User login
Online education program linked to less anxiety, medication use in colonoscopies
An online education program appeared to dampen anxiety levels in colonoscopy patients and even seemed to reduce the burden of the procedure in terms of time and medication used, a study showed.
“The program was very well received and generally improved patient’s anxiety,” said lead author Siddhartha Parker, MD, a gastroenterologist at Dartmouth-Hitchcock Medical Center, Lebanon, N.H. “Only 4% of patients described feeling more anxious after watching the program whereas 58% felt less anxious.”
“Patients who are afraid or anxious may require more sedation, experience more pain, and, most importantly, may be less likely to have a procedure done,” he said. “Some patients don’t schedule at all, while others may schedule and then cancel last minute or no-show an appointment, which has its own implications and costs to the health care system.”
In terms of anxiety prevention, he said, “primarily we rely on nurses and doctors to discuss the need for screening and provide reassurance about low risks and real benefits.”
For the current study, published in the Journal of Clinical Gastroenterology, researchers tested a Web-based colonoscopy education program from a company called Emmi Solutions.
The program is designed to provide details about the colonoscopy process. “It covers what to expect before, during, and after the procedure including possible findings (e.g., polyps),” Dr. Parker said. “It also covers risks and benefits with some tips on recovery after the procedure. I think having some dedicated time to understand the procedure is most helpful. It is rare that a patient gets 15-20 minutes to review any topic with their doctor, and the Emmi program has the added benefit of visual cues.”
The researchers recruited and randomly assigned 51 patients to a control group and 52 patients to the intervention. All were scheduled to undergo colonoscopies within at least 2 weeks.
The groups were similar: Most patients were white (100% of the control group, 89% of the intervention group), and most were female; the average age was 48 years The intervention group had more education, at 69% with college degrees vs. 43% in the control group (P = .01).
Patients in the control group received usual colonoscopy education materials, while those in the intervention group were also directed to the education website.
All participants took a survey immediately before their procedures and received a $10 gift card as reimbursement for their participation.
Researchers found that patients in the intervention group scored higher than did those in the control group on questions about colonoscopy knowledge (82% of questions correct vs. 74%, P less than .001).
Also, the education website may have convinced its viewers to worry less about the procedure and more about its ultimate findings. Compared with the control group, those in the intervention group were more likely to say they were most concerned about the findings (38.5% vs. 21.6%) and less likely to say they were more concerned about both the findings and the procedure (38.5% vs. 56.9%). Roughly 20% of those in both groups said they were most concerned about the procedure.
“Some patients report being more concerned about findings, e.g., cancer, before the program but more anxious about complications after the program,” Dr. Parker said. “I think that is a reasonable outcome as asymptomatic patients should have very little concern about findings, given the goal is to find and remove polyps.”
Researchers also tracked the use of sedatives during the procedure. Those in the intervention group required less midazolam (Versed) (average dose of 3.66 mg vs. 4.46 mg, P = .004). They also required less fentanyl (164 mcg vs. 186 mcg), but this finding was not statistically significant (P = .063).
“There was also a trend, not quite statistically significant, to improved prep quality,” Dr. Parker said. (In the intervention vs. the control group, 96% vs. 88% of subjects were rated as good or excellent in terms of prep quality, P = .27).
“This is important because a poor or inadequate prep leads to a need for a repeat procedure to ensure adequate screening,” he said. “If prep quality improves, that means fewer unnecessary repeated procedures.”
The researchers reported study limitations such as multiple endoscopists and sedation nurses and the variation in education levels of the participants.
The cost of the interactive program was not available.
According to Dr. Parker, Dartmouth-Hitchcock Medical Center now offers the education program to all colonoscopy patients, and a modified version is available for those undergoing upper endoscopies.
“Not all patients have adequate computer resources, and some patients simply choose not to watch,” he said. “But my impression is that those who watched the program are more relaxed during the informed consent process. However, we are not actively tracking outcomes such as procedure time or medication usage.”
No more studies of the program are planned at the institution, he said.
One of the study authors is an employee of Emmi Solutions, which provided the program for free for use in the study. The other authors reported no relevant disclosures. No study funding was reported.
SOURCE: Parker S et al. J Clin Gastroenterol. doi: 10.1097/MCG.0000000000000958
An online education program appeared to dampen anxiety levels in colonoscopy patients and even seemed to reduce the burden of the procedure in terms of time and medication used, a study showed.
“The program was very well received and generally improved patient’s anxiety,” said lead author Siddhartha Parker, MD, a gastroenterologist at Dartmouth-Hitchcock Medical Center, Lebanon, N.H. “Only 4% of patients described feeling more anxious after watching the program whereas 58% felt less anxious.”
“Patients who are afraid or anxious may require more sedation, experience more pain, and, most importantly, may be less likely to have a procedure done,” he said. “Some patients don’t schedule at all, while others may schedule and then cancel last minute or no-show an appointment, which has its own implications and costs to the health care system.”
In terms of anxiety prevention, he said, “primarily we rely on nurses and doctors to discuss the need for screening and provide reassurance about low risks and real benefits.”
For the current study, published in the Journal of Clinical Gastroenterology, researchers tested a Web-based colonoscopy education program from a company called Emmi Solutions.
The program is designed to provide details about the colonoscopy process. “It covers what to expect before, during, and after the procedure including possible findings (e.g., polyps),” Dr. Parker said. “It also covers risks and benefits with some tips on recovery after the procedure. I think having some dedicated time to understand the procedure is most helpful. It is rare that a patient gets 15-20 minutes to review any topic with their doctor, and the Emmi program has the added benefit of visual cues.”
The researchers recruited and randomly assigned 51 patients to a control group and 52 patients to the intervention. All were scheduled to undergo colonoscopies within at least 2 weeks.
The groups were similar: Most patients were white (100% of the control group, 89% of the intervention group), and most were female; the average age was 48 years The intervention group had more education, at 69% with college degrees vs. 43% in the control group (P = .01).
Patients in the control group received usual colonoscopy education materials, while those in the intervention group were also directed to the education website.
All participants took a survey immediately before their procedures and received a $10 gift card as reimbursement for their participation.
Researchers found that patients in the intervention group scored higher than did those in the control group on questions about colonoscopy knowledge (82% of questions correct vs. 74%, P less than .001).
Also, the education website may have convinced its viewers to worry less about the procedure and more about its ultimate findings. Compared with the control group, those in the intervention group were more likely to say they were most concerned about the findings (38.5% vs. 21.6%) and less likely to say they were more concerned about both the findings and the procedure (38.5% vs. 56.9%). Roughly 20% of those in both groups said they were most concerned about the procedure.
“Some patients report being more concerned about findings, e.g., cancer, before the program but more anxious about complications after the program,” Dr. Parker said. “I think that is a reasonable outcome as asymptomatic patients should have very little concern about findings, given the goal is to find and remove polyps.”
Researchers also tracked the use of sedatives during the procedure. Those in the intervention group required less midazolam (Versed) (average dose of 3.66 mg vs. 4.46 mg, P = .004). They also required less fentanyl (164 mcg vs. 186 mcg), but this finding was not statistically significant (P = .063).
“There was also a trend, not quite statistically significant, to improved prep quality,” Dr. Parker said. (In the intervention vs. the control group, 96% vs. 88% of subjects were rated as good or excellent in terms of prep quality, P = .27).
“This is important because a poor or inadequate prep leads to a need for a repeat procedure to ensure adequate screening,” he said. “If prep quality improves, that means fewer unnecessary repeated procedures.”
The researchers reported study limitations such as multiple endoscopists and sedation nurses and the variation in education levels of the participants.
The cost of the interactive program was not available.
According to Dr. Parker, Dartmouth-Hitchcock Medical Center now offers the education program to all colonoscopy patients, and a modified version is available for those undergoing upper endoscopies.
“Not all patients have adequate computer resources, and some patients simply choose not to watch,” he said. “But my impression is that those who watched the program are more relaxed during the informed consent process. However, we are not actively tracking outcomes such as procedure time or medication usage.”
No more studies of the program are planned at the institution, he said.
One of the study authors is an employee of Emmi Solutions, which provided the program for free for use in the study. The other authors reported no relevant disclosures. No study funding was reported.
SOURCE: Parker S et al. J Clin Gastroenterol. doi: 10.1097/MCG.0000000000000958
An online education program appeared to dampen anxiety levels in colonoscopy patients and even seemed to reduce the burden of the procedure in terms of time and medication used, a study showed.
“The program was very well received and generally improved patient’s anxiety,” said lead author Siddhartha Parker, MD, a gastroenterologist at Dartmouth-Hitchcock Medical Center, Lebanon, N.H. “Only 4% of patients described feeling more anxious after watching the program whereas 58% felt less anxious.”
“Patients who are afraid or anxious may require more sedation, experience more pain, and, most importantly, may be less likely to have a procedure done,” he said. “Some patients don’t schedule at all, while others may schedule and then cancel last minute or no-show an appointment, which has its own implications and costs to the health care system.”
In terms of anxiety prevention, he said, “primarily we rely on nurses and doctors to discuss the need for screening and provide reassurance about low risks and real benefits.”
For the current study, published in the Journal of Clinical Gastroenterology, researchers tested a Web-based colonoscopy education program from a company called Emmi Solutions.
The program is designed to provide details about the colonoscopy process. “It covers what to expect before, during, and after the procedure including possible findings (e.g., polyps),” Dr. Parker said. “It also covers risks and benefits with some tips on recovery after the procedure. I think having some dedicated time to understand the procedure is most helpful. It is rare that a patient gets 15-20 minutes to review any topic with their doctor, and the Emmi program has the added benefit of visual cues.”
The researchers recruited and randomly assigned 51 patients to a control group and 52 patients to the intervention. All were scheduled to undergo colonoscopies within at least 2 weeks.
The groups were similar: Most patients were white (100% of the control group, 89% of the intervention group), and most were female; the average age was 48 years The intervention group had more education, at 69% with college degrees vs. 43% in the control group (P = .01).
Patients in the control group received usual colonoscopy education materials, while those in the intervention group were also directed to the education website.
All participants took a survey immediately before their procedures and received a $10 gift card as reimbursement for their participation.
Researchers found that patients in the intervention group scored higher than did those in the control group on questions about colonoscopy knowledge (82% of questions correct vs. 74%, P less than .001).
Also, the education website may have convinced its viewers to worry less about the procedure and more about its ultimate findings. Compared with the control group, those in the intervention group were more likely to say they were most concerned about the findings (38.5% vs. 21.6%) and less likely to say they were more concerned about both the findings and the procedure (38.5% vs. 56.9%). Roughly 20% of those in both groups said they were most concerned about the procedure.
“Some patients report being more concerned about findings, e.g., cancer, before the program but more anxious about complications after the program,” Dr. Parker said. “I think that is a reasonable outcome as asymptomatic patients should have very little concern about findings, given the goal is to find and remove polyps.”
Researchers also tracked the use of sedatives during the procedure. Those in the intervention group required less midazolam (Versed) (average dose of 3.66 mg vs. 4.46 mg, P = .004). They also required less fentanyl (164 mcg vs. 186 mcg), but this finding was not statistically significant (P = .063).
“There was also a trend, not quite statistically significant, to improved prep quality,” Dr. Parker said. (In the intervention vs. the control group, 96% vs. 88% of subjects were rated as good or excellent in terms of prep quality, P = .27).
“This is important because a poor or inadequate prep leads to a need for a repeat procedure to ensure adequate screening,” he said. “If prep quality improves, that means fewer unnecessary repeated procedures.”
The researchers reported study limitations such as multiple endoscopists and sedation nurses and the variation in education levels of the participants.
