intervention directed toward early detection of pulmonary exacerbations using electronic home monitoring of spirometry and symptoms would result in slower decline in lung function.
Design. Multicenter, randomized, nonblinded 2-arm clinical trial.
Setting and participants. The study was conducted at 14 cystic fibrosis centers in the United States between 2011 and 2015. Cystic fibrosis patients (stable at baseline, FEV1 > 25% predicted) at least 14 years old (adolescent and adults) were included and randomized 1:1 to either an early intervention arm or usual care arm.
Intervention. The intervention arm used home-based spirometers and patient-reported respiratory symptoms using the Cystic Fibrosis Respiratory Symptoms Diary (CFRSD), which was to be completed twice weekly and collected by the central AM2 system. This AM2 system alerted sites to contact patients for an acute pulmonary exacerbation evaluation when FEV1 values fell by greater than 10% from baseline or CFRSD worsened from baseline in two or more of eight respiratory symptoms. The usual care arm patients had quarterly CF visits and/or acute visits based on their need.
Main outcome measures. The primary outcome variable was the 52-week change in FEV1 volume in liters. Secondary outcome variables were changes in CFQ-R (Cystic Fibrosis Questionnaire, revised), CFRSD, FEV1 % predicted, FVC in liters, FEF25-75%, time to first acute pulmonary exacerbation, time from first pulmonary exacerbation to subsequent pulmonary exacerbation, number of hospitalization days, number of hospitalizations, percent change in prevalence of Pseudomonas or Staphylococcus aureus and global assessment of protocol burden score.
Main results. A total of 267 patients were randomized. The results were analyzed using intention-to-treat analysis. There was no significant difference between study arms in 52-week mean change in FEV1 slope (mean slope difference, 0.00 L, 95% confidence interval, –0.07 to 0.07; P = 0.99). The early intervention arm subjects detected exacerbations sooner and more frequently than usual care arm subjects (time to first exacerbation hazard ratio, 1.45; 94% confidence interval, 1.09 to 1.93; P = 0.01). Adverse events were not significantly different between treatment arms.
Conclusion. An intervention of electronic home monitoring of patients with CF was able to detect more exacerbations than usual care, but this did not result in slower decline in lung function.
Commentary
Establishing efficacy and safety of home monitoring is a popular research topic in the current era of information technology. Most data to date has come from chronic adult disease such as heart failure, diabetes, or COPD [1]. While relatively rare, CF is a chronic lung disease that could potentially benefit from home monitoring. This is supported by previous evidence suggesting that up to a quarter of pulmonary exacerbations in CF patients result in worsened baseline lung function [2]. Close monitoring of symptoms and FEV1 using home monitoring was hypothesized to improve management and long-term function in this population. Indeed, in children with CF, electronic home monitoring of symptoms and lung function was able to detect pulmonary exacerbations early [3]. Frequency of monitoring is widely variable between centers, and some suggest aggressive monitoring of CF provides better clinical outcomes [4]. Current CF guidelines do not make specific recommendations regarding frequency of monitoring.
In this study, Lechtzin et al attempted to determine if the early detection of acute pulmonary exacerbations in CF patients by home monitoring and treatment would prevent progressive decline in lung function. This multicenter randomized trial was conducted at large CF centers in the US with a total cohort of 267 patients. The study had a mean follow-up time of 46.8 weeks per participant in the intervention arm and a mean follow-up time of 50.9 weeks per participant in the usual care arm. Given the predefined follow-up length (52 weeks) the primary outcome of FEV1 in liters was deemed sensitive enough to detect a decline of lung function. However the discrepancy between follow-up times with the intervention group having a 4.1-week shorter mean follow-up than the usual care could have influenced the interpretation of the results. Additionally, a large percentage of these patients were clinically stable at initial enrollment, with an average FEV1 % predicted of 79.5%. The stability of initial participants raises questions as to the efficacy of home monitoring in CF patient with moderate to severe lung disease. Mostly importantly, due to the nature of intervention the study could not be blinded, which could have substantially increased anxiety and self-awareness of patients in reporting their symptoms in the intervention arm.
Currently, an established consensus definition of pulmonary exacerbations of CF is lacking. Previous studies have proposed several different criteria of acute pulmonary exacerbations. Most proposed definitions depend on symptom changes such as cough, sputum, chest pain, shortness of breath, fatigue and weight-loss, making the definition less specific or objective.
The number of acute visits in the intervention arm was significantly higher than that in the usual care arm (153 vs 64). Despite a higher number of visits with intervention group, a significant number of these visits did not lead to a diagnosis of acute pulmonary exacerbation. Reportedly, 108 acute visits met protocol-defined pulmonary exacerbation and 29 acute visits did not meet protocol-defined pulmonary exacerbation in the intervention arm compared to 44 and 12 respectively in the usual care arm of the study. Given that the groups had similar baseline demographics and were randomized appropriately, one would expect that the number of acute visits severe enough to meet protocol-defined criteria as a pulmonary exacerbation would be similar in both groups. However, the absolute number of protocol-defined pulmonary exacerbations was far greater in the intervention group. Therefore, one could question the clinical significance of what was defined as acute pulmonary exacerbation. Potentially, the elevation of the absolute number of protocol-defined pulmonary exacerbations in the intervention group was simply due to increased surveillance. If the former were correct, one would expect the lack of identification/treatment of a significant number of pulmonary exacerbations in the usual care group would have led to a larger decline in FEV1 after 52 weeks than was seen in the results when compared to the intervention group. Given that the results of the study indicate no significant difference in change in FEV1 between study arms, perhaps the studied parameters in the intervention group were overly sensitive.
Of note, the usual care arm did have a statistically significant higher rate of hospitalizations and IV antibiotic use, suggesting that early identification of acute visits can identify patients earlier in the course of an acute pulmonary exacerbation and prevent higher level of care, though at the expense of more acute event “false positives,” or over-diagnosis. This trade-off may not result in cost saving, though this was not a consideration of this study. Additionally, there was likely difference in treatment, as treatment was not standardized, with potential implications for the validity of results.
The early intervention protocol was not only shown to lead to increased visits with no benefit in lung function decline, but as one may expect, also proved to be remarkably burdensome to many patients compared to the usual care protocol. Entering data on a weekly basis (or perhaps even monthly) was found to be burdensome in many remote-monitoring trials [5]. This may be especially apparent in a younger age group: in this study the average age of the study population was between 18 and 30 years of age. It can be hypothesized that this age group may not have enough responsibility, time, or enthusiasm to participate in home monitoring. Home monitoring maybe more effective in a disease condition where the average age is older or in a pediatric population in whom the parents oversee the care of the patient or have more time and receive subjective benefit from home monitoring services.
Less may be sufficient. The current study suggests that the home monitoring in CF may increase medical expense and unnecessary antibiotic use with no improvement in lung function. It is difficult to assess from this study the impact that the burden of home monitoring would have on clinical outcomes, however, previous meta-analysis of data studying COPD populations using home monitoring system, interestingly, also had increased health service usage and even led to increase in mortality in the intervention group compared with usual care group [1,6].
Perhaps the negative result of current study is due to the oftentimes variable definitions of and management algorithms for pulmonary exacerbations rather than the home monitoring system itself. Limited evidence exists for optimal threshold identification [7]. Aggregated, large amounts of data gathered by telemonitoring have not been proven to be used effectively. Moreover, as mentioned, a clear definition and management guidelines for pulmonary exacerbation are lacking. As a next step, studies are ongoing to evaluate how to use the collected data without increasing harm or cost. This could utilize machine learning or developing a more specific model defining and predicting pulmonary exacerbations as well as standardized indications for antibiotic therapy and hospitalization.
Applications for Clinical Practice
CF patients suffer from frequent pulmonary exacerbations and close monitoring and appropriate treatment is necessary to prevent progressive decline of lung function. This study has shown no benefit of electronic home monitoring in CF patients based on symptoms and spirometry over usual care. However, this negative outcome may be due to the limitation of the current definition of pulmonary exacerbation and lack of a consensus management algorithm. Optimizing the definition of pulmonary exacerbation and protocoling management based on severity may improve future evaluations of electronic home monitoring. Electronic home monitoring may help identify patients requiring evaluation; however, clinicians should continue to manage CF patients with conventional tools including regular follow-up visits, thorough history taking, and appropriate use of antibiotics based on their clinical acumen.
—Minkyung Kwon, MD, Joel Roberson, MD, Drew Willey, MD, and Neal Patel, MD (Mayo Clinic Florida, Jacksonville, FL, except for Dr. Roberson, of Oakland University/ Beaumont Health, Royal Oak, MI)
Study Overview
Objective. To determine if an intervention directed toward early detection of pulmonary exacerbations using electronic home monitoring of spirometry and symptoms would result in slower decline in lung function.
Design. Multicenter, randomized, nonblinded 2-arm clinical trial.
Setting and participants. The study was conducted at 14 cystic fibrosis centers in the United States between 2011 and 2015. Cystic fibrosis patients (stable at baseline, FEV1 > 25% predicted) at least 14 years old (adolescent and adults) were included and randomized 1:1 to either an early intervention arm or usual care arm.
Intervention. The intervention arm used home-based spirometers and patient-reported respiratory symptoms using the Cystic Fibrosis Respiratory Symptoms Diary (CFRSD), which was to be completed twice weekly and collected by the central AM2 system. This AM2 system alerted sites to contact patients for an acute pulmonary exacerbation evaluation when FEV1 values fell by greater than 10% from baseline or CFRSD worsened from baseline in two or more of eight respiratory symptoms. The usual care arm patients had quarterly CF visits and/or acute visits based on their need.