The cost of the interactive program was not available.
According to Dr. Parker, Dartmouth-Hitchcock Medical Center now offers the education program to all colonoscopy patients, and a modified version is available for those undergoing upper endoscopies.
“Not all patients have adequate computer resources, and some patients simply choose not to watch,” he said. “But my impression is that those who watched the program are more relaxed during the informed consent process. However, we are not actively tracking outcomes such as procedure time or medication usage.”
No more studies of the program are planned at the institution, he said.
One of the study authors is an employee of Emmi Solutions, which provided the program for free for use in the study. The other authors reported no relevant disclosures. No study funding was reported.
SOURCE: Parker S et al. J Clin Gastroenterol. doi: 10.1097/MCG.0000000000000958
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point:
Major finding: Among program users, 58% reported being less anxious and required less midazolam during their procedures (average dose of 3.66 mg vs. 4.46 mg, P = .004).
Study details: A randomized, prospective study of 51 patients (control group) and 52 patients assigned to visit a website for a multimedia education program prior to colonoscopy.
Disclosures: One study author is an employee of the company that provided the program for free. The other study authors reported no relevant disclosures. No study funding was reported.
Source: Parker S et al. J Clin Gastroenterology. doi: 10.1097/MCG.0000000000000958.
Watchman device PREVAILs for stroke prevention
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: All-cause mortality was reduced by 27% in patients randomized to left atrial appendage closure with the Watchman device, compared with those assigned to warfarin.
Study details: This patient-level meta-analysis included 5-year follow-up data on 1,114 patients with atrial fibrillation at increased stroke risk who were randomized 2:1 to the Watchman device or warfarin.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to study sponsor Boston Scientific.
Mutations linked to checkpoint inhibitor response in RCC
in a derivation and validation study involving a total of 98 patients.
This finding “has important implications as a molecular tool for considering immunotherapy responsiveness” in patients with ccRCC and possibly patients with other cancer types, wrote Eliezer M. Van Allen, MD, of Dana Farber Cancer Institute in Boston and coauthors.
The derivation cohort included 35 patients with metastatic ccRCC treated with nivolumab in a prospective clinical trial. Genome sequencing of pretreatment tumor specimens showed that improved survival after treatment was significantly linked with truncating mutations in a gene, PBRM1, that codes for a protein in the SWI/SNF chromatin-remodeling complex. Patients in the derivation cohort who had these mutations were nearly 13-fold more likely to have clinical benefit from treatment, compared with those without these mutations.
The validation study included specimens and treatment-outcome results from 63 patients with metastatic ccRCC treated with either nivolumab or a different checkpoint inhibitor, such as atezolizumab (Tecentriq). In the validation study, PBRM1 mutations linked with a sixfold higher rate of clinical benefit from treatment.
The researchers noted that the types of mutations they identified as likely involved occur in more than 20% of all cancer types. Results from mouse studies have suggested that tumor cells with these types of mutations are more sensitive to T cell–mediated cytotoxicity, an observation that “lends a mechanistic basis” to the observed findings.
The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
SOURCE: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951
in a derivation and validation study involving a total of 98 patients.
This finding “has important implications as a molecular tool for considering immunotherapy responsiveness” in patients with ccRCC and possibly patients with other cancer types, wrote Eliezer M. Van Allen, MD, of Dana Farber Cancer Institute in Boston and coauthors.
The derivation cohort included 35 patients with metastatic ccRCC treated with nivolumab in a prospective clinical trial. Genome sequencing of pretreatment tumor specimens showed that improved survival after treatment was significantly linked with truncating mutations in a gene, PBRM1, that codes for a protein in the SWI/SNF chromatin-remodeling complex. Patients in the derivation cohort who had these mutations were nearly 13-fold more likely to have clinical benefit from treatment, compared with those without these mutations.
The validation study included specimens and treatment-outcome results from 63 patients with metastatic ccRCC treated with either nivolumab or a different checkpoint inhibitor, such as atezolizumab (Tecentriq). In the validation study, PBRM1 mutations linked with a sixfold higher rate of clinical benefit from treatment.
The researchers noted that the types of mutations they identified as likely involved occur in more than 20% of all cancer types. Results from mouse studies have suggested that tumor cells with these types of mutations are more sensitive to T cell–mediated cytotoxicity, an observation that “lends a mechanistic basis” to the observed findings.
The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
SOURCE: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951
in a derivation and validation study involving a total of 98 patients.
This finding “has important implications as a molecular tool for considering immunotherapy responsiveness” in patients with ccRCC and possibly patients with other cancer types, wrote Eliezer M. Van Allen, MD, of Dana Farber Cancer Institute in Boston and coauthors.
The derivation cohort included 35 patients with metastatic ccRCC treated with nivolumab in a prospective clinical trial. Genome sequencing of pretreatment tumor specimens showed that improved survival after treatment was significantly linked with truncating mutations in a gene, PBRM1, that codes for a protein in the SWI/SNF chromatin-remodeling complex. Patients in the derivation cohort who had these mutations were nearly 13-fold more likely to have clinical benefit from treatment, compared with those without these mutations.
The validation study included specimens and treatment-outcome results from 63 patients with metastatic ccRCC treated with either nivolumab or a different checkpoint inhibitor, such as atezolizumab (Tecentriq). In the validation study, PBRM1 mutations linked with a sixfold higher rate of clinical benefit from treatment.
The researchers noted that the types of mutations they identified as likely involved occur in more than 20% of all cancer types. Results from mouse studies have suggested that tumor cells with these types of mutations are more sensitive to T cell–mediated cytotoxicity, an observation that “lends a mechanistic basis” to the observed findings.
The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
SOURCE: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951
FROM SCIENCE
Key clinical point: Mutations in PBRM1 linked with better survival after immune checkpoint inhibitor therapy.
Major finding: Patients with a PBRM1 mutation were 6- to 13-fold more likely to have clinical benefit from checkpoint inhibitor treatment.
Study details: Derivation and validation studies that included 98 total patients with metastatic clear cell renal cell carcinoma.
Disclosures: The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
Source: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951.
See for yourself
About 800 million radiology exams were performed in this country in the past year, and they generated approximately 60 billion images, according to an article published in 2017 in the Wall Street Journal (“No need for radiologists to be negative on AI,” by Greg Ip, Nov. 24, 2017). How many of those millions of radiology studies did you order? And how many of the scores of images you requested did you see with your own two eyes? In fact, how many of the radiologists’ reports that were sent to you did you read in their entirety? How often did you just skip over the radiologist’s CYA disclaimers and simply read the final summary, “exam negative”?
I enjoy the challenge of interpreting x-ray images. In fact, I toyed with becoming a pediatric radiologist, but that career path would have meant settling in or near a large city, a compromise my wife and I were unwilling to make. I hoped to continue my habit of looking at all my patients’ x-rays, but because my practice was not in or near the hospital, I reluctantly had to bend my rules and admit I didn’t see every image I had ordered. But, I did read every report in its entirety. In one case, an offhand comment buried in the middle of the radiologist’s report referring to the “residual barium from a previous study” caught my eye, because I knew the patient hadn’t had a previous contrast study. Unfortunately, the neuroblastoma that the radiologist had missed initially, and I had seen the next day, never responded to treatment.
Toward the end of my career, digital imagery allowed me to view my patients’ x-rays without having to leave my desk, which got me closer to my goal of seeing all my patients’ studies. However, the advent of computerized axial tomography and magnetic resonance imaging meant that an increasing number of studies pushed my anatomic knowledge beyond its limits.
I suspect that many of you benefited from the if-you-order-it-look-at-it mantra during your training. How many of you have continued to follow the dictum? With the advent of digitized imagery, there is really little excuse for not taking a minute or 2 to pull up your patients’ images on your desktop. One could argue that looking inside your patient is part of a complete exam. Forcing yourself to take that extra step and look at the study may nudge you into thinking twice about whether you really needed the information the imaging study might add to the diagnostic process. Was your click to order the study just a reflex subliminally related to the fear of a lawsuit? Was it important enough to deserve a firsthand look?
At the very least, being able to say, “I’ve looked at your x-rays myself, and they look fine” may be more comforting to your patient than a third-hand relay of a “negative reading” performed by someone whom they have likely never met.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
About 800 million radiology exams were performed in this country in the past year, and they generated approximately 60 billion images, according to an article published in 2017 in the Wall Street Journal (“No need for radiologists to be negative on AI,” by Greg Ip, Nov. 24, 2017). How many of those millions of radiology studies did you order? And how many of the scores of images you requested did you see with your own two eyes? In fact, how many of the radiologists’ reports that were sent to you did you read in their entirety? How often did you just skip over the radiologist’s CYA disclaimers and simply read the final summary, “exam negative”?
I enjoy the challenge of interpreting x-ray images. In fact, I toyed with becoming a pediatric radiologist, but that career path would have meant settling in or near a large city, a compromise my wife and I were unwilling to make. I hoped to continue my habit of looking at all my patients’ x-rays, but because my practice was not in or near the hospital, I reluctantly had to bend my rules and admit I didn’t see every image I had ordered. But, I did read every report in its entirety. In one case, an offhand comment buried in the middle of the radiologist’s report referring to the “residual barium from a previous study” caught my eye, because I knew the patient hadn’t had a previous contrast study. Unfortunately, the neuroblastoma that the radiologist had missed initially, and I had seen the next day, never responded to treatment.
Toward the end of my career, digital imagery allowed me to view my patients’ x-rays without having to leave my desk, which got me closer to my goal of seeing all my patients’ studies. However, the advent of computerized axial tomography and magnetic resonance imaging meant that an increasing number of studies pushed my anatomic knowledge beyond its limits.
I suspect that many of you benefited from the if-you-order-it-look-at-it mantra during your training. How many of you have continued to follow the dictum? With the advent of digitized imagery, there is really little excuse for not taking a minute or 2 to pull up your patients’ images on your desktop. One could argue that looking inside your patient is part of a complete exam. Forcing yourself to take that extra step and look at the study may nudge you into thinking twice about whether you really needed the information the imaging study might add to the diagnostic process. Was your click to order the study just a reflex subliminally related to the fear of a lawsuit? Was it important enough to deserve a firsthand look?
At the very least, being able to say, “I’ve looked at your x-rays myself, and they look fine” may be more comforting to your patient than a third-hand relay of a “negative reading” performed by someone whom they have likely never met.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
About 800 million radiology exams were performed in this country in the past year, and they generated approximately 60 billion images, according to an article published in 2017 in the Wall Street Journal (“No need for radiologists to be negative on AI,” by Greg Ip, Nov. 24, 2017). How many of those millions of radiology studies did you order? And how many of the scores of images you requested did you see with your own two eyes? In fact, how many of the radiologists’ reports that were sent to you did you read in their entirety? How often did you just skip over the radiologist’s CYA disclaimers and simply read the final summary, “exam negative”?
I enjoy the challenge of interpreting x-ray images. In fact, I toyed with becoming a pediatric radiologist, but that career path would have meant settling in or near a large city, a compromise my wife and I were unwilling to make. I hoped to continue my habit of looking at all my patients’ x-rays, but because my practice was not in or near the hospital, I reluctantly had to bend my rules and admit I didn’t see every image I had ordered. But, I did read every report in its entirety. In one case, an offhand comment buried in the middle of the radiologist’s report referring to the “residual barium from a previous study” caught my eye, because I knew the patient hadn’t had a previous contrast study. Unfortunately, the neuroblastoma that the radiologist had missed initially, and I had seen the next day, never responded to treatment.
Toward the end of my career, digital imagery allowed me to view my patients’ x-rays without having to leave my desk, which got me closer to my goal of seeing all my patients’ studies. However, the advent of computerized axial tomography and magnetic resonance imaging meant that an increasing number of studies pushed my anatomic knowledge beyond its limits.
I suspect that many of you benefited from the if-you-order-it-look-at-it mantra during your training. How many of you have continued to follow the dictum? With the advent of digitized imagery, there is really little excuse for not taking a minute or 2 to pull up your patients’ images on your desktop. One could argue that looking inside your patient is part of a complete exam. Forcing yourself to take that extra step and look at the study may nudge you into thinking twice about whether you really needed the information the imaging study might add to the diagnostic process. Was your click to order the study just a reflex subliminally related to the fear of a lawsuit? Was it important enough to deserve a firsthand look?
At the very least, being able to say, “I’ve looked at your x-rays myself, and they look fine” may be more comforting to your patient than a third-hand relay of a “negative reading” performed by someone whom they have likely never met.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
DNA sequencing could help identify relapse risk in treated AML
ATLANTA – Clinicians may be able to get a jump on identifying risk factors for relapse among adults with acute myeloid leukemia (AML) in first complete remission through the use of next-generation DNA sequencing, investigators reported.
Among 430 adults with AML with somatic driver mutations persistent in bone marrow during morphological complete remission (CR) following induction therapy, the presence of minimal residual disease (MRD) bearing specific disease-related mutations on next-generation sequencing (NGS) was significantly associated with both the cumulative incidence of relapse and with overall survival. Tim Grob, MD, of Erasmus University Medical Center in Rotterdam, the Netherlands, reported the findings during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
By excluding three common AML mutations in genes commonly associated with clonal hematopoiesis – DNMT3A, TET2, and ASXL1 (collectively, DTA) – Dr. Grob and his colleagues at centers in the Netherlands, Belgium, and Switzerland were able to demonstrate that non-DTA mutations present in the marrow of patients in CR are highly predictive for relapse within 5 years and for worse overall survival.
They also showed that mutations associated with clonal hematopoiesis (the presence of small, preleukemic clones) in CR is not significantly associated with risk of relapse.
More than 80% of patients with AML are able to have a CR after induction therapy, but a significant proportion of patients will also experience relapse. Investigators have yet to nail down which leukemia-specific mutations that linger in patients with CR may be responsible for subsequent relapses, Dr. Grob said.
To get a better handle on which residual mutations may signal the need for extra vigilance or additional therapy in patients in CR after two cycles of induction therapy, the investigators used targeted next-generation (high-throughput) sequencing at the time of diagnosis and first CR in 430 patients enrolled in joint Dutch/Swiss clinical trials. The median patient age was 51.
The investigators screened marrow samples using a commercially available gene panel (Illumina) covering 54 genes that are commonly mutated in myeloid malignancies.
They divided the patients into a training cohort (283 patients) and a validation set (147) for confirmation of results.
About half of all patients in the training cohort (51.4%) had persistent mutations in bone marrow that occurred with highly variable variant allele frequencies. The most common mutations were in the DTA group with the most frequently mutated gene being DNMT3A (78.7% variant allele frequency), followed by TET2 (54.2%) and ASXL1 (51.6%).
Mutations in DTA genes in this cohort were not associated with the incidence of relapse at any variant allele frequency cut-off point used, which indicated that these mutations represented a stage of clonal hematopoiesis rather than early relapse signals.
However, among patients who had persistent DTA mutations, there was significant correlation with a risk for relapse when they also had persistence of any other non-DTA mutations. The cumulative 5-year incidence of relapse in patients with both persistent DTA and non-DTA mutations was 76.4%, compared with 39.4% for those without other, non-DTA mutations (P = .002).
Also in the training cohort, persistent non-DTA mutations (NGS MRD) were found to be highly associated with the risk of relapse with a subdistribution hazard ratio (SHR) of 1.85 (P = .001). In the validation set the effect was even stronger, with an SHR or 2.81 (P less than .001).
When data from the training and validation cohort were combined, the 5-year cumulative incidence of relapse was 58.3% for non-DTA mutation, vs. 33.9% (P less than .001).
NGS MRD was also predictive of overall survival, with a hazard ratio in the training cohort of 1.64 (P = .012) and an HR in the validation cohort of 3.08 (P less than .001).
In multivariable analysis of all 430 patients, adjusted for age, white blood cell count, 2017 European LeukemiaNet risk category, and number of induction cycles need to achieve CR, NGS MRD was an independent prognostic factor for both relapse (SHR 1.89, P less than .001) and overall survival (HR 1.64 P = .003).
When the investigators conducted a sensitivity analysis with time-dependent correction for allogeneic stem cell transplantation, they found that NGS MRD was still significantly prognostic for both relapse and survival.
The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
SOURCE: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
ATLANTA – Clinicians may be able to get a jump on identifying risk factors for relapse among adults with acute myeloid leukemia (AML) in first complete remission through the use of next-generation DNA sequencing, investigators reported.
Among 430 adults with AML with somatic driver mutations persistent in bone marrow during morphological complete remission (CR) following induction therapy, the presence of minimal residual disease (MRD) bearing specific disease-related mutations on next-generation sequencing (NGS) was significantly associated with both the cumulative incidence of relapse and with overall survival. Tim Grob, MD, of Erasmus University Medical Center in Rotterdam, the Netherlands, reported the findings during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
By excluding three common AML mutations in genes commonly associated with clonal hematopoiesis – DNMT3A, TET2, and ASXL1 (collectively, DTA) – Dr. Grob and his colleagues at centers in the Netherlands, Belgium, and Switzerland were able to demonstrate that non-DTA mutations present in the marrow of patients in CR are highly predictive for relapse within 5 years and for worse overall survival.
They also showed that mutations associated with clonal hematopoiesis (the presence of small, preleukemic clones) in CR is not significantly associated with risk of relapse.
More than 80% of patients with AML are able to have a CR after induction therapy, but a significant proportion of patients will also experience relapse. Investigators have yet to nail down which leukemia-specific mutations that linger in patients with CR may be responsible for subsequent relapses, Dr. Grob said.
To get a better handle on which residual mutations may signal the need for extra vigilance or additional therapy in patients in CR after two cycles of induction therapy, the investigators used targeted next-generation (high-throughput) sequencing at the time of diagnosis and first CR in 430 patients enrolled in joint Dutch/Swiss clinical trials. The median patient age was 51.
The investigators screened marrow samples using a commercially available gene panel (Illumina) covering 54 genes that are commonly mutated in myeloid malignancies.
They divided the patients into a training cohort (283 patients) and a validation set (147) for confirmation of results.
About half of all patients in the training cohort (51.4%) had persistent mutations in bone marrow that occurred with highly variable variant allele frequencies. The most common mutations were in the DTA group with the most frequently mutated gene being DNMT3A (78.7% variant allele frequency), followed by TET2 (54.2%) and ASXL1 (51.6%).
Mutations in DTA genes in this cohort were not associated with the incidence of relapse at any variant allele frequency cut-off point used, which indicated that these mutations represented a stage of clonal hematopoiesis rather than early relapse signals.
However, among patients who had persistent DTA mutations, there was significant correlation with a risk for relapse when they also had persistence of any other non-DTA mutations. The cumulative 5-year incidence of relapse in patients with both persistent DTA and non-DTA mutations was 76.4%, compared with 39.4% for those without other, non-DTA mutations (P = .002).
Also in the training cohort, persistent non-DTA mutations (NGS MRD) were found to be highly associated with the risk of relapse with a subdistribution hazard ratio (SHR) of 1.85 (P = .001). In the validation set the effect was even stronger, with an SHR or 2.81 (P less than .001).
When data from the training and validation cohort were combined, the 5-year cumulative incidence of relapse was 58.3% for non-DTA mutation, vs. 33.9% (P less than .001).
NGS MRD was also predictive of overall survival, with a hazard ratio in the training cohort of 1.64 (P = .012) and an HR in the validation cohort of 3.08 (P less than .001).
In multivariable analysis of all 430 patients, adjusted for age, white blood cell count, 2017 European LeukemiaNet risk category, and number of induction cycles need to achieve CR, NGS MRD was an independent prognostic factor for both relapse (SHR 1.89, P less than .001) and overall survival (HR 1.64 P = .003).
When the investigators conducted a sensitivity analysis with time-dependent correction for allogeneic stem cell transplantation, they found that NGS MRD was still significantly prognostic for both relapse and survival.
The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
SOURCE: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
ATLANTA – Clinicians may be able to get a jump on identifying risk factors for relapse among adults with acute myeloid leukemia (AML) in first complete remission through the use of next-generation DNA sequencing, investigators reported.
Among 430 adults with AML with somatic driver mutations persistent in bone marrow during morphological complete remission (CR) following induction therapy, the presence of minimal residual disease (MRD) bearing specific disease-related mutations on next-generation sequencing (NGS) was significantly associated with both the cumulative incidence of relapse and with overall survival. Tim Grob, MD, of Erasmus University Medical Center in Rotterdam, the Netherlands, reported the findings during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
By excluding three common AML mutations in genes commonly associated with clonal hematopoiesis – DNMT3A, TET2, and ASXL1 (collectively, DTA) – Dr. Grob and his colleagues at centers in the Netherlands, Belgium, and Switzerland were able to demonstrate that non-DTA mutations present in the marrow of patients in CR are highly predictive for relapse within 5 years and for worse overall survival.
They also showed that mutations associated with clonal hematopoiesis (the presence of small, preleukemic clones) in CR is not significantly associated with risk of relapse.
More than 80% of patients with AML are able to have a CR after induction therapy, but a significant proportion of patients will also experience relapse. Investigators have yet to nail down which leukemia-specific mutations that linger in patients with CR may be responsible for subsequent relapses, Dr. Grob said.
To get a better handle on which residual mutations may signal the need for extra vigilance or additional therapy in patients in CR after two cycles of induction therapy, the investigators used targeted next-generation (high-throughput) sequencing at the time of diagnosis and first CR in 430 patients enrolled in joint Dutch/Swiss clinical trials. The median patient age was 51.
The investigators screened marrow samples using a commercially available gene panel (Illumina) covering 54 genes that are commonly mutated in myeloid malignancies.
They divided the patients into a training cohort (283 patients) and a validation set (147) for confirmation of results.
About half of all patients in the training cohort (51.4%) had persistent mutations in bone marrow that occurred with highly variable variant allele frequencies. The most common mutations were in the DTA group with the most frequently mutated gene being DNMT3A (78.7% variant allele frequency), followed by TET2 (54.2%) and ASXL1 (51.6%).
Mutations in DTA genes in this cohort were not associated with the incidence of relapse at any variant allele frequency cut-off point used, which indicated that these mutations represented a stage of clonal hematopoiesis rather than early relapse signals.
However, among patients who had persistent DTA mutations, there was significant correlation with a risk for relapse when they also had persistence of any other non-DTA mutations. The cumulative 5-year incidence of relapse in patients with both persistent DTA and non-DTA mutations was 76.4%, compared with 39.4% for those without other, non-DTA mutations (P = .002).
Also in the training cohort, persistent non-DTA mutations (NGS MRD) were found to be highly associated with the risk of relapse with a subdistribution hazard ratio (SHR) of 1.85 (P = .001). In the validation set the effect was even stronger, with an SHR or 2.81 (P less than .001).
When data from the training and validation cohort were combined, the 5-year cumulative incidence of relapse was 58.3% for non-DTA mutation, vs. 33.9% (P less than .001).
NGS MRD was also predictive of overall survival, with a hazard ratio in the training cohort of 1.64 (P = .012) and an HR in the validation cohort of 3.08 (P less than .001).