Main outcome measures. The primary outcome variable was the 52-week change in FEV1 volume in liters. Secondary outcome variables were changes in CFQ-R (Cystic Fibrosis Questionnaire, revised), CFRSD, FEV1 % predicted, FVC in liters, FEF25-75%, time to first acute pulmonary exacerbation, time from first pulmonary exacerbation to subsequent pulmonary exacerbation, number of hospitalization days, number of hospitalizations, percent change in prevalence of Pseudomonas or Staphylococcus aureus and global assessment of protocol burden score.
Main results. A total of 267 patients were randomized. The results were analyzed using intention-to-treat analysis. There was no significant difference between study arms in 52-week mean change in FEV1 slope (mean slope difference, 0.00 L, 95% confidence interval, –0.07 to 0.07; P = 0.99). The early intervention arm subjects detected exacerbations sooner and more frequently than usual care arm subjects (time to first exacerbation hazard ratio, 1.45; 94% confidence interval, 1.09 to 1.93; P = 0.01). Adverse events were not significantly different between treatment arms.
Conclusion. An intervention of electronic home monitoring of patients with CF was able to detect more exacerbations than usual care, but this did not result in slower decline in lung function.
Commentary
Establishing efficacy and safety of home monitoring is a popular research topic in the current era of information technology. Most data to date has come from chronic adult disease such as heart failure, diabetes, or COPD [1]. While relatively rare, CF is a chronic lung disease that could potentially benefit from home monitoring. This is supported by previous evidence suggesting that up to a quarter of pulmonary exacerbations in CF patients result in worsened baseline lung function [2]. Close monitoring of symptoms and FEV1 using home monitoring was hypothesized to improve management and long-term function in this population. Indeed, in children with CF, electronic home monitoring of symptoms and lung function was able to detect pulmonary exacerbations early [3]. Frequency of monitoring is widely variable between centers, and some suggest aggressive monitoring of CF provides better clinical outcomes [4]. Current CF guidelines do not make specific recommendations regarding frequency of monitoring.
In this study, Lechtzin et al attempted to determine if the early detection of acute pulmonary exacerbations in CF patients by home monitoring and treatment would prevent progressive decline in lung function. This multicenter randomized trial was conducted at large CF centers in the US with a total cohort of 267 patients. The study had a mean follow-up time of 46.8 weeks per participant in the intervention arm and a mean follow-up time of 50.9 weeks per participant in the usual care arm. Given the predefined follow-up length (52 weeks) the primary outcome of FEV1 in liters was deemed sensitive enough to detect a decline of lung function. However the discrepancy between follow-up times with the intervention group having a 4.1-week shorter mean follow-up than the usual care could have influenced the interpretation of the results. Additionally, a large percentage of these patients were clinically stable at initial enrollment, with an average FEV1 % predicted of 79.5%. The stability of initial participants raises questions as to the efficacy of home monitoring in CF patient with moderate to severe lung disease. Mostly importantly, due to the nature of intervention the study could not be blinded, which could have substantially increased anxiety and self-awareness of patients in reporting their symptoms in the intervention arm.
Currently, an established consensus definition of pulmonary exacerbations of CF is lacking. Previous studies have proposed several different criteria of acute pulmonary exacerbations. Most proposed definitions depend on symptom changes such as cough, sputum, chest pain, shortness of breath, fatigue and weight-loss, making the definition less specific or objective.
The number of acute visits in the intervention arm was significantly higher than that in the usual care arm (153 vs 64). Despite a higher number of visits with intervention group, a significant number of these visits did not lead to a diagnosis of acute pulmonary exacerbation. Reportedly, 108 acute visits met protocol-defined pulmonary exacerbation and 29 acute visits did not meet protocol-defined pulmonary exacerbation in the intervention arm compared to 44 and 12 respectively in the usual care arm of the study. Given that the groups had similar baseline demographics and were randomized appropriately, one would expect that the number of acute visits severe enough to meet protocol-defined criteria as a pulmonary exacerbation would be similar in both groups. However, the absolute number of protocol-defined pulmonary exacerbations was far greater in the intervention group. Therefore, one could question the clinical significance of what was defined as acute pulmonary exacerbation. Potentially, the elevation of the absolute number of protocol-defined pulmonary exacerbations in the intervention group was simply due to increased surveillance. If the former were correct, one would expect the lack of identification/treatment of a significant number of pulmonary exacerbations in the usual care group would have led to a larger decline in FEV1 after 52 weeks than was seen in the results when compared to the intervention group. Given that the results of the study indicate no significant difference in change in FEV1 between study arms, perhaps the studied parameters in the intervention group were overly sensitive.
Of note, the usual care arm did have a statistically significant higher rate of hospitalizations and IV antibiotic use, suggesting that early identification of acute visits can identify patients earlier in the course of an acute pulmonary exacerbation and prevent higher level of care, though at the expense of more acute event “false positives,” or over-diagnosis. This trade-off may not result in cost saving, though this was not a consideration of this study. Additionally, there was likely difference in treatment, as treatment was not standardized, with potential implications for the validity of results.
The early intervention protocol was not only shown to lead to increased visits with no benefit in lung function decline, but as one may expect, also proved to be remarkably burdensome to many patients compared to the usual care protocol. Entering data on a weekly basis (or perhaps even monthly) was found to be burdensome in many remote-monitoring trials [5]. This may be especially apparent in a younger age group: in this study the average age of the study population was between 18 and 30 years of age. It can be hypothesized that this age group may not have enough responsibility, time, or enthusiasm to participate in home monitoring. Home monitoring maybe more effective in a disease condition where the average age is older or in a pediatric population in whom the parents oversee the care of the patient or have more time and receive subjective benefit from home monitoring services.
Less may be sufficient. The current study suggests that the home monitoring in CF may increase medical expense and unnecessary antibiotic use with no improvement in lung function. It is difficult to assess from this study the impact that the burden of home monitoring would have on clinical outcomes, however, previous meta-analysis of data studying COPD populations using home monitoring system, interestingly, also had increased health service usage and even led to increase in mortality in the intervention group compared with usual care group [1,6].
Perhaps the negative result of current study is due to the oftentimes variable definitions of and management algorithms for pulmonary exacerbations rather than the home monitoring system itself. Limited evidence exists for optimal threshold identification [7]. Aggregated, large amounts of data gathered by telemonitoring have not been proven to be used effectively. Moreover, as mentioned, a clear definition and management guidelines for pulmonary exacerbation are lacking. As a next step, studies are ongoing to evaluate how to use the collected data without increasing harm or cost. This could utilize machine learning or developing a more specific model defining and predicting pulmonary exacerbations as well as standardized indications for antibiotic therapy and hospitalization.
Applications for Clinical Practice
CF patients suffer from frequent pulmonary exacerbations and close monitoring and appropriate treatment is necessary to prevent progressive decline of lung function. This study has shown no benefit of electronic home monitoring in CF patients based on symptoms and spirometry over usual care. However, this negative outcome may be due to the limitation of the current definition of pulmonary exacerbation and lack of a consensus management algorithm. Optimizing the definition of pulmonary exacerbation and protocoling management based on severity may improve future evaluations of electronic home monitoring. Electronic home monitoring may help identify patients requiring evaluation; however, clinicians should continue to manage CF patients with conventional tools including regular follow-up visits, thorough history taking, and appropriate use of antibiotics based on their clinical acumen.
—Minkyung Kwon, MD, Joel Roberson, MD, Drew Willey, MD, and Neal Patel, MD (Mayo Clinic Florida, Jacksonville, FL, except for Dr. Roberson, of Oakland University/ Beaumont Health, Royal Oak, MI)
1. Polisena J, Tran K, Cimon K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2010;16 :120–7.
2. Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627–32.
3. van Horck M, Winkens B, Wesseling G, et al. Early detection of pulmonary exacerbations in children with Cystic Fibrosis by electronic home monitoring of symptoms and lung function. Sci Rep 2017;7:12350.
4. Johnson C, Butler SM, Konstan MW, et al. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest 2003;123:20–7.
5. Ding H, Karunanithi M, Kanagasingam Y, et al. A pilot study of a mobile-phone-based home monitoring system to assist in remote interventions in cases of acute exacerbation of COPD. J Telemed Telecare 2014;20:128–34.
6. Kargiannakis M, Fitzsimmons DA, Bentley CL, Mountain GA. Does telehealth monitoring identify exacerbations of chronic obstructive pulmonary disease and reduce hospitalisations? an analysis of system data. JMIR Med Inform 2017;5:e8.
7. Finkelstein J, Jeong IC. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann N Y Acad Sci 2017;1387:153–65.
1. Polisena J, Tran K, Cimon K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2010;16 :120–7.
2. Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627–32.
3. van Horck M, Winkens B, Wesseling G, et al. Early detection of pulmonary exacerbations in children with Cystic Fibrosis by electronic home monitoring of symptoms and lung function. Sci Rep 2017;7:12350.
4. Johnson C, Butler SM, Konstan MW, et al. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest 2003;123:20–7.
5. Ding H, Karunanithi M, Kanagasingam Y, et al. A pilot study of a mobile-phone-based home monitoring system to assist in remote interventions in cases of acute exacerbation of COPD. J Telemed Telecare 2014;20:128–34.
6. Kargiannakis M, Fitzsimmons DA, Bentley CL, Mountain GA. Does telehealth monitoring identify exacerbations of chronic obstructive pulmonary disease and reduce hospitalisations? an analysis of system data. JMIR Med Inform 2017;5:e8.