In multivariable analysis of all 430 patients, adjusted for age, white blood cell count, 2017 European LeukemiaNet risk category, and number of induction cycles need to achieve CR, NGS MRD was an independent prognostic factor for both relapse (SHR 1.89, P less than .001) and overall survival (HR 1.64 P = .003).
When the investigators conducted a sensitivity analysis with time-dependent correction for allogeneic stem cell transplantation, they found that NGS MRD was still significantly prognostic for both relapse and survival.
The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
SOURCE: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: The presence of any non-DTA mutation after CR was an independent prognostic factor for relapse (SHR 1.89) and overall survival (HR 1.64).
Study details: Prospective analysis of bone marrow samples from 430 patients with AML at diagnosis and in first complete remission.
Disclosures: The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
Source: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
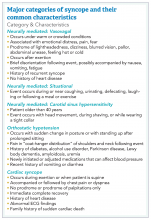
How to manage a patient presenting with syncope
Case
A 38-year-old construction worker without significant medical history presents following witnessed syncope at her job, after standing for at least 2 hours on a particularly warm day. She reported an episode of syncope under similar circumstances 2 months prior. With each episode, she experienced “tunneling” of peripheral vision, then loss of consciousness without palpitations or incontinence. Her physical exam, vital signs (including orthostatic blood pressures), labs, and ECG were unremarkable.
Brief overview
The American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines define syncope as “a symptom that presents with an abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery” with cerebral hypoperfusion as the presumed mechanism.1 Furthermore, “there should not be clinical features of other nonsyncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (that is, pseudosyncope).”1
A careful history revolving around the patient’s behavior prior to, during, and following the event, a thorough past medical history, and a review of current medications are essential. Potential obstacles in obtaining details of the event include lack of witnesses, patient’s inability to recall the experience, and inaccurate description of convulsive syncope as a “seizure” by bystanders.2
Overview of data
Obtaining a detailed history is crucial to understanding both the etiology of the syncopal event and determining which patients are at high risk for adverse outcomes. The etiology of syncope can be determined by history alone in 26% of patients younger than 65 years.3 Data on the prevalence of syncope by cause varies widely. As a general rule, in younger patients, especially those under 40 years of age, neurally mediated syncope is most common. As patients age, orthostatic hypotension and cardiac causes (including arrhythmias and structural diseases) occur more frequently, though neurally mediated syncope is still the most common.
Many of these predictors, however, would raise the clinical suspicion of most hospitalists for adverse outcomes in their hospitalized patients independent of the presence or absence of syncope. In fact, a meta-analysis has concluded that “None of the evaluated prediction tools (SFSR, EGSYS) performed better than clinical judgment in identifying serious outcomes during emergency department stay, and at 10 and 30 days after syncope.”6
Once the patient is hospitalized, further evaluation should be based on a careful history and physical examination. Standard evaluation also includes careful review of medications, an ECG to exclude findings suggestive of arrhythmias as well as structural or coronary artery disease, and orthostatic blood pressure measurements.1 Additional tests should be considered as deemed appropriate. For example, in patients over 40 years of age without history of carotid artery disease or stroke and in whom no carotid artery bruit is appreciated, a carotid sinus massage may be considered. The correct technique is to massage the sinus on the right then left, each for 5 seconds in both supine and standing positions with continuous heart rate and frequent blood pressure monitoring. Reproduction of syncope, especially concurrent with a cardiac pause of greater than 3 seconds and a systolic blood pressure drop of greater than 50 mmHg, is considered a positive test. Tilt-table testing should be considered in those for whom neurally mediated syncope is suspected but not confirmed, or in patients who might benefit from further elucidation of their prodromal symptoms.
Of note, a study recently published in the New England Journal of Medicine suggests that the prevalence of PE in patients (median age, 80 years) presenting with a first episode of syncope was 17%, a rate that is substantially higher than historically presumed.8 Although the prevalence of PE was highest among patients presenting with syncope of unclear origin (25%), nearly 13% of patients with other explanations for syncope also had PE.
Application of data
Bottom Line
Dr. Roberts, Dr. Krason, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Shen W-K et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;70(5):e39-e110.
2. Sheldon R. How to differentiate syncope from seizure. Cardiol Clin. 2015 Aug;33(3):377-85.
3. Del Rosso A et al. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431-5.
4. Blanc JJ. Syncope: Definition, epidemiology, and classification. Cardiol Clin. 2015 Aug;33(3):341-5.
5. Matthews IG et al. Syncope in the older person. Cardiol Clin. 2015 Aug;33(3):411-21.
6. Costantino G et al. Syncope risk stratification tools vs clinical judgment: An individual patient data meta-analysis. Am J Med. 2014 Nov;127(11):1126.e13-25.
7. Chiu DT et al. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47:113-8.
8. Prandoni P et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016 Oct;375(20):1524-31.
9. Sheldon RS et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011 Mar-Apr;27(2):246-253.
10. Moya A et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009 Nov;30(21):2631-71.
Additional reading
1. Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol. 2012 May; 59(18):1583-91.
2. Rosanio S et al. Syncope in adults: systematic review and proposal of a diagnostic and therapeutic algorithm. Int J Cardiol. 2013 Jan;162(3):149-57.
Case
A 38-year-old construction worker without significant medical history presents following witnessed syncope at her job, after standing for at least 2 hours on a particularly warm day. She reported an episode of syncope under similar circumstances 2 months prior. With each episode, she experienced “tunneling” of peripheral vision, then loss of consciousness without palpitations or incontinence. Her physical exam, vital signs (including orthostatic blood pressures), labs, and ECG were unremarkable.
Brief overview
The American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines define syncope as “a symptom that presents with an abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery” with cerebral hypoperfusion as the presumed mechanism.1 Furthermore, “there should not be clinical features of other nonsyncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (that is, pseudosyncope).”1
A careful history revolving around the patient’s behavior prior to, during, and following the event, a thorough past medical history, and a review of current medications are essential. Potential obstacles in obtaining details of the event include lack of witnesses, patient’s inability to recall the experience, and inaccurate description of convulsive syncope as a “seizure” by bystanders.2
Overview of data
Obtaining a detailed history is crucial to understanding both the etiology of the syncopal event and determining which patients are at high risk for adverse outcomes. The etiology of syncope can be determined by history alone in 26% of patients younger than 65 years.3 Data on the prevalence of syncope by cause varies widely. As a general rule, in younger patients, especially those under 40 years of age, neurally mediated syncope is most common. As patients age, orthostatic hypotension and cardiac causes (including arrhythmias and structural diseases) occur more frequently, though neurally mediated syncope is still the most common.
Many of these predictors, however, would raise the clinical suspicion of most hospitalists for adverse outcomes in their hospitalized patients independent of the presence or absence of syncope. In fact, a meta-analysis has concluded that “None of the evaluated prediction tools (SFSR, EGSYS) performed better than clinical judgment in identifying serious outcomes during emergency department stay, and at 10 and 30 days after syncope.”6
Once the patient is hospitalized, further evaluation should be based on a careful history and physical examination. Standard evaluation also includes careful review of medications, an ECG to exclude findings suggestive of arrhythmias as well as structural or coronary artery disease, and orthostatic blood pressure measurements.1 Additional tests should be considered as deemed appropriate. For example, in patients over 40 years of age without history of carotid artery disease or stroke and in whom no carotid artery bruit is appreciated, a carotid sinus massage may be considered. The correct technique is to massage the sinus on the right then left, each for 5 seconds in both supine and standing positions with continuous heart rate and frequent blood pressure monitoring. Reproduction of syncope, especially concurrent with a cardiac pause of greater than 3 seconds and a systolic blood pressure drop of greater than 50 mmHg, is considered a positive test. Tilt-table testing should be considered in those for whom neurally mediated syncope is suspected but not confirmed, or in patients who might benefit from further elucidation of their prodromal symptoms.
Of note, a study recently published in the New England Journal of Medicine suggests that the prevalence of PE in patients (median age, 80 years) presenting with a first episode of syncope was 17%, a rate that is substantially higher than historically presumed.8 Although the prevalence of PE was highest among patients presenting with syncope of unclear origin (25%), nearly 13% of patients with other explanations for syncope also had PE.
Application of data
Bottom Line
Dr. Roberts, Dr. Krason, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Shen W-K et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;70(5):e39-e110.
2. Sheldon R. How to differentiate syncope from seizure. Cardiol Clin. 2015 Aug;33(3):377-85.
3. Del Rosso A et al. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431-5.
4. Blanc JJ. Syncope: Definition, epidemiology, and classification. Cardiol Clin. 2015 Aug;33(3):341-5.
5. Matthews IG et al. Syncope in the older person. Cardiol Clin. 2015 Aug;33(3):411-21.
6. Costantino G et al. Syncope risk stratification tools vs clinical judgment: An individual patient data meta-analysis. Am J Med. 2014 Nov;127(11):1126.e13-25.
7. Chiu DT et al. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47:113-8.
8. Prandoni P et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016 Oct;375(20):1524-31.
9. Sheldon RS et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011 Mar-Apr;27(2):246-253.
10. Moya A et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009 Nov;30(21):2631-71.
Additional reading
1. Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol. 2012 May; 59(18):1583-91.
2. Rosanio S et al. Syncope in adults: systematic review and proposal of a diagnostic and therapeutic algorithm. Int J Cardiol. 2013 Jan;162(3):149-57.
Case
A 38-year-old construction worker without significant medical history presents following witnessed syncope at her job, after standing for at least 2 hours on a particularly warm day. She reported an episode of syncope under similar circumstances 2 months prior. With each episode, she experienced “tunneling” of peripheral vision, then loss of consciousness without palpitations or incontinence. Her physical exam, vital signs (including orthostatic blood pressures), labs, and ECG were unremarkable.
Brief overview
The American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines define syncope as “a symptom that presents with an abrupt, transient, complete loss of consciousness, associated with inability to maintain postural tone, with rapid and spontaneous recovery” with cerebral hypoperfusion as the presumed mechanism.1 Furthermore, “there should not be clinical features of other nonsyncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (that is, pseudosyncope).”1
A careful history revolving around the patient’s behavior prior to, during, and following the event, a thorough past medical history, and a review of current medications are essential. Potential obstacles in obtaining details of the event include lack of witnesses, patient’s inability to recall the experience, and inaccurate description of convulsive syncope as a “seizure” by bystanders.2
Overview of data
Obtaining a detailed history is crucial to understanding both the etiology of the syncopal event and determining which patients are at high risk for adverse outcomes. The etiology of syncope can be determined by history alone in 26% of patients younger than 65 years.3 Data on the prevalence of syncope by cause varies widely. As a general rule, in younger patients, especially those under 40 years of age, neurally mediated syncope is most common. As patients age, orthostatic hypotension and cardiac causes (including arrhythmias and structural diseases) occur more frequently, though neurally mediated syncope is still the most common.
Many of these predictors, however, would raise the clinical suspicion of most hospitalists for adverse outcomes in their hospitalized patients independent of the presence or absence of syncope. In fact, a meta-analysis has concluded that “None of the evaluated prediction tools (SFSR, EGSYS) performed better than clinical judgment in identifying serious outcomes during emergency department stay, and at 10 and 30 days after syncope.”6
Once the patient is hospitalized, further evaluation should be based on a careful history and physical examination. Standard evaluation also includes careful review of medications, an ECG to exclude findings suggestive of arrhythmias as well as structural or coronary artery disease, and orthostatic blood pressure measurements.1 Additional tests should be considered as deemed appropriate. For example, in patients over 40 years of age without history of carotid artery disease or stroke and in whom no carotid artery bruit is appreciated, a carotid sinus massage may be considered. The correct technique is to massage the sinus on the right then left, each for 5 seconds in both supine and standing positions with continuous heart rate and frequent blood pressure monitoring. Reproduction of syncope, especially concurrent with a cardiac pause of greater than 3 seconds and a systolic blood pressure drop of greater than 50 mmHg, is considered a positive test. Tilt-table testing should be considered in those for whom neurally mediated syncope is suspected but not confirmed, or in patients who might benefit from further elucidation of their prodromal symptoms.