7. Finkelstein J, Jeong IC. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann N Y Acad Sci 2017;1387:153–65.
Addition of Durvalumab After Chemoradiotherapy Improves Progression-Free Survival in Unresectable Stage III Non-Small-Cell Lung Cancer
Study Overview
Objective. To evaluate the efficacy of the PD-L1 antibody durvalumab in the treatment of patients with unresectable stage III non-small-cell lung cancer (NSCLC) following completion of standard chemoradiotherapy.
Design. Interim analysis of the phase III PACIFIC study, a randomized, double-blind, international study.
Setting and participants. A total of 709 patients underwent randomization between May 2014 and April 2016. Eligible patients had histologically proven stage III, locally advanced and unresectable NSCLC with no evidence of disease progression following chemoradiotherapy. The enrolled patients had received at least 2 cycles of platinum-based chemotherapy concurrently with definitive radiation therapy (54 Gy to 66 Gy). Initially, patients were randomized within 2 weeks of completing radiation; however, the protocol was amended to allow randomization up to 42 days following completion of therapy. Patients were not eligible if they had previous exposure to anti-PD-1 or PD-L1 antibodies or active or prior autoimmune disease in the last 2 years. All patients were required to have an WHO performance status of 0 or 1. The patients were stratified at randomization by age (< 65 or > 65 years), sex and smoking status. Enrollment was not restricted to level of PD-L1 expression.
Intervention. Patients were randomized in a 2:1 ratio to receive consolidation durvalumab 10 mg/kg or placebo every 2 weeks for up to 12 months. The intervention was discontinued if there was evidence of confirmed disease progression, treatment with an alternative anticancer therapy, toxicity or patient preference. The response to treatment was assessed every 8 weeks for the first year and then every 12 weeks thereafter.
Main outcome measures. The primary endpoints of the study were progression-free survival (PFS) by blinded independent review and overall survival (OS). Secondary endpoints were the percentage of patients alive without disease progression at 12 and 18 months, objective response rate, duration of response, safety, and time to death or metastasis. Patients were given the option to provide archived tumor specimens for PD-L1 testing.
Results. The baseline characteristics were balanced. The median age at enrollment was 64 years and 91% of the patients were current or former smokers. The vast majority of patients (> 99% in both groups) received concurrent chemoradiotherapy. The response to initial concurrent therapy was similar in both groups with complete response rates of 1.9% and 3% in the durvalumab and placebo groups, respectively, and partial response rates of 48.7% and 46.8%. Archived tumor samples showed ≥ 25% PD-L1 expression in 22.3% of patients (24% in durvalumab group versus 18.6% in placebo group) and < 25% in 41% of patients (39.3%% in durvalumab group versus 44.3% in placebo group). PD-L1 status was unknown in 36.7% of the enrolled patients. Of note, 6% of patients enrolled had EGFR mutations.
After a median follow-up of 14.5 months, the median PFS was 16.8 months with durvalumab versus 5.6 months with placebo (P < 0.001; hazard ratio [HR] 0.52, 95% confidence interval [CI] 0.42–0.65). The 12-month PFS rate was 55.9% and 35.3% in the durvalumab and placebo group, respectively. The 18-month PFS rate was 44.2% and 27% in the durvalumab and placebo group, respectively. The PFS results were consistent across all subgroups. The PFS benefit was observed regardless of PD-L1 expression. The median time to death or metastasis was 23.2 months in the durvalumab group versus 14.6 months with placebo (HR 0.52; 95% CI 0.39–0.69). The objective response rate was significantly higher in the durvalumab group (28.4% vs. 16%, P < 0.001). The median duration of response was longer with durvalumab. Of the patients who responded to durvalumab, 73% had ongoing response at 18 months compared with 47% in the placebo group. OS was not assessed at this interm analysis.
Adverse events (AE) of any grade occurred in over approximately 95% in both groups. Grade 3 or 4 AE occurred in 29.9% in the durvalumab group and 26.1% in the placebo group. The most common grade 3 or 4 AE was pneumonia, occurring in about 4% of patients in each group. More patients in the durvalumab group discontinued treatment (15.4% vs 9.8%). Death due to an AE occurred in 4.4% of the durvalumab group and 5.6% of the placebo group. The most frequent AE leading to discontinuation was pneumonitis or radiation pneumonitis and pneumonia. Pneumonitis or radiation pneumonitis occurred in 33.9% (3.4% grade 3 or 4) and 24.8% (2.6% grade 3 or 4) of the durvalumab and placebo groups, respectively. Immune-mediated AE of any grade were more common in the duvalumab group occurring in 24% of patients (vs. 8% in placebo). Of these, 14% of patients in the durvalumab group required glucocorticoids compared with 4.3% in the placebo group. The most AE of interest was diarrhea, which occurred in 18% of the patients in both groups.
Conclusion. The addition of consolidative durvalumab following completion of concurrent chemoradiotherapy in patients with stage III, locally advanced NSCLC significantly improved PFS without a significant increase in treatment-related adverse events.
Commentary
Pre-clinical evidence has suggested that chemotherapy and radiation therapy may lead to upregulation of PD-L1 expression by tumor cells leading to increased PD-L1 mediated T cell apoptosis [1,2]. Given prior studies documenting PD-L1 expression as a predictive biomarker for response to durvalumab, the authors of the current trial hypothesized that the addition of durvalumab after chemoradiotherapy would provide clinical benefit likely mediated by upregulation of PD-L1. The results from this pre-planned interim analysis show a significant improvement in progression-free survival with the addition of durvalumab with a 48% decrease in the risk of progression. This benefit was noted across all patient subgroups. In addition, responses to durvalumab were durable, with 72% of the patients who responded having an ongoing response at 18 months. Interestingly, the response to durvalumab was independent of PD-L1 expression, which is in contrast to previous studies showing PD-L1 expression to be a good biomarker for durvalumab response [3].
The results of the PACIFIC trial represent a clinically meaningful benefit and suggests an excellent option for patients with unresectable stage III NSCLC. One important point to highlight is that the addition of durvalumab was well tolerated and did not appear to significantly increase the rate of severe adverse events. Of particular interest is the similar rates of grade 3 or 4 pneumonitis, which appeared to be around 3% for each group. Overall survival data remain immature at the time of this analysis; however, given the acceptable toxicity profile and improved PFS this combination should be considered for these patients in clinical practice. Ongoing trials are underway to evaluate the role of single-agent durvalumab in the front-line setting for NSCLC.
Applications for Clinical Practice
In patients with unresectable stage III NSCLC who have no evidence of disease progression following completion of chemoradiotherapy, the addition of durvalumab provided a significant and clinically meaningful improvement in progression-free survival without an increase in serious adverse events. While the overall survival data is immature, the 48% improvement in progression-free survival supports the incorporation of durvalumab into standard practice in this patient population.
—Daniel Isaac, DO, MS
1. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest2014;124:687–95.
2. Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1 mediated T cell apoptosis. Mol Immun 2008;45:1470–6.
3. Antonia SJ, Brahmer JR, Khleif S, et al. Phase ½ [What should this be? 3?]study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Presented at the 41st European Society for Medical Oncology Annual Meeting, Copenhagen, October 7–11 2016.
Study Overview
Objective. To evaluate the efficacy of the PD-L1 antibody durvalumab in the treatment of patients with unresectable stage III non-small-cell lung cancer (NSCLC) following completion of standard chemoradiotherapy.
Design. Interim analysis of the phase III PACIFIC study, a randomized, double-blind, international study.
Setting and participants. A total of 709 patients underwent randomization between May 2014 and April 2016. Eligible patients had histologically proven stage III, locally advanced and unresectable NSCLC with no evidence of disease progression following chemoradiotherapy. The enrolled patients had received at least 2 cycles of platinum-based chemotherapy concurrently with definitive radiation therapy (54 Gy to 66 Gy). Initially, patients were randomized within 2 weeks of completing radiation; however, the protocol was amended to allow randomization up to 42 days following completion of therapy. Patients were not eligible if they had previous exposure to anti-PD-1 or PD-L1 antibodies or active or prior autoimmune disease in the last 2 years. All patients were required to have an WHO performance status of 0 or 1. The patients were stratified at randomization by age (< 65 or > 65 years), sex and smoking status. Enrollment was not restricted to level of PD-L1 expression.
Intervention. Patients were randomized in a 2:1 ratio to receive consolidation durvalumab 10 mg/kg or placebo every 2 weeks for up to 12 months. The intervention was discontinued if there was evidence of confirmed disease progression, treatment with an alternative anticancer therapy, toxicity or patient preference. The response to treatment was assessed every 8 weeks for the first year and then every 12 weeks thereafter.
Main outcome measures. The primary endpoints of the study were progression-free survival (PFS) by blinded independent review and overall survival (OS). Secondary endpoints were the percentage of patients alive without disease progression at 12 and 18 months, objective response rate, duration of response, safety, and time to death or metastasis. Patients were given the option to provide archived tumor specimens for PD-L1 testing.
Results. The baseline characteristics were balanced. The median age at enrollment was 64 years and 91% of the patients were current or former smokers. The vast majority of patients (> 99% in both groups) received concurrent chemoradiotherapy. The response to initial concurrent therapy was similar in both groups with complete response rates of 1.9% and 3% in the durvalumab and placebo groups, respectively, and partial response rates of 48.7% and 46.8%. Archived tumor samples showed ≥ 25% PD-L1 expression in 22.3% of patients (24% in durvalumab group versus 18.6% in placebo group) and < 25% in 41% of patients (39.3%% in durvalumab group versus 44.3% in placebo group). PD-L1 status was unknown in 36.7% of the enrolled patients. Of note, 6% of patients enrolled had EGFR mutations.
After a median follow-up of 14.5 months, the median PFS was 16.8 months with durvalumab versus 5.6 months with placebo (P < 0.001; hazard ratio [HR] 0.52, 95% confidence interval [CI] 0.42–0.65). The 12-month PFS rate was 55.9% and 35.3% in the durvalumab and placebo group, respectively. The 18-month PFS rate was 44.2% and 27% in the durvalumab and placebo group, respectively. The PFS results were consistent across all subgroups. The PFS benefit was observed regardless of PD-L1 expression. The median time to death or metastasis was 23.2 months in the durvalumab group versus 14.6 months with placebo (HR 0.52; 95% CI 0.39–0.69). The objective response rate was significantly higher in the durvalumab group (28.4% vs. 16%, P < 0.001). The median duration of response was longer with durvalumab. Of the patients who responded to durvalumab, 73% had ongoing response at 18 months compared with 47% in the placebo group. OS was not assessed at this interm analysis.
Adverse events (AE) of any grade occurred in over approximately 95% in both groups. Grade 3 or 4 AE occurred in 29.9% in the durvalumab group and 26.1% in the placebo group. The most common grade 3 or 4 AE was pneumonia, occurring in about 4% of patients in each group. More patients in the durvalumab group discontinued treatment (15.4% vs 9.8%). Death due to an AE occurred in 4.4% of the durvalumab group and 5.6% of the placebo group. The most frequent AE leading to discontinuation was pneumonitis or radiation pneumonitis and pneumonia. Pneumonitis or radiation pneumonitis occurred in 33.9% (3.4% grade 3 or 4) and 24.8% (2.6% grade 3 or 4) of the durvalumab and placebo groups, respectively. Immune-mediated AE of any grade were more common in the duvalumab group occurring in 24% of patients (vs. 8% in placebo). Of these, 14% of patients in the durvalumab group required glucocorticoids compared with 4.3% in the placebo group. The most AE of interest was diarrhea, which occurred in 18% of the patients in both groups.
Conclusion. The addition of consolidative durvalumab following completion of concurrent chemoradiotherapy in patients with stage III, locally advanced NSCLC significantly improved PFS without a significant increase in treatment-related adverse events.
Commentary
Pre-clinical evidence has suggested that chemotherapy and radiation therapy may lead to upregulation of PD-L1 expression by tumor cells leading to increased PD-L1 mediated T cell apoptosis [1,2]. Given prior studies documenting PD-L1 expression as a predictive biomarker for response to durvalumab, the authors of the current trial hypothesized that the addition of durvalumab after chemoradiotherapy would provide clinical benefit likely mediated by upregulation of PD-L1. The results from this pre-planned interim analysis show a significant improvement in progression-free survival with the addition of durvalumab with a 48% decrease in the risk of progression. This benefit was noted across all patient subgroups. In addition, responses to durvalumab were durable, with 72% of the patients who responded having an ongoing response at 18 months. Interestingly, the response to durvalumab was independent of PD-L1 expression, which is in contrast to previous studies showing PD-L1 expression to be a good biomarker for durvalumab response [3].
The results of the PACIFIC trial represent a clinically meaningful benefit and suggests an excellent option for patients with unresectable stage III NSCLC. One important point to highlight is that the addition of durvalumab was well tolerated and did not appear to significantly increase the rate of severe adverse events. Of particular interest is the similar rates of grade 3 or 4 pneumonitis, which appeared to be around 3% for each group. Overall survival data remain immature at the time of this analysis; however, given the acceptable toxicity profile and improved PFS this combination should be considered for these patients in clinical practice. Ongoing trials are underway to evaluate the role of single-agent durvalumab in the front-line setting for NSCLC.
Applications for Clinical Practice
In patients with unresectable stage III NSCLC who have no evidence of disease progression following completion of chemoradiotherapy, the addition of durvalumab provided a significant and clinically meaningful improvement in progression-free survival without an increase in serious adverse events. While the overall survival data is immature, the 48% improvement in progression-free survival supports the incorporation of durvalumab into standard practice in this patient population.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy of the PD-L1 antibody durvalumab in the treatment of patients with unresectable stage III non-small-cell lung cancer (NSCLC) following completion of standard chemoradiotherapy.
Design. Interim analysis of the phase III PACIFIC study, a randomized, double-blind, international study.
Setting and participants. A total of 709 patients underwent randomization between May 2014 and April 2016. Eligible patients had histologically proven stage III, locally advanced and unresectable NSCLC with no evidence of disease progression following chemoradiotherapy. The enrolled patients had received at least 2 cycles of platinum-based chemotherapy concurrently with definitive radiation therapy (54 Gy to 66 Gy). Initially, patients were randomized within 2 weeks of completing radiation; however, the protocol was amended to allow randomization up to 42 days following completion of therapy. Patients were not eligible if they had previous exposure to anti-PD-1 or PD-L1 antibodies or active or prior autoimmune disease in the last 2 years. All patients were required to have an WHO performance status of 0 or 1. The patients were stratified at randomization by age (< 65 or > 65 years), sex and smoking status. Enrollment was not restricted to level of PD-L1 expression.
Intervention. Patients were randomized in a 2:1 ratio to receive consolidation durvalumab 10 mg/kg or placebo every 2 weeks for up to 12 months. The intervention was discontinued if there was evidence of confirmed disease progression, treatment with an alternative anticancer therapy, toxicity or patient preference. The response to treatment was assessed every 8 weeks for the first year and then every 12 weeks thereafter.
Main outcome measures. The primary endpoints of the study were progression-free survival (PFS) by blinded independent review and overall survival (OS). Secondary endpoints were the percentage of patients alive without disease progression at 12 and 18 months, objective response rate, duration of response, safety, and time to death or metastasis. Patients were given the option to provide archived tumor specimens for PD-L1 testing.
Results. The baseline characteristics were balanced. The median age at enrollment was 64 years and 91% of the patients were current or former smokers. The vast majority of patients (> 99% in both groups) received concurrent chemoradiotherapy. The response to initial concurrent therapy was similar in both groups with complete response rates of 1.9% and 3% in the durvalumab and placebo groups, respectively, and partial response rates of 48.7% and 46.8%. Archived tumor samples showed ≥ 25% PD-L1 expression in 22.3% of patients (24% in durvalumab group versus 18.6% in placebo group) and < 25% in 41% of patients (39.3%% in durvalumab group versus 44.3% in placebo group). PD-L1 status was unknown in 36.7% of the enrolled patients. Of note, 6% of patients enrolled had EGFR mutations.
After a median follow-up of 14.5 months, the median PFS was 16.8 months with durvalumab versus 5.6 months with placebo (P < 0.001; hazard ratio [HR] 0.52, 95% confidence interval [CI] 0.42–0.65). The 12-month PFS rate was 55.9% and 35.3% in the durvalumab and placebo group, respectively. The 18-month PFS rate was 44.2% and 27% in the durvalumab and placebo group, respectively. The PFS results were consistent across all subgroups. The PFS benefit was observed regardless of PD-L1 expression. The median time to death or metastasis was 23.2 months in the durvalumab group versus 14.6 months with placebo (HR 0.52; 95% CI 0.39–0.69). The objective response rate was significantly higher in the durvalumab group (28.4% vs. 16%, P < 0.001). The median duration of response was longer with durvalumab. Of the patients who responded to durvalumab, 73% had ongoing response at 18 months compared with 47% in the placebo group. OS was not assessed at this interm analysis.
Adverse events (AE) of any grade occurred in over approximately 95% in both groups. Grade 3 or 4 AE occurred in 29.9% in the durvalumab group and 26.1% in the placebo group. The most common grade 3 or 4 AE was pneumonia, occurring in about 4% of patients in each group. More patients in the durvalumab group discontinued treatment (15.4% vs 9.8%). Death due to an AE occurred in 4.4% of the durvalumab group and 5.6% of the placebo group. The most frequent AE leading to discontinuation was pneumonitis or radiation pneumonitis and pneumonia. Pneumonitis or radiation pneumonitis occurred in 33.9% (3.4% grade 3 or 4) and 24.8% (2.6% grade 3 or 4) of the durvalumab and placebo groups, respectively. Immune-mediated AE of any grade were more common in the duvalumab group occurring in 24% of patients (vs. 8% in placebo). Of these, 14% of patients in the durvalumab group required glucocorticoids compared with 4.3% in the placebo group. The most AE of interest was diarrhea, which occurred in 18% of the patients in both groups.
Conclusion. The addition of consolidative durvalumab following completion of concurrent chemoradiotherapy in patients with stage III, locally advanced NSCLC significantly improved PFS without a significant increase in treatment-related adverse events.
Commentary
Pre-clinical evidence has suggested that chemotherapy and radiation therapy may lead to upregulation of PD-L1 expression by tumor cells leading to increased PD-L1 mediated T cell apoptosis [1,2]. Given prior studies documenting PD-L1 expression as a predictive biomarker for response to durvalumab, the authors of the current trial hypothesized that the addition of durvalumab after chemoradiotherapy would provide clinical benefit likely mediated by upregulation of PD-L1. The results from this pre-planned interim analysis show a significant improvement in progression-free survival with the addition of durvalumab with a 48% decrease in the risk of progression. This benefit was noted across all patient subgroups. In addition, responses to durvalumab were durable, with 72% of the patients who responded having an ongoing response at 18 months. Interestingly, the response to durvalumab was independent of PD-L1 expression, which is in contrast to previous studies showing PD-L1 expression to be a good biomarker for durvalumab response [3].