Of note, a study recently published in the New England Journal of Medicine suggests that the prevalence of PE in patients (median age, 80 years) presenting with a first episode of syncope was 17%, a rate that is substantially higher than historically presumed.8 Although the prevalence of PE was highest among patients presenting with syncope of unclear origin (25%), nearly 13% of patients with other explanations for syncope also had PE.
Application of data
Bottom Line
Dr. Roberts, Dr. Krason, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Shen W-K et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;70(5):e39-e110.
2. Sheldon R. How to differentiate syncope from seizure. Cardiol Clin. 2015 Aug;33(3):377-85.
3. Del Rosso A et al. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431-5.
4. Blanc JJ. Syncope: Definition, epidemiology, and classification. Cardiol Clin. 2015 Aug;33(3):341-5.
5. Matthews IG et al. Syncope in the older person. Cardiol Clin. 2015 Aug;33(3):411-21.
6. Costantino G et al. Syncope risk stratification tools vs clinical judgment: An individual patient data meta-analysis. Am J Med. 2014 Nov;127(11):1126.e13-25.
7. Chiu DT et al. Are echocardiography, telemetry, ambulatory electrocardiography monitoring, and cardiac enzymes in emergency department patients presenting with syncope useful tests? A preliminary investigation. J Emerg Med. 2014;47:113-8.
8. Prandoni P et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016 Oct;375(20):1524-31.
9. Sheldon RS et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011 Mar-Apr;27(2):246-253.
10. Moya A et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J. 2009 Nov;30(21):2631-71.
Additional reading
1. Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol. 2012 May; 59(18):1583-91.
2. Rosanio S et al. Syncope in adults: systematic review and proposal of a diagnostic and therapeutic algorithm. Int J Cardiol. 2013 Jan;162(3):149-57.
Risks identified for drug-resistant bacteremia in cirrhosis
In patients hospitalized with cirrhosis, biliary cirrhosis, recent health care exposure, nonwhite race, and cultures taken more than 48 hours after admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms (MDROs), according to a medical record review at CHI St. Luke’s Medical Center, an 850-bed tertiary care center in Houston.
“These variables along with severity of infection and liver disease may help clinicians identify patients who will benefit most from broader-spectrum empiric antimicrobial therapy,” wrote the investigators, led by Jennifer Addo Smith, PharmD, of St. Luke’s, in the Journal of Clinical Gastroenterology.
But local epidemiology remains important. “Although a gram-positive agent (e.g., vancomycin) and a carbapenem-sparing gram-negative agent (e.g., ceftriaxone, cefepime) are reasonable empiric agents at our center, other centers with different resistance patterns may warrant different empiric therapy. Given the low prevalence of VRE [vancomycin-resistant Enterococcus] in this study ... and E. faecium in other studies (4%-7%), an empiric agent active against VRE does not seem to be routinely required,” they said.
The team looked into the issue because there hasn’t been much investigation in the United States of the role of multidrug resistant organisms in bacteremia among patients hospitalized with cirrhosis.
Thirty patients in the study had bacteremia caused by MDROs while 60 had bacteremia from non-MDROs, giving a 33% prevalence of MDRO bacteremia, which was consistent with previous, mostly European studies.
Enterobacteriaceae (43%), Staphylococcus aureus (18%), Streptococcus spp. (11%), Enterococcus spp. (10%), and nonfermenting gram-negative bacilli (6%) were the main causes of bacteremia overall.
Among the 30 MDRO cases, methicillin-resistant S. aureus was isolated in seven (23%); methicillin-resistant coagulase-negative Staphylococci in four (13%); fluoroquinolone-resistant Enterobacteriaceae in nine (30%); extended spectrum beta-lactamase–producing Enterobacteriaceae in three (10%), and VRE in two (7%). No carbapenemase-producing gram-negative bacteria were identified.
The predictors of MDRO bacteremia emerged on multivariate analysis and included biliary cirrhosis (adjusted odds ratio, 11.75; 95% confidence interval, 2.08-66.32); recent health care exposure (aOR, 9.81; 95% CI, 2.15-44.88); blood cultures obtained 48 hours after hospital admission (aOR, 6.02; 95% CI, 1.70-21.40) and nonwhite race (aOR , 3.35; 95% CI, 1.19-9.38).
Blood cultures past 48 hours and recent health care exposure – generally hospitalization within the past 90 days – were likely surrogates for nosocomial infection.
The link with biliary cirrhosis is unclear. “Compared with other cirrhotic patients, perhaps patients with PBC [primary biliary cholangitis] have had more cumulative antimicrobial exposure because of [their] higher risk for UTIs [urinary tract infections] and therefore are at increased risk for MDROs,” they wrote.
The median age in the study was 59 years. Half of the patients were white; 46% were women. Hepatitis C was the most common cause of cirrhosis, followed by alcohol.
MDRO was defined in the study as bacteria not susceptible to at least one antibiotic in at least three antimicrobial categories; 90 cirrhosis patients without bacteremia served as controls.
The funding source was not reported. Dr. Addo Smith had no disclosures.
SOURCE: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
*This story was updated on 1/10/2018.
In patients hospitalized with cirrhosis, biliary cirrhosis, recent health care exposure, nonwhite race, and cultures taken more than 48 hours after admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms (MDROs), according to a medical record review at CHI St. Luke’s Medical Center, an 850-bed tertiary care center in Houston.
“These variables along with severity of infection and liver disease may help clinicians identify patients who will benefit most from broader-spectrum empiric antimicrobial therapy,” wrote the investigators, led by Jennifer Addo Smith, PharmD, of St. Luke’s, in the Journal of Clinical Gastroenterology.
But local epidemiology remains important. “Although a gram-positive agent (e.g., vancomycin) and a carbapenem-sparing gram-negative agent (e.g., ceftriaxone, cefepime) are reasonable empiric agents at our center, other centers with different resistance patterns may warrant different empiric therapy. Given the low prevalence of VRE [vancomycin-resistant Enterococcus] in this study ... and E. faecium in other studies (4%-7%), an empiric agent active against VRE does not seem to be routinely required,” they said.
The team looked into the issue because there hasn’t been much investigation in the United States of the role of multidrug resistant organisms in bacteremia among patients hospitalized with cirrhosis.
Thirty patients in the study had bacteremia caused by MDROs while 60 had bacteremia from non-MDROs, giving a 33% prevalence of MDRO bacteremia, which was consistent with previous, mostly European studies.
Enterobacteriaceae (43%), Staphylococcus aureus (18%), Streptococcus spp. (11%), Enterococcus spp. (10%), and nonfermenting gram-negative bacilli (6%) were the main causes of bacteremia overall.
Among the 30 MDRO cases, methicillin-resistant S. aureus was isolated in seven (23%); methicillin-resistant coagulase-negative Staphylococci in four (13%); fluoroquinolone-resistant Enterobacteriaceae in nine (30%); extended spectrum beta-lactamase–producing Enterobacteriaceae in three (10%), and VRE in two (7%). No carbapenemase-producing gram-negative bacteria were identified.
The predictors of MDRO bacteremia emerged on multivariate analysis and included biliary cirrhosis (adjusted odds ratio, 11.75; 95% confidence interval, 2.08-66.32); recent health care exposure (aOR, 9.81; 95% CI, 2.15-44.88); blood cultures obtained 48 hours after hospital admission (aOR, 6.02; 95% CI, 1.70-21.40) and nonwhite race (aOR , 3.35; 95% CI, 1.19-9.38).
Blood cultures past 48 hours and recent health care exposure – generally hospitalization within the past 90 days – were likely surrogates for nosocomial infection.
The link with biliary cirrhosis is unclear. “Compared with other cirrhotic patients, perhaps patients with PBC [primary biliary cholangitis] have had more cumulative antimicrobial exposure because of [their] higher risk for UTIs [urinary tract infections] and therefore are at increased risk for MDROs,” they wrote.
The median age in the study was 59 years. Half of the patients were white; 46% were women. Hepatitis C was the most common cause of cirrhosis, followed by alcohol.
MDRO was defined in the study as bacteria not susceptible to at least one antibiotic in at least three antimicrobial categories; 90 cirrhosis patients without bacteremia served as controls.
The funding source was not reported. Dr. Addo Smith had no disclosures.
SOURCE: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
*This story was updated on 1/10/2018.
In patients hospitalized with cirrhosis, biliary cirrhosis, recent health care exposure, nonwhite race, and cultures taken more than 48 hours after admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms (MDROs), according to a medical record review at CHI St. Luke’s Medical Center, an 850-bed tertiary care center in Houston.
“These variables along with severity of infection and liver disease may help clinicians identify patients who will benefit most from broader-spectrum empiric antimicrobial therapy,” wrote the investigators, led by Jennifer Addo Smith, PharmD, of St. Luke’s, in the Journal of Clinical Gastroenterology.
But local epidemiology remains important. “Although a gram-positive agent (e.g., vancomycin) and a carbapenem-sparing gram-negative agent (e.g., ceftriaxone, cefepime) are reasonable empiric agents at our center, other centers with different resistance patterns may warrant different empiric therapy. Given the low prevalence of VRE [vancomycin-resistant Enterococcus] in this study ... and E. faecium in other studies (4%-7%), an empiric agent active against VRE does not seem to be routinely required,” they said.
The team looked into the issue because there hasn’t been much investigation in the United States of the role of multidrug resistant organisms in bacteremia among patients hospitalized with cirrhosis.
Thirty patients in the study had bacteremia caused by MDROs while 60 had bacteremia from non-MDROs, giving a 33% prevalence of MDRO bacteremia, which was consistent with previous, mostly European studies.
Enterobacteriaceae (43%), Staphylococcus aureus (18%), Streptococcus spp. (11%), Enterococcus spp. (10%), and nonfermenting gram-negative bacilli (6%) were the main causes of bacteremia overall.
Among the 30 MDRO cases, methicillin-resistant S. aureus was isolated in seven (23%); methicillin-resistant coagulase-negative Staphylococci in four (13%); fluoroquinolone-resistant Enterobacteriaceae in nine (30%); extended spectrum beta-lactamase–producing Enterobacteriaceae in three (10%), and VRE in two (7%). No carbapenemase-producing gram-negative bacteria were identified.
The predictors of MDRO bacteremia emerged on multivariate analysis and included biliary cirrhosis (adjusted odds ratio, 11.75; 95% confidence interval, 2.08-66.32); recent health care exposure (aOR, 9.81; 95% CI, 2.15-44.88); blood cultures obtained 48 hours after hospital admission (aOR, 6.02; 95% CI, 1.70-21.40) and nonwhite race (aOR , 3.35; 95% CI, 1.19-9.38).
Blood cultures past 48 hours and recent health care exposure – generally hospitalization within the past 90 days – were likely surrogates for nosocomial infection.
The link with biliary cirrhosis is unclear. “Compared with other cirrhotic patients, perhaps patients with PBC [primary biliary cholangitis] have had more cumulative antimicrobial exposure because of [their] higher risk for UTIs [urinary tract infections] and therefore are at increased risk for MDROs,” they wrote.
The median age in the study was 59 years. Half of the patients were white; 46% were women. Hepatitis C was the most common cause of cirrhosis, followed by alcohol.
MDRO was defined in the study as bacteria not susceptible to at least one antibiotic in at least three antimicrobial categories; 90 cirrhosis patients without bacteremia served as controls.
The funding source was not reported. Dr. Addo Smith had no disclosures.
SOURCE: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
*This story was updated on 1/10/2018.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: In patients hospitalized with cirrhosis, nonwhite race, biliary involvement, recent health care exposure, and cultures taken more than 48 hours after hospital admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms.
Major finding: The predictors of multidrug-resistant organism bacteremia emerged on multivariate analysis and included biliary cirrhosis (aOR 11.75; 95% CI, 2.08-66.32); recent health care exposure (aOR 9.81; 95% CI, 2.15-44.88); and blood cultures obtained 48 hours after hospital admission (aOR 6.02; 95% CI, 1.70-21.40).
Study details: Review of 90 cirrhotic patients with bacteremia, plus 90 controls.
Disclosures: The lead investigator had no disclosures.
Source: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
Richner-Hanhart Syndrome (Tyrosinemia Type II)
To the Editor:
Richner-Hanhart syndrome, also known as tyrosinemia type II or oculocutaneous tyrosinemia, is a rare autosomal-recessive, childhood-onset, metabolic hereditary disease.1 A deficiency of tyrosine aminotransferase leads to an accumulation of tyrosine amino acid. It is characterized by the association of palmoplantar hyperkeratosis, bilateral keratitis, and neurological disorders.
An 18-month-old girl with recurrent warts of 6 months' duration was admitted to the dermatology department. She had been treated repeatedly with acyclovir for recurrent bilateral herpetic keratitis with major photophobia since 9 months of age with no response. Clinical presentation included punctate hyperkeratosis of the fingers and toes (Figure, A), severe photophobia with decreased visual acuity, and speech delay.
Her medical record showed a break of the growth curve with a weight of 9.25 kg (3rd percentile), a height of 80 cm (50th percentile), and a head circumference of 45 cm (50th percentile). Her parents were nonconsanguineous. The association of bilateral dendritic keratitis with punctate palmoplantar keratosis suggested a diagnosis of Richner-Hanhart syndrome. Diagnosis was confirmed by an elevated plasma level of tyrosine (1580 µmol/L; reference range, 40-80 µmol/L).
A low tyrosine and low phenylalanine diet (no animal proteins) was immediately introduced, with supplementation of amino acids, vitamins, and trace elements. After 8 days, the plasma level of tyrosinemia decreased by a factor of 4 (392 µmol/L). After 1 month, the cutaneous and ocular lesions completely resolved (Figure, B). Discrete psychomotor slowing still persisted for 1 year and then reached complete normalization. Genetic analysis showed a composite heterozygous mutation of the tyrosine aminotransferase gene, TAT, on chromosome 16. The mutation detected in the patient's mother was an A to V substitution at codon 147 (A147V). The second mutation was detected in the father; it was an 8 nucleotides duplication and then a substitution leading to a premature stop codon at codon 37 (R37X).
Richner-Hanhart syndrome is a rare autosomal-recessive disorder that is more common in Italy and in areas where inbreeding is prevalent1,2; however, no data are available on disease prevalence. It is caused by a homozygous mutation in the TAT gene located on chromosome 16q22.3 Tyrosine aminotransferase is an important enzyme involved in the tyrosine and phenylalanine metabolic degradation pathway located in the hepatic cytosol. Symptoms are due to the accumulation of tyrosine and its metabolite. Diagnosis is confirmed by an elevated plasma level of tyrosine (>500 µmol/L). This oculocutaneous syndrome is characterized by bilateral pseudodendritic keratitis, palmoplantar hyperkeratosis, and a variable degree of mental retardation.1 In contrast to tyrosinemia type II, types I and III do not affect the skin.
Intrafamilial and interfamilial phenotypic variability is reported. A large spectrum of mutations within the TAT gene have been reported.4-7 These mutations lead to a reduction or an absence in the activity of hepatic tyrosine aminotransferase. The degradation pathway of tyrosine involving TAT occurs mainly in the liver. This process also is present in the mitochondria where the enzyme is called aspartate aminotransferase.1,2 The mechanism by which Richner-Hanhart syndrome causes painful palmoplantar keratosis and keratitis remains unknown. It has been suggested that intracellular L-tyrosine crystals initiate an inflammation process resulting in the typical skin lesions and keratitis.8 There is some evidence that patients with higher values of tyrosine in early life are more likely to develop neurological problems.1 In addition, phenotype variability has been observed, even among individuals sharing the same pathogenic mutation.4
Tyrosinemia type II typically demonstrates ocular symptoms (75% of cases) that usually occur in the first year of life.8 They are characterized by photophobia, redness, and increase of lacrimation. Examination reveals a superficial and bilateral punctate keratosis with corneal dystrophy, often misdiagnosed as herpetic keratosis, as in our case, which may delay the diagnosis.9,10 Bilateral ocular lesions are suggestive, even if they are asymmetric.8,11 Furthermore, negative fluorescein staining, negative culture, and resistance to antiviral treatment exclude the diagnosis of herpetic keratosis.9,10
Skin lesions (85% of cases) typically appear in the first year of life. They are characterized by painful, irregular, limited, punctate hyperkeratosis on the palms and soles.1 They are more frequent in weight-bearing areas and tend to improve during summer, possibly due to a seasonal change in dietary behavior.4,12 Hyperkeratotic papules in a linear pattern also have been described on the flexor aspects of the fingers or toes.13 In our case, the lesions were misdiagnosed as warts for 6 months.
Retarded development affects 60% of patients with tyrosinemia type II. Expression of neurological symptoms is variable and could include mental retardation, nystagmus, tremors, ataxia, and convulsion.4 Lifetime follow-up of these patients is recommended.
Early initiation of a tyrosine-phenylalanine-restricted diet in infancy is the most effective therapy for Richner-Hanhart syndrome.13 The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into amino acid tyrosine. Thus, dietary treatment of Richner-Hanhart syndrome requires restricting or eliminating foods high in phenylalanine and tyrosine with protein "medical food" substitute. The dietary treatment allows resolution of both eye and skin symptoms after a few days or weeks and also may prevent mental retardation. It is effective in lowering the plasma level to less than 400 µmol/L. The diet must be introduced as soon as Richner-Hanhart syndrome is suspected. Supplementation with essential amino acids, vitamins, and trace elements is needed. Early screening of siblings in families with Richner-Hanhart syndrome history is recommended, even in the absence of clinical findings. Careful dietary control of maternal plasma tyrosine level must be considered during future pregnancy for women.4,14,15
Richner-Hanhart syndrome should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121-126.
- Meissner T, Betz RC, Pasternack SM, et al. Richner-Hanhart syndrome detected by expanded newborn screening. Pediatr Dermatol. 2008;25:378-380.
- Natt E, Kida K, Odievre M, et al. Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proc Natl Acad Sci USA. 1992;89:9297-9301.
- Charfeddine C, Monastiri K, Mokni M, et al. Clinical and mutational investigations of tyrosinemia type II in Northern Tunisia: identification and structural characterization of two novel TAT mutations. Mol Genet Metab. 2006;88:184-191.
- Legarda M, Wlodarczyk K, Lage S, et al. A large TAT deletion in a tyrosinaemia type II patient. Mol Genet Metab. 2011;104:407-409.
- Culic V, Betz RC, Refke M, et al. Tyrosinemia type II (Richner-Hanhart syndrome): a new mutation in the TAT gene. Eur J Med Genet. 2011;54:205-208.
- Pasternack SM, Betz RC, Brandrup F, et al. Identification of two new mutations in the TAT gene in a Danish family with tyrosinaemia type II. Br J Dermatol. 2009;160:704-706.
- Macsai MS, Schwartz TL, Hinkle D, et al. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132:522-527.
- Kymionis GD, Kankariya VP, Kontadakis GA, et al. Isolated corneal pseudodendrites as the initial manifestation of tyrosinemia type II in monozygotic twins. J Pediatr Ophthalmol Strabismus.2012;49:E33-E36.
- Iskeleli G, Bilgeç MD, Arici C, et al. Richner-Hanhart syndrome (tyrosinemia type II): a case report of delayed diagnosis with pseudodendritic corneal lesion. Turk J Pediatr. 2011;53:692-694.
- Rehák A, Selim MM, Yadav G. Richner-Hanhart syndrome (tyrosinaemia-II)(report of four cases without ocular involvement). Br J Dermatol. 1981;104:469-475.
- Viglizzo GM, Occella C, Bleidl D, et al. Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol. 2006;23:259-261.
- Machino H, Miki Y, Kawatsu T, et al. Successful dietary control of tyrosinemia II. J Am Acad Dermatol. 1983;9:533-539.
- el-Badramany MH, Fawzy AR, Farag TI. Familial Richner-Hanhart syndrome in Kuwait: twelve-year clinical reassessment by a multidisciplinary approach. Am J Med Genet. 1995;60:353-355.
- Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis. 2002;25:317-318.
To the Editor:
Richner-Hanhart syndrome, also known as tyrosinemia type II or oculocutaneous tyrosinemia, is a rare autosomal-recessive, childhood-onset, metabolic hereditary disease.1 A deficiency of tyrosine aminotransferase leads to an accumulation of tyrosine amino acid. It is characterized by the association of palmoplantar hyperkeratosis, bilateral keratitis, and neurological disorders.
An 18-month-old girl with recurrent warts of 6 months' duration was admitted to the dermatology department. She had been treated repeatedly with acyclovir for recurrent bilateral herpetic keratitis with major photophobia since 9 months of age with no response. Clinical presentation included punctate hyperkeratosis of the fingers and toes (Figure, A), severe photophobia with decreased visual acuity, and speech delay.

Her medical record showed a break of the growth curve with a weight of 9.25 kg (3rd percentile), a height of 80 cm (50th percentile), and a head circumference of 45 cm (50th percentile). Her parents were nonconsanguineous. The association of bilateral dendritic keratitis with punctate palmoplantar keratosis suggested a diagnosis of Richner-Hanhart syndrome. Diagnosis was confirmed by an elevated plasma level of tyrosine (1580 µmol/L; reference range, 40-80 µmol/L).
A low tyrosine and low phenylalanine diet (no animal proteins) was immediately introduced, with supplementation of amino acids, vitamins, and trace elements. After 8 days, the plasma level of tyrosinemia decreased by a factor of 4 (392 µmol/L). After 1 month, the cutaneous and ocular lesions completely resolved (Figure, B). Discrete psychomotor slowing still persisted for 1 year and then reached complete normalization. Genetic analysis showed a composite heterozygous mutation of the tyrosine aminotransferase gene, TAT, on chromosome 16. The mutation detected in the patient's mother was an A to V substitution at codon 147 (A147V). The second mutation was detected in the father; it was an 8 nucleotides duplication and then a substitution leading to a premature stop codon at codon 37 (R37X).
Richner-Hanhart syndrome is a rare autosomal-recessive disorder that is more common in Italy and in areas where inbreeding is prevalent1,2; however, no data are available on disease prevalence. It is caused by a homozygous mutation in the TAT gene located on chromosome 16q22.3 Tyrosine aminotransferase is an important enzyme involved in the tyrosine and phenylalanine metabolic degradation pathway located in the hepatic cytosol. Symptoms are due to the accumulation of tyrosine and its metabolite. Diagnosis is confirmed by an elevated plasma level of tyrosine (>500 µmol/L). This oculocutaneous syndrome is characterized by bilateral pseudodendritic keratitis, palmoplantar hyperkeratosis, and a variable degree of mental retardation.1 In contrast to tyrosinemia type II, types I and III do not affect the skin.