The results of the PACIFIC trial represent a clinically meaningful benefit and suggests an excellent option for patients with unresectable stage III NSCLC. One important point to highlight is that the addition of durvalumab was well tolerated and did not appear to significantly increase the rate of severe adverse events. Of particular interest is the similar rates of grade 3 or 4 pneumonitis, which appeared to be around 3% for each group. Overall survival data remain immature at the time of this analysis; however, given the acceptable toxicity profile and improved PFS this combination should be considered for these patients in clinical practice. Ongoing trials are underway to evaluate the role of single-agent durvalumab in the front-line setting for NSCLC.
Applications for Clinical Practice
In patients with unresectable stage III NSCLC who have no evidence of disease progression following completion of chemoradiotherapy, the addition of durvalumab provided a significant and clinically meaningful improvement in progression-free survival without an increase in serious adverse events. While the overall survival data is immature, the 48% improvement in progression-free survival supports the incorporation of durvalumab into standard practice in this patient population.
—Daniel Isaac, DO, MS
1. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest2014;124:687–95.
2. Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1 mediated T cell apoptosis. Mol Immun 2008;45:1470–6.
3. Antonia SJ, Brahmer JR, Khleif S, et al. Phase ½ [What should this be? 3?]study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Presented at the 41st European Society for Medical Oncology Annual Meeting, Copenhagen, October 7–11 2016.
1. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest2014;124:687–95.
2. Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1 mediated T cell apoptosis. Mol Immun 2008;45:1470–6.
3. Antonia SJ, Brahmer JR, Khleif S, et al. Phase ½ [What should this be? 3?]study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Presented at the 41st European Society for Medical Oncology Annual Meeting, Copenhagen, October 7–11 2016.
Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease
Study Overview
Objective. To determine the effect of mepolizumab on the annual rate of chronic obstructive pulmonary disease (COPD) exacerbations in high-risk patients.
Design. Two randomized double-blind placebo-controlled parallel trials (METREO and METREX).
Setting and participants. Participants were recruited from over 15 countries in over 100 investigative sites. Inclusion criteria were adults (40 years or older) with a diagnosis of COPD for at least 1 year with: airflow limitation (FEV1/FVC < 0.7); some bronchodilator reversibility (post-bronchodilator FEV1 > 20% and ≤ 80% of predicted values); current COPD therapy for at least 3 months prior to enrollment (a high-dose inhaled corticosteroid, ICS, with at least 2 other classes of medications, to obtain “triple therapy”); and a high risk of exacerbations (at least 1 severe [requiring hospitalization] or 2 moderate [treatment with systemic corticosteroids and/or antibiotics] exacerbations in past year).
Notable exclusion criteria were patients with diagnoses of asthma in never-smokers, alpha-1 antitrypsin deficiency, recent exacerbations (in past month), lung volume reduction surgery (in past year), eosinophilic or parasitic diseases, or those with recent monoclonal antibody treatment. Patients with the asthma-COPD overlap syndrome were included only if they had a history of smoking and met the COPD inclusion criteria listed above.
Intervention. The treatment period lasted for a total of 52 weeks, with an additional 8 weeks of follow-up. Patients were randomized 1:1 to placebo or low-dose medication (100 mg) using permuted-block randomization in the METREX study regardless of eosinophil count (but they were stratified for a modified intention-to-treat analysis at screening into either low eosinophilic count [< 150 cells/uL] or high [≥ 150 cells/uL]). In the METREO study, patients were randomized 1:1:1 to placebo, low-dose (100 mg), or high-dose (300 mg) medication only if blood eosinophilia was present (≥ 150 cells/uL at screening or ≥ 300 cells/uL in past 12 months). Investigators and patients were blinded to presence of drug or placebo. Sample size calculations indicated that in order to provide a 90% power to detect a 30% decrease in the rate of exacerbations in METREX and 35% decrease in METREO, a total of 800 patients and 660 patients would need to be enrolled in METREX and METREO respectively. Both studies met their enrollment quota.
Main outcome measures. The primary outcome was the annual rate of exacerbations that were either moderate (requiring systemic corticosteroids and/or antibiotics) or severe (requiring hospitalization). Secondary outcomes included the time to first moderate/severe exacerbation, change from baseline in the COPD Assessment Test (CAT) and St. George’s Respiratory Questionnaire (SGRQ), and change from baseline in blood eosinophil count, FEV1, and FVC. Safety and adverse events endpoints were also assessed.
A modified intention-to-treat analysis was performed overall and in the METREX study stratified on eosinophilic count at screening; all patients who underwent randomization and received at least one dose of medication or placebo were included in that respective group. Multiple comparisons were accounted for using the Benjamini-Hochberg Test, exacerbations were assumed to follow a negative binomial distribution, and Cox proportional-hazards was used to model the relationship between covariates of interest and the primary outcome.
Main results. In the METREX study, 1161 patients were enrolled and 836 underwent randomization and received at least 1 dose of medication or placebo. In METREO, 1071 patients were enrolled and 674 underwent randomization and received at least one dose of medication or placebo. In both studies the patients in the medication and placebo groups were well balanced at baseline across demographics (age, gender, smoking history, duration of COPD) and pulmonary function (FEV1, FVC, FEV1/FVC, CAT, SGRQ). In METREX, a total of 462 (55%) patients had an eosinophilic phenotype and 374 (45%) did not.
There was no difference between groups in the primary endpoint of annual exacerbation rate in METREO (1.49/yr in placebo vs. 1.19/yr in low-dose and 1.27/yr in high-dose mepolizumab, rate ratio of high-dose to placebo 0.86, 95% confidence interval [CI] 0.7–1.05, P = 0.14). There was no difference in the primary outcome in the overall intention-to-treat analysis in the METREX study (1.49/yr in mepolizumab vs. 1.52/yr in placebo, P > 0.99). Only when analyzing the high eosinophilic phenotype in the stratified intention-to-treat METREX group was there a significant difference in the primary outcome (1.41/yr in mepolizumab vs. 1.71/yr in placebo, P = 0.04, rate ratio 0.82, 95% CI 0.68–0.98).
There were no significant differences in any secondary endpoint in the METREO study. In the METREX study, mepolizumab treatment resulted in a significantly longer time to first exacerbation (192 days vs. 141 days, hazard ratio 0.75, 95% CI 0.60–0.94, P = 0.04) but no difference in the change in SGRQ (–2.8 vs. –3.0, P > 0.99) or CAT score (–0.8 vs. 0, P > 0.99). There was no significant difference in any measures of pulmonary function between the treatment and placebo groups (FEV1, FVC, FEV1/FVC). As expected, there was a significant decrease in peripheral blood eosinophil count in both studies in the medication arm. The incidence of adverse events and safety endpoints were similar between the trial groups in METREX and METREO.
Conclusions. In this pair of placebo-controlled double-blind randomized parallel studies, there was a significant decline in annual exacerbation rate in patients with an eosinophilic phenotype treated with mepolizumab in a stratified intention-to-treat analysis of one of two parallel studies (METREX). However, there was no significant difference in the primary outcome of the other parallel study (METREO), which included only those patients with an eosinophilic phenotype. Additionally, there was no significant difference in any secondary endpoints in either study. The medication was generally safe and well tolerated.
Commentary
Mepolizumab is a humanized monoclonal antibody that targets and blocks interleukin-5, a key mediator of eosinophilic activity. Due to its ability to decrease eosinophil number and function, it is currently approved as a therapy for severe asthma with an eosinophilic phenotype [1]. While asthma and COPD have historically been thought of as separate entities with distinct pathophysiologic mechanisms, recent evidence has suggested that a subset of COPD patients experience significant eosinophilic inflammation. This group may behave more like asthmatic patients, and may have a different response to medications such as inhaled corticosteroids, but the role of eosinophils to guide prognostication and treatment in this group is still unclear [2,3].
In this study, Pavord and colleagues investigated the use of the anti-IL5 drug mepolizumab in COPD patients at risk of exacerbations who demonstrated an eosinophilic phenotype. The physiologic rationale for the study was that eosinophilic inflammation is thought to be a driver of exacerbations in COPD patients with an eosinophilic phenotype, and therefore a decrease in eosinophilic number and function should result in a decrease in exacerbations. The authors conducted a well-designed placebo-controlled double-blind study with a clearly defined endpoint, met their enrollment goals as determined by their power calculations, and used COPD patients at high risk of exacerbations to enrich their study.
There was no difference in the primary outcome in the METREO arm of the study, which included patients with baseline eosinophilia (> 150 cells/uL) or in the overall intention-to-treat analysis in METREX (which did not screen patients on baseline eosinophil count). Only when stratified on baseline eosinophil count in the METREX study was a significant treatment effect found, where patients with high eosinophil count at baseline (> 150 cells/uL) had a decreased risk of exacerbations when treated with mepolizumab. Notably there was no difference in any secondary outcome in METREO or in METREX aside from a longer time to first exacerbation in METREX in the mepolizumab group. The authors use this data to conclude that mepolizumab treatment results in a lower rate of exacerbations and a longer time to the first exacerbation in COPD patients with an eosinophilic phenotype, and the extent of the treatment effect is related to blood eosinophil counts.