Intrafamilial and interfamilial phenotypic variability is reported. A large spectrum of mutations within the TAT gene have been reported.4-7 These mutations lead to a reduction or an absence in the activity of hepatic tyrosine aminotransferase. The degradation pathway of tyrosine involving TAT occurs mainly in the liver. This process also is present in the mitochondria where the enzyme is called aspartate aminotransferase.1,2 The mechanism by which Richner-Hanhart syndrome causes painful palmoplantar keratosis and keratitis remains unknown. It has been suggested that intracellular L-tyrosine crystals initiate an inflammation process resulting in the typical skin lesions and keratitis.8 There is some evidence that patients with higher values of tyrosine in early life are more likely to develop neurological problems.1 In addition, phenotype variability has been observed, even among individuals sharing the same pathogenic mutation.4
Tyrosinemia type II typically demonstrates ocular symptoms (75% of cases) that usually occur in the first year of life.8 They are characterized by photophobia, redness, and increase of lacrimation. Examination reveals a superficial and bilateral punctate keratosis with corneal dystrophy, often misdiagnosed as herpetic keratosis, as in our case, which may delay the diagnosis.9,10 Bilateral ocular lesions are suggestive, even if they are asymmetric.8,11 Furthermore, negative fluorescein staining, negative culture, and resistance to antiviral treatment exclude the diagnosis of herpetic keratosis.9,10
Skin lesions (85% of cases) typically appear in the first year of life. They are characterized by painful, irregular, limited, punctate hyperkeratosis on the palms and soles.1 They are more frequent in weight-bearing areas and tend to improve during summer, possibly due to a seasonal change in dietary behavior.4,12 Hyperkeratotic papules in a linear pattern also have been described on the flexor aspects of the fingers or toes.13 In our case, the lesions were misdiagnosed as warts for 6 months.
Retarded development affects 60% of patients with tyrosinemia type II. Expression of neurological symptoms is variable and could include mental retardation, nystagmus, tremors, ataxia, and convulsion.4 Lifetime follow-up of these patients is recommended.
Early initiation of a tyrosine-phenylalanine-restricted diet in infancy is the most effective therapy for Richner-Hanhart syndrome.13 The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into amino acid tyrosine. Thus, dietary treatment of Richner-Hanhart syndrome requires restricting or eliminating foods high in phenylalanine and tyrosine with protein "medical food" substitute. The dietary treatment allows resolution of both eye and skin symptoms after a few days or weeks and also may prevent mental retardation. It is effective in lowering the plasma level to less than 400 µmol/L. The diet must be introduced as soon as Richner-Hanhart syndrome is suspected. Supplementation with essential amino acids, vitamins, and trace elements is needed. Early screening of siblings in families with Richner-Hanhart syndrome history is recommended, even in the absence of clinical findings. Careful dietary control of maternal plasma tyrosine level must be considered during future pregnancy for women.4,14,15
Richner-Hanhart syndrome should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
To the Editor:
Richner-Hanhart syndrome, also known as tyrosinemia type II or oculocutaneous tyrosinemia, is a rare autosomal-recessive, childhood-onset, metabolic hereditary disease.1 A deficiency of tyrosine aminotransferase leads to an accumulation of tyrosine amino acid. It is characterized by the association of palmoplantar hyperkeratosis, bilateral keratitis, and neurological disorders.
An 18-month-old girl with recurrent warts of 6 months' duration was admitted to the dermatology department. She had been treated repeatedly with acyclovir for recurrent bilateral herpetic keratitis with major photophobia since 9 months of age with no response. Clinical presentation included punctate hyperkeratosis of the fingers and toes (Figure, A), severe photophobia with decreased visual acuity, and speech delay.

Her medical record showed a break of the growth curve with a weight of 9.25 kg (3rd percentile), a height of 80 cm (50th percentile), and a head circumference of 45 cm (50th percentile). Her parents were nonconsanguineous. The association of bilateral dendritic keratitis with punctate palmoplantar keratosis suggested a diagnosis of Richner-Hanhart syndrome. Diagnosis was confirmed by an elevated plasma level of tyrosine (1580 µmol/L; reference range, 40-80 µmol/L).
A low tyrosine and low phenylalanine diet (no animal proteins) was immediately introduced, with supplementation of amino acids, vitamins, and trace elements. After 8 days, the plasma level of tyrosinemia decreased by a factor of 4 (392 µmol/L). After 1 month, the cutaneous and ocular lesions completely resolved (Figure, B). Discrete psychomotor slowing still persisted for 1 year and then reached complete normalization. Genetic analysis showed a composite heterozygous mutation of the tyrosine aminotransferase gene, TAT, on chromosome 16. The mutation detected in the patient's mother was an A to V substitution at codon 147 (A147V). The second mutation was detected in the father; it was an 8 nucleotides duplication and then a substitution leading to a premature stop codon at codon 37 (R37X).
Richner-Hanhart syndrome is a rare autosomal-recessive disorder that is more common in Italy and in areas where inbreeding is prevalent1,2; however, no data are available on disease prevalence. It is caused by a homozygous mutation in the TAT gene located on chromosome 16q22.3 Tyrosine aminotransferase is an important enzyme involved in the tyrosine and phenylalanine metabolic degradation pathway located in the hepatic cytosol. Symptoms are due to the accumulation of tyrosine and its metabolite. Diagnosis is confirmed by an elevated plasma level of tyrosine (>500 µmol/L). This oculocutaneous syndrome is characterized by bilateral pseudodendritic keratitis, palmoplantar hyperkeratosis, and a variable degree of mental retardation.1 In contrast to tyrosinemia type II, types I and III do not affect the skin.
Intrafamilial and interfamilial phenotypic variability is reported. A large spectrum of mutations within the TAT gene have been reported.4-7 These mutations lead to a reduction or an absence in the activity of hepatic tyrosine aminotransferase. The degradation pathway of tyrosine involving TAT occurs mainly in the liver. This process also is present in the mitochondria where the enzyme is called aspartate aminotransferase.1,2 The mechanism by which Richner-Hanhart syndrome causes painful palmoplantar keratosis and keratitis remains unknown. It has been suggested that intracellular L-tyrosine crystals initiate an inflammation process resulting in the typical skin lesions and keratitis.8 There is some evidence that patients with higher values of tyrosine in early life are more likely to develop neurological problems.1 In addition, phenotype variability has been observed, even among individuals sharing the same pathogenic mutation.4
Tyrosinemia type II typically demonstrates ocular symptoms (75% of cases) that usually occur in the first year of life.8 They are characterized by photophobia, redness, and increase of lacrimation. Examination reveals a superficial and bilateral punctate keratosis with corneal dystrophy, often misdiagnosed as herpetic keratosis, as in our case, which may delay the diagnosis.9,10 Bilateral ocular lesions are suggestive, even if they are asymmetric.8,11 Furthermore, negative fluorescein staining, negative culture, and resistance to antiviral treatment exclude the diagnosis of herpetic keratosis.9,10
Skin lesions (85% of cases) typically appear in the first year of life. They are characterized by painful, irregular, limited, punctate hyperkeratosis on the palms and soles.1 They are more frequent in weight-bearing areas and tend to improve during summer, possibly due to a seasonal change in dietary behavior.4,12 Hyperkeratotic papules in a linear pattern also have been described on the flexor aspects of the fingers or toes.13 In our case, the lesions were misdiagnosed as warts for 6 months.
Retarded development affects 60% of patients with tyrosinemia type II. Expression of neurological symptoms is variable and could include mental retardation, nystagmus, tremors, ataxia, and convulsion.4 Lifetime follow-up of these patients is recommended.
Early initiation of a tyrosine-phenylalanine-restricted diet in infancy is the most effective therapy for Richner-Hanhart syndrome.13 The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into amino acid tyrosine. Thus, dietary treatment of Richner-Hanhart syndrome requires restricting or eliminating foods high in phenylalanine and tyrosine with protein "medical food" substitute. The dietary treatment allows resolution of both eye and skin symptoms after a few days or weeks and also may prevent mental retardation. It is effective in lowering the plasma level to less than 400 µmol/L. The diet must be introduced as soon as Richner-Hanhart syndrome is suspected. Supplementation with essential amino acids, vitamins, and trace elements is needed. Early screening of siblings in families with Richner-Hanhart syndrome history is recommended, even in the absence of clinical findings. Careful dietary control of maternal plasma tyrosine level must be considered during future pregnancy for women.4,14,15
Richner-Hanhart syndrome should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121-126.
- Meissner T, Betz RC, Pasternack SM, et al. Richner-Hanhart syndrome detected by expanded newborn screening. Pediatr Dermatol. 2008;25:378-380.
- Natt E, Kida K, Odievre M, et al. Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proc Natl Acad Sci USA. 1992;89:9297-9301.
- Charfeddine C, Monastiri K, Mokni M, et al. Clinical and mutational investigations of tyrosinemia type II in Northern Tunisia: identification and structural characterization of two novel TAT mutations. Mol Genet Metab. 2006;88:184-191.
- Legarda M, Wlodarczyk K, Lage S, et al. A large TAT deletion in a tyrosinaemia type II patient. Mol Genet Metab. 2011;104:407-409.
- Culic V, Betz RC, Refke M, et al. Tyrosinemia type II (Richner-Hanhart syndrome): a new mutation in the TAT gene. Eur J Med Genet. 2011;54:205-208.
- Pasternack SM, Betz RC, Brandrup F, et al. Identification of two new mutations in the TAT gene in a Danish family with tyrosinaemia type II. Br J Dermatol. 2009;160:704-706.
- Macsai MS, Schwartz TL, Hinkle D, et al. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132:522-527.
- Kymionis GD, Kankariya VP, Kontadakis GA, et al. Isolated corneal pseudodendrites as the initial manifestation of tyrosinemia type II in monozygotic twins. J Pediatr Ophthalmol Strabismus.2012;49:E33-E36.
- Iskeleli G, Bilgeç MD, Arici C, et al. Richner-Hanhart syndrome (tyrosinemia type II): a case report of delayed diagnosis with pseudodendritic corneal lesion. Turk J Pediatr. 2011;53:692-694.
- Rehák A, Selim MM, Yadav G. Richner-Hanhart syndrome (tyrosinaemia-II)(report of four cases without ocular involvement). Br J Dermatol. 1981;104:469-475.
- Viglizzo GM, Occella C, Bleidl D, et al. Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol. 2006;23:259-261.
- Machino H, Miki Y, Kawatsu T, et al. Successful dietary control of tyrosinemia II. J Am Acad Dermatol. 1983;9:533-539.
- el-Badramany MH, Fawzy AR, Farag TI. Familial Richner-Hanhart syndrome in Kuwait: twelve-year clinical reassessment by a multidisciplinary approach. Am J Med Genet. 1995;60:353-355.
- Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis. 2002;25:317-318.
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142C:121-126.
- Meissner T, Betz RC, Pasternack SM, et al. Richner-Hanhart syndrome detected by expanded newborn screening. Pediatr Dermatol. 2008;25:378-380.
- Natt E, Kida K, Odievre M, et al. Point mutations in the tyrosine aminotransferase gene in tyrosinemia type II. Proc Natl Acad Sci USA. 1992;89:9297-9301.
- Charfeddine C, Monastiri K, Mokni M, et al. Clinical and mutational investigations of tyrosinemia type II in Northern Tunisia: identification and structural characterization of two novel TAT mutations. Mol Genet Metab. 2006;88:184-191.
- Legarda M, Wlodarczyk K, Lage S, et al. A large TAT deletion in a tyrosinaemia type II patient. Mol Genet Metab. 2011;104:407-409.