The authors conducted a well-designed and rigorous study, and used robust and appropriate statistical analysis; however, significant questions remain regarding their conclusions. The primary concern is the role of mepolizumab in the treatment of COPD patients to decrease exacerbations may be overstated. When including only those with baseline eosinophilia in the METREO arm, there was no significant difference between placebo and low or high dose of mepolizumab; however, there was an appropriate and expected decrease in blood eosinophils, indicating the medication worked as intended. In the overall intention-to-treat analysis in the METREX arm, there was no difference between mepolizumab and placebo, and only in the analysis of METREX stratified to eosinophil count was there a significant difference (with an upper confidence interval rate ratio [0.98] approaching unity).
Additionally there was no significant difference between the 2 groups across a number of clinically important secondary endpoints, including pulmonary function measurements and symptomatic scores. Only the time to exacerbation was significantly longer in the mepolizumab group in METREX.
Taken together, this calls into question the conclusion that a decrease in eosinophil counts due to mepolizumab has resulted in a lower rate of exacerbations, particularly as a higher dose of mepolizumab did not result in a stronger effect. The lack of difference between groups in secondary endpoints is also concerning, as those would be expected to improve with a decrease in exacerbations [4,5]. As the authors point out, their evidence suggests that eosinophils may be an important biomarker in COPD and may aid in the therapeutic decision-making process. However, given the inconsistencies in the data as noted above, it would be difficult to rely on the evidence from this study alone to support their conclusion regarding the clinical utility of mepolizumab in COPD.
The authors discuss a number of limitations that may account for the lack of consistent effect seen in this study. Aside from the standard limitations applicable to any clinical trial, they note the potential confounding effect of previous oral glucocorticoid therapy in reducing eosinophil counts. This may have masked the eosinophilic phenotype in some study patients, leading to the attenuated effect of mepolizumab seen in this study.
The authors also note that information that might be potentially valuable for identifying treatment responders, such as a history of allergies and atopy, were not available. Inclusion of those patients may be helpful in enriching the trial with potential treatment-responders, and future studies may benefit from focusing on COPD patients with a more atopic phenotype who more closely resemble those with the asthma-COPD overlap syndrome.
A final limitation to discuss is the focus on blood eosinophilic counts. Due to the difficulty of measuring sputum eosinophils, and the reasonable degree of correlation between blood and sputum in asthmatic patients, blood eosinophils have largely supplanted sputum eosinophils as markers of TH2 CD4 T-cell activity in the pulmonary system [6]. This substitution is also used in the COPD population, however, due to the differences in pathophysiology it is unclear if eosinophils in asthmatic patients behave similarly to those in COPD patients [7]. Additionally, the cutoff of 150 cells/uL has been obtained primarily from sub-group analysis of previous studies on COPD patients, but it is unclear if this cutoff truly reflects elevated sputum eosinophilia. While there is likely some degree of correlation between blood and sputum eosinophilia in COPD patients, a lack of significant effect seen in this study may be due to an incorrect cutoff for elevated eosinophilia and a reliance on blood eosinophils over sputum counts. Further studies utilizing sputum eosinophils may be of value in addressing this limitation.
Applications for Clinical Practice
In this study, Pavord and colleagues found a potential benefit of mepolizumab treatment for reducing exacerbations in COPD patients with an eosinophilic phenotype. The conflicting results regarding the underlying physiology and the weak treatment effect suggest this medication may not be ready for use in clinical practice without additional supporting evidence. From a practical standpoint, the high cost of medication (~$2500 per month) and marginal benefit of treatment imply that treatment with mepolizumab in COPD patients may not be cost-effective, and even treatment in individual patients on a trial basis should be discouraged until additional supporting data becomes available. Of primary concern are the optimal selection of COPD patients that will achieve benefit with mepolizumab treatment, and the optimal dose of medication to achieve that benefit. The results presented here do not satisfactorily answer these questions, and additional studies are required.
—Arun Jose, MD, The George Washington University, Washington, DC
1. Pelaia C, Vatrella A, Busceti MT, et al. Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des Devel Ther 2017;11:3137–44.
2. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017;50:pii:1700853.
3. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med 2017;195:1189–97.
4. Halpin DMG, Decramer M, Celli BR, et al. Effect of a single exacerbation on decline in lung function in COPD. Respir Med 2017;128:85–91.
5. Rassouli F, Baty F, Stolz D, et al. Longitudinal change of COPD assessment test (CAT in a telehealthcare cohort is associated with exacerbation risk. Int J COPD 2017;12:3103–9.
6. Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med 2015;192:660–8.
7. Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J COPD 2016;11:1495–504.
Study Overview
Objective. To determine the effect of mepolizumab on the annual rate of chronic obstructive pulmonary disease (COPD) exacerbations in high-risk patients.
Design. Two randomized double-blind placebo-controlled parallel trials (METREO and METREX).
Setting and participants. Participants were recruited from over 15 countries in over 100 investigative sites. Inclusion criteria were adults (40 years or older) with a diagnosis of COPD for at least 1 year with: airflow limitation (FEV1/FVC < 0.7); some bronchodilator reversibility (post-bronchodilator FEV1 > 20% and ≤ 80% of predicted values); current COPD therapy for at least 3 months prior to enrollment (a high-dose inhaled corticosteroid, ICS, with at least 2 other classes of medications, to obtain “triple therapy”); and a high risk of exacerbations (at least 1 severe [requiring hospitalization] or 2 moderate [treatment with systemic corticosteroids and/or antibiotics] exacerbations in past year).
Notable exclusion criteria were patients with diagnoses of asthma in never-smokers, alpha-1 antitrypsin deficiency, recent exacerbations (in past month), lung volume reduction surgery (in past year), eosinophilic or parasitic diseases, or those with recent monoclonal antibody treatment. Patients with the asthma-COPD overlap syndrome were included only if they had a history of smoking and met the COPD inclusion criteria listed above.
Intervention. The treatment period lasted for a total of 52 weeks, with an additional 8 weeks of follow-up. Patients were randomized 1:1 to placebo or low-dose medication (100 mg) using permuted-block randomization in the METREX study regardless of eosinophil count (but they were stratified for a modified intention-to-treat analysis at screening into either low eosinophilic count [< 150 cells/uL] or high [≥ 150 cells/uL]). In the METREO study, patients were randomized 1:1:1 to placebo, low-dose (100 mg), or high-dose (300 mg) medication only if blood eosinophilia was present (≥ 150 cells/uL at screening or ≥ 300 cells/uL in past 12 months). Investigators and patients were blinded to presence of drug or placebo. Sample size calculations indicated that in order to provide a 90% power to detect a 30% decrease in the rate of exacerbations in METREX and 35% decrease in METREO, a total of 800 patients and 660 patients would need to be enrolled in METREX and METREO respectively. Both studies met their enrollment quota.
Main outcome measures. The primary outcome was the annual rate of exacerbations that were either moderate (requiring systemic corticosteroids and/or antibiotics) or severe (requiring hospitalization). Secondary outcomes included the time to first moderate/severe exacerbation, change from baseline in the COPD Assessment Test (CAT) and St. George’s Respiratory Questionnaire (SGRQ), and change from baseline in blood eosinophil count, FEV1, and FVC. Safety and adverse events endpoints were also assessed.
A modified intention-to-treat analysis was performed overall and in the METREX study stratified on eosinophilic count at screening; all patients who underwent randomization and received at least one dose of medication or placebo were included in that respective group. Multiple comparisons were accounted for using the Benjamini-Hochberg Test, exacerbations were assumed to follow a negative binomial distribution, and Cox proportional-hazards was used to model the relationship between covariates of interest and the primary outcome.
Main results. In the METREX study, 1161 patients were enrolled and 836 underwent randomization and received at least 1 dose of medication or placebo. In METREO, 1071 patients were enrolled and 674 underwent randomization and received at least one dose of medication or placebo. In both studies the patients in the medication and placebo groups were well balanced at baseline across demographics (age, gender, smoking history, duration of COPD) and pulmonary function (FEV1, FVC, FEV1/FVC, CAT, SGRQ). In METREX, a total of 462 (55%) patients had an eosinophilic phenotype and 374 (45%) did not.
There was no difference between groups in the primary endpoint of annual exacerbation rate in METREO (1.49/yr in placebo vs. 1.19/yr in low-dose and 1.27/yr in high-dose mepolizumab, rate ratio of high-dose to placebo 0.86, 95% confidence interval [CI] 0.7–1.05, P = 0.14). There was no difference in the primary outcome in the overall intention-to-treat analysis in the METREX study (1.49/yr in mepolizumab vs. 1.52/yr in placebo, P > 0.99). Only when analyzing the high eosinophilic phenotype in the stratified intention-to-treat METREX group was there a significant difference in the primary outcome (1.41/yr in mepolizumab vs. 1.71/yr in placebo, P = 0.04, rate ratio 0.82, 95% CI 0.68–0.98).
There were no significant differences in any secondary endpoint in the METREO study. In the METREX study, mepolizumab treatment resulted in a significantly longer time to first exacerbation (192 days vs. 141 days, hazard ratio 0.75, 95% CI 0.60–0.94, P = 0.04) but no difference in the change in SGRQ (–2.8 vs. –3.0, P > 0.99) or CAT score (–0.8 vs. 0, P > 0.99). There was no significant difference in any measures of pulmonary function between the treatment and placebo groups (FEV1, FVC, FEV1/FVC). As expected, there was a significant decrease in peripheral blood eosinophil count in both studies in the medication arm. The incidence of adverse events and safety endpoints were similar between the trial groups in METREX and METREO.
Conclusions. In this pair of placebo-controlled double-blind randomized parallel studies, there was a significant decline in annual exacerbation rate in patients with an eosinophilic phenotype treated with mepolizumab in a stratified intention-to-treat analysis of one of two parallel studies (METREX). However, there was no significant difference in the primary outcome of the other parallel study (METREO), which included only those patients with an eosinophilic phenotype. Additionally, there was no significant difference in any secondary endpoints in either study. The medication was generally safe and well tolerated.
Commentary
Mepolizumab is a humanized monoclonal antibody that targets and blocks interleukin-5, a key mediator of eosinophilic activity. Due to its ability to decrease eosinophil number and function, it is currently approved as a therapy for severe asthma with an eosinophilic phenotype [1]. While asthma and COPD have historically been thought of as separate entities with distinct pathophysiologic mechanisms, recent evidence has suggested that a subset of COPD patients experience significant eosinophilic inflammation. This group may behave more like asthmatic patients, and may have a different response to medications such as inhaled corticosteroids, but the role of eosinophils to guide prognostication and treatment in this group is still unclear [2,3].
In this study, Pavord and colleagues investigated the use of the anti-IL5 drug mepolizumab in COPD patients at risk of exacerbations who demonstrated an eosinophilic phenotype. The physiologic rationale for the study was that eosinophilic inflammation is thought to be a driver of exacerbations in COPD patients with an eosinophilic phenotype, and therefore a decrease in eosinophilic number and function should result in a decrease in exacerbations. The authors conducted a well-designed placebo-controlled double-blind study with a clearly defined endpoint, met their enrollment goals as determined by their power calculations, and used COPD patients at high risk of exacerbations to enrich their study.
There was no difference in the primary outcome in the METREO arm of the study, which included patients with baseline eosinophilia (> 150 cells/uL) or in the overall intention-to-treat analysis in METREX (which did not screen patients on baseline eosinophil count). Only when stratified on baseline eosinophil count in the METREX study was a significant treatment effect found, where patients with high eosinophil count at baseline (> 150 cells/uL) had a decreased risk of exacerbations when treated with mepolizumab. Notably there was no difference in any secondary outcome in METREO or in METREX aside from a longer time to first exacerbation in METREX in the mepolizumab group. The authors use this data to conclude that mepolizumab treatment results in a lower rate of exacerbations and a longer time to the first exacerbation in COPD patients with an eosinophilic phenotype, and the extent of the treatment effect is related to blood eosinophil counts.
The authors conducted a well-designed and rigorous study, and used robust and appropriate statistical analysis; however, significant questions remain regarding their conclusions. The primary concern is the role of mepolizumab in the treatment of COPD patients to decrease exacerbations may be overstated. When including only those with baseline eosinophilia in the METREO arm, there was no significant difference between placebo and low or high dose of mepolizumab; however, there was an appropriate and expected decrease in blood eosinophils, indicating the medication worked as intended. In the overall intention-to-treat analysis in the METREX arm, there was no difference between mepolizumab and placebo, and only in the analysis of METREX stratified to eosinophil count was there a significant difference (with an upper confidence interval rate ratio [0.98] approaching unity).
Additionally there was no significant difference between the 2 groups across a number of clinically important secondary endpoints, including pulmonary function measurements and symptomatic scores. Only the time to exacerbation was significantly longer in the mepolizumab group in METREX.
Taken together, this calls into question the conclusion that a decrease in eosinophil counts due to mepolizumab has resulted in a lower rate of exacerbations, particularly as a higher dose of mepolizumab did not result in a stronger effect. The lack of difference between groups in secondary endpoints is also concerning, as those would be expected to improve with a decrease in exacerbations [4,5]. As the authors point out, their evidence suggests that eosinophils may be an important biomarker in COPD and may aid in the therapeutic decision-making process. However, given the inconsistencies in the data as noted above, it would be difficult to rely on the evidence from this study alone to support their conclusion regarding the clinical utility of mepolizumab in COPD.
The authors discuss a number of limitations that may account for the lack of consistent effect seen in this study. Aside from the standard limitations applicable to any clinical trial, they note the potential confounding effect of previous oral glucocorticoid therapy in reducing eosinophil counts. This may have masked the eosinophilic phenotype in some study patients, leading to the attenuated effect of mepolizumab seen in this study.
The authors also note that information that might be potentially valuable for identifying treatment responders, such as a history of allergies and atopy, were not available. Inclusion of those patients may be helpful in enriching the trial with potential treatment-responders, and future studies may benefit from focusing on COPD patients with a more atopic phenotype who more closely resemble those with the asthma-COPD overlap syndrome.
A final limitation to discuss is the focus on blood eosinophilic counts. Due to the difficulty of measuring sputum eosinophils, and the reasonable degree of correlation between blood and sputum in asthmatic patients, blood eosinophils have largely supplanted sputum eosinophils as markers of TH2 CD4 T-cell activity in the pulmonary system [6]. This substitution is also used in the COPD population, however, due to the differences in pathophysiology it is unclear if eosinophils in asthmatic patients behave similarly to those in COPD patients [7]. Additionally, the cutoff of 150 cells/uL has been obtained primarily from sub-group analysis of previous studies on COPD patients, but it is unclear if this cutoff truly reflects elevated sputum eosinophilia. While there is likely some degree of correlation between blood and sputum eosinophilia in COPD patients, a lack of significant effect seen in this study may be due to an incorrect cutoff for elevated eosinophilia and a reliance on blood eosinophils over sputum counts. Further studies utilizing sputum eosinophils may be of value in addressing this limitation.
Applications for Clinical Practice
In this study, Pavord and colleagues found a potential benefit of mepolizumab treatment for reducing exacerbations in COPD patients with an eosinophilic phenotype. The conflicting results regarding the underlying physiology and the weak treatment effect suggest this medication may not be ready for use in clinical practice without additional supporting evidence. From a practical standpoint, the high cost of medication (~$2500 per month) and marginal benefit of treatment imply that treatment with mepolizumab in COPD patients may not be cost-effective, and even treatment in individual patients on a trial basis should be discouraged until additional supporting data becomes available. Of primary concern are the optimal selection of COPD patients that will achieve benefit with mepolizumab treatment, and the optimal dose of medication to achieve that benefit. The results presented here do not satisfactorily answer these questions, and additional studies are required.
—Arun Jose, MD, The George Washington University, Washington, DC
Study Overview
Objective. To determine the effect of mepolizumab on the annual rate of chronic obstructive pulmonary disease (COPD) exacerbations in high-risk patients.
Design. Two randomized double-blind placebo-controlled parallel trials (METREO and METREX).
Setting and participants. Participants were recruited from over 15 countries in over 100 investigative sites. Inclusion criteria were adults (40 years or older) with a diagnosis of COPD for at least 1 year with: airflow limitation (FEV1/FVC < 0.7); some bronchodilator reversibility (post-bronchodilator FEV1 > 20% and ≤ 80% of predicted values); current COPD therapy for at least 3 months prior to enrollment (a high-dose inhaled corticosteroid, ICS, with at least 2 other classes of medications, to obtain “triple therapy”); and a high risk of exacerbations (at least 1 severe [requiring hospitalization] or 2 moderate [treatment with systemic corticosteroids and/or antibiotics] exacerbations in past year).
Notable exclusion criteria were patients with diagnoses of asthma in never-smokers, alpha-1 antitrypsin deficiency, recent exacerbations (in past month), lung volume reduction surgery (in past year), eosinophilic or parasitic diseases, or those with recent monoclonal antibody treatment. Patients with the asthma-COPD overlap syndrome were included only if they had a history of smoking and met the COPD inclusion criteria listed above.
Intervention. The treatment period lasted for a total of 52 weeks, with an additional 8 weeks of follow-up. Patients were randomized 1:1 to placebo or low-dose medication (100 mg) using permuted-block randomization in the METREX study regardless of eosinophil count (but they were stratified for a modified intention-to-treat analysis at screening into either low eosinophilic count [< 150 cells/uL] or high [≥ 150 cells/uL]). In the METREO study, patients were randomized 1:1:1 to placebo, low-dose (100 mg), or high-dose (300 mg) medication only if blood eosinophilia was present (≥ 150 cells/uL at screening or ≥ 300 cells/uL in past 12 months). Investigators and patients were blinded to presence of drug or placebo. Sample size calculations indicated that in order to provide a 90% power to detect a 30% decrease in the rate of exacerbations in METREX and 35% decrease in METREO, a total of 800 patients and 660 patients would need to be enrolled in METREX and METREO respectively. Both studies met their enrollment quota.
Main outcome measures. The primary outcome was the annual rate of exacerbations that were either moderate (requiring systemic corticosteroids and/or antibiotics) or severe (requiring hospitalization). Secondary outcomes included the time to first moderate/severe exacerbation, change from baseline in the COPD Assessment Test (CAT) and St. George’s Respiratory Questionnaire (SGRQ), and change from baseline in blood eosinophil count, FEV1, and FVC. Safety and adverse events endpoints were also assessed.
A modified intention-to-treat analysis was performed overall and in the METREX study stratified on eosinophilic count at screening; all patients who underwent randomization and received at least one dose of medication or placebo were included in that respective group. Multiple comparisons were accounted for using the Benjamini-Hochberg Test, exacerbations were assumed to follow a negative binomial distribution, and Cox proportional-hazards was used to model the relationship between covariates of interest and the primary outcome.