- Culic V, Betz RC, Refke M, et al. Tyrosinemia type II (Richner-Hanhart syndrome): a new mutation in the TAT gene. Eur J Med Genet. 2011;54:205-208.
- Pasternack SM, Betz RC, Brandrup F, et al. Identification of two new mutations in the TAT gene in a Danish family with tyrosinaemia type II. Br J Dermatol. 2009;160:704-706.
- Macsai MS, Schwartz TL, Hinkle D, et al. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132:522-527.
- Kymionis GD, Kankariya VP, Kontadakis GA, et al. Isolated corneal pseudodendrites as the initial manifestation of tyrosinemia type II in monozygotic twins. J Pediatr Ophthalmol Strabismus.2012;49:E33-E36.
- Iskeleli G, Bilgeç MD, Arici C, et al. Richner-Hanhart syndrome (tyrosinemia type II): a case report of delayed diagnosis with pseudodendritic corneal lesion. Turk J Pediatr. 2011;53:692-694.
- Rehák A, Selim MM, Yadav G. Richner-Hanhart syndrome (tyrosinaemia-II)(report of four cases without ocular involvement). Br J Dermatol. 1981;104:469-475.
- Viglizzo GM, Occella C, Bleidl D, et al. Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol. 2006;23:259-261.
- Machino H, Miki Y, Kawatsu T, et al. Successful dietary control of tyrosinemia II. J Am Acad Dermatol. 1983;9:533-539.
- el-Badramany MH, Fawzy AR, Farag TI. Familial Richner-Hanhart syndrome in Kuwait: twelve-year clinical reassessment by a multidisciplinary approach. Am J Med Genet. 1995;60:353-355.
- Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis. 2002;25:317-318.
Practice Points
- Richner-Hanhart syndrome (tyrosinemia type II) should be suspected in patients demonstrating cutaneous lesions, especially palmoplantar keratosis associated with bilateral pseudodendritic corneal lesions unresponsive to antiviral therapy.
- Early diagnosis and initiation of a tyrosinephenylalanine–restricted diet in infancy is the most effective therapy to prevent mental retardation.
ISSOP’s Budapest Declaration: A call to action for children on the move
In late October 2017, pediatricians from over 25 countries gathered at the annual meeting of the International Society of Social Pediatrics and Child Health in Budapest to develop strategies to address the health and well-being of children on the move. With the staggering global increase in the displacement of children – due to conflict, climate change, natural disasters, and economic deprivation – there is a critical need for action.
An international slate of experts, including pediatricians who are responding to the needs of these children, provided an evidence base for intervention and action. These discussions informed the development of a consensus statement, the Budapest Declaration, that establishes a framework for a global response to the exigencies confronting these children and families.
The Budapest Declaration is grounded in children’s rights as guaranteed in the United Nation’s Convention on the Rights of the Child (CRC). All countries in the world have signed onto the CRC, with a notable exception being the United States. But leading American pediatric groups, including the American Academy of Pediatrics, have endorsed the CRC, making it a basis of organizational advocacy. The CRC entitles all children to optimal survival and development (Article 6) and optimal health and health care (Article 24).
As prescribed by these accepted legal rights for children, children and youth on the move are entitled to the same services, including health care, as resident populations of children, regardless of their legal status. Countries can be held accountable to ensure they receive the full rights due them as articulated in the CRC. Furthermore, efforts to do so should be assessed in national periodic reports to the Committee on the Rights of the Child to ensure accountability.
The relevance of the Budapest Declaration to the United States cannot be overstated. National and regional public policy throughout our country, and in particular along our Southern border, is having a devastating effect on the physical and mental health of children and youth on the move – and will continue to do so throughout their life course.
The involvement of pediatricians and other child health providers is essential to the planning and implementation of clinical and public health programs for children on the move. Child health professionals, in conjunction with their professional organizations, must be engaged in all aspects of local, national, and global responses. Leadership and contributions by pediatricians and pediatric societies and academic institutions are integral to the success of key partners, such as UNICEF, the World Health Organization, the International Organization for Migration, and the UN High Commissioner for Refugees.
Systems of care should be provided that address the physical, mental, and social health care needs of these children without bias and prejudice. Pregnant mothers on the move also should receive services to ensure the delivery of healthy newborns. Every nation should develop approaches and commitments to advance equity in the health and well-being for these children and families.
For much of the world, working within the realm of children’s rights provides a strategy and moral and legal basis for our efforts. Pediatricians need to work with colleagues in a transdisciplinary approach to ensure all children live in nurturing, rights-respecting environments. Our ongoing efforts should include encouraging academic institutions to assist with professional education, research, and evaluation in this regard. We need evaluations that contribute to continuous quality improvement in our efforts and integrate the metrics of child rights, social justice, and health equity into our care of children on the move.
We call on other national and international public, private, and academic sector organizations to advance the health and well-being of children and youth on the move. These children and families are depending on us to do so.
For the complete text of the Budapest Declaration, see www.issop.org/2017/11/10/budapest-declaration-rights-health-well-children-youth-move/.
Dr. Rushton is medical director of the South Carolina Quality Through Innovation in Pediatrics (SCQTIP) network. Dr. Goldhagen is president-elect, International Society for Social Pediatrics and Child Health, and professor of pediatrics, University of Florida, Jacksonville. Email them at pdnews@frontlinemedcom.com.
In late October 2017, pediatricians from over 25 countries gathered at the annual meeting of the International Society of Social Pediatrics and Child Health in Budapest to develop strategies to address the health and well-being of children on the move. With the staggering global increase in the displacement of children – due to conflict, climate change, natural disasters, and economic deprivation – there is a critical need for action.
An international slate of experts, including pediatricians who are responding to the needs of these children, provided an evidence base for intervention and action. These discussions informed the development of a consensus statement, the Budapest Declaration, that establishes a framework for a global response to the exigencies confronting these children and families.
The Budapest Declaration is grounded in children’s rights as guaranteed in the United Nation’s Convention on the Rights of the Child (CRC). All countries in the world have signed onto the CRC, with a notable exception being the United States. But leading American pediatric groups, including the American Academy of Pediatrics, have endorsed the CRC, making it a basis of organizational advocacy. The CRC entitles all children to optimal survival and development (Article 6) and optimal health and health care (Article 24).
As prescribed by these accepted legal rights for children, children and youth on the move are entitled to the same services, including health care, as resident populations of children, regardless of their legal status. Countries can be held accountable to ensure they receive the full rights due them as articulated in the CRC. Furthermore, efforts to do so should be assessed in national periodic reports to the Committee on the Rights of the Child to ensure accountability.
The relevance of the Budapest Declaration to the United States cannot be overstated. National and regional public policy throughout our country, and in particular along our Southern border, is having a devastating effect on the physical and mental health of children and youth on the move – and will continue to do so throughout their life course.
The involvement of pediatricians and other child health providers is essential to the planning and implementation of clinical and public health programs for children on the move. Child health professionals, in conjunction with their professional organizations, must be engaged in all aspects of local, national, and global responses. Leadership and contributions by pediatricians and pediatric societies and academic institutions are integral to the success of key partners, such as UNICEF, the World Health Organization, the International Organization for Migration, and the UN High Commissioner for Refugees.
Systems of care should be provided that address the physical, mental, and social health care needs of these children without bias and prejudice. Pregnant mothers on the move also should receive services to ensure the delivery of healthy newborns. Every nation should develop approaches and commitments to advance equity in the health and well-being for these children and families.
For much of the world, working within the realm of children’s rights provides a strategy and moral and legal basis for our efforts. Pediatricians need to work with colleagues in a transdisciplinary approach to ensure all children live in nurturing, rights-respecting environments. Our ongoing efforts should include encouraging academic institutions to assist with professional education, research, and evaluation in this regard. We need evaluations that contribute to continuous quality improvement in our efforts and integrate the metrics of child rights, social justice, and health equity into our care of children on the move.
We call on other national and international public, private, and academic sector organizations to advance the health and well-being of children and youth on the move. These children and families are depending on us to do so.
For the complete text of the Budapest Declaration, see www.issop.org/2017/11/10/budapest-declaration-rights-health-well-children-youth-move/.
Dr. Rushton is medical director of the South Carolina Quality Through Innovation in Pediatrics (SCQTIP) network. Dr. Goldhagen is president-elect, International Society for Social Pediatrics and Child Health, and professor of pediatrics, University of Florida, Jacksonville. Email them at pdnews@frontlinemedcom.com.
In late October 2017, pediatricians from over 25 countries gathered at the annual meeting of the International Society of Social Pediatrics and Child Health in Budapest to develop strategies to address the health and well-being of children on the move. With the staggering global increase in the displacement of children – due to conflict, climate change, natural disasters, and economic deprivation – there is a critical need for action.
An international slate of experts, including pediatricians who are responding to the needs of these children, provided an evidence base for intervention and action. These discussions informed the development of a consensus statement, the Budapest Declaration, that establishes a framework for a global response to the exigencies confronting these children and families.
The Budapest Declaration is grounded in children’s rights as guaranteed in the United Nation’s Convention on the Rights of the Child (CRC). All countries in the world have signed onto the CRC, with a notable exception being the United States. But leading American pediatric groups, including the American Academy of Pediatrics, have endorsed the CRC, making it a basis of organizational advocacy. The CRC entitles all children to optimal survival and development (Article 6) and optimal health and health care (Article 24).
As prescribed by these accepted legal rights for children, children and youth on the move are entitled to the same services, including health care, as resident populations of children, regardless of their legal status. Countries can be held accountable to ensure they receive the full rights due them as articulated in the CRC. Furthermore, efforts to do so should be assessed in national periodic reports to the Committee on the Rights of the Child to ensure accountability.
The relevance of the Budapest Declaration to the United States cannot be overstated. National and regional public policy throughout our country, and in particular along our Southern border, is having a devastating effect on the physical and mental health of children and youth on the move – and will continue to do so throughout their life course.
The involvement of pediatricians and other child health providers is essential to the planning and implementation of clinical and public health programs for children on the move. Child health professionals, in conjunction with their professional organizations, must be engaged in all aspects of local, national, and global responses. Leadership and contributions by pediatricians and pediatric societies and academic institutions are integral to the success of key partners, such as UNICEF, the World Health Organization, the International Organization for Migration, and the UN High Commissioner for Refugees.
Systems of care should be provided that address the physical, mental, and social health care needs of these children without bias and prejudice. Pregnant mothers on the move also should receive services to ensure the delivery of healthy newborns. Every nation should develop approaches and commitments to advance equity in the health and well-being for these children and families.
For much of the world, working within the realm of children’s rights provides a strategy and moral and legal basis for our efforts. Pediatricians need to work with colleagues in a transdisciplinary approach to ensure all children live in nurturing, rights-respecting environments. Our ongoing efforts should include encouraging academic institutions to assist with professional education, research, and evaluation in this regard. We need evaluations that contribute to continuous quality improvement in our efforts and integrate the metrics of child rights, social justice, and health equity into our care of children on the move.
We call on other national and international public, private, and academic sector organizations to advance the health and well-being of children and youth on the move. These children and families are depending on us to do so.
For the complete text of the Budapest Declaration, see www.issop.org/2017/11/10/budapest-declaration-rights-health-well-children-youth-move/.
Dr. Rushton is medical director of the South Carolina Quality Through Innovation in Pediatrics (SCQTIP) network. Dr. Goldhagen is president-elect, International Society for Social Pediatrics and Child Health, and professor of pediatrics, University of Florida, Jacksonville. Email them at pdnews@frontlinemedcom.com.
Cosmetic Corner: Dermatologists Weigh in on Pigment Correctors
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.