Main results. In the METREX study, 1161 patients were enrolled and 836 underwent randomization and received at least 1 dose of medication or placebo. In METREO, 1071 patients were enrolled and 674 underwent randomization and received at least one dose of medication or placebo. In both studies the patients in the medication and placebo groups were well balanced at baseline across demographics (age, gender, smoking history, duration of COPD) and pulmonary function (FEV1, FVC, FEV1/FVC, CAT, SGRQ). In METREX, a total of 462 (55%) patients had an eosinophilic phenotype and 374 (45%) did not.
There was no difference between groups in the primary endpoint of annual exacerbation rate in METREO (1.49/yr in placebo vs. 1.19/yr in low-dose and 1.27/yr in high-dose mepolizumab, rate ratio of high-dose to placebo 0.86, 95% confidence interval [CI] 0.7–1.05, P = 0.14). There was no difference in the primary outcome in the overall intention-to-treat analysis in the METREX study (1.49/yr in mepolizumab vs. 1.52/yr in placebo, P > 0.99). Only when analyzing the high eosinophilic phenotype in the stratified intention-to-treat METREX group was there a significant difference in the primary outcome (1.41/yr in mepolizumab vs. 1.71/yr in placebo, P = 0.04, rate ratio 0.82, 95% CI 0.68–0.98).
There were no significant differences in any secondary endpoint in the METREO study. In the METREX study, mepolizumab treatment resulted in a significantly longer time to first exacerbation (192 days vs. 141 days, hazard ratio 0.75, 95% CI 0.60–0.94, P = 0.04) but no difference in the change in SGRQ (–2.8 vs. –3.0, P > 0.99) or CAT score (–0.8 vs. 0, P > 0.99). There was no significant difference in any measures of pulmonary function between the treatment and placebo groups (FEV1, FVC, FEV1/FVC). As expected, there was a significant decrease in peripheral blood eosinophil count in both studies in the medication arm. The incidence of adverse events and safety endpoints were similar between the trial groups in METREX and METREO.
Conclusions. In this pair of placebo-controlled double-blind randomized parallel studies, there was a significant decline in annual exacerbation rate in patients with an eosinophilic phenotype treated with mepolizumab in a stratified intention-to-treat analysis of one of two parallel studies (METREX). However, there was no significant difference in the primary outcome of the other parallel study (METREO), which included only those patients with an eosinophilic phenotype. Additionally, there was no significant difference in any secondary endpoints in either study. The medication was generally safe and well tolerated.
Commentary
Mepolizumab is a humanized monoclonal antibody that targets and blocks interleukin-5, a key mediator of eosinophilic activity. Due to its ability to decrease eosinophil number and function, it is currently approved as a therapy for severe asthma with an eosinophilic phenotype [1]. While asthma and COPD have historically been thought of as separate entities with distinct pathophysiologic mechanisms, recent evidence has suggested that a subset of COPD patients experience significant eosinophilic inflammation. This group may behave more like asthmatic patients, and may have a different response to medications such as inhaled corticosteroids, but the role of eosinophils to guide prognostication and treatment in this group is still unclear [2,3].
In this study, Pavord and colleagues investigated the use of the anti-IL5 drug mepolizumab in COPD patients at risk of exacerbations who demonstrated an eosinophilic phenotype. The physiologic rationale for the study was that eosinophilic inflammation is thought to be a driver of exacerbations in COPD patients with an eosinophilic phenotype, and therefore a decrease in eosinophilic number and function should result in a decrease in exacerbations. The authors conducted a well-designed placebo-controlled double-blind study with a clearly defined endpoint, met their enrollment goals as determined by their power calculations, and used COPD patients at high risk of exacerbations to enrich their study.
There was no difference in the primary outcome in the METREO arm of the study, which included patients with baseline eosinophilia (> 150 cells/uL) or in the overall intention-to-treat analysis in METREX (which did not screen patients on baseline eosinophil count). Only when stratified on baseline eosinophil count in the METREX study was a significant treatment effect found, where patients with high eosinophil count at baseline (> 150 cells/uL) had a decreased risk of exacerbations when treated with mepolizumab. Notably there was no difference in any secondary outcome in METREO or in METREX aside from a longer time to first exacerbation in METREX in the mepolizumab group. The authors use this data to conclude that mepolizumab treatment results in a lower rate of exacerbations and a longer time to the first exacerbation in COPD patients with an eosinophilic phenotype, and the extent of the treatment effect is related to blood eosinophil counts.
The authors conducted a well-designed and rigorous study, and used robust and appropriate statistical analysis; however, significant questions remain regarding their conclusions. The primary concern is the role of mepolizumab in the treatment of COPD patients to decrease exacerbations may be overstated. When including only those with baseline eosinophilia in the METREO arm, there was no significant difference between placebo and low or high dose of mepolizumab; however, there was an appropriate and expected decrease in blood eosinophils, indicating the medication worked as intended. In the overall intention-to-treat analysis in the METREX arm, there was no difference between mepolizumab and placebo, and only in the analysis of METREX stratified to eosinophil count was there a significant difference (with an upper confidence interval rate ratio [0.98] approaching unity).
Additionally there was no significant difference between the 2 groups across a number of clinically important secondary endpoints, including pulmonary function measurements and symptomatic scores. Only the time to exacerbation was significantly longer in the mepolizumab group in METREX.
Taken together, this calls into question the conclusion that a decrease in eosinophil counts due to mepolizumab has resulted in a lower rate of exacerbations, particularly as a higher dose of mepolizumab did not result in a stronger effect. The lack of difference between groups in secondary endpoints is also concerning, as those would be expected to improve with a decrease in exacerbations [4,5]. As the authors point out, their evidence suggests that eosinophils may be an important biomarker in COPD and may aid in the therapeutic decision-making process. However, given the inconsistencies in the data as noted above, it would be difficult to rely on the evidence from this study alone to support their conclusion regarding the clinical utility of mepolizumab in COPD.
The authors discuss a number of limitations that may account for the lack of consistent effect seen in this study. Aside from the standard limitations applicable to any clinical trial, they note the potential confounding effect of previous oral glucocorticoid therapy in reducing eosinophil counts. This may have masked the eosinophilic phenotype in some study patients, leading to the attenuated effect of mepolizumab seen in this study.
The authors also note that information that might be potentially valuable for identifying treatment responders, such as a history of allergies and atopy, were not available. Inclusion of those patients may be helpful in enriching the trial with potential treatment-responders, and future studies may benefit from focusing on COPD patients with a more atopic phenotype who more closely resemble those with the asthma-COPD overlap syndrome.
A final limitation to discuss is the focus on blood eosinophilic counts. Due to the difficulty of measuring sputum eosinophils, and the reasonable degree of correlation between blood and sputum in asthmatic patients, blood eosinophils have largely supplanted sputum eosinophils as markers of TH2 CD4 T-cell activity in the pulmonary system [6]. This substitution is also used in the COPD population, however, due to the differences in pathophysiology it is unclear if eosinophils in asthmatic patients behave similarly to those in COPD patients [7]. Additionally, the cutoff of 150 cells/uL has been obtained primarily from sub-group analysis of previous studies on COPD patients, but it is unclear if this cutoff truly reflects elevated sputum eosinophilia. While there is likely some degree of correlation between blood and sputum eosinophilia in COPD patients, a lack of significant effect seen in this study may be due to an incorrect cutoff for elevated eosinophilia and a reliance on blood eosinophils over sputum counts. Further studies utilizing sputum eosinophils may be of value in addressing this limitation.
Applications for Clinical Practice
In this study, Pavord and colleagues found a potential benefit of mepolizumab treatment for reducing exacerbations in COPD patients with an eosinophilic phenotype. The conflicting results regarding the underlying physiology and the weak treatment effect suggest this medication may not be ready for use in clinical practice without additional supporting evidence. From a practical standpoint, the high cost of medication (~$2500 per month) and marginal benefit of treatment imply that treatment with mepolizumab in COPD patients may not be cost-effective, and even treatment in individual patients on a trial basis should be discouraged until additional supporting data becomes available. Of primary concern are the optimal selection of COPD patients that will achieve benefit with mepolizumab treatment, and the optimal dose of medication to achieve that benefit. The results presented here do not satisfactorily answer these questions, and additional studies are required.
—Arun Jose, MD, The George Washington University, Washington, DC
1. Pelaia C, Vatrella A, Busceti MT, et al. Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des Devel Ther 2017;11:3137–44.
2. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017;50:pii:1700853.
3. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med 2017;195:1189–97.
4. Halpin DMG, Decramer M, Celli BR, et al. Effect of a single exacerbation on decline in lung function in COPD. Respir Med 2017;128:85–91.
5. Rassouli F, Baty F, Stolz D, et al. Longitudinal change of COPD assessment test (CAT in a telehealthcare cohort is associated with exacerbation risk. Int J COPD 2017;12:3103–9.
6. Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med 2015;192:660–8.
7. Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J COPD 2016;11:1495–504.
1. Pelaia C, Vatrella A, Busceti MT, et al. Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des Devel Ther 2017;11:3137–44.
2. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017;50:pii:1700853.
3. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med 2017;195:1189–97.
4. Halpin DMG, Decramer M, Celli BR, et al. Effect of a single exacerbation on decline in lung function in COPD. Respir Med 2017;128:85–91.
5. Rassouli F, Baty F, Stolz D, et al. Longitudinal change of COPD assessment test (CAT in a telehealthcare cohort is associated with exacerbation risk. Int J COPD 2017;12:3103–9.
6. Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med 2015;192:660–8.
7. Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J COPD 2016;11:1495–504.